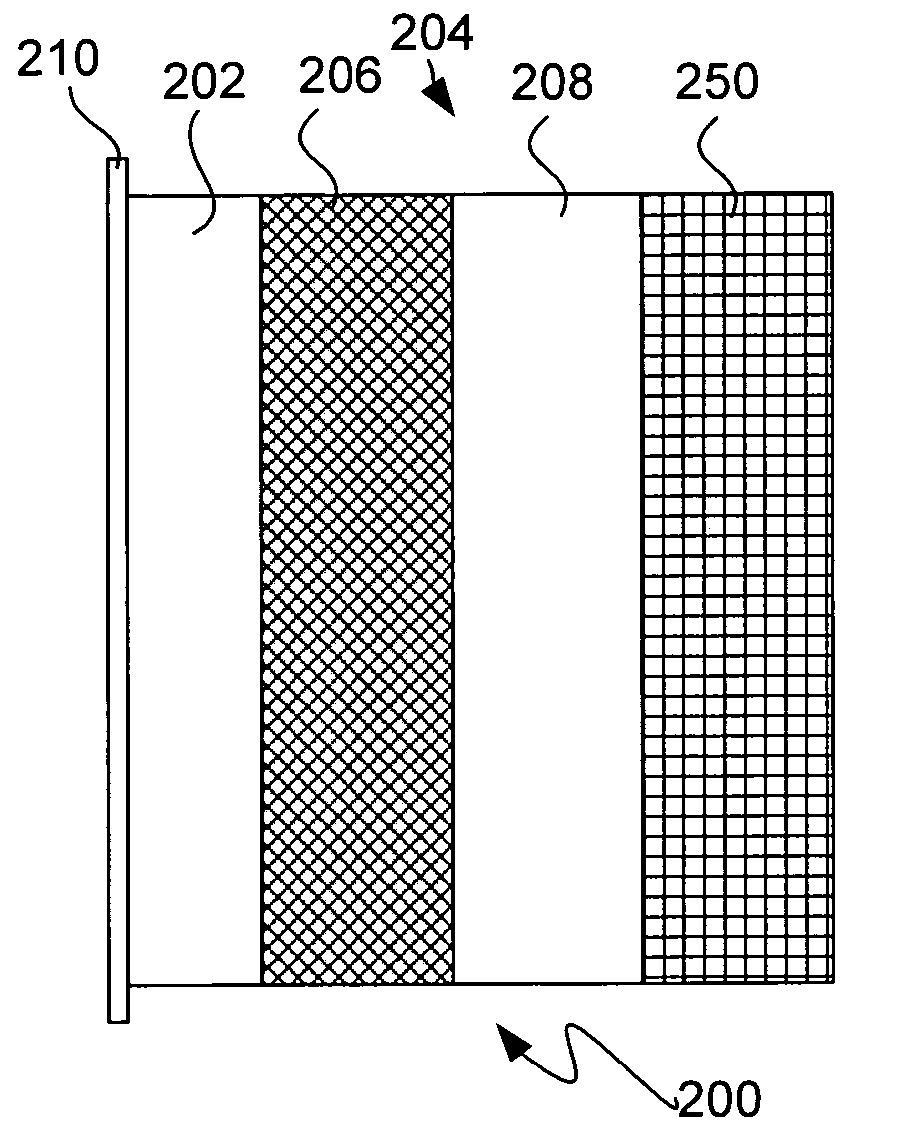
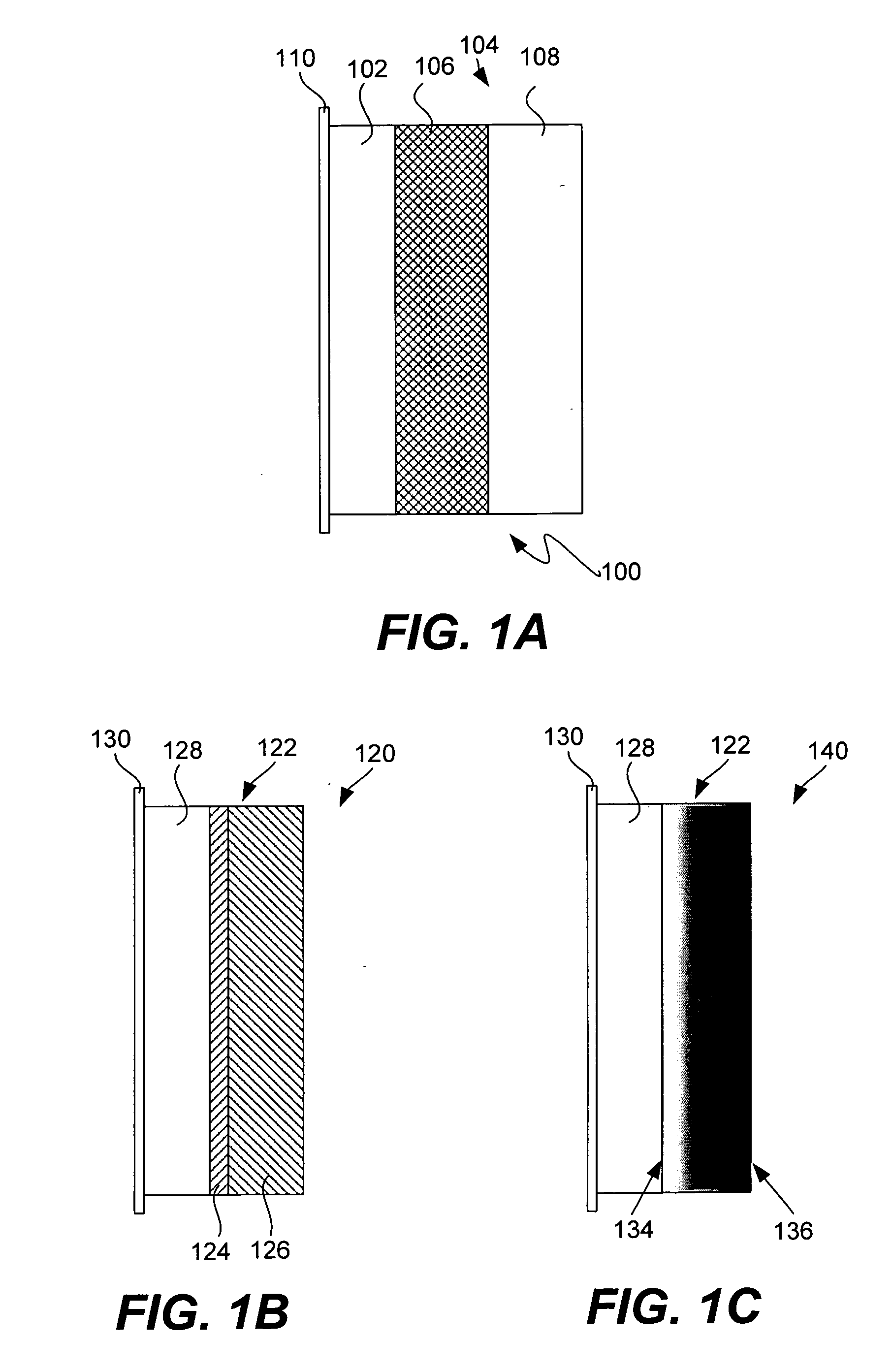
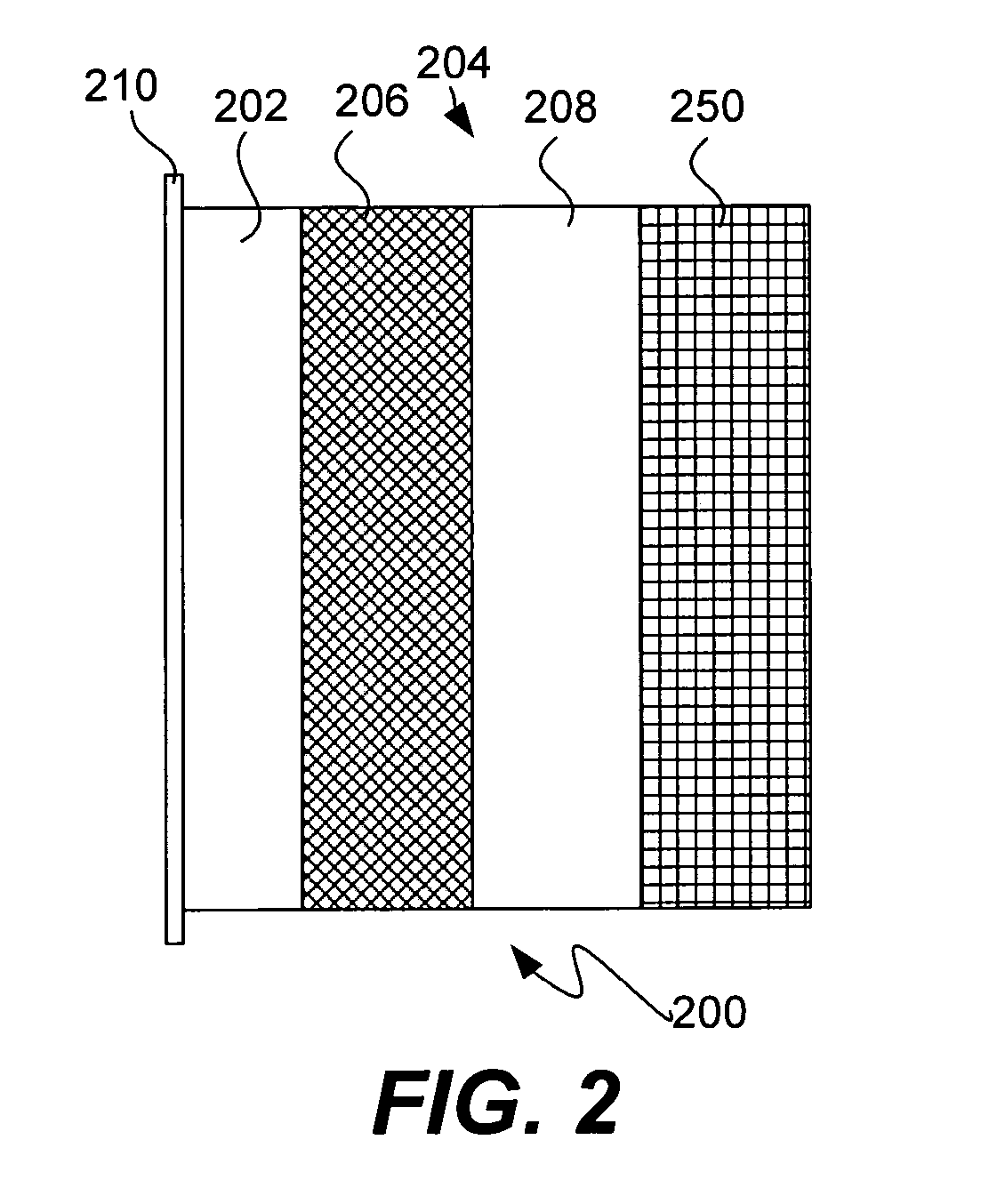
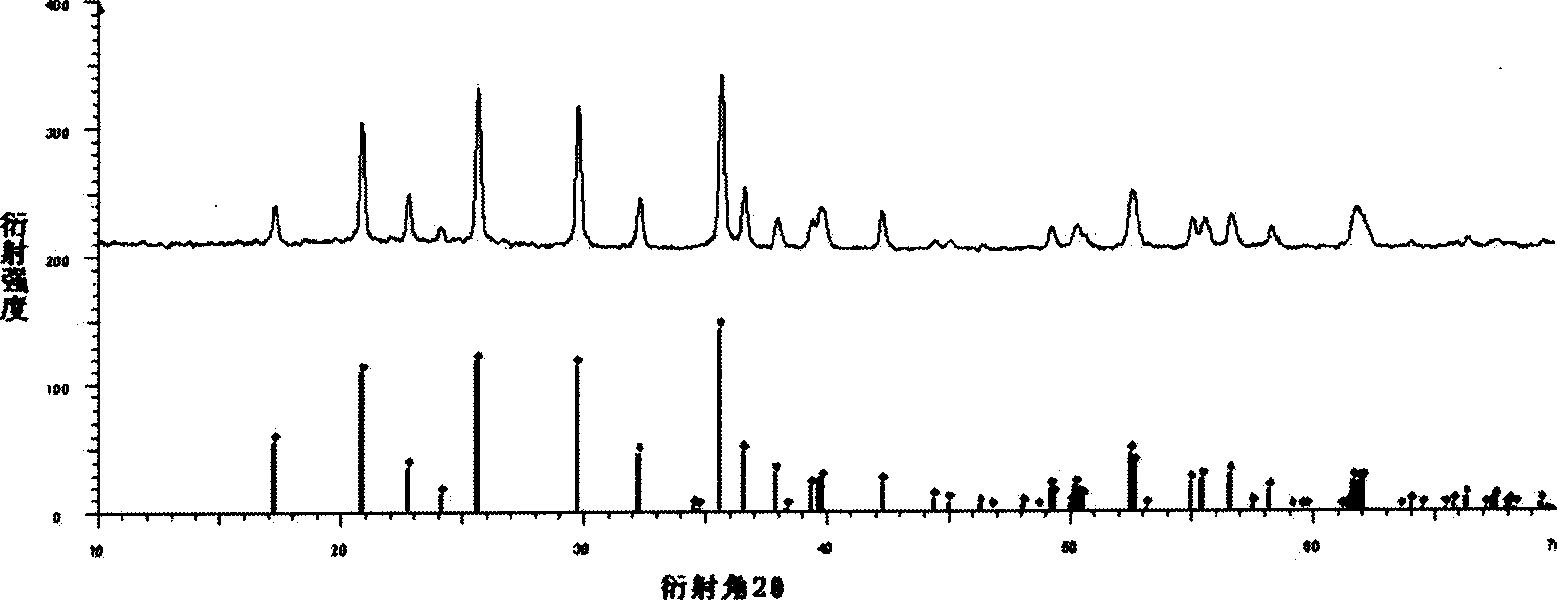
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1291 results about "LITHIUM PHOSPHATE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Conductive lithium storage electrode
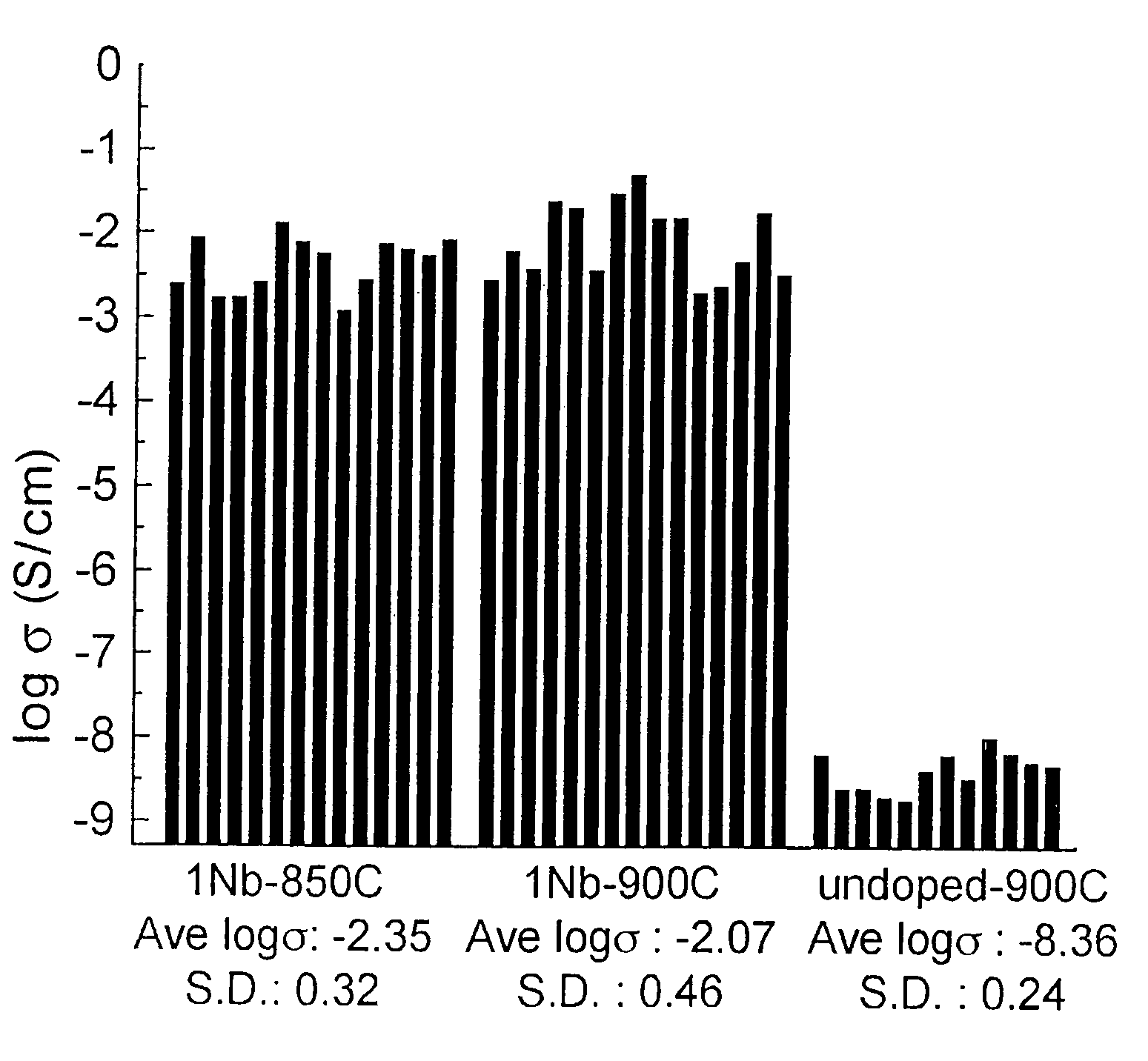
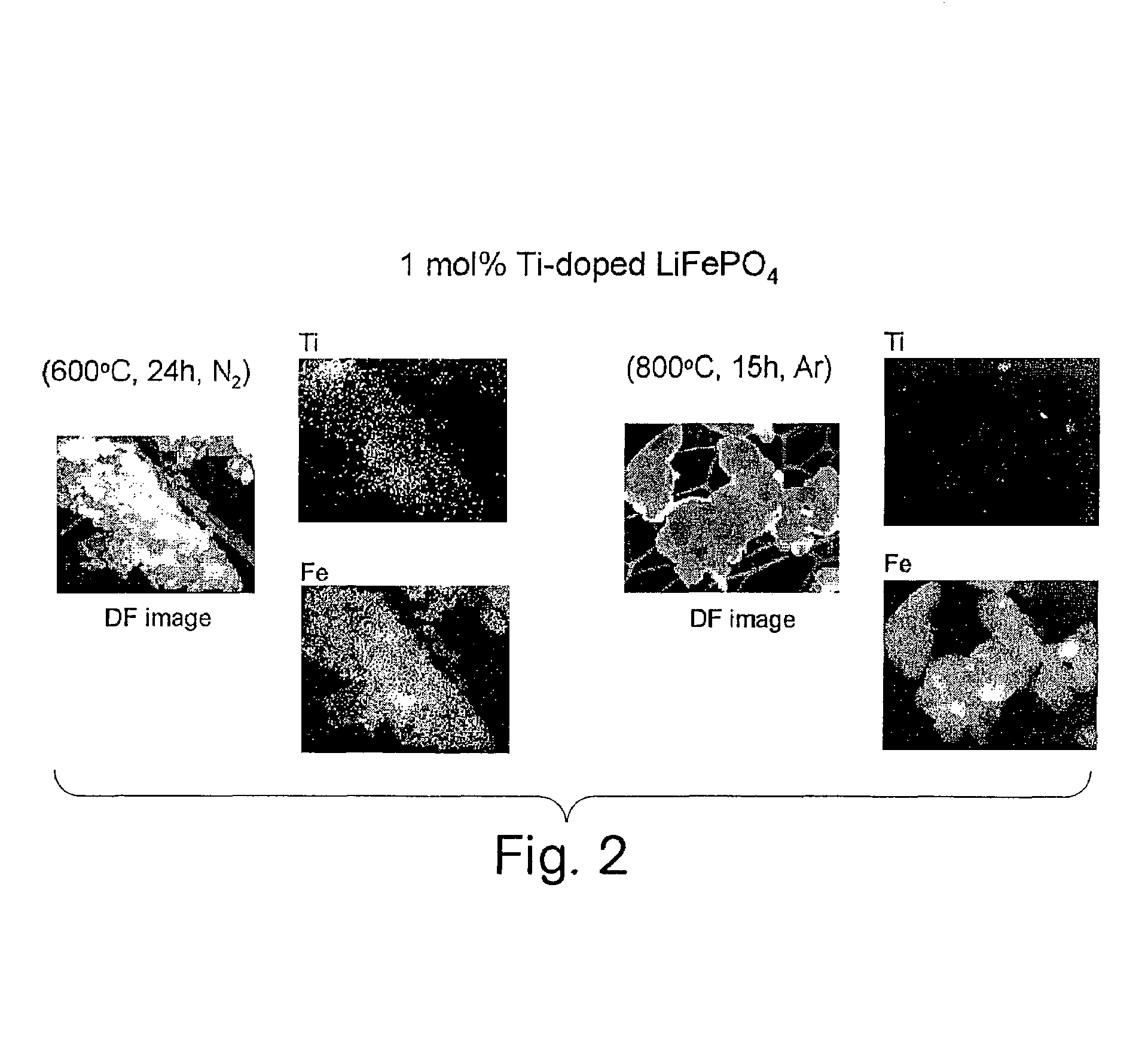
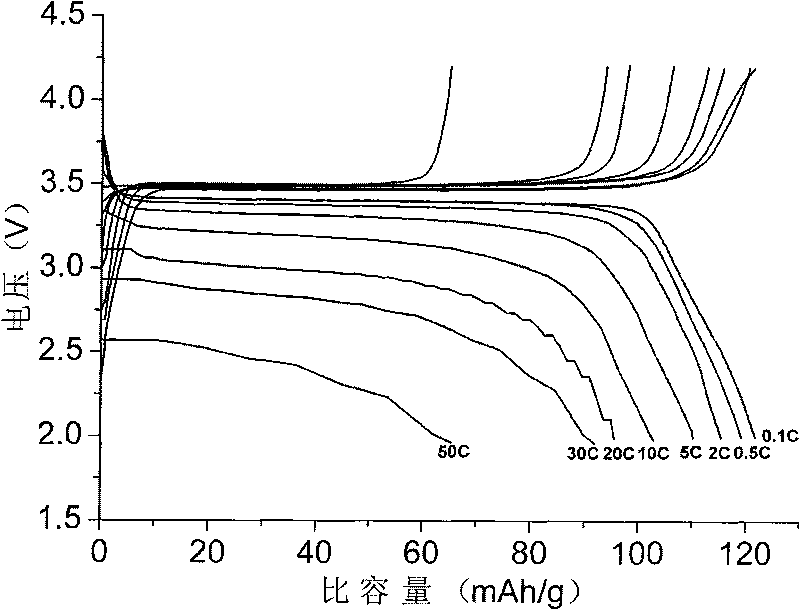
A compound comprising a composition Ax(M′1-aM″a)y(XD4)z, Ax(M′1-aM″a)y(DXD4)z, or Ax(M′1-aM″a)y(X2D7)z, and have values such that x, plus y(1-a) times a formal valence or valences of M′, plus ya times a formal valence or valence of M″, is equal to z times a formal valence of the XD4, X2D7, or DXD4 group; or a compound comprising a composition (A1-aM″a)xM′y(XD4)z, (A1-aM″a)xM′y(DXD4)z(A1-aM″a)xM′y(X2D7)z and have values such that (1-a)x plus the quantity ax times the formal valence or valences of M″ plus y times the formal valence or valences of M′ is equal to z times the formal valence of the XD4, X2D7 or DXD4 group. In the compound, A is at least one of an alkali metal and hydrogen, M′ is a first-row transition metal, X is at least one of phosphorus, sulfur, arsenic, molybdenum, and tungsten, M″ any of a Group IIA, IIIA, IVA, VA, VIA, VIIA, VIIIA, IB, IIB, IIIB, IVB, VB, and VIB metal, D is at least one of oxygen, nitrogen, carbon, or a halogen, 0.0001<a≦0.1, and x, y, and z are greater than zero. The compound can have a conductivity at 27° C. of at least about 10−8 S / cm. The compound can be a doped lithium phosphate that can intercalate lithium or hydrogen. The compound can be used in an electrochemical device including electrodes and storage batteries and can have a gravimetric capacity of at least about 80 mAh / g while being charged / discharged at greater than about C rate of the compound.
Owner:MASSACHUSETTS INST OF TECH
Graphite alkene iron lithium phosphate positive active material, preparing method thereof, and lithium ion twice battery based on the graphite alkene modified iron lithium phosphate positive active material
InactiveCN101752561AImprove conductivityImprove cycle stabilityLi-accumulatorsNon-aqueous electrolyte accumulator electrodesLithium-ion batteryCarbon coated
The present invention relates to graphite alkene iron lithium phosphate positive active material, a preparing method thereof, and a lithium ion twice battery based on the graphite alkene modified iron lithium phosphate positive active material. Graphite alkene and iron lithium phosphate are dispersed into water solution to be mixed evenly by stirring and ultra audible sound, then, are dried to obtain iron lithium phosphate material compounded by the graphite alkene and the iron lithium phosphate to be annealed by high temperature, and finally, the graphite alkene modified iron lithium phosphate positive active material is obtained. Compared with traditional carbon coated and conductive polymeric adulteration modified lithium batteries, the lithium ion twice battery based on the graphite alkene modified iron lithium phosphate positive active material has the advantages of high battery capacity, good charging-discharging circulating performance, long life and high circulating stability, and has great utility value.
