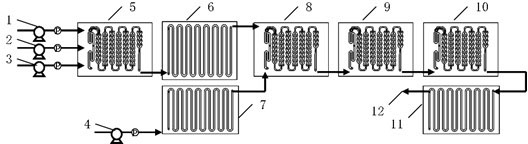
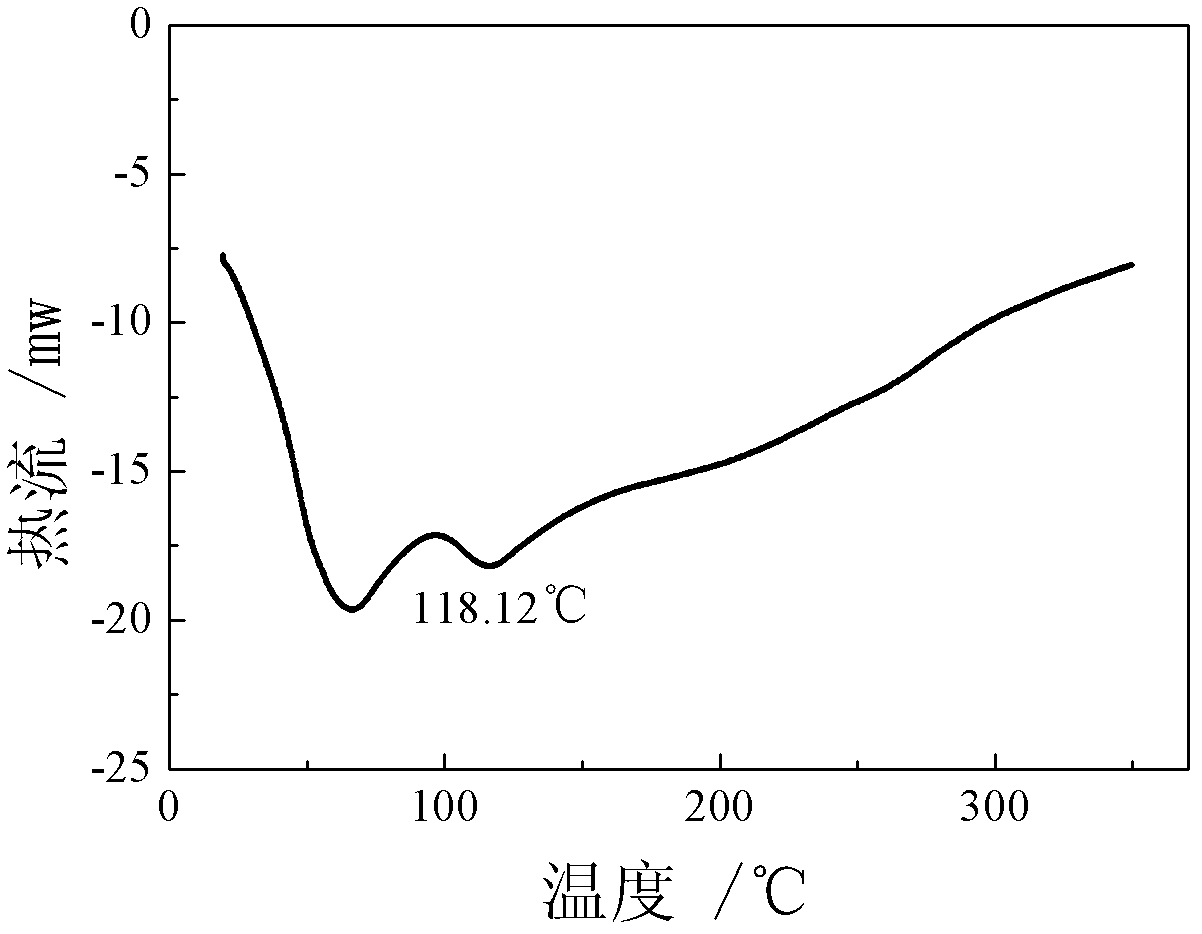
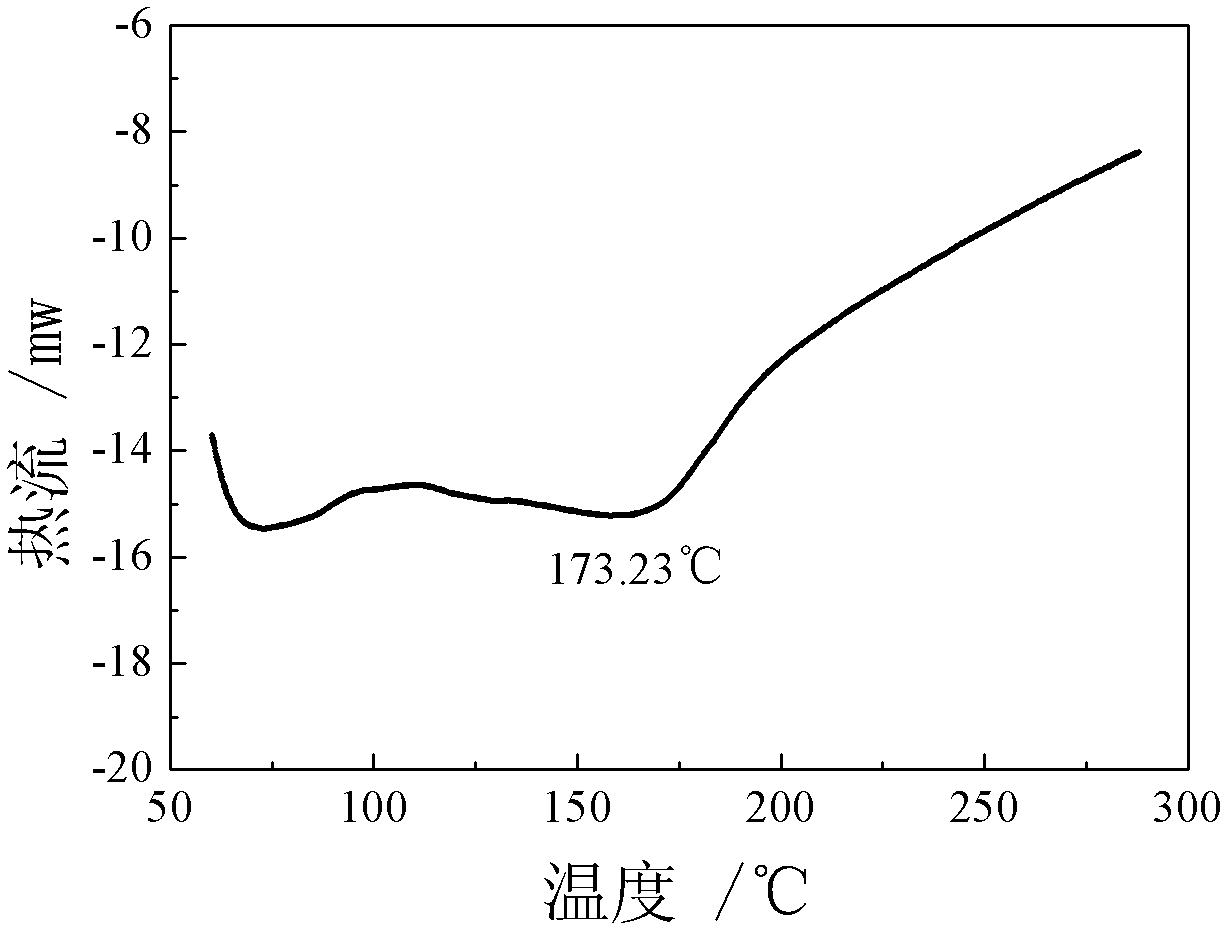
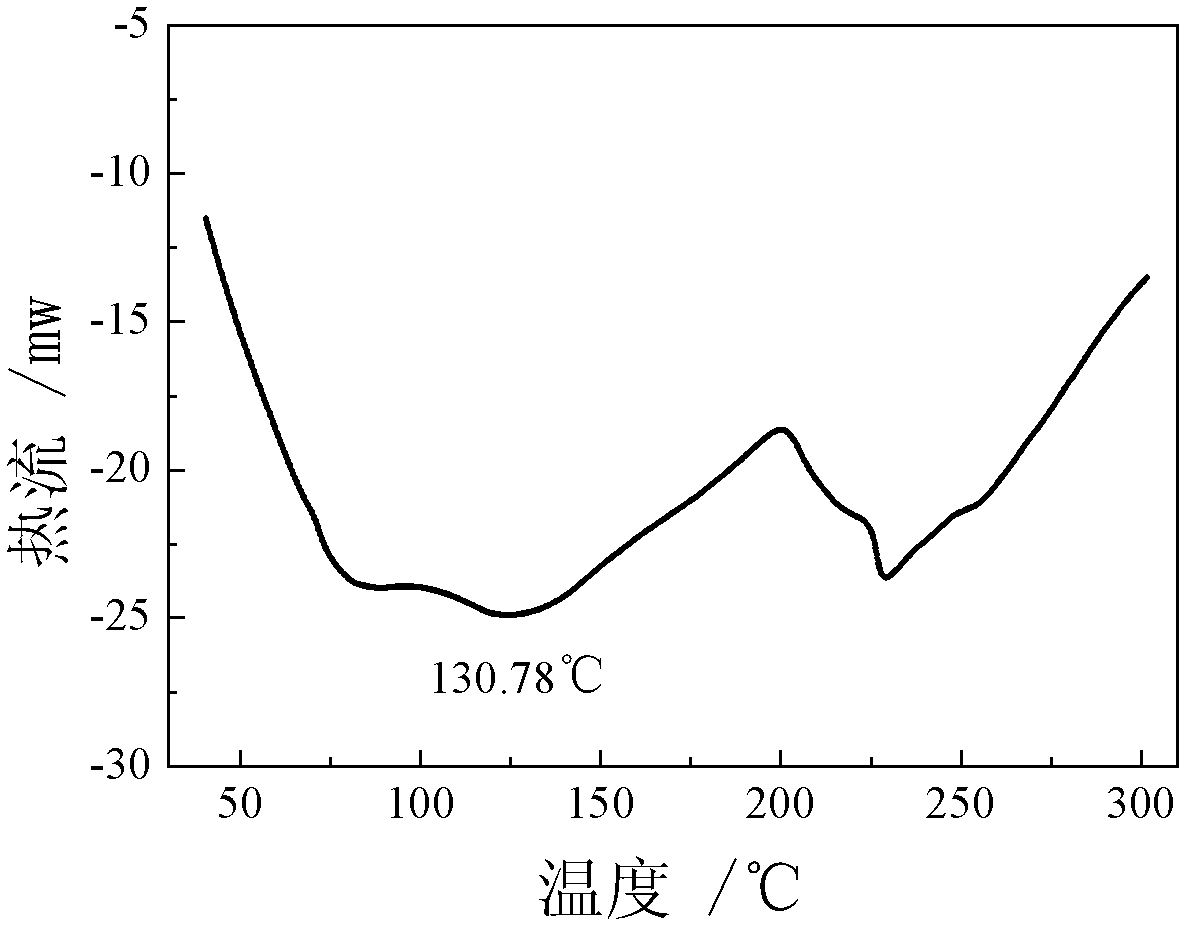
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
21565 results about "Nitric acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
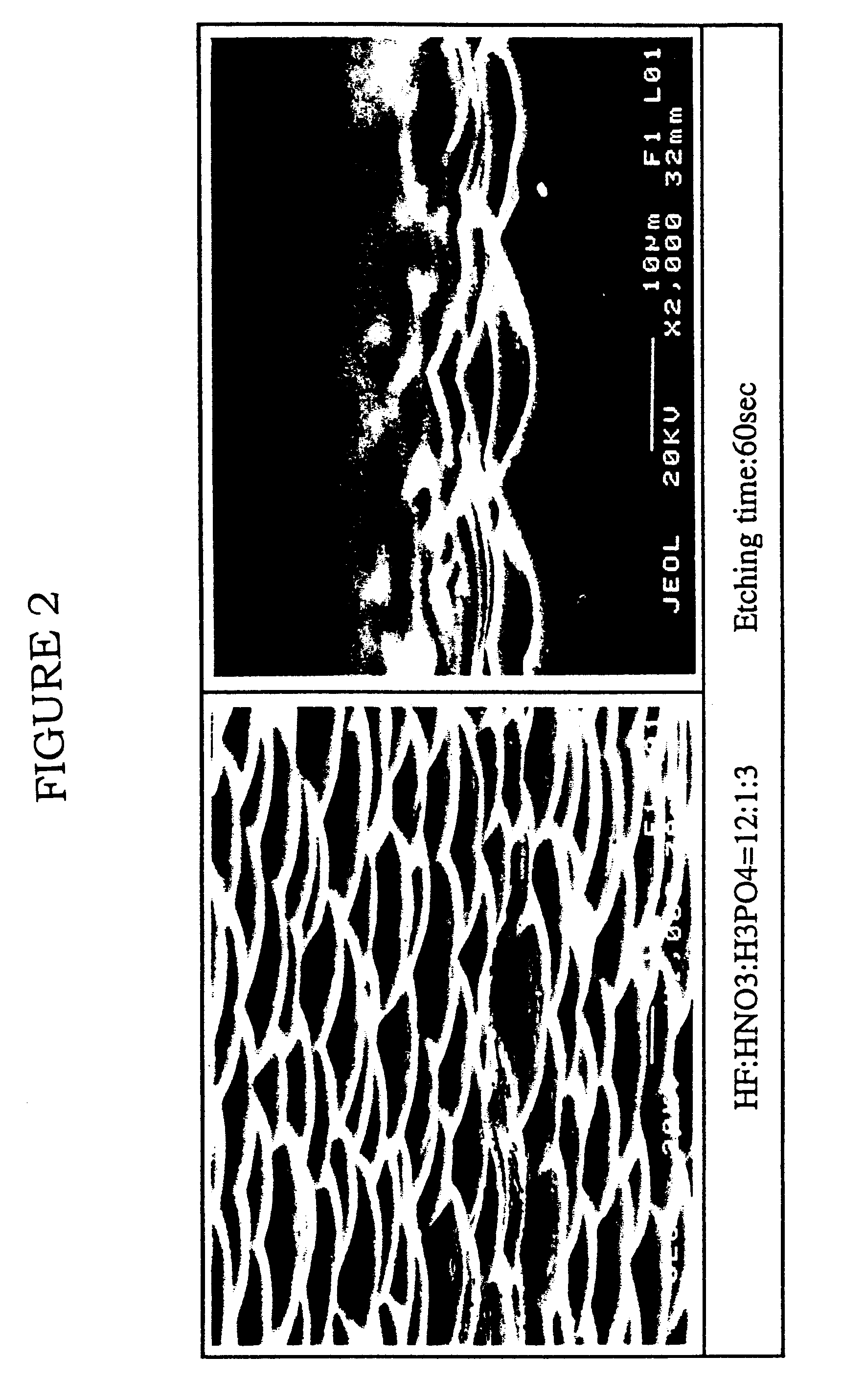
Nitric acid (HNO₃), also known as aqua fortis (Latin for "strong water") and spirit of niter, is a highly corrosive mineral acid. The pure compound is colorless, but older samples tend to acquire a yellow cast due to decomposition into oxides of nitrogen and water. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% HNO₃, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as white fuming nitric acid at concentrations above 95%, or red fuming nitric acid at concentrations above 86%.
