Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2296 results about "Digestive tract" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Digestive tract. noun. : the tubular passage typically extending from the mouth to the anus or cloaca that functions in digestion and absorption of food and elimination of residual waste and that in most mammals includes the mouth, pharynx, esophagus, stomach, intestine, and anus. — called also alimentary canal, alimentary tract.
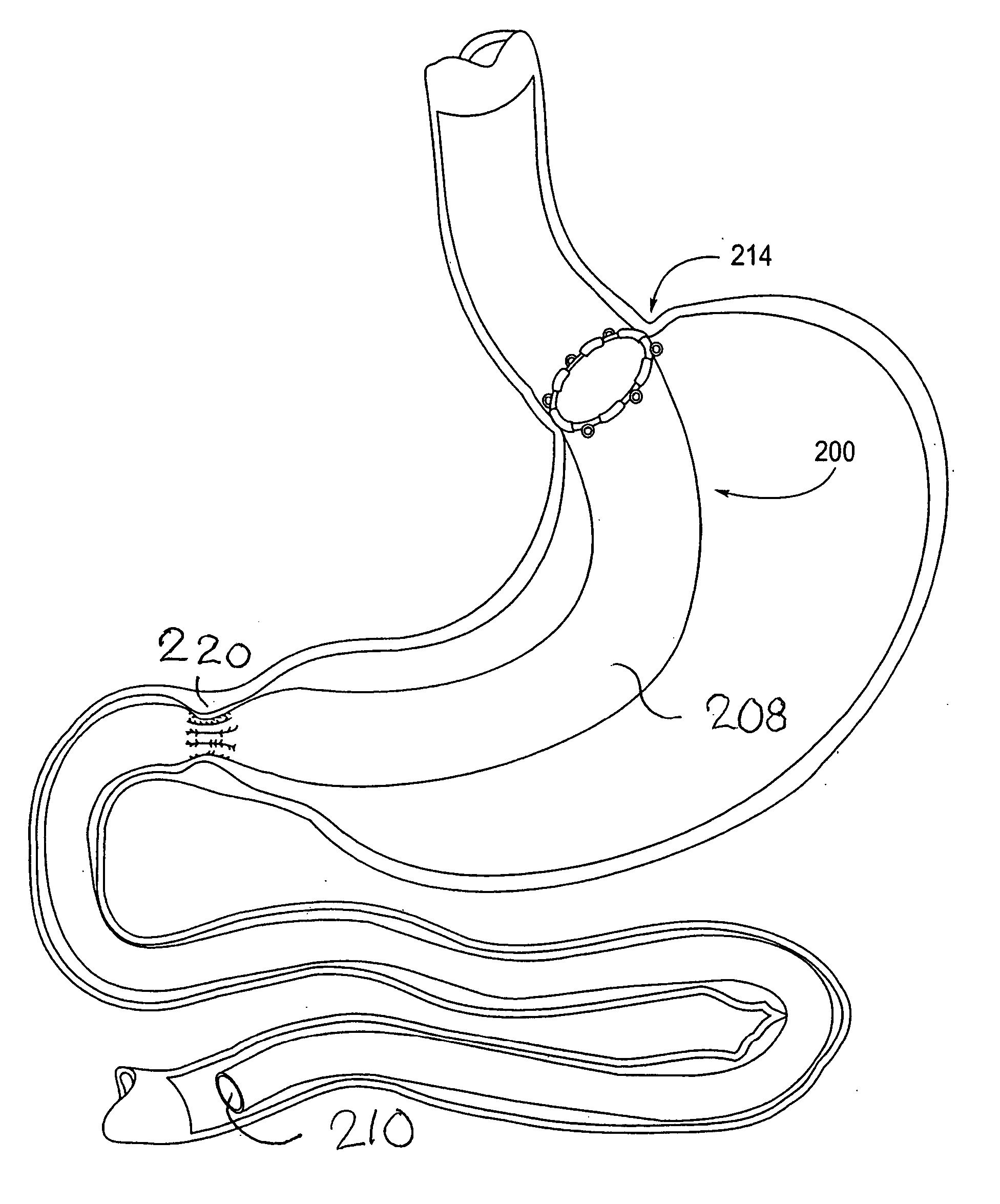
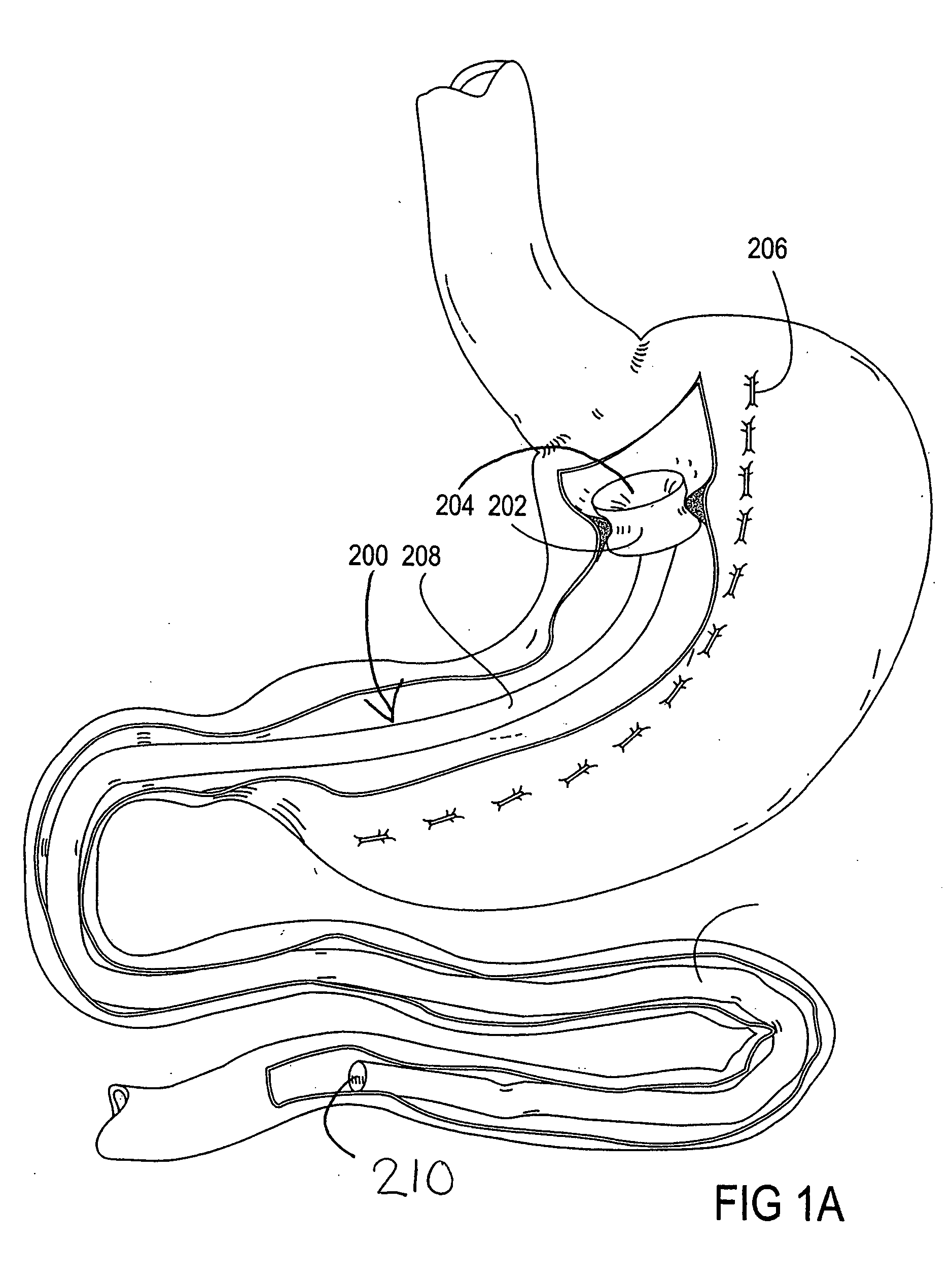
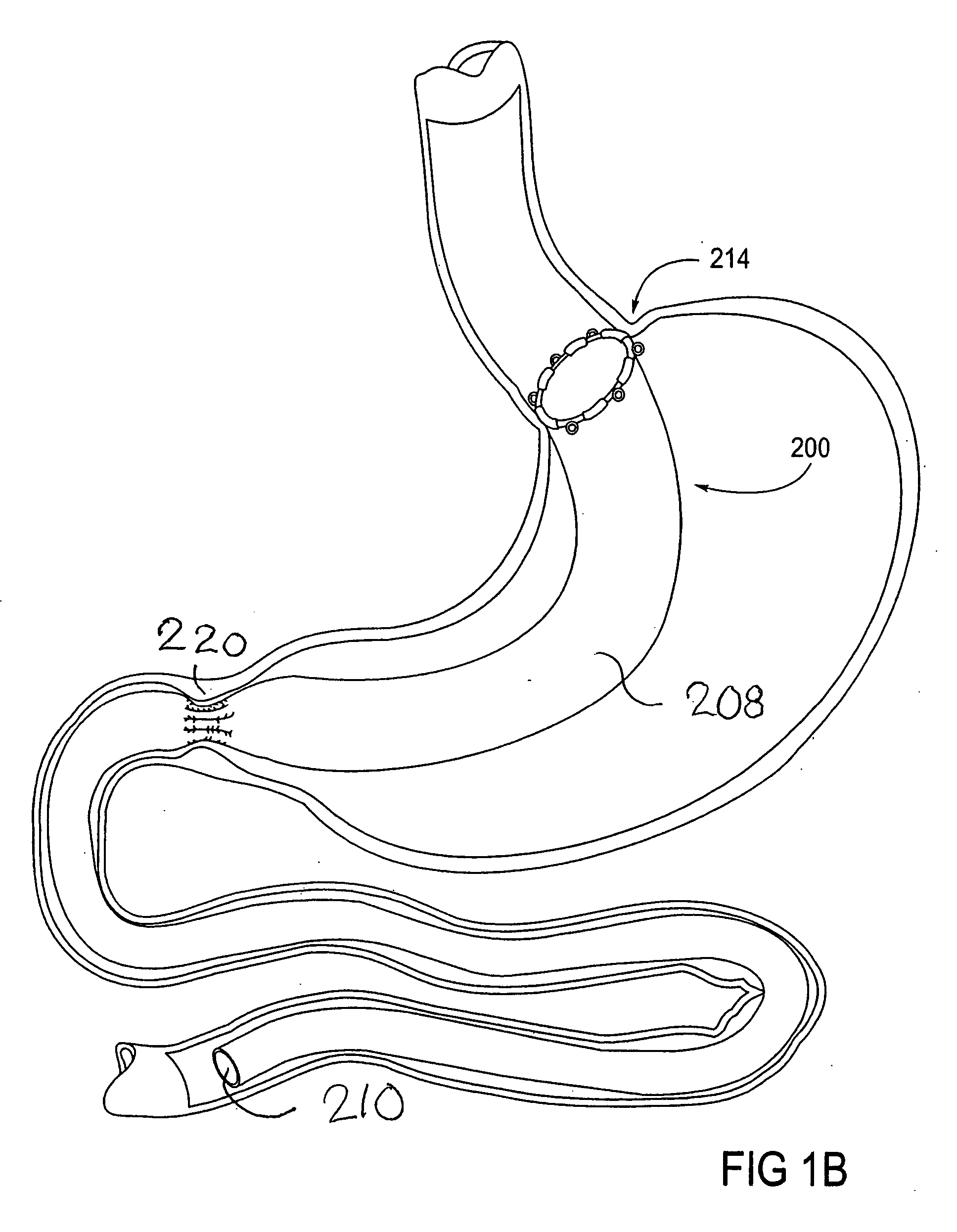
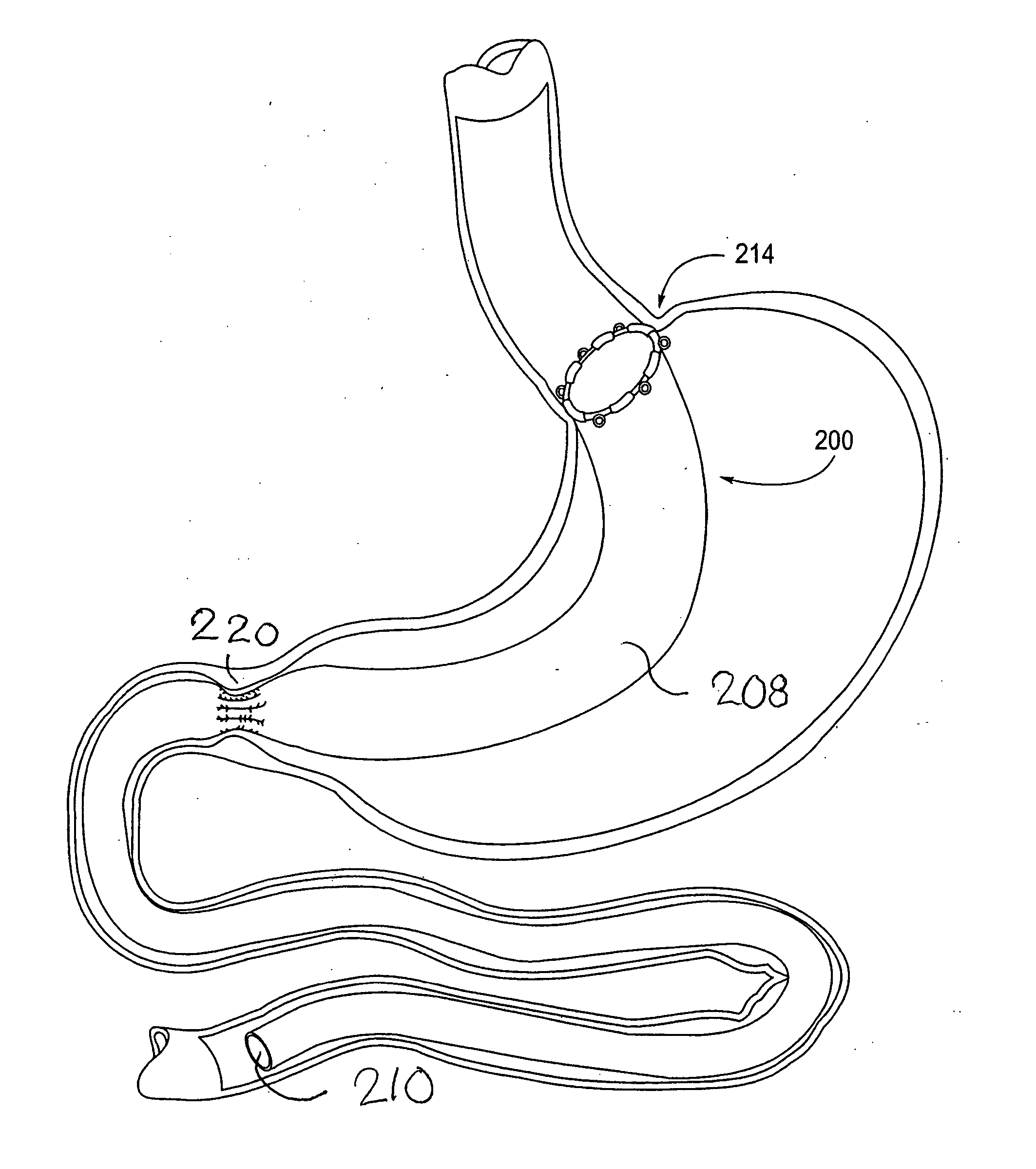
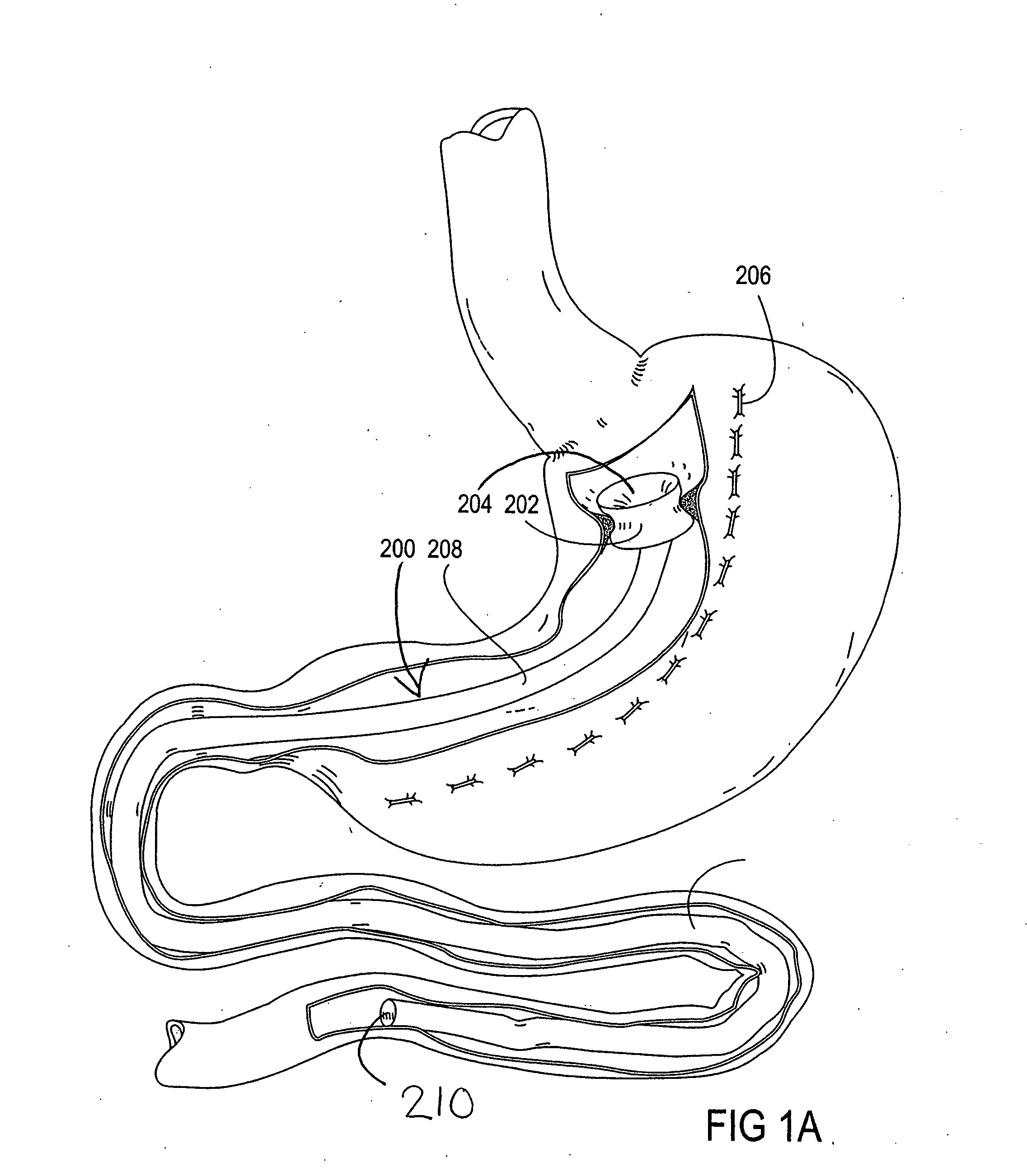
