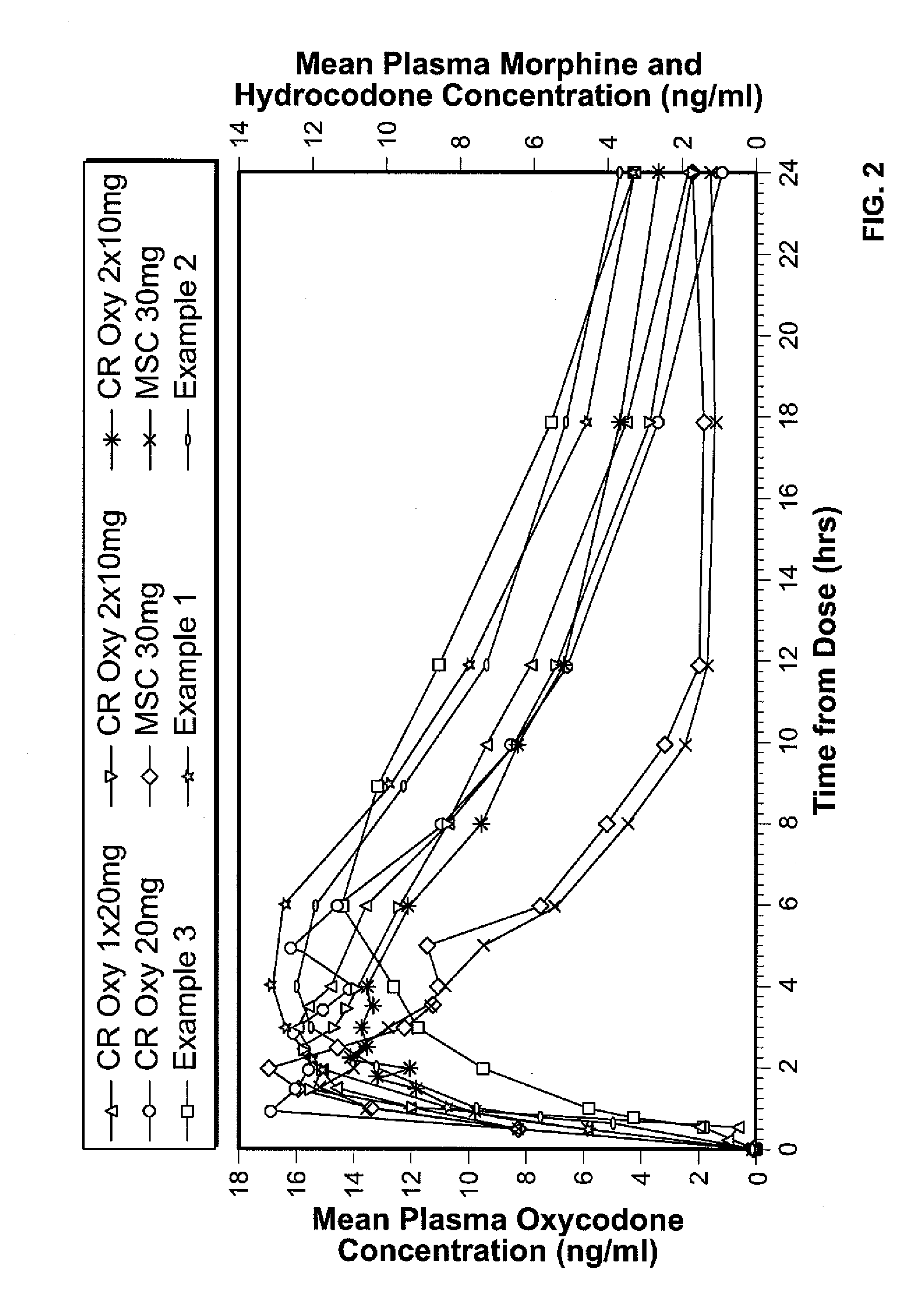
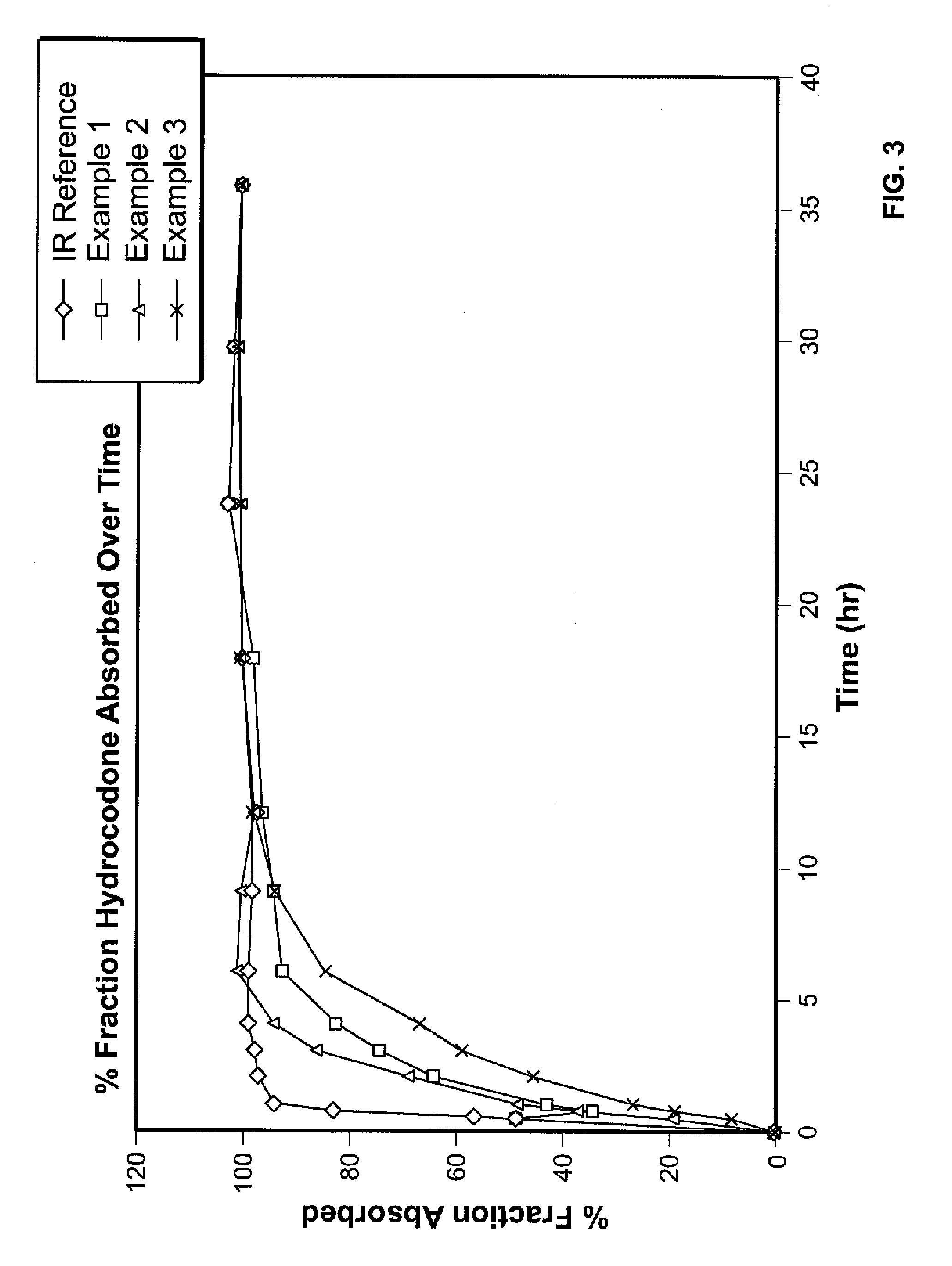
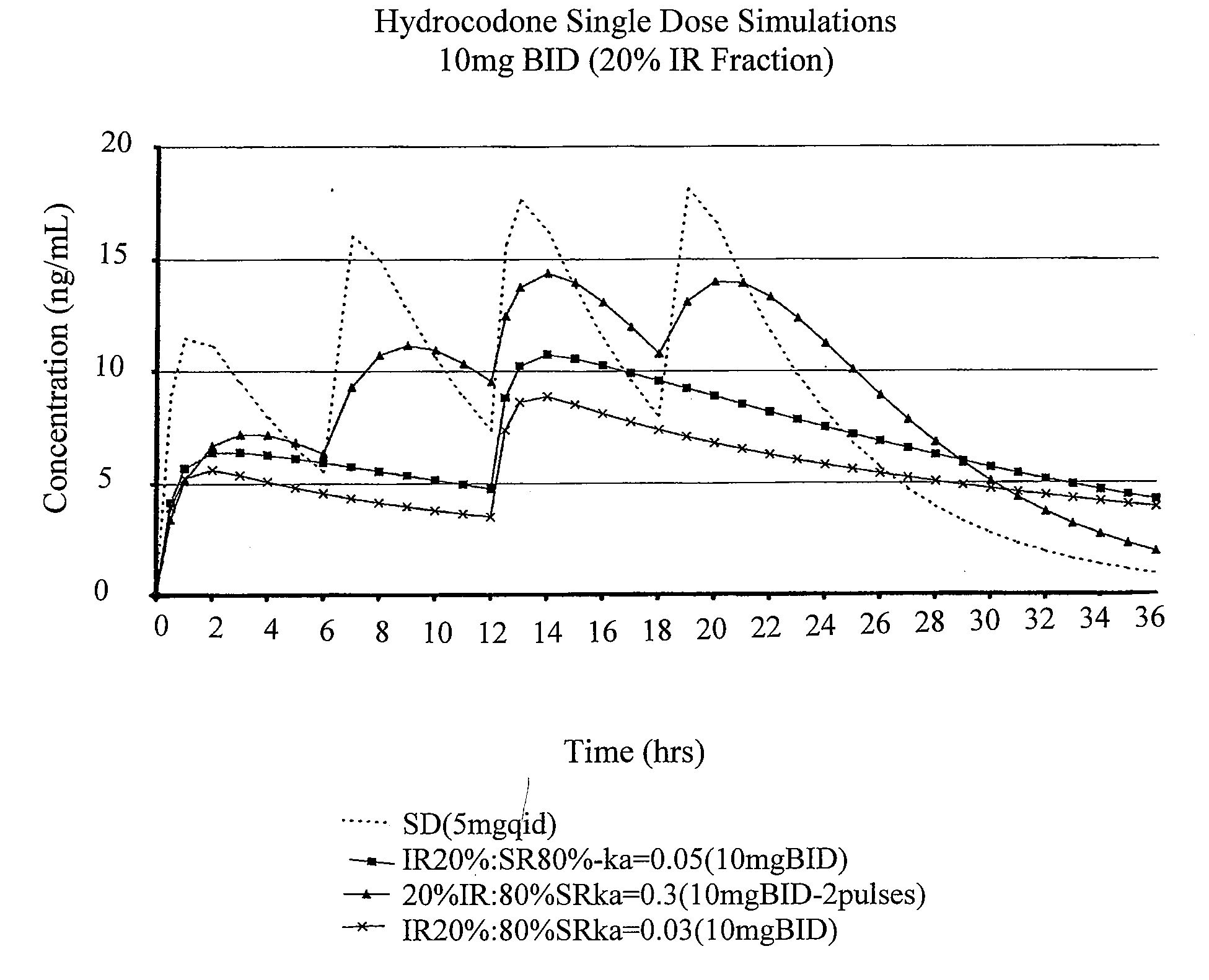
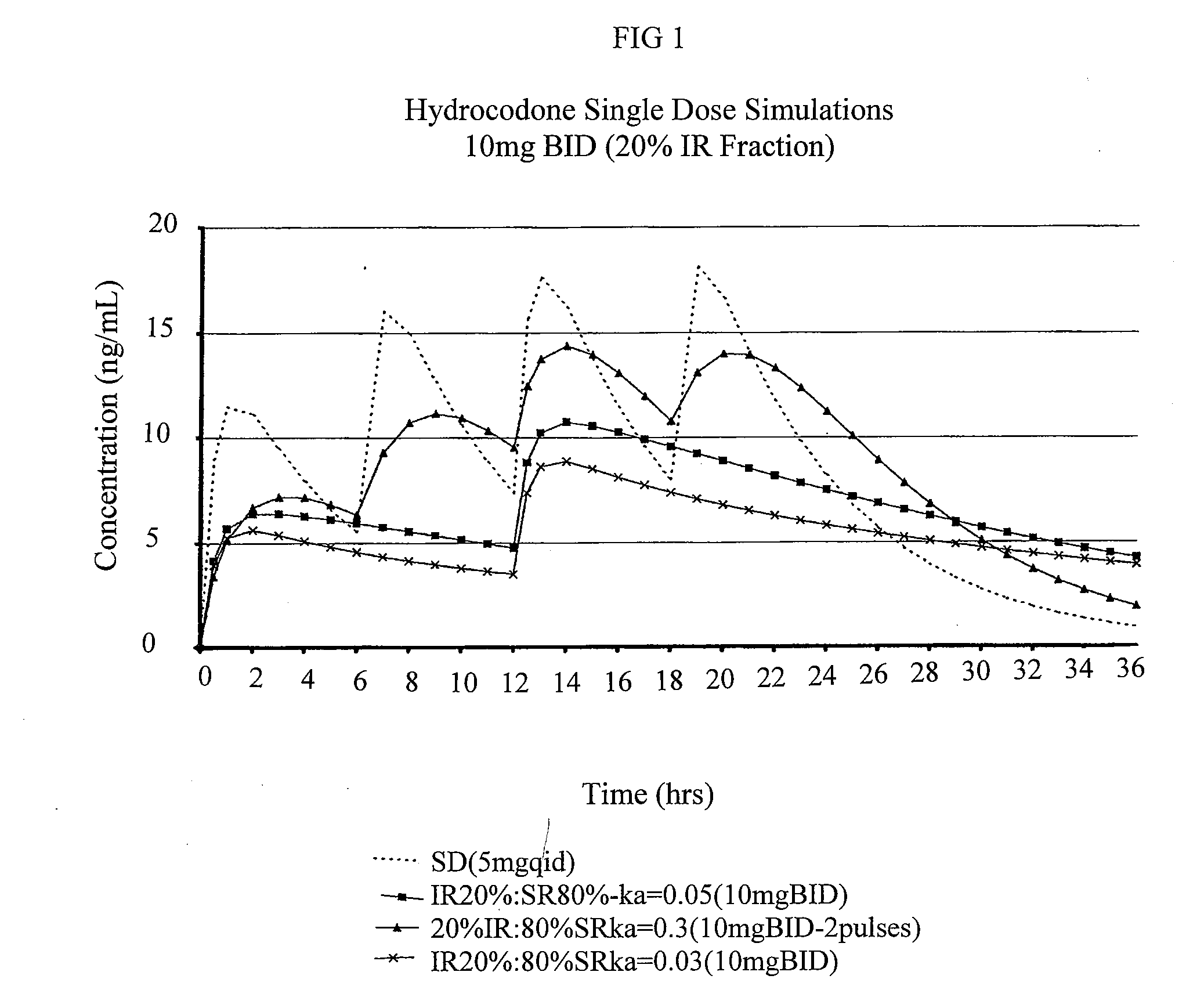
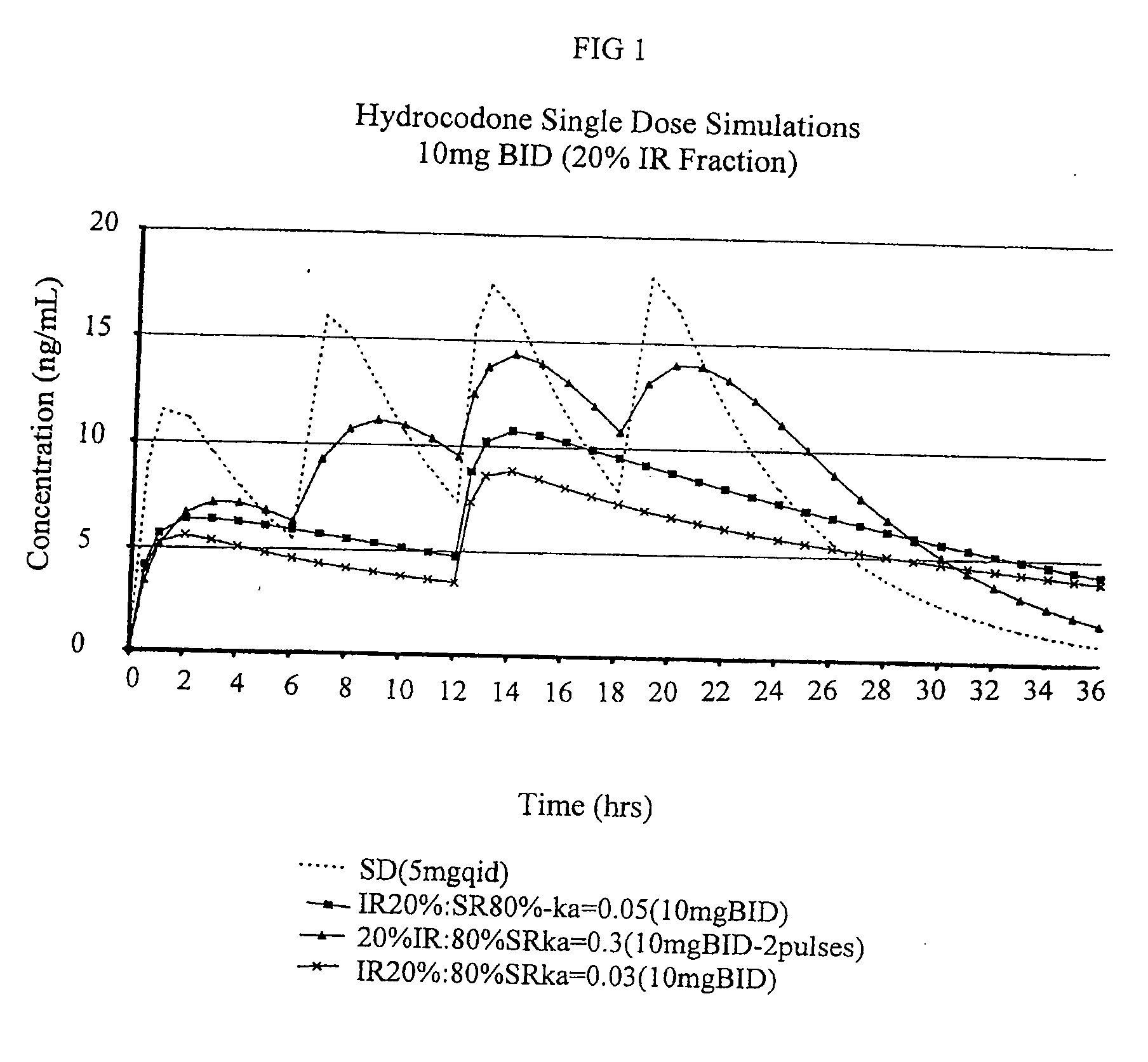
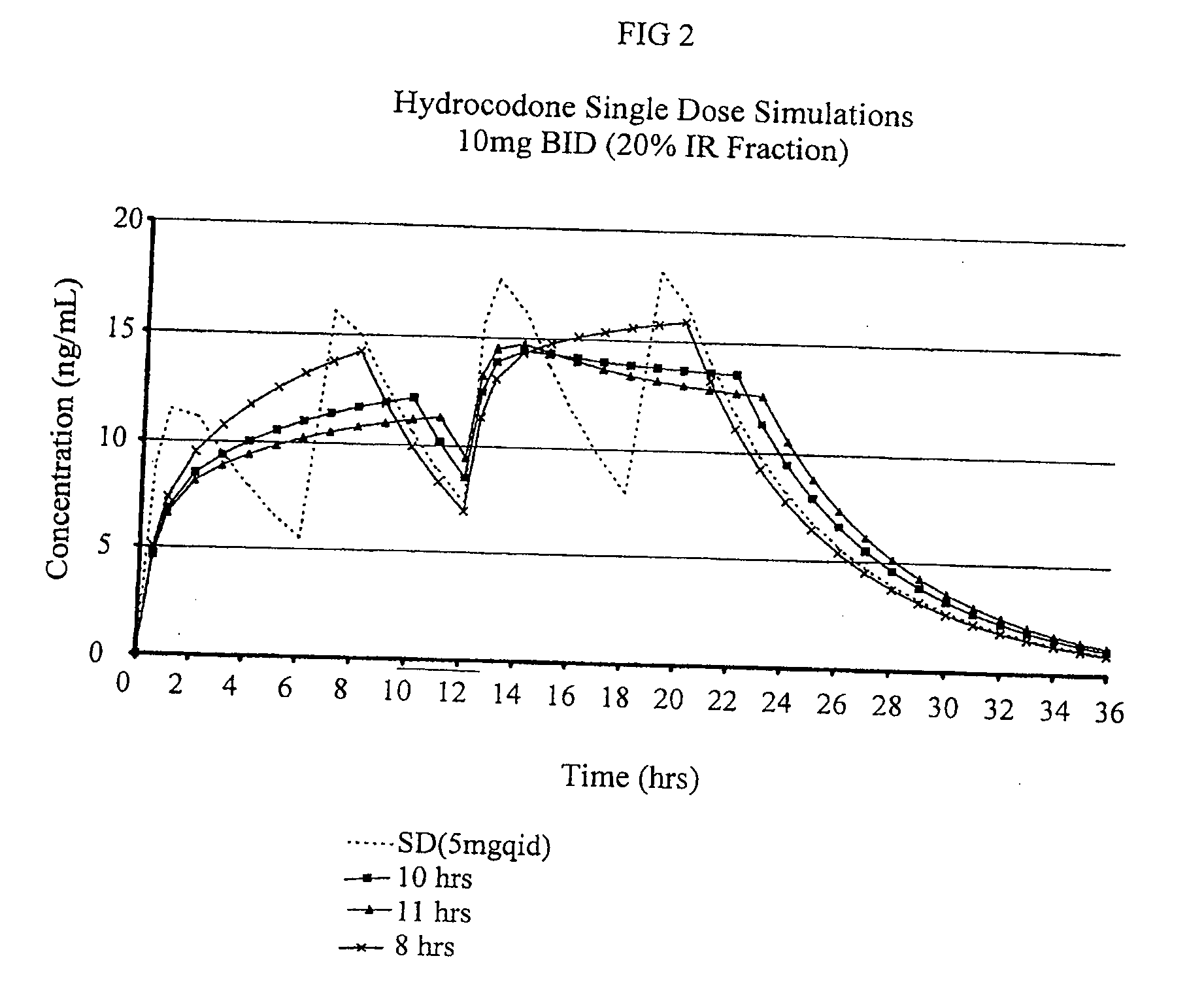
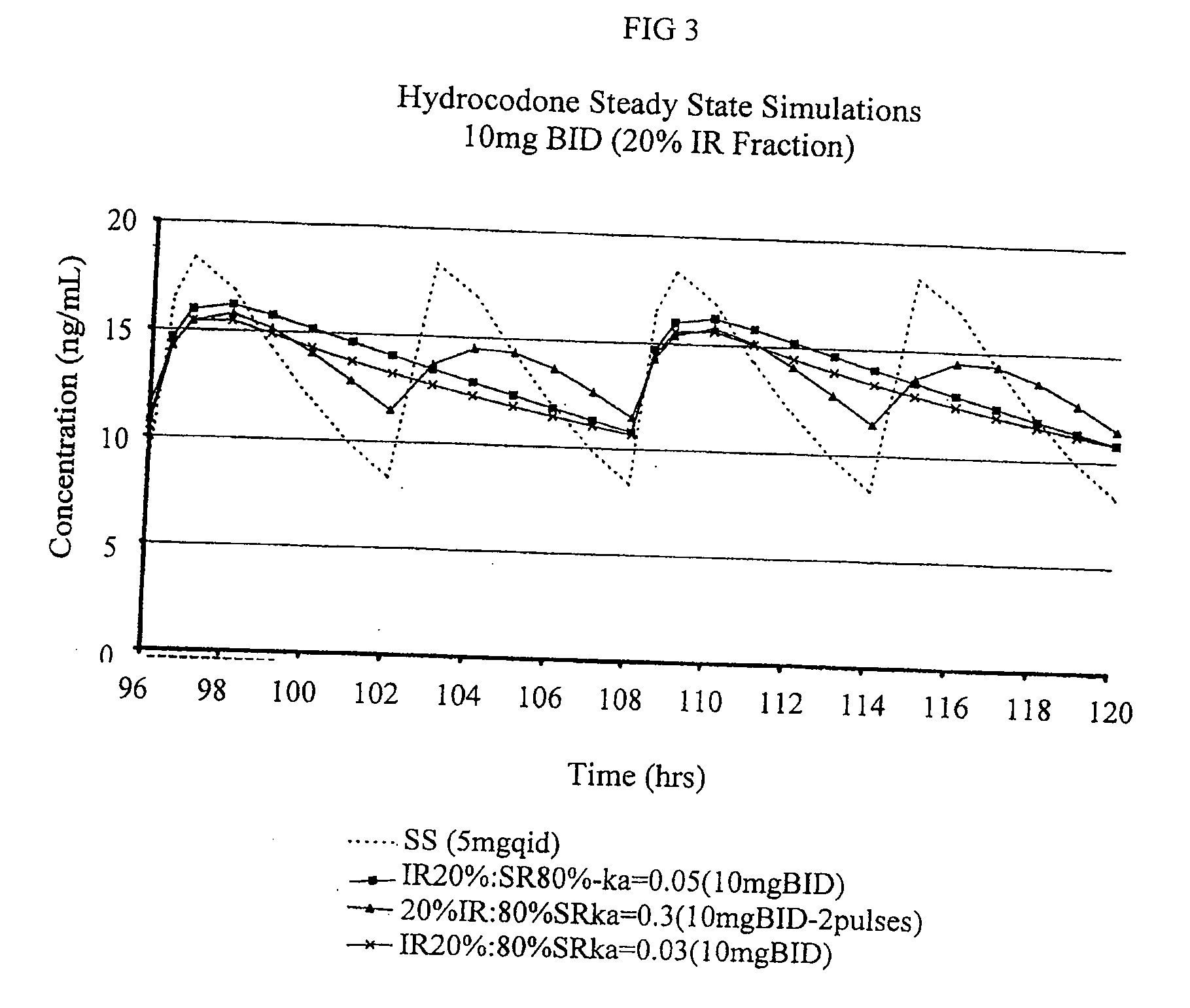
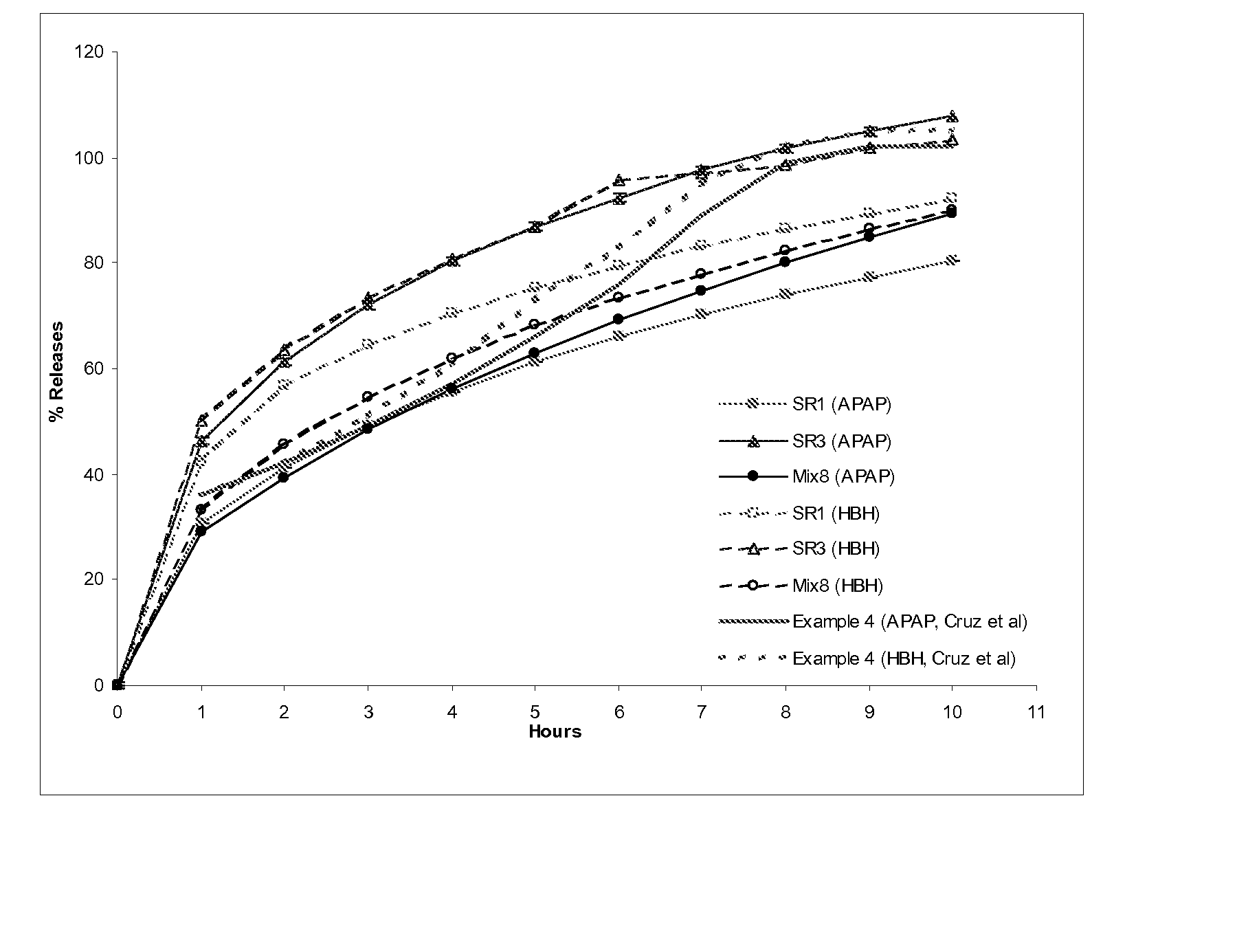
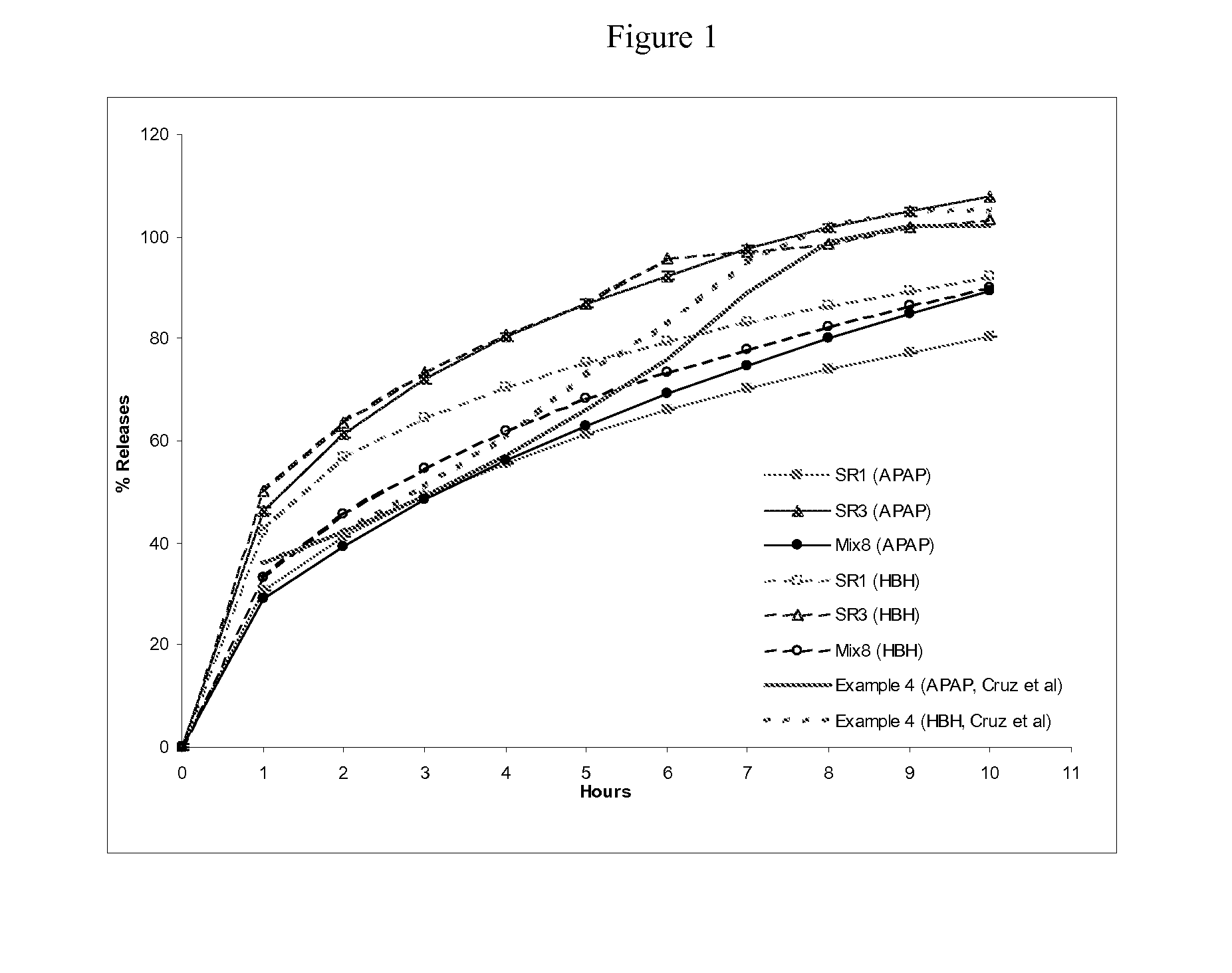
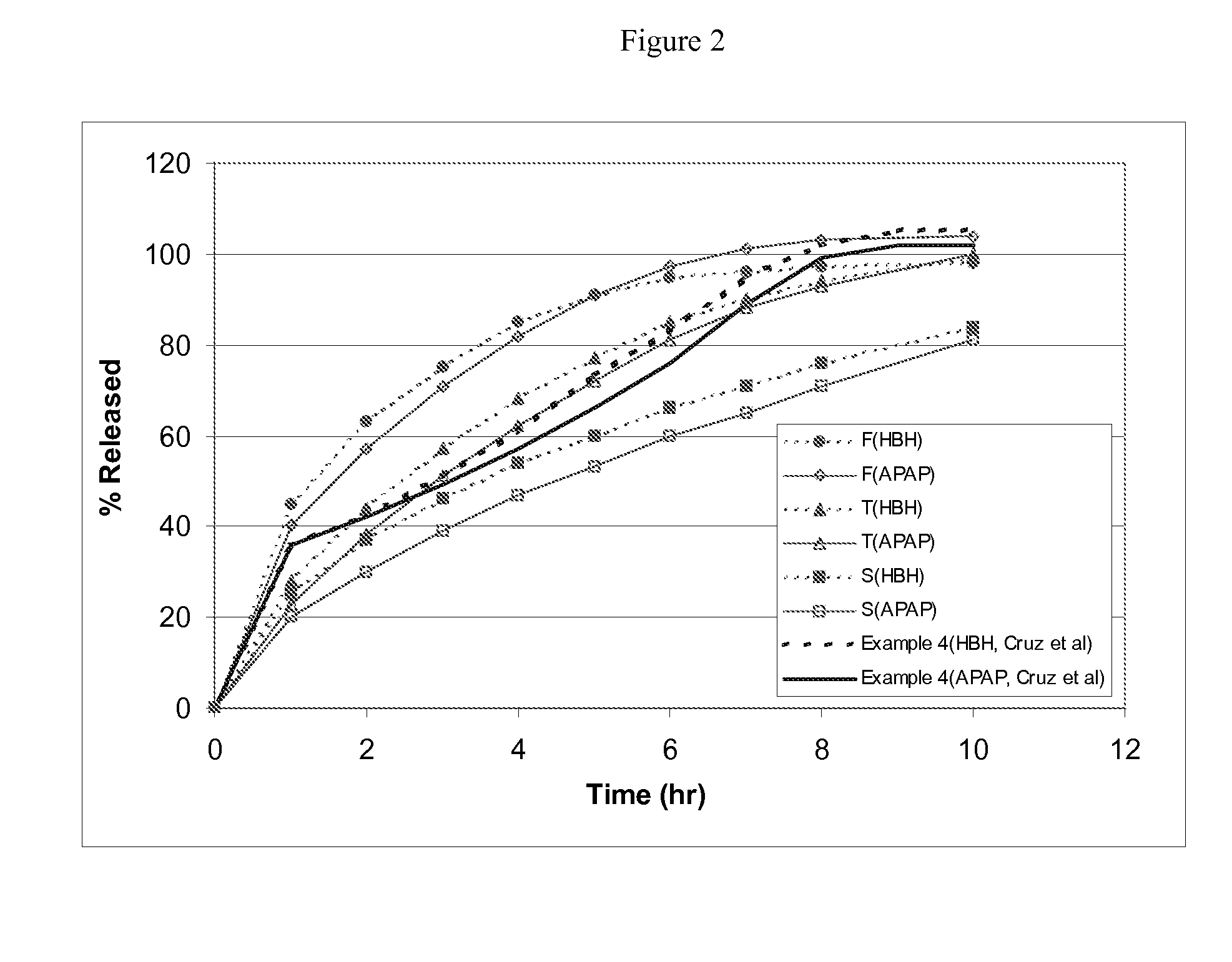
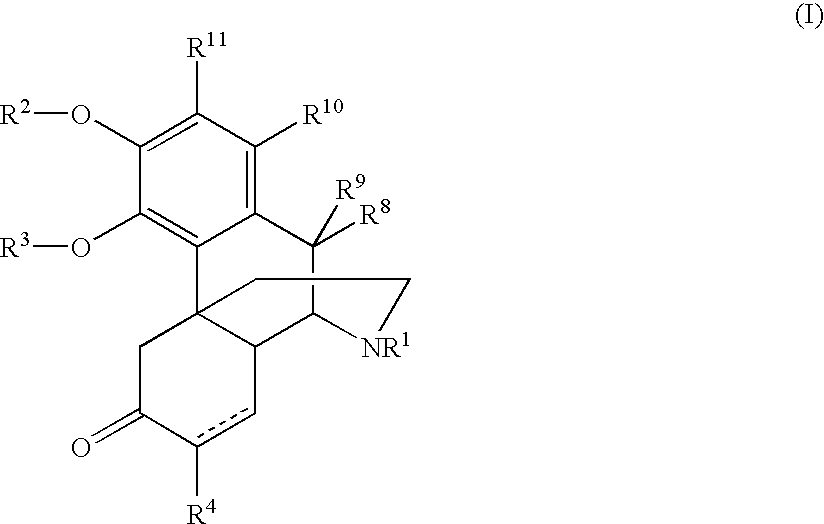
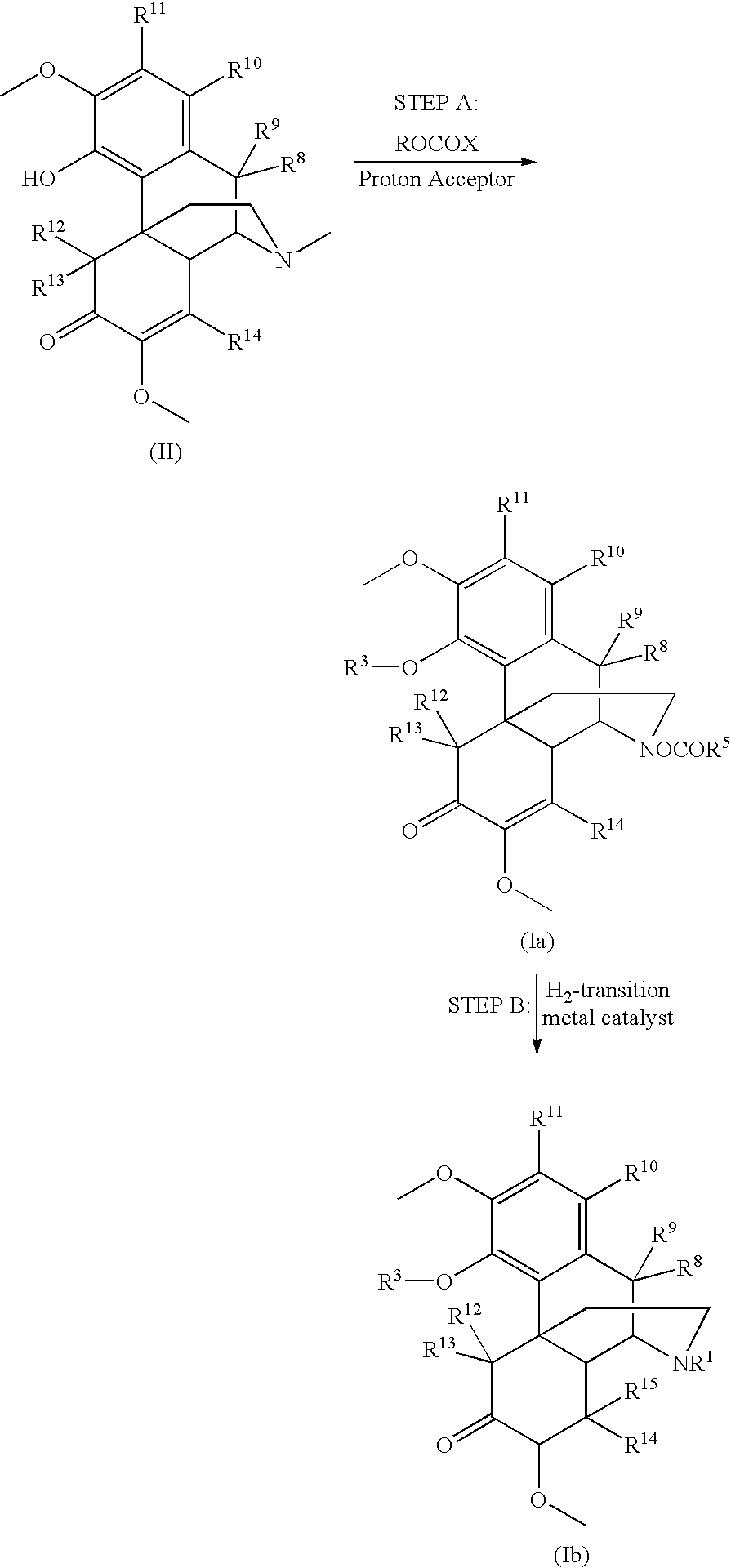
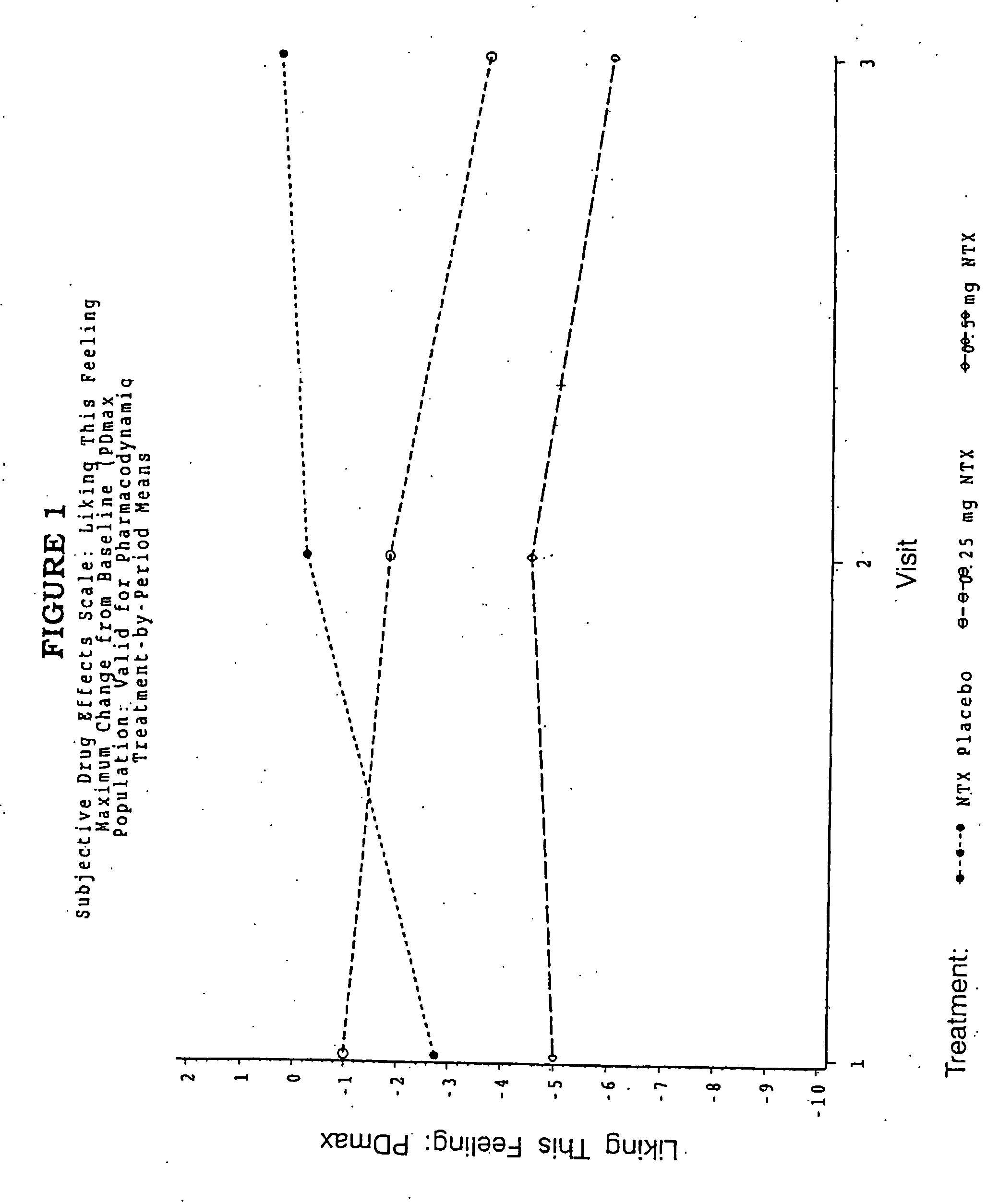
