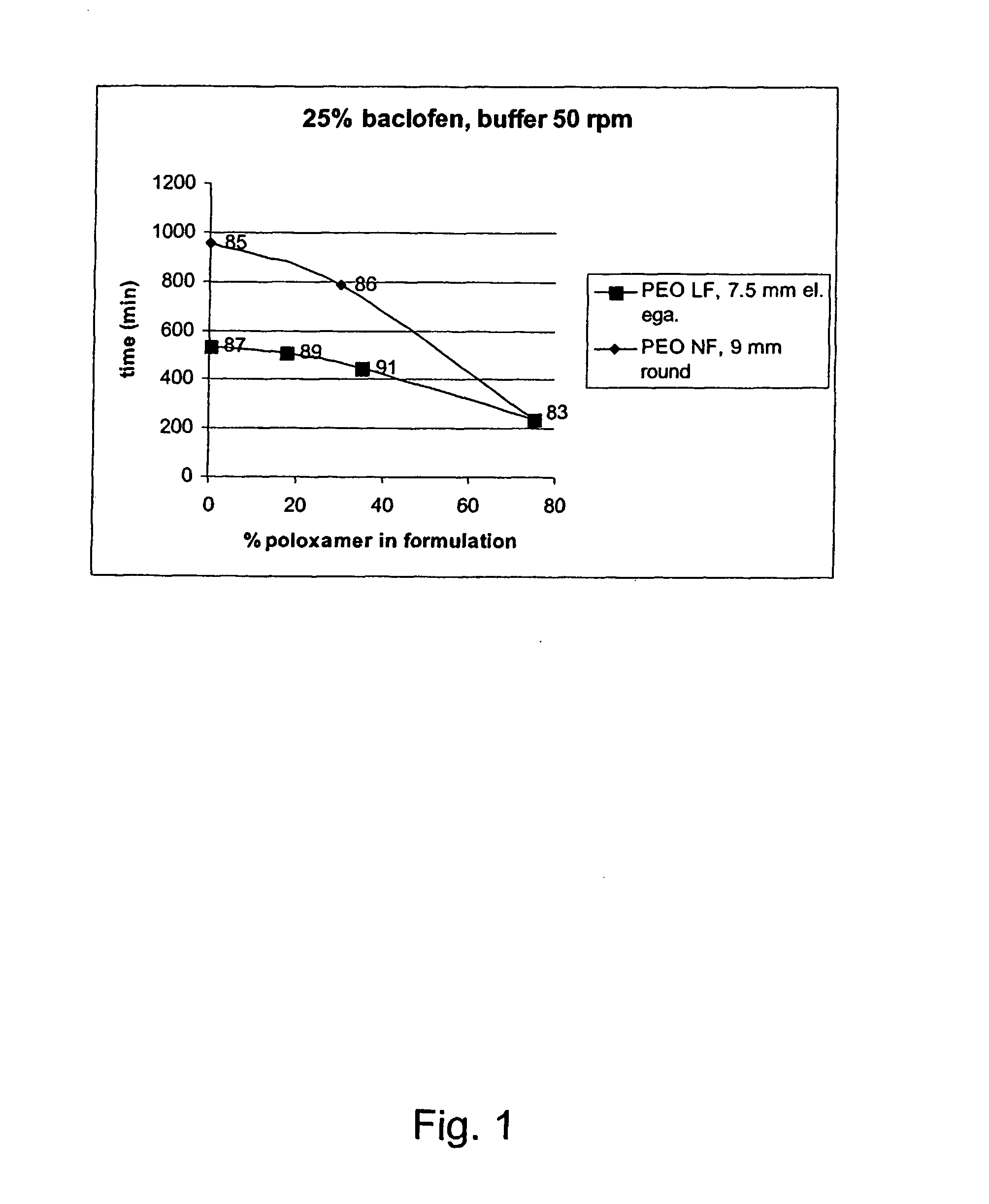
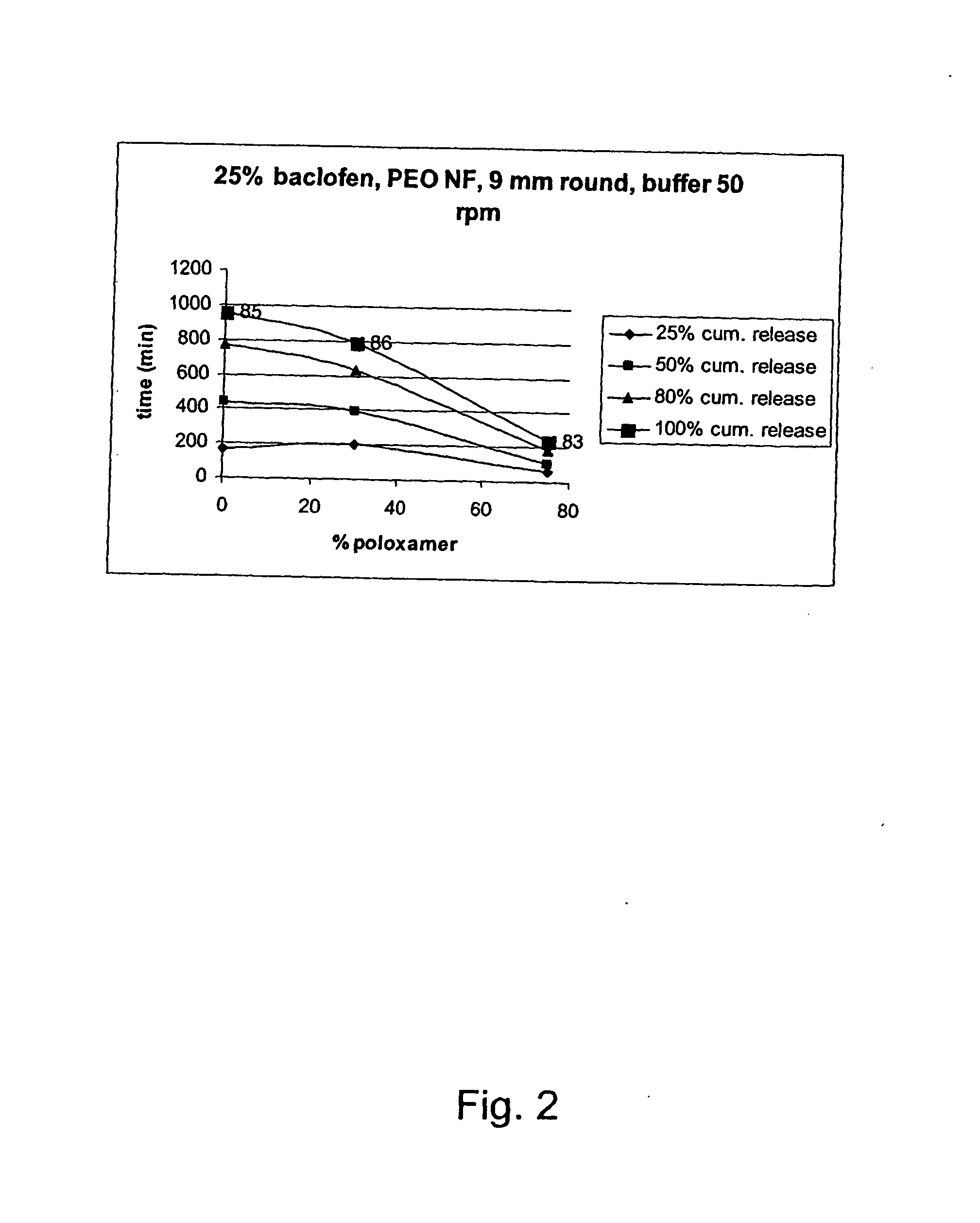
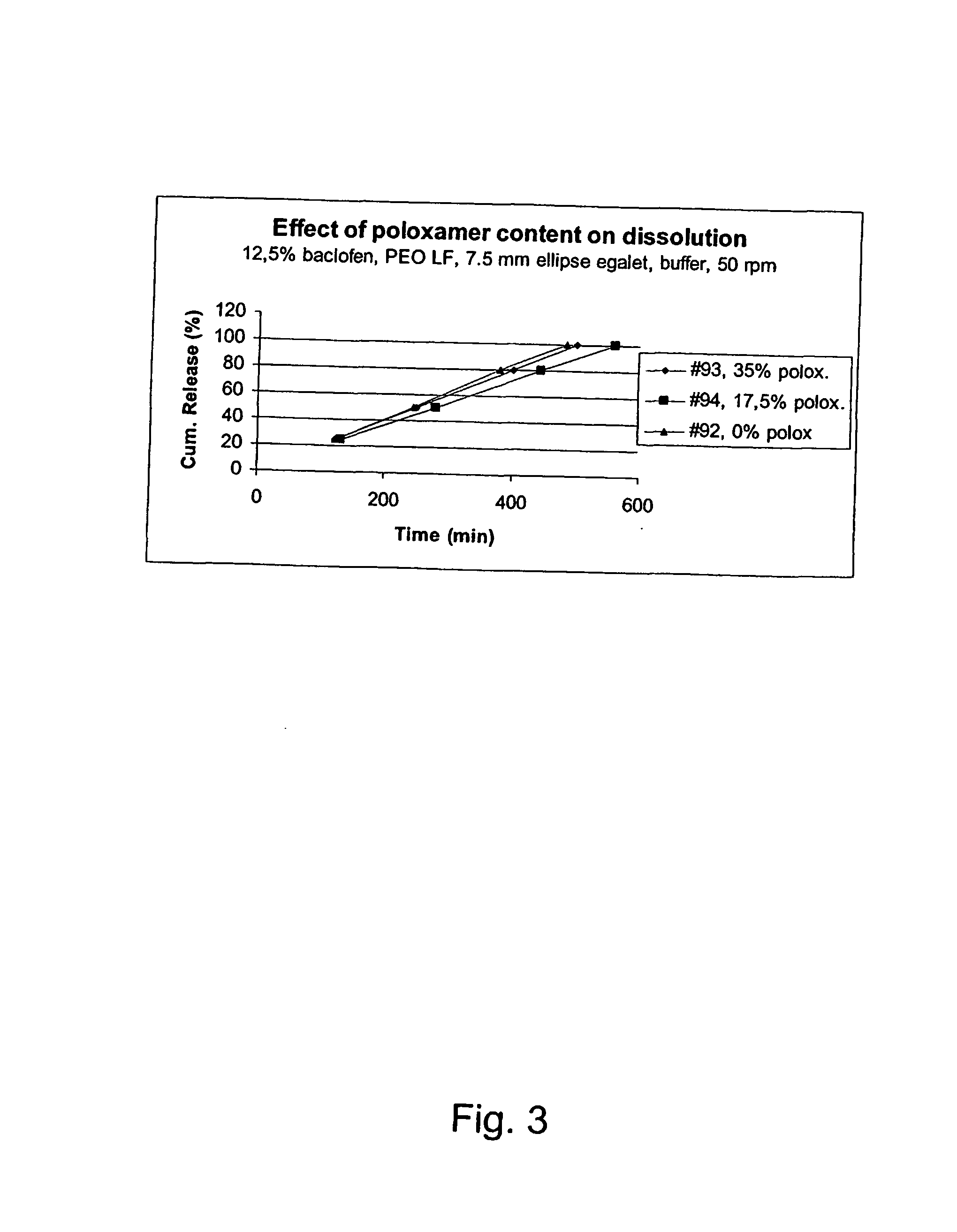
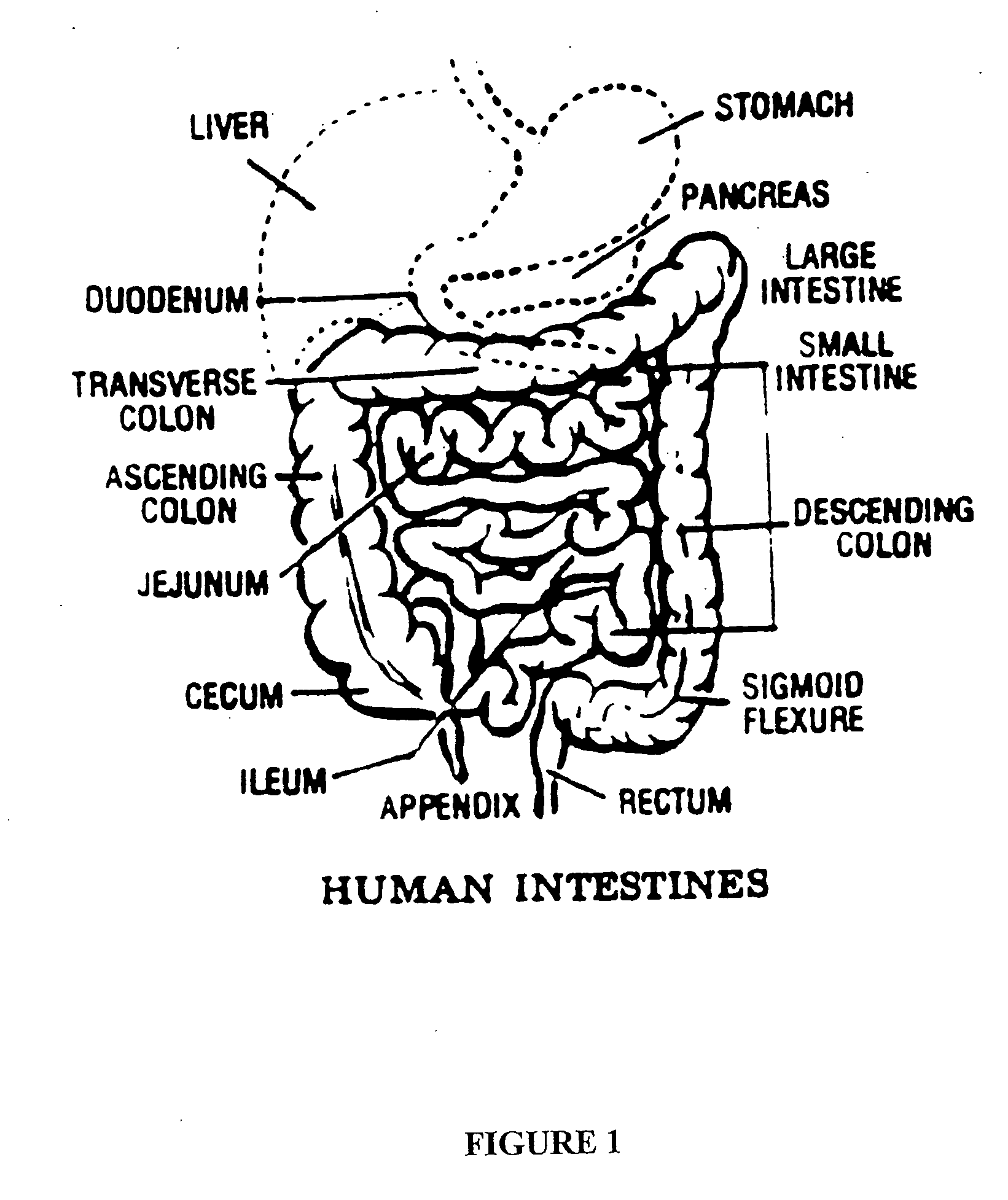
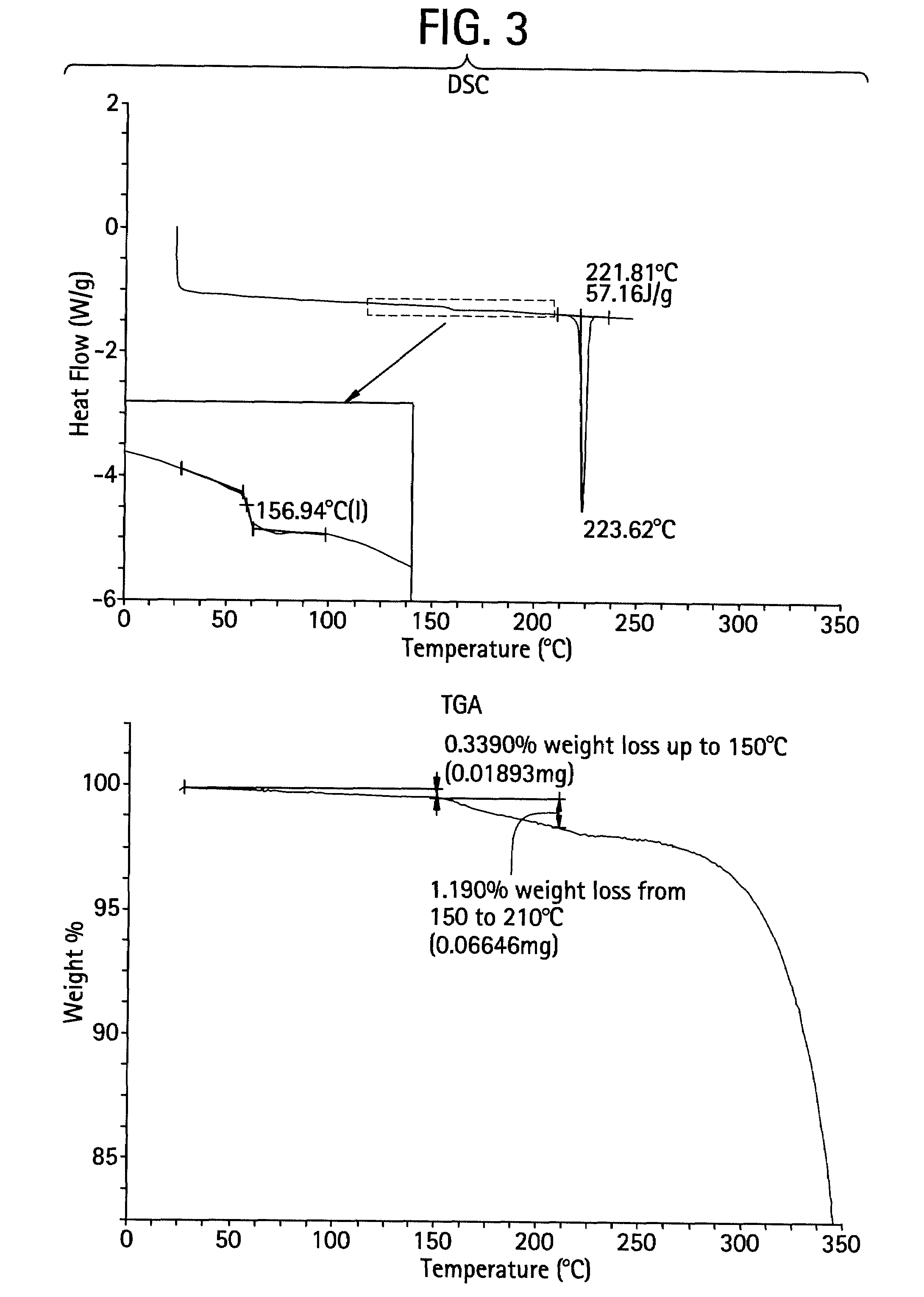
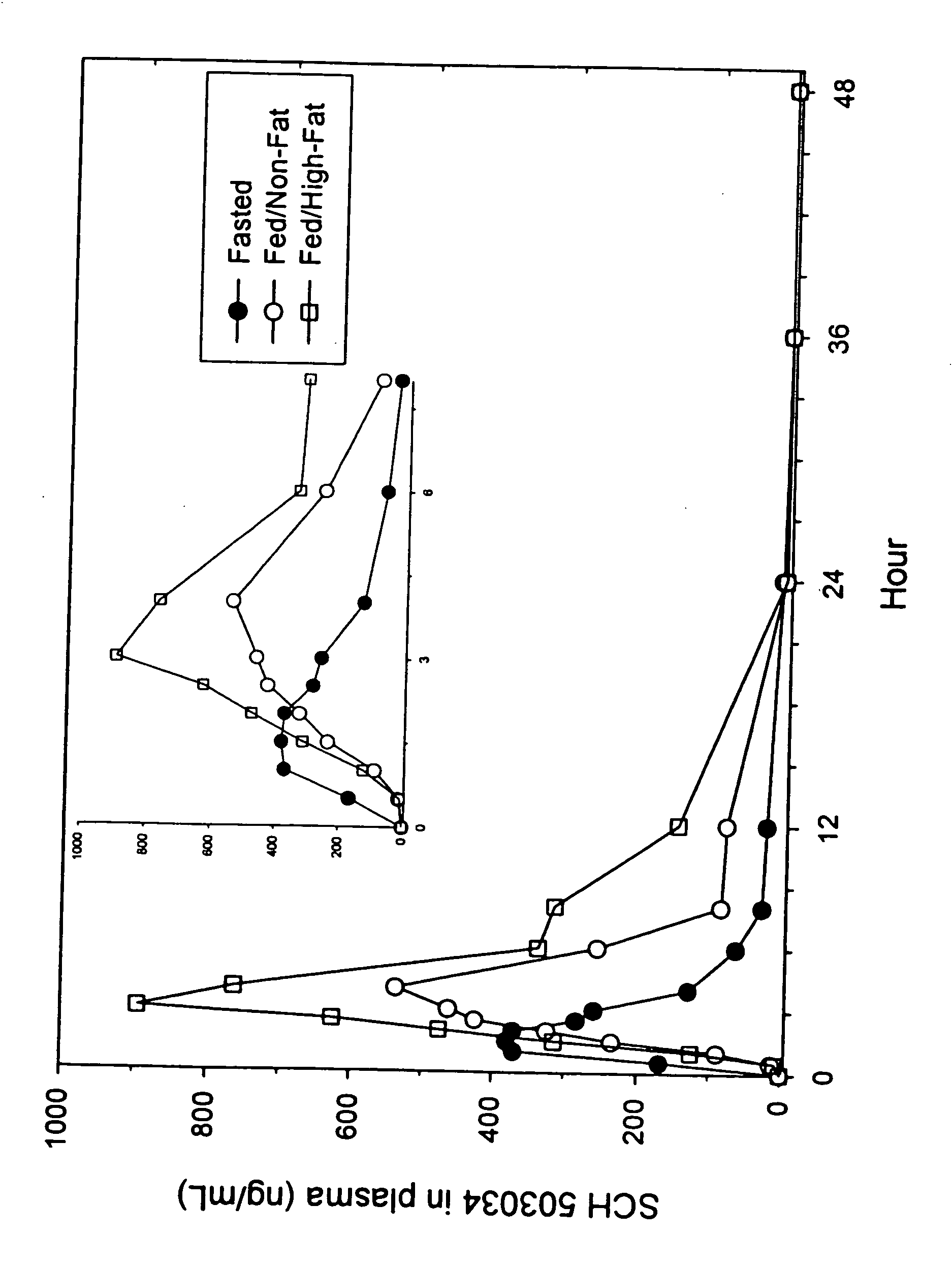
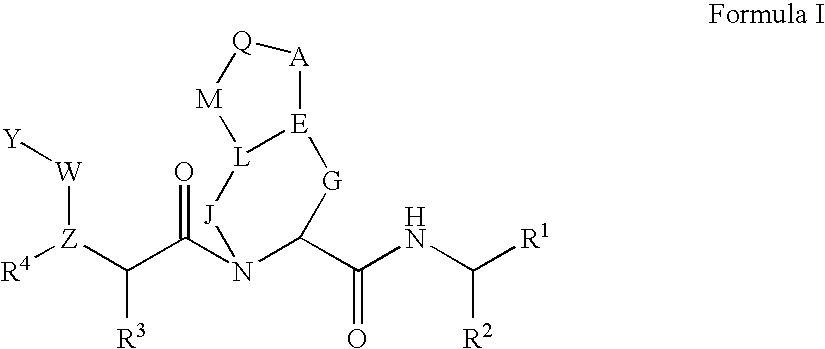
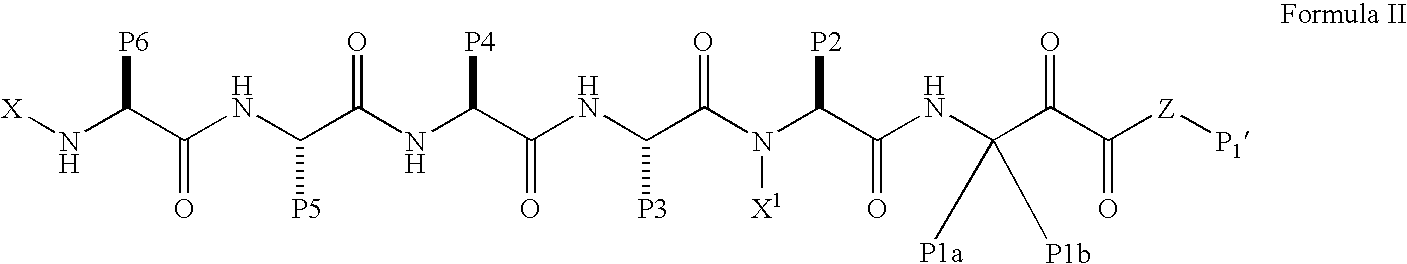
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1079 results about "Enhanced bioavailability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nano-Chinese medicinal biological product and its preparation
A nano-class biologic product of Chinese-medicinal material for higher biological utilization rate and target nature, lower toxic by-effect and improved curative effect is prepared through preparing the nanoparticles of Chinese-medicinal materials, and preparing nano-class (50-80 nm) biologic product.
