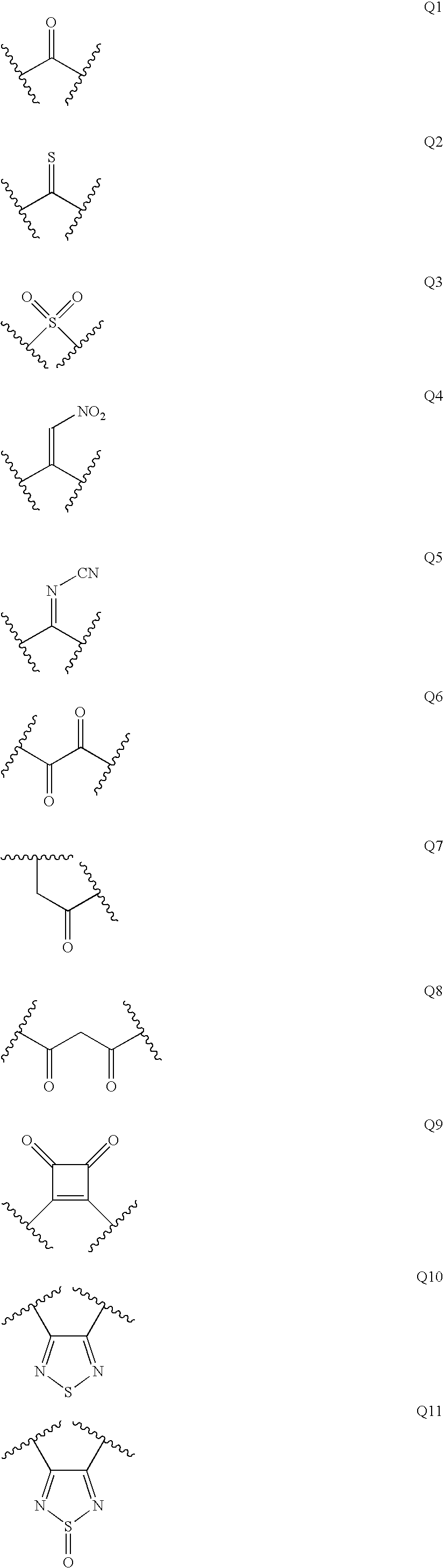
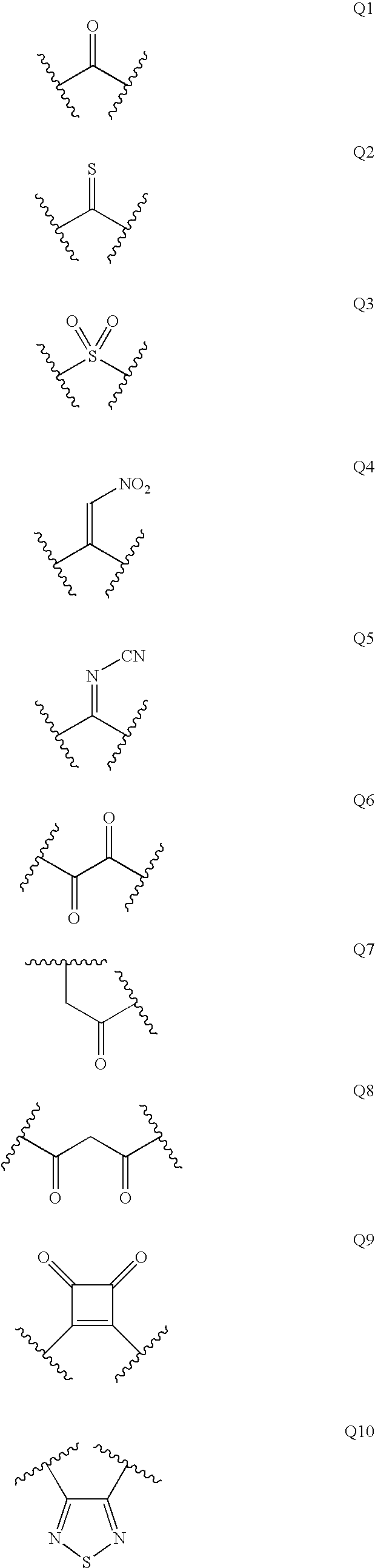
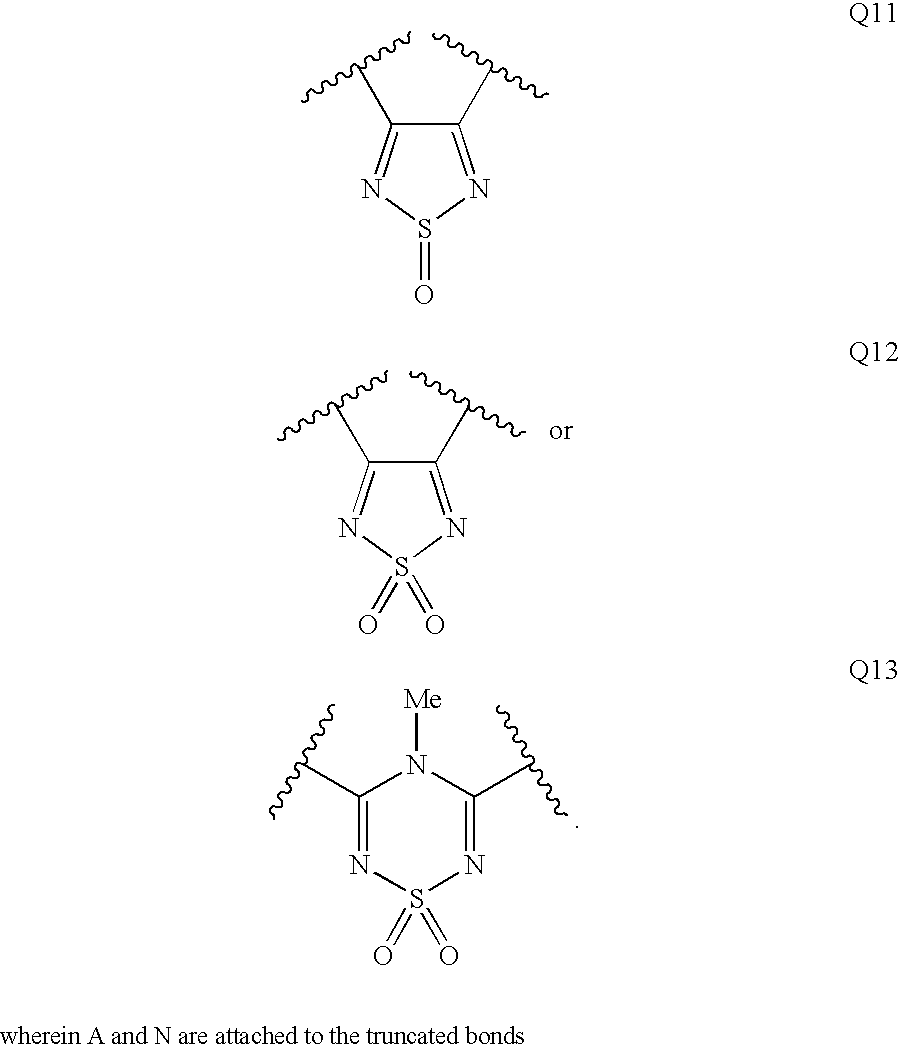
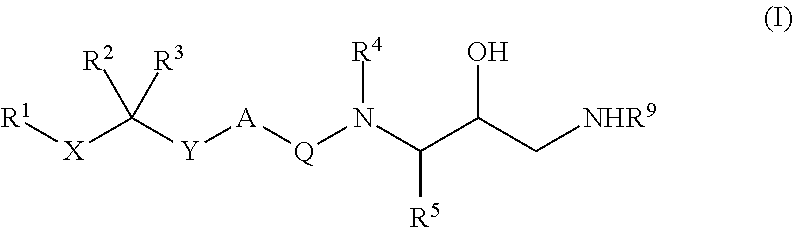
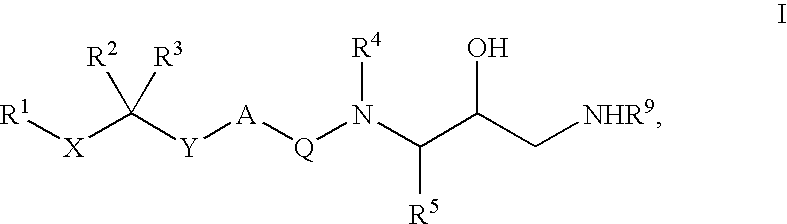
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
73 results about "Orally active" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The term “orally active” is of course a relative term. Lipophilically modified steroids are more orally active than the free parent steroids, however, they are no where near as active as the 17alpha-alkylated steroids.
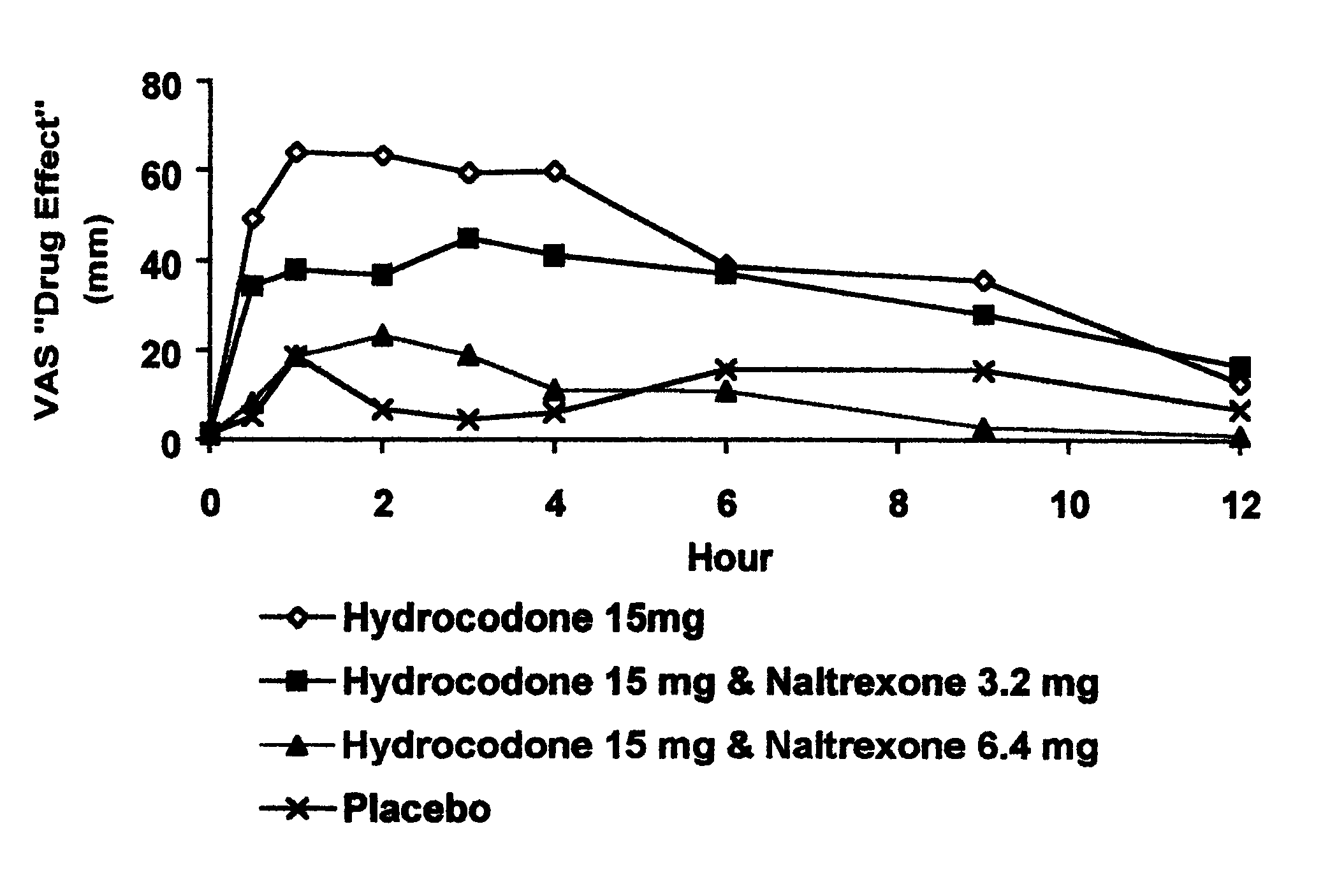
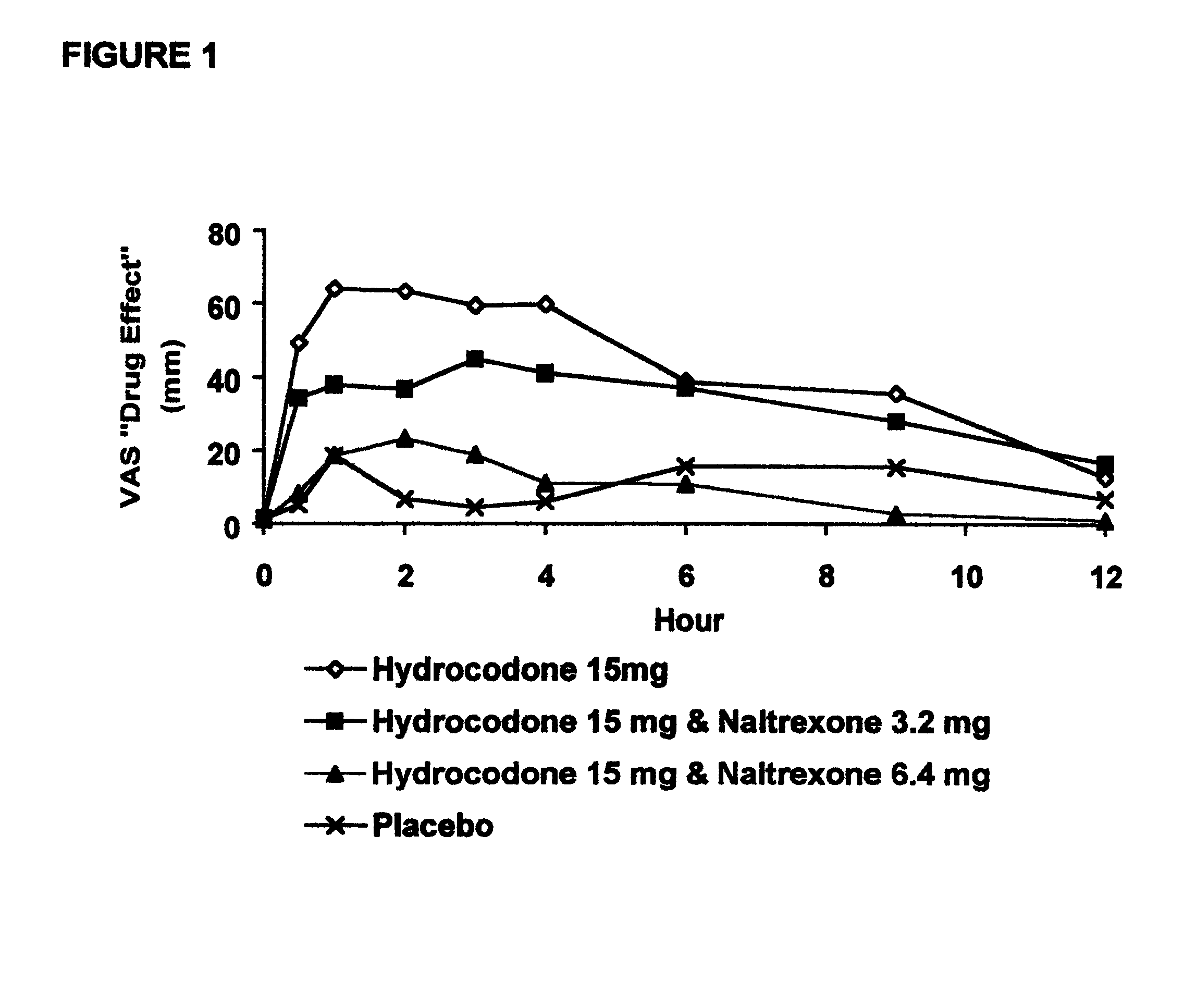
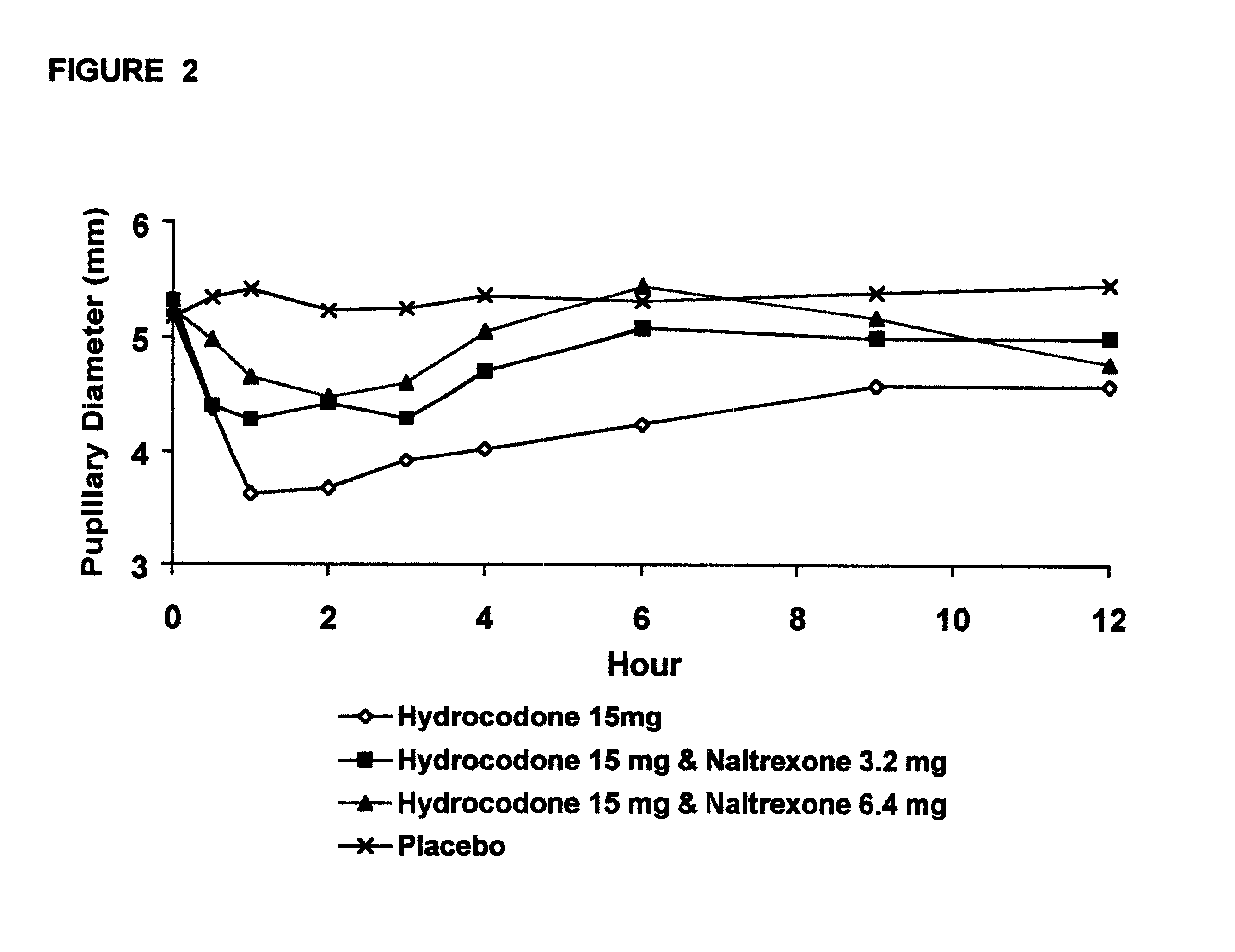
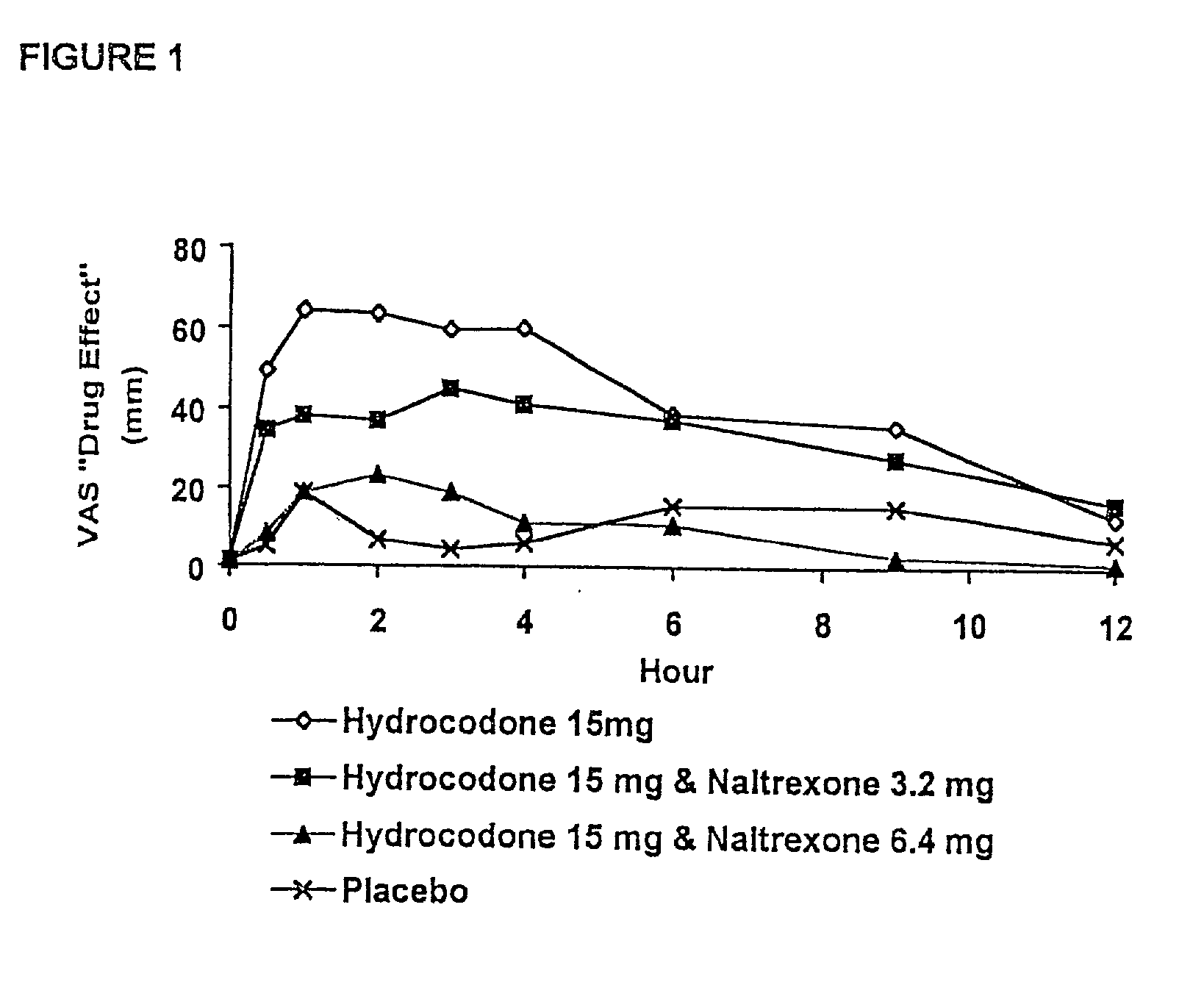
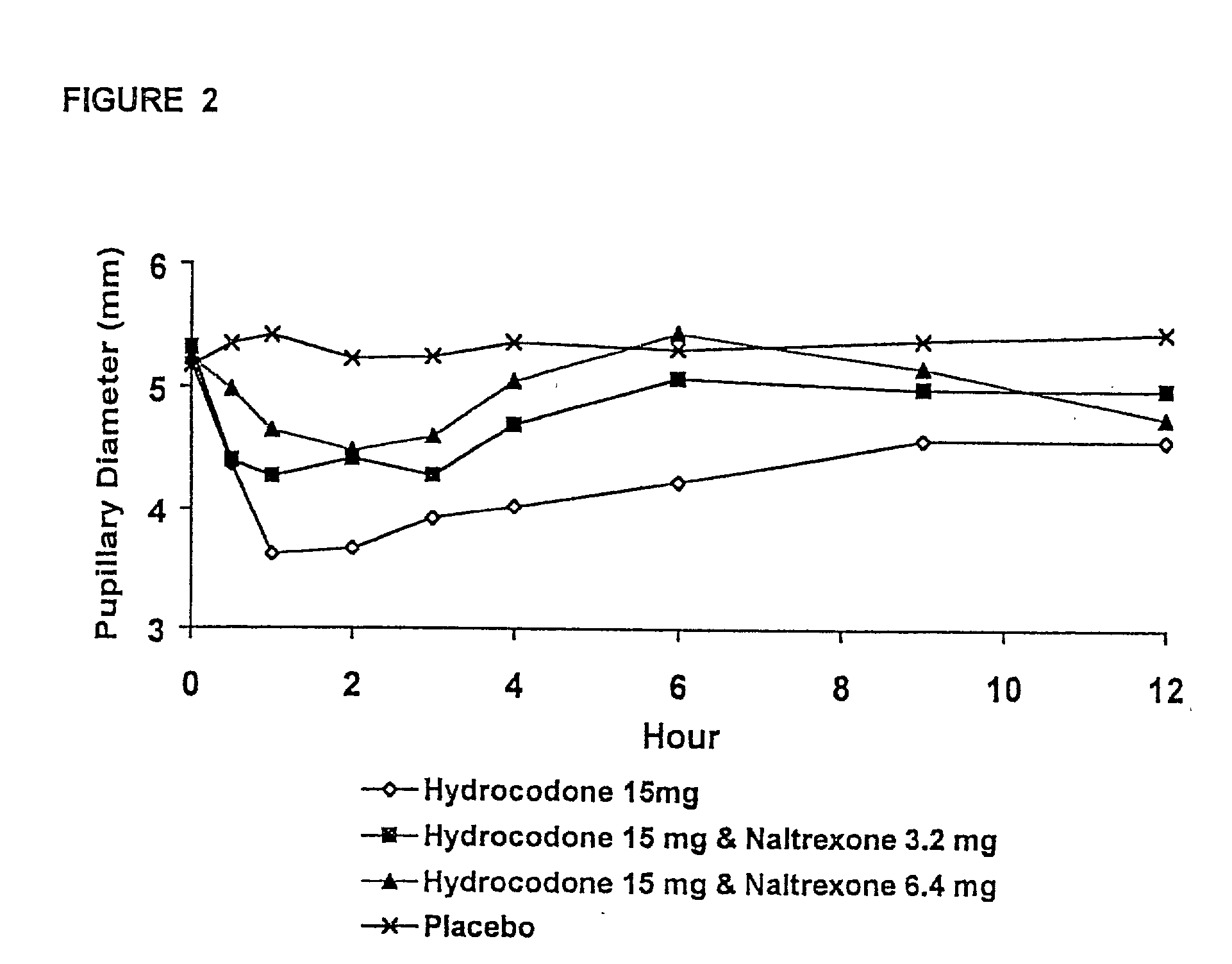
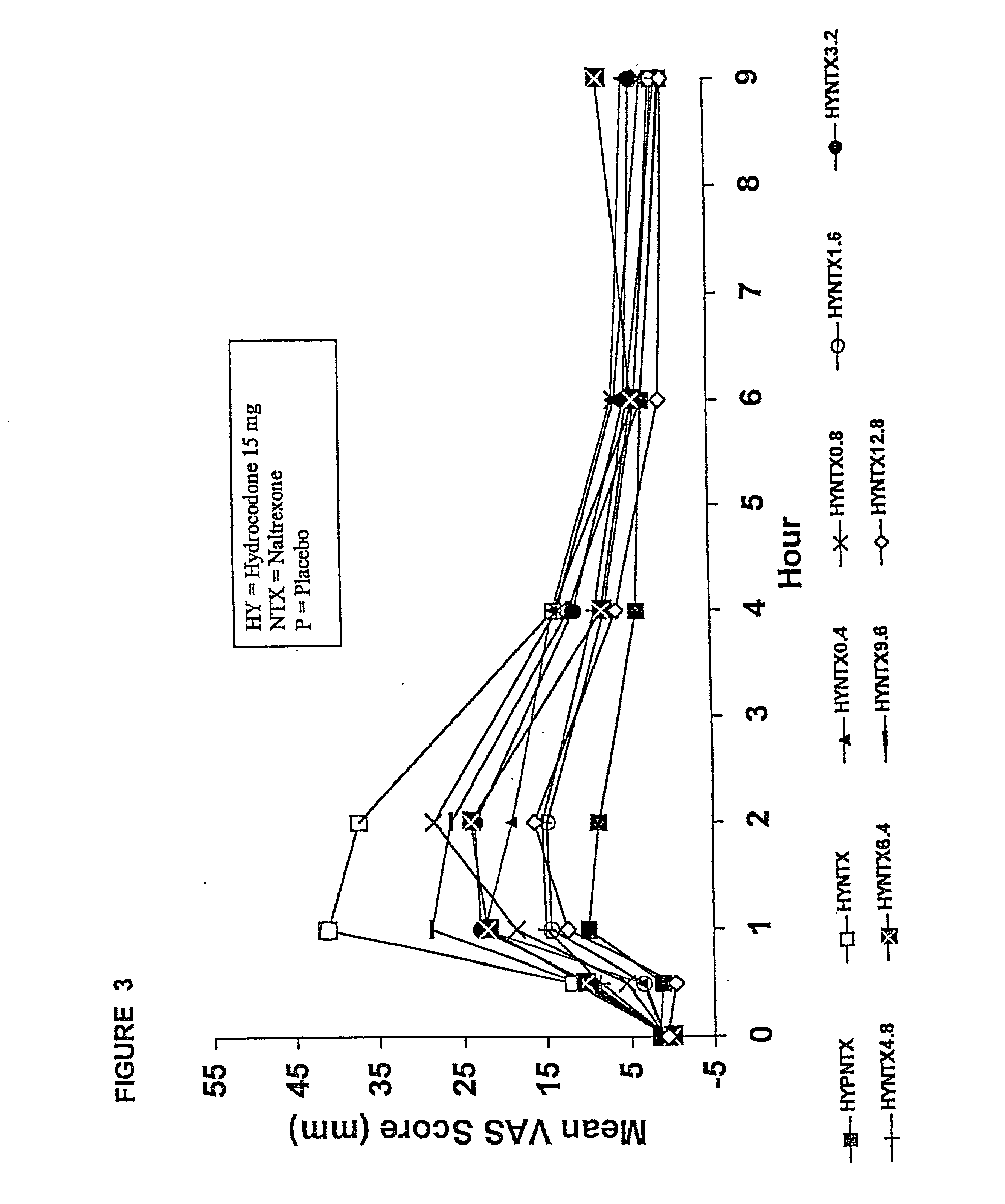