Method of manufacturing a contact interconnection layer containing a metal and nitrogen by atomic layer deposition for deep sub-micron semiconductor technology
ActiveUS7235482B2Good step coverageSafe handlingSemiconductor/solid-state device detailsSolid-state devicesAtomic layer depositionContamination
An atomic layer deposition method is used to deposit a TiN or TiSiN film having a thickness of about 50 nm or less on a substrat. A titanium precursor which is tetrakis(dimethylamido)titanium (TDMAT), tetrakis(diethylamido)titanium (TDEAT), or Ti{OCH(CH3)2}4 avoids halide contamination from a titanium halide precursor and is safer to handle than a titanium nitrate. After a monolayer of the titanium precursor is deposited on a substrate, a nitrogen containing reactant is introduced to form a TiN monolayer which is followed by a second purge. For TiSiN, a silicon source gas is fed into the process chamber after the TiN monolayer formation. The process is repeated several times to produce a composite layer comprised of a plurality of monolayers that fills a contact hole. The ALD method is cost effective and affords an interconnect with lower impurity levels and better step coverage than conventional PECVD or CVD processes.
Owner:TAIWAN SEMICON MFG CO LTD
Method for preparing lithium cobaltate by directly using invalid lithium ion battery
InactiveCN102030375AReduce dispersionHigh purityCell electrodesCobalt compoundsElectrical batteryPotassium hydroxide
The invention provides a method for preparing lithium cobaltate by directly using an invalid lithium ion battery. The method comprises the following steps: crushing the invalid lithium ion battery or scraps generated when a lithium cobaltate battery is produced by a mechanical crusher at normal temperature; adding water and one or more of acetic acid, sulfuric acid, hydrochloric acid or nitric acid to produce mixed aqueous solution of the battery scraps and acid; filling the mixed aqueous solution into a hermetic pressure reactor, and controlling the temperature in the reactor to be between 50 and 150 DEG C; introducing or adding one leaching additive of sulfur dioxide or hydrogen, or adding hydrazine hydrate; stirring and leaching, cooling, and filtering; adding one precipitator of sodium carbonate, potassium carbonate and ammonium carbonate, or adding composite precipitator consisting of one of the sodium carbonate, the potassium carbonate and the ammonium carbonate and one of sodium hydroxide and potassium hydroxide to obtain mixture of lithium carbonate, cobalt carbonate and cobalt hydroxide; drying and calcining at high temperature to produce a lithium cobaltate product. The method is particularly suitable for the treatment scale of medium-sized and small enterprises, and is an effective method for directly materializing cobalt secondary resources.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
Method for preparing humic acid and salt thereof by oxidation and degradation of brown coal
The invention discloses a method for producing humic acid and salt thereof through the oxidative degradation of young lignite. The method comprises the following steps: carrying out the oxidation reaction of the lignite containing the humic acid and aqueous hydrogen peroxide solution; after the reaction, obtaining water soluble fulvic acid through centrifugal separation, supernatant filtration, concentration and drying; adding alkali into the fulvic acid to prepare a fulvic acid salt product; carrying out the alkaline extraction and centrifugal separation of the residue deposit of the production of the fulvic acid, adding acid into the supernatant till the pH value is 1 to 2, carrying out a reaction at an increased temperature or room temperature, carrying out centrifugal separation after the reaction is finished, and obtaining purified ulmic acid after precipitation and drying; and directly concentrating and drying the supernatant in the previous step to obtain the humate. The method can improve the yield of the fulvic acid and total humic acid in the young lignite, and simultaneously increase the active group in the humic acid. The method can be used for producing fulvic acid, fulvic acid salt, ulmic acid and ulmic acid salt products. In particular, the method puts an end to the environmental pollution caused by the nitric acid which is taken as an oxidation degradation agent. In addition, the method has a short technological line, low cost, simple requirements on equipment, and moderate conditions. The method which can be applied to the industrialized production has good application prospect.
Owner:KUNMING UNIV OF SCI & TECH +4
Method of opening and filling carbon nanotubes
PCT No. PCT / GB95 / 02235 Sec. 371 Date Apr. 10, 1997 Sec. 102(e) Date Apr. 10, 1997 PCT Filed Sep. 20, 1995 PCT Pub. No. WO96 / 09246 PCT Pub. Date Mar. 28, 1996Method of making carbon nanotubes open on at least one end wherein capped nanotubes are treated with an oxidizing acid such as nitric acid. The treatment is effective to open at least 50% of the nanotubes on at least one end.
Owner:ISIS INNOVATION LTD
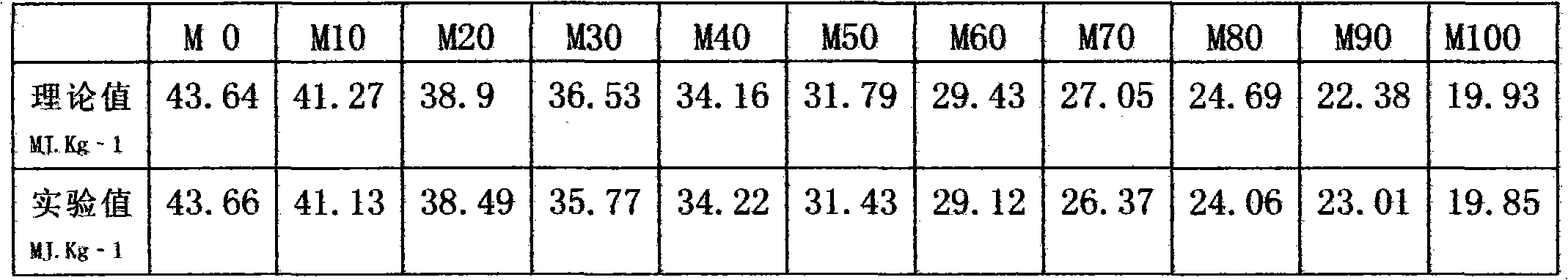
Methanol diesel oil manufacturing mode
The invention relates to a manufacturing mode of environment-friendly and energy-saving M15_M85 methanol diesel oil. The methanol diesel oil is composed of diesel oil, methanol, and additives. The methanol diesel oil is prepared with operations under normal temperatures and normal pressures. The invention discloses a plurality of common additives used for preparing methanol diesel oil in modern times. If explosives and aviation fuels are appropriately utilized, methanol defalcated heat value can be well compensated. The theory is tentatively considered as a gaseous detonation theory. The additives can be selected from nitric acid esters, nitrates, nitro compounds, non-aromatic compounds, peroxides, and the like.
Owner:陈若歆
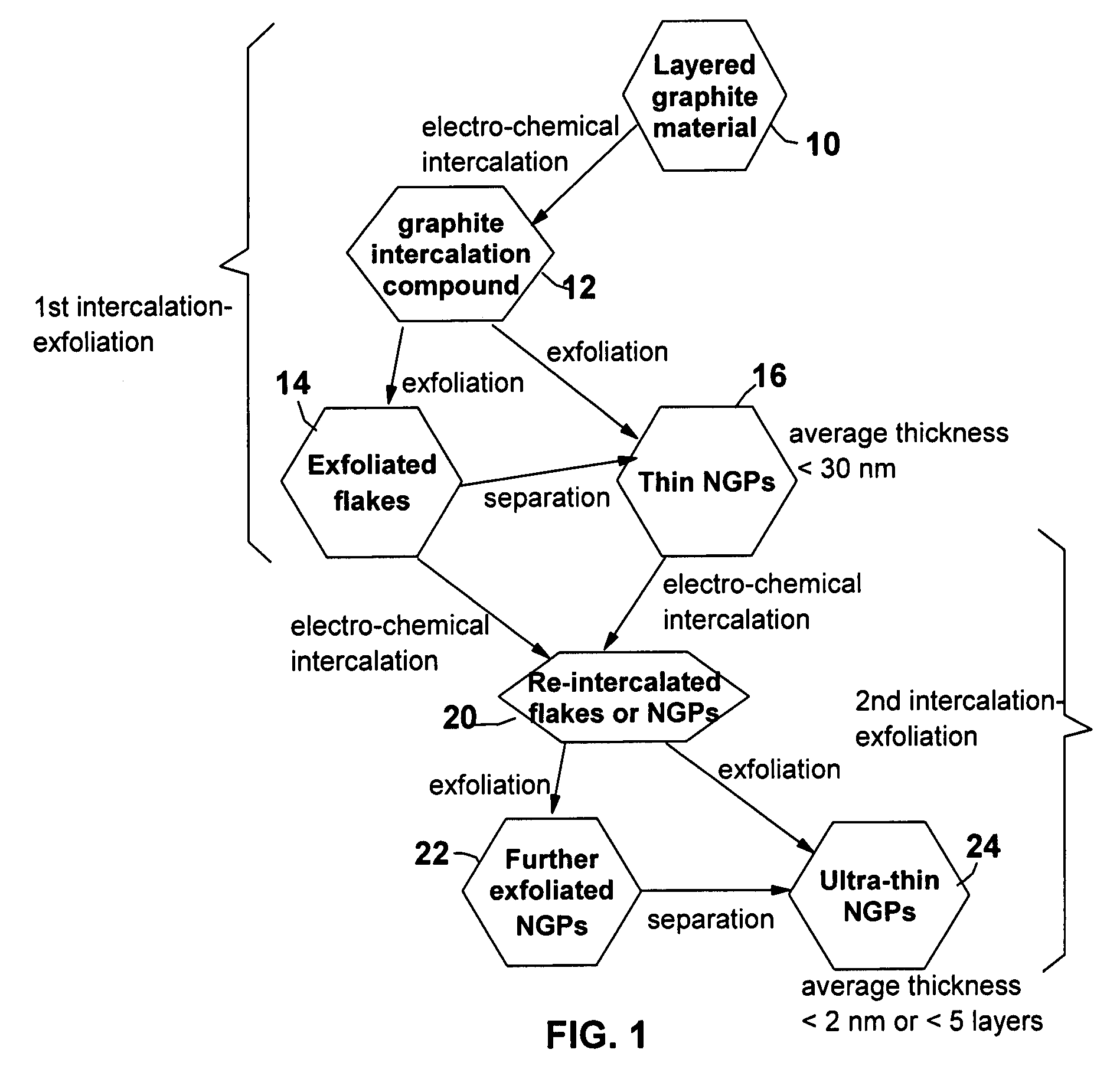
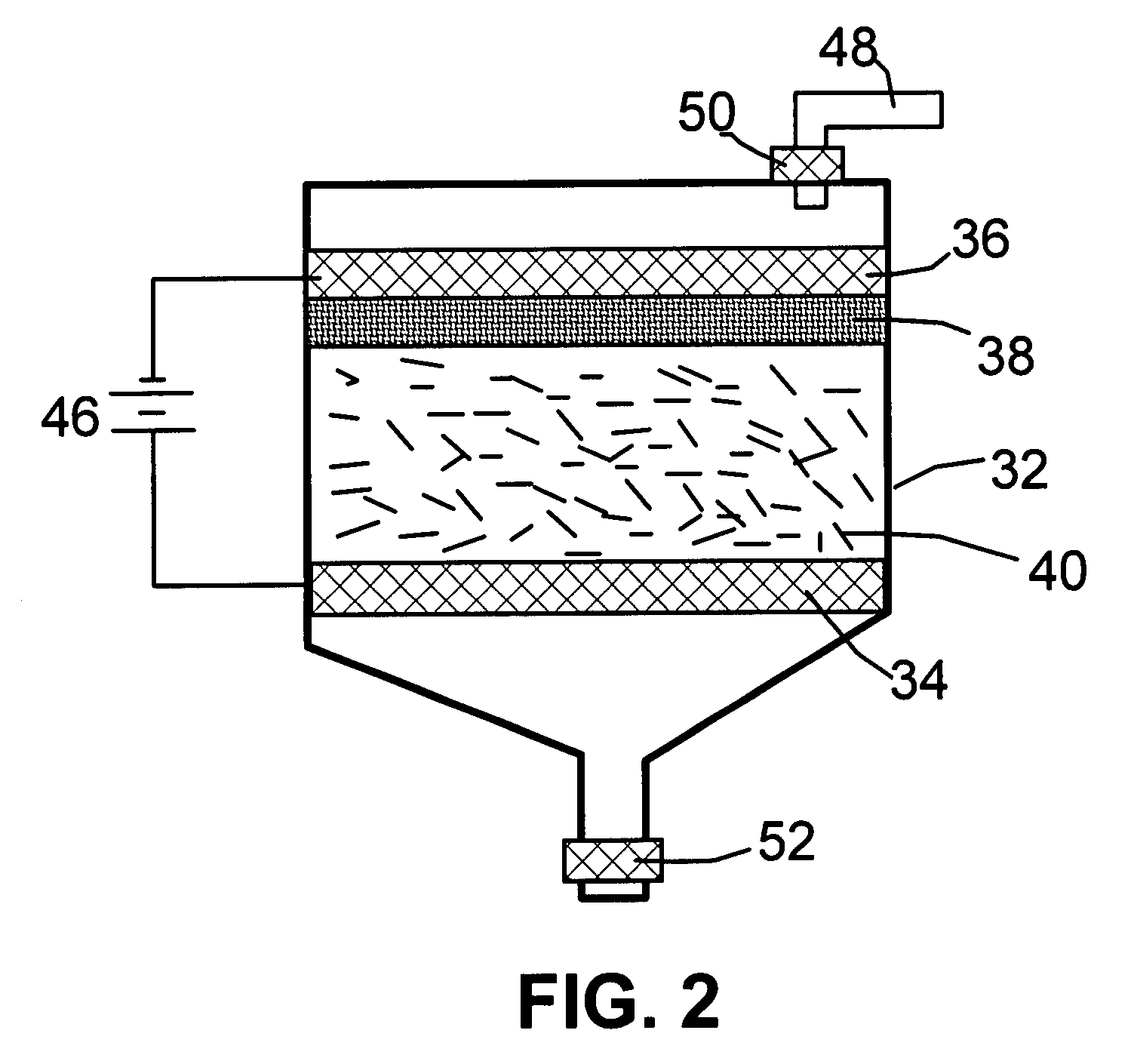
Electrochemical method of producing nano-scaled graphene platelets
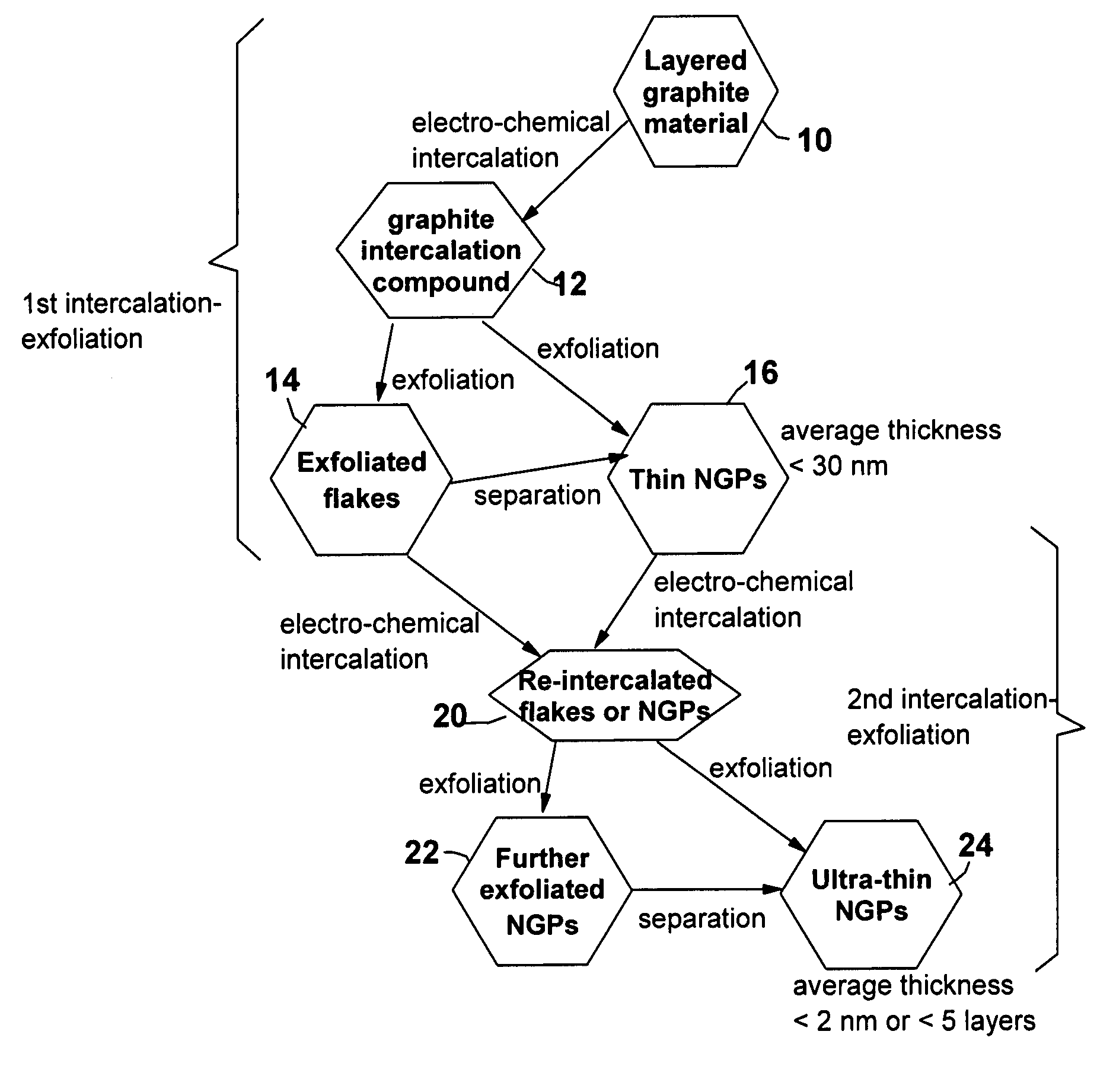
A method of producing nano-scaled graphene platelets with an average thickness smaller than 30 nm from a layered graphite material. The method comprises (a) forming a carboxylic acid-intercalated graphite compound by an electrochemical reaction which uses a carboxylic acid as both an electrolyte and an intercalate source, the layered graphite material as an anode material, and a metal or graphite as a cathode material, and wherein a current is imposed upon the cathode and the anode at a current density for a duration of time sufficient for effecting the electrochemical reaction; (b) exposing the intercalated graphite compound to a thermal shock to produce exfoliated graphite; and (c) subjecting the exfoliated graphite to a mechanical shearing treatment to produce the nano-scaled graphene platelets. Preferred carboxylic acids are formic acid and acetic acid. The exfoliation step in the instant invention does not involve the evolution of undesirable species, such as NOx and SOx, which are common by-products of exfoliating conventional sulfuric or nitric acid-intercalated graphite compounds. The nano-scaled platelets are candidate reinforcement fillers for polymer nanocomposites. Nano-scaled graphene platelets are much lower-cost alternatives to carbon nano-tubes or carbon nano-fibers.
