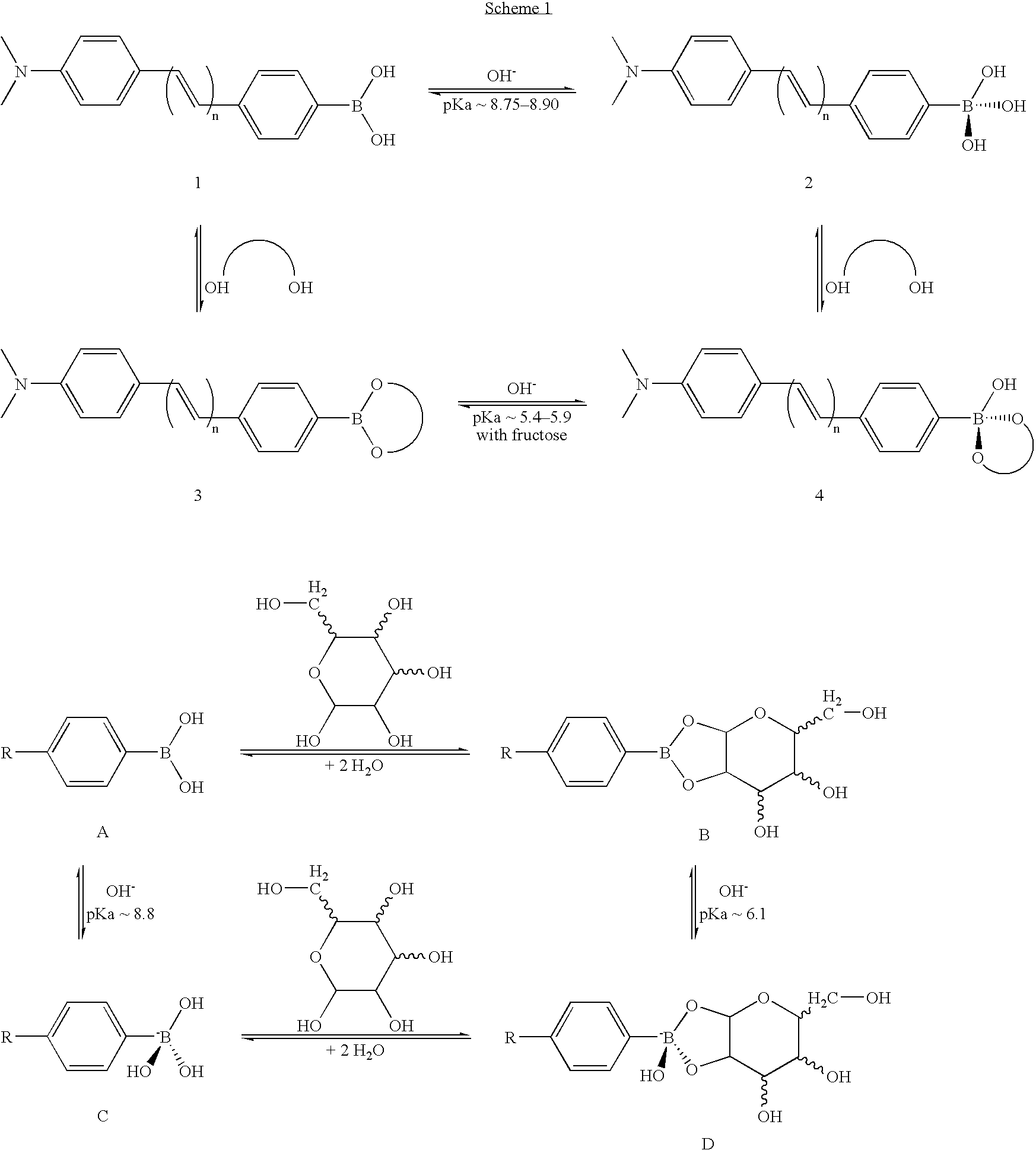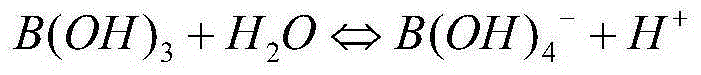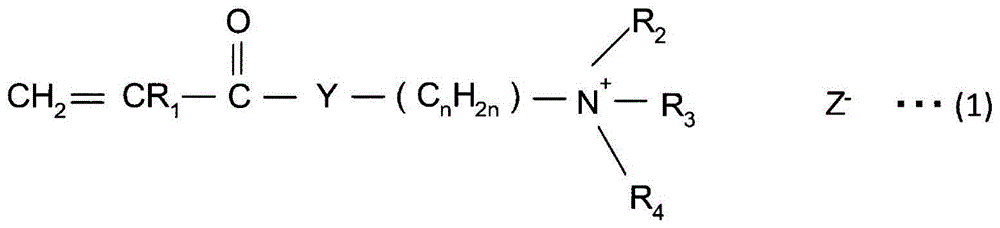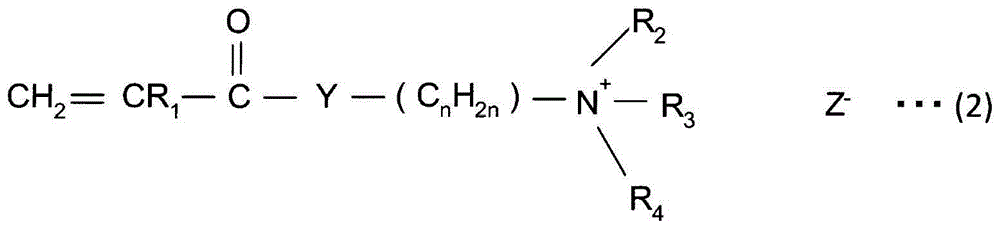Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
91 results about "Borate ion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
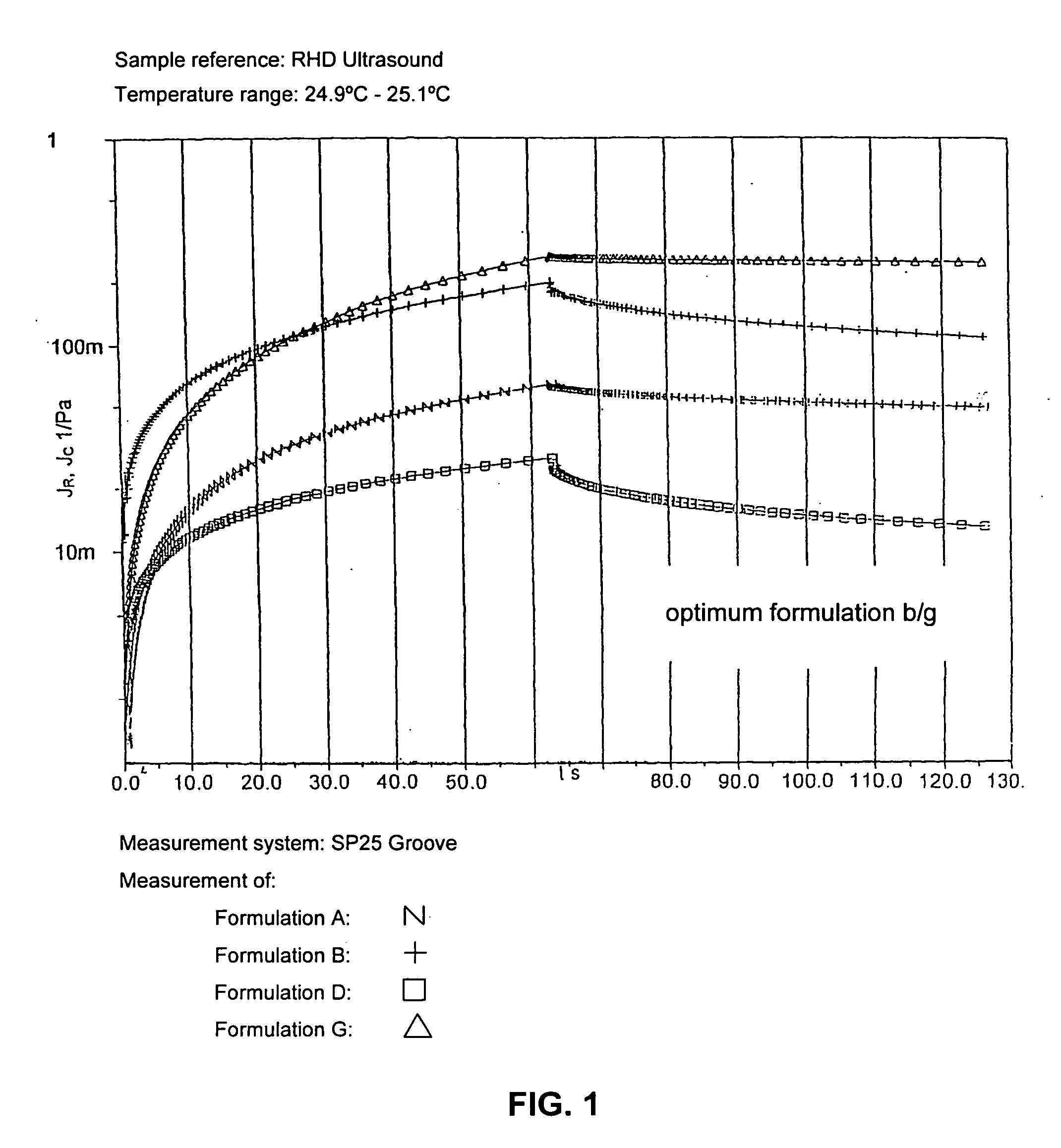
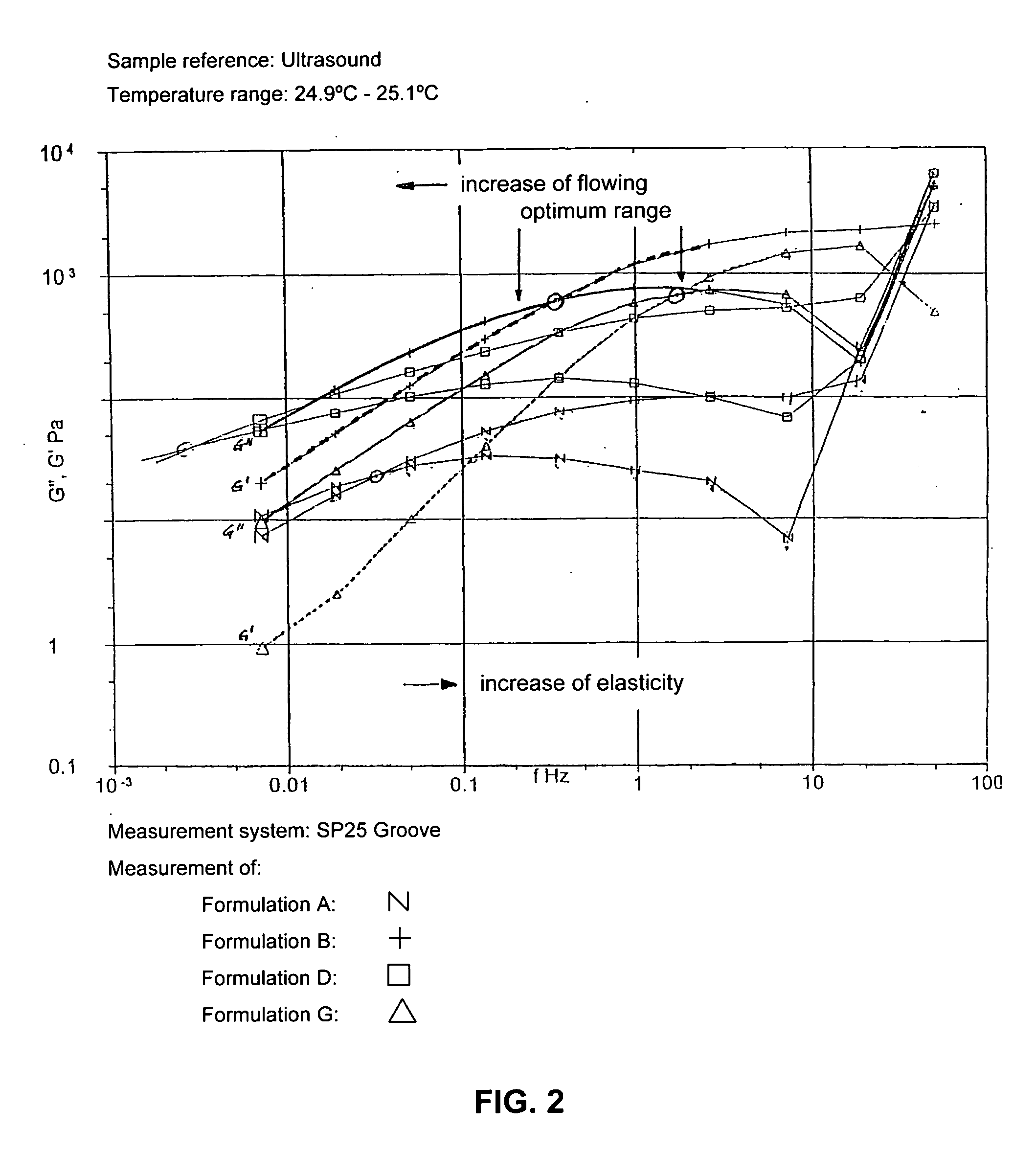
Ultrasound coupling medium for use in medical diagnostics
InactiveUS20070087060A1Avoid pollutionSuitable for usePowder deliveryEchographic/ultrasound-imaging preparationsPolyvinyl alcoholPreservative
A composition of an ultrasound coupling medium is provided. The composition comprises at least 90% water, at least one preservative, and at least one base substance, wherein the composition is extensible into a film with a thickness of up to 1 / 10 mm, wherein the composition can withstand a pressure of up to 30 kp without tearing, wherein the composition can adapt exactly to skin surface without causing any significant air pockets, and wherein the composition can be removed from skin with substantially no residue left behind. The at least one base substance may be a galactomannan, a polyvinyl alcohol (PVA), a complex formation of galactomannan and borate ions, or comninations thereof.