Owner:宁波艾能锂电材料科技股份有限公司
Solid electrolytes based on lithium hafnium phosphate for active metal anode protection
InactiveUS20060078790A1Avoid harmful reactionsCell electrodesNon-aqueous electrolyte cellsPhosphateOrganic liquids
Active metal electrochemical structure, in particular an active metal negative electrode (anode) protected with an ionically conductive protective architecture incorporating a glassy, ceramic or glass-ceramic solid electrolyte material based on lithium hafnium phosphate, and associated electrochemical devices and methods, provides advantages over conventional structures. The protective architecture prevents the active metal from deleterious reaction with the environment on the other (cathode) side of the architecture, which may include aqueous, air or organic liquid electrolytes and / or electrochemically active materials.
Owner:POLYPLUS BATTERY CO INC
Method for preparing lithiumion cell positive material Iron-lithium phosphate
ActiveCN1581537AAvoid synthetic stepsSolve the problem of impurityElectrode manufacturing processesLithium compoundsPhosphateNitrogen gas
Mechanical solid phase method for synthesizing lithium ferric phosphate includes following steps: mixing iron powder, ferric phosphate, lithium phosphate, doping elements of phosphate, conducting agent or predecessor of conducting agent according to proportion evenly; placing the mixed admixture into ball milling container with inert gases being filled and ball milling for 18-36 hours; then putting the produced result from ball milling into high-temperature furnace with inert gases such as nitrogen gas and argon gas being filled; heating up in 10-30 deg.C / minute heating rate, baking at constant temperature 450-750 deg.C for 10-60 minutes; then cooling at 10-30 deg.C / minutes cooling rate, cooling the admixture to room temperature so as to obtain powder of lithium ferric phosphate or powder of doped powder of lithium ferric phosphate. Advantages are: feasible, no pollution, high specific capacity and good cycle performance.
Owner:SHANGHAI SINOPOLY JIAHUA BATTERY TECH
Wet chemistry method for preparing lithium iron phosphate
InactiveCN1431147AEasy to controlEvenly distributedElectrode thermal treatmentLi-accumulatorsChemical compositionLithium iron phosphate
A wet chemical process for preparing iron lithium phosphate includes mixing the solution or suspensions of Li source compound, Fe source compound, P source compound, doping element compound or electric conducting agent, and precipitant, reaction at 5-120 deg.C for 0.5-24 hr while stirring, filtering, washing, baking to obtain nano precursor, quickly heating to 500-800 deg.C in non-air or non-oxidizing atmosphere, calcining for 5-48 hr, and cooling. Its advantages are easy control, high uniformity and electric conductivity.
