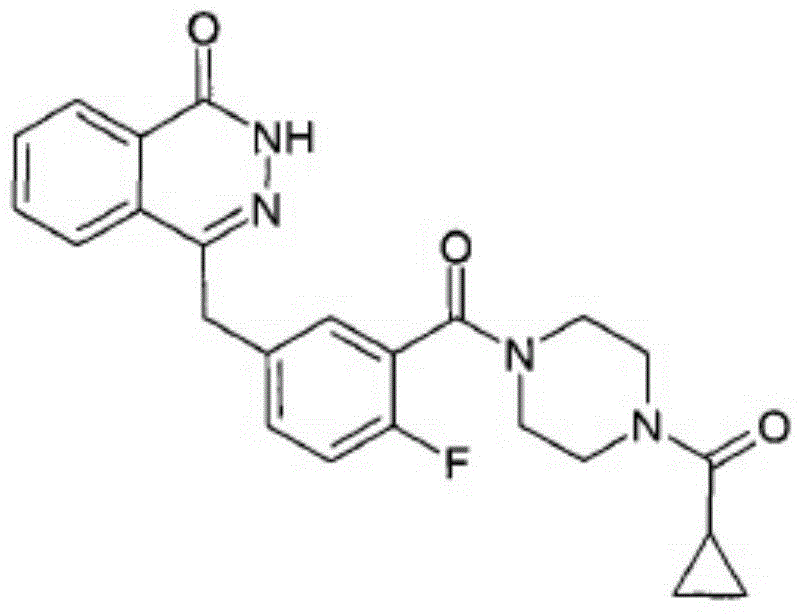
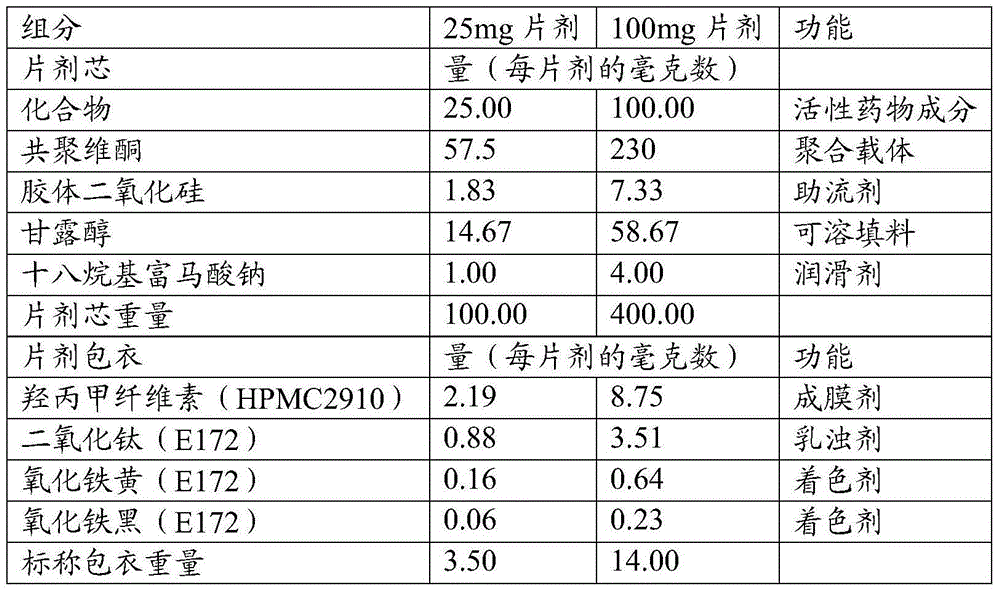
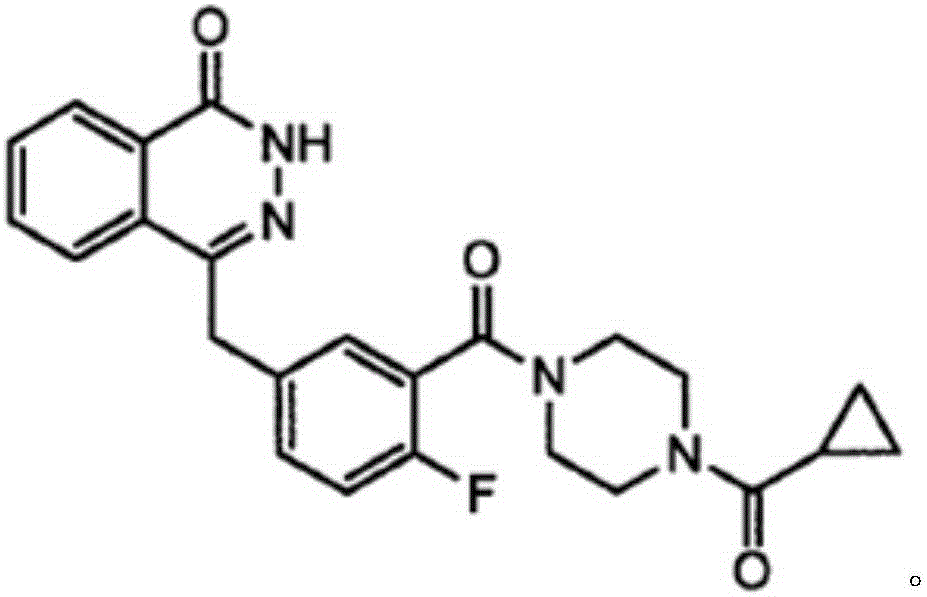
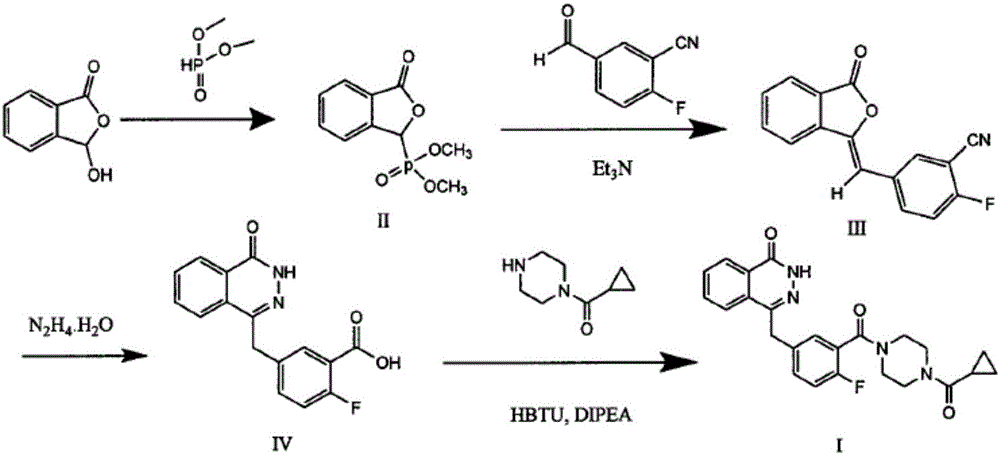
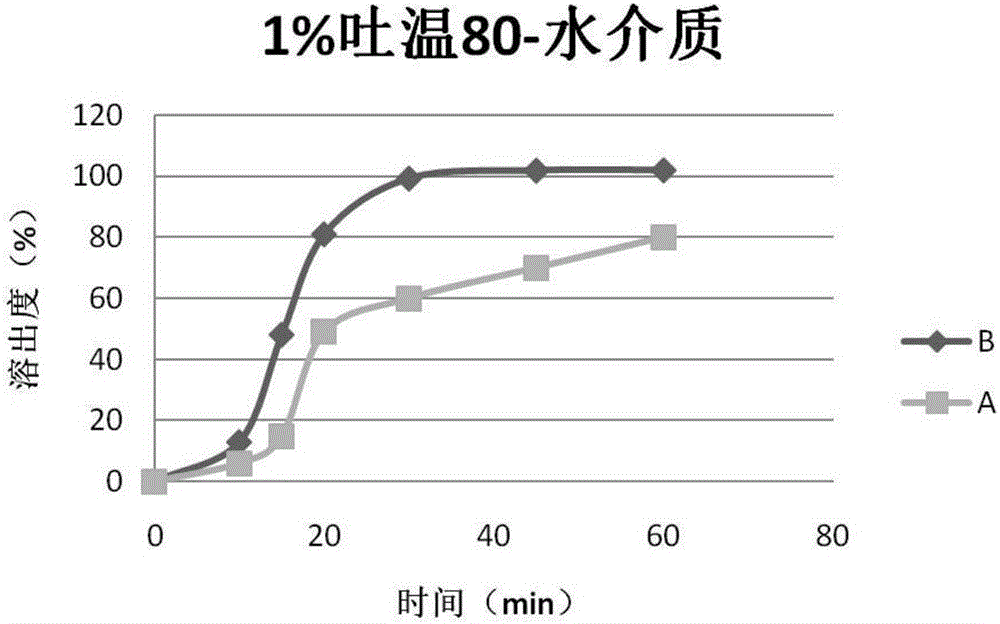
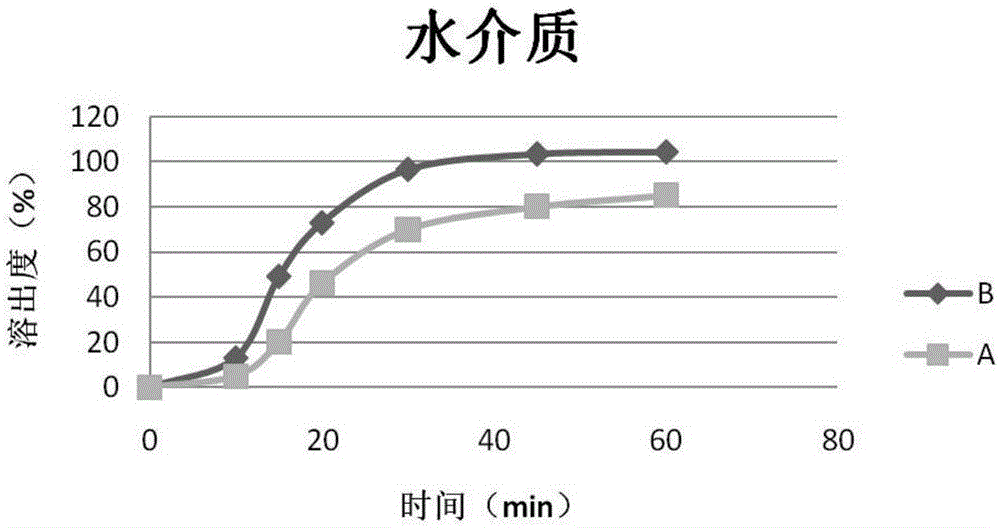
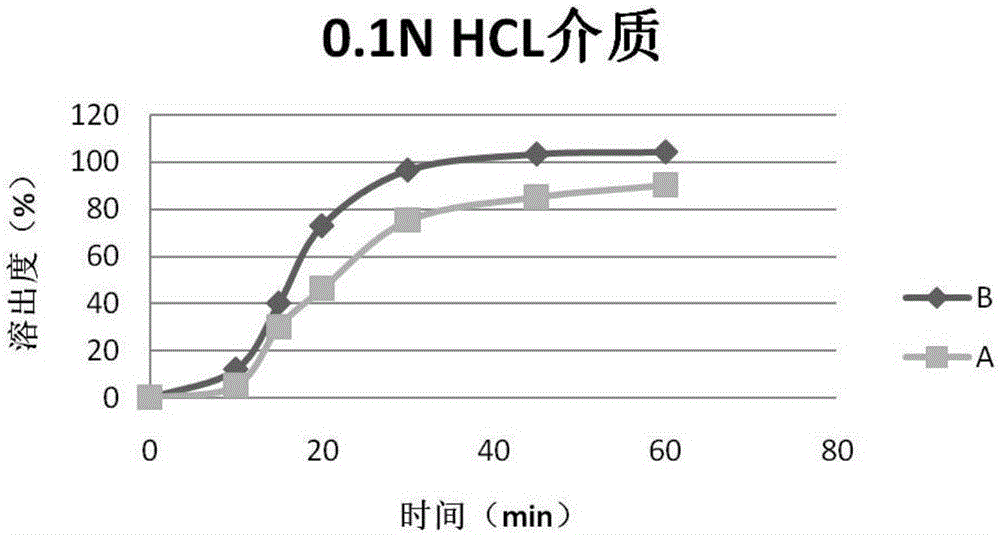
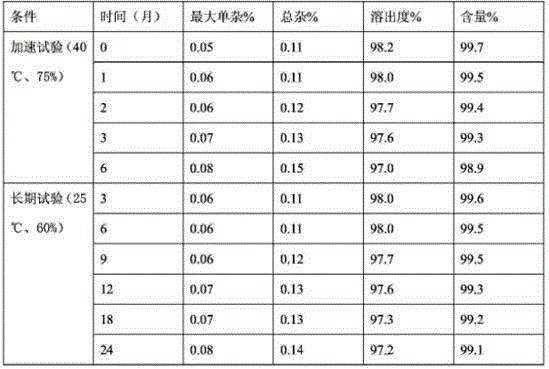
