Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
216 results about "Laxative" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Laxatives, purgatives, or aperients are substances that loosen stools and increase bowel movements. They are used to treat and prevent constipation. Laxatives vary as to how they work and the side effects they may have. Certain stimulant, lubricant and saline laxatives are used to evacuate the colon for rectal and bowel examinations, and may be supplemented by enemas under certain circumstances. Sufficiently high doses of laxatives may cause diarrhea.
Formulations of guanylate cyclase c agonists and methods of use
InactiveUS20100221329A1Minimize exposureReducing and eliminating degradationAntibacterial agentsBiocideDiseaseGastrointestinal cancer
The invention provides novel formulations of guanylate cyclase-C (“GCC”) agonist peptides and methods for their use in the treatment of gastrointestinal diseases and disorders, including gastrointestinal cancer. The GCC agonist formulations of the invention can be administered either alone or in combination with one or more additional therapeutic agents, preferably an inhibitor of cGMP-dependent phosphodiesterase or a laxative.
Owner:SYNERGY PHARMA
Salt solution for colon cleansing
The field of colonic diagnostic and surgical procedures is hampered by the lack of optimal means available to cleanse the colon. A compromise between convenient, distasteful, solid or low volume, hyperosmotic solutions which cause considerable fluid and electrolyte imbalances in patients and large volume, difficult to consume, iso-osmotic solutions has had to be made heretofore. This invention describes a low volume, hyper-osmotic solution consisting of sulfate salts with and with out polyethylene glycol. Unlike prior art, this composition is useful for the cleansing of the bowel and, in lower volumes, as a laxative, without producing clinically significant changes in bodily function.
Owner:BRAINTREE LAB
Administering osmotic colonic evacuant containing a picosulfate
Colonic evacuation, treatment of small bowel bacterial overgrowth or irritable bowel syndrome or treating acute or chronic bacterial bowel infection comprises administering an osmotic colonic evacuant in solid form, preferably a mixture of sodium dihydrogen phosphate and disodium hydrogen phosphate or a sulfate based laxative comprising a picosulfate, together with a diluent.
Owner:REDHILL BIOPHARMA +1
Electrolyte purgative
ActiveUS20050271749A1Reduce the amount requiredIncreased tonicityOrganic active ingredientsBiocideMagnesium saltPotassium
The invention relates to compositions for use in purgatives, to purgatives comprising such compositions, and to methods for inducing purgation of the colon. The composition may comprise at least one water-soluble sodium salt; at least one water-soluble minimally degradable sugar in an amount, by weight, of from about 1 to about 3 times the weight of sodium ions in said composition; at least one water-soluble potassium salt in an amount, by weight, of from about 0.05 to about 1 time the weight of said sodium salt in said composition; and at least one water-soluble magnesium salt, wherein the weight of magnesium ions in said composition is from 0.1 to about 10 times the weight of sodium ions in said composition.
Owner:RITE PREP PTY LTD AS TRUSTEE FOR THE RITE PREP UNIT TRUST
Nutritional dietary system, formulation, kit and method for use in preparing an individual for a predetermined activity
InactiveUS6866873B2Easy to prepareHigh complianceBiocideOrganic active ingredientsBiotechnologyReady to eat
Owner:BRACCO DIAGNOSTICS
Administering osmotic colonic evacuant containing a picosulfate
Colonic evacuation, treatment of small bowel bacterial overgrowth or irritable bowel syndrome or treating acute or chronic bacterial bowel infection comprises administering an osmotic colonic evacuant in solid form, preferably a mixture of sodium dihydrogen phosphate and disodium hydrogen phosphate or a sulfate based laxative comprising a picosulfate, together with a diluent.
Owner:REDHILL BIOPHARMA +1
Colonic purgative composition with soluble binding agent
ActiveUS20050129781A1Improve dosage form characteristicSimple preparation processBiocideInorganic phosphorous active ingredientsBowel cleansingTolerability
This invention relates to novel colonic purgative compositions in a solid dosage form, comprising at least one purgative and at least one soluble, or soluble, nonfermentable binder, such as polyethylene glycol. Further, this invention relates to methods of using the colonic purgative compositions. The present compositions and methods are designed to improve patient tolerance and compliance, while at the same time improving the quality of bowel cleansing. The formulations and methods of this invention are particularly useful to cleanse the bowel prior to diagnostic and surgical procedures and can also be employed in lower dosages as a laxative to promote elimination and / or to relieve constipation.
Owner:SALIX PHARMA INC
Nutritional preparations
InactiveUS20060217385A1Promote maturityReduce and alleviate fetusBiocideAnimal repellantsPregnancyAdditive ingredient
Compositions and methods for improving the nutritional and physiological status of a woman and her child during all stages of pregnancy are provided herein. This includes pre-conceptional women, pregnant women, and post-natal women (both lactating and non-lactating mothers). The compositions are particularly useful for the neurological, visual, and cognitive development of an embryo, fetus, or infant and the nutritional and physiological well-being of the mother, fetus, and infant. The compositions contain one or more folates, such as a reduced folate and / or folic acid, and one or more essential fatty acids (EFA), such as an omega-3 and / or omega-6 fatty acid. The addition of the essential fatty acid improves upon the folate containing nutritional preparations described in the prior art. The one or more folates and essential fatty acid may be administered together or in separate dosage units. The one or more folates may be selected from folic acid / folate, one or more reduced folates, or a combination of folic acid / folate and one or more reduced folates. The reduced folate is preferably 5-methyltetrahydrofolate, and most preferably 5-methyl-(6S)-tetrahydrofolic acid. The essential fatty acid is preferably an omega-3 fatty acid, and is preferably docosahexenoic acid (DHA) derived from a vegetarian or non-fish source. The compositions may optionally contain other vitamins, minerals, and ingredients, such as, emollient laxatives—all defined herein as “optional or other ingredients”.
