Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1130 results about "Human patient" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
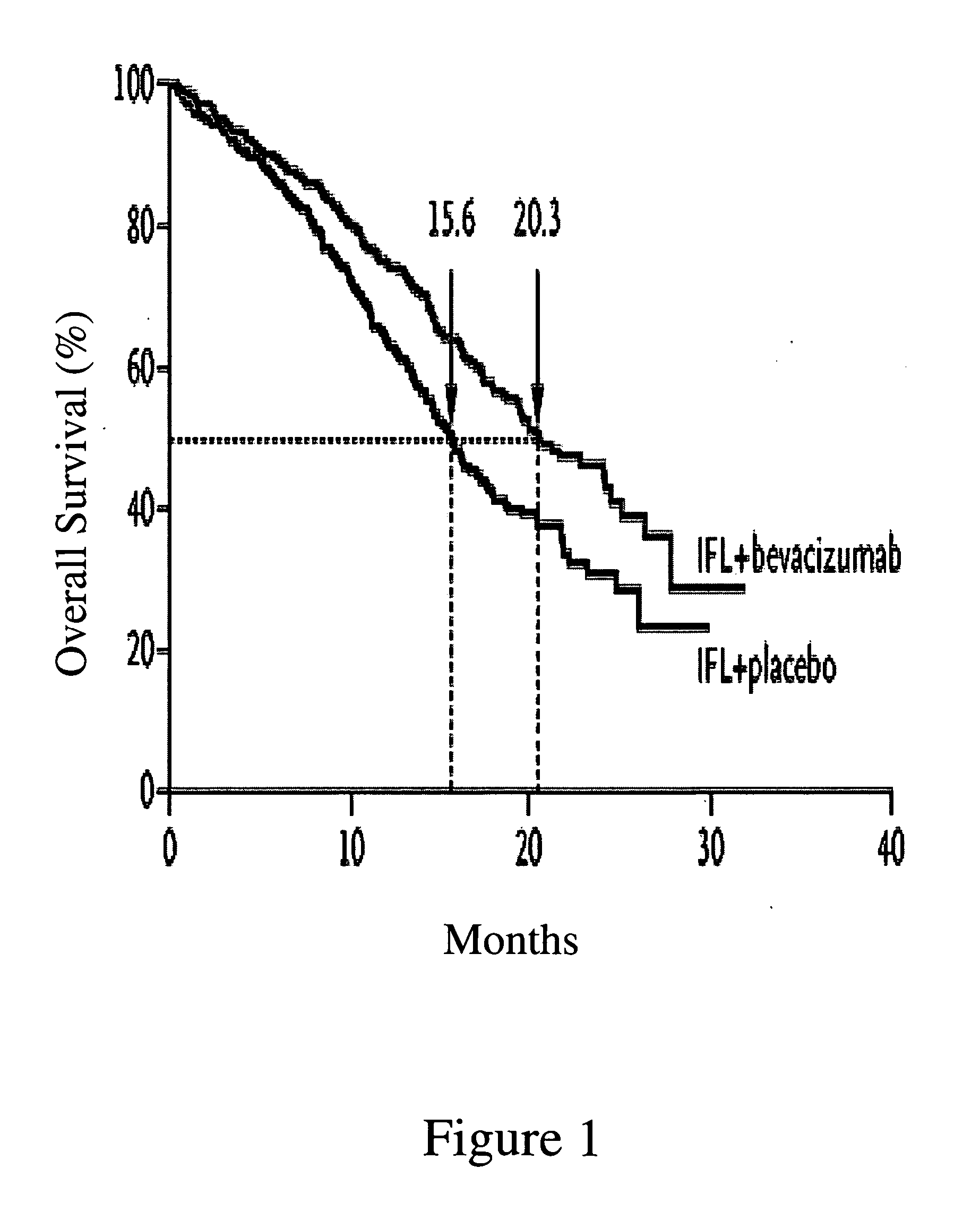
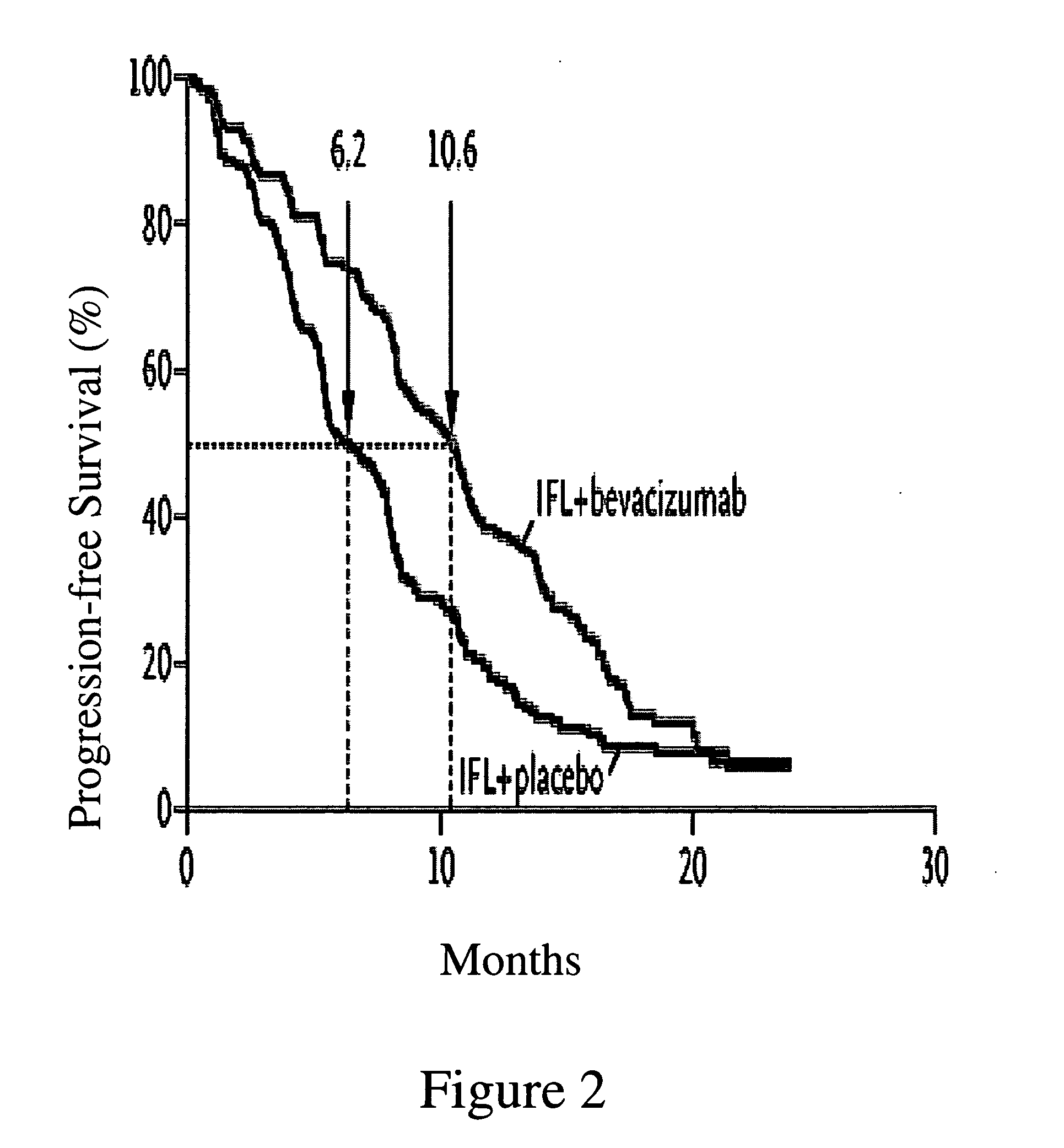
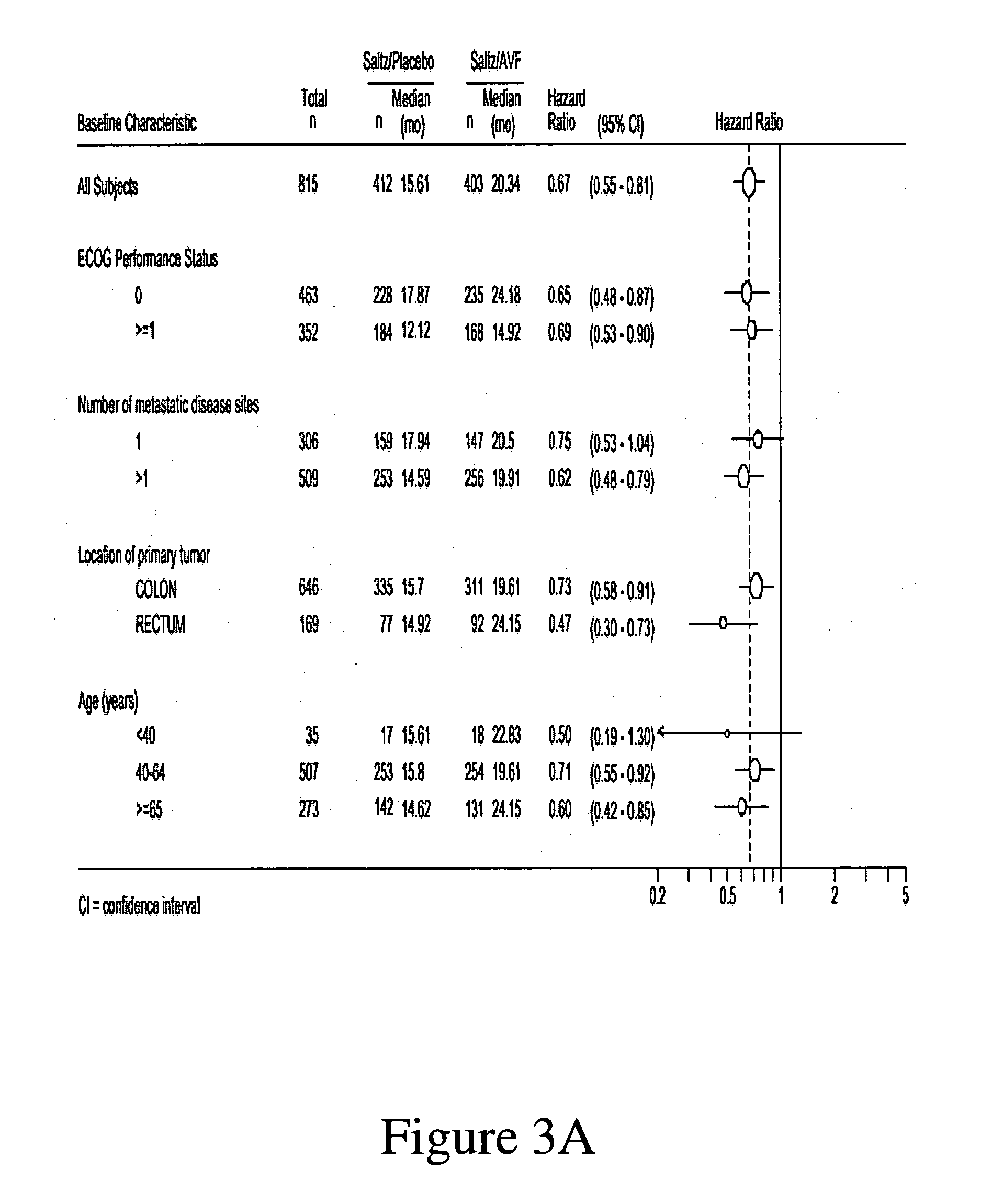
Treatment with anti-VEGF antibodies
InactiveUS20050186208A1Effective approachExtended durationBiocidePeptide/protein ingredientsAbnormal tissue growthAnti vegf antibody
This invention concerns in general treatment of diseases and pathological conditions with anti-VEGF antibodies. More specifically, the invention concerns the treatment of human patients susceptible to or diagnosed with cancer using an anti-VEGF antibody, preferably in combination with one or more additional anti-tumor therapeutic agents.
Owner:GENENTECH INC
Total disc implant
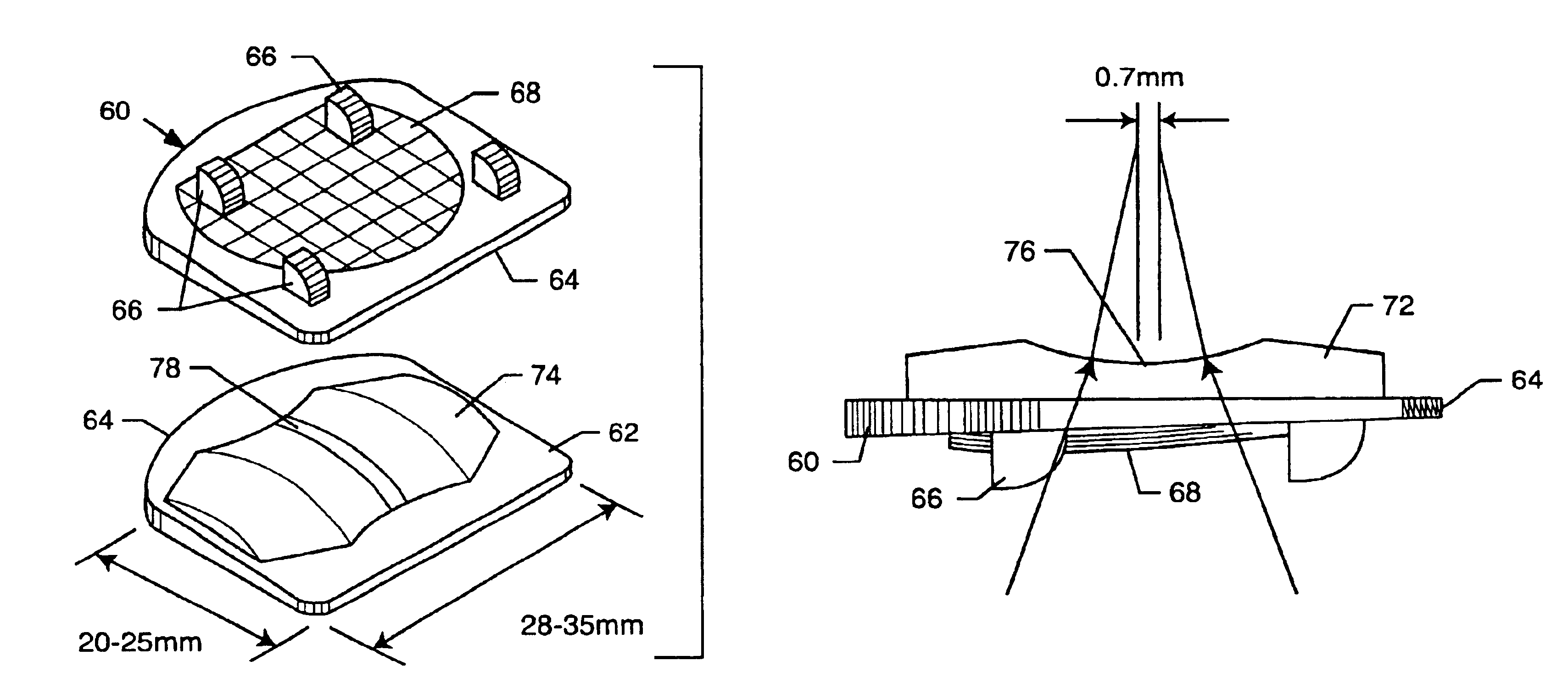
InactiveUS6994727B2Impressive mechanicalImpressive tribologicalInternal osteosythesisJoint implantsHuman patientIntervertebral disk
A total disc implant (TDI) is provided for total replacement of a spinal disc or discs in a human patient or other mammal, wherein the TDI is designed to maintain a substantially full range of natural motion (ROM) following implantation. The TDI generally comprises, in one preferred form, upper and lower end plates for affixation to adjacent vertebral bodies, with an intervening insert disposed therebetween. The end plates each include elongated part-cylindrical surfaces oriented generally perpendicular to each other, with one of said surfaces extending in an anterior-posterior direction and the other extending in a medial-lateral direction. The intervening insert defines concave upper and lower part-cylindrical seats oriented for respectively engaging these part-cylindrical surfaces, wherein these part-cylindrical seats are defined by offset radii to include a somewhat flattened central base region merging smoothly with upwardly curving radiused sides.
Owner:AMEDICA A DELAWARE
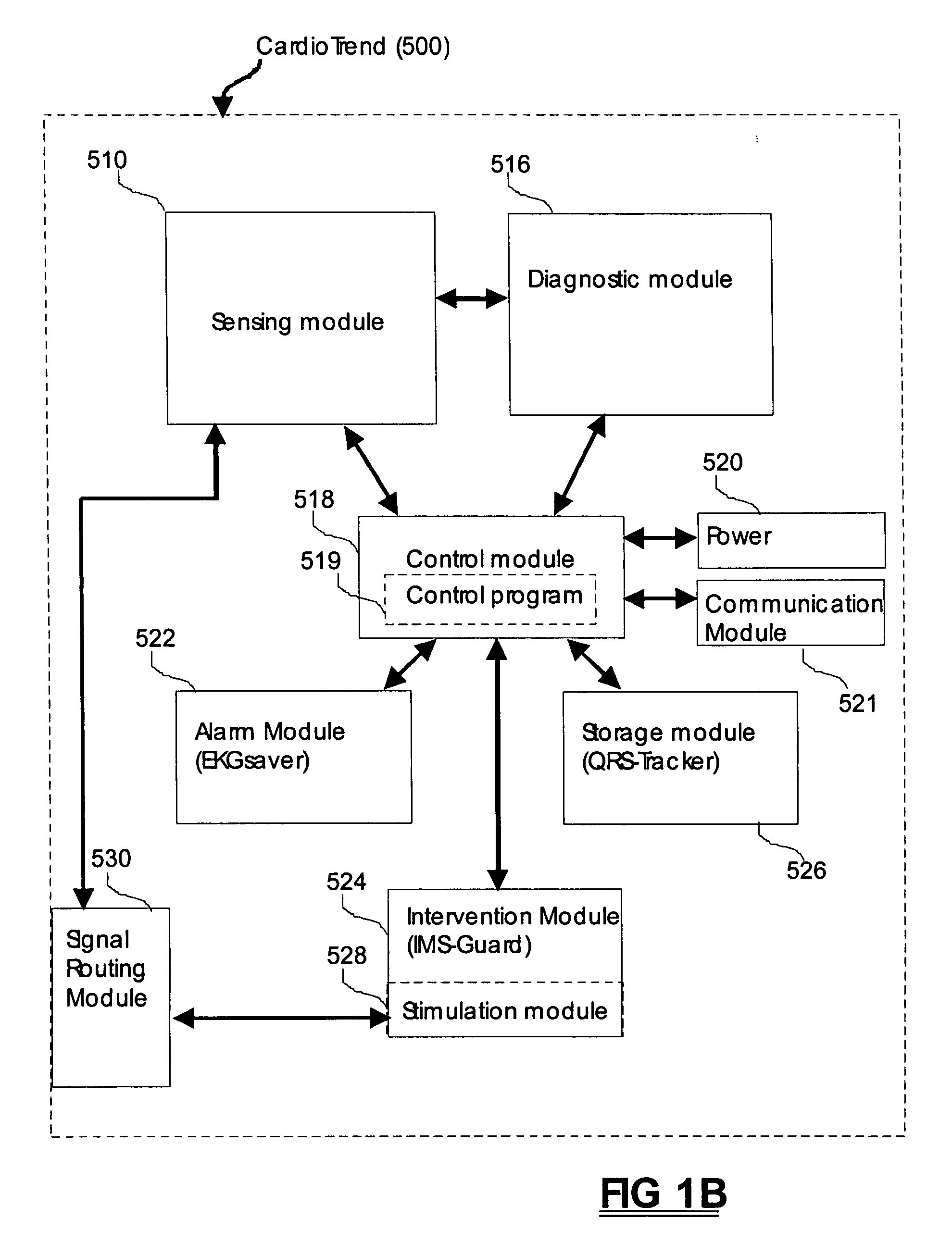
Systems and methods of medical monitoring according to patient state
A system for the detection of cardiac events occurring in a human patient is provided. At least two electrodes are included in the system for obtaining an electrical signal from a patient's heart. An electrical signal processor is electrically coupled to the electrodes for processing the electrical signal. The systems receives data regarding the patient's state (e.g. asleep, exercising). Patient state information is stored in a patient state array, thereby enabling the system to track the patient's state over time, and to select an appropriate test for detecting a cardiac event based on both past and present data regarding the patient's state.
Owner:ANGEL MEDICAL SYST
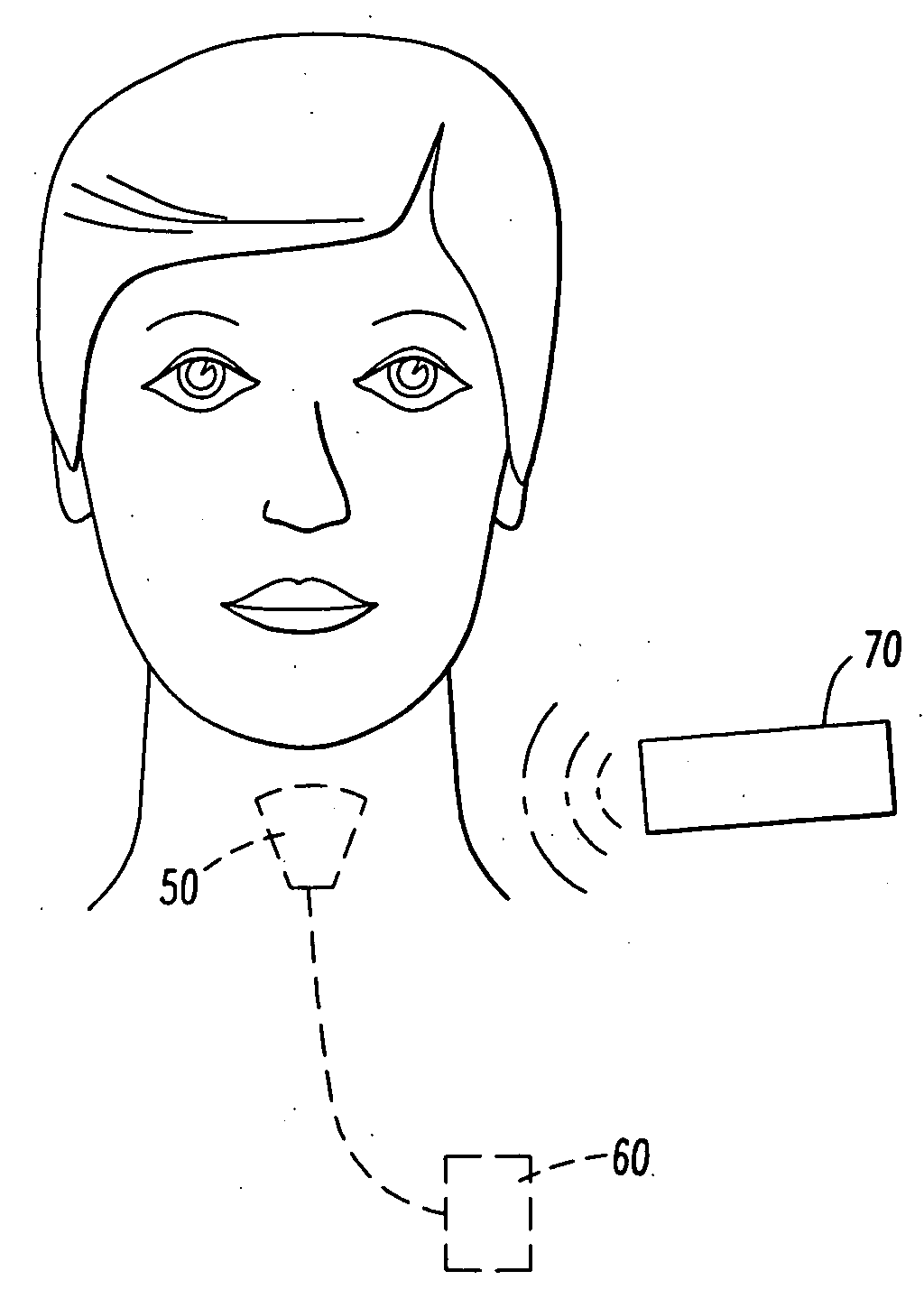
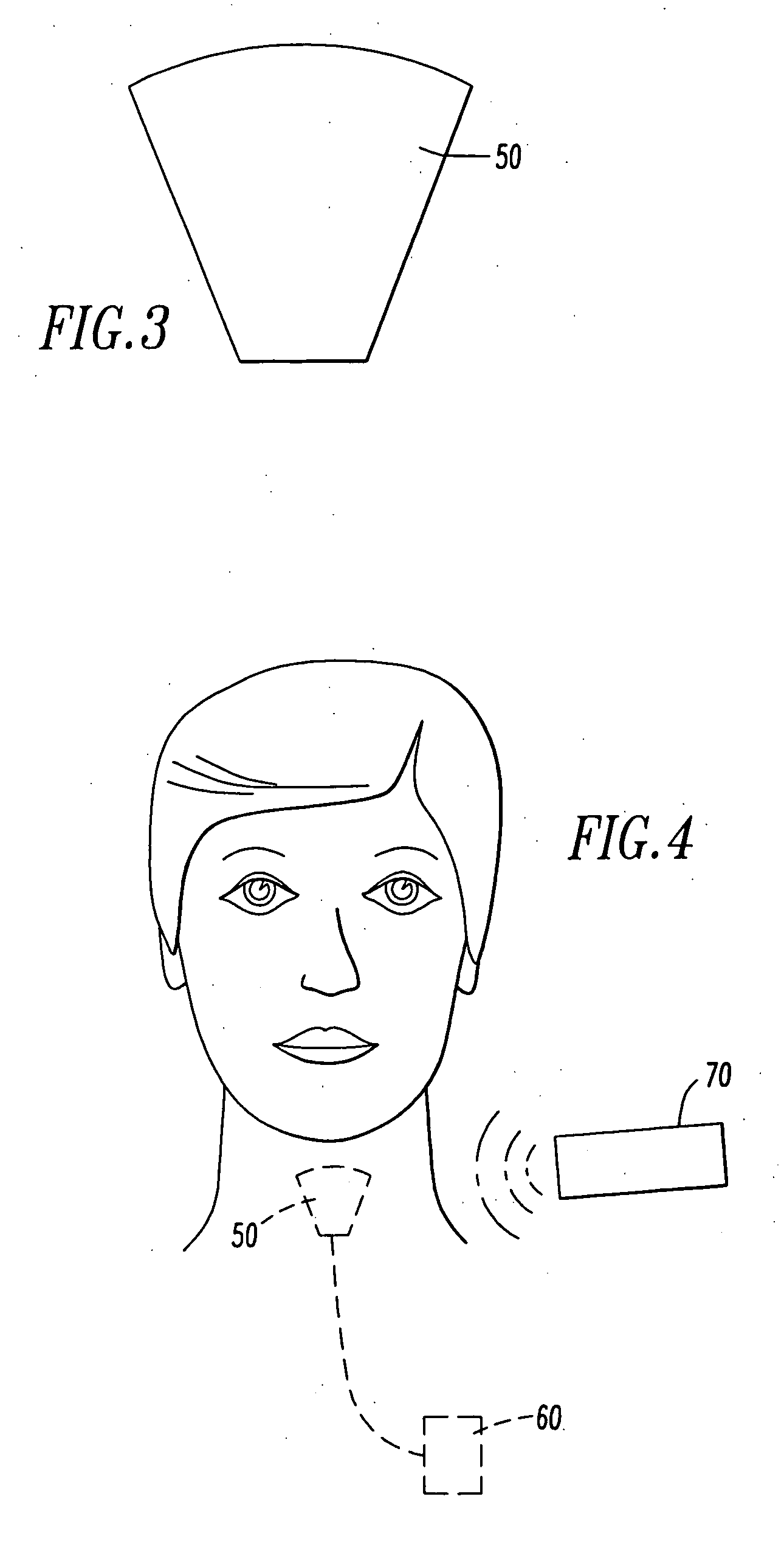
Method and stereotactic apparatus for locating intracranial targets guiding surgical instruments
A method and an instrument are presented for reliably, accurately and easily locating intracranial targets and guiding surgical instruments to intracranial targets in human patients. An adjustment apparatus and a portable guider 70 constitute main components of the invention. The method and instrument make use of images of natural reference points or fiducial markers found in CT / MRI images of patient's head. In accordance with the present method, after the adjustment apparatus is mechanically adjusted based on the information gathered from CT / MRI images, the adjustments are transferred by a special procedure to the portable guider. The portable guider is the only component employed during surgery, resulting in minimal general intrusion with the work of the surgeon.