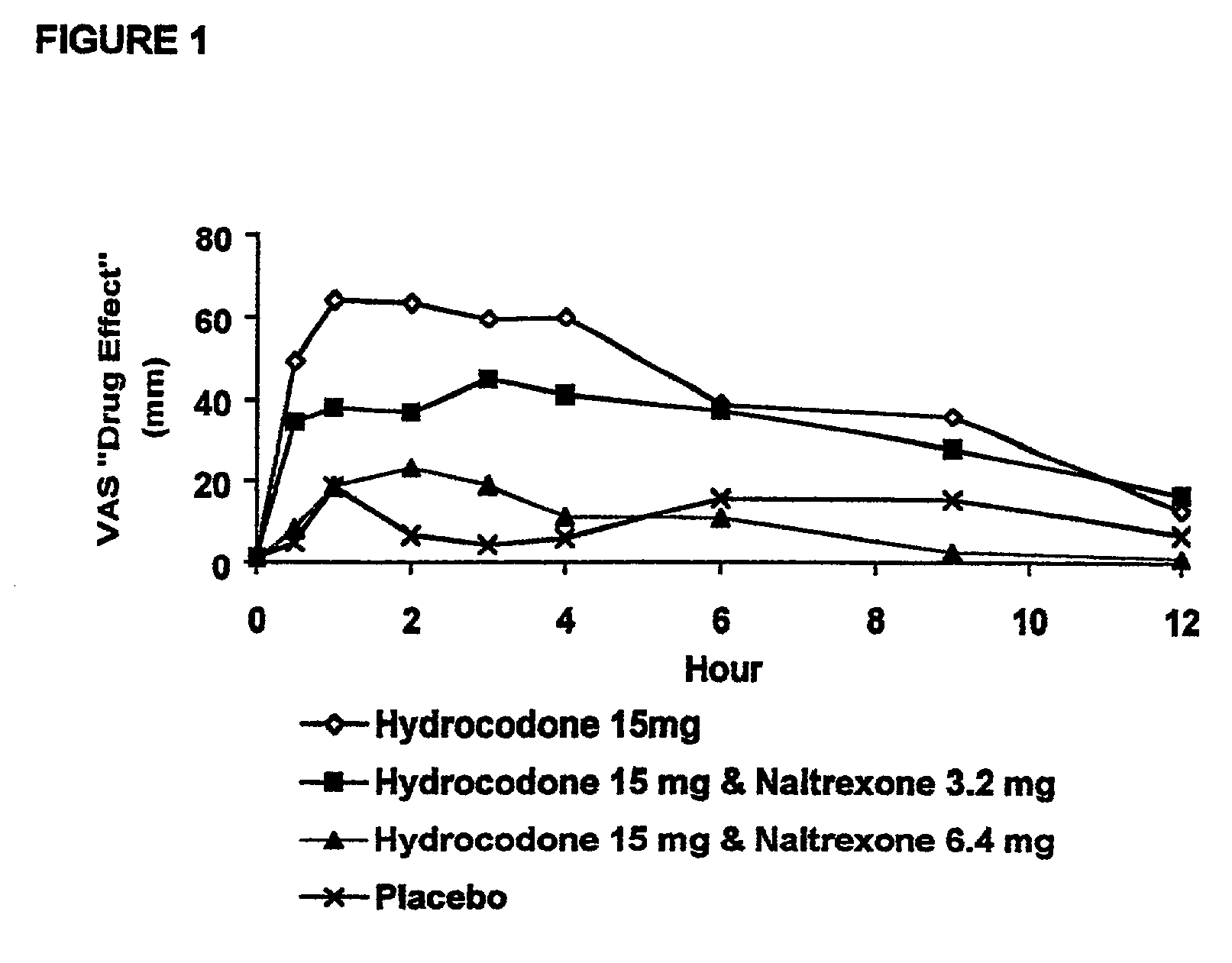
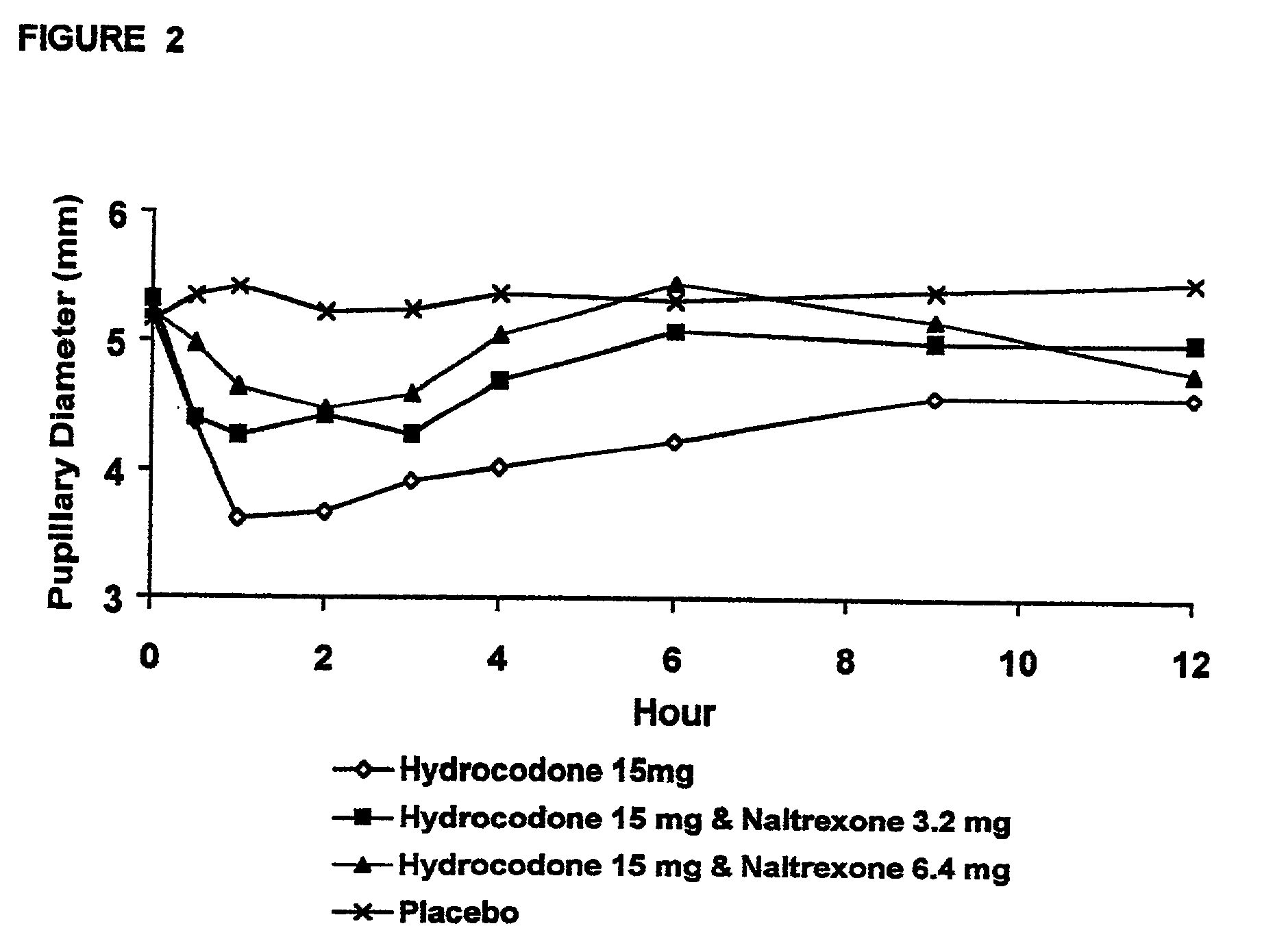
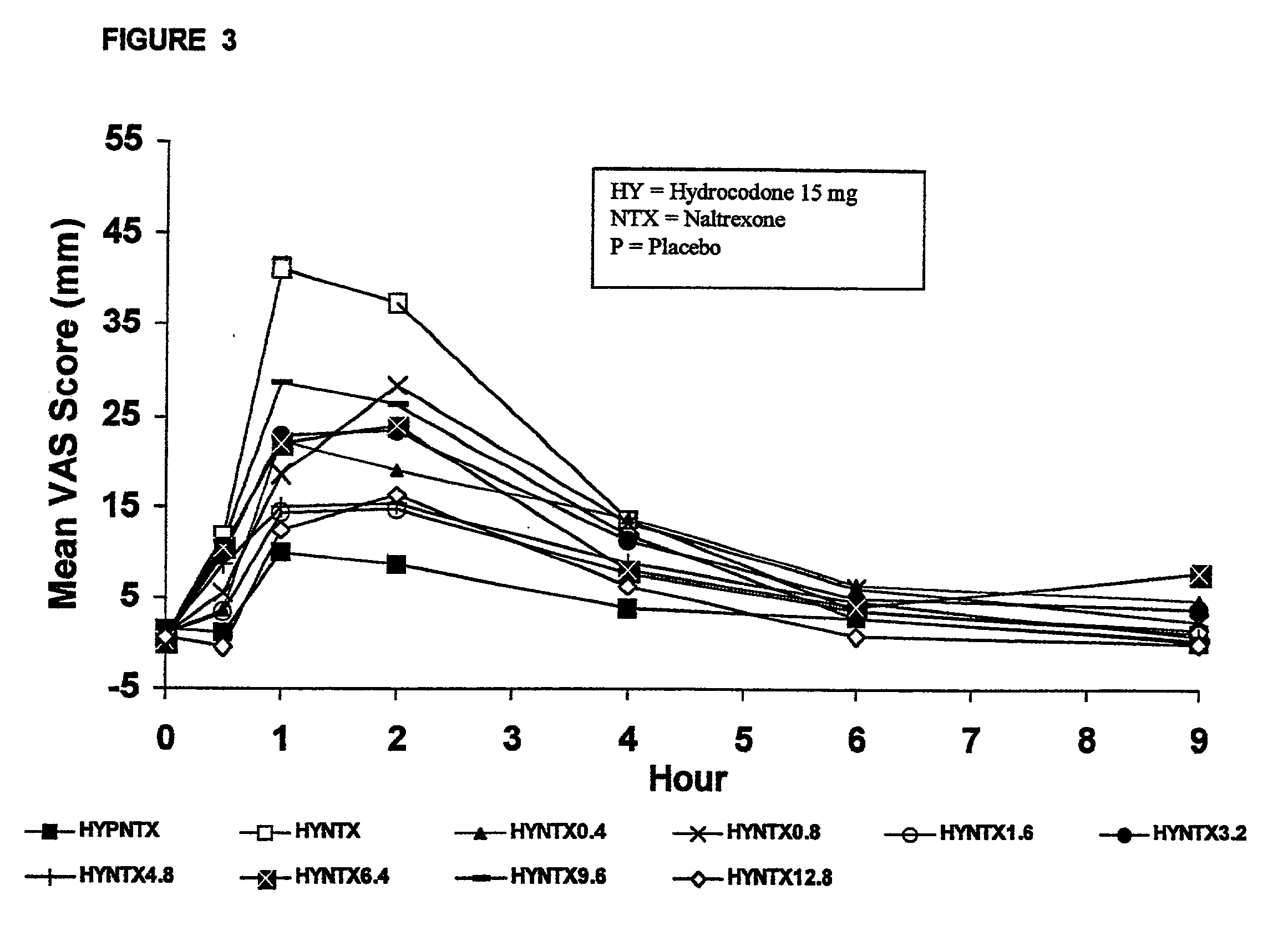
Method of preventing abuse of opioid dosage forms
InactiveUS6228863B1Reducing parenteral abuse potential of dosage formBiocideNervous disorderOpioid antagonistOpioid Agonist
The invention relates in part to a method of reducing the abuse potential of an oral dosage form of an opioid analgesic, wherein an analgesically effective amount of an orally active opioid agonist is combined with an opioid antagonist into an oral dosage form which would require at least a two-step extraction process to be separated from the opioid agonist, the amount of opioid antagonist including being sufficient to counteract opioid effects if extracted together with the opioid agonist and administered parenterally.
