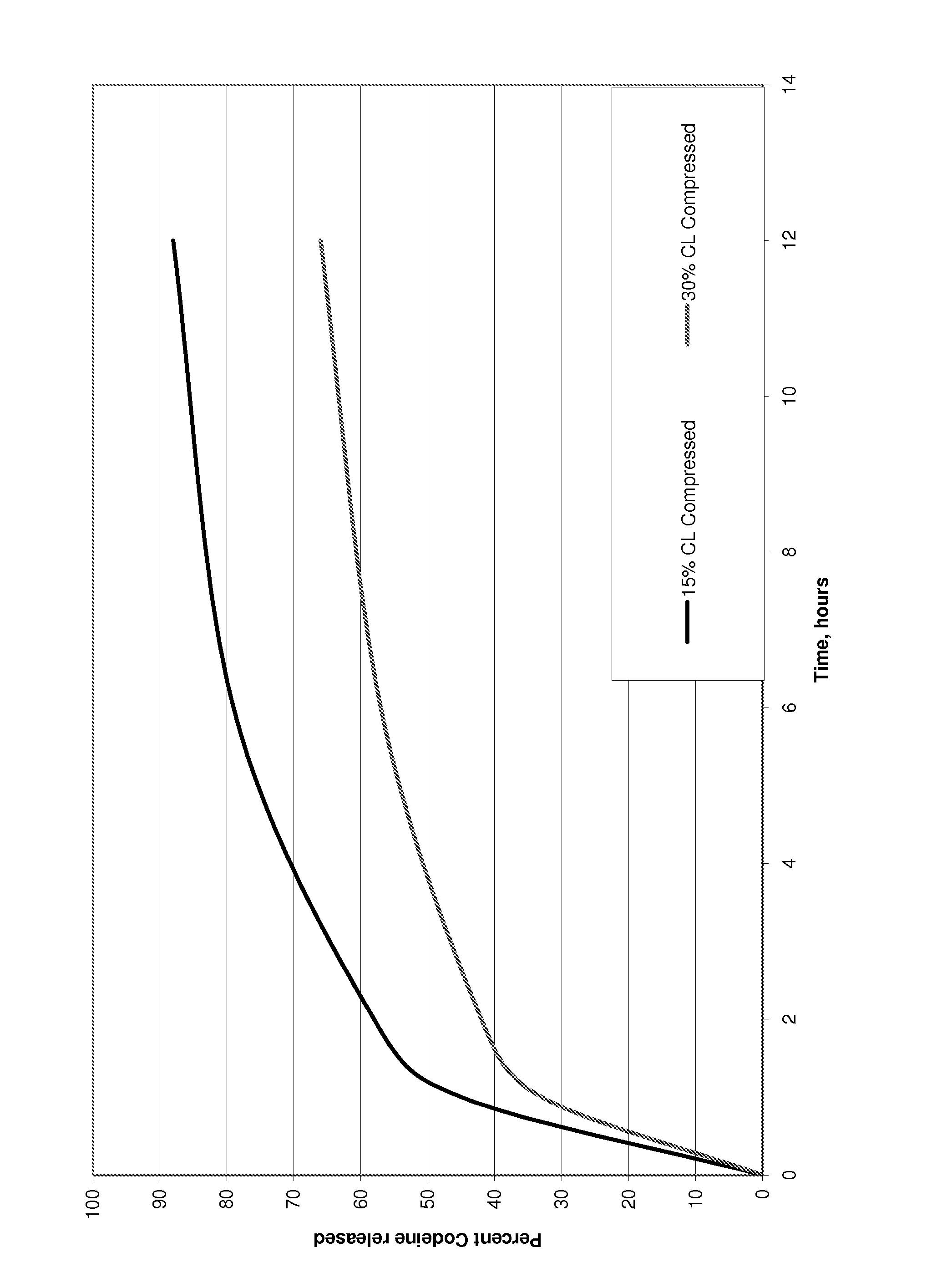
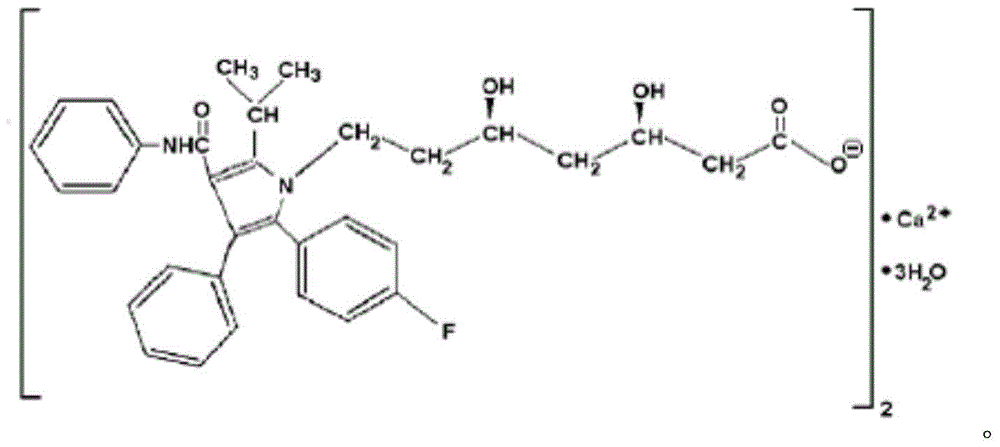
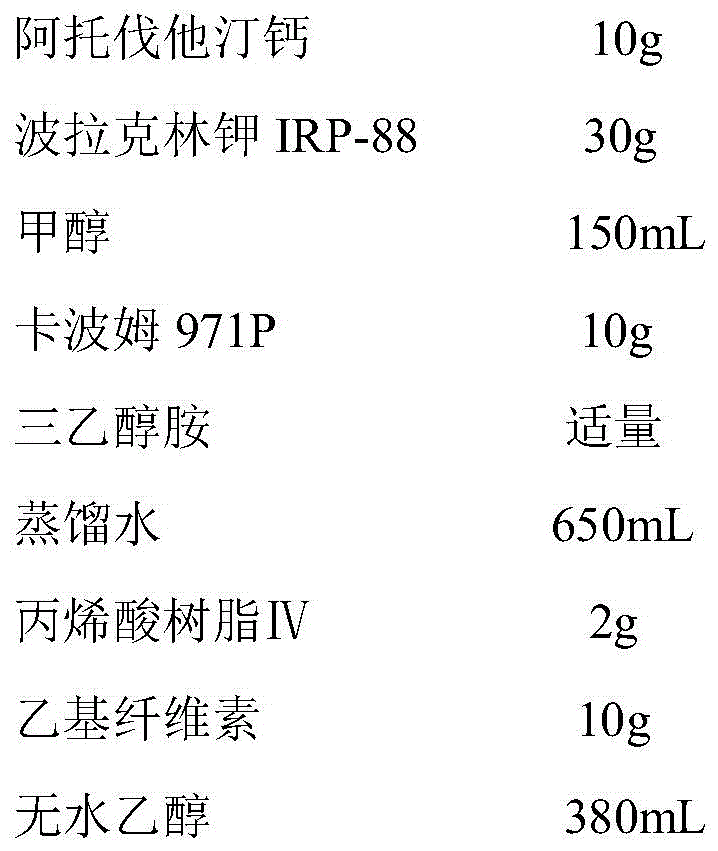
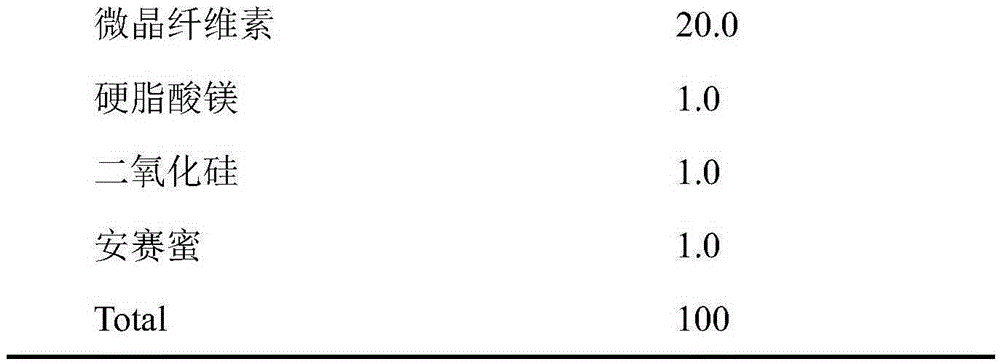Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "POLACRILIN POTASSIUM" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
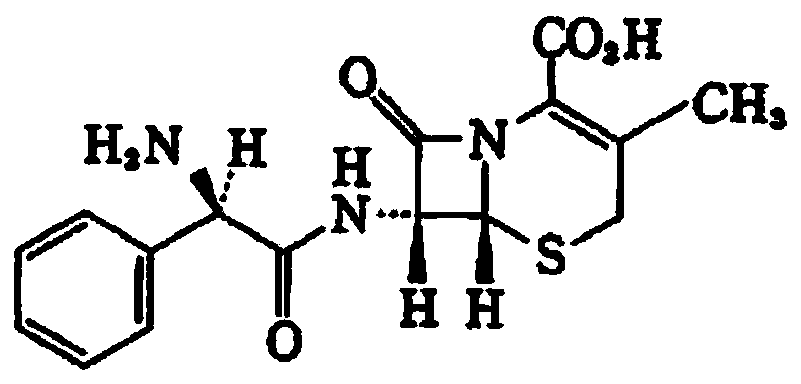
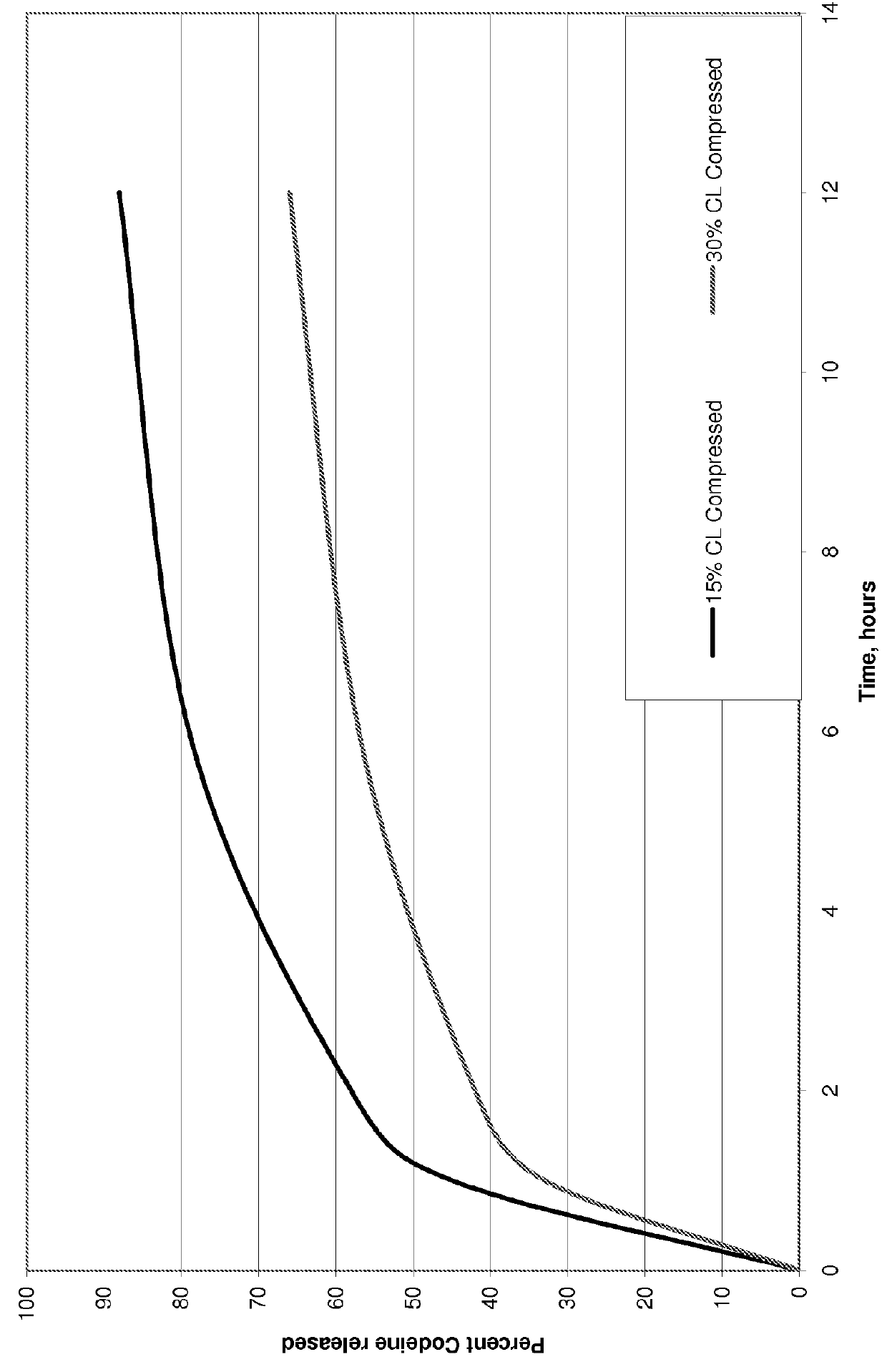
Polacrilin potassium is an ion exchange resin used in oral pharmaceutical formulations as a tablet disintegrant. It is a weakly acidic cation exchange resin. Chemically, it is a partial potassium salt of a copolymer of methacrylic acid with divinyl benzene.