Owner:OMURTAG AHMET +1
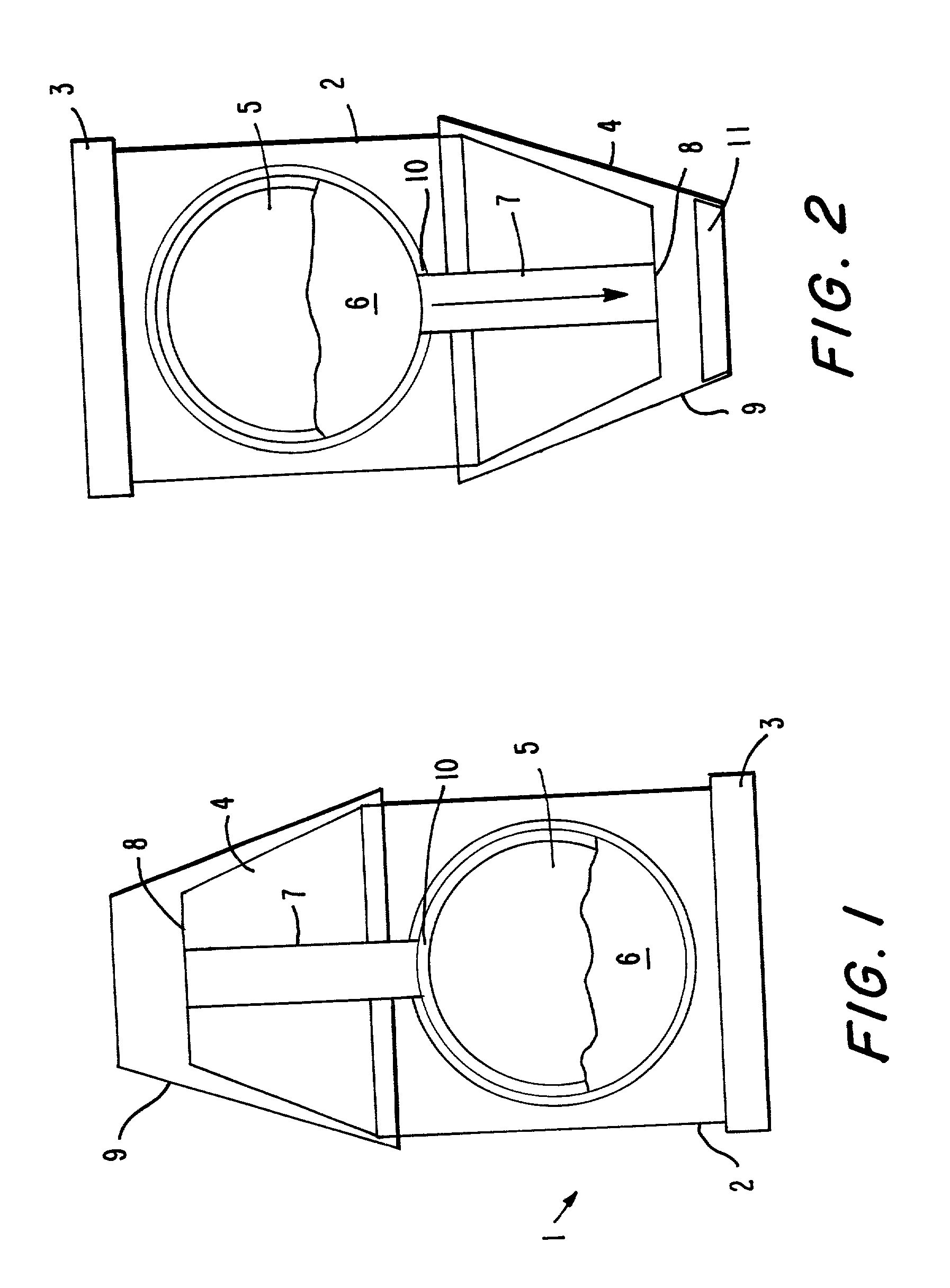
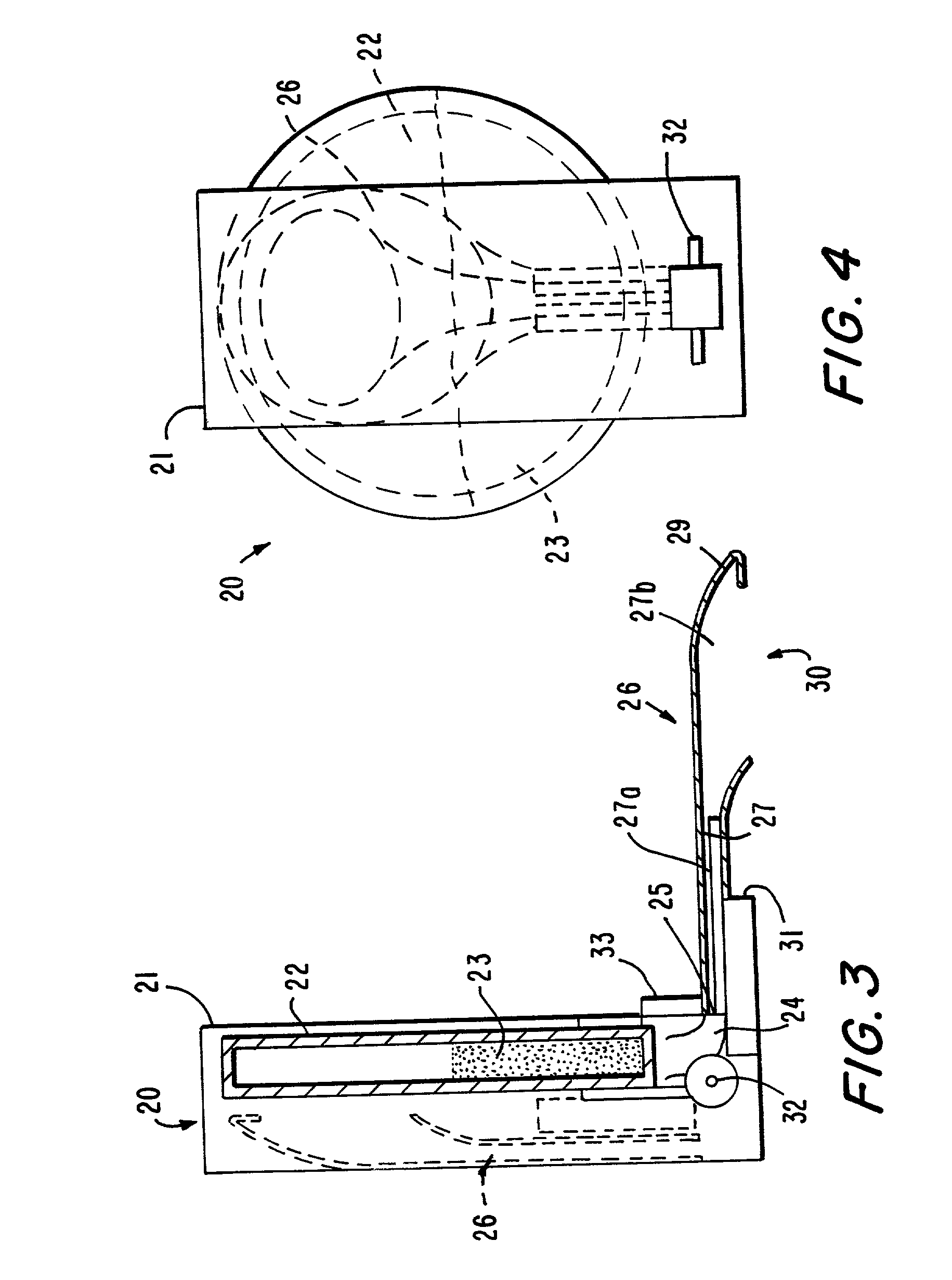
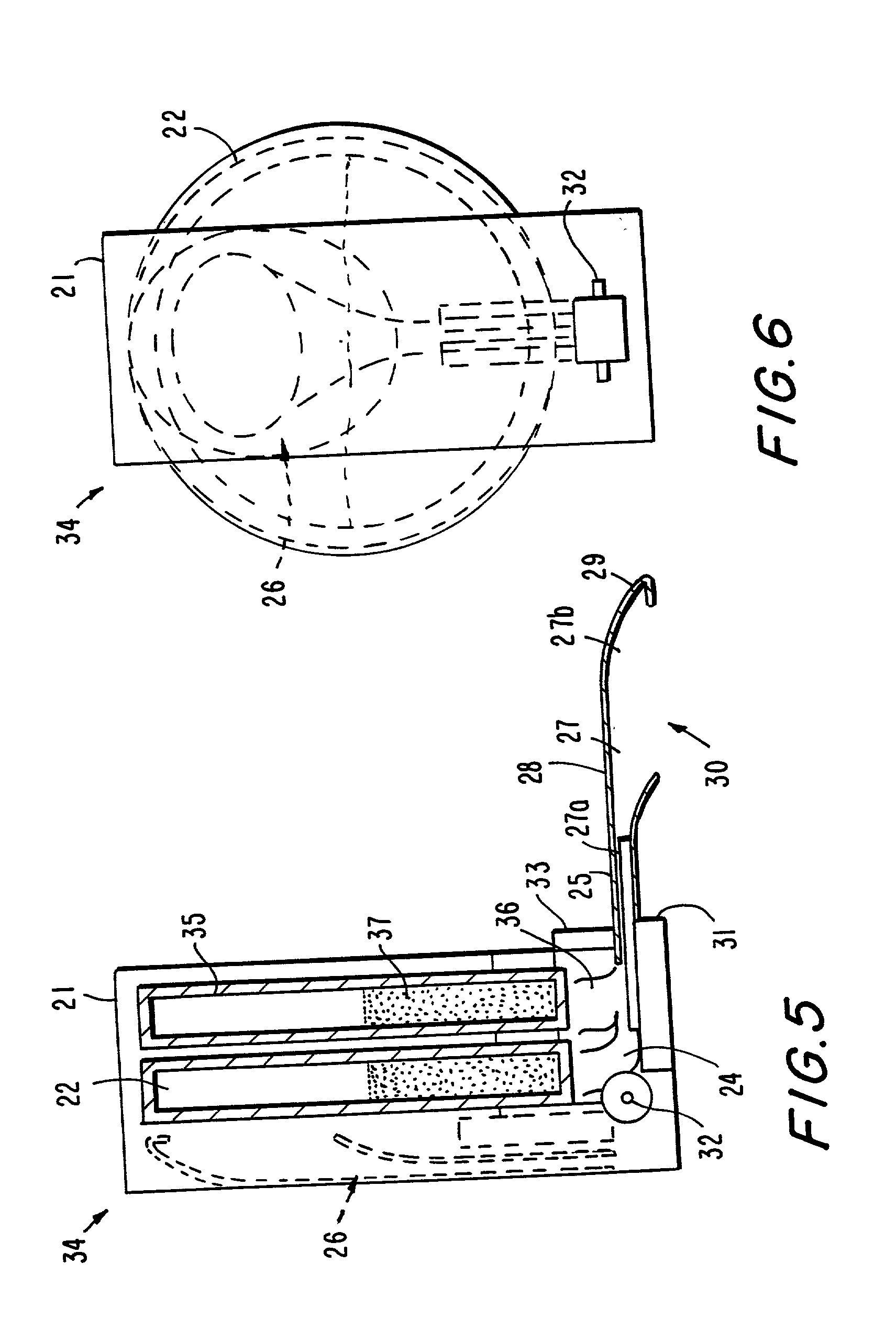
Delivery of oral drugs
InactiveUS20010020147A1Comfortable and convenient motionComfortable and convenient feelPowder deliveryLiquid surface applicatorsMean diameterHuman patient
Disclosed is a system for delivery of a drug comprising a multiple unit dosing device comprising a housing and an actuator, said device containing multiple doses of multiparticulates comprising drug particles, said device upon actuation delivering a unit dose of said multiparticulates, said drug particles having a mean diameter of greater than 10 mum to about 1 mm such that an effective dose of said drug cannot be delivered into the lower lung of a human patient. Also disclosed are novel methods, devices and dosage forms for delivering a drug.
Owner:PHARMAKODEX LTD
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS6716449B2Good curative effectPatient compliance is goodBiocideNervous disorderOpioid antagonistOpioid Agonist
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of the opioid antagonist effective to attenuate a side effect of the opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
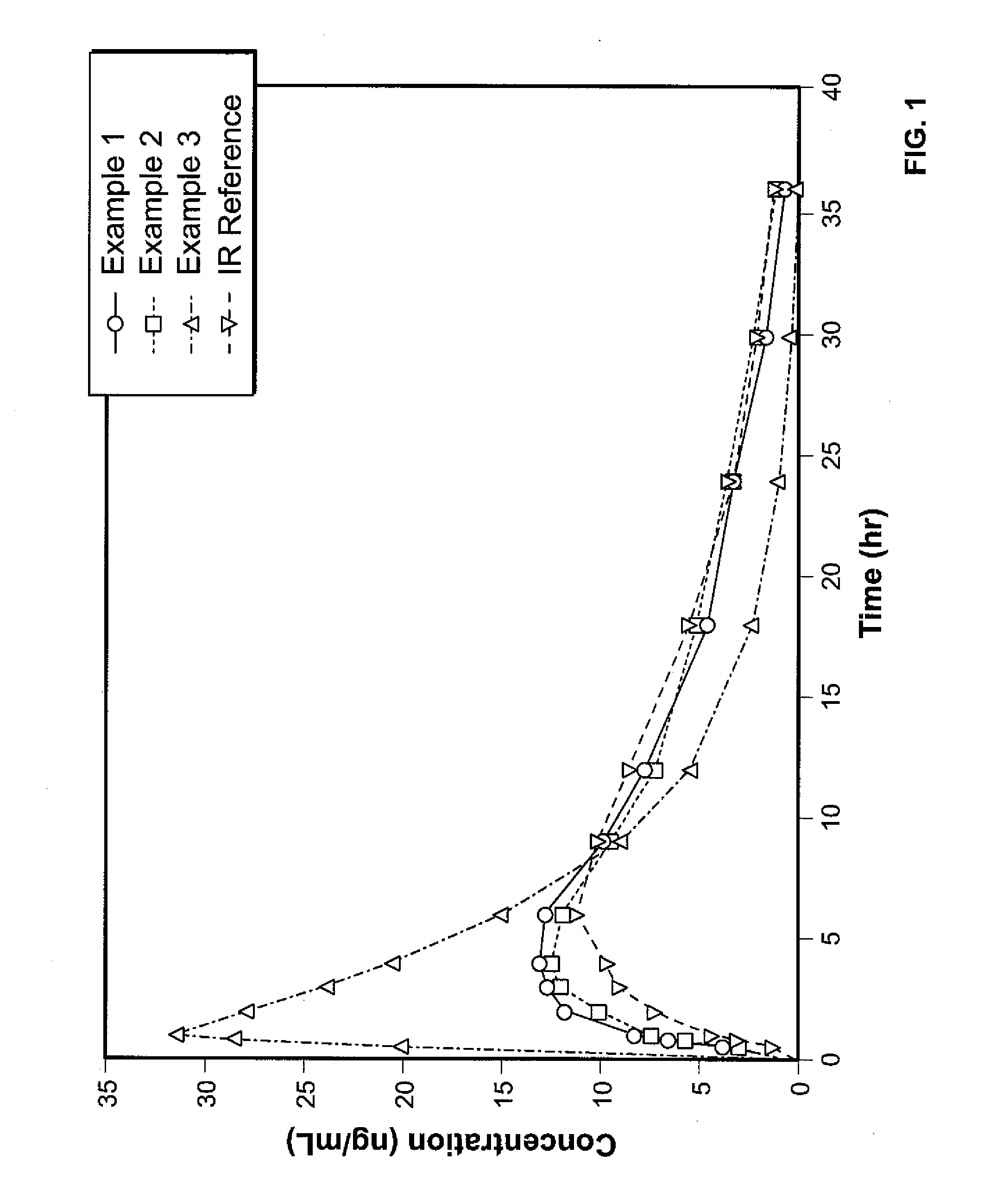
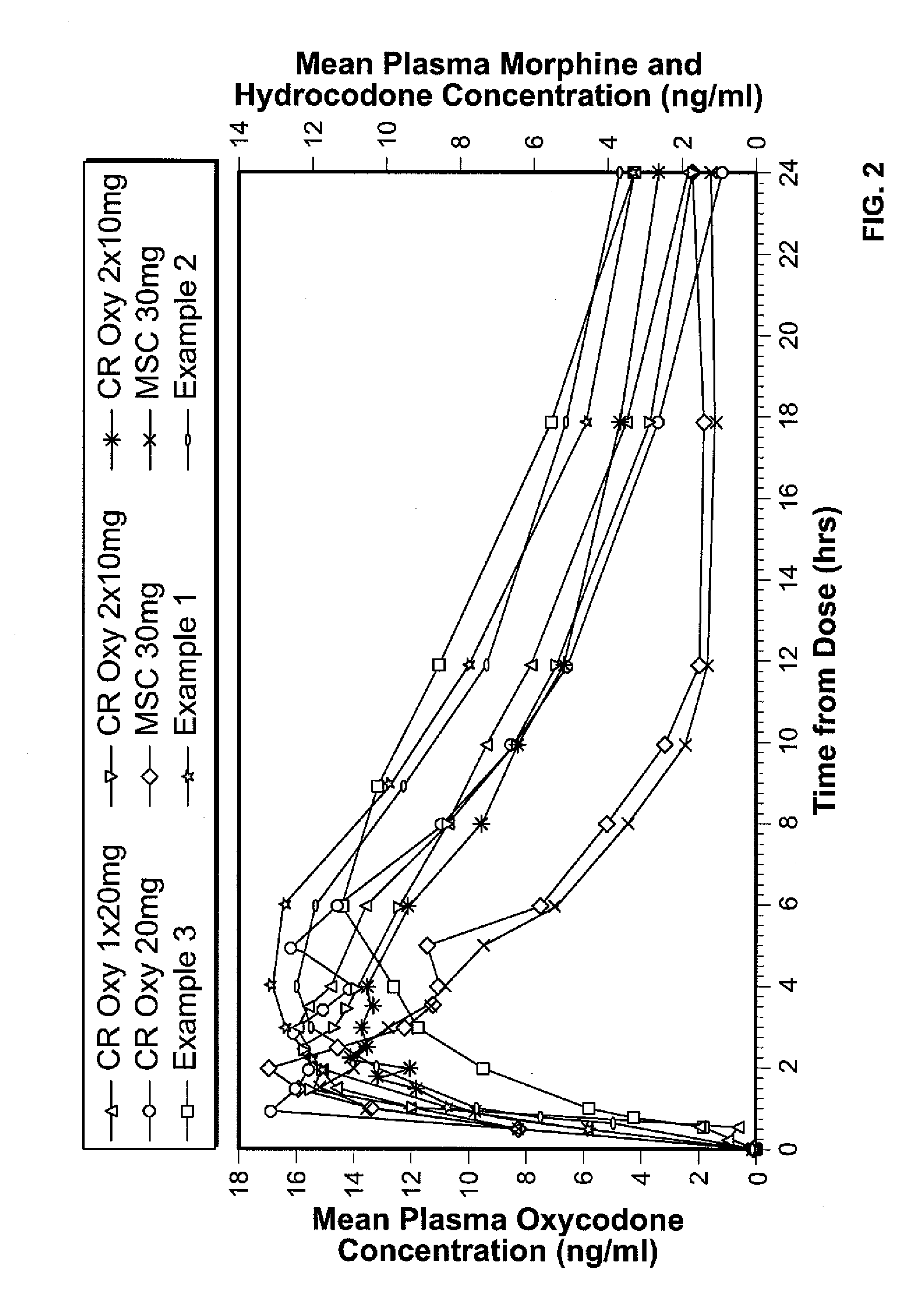
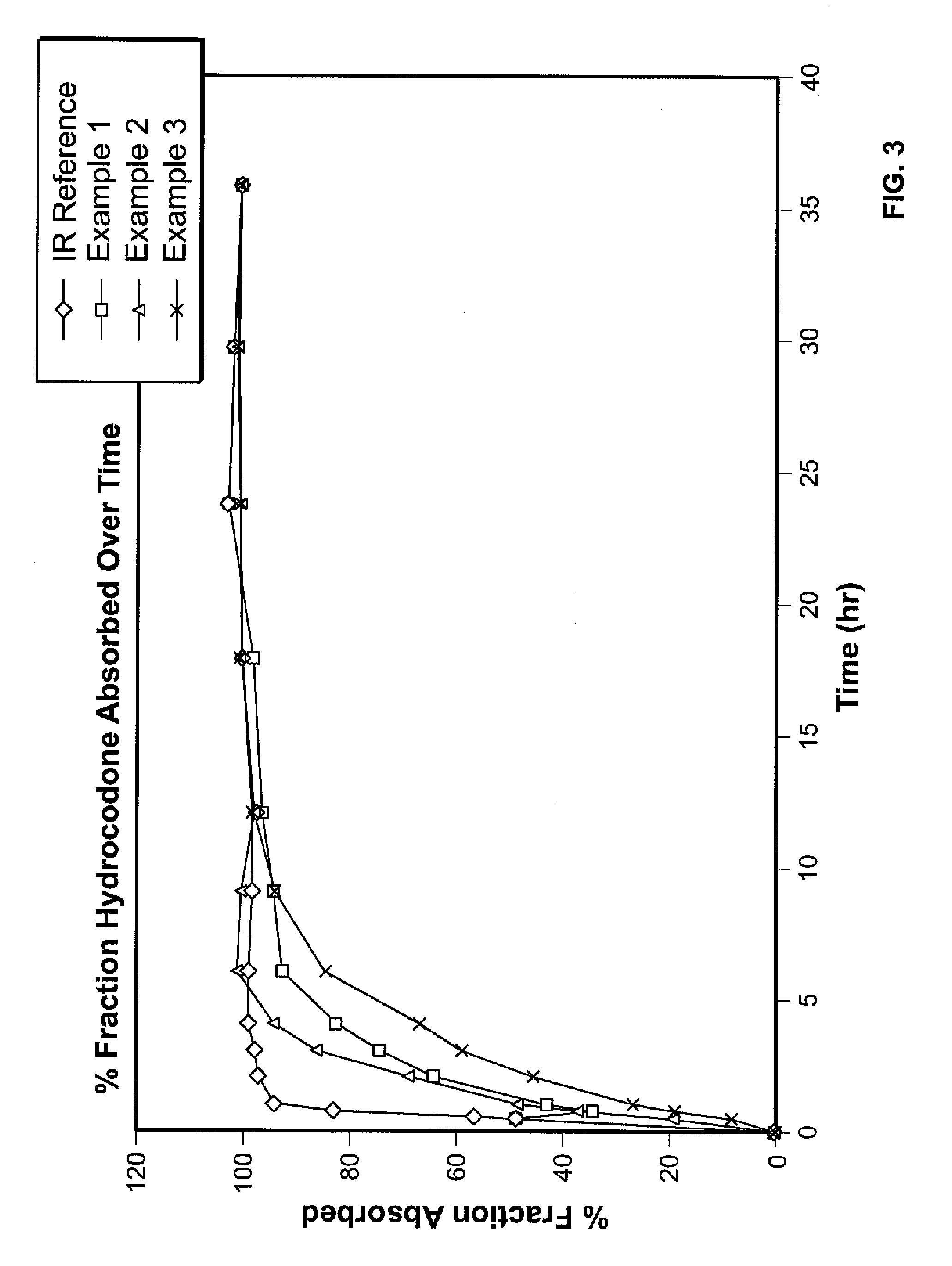
Controlled release formulations of opioid and nonopioid analgesics
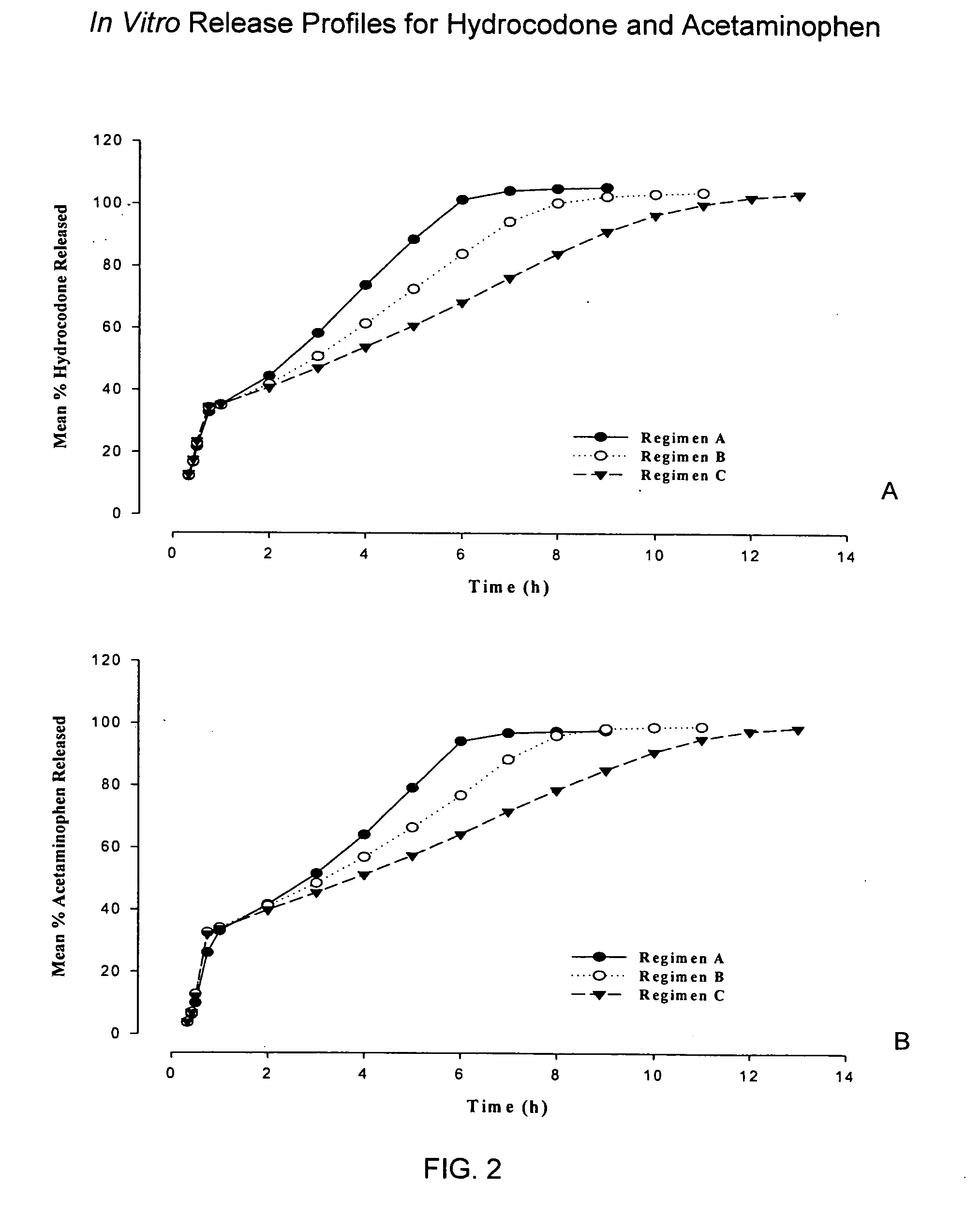
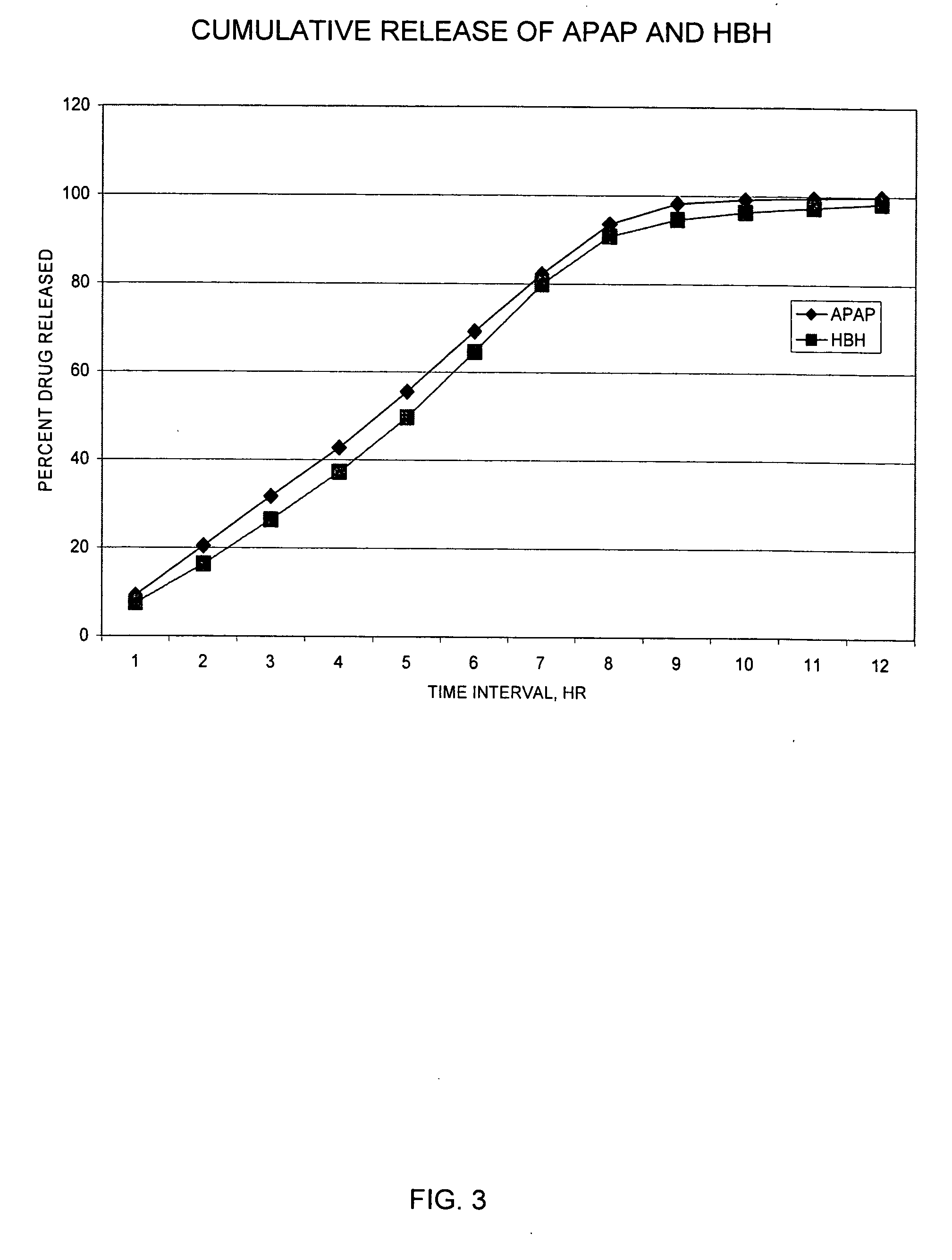
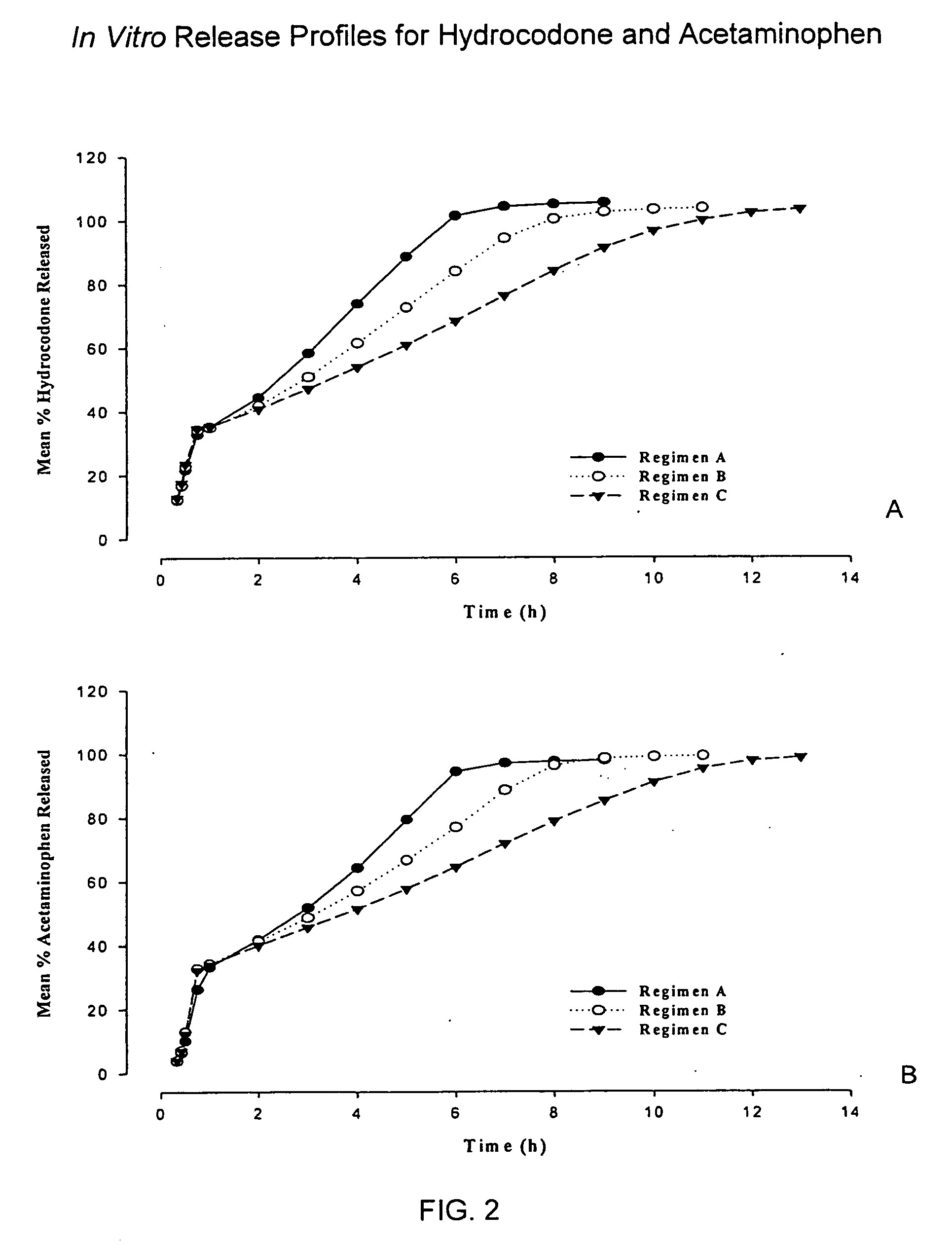
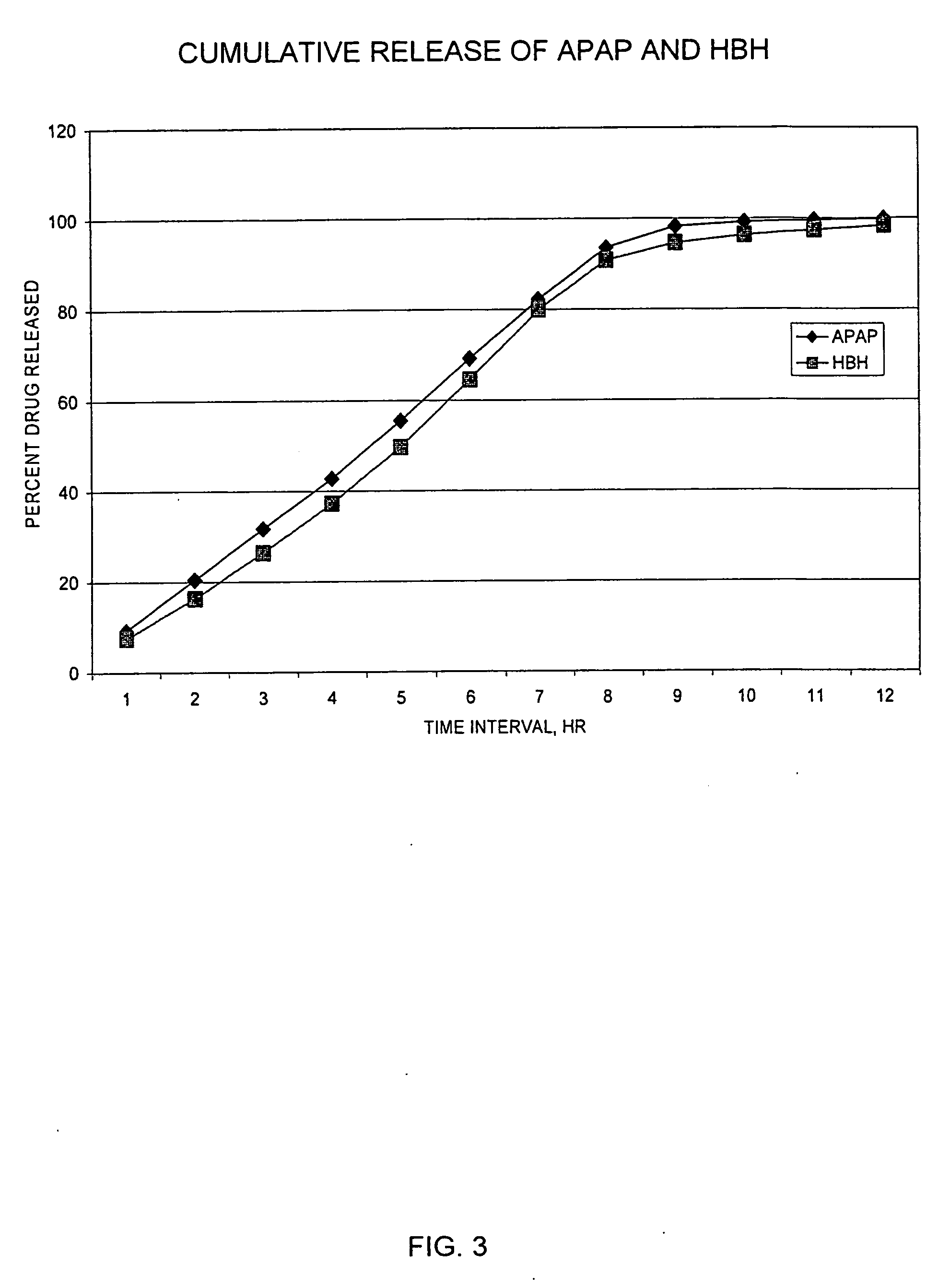
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
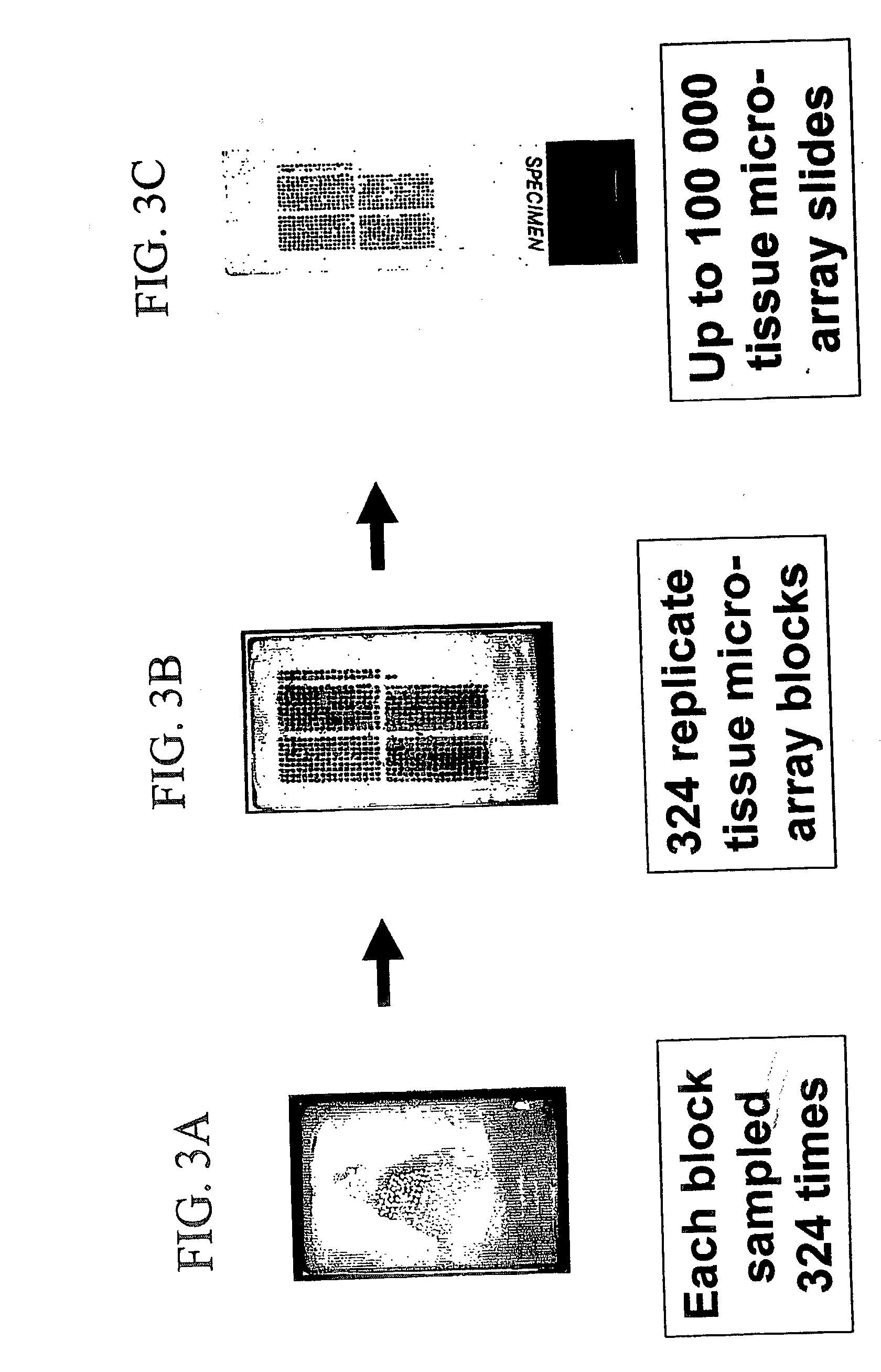
High-throughput tissue microarray technology and applications
InactiveUS20030215936A1Easy to analyzeGood for comparisonBioreactor/fermenter combinationsBiological substance pretreatmentsClinical informationTissue microarray
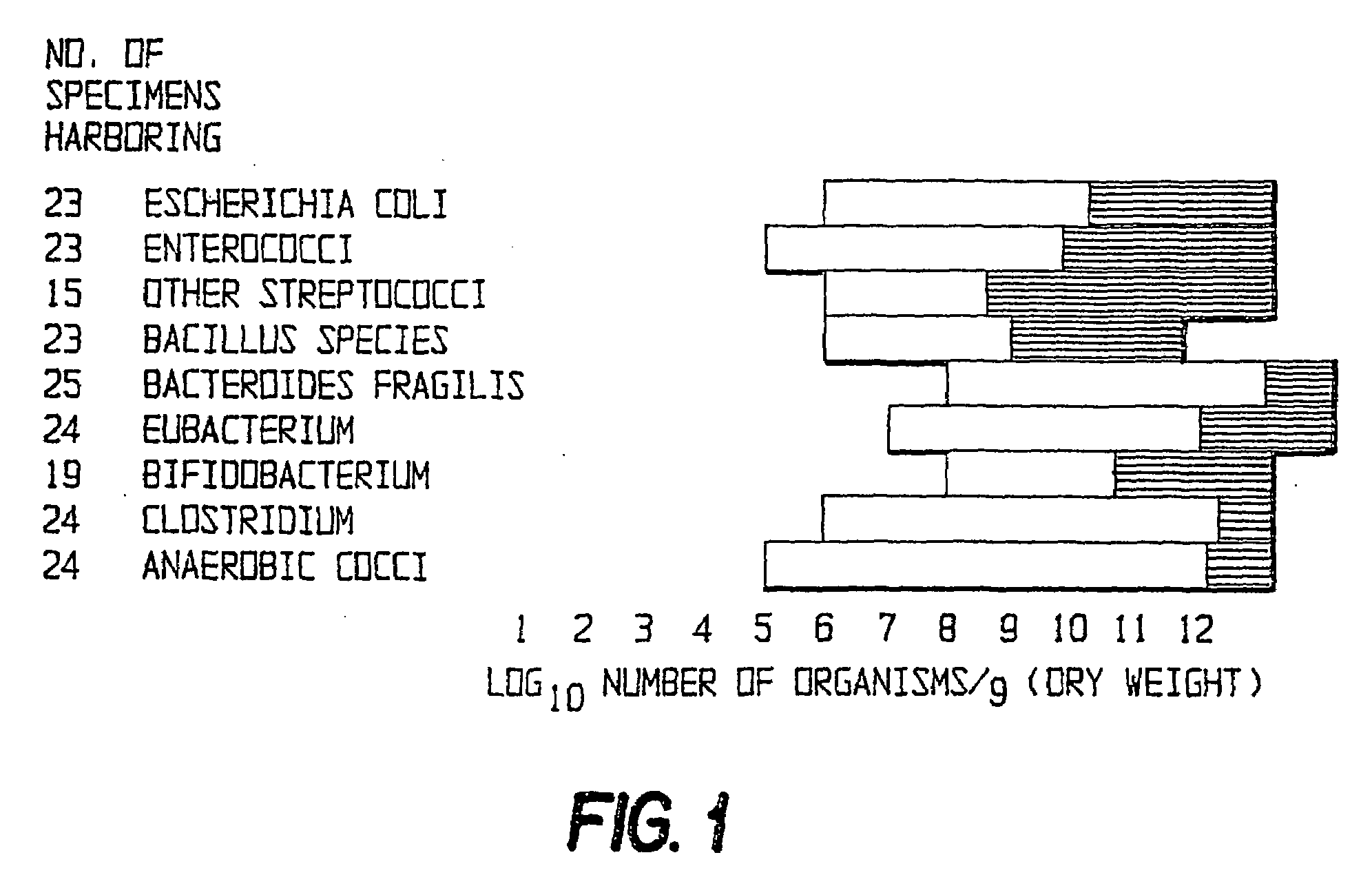
