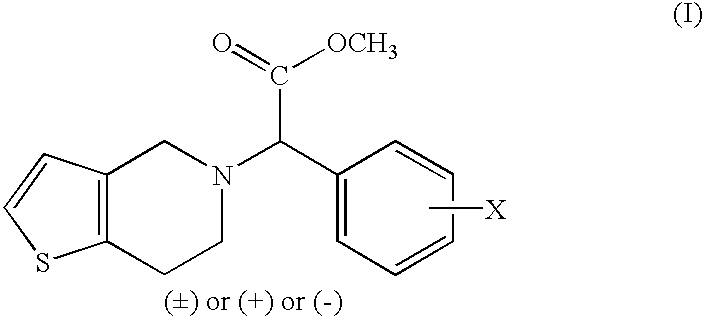
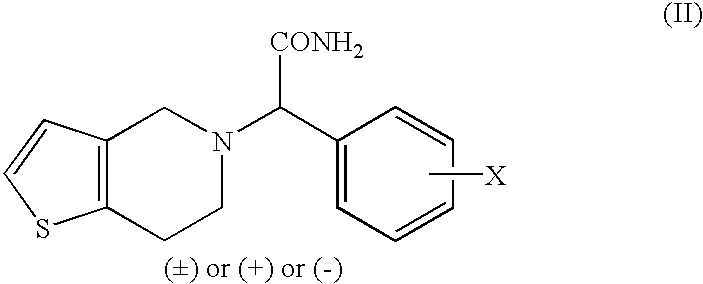
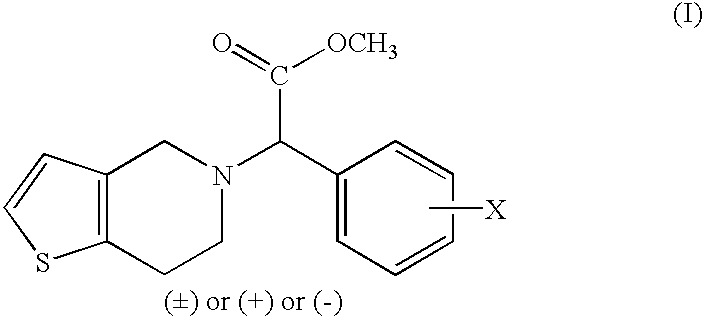
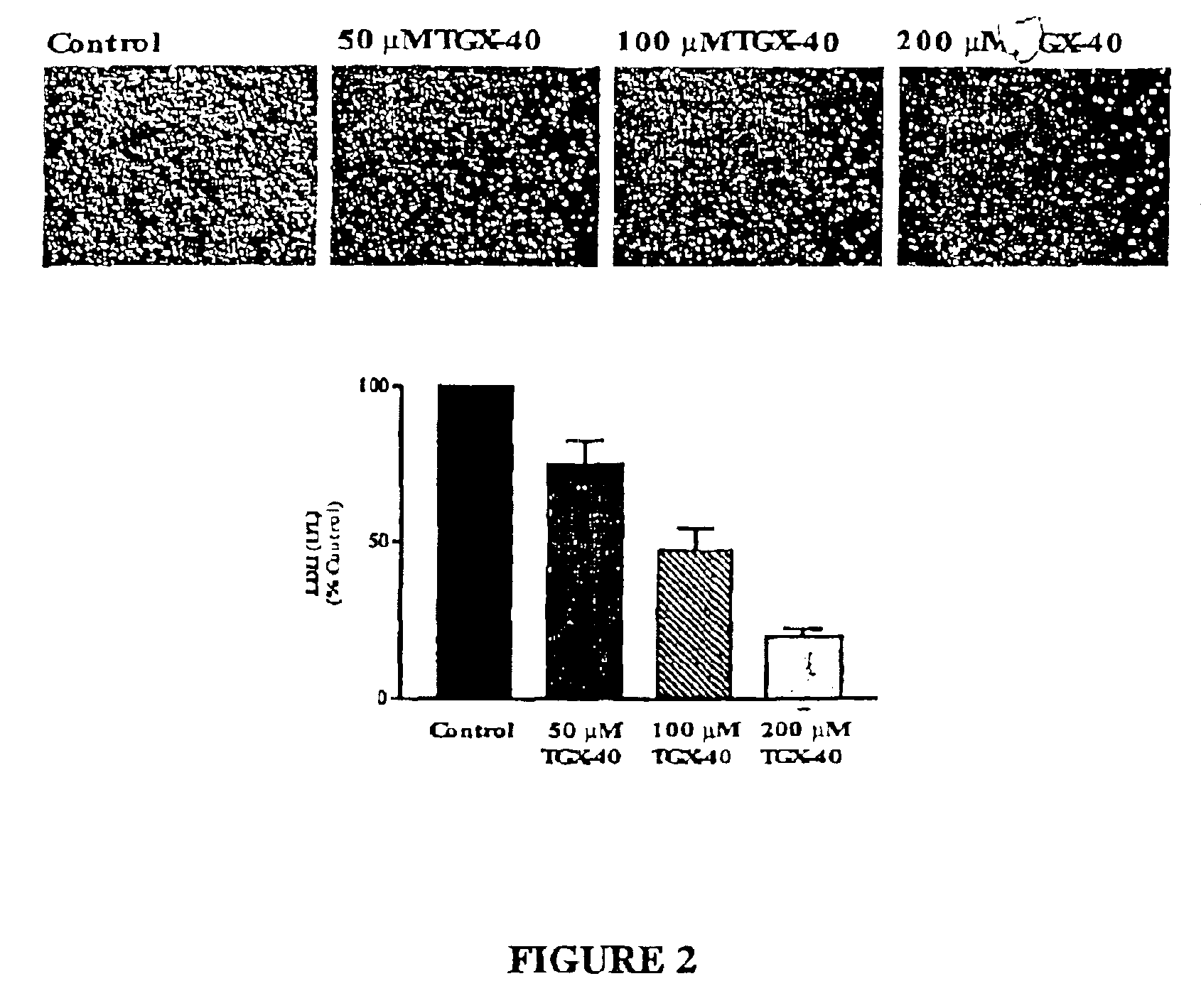
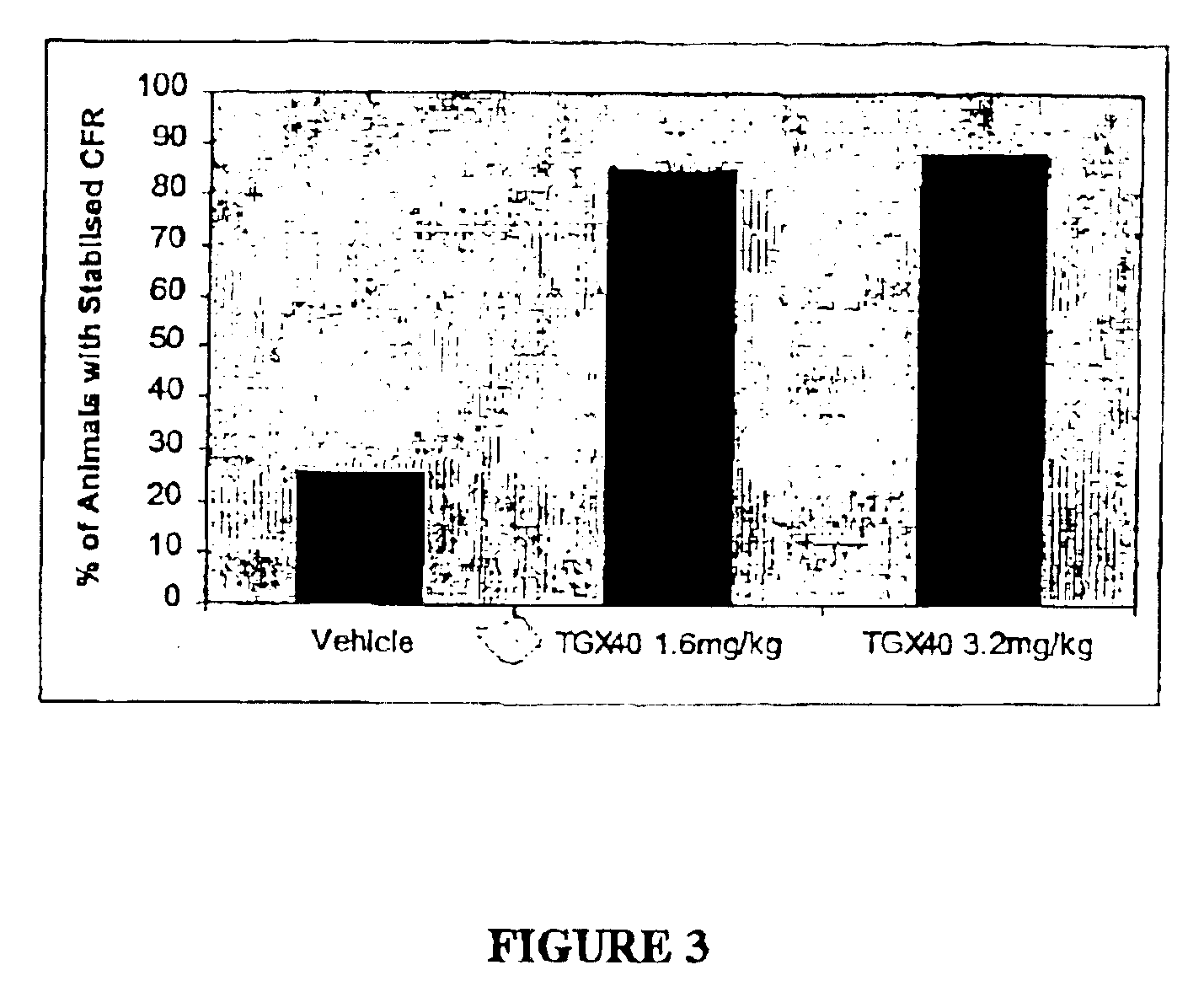
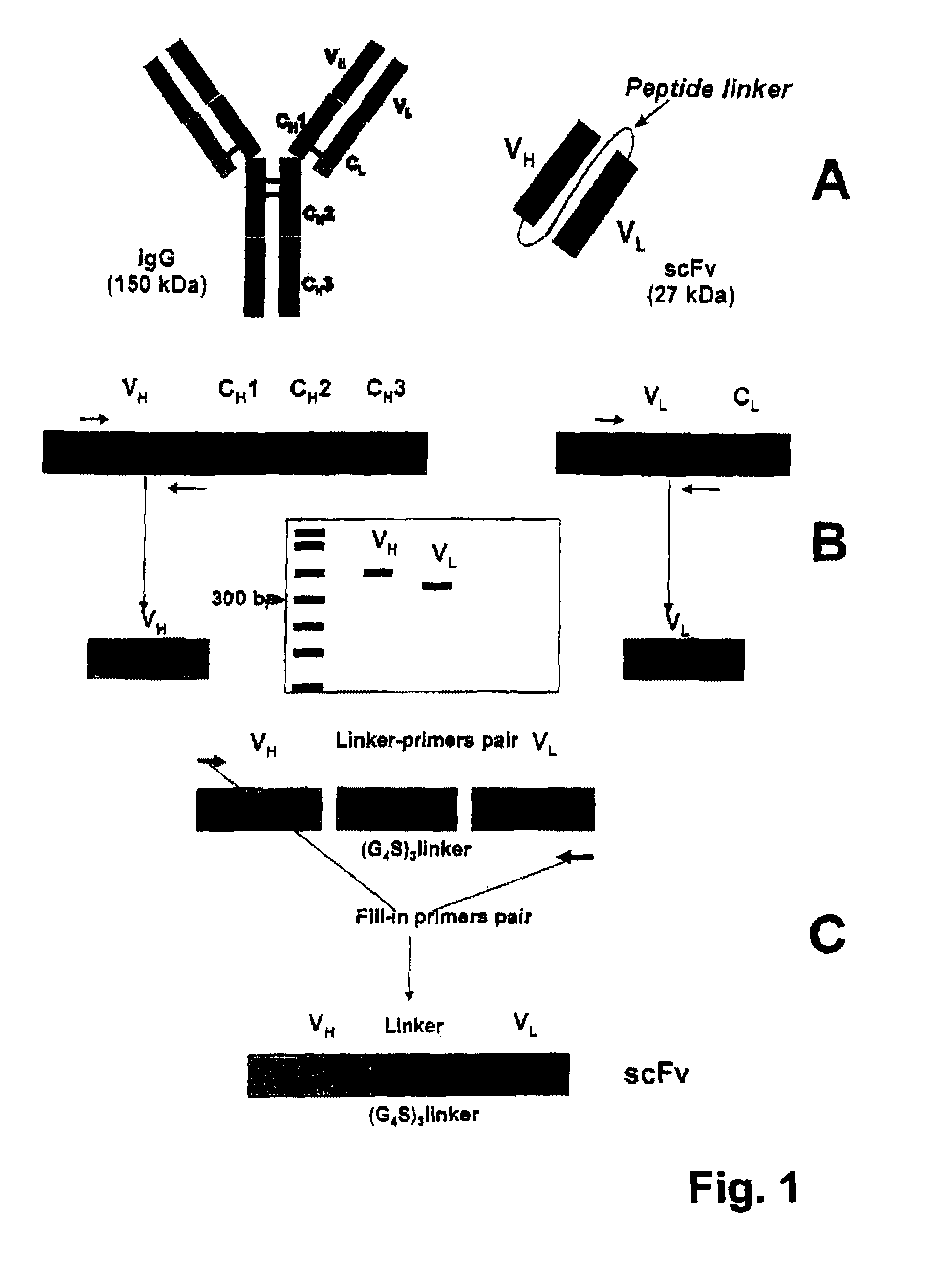
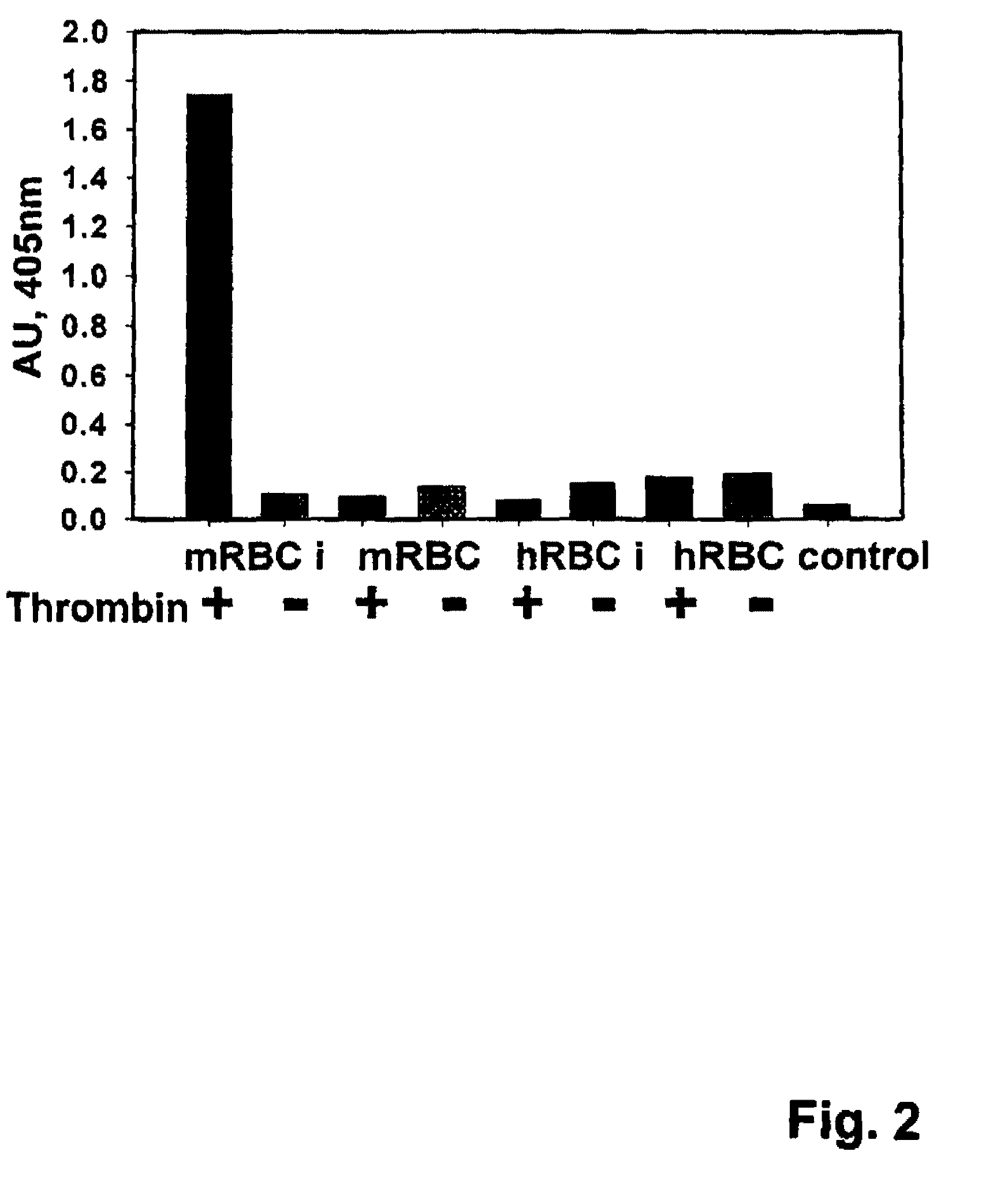
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
635 results about "Antithrombotic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An antithrombotic agent is a drug that reduces the formation of blood clots (thrombi). Antithrombotics can be used therapeutically for prevention (primary prevention, secondary prevention) or treatment of a dangerous blood clot (acute thrombus). In the U.S., the American College of Chest Physicians publishes clinical guidelines for clinicians for the use of these drugs to treat and prevent a variety of diseases.
