Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2049 results about "Hepatitis B virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
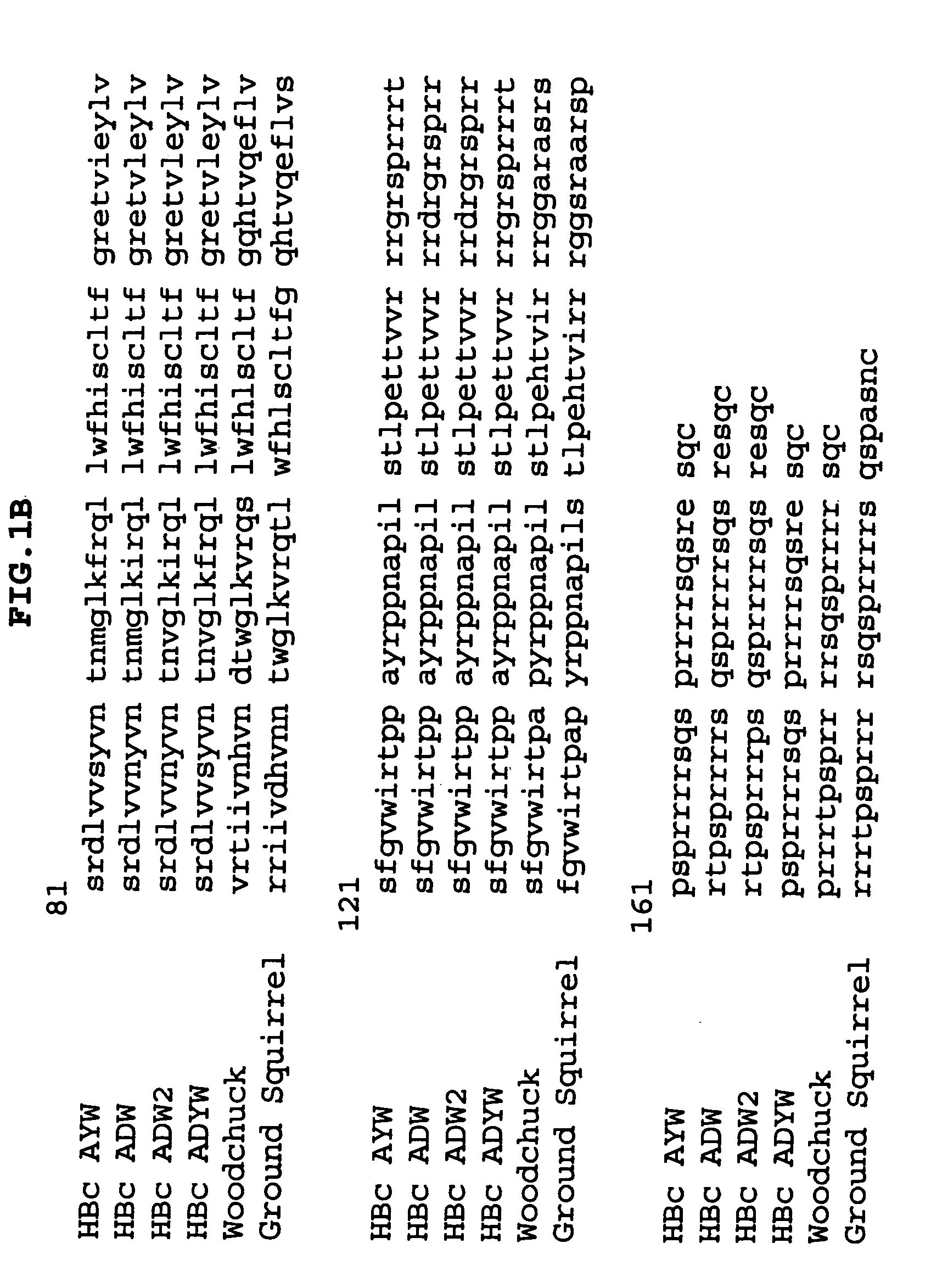
Hepatitis B virus, abbreviated HBV, is a partially double-stranded DNA virus, a species of the genus Orthohepadnavirus and a member of the Hepadnaviridae family of viruses. This virus causes the disease hepatitis B.
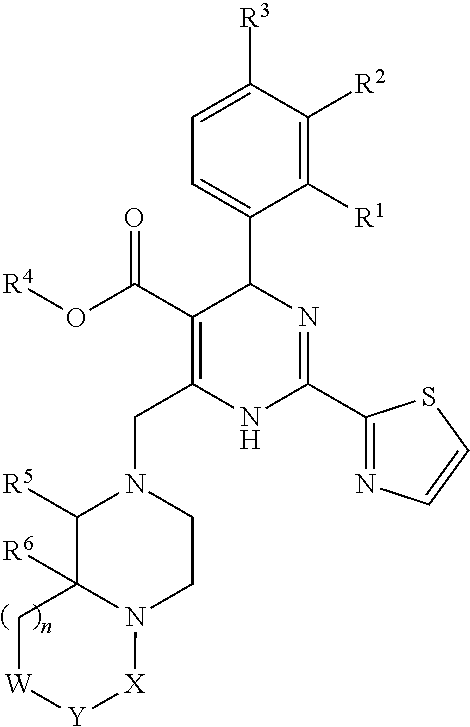
Novel 6-fused heteroaryldihydropyrimidines for the treatment and prophylaxis of hepatitis B virus infection
Owner:F HOFFMANN LA ROCHE INC
Novel 4-methyl-dihydropyrimidines for the treatment and prophylaxis of hepatitis b virus infection
Owner:GUO LEI +8
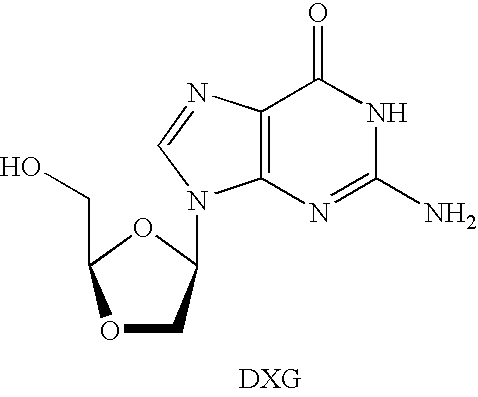
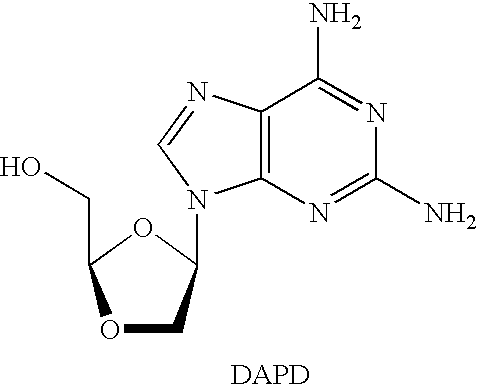
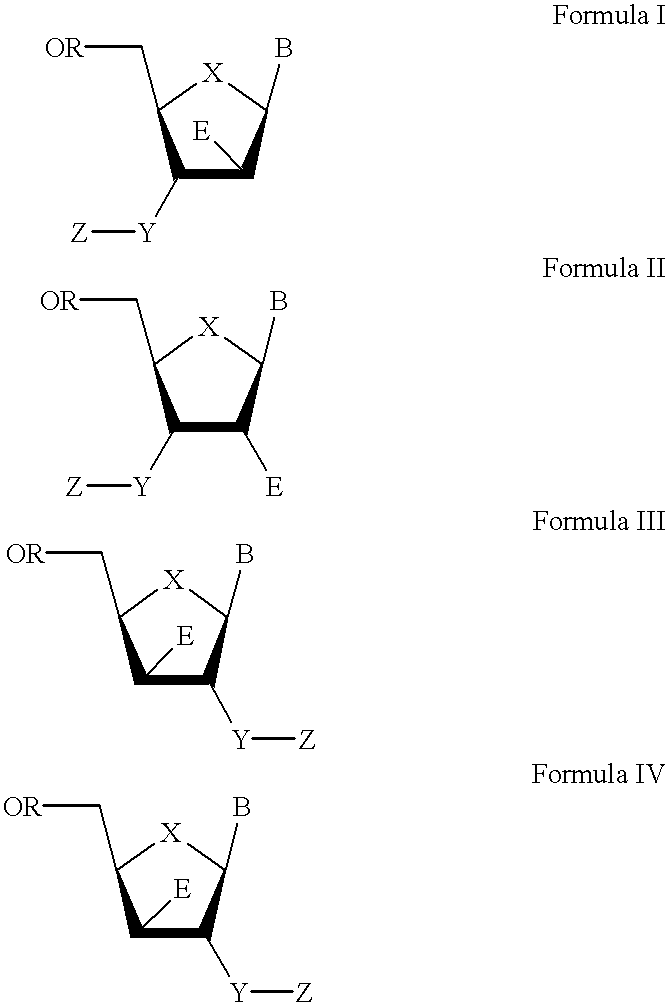
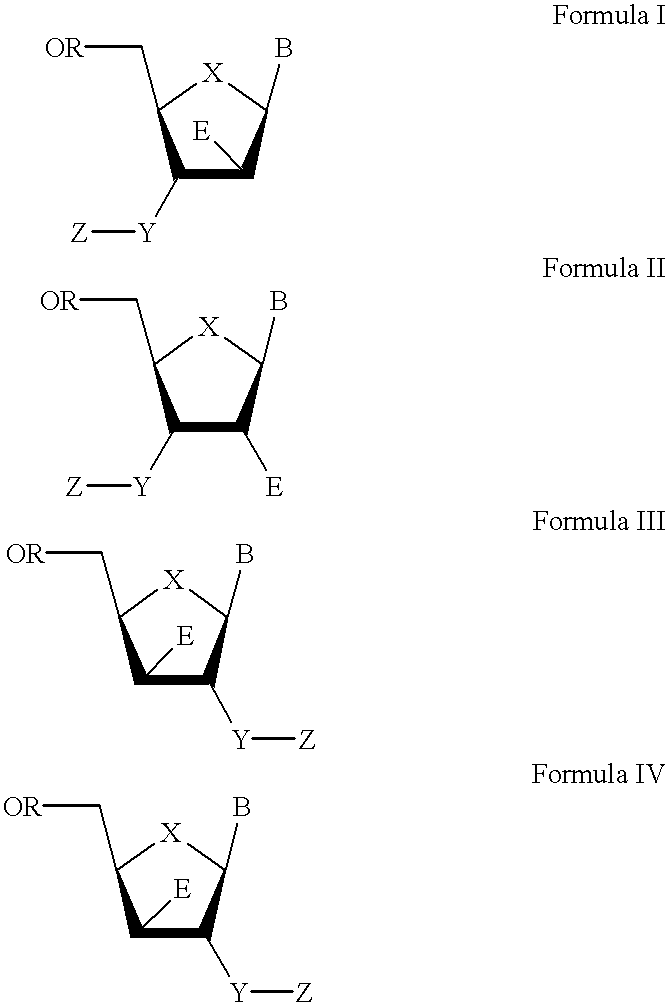
3′-or 2′-hydroxymethyl substituted nucleoside derivatives for treatment of hepatitis virus infections
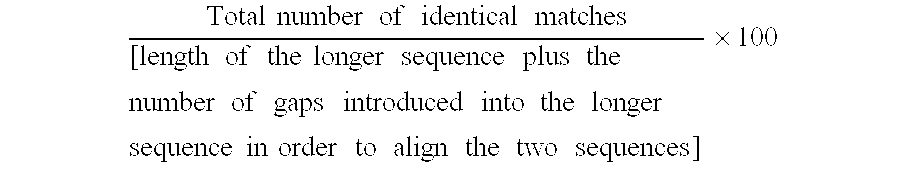
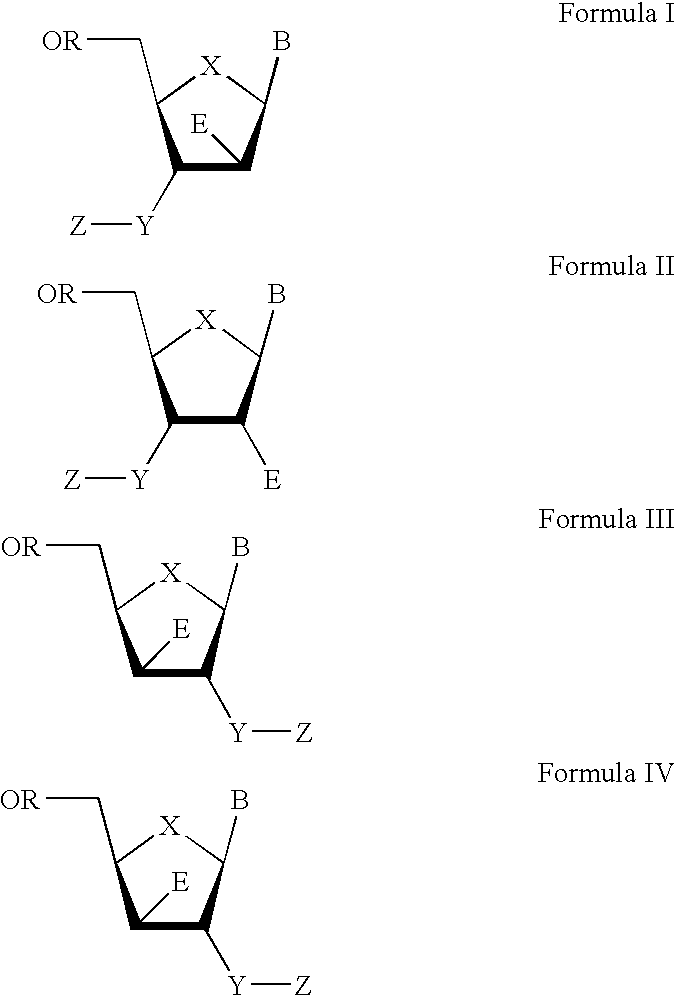
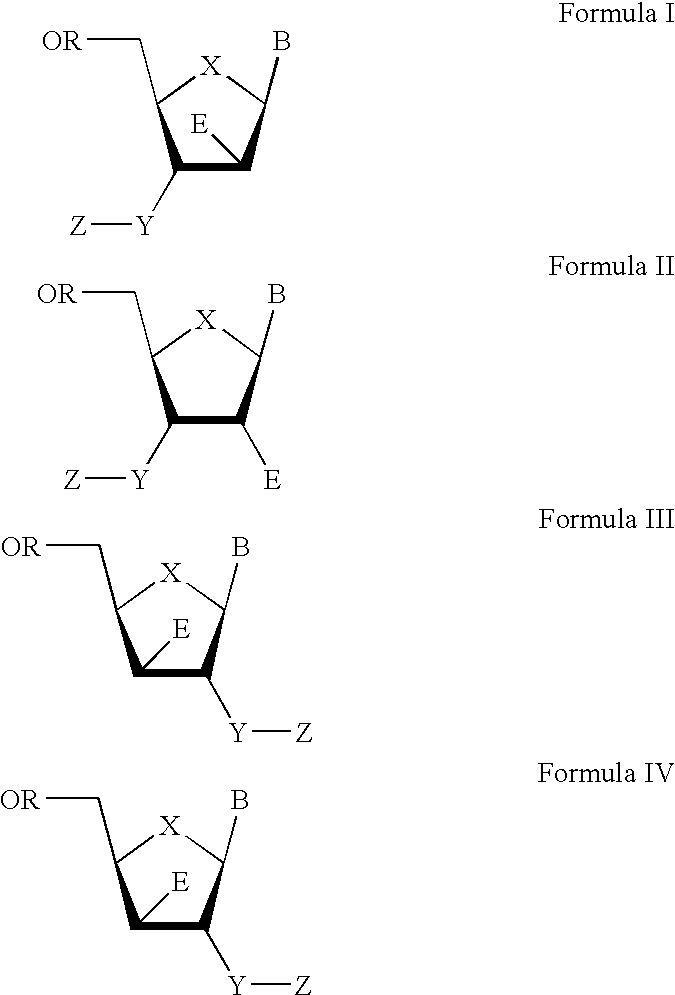
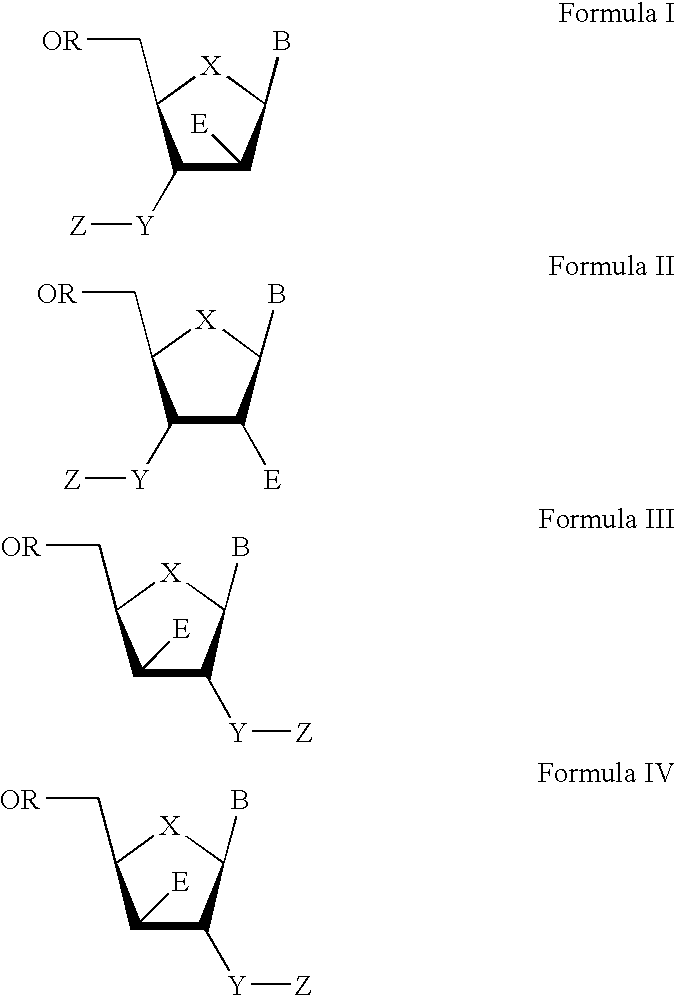
The present invention relates to a composition for and a method of treating hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, hepatitis D virus (HDV) infection or a proliferative disorder in a patient using an effective amount of a compound selected from the group consisting of formulas [I]–[IV] below and mixtures of two or more thereof:wherein the substituents are as defined herein. Pharmaceutical compositions comprising these compounds in combination with other HBV, HCV, or HDV agents is also disclosed.