Owner:SCIELE PHARMA CAYMAN
Methods and related systems and formulations to normalize and improve human body chemistry and healing ability
InactiveUS20080260708A1Improve the situationImprove abilitiesBiocidePeptide/protein ingredientsSodium BentoniteOlive leaf
Methods, systems and formulations for normalizing and improving human body chemistry and the body's natural ability to heal itself. In one embodiment a system including effective amounts of a digestive enzyme, soluble and insoluble fiber, laxative, probiotics, vitamin C, potassium, protease enzymes, lipase, lysine, taurine, proline, choline, inositol, inositol hexaphosphate, policosanol, charcoal, bentonite clay, thyme, ascorbic acid, magnesium citrate, calcium citrate, methylsulfonyl methane, cayenne pepper, magnesium, potassium, ester-c, ginger and niacin, lysine calcium, stevia leaf, citric acid, a tincture of bayberry bark, juniper berries, yam root, cramp bark, golden seal root, fennel seed, uva ursi leaves, ginger root, lobelia herb, catnip herb, and peppermint leaf, golden seal root, Echinacea angustifolia root, ginger root, and licorice root, a tincture of black walnut hulls, venus fly trap, chaparral, wormwood, licorice root, slippery elm, cloves and comfrey root, burdock root, sheep sorrel, rhubarb root, slippery elm, olive leaf and yarrow flower is provided.
Owner:HALL MICKEY A
Electrolyte purgative
ActiveUS7993682B2Simply performing their known purgative functionIncreased tonicityBiocideOrganic active ingredientsMagnesium saltPotassium
The invention relates to compositions for use in purgatives, to purgatives comprising such compositions, and to methods for inducing purgation of the colon. The composition may comprise at least one water-soluble sodium salt; at least one water-soluble minimally degradable sugar in an amount, by weight, of from about 1 to about 3 times the weight of sodium ions in said composition; at least one water-soluble potassium salt in an amount, by weight, of from about 0.05 to about 1 time the weight of said sodium salt in said composition; and at least one water-soluble magnesium salt, wherein the weight of magnesium ions in said composition is from 0.1 to about 10 times the weight of sodium ions in said composition.
Owner:RITE PREP PTY LTD AS TRUSTEE FOR THE RITE PREP UNIT TRUST
Edible gelatin bowel preparation and bowel cleansing method
The present invention relates to an eatable gelatin bowel preparation for use in preparing an individual for a medical procedure requiring a clean colon. Specifically, an individual is provided with an edible diet comprising a laxative and a gelatin based food.
Owner:GARREN MARY L +3
Nutritional preparations
InactiveUS20060217386A1Promote maturityReduce and alleviate fetusBiocideSkeletal disorderPregnancyAdditive ingredient
Compositions and methods for improving the nutritional and physiological status of a woman and her child during all stages of pregnancy are provided herein. This includes pre-conceptional women, pregnant women, and post-natal women (both lactating and non-lactating mothers). The compositions are particularly useful for the neurological, visual, and cognitive development of an embryo, fetus, or infant and the nutritional and physiological well-being of the mother, fetus, and infant. The compositions contain one or more folates, such as a reduced folate and / or folic acid, and one or more essential fatty acids (EFA), such as an omega-3 and / or omega-6 fatty acid. The addition of the essential fatty acid improves upon the folate containing nutritional preparations described in the prior art. The one or more folates and essential fatty acid may be administered together or in separate dosage units. The one or more folates may be selected from folic acid / folate, one or more reduced folates, or a combination of folic acid / folate and one or more reduced folates. The reduced folate is preferably 5-methyltetrahydrofolate, and most preferably 5-methyl-(6S)-tetrahydrofolic acid. The essential fatty acid is preferably an omega-3 fatty acid, and is preferably docosahexenoic acid (DHA) derived from a vegetarian or non-fish source. The compositions may optionally contain other vitamins, minerals, and ingredients, such as, emollient laxatives-all defined herein as “optional or other ingredients”.
Owner:SCIELE PHARMA CAYMAN
Nutritional dietary system, formulation, kit and method for use in preparing an individual for a predetermined activity
InactiveUS20030180393A1Enough caloriesWithout loss of nutritional benefitBiocideOrganic active ingredientsBiotechnologyReady to eat
This invention relates to a nutritional dietary system, formulation, kit and method for use in preparing an individual for a predetermined activity which requires a clean digestive tract, particularly the colon. Such predetermined activities include, but are not limited to, gastrointestinal surgery and colon screenings. Specifically, the present invention provides an individual low amounts of fat, dietary fiber and solid food content to minimize stool formation and / or facilitate removal of stool from the digestive tract prior to the predetermined activity. The present invention also provides the individual with sufficient calories and nutrition to enable the individual to conduct daily, routine activities while utilizing the present invention. In one alternative embodiment, the dietary regimen of the present invention provides a variety of pre-packaged, ready to eat or easy to prepare nutritional liquid or solid foods which, when coordinated with a laxative regimen, result in removal of residue such that a medically and / or diagnostically useful procedure can be performed on the digestive tract.
Owner:BRACCO DIAGNOSTICS
Chinese medicine prescription orally taken for curing constipation
The invention discloses a traditional Chinese medicine prescription for oral administration for treating constipation, which belongs to the field of traditional Chinese medicines. This kind of traditional Chinese medicine prescription for oral treatment of constipation, the raw materials of active ingredients are made into the following weights: 10 grams of raw Atractylodes macrocephala, 10 grams of raw white peony root, 20 grams of raw licorice, 6 grams of raw rhubarb, 20 grams of angelica, 12 grams of raw land, Hemp seed 15 grams, Cistanche 12 grams, Ophiopogon japonicus 10 grams, Astragalus 20 grams, Tangerine peel 10 grams, Codonopsis 10 grams, Citrus aurantium 10 grams. Decoction in water, one dose a day, one dose divided into two doses. Ten days is a course of treatment. The medicine of the present invention has the effects of nourishing qi, nourishing blood, moistening intestines and defecating. During the course of treatment for constipation treatment, the effect is remarkable.