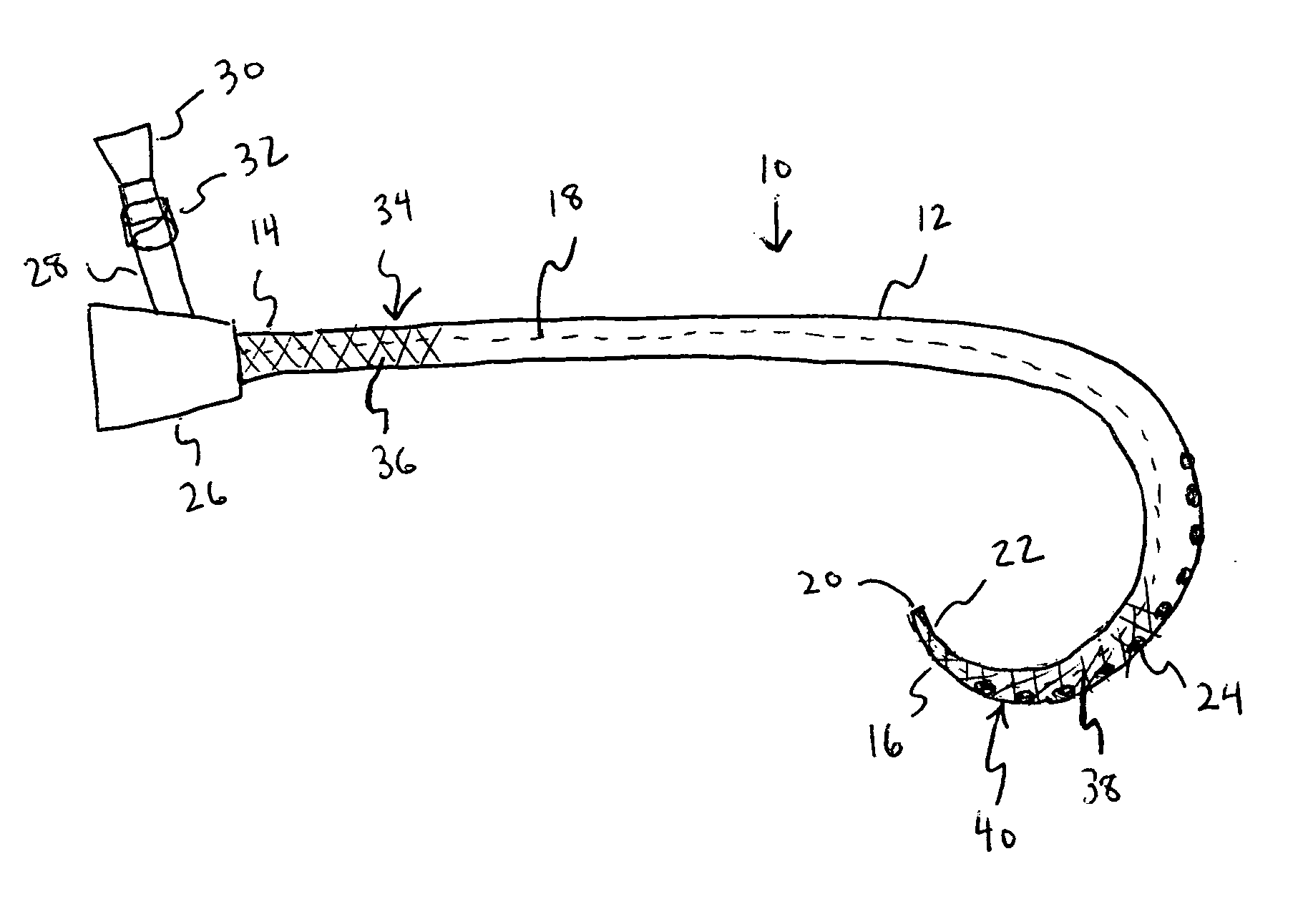
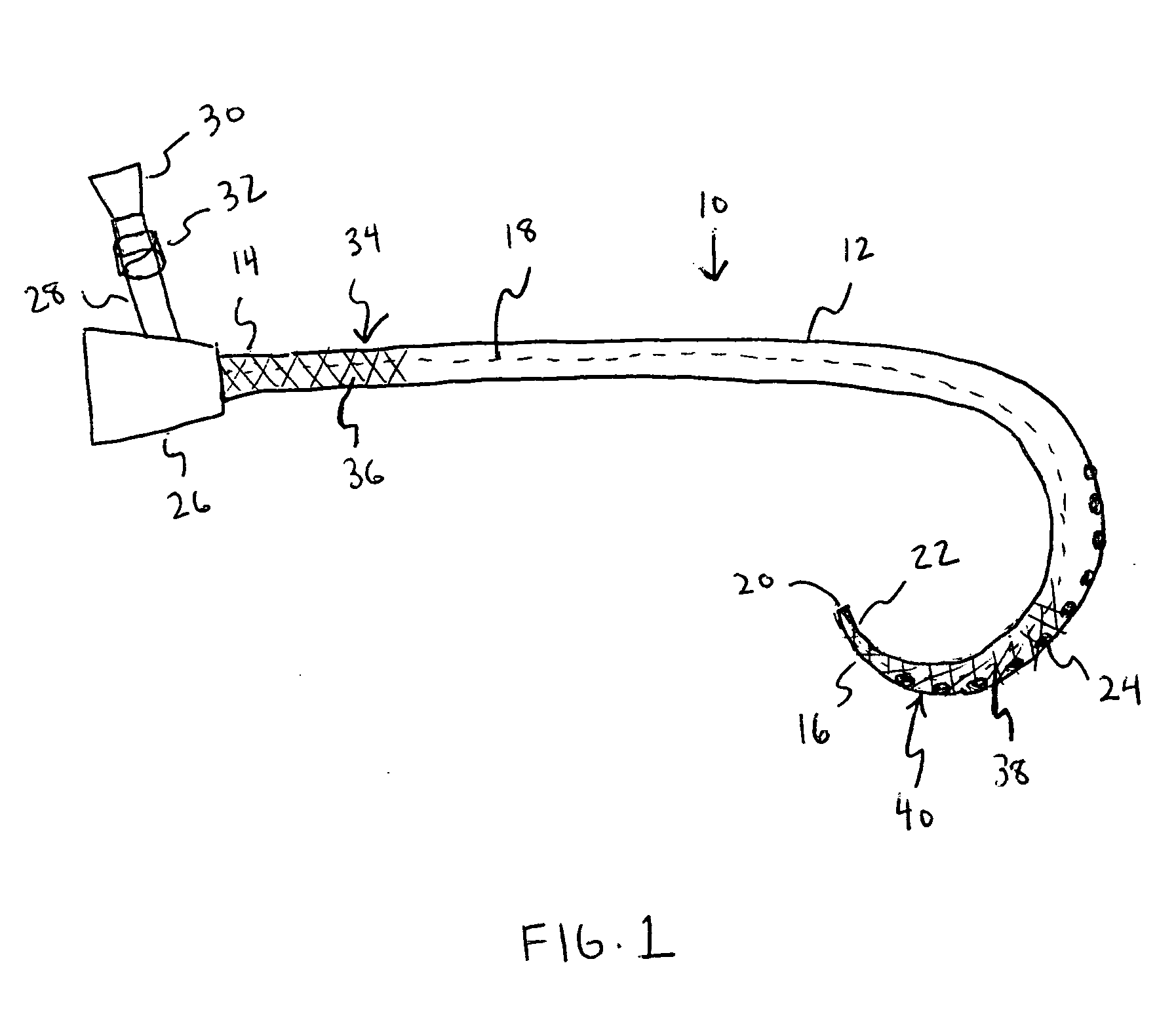
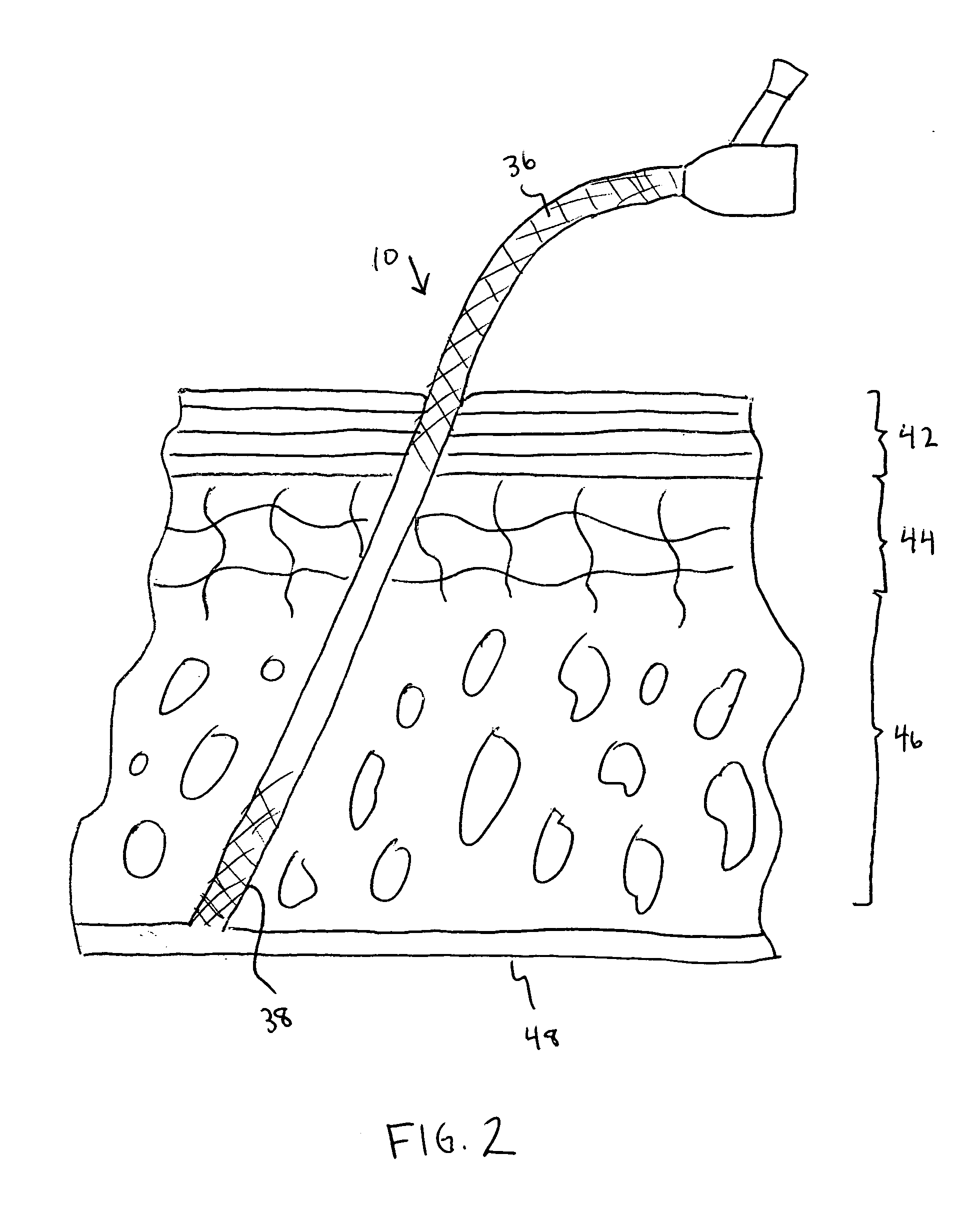
Catheter with polymeric coating
A catheter including a main body having a first end, a second end, a lumen extending between the first end and the second end, a first section located proximal the first end of the main body and second section located proximal the second end of the main body. An inhibitory polymer is disposed at the first section. The inhibitory polymer includes one or more members selected from the group consisting of antiproliferatives, antithrombotics, thrombolytics, and fibrinolytics. An antimicrobial agent is disposed at the second section. The main body has a length such that when the catheter is at least partially implanted the first end accesses a body vessel and at least a portion of the second section is disposed within a subcutaneous space of a patient.
Owner:TELEFLEX LIFE SCI LTD
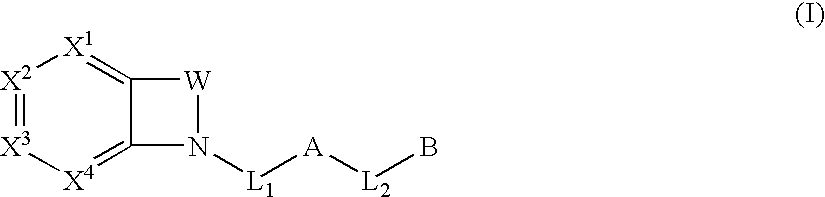
Biarylmethyl indolines and indoles as antithromboembolic agents
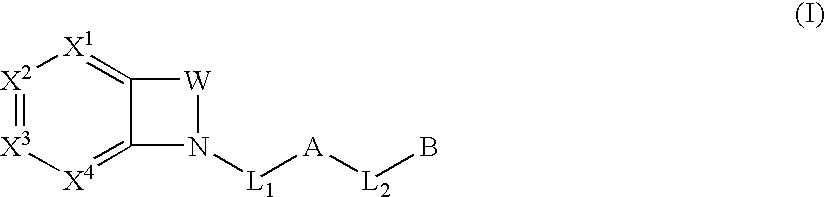
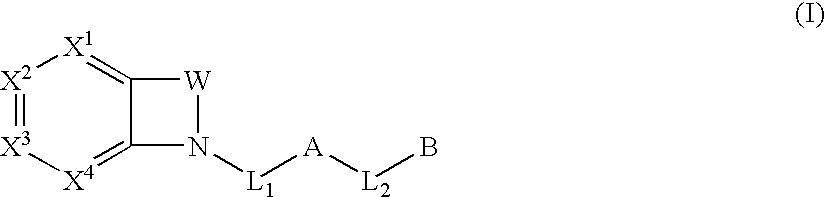
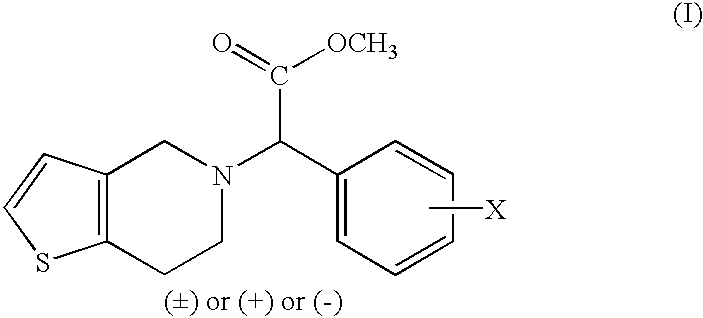
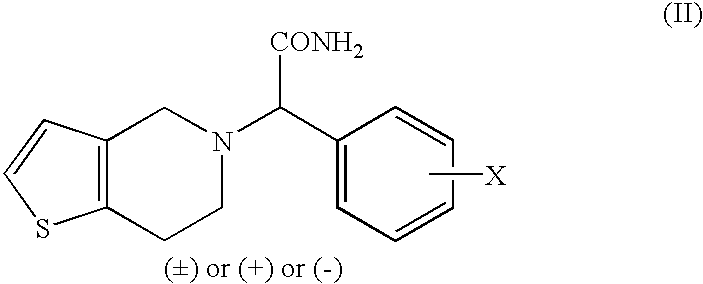
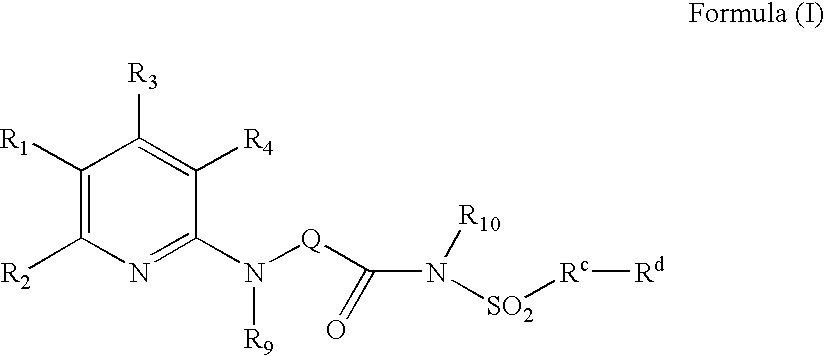
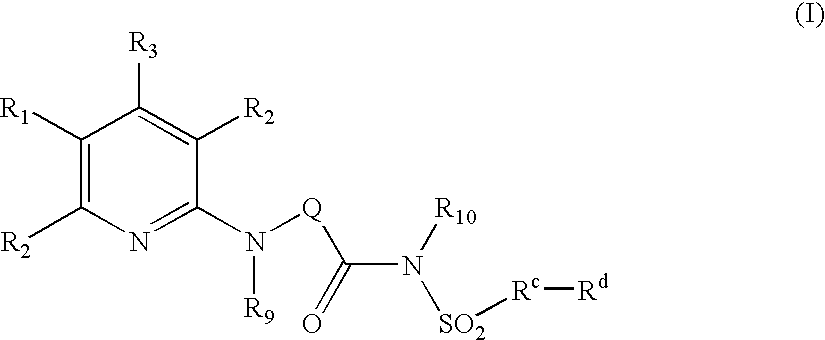
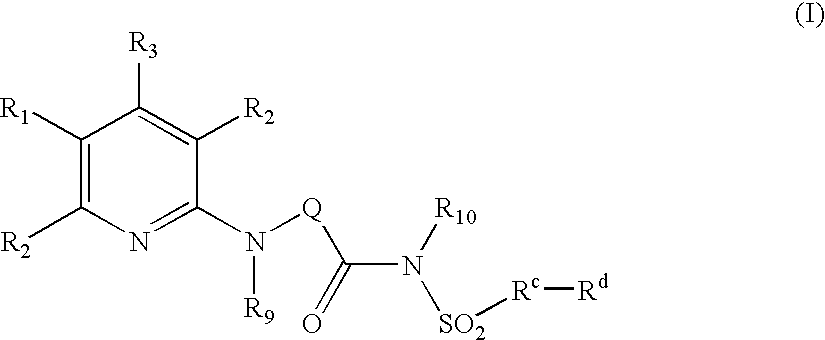
The present invention provides compounds of Formula (I):or a stereoisomer or pharmaceutically acceptable salt or hydrate form thereof, wherein the variables A, B, L1, L2, X1, X2, X3, X4 and W are as defined herein. The compounds of Formula (I) are useful as selective inhibitors of serine protease enzymes of the coagulation cascade and / or contact activation system; for example thrombin, factor Xa, factor XIa, factor IXa, factor VIIa and / or plasma kallikrein. In particular, it relates to compounds that are selective factor XIa inhibitors. This invention also relates to pharmaceutical compositions comprising these compounds and methods of treating thromboembolic and / or inflammatory disorders using the same.
