Laxative tablet
A technology for tablets and laxatives, which is applied in the directions of pill delivery, medical preparations of inactive ingredients, and drug combinations, etc., can solve the problems of water suspension being difficult to pass through nutrition tubes, etc., and achieves the advantages of short disintegration time and reducing the burden of taking. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
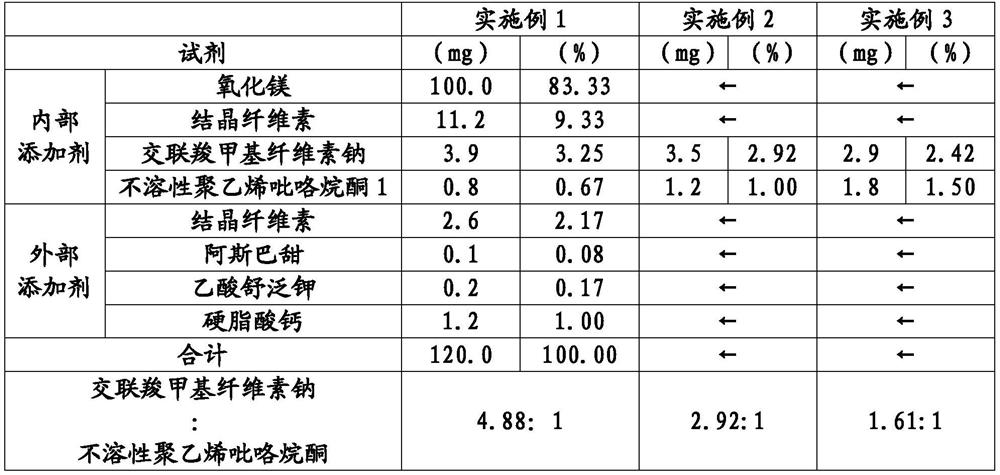
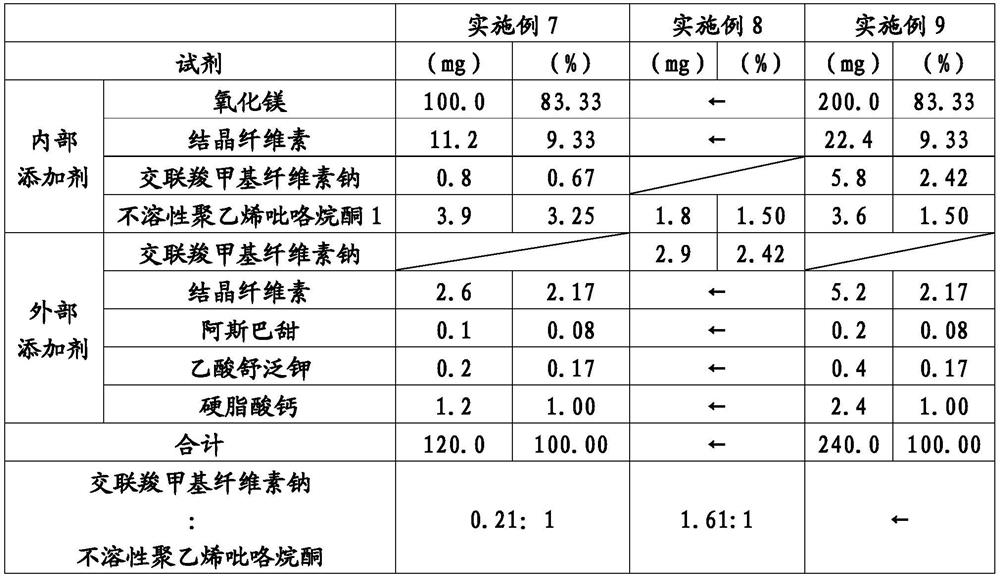
[0111] According to the formulation in Table 1, tablets for laxatives were produced by the following production method. In addition, in Tables 1-6, "internal additive" means the reagent used at the time of producing a granule, and "external additive" means the reagent further mixed with the granule obtained beforehand. In Tables 1 to 6, the expression "mg" represents the relative amount of each component when magnesium oxide is 100.0 mg.
[0112] Magnesium oxide with a volume basis 50% particle diameter (D50) of 6.5 μm: 1500 g, crystalline cellulose: 168 g, croscarmellose sodium: 58.5 g, volume basis 50% particle diameter ( D50) is 5.4 μm insoluble polyvinylpyrrolidone 1:12g mixed, and then granulated by a roll-forming dry granulator at a roll pressure of 5 MPa. The granulated matter is pulverized by an oscillating pulverizer to produce granulated matter. Calcium stearate: 16 g, crystalline cellulose: 34.6 g, aspartame: 1.3 g, and sulfame potassium acetate: 2.7 g were added ...
Embodiment 2
[0114] According to the formula of Table 1, the laxative tablet is manufactured. In Example 1, the addition amount of croscarmellose sodium was changed to 52.5g, and the addition amount of insoluble polyvinylpyrrolidone 1 was changed to 18g, except that tablets were produced in the same way, and the weight per tablet was obtained. 120mg, 6mm in diameter, 3.4mm in thickness magnesium oxide tablet. Table 7 shows the volume-based 50% particle size (D50), volume-based 90% particle size (D90), disintegration time, and tube permeability test results of the obtained magnesium oxide tablet after suspension in water.
Embodiment 3
[0116] According to the formula of Table 1, the laxative tablet is manufactured. In Example 1, the addition amount of croscarmellose sodium was changed to 43.5g, and the addition amount of insoluble polyvinylpyrrolidone 1 was changed to 27g, except that tablets were produced in the same manner, and the weight per tablet was obtained. 120mg, 6mm in diameter, 3.4mm in thickness magnesium oxide tablet. Table 7 shows the volume-based 50% particle size (D50), volume-based 90% particle size (D90), disintegration time, and tube permeability test results of the obtained magnesium oxide tablet after suspension in water.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com