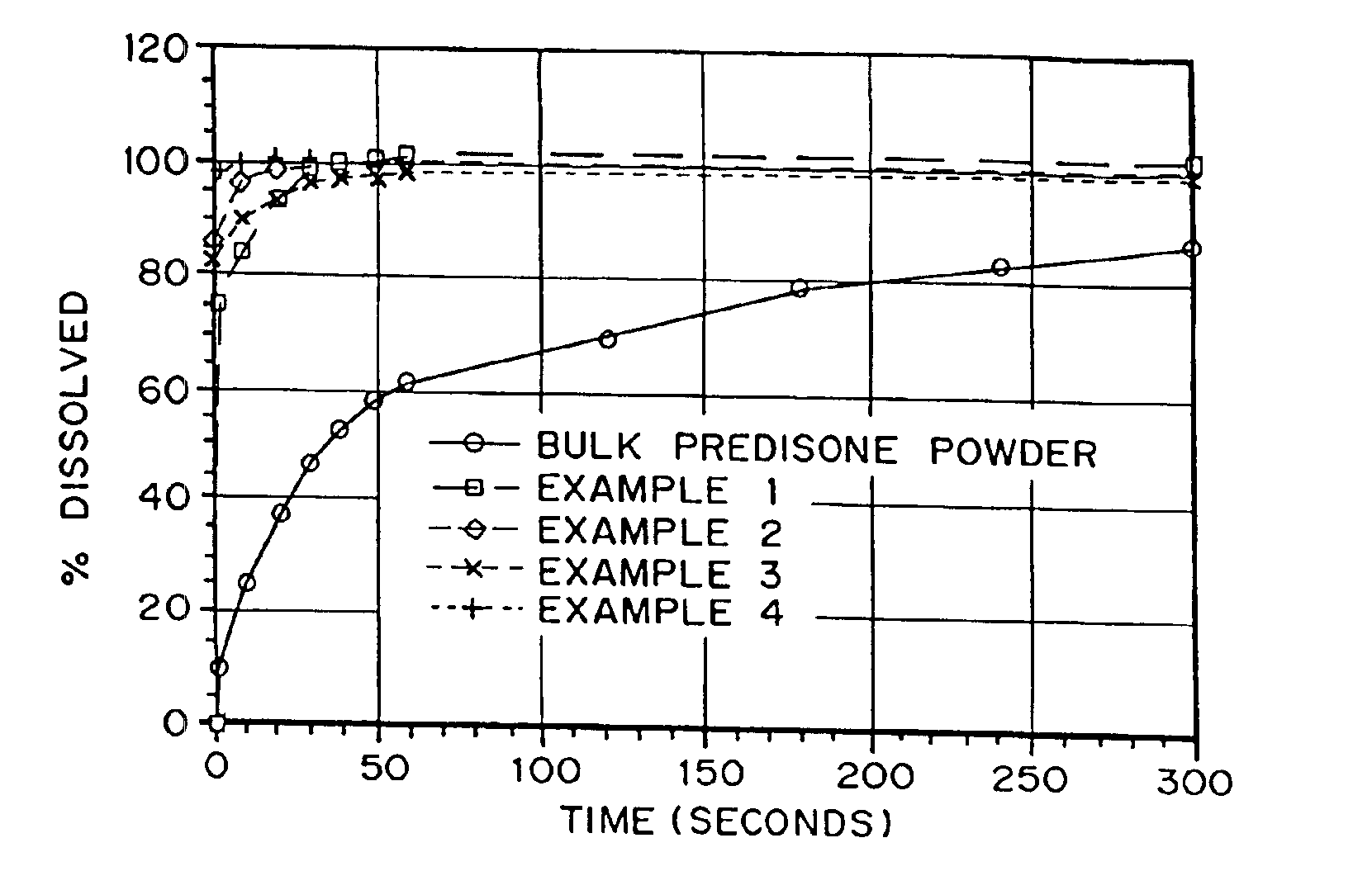
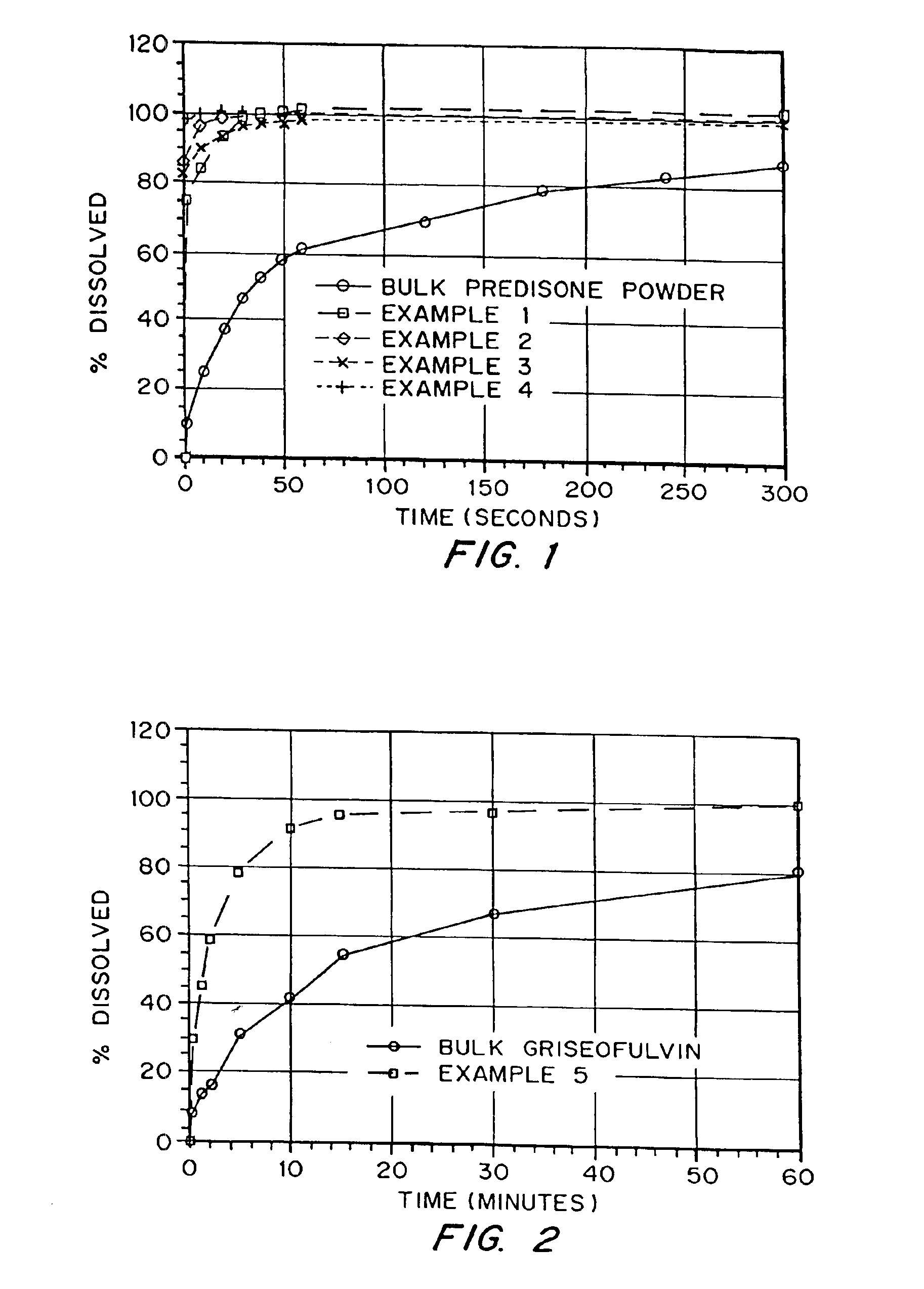
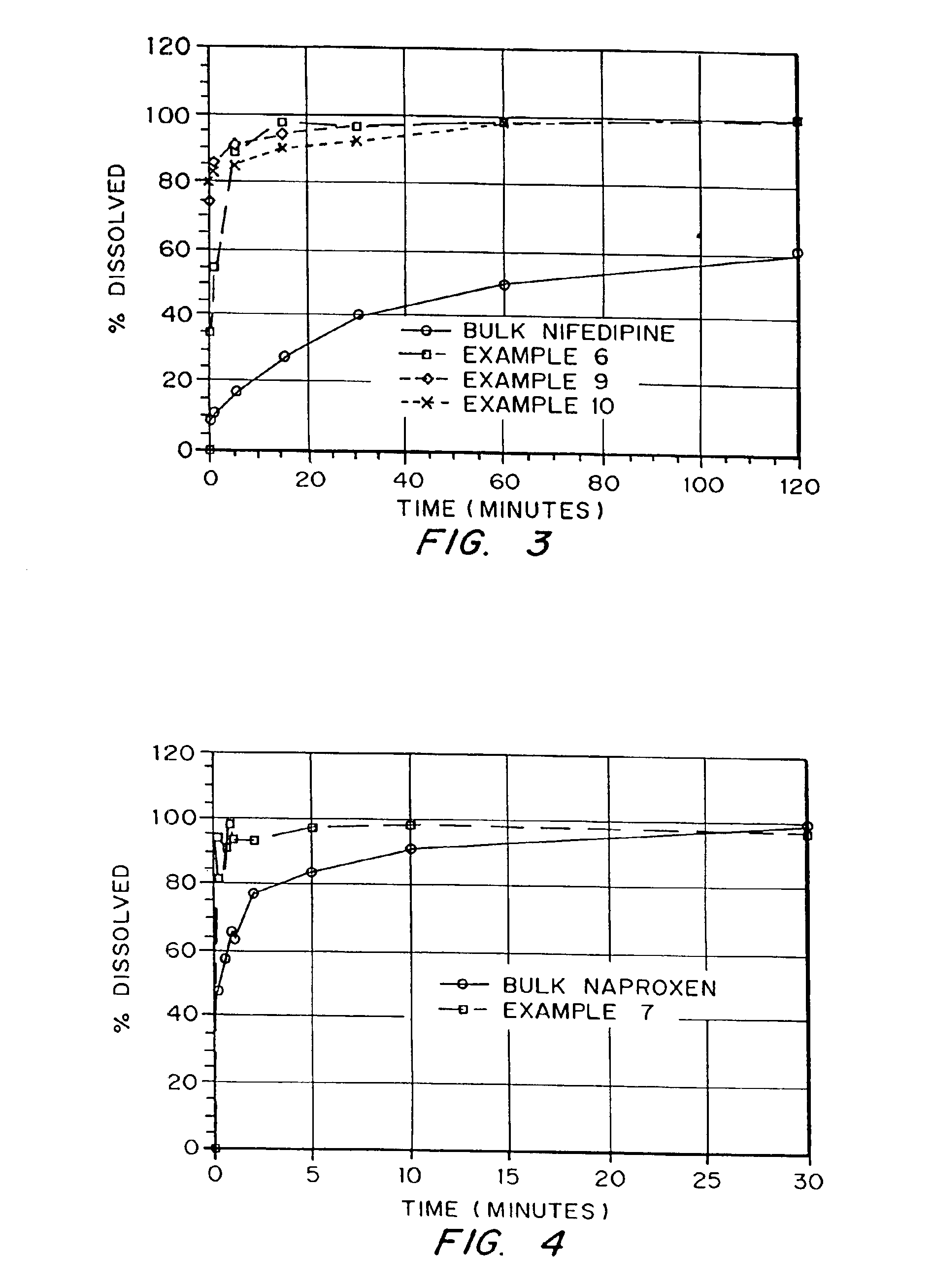
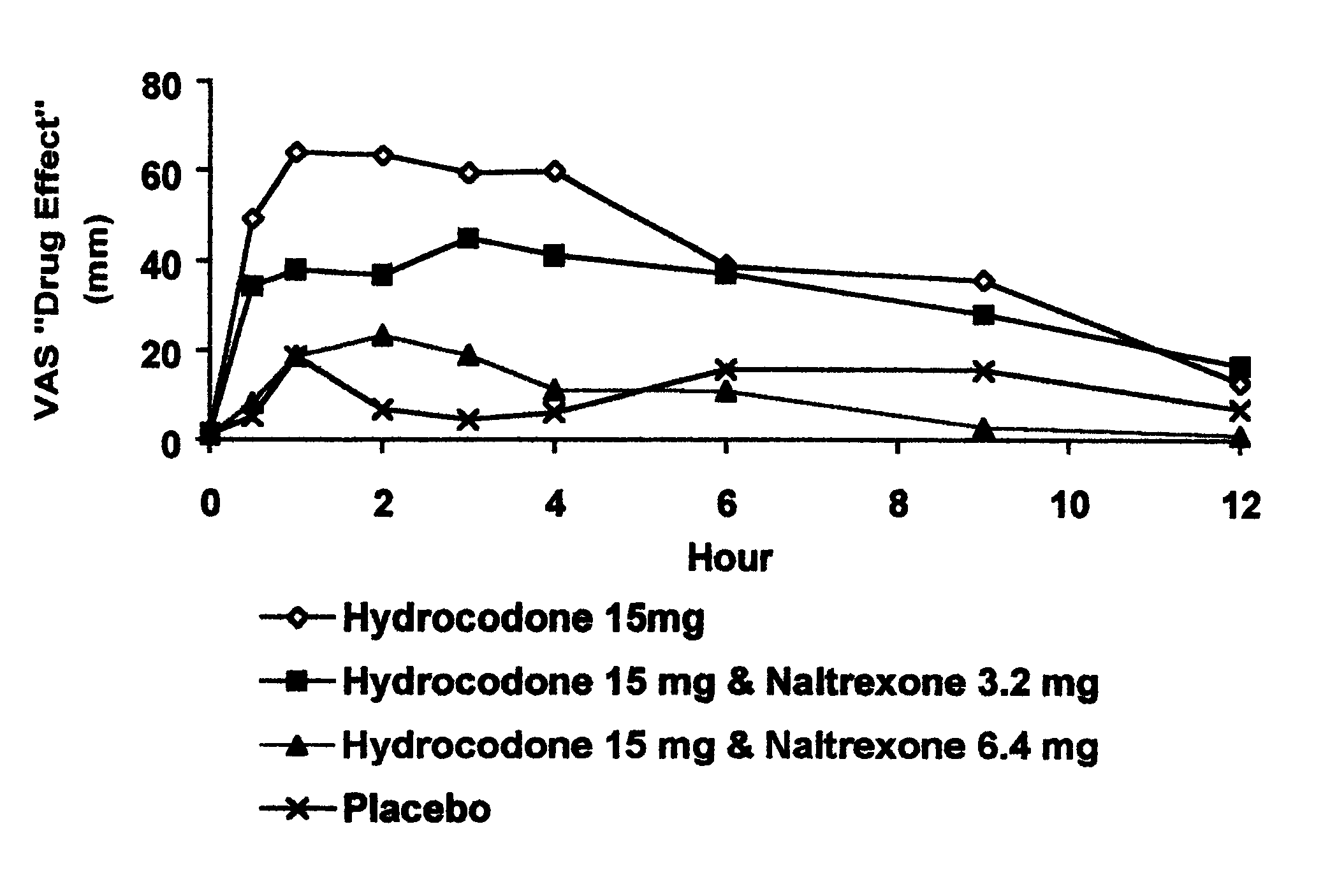
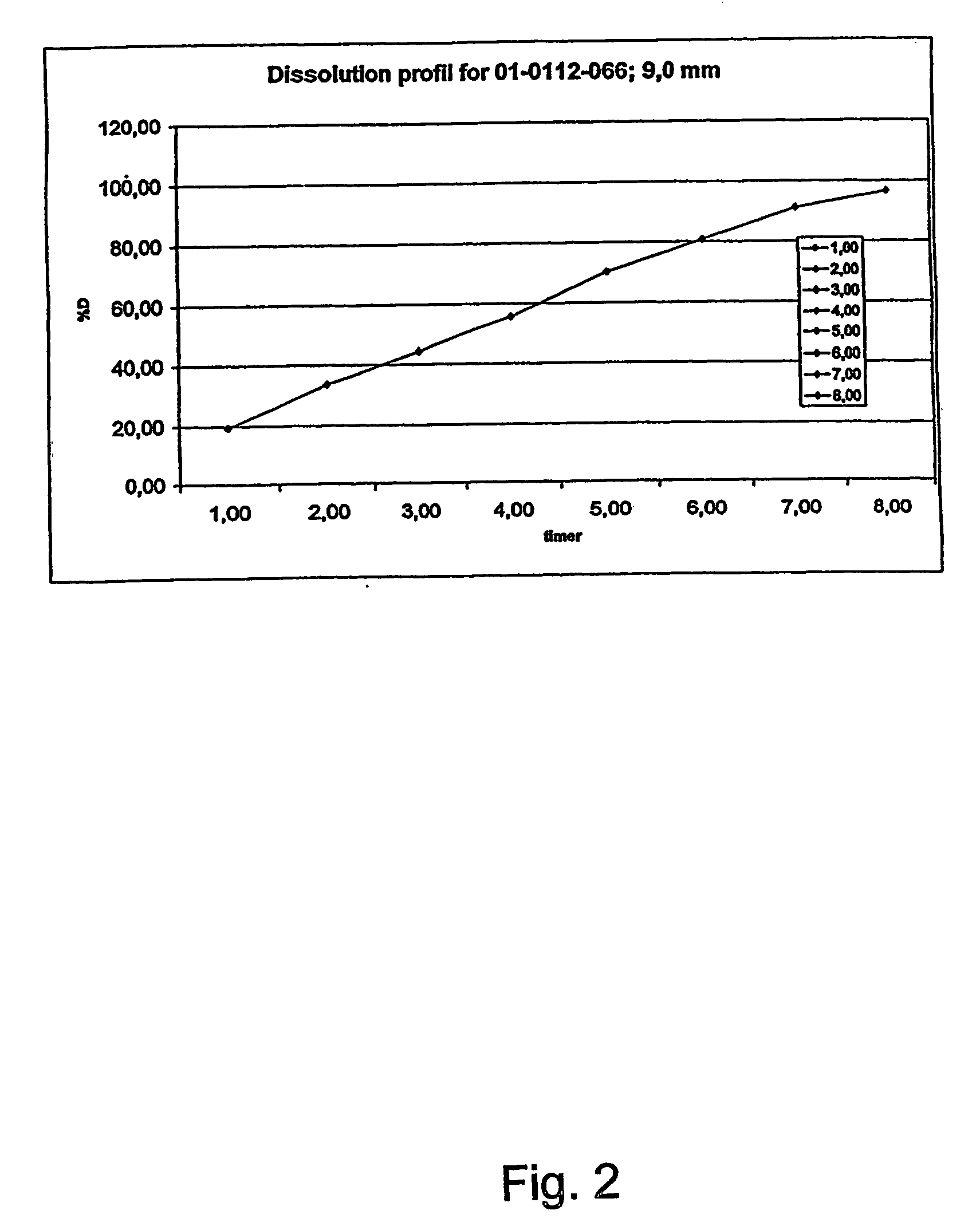
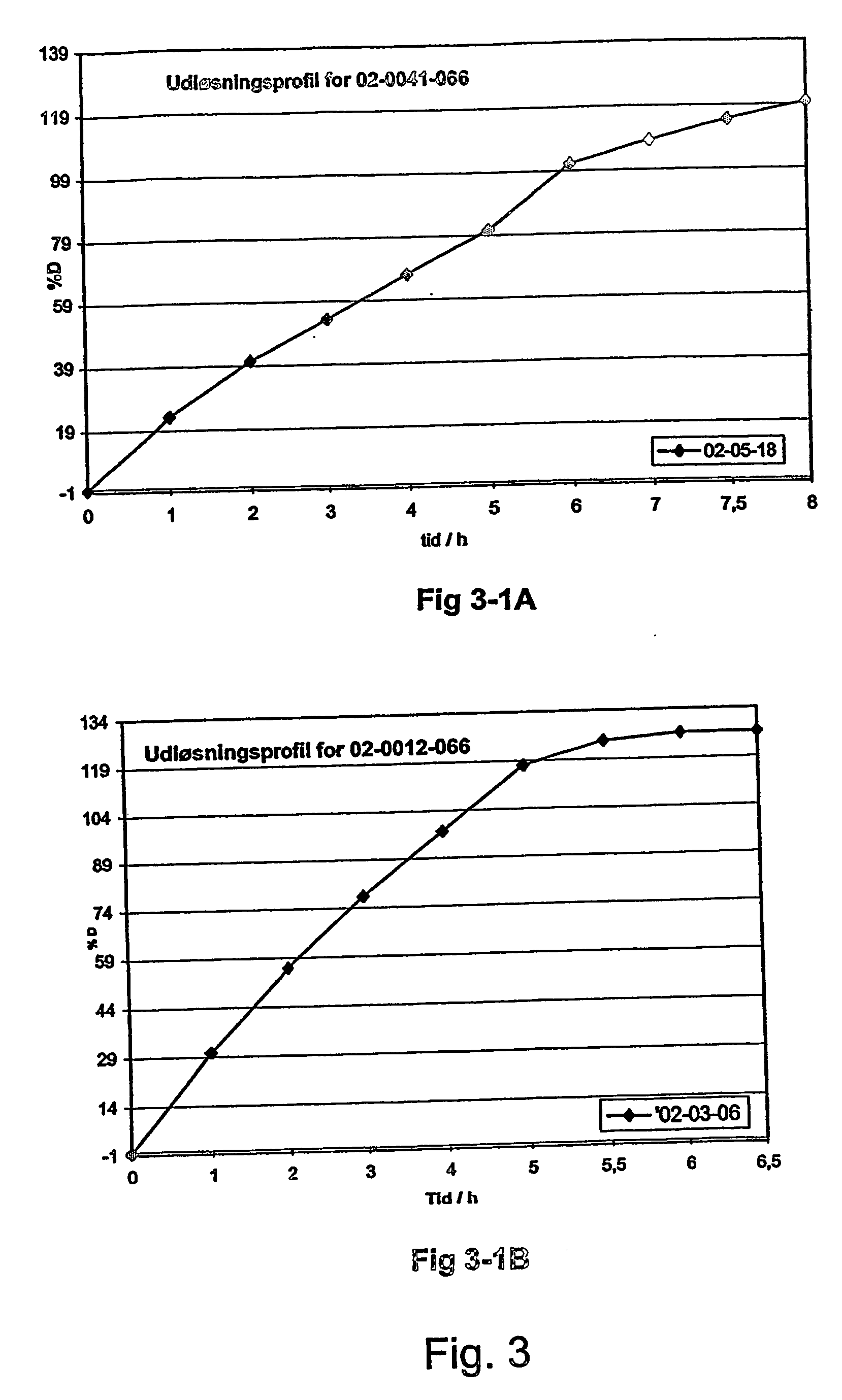
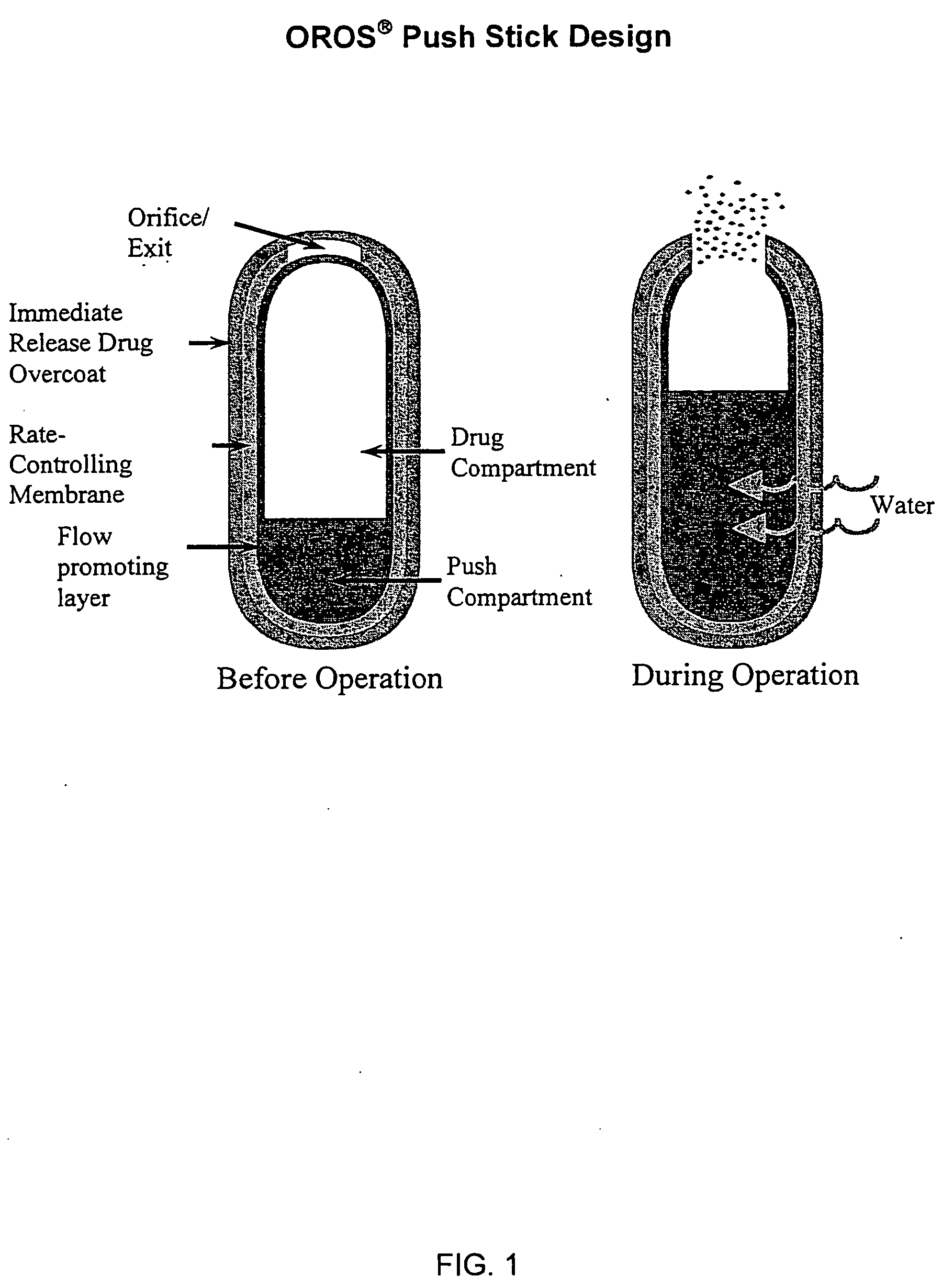
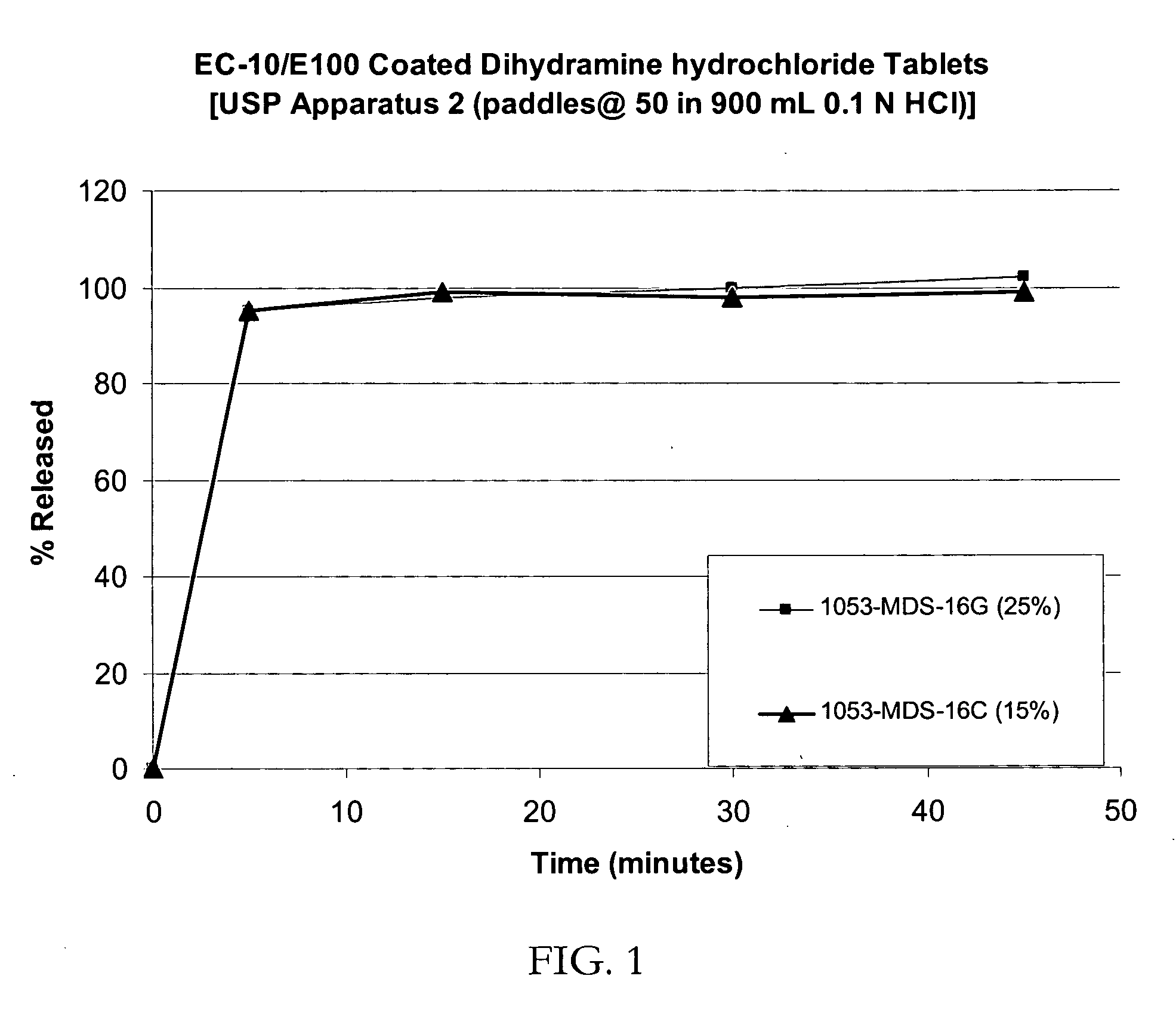
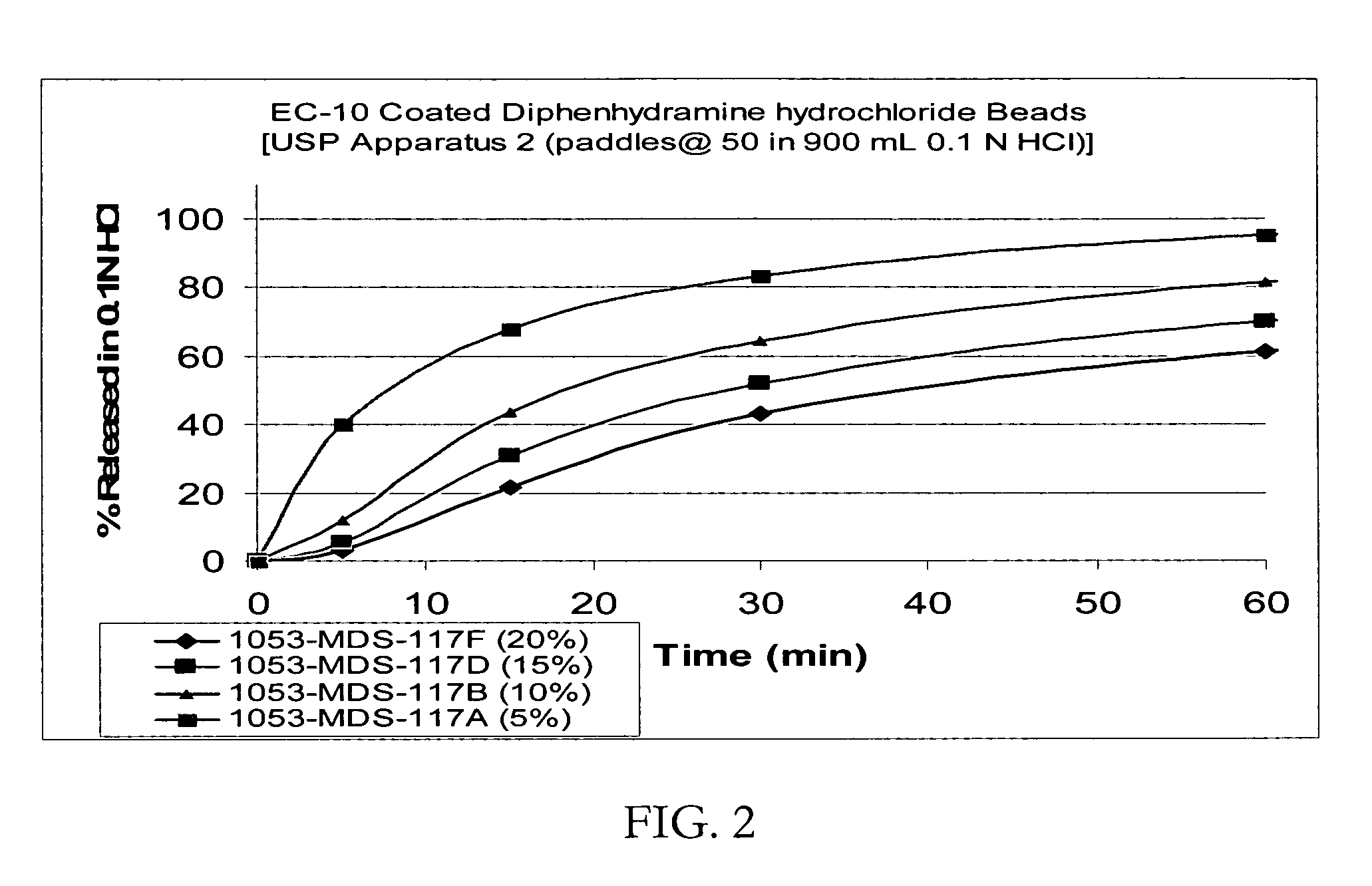
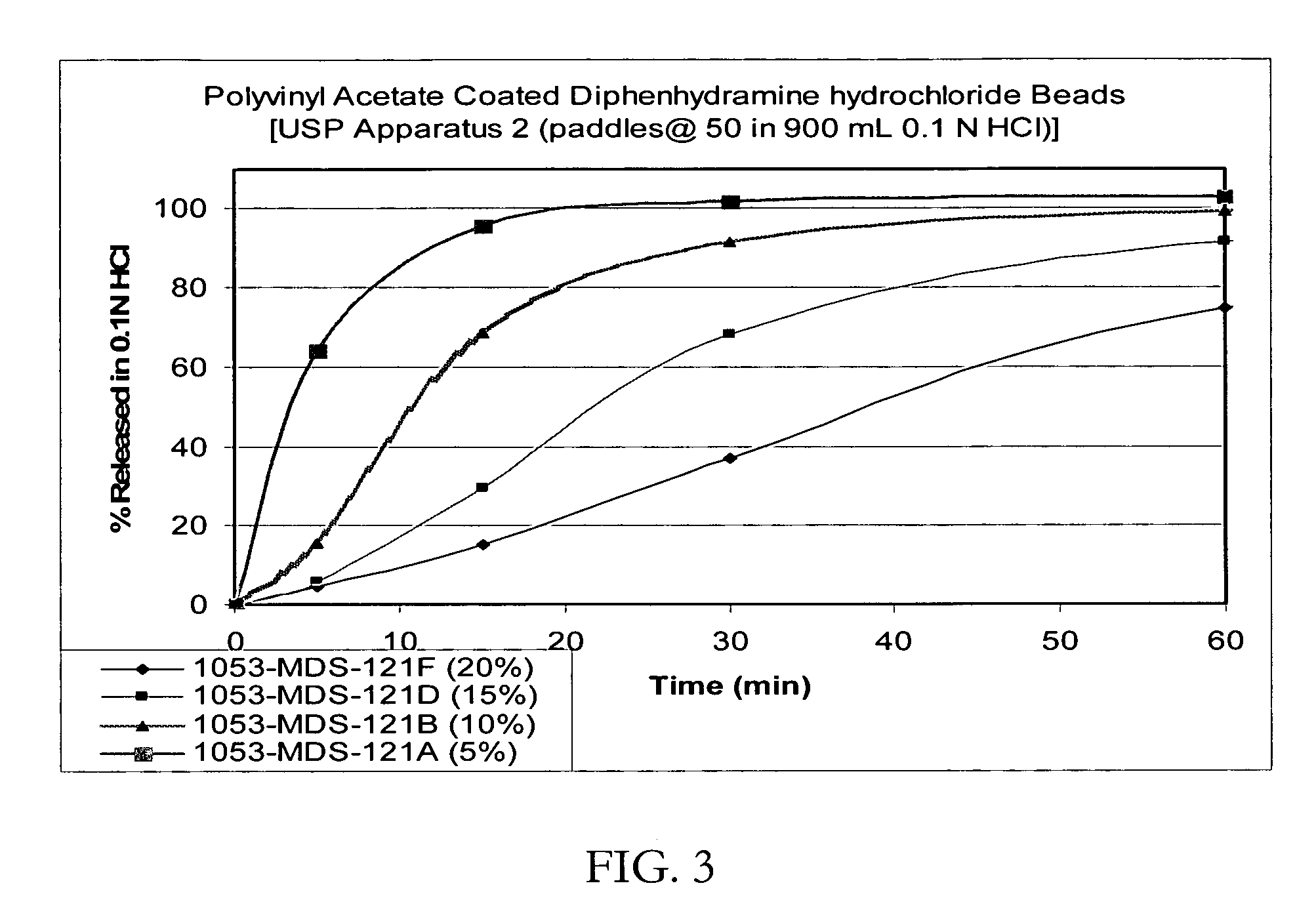
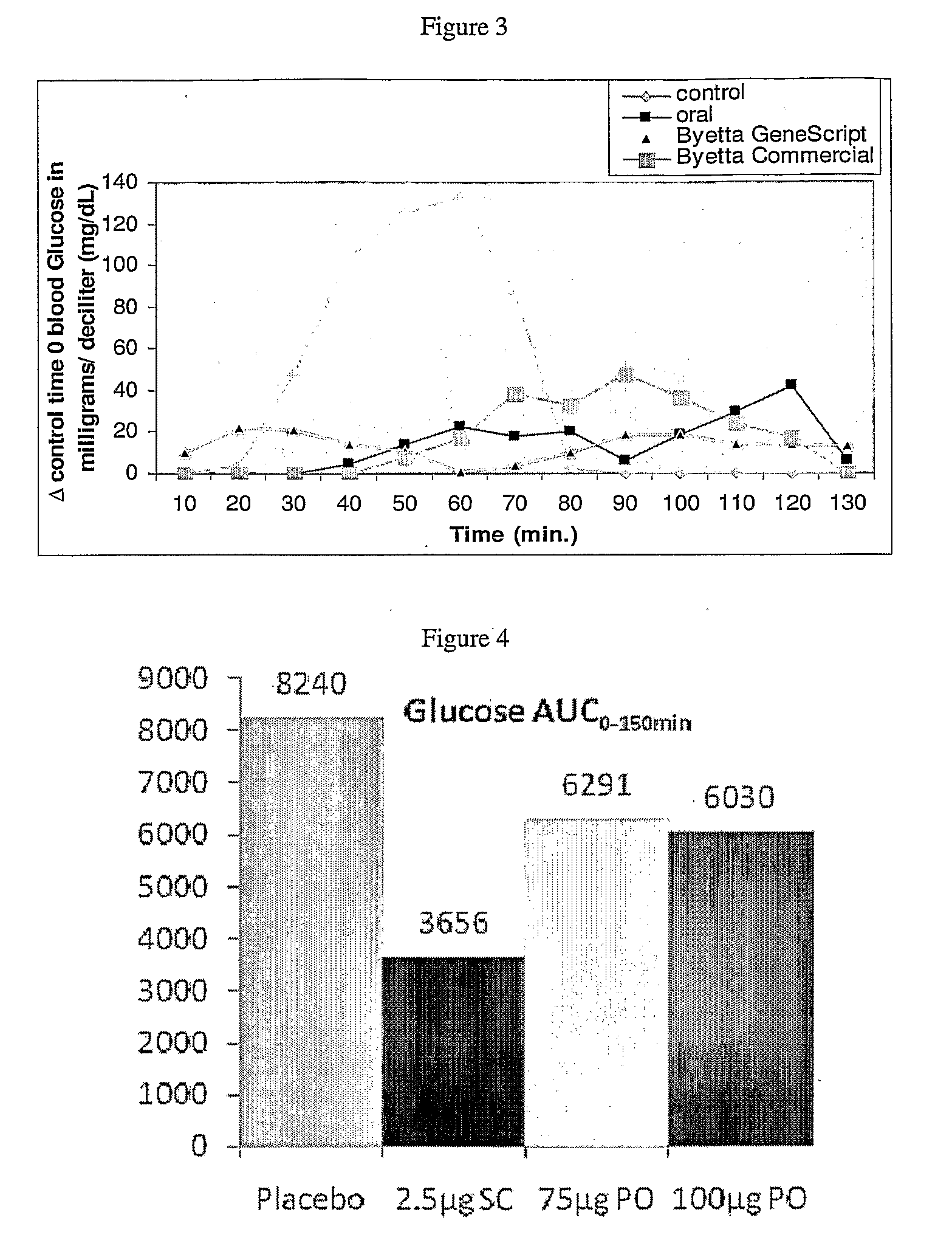
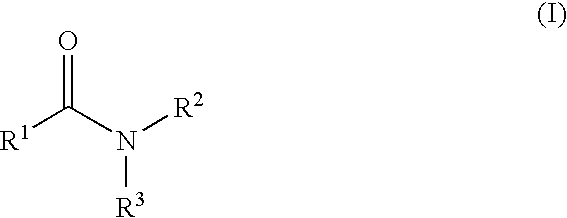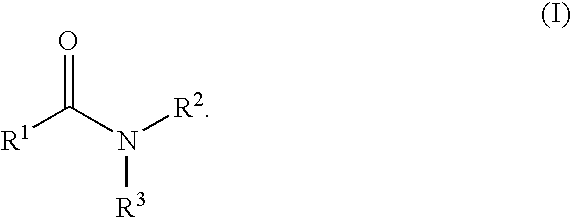Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
7368 results about "Oral medication" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oral administration is a route of administration where a substance is taken through the mouth. Per os (P.O.) is sometimes used as an abbreviation for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example.