Owner:陈永丽
Orally administrable composition capable of providing enhanced bioavailability when ingested
InactiveUS6054136AImprove solubilityImprove bioavailabilityCosmetic preparationsToilet preparationsFatty acid esterIngestion
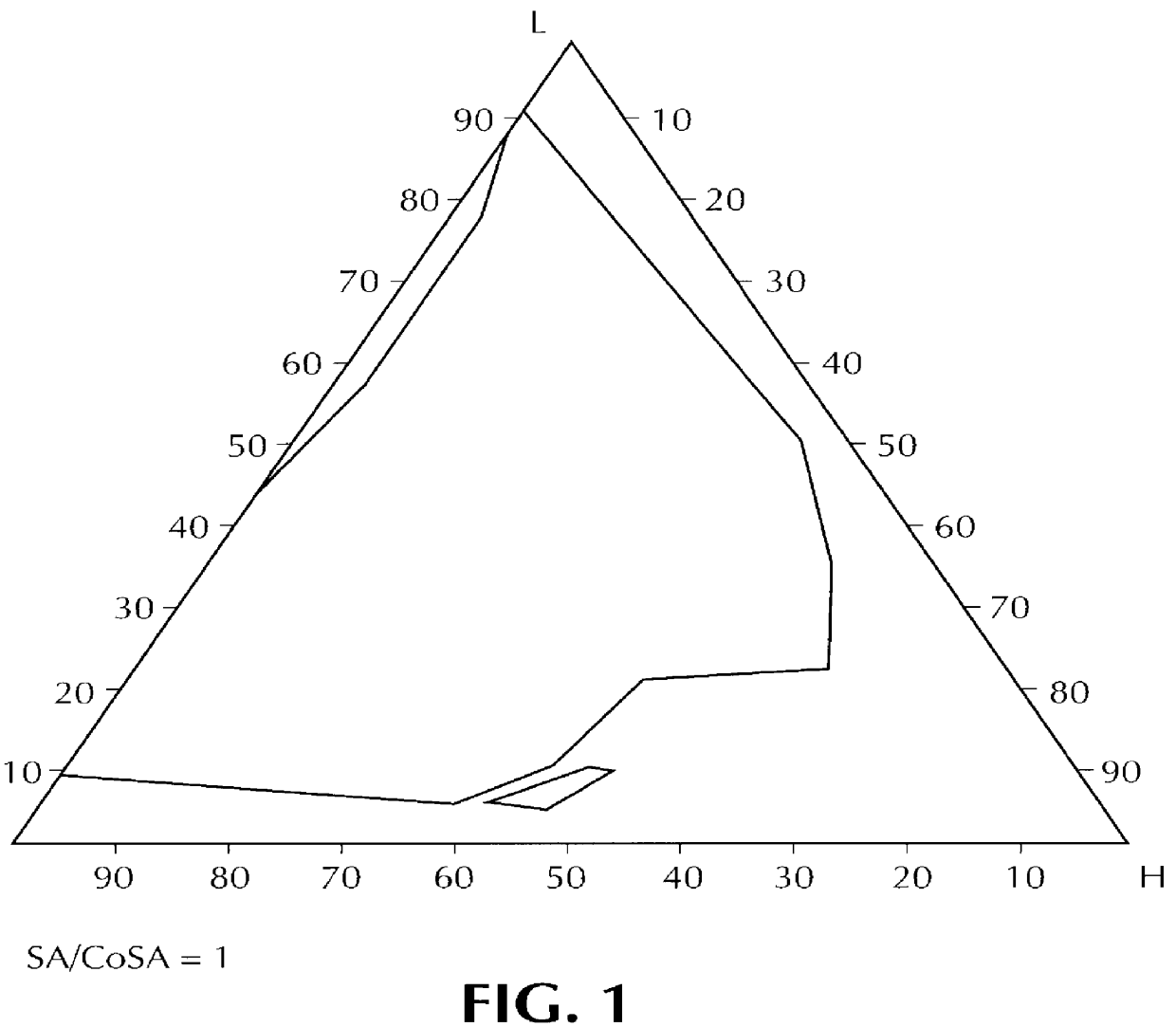
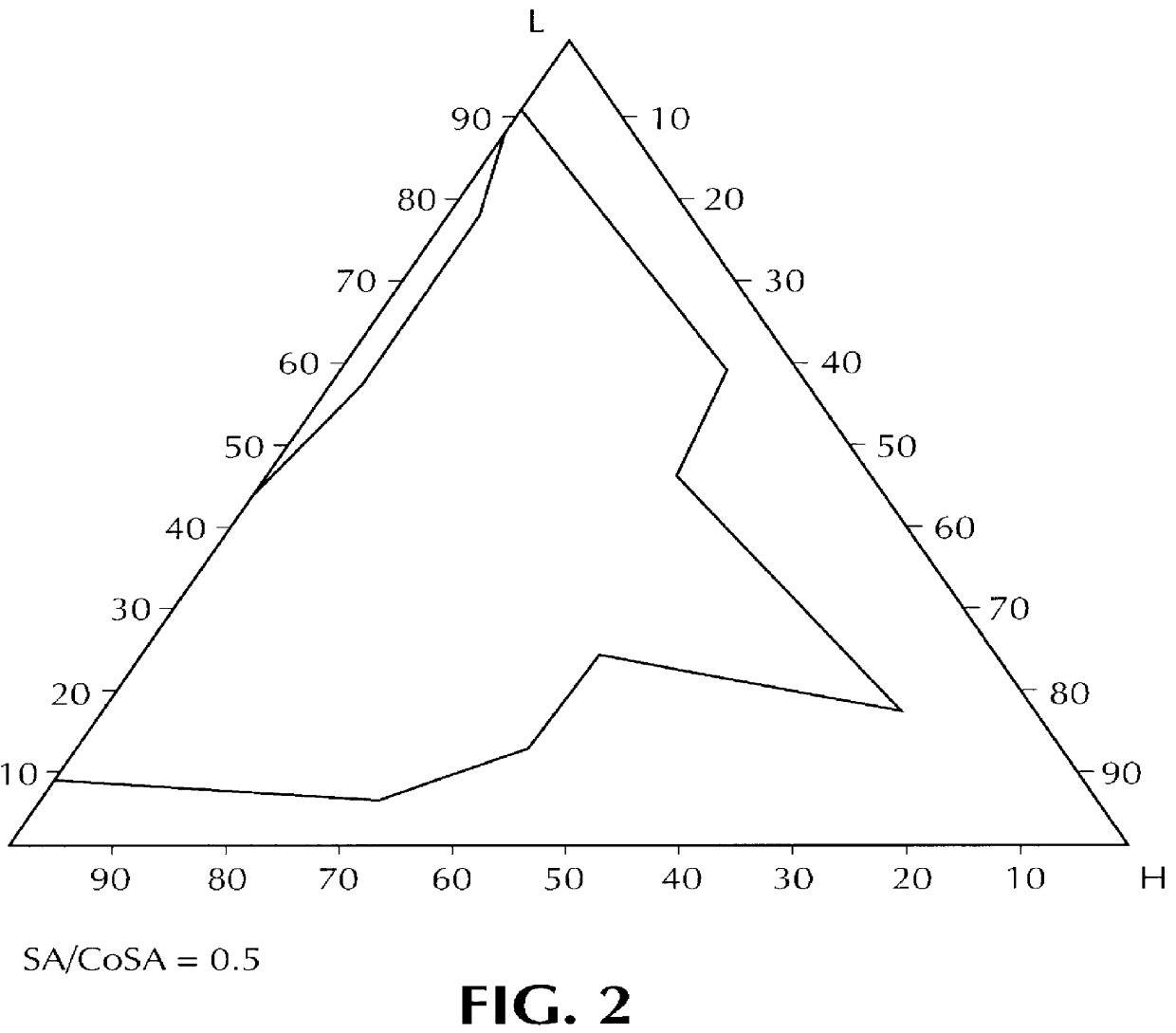
Composition for pharmaceutical or cosmetic use, capable of forming a microemulsion, comprising at least: an active principle, a lipophilic phase consisting of a mixture of fatty acid esters and glycerides, a surfactant (SA), a cosurfactant (CoSA), a hydrophilic phase, characterized: in that the lipophilic phase consists of a mixture of C8 to C18 polyglycolized glycerides having a hydrophilic-lipophilic balance (HLB) of less than 16, this lipophilic phase representing from 30 to 75% of the total weight of the composition; in that the surfactant (SA) is chosen from the group comprising saturated C8-C10 olyglycolized glycerides and oleic esters of polyglycerol, this surfactant having an HLB of less than 16; in that the cosurfactant (CoSA) is chosen from the group comprising lauric esters of propylene glycol, oleic esters of polyglycerol and ethyl diglycol; in that the SA / CoSA ratio is between 0.5 and 6; and in that the hydrophilic phase of the final microemulsion is supplied after ingestion by the physiological fluid of the digestive milieu.
Owner:GATTEFOSSE HLDG
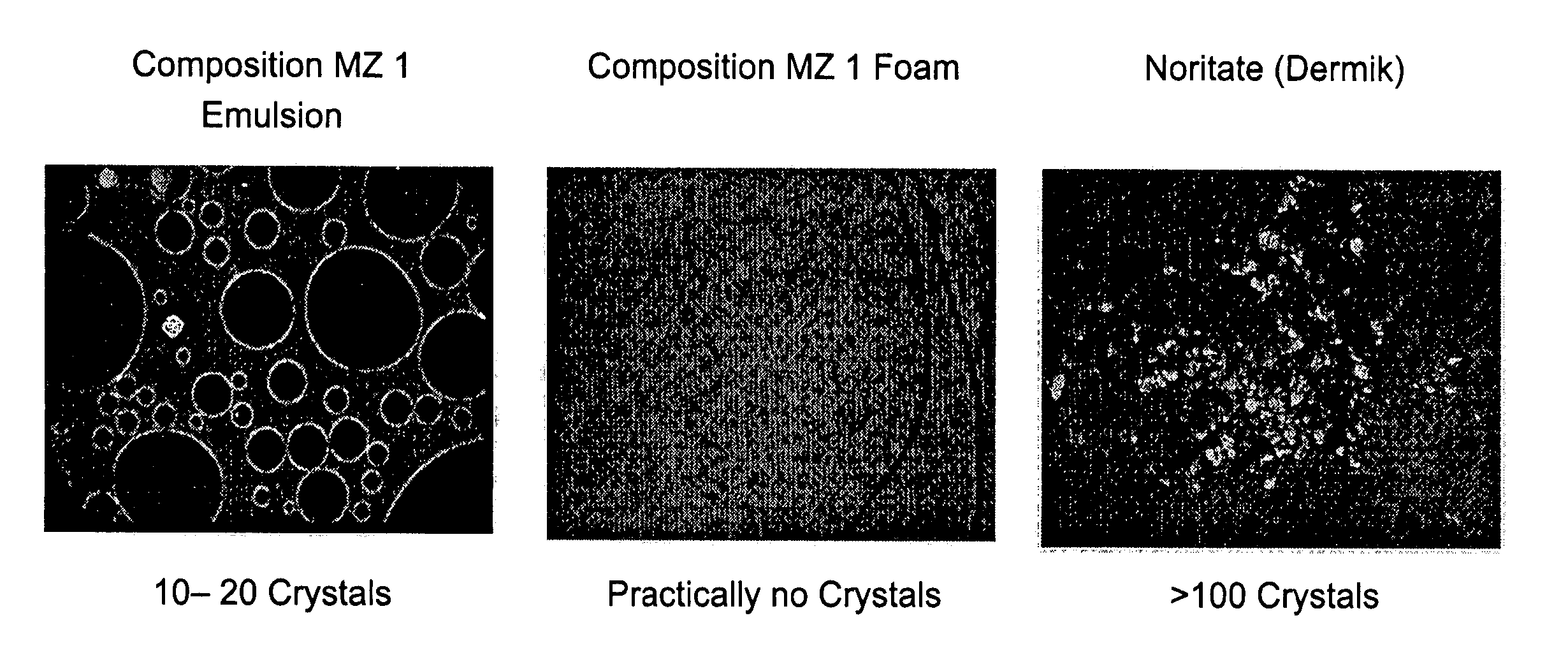
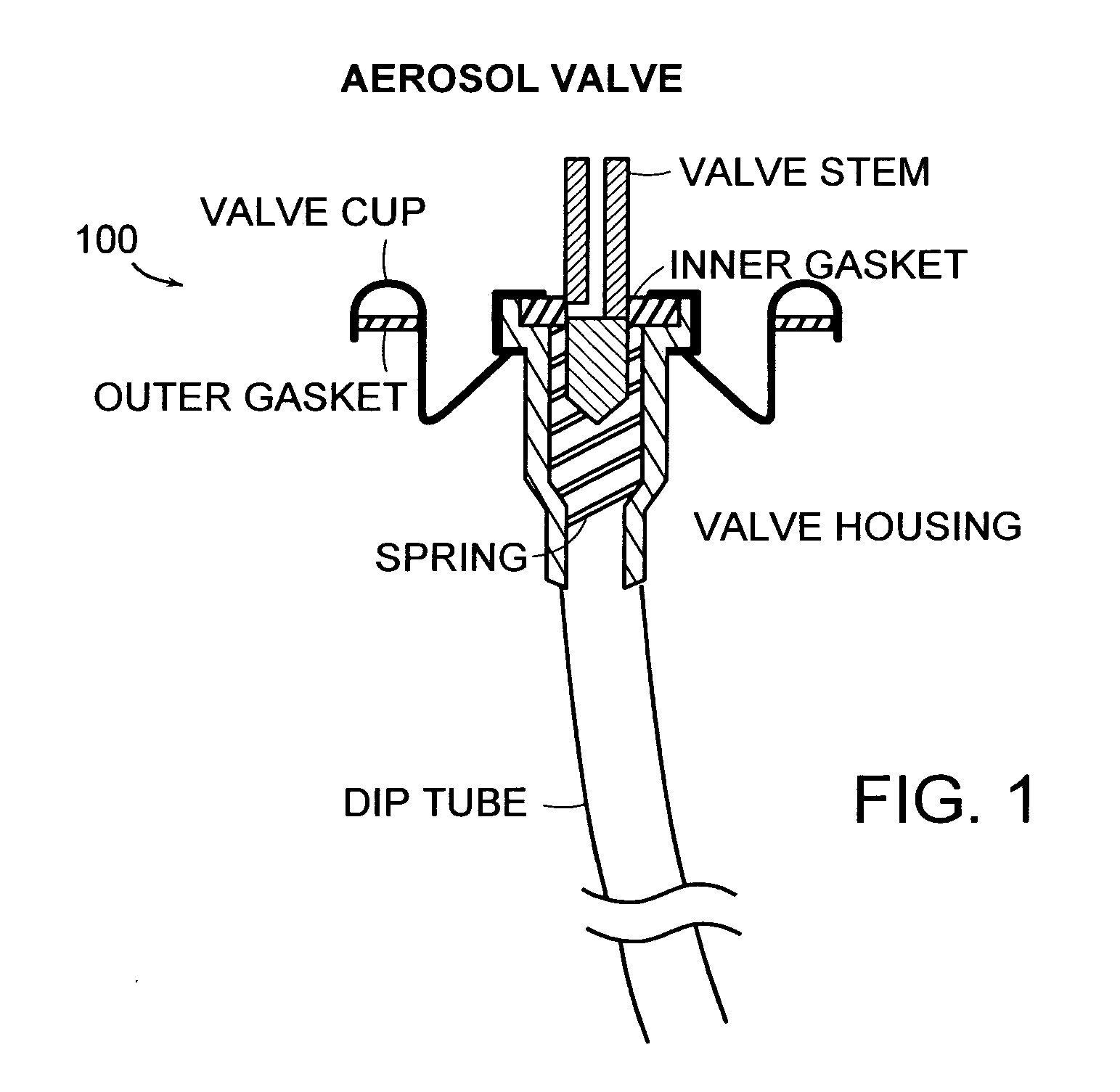
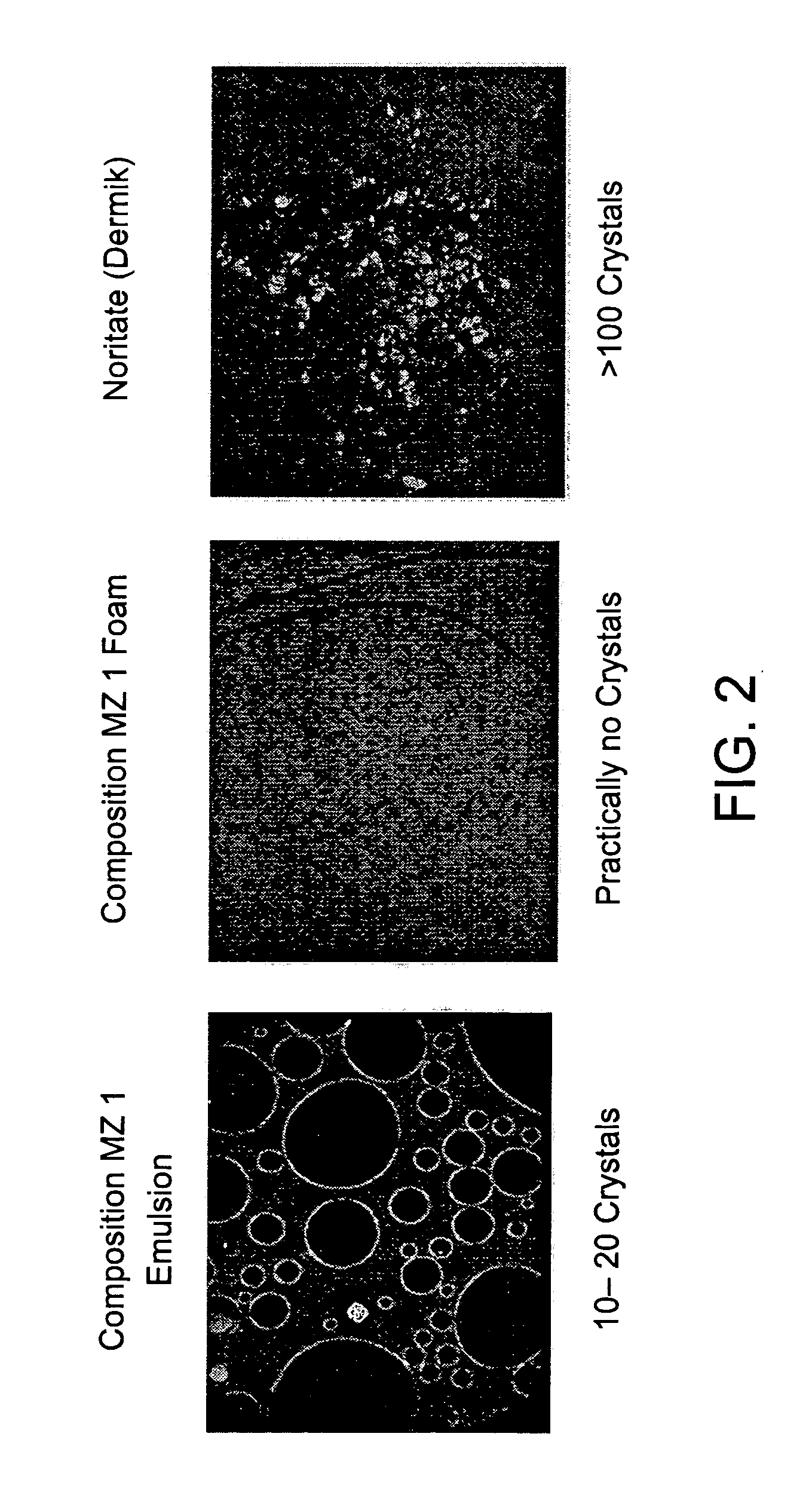
Kit and composition of imidazole with enhanced bioavailability
A composition and therapeutic kit provide a therapeutic azole with increased solubility. The kit includes an aerosol packaging assembly containing a container accommodating a pressurized product and an outlet capable of releasing the pressurized product as a foam. The pressurized product includes a foamable composition including: i. a therapeutic azole, wherein the solubility of the azole in the composition before foaming is less than the solubility of the azole in the composition after foaming; ii. at least one organic carrier selected from the group consisting of a hydrophobic organic carrier, a co-solvent, an emollient and mixtures thereof, at a concentration of about 2% to about 50% by weight; iii. a surface-active agent; iv. about 0.01% to about 5% by weight of at least one polymeric additive selected from the group consisting of a bioadhesive agent, a gelling agent, a film forming agent and a phase change agent; v. water; and vi. liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition.