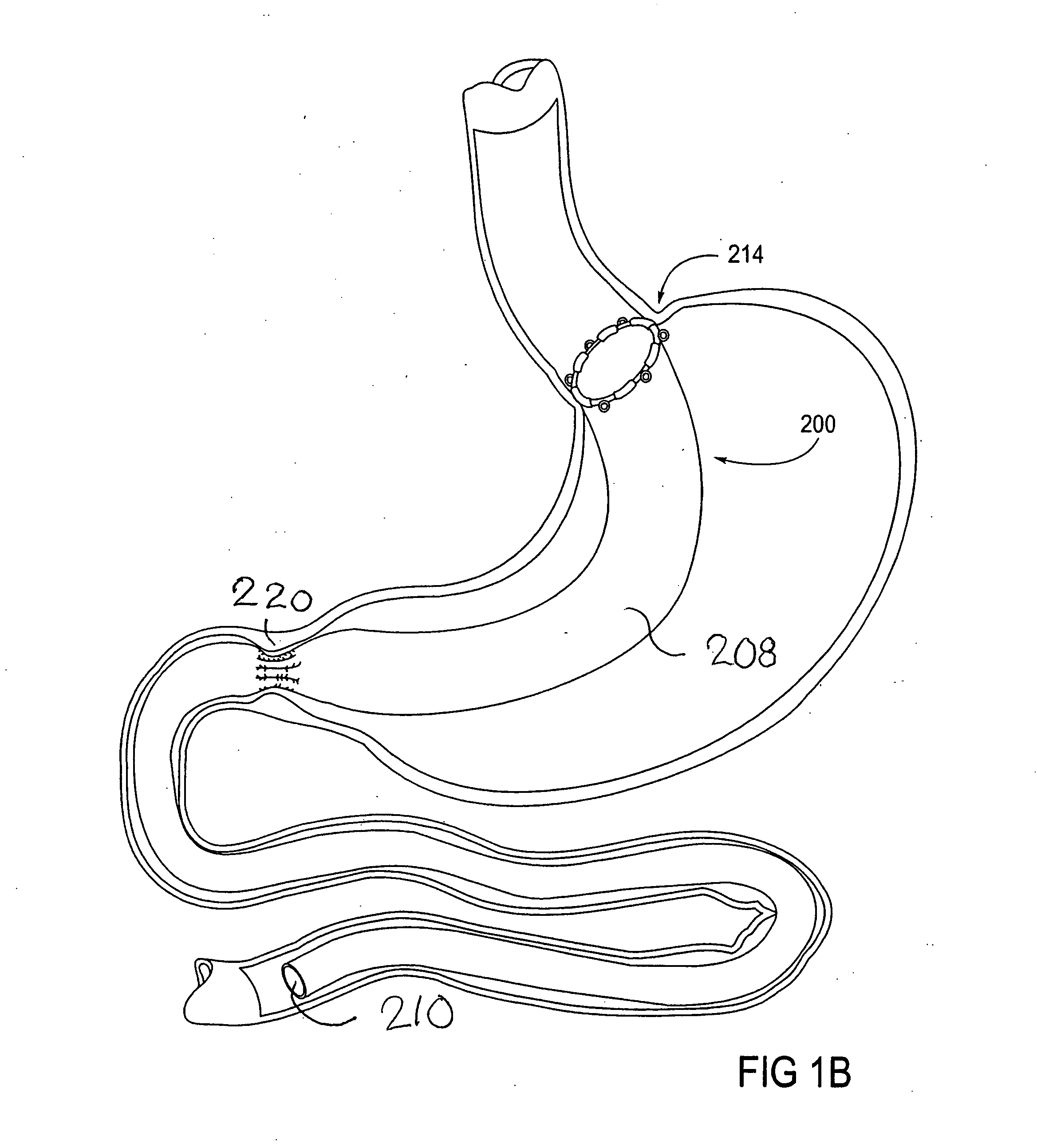
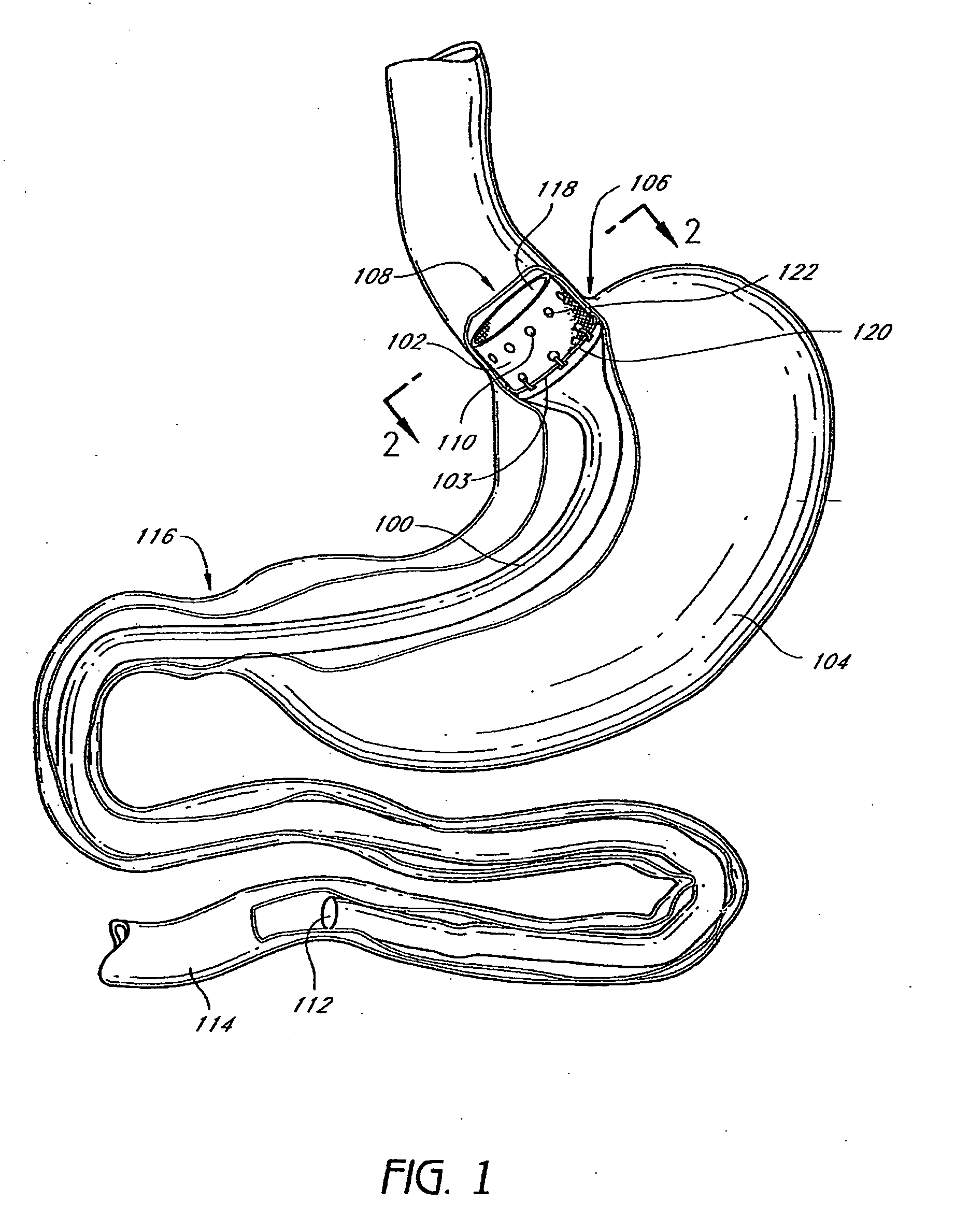
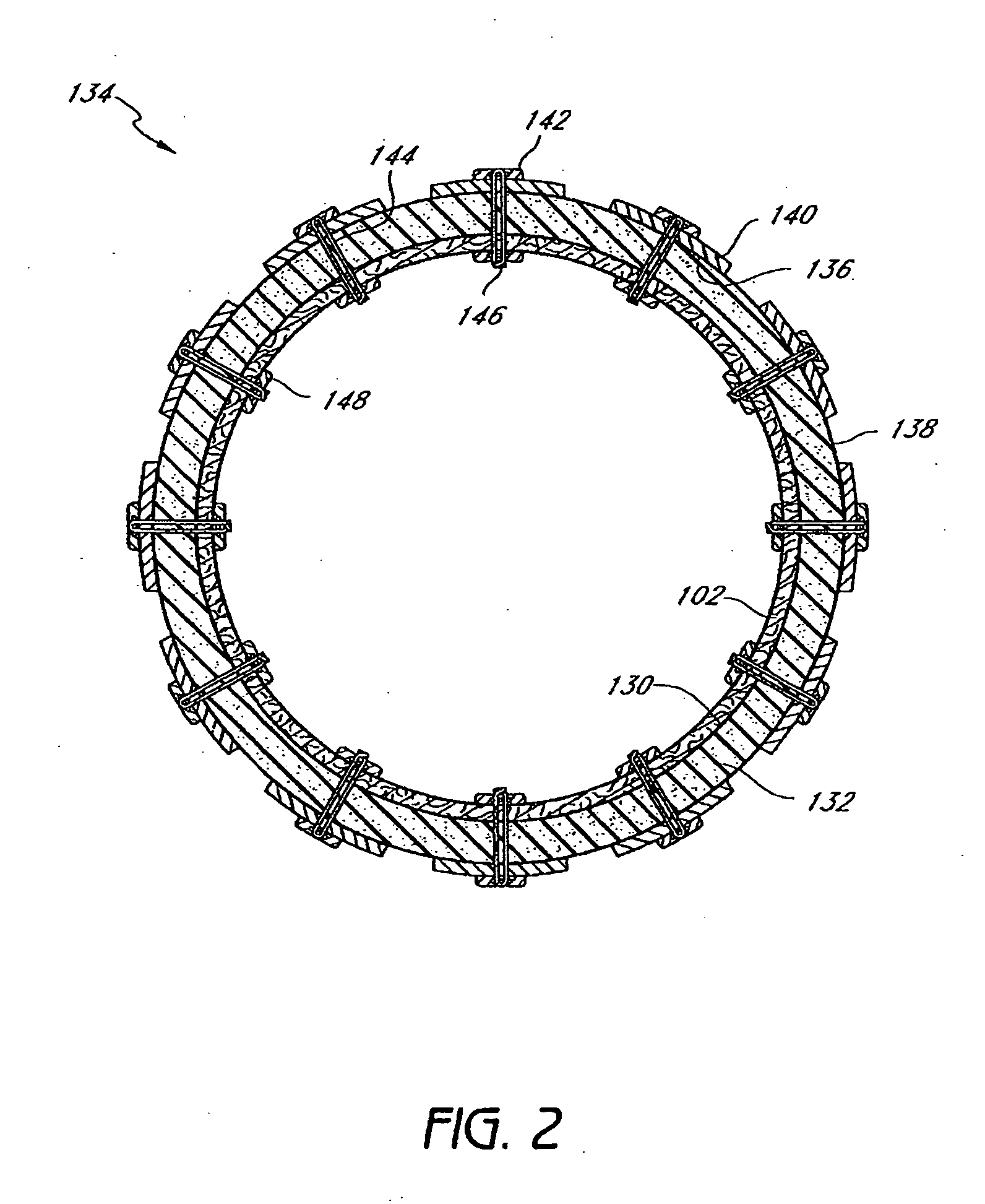
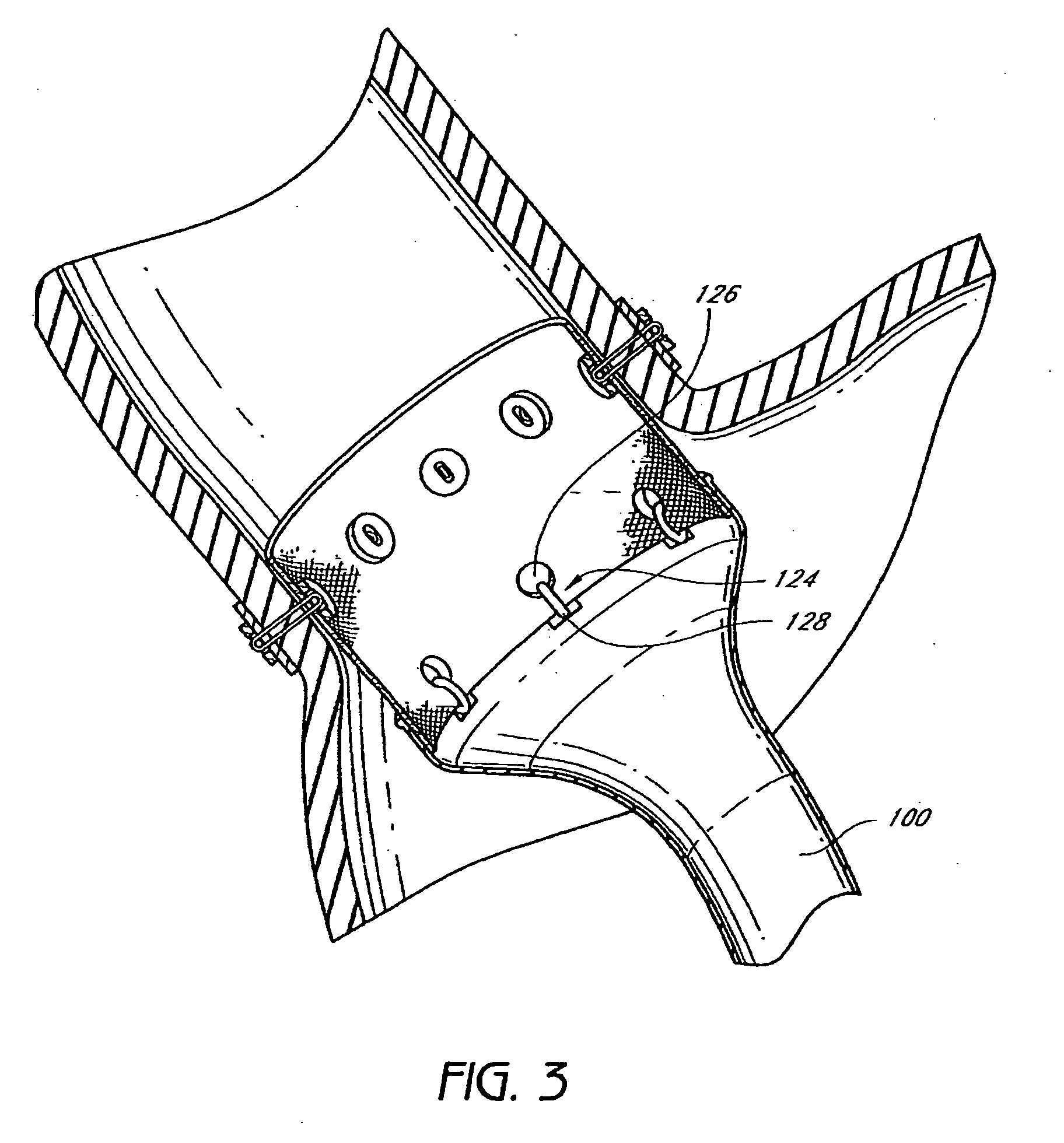
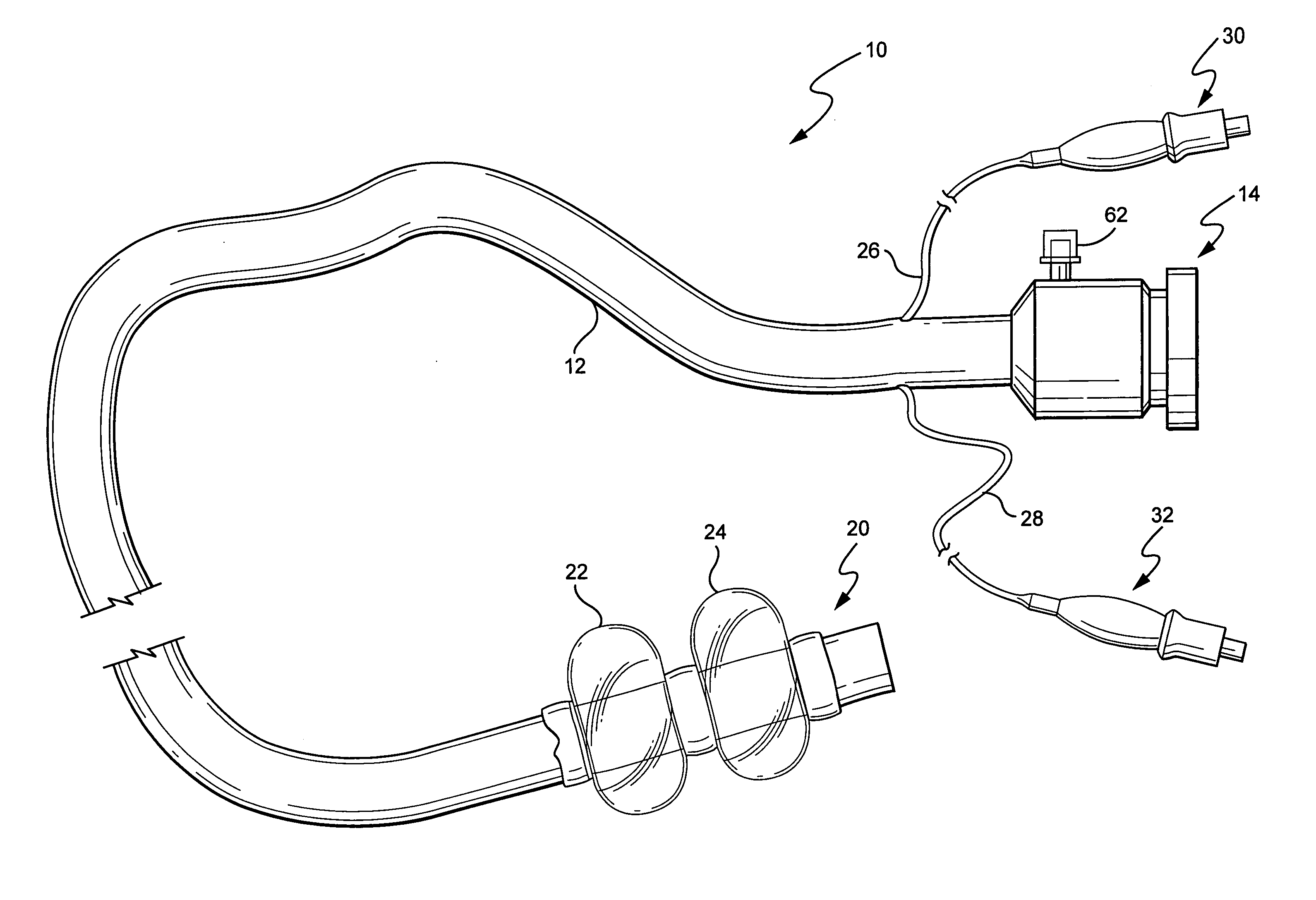
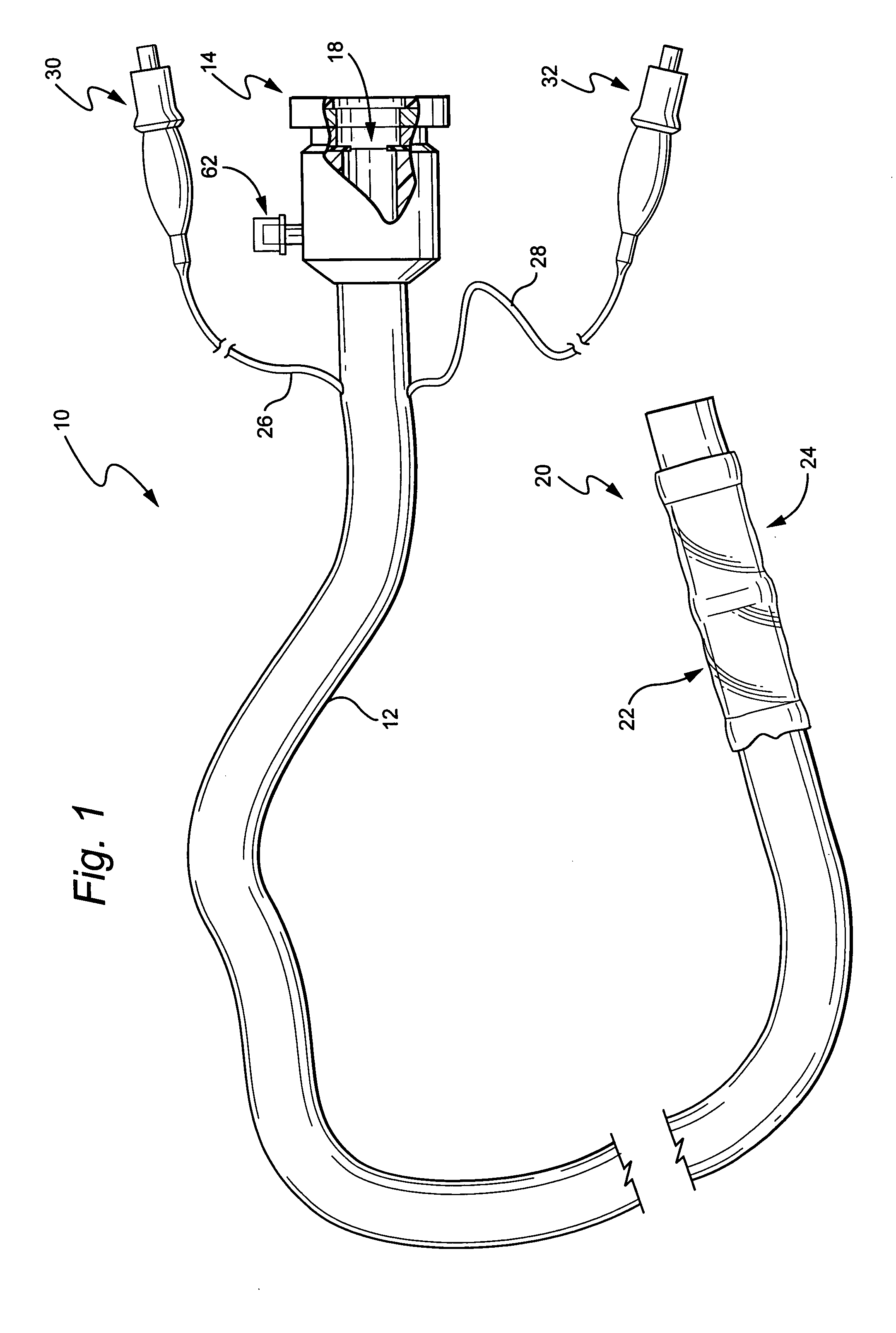
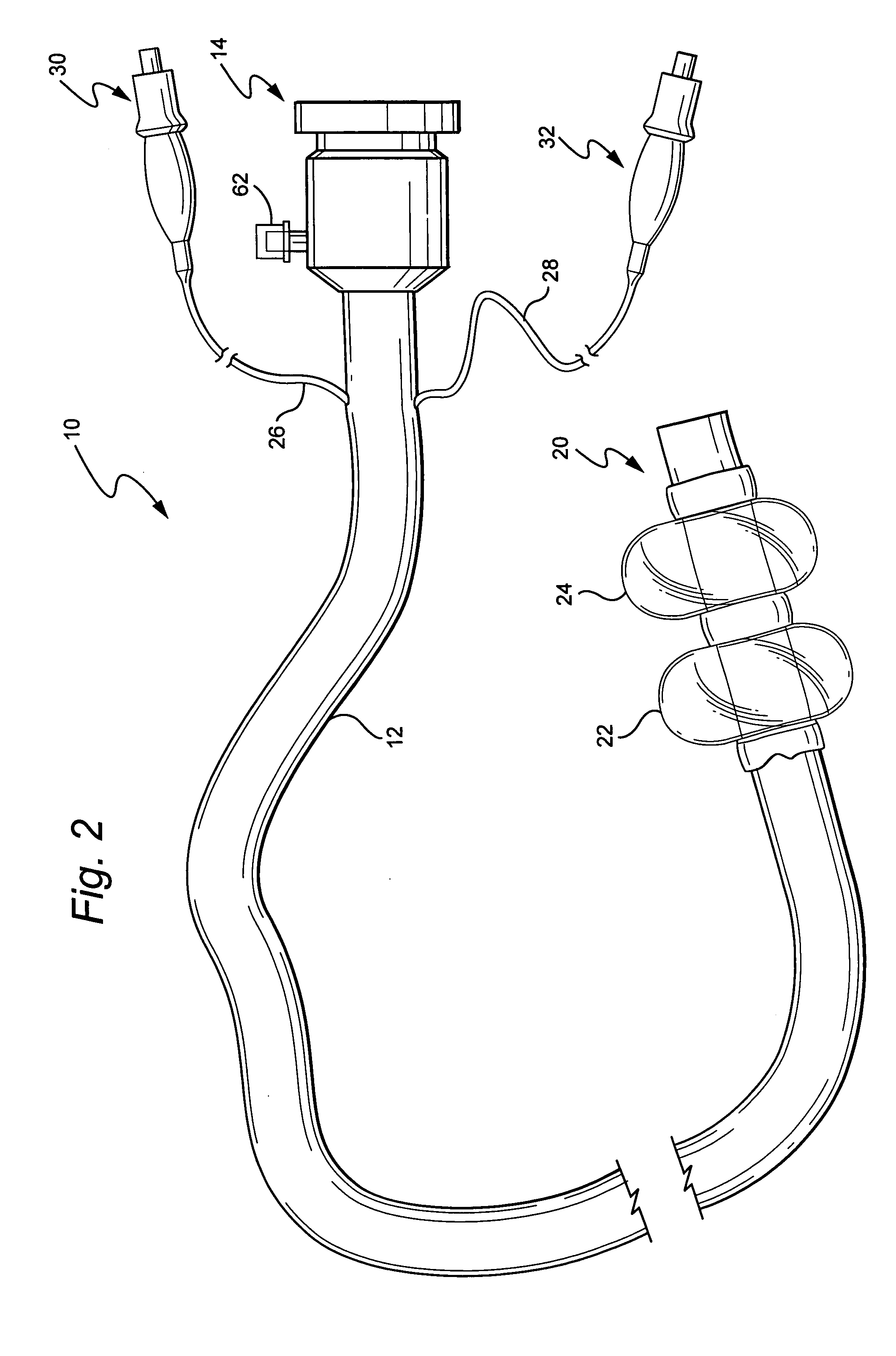
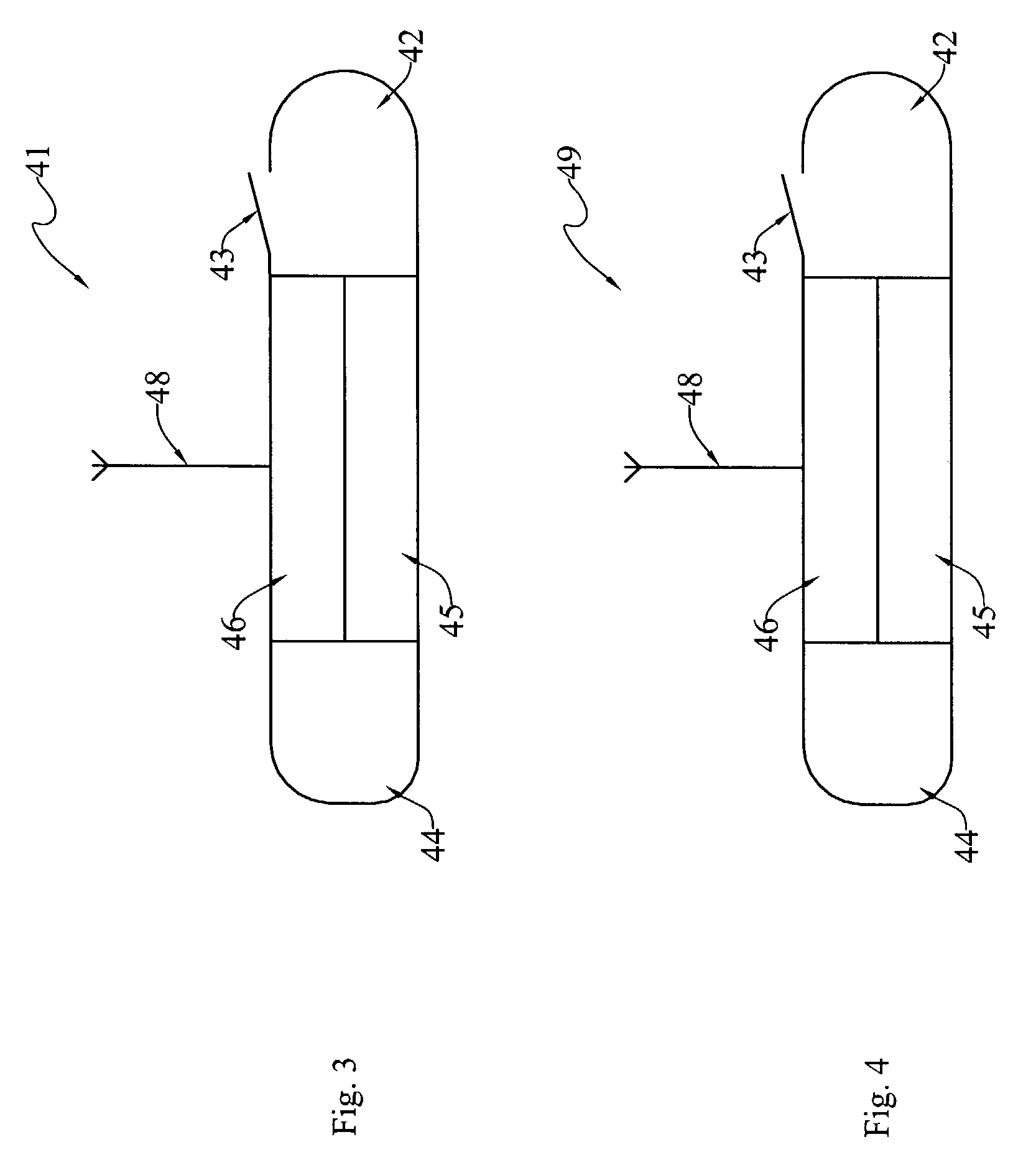
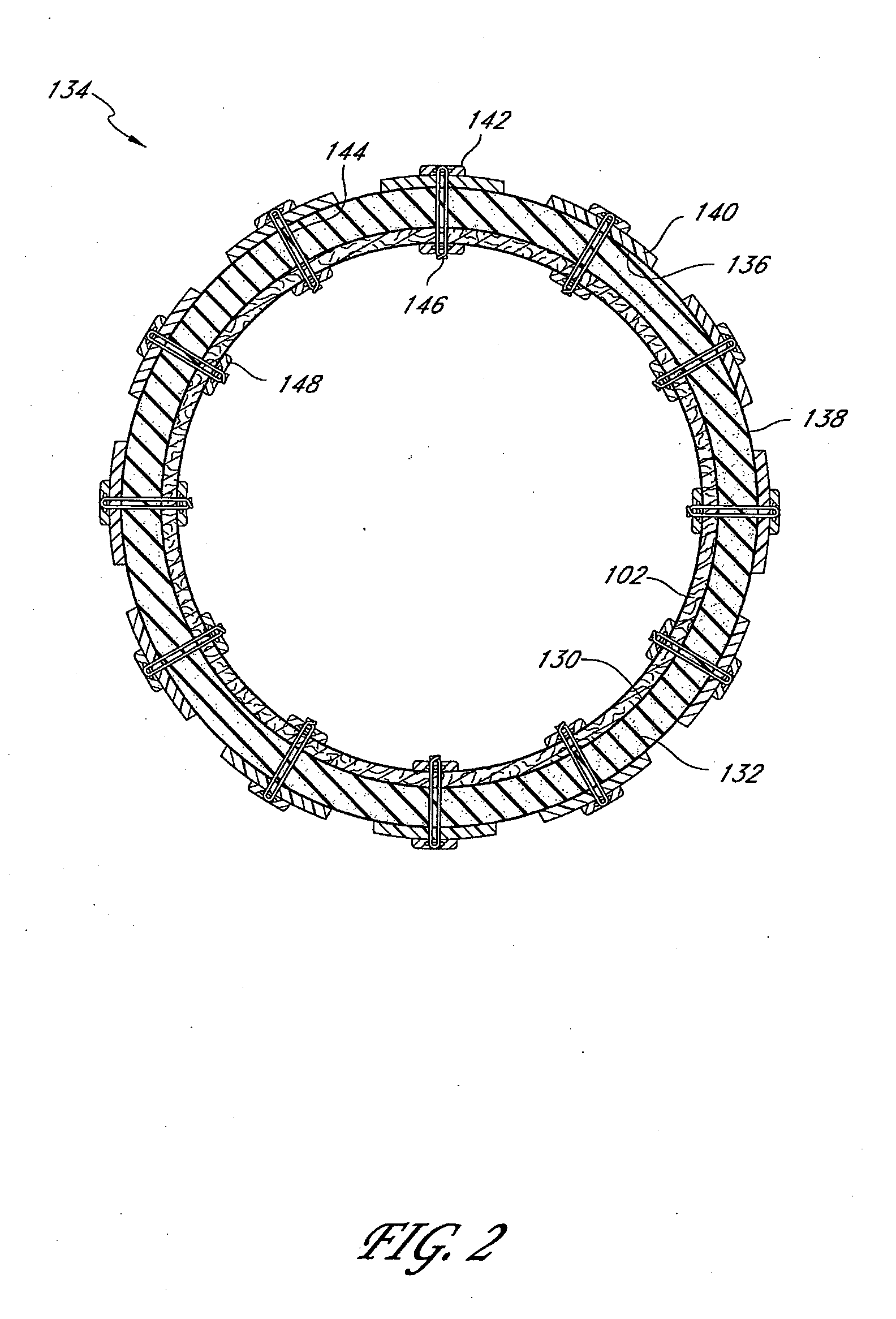
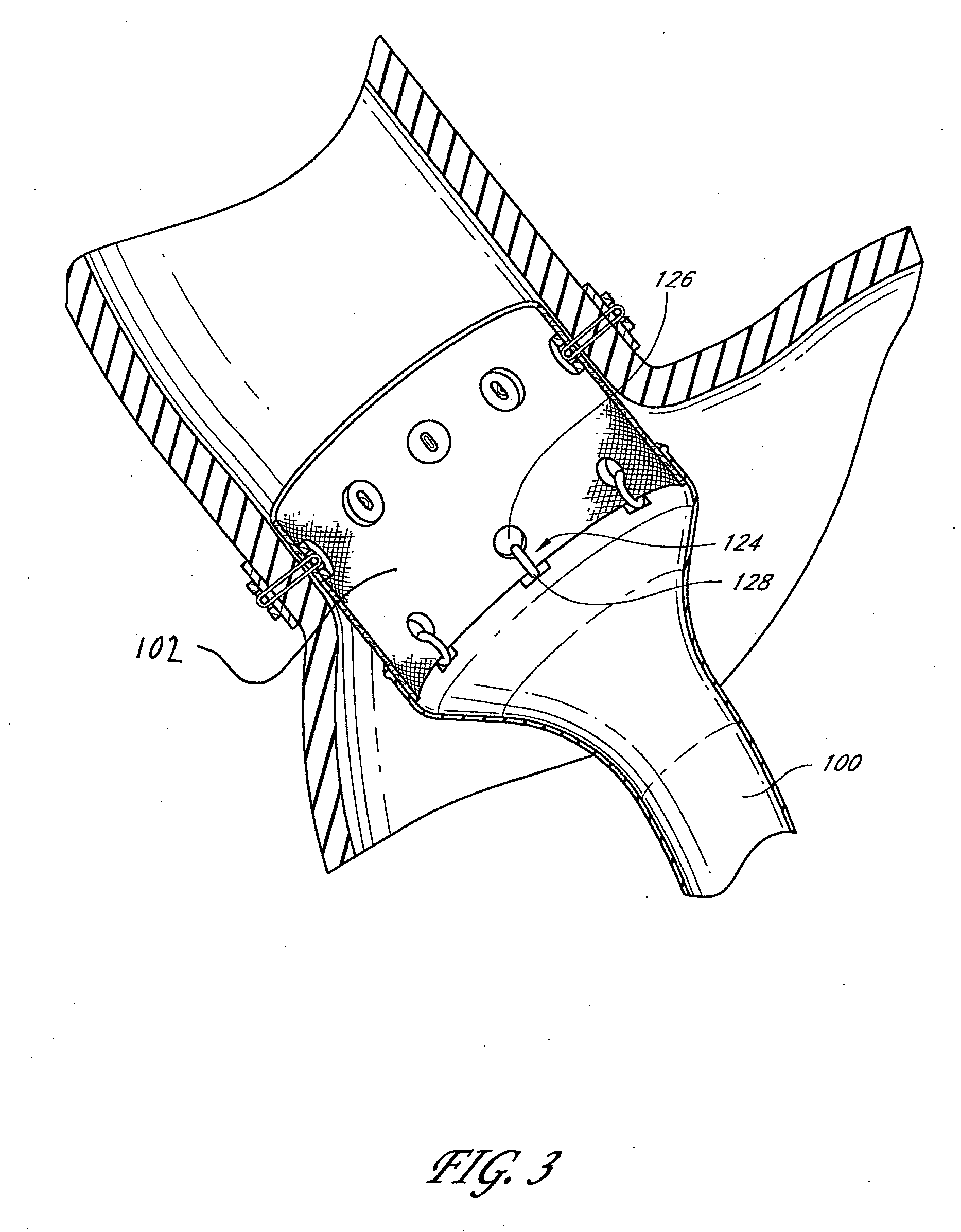
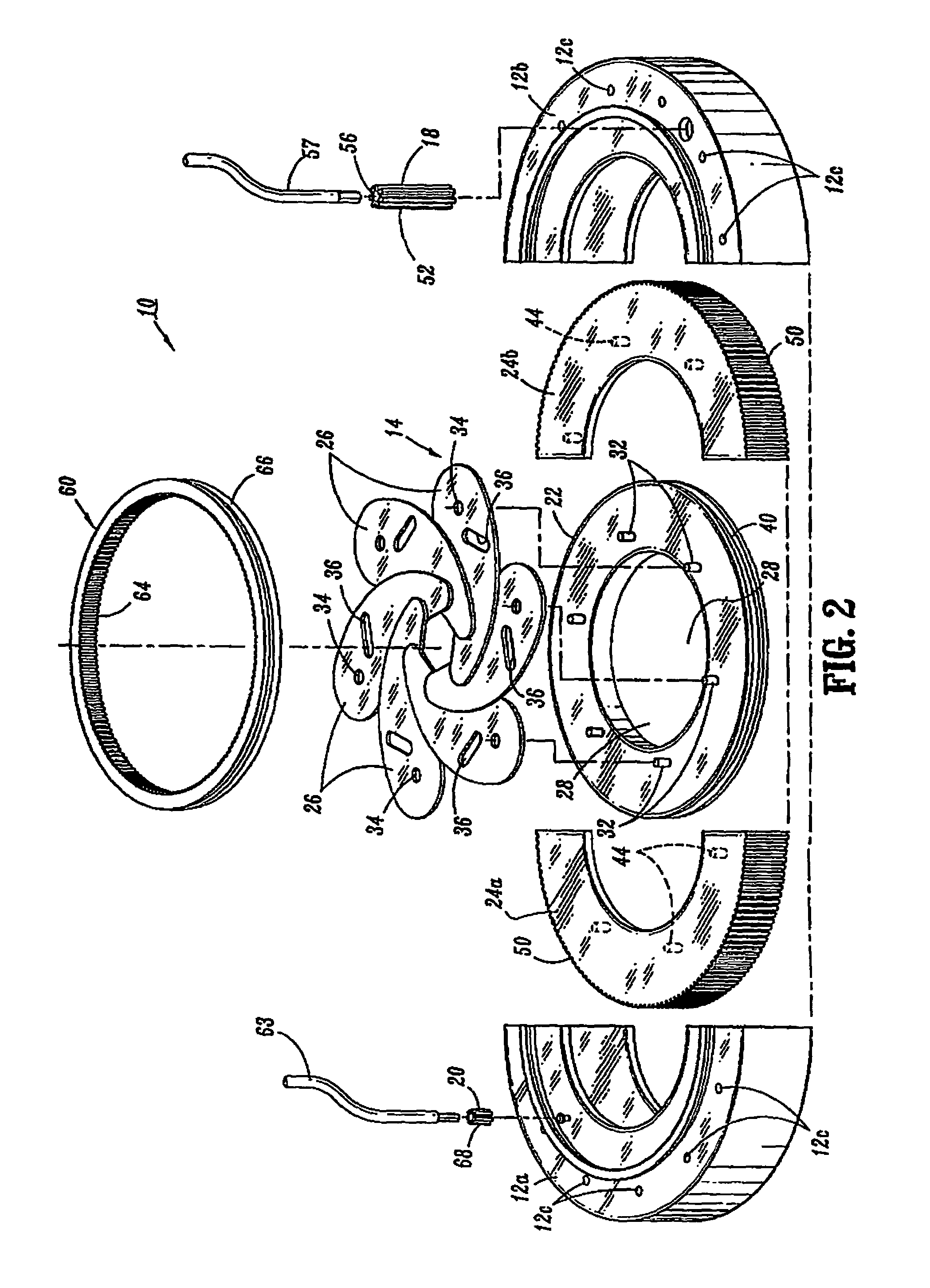
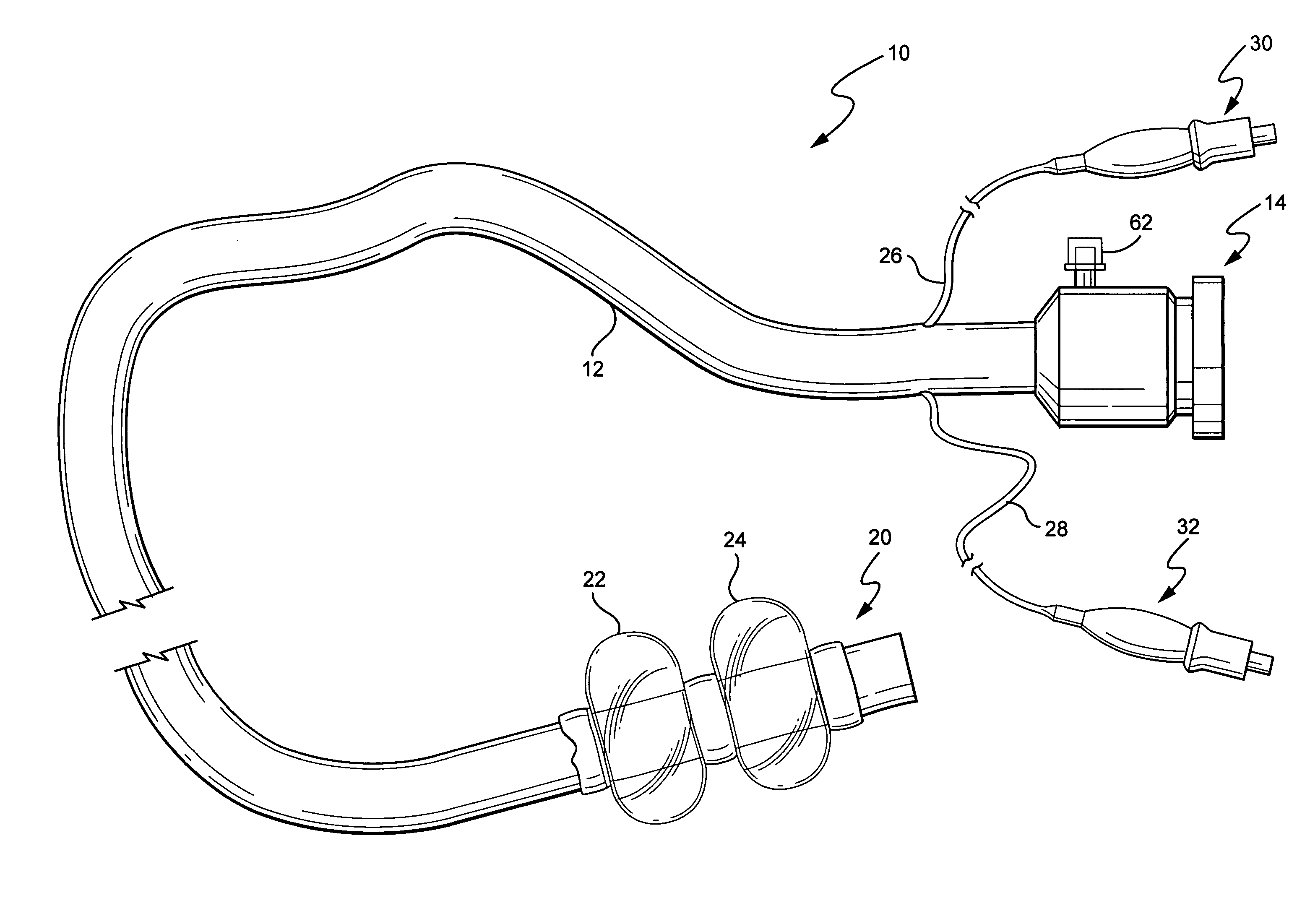
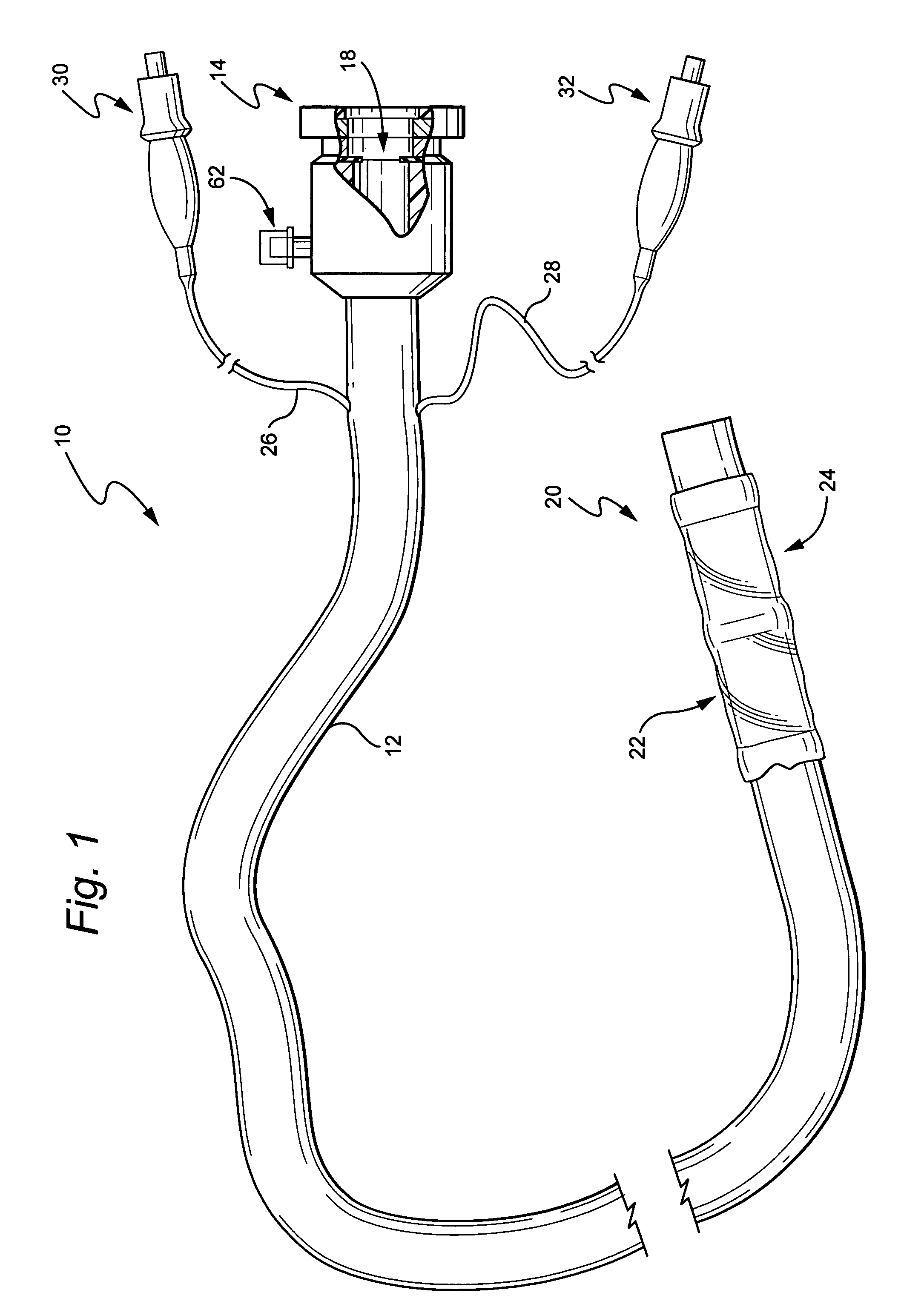
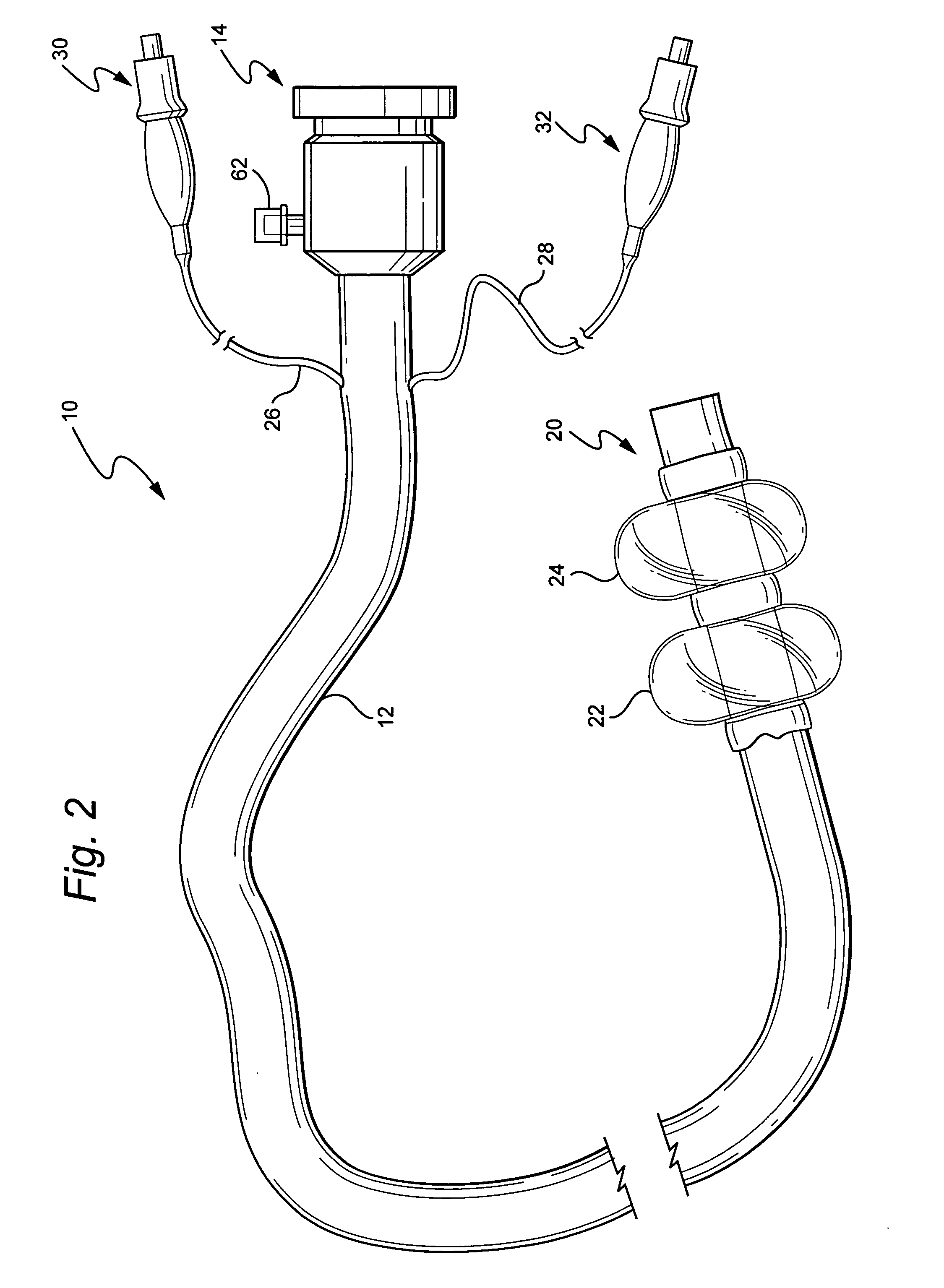
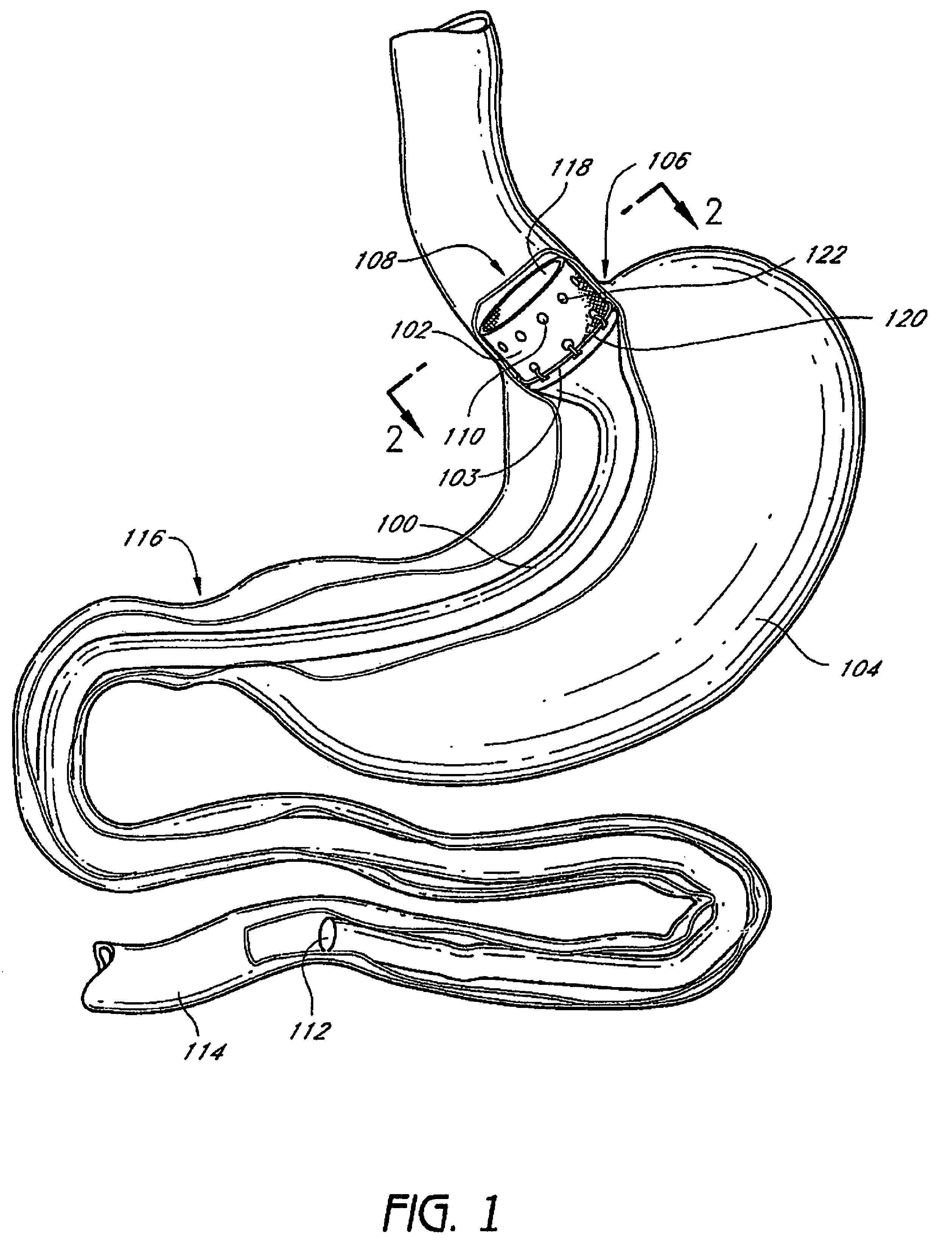
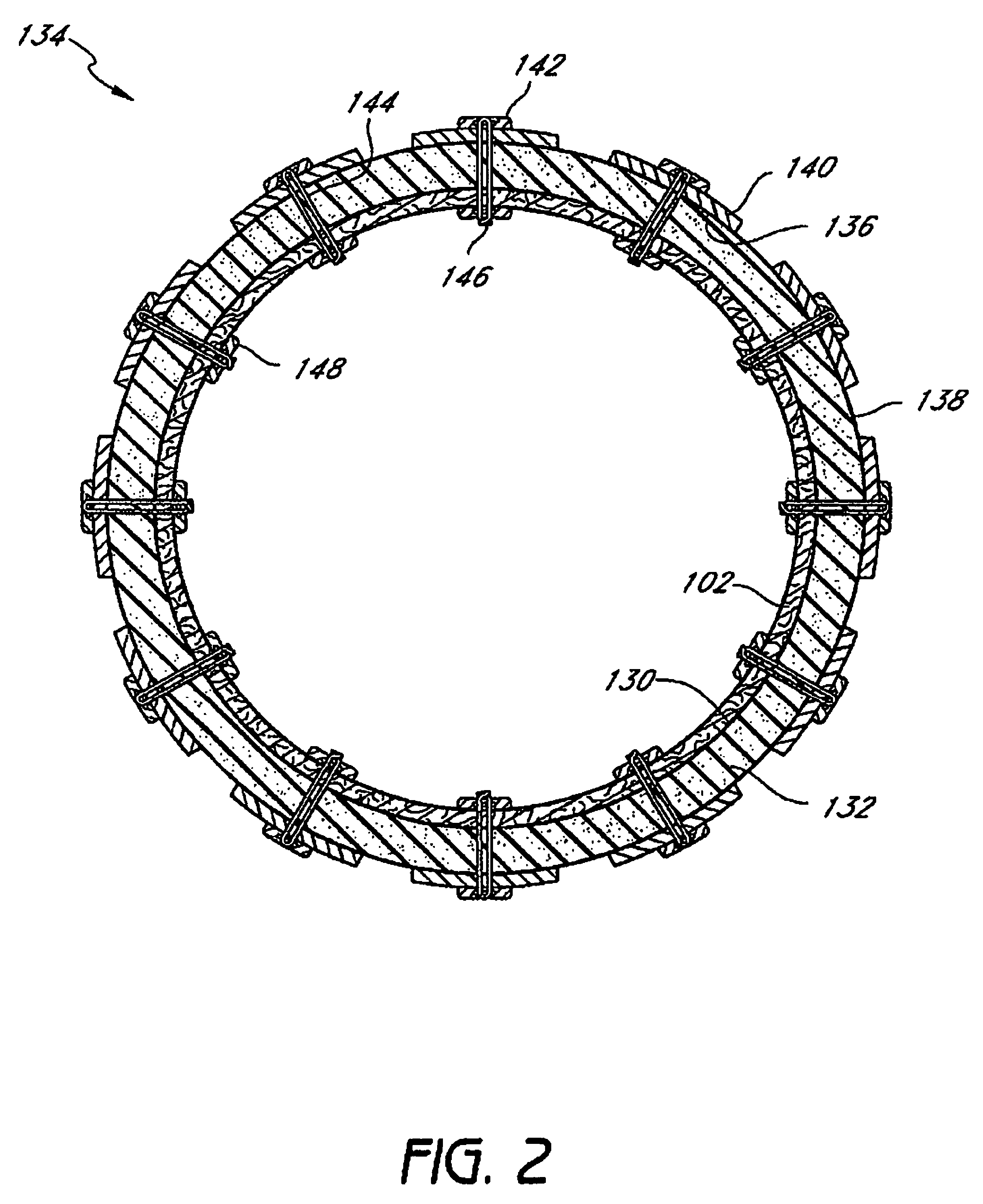
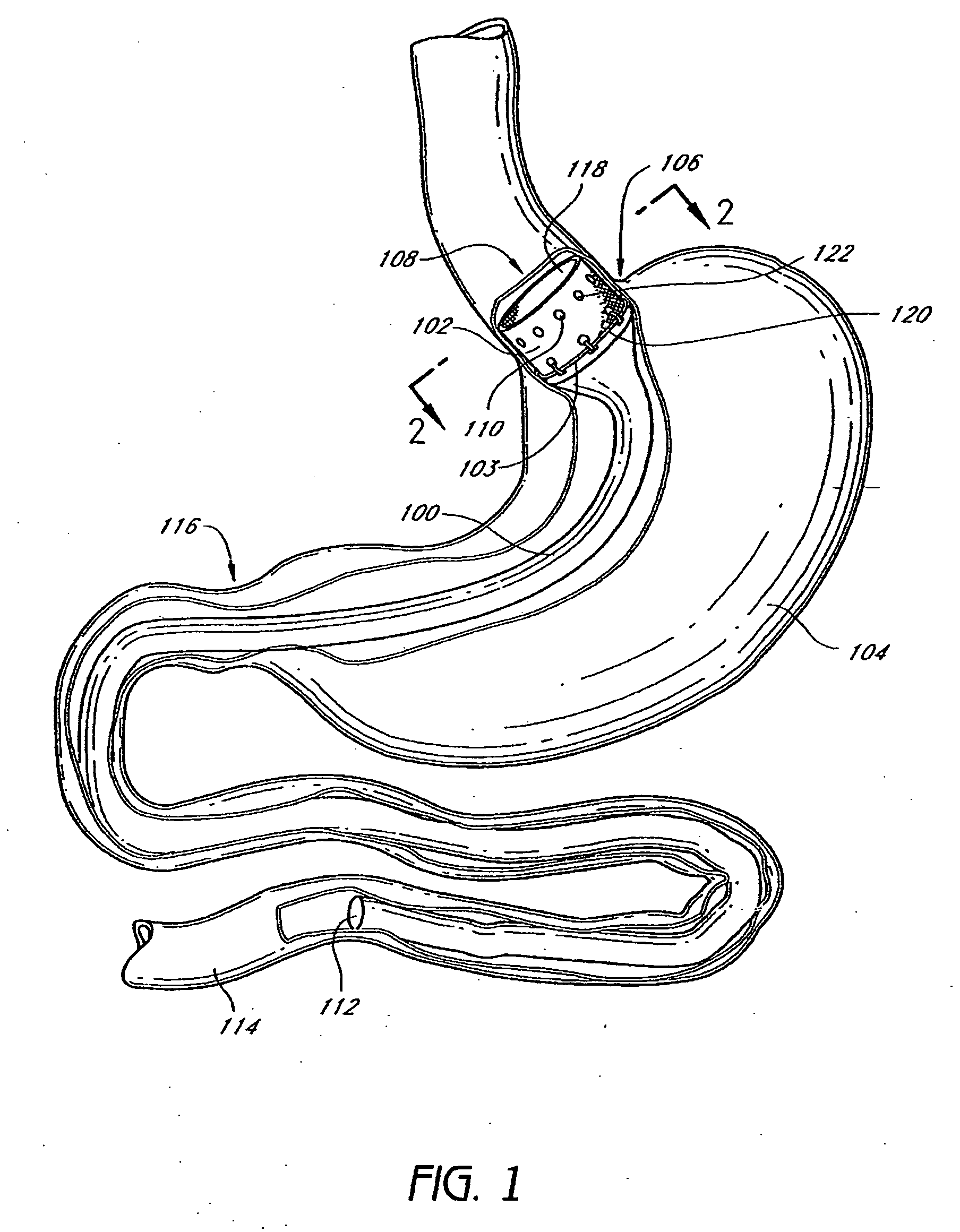