A method and apparatus are disclosed for a high-throughput, large-scale molecular profiling of tissue specimens by retrieving a donor tissue specimen from an array of donor specimens, placing a sample of the donor specimen in an assigned location in a recipient array, providing substantial copies of the array, performing a different biological analysis of each copy, and storing the results of the analysis. The results may be compared to determine if there are correlations or discrepancies between the results of different biological analyses at each assigned location, and also compared to clinical information about the human patient from which the tissue was obtained. The results of similar analyses on corresponding sections of the array can be used as quality control devices, for example by subjecting the arrays to a single simultaneous investigative procedure. Uniform interpretation of the arrays can be obtained, and compared to interpretations of different observers.
Owner:UNITED STATES OF AMERICA
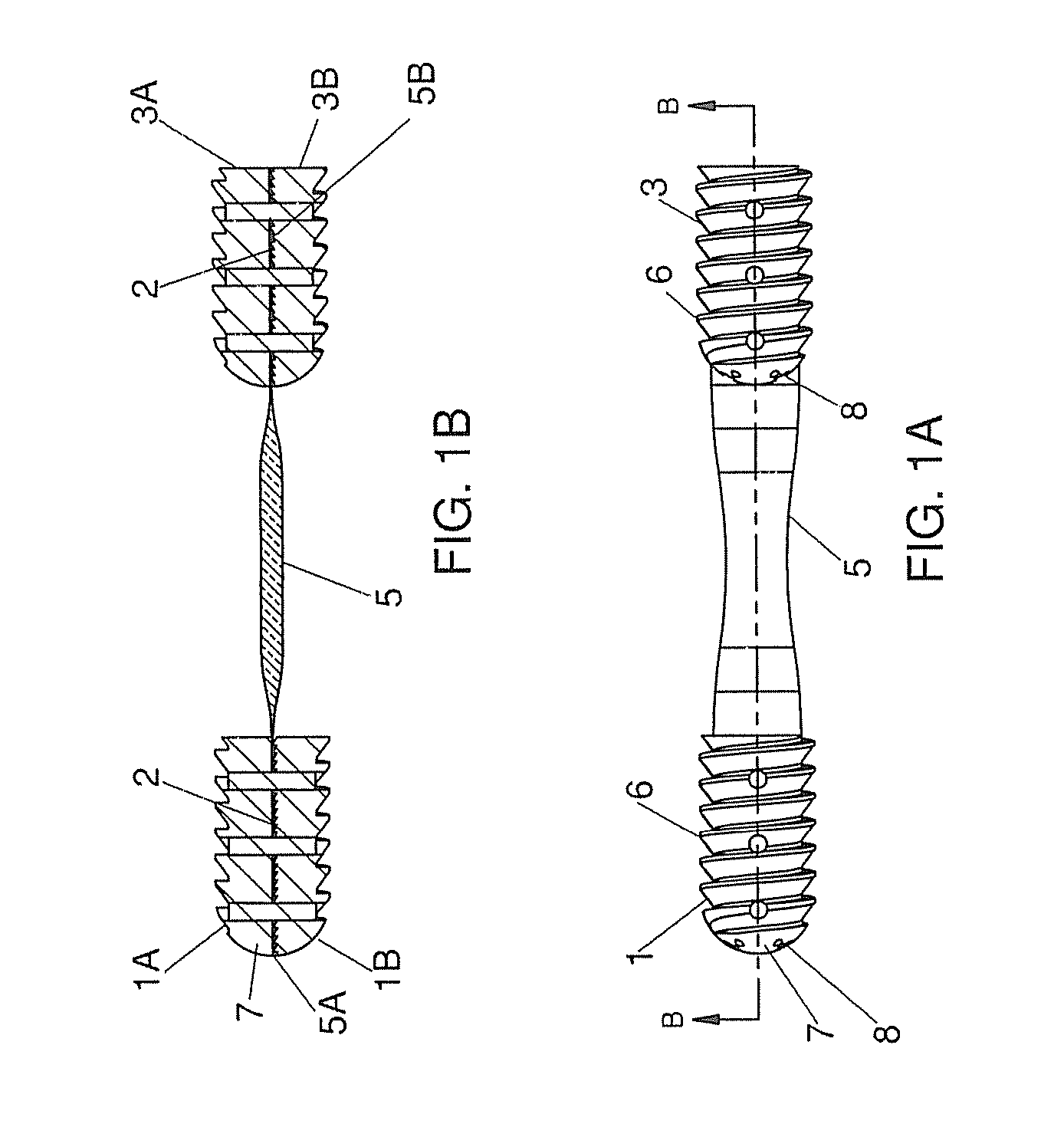
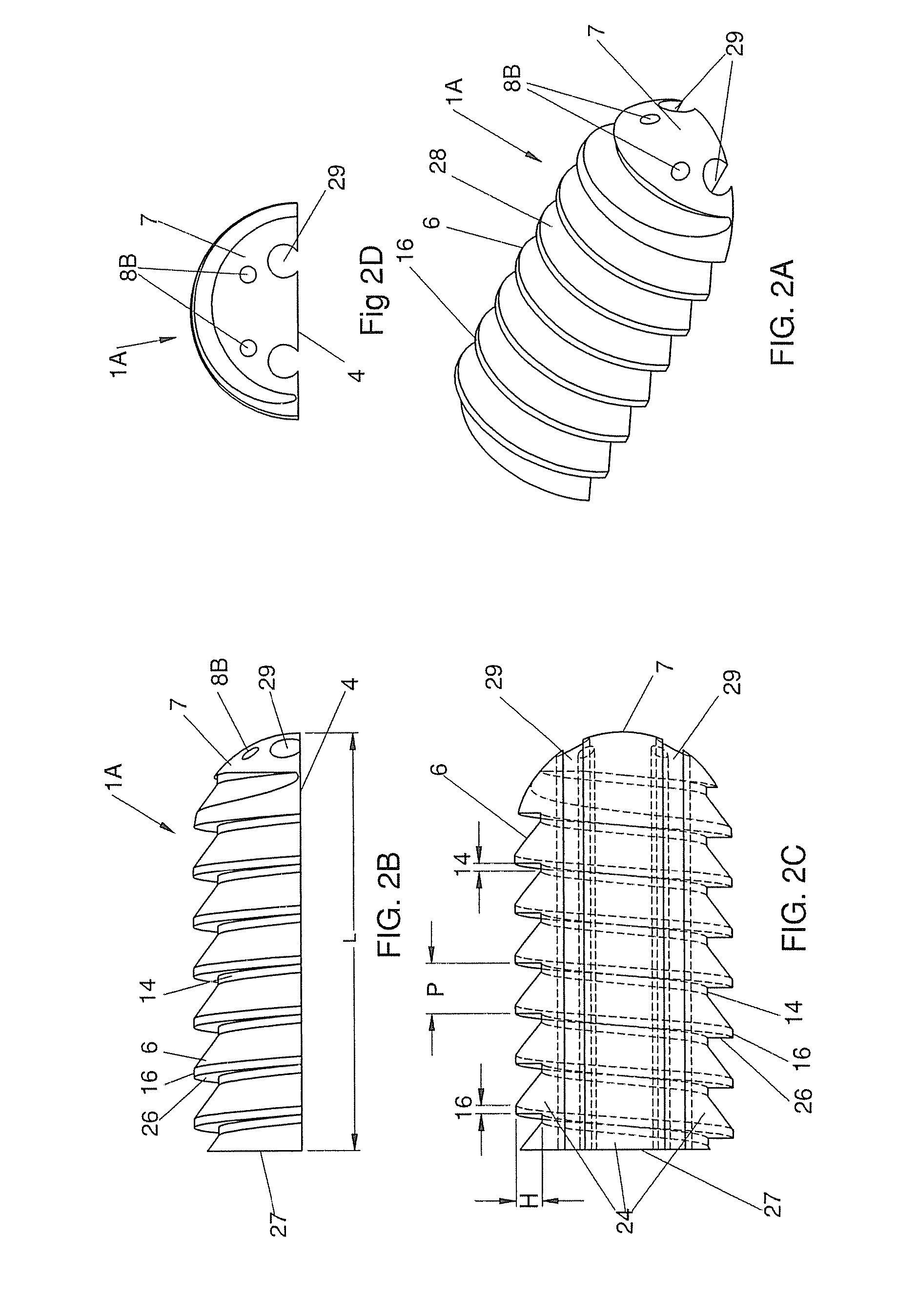
Self Fixing Assembled Bone-Tendon-Bone Graft
The present invention has multiple aspects relating to assembled self fixing bone-tendon-bone (BTB) grafts and BTB implants. A preferred application in which self fixing assembled bone-tendon-bone (BTB) grafts and implants of the present technology can be used is for ACL repairs in a human patient. In one embodiment, a self fixing BTB graft is characterized by the presence of threads along at least a portion of the exterior surface of one or both bone blocks. In another embodiment, a self fixing assembled bone-tendon-bone implant comprises a removable tendon tensioner which imparts a predetermined tension on the tendon of the BTB graft. In this embodiment, the tensioner can be narrower than the diameter of the bone blocks or can be threaded such that the threads are continuous with the threads of at least one of the bone blocks. The threads facilitate the simultaneous implantation of the leading and trailing bone blocks of the BTB graft in tapped (threaded) holes in the tibia and the femur of a recipient patient. The tensioner maintains the tension on the tendon and the spacing between the bone blocks until the leading bone block is fixed (threaded) in the tapped hole in the femur and the trailing bone block is fixed (threaded) into the tapped tibial bone tunnel. Once the bone blocks are substantially in their fixed positions, the tensioner is removed arthroscopically in the joint space between the tibia and the femur.