Owner:FOAMIX PHARMACEUTICALS LIMITED
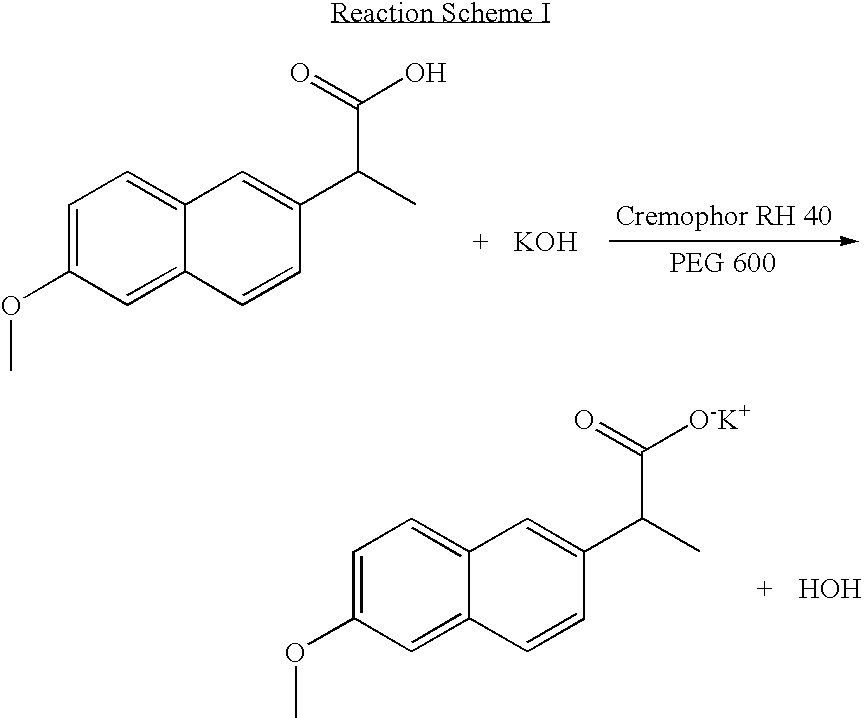
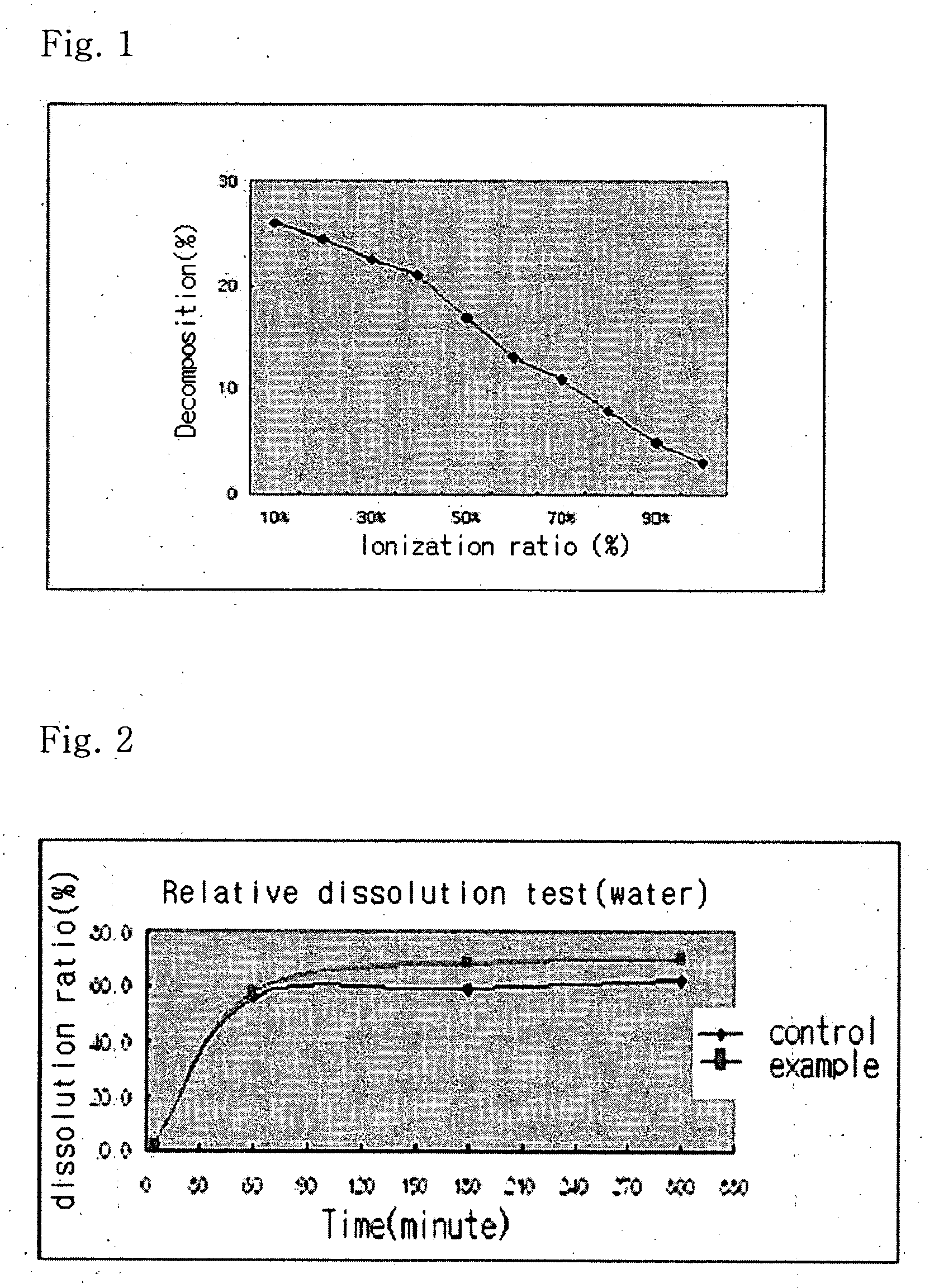
Solvent system of hardly soluble drug with improved dissolution rate
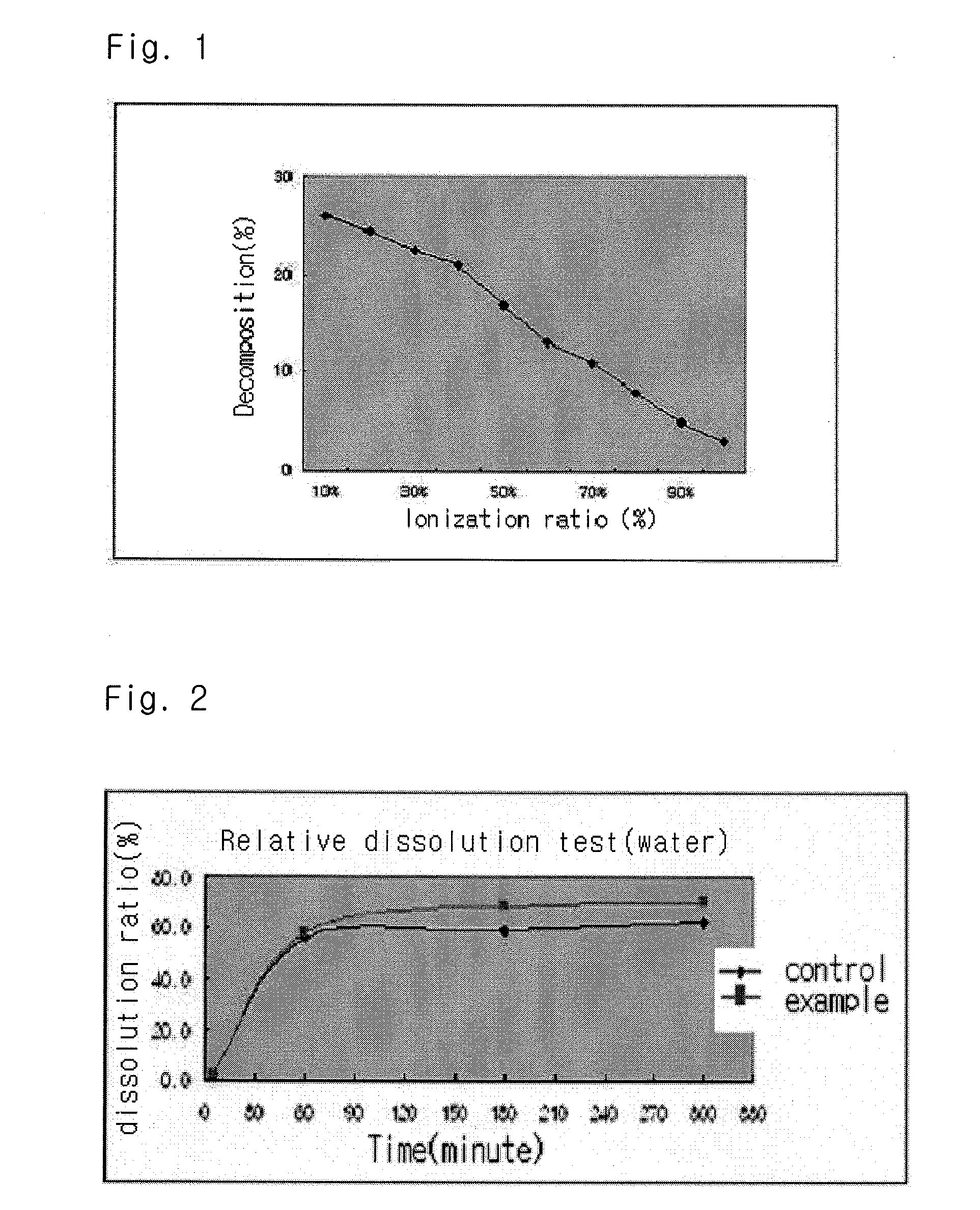
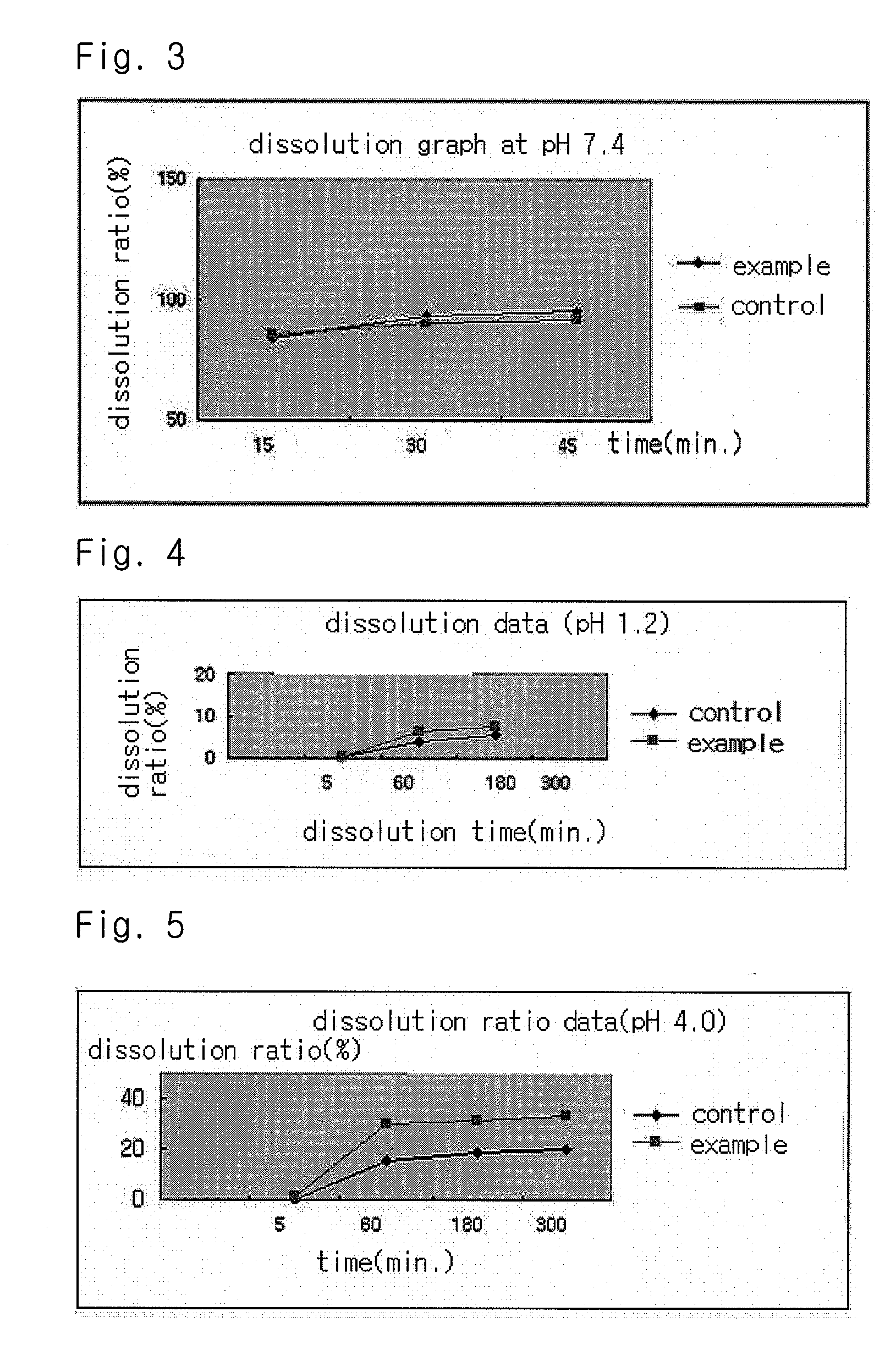
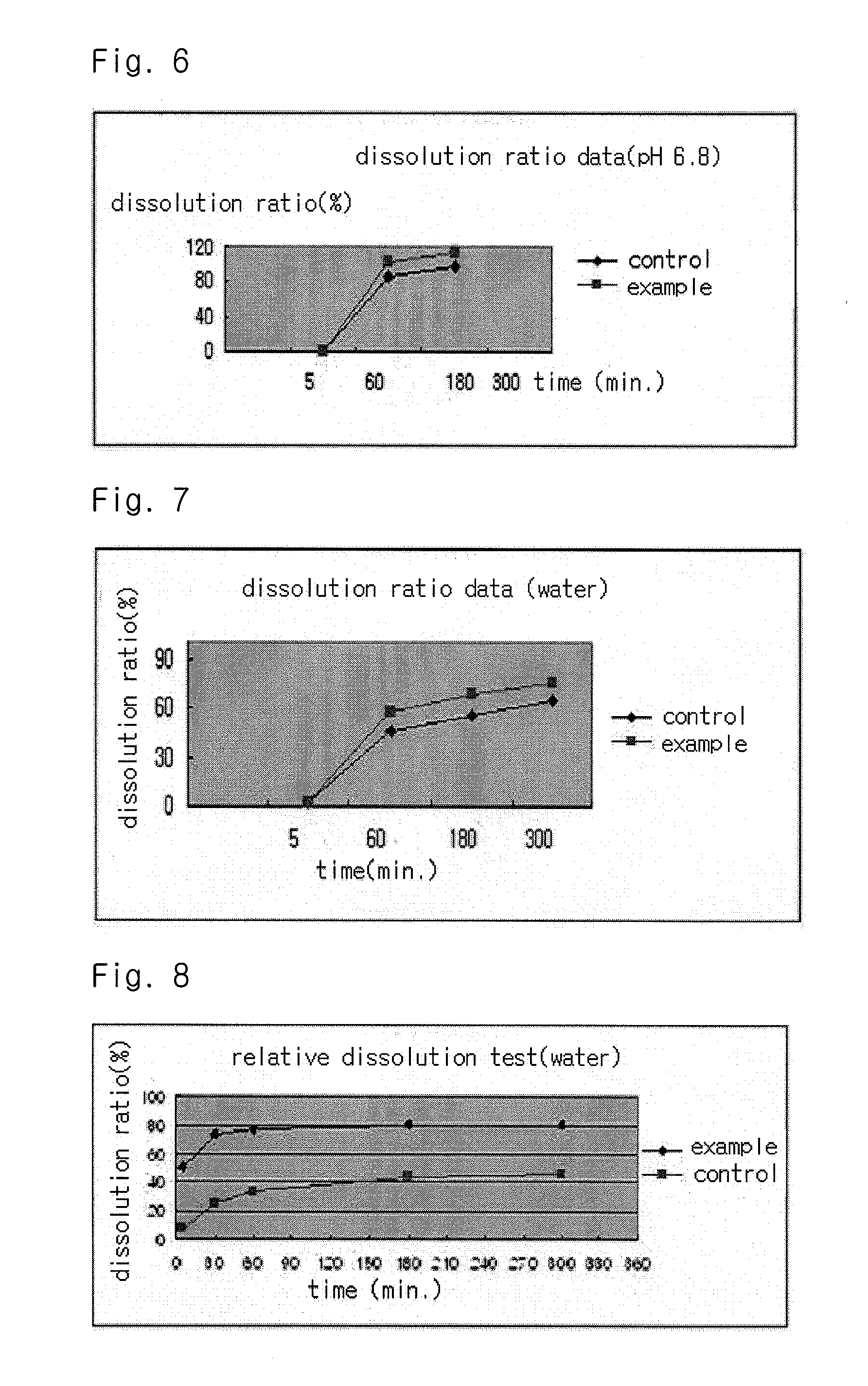
InactiveUS20040157928A1Good disintegrationPromote dissolutionBiocideAntipyreticDissolutionIonization
The present invention relates to a solvent system with improved disintegration degree and dissolution ratio of a hardly soluble drug by highly concentrating the drug through partial ionization, and by establishing optimal conditions for enhancing bioavailability of the drug, such as the co-relation between the acid drug and the accompanied components, ionization degree of a solvent system, use of an appropriate cation acceptance, water content, selection of optimal mixing ratio of the respective components and use of specific surfactants, and to a pharmaceutical preparation comprising the same. The solvent system of the invention has advantages in that it can enhance bioavailability by improving the disintegration degree and dissolution ratio of a hardly soluble drug and also provide a capsule with a sufficiently small volume to permit easy swallowing.
Owner:R & P KOREA
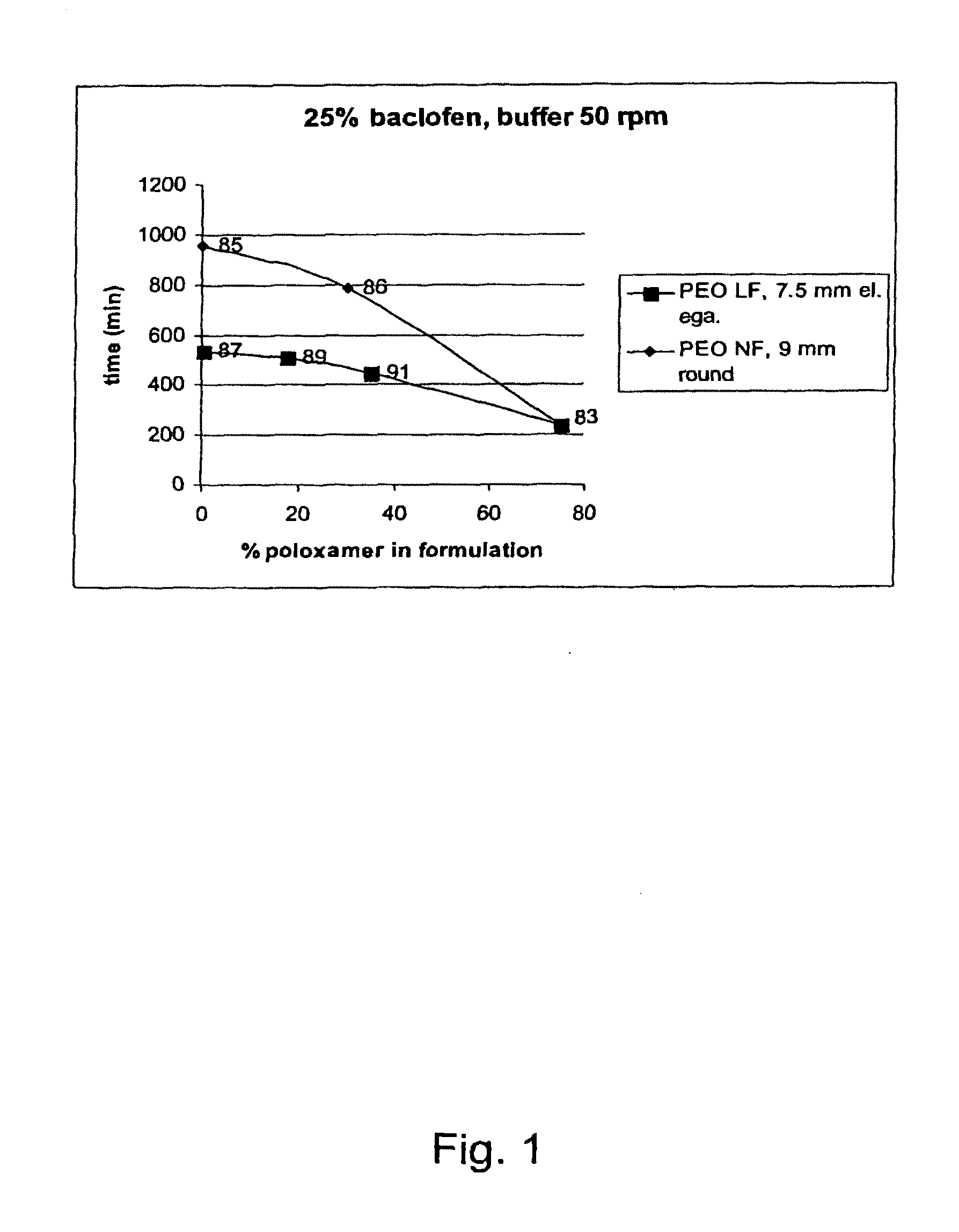
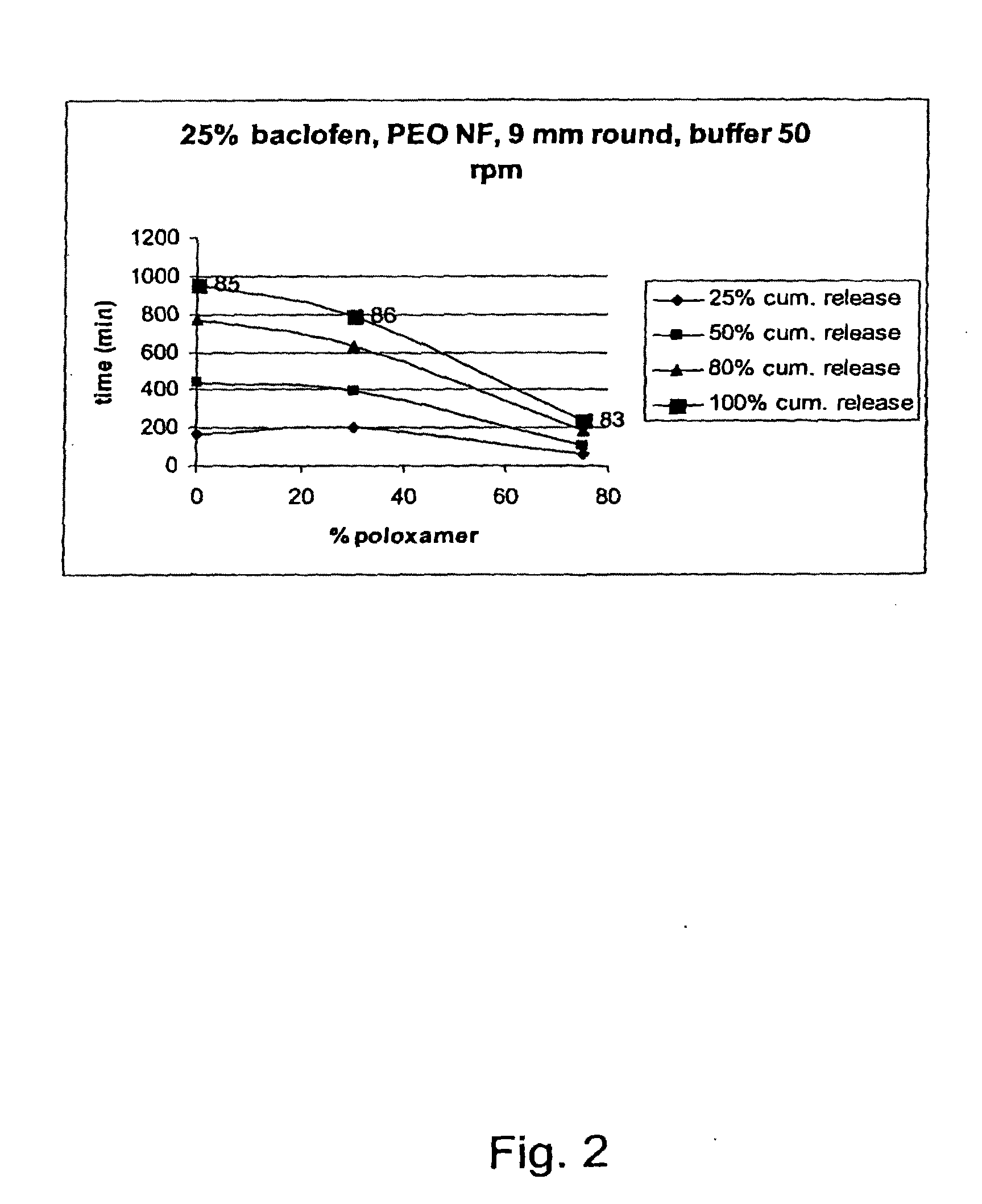
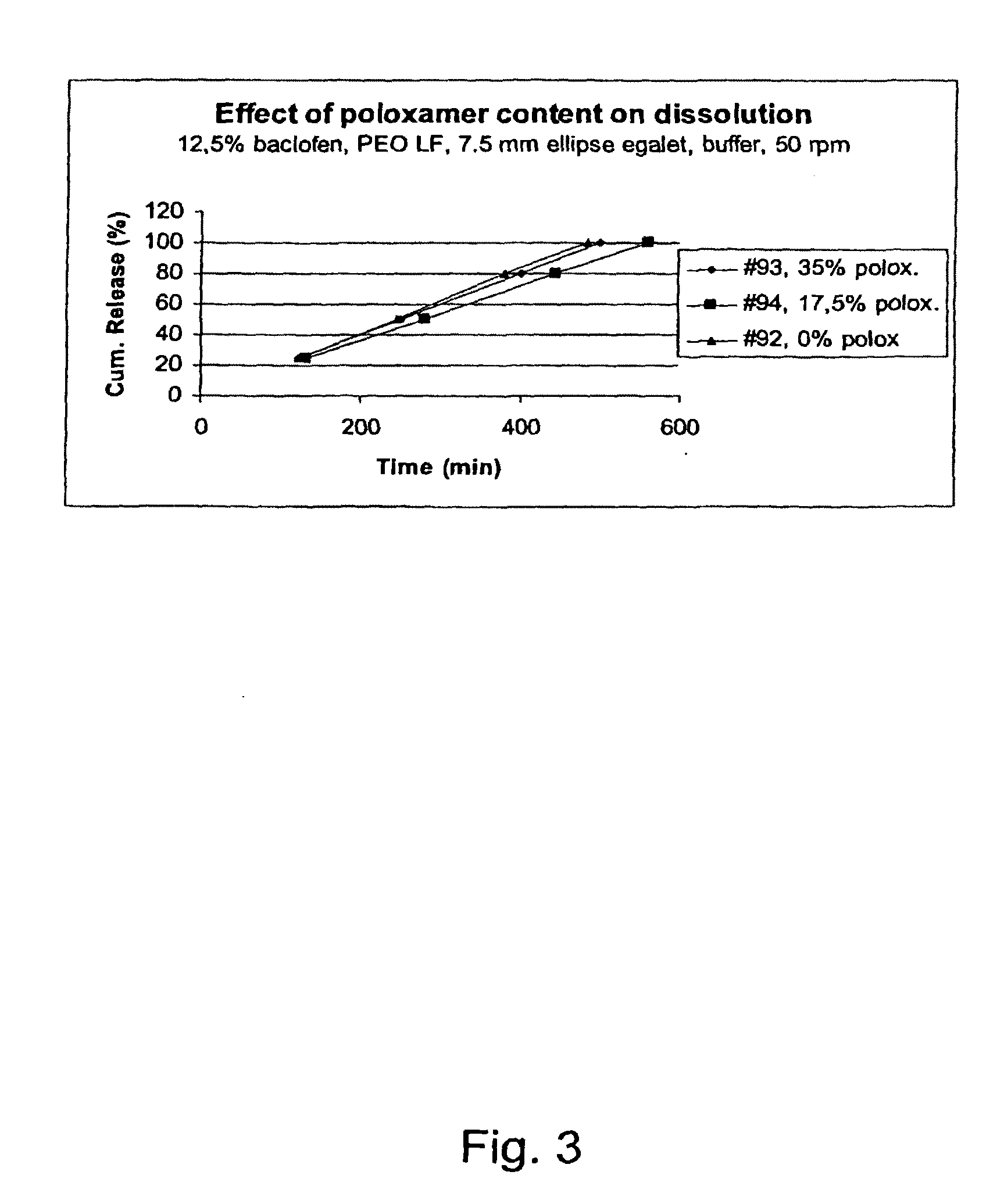
Matrix compositions for controlled delivery of drug substances
InactiveUS20070042044A1Improve solubilityImprove oral bioavailabilityBiocidePowder deliveryPolyethylene oxidePEG-PLGA-PEG
A novel matrix composition for pharmaceutical use. The matrix composition has been designed so that it is especially suitable in those situation where an improved bioavailability is desired and / or in those situation where a slightly or insoluble active substance is employed. Accordingly, a controlled release pharmaceutical composition for oral use is provided in the form of a coated matrix composition, the matrix composition comprising i) a mixture of a first and a second polymer that have plasticizing properties and which have melting points or melting intervals of a temperature of at the most 200° C., the first polymer being selected from the group consisting of polyethylene glycols and polyethylene oxides, and the second polymer being selected form block copolymer of ethylene oxide and propylene oxide including poly(ethylene-glycol-b-(DL-lactic acid-co-glycolic acid)-b-ethylene glycol (PEG-PLGA PEG), poly((DL-lactic acid-co-glycolic acid)-g-ethylene glycol) (PLGA-g-PEG), poloxamers and polyethylene oxide-polypropylene oxide (PEO-PPO), ii) a therapeutically, prophylactically and / or diagnostically active substance, the matrix composition being provided with a coating having at least one opening exposing at one surface of said matrix, wherein the active substance is released with a substantially zero order release.
