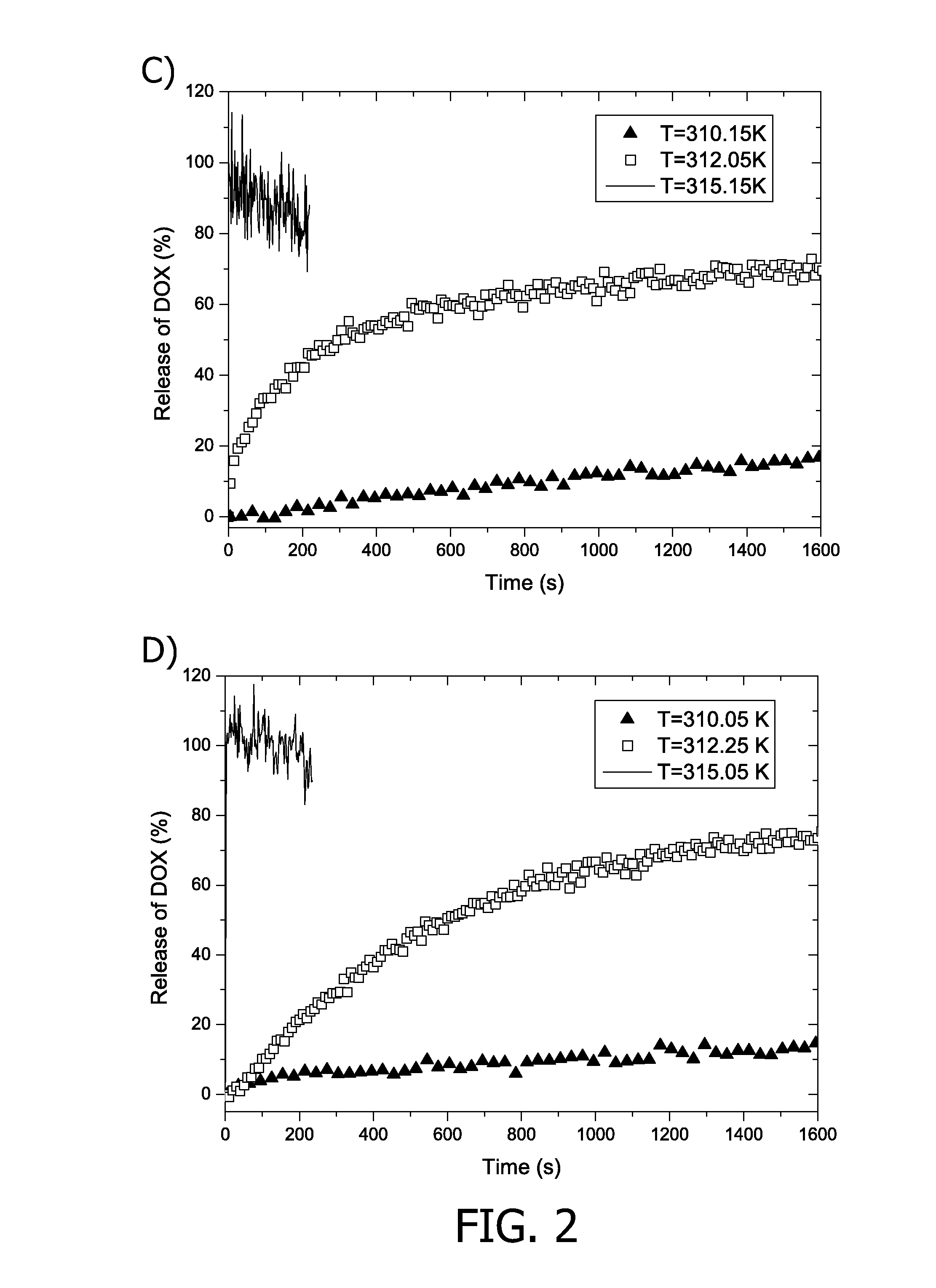
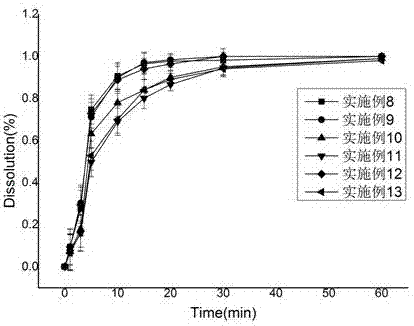
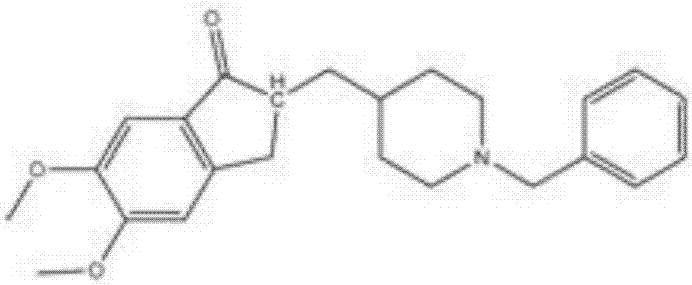
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
196results about How to "Rapid drug release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
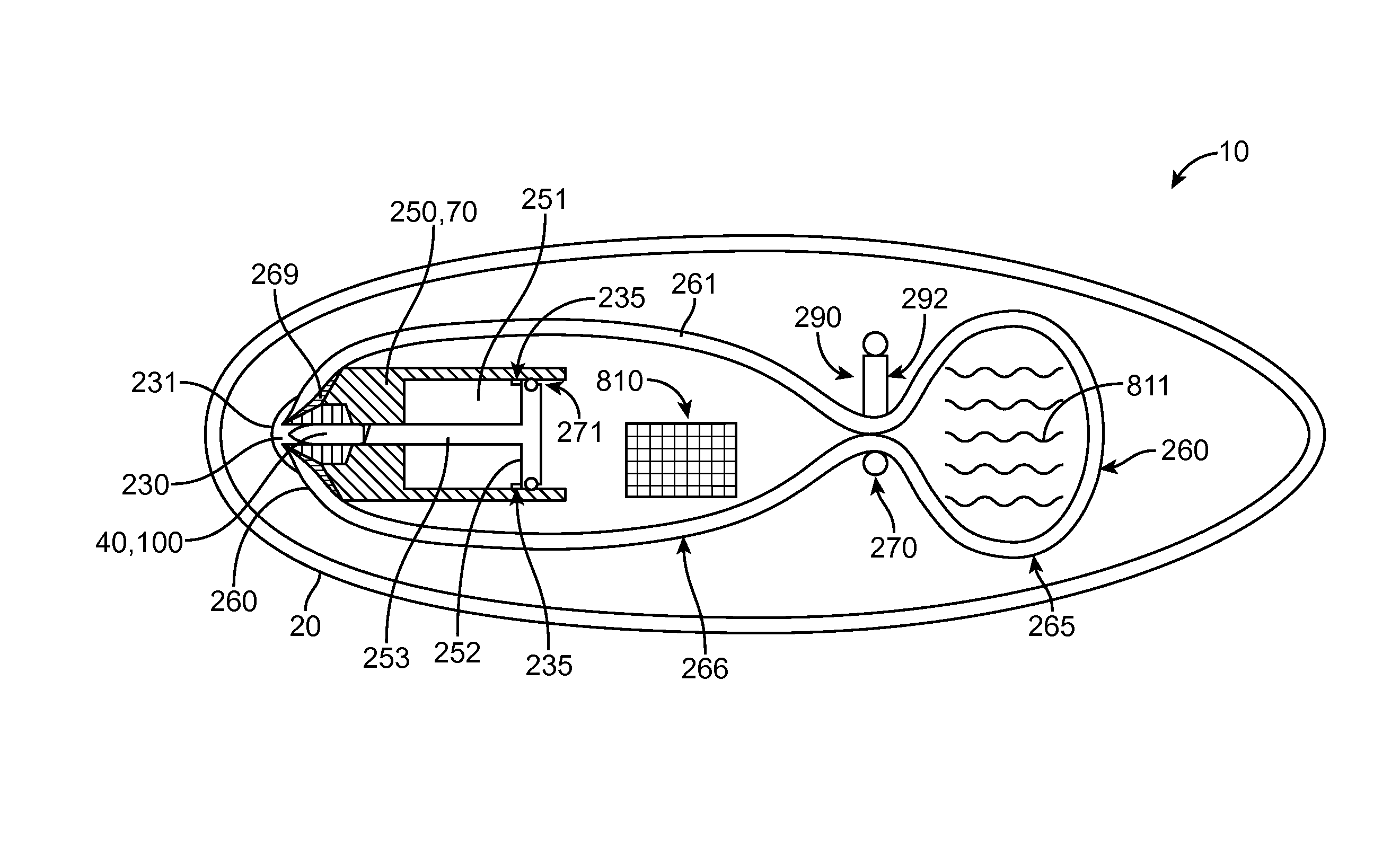
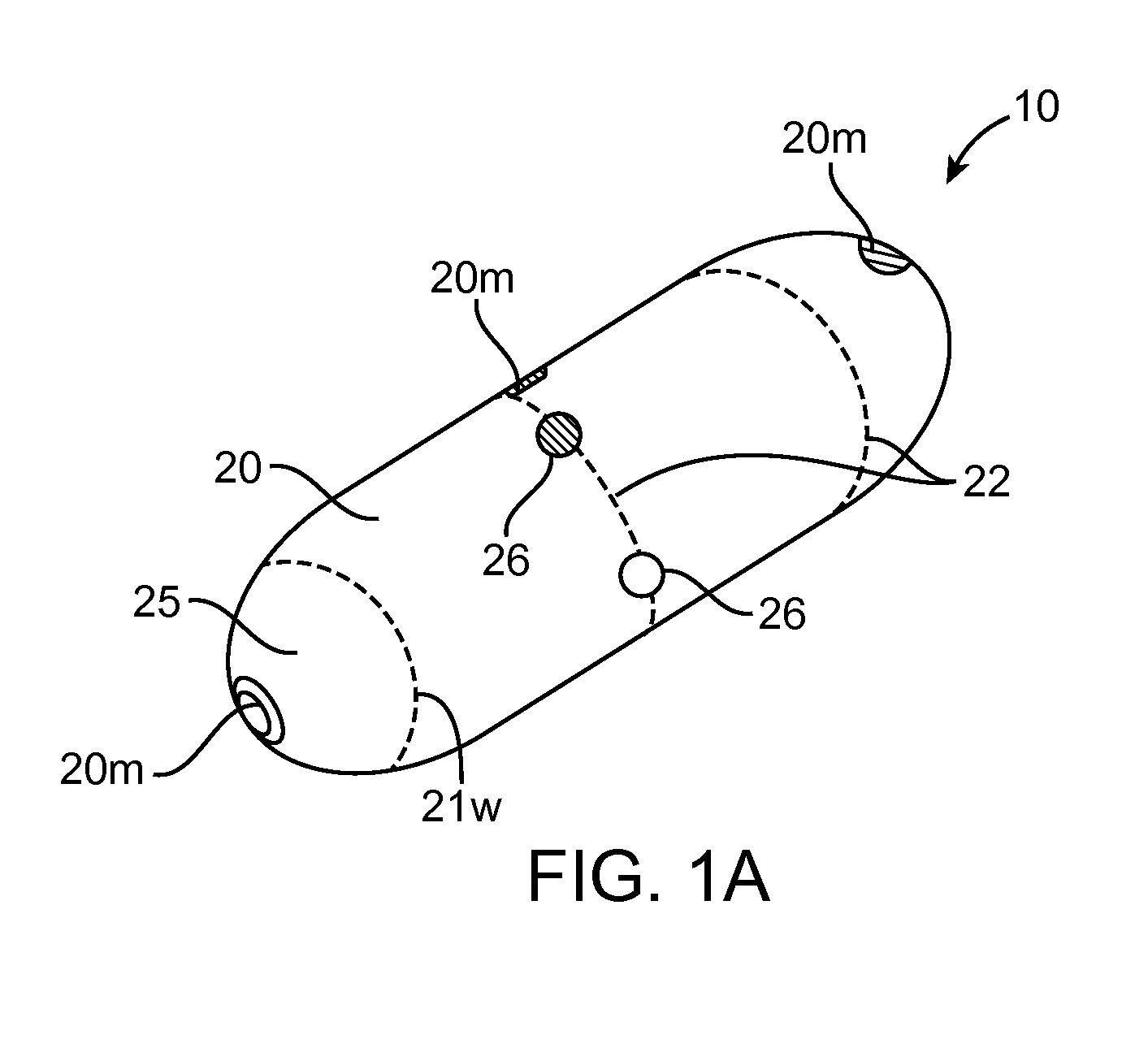
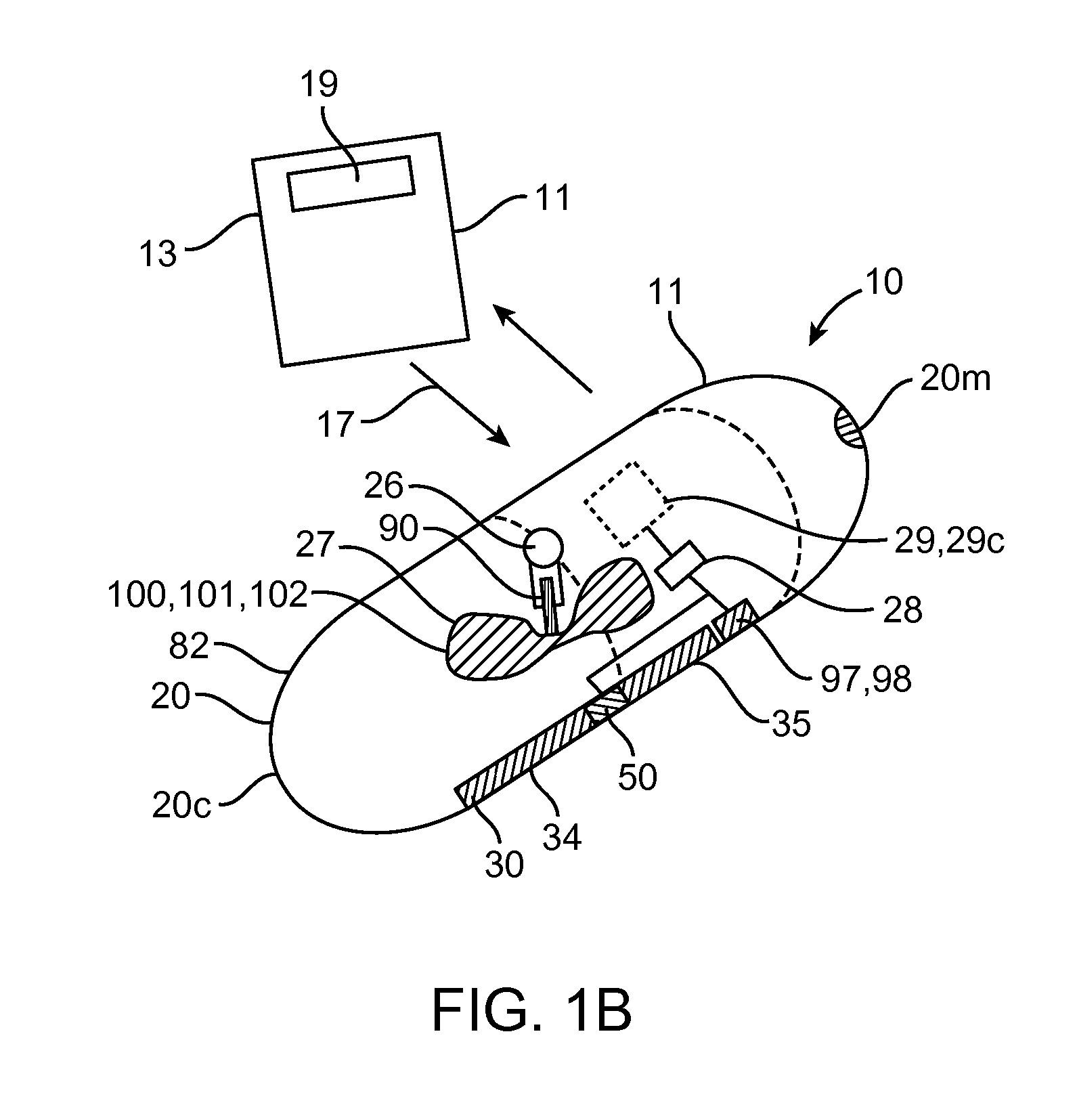
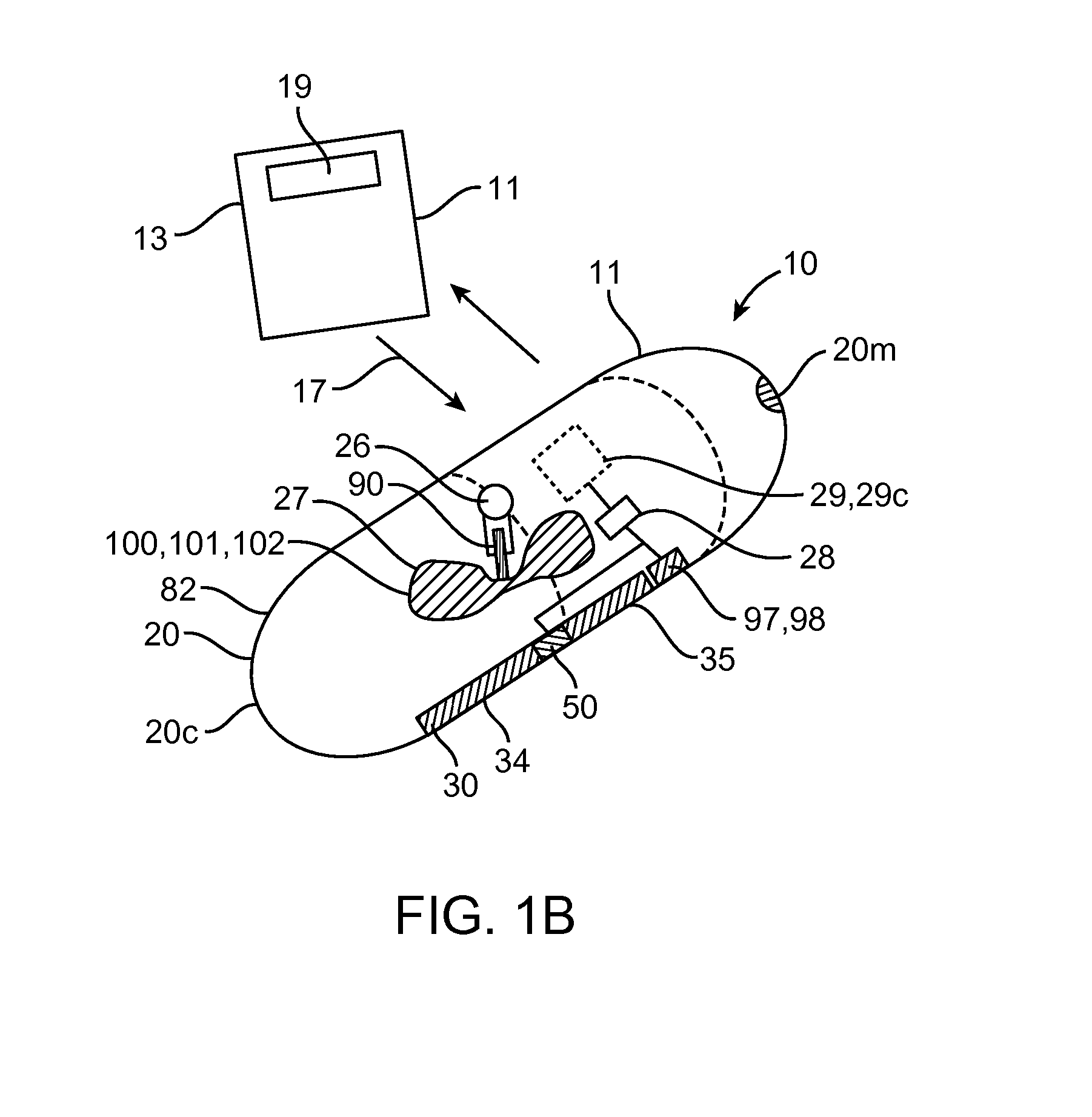
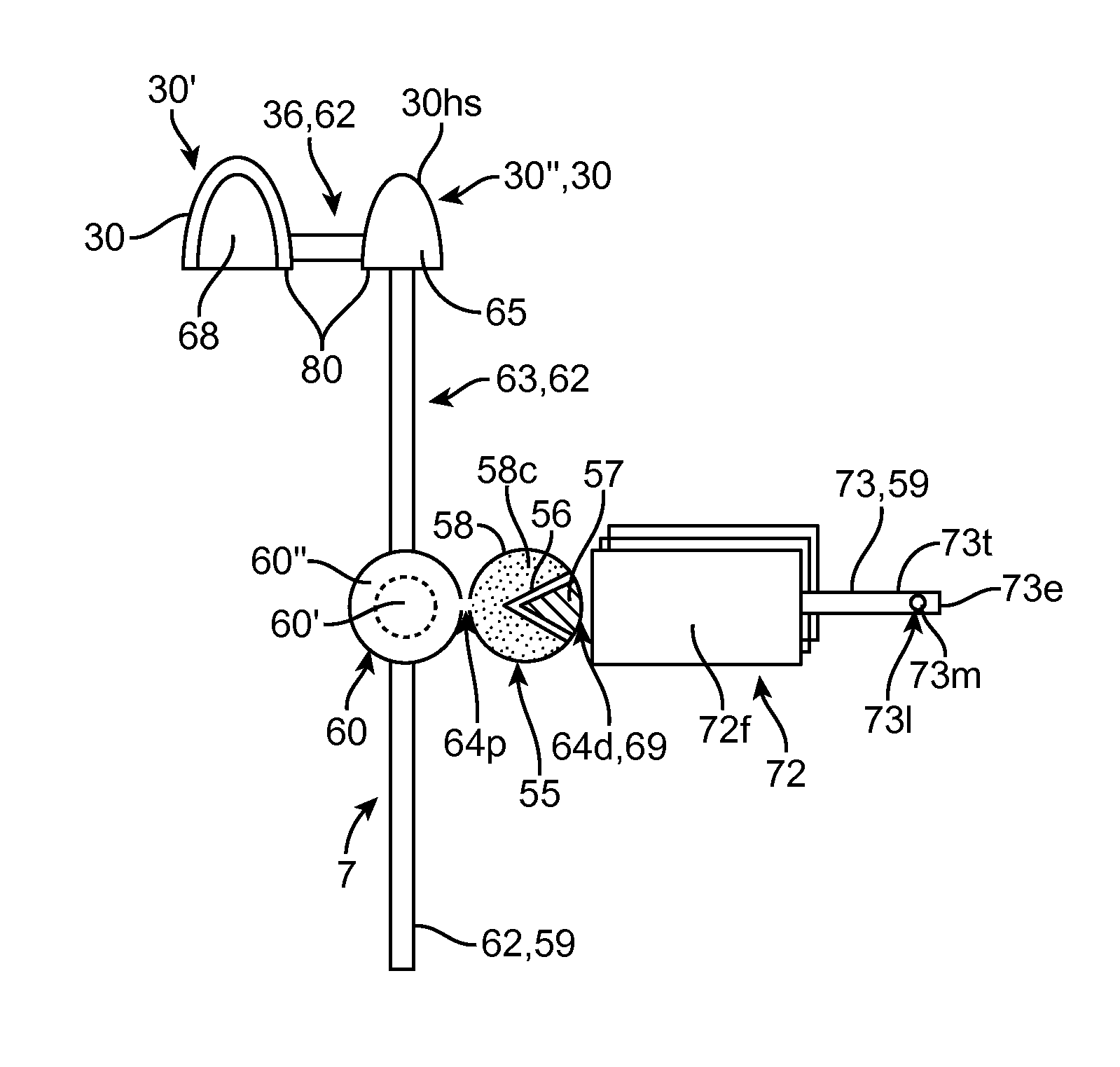
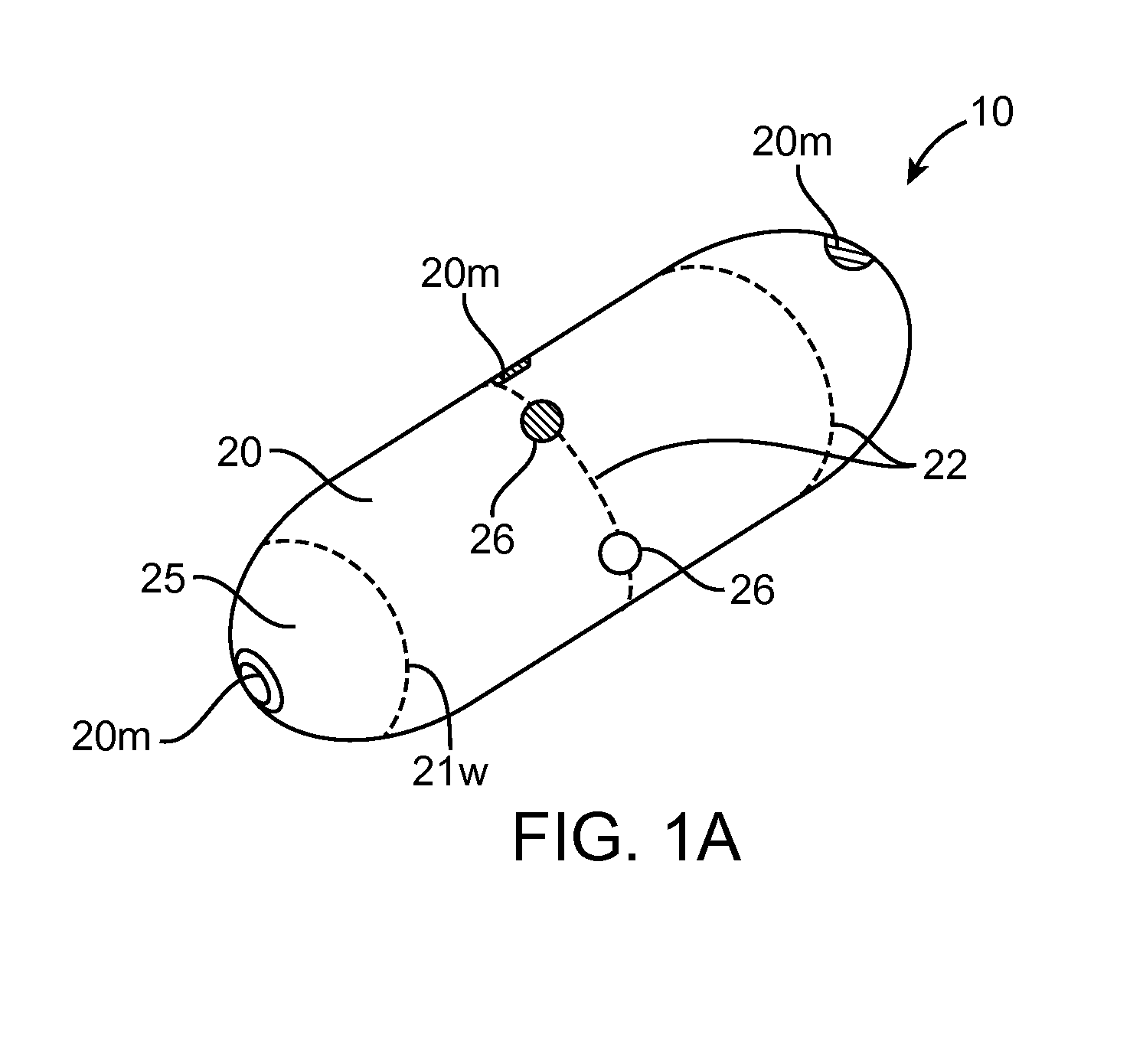
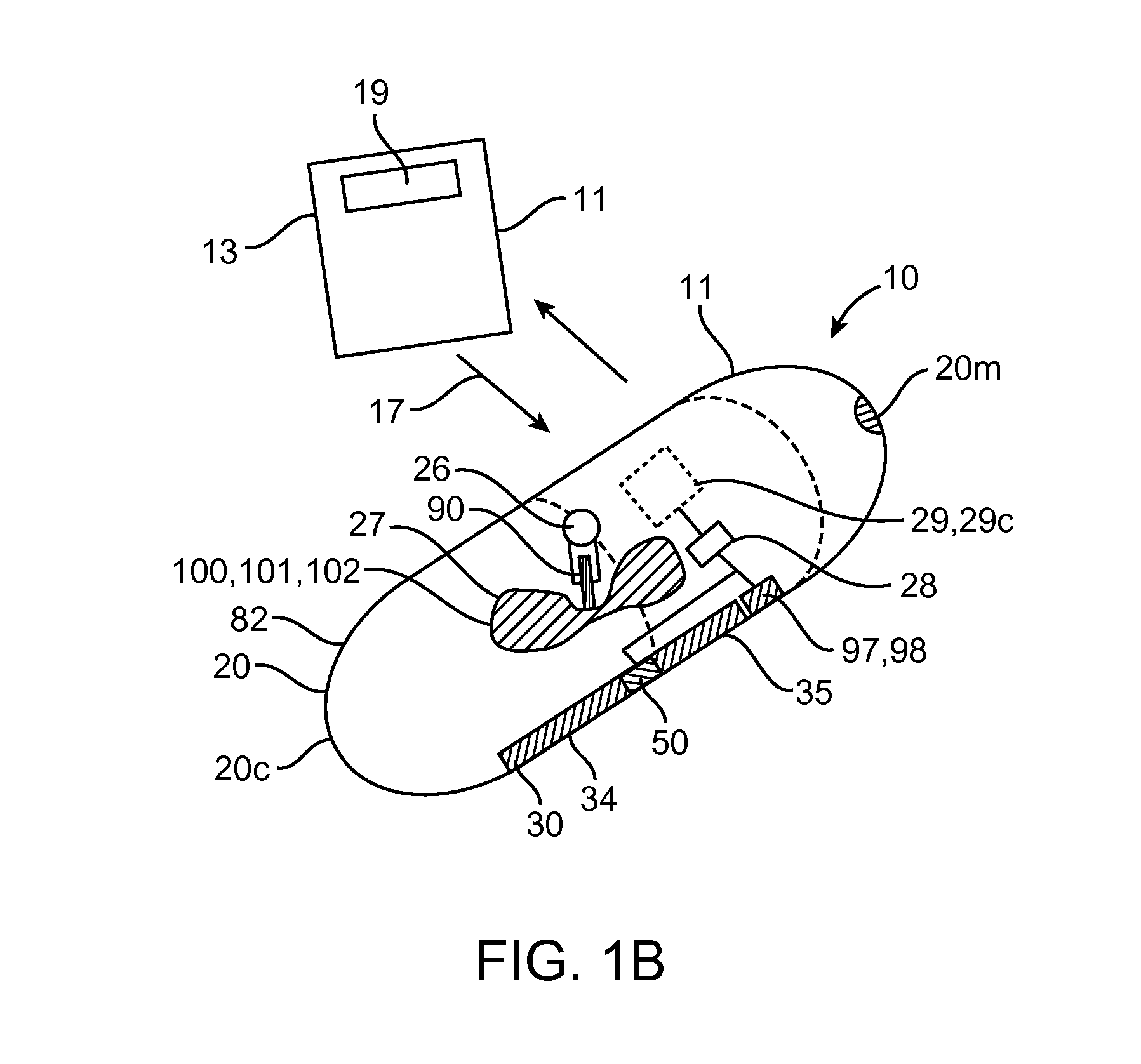
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS8734429B2Rapid drug releasePoor absorptionMedical devicesPressure infusionIntestinal wallsDrugs preparations
Owner:RANI THERAPEUTICS
Coating on a balloon comprising a polymer and a drug
ActiveUS20100063570A1Rapid drug releaseGood mechanical integrityBiocidePeptide/protein ingredientsMedicineMedical device
Owner:ABBOTT CARDIOVASCULAR
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS9149617B2Rapid drug releasePoor absorptionPeptide/protein ingredientsMedical devicesIntestinal wallsSmall intestine
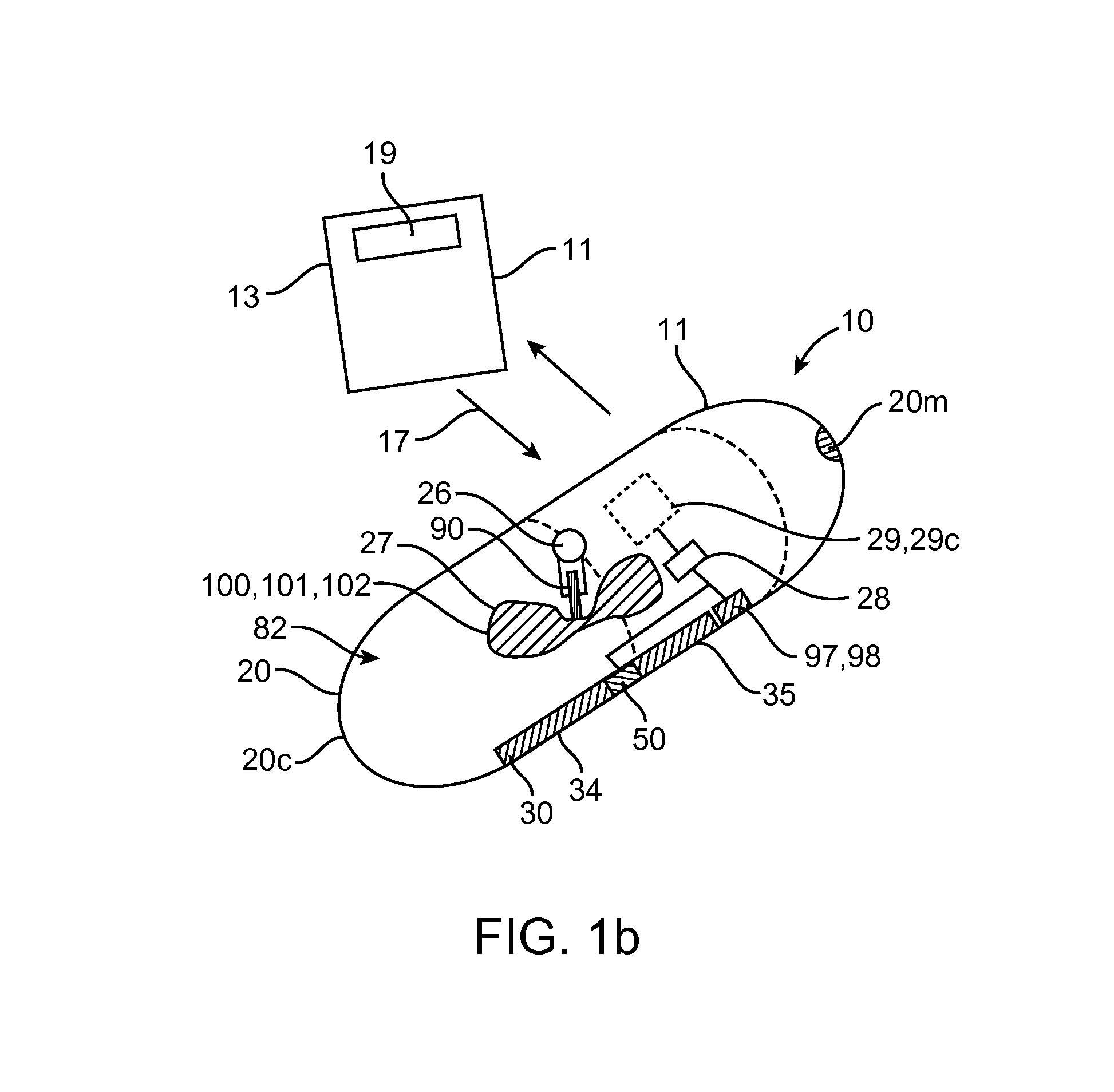
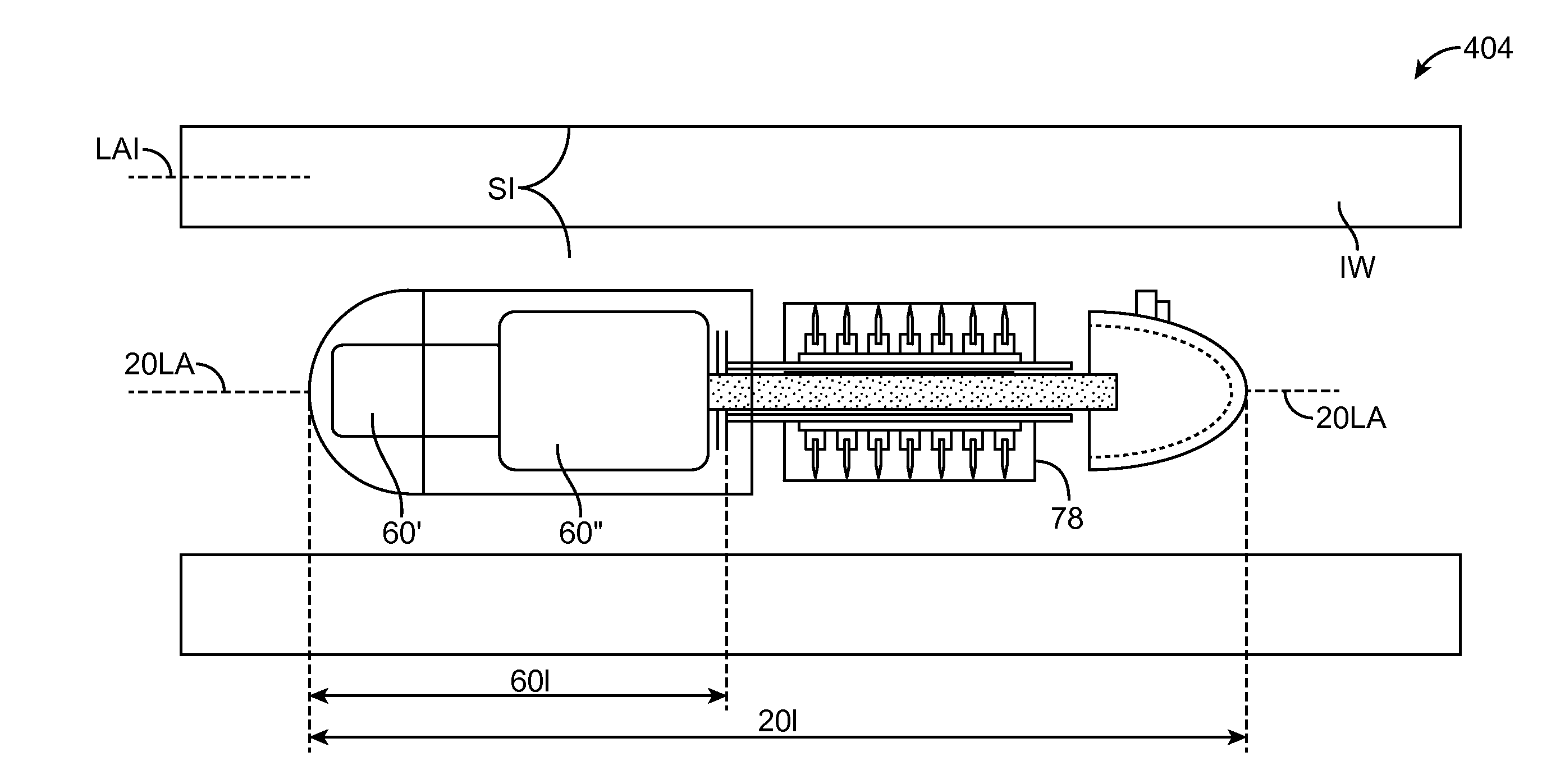
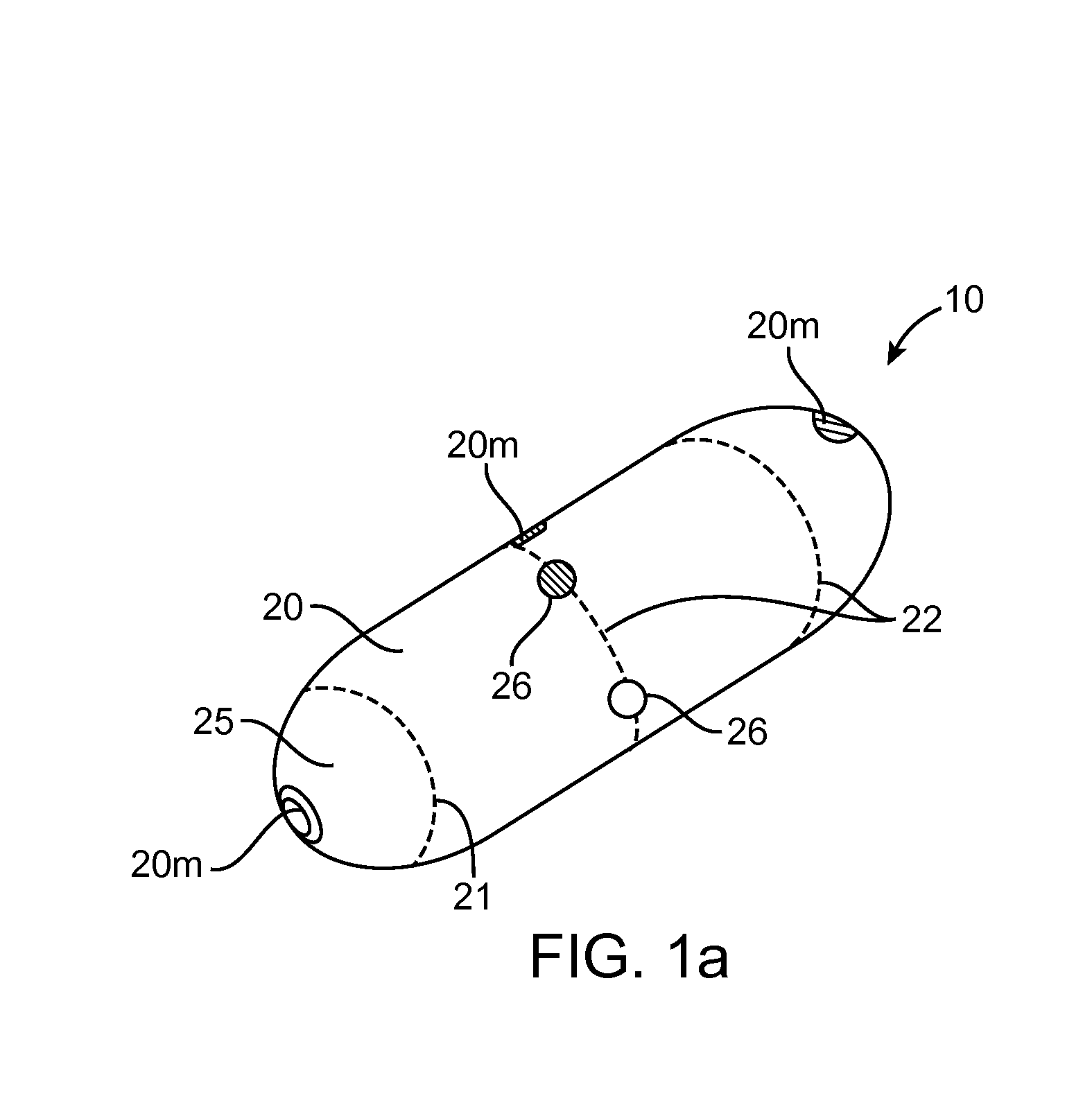
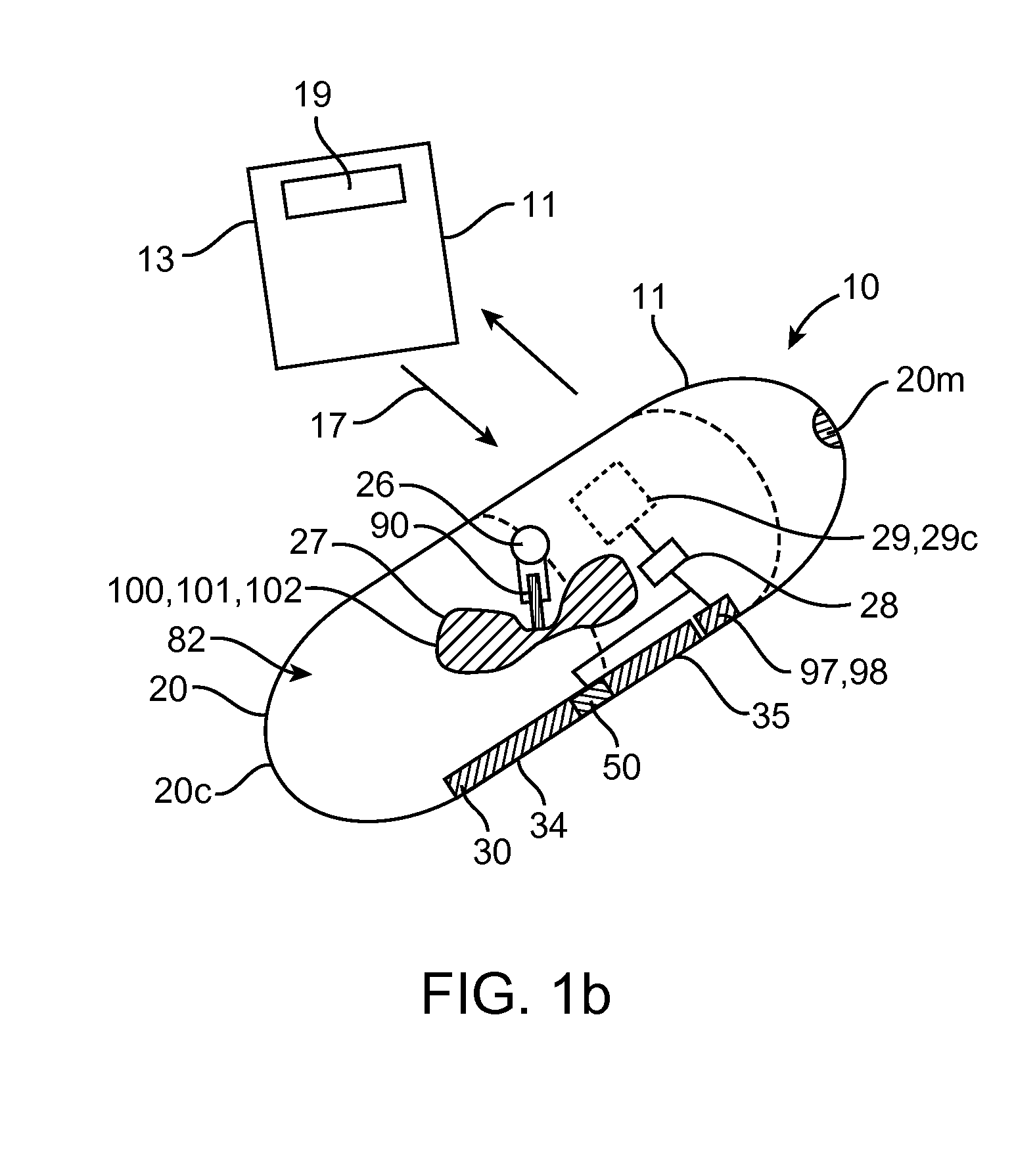
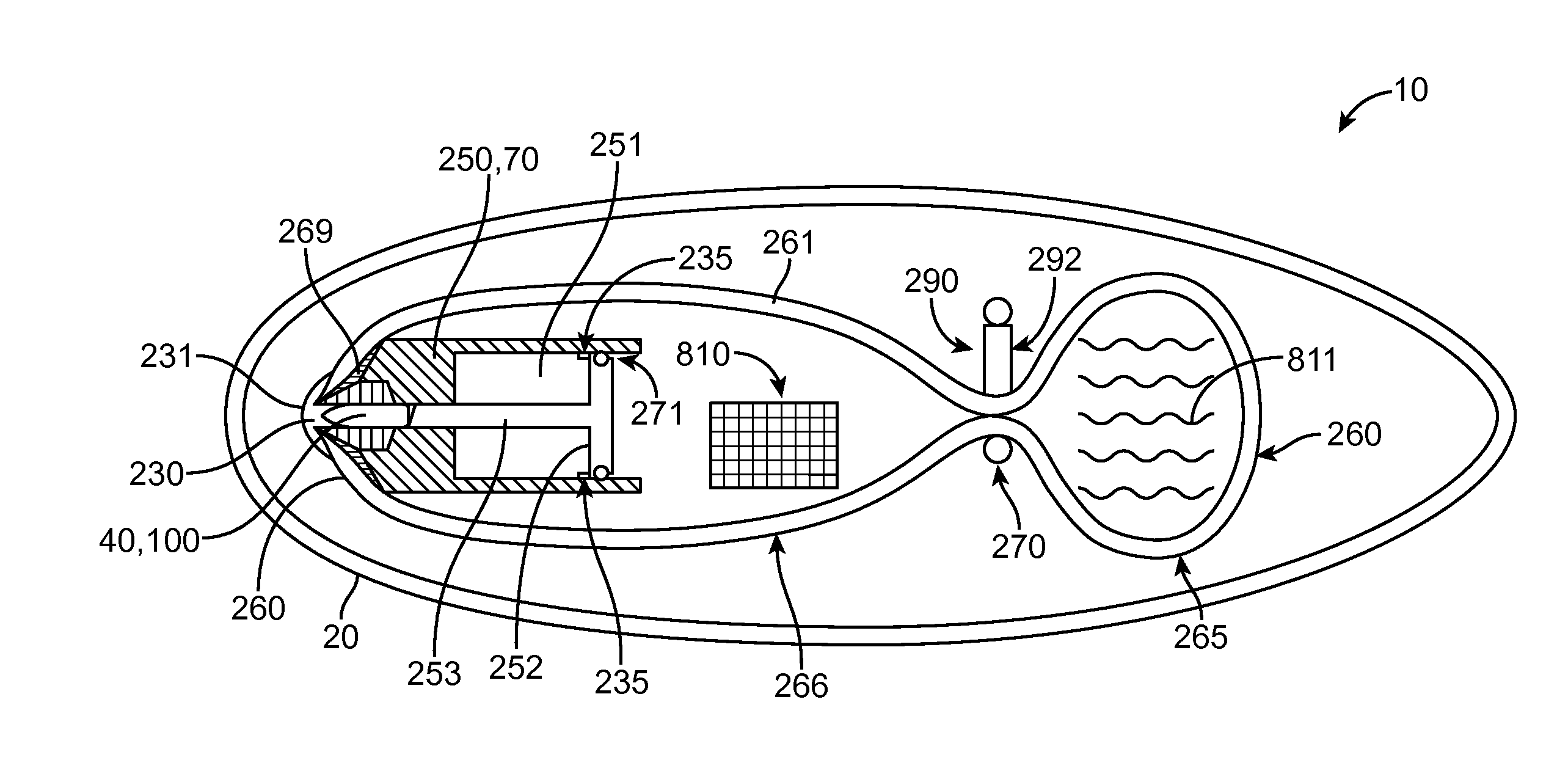
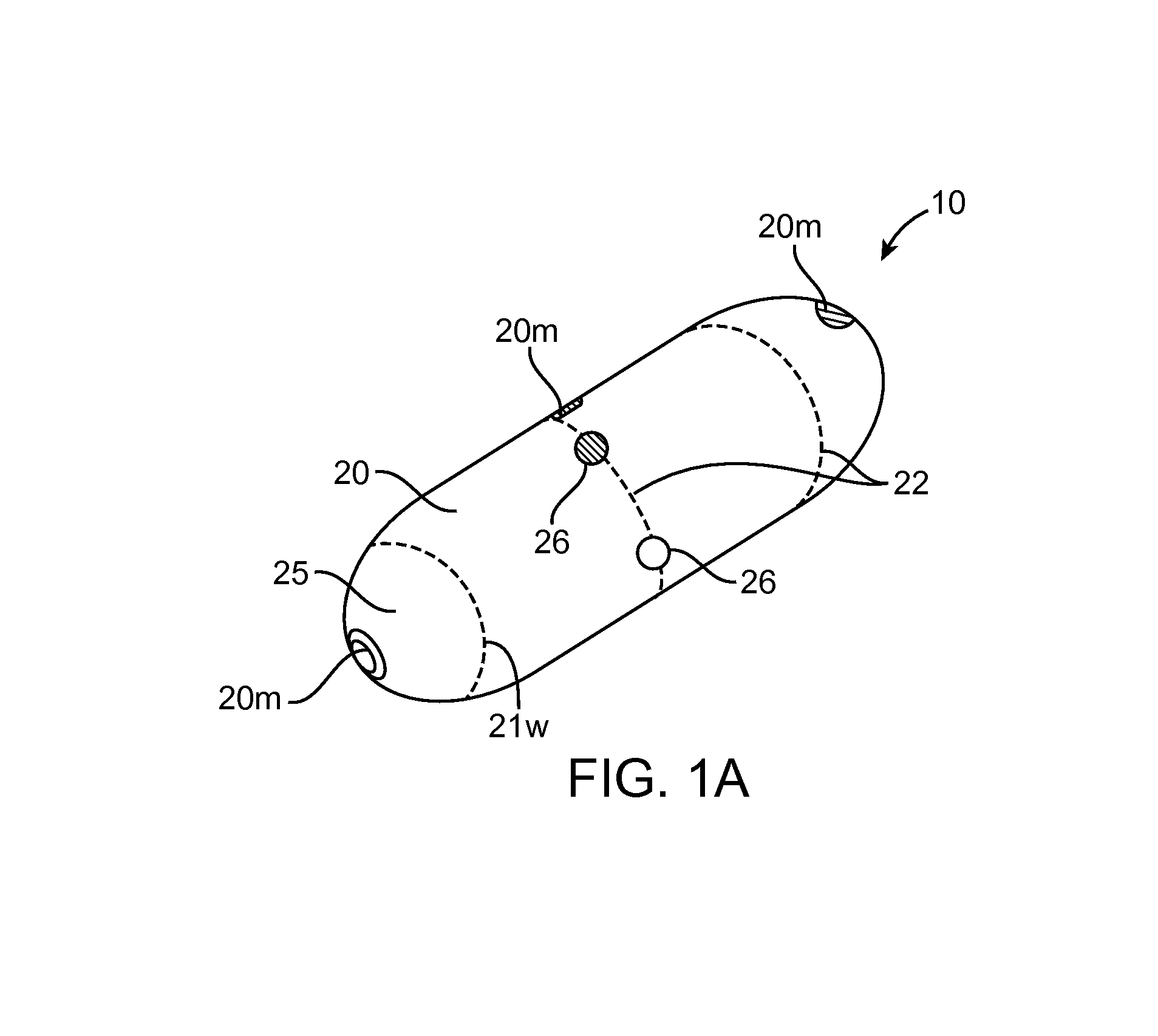