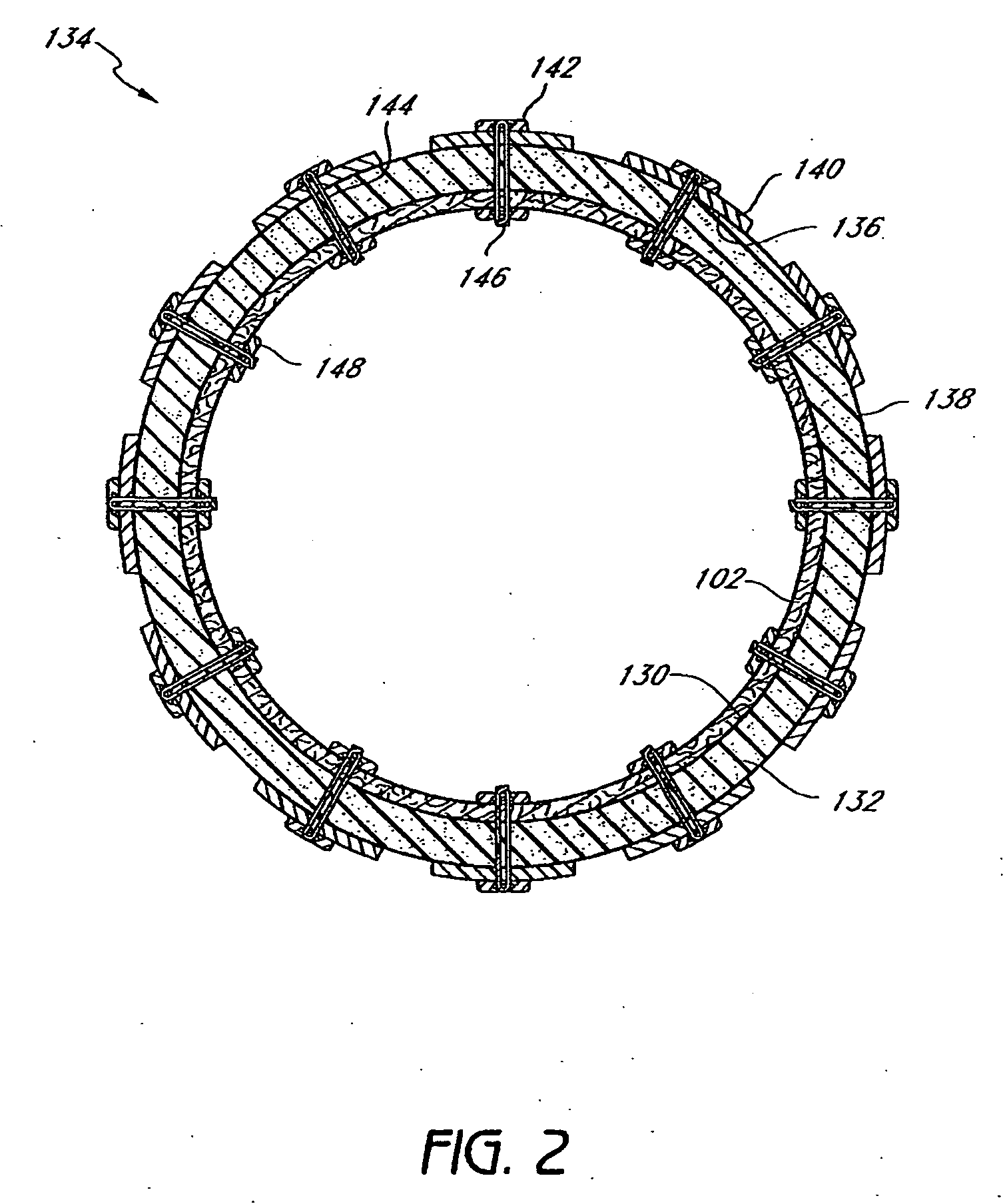
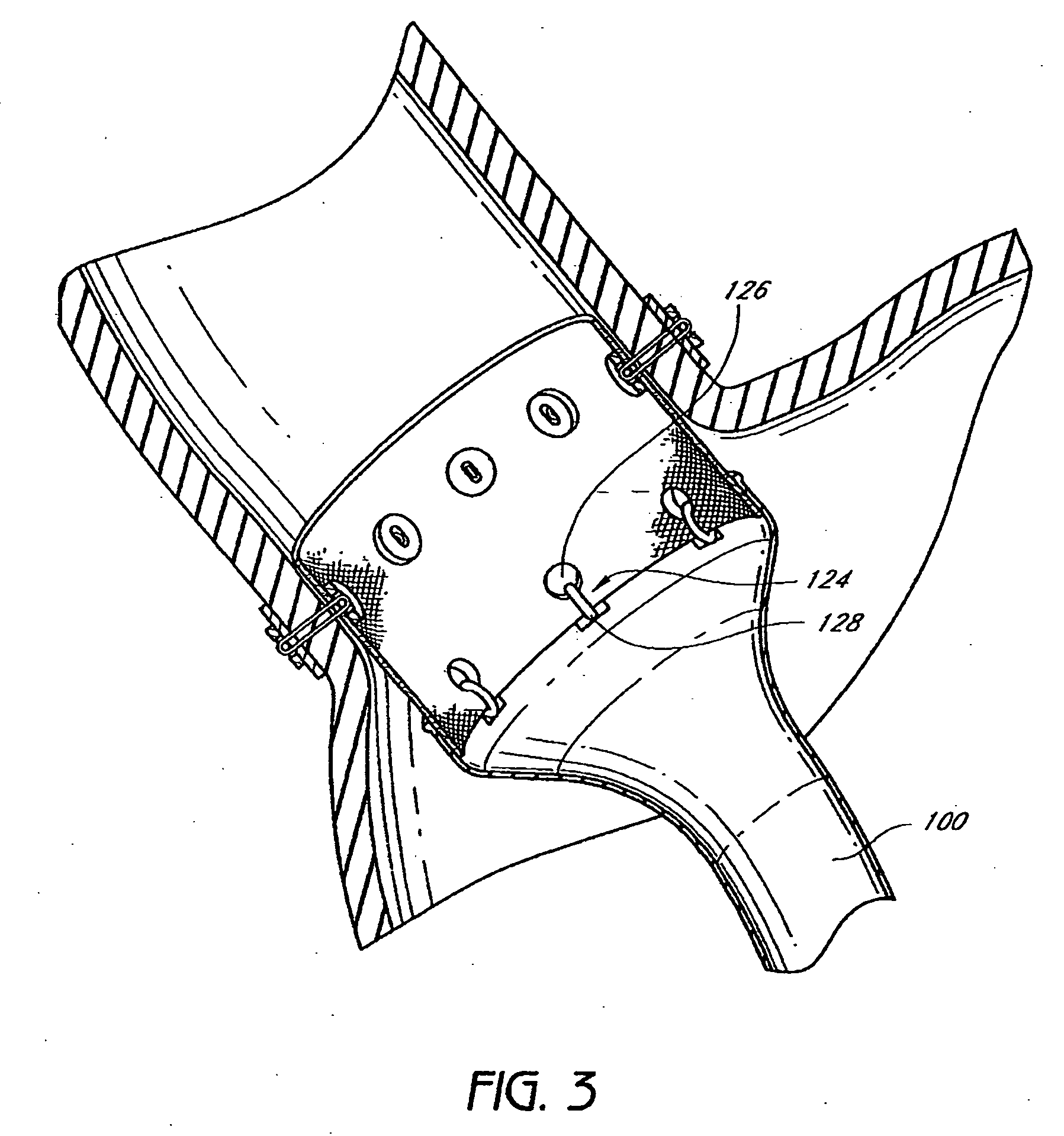
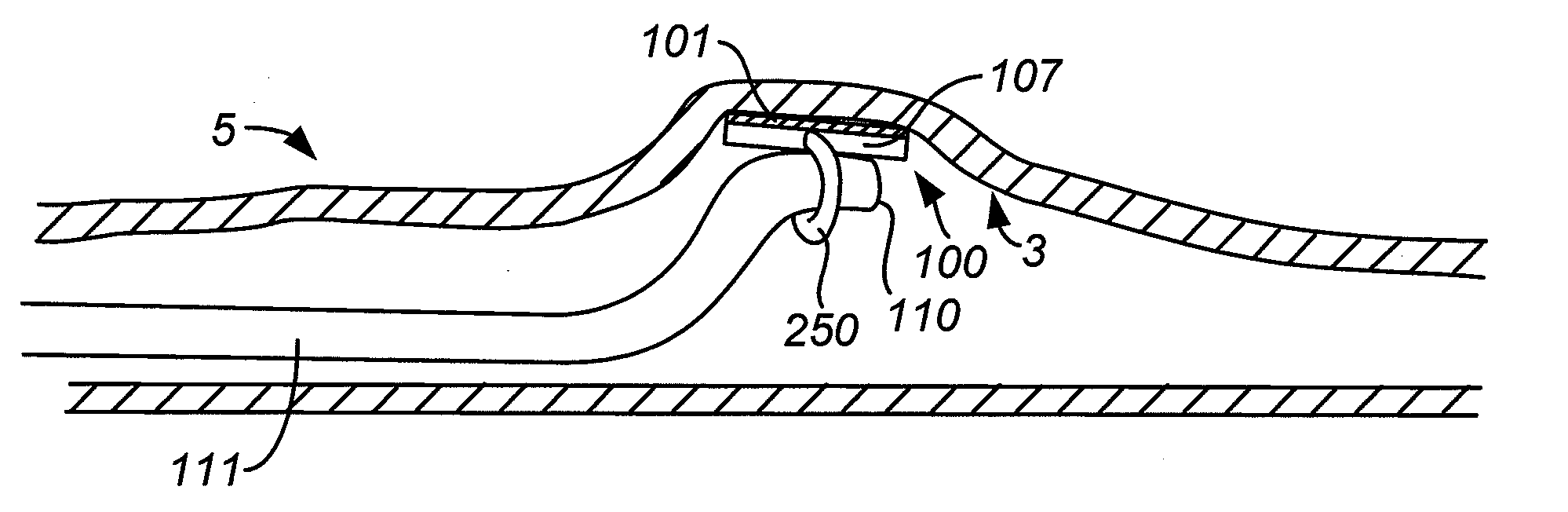
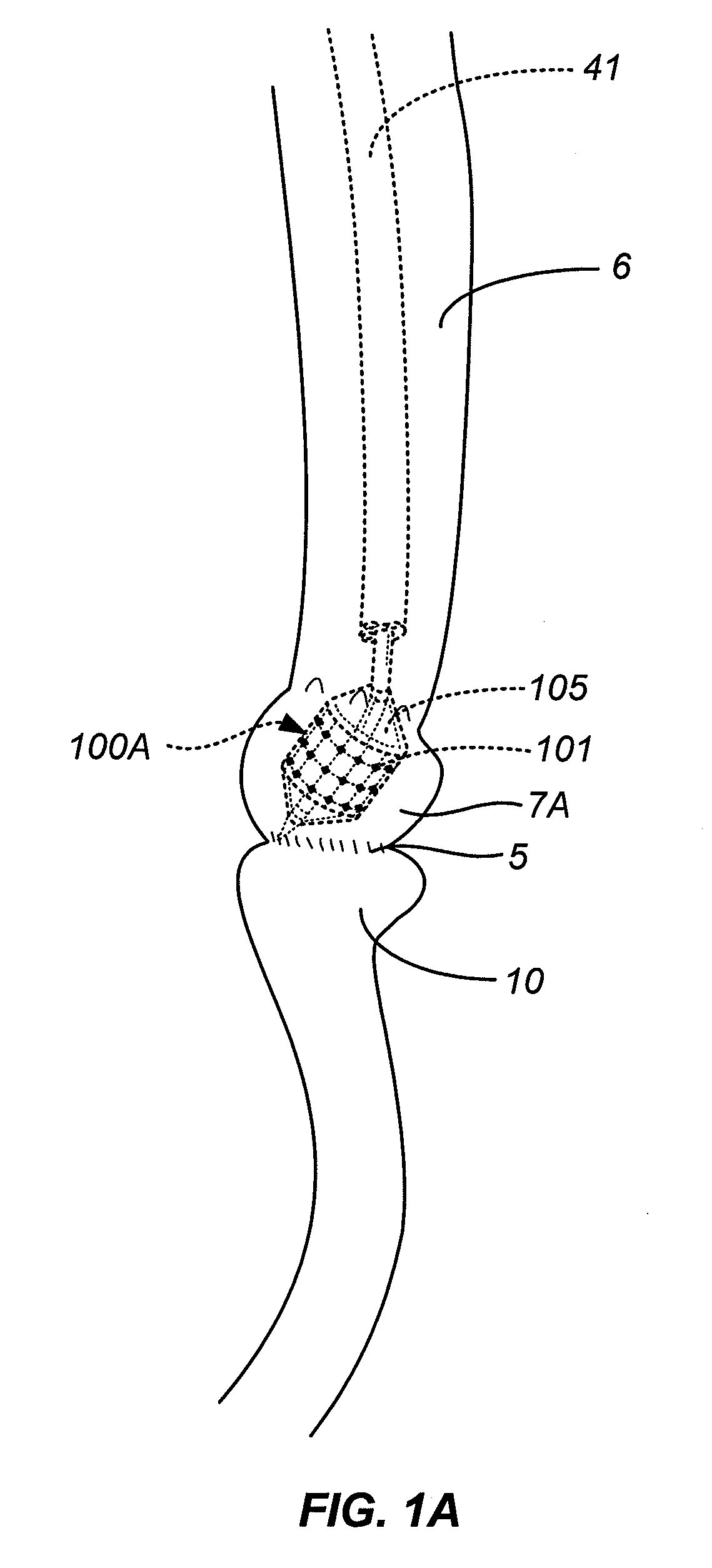
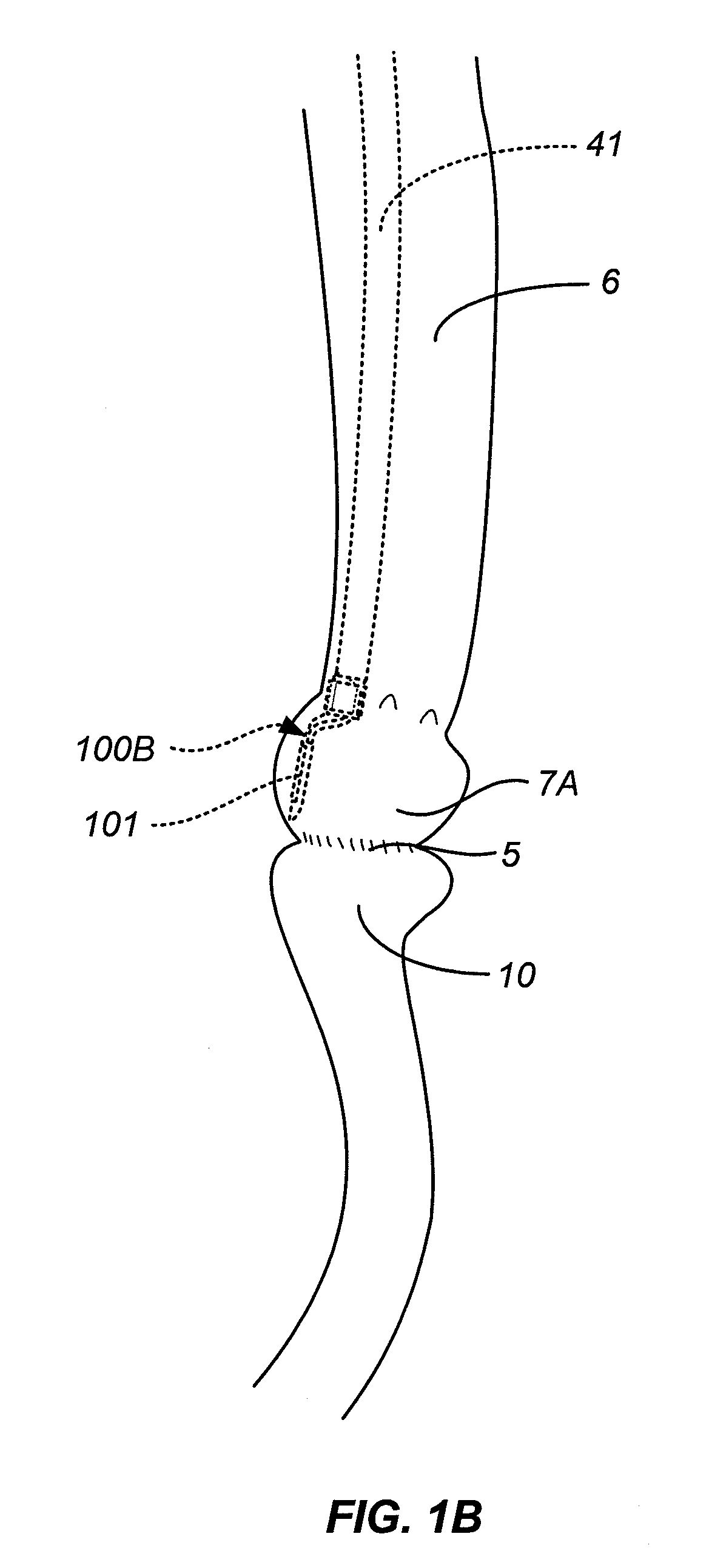
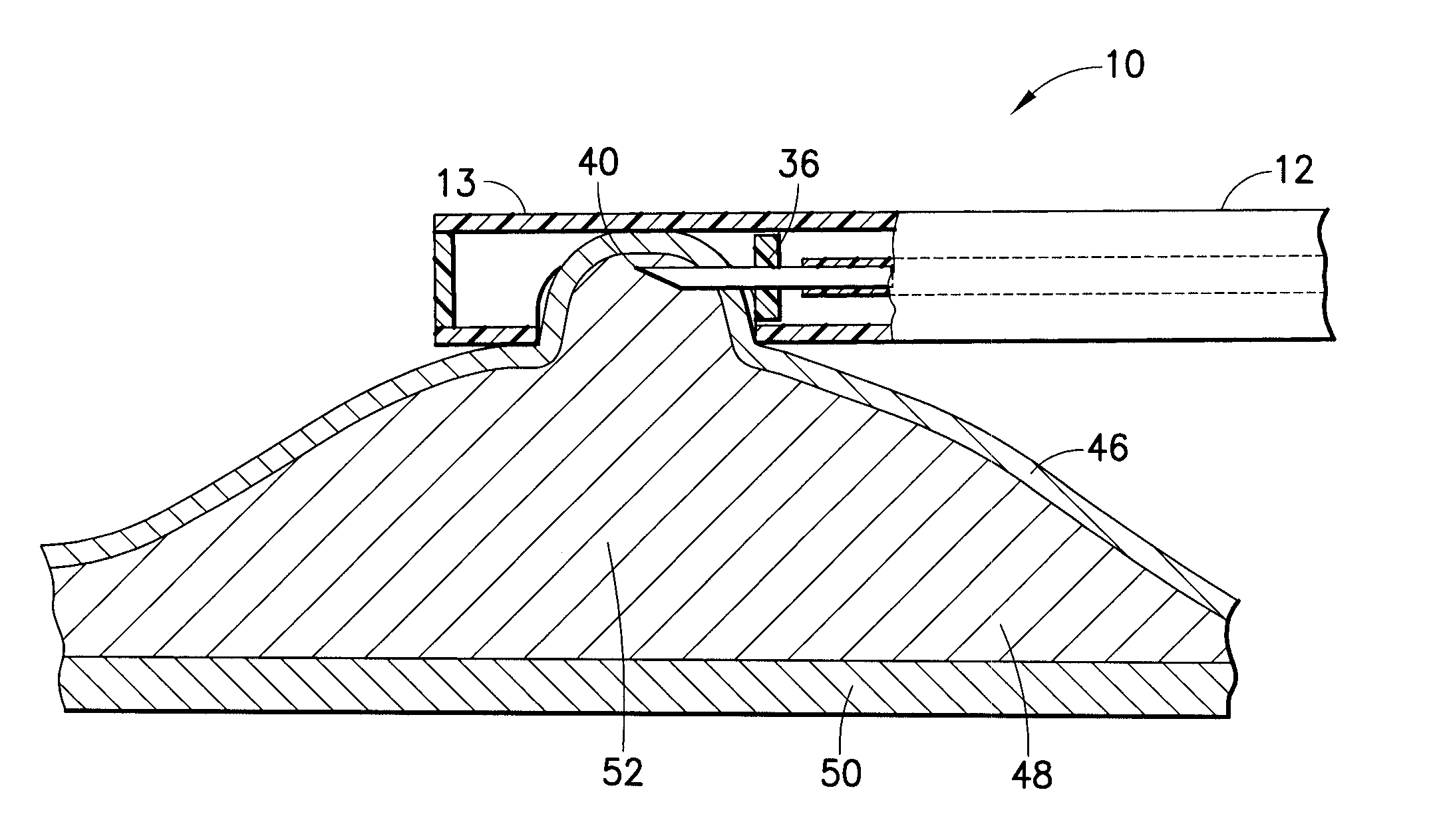
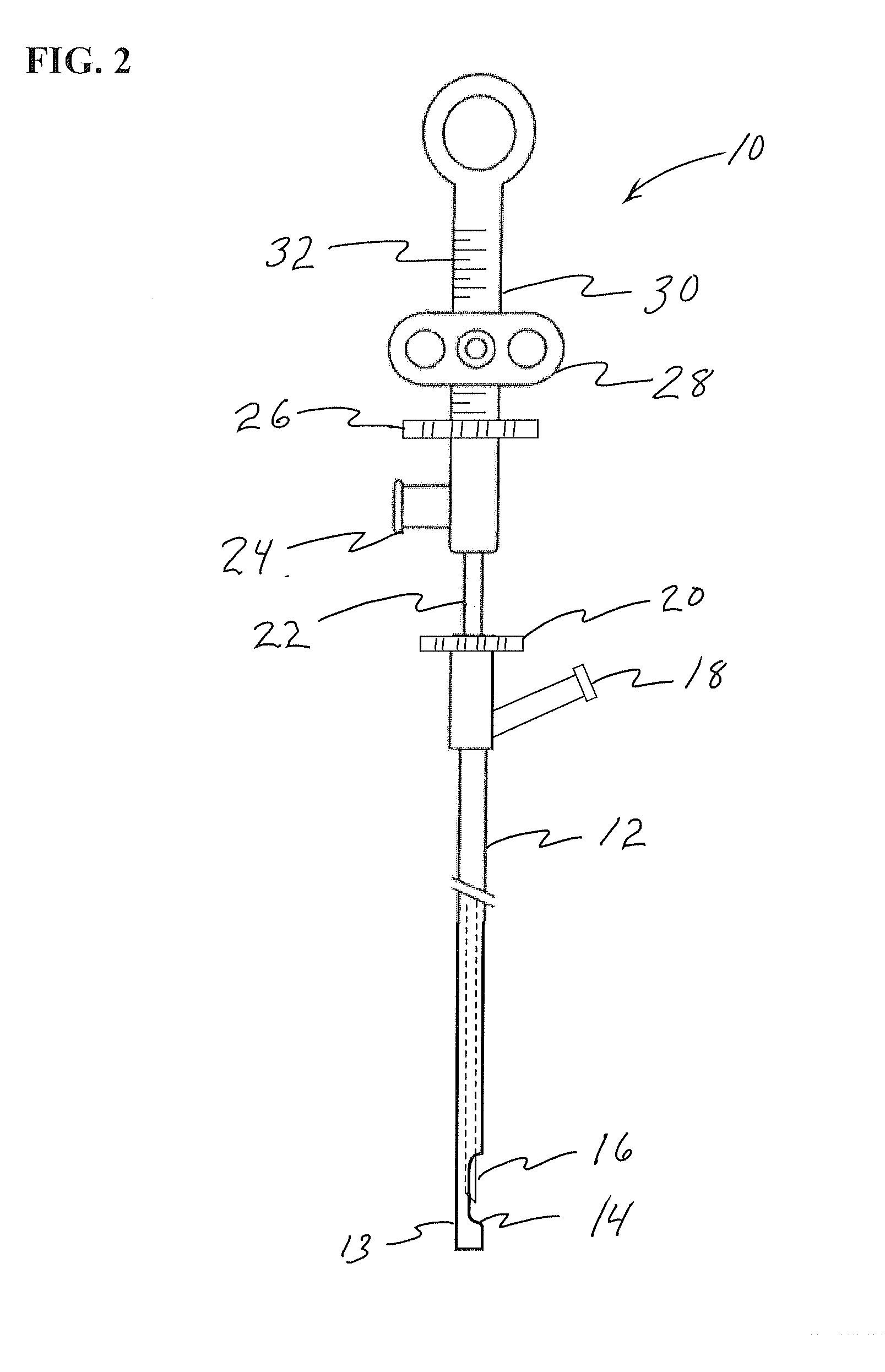
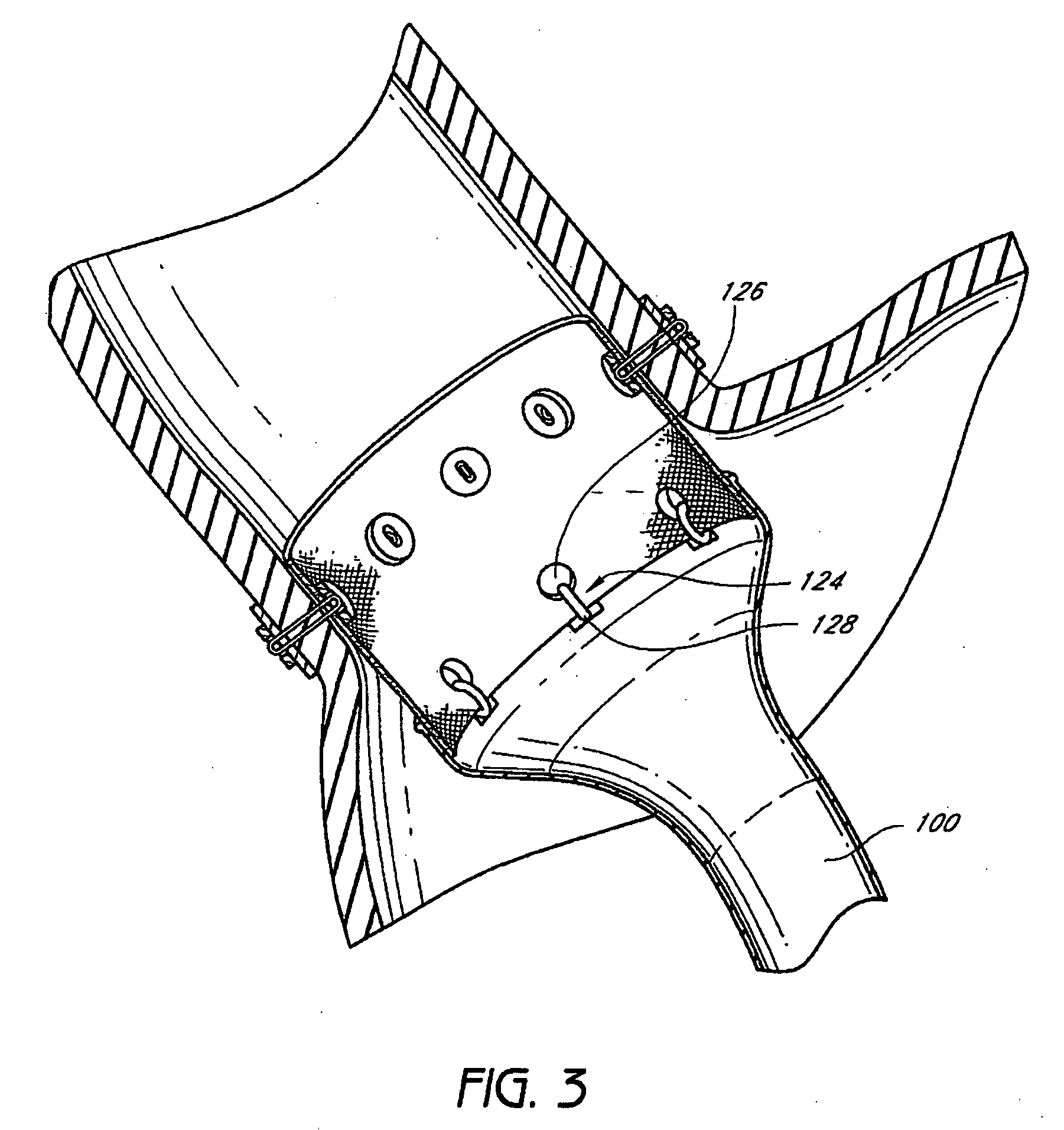
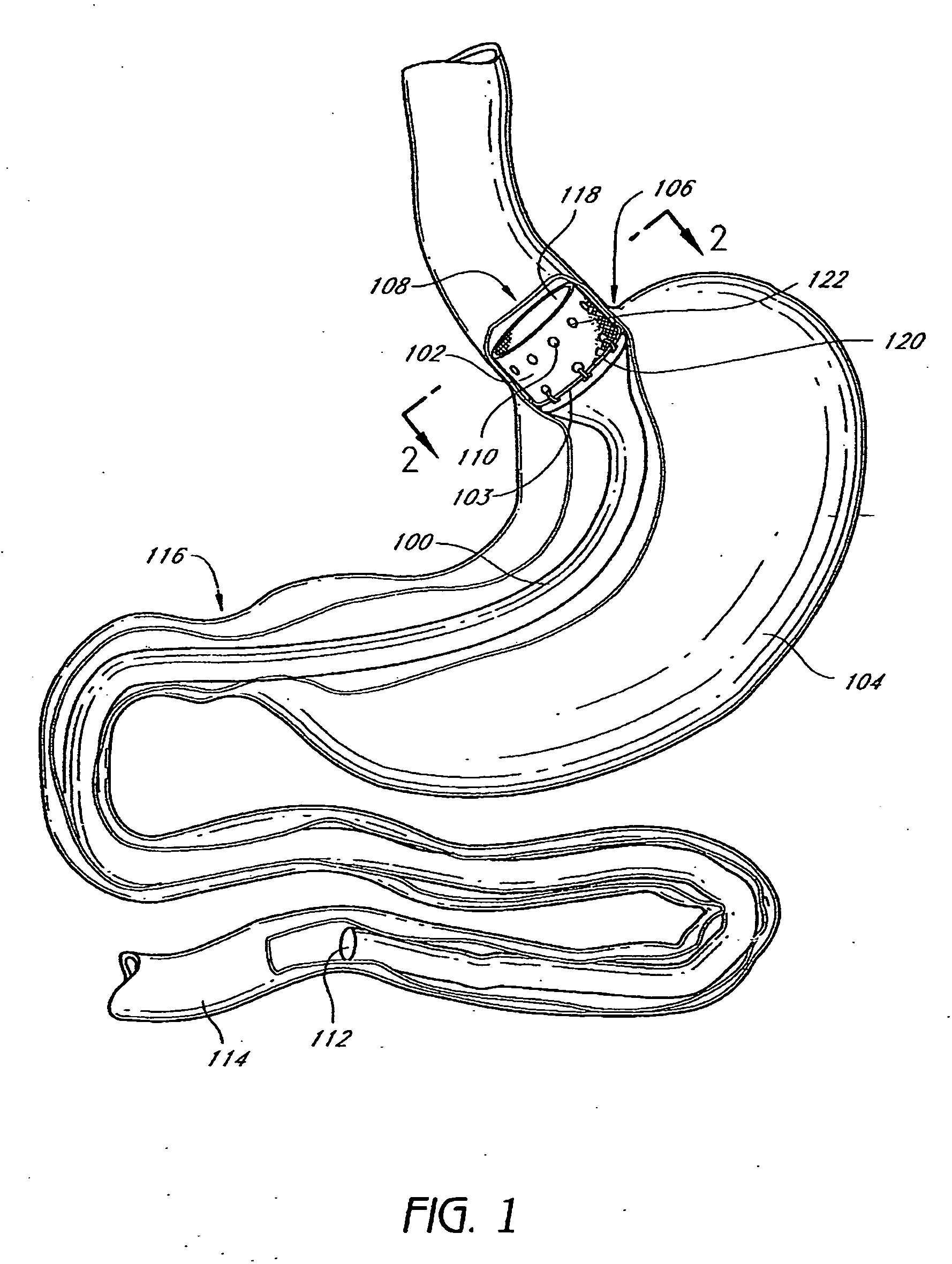
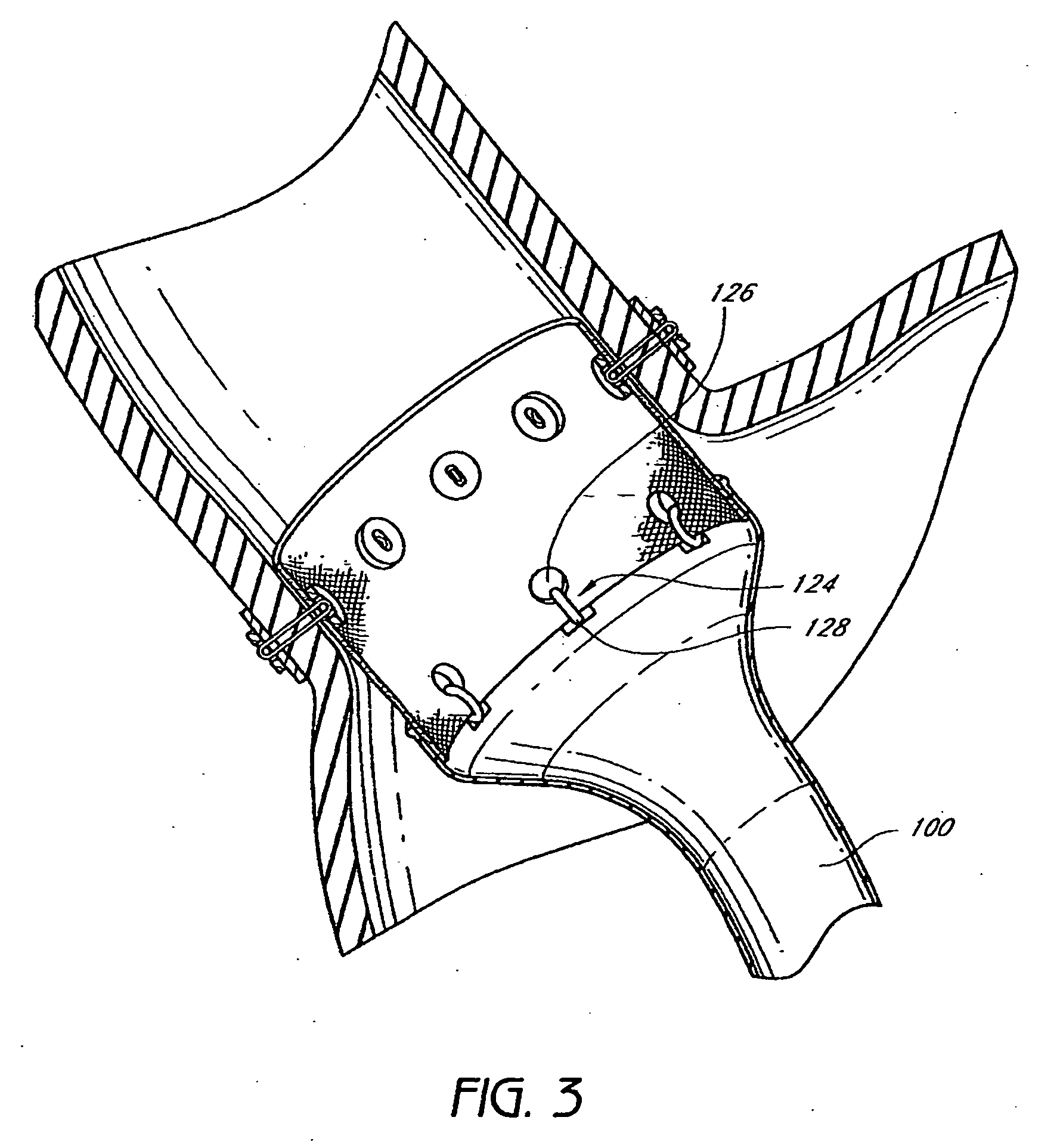
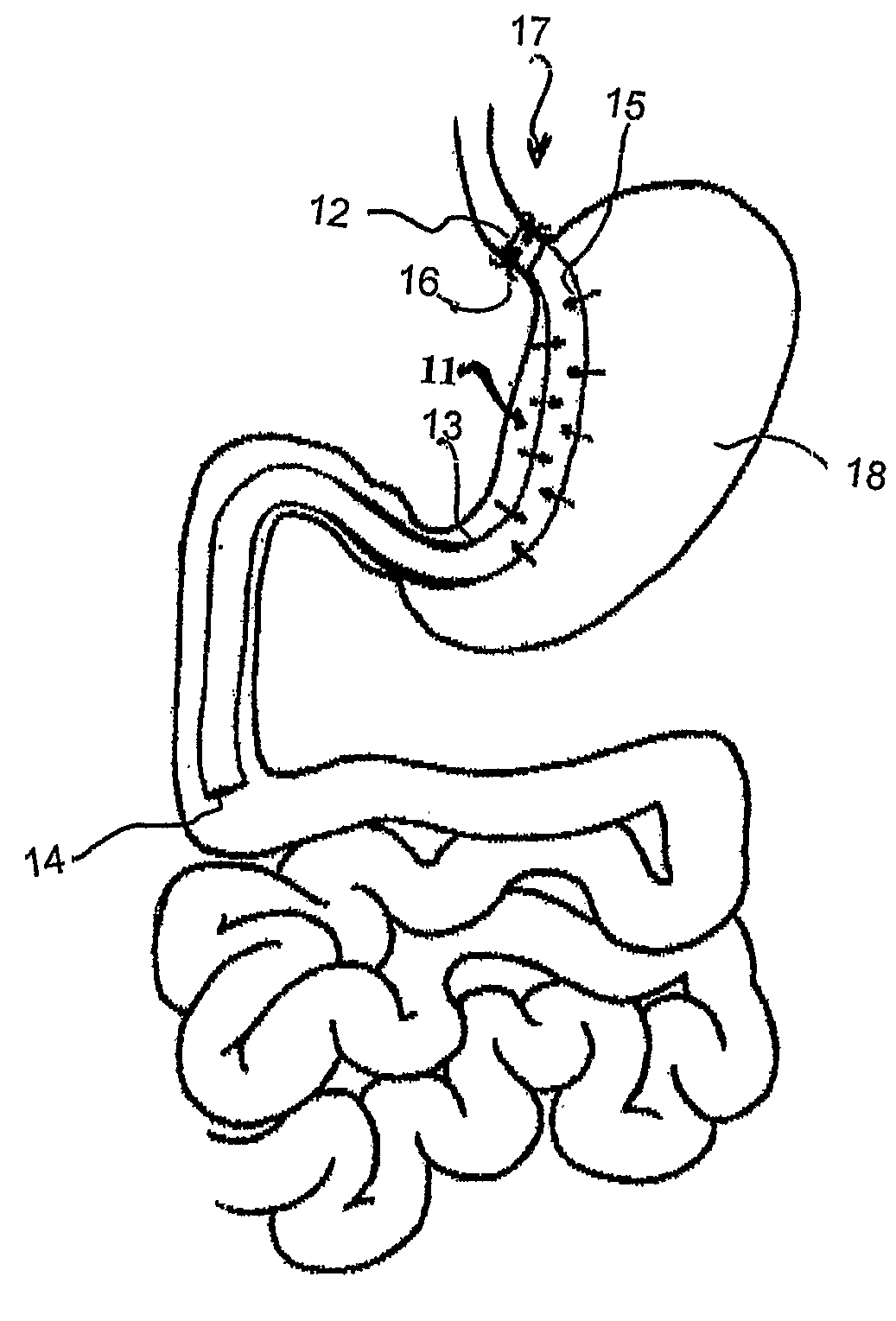
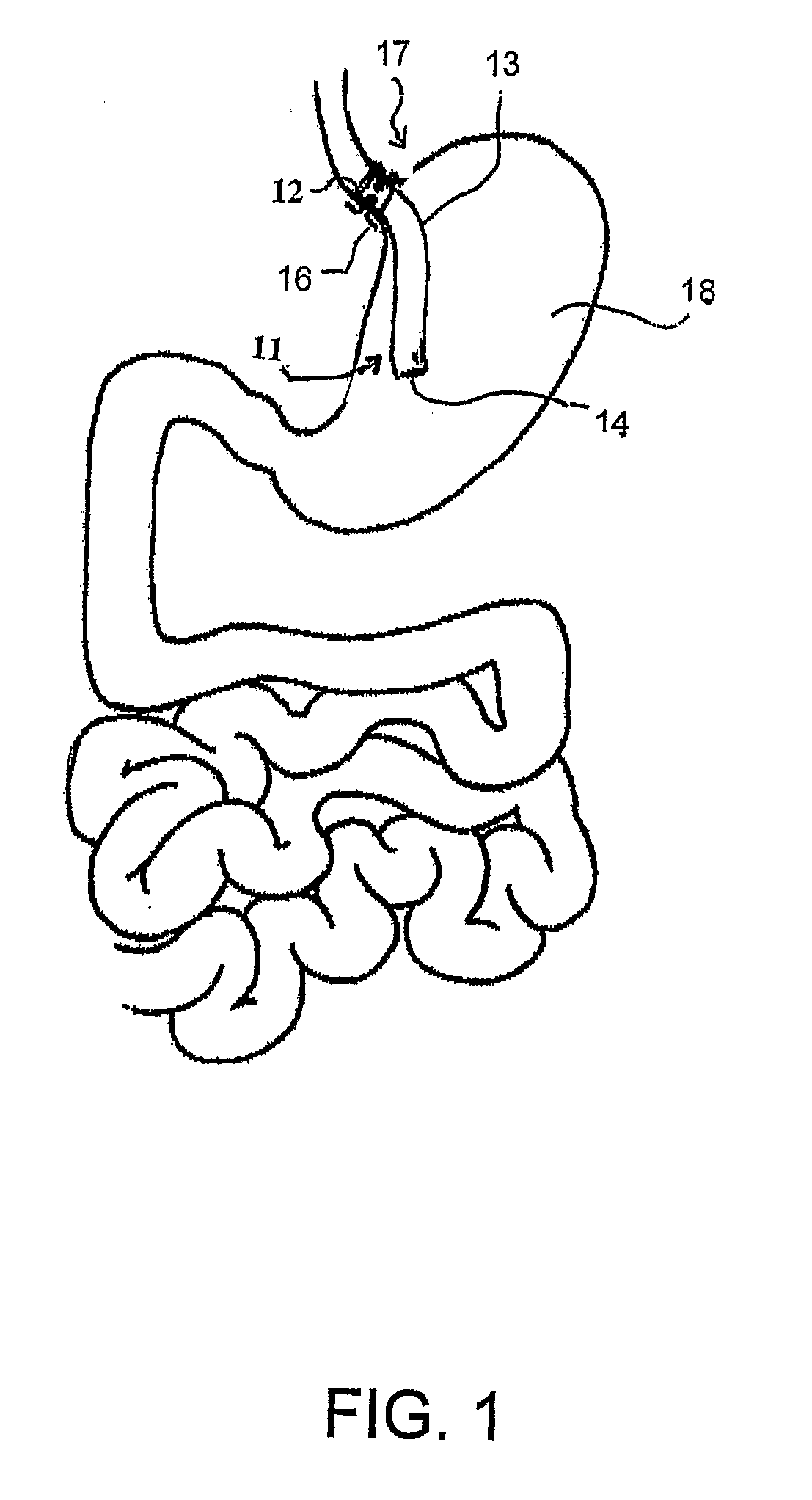
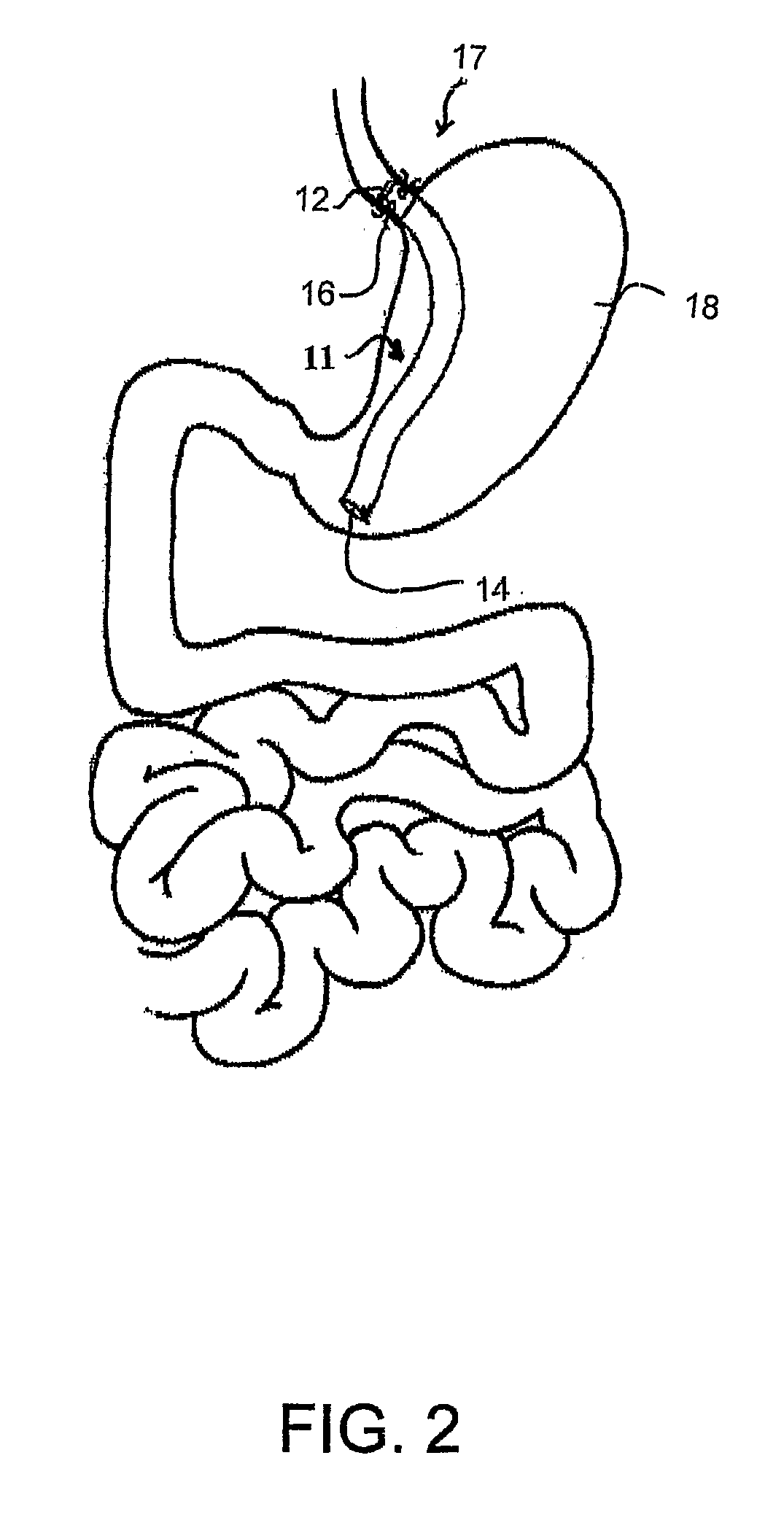
Devices and methods for treating morbid obesity
The present invention provides devices and methods for attachment of an implanted device, such as an artificial stoma device, a gastrointestinal sleeve device or an attachment cuff, within a patient's digestive tract for treatment of obesity. Special surgical fasteners provide a lasting and durable attachment to the gastrointestinal tissue without causing excessive pressure that could result in tissue erosion and detachment of the implanted device. Fastener delivery devices that facilitate peroral placement and deployment of fasteners and secondary devices are also provided. Also described are implantable devices and attachment means that avoid causing excessive pressure within the tissue by having compliance that is compatible with the gastrointestinal tissues where it is attached.