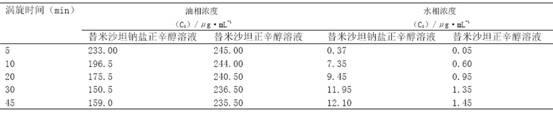
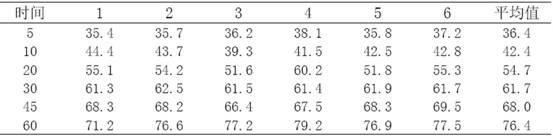
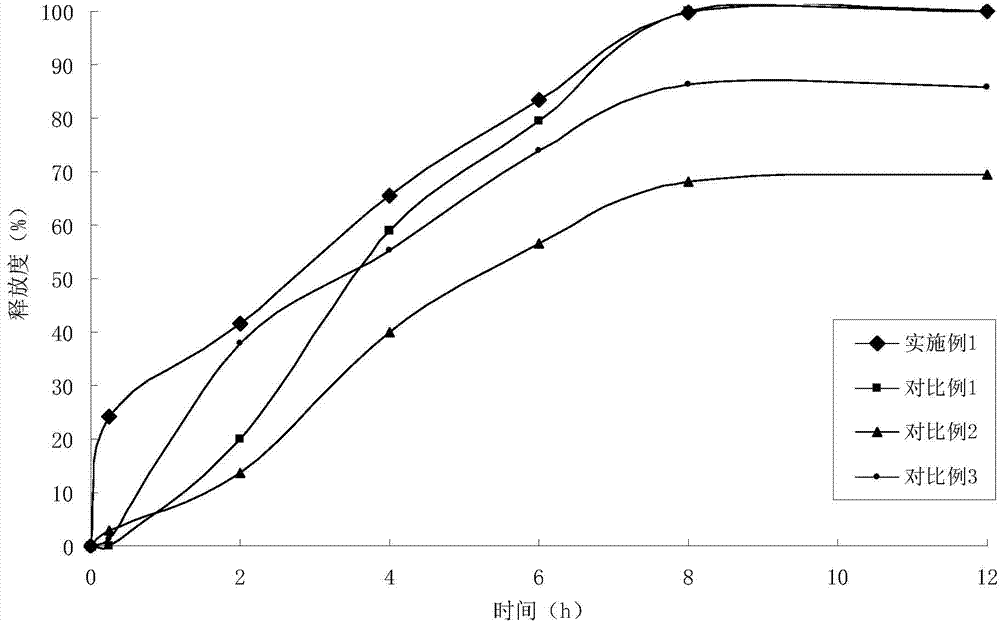
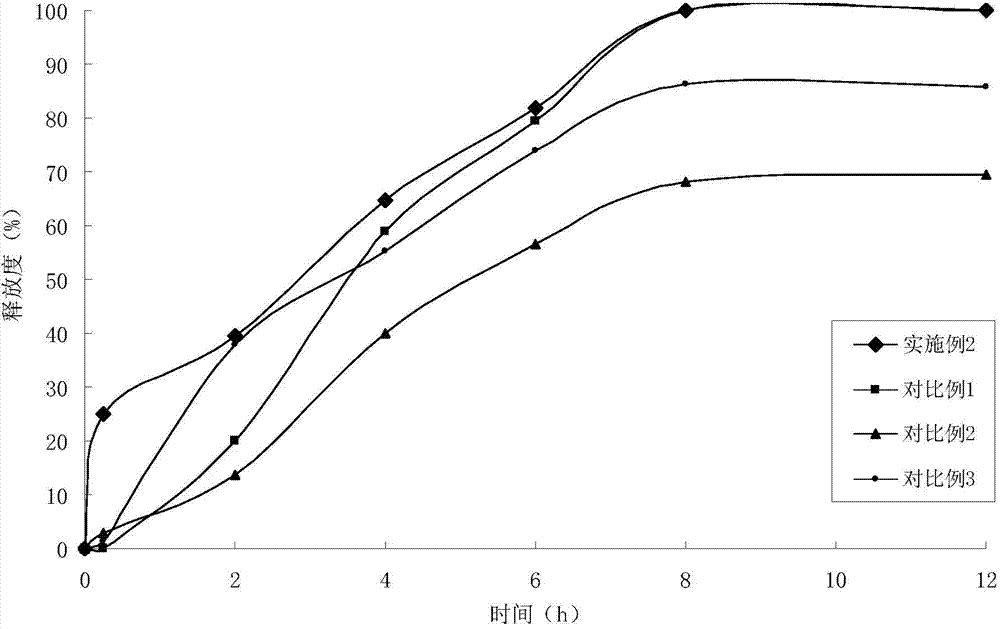
Telmisartan medicinal composition, telmisartan medicinal composition tablets and preparation method for telmisartan medicinal composition tablets
ActiveCN102526037AGood water solubilityHas a diuretic effectOrganic chemistryHydroxy compound active ingredientsTherapeutic effectMagnesium stearate
The invention provides a telmisartan medicinal composition, telmisartan medicinal composition tablets and a preparation method for the telmisartan medicinal composition tablets. The composition consists of the following components: telmisartan sodium salt, sorbitol, polacrilin potassium, anhydrous calcium phosphate, lactose, microcrystalline cellulose and magnesium stearate. The invention provides a preparation method for the telmisartan sodium salt, reaction conditions are mild, few side reactions are performed, and the generated sodium salt has higher water solubility; meanwhile, the sorbitol in the medicine has a diuresis effect, and has a synergistic antihypertensive effect with telmisartan in human bodies, so that the therapeutic effect of the product is strengthened; and after the telmisartan is taken, the urinary albumin excretion rate (UAER) of patients with early diabetic nephropathy is remarkably reduced, so the telmisartan has a kidney protection effect.
Owner:CHONGQING CONQUER PHARML
Pantoprazole sodium-containing enteric-coated tablet and preparation method thereof
ActiveCN103933005AReduce typesFacilitated releaseOrganic active ingredientsDigestive systemAlcoholFiller Excipient
The invention discloses a pantoprazole sodium-containing enteric-coated tablet and a preparation method thereof. The enteric-coated tablet is obtained by sequentially coating a tablet core with an isolating coating and an enteric coating, wherein the tablet core is formed by evenly mixing medicine-containing granules with a filler and a lubricant and then directly tabletting, and a preparation method of the medicine-containing granules comprises the following steps of: dissolving pantoprazole sodium into absolute ethyl alcohol, adding polacrilin potassium, drying and volatilizing to remove ethanol, and sieving dried materials. The varieties of auxiliary materials are fewer, the drug release is fast, the stability of the preparation is high, the production technology is simple, no sticking phenomenon exists in tabletting, and the industrial production is easy.
Owner:LIAONING NIRVANA PHARMA
Felodipine sustained-release tablet and preparation technology thereof
ActiveCN104758266ARapid drug releaseStable drug releaseOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsSustained Release Tablet
The invention discloses a felodipine sustained-release tablet and a preparation technology thereof. According to the preparation technology of the felodipine sustained-release tablet, fast-release solid dispersions, sustained-release micro-pills and pharmaceutically acceptable accessories are mixed and the mixture is compressed to obtain the preparation. The fast-release solid dispersions comprise felodipine, copovidone and polacrilin potassium; the sustained-release micro pills are obtained by coating the fast-release solid dispersions with the ethyl cellulose film with a pore-forming agent. The sustained-release tablet disclosed by the invention has the advantages of fast early-stage releasing speed and slow and stable later-stage releasing speed, so that the drug can be completely released and an effective blood drug concentration can be maintained for a long time; the felodipine sustained-release tablet is high in bioavailability, simple in preparation technology and applicable to industrial mass production.