Owner:JOKER AG
Fluorescent probes for saccharrides
InactiveUS20040087842A1Decrease of fluorescence emissionReduce sensitivityAnalysis using chemical indicatorsChemiluminescene/bioluminescenceFluoProbesTelluric acid
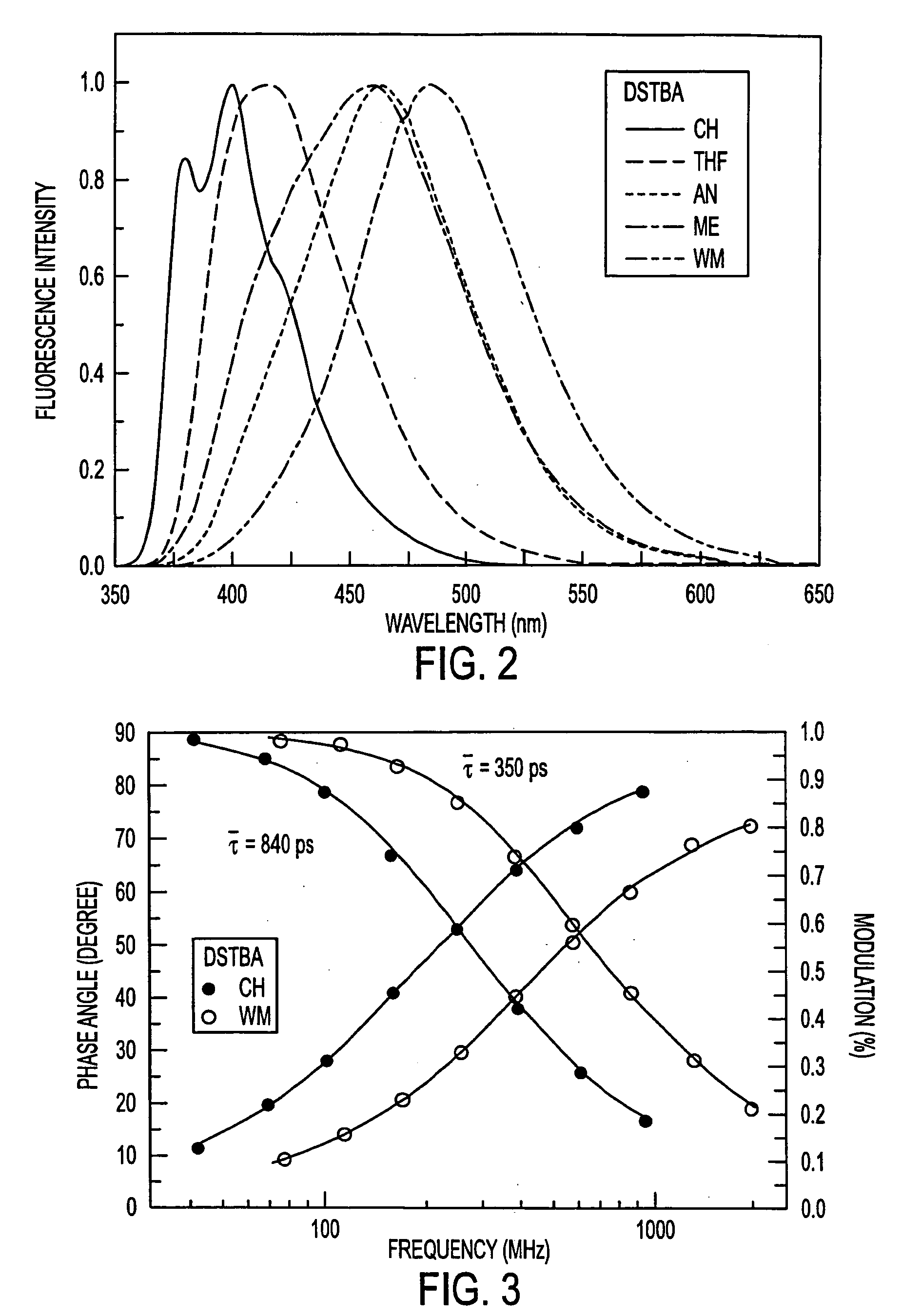
The spectroscopic and photophysical properties of fluorescent probes comprising donor-acceptor derivatives comprising the boric acid group or a derivative of boric acid, B(OH)3 (or borate ion, BO(OH)2<-1>), arsenious acid, H3 AsO3 (or arsenite ion, H2AsO3<-1>), telluric acid, H6TeO6 (or tellurate ion, H5 TeO6<-1>) or germanic acid, Ge(OH)6 (or germanate ion, GeO(OH)3<-1>) are described. Method of using said probes are also provided.
Owner:LAKOWICZ JOSEPH R +1
Magnesium ion-containing non-aqueous electrolyte and a production process thereof, as well as electrochemical device
ActiveUS20090068568A1Effective resourcesEfficient use ofCell electrodesOrganic electrolyte cellsAluminum IonPhosphate ion
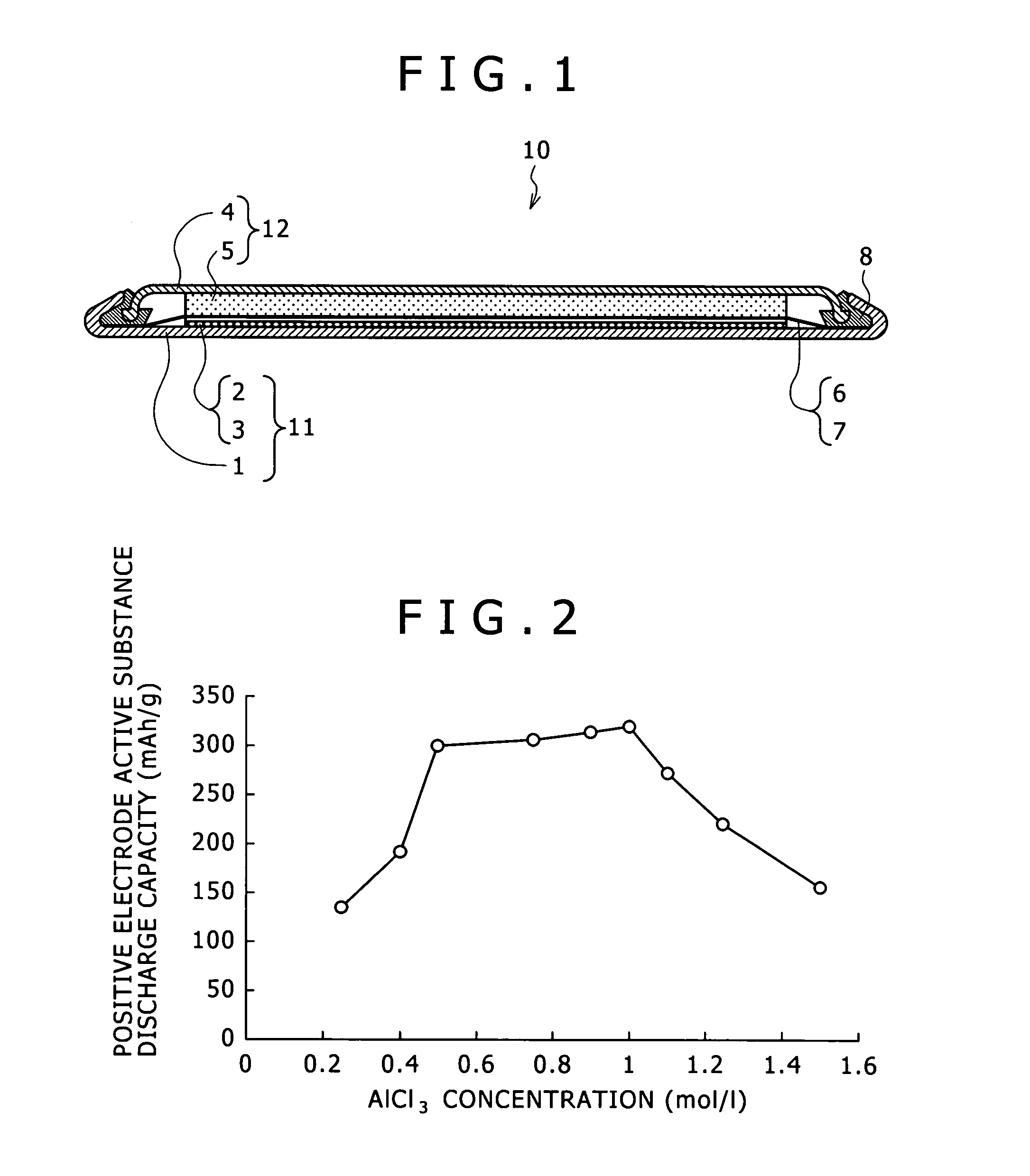
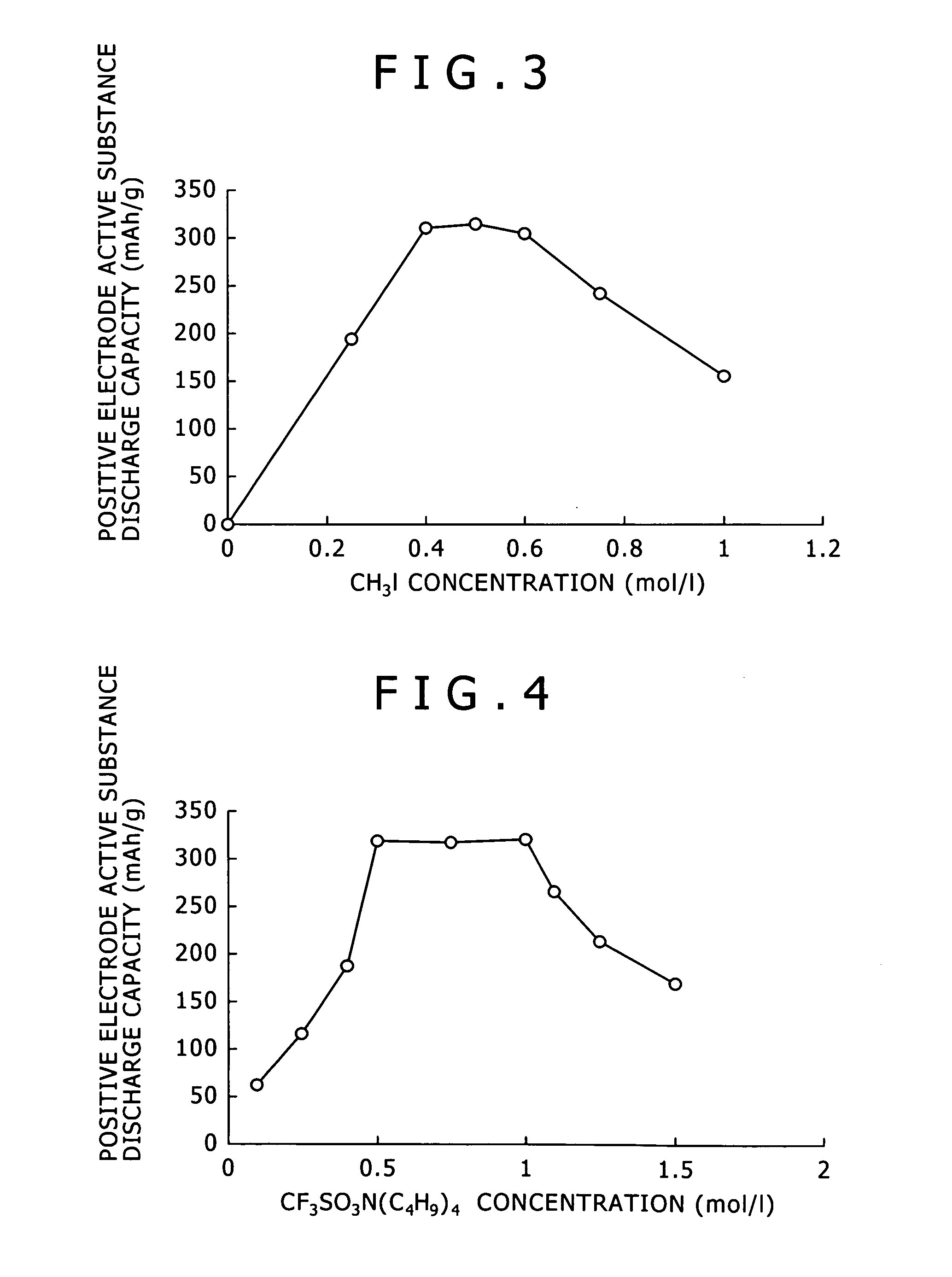
A magnesium ion containing non-aqueous electrolyte in which magnesium ions and aluminum ions are dissolved in an organic etheric solvent, and which is formed by: adding metal magnesium, a halogenated hydrocarbon RX, an aluminum halide AlY3, and a quaternary ammonium salt R1R2R3R4N+Z− to an organic etheric solvent; and applying a heating treatment while stirring them (in the general formula RX representing the halogenated hydrocarbon, R is an alkyl group or an aryl group, X is chlorine, bromine, or iodine, in the general formula AlY3 representing the aluminum halide, Y is chlorine, bromine, or iodine, in the general formula R1R2R3R4N+Z− representing the quaternary ammonium salt, R1, R2, R3, and R4 represent each an alkyl group or an aryl group, and Z− represents chloride ion, bromide ion, iodide ion, acetate ion, perchlorate ion, tetrafluoro borate ion, hexafluoro phosphate ion, hexafluoro arsenate ion, perfluoroalkyl sulfonate ion, or perfluoroalkyl sulfonylimide ion.