Owner:VALENTX
Devices and methods for treating morbid obesity
The present invention provides devices and methods for attachment of an implanted device, such as an artificial stoma device, a gastrointestinal sleeve device or an attachment cuff, within a patient's digestive tract for treatment of obesity. Special surgical fasteners provide a lasting and durable attachment to the gastrointestinal tissue without causing excessive pressure that could result in tissue erosion and detachment of the implanted device. Fastener delivery devices that facilitate peroral placement and deployment of fasteners and secondary devices are also provided. Also described are implantable devices and attachment means that avoid causing excessive pressure within the tissue by having compliance that is compatible with the gastrointestinal tissues where it is attached.
Owner:VALENTX
Methods and devices for diagnostic and therapeutic interventions in the peritoneal cavity
A novel approach to diagnostic and therapeutic interventions in the peritoneal cavity is described. More specifically, a technique for accessing the peritoneal cavity via the wall of the digestive tract is provided so that examination of and / or a surgical procedure in the peritoneal cavity can be conducted via the wall of the digestive tract with the use of a flexible endoscope. As presently proposed, the technique is particularly adapted to transgastric peritoneoscopy. However, access in addition or in the alternative through the intestinal wall is contemplated and described as well. Transgastric and / or transintestinal peritoneoscopy will have an excellent cosmetic result as there are no incisions in the abdominal wall and no potential for visible post-surgical scars or hernias.
Owner:APOLLO ENDOSURGERY INC
Intragastric volume-occupying device
A self-inflating and self-deflating orally ingestible device that is able to traverse the entirety of the alimentary canal is described. In one embodiment, the device includes a closed balloon having a surface separating an enclosed space internal to the balloon from a space external to the balloon, at least one self-sealing valve integrated with the surface of the balloon to provide access to the enclosed space internal to the balloon from the space external to the balloon, a vent comprised of a dissolvable seal integrated with the surface of the balloon, and a vessel located within the enclosed space internal to the balloon to separate internal contents of the vessel from the enclosed space internal to the balloon.
Owner:OBALON THERAPEUTICS
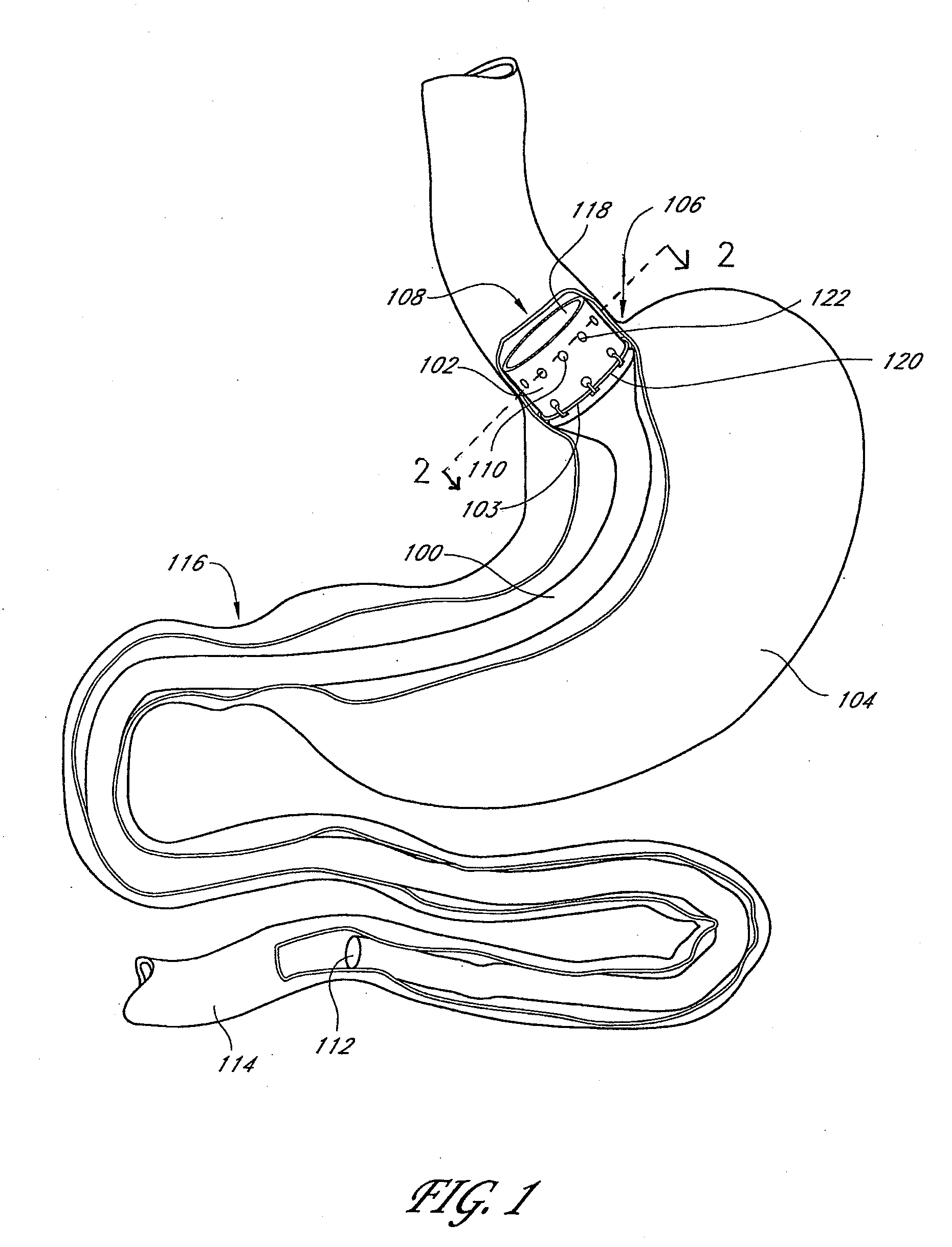
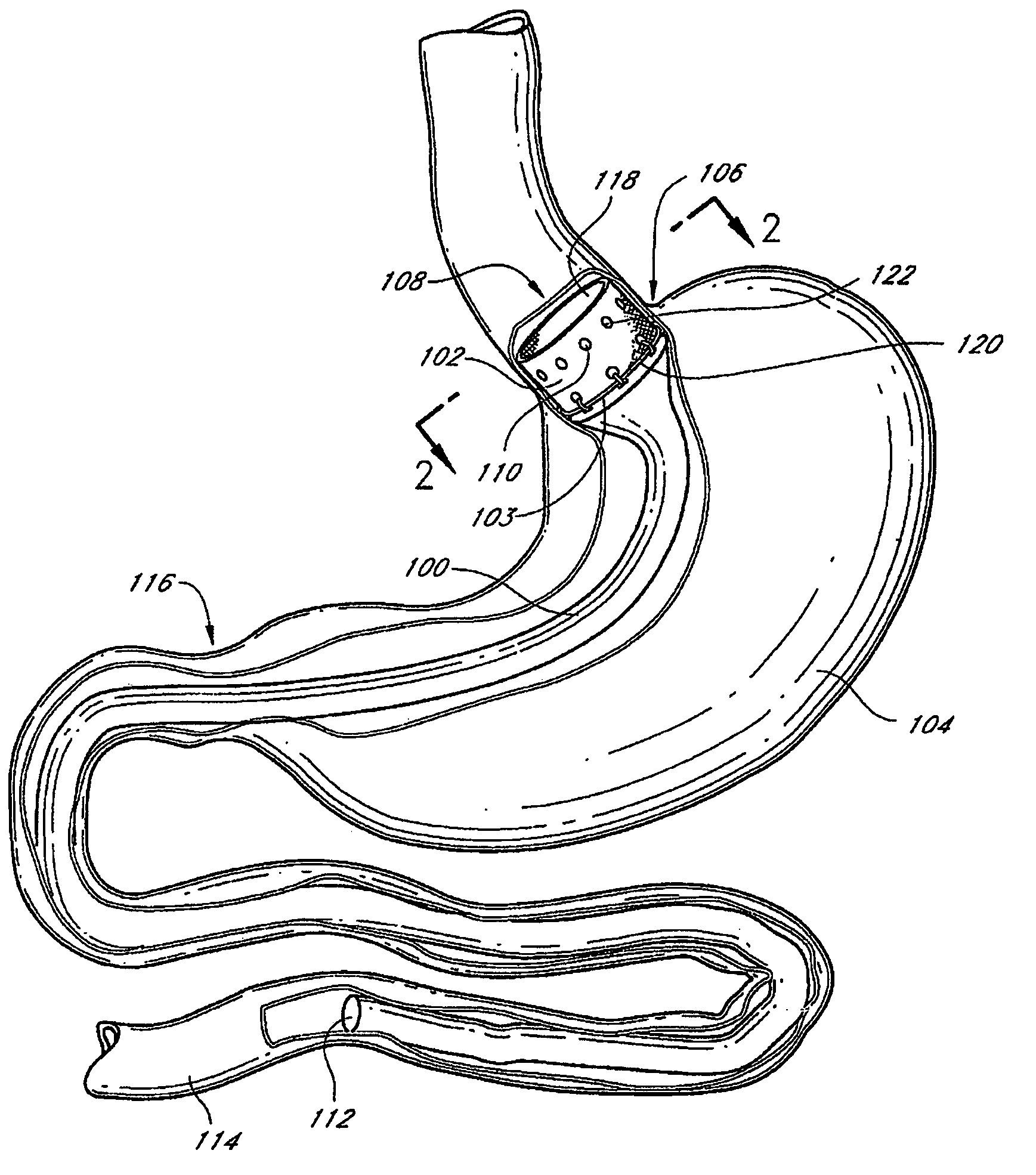
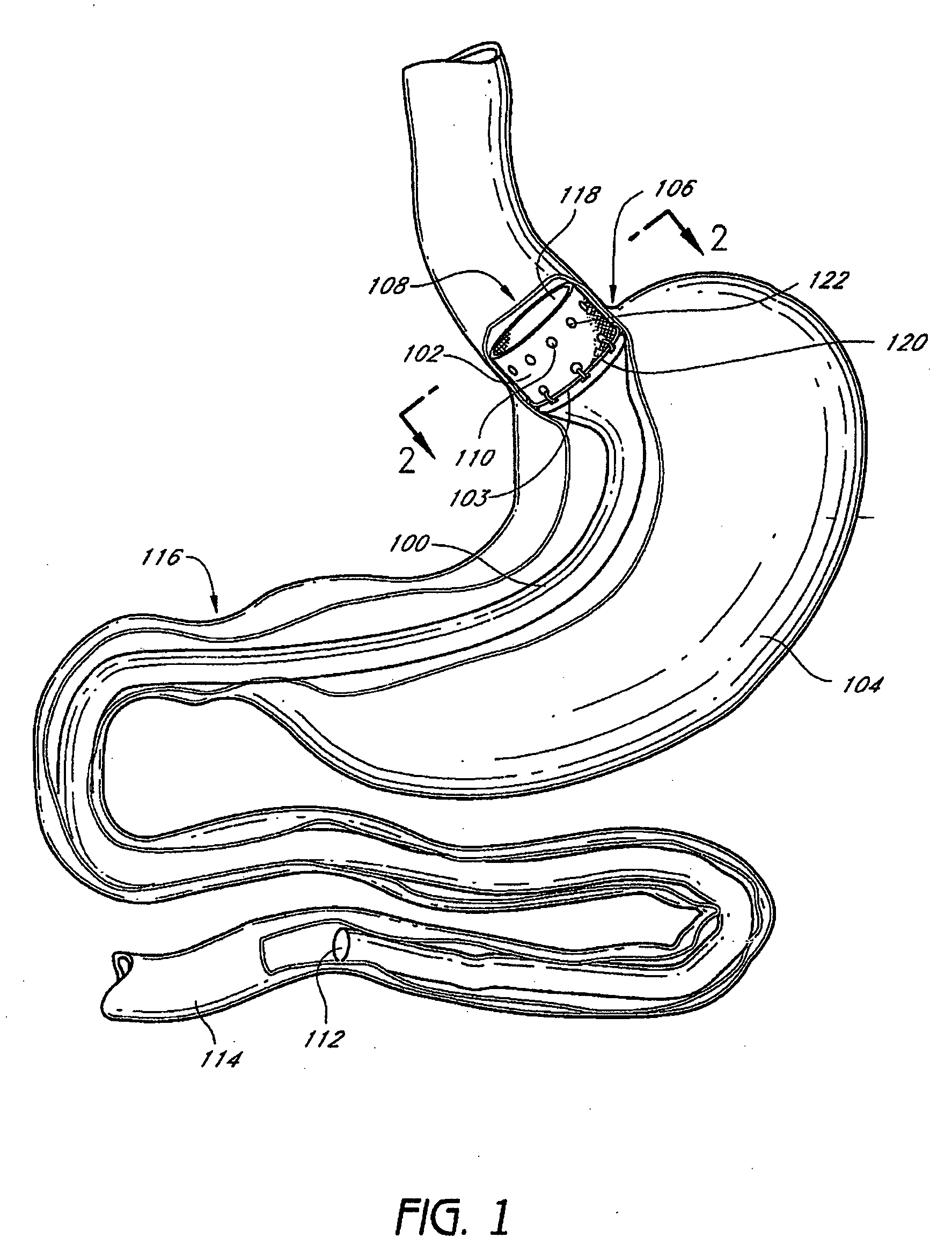
Gastrointestinal implant system
The present invention provides devices and methods for attachment of an endolumenal gastrointestinal device, such as an artificial stoma device, a gastrointestinal bypass sleeve or other therapeutic or diagnostic device, within a patient's digestive tract. In one application of the invention, an endolumenal bypass sleeve is removeably attached in the vicinity of the gastroesophageal junction to treat obesity and / or its comorbidities, such as diabetes. The bypass sleeve may be at least partially deployed by eversion.
Owner:DANN MITCHELL +4
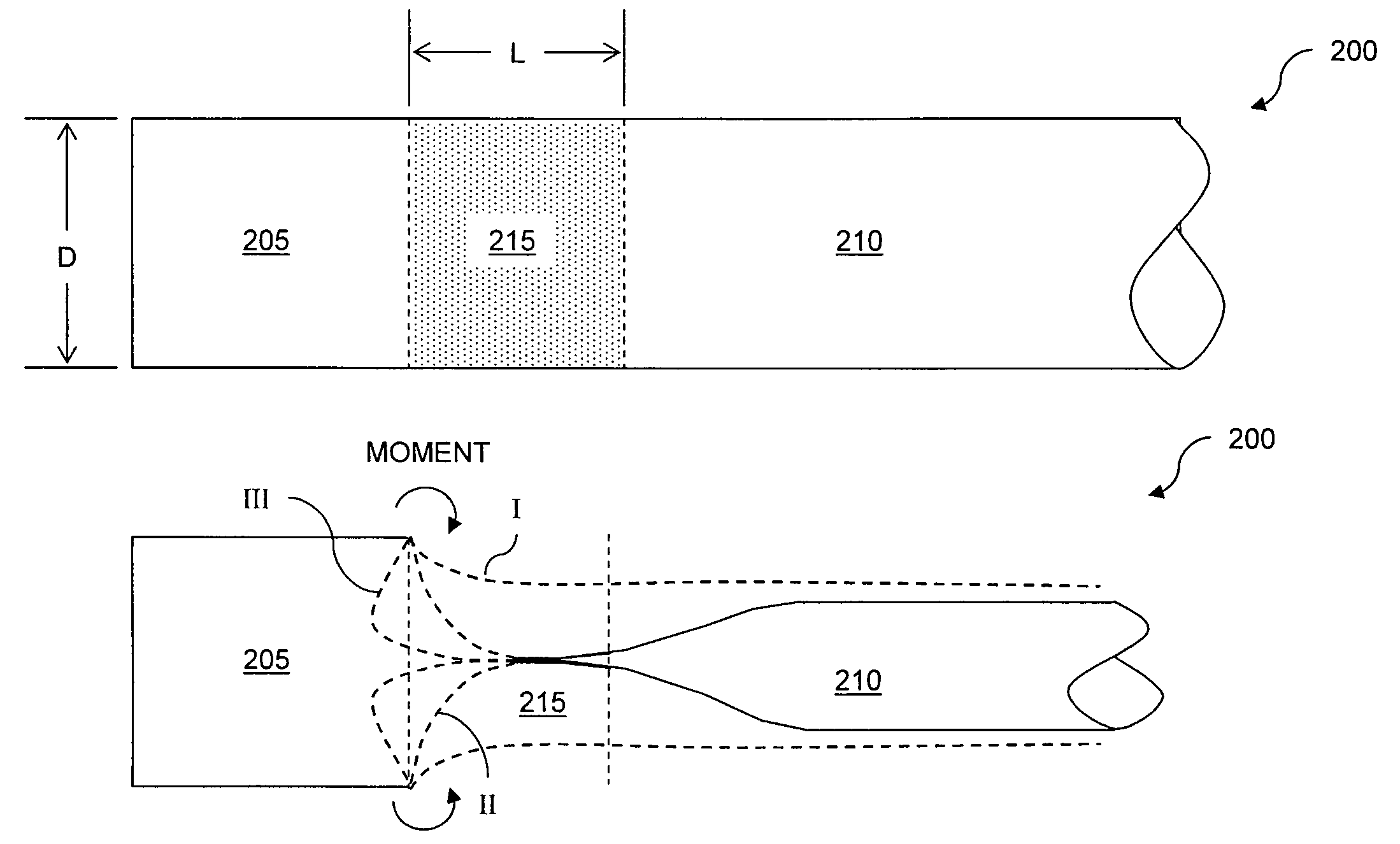
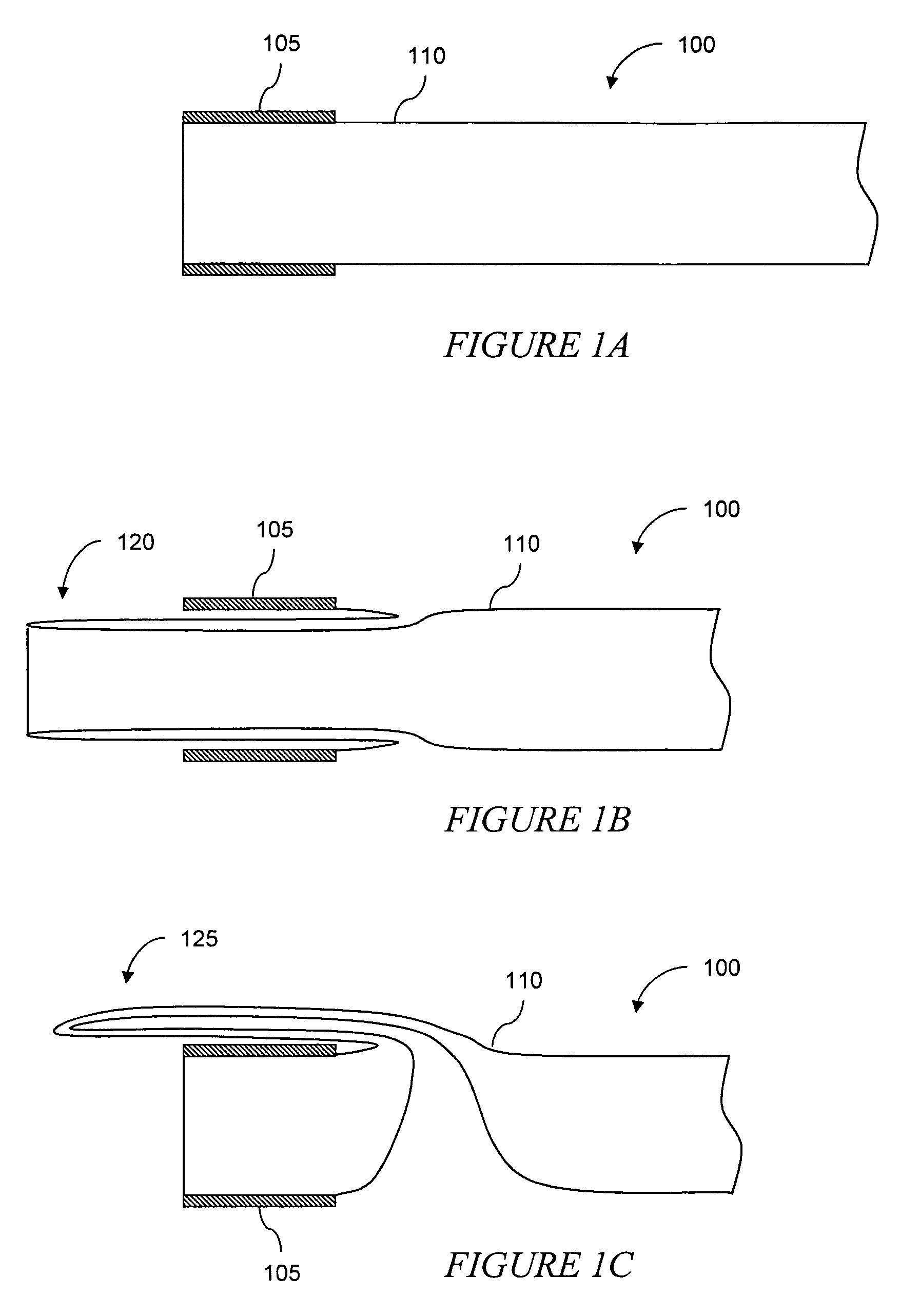
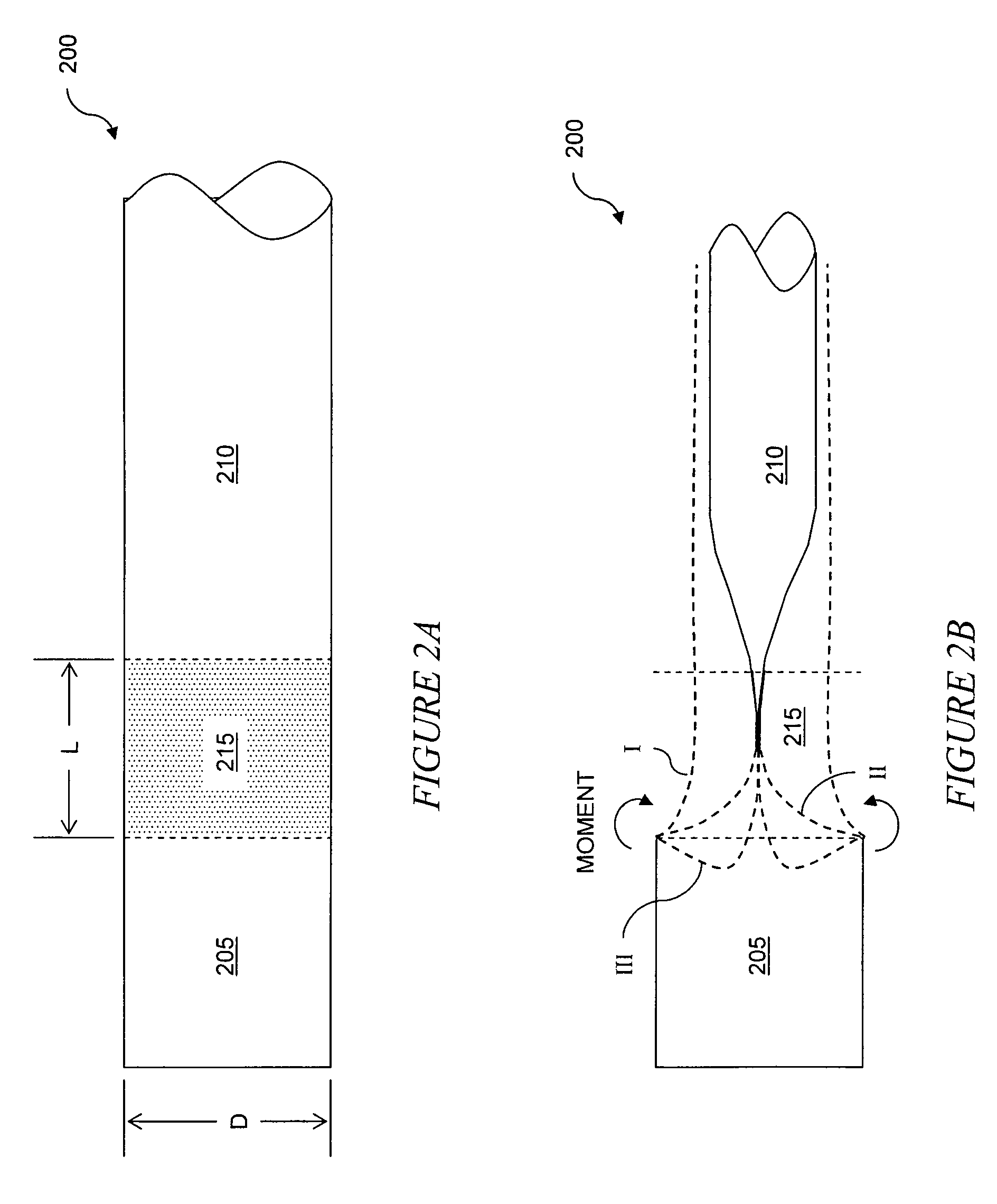
Eversion resistant sleeves
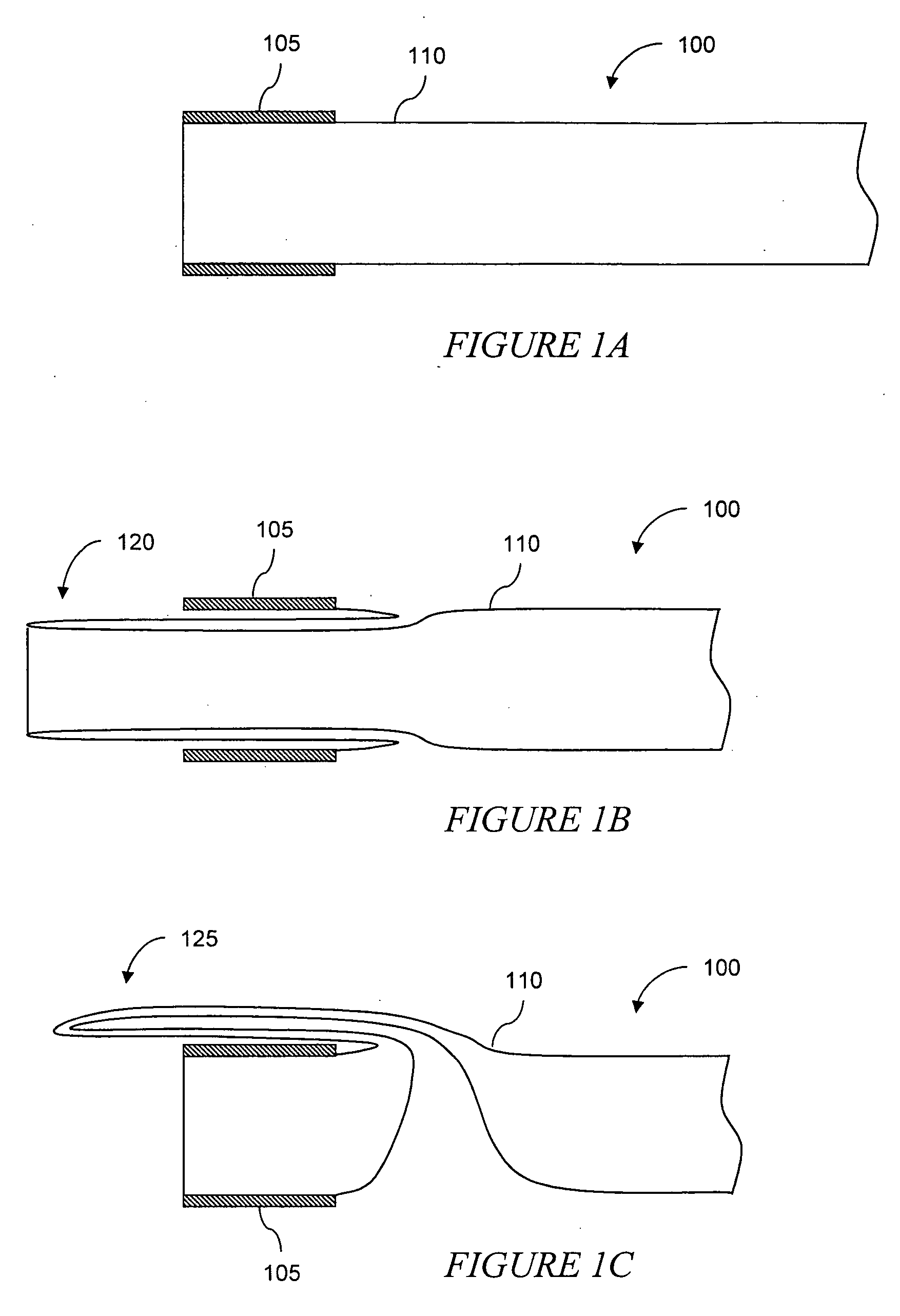
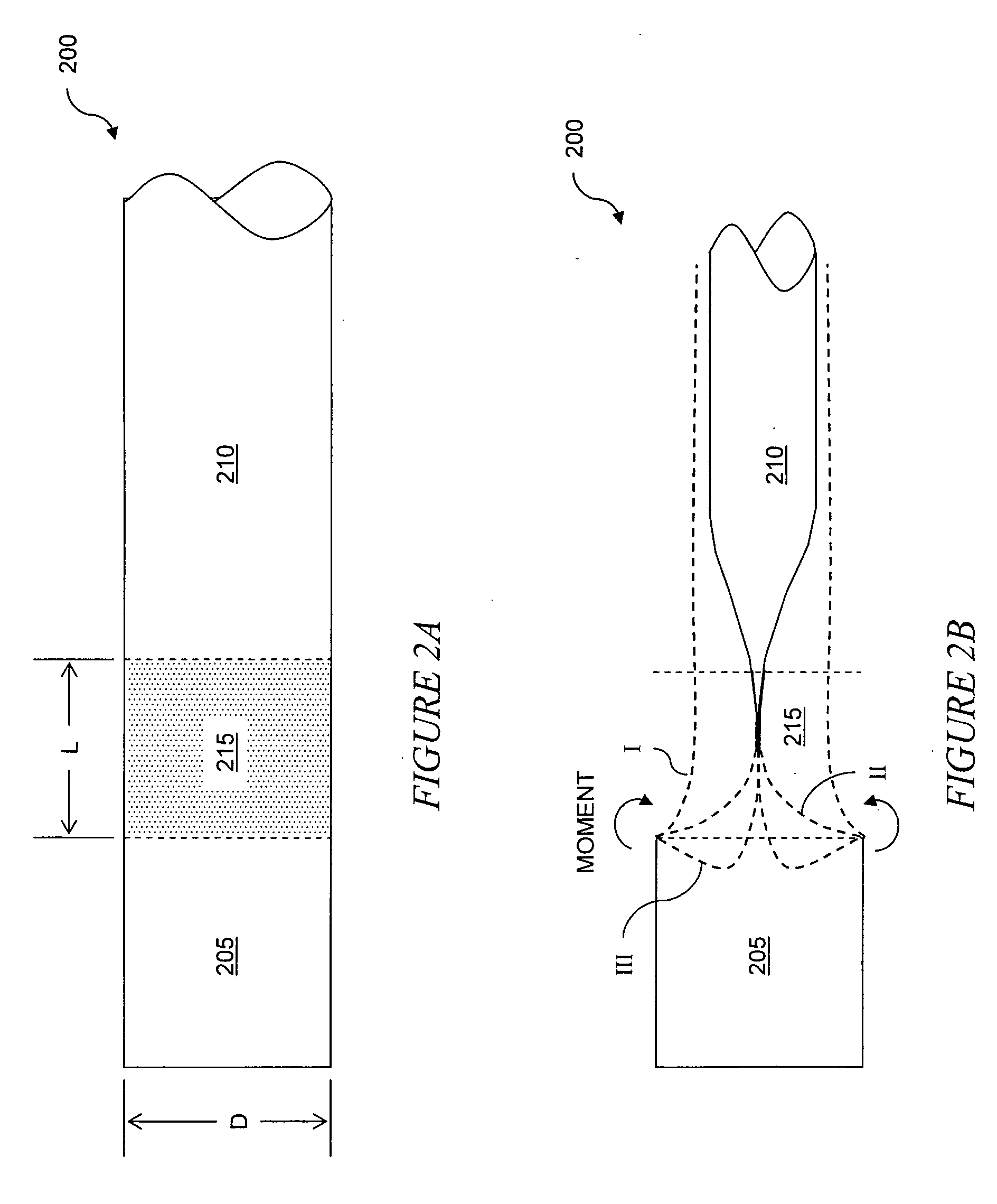
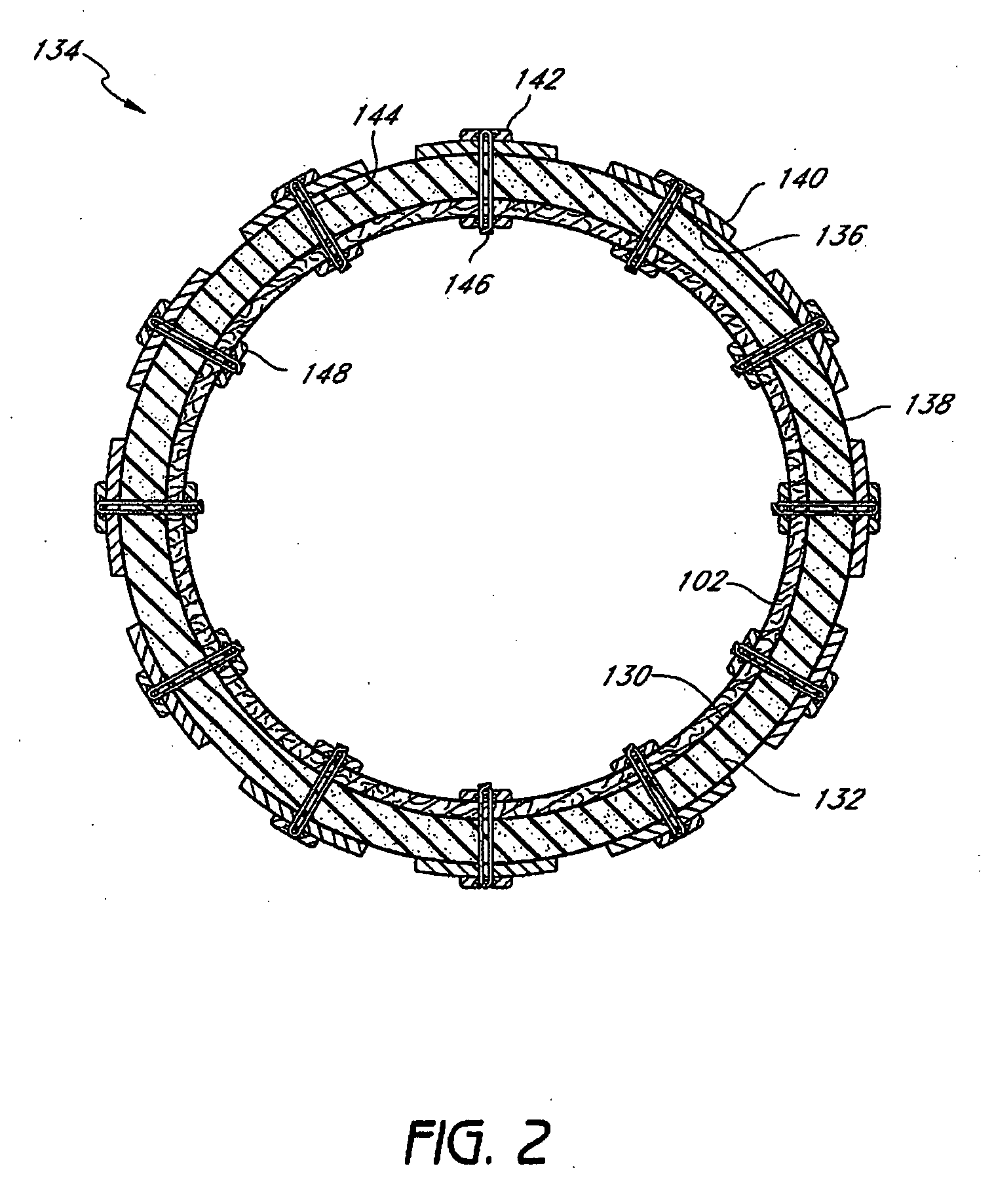
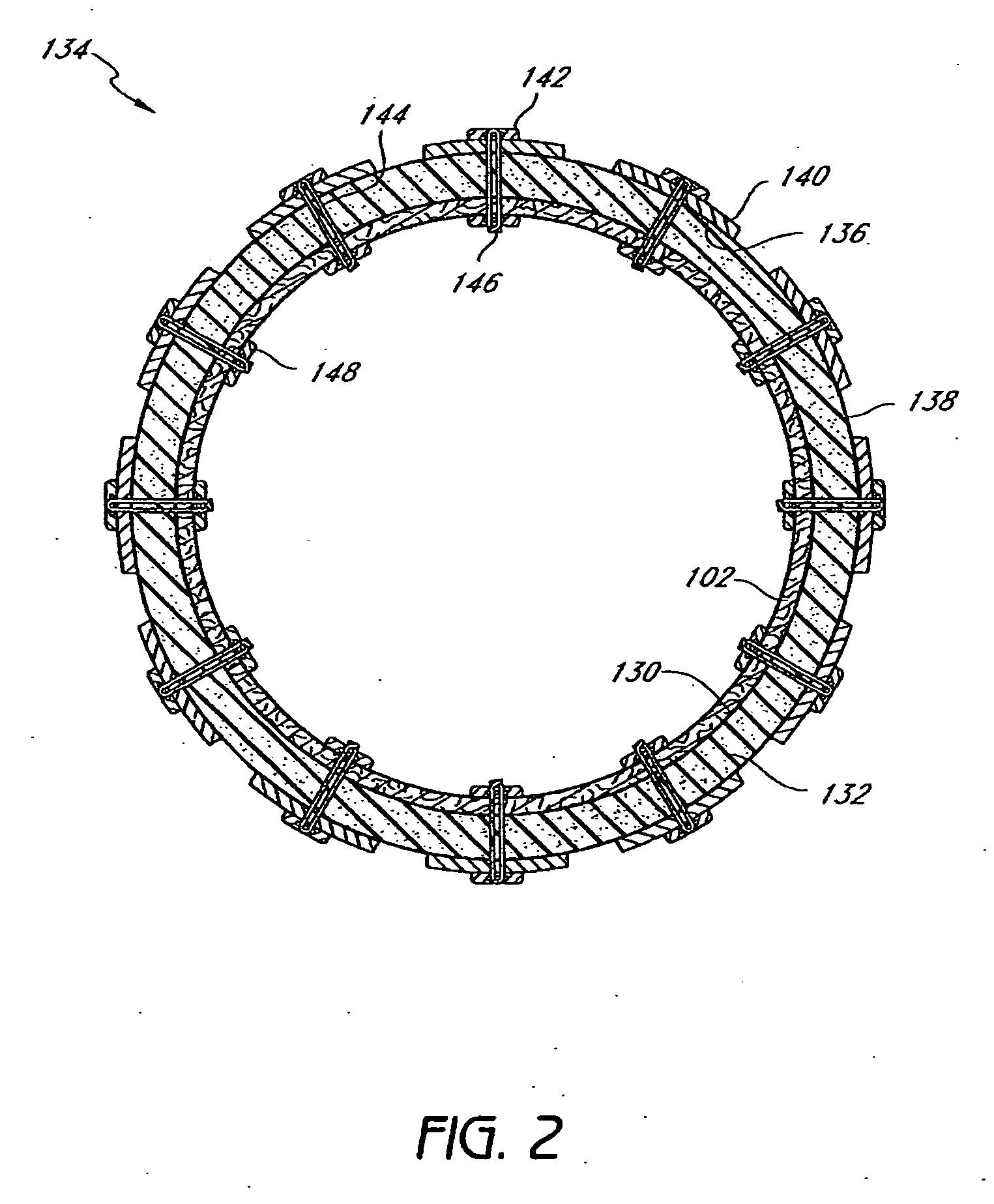
ActiveUS20060161187A1Reduce coefficient of frictionIncrease stiffnessOesophagiIntravenous devicesThin walledDigestive tract
The invention relates to improved means for preventing eversion and subsequent obstruction of thin-walled, floppy gastrointestinal liners implanted in the digestive tract of an animal. The implantable devices include an anchor adapted for attachment within a natural body lumen and a thin-walled, floppy sleeve open at both ends and defining a lumen therebetween. A substantial length of the sleeve has material characteristics that result in the sleeve being prone to eversion in the presence of retrograde pressures. Exemplary eversion-resistant features provide an increased stiffness and / or an increased friction coefficient between the anchor and the proximal end of the sleeve to resist eversion. In some embodiments, the eversion-resistant feature includes an anti-buckling element, such as a wire coupled along the substantial length of the sleeve.