Embodiments of the invention provide swallowable devices, preparations and methods for delivering drugs and other therapeutic agents within the GI tract. Particular embodiments provide a swallowable device such as a capsule for delivering drugs into the intestinal wall or other GI lumen. Embodiments also provide various drug preparations that are configured to be contained within the capsule, advanced from the capsule into the intestinal wall and degrade within the wall to release the drug to produce a therapeutic effect. The preparation can be coupled to a delivery mechanism having one or more balloons or other expandable devices which are expandable responsive to a condition in the small intestine or other GI lumen to advance the preparation out of the capsule into the intestinal wall. Embodiments of the invention are particularly useful for the delivery of drugs which are poorly absorbed, tolerated and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS20130165859A1Rapid drug releasePoor absorptionPeptide/protein ingredientsSurgeryIntestinal wallsSmall intestine
Embodiments of the invention provide swallowable devices, preparations and methods for delivering drugs and other therapeutic agents within the GI tract. Particular embodiments provide a swallowable device such as a capsule for delivering drugs into the intestinal wall or other GI lumen. Embodiments also provide various drug preparations that are configured to be contained within the capsule, advanced from the capsule into the intestinal wall and degrade within the wall to release the drug to produce a therapeutic effect. The preparation can be coupled to a delivery mechanism having one or more balloons or other expandable devices which are