Owner:BRISTOL MYERS SQUIBB CO
Drug-releasing stent with ceramic-containing layer
InactiveUS7713297B2Prevent proliferationPrevent restenosisStentsSurgeryEndoluminal stentAnti platelet
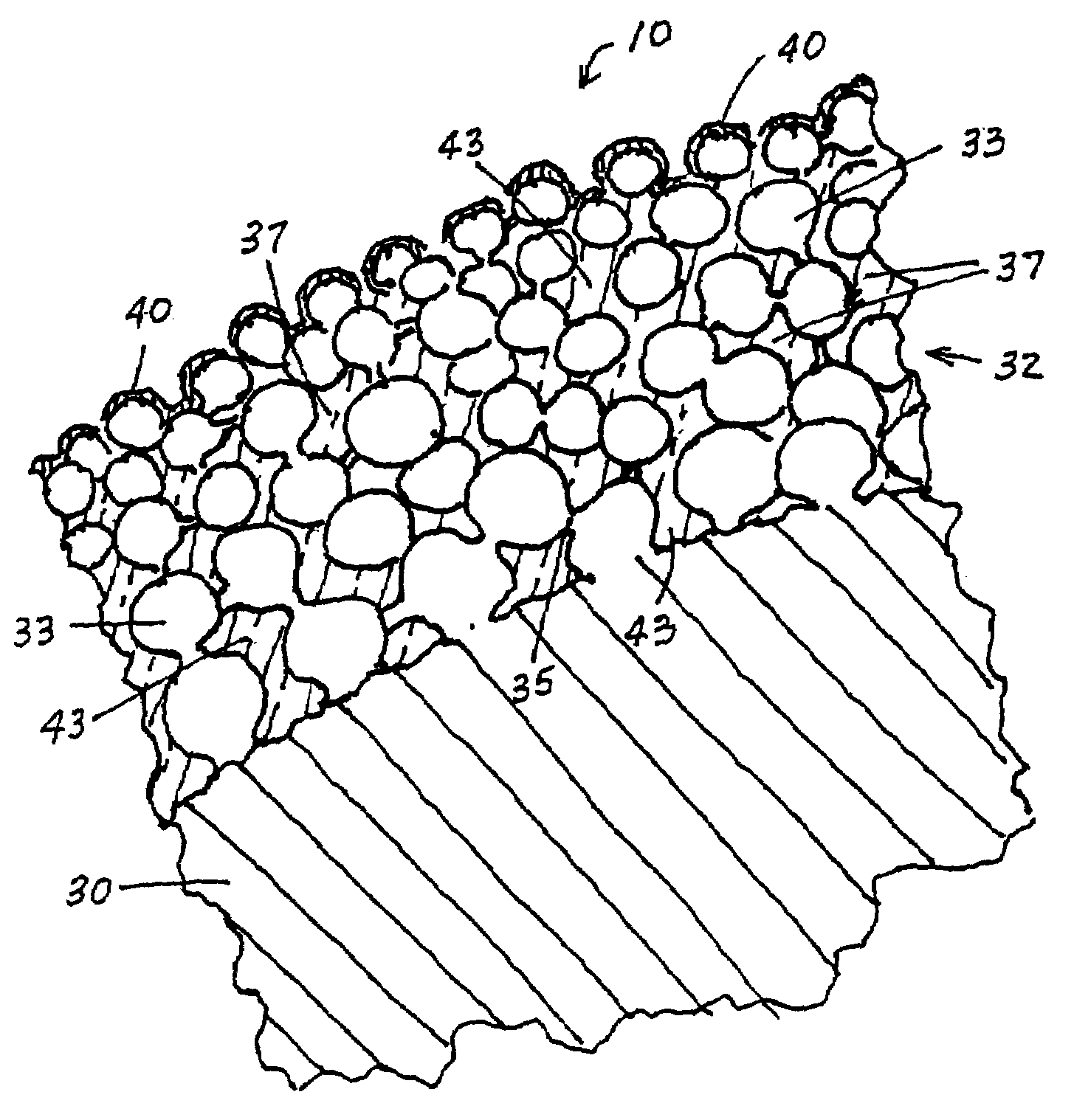
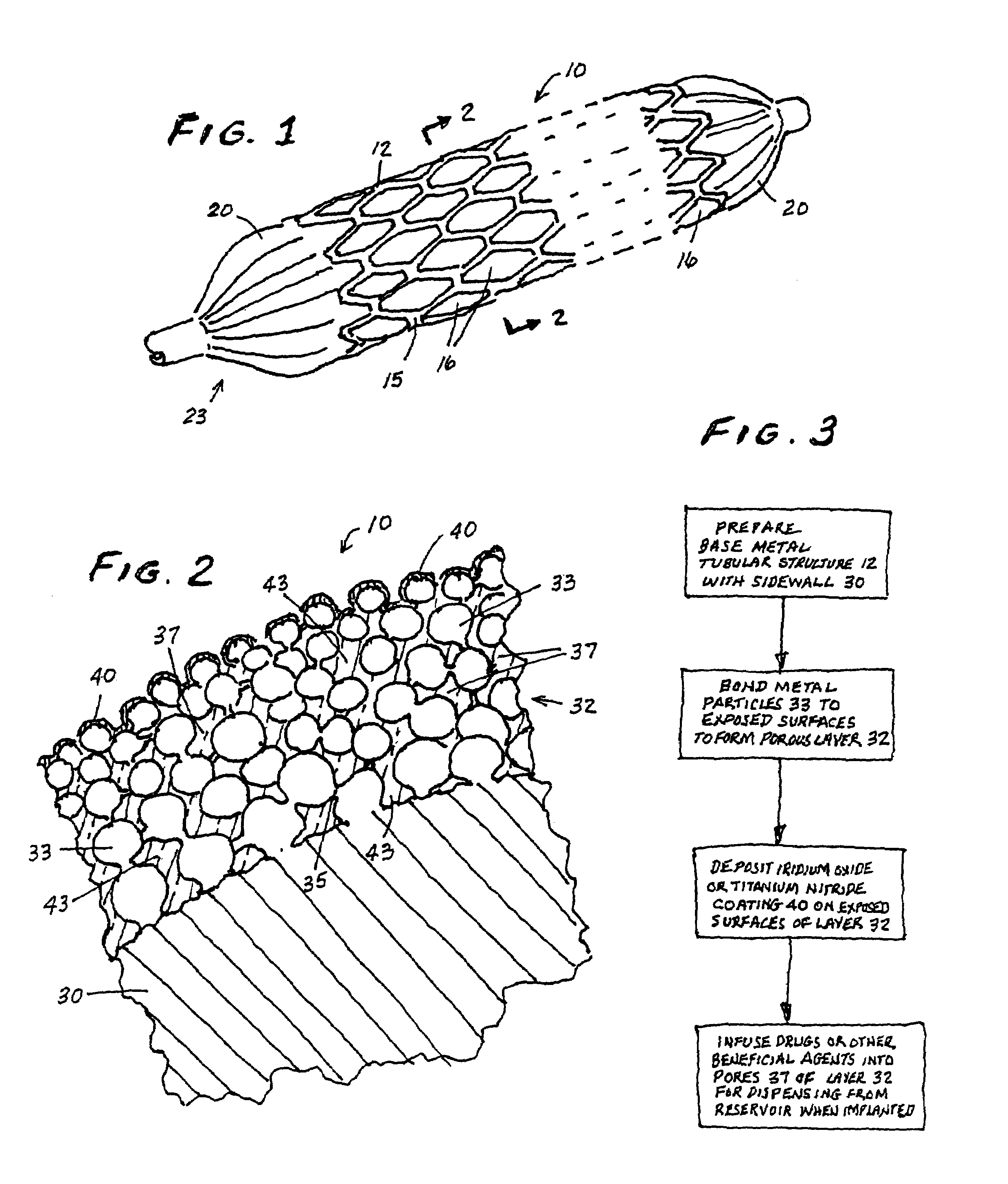
A vascular or endoluminal stent is adapted to be implanted in a vessel, duct or tract of a human body to maintain an open lumen at the site of the implant. The sidewall of the open-ended tubular structure of the stent is a base layer of a metal biologically compatible with blood and tissue of the human body. An intermediate metal particle layer of substantial greater radiopacity overlies the base layer, with particles bonded to the base layer and to each other to leave interstices therebetween as a repository for retaining and dispensing drugs or other agents for time release therefrom after the stent is implanted, to assist the stent in maintaining the lumen open. The particles are composed primarily of a noble metal—an alloy of platinum-iridium. The sidewall has holes extending therethrough, and the particle layer resides along the outward facing and inward facing surfaces, and the edges of the through holes and open ends of the sidewall. The larger particles are bonded to surfaces of the sidewall and progressively smaller particles are bonded to those and to each other up to the outer portion of the particle layer. Exposed surfaces of the particle layer are coated with ceramic-like iridium oxide or titanium nitrate, as a biocompatible material to inhibit irritation of tissue at the inner lining of the vessel when the stent is implanted. One or more anti-thrombotic, anti-platelet, anti-inflammatory and / or anti-proliferative drugs are retained in the interstices, together with a biodegradable carrier for time release therefrom. In an alternative embodiment, the intermediate layer is solid and the biodegradable carrier and drugs or agents therein are applied to the surface of the ceramic-like coating. Gene transfer is alternatively used to control tissue proliferation.
Owner:BOSTON SCI SCIMED INC
Process to prepare clopidogrel
InactiveUS6635763B2Increased cost-effectivenessOrganic chemistryThienopyridine DerivativesPharmacometrics
The present invention relates to a process for the preparation of thieno[3,2-c]pyridine derivatives having pharmacologically significant anti-aggregating and anti-thrombotic properties.
Owner:CADILA HEALTHCARE LTD
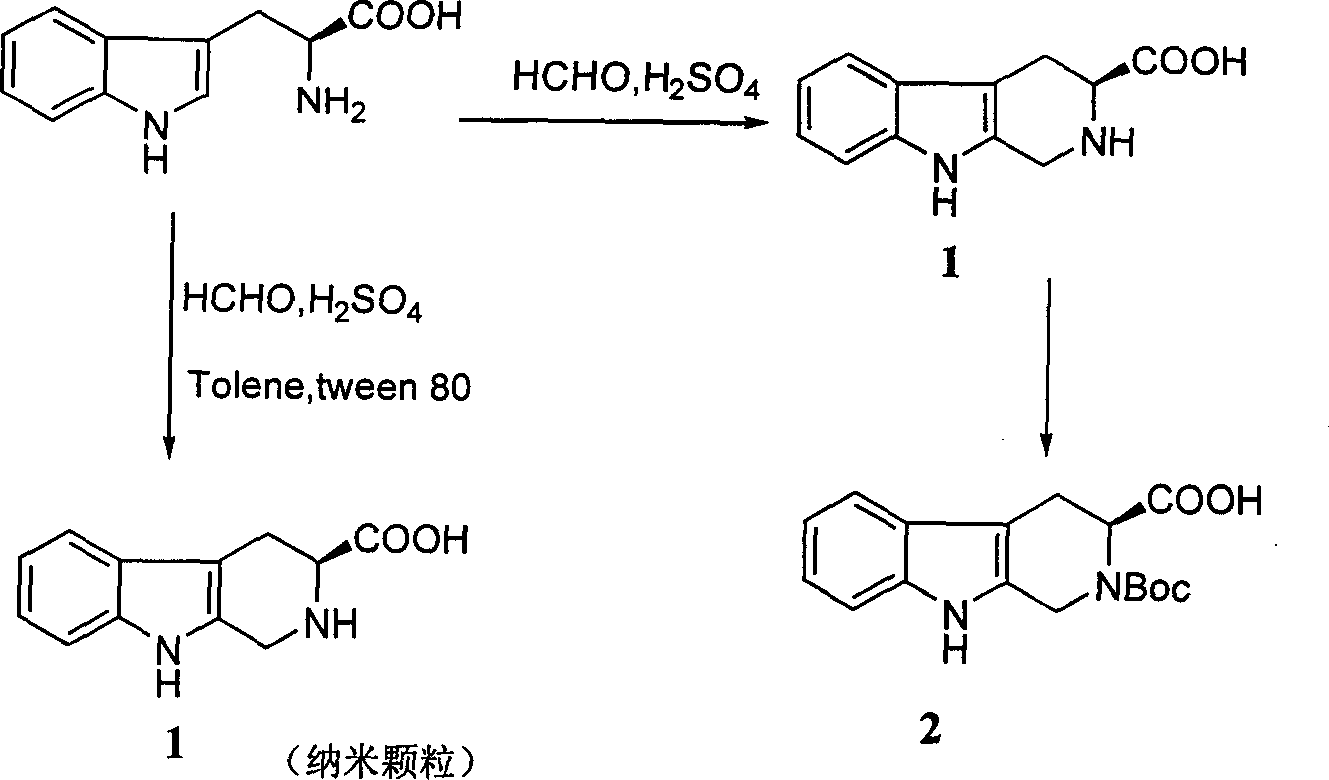
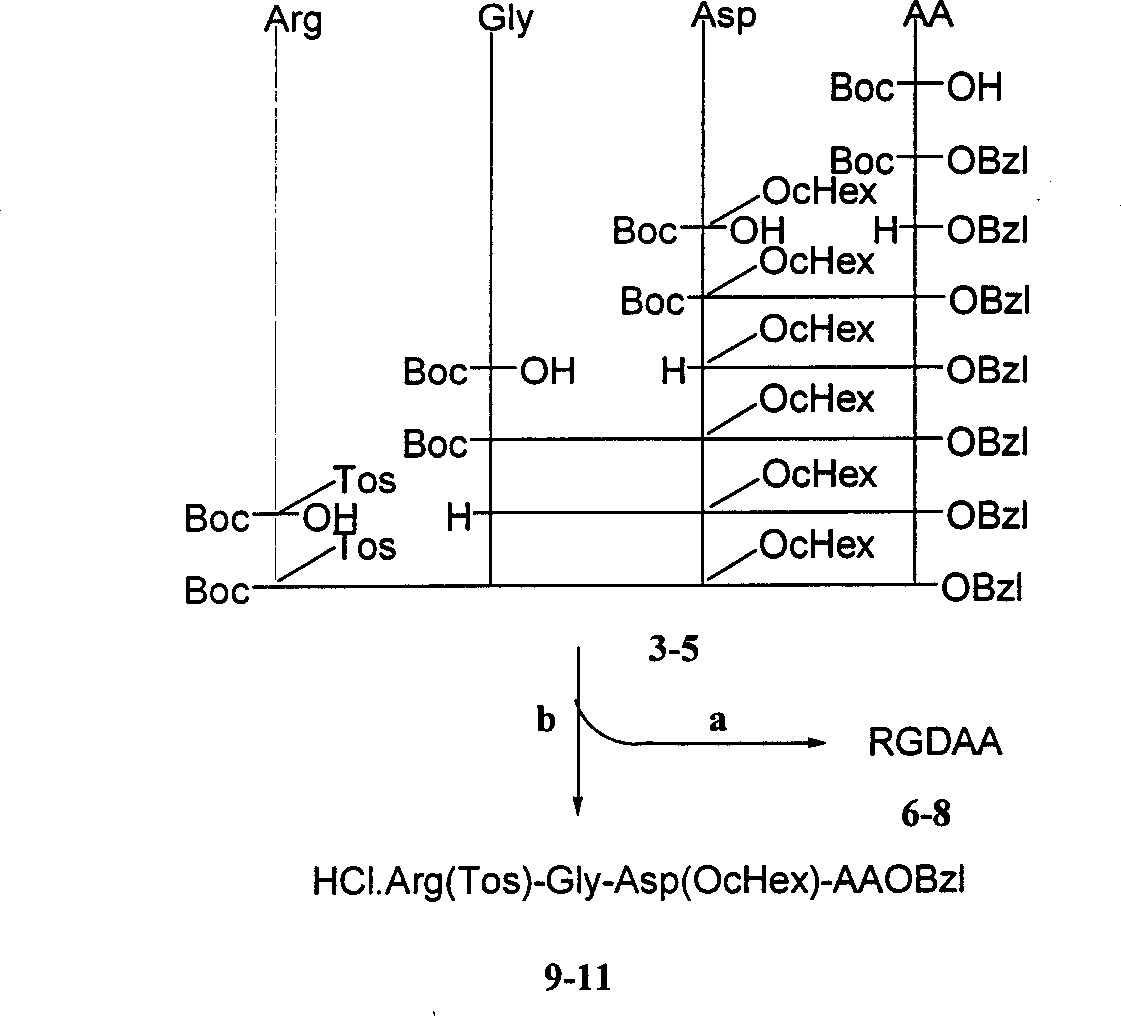
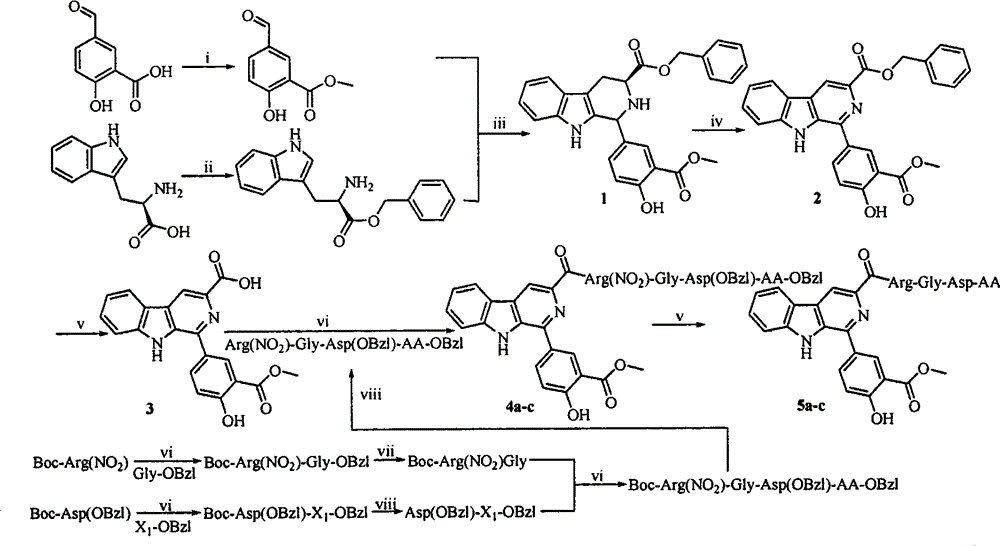
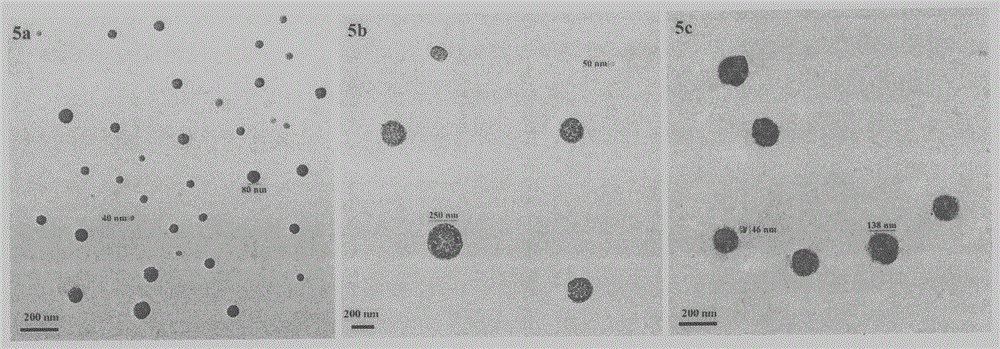
Beta-tetrahydro carboline carboxylic acid, its RGD conjugate, their synthesis and medical application
The present invention relates to the synthesis of beta-tetrahydro carboline carboxylic acid and its nanoparticle preparation; liquid phase process of gradually grafting peptide to obtain protected tetrapeptide intermediate, which, after eliminating its Boc, is condensated with N-Boc-beta-tetrahydro carboline carboxylic acid and further HF treated to eliminate its protection to obtain the said compound; the nanoparticle of tetrahydro carboline carboxylic acid and the in-vitro and in-vivo activity of said compound in-vitro and in-vivo experiments show that the compound of the present invention has excellent activity of resisting blood platelet aggregation, effect of resisting cell adhesion and effect of resisting thrombus.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
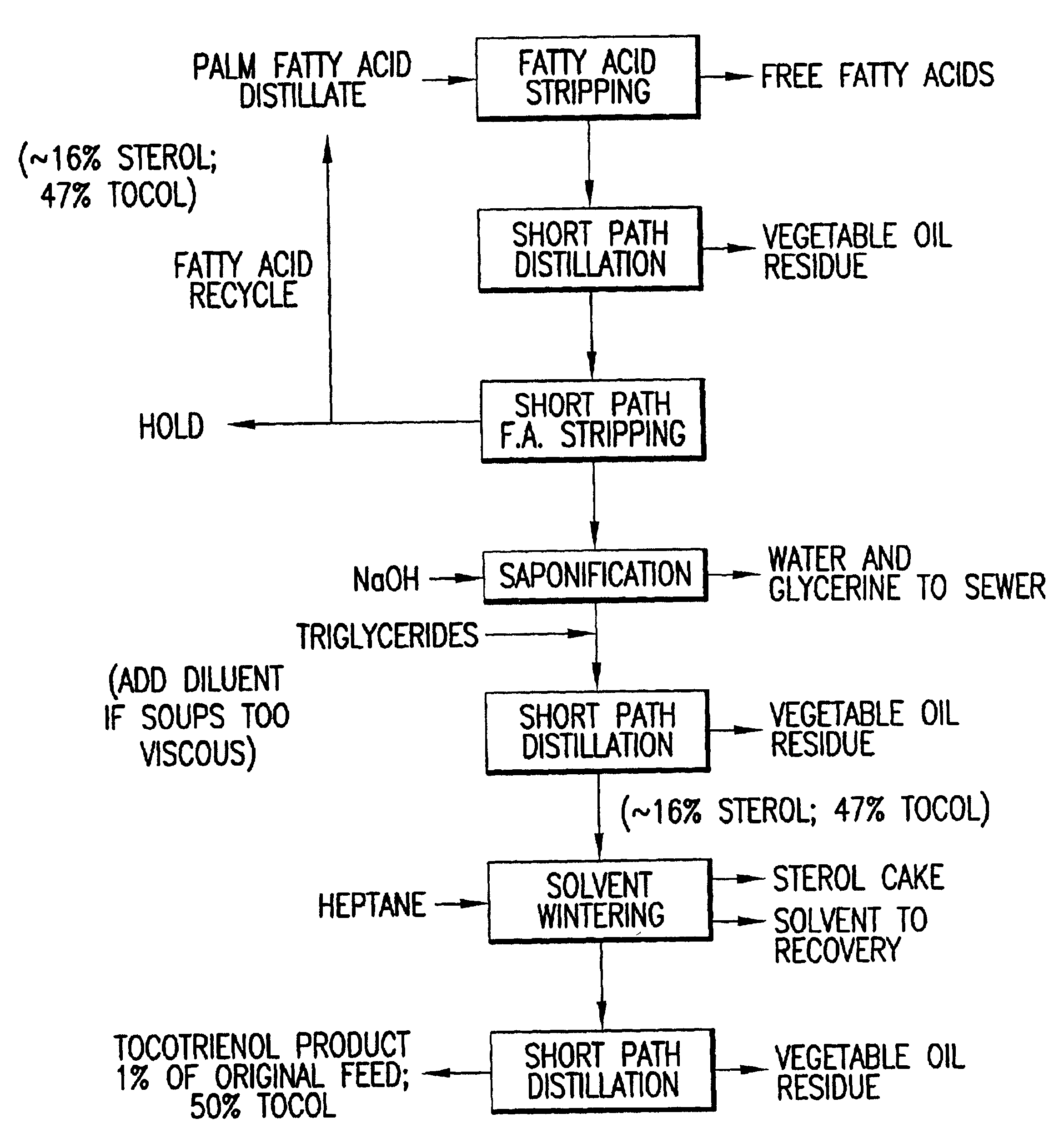
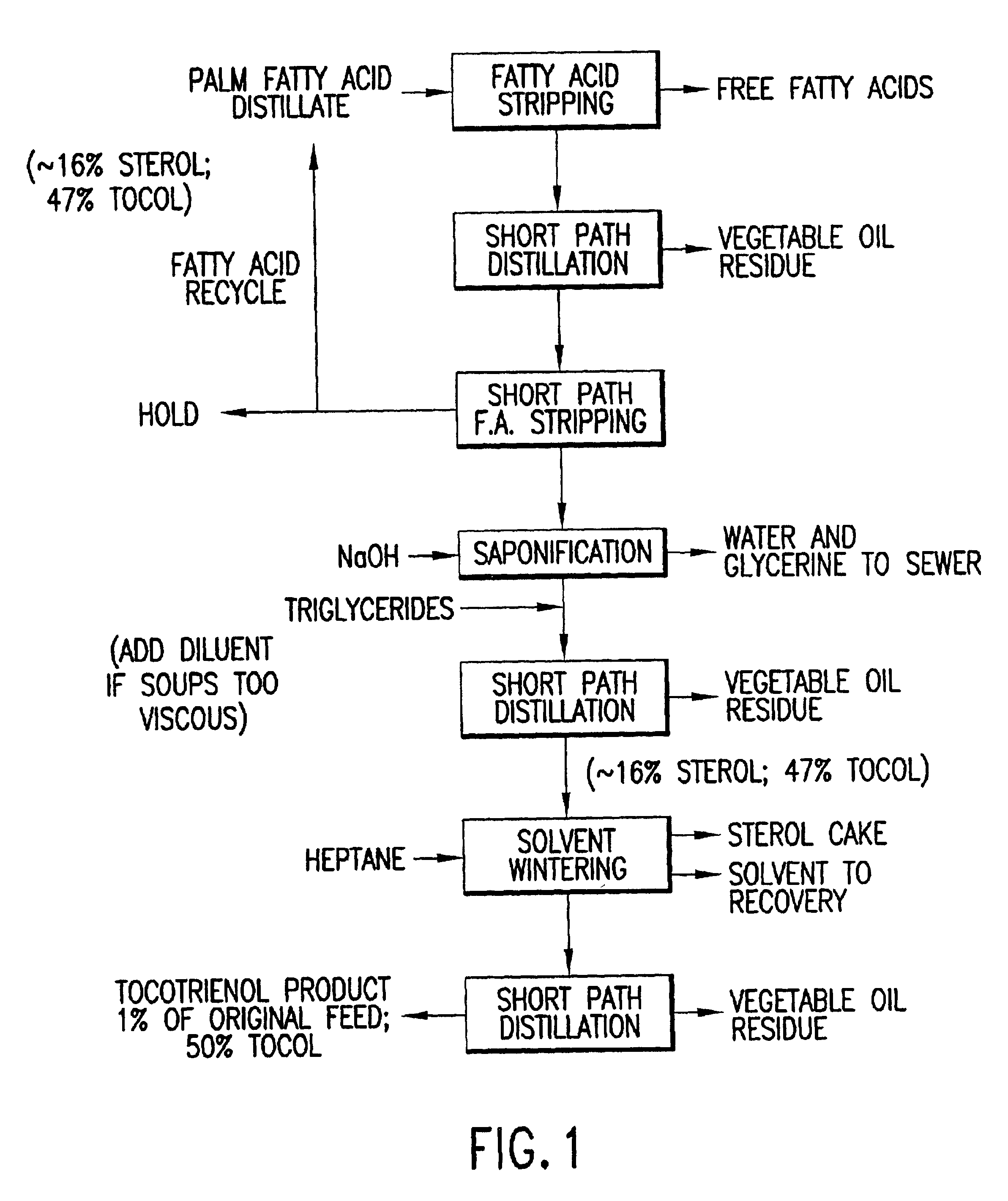
Process for the production of tocotrienols
This invention relates to processes for the production of tocotrienol compounds from biological sources such as palm oil, cereals, grains, and grain oils. The tocotrienol products are recovered in high yields. These tocotrienols are useful as pharmaceuticals, in foodstuffs and as dietary supplements. These compositions are hypocholesterolemic, antioxidizing, antithrombotic, antiatherogenic, antiinflammatory and immunoregulatory in nature. Tocotrienols are known to lower the levels of low density lipoproteins in the bloodstream.
