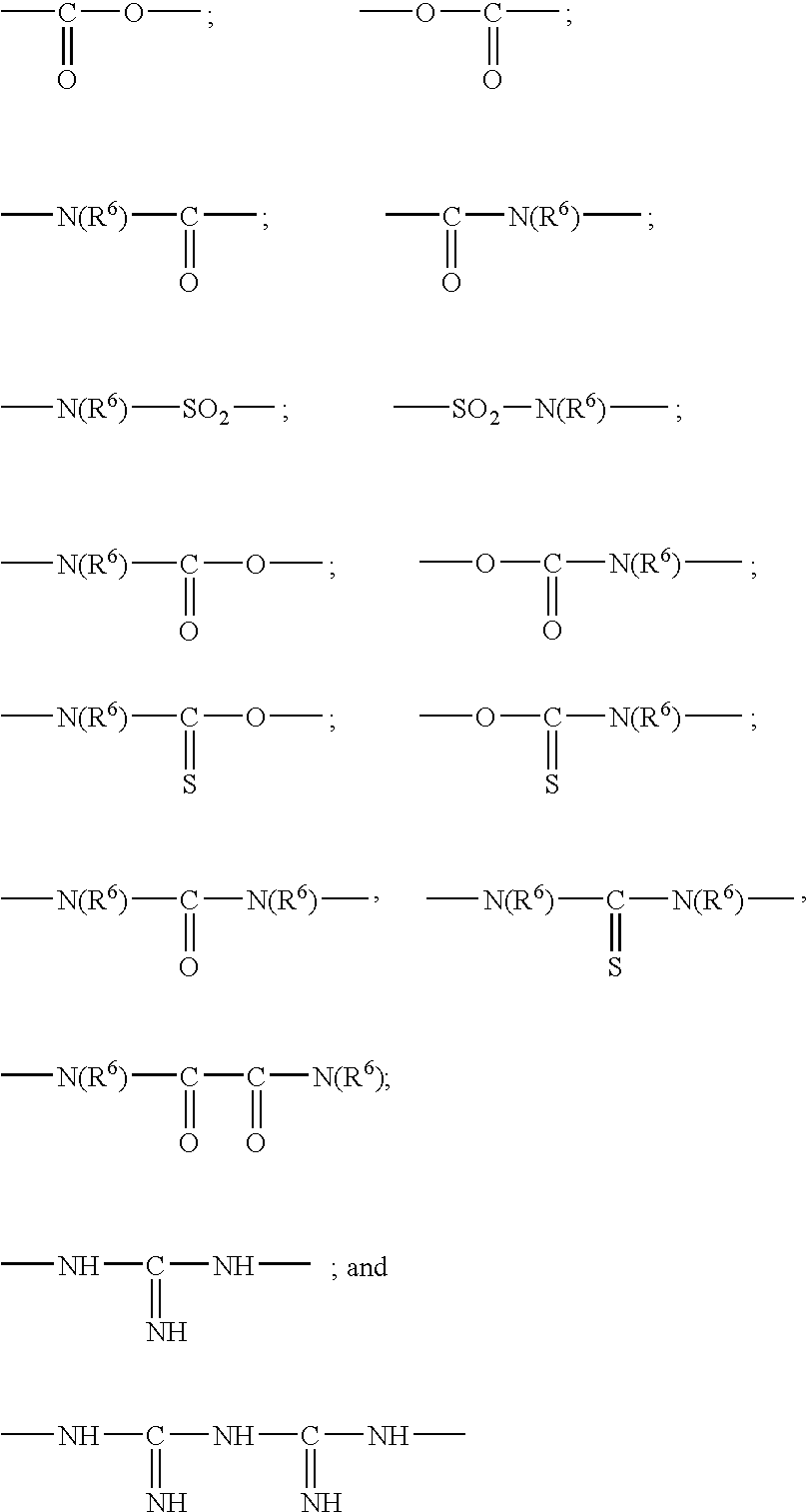Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
849results about How to "Good disintegration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
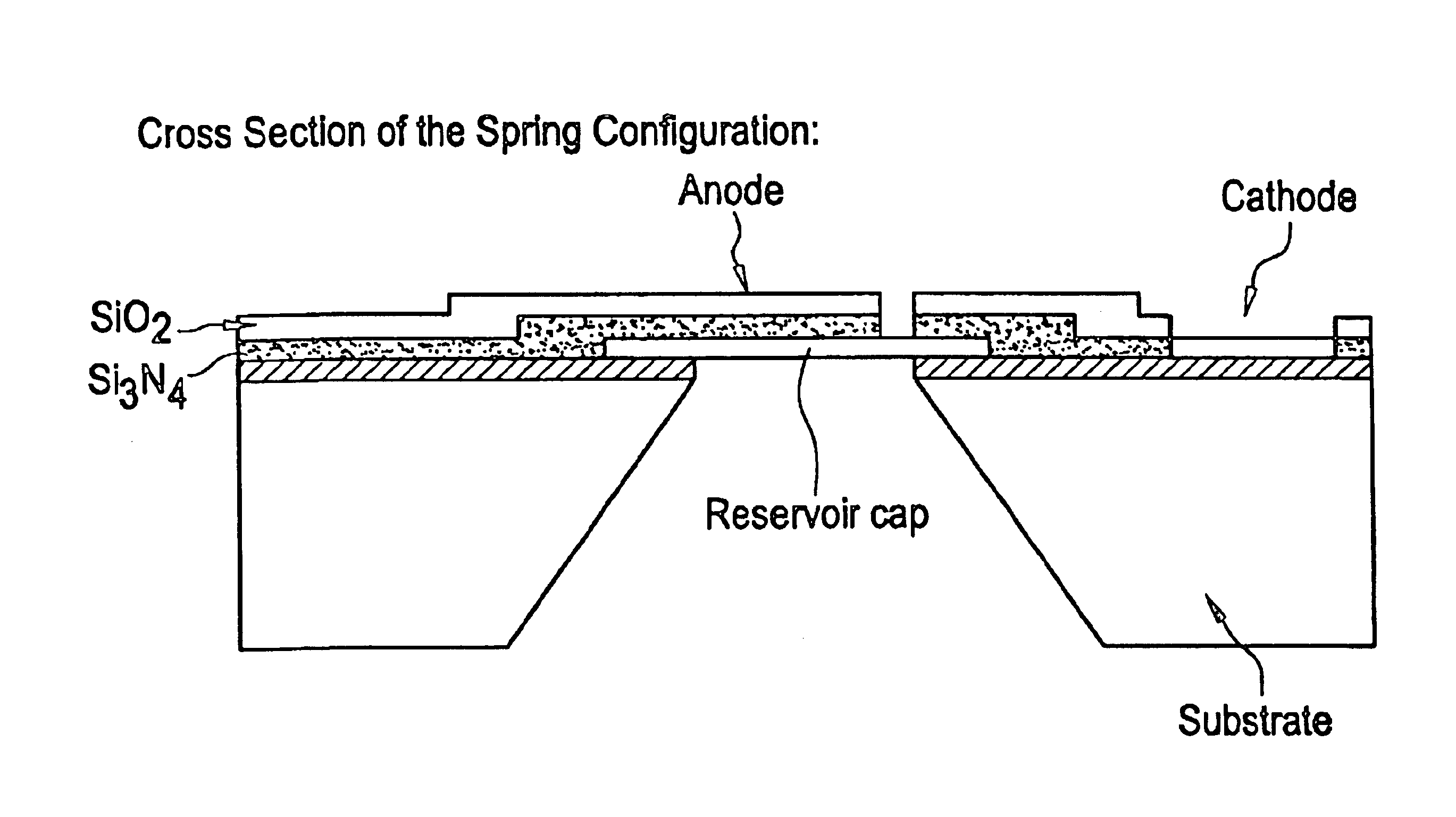
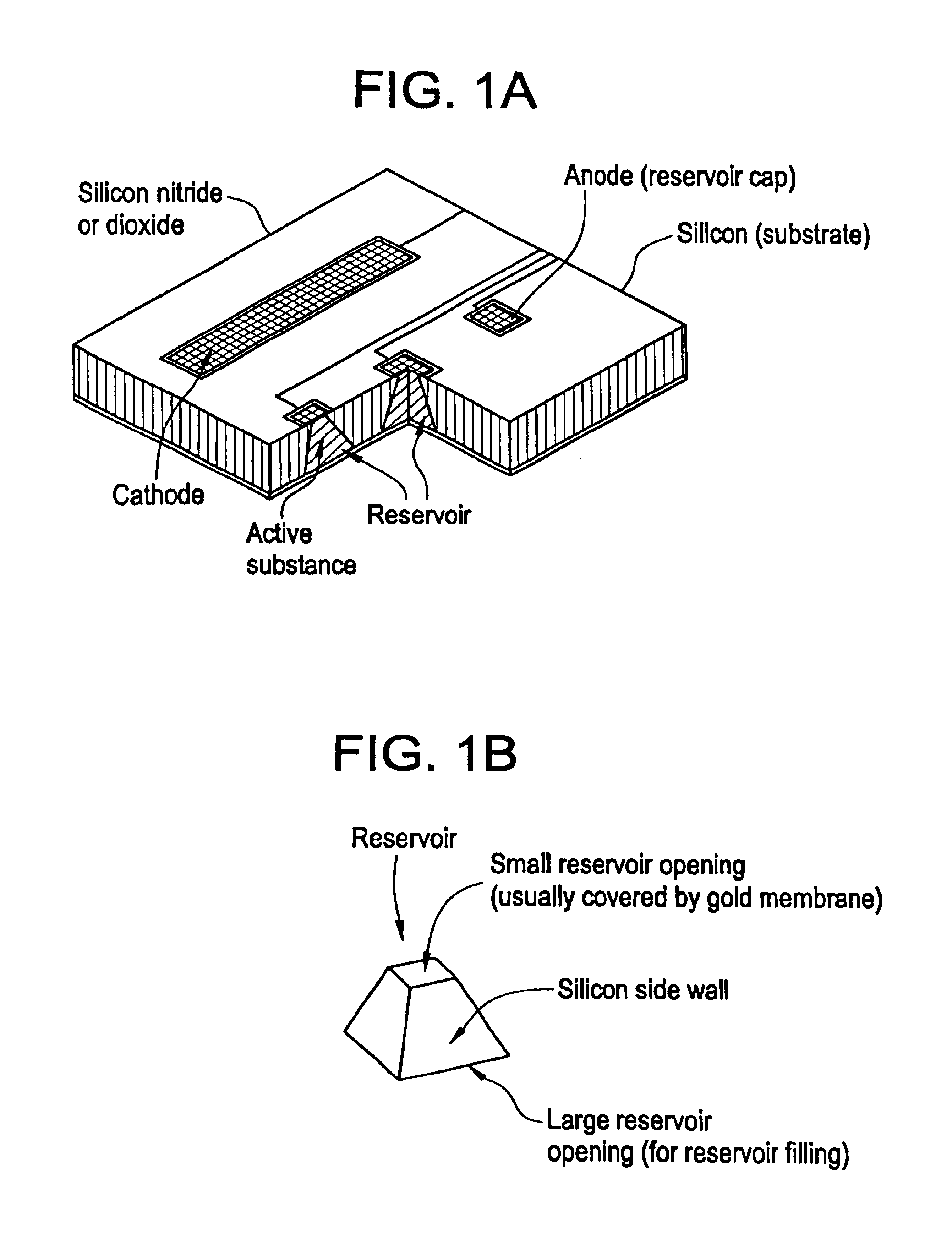
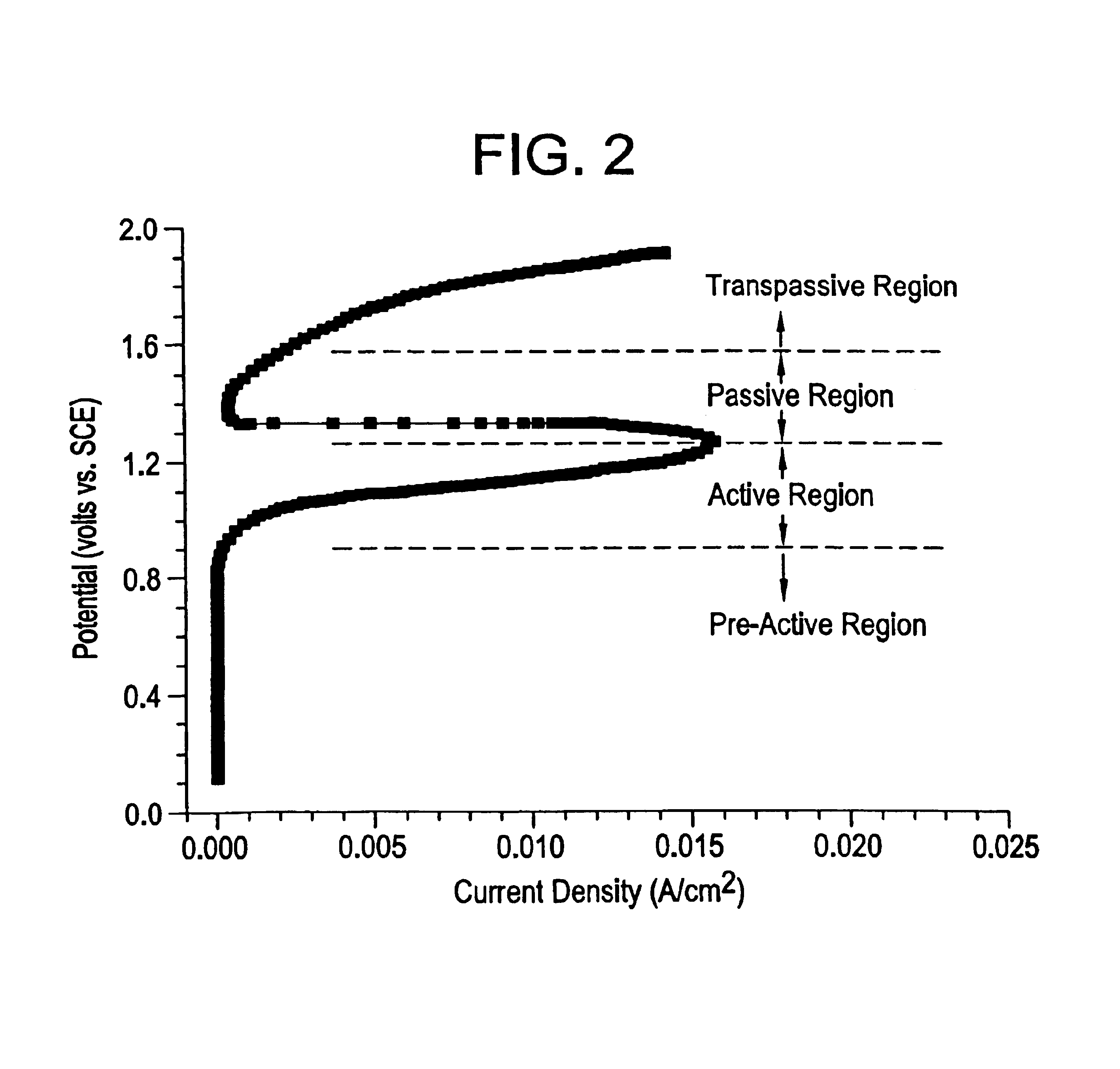
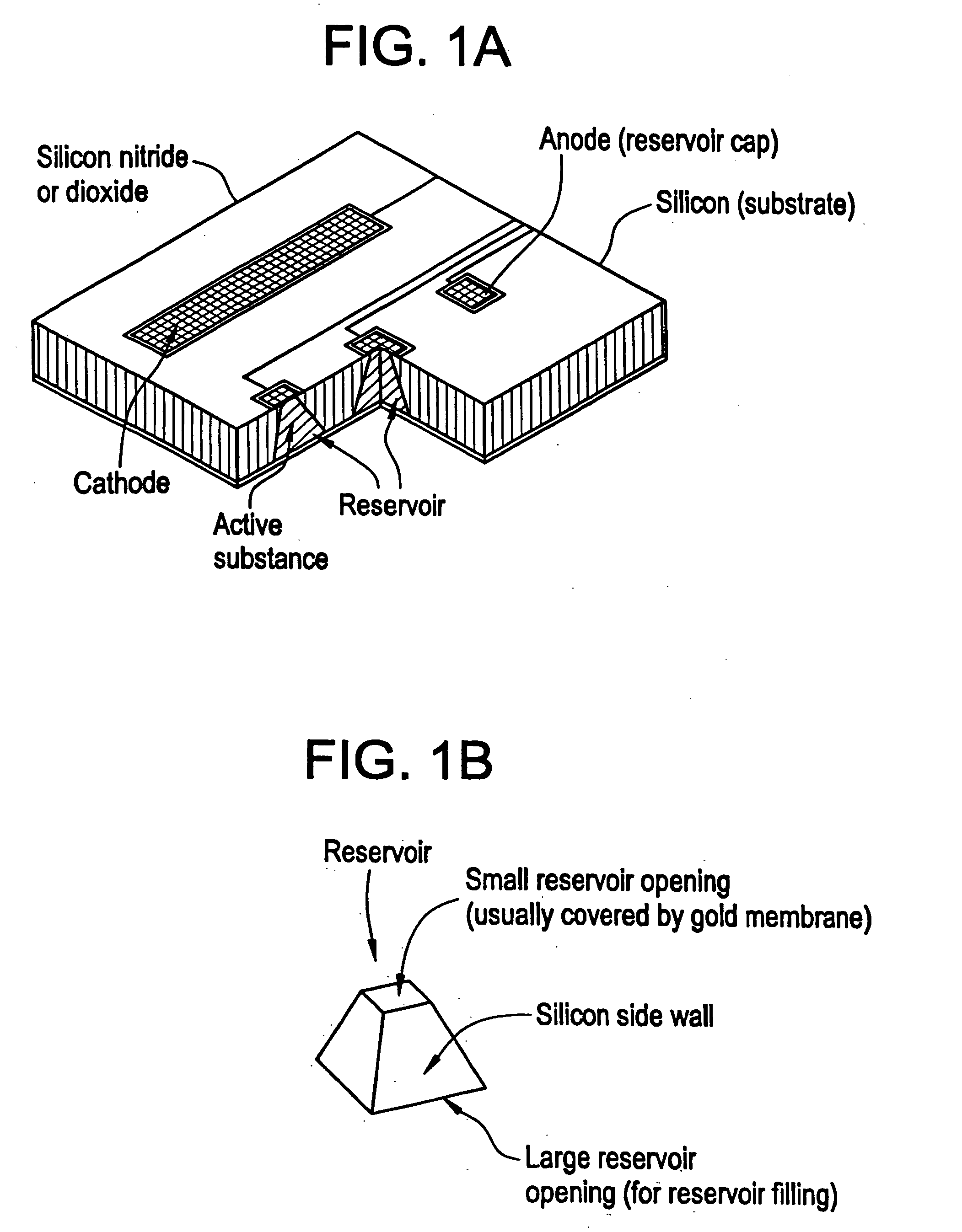
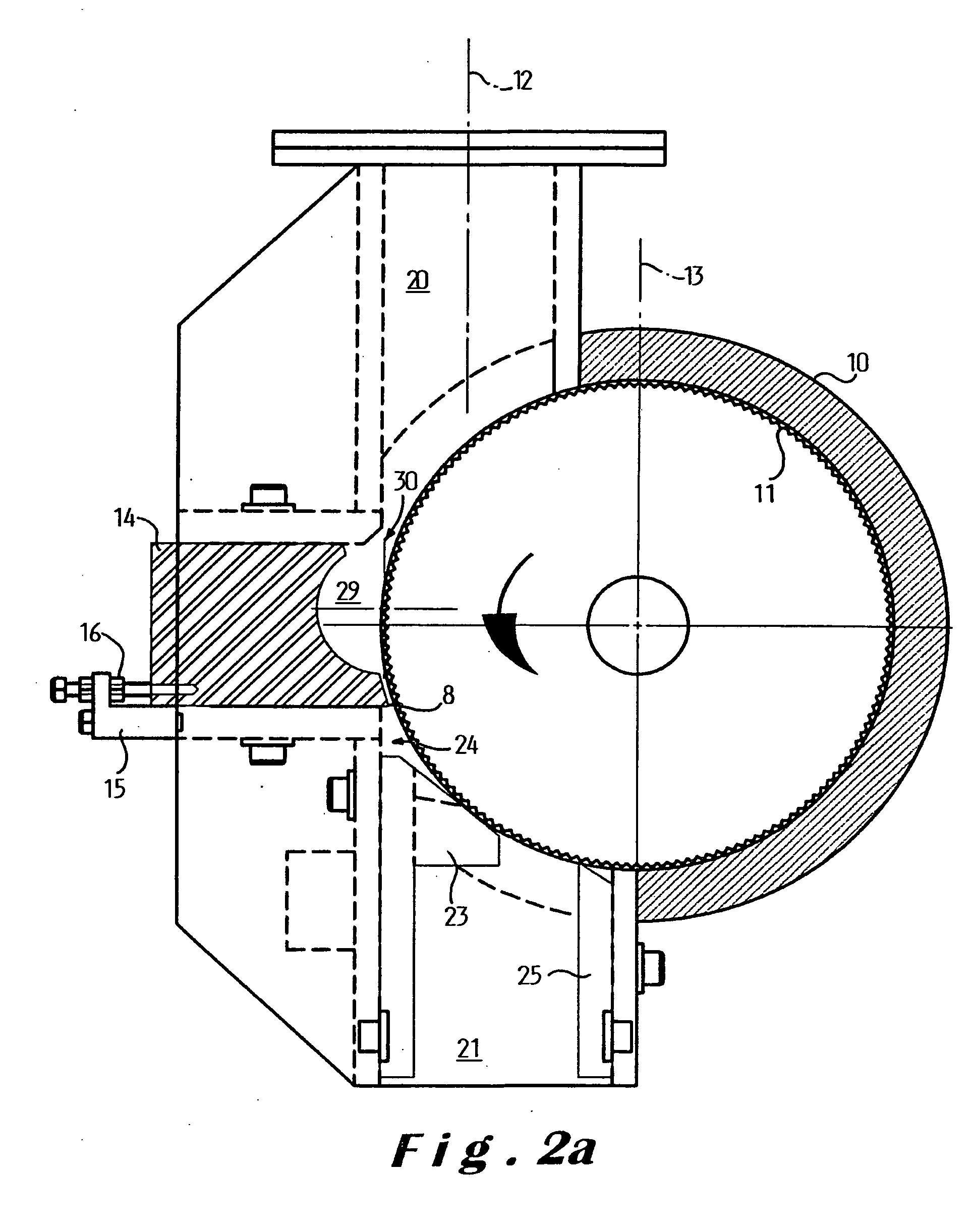
Microchip devices with improved reservoir opening