expandable responsive to a condition in the small intestine or other GI lumen to advance the preparation out of the capsule into the intestinal wall. Embodiments of the invention are particularly useful for the delivery of drugs which are poorly absorbed, tolerated and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
Controlled-release formulation of HCV protease inhibitor and methods using the same
InactiveUS20070237818A1Improve bioavailability and convenienceRapid release of drugBiocideOrganic chemistryChemistryHcv protease
Owner:SCHERING CORP
Oral pharmaceutical compositions in timed-release particle form and fast-disintegrating tablets containing this composition
InactiveUS20050287211A1Reduce solubilityProne to feverAntibacterial agentsOrganic active ingredientsWater solubleLag time
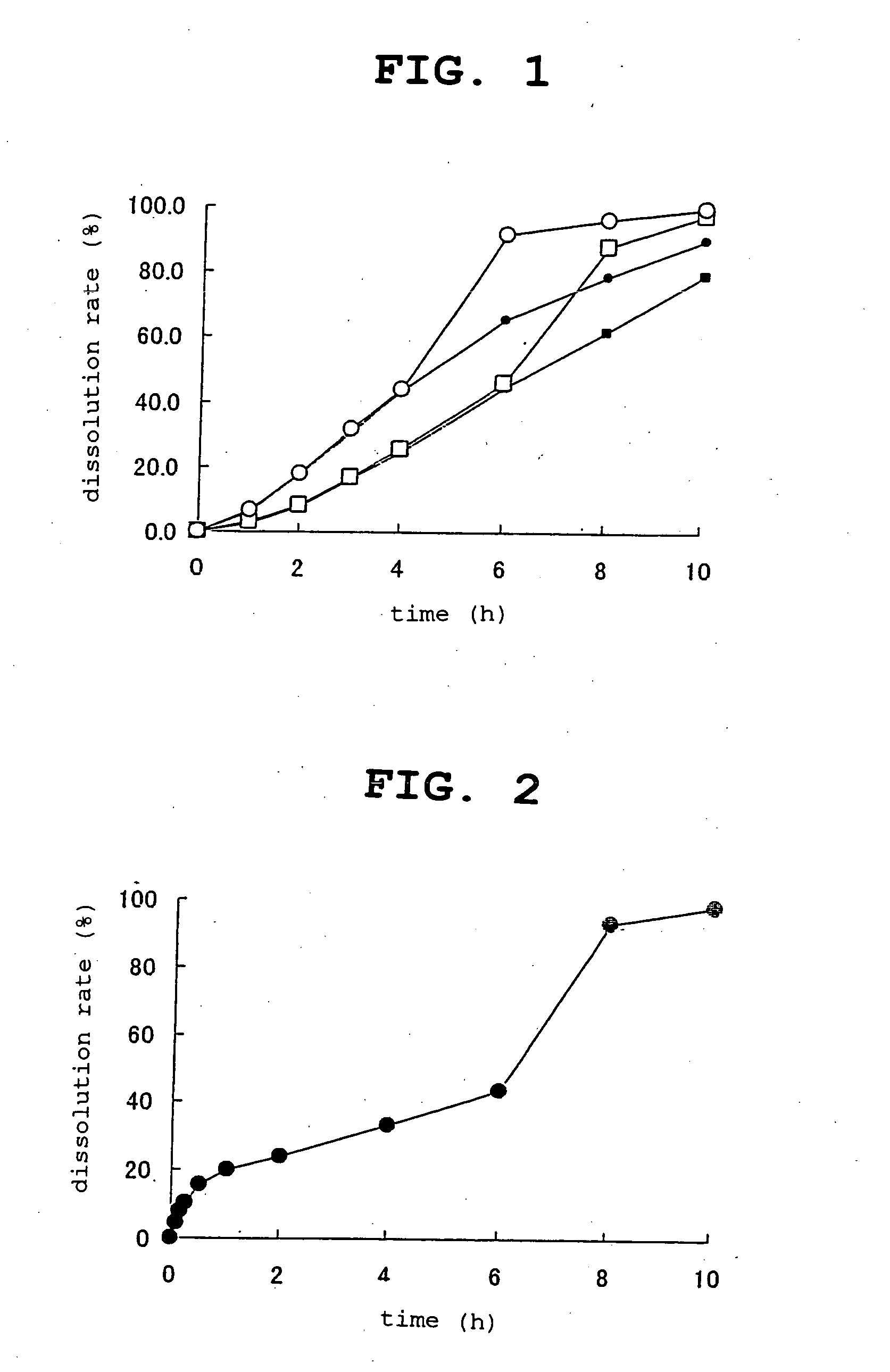
The present invention relates to an oral pharmaceutical composition in particle form, which comprises particles that contain a drug at the core of the pharmaceutical composition in particle form; a middle layer that contains two types of water-soluble components, an insolubilizer and an insolubilizing substance; and an outer layer for controlling water penetration that contains a water-insoluble substance. The present invention makes it possible to provide a pharmaceutical composition in particle form for oral use with which initial drug release is suppressed, the drug is quickly released thereafter, and lag time can be controlled as needed, and fast-disintegrating tablets containing this composition.