Owner:ARCHER DANIELS MIDLAND CO
Biological component comprising artificial membrane
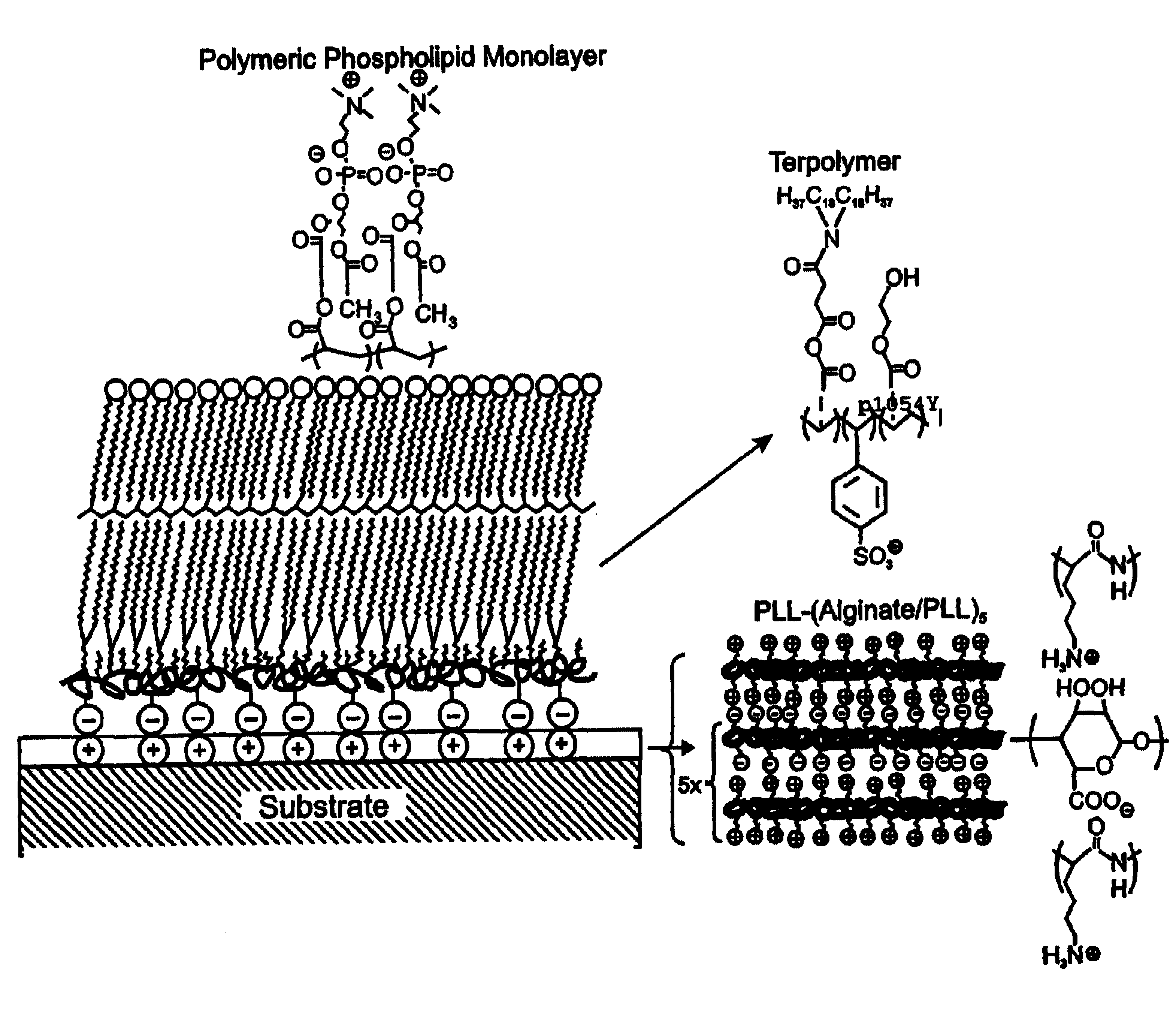
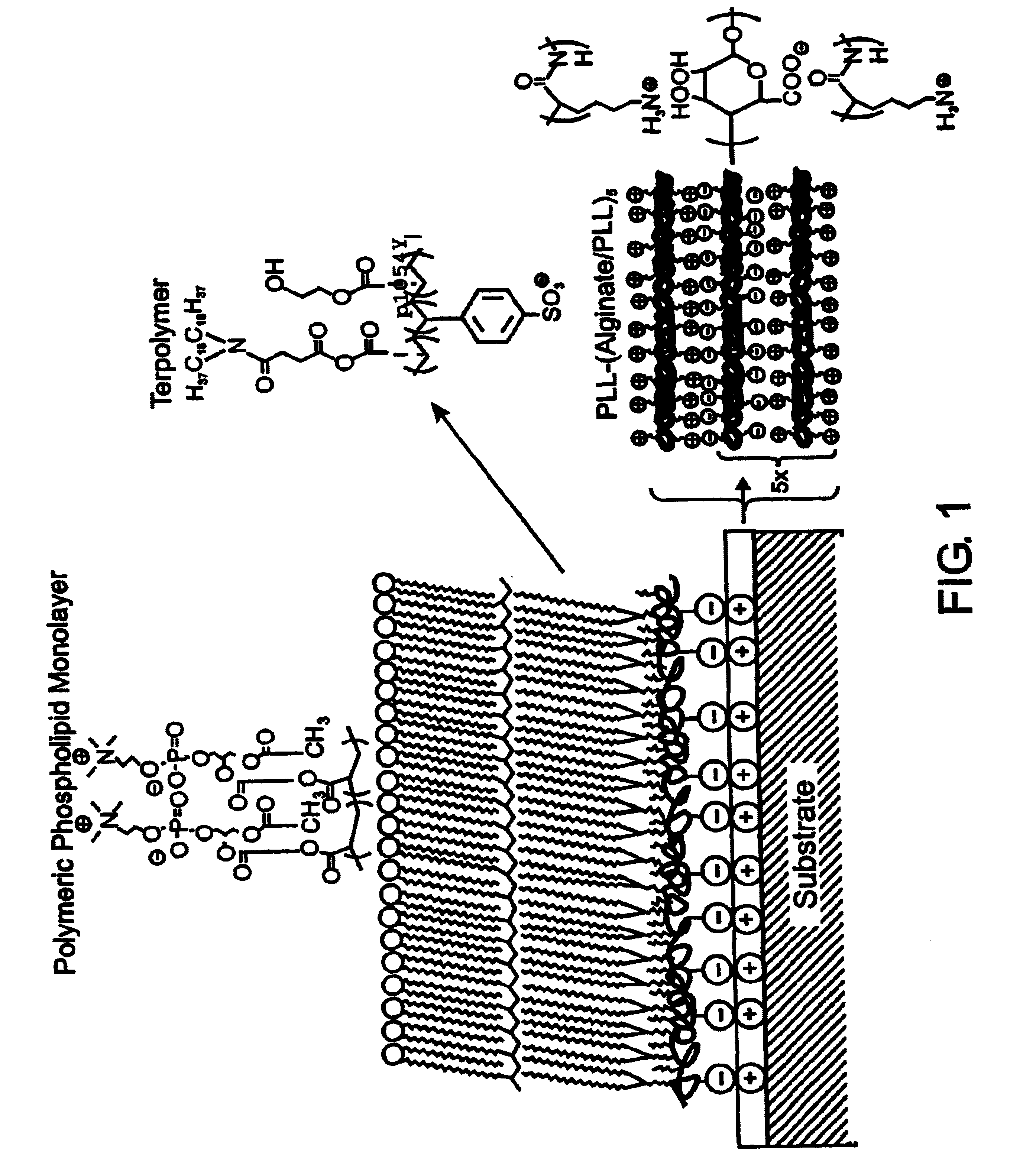
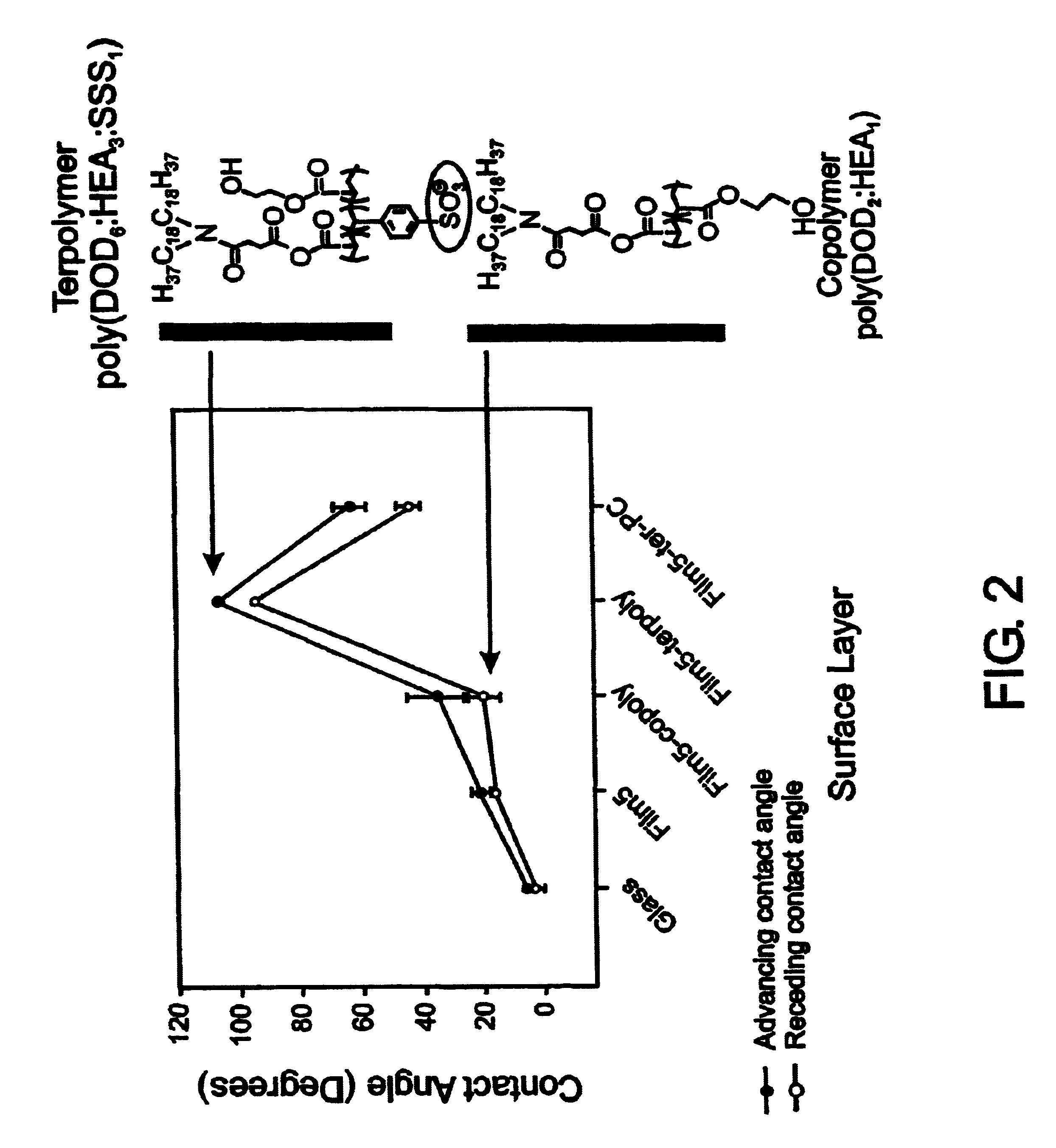
InactiveUS7713544B2Improved performance characteristicsMinimize adhesionMaterial nanotechnologyMicroorganismsAmount of substanceMembrane mimetic
A biocompatible biological component is provided comprising a membrane-mimetic surface film covering a substrate. Suitable substrates include hydrated substrates, e.g. hydrogels which may contain drugs for delivery to a patient through the membrane-mimetic film, or may be made up of cells, such as islet cells, for transplantation. The surface may present exposed bioactive molecules or moieties for binding to target molecules in vivo, for modulating host response when implanted into a patient (e.g. the surface may be antithrombogenic or antiinflammatory) and the surface may have pores of selected sizes to facilitate transport of substances therethrough. An optional hydrophilic cushion or spacer between the substrate and the membrane-mimetic surface allows transmembrane proteins to extend from the surface through the hydrophilic cushion, mimicking the structure of naturally-occurring cells. An alkylated layer directly beneath the membrane-mimetic surface facilates bonding of the surface to the remainder of the biological component. Alkyl chains may extend entirely through the hydrophilic cushion when present. To facilitate binding, the substrate may optionally be treated with a polyelectrolyte or alternating layers of oppositely-charged polyelectrolytes to facilitate charged binding of the membrane-mimetic film or alkylated layer beneath the membrane-mimetic film to the substrate. The membrane-mimetic film is preferably made by in situ polymerization of phospholipid vesicles.
