Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
118 results about "Expectorant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Any agent that promotes ejection of mucus or exudate from the lungs, bronchi and trachea by decreasing mucus viscosity or by increasing the secretion of mucus in a dry, unproductive cough.
Dosage form containing multiple drugs
A pharmaceutical dosage form comprising a first drug and a second drug, both of which are selected from decongestants, antitussives, expectorants, analgesics and antihistamines. The dosage form provides a plasma concentration within a therapeutic range of the second drug over a period which is coextensive with at least about 70% of a period over which the dosage form provides a plasma concentration within a therapeutic range of the first drug. This Abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Decongestant and expectorant tablets
The present invention relates to a sustained release oral pharmaceutical tablet formulation containing an expectorant and a decongestant.
Owner:ANDRX LABS
Sequential release pharmaceutical formulations
InactiveUS20070141147A1Efficient coordinationBiocideHydroxy compound active ingredientsControlled releaseImmediate release
A mixed-release tablet or capsule formulation including vehicles for the delivery of a plurality of drugs in various combinations of immediate release, extended release, and / or delayed release modes over a predetermined time period have been developed, which provide for controlled release not just of the drugs, but controlled release that is designed to create more effective coordination between the drugs being delivered. The drugs can be any medically and / or physiologically appropriate combination of drugs and active ingredients, preferably decongestant drugs, antihistamines, expectorants, antitussives, cough suppressants, and drying agents.
Owner:AURIGA LAB
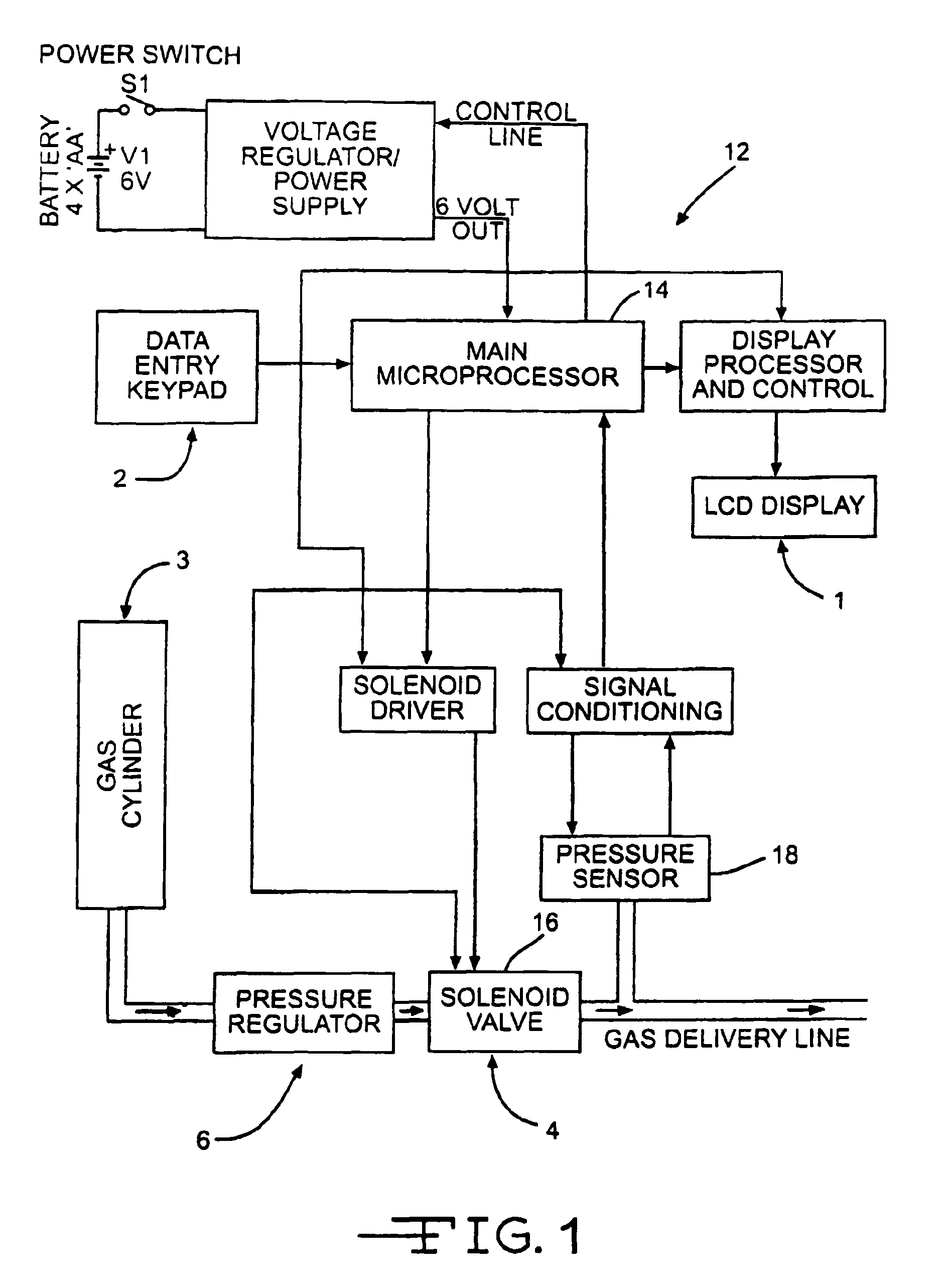
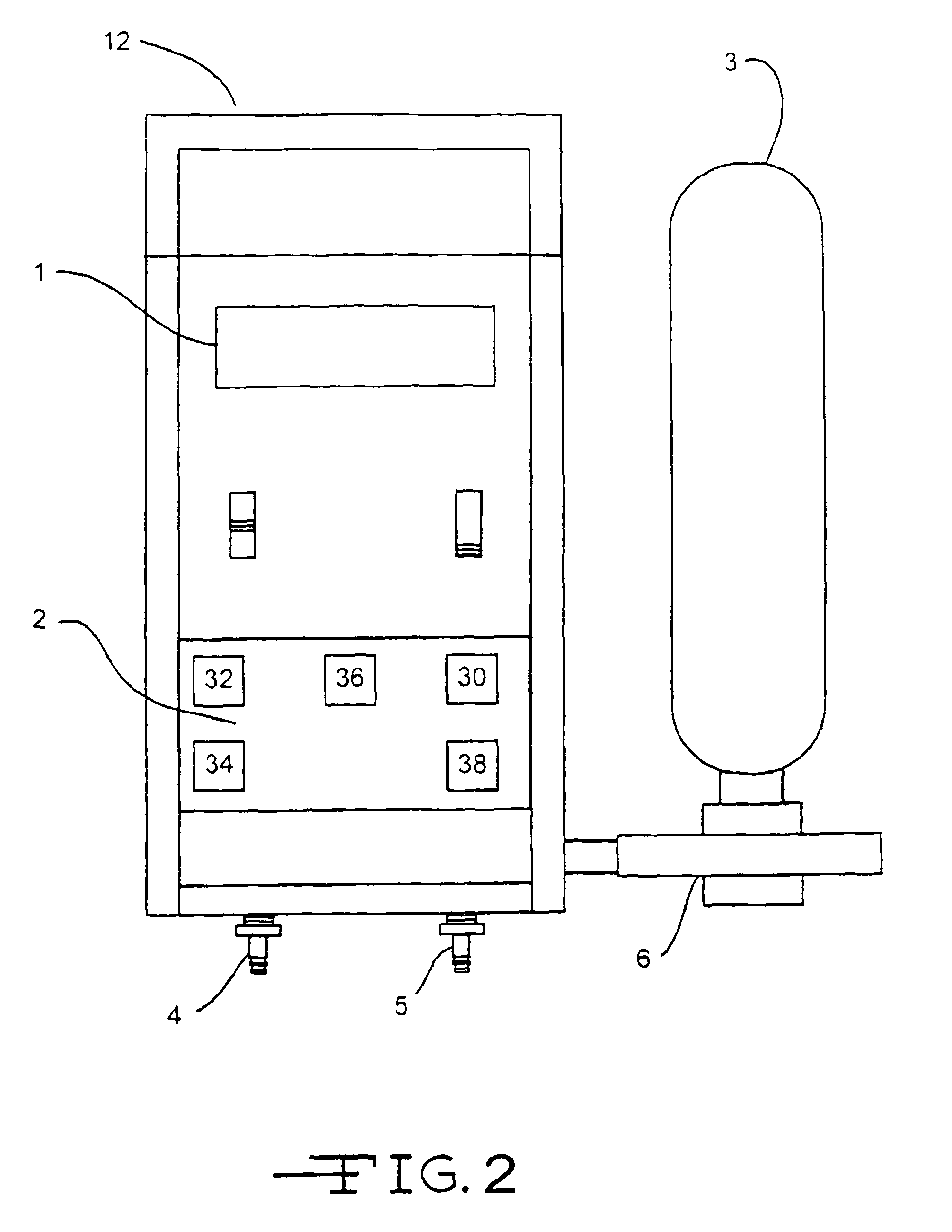
Use of inhaled gaseous nitric oxide as a mucolytic agent or expectorant
InactiveUS20070104653A1Increased mucociliary clearanceReduce severityRespiratorsBiocideInhalationCompound (substance)
Methods and devices for treating excess mucus accumulation in mammals by administering gaseous inhaled nitric oxide or nitric oxide releasing compounds as a mucolytic agent or expectorant are provided. Delivery of gaseous nitric oxide can be made nasally or orally and is preferably substantially coincident with inhalation of the mammal or based on a synchronous parameter of the mammal's respiratory cycle. Varying therapeutic profiles may be used for the delivery of gaseous nitric oxide depending on the severity of the excess mucus accumulation. Parameters for the therapeutic profiles may include flow rate of nitric oxide containing gas, duration of administration of nitric oxide containing gas, number of breaths for which nitric oxide containing gas is to be administered, and concentrations of therapeutic NO delivered to the airways.
Owner:BEYOND AIR LTD
Dynamic variable release
InactiveUS20050152967A1Improve efficiencyReduce in quantityBiocideOrganic active ingredientsDiseaseCommon cold
The present invention relates to novel mixed release pharmaceutical formulations that include a expectorant available for immediate release and a decongestant for extended release that provide for the symptomatic relief of cough associated with respiratory tract conditions such as the common cold, bronchial asthma, acute and chronic bronchitis.