Owner:PHARMASSET
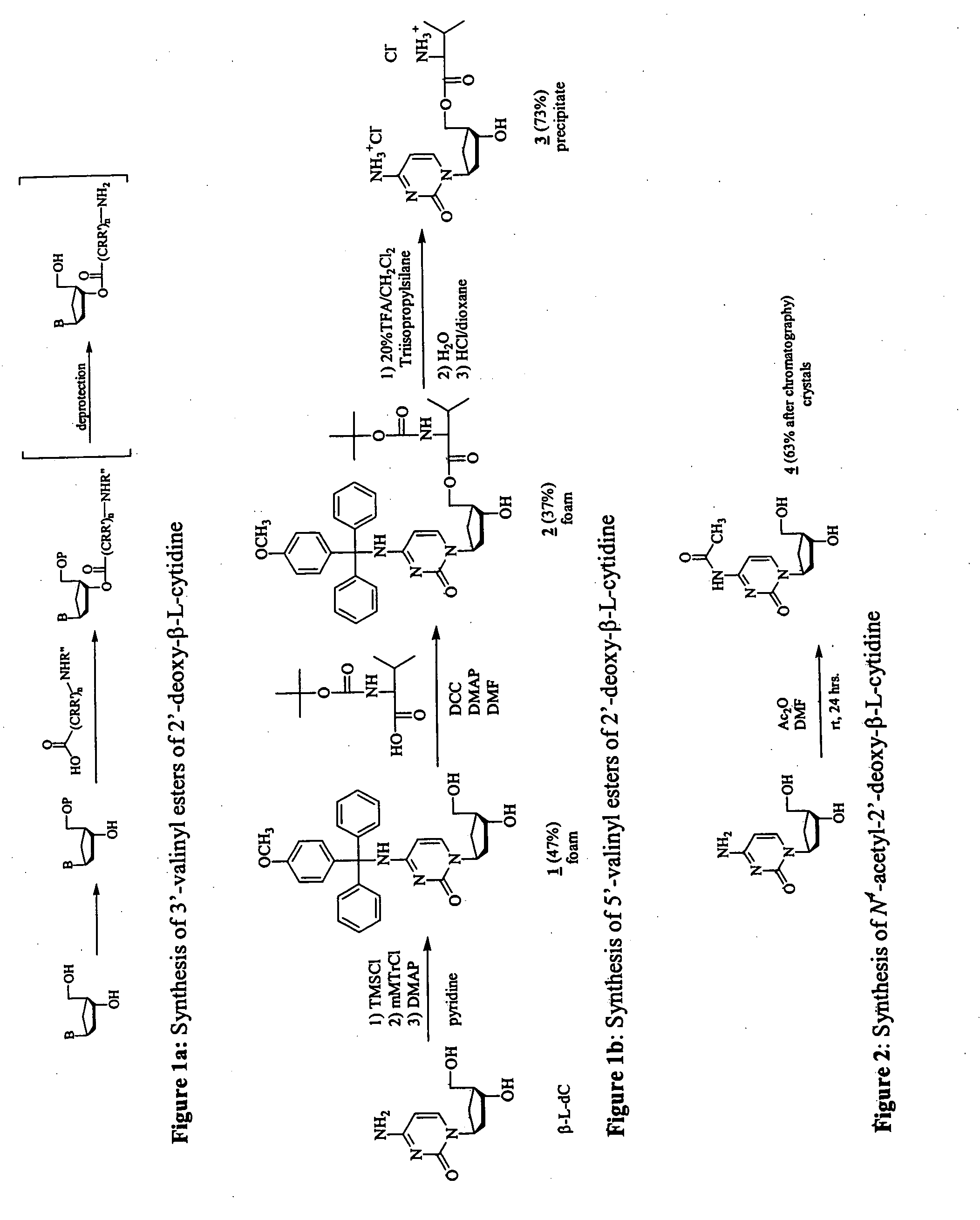
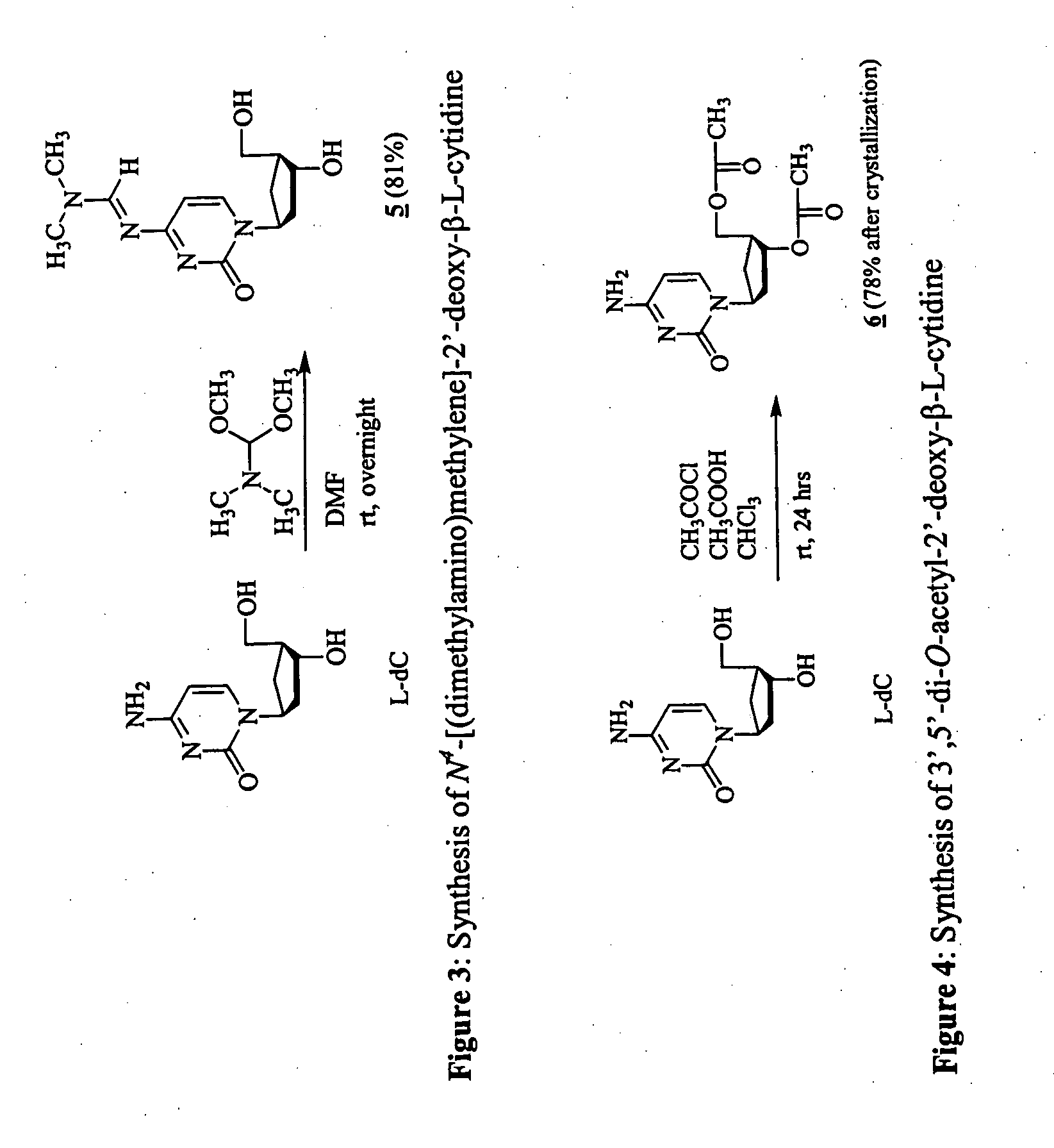
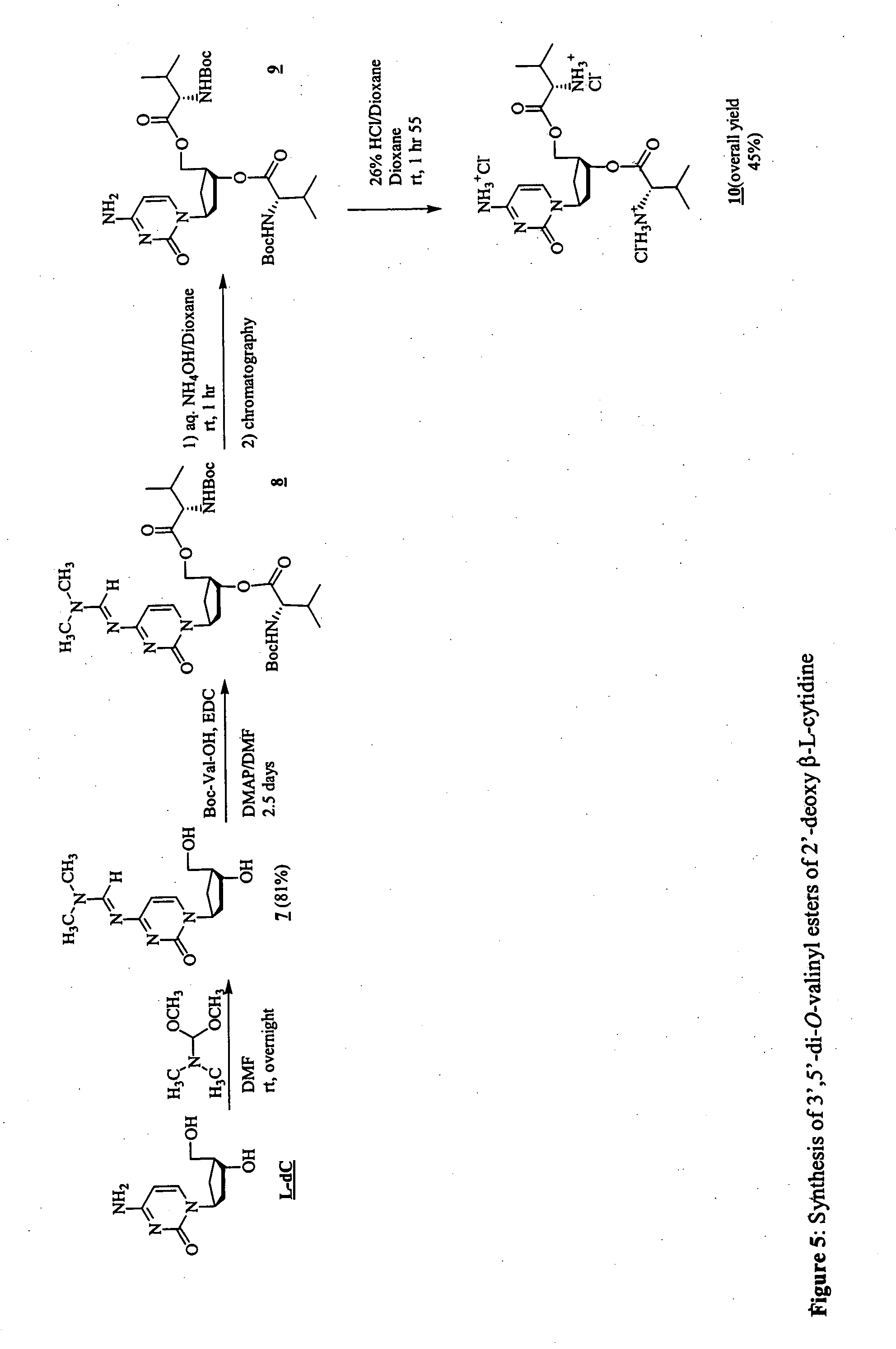
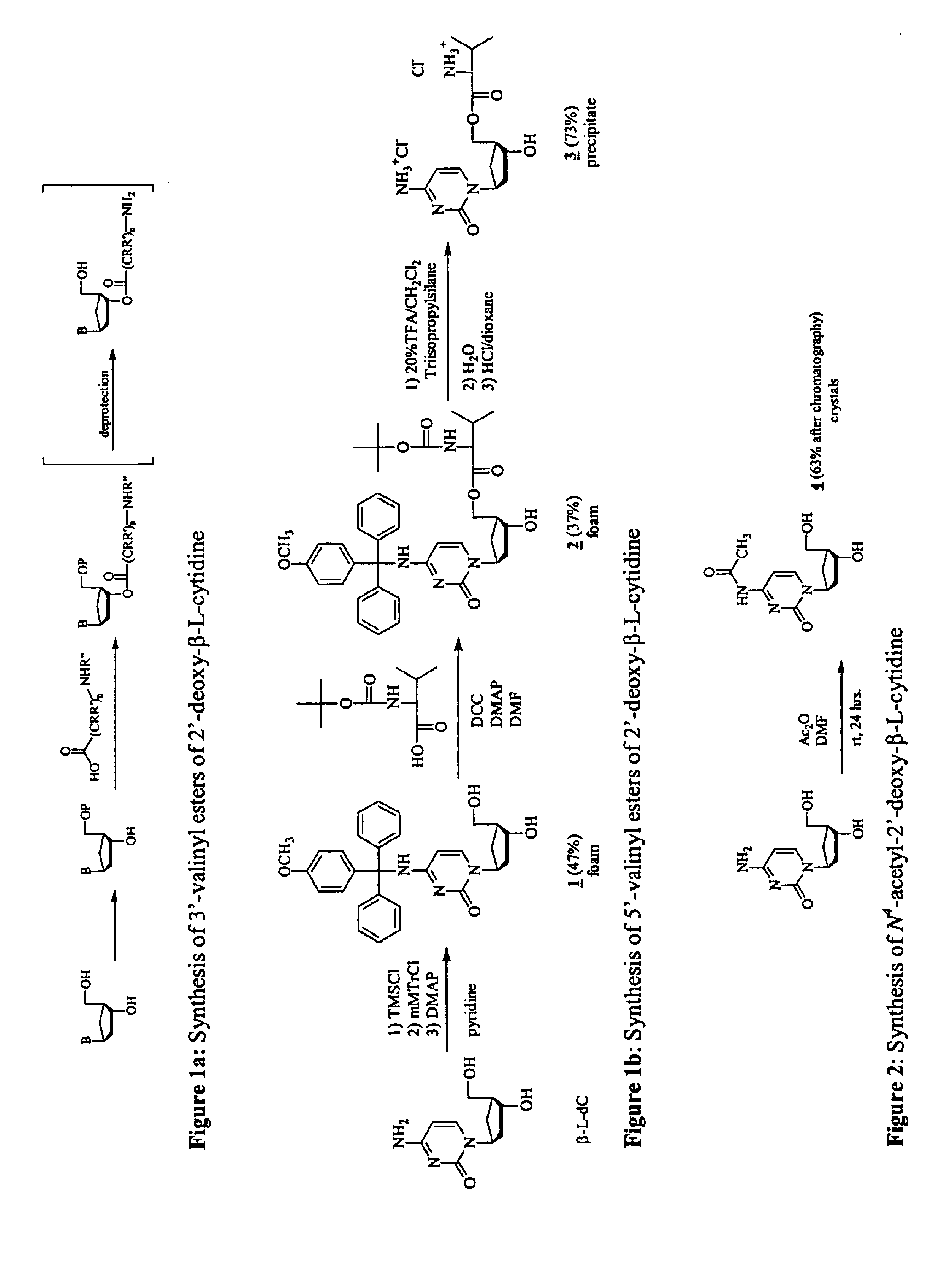
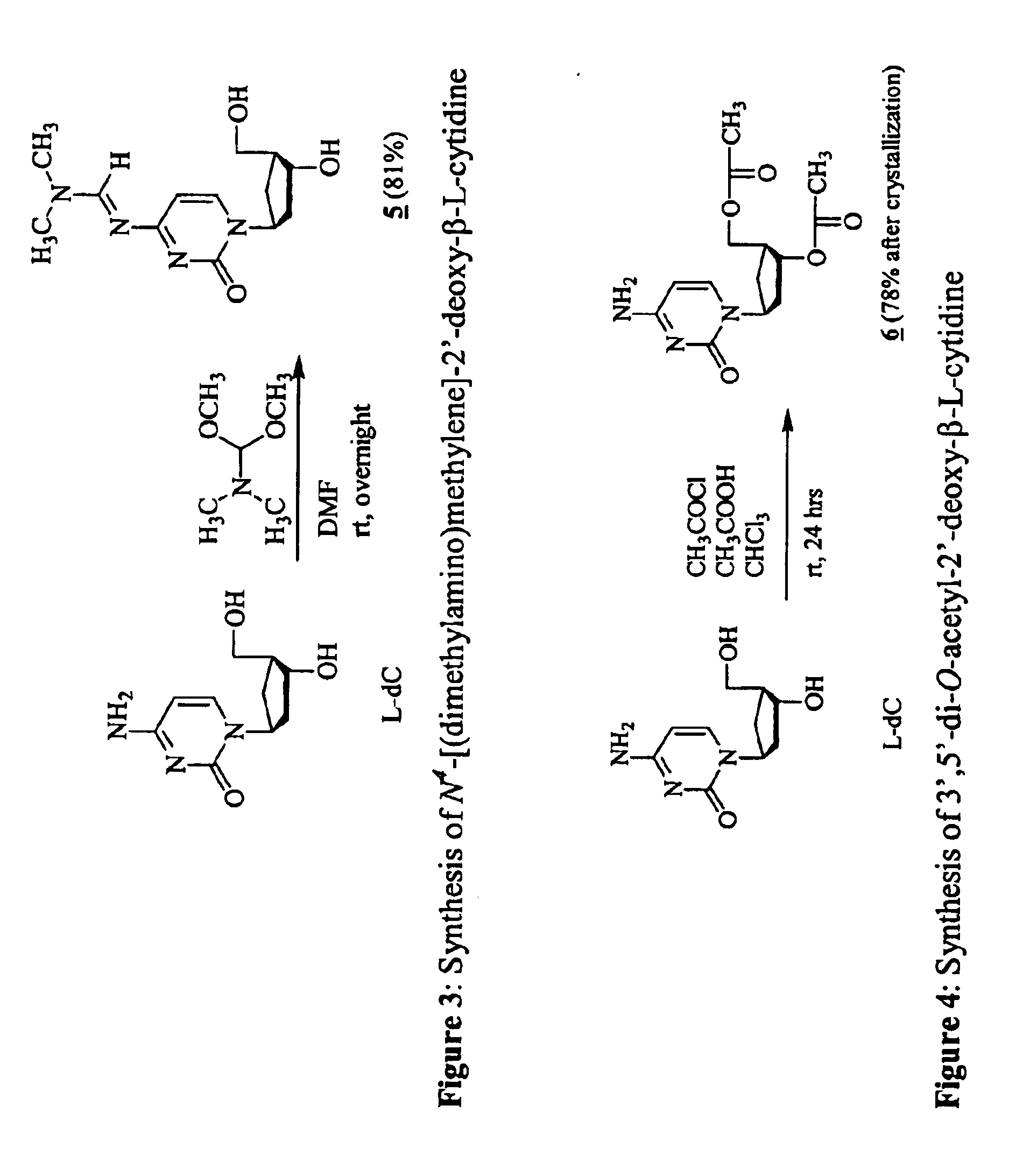
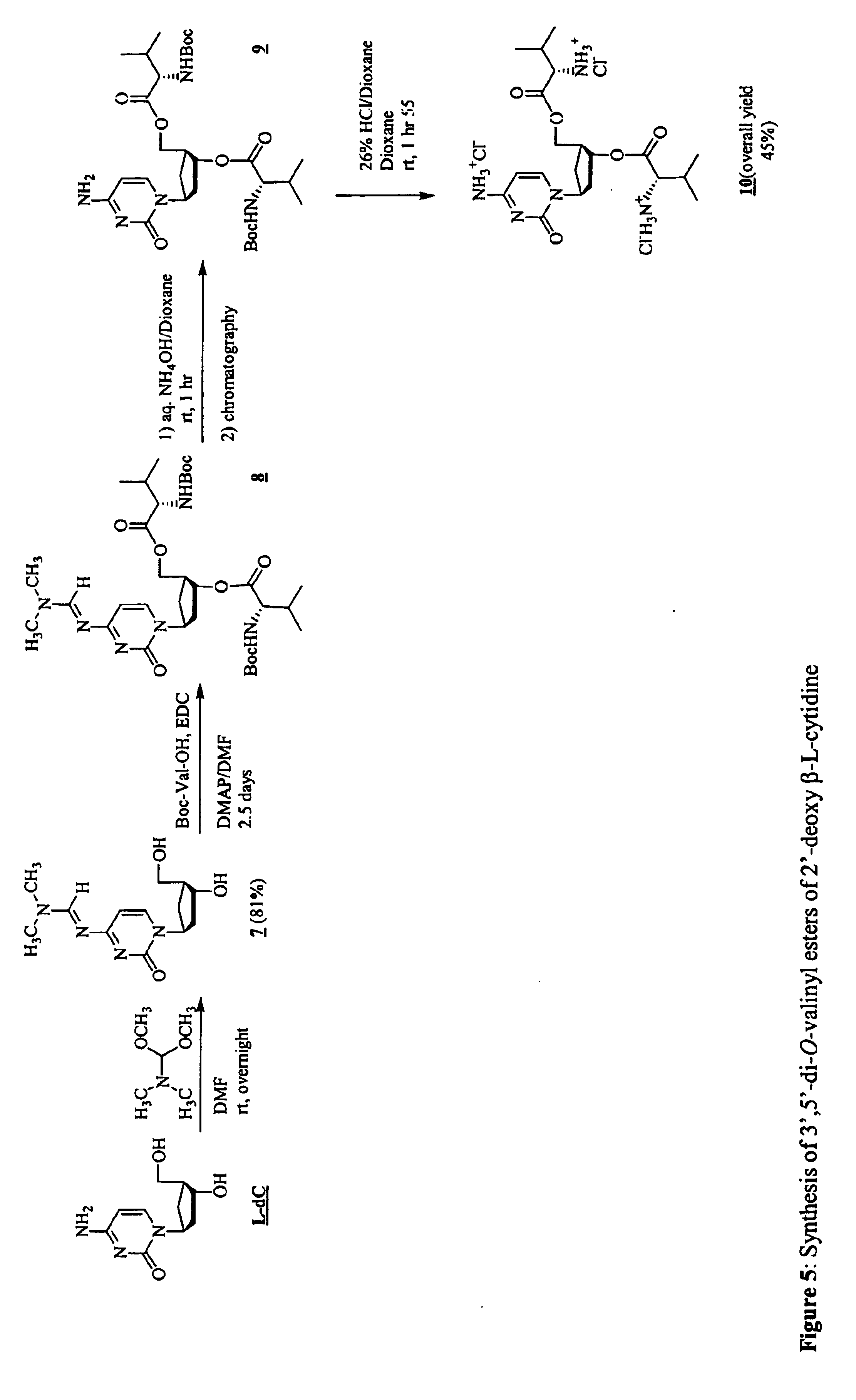
3'-prodrugs of 2'-deoxy-beta-L-nucleosides
The present invention relates to compounds, compositions and methods for the treatment of a host infected with a hepatitis B virus. Specifically, compound and compositions of 3′-esters of 2′-deoxy-β-L-nucleosides are disclosed, which can be administered either alone or in combination with other anti-hepatitis B agents. Compound and compositions of 3′,5′-diesters of 2′-deoxy-β-L-nucleosides are disclosed, which can be administered either alone or in combination with other anti-hepatitis B agents, are also disclosed.