Owner:RTI BIOLOGICS INC
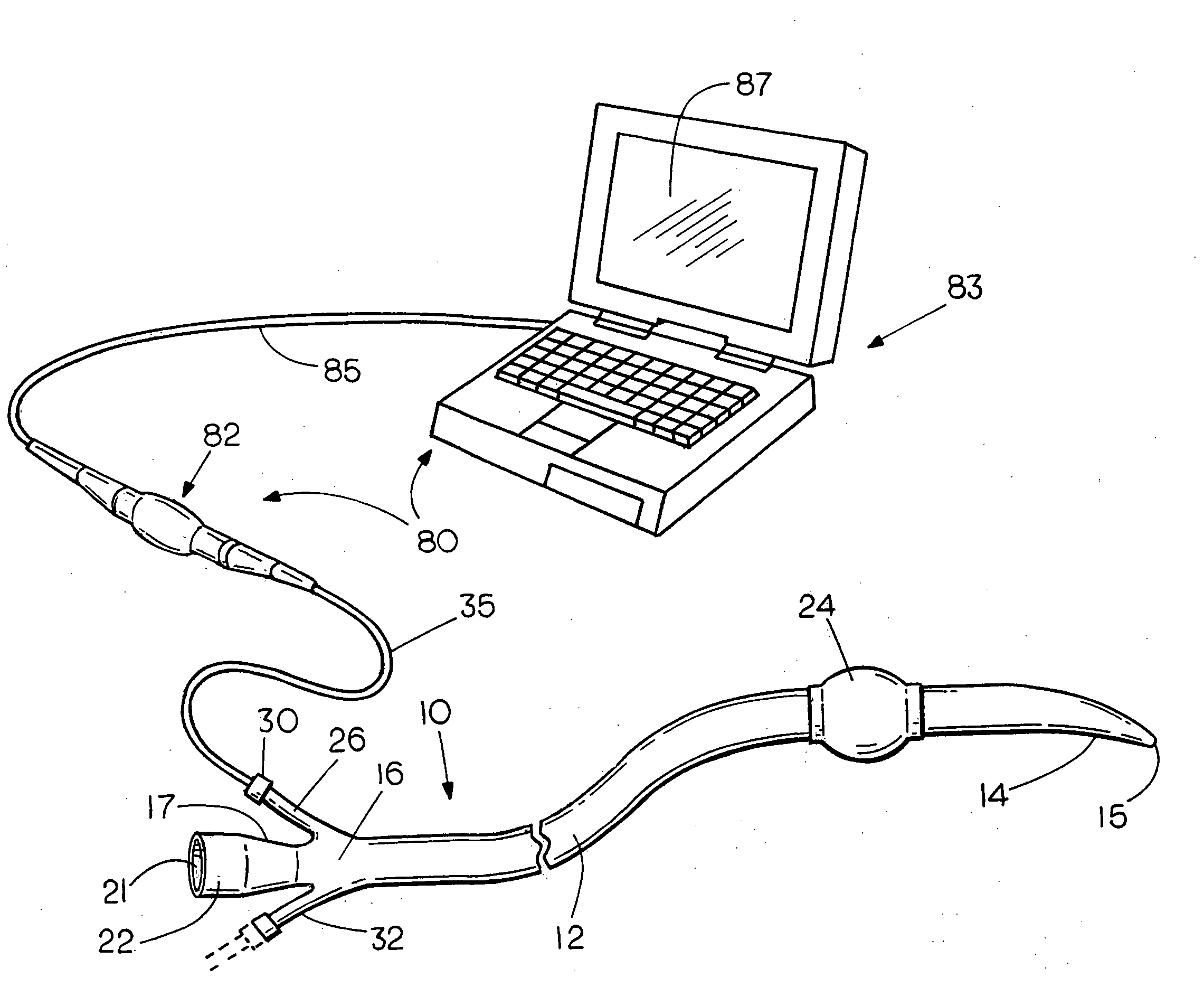
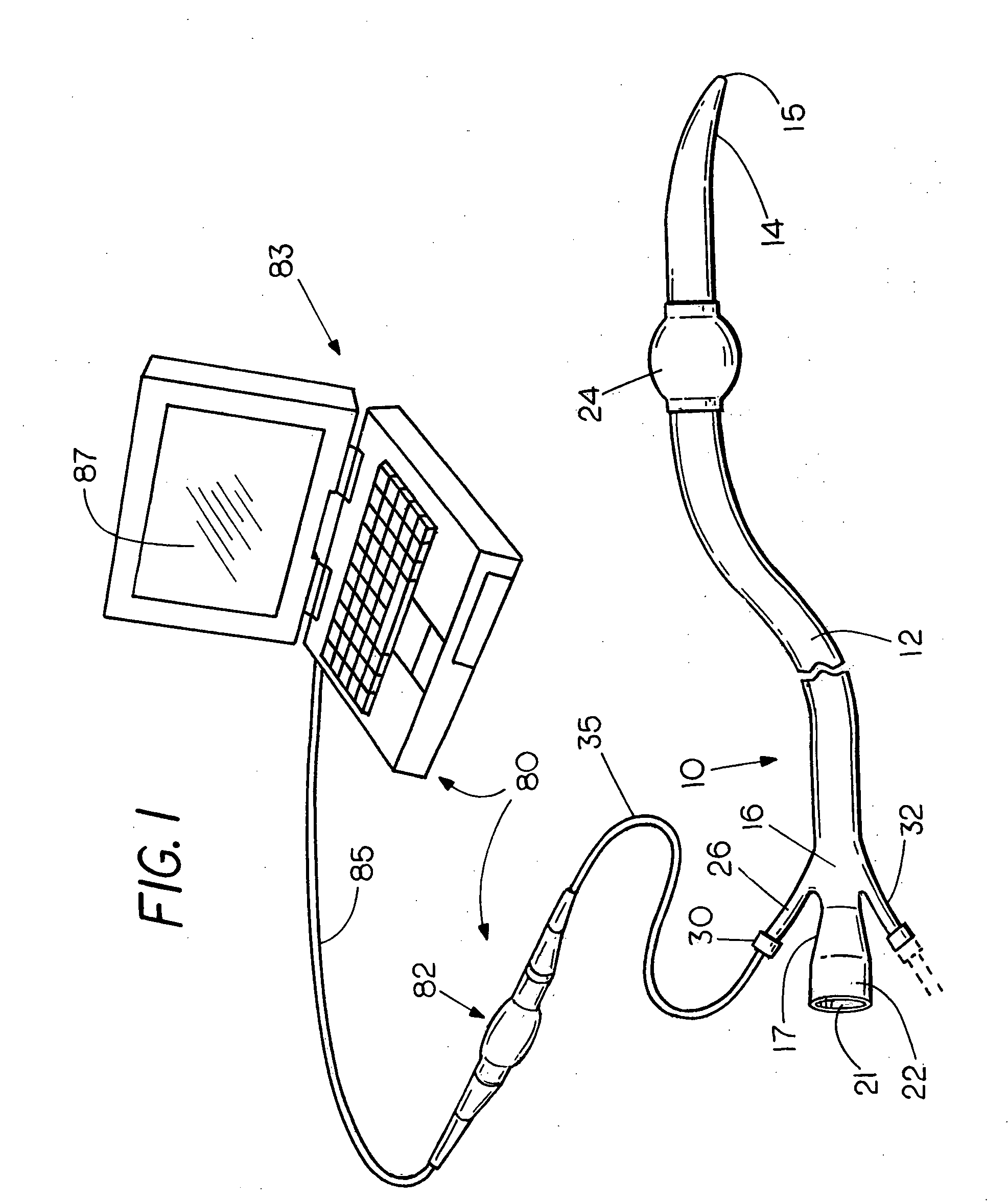
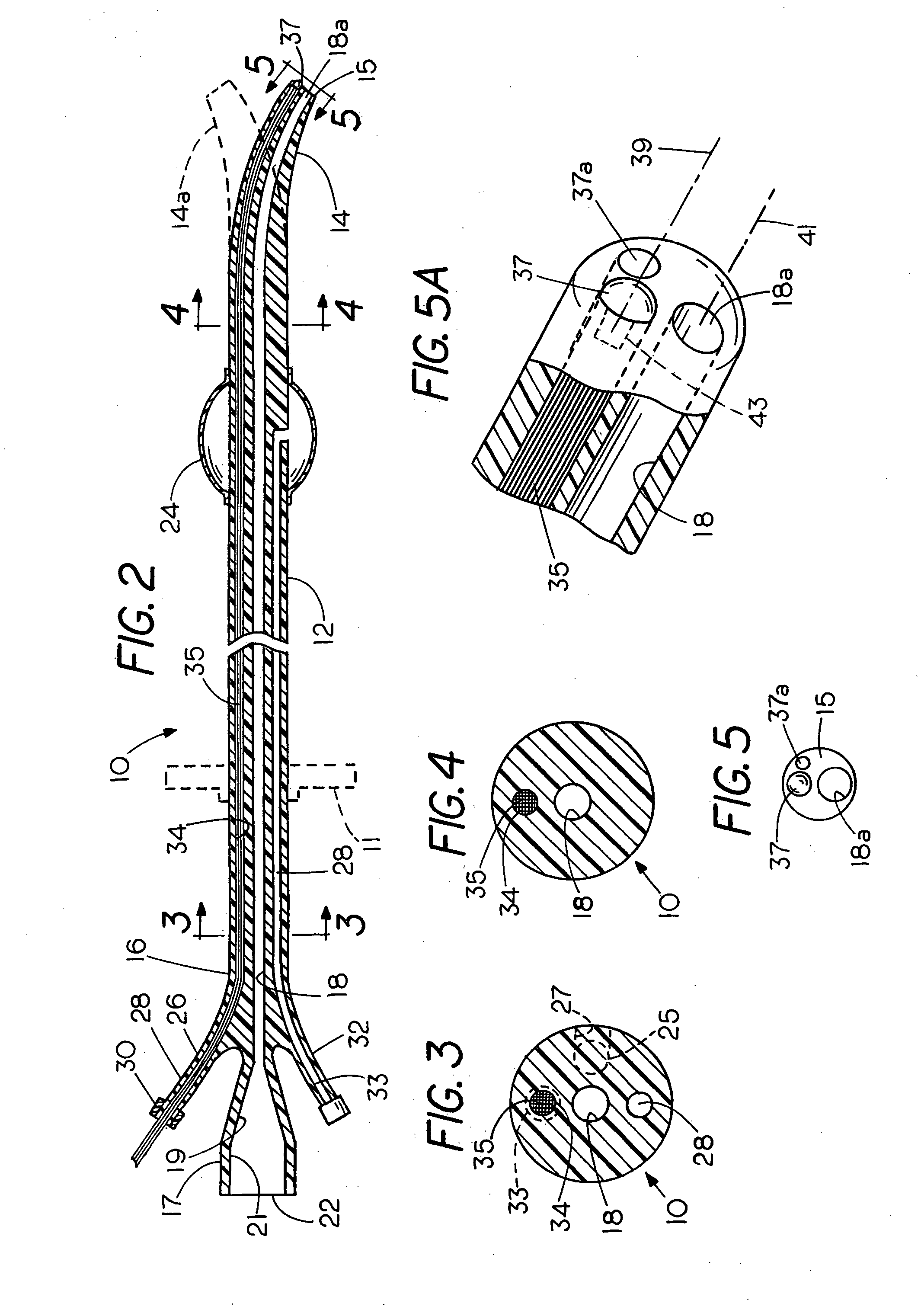
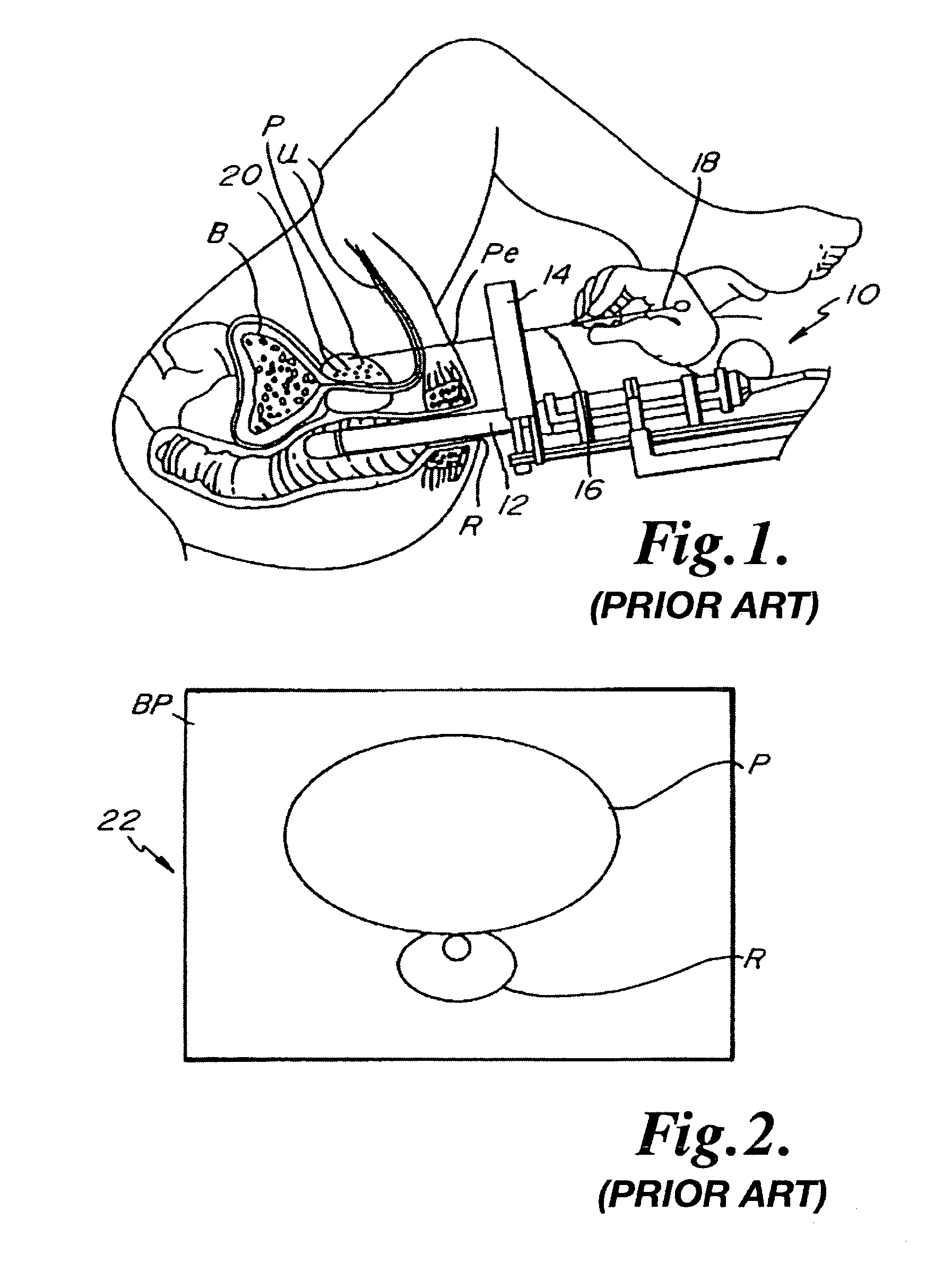
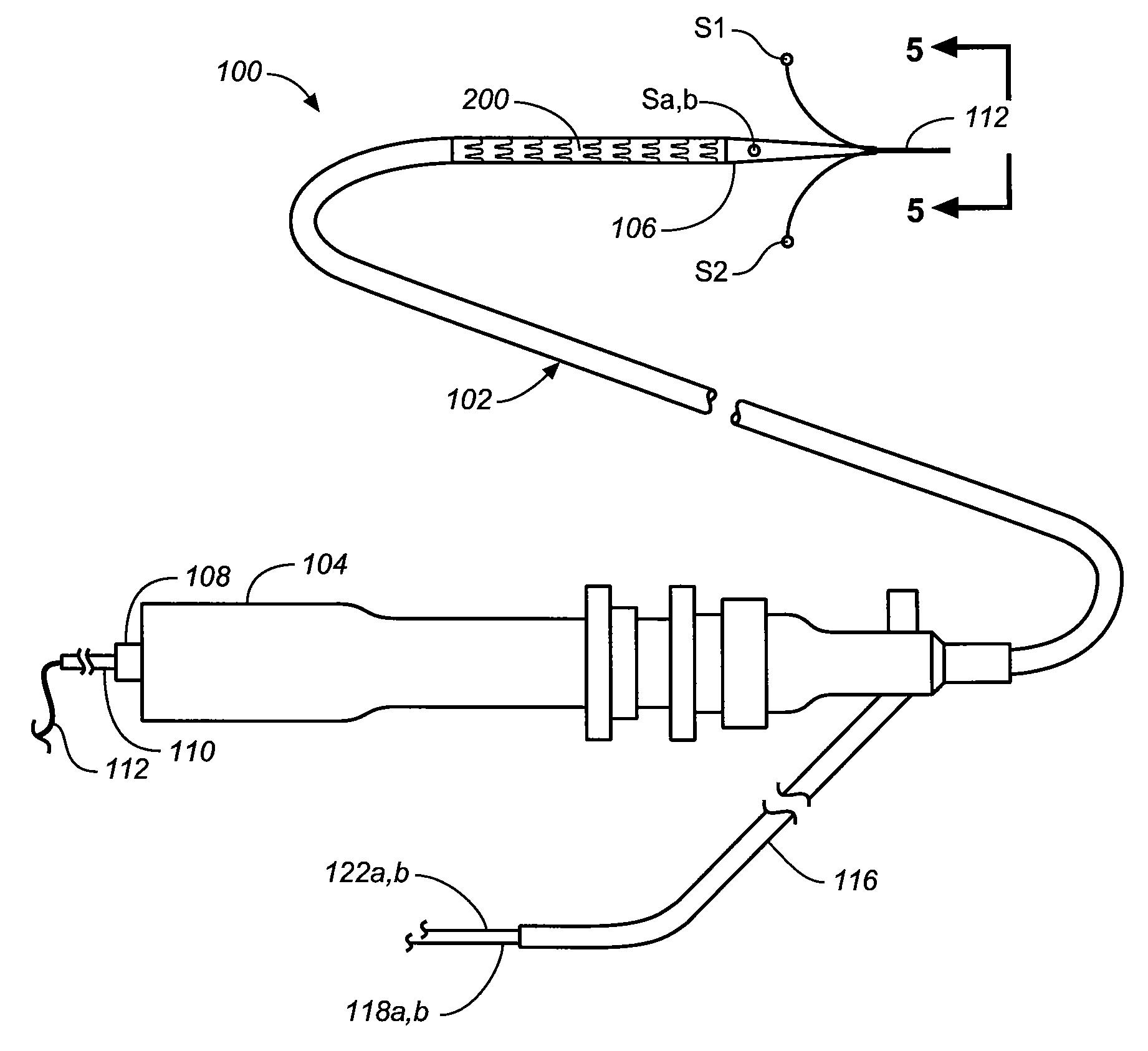
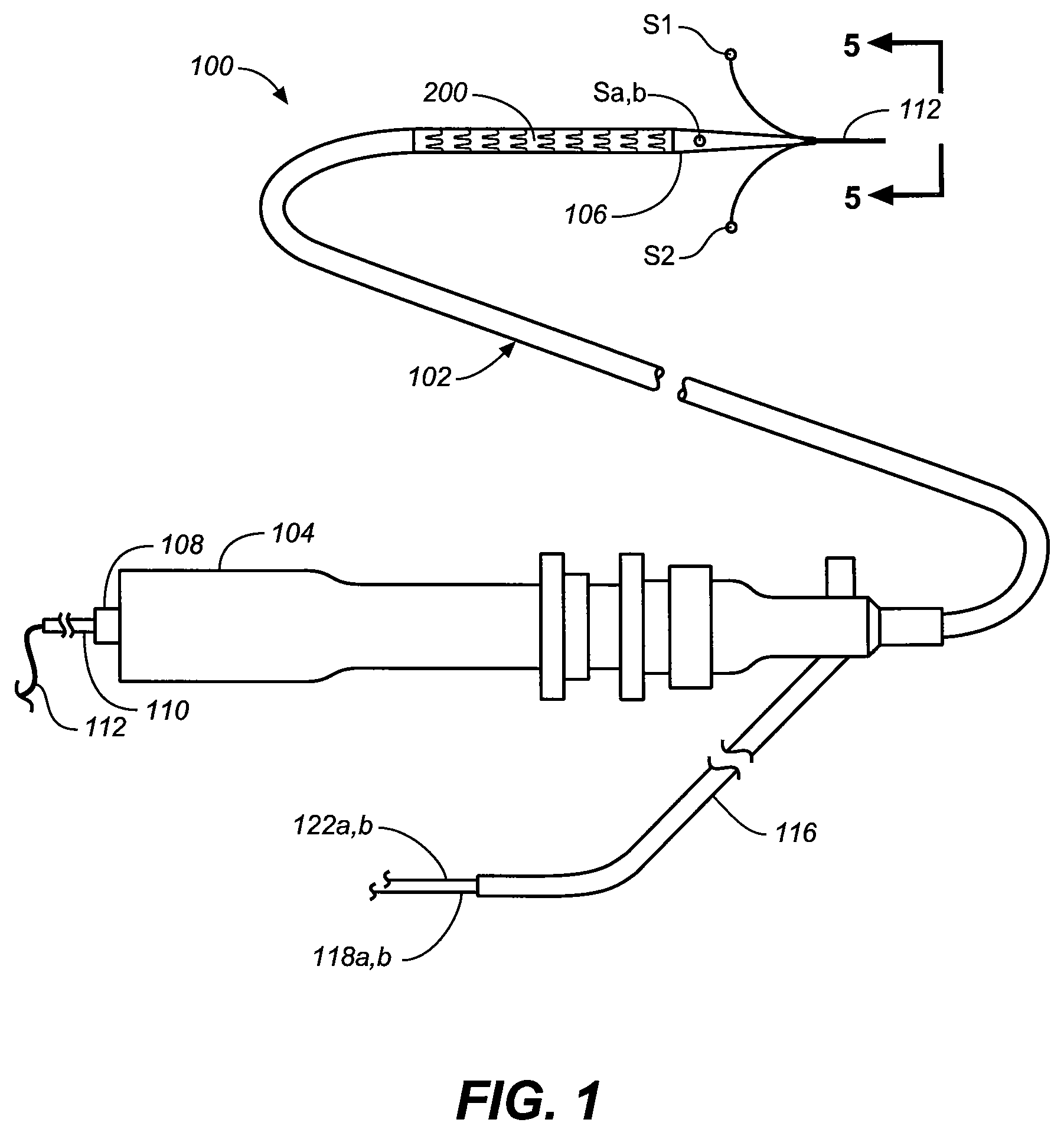
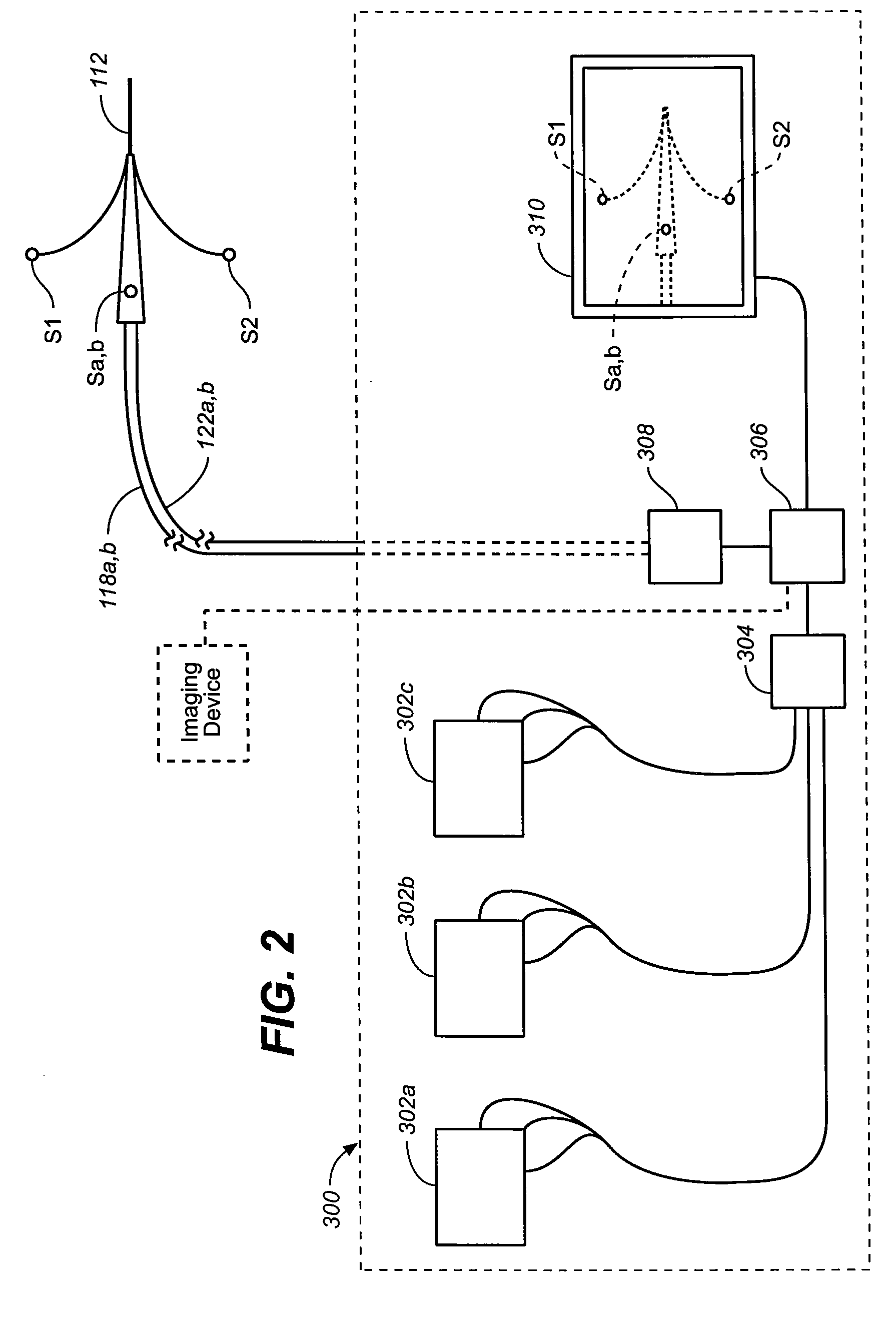
Flexible visually directed medical intubation instrument and method
Owner:PERCUVISION
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20060251721A1Improved ability to treat painLess attentionBiocideNervous disorderImmediate releasePharmaceutical medicine
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
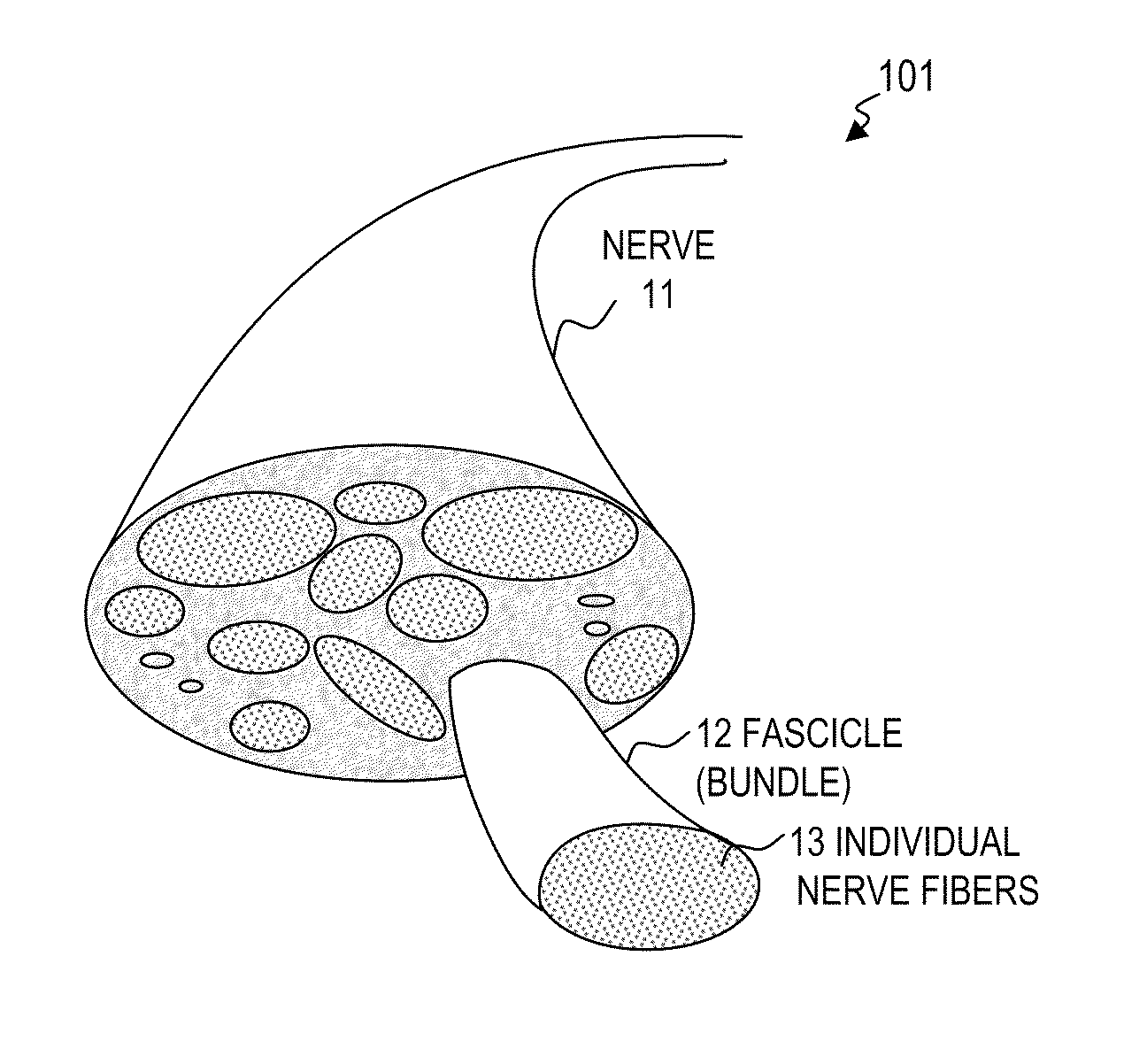
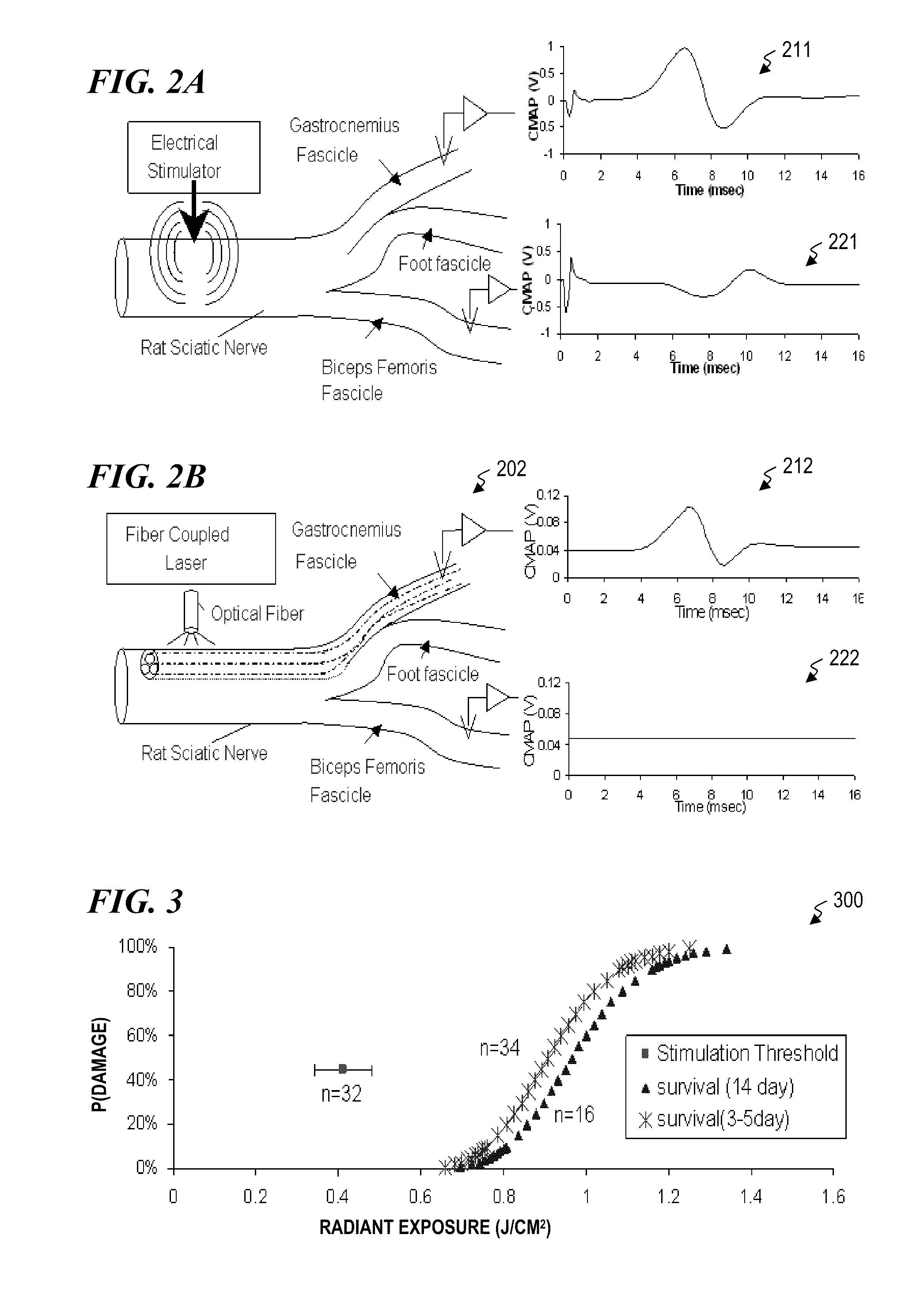
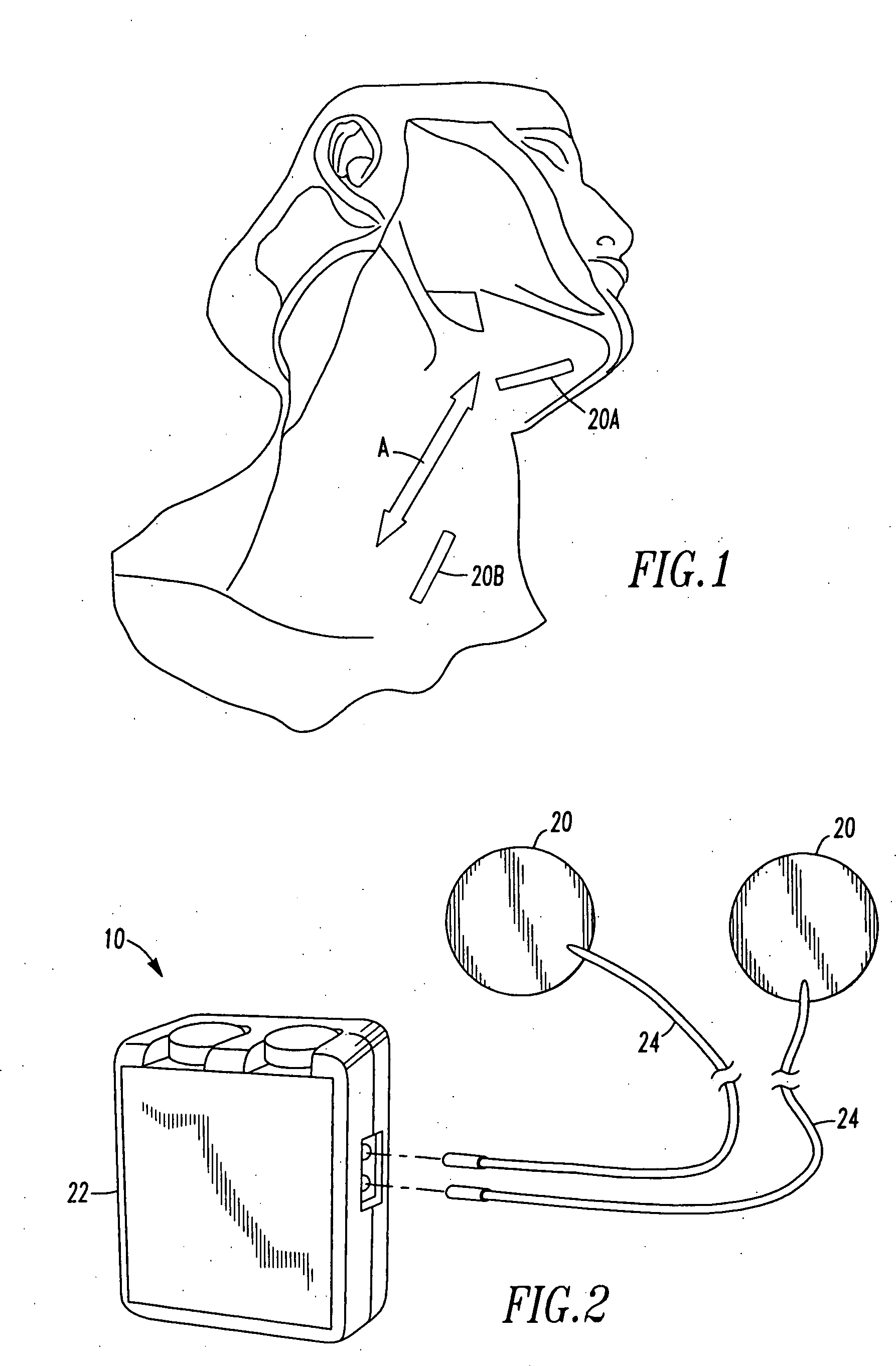
Nerve stimulator and method using simultaneous electrical and optical signals
ActiveUS20100114190A1Reduce amountLower Level RequirementsInternal electrodesImplantable neurostimulatorsEngineeringAction potential
An apparatus and method for stimulating animal tissue (for example to trigger a nerve action potential (NAP) signal in a human patient) by application of both electrical and optical signals for treatment and diagnosis purposes. The application of an electrical signal before or simultaneously to the application of a NAP-triggering optical signal allows the use of a lower amount of optical power or energy than would otherwise be needed if an optical signal alone was used for the same purpose and effectiveness. The application of the electrical signal may precondition the nerve tissue such that a lower-power optical signal can be used