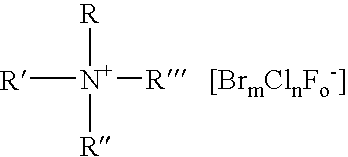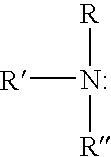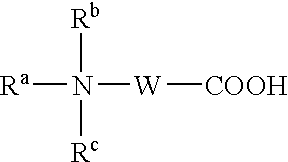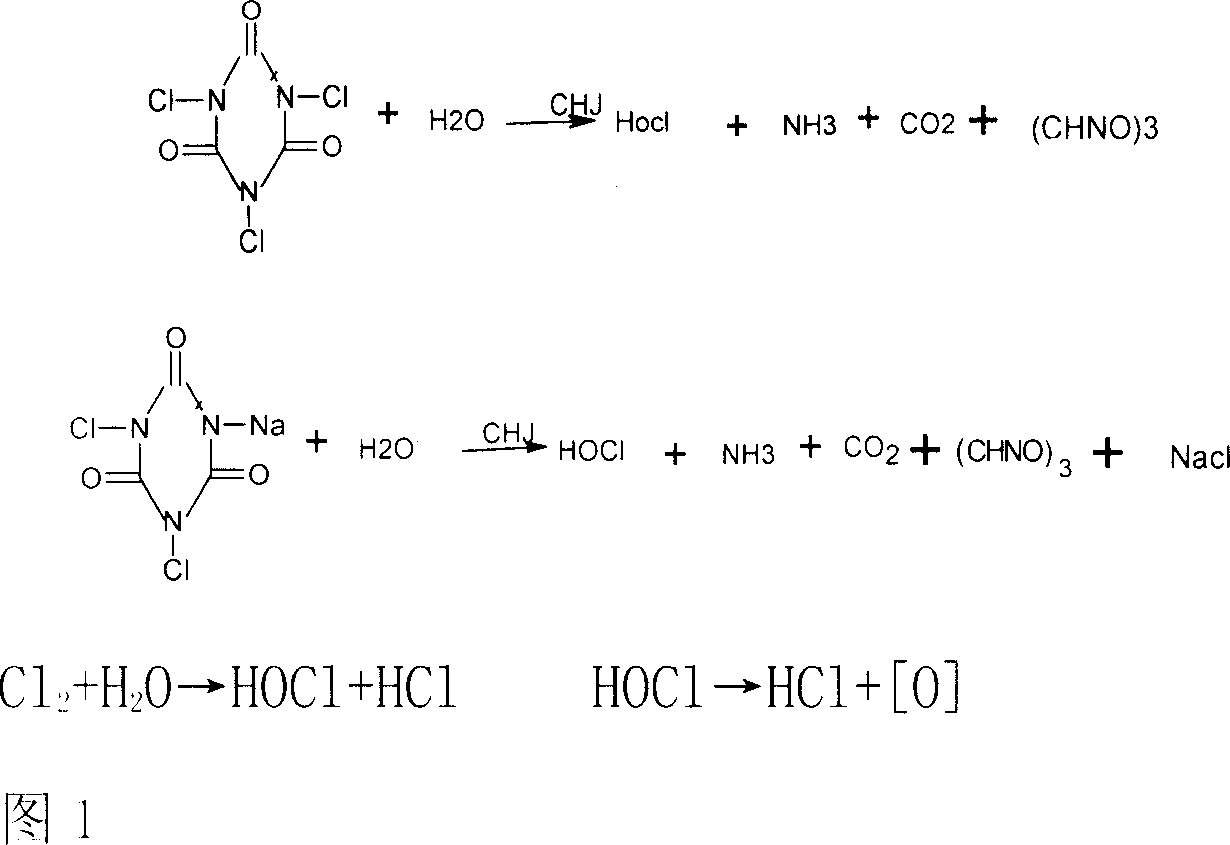Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
136 results about "Chlorate ion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chlorate is an ion. Its chemical formula is ClO3-. It contains chlorine in the +5 oxidation state. It is a strong oxidizing agent. It exists in chemical compounds such as potassium chlorate.
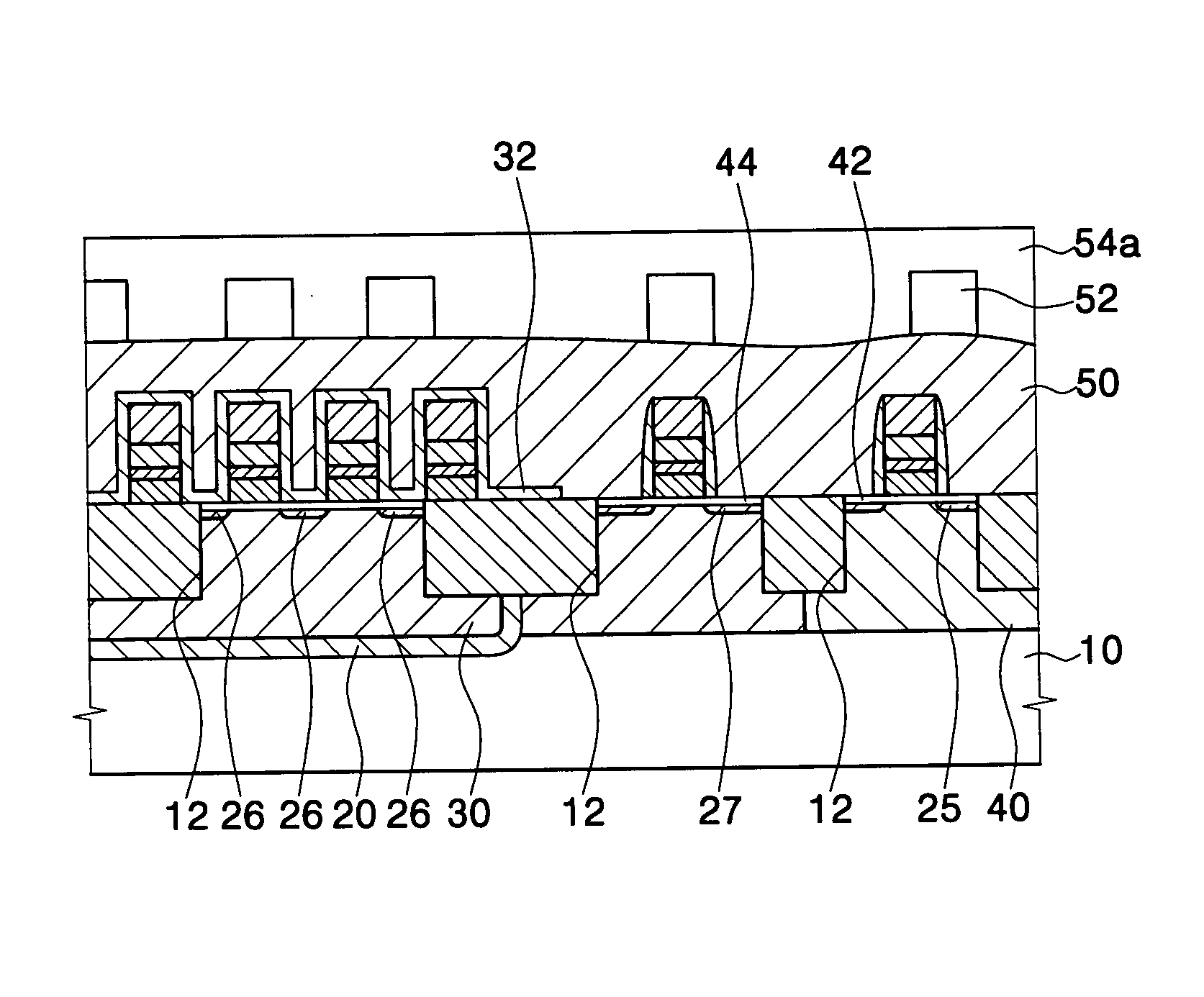
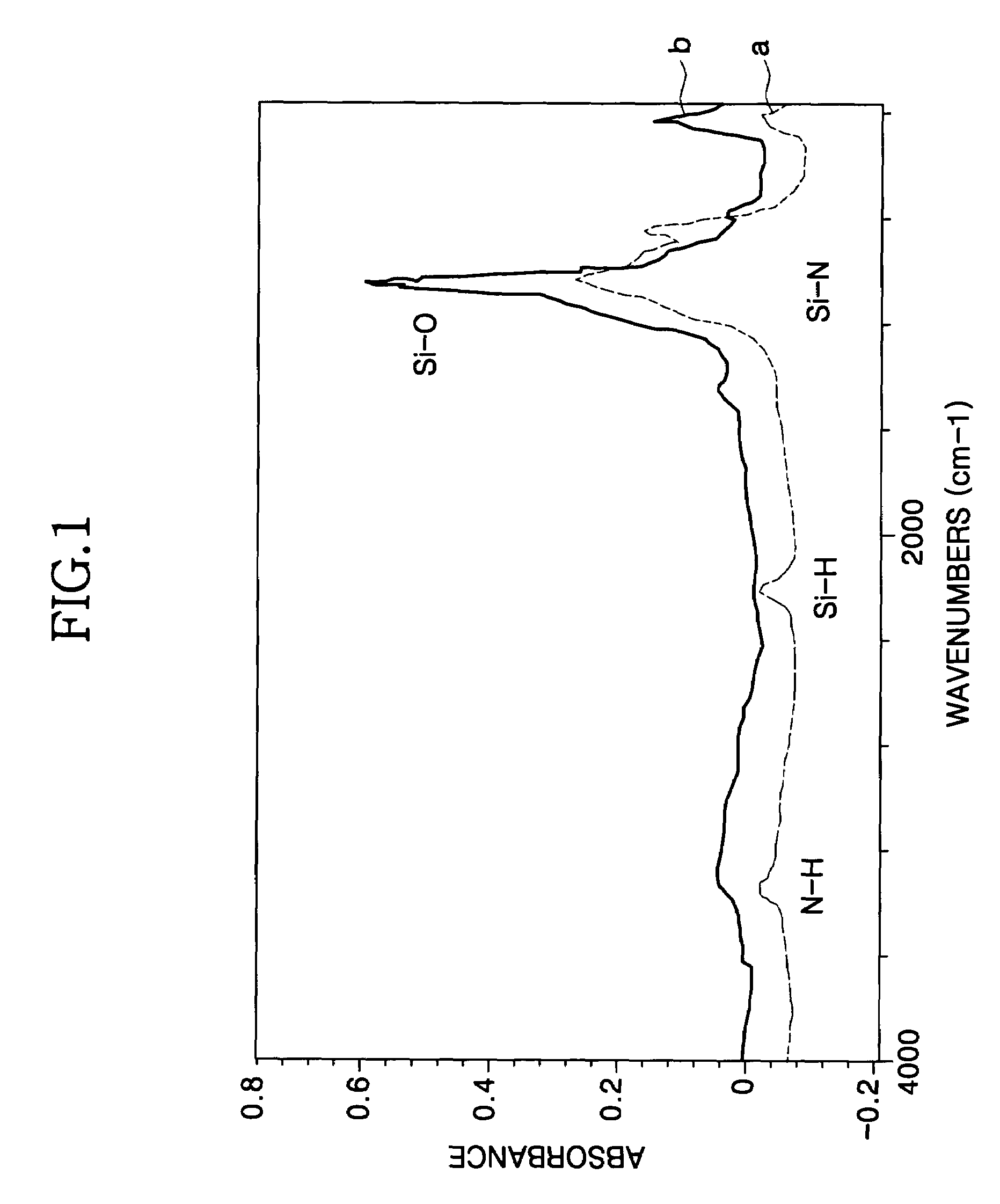
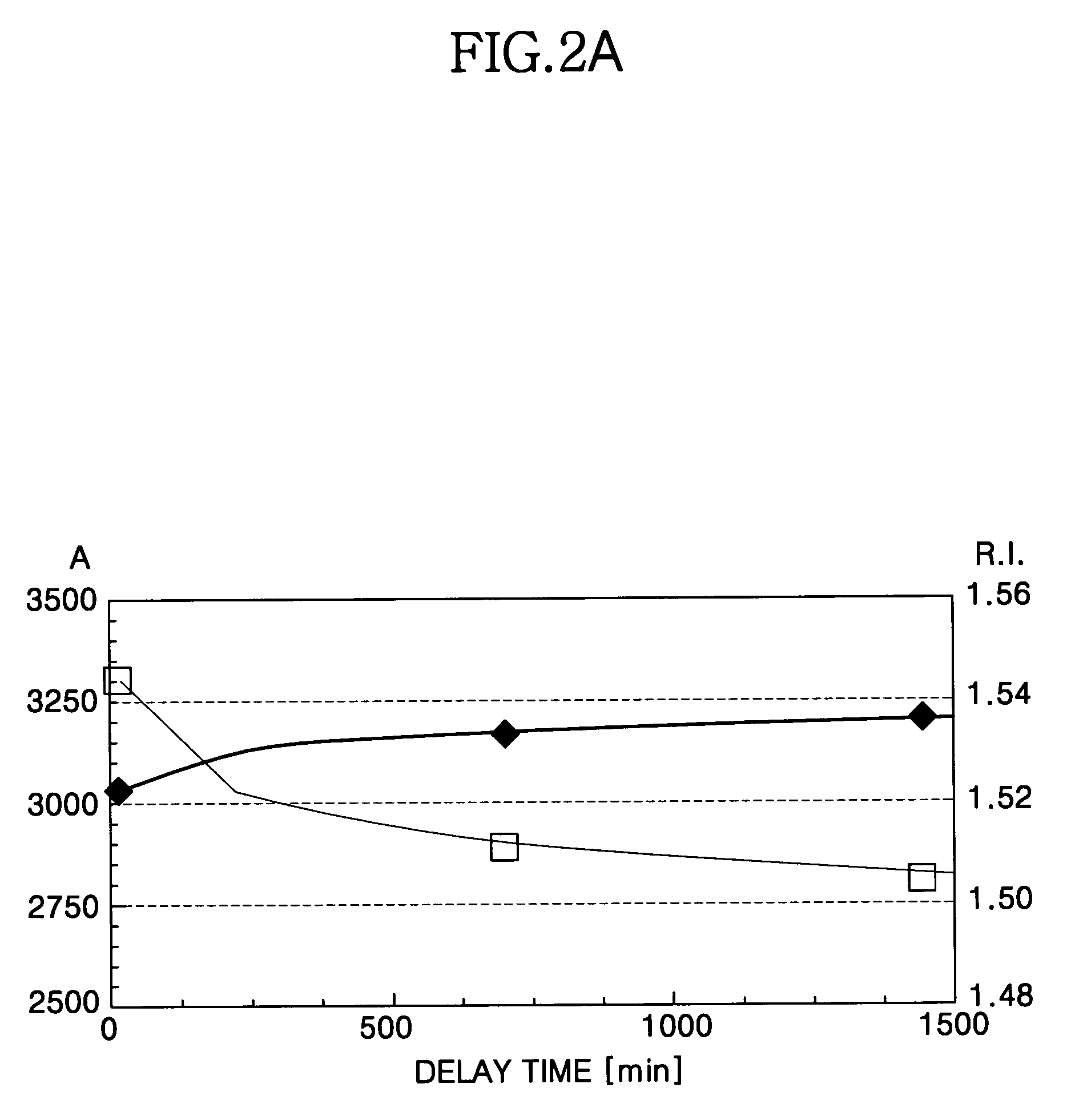
Method for forming a silicon oxide layer using spin-on glass
A method is provided for forming silicon oxide layers during the processing of semiconductor devices by applying a SOG layer including polysilazane to a substrate and then substantially converting the SOG layer to a silicon oxide layer using an oxidant solution. The oxidant solution may include one or more oxidants including, for example, ozone, peroxides, permanganates, hypochlorites, chlorites, chlorates, perchlorates, hypobromites, bromites, bromates, hypoiodites, iodites, iodates and strong acids.