Owner:INDENIX PHARM LLC +1
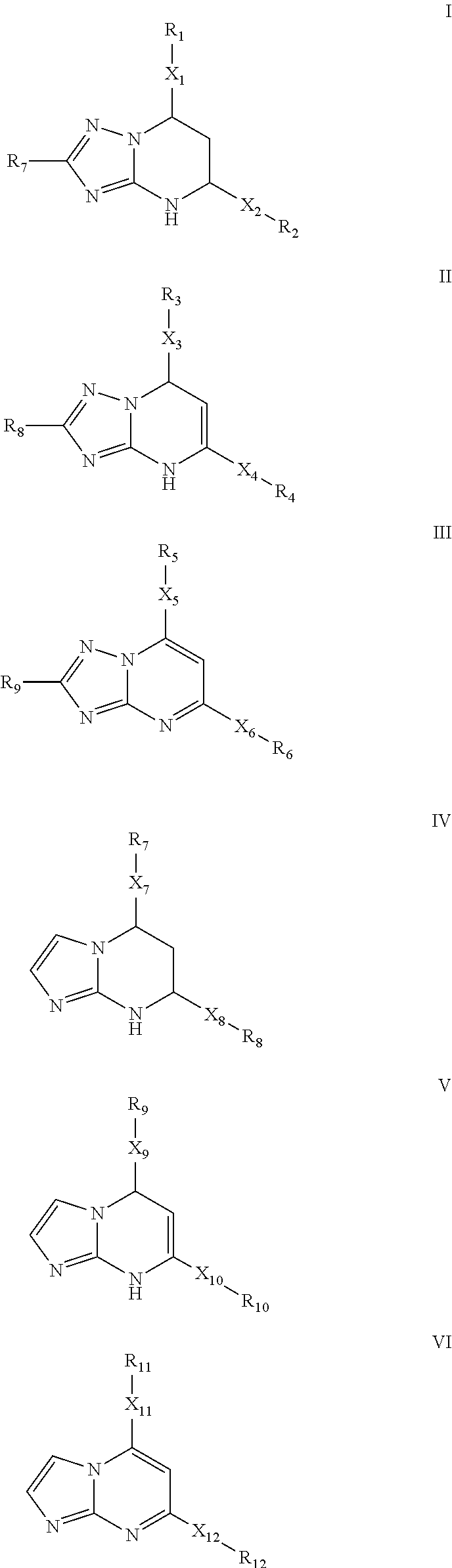
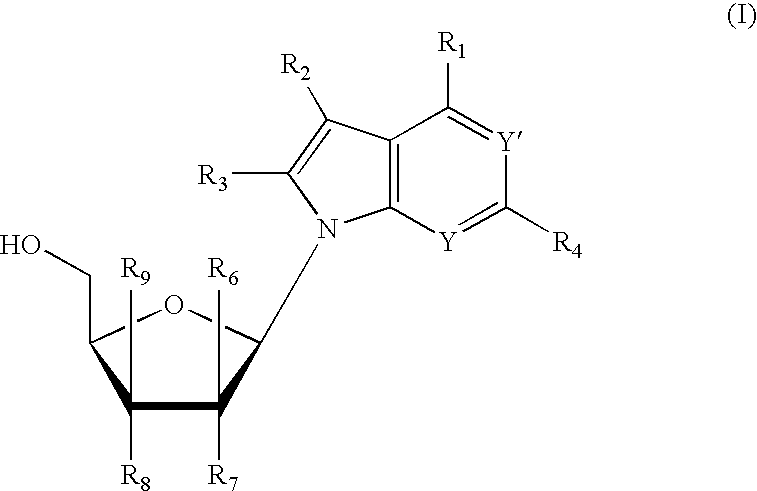
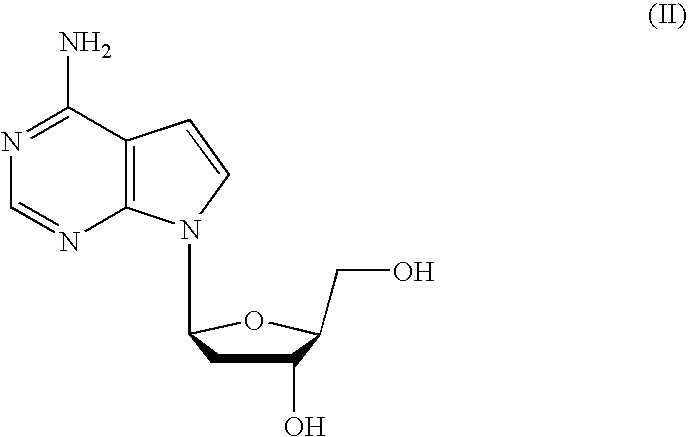
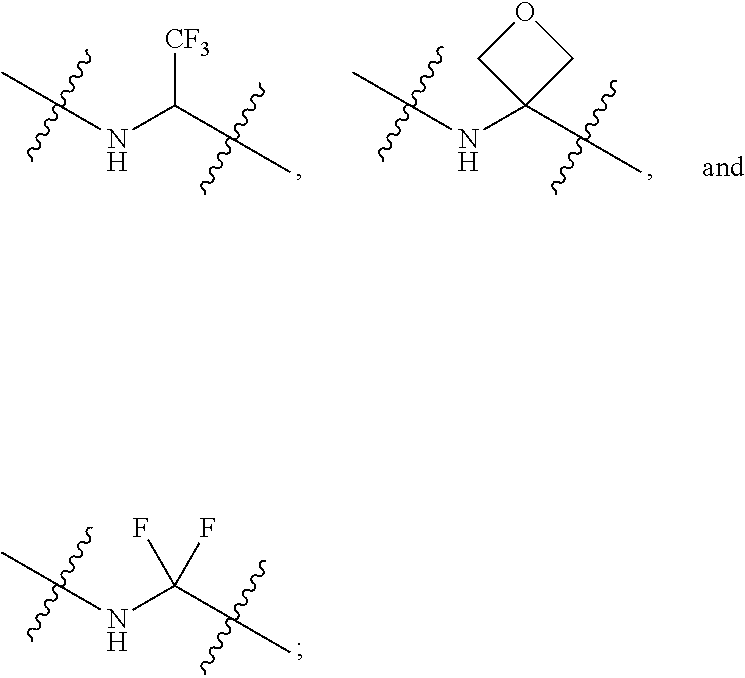
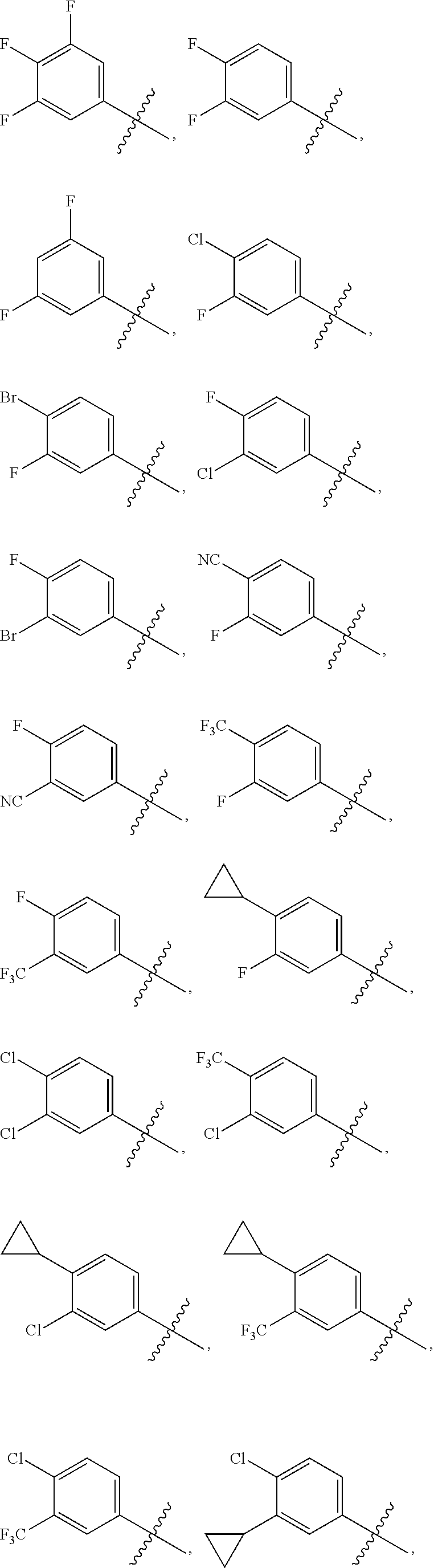
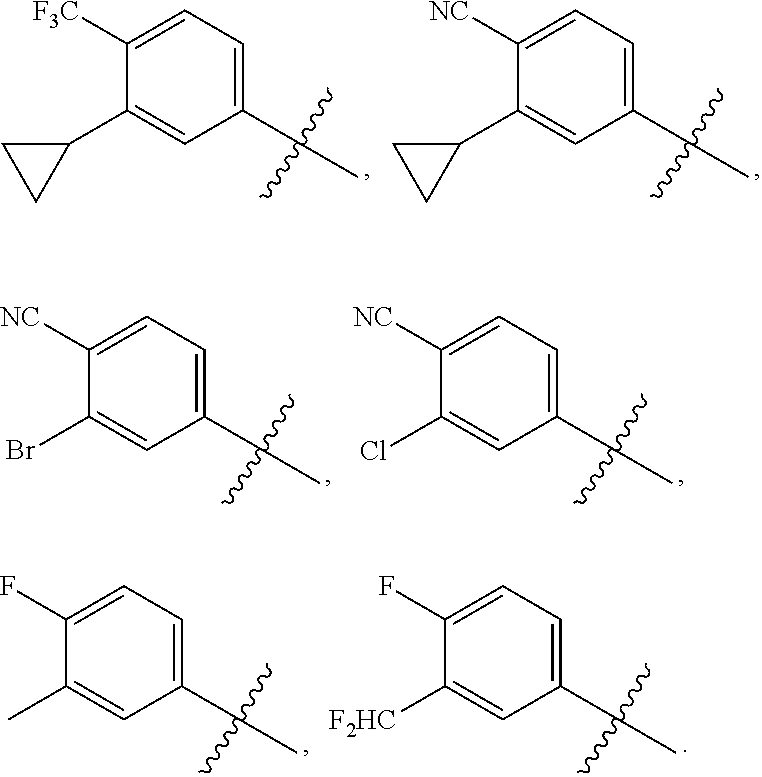
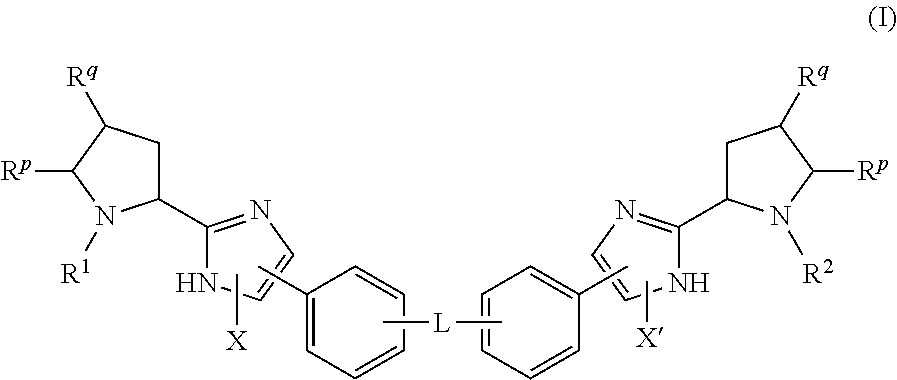
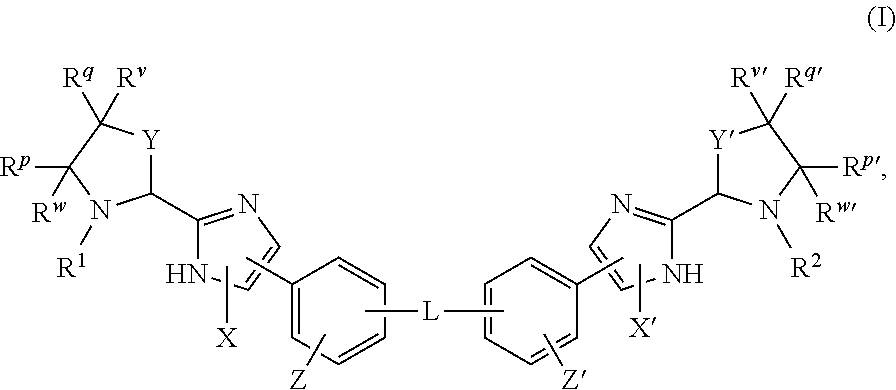
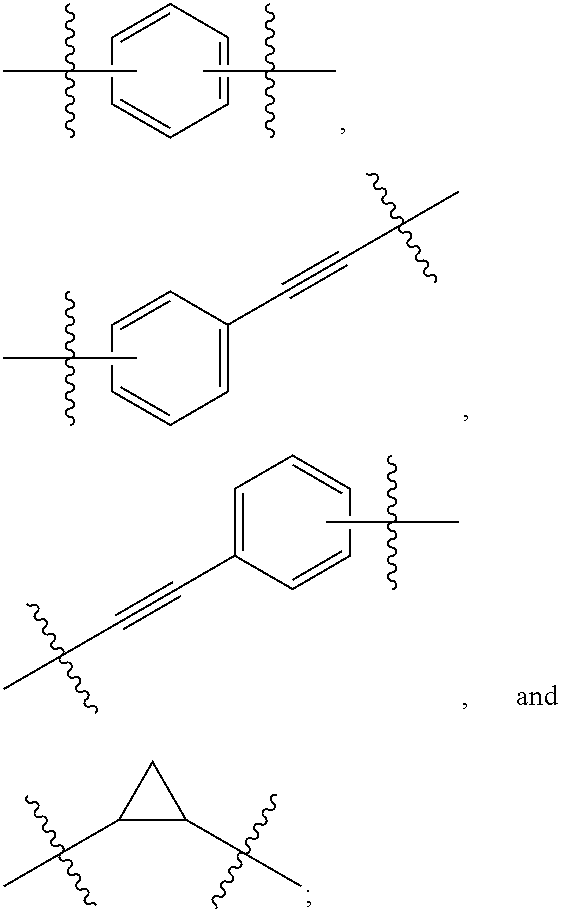
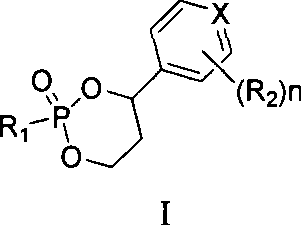
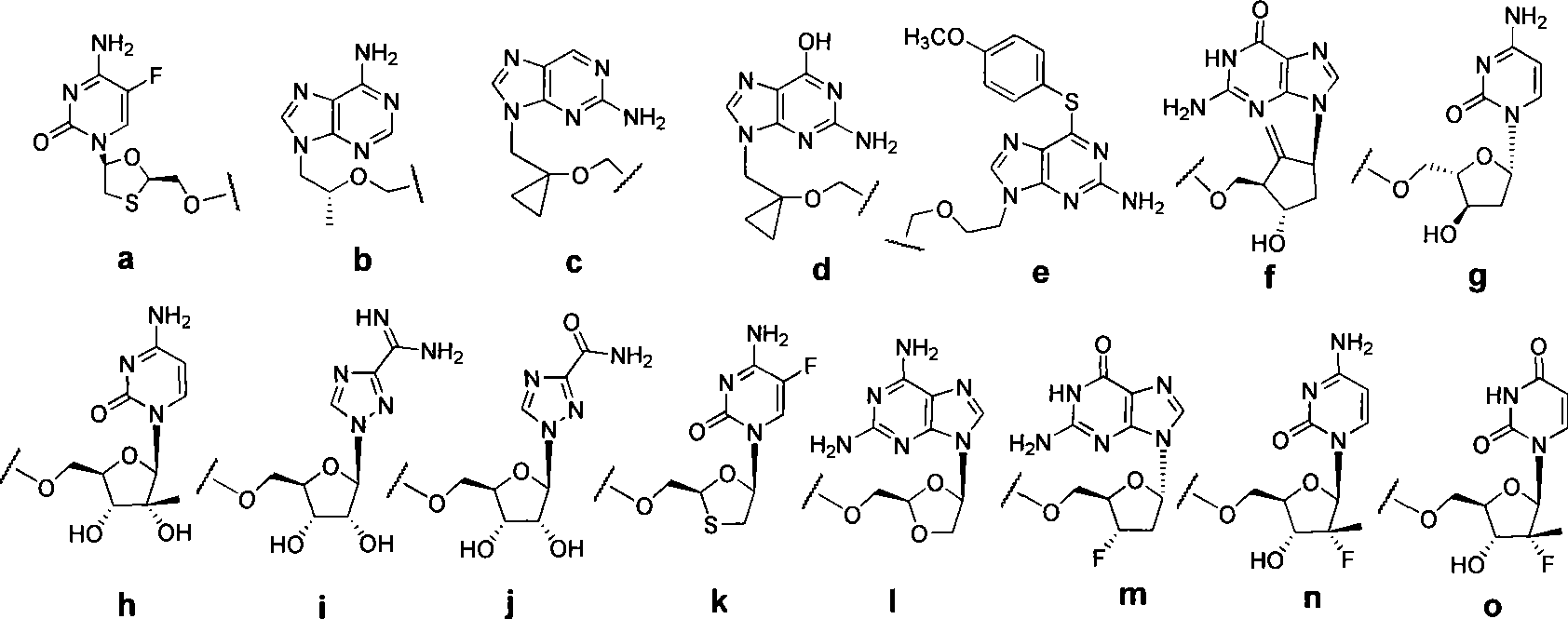
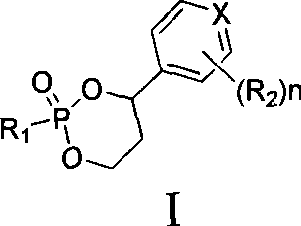
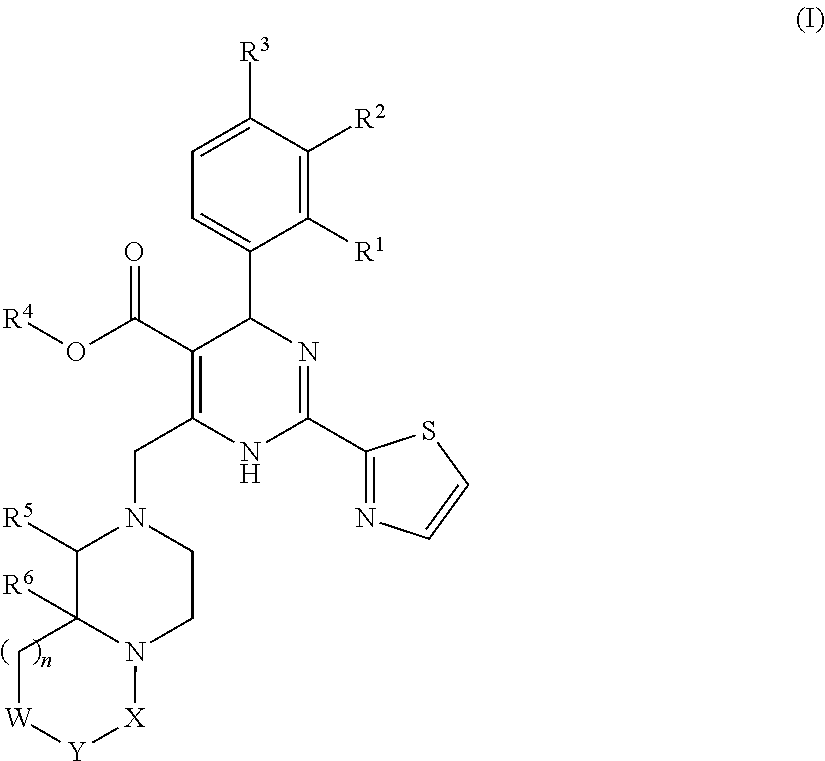
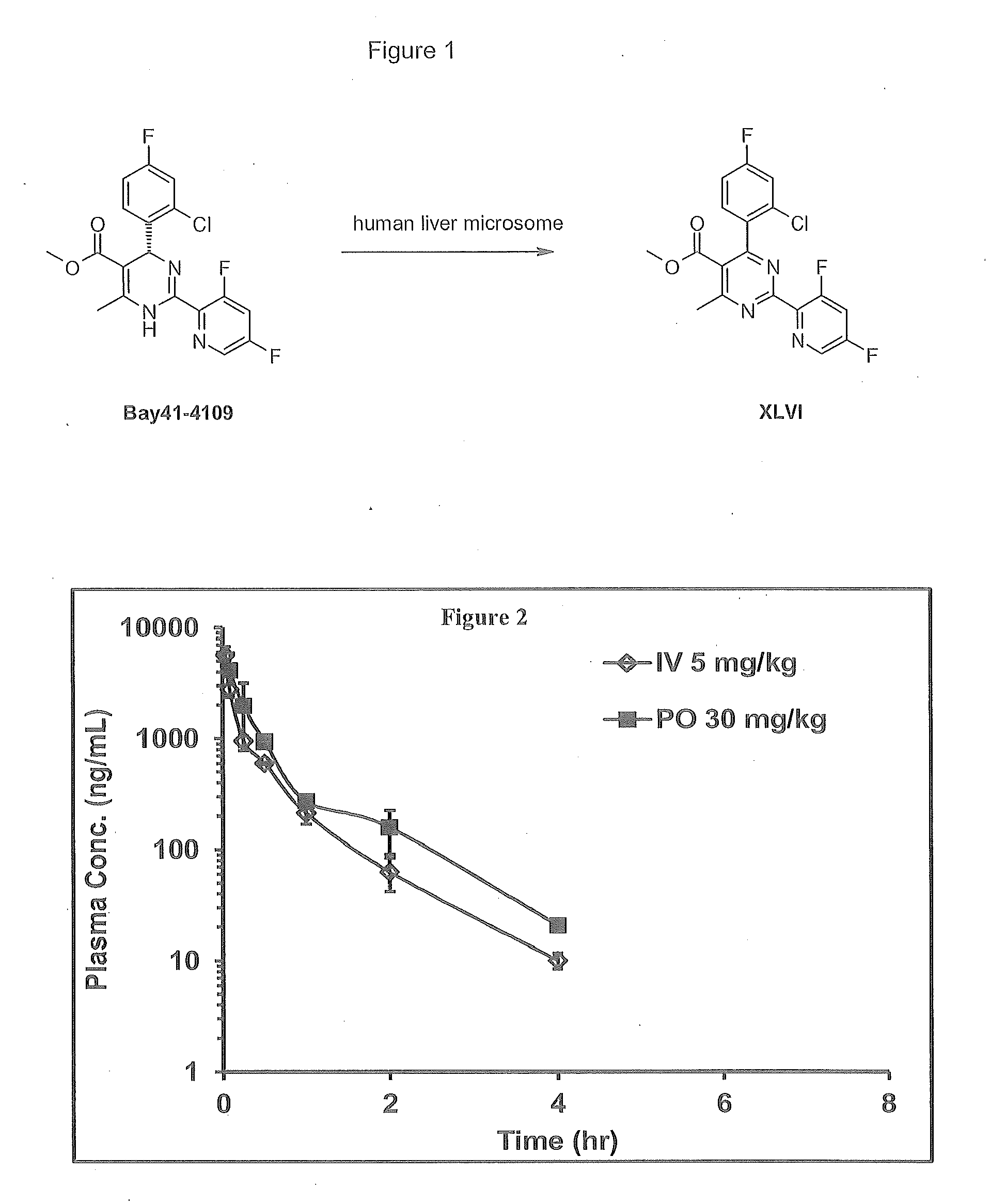
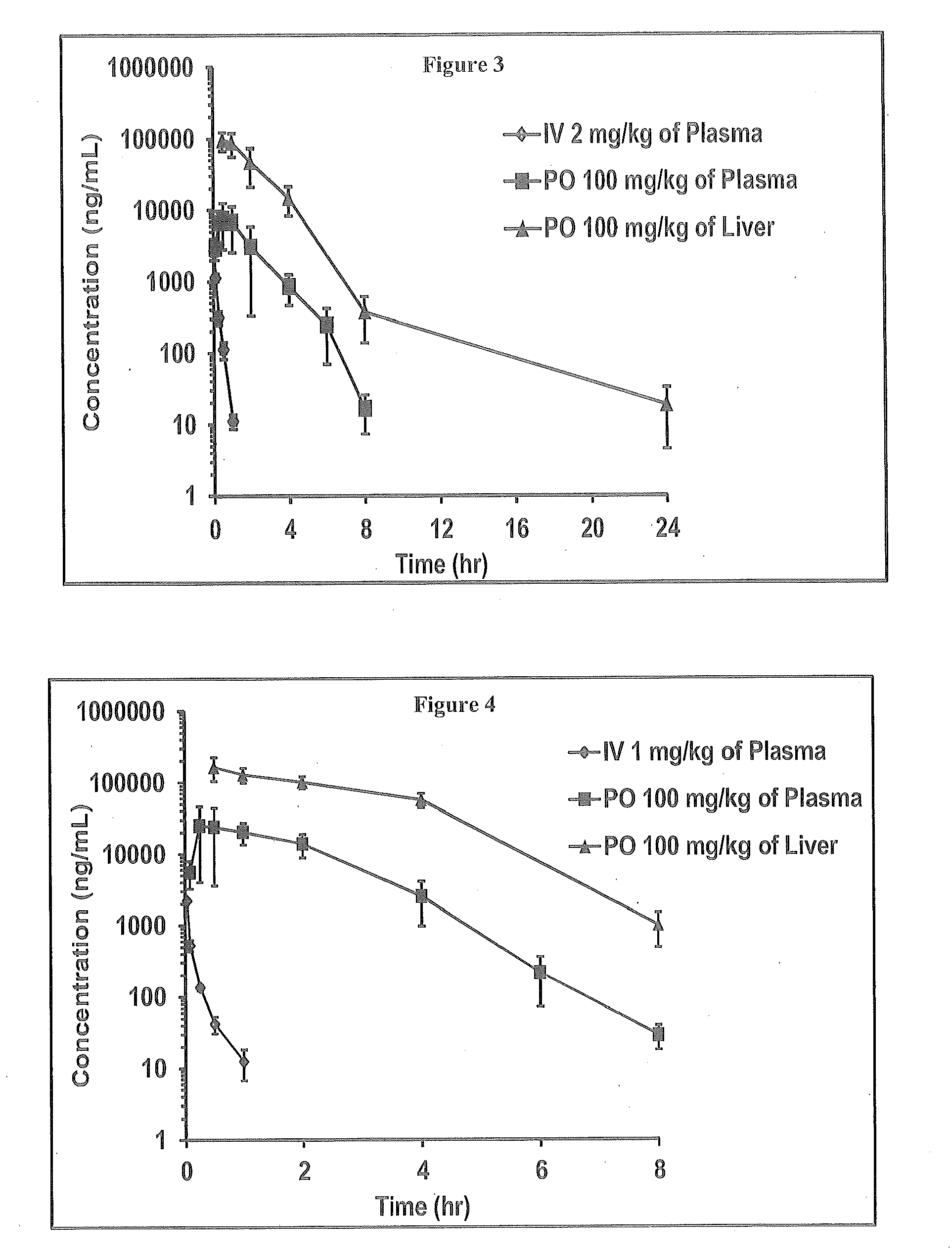
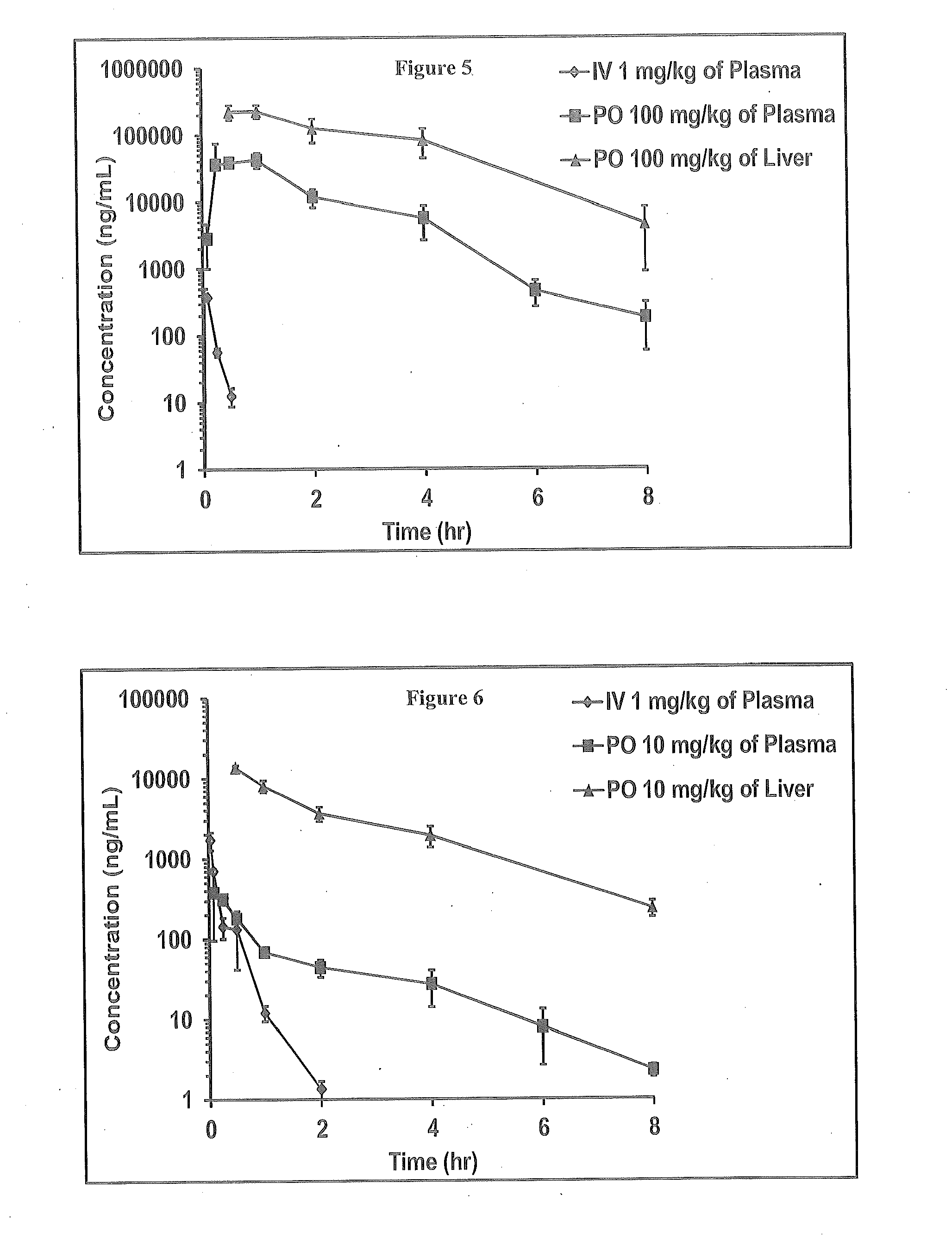
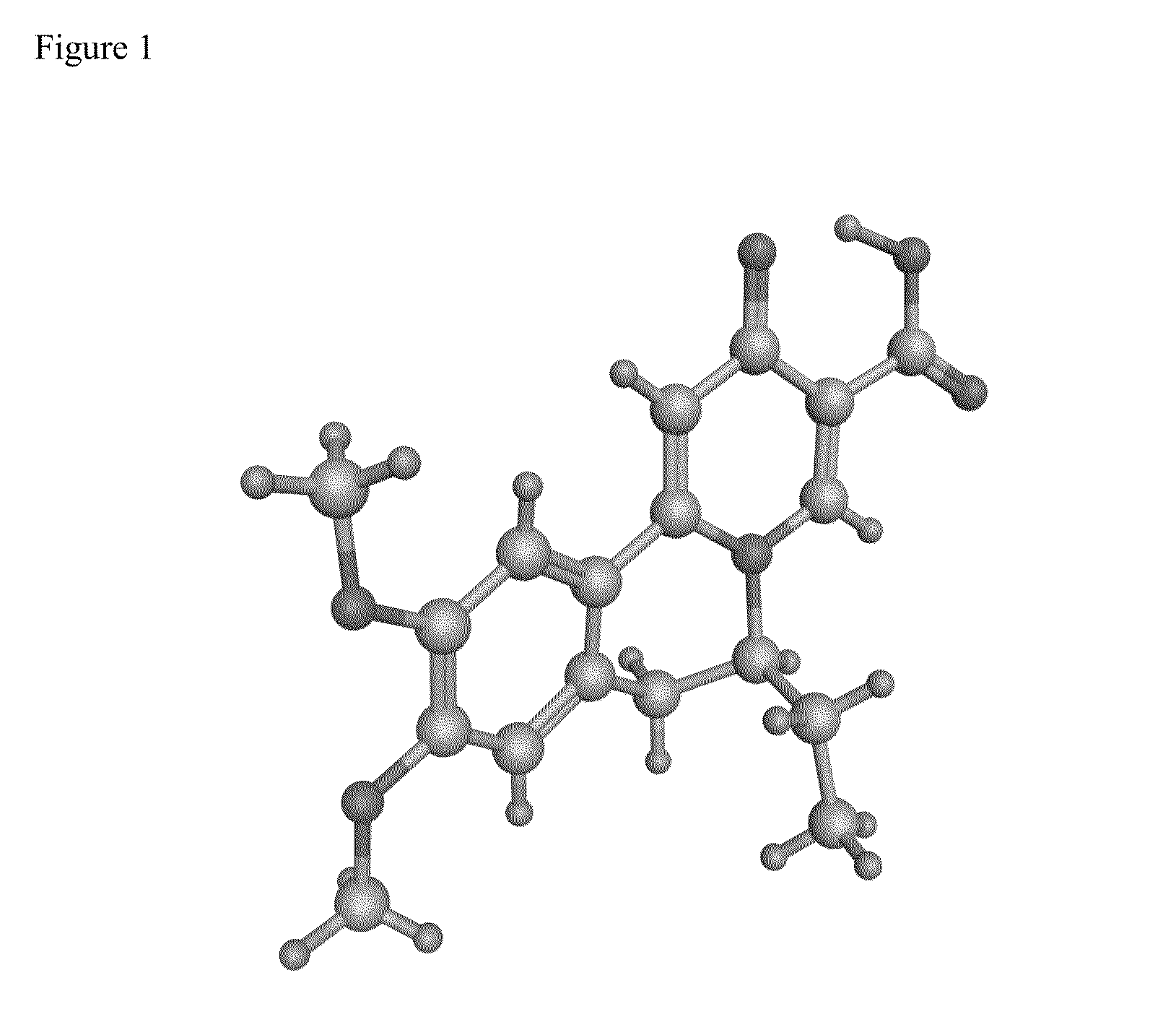
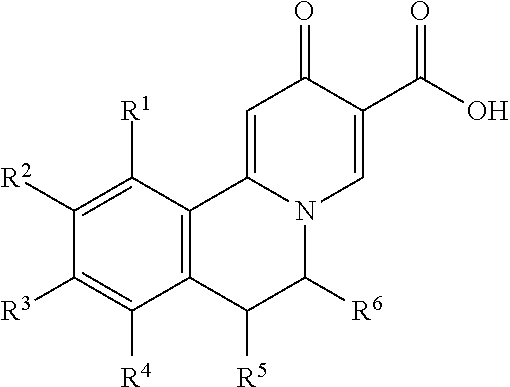
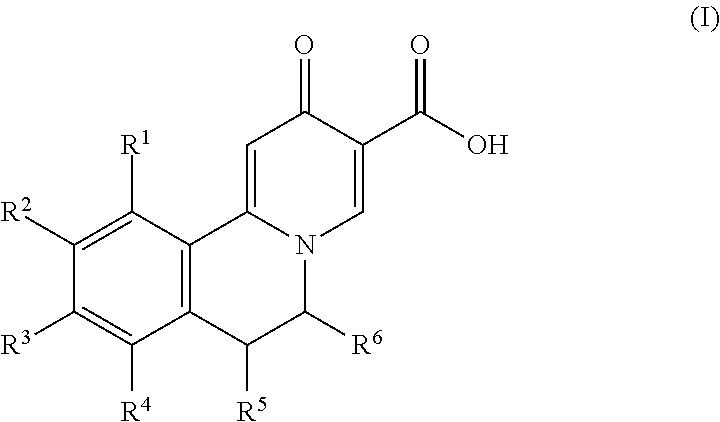
Novel 6,7-dihydrobenzo[a]quinolizin-2-one derivatives for the treatment and prophylaxis of hepatitis B virus infection
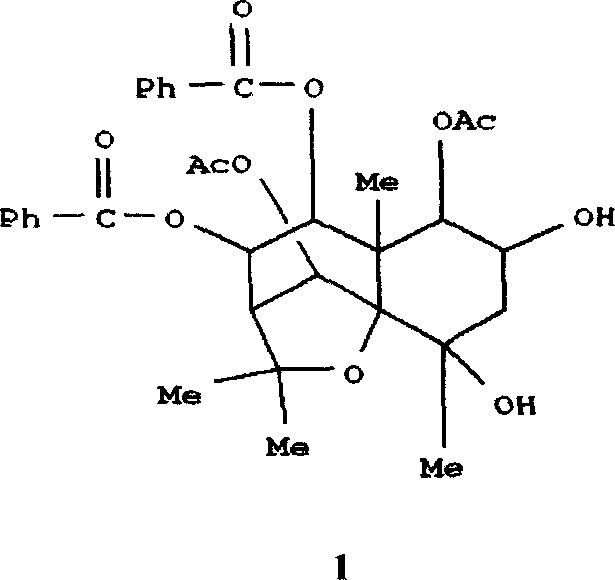
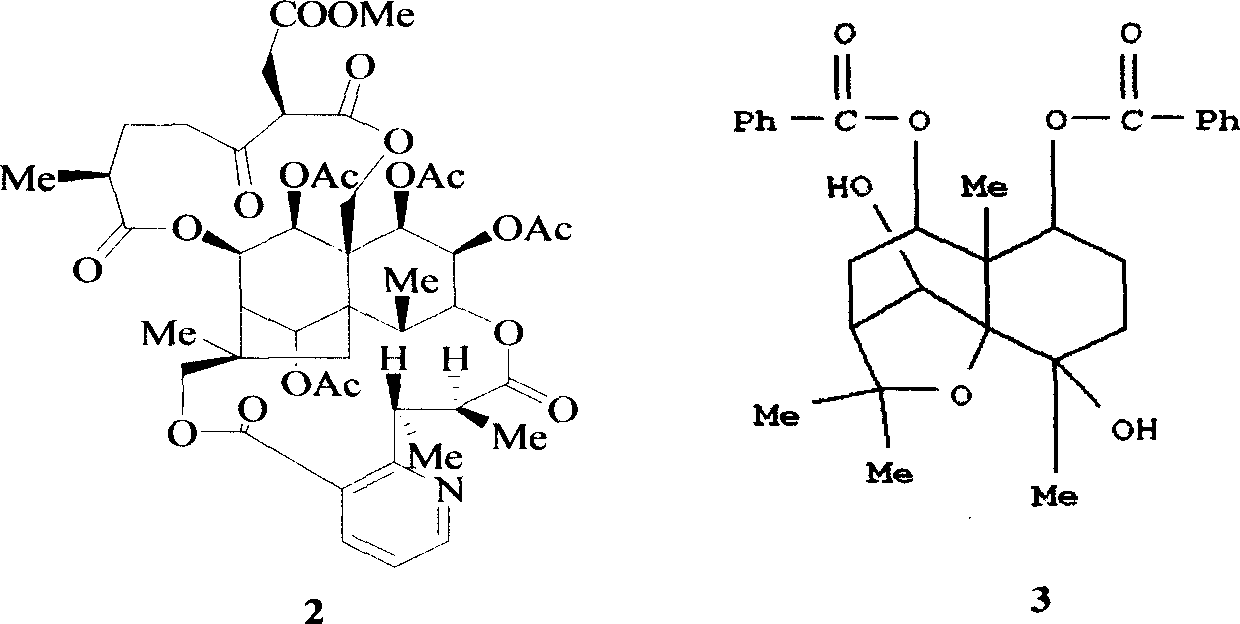
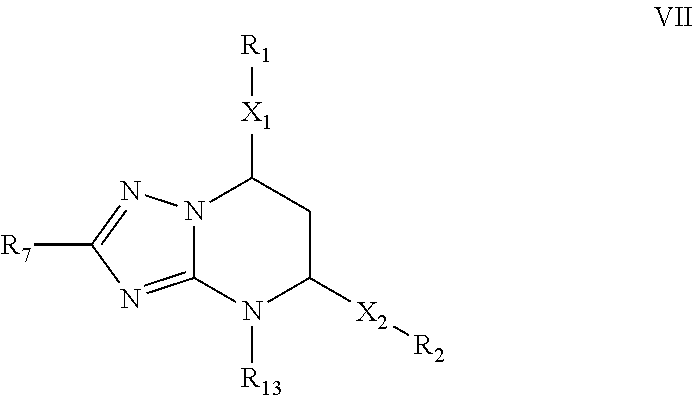
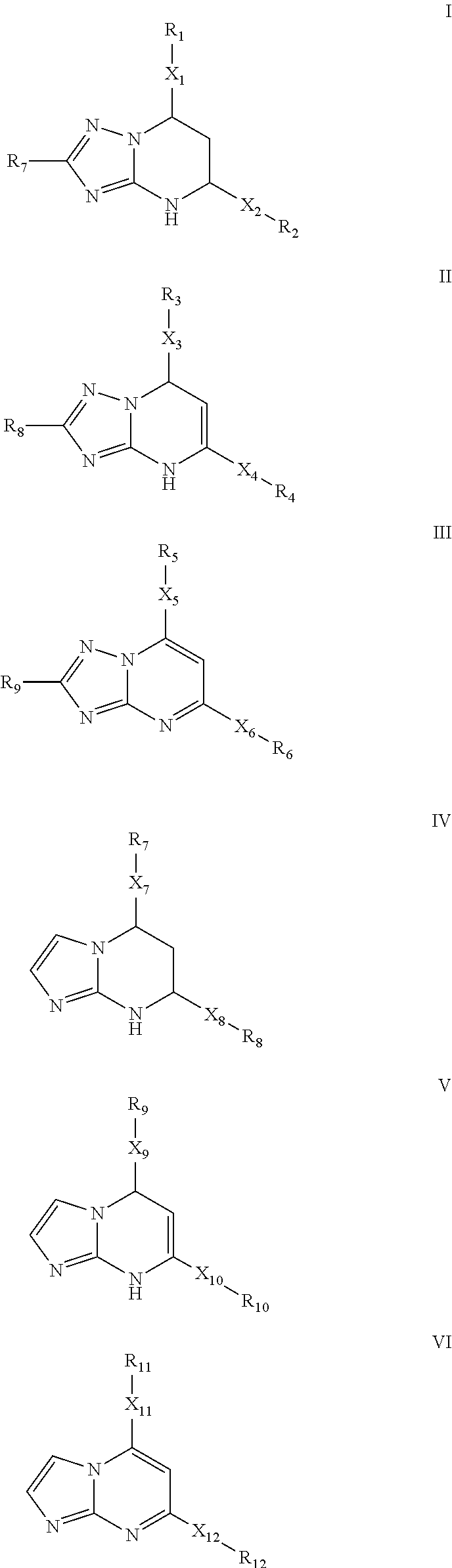
The invention provides novel compounds having the general formula:wherein R1 to R6, W and X are as described herein and their pharmaceutically acceptable salt, enantiomer or diastereomer thereof, and compositions including the compounds and methods of using the compounds.
Owner:F HOFFMANN LA ROCHE INC
Natural Juncao liver-nourishing and sobering-up agent
The invention describes a health product additive and preparation for sots or hepatitis B pathogen carriers By adding medicinal fungus fermentation liquor as additive and using effective components and fine powder extracted from plants and herbal medicines by solvent as adjuvant, the health product can be made into forms of effervescent tablet, buccal tablet, chewable tablet, oral taken tablet, candy, chocolate, chewing gum, oral liquid, particle, soluble granules, capsule, aerosol, liquid beverage and solid beverage. The product can relieve alcohol effect, nourish stomach, protect liver, and help to remit hepatitis B virus and hepatitis liver cancer patient condition, achieves a quite important effect for protecting the health of the sots or hepatitis B pathogen carriers in daily or social occasions. The invention provides a good idea to the utilization of the large amount of active fermentation liquid generated with the thallus pharmacy in the medicinal fungus fermentation industries, and also provides a effective approach for increasing the utilization value of the large amount of wild plant resources such as wild jujube and haw widely distributed in the north areas.