Owner:杨海新
Fatty acids for use as a medicament
ActiveUS20100113387A1Clear and significant laxative effectAntibacterial agentsBiocideDiseasePharmaceutical formulation
The invention relates to fatty acid stimulation of rectal mucosa initiating the process of defecation, acting as a laxative. Furthermore, the invention relates to the usage of free fatty acids, fatty acid mixtures and fatty acid extracts from marine lipids in pharmaceutical formulations such as suppositories, ointments, tablets and gelatin capsules for treatment and prevention of multiple disorders like constipation, hemorrhoids, bacterial infections (e.g. helicobacter pylori), viral infections (e.g. herpes simplex virus infections) and inflammations, as well as against fissura ani and pruritus ani.
Owner:LIPID PHARMA EHF
Method and system for production and collection of lavage induced stool (LIS) for chemical and biologic tests of cells
InactiveUS6447763B1Improve practicalityImprove cost effectivenessDigestive systemSurgeryDiseaseFeces
Beverages are provided and administered for producing LIS samples containing cells exfoliated from throughout the gut in sufficient numbers and free of interfering substances such as formed fecal particles for chemical assays and biologic assays for nucleic acid sequence information, and medical diagnosis. A kit is also provided for use by patients without assistance to produce a LIS sample suitable for analysis. A collection kit employs a sequence of the beverages and other ingested substances to produce preserved cells for medical diagnosis, allowing cytologic analysis of the LIS for diagnosis of