to trigger the desired NAP, which otherwise would take a higher-power optical signal were the electric signal not applied. Some embodiments include an implanted nerve interface having a plurality of closely spaced electrodes placed transversely and / or longitudinally to the nerve and a plurality of optical emitters.
Owner:NERVESENSE LTD
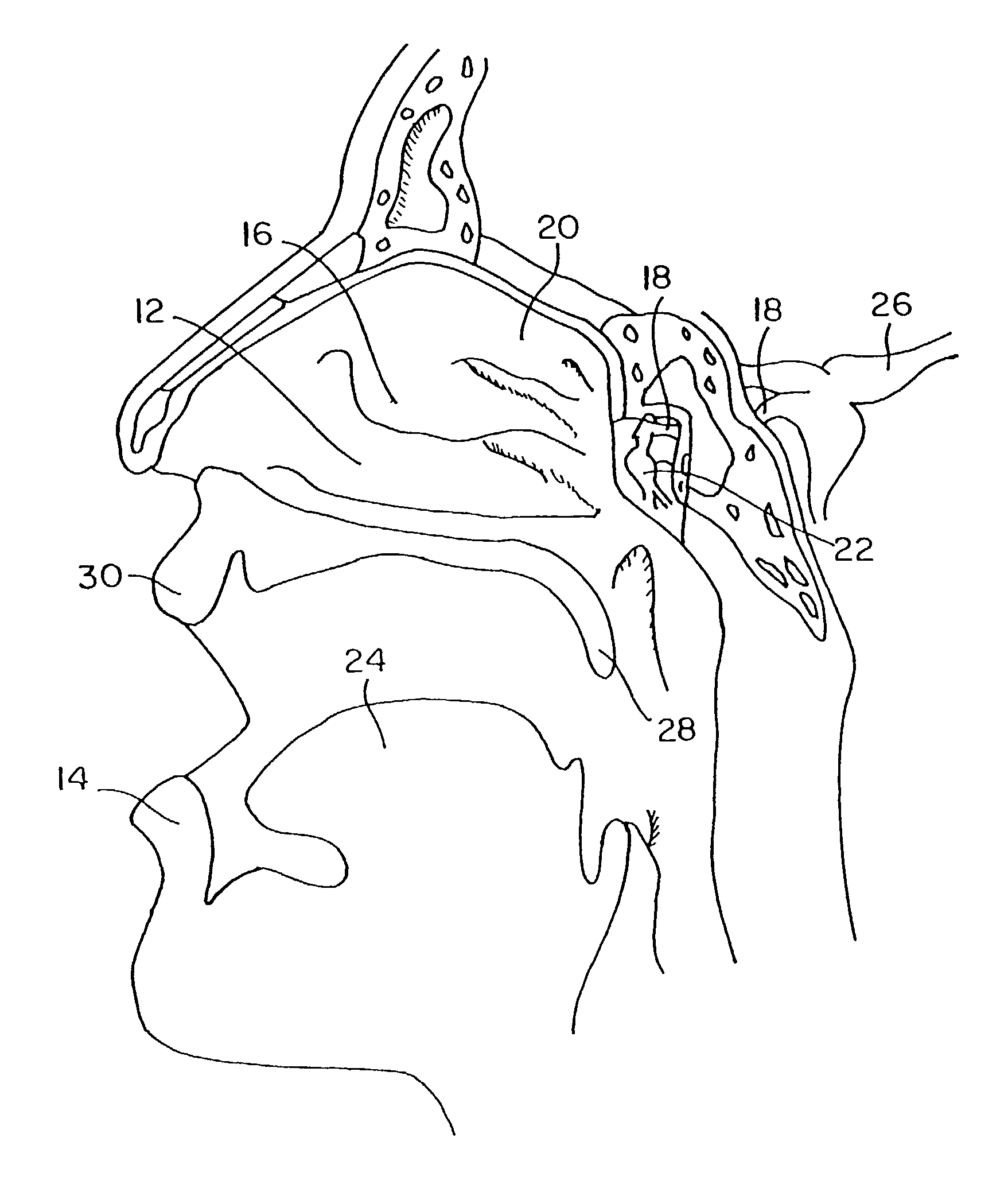
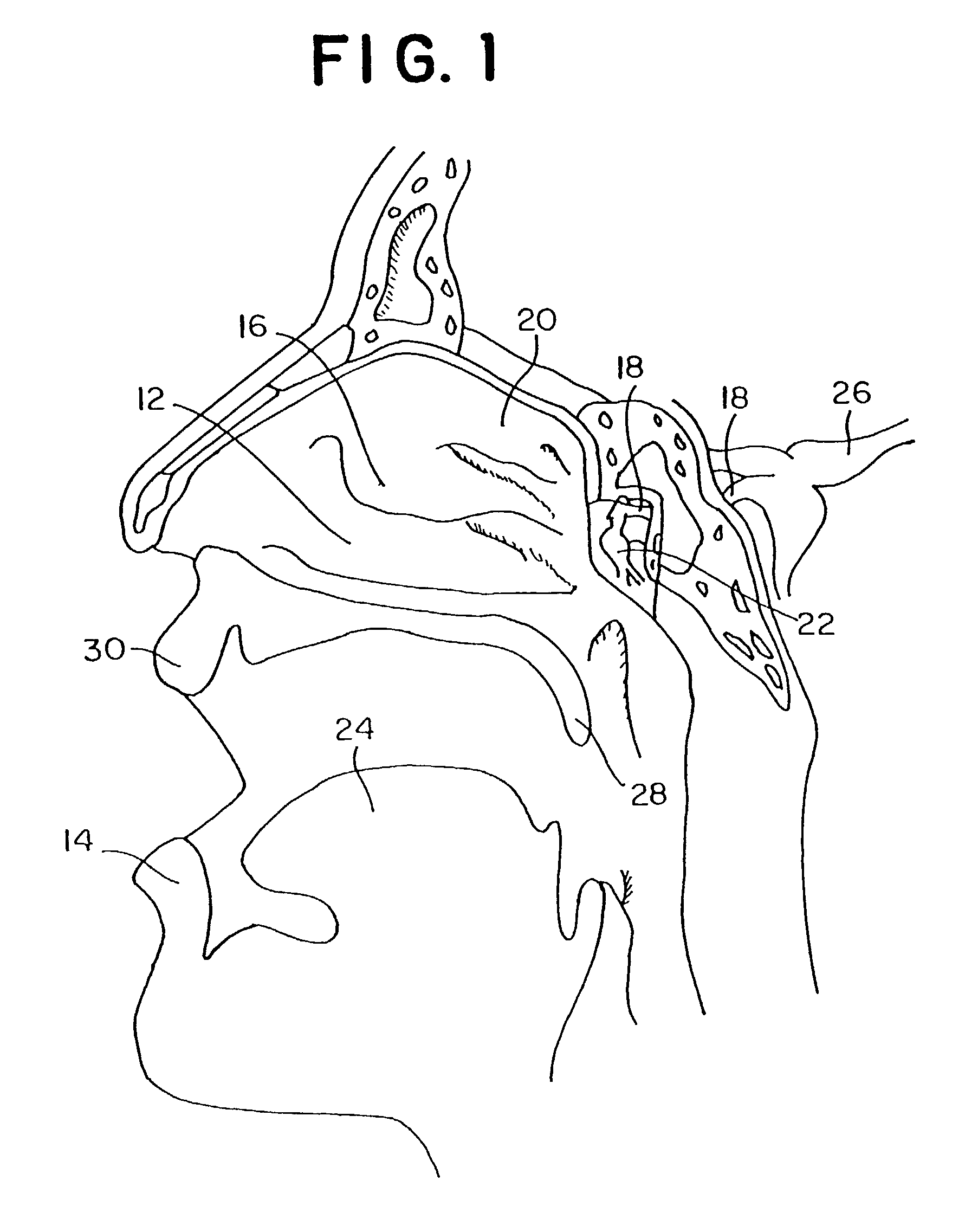
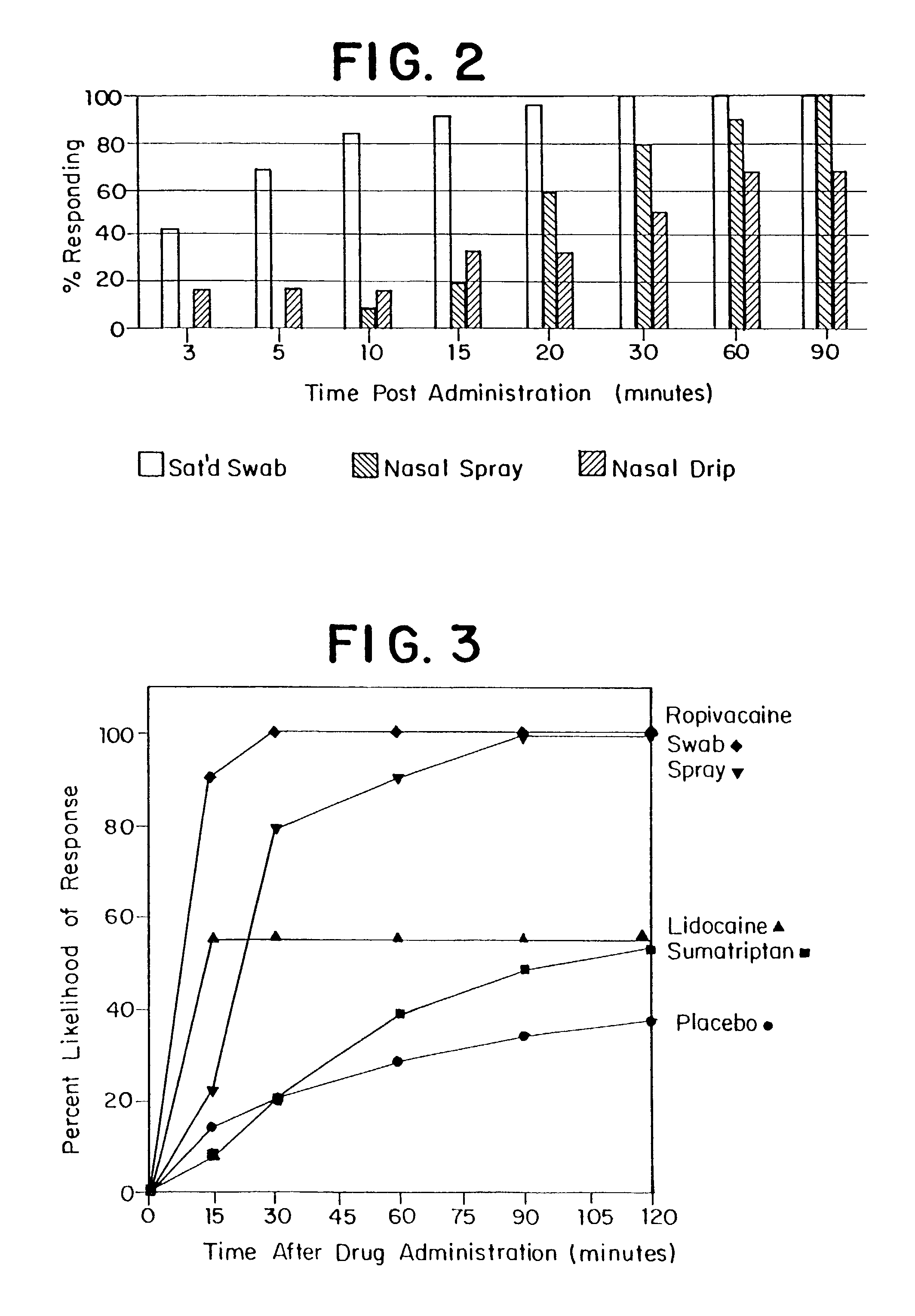
Method for directed intranasal administration of a composition
Methods, kits, apparatus, and compositions for inhibiting a cerebral neurovascular disorder, a muscular headache, or cerebral inflammation in a human patient are provided. The methods comprise intranasally administering to the patient a pharmaceutical composition comprising a local anesthetic, and preferably a long-acting local anesthetic ingredient. A composition useful for practicing the methods of the invention is described which comprises at least one local anesthetic in a pharmaceutically acceptable carrier, wherein the composition is formulated for intranasal delivery. Cerebral neurovascular disorders include migraine and cluster headache. Muscular headaches include tension headaches and muscle contraction headaches. A kit comprising the composition and an intranasal applicator and a method of systemically delivering a pharmaceutically active agent to an animal are also included in the invention. Apparatus for directed intranasal administration of the compositions of the invention and for performing the methods of the invention are also described.