InactiveUS6875208B2Improve uniformityImprove reliabilityMedical devicesMicromachined deliveryControlled releaseCurrent distribution
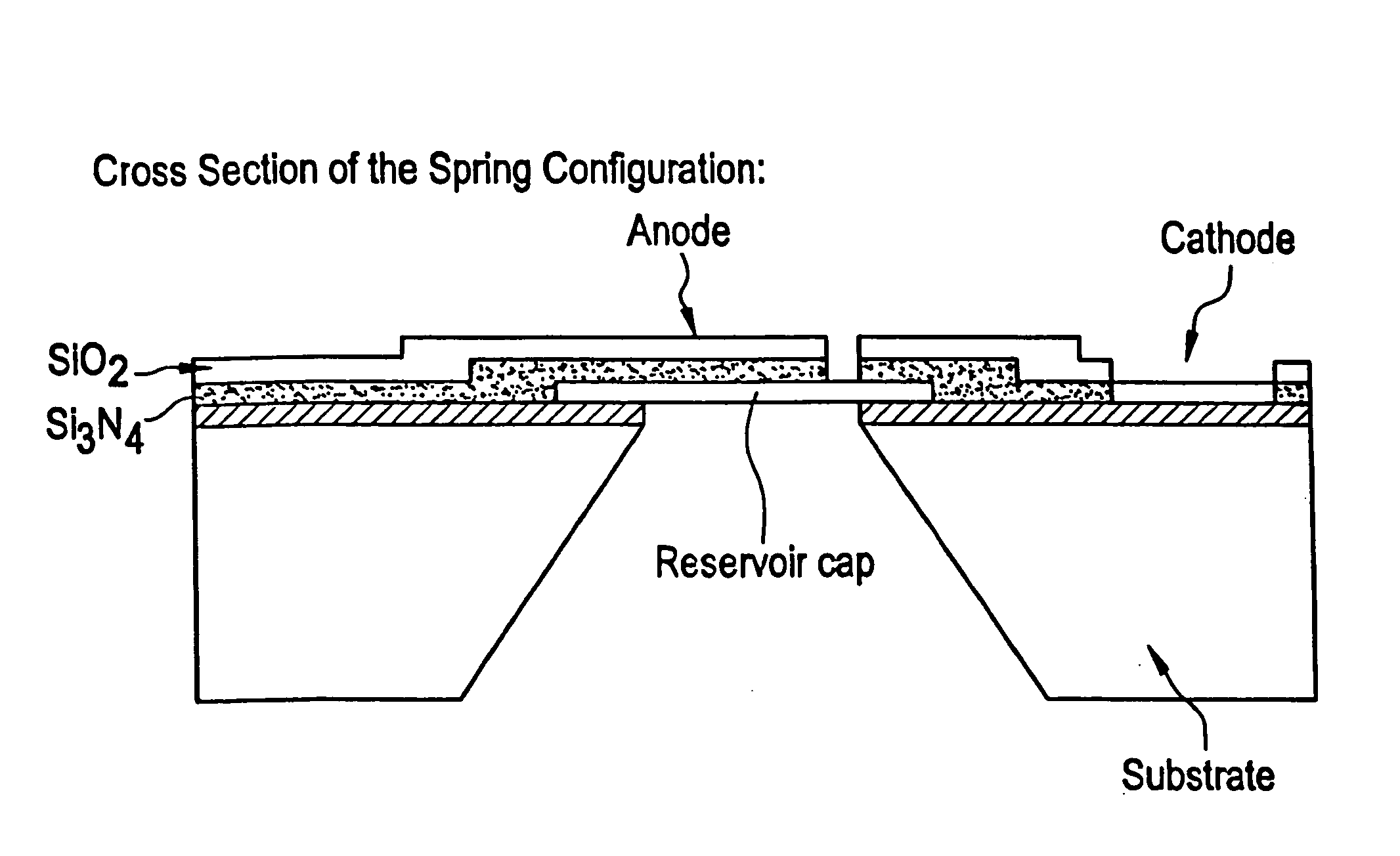
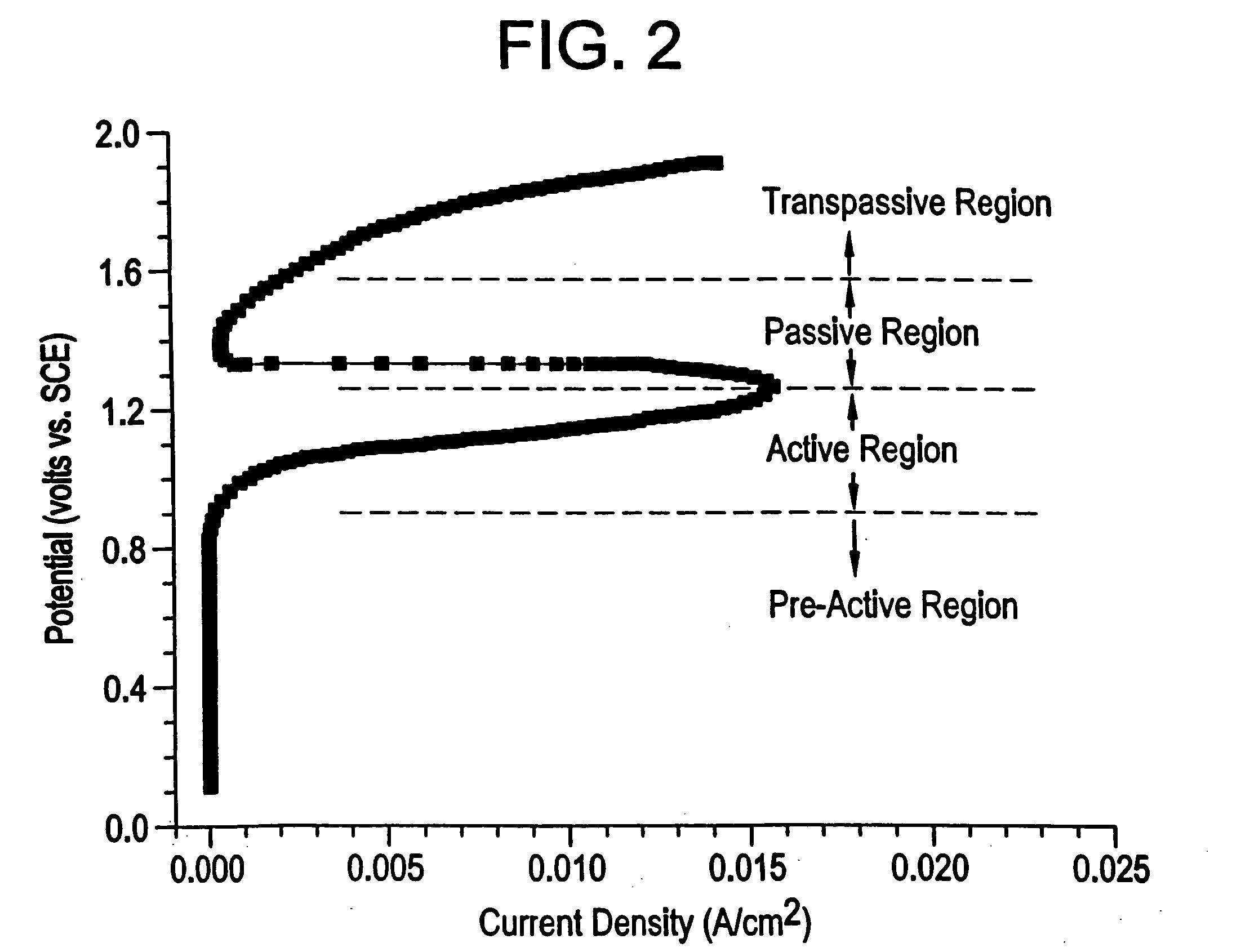
Microchip devices and methods of manufacture thereof are provided to increase the uniformity and reliability of active exposure and release of microchip reservoir contents. In one embodiment, the microchip device for the controlled release or exposure of molecules or secondary devices comprises: (1) a substrate having a plurality of reservoirs; (2) reservoir contents comprising molecules, a secondary device, or both, located in the reservoirs; (3) reservoir caps positioned on the reservoirs over the reservoir contents; (4) electrical activation means for disintegrating the reservoir cap to initiate exposure or release of the reservoir contents in selected reservoirs; and (5) a current distribution means, a stress induction means, or both, operably engaged with or integrated into the reservoir cap, to enhance reservoir cap disintegration.