Owner:GI DYNAMICS
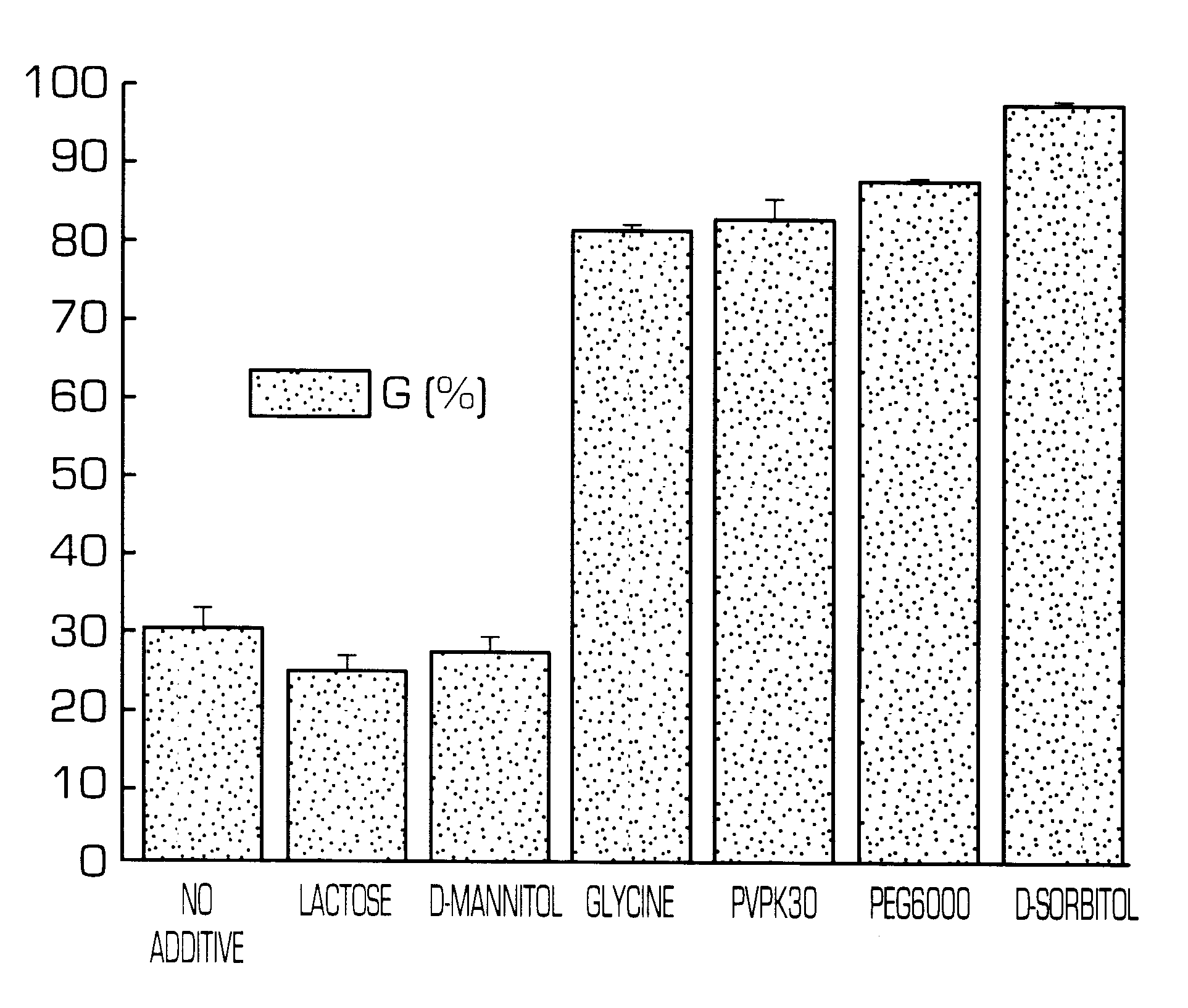
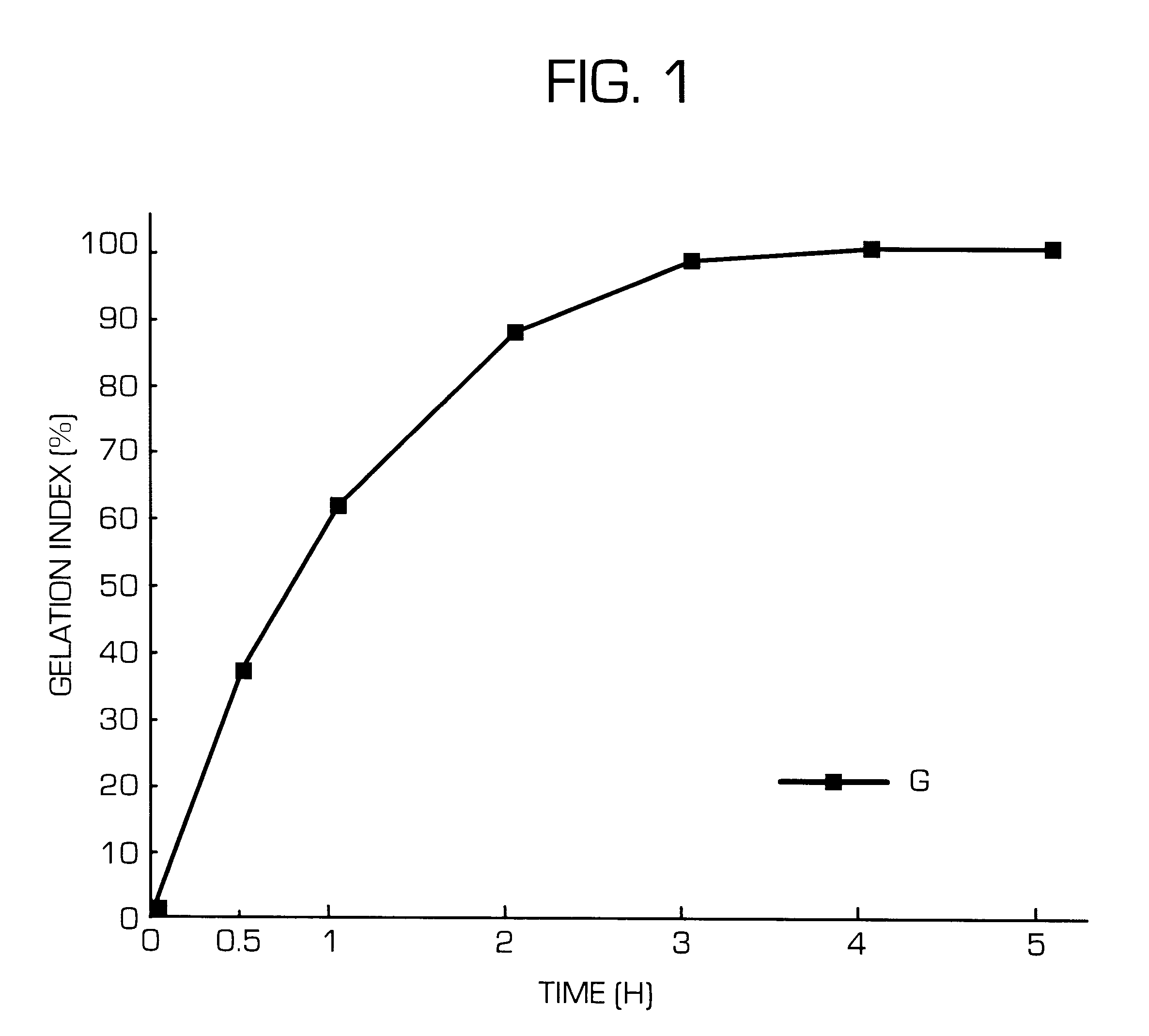
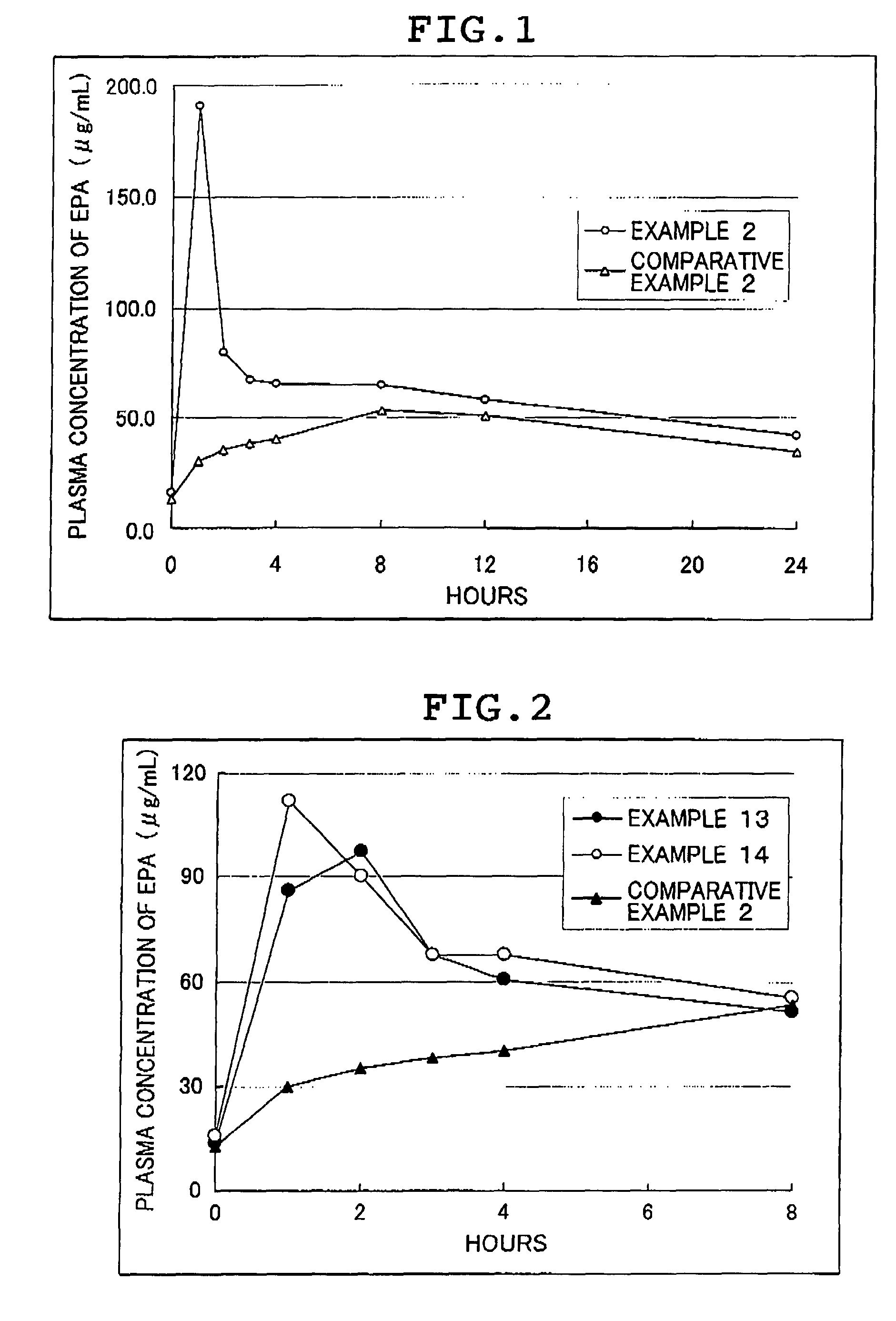
Hydrogel-forming sustained-release preparation
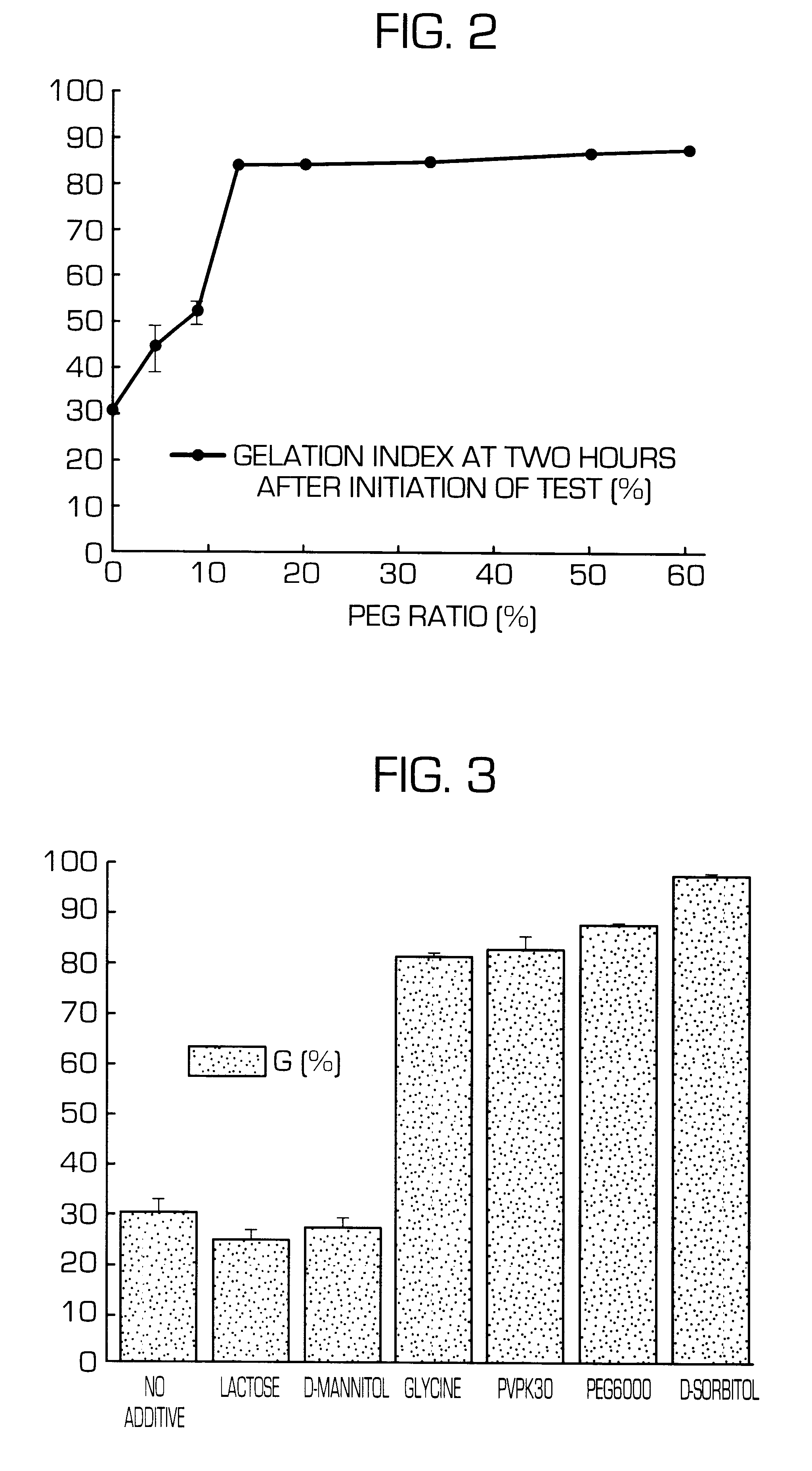
InactiveUS6436441B1Rapid drug releaseReduce the amount of solutionPowder deliveryPill deliverySmall intestinePolymer
The invention provides a hydrogel-type sustained-release preparation comprising (1) at least one drug, (2) an additive which insures a penetration of water into the core of the preparation and (3) a hydrogel-forming polymer, wherein said preparation is capable of undergoing substantially complete gelation during its stay in the upper digestive tract such as stomach and small intestine and is capable of releasing the drug in the lower digestive tract including colon.By the preparation of the invention, the drug is efficiently released and absorbed even in the colon so that a steady and sustained release effect can be achieved.
Owner:ASTELLAS PHARMA INC
Methods and devices for diagnostic and therapeutic interventions in the peritoneal cavity
A novel approach to diagnostic and therapeutic interventions in the peritoneal cavity is described. More specifically, a technique for accessing the peritoneal cavity via the wall of the digestive tract is provided so that examination of and / or a surgical procedure in the peritoneal cavity can be conducted via the wall of the digestive tract with the use of a flexible endoscope. As presently proposed, the technique is particularly adapted to transgastric peritoneoscopy. However, access in addition or in the alternative through the intestinal wall is contemplated and described as well. Transgastric and / or transintestinal peritoneoscopy will have an excellent cosmetic result as there are no incisions in the abdominal wall and no potential for visible post-surgical scars or hernias.
Owner:APOLLO ENDOSURGERY INC
Anti-buckling sleeve
The invention relates to improved means for preventing buckling and therefore eversion of thin-walled, flexible, floppy gastrointestinal liners implanted in the digestive tract of an animal. The implantable devices include an anchor adapted for attachment within a natural body lumen and a thin-walled, floppy sleeve open at both ends and defining a lumen therebetween. A substantial length of the sleeve has material characteristics that result in the sleeve being prone to buckling and therefore eversion in the presence of retrograde pressures. Exemplary anti-buckling mechanisms provide an increased stiffness and / or an increased friction coefficient between the anchor and the proximal end of the sleeve to resist buckling and therefore eversion. In some embodiments, the anti-buckling mechanism is as a wire coupled along the substantial length of the sleeve.