foregut and hindgut disease. A preliminary cathartic lavage is used to cleanse a patient's digestive tract; at least one stool induced by the preliminary cathartic lavage is collected; and a final cathartic lavage is used to exfoliate and preserve cells from a patient's digestive tract. Time release capsules containing a cathartic medicament can also be used after completing preliminary lavage administration. The kit also allows provides apparatus for collection, sealing, and packing of the collected LIS specimen for analysis.
Owner:GORDON IAN L
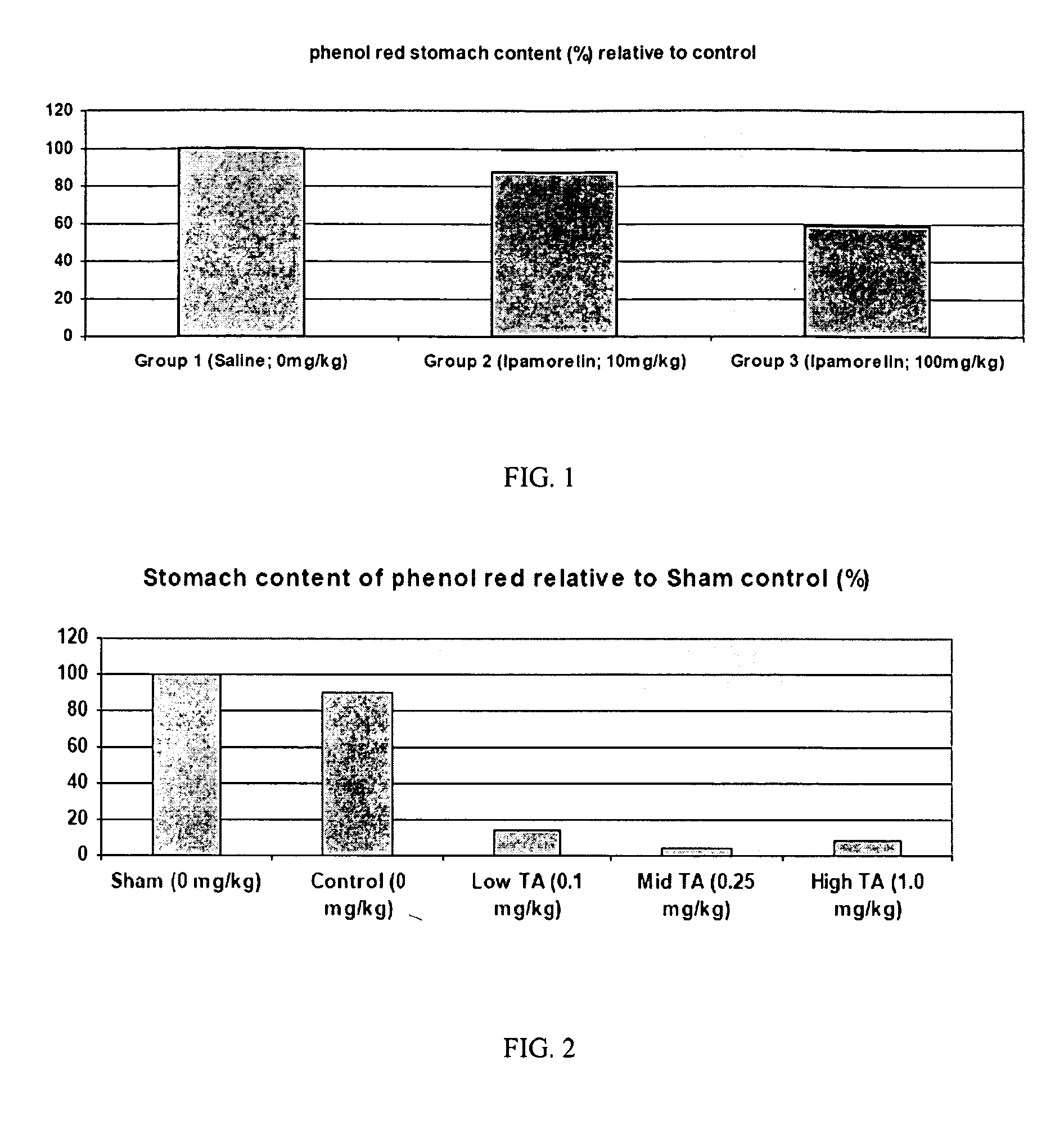
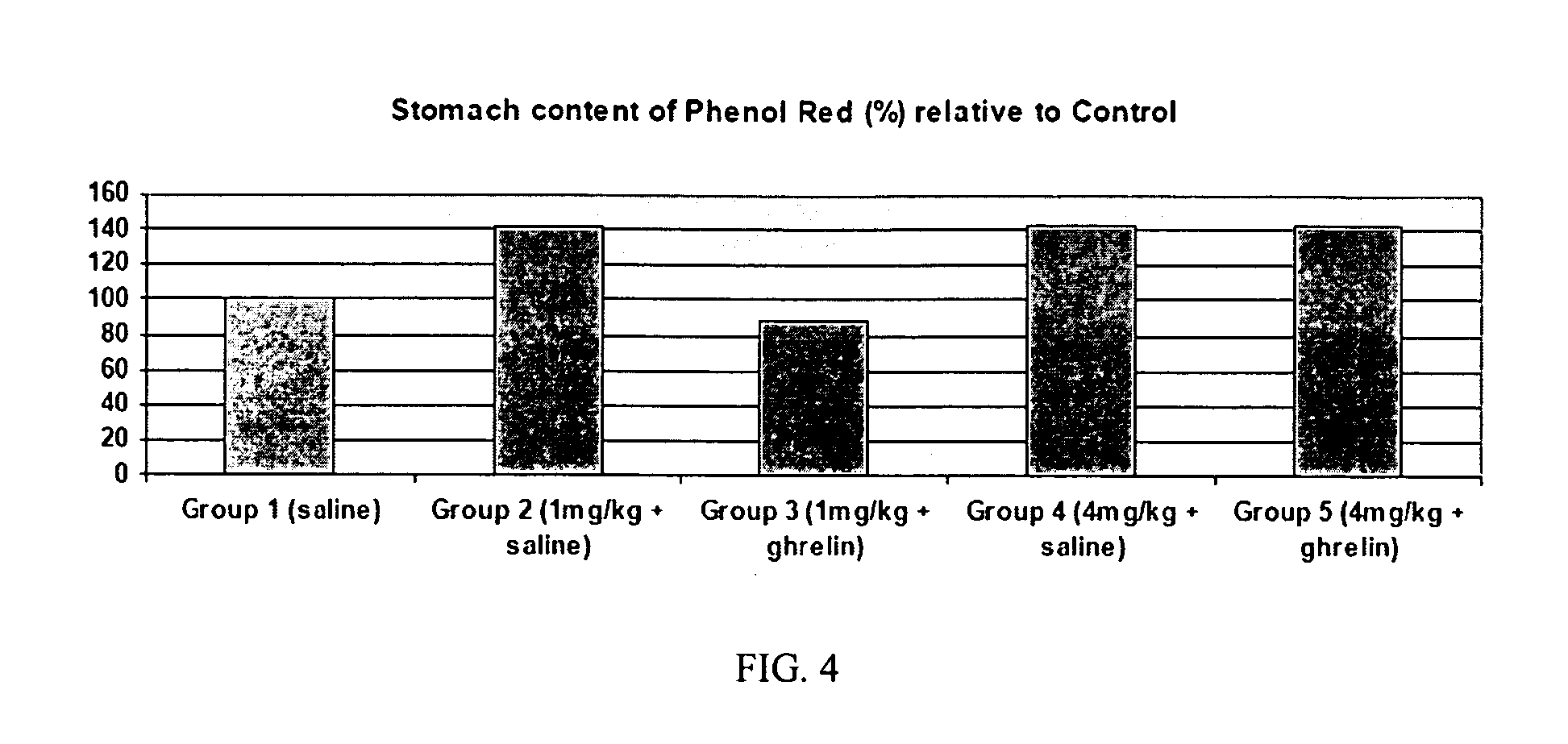
Method of stimulating the motility of the gastrointestinal system using ipamorelin
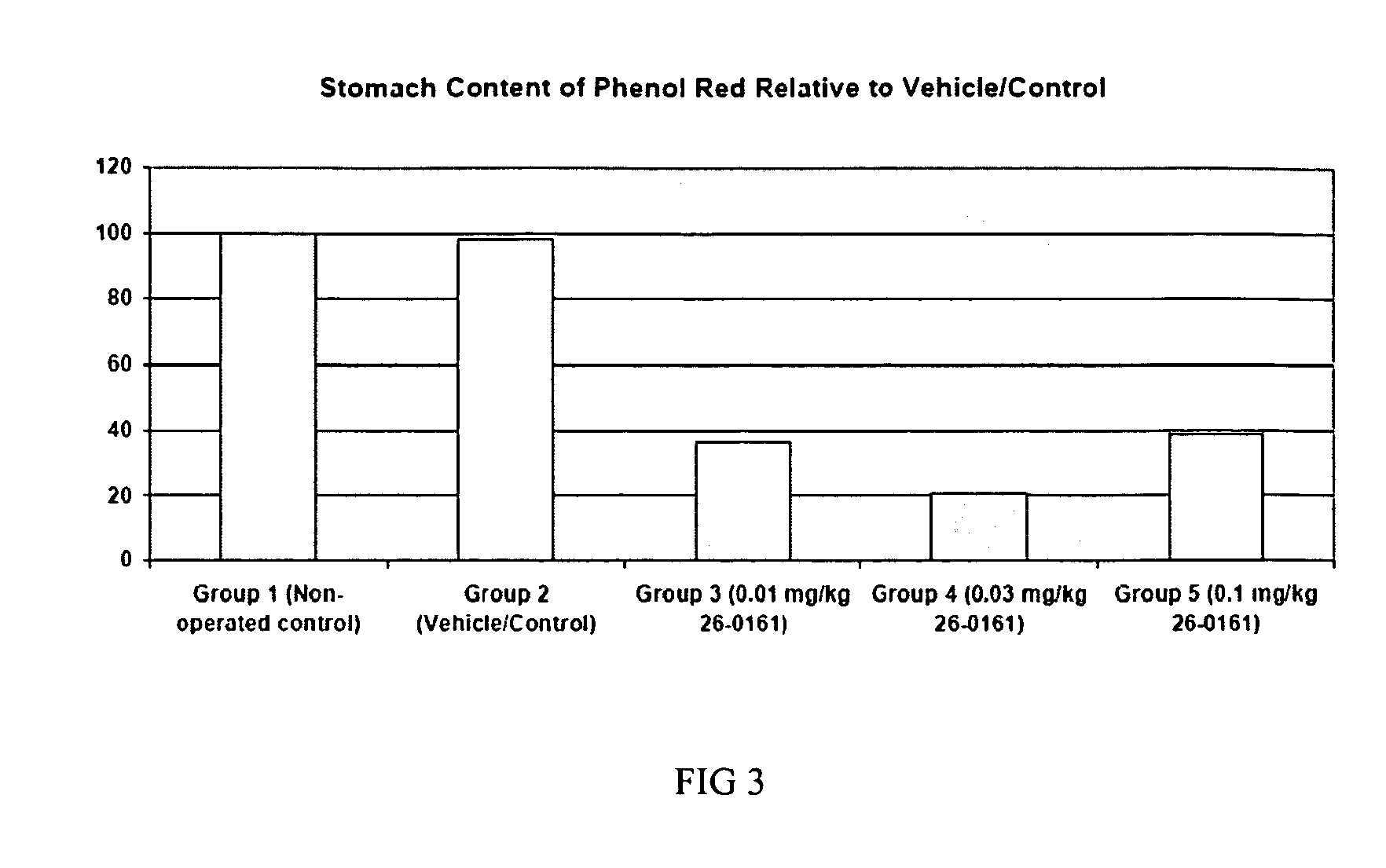
The present invention provides a method of stimulating the motility of the gastrointestinal system in a subject in need thereof, wherein the subject suffers from maladies (i.e., disorders, diseases, conditions, or drug- or surgery-induced dysfunction) of the gastrointestinal system, by administering to the subject a ghrelin mimetic, or pharmaceutically acceptable salt thereof. The invention also provides a method of treating a gastrointestinal malady by co-administering a ghrelin mimetic with a laxative, a H2 receptor antagonist, a serotonin receptor agonist, pure or mixed, an antacid, an opioid antagonist, a proton pump inhibitor, a motilin receptor agonist, dopamine antagonist, a cholinergic agonist, a cholinesterase inhibitor, somatostatin, octreotide, or any combination thereof.
Owner:HELSINN THERAPEUTICS (U S) INC
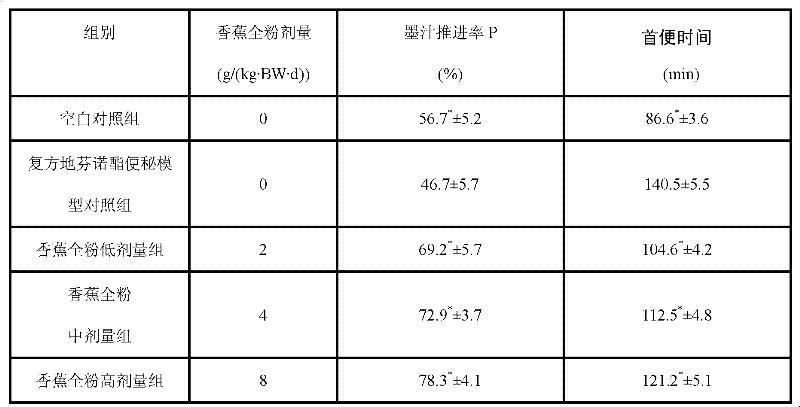