Owner:CHANGZHOU NO 4 PHARMA FACTORY +1
Anti-cancer drug erlotinib hydrochloride tablet and preparation method thereof
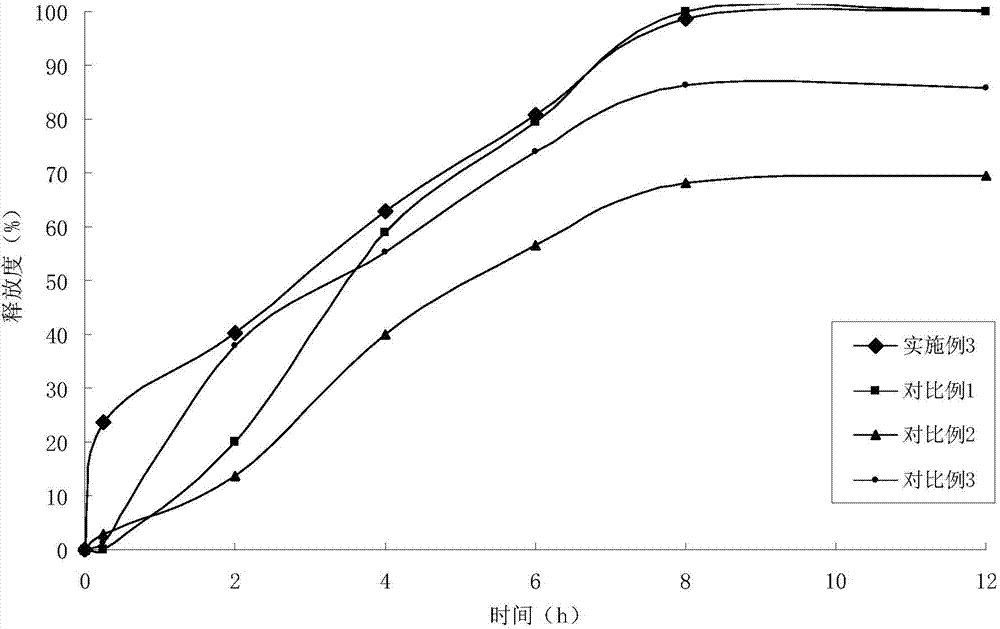
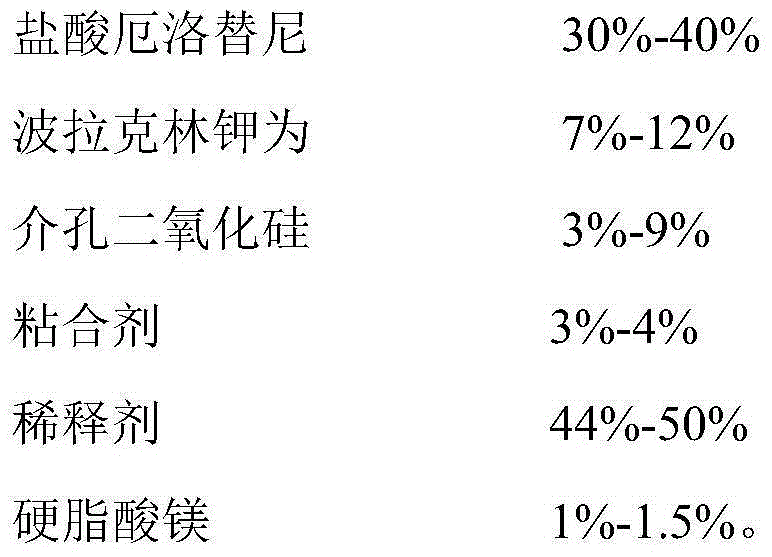
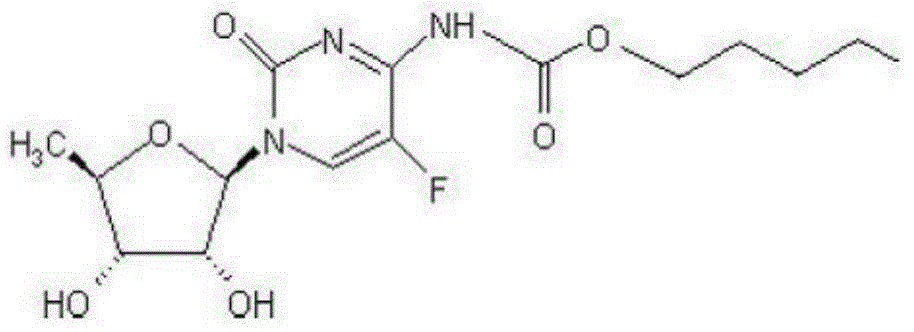
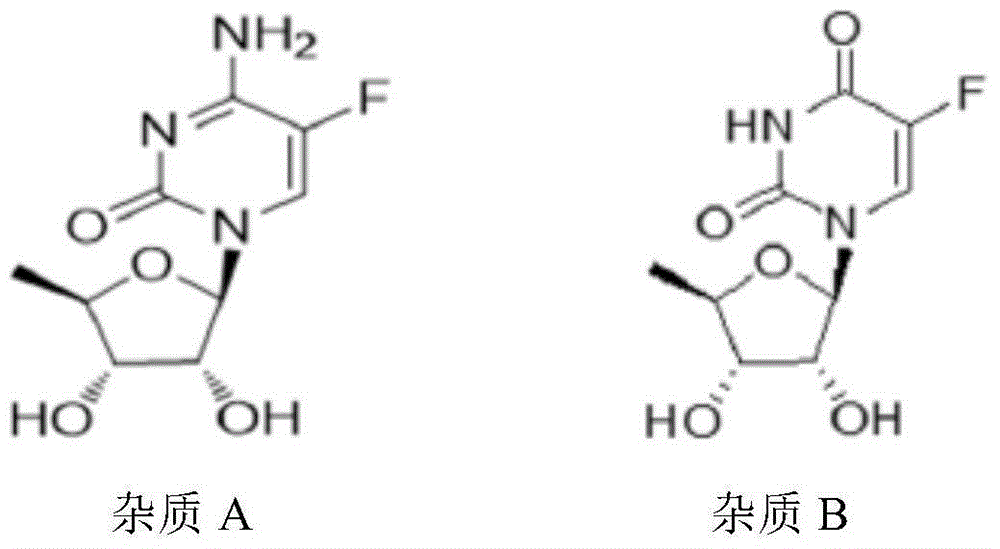
ActiveCN105030705AGuaranteed stabilityHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsPrillFluidized bed
The invention discloses an anti-cancer drug erlotinib hydrochloride tablet and a preparation method thereof. A preparation of the drug is prepared from raw materials and auxiliary materials. The auxiliary materials contain polacrilin potassium and mesoporous silicon dioxide. The preparation method comprises the following steps: (1) dissolving a binder in an acidic solution, adding erlotinib hydrochloride into the acidic solution, stirring and grinding for standby application; (2) measuring the polacrilin potassium, mesoporous silicon dioxide and a diluting agent, uniformly mixing in a fluidized bed, then spraying a mixed solution obtained in the step (1), granulating, drying, and size stabilizing; (3) uniformly mixing dry particles and a lubricating agent, and tabletting. The preparation has the advantages of fast dissolution, good dissolution stability and simple preparation process.
Owner:QINGDAO TUMOR HOSPITAL
High-stability capecitabine tablets and preparation method thereof
InactiveCN104997744APromote dissolutionDissolution will not decreaseOrganic active ingredientsPill deliveryPOLACRILIN POTASSIUMMethionine biosynthesis
The invention discloses high-stability capecitabine tablets and a preparation method thereof. The tablets contain L-methionine, polacrilin potassium and other auxiliary materials which are acceptable in pharmacy and low in hygroscopicity. The tables are prepared through the following steps: 1, weighing the L-methionine, and dissolving the L-methionine in the water for standby application; 2, smashing and sieving the capecitabine, weighing and mixing the capecitabine, the polacrilin potassium, filling agents and adhesion agents uniformly, adding an L-methionine aqueous solution for prilling, and conducting sieving, drying and granulating; 3, mixing dry particles and lubricating agents uniformly, and obtaining the capecitabine tablets through tabletting. The prepared tablets are small in related substance, good in stability, fast to dissolve, simple in preparation process and suitable for industrialized mass production.
