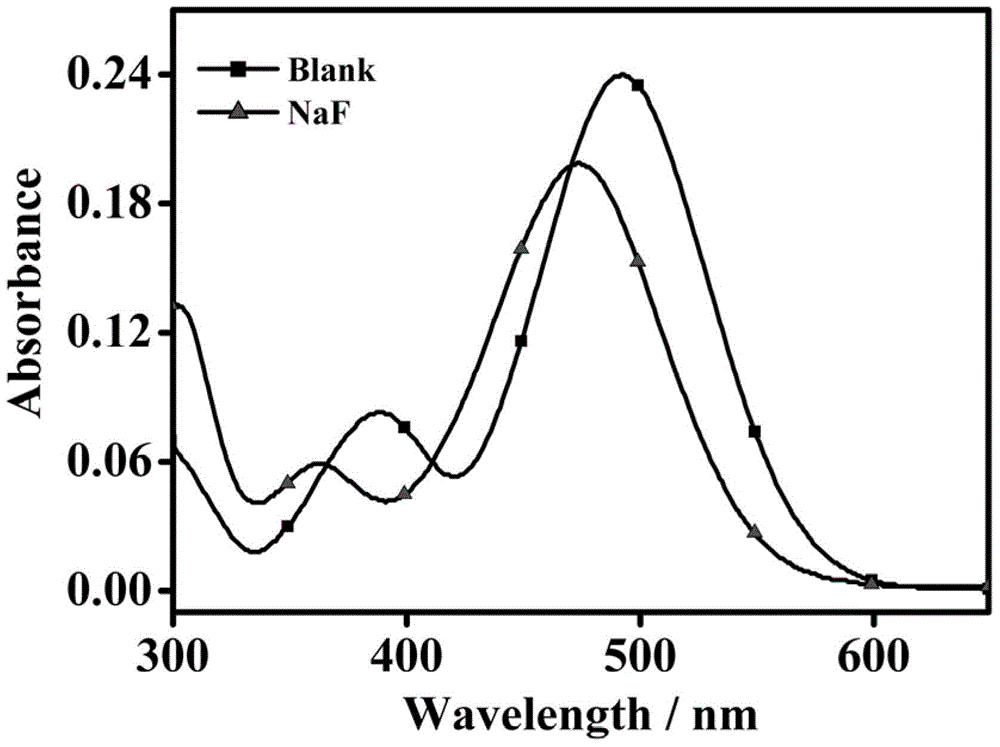
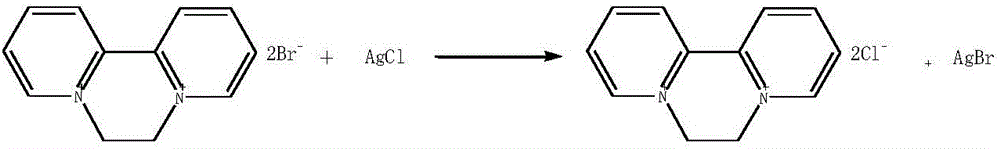
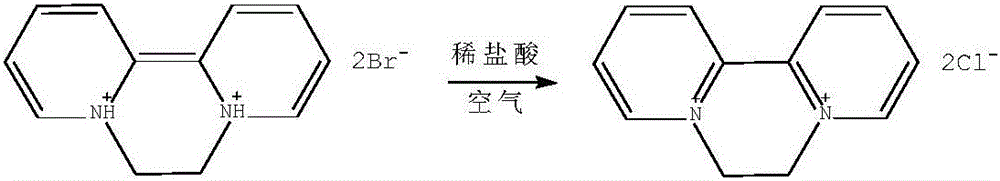
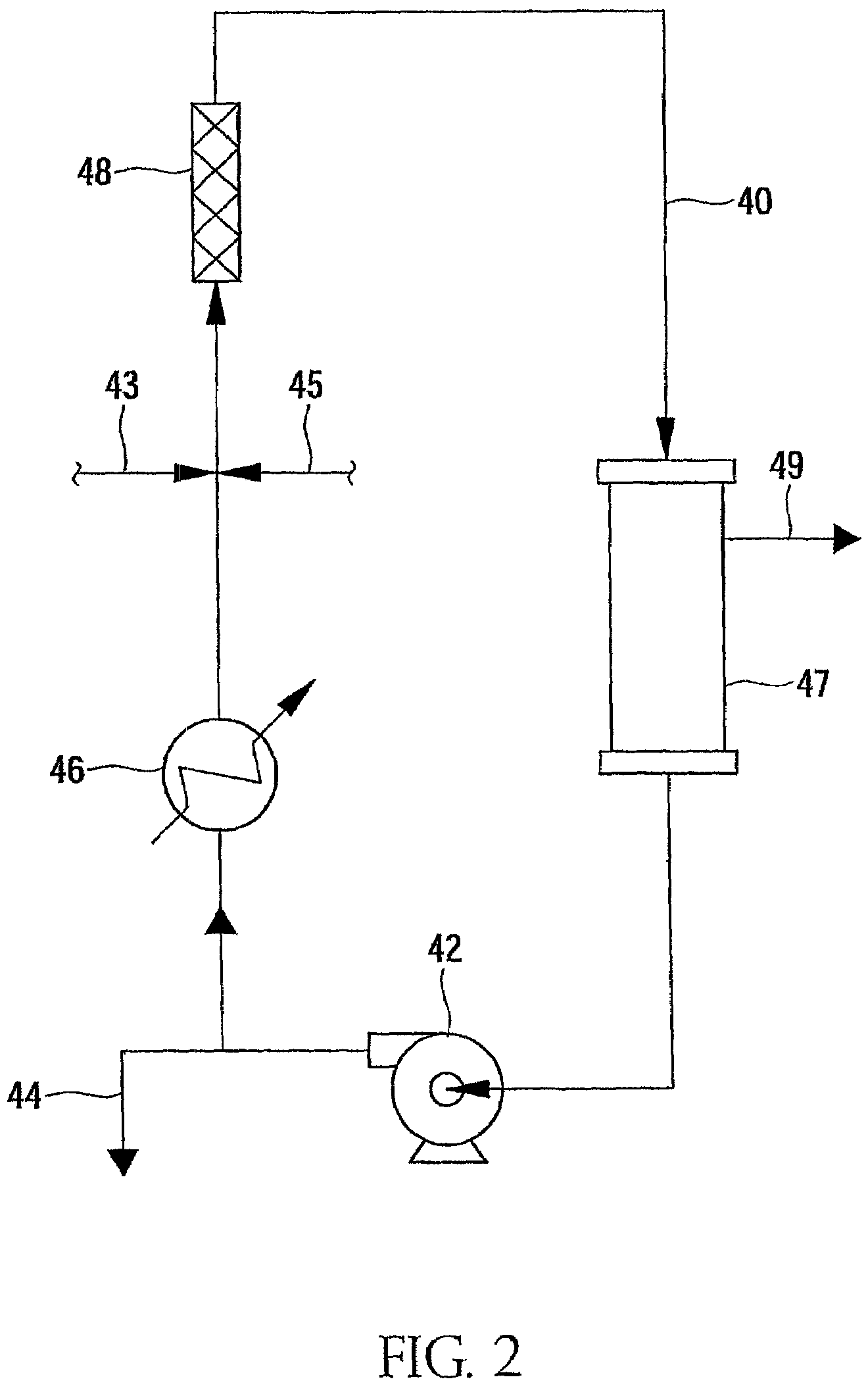
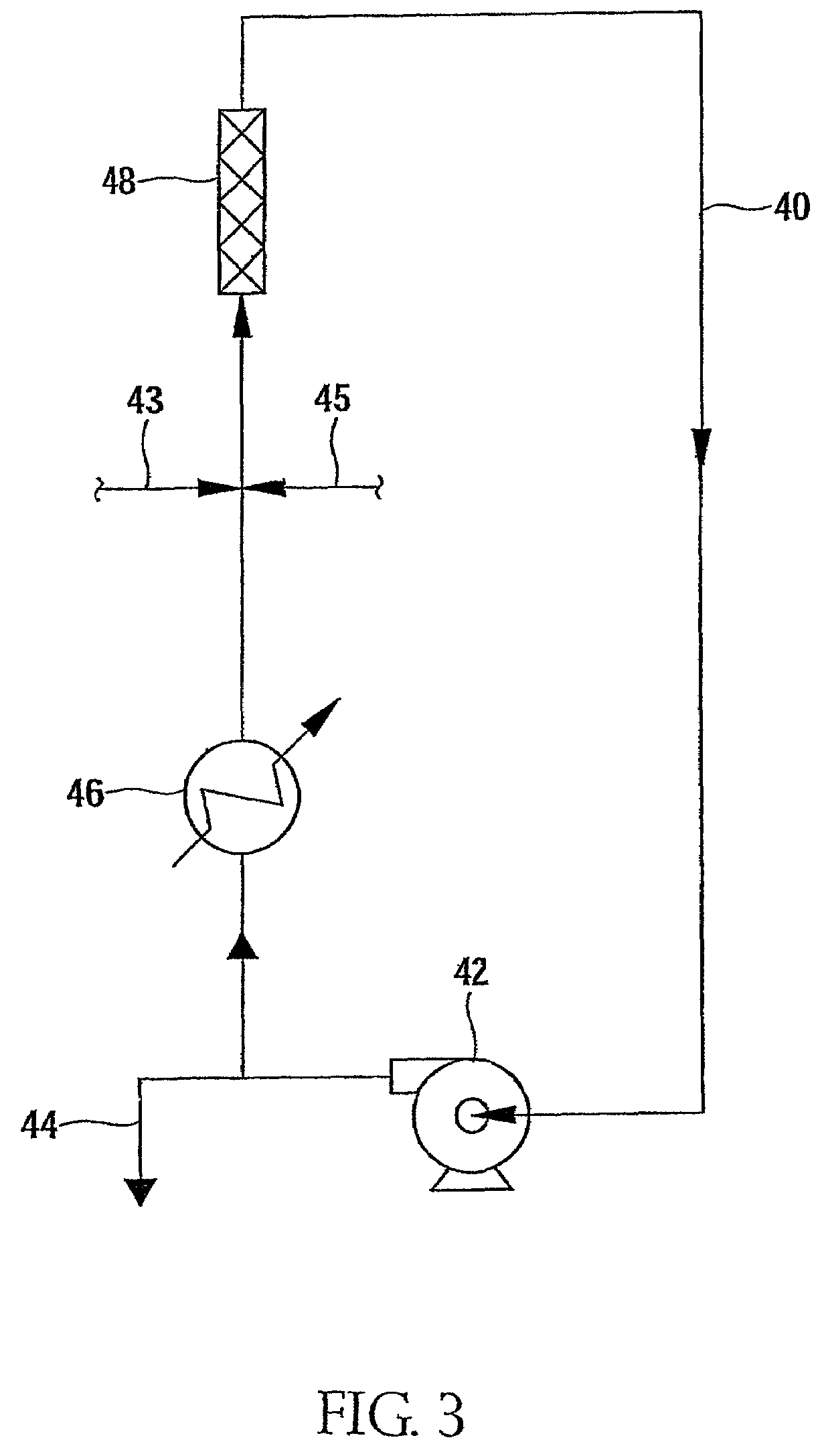
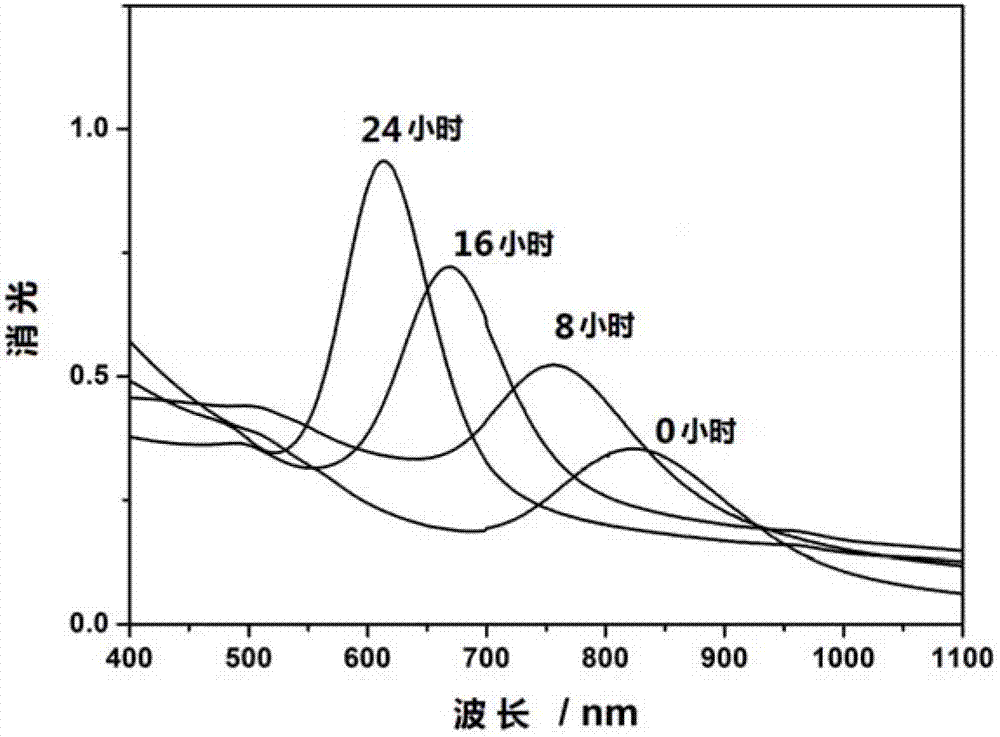
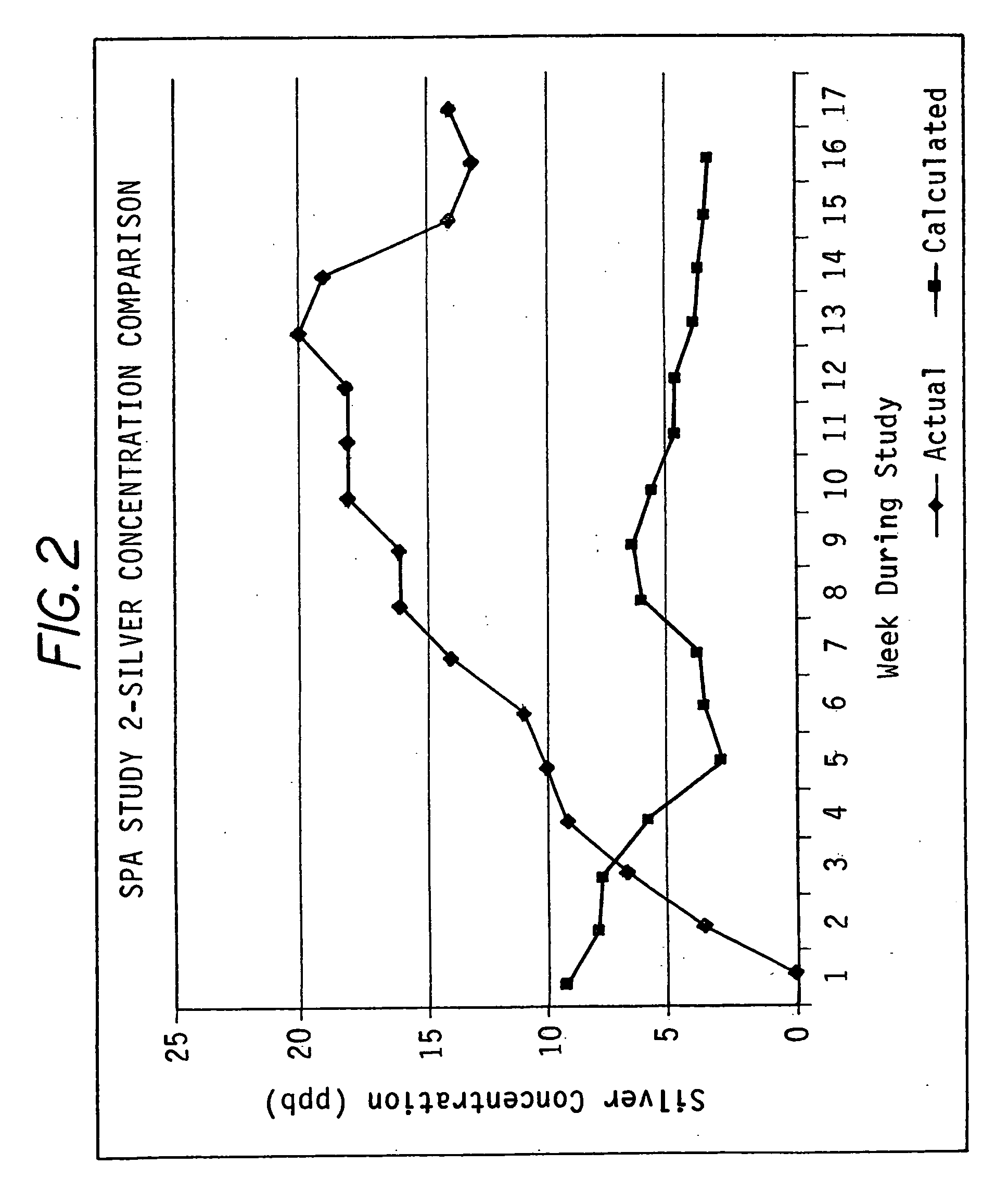
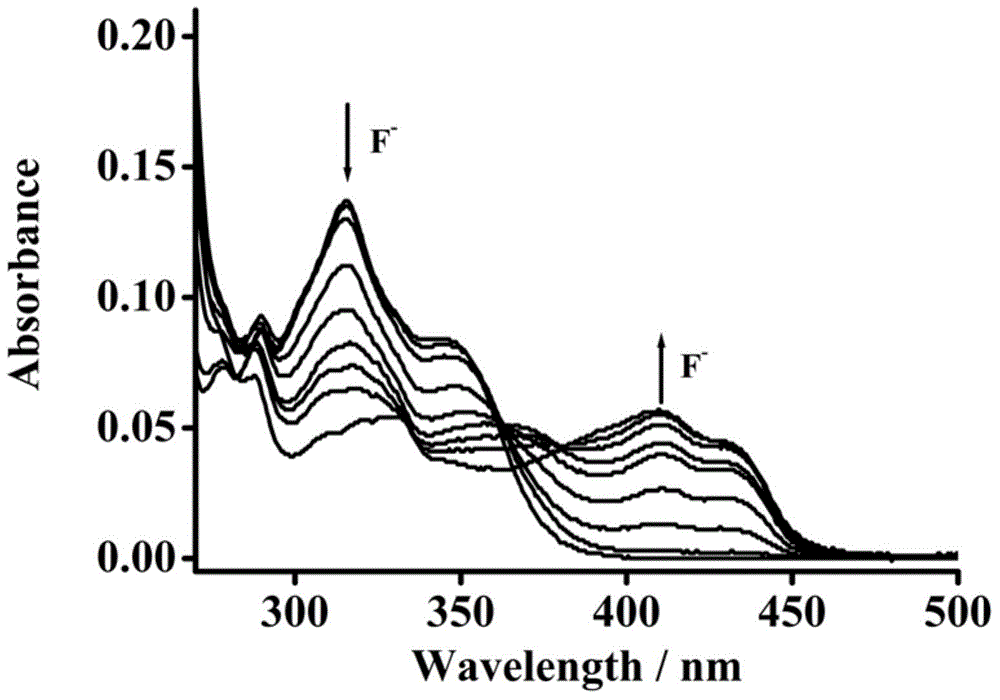
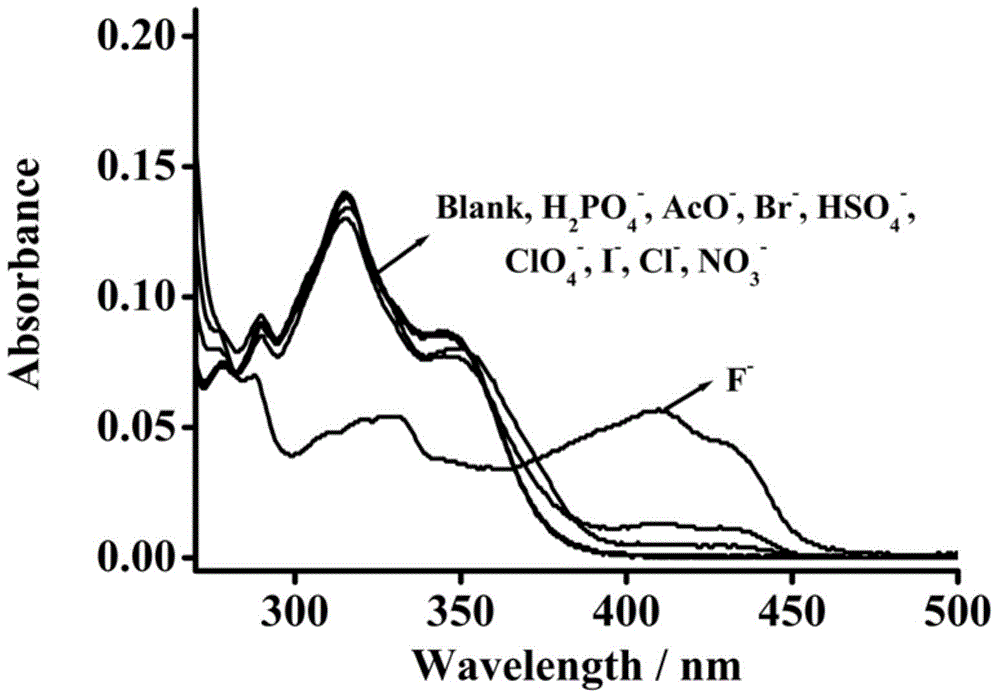
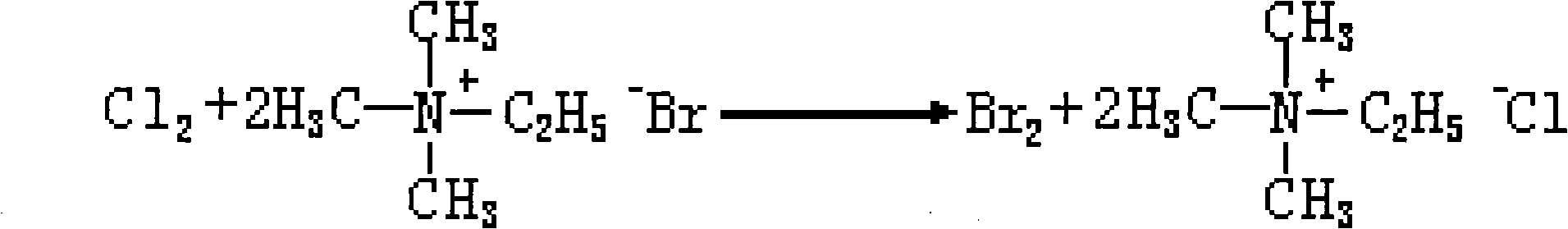Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
334 results about "Bromide ions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
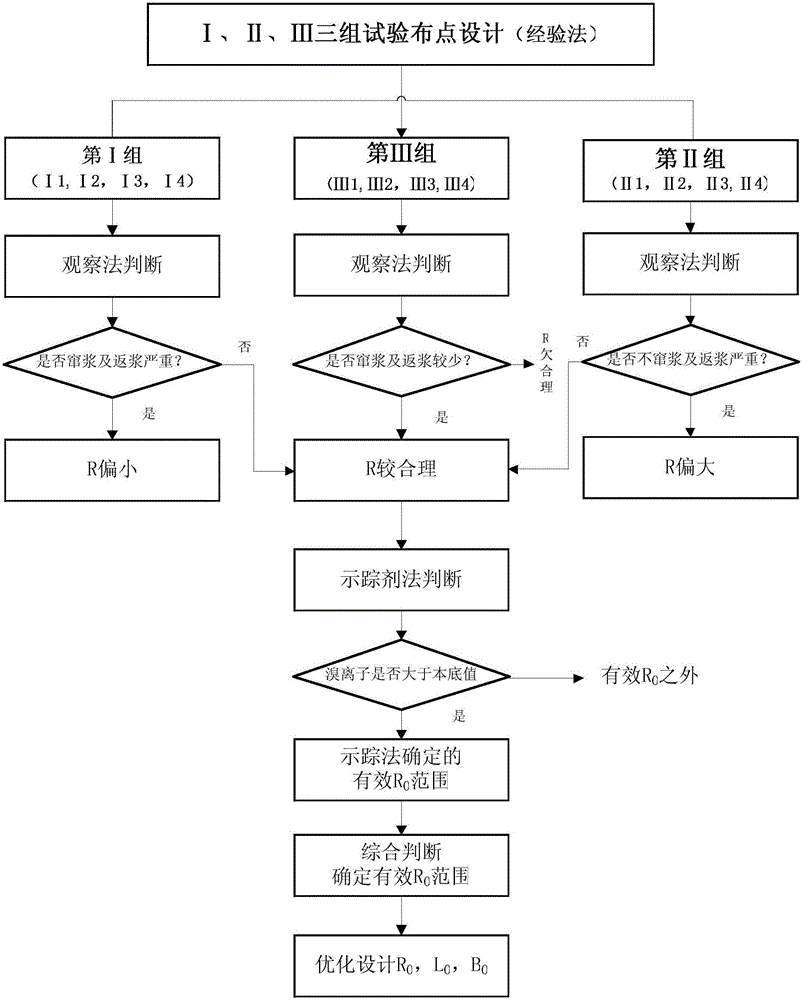
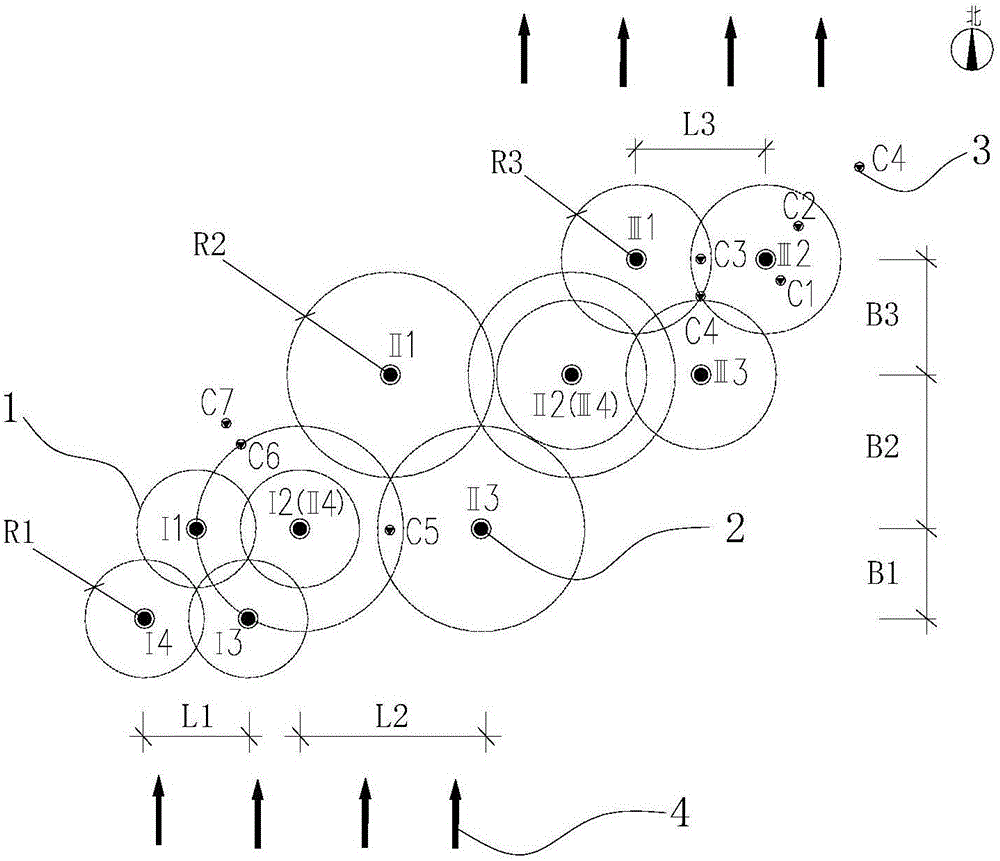
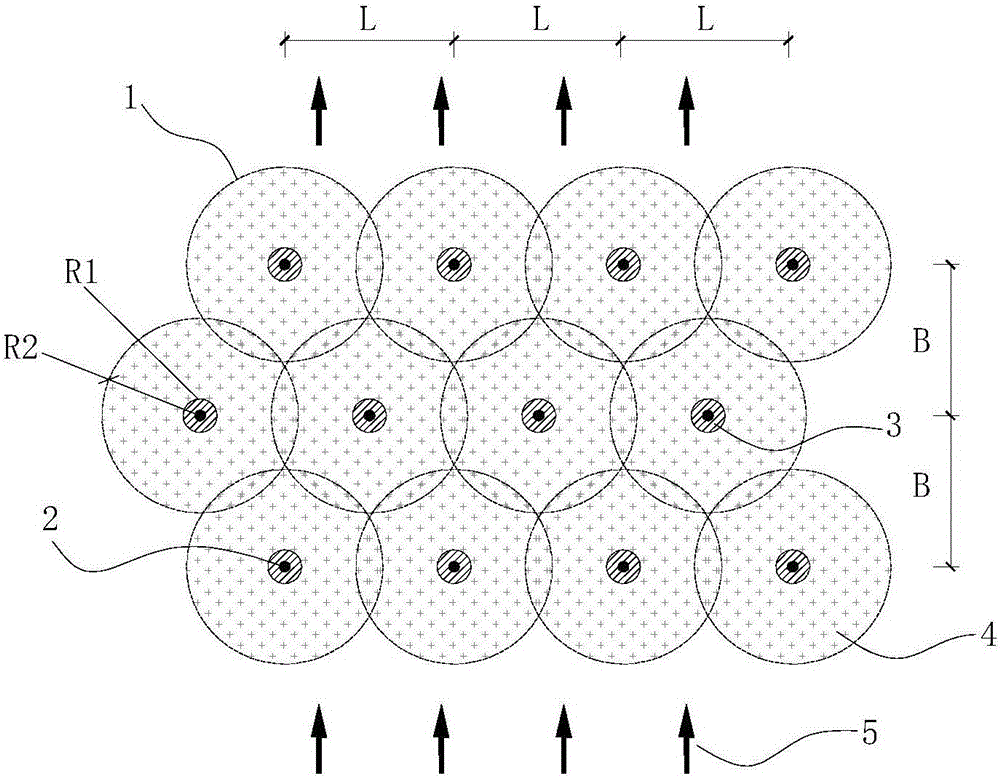
Method for determining in situ injection repair diffusion radiuses of contaminated soil and underground water
ActiveCN105973759ASolve the problem of back slurryEasy to operateConstructionsContaminated soil reclamationHigh pressureInjection test
The invention discloses a method for determining in situ injection repair diffusion radiuses of contaminated soil and underground water. According to the method, points are distributed according to a triangle method, pitch-row is designed to be perpendicular to the flowing direction of the underground water, array pitch is in the flowing direction of the underground water, and the flow expansion of a repair reagent in the underground water within the reaction effective time is taken into account. The method comprises three steps: firstly, observation method: the repair reagent and a definite proportion of bromide ion serving as tracers are simultaneously injected into an aqueous stratum in the condition of high pressure whirl spraying injection. The diffusion of the reagent is judged by observing the phenomena of grout return and grout leaking between adjacent injection points; secondly, bromide ion tracer method: after the completion of injection, underground water depth setting rapid sampling is performed, the concentration of the tracers is rapidly detected in field, and the concentration of the bromide ion in the underground water is compared with a background value; thirdly, bromide