Owner:MASSACHUSETTS INST OF TECH
Solvent system of hardly soluble drug with improved dissolution rate
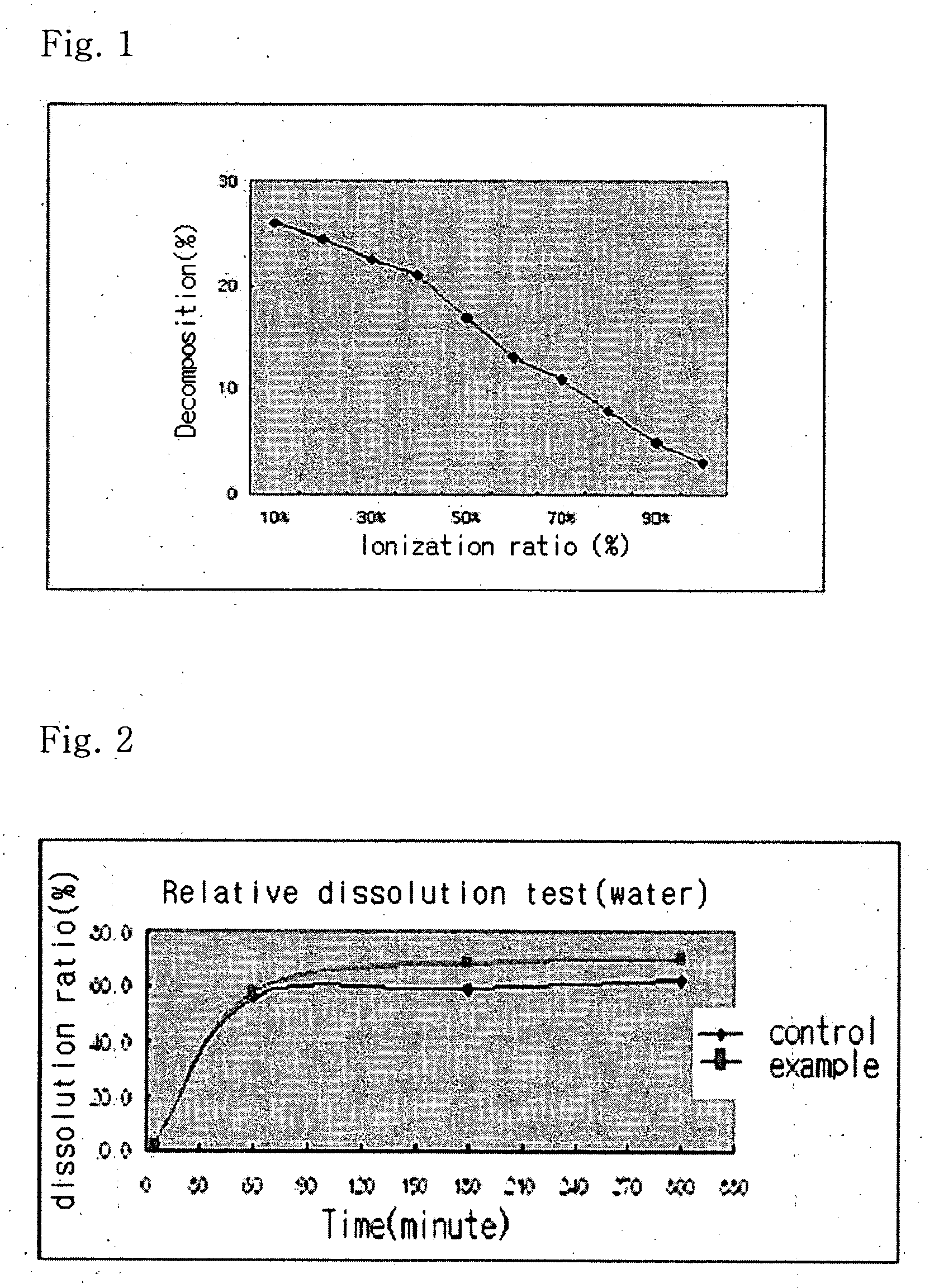
InactiveUS20040157928A1Good disintegrationPromote dissolutionBiocideAntipyreticDissolutionIonization
The present invention relates to a solvent system with improved disintegration degree and dissolution ratio of a hardly soluble drug by highly concentrating the drug through partial ionization, and by establishing optimal conditions for enhancing bioavailability of the drug, such as the co-relation between the acid drug and the accompanied components, ionization degree of a solvent system, use of an appropriate cation acceptance, water content, selection of optimal mixing ratio of the respective components and use of specific surfactants, and to a pharmaceutical preparation comprising the same. The solvent system of the invention has advantages in that it can enhance bioavailability by improving the disintegration degree and dissolution ratio of a hardly soluble drug and also provide a capsule with a sufficiently small volume to permit easy swallowing.
Owner:R & P KOREA
Orodispersible tablets
ActiveUS20100297031A1Short disintegration timeGood mechanical resistanceBiocideNervous disorderCalcium silicateOrally disintegrating tablet
This invention relates to a an orally disintegrating tablet obtainable by direct compression of a dry powdered mixture, said mixture comprising up to 15% by weight of calcium silicate, at least 50% of a diluent, a disintegrant agent and an active ingredient. It also relates to a process for preparing the tablets by homogeneous blending the specific excipients in powder form and subsequent direct compression of the mixture. Said tablets disintegrate quickly in the cavity of the mouth, in particular in less than 15 seconds.
Owner:LAB LESVI SL
Capsule Stable Against Mastication
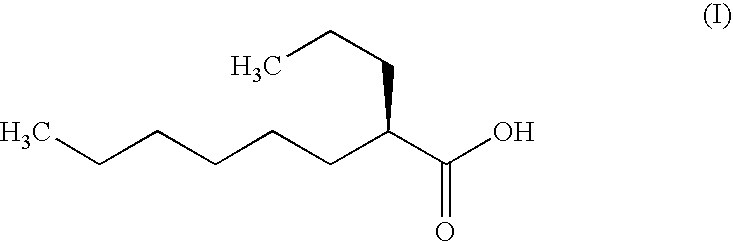
InactiveUS20080057115A1Good disintegrationExcellent pharmaceutical preparationAntibacterial agentsNervous disorderSlice thicknessMedicine
The present invention relates to a soft capsule which is easily disintegrated in the stomach, wherein the contents thereof are not easily leaked at the time of mastication, which is obtained by providing a soft capsule comprising (2R)-2-propyloctanoic acid or a salt thereof with at least one property, preferably all properties, selected from (A) wherein it has a strength of 150 to 400 N by a cracking test; (B) wherein it has a disintegration time of 3 to 10 minutes by the disintegration test stipulated in Japanese Pharmacopoeia; (C) wherein the capsule shell has a shell thickness of 0.05 to 0.50 mm; (D) wherein the capsule shell has a first seam thickness of 0.10 to 0.55 mm; (E) wherein the capsule shell has a second seam thickness of 0.05 to 0.50 mm; (F) wherein the capsule shell has a water content of 5.0 to 9.0%.
Owner:ONO PHARMA CO LTD
Jelly composition
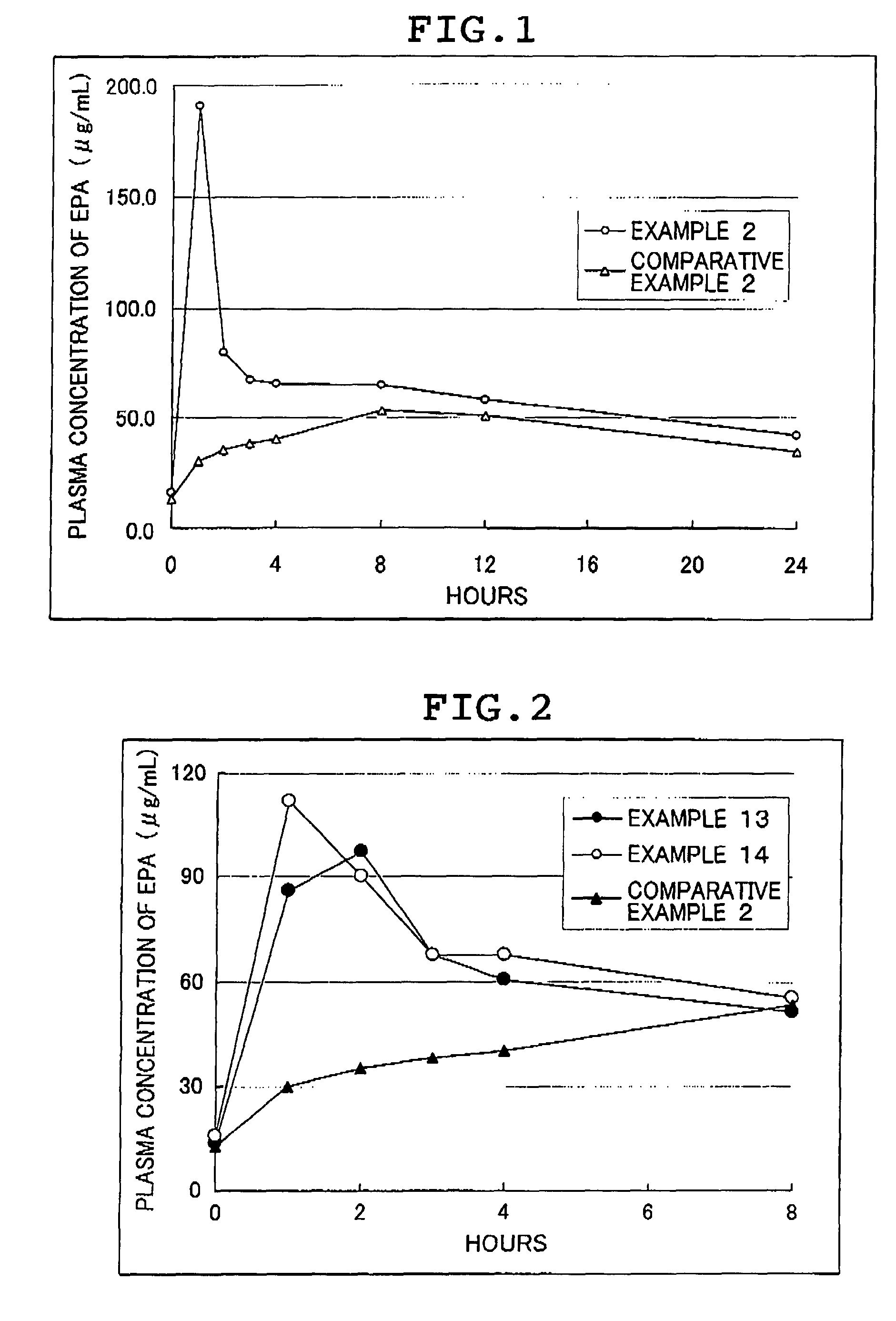
InactiveUS9452150B2Easy to carryEasily administered without waterBiocideAntipyreticBULK ACTIVE INGREDIENTActive ingredient
A composition in which a preparation of the composition itself has an excellent disintegratability in the right place and excellent active ingredient releasability within the digestive tract, the active ingredient and the preparation itself have long-term stability, excellent ease of operation such as manufacturability and filling into containers, an amount sufficient for achieving physiological effects can be easily taken and swallowed, and can be absorbed rapidly from the digestive tract, thus the physiological effect can be expected. The composition is an easy-release jelly composition containing an emulsified polyvalent unsaturated fatty acid or derivative thereof in an amount exceedingly 10 mass % and further containing an emulsifying agent and a gallant. The easy-release jelly composition includes an emulsified polyunsaturated fatty acid or derivative thereof in an amount greater than 10 wt %, an emulsifying agent, and a gelling agent.