Owner:MURATA MFG CO LTD
Processes for separating metals from metal salts
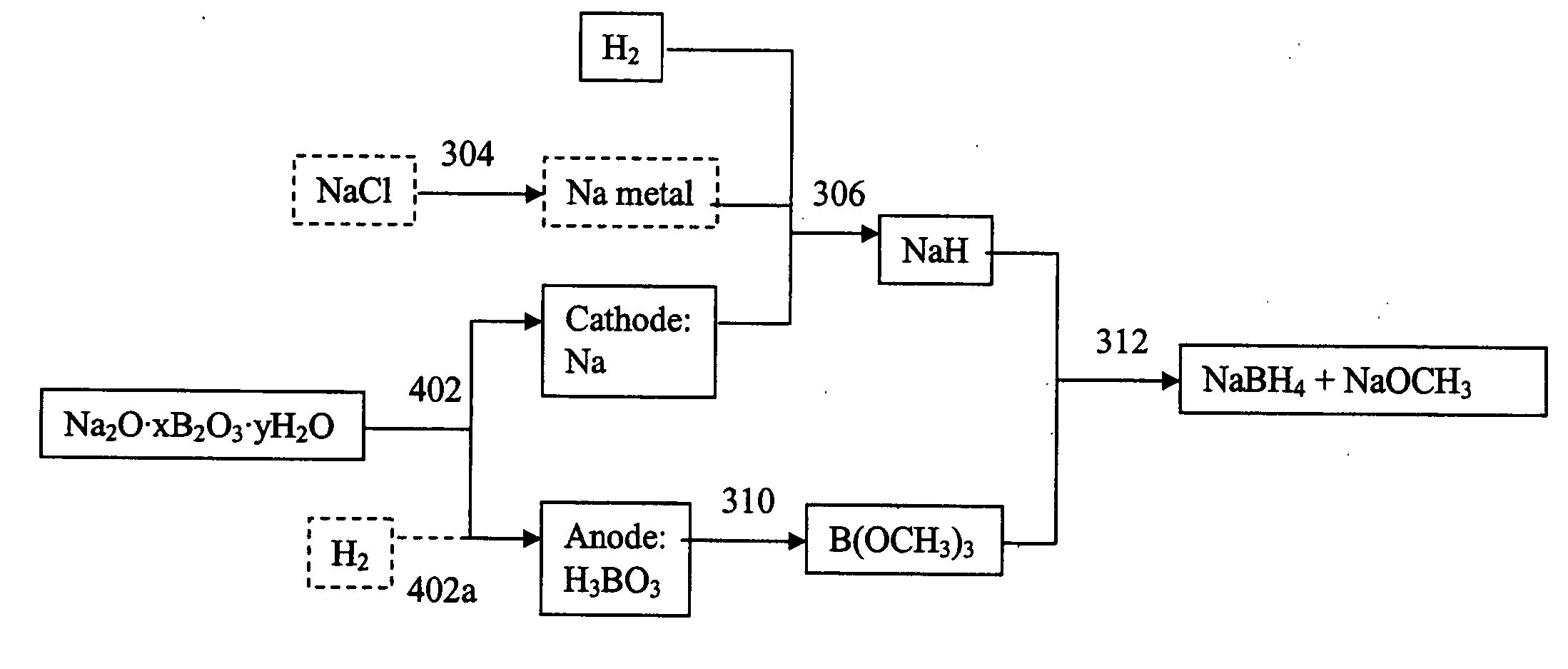
InactiveUS20060102491A1Electrolysis componentsGroup 3/13 element organic compoundsBorate ionSodium borohydride
Electrochemical processes and apparatus for obtaining metals from metal salts, particularly separating alkali metal and borate ions from alkali metal borate compounds, are disclosed. Aqueous solutions of metal borates or metal carbonates are converted to metals by preferred electrochemical processes. These electrochemical processes also may be integrated into processes for the production of borohydrides, such as sodium borohydride.
Owner:MILLENNIUM CELL
Detergent product
InactiveUS7179780B2Organic detergent compounding agentsSurface-active detergent compositionsPolyvinyl alcoholBorate ion
The present invention relates to an article comprising: (a) a liquid composition comprising: (i) enzyme; and (ii) from 0% to 10% (by weight of said liquid composition) free water, preferably 0% to 5% free water; and (iii) carboxylic acid comprising 5 carbon atoms or less, and 1 or 2 carboxy groups; and (iv) chelating agent; and (v) enzyme stabilizing metal ion system consisting of calcium ions and magnesium ions, present in a weight ratio of calcium ion to magnesium ion of from 1:1 to 4:1; and (vi) from 0% to 0.2% (by weight of said liquid composition) source of borate ions; and (b) a water-soluble polymeric material that is capable of being cross-linked by borate ions, preferably a water-soluble polymeric material comprising poly-vinyl alcohol.
Owner:THE PROCTER & GAMBLE COMPANY
Preparation method of low-surface-residual-alkali nickel cobalt manganese ternary positive electrode material
The invention relates to a preparation method of a low-surface-residual-alkali nickel cobalt manganese ternary positive electrode material and belongs to the field of chemical energy storage batteries. The preparation method includes that boric acid or citric acid is dissolved in alcohol, so that a certain amount of free-state hydrogen ions are contained in a solution; a nickel cobalt manganese ternary positive electrode material is added then, acid-alkali neutralization reaction between H+ and material surface residual alkali is utilized, and reaction intensity is regulated through stirring time, so that the residual alkali on the surface of the material can be reduced effectively, and pH value of the surface of the material is lowered; an alcohol solution is used for flushing then, so that the surface of the material is ensured to be free of residual borate ions or citrate ions; alcohol molecules probably left on the surface are removed through a two-time calcining method, so that uniformity and stability of the material are improved.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Preparation method of in-situ self-assembling N-doped super-hydrophilic carbon aerogel supercapacitor electrode material
InactiveCN105480962AHigh pseudocapacitanceImprove transmission performanceHybrid capacitor electrodesHybrid/EDL manufactureCapacitanceCross-link
The invention discloses a preparation method of an in-situ self-assembling N-doped super-hydrophilic carbon aerogel supercapacitor electrode material. The preparation method comprises the steps of introducing an N-containing conducting polymer and a borate radical framework in a gel three-dimensional net structure, and preparing a super-hydrophilic high-capacitance N-doped carbon aerogel supercapacitor electrode material which is of a porous structure after high-temperature activating, wherein N plays dual functions of increasing pseudocapacitance of the material and increasing the electronic transmission capacity, and borate radical ions are not only used as a cross-linking agent for maintaining a gel framework, but also have the effect of a self-assembling template so as to promote the generation of macroporous gaps. According to the preparation method of the in-situ self-assembling N-doped super-hydrophilic carbon aerogel supercapacitor electrode material, disclosed by the invention, excellent super-hydrophility can be shown without treatment of ultraviolet irradiation, or additional surface chemical modification and the like; solvent exchange needs not to be adopted, a freeze drying technology is used, the cost is low, the preparation method is simple and easy, the special three-dimensional net structure in gel is maintained, and environment friendliness is realized.
Owner:HENAN NORMAL UNIV
Preparation method of borate ion crosslinked conductive graphene paper
The invention discloses a preparation method of borate ion crosslinked conductive graphene paper. The preparation method comprises the following steps: preparing graphite oxide by adopting an improved Hummers method and ultrasonically stripping in deionized water to obtain graphite oxide hydrosol; then, adding a sodium hydroxide solution into the graphite oxide hydrosol to adjust the pH to 10-12; then adding boric acid; heating to 80-90 DEG C under a stirring condition; insulating heat for 3-5 hours; then naturally cooling; separating; washing; ultrasonically dispersing in deionized water; and finally filtering by a millipore filter and self-assembling layer by layer to obtain the borate ion crosslinked conductive graphene paper. The preparation method disclosed by the invention is simple, convenient to operate and low in cost, does not need special equipment and is easy for batch production. The obtained graphene paper has an excellent conductive performance and possibly can be used as an ideal conductive material for optoelectronic devices.