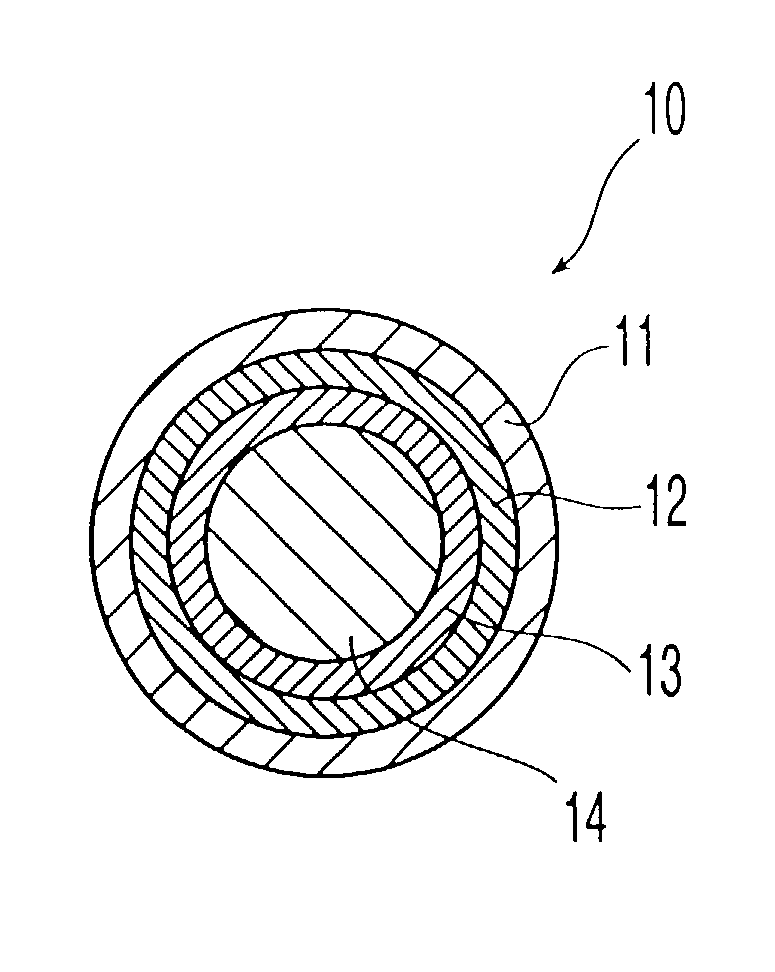
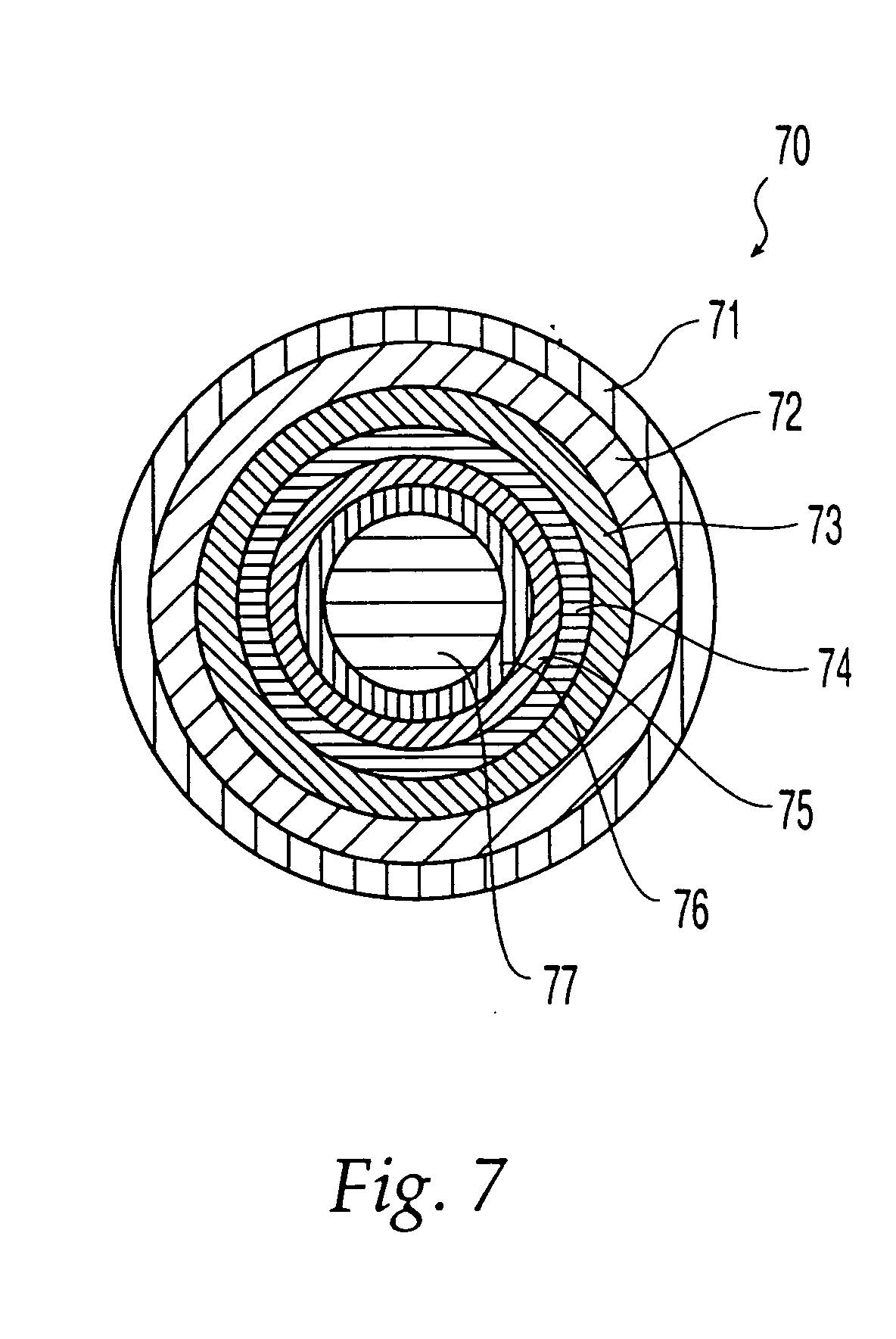
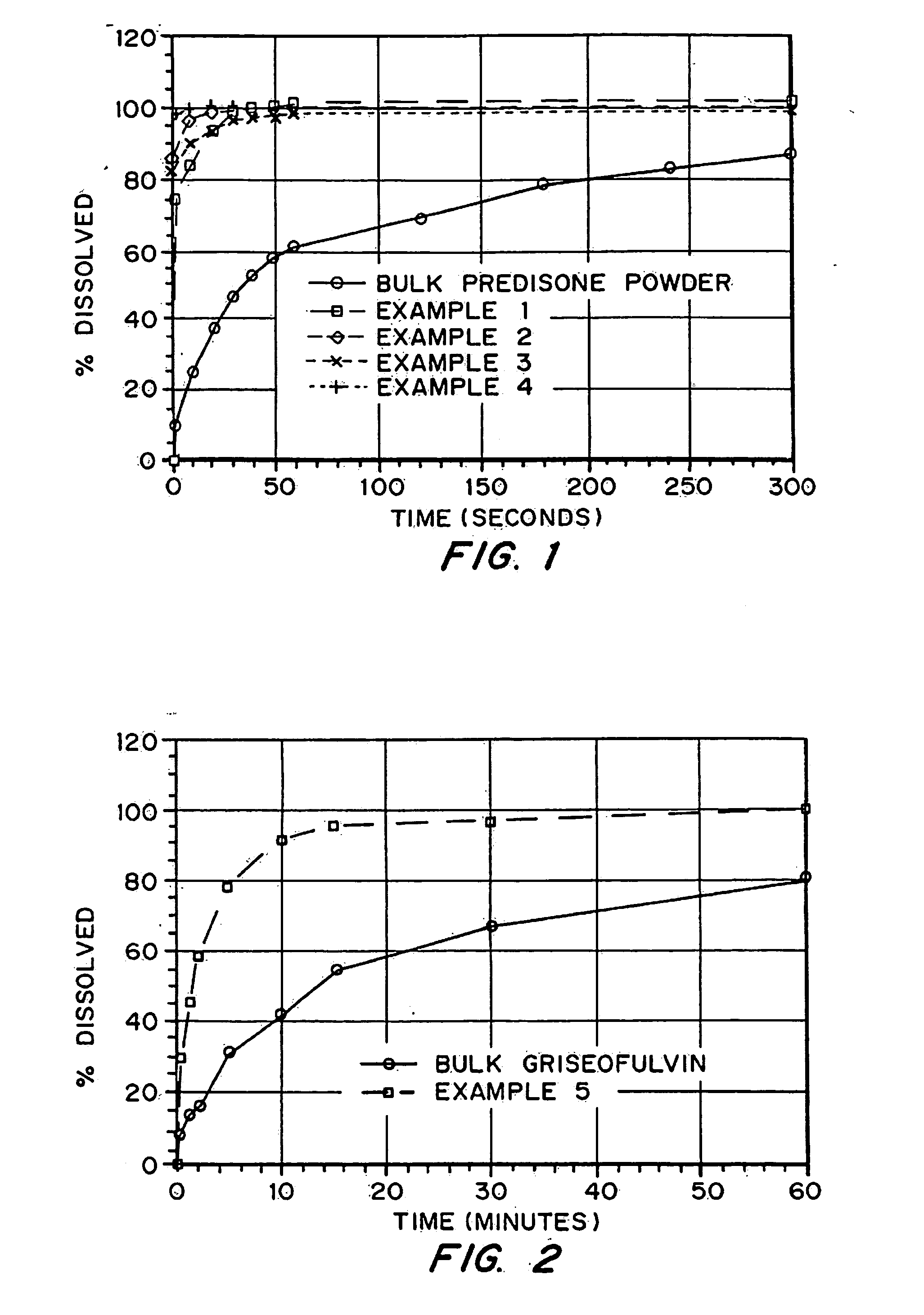
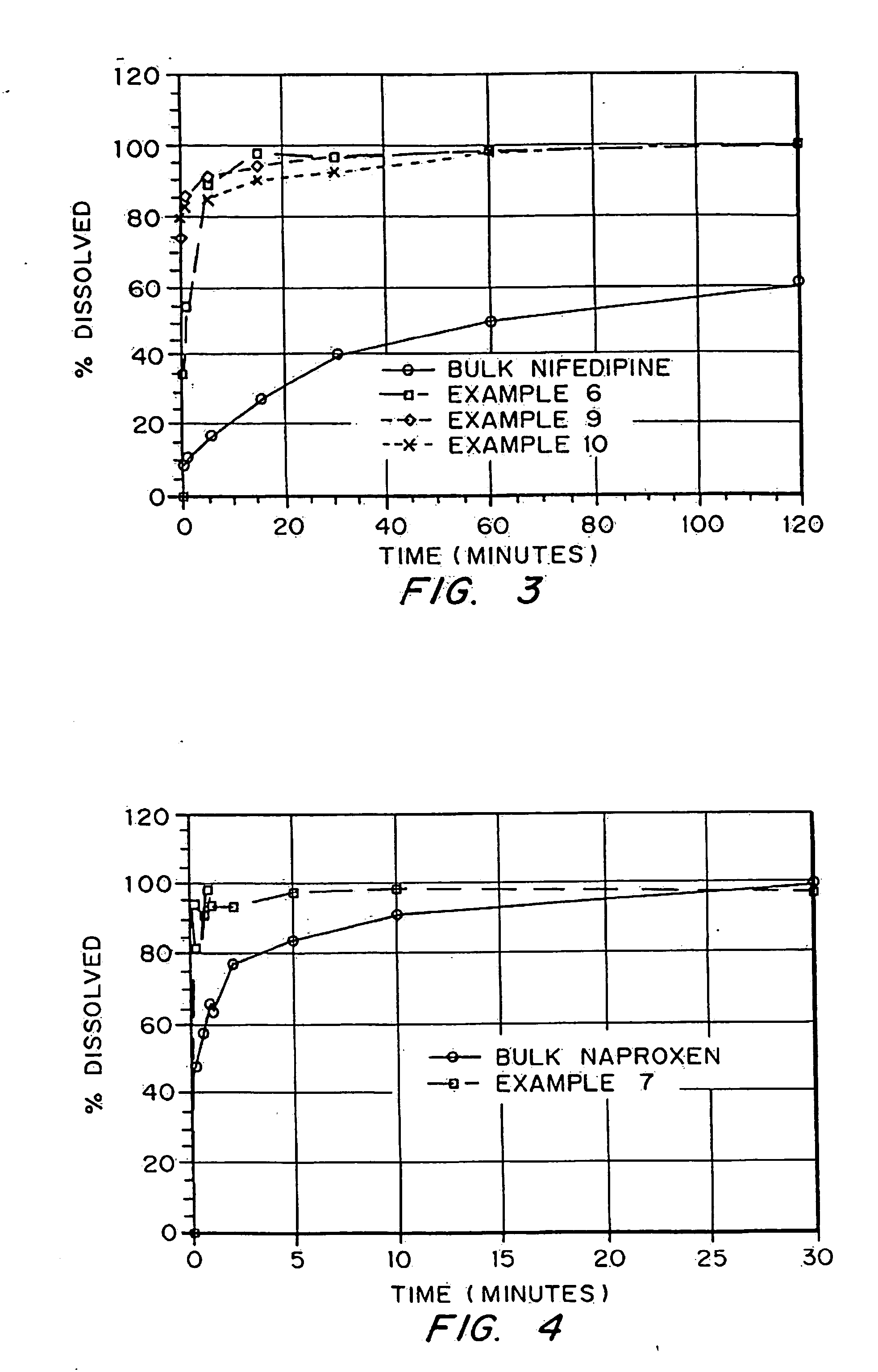
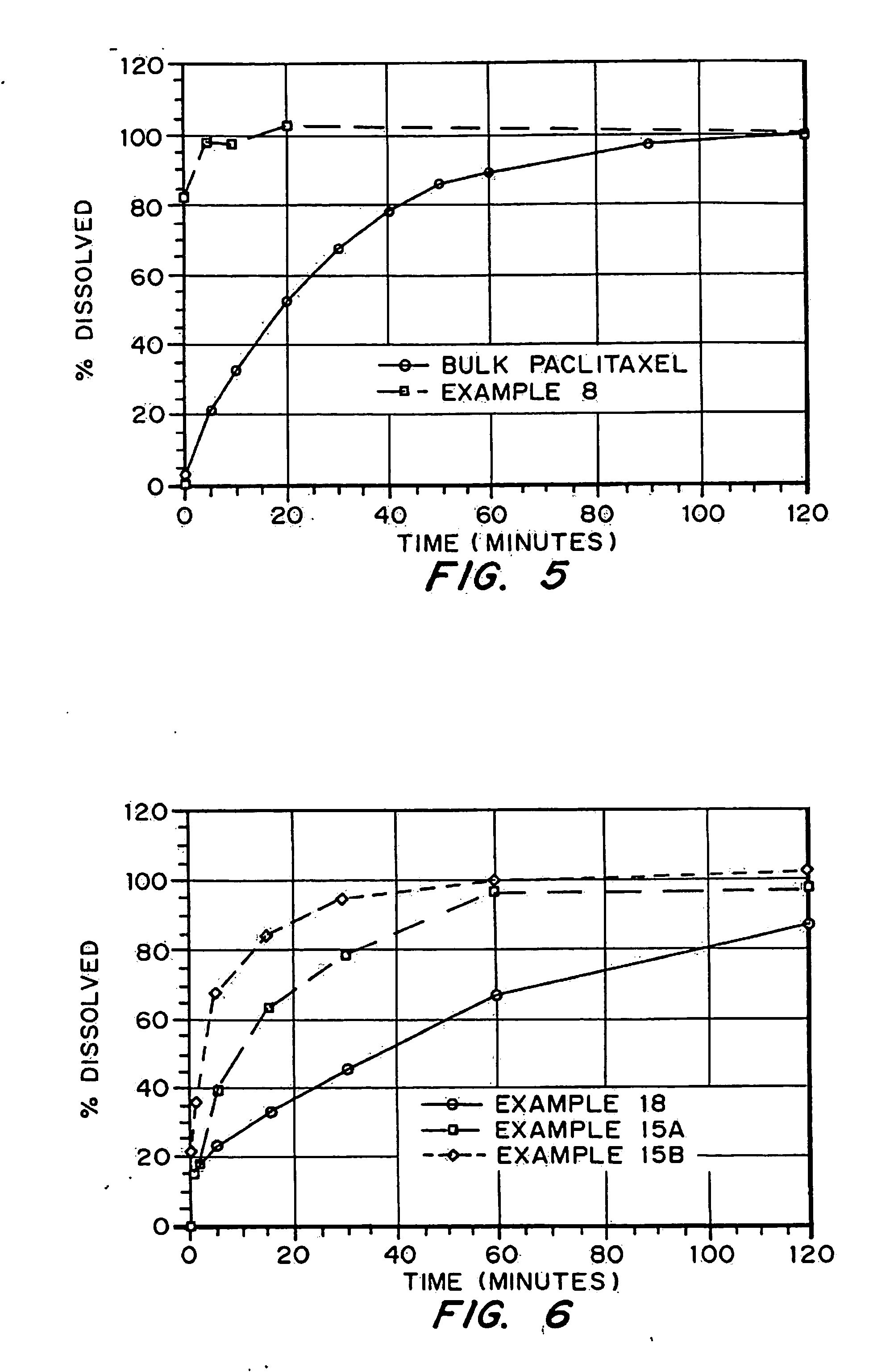
Porous drug matrices and methods of manufacture thereof
InactiveUS6932983B1Lower the volumePrevent precipitationPowder deliveryNanotechDrugs solutionWater soluble drug
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
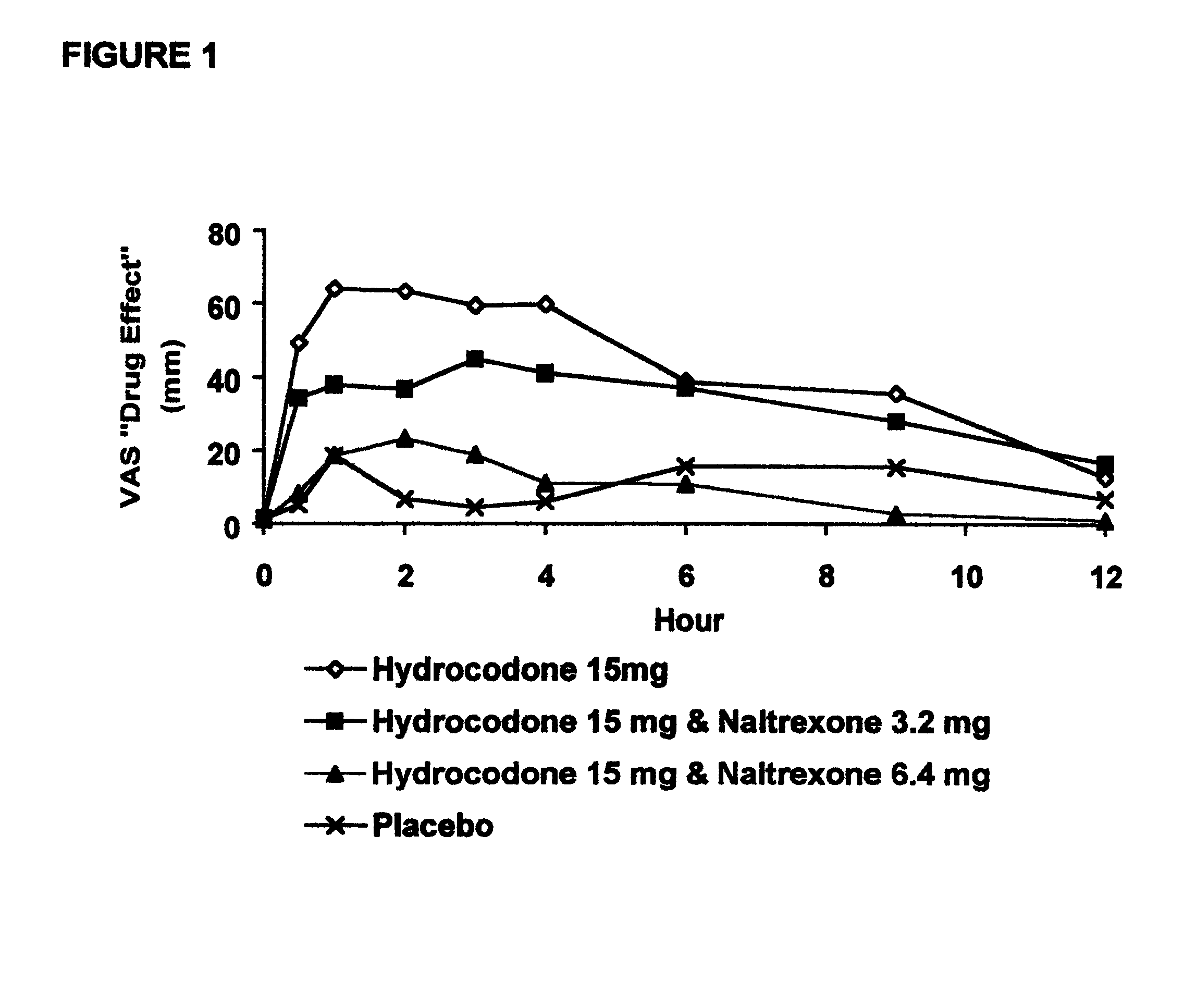
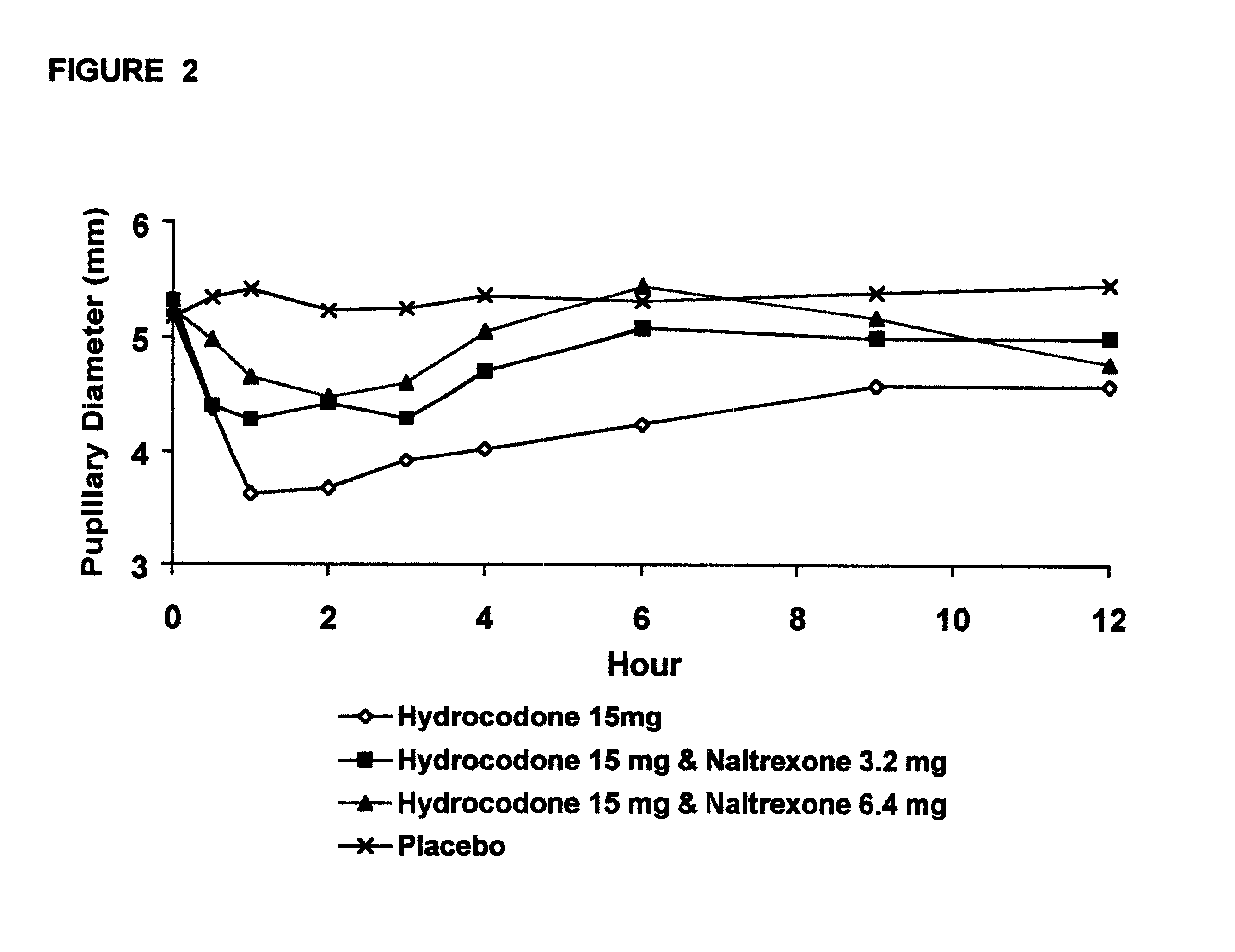
Opioid agonist/antagonist combinations
InactiveUS6277384B1Increase elasticityTrend downBiocideNervous disorderOpioid AgonistOpioid antagonist
The invention is directed in part to oral dosage forms comprising a combination of an orally analgesically effective amount of an opioid agonist and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Orally administrable opioid formulations having extended duration of effect
InactiveUS6294195B1Effective steady-state blood levelPowder deliveryBiocideBlood levelOral medication
Sustained release oral solid dosage forms of opioid analgesics are provided as multiparticulate systems which are bioavailable and which provide effective blood levels of the opioid analgesic for at least about 24 hours. A unit dose of the opioid analgesic contains a plurality of substrates including the opioid analgesic in sustained release form. The substrates have a diameter from about 0.1 mm to about 3 mm.