ion tracer method and observation method: the optimum diffusion radiuses are determined through synthetic judgment. The method is applied to optimal design of diffusion radiuses and hole arrangement parameters and monitoring of injection effect in soil and underground water in situ injection tests and repair construction.
Owner:BCEG ENVIRONMENTAL REMEDIATION CO LTD
Magnesium ion-containing non-aqueous electrolyte and a production process thereof, as well as electrochemical device
ActiveUS20090068568A1Effective resourcesEfficient use ofCell electrodesOrganic electrolyte cellsAluminum IonPhosphate ion
A magnesium ion containing non-aqueous electrolyte in which magnesium ions and aluminum ions are dissolved in an organic etheric solvent, and which is formed by: adding metal magnesium, a halogenated hydrocarbon RX, an aluminum halide AlY3, and a quaternary ammonium salt R1R2R3R4N+Z− to an organic etheric solvent; and applying a heating treatment while stirring them (in the general formula RX representing the halogenated hydrocarbon, R is an alkyl group or an aryl group, X is chlorine, bromine, or iodine, in the general formula AlY3 representing the aluminum halide, Y is chlorine, bromine, or iodine, in the general formula R1R2R3R4N+Z− representing the quaternary ammonium salt, R1, R2, R3, and R4 represent each an alkyl group or an aryl group, and Z− represents chloride ion, bromide ion, iodide ion, acetate ion, perchlorate ion, tetrafluoro borate ion, hexafluoro phosphate ion, hexafluoro arsenate ion, perfluoroalkyl sulfonate ion, or perfluoroalkyl sulfonylimide ion.
Owner:MURATA MFG CO LTD
Method of preparing a biocide comprising stabilized hypochlorite and a bromide ion source and a method of controlling microbial fouling using the same
InactiveUS20050147528A1Long retentionIncreased durabilityBiocideSpecific water treatment objectivesAlkaline earth metalBromide ions