Owner:GLOBAL GRAPHENE GRP INC
Low-temperature smoke denitration SCR (silicon controlled rectifier) catalyst and preparation method
ActiveCN102114424ARich pore structureLarge specific surface areaDispersed particle separationCatalyst activation/preparationSilicon-controlled rectifierManganese oxide
The invention relates to a low-temperature smoke denitration SCR (silicon controlled rectifier) catalyst, which comprises a carrier, a manganese oxide, and composite oxide of one or more of Ce, Zr, Ti, Co, Fe and Cu, the mass content of manganese is 0.1-66 percent, and the total mass content of the Ce, Zr, Ti, Co, Fe or / and Cu is 0-50 percent; and glass fiber and / or kieselguhr is used as the carrier, wherein the glass fiber of the carrier is calcined for 2-4 hours at temperature of 400-600 DEG C, then placed in a nitric acid, sulfuric acid or hydrochloric acid solution with mass concentration of 5-40 percent for acidizing for 1-8 hours, washed by distilled water to be neutered, dried at temperature of 80-120 DEG C, and crushed to have the fineness of 20-325 meshes. The catalyst uses the glass fiber and the kieselguhr as the carriers, so that the dispersion effect of nanoparticles and specific surface area of the catalyst are increased, the high adsorptive capacity and strong heat resistance and corrosion resistance capacity are achieved, stronger toxic resistance capacity to sulfur dioxide and stream contained in the smoke is realized, the invention can be used for 10-200 DEG C of low temperature smoke denitration, and has strong water resisting and sulphur toxic resisting capacities.
Owner:GUODIAN SCI & TECH RES INST +1
Composition for use in golf balls and sports equipment
The present invention relates to a novel blend composition suitable for use in sports equipment in general and in particular for use in golf ball manufacture. The composition is the reaction product of; A) a polymer of ethylene and / or one or more alpha olefins, and an acid, ester, or anhydride (“Component (A)”); and B) a compound comprising both an amine and a carboxylic acid in the same molecule which may be present in either a neutral or ionic or zwitterionic form (“Component (B)”); and C) a basic metal ion salt, capable of neutralizing the acid groups of Component (A) and / or Component (B). The metal ions including Li+, Na+, K+, Zn+, Co2+, Ca2+, Ni2+, Cu2+, Pb2+, and Mg2+, with Li+, Na+, Zn2+, Ca2+, and Mg2+ being preferred, and their salts include those of, for example, formic acid, acetic acid, nitric acid, sulfuric acid, carbonic acid, bicarbonic acid, as well as the metal oxides, hydroxides, and alkoxides (“Component (C)”). The present invention is also embodied in a blend composition including the reaction product of one or more ionomers and Component (B) which is a compound having a general formula (R2N)m—R′—(X(O)nORy)m, where R is either hydrogen, one or more C1-C20 aliphatic systems, one or more cycloaliphatic systems, one or more aromatic systems, or a combination of these. Also R′ is a bridging group comprising one or more unsubstituted C1-C20 straight chain or branched aliphatic or alicyclic groups, or one or more substituted straight chain or branched aliphatic or alicyclic groups, or one or more aromatic groups, one or more oligomers each containing up to 12 repeating units, and when X is C or S or P, m is 1-3. Also when X=C, n=1 and y=1, and when X=S, n=2 and y=1, and when X=P, n=2 and y=2. The present invention also resides in a golf ball including a core, an outer cover layer; and from 0 to 5 intermediate layers, wherein one or more of said core, outer cover, and / or intermediate layers, if present, includes the aforementioned blend compositions. Finally, the present invention is also embodied in sports equipment items comprising the aforementioned blend compositions.
Owner:TAYLOR MADE GOLF
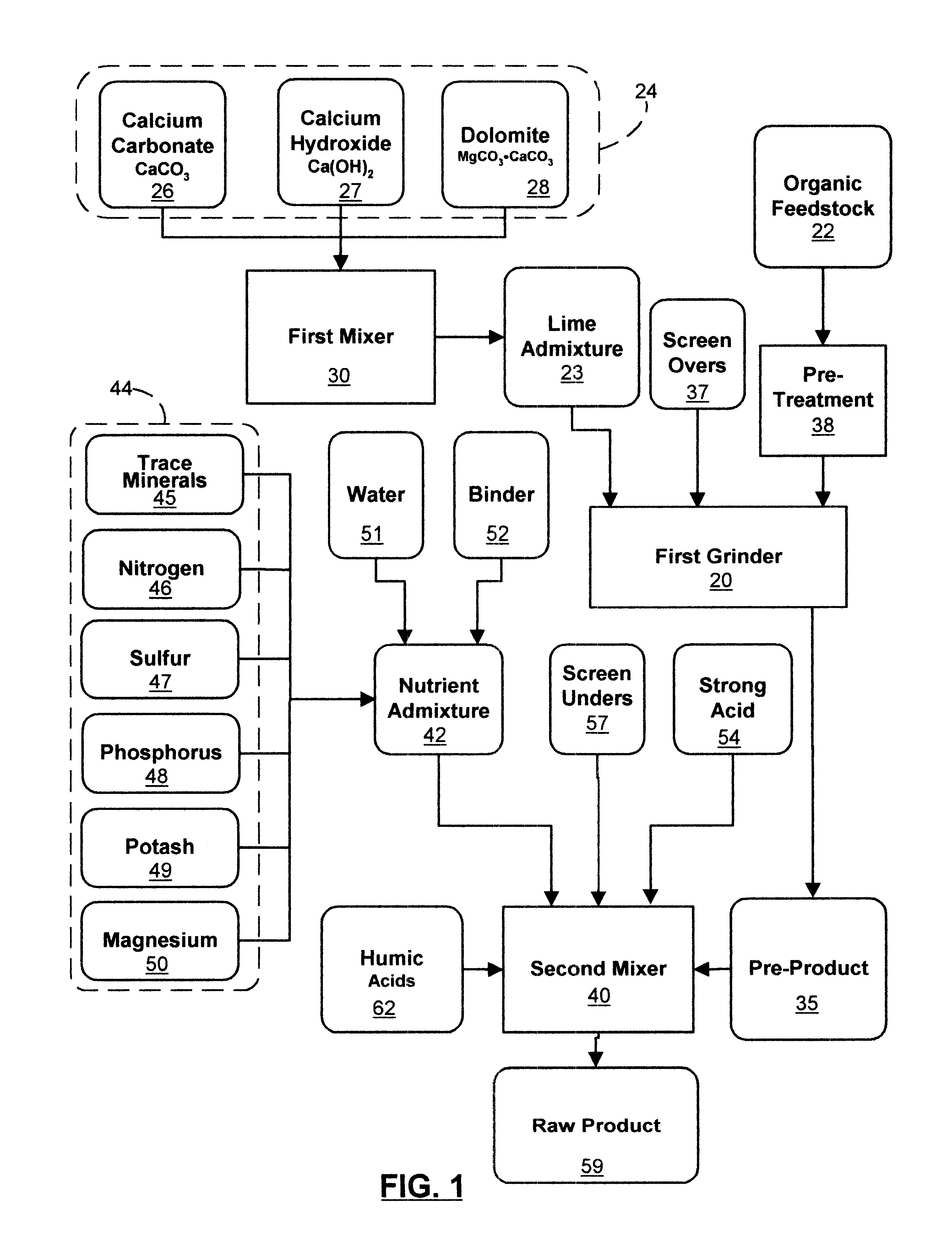
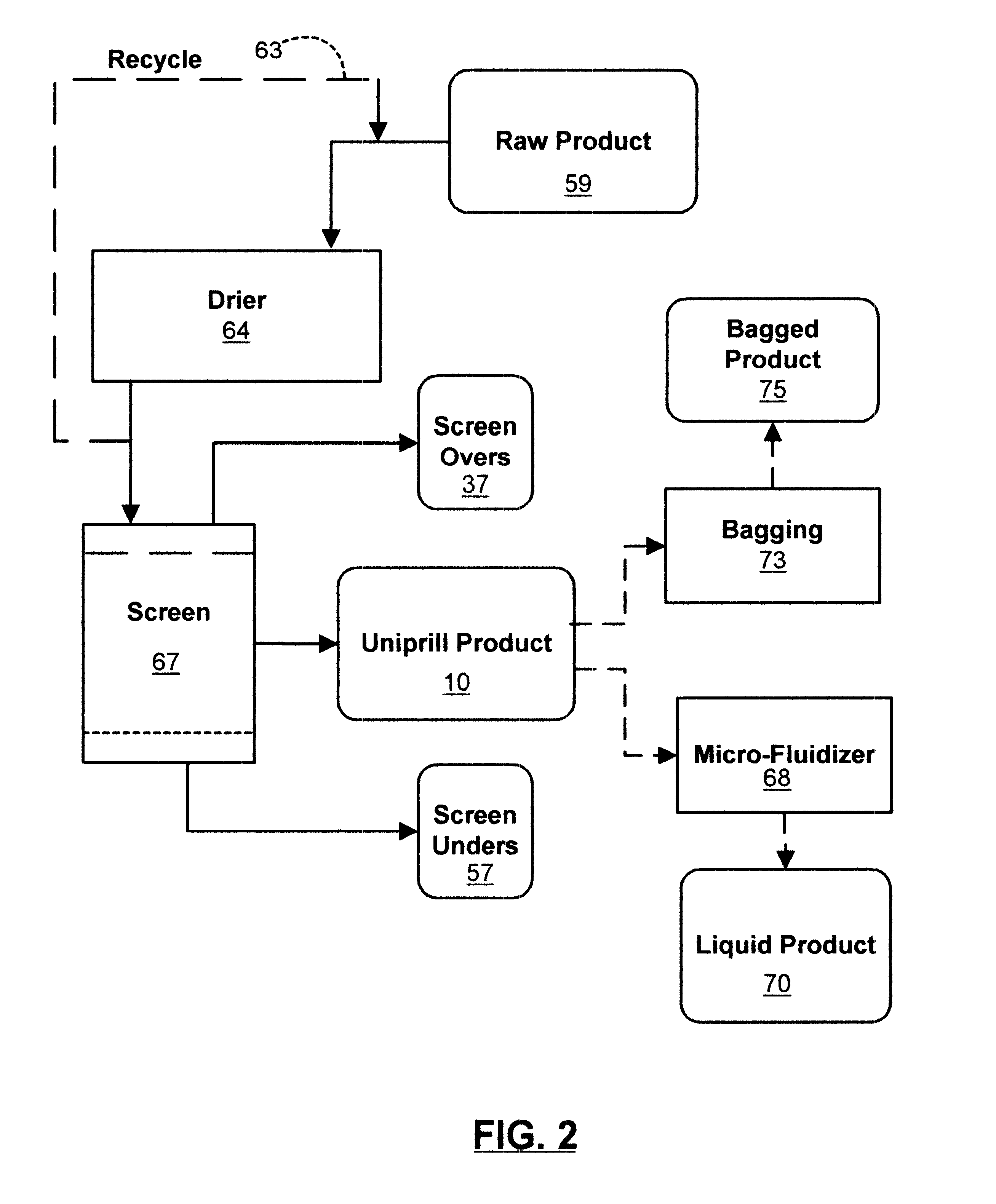
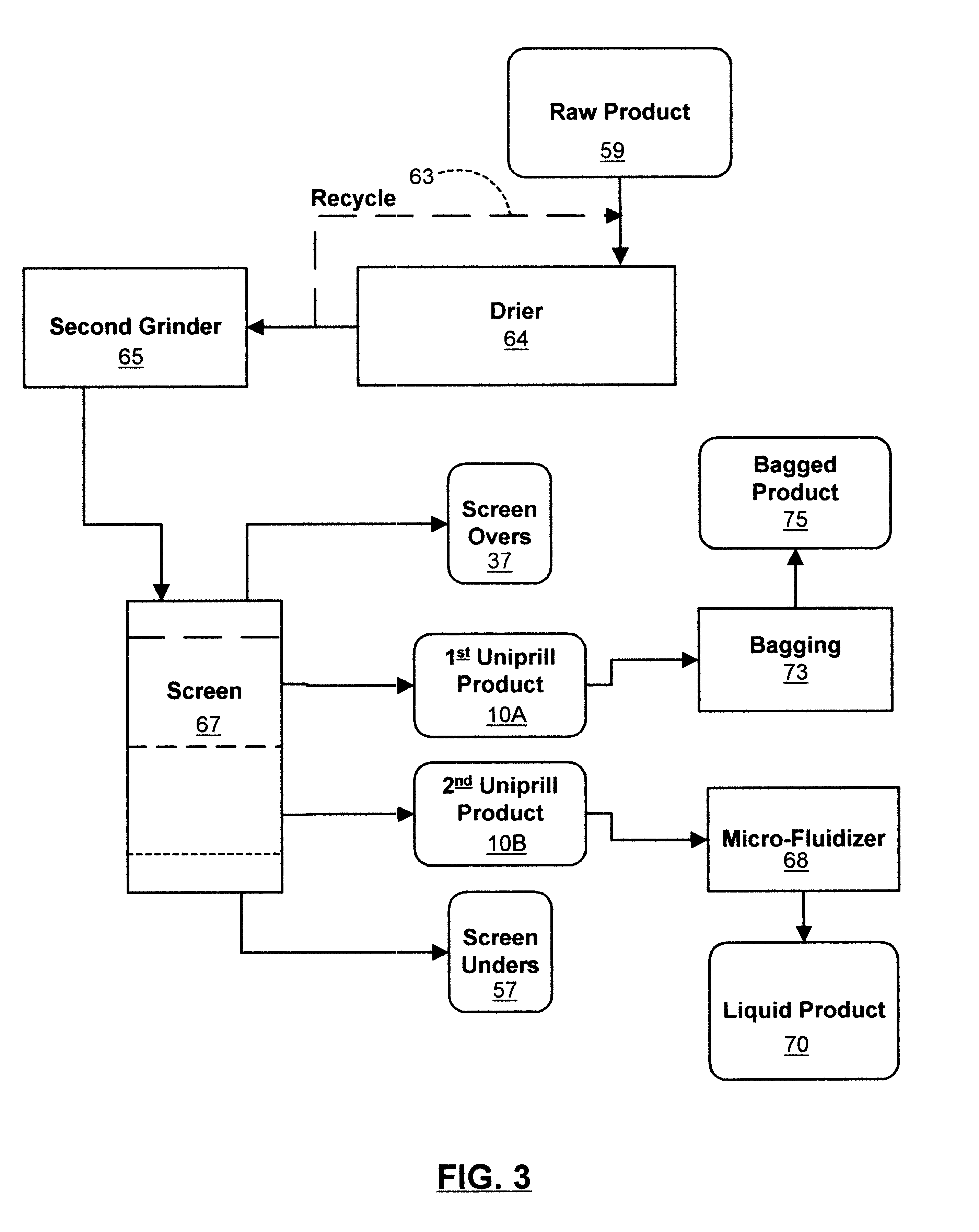
Organic material based uniprill fertilizer
InactiveUS6461399B1Reduce moisture contentIncrease speedCalcareous fertilisersBiocideOrganic basePhosphoric acid
An organic-based uniprill fertilizer is provided. To produce the fertilizer, organic matter is sequentially pre-treated by first mixing it in a first grinder with a lime admixture, then adding a slurry of reagents and binders, followed by a mixture of acids. The acids can include sulfuric and phosphoric acids, in addition to nitric acid and various organic acids such as citric and fulvic acid, depending on the end requirements of the fertilizer product. Following the mixing of the pre-product with the mixture of acids and reagents, the resultant raw product is preferably processed through a second grinder. This grinding further dries, mixes and granulates the raw product. The particle size of the completed fertilizer is reduced into a flowable, user safe uniprill product that can be further ground to reduce its moisture. Further drying may be necessary for bag or bulk product, or it can be liquified by high speed blending or micro-fluidized for sprinkler or drip applications. The uniprill fertilizer comprises small, preferably microscopic particles that are homogenous in nature, in that any single particle is substantially identical in composition to all other particles of the fertilizer. Additionally, each uniprill particle contains substantially all sixteen nutriments and minerals required for the growth of healthy plants.
