Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2729 results about "Polymer coating" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical devices having durable and lubricious polymeric coating
A medical device having a contact surface exposed repeatedly to bodily tissue is disclosed. The contact surface is coated with a silicone polymer and one or more non-silicone hydrophobic polymers. The preferred medical device is a surgical needle, and the preferred coating is a polydimethylsiloxane and polypropylene wax hydrocarbon mixture. The incorporation of the non-silicone hydrophobic polymer increases the durability of the coating on the device without sacrificing lubricity.
Owner:ETHICON INC
Controlled release bioactive agent delivery device
InactiveUS20050019371A1Minimize damageInterference minimizationOrganic active ingredientsSenses disorderControlled releaseMeth-
Owner:SURMODICS INC
Apparatus and methods for plasma enhanced chemical vapor deposition of polymer coatings
Apparatuses and methods are described that involve the deposition of polymer coatings on substrates. The polymer coatings generally comprise an electrically insulating layer and / or a hydrophobic layer. The hydrophobic layer can comprise fused polymer particles have an average primary particle diameter on the nanometer to micrometer scale. The polymer coatings are deposited on substrates using specifically adapted plasma enhanced chemical vapor deposition approaches. The substrates can include computing devices and fabrics.
Owner:LIQUIPEL IP
Organic/inorganic composite porous film and electrochemical device prepared thereby
ActiveUS20060046149A1Improve thermal safetySevere heat shrinkingLiquid surface applicatorsFinal product manufactureElectrochemistryPolymer coating
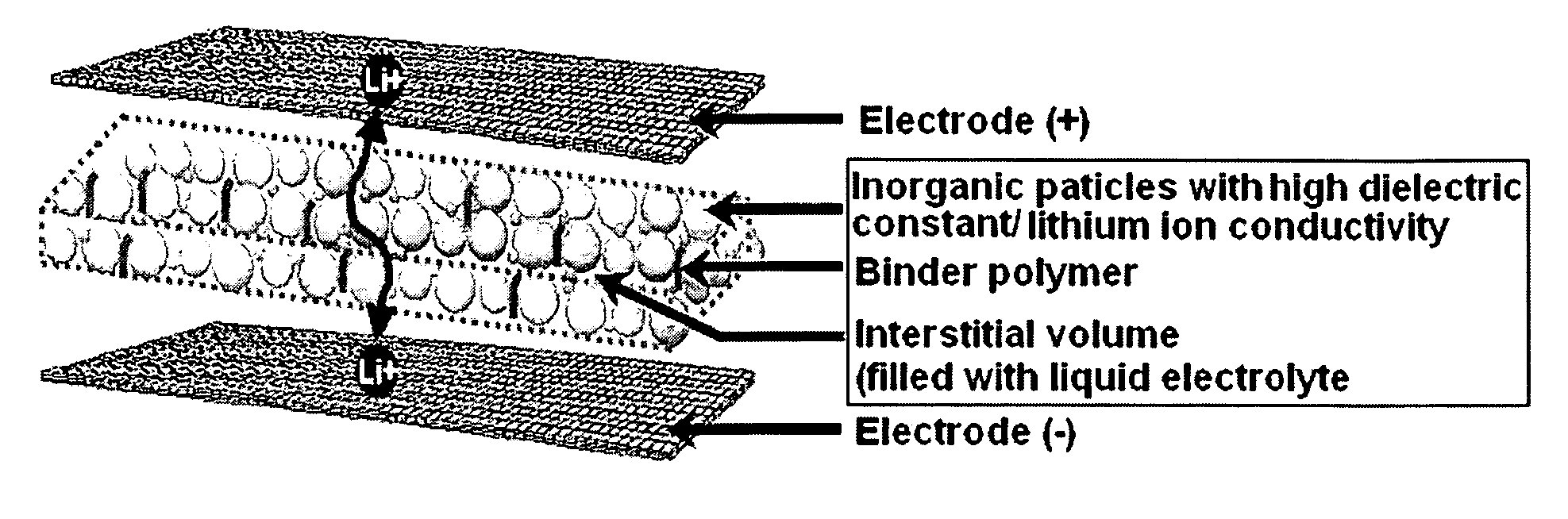
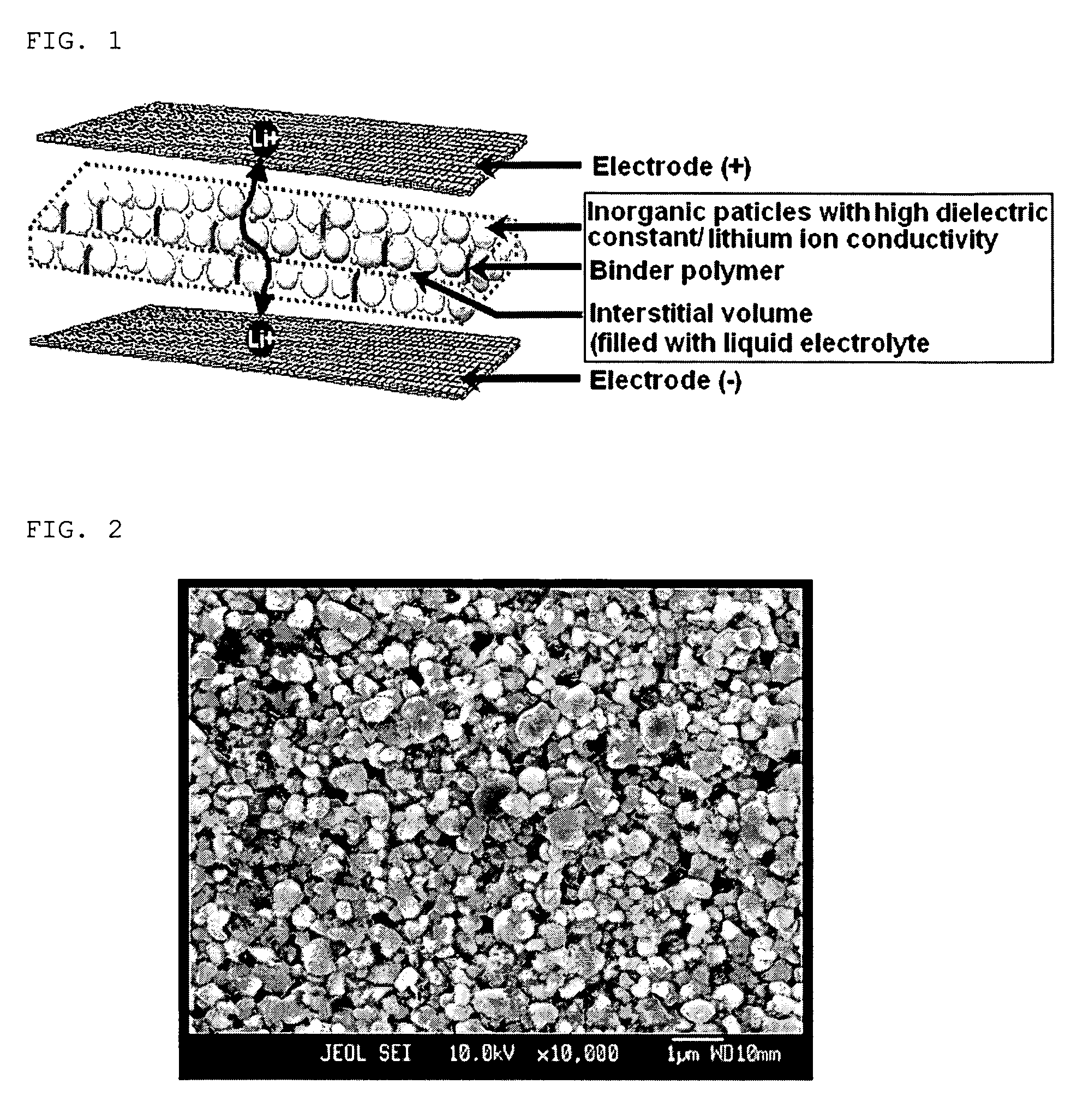
Disclosed is an organic / inorganic composite porous film comprising: (a) inorganic particles; and (b) a binder polymer coating layer formed partially or totally on surfaces of the inorganic particles, wherein the inorganic particles are interconnected among themselves and are fixed by the binder polymer, and interstitial volumes among the inorganic particles form a micropore structure. A method for manufacturing the same film and an electrochemical device including the same film are also disclosed. An electrochemical device comprising the organic / inorganic composite porous film shows improved safety and quality.
Owner:TORAY BATTERY SEPARATOR FILM +1
Polymer coating for medical devices
Coatings are provided in which surfaces may be activated by covalently bonding a silane derivative to the metal surface, covalently bonding a lactone polymer to the silane derivative by in situ ring opening polymerization, and depositing at least one layer of a polyester on the bonded lactone. Biologically active agents may be deposited with the polyester layers. Such coated surfaces may be useful in medical devices, in particular stents.
Owner:CV THERAPEUTICS INC
Medical device for delivery of a biologically active material to a lumen
An apparatus for delivery of biologically active materials comprises a catheter and balloon having micro-needles or pores. In the apparatus, the balloon can have a polymer coating containing the biologically active material, and the apparatus can include a sheath surrounding the balloon. In one embodiment the biologically active material is delivered through lumens in the micro-needles. Another embodiment of the invention is an apparatus for delivery of biologically active materials comprising a catheter with a balloon disposed thereon and a shockwave generator for producing a shockwave for delivering the biologically active material to a body lumen. Methods for delivery of biologically active materials are also disclosed.