Owner:PURDUE PHARMA LP
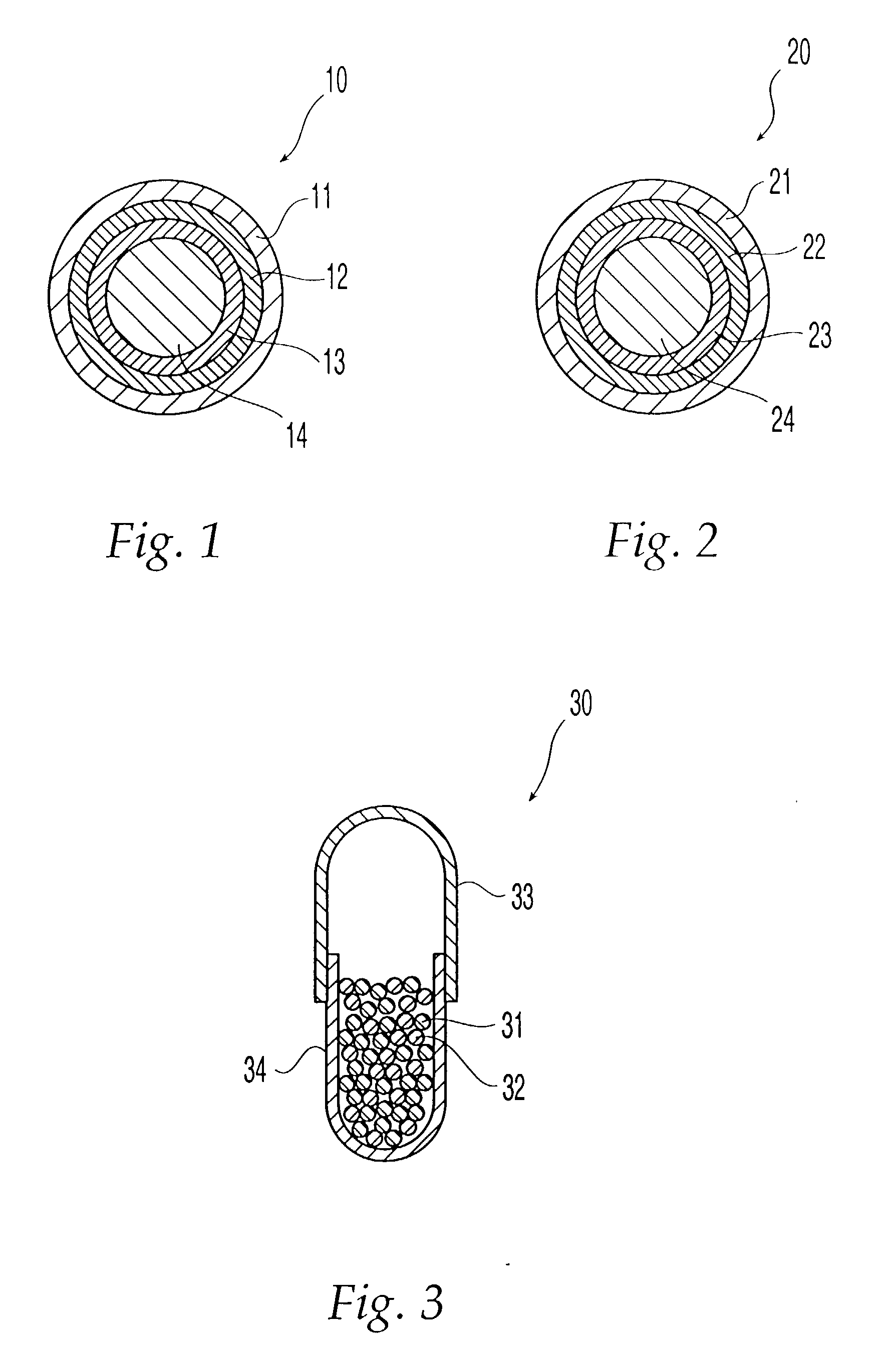
Non-gelatin substitutes for oral delivery capsules, their composition and process of manufacture
Gelatin-free capsule for use in oral administration of medicines, cosmetic or bath applications, or dietary supplements can be prepared from compositions comprisinga) 8-50% by weight of water-dispersible or water-soluble plasticizer,b) 0.5 to 12% by weight kappa-carrageenan,c) 0 to 60% dextrins, andd) 1% to 95% by weight water,with the kappa-carrageenan comprising at least 50% by weight of all gums forming or contributing to formation of thermoreversible gels in the composition. A capsule for oral administration or cosmetic application may comprise a fill material to be administered to a patient or subject and a capsule, the capsule comprising an aqueous based film comprisinga) water-dispersible or water-soluble plasticizer, andb) carrageenan,with the carrageenan comprising at least 50% or 75% by weight of kappa-carrageenan, and the carrageenan comprising at least 50% or 75% by weight of all gums which form or contribute to the formation of thermoreversible gels. A process for forming the capsules may comprise heating the composition, casting or extruding the composition into a film, gelling the composition by cooling, associating a fill material with the gelled composition (usually as a film) and sealing the film about the fill material.
Owner:PATHEON SOFTGELS INC
Sublingual buccal effervescent
A pharmaceutical dosage form adapted to supply a medicament to the oral cavity for buccal, sublingual or gingival absorption of the medicament which contains an orally administerable medicament in combination with an effervescent for use in promoting absorption of the medicament in the oral cavity. The use of an additional pH adjusting substance in combination with the effervescent for promoting the absorption drugs is also disclosed.
Owner:CEPHALON INC
Pharmaceutical compositions for lipophilic drugs
InactiveUS7070802B1Good self-emulsifying performanceShelf-stableCyclic peptide ingredientsCapsule deliveryMonoglycerideCyclosporins
Stable solutions of lipophilic drugs, such as cyclosporin, forming a polar lipid self-emulsifying drug delivery system. The solutions can include lipophilic drugs, such as cyclosporin, dissolved in a polar lipid, such as having a C6-C12 fatty acid monoglyceride content of at least about 50%, surfactants and triglycerides. The composition forms a fine emulsion on exposure to water. The encapsulated dosage form of this composition needs neither a hydrophilic component nor air-tight blister packaging, and is particularly suitable for oral administration.
Owner:WATSON LAB INC
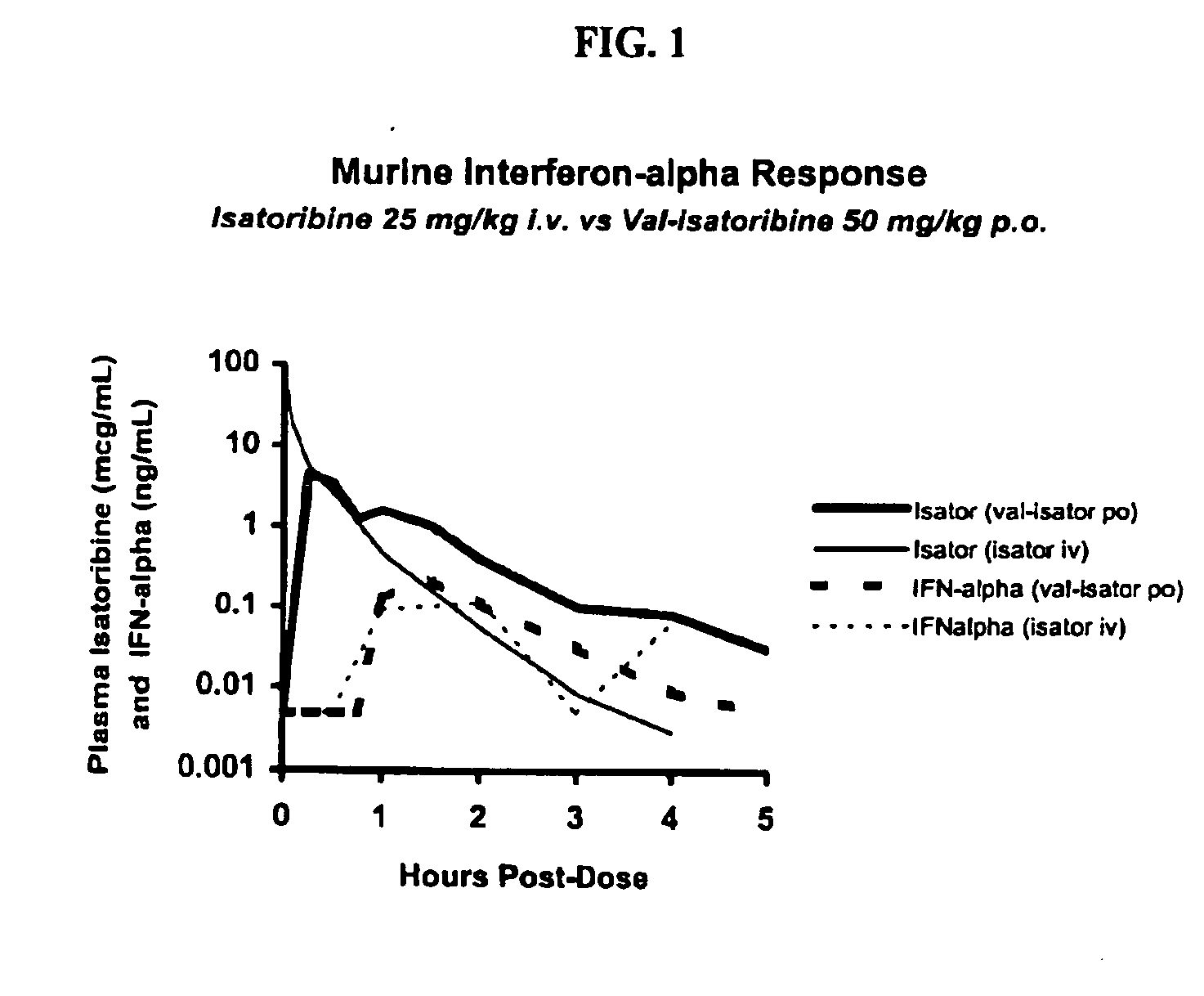
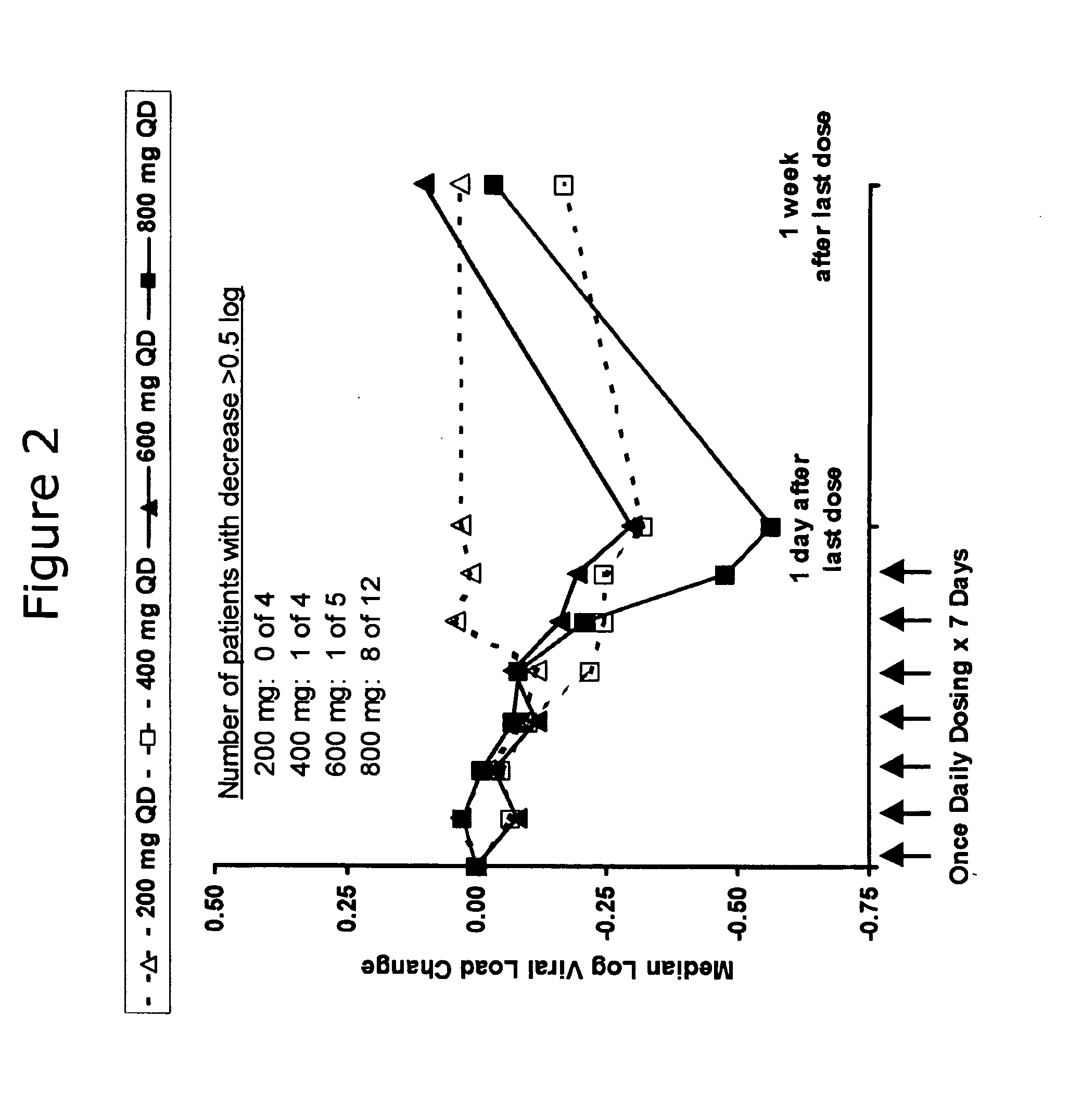
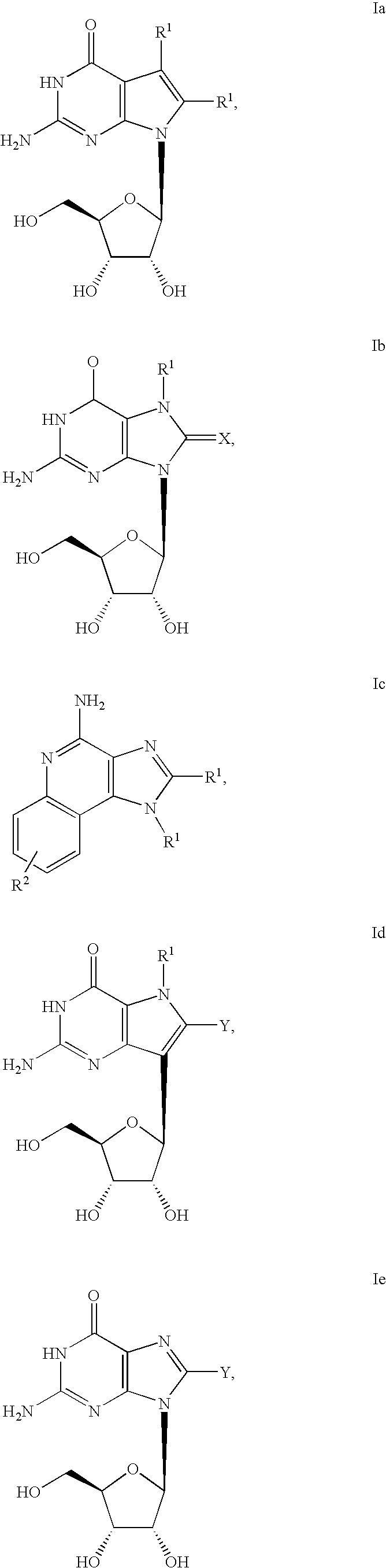
Administration of TLR7 ligands and prodrugs thereof for treatment of infection by hepatitis C virus
InactiveUS20050054590A1Reduce sensitivityAvoid spreadingBiocideDigestive systemHepatitis c viralSide effect
This invention relates to methods for treating or preventing hepatitis C virus infections in mammals using Toll-Like Receptor (TLR)7 ligands and prodrugs thereof. More particularly, this invention relates to methods of orally administering a therapeutically effective amount of one or more prodrugs of TLR7 ligands for the treatment or prevention of hepatitis C viral infection. Oral administration of these TLR7 immunomodulating ligands and prodrugs thereof to a mammal provides therapeutically effective amounts and reduced undesirable side effects.