Owner:郑绵平
Lithium titanate composite electrode material with surface coating layer
InactiveCN101764209AChange physical propertiesChange chemical propertiesCell electrodesMagnesium phosphateMagnesium orthophosphate
The invention relates to a battery electrode material, in particular to a lithium titanate composite electrode material with surface coating layer; in the lithium titanate composite electrode material with surface coating layer, the electrode material is composed of lithium titanate particles and a coating layer coated with the surface of the lithium titanate particles; the particle size of the lithium titanate particles is 100nm-95mum, the average thickness of the surface coating layer is 0.2nm-5m, and the particle diameter of the composite electrode material is 0.1-100mum; the material of the surface coating layer is one or mixture of more than one kind of insulation oxide, insulation composite oxide, aluminium phosphate, magnesium phosphate, lithium fluoride, lithium phosphate or LiMPO4, wherein M is magnesium, ferrum, cobalt, nickel, chromium, titanium or vanadium; in the invention, by carrying out surface coating treatment to the surfaces of the existing lithium titanate particles, a layer of protective film is formed on the surface, so as to change the physical and chemical characteristics of the surface of the lithium titanate active material, the surface can not be reacted with electrolyte even if under overpotential condition, so as to avoid ballooning and ensure the capacity and the circularity of the battery not to be reduced.
Owner:SUZHOU PHYLION BATTERY
Binary, ternary and quaternary lithium phosphates, method for the production thereof and use of the same
ActiveUS20040151649A1High specific capacitySimple and inexpensivePhosphatesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesRoom temperatureReducing atmosphere
The invention relates to binary, ternary and quaternary lithium phosphates of general formula Li(FexM<1>yM<2>z)PO4 wherein M<1 >represents at least one element of the group comprising Sc, Ti, V, Cr, Mn, Co, Ni, Cu, Zn, Be, Mg, Ca, Sr, Ba, Al, Zr, and La; M<2 >represents at least one element of the group comprising Sc, Ti, V, Cr, Mn, Co, Ni, Cu, Zn, Be, Mg, Ca, Sr, Ba, Al, Zr, and La; x=between 0.5 and 1, y=between 0 and 0.5, z=between 0 and 0.5, provided that x+y+z=1, or x=0, y =1 and z=0. The said lithium phosphates can be obtained according to a method whereby precursor compounds of elements Li, Fe, M<1 >and / or M<2 >are precipitated from aqueous solutions and the precipitation product is dried in an inert gas atmosphere or a reducing atmosphere at a temperature which is between room temperature and approximately 200° C. and tempered at a temperature of between 300° C. and 1000° C. The inventive lithium phosphates have a very high capacity when used as cathode material in lithium accumulators.
Owner:ZENT FUR SONNENENERGIE & WASSERSTOFF FORSCHUNG BADEN WURTTEMBERG GEMEINNUTZIGE STIFTUNG
Vapor deposition of metal oxides, silicates and phosphates, and silicon dioxide
InactiveUS6969539B2Good step coverageNarrow structureOxygen/ozone/oxide/hydroxideAluminium silicatesAlkylphosphatePhosphate
Metal silicates or phosphates are deposited on a heated substrate by the reaction of vapors of alkoxysilanols or alkylphosphates along with reactive metal amides, alkyls or alkoxides. For example, vapors of tris-(ter-butoxy)silanol react with vapors of tetrakis(ethylmethylamido)hafnium to deposit hafnium silicate on surfaces heated to 300° C. The product film has a very uniform stoichiometry throughout the reactor. Similarly, vapors of diisopropylphosphate react with vapors of lithium bis(ethyldimethylsilyl)amide to deposit lithium phosphate films on substrates heated to 250° C. supplying the vapors in alternating pulse produces these same compositions with a very uniform distribution of thickness and excellent step coverage.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
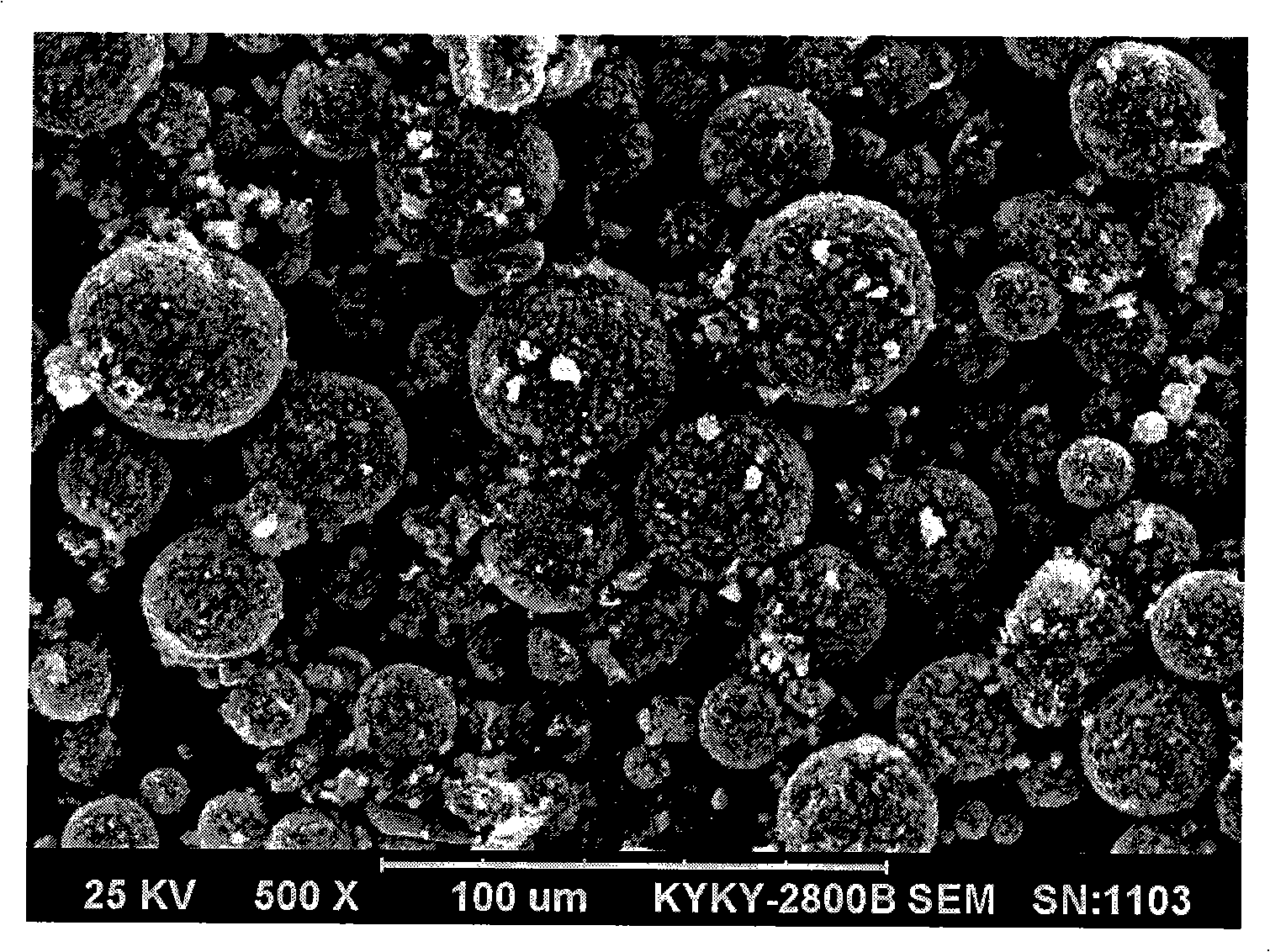
Method for preparing high-density spherical ferric lithium phosphate as anode material of lithium-ion battery
InactiveCN1635648AHigh bulk densityIncrease volume capacityElectrode manufacturing processesLithium compoundsPhosphateLithium-ion battery
This invention discloses the method for preparing high-density spherical lithium ferric phosphate used as the positive material of lithium ion cell, which contains synthesizing the ferric iron salt aqueous solution, phosphorus source aqueous solution and alkali aqueous solution to form spherical or spheroid ferric phosphate precursor, uniformly mixed with lithium source, carbon source and doped metal compound after being washed and dried, high temperature heat treating at 600-900 degree centigrade for 8-48 hr under inertia or reducing atmosphere protection to obtain lithium ferric phosphate with mean grain size of 7-12 micrometer,2.0-2.2g / cm3 of tap density, high buck density of 140-155mAh / g first discharge ratio capacity at normal temperature, and high volume ratio capacity.
Owner:TSINGHUA UNIV
Large-capacity high power polymer ferric lithium phosphate power cell and preparation method thereof
InactiveCN101409369AImprove securityIncrease capacityElectrode manufacturing processesFinal product manufactureSlurryElectric vehicle
The invention discloses a large-capacity high-power polymer lithium iron phosphate power battery. The weight ratio of anode slurry is as follows: 81 to 85 percent of lithium iron phosphate, 1 to 5.5 percent of superconduction carbon, 0 to 2.5 percent of conductive carbon soot, 0 to 4 percent of conductive black lead, 0 to 2.5 percent of crystalline flake graphite, 0 to 2 percent of carbon nanometer tube as well as 6 to 7.5 percent of polyvinylidene fluoride; the weight ratio of cathode slurry is as follows: 89 to 91 percent of cathode material, 1 to 3.5 percent of superconduction carbon, 0 to 2 percent of conductive carbon soot, 0 to 4 percent of conductive black lead, 2.5 to 3.5 percent of styrene-butadiene rubber as well as 1.5 to 2 percent of sodium carboxymethyl cellulose; the steps for preparing the battery are as follows: preparing slurry, coating the anode and the cathode, rolling and pressing a polar plate, transversely and separately cutting the polar plate, baking the polar plate, welding the polar ears of the anode and the cathode, preparing a battery cell, putting the electric core into a shell and sealing, baking the electric core, injecting liquid into the battery as well as forming the battery and dividing the volume of the battery. The invention relates to a lithium-ion secondary battery which can provide drive energies for electric tools, electric bicycles, motor cars and electric vehicles.
Owner:MCNAIR TECH
Thermoplastic monofilament fibers exhibiting low-shrink, high tenacity, and extremely high modulus levels
InactiveUS6759124B2High tensile strengthLow shrinkageSynthetic resin layered productsFilament/thread formingThermoplasticYarn
Unique thermoplastic monofilament fibers and yarns that exhibit heretofore unattained physical properties are provided. Such fibers are basically manufactured through the extrusion of thermoplastic resins that include a certain class of nucleating agent therein, and are able to be drawn at high ratios with such nucleating agents present that the tenacity and modulus strength are much higher than any other previously produced thermoplastic fibers, particularly those that also simultaneously exhibit extremely low shrinkage rates. Thus, such fibers require the presence of certain compounds that quickly and effectively provide rigidity to the target thermoplastic (for example, polypropylene), particularly after heat-setting. Generally, these compounds include any structure that nucleates polymer crystals within the target thermoplastic after exposure to sufficient heat to melt the initial pelletized polymer and allowing such an oriented polymer to cool. The compounds must nucleate polymer crystals at a higher temperature than the target thermoplastic without the nucleating agent during cooling. In such a manner, the "rigidifying" nucleator compounds provide nucleation sites for thermoplastic crystal growth. The preferred "rigidifying" compounds include dibenzylidene sorbitol based compounds, as well as less preferred compounds, such as [2.2.1]heptane-bicyclodicarboxylic acid, otherwise known as HPN-68, sodium benzoate, certain sodium and lithium phosphate salts [such as sodium 2,2'-methylene-bis-(4,6-di-tert-butylphenyl)phosphate, otherwise known as NA-11]. Specific methods of manufacture of such inventive thermoplastic fibers, as well as fabric articles made therefrom, are also encompassed within this invention.
Owner:MILLIKEN & CO
Rare earth doped carbon clad type nanometer anode material iron lithium phosphate and its preparation method
InactiveCN1830764AIncrease capacityImprove cycle lifeCell electrodesPhosphorus compoundsEpoxyRare earth
A carbon-coated RE-doped iron lithium phosphate nano-particle used for positive electrode of battery is prepared from FeC2H4, H2O, Li2CO3, NH4H2PO4, Y2O3, CeO2 and epoxy resin through proportional mixing, ball grinding, baking, sieving, calcining in N2 atmosphere at 350 deg.C and 600-750 deg.C respectively, and keeping the temp for a certain time.
Owner:TSINGHUA UNIV +1
Method for preparing iron lithium phosphate by recovering water-system waste lithium-ion power battery
ActiveCN101916889AReduce manufacturing costSolving Recycling ProblemsWaste accumulators reclaimingBattery recyclingPower batteryIron salts
The invention discloses a method for preparing iron lithium phosphate by recovering water-system waste lithium-ion power batteries, comprising the following steps: 1) cutting and brushing a water-system waste lithium-ion power battery, processing by deionized water, sieving and drying to recover the mixture of an electrode material and a conductive agent; 2) adding inorganic acid to the dried mixture of the electrode material and the conductive agent to process, filtering to obtain acid solution containing Li+, Fe2+ and PO43-; 3) adding lithium salt or iron salt to the acid solution containing Li+, Fe2+ and PO43-, adding ascorbic acid and stirring, controlling the pH value to equal to 3-7, and filtering to obtain precipitation; and 4) adding the crude product LiFePO4 obtained in step 3) to a water solution of cane sugar for ball milling, drying and calcining to obtain a regenerative LiFePO4 material. The method has low cost, simple operation and no secondary pollution.
Owner:长春劲能科技集团有限公司
Cathode active material, cathode, and nonaqueous electrolyte secondary battery
ActiveUS20090142668A1ResistanceReduce electrical conductivityFinal product manufacturePositive electrodesLITHIUM PHOSPHATEPore diameter
The present application provides a nonaqueous electrolyte secondary battery which includes a cathode having a cathode active material layer, an anode, and a nonaqueous electrolyte, wherein the cathode active material layer includes secondary particles of a lithium phosphate compound having olivine structure, an average particle diameter A of primary particles constituting the secondary particles is 50 nm or more and 500 nm or less, and a ratio B / A of a pore diameter B of the secondary particles to the average particle diameter A of the primary particles is 0.10 or more and 0.90 or less.