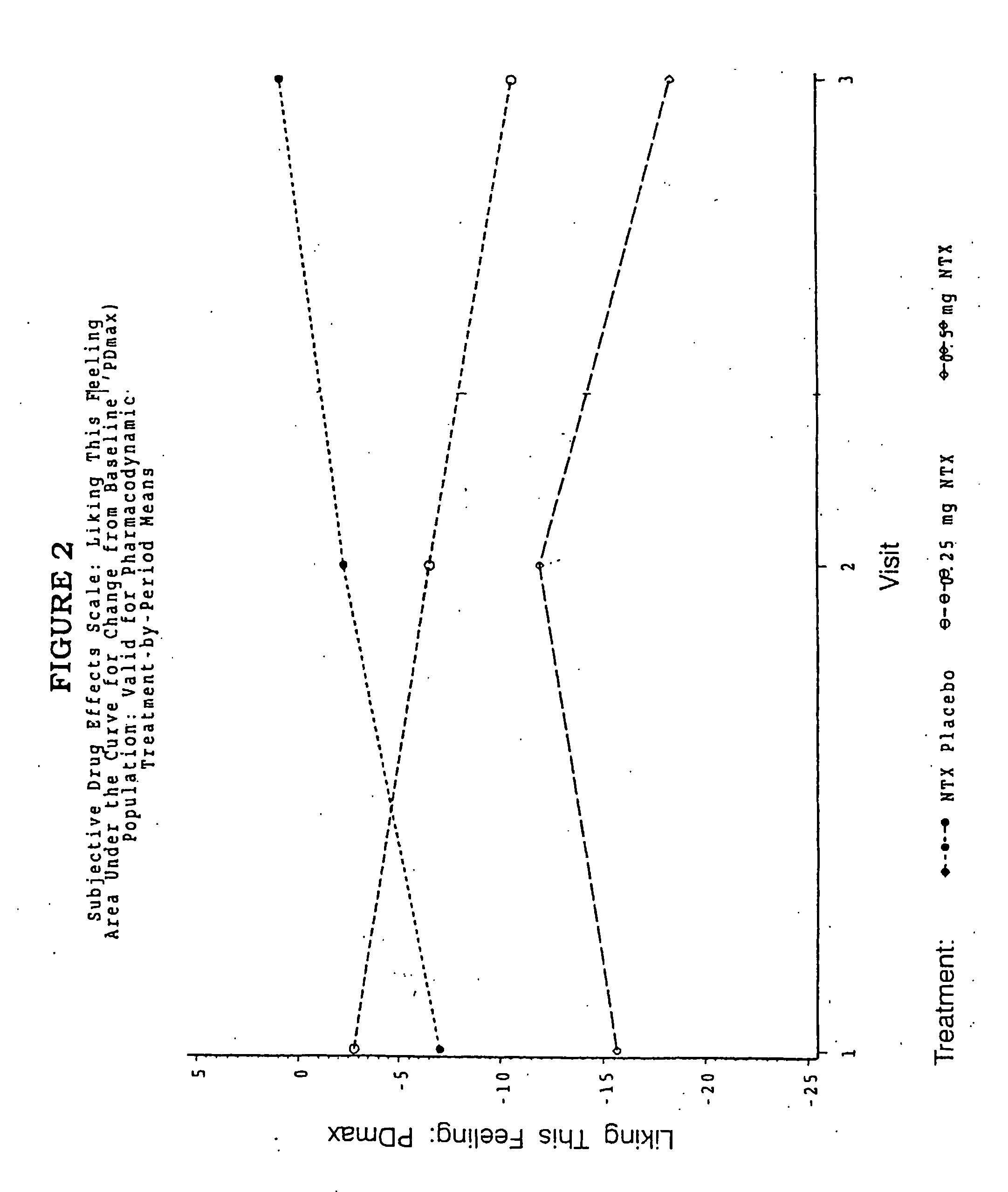
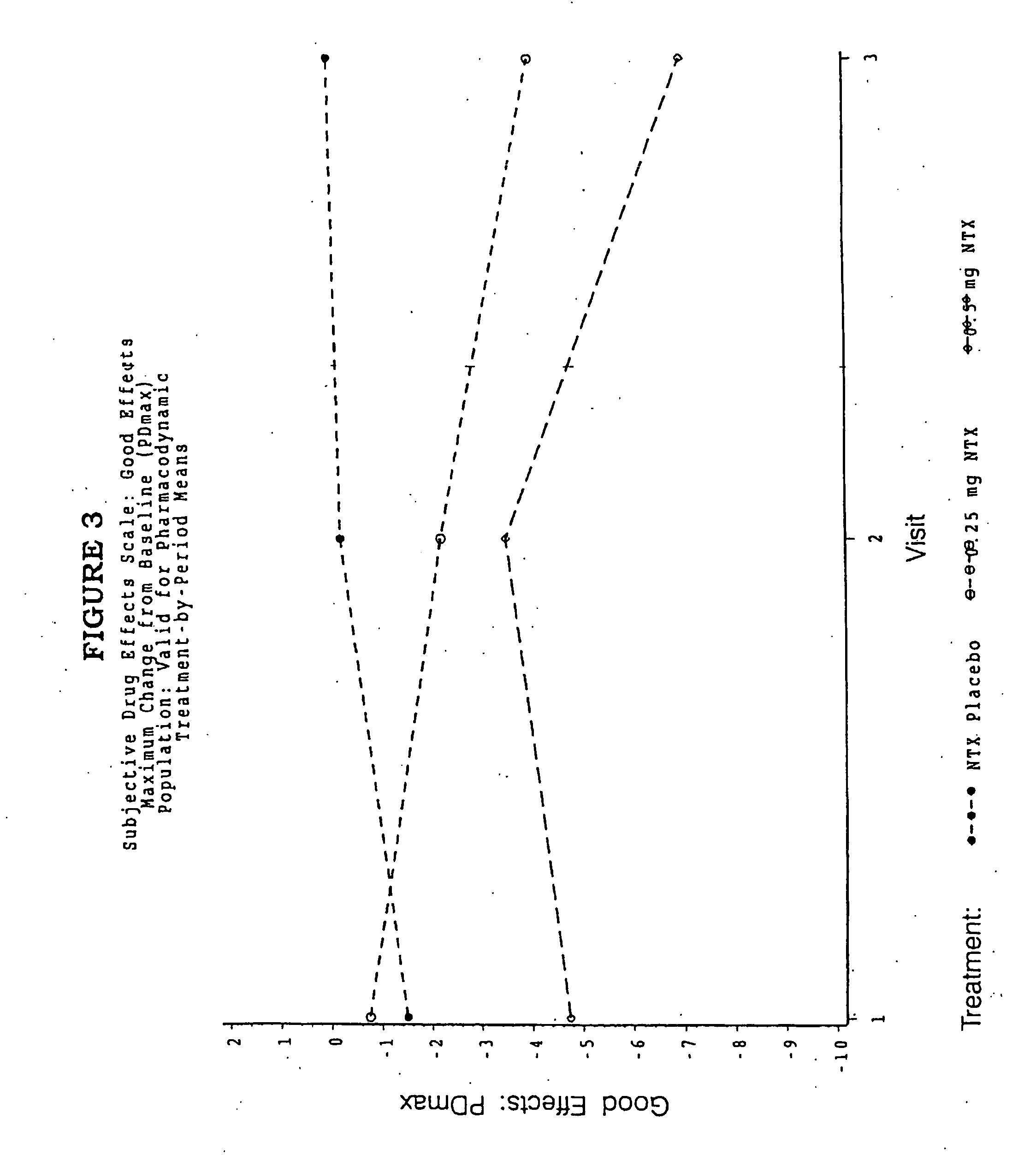
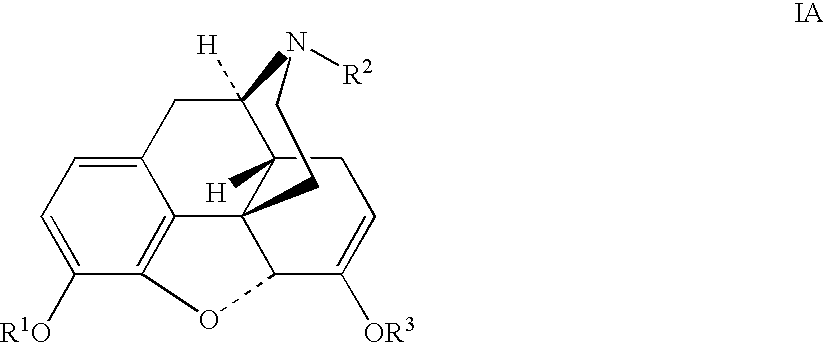
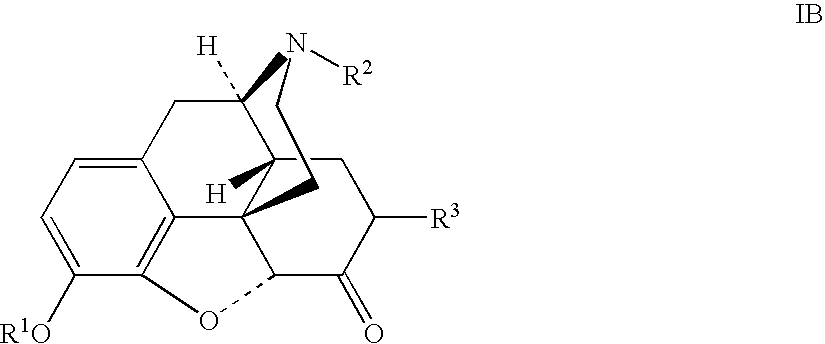
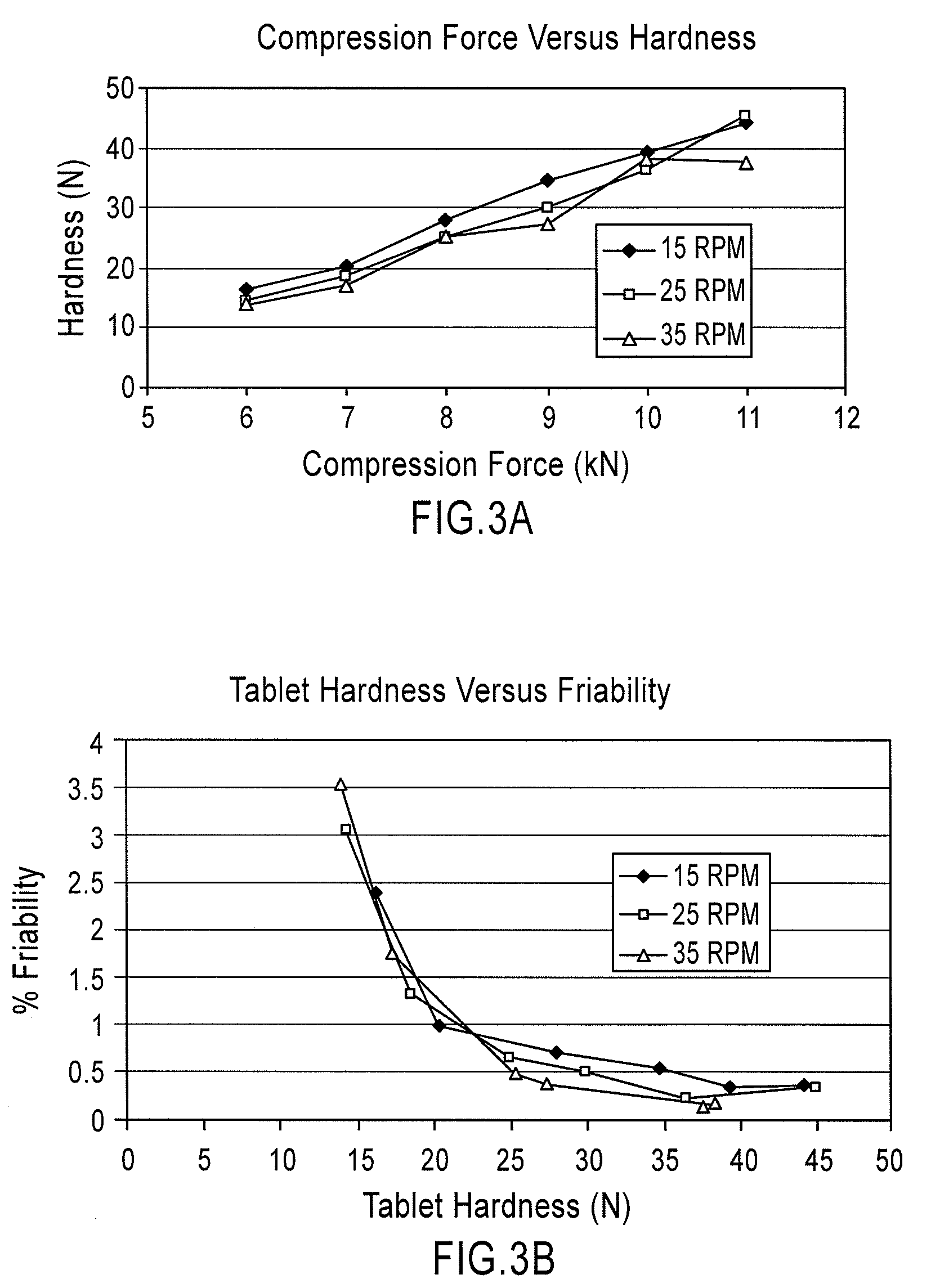
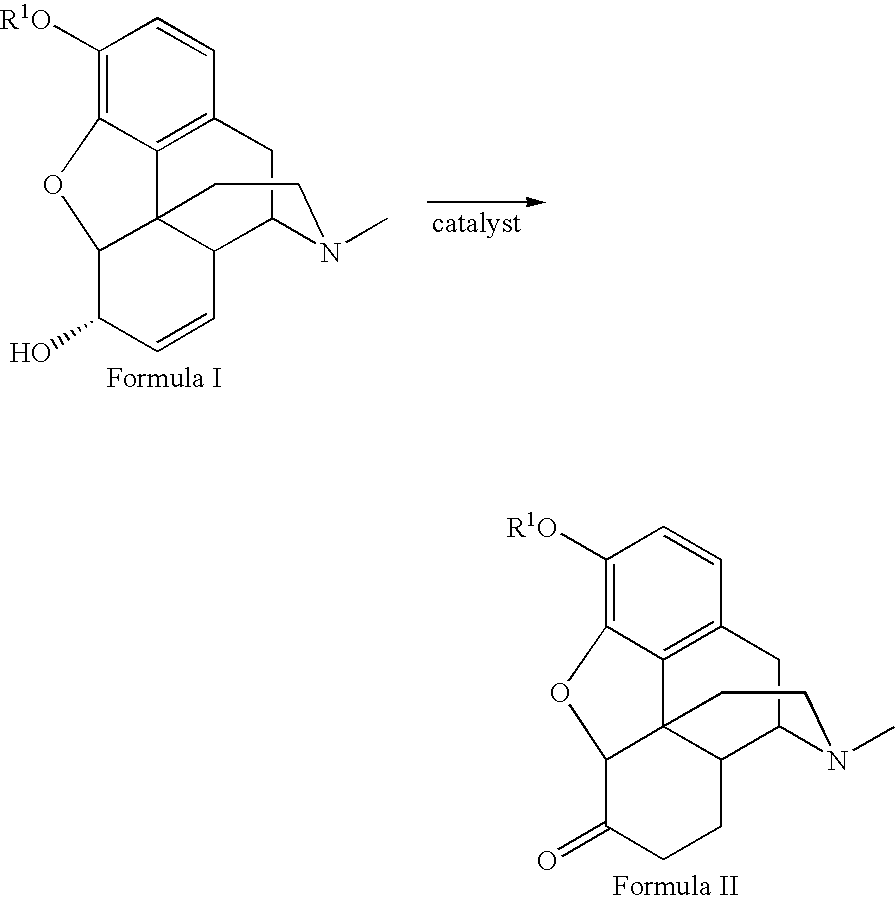
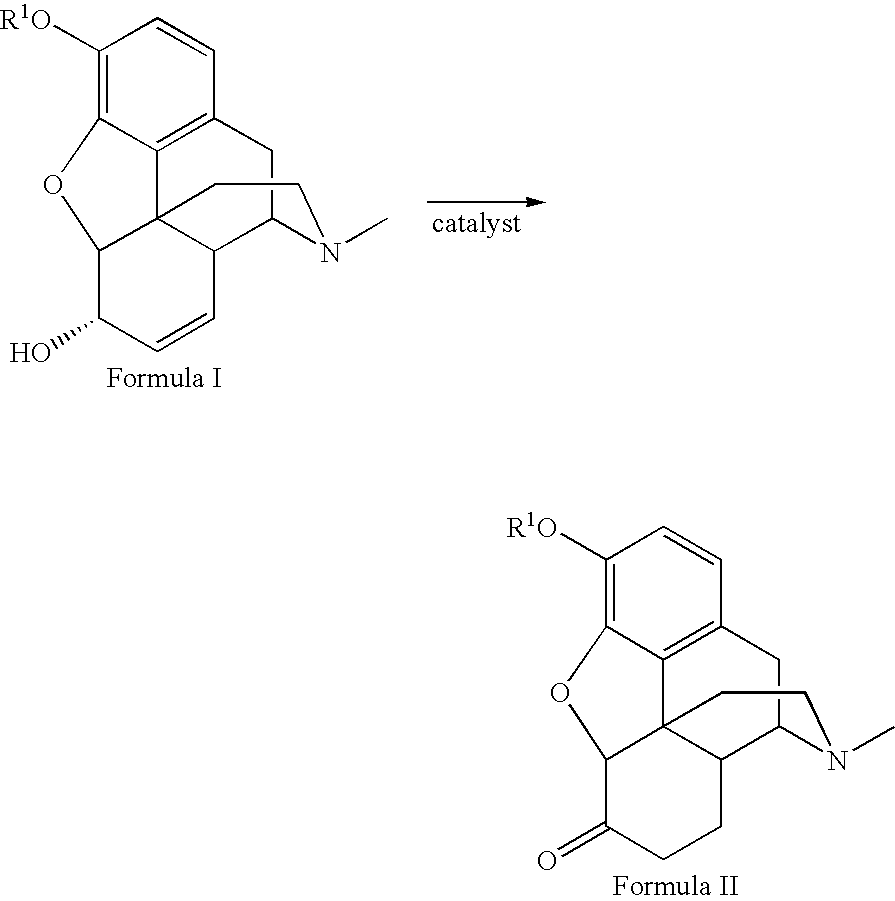
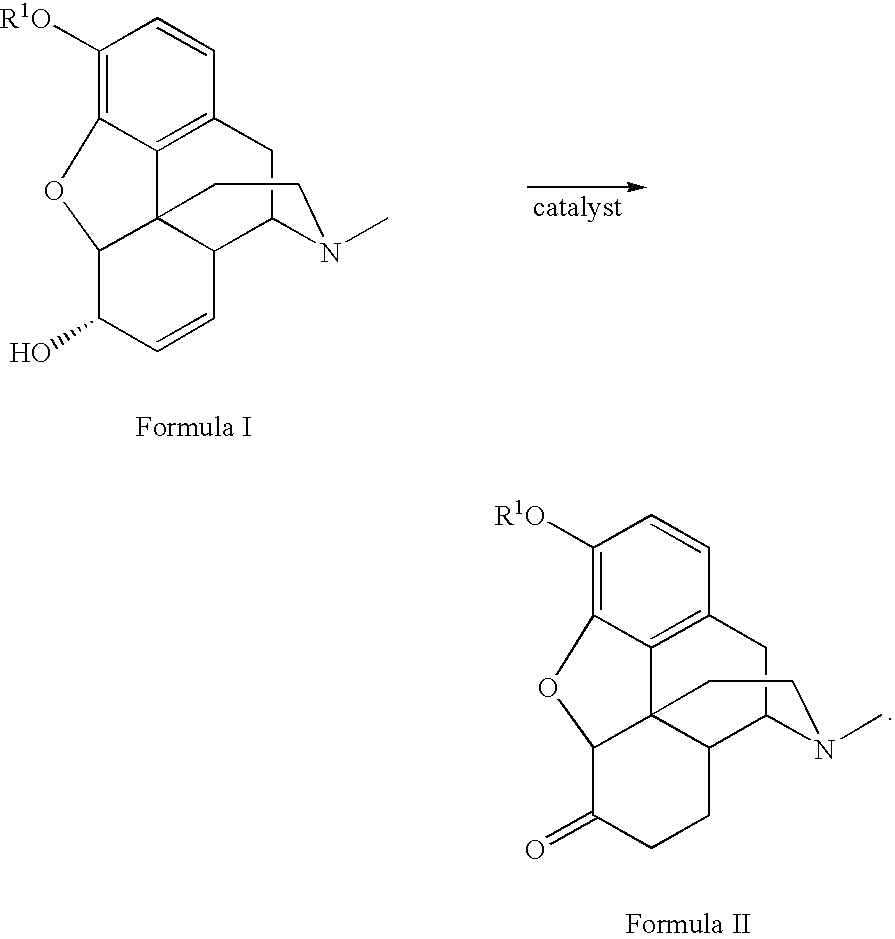
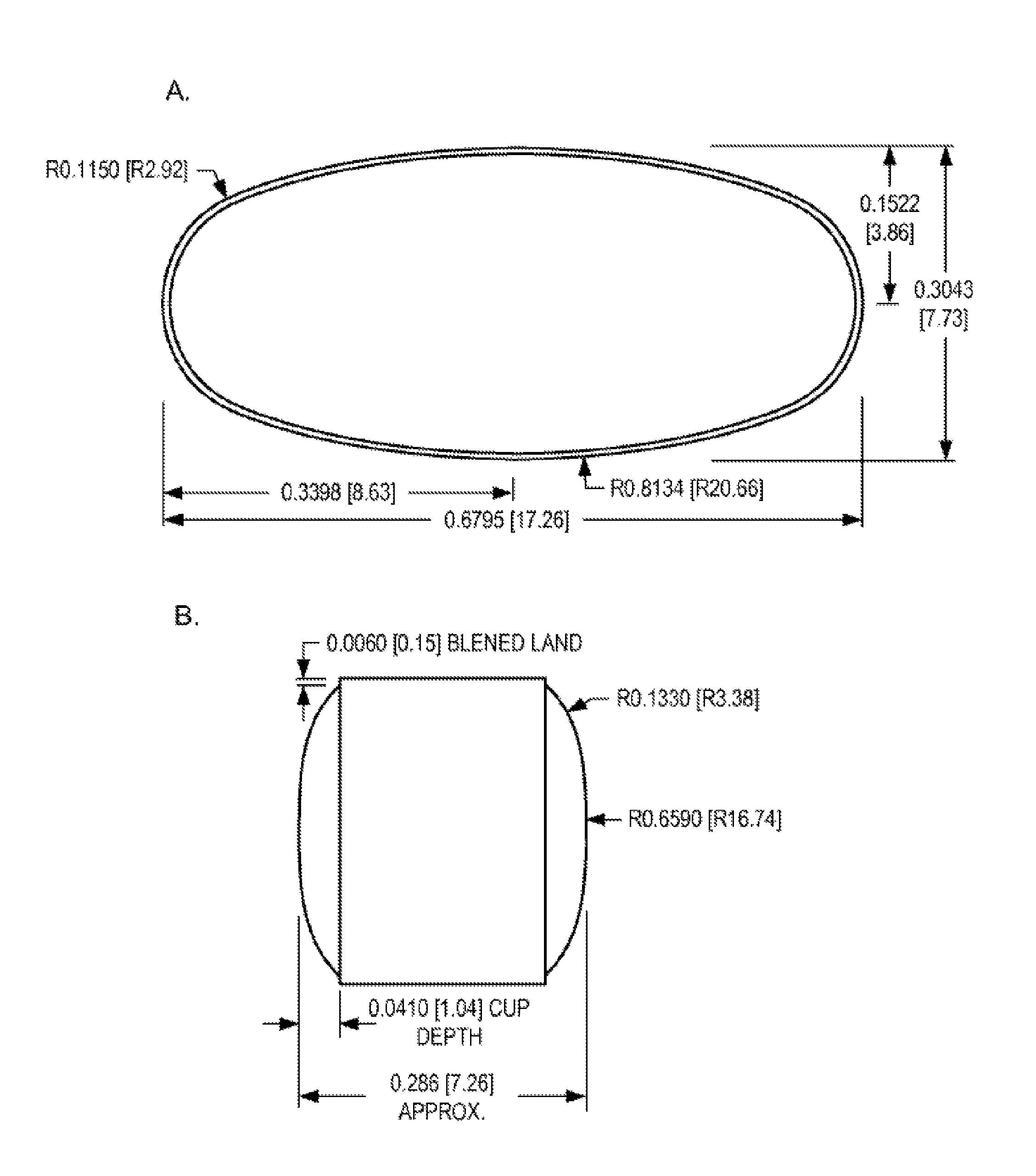
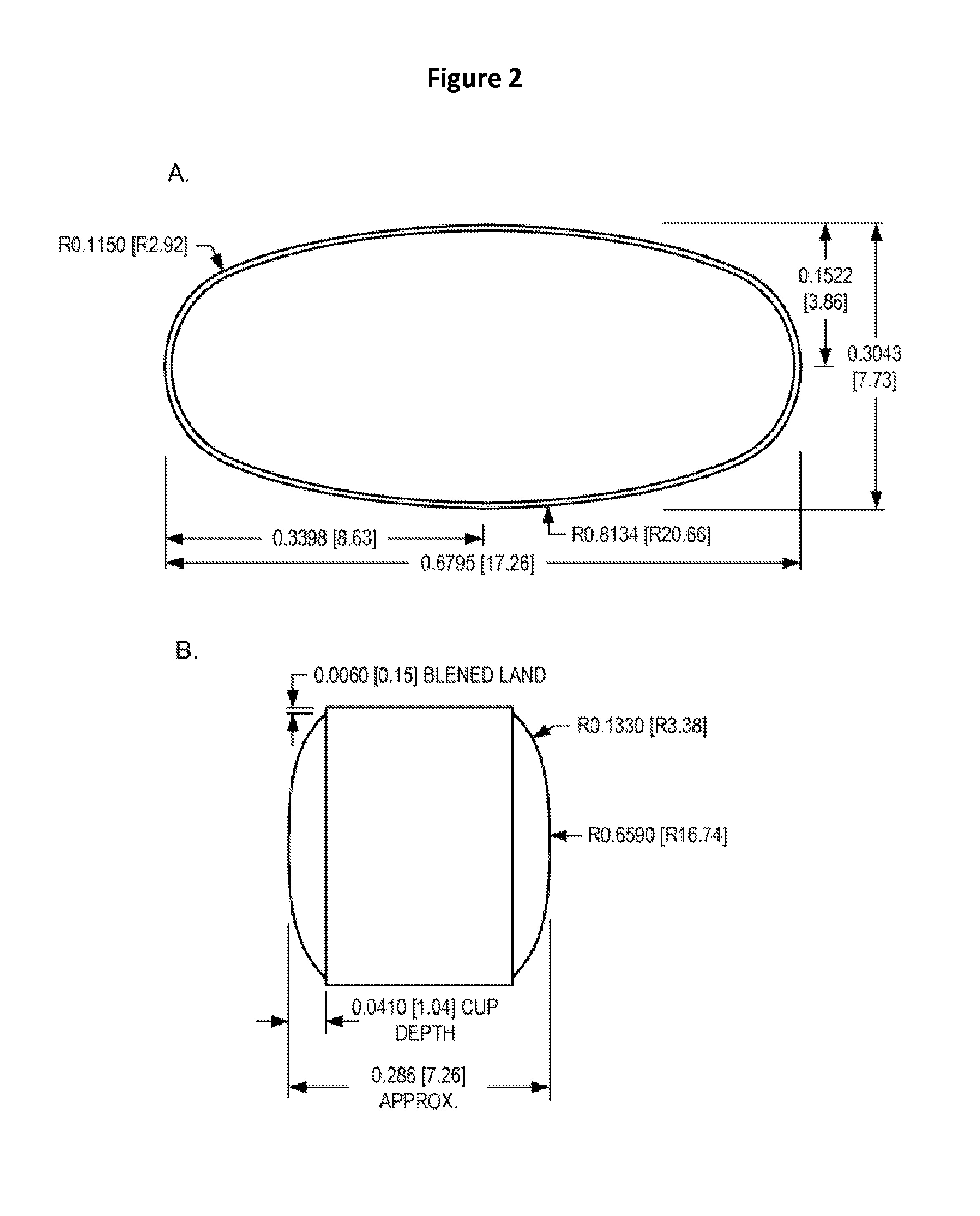
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
78 results about "Hydrocodone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to