Owner:NEOS THERAPEUTICS LP
Use of inhaled gaseous nitric oxide as a mucolytic agent or expectorant
InactiveUS8518457B2Effective treatmentReducing severity and pathologyRespiratorsBiocideMedicineInhalation
Methods and devices for treating excess mucus accumulation in mammals by administering gaseous inhaled nitric oxide or nitric oxide releasing compounds as a mucolytic agent or expectorant are provided. Delivery of gaseous nitric oxide can be made nasally or orally and is preferably substantially coincident with inhalation of the mammal or based on a synchronous parameter of the mammal's respiratory cycle. Varying therapeutic profiles may be used for the delivery of gaseous nitric oxide depending on the severity of the excess mucus accumulation. Parameters for the therapeutic profiles may include flow rate of nitric oxide containing gas, duration of administration of nitric oxide containing gas, number of breaths for which nitric oxide containing gas is to be administered, and concentrations of therapeutic NO delivered to the airways.
Owner:BEYOND AIR LTD
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
The present invention relates to compositions that comprise an expectorant, an extended release antitussive, and an extended release decongestant. Specifically, the compositions comprise guaifenesin, phenylephrine tannate, and dextromethorphan tannate. The present invention also includes methods for using these compositions for treatment of patients suffering from, for example and without limitation, coughing, sneezing, rhinorrhea, and / or nasal obstruction.
Owner:EVERETT LAB
Compositions and methods for tolerizing the immune system to allergens
InactiveUS20160263212A1Reduce development riskReduce riskHydroxy compound active ingredientsAllergen ingredientsIMMUNE SUPPRESSANTSBULK ACTIVE INGREDIENT
Compositions and methods can be used for tolerizing the immune system. The compositions can be physiologically acceptable and can include any of a wide variety of allergens that are designed to be administered in escalating doses to, for example, an infant. The compositions can include other active ingredients (e.g., one or more of a steroid, vitamin, mineral, vasodilator, hormone, decongestant, anticholinergic agent, leukotriene inhibitor, immunomodulator, mast cell stabilizer, expectorant, immune suppressant, anti-histamine, or anti-inflammatory agent) and / or a carrier.
Owner:STALLERGENES GREER PLC
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
The present invention relates to compositions comprising an antitussive, a decongestant and an expectorant, and in a specific embodiment comprising hydrocodone, phenylephrine hydrochloride and guaifenesin, wherein the composition may be substantially free of added sugar and added alcohol, and methods for using these compositions for the treatment of patients suffering from, for example and without limitation, coughing, sneezing, rhinorrhea, and / or nasal obstruction.
Owner:EVERETT LAB
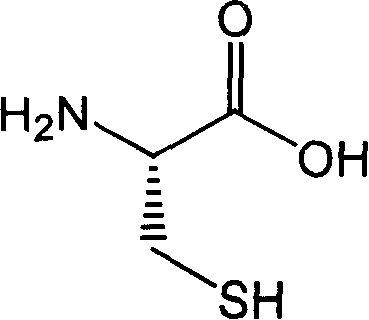
Process for preparing fudosteine
ActiveCN1840524AReduce usagePrevent neutralizationOrganic chemistryRespiratory disorderStructural formulaOrganic chemistry
The invention provides a process for preparing Fudosteine, which has the chemical structural formula (I) disclosed in the specification. The Fudosteine can be used as the medicament for relieving cough and expectorant.