Owner:ASTELLAS PHARMA INC
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS20140221927A1Rapid drug releasePoor absorptionMedical devicesPressure infusionIntestinal wallsSmall intestine
Embodiments of the invention provide swallowable devices, preparations and methods for delivering drugs and other therapeutic agents within the GI tract. Particular embodiments provide a swallowable device such as a capsule for delivering drugs into the intestinal wall or other GI lumen. Embodiments also provide various drug preparations that are configured to be contained within the capsule, advanced from the capsule into the intestinal wall and degrade within the wall to release the drug to produce a therapeutic effect. The preparation can be coupled to a delivery mechanism having one or more balloons or other expandable devices which are expandable responsive to a condition in the small intestine or other GI lumen to advance the preparation out of the capsule into the intestinal wall. Embodiments of the invention are particularly useful for the delivery of drugs which are poorly absorbed, tolerated and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
Sustained-release drug delivery compositions and methods
InactiveUS20100092562A1Improve stabilityReduce molecular weightPowder deliveryOrganic active ingredientsImmediate releaseDecongestant
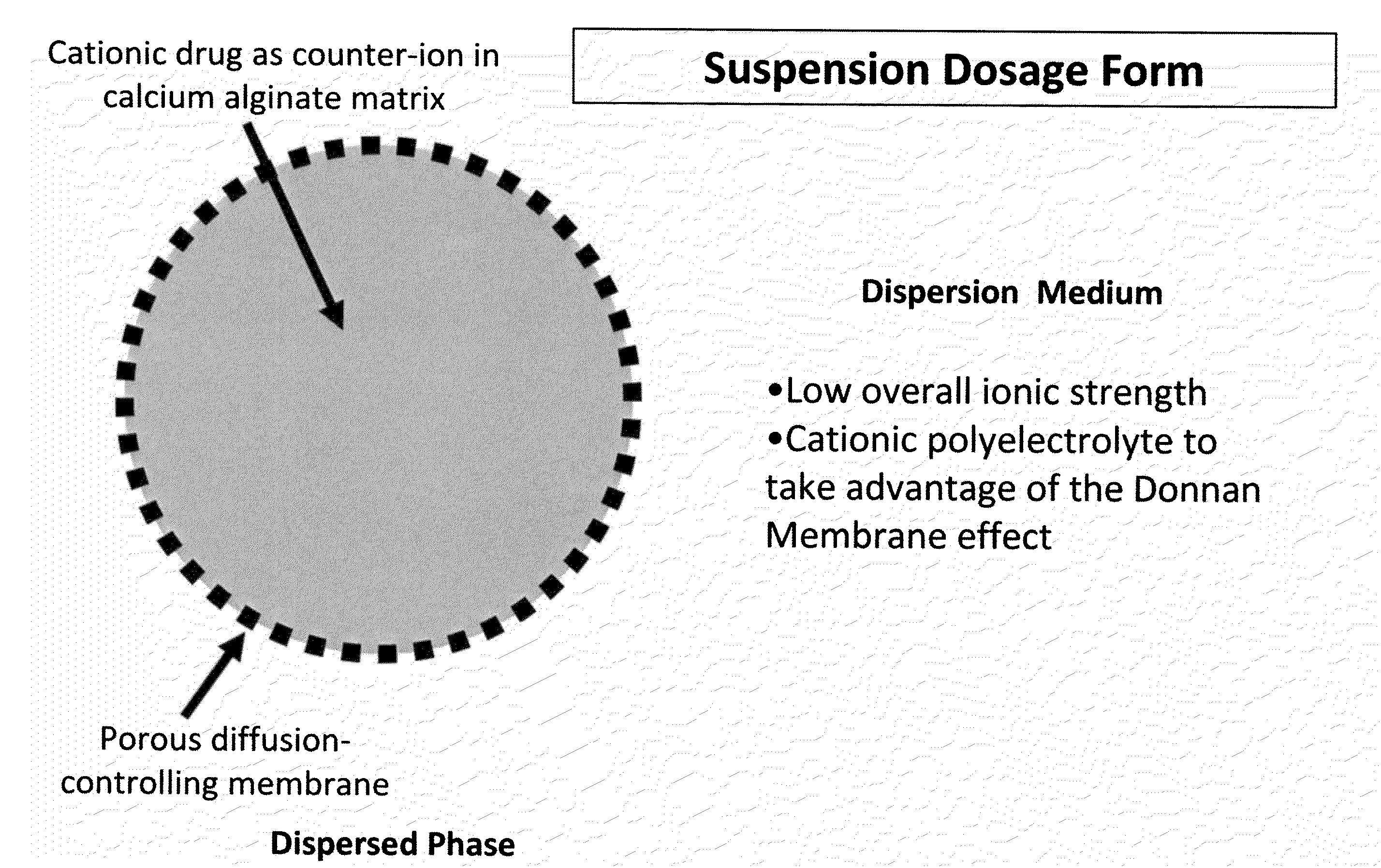
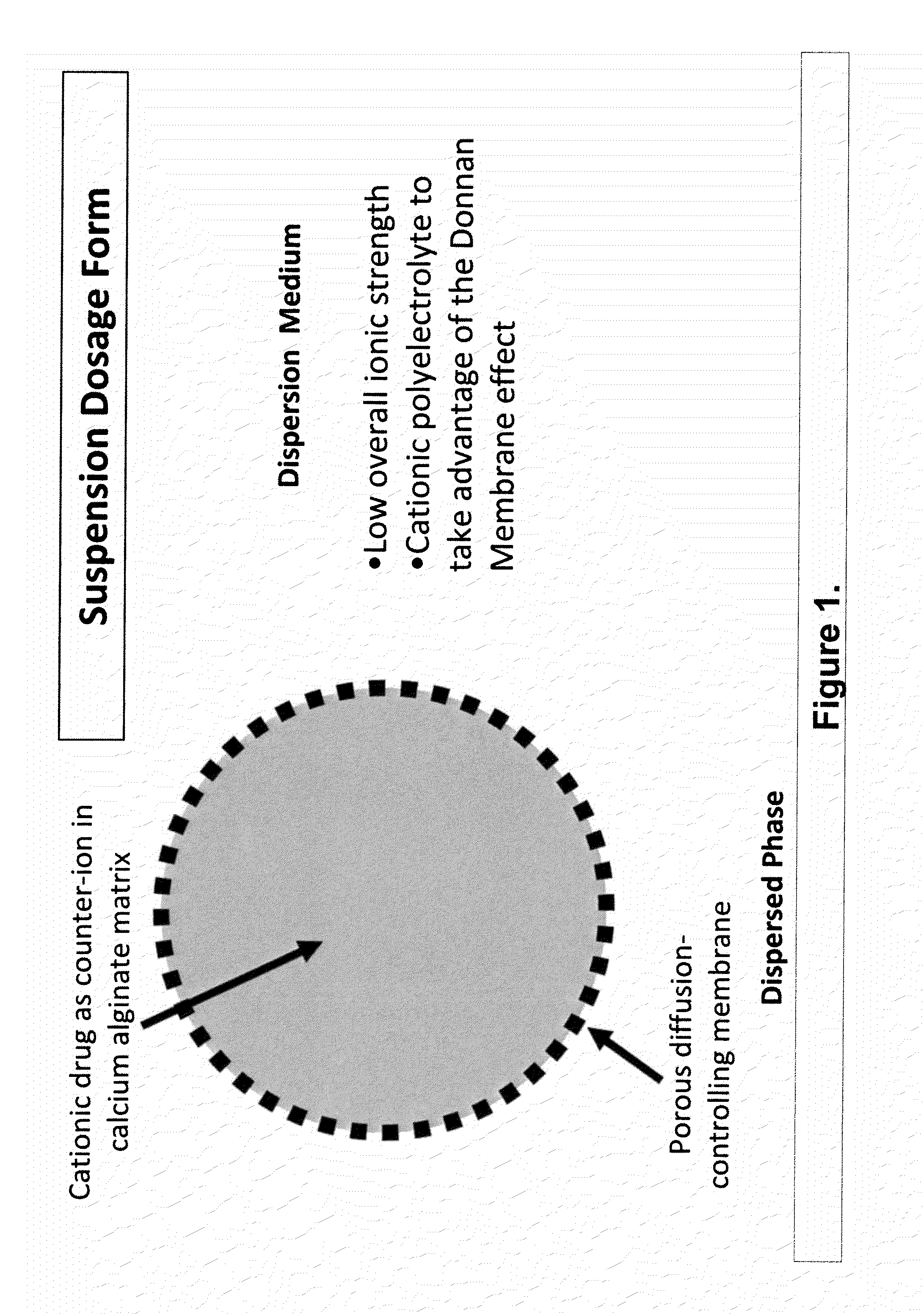
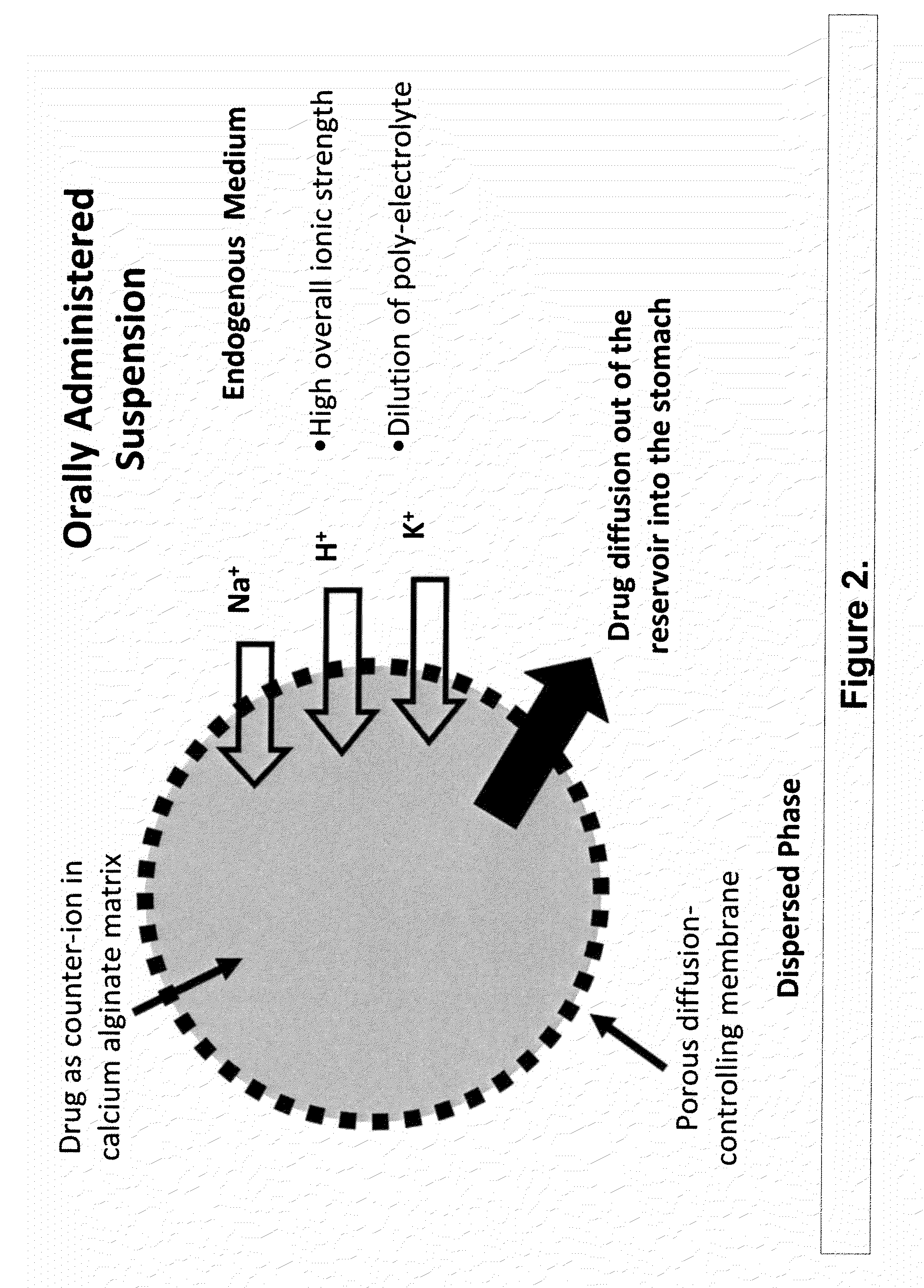
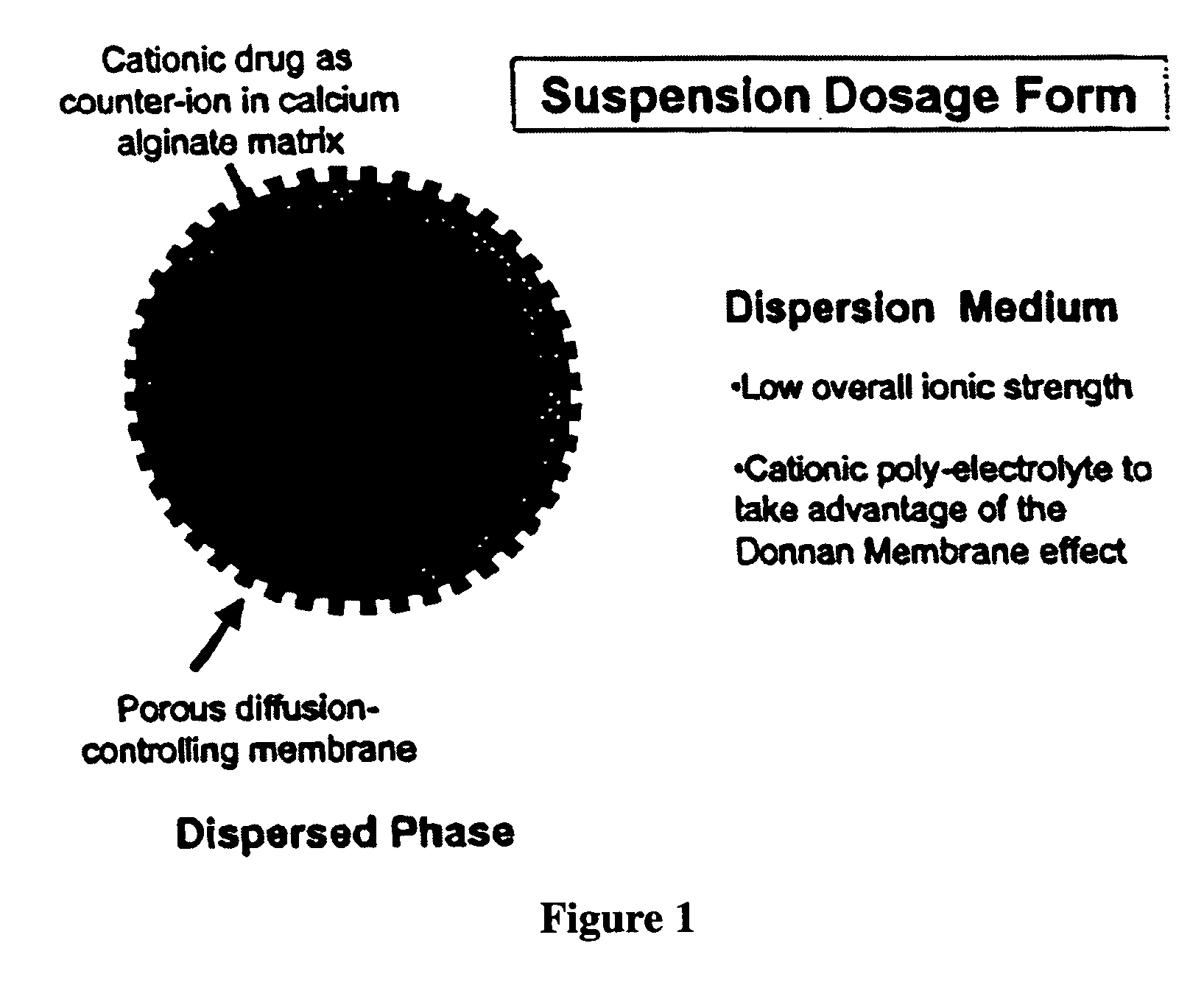
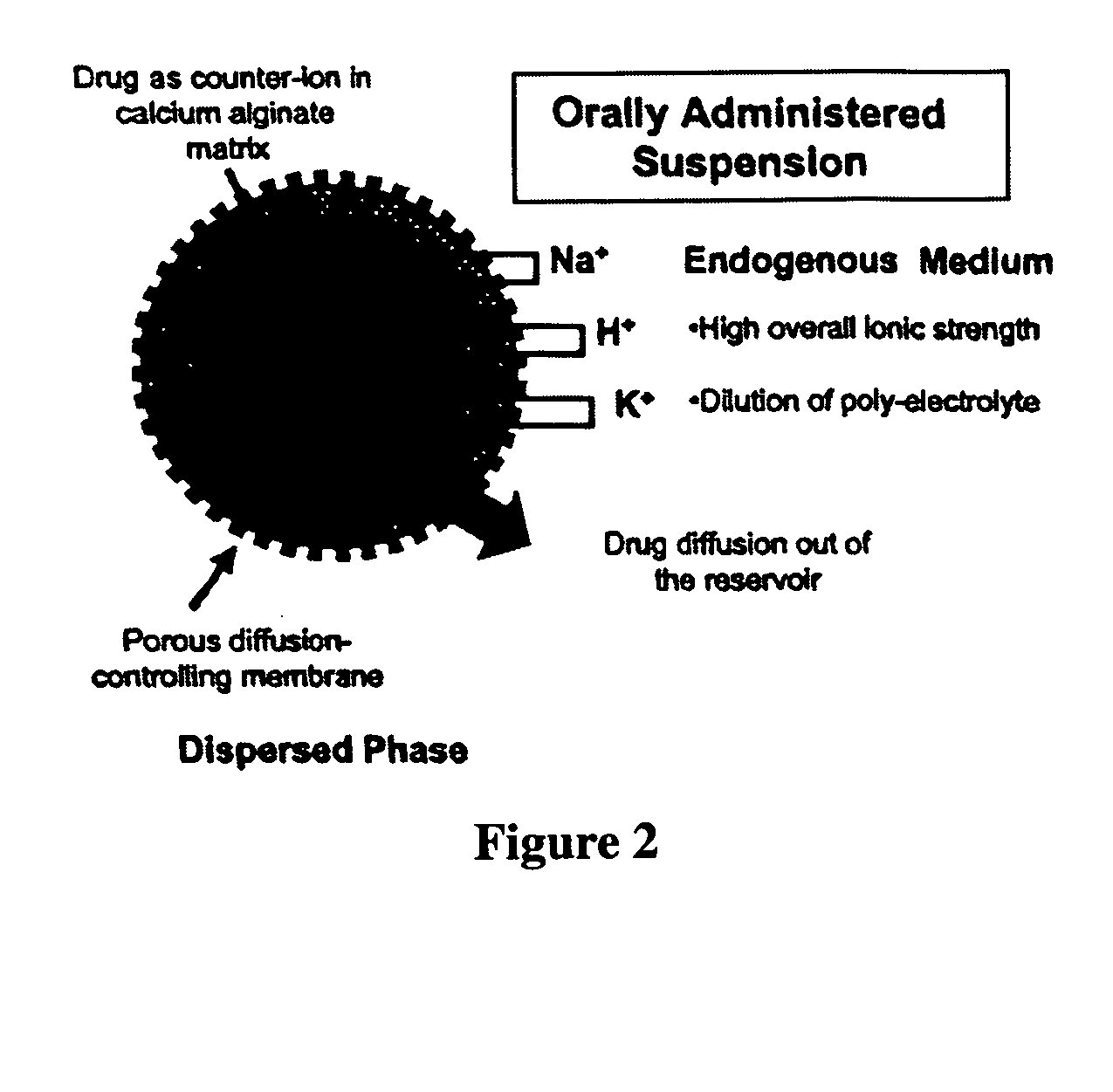
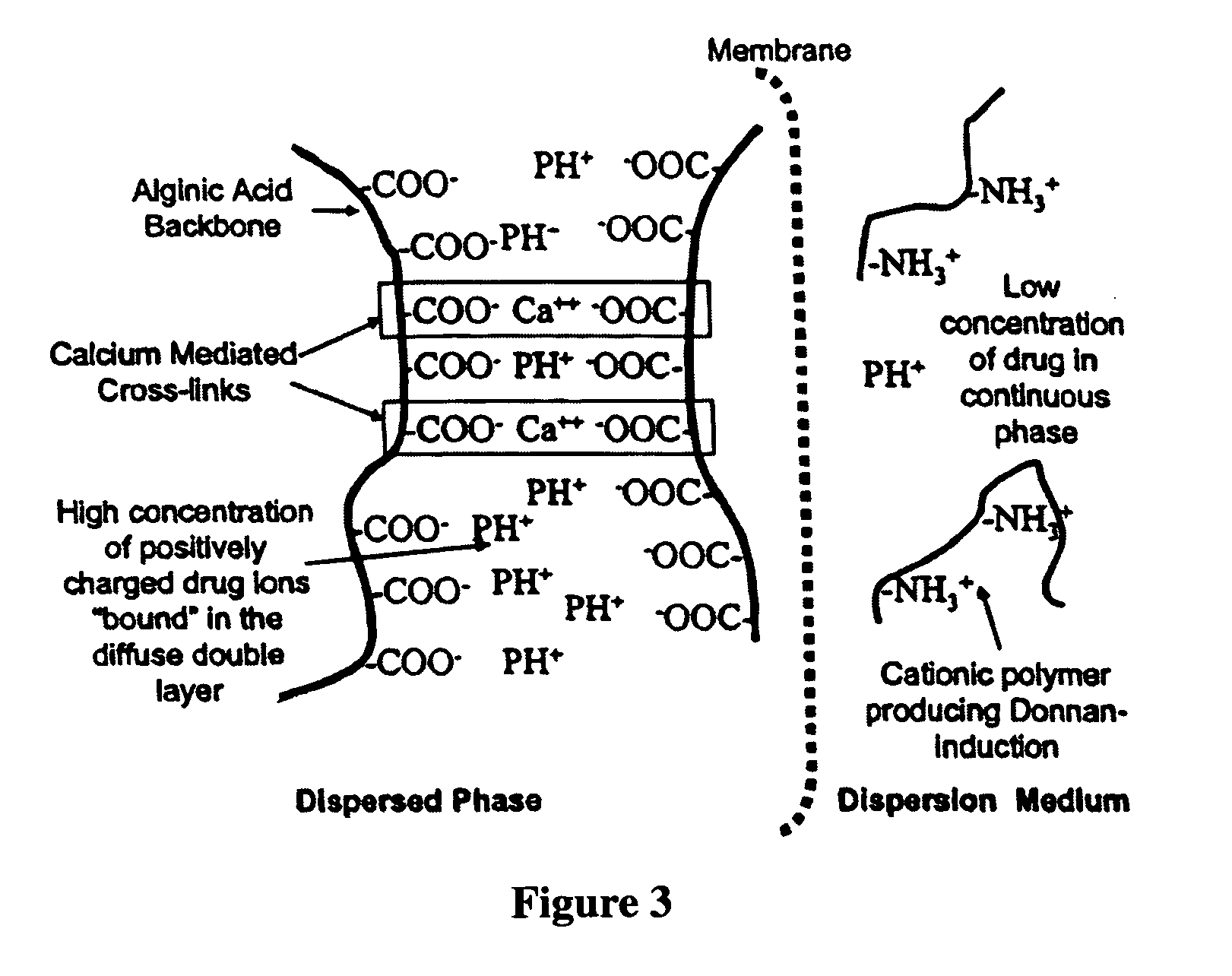
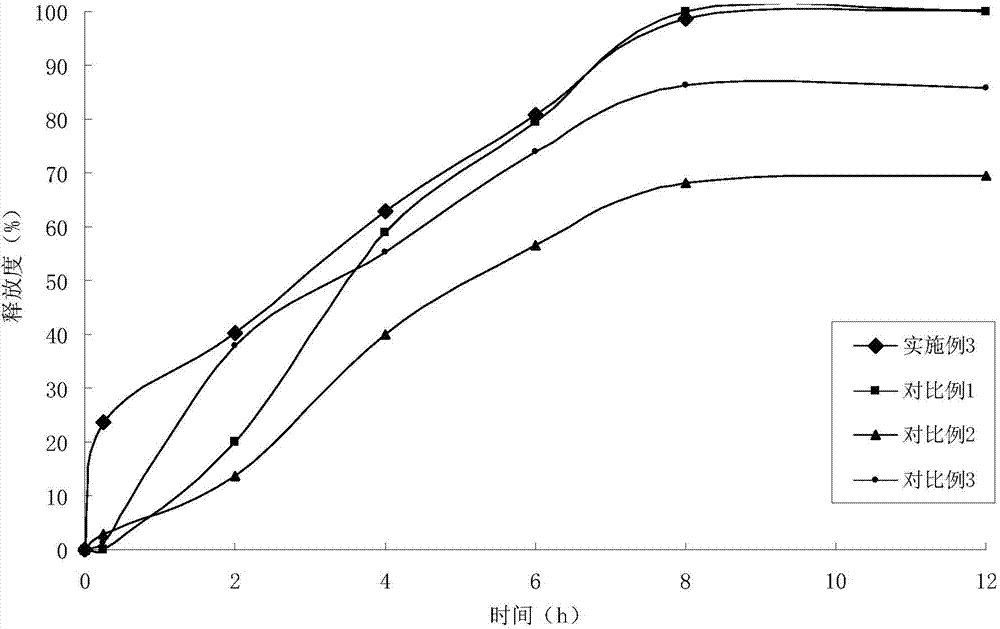
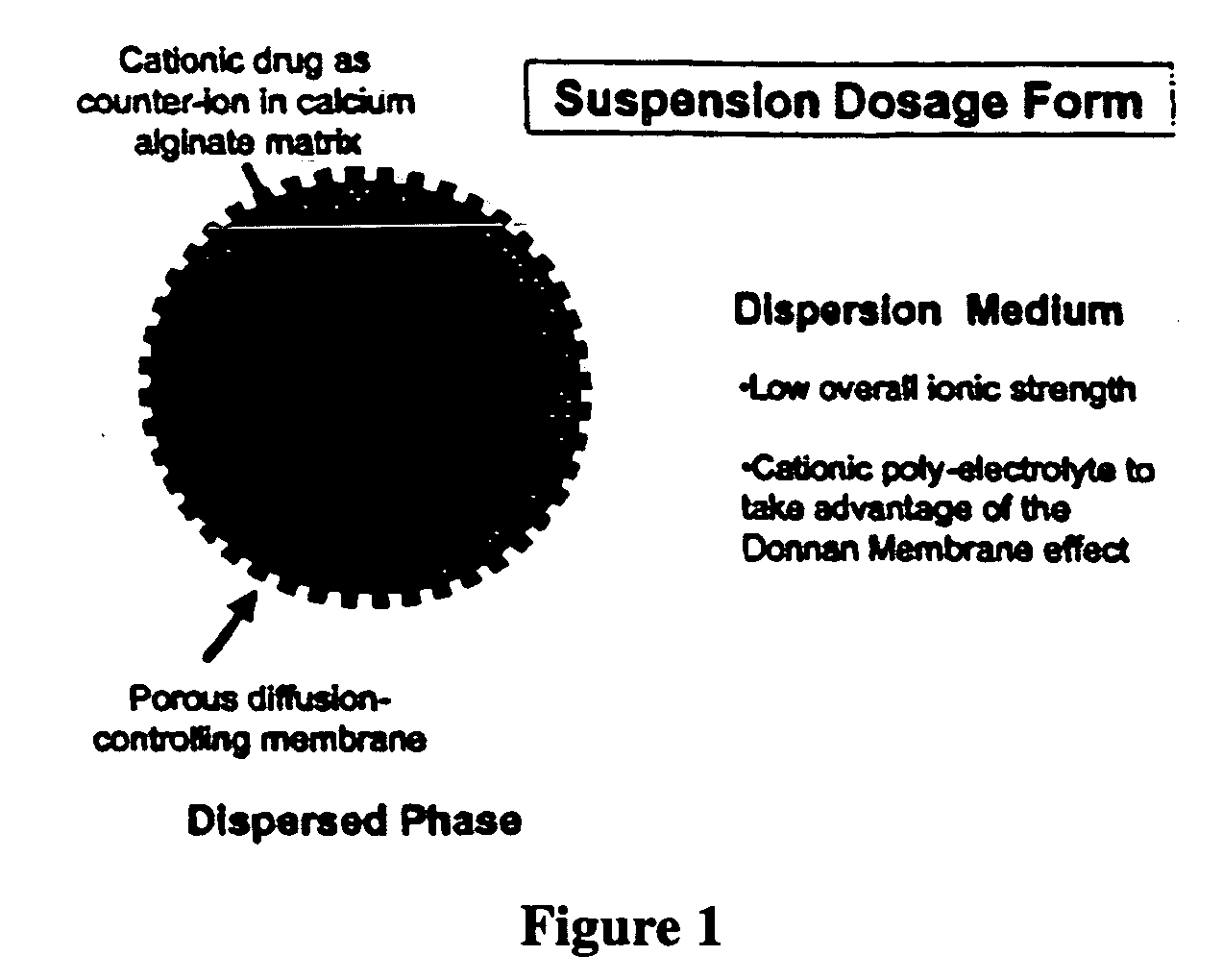
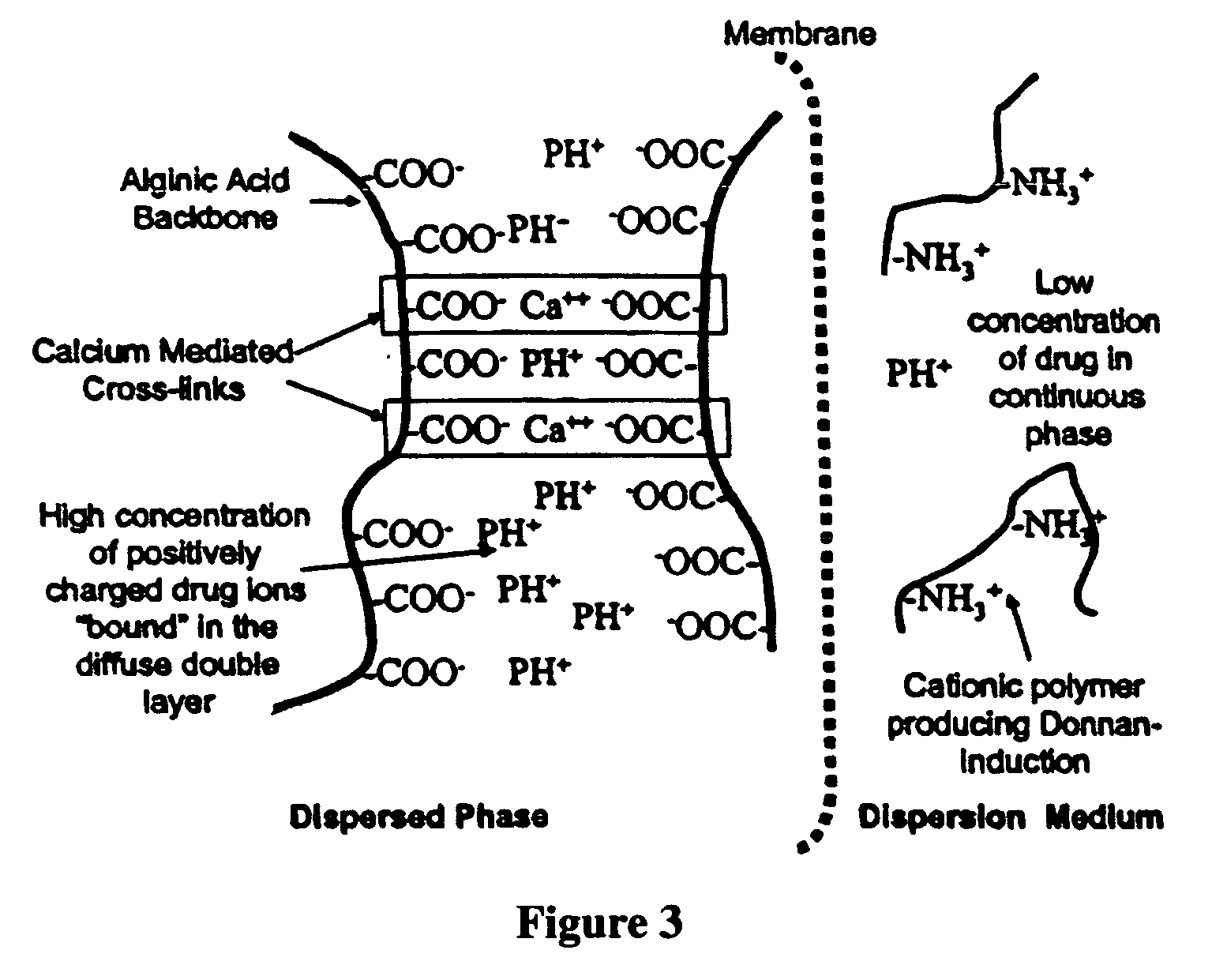
The present invention relates to liquid sustained release suspension dosage forms. In particular, the invention encompasses sustained release compositions comprising a dispersed phase, which contains an ion-exchange matrix drug complex, a diffusion controlling membrane coating and a dispersion medium comprising an excipient capable of impeding water activity such that drug dissolution is inhibited prior to administration. Further, the invention provides for compositions wherein several active ingredients associate in a single bead in the dispersed phase, such that the abuse potential of such active ingredients is reduced. The invention also encompasses sustained release formulations of combination drugs comprising an extended release phase and an immediate release phase. The formulations of the invention may be used to treat a variety of conditions and symptoms, including those that require administration of several drugs, such as cold and allergy symptoms. In one of the embodiments, the sustained release composition combines an antihistamine, an antitussive and a decongestant. The invention further provides for methods of making and using such formulations.
Owner:UPM PHARMA