Owner:ANDADYS PHARMA INC
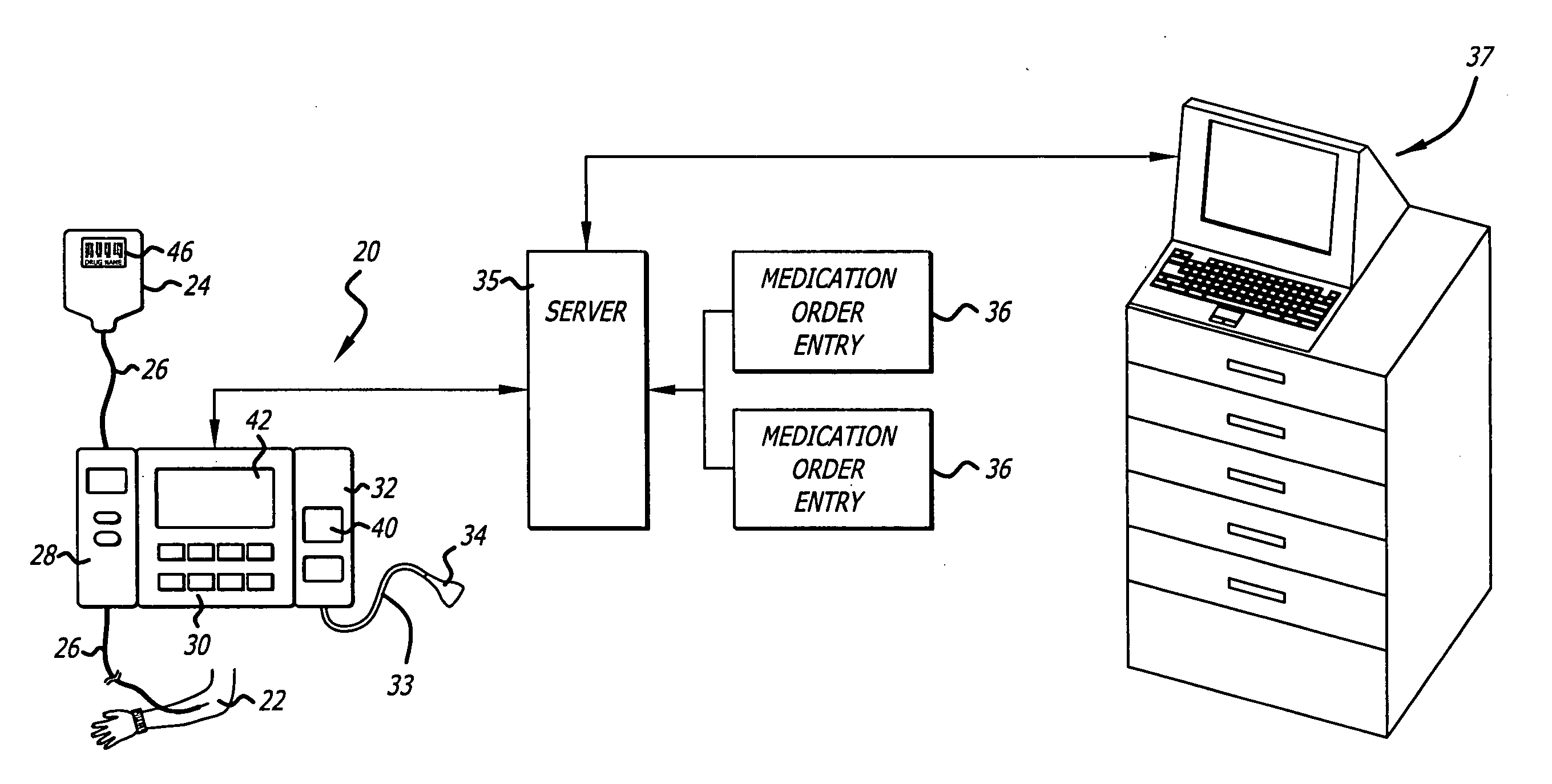
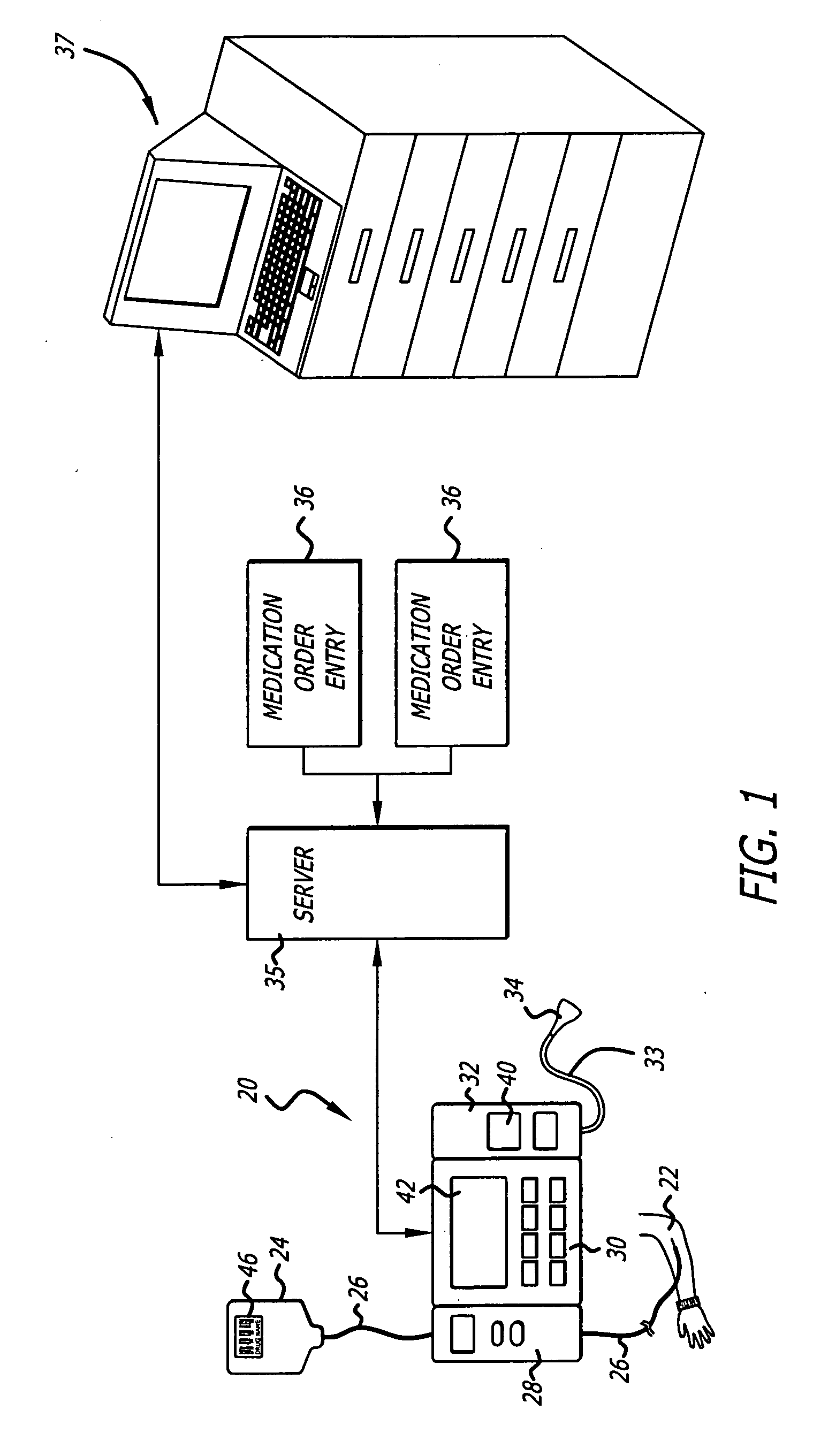
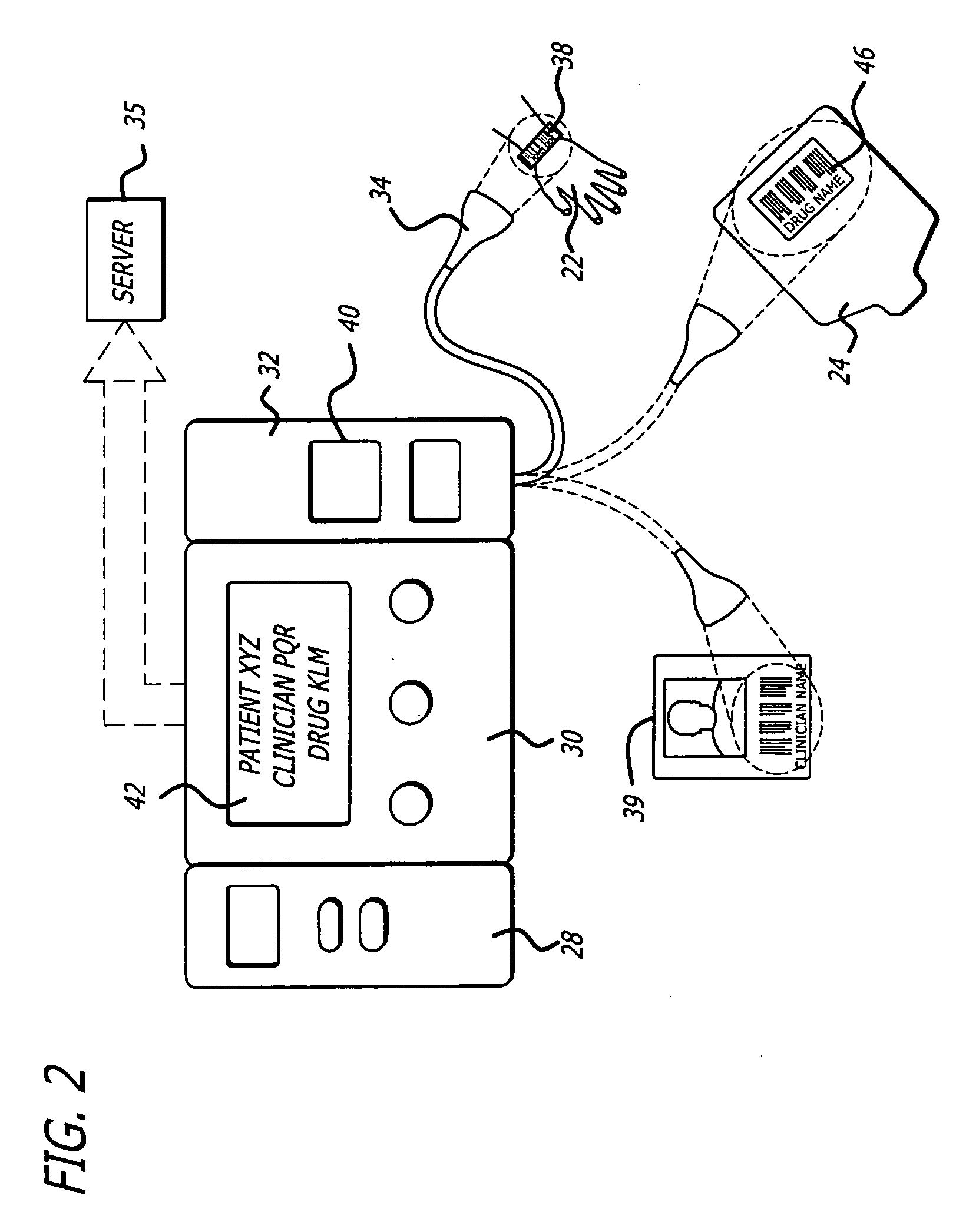
Management of pending medication orders
ActiveUS20060200369A1Data processing applicationsDrug and medicationsOral medicationAutomatic programming
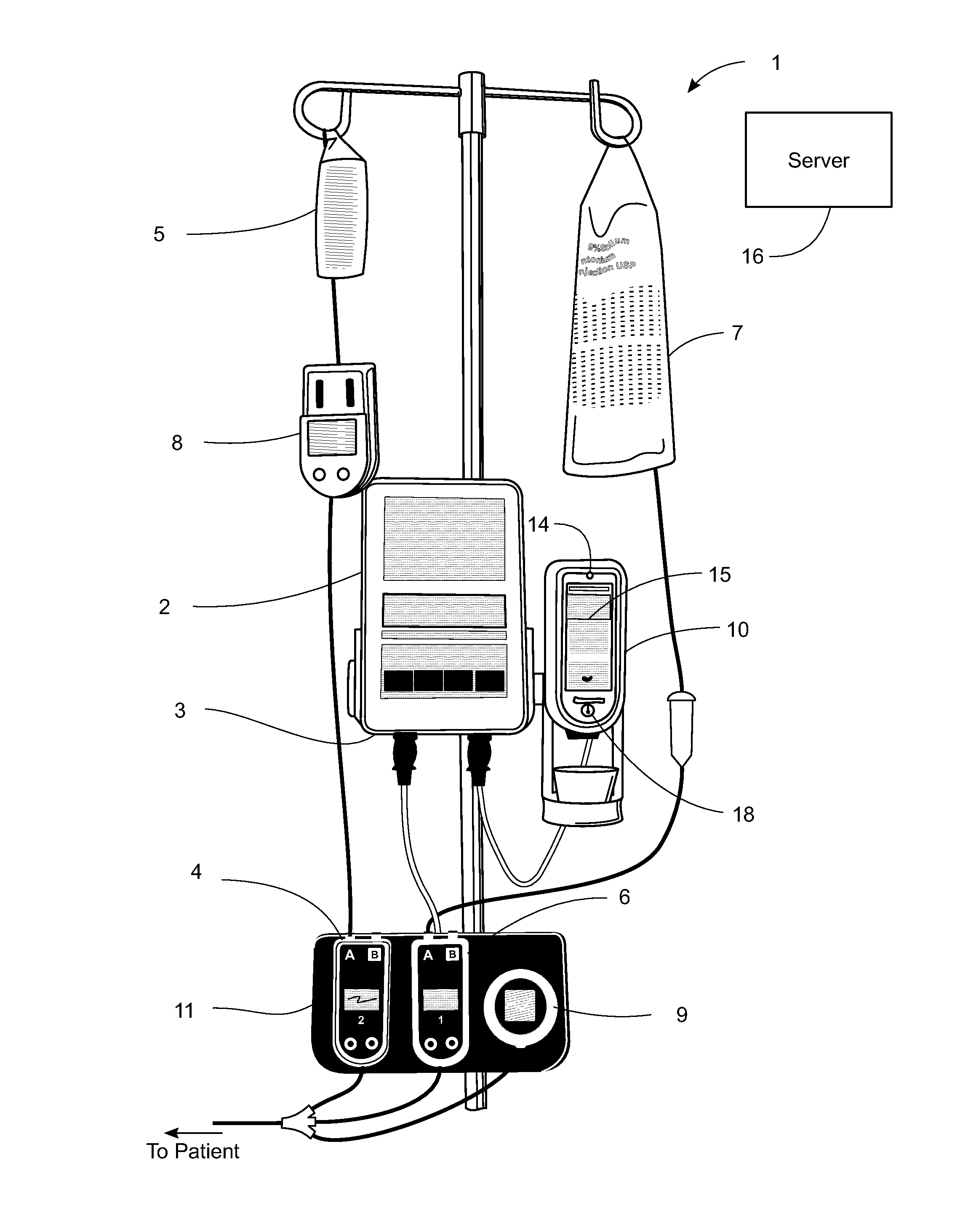
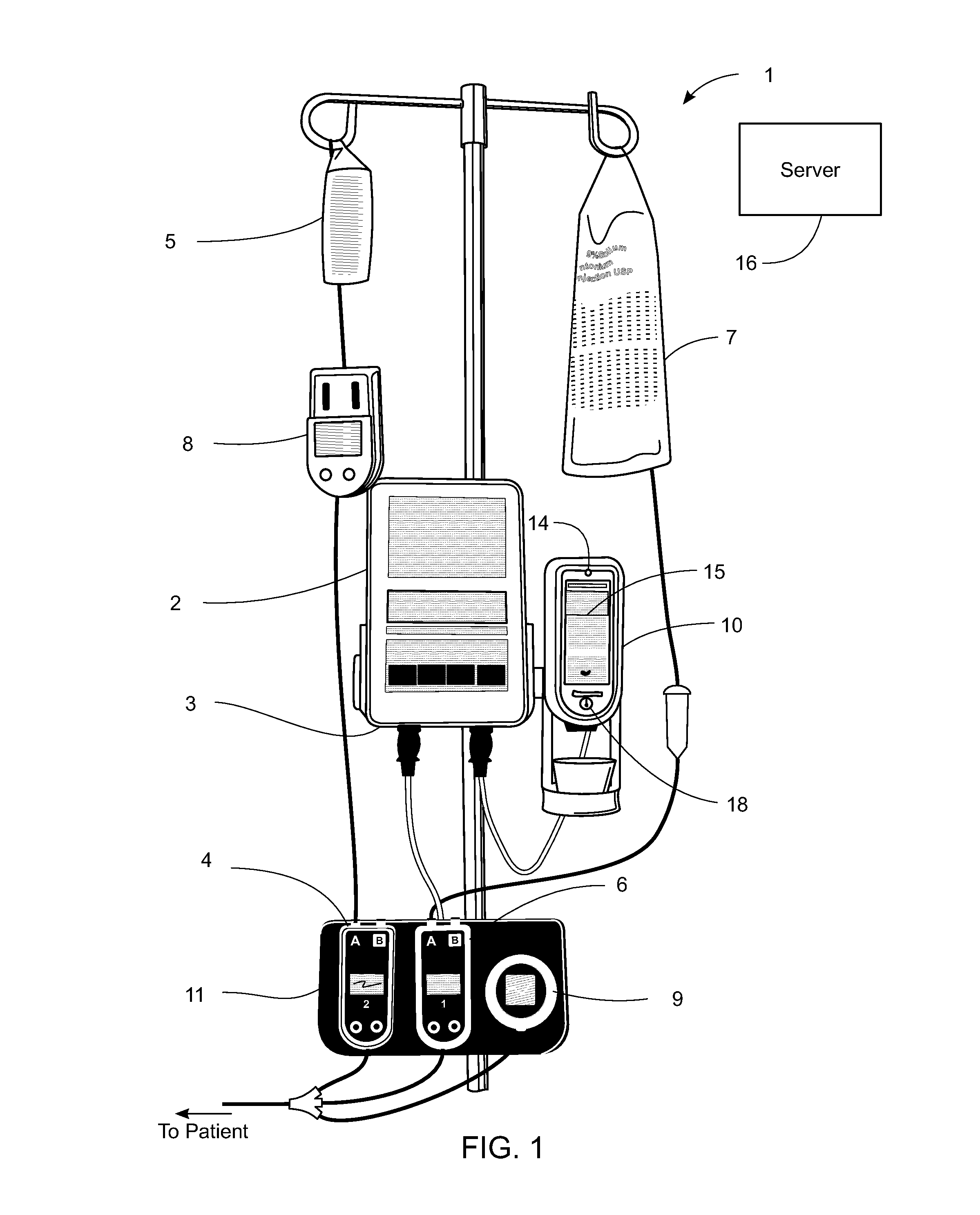
Pending medication orders are presented on the controller of an infusion pump to which a patient has been identified. All pending medication orders are displayed on the screen of the controller and any may be selected by the clinician. Selecting a pending order at the controller that involves an infusion to the patient also results in automatic programming of the infusion pump in accordance with that order. Selection of an oral medication at the controller results, as does selection of any other order, in notification to the healthcare facility server that the order is being administered. Such notification resolves open controlled item transactions as well as provides information that may be relevant to a patient's EMAR. Selection of a pending order may occur manually or automatically through identification of a medication.
Owner:CAREFUSION 303 INC
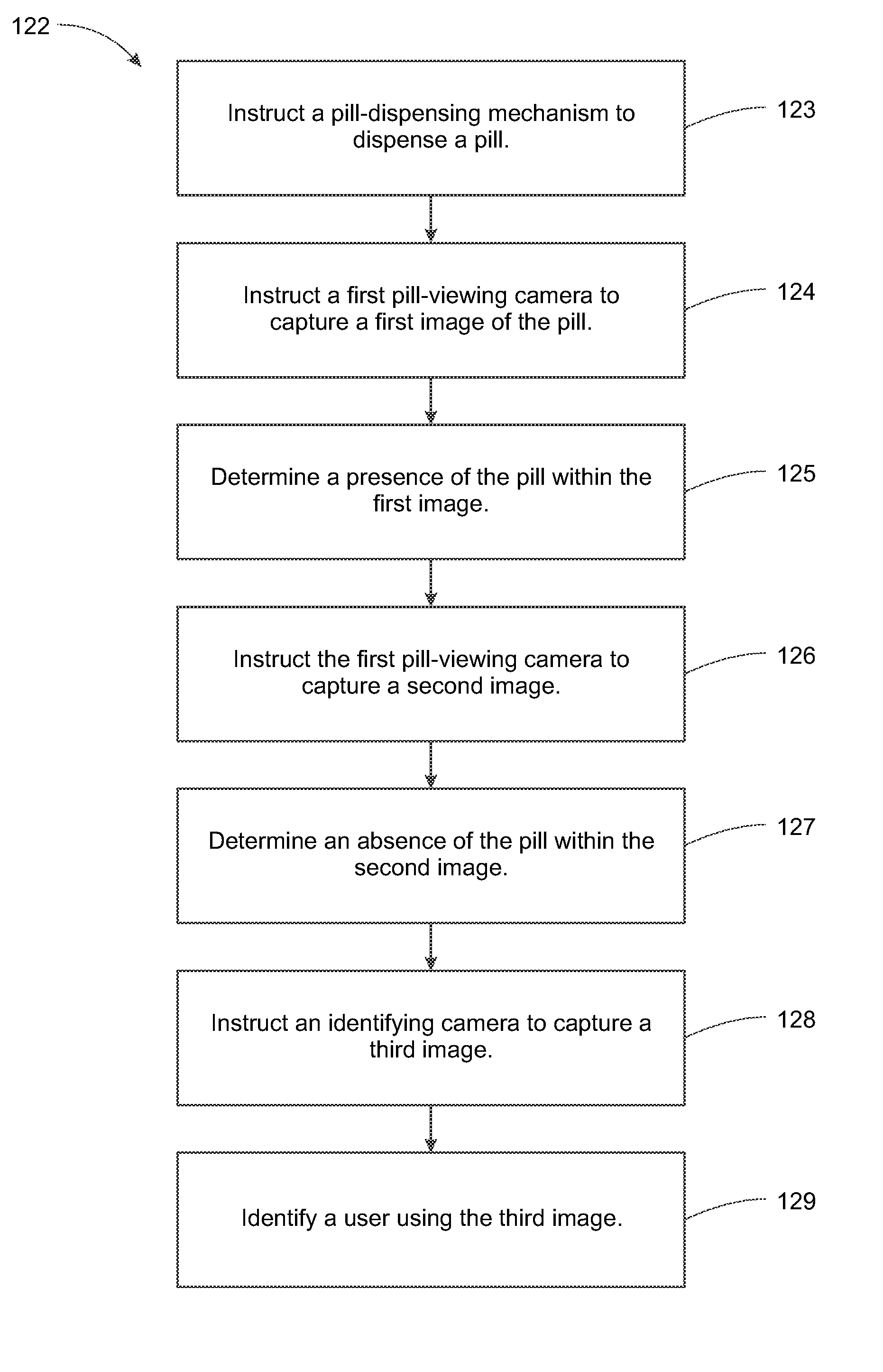
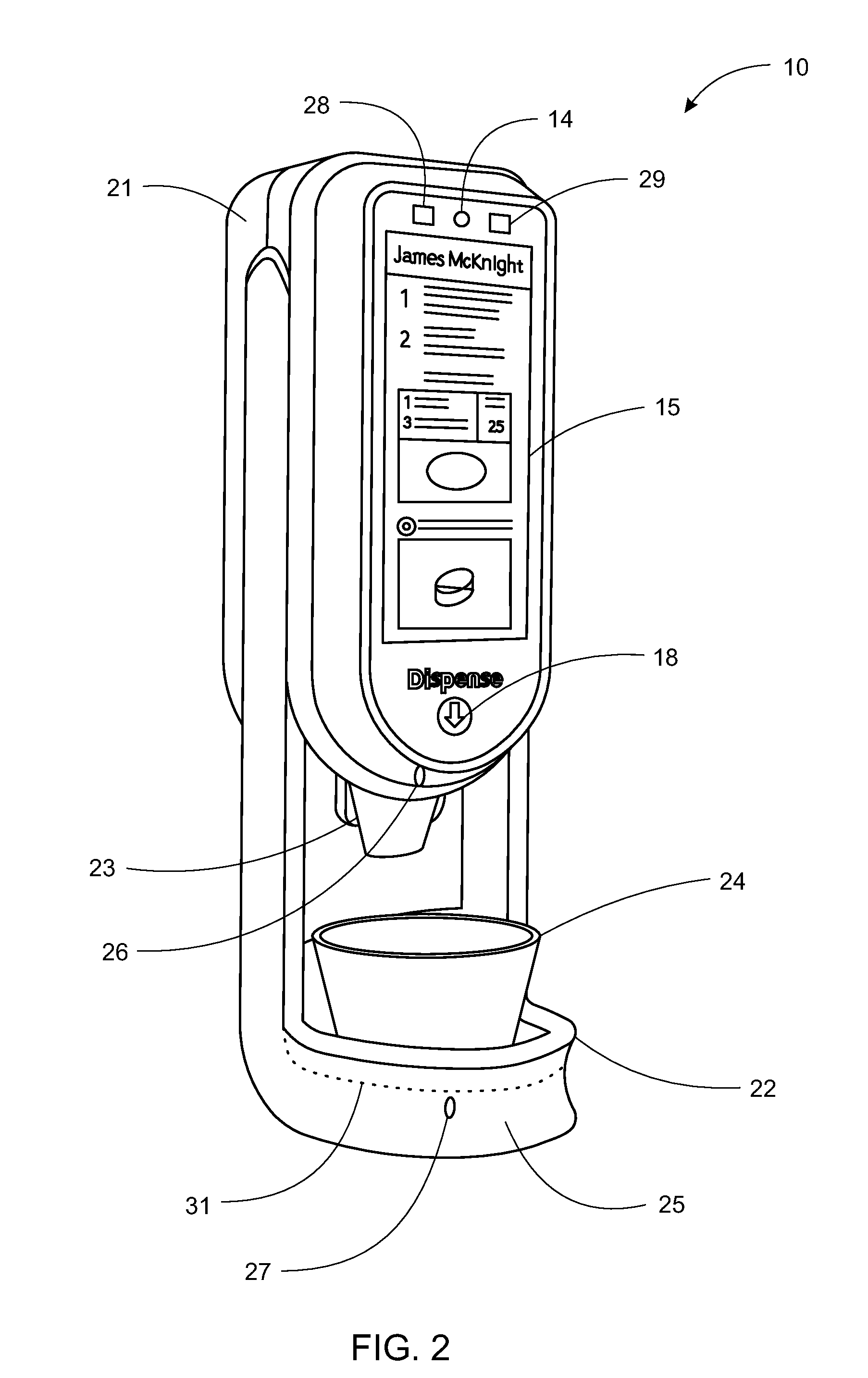
System, Method, and Apparatus for Dispensing Oral Medications
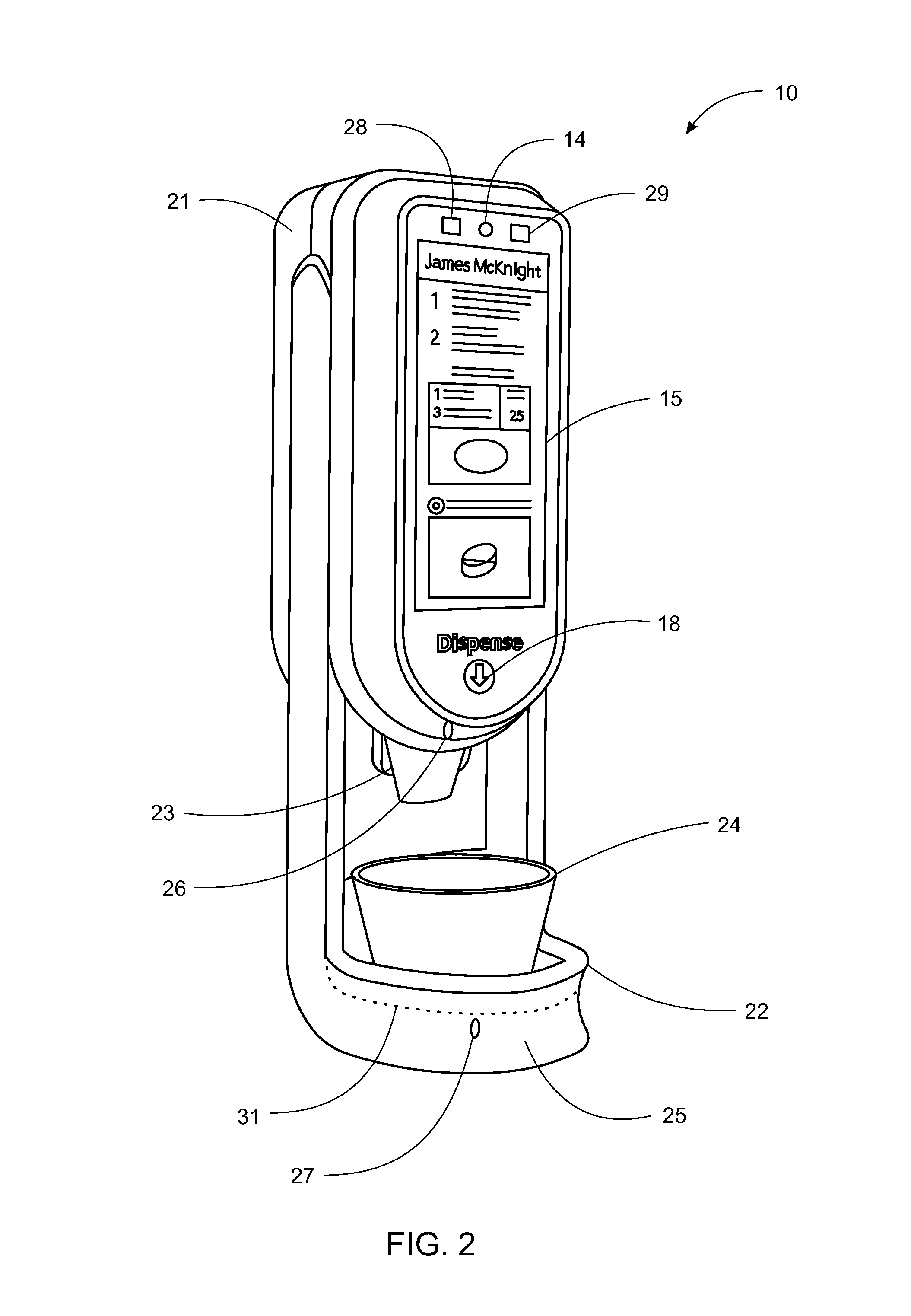
A pill dispenser includes a housing, a pill-dispensing mechanism, a receptacle, a pill-viewing camera, an identifying camera, one or more processors, and a storage medium (e.g., a memory). The pill-dispensing mechanism is coupled to an opening of the housing. The first pill-viewing camera is positioned to capture an image of the receptacle, and the identifying camera is positioned to capture an image of an area adjacent to the housing. The one or more processors are in operative communication with the pill-dispensing mechanism, the pill-viewing camera, and the identifying camera. The storage medium stores processor-executable instructions for: instructing the pill-dispensing mechanism to dispense a pill; instructing the pill-viewing camera to capture a first image of the pill to determine a presence of the pill; instructing the pill-viewing camera to capture a second image to determine an absence of the pill; and instructing the identifying camera to capture a third image.