Disclosed is a method of preparing a biocide having improved durability of its biocidal activity as well as disinfection efficiency at an initial stage, comprising the steps of: (a) preparing stabilized alkali or alkaline earth metal hypochlorite having a pH at least 11 by mixing a chlorine oxidant including alkali or alkaline earth metal hypochlorite with a stabilizer in an alkali solution; (b) preparing a bromide ion source; and (c) adding the bromide ion source prepared in step (b) into the stabilized alkali or alkaline earth metal hypochlorite prepared in step (a). Also, a method of controlling the growth of microorganisms using a biocide prepared by the method of the present invention is disclosed.
Owner:ACCU LAB CO LTD
Brominated anionic styrenic polymers and their preparation
Concurrently fed into a reaction zone held at about 10° C. or less are brominating agent, aluminum halide catalyst, and a solution of anionic styrenic polymer having a GPC Mn about 2000-30,000. The components are in at least two separate feed streams. The feeds are proportioned to maintain (a) the amount of aluminum halide being fed at about 0.8 mole percent or less based on the amount of aromatic monomeric units in the polymer being fed, and (b) amounts of brominating agent and unbrominated polymer in the reaction zone that produce a final washed and dried polymer product containing about 60-71 wt % bromine. The catalyst is deactivated, bromide ions and catalyst residues are washed away from the reaction mixture, and the brominated anionic styrenic polymer is recovered and dried. The dried polymer has a volatile bromobenzene content of about 600 ppm (wt / wt) or less as well as other beneficial properties.
Owner:ALBEMARLE CORP
Preparation method for ionic-liquid-modified carbon quantum dot
ActiveCN105315993AHigh yieldHigh fluorescence quantum yieldLuminescent compositionsModified carbonPhysical chemistry
The invention discloses a preparation method for an ionic-liquid-modified carbon quantum dot. The preparation method comprises dissolving citric acid and aminoimidazolium bromide in ultrapure water, and dewatering to obtain a gelatinoids, then stirring and refluxing, adding ultrapure water for once-more dispersing after cooling, and then dialyzing for 2-3 days, and drying to obtain a carbon quantum dot of which the