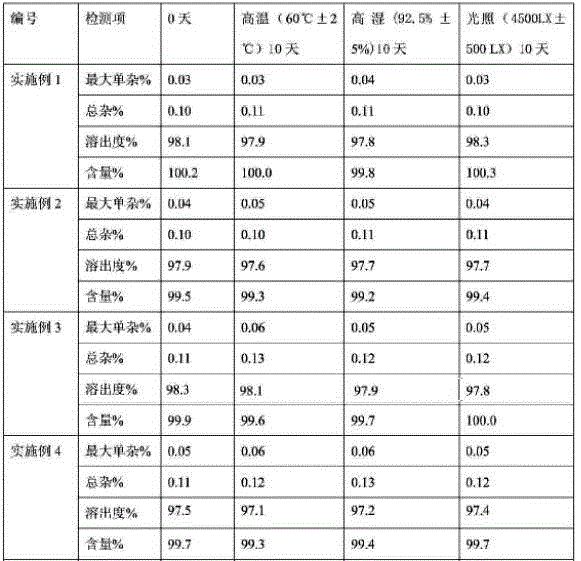
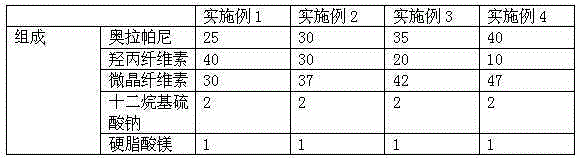
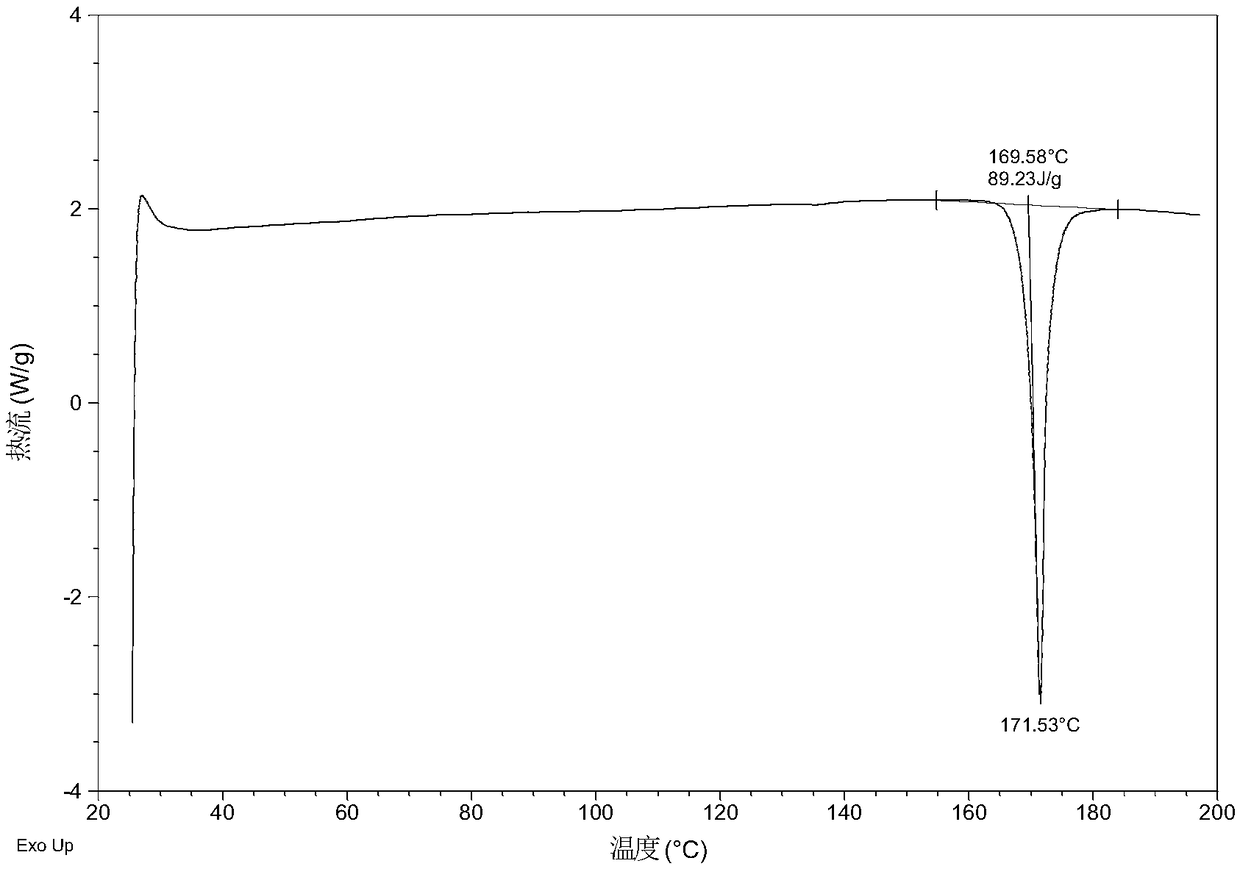
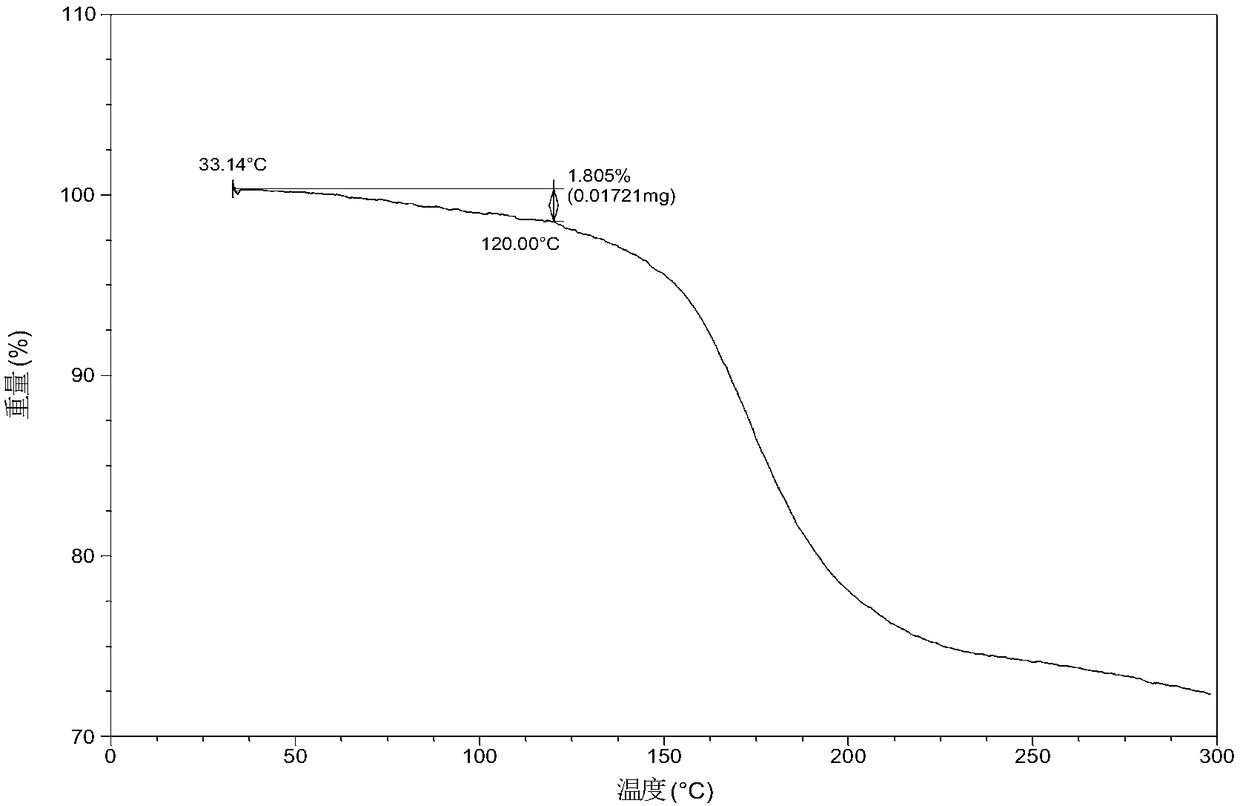
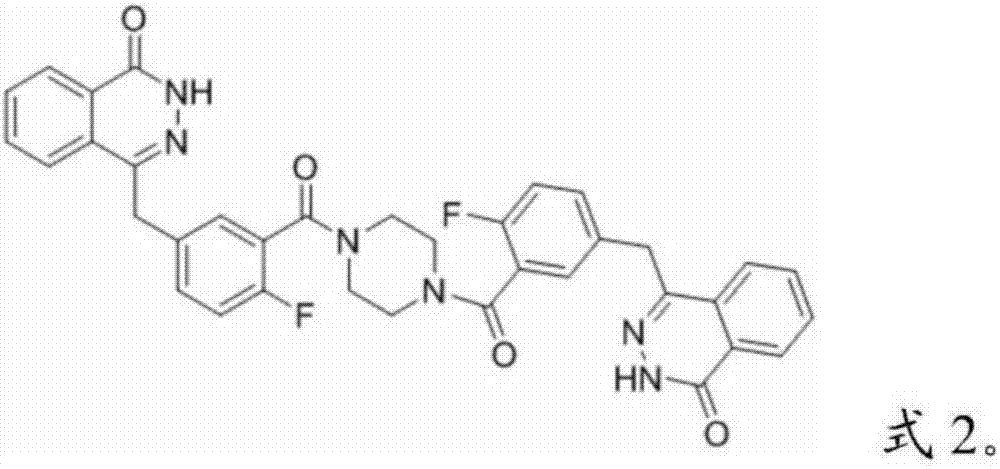
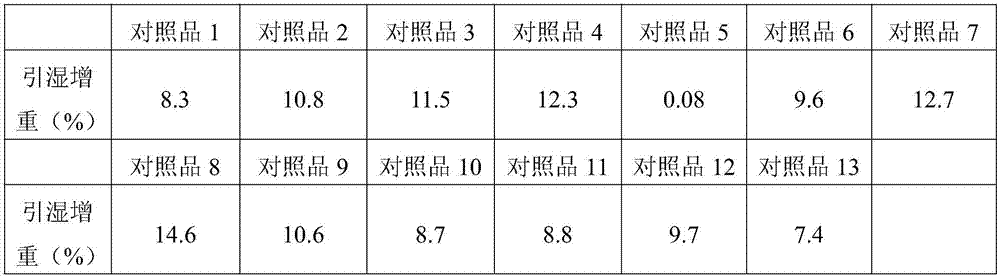
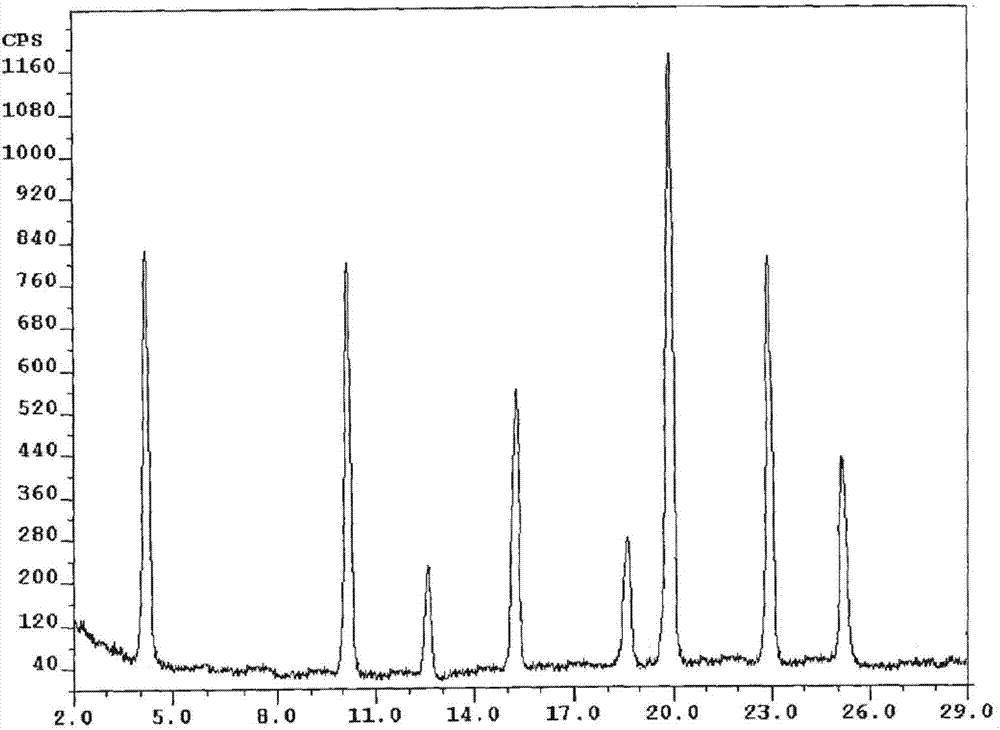