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS20130274659A1Rapid drug releasePoor absorptionSurgeryMedical devicesIntestinal wallsChemical compound
Embodiments of the invention provide swallowable devices, preparations and methods for delivering drugs and other therapeutic agents within the GI tract. Particular embodiments provide a swallowable device such as a capsule for delivering drugs into the intestinal wall or other GI lumen. Embodiments also provide various drug preparations that are configured to be contained within the capsule, advanced from the capsule into the intestinal wall and degrade within the wall to release the drug to produce a therapeutic effect. The preparation can be coupled to a delivery mechanism having one or more balloons or other expandable devices which are expandable responsive to a condition in the small intestine or other GI lumen to advance the preparation out of the capsule into the intestinal wall. Embodiments of the invention are particularly useful for the delivery of drugs which are poorly absorbed, tolerated and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
Aqueous sustained-release drug delivery system for highly water-soluble electrolytic drugs
InactiveUS20060134148A1Reduce molecular weightQuick releasePowder deliveryPharmaceutical non-active ingredientsElectrolysisIon exchange
Owner:HOLLENBECK R GARY
Lipid bilayer carrier for drugs or imaging agents
InactiveUS20130108551A1Better addressMaximize MR contrast enhancementOrganic active ingredientsDispersion deliveryChain lengthImaging agent
Disclosed are carriers for drugs and / or MR imaging agents having a lipid bilayer shell comprising a phospholipid having two terminal alkyl chains, one being a short chain having a chain length of at most seven carbon atoms, the other being a long chain having a chain length of at least fifteen carbon atoms. The mixed long / short chain phospholipids serve to tune the release properties of the carrier. Preferred phospholipids are phosphatidylcholines.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Coating compositions for bitterness inhibition
InactiveUS7294347B2Mask bitternessImprove palatabilityPowder deliveryInksPH-sensitive polymersPh independent
The present invention discloses coating compositions with taste masking property, comprising a blend of pH sensitive polymers and optionally a pH independent polymer or a blend of the pH sensitive polymer and pH independent polymer used for taste masking of highly bitter drugs. The pH sensitive polymers used comprise the acid soluble polymers and the enteric polymers. The process for the preparation of taste masked pharmaceutical compositions of the bitter drugs comprising the said coating compositions is disclosed. The concomitant use of the polymers inhibits the release of the bitter drug at the pH of saliva. The said coating compositions deliver substantial amount of the bitter drug immediately with improved palatability.