Owner:DEKA PROD LLP
Controlled release formulations of opioid and nonopioid analgesics
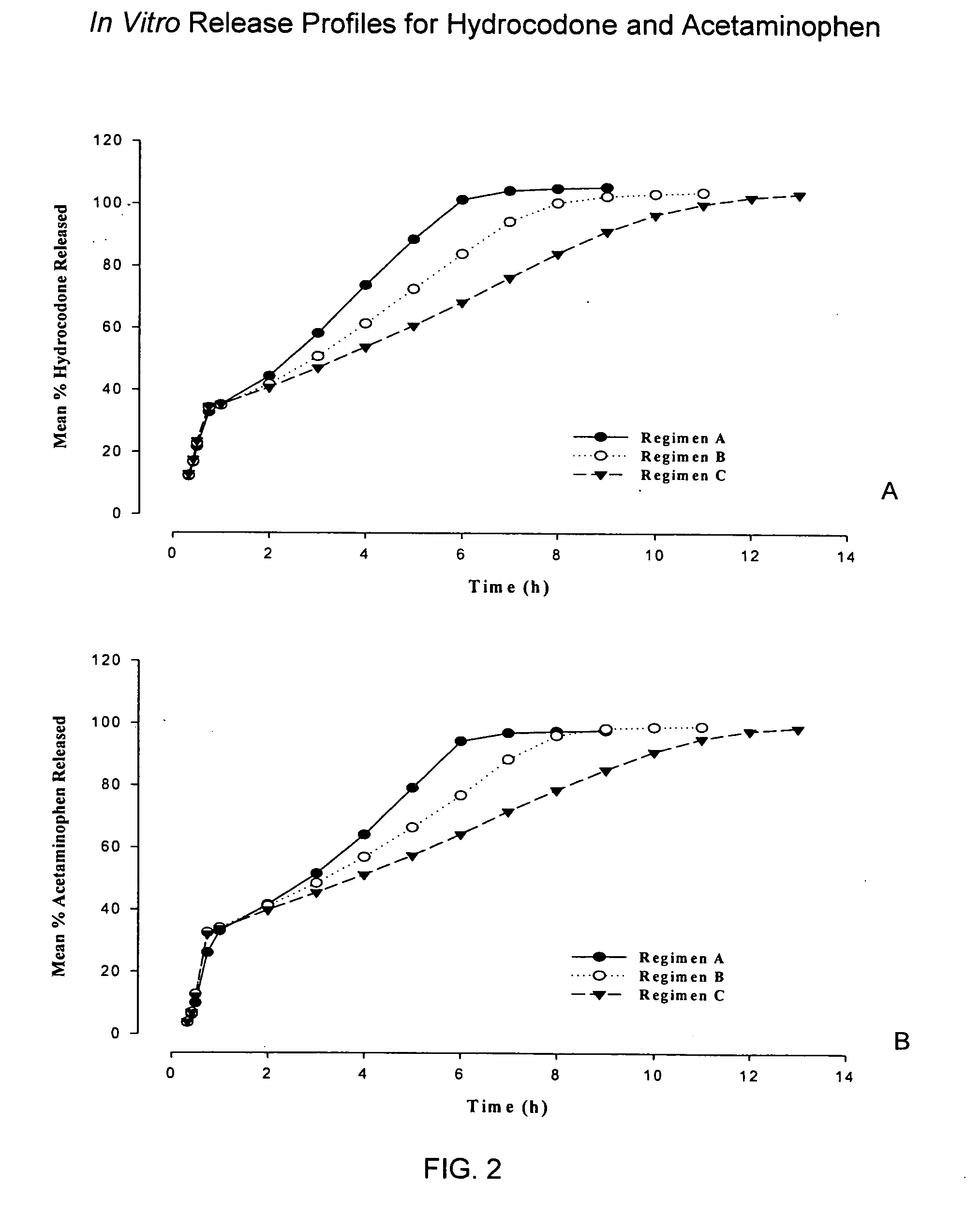
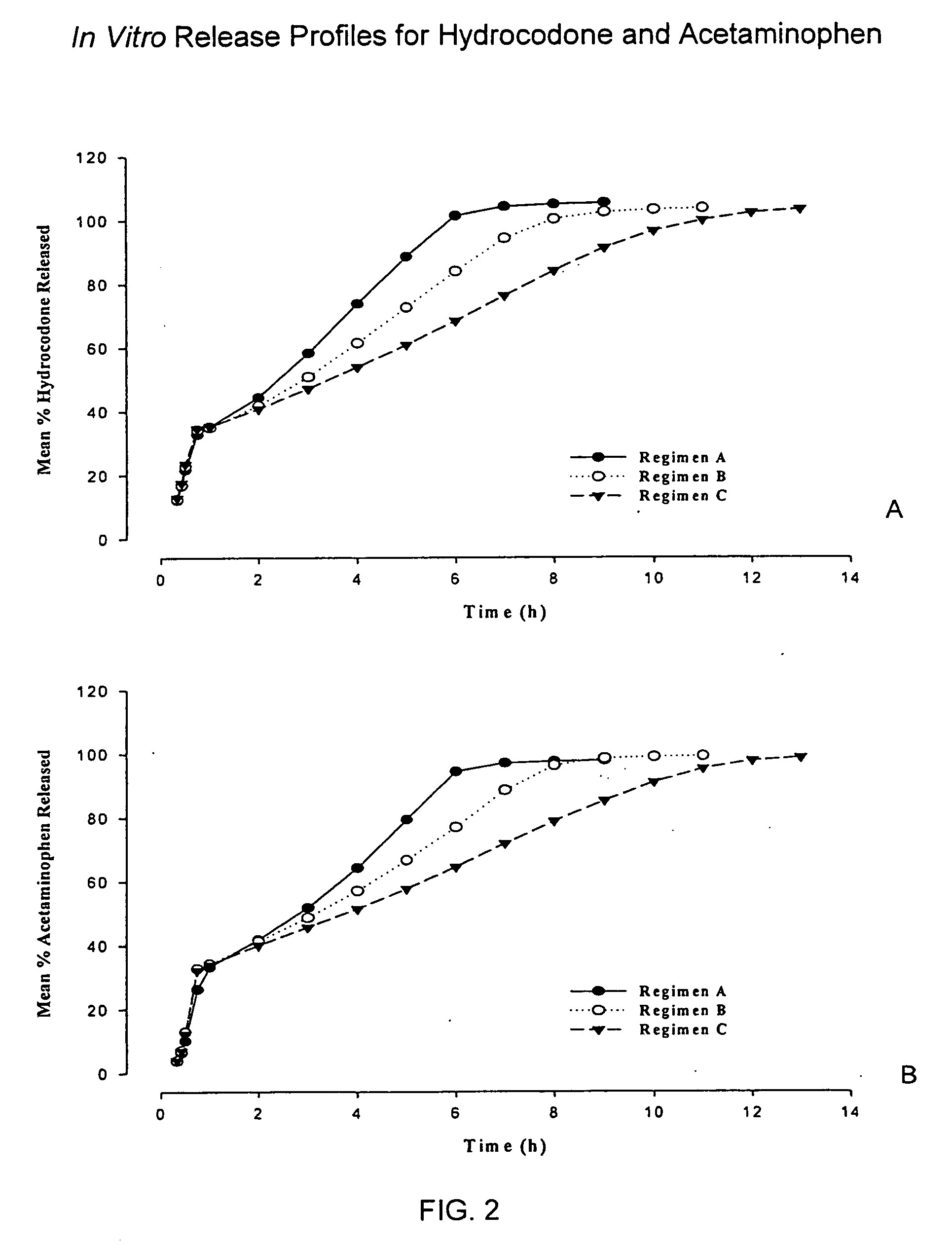
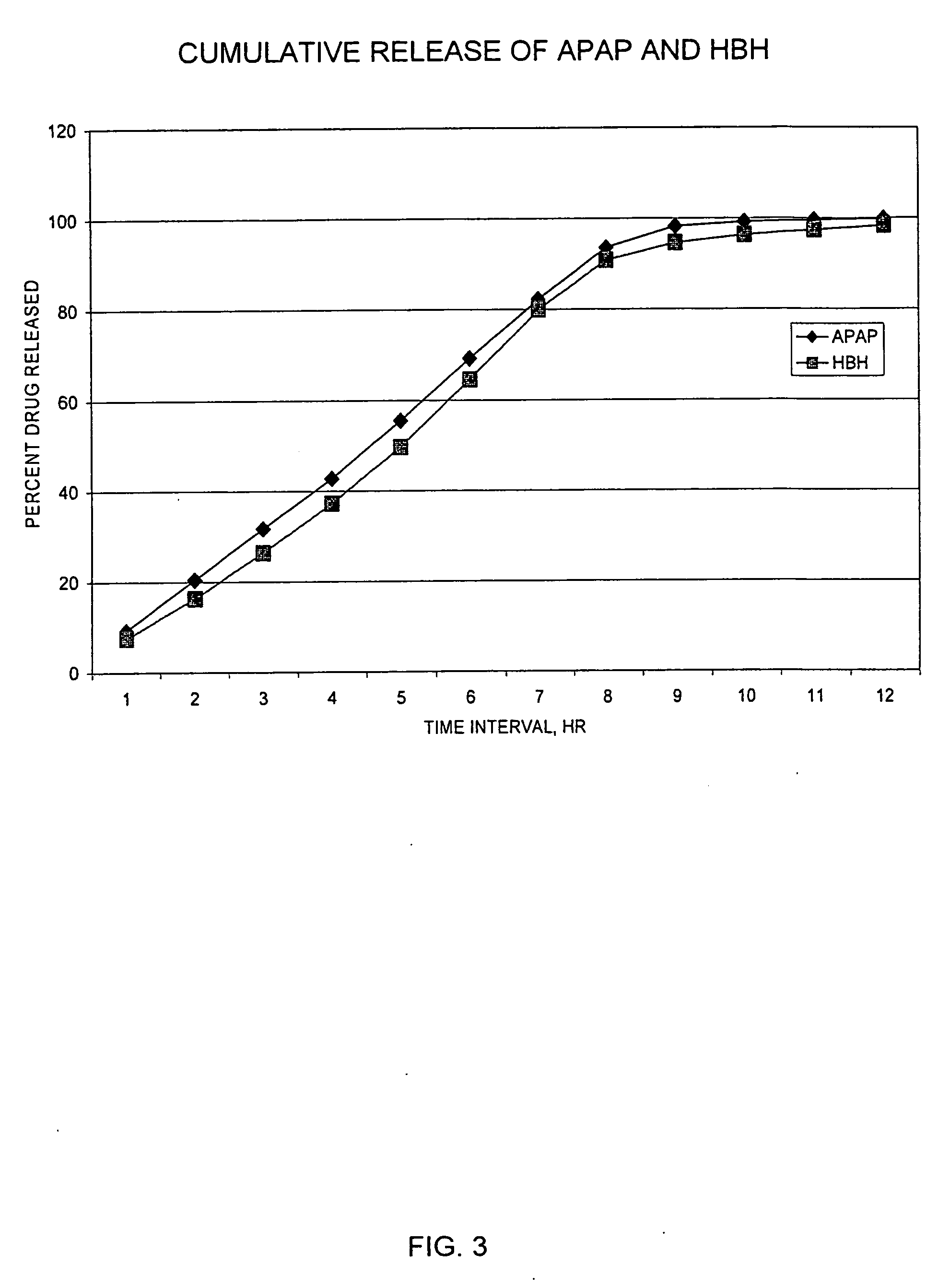
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Oral dosage form comprising a therapeutic agent and an adverse-effect agent
InactiveUS20030044458A1Reduce eliminateReduce and eliminate pharmacological effectOrganic active ingredientsNervous disorderOral medicationTherapeutic effect
The present invention provides an oral dosage form comprising a first composition and a second composition. The first composition comprises an effective amount of a therapeutic agent and the second composition comprises an effective amount of an adverse-effect agent. The adverse-effect agent is covered with a coating that is substantially insoluble in the gastrointestinal tract. In one embodiment, the adverse-effect agent is coated with an outer base-soluble layer and an inner acid-soluble layer. The therapeutic agent can be uncoated or can be coated with a coating having an outer acid-soluble layer and an inner base-soluble layer. The dosage form discourages administration of the therapeutic agent by other than oral administration.
Owner:PURDUE PHARMA LP
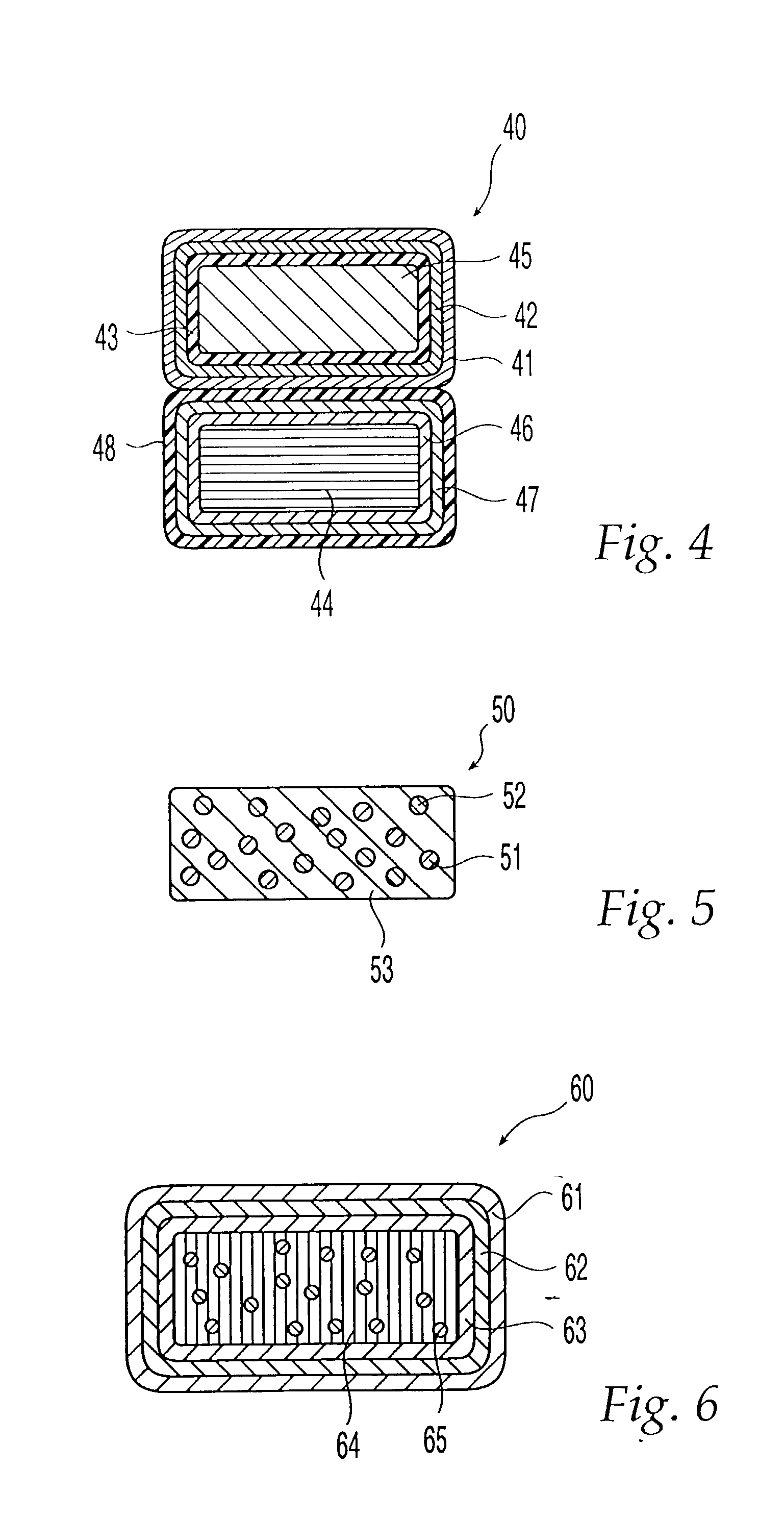
Dosage forms using drug-loaded ion exchange resins
InactiveUS20050181050A1Avoid breakingPharmaceutical non-active ingredientsPill deliveryOral medicationImmediate release
A multiparticulate, modified release composition for oral administration has been developed. The formulation is made by complexing a drug with an ion-exchange resin in the form of small particles, typically less than 150 microns. The present invention provides novel extended release coated ion exchange particles comprising drug-resin complexes, produced by binding the salt form of the drug, that do not require impregnating agents to insure the integrity of the extended release coat. To prepare a modified release formulation, one or more of the following types of particles are formulated into a final dosage form: (a) Immediate release particles, (b) Enteric coated particles, (c) Extended release particles, (d) Enteric coated-extended release particles; and (e) Delayed release particles. The various drug-containing particles described above can be further formulated into a number of different easy-to-swallow final dosage forms including, but not limited to, a liquid suspension, gel, chewable tablet, crushable tablet, rapidly dissolving tablet, or unit of use sachet or capsule for reconstitution
Owner:COLLEGIUM PHARMA INC
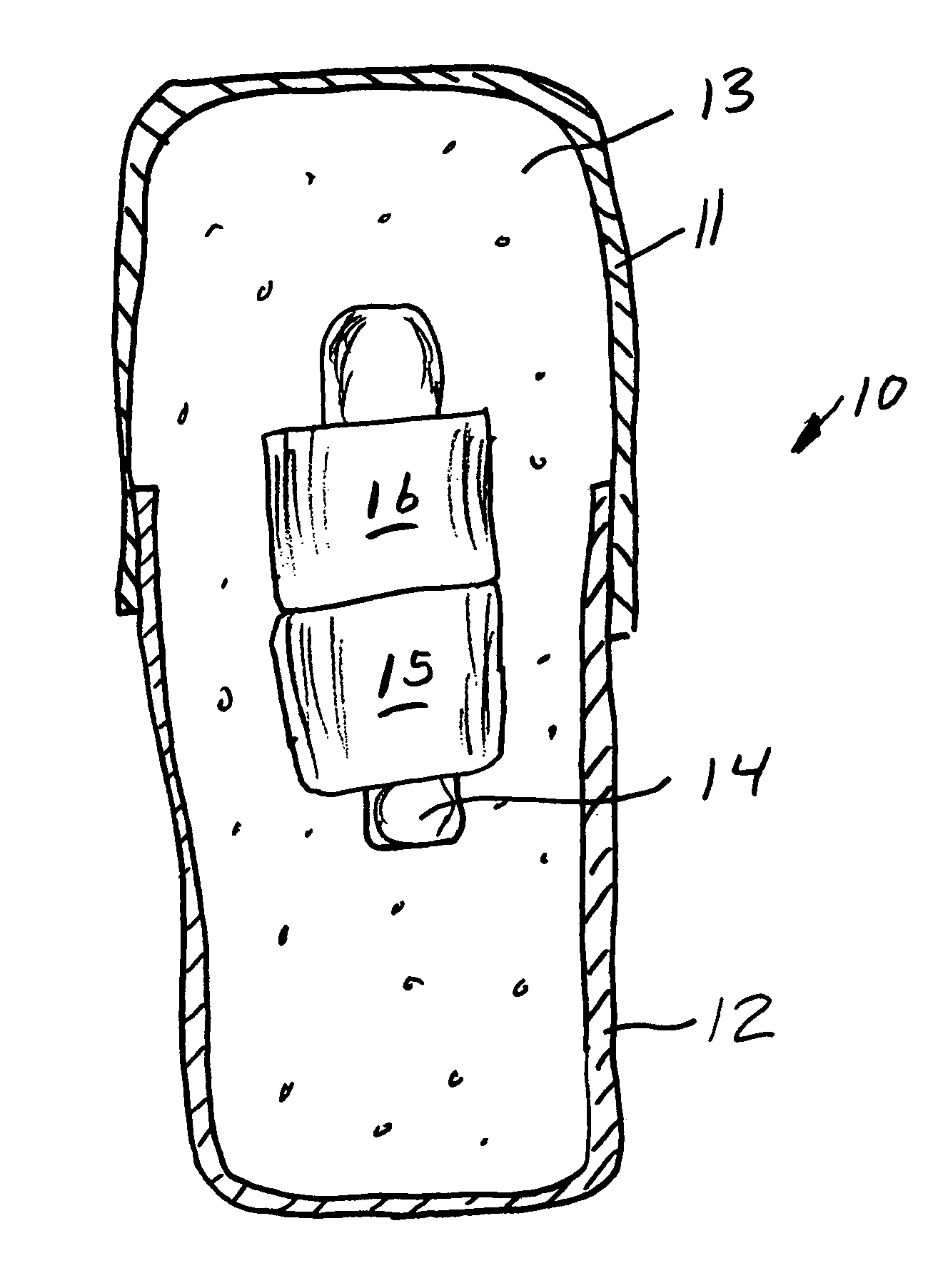
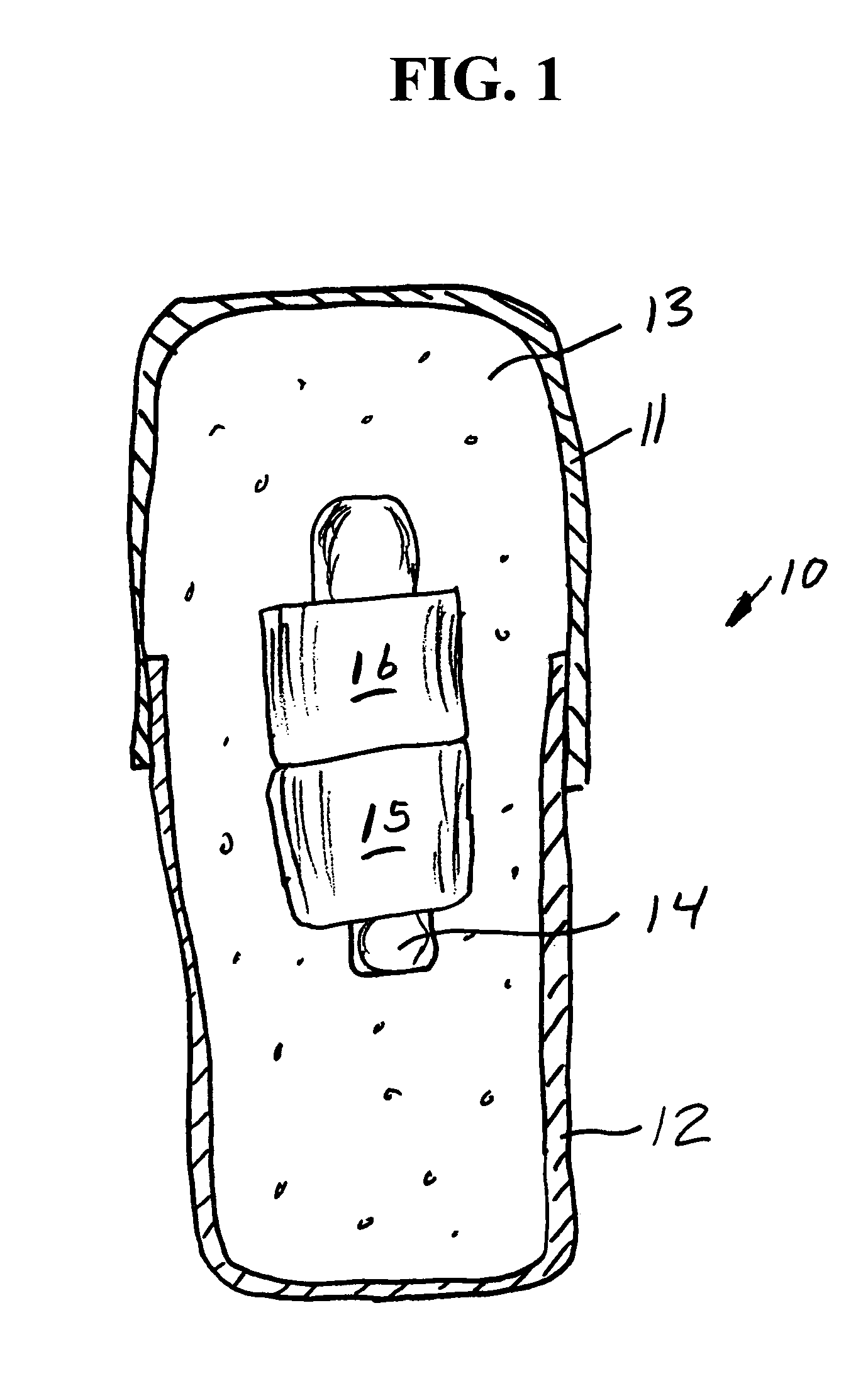
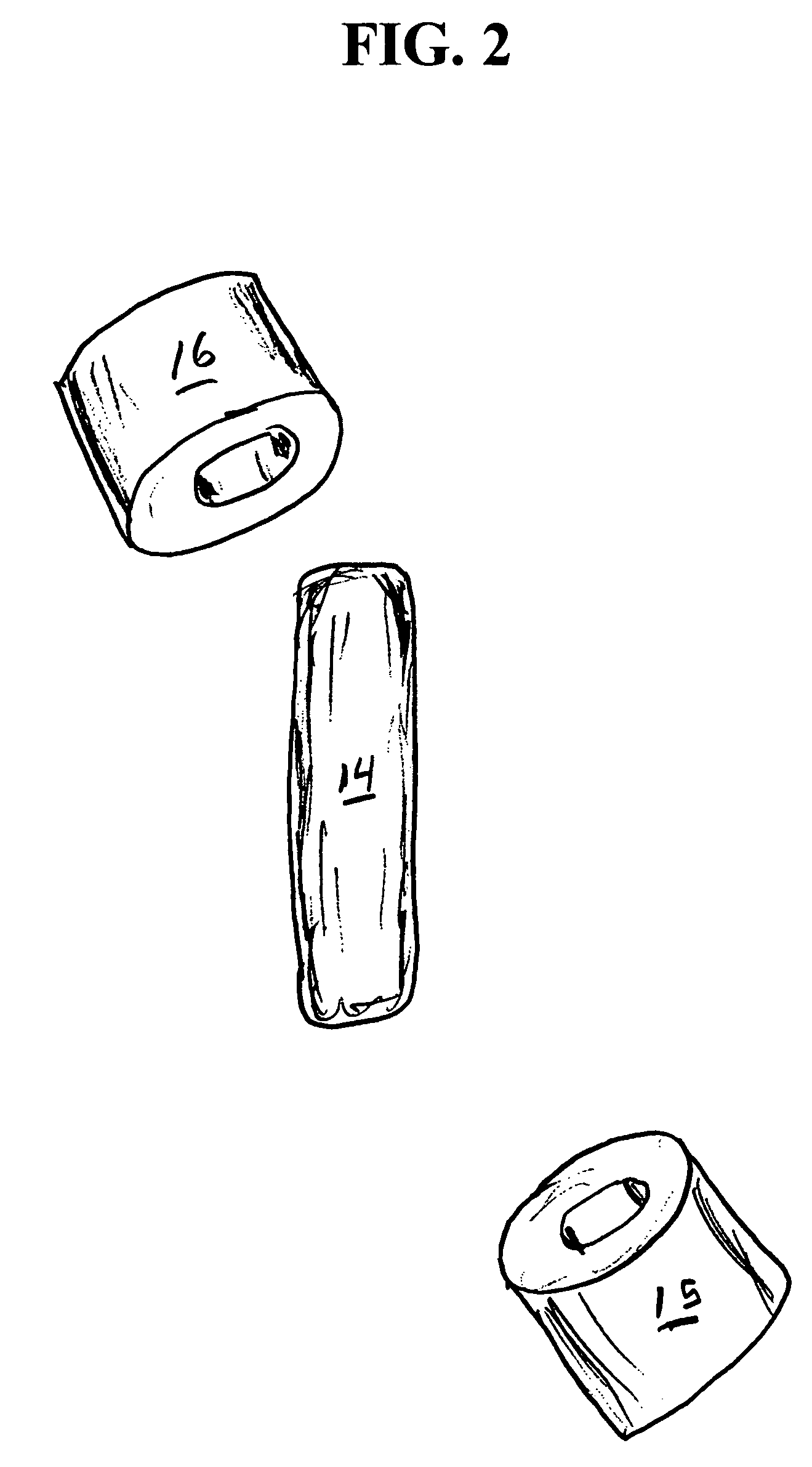
Oral drug compliance monitoring using radio frequency identification tags
A device useful for oral drug delivery device consisting of: (a) a capsule, tablet or pill designed to disperse in the gastrointestinal system; (b) an RFID tag positioned in the capsule, tablet or pill, the RFID tag comprising an antenna; (c) an object selected from the group consisting of a magnet, a ferromagnetic object, a ferrite object and an electromagnetic shielding object positioned within, over or adjacent the antenna of the RFID tag to alter the antenna characteristics of the RFID tag so that if the RFID tag is interrogated before the capsule, tablet or pill disperses in the gastrointestinal system, the response of the RFID tag is sufficiently altered or attenuated to determine that the capsule, tablet or pill has not dispersed in the gastrointestinal system and so that if the RFID tag is interrogated after the capsule, tablet or pill has dispersed in the gastrointestinal system, the object separates from the RFID tag so that the response of the RFID tag is sufficiently detectable to determine that the capsule, tablet or pill has dispersed in the gastrointestinal system. Alternatively, a switch can be used to signal ingestion of the device, and change the response of the device. In another embodiment, the instant invention is a device useful for oral drug delivery, consisting of: (a) a capsule, tablet or pill designed to disperse in the gastrointestinal system; (b) a first non-anti-collision RFID tag positioned in the capsule; (c) a second non-anti-collision RFID tag positioned in the capsule, so that if the RFID tags are interrogated by an RFID reader before the capsule, tablet or pill disperses in the gastrointestinal system, the response of the RFID tags collide and so that after the dispersible material of the capsule has dispersed in the gastrointestinal system thereby allowing the first and second non-anti-collision tags to separate from each other, then the response of the RFID tags is sufficiently different from each other to determine that the capsule has dispersed in the gastrointestinal system
Owner:DOW GLOBAL TECH LLC
Oral dosage form comprising a therapeutic agent and an adverse-effect agent
The present invention provides an oral dosage form comprising a first composition and a second composition. The first composition comprises an effective amount of a therapeutic agent and the second composition comprises an effective amount of an adverse-effect agent. The adverse-effect agent is covered with a coating that is substantially insoluble in the gastrointestinal tract. In one embodiment, the adverse-effect agent is coated with an outer base-soluble layer and an inner acid-soluble layer. The therapeutic agent can be uncoated or can be coated with a coating having an outer acid-soluble layer and an inner base-soluble layer. The dosage form discourages administration of the therapeutic agent by other than oral administration.