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
N4-acylcytosine-1,3-dioxolane nucleosides for treatment of viral infections
InactiveUS6908924B2Strong inhibitory activityBiocideOrganic active ingredientsImmunodeficiency virusHuman patient
The present invention is directed to a compound, method and composition of treating or preventing viral infections, in particular, human immunodeficiency virus (HIV) and hepatitis B virus (HBV) infections, in human patients or other animal hosts, comprising the administration of N4-acylcytosine-1,3-dioxolane and pharmaceutically acceptable salts, prodrugs, and other derivatives thereof.
Owner:GILEAD PHARMASSET LLC
Methods for treating viral infection using IL-28 and IL-29
ActiveUS7135170B2Reduction in viral infection levelReduce viral infectionBiocidePeptide/protein ingredientsInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
3'-or 2'-hydroxymethyl substituted nucleoside derivatives for treatment of hepatites virus infections
InactiveUS20020055483A1Good effectEasy to modifyBiocideSugar derivativesDiseaseHepatitis B immunization
The present invention relates to a composition for and a method of treating hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, hepatitis D virus (HDV) infection or a proliferative disorder in a patient using an effective amount of a compound selected from the group consisting of formulas [I]- [IV] below and mixtures of two or more thereof: wherein the substituents are as defined herein. Pharmaceutical compositions comprising these compounds in combination with other HBV, HCV, or HDV agents is also disclosed.
Owner:PHARMASSET
Pharmaceutical use of 1 beta-hydroxy ilexolic acid for inhibiting hepatitis virus
InactiveCN1935131APrevention and treatment of viral hepatitis BHBsAg reductionOrganic active ingredientsOrganic chemistryChemical structureDisease
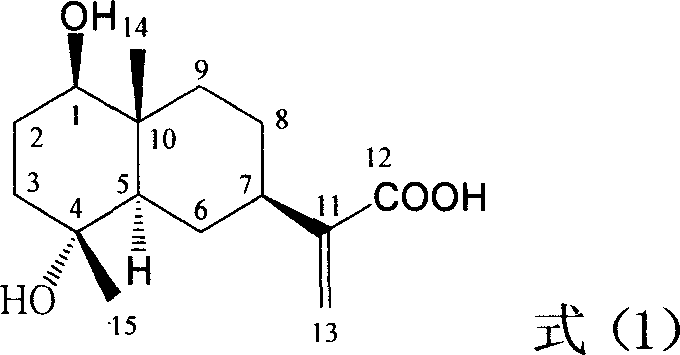