Owner:QINGDAO CENT HOSPITAL
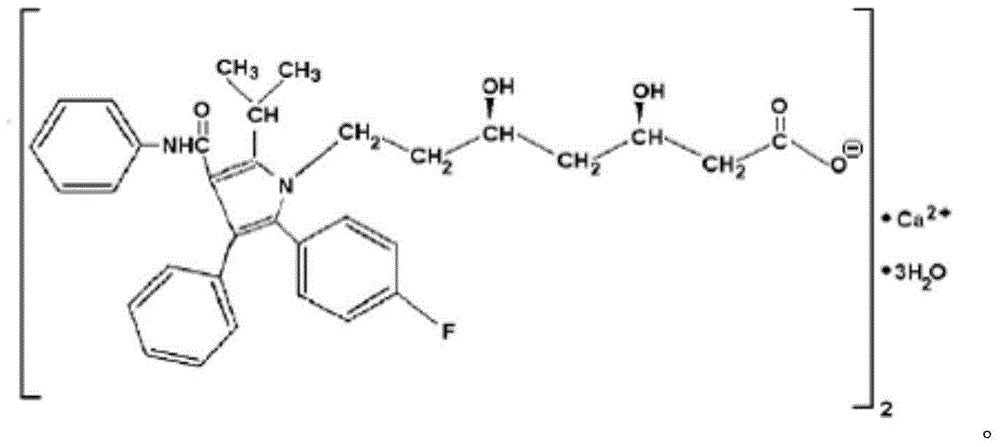
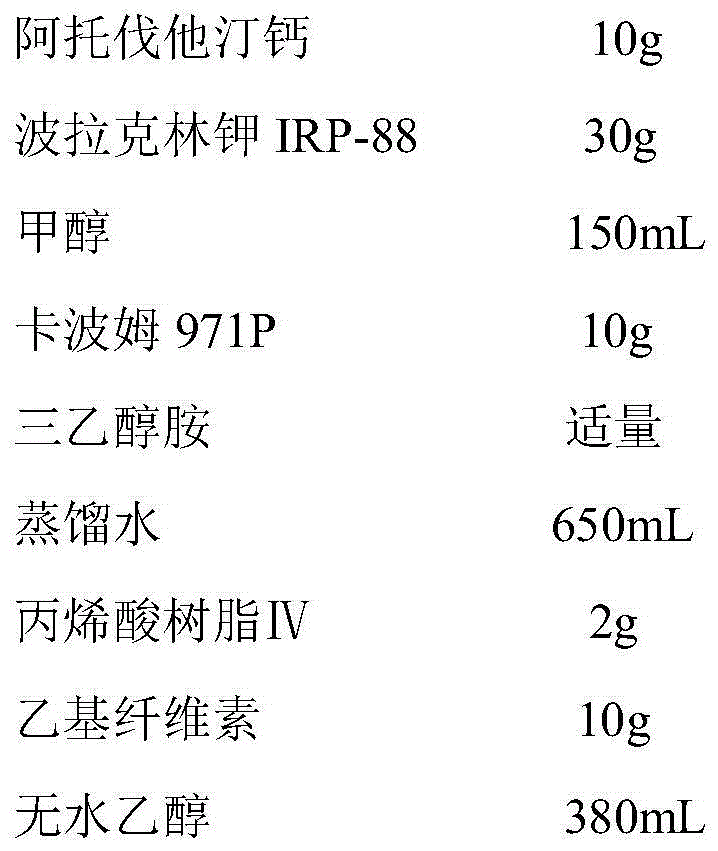
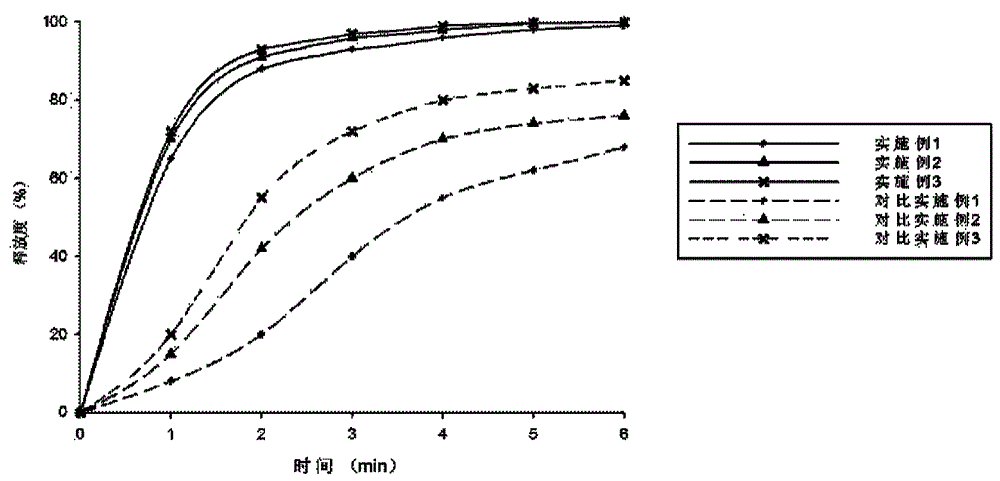
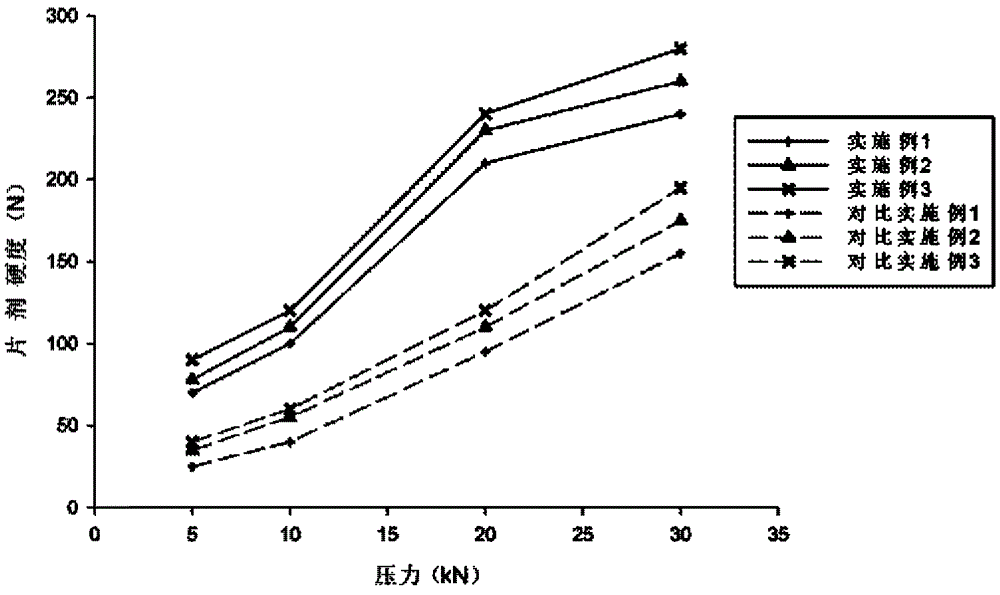
Atorvastatin calcium tablet and preparation method thereof
ActiveCN104306343AAdvantages and Significant AdvancementsFix stability issuesMetabolism disorderPharmaceutical non-active ingredientsSolubilityAcrylic resin
The invention discloses an atorvastatin calcium tablet and a preparation method thereof. The tablet is prepared from atorvastatin calcium, polacrilin potassium IRP-88, carbomer 971P, acrylic resin IV, ethyl cellulose and other pharmaceutically acceptable auxiliaries. According to the atorvastatin calcium tablet, the stability problem of atorvastatin calcium preparation and storage processes can be successfully solved, the atorvastatin calcium solid dispersion is prepared by adopting a solvent deposition technology, and the drug solubility can be greatly improved; and stomach discomfort and other side effects can be reduced by means of a semipermeable membrane coating.
Owner:NANJING CHIA TAI TIANQING PHARMA
Cinacalcet hydrochloride film-coated tablet composition
ActiveCN104721164AImprove solubilityPromote dissolutionOrganic active ingredientsPharmaceutical delivery mechanismPOLACRILIN POTASSIUMCinacalcet Hydrochloride
The invention provides a cinacalcet hydrochloride film-coated tablet composition and a preparation method thereof. The cinacalcet hydrochloride film-coated tablet comprises cinacalcet hydrochloride, polacrilin potassium, microcrystalline cellulose, silicon dioxide, magnesium stearate and a film coating. The cinacalcet hydrochloride film-coated tablet has the advantages of simple process, low cost and good digestion.
Owner:仁合益康集团有限公司
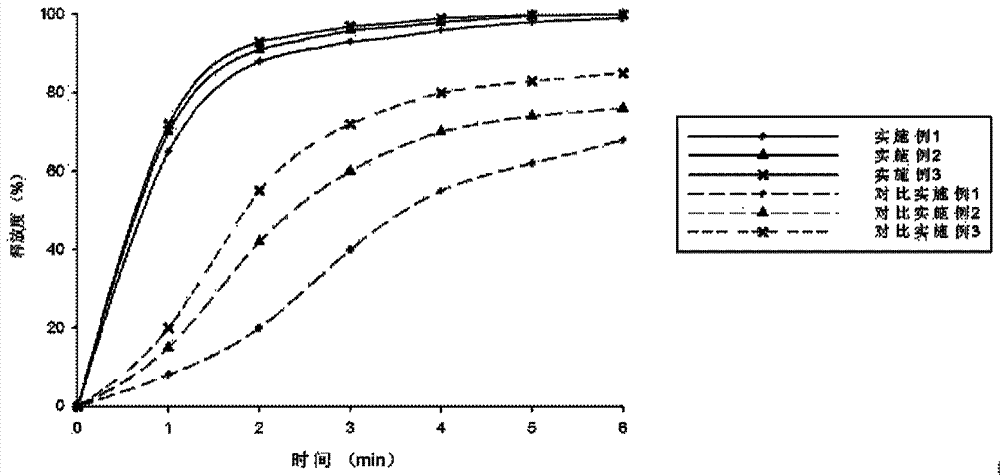
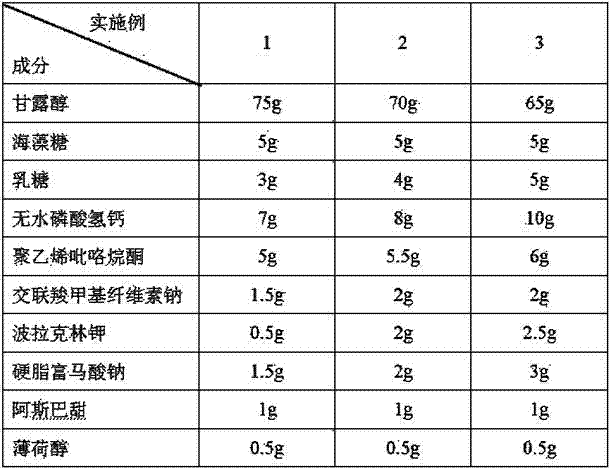
Premixed auxiliary material for preparing orally disintegrating tablet through direct compression
ActiveCN105012955AFacilitated releaseImprove the lubrication effectPharmaceutical non-active ingredientsPill deliveryHydrogen phosphateOrally disintegrating tablet
The invention provides a premixed auxiliary material for preparing an orally disintegrating tablet through direct compression. The auxiliary material is composed of the following auxiliary materials such as mannitol, trehalose, lactose, anhydrous sodium hydrogen phosphate, crosslinked carboxymethylcellulose sodium, microcrystalline cellulose, polacrilin potassium, polyvinylpyrrolidone and sodium stearyl fumarate. The premixed auxiliary material is obtained by re-crystallizing, grinding and screening by using a method according to respective parameters. The premixed auxiliary material is particularly suitable for dosage forms such as the orally disintegrating tablet and a buccal tablet.
Owner:HUNAN ER KANG PHARMA
Domperidone tablet and preparation process thereof
ActiveCN104127390AStir wellImprove solubilityOrganic active ingredientsDigestive systemAlcoholPOLACRILIN POTASSIUM
The invention discloses a domperidone tablet and a preparation process thereof. The preparation process comprises the following steps: dissolving domperidone in alcohol, adding hydrophilic auxiliary materials, dispersing, drying under reduced pressure to remove alcohol, adding tertiary butanol suspension of polacrilin potassium, volatilizing to remove tertiary butanol to obtain drug-containing solid sediment; and uniformly mixing with other pharmaceutically acceptable auxiliary materials and tabletting. The domperidone tablet disclosed by the invention can be dissolved out quickly; and the preparation process is simple and suitable for industrial production.
Owner:广东人人康药业有限公司
Orally disintegrating tablet containing sertraline hydrochloride and preparation method thereof
InactiveCN105616367ASimple processGood taste masking effectOrganic active ingredientsNervous disorderOrally disintegrating tabletPOLACRILIN POTASSIUM
The invention discloses a preparation method of an orally disintegrating tablet containing sertraline hydrochloride. The orally disintegrating tablet contains a sertraline hydrochloride-polacrilin potassium compound and other pharmaceutically acceptable auxiliary materials. An ion exchange technology is adopted to mask taste, and the orally disintegrating tablet is prepared by wet granulation and tabletting and can be disintegrated in the oral cavity without needing water, thereby effectively masking the bitter, pungent and spicy taste of the main active ingredient, namely sertraline hydrochloride. The preparation method is simple and convenient in process and suitable for industrial production.