Owner:AVENTIS PHARMA HAINAN
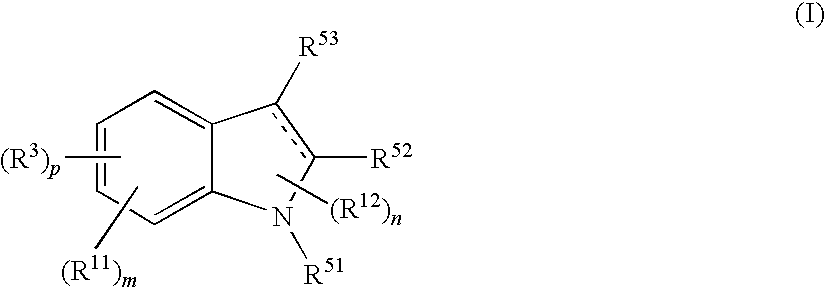
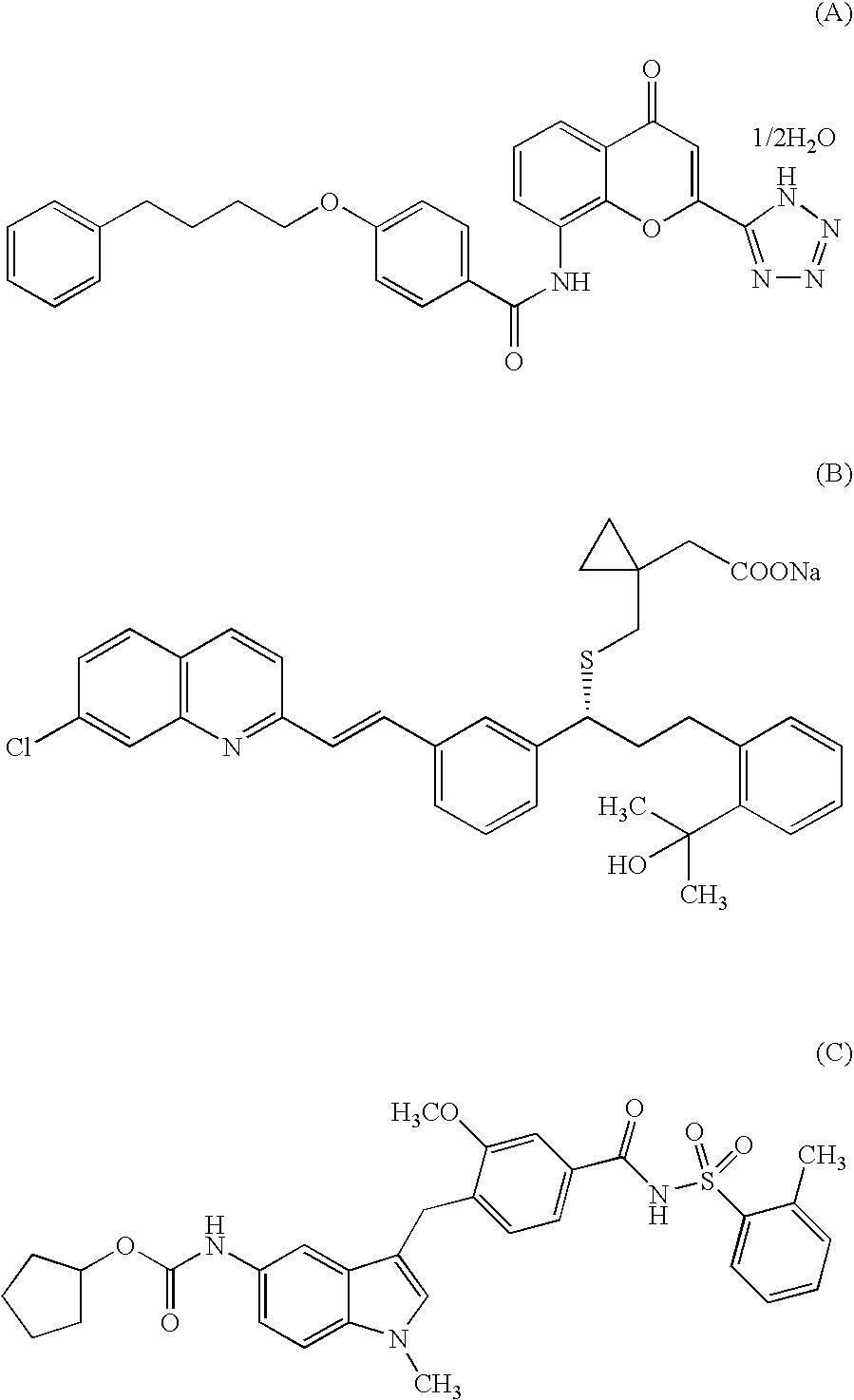
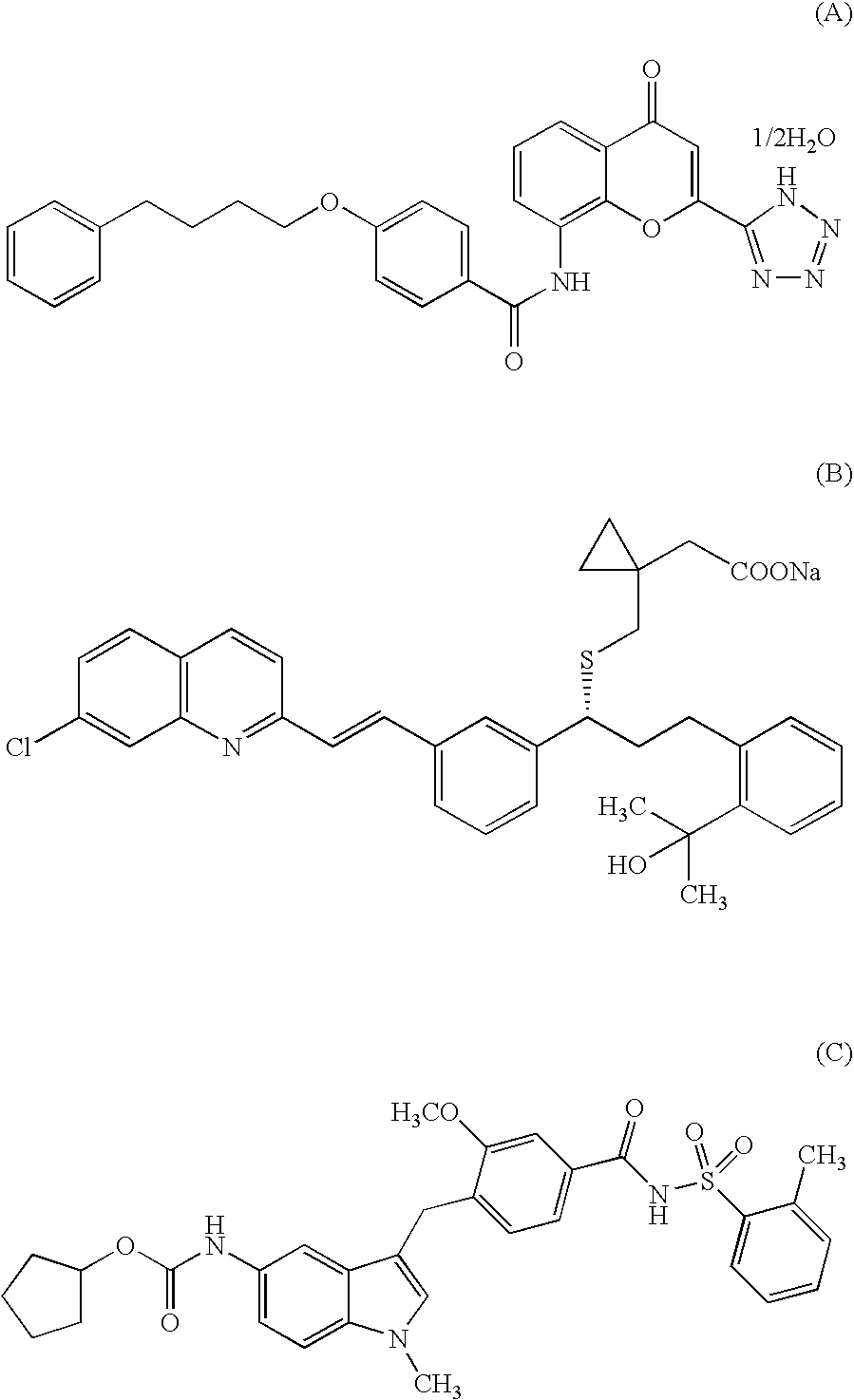
Indole compound and use thereof
InactiveUS7728023B2Increased airway hyperreactivityImprove respiratory functionBiocideSenses disorderClinical trialObstructive Pulmonary Diseases
The present invention relates to a compound represented by the formula (I),wherein all symbols are as defined in the description,a salt thereof, a solvate thereof, or a prodrug thereof, which has a leukotriene receptor antagonistic activity which is expected to be more effective than those of the leukotriene receptor antagonists currently used in clinical trials. Therefore, it is useful as an agent for the prevention and / or treatment of a leukotriene-mediated disease such as a respiratory diseases such as bronchial asthma, chronic obstructive pulmonary disease, pulmonary emphysema, chronic bronchitis, pneumonia (e.g. interstitial pneumonia etc.), severe acute respiratory syndrome (SARS), acute respiratory distress syndrome (ARDS), allergic rhinitis, sinusitis (e.g. acute sinusitis, chronic sinusitis, etc.), or the like, or as an expectorant or an antiitussive.
