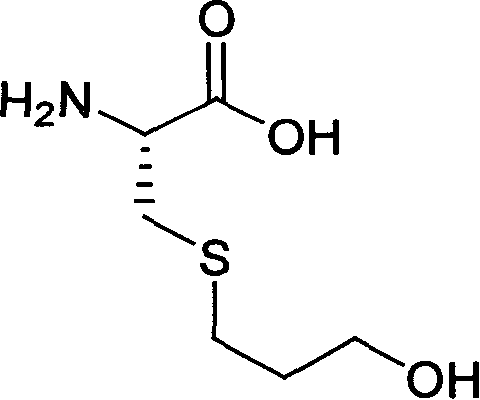
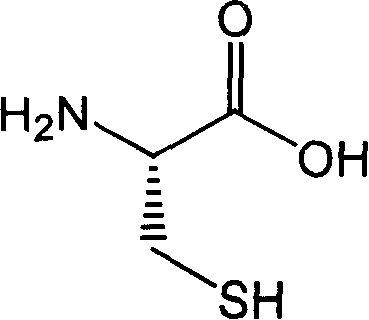
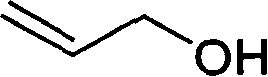
Process for preparing fudosteine
A compound, addition reaction technology, applied in respiratory diseases, organic chemistry, drug combination, etc., can solve the problems of high production equipment requirements, high cost, good solubility, etc., and achieve the effect of improving product quality and reducing requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0022] Dissolve 2.0 g (16.5 mmol) of L-cysteine in 20 ml of water, add 1.95 g (33 mmol) of allyl alcohol, and stir at room temperature. Add 200 mg of ferrous chloride and 200 mg of ammonium peroxodisulfate and continue stirring for 15 minutes. After the reaction, the solvent was evaporated under reduced pressure, ethanol (25%) was added to the residue, the insolubles were filtered off, the filtrate was concentrated under reduced pressure, and the residue was recrystallized from a water-ethanol system to obtain 2.3 g of colorless crystals with a yield of 80%. %.
Embodiment 3
[0024] Dissolve 2.0 g (16.5 mmol) of L-cysteine in 20 ml of water, add 1.95 g (33 mmol) of allyl alcohol, and stir at room temperature. Add 200 mg of ferrous chloride and 200 mg of ammonium peroxodisulfate, and irradiate with a low-pressure mercury lamp (250-310 nm, 10 W) for 30 minutes. After the reaction, the residue was washed with ethyl acetate, the aqueous phase was concentrated under reduced pressure, and the residue was recrystallized from a water-ethanol system to obtain 2.78 g of colorless crystals, with a yield of 80%.
[0025] Embodiment of this patent
Embodiment 1
[0027] Mix 8.5L distilled water and 1.9kg L-cysteine, there is a small amount of insoluble matter. Add 2.2L propenyl alcohol, and it becomes a white cloudy liquid. After the addition is complete, heat to 50-60°C and stir to react for more than 7 hours. Stirring was stopped, and 45 L of acetone was added dropwise to the reaction solution to precipitate a white solid. After dropping, continue to stir for 45 minutes, let stand for 0.5-1.0 hours, filter with suction to obtain a white solid, rinse the upper layer solid with 3-5L acetone. The obtained solid was dried at 45° C. for 24 hours to obtain 2.0 kg of product, with a yield of 80%.
[0028] Example 2:
[0029] Mix 4.3L distilled water and 1.0kg L-cysteine, there is a small amount of insoluble matter. Add 0.6L propenyl alcohol, and it becomes a white cloudy liquid. After the addition is complete, heat to 50-60°C and stir to react for more than 7 hours. Stirring was stopped, and 23 L of acetone was added dropwise to the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com