Owner:SOUTHEAST UNIV
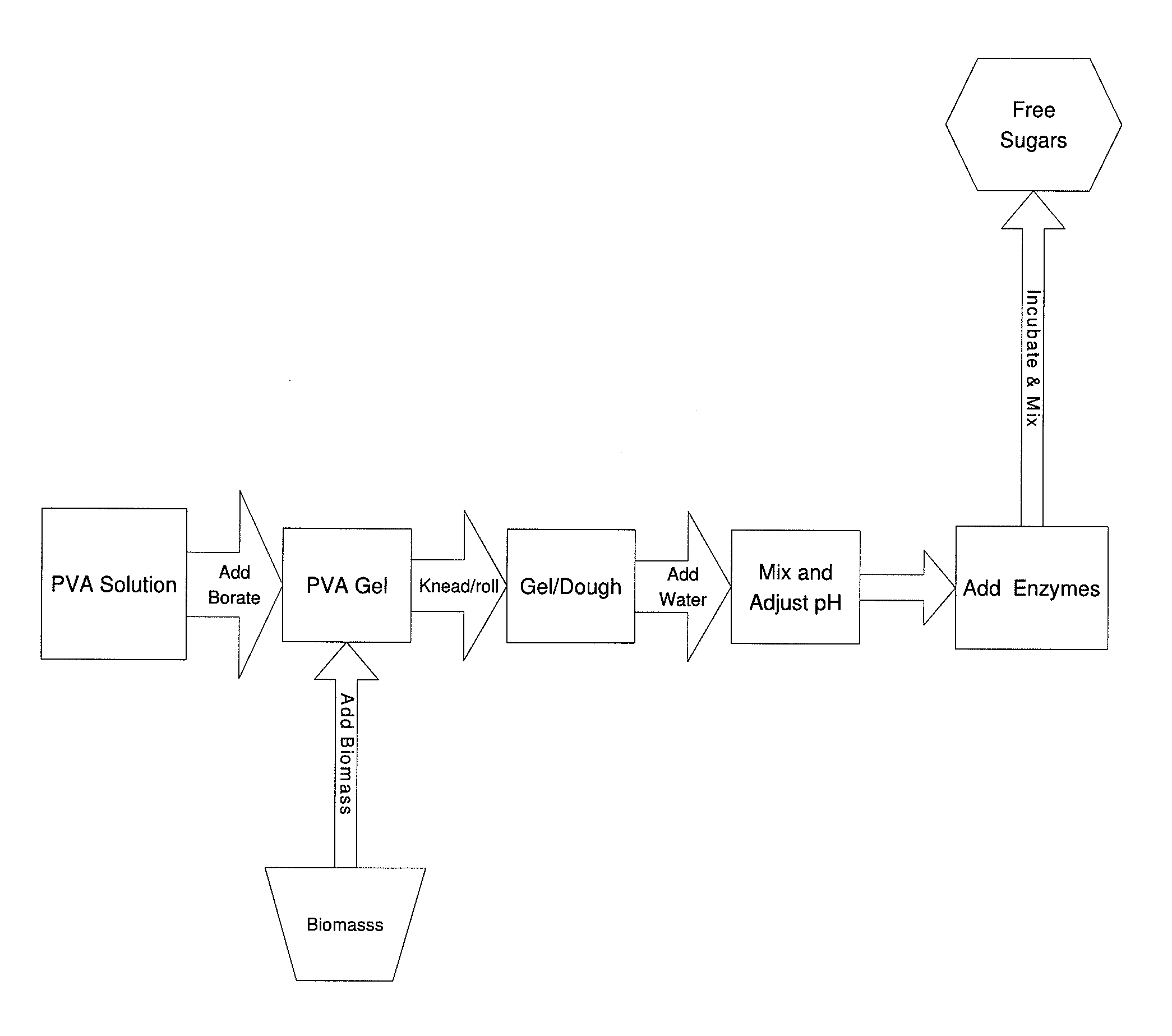
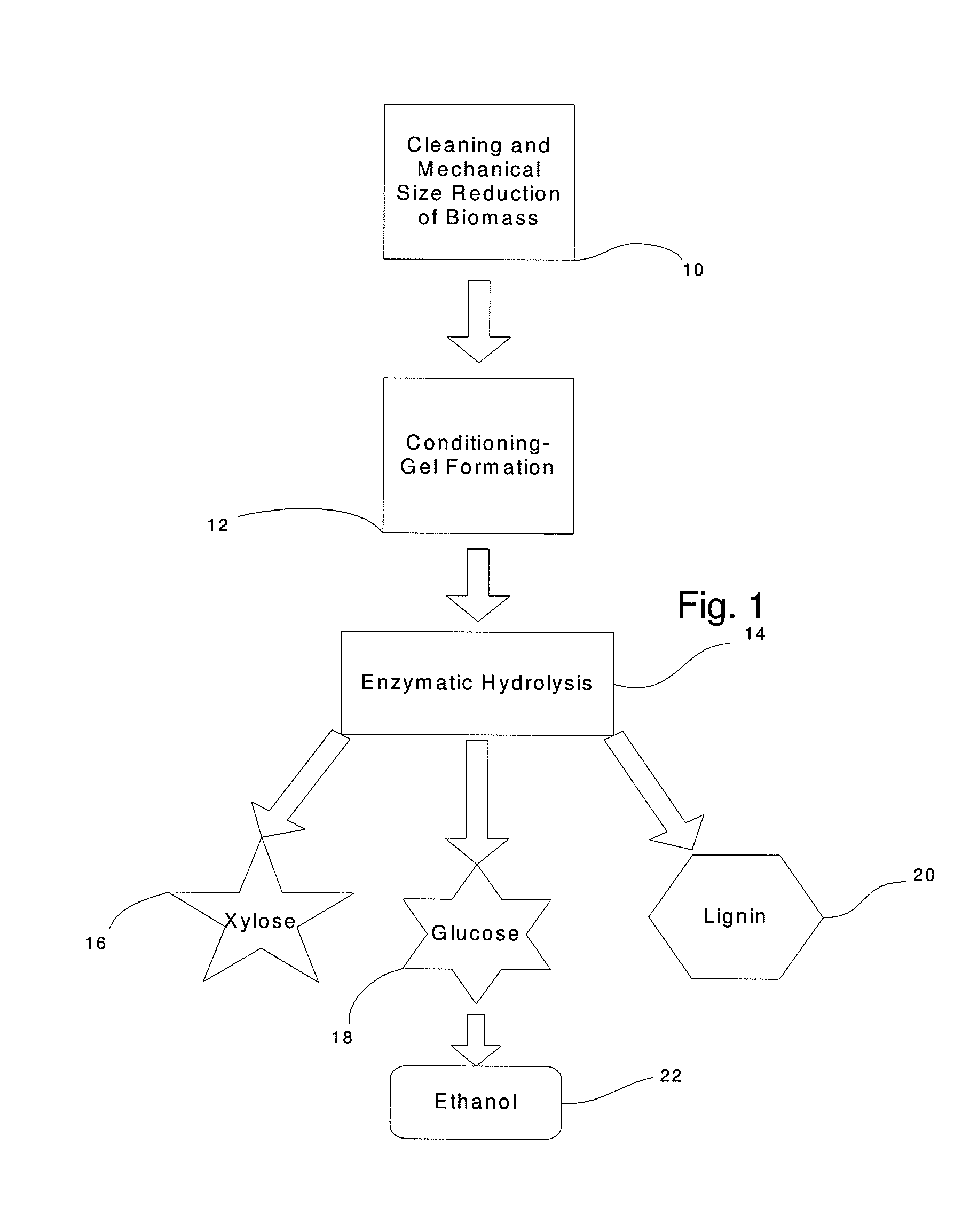
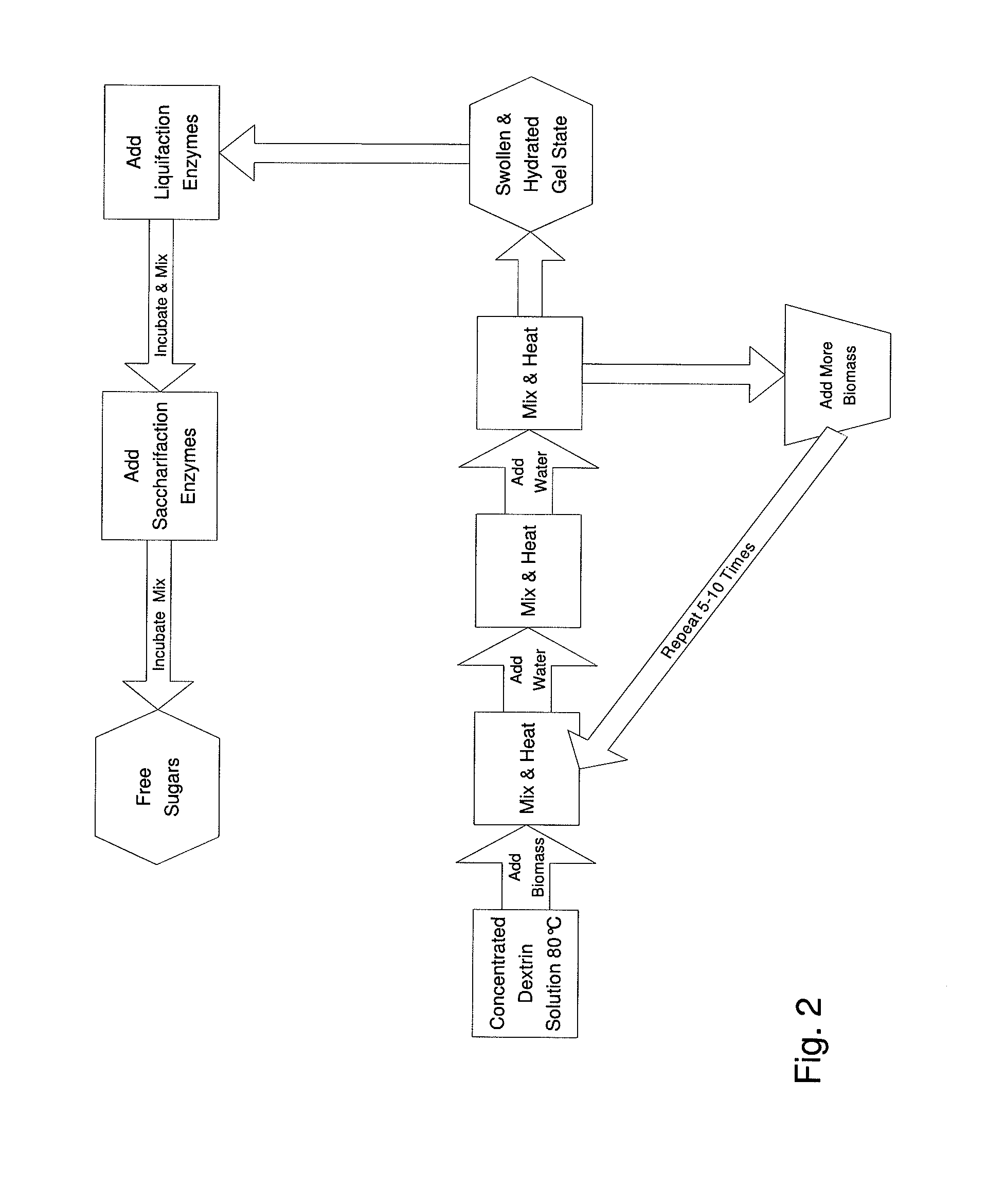
Simplified Method for Digestion of Cellulosic Biomass
InactiveUS20110020874A1Speed up the processLow viscosityCellulosic pulp after-treatmentPulping with organic compoundsHydrophilic polymersBorate ion
The inventive process converts cellulosic biomass into a gel-like state that is readily hydrolyzed by appropriate enzymes. First the biomass is mechanically reduced in size. The biomass is then mixed and kneaded with an aqueous solution of a hydrophilic polymer that acts as a conditioning agent or as a co-solvent. During mixing the cellulose (and hemicellulose) in the biomass swells and becomes hydrated forming a viscous gel-like material. The processed material can then be thinned through the addition of water whereupon hydrolytic enzymes are mixed into the material and rapid hydrolysis into free sugars takes place. Dextrins are effective hydrophilic polymers for conditioning biomass. Polyvinyl alcohol is a particularly effective conditioning agent for use with biomass when converted into a viscous gel by adding borate ions.
Owner:BIOMASS CONVERSIONS LLC
Fluorescent probes for saccharrides
The spectroscopic and photophysical properties of fluorescent probes comprising donor-acceptor derivatives comprising the boric acid group or a derivative of boric acid, B(OH)3 (or borate ion, BO(OH)2−1), arsenious acid, H3 AsO3 (or arsenite ion, H2AsO3−1), telluric acid, H6TeO6 (or tellurate ion, H5 TeO6−1) or germanic acid, Ge(OH)6 (or germanate ion, GeO(OH)3−1) are described. Method of using said probes are also provided.
Owner:LAKOWICZ JOSEPH R +1
Clean method for preparing borate intercalation hydrotalcite-like compound
The invention discloses a clean method for preparing a borate intercalation hydrotalcite-like compound. By using the adjustability of interlaminar anions of the hydrotalcite-like compound and based on atom economic reaction, the borate intercalation hydrotalcite-like compound with the interlaminar anions B(OH)4<-> and / or B3O3(OH)4<-> is generated by inserting borate ions into interlamination of the hydrotalcite-like compound. All atoms in raw materials enter in the reaction to generate the target product in the preparation process without byproducts; and a purified product can be obtained by directly filtering and drying the product without washing, so washing water is greatly saved and the environment is protected.
Owner:北京泰克来尔科技有限公司
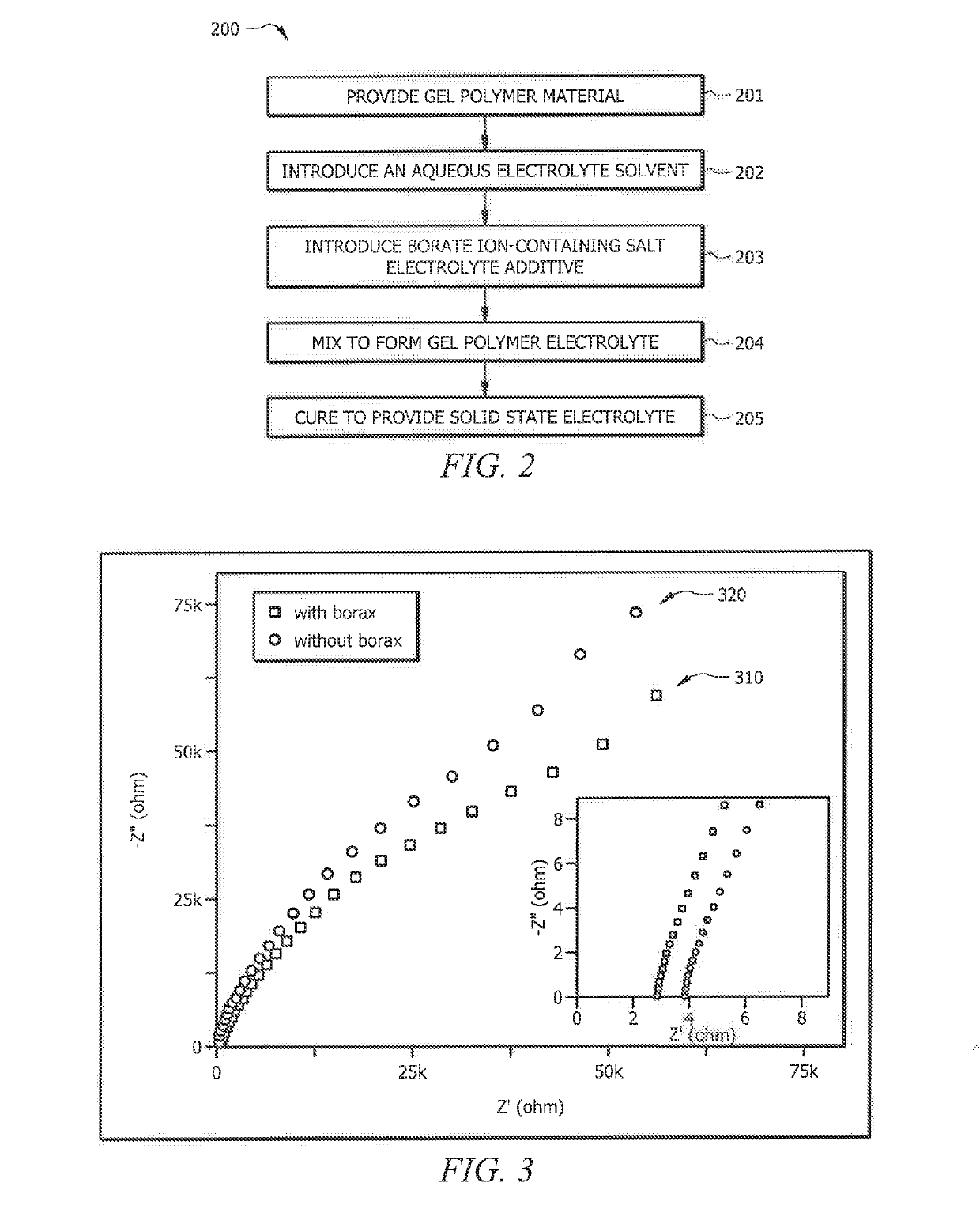
Gel polymer electrolytes comprising electrolyte additive
ActiveUS20190140317A1Improve performanceImprove ionic conductivityZinc oxides/hydroxidesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesPolymer electrolytesPolymer science
Systems and methods which provide an aqueous gel polymer electrolyte having one or more additive therein selected to configure the aqueous gel polymer electrolyte, and batteries formed therewith, for improved performance are described. Aqueous gel polymer electrolytes may, for example, have an additive compound including boron (e.g., a borate ion-containing salt) therein to configure batteries formed using the aqueous gel polymer electrolyte to increase the ionic conductivity of the gel polymer electrolyte. The addition of borax in Zinc-ion battery gel electrolytes of embodiments is configured to enhance the dissociation of zinc ions and anions, and subsequently release more mobile zinc ions. Furthermore, the interaction between borax and divalent transition metal (Zn) in electrolyte according to embodiments may enhance the transportation of mobile zinc ions.