Owner:ONO PHARMA CO LTD
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
Owner:EVERETT LAB
Kits for Prevention and Treatment of Rhinitis
Kits providing a combination of one or more pharmaceutical information comprising one or more agent(s) for the treatment or alleviation of symptoms commonly associated with a cold and an immunonutritional composition comprising immunonutritional agent and methods of using these kits are described . The kits provide both the pharmaceutical agent(s) and the immunonutritional agent in a convenient form for administration. The kit typically includes instruction for coordinating the administration of the pharmaceutical formulation with the administration of the immunonutritional composition. The preferred immunonutritional agents are compounds that contain a pharmaceutically acceptable form of zinc, such as zinc acetate, zinc gluconate, zinc gluconate glycine, and zinc sulfate. Preferably the kit contains multiple dosage forms containing the immunonutritional composition. In the most preferred embodiment, the immunonutritional composition is in the form of a lozenge. Suitable pharmaceutical agents include but are not limited to antihistamines, decongestants, anticholinergies, antitussives, analgestics, mucolytics, expectorants, and combinations thereof. The pharmaceutical formulations may be in any suitable dosage form, including forms which provide controlled release of the pharmaceutical agent, including immediate, sustained, modified, delayed or pulsed release pharmacokinetic mechanism or a combination thereof. The combined treatment requires administration of both the pharmaceutical formulation(s) for the treatment of symptoms commonly associated with a cold and the administration of the immunonutritional composition, which supplies nutritional support for the patient's innate immune response to the presence of infectious organisms.
Owner:AURIGA LAB
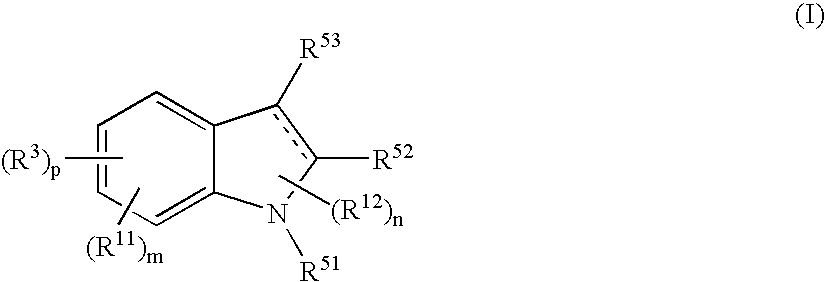
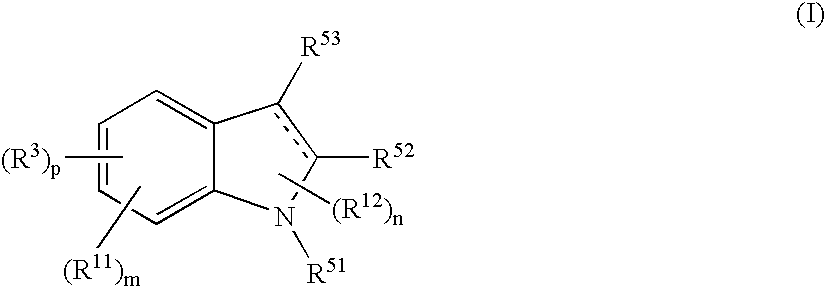
Indole Compound and Use Thereof
InactiveUS20080188532A1Increased airway hyperreactivityImprove respiratory functionBiocideSenses disorderDiseaseBronchial epithelium
The present invention relates to a compound represented by the formula (I),wherein all symbols are as defined in the description,a salt thereof, a solvate thereof, or a prodrug thereof, which has a leukotriene receptor antagonistic activity which is expected to be more effective than those of the leukotriene receptor antagonists currently used in clinical trials. Therefore, it is useful as an agent for the prevention and / or treatment of a leukotriene-mediated disease such as a respiratory diseases such as bronchial asthma, chronic obstructive pulmonary disease, pulmonary emphysema, chronic bronchitis, pneumonia (e.g. interstitial pneumonia etc.), severe acute respiratory syndrome (SARS), acute respiratory distress syndrome (ARDS), allergic rhinitis, sinusitis (e.g. acute sinusitis, chronic sinusitis, etc.), or the like, or as an expectorant or an antiitussive.
Owner:ONO PHARMA CO LTD
Vitex oil nanoemulsion and preparation method thereof
InactiveCN101773578AFix stability issuesGood biocompatibilityPharmaceutical non-active ingredientsRespiratory disorderPolymer scienceBiocompatibility Testing
The invention relates to a vitex oil nanoemulsion and a preparation method and application thereof. The preparation method is characterized in that the advanced nanotechnology is used for the formula and process design of the vitex oil nanoemulsion. The preparation method comprises the steps that: vitex oil, medium chain fatty acid triglyceride, soy lecithin, ethanol and the like are evenly mixed to be used as an oil phase; poloxamer is mixed with water and the like to be used as a water phase; the oil phase is added to the water phase to be mixed and emulsified at a temperature of 40 to 60 DEG C and be treated by a two-step high-pressure homogenizer to obtain the milk-white vitex oil nanoemulsion with the average particle size of less than 100nm. The vitex oil nanoemulsion prepared by the invention has high quality, reliable quality stability and good biocompatibility, and simultaneously has good expectorant, antitussive and antiasthmatic effects.