Preparation method of banana powder with laxative and weight-reducing functions
The invention discloses a preparation method of a banana powder product with functions of relaxing bowel and losing weight. The product is mainly prepared from whole banana powder and banana resistant starch in different ratio. The production method comprises the following steps of: preparing mature bananas with full yellow skin into the whole banana powder by the steps of peeling, color protection, pulping, vacuum freeze drying and the like; and preparing raw bananas with dark green skin into the banana resistant starch by the steps of peeling, pulping, enzymolysis, drying with a heat pump and the like. The whole banana powder has fragrant and sweet mouthfeel and has the function of relaxing bowel; the banana resistant starch has no smell or no taste, and has the functions of increasing satiety and losing weight; and the whole banana powder and the banana resistant starch can be blended and taken in a ratio of 1:9-9:1 according to the requirement of a user, so that the effect of relaxing bowel or losing weight is achieved.
Owner:SOUTH CHINA UNIV OF TECH
Dried fruit composition with laxative effects and method for administration thereof
InactiveUS20060045924A1Good laxative effectImprove Gut HealthBiocideAnimal repellantsOral medicationMedicine
A dried fruit composition enhanced with herbal extracts and a healthful dose of bacteria for enhanced laxative effects and appetite suppression. Preferred methods of manufacture and methods for oral administration of the dried fruit composition are further disclosed.