Owner:PURDUE PHARMA LP
Opioid agonist/antagonist combinations
InactiveUS6277384B1Increase elasticityTrend downBiocideNervous disorderOpioid AgonistOpioid antagonist
The invention is directed in part to oral dosage forms comprising a combination of an orally analgesically effective amount of an opioid agonist and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Opioid agonist/opioid antagonist/acetaminophen combinations
InactiveUS20020058673A1Reducing oral abuse potential of dosage formImprove subjective experienceBiocideDrug compositionsOpioid antagonistOpioid Agonist
The invention is directed in part to oral dosage forms comprising a combination of an opioid agonist, acetaminophen and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
As-needed administration of an androgenic agent to enhance female desire and responsiveness
InactiveUS20050070516A1Improving tissue healthImprove responsivenessElcosanoid active ingredientsNanomedicineOral medicationUrethra
A method is provided for enhancing a female individual's sexual desire and responsiveness. The method involves administration of a pharmaceutical formulation containing an effective amount of an androgenic agent, wherein administration is on an as-needed basis rather than involving chronic pharmacotherapy. Local delivery may be accomplished via administration to the vagina, vulvar area or urethra of the individual, although oral administration is preferred for those androgenic agents that are orally active. Formulations and kits for carrying out the method are provided as well.
Owner:VIVUS
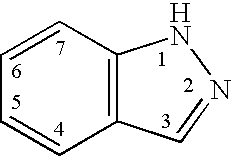
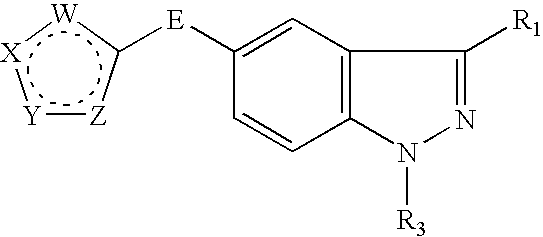
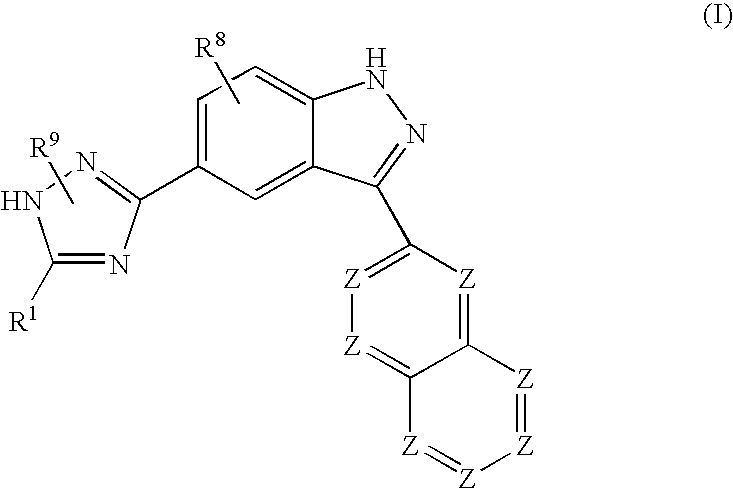
Indazole compounds and methods of use thereof
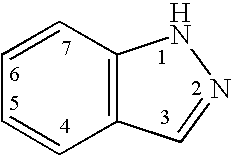
InactiveUS20060004043A1Modulating levelUseful in treatmentAntibacterial agentsBiocideAutoimmune conditionProtein kinase A signaling
This invention is directed to Indazole Compounds or pharmaceutically acceptable salts, solvates and hydrates thereof. The Indazole Compounds have utility in the treatment or prevention of a wide range of diseases and disorders that are responsive to the inhibition, modulation or regulation of kinases, such as inflammatory diseases, abnormal angiogenesis and diseases related thereto, cancer, atherosclerosis, a cardiovascular disease, a renal disease, an autoimmune condition, macular degeneration, disease-related wasting, an asbestos-related condition, pulmonary hypertension, diabetes, obesity, pain and others. Thus, methods of treating or preventing such diseases and disorders are also disclosed, as are pharmaceutical compositions comprising one or more of the Indazole Compounds. This invention is based, in part, upon the discovery of a novel class of 5-triazolyl substituted indazole molecules that have potent activity with respect to the modulation of protein kinases. Thus, the invention encompasses orally active molecules as well as parenterally active molecules which can be used at lower doses or serum concentrations for treating diseses or disorders associated with protein kinase signal transduction.
Owner:BHAGWAT SHRIPAD S +10
Heterocyclic receptor agonists for the treatment of diabetes and metabolic disorders
Compounds and methods are provided for the treatment of, inter alia, Type II diabetes and other diseases associated with poor glycemic control. The compounds of the invention are orally active.
Owner:CYMABAY THERAPEUTICS
Solid, Oral Drug Form Which Has Been Designed to Prevent Misuse
InactiveUS20080193540A1Avoid misuseEasy to getBiocideOrganic active ingredientsOral medicationPublic health
The invention relates to the field of solid medicaments that are intended for the oral administration of active ingredients. The aim of the invention is to prevent the improper use of solid, oral medicaments for any user other than the therapeutic use(s) officially approved by the appropriate public health authorities. More specifically, the invention relates to a solid, oral drug form which is characturised in that it comprises: A) at least one caking agent; and B) at least one viscosifying agent, such as to prevent the misuse thereof.
Owner:FLAMEL TECHNOLOGIES
Orally Active Purine-Based Inhibitors of Heat Shock Protein 90
Novel purine compounds and tautomers and pharmaceutically acceptable salts thereof are described, as are pharmaceutical compositions comprising the same, complexes comprising the same, e.g., HSP90 complexes, and methods of using the same. Methods of using the novel purine compounds of the invention, and tautomers and pharmaceutically acceptable salts thereof, include their use in inhibiting heat shock protein 90's (HSP90's) to thereby treat or prevent HSP90-dependent diseases, e.g., proliferative disorders such as breast cancer.
Owner:CONFORMAL THERAPEUTICS CORP (US)
Orally active fraction of momordica charactia, active peptides thereof, and their use in the treatment of diabetes
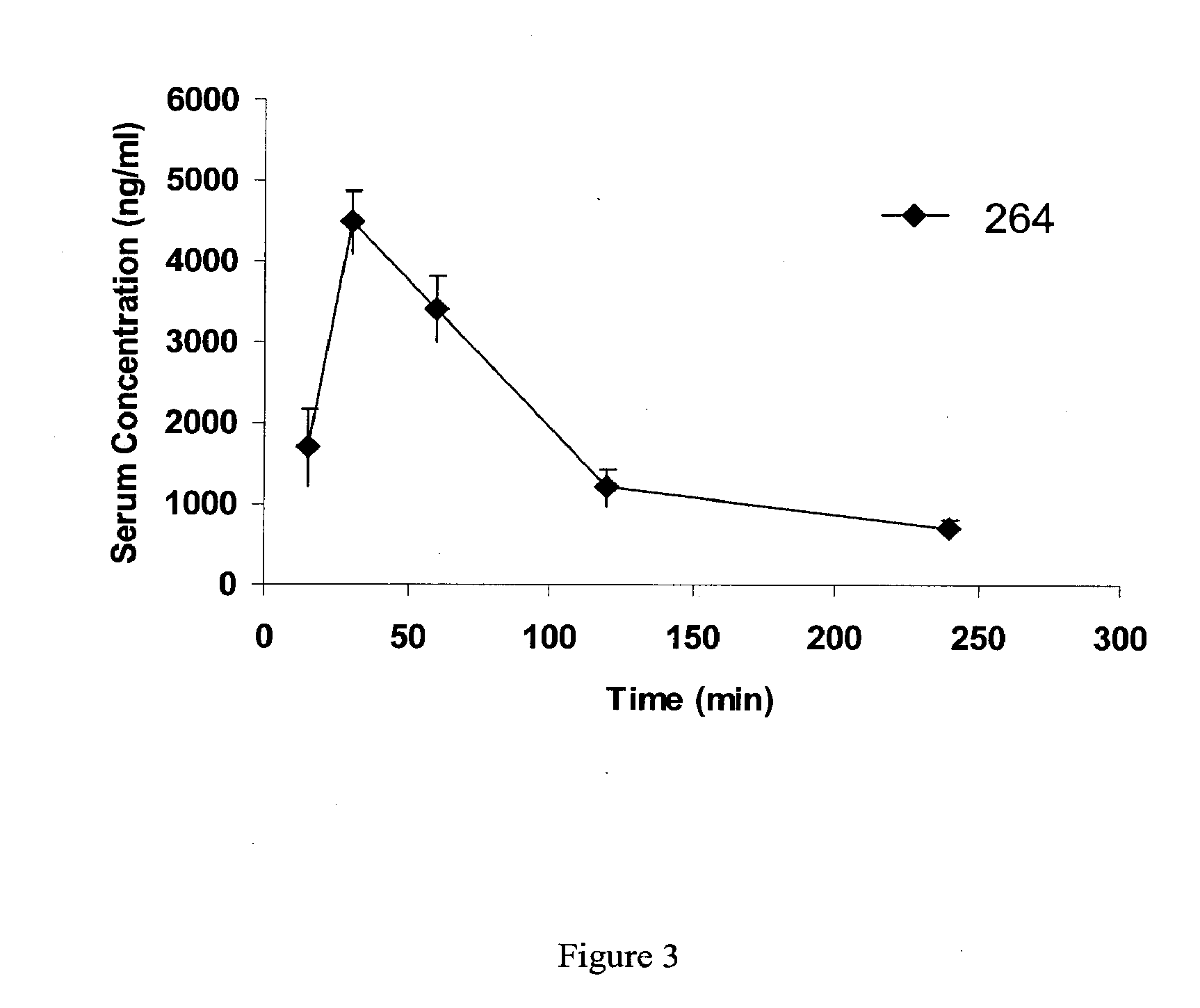
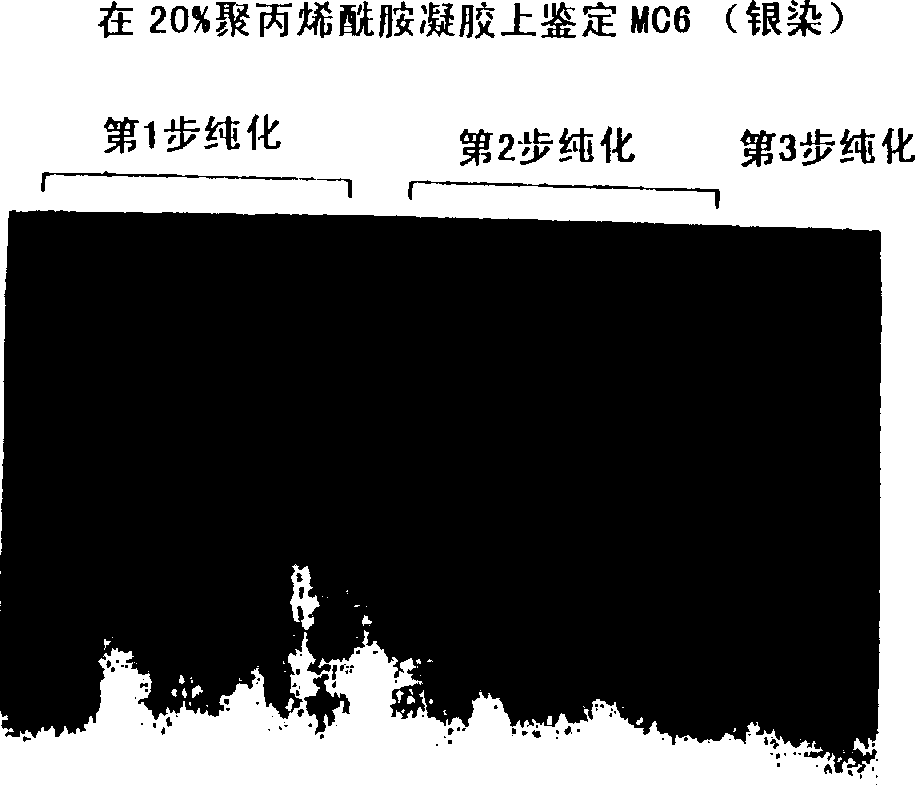
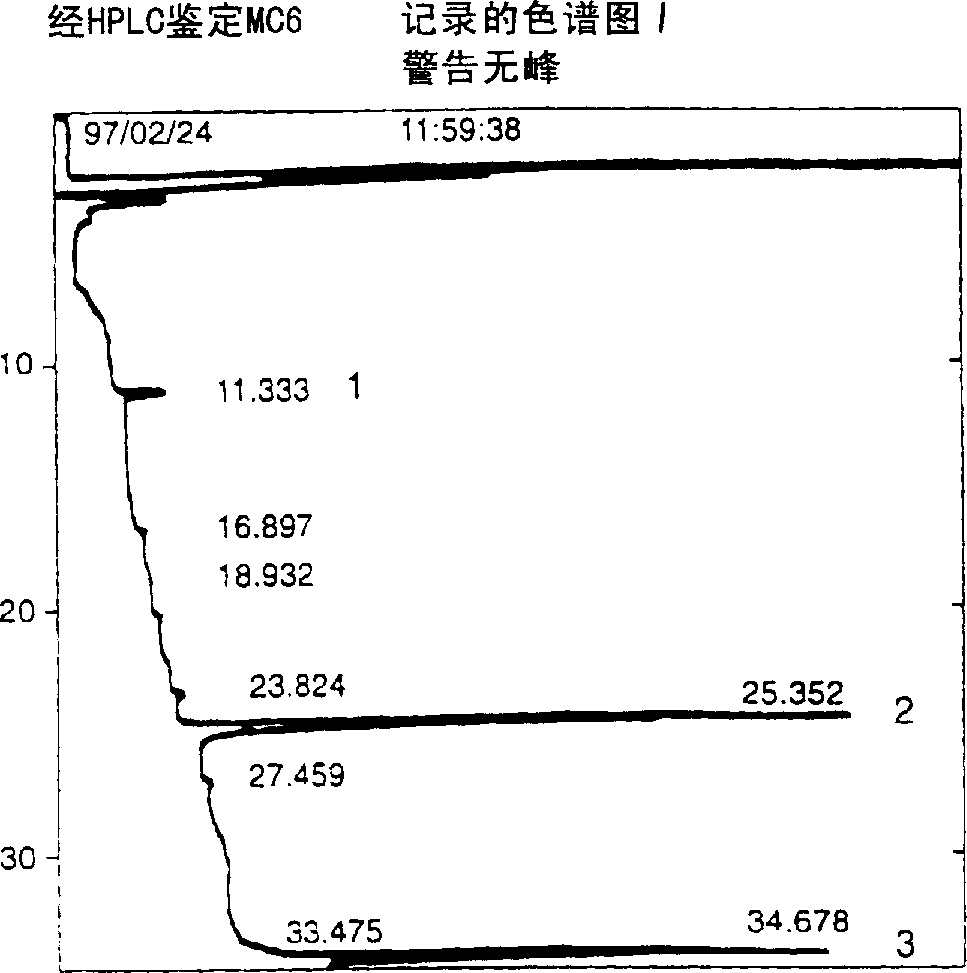
A water soluble extract of M.charantia named MC6, methods for its preparation and methods for its use in the treatment of hyperglycemic disorders are provided. The active MC6 is characterized by moving as a single band on SDS-PAGE having a molecular weight of less than 10 kDal, and by comprising three peptides. Also provided is a peptide component of MC6 named MC6.1, as well as analogues and mimetics thereof. The active MC6, MC 6.1, MC6.2, and MC6.3 exhibit hypoglycemic activity, even following oral administration. Also provided are methods of using the active agents to treat hyperglycemic disorders, particularly diabetes, where the active agents are preferably orally administered.