Owner:EMORY UNIVERSITY
Halogenated 2-(a-hydroxyl pentyl) benzoate, production method and uses thereof
ActiveCN101402565ASuitable for storageSuitable for processingOrganic active ingredientsNervous disorderDiseaseBenzoic acid
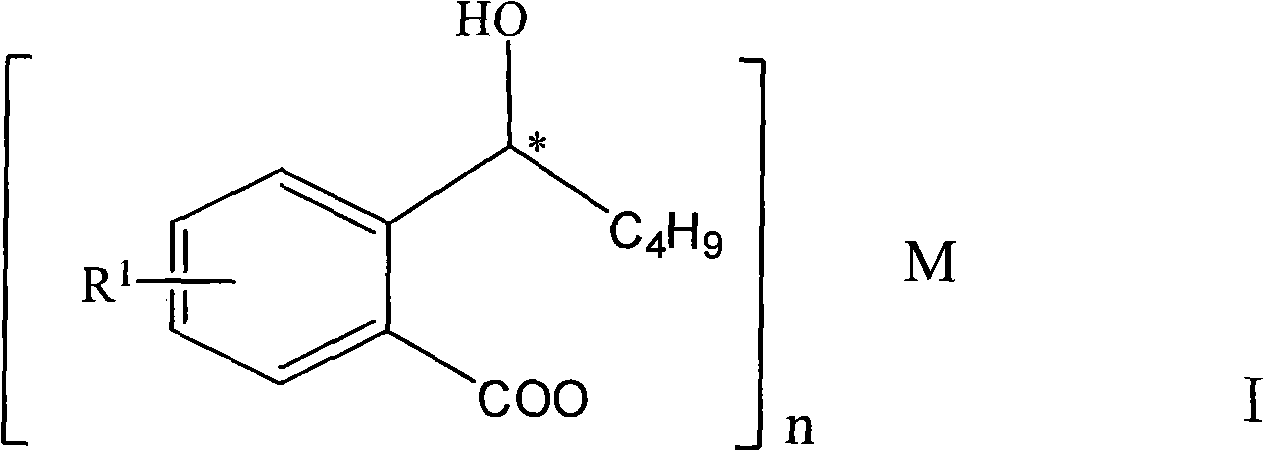
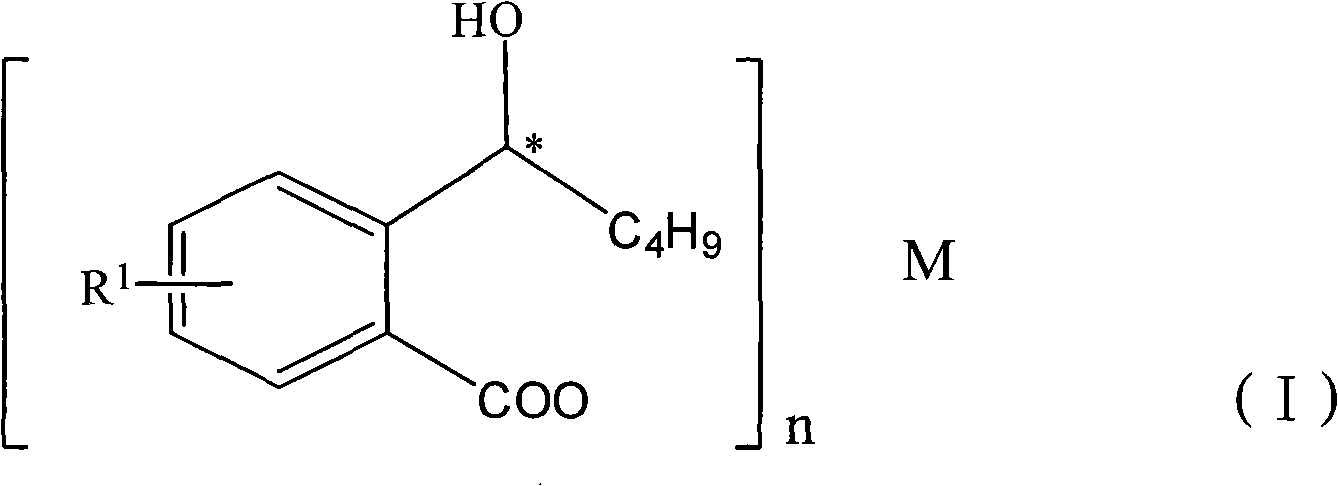
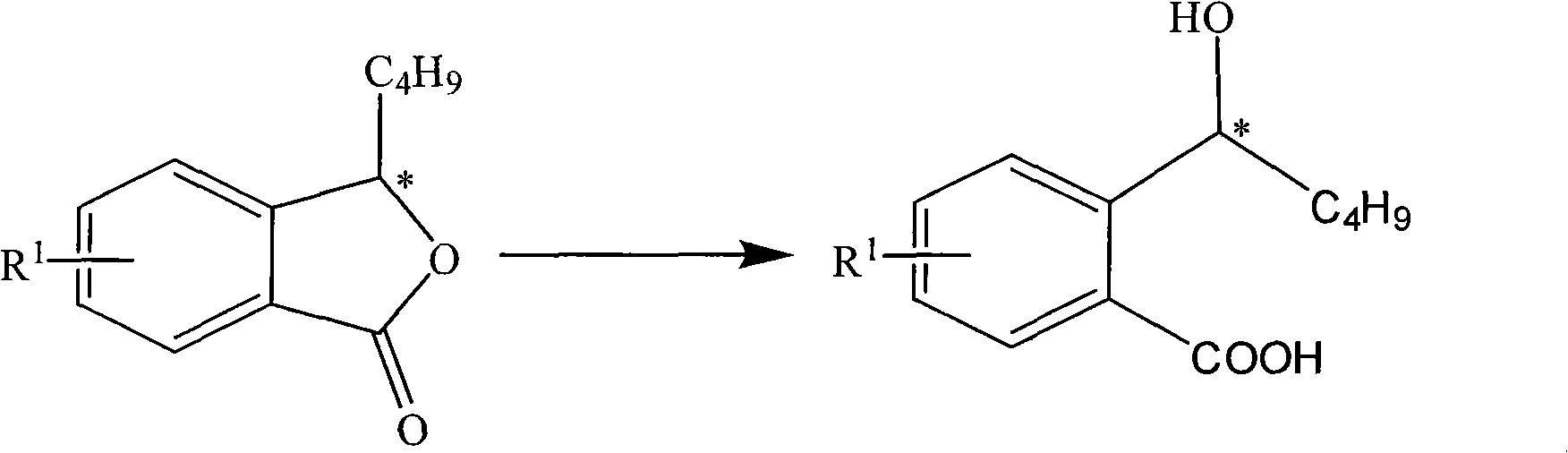
The invention discloses a halogen substituted 2-(a-hydroxyl amyl) benzoate compound, a preparation method and medicinal application thereof, which belong to the technical field of organic chemical synthesis. The structural formula of the compound which is showed as above has an antimer structure, wherein <R1> represents a halogen atom; n is equal to 1 to 3; and M represents a univalent metallic ion or a bivalent metallic ion or a trivalent metallic ion or an organic base. The compound has the advantages of better activity on preventing and treating cardio-cerebral ischemia diseases, improving cardio-cerebral circulation disturbance and resisting thrombus, and the like.
Owner:ZHEJIANG AUSUN PHARMA
Technique for producing ultra-low molecular heparin sodium (calcium)
InactiveCN101519459AImprove securityGood and long-lasting antithrombotic effectPulmonary artery embolismDisease
Aiming at the conditions that heparin has severe bleeding side effects in clinical practice and clinical application thereof is restricted, the invention discloses a technique for producing ultra-low molecular heparin sodium (calcium). The technique comprises the following steps of: taking heparin sodium solution, adding sodium nitrite solution for cracking; adjusting the lysis buffer by using alkaline; absorbing impurities by using an anion-exchange column; washing for obtaining ultra-low molecular heparin calcium; carrying out filtration by using an ultrafiltration membrane and obtaining a precipitate by using alcohol; and after desalting, dehydration, re-precipitation, cooling and drying, obtaining a finished product of ultra-low molecular heparin calcium. The product has better and safer antithrombotic effect under low level of anticoagulation, and can be widely used for preventing and treating diseases such as deep vein thrombosis, pulmonary embolism, disseminated intravascular coagulation, and the like.
Owner:SUZHOU FAST BIOLOGICAL PHARMACY TECH
Therapeutic morpholino-substituted compounds
Morpholino-substituted pyridopyrimidine, quinolone, and benzopyranone derivatives inhibit phosphoinositide (PI) 3-kinase, an enzyme that regulates platelet-adhesion processes. As a consequence, the compounds in question have anti-thrombotic activity, as well as other pharmaceutical properties. The compounds claimed are represented by formula (I), (II) and (III). PI 3-kinase generates 3-phosphorylated PI second messengers which stimulate platelet adhesion under blood-flow conditions. Because platelet adhesion is a necessary step in the formation of a thrombus, inhibition by these compounds of PI 3-kinase under such conditions inhibits or prevents thrombus formation. The compounds are useful in treating PI 3-kinase-dependent conditions including cardiovascular diseases such as coronary artery occlusion, stroke, acute coronary syndrome, acute myocardial infarction, vascular restenosis, atherosclerosis, and unstable angina; respiratory diseases such as asthma, chronic obstructive pulmonary diseases (COPD), and bronchitis; inflammatory disorders; neoplasms including cancers such as glioma, prostate cancer, small cell lung cancer, and breast cancer, and diseases linked to disordered white blood cell function, such as autoimmune and inflammatory diseases.
Owner:ASTRAZENECA AB
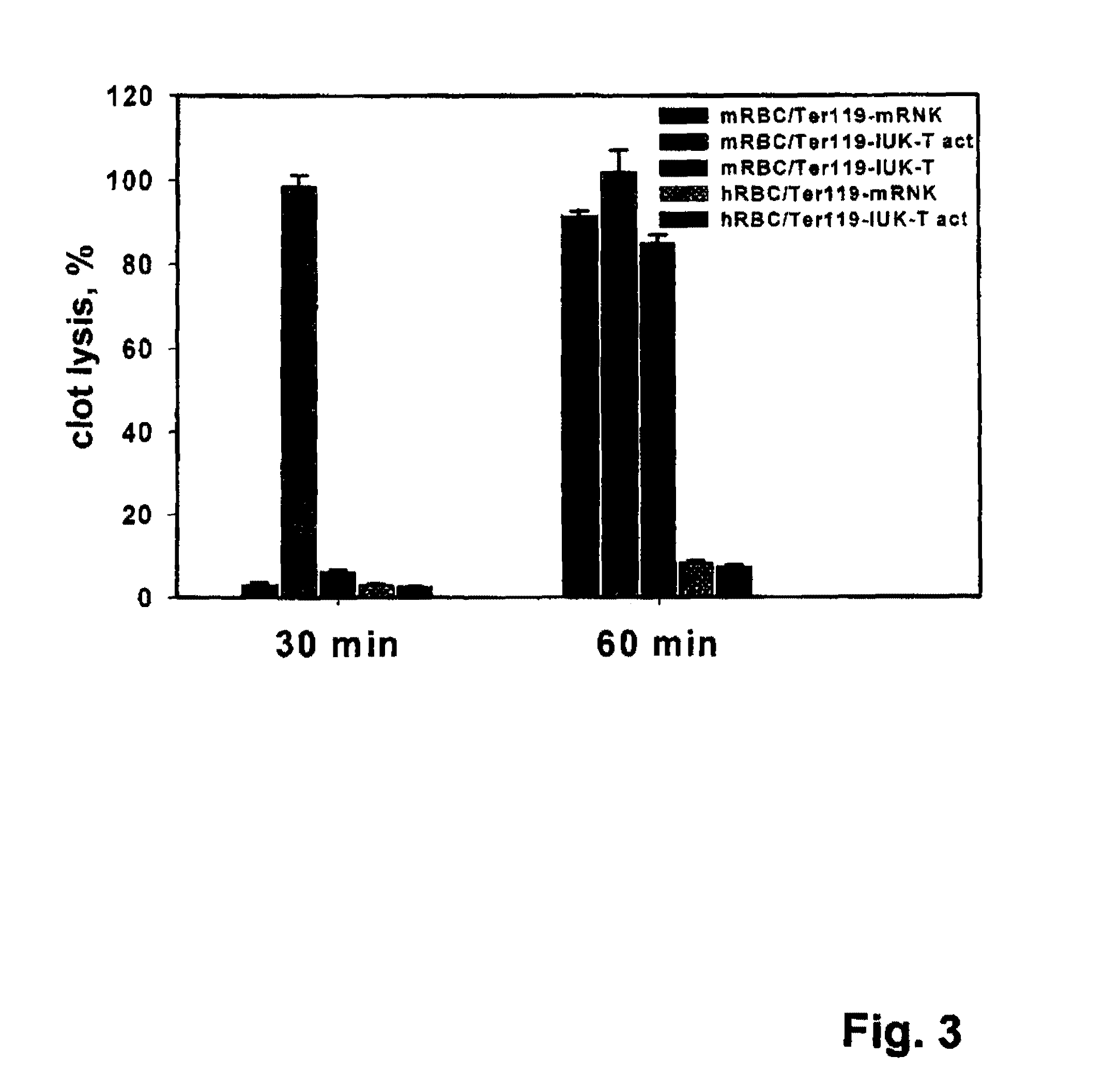
Targeting recombinant therapeutics to circulating red blood cells
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Implantable vascular access system
ActiveUS20120245536A1Easy to moveAvoiding operator errorMedical devicesIntravenous devicesAntithromboticReoperative surgery
The invention relates to implantable vascular access ports that can release agents such as antibiotics, anti-thrombogenics and anti-proliferatives. The invention also relates to ports having locking mechanisms that prevent accidental disengagement, and structures that facilitate surgical implantation and provide for long-term stability and use of the port.
Owner:MOZARC MEDICAL US LLC
Devices with Anti-thrombogenic and Anti-microbial treatment
PendingUS20160015863A1Reduce microbial activityReducing a thrombogenic eventBiocideOrganic active ingredientsMedicineMedical device
A medical device adapted for contact with a vessel or cavity in the body including a tubular portion is provided. The device has an external surface including an external substance that is at least one of a coating or an impregnation, comprising alexidine in an amount that is both anti thrombogenically effective and anti microbially effective. The device also has an internal surface including an internal substance that is at least one of a coating or an impregnation, comprising alexidine in an amount that is both anti thrombogenically effective and anti microbially effective.
Owner:TELEFLEX MEDICAL INC
Chinese medicine extract and medicine use thereof
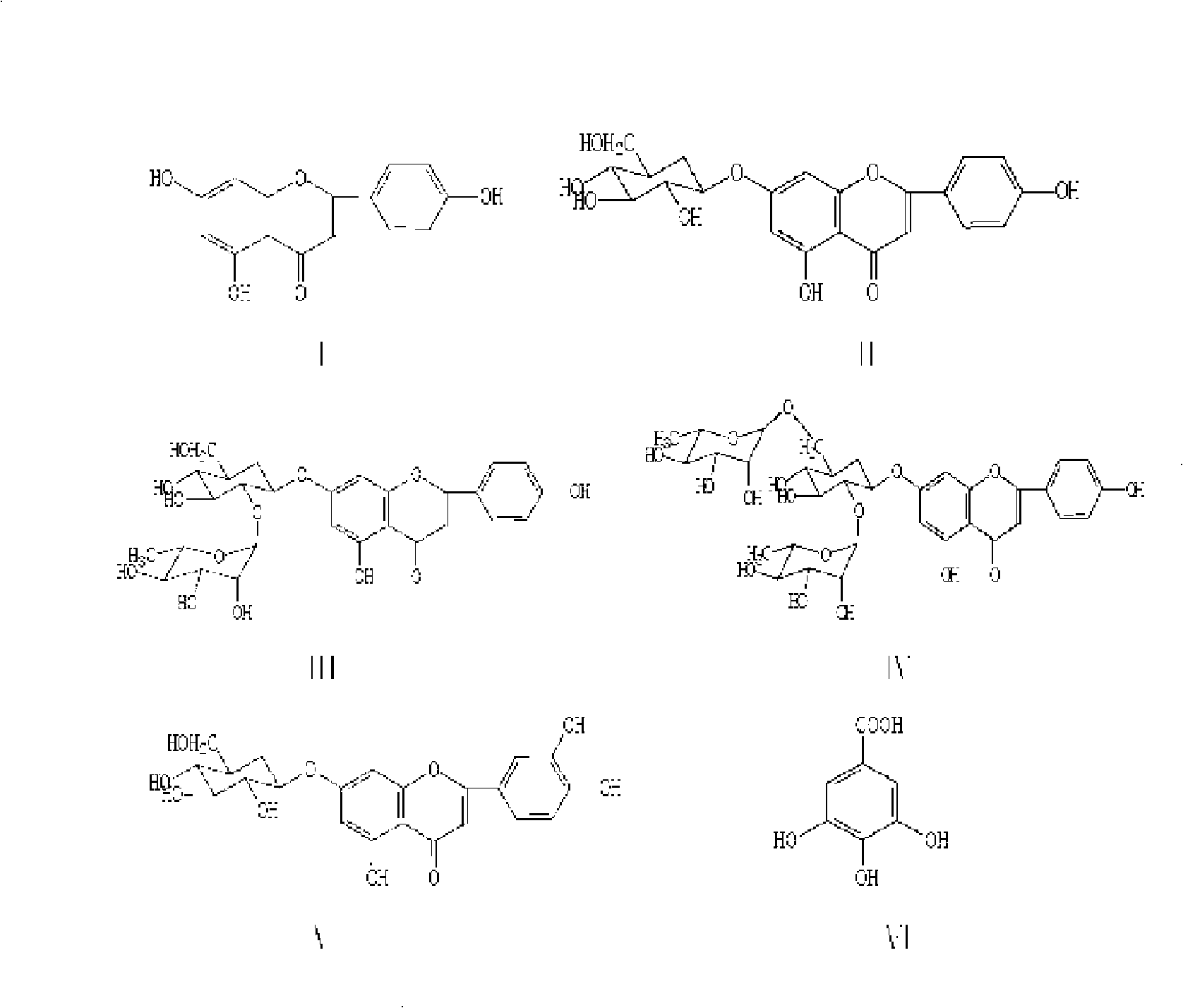
The invention belongs to the traditional Chinese medicinal material field, and relates to a Turpinia arguta leaf extract, which contains a flavonoid chemical constituent and acceptable salt in the medicine thereof. The content of total flavonoids is more than 15 percent; the content of apigenin aglycon and a flavone glycoside constituent which uses apigenin as aglycon is no less than 1.5 percent measured in the apigenin; a flavonoid chemical constituent contains one or more of the apigenin, apigenin-7-O-Bata-neohesperidoside, apigenin-7-O-2<1>-Bata-rhamanopyranosyl rutinoside, apigenin-7-O-Bata-glucoside, luteolin-7-O-Bata-glucoside. The invention furhter provides a method for preparing the extract thereof, a method for controlling the quality of the extract, and the application of the extract for preparing the medicines such as antibiosis, oxidation resistance, anti-mutation, antitumor, hepatic protection, anti-hepatitis b virus, anti-thrombosis, anti-arteriosclerosis, etc.
Owner:胡军
Process to prepare clopidogrel
The present invention relates to a process for the preparation of thieno[3,2-c]pyridine derivatives having pharmacologically significant anti-aggregating and anti-thrombotic properties.
Owner:CADILA HEALTHCARE LTD
Shanxiangyuan leaf extract, preparation method and uses thereof
The invention relates to a method for preparing the suaveolic round leaf extractive which contains celery element or the chromocor compound with celery element as corn, or relative salt. The invention also provides relative quality control method, while said extractive can be used to prepare the drug for treating virus B hepatitis, thrombus, cancer, or the like.