Owner:MURATA MFG CO LTD
Battery
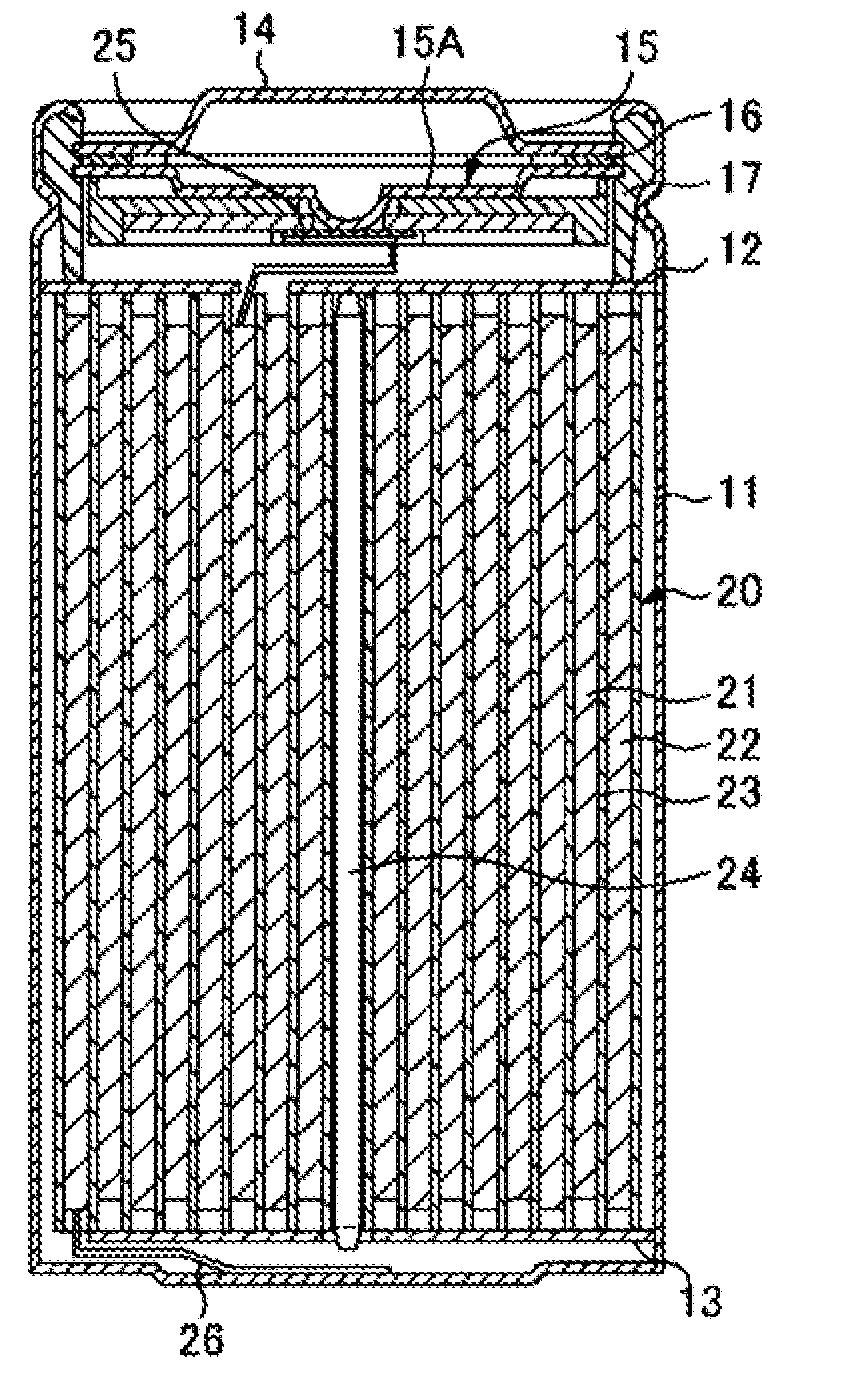
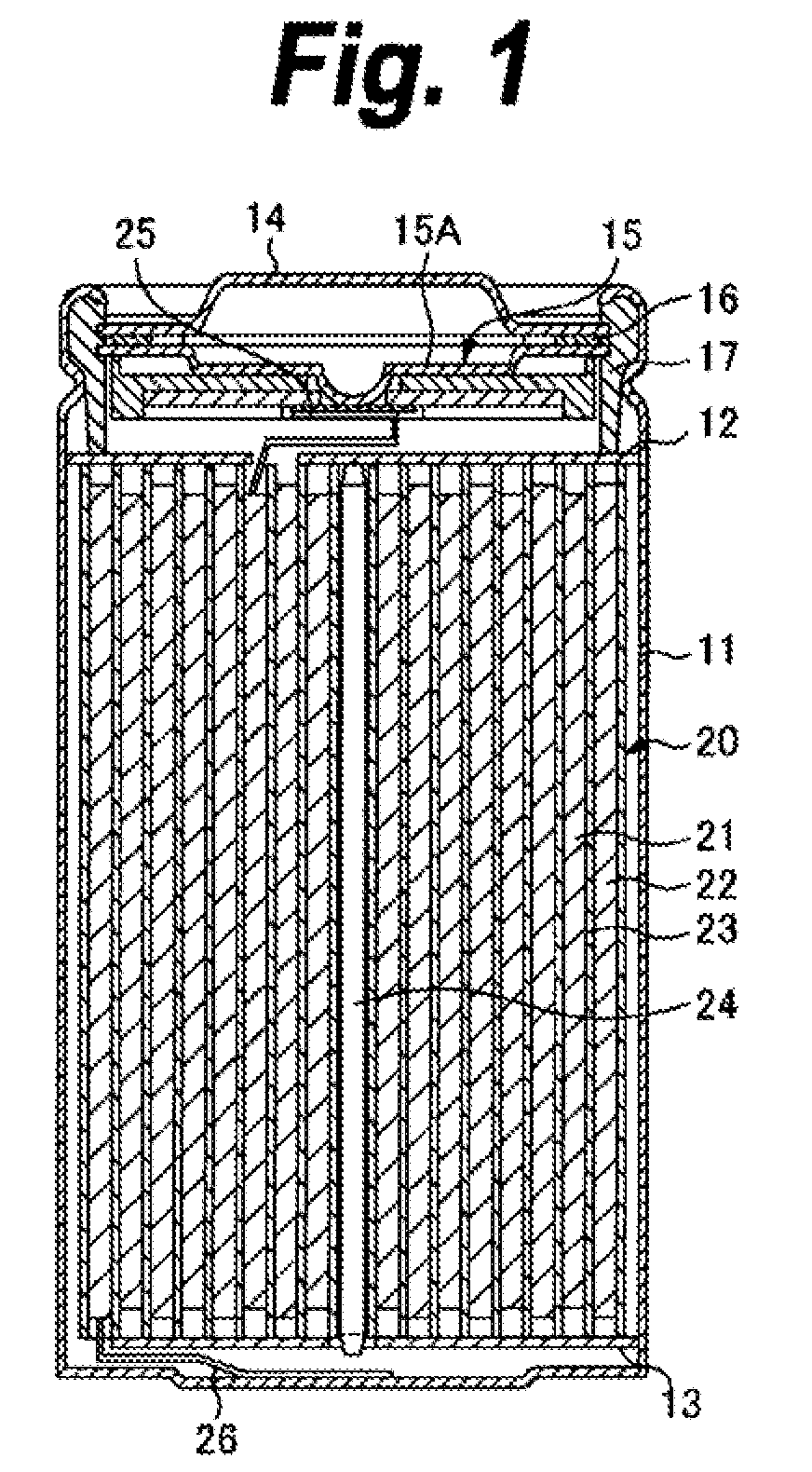
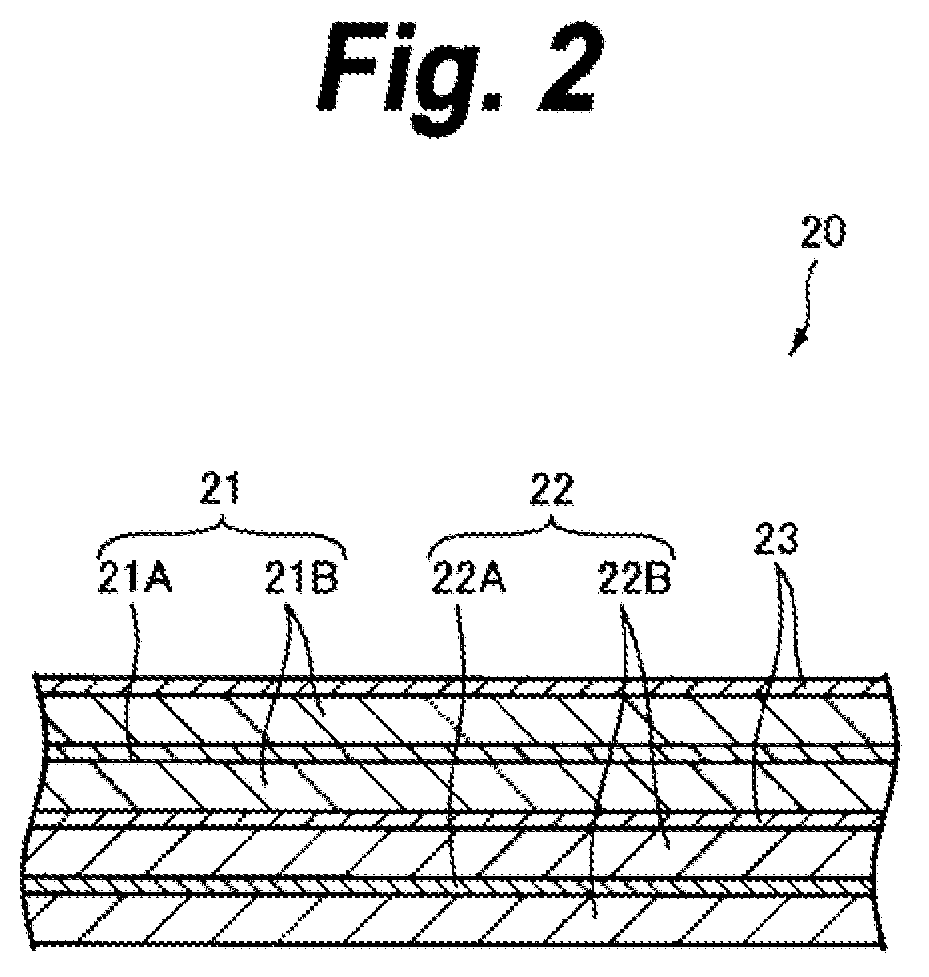
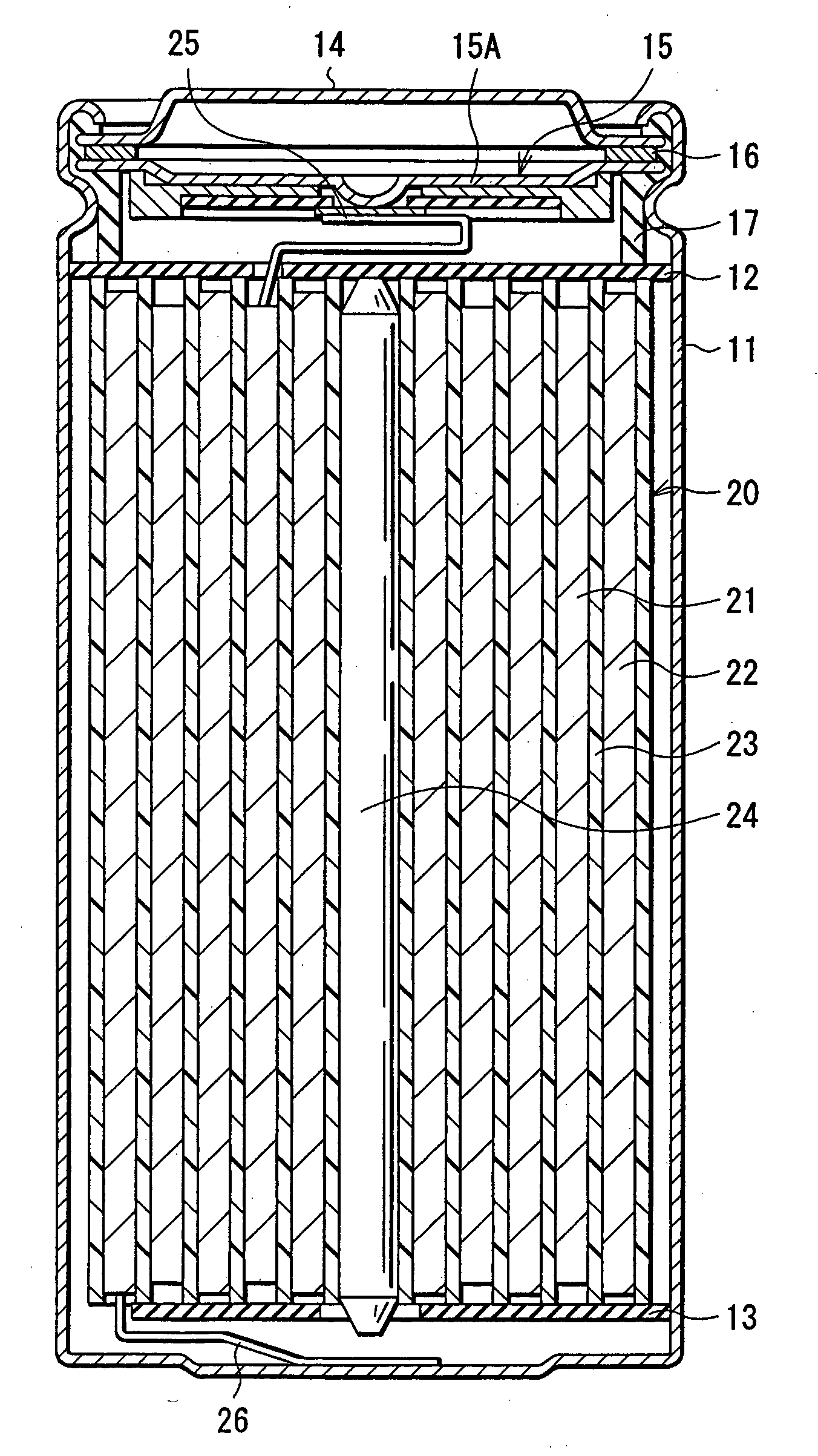
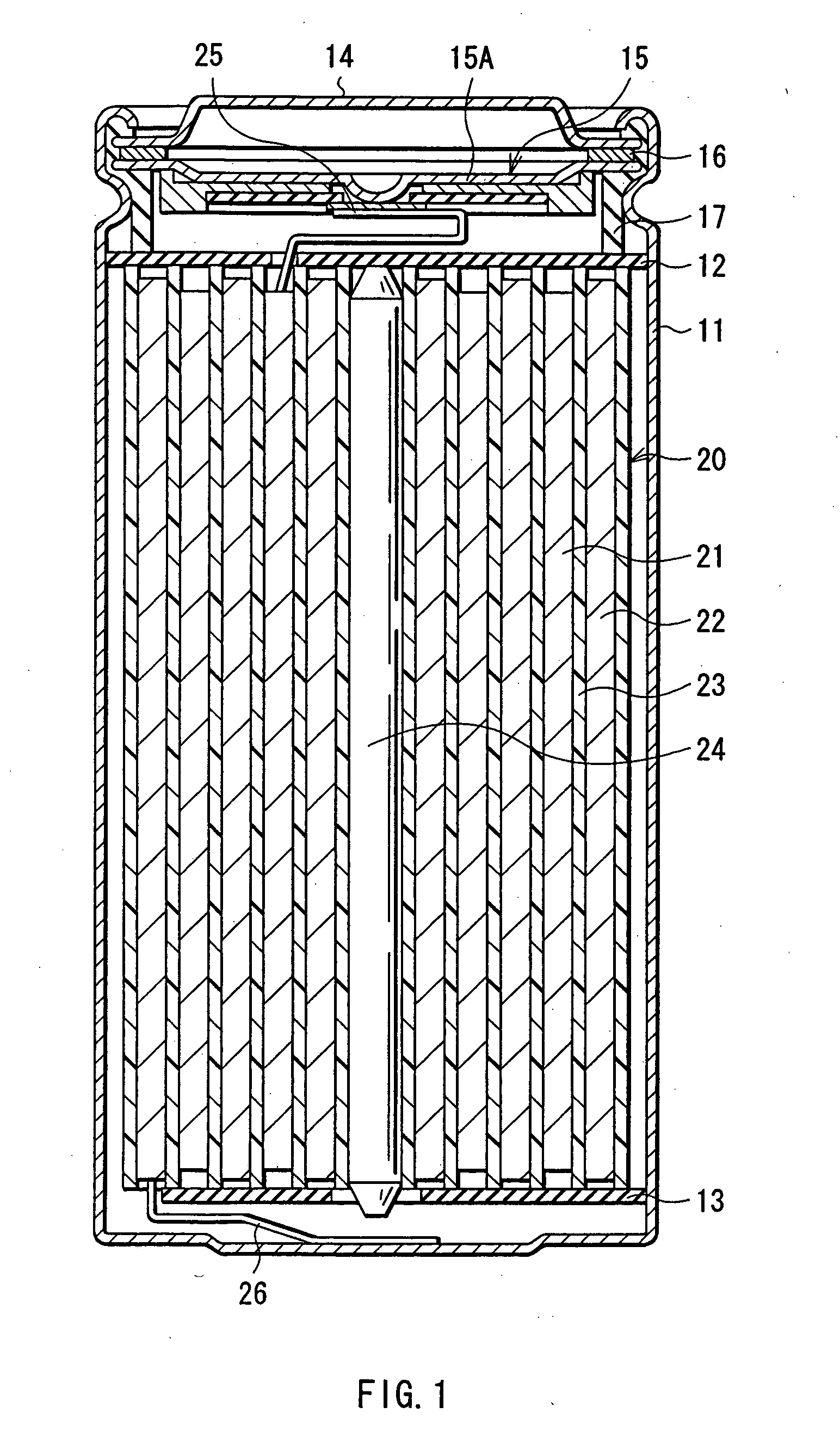
ActiveUS20050095503A1Large capacityImprove featuresElectrode carriers/collectorsNon-aqueous electrolyte accumulator electrodesDecompositionAlloy
A battery is provided which has a high capacity and can improve battery characteristics, such as cycle characteristics. The battery includes a spirally wound electrode body, wherein a cathode and an anode are wound with a separator in between. The anode includes, for example, simple substances, alloys, compounds of metal elements or metalloid elements capable of forming an alloy with Li, the like and combinations thereof. An electrolytic solution wherein an electrolyte salt is dissolved in a solvent is impregnated in the separator. For the electrolyte salt, a light metallic salt having B—O bond or P—O bond, such as difluoro[oxalato-O,O′]lithium borate and tetra fluoro[oxalato-O,O′]lithium phosphate, can be used. By forming a stable coating, decomposition reaction of the solvent can be inhibited, and reaction between the anode and the solvent can be prevented.
Owner:SONY CORP +1
Lithium ion battery anode material manganese lithium phosphate and preparation method thereof
ActiveCN101320809AImprove electronic conductivityEvenly dispersedElectrode manufacturing processesPhosphorus compoundsCapacitanceLithium iron phosphate
The invention discloses a manganese / lithium phosphate of lithium iron battery positive pole material and a production method thereof, the technical issue to be solved is to improve electrochemical performances of the positive pole material. The material of the invention includes substrates of manganese / lithium phosphate which are covered by a carbon material covering layer, the lithium covering the manganese / lithium phosphate behind the carbon material covering layer is spherical and has microscopic characteristics of being near spherical, rhombic, tapered, tabular, layered or / and block-shaped as well as of having 0.5-30 mum long and short axles. The production method comprises the following steps of: production of nanometer particles, liquid phase mixed reaction, production of precursor, sintering treatment, covering organic substances. Compared with the prior art, the invention improves the electron conductivity of the manganese / lithium phosphate by covering with carbon liquid phase, the carbon sufficiently covers active materials to efficiently prevent particle aggregation, the invention has the characteristics of about 4V of discharge voltage, high discharge and charge capacitance, excellent circulation stability, high safety, simple process, low cost and little influence on the environment.
Owner:SHENZHEN CITY BATTERY NANOMETER TECH
Low-shrink polypropylene fibers
This invention relates to improvements in preventing heat- and moisture-shrink problems in specific polypropylene fibers. Such fibers require the presence of certain compounds that quickly and effectively provide rigidity to the target polypropylene fiber after heat-setting. Generally, these compounds include any structure that nucleates polymer crystals within the target polypropylene after exposure to sufficient heat to melt the initial pelletized polymer and upon allowing such a melt to cool. The compounds must nucleate polymer crystals at a higher temperature than the target polypropylene without the nucleating agent during cooling. In such a manner, the "rigidifying" nucleator compounds provide nucleation sites for polypropylene crystal growth. After drawing the nucleated composition into fiber form, the fiber is then exposed to sufficient heat to grow the crystalline network, thus holding the fiber in a desired position. The preferred "rigidifying" compounds include dibenzylidene sorbitol based compounds, as well as less preferred compounds, such as sodium benzoate, certain sodium and lithium phosphate salts (such as sodium 2,2'-methylene-bis-(4,6-di-tert-butylphenyl)phosphate, otherwise known as NA-11). Specific methods of manufacture of such fibers, as well as fabric articles made therefrom, are also encompassed within this invention.
Owner:MILLIKEN & CO
Preparation method of transition element doped iron lithium phosphate powder
InactiveCN1785799AIncrease capacityImprove capacity cycling performancePhosphorus compoundsMaterials preparationLithium iron phosphate
The present invention discloses a preparation method of transition element Mn, Co and Ni doped iron lithium phosphate powder body, belonging to the field of electrochemical power supply material preparation technology. Its molecular formula is Li1-x TRxFePO4, and its preparation method includes the following steps: weighting lithium salt, ferrous salt, phosphate and adulterant according to mole ratio, mixing them, drying, low-temperature prebaking and high-temperature secondary calcining so as to obtain the invented product which can be used as positive electrode material of lithium ion cell.
Owner:TSINGHUA UNIV
Process for producing carbon coated iron lithium phosphate
InactiveCN101172599AElectrode manufacturing processesPhosphorus compoundsShortest distancePhosphate ion