Owner:BOSTON SCI SCIMED INC
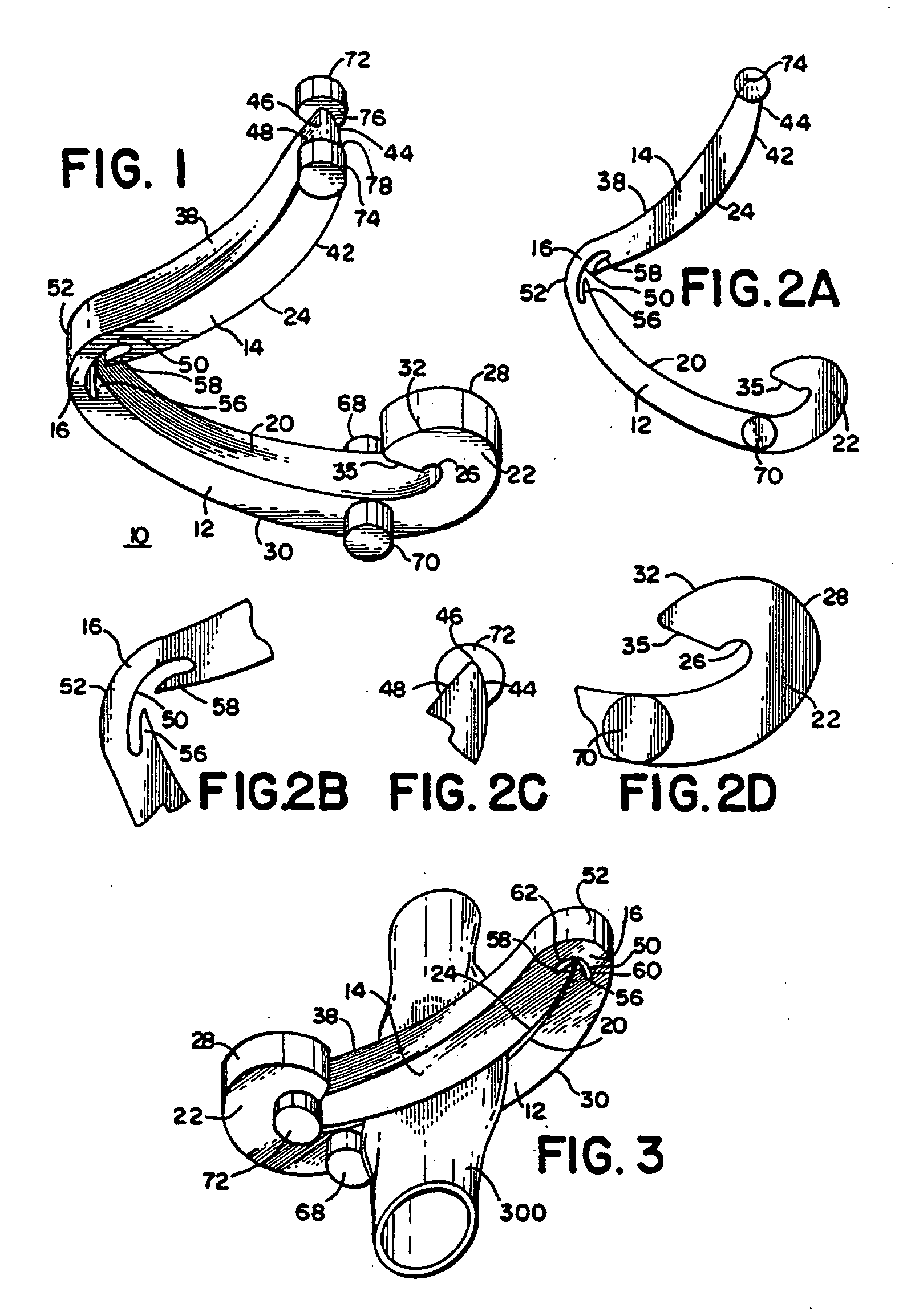
Coated ligating clip
A polymeric, surgical clip having first and second curved leg members joined at their proximal end by a hinge portion and movable from an open position to a closed position for clamping a vessel between curved opposing inner surfaces. The clip includes a coating on its exterior which reduces the friction between the leg members and complementary locking mechanisms disposed at the distal end portions of the leg members, and stabilizes closing of the clip. The coating can be any coating, including a polymer coating such as one which includes polytetrafluoroethylene. The coating may be applied using a solvent such as HFC43-10.
Owner:PILLING WECK INC
Stents and methods for preparing stents from wires having hydrogel coating layers thereon
InactiveUS20050251250A1Improved propertyReduce surface roughnessStentsSurgeryActive agentInsertion stent
Radially expandable stents having hydrogel coating layers thereon, and methods of preparing such stents are disclosed. The methods include coating a wire with a solution that includes a solvent and a water soluble polymer in the solvent, evaporating the solvent to provide a polymeric coating on the wire, and crosslinking the polymeric coating to provide a hydrogel coating layer on the wire. The coated wire can be fabricated into stents, which preferably have substantially uniform coatings with low surface roughness. Preferably the coatings have hydrophilic properties and provide a biocompatible surface. The coatings may also provide for the delivery of biologically active agents into the body.
Owner:MEDTRONIC INC
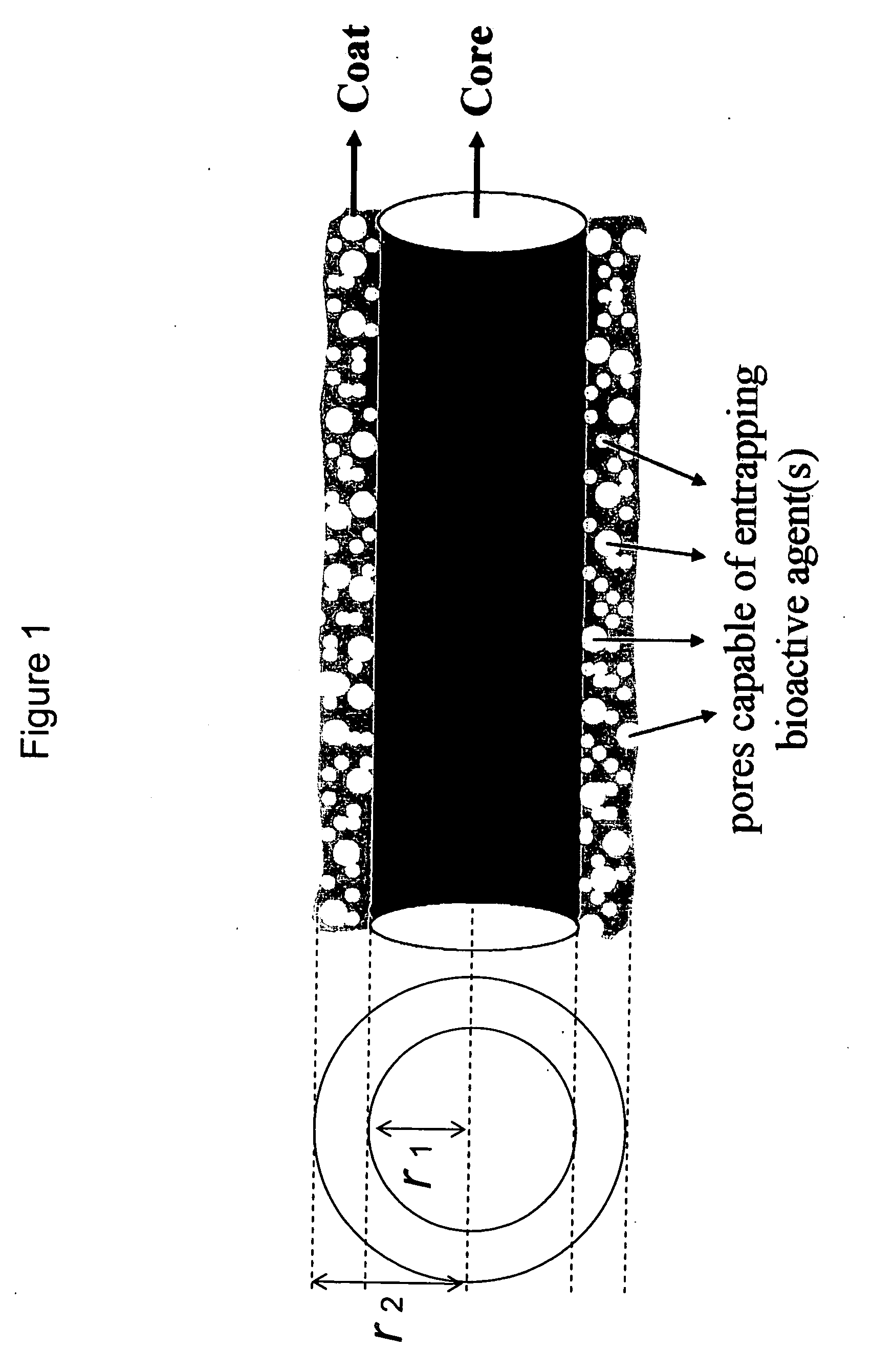
Drug-delivering composite structures
Composite structures composed of a fibril core and a polymeric coat and designed capable of encapsulating both hydrophobic and hydrophilic bioactive agents while retaining the activity of these agents are disclosed. Further disclosed are processes of preparing such composite structures, and medical devices and disposable articles made therefrom.
Owner:ZILBERMAN MEITAL
Nanoparticle-based controlled release polymer coatings for medical implants
InactiveUS20050095267A1Improve solubilityFacilitated releaseStentsSurgeryControlled releaseSolubility
Implantable medical devices having a nanoparticle coating applied thereon. The nanoparticle coating comprises nanopulverized antiproliferative compounds. More specifically, the nanoparticulate antiproliferative compounds comprise particles less than 500 nm in size. The nanoparticle size of the compounds improves compounds' solubility. In addition to improving the solubility of otherwise insoluable antiproliferative compounds, the nanoparticle size minimizes the preparation and formulation required to prepare a dose of the antiproliferative compounds.