Owner:SAMSUNG ELECTRONICS CO LTD
Composition for the production of chlorine dioxide using non-iodo interhalides or polyhalides and methods of making and using the same
ActiveUS7087190B2Reduce microbial countQuick buildBiocideLiquid degasificationChlorine dioxideCHLORITE ION
A composition for the generation of chlorine dioxide including at least one non-iodo interhalide, polyhalide or salt thereof having the formulaBrmClnFoXpwherein m=0–3, n=0–4, o=0–3, p=0–2, X is a cationic moiety and with the provisos that m+n+o cannot be zero; if m+n+p<2, or mixtures thereof, and at least one source of chlorite ions.
Owner:ECOLAB USA INC
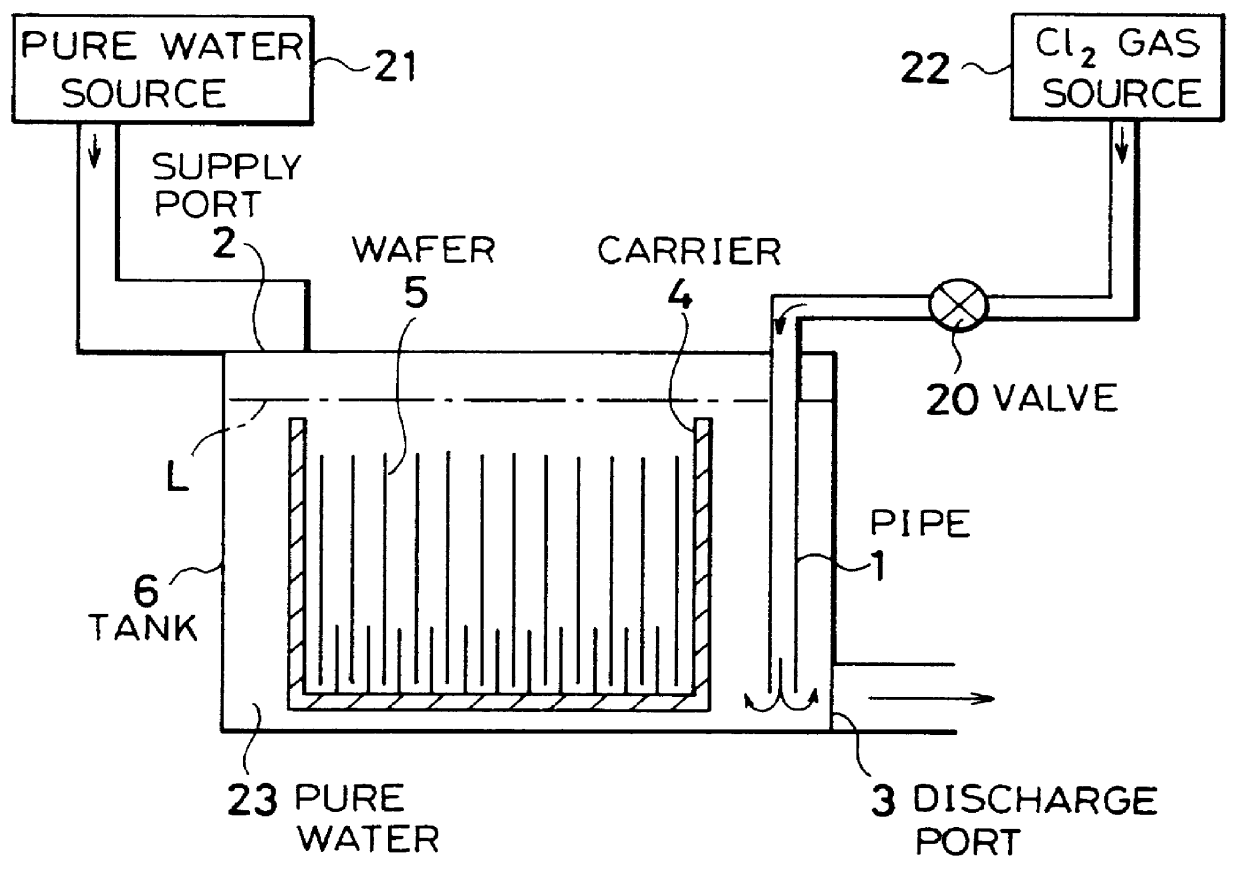
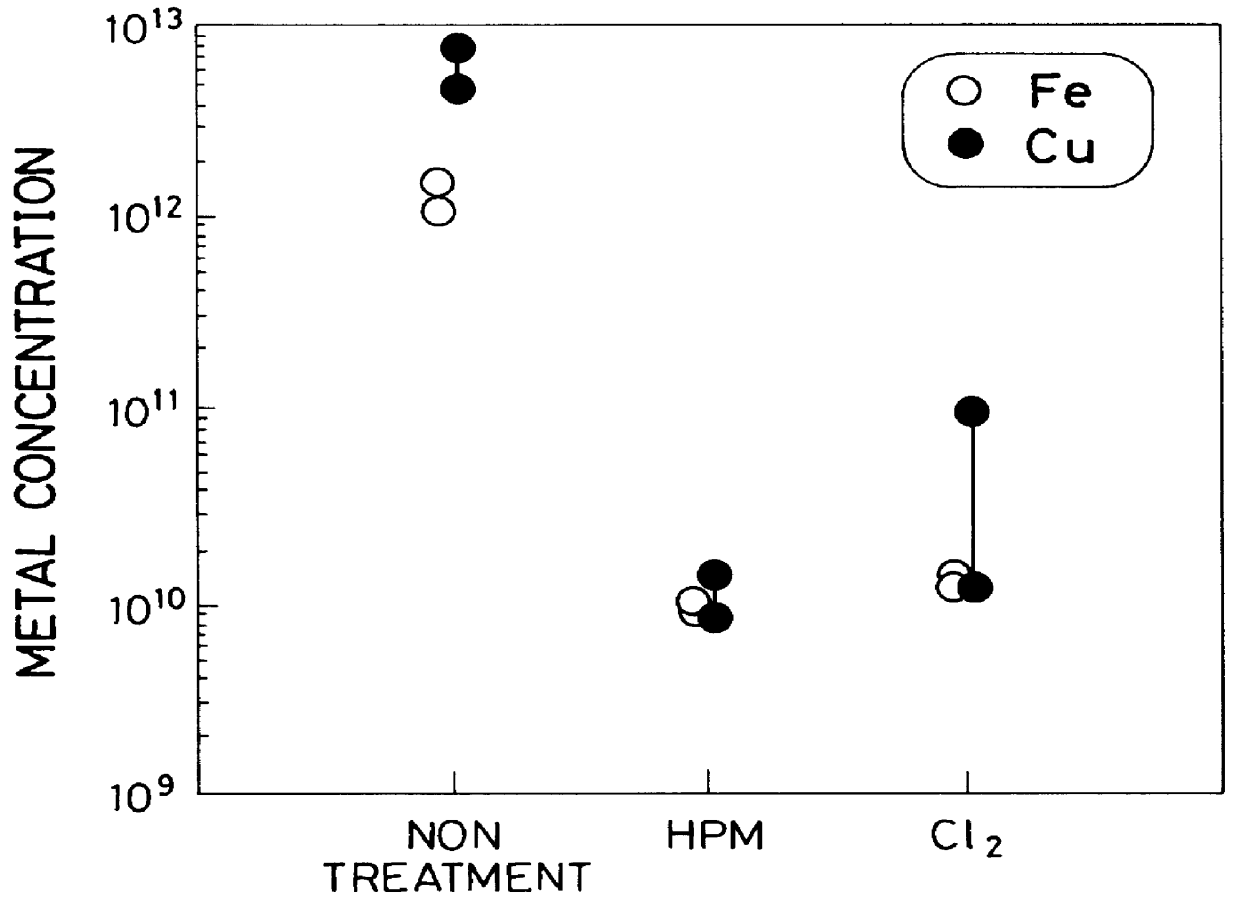
Cleaning method and system of semiconductor substrate and production method of cleaning solution
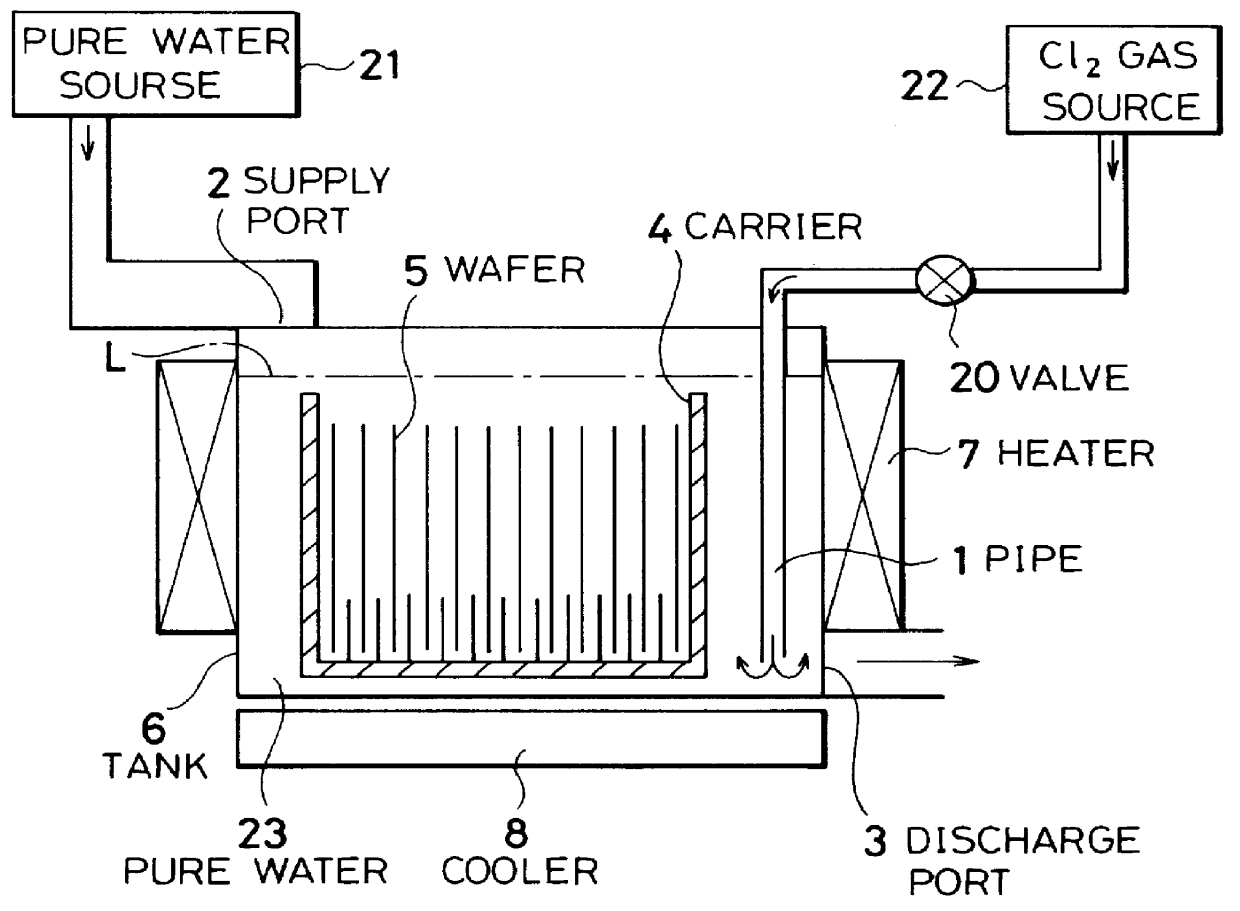
InactiveUS6116254AMinimize power consumptionReduce adverse effectsNon-surface-active detergent compositionsSemiconductor/solid-state device manufacturingChlorate ionHypochlorite
A cleaning method for a semiconductor substrate is provided. After pure water is supplied to a cleaning tank, a chlorine gas is supplied to the pure water to thereby generate chloride ions, hypochlorite ions, chlorite ions, and chlorate ions in the pure water. Then, a semiconductor substrate is immersed into the pure water containing the chloride ions, hypochlorite ions, chlorite ions, and chlorate ions. The fabrication cost of a semiconductor device and adverse effects on the earth environment can be reduced. The concentration of the dissolved chlorine gas in the pure water is preferably in the range from 0.003 to 0.3% by weight.
