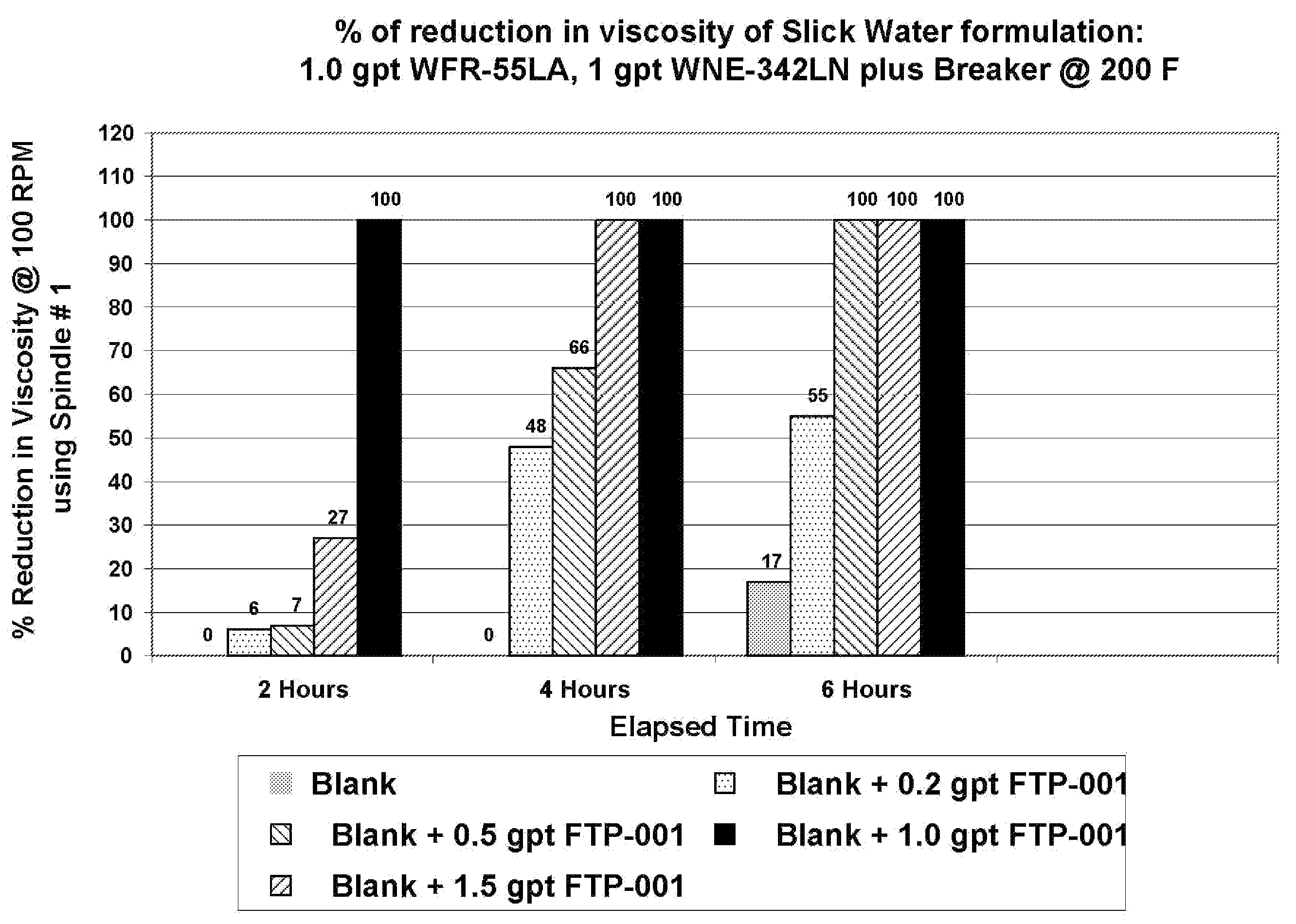
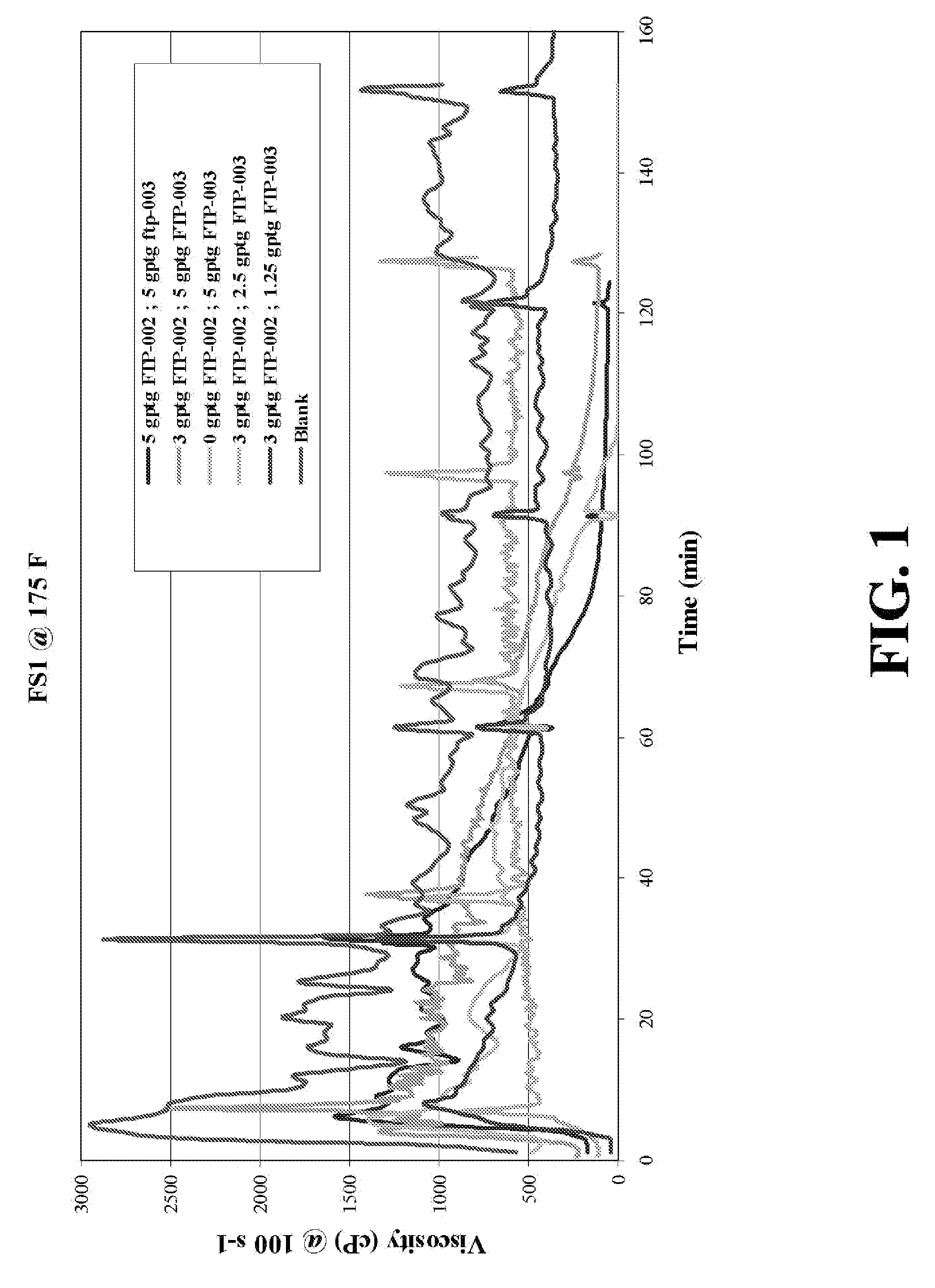
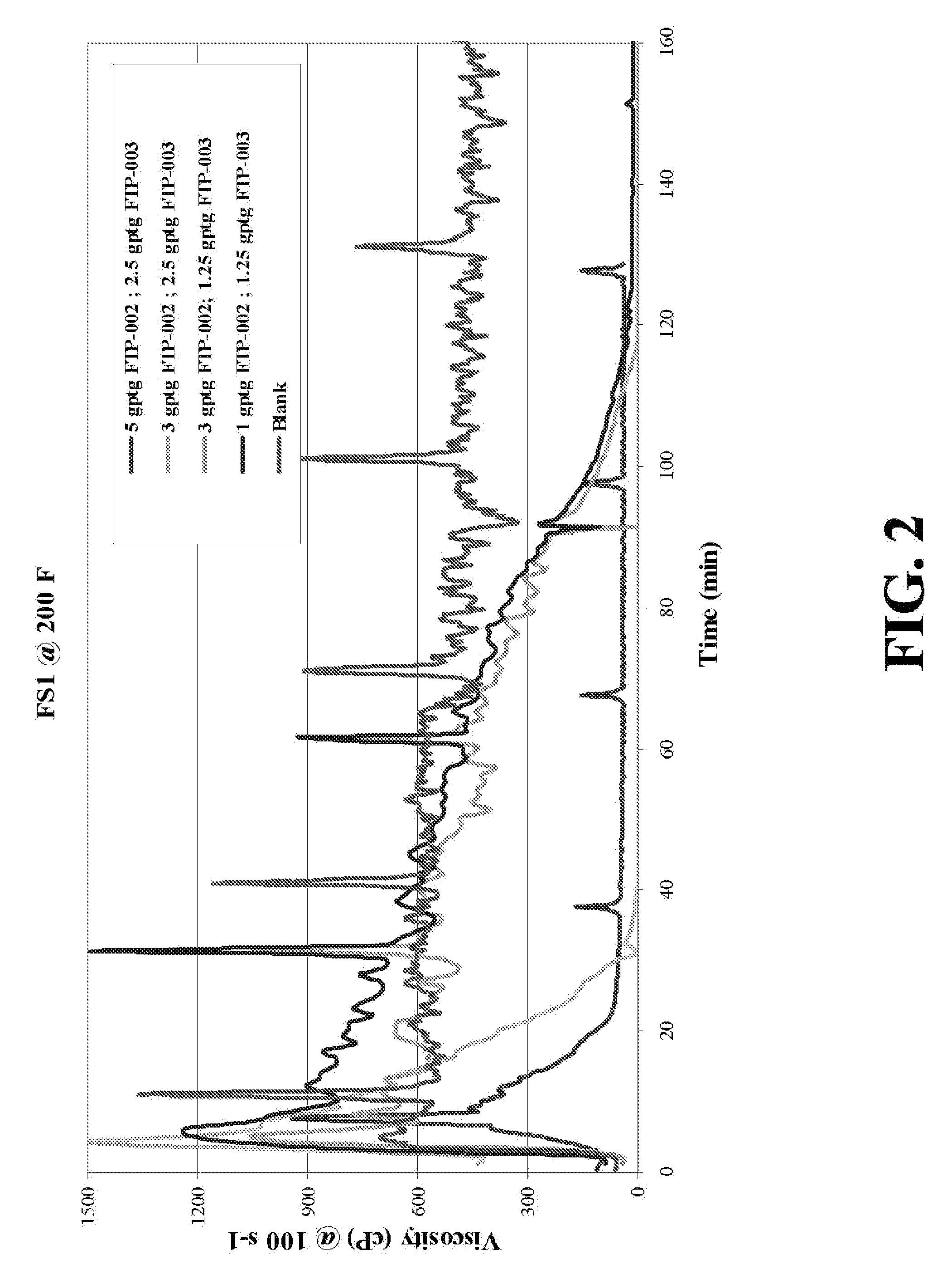
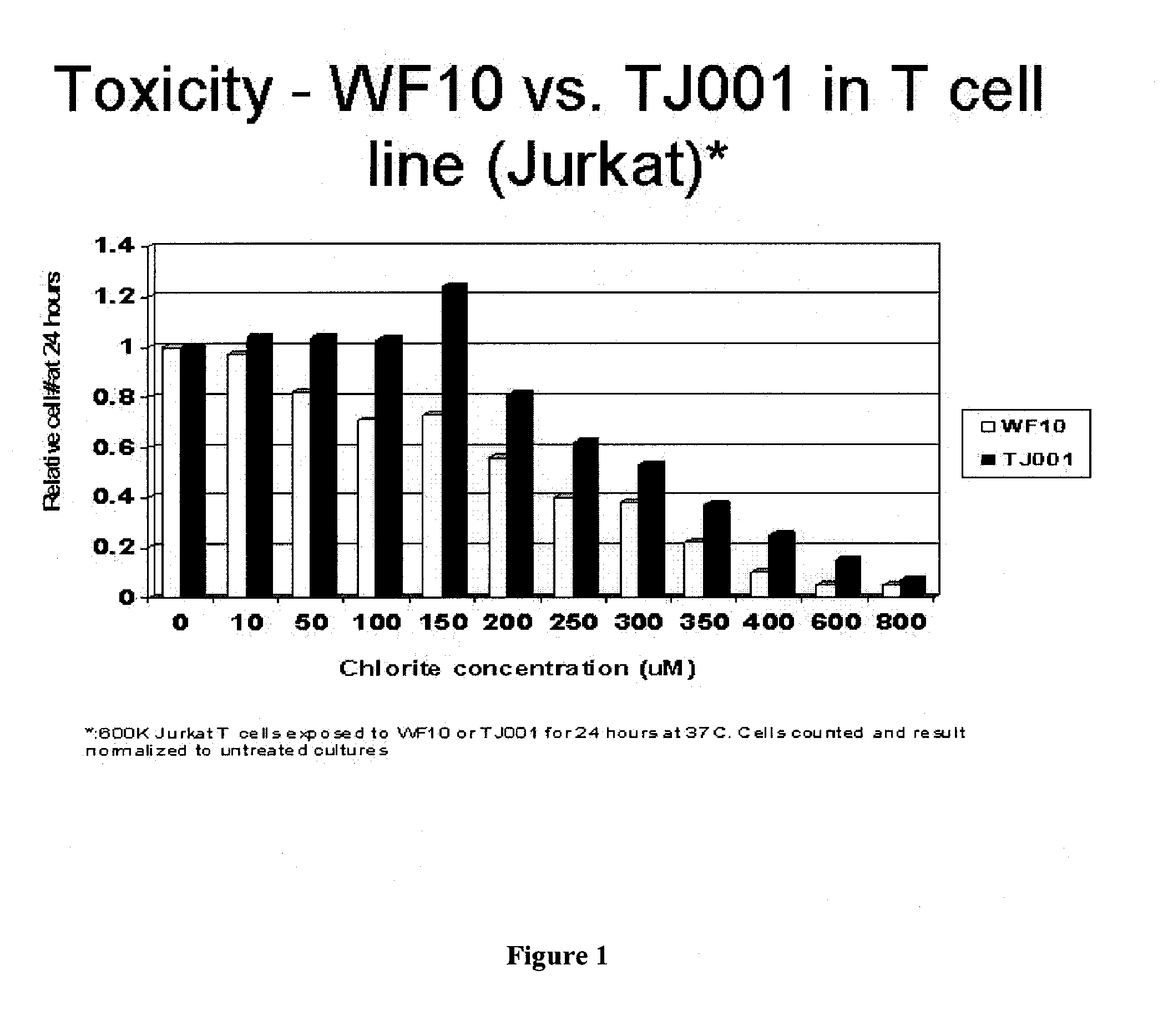
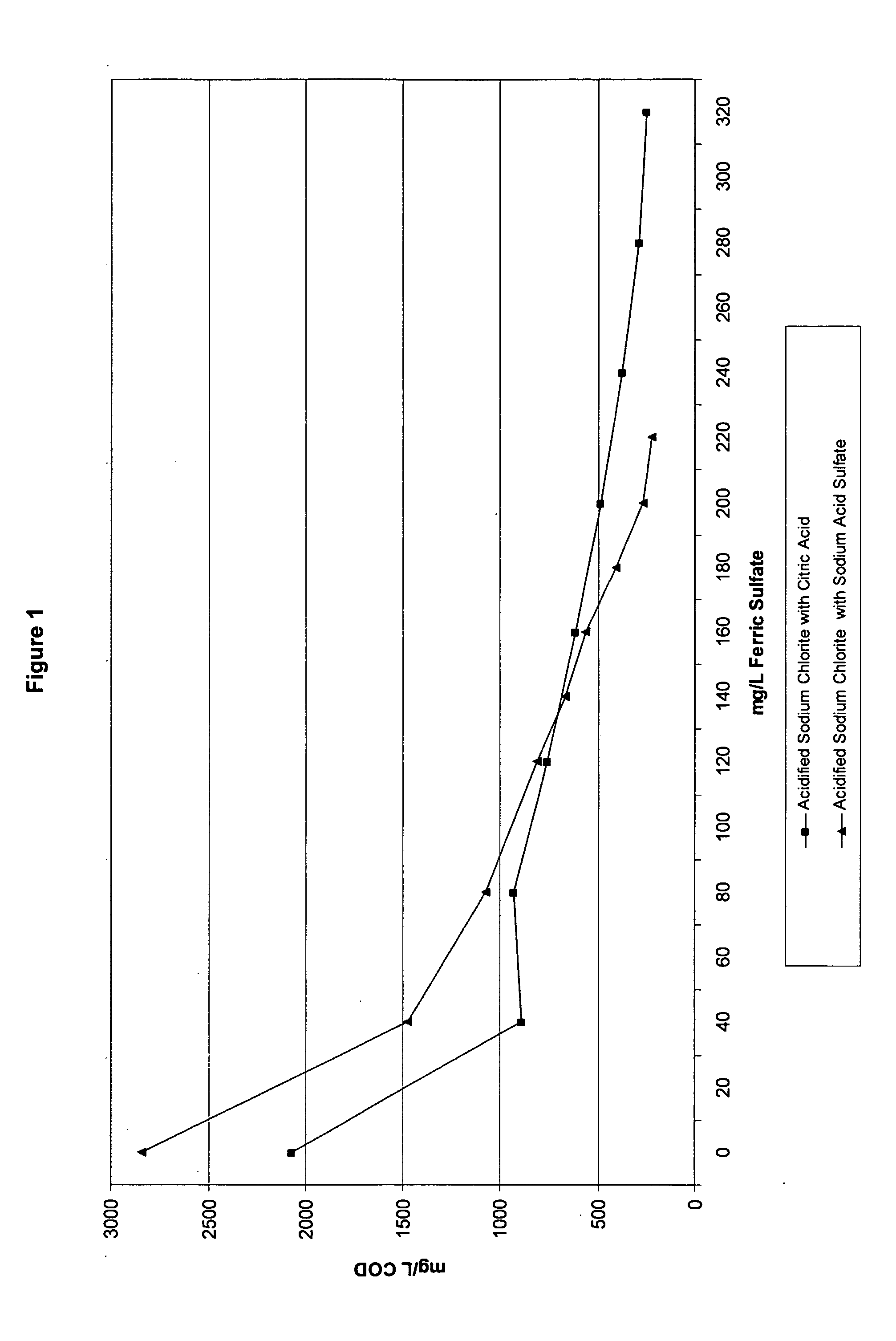
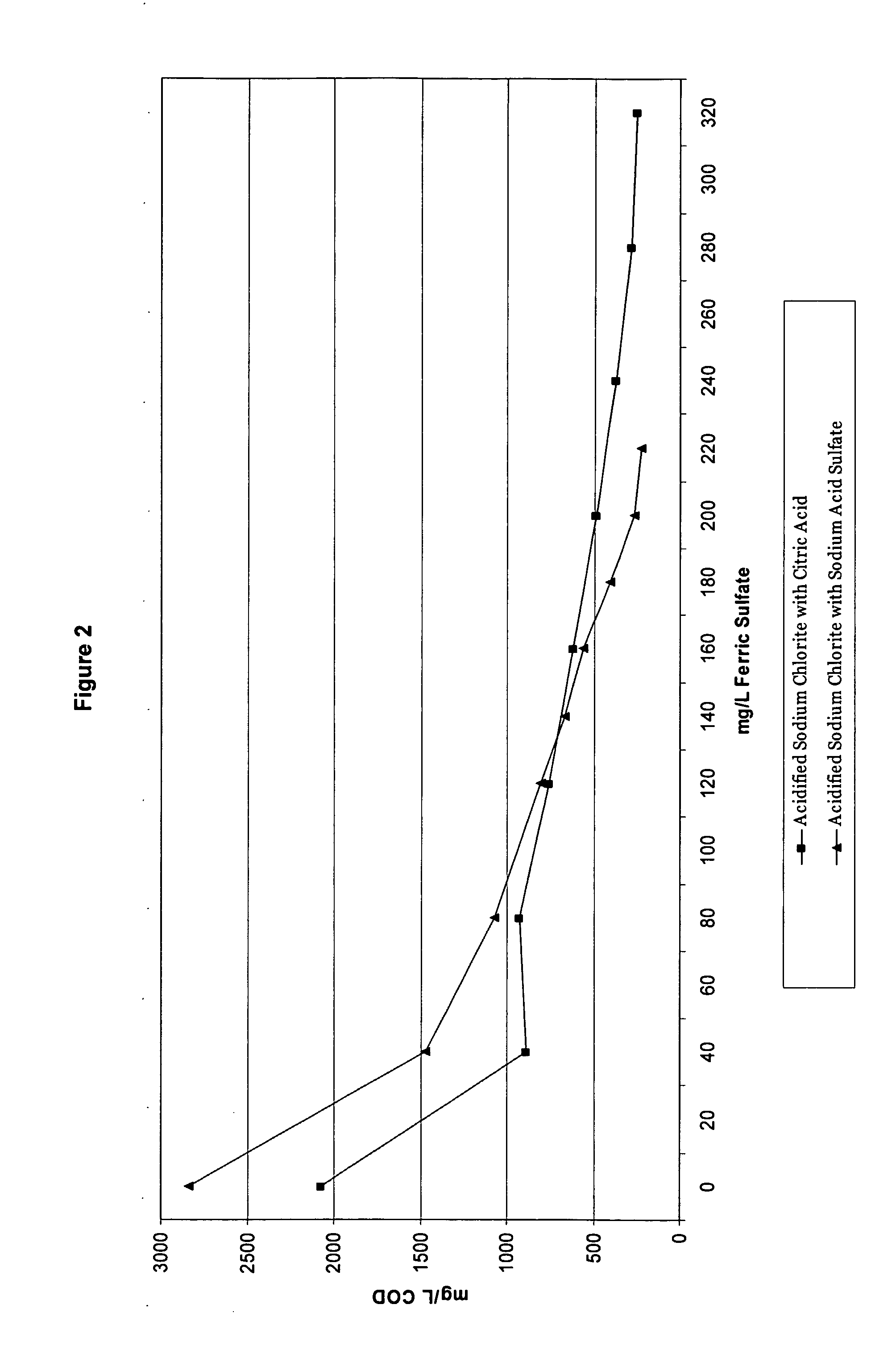
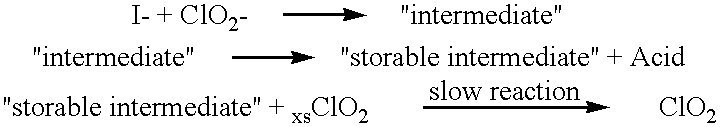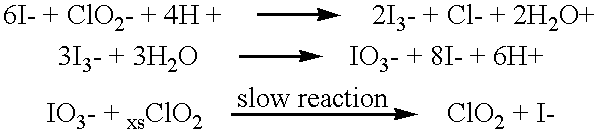Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
713 results about "Chlorite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The chlorite ion, or chlorine dioxide anion, is the halite with the chemical formula of ClO⁻₂. A chlorite (compound) is a compound that contains this group, with chlorine in the oxidation state of +3. Chlorites are also known as salts of chlorous acid.