Owner:江苏吴中苏药医药开发有限责任公司
New Application of Alkaloid Compounds of Fritillaria sichuanensis
InactiveCN102283851AEnhance anti-inflammatoryObvious antitussiveOrganic active ingredientsAntipyreticPhosphoric acidKetone
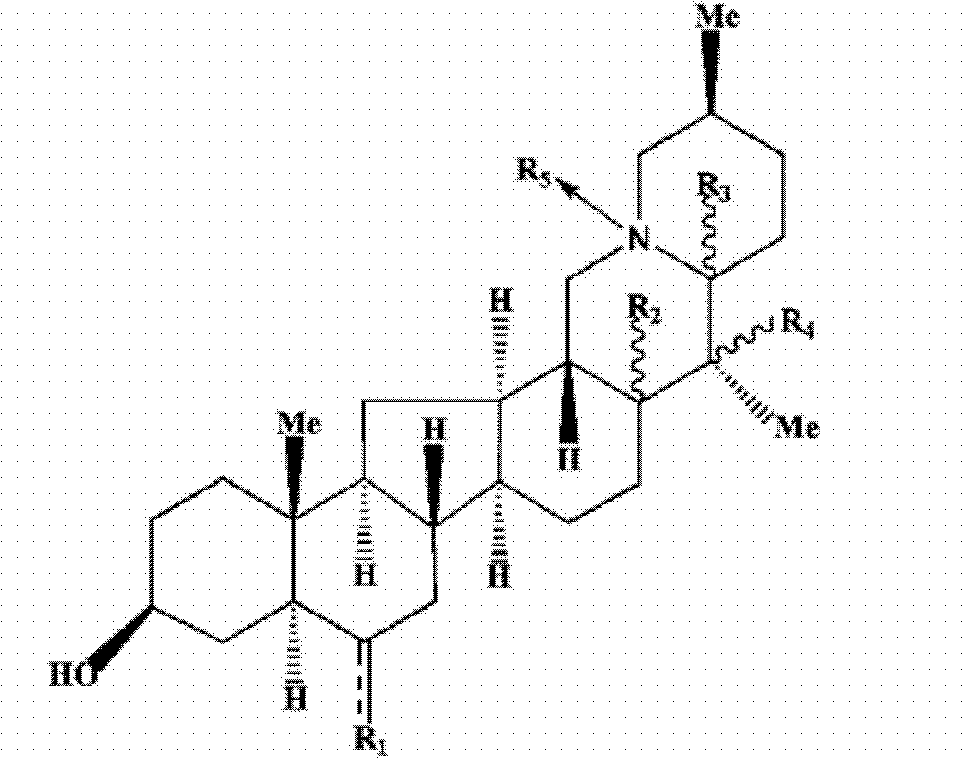
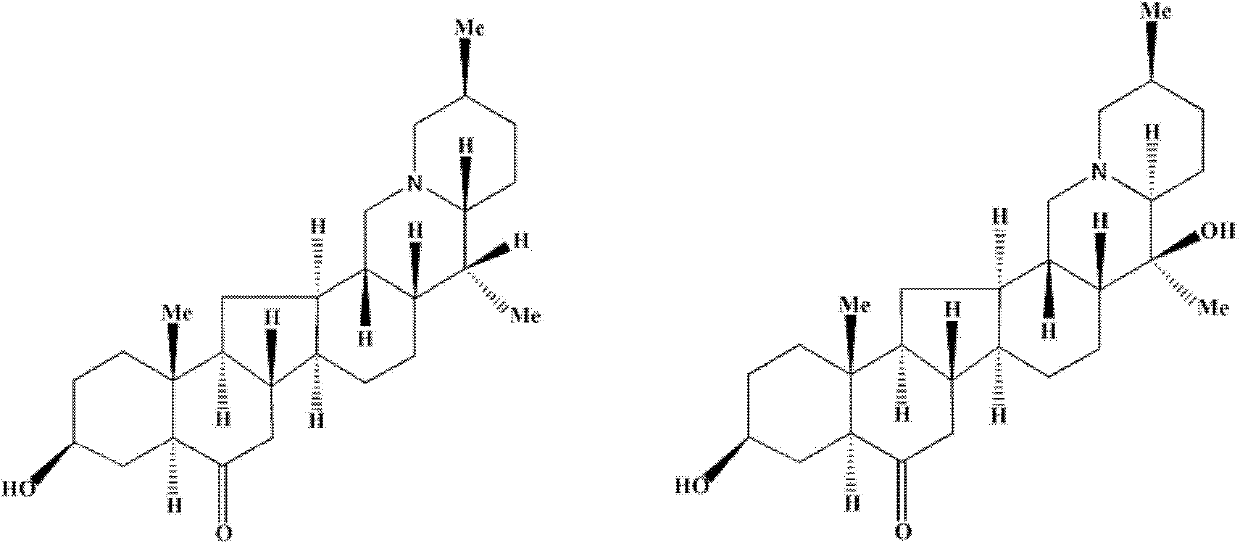
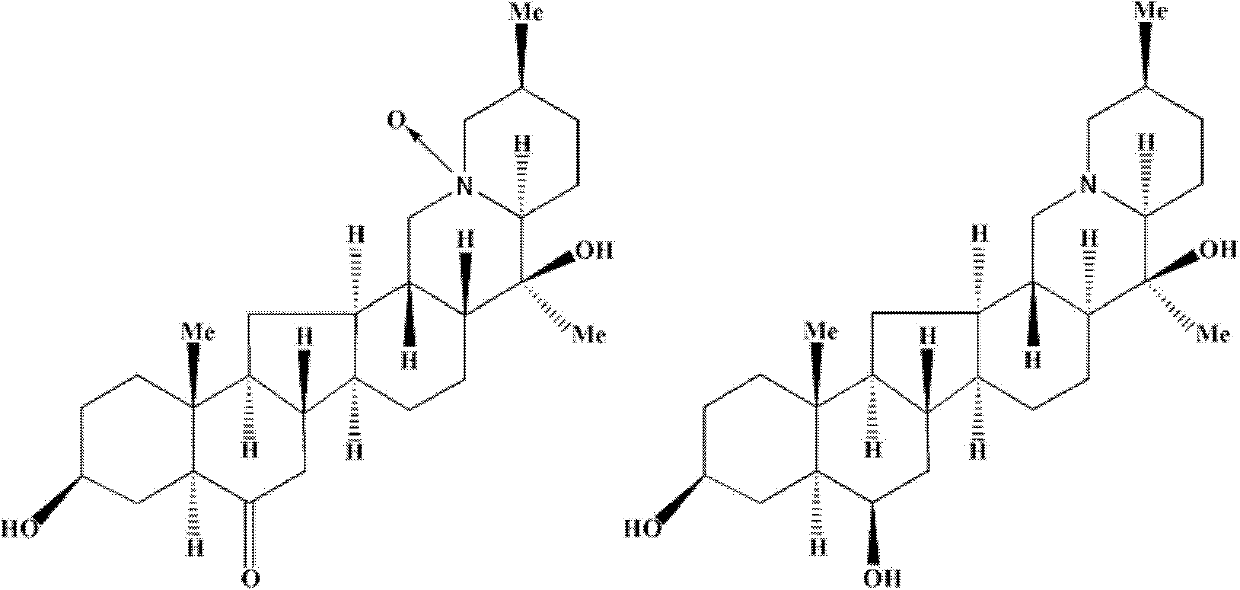
The present invention discloses the use of Fritillaria fricarifolia alkaloid compounds represented by general formula I in the preparation of antitussive, expectorant and / or anti-inflammatory drugs, wherein R1 is: =O or R2 is: H; R3 is: H; R4 is H or OH; R5 is: O or none. Among them, Sibetine, Tribenidone, Sibetine Nitrogen Oxides, Isofibesine, Isofibesine Nitrogen Oxides and 10 times the amount of codeine phosphate have similar antitussive effects, and the 5 The antitussive and anti-inflammatory effects of this monomer are also superior to peiminine and peiminine; among them, peiminine, peiminine, sibenetine, sibemine nitrogen oxide, and isobeamine Nitrogen oxides have a good expectorant effect. The invention also discloses the total alkaloid extract of Fritillaria wabu and its preparation method and application. Formula I
Owner:SICHUAN UNIV
Composition for treating chicken mycoplasma respiratory infection and preparing method thereof
InactiveCN103417571AEasy dischargeEasy to removeAntibacterial agentsOrganic active ingredientsDiseaseRespiratory disease
The invention discloses a composition for treating chicken mycoplasma respiratory infection and a preparing method of the composition. The composition comprises, by weight, 1-10% of erythromycin thiocyanate, 40-90% of ammonium chloride and the balance anhydrous dextrose. According to the composition, the erythromycin thiocyanate is used as a main ingredient for resisting mycoplasmas, is effective in various mycoplasmas causing the chicken respiratory infection, is taken orally and can be absorbed, and can effectively treat mycoplasma infection of respiratory tracts; the ammonium chloride is an expectorant and enables sputum to be discharged easily through increase of sputum quantity, thereby facilitating removal of phlegm, enabling cheesy material and phlegm in tracheas to be diluted, dissolved and discharged out of the body, and keeping smooth breath. Through combination of the two medicines, the composition aims at pathogeny and also aims at symptomatic treatment, can effectively improve the cure rate, and reduces losses caused by respiratory disease characteristics.