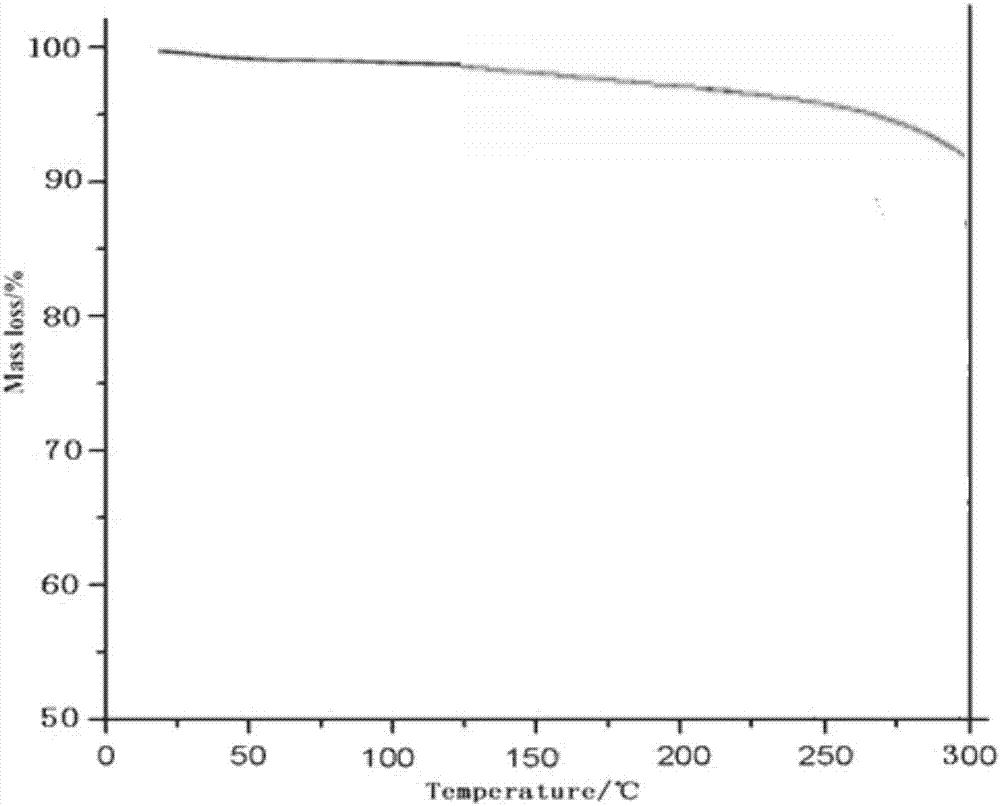
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
114 results about "Olaparib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
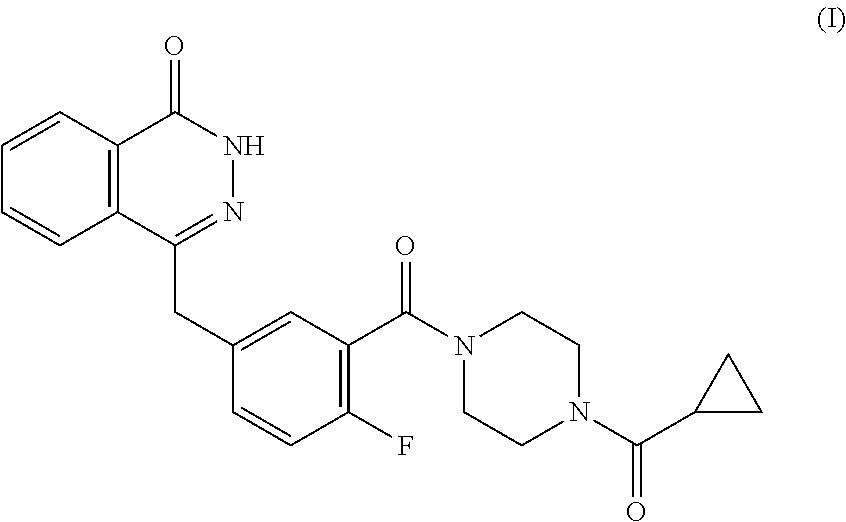
This medication is used to treat certain cancers (such as breast, ovarian, fallopian tube, or peritoneal cancer).