anion is bromide ion; dissolving the carbon quantum dot in ultrapure water, introducing a specific anion (such as N(CF3SO2)<2->) and an oil phase (such as ethyl acetate) into the carbon-quantum-dot-dissolved ultrapure water, so as to enable the carbon quantum dot to have anion exchange and be transferred from the ultrapure water phase to the oil phase, and separating the oil phase, so as to obtain the carbon quantum dot of which the anion is N(CF3SO2)<2->; and continuing introducing other anions(such as Cl<->), so as to enable the carbon quantum dot to be transferred into the ultrapure water phase from the oil phase. Through once or multitime phase transfer and separation of the oil phase and the ultrapure water phase, a series of high-purity carbon quantum dots which contain different anions and are adjustable in dissolvability and fluorescence property are obtained.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Developing roller, electrophotographic process cartridge, and electrophotographic image forming apparatus
ActiveUS8913930B2Improve charging effectHigh quality imagingSynthetic resin layered productsElectrographic process apparatusHydrogen atomEngineering
Provided is the following developer carrying member. The member has high charge-providing performance even under a high-temperature, high-humidity environment, and its surface layer hardly peels off its elastic layer even after long-term standing under the high-temperature, high-humidity environment. The developer carrying member comprises a mandrel, an elastic layer including a silicone rubber, and a surface layer covering a surface of the elastic layer, and the surface layer comprises a binder resin, and a copolymer having structural units of formula (1) and formula (2). R1 represents an alkyl group having 10-18 carbon atoms, R2 represents a methyl group or a hydrogen atom, R3 represents an alkylene group having 1-4 carbon atoms, X− represents a chloride ion, a bromide ion, or a p-toluenesulfonic acid ion, R4 represents a methyl group or a hydrogen atom, and R5 represents an alkylene group having 1-4 carbon atoms.