The present invention relates to an eudesmane type sesquiterpene derivative 1 beta-hydroxyilicic acid, namely 1 beta-hydroxy-5 alpha H-eudesmane-11 (13)-ethylene-12-acid, its medicineal salt or solvent compound and its medicine composition and medicinal application for preparing medicine capable of curing hepatitis B virus infective disease and resisting hepatitis B virus. Said invention also provides its chemical structure formula.
Owner:WENZHOU MEDICAL UNIV
Use of laggera plant abstract in inhibiting herpes simplex virus and hepatitis B virus
InactiveCN1989989AReduced expression functionDigestive systemPharmaceutical delivery mechanismDiseaseCaffeoylquinic acid
The invention involves novel drug use of six-rowed chrysanthemum plant extracts which is used to treating herpes simplex virus (type 1 and / or type 2) and various disease caused by hepatitis B virus infection. The six-rowed chrysanthemum plant extracts is prepared by six-rowed chrysanthemum plant fresh or dry goods through the refining of alcohol-water extraction, column chromatography, alcohol solvent elution, the amount of caffeoyl guinic acid chemical compound is below 30%. The six-rowed chrysanthemum plant extracts prepared in the invention has significant function of inhibiting herpes simplex virus with type 1 (HSV-1), herpes simplex virus type 2 (HSV-2) and hepatitis B virus (HBV) replication, and can reduce effectiveness of HBV e antigen (HBeAg) in the HepG 2.2.15 cell lines, it can be used for treatment various disease caused by said correlate virus infection.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Influenza immunogen and vaccine
InactiveUS20060115489A1High antibody titerEasy to prepareSsRNA viruses negative-senseAntibody mimetics/scaffoldsHepatitis B immunizationHepatitis B virus
A chimeric, carboxy-terminal truncated hepatitis B virus nucleocapsid (HBc) protein is disclosed that contains an immunogen for inducing the production of antibodies to the influenza M2 protein. An immunogenic influenza sequence in two to four copies is preferably expressed at or near the N-terminus or in the HBc immunogenic loop sequence. The HBc chimer preferably contains an influenza-specific T cell epitope and is preferably engineered for both enhanced stability of self-assembled particles and enhanced yield of those chimeric particles. Methods of making and using the chimers are also disclosed.