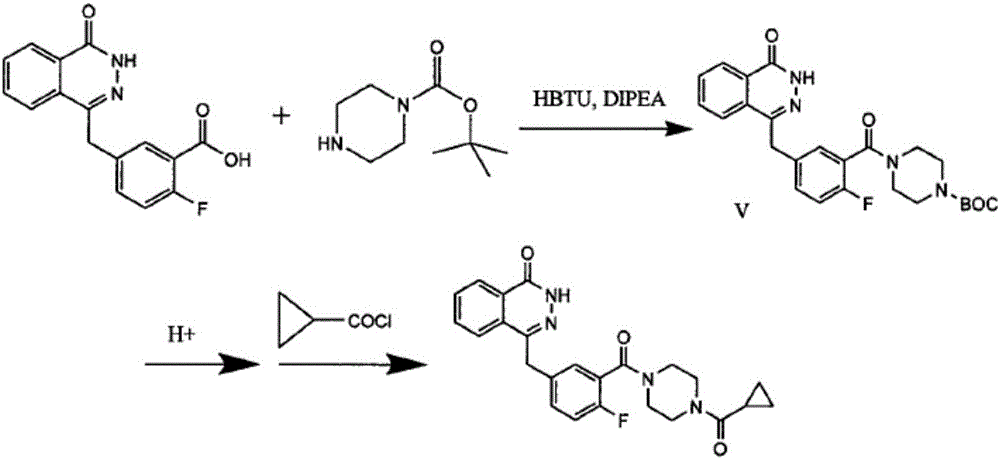
Olaparib solid dispersion preparation and preparation method thereof
ActiveCN104434809AWide variety of sourcesEase of industrial productionPowder deliveryOrganic active ingredientsBioavailabilityLubricant
The invention relates to the field of a pharmaceutical preparation, and in particular discloses an olaparib solid dispersion preparation and a preparation method thereof. The olaparib solid dispersion preparation consists of olaparib, povidone, a special lubricating agent, a special disintegrating agent and a thinner. According to the olaparib solid dispersion preparation disclosed by the invention, povidone, replacing the existing copovidone, is used as a matrix polymer, and appropriate auxiliary materials are added, so that the povidone, as the matrix polymer of the olaparib solid dispersion preparation, is wide in source, low in cost and clear in quality standard in accordance with Chinese Pharmacopoeia; meanwhile, the dissolution effect, bioavailability and stability of the preparation are guaranteed; and the industrial production of the olaparib solid dispersion preparation is facilitated.
Owner:BEIJING COLLAB PHARMA
Preparing method for Olaparib
ActiveCN105820126AEasy to operateSimple processing capacityOrganic chemistryCatecholboraneBoric acid
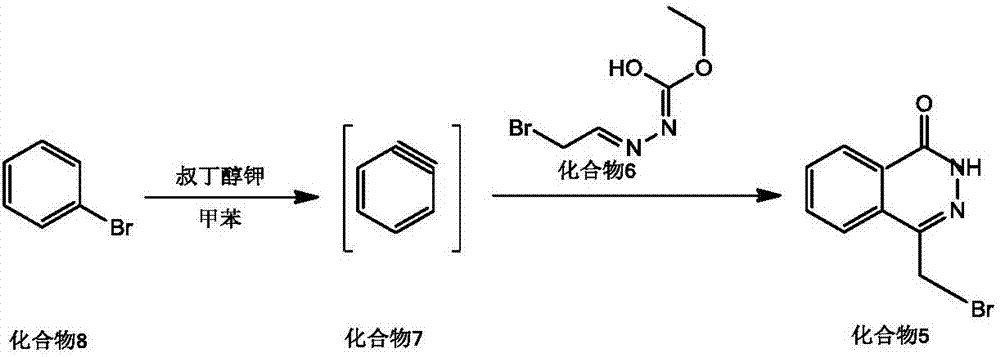
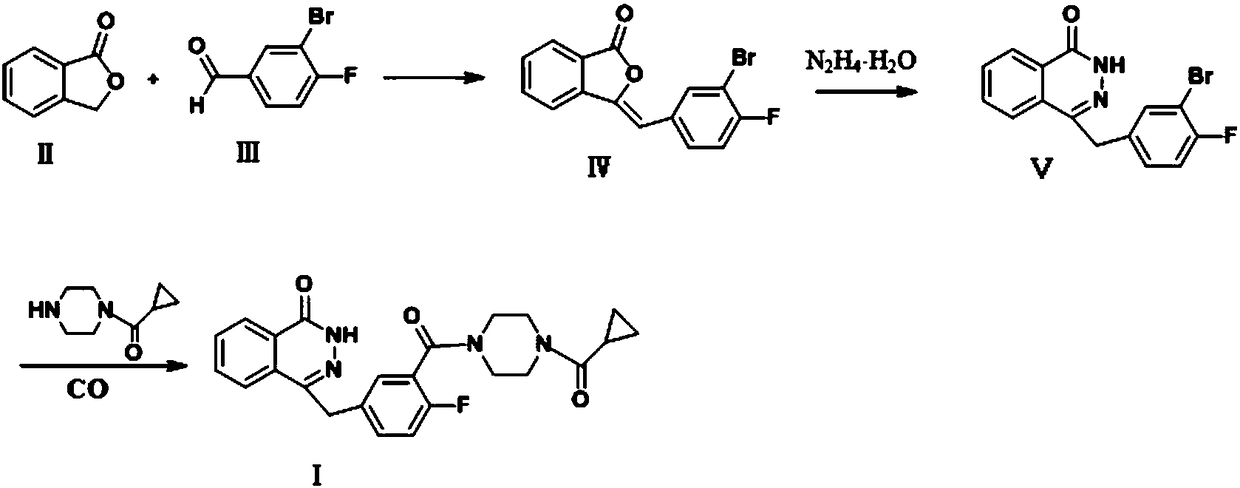
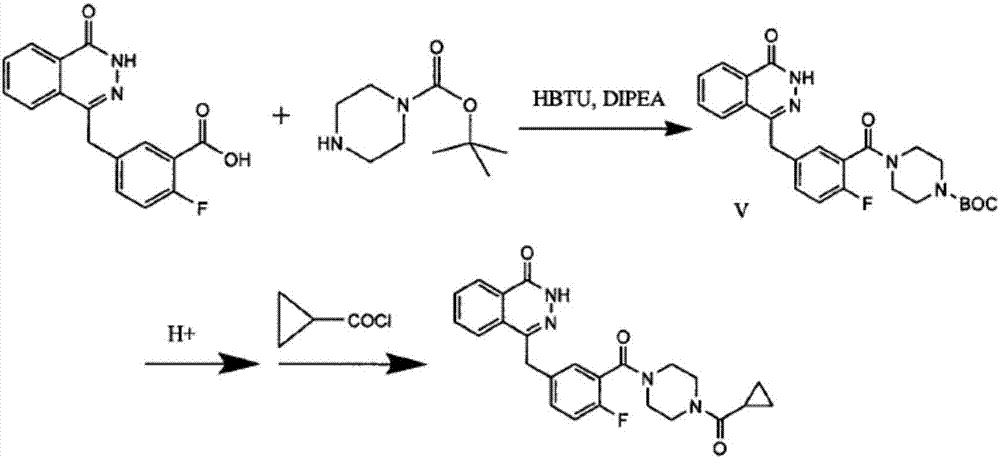
The invention discloses a preparing method for Olaparib. 5-bromomethyl-2-fluorobenzaote serves as a raw material and is subjected to a boric acid reaction with catecholborane, and a compound 3 is obtained; the compound 3 is subjected to a Suzuki coupling reaction, and a compound 5 is obtained; the compound 5 is subjected to a hydrolysis reaction, and a compound 6 is obtained; the compound 6 reacts with a compound 7 under the action of a CDI catalyst, and Olaparib is obtained. According to the preparing method, the raw material is easy to obtain, the course is short, operation and posttreatment are simple, the reaction conditions in all the steps are mild, the reaction yields of all the steps reach 90% or above, the total yield is increased to 82.3% from 49% achieved in the prior art, and the preparing method is environmentally friendly and suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
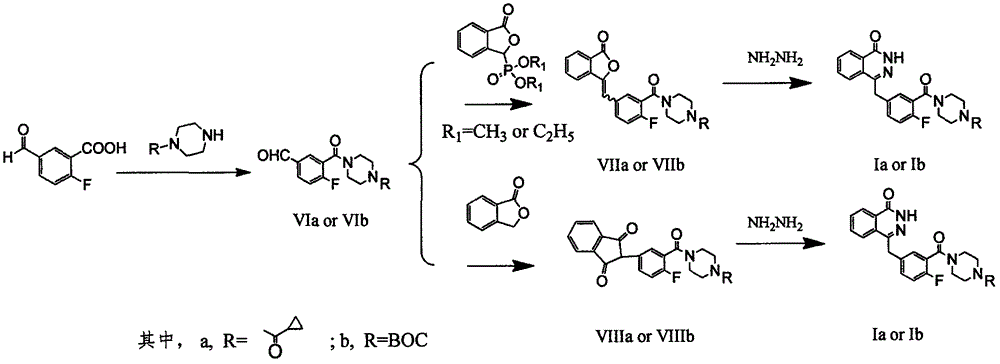
Preparation method of Olaparib and analogue of Olaparib
InactiveCN105085407ARaw materials are easy to getSimple processOrganic chemistryBenzoic acidIsobenzofuran
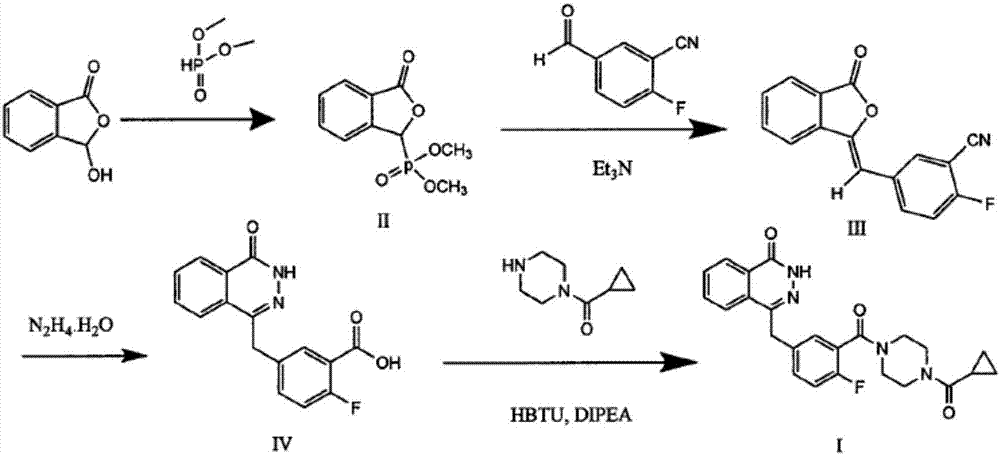
The invention discloses a preparation method of Olaparib and an analogue of the Olaparib. 2-fluoro-5-formyl benzoic acid is taken as a raw material and reacts with 1-substitutent piperazine to produce 3-(4-substitutent)piperazine-1-carbonyl)-4-fluorobenzalde which reacts with (3-oxo-1,3-dihydro-isobenzofuran-1-yl)dialkyl phosphate to produce 1-(substitutent)-4-[5-(3-oxo-3H-isobenzofuran-1-yl-methylene)-2-fluorobenzoyl]piperazine, then the 1-(substitutent)-4-[5-(3-oxo-3H-isobenzofuran-1-yl-methylene)-2-fluorobenzoyl]piperazine reacts with hydrazine hydrate to produce the Olaparib (Ia,R=cyclopropyl formyl) and the analogue (Ib,R=BOC) of the Olaparib; or 3-(4-substitutent)paperazine-1-carbonyl)-4-fluorobenzalde reacts with phthalide to produce 1-(substituent)-4-[5-(2,3-dihydro-1,3-dioxo-1H-indene-2-yl)-2-fluorobenzoyl]piperazine which reacts with the hydrazine hydrate to produce the Olaparib (Ia,R=cyclopropyl formyl) and the analogue (Ib,R=BOC) of the Olaparib.