Owner:RENESAS ELECTRONICS CORP
Use of an ammonia storage device in production of energy
An electric power generating unit comprising (i) an ammonia storage device in the form of a container comprising an ammonia absorbing and releasing salt of the general formula: Ma(NH3)nXz, wherein M is one or more cations selected from alkali metals, alkaline earth metals, and transition metals such as Li, K, Mg, Ca, V, Cr, Mn, Fe, Co, Ni, Cu or Zn, X is one or more anions selected from fluoride, chloride, bromide, iodide, nitrate, thiocyanate, sulphate, molybdate, phosphate, and chlorate ions, a is the number of cations per salt molecule, Z is the number of anions per salt molecule, and n is the coordination number of 2 to 12. (ii) means for heating said container and ammonia absorbing and releasing salt for releasing ammonia gas and (iiia) a fuel cell for converting ammonia directly into electric power; or (iiib1) a reactor for dissociating ammonia into hydrogen and nitrogen and (iiib2) a fuel cell for converting hydrogen into electric power.
Owner:AMMINEX
Use Of An Ammonia Storage Device In Production Of Energy
InactiveUS20070207351A1Reactant parameters controlMagnesium halidesAlkaline earth metalAmmonia storage
An electric power generating unit comprising (i) an ammonia storage device in the form of a container comprising an ammonia absorbing and releasing salt of the general formula: Ma(NH3)nXz, wherein M is one or more cations selected from alkali metals, alkaline earth metals, and transition metals such as Li, K, Mg, Ca, V, Cr, Mn, Fe, Co, Ni, Cu or Zn, X is one or more anions selected from fluoride, chloride, bromide, iodide, nitrate, thiocyanate, sulphate, molybdate, phosphate, and chlorate ions, a is the number of cations per salt molecule, Z is the number of anions per salt molecule, and n is the coordination number of 2 to 12. (ii) means for heating said container and ammonia absorbing and releasing salt for releasing ammonia gas and (iiia) a fuel cell for converting ammonia directly into electric power; or (iiib1) a reactor for dissociating ammonia into hydrogen and nitrogen and (iiib2) a fuel cell for converting hydrogen into electric power is useful for large stationary energy producing facilities, but also for use for is useful for large stationary energy producing facilities, but also for use for small rechargeable and / or replaceable power supply units for micro-fabricated or miniaturized ammonia decomposition reactors for use in mobile units and portable devices may be used for large energy producing facilities, and by use of small rechargeable and / or replaceable ammonia storage decomposition reactors, it is also possible to provide energy for mobile units and portable devices.
Owner:AMMINEX
Method of preparing a biocide comprising stabilized hypochlorite and a bromide ion source and a method of controlling microbial fouling using the same
InactiveUS20050147528A1Long retentionIncreased durabilityBiocideSpecific water treatment objectivesAlkaline earth metalBromide ions
Disclosed is a method of preparing a biocide having improved durability of its biocidal activity as well as disinfection efficiency at an initial stage, comprising the steps of: (a) preparing stabilized alkali or alkaline earth metal hypochlorite having a pH at least 11 by mixing a chlorine oxidant including alkali or alkaline earth metal hypochlorite with a stabilizer in an alkali solution; (b) preparing a bromide ion source; and (c) adding the bromide ion source prepared in step (b) into the stabilized alkali or alkaline earth metal hypochlorite prepared in step (a). Also, a method of controlling the growth of microorganisms using a biocide prepared by the method of the present invention is disclosed.
Owner:ACCU LAB CO LTD
Chemical method
InactiveUS6322690B1Avoiding net productionEliminate needElectrolysis componentsChlorine dioxideChlorate ionChlorine dioxide
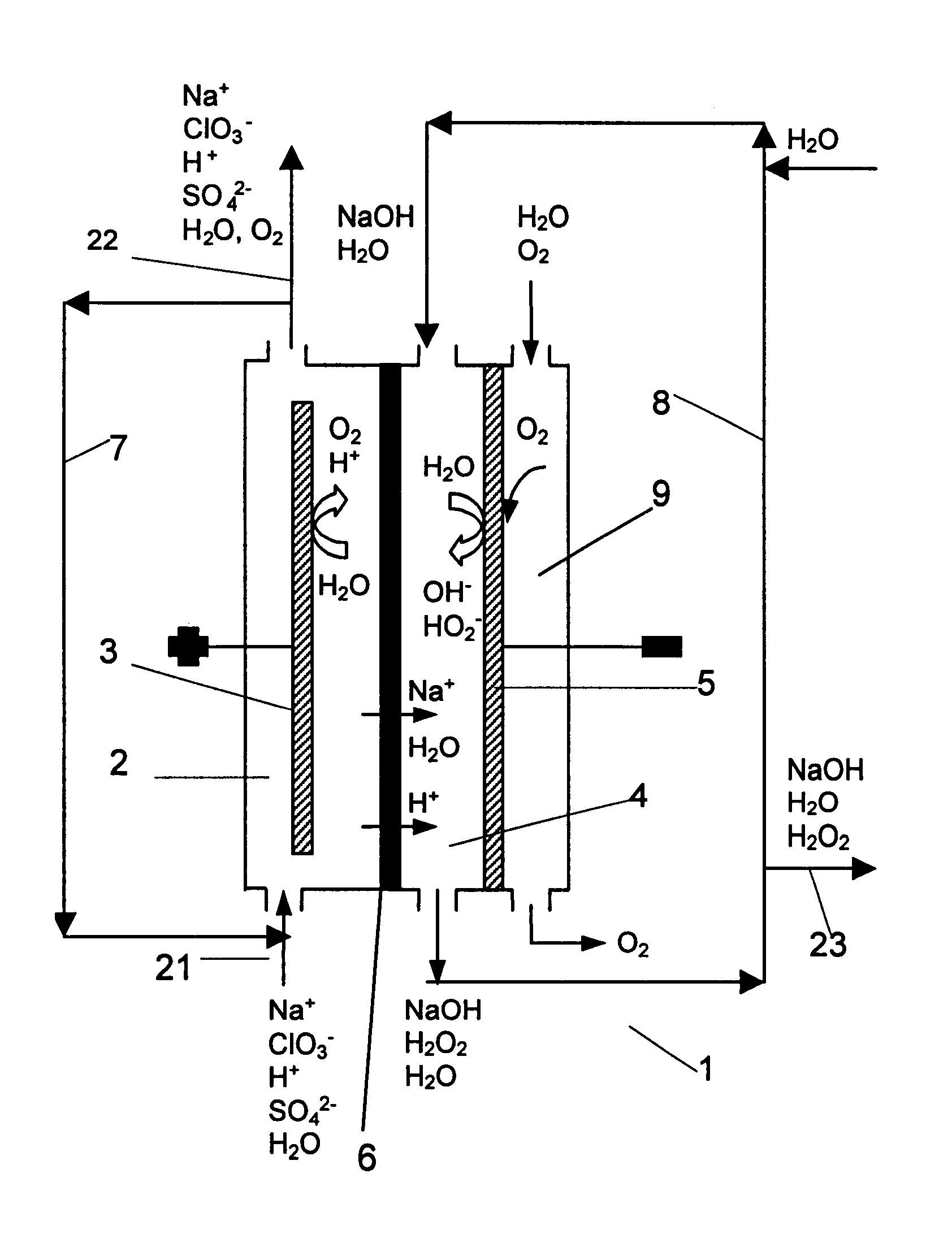
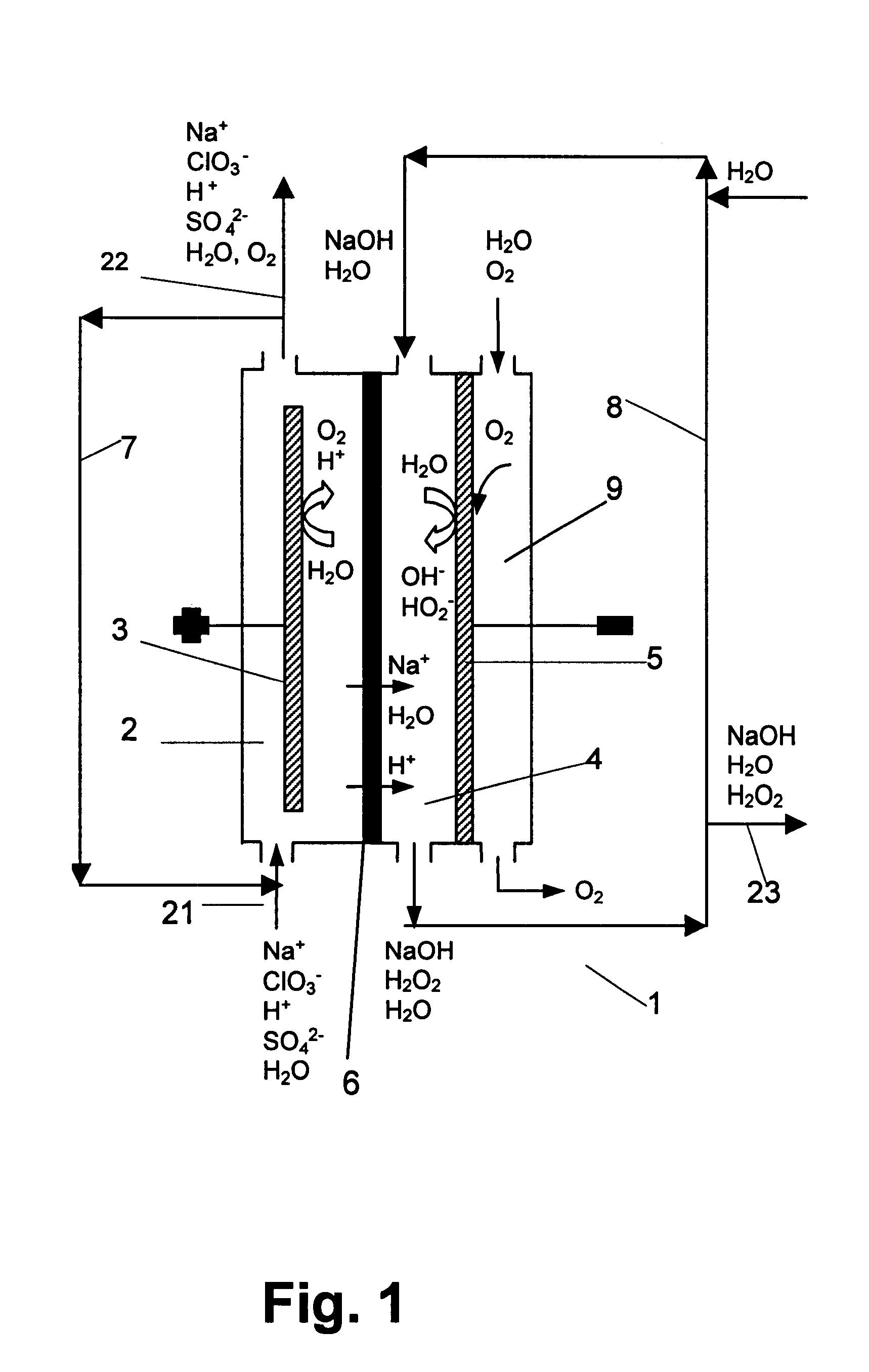
The invention relates to a process for production of an alkaline hydrogen peroxide solution and an acidified alkali metal salt solution containing chlorate in an electrochemical cell including an anode compartment provided with an anode and a cathode compartment provided with an oxygen reducing cathode. In the cathode compartment an aqueous alkali metal hydroxide and hydrogen peroxide is formed, wherein the molar ratio MOH:H2O2 for the net production thereof is maintained from about 0.1:1 to about 2:1. Also a process for simultaneous production of chlorine dioxide is disclosed.