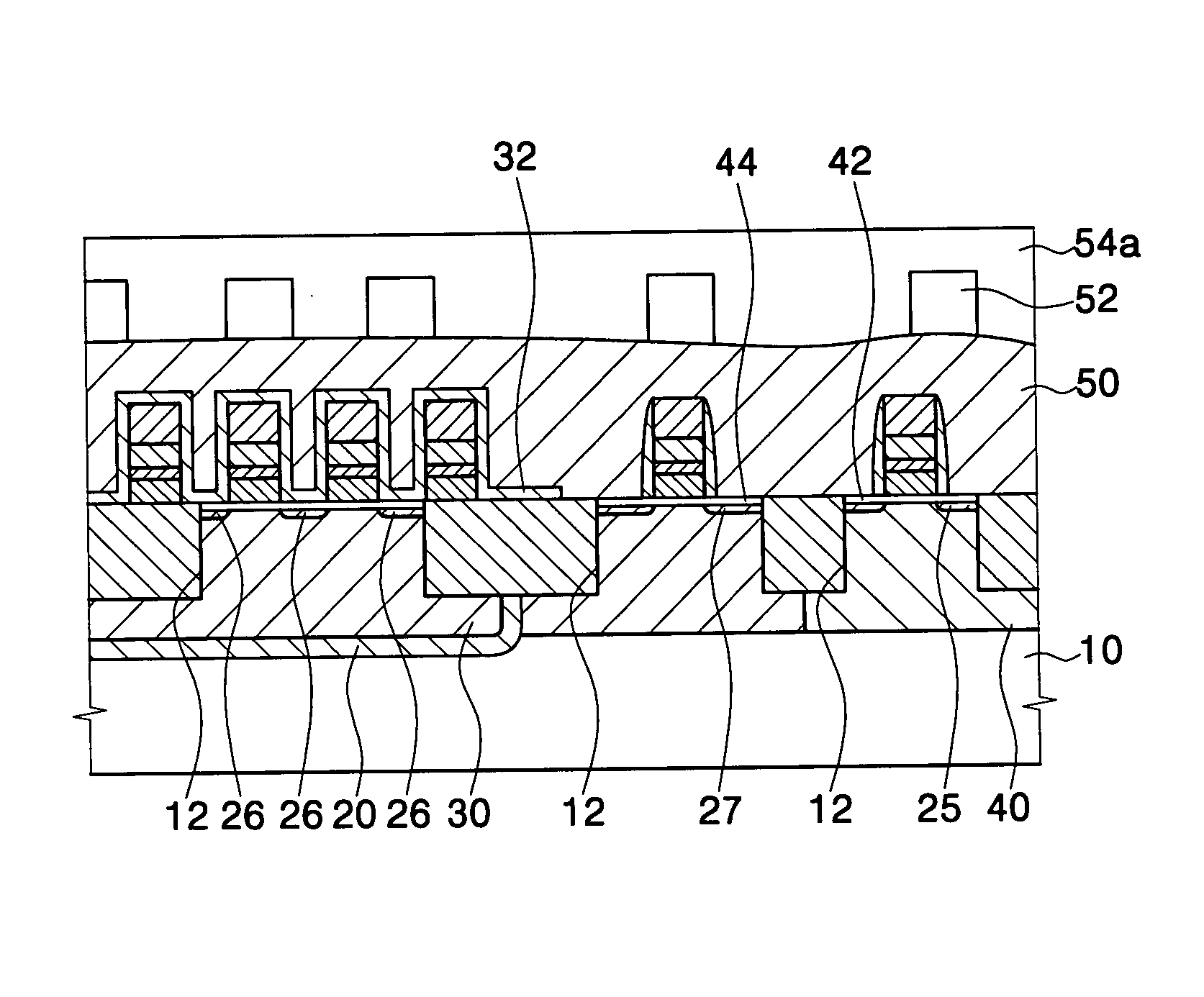
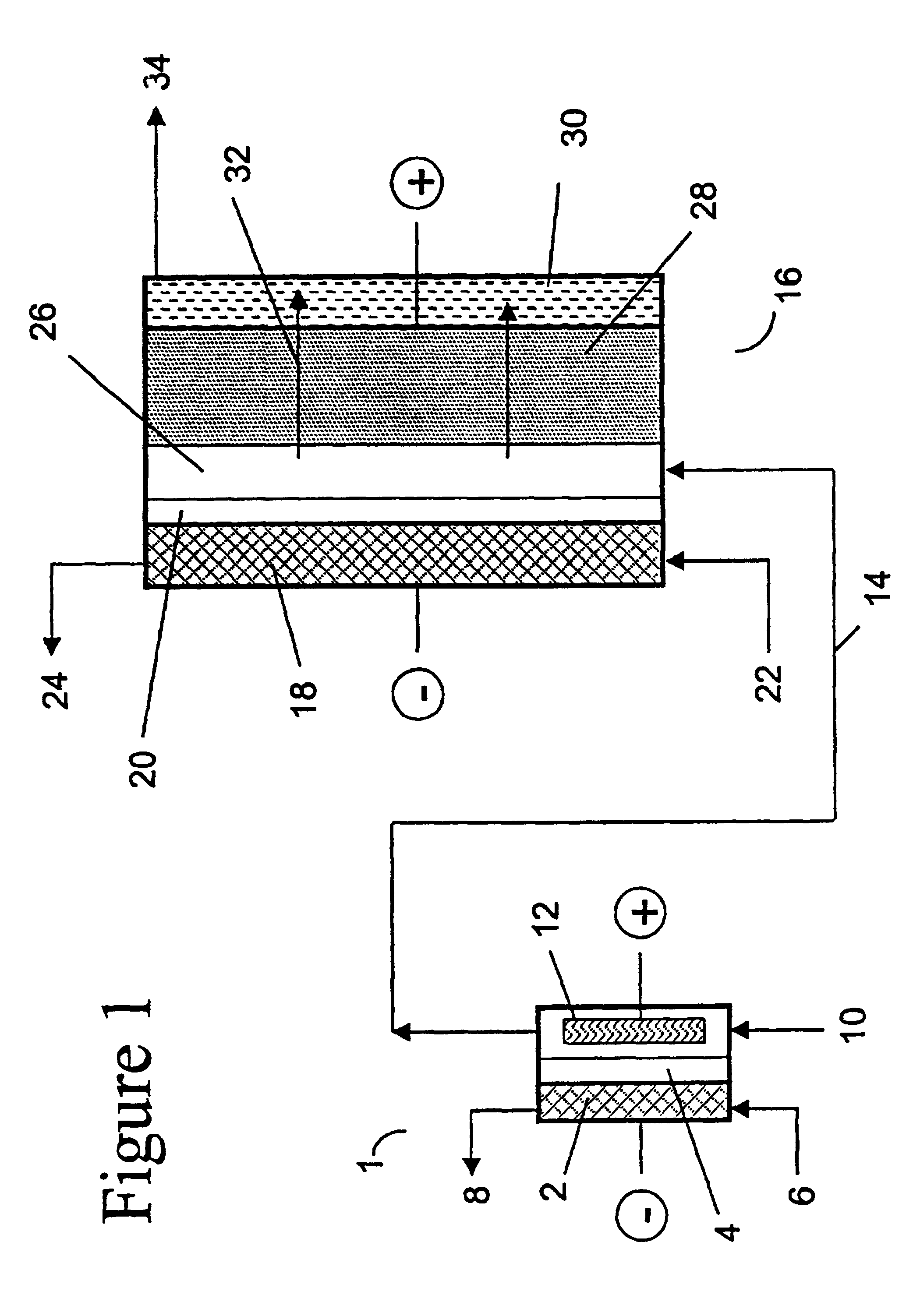
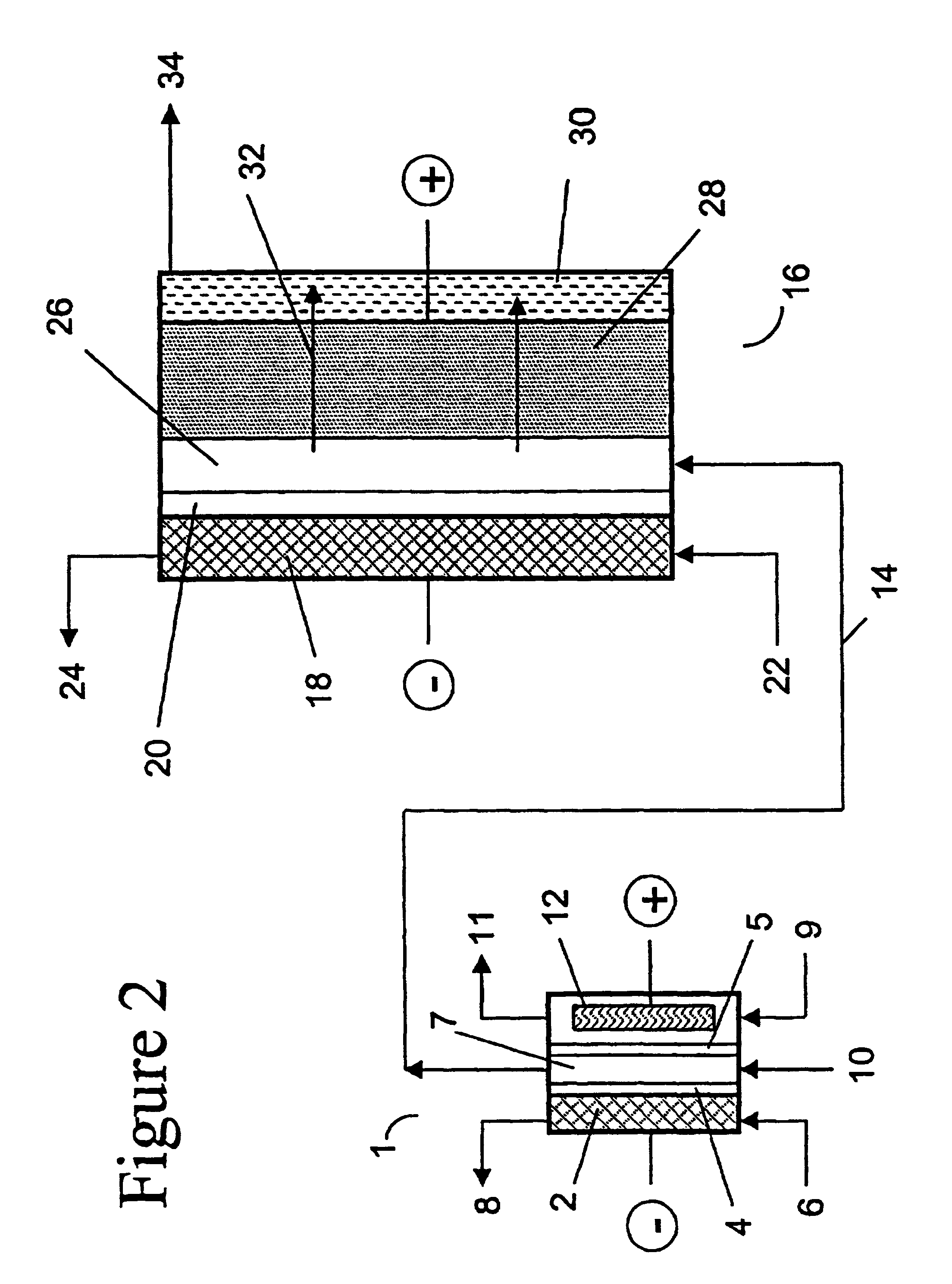
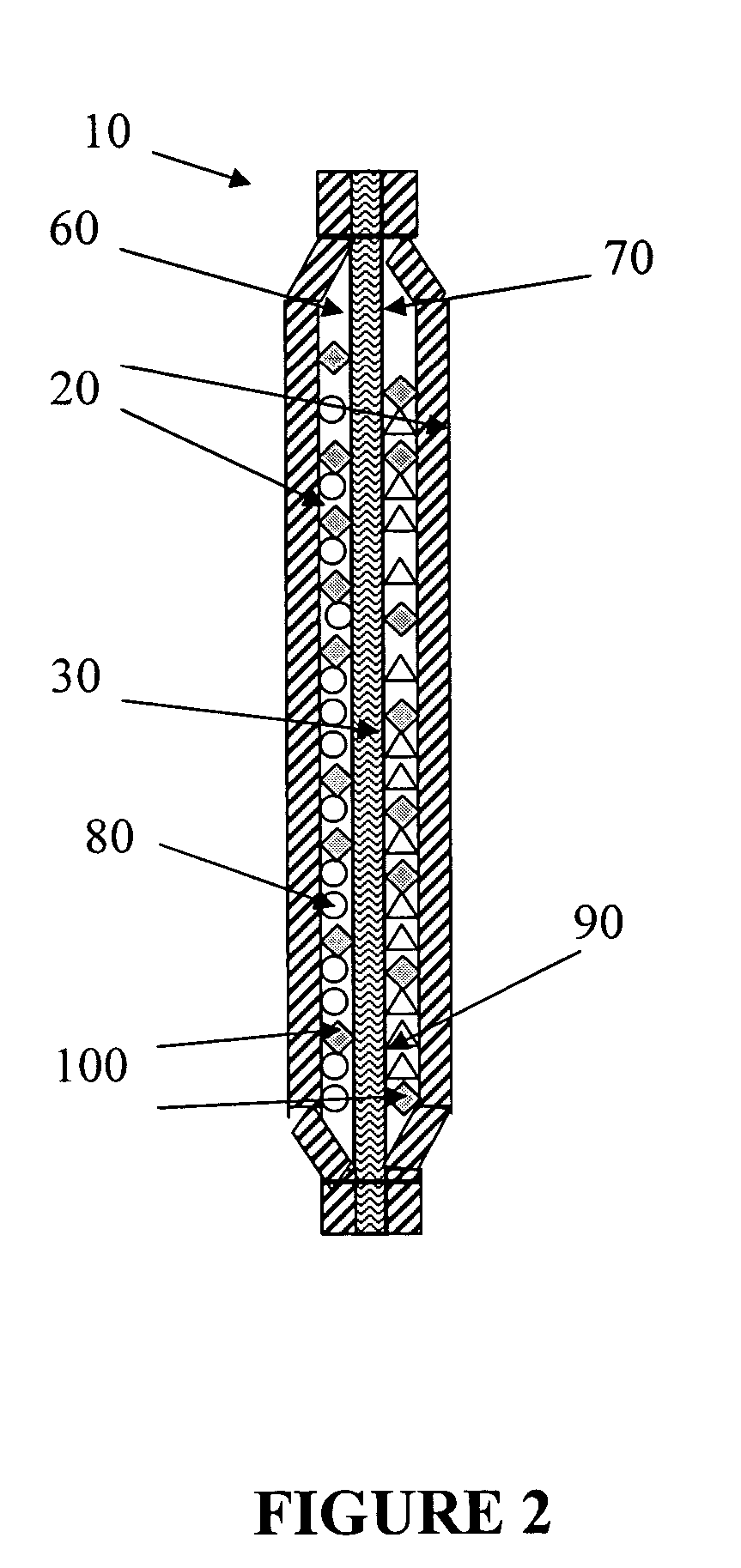
Method for forming a silicon oxide layer using spin-on glass
A method is provided for forming silicon oxide layers during the processing of semiconductor devices by applying a SOG layer including polysilazane to a substrate and then substantially converting the SOG layer to a silicon oxide layer using an oxidant solution. The oxidant solution may include one or more oxidants including, for example, ozone, peroxides, permanganates, hypochlorites, chlorites, chlorates, perchlorates, hypobromites, bromites, bromates, hypoiodites, iodites, iodates and strong acids.