Owner:GUANGZHOU YOUMIJIAN PHARMA TECH CO LTD
Preparation method of high-purity olaparib
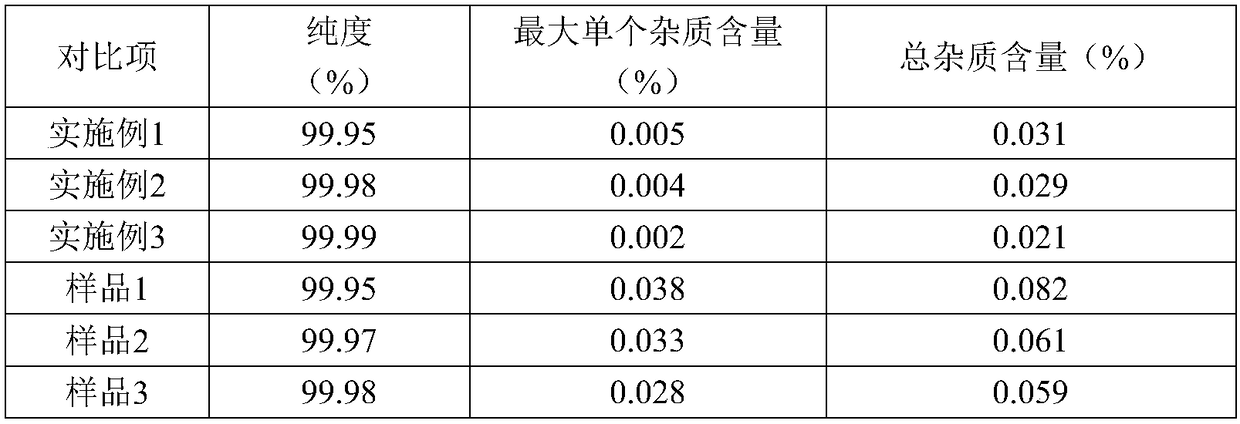
The invention discloses a preparation method of high-purity olaparib. The preparation method comprises: subjecting 2-fluoro-5-[(4-oxo-3,4-dihydrophthalazine-1-yl)methyl] benzoic acid as a starting material to activation and aminolysis crystallization to obtain high-purity olaparib, wherein the activation refers to adding carbonyldiimidazole activating agent into a solution containing 2-fluoro-5-[(4-oxo-3,4-dihydrophthalazine-1-yl)methyl] benzoic acid to obtain active amide intermediate; with separation and purification, subjecting the active amide intermediate to direct aminolysis crystallization with 1-(cyclopropanecarbonyl)piperazine to obtain the olaparib. The purity of the olaparib prepared by the method is greater than 99.8 %, and the process is simple, high in yield, low in cost and more suitable for industrial production.
Owner:合肥启旸生物科技有限公司
Crystalline form I of Olaparib and preparation method therefor
InactiveCN105439961ADevelopment is of great significanceImprove stabilityOrganic active ingredientsOrganic chemistryX-rayPowder diffraction
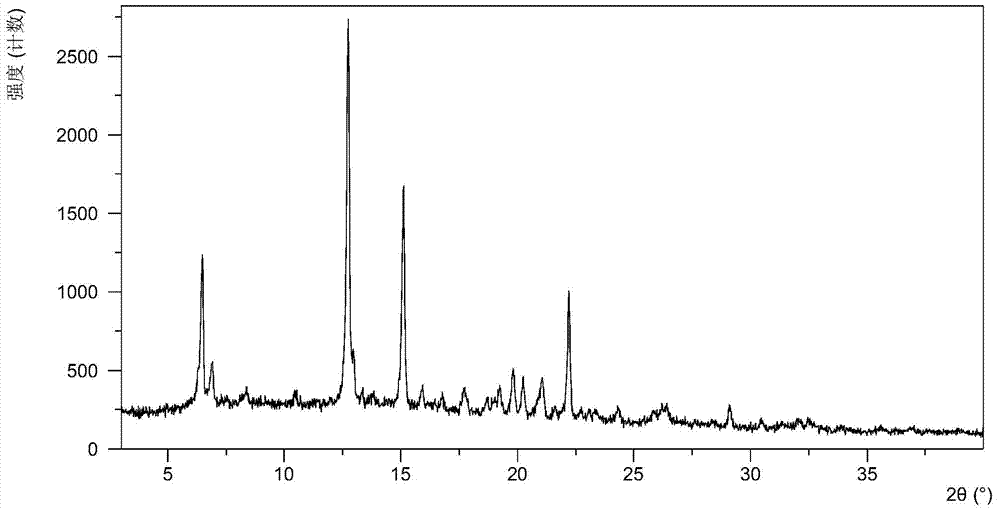
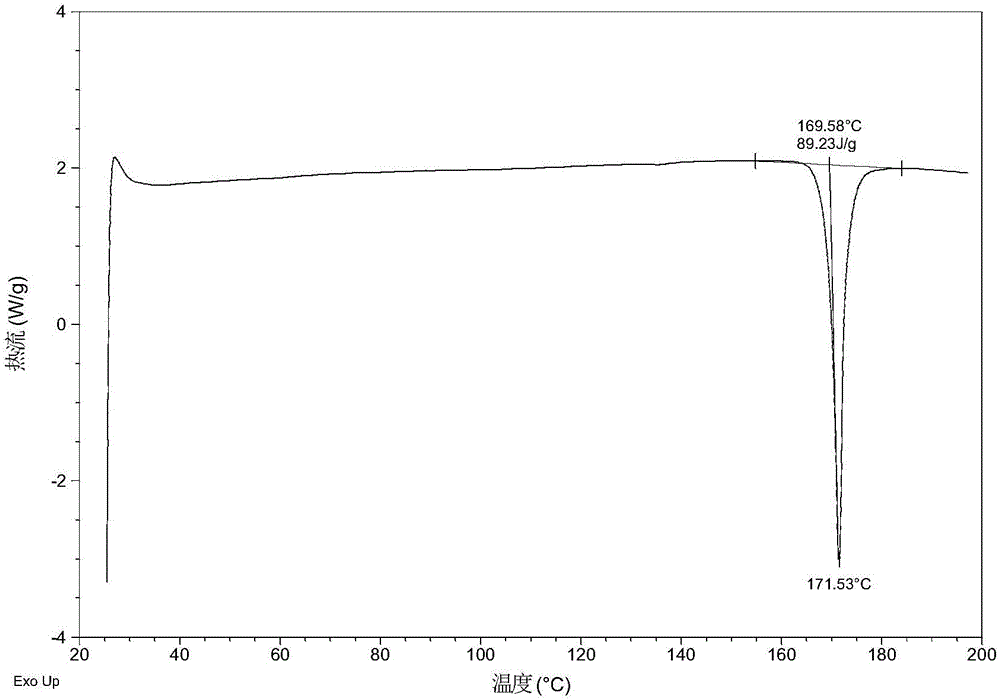
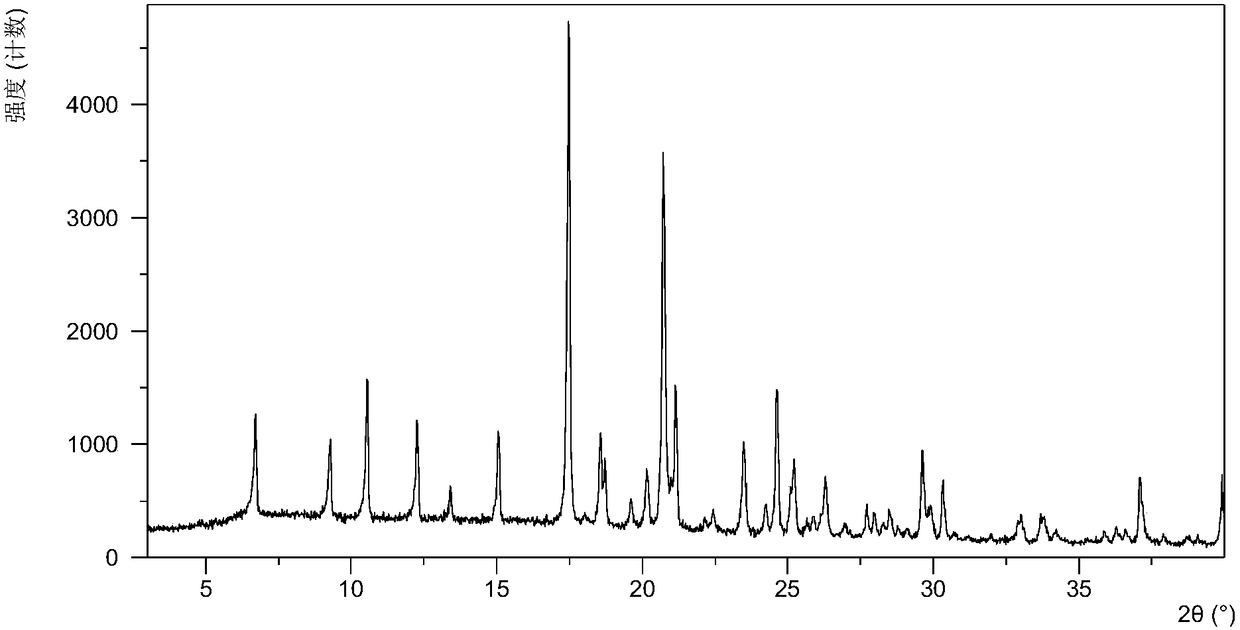
The present invention relates to a crystalline form I of Olaparib and a preparation method therefor. The present invention particularly provides a crystalline form I. The crystalline form I is characterized in that the X-ray powder diffraction pattern of the crystalline form I has characteristic peaks when the 2 theta value is 6.4 degrees + / - 0.2 degree, 12.7 degrees + / - 0.2 degree and 15.1 degrees + / - 0.2 degree. The crystalline form I provided by the present invention is better in stability, and has important values for optimization and development of the drug in the future.