Owner:胡军
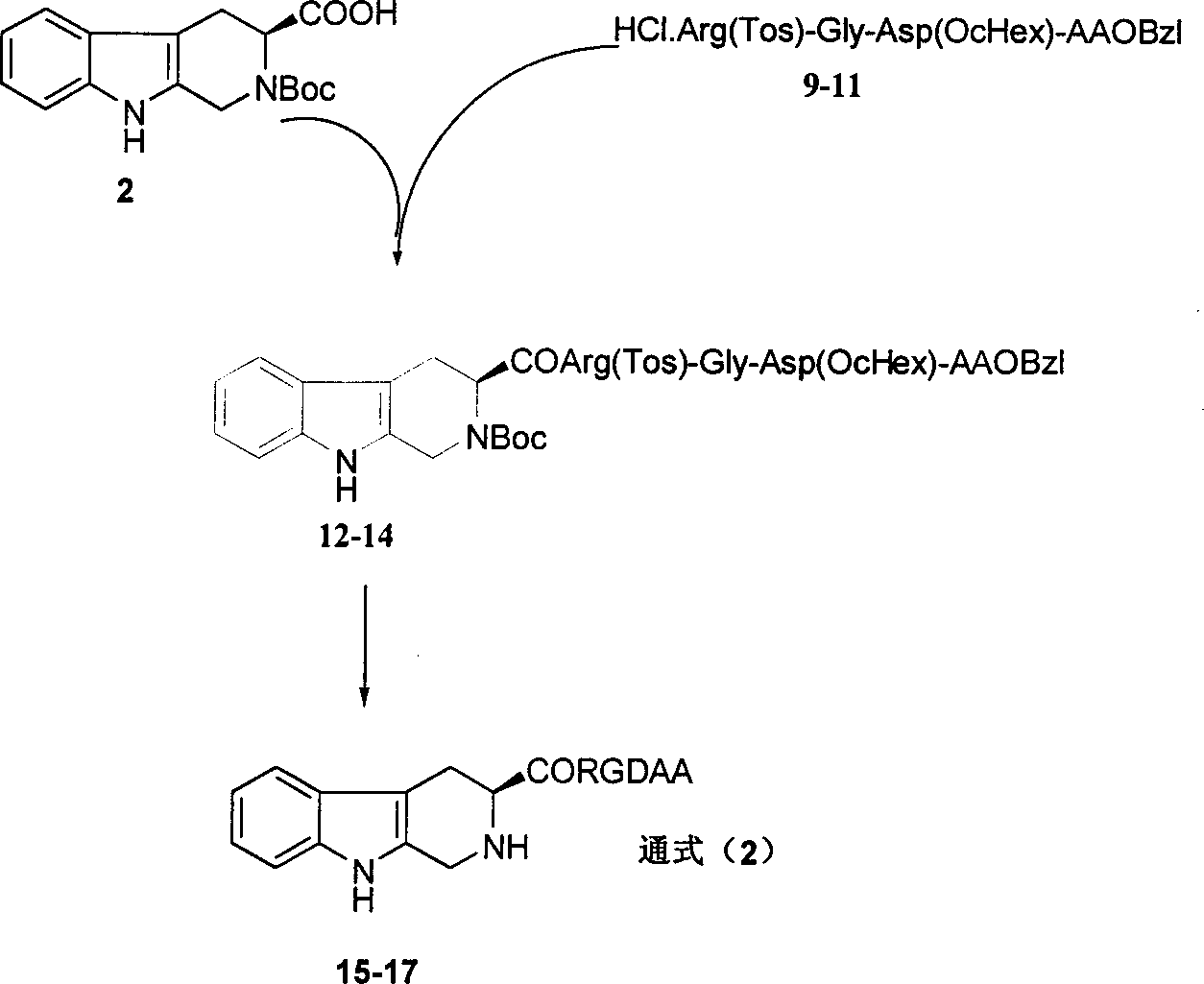
RGD tetrapeptide-modified beta-carboline, preparation, activity and application thereof
The invention discloses 1-(4-hydroxy-3-methoxycarbonylphenyl)-beta-carboline-3-formyl-RGD tetrapeptide with a structure as shown in the formula. The invention also discloses a preparation method of the compound, a nano-structure of the compound, an anti-tumor effect of the compound, an effect of the compound in inhibiting adhesion, invasion and migration of tumor cells, further discloses anti-inflammatory and antithrombotic effects of the compound and expounds an application of the compound in medical science.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
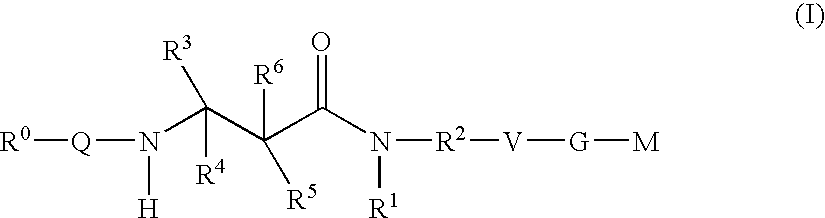
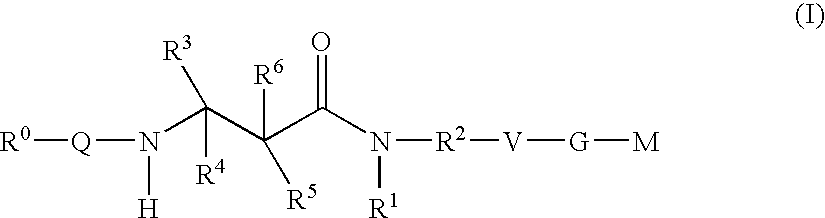
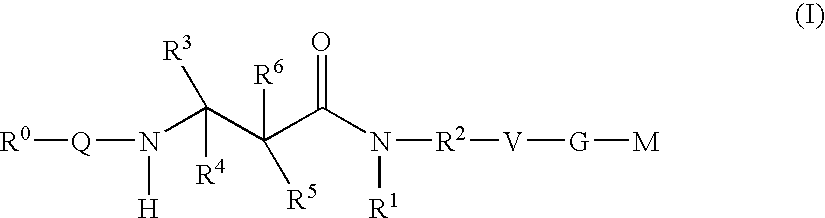
Beta-Aminoacid-Derivatives As Factor Xa Inhibitors
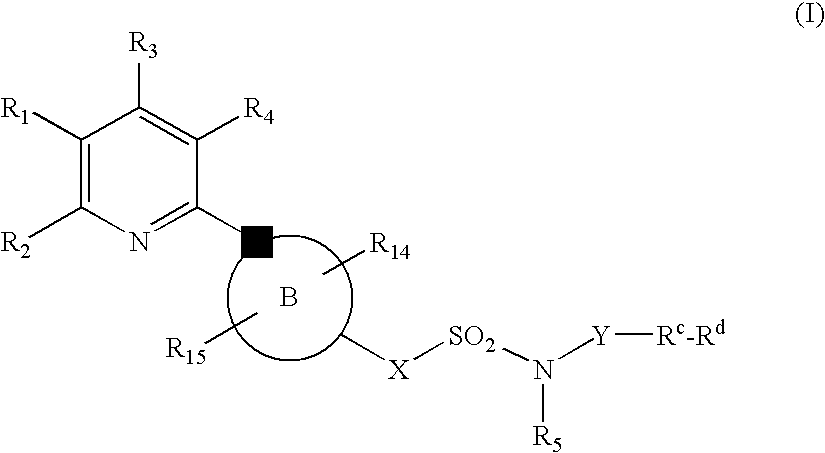
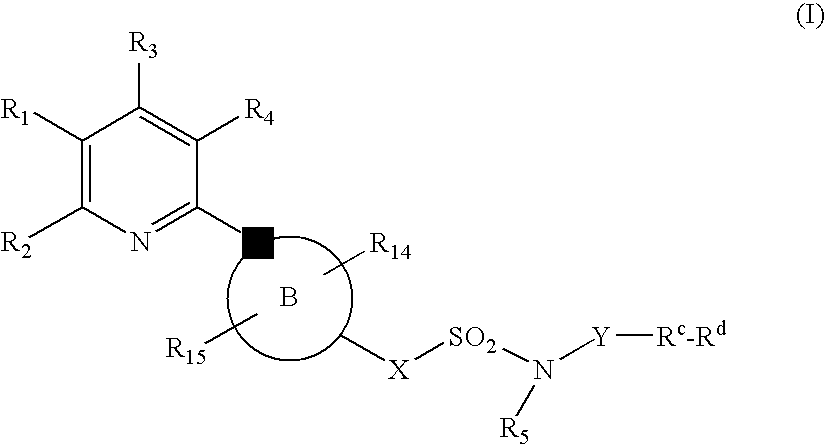
The present invention relates to compounds of the formula I, in which R0; R1; R2; R3; R4; R5, R, Q; V, G and M have the meanings indicated in the claims. The compounds of the formula I are valuable pharmacologically active compounds. They exhibit a strong antithrombotic effect and are suitable, for example, for the therapy and prophylaxis of cardiovascular disorders like thromboemboic diseases or restenoses. They are reversible inhibitors of the blood clotting enzymes factor Xa (FXa) and / or factor VIIa (FVIIa), and can in general be applied in conditions in which an undesired activity of factor Xa and / or factor VIIa is present or for the cure or prevention of which an inhibition of factor Xa and / or factor VIIa is intended. The invention furthermore relates to processes for the preparation of compounds of the formula I, their use, in particular as active ingredients in pharmaceuticals, and pharmaceutical preparations comprising them.
Owner:SANOFI AVENTIS DEUTSCHLAND GMBH
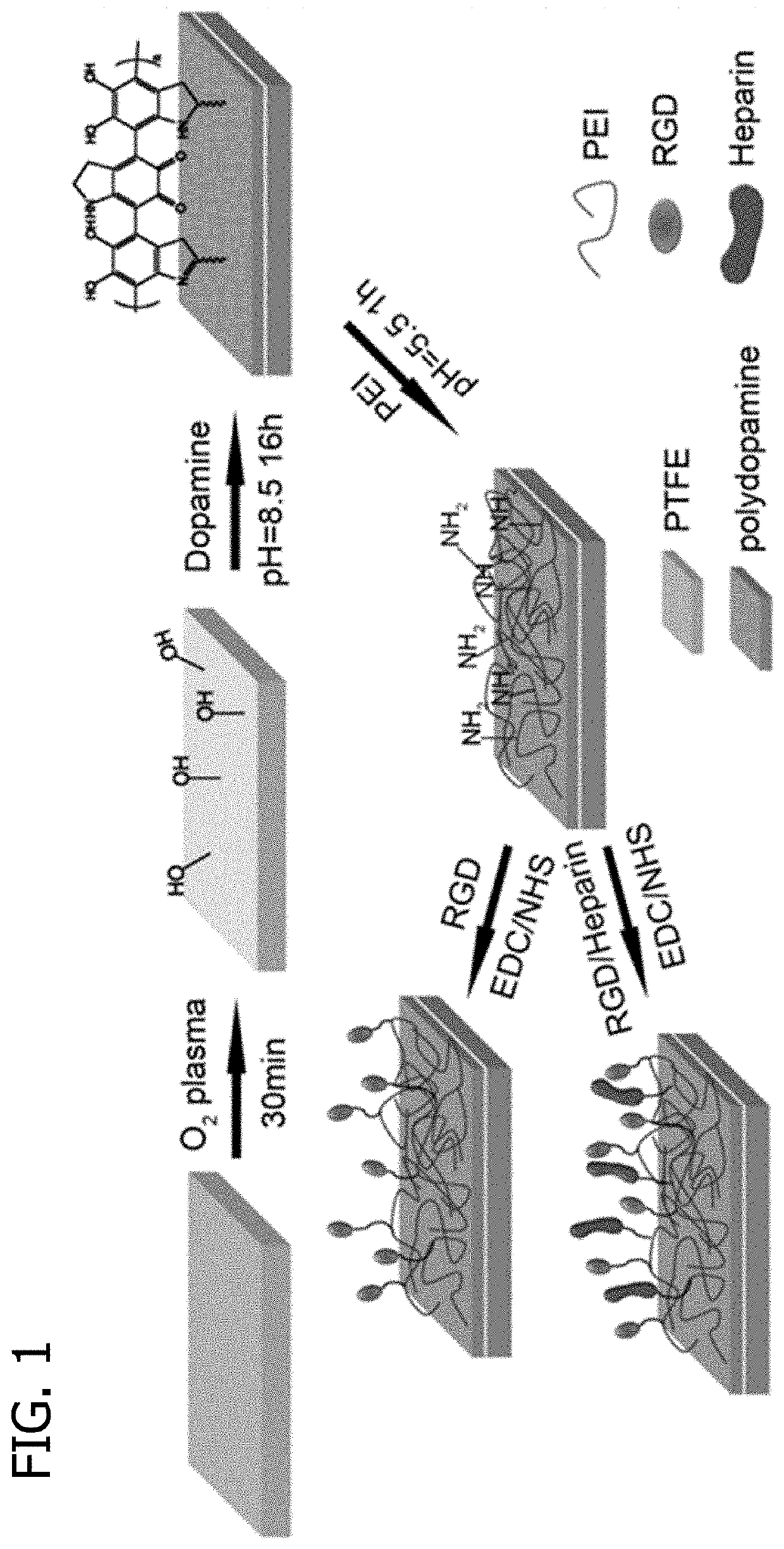
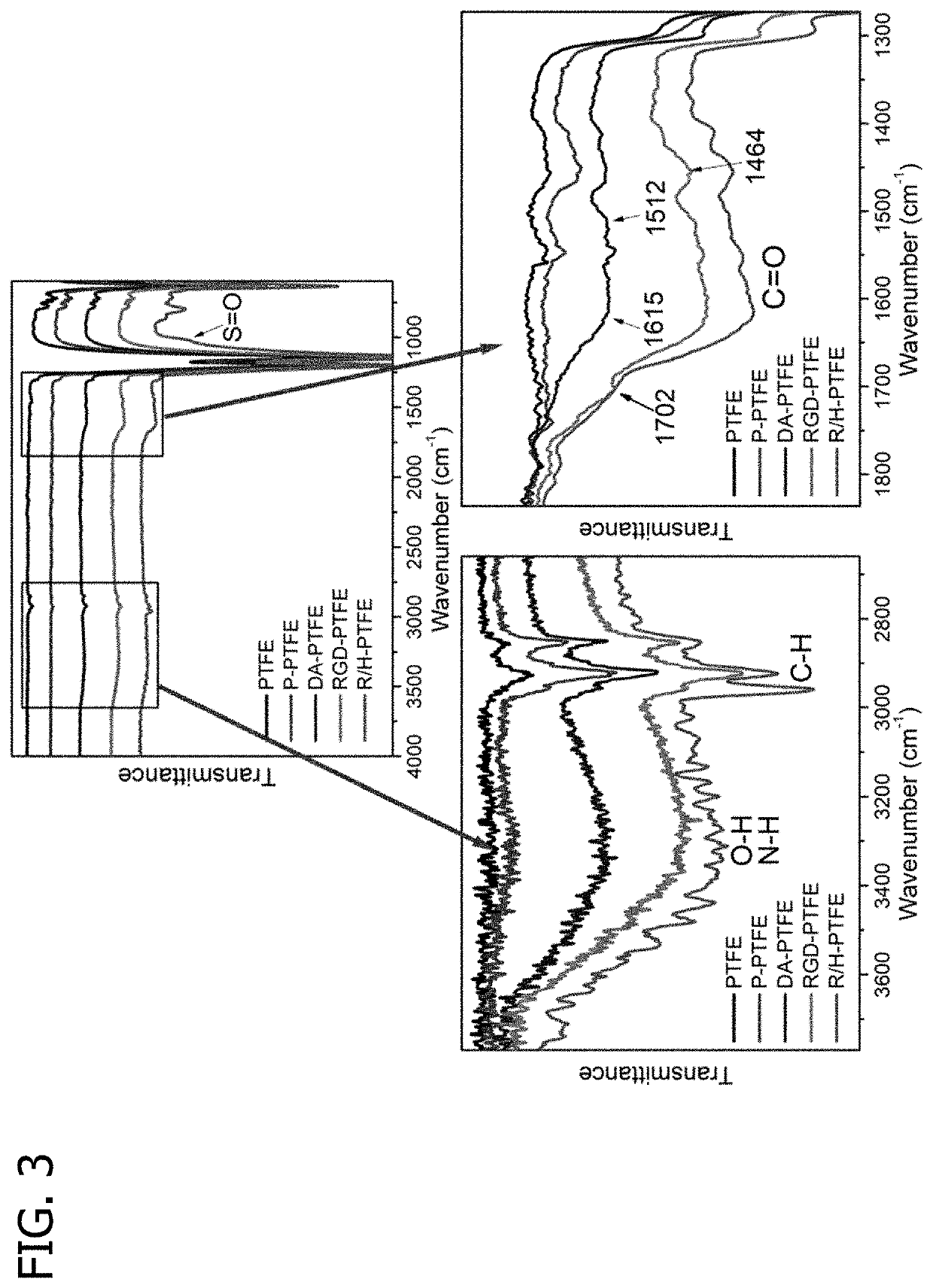
Promoting endothelial cell affinity and antithrombogenicity of polytetrafluoroethylene (PTFE) by mussel-inspired modification and rgd/heparin grafting
ActiveUS20190365954A1Promote cell affinityReduce thrombosisPharmaceutical containersPharmaceutical delivery mechanismCrystallographyThrombogenicity
Disclosed herein are methods for modifying a substrate having a hydrophobic surface. Also disclosed are modified hydrophobic substrates. The modified hydrophobic substrates and methods disclosed herein advantageously improve cell affinity and antithrombogenicity of hydrophobic surfaces.