Owner:COUNCIL OF SCI & IND RES
Slow/controlled release pellet composition containing ginkgo leaf extracts and preparation method thereof
InactiveCN101375869ASmall toxicityStable blood concentrationGranular deliveryGinkgophyta medical ingredientsSustained release pelletsHard Capsule
The invention belongs to the field sustained / controlled-release preparations, in particular to an oral sustained / controlled-release pellet combination containing ginkgo biloba extract and a preparation method. The oral sustained / controlled-release pellet combination is composed of (A) a core containing a pill; (B) an insulating coating layer; (C) a sustained-release coating layer; (D) and an enteric-coated coating layer. The invention is the traditional Chinese medicine multi-component sustained-release pellet combination which is taken once by 24 hours and the multi-unit sustained-release pellet combined preparation with the different drug release systems, the core containing the pill is prepared by adopting the extrusion pill rolling method, a novel sustained-release multi-layer coating technology and a fluidized bed are utilized for coating the sustained-release pellet, the rapid-release part and the sustained-release part of the coated pellet are mixedly filled into a hard capsule or pressed into a pellet tablet. The sustained-release pellet has stable coating process and good reproducibility, thereby being applicable to the industrial mass production; and the drug quality of the preparation is stable through the long-term storage. The in vitro release test shows that the multiple components of the traditional Chinese medicine can achieve the sustained-release role, the sustained-release preparation can significantly increase the transmembrane absorption and the stability of various effective active ingredients by oral drug administration, the curve of plasma drug concentration in vivo is smooth, and the design purpose of 24-hour sustained-release is achieved.
Owner:CHINA PHARM UNIV
Controlled release composition
ActiveUS20060177509A1Reduce the amount requiredIncrease release rateDigestive systemPill deliveryControl releaseMedicine
The present invention provides a controlled release composition showing release of an active ingredient (proton pump inhibitor) controlled in two or more steps at different release rates, which contains 1) a release-controlled part A capable of controlling release of the active ingredient to occur at a predetermined rate, 2) a release-controlled part B capable of controlling release of the active ingredient to occur at a predetermined rate lower than the release rate of the release-controlled part A, and where necessary, 3) a release-controlled part C capable of controlling release of the active ingredient to occur at a predetermined rate faster than the release rate of the release-controlled part B, wherein the release of the active ingredient from the release-controlled part B precedes the release of the active ingredient from the release-controlled part A (when release-controlled part C is contained, the release of the active ingredient from the release-controlled part C precedes the release of the active ingredient from the release-controlled part B).
Owner:TAKEDA PHARMA CO LTD
Coating compositions for bitterness inhibition
InactiveUS20050281874A1Improve protectionMask bitternessPowder deliveryInksPH-sensitive polymersBitter taste
The present invention discloses coating compositions with taste masking property, comprising a blend of pH sensitive polymers and optionally a pH independent polymer or a blend of the pH sensitive polymer and pH independent polymer used for taste masking of highly bitter drugs. The pH sensitive polymers used comprise the acid soluble polymers and the enteric polymers. The process for the preparation of taste masked pharmaceutical compositions of the bitter drugs comprising the said coating compositions is disclosed. The concomitant use of the polymers inhibits the release of the bitter drug at the pH of saliva. The said coating compositions deliver substantial amount of the bitter drug immediately with improved palatability.
Owner:COUNCIL OF SCI & IND RES
Pharmaceutical composition for improving palatability of drugs and process for preparation thereof
InactiveUS20060141053A1Reduce the amount requiredSuppress bitternessBiocidePowder deliveryLipid formationGastric ph
The present invention discloses compositions, comprising a lipid-polymer matrix to mask the bitter or unpleasant taste of the medicament. The lipid or a blend of lipids, are used in combination with the pH dependent polymer where the said polymer is acid soluble or swellable The process for the preparation of taste masked pharmaceutical compositions of the bitter drugs comprising the said lipid-polymer compositions are disclosed. The concomitant use of the acid soluble polymer, which remains collapsed at the pH of saliva, inhibits the release of drug at that pH and hence they further help in bitterness inhibition. The said compositions deliver substantial amount of the bitter drug immediately at the gastric pH with improved palatability.
Owner:COUNCIL OF SCI & IND RES
Mussel mucoprotein gel for wound repair, and preparation method and application thereof
InactiveCN104645320ABroaden applicationGood adhesionPeptide/protein ingredientsAerosol deliveryIrritationPreservative
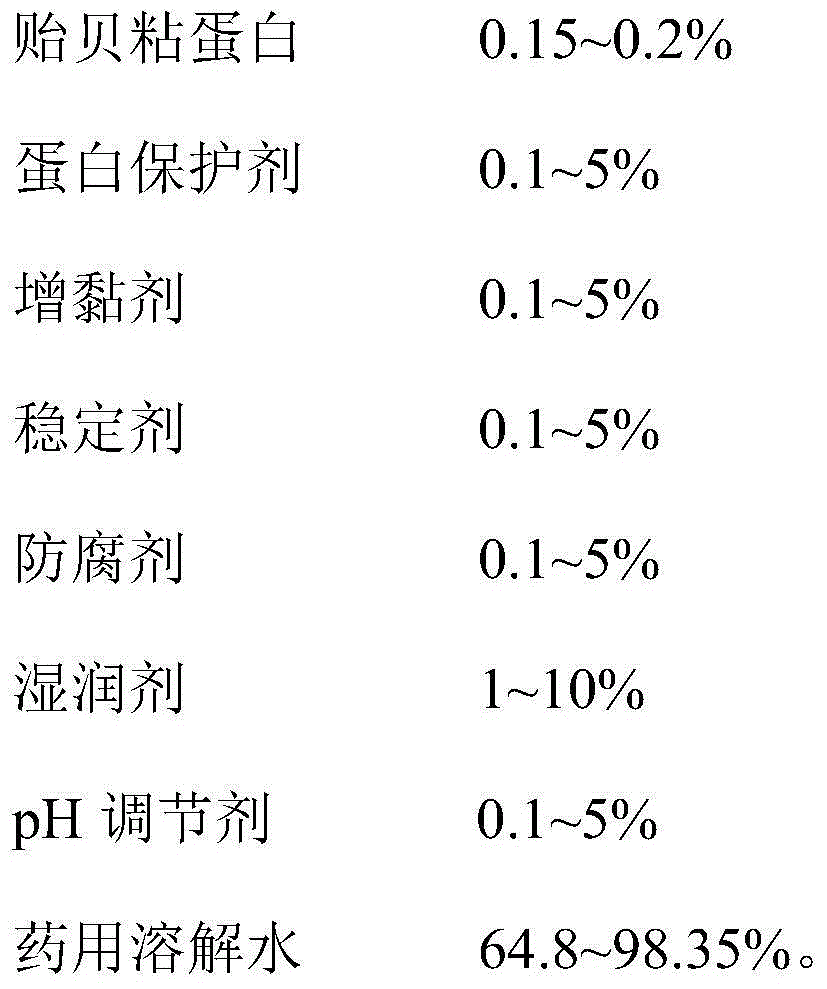
The invention provides a mussel mucoprotein gel for wound repair, and a preparation method and an application thereof. The mussel mucoprotein gel for wound repair comprises the following raw materials in percentage by weight: 0.15-0.2% of mussel mucoprotein, 0.1-5% of protein protector, 0.1-5% of tackifier, 0.1-5% of stabilizer, 0.1-5% of preservative, 1-10% of wetting agent, 0.1-5% of pH (potential of hydrogen), and 64.8-98.35% of medical dissolving water. With Carbomer as a substrate, the mussel mucoprotein gel has good extending property, is easy to coat and adhere on a skin, has no irritation to skin and mucosa and can absorb tissue penetrating fluid, so secretions can be exhausted conveniently; the gel is not greasy, the medicines are released fast, and the coupling effect with skin tissues is excellent.
Owner:NANJING UNIV OF AERONAUTICS & ASTRONAUTICS
Plant mass cement for preparing hollow capsule, as well as hollow capsule of plant mass cement
ActiveCN103933011AGood water solubilityBreak the dense structurePharmaceutical non-active ingredientsCapsule deliveryMethyl celluloseAqueous solution
The invention relates to plant mass cement for manufacturing hollow capsules of foods, medicines and the like, and a hollow capsule which is prepared from the plant mass cement and can be quickly disintegrated. The plant mass cement for preparing the hollow capsule is prepared by mixing the following components in percentage by weight: 12-25 percent of hydroxypropyl methyl cellulose, 0.1-9 percent of hydroxypropyl starch or acidified hydroxypropyl starch, 0.4-2 percent of a gelatinizing agent and the balance of water, wherein the aqueous solution of the 2wt% hydroxypropyl starch at 20 DEG C has a viscosity of 3-7mpa.s; the substitution degree DS of the hydroxypropyl starch or acidified hydroxypropyl starch is in a range of 0.004-0.55. The plant mass cement is prepared by adopting a conventional capsule preparation process for preparing the hollow capsule can be used for preparing a hydroxypropyl methyl cellulose hollow capsule which can be quickly disintegrated.
Owner:江苏崇尚生物科技有限公司
Zwitter-ion-contained multiple acid-sensitive anti-tumor drug-loading micelle and preparation method and application thereof
InactiveCN105968373AHigh sensitivity and responsivenessImprove anti-tumor efficiencyOrganic active ingredientsPharmaceutical non-active ingredientsIonDialysis method
The invention discloses a zwitter-ion-contained multiple acid-sensitive anti-tumor drug-loading micelle, and a preparation method and an application thereof, and belongs to the field of biological materials. The zwitter-ion-contained multiple acid-sensitive anti-tumor drug-loading micelle comprises a zwitter-ion hydrophilic shell and a lyophobic core containing two acid-sensitive bonds. The method includes the steps: separating methanol from hydroxyethyl acrylate and 2,2-dimethoxypropane and synthesizing 2,2-di(acryloyloxy-1-oxethyl) propane by an alcohol exchange method; connecting open-loop beta-propiolactone with 2-(dimethylamino) ethyl methacrylate to synthesize carboxybetaine methacrylate; obtaining a carboxybetaine methacrylate monomer polymer by an atom transfer free radical polymerization method; synthesizing triblock amphiphilic polymers by the atom transfer free radical polymerization method and a Michael addition one-pot method, and preparing the drug-loading nano-micelle by an ultrasonic drop method and a dialysis method. By aid of the drug-loading micelle, anti-tumor effects of chemotherapeutic drugs can be improved.
Owner:SICHUAN UNIV
Tumor targeted therapy sustained release preparation and preparation method thereof
InactiveCN108273058AResponsive to near-infrared photothermal stimulationHigh drug loadingOrganic active ingredientsHeavy metal active ingredientsTumor targetPolyethylene glycol
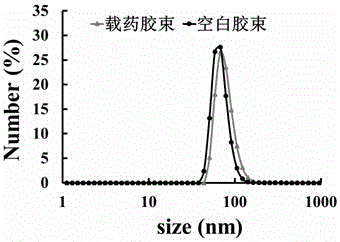
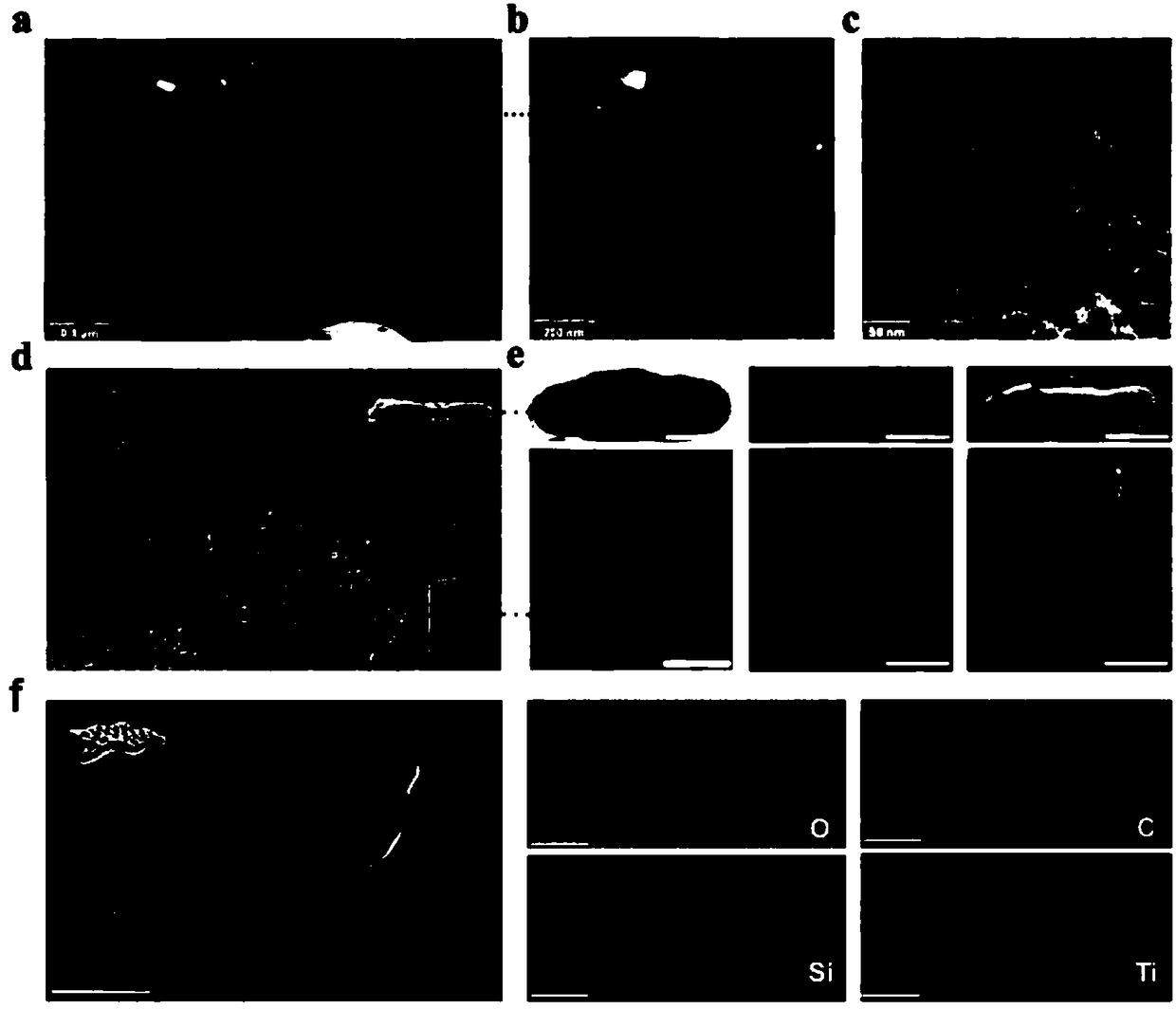
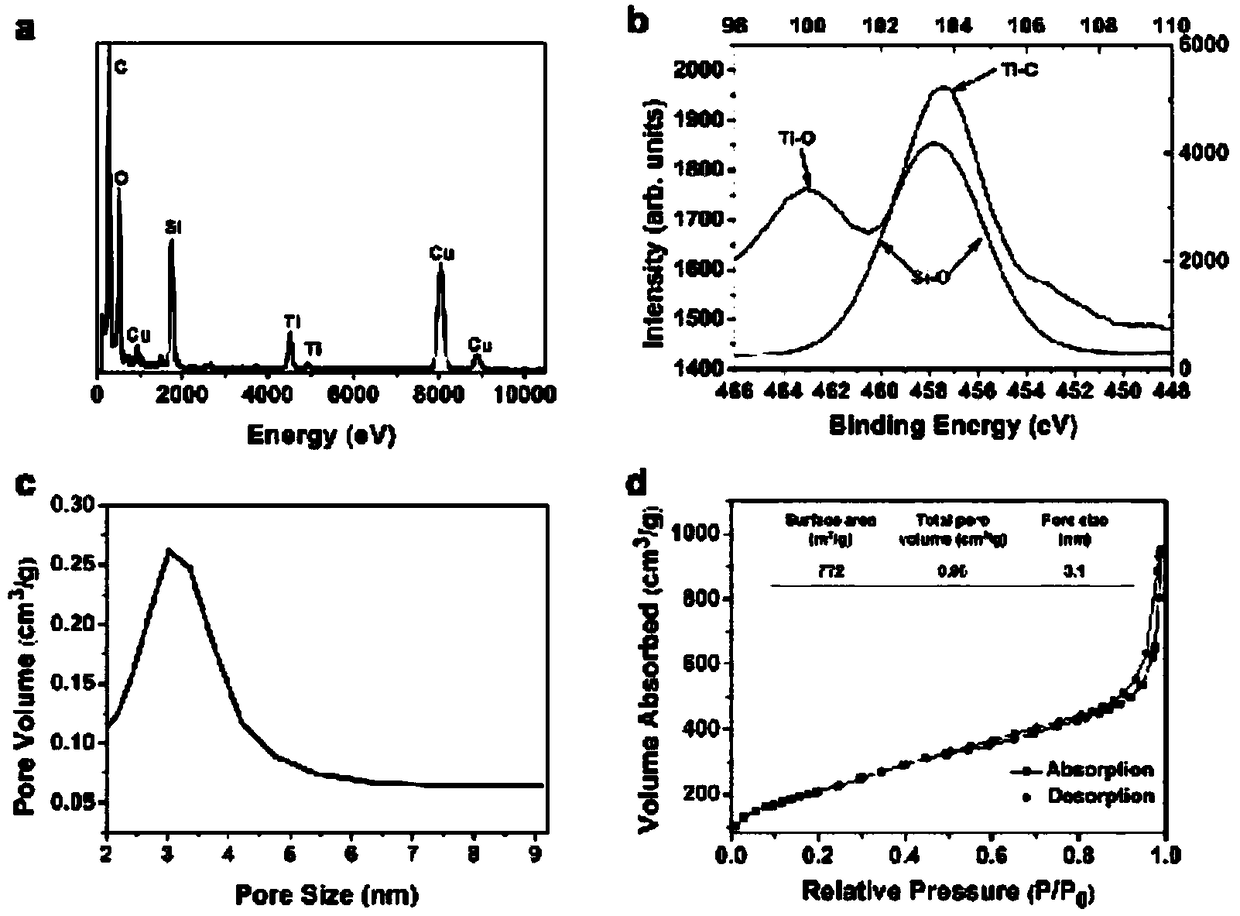
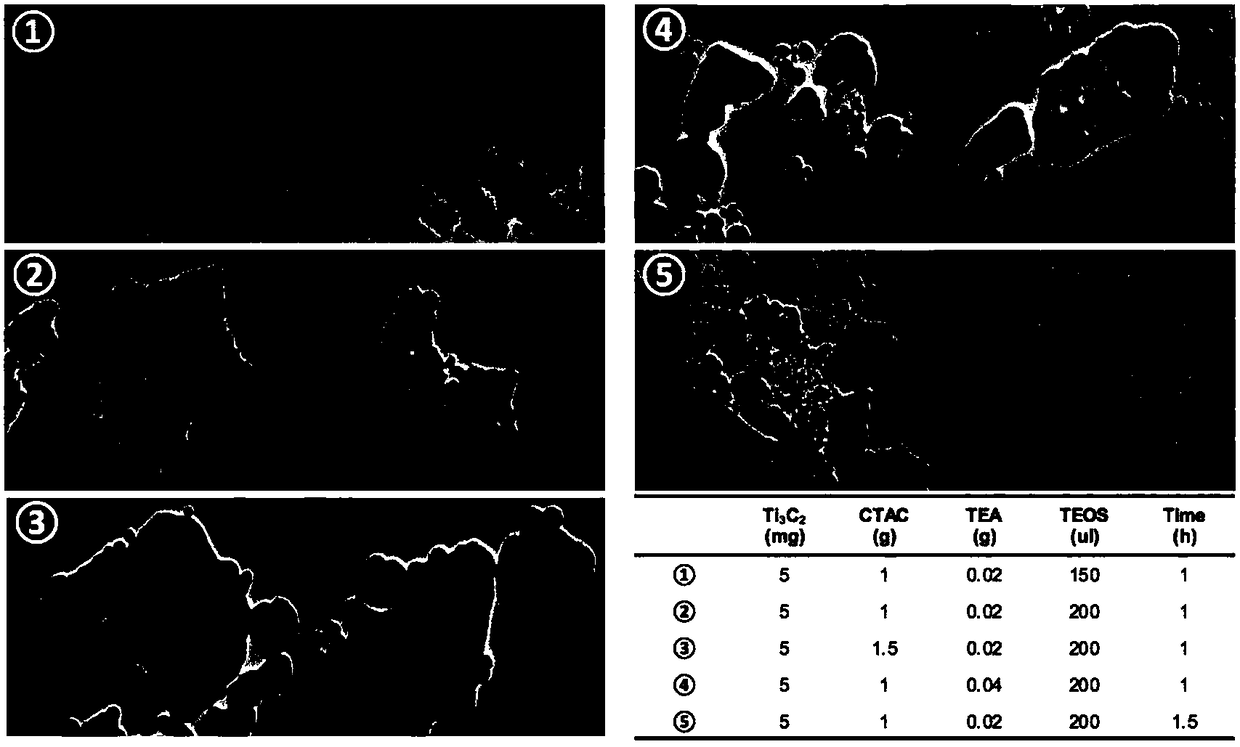
The invention provides a preparation method of a tumor targeted therapy sustained release preparation. The preparation method comprises the following steps: (1) mixing titanium powder, aluminum powderand graphite powder, ball-milling and pressing, and performing high-temperature sintering under the condition of importing argon gas, thereby obtaining a Ti3AlC2 ceramic material; (2) crushing the material obtained in the step (1) into powder, placing in hydrofluoric acid to react, centrifugally washing, placing in a tetrapropylammonium hydroxide aqueous solution to react, and centrifugally washing to obtain a Ti3C2MXenes material; (3) dropping an aqueous solution of the Ti3C2MXenes material in a mixed aqueous solution of CTAC and TEA to react; and then adding TEOS, reacting under 80 DEG C, and centrifugally washing to obtain an MXene nano-sheet covered by mesoporous silica; and (4), performing polyethylene glycol surface modification on a material obtained in the step (3), covalently combining by using RGD polypeptide, and loading a medicine to obtain the preparation. The tumor targeted therapy sustained release preparation provided by the invention can realize the targeted therapy on tumor and acquire a good tumor-inhibiting effect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY +1
Albumin binding type anti-tumor drug-maleimide molecule prodrug
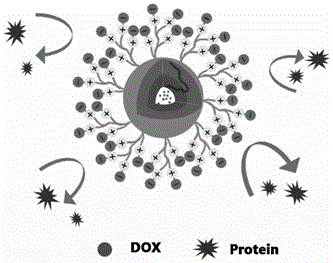
ActiveCN108187063AExtended stayRapid drug releaseOrganic active ingredientsOrganic chemistryDocetaxelTumor cells
The invention belongs to the technical field of medicines, and relates to a redox sensitive albumin binding prodrug with a series of tumor tissue specific responses. The albumin binding prodrug is ananti-tumor drug-maleimide molecule prodrug particularly including three docetaxel-maleimide small molecule prodrugs; by utilizing a maleimide ring in a prodrug structure as a target head of plasma albumin 34-bit free sulfydryl in a binding body, so that after a drug enters into a body through intravenous injection, the drug is quickly and specifically bound with albumin to form an albumin-drug composition; therefore, the drug stability is enhanced, the circulation time of the drug in the boy is obviously prolonged, and accumulation of the drug on tumor tissues is realized by utilizing an EPR (Enhanced Permeability and Retention) effect. In addition, in the prodrug structure, by using a redox sensitive key as a binding key, quick release of the drug in tumor cells is promoted, a toxic effect of the drug on normal cells also can be alleviated, the prodrug has high selectivity, the aims of increasing an effect and reducing the toxicity of docetaxel are fulfilled, and a greater market application prospect is achieved.