Owner:CALYX THERAPEUTICS
Hydrogel sheets and shapes for oral care
InactiveUS20070122362A1Enhanced inhibitory effectReduce releaseCosmetic preparationsToilet preparationsCross-linkBeam energy
A hydrogel for use in oral care. The hydrogel is ion beam cross-linked, the hydrogel is adapted to be disposed in the oral cavity and may be adapted to provide a denture fixative or may be loaded with a whitening agent for use in whitening one or more teeth and disposed on or adjacent one or more teeth and the whitening agent is slow-released to whiten the one or more teeth. Other loading materials may include materials for treatment of alveolitis or malodor, inter alia. The present invention is a new hydrophilic oral and dental cohesive hydrogel sheet designed to securely grip and cushion prosthetic devices in the human mouth with the further ability to slow release antimicrobial or other orally desirable agents. Additionally, the invention also provides a method of making an orally cohesive device that: 1) is easily adapted and applied to a removable dental prosthesis; 2) bonds well to alveolar ridge / palatal mucosa and denture acrylic materials; and 3) releases cleanly, with no tacky or thixotropic residue when the prosthesis is removed. The cohesive hydrogel gel device is a hydrogel-forming polymer mixed with water, optionally surrounding an internal scrim, and uses an electron-beam energy source to cause cross-linking. The method does not need any chemical additive to affect the cross-linking. Furthermore the beam energy can be adjusted to optimize the cohesive properties of either side of the device, as well as to compensate for addition of orally active agents, if any are chosen. The hydrogel sheets are pre-cut to fit most sizes of maxillary and mandibular full denture prostheses, but can be easily trimmed with a scissors by the end user for the ideal custom fit of any full or partial denture, in either arch.
Owner:GINIGER MARTIN S +1
Indazole compounds and methods of use thereof
This invention is directed to Indazole Compounds or pharmaceutically acceptable salts, solvates and hydrates thereof. The Indazole Compounds have utility in the treatment or prevention of a wide range of diseases and disorders that are responsive to the inhibition, modulation or regulation of kinases, such as inflammatory diseases, abnormal angiogenesis and diseases related thereto, cancer, atherosclerosis, a cardiovascular disease, a renal disease, an autoimmune condition, macular degeneration, disease-related wasting, an asbestos-related condition, pulmonary hypertension, diabetes, obesity, pain and others. Thus, methods of treating or preventing such diseases and disorders are also disclosed, as are pharmaceutical compositions comprising one or more of the Indazole Compounds. This invention is based, in part, upon the discovery of a novel class of 5-triazolyl substituted indazole molecules that have potent activity with respect to the modulation of protein kinases. Thus, the invention encompasses orally active molecules as well as parenterally active molecules which can be used at lower doses or serum concentrations for treating diseases or disorders associated with protein kinase signal transduction.
Owner:BHAGWAT SHRIPAD S +10
Methods for oral administration of active drugs
InactiveUS20090155363A1Oral administration is convenientBiocidePowder deliveryCo administrationOral medication
The present invention relates to methods that facilitate the oral administration of active drugs to a patient. Specifically, the methods of the present invention may utilize compositions comprising an active drug and a gelling agent that provides an easily consumable gel dosage form and the active drug is homogenously mixed within the gel.
Owner:MAIBACH TODD
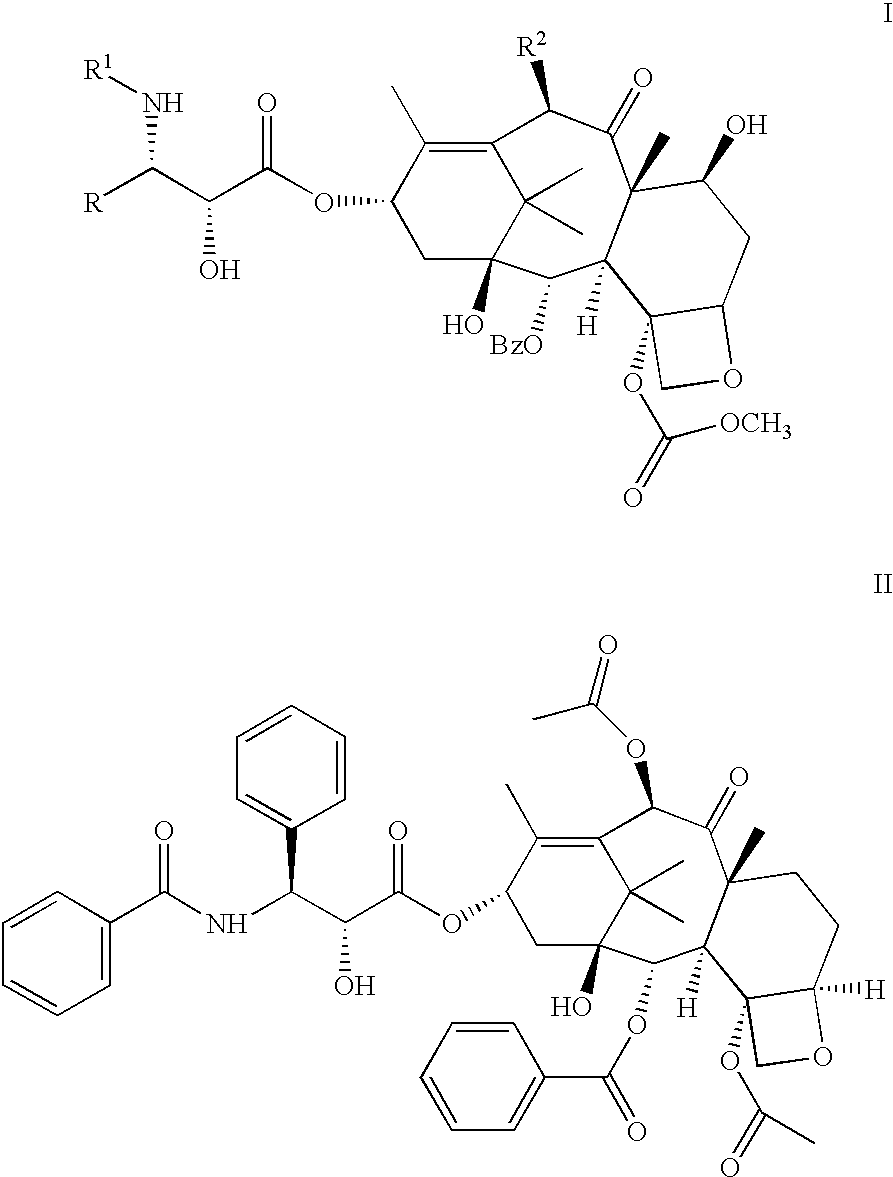
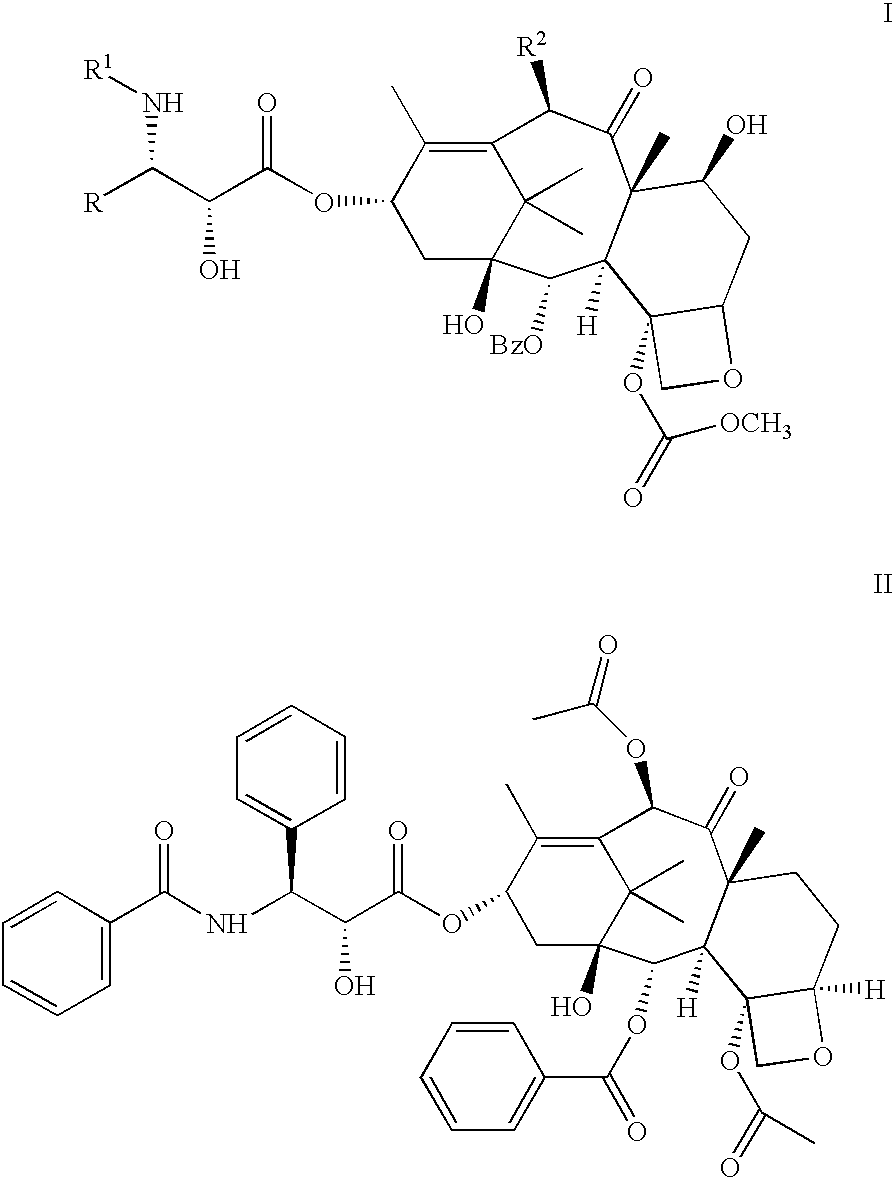
Pharmaceutical compositions of orally active taxane derivatives having enhanced bioavailability
Owner:BRISTOL MYERS SQUIBB CO
Chewing gum compositions
InactiveUS6926916B1Shorten the timeHigh smooth teeth feelCosmetic preparationsToilet preparationsParticulatesPolymeric surface
The present invention relates to chewing gum composition comprising a polymeric surface active agent. A second aspect of this invention relates to a crunchy chewing gum wherein the crunchy texture is provided by particulate polyphosphate particles within the formulation and which lasts through the initial minutes of mastication. The chewing gum composition may also contain a cationic material and or an orally active metallic ion. The chewing gum composition will provide surface conditioning effects on a subject's teeth and or oral mucosa and the crunchy texture is used as a sensate to reinforce these effects. The surface conditioning effects can be measured through in vitro or in vivo testing. The in vitro testing shows a total surface energy and or a lewis base score to increase immediately after treatment with the chewing gum and then decrease over time. The in vivo testing shows a water contact angle of the oral mucosa to decrease after treatment with the chewing gum composition and / or a significantly higher smooth teeth feel relative to other chewing gum compositions. The present invention also relates to methods of providing surface conditioning effects to a subject comprising administering to the subject a chewing gum comprising a polymeric surface active agent. The present invention also relates to methods of reducing astringency of a chewing gum containing an orally active metallic ion without significantly reducing the efficacy of the metallic ion.
Owner:THE PROCTER & GAMBLE COMPANY
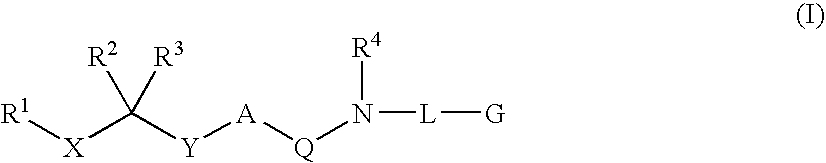
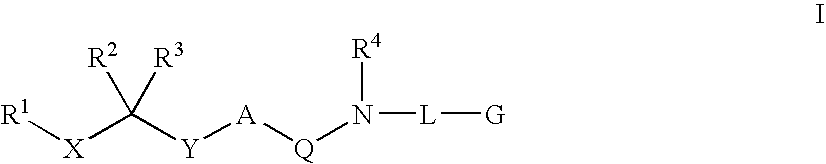
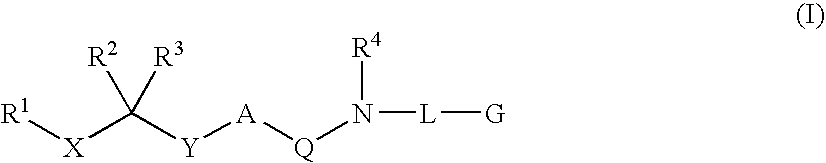
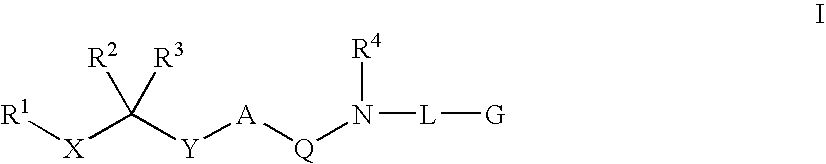
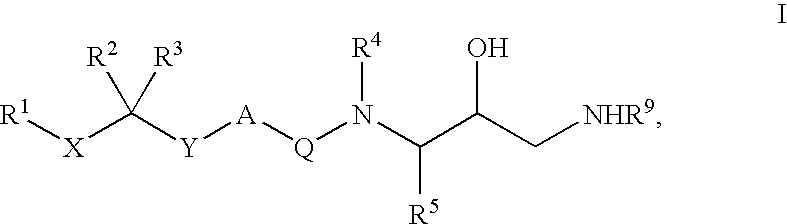
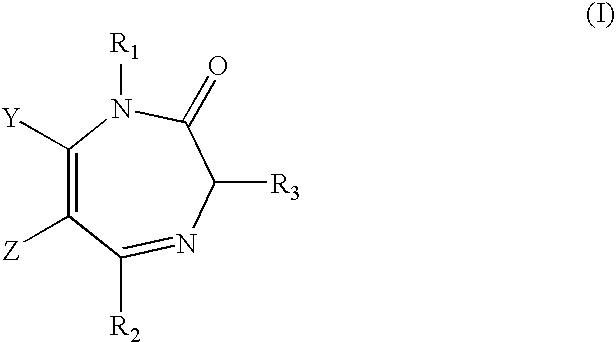
Diaminoalkane Aspartic Protease Inhibitors
Diaminoalkanes of Formula I have now been found which are orally active and bind to aspartic proteases to inhibit their activity. They are useful in the treatment or amelioration of diseases associated with elevated levels of aspartic protease activity. The invention also relates to a method for the use of the compounds of Formula I in ameliorating or treating aspartic protease related disorders in a subject in need thereof comprising administering to said subject an effective amount of a compound of Formula I.
Owner:VITAE PHARMA INC
Heterocyclic receptor agonists for the treatment of diabetes and metabolic disorders
Compounds and methods are provided for the treatment of, inter alia, Type II diabetes and other diseases associated with poor glycemic control. The compounds of the invention are orally active.
Owner:CYMABAY THERAPEUTICS
Method of preventing abuse of opioid dosage forms
InactiveUS20020004509A1Reducing parenteral abuse potential of dosage formBiocideNervous disorderOpioid antagonistOpioid Agonist
The invention relates in part to a method of reducing the abuse potential of an oral dosage form of an opioid analgesic, wherein an analgesically effective amount of an orally active opioid agonist is combined with an opioid antagonist into an oral dosage form which would require at least a two-step extraction process to be separated from the opioid agonist, the amount of opioid antagonist including being sufficient to counteract opioid effects if extracted together with the opioid agonist and administered parenterally.