Owner:EGALET LTD
Pharmaceutical formulations targeting specific regions of the gastrointesinal tract
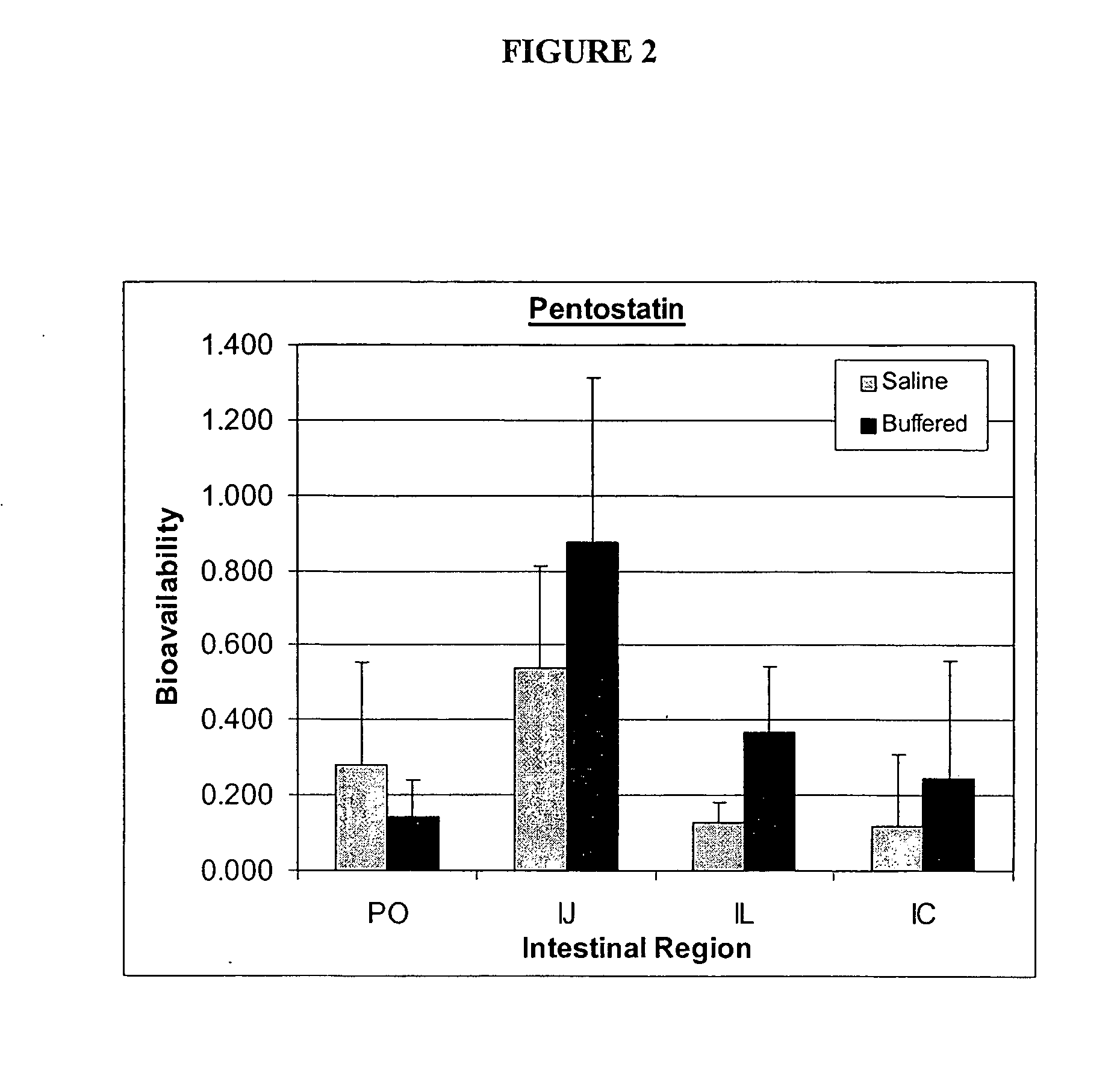
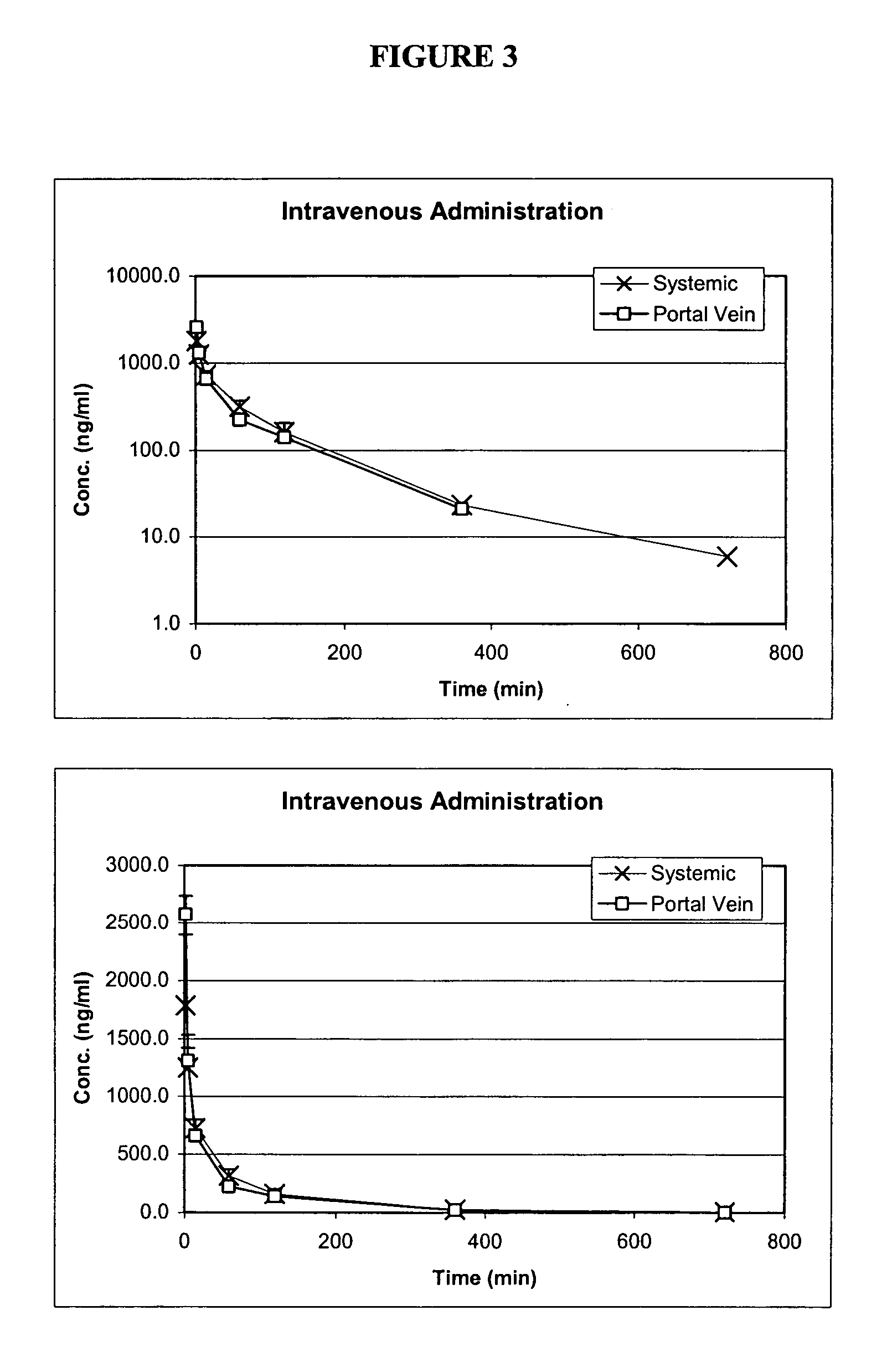
Oral formulations of pharmaceuticals are provided with enhanced bioavailability by targeting specific regions of the gastrointestinal tract. Particularly, water soluble and acid-labile drugs such as cytidine analogs (e.g., decitabine) and 2'-deoxyadenosine analogs (e.g., pentostatin) are formulated with pH-sensitive polymers so that these drugs are preferably absorbed in the upper regions of the small intestine, such as the jejunum. In addition, drugs with poor oral bioavailability such as camptothecin compounds (e.g., 9-nitro-camptothecin) can also be formulated using similar strategies in order to significantly improve their oral bioavailability. These formulations can be used to treat a wide variety of diseases or conditions, such hematological disorders, benign tumors, cancer, restenosis, inflammatory diseases, and autoimmune diseases.
Owner:MAYNE PHARMA USA
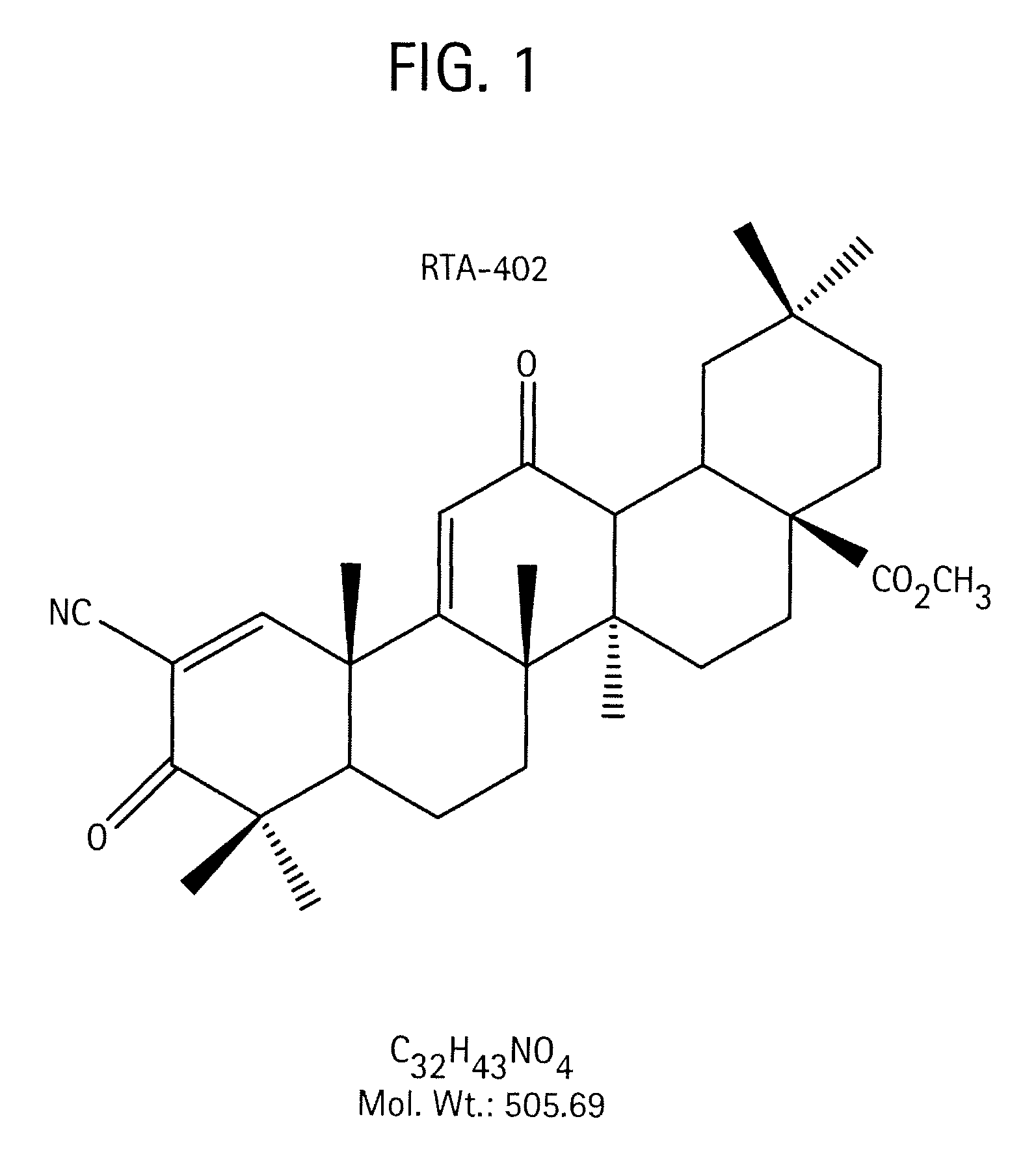
Novel forms of cddo methyl ester
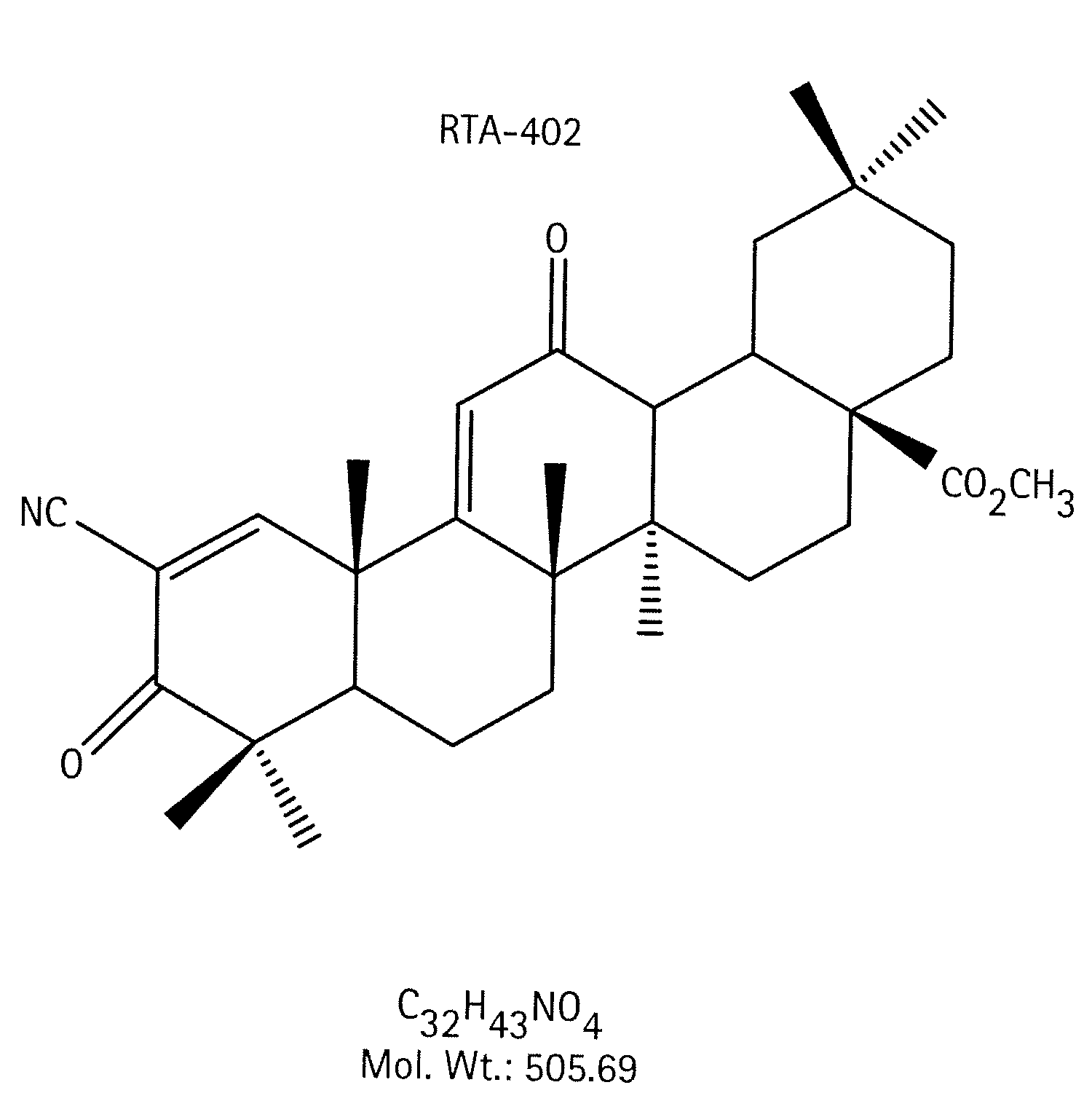
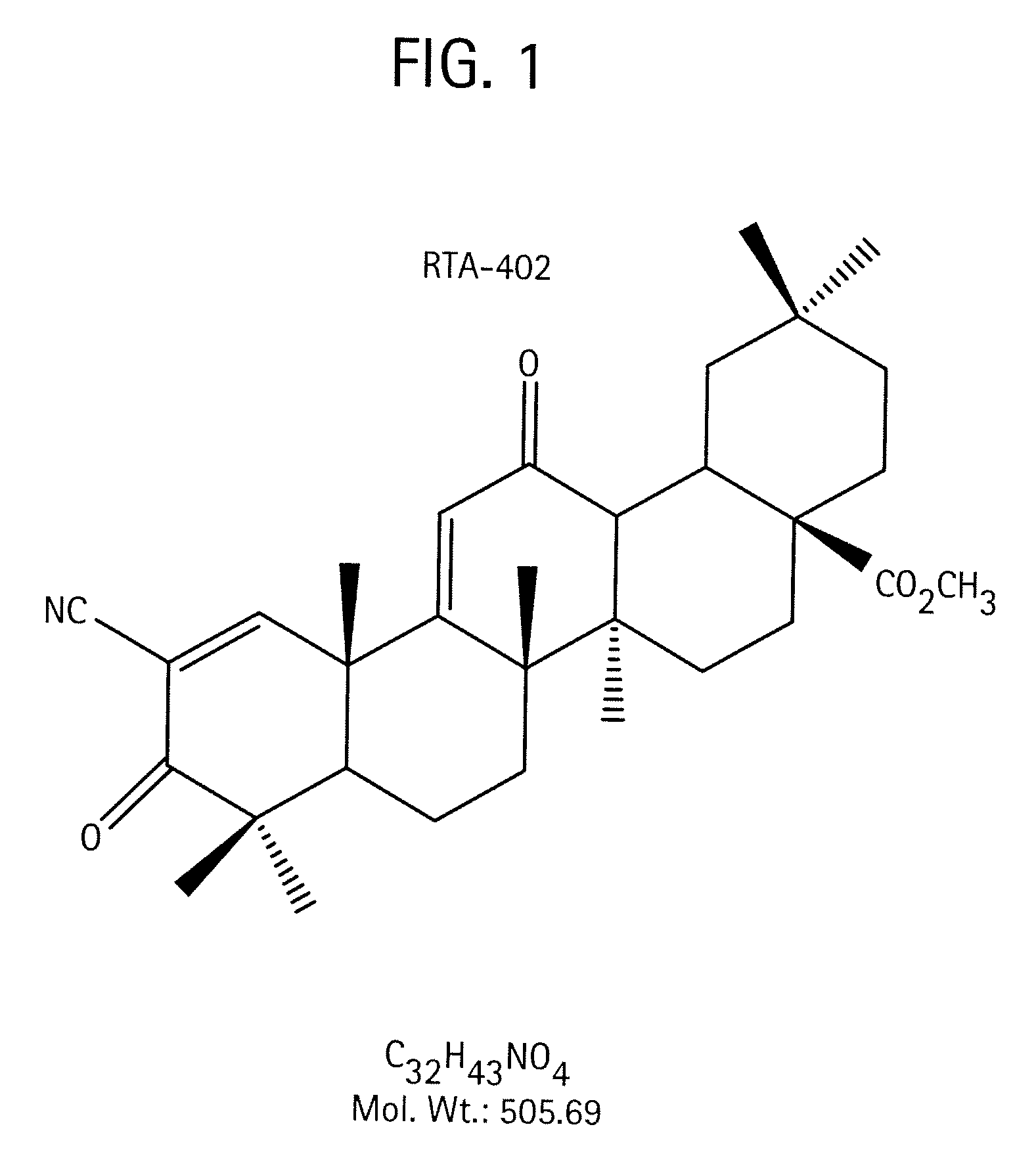
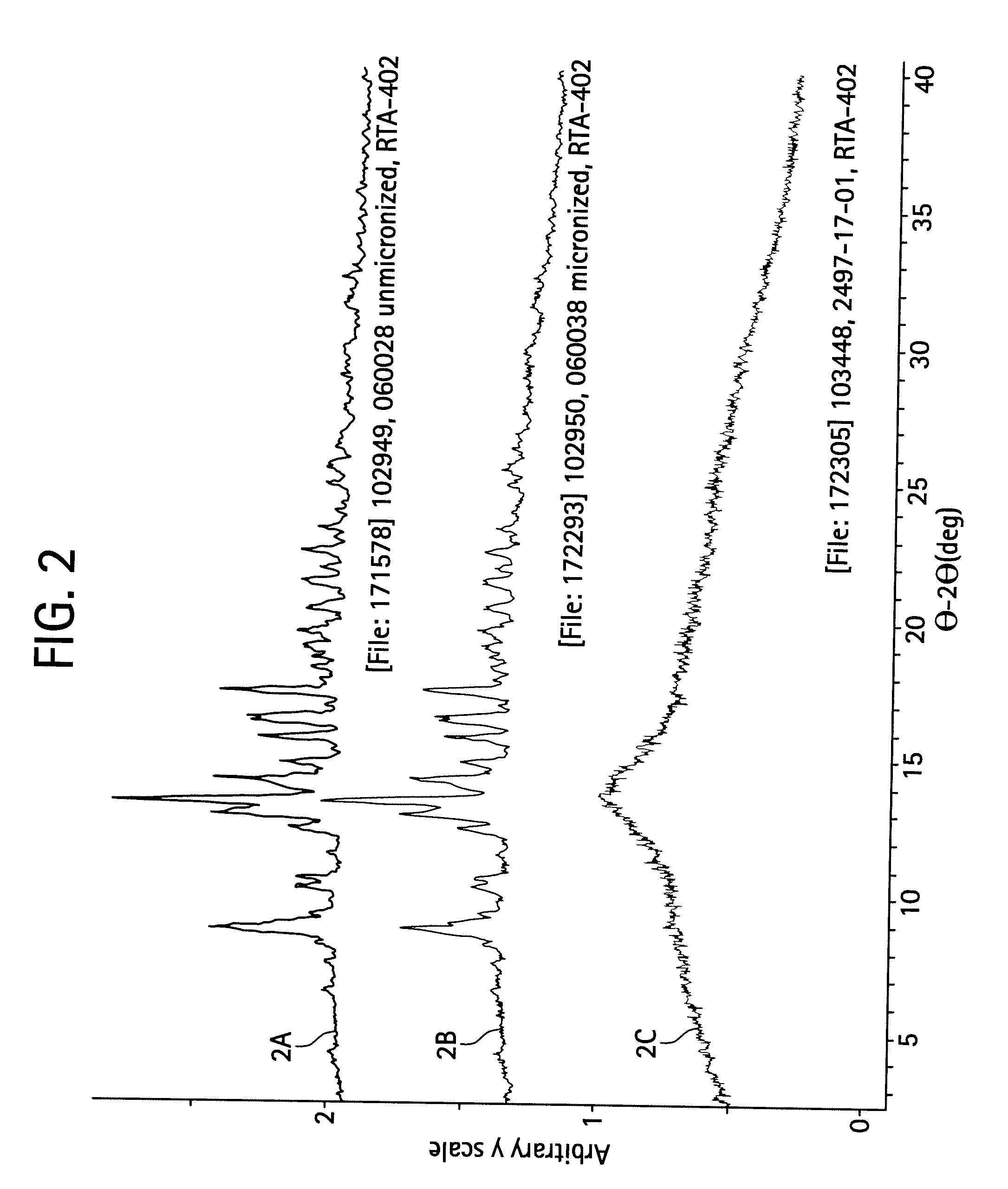
A triterpenoid compound, methyl 2-cyano-3,12-dioxoleana-1,9(11)-dien-28-oate (CDDO methyl ester), has a non-crystalline, glassy solid form and a non-hydrous crystalline form that can prepared, for example, from a saturated methanol solution. The glassy form displays an enhanced bioavailability over the non-hydrous crystalline form. Each form of CDDO methyl ester is a superior candidate for use, typically in solid dosage form, for treating a variety of disease states, generally associated with inflammation.