Owner:CHEN LILY +1
Yogurt containing coix seed and lily
InactiveCN102007967AFull of nutritionNutritional balanceMilk preparationFood preparationBiotechnologyJoint arthralgia
The invention discloses yogurt containing coix seed and lily, comprising the following raw materials in percentage by weight: 50-65 percent of fresh milk, 7-12 percent of lily juice, 10-18 percent of coix seed pulp, 3-10 percent of medlar juice, 5-10 percent of white granulated sugar, 0.6-1 percent of emulsion stabilizer, 2-6 percent of strain and 0.01-0.02 percent of ethyl maltol. The yogurt has abundant and balanced nutrition and the efficacies of tranquilizing by nourishing the heart, moistening the lung and clearing away the heart-fire, inducing diuresis for removing edema, resolving dampness by tonifying spleen, relieving arthralgia by relaxing muscles and tendons, clearing away heat and eliminating pus, tendering the skin, eliminating acne, helping produce saliva and slake thirst, reinforcing insufficiency and promoting appetite, relieving constipation with laxatives, reducing blood fat and resisting cancer.
Owner:凌一
Chinese medicine for treating constipation for children
ActiveCN101062234AEnhance peristalsisRelieve clinical symptomsDigestive systemSolution deliveryAdditive ingredientLarge intestine
The invention provides a Chinese medicament for treating children's constipation which comprises the following main constituents (by weight portions): white atractylodes rhizome 6-18, bitter orange 3-10 parts, apricot kernel 6-10, magnolia bark 3-10, radish seeds 6-15, cassia seed 6-12 and aloe 1-6. The medicament can be prepared into particles, oral liquids, capsules, tablets and bag infusions.
Owner:JUMPCAN PHARMA GRP
Composition and method of producing a taste masking formulation of laxatives for bowel cleaning preparation prior to colonoscopy
This invention relates to a solid taste masking formulation of laxatives which can be dispersed in water for oral use for bowel cleaning preparation prior to colonoscopy procedures. This invention also relates to the methods to produce the taste masking laxative formulations.
Owner:CHEN JINLING +1
Traditional Chinese medicinal prepn. for treating constipation of slow-remove type, and its prepn. method
InactiveCN1724030AWrigglyPromote peristalsisDigestive systemCapsule deliveryCurative effectTangerine Peel
A Chinese medicine for treating the slow-transmission constipation is prepared from 13 Chinese-medicinal materials including tangerine peel, astragalus root, Chinese angelica root, rehmannia root, etc through proportional mixing, distilling, extracting, concentrating, drying and loading in capsules.
Owner:李红岩
Medicine for preventing and curing middle and old aged constipation
The invention discloses a medicine for preventing and curing middle and old aged constipation, comprising the following raw medicines in parts by weight: 10-60 parts of radix rehmanniae, 5-40 parts of semen cassiae torae, 5-40 parts of milk vetch, 5-40 parts of fructus aurantii, 5-40 parts of semen pruni, 50-40 parts of Cistanche salsa, 5-40 parts of angelica, and 5-40 parts of semen cannabis. The preparation method of the medicine comprises the following steps: weighing and mixing the raw medicines according to weight ratio, and then filtering through a sieve of at least 80 meshes to obtain filtered powder; uniformly stirring the obtained powder and then filling, and packaging into vacuum packets through normal sterilization and checking. The medicine has the functions of relieving constipation with laxatives and clearing away heat and eliminating of toxicant, and has very good effect for preventing and curing middle and old aged constipation without toxic and side effect; and the medicine has short course of treatment, low cost, quick production and good economic benefit.
Owner:NORTHWEST A & F UNIV
Bowel purgative and uses thereof
The present invention is directed to a dry composition for admixture with water for oral administration to a mammal and methods of using the composition for cleansing the bowel of a mammal in need thereof.
Owner:THOMAS JEFFERSON UNIV
Chinese medicine composition orally taken for curing constipation
The invention discloses a traditional Chinese medicine composition for oral administration for treating constipation, which belongs to the field of traditional Chinese medicines. This kind of traditional Chinese medicine composition for oral treatment of constipation consists of 20 grams of Angelica, 12 grams of Rehmannia, 15 grams of Hemp Seed, 12 grams of Cistanche, 20 grams of Astragalus, 10 grams of Raw Atractylodes Rhizoma, 10 grams of Tangerine Peel, 10 grams of Codonopsis, and 10 grams of Fructus Aurantii. Prepared with 6 grams of raw rhubarb. Decoction in water, one dose a day, one dose divided into two doses. The medicine of the present invention has the effects of nourishing blood, nourishing qi, moistening intestines and laxative. During the course of treatment for constipation treatment, the effect is remarkable.
Owner:辛峰
Solid, edible, chewable laxative composition
InactiveUS20140235730A1Great tastePatient compliance is goodBiocideInorganic active ingredientsMedicineFiller Excipient
The invention is directed to an edible solid composition having laxative properties. The composition has a smooth mouth feel, in non-gritty and has a pleasant taste. It is prepared by melt-combining together the ingredients including a bulking agent and laxative as well as an optional flavor agent and electrolyte. The melt is a dispersion containing a substantially homogeneous combination of ingredients. The melt is cooled to form an amorphous solid dispersion or solution of the ingredients. The ingredients are mutually compatible so that upon cooling they do not separately crystallize to form a gritty mouth feel product.