Owner:AKZO NOBEL NV
Process for production of chlorine dioxide
InactiveUS20070116637A1Easy to useEasy to manufactureGaseous chemical processesLiquid-gas reaction of thin-film typeChlorate ionVapor–liquid separator
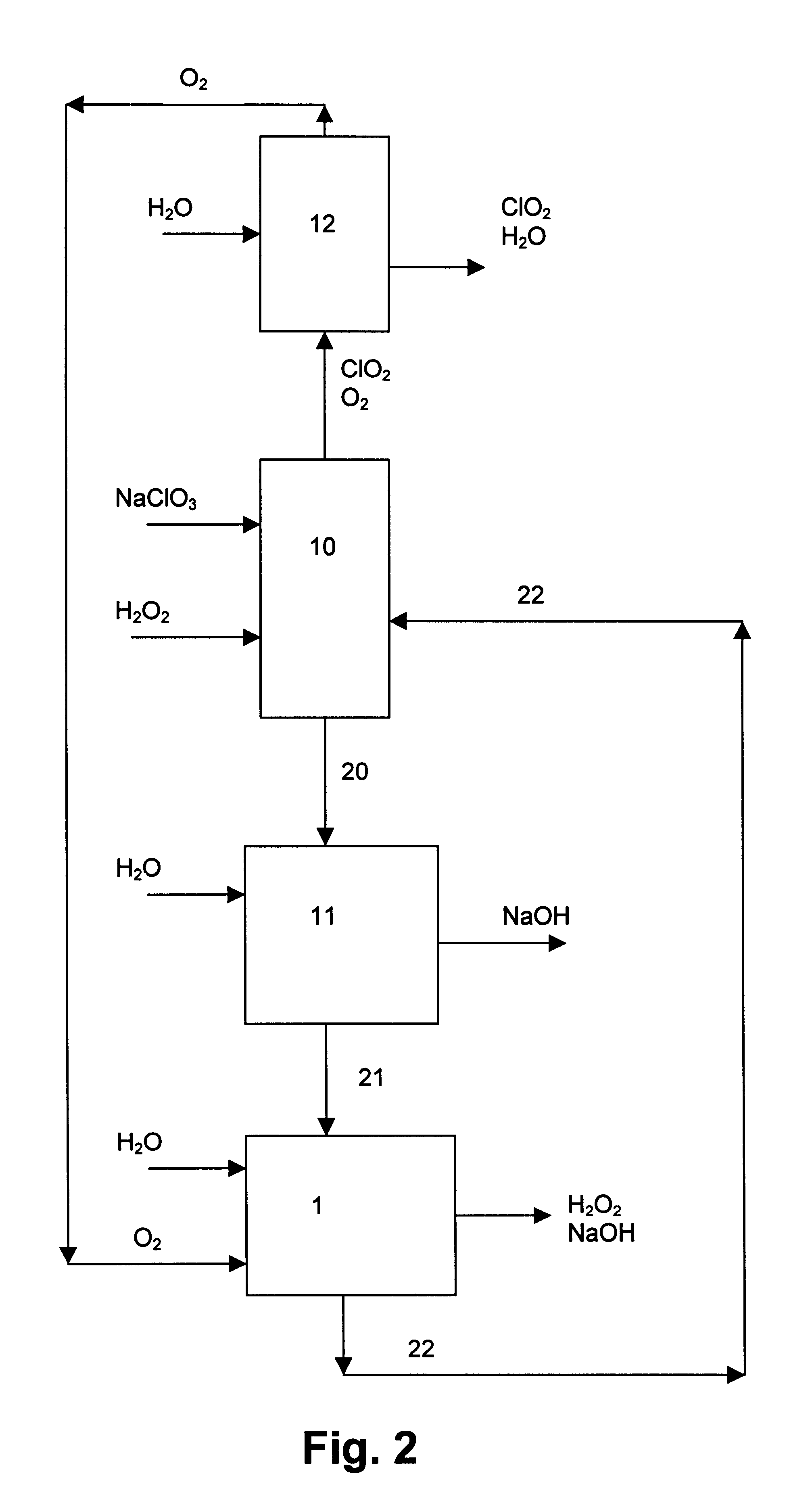
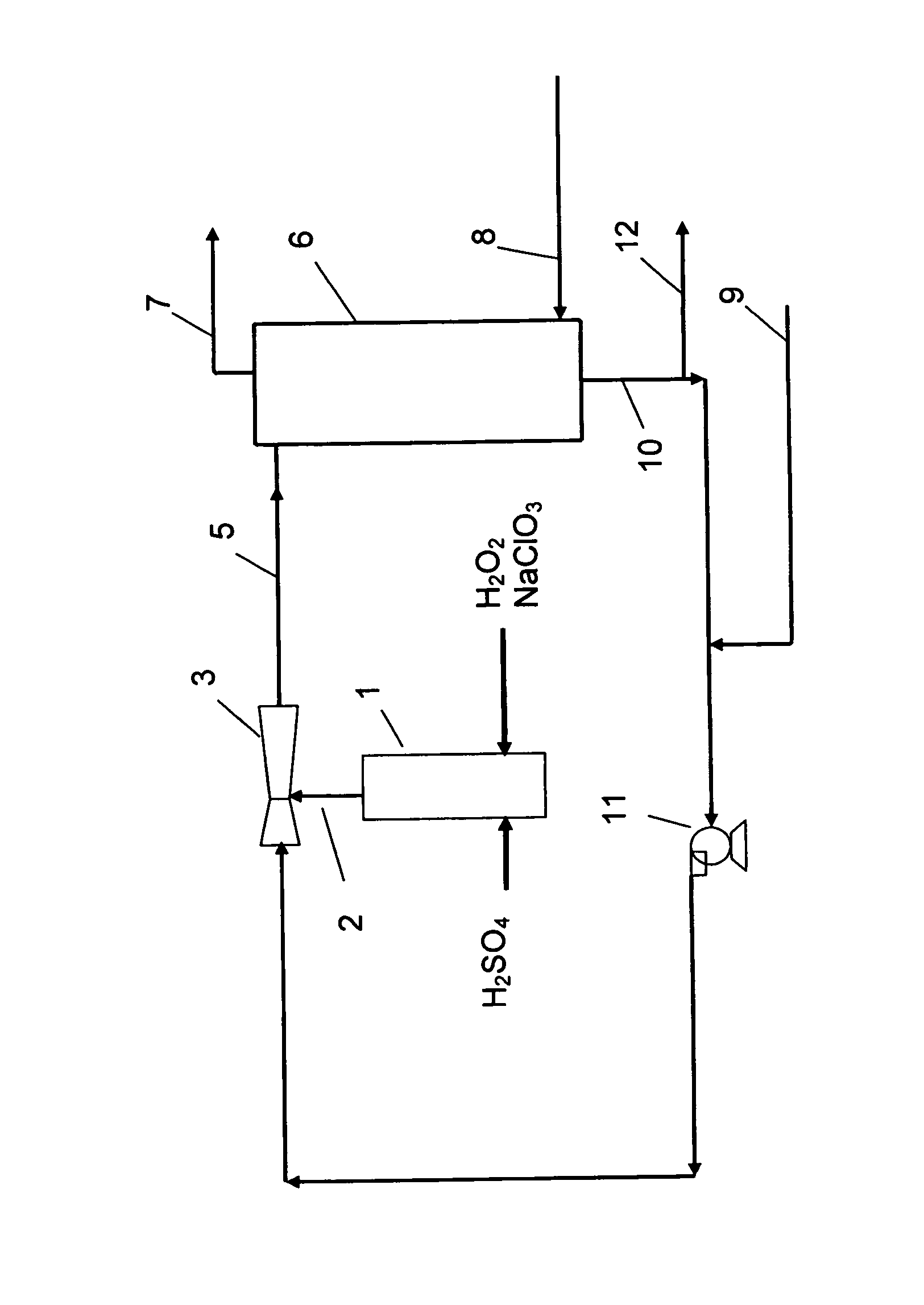
The present invention relates to a process for the production of chlorine dioxide, said process comprising the steps of continuously: (a) feeding to a reactor an acid, alkali metal chlorate and a reducing agent; (b) reacting the alkali metal chlorate with the acid and the reducing agent to form a product stream comprising chlorine dioxide and alkali metal salt of the acid; (c) bringing the product stream from the reactor to an eductor and mixing it with motive fluid fed to the eductor and thereby forming a diluted product stream; (d) bringing the diluted product stream to a gas-liquid separator where gas is separated from liquid therein; (e) withdrawing a gaseous product stream comprising chlorine dioxide and inert gas from said gas-liquid separator; and, (f) withdrawing a liquid phase from the gas-liquid separator. The invention also relates to a production unit to produce chlorine dioxide.
Owner:ECOLAB USA INC +1
Methods of simultaneously cleaning and disinfecting industrial water systems
InactiveUS20050150520A1Reduced pHDetergent bleaching agentsWater/sewage treatment by neutralisationChlorine dioxideOnline and offline
On-Line and Off-Line methods of simultaneously cleaning and disinfecting an industrial water system are described and claimed. The methods involve the addition to the water of the industrial water system of a Compound selected from the group consisting of the alkali salts of chlorite and chlorate and mixtures thereof; and an acid, followed by allowing the water in the industrial water system to circulate for several hours. The reaction of the alkali salts of chlorite and chlorate and acid produces chlorine dioxide in-situ in the water of the industrial water system. The chlorine dioxide kills microorganisms and the acid acts to remove deposits upon the water-contact surfaces of the equipment. An alternative method involves the use of a chelating agent and a biocide. Other possible cleaning and disinfection reagents may be added as needed including corrosion inhibitors, chelating agents, biocides, surfactants and reducing agents. These cleaning and disinfecting methods work in a variety of industrial water systems including cooling water and boiler water systems.
Owner:ECOLAB USA INC
Method for forming a silicon oxide layer using spin-on glass
A method is provided for forming silicon oxide layers during the processing of semiconductor devices by applying a SOG layer including polysilazane to a substrate and then substantially converting the SOG layer to a silicon oxide layer using an oxidant solution. The oxidant solution may include one or more oxidants including, for example, ozone, peroxides, permanganates, hypochlorites, chlorites, chlorates, perchlorates, hypobromites, bromites, bromates, hypoiodites, iodites, iodates and strong acids.
Owner:SAMSUNG ELECTRONICS CO LTD
Compositions providing frost appearance for printing on glass or ceramic substrates and methods for the use thereof
A method of producing a substrate having a frosted appearance, an article having a frosted appearance and frost-imparting compositions are disclosed. The method includes applying a frost-imparting composition onto a ceramic substrate and firing the coated substrate above 400° C. to impart the frost appearance. The frost-imparting composition comprises a liquid vehicle, 20% to 90% by weight of glass frit particles and less than 10% by weight of at least one gas-releasing material, inactive below an activation temperature of at least 400° C. The gas releasing material may include carbonate, nitrate, iodate, bromate, chlorate, fluoride, manganate, dimanganate and sulfate compounds. The firing temperature is chosen to be above the activation temperature of the gas-releasing material, above which the gas-releasing material yields gas bubbles and above a temperature that causes the glass frit particles to behave as a viscous liquid.
Owner:DIP TECH
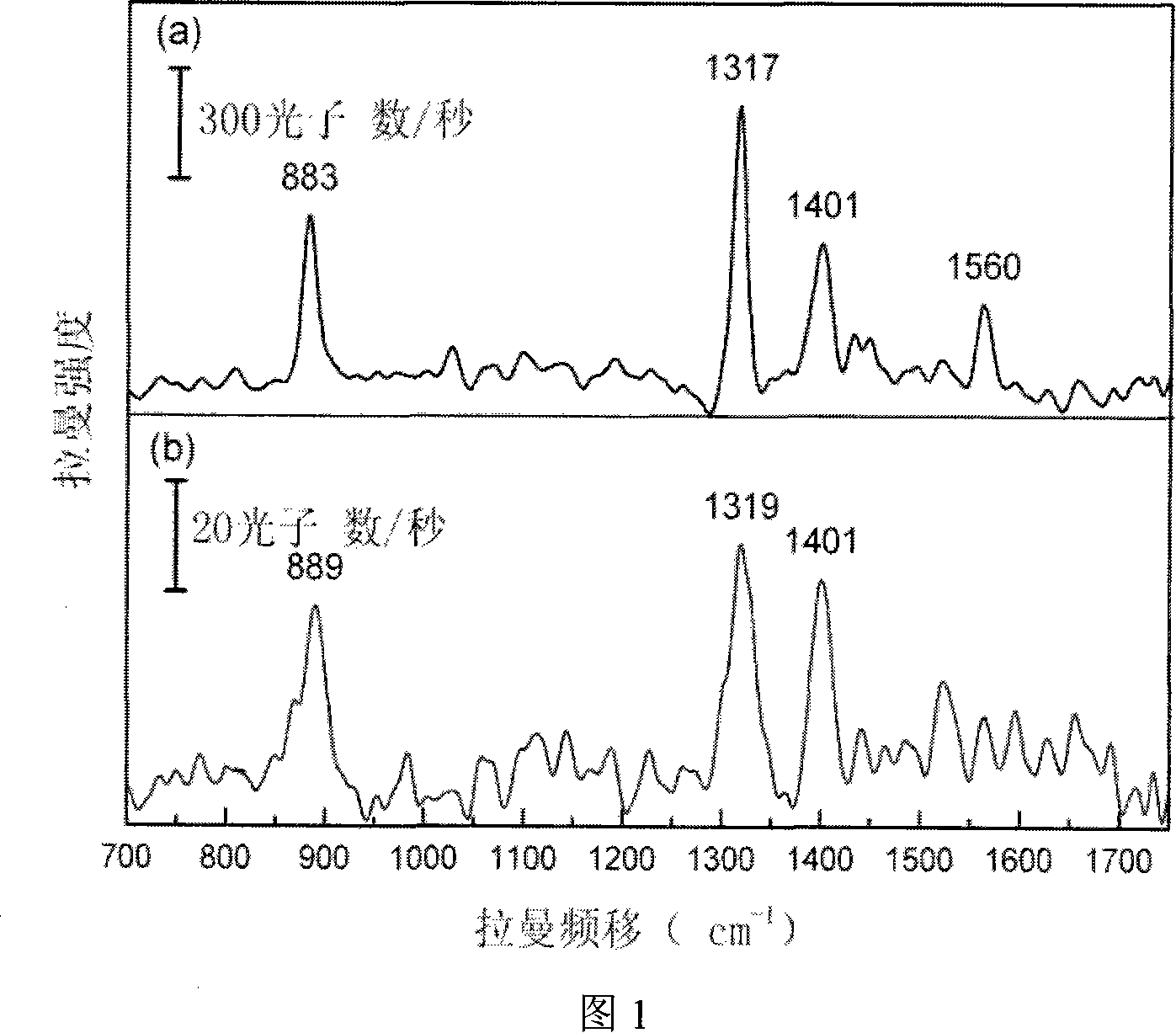
Preparation method of surface reinforced Raman diffuse transmission substrate
A method to produce surface enhanced Raman substrate (SERS) focalizes a laser beam with pulse width of 10 swung dash 500femtosecond, power of 1 swung dash 100milliwatt and peak power of 2 is multiplied by 109 swung dash 5 is multiplied by 1012W to form a light spot with a diameter of several microns, thus projecting the focalized light spot of the femtosecond laser beam onto a silicon glass surface mixed with precious metals, synchronously scanning the focalized light spot and forming a silicon glass substrate with nanometer roughness precious metal grains on its surface. And then the substrate is positioned to silver nitrate or aurum chlorate for steeping to form a precious metal film layer with nanometer roughness and obtaining a SERS. Relevant experiments indicate that the SERS produced according to the present invention is provided with a remarkably enhanced surface Raman scattering effect.