Owner:WISCONSIN ALUMNI RES FOUND
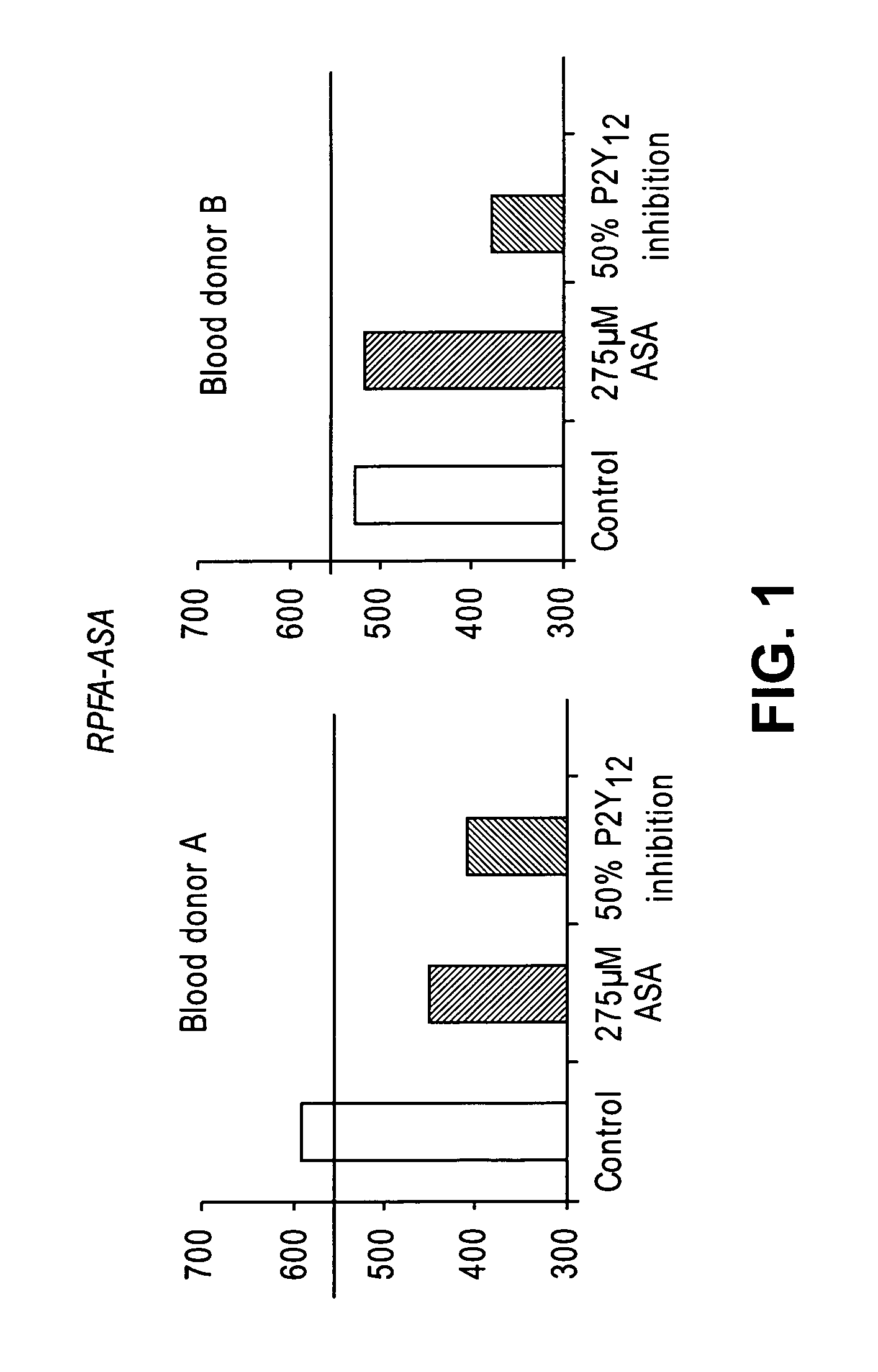
Methods for determining patient response to Anti-platelet aggregation therapy
ActiveUS20140200240A1Small particle sizeDetect presenceBiocideBiological testingThrombusAnti platelet
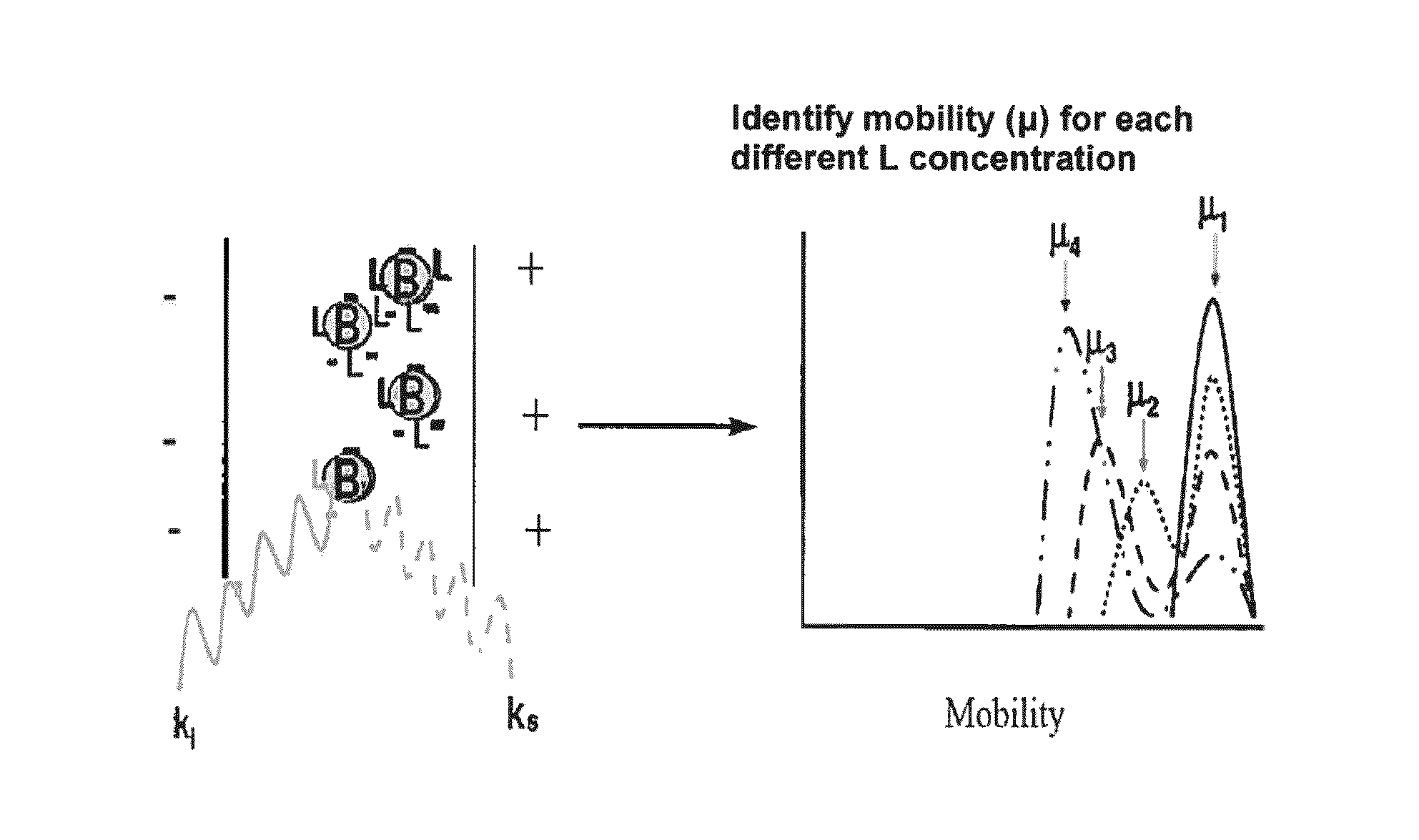
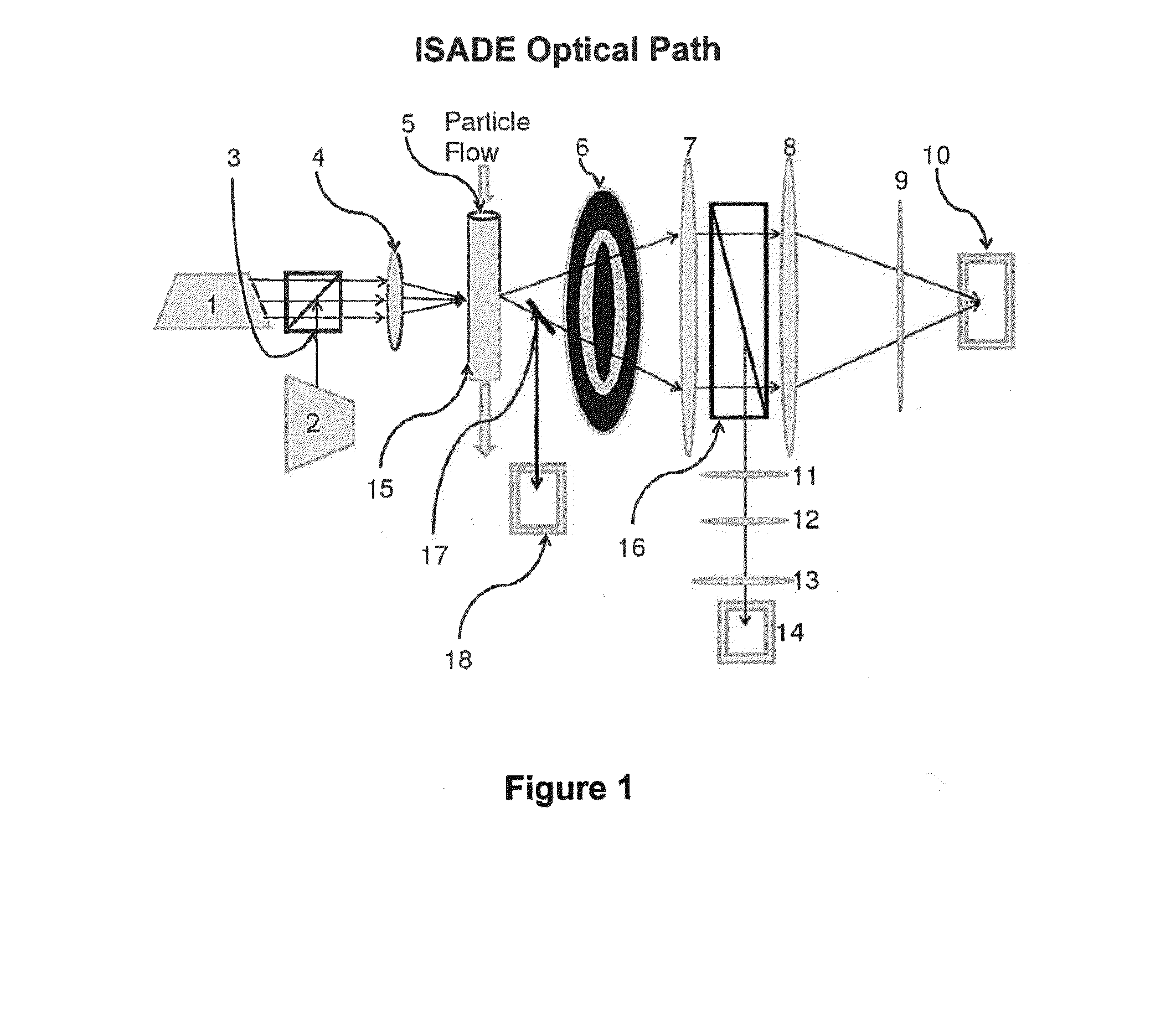
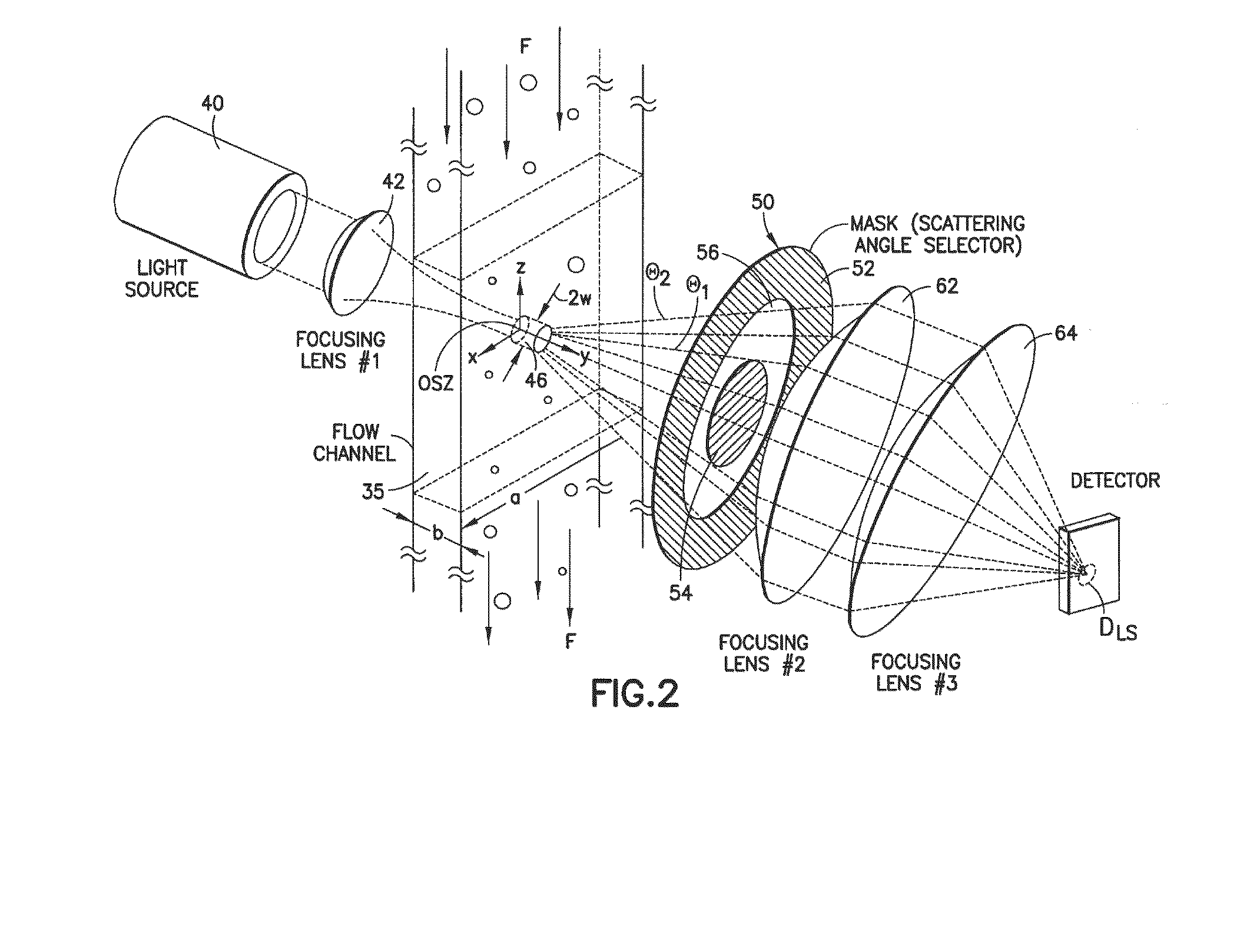
Diagnostic methods for determining whether an individual will benefit from a particular anti-thrombotic therapeutic agent are disclosed. The methods involve obtaining a biological sample that comprises platelets, from a patient who has been pre-administered a particular therapeutic agent, which is an antagonist of a receptor associated with the biochemical pathways involved in platelet aggregation, and exposing the platelets to an agonist of the receptor. If the antagonist is ineffective, the platelets will eject microparticles, will have a different size distribution than platelets not exposed to the agonist, and will experience a change in their surface charge. In one embodiment, the diagnostic methods involve using single particle optical sizing techniques to determine the presence of such ejected microparticles, or a change in platelet size due to its activation by the agonist. In another embodiment, electrophoretic quasi-elastic light scattering techniques are used to determine the presence of a change in surface charge on the platelets. Once an effective therapeutic agent, or an effective dosage of such therapeutic agent, has been identified, the patient can begin therapy knowing that the agent will be effective.
Owner:INVITROX
Method of thrombolysis by local delivery of reversibly inactivated acidified plasmin
InactiveUS6964764B2Formula stableReduce capacityPeptide/protein ingredientsInorganic non-active ingredientsWhole bodyThrombus
Methods of thrombolysis that allow the use of a fibrinolytic composition comprising reversibly inactivated acidified plasmin and the localized delivery of the plasmin to a vascular thrombotic occlusion are disclosed. Further disclosed is a method for administering a therapeutic dose of a fibrinolytic composition substantially free of plasminogen activator to a human or animal having a vascular thrombotic occlusion. The fibrinolytic composition includes a reversibly inactivated acidified plasmin substantially free of plasminogen activator. Intravascular catheter delivery of the fibrinolytic composition directly into or in the immediate vicinity of the thrombus is disclosed to minimize the systemic degradation of fibrin while retaining the maximum plasmin activity against the thrombus.
Owner:GRIFOLS THERAPEUTICS LLC
Device and methods for identifying and treating aspirin non-responsive patients
InactiveUS20060160165A1Reduce post-acute myocardial infarction (AMI) cardiovascular eventIncreased riskMicrobiological testing/measurementBiological testingO-acetylsalicylic acidMedicine
The present invention relates to methods and compositions for identifying and treating subjects in need of antithrombotic therapies but who are not responsive to aspirin.
Owner:ALEXION PHARMA INC
Pyridine Analogues VI
InactiveUS20080045494A1Improve performanceHigh selectivityBiocideOrganic active ingredientsVascular diseasePyridine
Owner:ASTRAZENECA AB
Combination drug therapy for reducing scar tissue formation
InactiveUS20080039362A1Reduce spreadPrevents a permanent catheter tip splayBiocidePeptide/protein ingredientsCombination drug therapySurgical site
The present invention describes various devices and methods wherein a cytostatic antiproliferative drug, either alone or in combination with other drugs, is placed between internal body tissues to prevent the formation of scar tissue and / or adhesions during healing of a wound or surgical site. Specific devices to achieve this administration include, but are not limited to, a permanent implant or a biodegradable material having an attached antiproliferative drug such as sirolimus. These antiproliferative drugs may be combined with other drugs including, but not limited to, antiplatelets, antithrombotics or anticoagulants. The present invention also contemplates methods to a reduce scar tissue and / or adhesions or adhesion formation at an anastomosis site. In particular, a cytostatic antiproliferative drug is administered to an arteriovenous shunt anastomoses in patients having end-stage renal disease.
Owner:AFMEDICA INC
Device and methods for identifying and treating aspirin non-responsive patients
The present invention relates to methods and compositions for identifying and treating subjects in need of antithrombotic therapies but who are not responsive to aspirin.
Owner:ALEXION PHARMA INC
Drug/gene eluting stent
InactiveUS20070077266A1Improve gene transfer efficiencyReduce the given dosesPeptide/protein ingredientsGenetic material ingredientsEndothelial regenerationThrombus
The present invention provides a more safe and highly effective stent having functions such as anti-inflammatory action, antithrombotic action, maintenance of tissue restoration response and maintenance of endothelial regeneration. More specifically, the drug / gene eluting stent has a layer containing a gene encoding a hybrid polypeptide on the surface. The hybrid polypeptide is preferably a bound of fibronectin-derived collagen binding domain (FNCBD) polypeptide and an anti-inflammatory factor or an angiogenic factor. The uniform fine particle size capsules can directly deliver the gene encoding the hybrid polypeptide to the lesion and have benefits of reducing the given doses, and thus improving safety and efficacy and further maintaining the efficacy for a long period.
Owner:EGASHIRA KENSUKE
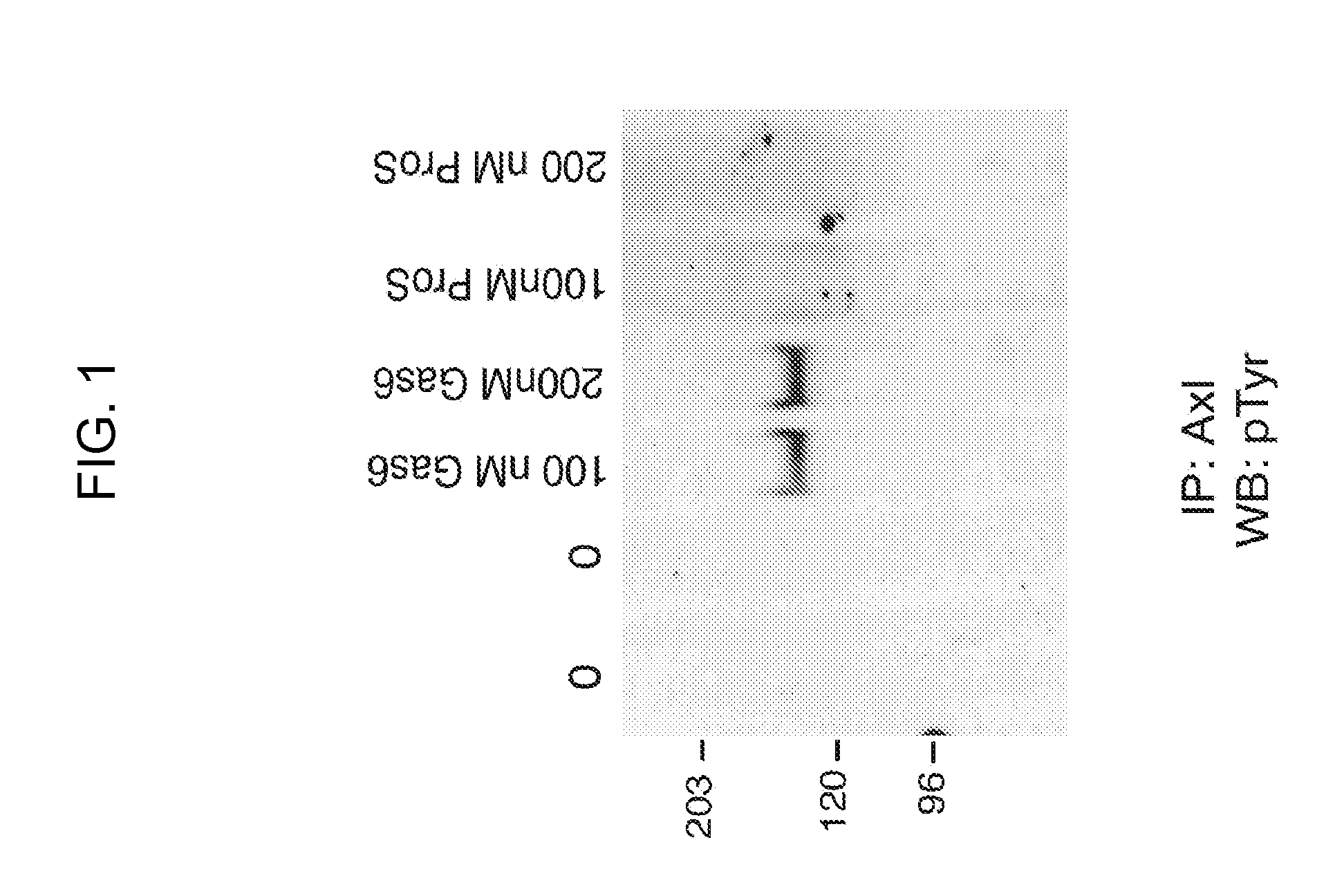
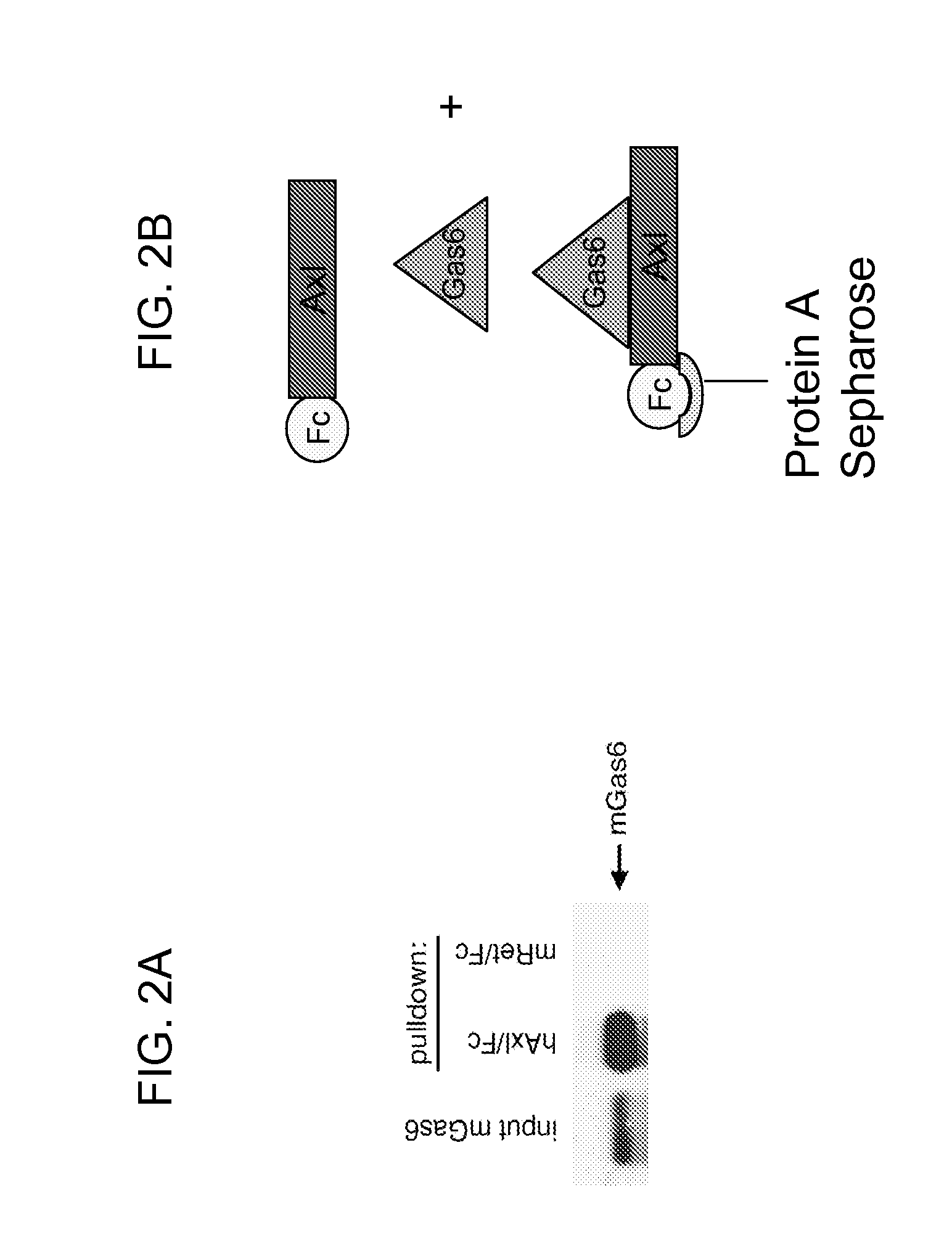
Axl tyrosine kinase inhibitors and methods of making and using the same
ActiveUS20110014173A1Increased riskPeptide/protein ingredientsAntibody mimetics/scaffoldsTyrosine-kinase inhibitorCancer therapy
Disclosed are novel inhibitors of the AxI receptor tyrosine kinase (RTK) and methods of using such inhibitors in a variety of therapeutic approaches in the areas of cancer therapy and anti-thrombosis (anti-clotting) therapy.