Owner:SANOFI PASTEUR BIOLOGICS CO +1
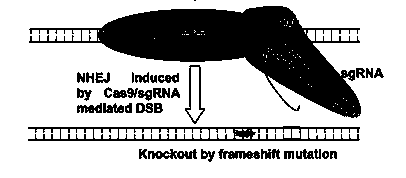
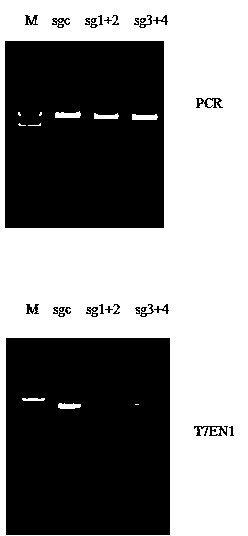
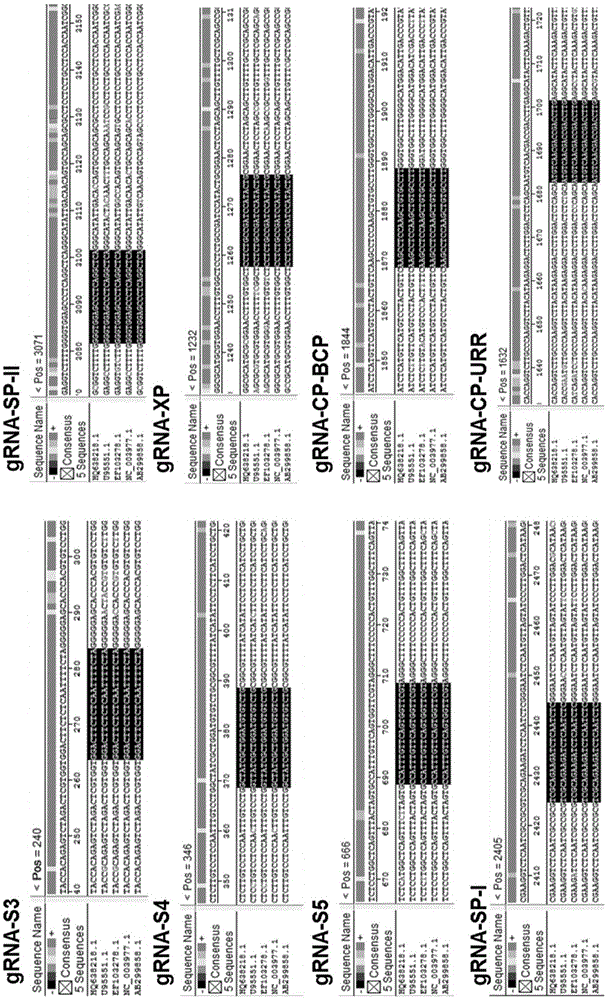
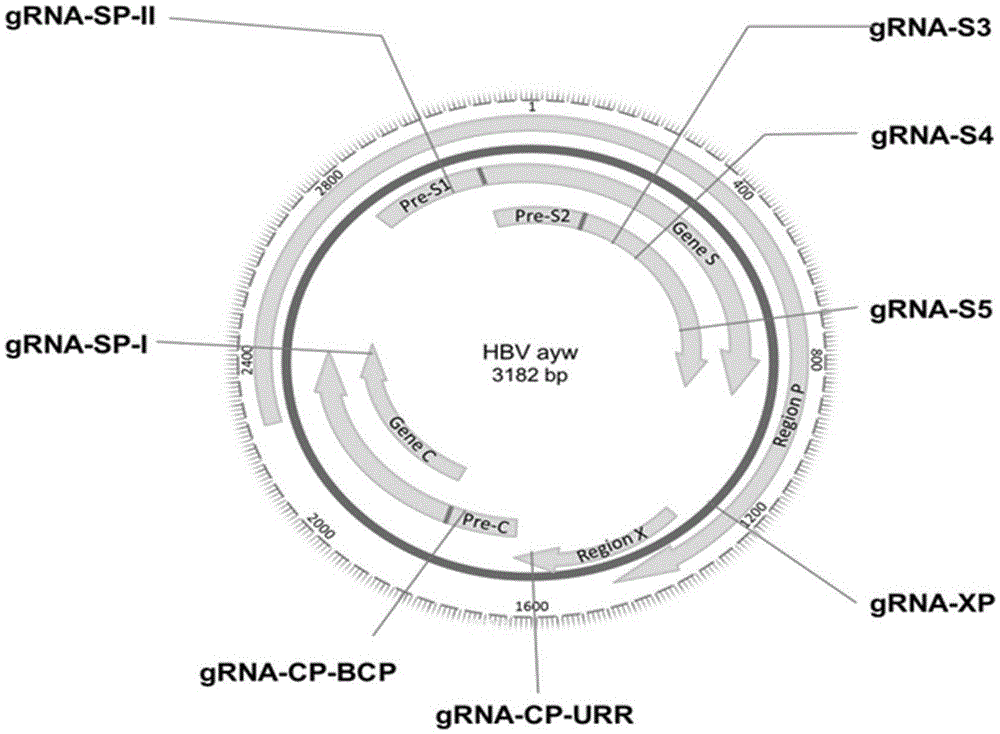
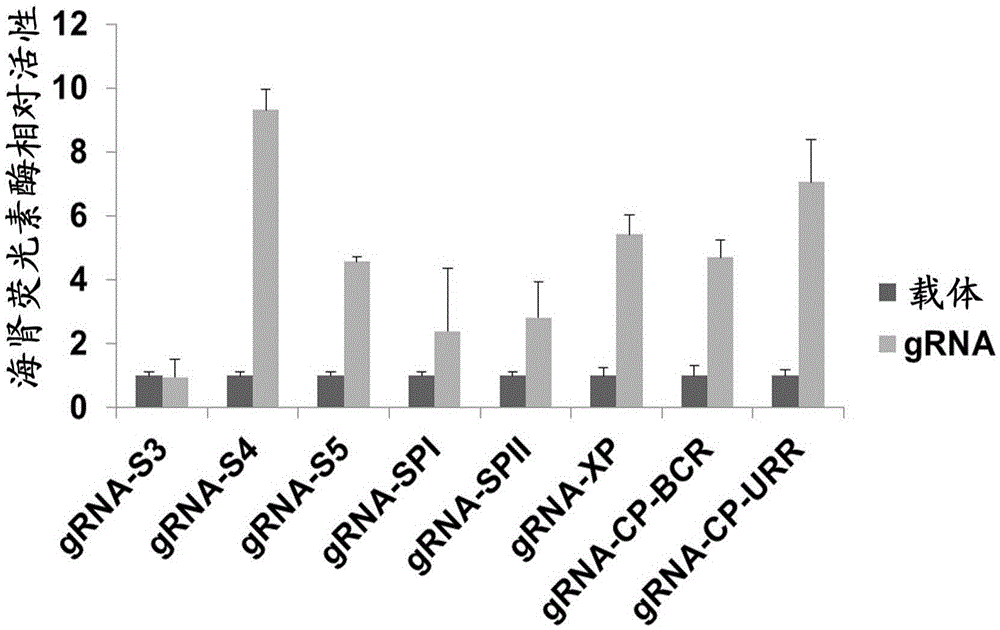
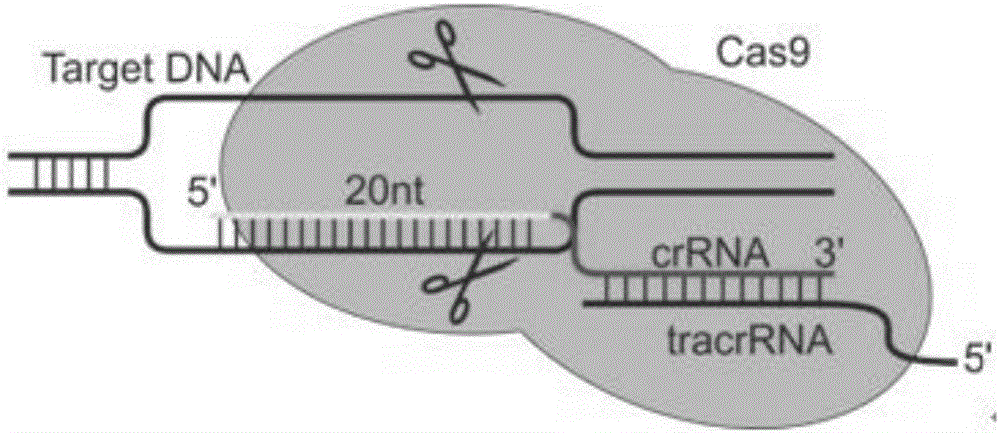
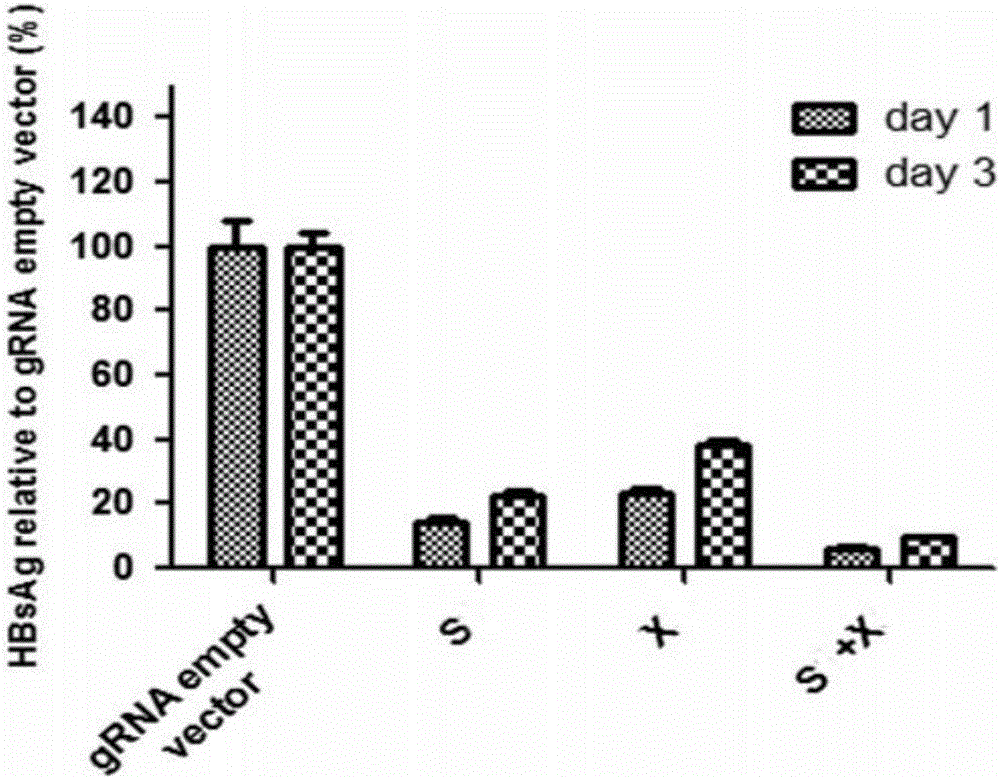
CRISPR-Cas9 targeted knockout hepatitis b virus cccDNA and specific sgRNA thereof
The invention belongs to the field of genetic engineering, and particularly relates to a method for specifically knocking out hepatitis b virus cccDNA by using CRISPR-Cas9 and sgRNA for specifically targeting the hepatitis b virus cccDNA. The invention provides a method for specifically knocking out hepatitis b virus cccDNA by using CRISPR-Cas9 and sgRNA for specifically targeting the hepatitis b virus cccDNA. The sgRNA of specific targeted hepatitis b virus cccDNA prepared according to the invention can precisely target hepatitis b virus cccDNA and realize gene knockout. A preparation method is simple in steps and good in sgRNA targeting, and the knockout efficiency of a CRISPR-Cas9 system is high.
Owner:AOMIAO BIOTECH GUANGZHOU CO LTD
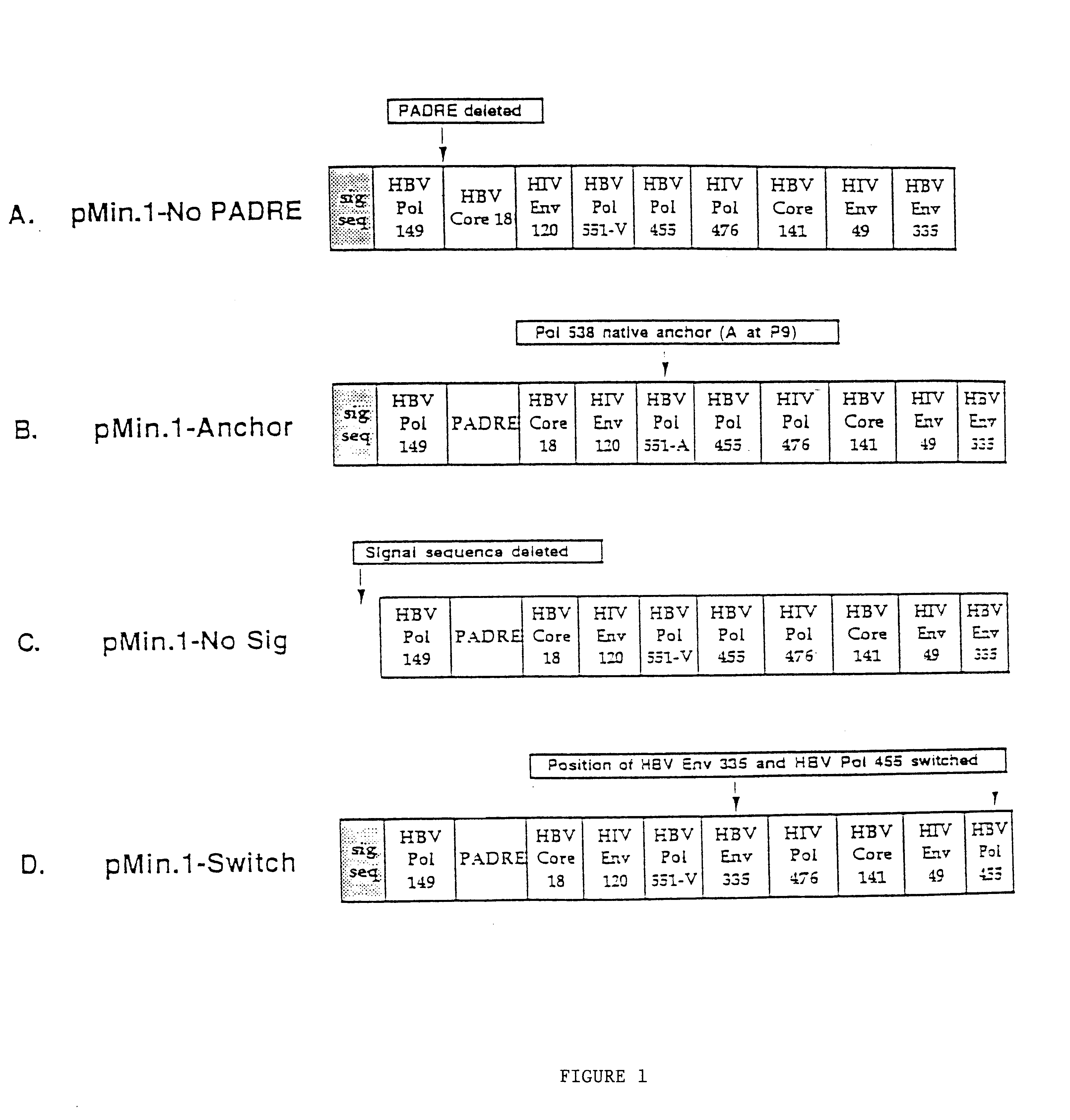
Inducing cellular immune responses to hepatitis B virus using peptide and nucleic acid compositions
This invention uses our knowledge of the mechanisms by which antigen is recognized by T cells to develop epitope-based vaccines directed towards HBV. More specifically, this application communicates our discovery of pharmaceutical compositions and methods of use in the prevention and treatment of HBV infection.
Owner:PHARMEXA
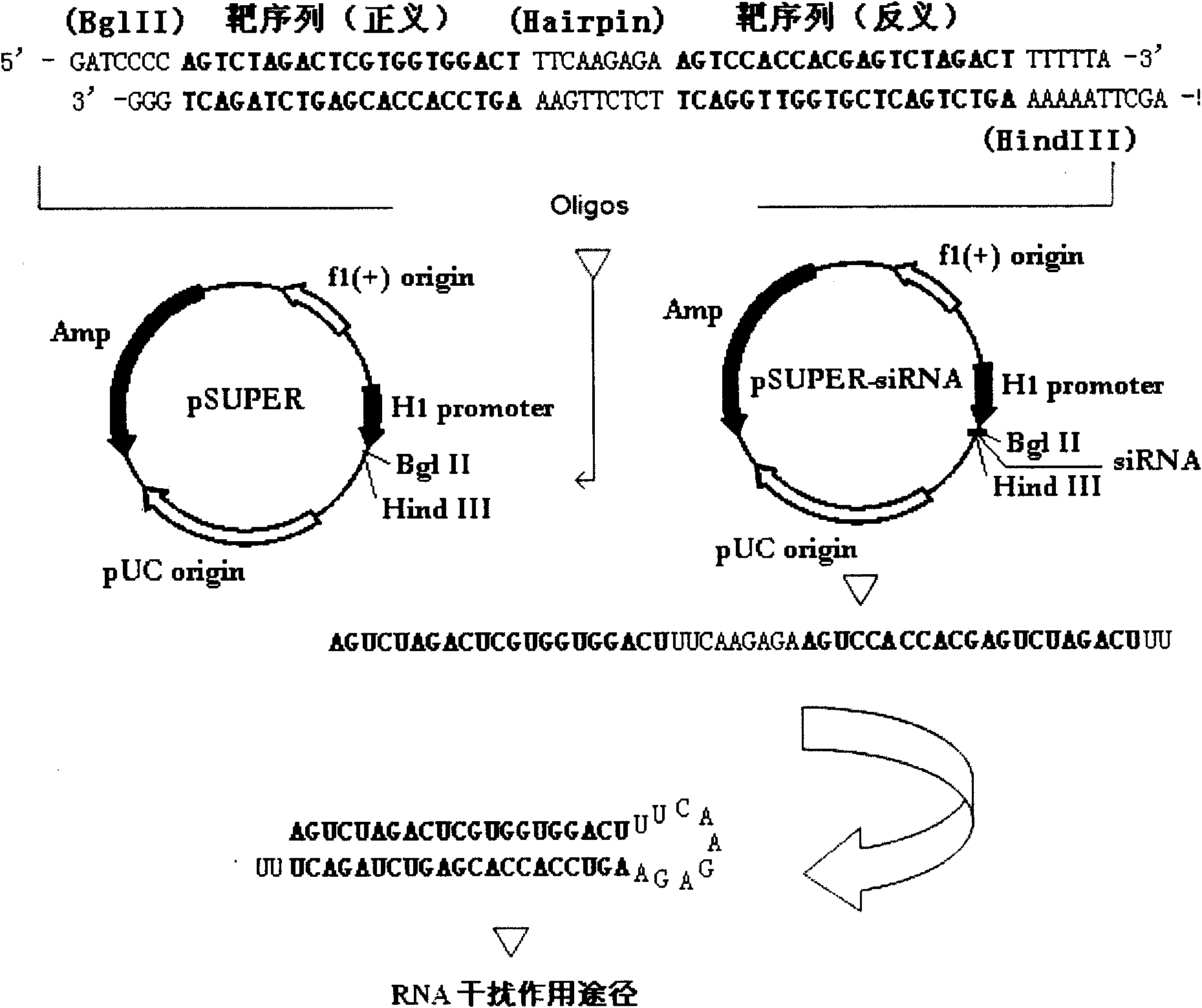
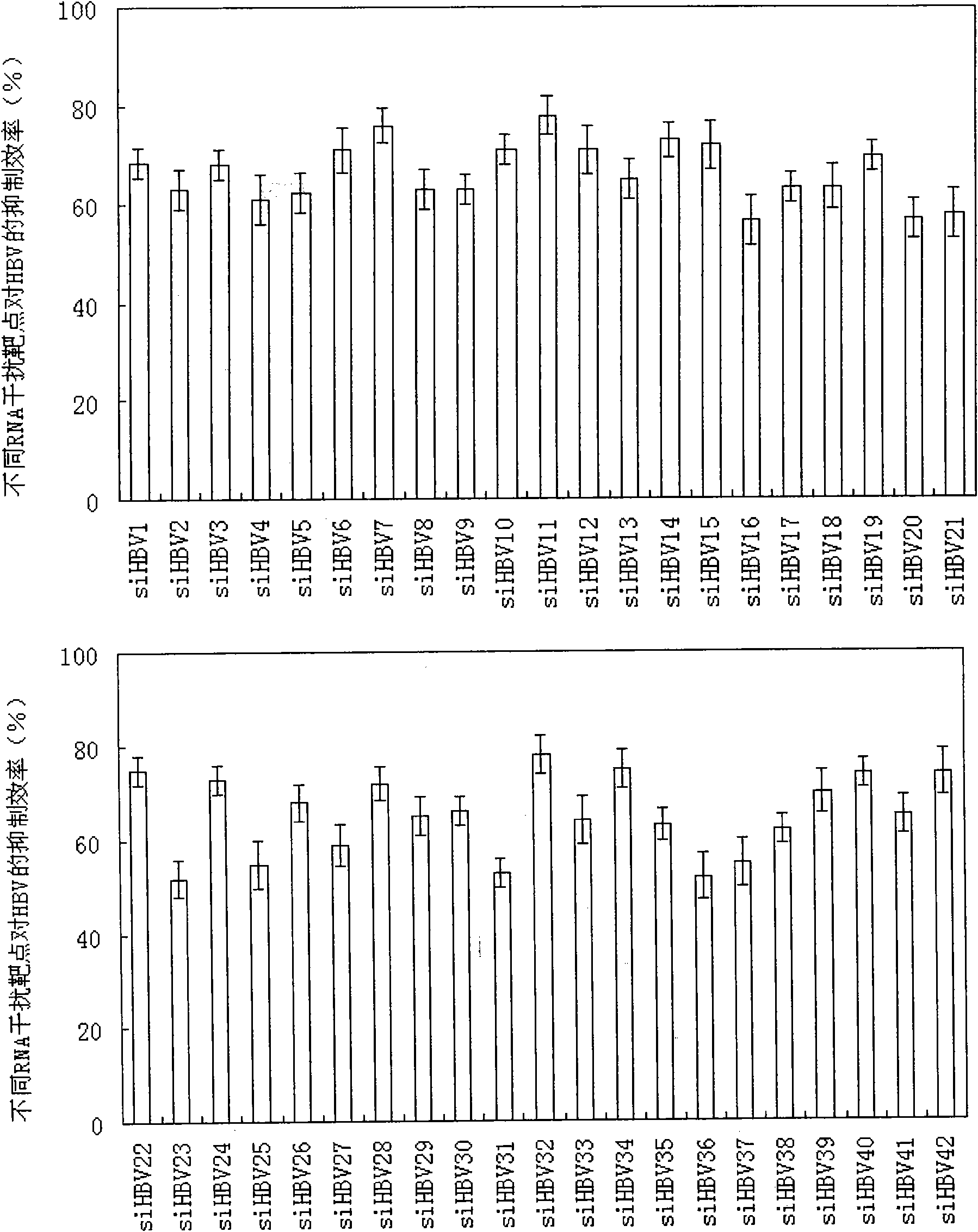