Owner:REATA PHARM HLDG LLC
Bioadhesive drug formulations for oral transmucosal delivery
Formulations for controlled delivery of oral transmucosal medications are provided. The formulations are characterized as hydrogel-forming or eroding-types which are bioadhesive and provide for controlled and sustained release of the medication such that enhanced bioavailability and efficacy is provided.
Owner:VERTICAL PHARMA
Solvent system of hardly soluble drug with improved dissolution rate
InactiveUS20090318558A1Good disintegrationPromote dissolutionBiocideAntipyreticActive agentDissolution
The present invention relates to a solvent system with improved disintegration degree and dissolution ratio of a hardly soluble drug by highly concentrating the drug through partial ionization, and by establishing optimal conditions for enhancing bioavailability of the drug, such as the co-relation between the acid drug and the accompanied components, ionization degree of a solvent system, use of an appropriate cation acceptor, water content, selection of optimal mixing ratio of the respective components and use of specific surfactants, and to a pharmaceutical preparation comprising the same. The solvent system of the invention has advantages in that it can enhance bioavailability by improving the disintegration degree and dissolution ratio of a hardly soluble drug and also provide a capsule with a sufficiently small volume to permit easy swallowing.
Owner:R & P KOREA
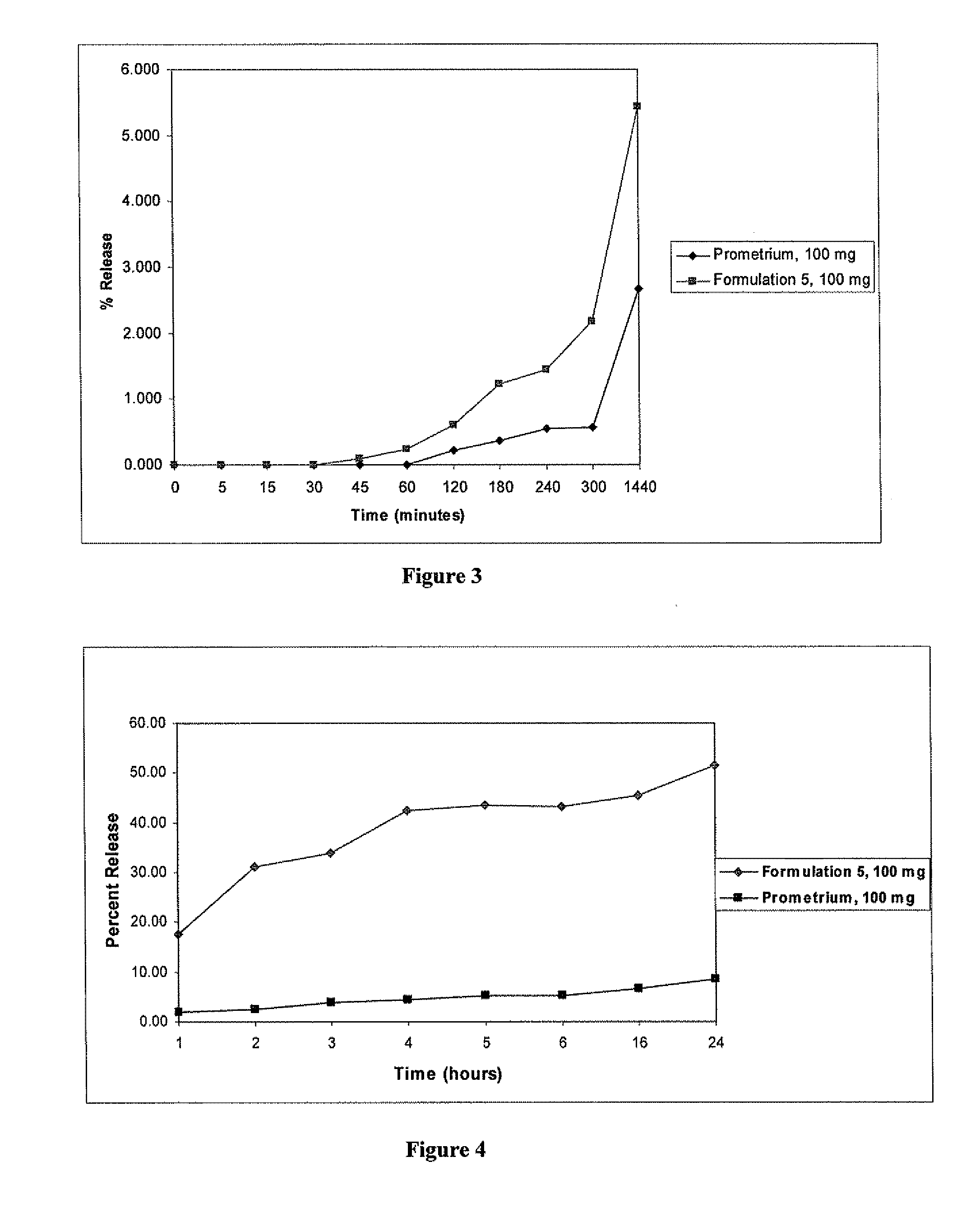
Progesterone Solutions for Increased Bioavailability
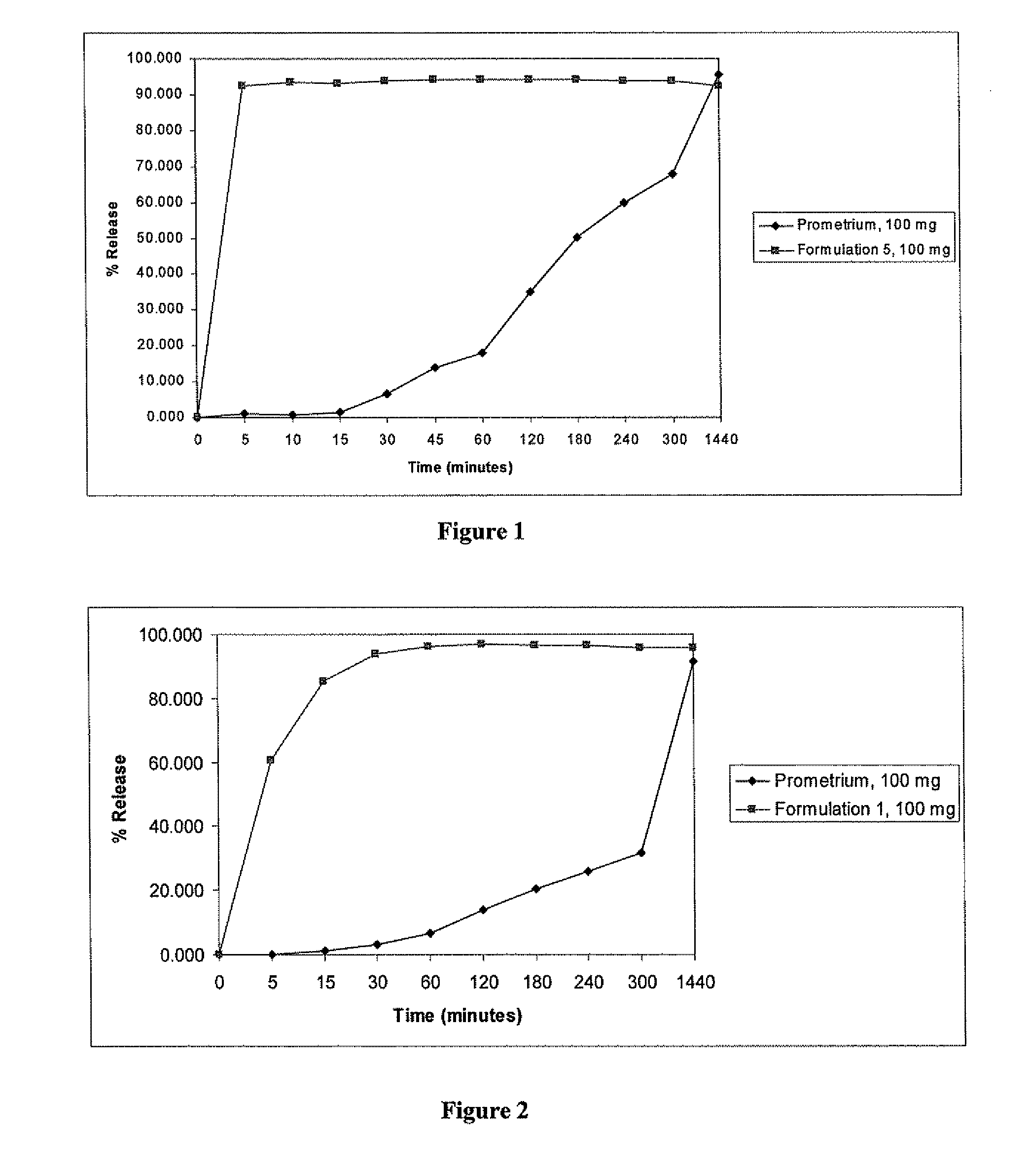
ActiveUS20100255085A1Improve bioavailabilityHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsDissolutionProgesterones
Fill materials for hydrophobic drugs, such as progesterone, and methods of making and using thereof are described herein. The fill material contains the hydrophobic drug dissolved in one or more fatty acids. The concentration of the hydrophobic drug is typically from about 7% to about 50% by weight of the fill material. The concentration of the one or more fatty acids is from about 60% to about 95% by weight of the carrier. The formulation also contains an organic acid and one or both of one or more pharmaceutically acceptable alcohols and one or more pharmaceutically acceptable mono-, di-, or triesters of medium or long chain fatty acids. The fill material can be encapsulated in a hard or soft capsule. The formulations described herein have a higher dissolution rate and faster onset of dissolution compared to micronized progesterone suspended in an oil and thus should have increased bioavailability in vivo.
Owner:PATHEON SOFTGELS INC
Chemical Combination and Method for Increasing Delivery of Coenzyme Q10
InactiveUS20110318405A1Reduce deliveryOvercome problemsBiocidePeptide/protein ingredientsTransdermal patchMedicine
The present invention relates to a chemical combination and method for increasing delivery of Coenzyme Q10. The chemical combination comprises Coenzyme Q10 mixed with at least one chemical. The at least one chemical includes cyclic terpene containing essential oil(s) that permit unprecedented levels of Coenzyme Q10 to be made available for delivery and absorption, increasing bioavailability, as well as overcoming the previous limits. A transdermal patch including a layer containing Coenzyme Q10 is also provided.
Owner:ERWIN CHARLES
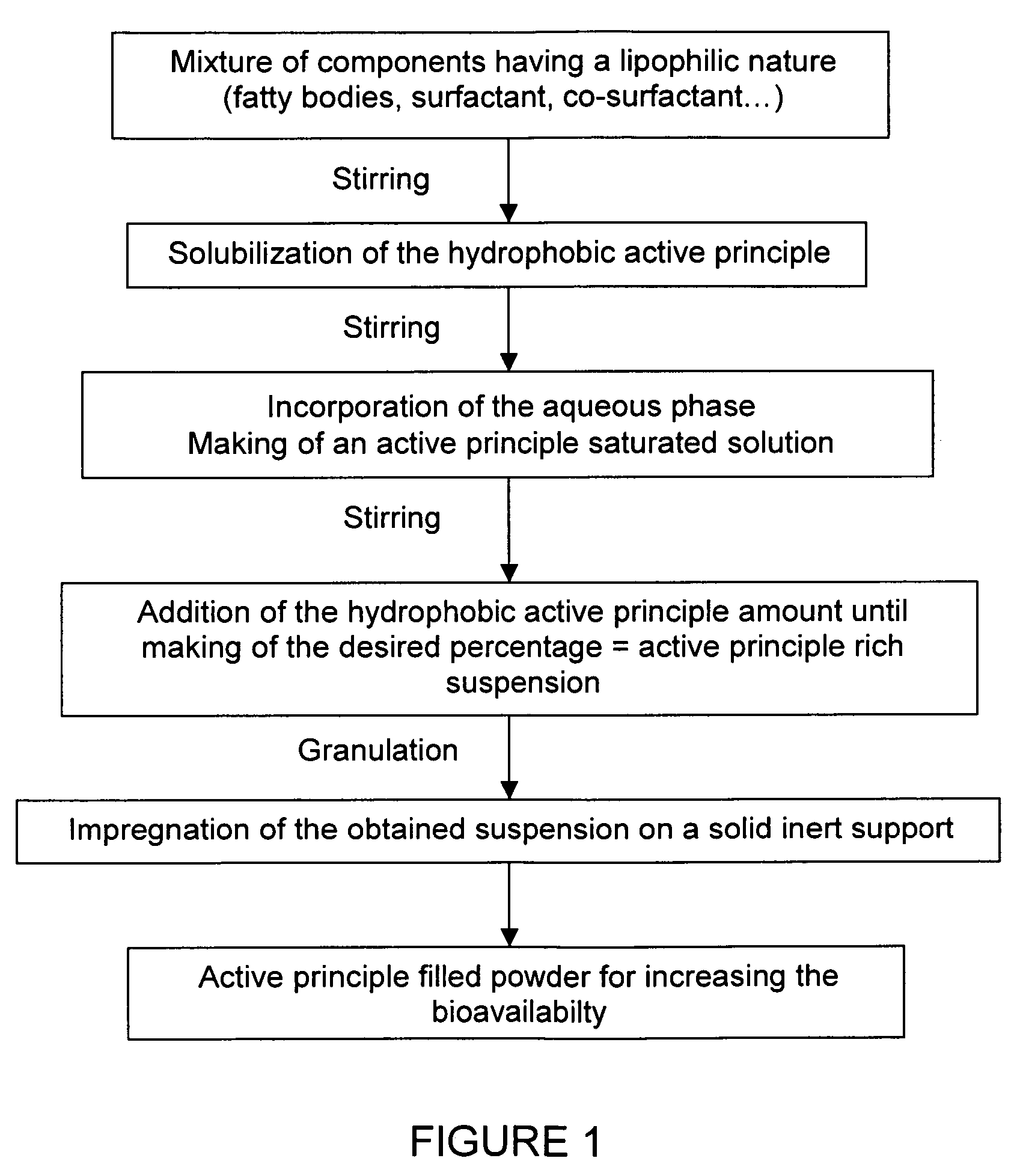
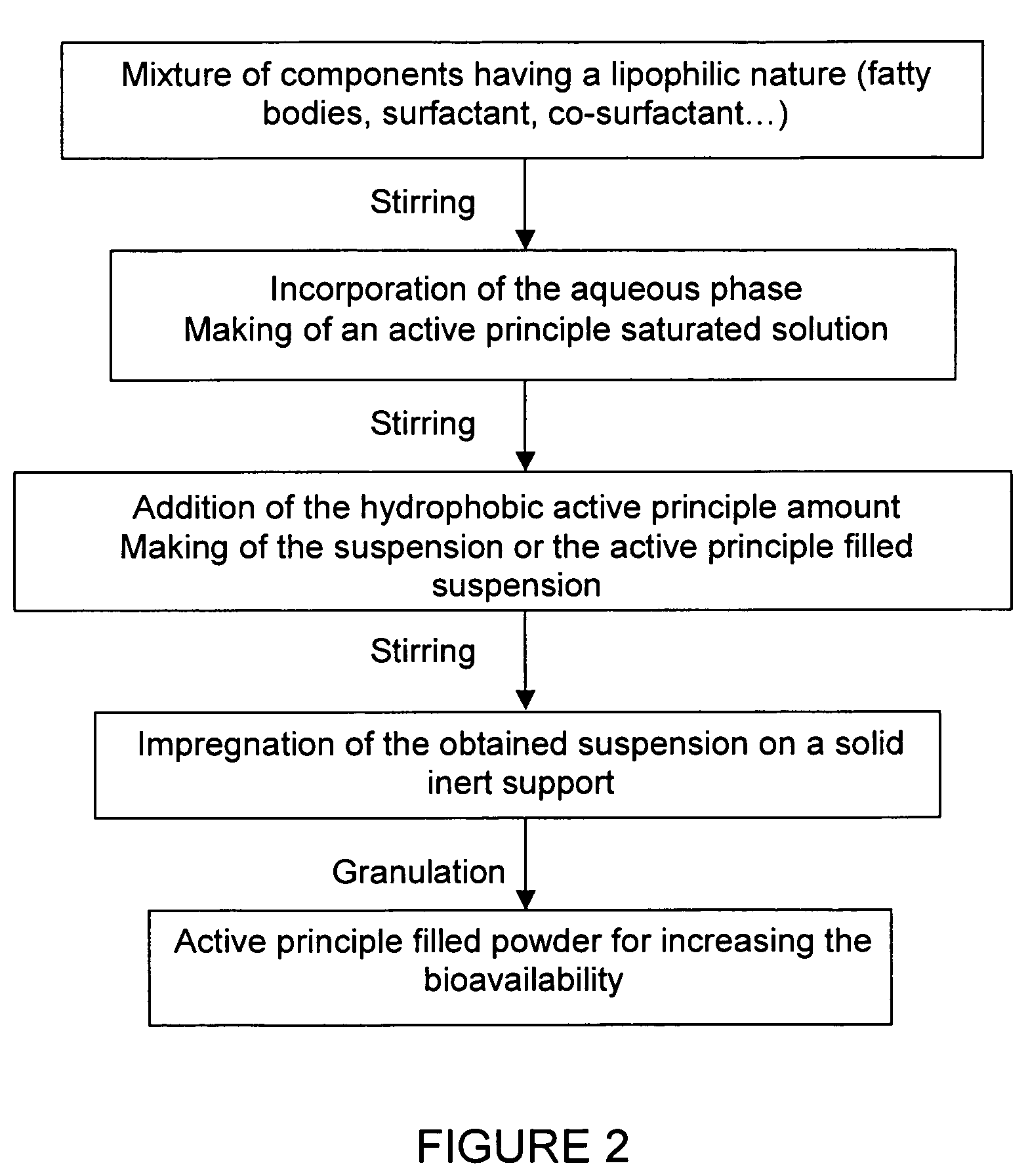
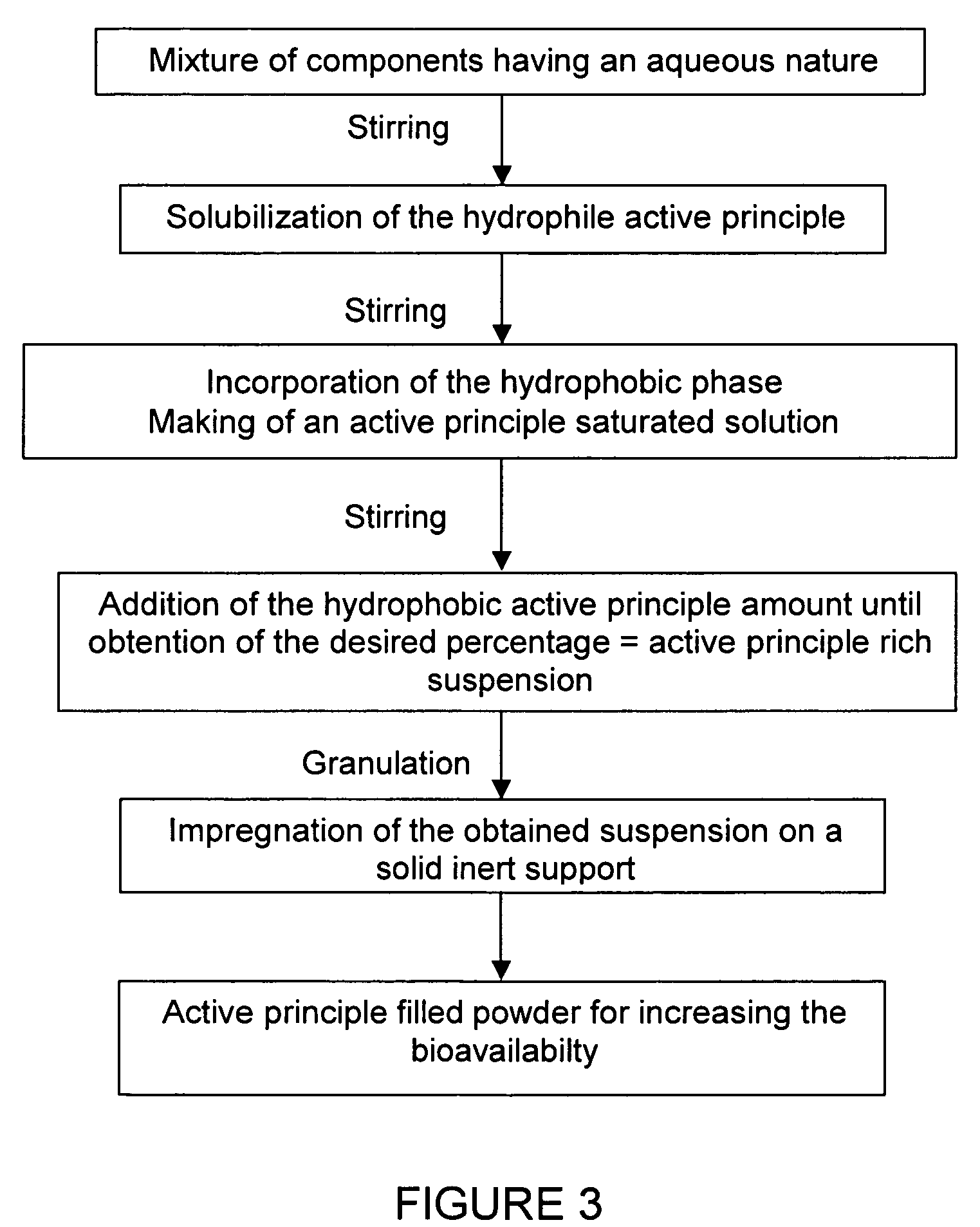
Impregnated powder improving bioavailability and/or solubility and method of production
This invention relates to an impregnated powder for increasing the bioavailabilty and / or the solubility of at least one active principle comprising a solid, inert support in a particle form impregnated by a liquid medium comprising a hydrophobic phase and optionally a hydrophilic phase, at least one surfactant and at least one active principle dissolved in at least one of said phases, wherein said active principle(s) is(are) also present in at least one of said phases in the form of a suspension.Such an impregnated powder is used as a base for various preparations in the pharmaceutical, parapharmaceutical and cosmetic field, in the food complement field and in the food processing industry.