relieve a dry cough usually caused by the common cold or other conditions as prescribed by your doctor.
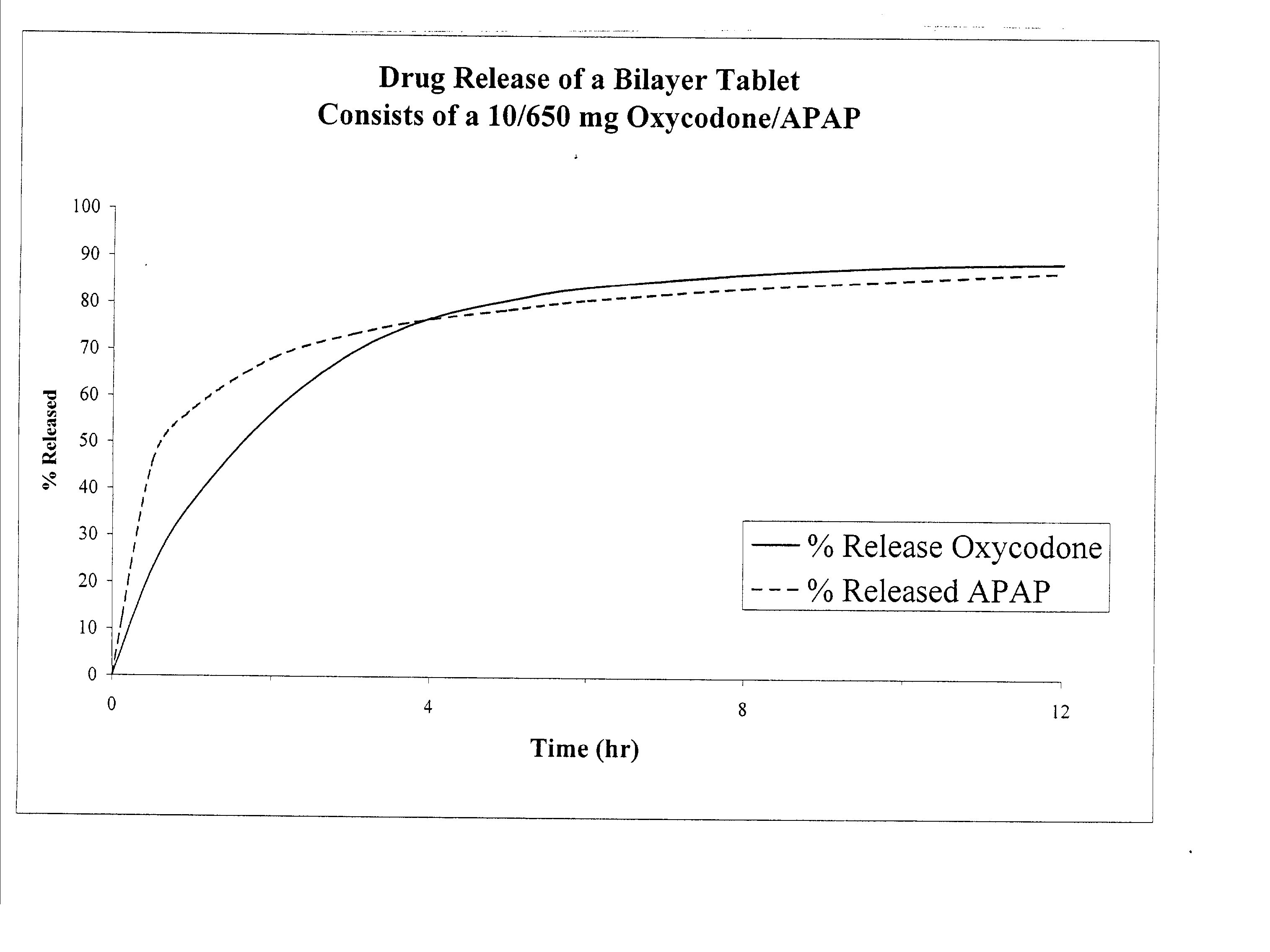
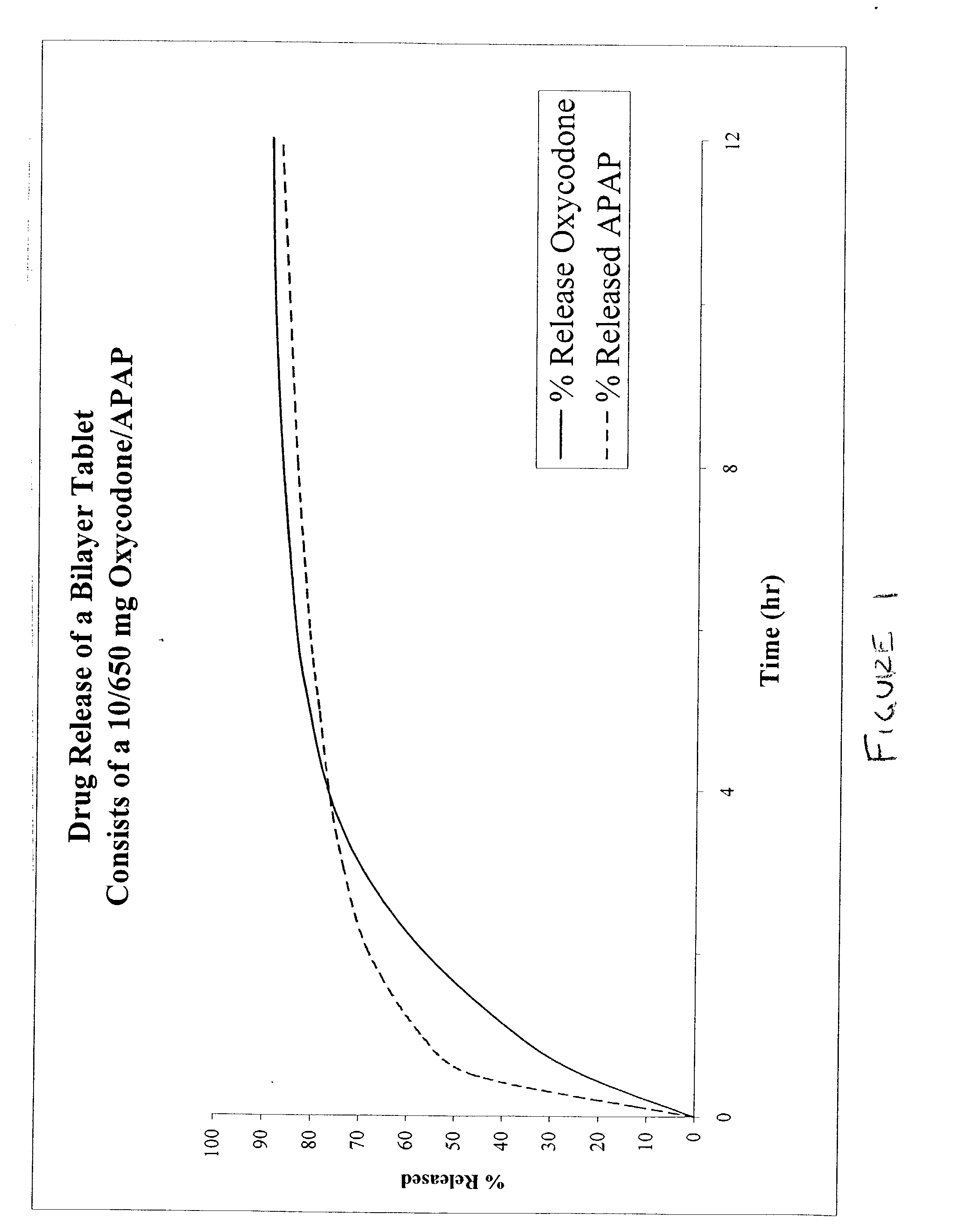
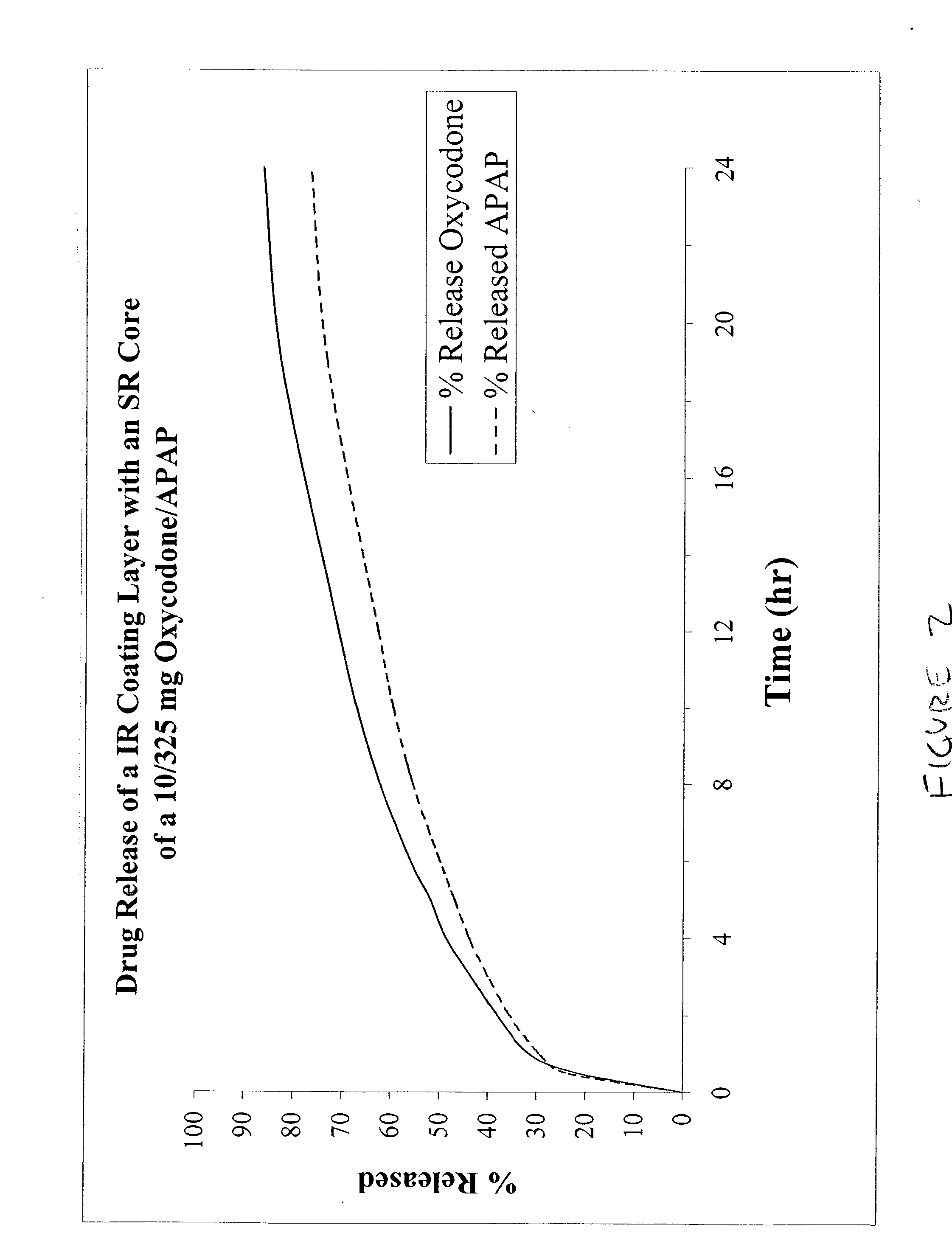
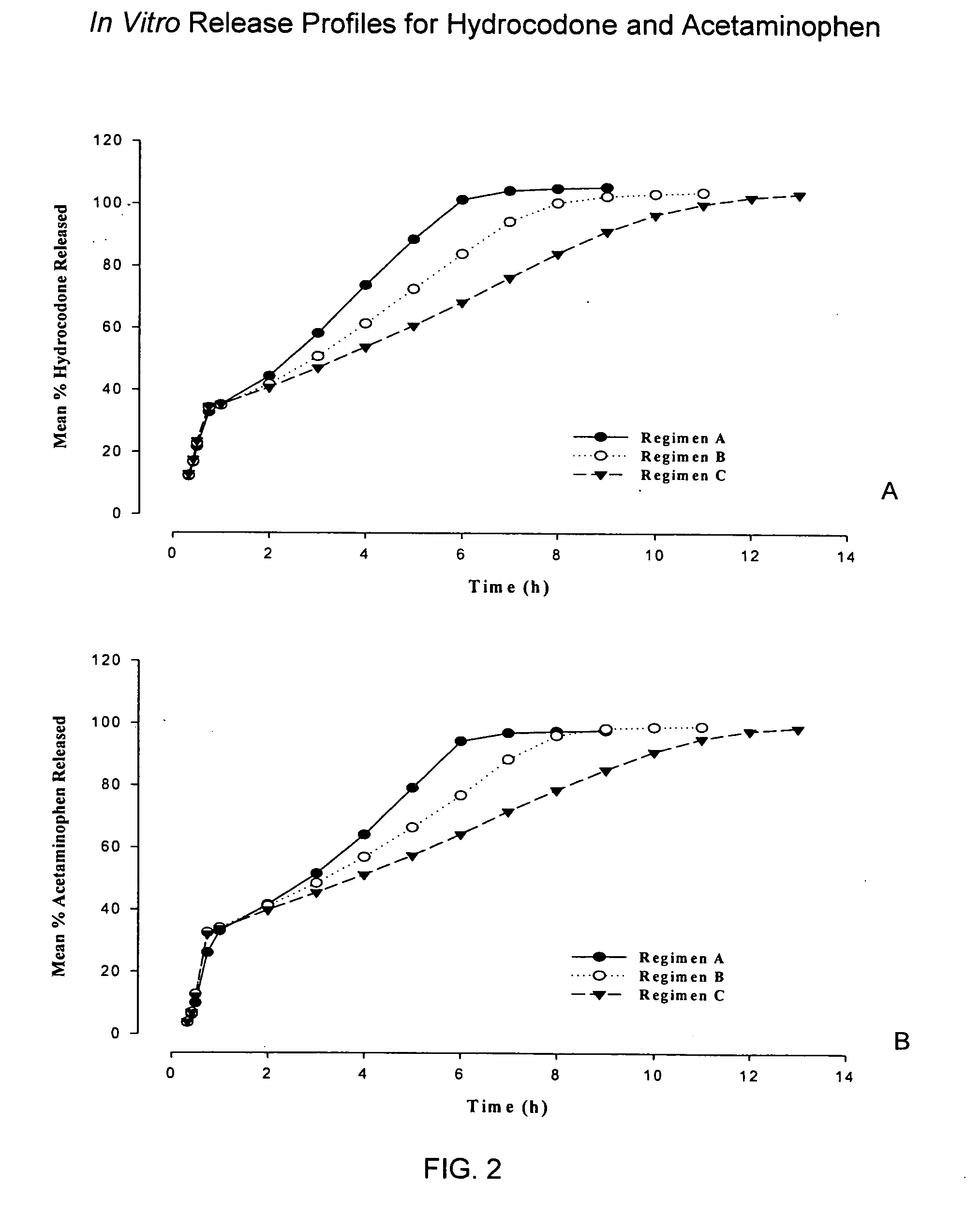
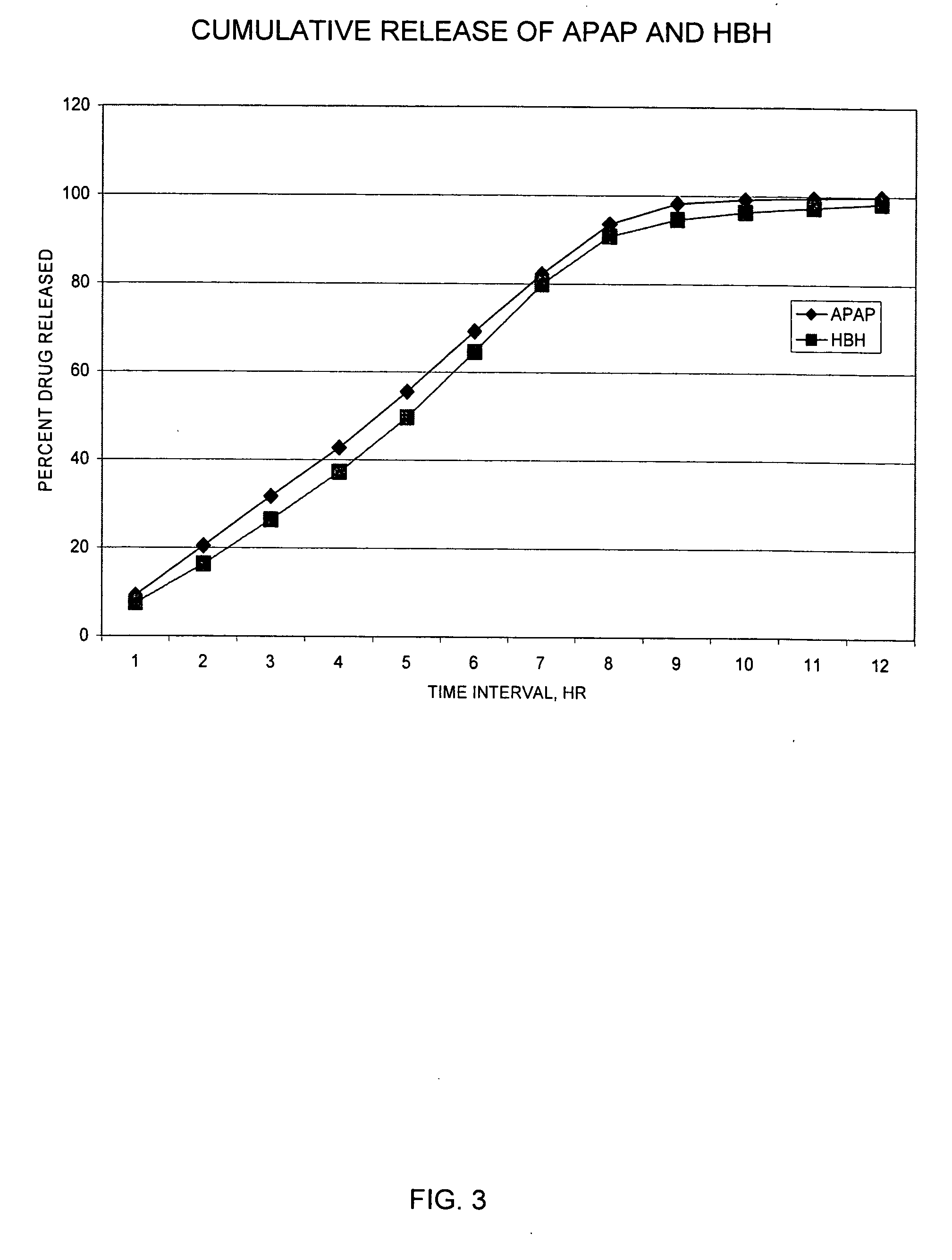
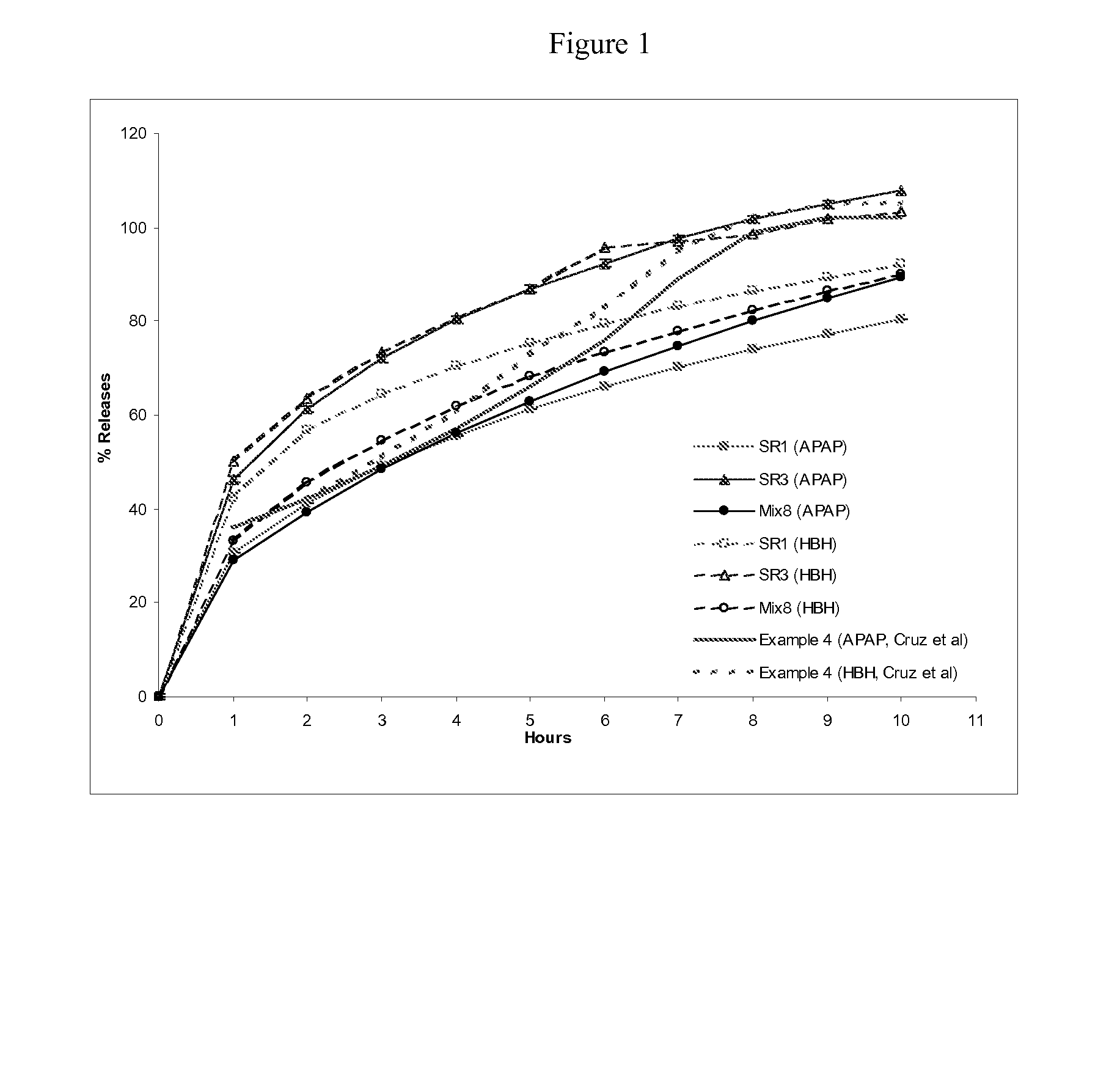
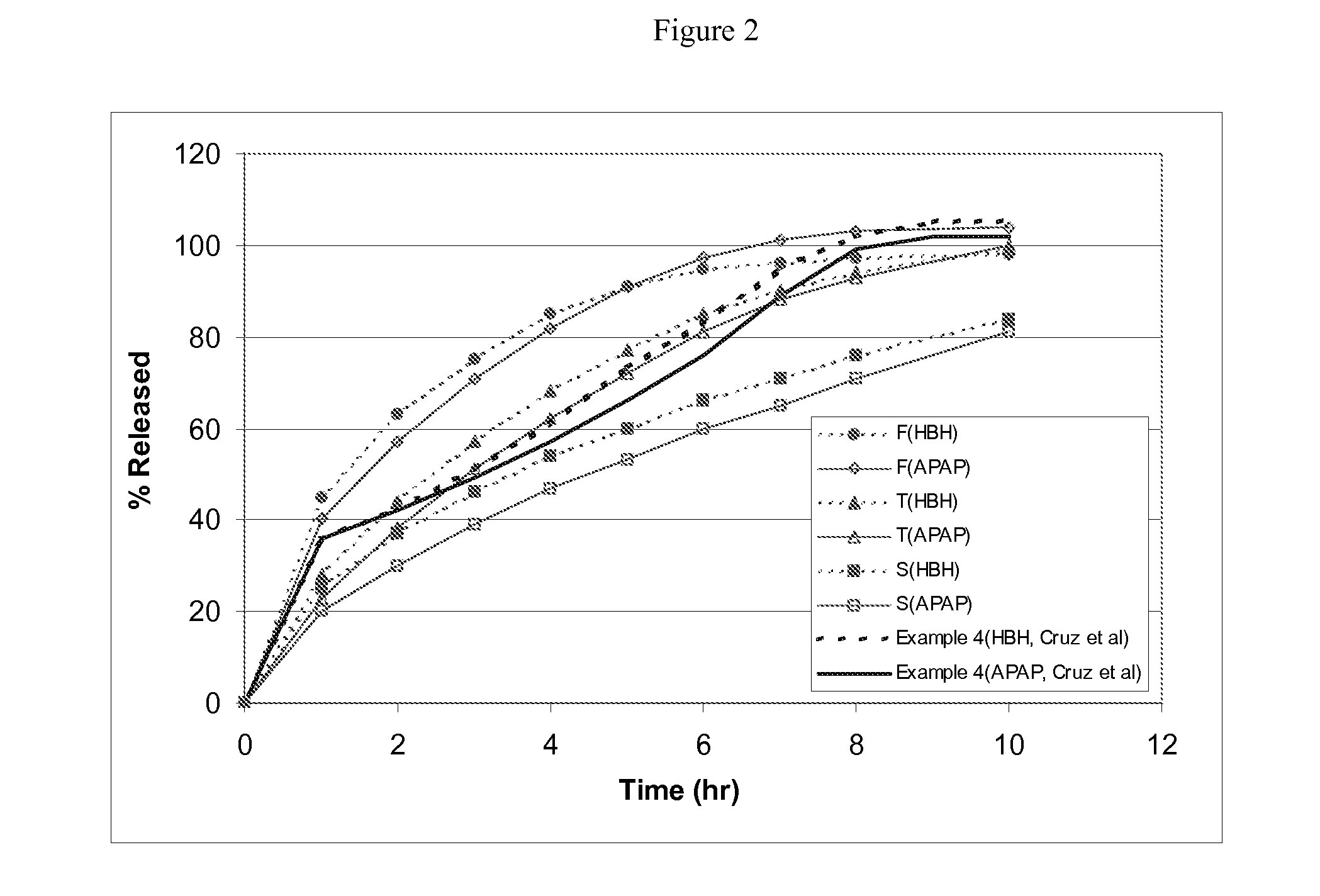
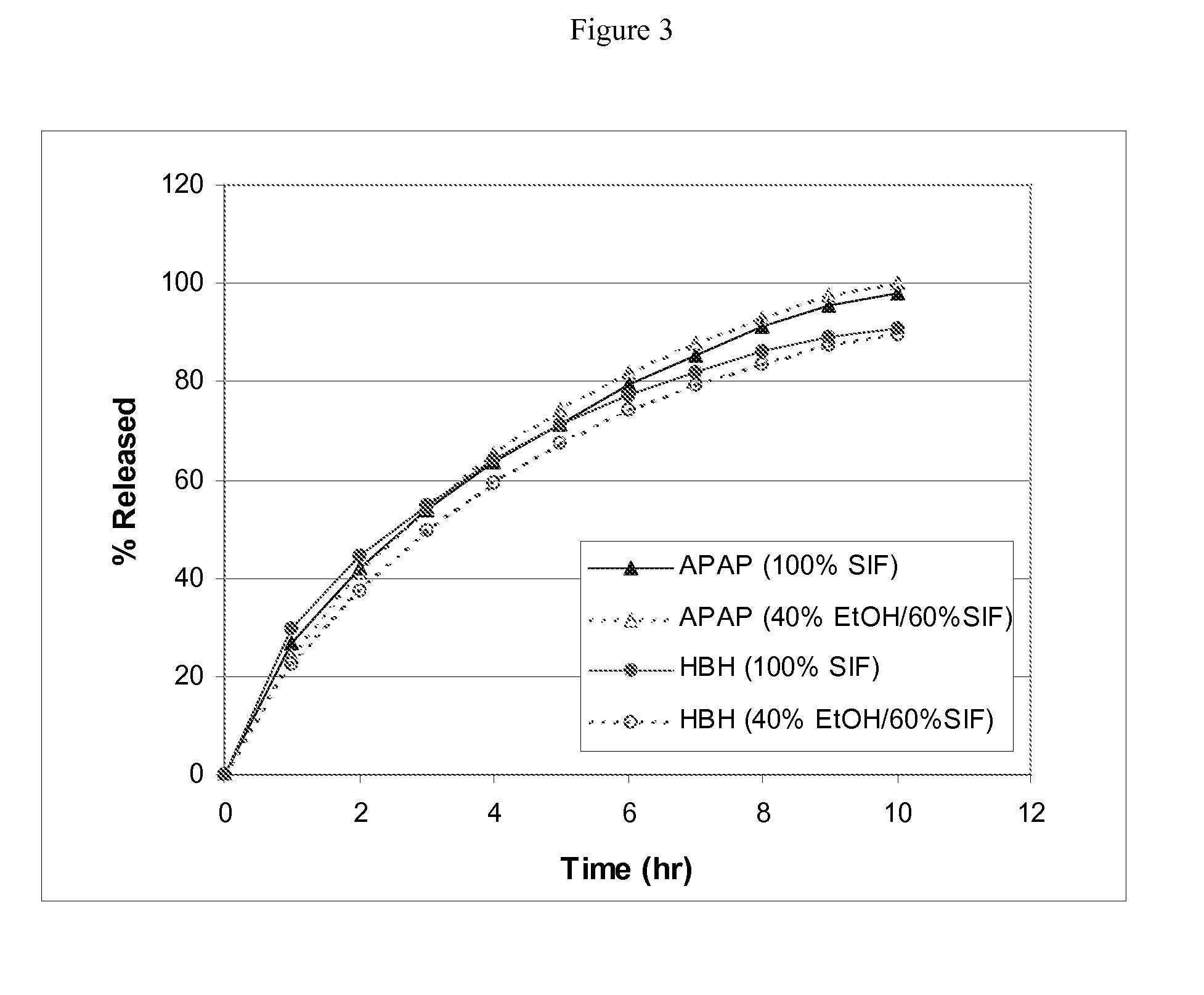
Combination sustained release-immediate release oral dosage forms with an opioid analgesic and a non-opioid analgesic
InactiveUS20030092724A1Long durationConstant plasma levels of opioid and non-opioid analgesicsBiocidePill deliveryImmediate releaseTherapeutic effect
The present invention relates to new and useful oral tablet compositions which include an immediate release portion having an opioid analgesic and a non-opioid analgesic, providing for a rapid onset of therapeutic effect, and a sustained release portion of an opioid analgesic and a non-opioid analgesic, providing for a relatively longer duration of therapeutic effect. A multilayer oral dosage form containing a sustained release layer, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release layer containing the same active ingredients as the sustained release layer, is also disclosed. Also disclosed are oral tablet compositions, containing a sustained release core, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release coating containing the same active ingredients as the sustained release core, are also disclosed. In addition, methods of making and using such oral tablet compositions are disclosed.