Owner:SAMSUNG ELECTRONICS CO LTD
Method of making molecular chlorine dioxide
A method for manufacturing molecular chlorine dioxide, by the addition of potassium iodide to a solution of alkali metal chlorite. The metal chlorite and the potassium iodide are kept separate, until the need for the generation of chlorine dioxide arises-to ensure long-shelf life. After initiation or activation of the chlorite anion to form chlorine dioxide, the beneficial properties of chlorine dioxide can be used, for different health and cosmetic purposes. Such uses include the treatment of herpes, dandruff, acne, skin rashes (e.g. poison ivy), ulcers, bed sores, warts, nail fungus, athletes foot, sun burn and gum disease; and as an antiseptic, disinfectant, and general deodorant form refrigerator sprays to oral mouthrinses.
Owner:MADRAY GEORGE
Methods of simultaneously cleaning and disinfecting industrial water systems
InactiveUS6840251B2Reduce Microbial ContaminationReduce removalDetergent bleaching agentsWater/sewage treatment by neutralisationChlorine dioxideOnline and offline
On-Line and Off-Line methods of simultaneously cleaning and disinfecting an industrial water system are described and claimed. The methods involve the addition to the water of the industrial water system of a Compound selected from the group consisting of the alkali salts of chlorite and chlorate and mixtures thereof; and an acid, followed by allowing the water in the industrial water system to circulate for several hours. The reaction of the alkali salts of chlorite and chlorate and acid produces chlorine dioxide in-situ in the water of the industrial water system. The chlorine dioxide kills microorganisms and the acid acts to remove deposits upon the water-contact surfaces of the equipment. An alternative method involves the use of a chelating agent and a biocide. Other possible cleaning and disinfection reagents may be added as needed including corrosion inhibitors, chelating agents, biocides, surfactants and reducing agents. These cleaning and disinfecting methods work in a variety of industrial water systems including cooling water and boiler water systems.
Owner:ECOLAB USA INC
Oxidative systems for breaking polymer viscosified fluids
A fluid breaking composition including an effective amount of an alkali chlorite and / or hyperchlorite is disclosed, where the composition reduces a viscosity of a polymer viscosified fluid to a desired low value at down hole conditions within a time period coincident with a formation stimulation time, generally between about 30 minutes and 195 minutes. Methods for making and using the breaking composition are also disclosed.
Owner:WEATHERFORD TECH HLDG LLC
Thickened fluid composition comprising chlorine dioxide
This invention relates to a stable composition and method of making a thickened fluid composition comprising chlorine dioxide. The stable composition includes a mixture containing a chlorite, an acid source, and a thickener component. At least one of the chlorite, the acid source and the thickener component is in particulate form. In an embodiment, the mixture may be a unitary solid body wherein chlorine dioxide is generated upon interaction with an aqueous medium, such as water. A free halogen source may also be added to the mixture in some cases.
Owner:ENGELHARD CORP
Massive bodies containing free halogen source for producing highly converted thickened solutions of chlorine dioxide
A massive body, e.g., a tablet, for producing a thickened solution of chlorine dioxide when the massive body is added to liquid water is disclosed. The massive body comprises a metal chlorite, an acid source and a thickener (incorporated directly into the massive body or added as a component separate from the massive body) and optionally a source of free halogen. The concentration of free chlorine in the solution will be: (a) less than the concentration of chlorine dioxide in said solution on a weight basis and the ratio of the concentration of chlorine dioxide to the sum of the concentrations of chlorine dioxide and chlorite anion in said solution is at least 0.25:1 by weight; or (b) equal to or greater than the concentration of chlorine dioxide in said solution on a weight basis and the ratio of the concentration of chlorine dioxide to the sum of the concentrations of chlorine dioxide and chlorite anion in said solution is at least 0.50:1 by weight.
Owner:BASF AG
Use of xanthan gum for gelling CIO2 and related species
The invention described relates to gelled chlorine dioxide compositions comprising water, an acid, a water-soluble chlorite salt and a gelling agent in two parts A and B, wherein the gelling agent is xanthan gum or a mixture of xanthan gum and an acid stable gelling agents. Compositions according to the invention are storage stable for at least 6 months, preferably at least about year.
Owner:ALLIGER HOWARD
Treatment of macrophage-related disorders
The present invention provides a method of treating a macrophage related disease comprising administering to a subject in need thereof an effective amount of an oxidative agent or an immunosuppressive agent. The present invention also provides a method of modulating macrophage accumulation or activation comprising administering to a subject in need thereof an effective amount of an oxidative agent or an immunosuppressive agent. The oxidative agent can be chlorite or a chlorite containing compound.
Owner:RGT UNIV OF CALIFORNIA +1
Oral composition for reducing plaque and microbial infections and whitening teeth
InactiveUS20060045855A1Reduce riskWhitening teethCosmetic preparationsOrganic active ingredientsAdditive ingredientMicrobial agent
A dental mouthwash product, and method of use, comprising a water-soluble chlorite compound in a first solution, a hydrogen peroxide and a physiologically acceptable readily water-soluble oxidizing agent in a second solution, or optionally the oxidizing agent separate from the second solution as a powder, tablet, gel capsule, or liquid, wherein said oxidizing agent oxidizes chlorite to ClO2 within seconds, wherein the three ingredients are mixed to provide the mouthwash product. The mouthwash product may be used for any one or more of the following: as an antimicrobial agent for treating dental disease such as dental caries, plaque, gum disease, and / or bad breath, for strengthening gum and ligament attachments to the teeth, or for whitening teeth.
Owner:SASSON J ALAN
Electrolytic process for producing chlorine dioxide
InactiveUS6203688B1Improved conversion efficiency per passMinimize or prevent any side anodic oxidation reactionsCellsChlorine dioxideElectrochemical cell
A process for converting in a single pass an aqueous alkaline pH, alkali metal chlorite solution into an aqueous chlorine dioxide-containing solution that involves the combination of (1) using an electrochemical acidification cell to lower the pH value of the aqueous alkali metal chlorite feed before it enters the anode compartment of an electrochemical oxidation cell where the chlorite is converted to chlorine dioxide with (2) using an anolyte flow pattern where the anolyte passes through a porous, high surface area electrode. This process results in a substantially improved conversion efficiency per pass.
Owner:STERLING PULP CHEM +2
Massive bodies containing free halogen source for producing highly converted solutions of chlorine dioxide
InactiveUS7182883B2Increase conversion rateImprove permeabilityBiocideOrganic chemistryHalogenLiquid water
A massive body, e.g., a tablet, for producing a solution of chlorine dioxide when the massive body is added to liquid water. The massive body comprises a metal chlorite such as sodium chlorite, an acid source such as sodium bisulfate and a source of free halogen such as the sodium salt of dichloroisocyanuric acid or a hydrate thereof. The concentration of free halogen in the solution will be:(a) less than the concentration of chlorine dioxide in said solution on a weight basis and the ratio of the concentration of chlorine dioxide to the sum of the concentrations of chlorine dioxide and chlorite anion in said solution is at least 0.25:1 by weight; or(b) equal to or greater than the concentration of chlorine dioxide in said solution on a weight basis and the ratio of the concentration of chlorine dioxide to the sum of the concentrations of chlorine dioxide and chlorite anion in said solution is at least 0.50:1 by weight.
Owner:ENGELHARD CORP
Unitary solid chlorine dioxide effervescent tablet and preparation method thereof
InactiveCN101228868AFast dissolutionImprove chemical yieldBiocideDisinfectantsEffervescent tabletChlorine dioxide
The invention discloses a one-variable solid effervescent tablet and a preparation method for the tablet. The material system of the effervescent tablet comprises chlorite, solid acid source, free halogen source, activated Promoter, desiccant, bond, release agent, effervescent and surfactant. The preparation technique includes: after the coating processing with chlorite grains, drying and mixing well with other raw materials according to a specific proportion and technique requirements to prepare compound raw materials, and then pressing into tablets with different specification and shaping on the tablet machine to fit for application in various occasions. The effervescent tablet can quickly release chlorine dioxide and acquire pellucid water solution of chlorine dioxide while dissolving in the water, which is high in stability, rapid in dissolving rapid and strong in bactericidal ability.
Owner:石家庄卫科生物科技有限公司
Methods and viscosified compositions for treating wells
The present invention relates to methods of treating subterranean formations with viscosified aqueous well treating compositions which break into thin fluids at static temperatures in the range of from about 150° F. to about 200° F. A breaker system is included in the compositions comprised of an alkali metal or ammonium persulfate breaker and a breaker activity delaying agent comprised of an alkali metal chlorite or hypochlorite.
Owner:HALLIBURTON ENERGY SERVICES INC
Method and system for the controlled release of chlorine dioxide gas
Method, composition and system for generating chlorine dioxide gas in a controlled release manner by combining at least one metal chlorite and a dry solid hydrophilic material that reacts with the metal chlorite in the presence of water vapor, but does not react with the metal chlorite in the substantial absence of liquid water or water vapor to produce chlorine dioxide gas in a sustained amount of from about 0.001 to 1,000 ppm.
Owner:BASF CATALYSTS LLC
Methods for whitening teeth
Method for whitening teeth are provided. The methods include the steps of providing a strip of material and applying a thin layer of a tooth whitening substance having a whitening active selected from the group consisting of peroxides, metal chlorites, perborates, percarbonates, peroxyacids, hypochlorites, and combinations thereof to a front surface of a plurality of teeth, wherein the amount of the tooth whitening substance is between about 0.05 grams and about 0.4 grams. The method further includes the steps of conforming the strip of material to the front surface of the plurality of teeth and removing the strip of material, wherein the front surface of the plurality of teeth have between a 1 and a 4 VITA LUMIN shade guide improvement in color.