Owner:TIANJIN SHENGJI GRP CO LTD
Expectorant and asthma-curing tea
InactiveCN101036491AHeat-clearingWith phlegmPre-extraction tea treatmentAntipyreticAster tataricusColtsfoots
The invention discloses an apophlegmatic and antasthmatic tea, which is made of 2% to 4% of coltsfoot flower, 8% to 16% of dahurian rhododendron leaf, 5% to 8% of guangdong earthworm, 5% to 8% of Aster tataricus tatarian aster, and 5% to 8% oftea leaf. The preparation process comprises the steps of selecting the blending materials according to said proportion, mixing, cleaning, crushing, drying, filling the nonwoven-fabrics inner bag with the blending materials, filling the aluminum foil outer bag with the inner bag, and hot pressing and sealing.
Owner:范传玲
Pharmaceutical formulation
A formulation for oral administration comprises an expectorant, an analgesic, and at least one additional active ingredient having a modified release providing a therapeutic effect for each of the active ingredients for up to 12 hours.
Owner:RB HEALTH US LLC
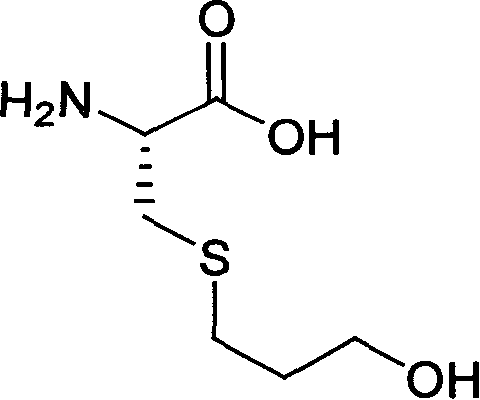
Method for preparing expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol
InactiveCN102050748APreparation method greenEconomical method of preparationOrganic compound preparationAmino-hyroxy compound preparationNitrogenCyclohexanol
The invention relates to a method for preparing an expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol. Nitrobenzaldehyde and trans-4-amino cyclohexanol are subjected to the combination reaction of condensation, carbon and nitrogen double-bond hydrogenation and nitro reduction to prepare the trans-4-[(2-amino benzyl) amino]-cyclohexanol in a high-yield mode. The method has the advantages of reasonable process route design, simple and convenient process, high reaction yield, low raw material cost and no harsh reaction conditions and is easy for scale production.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Appetite control compositions and methods of use