Owner:GREEN TRIANGLE
Method for producing metal fibers
InactiveUS20050000321A1Transportation and packagingMetal-working apparatusO-Phosphoric AcidMetal fibers
A method of producing metal fibers including melting a mixture of at least a fiber metal and a matrix metal, cooling the mixture to form a bulk matrix comprising at least a fiber phase and a matrix phase and removing at least a substantial portion of the matrix phase from the fiber phase. Additionally, the method may include deforming the bulk matrix. In certain embodiments, the fiber metal may be at least one of niobium, a niobium alloy, tantalum and a tantalum alloy and the matrix metal may be at least one of copper and a copper alloy. The substantial portion of the matrix phase may be removed, in certain embodiments, by dissolving of the matrix phase in a suitable mineral acid, such as, but not limited to, nitric acid, sulfuric acid, hydrochloric acid and phosphoric acid.
Owner:ATI PROPERTIES
Purification of carbon dioxide
SO2 and / or NOx are removed from gaseous CO2 at elevated pressure(s) in the presence of molecular oxygen and water and, when SO2 is to be removed, NOx, to convert SO2 to sulfuric acid and / or NOx to nitric acid. The sulfuric acid and / or nitric acid is / are then removed from the gaseous carbon dioxide to produce SO2-free, NOx-lean carbon dioxide gas. The invention has particular application in the removal of SO2 and / or NOx from carbon dioxide flue gas produced in an oxyfuel combustion process, for example, in a pulverized coal fired power station.
Owner:AIR PROD & CHEM INC
Synthesis process of nanostring and nanopowder of RE hydroxide or oxide
InactiveCN1403375ALarge specific surface areaSimple processRare earth metal compoundsRare-earth elementNanowire
Owner:TSINGHUA UNIV
Methods of forming semiconductor constructions and capacitors
InactiveUS20070048976A1Solid-state devicesSemiconductor/solid-state device manufacturingTetramethylammonium hydroxideOxygen compound
The invention includes methods in which silicon is removed from titanium-containing container structures with an etching composition having a phosphorus-and-oxygen-containing compound therein. The etching composition can, for example, include one or both of ammonium hydroxide and tetra-methyl ammonium hydroxide. The invention also includes methods in which titanium-containing whiskers are removed from between titanium-containing capacitor electrodes. Such removal can be, for example, accomplished with an etch utilizing one or more of hydrofluoric acid, ammonium fluoride, nitric acid and hydrogen peroxide.
Owner:MICRON TECH INC
Method for producing metal fibers
Owner:ATI PROPERTIES
Stainless steel cleaning agent
InactiveCN101135056AImprove cleaning efficiencySimple cleaning processPhosphoric acidCleansing Agents
The stainless steel detergent for washing stainless steel product is prepared with acids, basic salts, surfactant, assistant, stabilizer and water, and through stirring and reaction at normal temperature. Specifically, it consists of sodium carbonate, sodium tripolyphosphate, tartaric acid, citric acid, hydrofluoric acid, nitric acid, phosphoric aicd, JFC, OP-10, triethanolamine, urotropin, trisodium phosphate, acetic acid, alcohol and water in certain weight proportion. It has the features of simple preparation process, environment friendship, high cleaning efficiency, low cleaning cost, etc.
Owner:吴铭鑫
Process and apparatus for the generation of chlorine dioxide using a replenished foam system
InactiveUS20030031621A1Reduce operating costsReadily availableChlorine dioxideChlorine dioxideAlkali metal
An aqueous solution of metal chlorate, mineral acid and a reducing agent are continuously or intermittently sprayed, in a pattern to achieve intimate mixing, into a spherical chamber creating an aqueous foam reaction mixture generating chlorine dioxide which is removed in a direction 90 degrees to the axis of the spray nozzles. A baffle plate may be used to reduce the open cross sectional area of the exit port to increase reaction efficiency. The reactants are a mineral acid and an alkali metal chlorate or chloric acid and a reducing agent such as hydrogen peroxide. The mineral acid is either diluted or concentrated sulfuric acid, hydrochloric acid, acetic acid, nitric acid or a blend thereof. The ratio of acid is greater than one and less than 3 kg acid per kg of ClO2 formed. The chlorine dioxide may be removed with a stripper column.