Owner:MEDTRONIC VASCULAR INC
Hydrophobic biologically absorbable coatings for drug delivery devices and methods for fabricating the same
Owner:ABBOTT CARDIOVASCULAR
Methods and apparatus for treatment of aneurysms adjacent branch arteries
Owner:MEDTRONIC VASCULAR INC
Electrophoretic particles, and processes for the production thereof
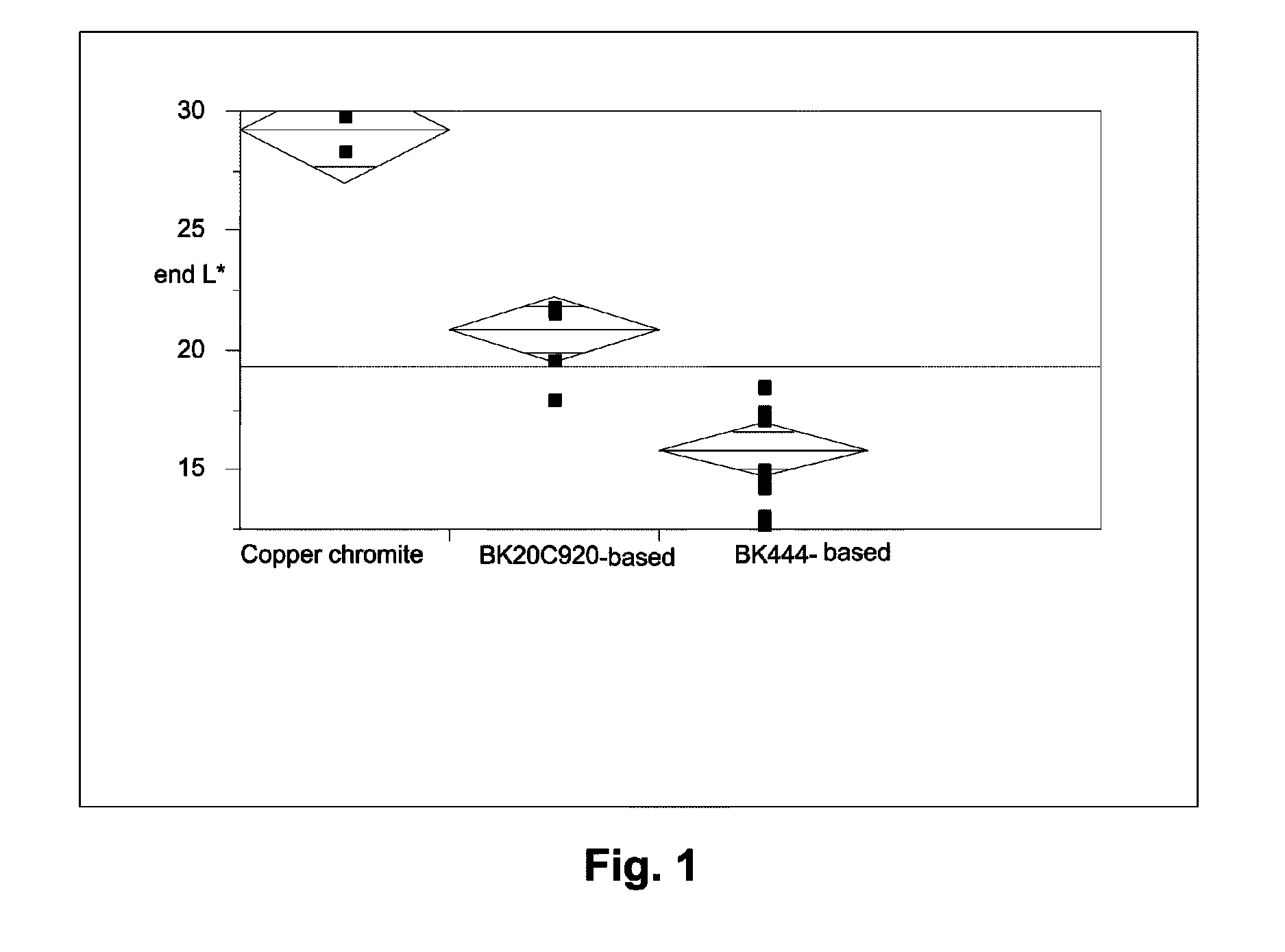
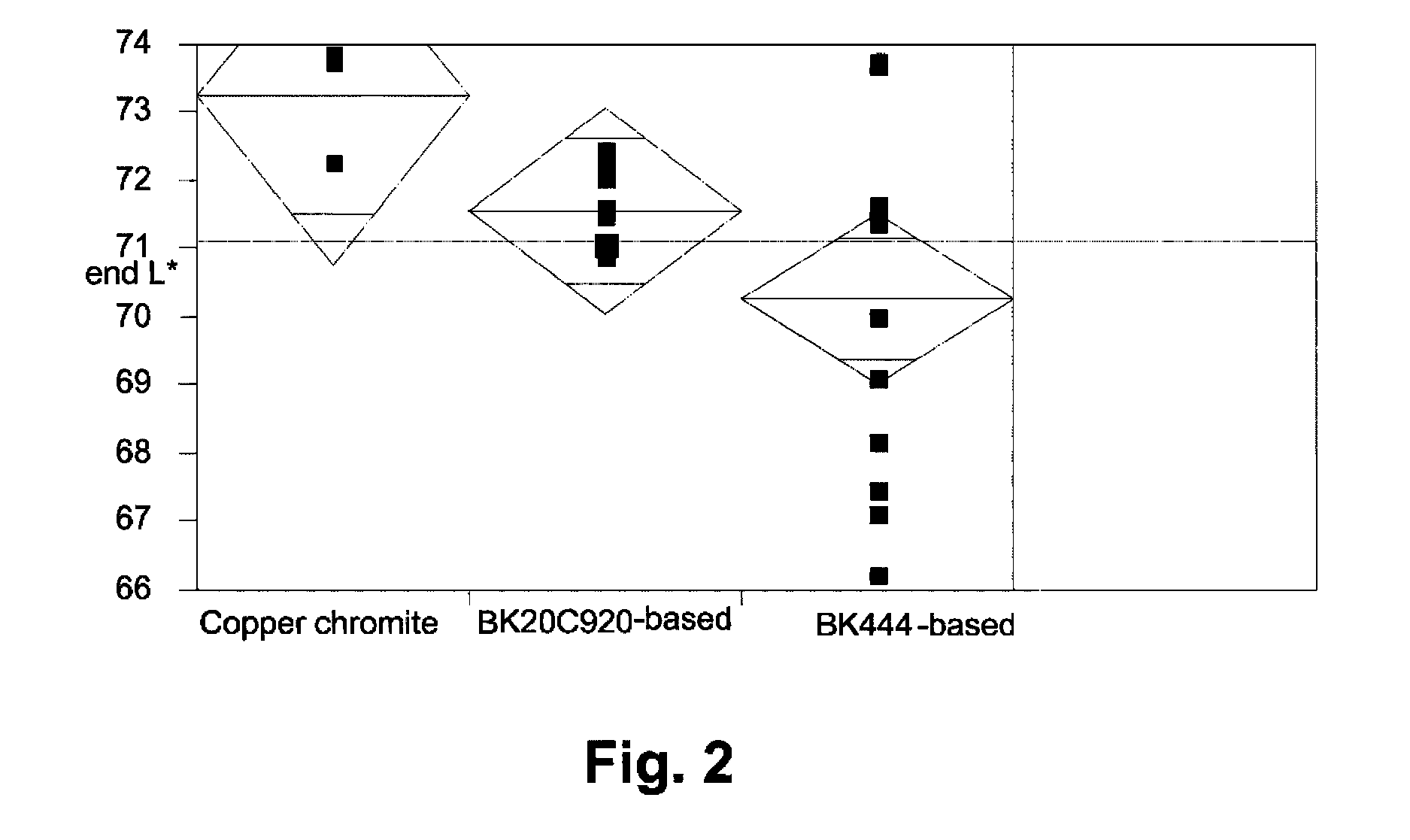
InactiveUS20050000813A1Volume/mass flow measurementFluid pressure measurement by electric/magnetic elementsElectrophoresisCopper chromite
Owner:E INK CORPORATION
Loading and release of water-insoluble drugs
Owner:BOSTON SCI SCIMED INC
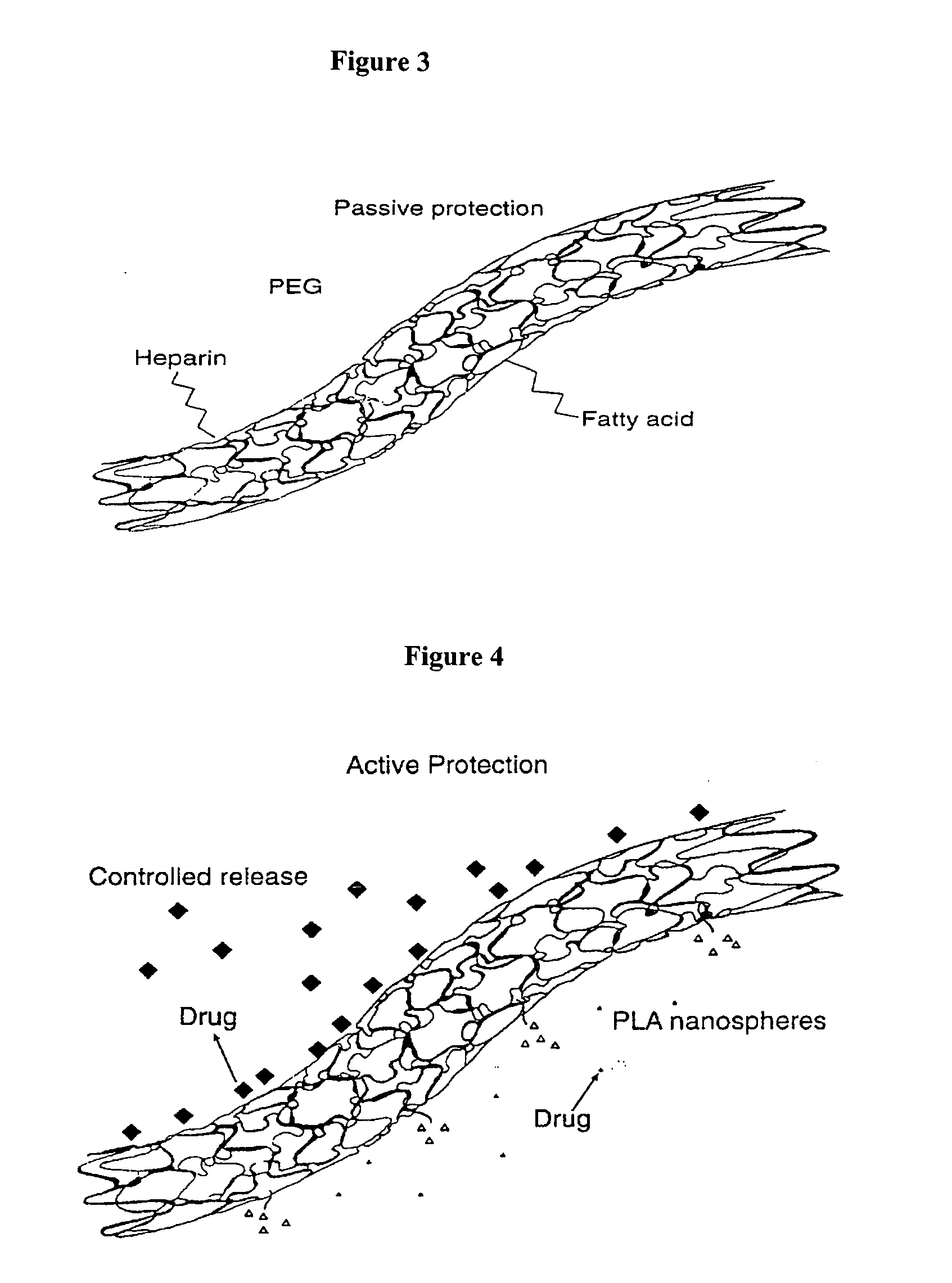
Biocompatible controlled release coatings for medical devices and related methods
ActiveUS20050084515A1High elasticity/ductilityResist erosionSurgeryMedical preparationsSolubilityBiocompatible coating
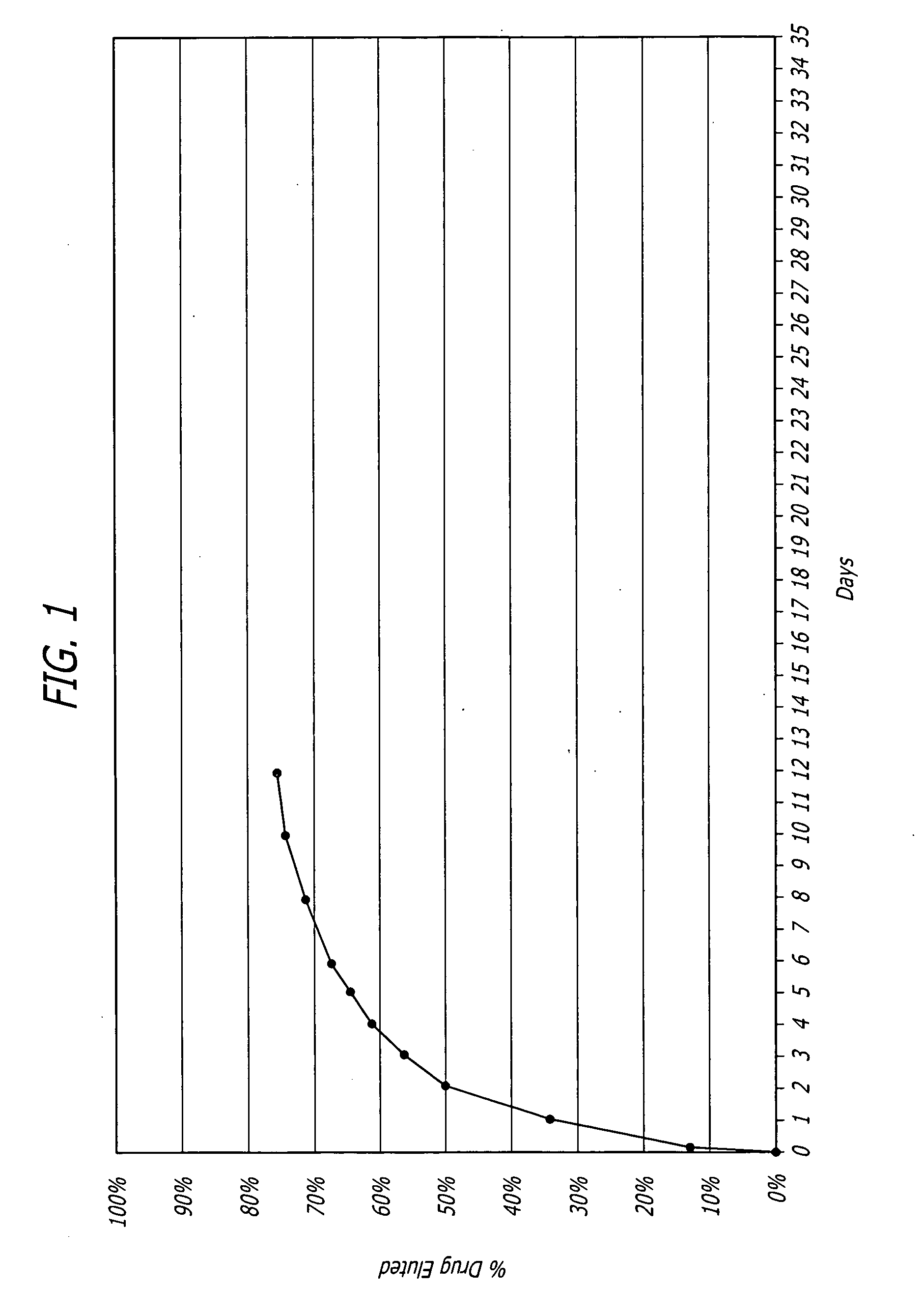
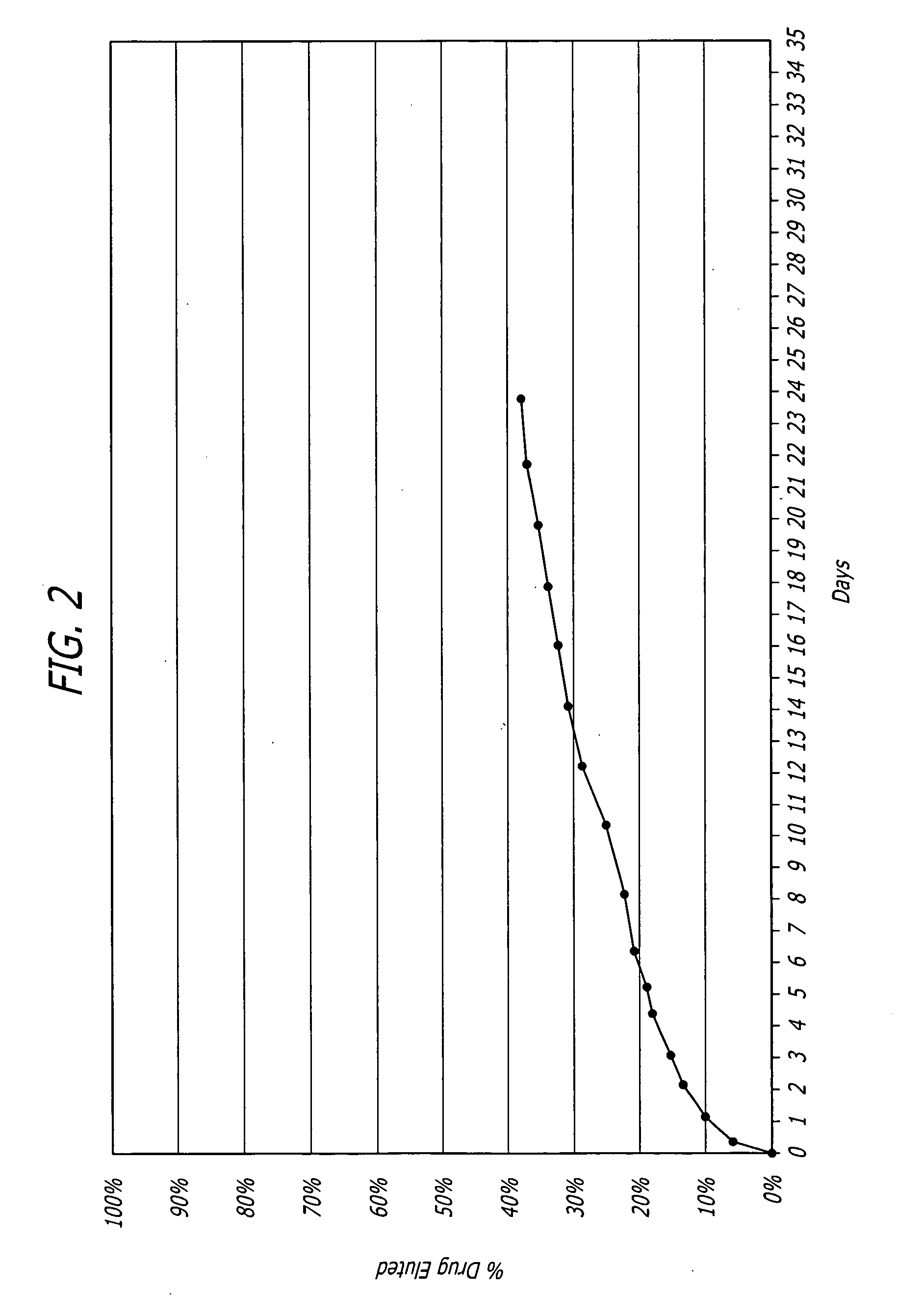