Owner:SHANGHAI INST OF OPTICS & FINE MECHANICS CHINESE ACAD OF SCI
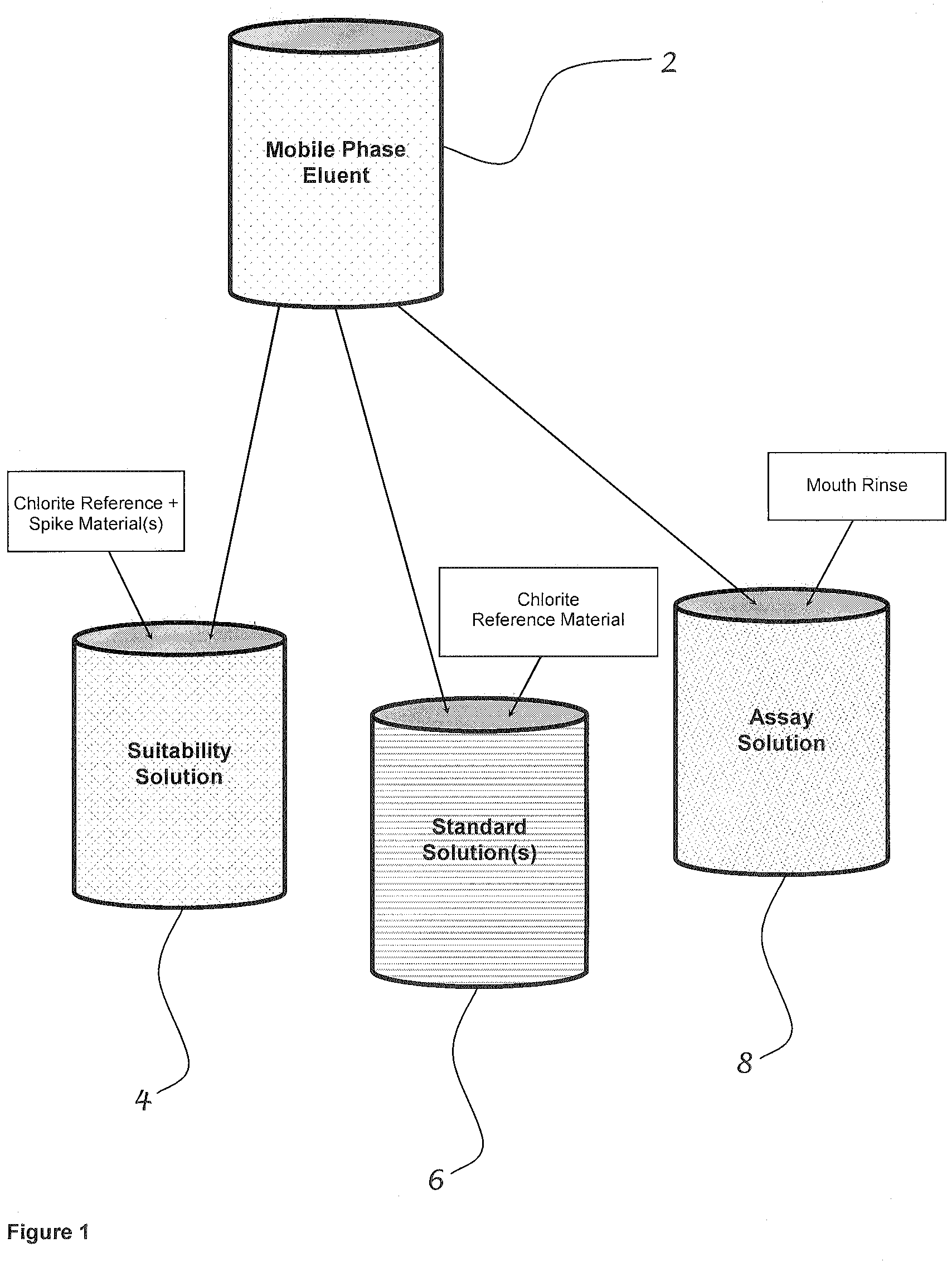
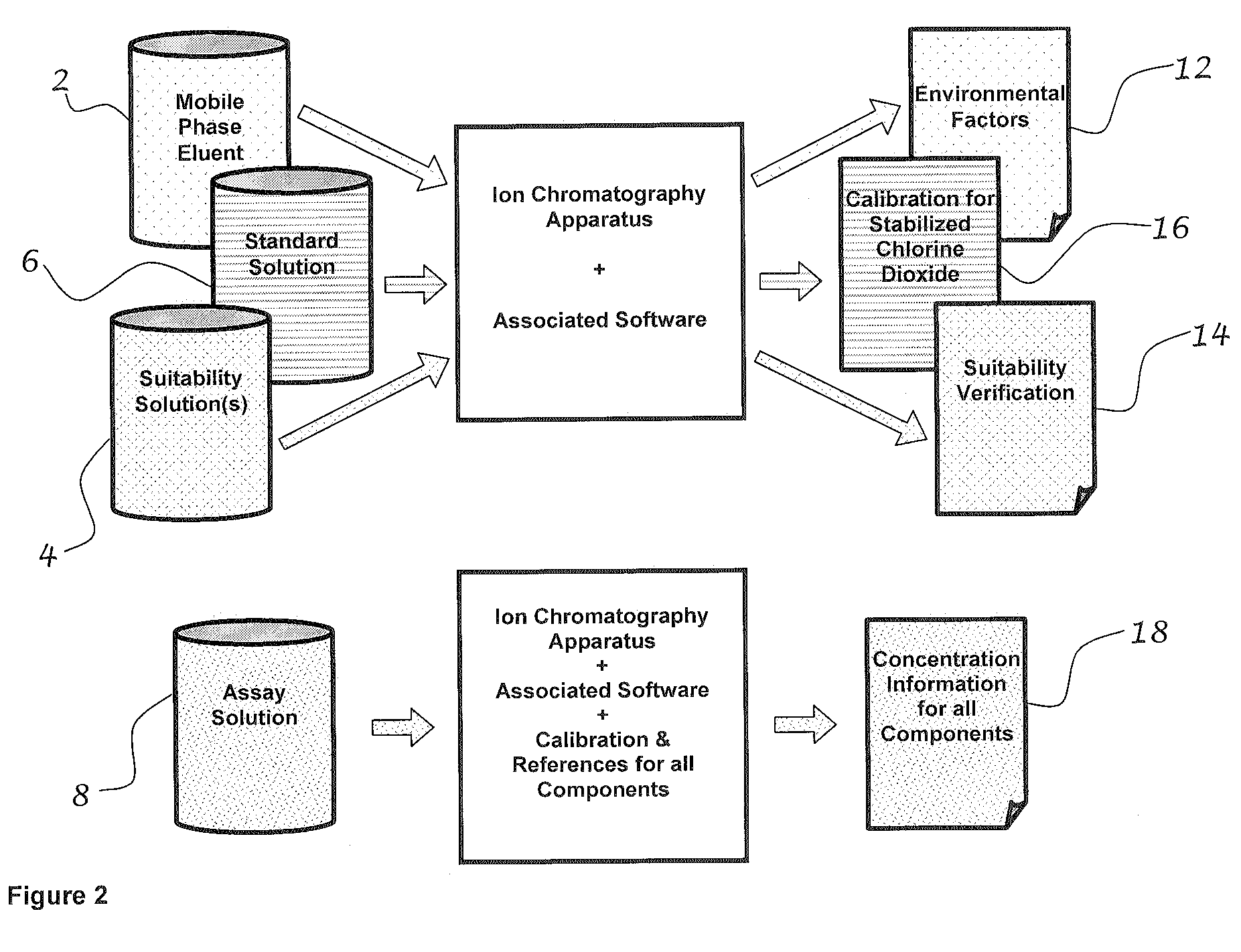
Method for determining the effectiveness of stabilized chlorine dioxide in a mouth rinse
ActiveUS7875460B2Accurate measurementCosmetic preparationsComponent separationChlorine dioxideDelivery system
A method for determining the available treatment dosage of stabilized chlorine dioxide in the prevention and the treatment of plaque accumulation, volatile sulfur compound production, gingivitis and periodontitis, and for differentiating the treatment dosage from other chlorine-containing compounds that may not have such beneficial effects is disclosed. When in solution as stabilized chlorine dioxide, the presence of other ions such as chlorate and chloride may not only obscure results as to the concentration of stabilized ClO2, but also reduce the predicted effectiveness. The present invention uses validated analytical methods to predict the effectiveness of stabilized ClO2 by more precisely measuring its concentration in solution. Such measurement renders precision at a level required of food-grade and pharmacy-grade chemotherapeutic agents in the oral cavity. Preferred concentrations are within the range of about 0.005 to about 2% (w / v) stabilized chlorine dioxide. The solution may be in the form of wash, rinse, soak, paste, gel, aerosol spray, or other suitable delivery system.
Owner:MICROPURE INC
Disinfectant composition with function of deodorization and bleaching effects and its prepn. method
A deodoring, bleeching and disintifecting composition in the form of powder, particle, lozenge, tablet, effervescent agent, liquid, colloid, or aerosol for various purposes is prepared from chlorinated isocyanuric acid, bromochloro hydrantoin, dibromo hydrantoin, or hypochlorite for providing hypochlorous acid component through reacting on chlorate, chlorite and tabilizer to obtain stable ClO2 system, and adding the corrosion inhibitor of metal.
Owner:高旭
Solid chlorine dioxide anti septic
A solid ClO2 disinfectant for the drinking water, food and medical apparatus is prepared through mixing oxalic acid with citric acid, grinding, adding dextrin, activating, mixing with sodium chlorite, aluminium chlorate, glucose and sodium chloride, and packing.
Owner:兰州金陵石化有限责任公司
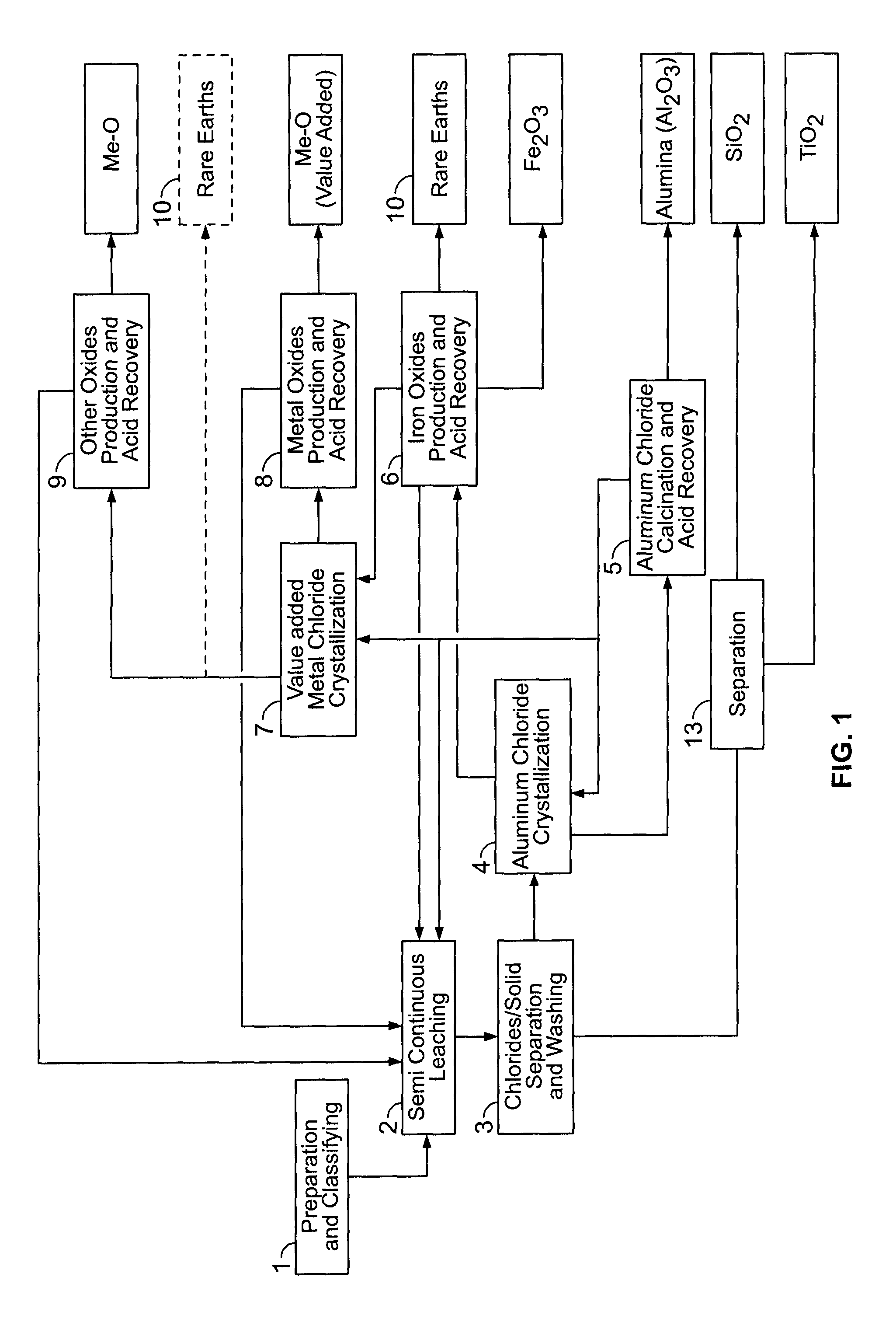
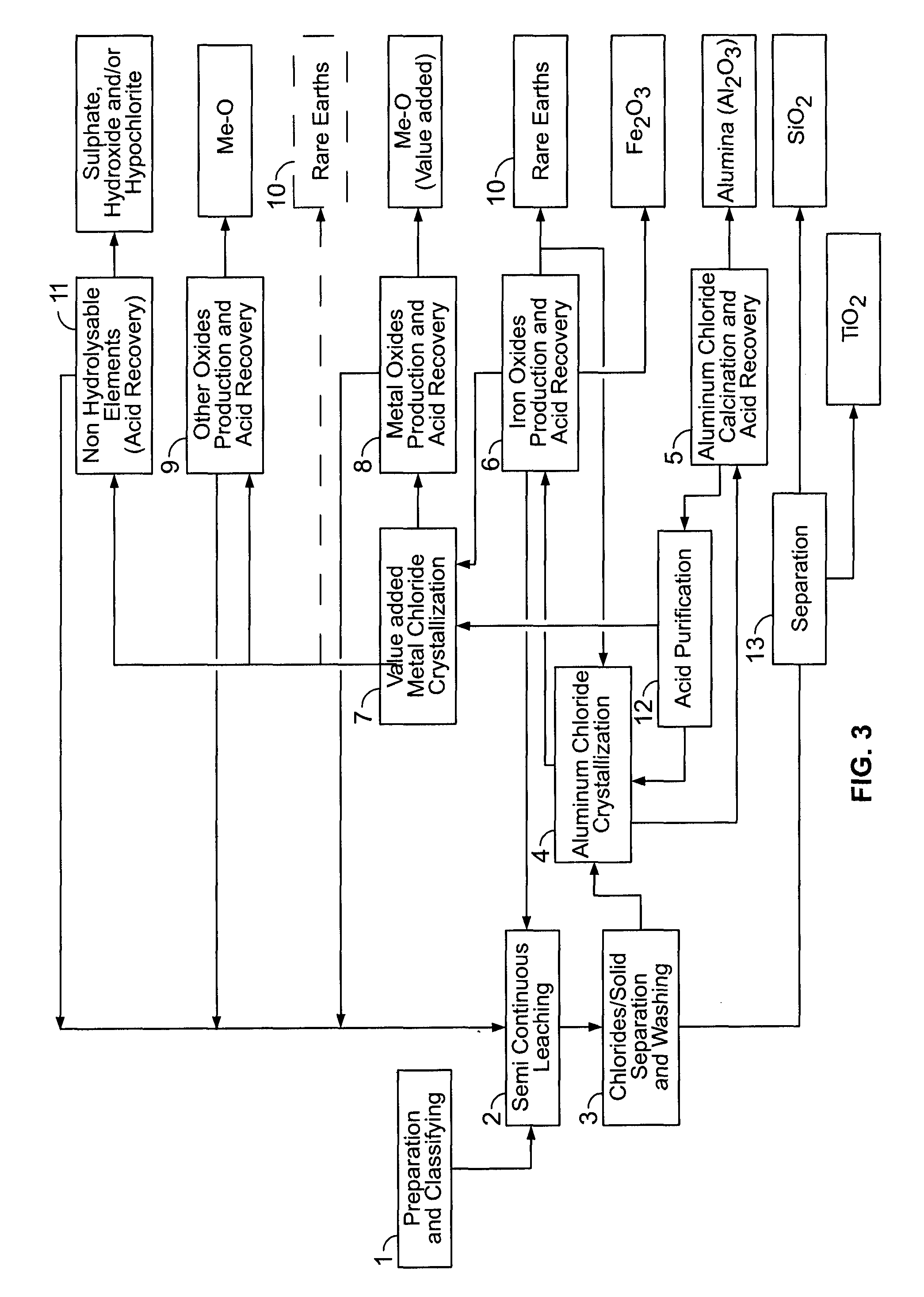
PROCESSES FOR PREPARING ALUMINA AND MAGNESIUM CHLORIDE BY HCl LEACHING OF VARIOUS MATERIALS
The disclosed processes can be effective for treating various materials comprising several different metals. These materials can be leached with HCl for obtaining a leachate and a solid. Then, they can be separated from one another and a first metal can be isolated from the leachate. Then, a second metal can further be isolated from the leachate. The first and second metals can each be substantially selectively isolated from the leachate. This can be done by controlling the temperature of the leachate, adjusting pH, further reacting the leachate with HCl, etc. The metals that can be recovered in the form of metal chlorides can eventually be converted into the corresponding metal oxides, thereby allowing for recovering HCl. The various metals can be chosen from aluminum, iron, zinc, copper, gold, silver, molybdenum, cobalt, magnesium, lithium, manganese, nickel, palladium, platinum, thorium, phosphorus, uranium, titanium, rare earth element and rare metals.