Owner:THE PROCTER & GAMBLE COMPANY
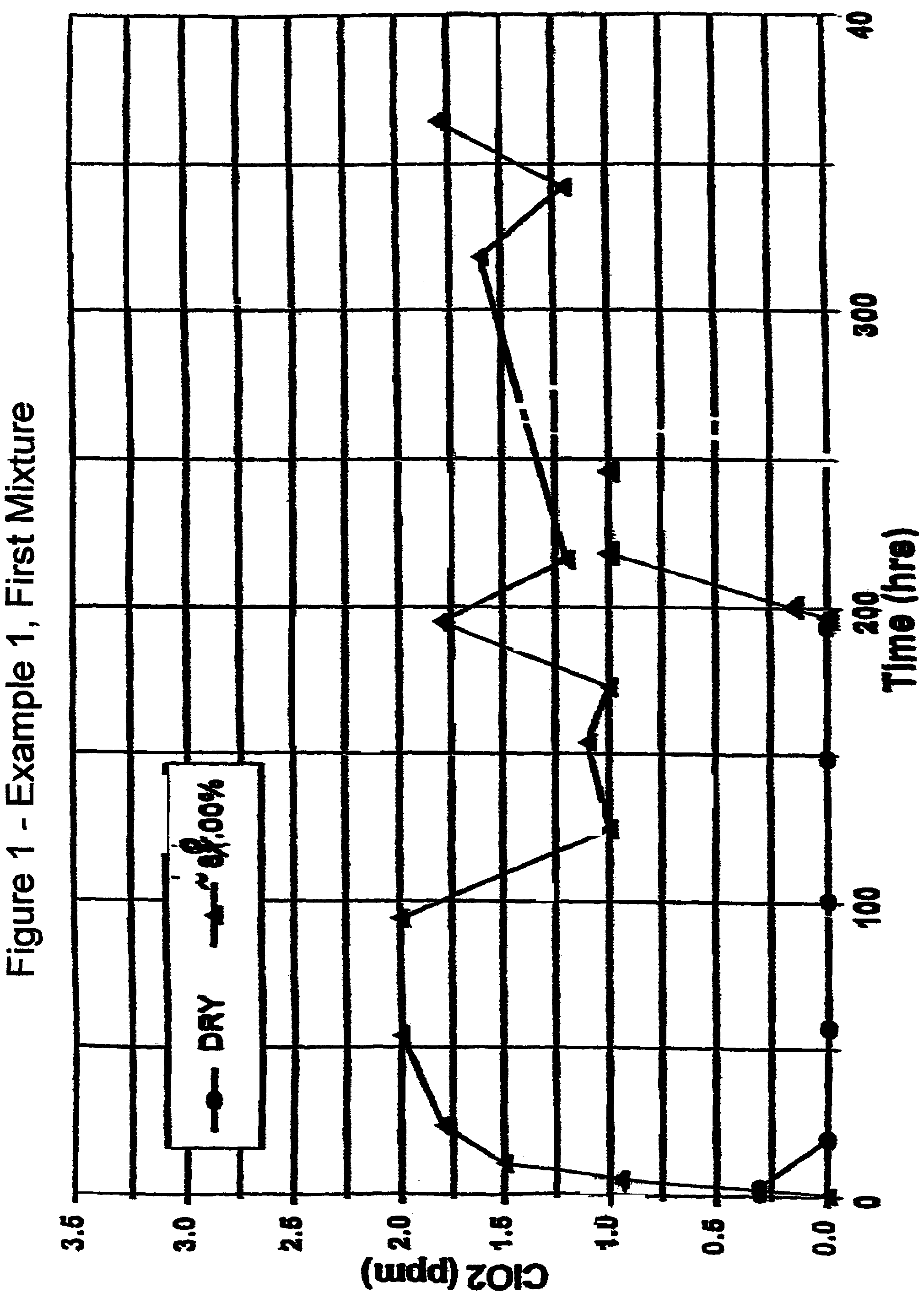
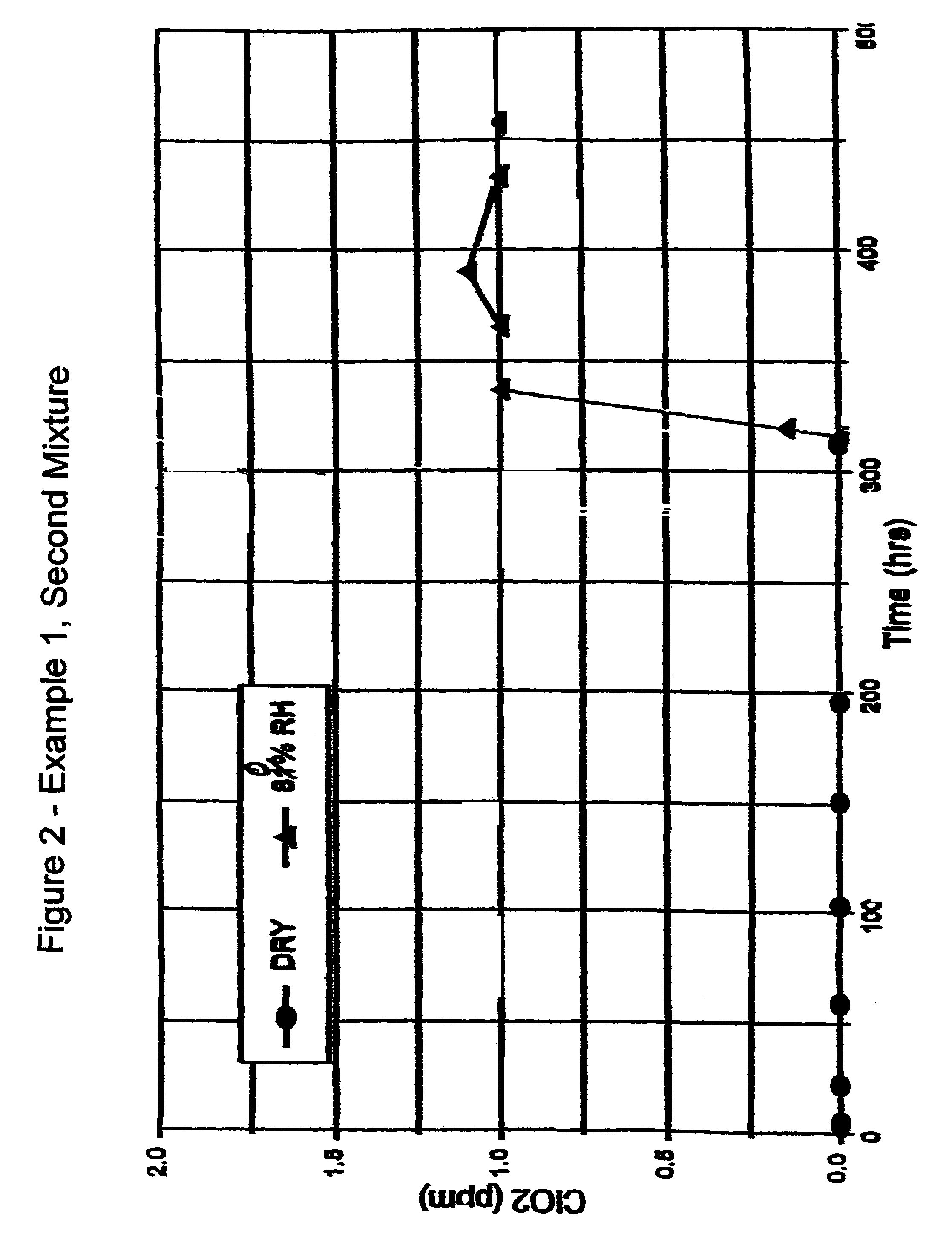
Compositions and methods for storing aqueous chlorine dioxide solutions
InactiveUS6284152B1Little and no diffusional lossLoss and degradationCosmetic preparationsBiocideDiffusionChlorine dioxide
The present invention is directed to both physical and chemical means for maintaining continuous high levels of chlorine dioxide in aqueous solutions for extended periods, with minimum loss through degradation in the solution and dissipation through the walls of the containing vessel within which the solution is stored. The ClO2 may be maintained even in the absence of very high levels of chlorite, from which supplemental, offsetting levels of ClO2 may be generated. The present invention relates to the discovery that certain types of plastic and glass containers have the ability to maintain relatively constant levels of ClO2 in their contained solutions over extended periods, particularly in aqueous compositions comprising chlorite / ClO2 ratios sufficient for a stabilizing complex of the two, presumably Cl204-, to suppress the ClO2 loss. Of particular value is certain high-density polyethylene terephthalate (PETE) plastic bottling and plastic containers which have been initially exposed to a fluorinating environment. Specifically, in the latter regard, it has been discovered that certain plastic containers which ordinarily are incapable of maintaining aqueous ClO2 levels can be treated with fluorine gas to transform their surfaces so as to effectively suppress ClO2 diffusion therethrough.
Owner:PROFRESH PROPERTIES
Electrochemical fabrication methods with enhanced post deposition processing
InactiveUS20050029225A1Improved post deposition processingDecorative surface effects3D structure electroformingAlcoholNickel alloy
An electrochemical fabrication process for producing three-dimensional structures from a plurality of adhered layers is provided where each layer comprises at least one structural material (e.g. nickel or nickel alloy) and at least one sacrificial material (e.g. copper) that will be etched away from the structural material after the formation of all layers have been completed. An etchant containing chlorite (e.g. Enthone C-38) is combined with a corrosion inhibitor (e.g. sodium nitrate) to prevent pitting of the structural material during removal of the sacrificial material. A simple process for drying the etched structure without the drying process causing surfaces to stick together includes immersion of the structure in water after etching and then immersion in alcohol and then placing the structure in an oven for drying.
Owner:UNIV OF SOUTHERN CALIFORNIA
Electrolytic process for generating chlorine dioxide
An electrolytic process for generating chlorine dioxide. An aqueous feed stream of an alkali metal chlorite solution is treated with chlorine gas or a mixture of hydrogen chloride and hypochlorous acid formed in an anode compartment from, an aqueous alkali metal chloride solution and subsequently electrolyzed to form a chlorine dioxide effluent.
Owner:NALCO CO +1
Device and methods for the production of chlorine dioxide vapor
InactiveUS20060039840A1High moisture levelRapid productionDeodrantsGas generation devicesChlorine dioxideLiquid water
A device for producing chlorine dioxide vapor in a time release manner when exposed to ambient moisture is provided. The device comprises two outer membranes and an inner membrane. The outer and inner membranes are sealed together along the edges of the device. The outer and inner membranes together form a pouch comprising two separate compartments separated by the inner membrane. Each compartment is provided with a dry reactant (e.g. an acid component and a metal chlorite component) for producing chlorine dioxide vapor. The outer membranes are permeable to moisture and chlorine dioxide vapor, and impervious to liquid water and the dry reactants. The acid component in one of the compartments is hydrated by the moisture penetrated through the outer membranes, and the hydrated acid component is absorbed by the inner membrane and transported across the inner membrane to come into contact with the metal chlorite component in the other compartment to produce chlorine dioxide vapor. The chlorine dioxide vapor eventually slowly diffuses out of outer membranes into the surrounding environment.
Owner:AVANTEC TECH
Methods and Solid Compositions for Generating Soapy and Non-Soapy Aqueous Solutions Containing Free Chlorine Dioxide
InactiveUS20080067470A1Improve stabilityThe result is stableOther chemical processesChlorine dioxideChlorine dioxideSolid acid
Some embodiments of the invention provide solid compositions that, when exposed to or otherwise placed in an aqueous solution, will release chlorine dioxide and surfactants, producing a soapy, aqueous solution containing chlorine dioxide. These solid compositions comprise an alkali chlorite salt, a solid acid source, and a surfactant. In some other embodiments, the solid compositions produce a non-soapy aqueous solution containing chlorine dioxide. These solid compositions comprise an alkali chlorite salt and a solid acid source.
Owner:SIPKA
Methods of simultaneously cleaning and disinfecting industrial water systems
InactiveUS20050150520A1Reduced pHDetergent bleaching agentsWater/sewage treatment by neutralisationChlorine dioxideOnline and offline
On-Line and Off-Line methods of simultaneously cleaning and disinfecting an industrial water system are described and claimed. The methods involve the addition to the water of the industrial water system of a Compound selected from the group consisting of the alkali salts of chlorite and chlorate and mixtures thereof; and an acid, followed by allowing the water in the industrial water system to circulate for several hours. The reaction of the alkali salts of chlorite and chlorate and acid produces chlorine dioxide in-situ in the water of the industrial water system. The chlorine dioxide kills microorganisms and the acid acts to remove deposits upon the water-contact surfaces of the equipment. An alternative method involves the use of a chelating agent and a biocide. Other possible cleaning and disinfection reagents may be added as needed including corrosion inhibitors, chelating agents, biocides, surfactants and reducing agents. These cleaning and disinfecting methods work in a variety of industrial water systems including cooling water and boiler water systems.
Owner:ECOLAB USA INC
Acidified chlorite disinfectant compositions with olefin stabilizers
InactiveUS20050184273A1Reducing chlorine dioxide generationReduce drynessBiocideCosmetic preparationsDisinfectantCompound (substance)
A two-part disinfecting systems, as well as disinfecting compositions and methods for making and using the same. The two-part disinfecting system contains a first part and a second part adapted to be mixed to yield an aqueous disinfecting composition, wherein the first part comprises a chlorite and the second part comprises an acid, and wherein the first part, the second part, or both the first and second parts comprise an olefin compound.
Owner:ECOLAB USA INC
Method for forming a silicon oxide layer using spin-on glass
A method is provided for forming silicon oxide layers during the processing of semiconductor devices by applying a SOG layer including polysilazane to a substrate and then substantially converting the SOG layer to a silicon oxide layer using an oxidant solution. The oxidant solution may include one or more oxidants including, for example, ozone, peroxides, permanganates, hypochlorites, chlorites, chlorates, perchlorates, hypobromites, bromites, bromates, hypoiodites, iodites, iodates and strong acids.
Owner:SAMSUNG ELECTRONICS CO LTD
Compound microbiological oxygenate for removing ammonia nitrogen in aquatic water and preparation method of compound microbiological oxygenate
ActiveCN104671433AImprove solubilityQuick breakdownWater contaminantsBiological water/sewage treatmentDiseaseWater quality
The invention belongs to the technical field of aquiculture and discloses a compound microbiological oxygenate for removing ammonia nitrogen in aquatic water and a preparation method of the compound microbiological oxygenate. The oxygenate is prepared from the following raw material components in parts by weight: 15-25 parts of zeolite powder, 10-15 parts of chlorite, 10-15 parts of citric acid, 15-20 parts of a powdery bacillus subtilis, photosynthetic bacterium and EM bacterium mixture, 5-15 parts of a proteolytic enzyme, 5-15 parts of silicate, 20-25 parts of sodium percarbonate, 5-10 parts of quicklime, 1-4 parts of ferrous sulfate, 3-5 parts of dextrin, 3-5 parts of starch slurry and 15-20 parts of a protective agent. The oxygenate disclosed by the invention is suitable for sterilizing, oxygenating and preventing and treating diseases in aquiculture and can be used for improving the water quality, reducing the ammonia nitrogen content in a water area, improving the water level transparency, improving the substrate and purifying water.
