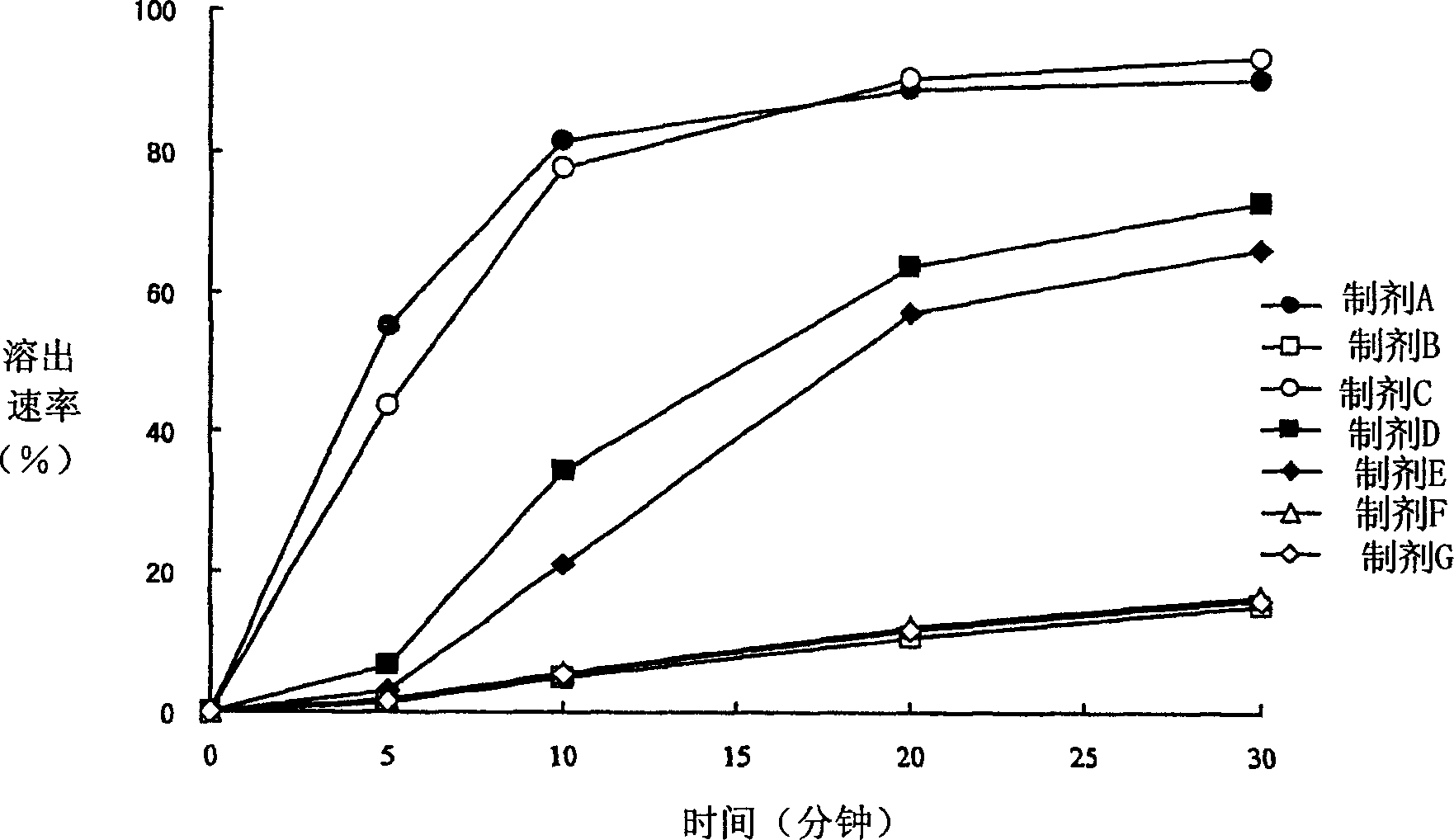
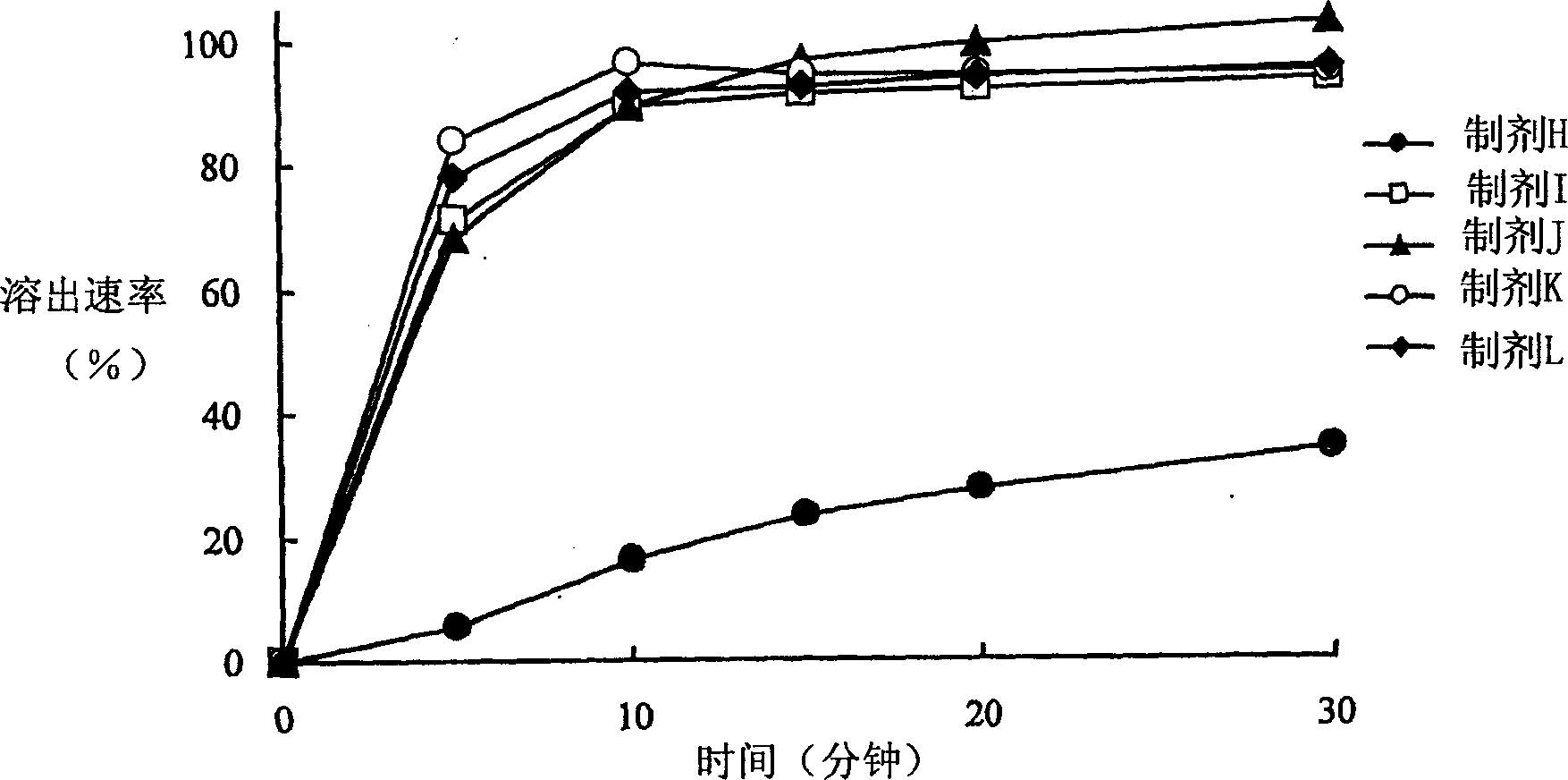
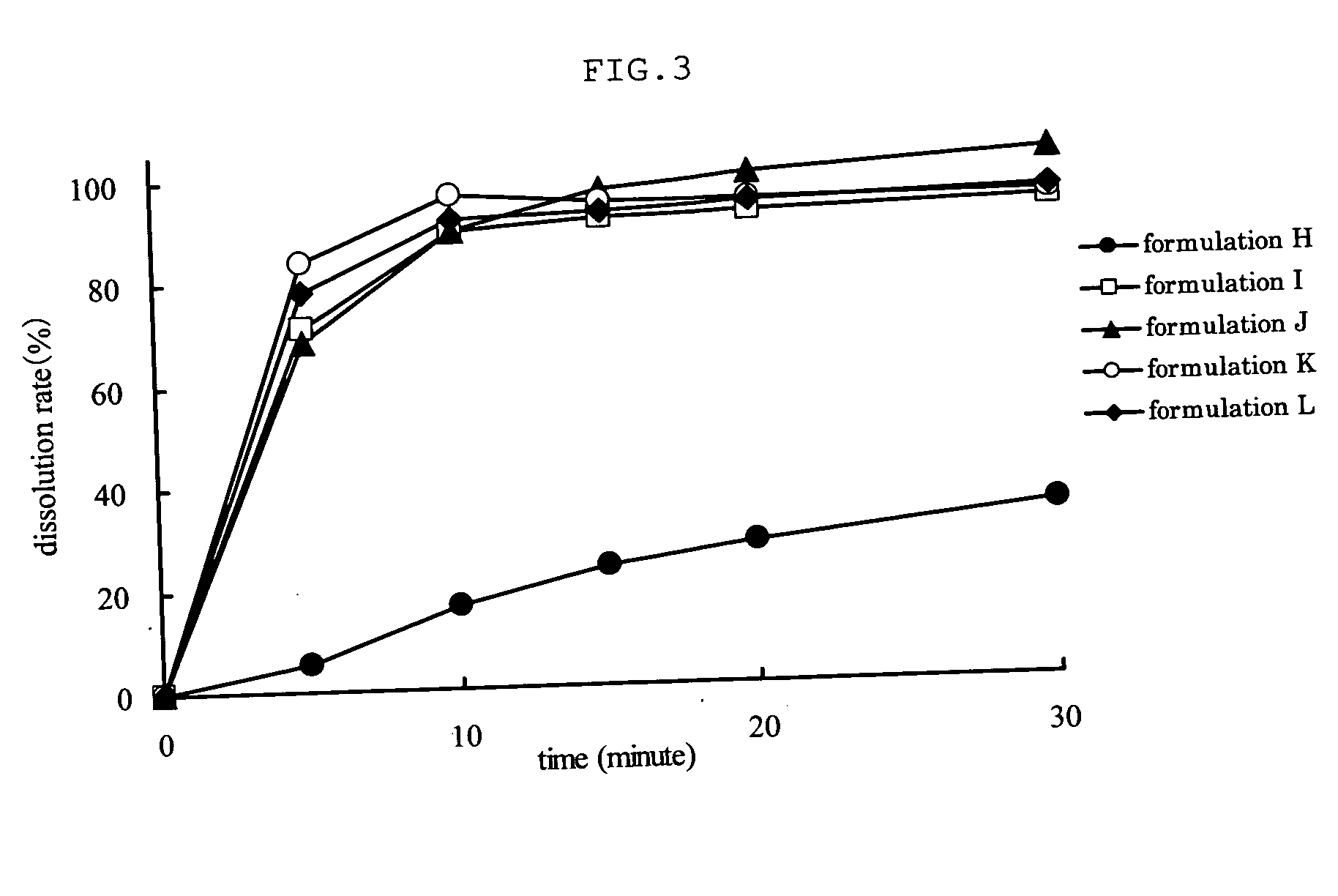
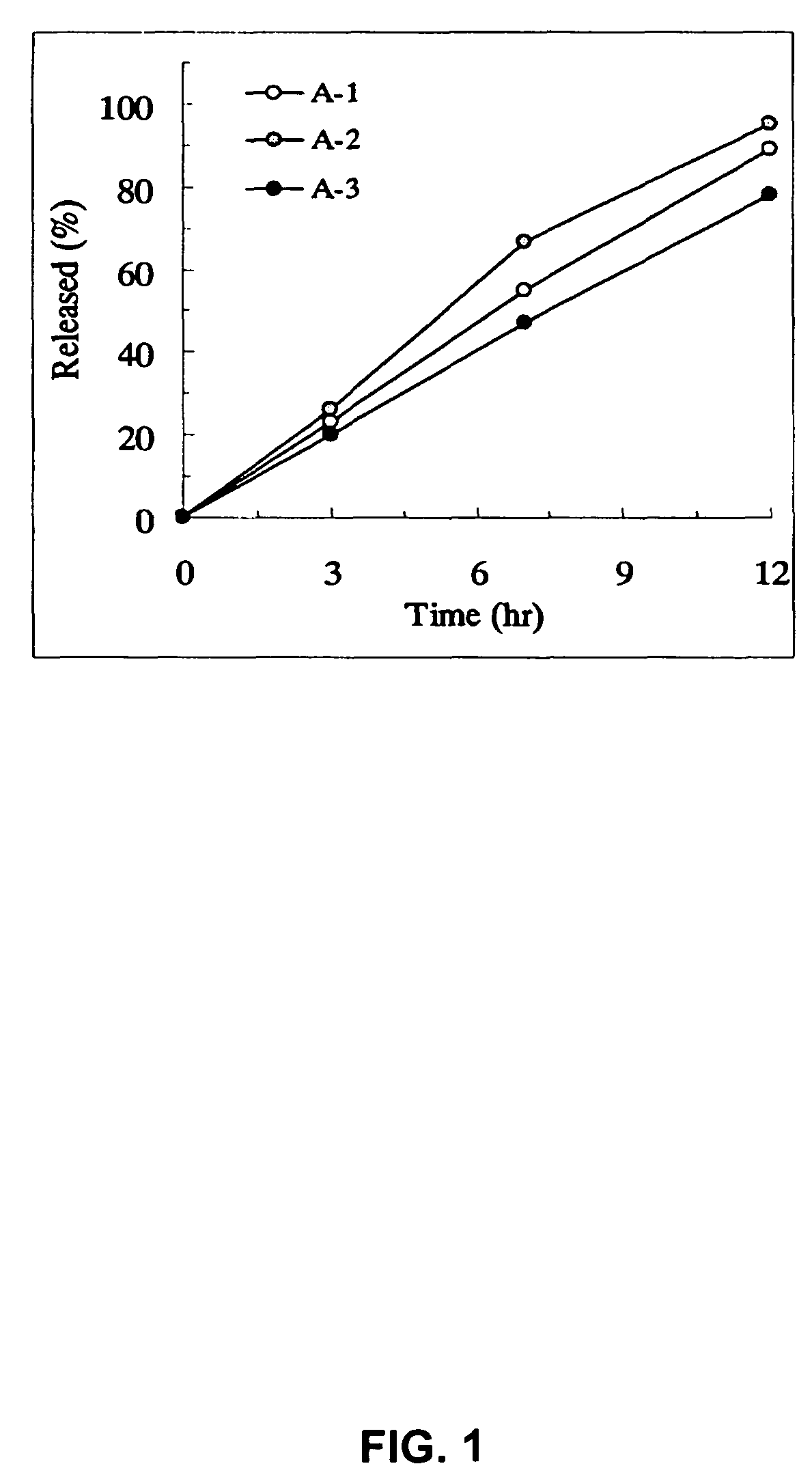
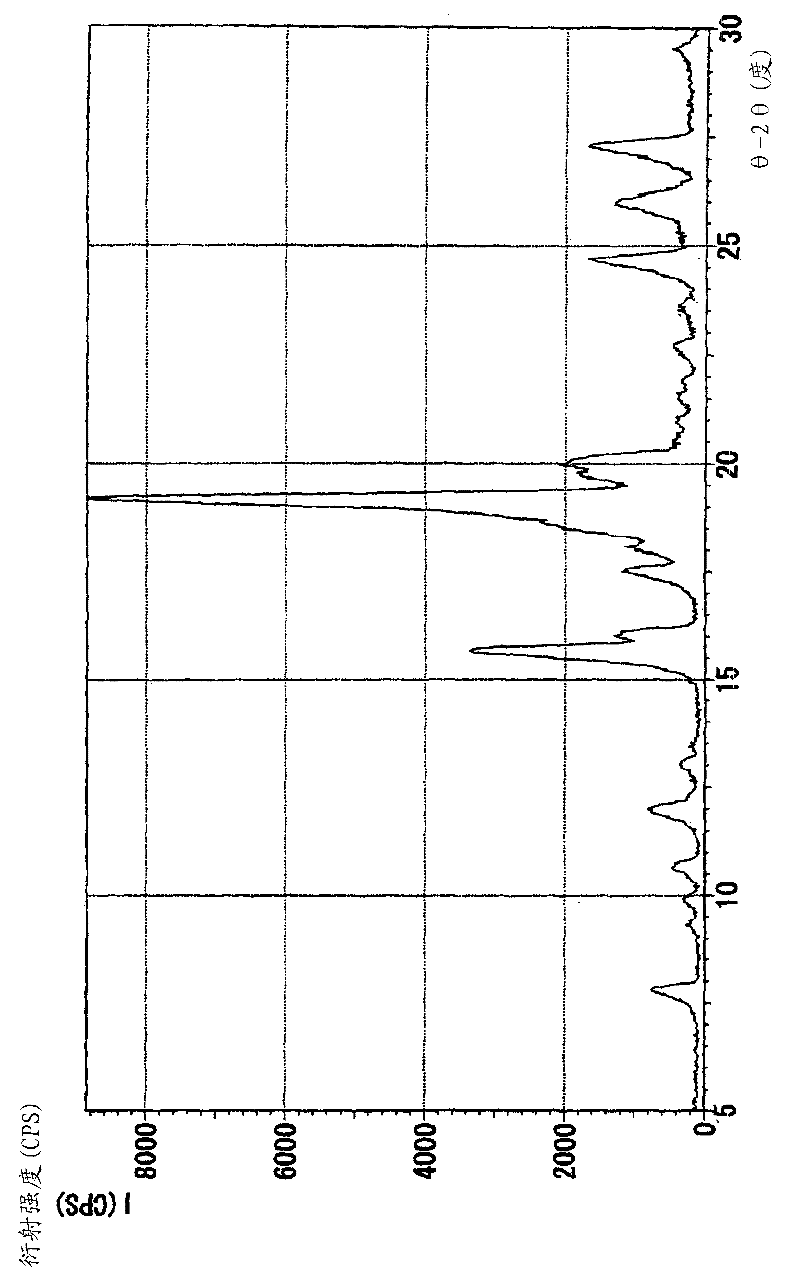
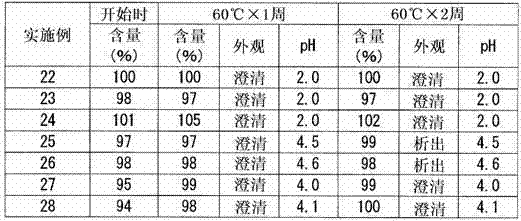
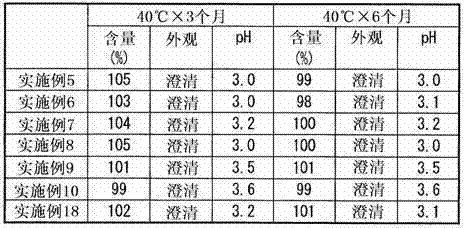
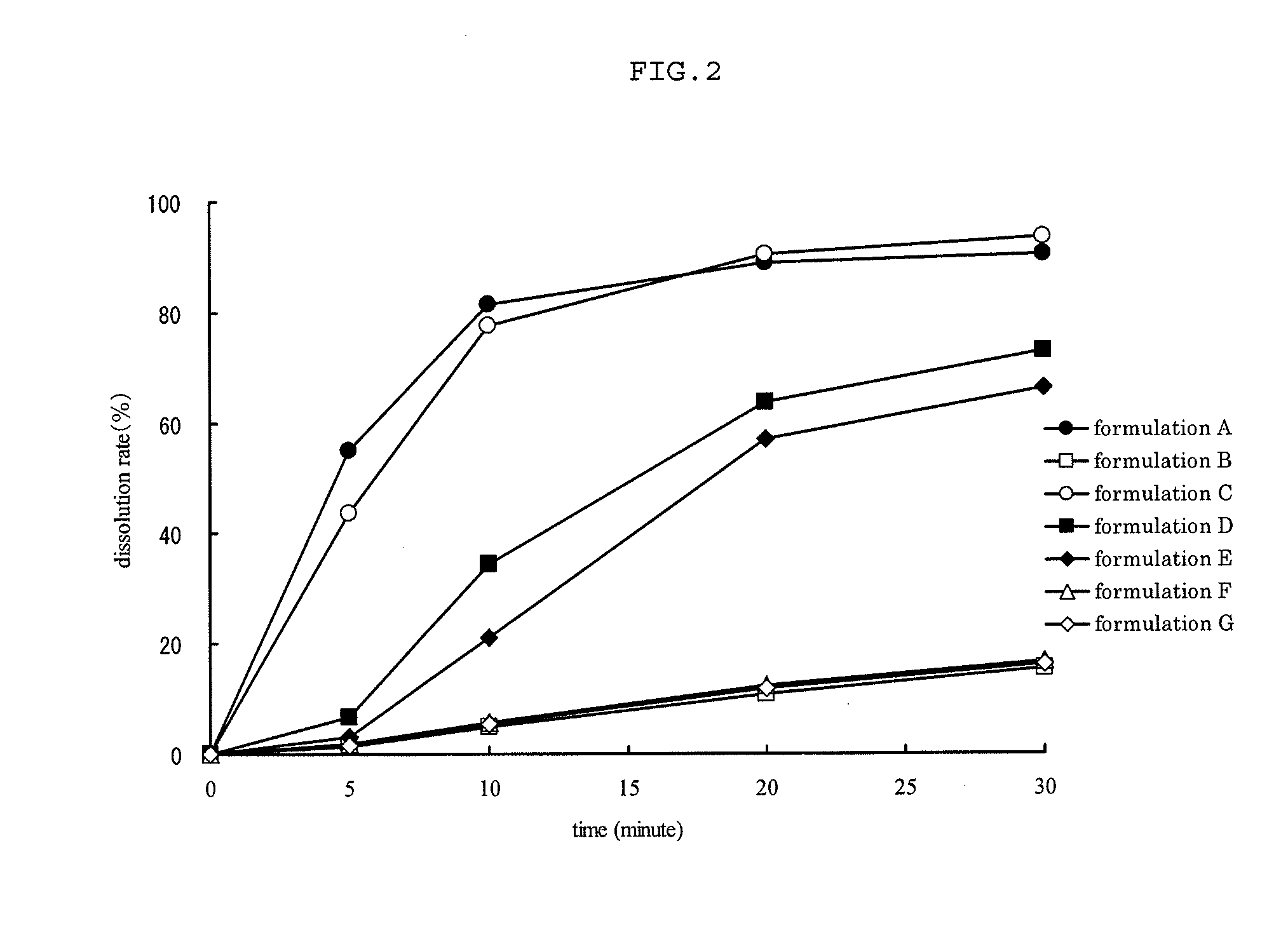
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Japanese Pharmacopoeia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Japanese Pharmacopoeia (日本薬局方) is the official pharmacopoeia of Japan. It is published by the Pharmaceuticals and Medical Devices Agency (独立行政法人 医薬品医療機器総合機構) under the authority of the Ministry of Health, Labour and Welfare. The first edition was published on 25 June, 1886, with revisions being issued from time to time. The current revision is number 17, issued electronically in English language on 7 March 2016.