Owner:CRYSTAL PHARMA CO LTD
Preparation method for olaparib
The invention discloses a preparation method for olaparib. The preparation method comprises the following steps: subjecting 4-(4-fluoro-3-(piperazine-1-carbonyl)benzyl)phthalazin-1(2H)-one, a condensing agent, cyclopropanecarboxylic acid, alkali and a polar organic solvent to a reaction at 0 to 120 DEG C for 2 to 8 h; adding water; allowing a solid to be precipitated; and carrying out pumping filtration, washing and drying so as to obtain olaparib. According to the invention, cyclopropanecarboxylic acid is used as a raw material; reaction conditions are mild; operational safety is good; organic solvents like dichloromethane is not needed for aftertreatment; separation and purification steps are simple; the yield of olaparib is high, as high as 92% or above; raw materials are easily available; the method is simple; side reactions are few; the chromatographic purity of olaparib is high; industrial scale-up production can be easily implemented; and the method has good industrial application prospects.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Olaparib and urea eutectic and preparation method thereof
ActiveCN105753789AGood eutectic stabilityReduce humidityUrea derivatives preparationOrganic active ingredientsSolubilityX-ray
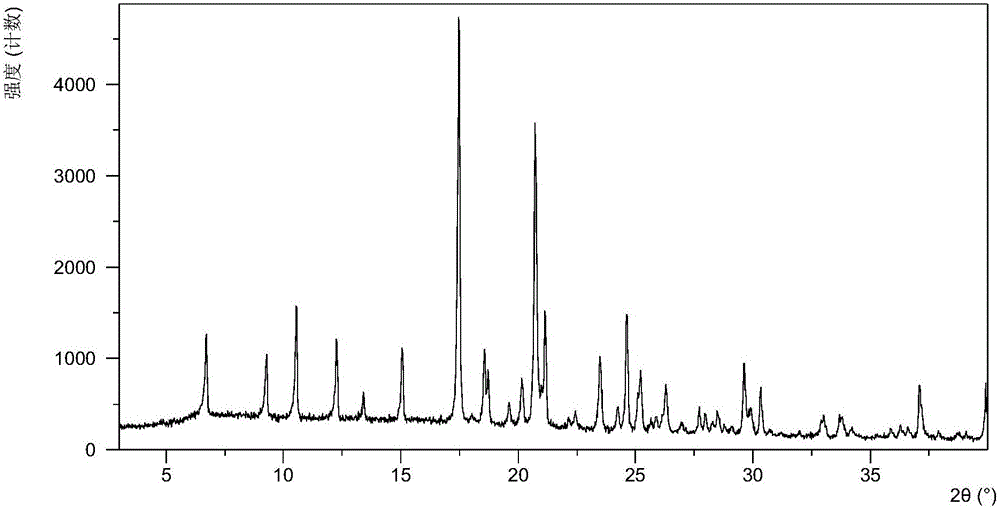
The invention relates to olaparib and urea eutectic and a preparation method thereof and particularly provides a eutectic form A, an X-ray powder diffraction of the crystal form shows characteristic peaks at 17.4 DEG + / - 0.2 DEG, 20.7 DEG + / - 0.2 DEG and 10.5 DEG + / - 0.2 DEG of value 2theta.The eutectic has better stability, lower hygroscopicity and higher solubility than existing olaparib free base crystal forms and is of significant value to the optimization and development of its drugs in future.
Owner:CRYSTAL PHARMATECH CO LTD
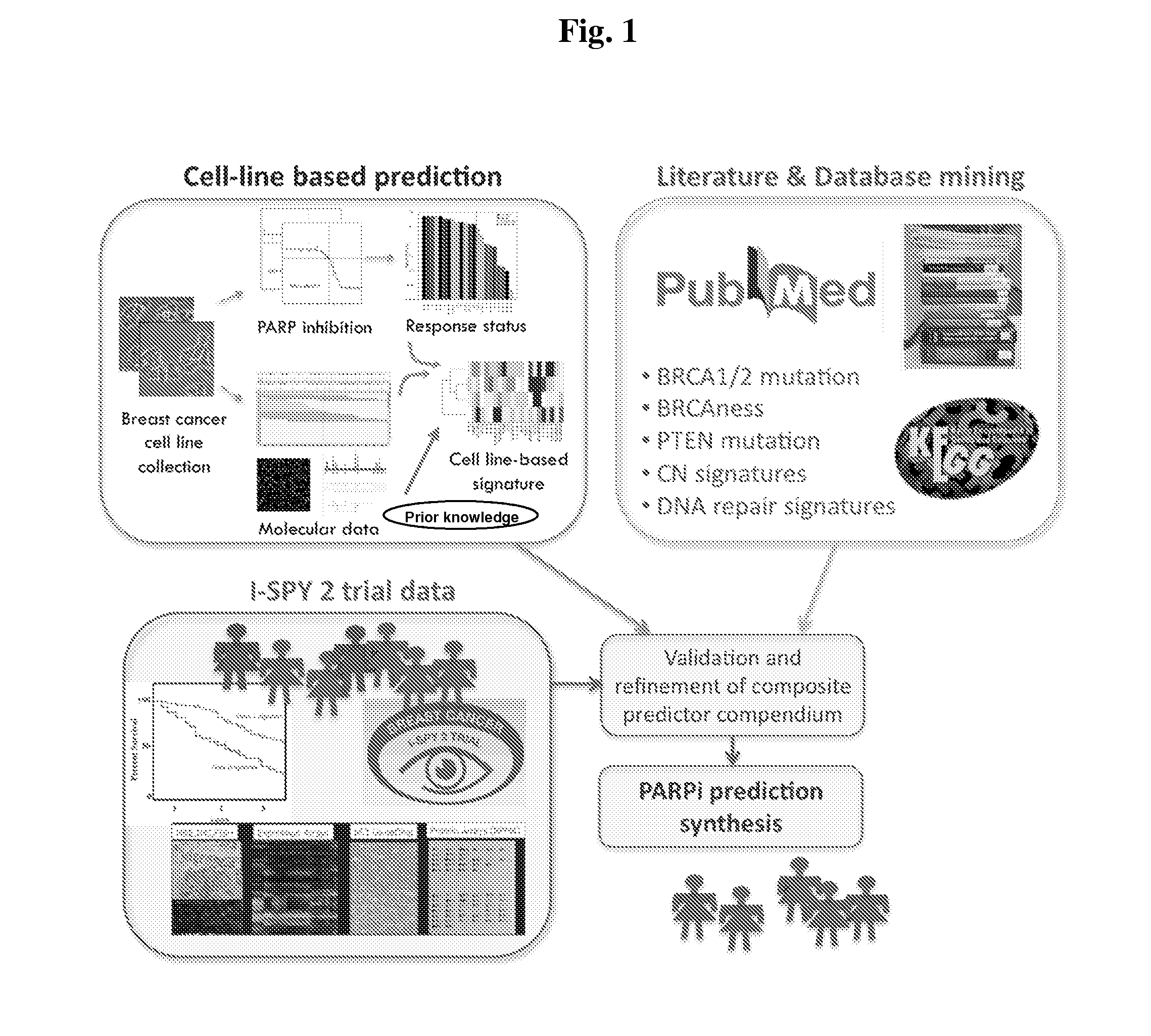
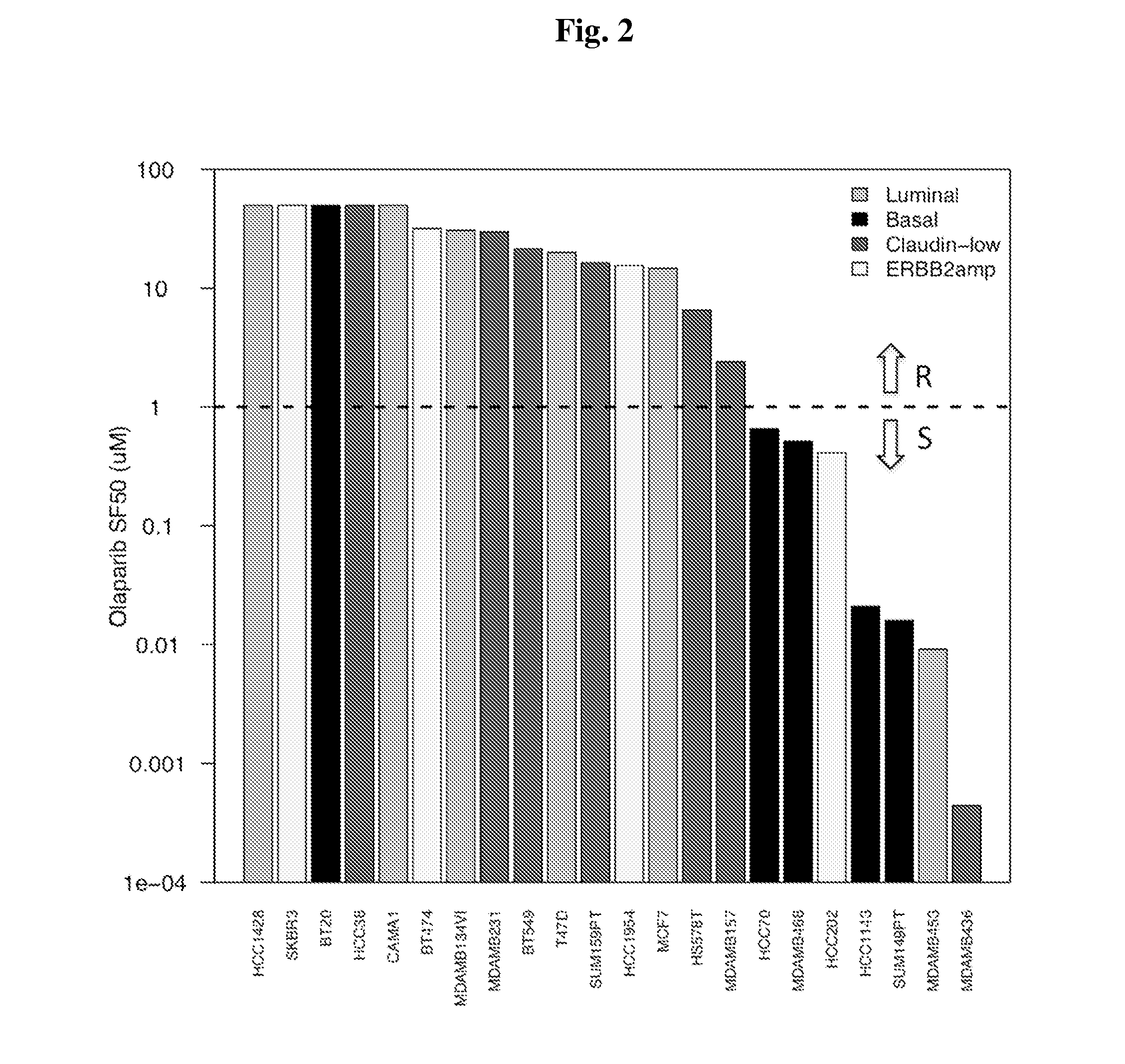
Biomarkers for Prediction of Response to PARP Inhibition in Breast Cancer
InactiveUS20140364434A1High expressionReduce expressionBiocideMicrobiological testing/measurementPARP inhibitorBiomarker (petroleum)
Owner:RGT UNIV OF CALIFORNIA
Olaparib co-precipitate and preparation method thereof
The present invention relates to co-precipitates of olaparib and an ionic polymer and pharmaceutical composition containing the co-precipitates. Further, the present invention relates to a method of treating disorders in a patient in need thereof, comprising administering a therapeutically effective amount of said composition.
Owner:CADILA HEALTHCARE LTD +1
Preparation method of olaparib solid dispersoid and product thereof
InactiveCN106692066ADissolution rate is fastLow costPowder deliveryOrganic active ingredientsSolventPharmaceutical formulation