Owner:EURO-CELTIQUE SA
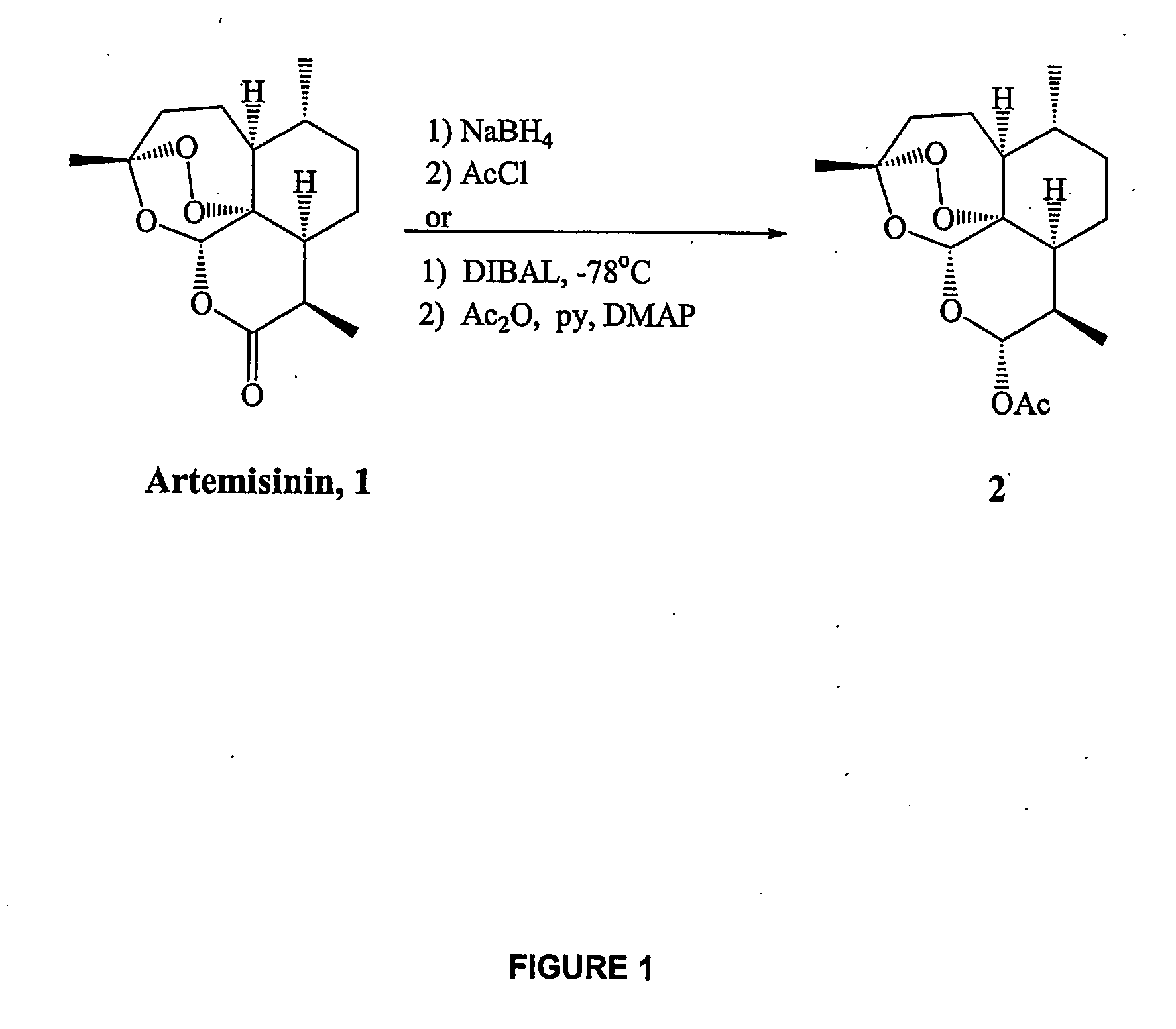
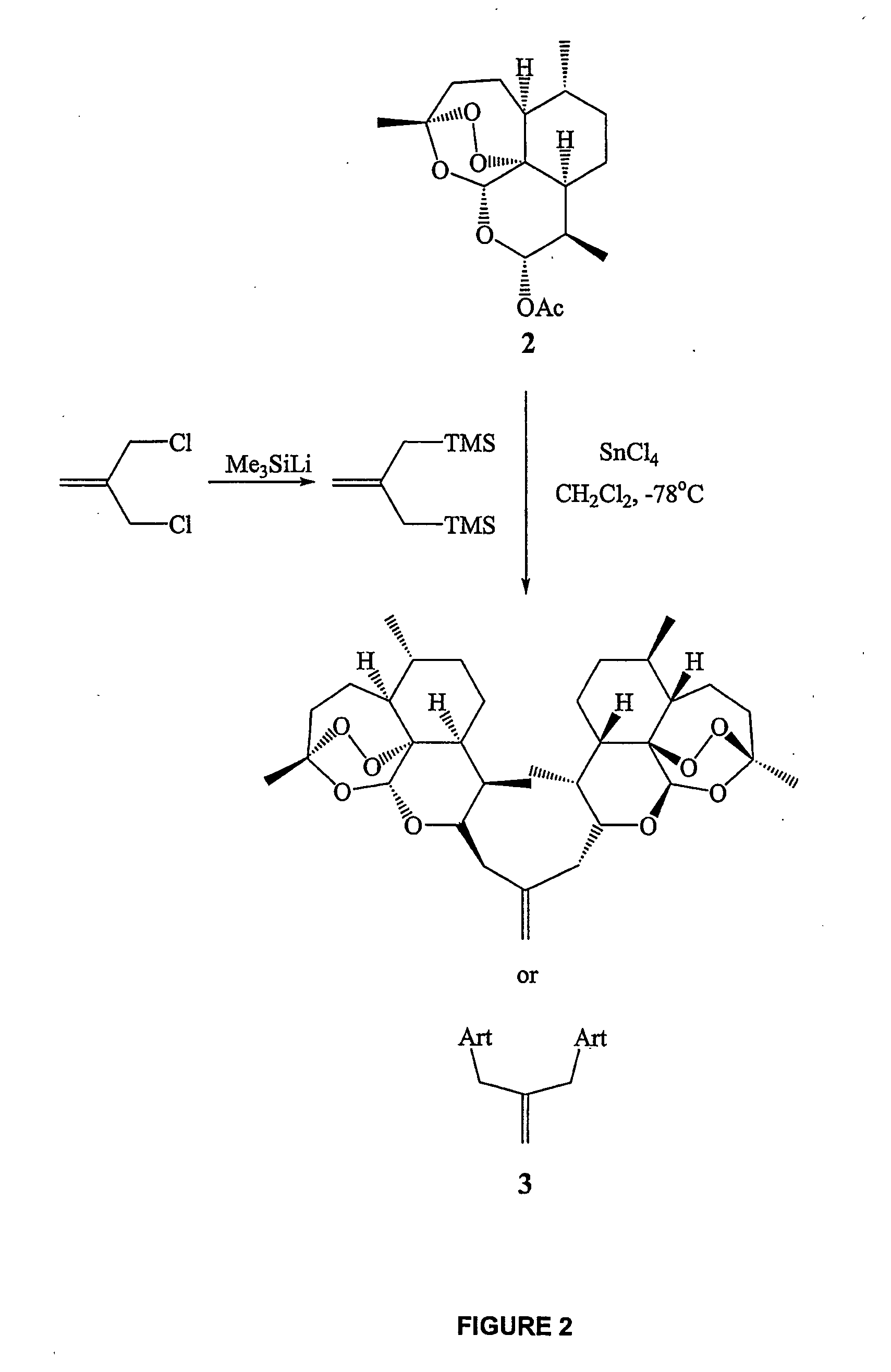
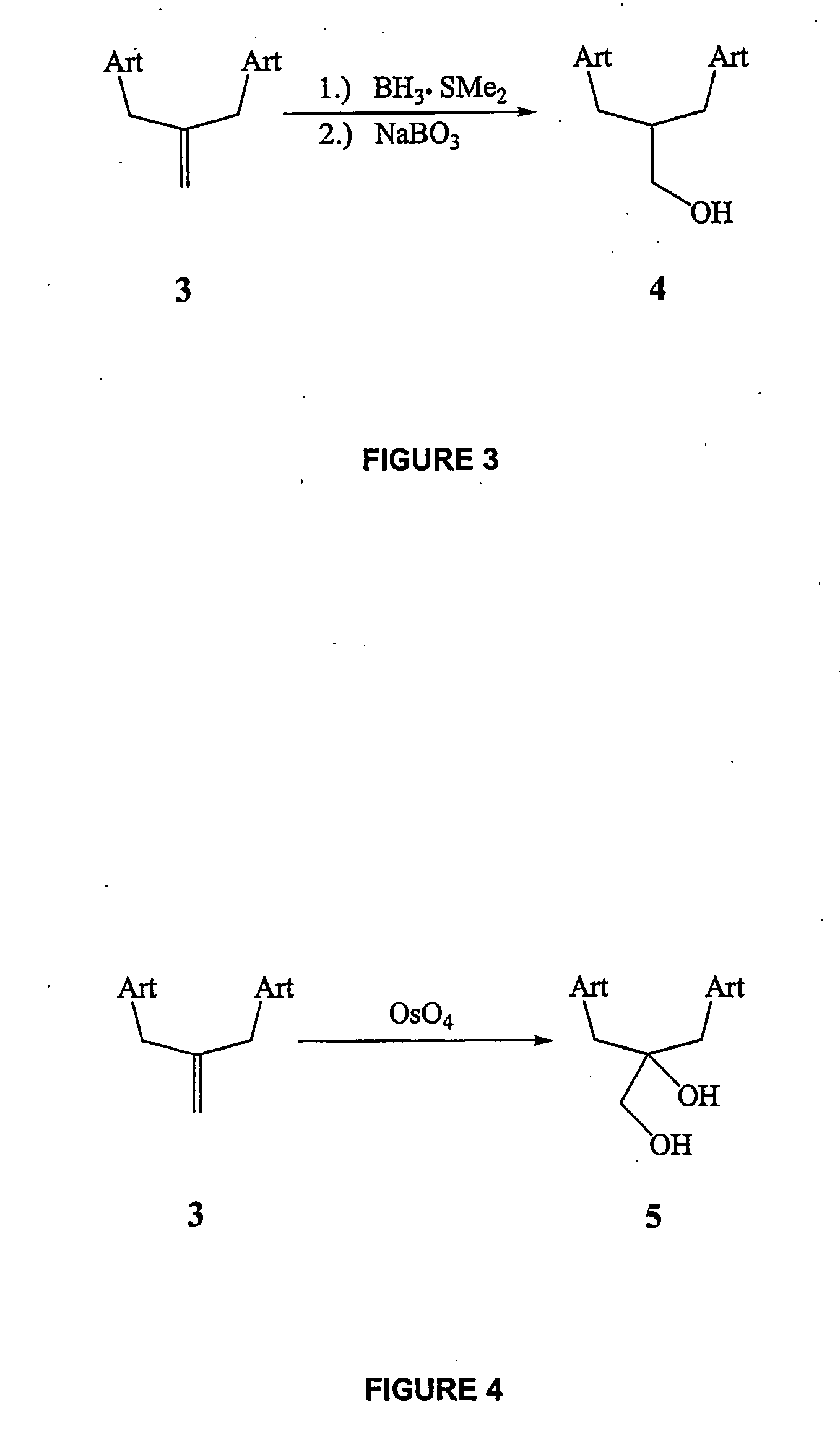
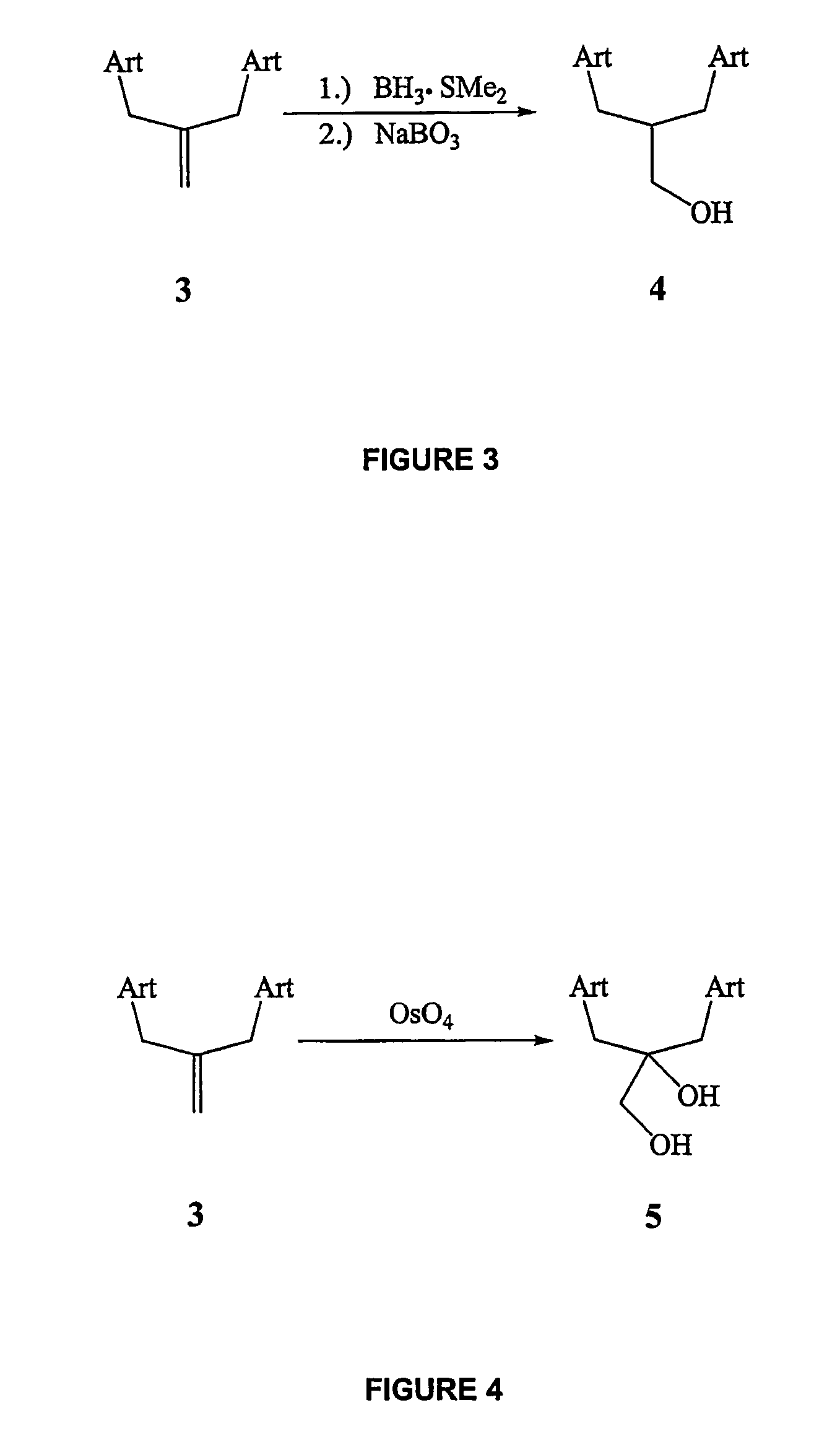
Orally active, antimalarial, anticancer, artemisinin-derived trioxane dimers with high selectively, stability and efficacy and methods of making the same
ActiveUS20060142377A1Easy to transformPotent and selective anticancer activityOrganic active ingredientsBiocideO-Phosphoric AcidCancer cell
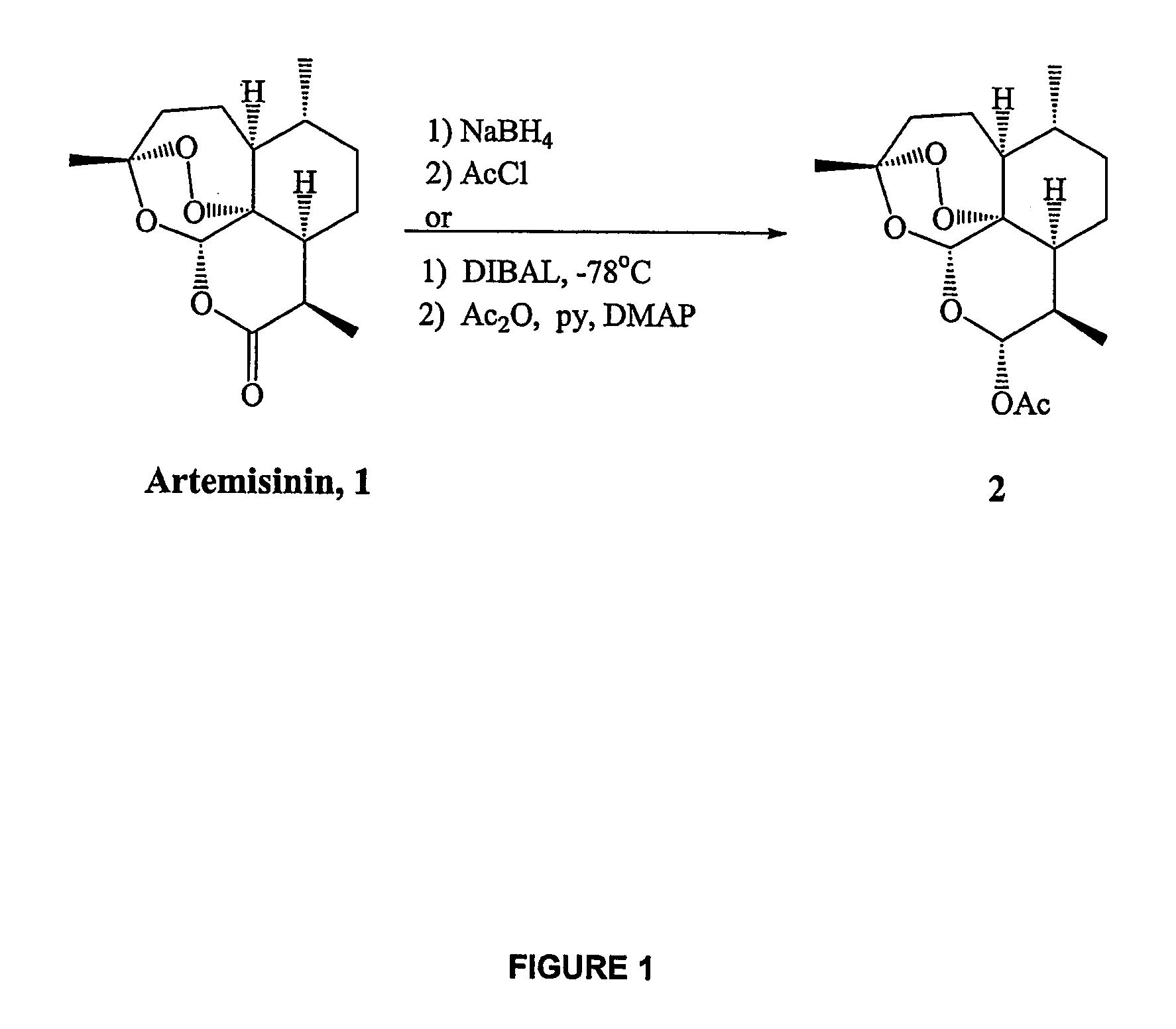
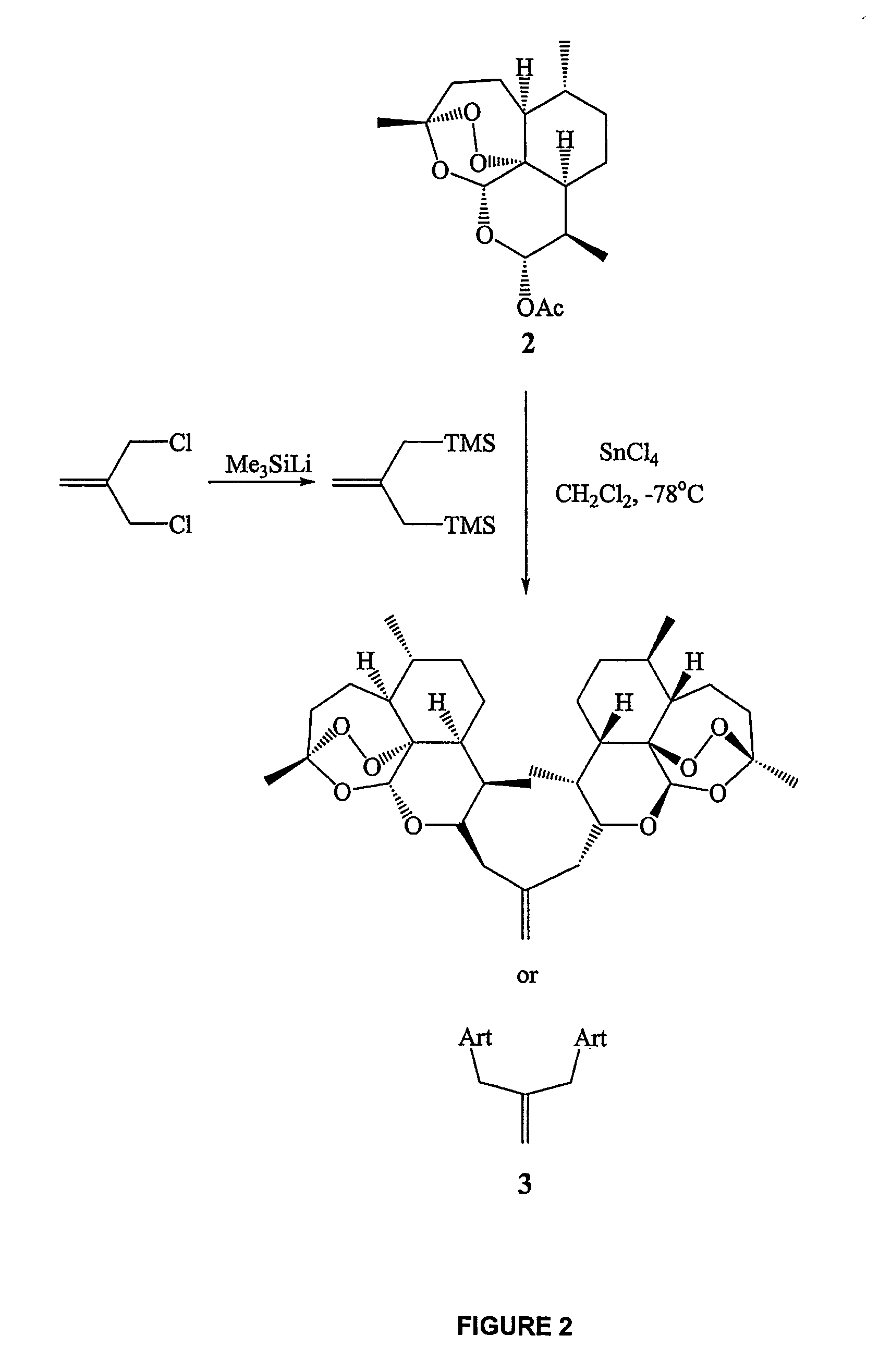
In only two steps and in 65% overall yield, natural trioxane artemisinin (I) was converted on gram scale into C-10-carba trioxane dimer (3). This new, very stable dimer was then transformed easily in one additional step into four different dimers (4-7). Alcohol and diol dimers (4 and 5) and ketone dimer (7) are 10 times more antimalarially potent in vitro than artemisinin (1), and alcohol and diol dimers (4 and 5) are strongly inhibitory but not cytotoxic toward several human cancer cell lines. Water-soluble carboxylic acid derivatives (8a-10c and 12) were easily prepared from dimers (4-6); they are thermally stable even at 60° C. for 24 hours, are more orally efficacious as antimalarials than either artelinic acid or sodium artesunate, and have potent and selective anticancer activities. Further derivitization of the alcohol dimers (4 and 17), diol dimer (5) and ketone (7) has produced a number of analogs also antimalarially active in vitro at sub-nanomolar concentrations (most notably: pyridine N-oxides (13, 15, 18, 23, 24 and 25), phosphoric acid triesters (26 and 27), sulfonamide (40) and cyclic carbonate (41)). In addition, dimers (13 and 19) are more efficacious (when administered both orally and i.v.) and less toxic (when administered intraperitoneally to mice as a single dose) than clinically-used sodium artesunate, thereby giving them a better antimalarial therapeutic index than sodium artesunate.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
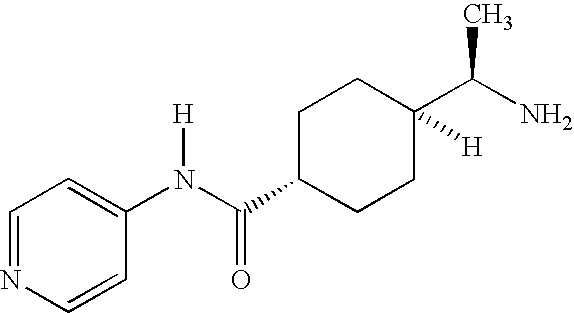
Renin inhibitors
Disclosed are aspartic protease inhibitors represented by the following structural formula: and pharmaceutically acceptable salts thereof. These compounds are orally active and bind to aspartic proteases to inhibit their activity. They are useful in the treatment or amelioration of diseases associated with aspartic protease activity. The present invention is also directed to pharmaceutical compositions comprising a compound described herein or enantiomers, diastereomers, or salts thereof and a pharmaceutically acceptable carrier or excipient.