Owner:ENDO PHARMA INC
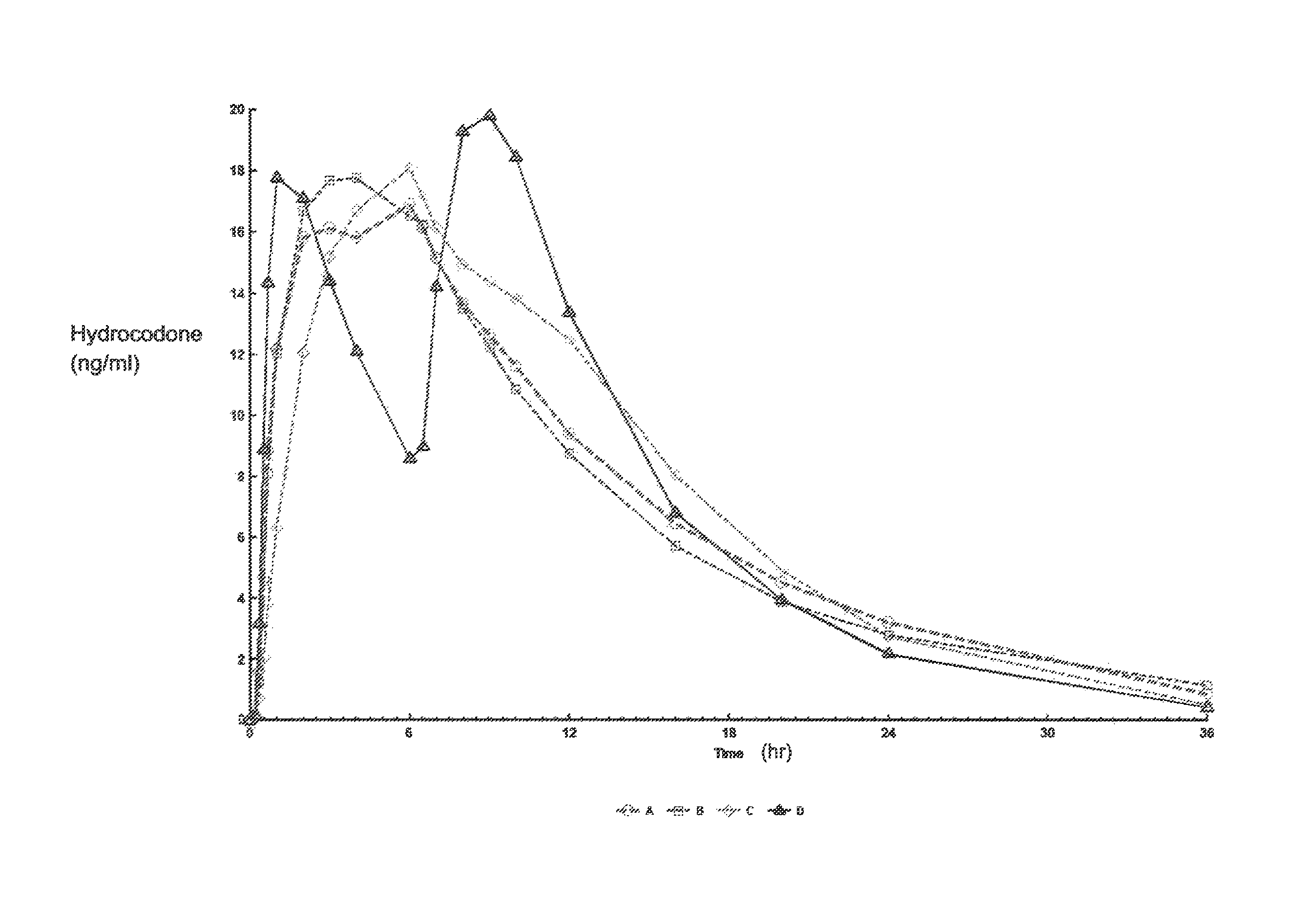
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
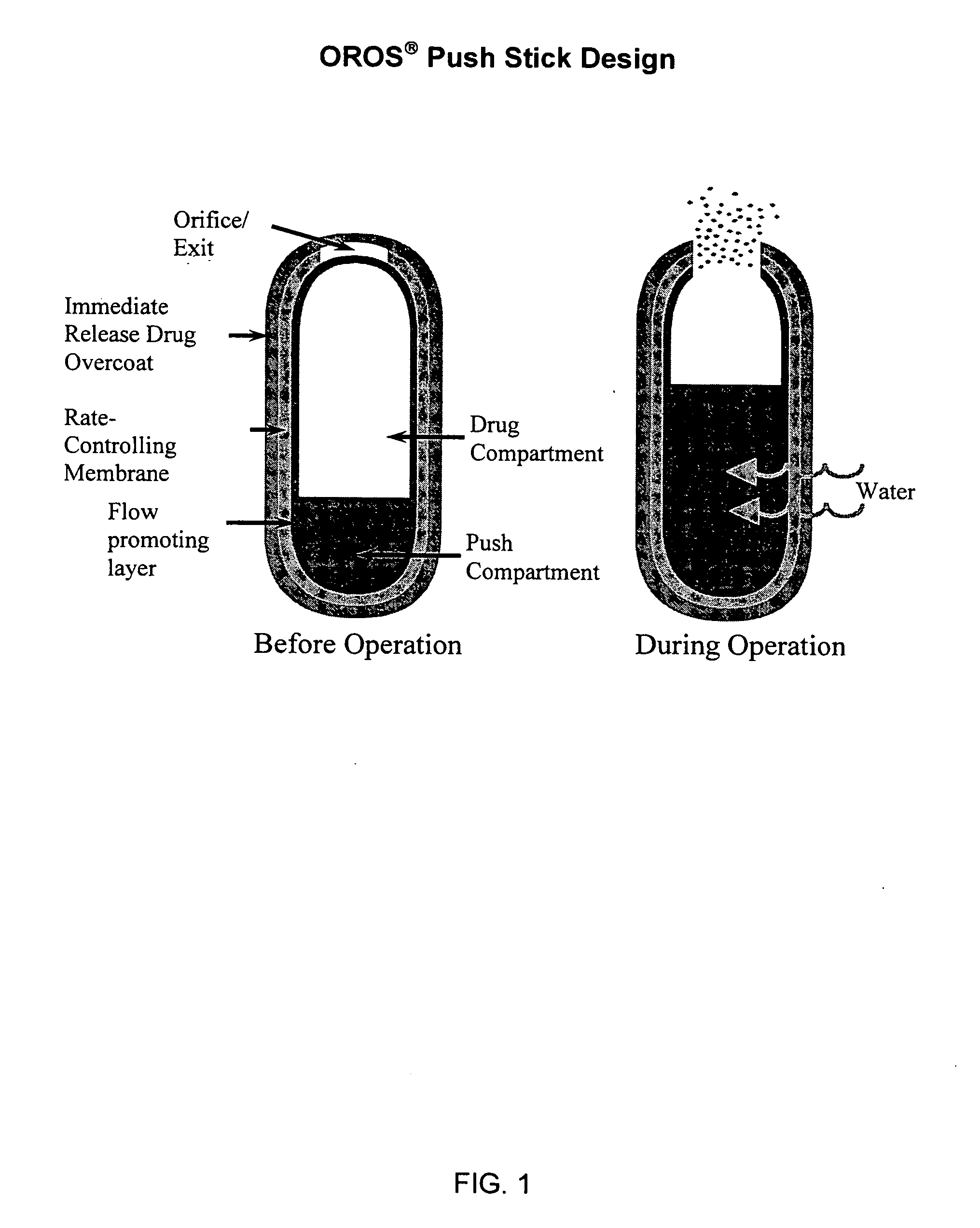
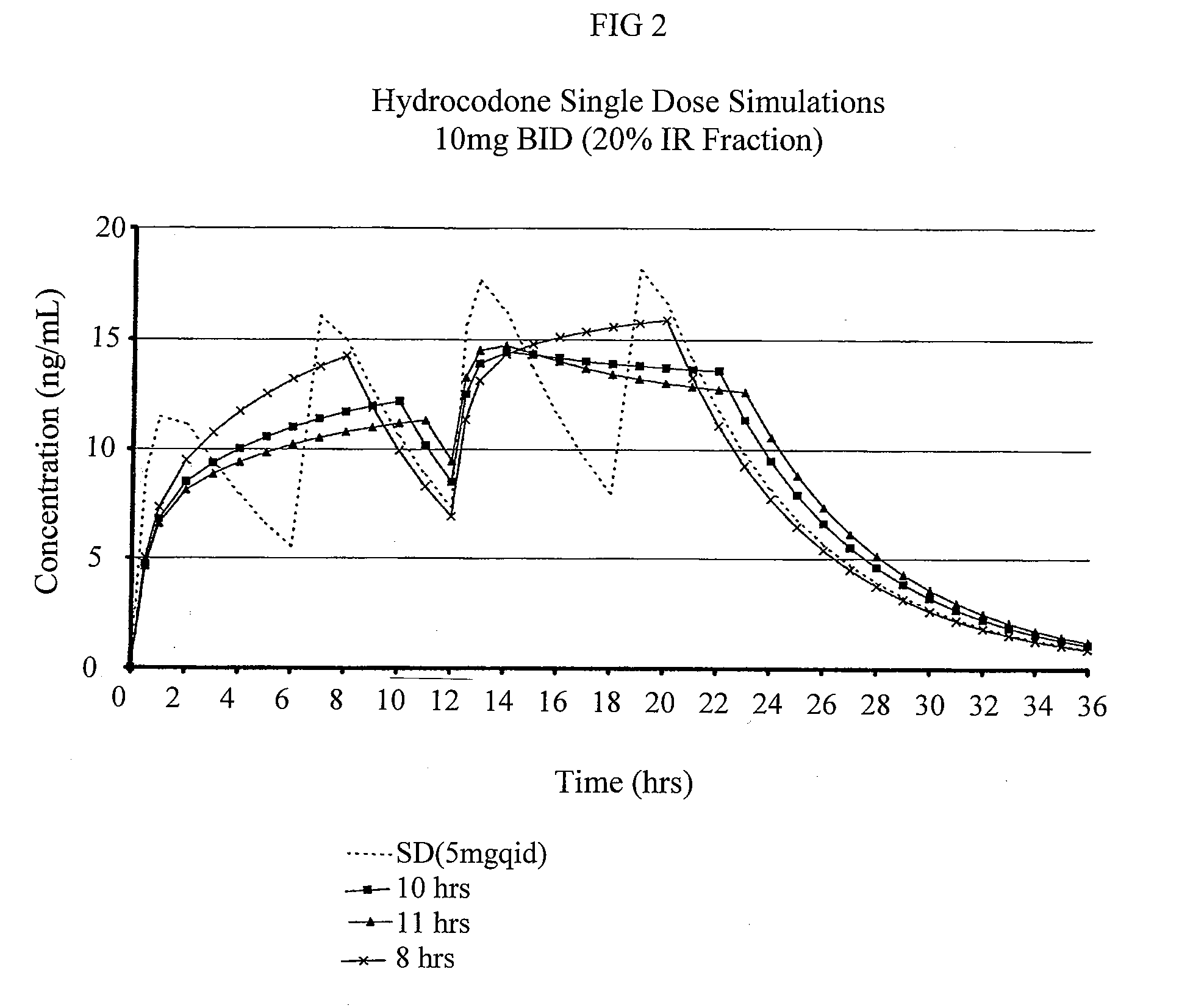
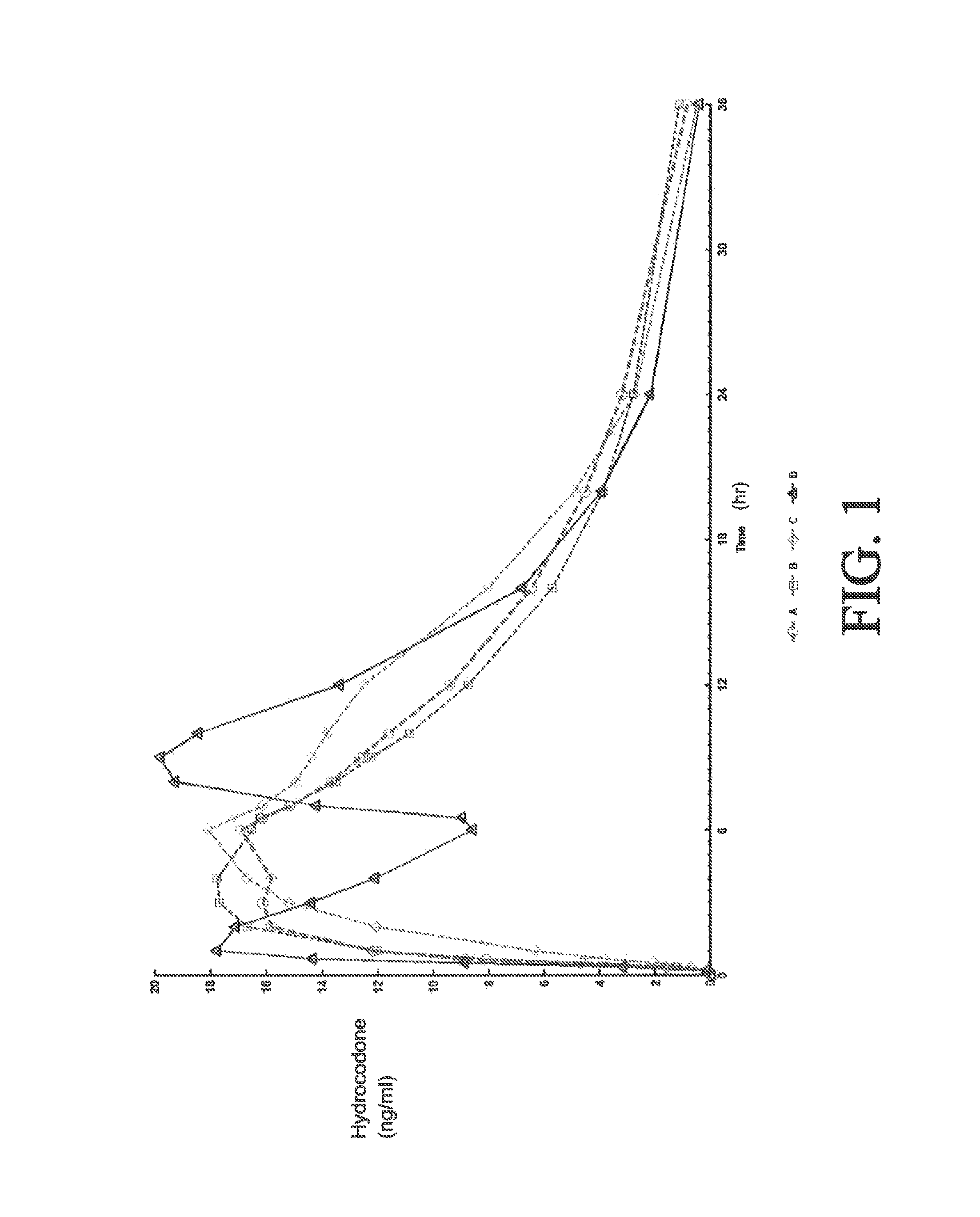
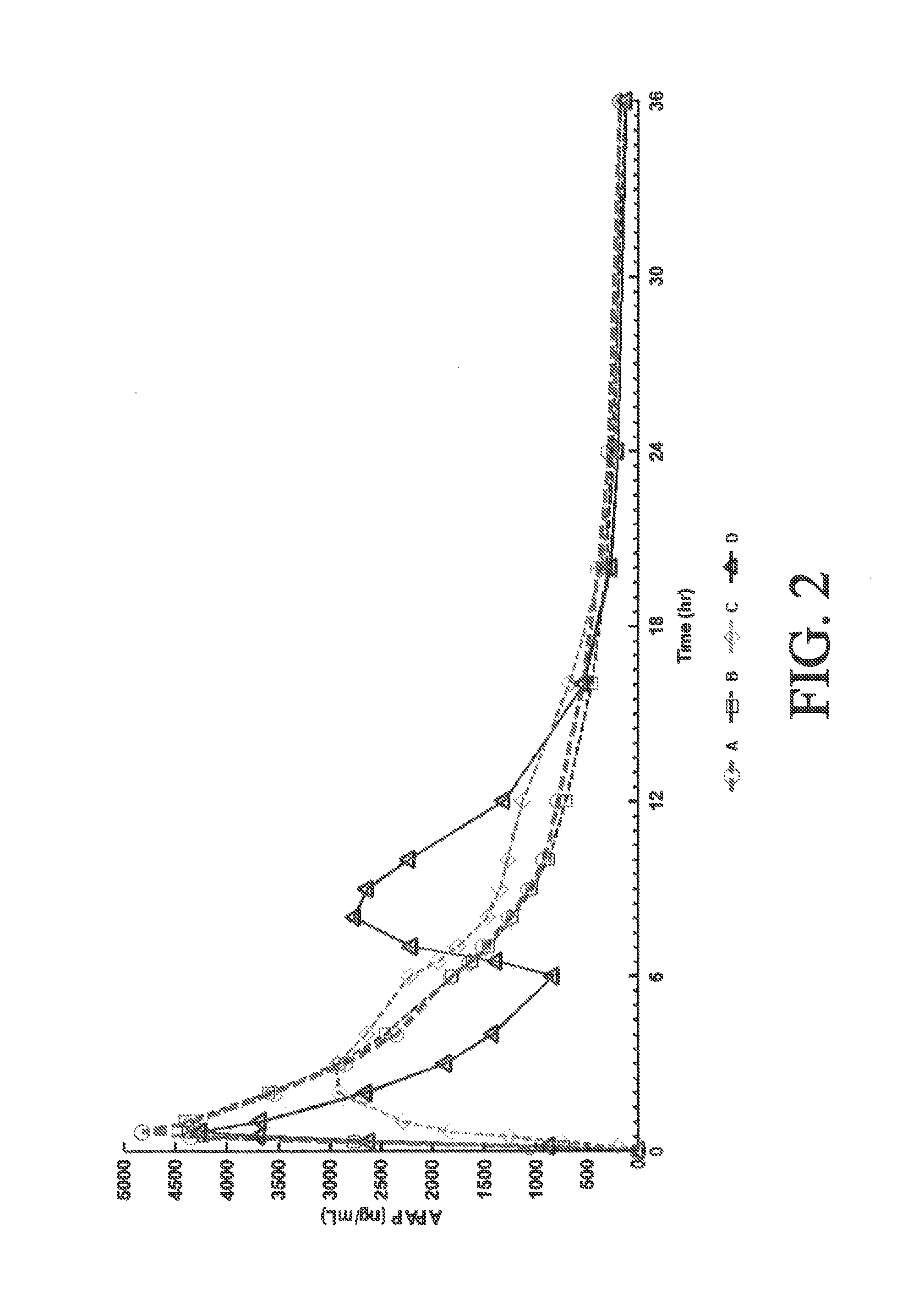
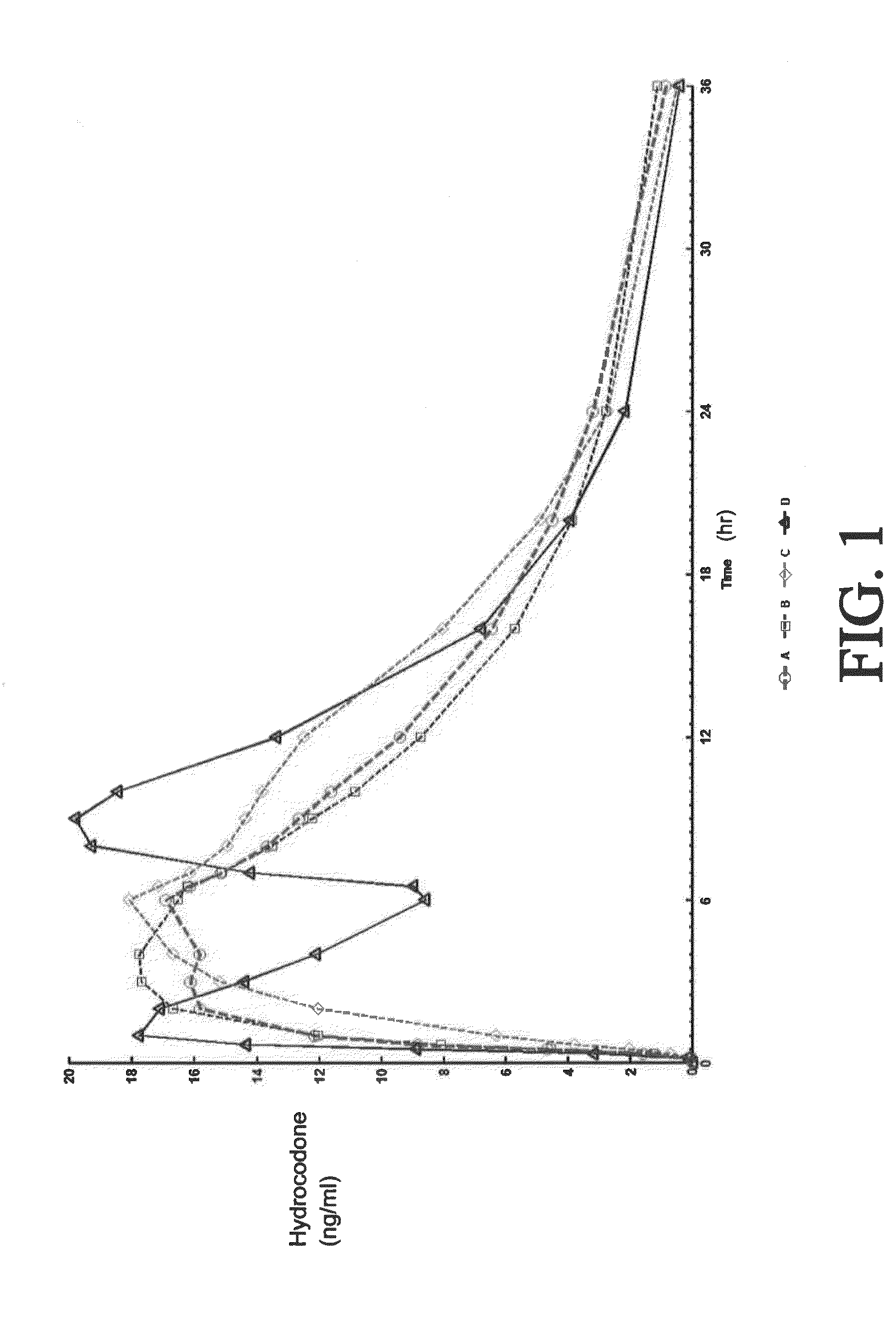
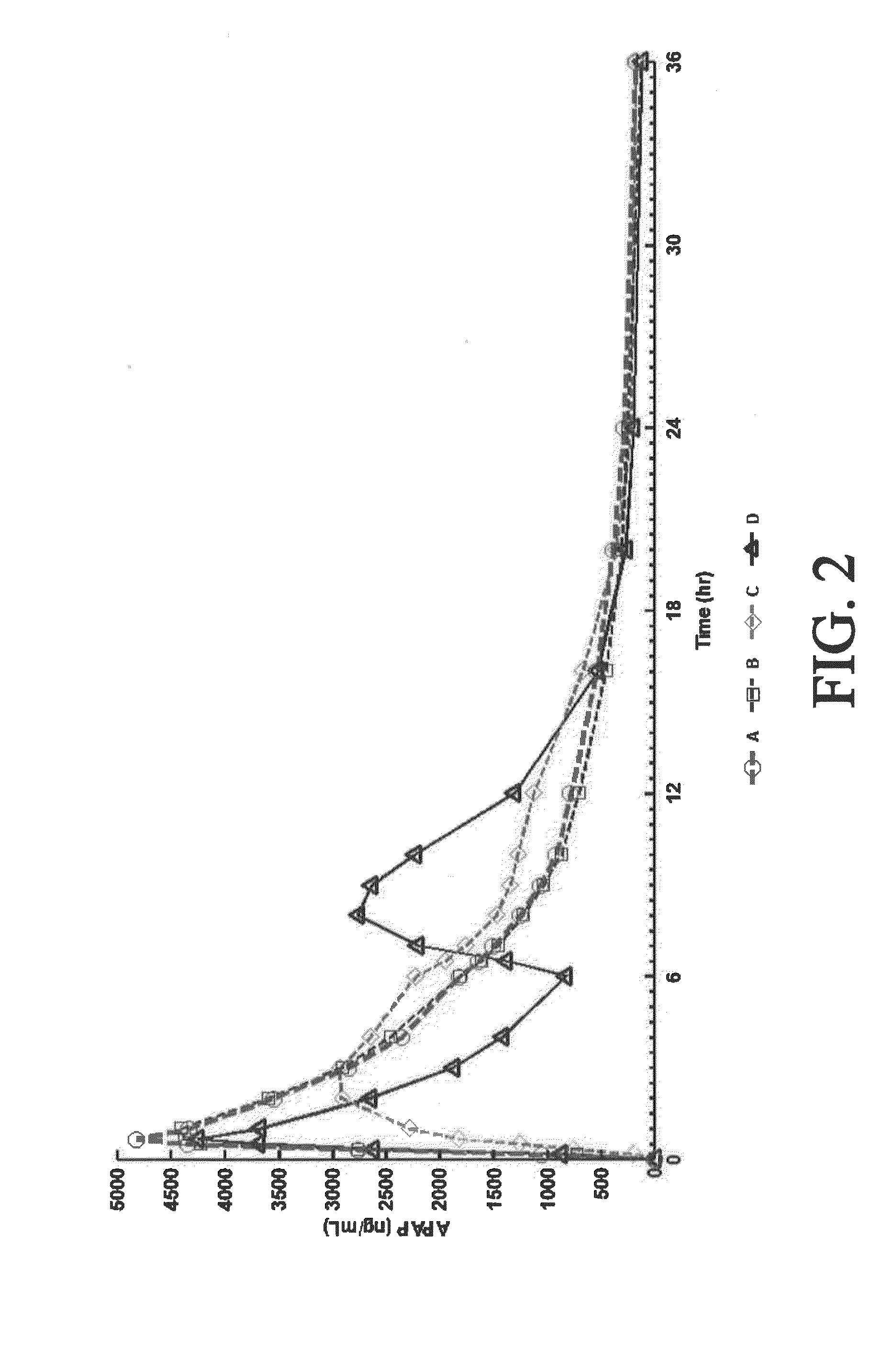
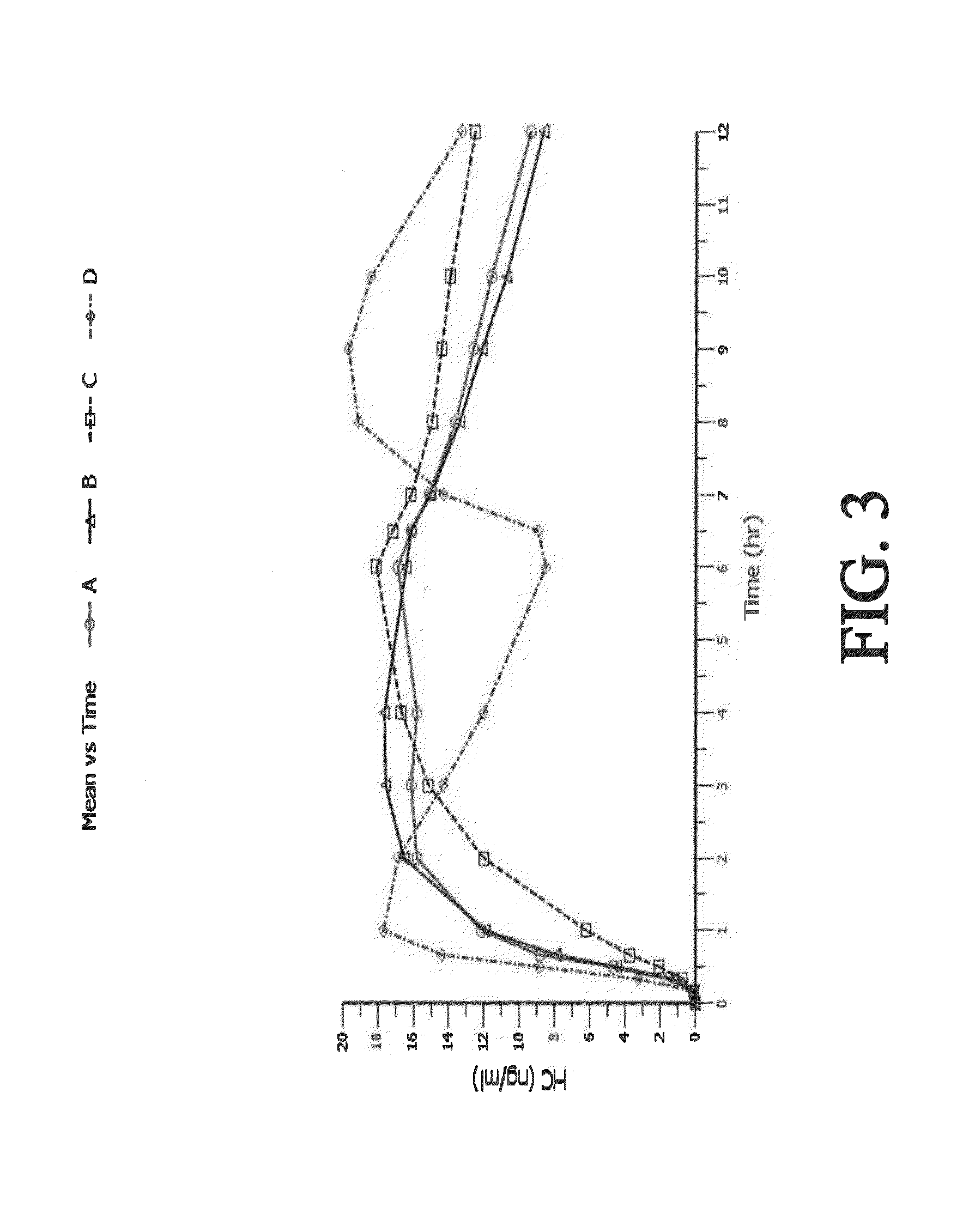
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Formulations of nonopioid and confined opioid analgesics
The preferred exemplary embodiments in the present application provide formulations and methods for the delivery of drugs, particularly drugs of abuse, having an abuse-relevant drug substantially confined in the core and a non-abuse relevant drug in a non-core region. These formulations have reduced potential for abuse. In the formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20060251721A1Improved ability to treat painLess attentionBiocideNervous disorderImmediate releasePharmaceutical medicine
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Abuse resistant melt extruded formulation having reduced alcohol interaction
InactiveUS20090317355A1Reduced and limited dose-dumping effectReduce interactionBiocideNervous disorderVerapamilOral medication
The present invention relates to compositions for oral administration. The invention preferably comprises at least one abuse-resistant drug delivery composition for delivering a drug having potential for dose dumping in alcohol, related methods of preparing these dosage forms, and methods of treating a patient in need thereof comprising administering the inventive compositions to the patient. Most preferably, the dosage form includes verapamil. These formulations have reduced potential for abuse. In another formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Controlled Release Hydrocodone Formulations
InactiveUS20110262532A1Improve efficiency and qualityGood effectPowder deliveryBiocideControlled releaseHuman patient
A solid oral controlled-release oral dosage form of hydrocodone is disclosed. The dosage form comprising an analgesically effective amount of hydrocodone or a pharmaceutically acceptable salt thereof, and a sufficient amount of a controlled release material to render the dosage form suitable for twice-a-day administration to a human patient, the dosage form providing a C12 / Cmax ratio of 0.55 to 0.85, said dosage form providing a therapeutic effect for at least about 12 hours.