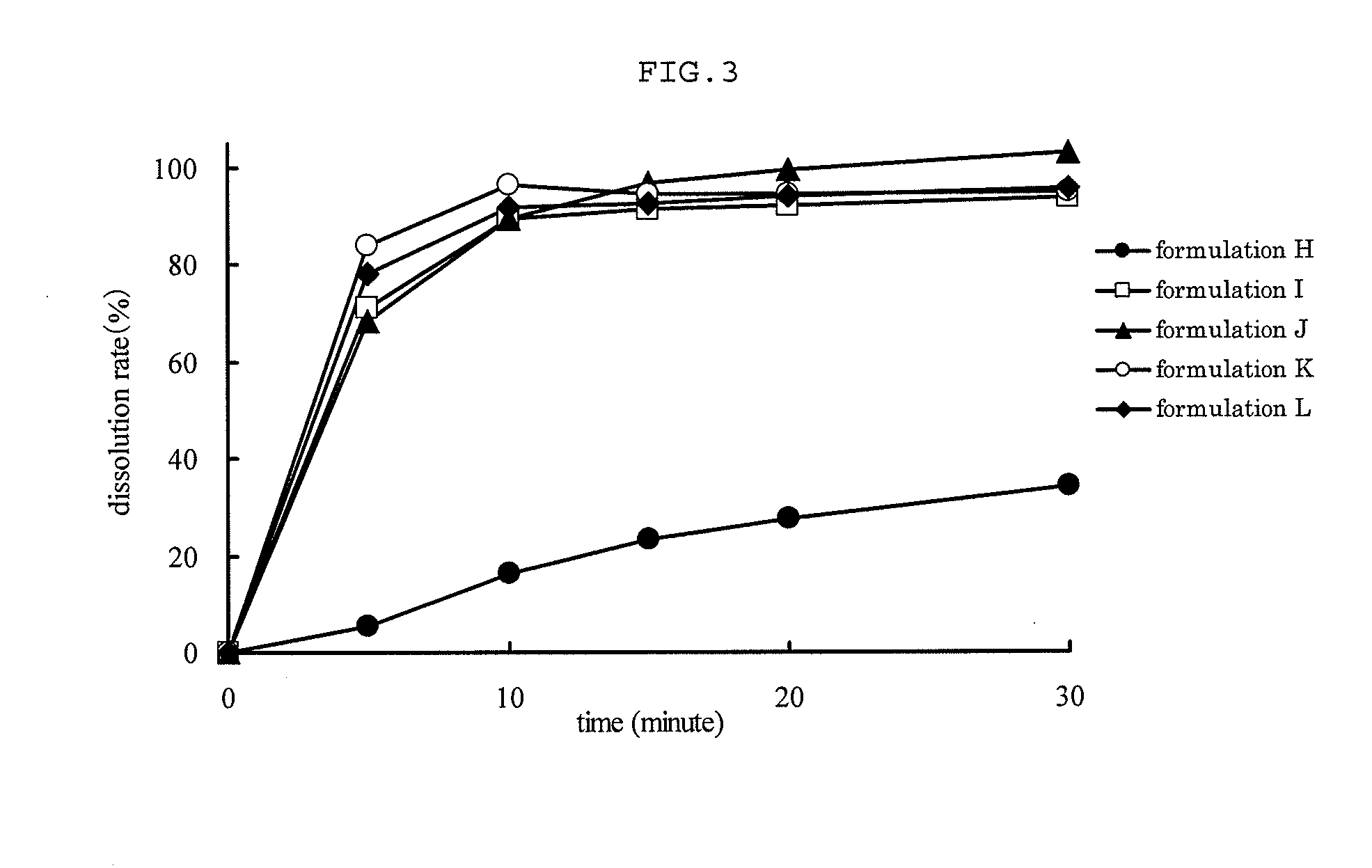
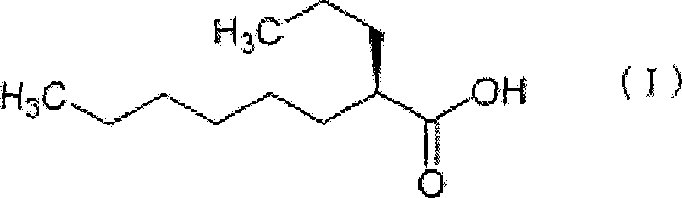
Capsule Stable Against Mastication
InactiveUS20080057115A1Good disintegrationExcellent pharmaceutical preparationAntibacterial agentsNervous disorderSlice thicknessMedicine
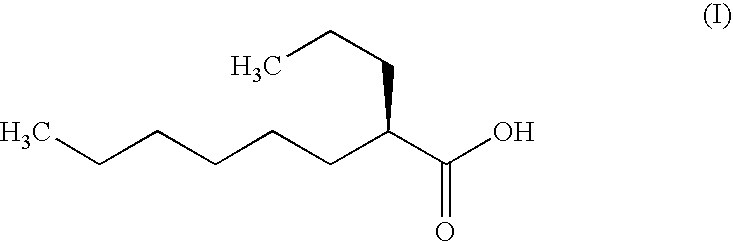
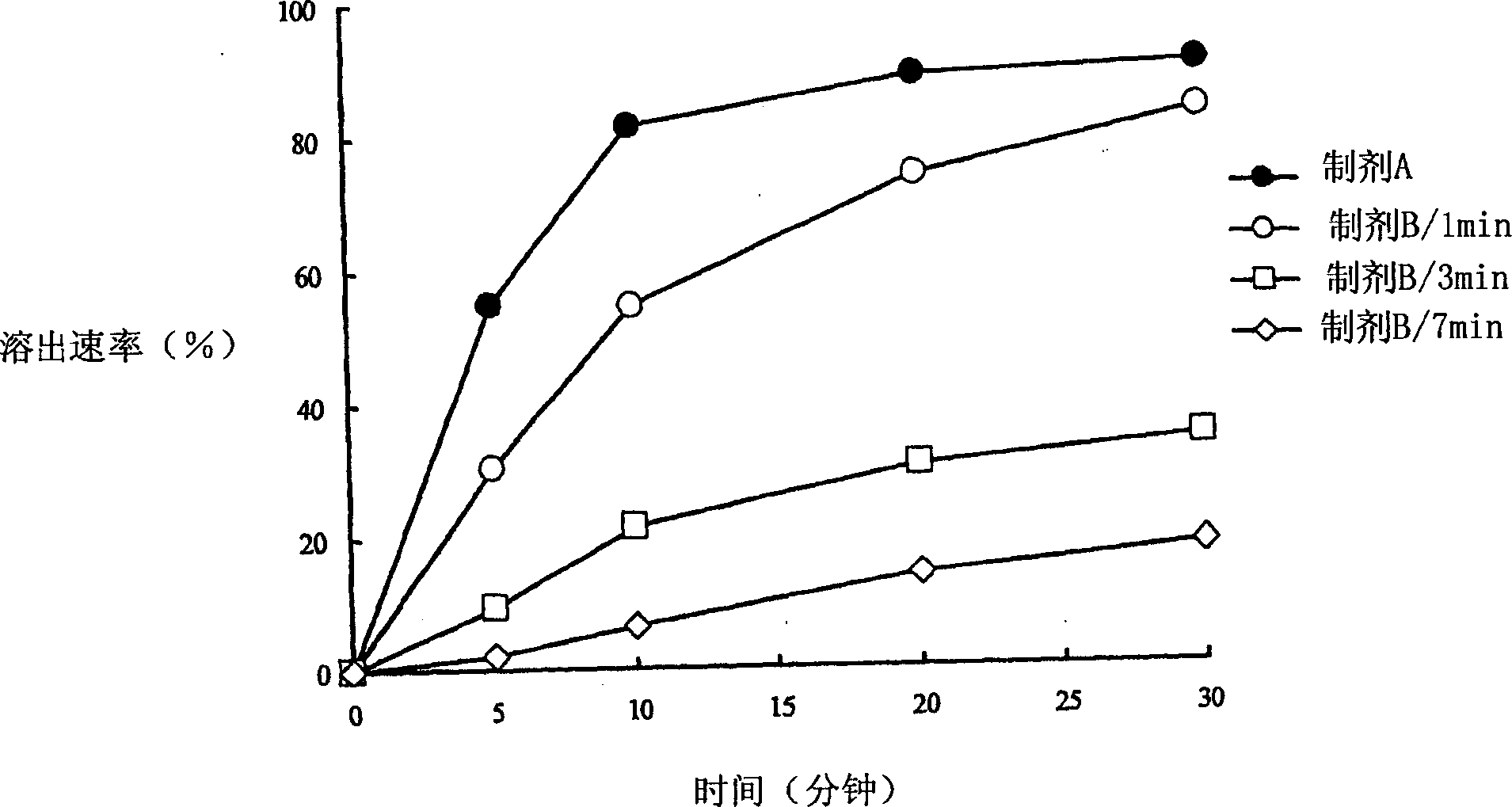
The present invention relates to a soft capsule which is easily disintegrated in the stomach, wherein the contents thereof are not easily leaked at the time of mastication, which is obtained by providing a soft capsule comprising (2R)-2-propyloctanoic acid or a salt thereof with at least one property, preferably all properties, selected from (A) wherein it has a strength of 150 to 400 N by a cracking test; (B) wherein it has a disintegration time of 3 to 10 minutes by the disintegration test stipulated in Japanese Pharmacopoeia; (C) wherein the capsule shell has a shell thickness of 0.05 to 0.50 mm; (D) wherein the capsule shell has a first seam thickness of 0.10 to 0.55 mm; (E) wherein the capsule shell has a second seam thickness of 0.05 to 0.50 mm; (F) wherein the capsule shell has a water content of 5.0 to 9.0%.
Owner:ONO PHARMA CO LTD
Sustained release pharmaceutical composition
InactiveUS20050100602A1Reduces adverse eventImprove featuresPowder deliveryBiocideTamsulosin hclJapanese Pharmacopoeia
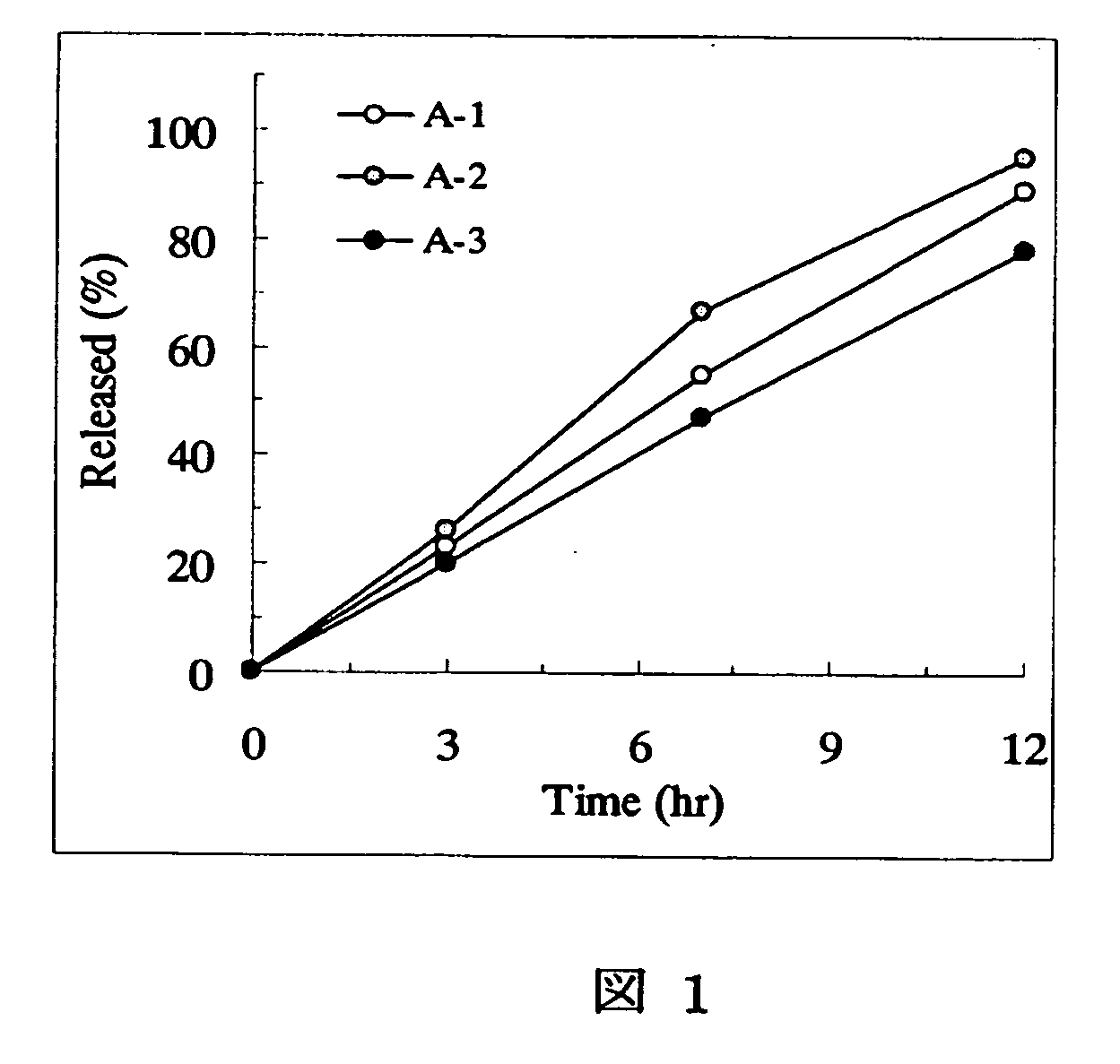
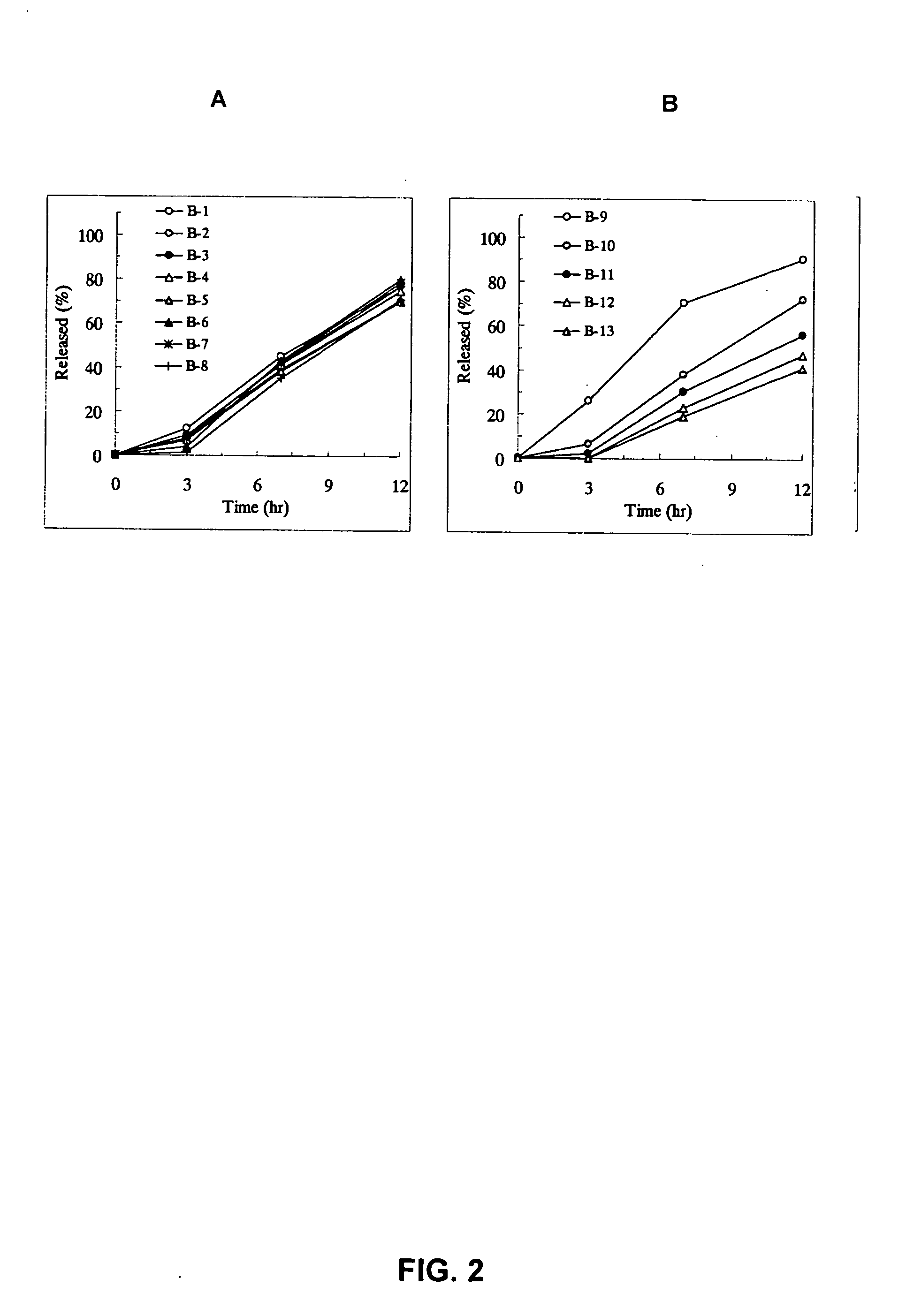
[Problem] As compared with the current oral sustained-release preparation containing tamsulosin hydrochloride which have been supplied to the medical setting, there is a problem to provide a sustained-release pharmaceutical composition in which efficacy is equivalent or even better, adverse events such as adverse reactions (e.g., postural hypotension) are reduced, dose can be increased and, if desired, ingestion of food is not limited. [Means for Resolution] A sustained-release pharmaceutical composition, characterized in that, there are contained tamsulosin or a pharmaceutically acceptable salt thereof and a carrier for a sustained-release pharmaceutical composition and, when dissolution test is carried out according to Japanese Pharmacopoeia Dissolution Test Method 2, the tamsulosin release after 7 hours from the start of the dissolution is about 20 to about 85%.
Owner:ASTELLAS PHARMA INC
Solid drug for oral use
Owner:KISSEI PHARMA
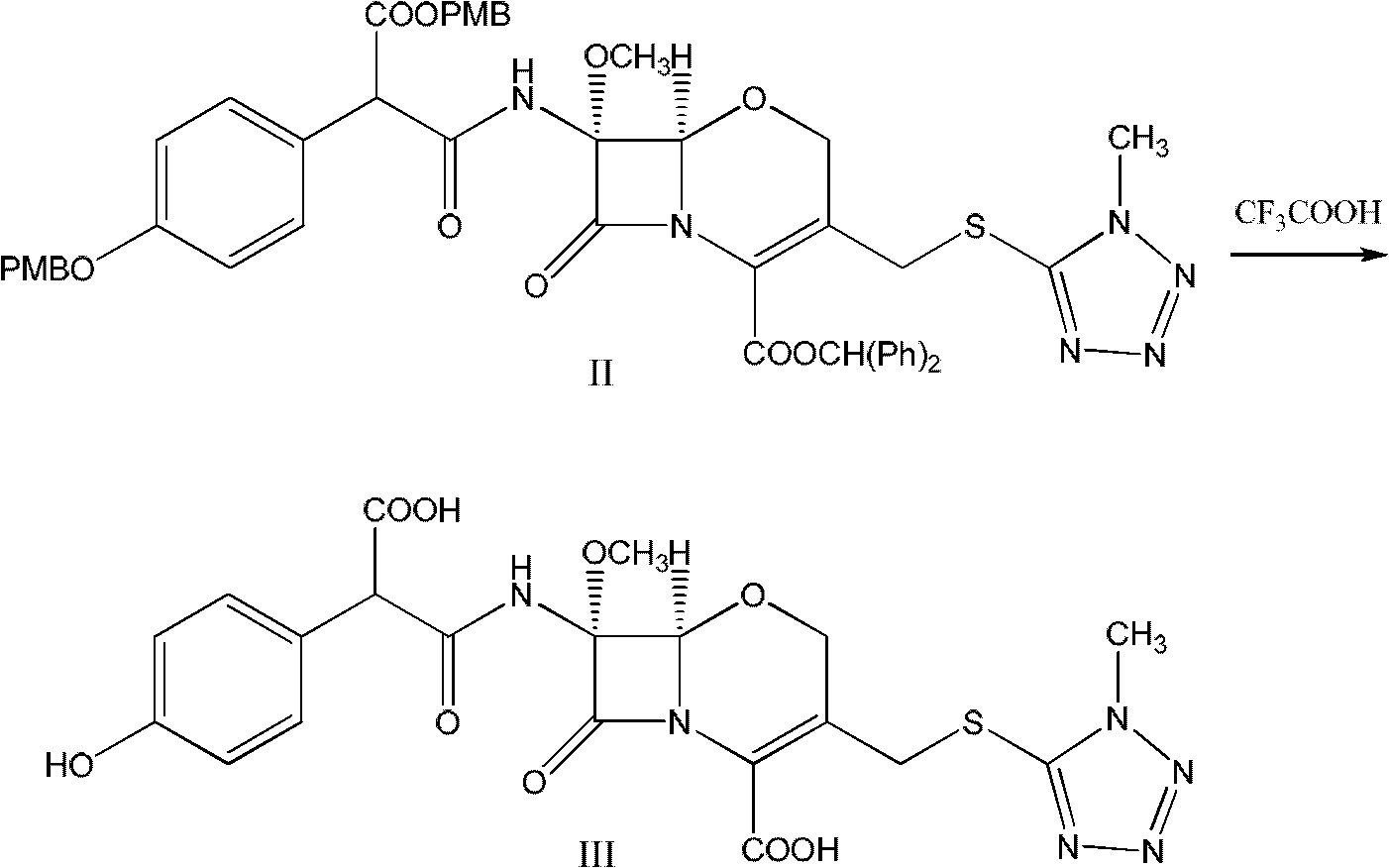
Preparation technology of high-purity flomoxef sodium
The invention discloses a preparation technology of high-purity flomoxef sodium. The preparation technology includes following steps: on the basis of a flomoxef intermediate, performing an acidifying reaction to obtain a reaction liquid; performing a water washing process, an extraction process, an aseptic filtration process and the like to the reaction liquid; and performing a solventing-out crystallization step through a one-step crystallization process to obtain a flomoxef sodium product with a purity being higher than 99.90%, wherein key points in the solventing-out crystallization step are selection and proportion of a solventing agent and dropwise addition of a salifying agent and the solventing agent at the same time with crystal growing. The preparation technology overcomes problems which are difficult to control during the solventing-out crystallization step of the flomoxef sodium. The flomoxef sodium is prepared in one step with the flomoxef intermediate as a raw material and the purity of the product of the flomoxef sodium reaches higher than 99.90% just through one crystallization step, which is cannot be achieved through a freeze-drying method or a common solventing-out crystallization process in the prior art. Quality of the product is in conformity with or even exceeds a standard in japanese pharmacopoeia JP16. The preparation technology has a more wide application prospect.