Owner:MOCHIDA PHARM CO LTD
Solid pharmaceutical preparation
InactiveUS6299904B1Good disintegrationPromote dissolutionOrganic active ingredientsBiocideLow-substituted hydroxypropylcelluloseMaltitol
A solid preparation which comprises (i) a pharmaceutically active ingredient, (ii) one or more water-soluble sugar alcohols selected from the group consisting of sorbitol, maltitol, reduced starch saccharide, xylitol, reduced palatinose and erythritol, and (iii) low-substituted hydroxypropylcellulose having hydroxypropoxyl group contents of 7.0 to 9.9 percent by weight; which exhibits excellent buccal disintegration and dissolution and also appropriate strength.
Owner:TAKEDA PHARMA CO LTD
Orally disintegrable tablets
InactiveUS20020142034A1High strengthGood disintegrationBiocidePowder deliveryDiseaseOrally disintegrating tablet
An orally disintegrable tablet, of the present invention, which comprises (i) fine granules having an average particle diameter of 400 mum or less, which fine granules comprise a composition coated by an enteric coating layer, said composition having 10 weight % or more of an acid-labile physiologically active substance and (ii) an additive, has superior disintegrability or dissolution in the oral cavity so that it can be used for treatment or prevention of various diseases, as an orally disintegrable tablet capable of being administered to the aged or children and easily administered without water. Also, because the tablet of the present invention contains fine granules having the average particle diameter such that it will not impart roughness in mouth, it can be administered easily without discomfort at the administration.
Owner:TAKEDA PHARMA CO LTD
Orally disintegrable tablets
InactiveUS7431942B2High strengthGood disintegrationPowder deliveryOrganic active ingredientsOrally disintegrating tabletEngineering
An orally disintegrable tablet of the present invention, which comprises (i) fine granules having an average particle diameter of 400 μm or less, which fine granules comprise a composition coated by an enteric coating layer, said composition having 10 weight % or more of an acid-labile physiologically active substance and (ii) an additive, has superior disintegrability or dissolution in the oral cavity so that it can be used for treatment or prevention of various diseases, as an orally disintegrable tablet capable of being administered to the aged or children and easily administered without water. Also, because the tablet of the present invention contains fine granules having the average particle diameter such that it will not impart roughness in mouth, it can be administered easily without discomfort at the administration.
Owner:TAKEDA PHARMA CO LTD
Sublingual buccal effervescent
InactiveUS20030091629A1Stimulates additional salivationGood disintegrationOrganic active ingredientsPharmaceutical delivery mechanismOral medicationAbsorption drugs
A pharmaceutical dosage form adapted to supply a medicament to the oral cavity for buccal, sublingual or gingival absorption of the medicament which contains an orally administrable medicament in combination with an effervescent for use in promoting absorption of the medicament in the oral cavity. The use of an additional pH adjusting substance in combination with the effervescent for promoting the absorption drugs is also disclosed.
Owner:CIMA LABS
Oligosaccharide-supplying compositions
InactiveUS6750331B1Easy to useEvenly dispersedSugar productsAntibiotics chemistryOligosaccharideNuclear chemistry
The present invention provides an oligosaccharide-supplying composition which contains 10 to 80% by weight of oligosaccharide, 0.3 to 10% by weight of a foaming component and 0.9 to 30% by weight of a neutralizing component and is in the form of foaming chewable tablets. The composition is highly useful in practice, has a uniform composition, excellent texture and high stability and can be easily taken.
Owner:OTSUKA PHARM CO LTD
Compositions and methods for sublingual formulations of dihydroergotamine for the treatment of migraine
InactiveUS20030022910A1Stimulates additional salivationGood disintegrationBiocidePharmaceutical delivery mechanismDihydroergotamineSide effect
The present invention is an improvement in the treatment of migraine headaches. By administering dihydroergotamine across the oral mucosa, major limitations of past treatments are circumvented thereby allowing for higher efficacy and fewer side effects of treatment at lower doses.
Owner:ALAMO PHARMA
Solid preparation and preparation method thereof
ActiveCN101987082AHigh humidity stabilityAvoid crackingInorganic non-active ingredientsPharmaceutical delivery mechanismActive componentAdhesive
The invention discloses a solid preparation. The solid preparation comprises a wet unstable substance, an enough amount of non-hygroscopic sugar alcohol (I) at the normal temperature, an enough amount of hydrophilic non-hygroscopic melt adhesive at the normal temperature and / or pharmaceutically acceptable additive and / or active component, wherein, the melting point of the melt adhesive is lower than that of the sugar alcohol (I); the melt adhesive melts, solidifies and adheres to the sugar alcohol (I) to form a bridging substance which leads the solid preparation to be completely or basicallycompletely insulated from the external environment. The invention also discloses a preparation method of the solid preparation; and the solid preparation has extremely high wet stability, higher hydrophilism, better disintegrating or releasing performance, and better mechanical performance, and insulates the external environment in a high degree, can insulate bad flavor or taste, and can be produced by a fully dry method.
Owner:北京中关科城科技股份有限公司
A kind of gefitinib dispersible tablet and its preparation method and application
ActiveCN102266300AImprove bioavailabilityPromote dissolutionOrganic active ingredientsPill deliveryMedicineActive agent
The invention discloses a gefitinib dispersible tablet, a preparation method and an application thereof. The gefitinib dispersible tablet of the invention comprises the following components by weight: 10-65% of gefitinib, 1-30% of fillers, 10-50% of disintegrants, 1-60% of acidifiers, 0.1-20% of adhesives, and 0.1-30% of lubricants and glidants. According to the invention, gefitinib is wrapped bythe acidifier or gefitinib and the acidifier are wrapped with each other so as to reach the embedding effect. Compared with commercially available common tablets, the gefitinib dispersible tablet of the invention does not contain surfactants, has good dissolvability, dispersibility and disintegrability, and can be disintegrated completely within one minute. The gefitinib dispersible tablet prepared by the method of the invention has a high dissolution rate, good bioavailability, rapid distribution in vivo, and stable quality, and the preparation method is simple and practical, and is applicable to industrial production.
Owner:GUANGDONG PHARMA UNIV
Care and/or make-up cosmetic composition structured with silicone polymers and organogelling agents, in rigid form
InactiveUS20050245673A1Easy to disperseImprove staminaCosmetic preparationsMake-upHydrogenRoom temperature
The invention relates to a care and / or make-up cosmetic composition comprising a liquid fatty phase comprising at least one silicone oil, structured with a gelling system comprising 1) at least one polymer having a weight-average molecular mass ranging from 500 to 500 000, containing at least one moiety comprising: at least one polyorganosiloxane group consisting of 1 to 1 000 organosiloxane units in the chain of the moiety or in the form of a graft, and at least two groups capable of establishing hydrogen interactions, the polymer being solid at room temperature and soluble in the liquid fatty phase at a temperature of 25 to 250° C., and 2) at least one non-polymeric organogelling agent.
Owner:LOREAL SA
Low-substituted hydroxypropylcellulose powder and method for producing the same
ActiveUS20080039621A1Improve compression performanceGood lowabilityPill deliveryGranular deliveryAqueous sodium hydroxideLow-substituted hydroxypropylcellulose
Provided are a low-substituted hydroxypropylcellulose powder having high compressibility, good flowability and excellent disintegration, and a method for producing the same. More specifically, provided is a method for producing a low-substituted hydroxypropylcellulose powder having a molar substitution number per anhydrous glucose unit of 0.05 to 1.0, which is insoluble in water and swollenable by absorbing water, comprising the steps of: adding an aqueous sodium hydroxide solution to powdered pulp in such a manner that weight ratio of sodium hydroxide with respect to anhydrous cellulose is 0.1 to 0.3 so as to produce alkali cellulose; etherifying the obtained alkali cellulose to obtain a crude product; neutralizing the sodium hydroxide contained in the obtained crude reaction product; washing the resultant; drying; and pulverizing using by compaction-grinding.