Owner:GRAVITT ALAN +1
Method for leaching anode and cathode mixed material of waste lithium nickel manganese cobalt battery
InactiveCN101619394AAvoid it happening againSave pollution control costsProcess efficiency improvementLithiumManganese
The invention relates to a method for leaching an anode and cathode mixed material of a waste lithium nickel manganese cobalt battery, which comprises the following steps: putting an anode and cathode mixed material separated out of a waste lithium nickel manganese cobalt battery in a container which is resistant to pressure and the corrosion of sulphuric acid and nitric acid; then sealing the container and pumping the sulphuric acid and the nitric acid into the container; and leading industrial pure oxygen into the container to leach out the anode and cathode mixed material of the waste lithium nickel manganese cobalt battery, wherein leaching temperature is 20-100 DEG C; leaching pressure is 0.05-0.5MPa; the initial concentration of the leaching sulphuric acid is 1-5mol / L; the initial concentration of the nitric acid is 5-20g / L; reaction time is 1-5 hours; stirring is carried out in a reaction process, and stirring speed is 30-100r / min; and the addition of the sulphuric acid is 101-200 percent of the theoretical sulphuric acid consumption of all leached metal in the anode and cathode mixed material added into the container.
Owner:SICHUAN NORMAL UNIVERSITY
Acidic solution for treating surface of magnesium alloy and processing method
InactiveCN101285193AFully activatedHigh activityMetallic material coating processesGlutaric acidBinding force
The invention relates to acid solution used for processing the magnesium alloy surface. The acid solution is water solution which contains acids, inhibitor and wetting agent, wherein, the acids are first acids or mixture of first acids and second acids; the first acids are selected from one type or a plurality of types among citric acids, oxalic acids, tartaric acids, methanoic acids, acetic acids, metacetonic acids, butyric acids, glutaric acids, phenylformic acids, benzene dicarboxylic acids, lactic acids, glycolic acids, glyoxylic acids and amino acids; and the second acids are hydrochloric acids and / or nitric acids. By adoption of the acid solution, the magnesium alloy surface can be fully activated; the membranous layer of a converting film which is formed on the magnesium alloy surface after chemical conversion process is compact, has erosion resistance and good binding force with a paint film. Moreover, the method is a environment-friendly method for processing the magnesium alloy surface.
Owner:BYD CO LTD
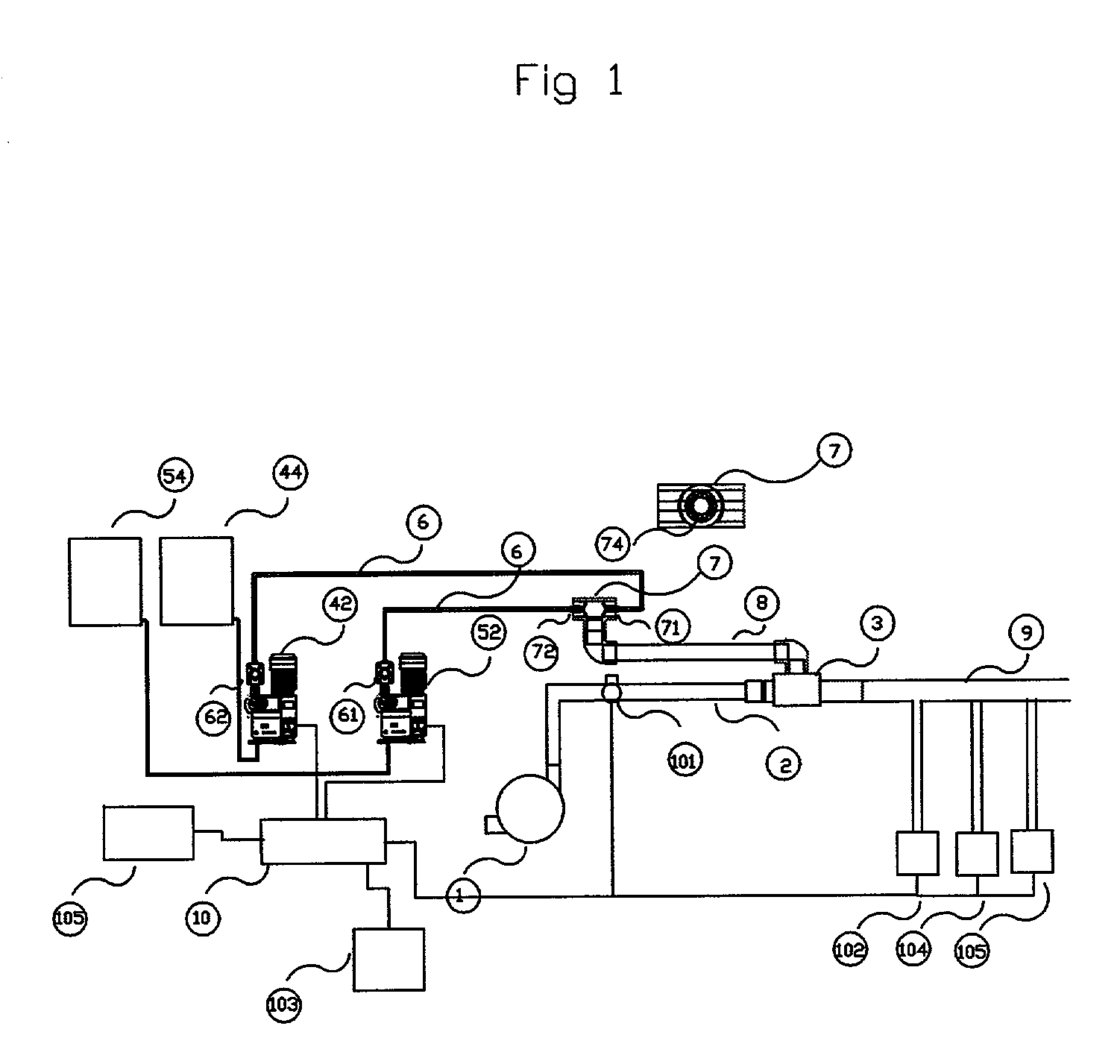
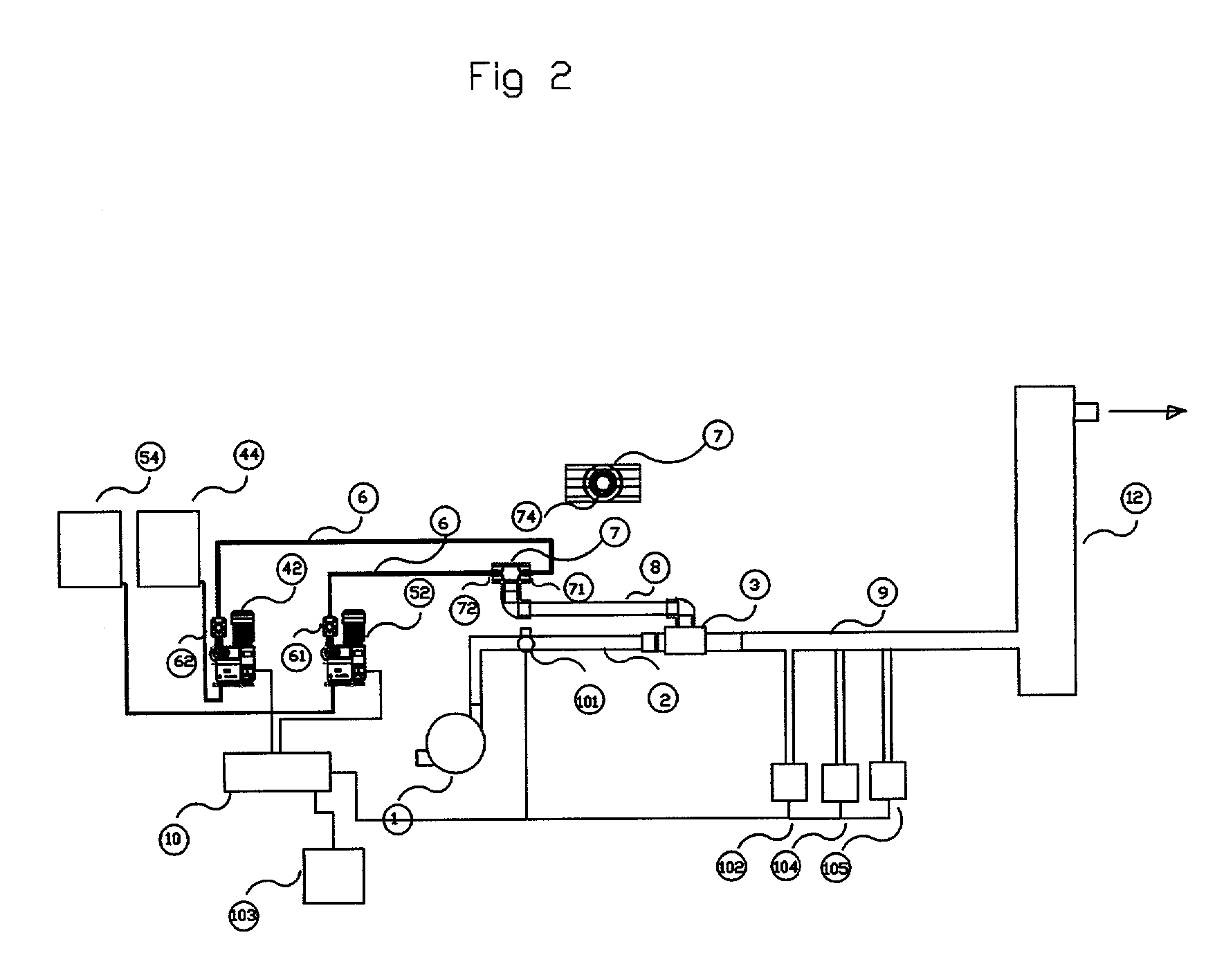
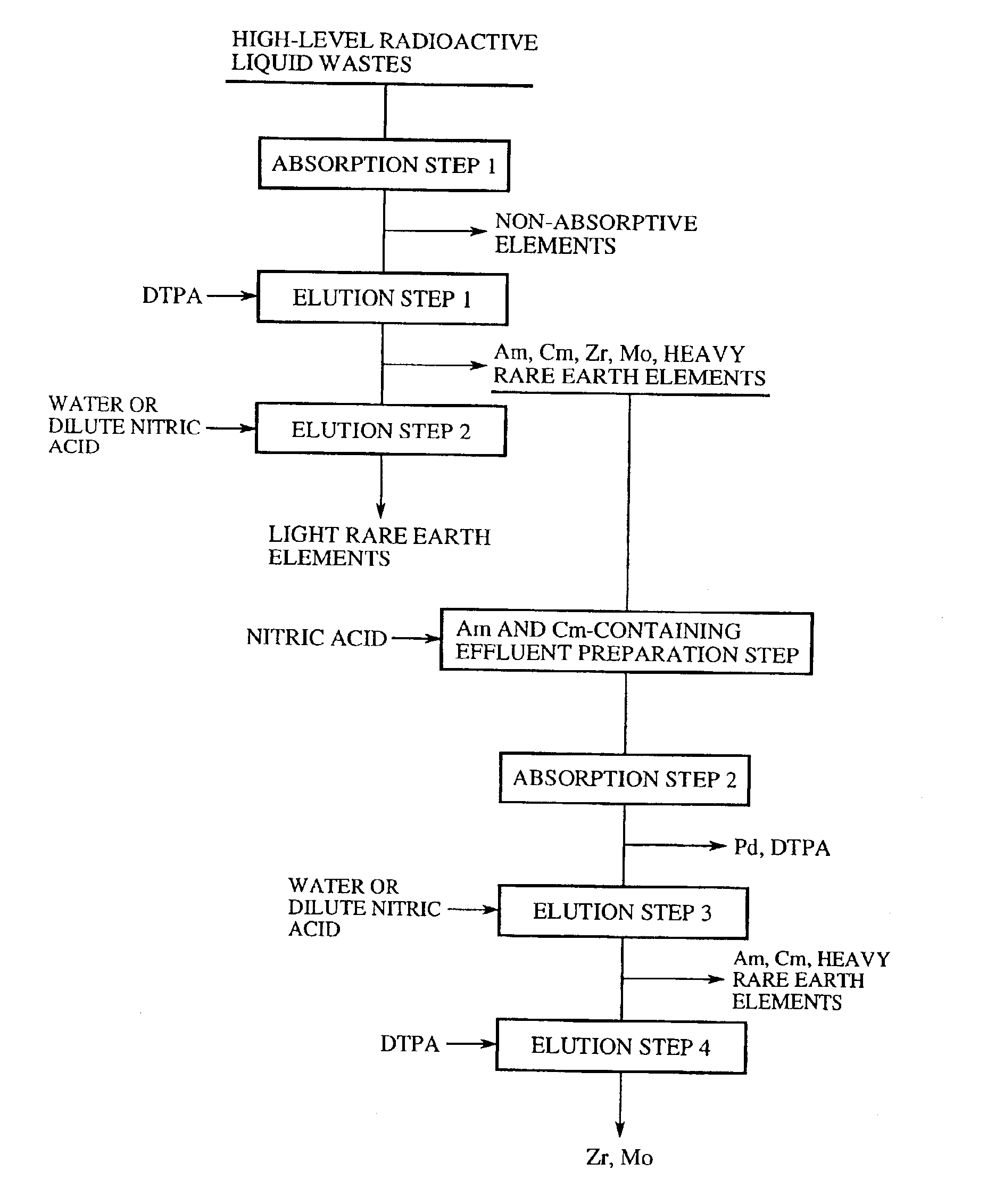
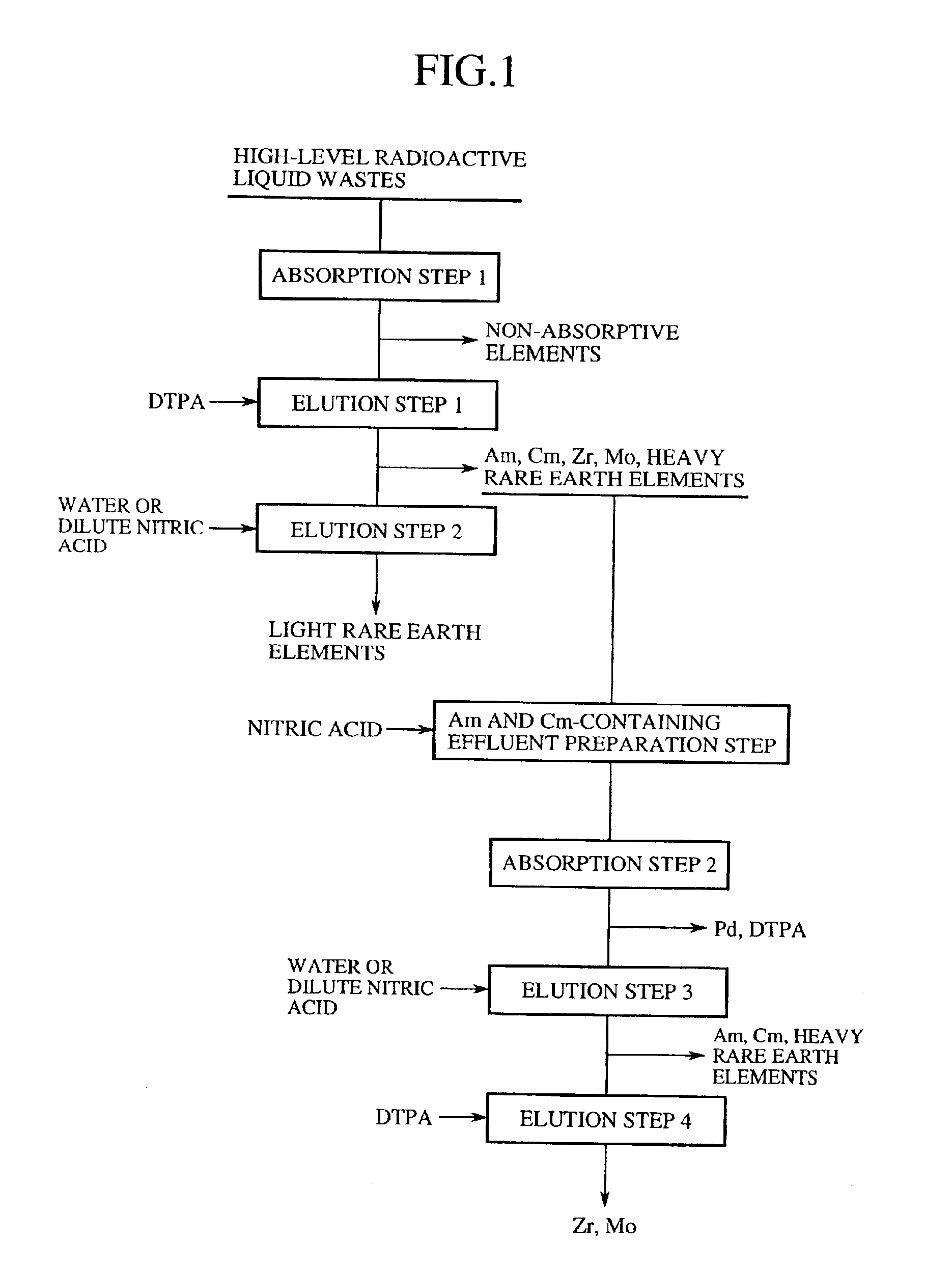
Method of separation and recovery of elements from radioactive liquid wastes
InactiveUS6843921B2Efficient elutionImprove efficiencyOther chemical processesSolid sorbent liquid separationRare-earth elementElution
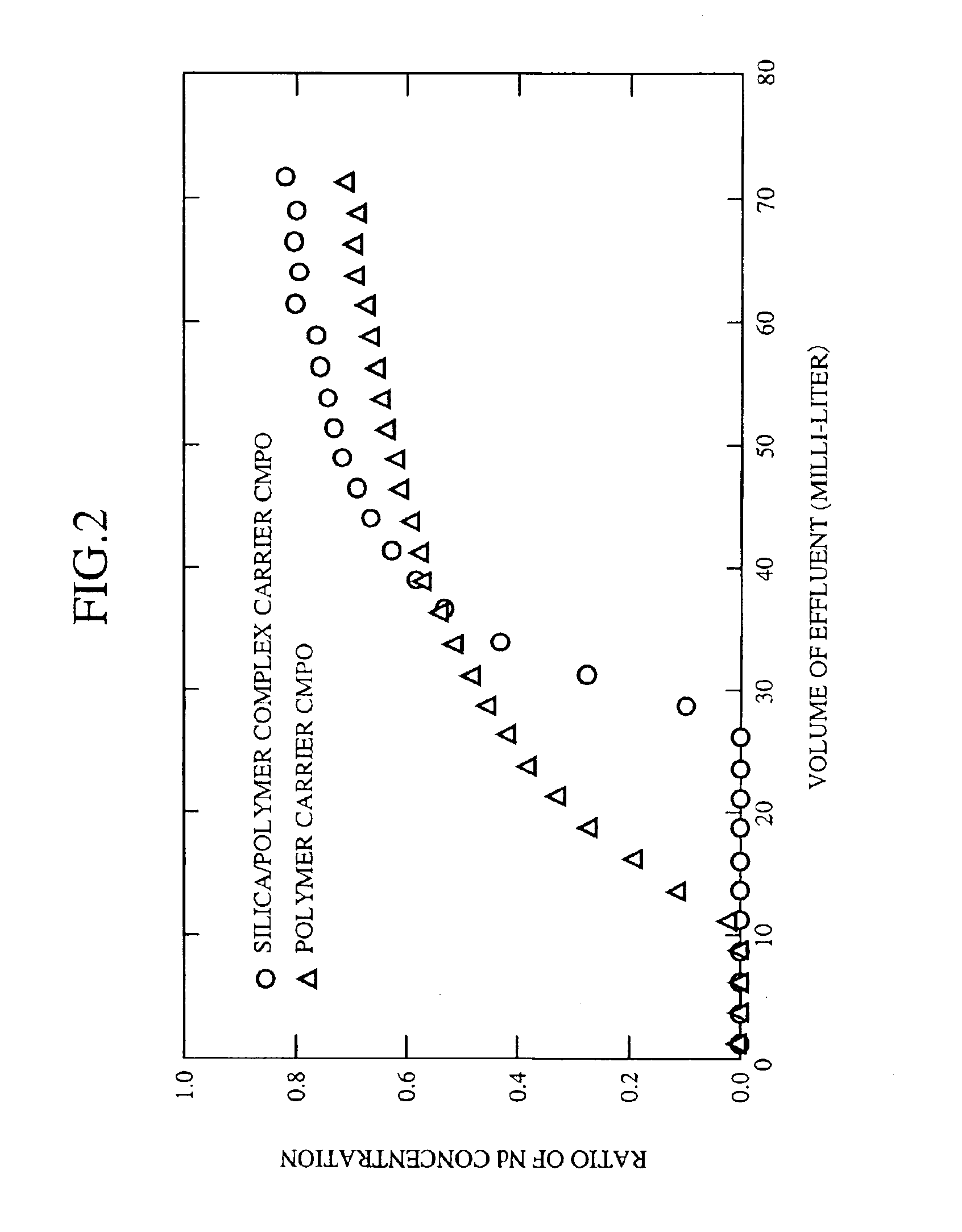
A method of separation and recovery of elements from radioactive liquid wastes, includes a step of bringing into contact a high-level radioactive liquid waste containing separation target elements including Americium, Curium, Zirconium, Molybdenum, Palladium and rare earth elements with solid absorbent containing organophosphorus compounds so that the separation target elements are absorbed in the solid absorbent, a step of bringing into contact the solid absorbent with an acidic solution containing diethylenetriaminepentaacetic acid so that Americium, Curium, Zirconium, Molybdenum, Palladium and heavy rare earth elements are eluted from the solid absorbent, and a step of bringing into contact the solid absorbent underwent the first elution step with water or dilute nitric acid so that light rare earth elements are eluted from the solid absorbent. With the method, elements, which include Americium, Curium, Zirconium, Molybdenum, Palladium and rare earth elements, are efficiently and economically separated and recovered from the radioactive liquid waste.