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Vulcanizing system and medical chlorinated butyl rubber plug
ActiveCN102702574ARaw materials are easy to getSimple processPharmaceutical containersMedical packagingPolymer sciencePtru catalyst
The invention discloses a vulcanizing system, which consists of the following components in parts by weight: 0.5-5 parts of a vulcanizing agent, 0.1-2 parts of a catalyst and 0.5-6 parts of an activating agent, wherein the vulcanizing agent is a silane coupling agent of which the general formula is shown as Y(CH2)nSiX3; in the formula, Y represents alkyl, vinyl, an epoxy group, amino or sulfydryl, and X represents chloro or alkoxyl; the catalyst is thiolimidazoline or 2-thiolbenzimidazole; and the activating agent is zinc oxide or magnesium oxide. A medical chlorinated butyl rubber plug has the advantages of readily-available raw materials and simple process. The medical chlorinated butyl rubber plug prepared by adopting the vulcanizing system can meet the requirements of standards YBB00042005 and / or YBB00052005 issued by the State Food and Drug Administration, can meet the requirements of the Japanese Pharmacopoeia, can meet the requirement of compatibility of special medicaments simultaneously, and is equivalent to the quality of national and international products of the same type.
Owner:郑州翱翔医药科技股份有限公司
Preparation method of flomoxef sodium
The invention discloses a preparation method of flomoxef sodium. The preparation method comprises the following steps of dropwise adding a sodium bicarbonate solution into flomoxef acid while stirring at the temperature of 0-10 DEG C until the pH value of a reaction solution is up to 4.2-5.2, and after flomoxef acid is completely dissolved, extracting, decoloring and removing a decolorizing agent to obtain flomoxef sodium. By using the preparation method of flomoxef sodium, provided by the invention, flomoxef sodium can be effectively and uniformly produced in real time, the residues of sodium salt and flomoxef acid in a product are avoided, the purity of obtained flomoxef sodium is larger than or equal to 99%, and the residual quantity of 1-ethoxyl-5-thiol-1H-tetrazole is smaller than or equal to 0.2%, therefore, the standard requirement of Japanese pharmacopoeia JP16 is completely met.
Owner:SHANGHAI LONGXIANG BIO MEDICINE DEV CO LTD
Solid drug for oral use
InactiveUS20060018959A1Improve accuracyImprove stabilityAntibacterial agentsBiocideIndolineDissolution
The present invention provides a solid oral dosage form pharmaceutical for the treatment of dysuria, which comprises, as an active ingredient, an indoline compound having an α1-adrenoceptor blocking activity and represented by the formula: prodrug, pharmaceutically acceptable salt or pharmaceutically acceptable solvate thereof, wherein said pharmaceutical is prepared to have 85% dissolution time of not more than 60 minutes in a dissolution test according to method 2 (paddle method) of Japanese pharmacopoeia in a condition using water.