The invention belongs to the field of pharmaceutical preparations and relates to a preparation method of an olaparib solid dispersoid and a product thereof. The preparation method comprises the following steps: 1) sieving olaparib and a hydrophilic high-molecular compound respectively and uniformly mixing the sieved olaparib and the sieved hydrophilic high-molecular compound at a ratio, so as to obtain a raw auxiliary material mixture; 2) adding the raw auxiliary material mixture into an extruding machine achieving the extruding temperature, and fusing and extruding the raw auxiliary material mixture, so as to obtain strip extrudate; 3) cooling, smashing and sieving the strip extrudate, so as to obtain the olaparib solid dispersoid. The solid dispersoid prepared with the method can effectively improve the dissolving-out speed of drugs, the crude drugs do not needs to be micronized, and no special equipment is required to be used, therefore, the process is simple and easy, the cost is low, the energy consumption is less, no solvent residue exists, other impurities are not introduced in the whole process, and continuous mass production is realized easily.
Owner:江苏开元医药有限公司 +1
Olaparib capsule and preparation method thereof
InactiveCN106551916AEasy to absorb moisture and agglomerateQuality improvementOrganic active ingredientsPharmaceutical non-active ingredientsBULK ACTIVE INGREDIENTActive ingredient
The invention relates to an olaparib capsule and a preparation method thereof. The olaparib capsule comprises olaparib which is an active ingredient, and hydroxypropyl cellulose serving as a disintegrating agent and a bonding agent. The olaparib capsule can be rapidly disintegrated and resolved out, the process is simple, and quality is stable.
Owner:TIANJIN HANKANG PHARMA BIOTECH
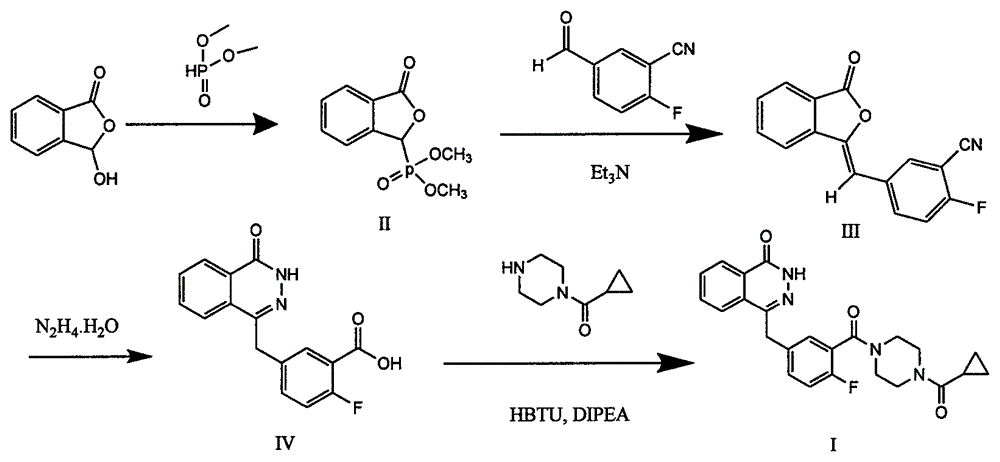
Preparation method of Olaparib intermediate
InactiveCN105085408ARaw materials are easy to getSimple processOrganic chemistryBenzoic acidIsobenzofuran
The invention relates to a preparation method of an Olaparib intermediate (IV), namely, 2-fluoro-5-[(4-oxo-3,4-dihydrophthalazin-1-yl)methyl]benzoic acid. The method comprises steps as follows: 1, 2-fluoro-5-formyl benzoic acid is used as a raw material and reacts with (3-oxo-1,3-dihydro-isobenzofuran-1-yl)dialkyl phosphate to produce 2-fluoro-5-(3-oxo-3H-isobenzofuran-1-yl-methylene)benzoic acid, namely, an intermediate (V); or 2-fluoro-5-formyl benzoic acid reacts with phthalide to produce 5-(2,3-dihydro-1,3-dioxo-1H-indene-2-yl)-2-fluobenzoic acid, namely, an intermediate (VI); 2, the intermediate V or the intermediate VI reacts with hydrazine hydtaye to produce an Olaparib intermediate (IV). The preparation method is concise in process, environment-friendly, economical and suitable for the industrial key Olaparib intermediate, raw materials are easy to obtain, and purification is easy.