Owner:JAPAN ATOMIC ENERGY AGENCY INDEPENDANT ADMINISTRATIVE CORP +1
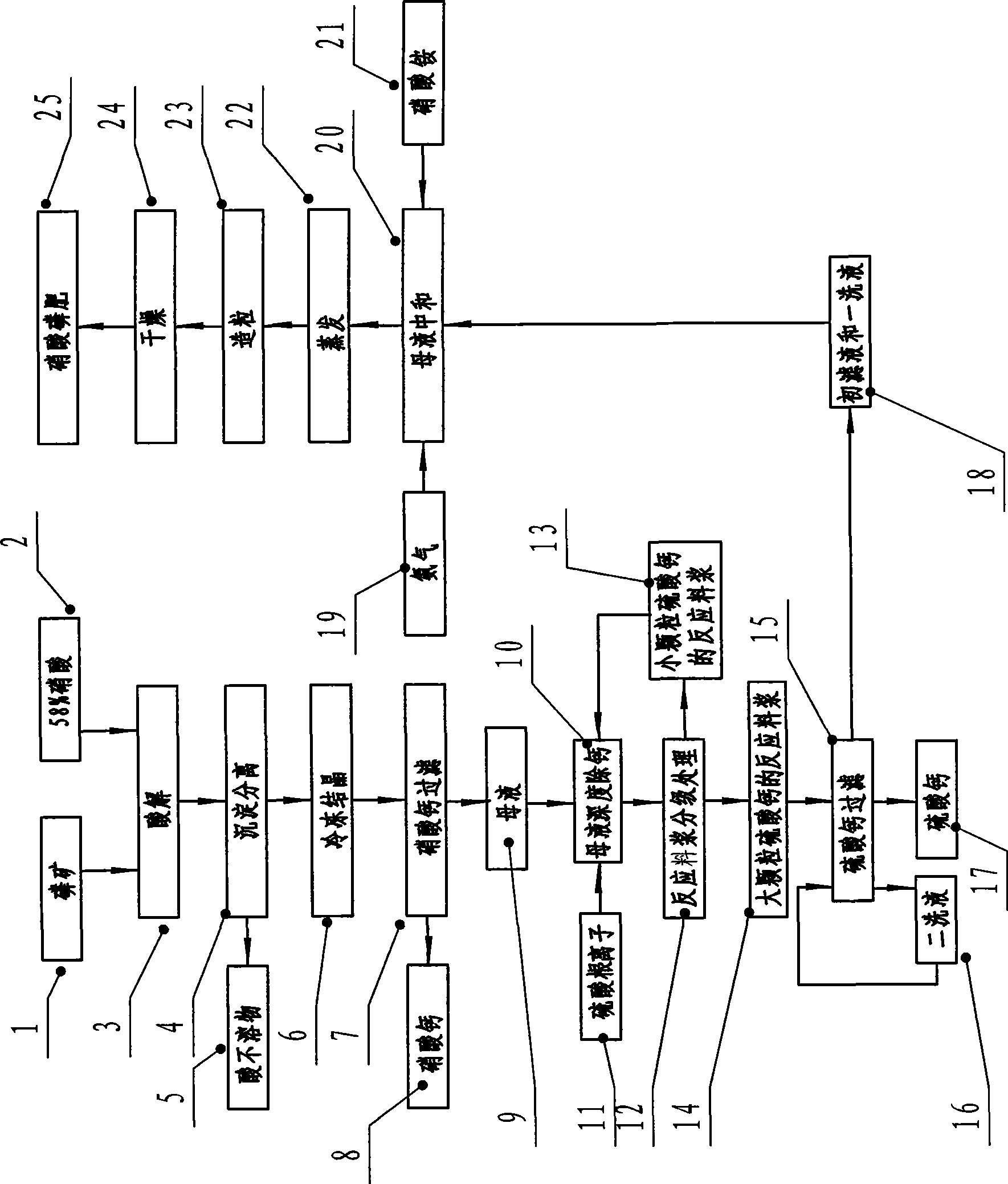
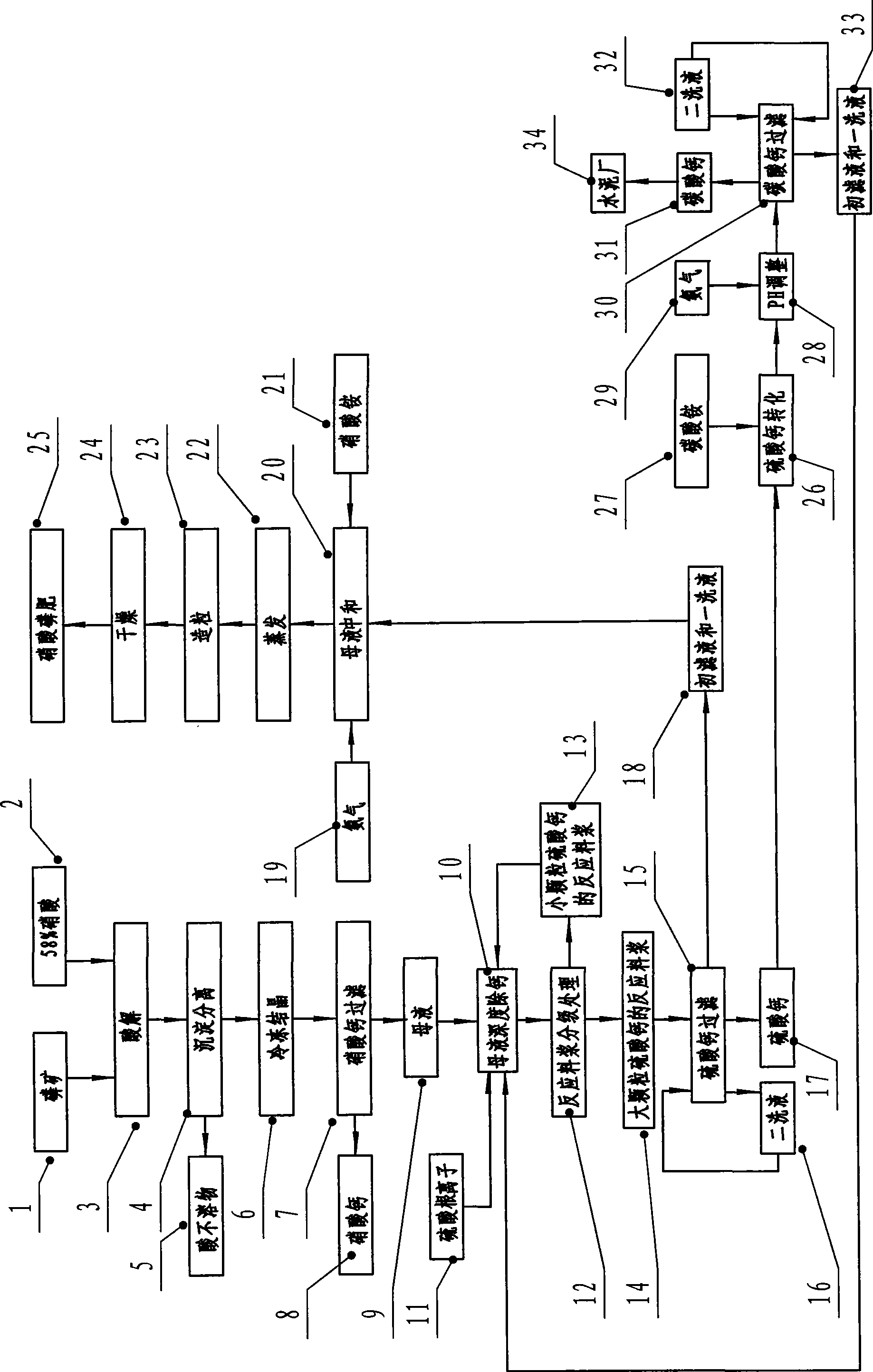
Technological process for producing high concentration nitric-phosphate fertilizer
The invention discloses a technique method for producing high-concentration nitric phosphate, which comprises the following steps of: adding nitric acid into phosphorus ore for acidolysis, depositing and separating acid non-soluble substance, freezing and crystallizing calcium nitrate, filtering the calcium nitrate, neutralizing the mother liquid, vaporization, pelleting and drying. The invention is characterized in that: a step of thoroughly removing the calcium of the mother liquid is arranged between the steps of filtering the calcium nitrate, and neutralizing the mother liquid. The steps comprise the following steps of: I) thoroughly removing the calcium of the mother liquid and adding sulfuric acid or ammonium sulfate; the calcium ions and the sulfate ions in the mother liquid generate dihydrate calcium sulfate crystal; II) the grading processing of reaction slurry: employing a grading device to carry out grading processing to the reaction slurry; returning the reaction slurry provided with small grain calcium sulfate to the mother liquid for thoroughly removing the calcium, and feeding the reaction slurry provided with large grain calcium sulfate to the calcium sulfate for filtering; and III) filtering and washing the filtering reaction slurry of calcium sulfate by a filter, feeding the primary filtrate and the primary lavage fluid into the working procedure of neutralizing, and returning the secondary lavage fluid to the filter. The invention has the advantage of using middle-low quality phosphorite to prepare the high-concentration nitric phosphate.
Owner:TIANJI COAL CHEM IND GROUP +1
Analyses testing method of aluminum, calcium, iron, molybdenum, niobium, titanium, tungsten impurity elements in chromium carbide
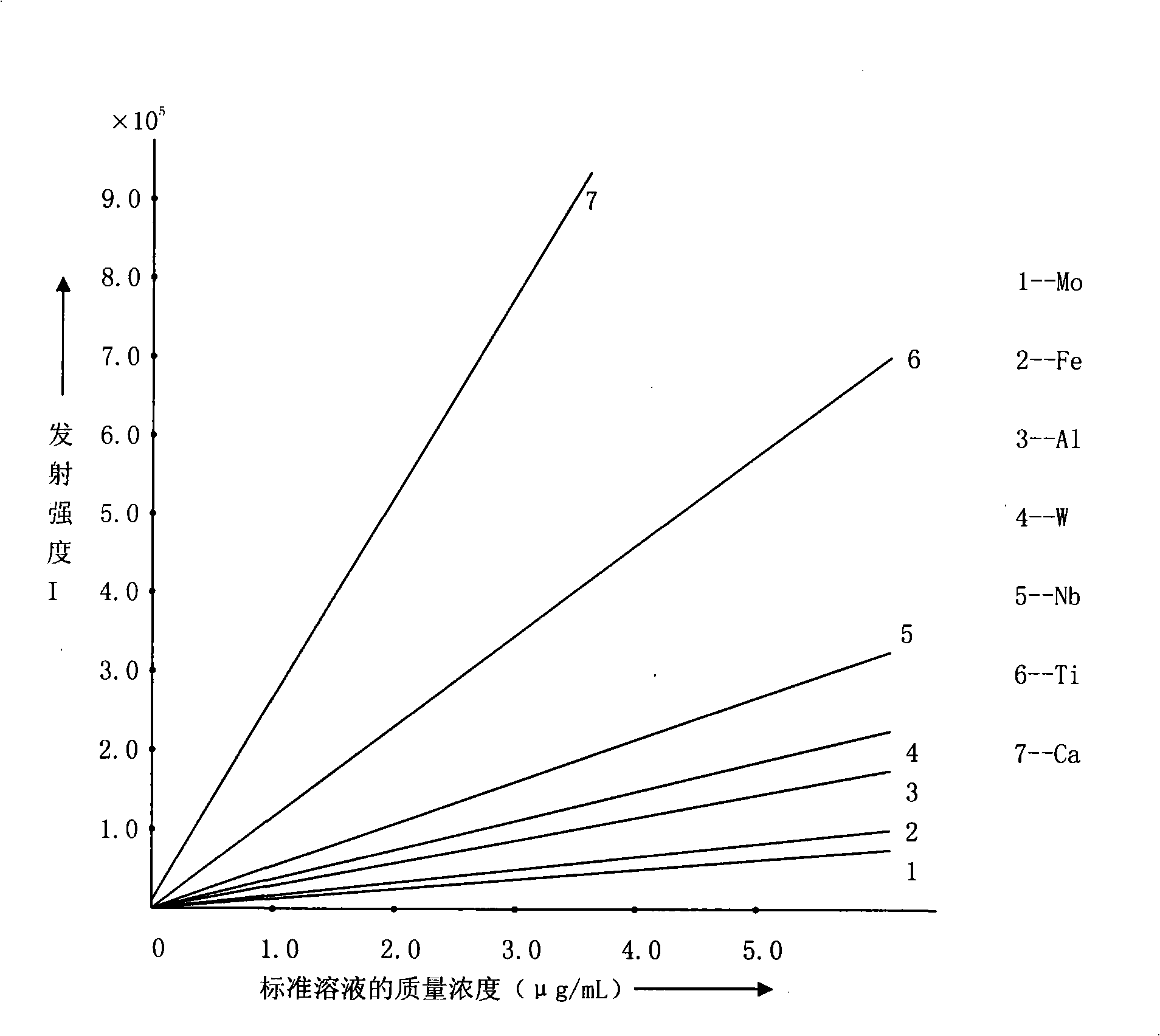
ActiveCN101303307ASolve difficult technical problemsImprove measurement accuracyPreparing sample for investigationAnalysis by thermal excitationNiobiumDecomposition
The invention discloses an analysis and detection method for impurity elements such as aluminum, calcium, ion, molybdenum, niobium, titanium, tungsten and the like in chromium carbide. The method comprises adding a chromium carbide sample into a dissolving cup, adding hydrofluoric acid, sulphuric acid and nitric acid sequentially, stirring, charging into a sealed high-pressure jar; putting the sealed high-pressure jar into a microwave extinguishing instrument for two times of microwave extinguishment; taking the high-pressure jar out of the microwave extinguishing instrument for cooling, transferring the dissolved chromium carbide liquid sample into a volumeric flask, diluting to a predetermined index, stirring; preparing a chromium substrate matched mixed standard solution series of aluminum, calcium, iron, molybdenum, niobium, titanium and tungsten; measuring element emission power of aluminum, calcium, iron, molybdenum, niobium, titanium, tungsten or the like in a blank liquid sample, a chromium carbide liquid sample and the prepared series mixed standard solution by an inductively coupled plasma atomic emission spectrometer in the same time, obtaining the analysis result by checking a standard working curve or by linear equation calculation. The invention adopts two times of microwave extinguishment using the mixed acid, solves the problem of hardness in chromium carbide decomposition, having a measurement range from 0.010% to 1.00%, which is high in accuracy, and good in precision.
Owner:ZHUZHOU HARD ALLOY GRP CO LTD
High tensile strength carbon nanotube film and process for making the same
InactiveUS20060029537A1Material nanotechnologyArtificial filament chemical after-treatmentOxidizing agentNanotube
A conductive carbon nanotube film having high tensile strength and initial tensile modulus comprises primarily oxidized small-diameter carbon nanotubes wherein the diameter of the small-diameter carbon nanotubes are at most about 3 nm. A method for making the film comprises refluxing an aqueous mixture comprising carbon nanotubes and an oxidizing agent to form a refluxed nanotube dispersion; forming a carbon nanotube film from the refluxed carbon nanotube dispersion; optionally removing nitric acid or other oxidizing agent from the carbon nanotube film; drying the carbon nanotube film; and heat-treating the carbon nanotube film to form a heat-treated carbon nanotube film. The method can also comprise sonicating the nanotubes prior to or after refluxing. A heat-treated small-diameter carbon nanotube film can have a tensile strength of over 70 MPa and an initial tensile modulus of about 5 GPa.