Owner:CANON KK
Method for preparing high-purity tetrabutyl ammonium hydroxide by continuous electrolysis
InactiveCN104278288ALow costNot prone to lifeElectrolysis componentsElectrolytic organic productionElectrolysisWastewater
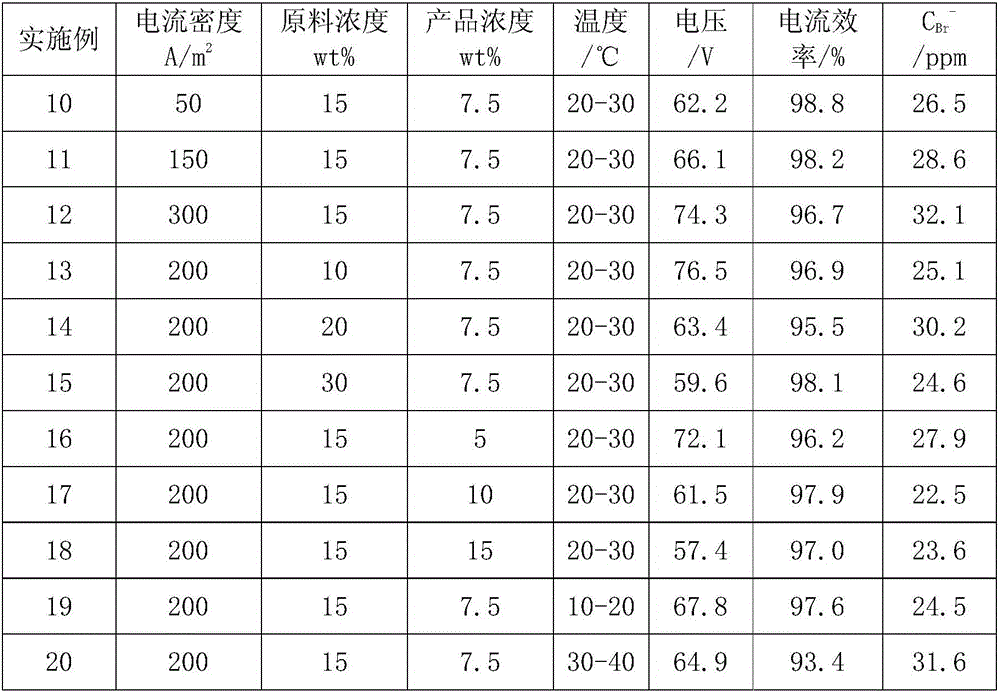
The invention relates to a method for preparing high-purity tetrabutyl ammonium hydroxide by continuous electrolysis, particularly relates to a method for preparing high-purity tetrabutyl ammonium hydroxide by using tetrabutyl ammonium bromide as a raw material and adopting a three-compartment bipolar membrane electrodialysis device, and belongs to the field of organic chemistry. The method is characterized by comprising the following step: by adopting the three-compartment bipolar membrane electrodialysis device, continuously preparing 5-20% tetrabutyl ammonium hydroxide by using a 15-35% tetrabutyl ammonium bromide aqueous solution as a raw material under the condition that the reaction temperature is 30-60 DEG C, the current density is 200-600A / m<2> and the flow rate of the raw materials is 500-2000L / h, wherein the concentration of bromine ions in the prepared tetrabutyl ammonium hydroxide is less than 100ppm. According to the method provided by the invention, the materials in the compartments are relatively constant in concentration, the temperature is relatively constant, an ionic membrane is not easy to swell or shrink, the membrane cost is saved, the current efficiency and the product conversion ratio are improved and the energy consumption is reduced, and moreover, the product quality is further improved. The method is suitable for industrial safe production on a large scale, clean in production process and free of discharge of wastewater and solid wastes.
Owner:赵文洲
Fluorescent molecular probe for detecting fluoride ions in aqueous solutions as well as synthesis method and application thereof
ActiveCN104418874AEasy to synthesizeMild reaction conditionsGroup 4/14 element organic compoundsFluorescence/phosphorescenceHydrogen SulfateSulfate radicals
The invention relates to a preparation method of a fluorescent molecular probe for detecting fluoride ions in aqueous solutions through fluorescence enhancement and an application of the fluorescent molecular probe to detecting fluoride ions. The fluorescent molecular probe is prepared by protecting 1,4-diethyl-1,2,3,4-tetrahydro-7-hydroxyquinoxaline-6-aldehyde taken as a raw material with silane and then condensing the raw material and malononitrile. The fluorescent molecular probe is simple and convenient to synthesize, and reaction conditions are mild. The fluorescent molecular probe has the specific characteristics that the probe molecule has stable optical properties and higher synthetic yield; the probe molecule has high sensitivity of detection of fluoride ions in the aqueous solutions and low lower limit of detection, and the limit of detection is 5.4mu M; the response range is 0-1mM and the detection range is wide; the probe molecule has good selectivity and has no responses to anions, such as chloride ions, bromide ions, iodide ions, tetrabutylammonium cyanide, nitrates radicals, hydrosulfate radicals, perchlorate radicals, acetate radicals, thiocyanate radicals, azide radicals, cysteine, bovine serum albumin, carbonate radicals, sulfate radicals and reduced glutathione; the fluorescent molecular probe has practical application values in the fields of biochemistry, environmental sciences and the like.
Owner:SUZHOU ROWLAND BIOTECH
Method of controlling the growth of microorganisms
InactiveUS7341671B2Low level of freeLong retentionBiocideSpecific water treatment objectivesMicroorganismHypochlorite
Disclosed is a method of preparing a biocide having improved durability of its biocidal activity as well as disinfection efficiency at an initial stage, comprising the steps of: (a) preparing stabilized alkali or alkaline earth metal hypochlorite having a pH at least 11 by mixing a chlorine oxidant including alkali or alkaline earth metal hypochlorite with a stabilizer in an alkali solution; (b) preparing a bromide ion source; and (c) adding the bromide ion source prepared in step (b) into the stabilized alkali or alkaline earth metal hypochlorite prepared in step (a). Also, a method of controlling the growth of microorganisms using a biocide prepared by the method of the present invention is disclosed.
Owner:ACCU LAB CO LTD
Preparation method of aquacide dichloride
InactiveCN106220629AOvercome the problem of high production costReduce manufacturing costOrganic chemistryWater vaporBromine
The invention discloses a preparation method of aquacide dichloride. Bromide ions are oxidized by taking an aquacide dibromo salt aqueous solution as a raw material and taking chlorine as an oxidant to generate bromine and aquacide dichloride, wherein bromine is recycled by distillation and condensation; residual bromine and chlorine are purged by introducing air, oxygen, water vapor or inert gas to obtain aquacide dichloride. According to preparation of the aquacide dichloride, the aquacide dichloride is obtained by taking the aquacide dibromo salt aqueous solution as the raw material and by oxidizing by cheap chlorine, so that the preparation method of aquacide dichloride has the advantages of low production cost, simple reaction and high product yield, and is suitable for industrialized production. More importantly, expensive bromine is obtained by replacement, and bromine is a chemical raw material applied widely, can serve as a product to be sold directly and can also be circularly used for preparation of aquacide dichloride, so that the problem that existing herbicide aquacide has high production cost is solved and significant economic benefit is achieved.
Owner:NANJING REDSUN BIOCHEM CO LTD
Brominated anionic styrenic polymers and their preparation
Concurrently fed into a reaction zone held at about 10° C. or less are brominating agent, aluminum halide catalyst, and a solution of anionic styrenic polymer having a GPC Mn about 2000-30,000. The components are in at least two separate feed streams. The feeds are proportioned to maintain (a) the amount of aluminum halide being fed at about 0.8 mole percent or less based on the amount of aromatic monomeric units in the polymer being fed, and (b) amounts of brominating agent and unbrominated polymer in the reaction zone that produce a final washed and dried polymer product containing about 60-71 wt % bromine. The catalyst is deactivated, bromide ions and catalyst residues are washed away from the reaction mixture, and the brominated anionic styrenic polymer is recovered and dried. The dried polymer has a volatile bromobenzene content of about 600 ppm (wt / wt) or less as well as other beneficial properties.
Owner:ALBEMARLE CORP
Process for electrochemical oxidation of bromide to bromine
InactiveUS20040188271A1Eliminate the problemSave energyElectrolysis componentsPhotography auxillary processesBromineElectrochemistry
The present invention relates to a process for electrochemical oxidation of bromide to bromine, more particularly to oxidation of bromide ions in brine, bittern and effluents using an indigenous cation exchange membrane flow cell.
Owner:COUNCIL OF SCI & IND RES
Method for extracting bromine by industrial wastewater rich in Br-
InactiveCN102556972AReduce manufacturing costProductiveWater/sewage treatmentBromine/hydrogen-bromideElectrolysisHypochlorite
The invention discloses a method for extracting bromine by industrial wastewater rich in Br-, and is characterized in that bromine is obtained by electrochemical oxidation, blow-out, and collection of industrial wastewater which is pretreated by purification and is rich in Br-; in the electrochemical oxidation, an electrolytic tank is partitioned into a cathode chamber and an anode chamber by an ion exchange membrane; the anode electrolyte is an aqueous solution which is rich in Br- and is obtained by purification treatment of the industrial wastewater rich in Br-; the cathode electrolyte is an acid solution with a pH of 1-4; the temperature of the electrolytes is controlled at 25-45 DEG C; the current density is 0.01-0.1 A / cm2; or a constant voltage is 0.5-6.0 V; after electrolytic balance is reached, the anode electrolyte is removed, and bromine is obtained by air blow-out, condensation, gas-liquid separation, and water-bromine separation. The method of the invention overcomes the disadvantages of bromine preparation by traditional oxidation process through chlorine, hypochlorite, and the like; the method is applicable to the treatment of industrial wastewater with a bromide ion concentration of 0.02-11.5 mol / L, and the process is simple and environment-friendly.