Owner:GALENIX INNOVATIONS
Solid pharmaceutical dispersions with enhanced bioavailability
InactiveUS8263128B2Improve bioavailabilityPreventing and retarding ratePowder deliveryBiocideAcetic acidHydroxypropylmethylcellulose acetate succinate
Spray dried solid dispersions comprising a sparingly soluble drug and hydroxypropylmethylcellulose acetate succinate (HPMCAS) provide increased aqueous solubility and / or biavailability in a use environment.
Owner:BEND RES
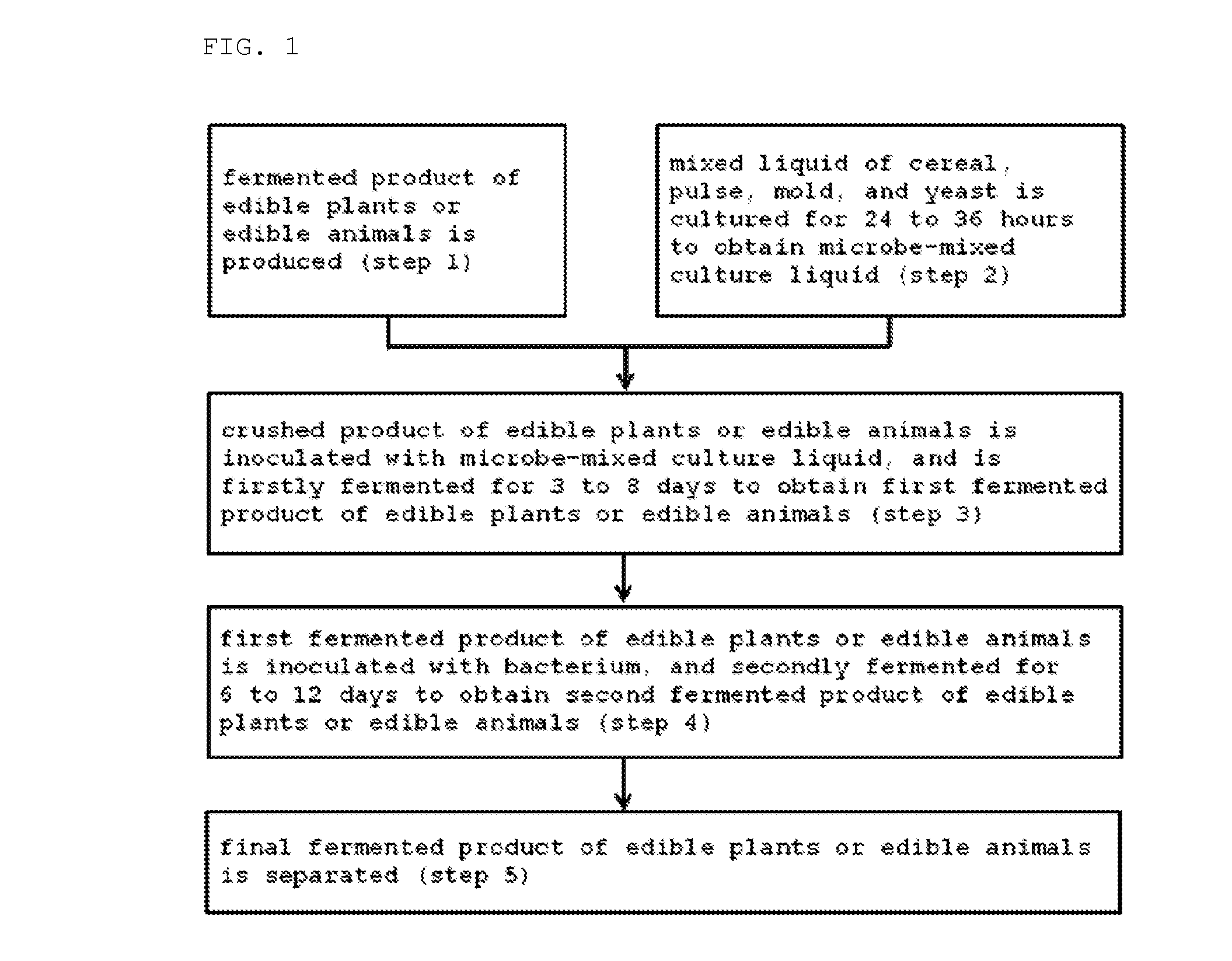
Method for producing fermented edible plants or edible animal/plants, fermented edible plants or edible animal/plants produced by same, and foods containing same
ActiveUS20100316763A1Increase heightPrevent invasionDough treatmentEdible seed preservationFood flavorOrganism
The present invention relates to a method for producing fermented edible plants or edible animal / plants, to fermented edible plants or edible animal / plants produced by same, and to foods containing same. The method for producing fermented edible plants or edible animal / plants includes the steps of: producing crushed edible plants or edible animal / plants; culturing a liquid mixture of grains, saccharides, filamentous fungi, and yeast for 24 to 36 hours to produce a mixed microbial broth; inoculating the edible plants or edible animal / plants with the mixed microbial broth, and firstly fermenting the edible plants or edible animal / plants for 3 to 8 days to produce first fermented edible plants or edible animal / plants; and inoculating the first fermented edible plants or edible animal / plants with bacteria, and secondly fermenting the first fermented edible plants or edible animal / plants for 6 to 12 days to produce second fermented edible plants or edible animal / plants. Whereby, a fermentation period can be shortened, and food deterioration and the growth of pathogenic microorganisms can be suppressed. Further, adding the fermented edible plants or edible animal / plants produced by the above-described method into foods can provide storage stability, increase bioavailability, and improve flavor.
Owner:PHARVIS R&D KOREA
Ascorbic acid salts of organic bases with enhanced bioavailability for synergictic anti-aging and skin protective cosmetic compositions
This invention relates to in-situ preparation, and stable topical delivery systems of ascorbic acid salts of organic bases that provide skin beneficial properties, including reduction in signs of skin aging, anti-wrinkle, anti-oxidant, and photo-protection from UV and sunlight. The formulation avoids the use of oils, minimizes the importance of the pH of the formulation, allows the incorporation of an aqueous solution of ascorbic acid or alkali metal salts of ascorbic acid in the formulation, does not require packaging the formulation in air tight containers, allows the use of large amounts of ascorbic acid, its salts, and its derivatives, and does not require the use of expensive coatings. Moreover, several ascorbic acid derivatives of different chemical composition can be made in a stable topical formulation by the in-situ combination of readily available starting materials in a water solution, despite the understanding well known in the prior art that such compositions in water are inherently unstable. The in-situ method also permits the preparation of novel ascorbic acid derivatives that are not known in the prior art.
Owner:GUPTA SHYAM K
Solid pharmaceutical dispersions with enhanced bioavailability
InactiveUS8257741B2Improve solubilityEffective dispersionPowder deliveryBiocideAcetic acidHydroxypropylmethylcellulose acetate succinate
Spray dried solid dispersions comprising a sparingly soluble drug and hydroxypropylmethylcellulose acetate succinate (HPMCAS) provide increased aqueous solubility and / or biavailability in a use environment.
Owner:BEND RES
Bioavailable curcuminoid formulations for treating alzheimer's disease and other age-related disorders
ActiveUS20090324703A1Improve bioavailabilityBiocideNervous disorderGlucuronide metabolismAntioxidant
Curcuminoid formulations having enhanced bioavailability are provided and comprise a curcuminoid, antioxidant, glucuronidation inhibitor, and water-soluble, pharmaceutically acceptable inhibitor. A method of treating Alzheimer's and other age-related diseases by administering such a composition is also provided.
Owner:RGT UNIV OF CALIFORNIA +1
Forms of CDDO methyl ester
A triterpenoid compound, methyl 2-cyano-3,12-dioxoleana-1,9(11)-dien-28-oate (CDDO methyl ester), has a non-crystalline, glassy solid form and a non-hydrous crystalline form that can prepared, for example, from a saturated methanol solution. The glassy form displays an enhanced bioavailability over the non-hydrous crystalline form. Each form of CDDO methyl ester is a superior candidate for use, typically in solid dosage form, for treating a variety of disease states, generally associated with inflammation.
Owner:REATA PHARM HLDG LLC
Administration of HCV protease inhibitors in combination with food to improve bioavailability
InactiveUS20060281688A1Improve bioavailabilityBiocideDipeptide ingredientsEnzyme Inhibitor AgentEnhanced bioavailability
Methods of treating, preventing or ameliorating one or more symptoms of hepatitis C in a subject comprising the step of administering at least one HCV protease inhibitor in combination with food are provided. Also provided are methods of increasing bioavailability of an HCV protease inhibitor and methods of increasing serum levels of an HCV protease inhibitor in a subject. All methods comprise adminstering at least one HCV protease inhibitor in combination with food, the at least one HCV protease inhibitor selected from the group consisting of compounds of Formulae I-XXVI, described herein. Administration of compounds of the present invention in combination with food provides improved bioavailability and increased peak serum levels of the compounds as compared to administration without food.
Owner:MERCK SHARP & DOHME CORP
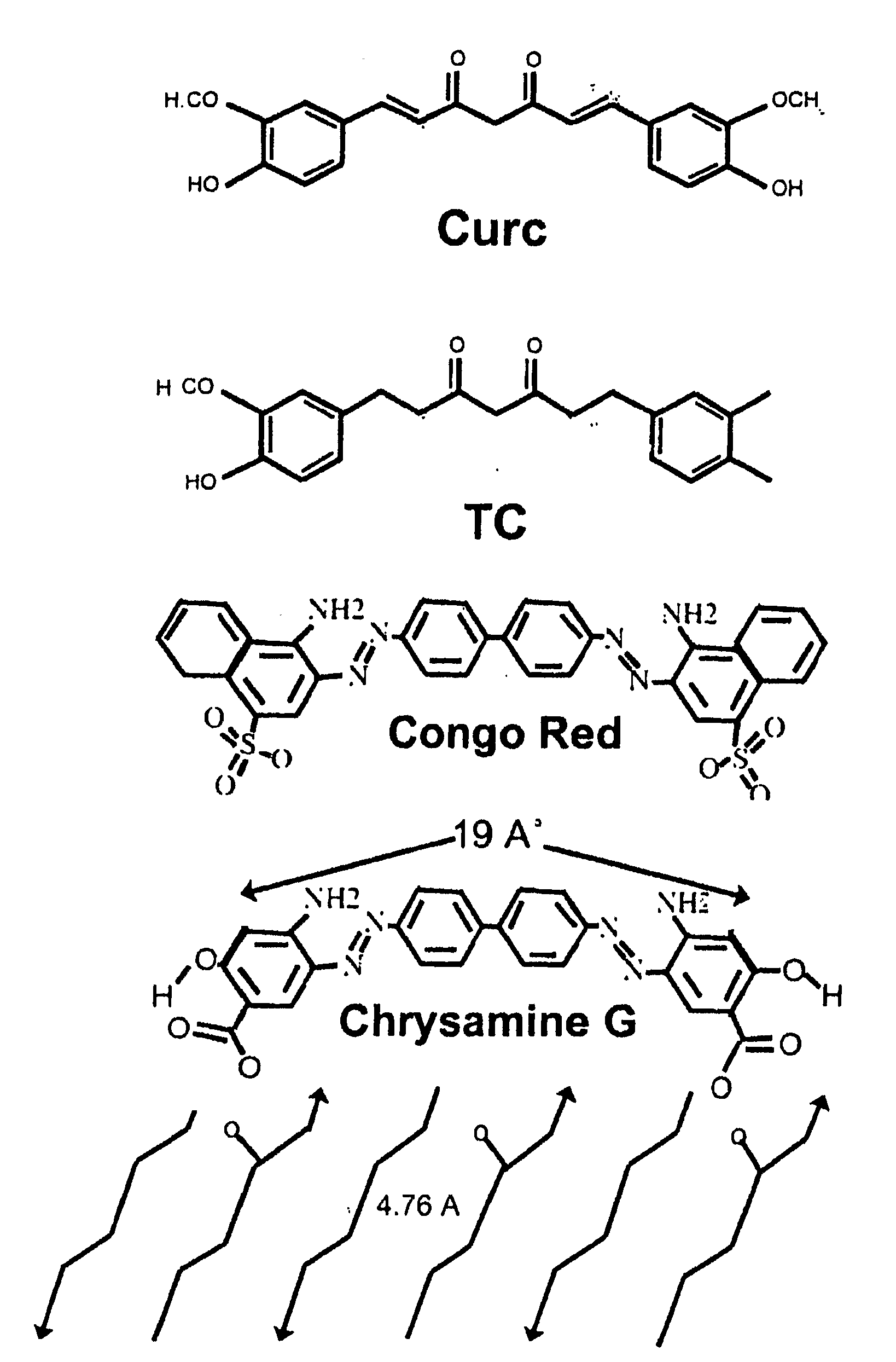
Curcumin cyclodextrin combination for preventing or treating various diseases
Curcumin has shown anti-inflammatory and anti-angiogenic properties that could be useful in treating various diseases such as those of rheumatology and oncology. However, curcumin is very poorly absorbed and has a very low bioavailability. This patent describes a method of increasing the delivery of curcumin by complexing it with cyclodextrins. Cyclodextrins are well known in the food industry and have been used to carry other drugs to increase bioavailability. The new combination of cyclodextrins and curcumin has been tested in pre-clinical inflammation models where it has demonstrated efficacy superior to both the positive control and curcumin.
Owner:DESAI KETAN
Novel forms of cddo methyl ester
A triterpenoid compound, methyl 2-cyano-3,12-dioxoleana-1,9(11)-dien-28-oate (CDDO methyl ester), has a non-crystalline, glassy solid form and a non-hydrous crystalline form that can prepared, for example, from a saturated methanol solution. The glassy form displays an enhanced bioavailability over the non-hydrous crystalline form. Each form of CDDO methyl ester is a superior candidate for use, typically in solid dosage form, for treating a variety of disease states, generally associated with inflammation.
Owner:REATA PHARM HLDG LLC
Water-soluble formulations of fat soluble vitamins and pharmaceutical agents and their applications
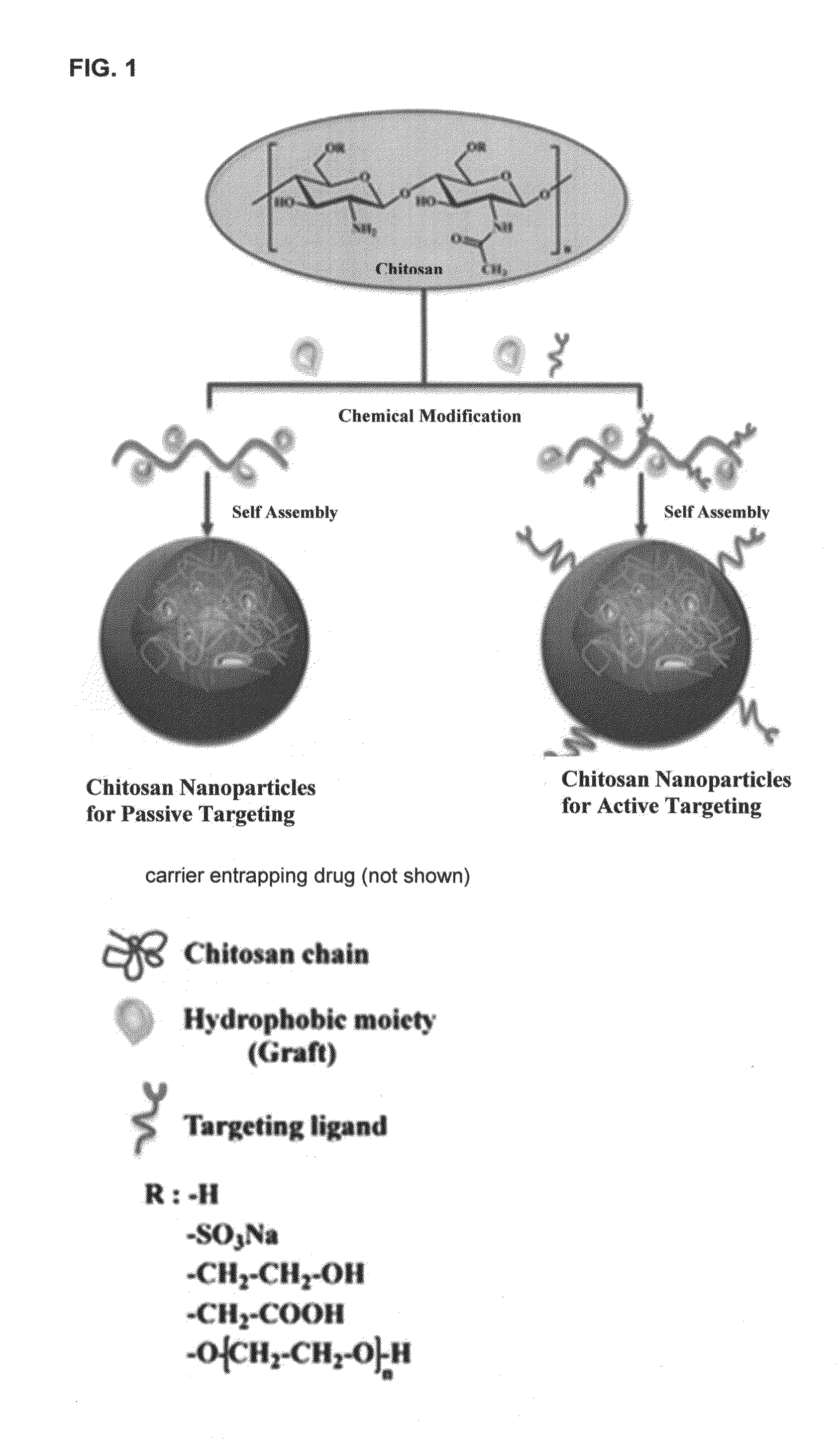
Aqueous liquid or gel formulations of fat soluble vitamins, essential nutrients and other pharmaceutical agents have enhanced concentration of the active components relative to known compositions and therefore have enhanced bioavailability. The aqueous solutions or gels form a free flowing powder when they are absorbed on a suitable pharmaceutically acceptable solid carrier, such as silicon dioxide, maltodextrin, magnesium oxide, aluminum hydroxide, magnesium trisilicate, starch or sugars, or encapsulated by polymers such as gelatin, pectin, chitosan and the like.