Owner:ORBITE ALUMINAE INC
Process for processing acidic arsenic-containing waste water
ActiveCN106517577AOxidation effect is thoroughReduce dosageWater contaminantsMultistage water/sewage treatmentChlorate ionWastewater
The invention provides a process for processing acidic arsenic-containing wastewater. According to the process, chlorate or / and perchlorate is adopted as an oxidizing agent to oxidize trivalent arsenic into pentavalent arsenic, and inorganic flocculating agent and organic flocculating agent are sequentially added for flocculating, precipitating and removing arsenic. By adopting the process, a solution can be oxidized more completely by adopting the oxidizing agent, so that the dosage of inorganic flocculating agent can be reduced, the amount of arsenic-containing residue can be reduced, and subsequent arsenic-containing residue treatment cost can be reduced. The process is simple, has the advantages of low cost, high arsenic removing rate and wide application range, enables the arsenic-containing amount of treated wastewater to be less than 50ppb, and can meet the emission requirement of different emission standards.
Owner:昆明先导新材料科技有限责任公司
ABC dry powder extinguishing agent
The invention relates to an ABC dry powder extinguishing agent. An extinguishing base material of the extinguishing agent is formed by nitrate (perchlorate) of <+2> valent or <+1> valent metal ions, or is formed by a mixture of the nitrate (perchlorate) with an imidazole compound; and the ABC dry powder extinguishing agent can be widely applied to extinguishing various combustible solids, liquid and gas fire disasters and can also be used for putting out electric fire disasters. Compared with an existing commonly-used ammonium phosphate ABC dry powder extinguishing agent, the ABC dry powder extinguishing agent has higher extinguishing efficiency.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Method for preparing a chlorine dioxide block-removing agent in oil wells
InactiveUS7131495B2Low viscosityFlows out easily and quickly kills variousSurveyCleaning apparatusChlorine dioxideAcid substances
A method to synthesize a chlorine dioxide block remover in a well is described. The method includes dissolving a chlorate aqueous solution and some acidic substances in water and to produce a hydrogen ion. The chlorate aqueous solution and acidic substances are quickly injected into the well by an injection pump, so they react with each other and produce chlorine dioxide. The synthesis of the chlorine block remover in the well removes oil layer blocks induced by polymers and microbes, while reducing the risk of explosion caused by leaked chlorine dioxide, and reducing the corrosion of equipment and pipes caused by chlorine dioxide.
Owner:HAO ZHANYUAN +1
Aluminum chlorate of polymerized silicic acid and preparation method
InactiveCN1915852ASolve pollutionAchieve recyclingWater/sewage treatment by flocculation/precipitationLiquid productAluminium chloride
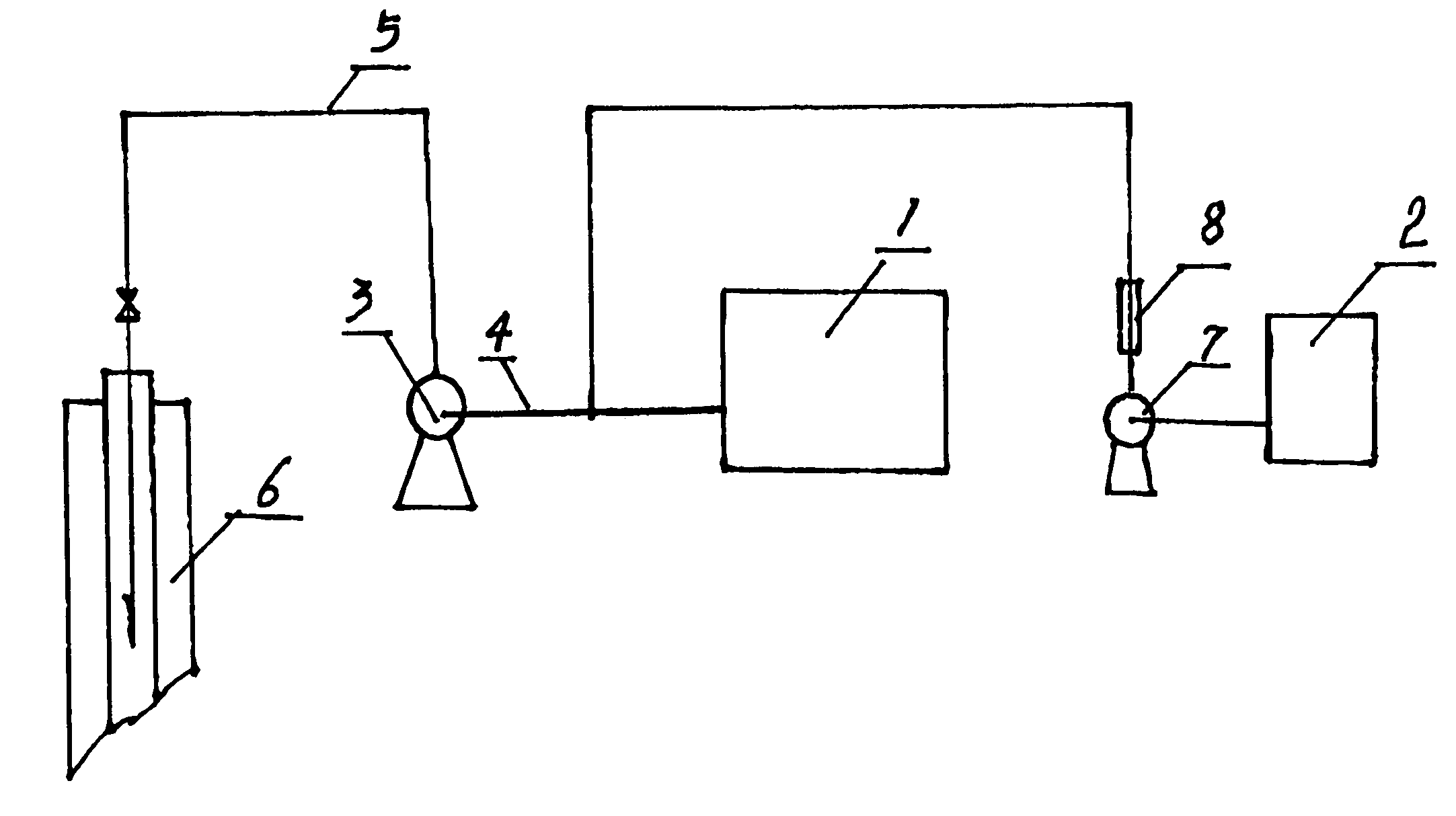
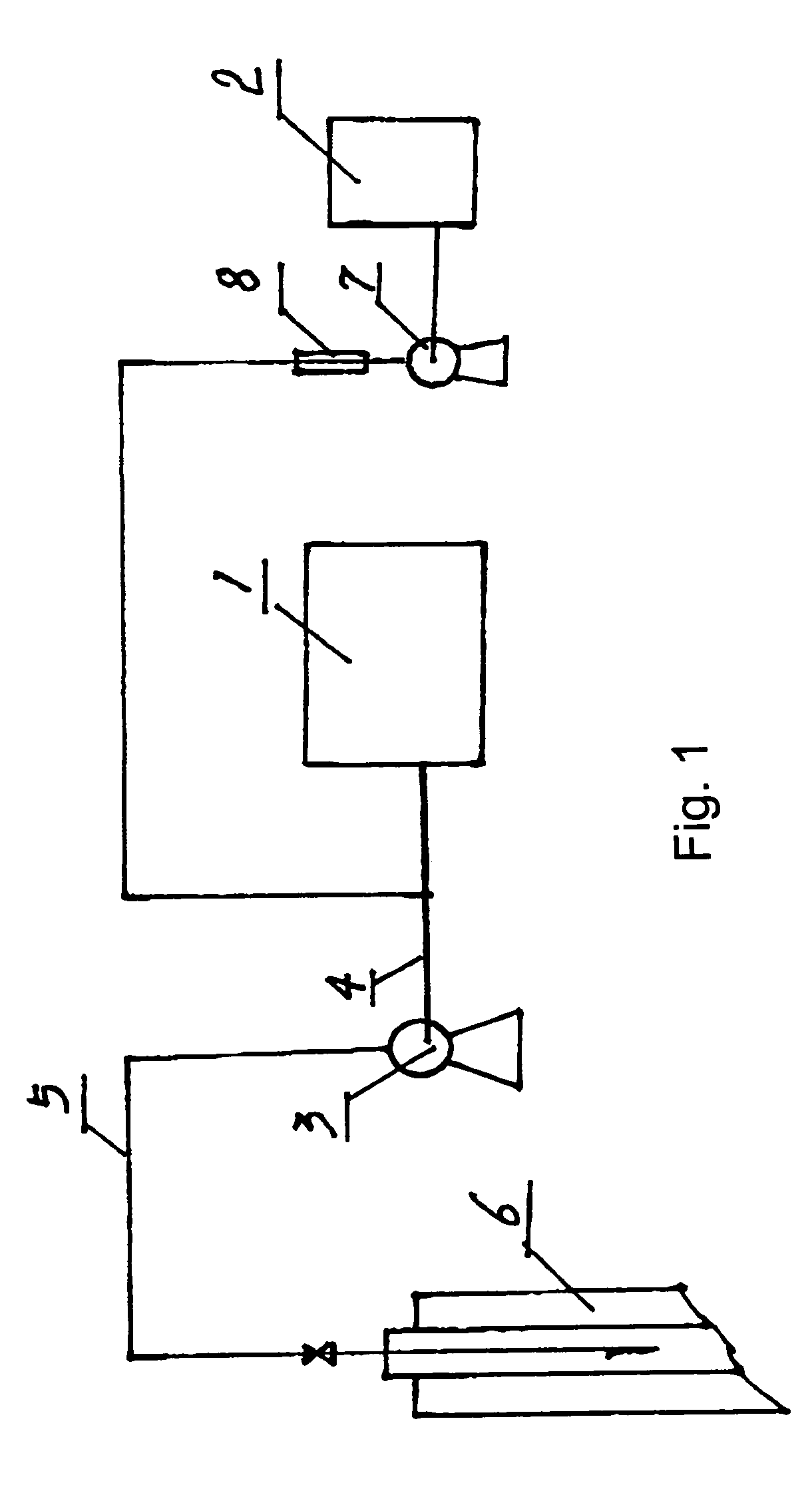
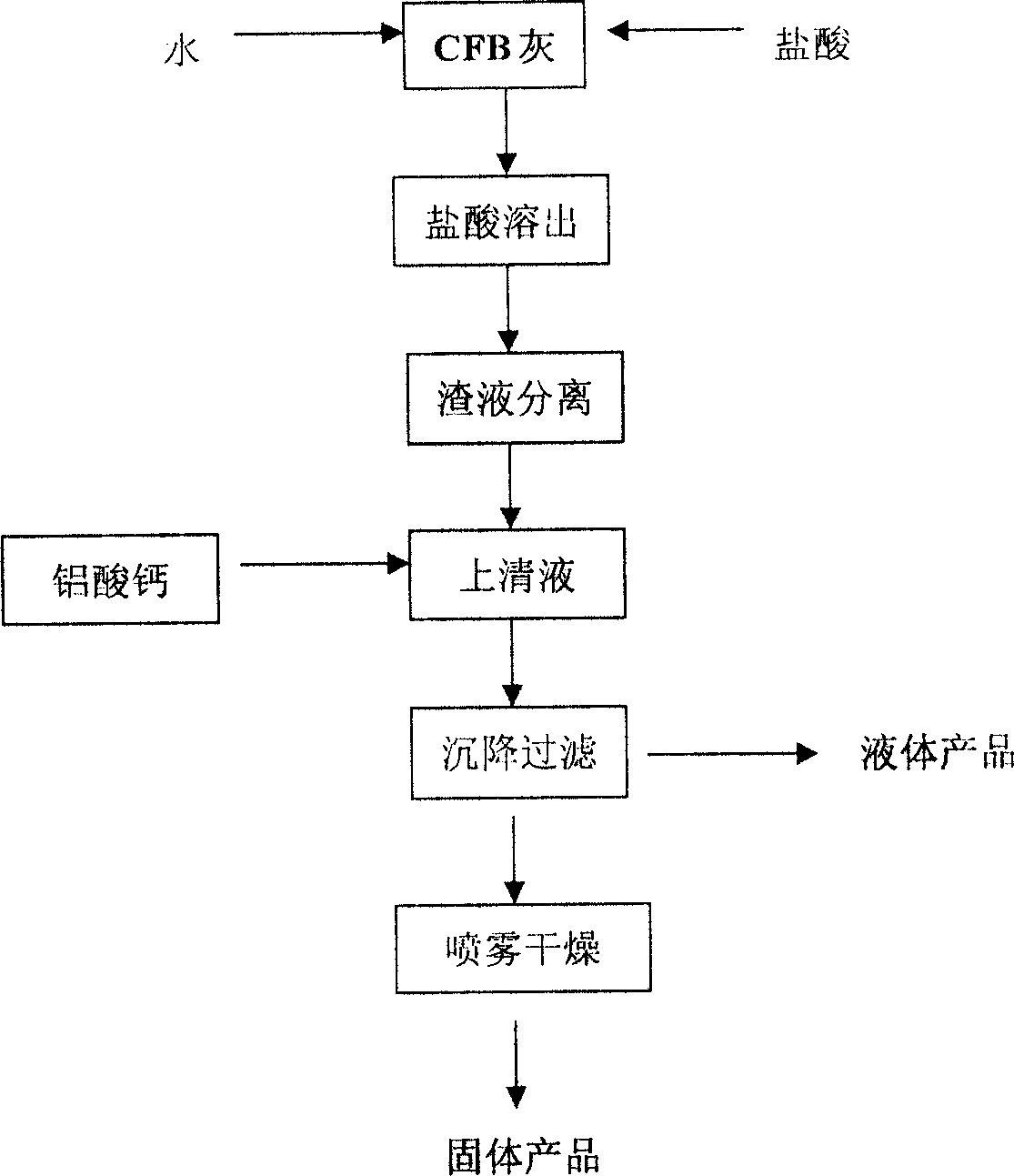
This invention relates to a method for preparing water purifier of polyaluminum ferrum chloride with fly ashes derived from circulating fluidized bed as the raw material. The method comprises: dissolving fly ashes derived from circulating fluidized bed in an acid to prepare aluminum ferrum chloride solution, and adjusting the base saturability to obtain the water purifier. The water purifier can satisfy related national standards. Compared with other water purifiers, the water purifier in this invention has such advantages as simple process, low cost and good water purification ability. The method realizes recovery and recycle of solid wastes, and solves the problem of fly ashes pollution.
Owner:JILIN UNIV
Method for extracting copper from organic silicon chemical waste residue leaching liquid of chloride and mixed salts containing copper
InactiveCN1844422AGuaranteed separation effectLow costPhotography auxillary processesCopper oxides/halidesChemical industryDecomposition
The invention selects oxalic acid solution to act as the precipitating agents of organosilicon chemical industry waste slag leachate of copper chlorate or mixture salt system, after filtering, washing and drying of the process residue of copper oxalic acid, heat decomposition in oxidizing atmosphere and at the temperature of 500~900 centigrade, the production of copper oxide is obtained by product yield cool-down. The yield acid of oxalic acid precipitation process is retuned organosilicon chemical industry waste slag leachate system to act as infusion. After the heat decomposition copper oxide dissolves in sulphuric acid, the solution can be used in electrodepositing cathode copper or regenerating copper sulphate, also can be used to produce cathode copper by copper electrodepositing solution dissolution regeneration electrodeposition, also sell as commercial copper oxide.