Appetite control compositions comprise Gymnema sylvestre and an expectorant. In some embodiments, the appetite control compositions contain from about 3 mg to about 50 mg of gymnemic acid (an extract of Gymnema sylvestre), from about 10 mg to about 80 mg of glycyrrhizin (an extract of Glycyrrhiza glabra), high impact flavor, and a sweetening agent in a non-traditional dosage form. A non-traditional dosage form provides for the topical application of medicaments to the tissues of the mouth and tongue, more specifically, to the sweetness taste receptors of the tongue. By delivering gymnemic acid to the sweetness receptors of the tongue, the sensation of sweetness is blocked, thereby providing appetite control.
Owner:CRAVE BUSTERS
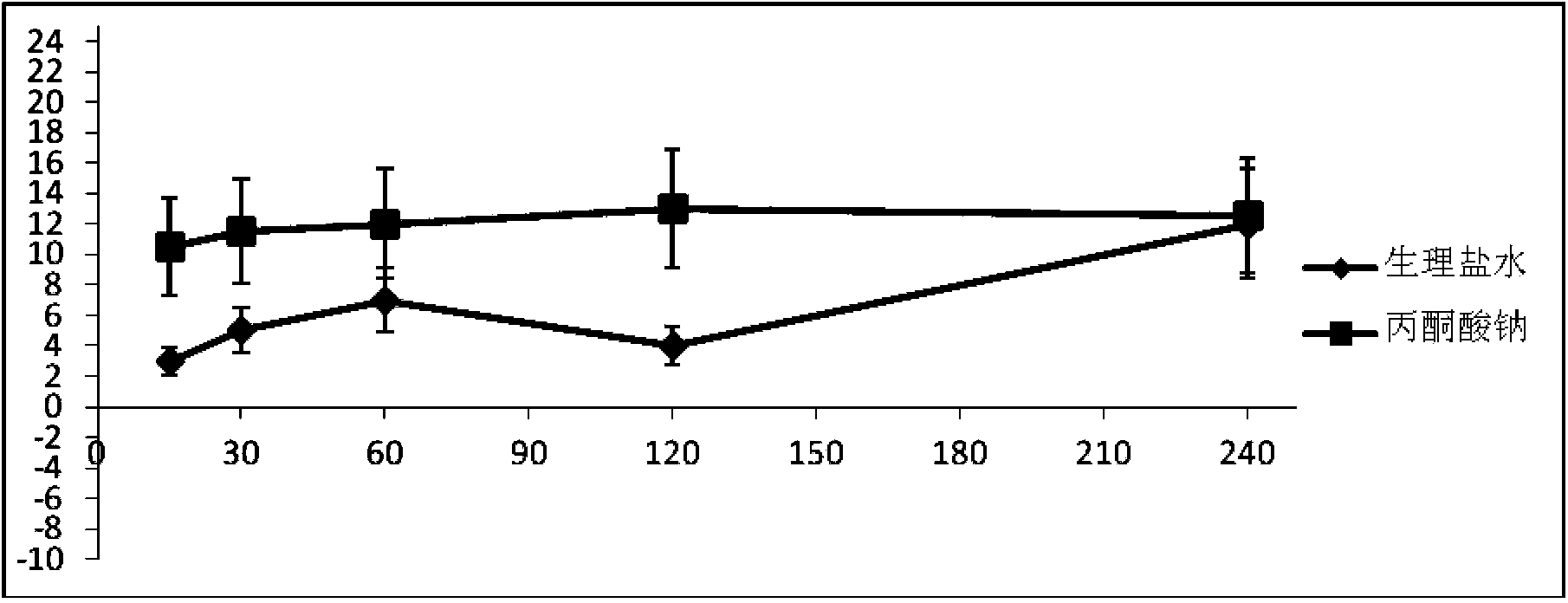
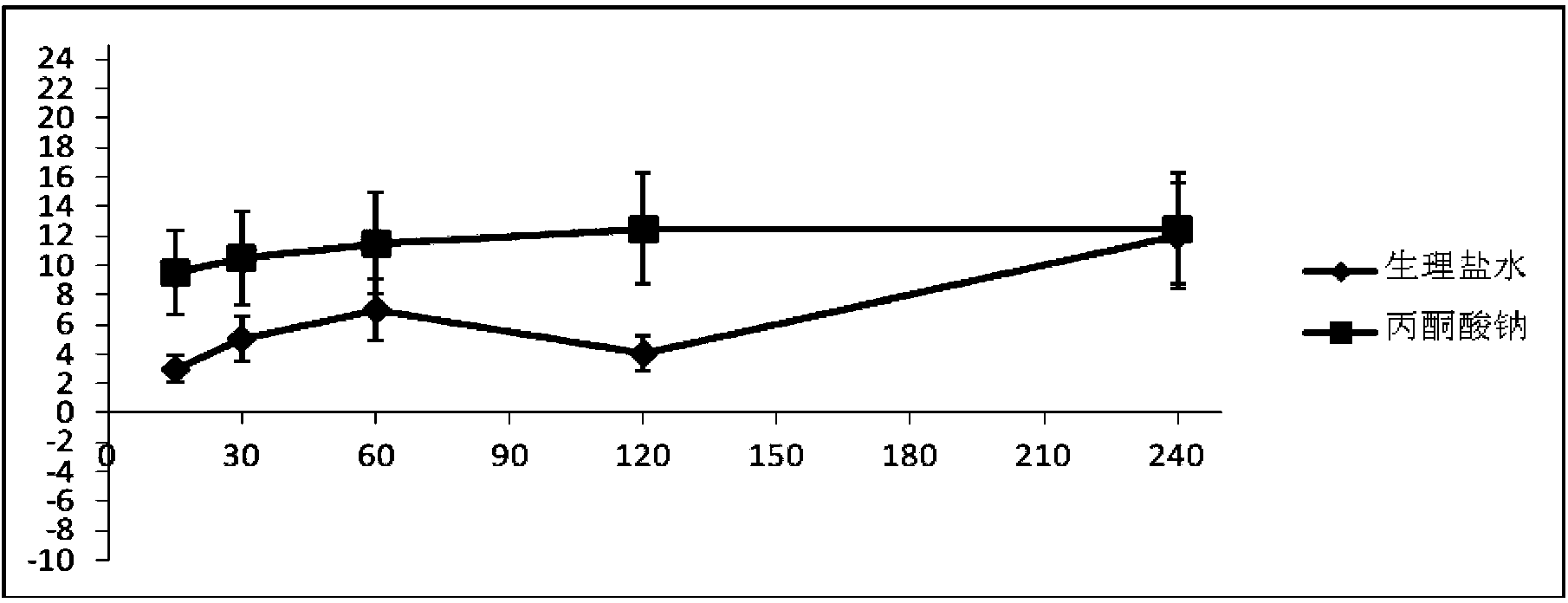
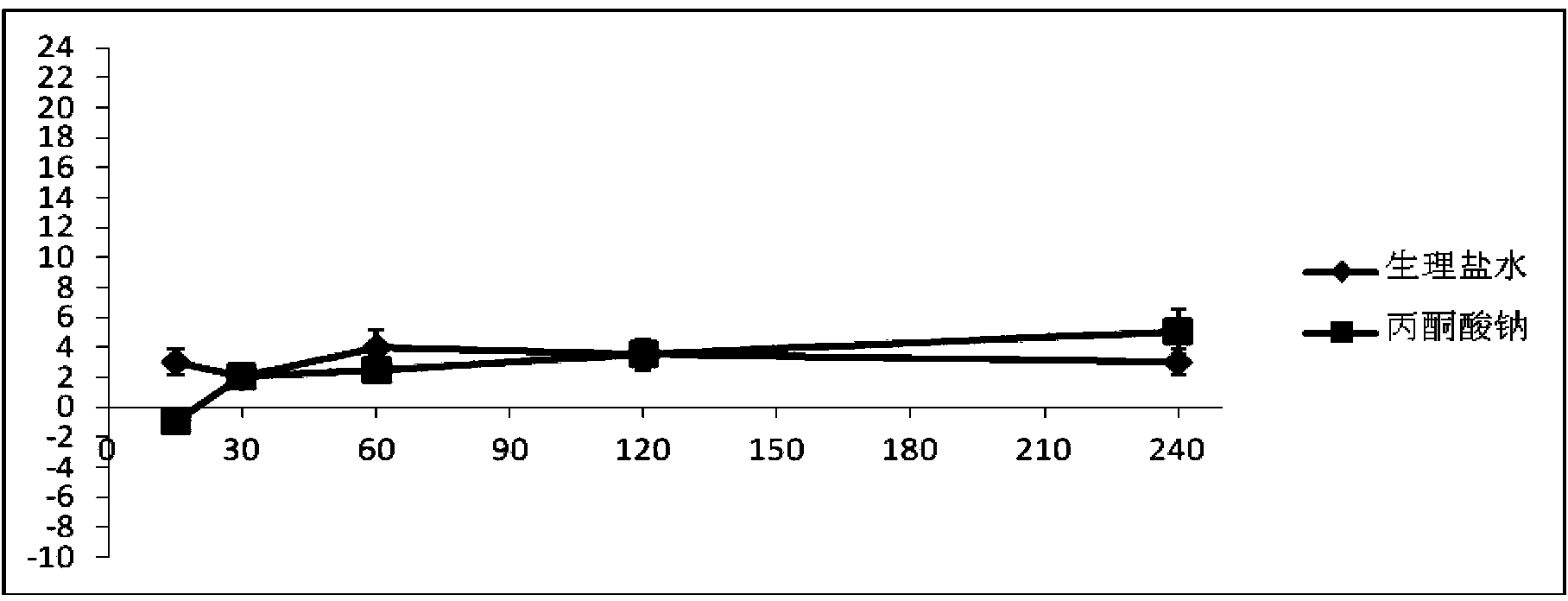
Pyruvic acid medicinal composition and application thereof in preparation of medicament for treating chronic obstructive pulmonary diseases
InactiveCN103720682AImprove targetingTargetedAntinoxious agentsPharmaceutical delivery mechanismSide effectWhole body
The invention provides an inhalant composition for treating chronic obstructive pulmonary diseases. Active ingredients in the composition include pyruvic acid, medicinal pyruvate, a pyruvic acid medicinal precursor or a composition thereof. The chronic obstructive pulmonary diseases include chronic bronchitis and emphysema. Compared with the prior art, the inhalant composition used for treating chronic obstructive pulmonary diseases has the advantages that 1, the inhalant can directly enter the lung in a highly targeting manner, can directly act on the focus of the lung in a highly targeting manner, and does not participate in body metabolism; 2, the medicinal pyruvate, the pyruvic acid precursor and the pyruvic acid in the composition are nutrient substances, and are abundant in the blood, so that the toxic and side effects are reduced, and higher safety and medicament stability are achieved; 3, as an antioxidant, sodium pyruvate differs from antibiotics, expectorants and sterol medicaments, acts more comprehensively, and is better in effect.