Owner:SHIN ETSU CHEM IND CO LTD
Solid preparation
InactiveUS20010009678A1Good disintegrationPromote dissolutionBiocideMetabolism disorderLow-substituted hydroxypropylcelluloseMaltitol
A solid preparation which comprises (i) a pharmaceutically active ingredient, (ii) one or more water-soluble sugar alcohol selected from the group consisting of sorbitol, maltitol, reduced starch saccharide, xylitol, reduced palatinose and erythritol, and (iii) low-substituted hydroxypropylcellulose having hydroxypropoxyl group contents of 7.0 to 9.9 percent by weight; which exhibits excellent buccal disintegration and dissolution and also appropriate strength.
Owner:TAKEDA PHARMA CO LTD
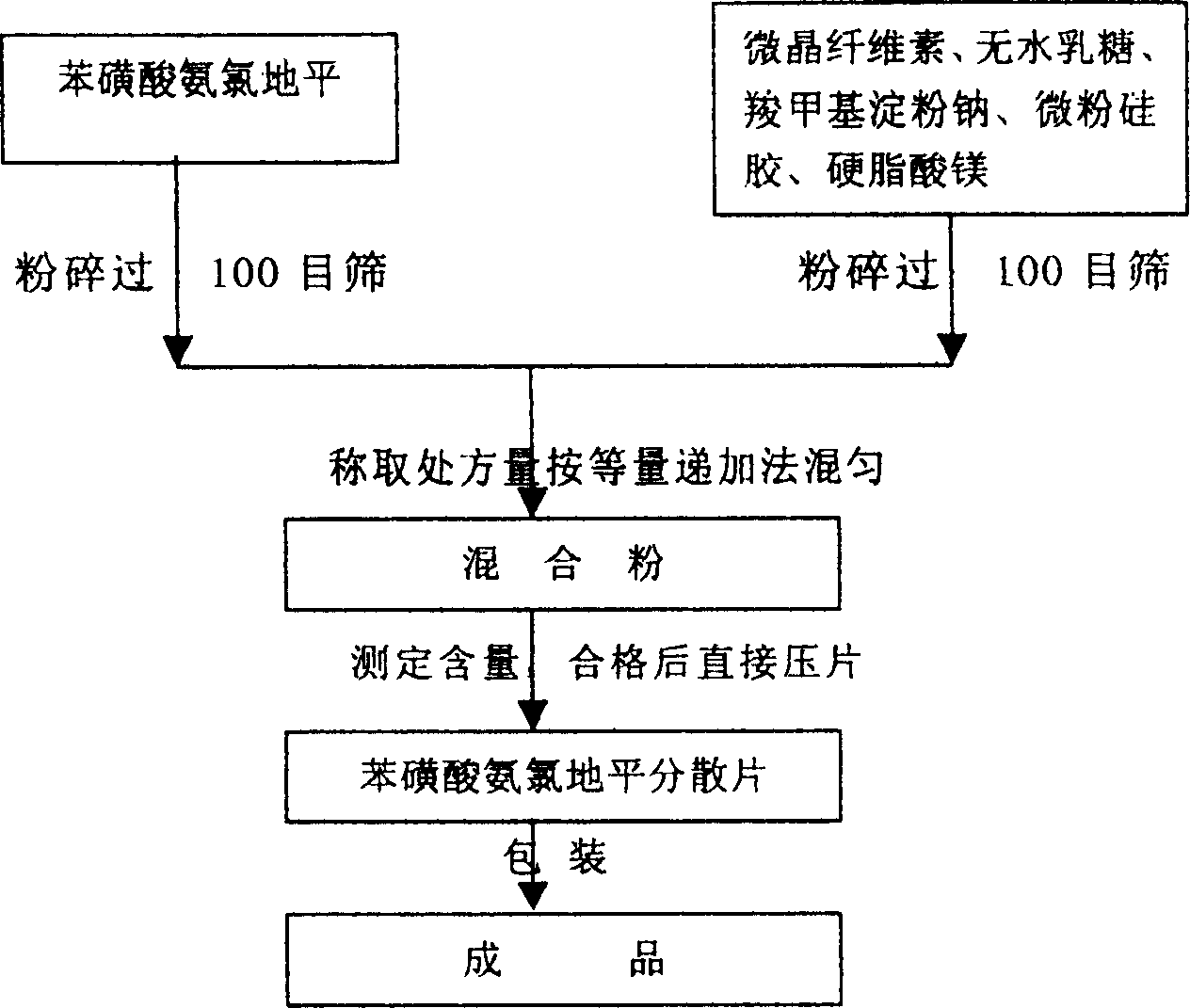
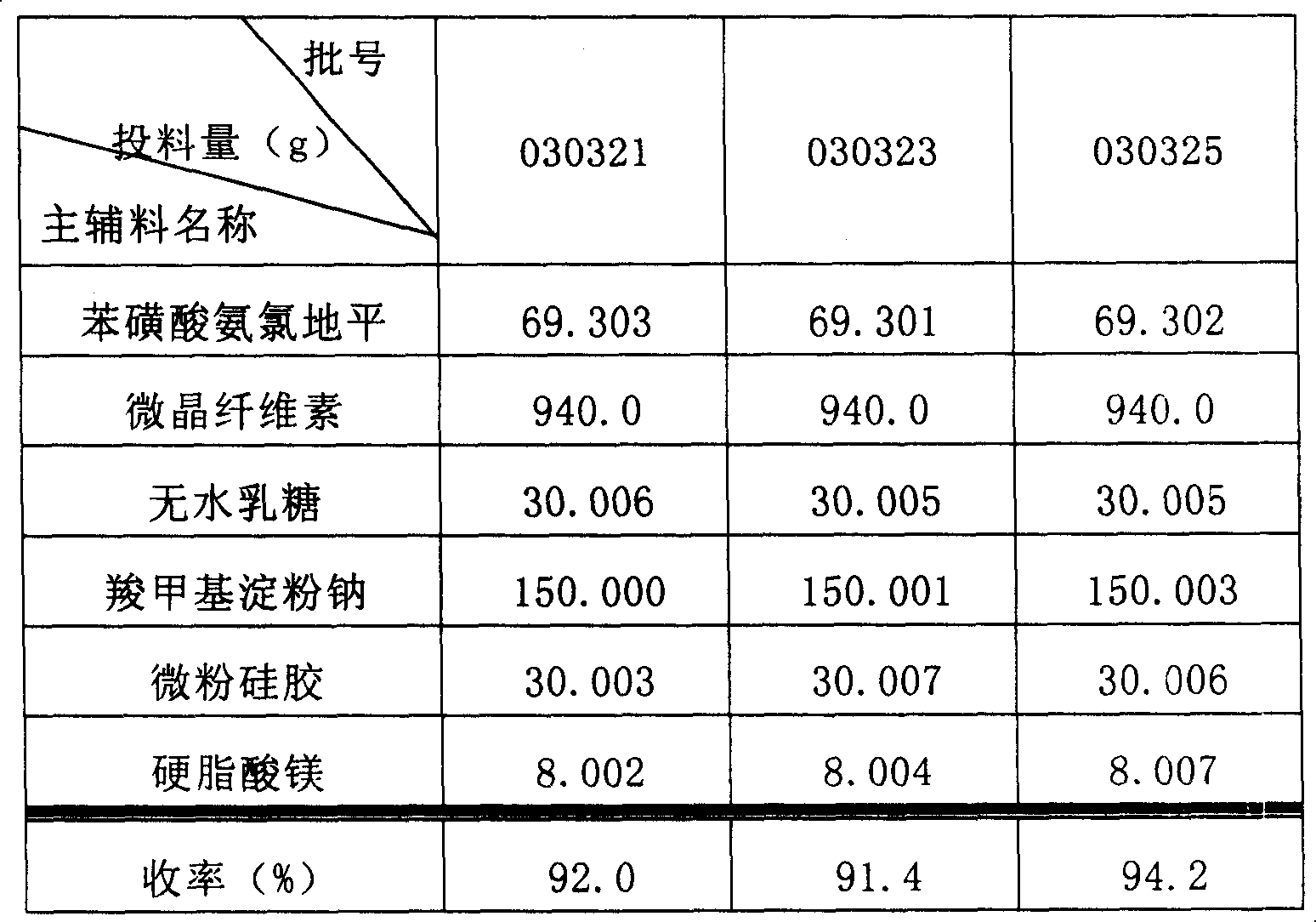
Phenylsulfonic acid amido chloro diping dispersion tablet and its preparation method
InactiveCN1686121AEasy to takeTake fastOrganic active ingredientsPill deliveryCarboxymethyl starchAmlodipine besilate
A dispersing table of amlodipine benzosulfonate for treating hypertension is proportionally prepared from amlodipine benzosulfonate, microcrystalline cellulose, anhydrous lactose, carboxymethyl starch sodium, micropowdered silica gel, and magnesium stearate.