Owner:AVENTIS PHARMA HAINAN
Orally disintegrating tablet of ambroxol hydrochloride composition and preparation method of same
InactiveCN102552391ARelieve discomfortSolve the problem of using together to treat throat diseasesOrganic active ingredientsPill deliveryOrally disintegrating tabletEfficacy
The invention provides an orally disintegrating tablet of ambroxol hydrochloride composition which comprises ambroxol hydrochloride, menthol, sodium chloride, polacrilin potassium, colloidal silicon dioxide, xylitol, trichloromethyl and Magnesium stearate, wherein the weight ratio of ambroxol hydrochloride and menthol as major active components is (15-45): (4-15). The orally disintegrating tablet of ambroxol hydrochloride composition has expectorant and antitussive activities, can be used for treatment of pharyngolaryngitis, has no problems occurring during throat disease treatment with the existing ambroxol hydrochloride medicaments in combination with other medicaments, and has excellent efficacy. Besides, the disintegration time of the orally disintegrating tablet of ambroxol hydrochloride composition is greatly reduced, and the orally disintegrating tablet is well accepted by patients.
Owner:CHONGQING CONQUER PHARML
Compound cefalexin capsule and preparation method thereof
InactiveCN104606166ASmall molecular weightGood water solubilityAntibacterial agentsOrganic active ingredientsCefalexinPOLACRILIN POTASSIUM
The invention relates to a compound cefalexin capsule and a preparation method thereof. The compound cefalexin capsule is prepared from the following raw materials in parts by weight: 220-270 parts of cefalexin, 2-20 parts of ambroxol, 25-125 parts of polacrilin potassium, 50-100 parts of chito-oligosaccharide, 5-50 parts of talc and 10-75 parts of starch. The compound cefalexin capsule is reasonable in formula, high in drug dissolution, simple in preparation method without pollution and remarkable in efficacy without adverse reaction.
Owner:SHANGHAI HUAYUAN ANHUI RENJI PHARMA
Pharmaceutical composition for treating breast cancer and preparation method of pharmaceutical composition
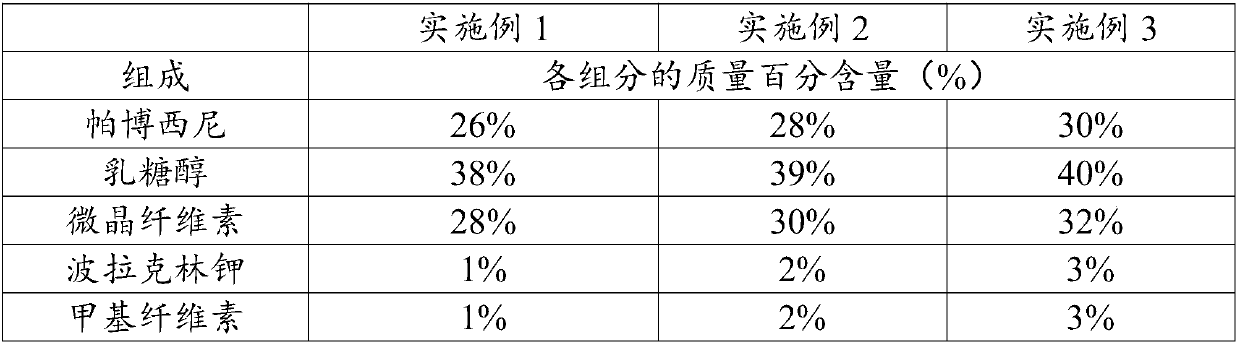
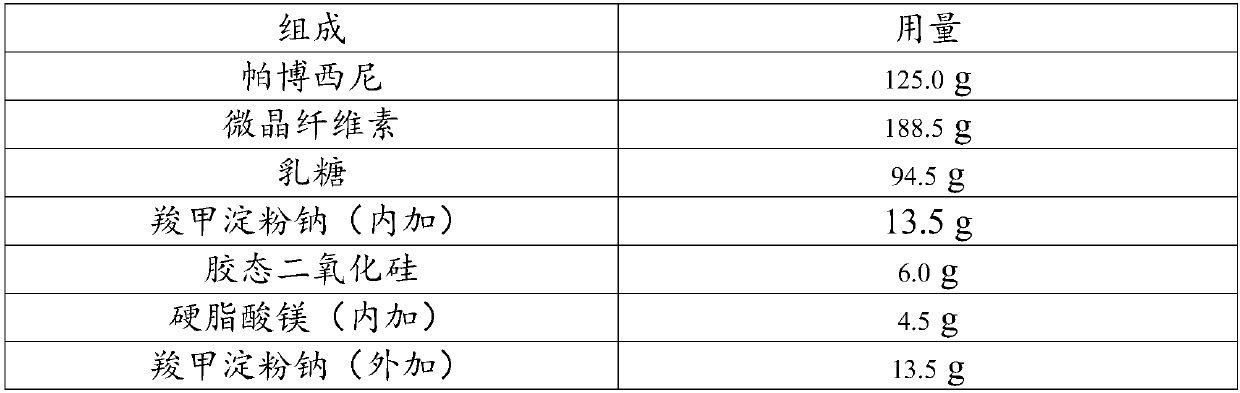
InactiveCN108014343AHigh dissolution rateLittle difference in tablet weightOrganic active ingredientsPharmaceutical non-active ingredientsPOLACRILIN POTASSIUMDissolution
The invention relates to the field of pharmaceutical preparations and particularly discloses pharmaceutical composition for treating breast cancer and a preparation method of the pharmaceutical composition. The pharmaceutical composition is prepared from components in percentage by mass as follows: 26%-30% of palbociclib, 38%-40% of lactitol, 28%-32% of microcrystalline cellulose, 1%-3% of polacrilin potassium and 1%-3% of methylcellulose. The composition is suitable for wet granulation, and tablets prepared through wet granulation have high dissolution rate, low impurity content, good fluidity, small tablet weight difference and good stability.
Owner:孟斯琴
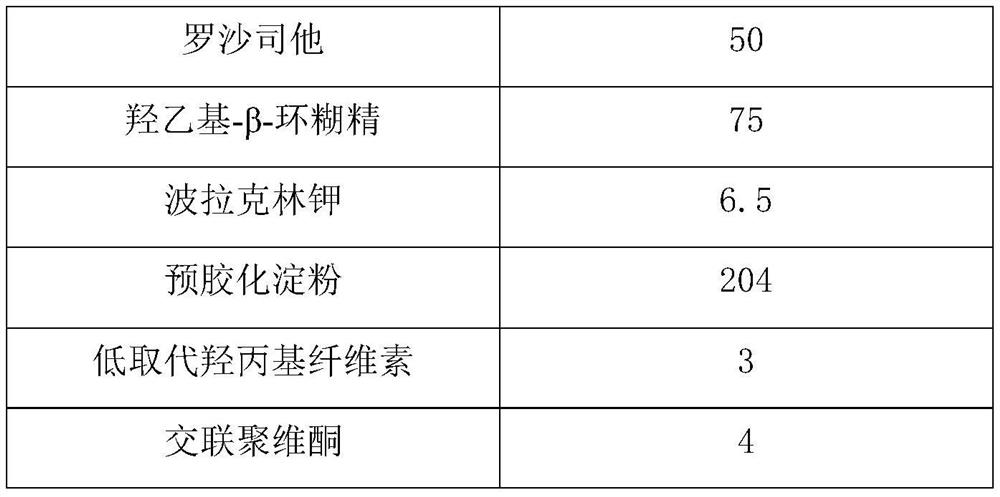
Roxadustat pharmaceutical preparation
ActiveCN113855638ASolve the problem of poor dissolution effectPromote dissolutionOrganic active ingredientsPill deliveryPOLACRILIN POTASSIUMCurative effect
The invention relates to a roxadustat pharmaceutical preparation. The pharmaceutical preparation specifically comprises roxadustat, a cyclodextrin derivative, polacrilin potassium, a diluent and a disintegrating agent. The particle size d0.9 of the roxadustat is not greater than 30 [mu] m. The roxadustat pharmaceutical preparation prepared by the invention has better dissolution rate, thereby ensuring quality and curative effect of the medicine.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Preparation method of tedizolid phosphate composition tablets
ActiveCN107625737AImprove stabilityExcellent curative effectAntibacterial agentsOrganic active ingredientsSucrosePhosphate
The invention belongs to the field of medical preparation, and more specifically discloses a preparation method of tedizolid phosphate composition tablets. The preparation method comprise following steps: tedizolid phosphate, pregelatinized starch, sucrose, tyrosine, polacrilin potassium, and sodium lauryl sulfate are added into a 5% polyvinylpyrrolidone K30 solution, and wet granulation sodium lauryl sulfate is adopted so as to obtain the tedizolid phosphate composition tablets. The stability of the tedizolid phosphate composition tablets is improved obviously via screening on the accessory ingredients and controlling on the amount; and drug crystal transformation technical problem in wet granulation is solved via screening on the kind and the amount of a binding agent. It is found via experiments that, compared with the prior art, the preparation method possesses following advantages: the curative effect is better, the adverse reaction is reduced, and antimicrobial activity on MRSA is increased obviously.
Owner:SHANDONG YUXIN PHARMA CO LTD
Pharmaceutical Formulation
InactiveUS20160228386A1Observed effectCompatibility concernEther/acetal active ingredientsPill deliveryImmediate releasePOLACRILIN POTASSIUM
A pharmaceutical composition in the form of a tablet including a first portion and a second portion, wherein the first portion includes guaifenesin having an immediate release profile and a second drug having a sustained release profile, and wherein the second portion includes guaifenesin having a sustained release profile. The second drug can be in the form of a drug-resin complex. The second drug can be either an anti-tussive or a decongestant. The drug-resin complex includes a drug complexed to an ion exchange resin. The ion exchange resin can be a polystyrene sulfonate resin, polacrilex resin, polacrilin potassium, cholestyramine resin, or a colestyramine resin. The drug-resin complex can be provided with a coating, the coating thickness being selected to obtain the desired release profile. The drug-resin complex can be provided with a coating level of from 5% to 50%. The coating level can be from 10% to 35%.
Owner:RB HEALTH (US) LLC
A kind of atorvastatin calcium tablet and preparation method thereof
ActiveCN104306343BAdvantages and Significant AdvancementsFix stability issuesMetabolism disorderPharmaceutical non-active ingredientsSolubilitySide effect
The invention discloses an atorvastatin calcium tablet and a preparation method thereof. The tablet contains atorvastatin calcium, polacrilin potassium IRP-88, carbomer 971P, acrylic resin IV, and ethyl cellulose and other pharmaceutically acceptable excipients. The present invention successfully solves the stability problem during the preparation and storage of atorvastatin calcium, and prepares the solid dispersion of atorvastatin calcium by using solvent deposition technology, which greatly improves the solubility of the drug; at the same time, through semi-permeable membrane coating, Side effects such as upset stomach are reduced.