Owner:CITY UNIVERSITY OF HONG KONG
Developing solution and method for production of finely patterned material
InactiveCN101641647AShorten development timePhotosensitive material processingOptical record carrier manufacturePhosphate ionBorate ion
Disclosed is a developing solution comprising an aqueous alkali solution, at least one anion selected from a silicate ion, a carbonate ion, a borate ion and a phosphate ion, and at least one cation selected from an ammonium ion, an organic ammonium ion and an alkali metal ion. The aqueous alkali solution may be an aqueous solution of a tetra-alkyl ammonium hydroxide.
Owner:SONY CORP
Preparation method of lithium phosphate catalysts
A new method for preparing lithium phosphate catalysts is disclosed. The method comprises precipitating a lithium phosphate from a mixture comprising a first aqueous solution which contains lithium and sodium ions and a second aqueous solution which contains phosphate and borate ions. The resultant lithium phosphate catalyst has increased activity and selectivity in the isomerization of an alkylene oxide to the corresponding allylic alcohol.
Owner:LYONDELL CHEM TECH LP
Regenerated solution of alkaline copper etching solution and method for increasing etching speed thereof
The invention provides a regenerated solution of alkaline copper etching solution and a method for increasing the etching speed thereof. The regenerated solution of alkaline copper etching solution contains a first accelerating agent and a second accelerating agent, wherein the first accelerating agent contains an organic thio-acid compound with a NH2-CS-NH-group, and the second accelerating agent is selected from at least one of a chlorition compound and a perboric acid ion compound. According to the method for increasing the etching speed of the regenerated solution of alkaline copper etching solution, an effective amount of second accelerating agent is added to the regenerated solution of alkaline copper etching solution, wherein the second accelerating agent is selected from at least one of the chlorition compound and the perboric acid ion compound. The regenerated solution of alkaline copper etching solution provided by the invention can effectively improve the etching speed of the alkaline etching solution, and can keep the etching speed stable, thereby prolonging the service life of the etching regenerated solution.
Owner:SHENZHEN JECH TECH
Tetrafluoro-borate ion crosslinking hydroxyl polymer binding agent, preparation method thereof, secondary battery and negative electrode and negative paste of secondary battery
InactiveCN108417838AImprove mechanical propertiesHigh elongation at breakElectrode collector coatingNon-aqueous electrolyte accumulator electrodesBorideBorate ion
The invention provides a tetrafluoro-borate ion crosslinking hydroxyl polymer binding agent and a preparation method thereof. A polymer with a crosslinking network structure is formed by condensationreaction of tetrafluoro-borate ions formed by hydrolysis of a boride and hydroxyl on a hydroxyl polymer. The binding agent is high in tensile strength and high in elastic modulus and can bear stress generated by volume expansion and shrinkage of a negative active material of a secondary battery, and the volume change is effectively reduced; the active material and conductive additive particles canbe effectively coated by a three-dimensional network structure of the binding agent, favorable contact of the active material and the conductive additive is ensured, and the conductivity of an electrode plate is further ensured; and a hydroxyl group on the binding agent and the active material such as silicon can form a chemical bond, so that the bonding strength of the binding agent and the active material is improved. The invention also provides secondary battery negative paste, a secondary battery negative electrode and the secondary battery which are prepared by employing the tetrafluoro-borate ion crosslinking hydroxyl polymer binding agent.
Owner:XI AN JIAOTONG UNIV
Graphene hybrid containing boron, nitrogen, cobalt, zinc and silicon and preparation method of graphene hybrid
The invention belongs to the technical field of flame retardance and smoke suppression, and particularly relates to a graphene hybrid containing boron, nitrogen, cobalt, zinc and silicon, and the graphene hybrid is prepared by carrying out hybridization on KH550, graphene oxide, Co / Zn-ZIF and sodium tetraborate decahydrate. The invention also relates to a preparation method of the graphene hybrid.Through the preparation method, KH550 is introduced to the surface of graphene through an amidation reaction of the KH550 and oxygen-containing functional groups on the surface of the graphene oxide;Co / Zn-ZIF grows on the surface of graphene through electrostatic interaction; and borate ions are combined on the surface of Co / Zn-ZIF through covalent bond acting force, so that the graphene hybridcontaining boron, nitrogen, cobalt, zinc and silicon is prepared. Through addition of the hybrid, flame retardancy, smoke suppression and mechanical properties of a composite material can be significantly improved.
Owner:ANHUI UNIVERSITY OF ARCHITECTURE
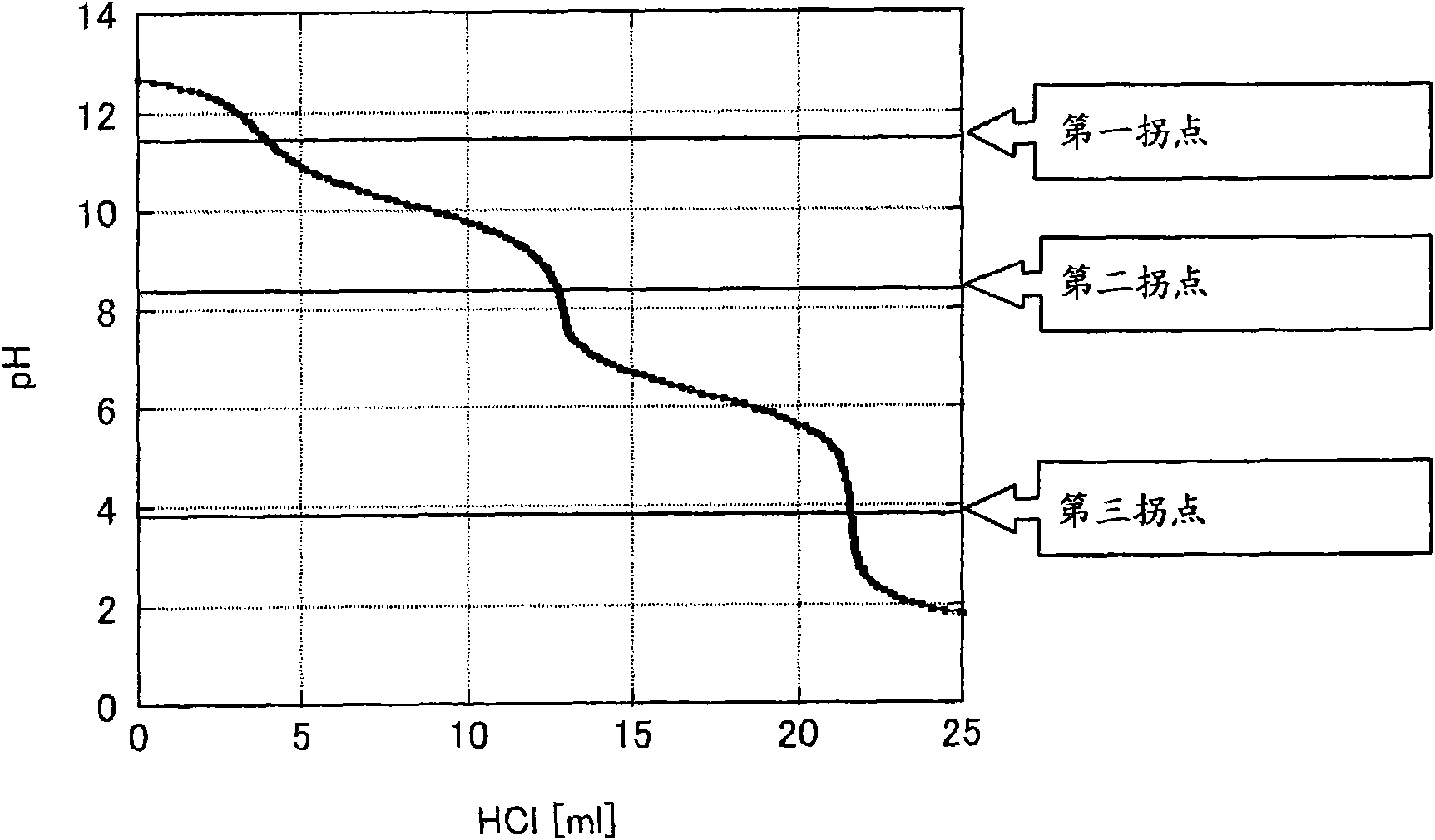
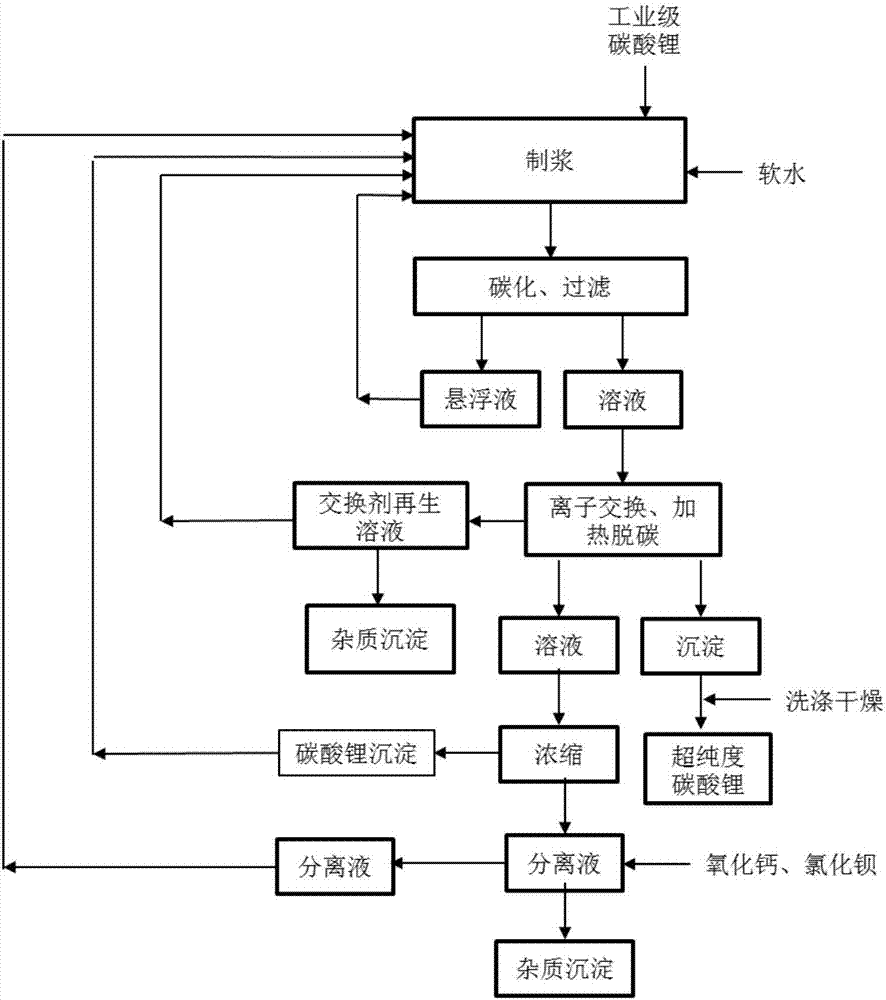
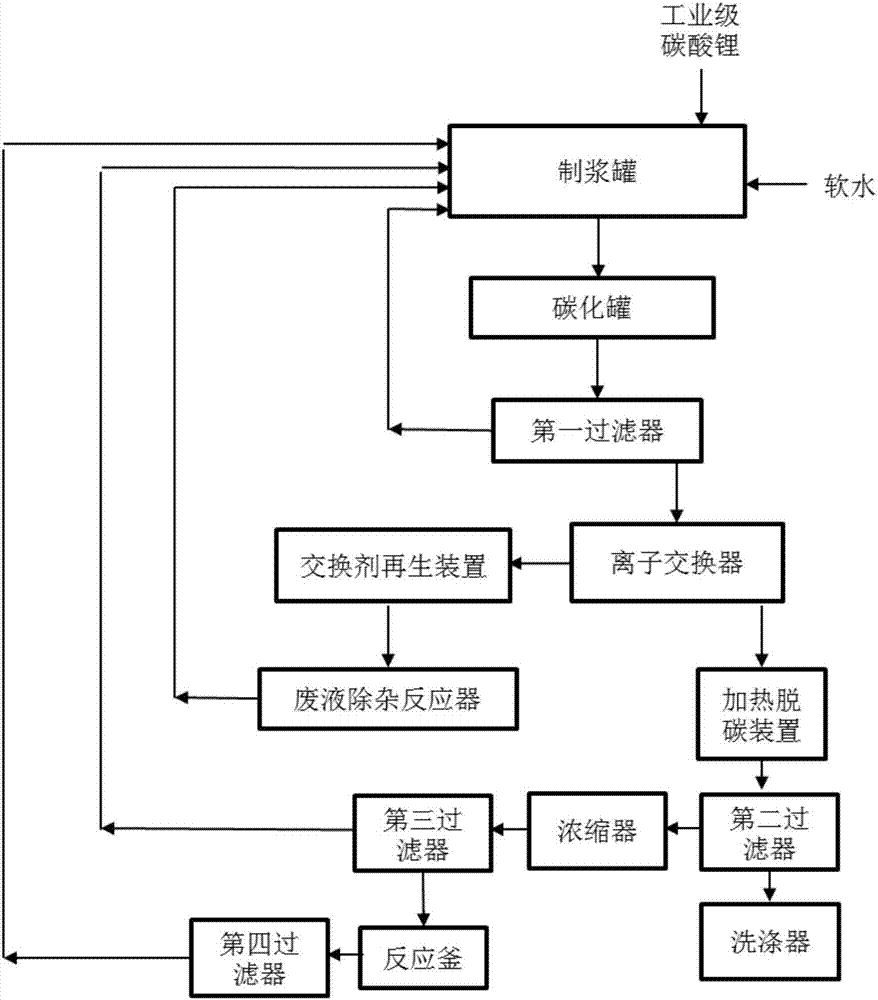
Method and device for extracting ultrapure lithium carbonate from industrial-grade lithium carbonate
The invention relates to a method for extracting ultrapure lithium carbonate from industrial-grade lithium carbonate. The method comprises the following steps: A, preparing a lithium hydrogen carbonate solution; B, performing ion exchange impurity removal; C, performing impurity removal on an ion exchange resin regeneration solution; D, performing lithium hydrogen carbonate heating decarbonization: heating the lithium hydrogen carbonate solution subjected to ion exchange impurity removal, so as to convert lithium hydrogen carbonate into lithium carbonate precipitates, and filtering the solution to obtain the lithium carbonate precipitates and a separate solution; E, concentrating the separate solution in Step D to obtain the lithium carbonate precipitates and a separate solution; F, adding an impurity removal reagent into the separate solution in Step E to remove sulfate radical and borate ions, filtering to obtain precipitates and a separate solution, and returning the separate solution as a pulping raw material. The method comprises few34 procedures, and is pollution-free, and a lithium utilization rate is high.