Owner:JIANG SU PHARMAMAXCORP
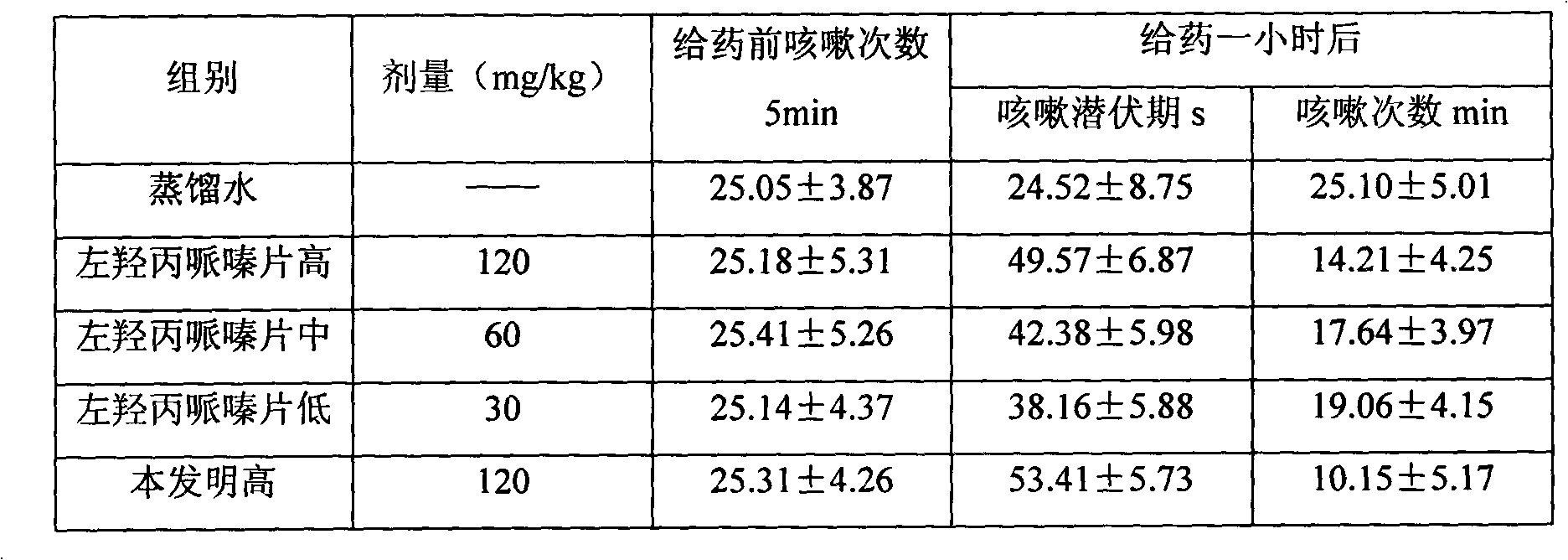
Compound pharmaceutical chemical acting on respiratory diseases and preparation process and application thereof
The invention provides pharmaceutical composition for respiratory diseases and a preparation process thereof, in particular to pharmaceutical composition and a preparation process and application thereof. The pharmaceutical composition comprises active ingredients, namely levodropropizine and carbocysteine, and pharmaceutically acceptable accessories. Compared with commonly used existing antitussive drugs or expectorants in the market, the pharmaceutical composition has more evident effect of cough relieving and fewer adverse reactions and has certain social benefit and economic benefit.
Owner:HUNAN JIUDIAN PHARMA
Preparation method and clinical application of acetylcysteine powdered injection and acetylcysteine infusion
The acetylcysteine has always been widely used as expectorant in clinic in domestic, with the using form being spray (powder spray) and effervescence tablet, the invention relates to a method for preparing the powder injection of acetylcysteine (comprising freeze-drying injection, dissolvent crystalline powder, un-dissolvent crystalline powder, etc) and large fluid infusion and its clinical application, the powder injection of acetylcysteine can not only be used in form of injection and spray for removing the phlegm, but most importantly it can be used in form of injection for treating various hepatitis, especially the light or heavy chromic, acute hepatitis B, and etc.
Owner:姜建国 +1
Ibuprofen solutions for capsule-filling and capsule preparations
InactiveCN1471389AAvoid bitternessQuick-actingNervous disorderHydroxy compound active ingredientsPh controlStimulant
Capsule preparations are produced by using solutions for capsule-filling which contain ibuprofen, polyethylene glycol, water and terpenes (menthol, limonene, borneol, dl-camphor, mentha oil, etc.) optionally together with one or more drugs selected from among antipyretic analgesics, antihistamines, antitussives, expectorants, sympathetic stimulant, analeptics, hypnotic sedatives and anti-inflammatory agents, solubilizers, thickeners, pH controlling agents, colorants, etc. Thus, it is attempted to relieve the bitterness of ibuprofen and establish an immediate action at the same time.
Owner:KOWA CO LTD
Kits for prevention and treatment of rhinitis
Owner:HALL MISCHELLE +2
Extended release formulations of guaifenesin
InactiveUS20090202633A1BiocideEther/acetal active ingredientsExtended Release FormulationsGuaifenesin
The present invention relates to extended release formulations comprising expectorant. More particularly, the present invention relates to extended release formulations comprising guaifenesin. The present invention also relates to a process for the preparation of extended release formulations comprising guaifenesin.
Owner:AUROBINDO PHARMA LTD
Green bamboo health-care beverage and preparation method thereof
InactiveCN101416722AInhibit peroxidationScavenge active oxygen free radicalsFood preparationEnzyme digestionReactive oxygen radicals
A green bamboo health-care drink and a preparation method thereof, characterized by comprising the following dry raw materials: 70-90 parts of green bamboo leaves, 4-15 parts of hericium erinaceus or dictyophora phalloidea, 5-10 parts of chrysanthemum or grass jelly, and appropriate amount of sugar and purified water. Conventional microwave extraction method and\or compound enzyme digestion method, or such conventional extraction methods as hot water extraction method or ethanol extraction method; total flavonoid or other effective active materials are extracted from green bamboo leaves. The finished products can be green bamboo juice health-care drinks and green bamboo leaf flavone oral liquid drinks. The green bamboo health-care drink has all-round nutrition, can eliminate many kinds of active oxyradical, prevents nitrosation reaction and has the functions of protecting blood vessel of brain, fatigue, ageing and bacterial resistance, anti-inflammation, antiviral, decreasing triglyceride, relieving cough and expectorant, and heat-clearing and detoxifying material.
Owner:福建国鑫绿色食品发展有限公司
Polygala dropping balls and preparation thereof
ActiveCN1679669AIncrease surface areaHas a wetting effectUnknown materialsRespiratory disorderMedicinePolygala
An expectorant in the form of dripping pill is prepared from polygala root. It is based on the polygala syrup.
Owner:JIANGXI JIMINKEXIN PHARMA
A kind of traditional Chinese medicine composition for treating vertigo
InactiveCN102293946AEffective and reliableMedicinal and calmAnthropod material medical ingredientsDigestive systemSide effectGastrodia
The invention relates to a traditional Chinese medicine composition for treating vertigo, which is composed of the following traditional Chinese medicine raw materials in parts by weight: 10-40 parts of astragalus, 5-30 parts of angelica, 5-25 parts of Gastrodia elata, 10-40 parts of Codonopsis pilosula, 5 parts of Fushen ~30 servings, 5-25 servings of polygala, 5-25 servings of yuan meat, 10-40 servings of fried jujube kernels, 10-40 servings of dragon teeth, 5-30 servings of night vine, 5-25 servings of taro meat, 5-25 servings of yam 30 parts, Rehmannia glutinosa 10-40 parts, Guizhi 5-25 parts, Aconite 5-20 parts, Cuscuta 5-30 parts, Angelica dahurica 5-20 parts, Schisandra chinensis 5-25 parts, mulberry octopus 5-30 parts, roses 5 to 25 servings. The composition of the invention has the effects of calming the liver and quenching the wind, removing phlegm and reducing turbidity, nourishing the heart and spleen, nourishing the liver and kidney, and benefiting qi and generating essence. It has a remarkable and reliable curative effect on treating vertigo. Advantages of toxic side effects.
Owner:荣成市崖头美全口腔诊所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![Method for preparing expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol Method for preparing expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/610db442-4a3f-42b8-80bc-143c958c71a7/BSA00000368100100011.PNG)
![Method for preparing expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol Method for preparing expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/610db442-4a3f-42b8-80bc-143c958c71a7/BSA00000368100100021.PNG)
![Method for preparing expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol Method for preparing expectorant, namely ambroxol key intermediate trans-4-[(2-amino benzyl) amino]-cyclohexanol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/610db442-4a3f-42b8-80bc-143c958c71a7/BSA00000368100100031.PNG)