Owner:HUAIBEI NORMAL UNIVERSITY +2
Discoloration indicator for shelf life of perishable product and preparation method thereof
ActiveCN102735796ADistinguishable color changeAdjustable color change rateAnalysis using chemical indicatorsGold nanorodExtinction
The invention provides a discoloration indicator for shelf life of a perishable product and a preparation method thereof. The preparation method comprises the following steps: 1) preparing a surfactant aqueous solution containing chloride or bromide ions, a soluble ascorbate aqueous solution, a soluble weak acid or weak acid salt aqueous solution and a soluble silver salt solution, wherein concentration of each of the four aqueous solutions is not less than 0.1 mM; and 2) mixing gold nanorod with a largest extinction wavelength not less than 700nm with the four solutions in the step 1) to obtain the indicator, and conducting a metachromatism. During mixing, concentration of gold nanorod in the indicator needs to be adjusted to realize an optical density of the solution no less than 0.1cm<-1> at 500-520nm; usage amount of soluble silver salt in the indicator is no less than 4 times an amount of substance of gold in the gold nanorod solution matter; and usage amount of other reagent is adjusted according to shelf life of an indicating object. According to the invention, discoloration process of the perishable product with change of temperature is tracked and recorded to simulate a metamorphic process of the product to be indicated, and quality and shelf life of the product are visually indicated through the color.
Owner:PEKING UNIV
Etching liquid composition for copper/molybdenum film or copper/molybdenum alloy film
The present invention relates to an etching liquid composition for a copper / molybdenum or a copper / molybdenum alloy film. As for the overall weight of the composition which is of 100% weight, the etching liquid composition contains 5-40% weight of hydrogen peroxide, 0.1-5% weight of an etching inhibitor, 0.1-5% weight of a chelating agent, 0.1-5% weight of an etching additive, 0.01-2% weight of fluoride, 0.01-2% weight of a hydrogen peroxide stabilizer, and 0.1-5% weight of a bromide ion compound, the balance being water. The bromide ion compound is a bromine inorganic acid salt, a nitride bromide, a sulfide bromide, a bromine fluoride, a bromine chloride or a mixture thereof. When etching is repeatedly performed and thus the content of metal ions in the etching liquid is high, with adoption of the etching liquid composite, the etching characteristics such as the etching taper angle, etching deviation and etching straightness are maintained, and the composition can also be used as a lower film and applied to TFT-LCD display electrode manufacture.
Owner:ENF TECH
Method for recycling bromine from bromine containing waste water
ActiveCN109371416ASimple processSmall footprintElectrolysis componentsWater/sewage treatmentElectrolysisHydrogen
The invention discloses a device and method for recycling bromine from bromine containing waste water. The method comprises the following steps that a, pre-treated bromine containing waste water is led into an electrolysis device, direct current passes through the bromine containing waste water in the electrolysis device, and bromide ions have the electrolytic reaction to generate elemental bromide; b, an extraction agent and an electrolyzed water solution make contact, and the elemental bromide is recycled from an extraction phase; c, the extracted water phase is subjected to steam stripping,the extraction agent is recycled, and then the water phase enters a subsequent waste water treatment unit. The oxidizing agent safety problem brought by a traditional oxidation method can be solved.The recycled molecular bromine can directly return to the system to be applied, the bromine consumption is reduced. The high-added-value hydrogen resources can be recycled.
Owner:ZHEJIANG UNIV
Binuclear cuprous complex luminescent material and preparation method thereof
InactiveCN103626789AImprove luminous efficiencyStrong green emission characteristicGroup 5/15 element organic compoundsCopper organic compoundsPhotoluminescenceGreen-light
The invention discloses a binuclear Cu(I) complex luminescent material capable of emitting green light and a preparation method of the binuclear Cu(I) complex luminescent material. The green luminescent complex is obtained by complexing cuprous bromide and a ligand, and the molecular structure is [Cu(POP)Br]2, wherein the POP in the formula is an electric neutrality ligand bis(2-diphenyl phosphorus phenyl)ether, and the binuclear structure molecule is formed by coupling a bridging ligand bromide ion with two metal ions. The complex has the advantages that the micromolecule is easy to purify and the luminous efficiency is high, and the heat stability is high. The material is obtained by directly mixing and reacting the cuprous bromide with a dichloromethane solution of the ligand, the process is simple and convenient, the equipment is simple, the raw material is easy to obtain and the cost is low. The material can be used as a photoluminescence green material, and can be further used as a luminescent layer green material in an electroluminescent device composed of multilayer organic materials.
Owner:CHINA JILIANG UNIV
Method for treating profenofos synthetic wastewater by using hydrogen peroxide
InactiveCN101525195ASimple processImprove stabilityMultistage water/sewage treatmentNature of treatment waterHydrogen-Ion ConcentrationsPhosphoric acid
The invention relates to a method for treating profenofos synthetic wastewater by using hydrogen peroxide, which comprises the following steps: after a proper amount of sulphuric acid is added to the wastewater to regulate the acidity of the wastewater, the hydrogen peroxide is added to the wastewater for heating reaction, bromide ions are oxidized into bromine, other organic matters are oxidized, the bromine is separated from the wastewater, and the wastewater from which the bromine is separated is neutralized; the temperature of oxidation reaction is 0 to 105 DEG C, sulphuric acid, hydrochloric acid, nitric acid, phosphoric acid or a mixture of the same can be added to the wastewater, the proper amount of acid is added for regulating the acidity of the wastewater until the concentration of hydrogen ions is 0.01 to 10.0 mol / L, the concentration of the hydrogen peroxide is 7 to 50 percent, the mol ratio of the hydrogen peroxide to the bromine in the wastewater is from 1:0.5-1:4, the hydrogen peroxide is added to the wastewater for reaction of 0.5 to 5.0 h, the wastewater introduced by a connecting pipe of a wastewater overhead tank and the hydrogen peroxide introduced by a connecting pipe of a hydrogen peroxide overhead tank are collected into an oxidation tower to for oxidation reaction, the upper end of a distillating tower is connected with a condensator by a pipeline, and a fine bromine output pipe is arranged after the condensation of the product of the oxidation reaction through a primary rectifying tower and a secondary rectifying tower. The invention has simple integral technology, good stability, high reliability and low operating cost.
Owner:吴秀玲
Device and method for preparing high-purity tetrapropylammonium hydroxide and co-producing bromine through electrolysis
ActiveCN104073839ALow costImprove qualityCellsElectrolytic organic productionPropyl bromideBromide ions
The invention discloses a device and a method for preparing high-purity tetrapropylammonium hydroxide and co-producing bromine through electrolysis. The method comprises the following steps: pumping and putting a tetrapropyl ammonium bromide solution of which the mass concentration is 45-55% in a raw material circulation tank into an anode chamber, meanwhile blowing air into the anode chamber through an air compressor, dehydrating the tetrapropyl ammonium bromide in the anode chamber to generate cation (CH3CH2CH2)4N<+> and anion Br<->, feeding the cation into a cathode chamber through a cation exchanger membrane, feeding the anion to an anode plate to generate gas phase bromine, feeding the gas phase bromine together with diluted tetrapropyl ammonium bromide into a gas-liquid separator for gas-liquid separation, feeding the diluted tetrapropyl ammonium bromide in the gas-liquid separator and separated liquid bromine into two bromine settling tank for switching and settling, mixing the diluted tetrapropyl ammonium bromide which contains a small amount of bromine at an upper layer with concentrated tetrapropyl ammonium bromide which is discharged from a concentrated tetrapropyl ammonium bromide tank into tetrapropyl ammonium bromide of which the mass concentration is 45-55%, and feeding the tetrapropyl ammonium bromide into the raw material circulation tank for further recycling and use, wherein the ionic concentration of the tetrapropylammonium hydroxide is less than 100ppm, and the content of metal ions is less than 20ppb.
Owner:镇江润晶高纯化工科技股份有限公司
Process for treating waste water in chlorfluazuron synthesis
InactiveCN101274795AGroup 5/15 element organic compoundsBromine/hydrogen-bromideBromineEconomic benefits
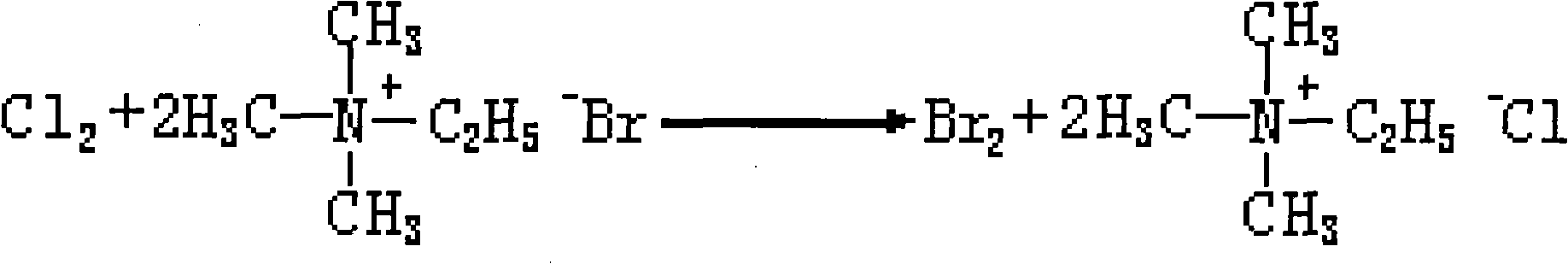
The invention provides a method for treating profenofos synthetic wastewater. A certain amount of chlorine is led into the wastewater produced in the synthesis process of profenofos, bromide ion in the wastewater is replaced by bromine and trimethyl ethyl ammonium chloride is generated, the generated bromine is separated with the wastewater, and then the wastewater after the bromine is separated is used for preparing the trimethyl ethyl ammonium chloride. The method can be used for treating the waste water produced in the synthesis process of the profenofos to recover valuable resource, thereby having better social and economic benefits.