Owner:深圳市聚能永拓科技开发有限公司
Method for preparing boron-doped carbon nanospheres from lignin, and product
The invention belongs to the field of lignin utilization, and discloses a method for preparing boron-doped carbon nanospheres from lignin, and a product. The method comprises the following steps: S1,dissolving lignin and a boron-based additive in water according to a preset mass ratio to prepare a mixed solution; S2, carrying out ultrasonic treatment on the mixed solution to hydrolyze the boron additive to generate tetrahydroxy borate ions, and carrying out a cross-linking reaction on the tetrahydroxy borate ions and hydroxyl functional groups to obtain a lignin solution; S3, drying and grinding the lignin solution to obtain lignin powder; and S4, pyrolyzing and cooling the lignin powder to obtain the boron-doped carbon nanospheres. The boron additive reacts with lignin macromolecular functional groups to prevent the lignin macromolecules from being agglomerated and caked in a pyrolytic reaction, meanwhile, the additive has the functions of a plasticizer and a doping agent, obtained lignin coke is in a nanoscale spherical shape microcosmically, boron elements are uniformly embedded into a coke skeleton, and the lignin coke is a boron-doped carbon material with high additional value and good adsorption and catalysis carriers and energy storage materials.
Owner:HUAZHONG UNIV OF SCI & TECH
Method for detecting perboric acid ion
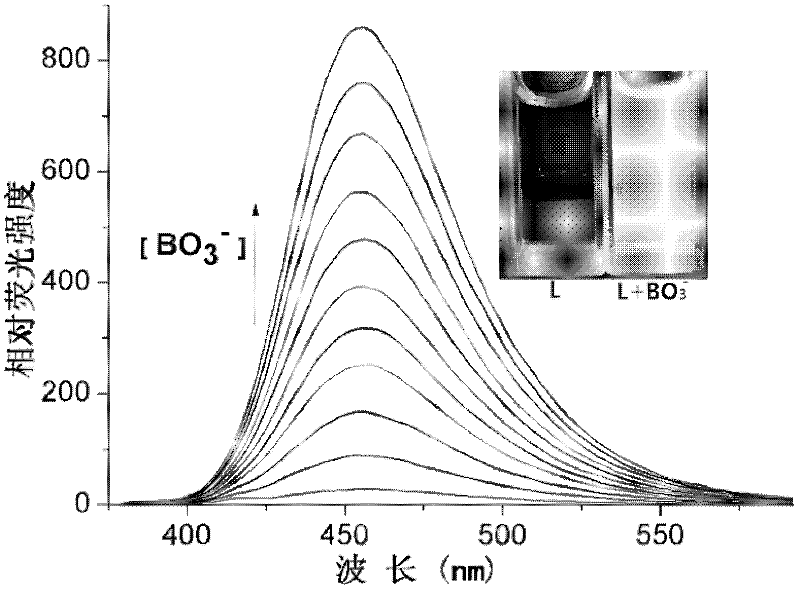
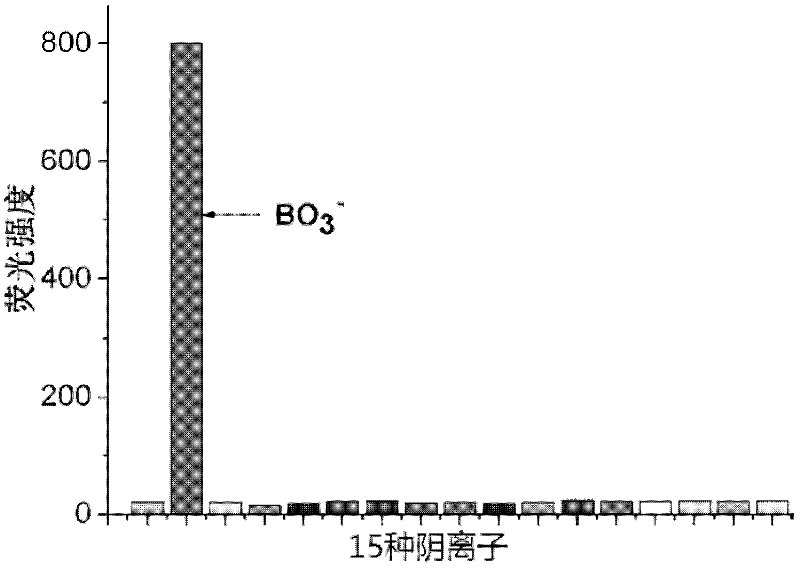
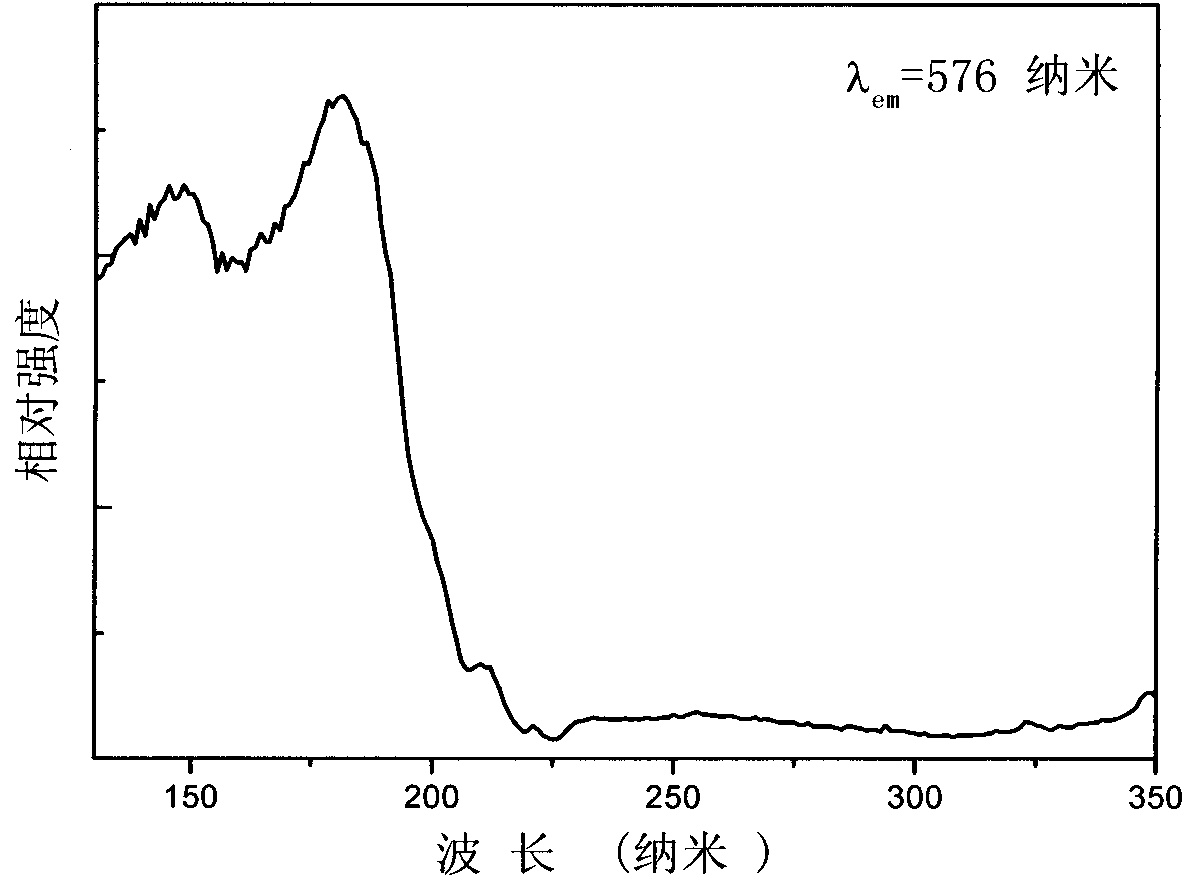
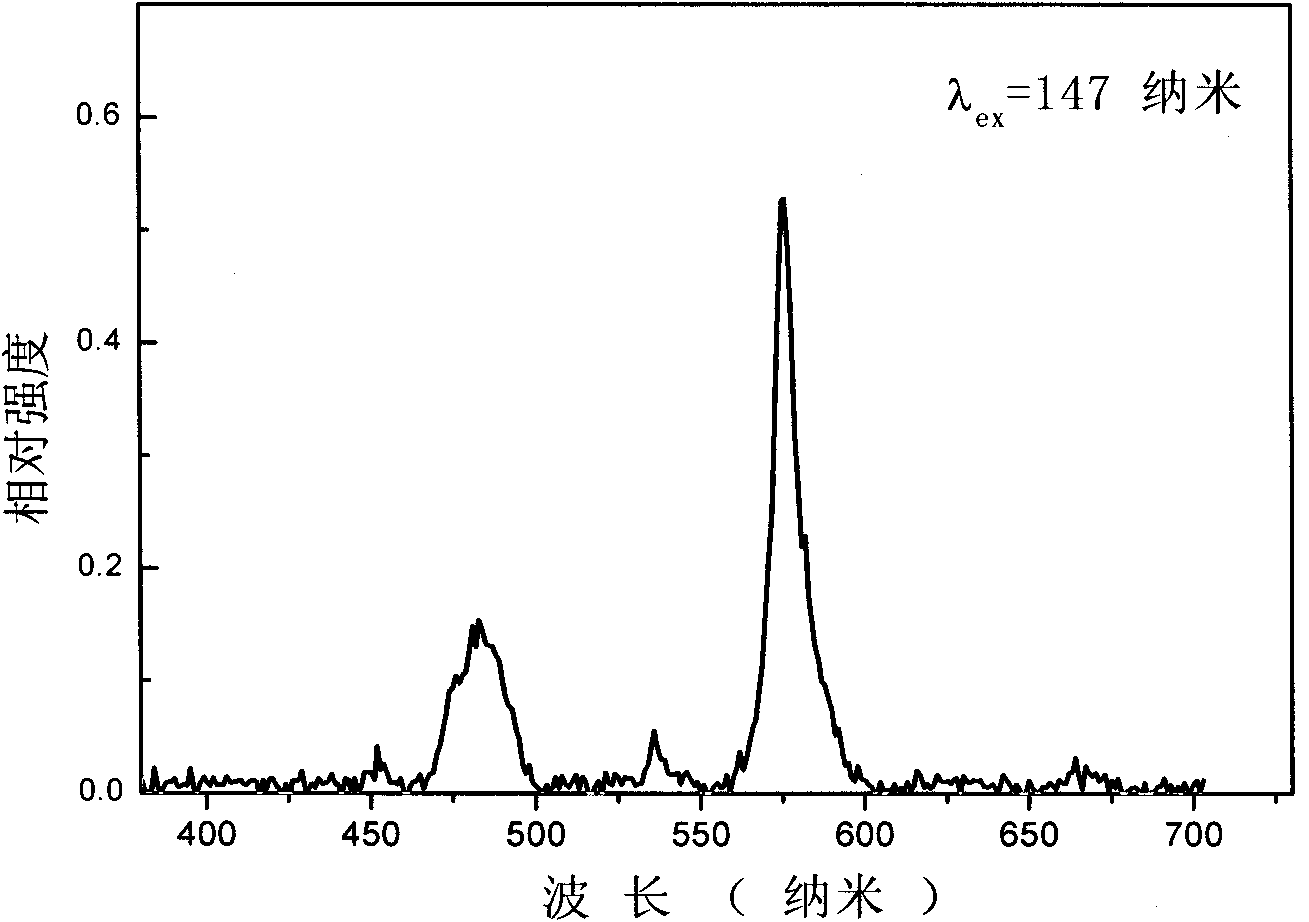
InactiveCN102590162ALow costUndisturbedFluorescence/phosphorescenceFluorescence spectrometryBorate ion
The invention provides a method for detecting perboric acid ion BO<3->, and in particular relates to a method for quantitatively detecting the BO<3-> based on a coumarin derivative acetoxycoumarin (AC). Particularly, the content of the BO<3-> is quantitatively detected by the AC in aid of fluorescence spectrum in a 10mM 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer solution with pH of 5.0. The method has the characteristics of high sensitivity and selectivity to the BO<3->, convenient, sensitive and quick detection process and accurate detection result.