Owner:PURDUE PHARMA LP
Oral drug compliance monitoring using radio frequency identification tags
A device useful for oral drug delivery device consisting of: (a) a capsule, tablet or pill designed to disperse in the gastrointestinal system; (b) an RFID tag positioned in the capsule, tablet or pill, the RFID tag comprising an antenna; (c) an object selected from the group consisting of a magnet, a ferromagnetic object, a ferrite object and an electromagnetic shielding object positioned within, over or adjacent the antenna of the RFID tag to alter the antenna characteristics of the RFID tag so that if the RFID tag is interrogated before the capsule, tablet or pill disperses in the gastrointestinal system, the response of the RFID tag is sufficiently altered or attenuated to determine that the capsule, tablet or pill has not dispersed in the gastrointestinal system and so that if the RFID tag is interrogated after the capsule, tablet or pill has dispersed in the gastrointestinal system, the object separates from the RFID tag so that the response of the RFID tag is sufficiently detectable to determine that the capsule, tablet or pill has dispersed in the gastrointestinal system. Alternatively, a switch can be used to signal ingestion of the device, and change the response of the device.
Owner:DOW GLOBAL TECH LLC
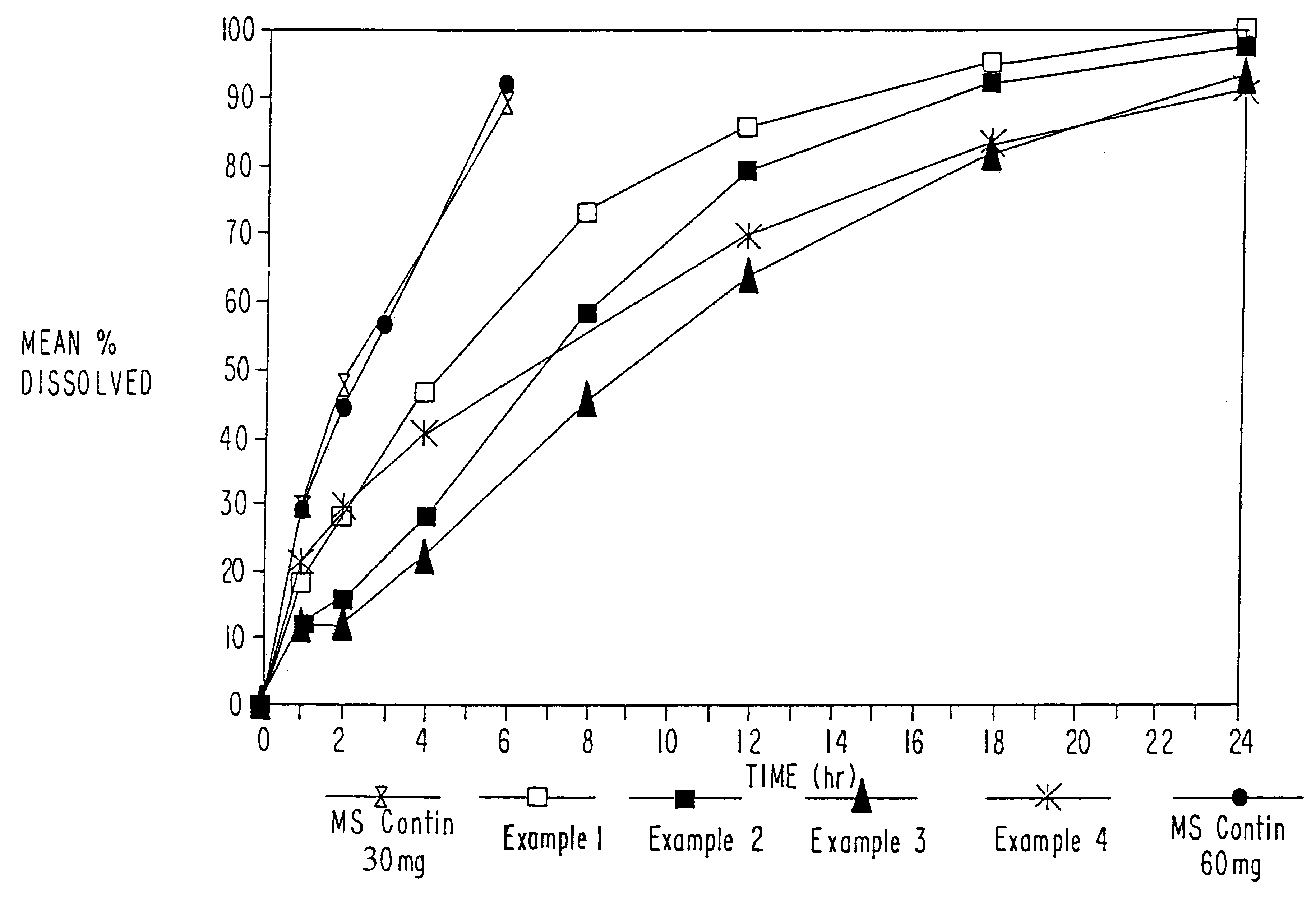
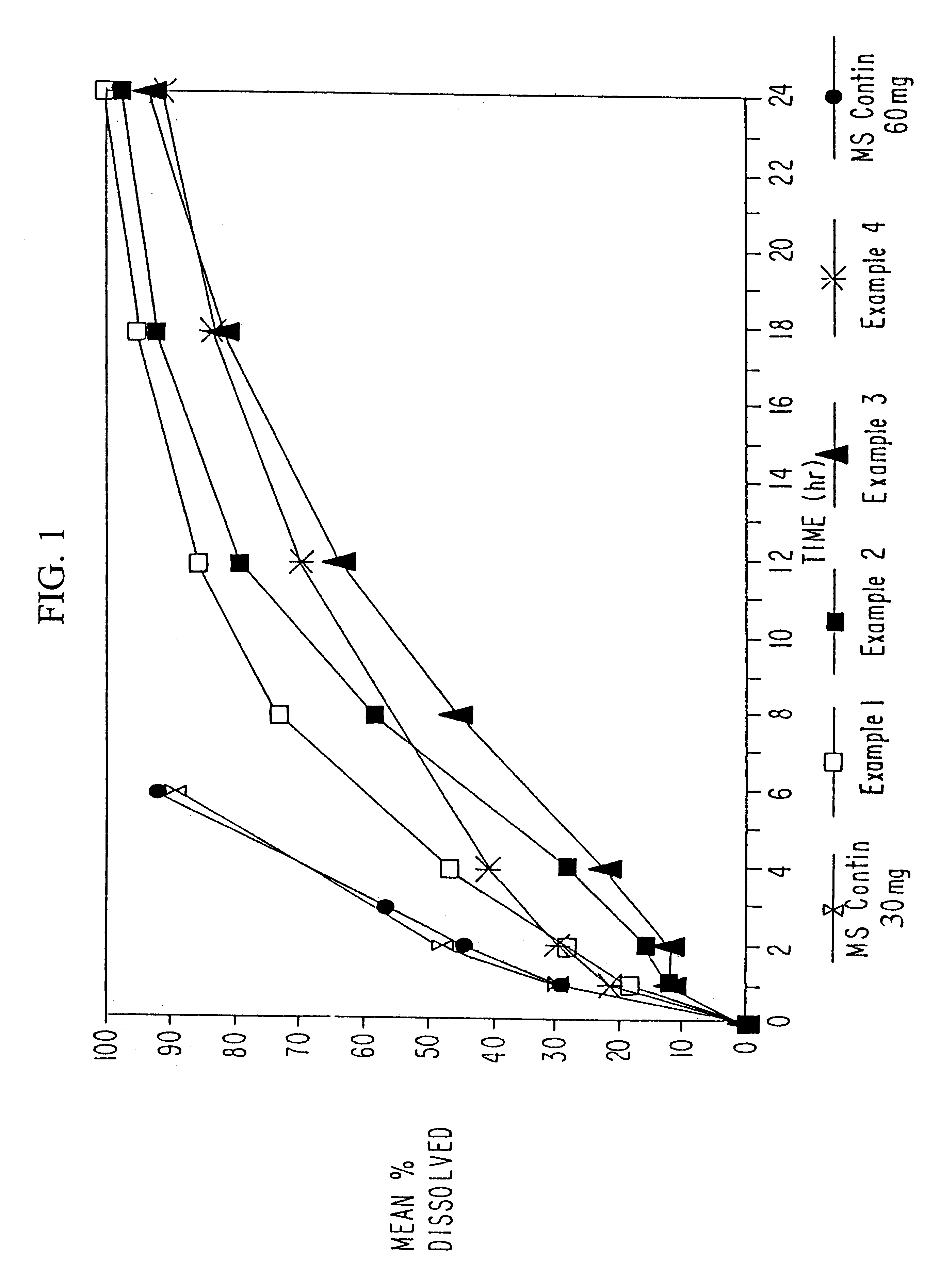
Morphine controlled release system
InactiveUS20070003617A1Low administration frequencyAffecting extent of drug bioavailabilityBiocideNervous disorderMorphineDissolution
A composition for controlled release of an opioid from a pharmaceutical composition, the method comprises controlling the release of at least one opioid into an aqueous medium by erosion of at least one surface of a pharmaceutical composition comprising I) a matrix composition comprising a) polymer or a mixture of polymers, b) an opioid and, optionally, c) one or more pharmaceutically acceptable excipients, and (i) a coating. The matrix composition has a conus-like shape so the surface area exposed to the aqueous medium increases at least during initial erosion of the matrix composition, and the dissolution of the opioid-when tested in a Dissolution Test as described herein with or without application of sinkers-results in a zero order release of at least 80% of the opioid contained in the composition. Such compositions are especially suitable for controlled release of an opioid to obtain a delayed pead concentration and a prolonged therapeutically effective plasma concentration upon oral administration. Once or twice daily administration is possible. The matrix typically comprises PEO and the active substance is typically an opioid such as morphine or a glucuronide thereof.
Owner:EGALET LTD
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20060251721A1Improved ability to treat painLess attentionBiocideNervous disorderImmediate releasePharmaceutical medicine
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Multi-particulate, modified-release composition
A multi-particulate, modified-release pharmaceutical composition for the oral administration of an active ingredient to the colon, wherein said particles comprise: (a) a core comprising an active ingredient or a pharmaceutically acceptable salt or ester thereof, and optionally one or more excipients; (b) a first coating applied to the surface of the core, wherein said first coating is insoluble in gastric juice and in intestinal juice below pH 7, but soluble in colonic intestinal juice; and (c) a second coating applied to the surface of the first coating.
Owner:SANDOZ AG
Abuse resistant melt extruded formulation having reduced alcohol interaction
InactiveUS20090317355A1Reduced and limited dose-dumping effectReduce interactionBiocideNervous disorderVerapamilOral medication
The present invention relates to compositions for oral administration. The invention preferably comprises at least one abuse-resistant drug delivery composition for delivering a drug having potential for dose dumping in alcohol, related methods of preparing these dosage forms, and methods of treating a patient in need thereof comprising administering the inventive compositions to the patient. Most preferably, the dosage form includes verapamil. These formulations have reduced potential for abuse. In another formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Porous drug matrices and methods of manufacture thereof
InactiveUS20050048116A1Fast dissolutionHigh dissolution ratePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid-compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
System, method, and apparatus for dispensing oral medications
A pill dispenser includes a housing, a pill-dispensing mechanism, a receptacle, a pill-viewing camera, an identifying camera, one or more processors, and a storage medium (e.g., a memory). The pill-dispensing mechanism is coupled to an opening of the housing. The first pill-viewing camera is positioned to capture an image of the receptacle, and the identifying camera is positioned to capture an image of an area adjacent to the housing. The one or more processors are in operative communication with the pill-dispensing mechanism, the pill-viewing camera, and the identifying camera. The storage medium stores processor-executable instructions for: instructing the pill-dispensing mechanism to dispense a pill; instructing the pill-viewing camera to capture a first image of the pill to determine a presence of the pill; instructing the pill-viewing camera to capture a second image to determine an absence of the pill; and instructing the identifying camera to capture a third image.