Owner:KISSEI PHARMA
Sustained release pharmaceutical composition
InactiveUS8197846B2Equivalent orNo less efficacyBiocidePowder deliveryDissolutionJapanese Pharmacopoeia
The present invention provides a sustained-release pharmaceutical composition, characterized in that, there are contained tamsulosin or a pharmaceutically acceptable salt thereof and a carrier for a sustained-release pharmaceutical composition and, when a dissolution test is carried out according to Japanese Pharmacopoeia Dissolution Test Method 2, the tamsulosin release after 7 hours from the start of the dissolution is about 20 to about 85%.
Owner:ASTELLAS PHARMA INC
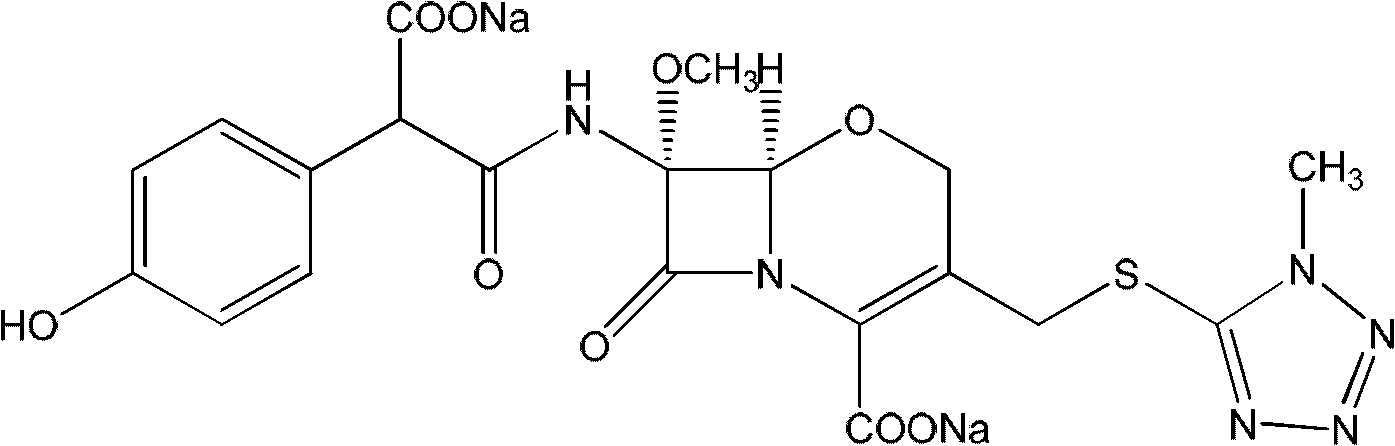
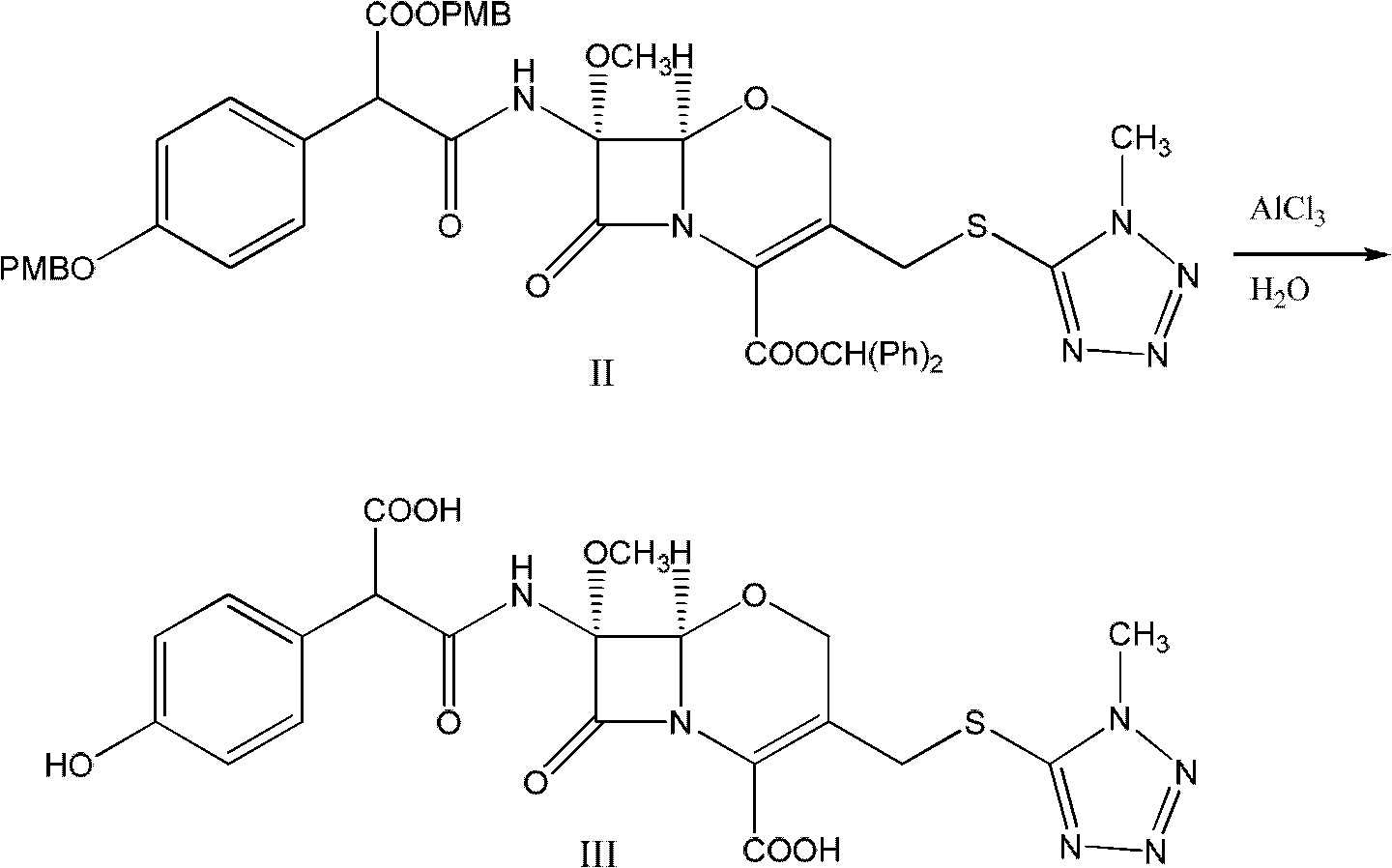
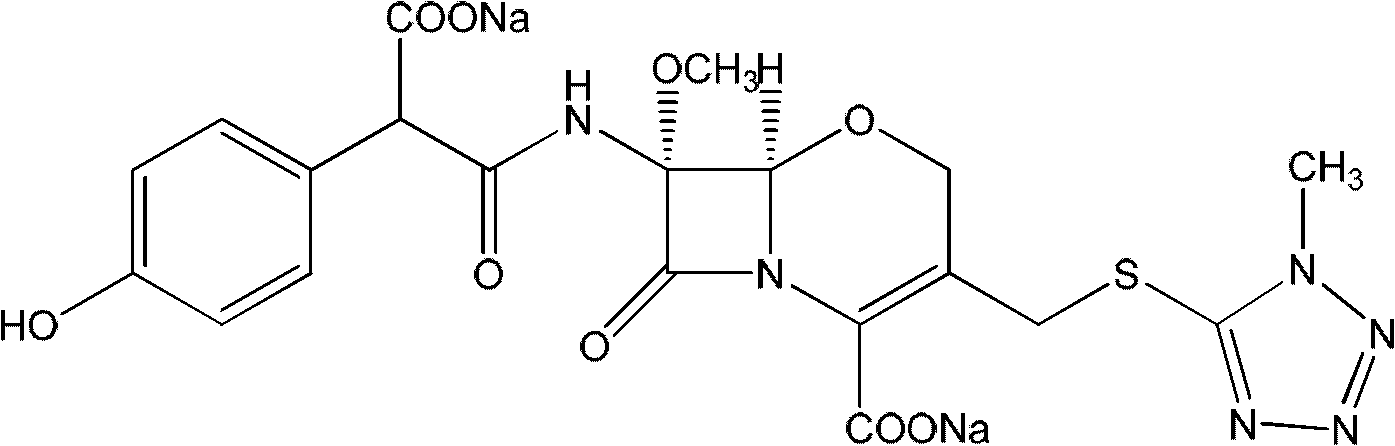
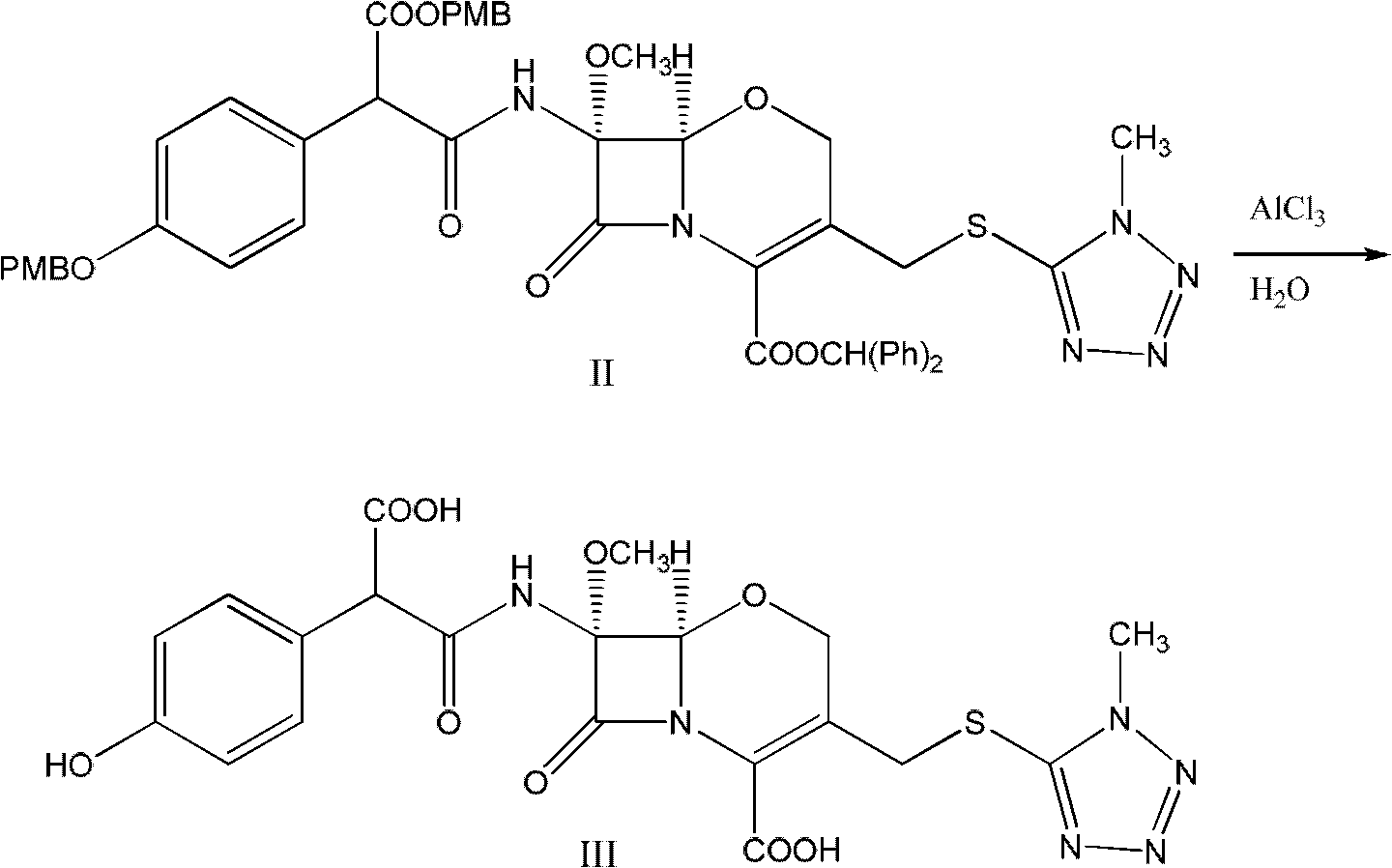
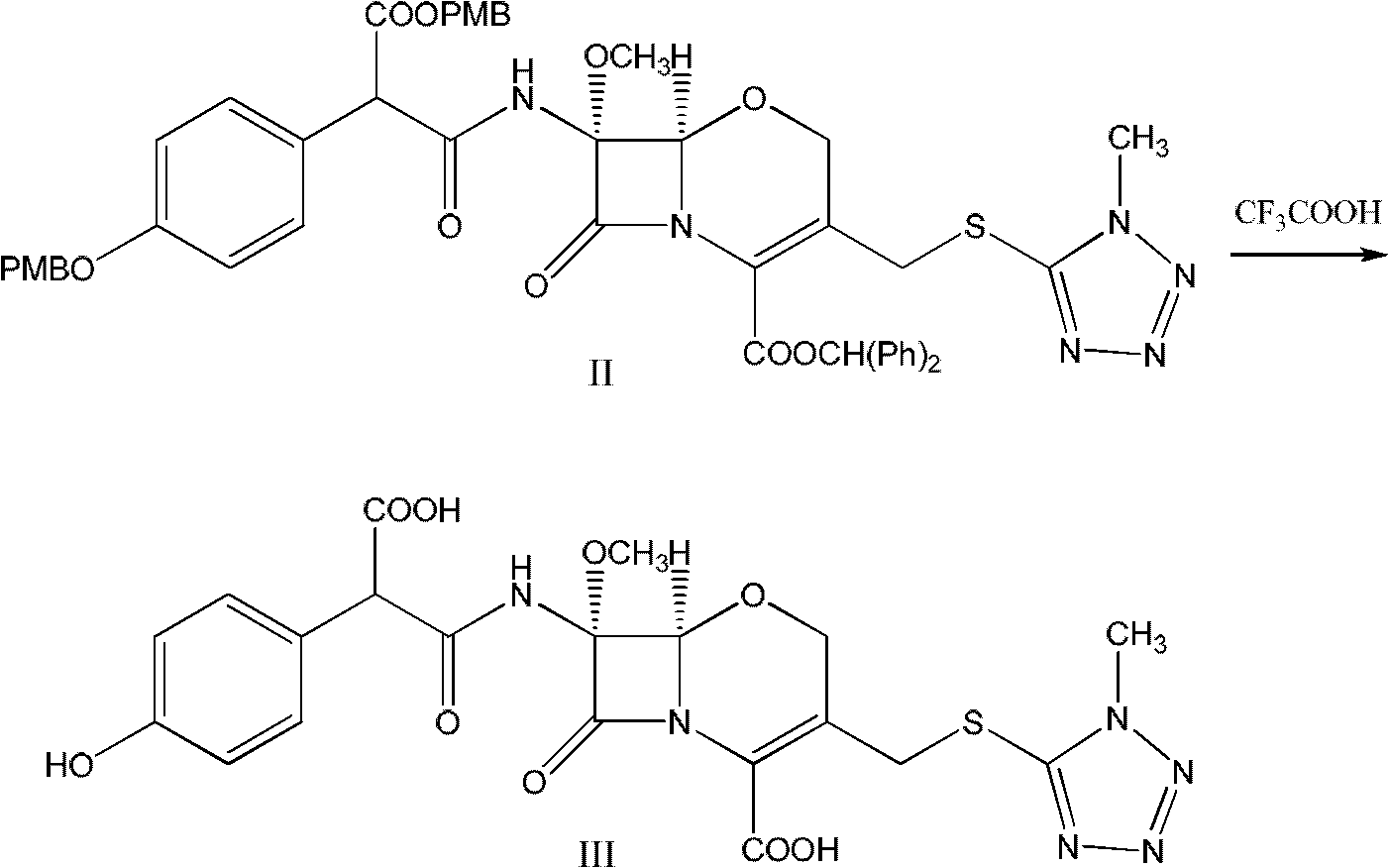
Latamoxef aluminium chloride (stannum)-anisole complex, as well as preparation method and application thereof
ActiveCN102603784AEasy to prepareEasy to separateTin organic compoundsGroup 3/13 element organic compoundsAluminium chlorideJapanese Pharmacopoeia
The invention relates to an intermediate for synthesizing latamoxef and a preparation method of the intermediate. The intermediate provided by the invention is a latamoxef aluminium chloride (stannum)-anisole complex which is simple and convenient in preparation method, easy to separate, high in content and yield and stability and low in cost and can be conveniently converted to latamoxef acid (sodium) for current use, so that the difficulty that purity of latamoxef acid (sodium) synthesized by the prior art is inadequate can be overcome. The latamoxef sodium prepared by using the complex as raw material and hydrolyzing has a purity of greater than 99%, and the purity is considerably higher than the standard of the Chinese pharmacopoeia (single impurity is not higher than 2% and total impurities are not higher than 5%) and that of the Japanese pharmacopoeia.
Owner:ZHEJIANG WHITESON PHARMA
Solid pharmaceutical preparation dissolved in oral cavity
A solid pharmaceutical preparation dissolved in oral cavity, comprising part containing an intraoral antiinflammatory ingredient of 45 sec to 2 min disintegration time, exhibited in a disintegration test according to the Japanese Pharmacopoeia disintegration test, and part containing an intraoral antimicrobial ingredient of > 3 min disintegration time. This solid pharmaceutical preparation enables to easily realize simultaneous accomplishment of treatment of throat pain and intraoral sustained sterilization by means of a single pharmaceutical preparation.
Owner:KOWA CO LTD
Purification method for obtaining high-purity recombinant human serum albumin
PendingCN110092827AEfficient separationFacilitate efficient separationSerum albuminPeptide preparation methodsPurification methodsUltrafiltration