Biocompatible coatings for medical devices are disclosed. Specifically, polymer coatings designed to control the release of bioactive agents from medical devices in vivo are disclosed wherein the solubility parameters of polymers and drugs are closely matched to control elute rate profiles. The present application also discloses providing vascular stents with controlled release coatings and related methods for making these coatings.
Owner:MEDTRONIC VASCULAR INC
Controlled release bioactive agent delivery device
InactiveUS20050271706A1Minimizes damage interferenceRisks moreOrganic active ingredientsSenses disorderControlled releaseActive agent
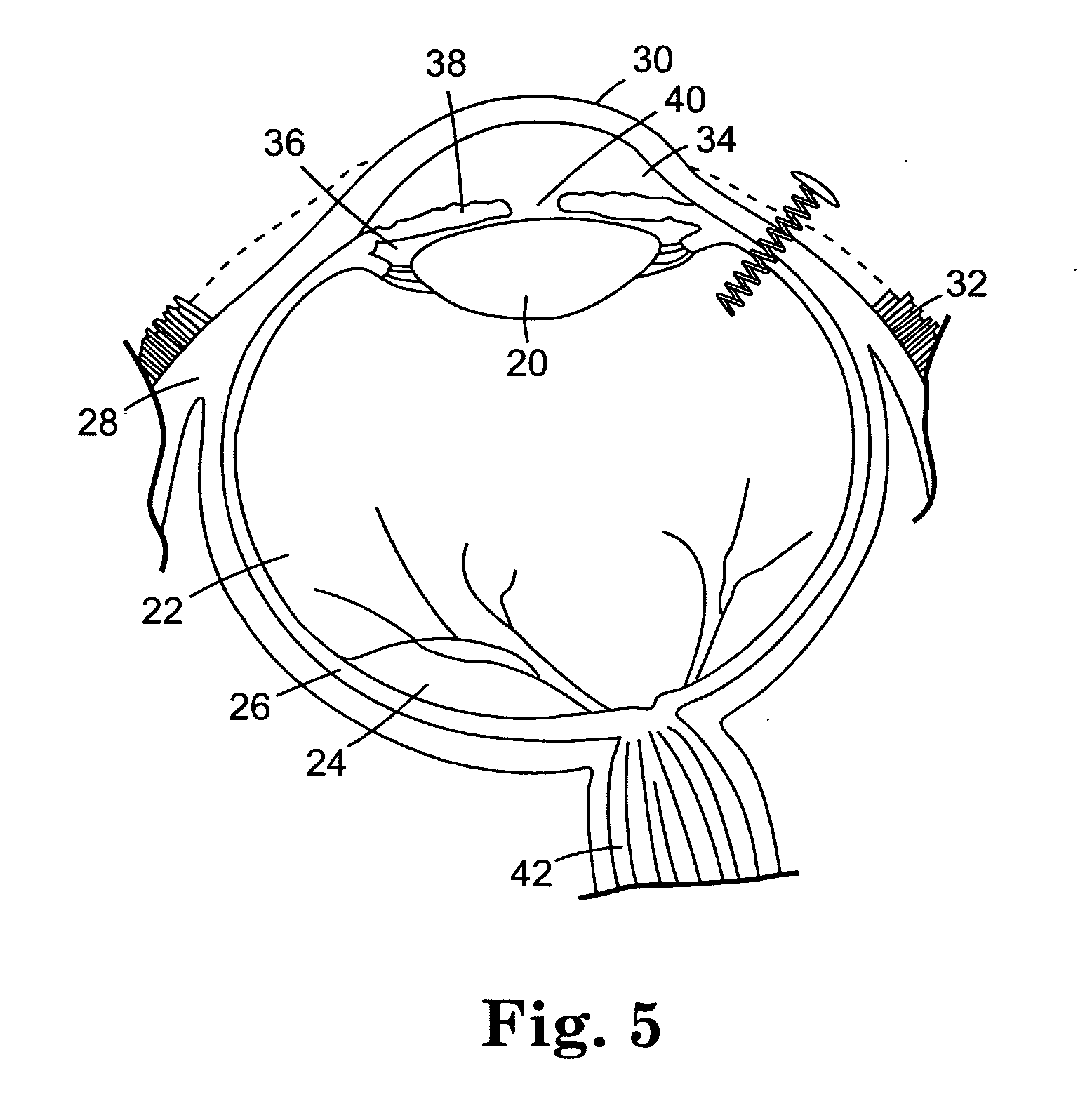
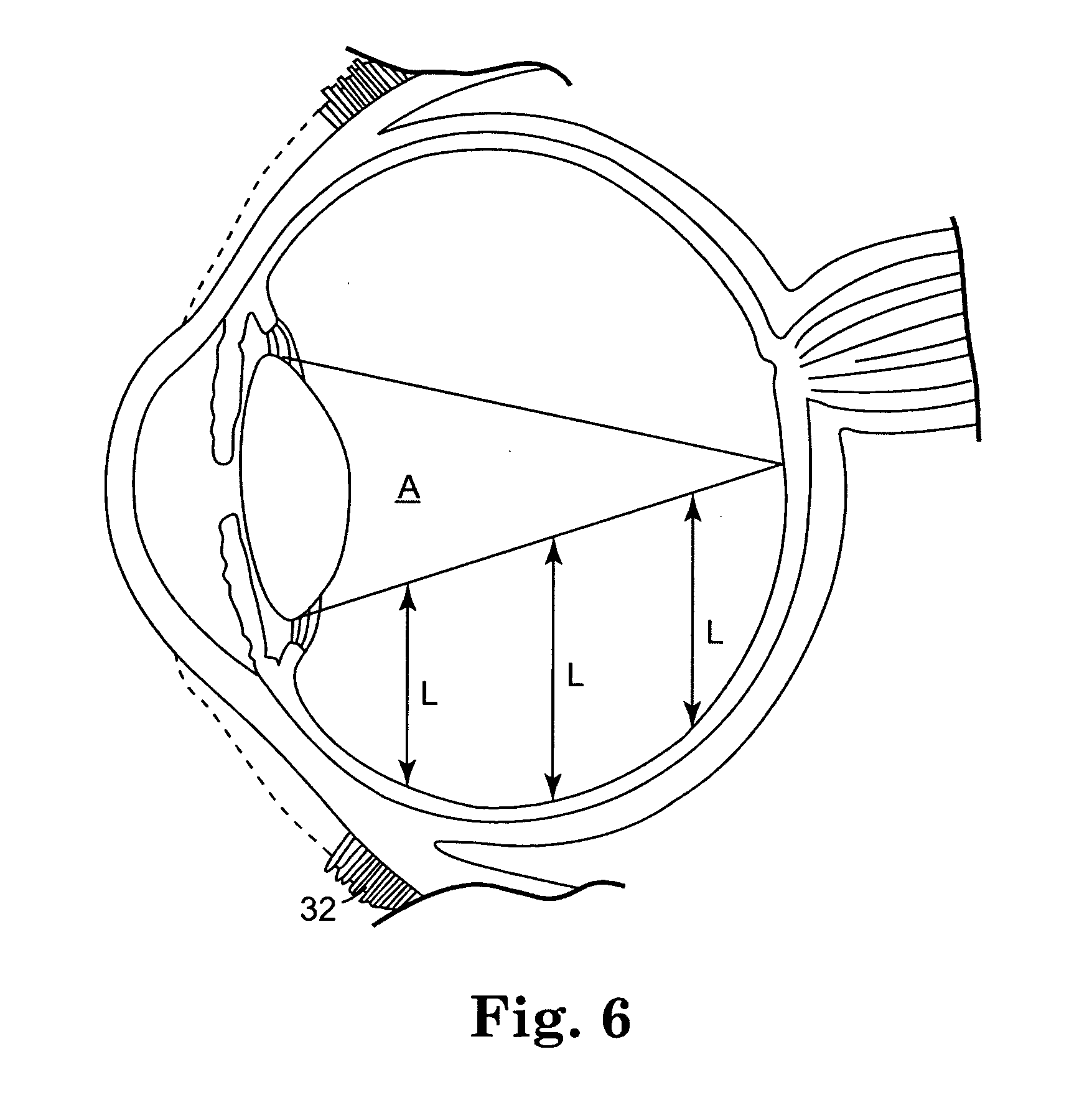
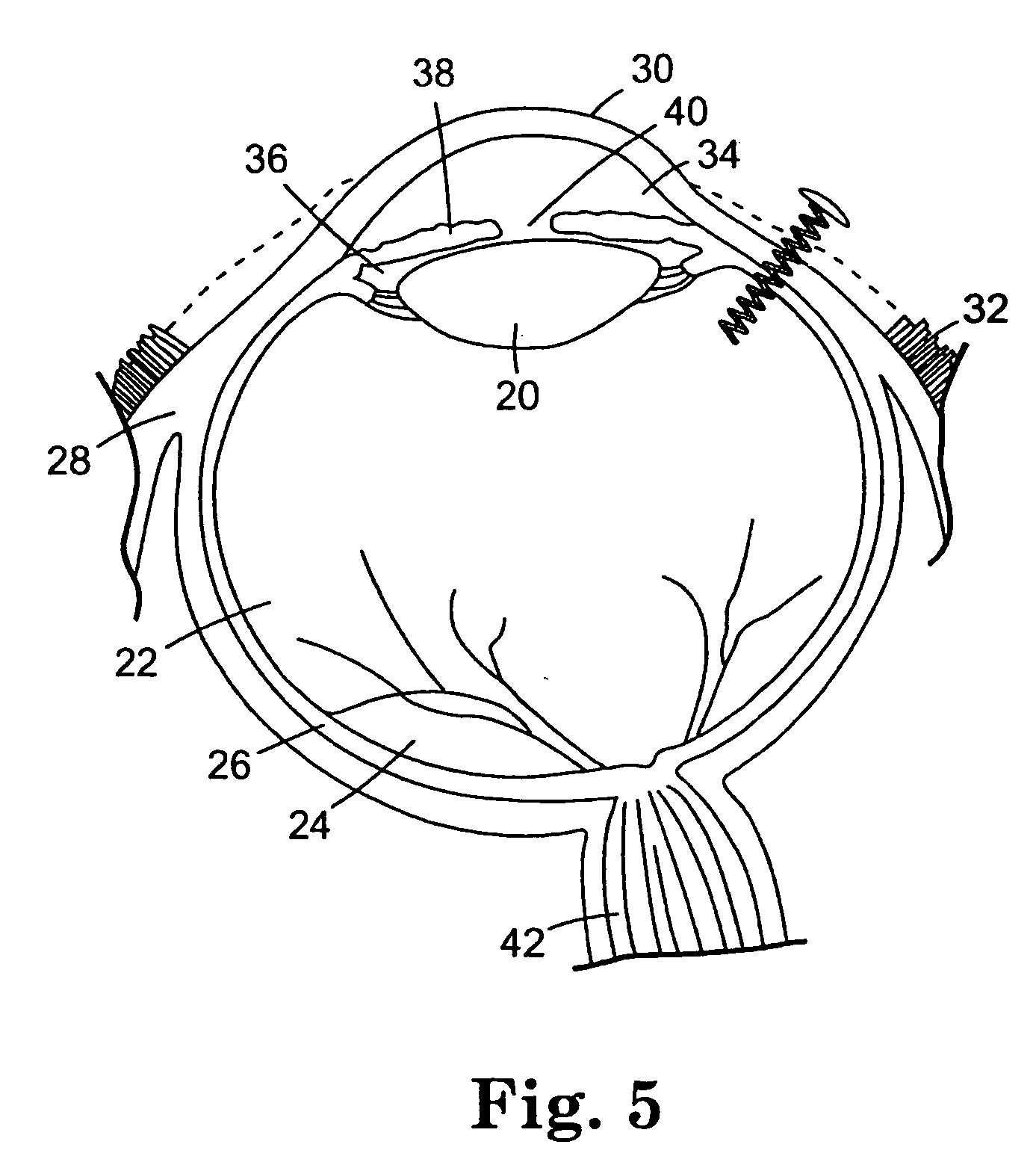
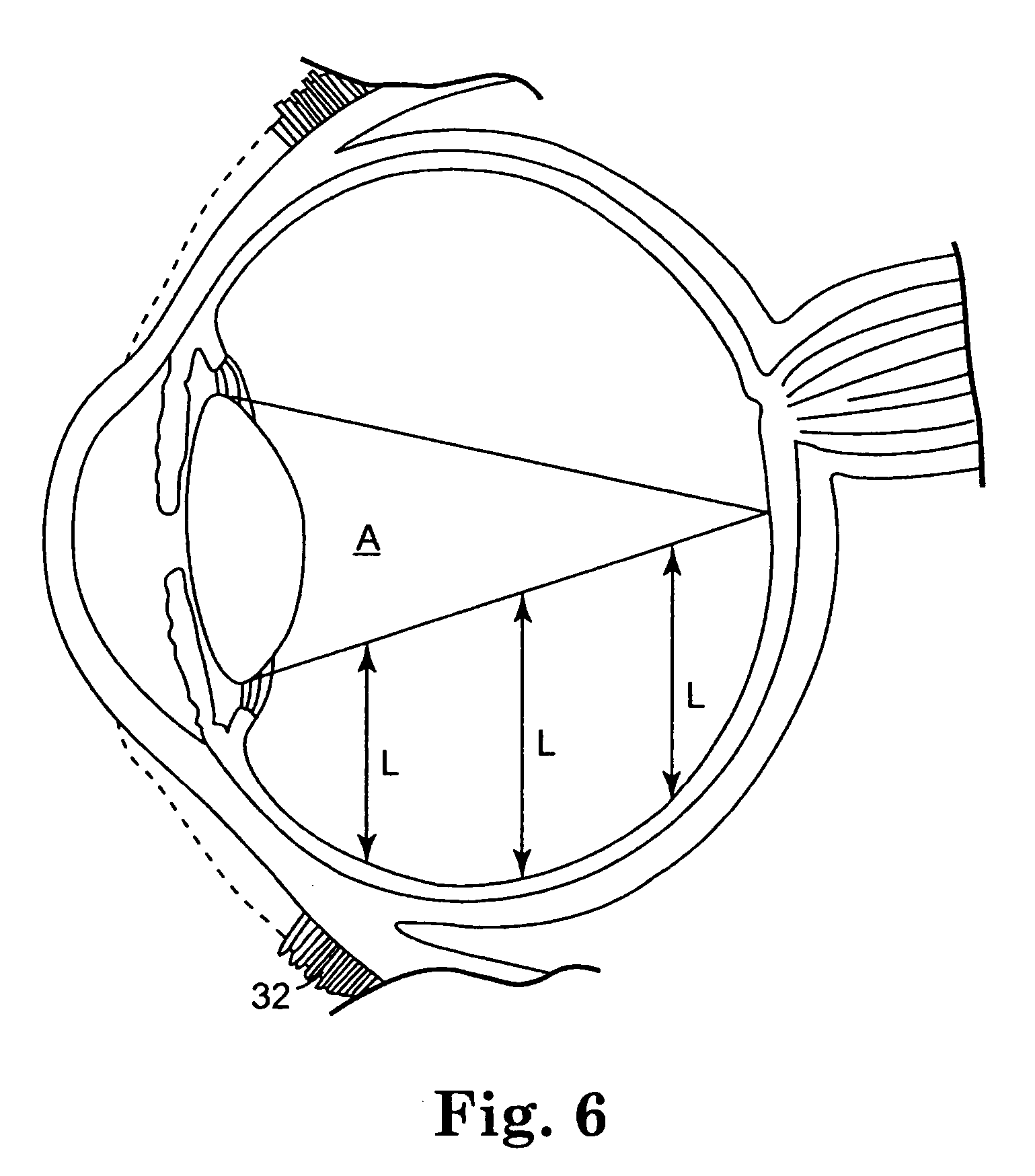
The invention provides methods for delivering bioactive agent to an eye, the methods including steps of providing a device at an implantation site within the eye, and maintaining the device at the implantation site to provide a therapeutically effective amount of the bioactive agent to the eye. The device includes a body member having a direction of extension, a longitudinal axis along the direction of extension, and a proximal end and a distal end, wherein at least a portion of the body member deviates from the direction of extension. A polymeric coated composition is provided in contact with a surface of the body member, the polymeric coated composition including a first polymer, a second polymer, and a bioactive agent. The invention also provides methods of administering a therapeutically effective amount of bioactive agent to a posterior segment of an eye.
Owner:ANDERSON ARON B +5
Polymer coating for medical devices
Coatings are provided in which surfaces may be activated by covalently bonding a silane derivative to the metal surface, covalently bonding a lactone polymer to the silane derivative by in situ ring opening polymerization, and depositing at least one layer of poly(lactide-co-caprolactone) copolymer on the bonded lactone. Biologically active agents may be disposed with the poly(lactide-co-caprolactone) copolymer layers. Such coated surfaces may be useful in medical devices, in particular stents.