Owner:PURDUE PHARMA LP
Compositions comprising nanoparticulate meloxicam and controlled release hydrocodone
InactiveUS20080102121A1Increasing patient convenienceImprove complianceBiocidePowder deliveryMeloxicamControl release
The invention relates to a compositions comprising a nanoparticulate meloxicam composition in combination with a multiparticulate modified release hydrocodone composition that, upon administration to a patient, delivers a hydrocodone in a bimodal or multimodal manner. The multiparticulate modified release composition comprises a first component and at least one subsequent component; the first component comprising a first population of hydrocodone-comprising particles and the at least one subsequent component comprising a second population of hydrocodone-comprising particles, wherein the combination of the components exhibit a bimodal or multimodal release profile. The invention also relates to a solid oral dosage form comprising such a combination composition.
Owner:ELAN PHRMA INT LTD
Abuse resistant melt extruded formulation having reduced alcohol interaction
InactiveUS20100172989A1Reduced and limited dose-dumping effectReduce interactionPowder deliveryBiocideVerapamilOral medication
The present invention relates to compositions for oral administration. The invention preferably comprises at least one abuse-resistant drug delivery composition for delivering a drug having potential for dose dumping in alcohol, related methods of preparing these dosage forms, and methods of treating a patient in need thereof comprising administering the inventive compositions to the patient. Most preferably, the dosage form includes verapamil. These formulations have reduced potential for abuse. In another formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG +1
Compositions comprising nanoparticulate naproxen and controlled release hydrocodone
InactiveUS20080113025A1Reduces and eliminates developmentImprove complianceBiocidePowder deliveryControl releaseHydrocodone
The invention relates to a compositions comprising a nanoparticulate naproxen composition in combination with a multiparticulate modified release hydrocodone composition that, upon administration to a patient, delivers a hydrocodone in a bimodal or multimodal manner. The multiparticulate modified release composition comprises a first component and at least one subsequent component; the first component comprising a first population of hydrocodone-comprising particles and the at least one subsequent component comprising a second population of hydrocodone-comprising particles, wherein the combination of the components exhibit a bimodal or multimodal release profile. The invention also relates to a solid oral dosage form comprising such a combination composition.
Owner:ELAN PHRMA INT LTD
Sustained release monoeximic formulations of opioid and nonopioid analgesics
InactiveUS20080031901A1Improve abilitiesSimple compositionBiocideAntipyreticHydrocodoneAnalgesic agents
Owner:ABBOTT LAB INC
Opiod tannate compositions
A composition comprising the tannate of an opioid. Suitable opioids include alfentanil, buprenorphine, butorphanol, carfentanil, cocaine, codeine, dezocine, diacetylmorphine, dihydrocodeine, dihydromorphine, diphenoxylate, diprenorphine, etorphine, fentanyl, heroin, hydrocodone, hydromorphone, beta-hydroxy-3-methylfentanyl, levo-alpha-acetylmethadol, levorphanol, lofentanil, meperidine, methadone, morphine, nalbuphine, nalmefene, o-methylnaltrexone, naloxone, naltrexone, oxycodone, oxymorphone, pentazocine, pethidine, propoxyphene, remifentanil, sufentanil, tilidine and tramadol. The opioid tannate may be readily prepared by reacting an opioid free base with tannic acid, either neat or in the presence of up to about 30 wt. % water, at a temperature of about 60 to about 150° C. and thereafter recovering the resultant opioid tannate. The opioid tannate may also be prepared by an alternative process that involves reacting the opioid free base with water at a temperature such that not more than about 10 wt. % of the opioid tannate will be decomposed and thereafter removing the water by freeze-drying.
Owner:JAME FINE CHEM
Process and compounds for the production of (+)opiates
The invention generally provides processes and intermediate compounds useful for the production of (+)-opiates. Non-limiting examples of (+) opiates that may be derived from one or more compounds of the invention include (+)-noroxymorphone, (+)-naltrexone, (+)-naloxone, (+)-N-cyclopropylmethylnorhydrocodone, (+)-N-cycloproylmethylnorhydromorphone, (+)-N-allylnorhydrocodone, (+)-N-allylnorhydromorphone, (+)-noroxycodone, (+)-naltrexol, (+)-naloxol, and (+)-3-O-methyl-naltrexone.
Owner:SPECGX LLC
Pharmaceutical combinations of hydrocodone and naltrexone
InactiveUS20060194826A1Reducing abuse potential of dosage formLess abuse potentialBiocideNervous disorderHydrocodoneNaltrexone
Disclosed is a pharmaceutical composition comprising from about 5 to about 20 mg of hydrocodone or a pharmaceutically acceptable salt thereof and from 0.055 to about 0.56 mg naltrexone or pharmaceutically acceptable salt thereof.
Owner:PURDUE PHARMA LP
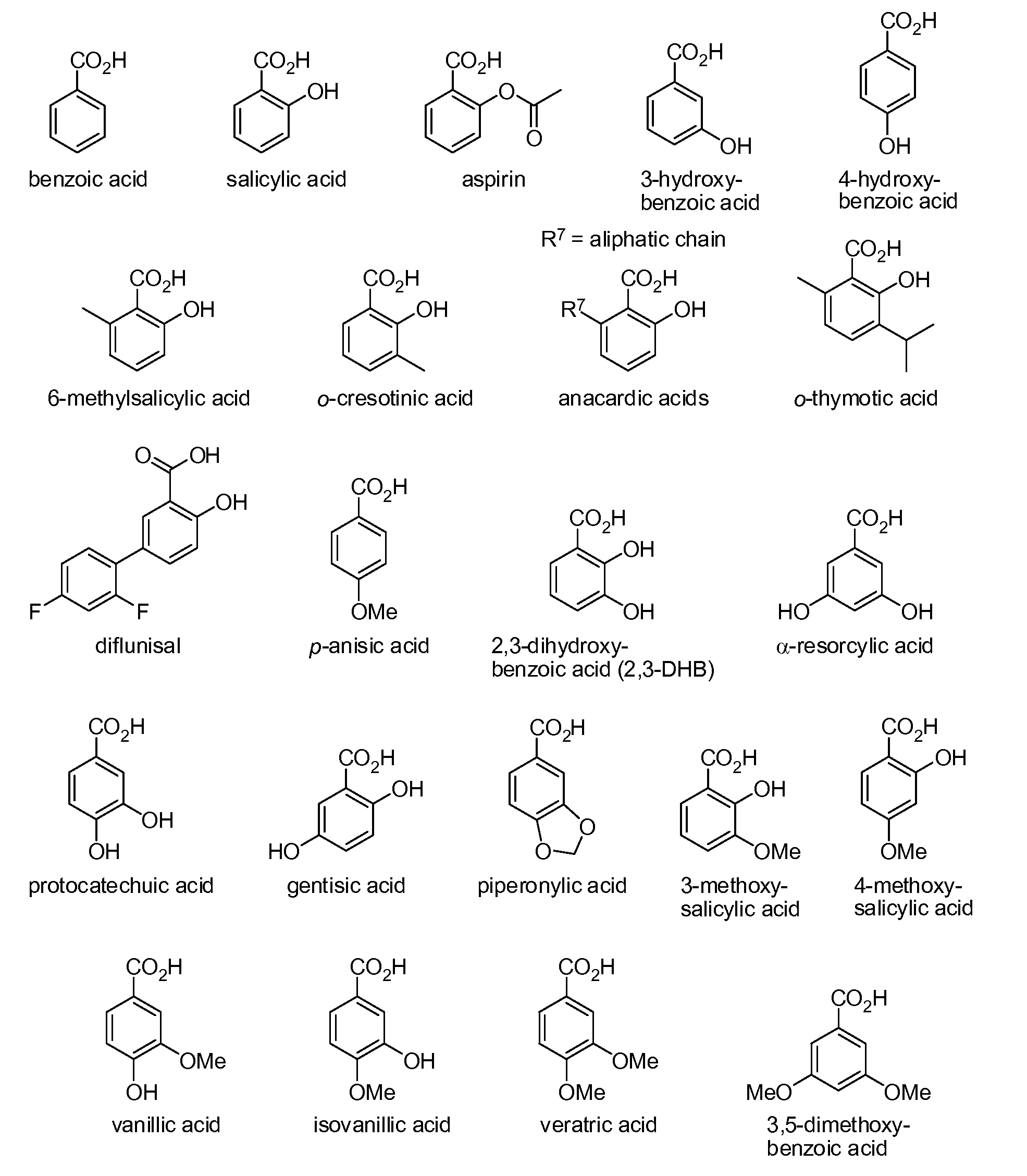
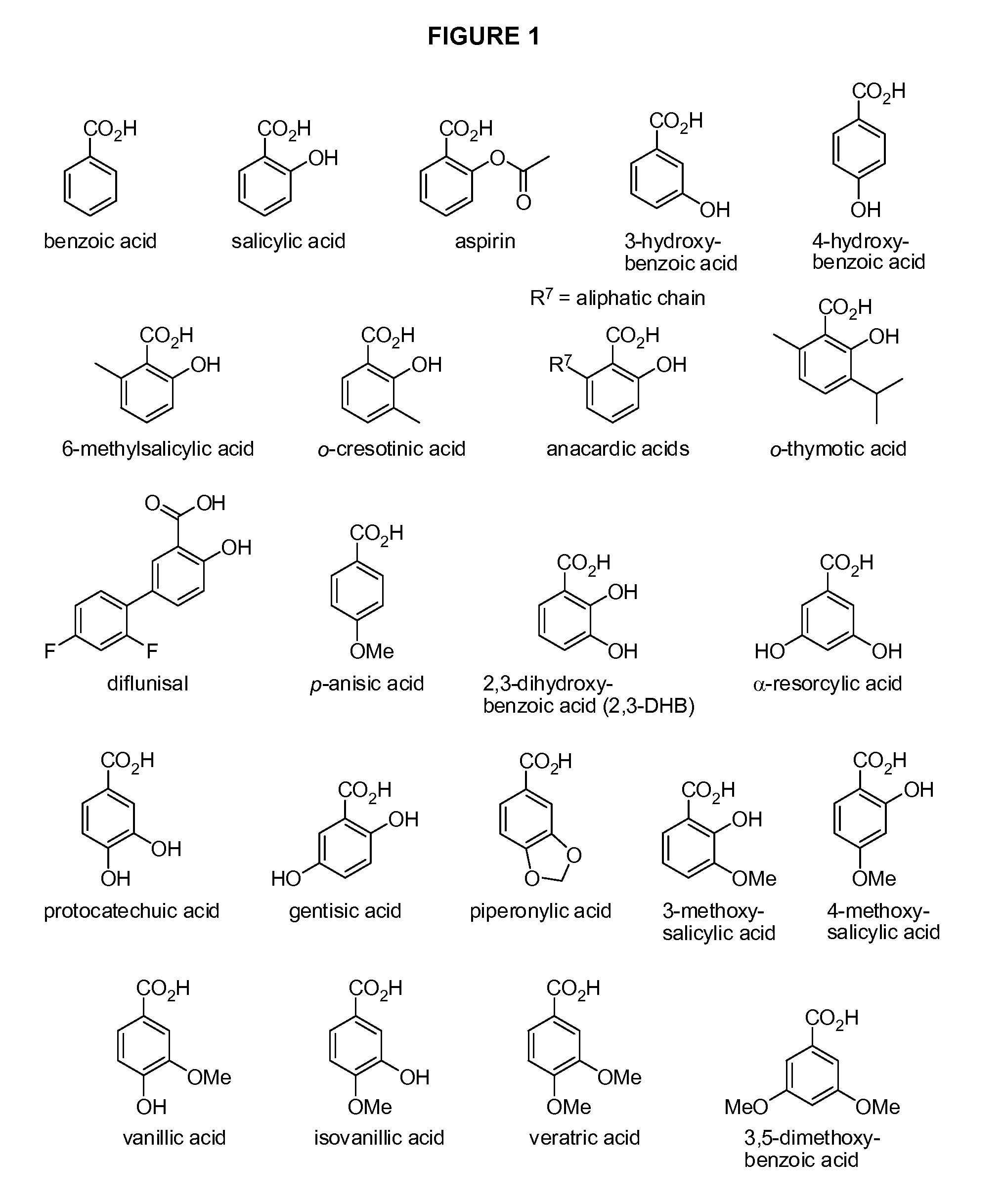
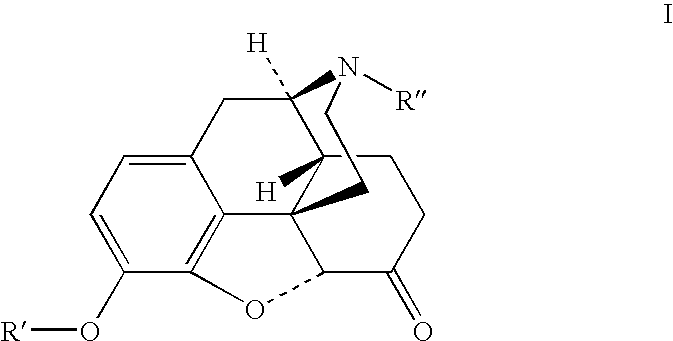
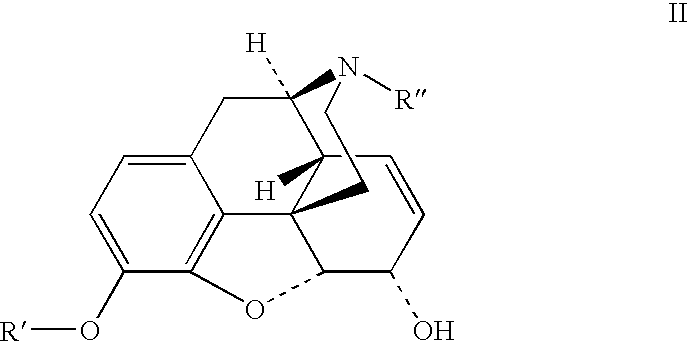
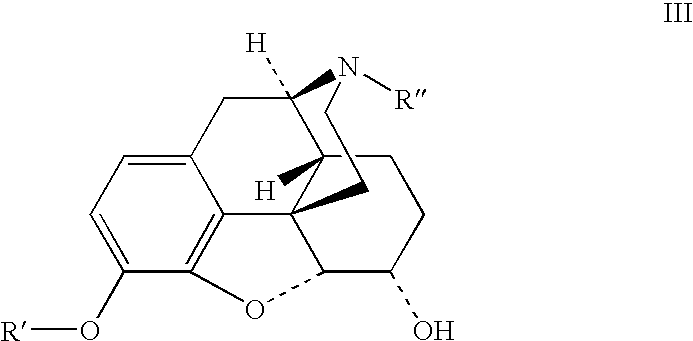
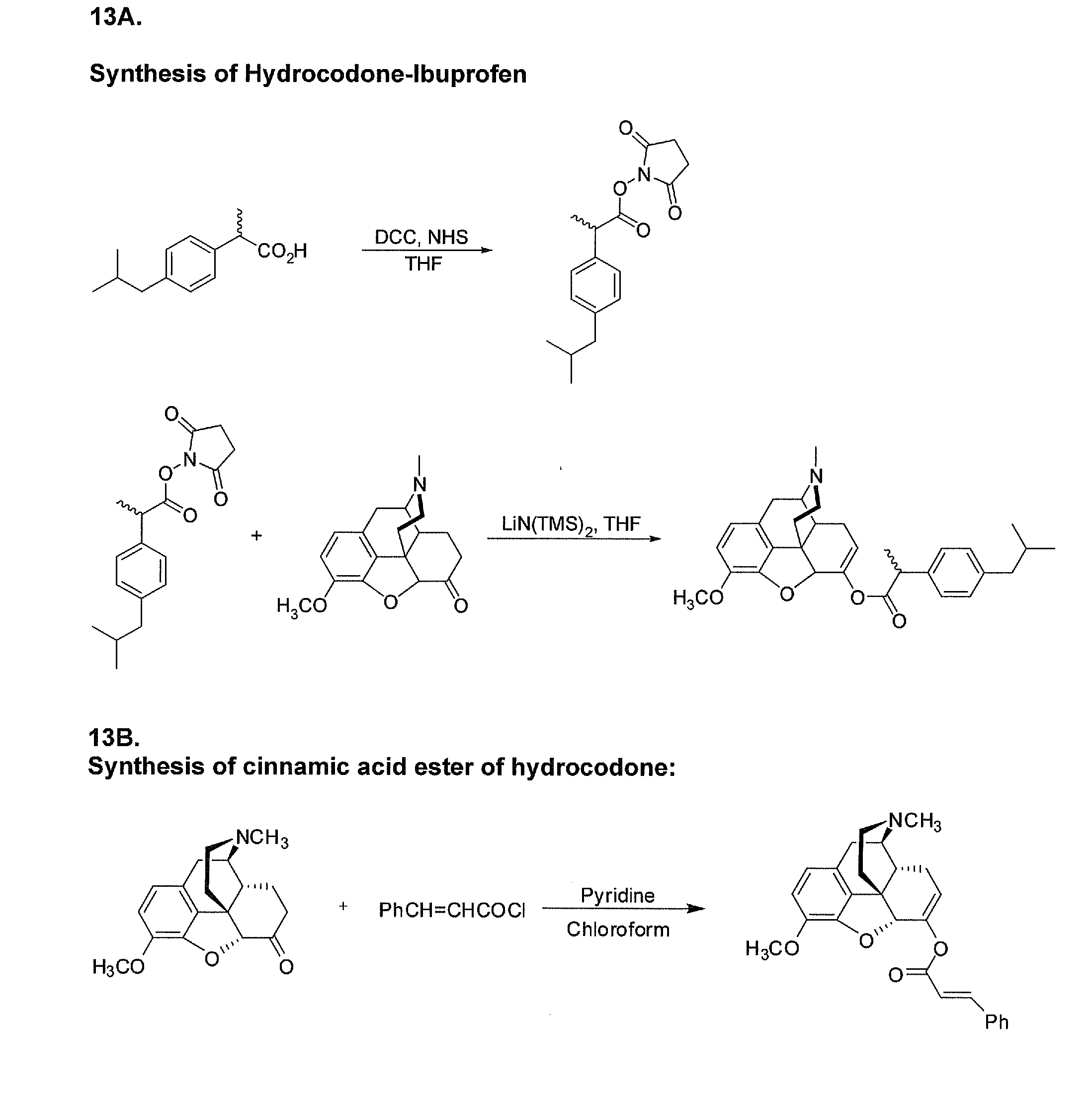
Benzoic acid, benzoic acid derivatives and heteroaryl carboxylic acid conjugates of hydrocodone, prodrugs, methods of making and use thereof
The presently described technology provides compositions comprising aryl carboxylic acids chemically conjugated to hydrocodone (morphinan-6-one, 4,5-alpha-epoxy-3-methoxy-17-methyl) to form novel prodrugs / compositions of hydrocodone, including benzoates and heteroaryl carboxylic acids, which have a decreased potential for abuse of hydrocodone. The present technology also provides methods of treating patients, pharmaceutical kits and methods of synthesizing conjugates of the present technology.