Owner:SHANXI UNIV
Borofluoride white light emitting material and preparation method thereof
InactiveCN102140347AEasy to prepareIncrease productionGas discharge lamp usageLuminescent compositionsAlkaline earth metalChemical composition
The invention relates to a borofluoride white light emitting material. The chemical composition formula of the borofluoride white light emitting material is M5-2x(BO3)5F:Dyx, Rx; and M is alkaline earth metal ions, Dy is trivalent activated ions, R is alkali metal ions, the molar percentage content of the Dy relative to the alkali metal ion R is x, and the x is more than or equal to 0.01 and less than or equal to 0.2. Moreover, the invention also relates to a method for preparing the material. The method comprises the following steps of: 1, selecting a source compound of the alkaline earth metal ions, a source compound of borate radical ions, a source compound of the alkali metal ions, a source compound of dysprosium ions and a source compound of fluorine according to a stoichiometric ratio, wherein the stoichiometric ratio of the source compounds is a molar ratio of corresponding elements in the M5-2x(BO3)5F:Dyx, Rx, the x is the molar percentage content of the dysprosium ions relative to the alkali metal ion R, and the x is more than or equal to 0.01 and less than or equal to 0.2; 2, grinding and mixing the source compounds; and 3, sintering the mixture in a high-temperature furnace, and then cooling and grinding to obtain the borofluoride white light emitting material.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +1
Method for oxidatively removing hydrogen sulfide
InactiveCN108889100AReduce usageComplex structureGas treatmentDispersed particle separationCarbanionPhosphate ion
The invention relates to a method for oxidatively removing hydrogen sulfide. The method adopts peroxide, or peroxide and oxygen, or mixture of peroxide and air as an oxidant and a compound with an activating effect, namely the compound which can generate carbanions, bicarbonate ions, sulfate ions, borate ions, hydroborate ions, phosphate ions, monohydrogenphosphate ions and dihydrogen phosphate ions in an aqueous solution, as an activator. The method comprises the following steps: mixing the activator and the oxidant, standing at negative 15-85 DEG C for 1-45 minutes, mixing with a hydrogen sulfide solution, stirring to react, or introducing the hydrogen sulfide gas into a hydrogen sulfide absorption liquid to react. Under a weak base condition, the hydrogen sulfide removal rate by the method can be 96-99 percent or above, the corrosion degree on equipment is relatively small, and the reacted product is safe and pollution-free.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for converting carbonic acid type salt lake brine into chloride type brine
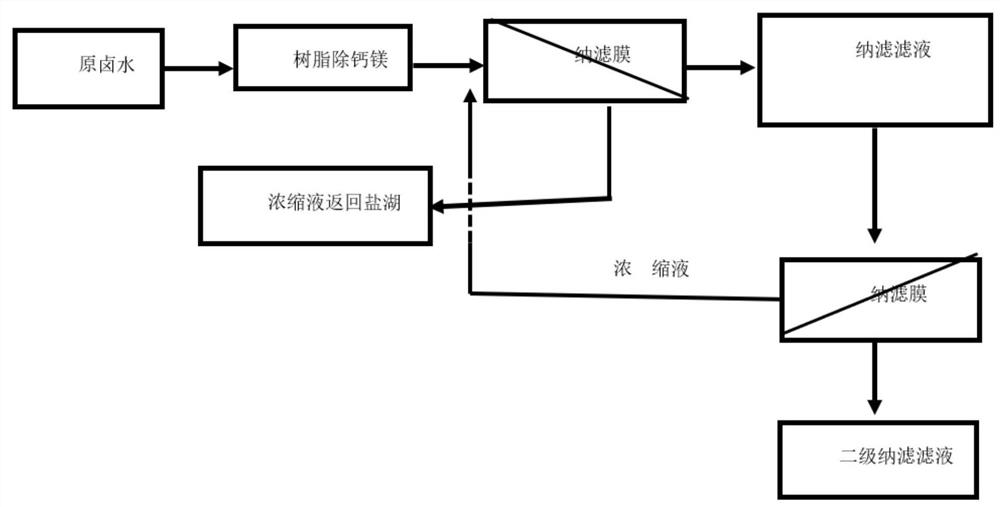
PendingCN111606334AImprove radiation efficiencyHigh yieldLithium halidesAlkali metal chloridesSulfate radicalsIon exchange
The invention discloses a method for converting carbonic acid type salt lake brine into chloride type brine. The method comprises the following steps that: enabling carbonic acid type salt lake brineto pass through ion exchange resin to remove trace calcium and magnesium ions in the brine and obtain softened brine; pumping softened brine into a first-stage nanofiltration membrane system, applyingpressure to the two sides of a first-stage nanofiltration membrane, to form a pressure difference; enabling parts of water, sodium ions, potassium ions, lithium ions and chloride ions in the softenedbrine to migrate to a low-pressure side from a high-pressure side through the first-stage nanofiltration membrane, enriching the high-valence anions on the high-pressure side to obtain brine rich inthe high-valence anions, pumping the brine rich in the high-valence anions back to the salt lake, wherein the high-valence anions comprise sulfate ions, carbonate ions and polyborate ions; and obtaining chloride type brine containing a small amount of high-valence anions on the low-pressure side.
Owner:BEIJING QINGYUAN POWERISE TECH CO LTD +1
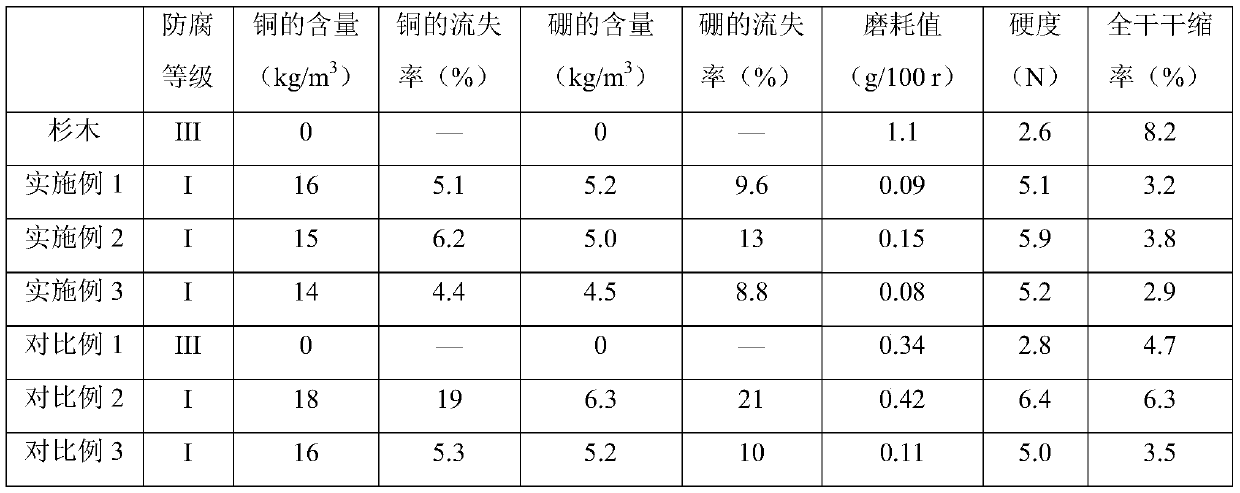
Chinese fir anticorrosion modification agent and preparing method and constructing process thereof
ActiveCN110116444AGood dispersionGive full play to mechanical propertiesWood treatment detailsPressure impregnationSocial benefitsBorate ion
The invention discloses a Chinese fir anticorrosion modification agent and a preparing method and constructing process thereof, and belongs to the technical field of wood anticorrosion treatment. Theanticorrosion modification agent is prepared by adding copper-ion-loaded and borate-ion-loaded graphene oxide, waterborne polyurethane, a dispersing agent and a defoaming agent to water for mechanicalstirring and ultrasonic processing. The constructing process includes the steps of conducting impregnation treatment on Chinese fir through the prepared anticorrosion modification agent and conducting thermal treatment on the Chinese fir through a superheated steam drying box. By means of the prepared anticorrosion modification agent and the constructing process, the anticorrosion property and anticorrosion stability of the Chinese fir can be remarkably improved, the hardness, wear resistance and size stability of the Chinese fir can be greatly improved, and the Chinese fir anticorrosion modification agent has the remarkable economic value and social benefits.
Owner:福建省顺昌县升升木业有限公司
Preparation method of hollow BiVO4 micron sheet photocatalyst
InactiveCN107486213ARealize large-scale preparationAchieve co-modificationWater/sewage treatment by irradiationWater treatment compoundsSynthesis methodsBorate ion
The invention discloses a preparation method of a hollow BiVO4 micron sheet photocatalyst. The preparation method comprises the steps of synthesizing a hollow BiVO4 micron sheet and preparing a boric acid ion doped and cobaltous oxide nano particle co-modified hollow BiVO4 micron sheet. The method has the benefits that a required raw material source is rich; a synthesis method is simple; large scale preparation can be achieved; the repeatability is good; the material stability is high; the material prepared by the method can be used for photocatalytic degradation of organic pollutants and photocatalytic decomposition of water produced oxygen, and has very good practical values and application prospects.
Owner:WENZHOU UNIVERSITY
Fire retardant compositions and methods of use
InactiveUS20080099736A1Improve the level ofLevel of easeFireproof paintsOther chemical processesBorate ionMildew
The present invention provides fire retardant compositions that have the further advantage of conferring resistance to insects, mould, mildew and fungus species. The composition includes a metal ion, a borate ion, and a borate ion-complexing species. Also provide are methods for producing a fire retardant composition as described in the specification.