Owner:SHENYANG PHARMA UNIVERSITY
Tetrodotoxin quick-release pellet preparation and its preparation method and use
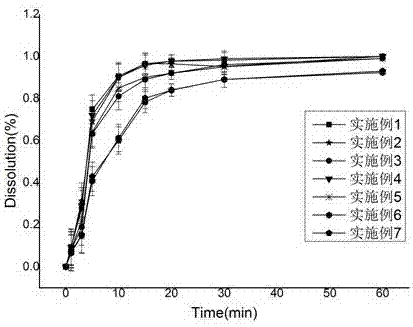
ActiveCN106063780AHigh rate of drug useGood content uniformityOrganic active ingredientsNervous disorderDrug release rateOral medication
The invention discloses a tetrodotoxin quick-release pellet preparation and its preparation method and use and relates to the field of a medicinal preparation technology and use. The tetrodotoxin quick-release pellet preparation is suitable for oral administration and utilizes a blank pellet core as a carrier and a tetrodotoxin aqueous solution containing a cosolvent and a binder as a drug feeding solution. A weight ratio of the tetrodotoxin to the pellet core is 0.0025%-0.3% and an oral dosage of the tetrodotoxin is less than or equal to 300 micrograms. The tetrodotoxin quick-release pellet preparation has the advantages of high superimpose rate, good content uniformity, fast drug release rate and fast pain easing. The tetrodotoxin quick-release pellet preparation has the characteristics of good clinical use compliance and high safety. The blank pellet core fluidized bed-based drug superimpose method is suitable for preparation of a very low specification tetrodotoxin oral preparation.
Owner:XIAMEN ZHAOYANG BIOLOGICAL ENG
An wind-expelling ointment and method for preparing same
ActiveCN1879833AEasy to carrySafe to carryHydroxy compound active ingredientsAntipyreticCentipedeMyrrh
The invention relates to a rheumatalgia-relieving plaster comprising the following constituents (by weight ratio): radix aconiti kusnezoffii 10-120, Chinese ephedra 10-120, asarum herb 10-120, notopterygium root 10-120, spicebush root 10-120, dahurian angelica root 10-120, galange oil 10-120, levisticum 10-120, Clematis chinensis 10-120, radix aconiti 1-30, Cinnamomum cassia 10-100, safflower 10-100, peach kernel 10-100, sappan wood 1-30, red peony root 10-100, frankincense 1-20, myrrh 1-20, Chinese angelica root 10-100, centipede 1-30, snake slough 1-30, futokadsura stem 10-100, achyranthes and cyathula root 10-100, dipsacus root 1-30, cortex acanthopanacis 1-30, knoxia root 10-100, musk ketone 0.01-0.5, dragon's blood resin 1-30, oil of Chinese cinnamon 1-30, borneol 0-120, menthol 0-120, hot pepper 10-200, clove sweet basil oil 0-100, Laurocapram 0-30, camphor 0-120, and methyl salicylate 0-100.
Owner:桂林华润天和药业有限公司
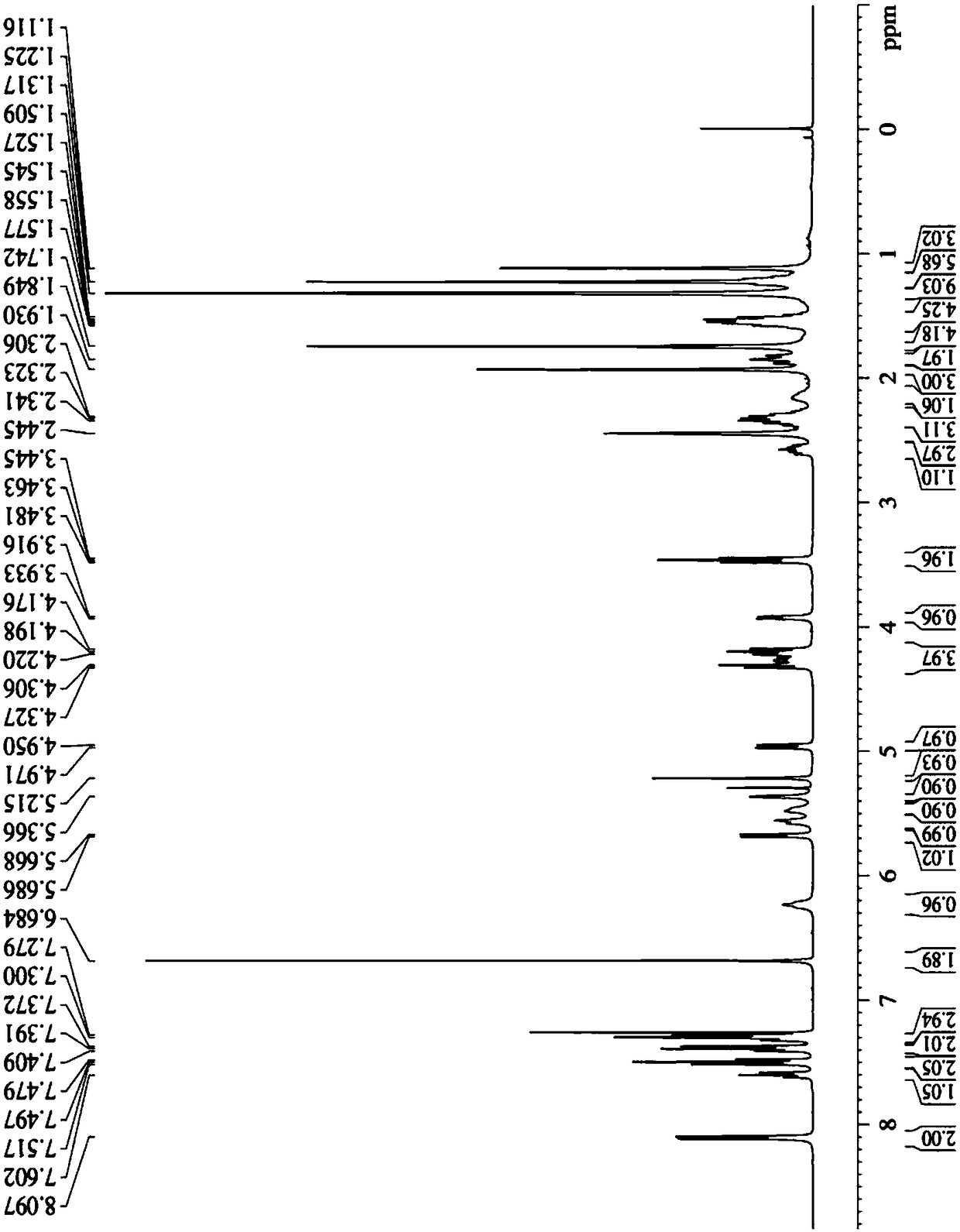
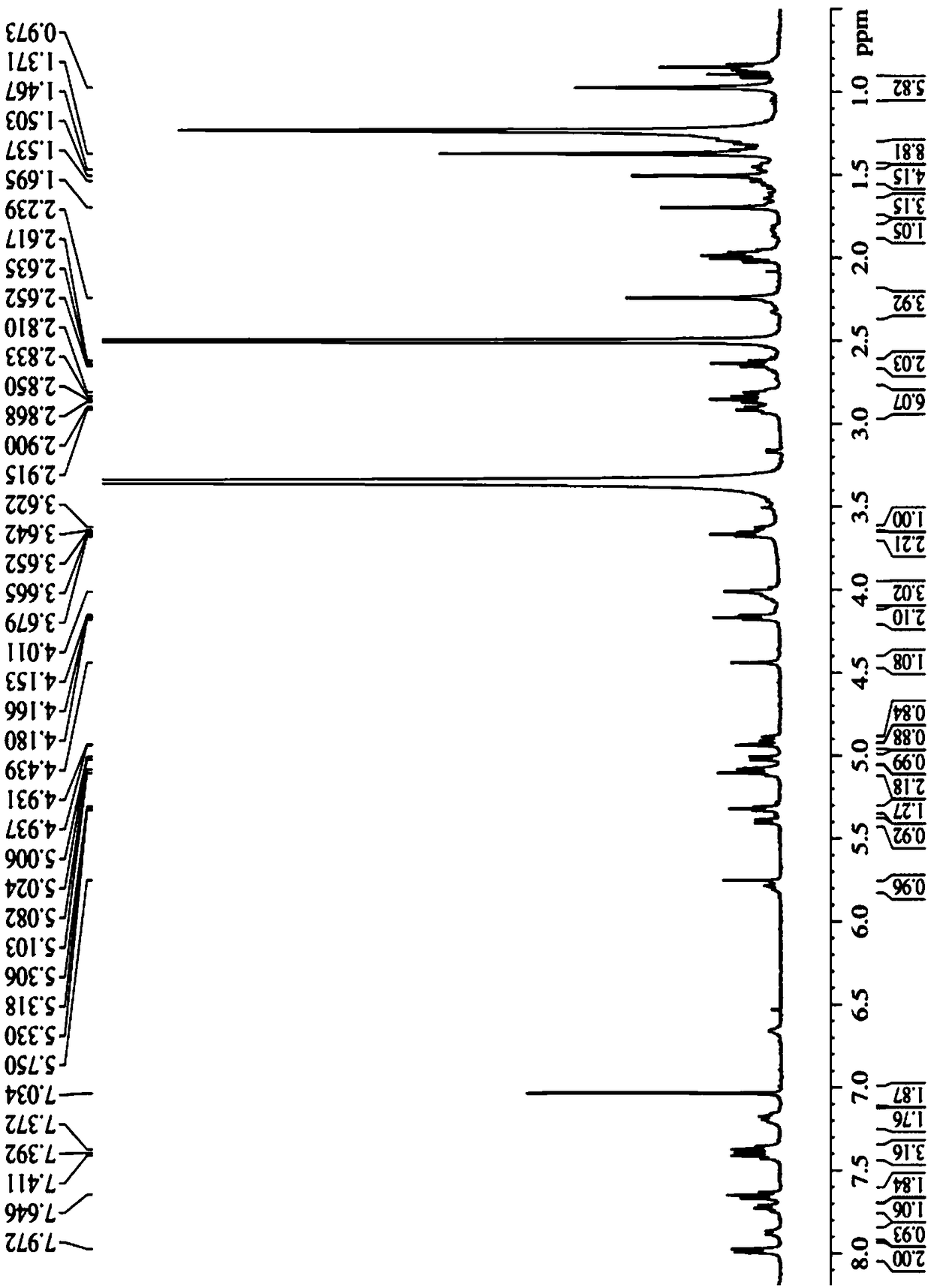
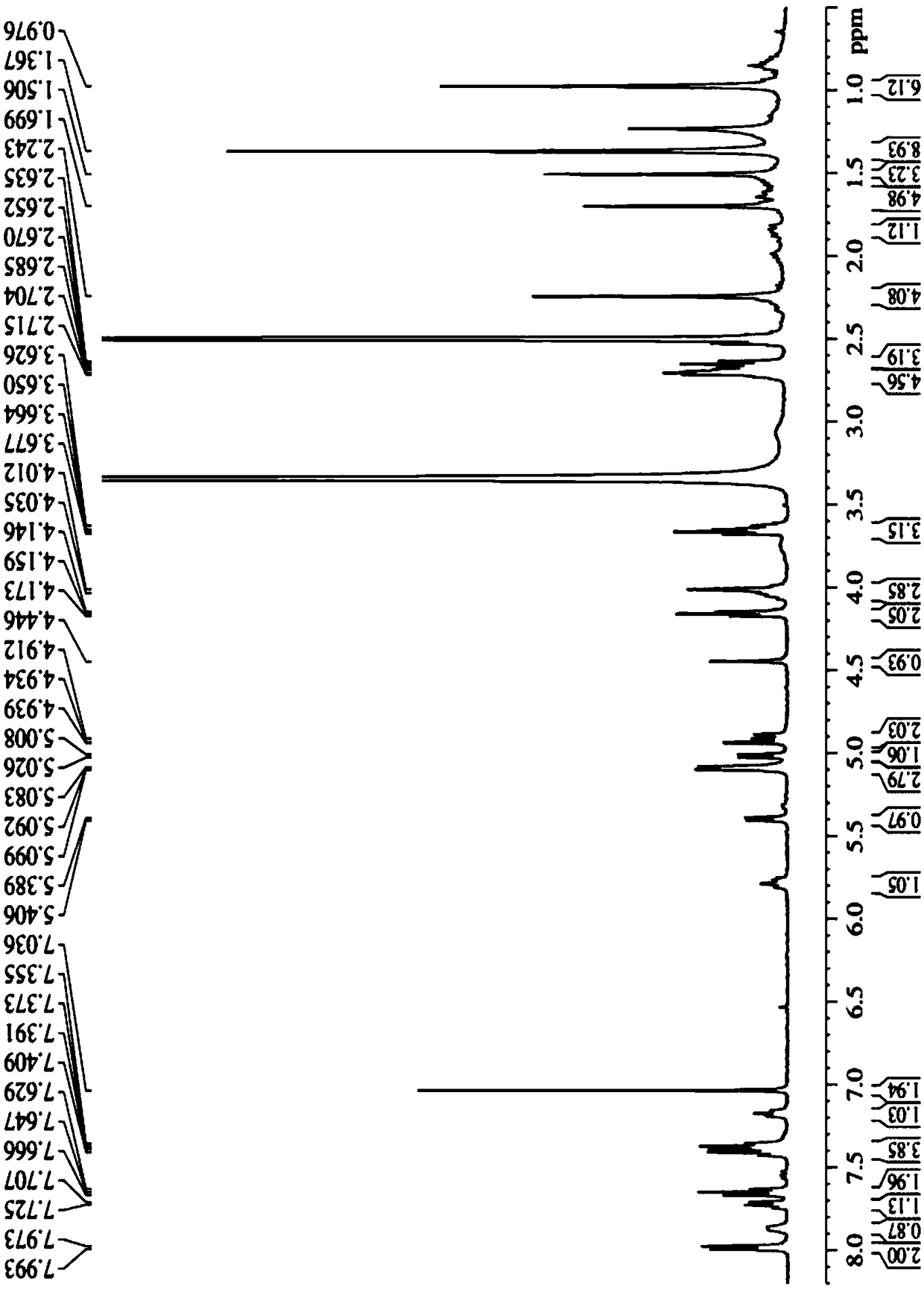
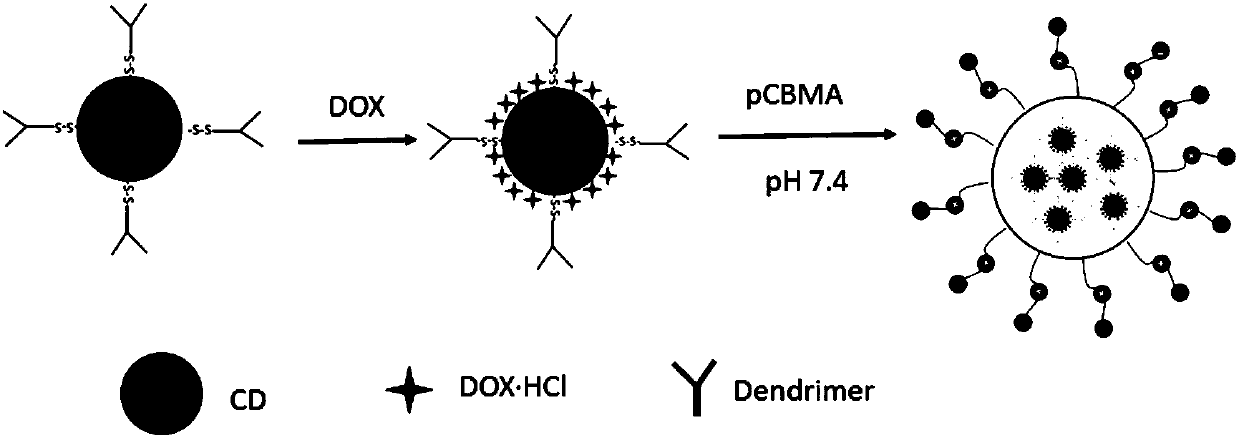
Tumor microenvironment response nanoparticle based on peptides dendrimer modified fluorescence carbon dots and preparation method of tumor microenvironment response nanoparticle
InactiveCN107802840AEffectively play the role of medicineReduced scavengingOrganic active ingredientsInorganic non-active ingredientsHigh concentrationDendrimer
The invention discloses a tumor microenvironment response nanoparticle based on peptides dendrimer modified fluorescence carbon dots. A preparation method of the tumor microenvironment response nanoparticle comprises the following steps: (1) preparation of nanometer fluorescence carbon dots; (2) surface sulfhydrylation modification of the fluorescence carbon dots; (3) preparation of second-generation peptides dendrimer grafted by arginine-lysine; (4) surface modification of the second-generation peptides dendrimer with fluorescence carbon dots; (5) preparation of a zwitterionic polymer polycarboxylate betaine methacrylate; (6) preparation of drug-loading carbon dots; (7) preparation of a drug-loading nanoparticle. The drug-loading nanoparticle prepared with the method has the specific fluorescent property of the carbon dots and dual high-sensitive responsiveness for an acid environment of a tumor site and high-concentration glutathione, high-selectivity rapid drug release in tumor cells can be achieved, and the drug-loading nanoparticle is high in anti-tumor efficiency and good in safety; in addition, integration of diagnosis and treatment of tumors is expected to achieve.