Owner:GI DYNAMICS
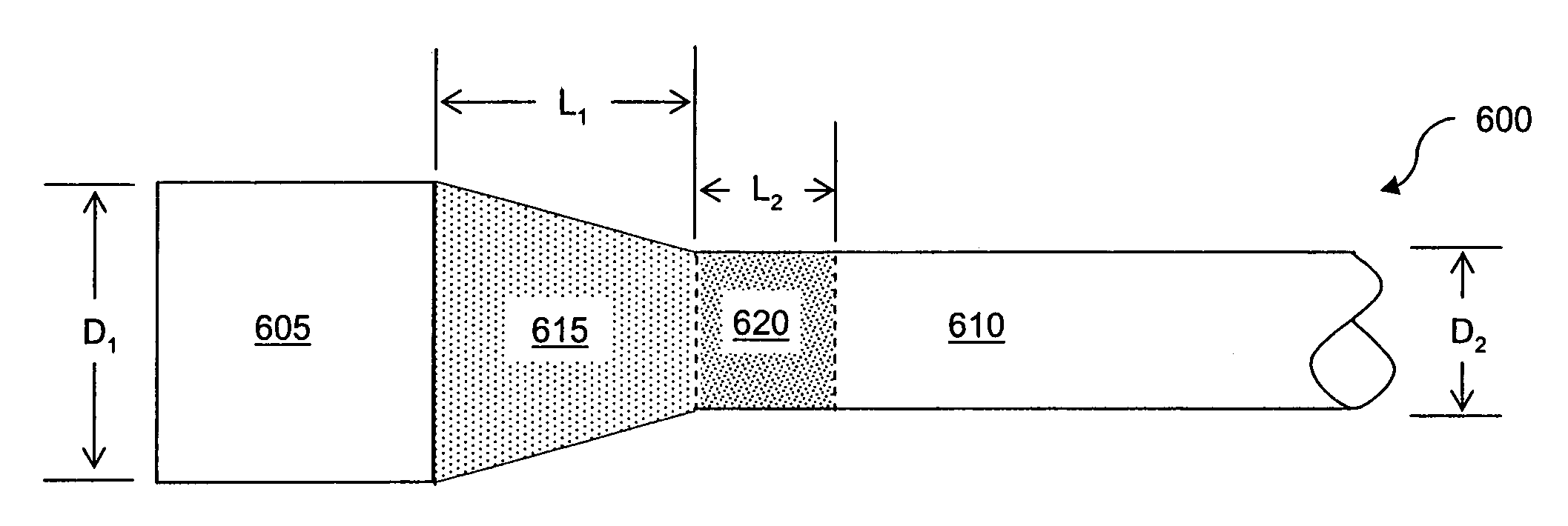
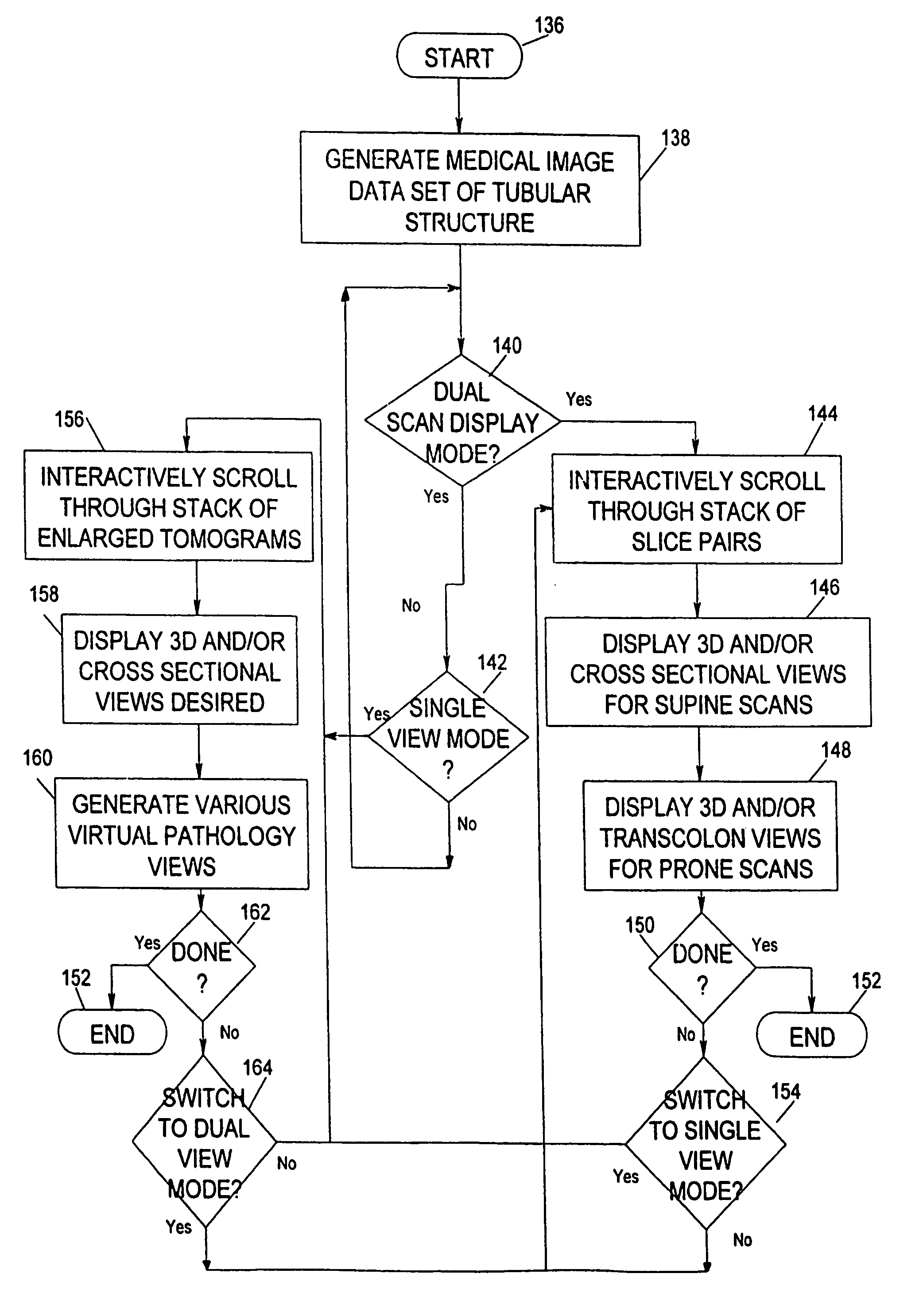
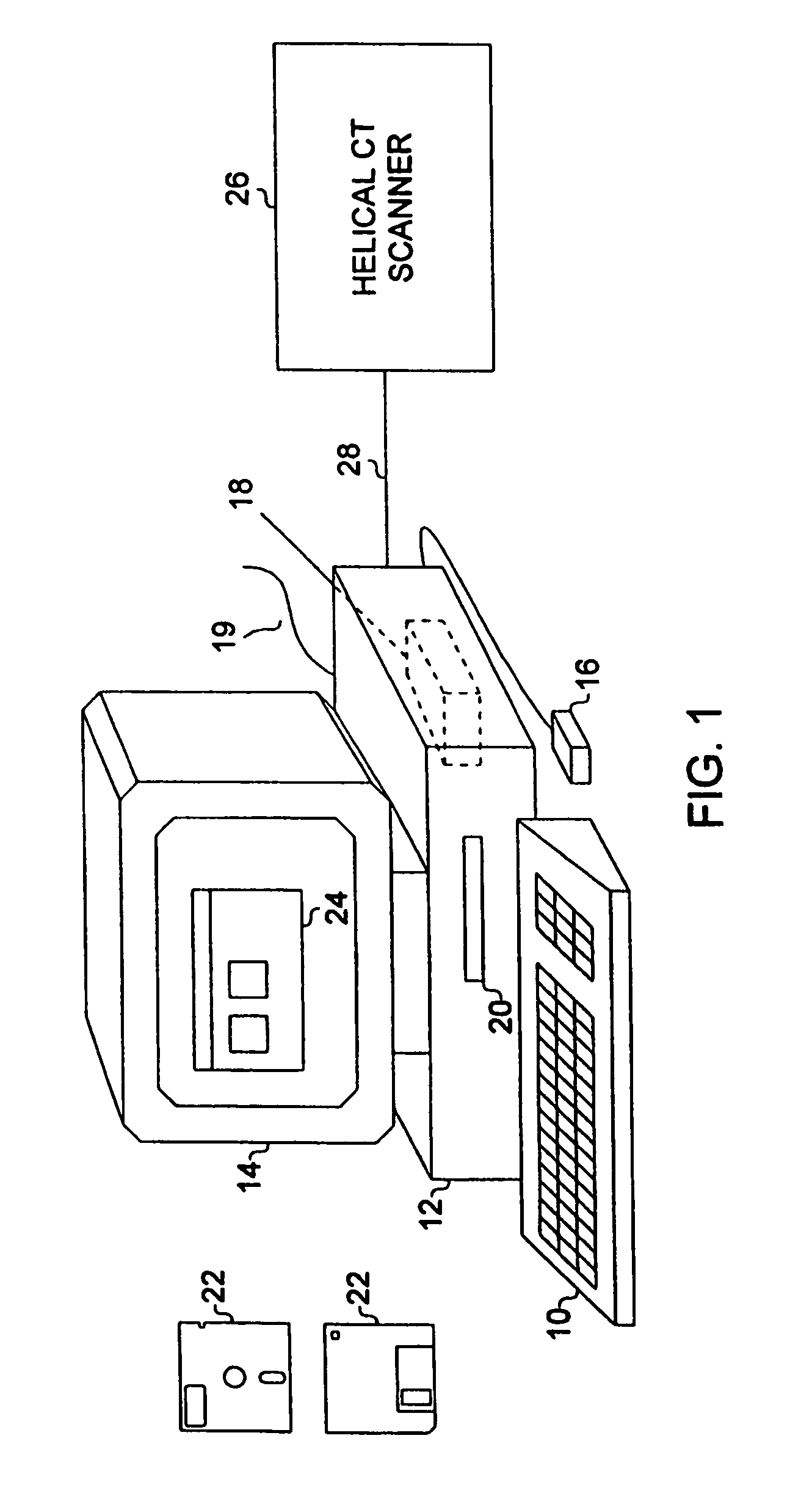
System for two-dimensional and three-dimensional imaging of tubular structures in the human body
InactiveUS6928314B1Interpretation time is not improvedStrong computing abilityImage enhancementImage analysisHuman bodyViewpoints
This invention is a system, method, and article of manufacture for imaging tubular structures of the human body, such as the digestive tract of a living person, with a medical imaging device such as a computed tomography (CT) scanner and a computer work station. The system comprises receiving a first image data set representative of a portion of the colon in a prone position and a second image data set representative of a portion of the colon in a supine position, at a series of viewpoints. At each of the viewpoints, an image is generated of the colon in the prone and supine positions. The prone and supine images of the colon are simultaneously displayed on a screen display in a dual view mode.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
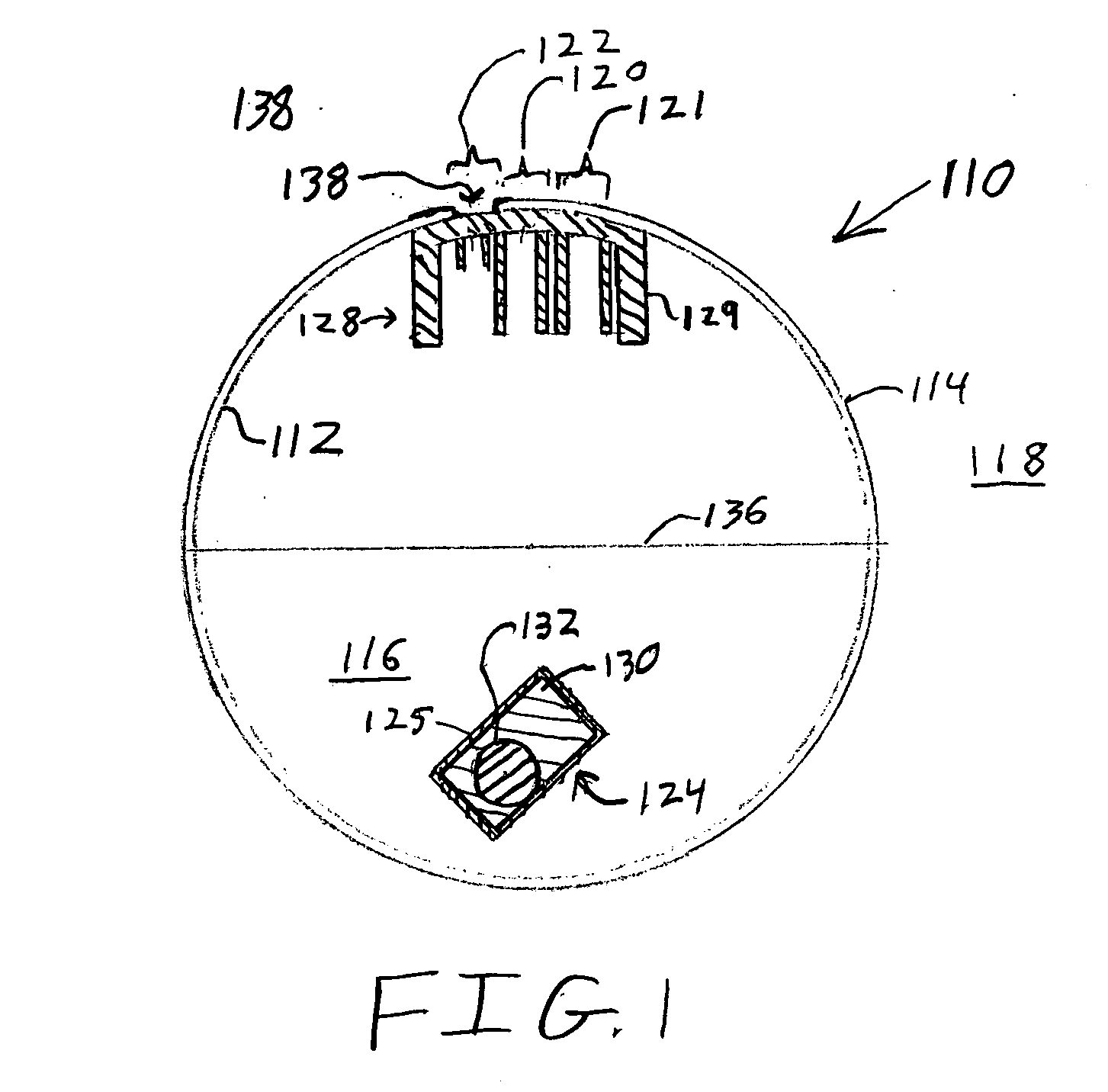
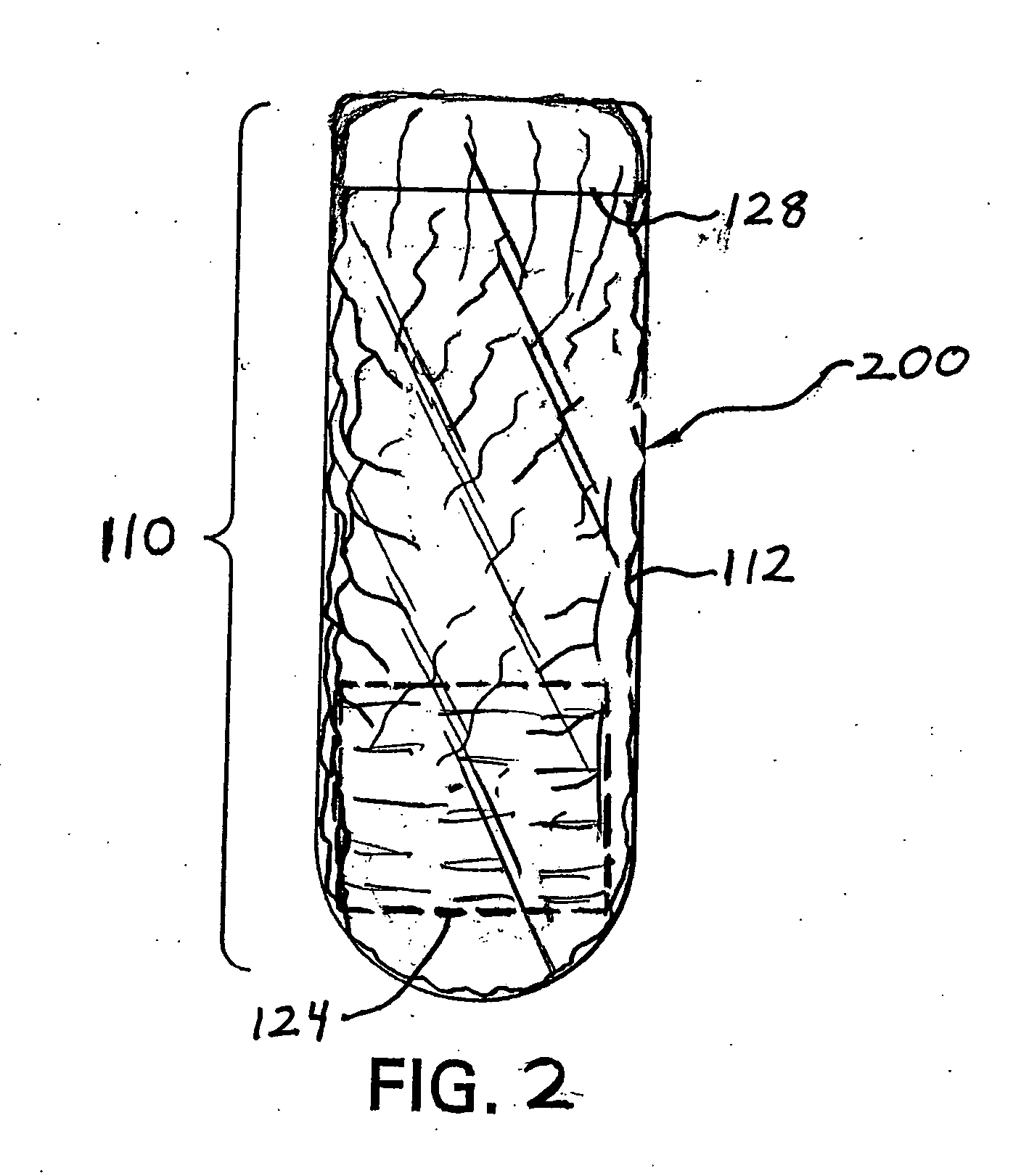
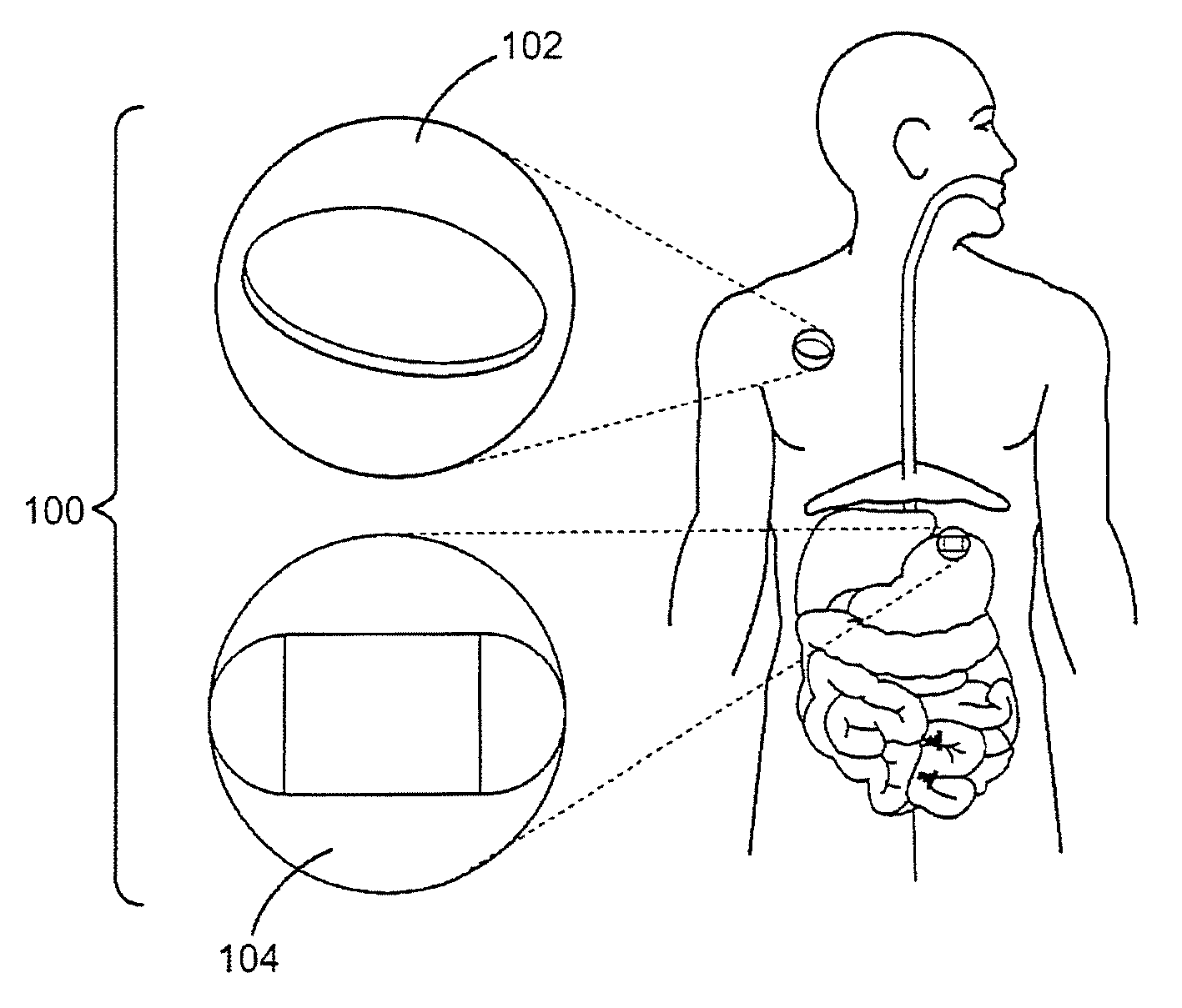
Ingestible event marker systems
ActiveUS20100185055A1Rapid and simple notationPrimary cell manufactureSurgeryComputer scienceDigestive tract
Ingestible event marker systems that include an ingestible event marker (i.e., an IEM) and a personal signal receiver are provided. Embodiments of the IEM include an identifier, which may or may not be present in a physiologically acceptable carrier. The identifier is characterized by being activated upon contact with a target internal physiological site of a body, such as digestive tract internal target site. The personal signal receiver is configured to be associated with a physiological location, e.g., inside of or on the body, and to receive a signal the IEM. During use, the IEM broadcasts a signal which is received by the personal signal receiver.
Owner:PROTEUS DIGITAL HEALTH INC
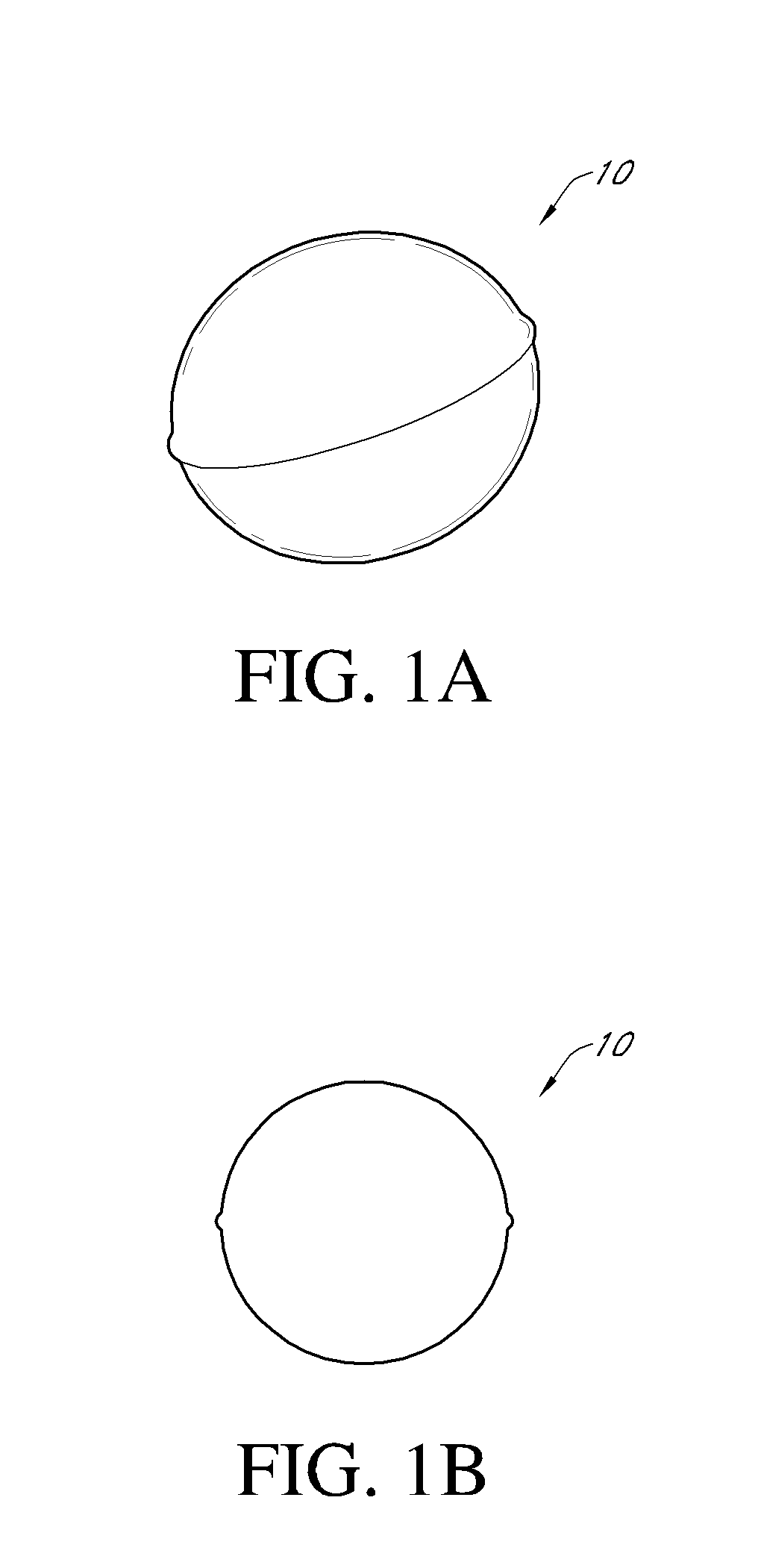
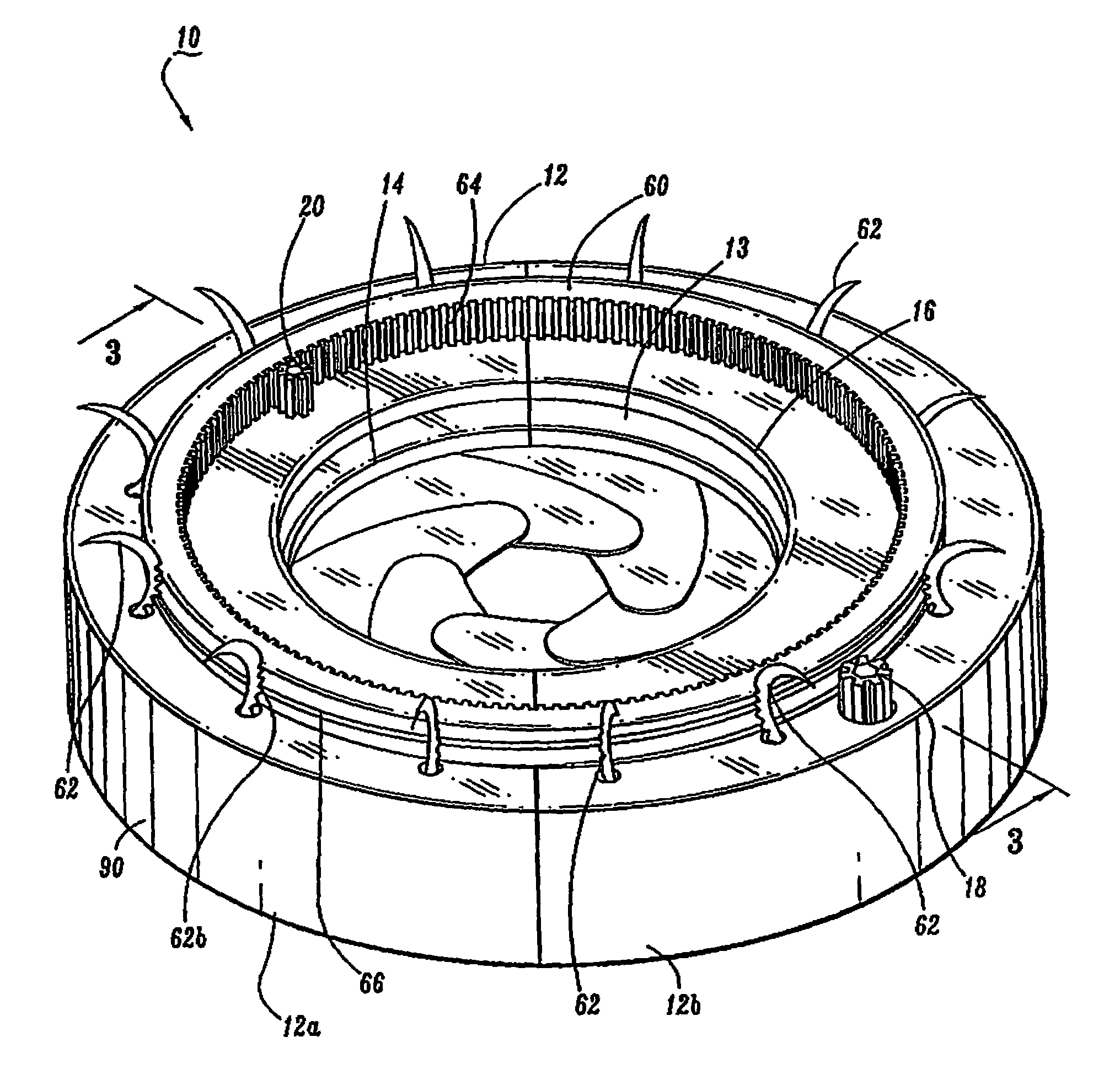
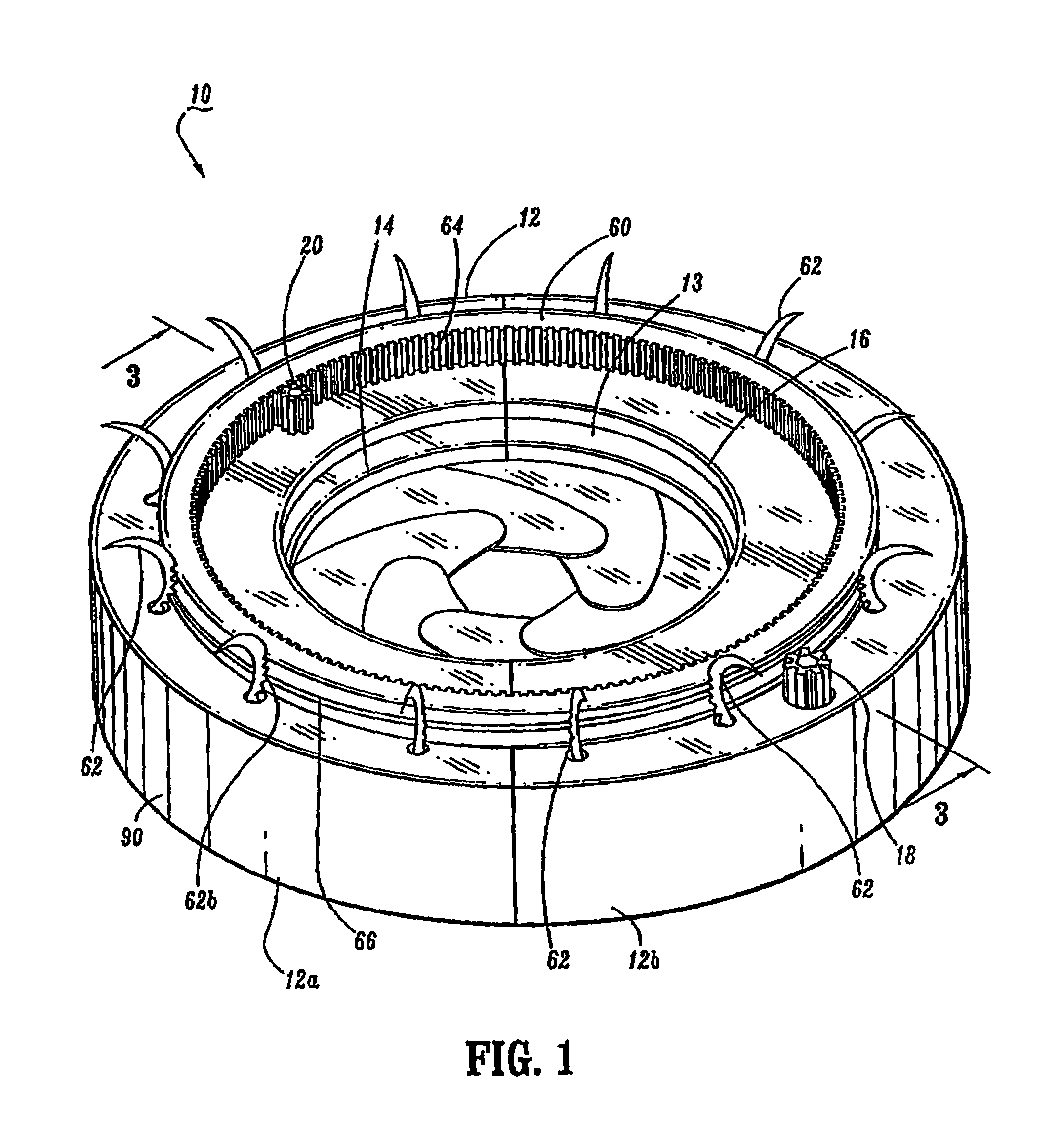
Intragastric device
An implant configured for ingestion by a patient. After the implant has been swallowed by the patient and is disposed within the target location, e.g. the patient's stomach, an inflation subcomponent causes the implant to expand from a compact delivery state to an expanded, volume-occupying, deployed state. In the deployed state the implant creates a sensation of satiety in the patient stomach and thereby aids in limiting food intake and obesity. After a predetermined time a deflation subcomponent is actuated and the implant reduces in size so as to allow it to pass through the remainder of the patient's digestive track. The device may further incorporate tracking and visualization subcomponents, as well as pharmaceutical delivery subcomponents.
Owner:RESHAPE LIFESCIENCES INC
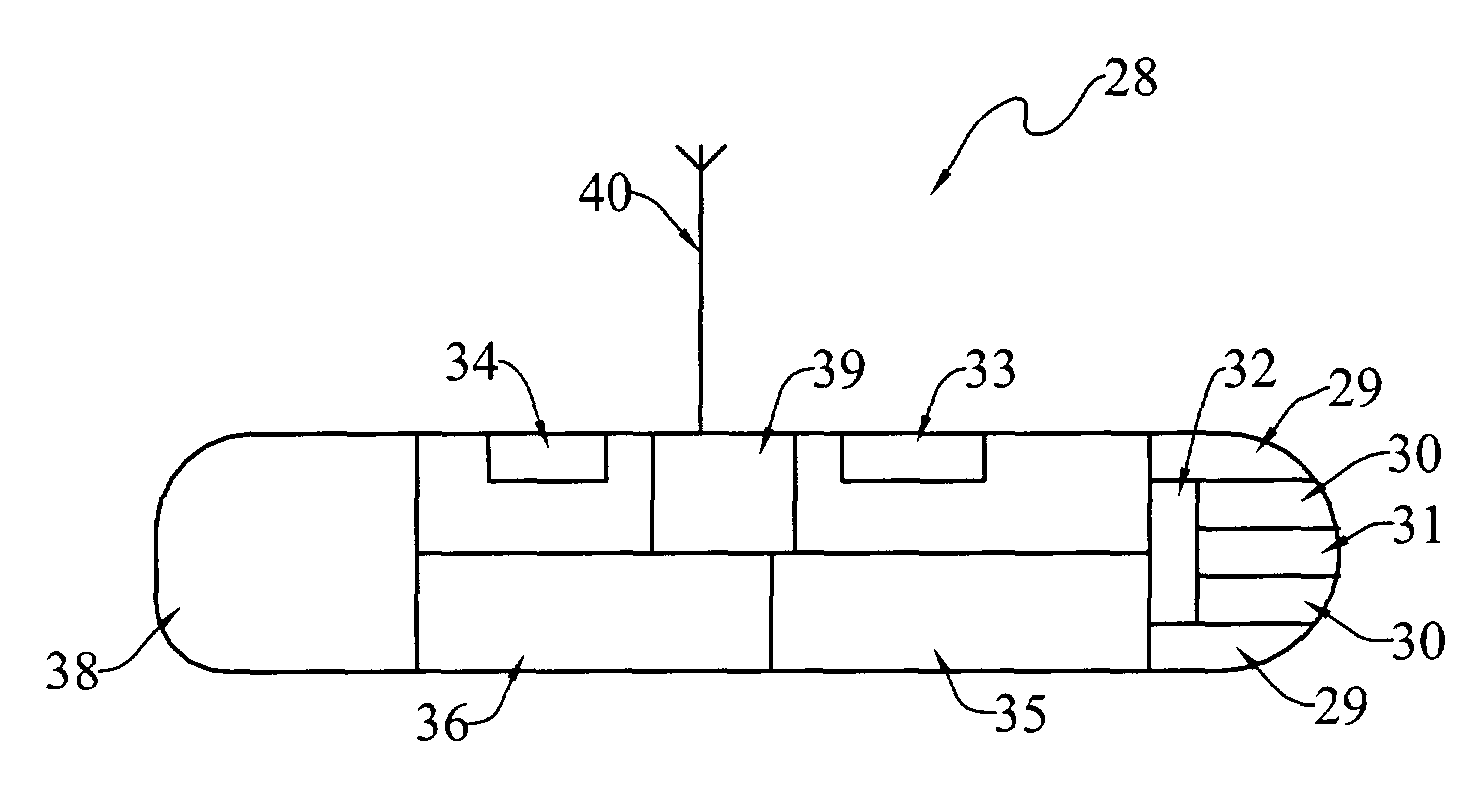
Method of using, and determining location of, an ingestible capsule
The present invention broadly provides an improved ingestible capsule (28) that is arranged to sense one or more physiological parameters within a mammalian body, an to transmit such parameters to an extra-corporeal receiver (50). In use, the capsule and receiver perform the method of determining the real-time location of the capsule within a tract of a mammal. This method includes the steps of providing the capsule, the capsule having one or more sensors, ingesting the capsule, transmitting a signal from the capsule, receiving the transmitted signal, and determining the real-time location of the capsule within the tract as a function of the received signal. The received signal may also indicate the value of one or more sensed parameters.
Owner:GIVEN IMAGING INC
Intragastric device
An implant configured for ingestion by a patient. After the implant has been swallowed by the patient and is disposed within the target location, e.g. the patient's stomach, an inflation subcomponent causes the implant to expand from a compact delivery state to an expanded, volume-occupying, deployed state. In the deployed state the implant creates a sensation of satiety in the patient stomach and thereby aids in limiting food intake and obesity. After a predetermined time a deflation subcomponent is actuated and the implant reduces in size so as to allow it to pass through the remainder of the patient's digestive track. The device may further incorporate tracking and visualization subcomponents, as well as pharmaceutical delivery subcomponents.