The invention relates to a preparation method of lithium iron phosphate for the carbon cladding of a lithium ion battery. The prior lithium iron phosphate preparation technical art is complex, and has high cost. The invention has the synthetic process that: ferric oxide, phosphoric acid, simple organics and doped element compound are mixed and dried, the mol ratio of phosphate radical ion, ferric ion and doped element ion is 1:y:z, wherein, y is larger than or equal to 0.95 and smaller than or equal to 1, and y plus z is equal to 1; the mixture is added with lithium source compound, added with water to be mixed and dried, the mol ratio of lithium ion and phosphate radical ion is x:1, and x is larger than or equal to 0.95 and smaller than or equal to 1.05; the mixture which reacts for 2 to 20 hours under 500 to 800 DEG C is cooled in a furnace. The invention finally produces the precursor uniformly mixed with superfine crystal grain, and during the subsequent high temperature solid phase reaction, the end product lithium iron phosphate can be produced through shorter distance diffuseness of atoms. The end product has high purity, the crystallisation is good, the capacity is high, and the cycle stability is good.
Owner:HANGZHOU DIANZI UNIV
Power lithium ion battery electrolyte and power lithium ion battery
InactiveCN106099171AImprove charge and discharge efficiencyImprove cycle performanceSecondary cellsOrganic solventHigh energy
The invention discloses power lithium ion battery electrolyte and a power lithium ion battery. The power lithium ion battery electrolyte is prepared from a non-aqueous organic solvent, lithium salt, an additive A and an additive B, wherein the additive A is selected from at least one of difluoro lithium phosphate, difluoro sulfimide lithium and lithium difluoroborate, and the additive B is selected from at least one sulfide which has a first structural formula or a second structural formula or a third structural formula. According to the power lithium ion battery electrolyte, the additive A or the additive B with low impedance is blended, meanwhile, the dosages of high-impedance additives including vinylene carbonate, 1,3-propane sultone and the like are controlled, and an SEI (Solid Electrolyte Interface) membrane with low impendence and a stable structure can be formed on the surface of an electrode; the prepared power lithium ion battery has the advantages of long cycle life, high energy density, high output power, good low-temperature performance and the like.
Owner:GUANGZHOU TINCI MATERIALS TECH
Method for preparing equal dispersion ferric phosphate lithium nano crystal by hydrothermal synthetis method
InactiveCN101047242AExcellent inert environmentElectrode manufacturing processesPhosphateFerrous salts
A method for preparing uniformly scattered nanocrystal of iron-lithium phosphate by hydrothermal synthesis includes using ferrous salt and phosphoric acid as well as lithium hydroxide as raw materials to obtain reaction pioneer matter under temperature of 40-100deg.c first, then reacting on obtained pioneer matter in high pressure reactor with temperature of 150-200deg.c under hydrothermal condition and processing obtained product by high temperature under protection of inert gas to finally obtain said uniformly scattered nanocrystal with average particle diameter of 0.2-0.5micron.
Owner:胜利油田华鑫石油材料有限公司
Anode material of Li-ion secondary battery and battery containing the same
InactiveCN101212048AIncrease capacityImprove cycle performanceCell electrodesSecondary cellsHigh temperature storagePhosphoric acid
The invention relates to an anode material for a lithium ion secondary battery. The anode material comprises anode active substance, a conductive agent and a caking agent, wherein the anode active substance comprises material A and material B. The material B is the oxide C of plated metal lithium and / or the oxide D of plated metal lithium which are coated and processed by the material A. The material A is the lithium phosphate slat with a olivine structure. The anode material can obviously improve the safety performance of the lithium ion secondary battery; the battery has a big capacity; the anode material has a good performance of charge and discharge for a big current, circulation and high temperature storage.
Owner:BYD CO LTD
Method for treating lithium iron phosphate cathode material of waste and old power lithium battery of automobile
ActiveCN102956936AMaximizeEliminate distractionsWaste accumulators reclaimingBattery recyclingImpurityCalcination
The invention provides a method for treating a lithium iron phosphate cathode material of a waste and old power lithium battery of an automobile. The method comprises the following steps of calcination, acid leaching, alkaline leaching, and recovery and recycle of valuable metals. The method avoids the interference of iron, copper and aluminum impurities in a lithium iron phosphate cathode material on lithium recovery thereby realizing production of a pure lithium phosphate product. The method realizes the greatest degree of integrated utilization of a lithium iron phosphate cathode material, is reasonable, is realized easily, has a low cost, is environmentally friendly, is suitable for industrialization, and has high economic benefits and social benefits.