Owner:MICELLE PRODS
Matrix compositions for controlled delivery of drug substances
InactiveUS20100166866A1Improve solubilityImprove oral bioavailabilityPowder deliveryBiocidePolyethylene oxidePEG-PLGA-PEG
Owner:EGALET LTD
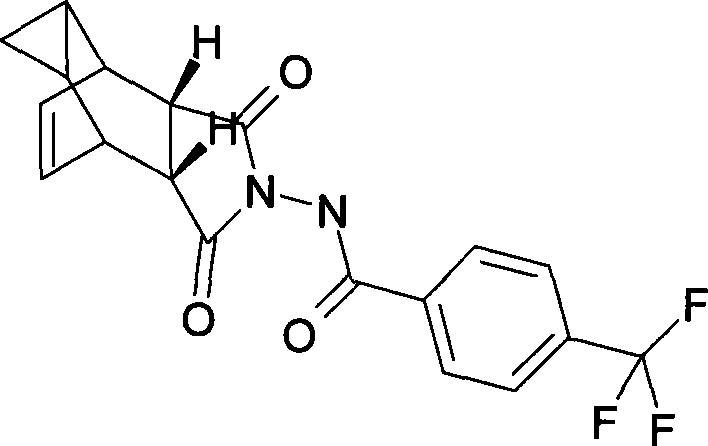
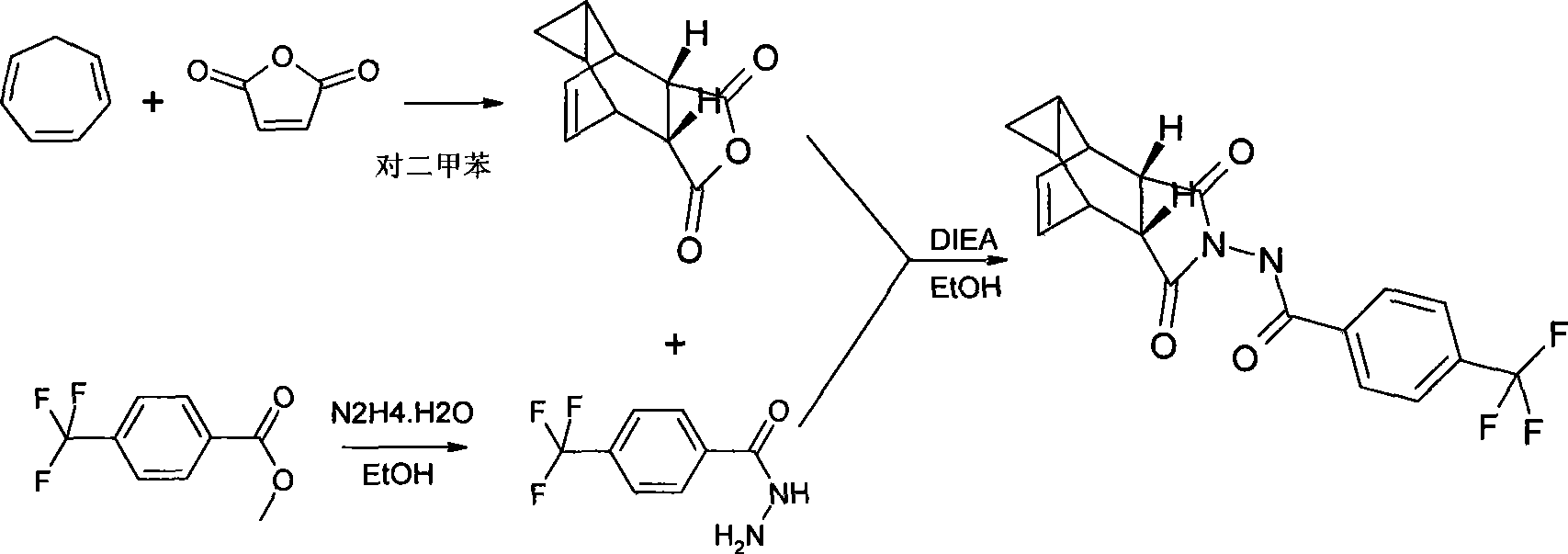
Compound ST-246 containing a crystal water, crystal thereof and preparation method thereof
ActiveCN101445478AStable structurePromote crystallizationOrganic active ingredientsOrganic chemistryOrganic baseTropane
The invention discloses a compound ST-246 containing a crystal water, known as ST-246.H2O. The ST-246.H2O is prepared according to the following method: in the presence of organic base and organic solvent and being under protection of nitrogen, tropane anhydride and p-trifluoromethyl benzoylhydrazine are heated and return flow and reaction solution is cooled and filtered, thereby obtaining the ST-246.H2O. The ST-246.H2O prepared by the method is steady at room temperature, is difficult to lose the crystal water or absorb moisture, is difficult to agglomerate after micronization and is beneficial for improving bioavailability. The compound can be used for preparing anti-poxvirus medicines.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Self microemulsifying preparation of curcumin and its preparing process
ActiveCN1682701AImprove solubilityPromote absorptionOrganic active ingredientsAntipyreticSolubilitySorbent
The self microemulsified curcumin preparation consists of curcumin, surfactant, co-surfactant, oil phase and solid adsorbent. The self microemulsified curcumin preparation may be prepared into capsule or granule for orally taking, and the capsule or granule under the action of gastrointestinal fluid may be self microemulsified to form liquid drops with size below 100 nm. Therefore, the present invention has increased curcumin solubility and absorption in gastrointestinal tract and raised bioavailability.
Owner:山东淄博新达制药有限公司
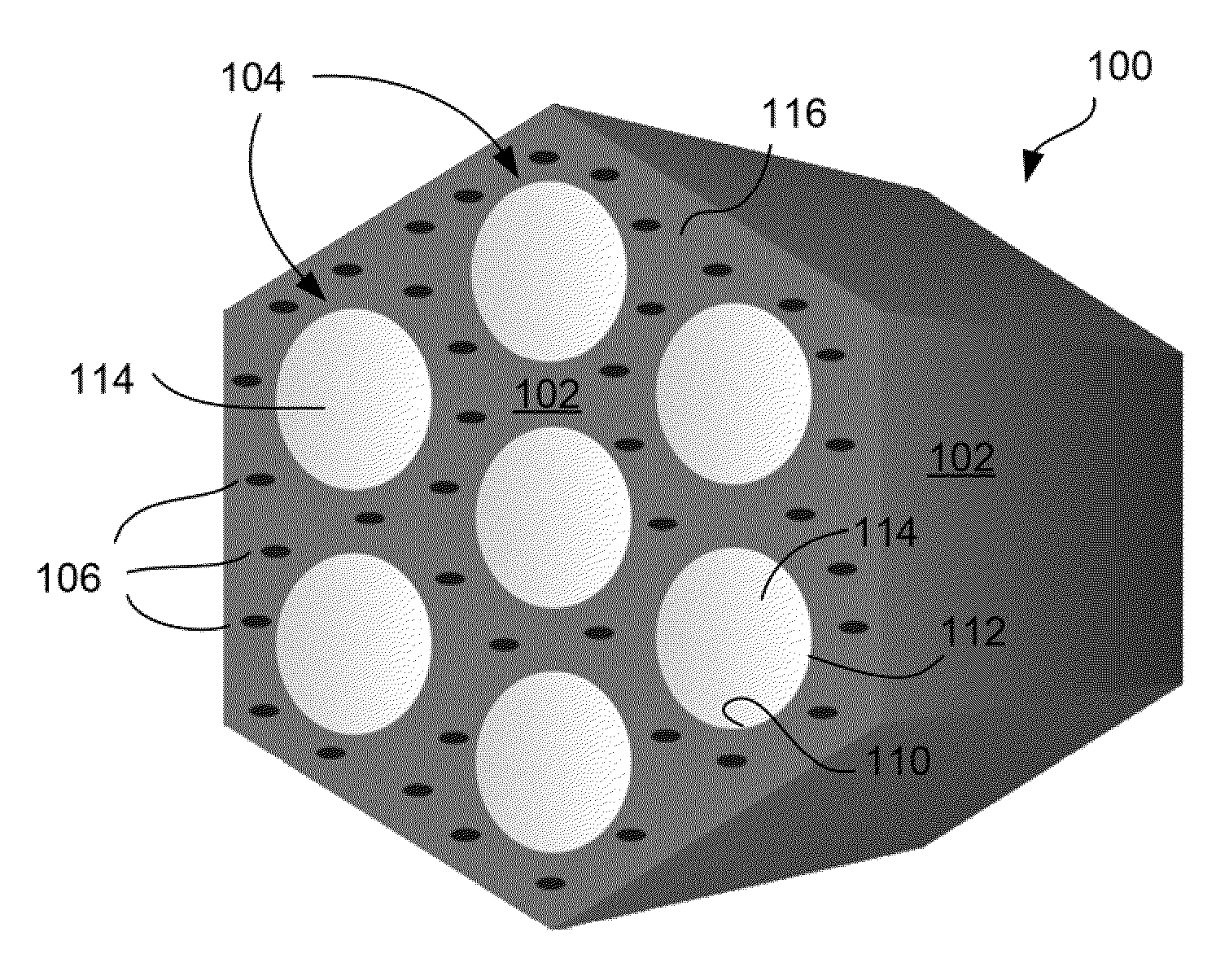
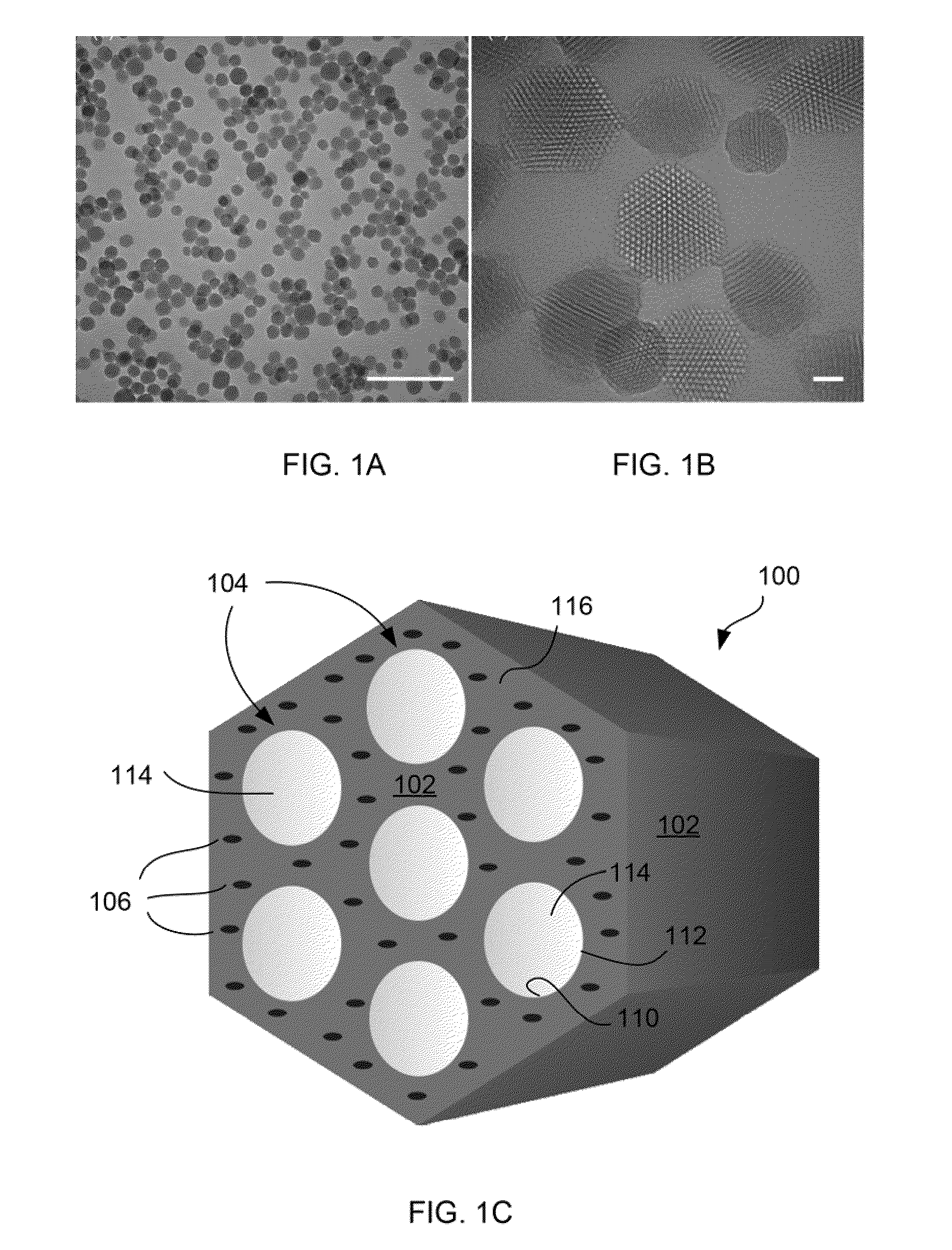
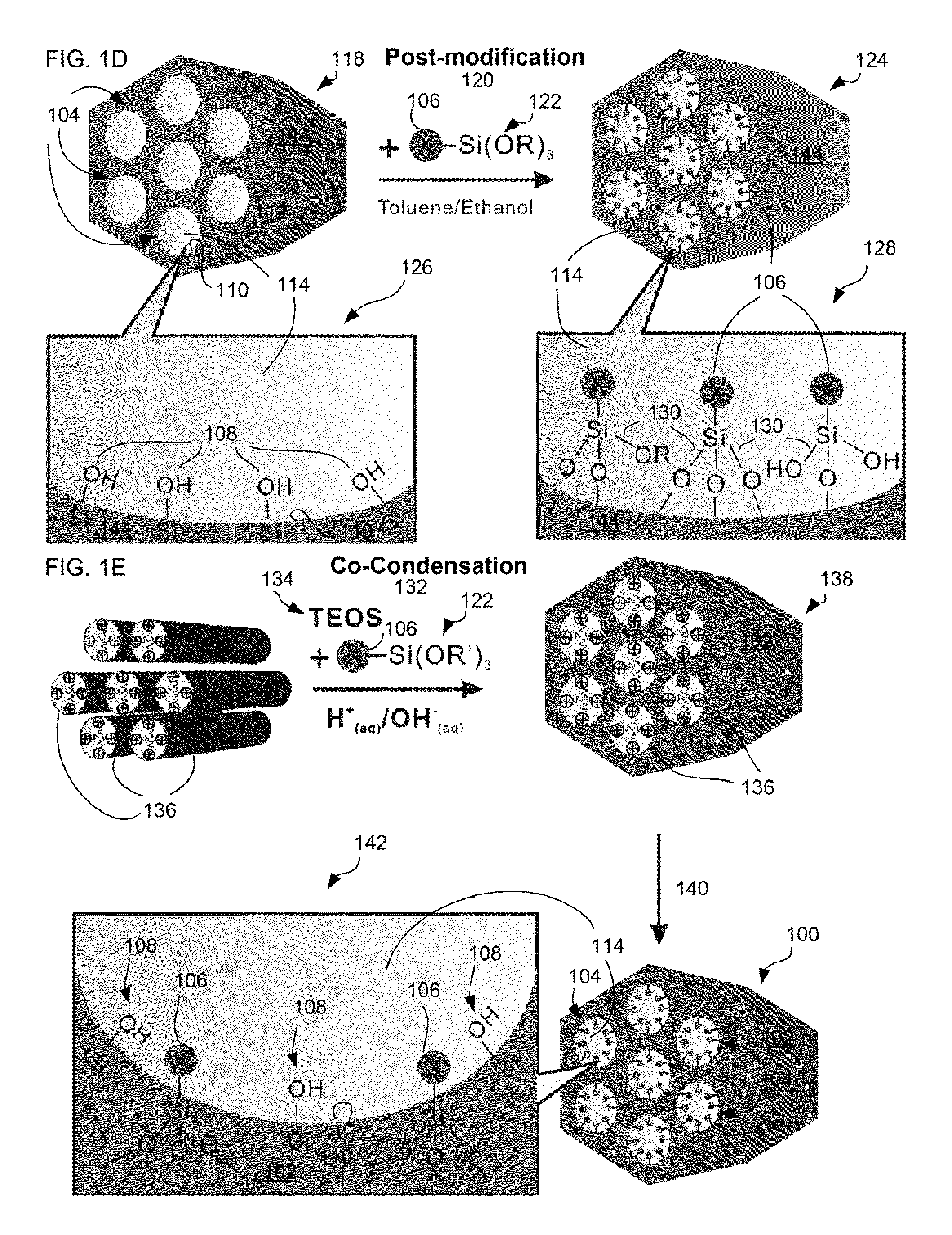
Charged mesoporous silica nanoparticle-based drug delivery system for controlled release and enhanced bioavailability
A charged mesoporous silica nanoparticle (MSN)-based drug delivery system for controlled release and enhanced bioavailability is disclosed. The system comprises a positively charged MSN, which has a silica matrix and an array of pores and / or nanochannels in the matrix. The entire substance of the matrix, all the surfaces and the pores and / or nanochannels comprise a plurality of silanol (Si—OH) and quaternary ammonium functional groups. The bioavailability of a negatively charged bioactive compound can be increased by loading it into the pores and / or nanochannels. The silanol (Si—OH) functional groups on the surfaces lining the walls of the pores and / or nanochannels are free to deprotonate in a fluid having pH above the pI of the positively charged MSN and lead to a sustained release of the negatively charged drug from the pores and / or nanochannels, and thereby enhance the bioavailability of the drug.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
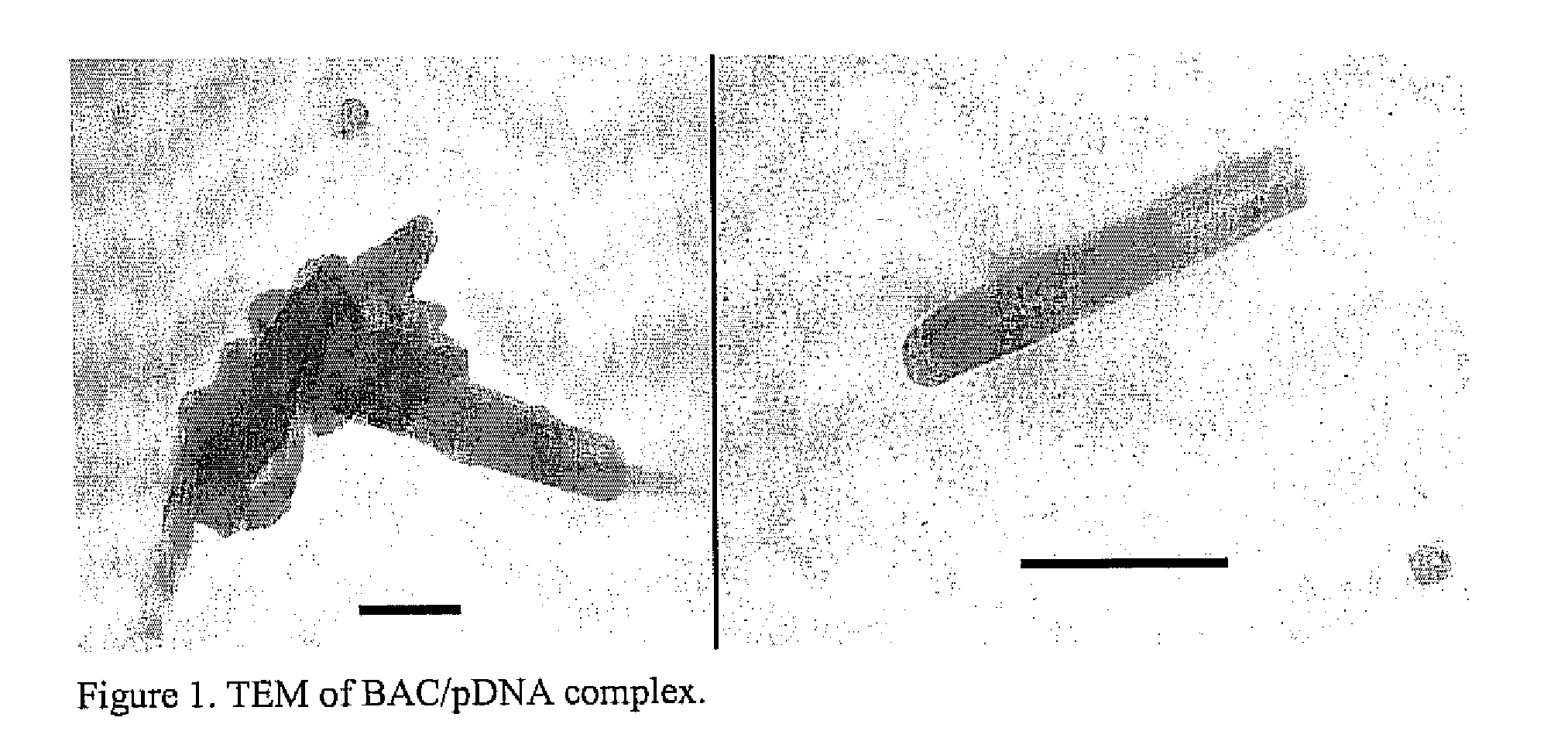
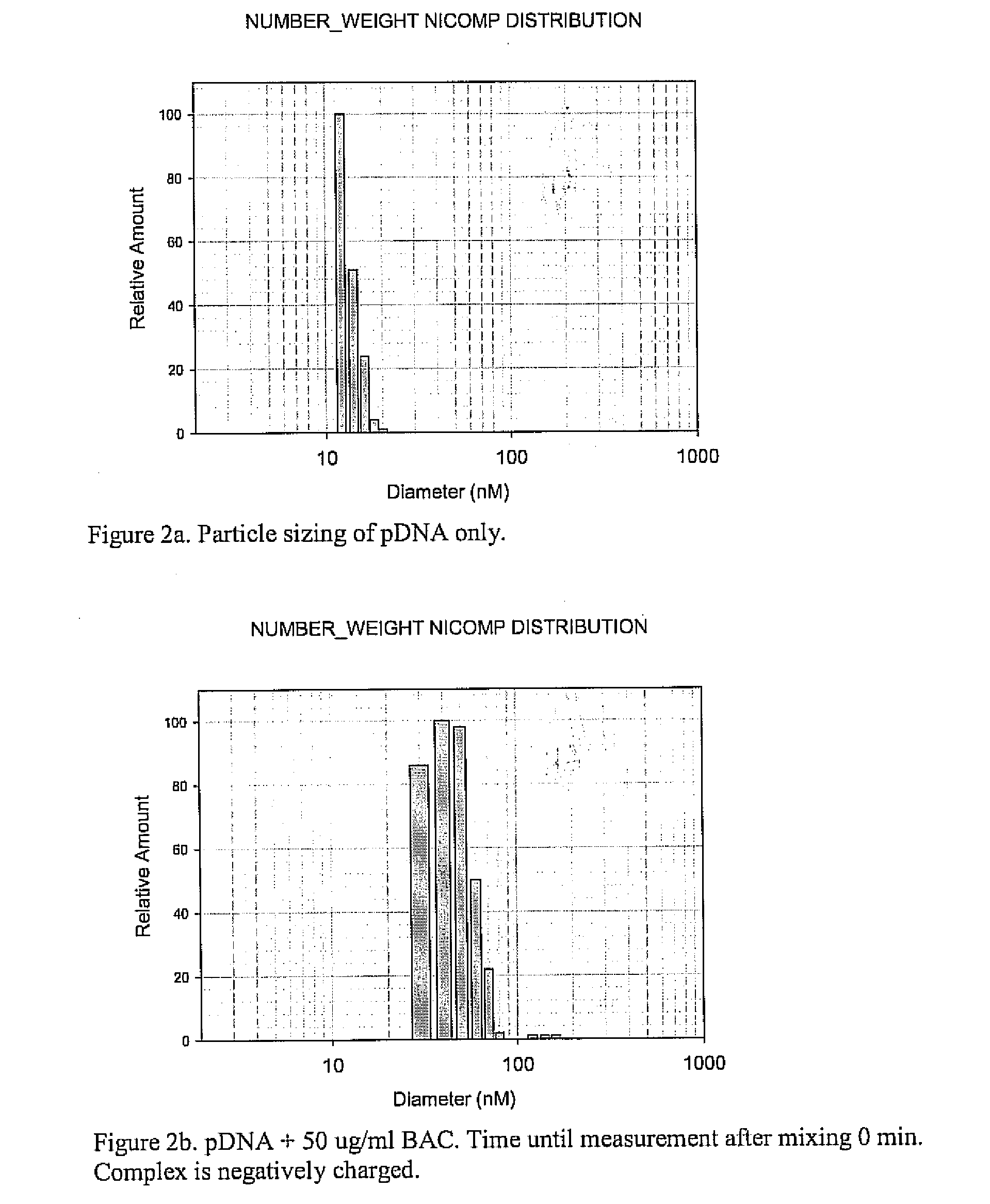
Methods and compositions of gene delivery agents for systemic and local therapy
A method is provided for the delivery of a therapeutic to epithelial cells through the use of a bile acid conjugated to a peptide, the peptide being ionically charged at physiological pH. The complex is well suited for oral and other forms of therapeutic administration of therapeutic drugs known in the art in order to exact systemic and / or localized effect. Intestinal epithelial cells, as well as non-epithelial cells within the gastrointestinal tract and other target cells receive with greater efficiency a charged therapeutic when delivered with an oppositely charged bile acid conjugate (BAC) through oral administration, direct injection, or infusive administrations, thereby increasing bioavailability.