Owner:LUPIN ATLANTIS HLDG
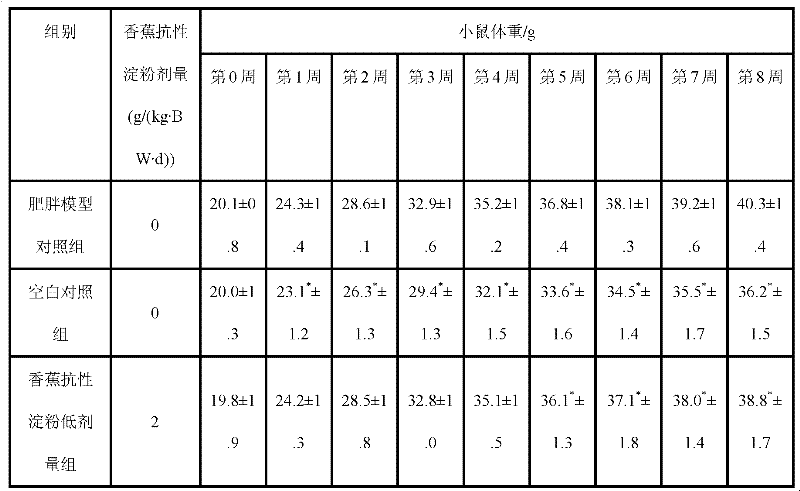
Composition of banana oligosaccharide and dietary fibre, preparation method and application thereof
InactiveCN101715910AImprove water holding capacityStrong binding hydraulicDigestive systemFood preparationSide effectFreeze-drying
The invention discloses a composition of banana oligosaccharide and dietary fibre, a preparation method and an application thereof. The composition comprises banana oligosaccharide, soluble dietary fibre and insoluble dietary fibre, wherein the proportion of the banana oligosaccharide to the soluble dietary fibre to the insoluble dietary fibre is 2.5:1:2 or 2:2:2.In the invention, the banana oligosaccharide is obtained by pulping the fruit flesh of bananas, leaching by water, precipitating by ethanol and freeze-drying in vacuum; the soluble and insoluble dietary fibre of the bananas is obtained by carrying out zymohydrolysis on the fruit residues of the bananas. The composition has the effect of relaxing bowels of a common purge, is mild, does not have stimulation property, overcomes side effects caused by common rhubarb and anthraquinone class purges, can exert the physiological function of preventing colonic and rectal cancers, cardiovascular diseases and the like of the dietary fibre and achieves the aims of retaining water, lubricating and keeping high penetrability; because the composition is natural, safe and effective, the composition is especially suitable for relieving the constipation symptoms of dry, dense and clotty stool and difficult defecation of infants because of eating powdered milk.
Owner:SOUTH CHINA AGRI UNIV
Quick dissolving agglomerated soluble fiber compositions and the process for making the same