Owner:GEORGIA TECH RES CORP
Preparation for composite material with nanometal or metal oxide distributed on surface of carbon nanotube uniformly
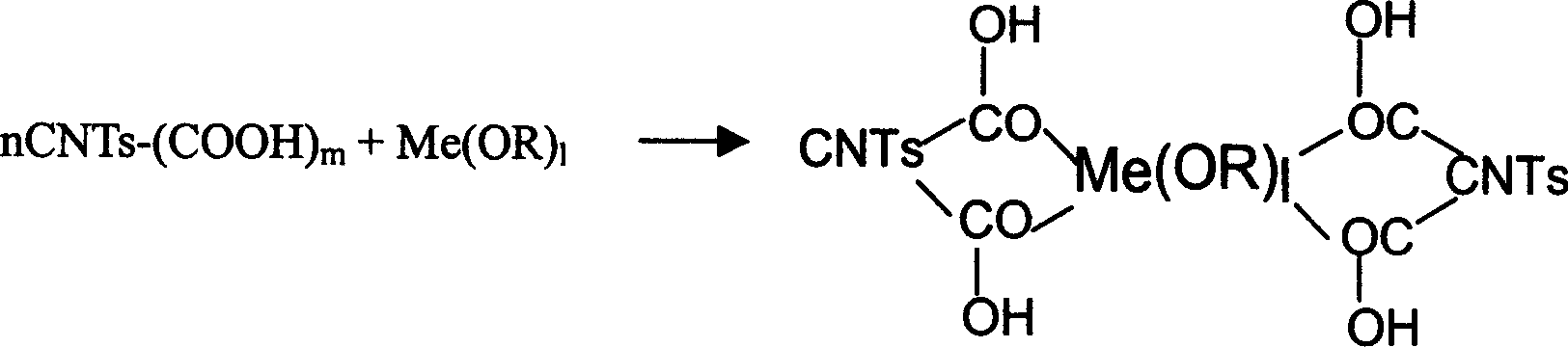
The invention discloses the preparation for composite material with nanometal or metal oxide distributed on surface of carbon nanotube uniformly which comprises, stirring and stewing the precursor through long time supersound treatment, the precursor being metal organic compound and concentrated nitric acid treated carbon nano tube, thus resulting in coordination reaction between the metal organic compound and carbon nano tube surface carboxyl and / or carbonyl and / or hydroxyl with the presence of the alcohol solvent action, and forming cores on the carbon nano tube walls so as to form the composite material.
Owner:HUAZHONG NORMAL UNIV
Process for solvent extraction separation purification of rare earth element
ActiveCN101319275AStripping is easyReduce acid and alkali consumptionProcess efficiency improvementRare-earth elementNitrate
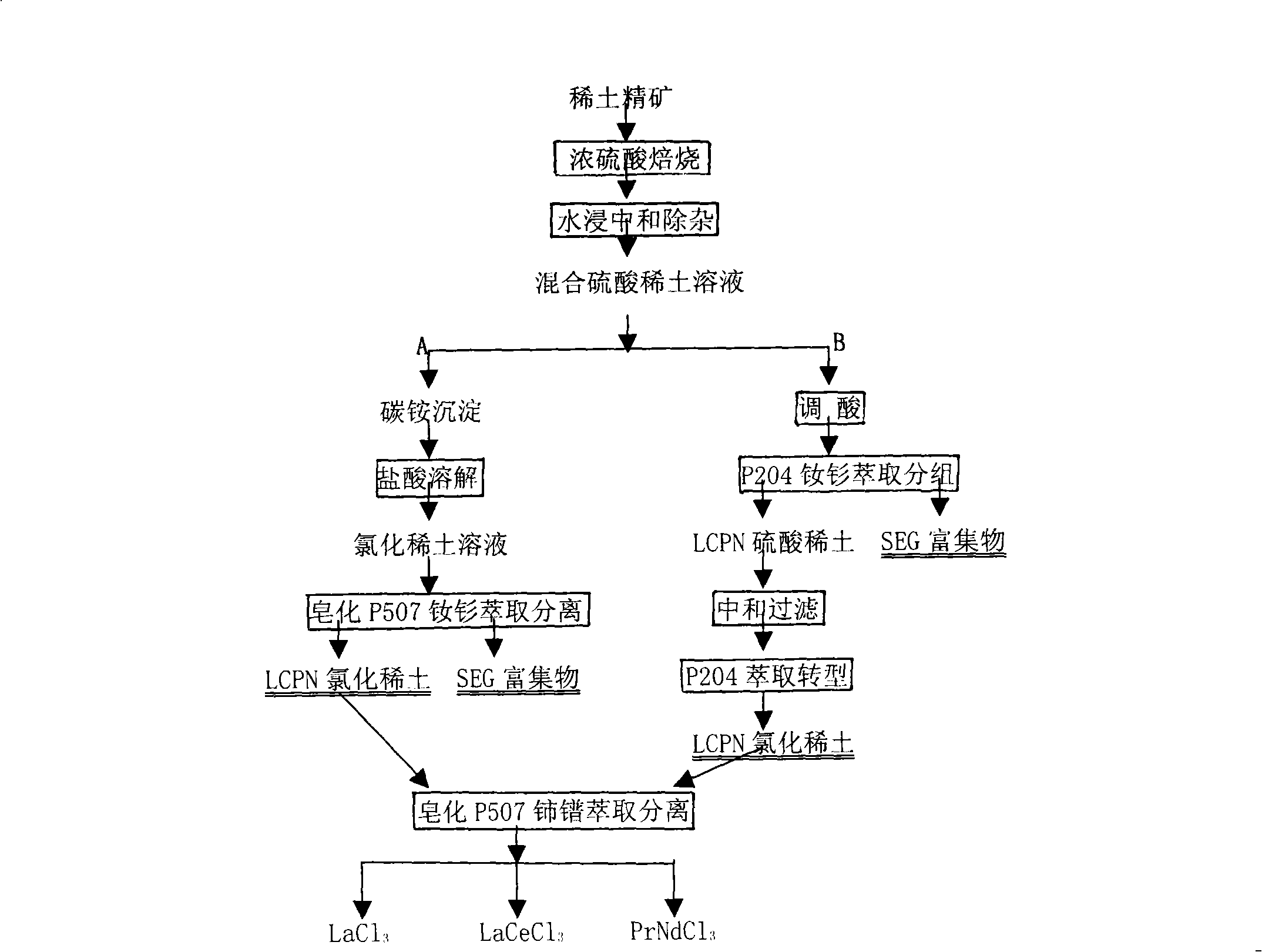
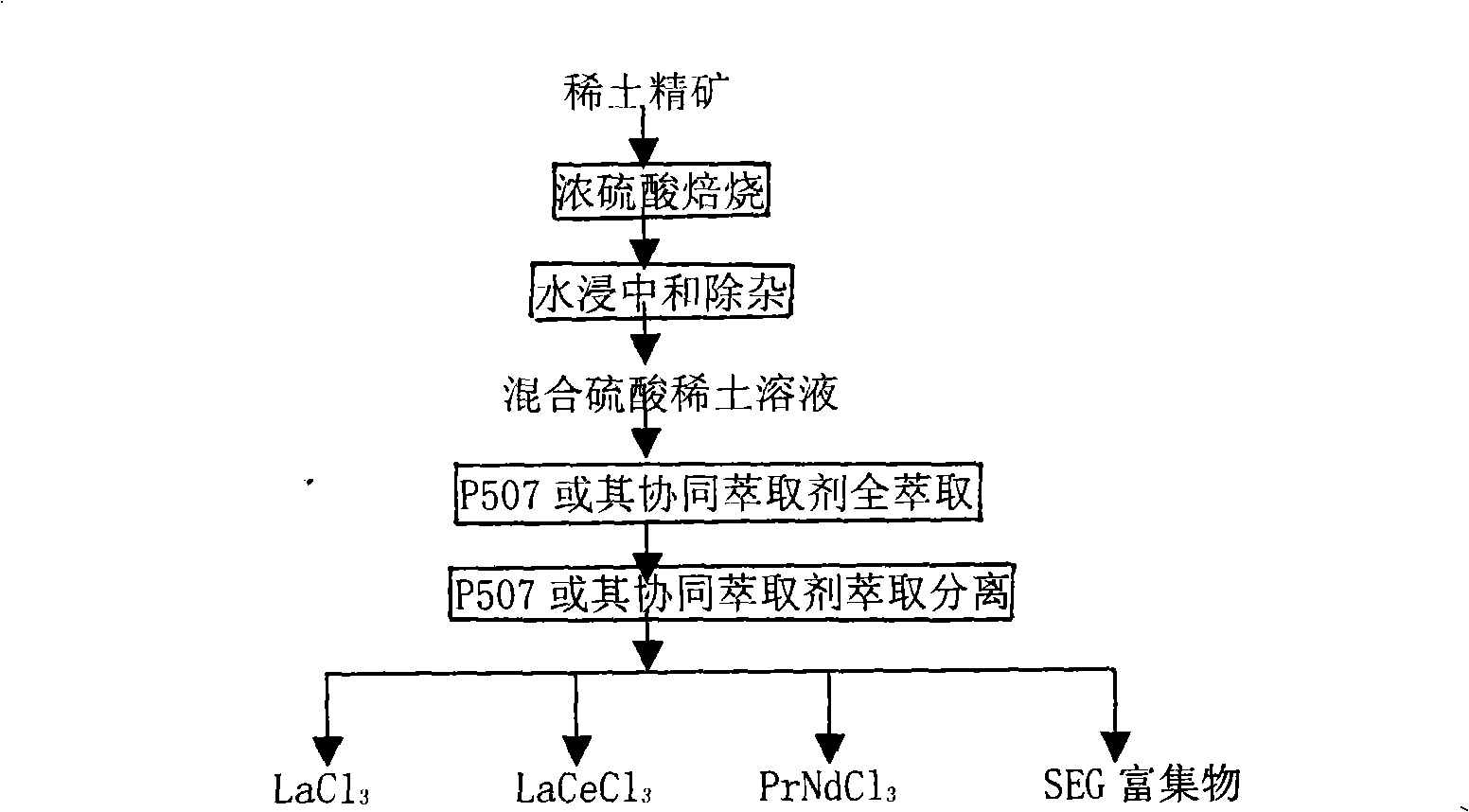
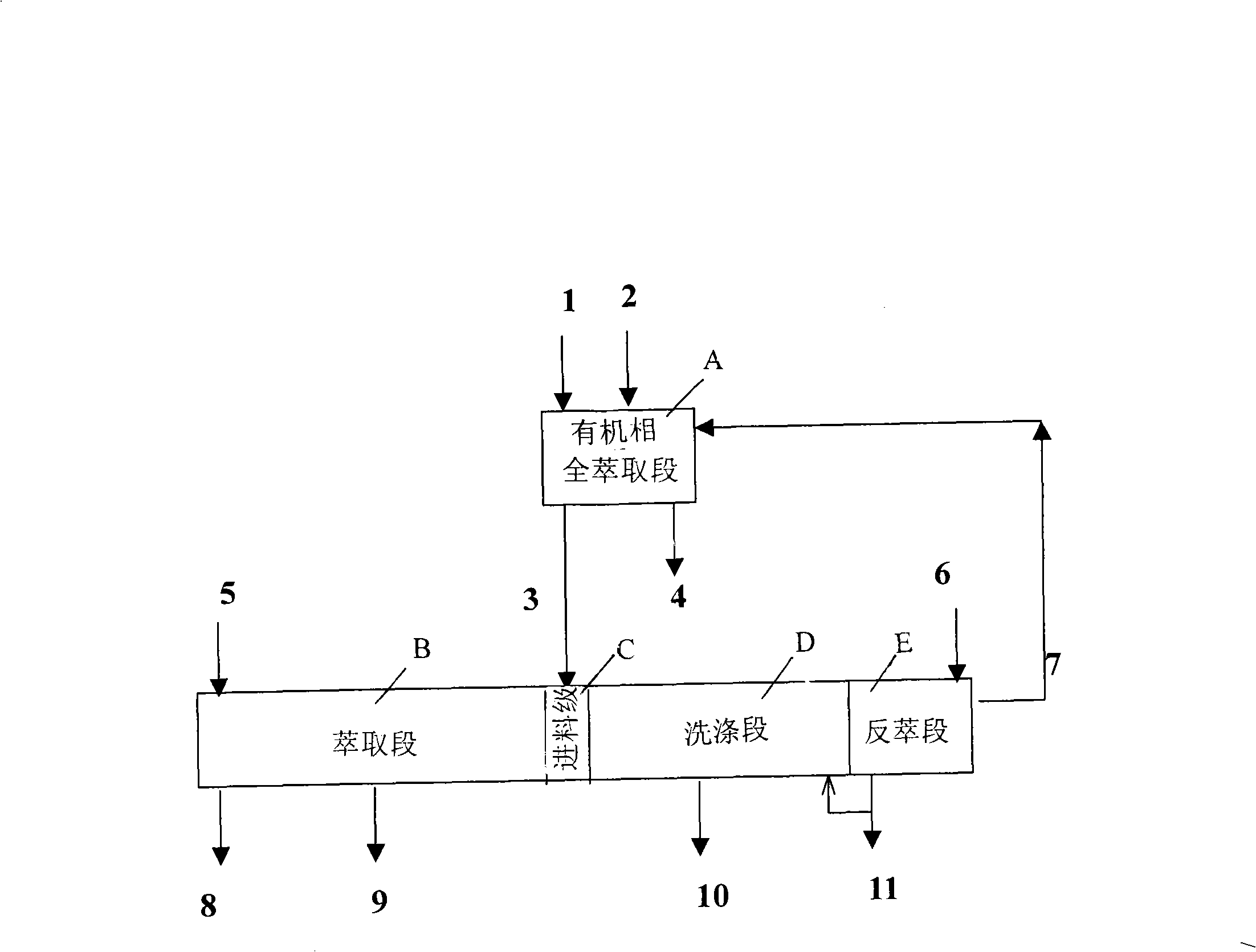
The invention provides a process method for separating and purifying rare earth elements by solvent extraction. The method takes a mixed rare earth sulfate solution obtained from a rare earth ore treated by sulphuric acid as a raw material, directly adopts a nonsaponifiable P507 extraction agent or a synergistic extraction agent containing the P507 to completely extract rare earth in a material liquid into an organic phase, and then takes the organic phase as a rare earth material liquid to extract, separate and purify the rare earth or directly backextract to produce mixed chlorinated rare earth or rare earth nitrate. The process method is simple and flexible, does not saponify the organic phase during the extraction and separation process, does not produce ammonia nitrogen waste water, is easy to backextract middle and heavy rare earths, and has less consumption of acid and base and low production cost.
Owner:GRIREM ADVANCED MATERIALS CO LTD
Treating solution for surface treatment of metal and a method for surface treatment
InactiveUS20060185769A1Improve corrosion resistanceAnodisationElectrolytic inorganic material coatingSurface-active agentsOxygen
Treating solution for surface treatment of metal to treat one metal material selected from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material or to treat two or more metal materials selected from the group consisting of ferriferous material, zinciferous material, aluminiferous material and magnesiferous material at the same time, wherein the aqueous surface treating solution contains from 5 to 5000 ppm of at least one compound selected from the group consisting of zirconium compound and titanium compound by the metal element, and from 0.1 to 100 ppm of free fluorine ion, further the pH of the treating solution is from 2 to 6. To the treating solution, calcium compound, magnesium compound, strontium compound, nitric acid group, oxygen acid and / or salt of oxygen acid, polymer compound and surface active agent can be added.
Owner:NIHON PARKERIZING +2
Method for undergoing chlorobenzene nitration reaction by using micro-channel reactor
ActiveCN102432471APrevent leakageAvoid dangerNitro compound preparationTemperature controlChlorobenzene
The invention relates to a method for undergoing a chlorobenzene nitration reaction by using a micro-channel reactor, belonging to the technical field of application of organic synthesis. In the method, nitric acid, sulfuric acid, water and chlorobenzene are taken as initial reaction raw materials, and processes such as mixed acid preparation, mixed acid and chlorobenzene preheating, mixed acid and chlorobenzene reacting and the like are completed in a micro-channel reactor system. In the reaction, nitro-sulfuric mixed acid is taken as a nitrating agent, the effective concentration of sulfuric acid in the mixed acid is 50-90 percent, the molar ratio of the nitric acid to the sulfuric acid in the mixed acid is 1:1-1:10, the molar ratio of the chlorobenzene to the nitric acid is 1:1.0-1:2.0, the reaction temperature is 50-100 DEG C, and the reaction time is 30-120 seconds. The chlorobenzene transformation ratio is up to 97 percent, the selectivity of nitrochlorobenzene serving as a product is over 96.5 percent, and the ratio of ortho-para nitrochlorobenzene is over 0.6. A strengthened mixed micro-channel reactor adopted in the invention is particularly suitable for undergoing a continuous nitration reaction, and has the characteristics of stable temperature control and safe process.