The invention relates to a purification method for obtaining high-purity recombinant human serum albumin. The method comprises the following steps: centrifugal ultrafiltration and secondary ultrafiltration are carried out after centrifuging and heating the fermentation liquid, the impurities encapsulated in the recombinant human serum albumin are then removed using 30% of alcohol and 6 of mol / L urea, the protein is renatured, eluted, and dialyzed to obtain the decontamination liquid, finally, the obtained decontamination liquid is sequentially subjected to cation exchange chromatography, anionexchange chromatography, and hydrophobic chromatography to obtain the high-purity recombinant human serum albumin. The recombinant human serum albumin obtained by the method has the advantages of high purity, less impurities, low degradation, high stability, and the like, and has the good application prospect. The final obtained recombinant human serum albumin reaches the level of pharmaceuticalgrade (the purity is greater than 99.999999%), which fully meets the requirements of the US Pharmacopoeia and the Japanese Pharmacopoeia for the injection-level recombinant human serum albumin.
Owner:山东健通生物科技有限公司
Resin molded article
The present invention pertains to a resin formed article obtained by forming a resin composition that comprises a cycloolefin resin and a styrene-based thermoplastic elastomer, the styrene-based thermoplastic elastomer having a weight average molecular weight of 20,000 to 150,000, and having a difference in refractive index (ΔnD) of more than −0.002 to less than +0.002 with respect to the cycloolefin resin, the resin composition having a residual ratio of 0.10 wt % or less when analyzed based on the residue on ignition test method specified in the Japanese Pharmacopeia, the resin composition having a light transmittance (optical path length: 3 mm) of 55% or more with respect to light having a wavelength of 450 nm when the resin composition is formed in a shape of a sheet having a thickness of 3.0 mm, and subjected to light transmittance measurement, and the resin composition having a Charpy impact strength of 5 to 40 kJ / m2 when the resin composition is formed to have a thickness of 4.0 mm, a length of 80.0 mm, and a width of 10.0 mm, and subjected to the notched Charpy impact test specified in JIS K 7111-1 at 23° C.
Owner:ZEON CORP
Sustained release preparation
InactiveUS20090148480A1Sufficient gastric residence timeEasy to prepareOrganic active ingredientsDigestive systemPharmaceutical drugJapanese Pharmacopoeia
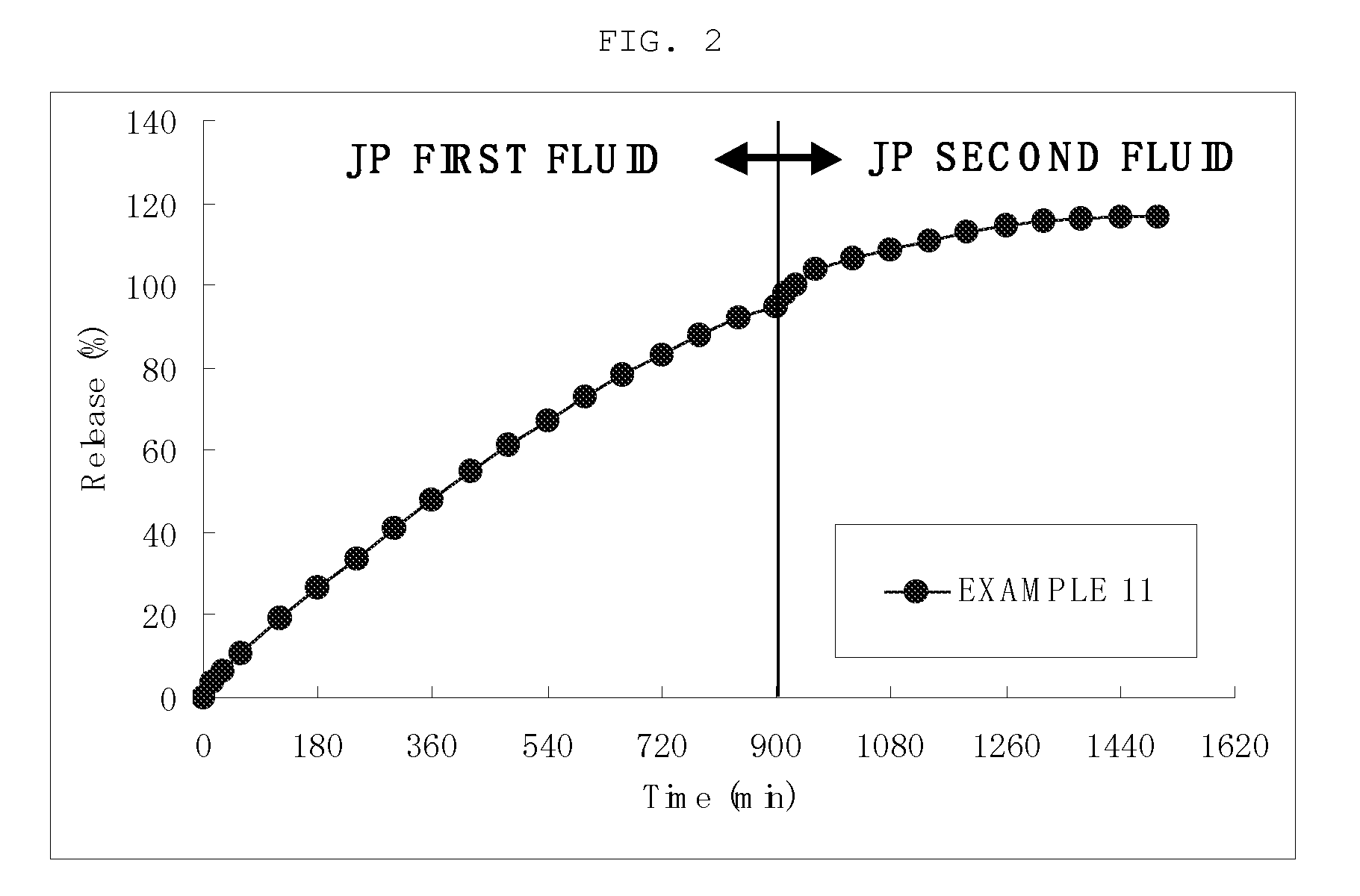
The invention provides a preparation which shows a satisfactory gastric residence time, has such a size that allows for easy ingestion, can quickly disintegrate after expelled from the stomach, and can be prepared readily in an industrial scale. A gastric retentive preparation having a gastric resident layer and a drug release layer is provided, wherein the gastric resident layer does not disintegrate in the stomach and disintegrates in the intestine. Preferably, the gastric resident layer has a minimum diameter of 7 mm or more as measured after stirring the preparation in the first fluid at 200 rpm at 37° C. for 15 hours under the conditions of the paddle method in the dissolution test in accordance with Japanese Pharmacopoeia and has a maximum diameter of 6 mm or less as measured after further stirring the preparation in the second fluid at 200 rpm at 37° C. for 9 hours under the same conditions.