Owner:KEMPHARM INC
Sustained release formulations of opioid and nonopioid analgesics
ActiveUS20070281018A1Improve abilitiesSimple compositionBiocideAnimal repellantsHydrocodoneSustained Release Formulations
The present invention relates to SRSR solid dosage forms for administering pharmaceutical agents, particularly Hydrocodone and acetaminophen, methods for preparing said dosage forms, and methods for providing therapeutic agents to patients in need of treatment.
Owner:ABBVIE INC
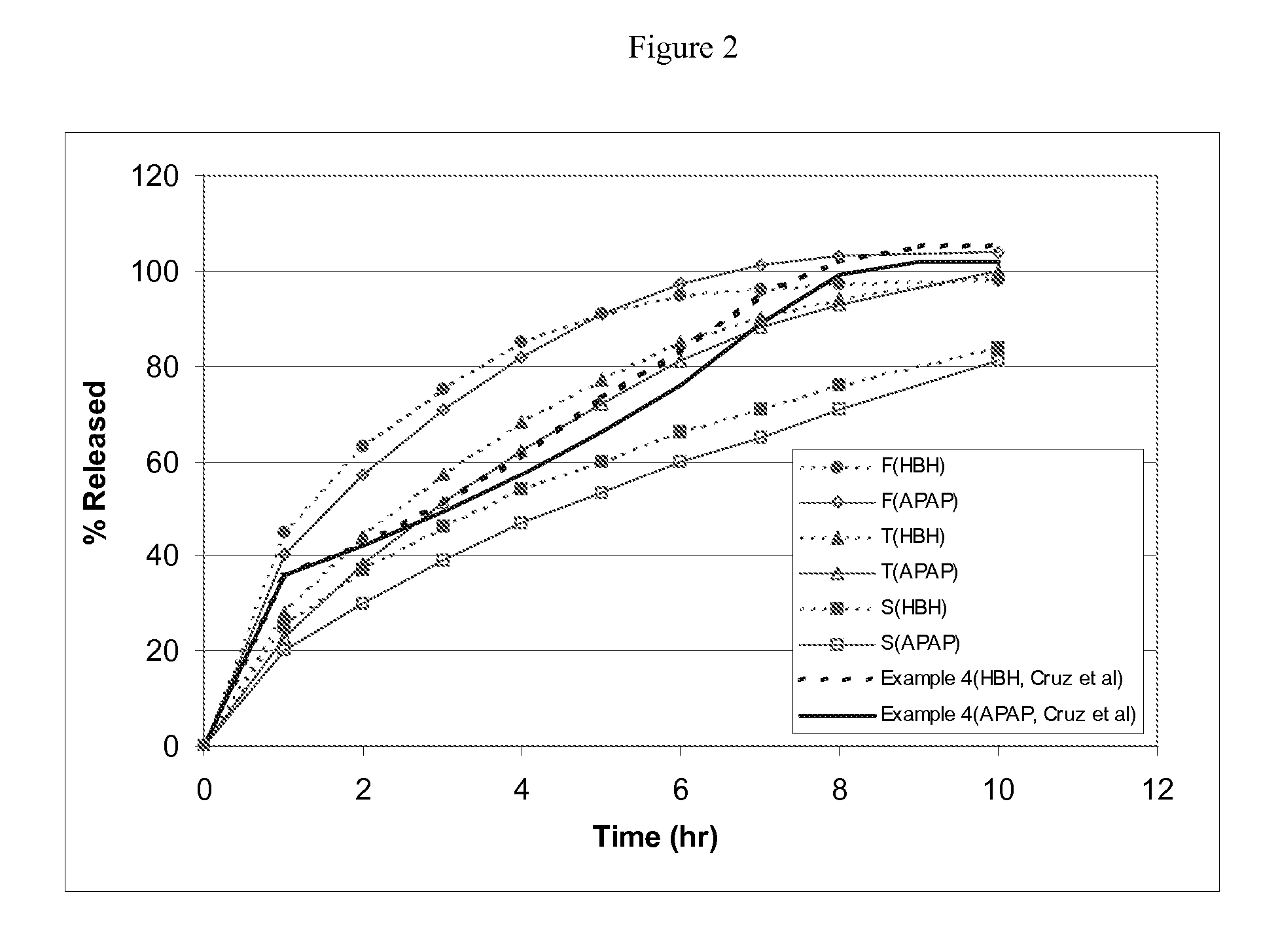
Tamper resistant composition comprising hydrocodone and acetaminophen for rapid onset and extended duration of analgesia
The present disclosure provides an extended release pharmaceutical composition comprising hydrocodone and acetaminophen that provides a rapid onset of analgesia, and reduced levels of acetaminophen near the end of the dosing interval. Also provided are methods for reducing the risk of acetaminophen-induced hepatic damage in a subject being treated with an acetaminophen containing composition, as well as methods for treating pain in a subject in need thereof.
Owner:MALLINCKRODT INC
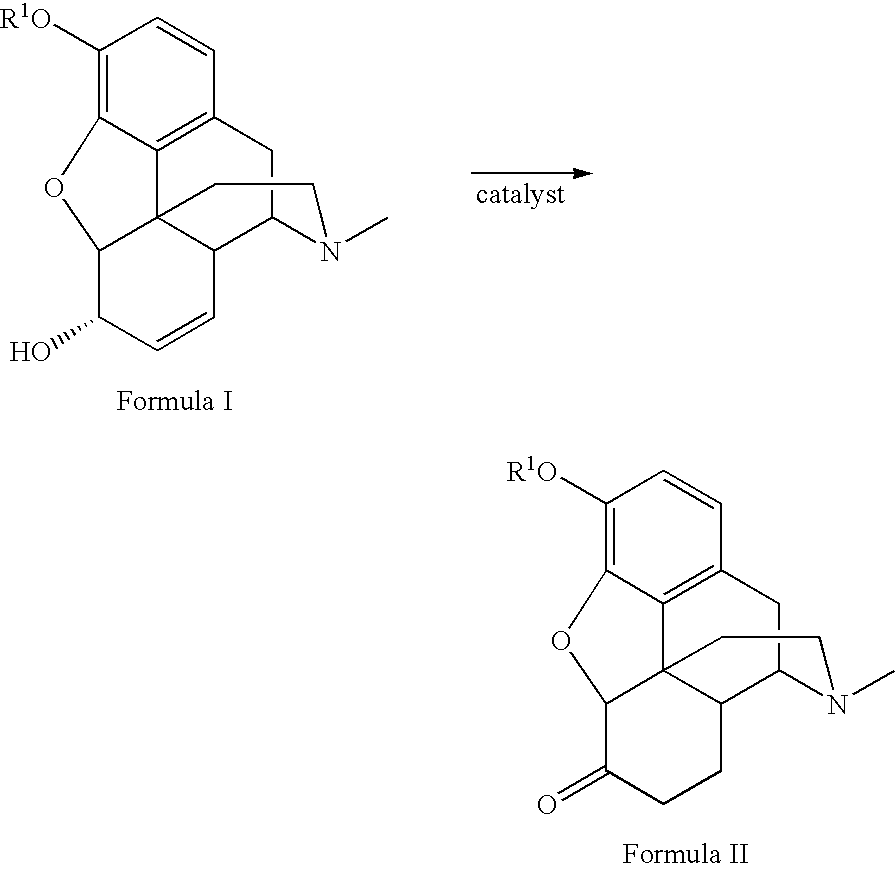
Preparation of opioid analgesics by a one-pot process
A one-pot process for preparing opioid analgesics such as hydrocodone, hydromorphone, and analogues thereof by reacting codeine, morphine, and analogues thereof with hydrogen in a solvent system of benzophenone and neutral solvent in the presence of a metal catalyst followed by oxidation in the presence of potassium tert-alkylate.
Owner:ACURA PHARMA
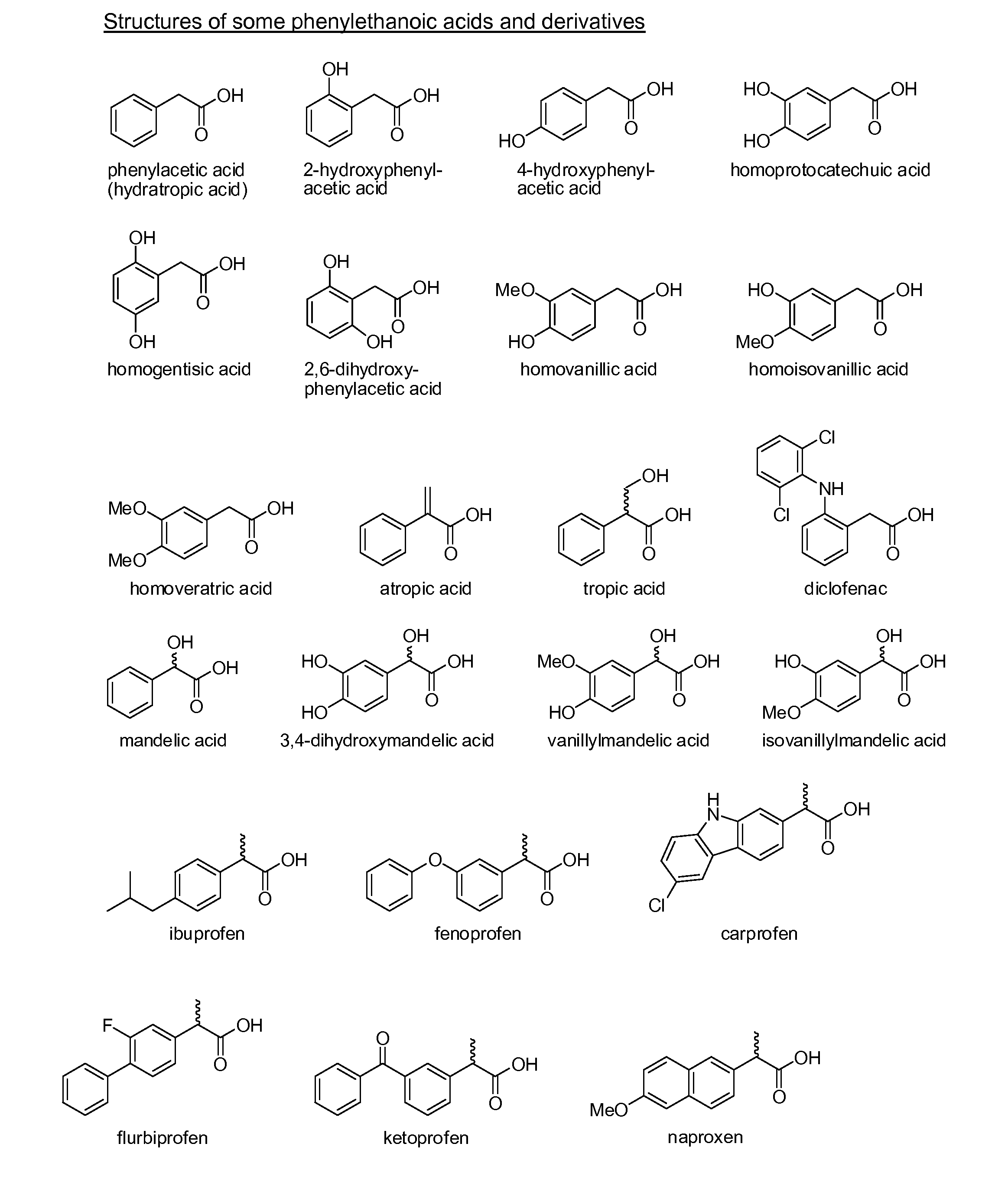
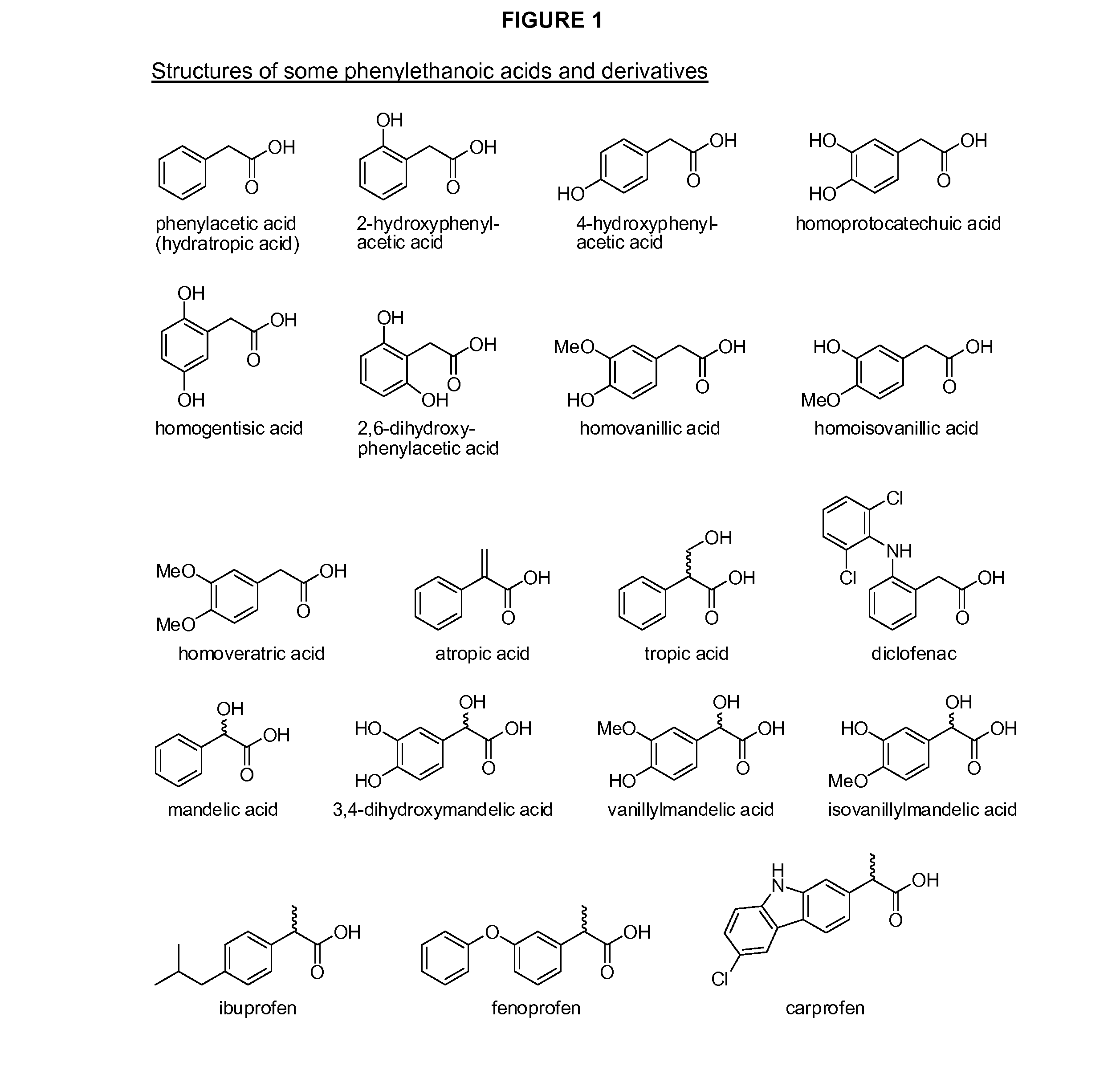
Phenylethanoic acid, phenylpropanoic acid and phenylpropenoic acid conjugates and prodrugs of hydrocodone, method of making and use thereof
ActiveUS20110002991A1Lower potentialReduce addictionBiocideNervous disorder3-phenylpropanoic acidEpoxy
The presently described technology provides phenylethanoic acid, phenylpropanoic acid, phenylpropenoic acid, a salt thereof, a derivative thereof or a combination thereof chemically conjugated to hydrocodone (morphinan-6-one, 4,5-alpha-epoxy-3-methoxy-17-methyl) to form novel prodrugs or compositions of hydrocodone which have a decreased potential for abuse of hydrocodone. The present technology also provides methods of treating patients, pharmaceutical kits and methods of synthesizing conjugates of the present technology.
Owner:KEMPHARM INC
Formulations of nonopioid and confined opioid analgesics
The preferred exemplary embodiments in the present application provide formulations and methods for the delivery of drugs, particularly drugs of abuse, having an abuse-relevant drug substantially confined in the core and a non-abuse relevant drug in a non-core region. These formulations have reduced potential for abuse. In the formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Tamper Resistant Composition Comprising Hydrocodone And Acetaminophen For Rapid Onset And Extended Duration Of Analgesia
The present disclosure provides an extended release pharmaceutical composition comprising hydrocodone and acetaminophen that provides a rapid onset of analgesia, and reduced levels of acetaminophen near the end of the dosing interval. Also provided are methods for reducing the risk of acetaminophen-induced hepatic damage in a subject being treated with an acetaminophen containing composition, as well as methods for treating pain in a subject in need thereof.
Owner:MALLINCKRODT INC
Stabilized hydrocodone pharmaceutical compositions with ethylenediaminetetraacetic acid
InactiveUS20060205752A1Effective stabilizing amountBiocideDispersion deliveryEthylenediamineAcetic acid
Hydrocodone pharmaceutical formulations are stabilized with a stabilizing effective amount of ethylenediaminetetraacetic compound.
Owner:WHITEHEAD KEITH
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
The present invention relates to compositions comprising an antitussive, a decongestant and an expectorant, and in a specific embodiment comprising hydrocodone, phenylephrine hydrochloride and guaifenesin, wherein the composition may be substantially free of added sugar and added alcohol, and methods for using these compositions for the treatment of patients suffering from, for example and without limitation, coughing, sneezing, rhinorrhea, and / or nasal obstruction.
Owner:EVERETT LAB
Pharmaceutical composition
A pharmaceutical composition comprising an analgesic or analgesic combination and a stool softener is disclosed. The analgesic is selected from morphine, meperidine, fentanyl, hydromorphone, oxymorphone, oxycodone, hydrocodone, methadone, propoxyphene, pentazocine, levorphanol, codeine, acetaminophen and combinations of these analgesics. The composition is formulated for oral administration as a liquid or solid dosage form for immediate, slow, delayed or sustained-release characteristics.
Owner:BRANDED PRODS FOR THE FUTURE
Phenylethanoic Acid, Phenylpropanoic Acid and Phenylpropenoic Acid Conjugates and Prodrugs of Hydrocodone, Methods of Making and Use Thereof
Owner:KEMPHARM INC
Production of opioid analgesics
The present invention includes a process for the manufacture of dihydrothebaine, dihydrocodeinone enol acetate, hydrocodone, and analogs thereof by reacting dihydrocodeine or analogs thereof with benzophenone in the presence of potassium tert-alkylate in a hydrocarbon solvent to generate a reaction mixture containing an enolate of the corresponding ketone, followed by addition of the reaction mixture to the electrophilic agent and isolation of the product.
Owner:ACURA PHARMA
Orally disintegrating tablets comprising diphenhydramine
The compositions of the present invention comprise a therapeutically effective amount of particles consisting of diphenhydramine or pharmaceutically acceptable salts thereof, optionally in combination with another drug such as pseudoephedrine, or phenylephrine and hydrocodone, in combination with rapidly-dispersing microgranules comprising a disintegrant and a sugar alcohol and / or a saccharide. These compositions are useful in treating the symptoms of one or more diseases or conditions in which diphenhydramine (alone or in combination with one or two other drugs) is a therapeutically effective, e.g. allergic rhinitis, sinusitis, upper respiratory tract infections, motion sickness, Parkinson's disease, insomnia, the common cold, and nighttime pain management, particularly for subjects or patients with dysphagia, and people ‘on the move’.
Owner:ADARE PHARM INC
Method for the catalytic production of hydrocodone and hydromorphone
A method for the catalytic conversion of codeine, morphine or analogs thereof into hydrocodone, hydromorphone or analogs thereof utilizing a transition metal complex of a tertiary phosphine halide as catalyst.
Owner:MALLINCKRODT INC
Sustained release monoeximic formulations of opioid and nonopioid analgesics
InactiveUS20110166171A1Improve abilitiesSimple compositionBiocideAntipyreticHydrocodoneSolid Dose Form
The present invention relates to monoeximic solid dosage forms for administering pharmaceutical agents, particularly Hydrocodone and acetaminophen, methods for preparing said dosage forms, and methods for providing therapeutic agents to patients in need of treatment.
Owner:ABBOTT LAB INC
Method for the catalytic production of hydrocodone and hydromorphone
InactiveUS7321038B2Organic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsArylHydrocodone
A method for the catalytic conversion of codeine, morphine or analogs thereof into hydrocodone, hydromorphone or analogs thereof utilizing a transition metal catalyst of the formula [M(PR3R4R5)nXm]p; wherein R1 is H, alkyl, aryl or acyl; M is a Group VIII transition metal; R3, R4 and R5 are selected from the group consisting of alkyl, aryl, alkoxyl, phenoxyl and combinations thereof; X is a halide or an anion; n is 1, 2, 3 or 4; m is 1 or 2; and p is at least 1.
Owner:MALLINCKRODT INC
Pharmaceutical compositions for treating or preventing pain
Methods and compositions are provided which comprise effective amounts of one or more analgesics, such as hydrocodone or acetaminophen, and an antiemetic, such as promethazine, to treat a subject, including reducing or eliminating an adverse effect associated with the analgesics.
Owner:LOCL PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com