Owner:GEM CO LTD
Non-aqueous electrolyte battery
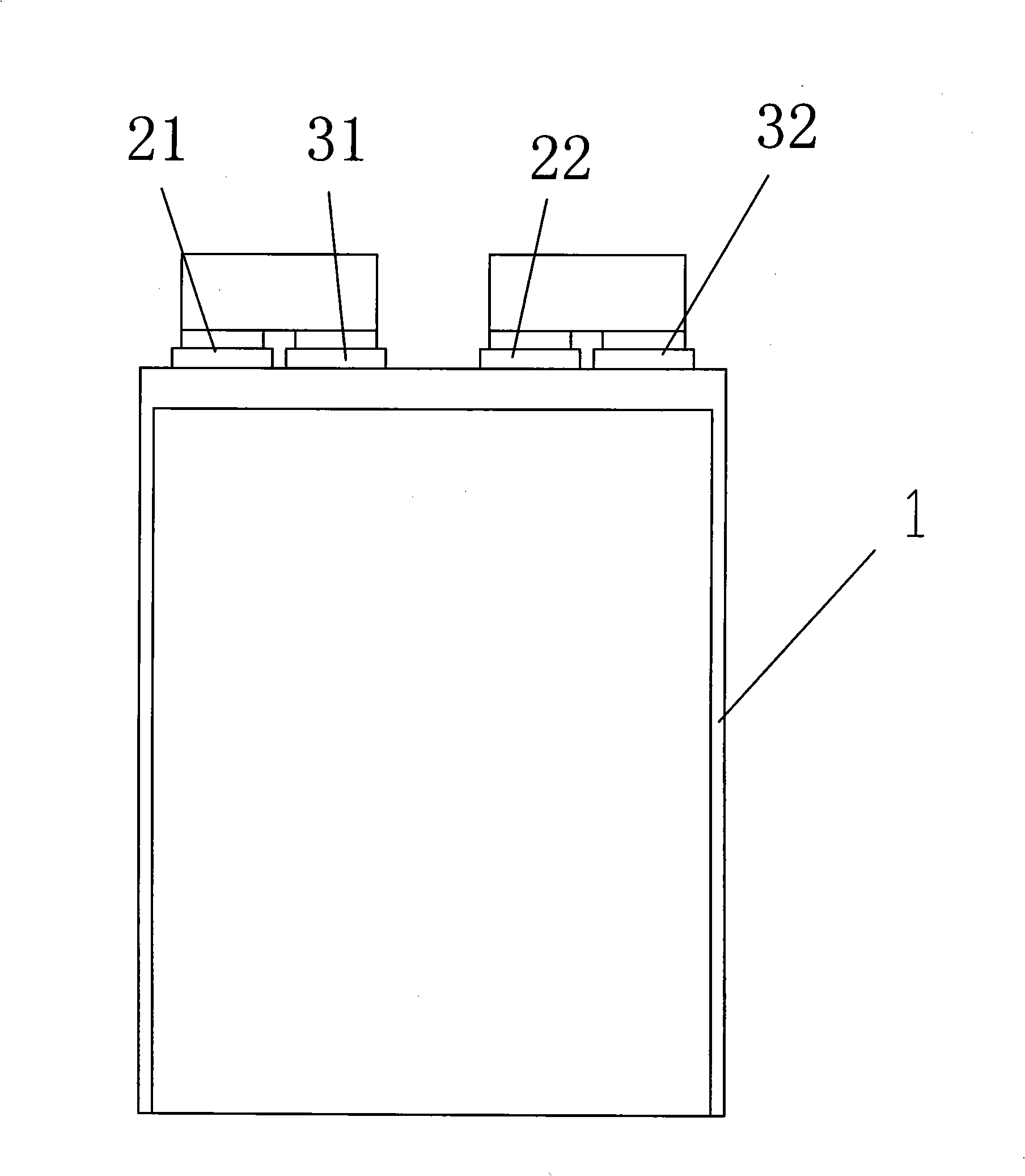
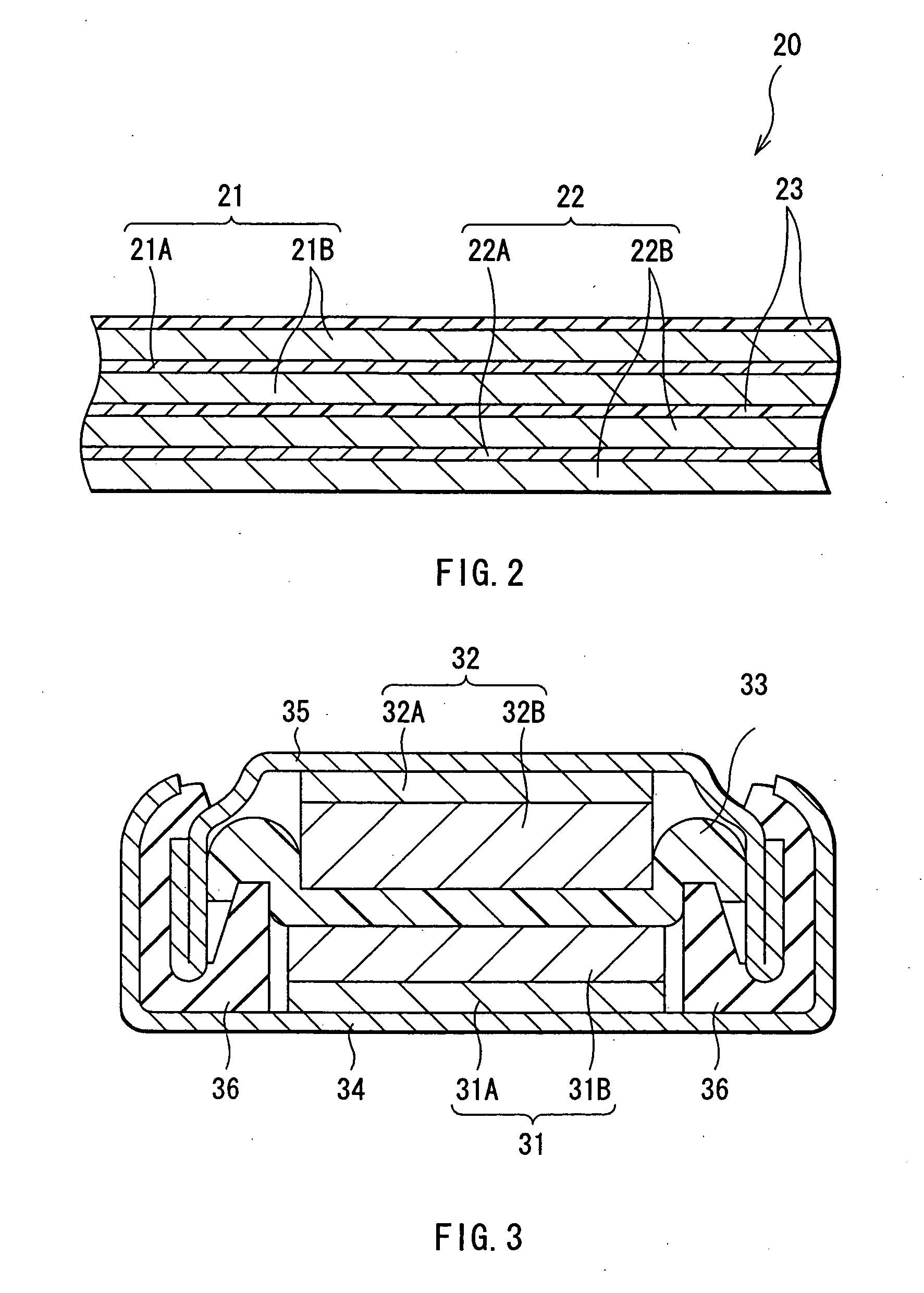
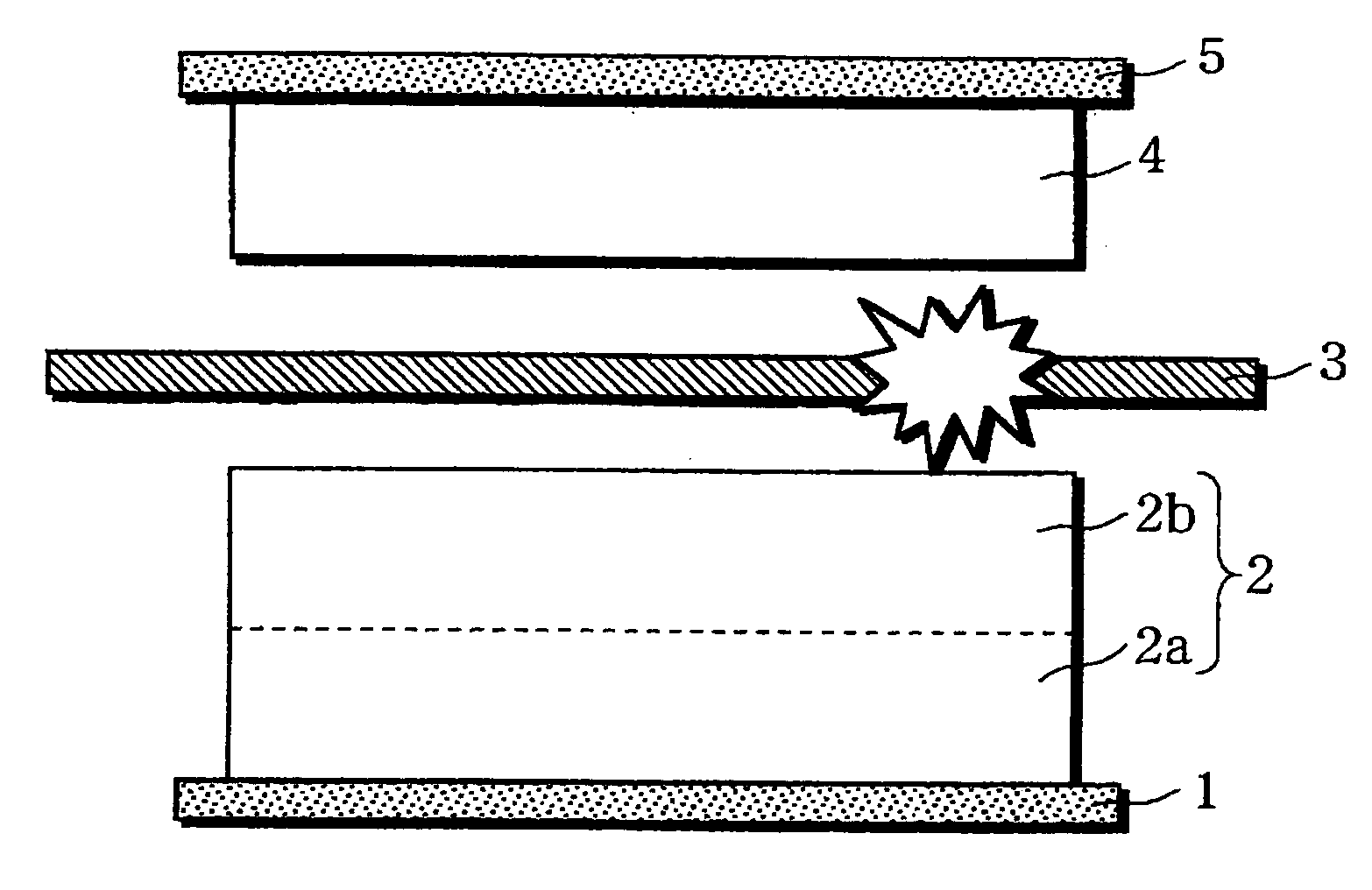
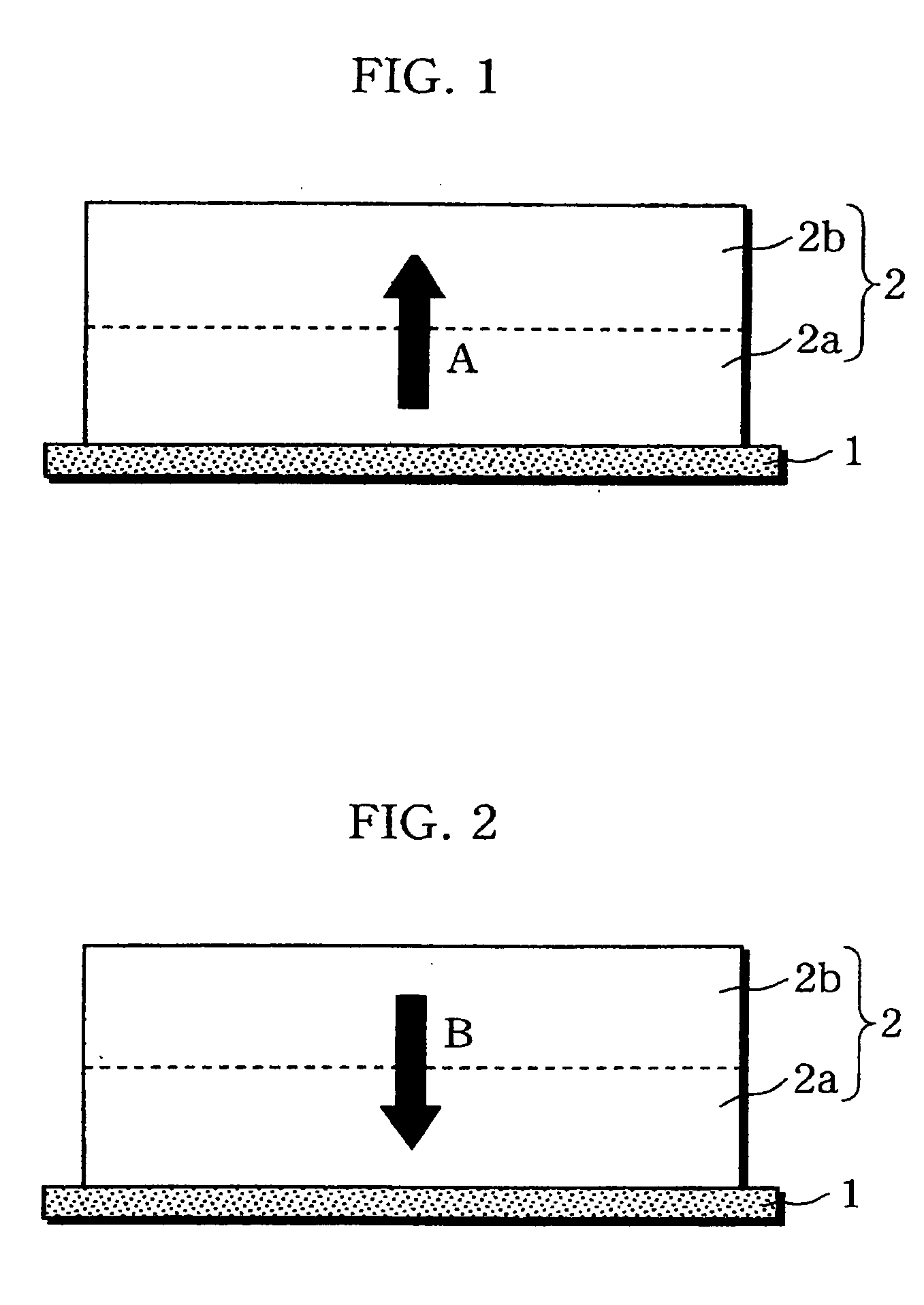
InactiveUS20060019151A1Avoid depositionIncrease probabilityFinal product manufactureCell temperature controlSpinelEngineering
A non-aqueous electrolyte battery is provided that is capable of improving safety, particularly the tolerance of the battery to overcharging, without compromising conventional battery constructions considerably. A non-aqueous electrolyte battery is furnished with a positive electrode including a positive electrode active material-layer (2) containing a plurality of positive electrode active materials and being formed on a surface of a positive electrode current collector (1), a negative electrode including a negative electrode active material layer (4), and a separator (3) interposed between the electrodes. The positive electrode active material-layer (2) is composed of two layers (2a) and (2b) having different positive electrode active materials, and of the two layers (2a) and (2b), the layer (2b) that is nearer the positive electrode current collector contains, as its main active material, a spinel-type lithium manganese oxide or an olivine-type lithium phosphate compound.