Owner:BHL PATENT HLDG
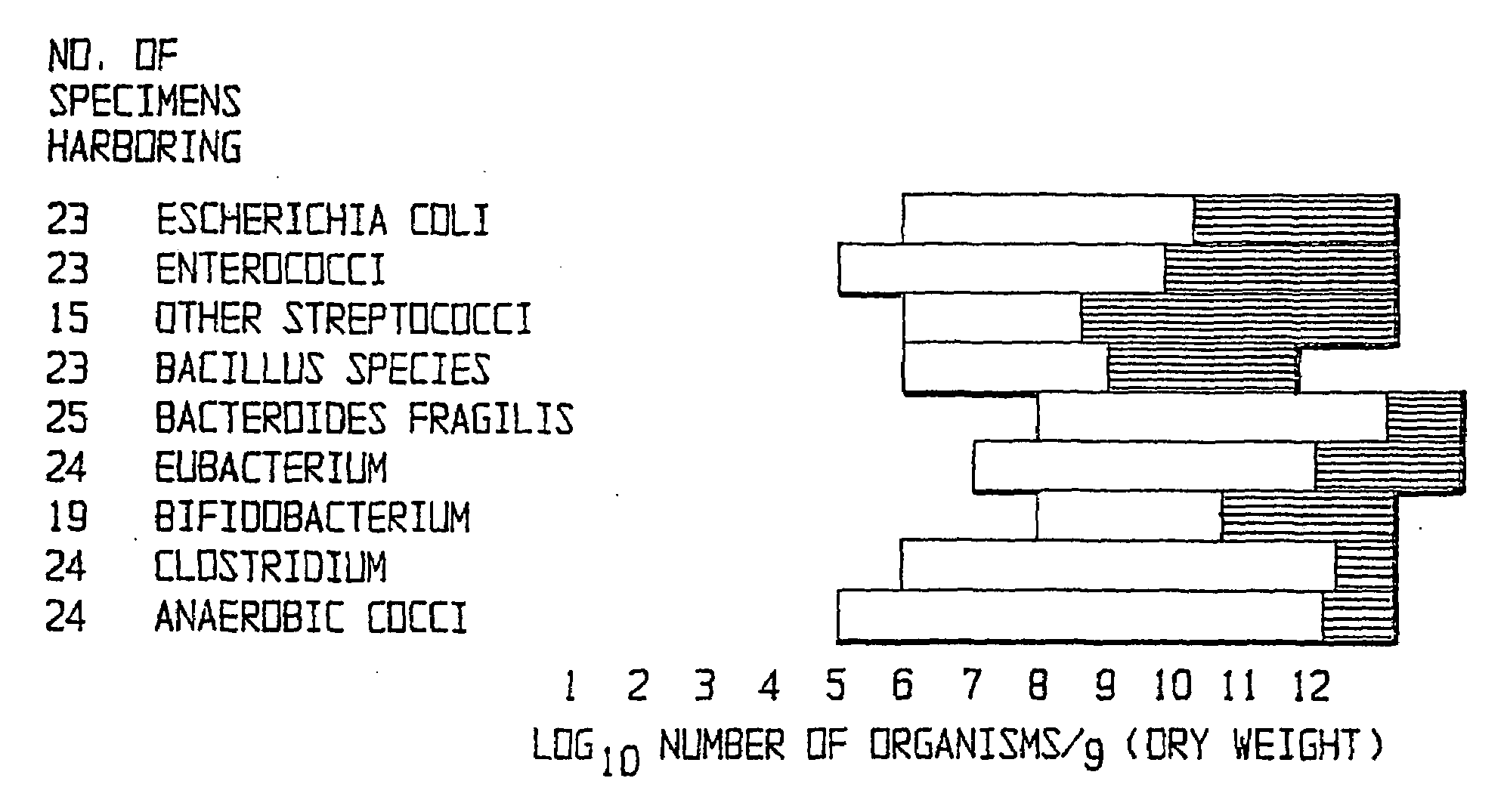
Method of treating gastrointestinal diseases associated with species of genus clostridium
The invention includes a method of treating gastrointestinal diseases associated with species of genus Clostridium such as clostridium deficit in human patients with gastrointestinal disorders having an etiological component such as a microbial agent producing a toxin where treated with an antimicrobial composition an amount effective to inhibit or eliminate the microbial agent. The antimicrobial composition in a form of probiotic mixture can be administrated alone or in combination with an antimicrobial agent, such as a bacteriophage which is specific for a bacterium producing toxin or antibiotics which are then used to eliminate or inhibit the clostridial species overgrown in a patient's gastrointestinal tract. Disorders that can be treated by the method of the invention include diarrhea or inflammatory bowel diseases such as colitis or Crohn's disease.
Owner:UNITED STATES OF AMERICA
Intraoral apparatus for enhancing airway patency
InactiveUS6877513B2Enhancing upper airway stabilityEnhanced upper airway patencyTracheal tubesOperating means/releasing devices for valvesPositive airway pressureInstability
An apparatus for selectively positioning intraoral anatomic features of a human patient to enhance upper airway stability for use alone or in combination with positive airway pressure as therapeutic treatment for obstructive sleep apnea and other conditions, such as snoring, which are symptomatic of upper airway instability.
Owner:RIC INVESTMENTS LLC
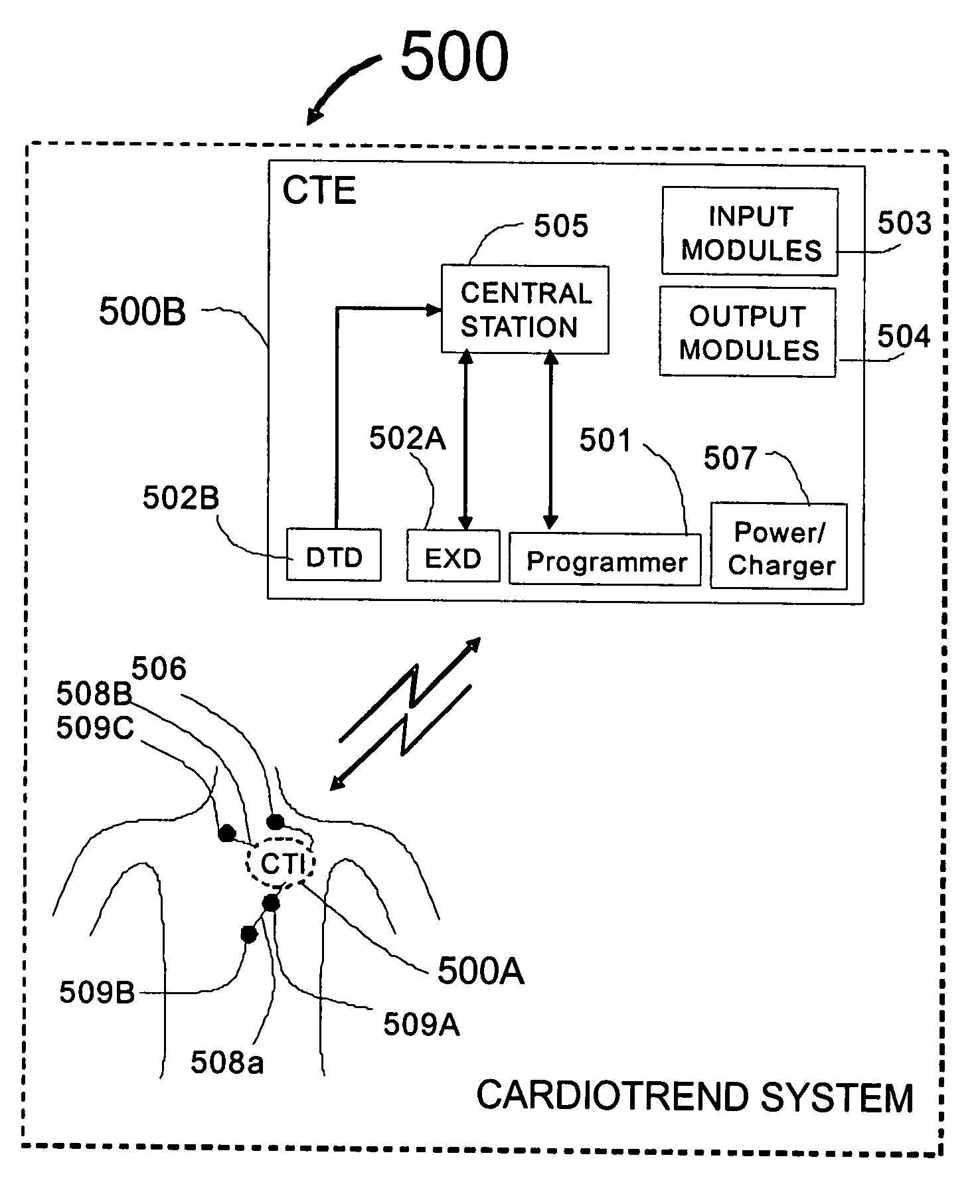
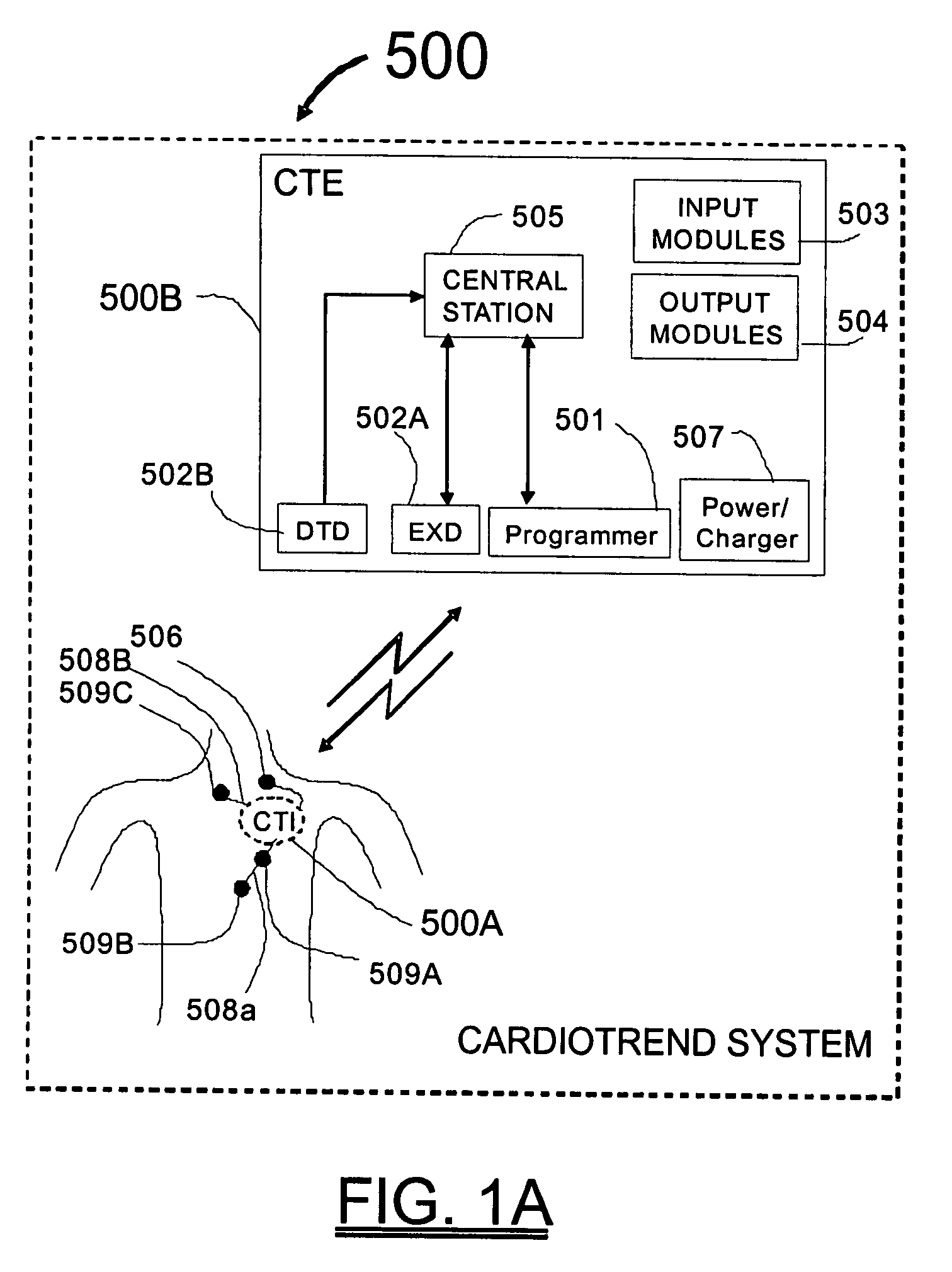
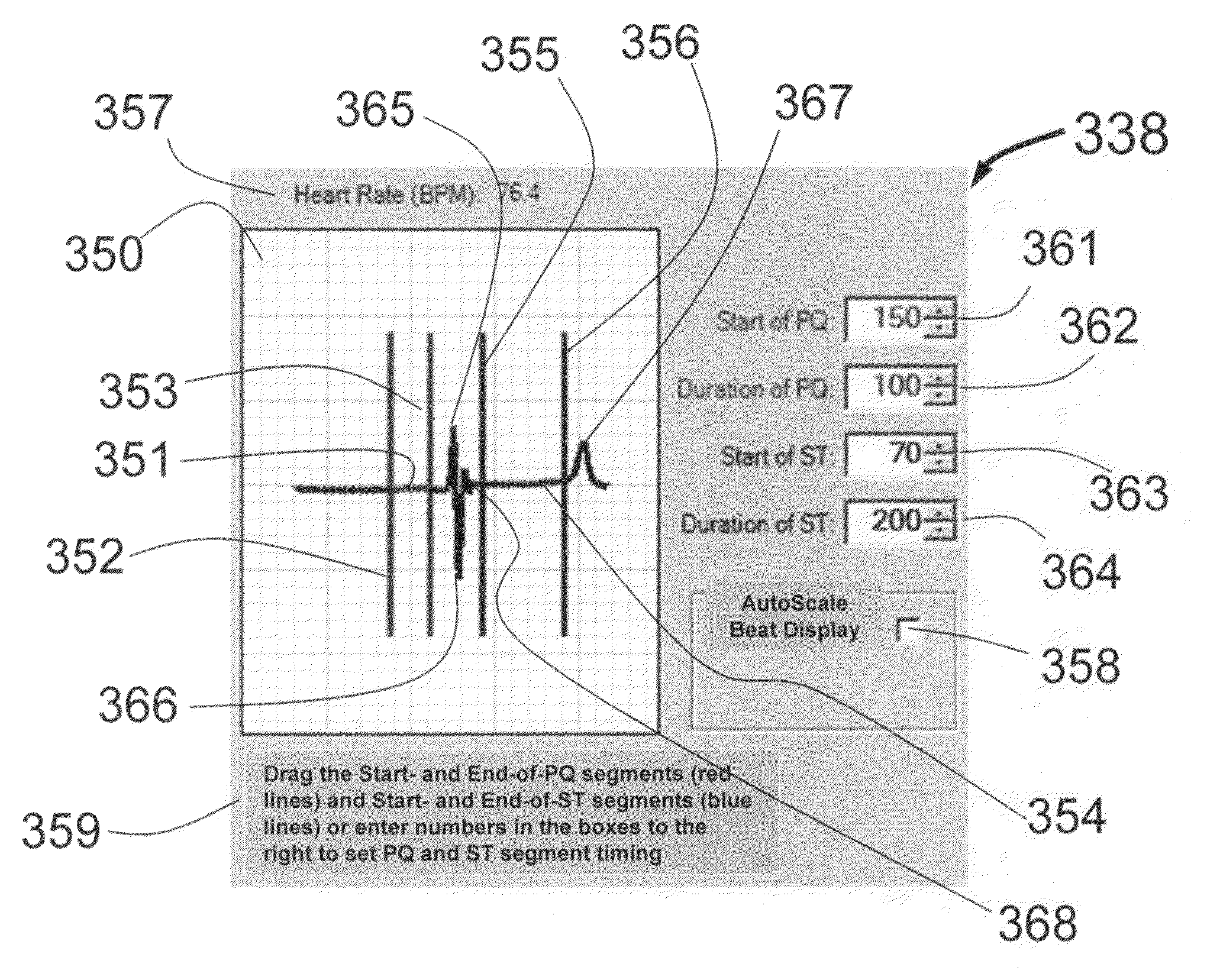
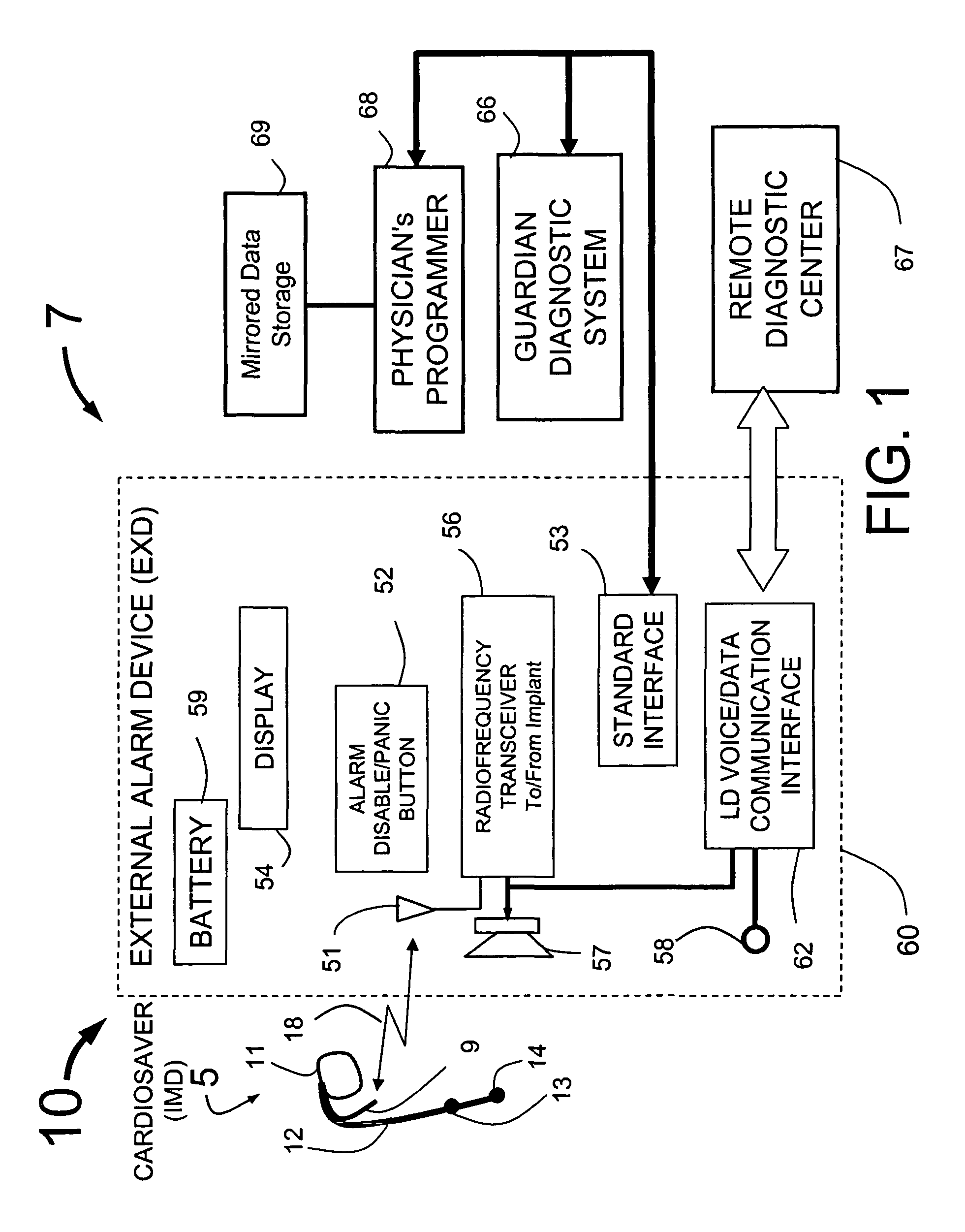
Physician's programmer for implantable devices having cardiac diagnostic and patient alerting capabilities
InactiveUS7801596B2Easy parameter settingElectrotherapyDiagnostic recording/measuringGraphicsGraphical user interface
A programmer is provided for an implantable medical device capable of detecting cardiac events in a human patient. The programmer has a two-way wireless data communication mechanism with the implantable medical device and a graphical user interface is included which has a display and input mechanism designed for use in programming patient specific parameters for the detection of ST shift related cardiac events.
Owner:ANGEL MEDICAL SYST
System and method for monitoring a physiological condition
Owner:HEALTH HERO NETWORK
Method of relieving analgesia and reducing inflamation using a cannabinoid delivery topical liniment
InactiveUS6949582B1Good effectSafe and effectiveBiocideHydroxy compound active ingredientsSide effectCannabinoid receptor
A method of relieving analgesia and reducing inflammation using a cannabinoid delivery topical liniment composition containing from about 97.5% to about 99.5% by weight a 70% monohydric alcohol solution, and from about 0.5% to about 2.5% by weight of a synergistic cannabinoid mixture extracted from the female plant Cannabis sativa L, including in combination: 9-Tetrahydrocannabinol (delta-9-THC), 9-THC Propyl Analogue (THC-V), Cannabidiol (CBD), Cannabidiol Propyl Analogue (CBD-V), Cannabinol (CBN), Cannabichromene (CBC), Cannabichromene Propyl Analogue (CBC-V), Cannabigerol (CBG), terpenoids, and flavonoids. The liniment is applied topically, preferably by spraying, and the constituents of the mixture are absorbed through the skin and interact with cannabinoid receptors in the body and tissues of a human patient to produce therapeutic analgesic and anti-inflammatory effects without undesirable psychotropic side effects.
Owner:WALLACE WALTER H
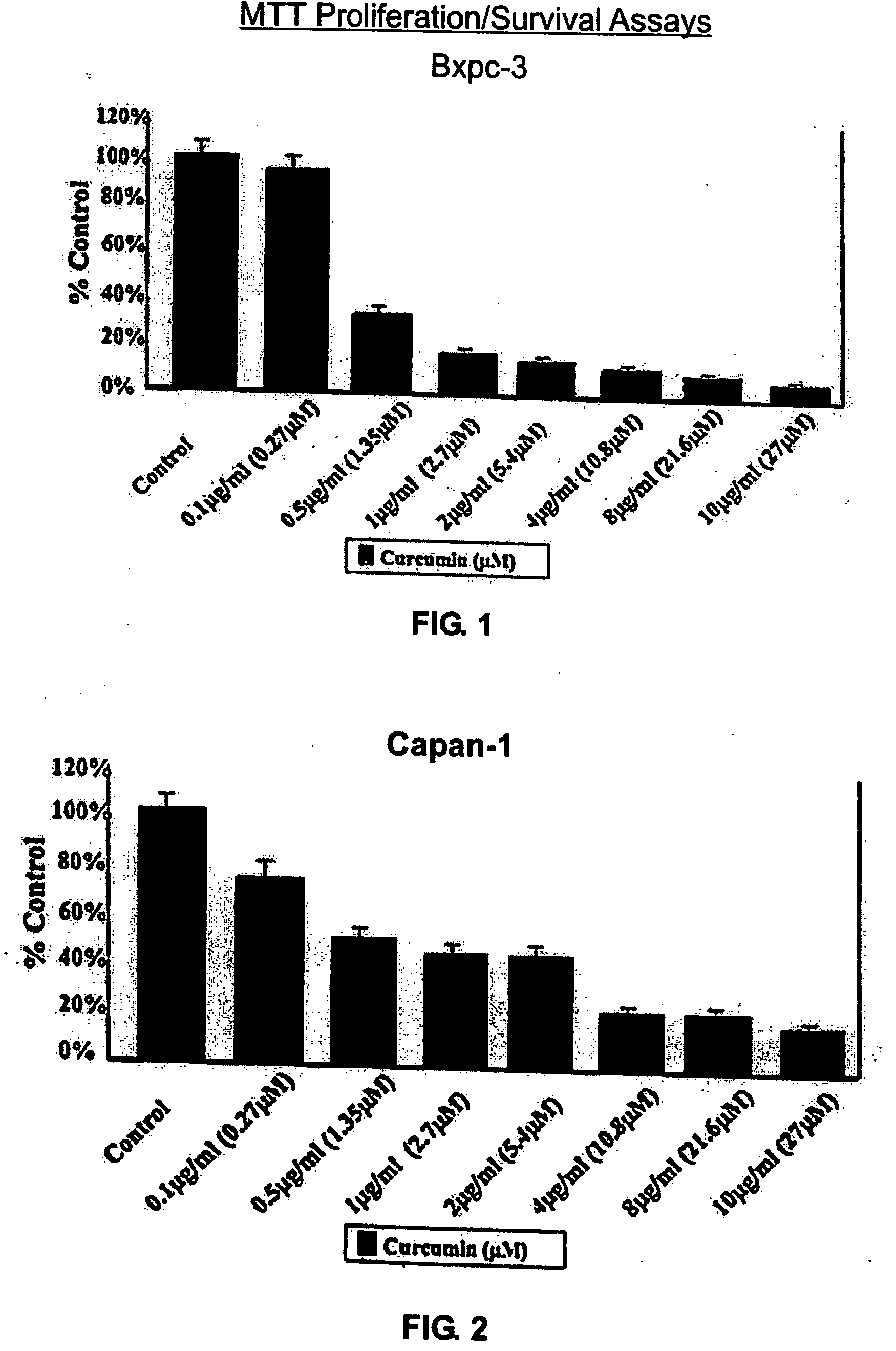
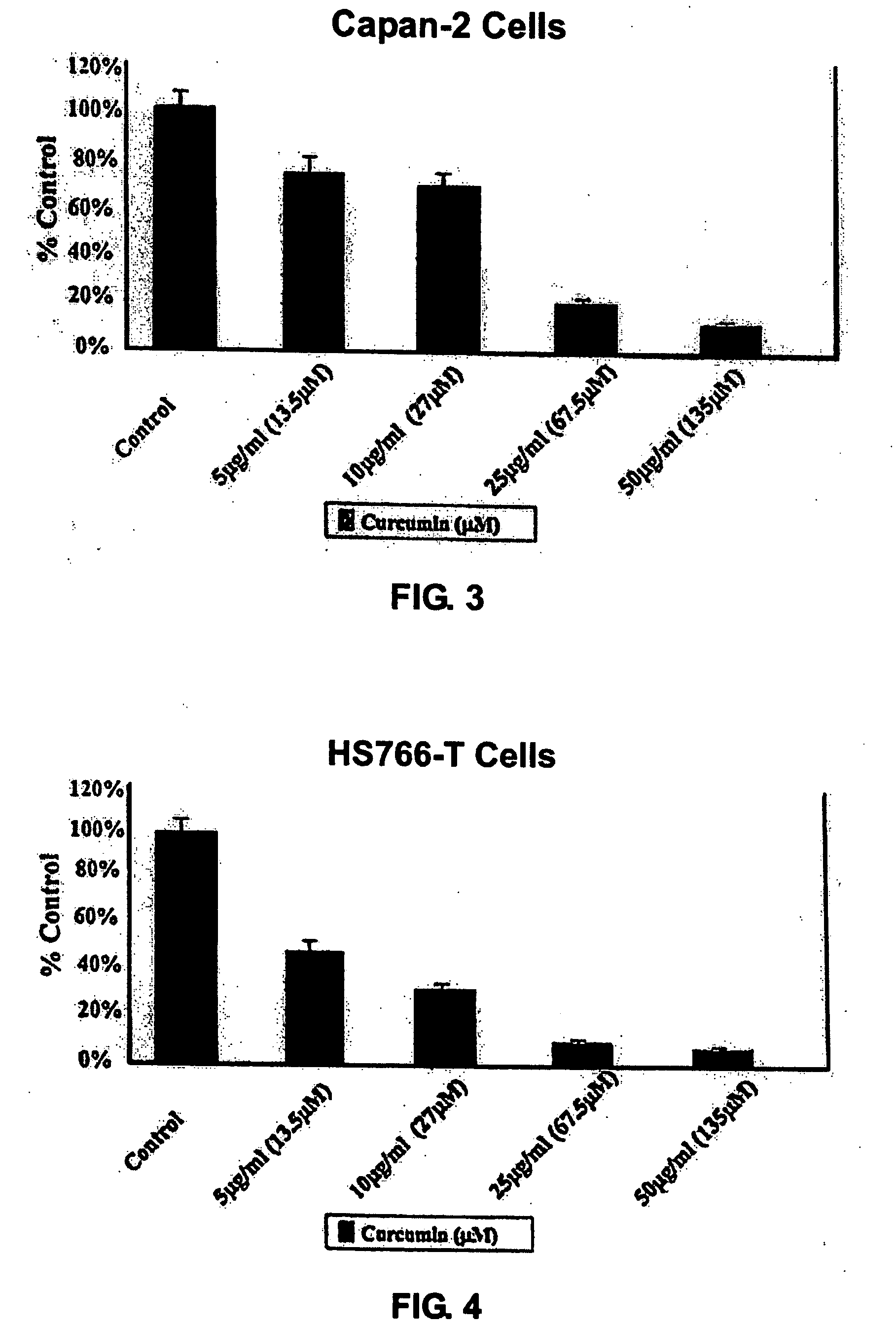
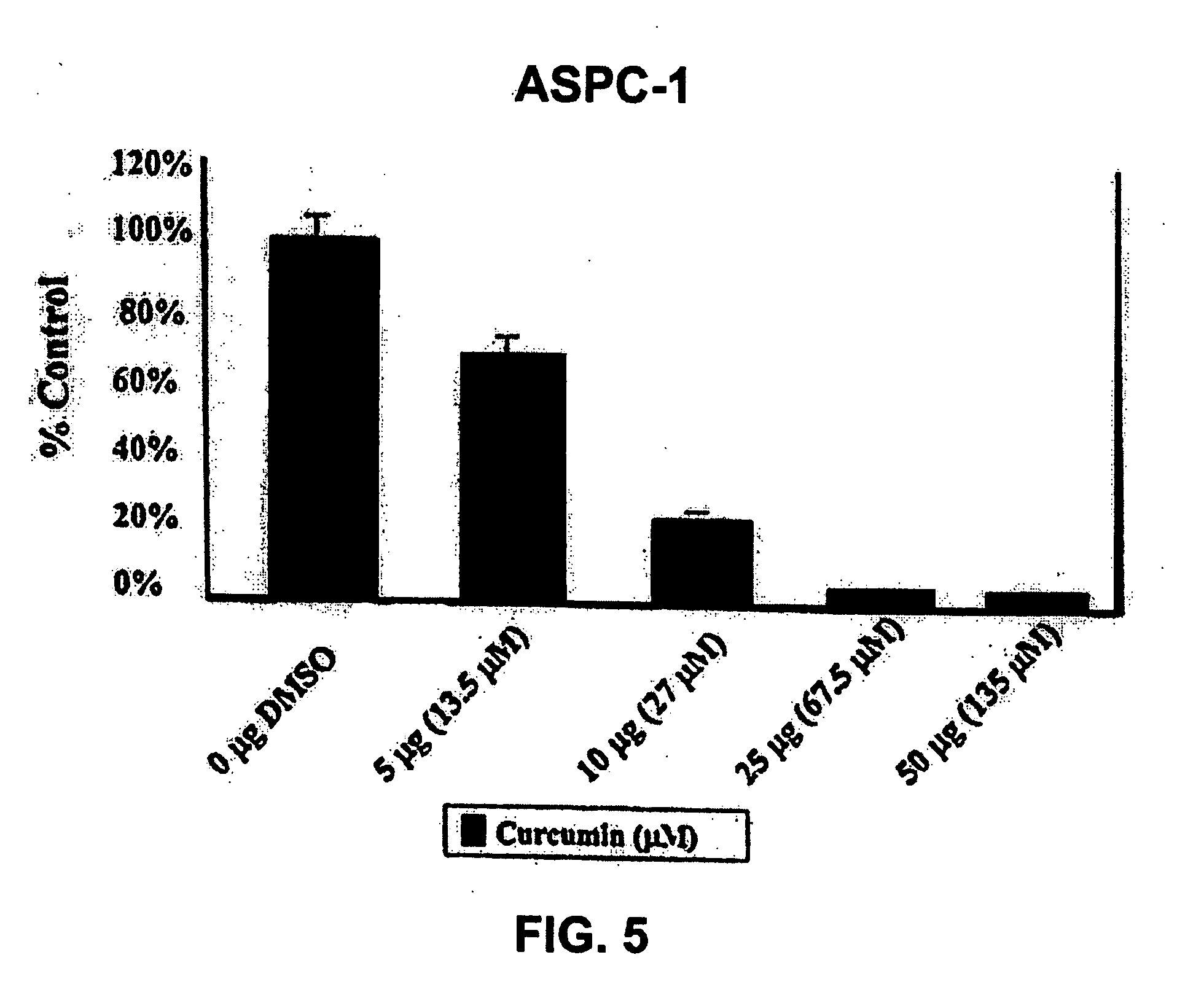
Liposomal curcumin for treatment of cancer
The present invention provides a compositions and methods for the treatment of cancer, including pancreatic cancer, breast cancer and melanoma, in a human patient. The methods and compositions of the present invention employ curcumin or a curcumin analogue encapsulated in a colloidal drug delivery system, preferably a liposomal drug delivery system. Suitable colloidal drug delivery systems also include nanoparticles, nanocapsules, microparticles or block copolymer micelles. The colloidal drug delivery system encapsulating curcumin or a curcumin analogue is administered parenterally in a pharmaceutically acceptable carrier.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Intraoral apparatus for enhancing airway patency
InactiveUS20010047805A1Eliminate the obstructive/restrictive episodesImprove pressure resistanceTracheal tubesOperating means/releasing devices for valvesPositive airway pressureInstability
An apparatus for selectively positioning intraoral anatomic features of a human patient to enhance upper airway stability for use alone or in combination with positive airway pressure as therapeutic treatment for obstructive sleep apnea and other conditions, such as snoring, which are symptomatic of upper airway instability.