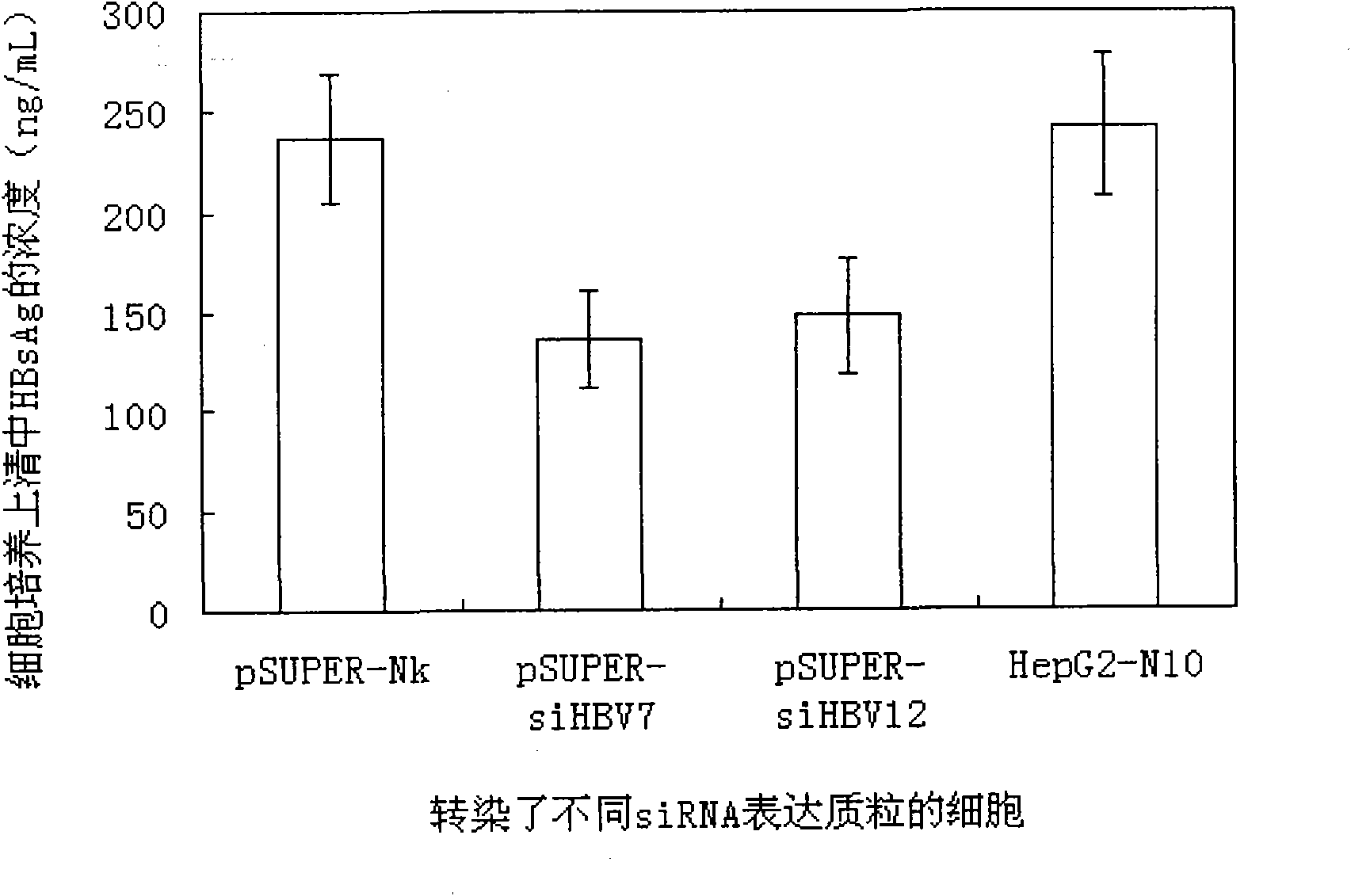
RNA interference target for treating hepatitis B virus infection
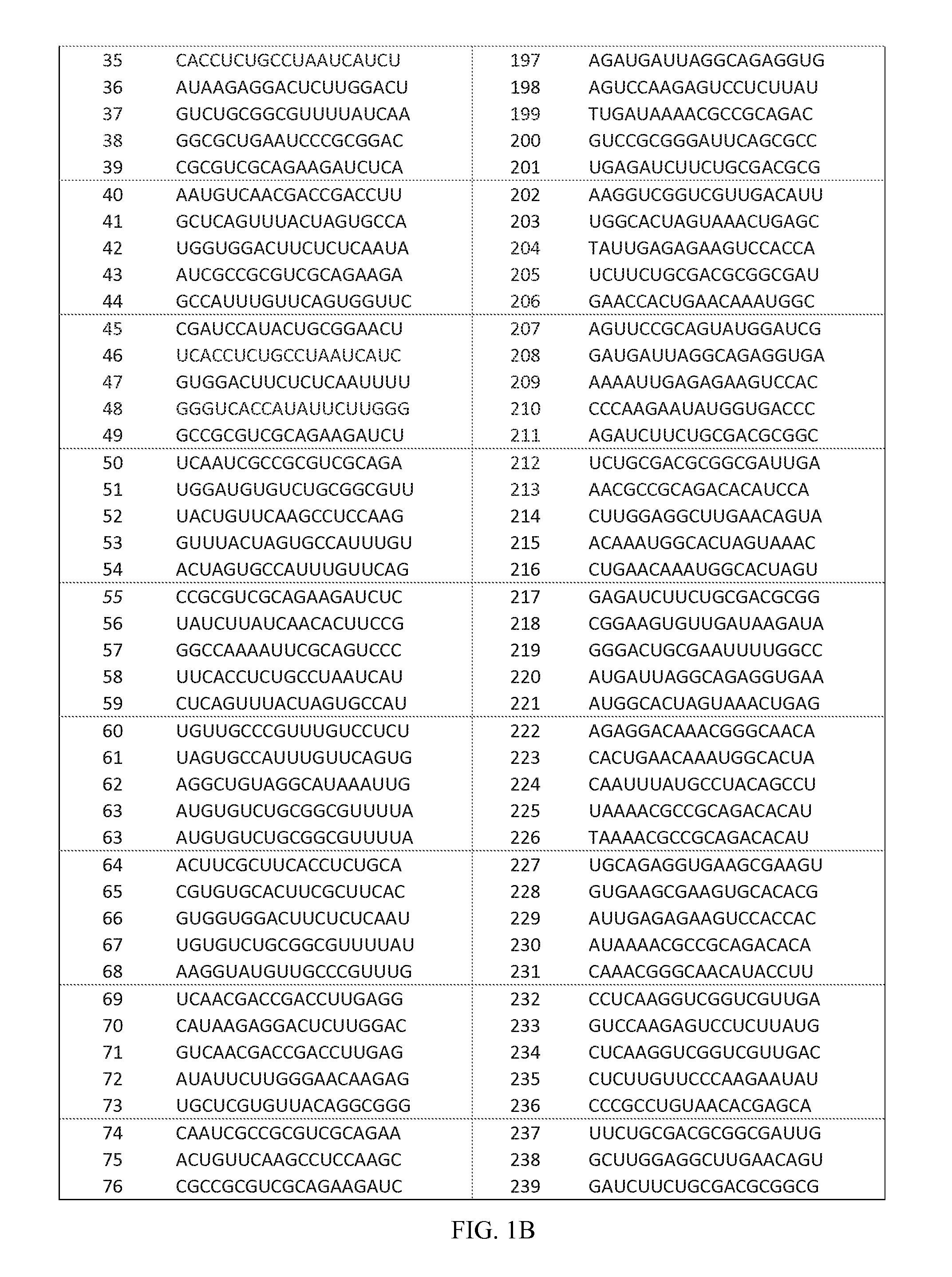
The invention relates to an RNA interference target for treating hepatitis B virus infection with 42 different targeting hepatitis B viruses (HBV), which can be used for preparing a medicament for treating the hepatitis B virus infection. The invention provides a recombinant expression vector of siRNA and / or miRNA and / or ribozyme and / or antisense oligonucleotide, which can be used for expressing targeting HBV. The invention relates to a cell which has the function of inhibiting the expression of HBV virus gene and can express and / or is induced with the siRNA and / or the miRNA and / or the ribozyme and / or the antisense oligonucleotide and / or the medication obtained according to the RNA interference target.
Owner:XIAMEN UNIV +1
Novel inhibitors of secretion of hepatitis b virus antigens
InactiveUS20130303552A1Low serum levelsBiocideOrganic active ingredientsAntigenHepatitis B Virus Antigen
Owner:DREXEL UNIV +2
Compositions and methods for inhibiting gene expression of hepatitis B virus
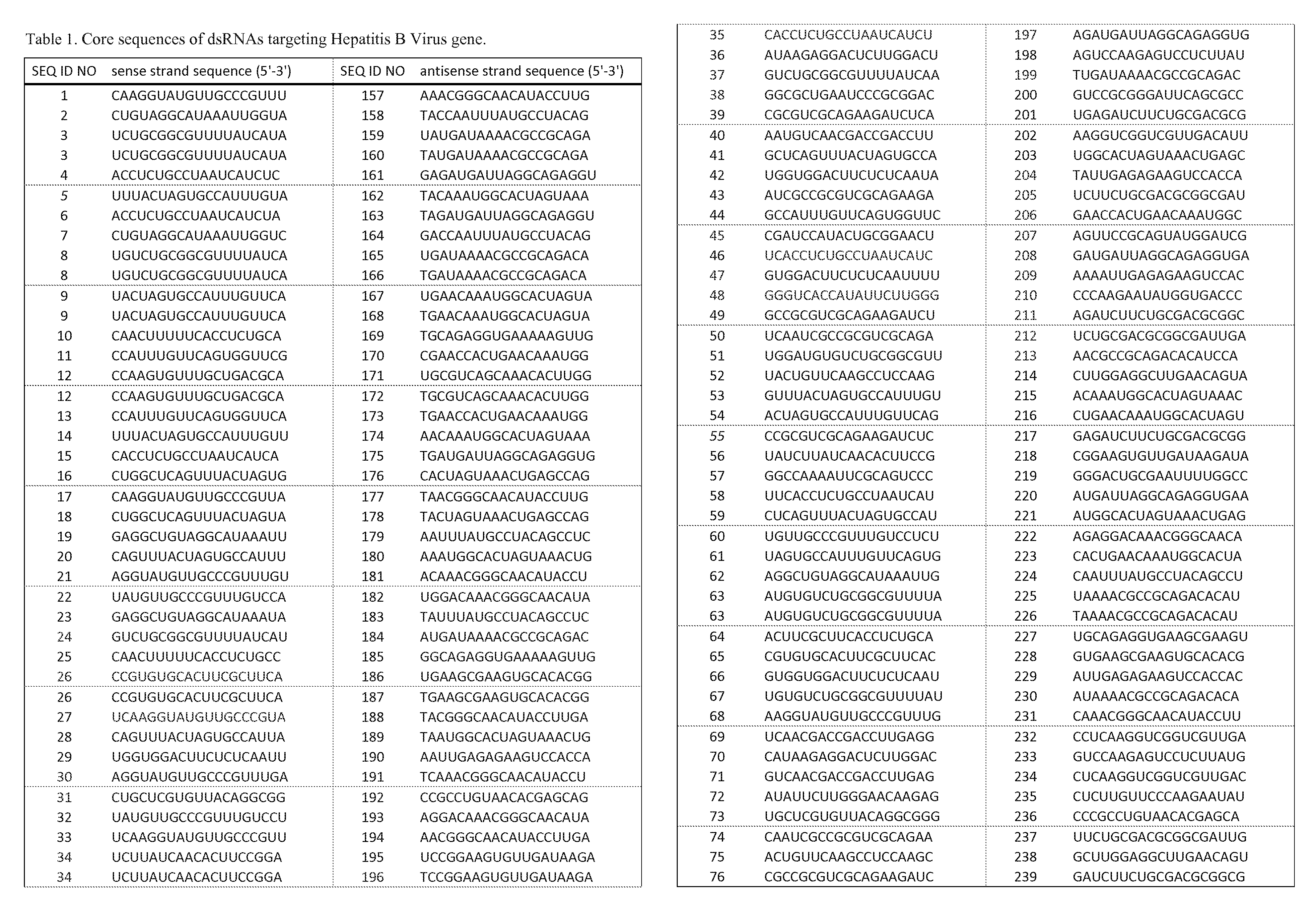
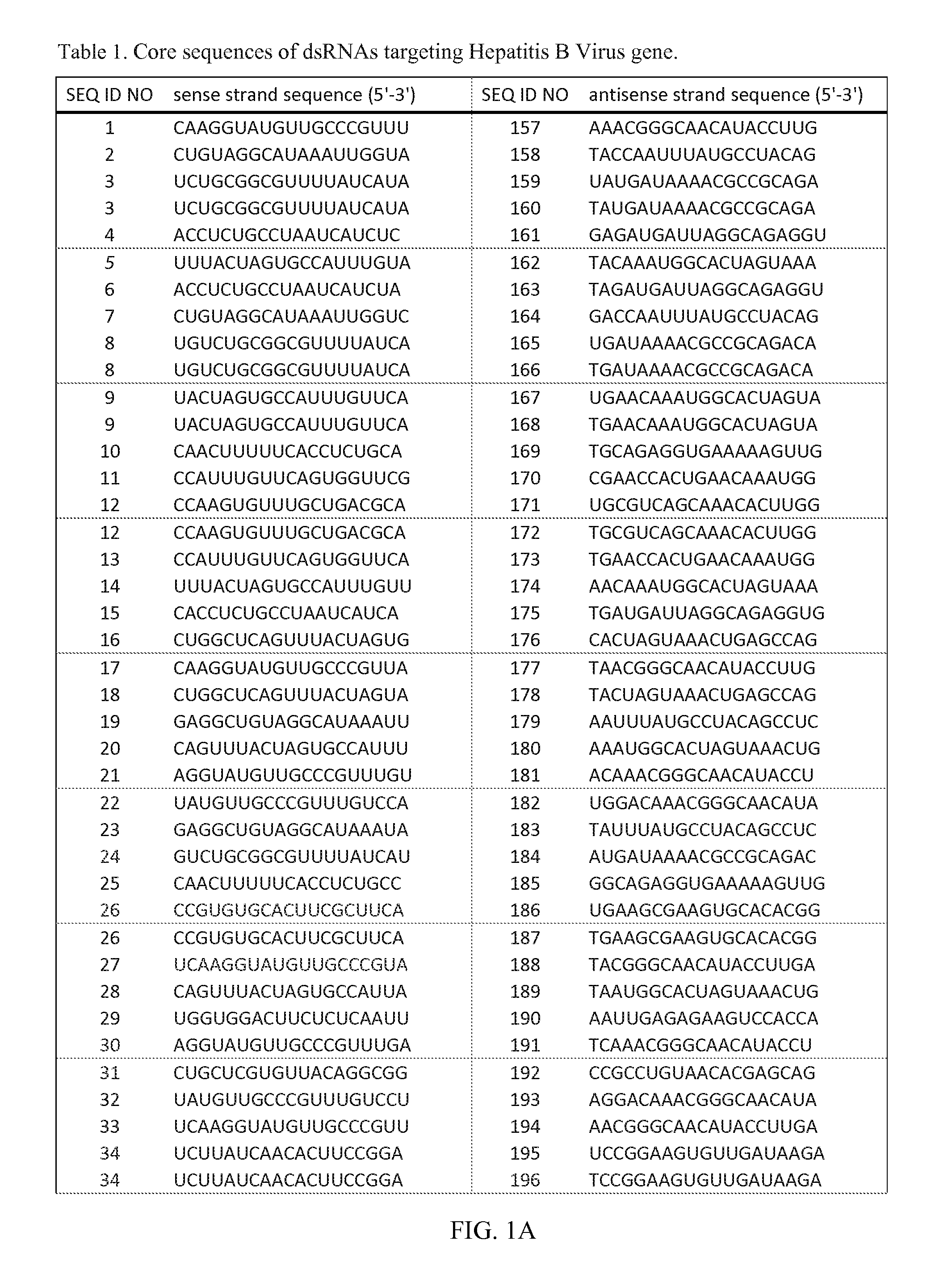
The invention relates to a double-stranded ribonucleic acid (dsRNA) for inhibiting the expression of a Hepatitis B Virus gene. The invention also relates to a pharmaceutical composition comprising the dsRNA or nucleic acid molecules or vectors encoding the same together with a pharmaceutically acceptable carrier; methods for treating diseases caused by Hepatitis B Virus infection using said pharmaceutical composition; and methods for inhibiting the expression of a Hepatitis B Virus gene in a cell.