Owner:SANYO ELECTRIC CO LTD
Iron lithium phosphate material used for lithium ion battery and its manufacturing method
InactiveCN1753216AReduce contentAvoid puffinessElectrode manufacturing processesLithium compoundsSodium-ion batteryMetal fibers
The invention relates to a LiFePO4 material for Li-ion battery and the making method thereof. The method controls diameter and length of C or metallic fiber at 100-300 nm and 10-20 mum, respectively and dopes ions with valences greater than +2-+4 in Li situ of LiFePO4, in the raw material composed of Li source, ion-doped compound, trivalent Fe source, phosphate radical, charcoal material, and C / metallic fiber, so as to make the LiFePO4 material, largely reducing the charcoal material content of the made LiFePO4 material on the premise of not reducing electron conductivity; and because the specific surface area of the charcoal material is selected at 30-80 sq m / g, effectively raising the tap density of material so as to bring a great convenience to the follow-up electrode smearing process. And the prepared LiFePO4 material has low cost and simple operating process, easy to large-scale production.
Owner:CHINA ELECTRONIC TECH GRP CORP NO 18 RES INST
Method for preparing spherical ferric lithium phosphate by oxidation control crystal-carbon thermal reduction method
InactiveCN101337666AWide variety of sourcesLow costCell electrodesPhosphorus compoundsElectric dischargePhosphoric acid
The invention discloses a method for preparing spherical lithium iron phosphate by adopting oxidation control crystallization-carbon thermal reduction. The preparation method comprises the steps of conducting reaction among a mixed water solution of bivalence malysite and phosphoric acid, an aqueous solution of ammonia and an oxidant; synthesizing a spherical precursor of a spherical hydration iron phosphate through the process of oxidation control crystallization; washing; drying; pre-burning for dewatering; mixing with lithium carbonate and a carbon source; and obtaining the lithium iron phosphate through high temperature carbon thermal reduction under the protection of inertial or reducing atmosphere. By using the preparation method, the spherical lithium iron phosphate, an anode material of a lithium ion battery, is prepared, and has high bulk density and high volume specific capacity. The average grain size of the spherical lithium iron phosphate is between 6 and 8 mum, the electric discharge specific capacity thereof for the first time can reach up to 145-150mAh / g at the room temperature of 0.5C.
Owner:TSINGHUA UNIV
Methods of making low-shrink polypropylene fibers
InactiveUS6656404B2Monocomponent polypropylene artificial filamentWoven fabricsPhosphateLITHIUM PHOSPHATE
Improved polypropylene fibers exhibiting greatly reduced heat- and moisture-shrink problems and including certain compounds that quickly and effectively provide rigidity to the target polypropylene fiber after heat-setting are disclosed herein. In such a manner, the "rigidifying" compounds provide nucleation sites for polypropylene crystal growth. After drawing the nucleated composition into fiber form, the fiber is then exposed to sufficient heat to grow the crystalline network, thus holding the fiber in a desired position. The preferred "rigidifying" compounds include dibenzylidene sorbitol based compounds, as well as less preferred compounds, such as sodium beuzoate, certain sodium and lithium phosphate salts (such as sodium 2,2'-methylene-bis-(4,6-di-tert-butylphenyl)phosphate, otherwise known as NA-11).
Owner:MILLIKEN & CO
Non-aqueous electrolyte battery
InactiveUS20070254209A1Good benefitImprove stabilityCell seperators/membranes/diaphragms/spacersFinal product manufactureElemental compositionInorganic particle
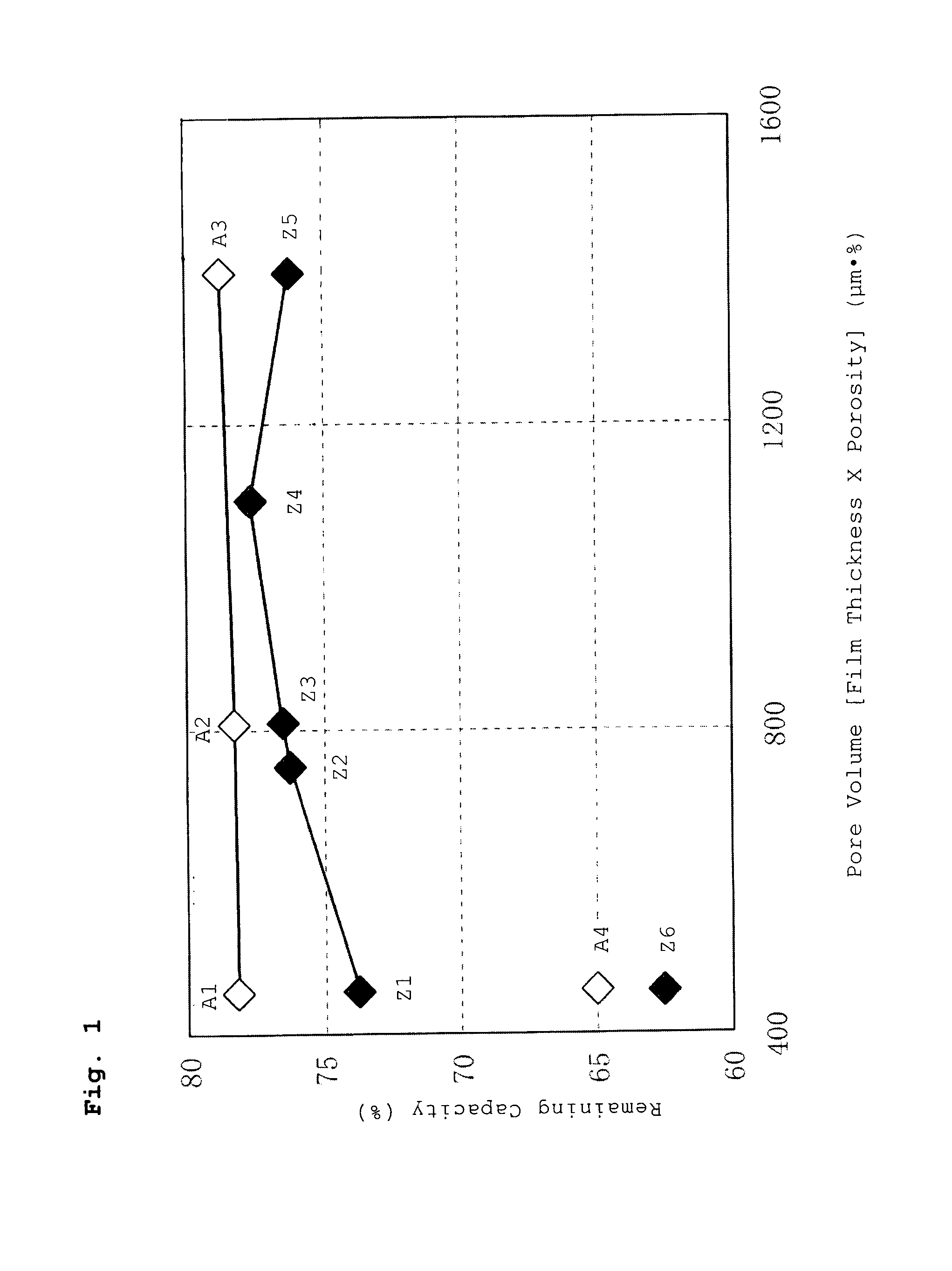
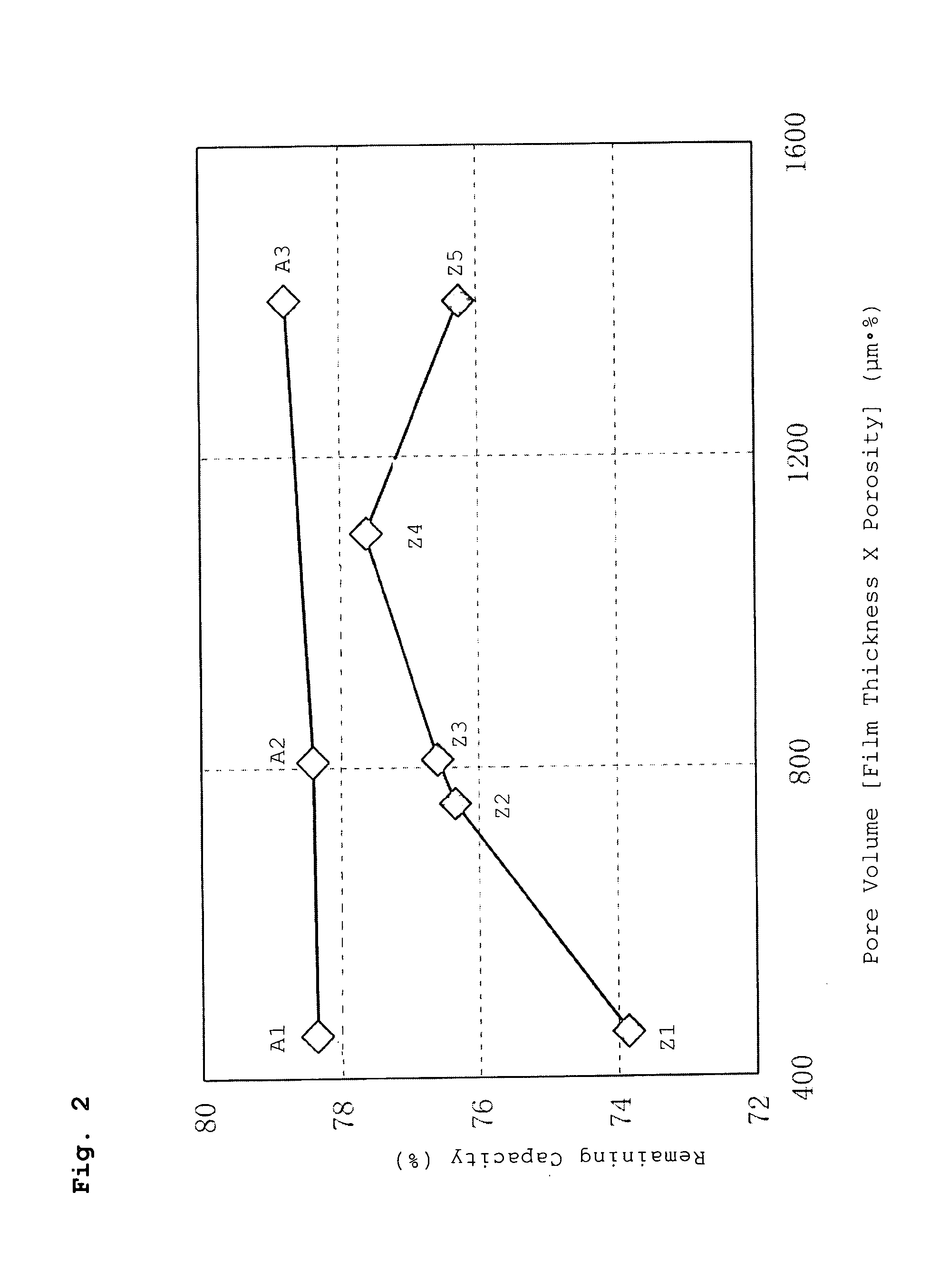
A battery has a positive electrode active material containing an olivine lithium phosphate-based compound having an elemental composition represented as LiMPO4, where M is a transition metal including at least Fe. The product of a separator thickness x (μm) and a separator porosity y (%) is controlled to be equal to or less than 1500 (μm·%). A porous layer containing inorganic particles and a binder is disposed between the separator and the positive electrode and / or between the separator and the negative electrode.
Owner:SANYO ELECTRIC CO LTD
Process for producing iron phosphate for producing iron lithium phosphate material
The preparation method of iron phosphate used for preparing lithium iron phosphate material in the present invention relates to heavy metal phosphate, and the steps are: dissolving analytically pure soluble iron salt in distilled water to prepare a 0.05-5M aqueous solution, and the added mass is the iron salt mass 0.01~3% of anionic surfactant, then add analytically pure phosphoric acid according to the molar ratio of Fe3+:PO43-=1:0.8~1.2 and stir evenly, and slowly add alkaline solution with a concentration of 1~9M under stirring , the feeding time is more than 1 hour, until the pH value of the solution reaches 6-7, the iron phosphate precipitate is filtered, and the filtered iron phosphate is washed 3-5 times with distilled water 2-5 times its weight. Dry at ~90°C to obtain the product FePO4·2H2O powder. The iron phosphate product with two crystal waters prepared by the method of the invention has high reactivity, and the performance of the lithium iron phosphate material made by using it is better than that of the lithium iron phosphate material made by commercially available iron phosphate products.