Owner:RIC INVESTMENTS LLC
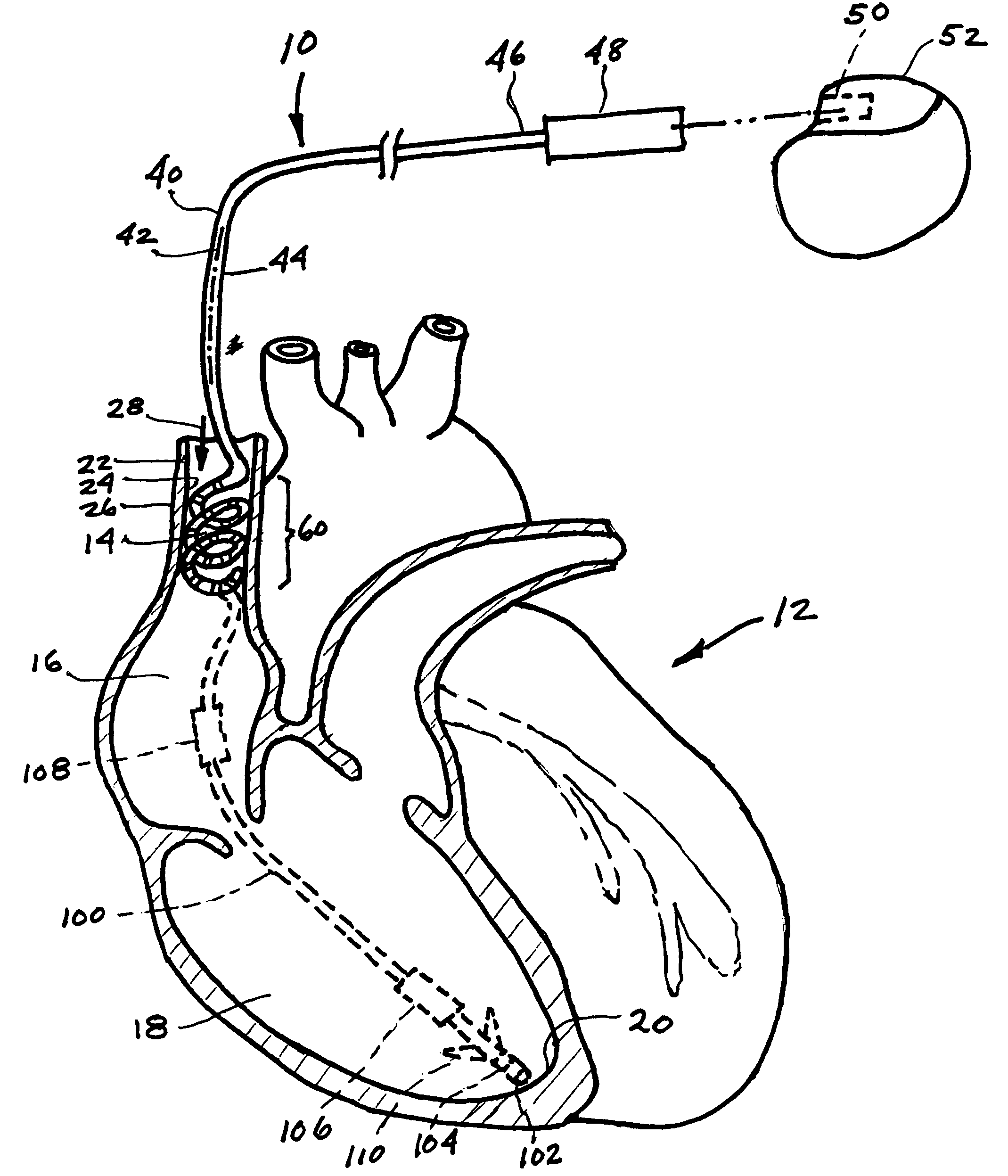
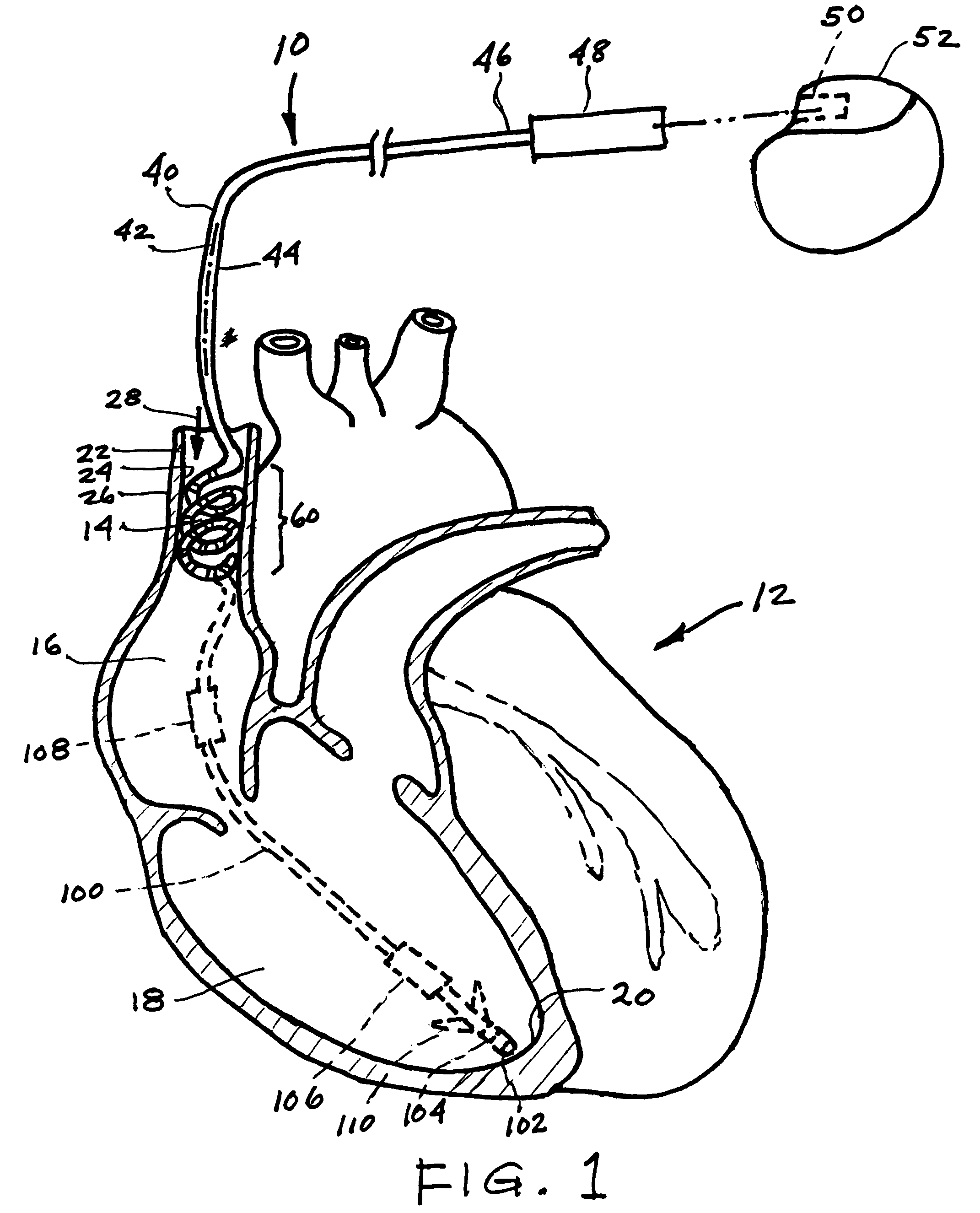
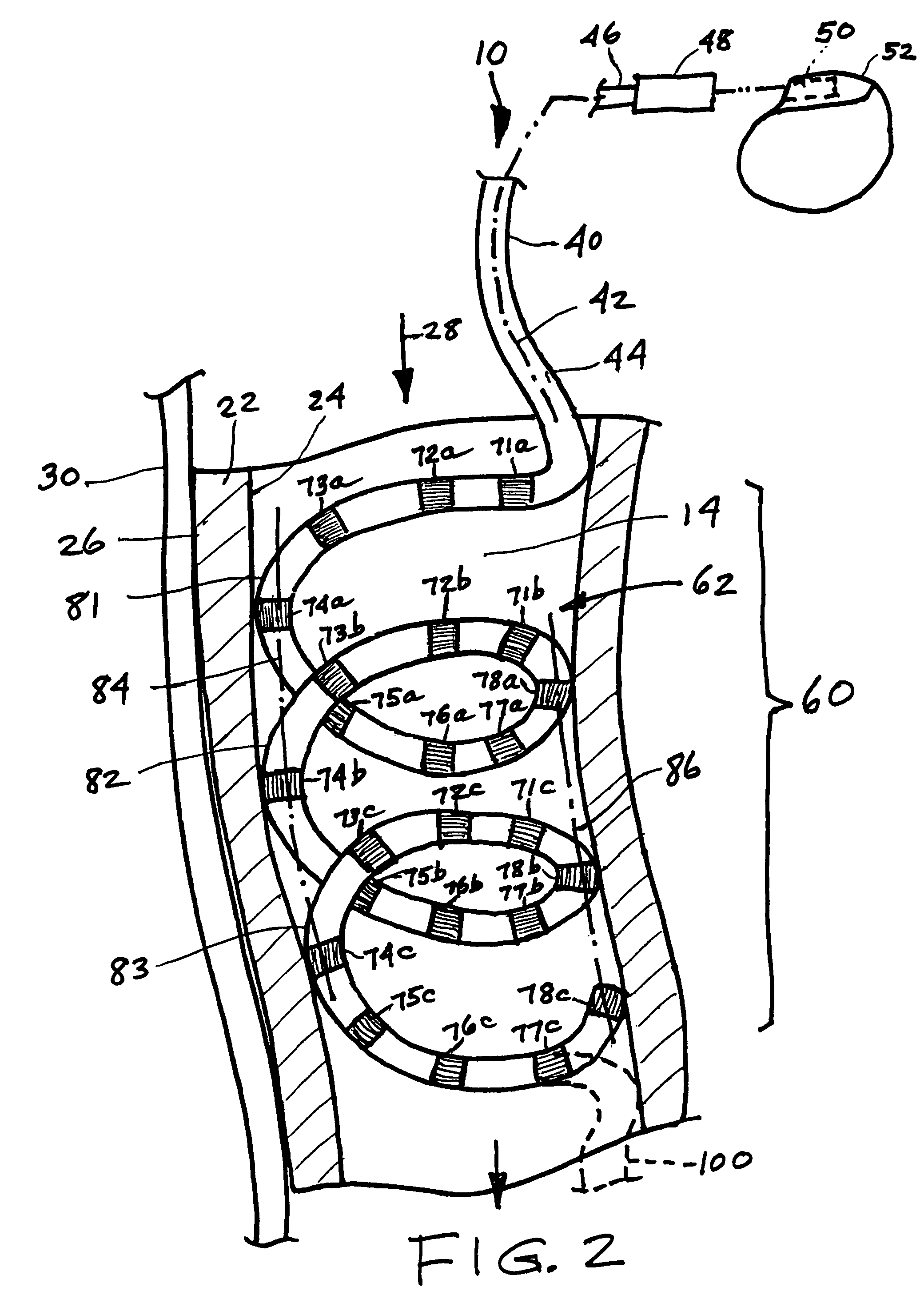
Endovascular lead for chronic nerve stimulation
A lead of the present invention comprises an electrode array adapted to be stably anchored at a selected location within the vena cava of a human patient. The electrode array may take various shapes, including helical, annular and linear. The electrode array is connectable to an electrical stimulation means such as an implantable pulse or signal generator. Electrical stimulation applied to a selected region of the vena cava and across the wall of the vein, that is, transvascularly, to the vagus nerve or branches thereof, depolarizes the nerve to thereby effect control of the heart rate.
Owner:PACESETTER INC
Method and device for the electrical treatment of sleep apnea and snoring
InactiveUS20080021506A1Minimal discomfortPrevent snoringRespiratorsElectrotherapyThroatCardiac pacemaker electrode
A device and method for the treatment of sleep apnea and / or snoring in a human patient includes at least one electrode stimulator for providing direct electrical stimulation to a throat and / or laryngeal muscles of the patient. The stimulator is constructed and arranged to be positioned at the throat and / or laryngeal muscles of the patient, either on the surface or subcutaneously. A power source provides a continuous electrical signal to the stimulator so that the throat and / or laryngeal muscles of the patient are contracted to open the airway of the patient. The power source can be a portable source remote from the electrode or a pacemaker unit also implanted in the patient.
Owner:GRINDSTONE MEDICAL LLC A LIMITED LIABILITY OF THE STATE OF MASSACHUSETTS
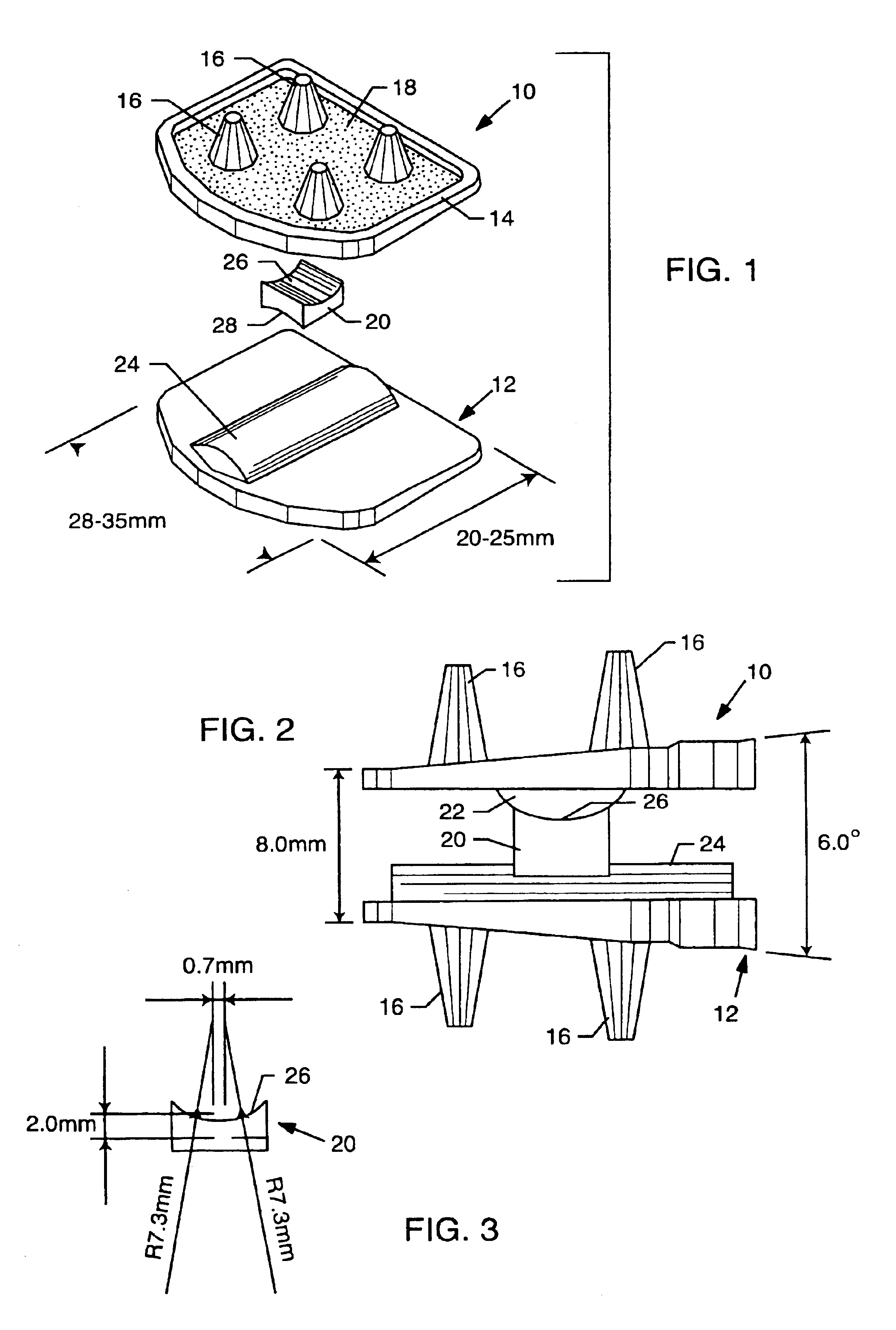
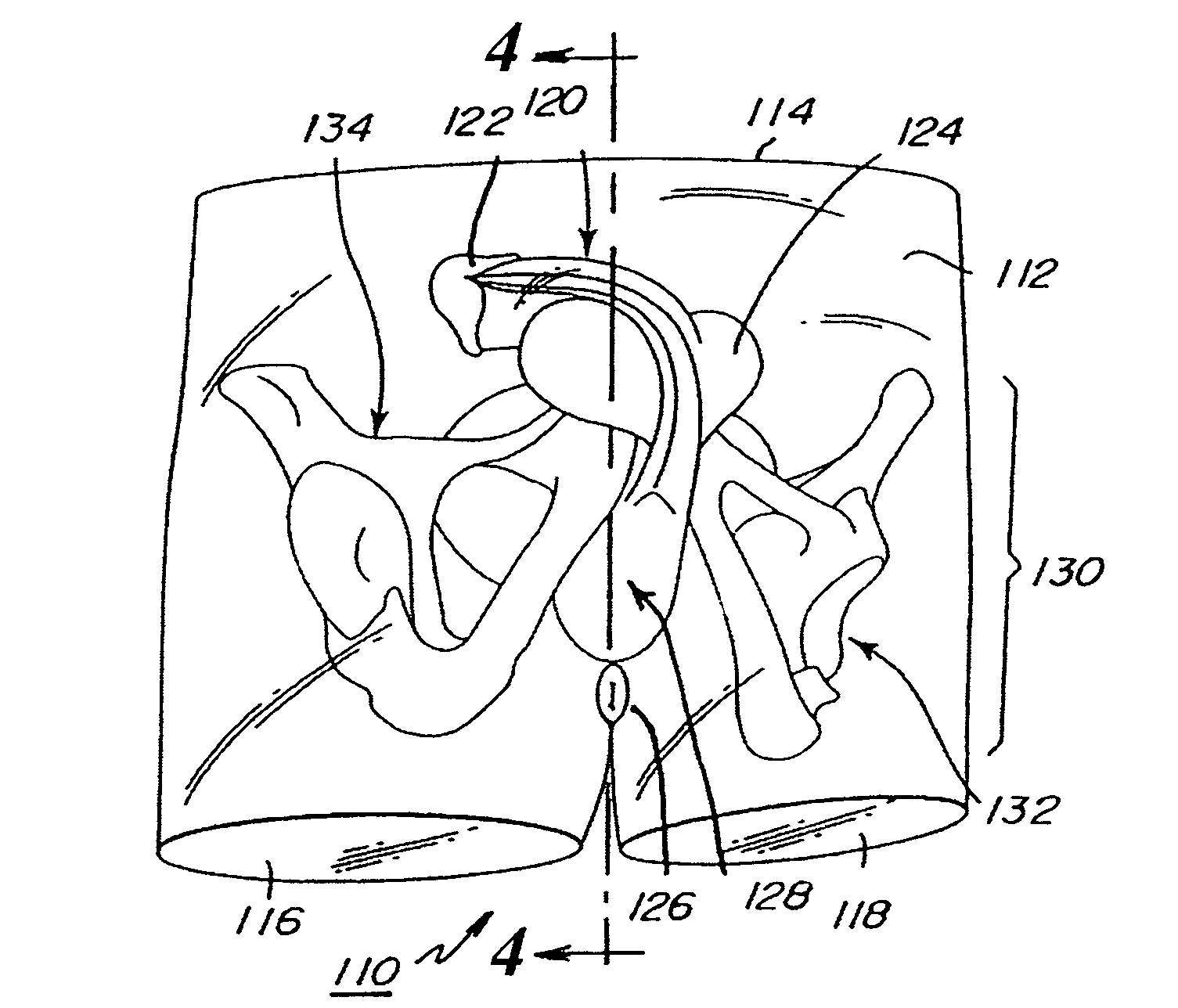
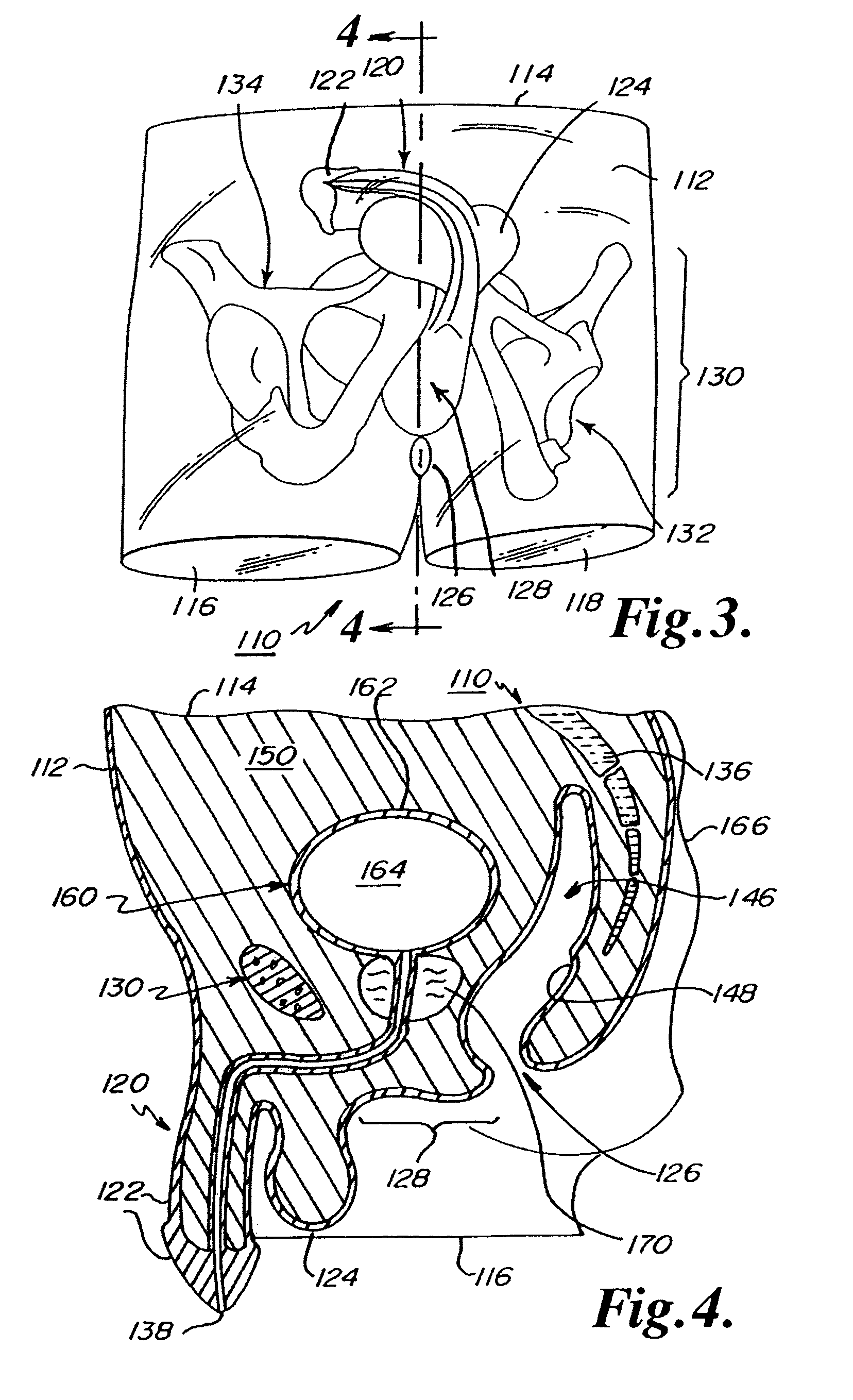
Abdominopelvic region male anatomic model
Anatomic models of the abdominopelvic region of a male human patient's body to assist in demonstrating or in training medical personnel in microsurgical techniques are disclosed. The anatomic models preferably demonstrate the passage of elongated medical instruments through the perineum to or into the prostate. The anatomic models preferably comprise a substantially transparent elastomer body mass encasing a relatively rigid skeletal frame and at least a prostate model. The elastomer mass is shaped to simulate the perineum overlying the prostate between a penis model and an anal opening.