Owner:SOUTH CHINA UNIV OF TECH
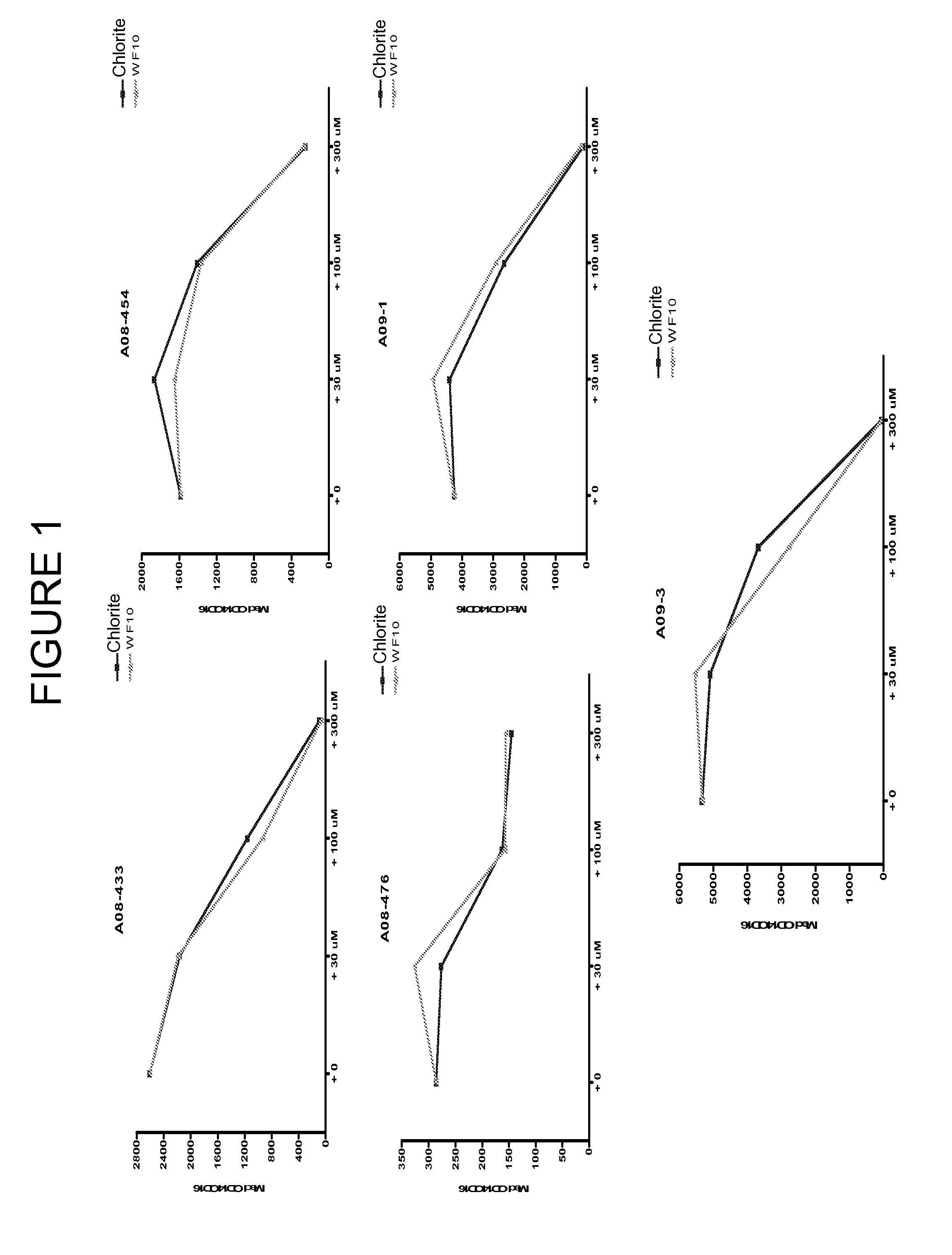
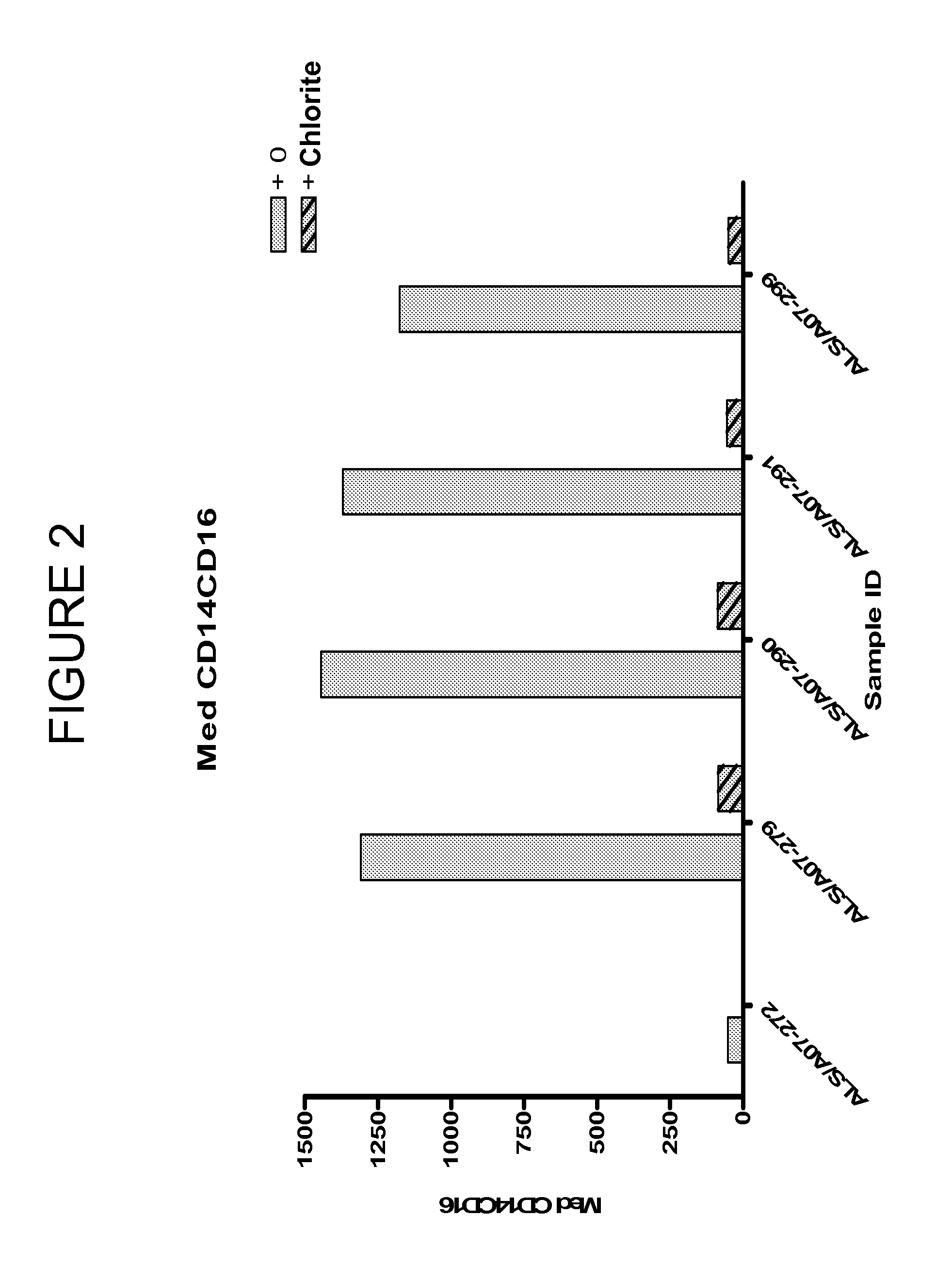
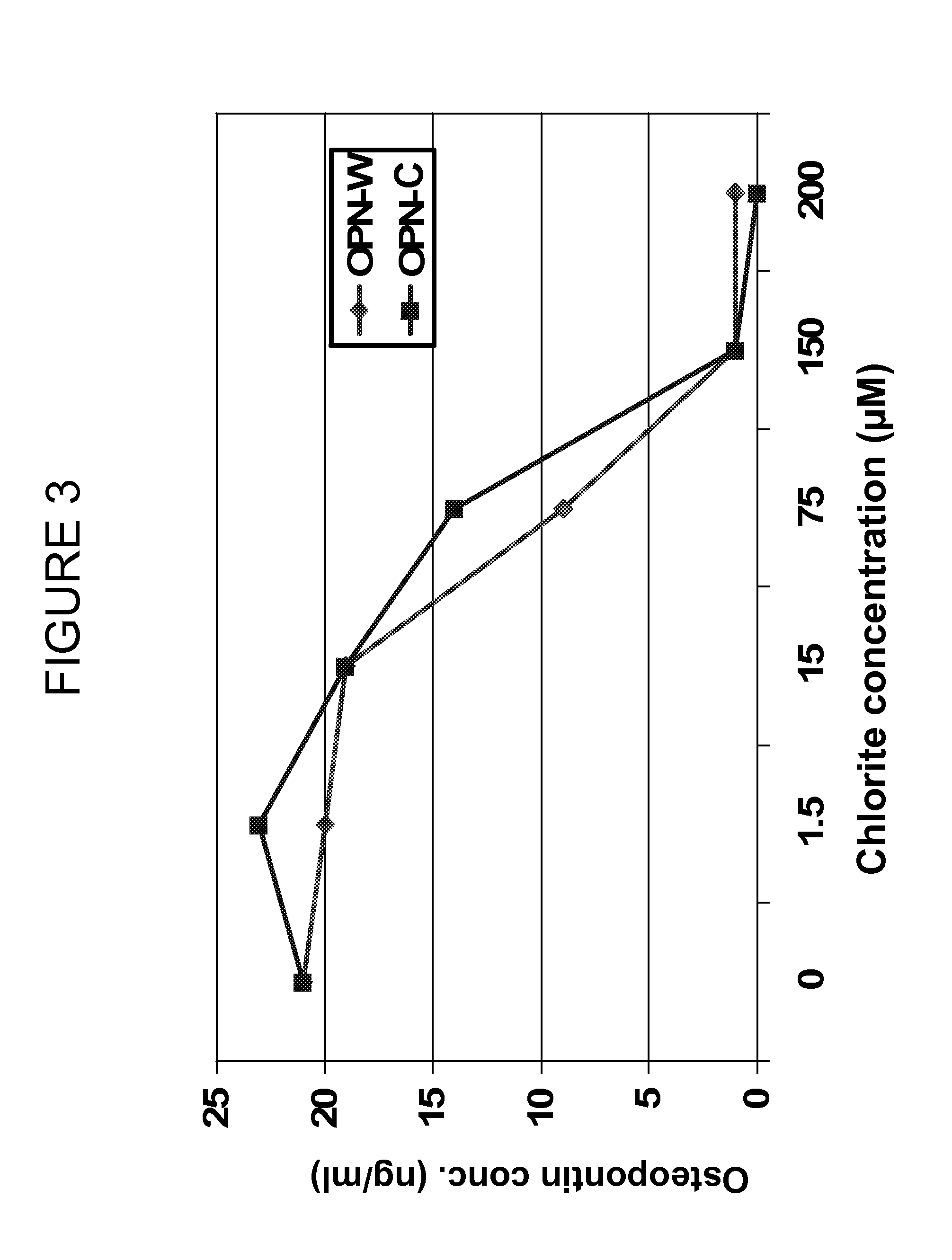
Chlorite in the treatment of neurodegenerative disease
ActiveUS20060159775A1Reduce activationBiocideNervous disorderAmyotrophic lateral sclerosisNeuro-degenerative disease
The invention features methods of treating a macrophage-associated neurodegenerative disease such as amyotrophic lateral sclerosis (ALS), Alzheimer's disease (AD), or multiple sclerosis (MS) in a subject by administering chlorite in an amount effective to decrease blood immune cell activation. The invention also features methods of monitoring therapy by assessing blood immune cell activation before and after therapy.