Owner:CLARKE COLIN EDWARD +1
Method for separating boron and radionuclides in radioactive wastewater by using long flow passage
InactiveCN105253966AHigh in boronLow radioactive contentDispersed particle separationRadioactive decontaminationBorate ionIon-exchange resin
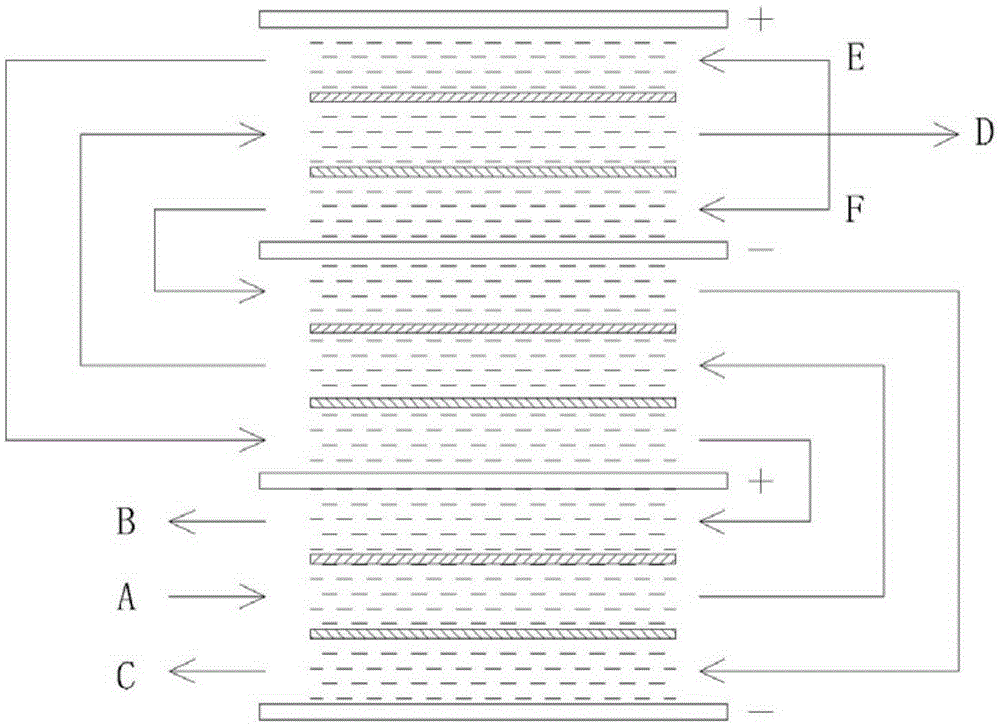
The invention discloses a method for separating boron and radionuclides in radioactive wastewater by using a long flow passage. The method comprises the following steps: (1) setting a membrane stack, alternately arranging a plurality of anodes and cathodes in the membrane stack, and setting a cation exchange membrane and an anion exchange membrane between the anodes and the cathodes to form cathode chambers, anode chambers and fresh water chambers, wherein the adjacent anodes and cathodes form an electric field; (2) filling ion exchange resin into the anode chambers, the cathode chambers and the fresh water chambers; and (3) connecting the cathode chambers, the anode chambers and the fresh water chambers in series respectively to form three flow passages, and introducing the radioactive wastewater into the three flow passages, wherein borate ions in the fresh water chambers are migrated into the anode chambers under the action of the electric field and nuclide ions in the fresh water chambers are migrated into the cathode chambers, thereby separating boron and radionuclides in the radioactive wastewater in the fresh water chambers; and during separation, the ratio of the mean concentrations of boron in the anode chambers and the fresh water chambers in the same electric field is controlled to be not more than 35.
Owner:SHANGHAI NUCLEAR ENG RES & DESIGN INST CO LTD +1
Nutrient salt balancing method for low-salinity circulating water used for penaeus vannamei culture
InactiveCN110839574AGood experimental foundationGood scientific basisClimate change adaptationPisciculture and aquariaSulfate radicalsBorate ion
The invention discloses a nutrient salt balancing method for low-salinity circulating water used for penaeus vannamei culture. The nutrient salt balancing method is realized by adjusting the concentration of main ions. The main ion concentrations of the water body are: 0.2-0.4g / L of calcium ion, 0.6-1.5g / L of magnesium ion, 0.07-0.15g / L of potassium ion, 3-5g / L of sodium ion, 2-5g / L of chloride ion, 0.5-1g / L of sulfate ion, 0.02-0.04g / L of carbonate ion, 0.2-0.5g / L of bicarbonate ion, 0.02-0.04g / L of bromide ion and 0.05-0.1g / L of borate ion; and the ratio of the calcium ion to magnesium ion is 1: 3-5. The nutrient salt balancing method of the low-salinity circulating water is used for penaeus vannamei culture, the yield of the penaeus vannamei reaches 6-7kg / m3, the survival rate of the penaeus vannamei is high, the growth is fast, and the culture range of the penaeus vannamei is effectively widened, so that the method plays a greater role in promoting the development of the culture ofthe penaeus vannamei.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY +1
Method for producing paper
ActiveCN103069074APrevent proliferationReduce paper qualityNon-fibrous pulp additionPaper/cardboardCardboardPhosphate ion
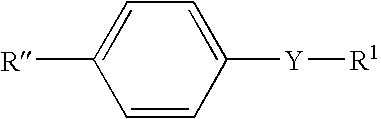
A method for producing paper that reduces the load on drainage and that obtains cardboard and paper having a fixed quality is provided. A slime control agent and a polymer having a cationic functional group are added to papermaking-step water containing starch. At that time, as the polymer containing a cationic functional group, a compound is used that is a polymer of solely a monomer represented by chemical formula (A) or a copolymer of said monomer and acrylamide or styrene, contains 20-100 mol% of a monomer unit derived from the monomer represented by chemical formula (A), is at least one of a diallyl dimethyl ammonium halide polymer, a polyethylenimine, or an epichlorohydrin polymer, and has an intrinsic viscosity (?) at 25 DEG C in a 1 N sodium chloride solution of 0.05-5 dl / g. (In the formula: R1 represents a hydrogen atom or a methyl group; R2 and R3 each represent a C1-4 alkyl group; R4 represents a hydrogen atom, a C1-4 alkyl group, or a benzyl group; Y represents O or NH; n represents 2-5; and Z- represents a halogen ion, a sulfate ion, a phosphate ion, a borate ion or an organic acid anion.).
Owner:KURITA WATER INDUSTRIES LTD
Ternary borate hydrotalcite flame retardant, preparation method and application thereof
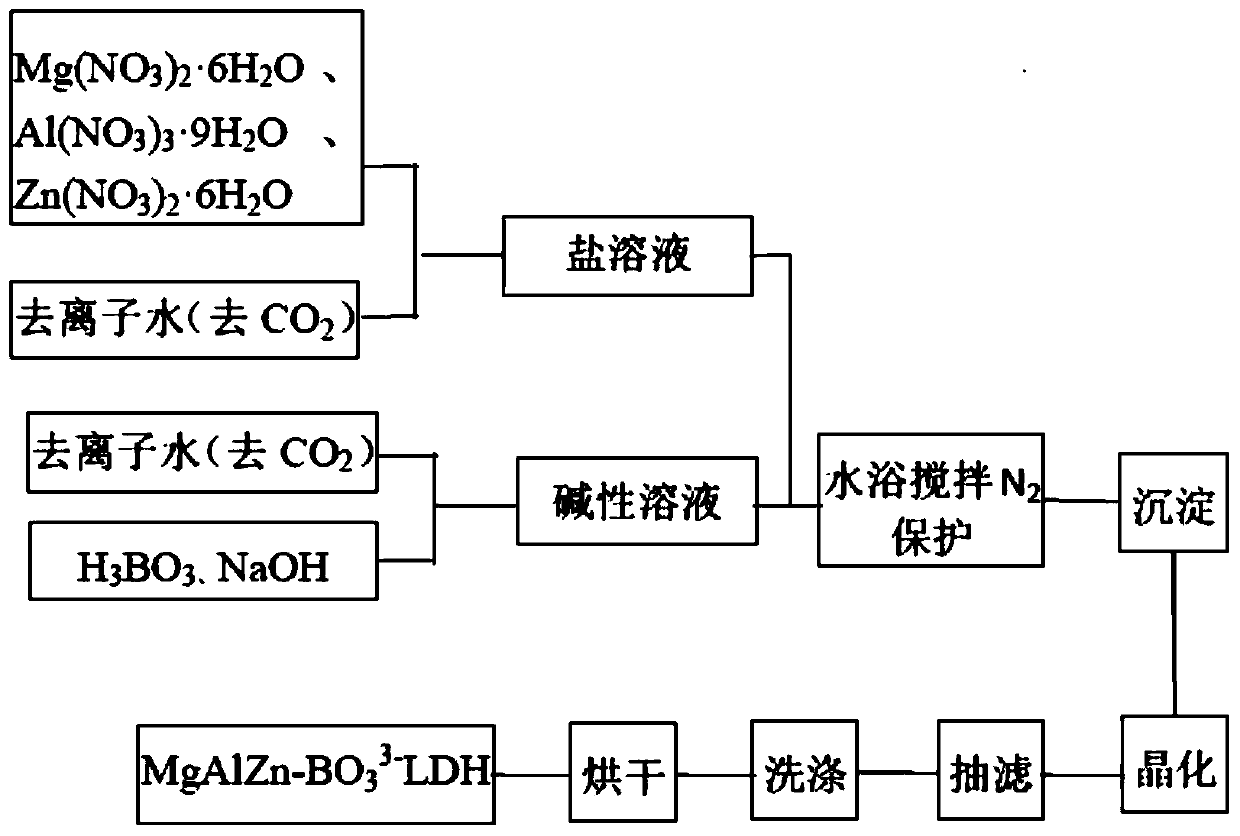
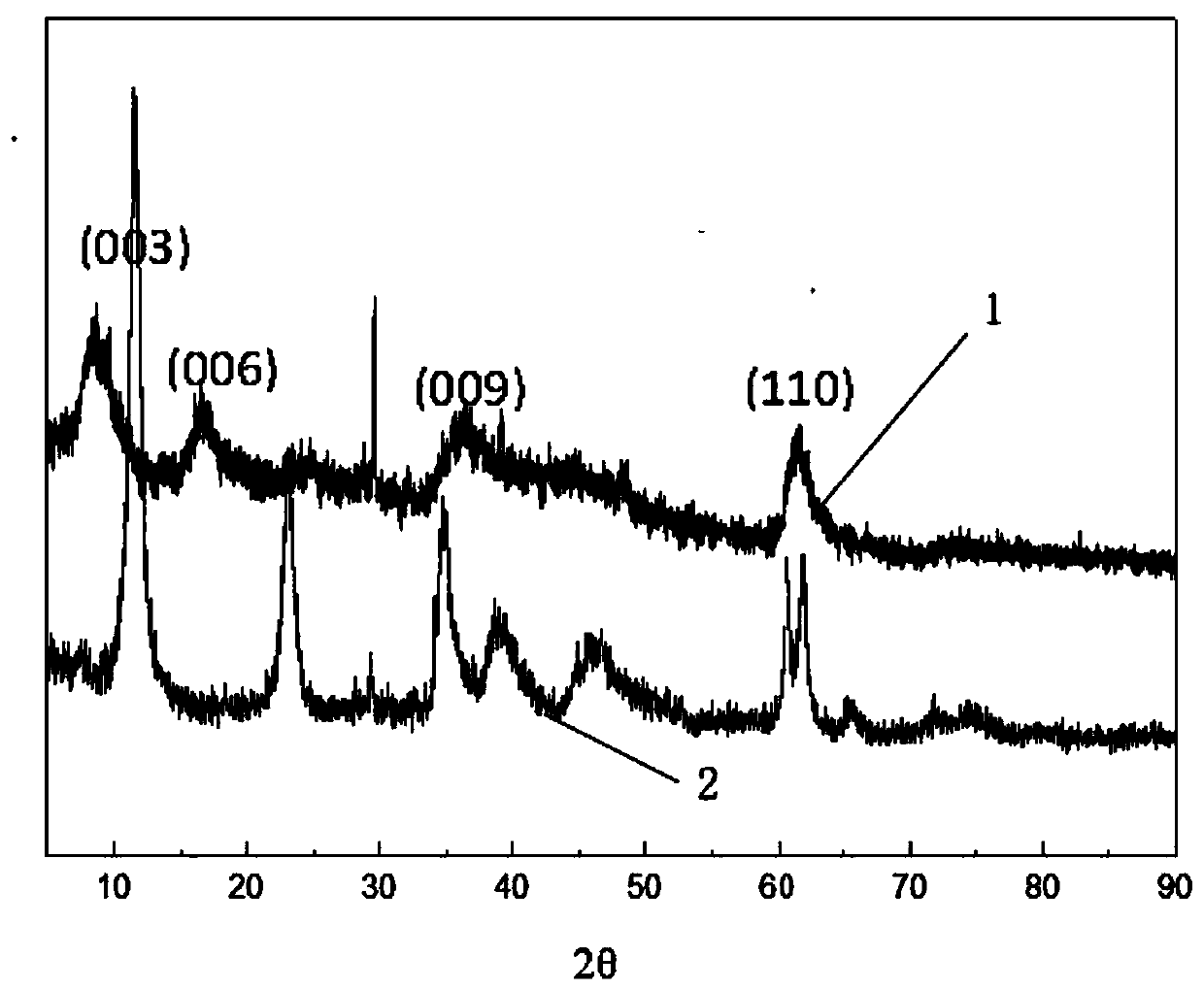
ActiveCN110627079AEasy to operateThe reaction is fully and evenlyZinc compoundsBoratesMaterials preparationPolymer science
Belonging to the technical field of plastic flame retardant material preparation, the invention discloses a ternary borate hydrotalcite flame retardant, a preparation method and application thereof. Specifically, the three salts Mg<2+>, Al<3+> and Zn<2+> are adopted as the lamellar main body, in the interlayer of nitrate radicals employing anions, a double-drop coprecipitation method is adopted toreplace nitrate ions with borate ions, thus obtaining the ternary borate hydrotalcite flame retardant, i.e. MgAlZn-BO3-LDHs. According to the preparation method and application method of the flame retardant provided by the invention, the obtained flame retardant is uniform in structure, has mild operation conditions, is energy saving and environment-friendly, and has great development prospects in the aspect of material preparation. The obtained ternary borate hydrotalcite flame retardant has good flame retardant effect, and has wide application prospects.
Owner:GUANGXI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com