InactiveUS20060286260A1Reduce molecular weightImprove solubilityFood shapingFood ingredient functionsSoluble fiberHigh doses
A rapidly dispersible, non-gelling, non-gritty low-viscosity producing powder having use as a laxative and fiber supplement essentially containing non-digestible maltodextrin agglomerated with natural gums is disclosed. Non-digestible maltodextrins containing 30-40% hydro-alcohol precipitable fiber content are agglomerated in a fluid bed agglomerator, utilizing a solution of gums like guar and acacia having greater than 70% hydro-alcohol precipitable fiber content. These fiber agglomerates when added to water or liquid beverages disperse within few seconds without needing any mechanical stirring. Fiber powder agglomerates produced utilizing the current invention dispersed within seconds without forming any lumps in soft foods like yogurt, soft ice cream, pudding, thick soups, sauce, and fruit puree. Agglomerated powders enriched a variety of soft foods and beverages with high doses of fiber, without significantly altering the taste or texture.
Owner:NAYAK VIN +2
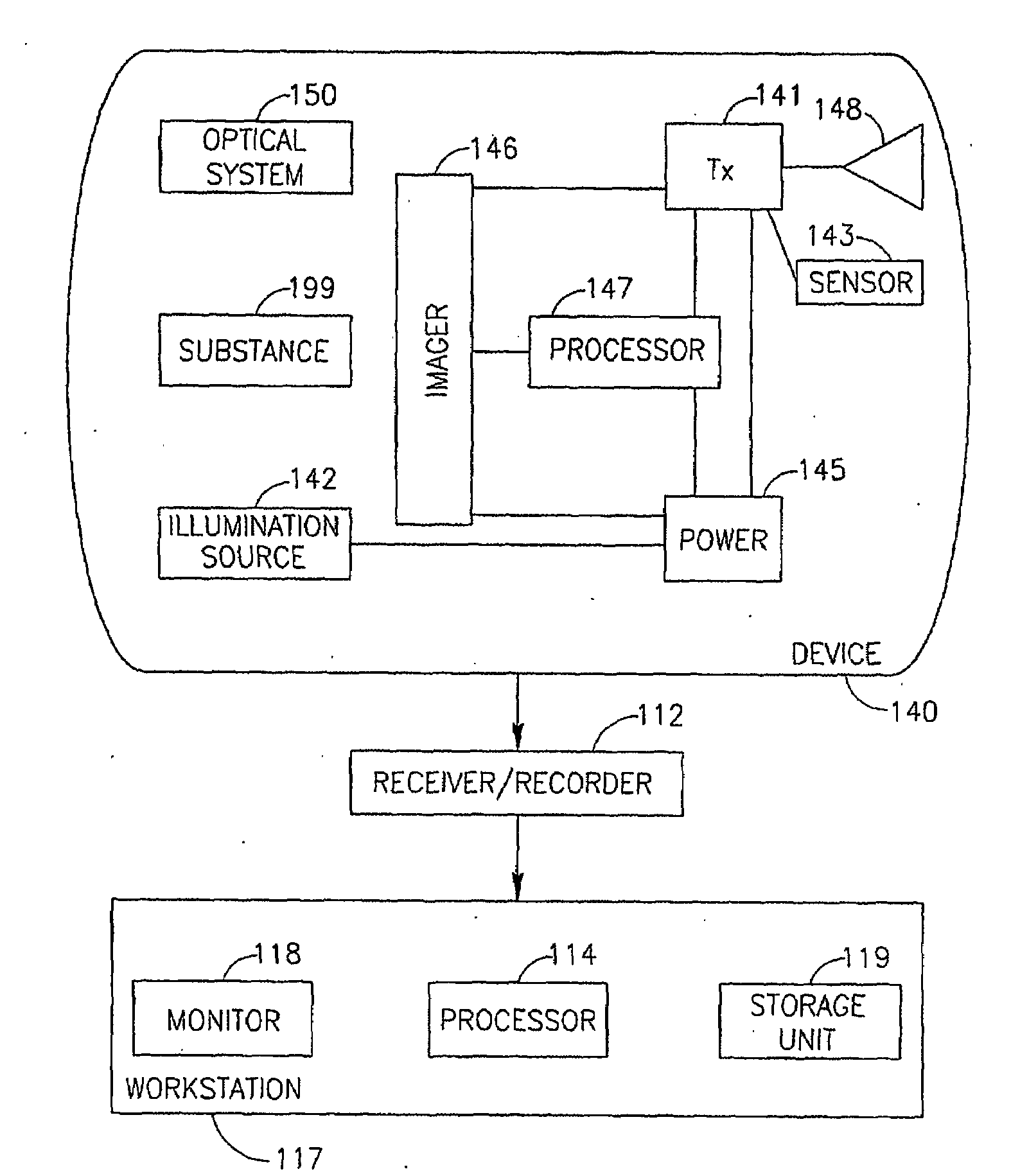
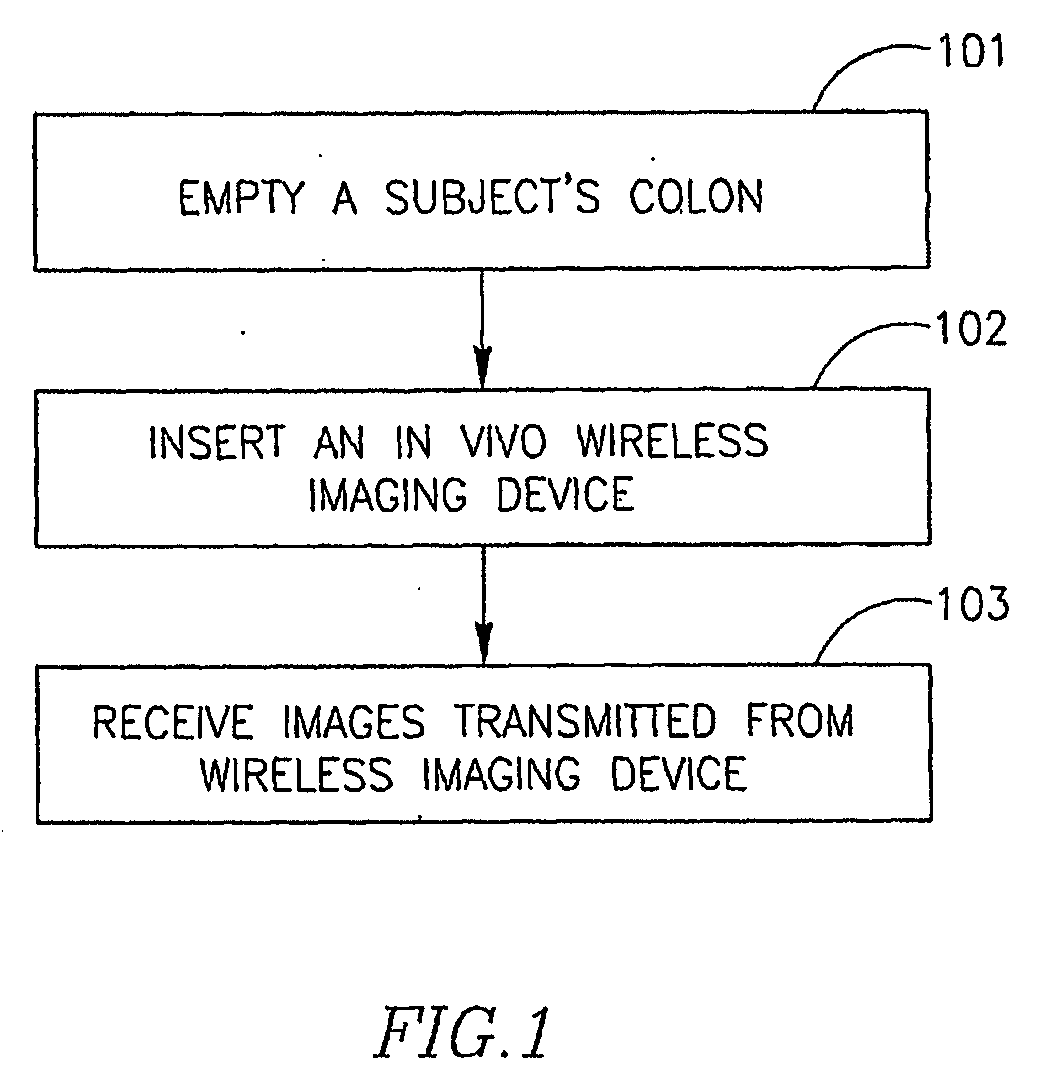
Device, System and Method for In-Vivo Examination
InactiveUS20090105537A1Effective timePatient compliance is goodBiocideInorganic phosphorous active ingredientsStimulantRadiology
A device, a system and a method for in-vivo examination. A method for in-vivo examination includes substantially emptying a subject's colon from content, and inserting an autonomous in-vivo imaging device into the subject's gastrointestinal tract. An in-vivo examination kit includes an autonomous in-vivo imaging device, a wireless receiver, an antenna set, a laxative, and optionally a stimulant and an instructions leaflet.
Owner:GIVEN IMAGING LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com