Owner:UNIV OF COLORADO THE REGENTS OF
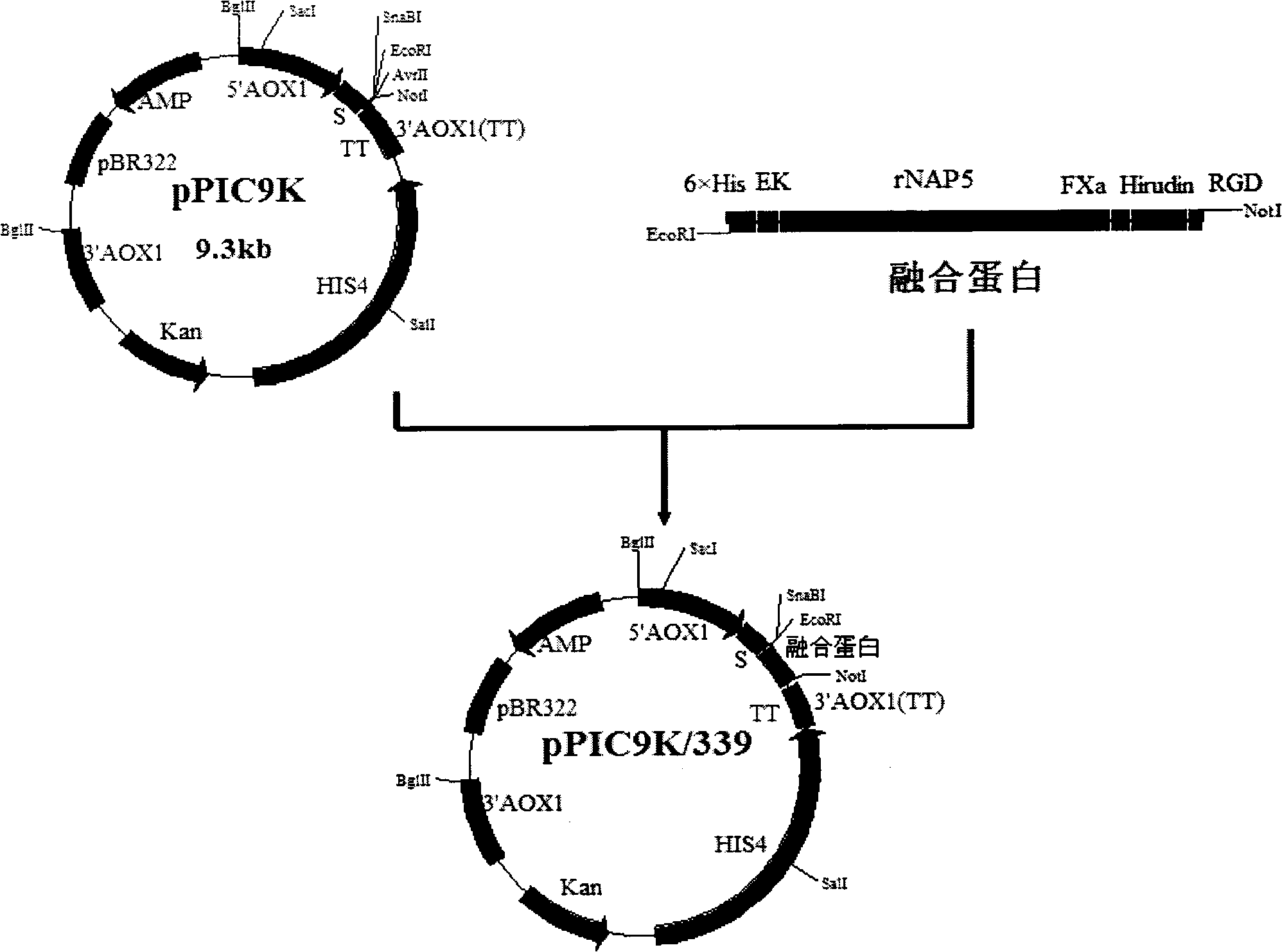
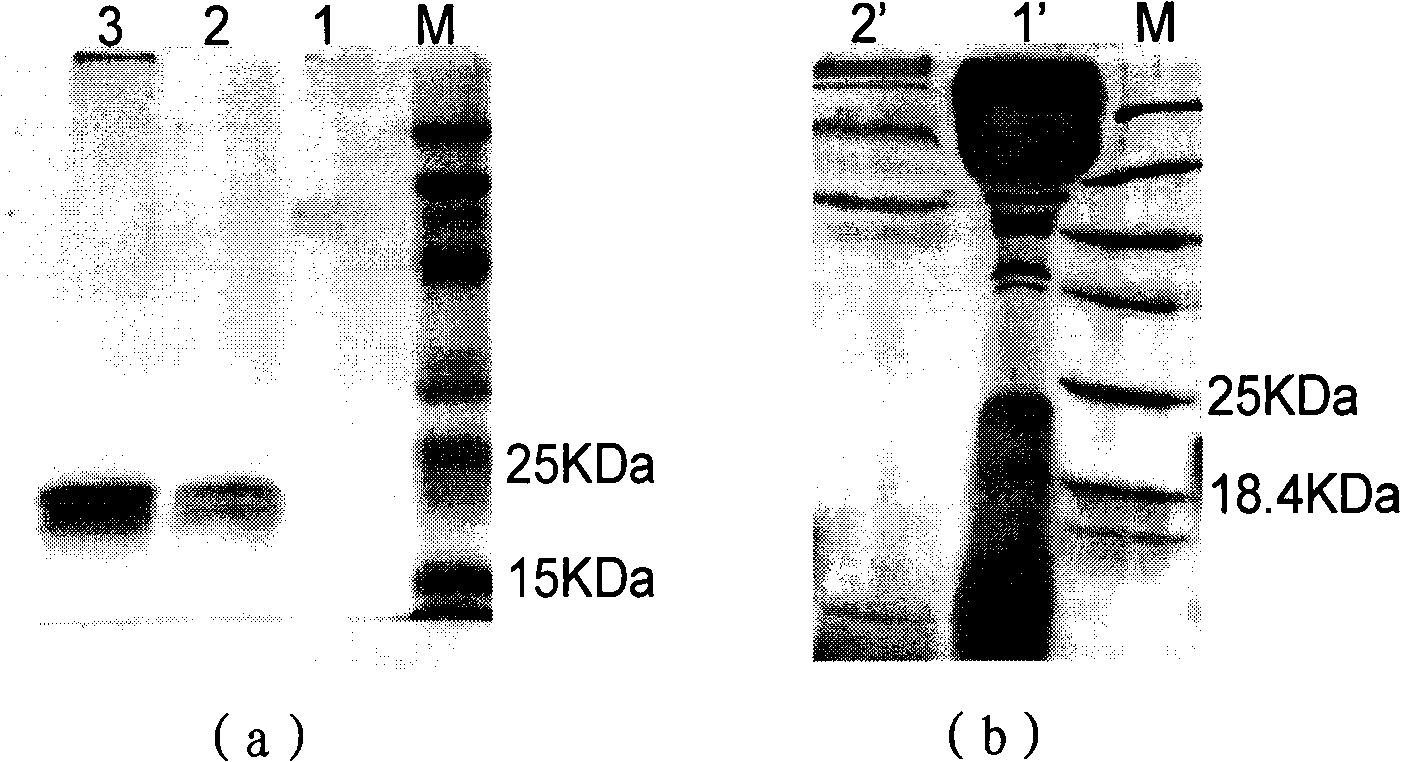
Fusion protein for resisting formation of thrombus targetedly and preparation method and application thereof
The invention discloses a fusion protein for resisting formation of thrombus targetedly, which comprises the following parts: (a) protein domains of nematode anticoagulant peptide 5, of which the amino acid sequence has at least 80 percent of similarity with a sequence shown as SEQ ID No.1; (b)protein domains of Hirulog, of which the amino acid sequence has at least 80 percent of similarity with a sequence shown as SEQ ID No.2; (c) protein domains of RGD peptide, consisting of 3 to 10 amino acids and containing an Arg-Gly-Asp sequence; and (d) human coagulation factor X a recognition sites, of which the amino acid sequence is shown as SEQ ID No.3. The invention also discloses a gene encoding the fusion protein, a recombined expression vector containing the gene, a transformant containing the recombined expression vector, and a method for preparing the fusion protein. The prepared fusion protein can inhibit formation and development of thrombus from a plurality of approaches and take effect on thrombus positions targetedly, and is suitable for preventing and treating thrombotic disorders.
Owner:CHONGQING UNIV
New Pyridine Analogues IV
InactiveUS20080009523A1Improve performanceHigh selectivityBiocideOrganic chemistryVascular diseasePyridine
Owner:ASTRAZENECA AB
Method for evaluating chemical composition of Rosa xanthina on basis of antithrombotic spectrum-effect relationship
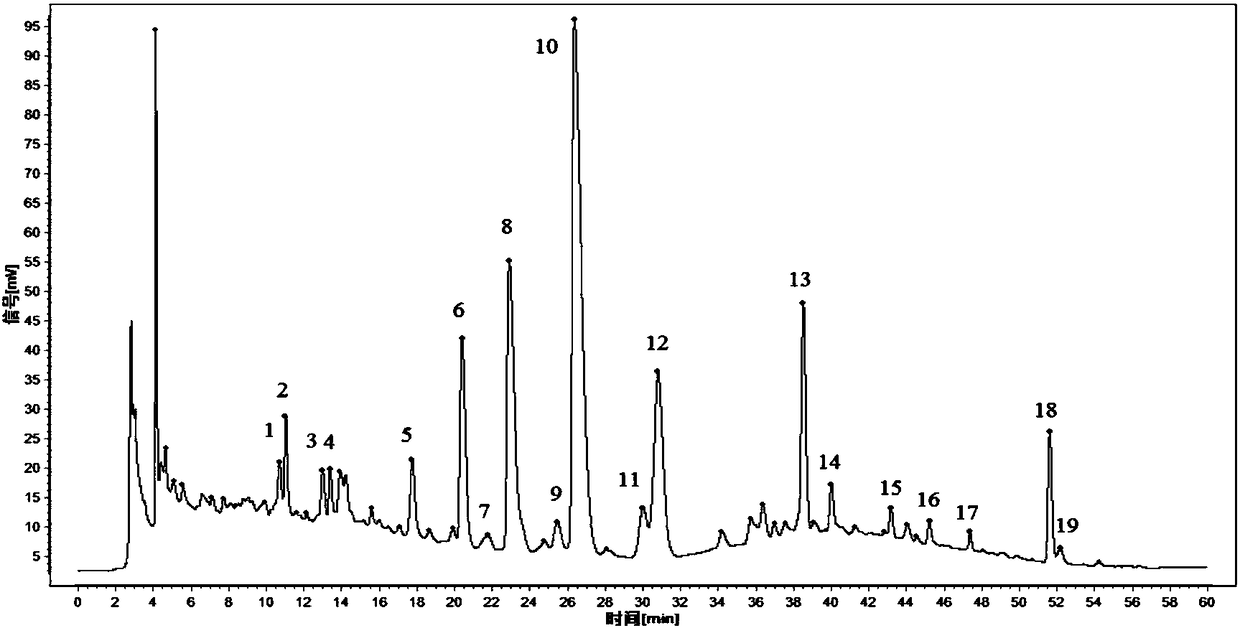
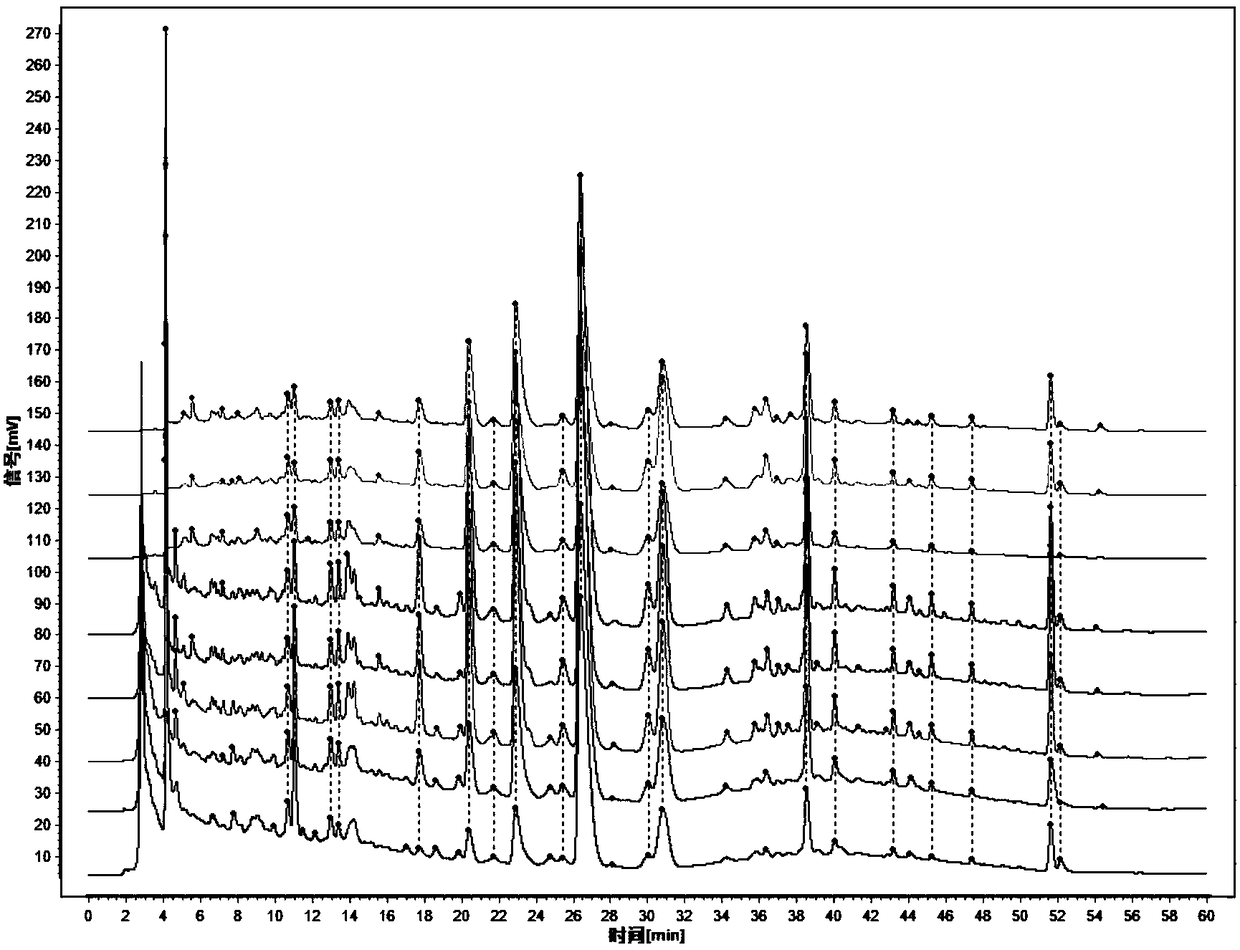
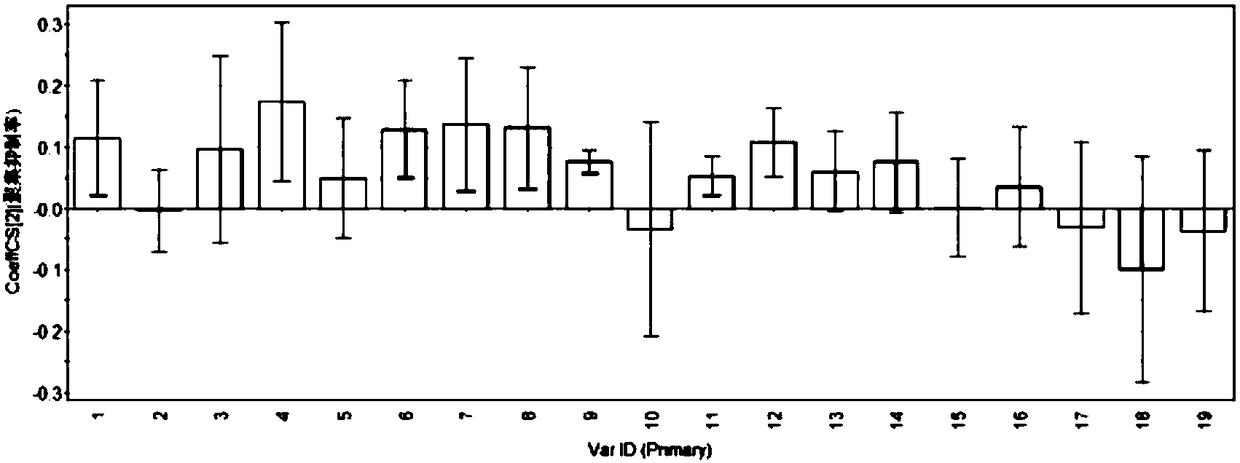
ActiveCN108195989AComprehensive and accurate spectrum effect basisClear chemical compositionComponent separationMathematical modelSeparation technology
The invention discloses a method for evaluating chemical composition of Rosa xanthina on the basis of antithrombotic spectrum-effect relationship. The method comprises the following steps: preparing extract of different polar components of the Rosa xanthina with a modern separation technology; establishing fingerprint of extract of each component with high-performance liquid chromatography, and calibrating characteristic peaks; evaluating antithrombotic activity of different extract on the basis of platelet aggregation inhibition rate, prothrombin time, thrombin time and activated partial thromboplastin time as indexes; substituting fingerprint characteristic peak data and pharmacodynamical activity data into a mathematical model for spectrum-effect correlation analysis, and evaluating pharmacodynamical activity of the characteristic peaks. With adoption of the method for evaluating the chemical composition of the Rosa xanthina on the basis of the antithrombotic spectrum-effect relationship, the antithrombotic chemical composition in the Rosa xanthina can be evaluated rapidly and accurately, a scientific and effective method is provided for research of pharmacodynamic material basis and quality control of the Rosa xanthina, and reference is provided for further development of Rosa xanthina drugs or health care products for treating thrombotic diseases.
Owner:山西省医药与生命科学研究院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com