Owner:HEBEI UNIV OF TECH
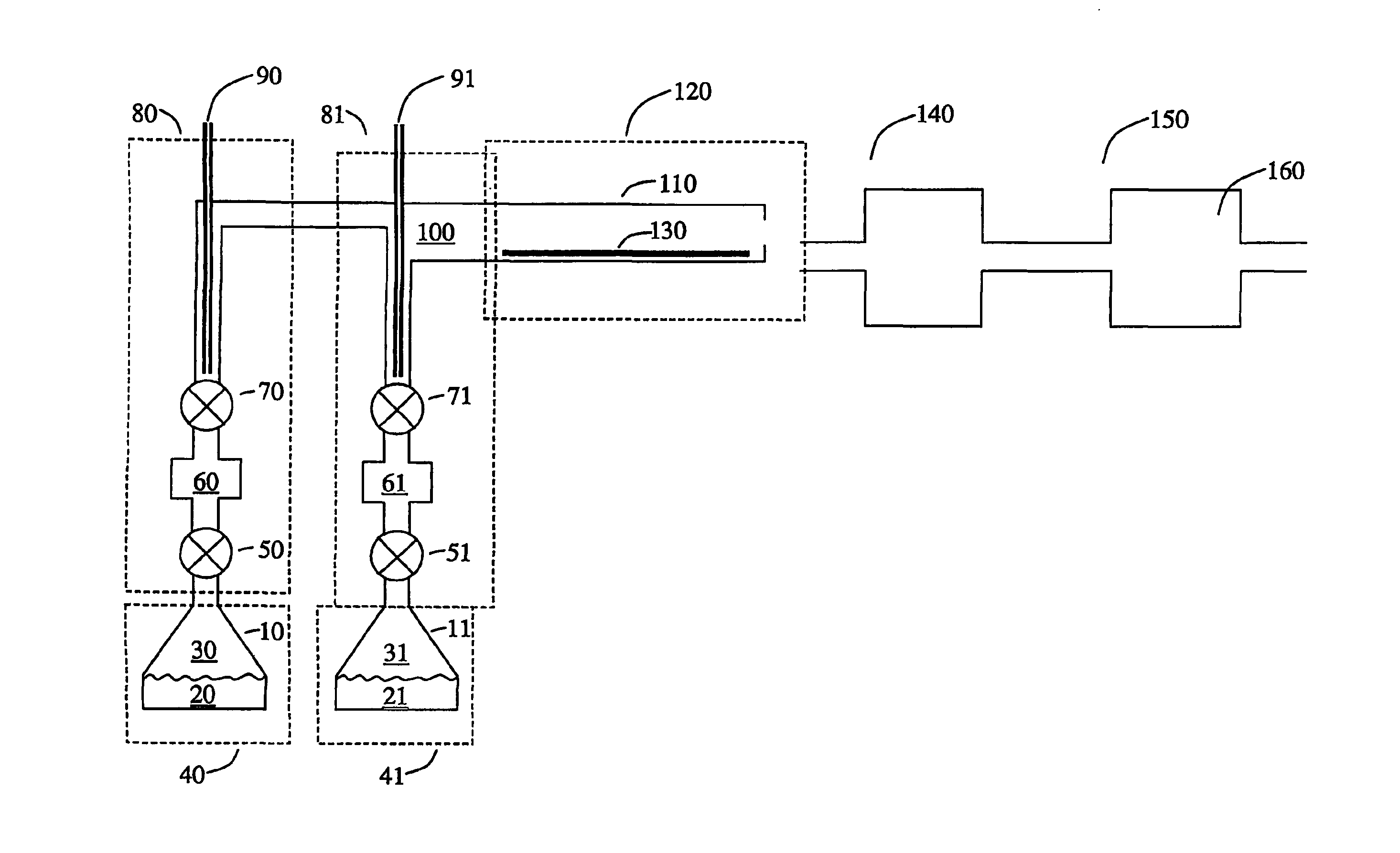
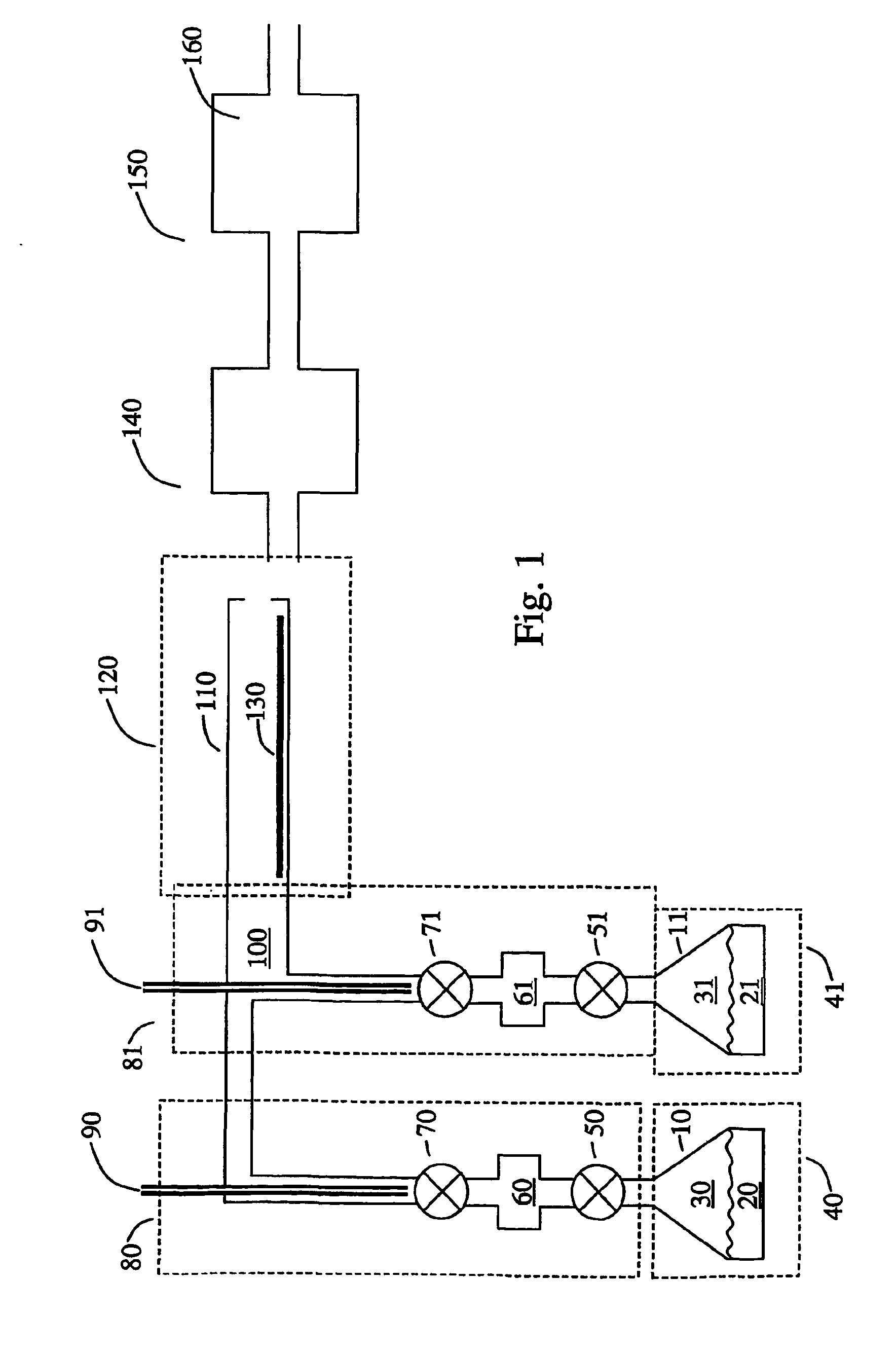
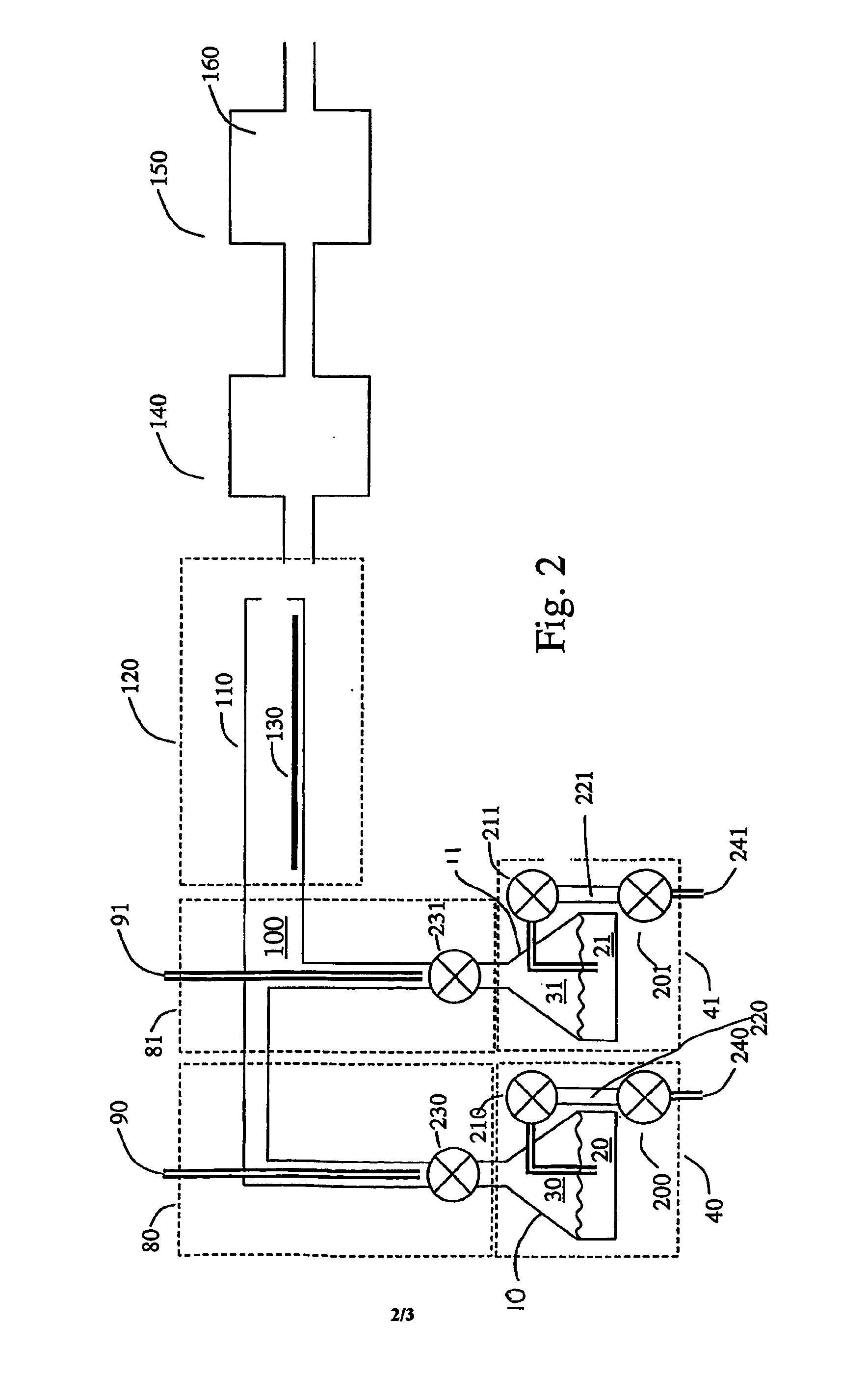
Method and system for estimating state of charge of a lithium electrochemical cell having a lithium phosphate type positive electrode
ActiveUS20130229154A1Accurately determinedPrevent over-chargingBatteries circuit arrangementsCell electrodesCurrent sensorPhosphoric acid
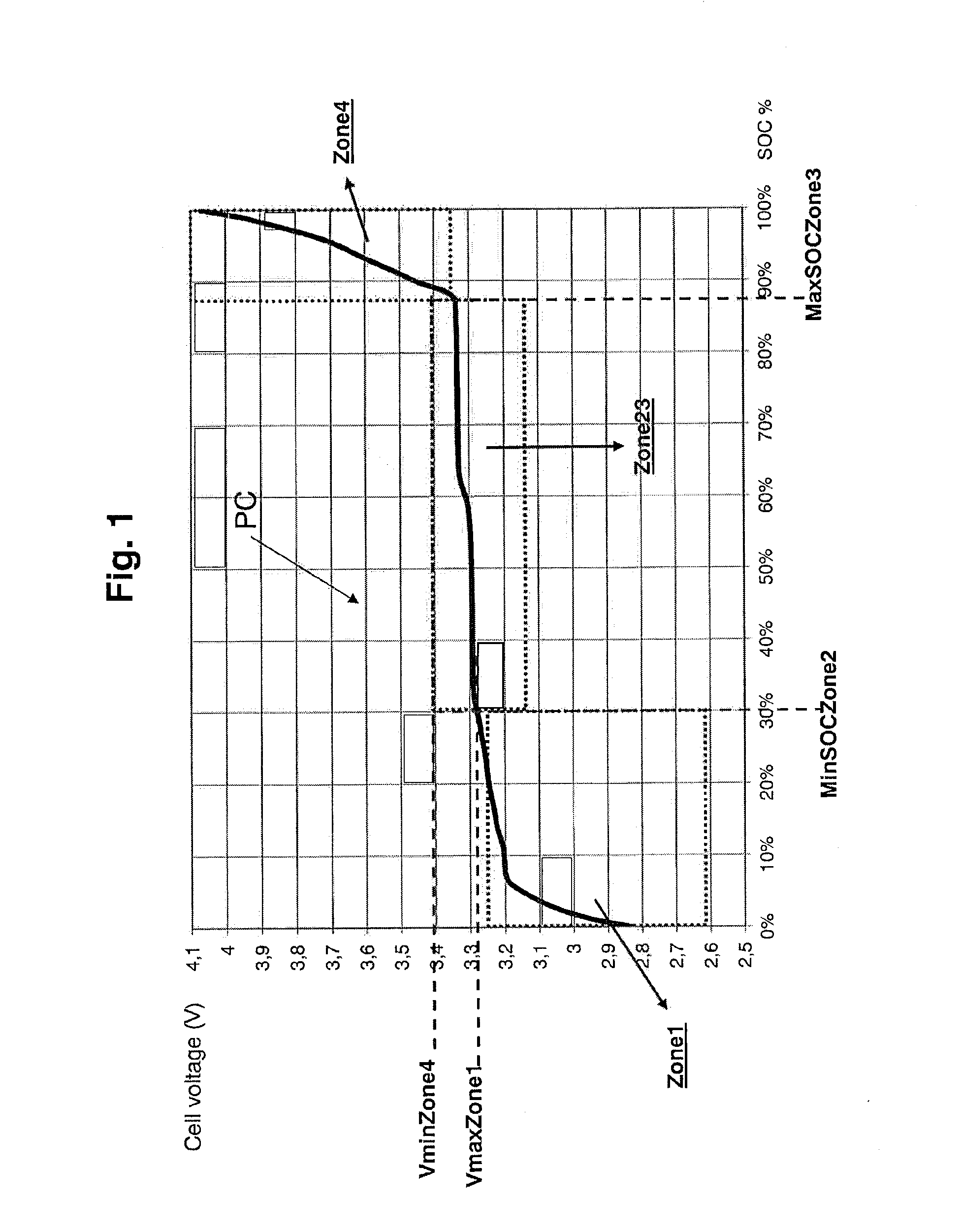
A method and system are provided for estimating state of charge of a cell having a positive electrode, for example of a lithium phosphate type, the charge profile of which includes a range of state of charge between about 30 and about 90% in which variation of voltage is 10 times less rapid than for a state of charge higher than 90%. Determination of state of charge for a state of charge greater than 90% is based on either the use of a calibration relationship between cell voltage and state of charge when a current is passing through the cell that is less than a predetermined threshold value and its voltage has stabilized, or on the use of coulometry the result of which is corrected taking into account the accuracy of the current sensor.
Owner:SAFT GRP SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com