Owner:CHANGZHOU UNIV
Nitrate molten salt heat transferring and reserving medium and preparation method and application thereof
InactiveCN102533226AWide operating temperature rangeImprove thermal stabilityHeat-exchange elementsDecompositionInstability
The invention discloses a nitrate molten salt heat transferring and reserving medium and a preparation method and application thereof. The nitrate molten salt heat transferring and reserving medium is prepared with 5 to 40 percent of potassium nitrate, 5 to 25 percent of sodium nitrate, and 10 to 70 percent of calcium nitrate. The melting point of the nitrate molten salt heat transferring and reserving medium can be as low as 120 DEG C, and the upper limit of temperature for use can reach 550 DEG C, the nitrate molten salt heat transferring and reserving medium has a wide temperature scope of application, can work normally at the temperature scope of 180 DEG C to 550 DEG C, and has good thermal stability; the shortcoming of high melting point of binary nitrate molten salt system can be overcome, and the problem of instability caused by easy oxidative decomposition at high temperature of NaNO2 in the Chinese patent 200110027954.1 and ternary nitrate salt system can be solved, and the problems of corrosion and cost increase caused by the existing LiNO3 in the Chinese patent 00111406.9 and the American patent US007588694B1 can also be solved.
Owner:SUN YAT SEN UNIV +1
Purification of carbon dioxide
SO2 and / or NOx are removed from gaseous CO2 at elevated pressure(s) in the presence of molecular oxygen and water and, when SO2 is to be removed, NOx, to convert SO2 to sulfuric acid and / or NOx to nitric acid. The sulfuric acid and / or nitric acid is / are then removed from the gaseous carbon dioxide to produce SO2-free, NOx-lean carbon dioxide gas. The invention has particular application in the removal of SO2 and / or NOx from carbon dioxide flue gas produced in an oxyfuel combustion process, for example, in a pulverized coal fired power station.
Owner:AIR PROD & CHEM INC
Solar cell, a method of producing the same and a semiconductor producing apparatus
InactiveUS6340640B1Final product manufactureSemiconductor/solid-state device manufacturingPhosphoric acidCarboxylic acid
In preparing a solar cell, minute projections and recesses are uniformly formed in a surface of a single crystal silicon substrate or a polycrystal silicon substrate by dipping the substrate in an etching liquid of a mixed acid including a hydrofluoride acid, a nitric acid and an adjusting agent containing at least a phosphoric acid or a water-soluble carboxylic acid having a higher molecular weight than acetic acid for adjusting the etching rate of the etching liquid. A solar cell having a substrate in which spherical projections and recesses are formed in a surface thereof to which light is incident; an apparatus for producing a solar cell, and a wet etching apparatus to maintain a constant concentration of a nitric acid, are provided.
Owner:MITSUBISHI ELECTRIC CORP
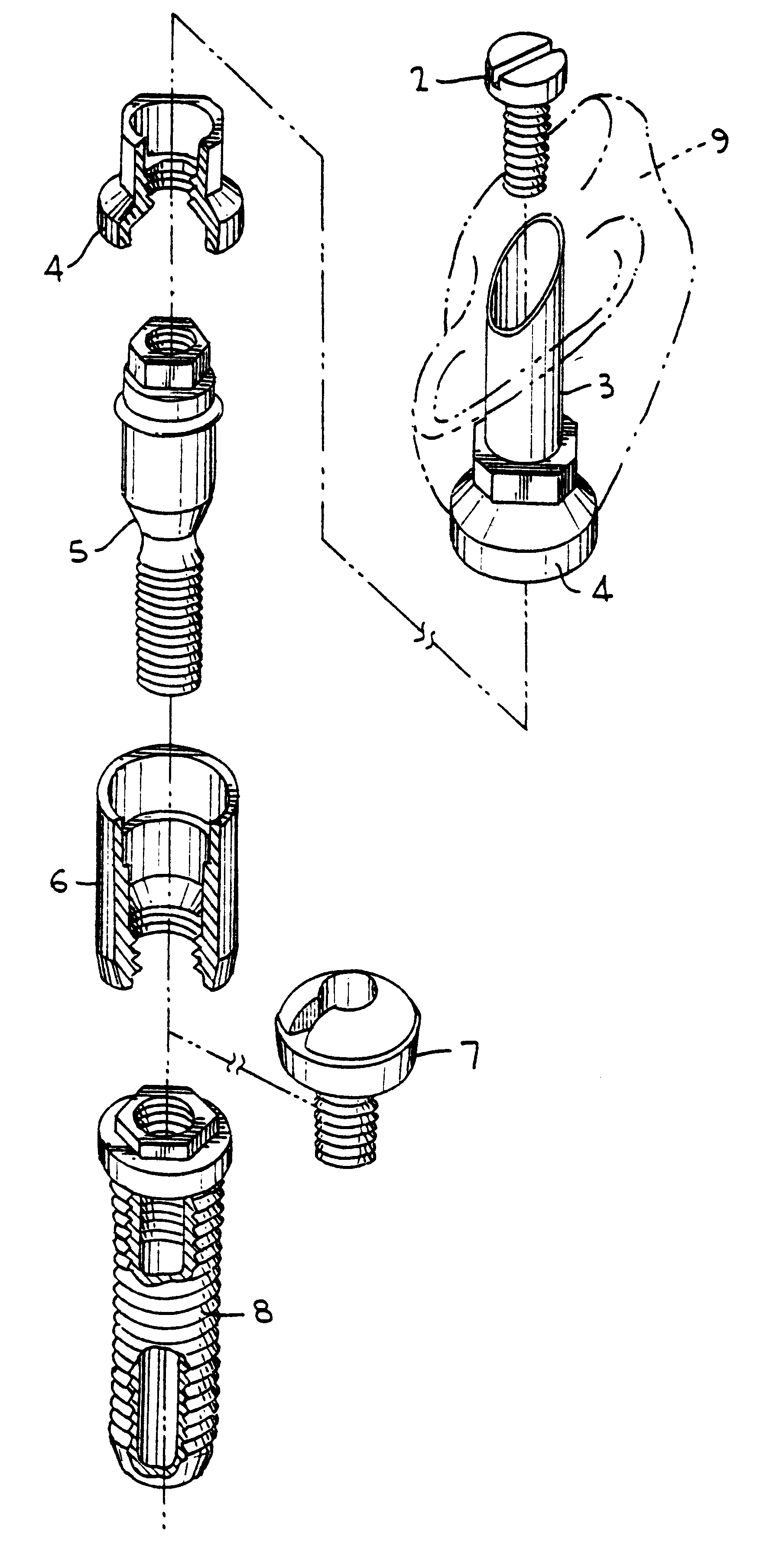
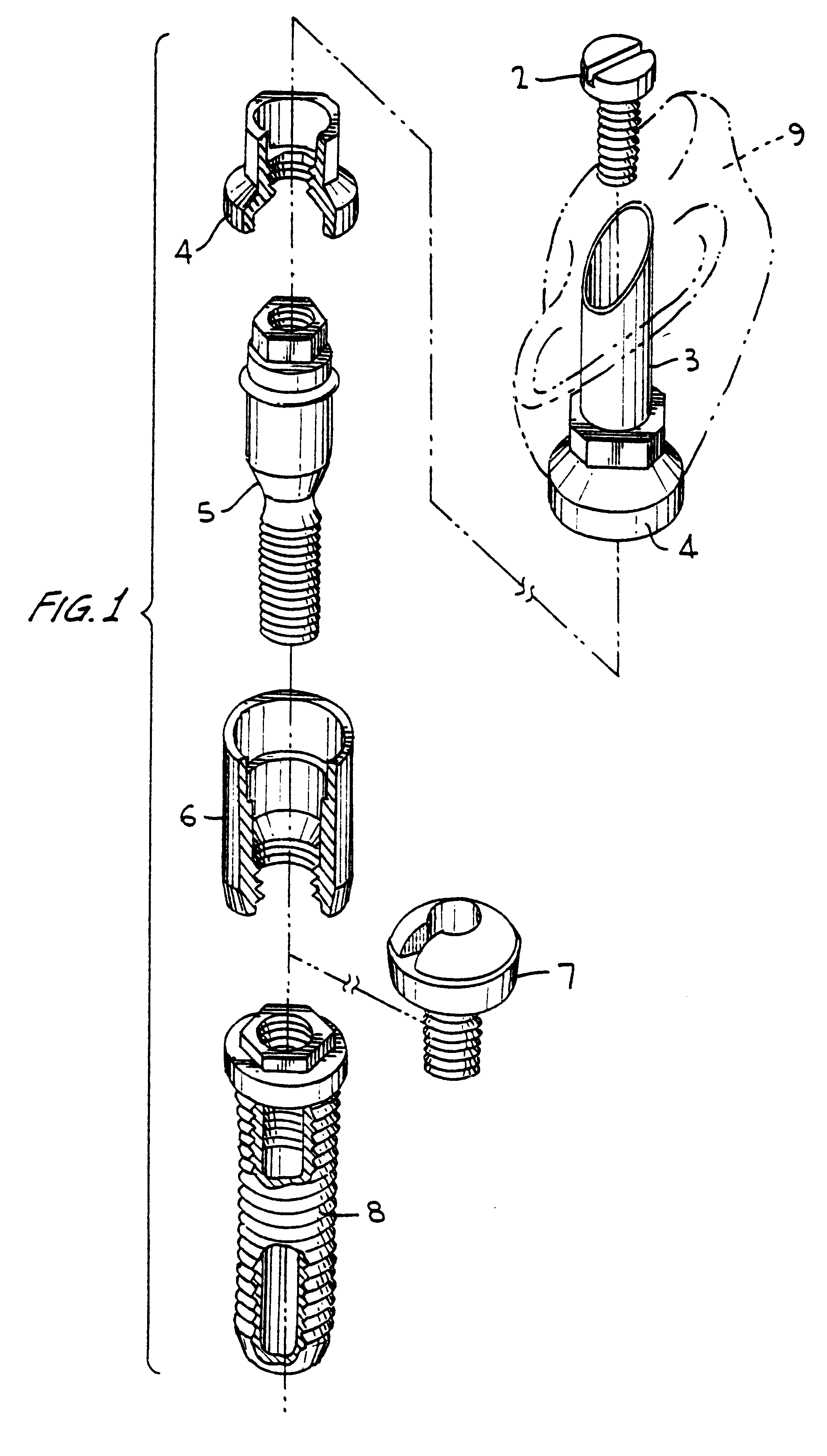
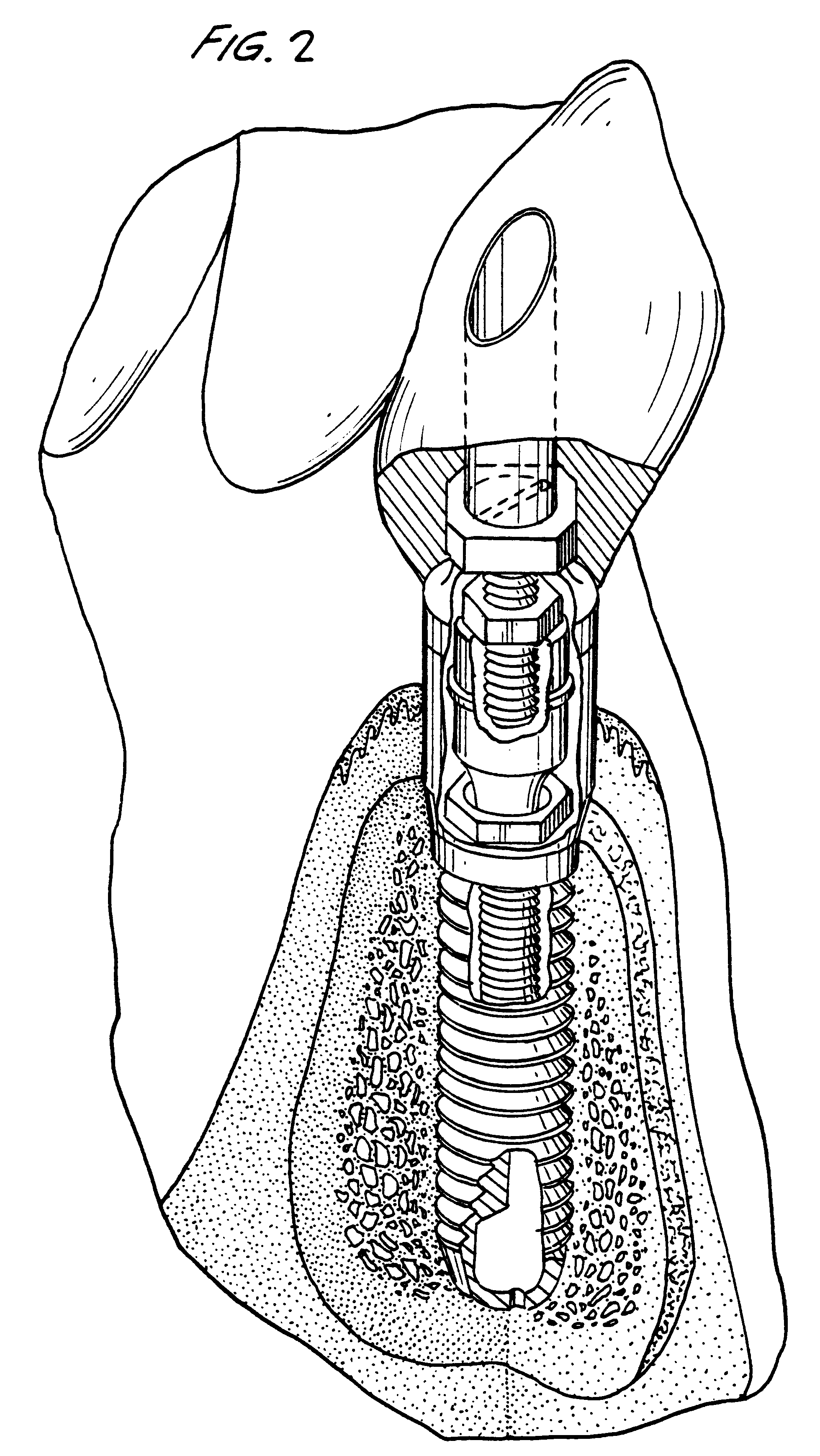
Titanium material implants
One of many universal requirements of dental or orthopedic implants, wherever they are used in the vital body, is that the implant system should be biologically functioning. To achieve the biological functionality, the implant should meet several requirements for compatibility. These include biological compatibility and mechanical compatibility. It has now been recognized that morphological compatibility and crystallographic compatibility should be added to these two compatibility requirements. Hence, the present invention provides a method of forming a certain type of crystalline structure of titanium oxide and controlled surface roughness to meet both morphological and crystallographic compatibilities. It has been further determined that a chemical treatment (using sodium hydroxide) alone or followed by in-air oxidation, or acid treatment (a mixed aqueous solution of hydrofluoric acid and nitric acid), followed by sodium hydroxide treatment, furthermore followed by in-air oxidation provide for advantageous surface modifications to create a complex mixture of rutile with anatase and / or brookite types of titanium oxide and provide a most favorable surface for wettability and an acceptable range of surface roughness.
Owner:OSHIDA YOSHIKI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com