Owner:VITAE PHARMA INC
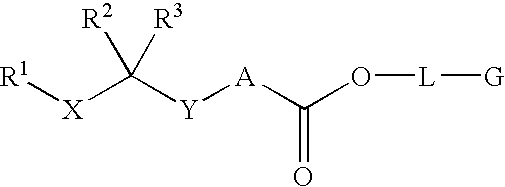
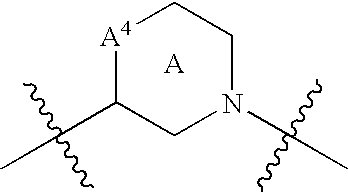
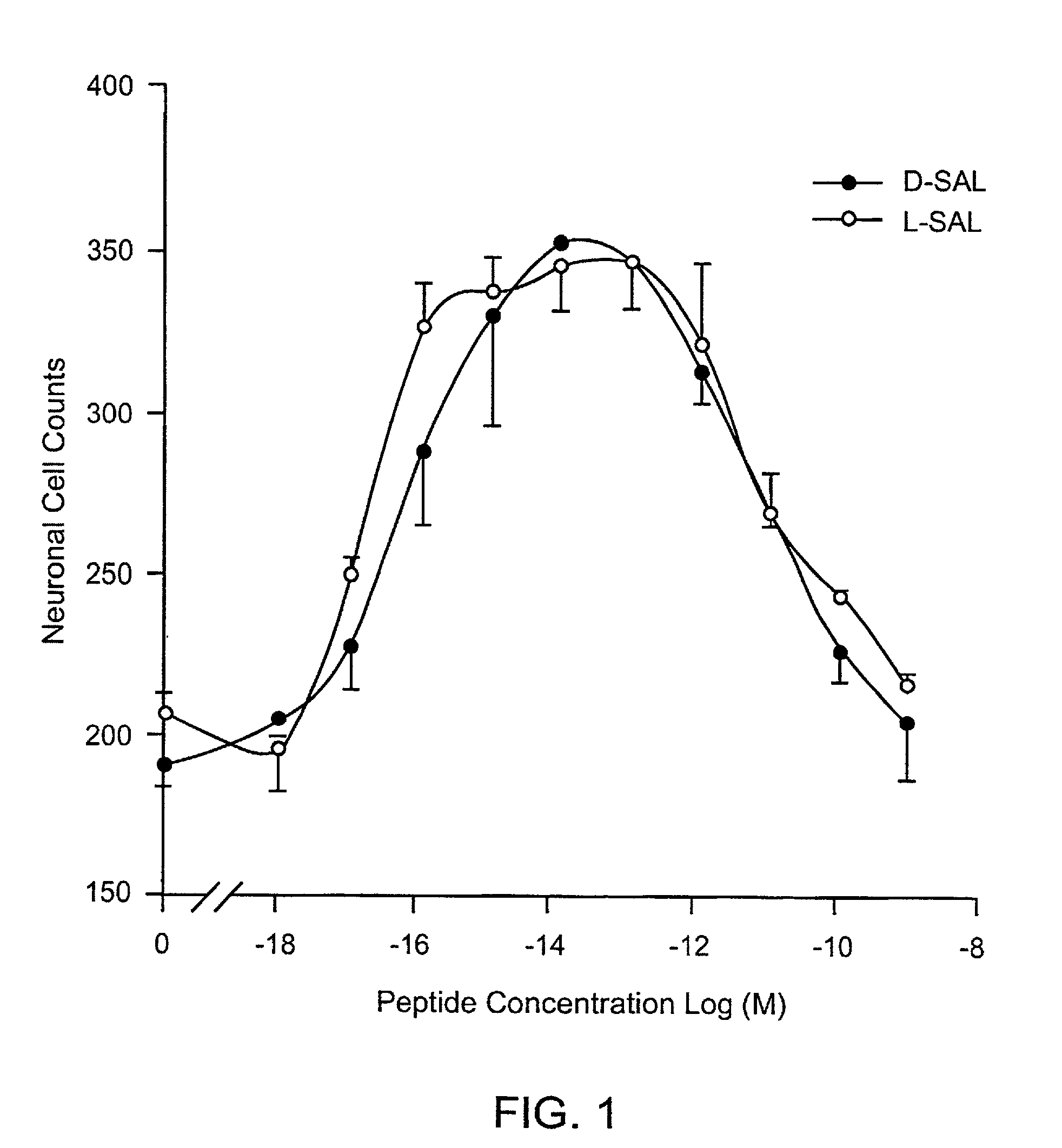
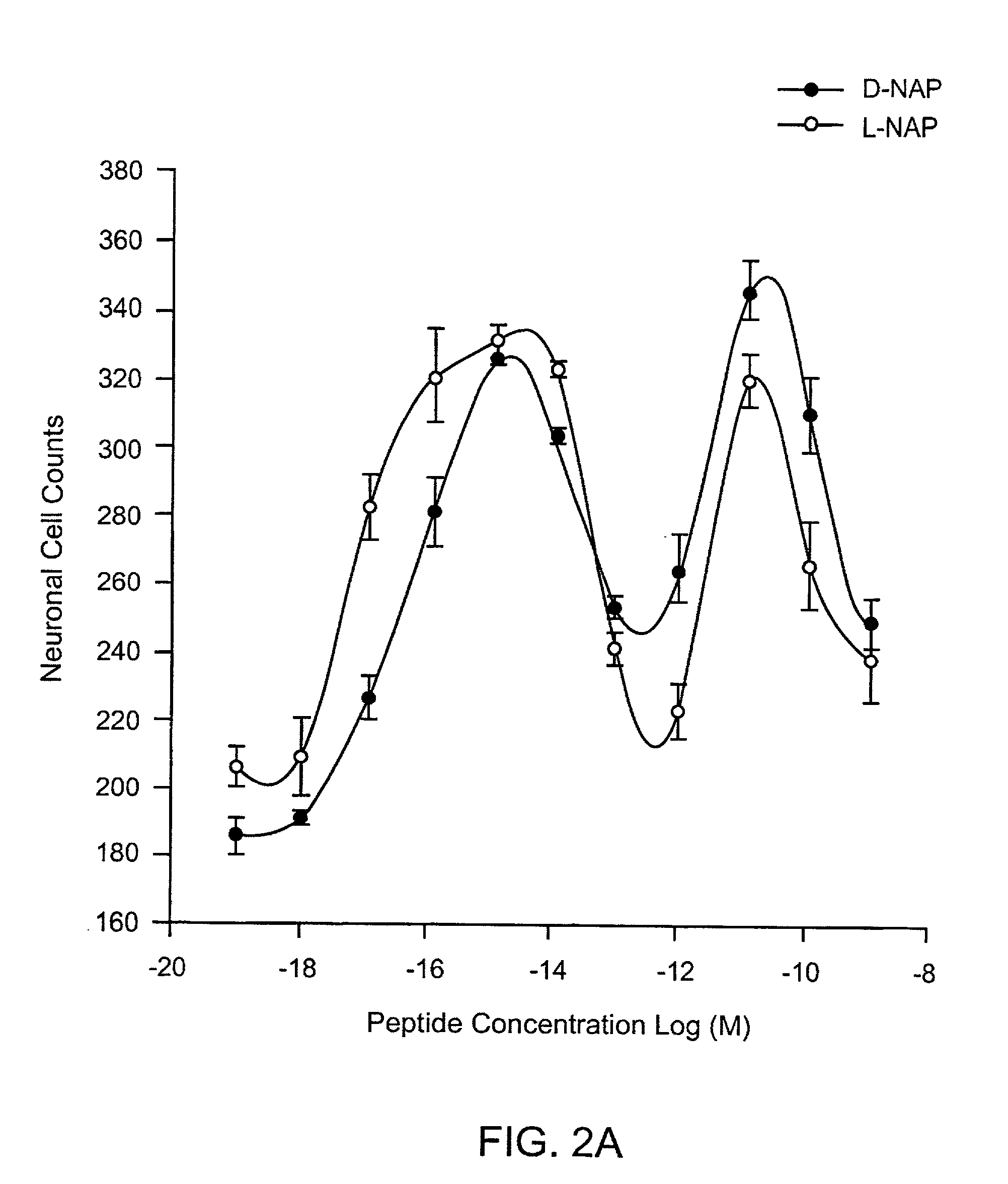
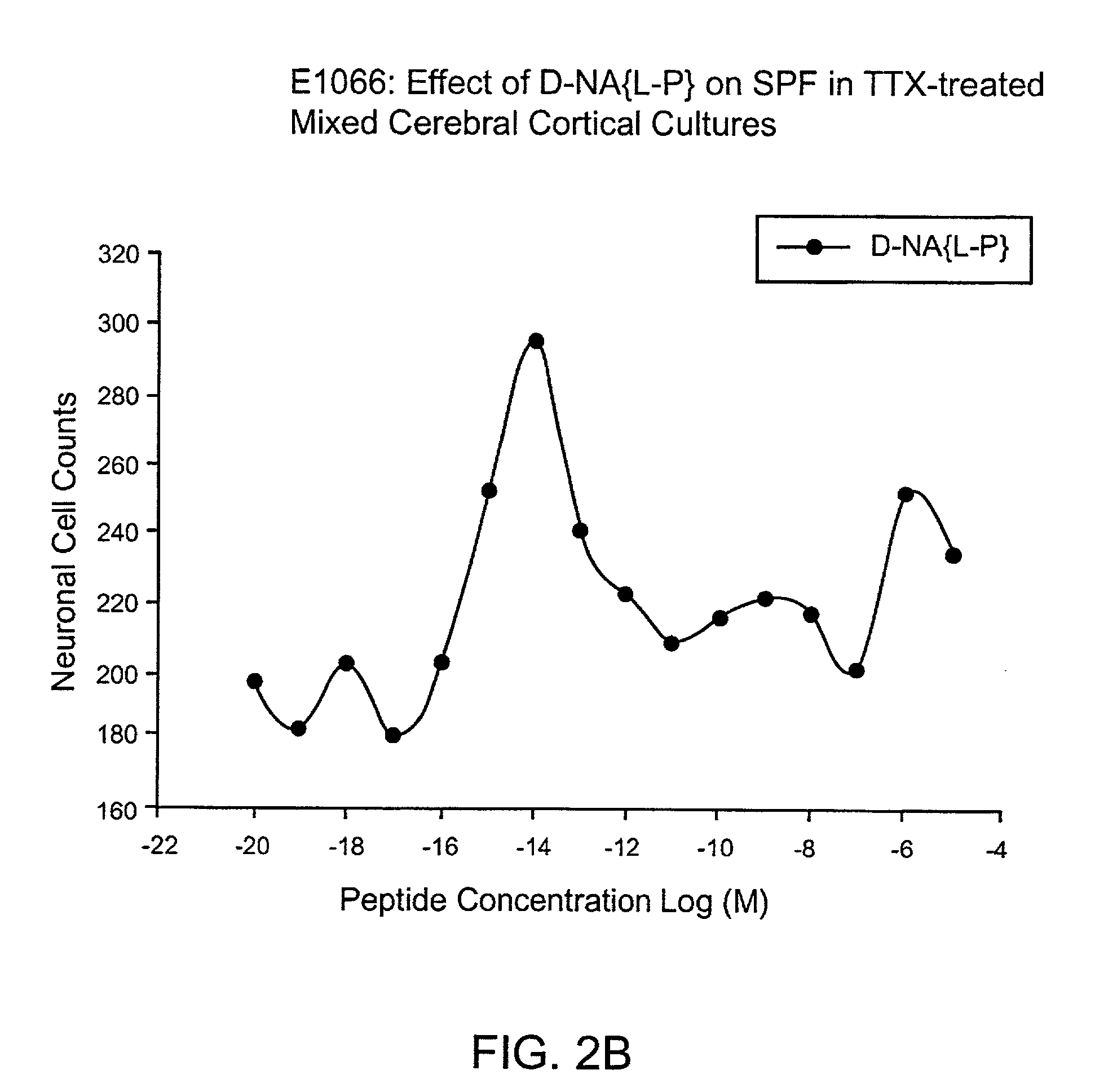
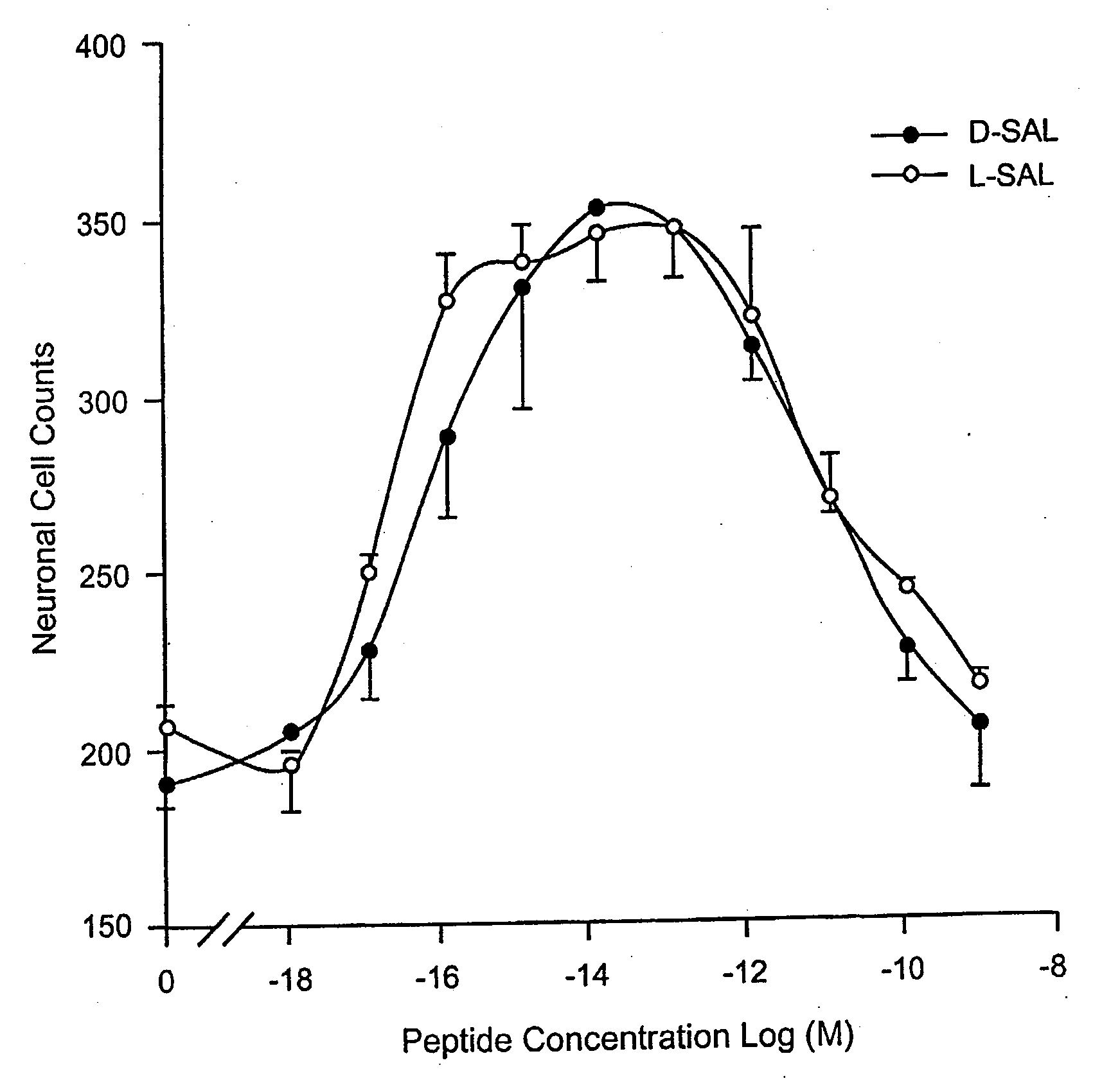
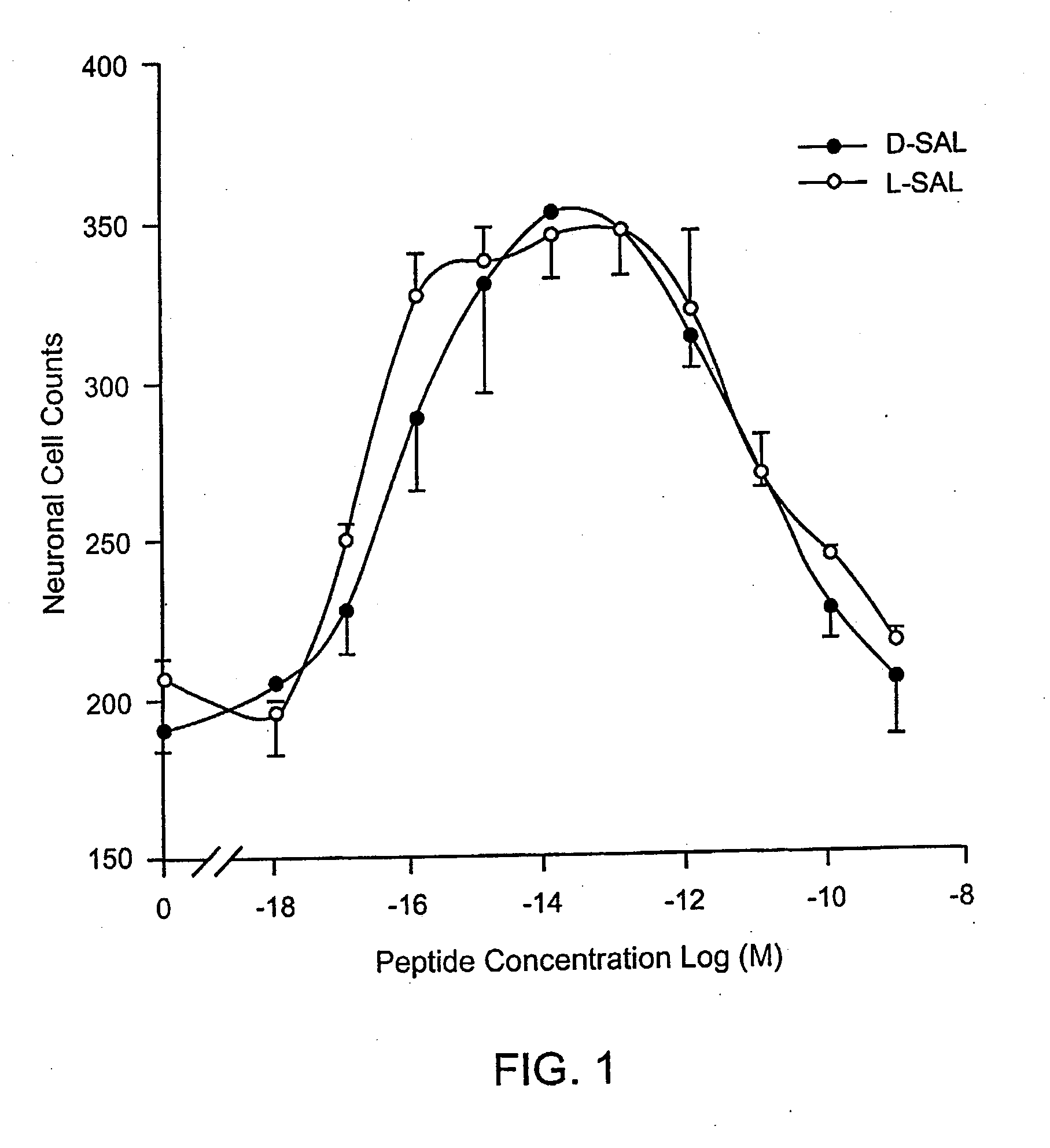
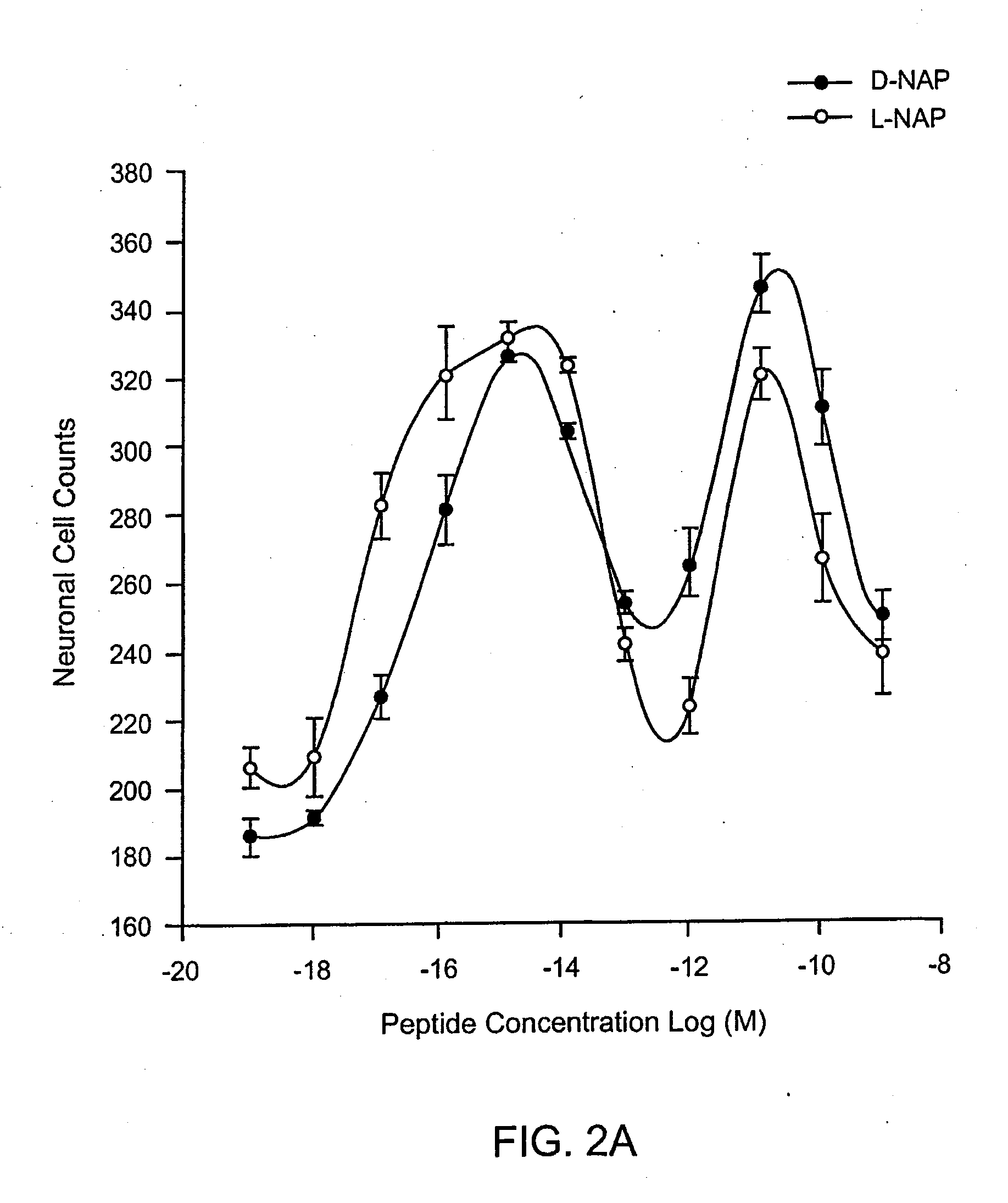
Orally active peptides that prevent cell damage and death
This invention provides an ADNF polypeptide comprising an active core site, the active core site comprising at least one D-amino acid. The invention also provides a pharmaceutical composition comprising an ADNF polypeptide comprising an active core site, the active core site comprising at least one D-amino acid. In particular, the pharmaceutical composition of the invention is orally active. The invention further provides methods for reducing neuronal cell death, methods for reducing oxidative stress, and methods for reducing a condition associated with fetal alcohol syndrome using the ADNF polypeptides and the pharmaceutical compositions of the invention.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
Diaminoalkane aspartic protease inhibitors
Diaminoalkanes of Formula I have now been found which are orally active and bind to aspartic proteases to inhibit their activity. They are useful in the treatment or amelioration of diseases associated with elevated levels of aspartic protease activity. The invention also relates to a method for the use of the compounds of Formula I in ameliorating or treating aspartic protease related disorders in a subject in need thereof comprising administering to said subject an effective amount of a compound of Formula I.
Owner:VITAE PHARMA INC
Orally active peptides that prevent cell damage and death
This invention provides an ADNF polypeptide comprising an active core site, the active core site comprising at least one D-amino acid. The invention also provides a pharmaceutical composition comprising an ADNF polypeptide comprising an active core site, the active core site comprising at least one D-amino acid. In particular, the pharmaceutical composition of the invention is orally active. The invention further provides methods for reducing neuronal cell death, methods for reducing oxidative stress, and methods for reducing a condition associated with fetal alcohol syndrome using the ADNF polypeptides and the pharmaceutical compositions of the invention.
Owner:RAMOT AT TEL AVIV UNIV LTD +1
Diaminopropanol Renin Inhibitors