Owner:NANJING CHIA TAI TIANQING PHARMA
Ubenimex capsule composition and preparation method thereof
ActiveCN103142544APromote dissolutionImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsSulfatePOLACRILIN POTASSIUM
The invention discloses an ubenimex capsule composition. Each unit preparation of ubenimex capsule consists of 10-30mg of ubenimex, 85-105mg of lactose, 95-118mg of microcrystalline cellulose, 8-10mg of croscarmellose sodium, 6-7mg of polacrilin potassium, 4.5-5.5mg of povidone, 1-2.5mg of sodium lauryl sulfate, 0.2-0.7mg of sucrose stearate and 3-4mg of magnesium stearate. According to the invention, the prepared ubenimex capsule can be used for effectively promoting the dissolution of ubenimex and improving the bioavailability; spray drying at 20-25 DEG C is adopted for granulation, the granulation efficiency is improved, the damage of ubenimex caused by high temperature is avoided, and the drug stability is improved; by combining an inner disintegrating agent and an outer disintegrating agent, the disintegrating speed of capsule is further increased, and the shortcomings of common capsule dissolution and disintegrating lag are overcome; and compared with prior art, the method is more suitable for industrial mass production.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
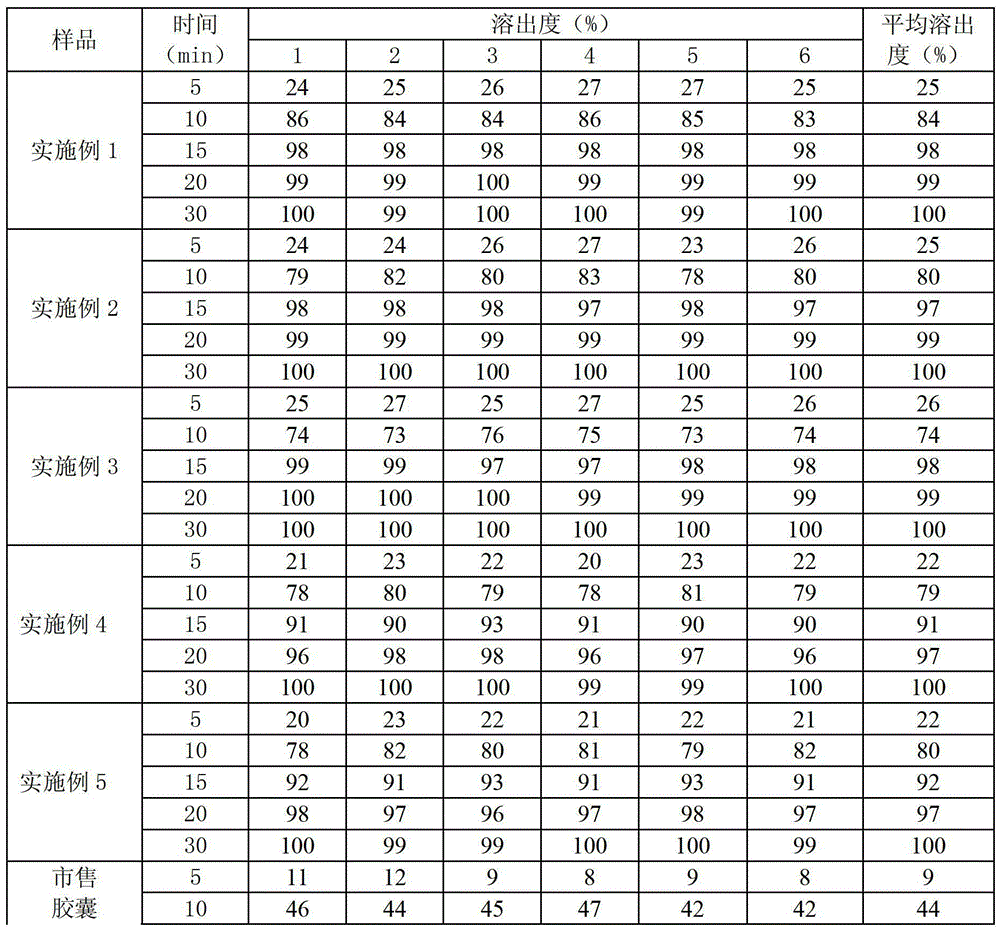
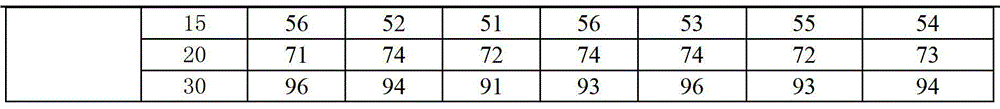
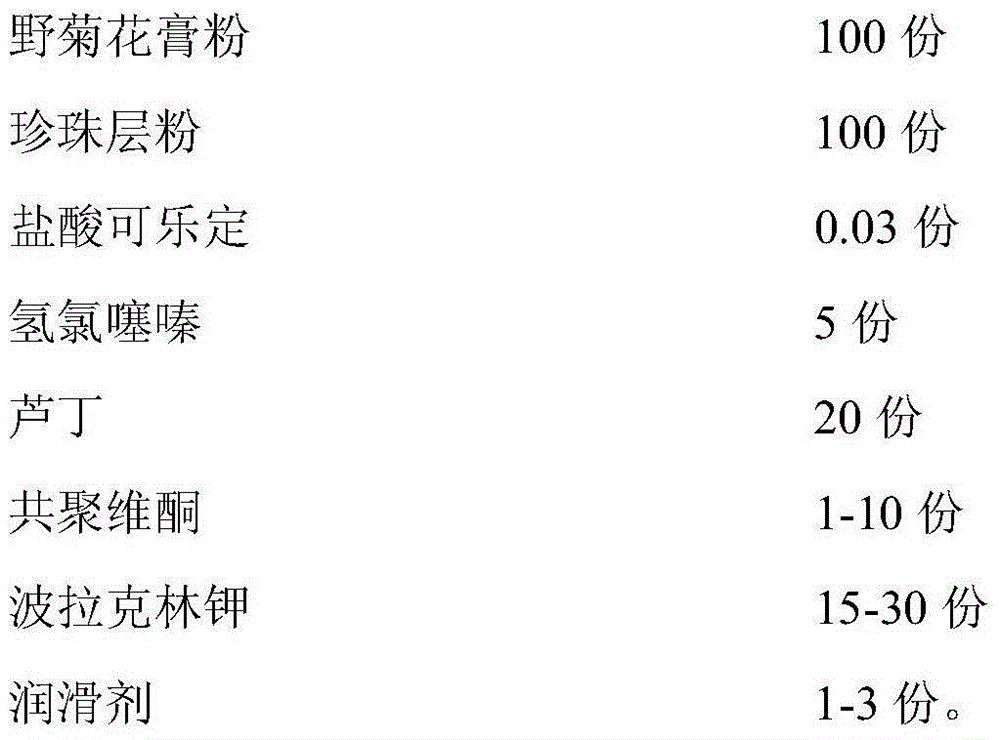
Rapidly-dissolved Zhenju antihypertension tablet and preparation process thereof
InactiveCN104784239AGood compressibilityDisintegrates quicklyOrganic active ingredientsPill deliveryDissolutionCompressibility
The invention discloses a rapidly-dissolved Zhenju antihypertension tablet and a preparation process thereof. The preparation contains copovidone and polacrilin potassium. The preparation process comprises the following steps: (1) placing the copovidone into pure water, stirring the pure water until the copovidone is dissolved, then adding hydrochlorothiazide and clonidine hydrochloride, and stirring until the hydrochlorothiazide and clonidine hydrochloride are dissolved for standby use; (2) weighing wild chrysanthemum flower cream powder, pearl layer powder, rutin and polacrilin potassium, filtering and uniformly mixing to obtain a mixture, spraying a mixed solution prepared in the step (1) by adopting a fluidized bed spray method, and carrying out the granulation and drying; and (3) weighing dry particles prepared in the step (2), adding the lubricating agent, uniformly mixing, and tabletting. The rapidly-dissolved Zhenju antihypertension tablet is good in compressibility, rapid to disintegrate and high in dissolution rate and has the advantages of simple preparation process and easiness in operation.
Owner:THE FIRST AFFILIATED HOSPITAL OF XINXIANG MEDICAL UNIV
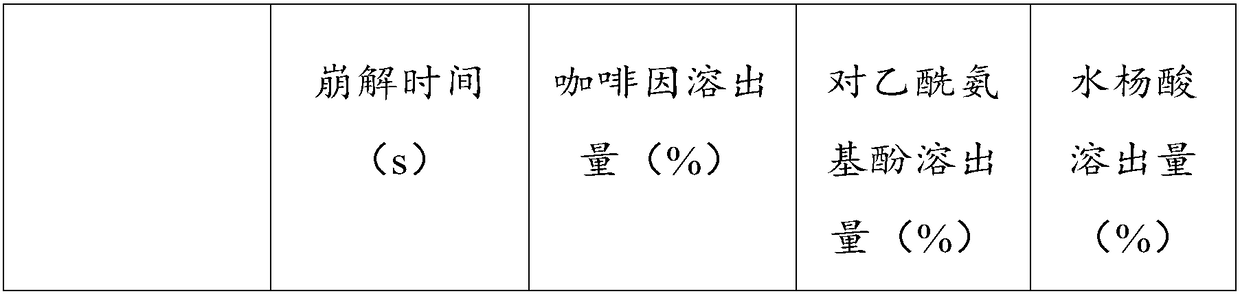
Compound paracetamol tablet and preparation method thereof
InactiveCN108272761AImprove the disintegration effectImprove efficacyAntipyreticAnalgesicsPharmacyCellulose
The invention relates to the field of pharmacy, in particular to a compound paracetamol tablet and a preparation method thereof. The compound paracetamol tablet is prepared from various raw and auxiliary materials including 60-70 parts of paracetamol, 35-40 parts of acetylsalicylic acid, 8-12 parts of caffeine, 2-4 parts of compound disintegrant, 0.5-1 part of inclusion adhesive and 35.5-54 partsof auxiliary material, wherein 2-4 parts of compound disintegrant and 0.5-1 part of inclusion adhesive are 1-2 parts of cellulose type disintegrant and 1-2 parts of polacrilin potassium. The compoundparacetamol tablet also has a good dissolution and disintegration effect after being stored for a long time and is stable in relative substances and contents.