Owner:RESHAPE LIFESCIENCES INC
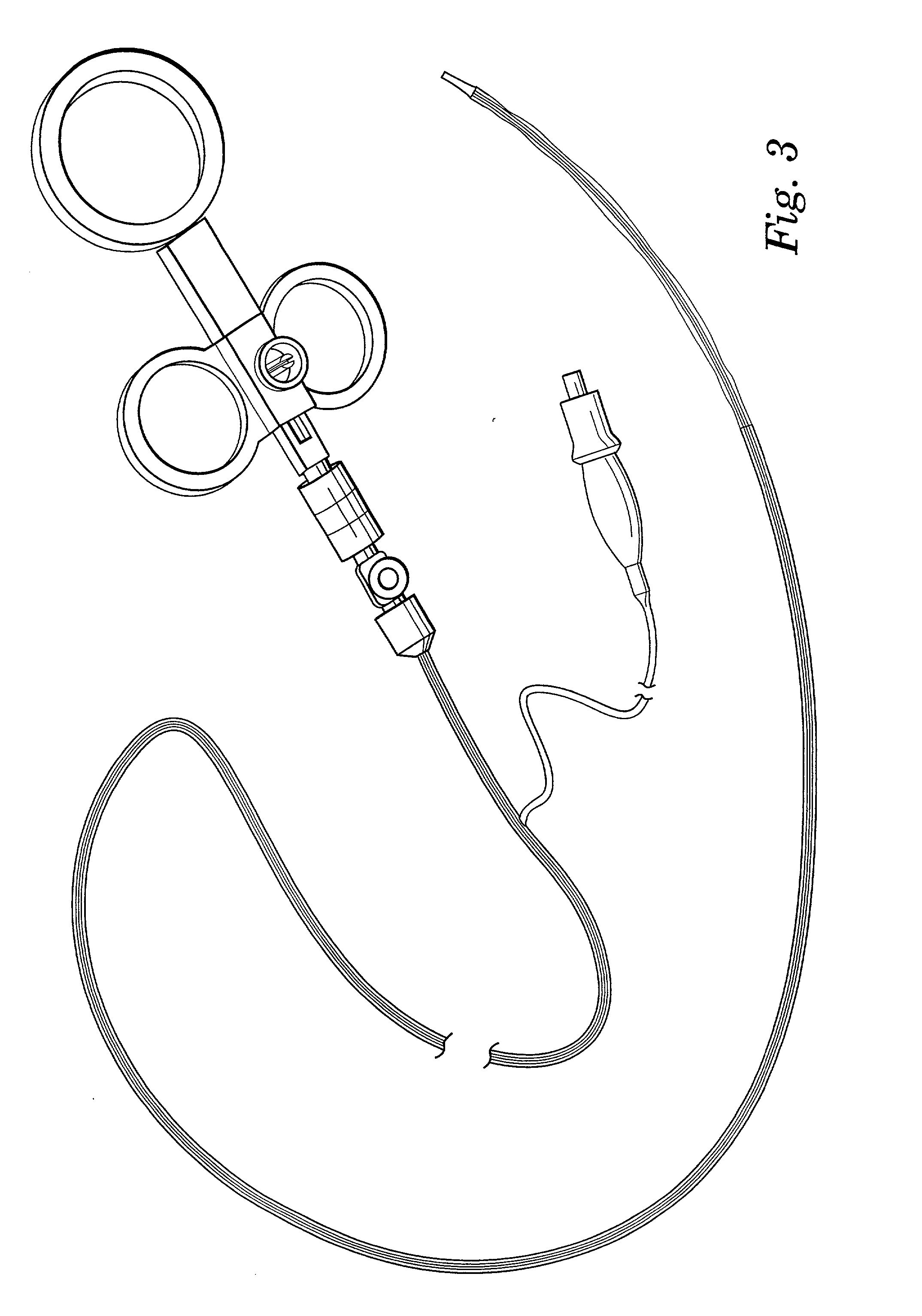
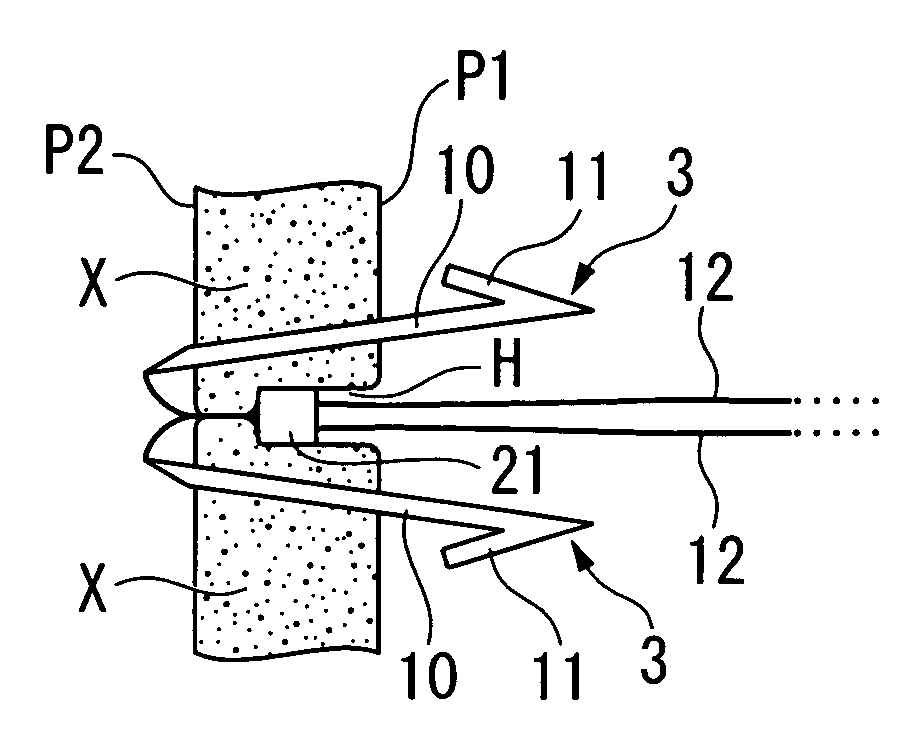
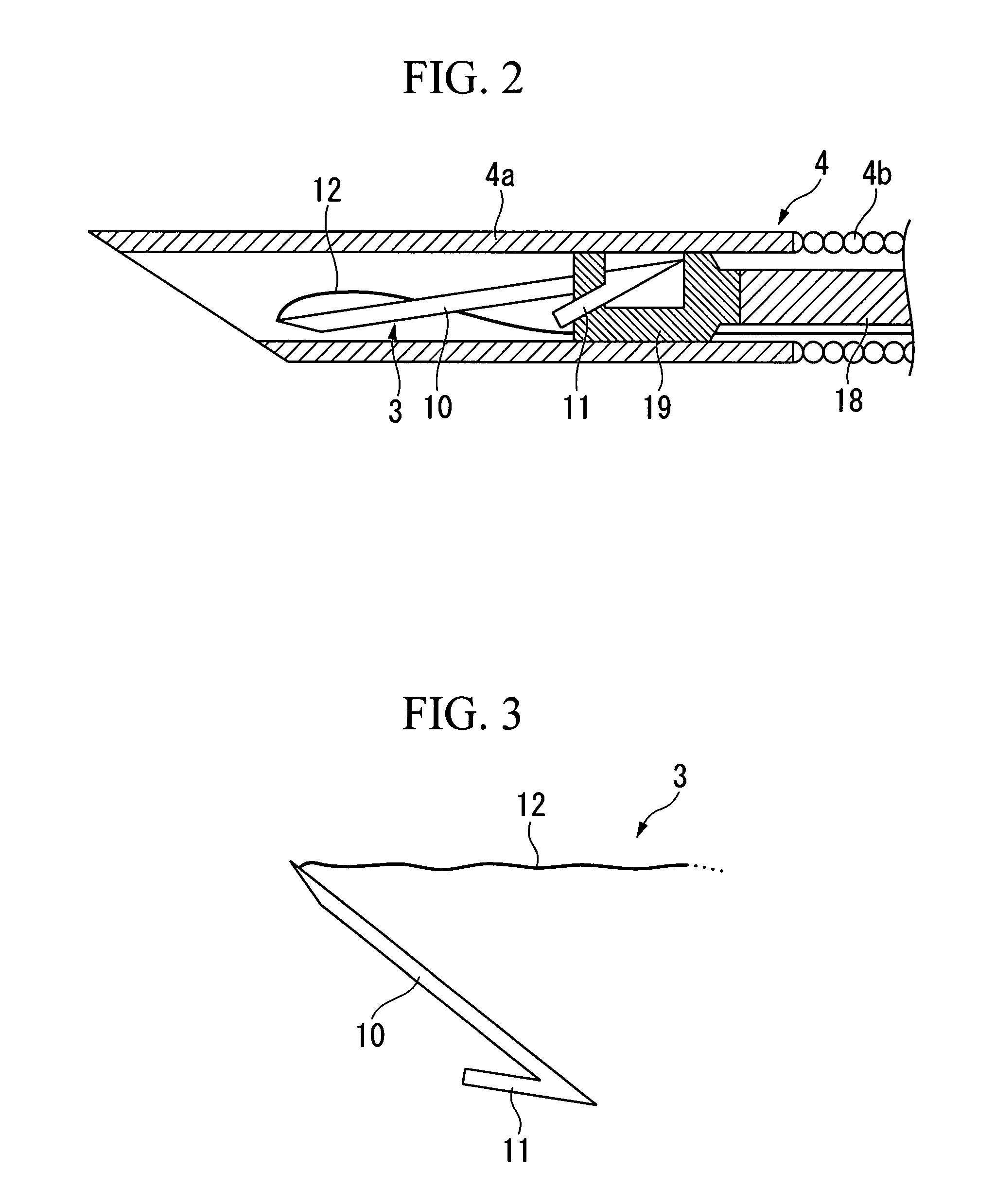
Perforation suture clip and clip device
InactiveUS20080114378A1Improve performanceSuture equipmentsSurgical needlesEndoscopeMaterial Perforation
A perforation suture clip is used in combination with an endoscope and sutures a perforation occurring in a digestive tract, and includes: a rod portion whose two ends are sharpened; a hook portion that is bent back from one end side of the rod portion at a predetermined opening angle towards the other end side of the rod portion; and a traction portion that is fixed to the other end side of the rod portion and drags the other end side of the rod portion.
Owner:OLYMPUS MEDICAL SYST CORP
Dosage forms of bisphosphonates
ActiveUS20050260262A1Effective absorptionReduce interactionBiocideMetabolism disorderBisphosphonate therapyUpper gastrointestinal
Oral dosage forms of a bisphosphonate comprised of a safe and effective amount of a pharmaceutical composition comprising a bisphosphonate, a chelating agent, and, means for effecting delayed release of the bisphosphonate and the chelating agent in the lower gastrointestinal tract provide delivery of the pharmaceutical composition to the lower gastrointestinal tract of the mammal subject and pharmaceutically effective absorption of the bisphosphonate with or without food or beverages. The present invention substantially alleviates the interaction between bisphosphonates and food or beverages, which interaction results in the bisphosphonate active ingredient not being available for absorption. The resulting oral dosage form may thus be taken with or without food. Further, the present invention effects delivery of the bisphosphonate and the chelating agent to the lower GI tract, substantially alleviating the upper GI irritation associated with bisphosphonate therapies. These benefits simplify previously complex treatment regimens and can lead to increased patient compliance with bisphosphonate therapies.
Owner:APTALIS PHARMA
Gastric restrictor assembly and method of use
Owner:TYCO HEALTHCARE GRP LP
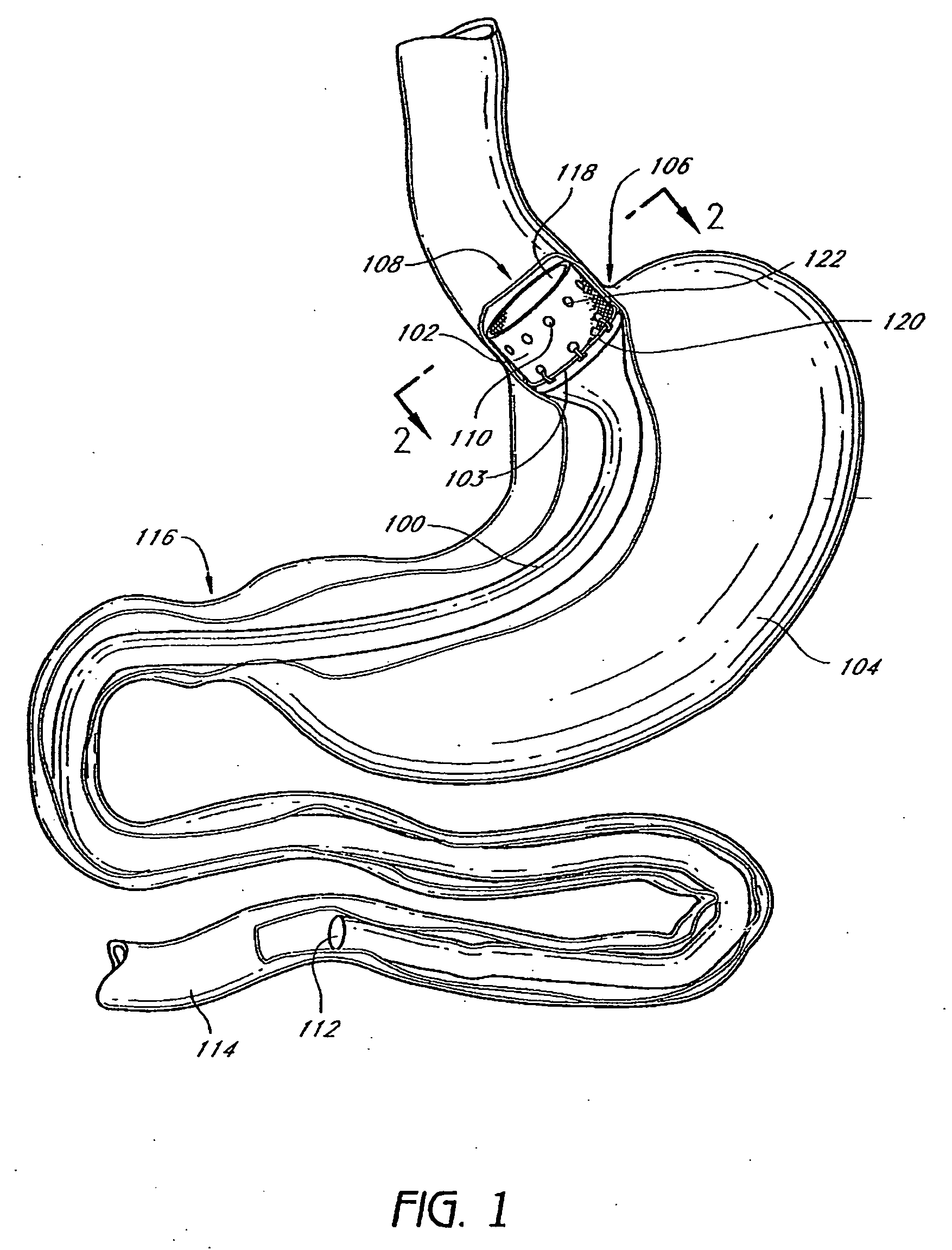
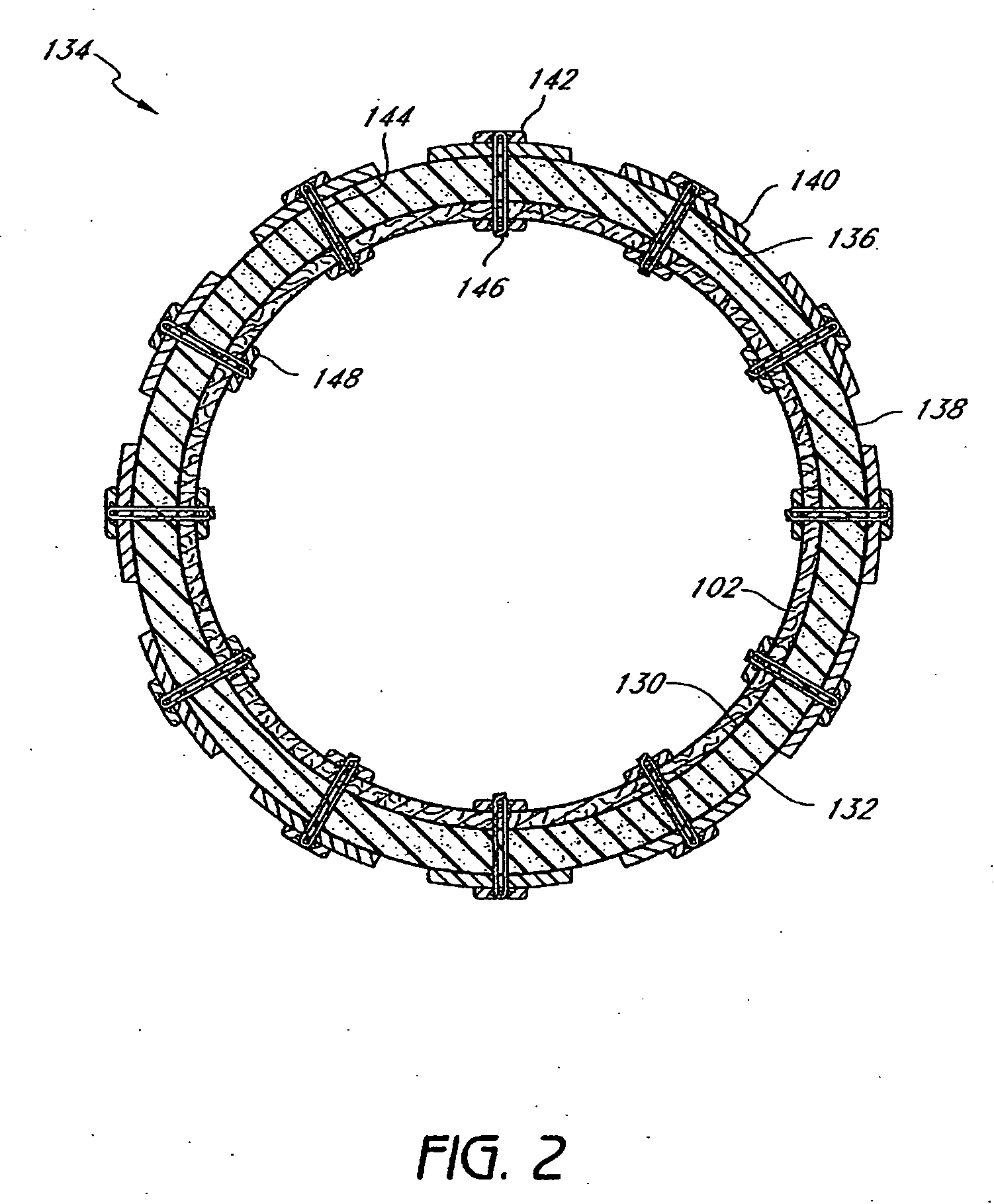
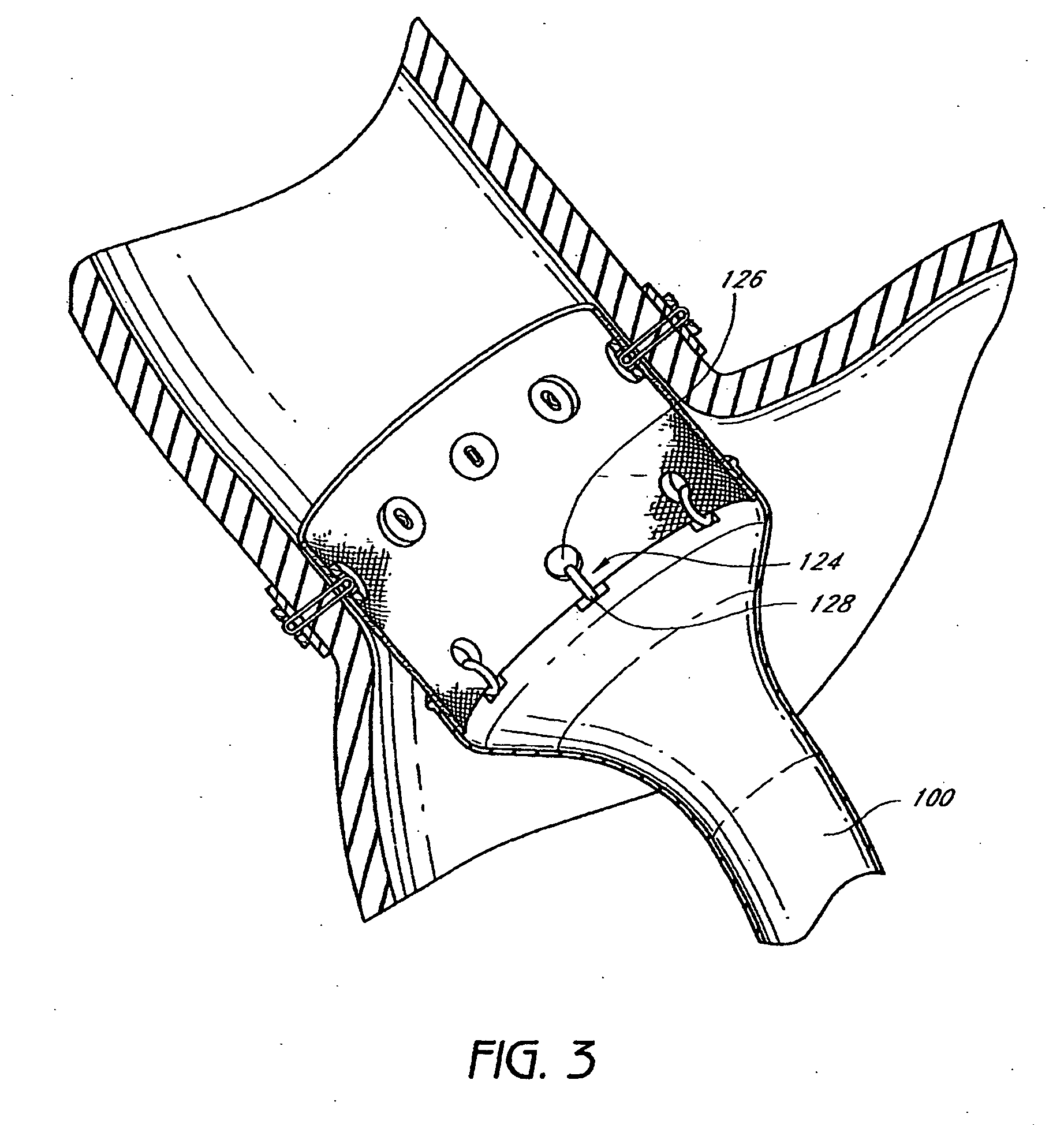
Everting gastrointestinal sleeve
The present invention provides devices and methods for attachment of an endolumenal gastrointestinal device, such as an artificial stoma device, a gastrointestinal bypass sleeve or other therapeutic or diagnostic device, within a patient's digestive tract. In one application of the invention, an endolumenal bypass sleeve is removeably attached in the vicinity of the gastroesophageal junction to treat obesity and / or its comorbidities, such as diabetes. The bypass sleeve may be at least partially deployed by eversion.
Owner:DANN MITCHELL +4
Methods and devices for diagnostic and therapeutic interventions in the peritoneal cavity
A novel approach to diagnostic and therapeutic interventions in the peritoneal cavity is described. More specifically, a technique for accessing the peritoneal cavity via the wall of the digestive tract is provided so that examination of and / or a surgical procedure in the peritoneal cavity can be conducted via the wall of the digestive tract with the use of a flexible endoscope. As presently proposed, the technique is particularly adapted to transgastric peritoneoscopy. However, access in addition or in the alternative through the intestinal wall is contemplated and described as well. Transgastric and / or transintestinal peritoneoscopy will have an excellent cosmetic result as there are no incisions in the abdominal wall and no potential for visible post-surgical scars or hernias.
Owner:APOLLO ENDOSURGERY INC
Attachment cuff for gastrointestinal implant
The present invention provides devices and methods for attachment of an endolumenal gastrointestinal device, such as an artificial stoma device, a gastrointestinal bypass sleeve or other therapeutic or diagnostic device, within a patient's digestive tract. In one application of the invention, an endolumenal bypass sleeve is removeably attached in the vicinity of the gastroesophageal junction to treat obesity and / or its comorbidities, such as diabetes. The bypass sleeve may be at least partially deployed by eversion.
Owner:VALENTX
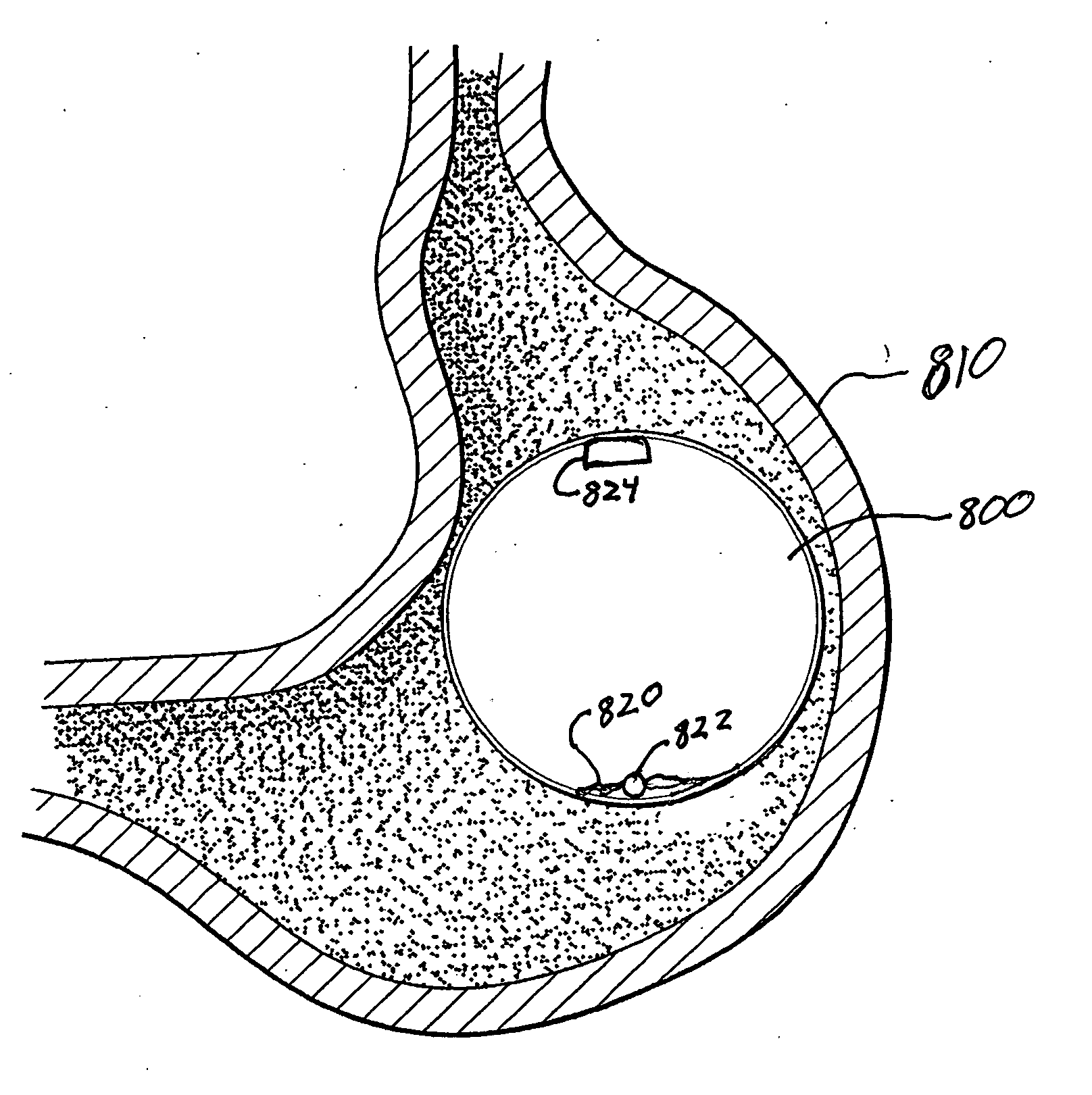
Method and Apparatus for Gastrointestinal Tract Ablation to Achieve Loss of Persistent and/or Recurrent Excess Body Weight Following a Weight-Loss Operation
ActiveUS20090012512A1Reduce complianceSmall sizeSurgical instruments for heatingSurgical instruments for coolingStomaGastrectomy
Devices and methods are provided for ablational treatment of regions of the digestive tract in post-bariatric surgery patients who fail to achieve or maintain the desired weight loss. Bariatric procedures include Roux-en-Y gastric bypass, biliopancreatic diversion, and sleeve gastrectomy. These procedures reconstruct gastrointestinal tract features, creating pouches, stoma, and tubes that restrict and / or divert the digestive flow. Post-surgical dilation of altered structures is common and diminishes their bariatric effectiveness. Ablation of compromised structures can reduce their size and compliance, restoring bariatric effectiveness. Ablation, as provided the invention, starts at the mucosa and penetrates deeper into the gastrointestinal wall in a controlled manner. Control may also be provided by a fractional ablation that ablates some tissue within a target region and leaves a portion substantially unaffected. Embodiments of the device include an ablational electrode array that spans 360 degrees and an array that spans an arc of less than 360 degrees.
Owner:TYCO HEALTHCARE GRP LP
Methods and Systems for Performing Submucosal Medical Procedures
InactiveUS20100217151A1Avoid damageSurgical needlesBlunt dissectorsMucosal resectionSubmucosal dissection
Instruments, systems and methods are provided for performing submucosal medical procedures in a desired area of the digestive tract using endoscopy. Instruments include safe access needle injection instruments incorporating electrosurgical capability, a submucosal tunneling instrument, a submucosal dissection instrument, a mucosal resection device and submucosal biopsy system. Systems include a combination of one or more of such instruments with or without injectable agents. Embodiments of various methods for performing the procedures are also provided.
Owner:APOLLO ENDOSURGERY INC
Devices and methods for endolumenal gastrointestinal bypass
The present invention provides devices and methods for attachment of an endolumenal gastrointestinal device, such as an artificial stoma device, a gastrointestinal bypass sleeve or other therapeutic or diagnostic device, within a patient's digestive tract. In one application of the invention, an endolumenal bypass sleeve is removeably attached in the vicinity of the gastroesophageal junction to treat obesity and / or its comorbidities, such as diabetes. The bypass sleeve may be at least partially deployed by eversion.
Owner:VALENTX
Cuff and sleeve system for gastrointestinal bypass
Owner:VALENTX
Eversion resistant sleeves
ActiveUS7766973B2Reduce coefficient of frictionIncrease stiffnessOesophagiIntravenous devicesImplanted deviceSurgery
The invention relates to improved means for preventing eversion and subsequent obstruction of thin-walled, floppy gastrointestinal liners implanted in the digestive tract of an animal. The implantable devices include an anchor adapted for attachment within a natural body lumen and a thin-walled, floppy sleeve open at both ends and defining a lumen therebetween. A substantial length of the sleeve has material characteristics that result in the sleeve being prone to eversion in the presence of retrograde pressures. Exemplary eversion-resistant features provide an increased stiffness and / or an increased friction coefficient between the anchor and the proximal end of the sleeve to resist eversion. In some embodiments, the eversion-resistant feature includes an anti-buckling element, such as a wire coupled along the substantial length of the sleeve.
Owner:GI DYNAMICS INC
Jelly composition
InactiveUS9452150B2Easy to carryEasily administered without waterBiocideAntipyreticBULK ACTIVE INGREDIENTActive ingredient
A composition in which a preparation of the composition itself has an excellent disintegratability in the right place and excellent active ingredient releasability within the digestive tract, the active ingredient and the preparation itself have long-term stability, excellent ease of operation such as manufacturability and filling into containers, an amount sufficient for achieving physiological effects can be easily taken and swallowed, and can be absorbed rapidly from the digestive tract, thus the physiological effect can be expected. The composition is an easy-release jelly composition containing an emulsified polyvalent unsaturated fatty acid or derivative thereof in an amount exceedingly 10 mass % and further containing an emulsifying agent and a gallant. The easy-release jelly composition includes an emulsified polyunsaturated fatty acid or derivative thereof in an amount greater than 10 wt %, an emulsifying agent, and a gelling agent.
Owner:MOCHIDA PHARM CO LTD
Medical Device and Method For Controlling Obesity
InactiveUS20080249533A1Slow down passage of foodEat more slowlyEar treatmentOesophagiPyloric orificeDiaphragm muscle
A method of, and device for, slowing the passage of food through a digestive tract of a patient and thereby treating obesity. The device is an obesity tube comprising (A) an upper ring of a size corresponding to a point under a patient's esophagus and above the patient's diaphragm muscle, and (B) a lower tube having a length and a distal opening. The method comprises stapling the upper ring under the patient's esophagus, above the patient's diaphragm muscle, and placing the lower tube distal to the upper ring. The length of the lower tube depends on whether the tube is to terminate distally in the stomach or terminate past the pylorus, in which case a section can be provided which is thick enough to resist collapsing under pylorus pressure. The lower tube can be entirely or partially non-permeable or semi-permeable. Semi-permeable tubes or sections thereof have walls which permit the passage of gastric hydrochloric acid but not food.
Owner:KS BIOMEDIX LTD
Ingestible event marker systems
Ingestible event marker systems that include an ingestible event marker (i.e., an IEM) and a personal signal receiver are provided. Embodiments of the IEM include an identifier, which may or may not be present in a physiologically acceptable carrier. The identifier is characterized by being activated upon contact with a target internal physiological site of a body, such as digestive tract internal target site. The personal signal receiver is configured to be associated with a physiological location, e.g., inside of or on the body, and to receive a signal the IEM. During use, the IEM broadcasts a signal which is received by the personal signal receiver.
Owner:PROTEUS DIGITAL HEALTH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com