Described are diaminopropanols of which are orally active and bind to renin to inhibit its activity. They are useful in the treatment or amelioration of diseases associated with elevated levels of renin activity or in the treatment of aspartic protease mediated disorders. Also described is a method for the use of the diaminopropanols in ameliorating or treating renin related disorders in a subject in need thereof.
Owner:VITAE PHARMA INC
Diaminopropanol renin inhibitors
Described are diaminopropanols of which are orally active and bind to renin to inhibit its activity. They are useful in the treatment or amelioration of diseases associated with elevated. levels of renin activity or in the treatment of aspartic protease mediated disorders. Also described is a method for the use of the diaminopropanols in ameliorating or treating renin related disorders in a subject in need thereof.
Owner:VITAE PHARMA INC
Opioid agonist /antagonist combinations
InactiveUS20020013301A1Reducing oral abuse potential of dosage formBiocideNervous disorderOpioid AgonistOpioid antagonist
The invention is directed in part to oral dosage forms comprising a combination of an orally analgesically effective amount of an opioid agonist and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Alpha-fetoprotein peptides and uses thereof
ActiveUS7122522B2Antibody mimetics/scaffoldsGeneral/multifunctional contrast agentsCancer preventionTamoxifen therapy
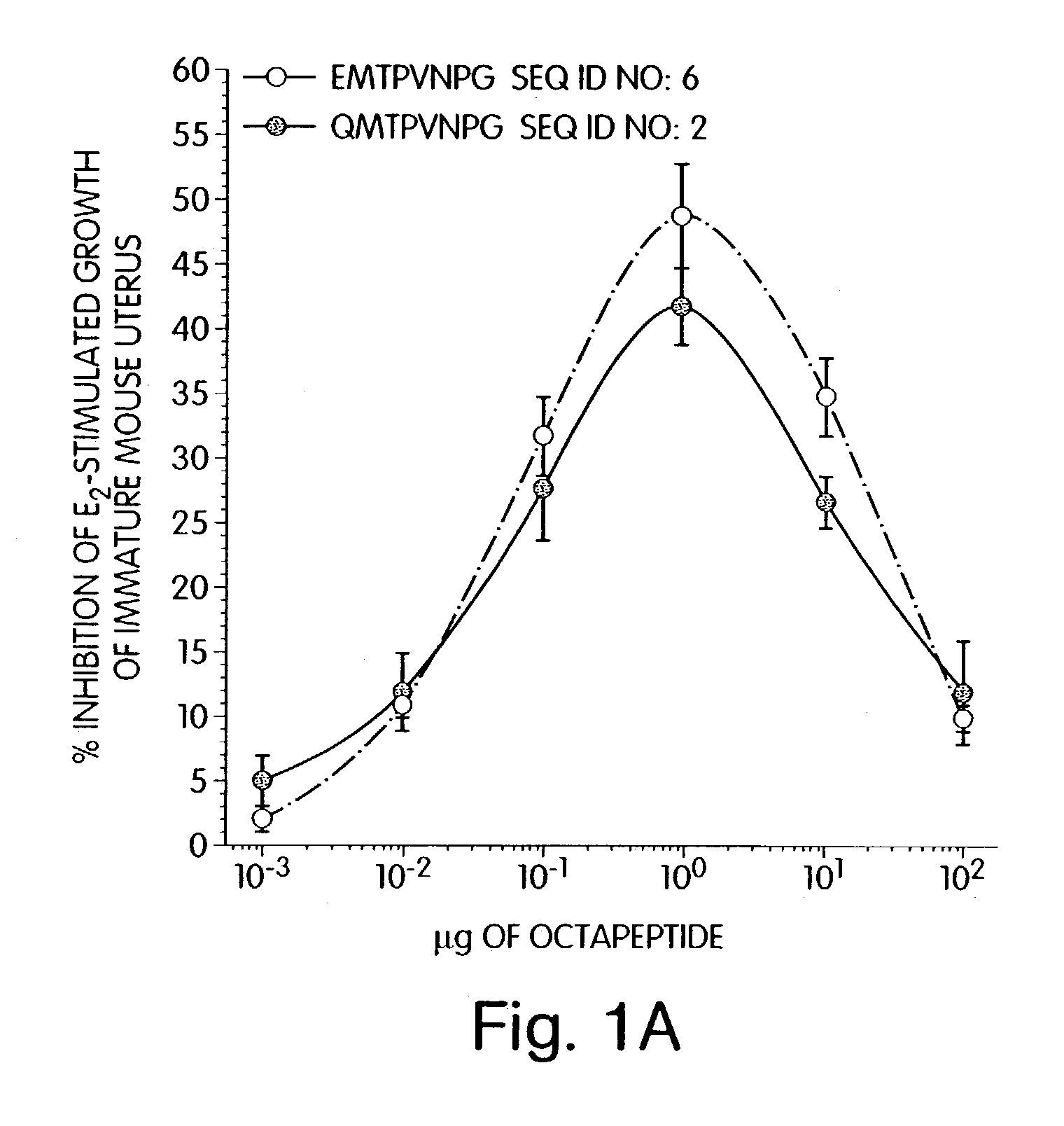
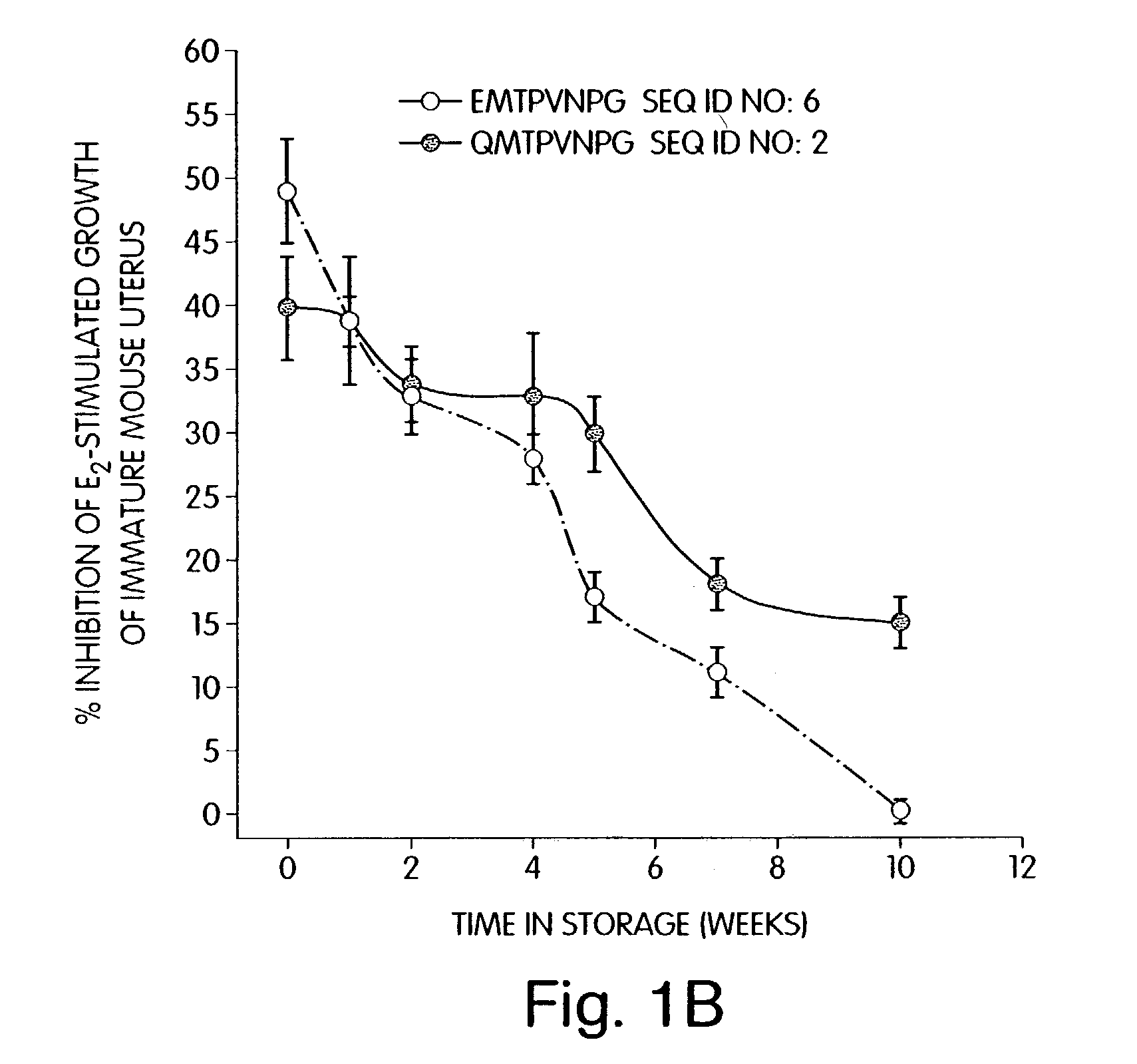
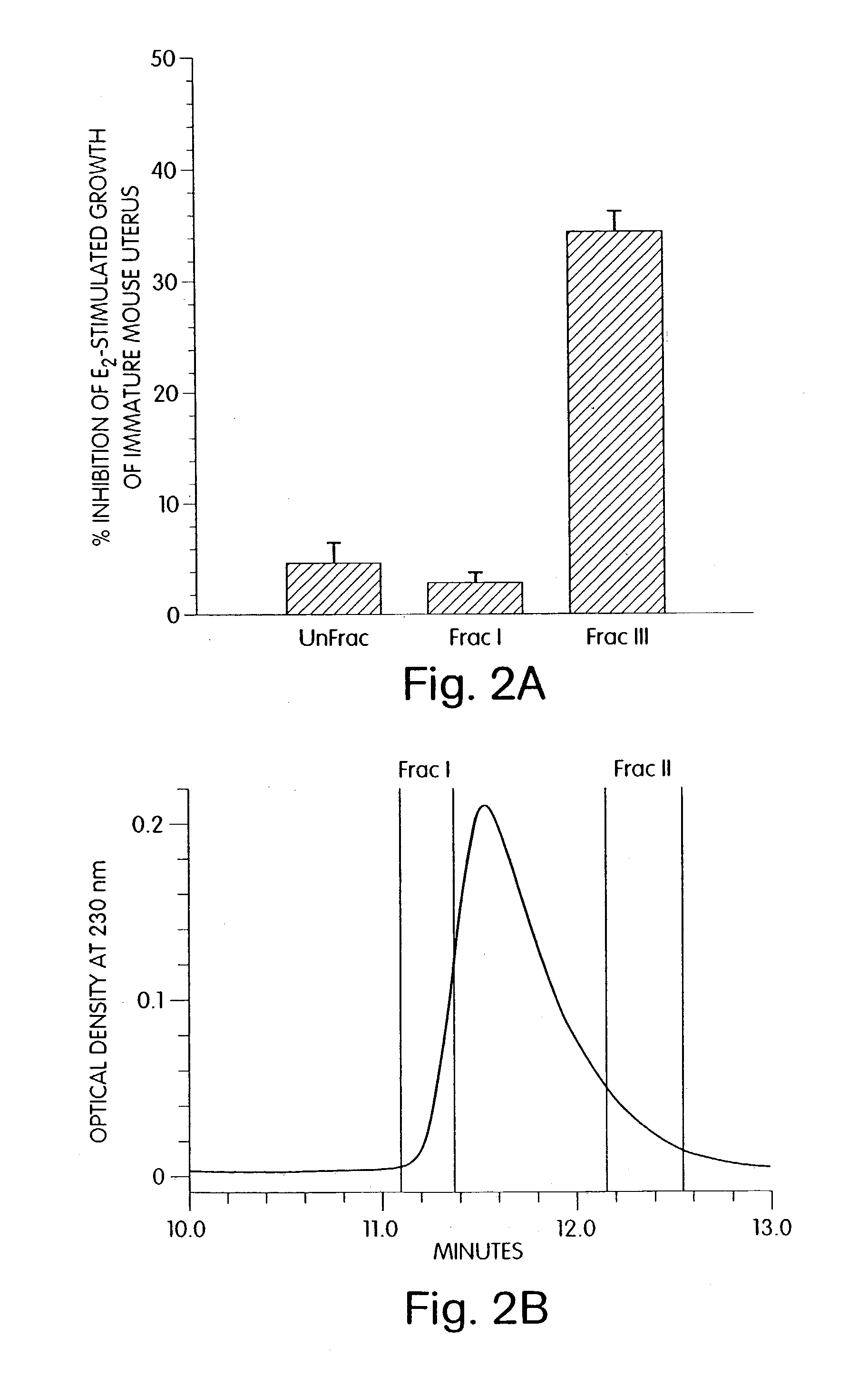
Therapeutic compounds which are cell proliferation modulators, preferably inhibitors. These modulators contain amino acid structures that are arranged as a hydrophilic analog of an alpha-fetoprotein. The modulator may be a peptide itself, e.g., an octapeptide like that of SEQ ID NO: 5; a peptidomimetic; or may be in the form of a pharmaceutically acceptable scaffold, such as a polycyclic hydrocarbon to which is attached the necessary amino acid structures for biological and / or chemical activity. The modulators of the invention are distinguished in one aspect over previous compounds in that they are orally active, and therefore do not have to be injected into the patient. The compositions and methods are useful for reducing estrogen-stimulated growth of cells, and treating or preventing cancer, such as breast cancer. The treatment or prevention methods can include the use of tamoxifen therapy in combination with the peptide therapy.