Owner:CV THERAPEUTICS INC
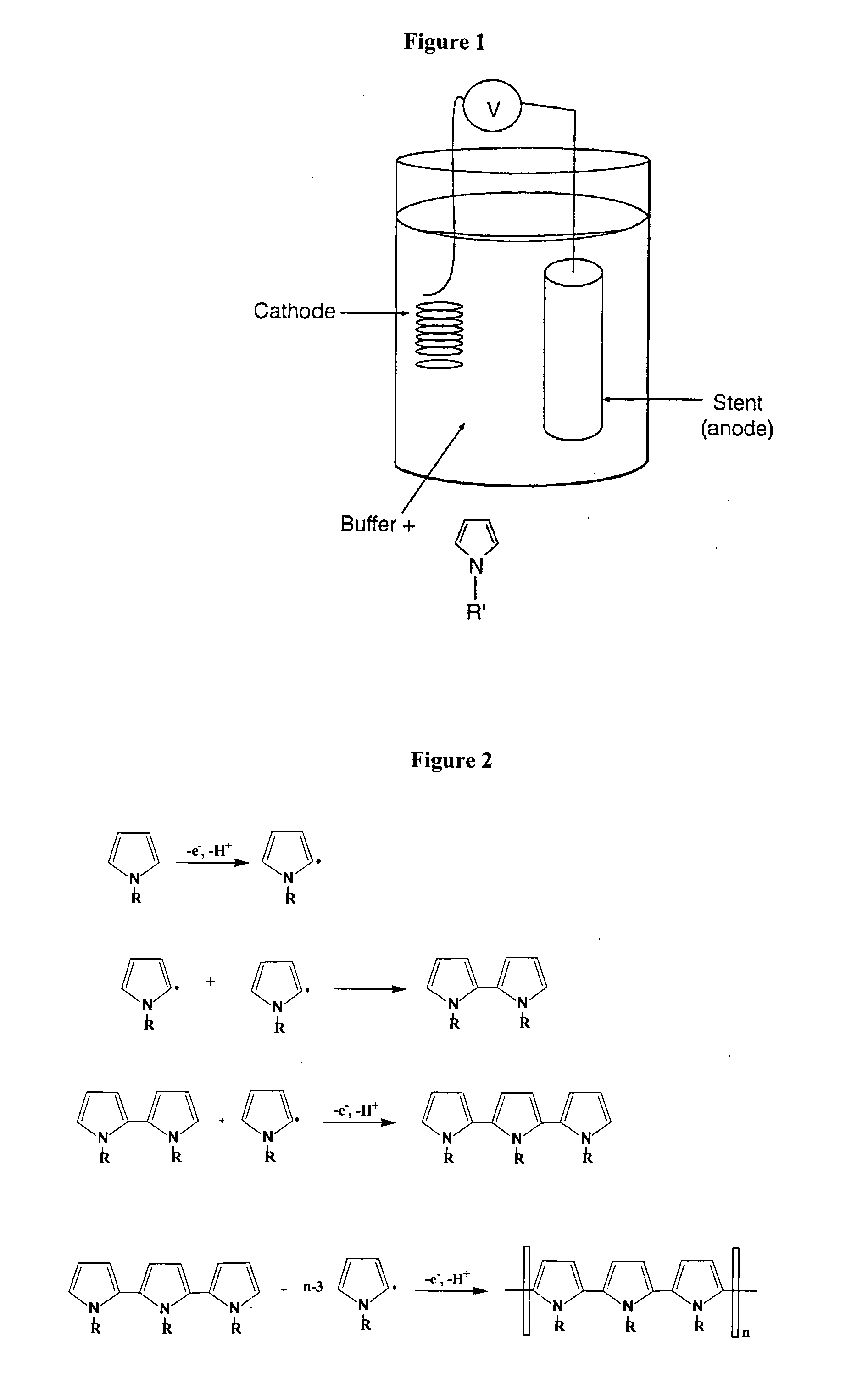
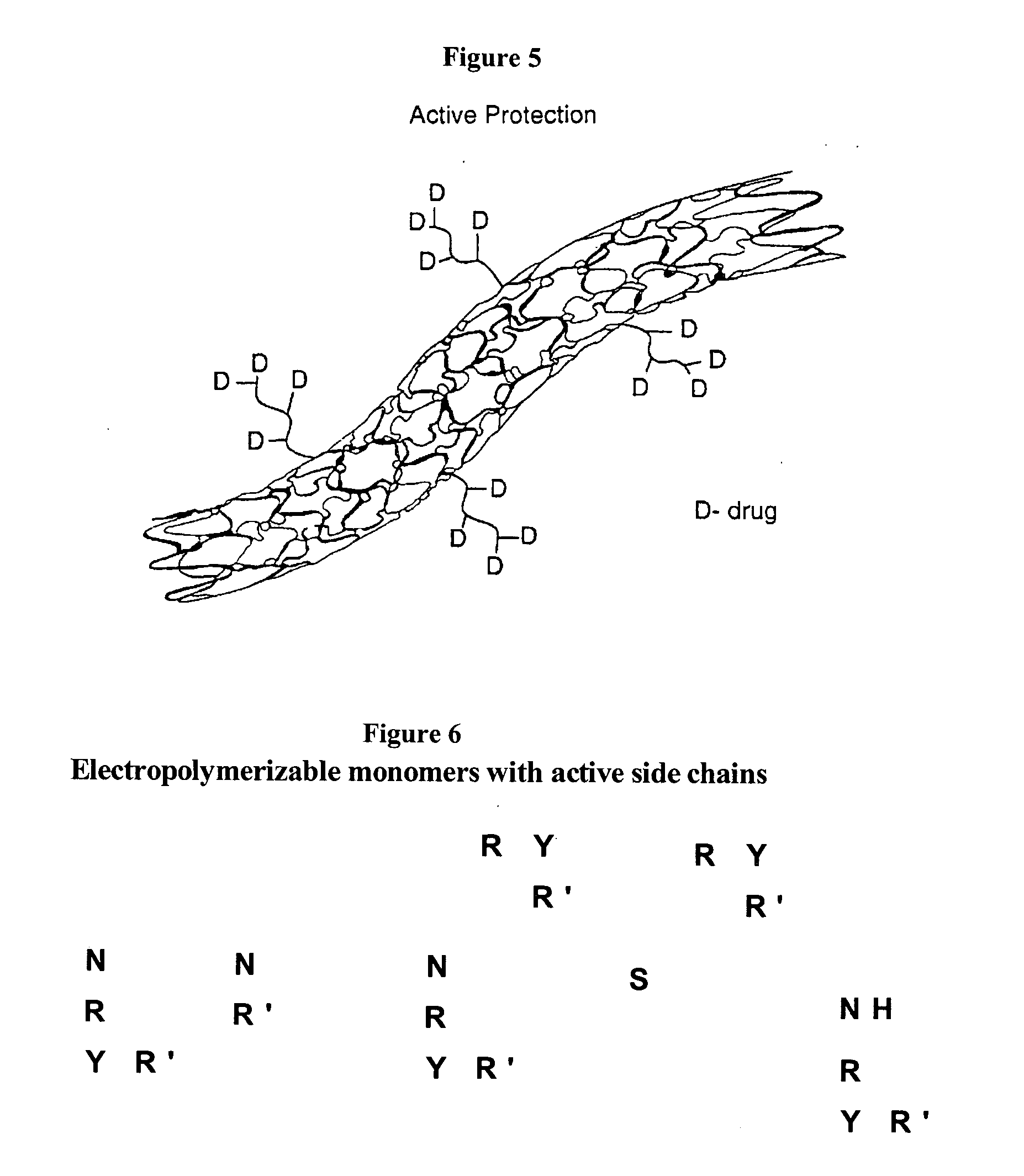
Electropolymerizable monomers and polymeric coatings on implantable devices prepared therefrom
InactiveUS20060013850A1Improve adhesionIncrease flexibilityPowder deliveryOrganic chemistryImplanted deviceMedical device
Owner:ELUTEX
Controlled release bioactive agent delivery device
InactiveUS20050271703A1Minimizes damage interferenceRisks moreOrganic active ingredientsSenses disorderControlled releaseActive agent
The invention provides implantable sustained release bioactive agent delivery devices that include a body member having a direction of extension, a longitudinal axis along the direction of extension, and a proximal end and a distal end, wherein at least a portion of the body member deviates from the direction of extension; and a polymeric coated composition in contact with a surface of the body member, the polymeric coated composition including a first polymer, a second polymer, and a bioactive agent. The polymeric coated composition is formulated to provide controlled release of bioactive agent over time when introduced into physiological conditions. Methods of preparing implantable devices configured and formulated to provide controlled release of bioactive agent are also provided.
Owner:SURMODICS INC
Calcium phosphate polymer composite and method
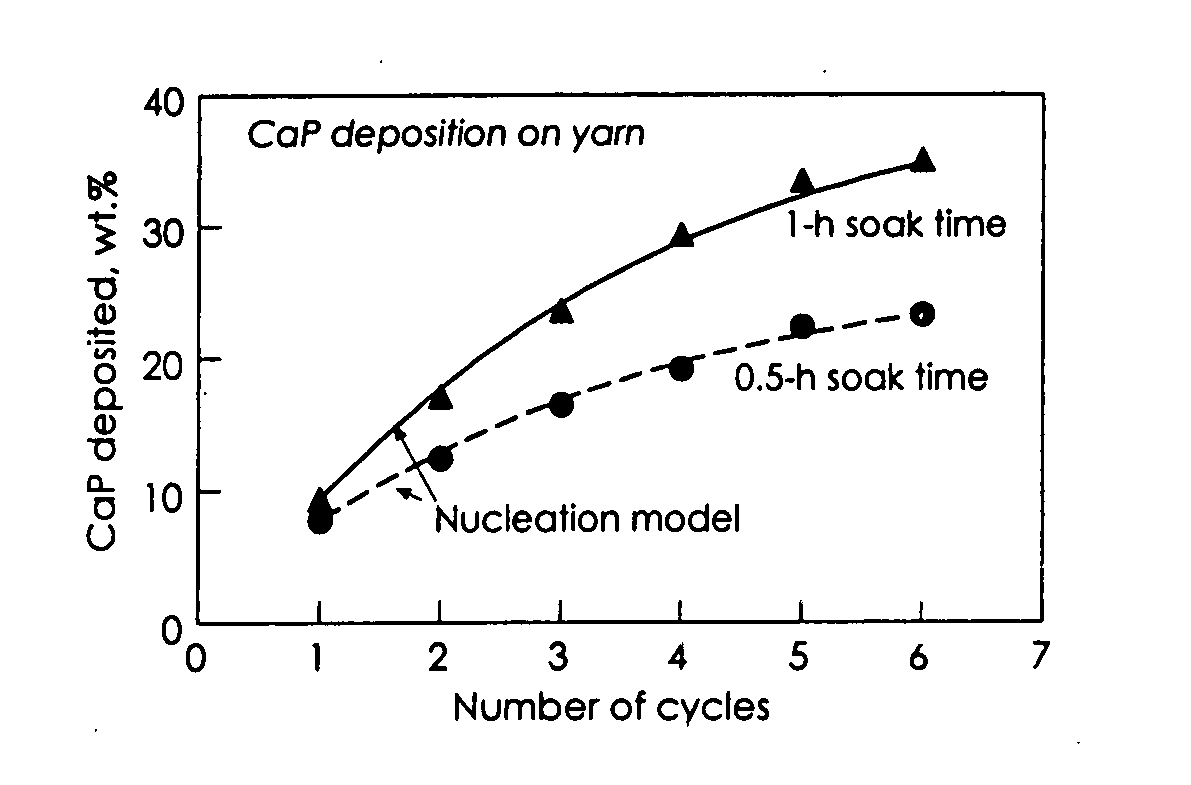
A bone-repair composite includes a core and a sheath. The core is a first primary unit including a combination of a first set of yarns coated with a calcium phosphate mineral layer. The first set of yarns being made from a first group of one ore more polymers. The sheath is a second primary unit a combination of a second set of yarns or one or more polymer coatings. The second set of yarns being made from a second group of one or more polymers, wherein the composite is made by covering the core with the sheath, and the composite is compression molded to allow the sheath to bond to the core. The bone-repair composite has a bending modulus comparable to that of a mammalian bone, such that the ratio of the core to the sheath is provided to maximize the mechanical strength of the bone-repair composite to mimic the mammalian bone.