Owner:RGT UNIV OF CALIFORNIA
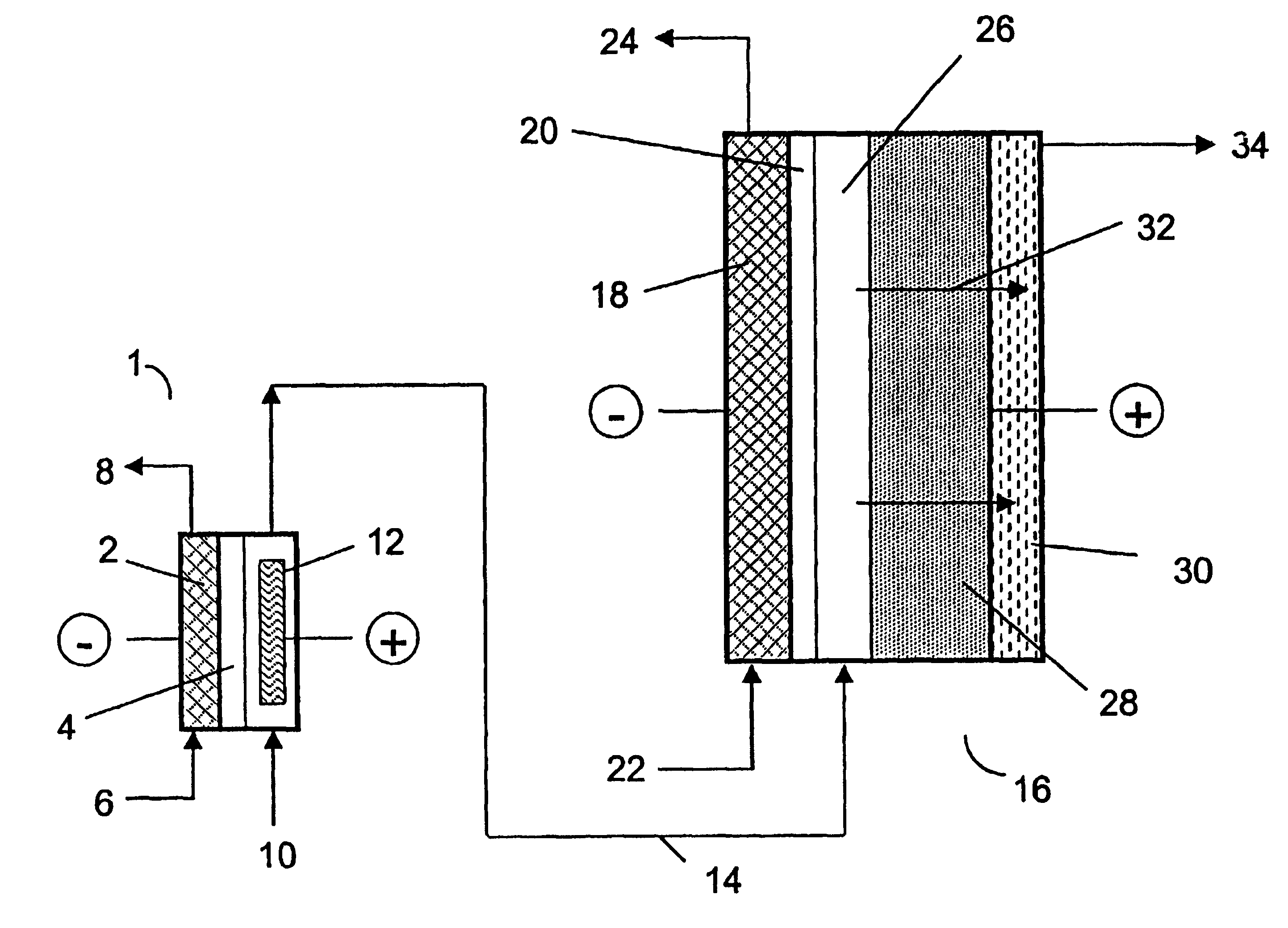
Chlorite Formulations, and Methods of Preparation and Use Thereof
Described herein are chlorite formulations having a pH between about 7 and about 8.5, wherein the chlorite formulations are substantially free of deleterious non-chlorite components. Described herein are chlorite formulations, including pharmaceutical formulations, which are formulated for systemic, parenteral, or intravenous administration. Described herein are methods of preparing and methods of using the chlorite formulations described herein.
Owner:NEUVIVO INC
Oxidation method and compositions therefor
InactiveUS20070042094A1Great range of controlIncrease flexibilityBiocideMilk preservationSulfateSodium sulfate
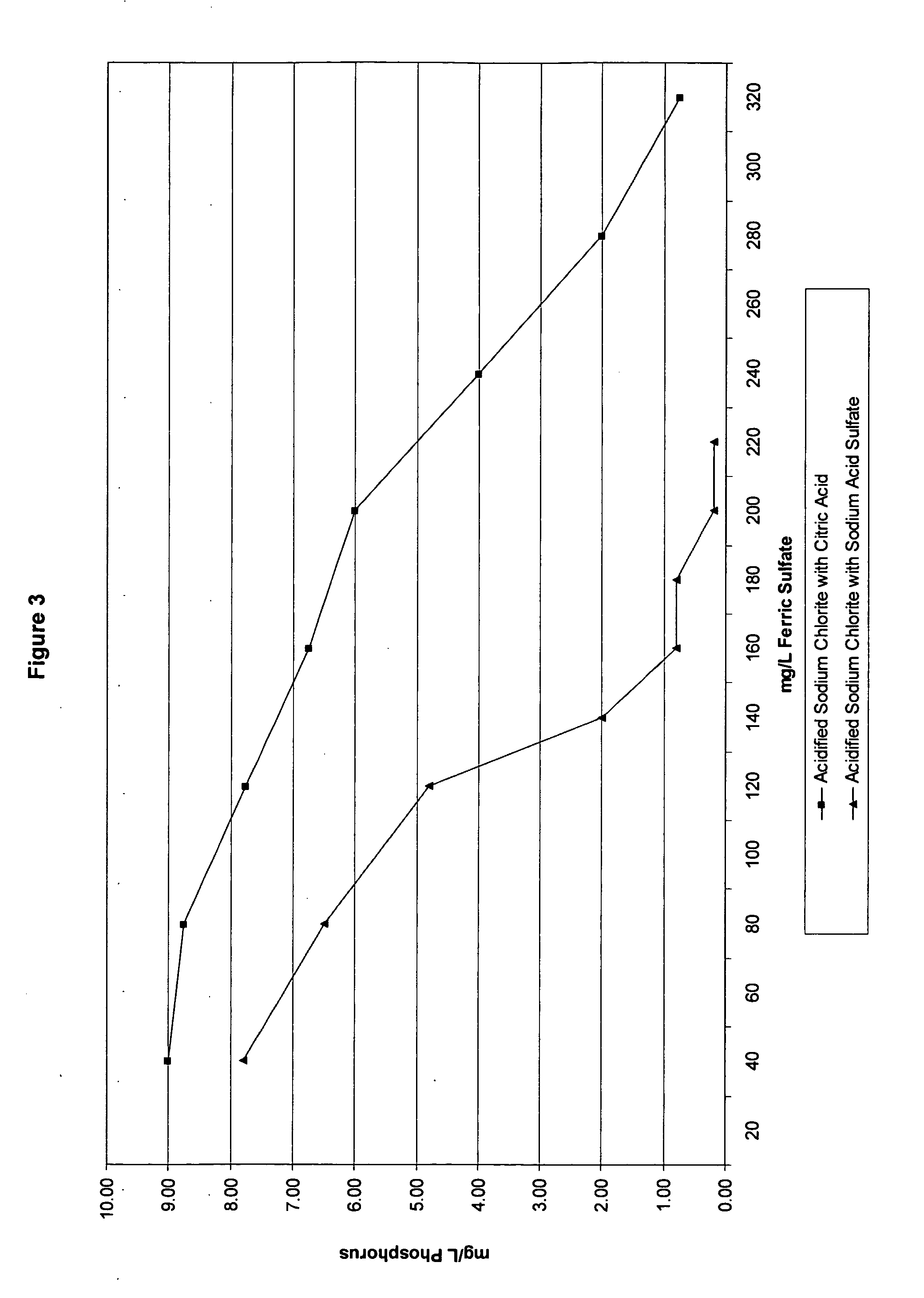
The present invention generally relates to an improved two-part oxidizing system, as well as oxidizing compositions and methods for making and using the same, and in a particular embodiment to a two-part oxidizing system that, when mixed, yields an oxidizing composition. The two-part oxidizing system includes a metal chlorite first part, and an acid second part where the acid is sodium acid sulfate or a derivative thereof.
Owner:ALCIDE CORP
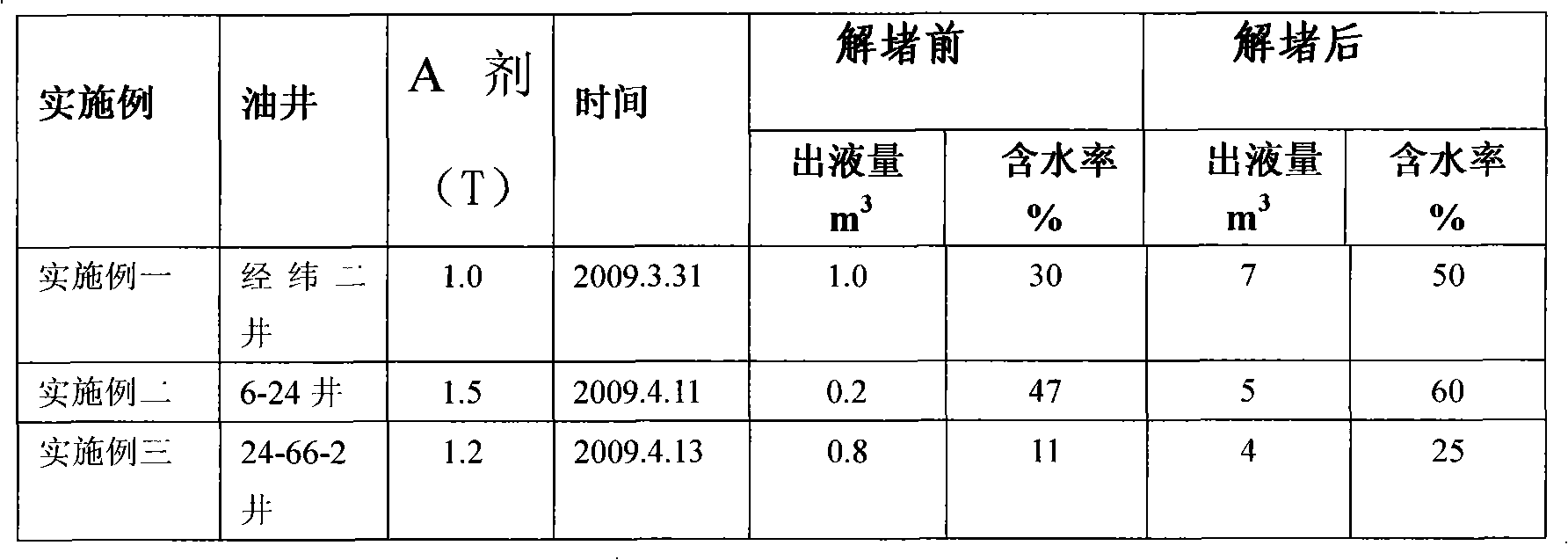
Compound blockage relieving agent and using method thereof
InactiveCN101608110AGuaranteed effective concentrationSimple processCleaning apparatusDrilling compositionHigh concentrationChlorine dioxide
The invention provides a compound blockage relieving agent, which comprises an A agent, a B agent and a C agent. The A agent is chlorine dioxide solution, and is chlorite or chlorate; the B agent is in a non-acid solid active agent system, and comprises organic oisocyanuric acid salt, inorganic sulfate and chlorate; and the C agent is an organic inhibitor for resisting oxidation corrosion of chlorine dioxide, and is organic phosphine. A using method comprises the following steps: the compound blockage relieving agent which is generated through fast activation aboveground and is based on the chlorine dioxide is wholly injected into an oil well formation fin one step through a bushing, then water is injected through the bushing, the chlorine dioxide compound blockage relieving agent with higher concentration after being activated is extruded into a perforation section, polymer of an oil layer is in oxidative degradation, and various bacterial microorganisms bred by raw oil and sulfide are oxidized and completely killed, thereby the invention achieves the purpose of eliminating blockage, has the characteristic that faint yellow liquid based on the chlorine dioxide of high concentration is generated through the fast activation, has no large quantity of chlorine dioxide gas escape, and can be widely used for fields such as oil well blockage relieving, water well blockage relieving, and the like.