Owner:KISSEI PHARMA
Production method of medicinal rubber plug
ActiveCN105111488ASealing is not affectedMeet the standard YBB00042005OfficinalBiochemical engineering
The invention discloses a production method of a medicinal rubber plug. The production method includes: thermally treating a rubber plug under a vacuum condition; using water to rinse the rubber plug which is subjected to vacuum thermal treatment; subjecting the rubber plug which is rinsed to siliconization and drying to obtain the medicinal rubber plug. The production method is simple in process, pollution-free, easy for industrialization, energy saving and environment friendly. Extractable matter and extract in the medicinal butyl rubber plug which is treated by the production method are reduced to great extent, sealability of an original butyl rubber plug is unaffected, and the medicinal rubber plug can meet requirements of Standards YBB00042005 and / or YBB00052005 issued by the State Food and Drug Administration, can meet requirements on compatibility of special drug and can reach requirements of the Japanese Pharmacopoeia.
Owner:郑州翱翔医药科技股份有限公司
Oral liquid preparation
InactiveCN102458406APromote dissolutionOrganic active ingredientsNervous disorderPyridineBULK ACTIVE INGREDIENT
Disclosed is an oral liquid preparation which contains 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10- hexahydrocycloocta[b] pyridine (an active ingredient) stably and can exhibit good elution behavior of the active ingredient in a wide pH range. The oral liquid preparation comprises the active ingredient and a dissolution aid for the active ingredient, and has an elution rate of 50% or more in an elution test in accordance with the Japanese pharmacopoeia 15th edition 15 minutes after the start of the test in a second elution test solution.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Solid drug for oral use
InactiveUS20120064154A1Improve accuracyImprove stabilityAntibacterial agentsOrganic active ingredientsIndolineDissolution
The present invention provides a solid oral dosage form pharmaceutical for the treatment of dysuria, which comprises, as an active ingredient, an indoline compound having an α1-adrenoceptor blocking activity and represented by the formula:prodrug, pharmaceutically acceptable salt or pharmaceutically acceptable solvate thereof, wherein said pharmaceutical is prepared to have 85% dissolution time of not more than 60 minutes in a dissolution test according to method 2 (paddle method) of Japanese pharmacopoeia in a condition using water.
Owner:KISSEI PHARMA
The preparation method of fluoxefor sodium
The invention discloses a preparation method of flomoxef sodium. The preparation method comprises the following steps of dropwise adding a sodium bicarbonate solution into flomoxef acid while stirring at the temperature of 0-10 DEG C until the pH value of a reaction solution is up to 4.2-5.2, and after flomoxef acid is completely dissolved, extracting, decoloring and removing a decolorizing agent to obtain flomoxef sodium. By using the preparation method of flomoxef sodium, provided by the invention, flomoxef sodium can be effectively and uniformly produced in real time, the residues of sodium salt and flomoxef acid in a product are avoided, the purity of obtained flomoxef sodium is larger than or equal to 99%, and the residual quantity of 1-ethoxyl-5-thiol-1H-tetrazole is smaller than or equal to 0.2%, therefore, the standard requirement of Japanese pharmacopoeia JP16 is completely met.
Owner:SHANGHAI LONGXIANG BIO MEDICINE DEV CO LTD
Preparation process of high-purity fluoxefom sodium
The invention discloses a preparation technology of high-purity flomoxef sodium. The preparation technology includes following steps: on the basis of a flomoxef intermediate, performing an acidifying reaction to obtain a reaction liquid; performing a water washing process, an extraction process, an aseptic filtration process and the like to the reaction liquid; and performing a solventing-out crystallization step through a one-step crystallization process to obtain a flomoxef sodium product with a purity being higher than 99.90%, wherein key points in the solventing-out crystallization step are selection and proportion of a solventing agent and dropwise addition of a salifying agent and the solventing agent at the same time with crystal growing. The preparation technology overcomes problems which are difficult to control during the solventing-out crystallization step of the flomoxef sodium. The flomoxef sodium is prepared in one step with the flomoxef intermediate as a raw material and the purity of the product of the flomoxef sodium reaches higher than 99.90% just through one crystallization step, which is cannot be achieved through a freeze-drying method or a common solventing-out crystallization process in the prior art. Quality of the product is in conformity with or even exceeds a standard in japanese pharmacopoeia JP16. The preparation technology has a more wide application prospect.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Capsule stable against mastication
InactiveCN101001622ASafe to takePromote absorptionNervous disorderCapsule deliveryJapanese PharmacopoeiaUltimate tensile strength
Soft capsules being easily disintegratable in the stomach and avoiding any ready leakage of contents at mastication, which soft capsules encapsulate (2R)-2-propyloctanoic acid or its salt and are characterized in that there is realized at least one property, preferably all thereof, selected from among (A) strength of 150 to 400 N as measured by a cracking load test; (B) disintegration time of 3 to 10 min as measured by a disintegration testing method stipulated in Japanese Pharmacopoeia; (C) thickness of 0.05 to 0.50 mm with respect to the central part of capsule shell; (D) thickness of 0.10 to 0.55 mm with respect to the first joint part of capsule shell; (E) thickness of 0.05 to 0.50 mm with respect to the second joint part of capsule shell; and (F) capsule shell water content of 5.0 to 9.0%.
Owner:ONO PHARMA CO LTD
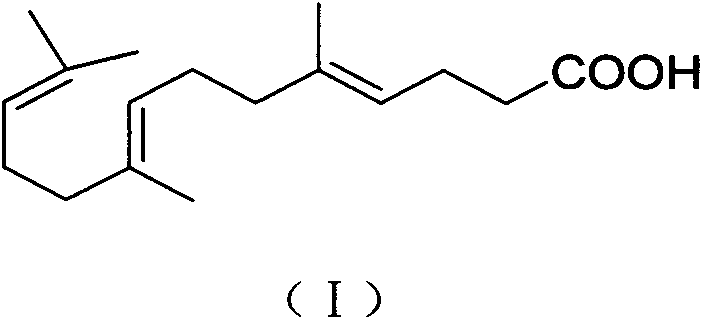
Gefarnate key intermediate refining or reaction solution direct post-processing method
InactiveCN104370731AGood effectEfficient removalCarboxylic compound separation/purificationOrganic solventGeraniol
The invention discloses a gefarnate key intermediate (E-farnesyl acid) refining or reaction solution post-processing method, and belongs to medicines. The method is as follows: according to the fact that E-farnesyl acid is water-soluble in alkaline conditions and insoluble in water in acidic conditions, through washing and filtering, fat soluble impurities can be removed in alkaline conditions, and water soluble impurities can be removed in acidic conditions; and the step is as follows: dissolving the E-farnesyl acid with an alkali solution, washing and filtering with an organic solvent, then adjusting filtrate to acidic, adding an organic solvent for extraction, and concentrating to obtain high purity E-farnesyl acid. Finally, gefarnate is prepared from the E-farnesyl acid and geraniol by the method well-known by technical personnel in the field. The gefarnate key intermediate (E-farnesyl acid) refining method can well improve the E-farnesyl acid purity, and especially removes the fat soluble impurities to enable finished product gefarnate after esterification reaction to fully meet and be higher than Japanese Pharmacopoeia standards, the purity can reach 99.5%, and the method is simple in operation, and is more suitable for industrial production.
Owner:NANJING REAL PHARMA
Particle coating preparation
InactiveCN102958522AEasy to takeDoes not cause irritationOrganic active ingredientsPill deliveryIrritationCarboxylic acid
Disclosed is a preparation which, in an orally fast-disintegrating tablet having as an active ingredient 2-(3-cyano-4-isobutyloxyphenyl)-4-methyl-5-thiazole carboxylic acid, causes basically no irritation in the mouth or throat, retains good dissolvability and oral disintegration even when stored under high temperature or high humidity conditions. The disclosed orally fast-disintegrating tablet is formed to contain particles comprising nuclear particles which contain 2-(3-cyano-4-isobutyloxyphenyl)-4-methyl-5-thiazole carboxylic acid, which are covered by a layer containing a methacrylic acid copolymer, and which are further coated with an outer layer containing water-soluble sugars, such that after two weeks of storage under conditions with the cap open at 40C / 75% RH, a dissolution test performed according to the Japanese Pharmacopoeia Paddle Method at 50 revolutions per minute (test solution: pH6.0McIlvaine buffer) evaluates the dissolution rate of the 2-(3-cyano-4-isobutyloxyphenyl)-4-methyl-5-thiazole carboxylic acid at 70% or greater after 10 minutes.
Owner:TEIJIN LTD
Composition containing swelling agent comprising swellable water-soluble polysaccharide and food, obesity preventive, and constipation alleviator each containing the composition
InactiveCN101022814AOrganic active ingredientsMetabolism disorderIntestinal structureWater soluble polysaccharides
To provide: a composition containing a swelling agent which less imposes a burden on the stomach or intestines even when the composition is taken in excess; and a food, obesity preventive, and constipation alleviator each containing the composition. [MEANS FOR SOLVING PROBLEMS] The composition is obtained by mixing the Japanese-Pharmacopoeia first liquid or second liquid, a swelling agent comprising a water-soluble polysaccharide swellable in water, and a swelling inhibitor which controls swelling of the swelling agent, bringing the mixture into a homogeneous solution state, and then drying it.
Owner:INA FOOD IND
Test method for endotoxin
ActiveUS20090076054A1Accurately detected and quantifiedBiocideMicrobiological testing/measurementJapanese PharmacopoeiaToxin
An object of the present invention is to provide a method capable of detecting and quantifying endotoxin in a sample in which endotoxin derived from gram-negative bacteria cannot be accurately detected or quantified by the method described in Commentary of the Japanese Pharmacopoeia Fourteenth Edition, Hirokawa Publishing Co. 2001 B-63. It has been found that the above object can be achieved by performing an endotoxin test using a lysate reagent in which the lysate reagent is added into a sample in the presence of albumin and / or globulin.
Owner:DAIICHI SANKYO CO LTD
Vulcanizing system and medical chlorinated butyl rubber plug
ActiveCN102702574BRaw materials are easy to getSimple processPharmaceutical containersMedical packagingPolymer sciencePtru catalyst
The invention discloses a vulcanizing system, which consists of the following components in parts by weight: 0.5-5 parts of a vulcanizing agent, 0.1-2 parts of a catalyst and 0.5-6 parts of an activating agent, wherein the vulcanizing agent is a silane coupling agent of which the general formula is shown as Y(CH2)nSiX3; in the formula, Y represents alkyl, vinyl, an epoxy group, amino or sulfydryl, and X represents chloro or alkoxyl; the catalyst is thiolimidazoline or 2-thiolbenzimidazole; and the activating agent is zinc oxide or magnesium oxide. A medical chlorinated butyl rubber plug has the advantages of readily-available raw materials and simple process. The medical chlorinated butyl rubber plug prepared by adopting the vulcanizing system can meet the requirements of standards YBB00042005 and / or YBB00052005 issued by the State Food and Drug Administration, can meet the requirements of the Japanese Pharmacopoeia, can meet the requirement of compatibility of special medicaments simultaneously, and is equivalent to the quality of national and international products of the same type.
Owner:郑州翱翔医药科技股份有限公司
Composition for forming hydrogel, hydrogel, and method for producing composition for forming hydrogel
Provided is a hydrogel-forming composition capable of forming a sterilized hydrogel having a high mechanical strength. Also provided are a hydrogel using the hydrogel-forming composition, and a method for producing the hydrogel-forming composition. The hydrogel-forming composition contains a vinyl alcohol polymer having an ethylenically unsaturated group and having a polymerization degree of 450 or more, in which the ethylenically unsaturated group introduction ratio is 0.01 to 10 mol % in all the structural units constituting the vinyl alcohol polymer, and in which no microorganisms detectable by the sterility test method (direct method) specified in the general test methods of the Japanese Pharmacopoeia are present.
Owner:KURARAY CO LTD
Methods for detecting an endotoxin with a lysate reagent
ActiveUS7833747B2Accurately detected and quantifiedBiocideMicrobiological testing/measurementJapanese PharmacopoeiaToxin
An object of the present invention is to provide a method capable of detecting and quantifying endotoxin in a sample in which endotoxin derived from gram-negative bacteria cannot be accurately detected or quantified by the method described in Commentary of the Japanese Pharmacopoeia Fourteenth Edition, Hirokawa Publishing Co. 2001 B-63. It has been found that the above object can be achieved by performing an endotoxin test using a lysate reagent in which the lysate reagent is added into a sample in the presence of albumin and / or globulin.