Owner:BOSTON SCI SCIMED INC
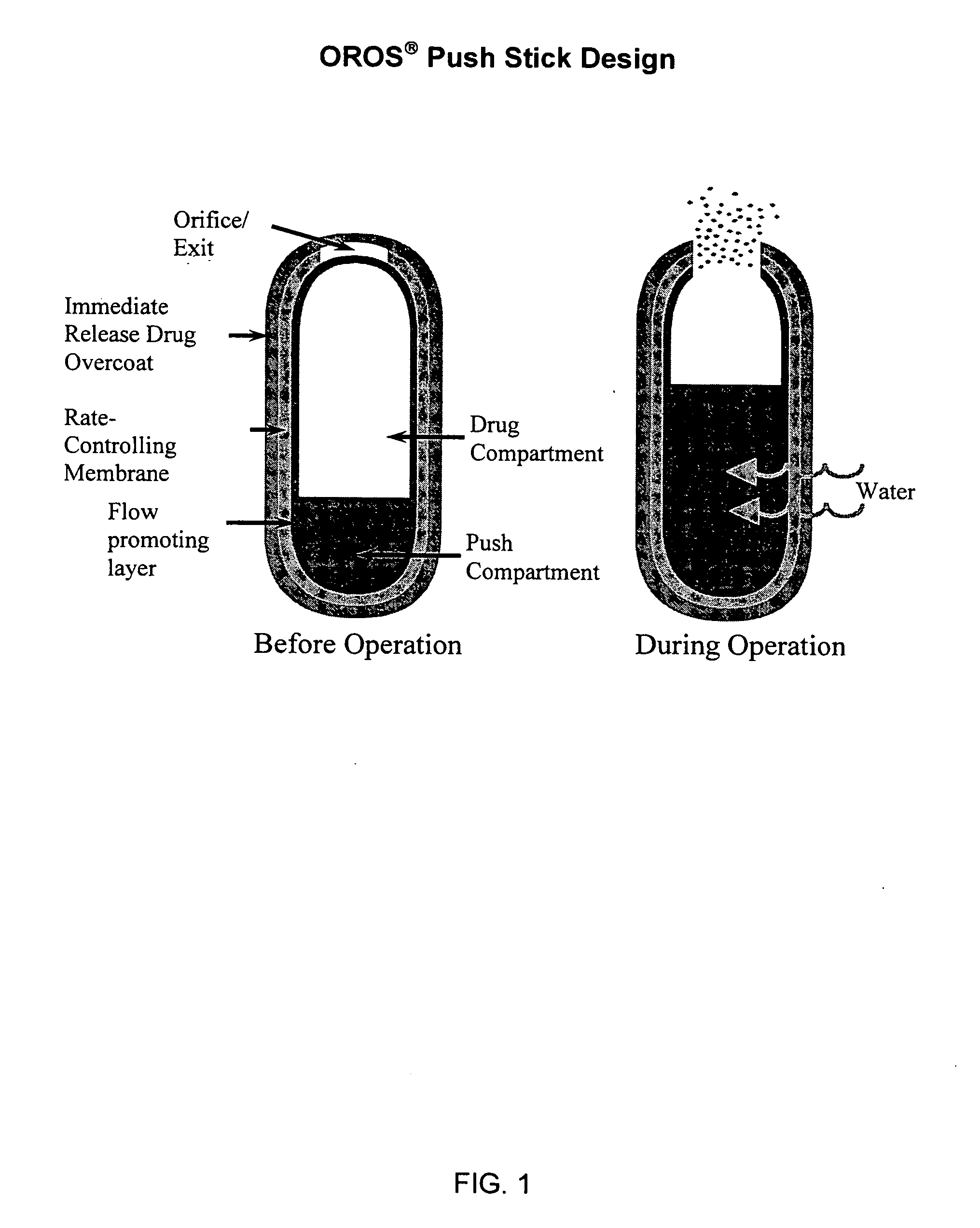
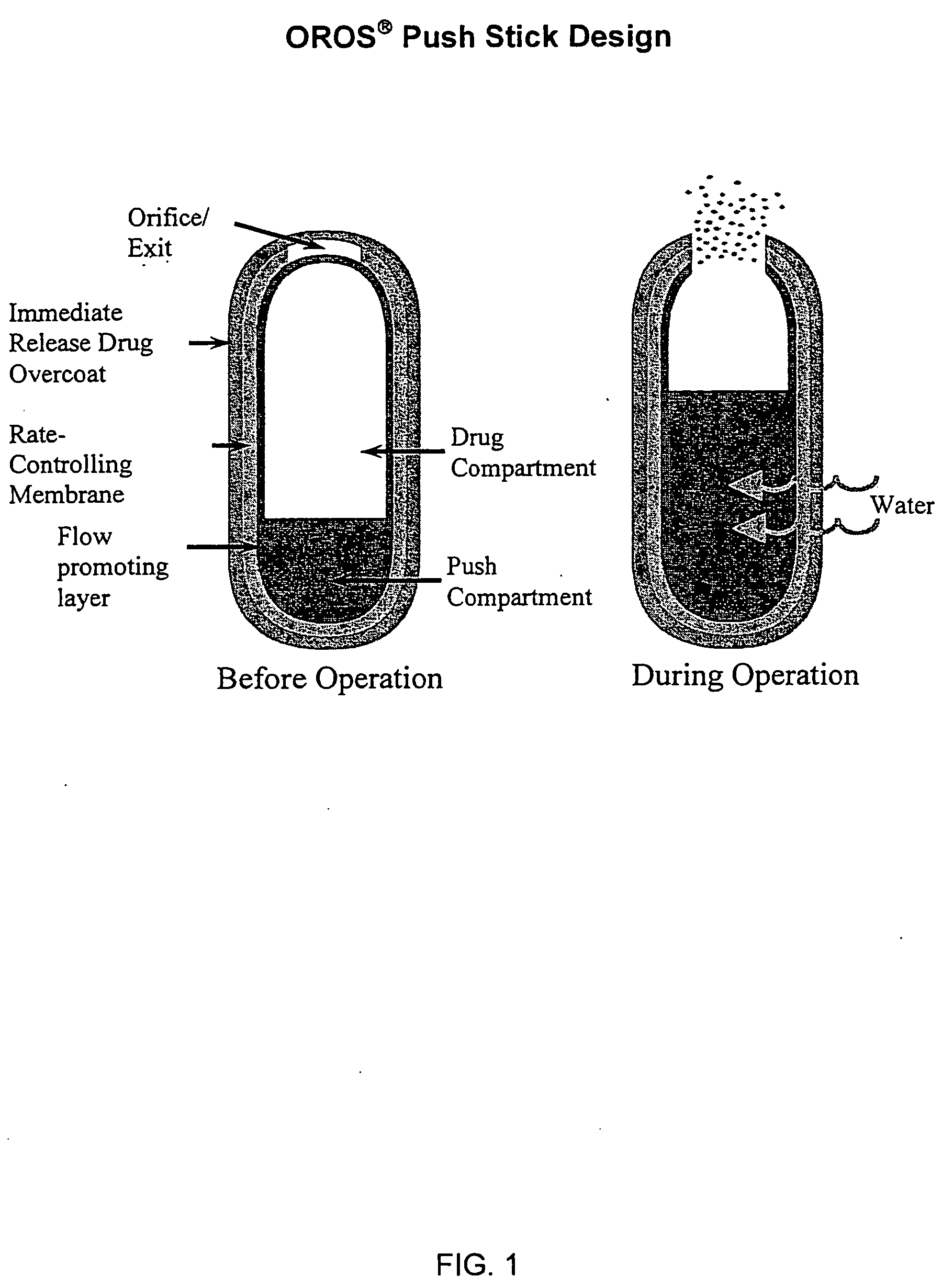
Controlled Release Hydrocodone Formulations
InactiveUS20110262532A1Improve efficiency and qualityGood effectPowder deliveryBiocideControlled releaseHuman patient
A solid oral controlled-release oral dosage form of hydrocodone is disclosed. The dosage form comprising an analgesically effective amount of hydrocodone or a pharmaceutically acceptable salt thereof, and a sufficient amount of a controlled release material to render the dosage form suitable for twice-a-day administration to a human patient, the dosage form providing a C12 / Cmax ratio of 0.55 to 0.85, said dosage form providing a therapeutic effect for at least about 12 hours.
Owner:PURDUE PHARMA LP
Non-invasive method and device to monitor cardiac parameters
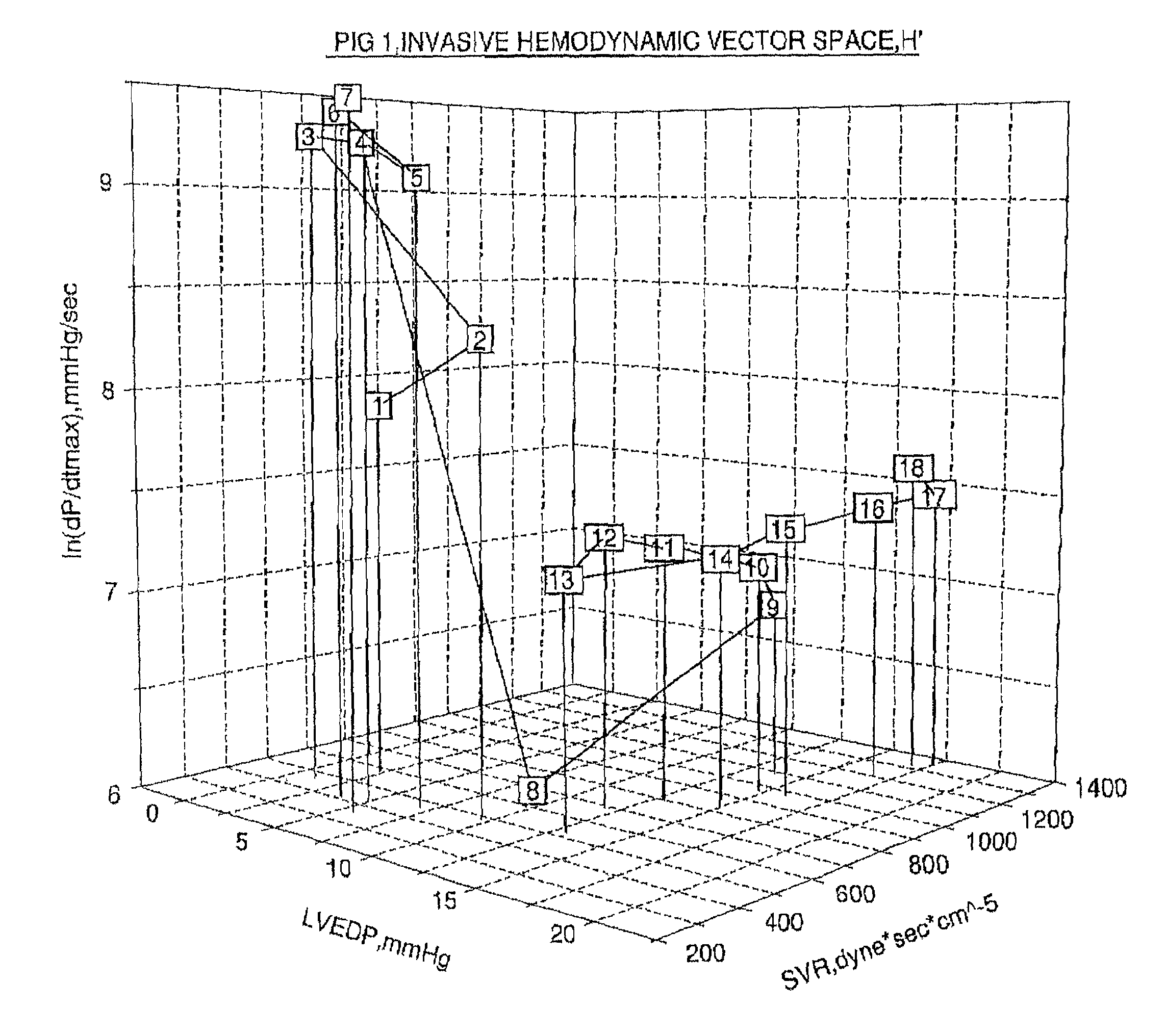
A method of and a device for non-invasively measuring the hemodynamic state of a subject or a human patient involve steps and units of non-invasively measuring cardiac cycle period, electrical-mechanical interval, mean arterial pressure, and ejection interval and converting the measured electrical-mechanical interval, mean arterial pressure and ejection interval into the cardiac parameters such as Preload, Afterload and Contractility, which are the common cardiac parameters used by an anesthesiologist.The converted hemodynamic state of a patient is displayed on a screen as a three-dimensional vector with each of its three coordinates respectively representing Preload, Afterload and Contractility. Therefore, a medical practitioner looks at the screen and quickly obtains the important and necessary information.
Owner:HIRSH ROBERT
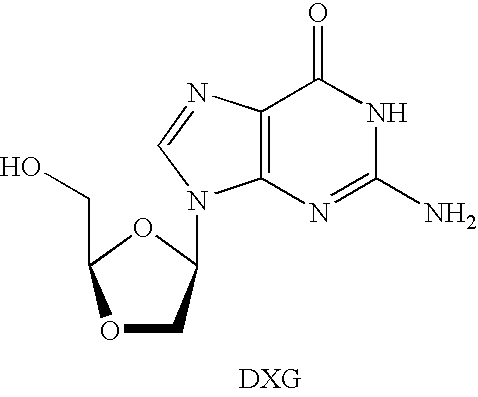
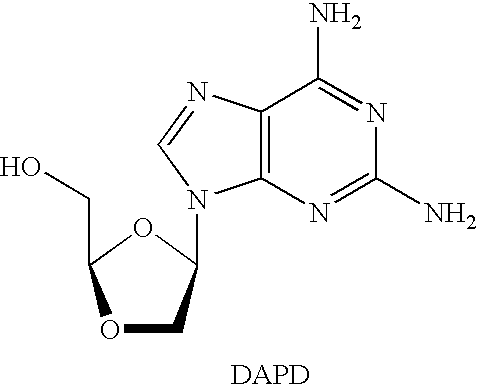
N4-acylcytosine-1,3-dioxolane nucleosides for treatment of viral infections
InactiveUS6908924B2Strong inhibitory activityBiocideOrganic active ingredientsImmunodeficiency virusHuman patient
The present invention is directed to a compound, method and composition of treating or preventing viral infections, in particular, human immunodeficiency virus (HIV) and hepatitis B virus (HBV) infections, in human patients or other animal hosts, comprising the administration of N4-acylcytosine-1,3-dioxolane and pharmaceutically acceptable salts, prodrugs, and other derivatives thereof.
Owner:GILEAD PHARMASSET LLC
Cardiac event microrecorder and method for implanting same
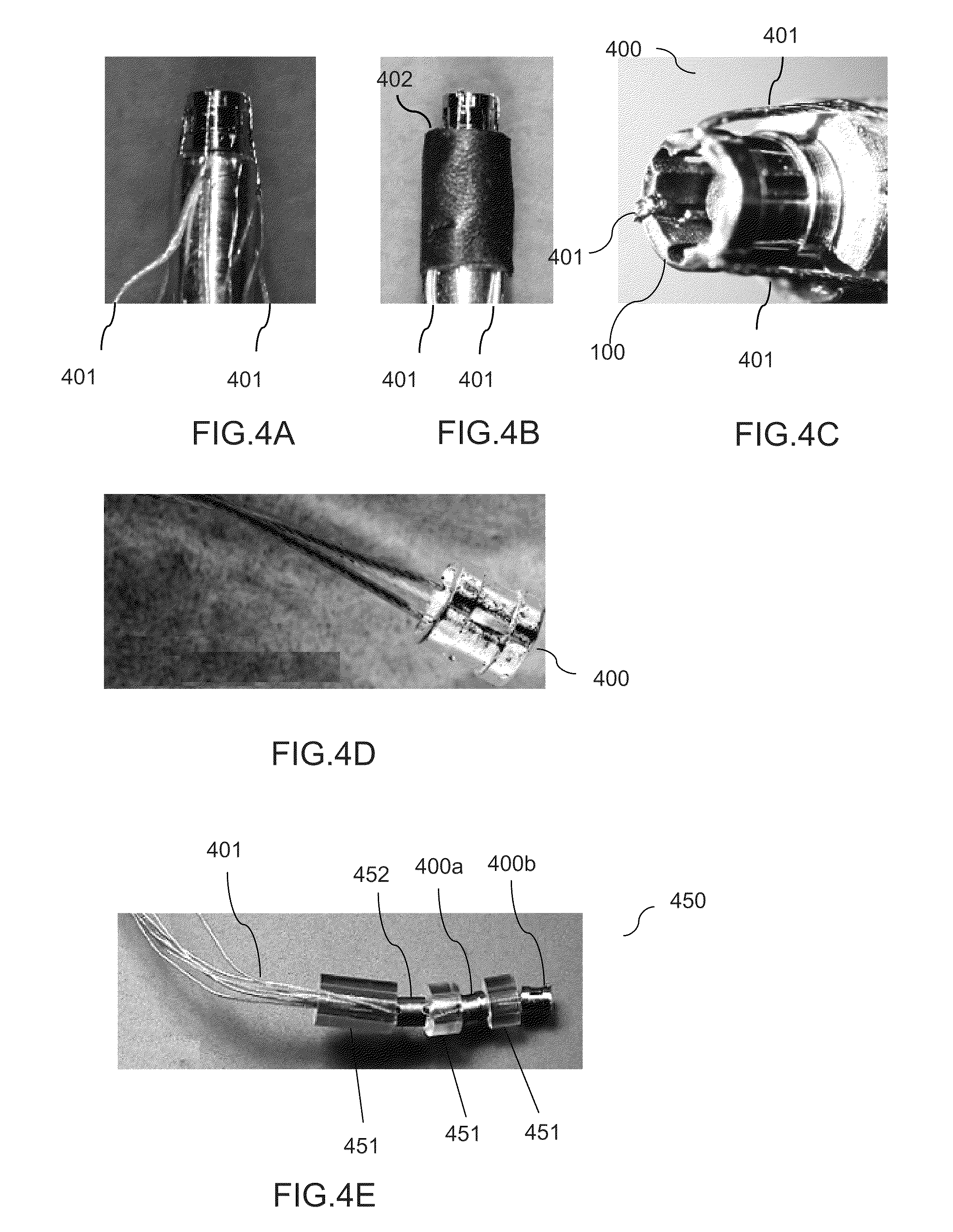
ActiveUS7294108B1Facilitate subcutaneous implantationElectrocardiographyHeart defibrillatorsElectricityHypodermic needle
A cardiac event microrecorder comprising a hermetically sealed housing is shaped and dimensioned to facilitate the subcutaneous implantation of the microrecorder in a human patient by injection through the lumen of a hypodermic needle. The housing comprises a tubular central section of an electrically insulating material, the central section having spaced apart extremities. An electrically conductive sense electrode is secured to and seals each extremity. The housing may have an interior enclosing (1) electrical circuitry for processing, storing and telemetering data representing physiologic information detected by the sense electrodes; (2) a primary cell or rechargeable battery for electrically powering the electrical circuitry; and (3) an inductively couplable charger where a rechargeable battery is used. Also disclosed are a method of subcutaneously implanting a cardiac event recorder in a patient's thoracic region, and a handheld mapping device for determining an optimum location and orientation for the cardiac event microrecorder to be implanted.
Owner:PACESETTER INC
Vascular Position Locating and/or Mapping Apparatus and Methods
A branch vessel in a human patient is located or mapped using in vivo tracked field sensors where in one variation the sensor positions can be located by determining the positions of the sensors relative to a plurality of magnetic field sources of known location. This approach is used, for example, in locating the opening in a renal artery and positioning the proximal end of the AAA stent-graft adjacent to the opening. In another example, the sensors are tracked along the inner wall of an aneurysm and the acquired sensor location data processed to map the contour of the aneurysm to size a prostheses for spanning the aneurysm. The portions of the vessel adjacent the aneurysm also can be mapped. In a further embodiment, an in vivo sensor is positioned in a deployed prosthesis to create a reference for a prosthetic member having a sensor to track to during cannulation of the deployed prosthesis with the prosthetic member.
Owner:MEDTRONIC VASCULAR INC
Medical leads with segmented electrodes and methods of fabrication thereof
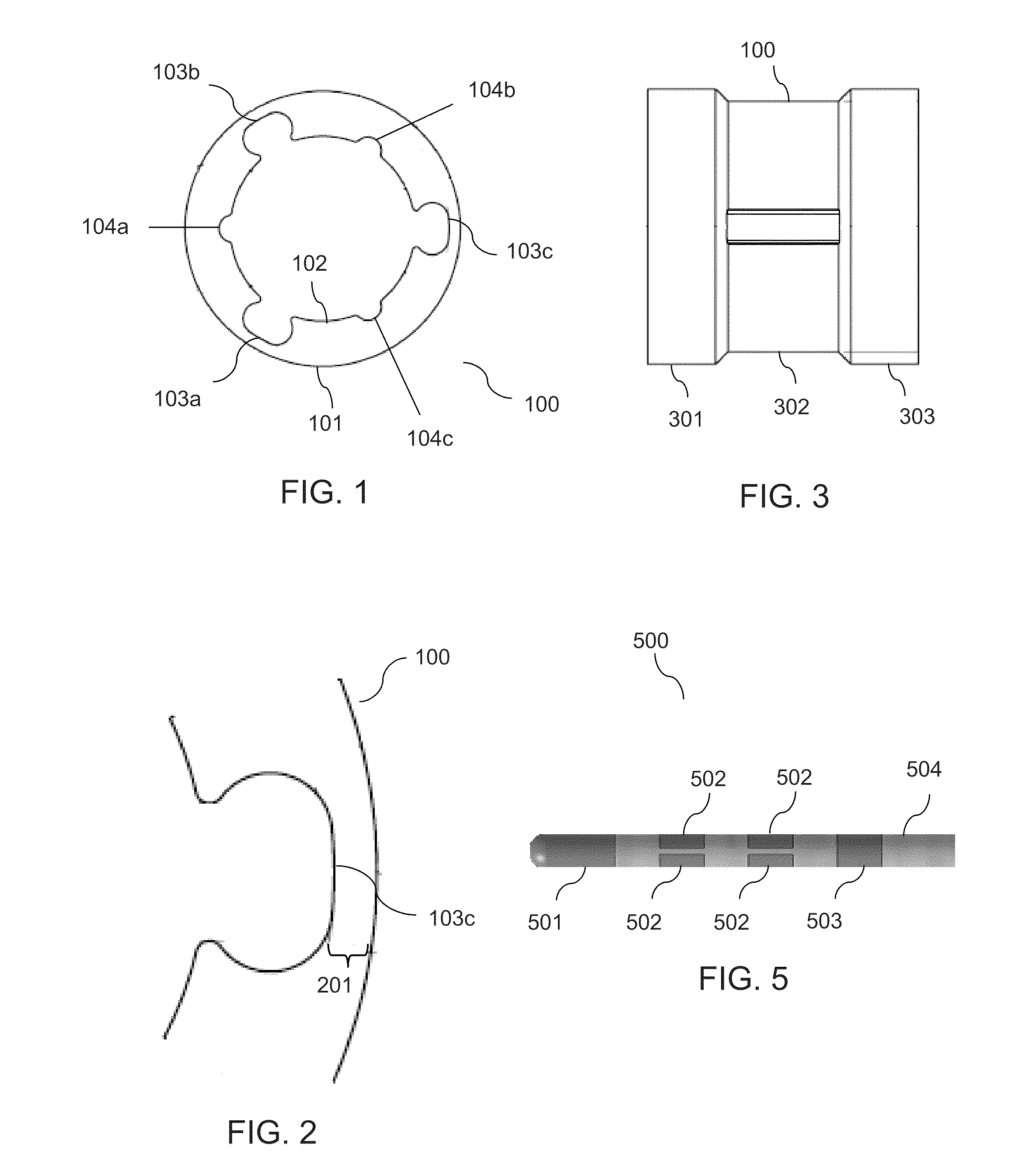
In one embodiment, a method of fabricating a segmented electrode stimulation lead for implantation within a human patient for stimulation of tissue of the patient, the method comprises: providing a conductive ring, the conductive ring comprising an inner surface and an outer surface, the conductive ring comprising a plurality of grooves provided in the inner surface; electrically coupling a plurality of wires to the conductive ring; forming a stimulation assembly of the lead including the conductive ring and the plurality of wires; and grinding down the outer surface of the stimulation assembly of the lead at least until reaching the plurality of grooves to separate the conductive ring into a plurality of electrically isolated segmented electrodes.
Owner:ADVANCED NEUROMODULATION SYST INC
Method and device to administer anesthetic and or vosactive agents according to non-invasively monitored cardiac and or neurological parameters
InactiveUS20090124867A1Efficiently safely teachingPumping capacity is overwhelmedMedical simulationRespiratorsCardiac cycleWhole body
A method of and a device for non-invasively measuring the neurological depressed state and the hemodynamic state of a human patient and involving steps and units of non-invasively measuring EEG, cardiac cycle period, electrical-mechanical interval, mean arterial pressure, and ejection interval and converting the EEG into a neurological index as well as converting the measured electrical-mechanical interval, mean arterial pressure and ejection interval into the cardiac parameters such as Preload, Afterload and Contractility, which are the common cardiac parameters used by an anesthesiologist. A general anesthetic is administered based upon the converted neurological index. A vasoactive agent is independently administered based upon the converted cardiac parameters as necessary in order to restore cardiovascular homeostasis in the patient. The converted neurological and hemodynamic state of a patient are displayed on a screen as an index value and a three-dimensional vector with each of its three coordinates respectively representing Preload, Afterload and Contractility. Therefore, a medical practitioner looks at the screen and quickly obtains the important and necessary information.
Owner:THE COOPER HEALTH SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com