Owner:TELEFLEX MEDICAL INC +1
Reflowed drug-polymer coated stent and method thereof
The present invention provides a method of forming a drug-polymer coated stent. A polymeric coating is applied onto at least a portion of a stent framework and dried. A jet of heated gas is directed towards excess coating portions of the dried polymeric coating. The excess coating portions, which extend into apertures of the stent framework, are removed from the apertures of the stent framework by reflowing the polymeric coating with the directed jet of heated gas. A drug-polymer coated stent with a reflowed drug-polymer coating and a system for treating a vascular condition are also disclosed.
Owner:MEDTRONIC VASCULAR INC
Solid electrolytic capacitor containing a conductive polymer
ActiveUS20080232037A1Hybrid capacitor electrolytesSolid electrolytic capacitorsOligomerConductive polymer
A method for forming an electrolytic capacitor is disclosed. The method includes forming a conductive polymer coating by polymerizing a monomer in the presence of less than a stoichiometric amount of an oxidative polymerization catalyst. The present inventor has found that the use of less than the stoichiometric amount of the oxidative polymerization catalyst per mole of monomer can slow the polymerization of the monomer, creating oligomers that are shorter in length than if fully polymerized into a polymer. Without wishing to be bound by theory, it is believed that these shorter oligomers provide better penetration into the porous anode. Thus, the resulting conductive polymer layer can be more intimately positioned with respect to the anode. As a result, the formed capacitor can exhibit better performance.
Owner:KYOCERA AVX COMPONENTS CORP
Injectable sustained release delivery devices
ActiveUS20040176341A1Organic active ingredientsCosmetic preparationsControlled releasePolymer coatings
An injectable drug delivery device includes a core containing one or more drugs and one or more polymers. The core may be surrounded by one or more polymer outer layers (referred to herein as "coatings," "skins," or "outer layers"). In certain embodiments, the device is formed by extruding or otherwise preforming a polymeric skin for a drug core. The drug core may be co-extruded with the skin, or inserted into the skin after the skin has been extruded, and possibly cured. In other embodiments, the drug core may be coated with one or more polymer coatings. These techniques may be usefully applied to fabricate devices having a wide array of drug formulations and skins that can be selected to control the release rate profile and various other properties of the drugs in the drug core in a form suitable for injection using standard or non-standard gauge needles. The device may be formed by combining at least one polymer, at least one drug, and at least one liquid solvent to form a liquid suspension or solution wherein, upon injection, such suspension or solution under goes a phase change and forms a gel. The configuration may provide for controlled release of the drug(s) for an extended period.
Owner:EYEPOINT PHARMA INC
Particles for use in electrophoretic displays
ActiveUS20080013156A1Liquid surface applicatorsSynthetic resin layered productsPolymer coatingsInorganic pigments
A particle for use in an electrophoretic display comprises a light-scattering inorganic core and a light-transmissive colored shell of an organic pigment. The core may be titania and the shell may be formed of particles having an average particle size less than 700 nm. The particles are produced by treating a light-scattering inorganic pigment with a polymer which adsorbs on both the inorganic pigment and an organic pigment; and adding the organic pigment and allowing the organic pigment to mix with the polymer-coated inorganic pigment. The particles may have a polymer coating.
Owner:E INK CORPORATION
Polymer coating/encapsulation of nanoparticles using a supercritical antisolvent process
InactiveUS20050191491A1Speed up the processGranule coatingPretreated surfacesSolventSupercritical carbon dioxide
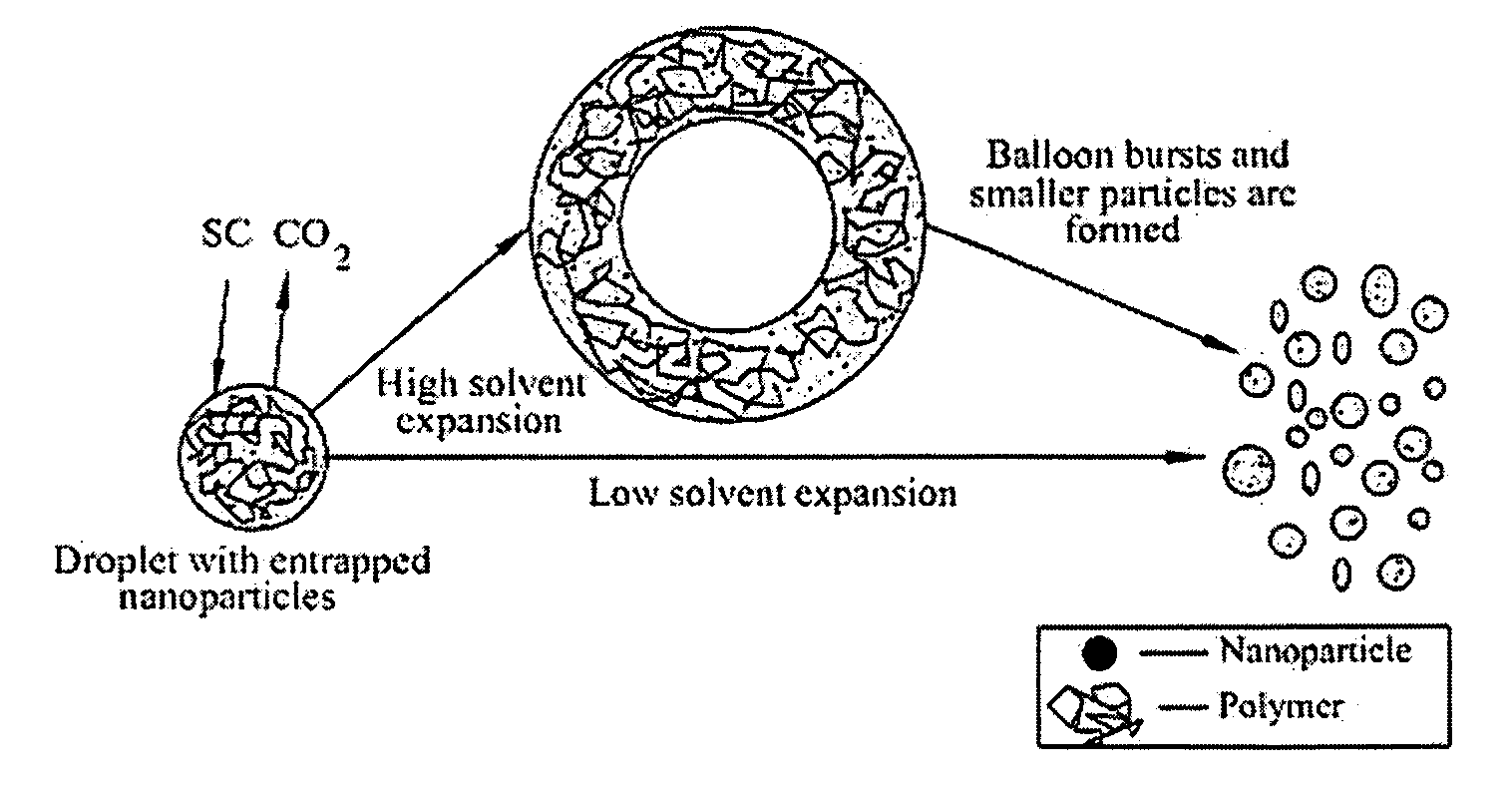
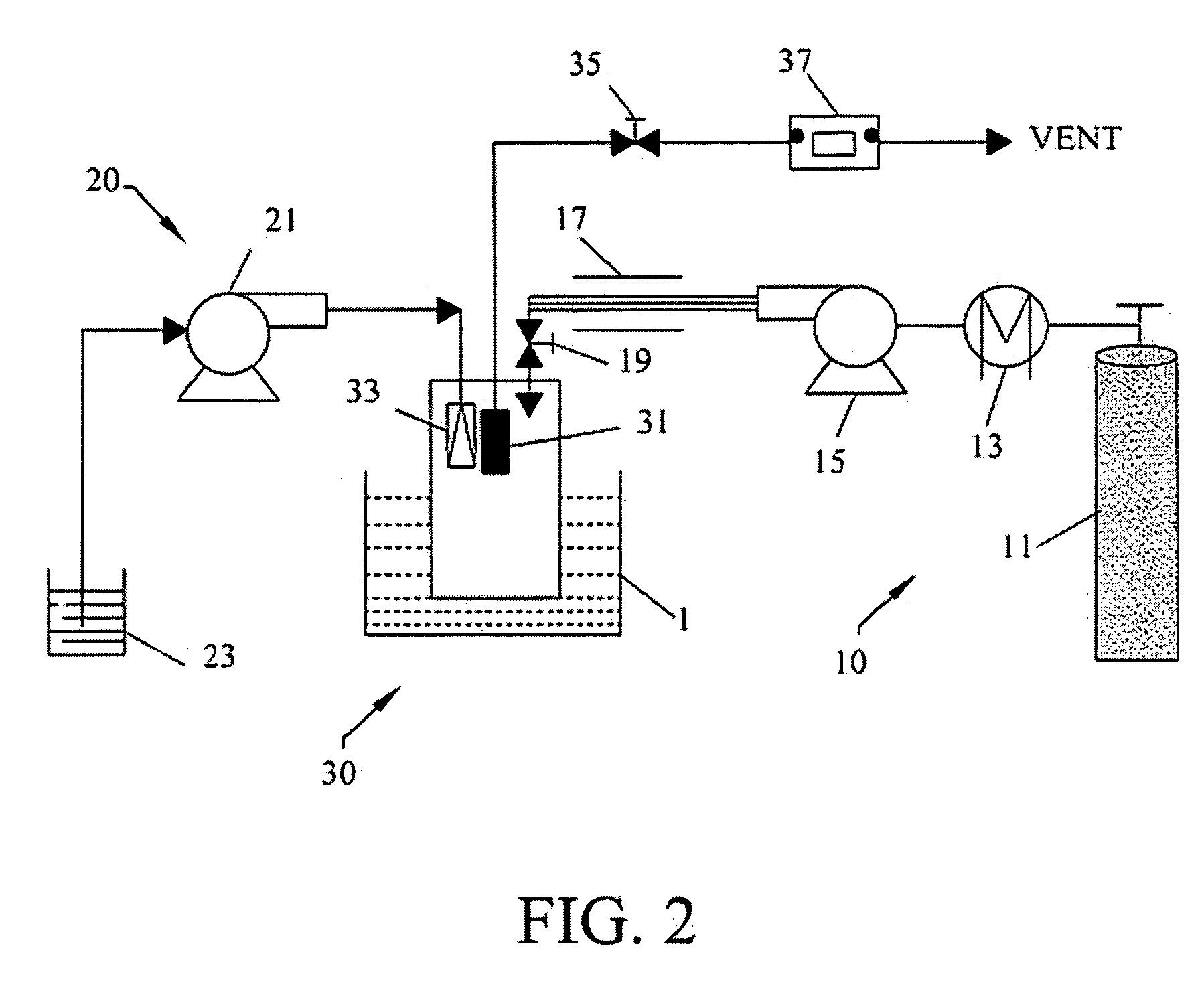
A process, method and / or system for preparing polymer-coated nanoparticles and / or other ultrafine particles utilizing a supercritical fluid, e.g., supercritical carbon dioxide (SC CO2), as an antisolvent that may be added to a solution of a polymer and an organic solvent in which insoluble nanoparticles or the like are suspended. The coating process occurs when the supercritical fluid (e.g., SC CO2) and the nanoparticle-containing suspension are combined to cause the suspended nanoparticles to precipitate as coated nanoparticles. Processing parameters for optimizing and / or enhancing the efficacy and / or efficiency of the coating process, method and / or system and for controlling the coating and / or agglomeration of coated particles are also described. The process, method and / or system has wide ranging applicability, e.g., for coating and / or encapsulation of pharmaceuticals, cosmetics, food products, chemicals, agrochemicals, pesticides, polymers, coatings, catalysts and the like.