Owner:HILFINGER JOHN +2
Capsule containing active substance pellets
InactiveUS20050058704A1Reduced activityConvenient and comfortable for consumerMetabolism disorderMicrocapsulesControlled releaseMedicine
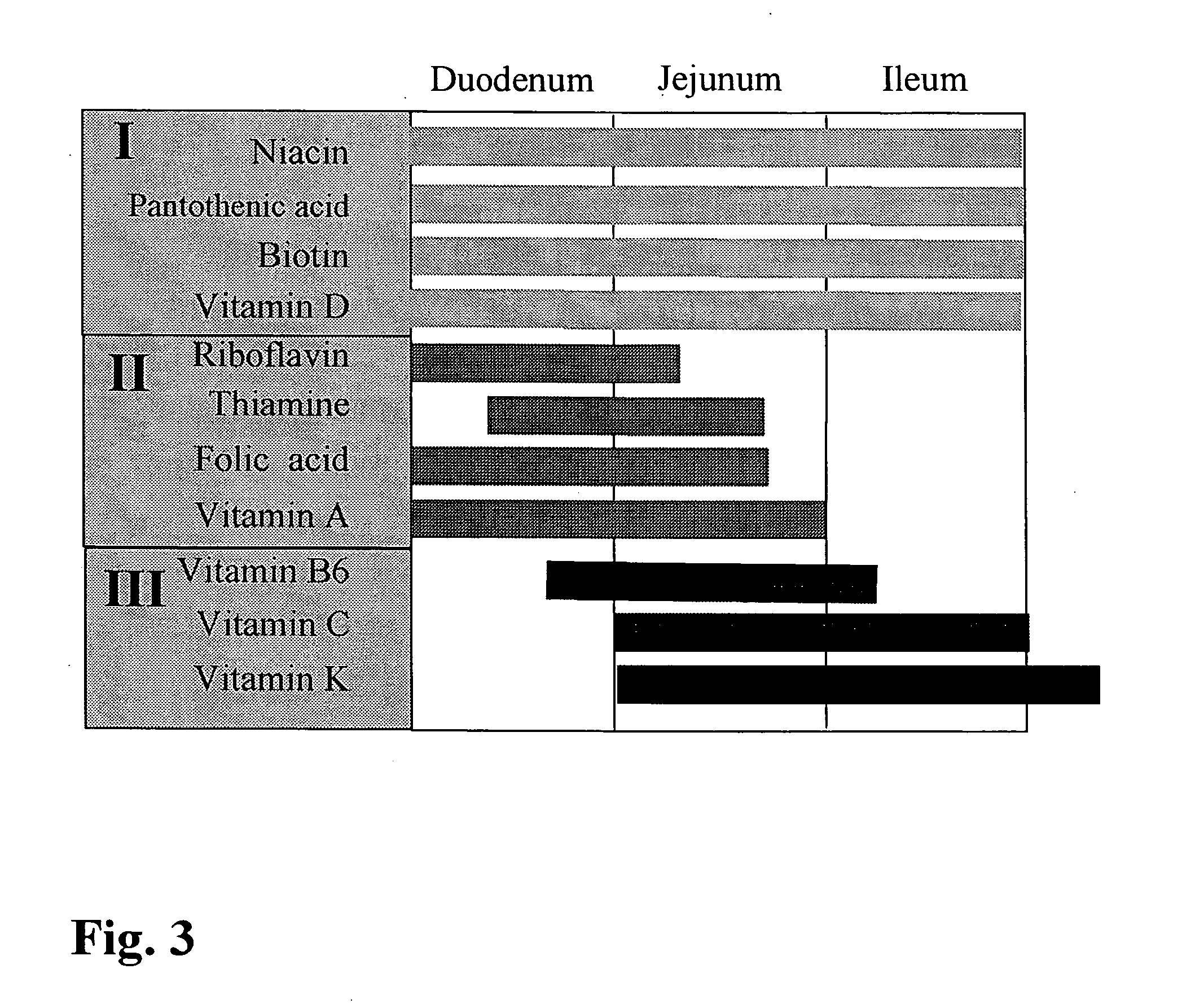
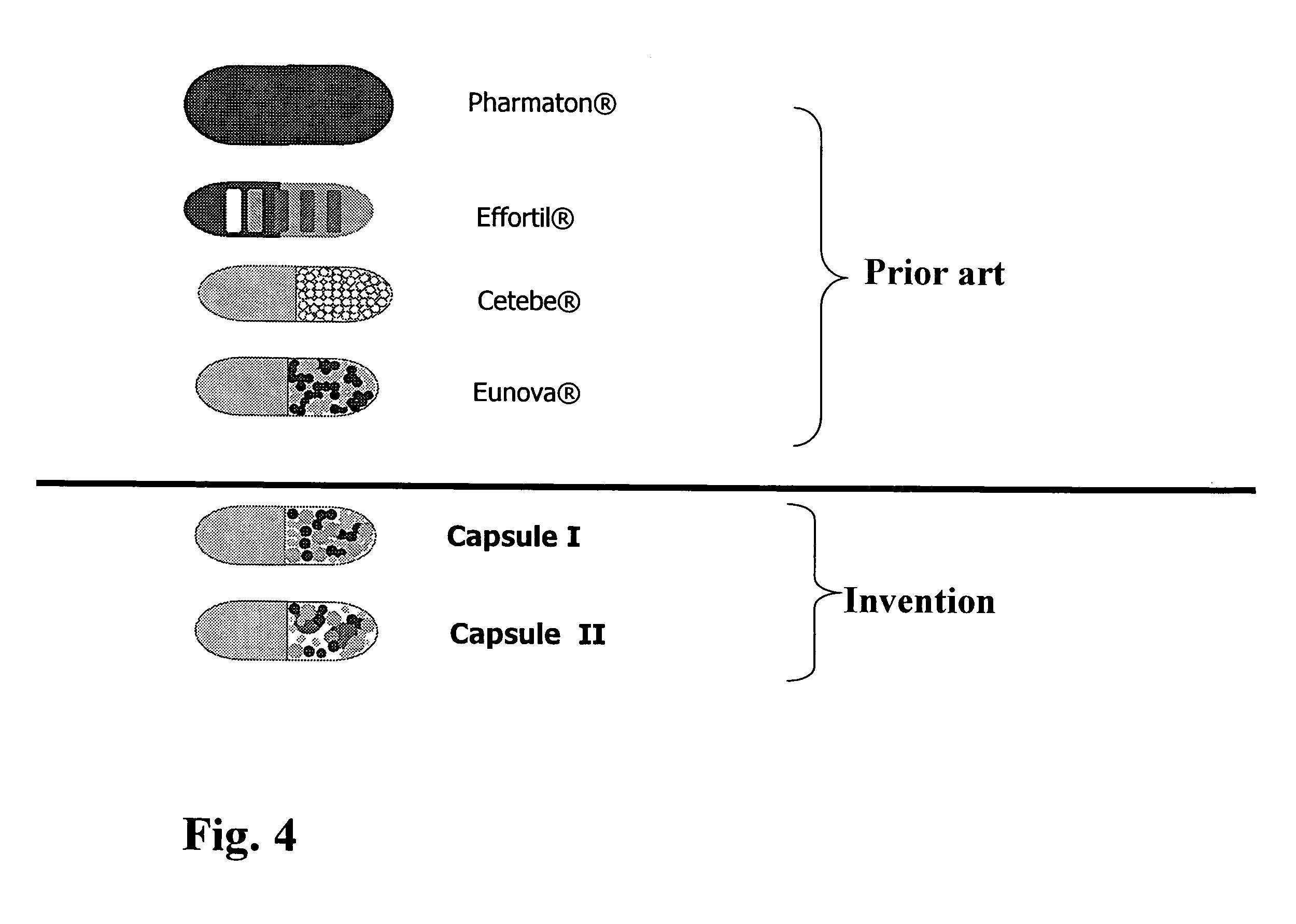
The invention relates to a capsule containing different active substance pellets with at least two different active substances which differ in their release profile in the gastro-intestinal tract, these active substances being selected from among the vitamins, minerals, trace elements, unsaturated fatty acids, amino acids and / or plant extracts and substances. The different release profiles represent rapid, moderate and / or slow dissolving of the active substance pellets. This controlled release leads to a targeted absorption of the active substances in the different absorption areas of the gastro-intestinal tract. Using the preparation provided according to the invention it is also possible to improve bioavailability to a high degree even when a large number of active substances are present.
Owner:PHARMATON
Oral formulations of chemotherapeutic agents
InactiveUS20150231069A1Increase probabilityEffectively infiltrate across the inflamed leakyPowder deliveryOrganic active ingredientsCyclosporinsEnhanced absorption
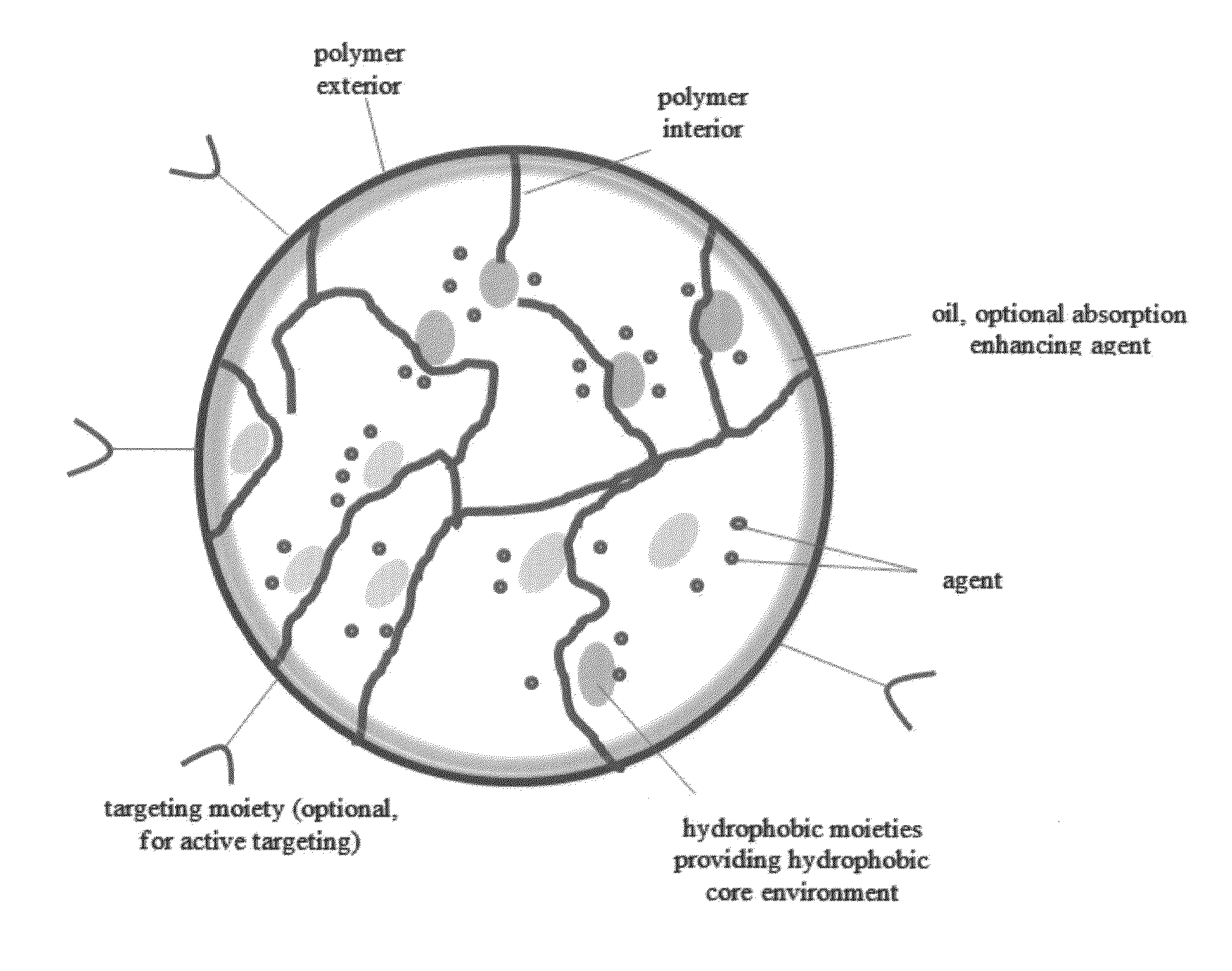
A composition and method of using the composition for treating a patient in need thereof, the composition comprising an oral formulation for enhanced bioavailability of therapeutic agents such as the taxane chemotherapeutic agents. The composition comprises the therapeutic agent and an absorption enhancing agent either co-administered with the agent or administered separately, the therapeutic agent in a polymer matrix resulting in a microbead and including an edible oil resulting in an emulsion. The absorption enhancing agent is a cyclosporin in one embodiment. The absorption enhancing agent is a P glycoprotein inhibitor in another embodiment.
Owner:MODI PANKAJ
Micelle encapsulation of therapeutic agents
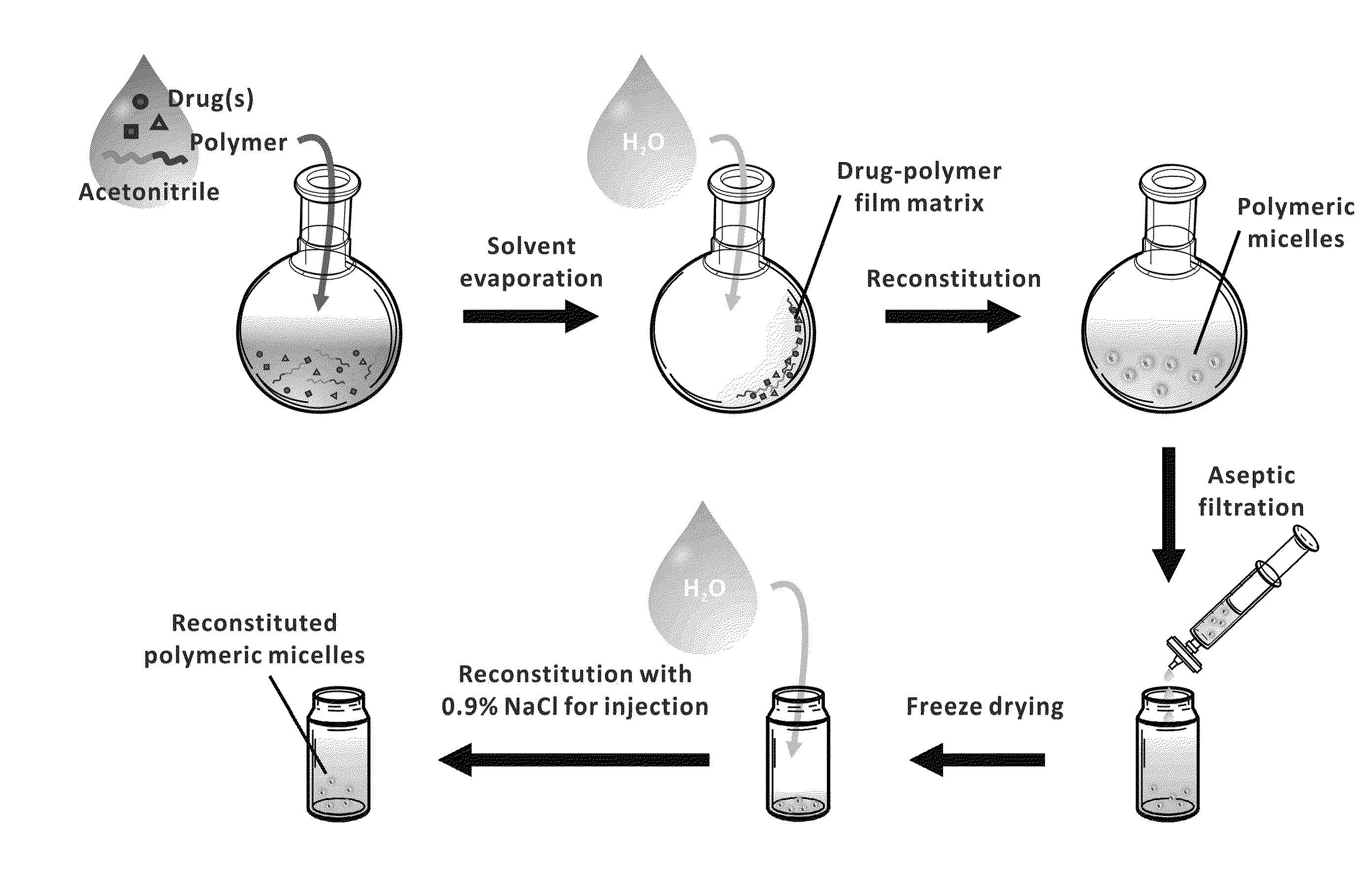
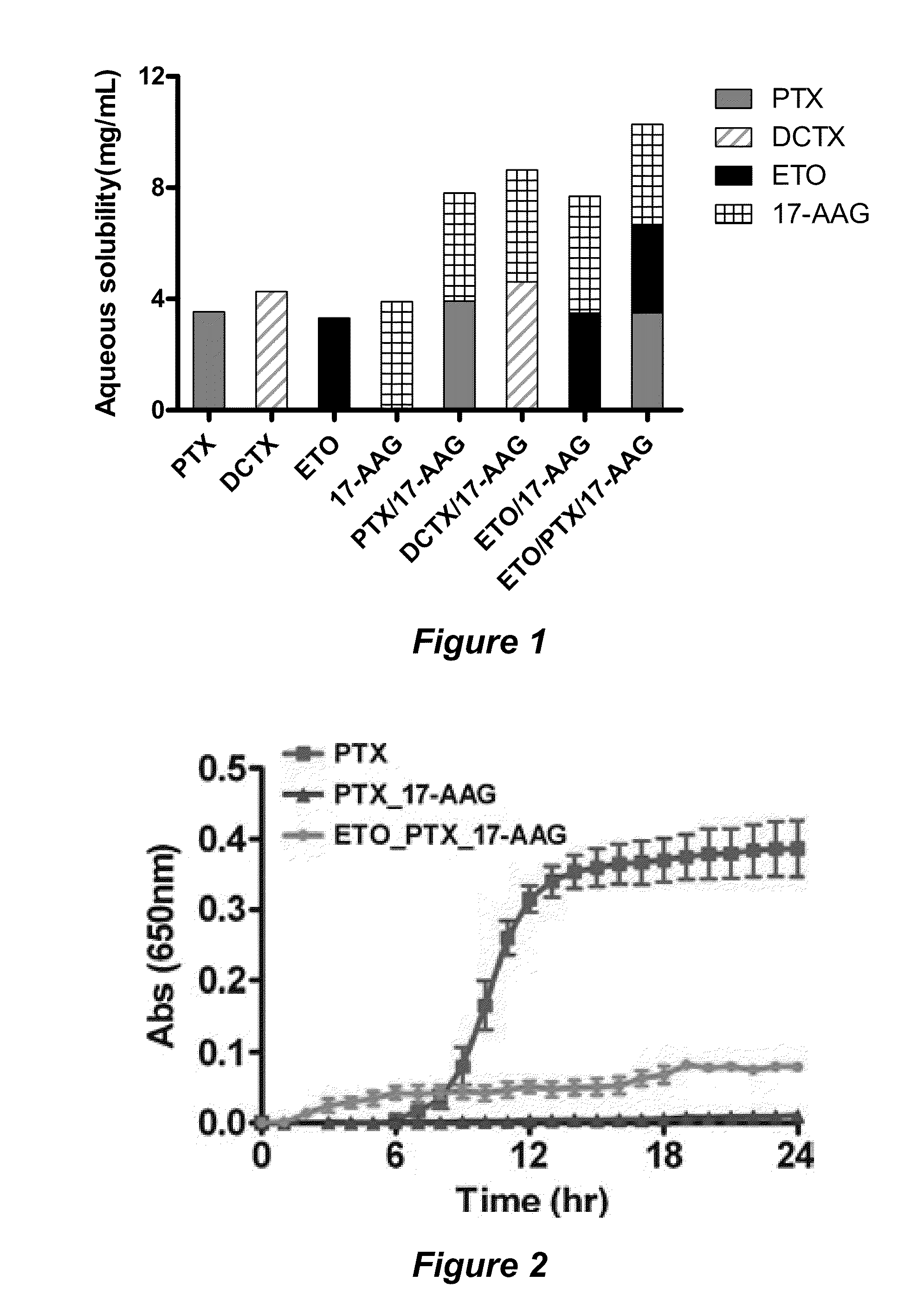
ActiveUS20110076308A1Effective dissolutionToxic reductionBiocideDispersion deliveryLarge doseActive agent
The invention provides active agents, such as paclitaxel, rapamycin, or 17-AAG, encapsulated by safe poly(ethylene glycol)-block-poly(lactic acid) (“PEG-b-PLA”) micelles. The compositions provide effective solubilization of drug combinations, such as paclitaxel, rapamycin, and 17-AAG, as well as others described herein. A significant advantage of PEG-b-PLA as a carrier is that it is less toxic than Cremophor® EL or DMSO, which are used in currently known compositions. Additionally, PEG-b-PLA micelles are easier to handle than DMSO and they do not possess a foul odor, which is a problem with formulations currently in clinical trials. Accordingly, the invention provides stable and biocompatible drug formulations that improve bioavailabilty without causing toxicity. It was also found that larger doses of individual drugs in micelle formulations can be administered compared to non-micelle formulations.
Owner:WISCONSIN ALUMNI RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com