Owner:SICHUAN UNIV
Oral delivery of proteins and peptides
InactiveUS20100303901A1Improve bioavailabilityRapid drug releasePeptide/protein ingredientsCalcitoninsPeptide drugFast release
Enteric coated capsules or tablets for oral delivery of a protein, polypeptide or peptide drug, in particular for oral delivery of insulin, are provided, comprising microparticles of the protein, polypeptide or peptide drug, microparticles of a protease inhibitor and, optionally, microparticles of an absorption enhancer. The protease inhibitor and the absorption enhancer may be together in the same microparticles. The microparticles of each component are embedded in an enteric polymer matrix. The enteric coated tablet or capsule of the invention enables fast release of the protein, polypeptide or peptide drug at different times at desired loci in the gastrointestinal tract
Owner:TECHNION RES & DEV FOUND LTD
Pharmaceutical tablet compositions containing irbesartan
InactiveUS20090030052A1Rapid and complete drug releaseMaintain good propertiesBiocidePill deliveryIrbesartanLactose
A pharmaceutical tablet composition comprising irbesartan and lactose, said composition being essentially free of surfactant.
Owner:ALEMBIC LTD
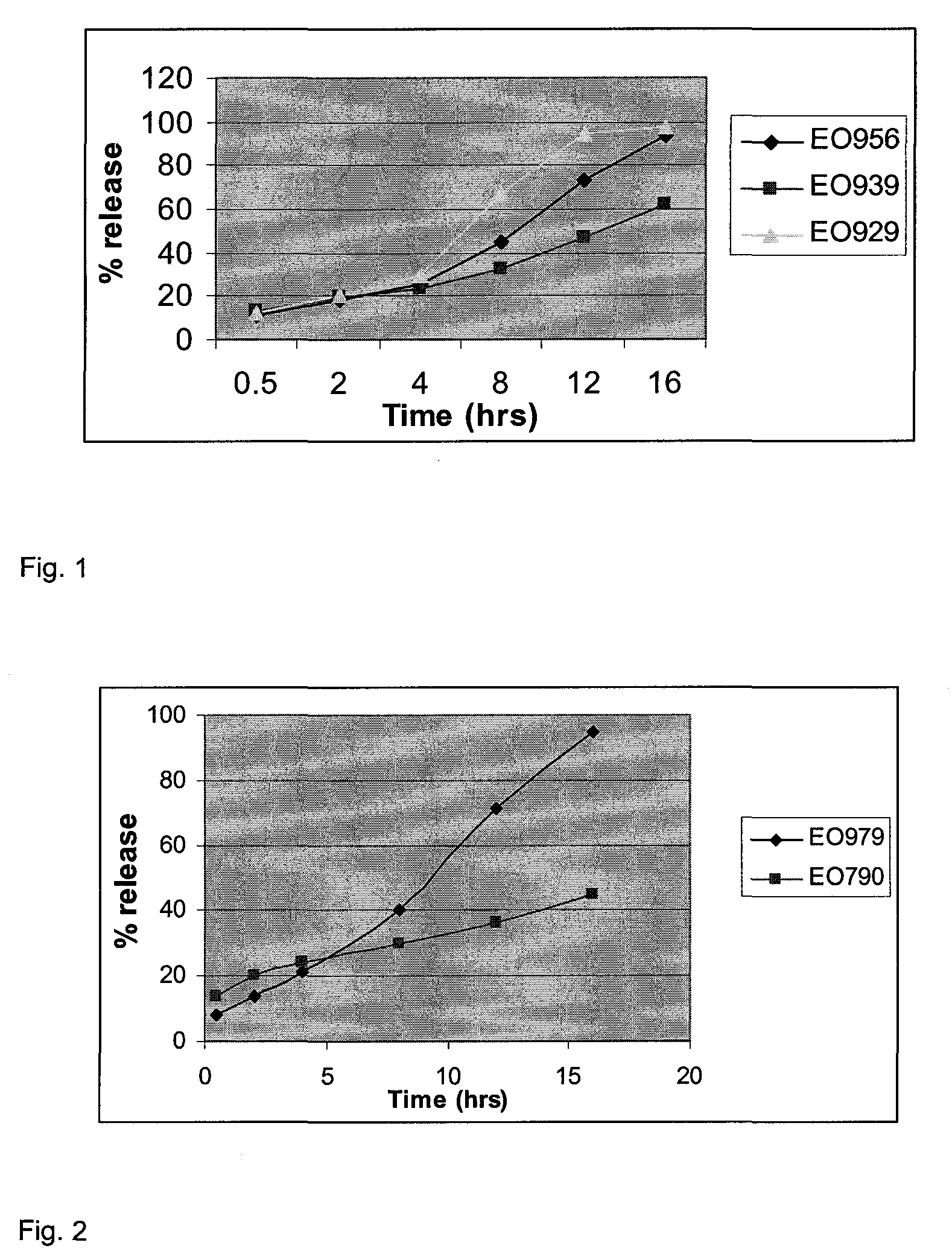
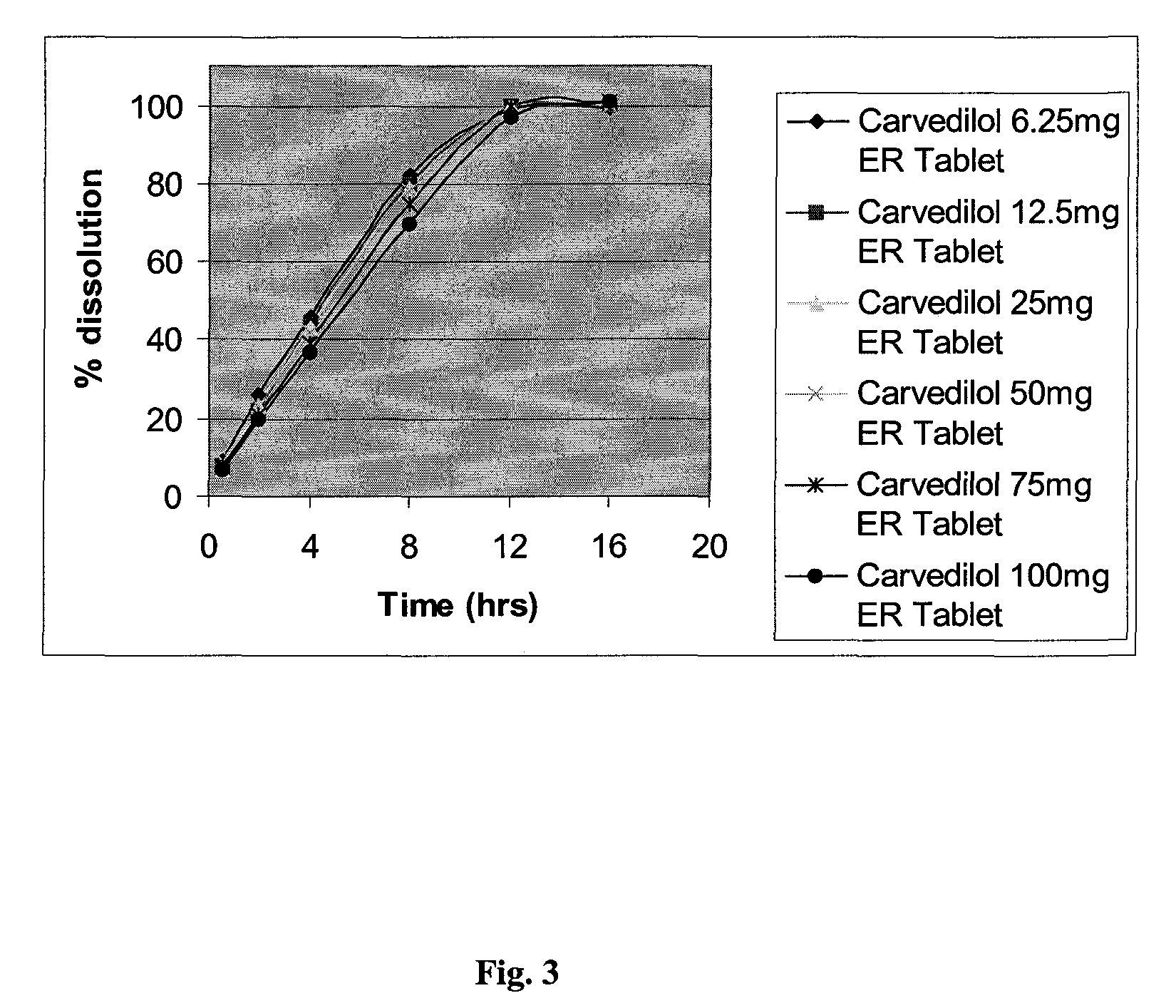
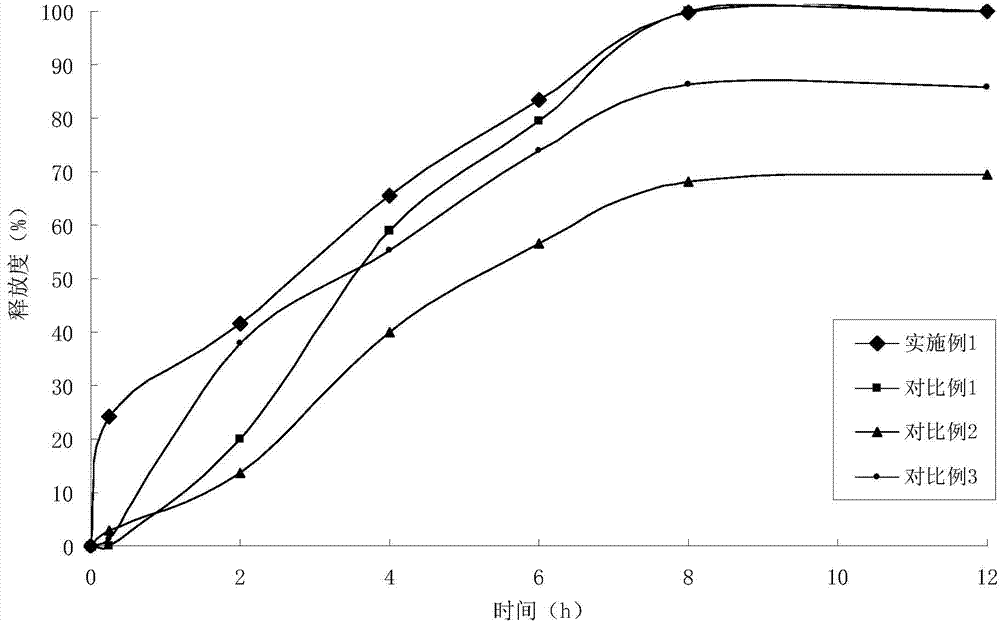
Extended release formulations of carvedilol
InactiveUS20080138404A1Dissolve fastEnhancing rate and extent of releaseBiocidePill deliveryHydrophilic polymersCarvedilol
An improved controlled release dosage form for once-daily administration of carvedilol is described. The controlled release dosage form comprises a therapeutically effective amount of carvedilol and / or a pharmaceutically acceptable salt thereof; one or more hydrophilic polymers; one or more pharmaceutically acceptable excipients; and a polyoxyalkylene block copolymer, a solid dispersion of carvedilol and an extrusion material or a combination of a polyoxyalkylene block copolymer, a solid dispersion of carvedilol and an extrusion material.
Owner:BIOVAIL LAB INT SRL
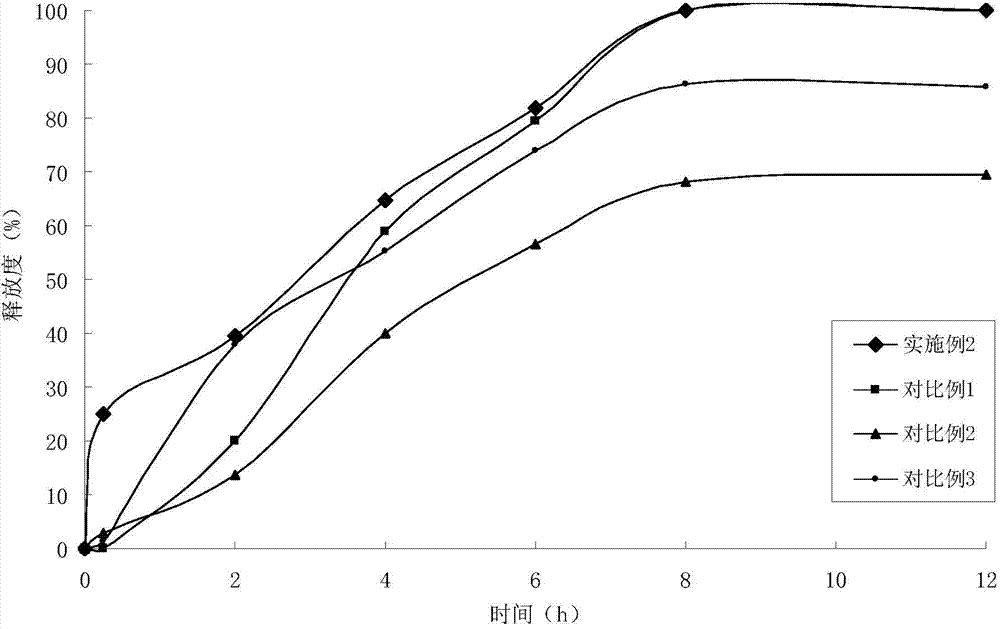
Felodipine sustained-release tablet and preparation technology thereof
ActiveCN104758266ARapid drug releaseStable drug releaseOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsSustained Release Tablet
The invention discloses a felodipine sustained-release tablet and a preparation technology thereof. According to the preparation technology of the felodipine sustained-release tablet, fast-release solid dispersions, sustained-release micro-pills and pharmaceutically acceptable accessories are mixed and the mixture is compressed to obtain the preparation. The fast-release solid dispersions comprise felodipine, copovidone and polacrilin potassium; the sustained-release micro pills are obtained by coating the fast-release solid dispersions with the ethyl cellulose film with a pore-forming agent. The sustained-release tablet disclosed by the invention has the advantages of fast early-stage releasing speed and slow and stable later-stage releasing speed, so that the drug can be completely released and an effective blood drug concentration can be maintained for a long time; the felodipine sustained-release tablet is high in bioavailability, simple in preparation technology and applicable to industrial mass production.
Owner:CHANGZHOU NO 4 PHARMA FACTORY +1
Aqueous sustained-release drug delivery system for highly water-soluble electrolytic drugs
InactiveUS20060018972A1Lower drug concentrationReduce molecular weightPowder deliveryPharmaceutical non-active ingredientsElectrolysisIon exchange
The present invention relates to liquid sustained release suspension dosage forms comprising ionized forms of water-soluble drugs. In particular, the invention encompasses a liquid form controlled release drug composition comprising a dispersed phase comprising an ion-exchange matrix drug complex comprising a pharmaceutically acceptable ion-exchange matrix and a water-soluble electrolytic drug associated with the ion-exchange matrix, wherein the surface charge of the ion-exchange matrix is opposite that of the electrolytic drug wherein the dispersed phase further comprises a non-electrolytic, soluble component having low molecular weight and a diffusion controlling membrane and a dispersion medium substantially free of diffusible counterions, further comprising an excipient capable of associating with water and impeding water activity such that drug dissolution is inhibited prior to administration. The invention also provides methods for preparing such compositions and methods of treatment.
Owner:UPM PHARMA
Donepezil cyclodextrin inclusion compound and oral instant membrane containing same
ActiveCN107375945AGreat tasteDisintegrate fastNervous disorderPharmaceutical non-active ingredientsDonepezilAdditive ingredient
The invention relates to a taste-masked donepezil cyclodextrin inclusion compound and an oral instant membrane containing the same, belonging to the technical field of medicine. The oral instant membrane comprises the donepezil cyclodextrin inclusion compound, a film-forming material, a plasticizer, a disintegrating agent and other excipients. Specifically, the oral instant membrane is prepared by including donepezil free alkali in cyclodextrin or a derivative thereof, then dissolving the excipients such as the film-forming material in distilled water, adding an appropriate amount of the donepezil cyclodextrin inclusion compound and preparing a homogeneous solution. The donepezil cyclodextrin inclusion compound provided by the invention completely masks the bad taste of donepezil; the oral instant membrane prepared from the donepezil cyclodextrin inclusion compound has good taste, is rapid in drug release and greatly improves patient compliance; moreover, the taste-masked donepezil cyclodextrin inclusion compound can be applied to other oral dosage forms such as tablets and solutions.
Owner:SHENYANG PHARMA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com