Owner:ARROWHEAD PHARMA INC
IL28 and IL29 TRUNCATED CYSTEINE MUTANTS AND ANTIVIRAL METHODS OF USING SAME
InactiveUS20070053933A1Organic active ingredientsPeptide/protein ingredientsInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
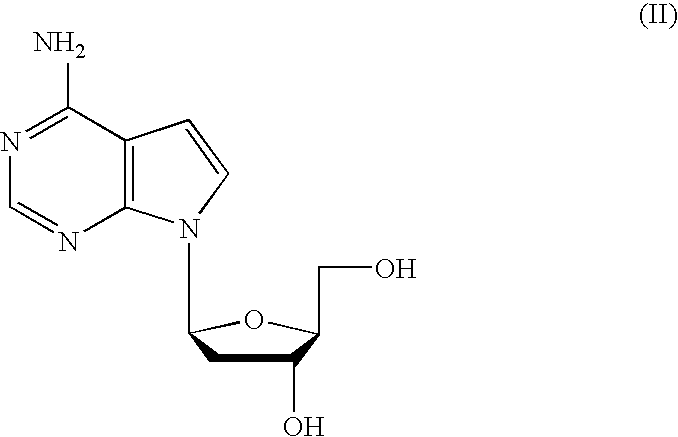
Anti-viral 7-deaza D-nucleosides and uses thereof
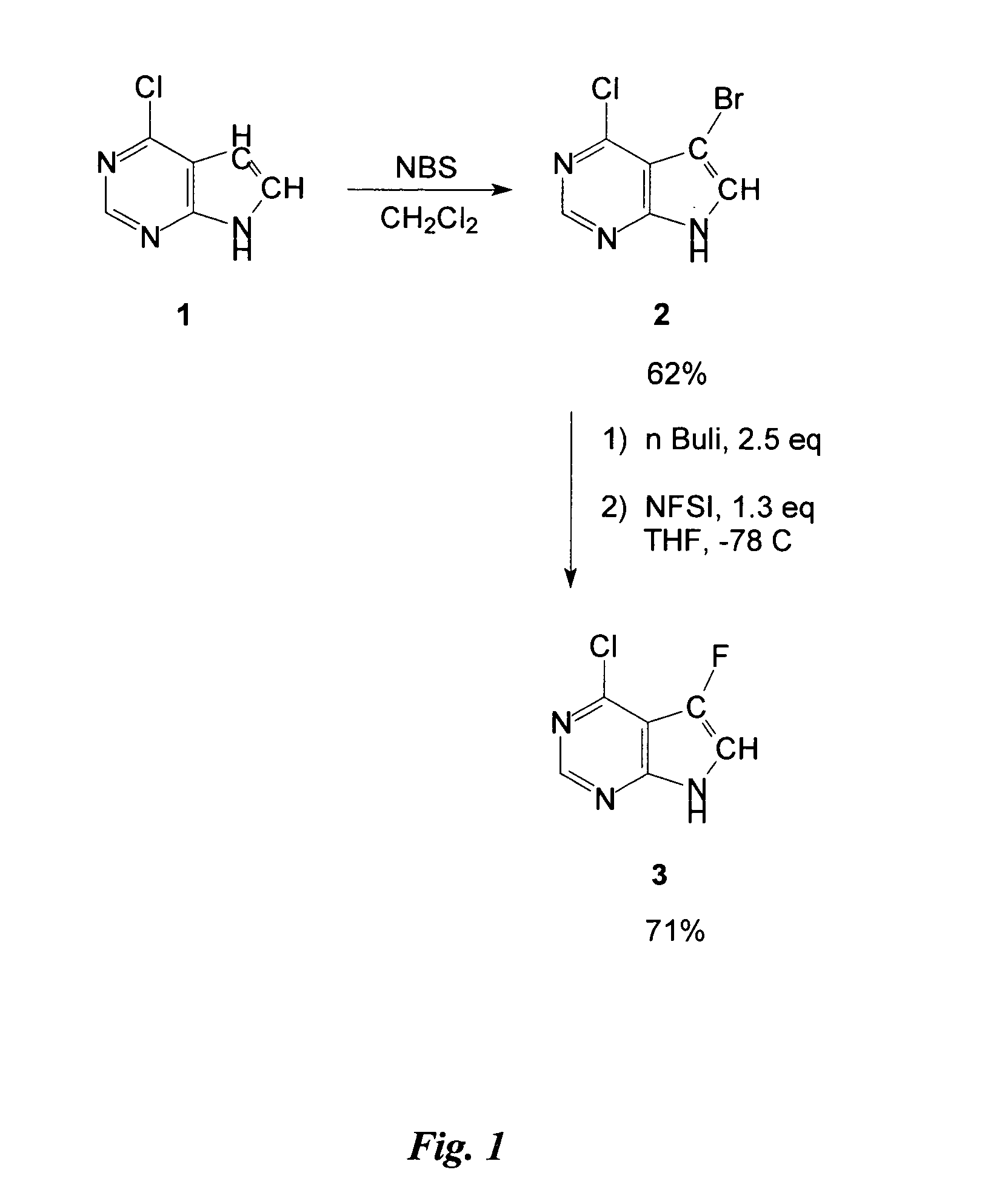
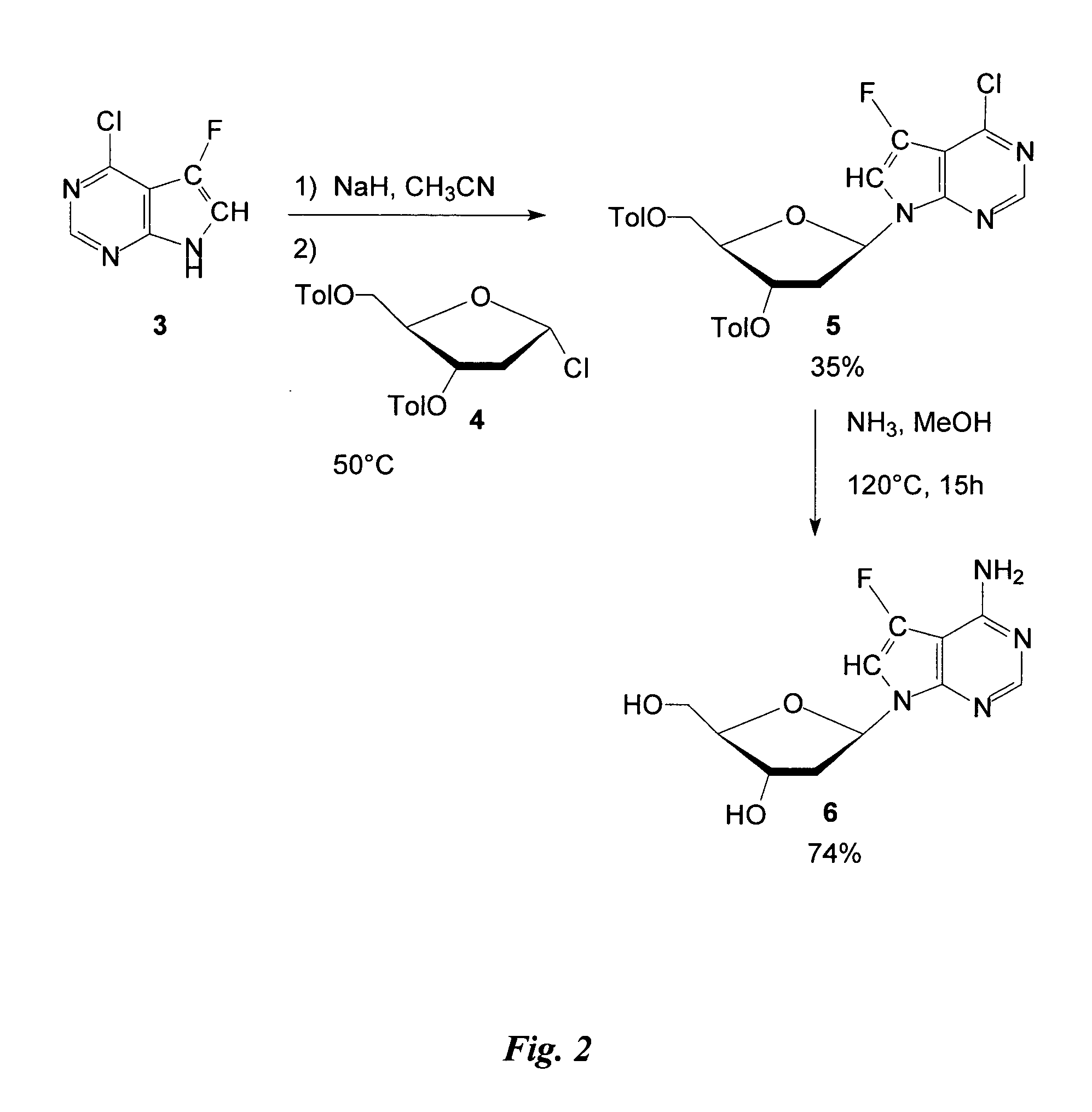
The present invention relates generally to anti-viral compounds, particularly anti-viral 7-deaza D-nucleosides and analogues, or derivatives thereof. The invention also relates to the use of such compounds to treat or prevent hepatitis B virus (HBV) infections, and to the use of such compounds to examine the biological mechanisms of HBV infection.
Owner:MIGENIX INC (CA)
Enantiomorphous eremophilanic acid and its medical use for inhibiting hepatitis B surface antigen
The invention relates to the medicine technical field and concretely relates to a mixture formed by two enantiomorphous eremophilane acids separated from murrey ligularia and the officinal salt as well as the medicine combined material. The enantiomorphous eremophilane acid can be used for preparing the medicine curing hepatitis B virus infectious diseases due to the ability of suppressing the antigenic activity on the surface of hepatitis B virus.
Owner:WENZHOU MEDICAL UNIV
Application of CRISPR-Cas9 system based on new gRNA (guide ribonucleic acid) sequence in preparing drugs for treating hepatitis B
PendingCN105647922AInhibition of replicationInhibit expressionOrganic active ingredientsVectorsHepatitis B virusHbv replication
The invention discloses a gRNA (guide ribonucleic acid) sequence. The sequence can be used for DNA (deoxyribonucleic acid) sequence edition by using a hepatitis B virus genome S gene conserved region site as a target sequence, can successfully destroy the HBV (hepatitis B virus) cccDNA and integrated-state HBV DNA in a HBV stable cell model and an HBV hydrodynamic mouse model, and obtains obvious effects on inhibiting HBV replication and expression. The invention also discloses application of the CRISPR-Cas9 system containing the gRNA sequence in preparing drugs for treating hepatitis B.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Anti-viral 7-deaza L-nucleosides
The present invention comprises 7-deaza L-nucleosides having unexpectedly high inhibitory activity against the hepatitis B virus. The invention further comprises pharmaceutical compositions comprising such compounds as well as methods of treating mammals, particularly humans, infected with HBV and other viral infections.
Owner:MICROLOGIX BIOTECH INC
Methods for treating viral infection using il-28 and il-29 cysteine mutants
InactiveUS20080075693A1Prolonged Circulatory Half-LifeLow immunogenicityPeptide/protein ingredientsAntipyreticInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
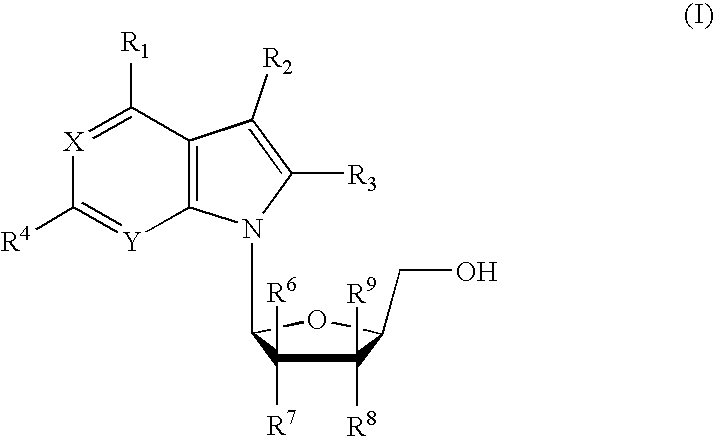
Hepatitis b antiviral agents
ActiveUS20170253609A1AntiviralsAmide active ingredientsHepatitis B immunizationPharmaceutical medicine
The present invention discloses compounds of Formula (I), or pharmaceutically acceptable salts, esters, or prodrugs thereof:X-A-Y-L-R (I)which inhibit the protein(s) encoded by hepatitis B virus (HBV) or interfere with the function of the HBV life cycle of the hepatitis B virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HBV infection. The invention also relates to methods of treating an HBV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Hepatitis C Virus Inhibitors
This disclosure concerns novel compounds of Formula (I) as defined in the specification and compositions comprising such novel compounds. These compounds are useful antiviral agents, especially in inhibiting the function of the NS5A protein encoded by Hepatitis C virus (HCV). Thus, the disclosure also concerns a method of treating HCV related diseases or conditions by use of these novel compounds or a composition comprising such novel compounds.
Owner:BRISTOL MYERS SQUIBB CO
Specific sgRNA combined with immunogene to inhibit HBV replication, expression vector thereof, and application of specific sgRNA and expression vector
ActiveCN105821039AImprove targetingHigh knockout efficiencyOrganic active ingredientsGenetic material ingredientsHepatitis B Virus AntigenIn vivo
The invention provides a specific sgRNA combined with an immunogene to inhibit HBV replication, an expression vector thereof, and an application of the specific sgRNA and the expression vector. The sgRNA sequences of a human hepatitis B virogene suitable for CRISPR-Cas9 targeting editing and a PD-1 gene are designed, the plasmid vector of the sgRNA inhibiting HBV and PD-1 genes is constructed, and the plasmid vector and a nuclease gene expression vector are transferred to HBV transgenic mice, and can obviously inhibit HBV DNA replication. The gene expression vector prepared in the invention has the advantages of simple method steps, good sgRNA targeting property, and high CRISPR-Cas9 knockout efficiency. The sgRNAs specifically targeting the HBV and PD-1 genes can accurately splice the HBV and PD-1 genes, inhibit in vivo hepatitis B virus replication, and reduce the hepatitis B virus antigen expression.
Owner:李旭
3'-Prodrugs of 2'-deoxy-beta-L-nucleosides
The present invention relates to compounds, compositions and methods for the treatment of a host infected with a hepatitis B virus. Specifically, compound and compositions of 3′-esters of 2′-deoxy-β-L-nucleosides are disclosed, which can be administered either alone or in combination with other anti-hepatitis B agents. Compound and compositions of 3′,5′-diesters of 2′-deoxy-β-L-nucleosides are disclosed, which can be administered either alone or in combination with other anti-hepatitis B agents, are also disclosed.
Owner:INDENIX PHARM LLC +2
Liver targeted antivirus precursor medicament annular phosphoester and use thereof
InactiveCN101475594AFew synthetic stepsEasy to operateOrganic active ingredientsGroup 5/15 element organic compoundsDrugPhosphate
The invention provides a prodrug of an antiviral drug for use in liver of cyclic phosphate of a general formula (I) and isomers, pharmaceutical salts, hydrates, solvates and pharmaceutical compositions of the same. The invention provides uses of the compounds singly or together with other antiviral drugs in the treatment of viruses, in particular of hepatitis B viruses (HBV), hepatitis C virus (HCV), HIV viruses and / or human cytomegaloviruses (HCMV).
Owner:廖国超
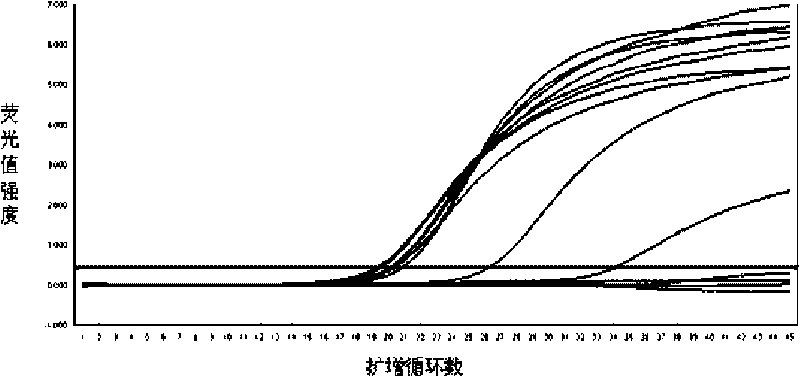
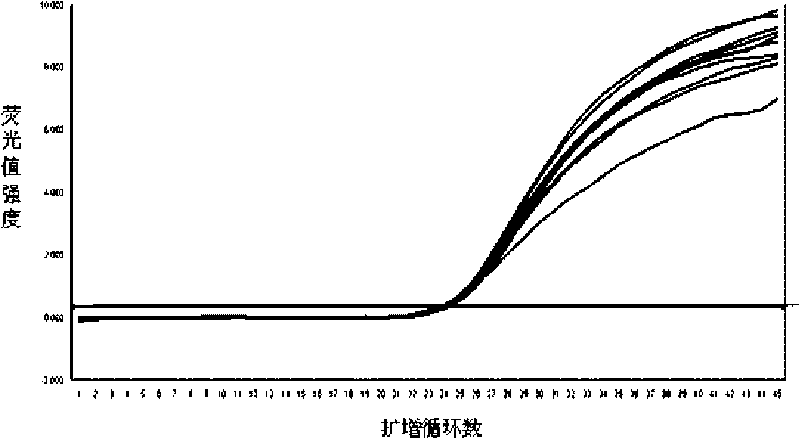
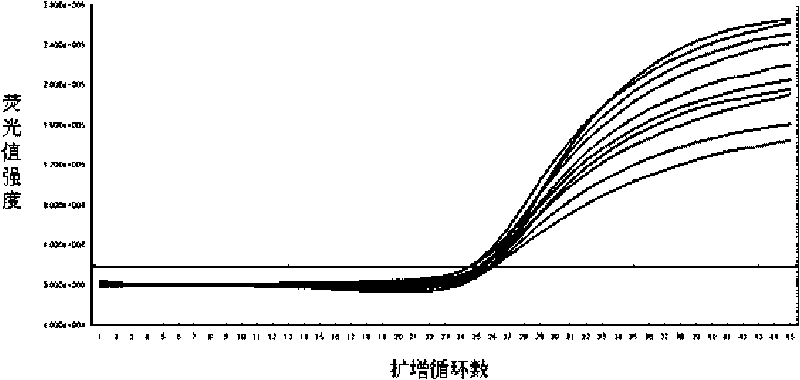
Fluorescence quantitative PCR detection kit of hepatitis B virus and application thereof
ActiveCN101701267AStrong specificityHigh purityMicrobiological testing/measurementMicroorganism based processesPositive controlFluorescence
The invention discloses a fluorescence quantitative PCR detection kit of hepatitis B virus and an application thereof. The kit is composed of the following independent components: DNA extraction solution I, DNA extraction solution II, DNA extraction solution III, DNA extraction solution IV, positive control interior label, PCR reaction liquid, probe HBV-SP, enzyme mixed liquor containing heat resistant DNA polyase and uracil DNA glycosylase, quantitative hepatitis B virus reference material, hepatitis B virus positive control serum and hepatitis B virus negative control serum, wherein DNA extraction solution I contains 0.2-1.0% of lauryl sodium sulphate (mass / volume), 1.0-4.0% of Triton (volume / volume) and 0.2-1.0mol / L of guanidinium isothiocyanate; DNA extraction solution II contains 100-300mmol / L of 4-HEPES, 100-300mmol / L of sodium chloride with pH of 6.5+ / -0.2 and 100-400 mu g / ml of magnetic beads; DNA extraction solution III contains 0.1-1.0% of Triton (volume / volume) and 100-300mmol / L of sodium chloride; DNA extraction solution IV contains mineral oil. The fluorescence quantitative PCR detection kit of hepatitis B virus of the invention can be used for detecting the HBV-DNA concentration in samples of serum, blood plasma or latex and the like.
Owner:SANSURE BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Novel 6,7-dihydrobenzo[a]quinolizin-2-one derivatives for the treatment and prophylaxis of hepatitis B virus infection Novel 6,7-dihydrobenzo[a]quinolizin-2-one derivatives for the treatment and prophylaxis of hepatitis B virus infection](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/22766c19-01fe-40a6-8b2e-6bdb5566cfb0/US20160122344A1-20160505-C00001.PNG)
![Novel 6,7-dihydrobenzo[a]quinolizin-2-one derivatives for the treatment and prophylaxis of hepatitis B virus infection Novel 6,7-dihydrobenzo[a]quinolizin-2-one derivatives for the treatment and prophylaxis of hepatitis B virus infection](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/22766c19-01fe-40a6-8b2e-6bdb5566cfb0/US20160122344A1-20160505-C00002.PNG)
![Novel 6,7-dihydrobenzo[a]quinolizin-2-one derivatives for the treatment and prophylaxis of hepatitis B virus infection Novel 6,7-dihydrobenzo[a]quinolizin-2-one derivatives for the treatment and prophylaxis of hepatitis B virus infection](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/22766c19-01fe-40a6-8b2e-6bdb5566cfb0/US20160122344A1-20160505-C00003.PNG)