Owner:YUNNAN BAIYAO GRP HEALTH PROD CO LTD
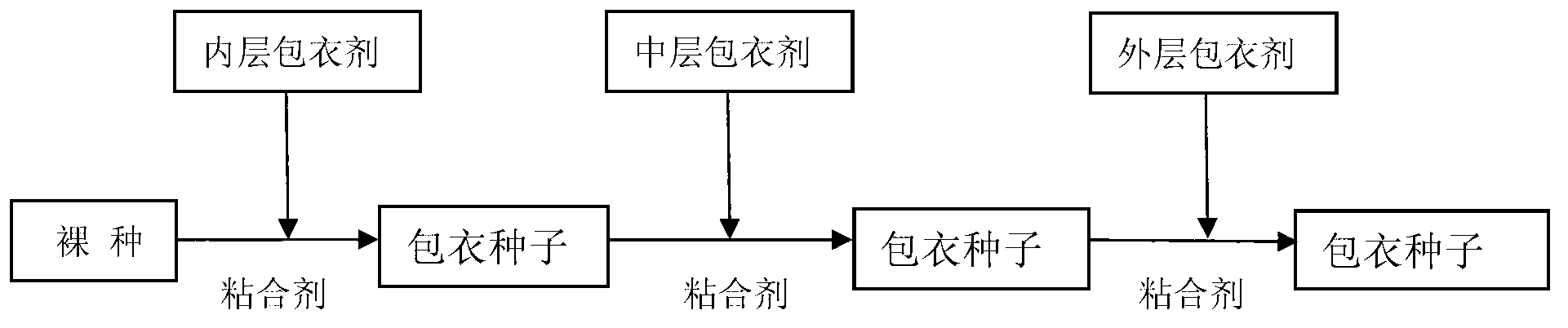
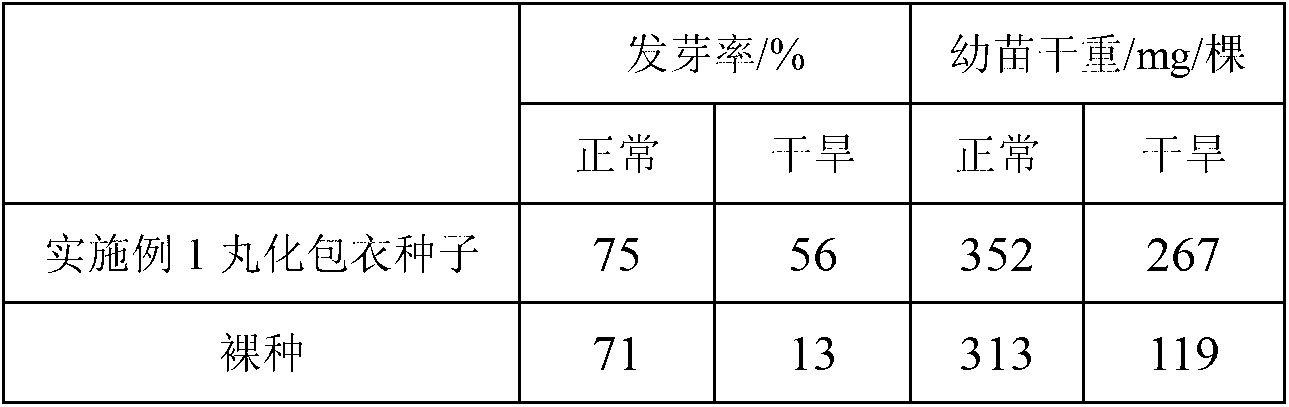
Seed coating agent and coated seeds for desert control and aerial seeding, and preparation method of agent
ActiveCN103058777AHigh single seed rateHigh seed yieldClimate change adaptationAfforestationNutrientGermination
The invention relates to a seed coating agent and coated seeds for desert control and aerial seeding, and a preparation method of the agent. The seeding coating agent mainly comprises an inner layer coating agent, a middle layer coating agent and an outer layer coating agent. As the coating method provided by the invention adopts a layered coating technology, not only is the mutual influence among different ingredients of the coating agent alleviated, but also the difficulty caused by the swelling and the adhesion of a water retaining agent which absorbs water in a coating process is avoided; special drying equipment is not needed after the coating is finished, so that the dependence of a traditional coating operation on the drying equipment is avoided; a repellent for birds, rabbits, mice and the like is added to the coating agent, so that the seeds are protected from being eaten while no harm is caused to the environment; and the coated seeds have a high germination rate and can take root rapidly under the action of the water retaining agent and nutrients, so that the seeds for desert control and aerial seeding are promoted to germinate and grow rapidly, and the survival rate of seedlings is remarkably improved.
Owner:BEIJING FORESTRY UNIVERSITY
Device for controlled reservoir opening with reinforced reservoir caps
InactiveUS20050143715A1Increase uniformity and reliabilityGood disintegrationValve arrangementsMedical devicesControl releaseControl circuit
Owner:MASSACHUSETTS INST OF TECH
Methods for treating early morning pathologies
InactiveUSRE39239E1Effective treatmentGood disintegrationNervous disorderPeptide/protein ingredientsControl releaseActive agent
The present invention provides methods of treating early morning pathologies using a time-specific controlled release dosage formulation which is administered prior to sleep, and which permits or achieves delivery of a pharmaceutically active agent effective for the treatment of the specific early morning pathology to be treated, at about the time of awakening. The time-specific controlled release dosage formulation comprises (1) a core including the pharmaceutically active agent(s) effective for the treatment of the early morning pathology, and (2) a swellable polymeric coating layer substantially surrounding the core. The swellable polymeric coating layer delays the release of the pharmaceutically active agent from the core for a predetermined period of time dependent upon the thickness of the swellable polymeric coating layer, to effect delivery of the pharmaceutically active agent at about the time of awakening.
Owner:POLICHEM SA
Tablets quickly disintegrating in oral cavity
InactiveUS20050147666A1Disintegrates quicklyLoses its shapeMetabolism disorderInorganic non-active ingredientsCompression moldingD-mannitol
According to the present invention, provided are an intraorally rapidly disintegrable tablet which comprises D-mannitol and a disintegrator in addition to fine granules prepared by granulating a mixture of a water-soluble pharmacologically active ingredient and an adsorbent; a process for producing an intraorally rapidly disintegrable tablet which comprises mixing D-mannitol and a disintegrator with fine granules prepared by granulating a mixture of a water-soluble pharmacologically active ingredient and an adsorbent to yield a material for compression molding, and subjecting the material to compression molding; and an intraorally rapidly disintegrable tablet which is prepared by mixing D-mannitol and a disintegrator with fine granules prepared by granulating a mixture of a water-soluble pharmacologically active ingredient and an adsorbent to yield a material for compression molding, subjecting the material to compression molding.
Owner:KYOWA HAKKO KIRIN CO LTD
A1 composite material being crumbled with water, a1 film and a1 power comprising the material and methods for preparation thereof, constitutional member for film-forming chamber method for recovering film-forming material
ActiveUS20060240271A1Efficiently be disintegratedEfficiently be collapsedLiquid surface applicatorsMolten spray coatingAl powderMetallurgy
In An Al composite material collapsible in the presence of moisture, the external surface of small pieces or powder constructed from a single or a plurality of crystalline grains of Al or an Al alloy is covered with a film of a low melting point metal or alloy selected from the group consisting of In, Sn, combinations of In and Sn, and alloys thereof. The content of the foregoing low melting point metal or alloy ranges from 0.1 to 20% by mass on the basis of the total mass of the composite material. A material obtained by adding a low melting point metal in an amount specified above to, for instance, Al and then fusing and melting the resulting mixture is quenched and solidified within a non-oxidizing atmosphere to thus form an Al composite material. An Al film, an Al spray-coated film and Al powder can be prepared from the foregoing Al composite material. A component member for a film-forming chamber is also provided, which is provided with a water-collapsible Al film on the surface thereof. Film-forming operations are continued over a long period of time using the component member for a film-forming chamber provided with the water-collapsible Al film and then film-forming materials can be recovered from the component member on which the film-forming materials are deposited in a substantial thickness.
Owner:ULVAC INC
Improved-performance tablet and preparation method thereof
InactiveCN103432091AMaintain integrityPrevent or reduce cracksPill deliveryPorosityWeather resistance
The invention discloses an improved-performance tablet which comprises an active component A1, a hydrophilic diluent B1 and a meltable solid dispersion and / or a solid coating C1, wherein the diluent B1 and / or the active component A1 are / is bonded and bridged by the solidified melt of the meltable solid dispersion and / or solid coating C1; and / or the tablet comprises a hydrophilic diluent B2 and a meltable solid dispersion containing an active component A2 and / or a solid coating C2, wherein the diluent B2 is bonded and bridged by the solidified melt of the meltable solid dispersion and / or solid coating C2. The invention also discloses a preparation method of the tablet. The tablet has stronger mechanical performance and / or better weather resistance and / or better hydrophilcity or better disintegration property or better medicine dissolubility and higher porosity.
Owner:钟术光
Coreless roll of absorbent sheet and method for manufacturing the same
ActiveUS20170280946A1Improve the immunityGood disintegrationMechanical working/deformationDomestic applicationsAbsorbent materialToilet paper
A coreless roll of an absorbent sheet product, such as napkins, toilet paper, towels etc., including a spirally wound continuous web of absorbent material having a first end and a second end and a coating composition comprising a specific polymer coated onto the second end is disclosed. The coreless roll has excellent resistance to collapsing, as well as excellent flexibility and elasticity. Moreover, the coreless roll has excellent disintegrability in water and can be used along its whole length. Also disclosed is a process for the manufacture of the coreless roll.
Owner:ESSITY OPERATIONS FRANCE
Electrical bypass element, in particular for storage cells of an energy storage device
ActiveUS20130252039A1Increased power lossGood disintegrationProtecting/adjusting hybrid/EDL capacitorEmergency protective circuit arrangementsThermal energyElectricity
An electrical bypass element, suitable for bypassing defective storage cells in energy storage devices includes two electrical conductors between which is formed a layer sequence with at least one electrical insulation layer and one or more reactive layer stacks, in which an exothermic reaction can be triggered. The reactive layer stacks and the insulation layer are matched to one another such that the insulation layer disintegrates as a result of the thermal energy released during the exothermic reaction and an electrical connection is produced between the electrical conductors. The electrical bypass element can be actively triggered even before the ultimate failure of a storage cell so that higher power losses in the energy storage device can be avoided.
