Graphite alkene iron lithium phosphate positive active material, preparing method thereof, and lithium ion twice battery based on the graphite alkene modified iron lithium phosphate positive active material
A cathode active material, graphene modification technology, applied in secondary batteries, lithium batteries, battery electrodes, etc., can solve the problems of low conductivity, limited direct application, etc., to achieve good conductivity, excellent cycle stability, high The effect of rate charge and discharge performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
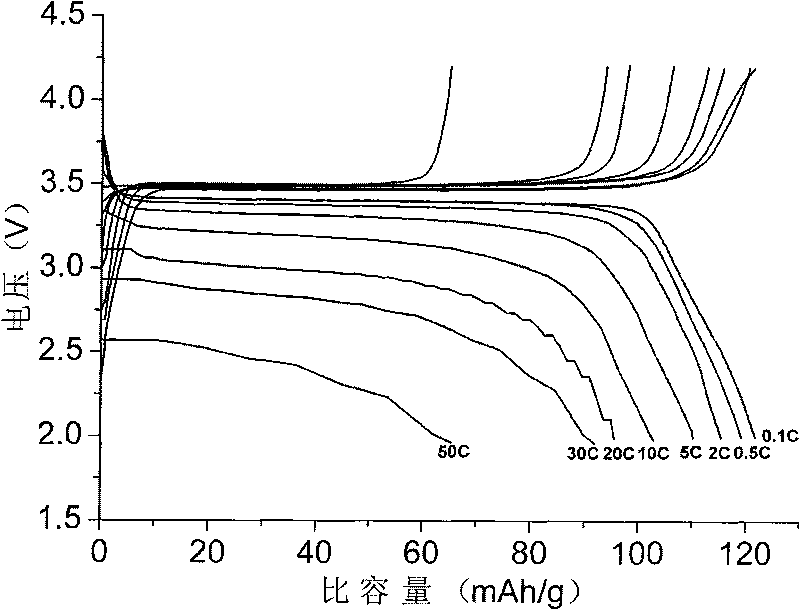
Image
Examples
preparation example Construction
[0025] The first step, the preparation of graphene
[0026] Graphite is placed in a strong oxidant and stirred for 2-8 hours under the heat of reaction to obtain graphite oxide. Washing with water to pH=4-5, exfoliating graphite oxide single layer by stirring, shaking, ultrasonic and other means to obtain graphene oxide. Graphene is then obtained by reducing graphene oxide. Wherein the strong oxidant can be a mixed system of potassium permanganate, concentrated sulfuric acid, potassium nitrate, or a mixed system of fuming nitric acid and sodium chlorate (or potassium chlorate), or a mixed system of fuming nitric acid, concentrated sulfuric acid and sodium chlorate (or potassium chlorate) mixed system. The reduction can be realized by using a water-soluble strong reducing agent (such as hydrazine hydrate, sodium borohydride, etc.) in the aqueous phase, or by high-temperature annealing.
[0027] The second step, the preparation of lithium iron phosphate
[0028] Preparation ...
Embodiment 1
[0038] In the first step, 1.2 g of potassium nitrate was weighed, added to 46 ml of concentrated sulfuric acid (96-98wt.%), 1.0 g of graphite was added thereto, and after mixing evenly, 6.0 g of potassium permanganate was slowly added under stirring. The system was then heated to 40°C and stirring was continued for 6 hours. Then 80 ml of water was slowly added dropwise, while the temperature of the system was raised to 70° C. and kept stirring for 30 minutes. Add 200ml of water and 6ml of hydrogen peroxide (30%), and stop stirring after 5 minutes. After the graphite oxide particles settle, remove the supernatant. Add water to the primary product and wash it several times until the pH value of the system reaches about 5 to obtain a pure graphite oxide mother liquor. The graphite oxide mother liquor was ultrasonically treated for 2 hours to obtain a single-layer exfoliated graphene oxide sol.
[0039] In the second step, according to the stoichiometric ratio, a certain amount...
Embodiment 2
[0043] The first step is the same as the first step in Example 1, and a graphene oxide sol is prepared.
[0044] In the second step, ferrous salt (such as ferrous oxalate), lithium salt (such as lithium chloride) and phosphorus source (such as ammonium dihydrogen phosphate) are dissolved in the aqueous solution according to the stoichiometric ratio, and stirred at room temperature until it is a uniform sol, Elevate the temperature and age until it becomes a gel. After drying, it is annealed at high temperature (400-700° C.) and protected by argon for 4-20 hours to obtain a lithium iron phosphate material.
[0045] Subsequent steps are the same as the third and fourth steps in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com