Owner:DEKA PROD LLP
Aromatic amides and ureas and their uses as sweet and/or umami flavor modifiers, tastants and taste enhancers
InactiveUS20060045953A1Improve responseInduce savory taste perceptionFood preparationMonosodium glutamateSalty taste
The inventions disclosed herein relate to non-naturally occurring amide compounds that are capable, when contacted with comestible food or drinks or pharmaceutical compositions at concentrations preferably on the order of about 100 ppm or lower, of serving as savory (“umami”) or sweet taste modifiers, savory or sweet flavoring agents and savory or sweet flavor enhancers, for use in foods, beverages, and other comestible or orally administered medicinal products or compositions, optionally in the presence of or in mixtures with conventional flavoring agents such as monosodium glutamate or known natural and artificial sweeteners.
Owner:SENOMYX INC
Pharmaceutical Dosage Form For Oral Administration Of Tyrosine Kinase Inhibitor
InactiveUS20090203709A1BiocideOrganic non-active ingredientsOral medicationTyrosine-kinase inhibitor
A pharmaceutical dosage form comprises a solid dispersion product of at least one tyrosine kinase inhibitor, at least one pharmaceutically acceptable polymer, and at least one pharmaceutically acceptable solubilizer.
Owner:ABBVIE INC
Dual controlled release dosage form
A dosage form that provides a controlled release of at least two different active agents is provided. Particular embodiments include a dosage form that provides therapeutically effective levels of a first active agent and a second active agent in a mammal for an extended period of time following oral administration. An osmotic device containing a bi-layered core is provided. The osmotic device provides a dual controlled release of both drugs from the core. The layers of the core are in stacked, substantially concentric or substantially eccentric arrangement.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
Aripiprazole oral solution
The present invention provides for a pharmaceutical solution suitable for oral administration comprising aripiprazole, a pharmaceutically suitable solvent system, one or more taste-enhancing / masking agents and one or more agents selected from the group consisting of lactic acid, acetic acid, tartaric acid and citric acid, wherein said solution has a pH from 2.5 to 4.5.
Owner:OTSUKA PHARM CO LTD
Taste-masked pharmaceutical compositions
ActiveUS20060078614A1Effective taste-maskingRapid/complete releasePill deliveryAdditive ingredientOrally disintegrating tablet
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredient(s), rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates on contact with saliva in the buccal cavity in about 60 seconds forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing the active) applied with a taste-masking membrane comprising a combination of water-insoluble and gastrosoluble polymers release not less than about 60% of the dose is in the stomach in about 30 minutes, thus maximizing the probability of achieving bioequivalence to the reference IR product having rapid onset of action (short Tmax). A process for preparing such compositions for oral administration using conventional fluid-bed equipment and rotary tablet press is also disclosed.
Owner:ADARE PHARM INC
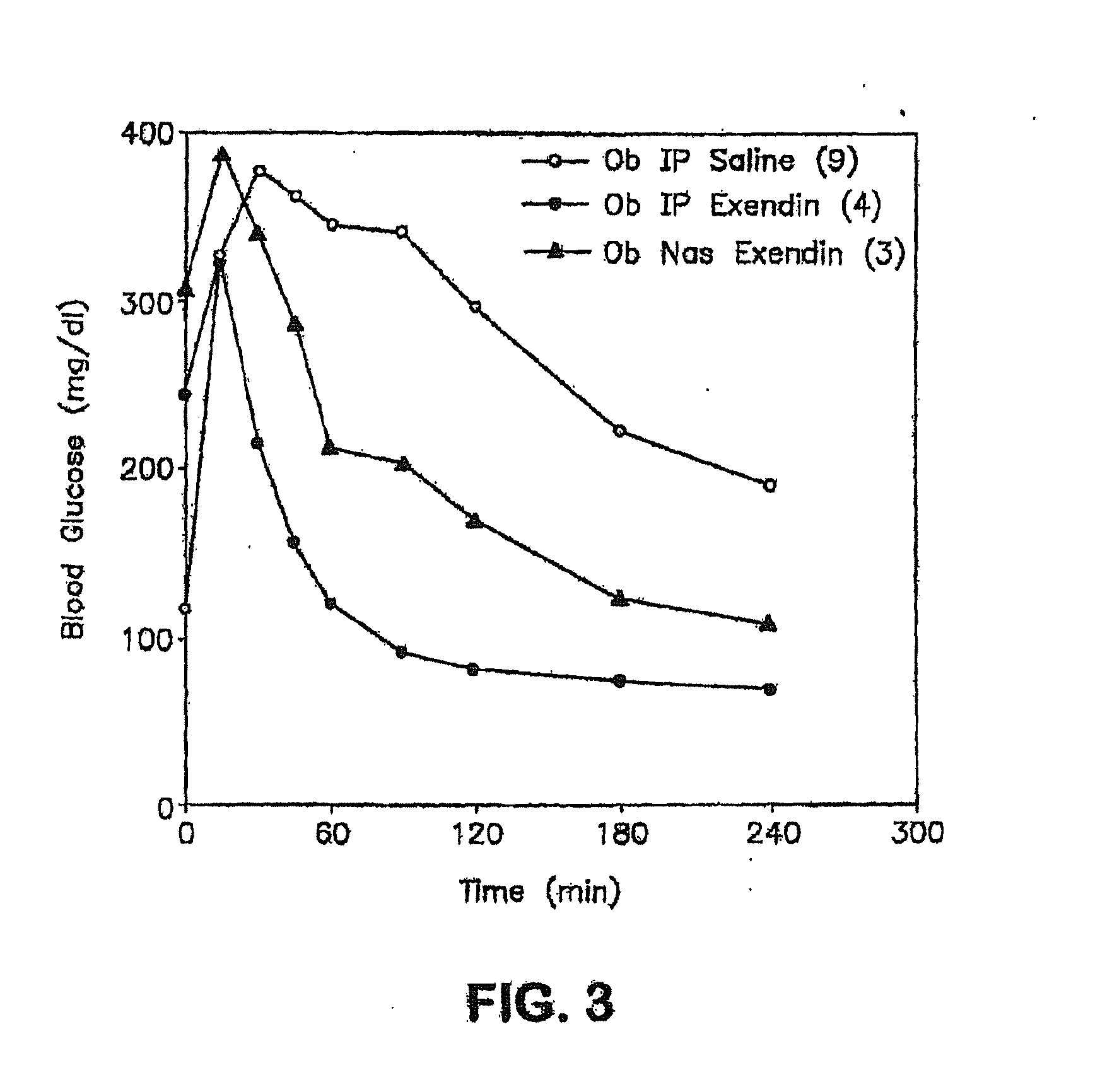
Methods and compositions for oral administration of exenatide
InactiveUS20110046053A1Reducing food intakeDecreased gastric motilityPeptide/protein ingredientsMetabolism disorderDiabetes mellitusOral medication
This invention provides compositions comprising a byetta, fish oil, and a protease inhibitor, method for treating diabetes mellitus, comprising administering same, and methods for oral or rectal administration of a byetta.
Owner:ORAMED
Compositions for oral drug administration
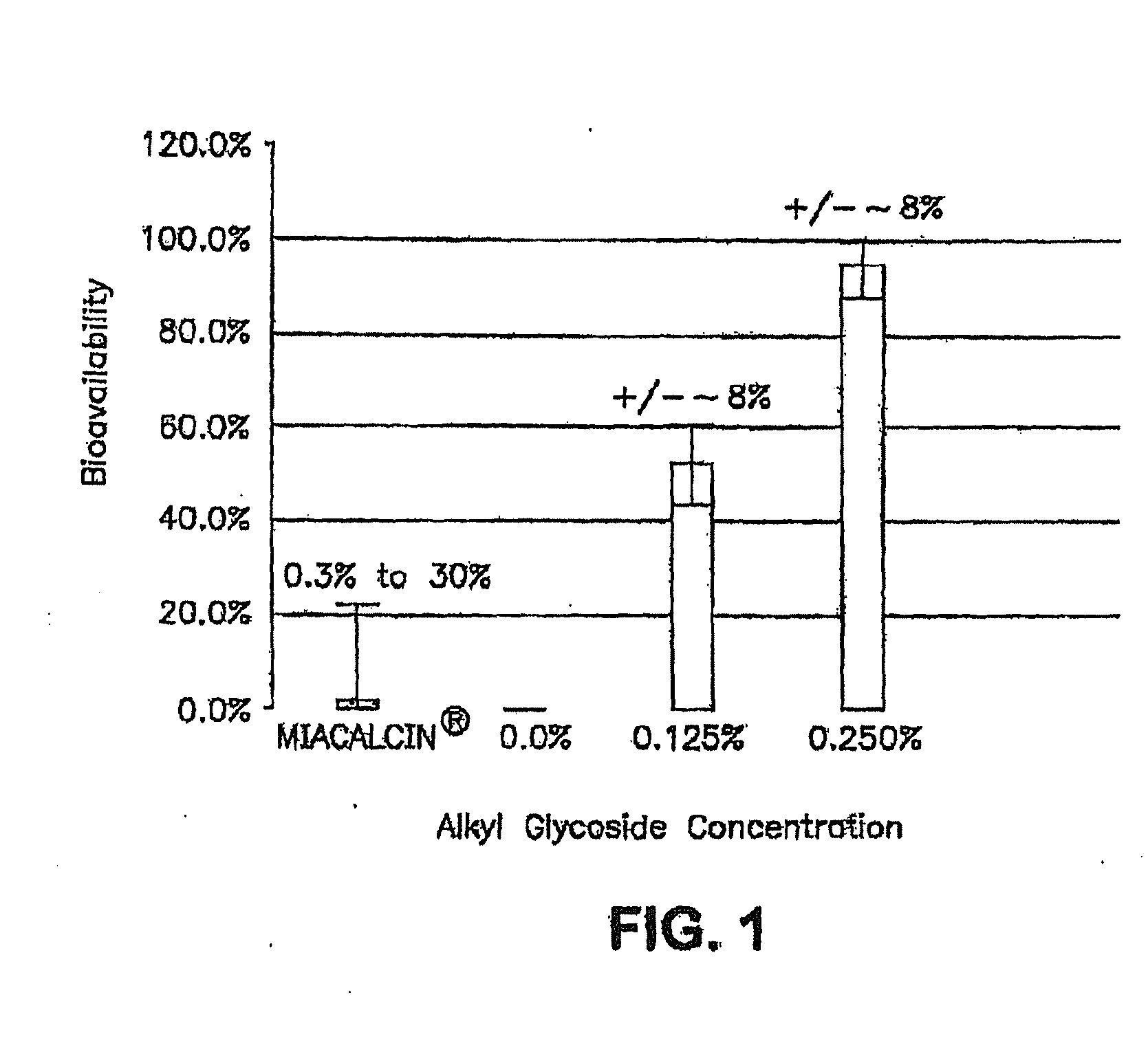
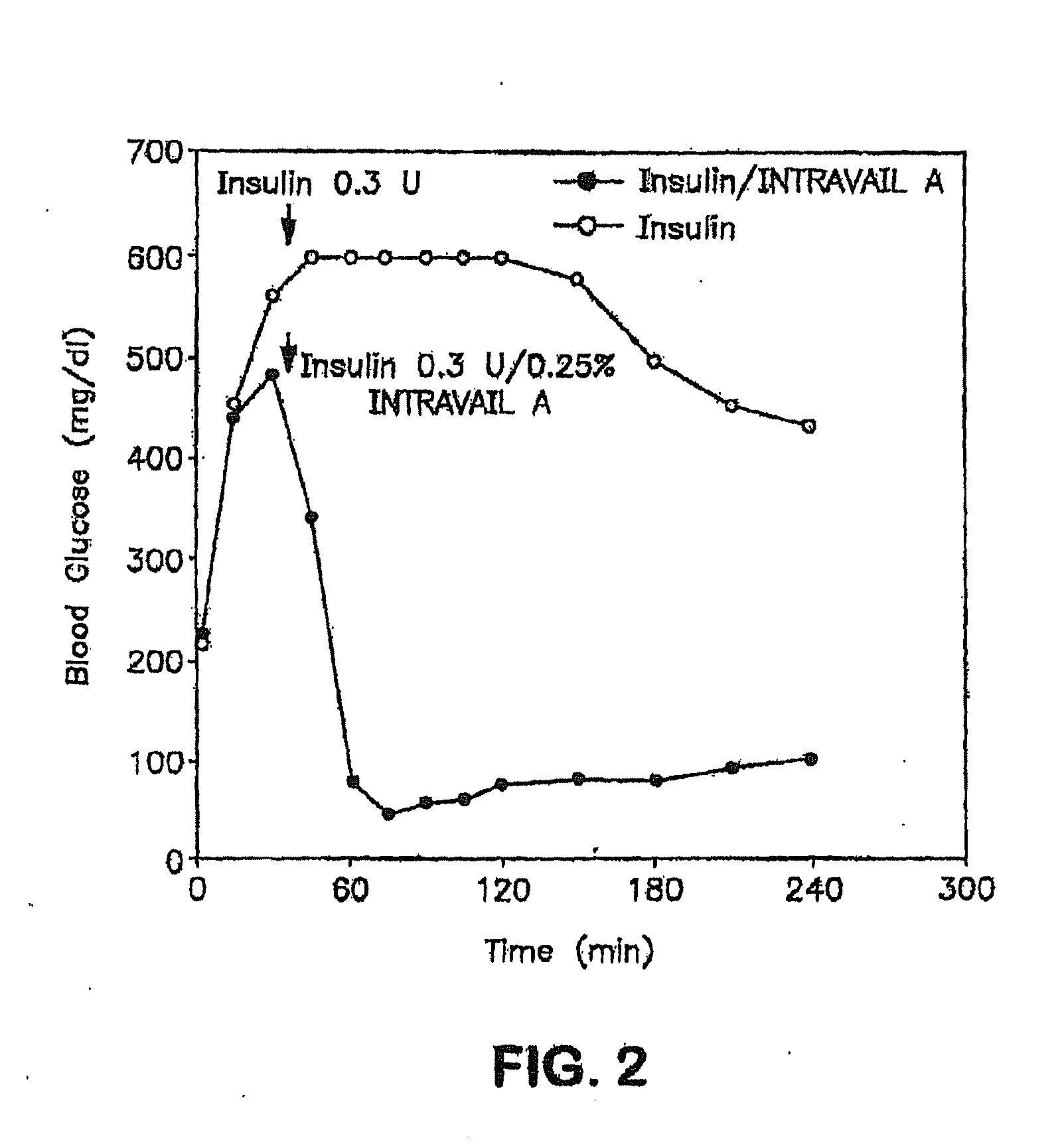
InactiveUS20140162965A1Promote absorptionImprove bioavailabilityBiocideCarbohydrate active ingredientsGlycosideOral medication
The present invention provides compositions and methods and for increasing the bioavailability of therapeutic agents in a subject. The compositions include at least one alkyl glycoside and at least one therapeutic agent, wherein the alkylglycoside has an alkyl chain length from about 10 to about 16 carbon atoms. In various aspects, the invention provides compositions and methods for oral delivery in the form of a tablet.
Owner:AEGIS THERAPEUTICS LLC
Porous drug matrices and methods of manufacture thereof
InactiveUS20050058710A1Fast dissolutionExtended half-lifePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
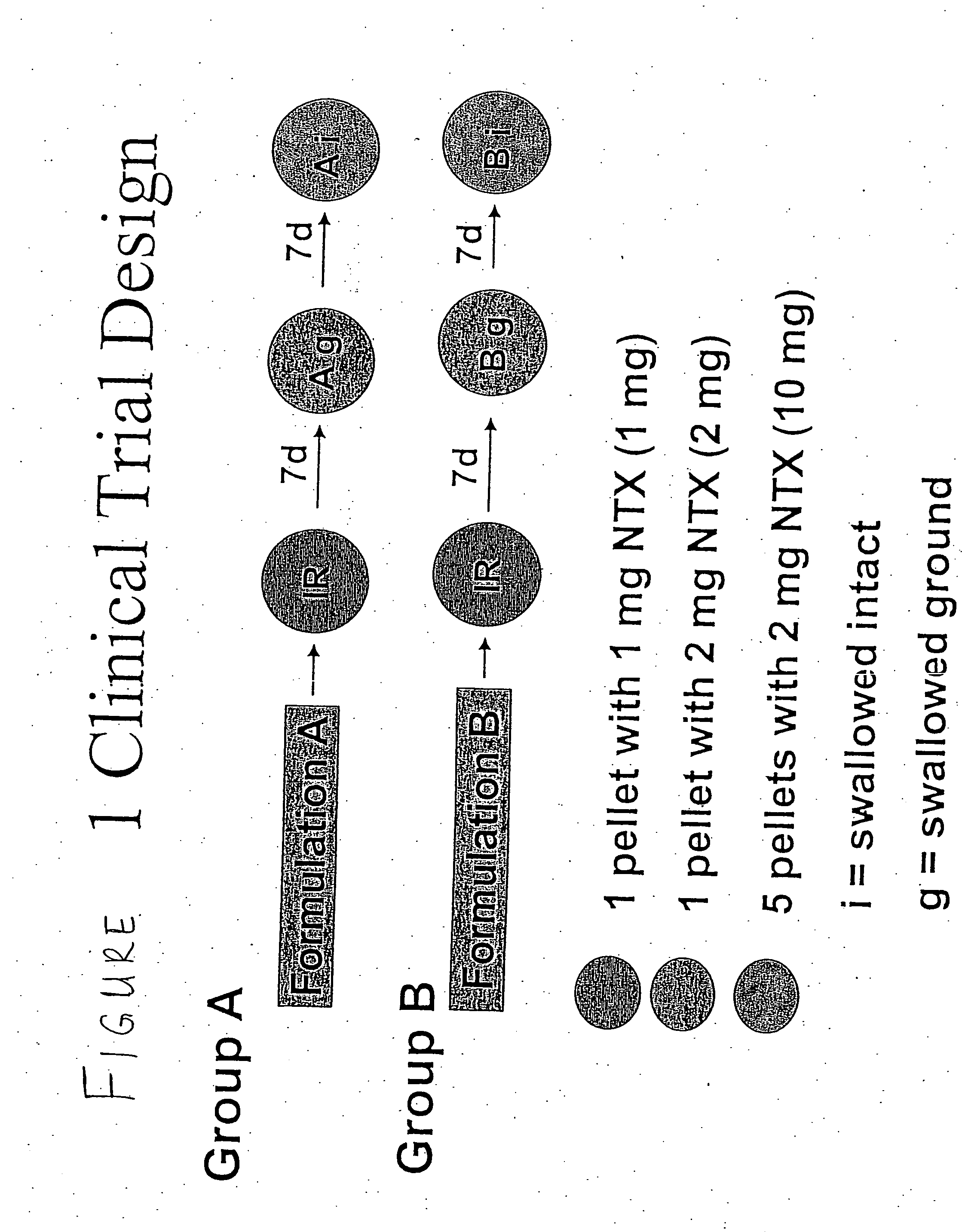
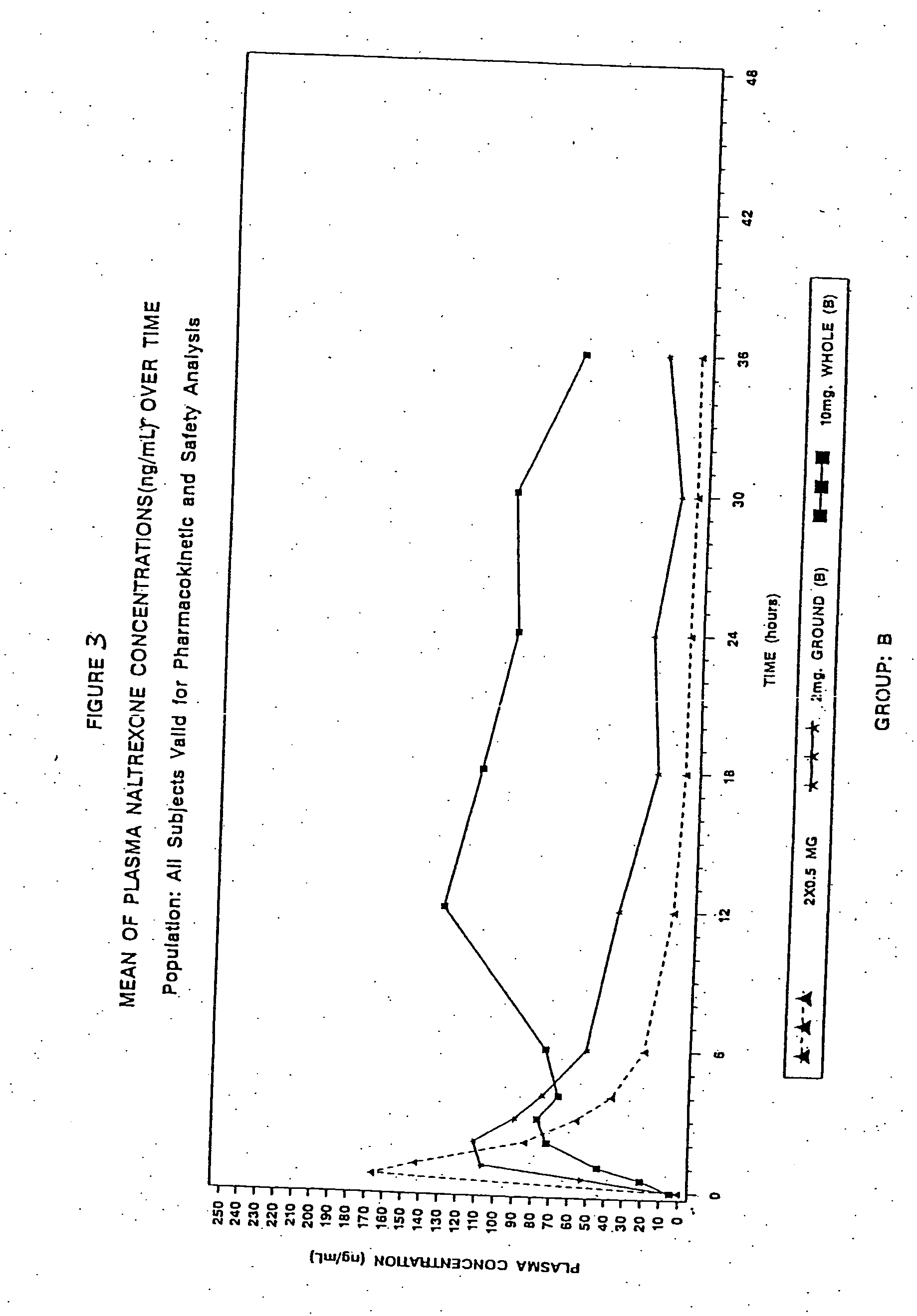
Sequestered antagonist formulations
InactiveUS20060182801A1Lower potentialIncurs riskBiocidePowder deliveryOpioid antagonistOpioid Agonist
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the mean Cmax of the antagonist after single dose oral administration of the dosage form after tampering to the mean Cmax of antagonist after single dose oral administration of an intact dosage form is at least 1.5:1.
Owner:PURDUE PHARMA LP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com