Owner:XIAN JINGDA CHEM
Method and system for treating oxidized contaminant-containing matrix
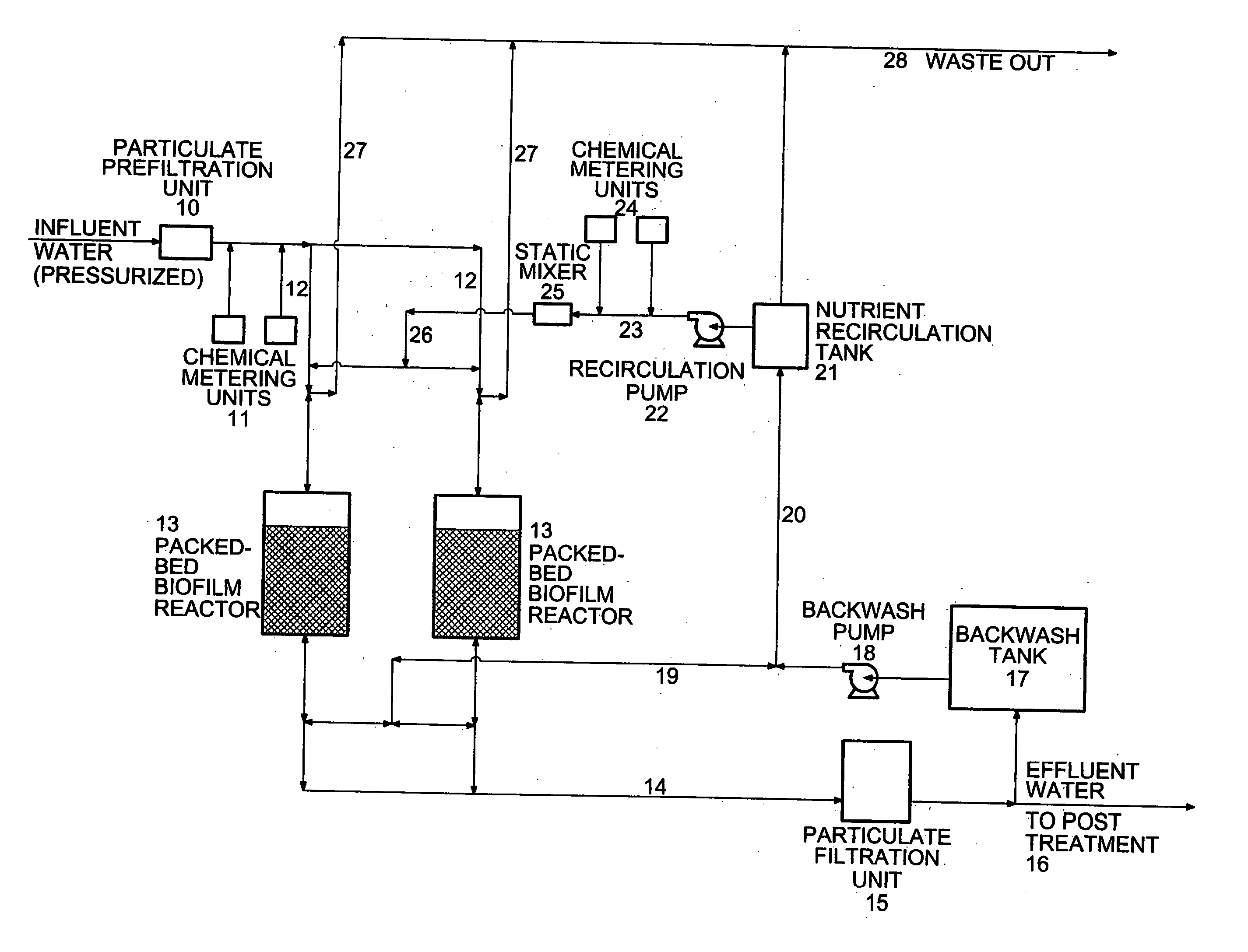
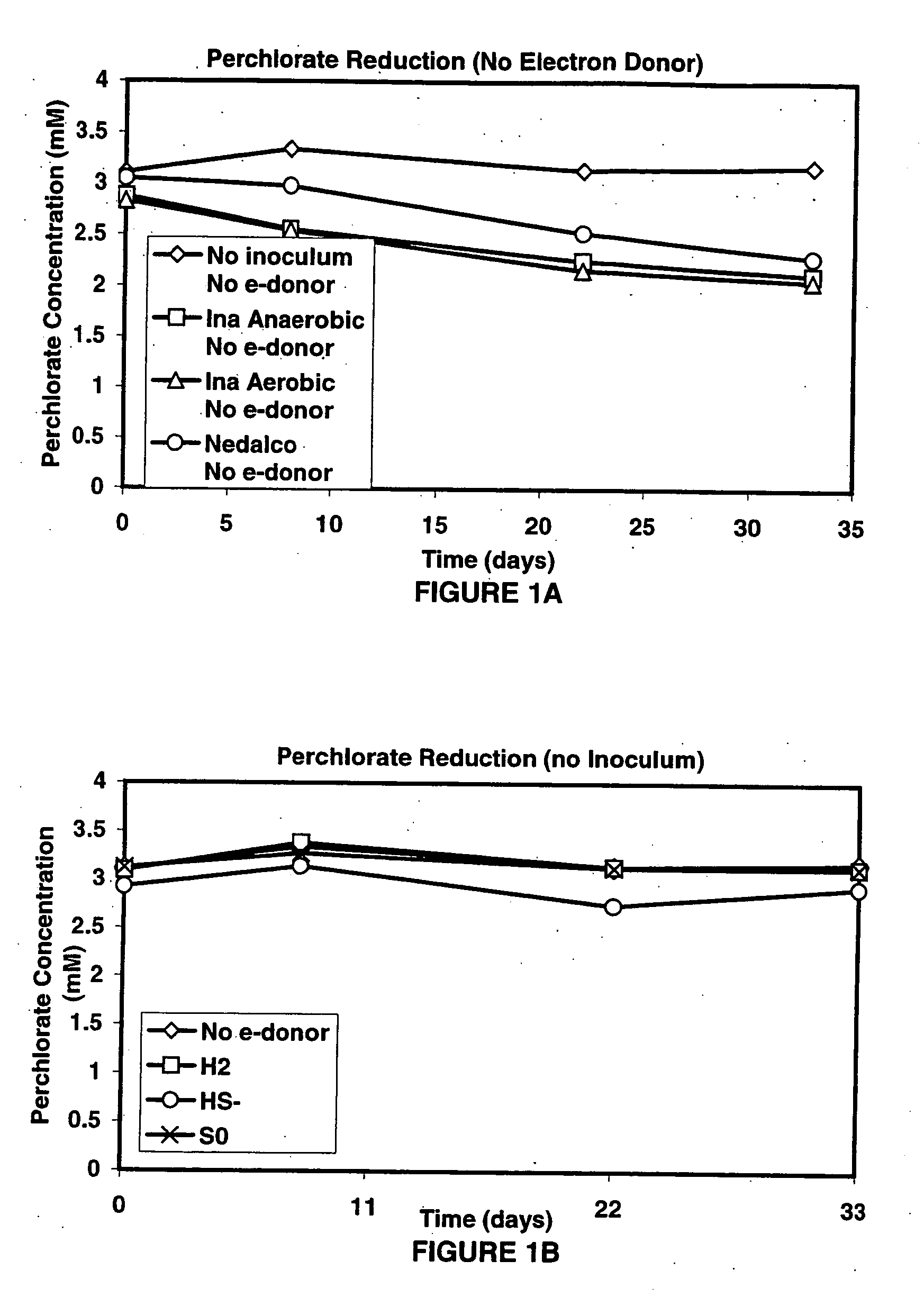
InactiveUS20060292684A1Reduce maintenanceHigh degreeWater treatment compoundsWater contaminantsSulfurElectron donor
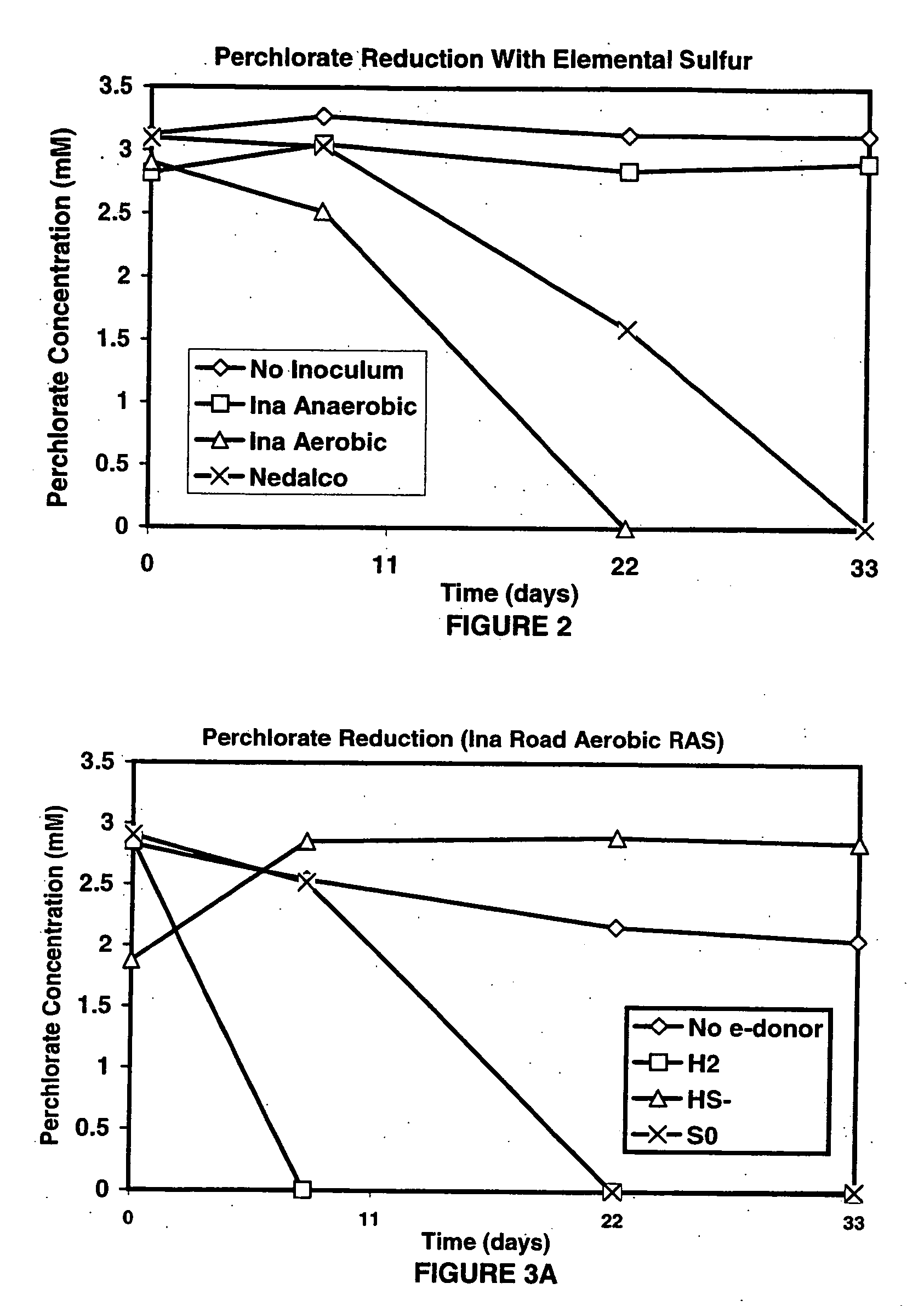
A new and useful way of treating oxidized-contaminant-containing water, soil, rock, other geological or non-geological matrix formation (such as a landfill) is provided. A bioreactor is provided that includes elemental sulfur and a microbial population capable of oxidizing sulfur and reducing pertechnate (TcO4−), arsenate (H2AsO4−), chromate (CrO42−), bromate (BrO3−), chlorite (ClO2−), chlorate (ClO3−), perchlorate (ClO4−), and uranium(VI) oxide, with biological reduction of these oxidized contaminants in the matrix containing the oxidized contaminant performed by the bioreactor, with the elemental sulfur as the electron donor.
Owner:HYDRO GEO CHEM
Microbial and odor control using amorphous calcium silicate impregnated with sodium chlorite
InactiveUS20030021819A1Long time-rangeIncrease time scaleHeavy metal active ingredientsBiocideCalcium silicateDisinfectant
The present invention is directed to the a composition and method for microbial and odor control. In one embodiment, the method includes mixing amorphous calcium silicate impregnated with a chlorite salt to form a reactant, combining the reactant with an activator to form the composition, and applying the composition to the treatment area. The present invention also includes the preparation of a product usable as a disinfectant and deodorizer, wherein the product includes a mixture of an amorphous and a chlorite salt and wherein the product is packaged as a tablet, permeable sachet, or a permeable patch attachable to a plastic bag. The present invention may also be applied using several methods to reduce the spoilage of produce. The present invention further includes a method for producing chlorine dioxide in accordance with a step-function release profile.
Owner:BIO CIDE INT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com