Owner:王永昌
System and method for preparing high-purity tetrapropylammonium hydroxide through electrolysis method
ActiveCN106801233AReduce contentAvoid containingCellsElectrolytic organic productionElectrolysisNitrogen gas
The invention provides a system and method for preparing high-purity tetrapropylammonium hydroxide through an electrolysis method. A 5wt%-25wt% tetrapropylammonium bromide solution serves as a raw material, a two-membrane three-chamber electrolyzer is adopted, tetrapropylammonium bromide in a middle chamber is dissociated to generate (CH3CH2CH2)4N+ and Br-, the cation (CH3CH2CH2)4N+ selectively penetrates a cation exchange film to form tetramethyl ammonium hydroxide together with hydroxyl in a cathode chamber; and the anion Br- selectively penetrates an anion exchange membrane to enter the cathode chamber to be oxidized to generate bromine, the bromine then reacts with ammonium hydroxide to generate ammonium bromide and nitrogen, and a 5wt%-15wt% high-purity tetrapropylammonium hydroxide solution is prepared through continuous current electrolysis according to a continuous or discontinuous method. The electrolysis process overcomes the defect that a product produced through an ion exchange method contains sodion, the beneficial effects of being low in energy consumption, high in product purity and high in current efficiency are achieved, and the bromide ion content in the product is smaller than 50 PPM.
Owner:ZHEJIANG UNIV OF TECH
Resonance Rayleigh scattering (RRS) method for measuring bromide ion
InactiveCN103604792ASimple and fast operationHigh sensitivityFluorescence/phosphorescenceRayleigh scatteringRayleigh Light Scattering
The invention discloses a resonance Rayleigh scattering (RRS) spectrum method for measuring Br<->. The RRS spectrum method comprises the following steps: (1) preparing a Br<-> standard solution system; (2) preparing a blank control solution system; (3) respectively measuring an RRS peak intensity value Istandard and an RRS peak intensity value Iblank of the Br<-> standard solution system and the blank control solution system and computing deltaI=Istandard-Iblank; (4) working out a working curve according to the concentration relation between deltaI and Br<->; (5) measuring a sample of a measured material and computing deltaIsample=Isample-Iblank; and (6) checking the working curve in the step (4) according to the measured deltaIsample of the sample and computing the content of Br<-> in the measured material. The measurement method has the advantages of simple instruments, quickness in operation, high sensitivity and good selectivity.
Owner:GUANGXI NORMAL UNIV
Bromate suppression
InactiveUS20090272698A1Enhance metal ion concentrationPrevent oxidationSpecific water treatment objectivesSolid sorbent liquid separationScavengerBromine
A apparatus and method for killing microorganisms in a body of water that has been treated with ozone in the presence of bromide ions with the method comprising the steps of carrying out the ozonization of a body of water in the presence of bromide ions, adding a metal ion donor to the body of water, and adding a hypobromite ion scavenger to the body of water to interact with the metal ion donor to enhance a metal ion concentration in the body of water while suppressing the oxidization of the bromide by the Ozone to produce bromate.
Owner:KING TECH
Fluorescent molecular probe for detecting fluoride ions as well as synthesis method and application thereof
InactiveCN104418875AHigh sensitivityStable fluorescenceGroup 4/14 element organic compoundsMaterial analysis by observing effect on chemical indicatorHigh fluoridePhosphate
The invention relates to a preparation method of a fluorescent molecular probe for detecting fluoride ions through colorimetric detection and fluorescence enhancement and an application of the fluorescent molecular probe to detecting fluoride ions. The fluorescent molecular probe is prepared by protecting 2-hydroxyl-1-naphthaldehyde taken as a raw material with silane and then condensing the raw material and malononitrile. The fluorescent molecular probe is simple and convenient to synthesize, and reaction conditions are mild. The fluorescent molecular probe has the specific characteristics that the probe molecule has stable optical properties and higher synthetic yield; the probe molecule has high fluoride ion detection sensitivity and low lower limit of detection, and the limit of detection is 0.52mu M; the response range is 0-100mu M and the detection range is wide; the probe molecule has good selectivity and has no responses to anions, such as dihydrogen phosphate radicals, acetate radicals, bromide ions, hydrosulfate radicals, chlorate radicals, iodide ions, chloride ions and nitrate radicals; the probe molecule is suitable for colorimetric detection; the fluorescent molecular probe has practical application values in the fields of biochemistry, environmental sciences and the like.
Owner:SUZHOU ROWLAND BIOTECH
Preparation method of near-infrared cyanine dye
ActiveCN109054428AImprove photostabilityMethine/polymethine dyesPhotodynamic therapyCarboxyl radicalPhoto stability
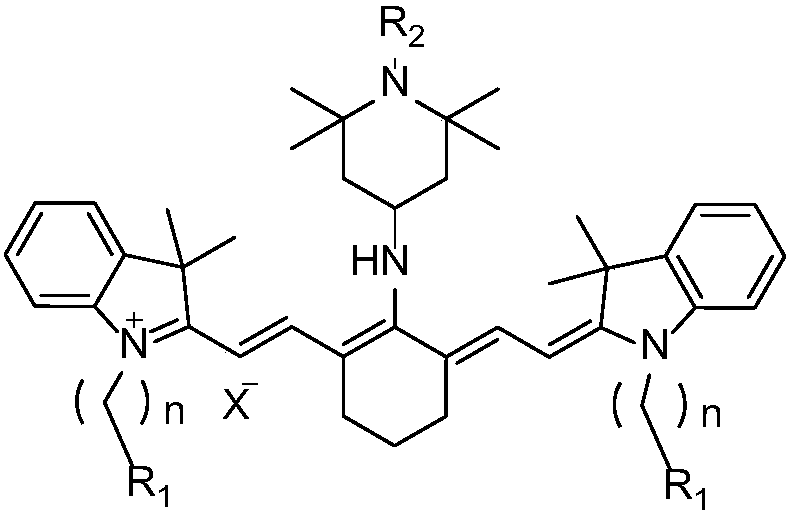
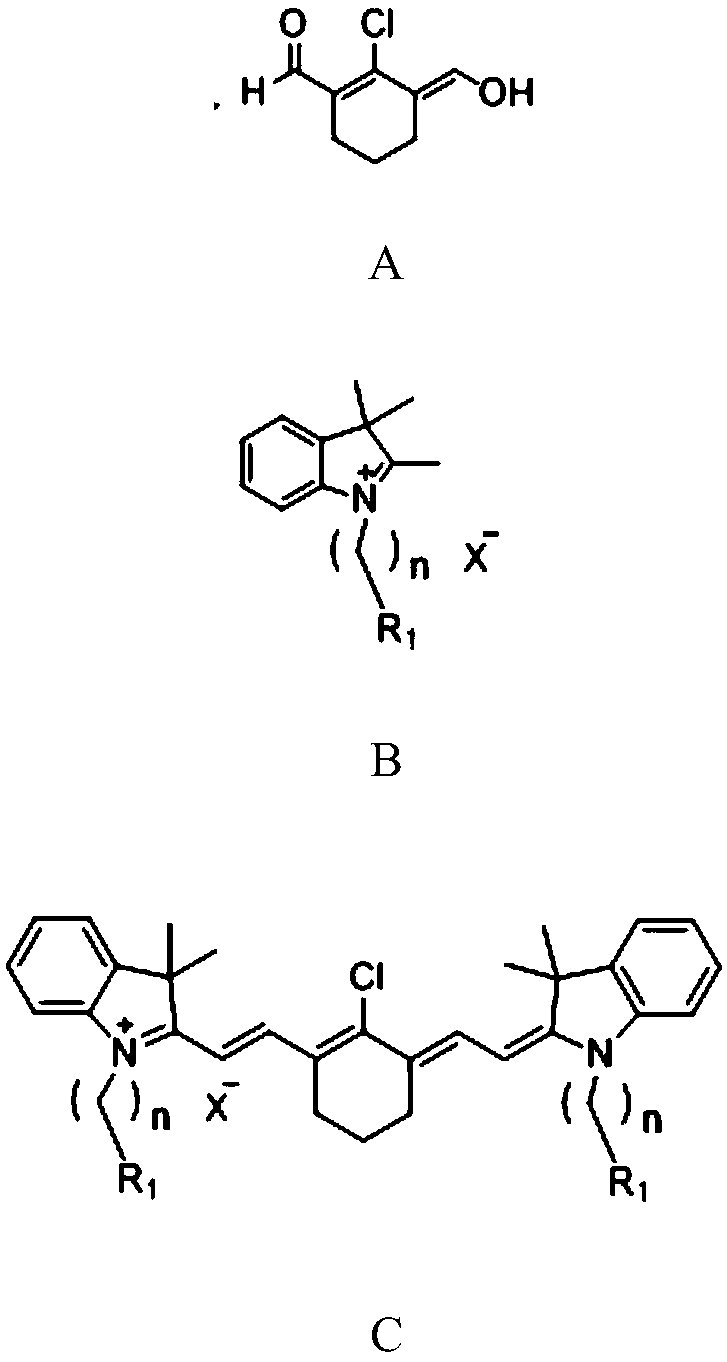
The invention discloses a preparation method and application of a near-infrared cyanine dye with high photo-stability. A structural formula of the dye is shown in the formula I as shown in the specification, wherein R1 is any of hydrogen, methyl and carboxyl, R2 is any of hydrogen, methyl and alkoxy, X<-> is any of an iodide ion and a bromide ion, n is any integer between 1 and 5. According to thedye, a hindered piperidine structure unit is introduced into a heptamethine indocyanine matrix through chemical bonding, so that the obtained dye is good in photo-stability and capable of absorptionand emission in a near-infrared region, has good photo-sensitive and photo-thermal properties, and further has good application prospects in fields such as textile coloring, near-infrared fluorescenceimaging, photo-dynamic therapy and photo-thermal therapy.