Owner:范有志
Tungsten polishing solution
InactiveUS7132058B2Other chemical processesSemiconductor/solid-state device manufacturingChlorate ionBromate
A tungsten CMP solution for planarizing semiconductor wafers includes a primary oxidizer having a sufficient oxidation potential for oxidizing tungsten metal to tungsten oxide; and the tungsten CMP solution has a static etch rate for removing the tungsten metal. A secondary oxidizer lowers the static etch rate of the tungsten CMP solution. The secondary oxidizer is selected from the group consisting of bromates and chlorates. Optionally the tungsten CMP contains 0 to 50 weight percent abrasive particles; and it contains a balance of water and incidental impurities.
Owner:ROHM & HAAS ELECTRONICS MATERIALS CMP HLDG INC
Method and process for synchronously removing perchlorate and nitrate in drinking water
InactiveCN103613210ASolve the problem of low pHSolve SO
<sub>4</sub>
<sup>2-</sup>
The problem of exceeding the standardWater contaminantsBiological water/sewage treatmentElectron donorNitrogen gas
The invention provides a method and process for synchronously removing perchlorate and nitrate in drinking water. In the method, perchlorate and nitrate in water are synchronously reduced through sulfur autotrophy and electrochemical hydrogen autotrophy coupling. The wastewater containing perchlorate and nitrate goes through a bio-membrane reactor taking sulfur particles as filler, and perchlorate and nitrate are partially reduced into chloride ions and nitrogen by taking elemental sulfur as an electron donor while H<+> and SO4<2-> are generated. A complex three-dimensional electrode-bio-membrane reactor taking a carbon medium as filler reduces H<+1> into H2 by use of an electrochemical process between a carbonaceous material and an electrode, and the rest perchlorate and nitrate are reduced into chloride ions and nitrogen by taking hydrogen as an electron donor. The method and process for synchronously removing perchlorate and nitrate in drinking water, provided by the invention, are economical and efficient and simple to operate.
Owner:OCEAN UNIV OF CHINA
Gas generator composition and molding thereof
InactiveUS6505562B1Improve combustion efficiencyReduce riskPedestrian/occupant safety arrangementNon-explosive/non-thermic compositionsKetoneOxygen
A gas generating composition for air bags to be used as occupant crash protection systems in automobiles, which is less toxic or dangerous, can be easily handled, has a high combustion efficiency and a high gas generation efficiency, produces few residue during combustion, can be safely manufactured and exhibits a high molding strength in the molding step; and a molded article thereof. The invention provides a gas generating composition containing (a) a fuel comprising at least one polymer compound selected from polyacrylic polymer compounds, polyacetal, urea resins, melamine resins, ketone resins and cellulose-based polymer compounds; (b) an oxidizing agent selected from ammonium nitrate and phase-stabilized ammonium nitrate; and (c) at least one combustion accelerator selected from oxyacid salts such as metal nitrates, metal nitrites, perchlorates and chlorates; a molded article thereof; and an inflator for air bags with the use of the same.
Owner:DAICEL CHEM IND LTD
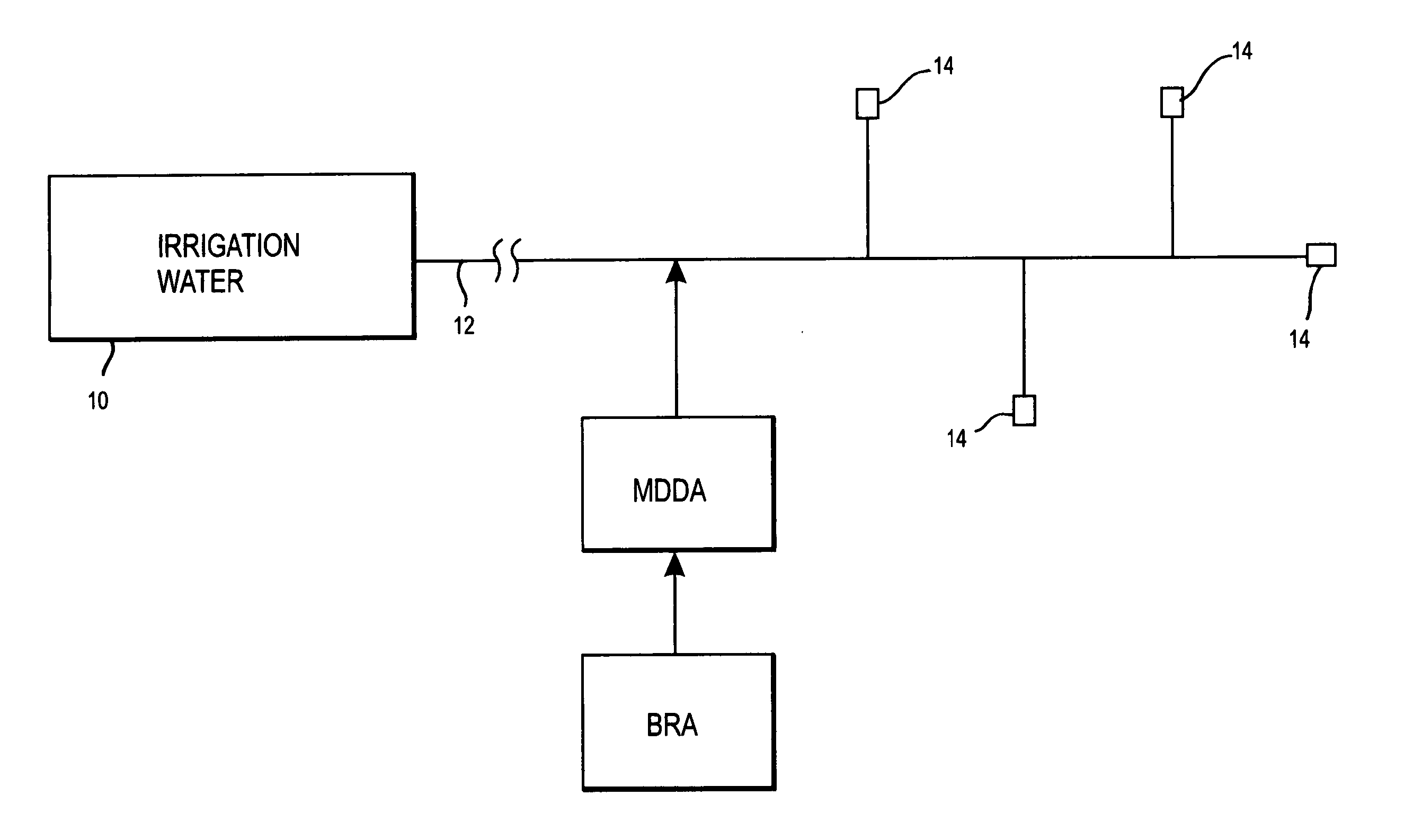
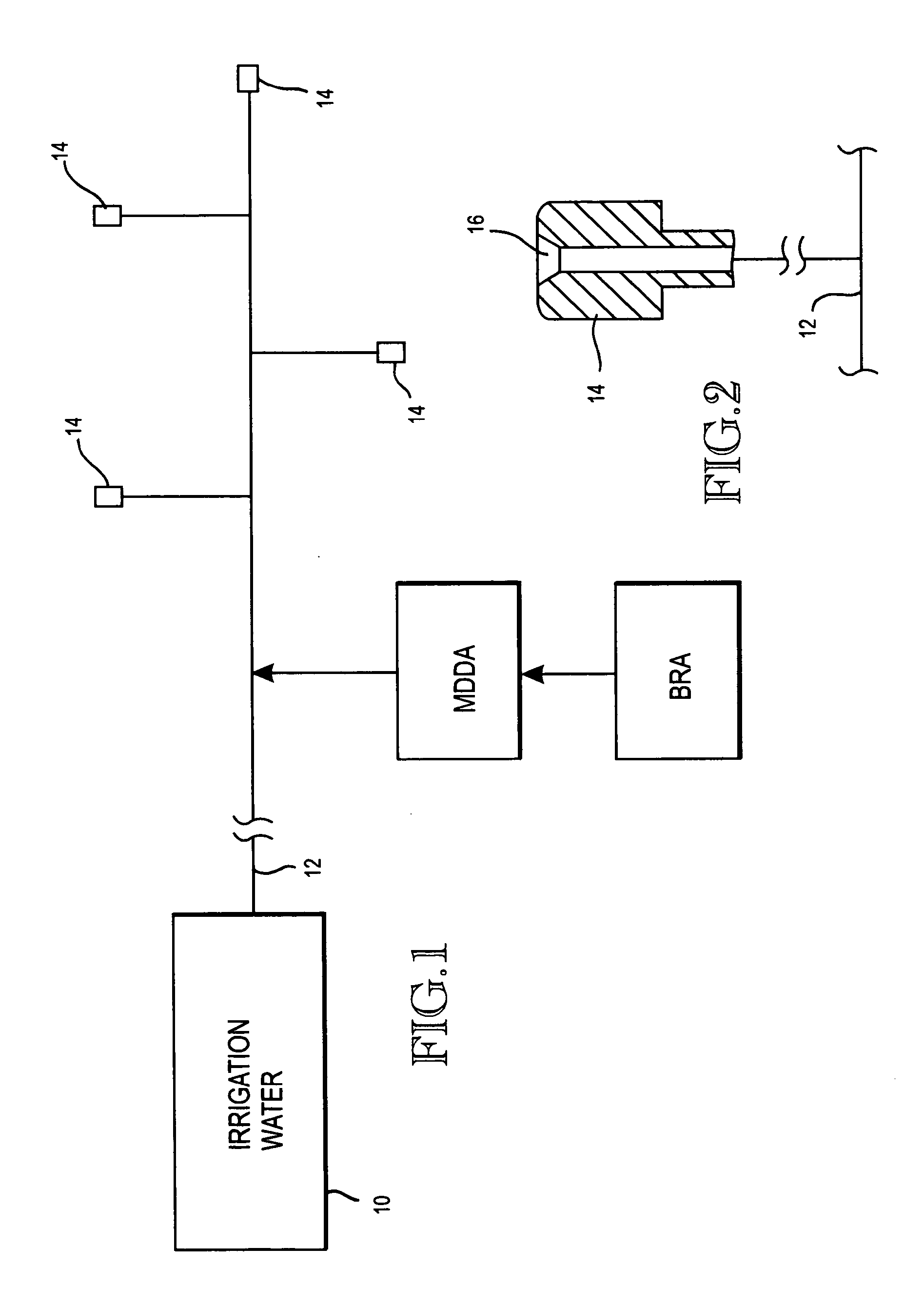
Method of promoting unrestricted flow of irrigation water through irrigation networks
ActiveUS20070257127A1Preserving unrestricted flowEliminates biofilm formationWatering devicesDifferential sedimentationHypochloriteChlorine dioxide
A biofilm reducing agent (BRA) and a mineral deposit distorting agent (MDDA) are admixed to irrigation water in amounts sufficient to substantially eliminate biofilm formation in the emitters (14) of an irrigation system (10) and produce amorphous mineral deposits in the emitters that are easily washed away by the irrigation water as it flows through the emitters (14). The BRA may be an oxidizer selected from the group consisting of chlorine, ozone, chlorine dioxide, hydrogen peroxide, hydroxy peracitic acid, iodine, bromine, hydrogen dioxide, chlorate salts, chlorite salts and hypochlorite compounds and mixtures thereof. The MDDA is a phosphonate selected from the group comprising AMP, ATMP, HEDP, EDTMPA, HMDTMPA, DETPMPA, BHMPTMPA, PBTC, HPA, PCA, NTMP, and DTPMP.