Owner:DAIICHI SANKYO CO LTD
Laxative tablet
ActiveCN113015555AFine disintegration timeShort disintegration timeDispersion deliveryDigestive systemMedicinePhysical chemistry
Provided is a laxative tablet which includes magnesium oxide as a main constituent, wherein (1) the particle size by volume of 50% (D50) of particles which emerge when the tablet is suspended in water is 70 mum or less as determined by laser diffractometry, (2) the particle size by volume of 90% (D90) of the particles which emerge when the tablet is suspended in water is 130 mum or less as determined by laser diffractometry, and (3) the dissolution time of the tablet when the tablet is suspended in water according to the Japanese Pharmacopoeia - general testing methods - dissolution testing methods is 10 seconds or less.
Owner:SETOLAS HLDG INC
Latamoxef aluminium chloride (stannum)-anisole complex, as well as preparation method and application thereof
ActiveCN102603784BEasy to prepareEasy to separateTin organic compoundsGroup 3/13 element organic compoundsAluminium chlorideJapanese Pharmacopoeia
The invention relates to an intermediate for synthesizing latamoxef and a preparation method of the intermediate. The intermediate provided by the invention is a latamoxef aluminium chloride (stannum)-anisole complex which is simple and convenient in preparation method, easy to separate, high in content and yield and stability and low in cost and can be conveniently converted to latamoxef acid (sodium) for current use, so that the difficulty that purity of latamoxef acid (sodium) synthesized by the prior art is inadequate can be overcome. The latamoxef sodium prepared by using the complex as raw material and hydrolyzing has a purity of greater than 99%, and the purity is considerably higher than the standard of the Chinese pharmacopoeia (single impurity is not higher than 2% and total impurities are not higher than 5%) and that of the Japanese pharmacopoeia.
Owner:ZHEJIANG WHITESON PHARMA
Method for detecting arbekacin sulfate and impurities thereof
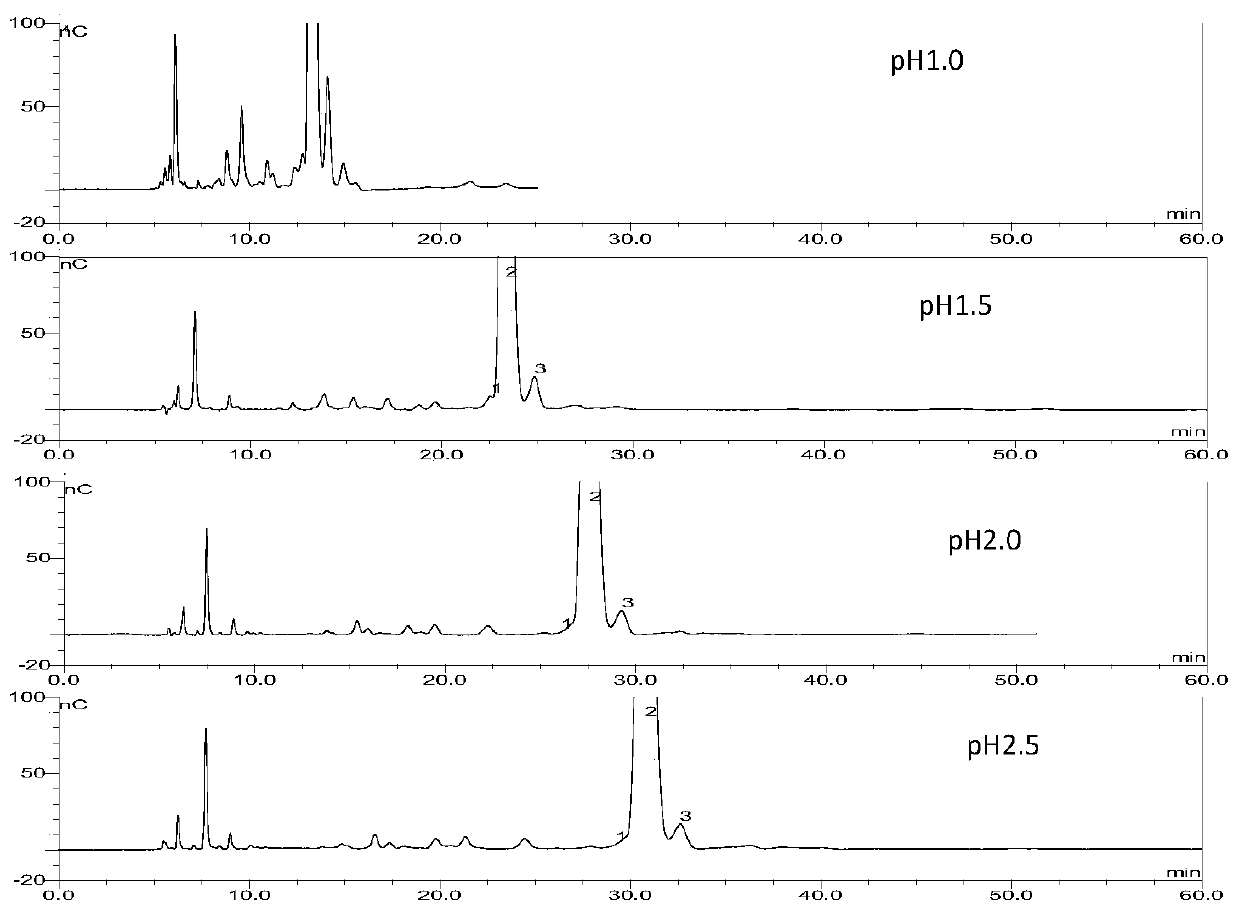
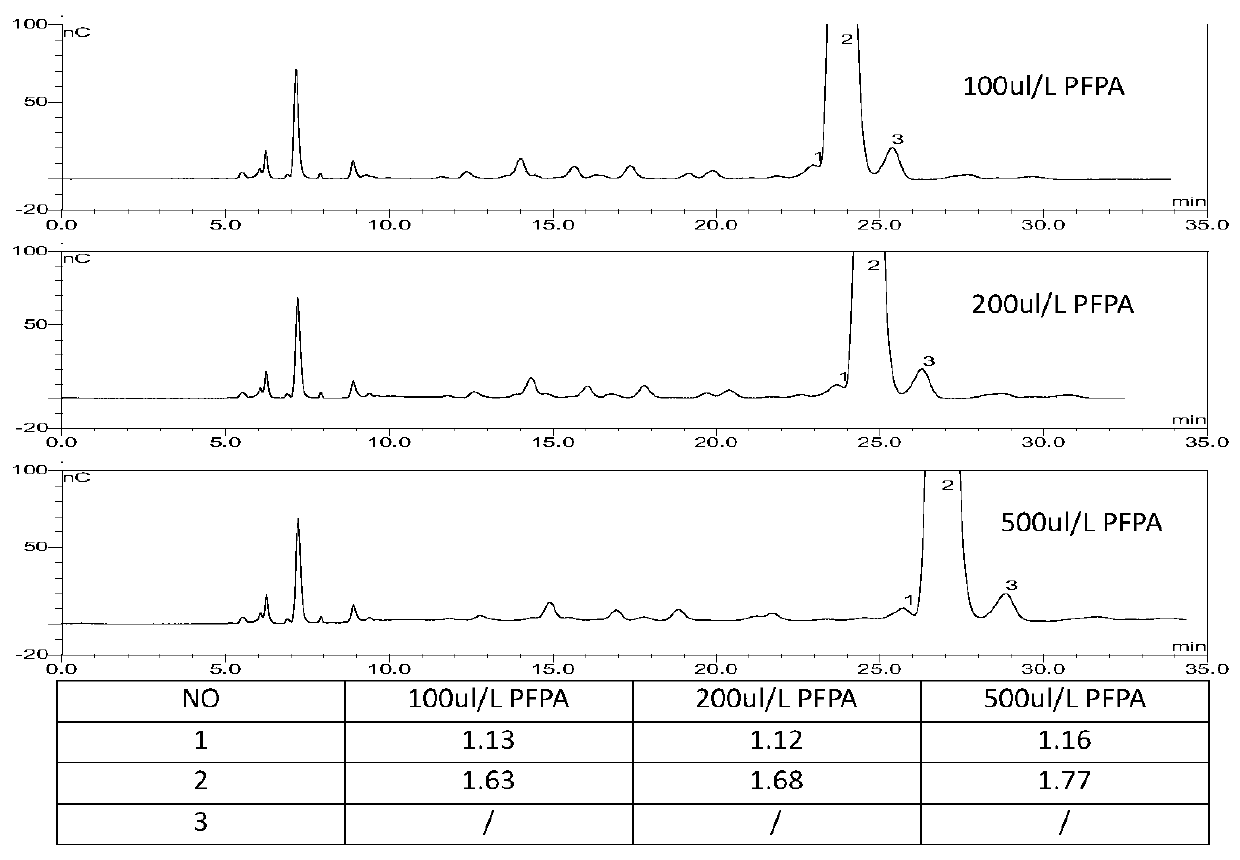
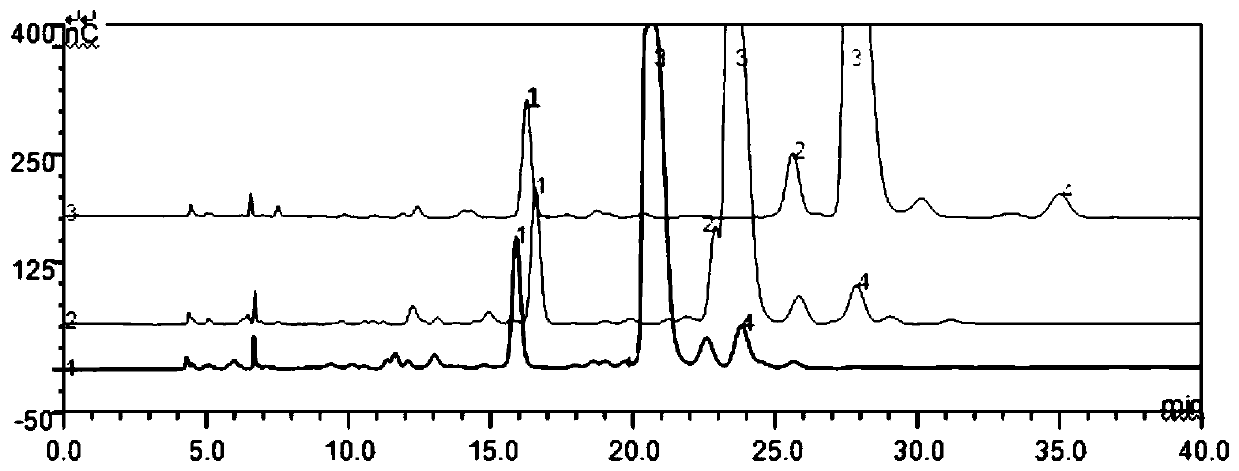
The invention relates to Arbekacin sulfate, in particular to a method for detecting Arbekacin sulfate and impurities thereof. The method for detecting the Arbekacin sulfate and the impurities of the Arbekacin sulfate is obtained through optimization. The HPLC-PAD method for determining the content of the arbekacin sulfate and the related substances is established for the first time, and compared with a Japanese pharmacopeia loading method, the specificity is good, and the method is durable.
Owner:江苏省食品药品监督检验研究院 +1
Method for preparing cation exchange resin of calcium polystyrolsulfon acid
The invention relates to a polystyrene sulfonic acid sodium cation exchange resin which is copolymerization prepared by the styrene-two ethylene benzene, through the compound which contains calcium ion transform, it purifies to the polystyrene sulfonic acid calcium resin, the compound which contains calcium ion is composed of calcium ion and its balance ion, the complexing agent, the acid alkalinity regulator and water. The balance ion is the chlorine ion; the complexing agent is: one kinds, two kinds or more than two kinds of the ethylene diamine four ethanoic acid, the second two support three amine five ethanoic acid, the ammonia three acetic acid, the citric acid and the calcium salt or sodium salt; the acid alkalinity regulator is hydrochloric acid and sodium hydroxide or hydrogen calcium oxide and so on abio-acid and alkali. The invention technical index has achieved to the standard of outward appearance,the calcium content, the potassium exchange quantity and the impurity (ammonium, heavy metal, arsenic, styrene and sodium) in the Japanese pharmacopoeia (JP14 e), the residual 1,2- dichloroethane content conforms to the standard about the organic solvent in the Chinese pharmacopoeia (2005 editions).
Owner:JIANGSU POLYTECHNIC UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com