Owner:BEIJING TIANGANG AUX CO LTD
Method for large-batch synthesizing and high-efficiency purifying of superfine silver nano wires
The invention relates to the field of nano material synthesizing and application and aims at providing a method for large-batch synthesizing and high-efficiency purifying of superfine silver nano wires. The method includes the steps that polyvinylpyrrolidone and AgNO3 are added into glycol under the stirring condition and are evenly mixed and dissolved; a chloridion halogeno salt-ethylene glycol solution and a bromide ion halogeno salt-ethylene glycol solution are added continuously, and a reaction is carried out in the N2 atmosphere; supernatant liquor is taken centrifugally, acetone is addedunder the stirring condition, and standing is conducted so that flocculent solids can be deposited; ionized water is used for dispersing, and a uniform dispersion solution is formed; and acetone is added under the stirring condition, and the adding action is stopped when flocculent solids occur again, the operation is repeated for 6 times to 10 times, and the high-purity superfine silver nano wires are obtained. By means of the method, the processes of Ag crystal nucleus forming, crystal growth and the like can be controlled, and large-batch and stable preparing of the superfine AgNWs is achieved. Large-particle-size nano particle impurities are removed completely, and small-particle-size nano particles are separated from the AgNWs; the prepared silver nano wires do not have silver nano particle impurities, and the purity is nearly 100%; and the diameter of the silver nano wires is smaller than or equal to 40.0 nm.
Owner:TAIZHOU BRANCH ZHEJIANG-CALIFORNIA INT NANOSYSTEMS INST +1
A process for electrochemical oxidation of bromide to bromine
InactiveCN1771353ASimple methodEcologically pleasantElectrolysis componentsWater contaminantsSaline waterBromine
The present invention relates to a process for electrochemical oxidation of bromide to bromine, more particularly to oxidation of bromide ions in brine, bittern and effluents using an indigenous cation exchange membrane flow cell.
Owner:科学和工业研究委员会
Method for extracting bromine from bromine-containing wastewater
InactiveCN103613073ALow costReduce usageMultistage water/sewage treatmentBromine/hydrogen-bromideN-dodecaneBromine
The invention relates to a method for extracting bromine from bromine-containing wastewater, which comprises the following steps: pretreatment: filtering the bromine-containing wastewater so that the turbidity of the filtered bromine-containing wastewater is lower than the preset value; acidification: adding hydrochloric acid or sulfuric acid into industrial wastewater containing bromide ions to adjust the pH value of the industrial wastewater to 3-5; oxidation: adding an oxidizing agent into the acidified industrial wastewater for oxidation to generate bromine; extraction: adopting n-dodecane as an extraction agent, and extracting bromine from an aqueous phase to an n-dodecane phase; liquid separation: performing liquid separation on the two-phase solution added with the extraction agent, wherein the upper layer is an oil phase, and the lower layer is an aqueous phase; separating the aqueous phase from the oil phase; rectification: rectifying the n-dodecane and bromine solution obtained by the liquid separation so that bromine is separated from n-dodecane to obtain a bromine product. In the method provided by the invention, bromine is extracted by a solvent extraction process; compared with the traditional technology, the use of steam is greatly saved, the cost of whole bromine extraction is remarkably reduced, and better economic benefits are obtained.
Owner:ZHEJIANG SHUANGYI ENVIRONMENTAL PROTECTION TECH DEV
Application of tri (alkylamino) phosphonic compound, lithium ion battery, electrolyte and electrolyte additive
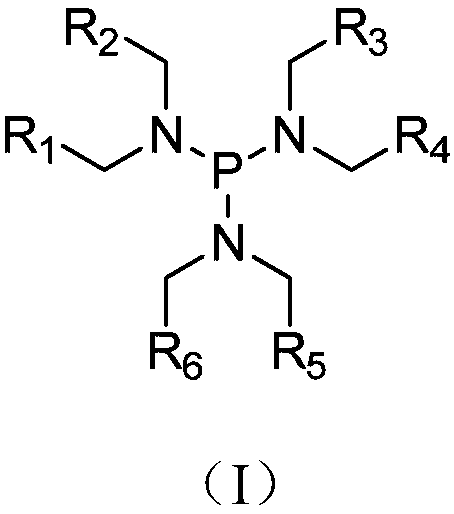
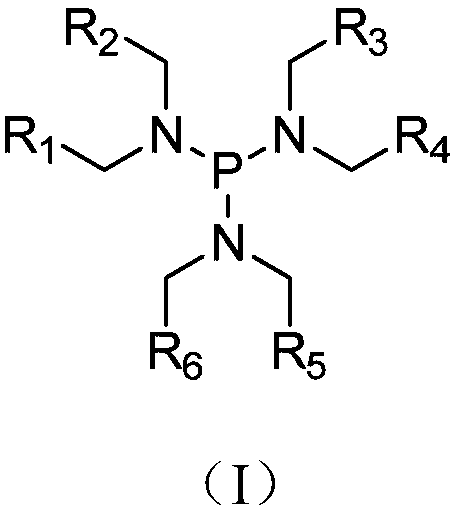
InactiveCN109585924AImprove cycle performanceAvoiding the Problem of Accelerated CorrosionSecondary cellsOrganic electrolytesHalogenDecomposition
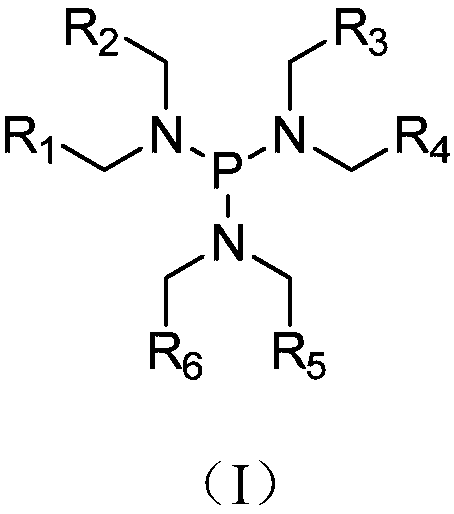
The invention relates to application of a tri (alkylamino) phosphonic compound, a lithium ion battery, an electrolyte and an electrolyte additive. The tri (alkylamino) phosphonic compound has a structure shown in a general formula (I), wherein R1-R6 are respectively selected from atom-substituted or non-substituted naphthenic base or halogen atom-substituted or non-substituted alkyl having 1-5 C atoms. P atoms in the tri (alkylamino) phosphonic compound can be combined with halide ions such as chloride ions or bromide ions, the problem that the halide ions such as the chloride ions or the bromide ions acts with an Al positive electrode collector to form [AlCl4]<-> or [AlBr4]<-> so as to cause corrosion acceleration of an oxide layer Al2O3 is prevented; and moreover, the tri (alkylamino) phosphonic compound shows alkalescence, a certain neutralization effect on a slight amount of HF generated by decomposition is achieved, so that the problem of corrosion of an electrolyte lithium salt on an aluminum foil of the positive electrode collector is prevented, and the cycle property of the lithium ion battery is further improved.
Owner:SOUNDON NEW ENERGY TECH CO LTD
Method for controlling formation quantity of bromate in ozone oxidized water treatment process by TiO2
InactiveCN102616916AReduce generationPromote degradationWater/sewage treatment by oxidationBromineWater quality
The invention relates to a method for controlling the formation quantity of bromate in an ozone oxidation process of drinking water by introducing a nano TiO2 catalyst, which avoids negative effects brought to the safety and the reliability of the drinking water of the traditional method for controlling the bromate by adding chemical drugs. According to the method, settled water treated by a conventional method is directly filled into a nano TiO2 catalyst bed for ozone contact and catalytic ozone oxidation, or after the settled water is subjected to ozone contact and catalytic ozone oxidation in a primary nano TiO2 catalyst bed, water still containing ozone is filled into a secondary nano TiO2 catalyst bed for carrying out a catalytic reaction, wherein the contact oxidation time is 10-20min. The method has the advantages that the nano TiO2 has obvious efficiency of reducing the formation quantity of the bromate in the ozone oxidation process and also has obvious control efficiency to the formation quantity of the bromate in the ozone oxidation process of water with high bromide ion concentration, the formation potential to trihalomethane and the reduction efficiency to DOC of individual ozone oxidation can be improved, and other toxic and side effects cannot be caused to the quality of the drinking water.
Owner:NANKAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com