Owner:NEW JERSEY INSTITUTE OF TECHNOLOGY
Electrophoretic particles, and processes for the production thereof
ActiveUS8270064B2Easy to useGood color saturationPigmenting treatmentStatic indicating devicesMagnetiteManganese oxide
An electrophoretic medium comprises at least one electrically charged particle dispersed posed in a fluid. The electrically charged particle comprises an inorganic black pigment having a surface area of at least about 7 m2 / g. Preferred pigments are magnetite and mixed metal oxides containing two or more of iron, chromium, nickel, manganese, copper and cobalt, for example copper iron manganese oxide spinel and copper chromium manganese oxide spinel. The inorganic black pigment may bear a polymer coating.
Owner:E INK CORPORATION +1
Electrophoretic medium and display with improved image stability
ActiveUS7903319B2Static indicating devicesElectrographic processes using charge patternPolymer scienceElectrophoresis
An electrophoretic medium comprises a fluid and a plurality of electrically charged particles disposed in the fluid and capable of moving therethrough on application of an electrical field to the medium. Each of the charged particles has a polymer coating comprising a first group. A polymer is dispersed in the fluid, this polymer having a plurality of second groups capable of attracting the first groups on the particles so that the polymer in the fluid forms a complex with the electrophoretic particles.
Owner:E INK CORPORATION
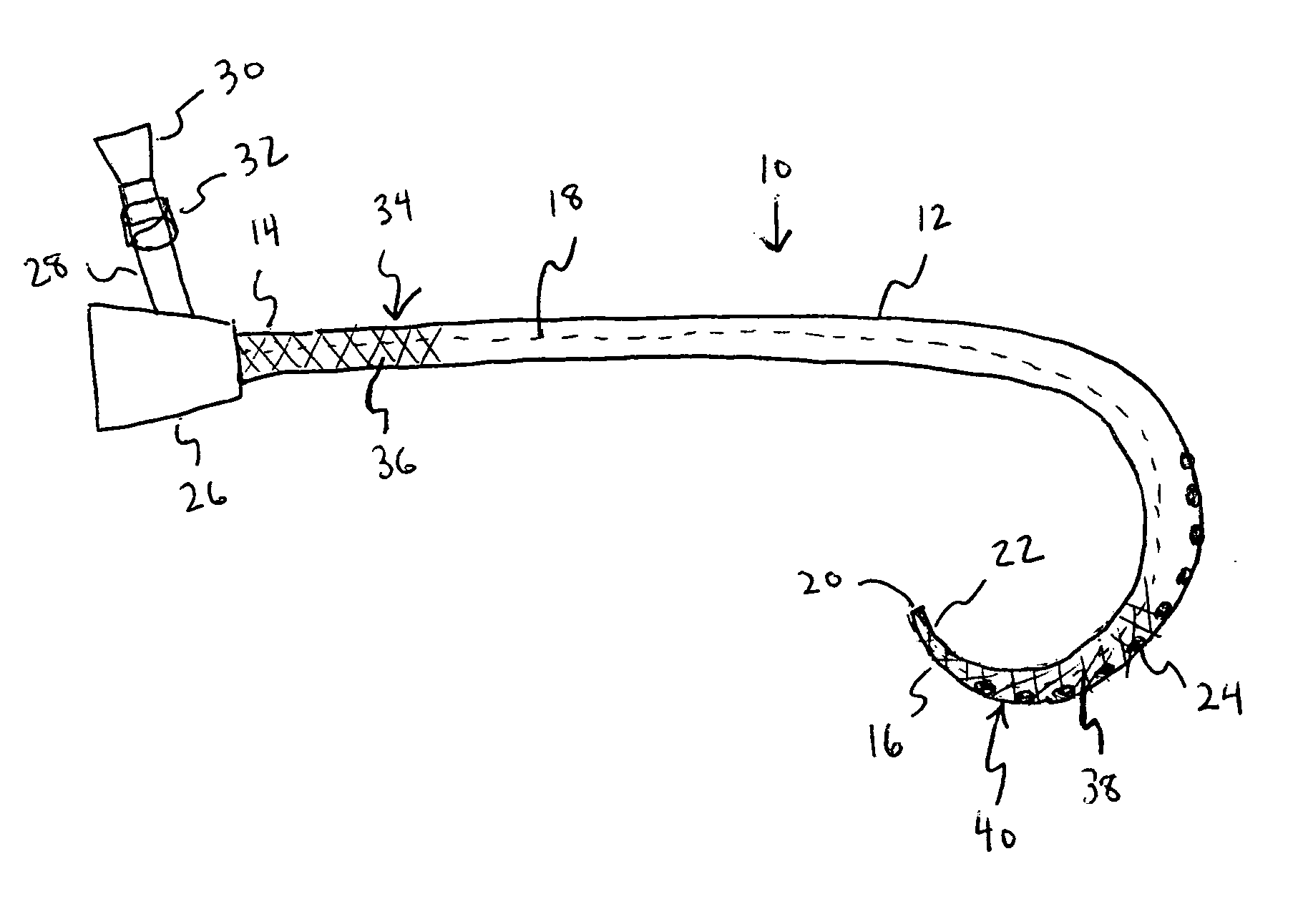
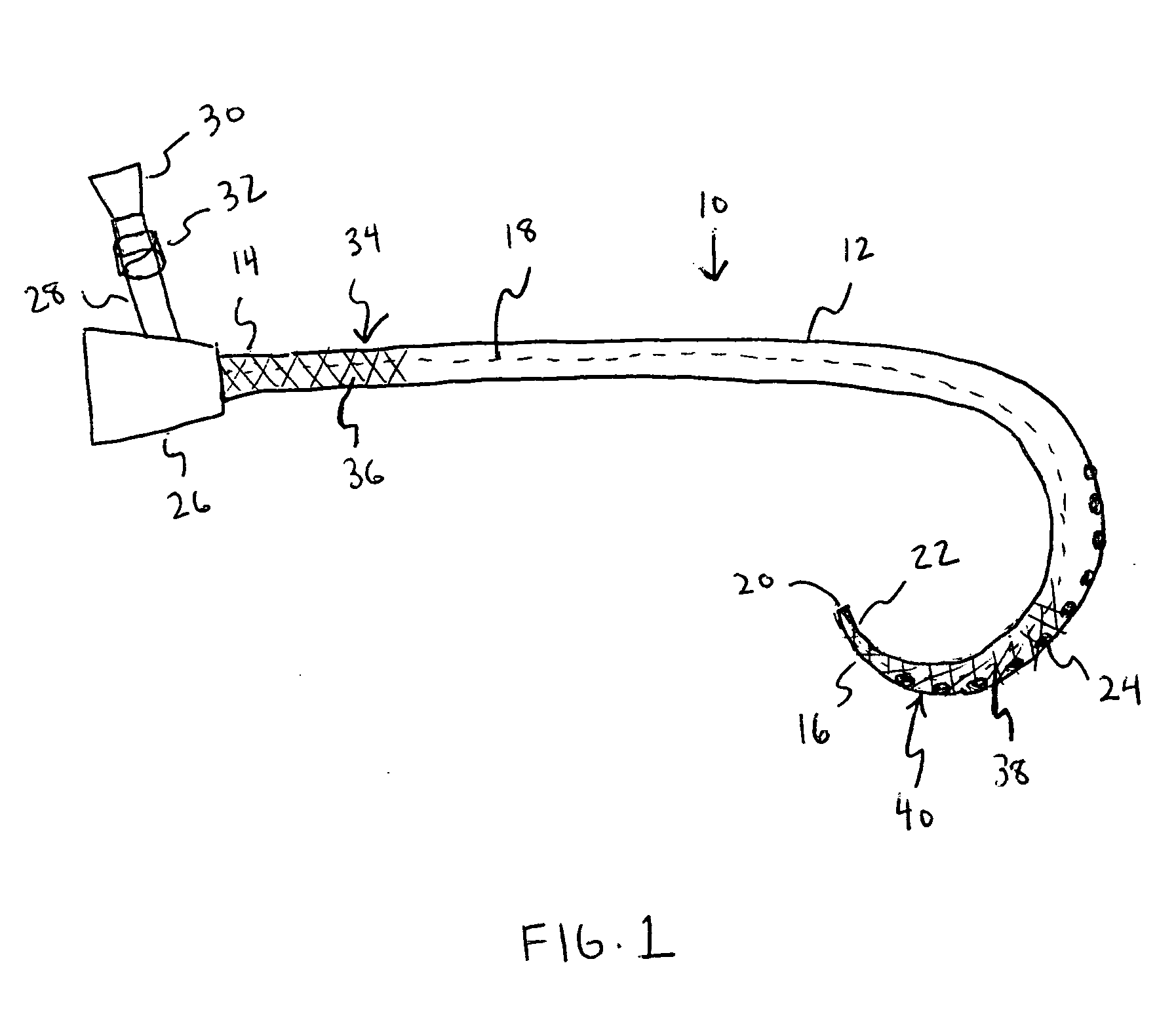
Catheter with polymeric coating
A catheter including a main body having a first end, a second end, a lumen extending between the first end and the second end, a first section located proximal the first end of the main body and second section located proximal the second end of the main body. An inhibitory polymer is disposed at the first section. The inhibitory polymer includes one or more members selected from the group consisting of antiproliferatives, antithrombotics, thrombolytics, and fibrinolytics. An antimicrobial agent is disposed at the second section. The main body has a length such that when the catheter is at least partially implanted the first end accesses a body vessel and at least a portion of the second section is disposed within a subcutaneous space of a patient.
Owner:TELEFLEX LIFE SCI LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com