Owner:ALBANY MEDICAL COLLEGE
Anxiolytic agents with reduced sedative and ataxic effects
ActiveUS7119196B2Less side effectsMedication convenienceBiocideNervous disorderBenzodiazepineMuscle relaxant
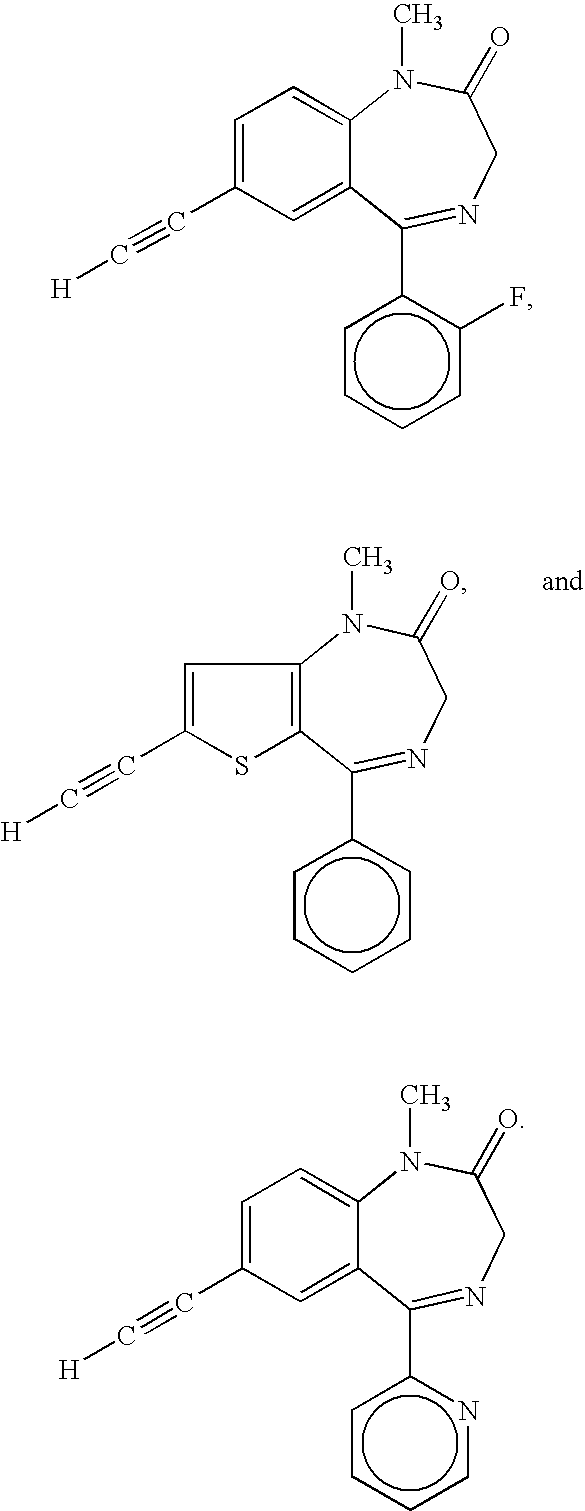
Orally active benzodiazepine derivatives and their salts are disclosed. These compounds and their salts have anxiolytic and anticonvulsant activity with reduced sedative / hypnotic / muscle relaxant / ataxic effects.
Owner:WISYS TECH FOUND
Orally active, antimalarial, anticancer, artemisinin-derived trioxane dimers
InactiveUS7417156B2Easy to transformPotent anticancer activityBiocideAnimal repellantsCancer cellPhosphoric acid
In only two steps and in 65% overall yield, natural trioxane artemisinin (I) was converted on gram scale into C-10-carba trioxane dimer (3). This new, very stable dimer was then transformed easily in one additional step into four different dimers (4-7). Alcohol and diol dimers (4 and 5) and ketone dimer (7) are 10 times more antimalarially potent in vitro than artemisinin (I), and alcohol and diol dimers (4 and 5) are strongly inhibitory but not cytotoxic toward several human cancer cell lines. Water-soluble carboxylic acid derivatives (8a-10c and 12) were easily prepared from dimers (4-6); they are thermally stable even at 60° C. for 24 hours, are more orally efficacious as antimalarials than either artelinic acid or sodium artesunate, and have potent and selective anticancer activities. Further derivitization of the alcohol dimers (4 and 17), diol dimer (5) and ketone (7) has produced a number of analogs also antimalarially active in vitro at sub-nanomolar concentrations (most notably: pyridine N-oxides (13, 15, 18, 23, 24 and 25), phosphoric acid triesters (26 and 27), sulfonamide (40) and cyclic carbonate (41)). In addition, dimers (13 and 19) are more efficacious (when administered both orally and i.v.) and less toxic (when administered intraperitoneally to mice as a single dose) than clinically-used sodium artesunate, thereby giving them a better antimalarial therapeutic index than sodium artesunate.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Formulations for oral administration of active agents
ActiveUS20180021272A1BioavailabilityPeptide/protein ingredientsHydroxy compound active ingredientsCo administrationActive agent
A pharmaceutical composition for oral administration is disclosed herein, comprising a therapeutically active agent, SNAC and at least one antacid compound. Further disclosed herein is a pharmaceutical composition unit dosage form for oral administration of a therapeutically active agent is provided herein, the unit dosage form comprising: a core comprising the therapeutically active agent and SNAC (sodium 8-N-(2-hydroxybenzoyl)aminocaprylate); and an external layer comprising at least one protective agent selected from the group consisting of an antacid compound and a protease inhibitor. Methods and uses utilizing the aforementioned pharmaceutical compositions, as well as methods and uses utilizing co-administration, by oral administration, of at least one antacid composition, and a composition comprising the therapeutically active agent and SNAC, are further disclosed herein, for use in treating a condition treatable by oral administration of the therapeutically active agent.
Owner:ENTERA BIO LTD
Hsp therapy in conjunction with a low antigenicity diet
InactiveUS20070060519A1Improve the protective effectPeptide/protein ingredientsMetabolism disorderHeat shockHSP60
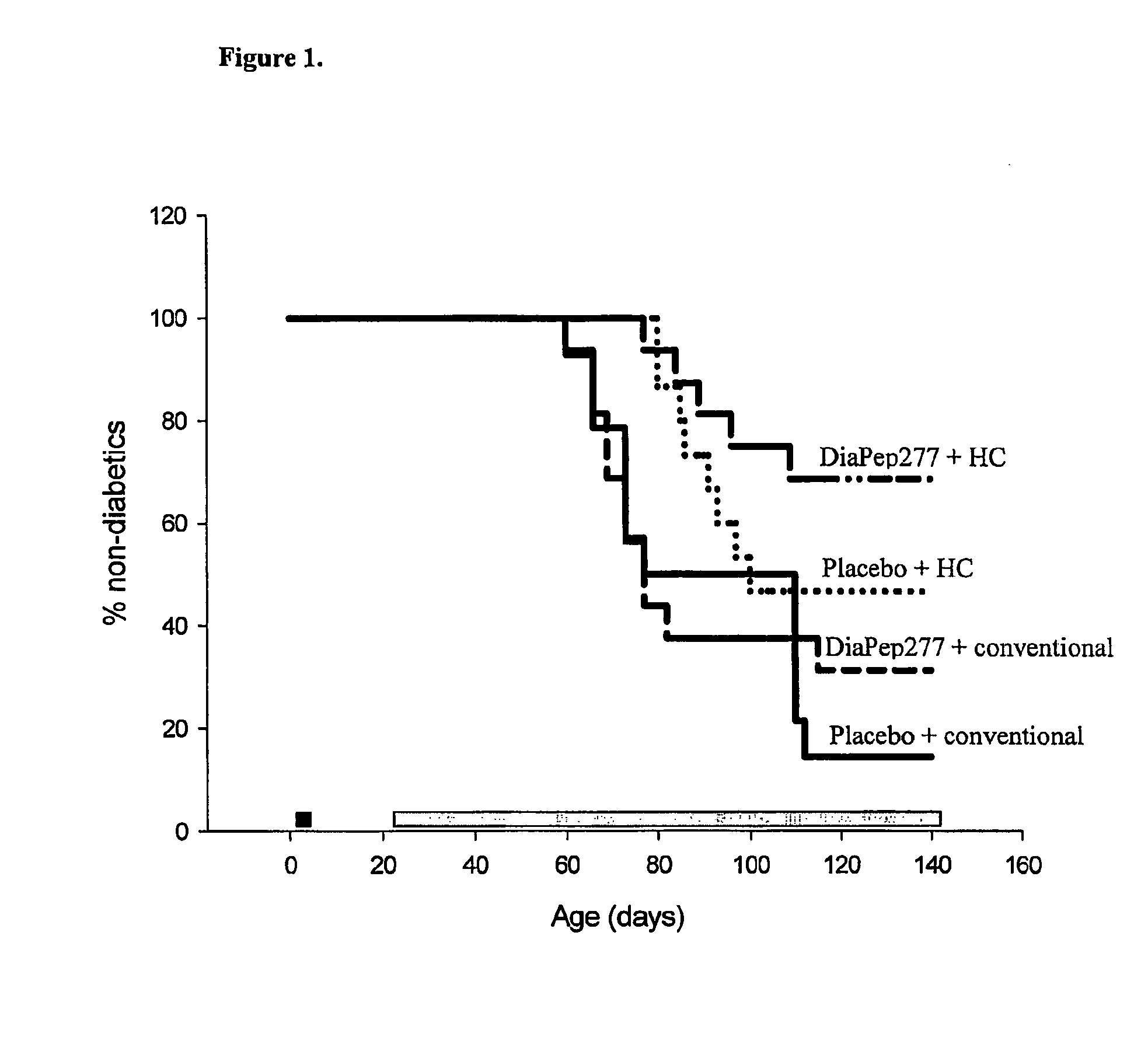
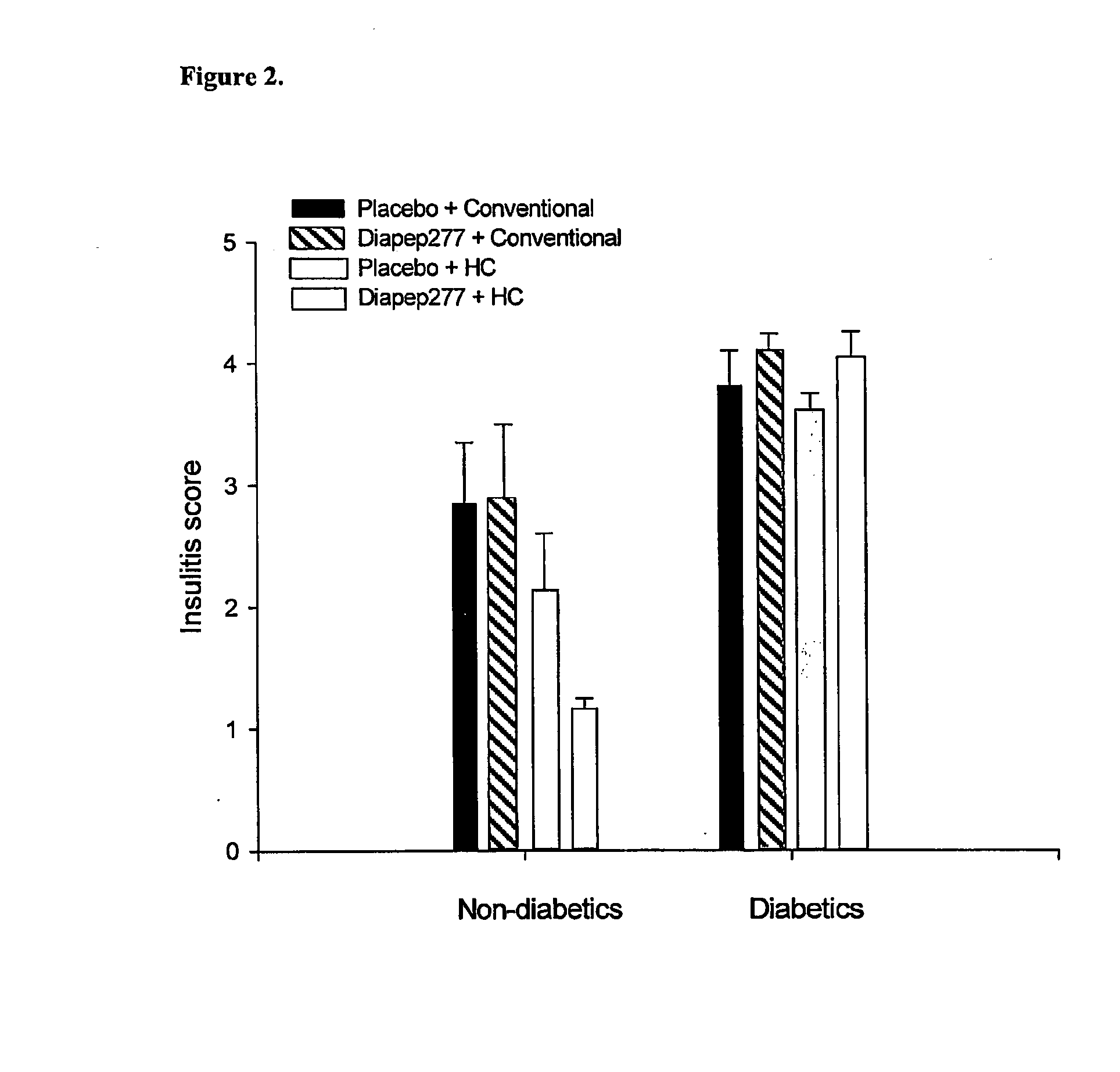
The present invention fragments and analogs of heat shock proteins in combination with a low antigenicity diet for suppression, prevention and treatment of diabetes. The invention is exemplified using DiaPep277™, an analog of residues 437-460 of human Hsp60 in combination with a hydrolyzed casein diet. The invention further relates to orally active pharmaceutical compositions comprising Hsp60 fragments and analogs, such as DiaPep277, useful for suppression, prevention or treatment of diabetes and to a regimen for delaying the onset of type 1 diabetes and for inhibition of insulitis.
Owner:ANDROMEDA BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com