Owner:重庆希尔安药业有限公司
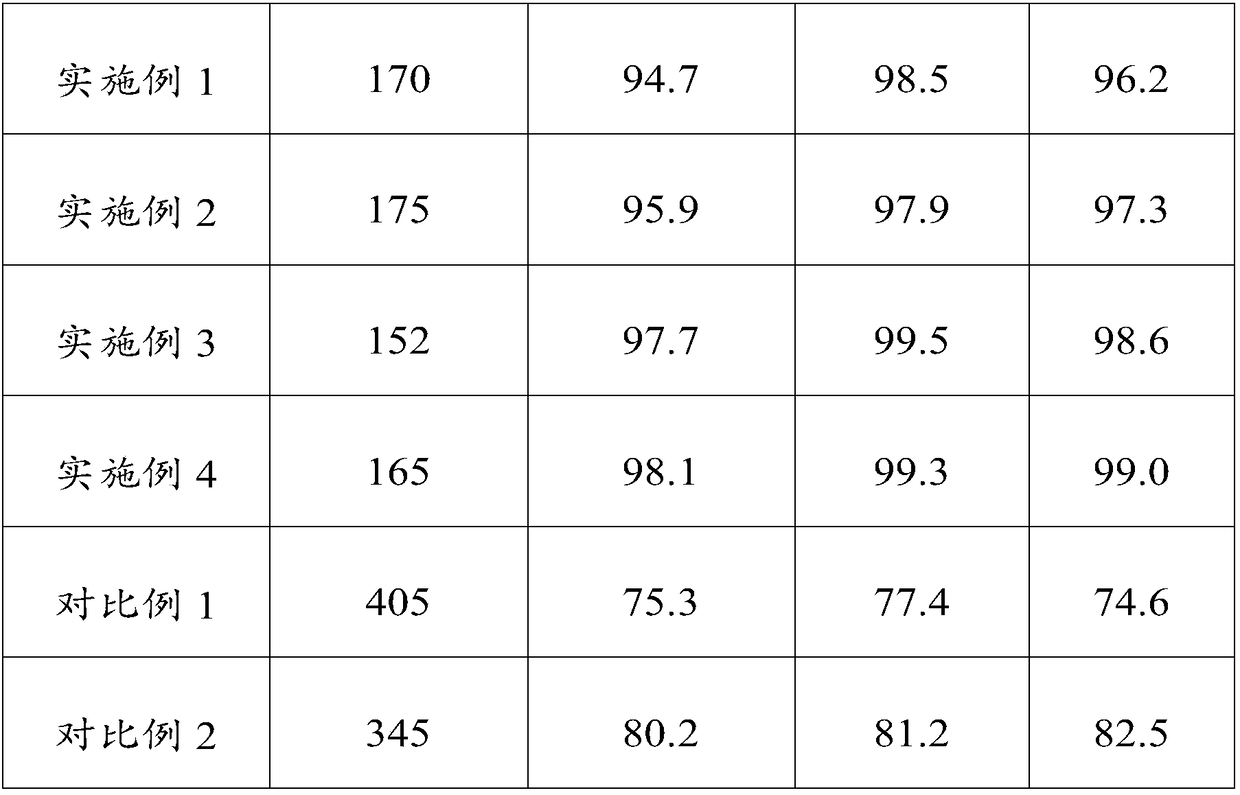
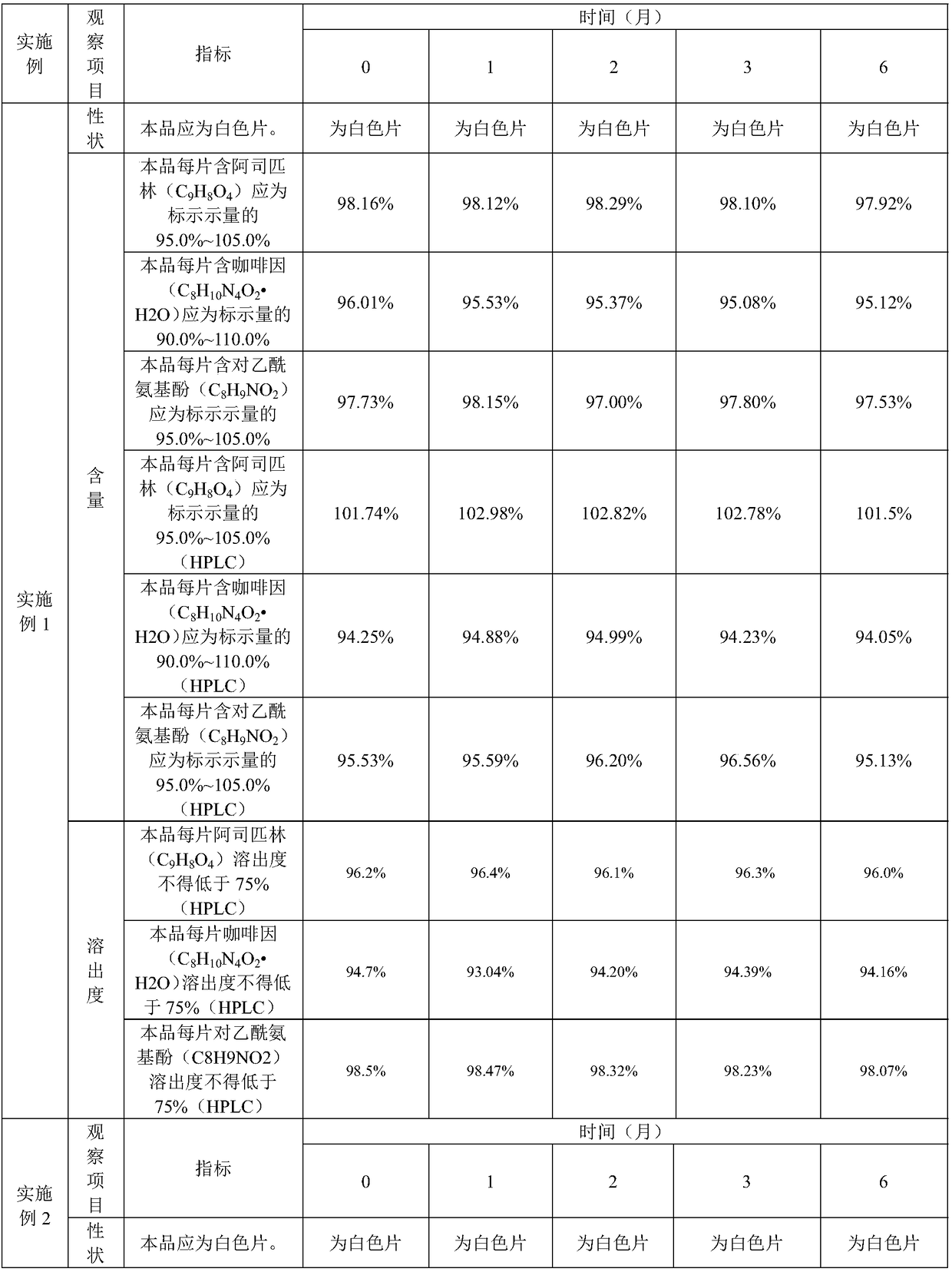
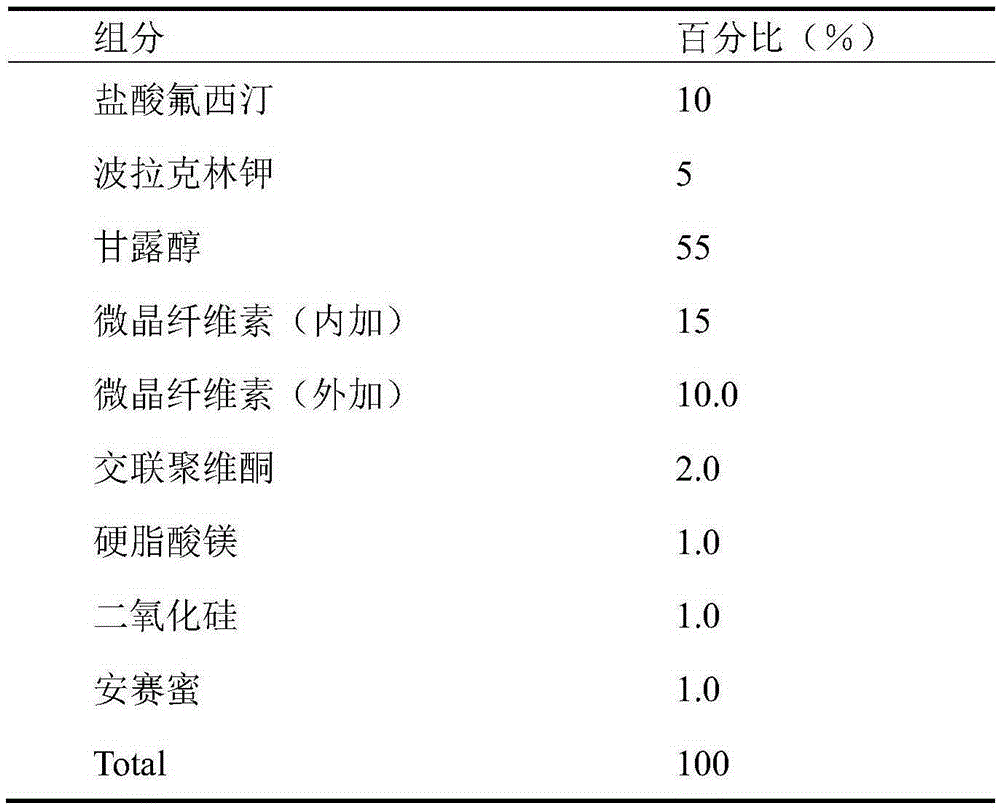
Orally disintegrating tablet containing polacrilin potassium-fluoxertine hydrochloride compound and preparation method of orally disintegrating tablet
The invention discloses an orally disintegrating tablet containing polacrilin potassium-fluoxertine hydrochloride compound and a preparation method of the orally disintegrating tablet. The orally disintegrating tablet is characterized in that fluoxertine hydrochloride and polacrilin potassium are prepared into an ion exchange compound through a precipitation method, and the ion exchange compound is prepared into the orally disintegrating tablet through a wet granulation technology. Through the technology, the mouthfeel of the medicine can be obviously improved, and meanwhile, an organic solvent is added in the preparation process, so that the water content of the compound is low, the yield of the compound and the drying efficiency in the preparation process can be obviously improved, the occurrence of the sticking problem in the tabletting process is prevented, and the stability is improved.