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
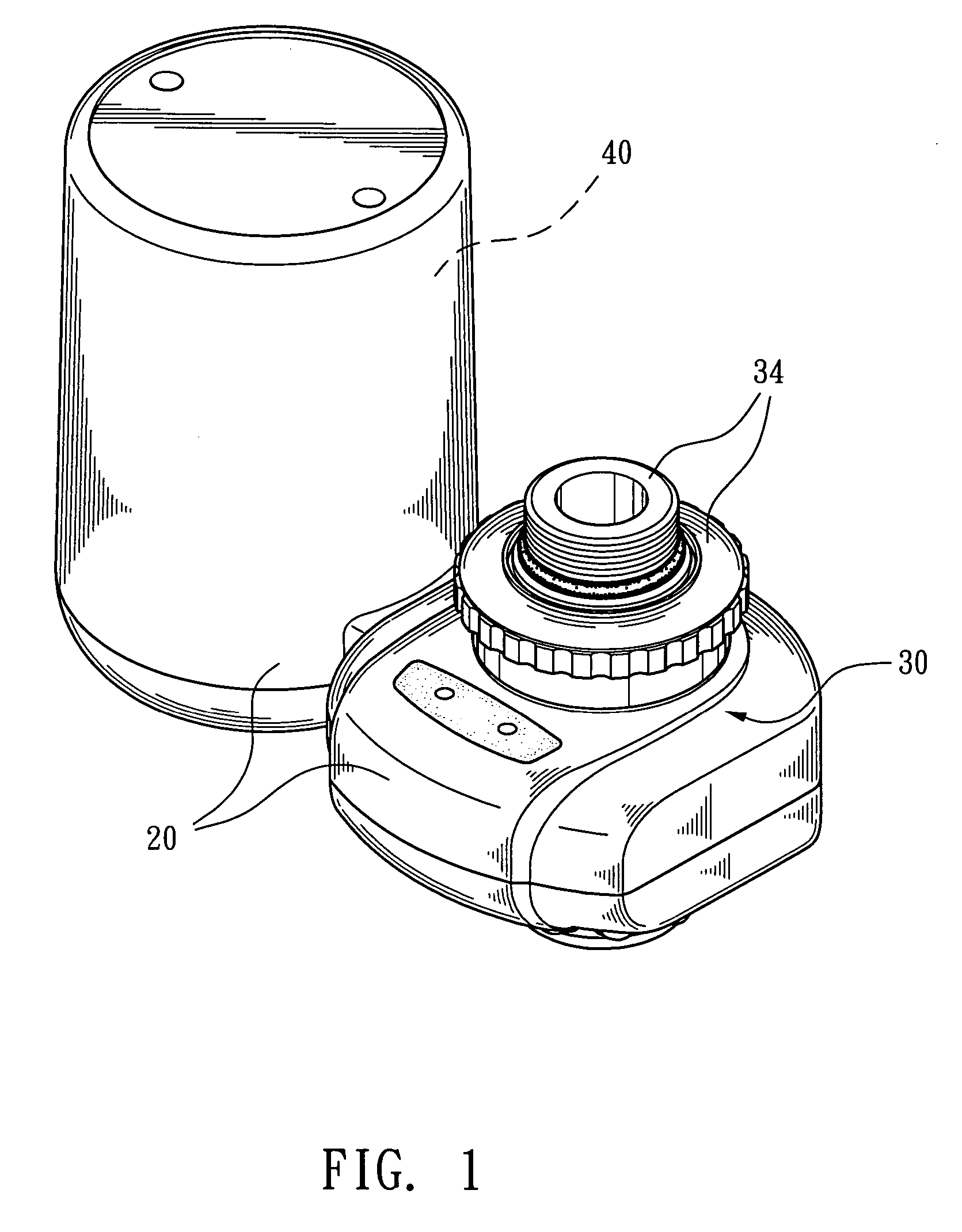
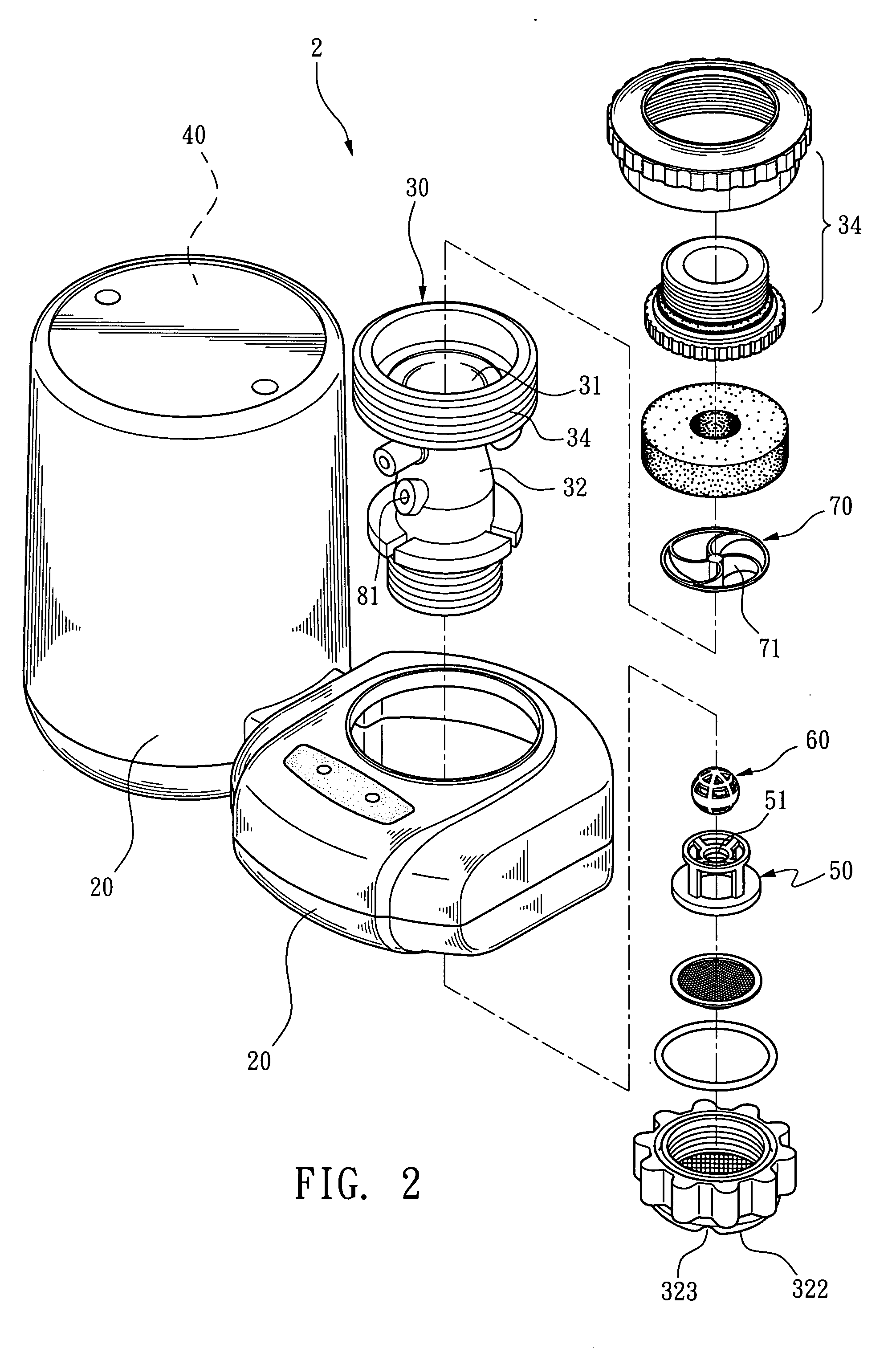
Ozone water faucet
InactiveUS20060266683A1Good disintegrationEfficient mixingOther chemical processesSolid sorbent liquid separationOzone generatorWater flow
An ozone water faucet having an ozone supplier disposed on an outcome of a faucet is disclosed. The ozone supplier consists of a gas-liquid mixing tube and an ozone generator. The gas-liquid mixing tube is composed by a water intake section, a water drainage section, and a diameter-reducing throat therebetween. The ozone from the ozone generator flows through the diameter-reducing throat, into the gas-liquid mixing tube. A cut out bearing is arranged inside the water drainage section for mounting a cut out stirring ball that moves freely. When water flows through the diameter-reducing throat, into the water drainage section, it flushes the cut out stirring ball as well as the bearing and drives the cut out stirring ball moving upwards and downwards so as to have better disintegration effect on the water.
Owner:SUNG WEI MING
Unit and method for recycling a bituminous membrane
ActiveUS20050263625A1Simpler and less-expensive constructionEasy to usePlastic recyclingWorking-up pitch/asphalt/bitumen by solidifying/disintegratingMembrane methodTrituration
Method and unit for recycling a bituminous membrane provided with at least one reinforcement, which membrane is reduced to pieces which are introduced into a recycling unit, provided with a first rotor, housed in a first stator, and where they are heated and ground, said pieces being conveyed into a chamber, delimited by an external wall of the first rotor and a recess arranged in a counter-element mounted on the stator, where they are subjected to a trituration, the pieces thus triturated then being discharged from the chamber by flowing along said external wall of the rotor.
Owner:IMPERBEL
Bio-enzyme degerming and cleaning effervescent tablet for clothes, and preparation method and application thereof
InactiveCN106753932AGood cleaning effectFast effervescent timeInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsSurface-active agentsNuclear chemistry
The invention discloses a bio-enzyme degerming and cleaning effervescent tablet for clothes. The bio-enzyme degerming and cleaning effervescent tablet for the clothes is prepared from the following components in percentage by weight: 20 percent to 55 percent of alkaline effervescing agent, 15 percent to 40 percent of surface active agent, 10 percent to 30 percent of acid effervescing agent, 2 percent to 10 percent of adhesion agent, 1 percent to 5 percent of complex enzyme preparation, 0.5 percent to 3 percent of decolorizer, 0.5 percent to 5 percent of disintegrating agent, and 0.5 percent to 2.5 percent of enzyme stabilizer. The bio-enzyme degerming and cleaning effervescent tablet for the clothes is good in cleaning effect, fast in effervescence time and intense in sense effect; when being used for cleaning the clothes, the components of the bio-enzyme degerming and cleaning effervescent tablet for the clothes are synergized, and especially the enzyme preparation, the surface active agent and various additives are synergized, so that various stubborn stains in the clothes can be quickly removed, the surface active agent has no residue after cleaning, and the bio-enzyme degerming and cleaning effervescent tablet for the clothes is healthy and safe.
Owner:深圳市美益洁生物科技有限公司
Orally disintegrating tablet
ActiveUS8377995B2Solve the lack of hardnessDisintegrates quicklyOrganic active ingredientsBiocideCelluloseHydrogen phosphate
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Chewing gum base composition
InactiveUS20060141094A1Excellent chewing texture and flavor lasting qualityExcellent in disintegratability and biodegradabilityContainers for annular articlesChewing gumPlasticizerPolylactic acid
The invention provide a gum base composition which has an excellent chewing texture and is disintegratable and biodegradable comprising biodegradable ingredients, including a lactic acid polymer comprising a poly-L-lactic acid polymer and / or other lactic acid polymers having a glass transition temperature higher than 50° C. in an amount of from 5% by weight to 60% by weight, and an emulsifying plasticizer in an amount of from 1% by weight to 20% by weight. The weight average molecular weight of the lactic acid polymer to be used is preferably 50,000 to 200,000.
Owner:FLAVOR FRAGRANCE & FOODTECH ACAD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com