Owner:CH20
Method for preparing perchlorate by electrolysis of chlorate
InactiveCN1508291AIncrease productionImprove current efficiencyElectrolysis componentsChlorate ionElectrolysis
The invention discloses a method to prepare perchlorate by electrolyzation. Make the electrobaths is greater than or equal to 3 in series become a group and arrange according to graded position difference, make the electrolyte flow from the high-position electrobath to the highest-position one, after electrolyzed, overflow into next one for electrolyzation, then overflow into next one, and electrolyzed in the final one to obtain the finished electrolyte, and control the conversion within 65-85%, to use the finished electrolyte to produce perchlorate product. It improves the current efficiency by above 15%, each ton of perchlorate can reduce electric consumption by 800kw.h, and the output of the perchlorate can be raised by 15% above.
Owner:吴建国 +2
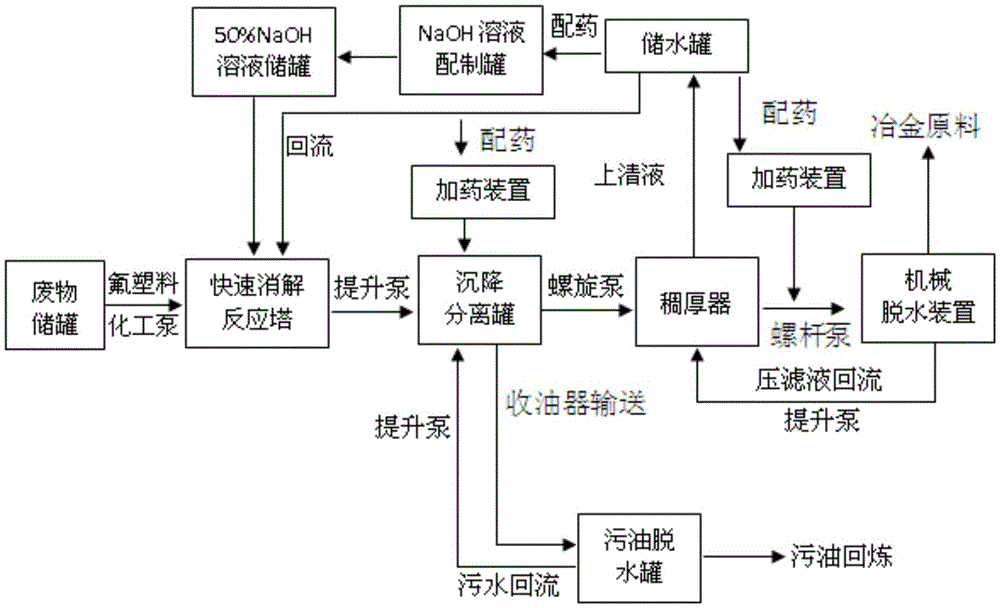
Method and system for processing aluminum chlorate class ionic liquid dead catalysts
The invention provides a method and system for processing aluminum chlorate class ionic liquid dead catalysts. The method includes the steps that a three-phase mixture is obtained through a degradation-neutralization reaction; the three-phase mixture and organic coagulant aids are mixed thoroughly and subjected to settling separation, and an acid soluble oil phase and a floc phase are collected respectively; the floc is condensed to form condensed slurry, part of aqueous solution separated out of condensation provides reaction medium for the degradation-neutralization reaction, and part of the aqueous solution is used for preparing an alkali solution and the organic coagulant aids; the condensed slurry is subjected to flocculation again and dehydrated to form dry residues, and the aqueous solution separated out after dehydration takes part in condensation treatment again. The acid soluble oil phase is dehydrated to obtain dehydrated sump oil, and discharged water is all returned. The processing system at least comprises a degradation reactor, a pipeline mixture, a settling separation device, a thickening device, a sump oil degradation tank and a slurry degradation device. The method and system for processing the aluminum chlorate class ionic liquid dead catalysts can lower the activity of catalyst residues. Oil and metal resources can be recycled. Application and popularization of an ionic liquid catalyzing process are promoted.
Owner:CHINA UNIV OF PETROLEUM (BEIJING)
Method for simultaneously removing sulfur dioxide and nitrogen oxide in exhaust air
InactiveCN101274208AReduce governance costsSimple methodDispersed particle separationAir quality improvementChlorate ionNitrogen oxides
The invention provides a wet-method oxidation method for removing SO2 and nitrogen oxides in exhaust gases, which is characterized in that in a routine reactor, water solution of chlorate and desulfurizer is reacted with flue gas containing the nitrogen oxides and SO2, so as to remove the nitrogen oxides and SO2 in the exhaust gas. The wet-method oxidation method of the invention is simple, economical and reasonable, and greatly reduces the disposal cost of the wet-method desulphurization and denitration.
Owner:EAST CHINA UNIV OF SCI & TECH
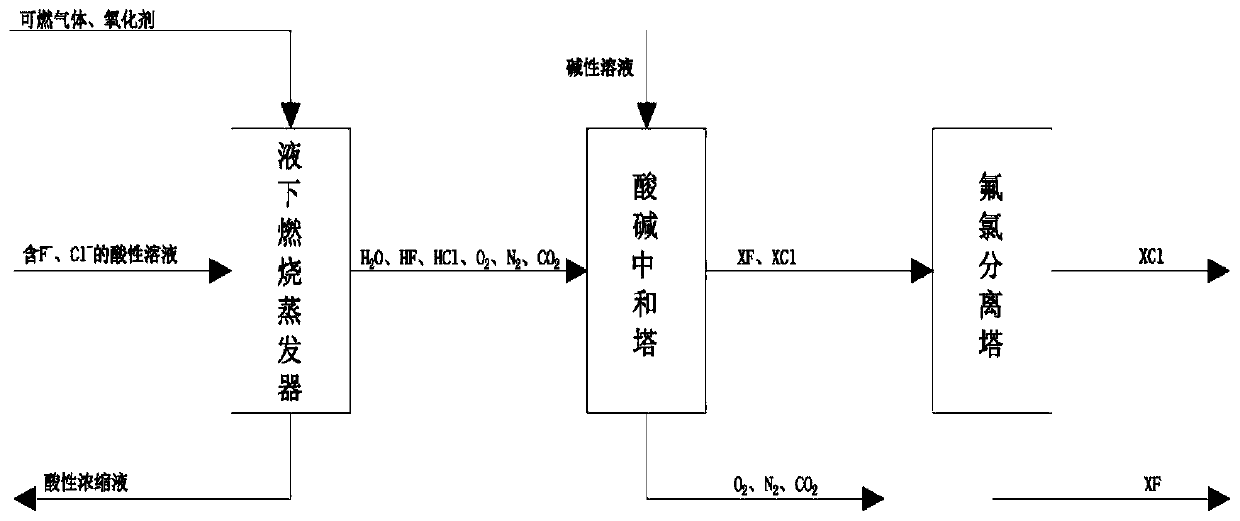
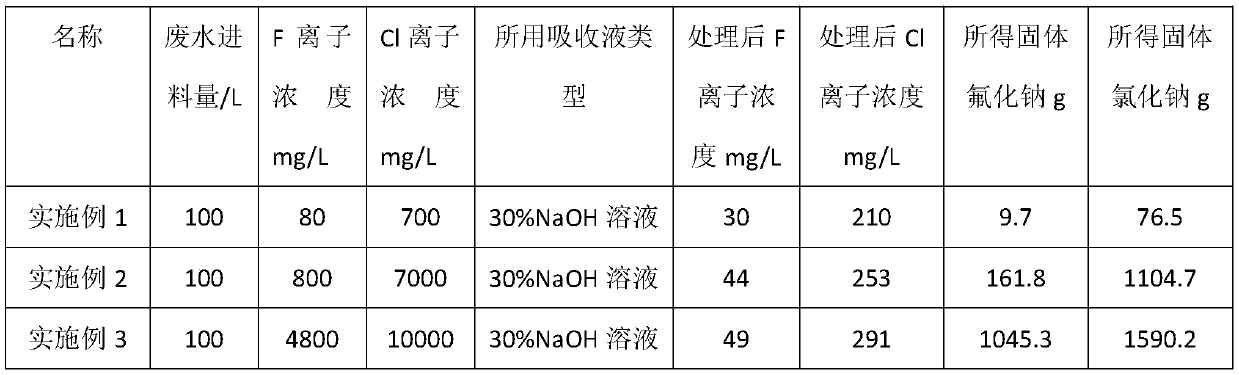
Method for treating fluorine ions and chlorine ions in acidic wastewater solution
InactiveCN110194557ASolve processingSolve the problem of energy consumptionWater contaminantsTreatment involving filtrationSolubilityHigh energy
The invention relates to a method for treating fluorine ions and chlorine ions in an acidic wastewater solution. The method comprises: carrying out evaporation concentration, wherein an acidic wastewater solution containing fluorine ions and chlorine ions is introduced into a submerged combustion evaporator, the acidic wastewater solution is subjected to evaporation concentration by using combustion hot flue gas, and the fluorine ions and the chlorine ions are taken out in the forms of gases of HF and HCl; carrying out acid-alkali neutralization, wherein the HF, the HCl and other gases evaporated in the previous step are introduced into an acid-alkali neutralization tower, and an alkaline solution downward sprayed by the tower top and the rising gases of HF and HCl are subjected to countercurrent contact so as to be neutralized, such that a fluoride salt solution and a chlorate solution are converted; and introducing the fluoride salt solution and the chlorate solution obtained by theacid-alkali neutralization tower into a fluorine-chlorine separation tower, and carrying out re-crystallizing separation based on the difference in solubility between fluorine and chlorine to obtaina solid fluoride and a chloride. According to the present invention, the method has advantages of simple process, convenient operation and low processing cost, and can solve the to-be-solved problemsof difficult treatment of fluorine ions and chlorine ions in acidic wastewater, high energy consumption of multi-effect evaporation, and the like in the current metallurgical industry are solved.
Owner:YANTAI UNIV
Long-acting controllable chlorine dioxide sustained release agent
InactiveCN104255785AReduce manufacturing costSimple manufacturing stepsBiocideFruit and vegetables preservationChlorate ionChlorine dioxide
The invention belongs to the field of chemical products, and specifically relates to a long-acting controllable chlorine dioxide sustained release agent. The chlorine dioxide sustained release agent is composed of a component A, a component B, and a component C according to a mass ratio of (1-10):(0.1-5):(10-1); wherein component A is a mixture of chlorate and halide, component B is an organic carboxylic acid, and component C is an absorbing medium. The chloride dioxide sustained release agent obtained by the provided optimal formula and manufacturing method has the advantages of low manufacture cost, simple manufacture steps, long slow release time, and controllability.
Owner:艾波
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com