Owner:万全万特制药江苏有限公司
Tedizolid phosphate composition tablet
ActiveCN107625738AGood curative effectHigh antibacterial activityAntibacterial agentsOrganic active ingredientsSucrosePhosphate
The invention belongs to the field of medical preparation, and more specifically discloses a tedizolid phosphate composition tablet. The tedizolid phosphate composition tablet comprises tedizolid phosphate, pregelatinized starch, sucrose, tyrosine, polacrilin potassium, and sodium lauryl sulfate. The stability of the tedizolid phosphate composition tablet is improved obviously. It is found via experiments that, compared with the prior art, the tedizolid phosphate composition tablet possesses following advantages: the curative effect is better, the adverse reaction is reduced, and antimicrobialactivity on MRSA is increased obviously.
Owner:SHANDONG YUXIN PHARMA CO LTD
Doxofylline sustained release tablet and preparation method thereof
InactiveCN110575443ASimple preparation processImprove quality stabilityPharmaceutical non-active ingredientsPill deliveryDoxofyllineSustained Release Tablet
The invention relates to a doxofylline sustained release tablet. The doxofylline sustained release tablet is characterized in that each unit preparation is prepared from the components by weight: 0.1g-0.3g of doxofylline, 0.05g-0.15g of a compound ODT direct pressure filler, 0.05g-0.15g of lactose, 0.05g-0.2g of cholestyramine resin, 0.05g-0.15g of sodium carboxymethylcellulose, 0.05g-0.10g of polacrilin potassium and 0.005g-0.02g of magnesium stearate. The invention further provides a preparation method of the doxofylline sustained release tablet. According to the doxofylline sustained release tablet and the preparation method thereof, components synergistically work, the finally prepared composition is good in the sustained release effect, and the property is stable.
Owner:重庆健能医药开发有限公司
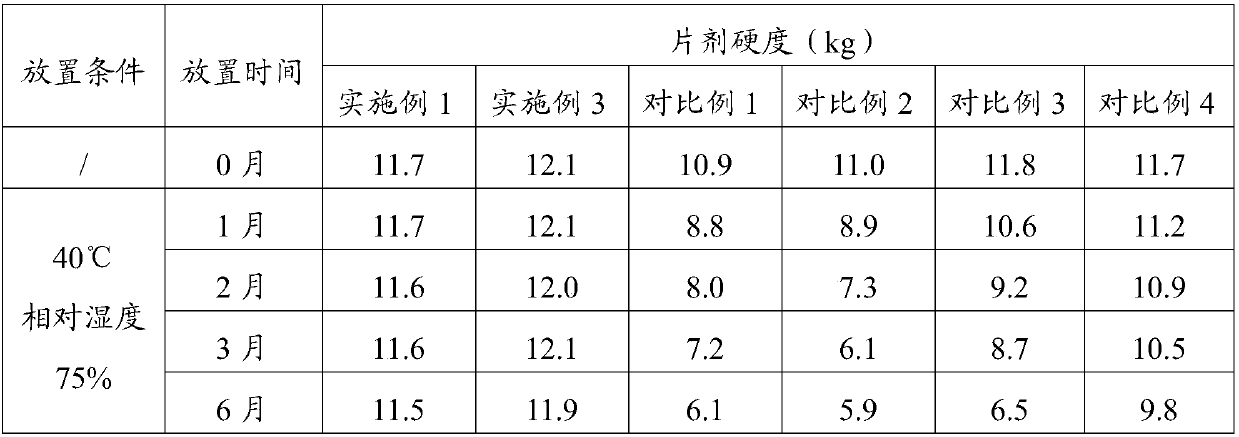
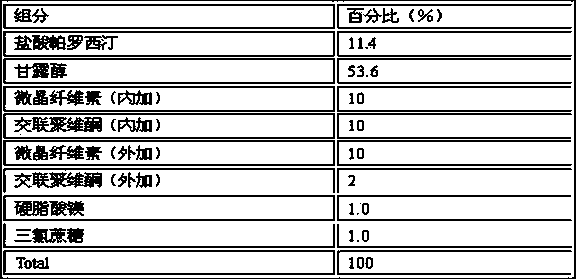
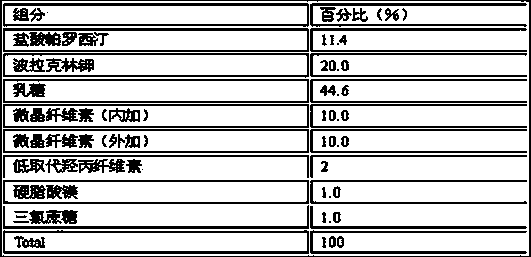
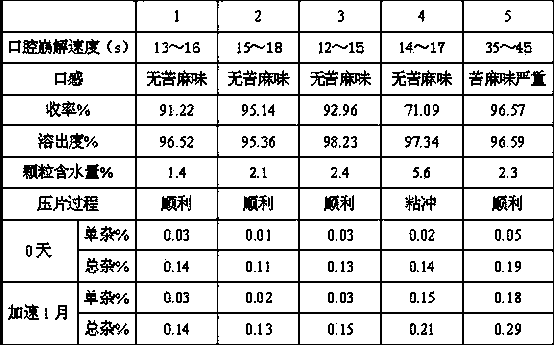
Compound containing polacrilin potassium-paroxetine
InactiveCN104382870ANo bitternessGreat tasteOrganic active ingredientsNervous disorderOrally disintegrating tabletIon exchange
The invention discloses an orally disintegrating tablet of a compound containing polacrilin potassium-paroxetine, and a preparation method of the orally disintegrating tablet. The orally disintegrating tablet is characterized in that an ion exchange compound is prepared from paroxetine hydrochloride and polacrilin potassium by adopting a precipitation method; and the orally disintegrating tablet is prepared by adopting a wet granulation process. Through the process, the taste of the medicine can be significantly improved, meanwhile, an organic solvent is added to the preparation process, so that the compound is low in moisture content, and is capable of effectively improving the yield of the compound and the drying efficiency in the preparation process, preventing the sticking problem in the tabletting process, and improving the stability.
Owner:万全万特制药江苏有限公司
Hypolycemic pharmaceutical composition and preparation method thereof
InactiveCN107898788AGood curative effectEffective blood sugar controlOrganic active ingredientsMetabolism disorderWeight decreasingMetformin Hydrochloride
The invention belongs to the technical field of medicines, and in particular discloses a hypolycemic pharmaceutical composition and a preparation method thereof. The hypolycemic pharmaceutical composition consists of the following raw materials in parts by weight: 5-10 parts of repaglinide, 15-20 parts of metformin hydrochloride, 5-10 parts of pioglitazone, 10-15 parts of sorbitol, 0.1-0.5 part ofsuperfine silica powder, 1-5 parts of polacrilin potassium resin (IRP88), 0.1-0.5 part of stevioside and 5-10 parts of ethanol. According to the hypolycemic pharmaceutical composition provided by theinvention, various hypolycemic medicines, which are different in action, are combined, and on the basis of a synergistic effect between the hypolycemic medicines which complement each other's advantages, a curative effect is enhanced, blood glucose is effectively controlled and complications are reduced; and the hypolycemic pharmaceutical composition, when used for reducing the blood glucose, canalso effectively reduce body weight, lower blood lipids and the like.
Owner:罗昌兴
Cefalexin capsule and preparation process thereof
ActiveCN104127392ALow polymer contentSimple preparation processAntibacterial agentsOrganic active ingredientsCefalexinSide effect
The invention discloses a cefalexin capsule and a preparation process thereof. The capsule is formed by filling content in a capsule shell, wherein the content contains cefalexin, polacrilin potassium IRP-88 and chitosan. The cefalexin capsule prepared by the invention is low in high polymer content, high in preparation stability, simple in preparation process and small in toxic and side effects.
Owner:ZHONGSHAN NIKEMEI PHARMA
A kind of tedizolid phosphate composition tablet
ActiveCN107625738BGood curative effectHigh antibacterial activityAntibacterial agentsOrganic active ingredientsSucrosePOLACRILIN POTASSIUM
The invention belongs to the field of medical preparation, and more specifically discloses a tedizolid phosphate composition tablet. The tedizolid phosphate composition tablet comprises tedizolid phosphate, pregelatinized starch, sucrose, tyrosine, polacrilin potassium, and sodium lauryl sulfate. The stability of the tedizolid phosphate composition tablet is improved obviously. It is found via experiments that, compared with the prior art, the tedizolid phosphate composition tablet possesses following advantages: the curative effect is better, the adverse reaction is reduced, and antimicrobialactivity on MRSA is increased obviously.
Owner:SHANDONG YUXIN PHARMA CO LTD
Pharmaceutical formulation
A pharmaceutical composition in the form of a tablet including a first portion and a second portion, wherein said first portion includes guaifenesin having an immediate release profile and a second drug having a sustained release profile, and wherein the second portion includes guaifenesin having a sustained release profile. The second drug can be in the form of a drug-resin complex. The second drug can be either an anti-tussive or a decongestant. The drug-resin complex includes a drug complexed to an ion exchange resin. The ion exchange resin can be a polystyrene sulfonate resin, polacrilex resin, polacrilin potassium, cholestyramine resin, or a colestyramine resin. The drug-resin complex can be provided with a coating, the coating thickness being selected to obtain the desired release profile. The drug-resin complex can be provided with a coating level of from 5% to 50%. The coating level can be from 10% to 35%.
Owner:RB HEALTH US LLC
Olaparib composition capsules
InactiveCN107375232AImprove liquidityHigh dissolution rateOrganic active ingredientsInorganic non-active ingredientsACETYLATED MONOGLYCERIDEMANNITOL/SORBITOL
The invention relates to the field of pharmaceutical preparations, and specifically discloses an olaparib composition capsule. The olaparib composition capsule of the present invention comprises olaparib, mannitol, dextrin, potassium metaphosphate, polacrilin potassium, and acetylated monoglyceride. The present invention preferably uses olaparib, mannitol, dextrin, potassium metaphosphate, polacrilin potassium, and acetylated monoglyceride as the composition of olaparib capsules, and the mutual synergy improves the stability of olaparib, Fluidity and dissolution rate, reducing water content, is conducive to the safe use and long-term storage of clinical drugs.
Owner:HUNAN QIWEI TECH CO LTD
A kind of premixed excipient for preparing orally disintegrating tablets by direct compression
ActiveCN105012955BFacilitated releaseImprove the lubrication effectPharmaceutical non-active ingredientsPill deliveryHydrogen phosphatePOLACRILIN POTASSIUM
The invention provides a premixed auxiliary material which can be used for direct tablet compression. The auxiliary material is composed of mannitol, trehalose, lactose, anhydrous sodium hydrogen phosphate, croscarmellose sodium, microcrystalline cellulose, polacrilin potassium, polyvinylpyrrolidone, sodium stearyl fumarate and other auxiliary materials. The respective parameters are obtained by recrystallizing powder and sieving through a method. It is especially suitable for dosage forms such as orally disintegrating tablets and buccal tablets.
Owner:HUNAN ER KANG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com