Owner:GUANGZHOU YOUMIJIAN PHARMA TECH CO LTD
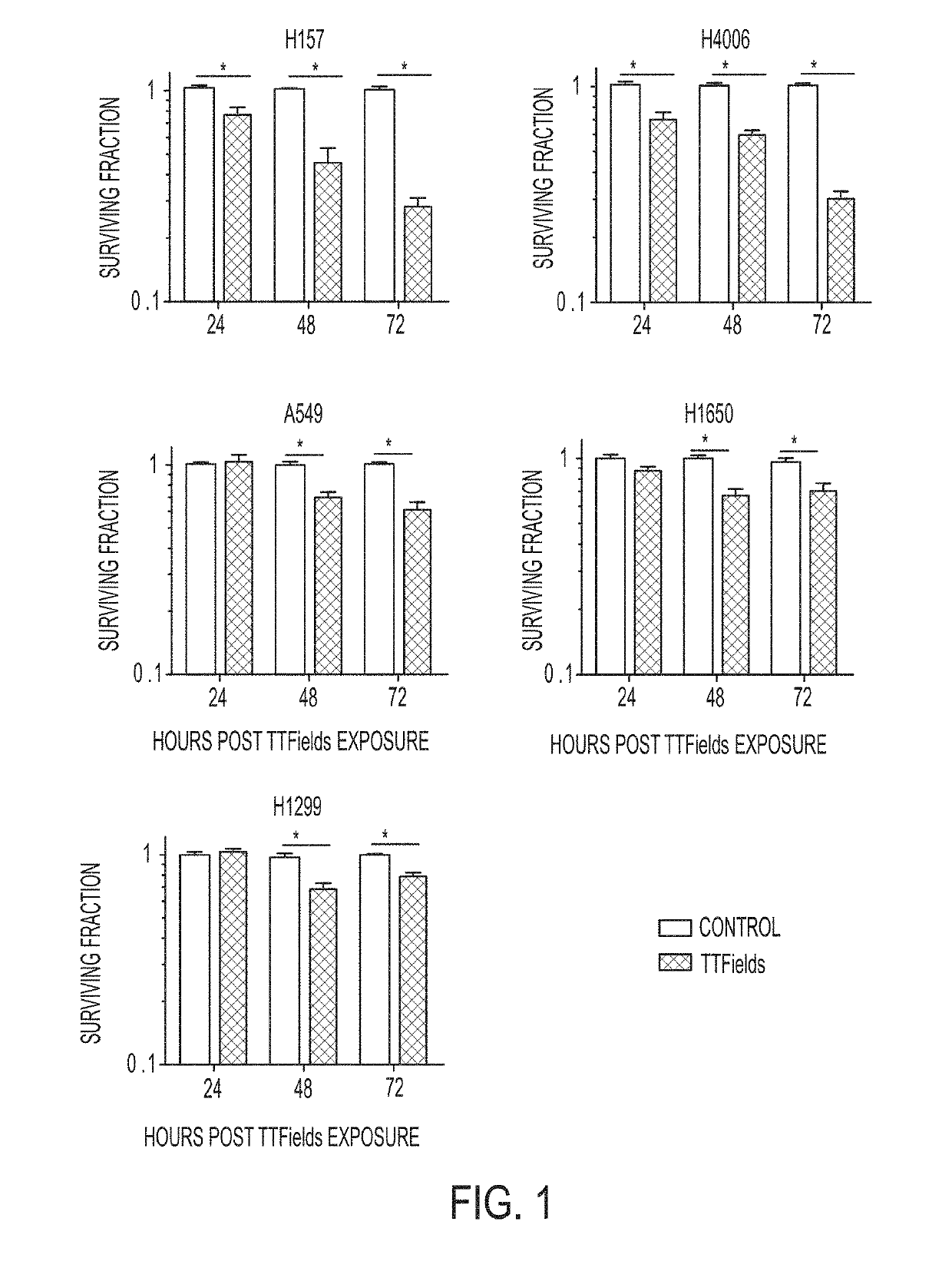
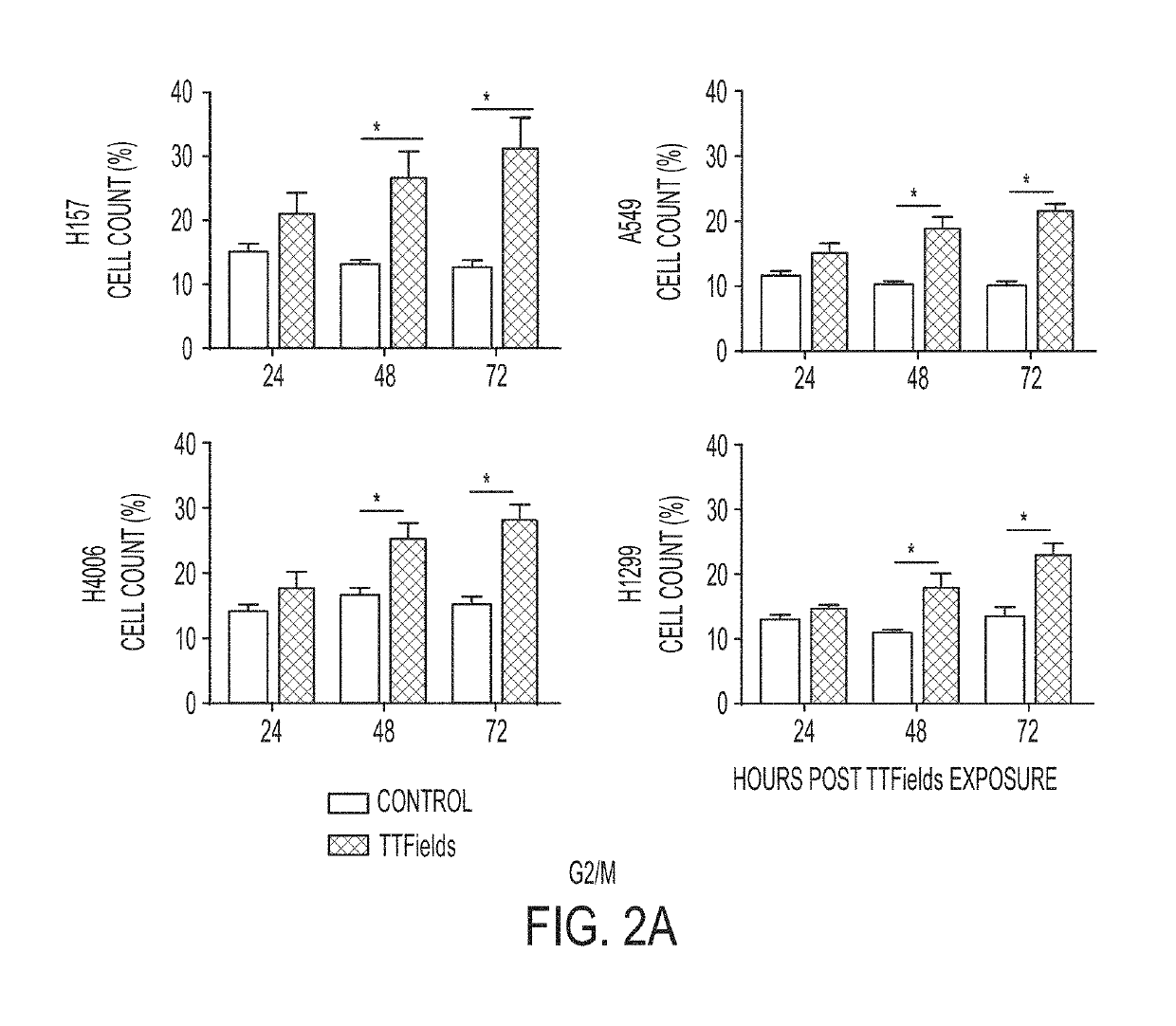
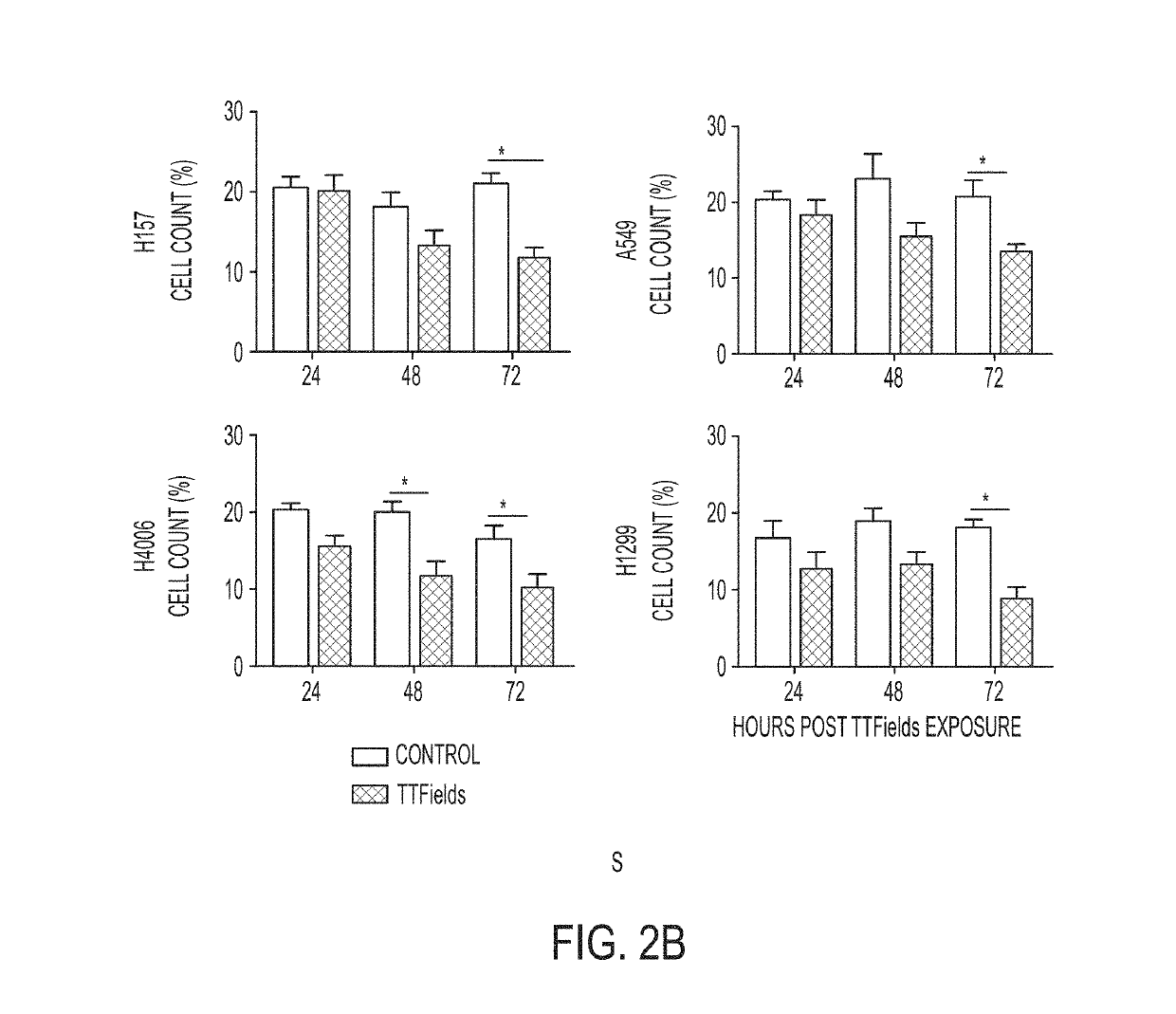
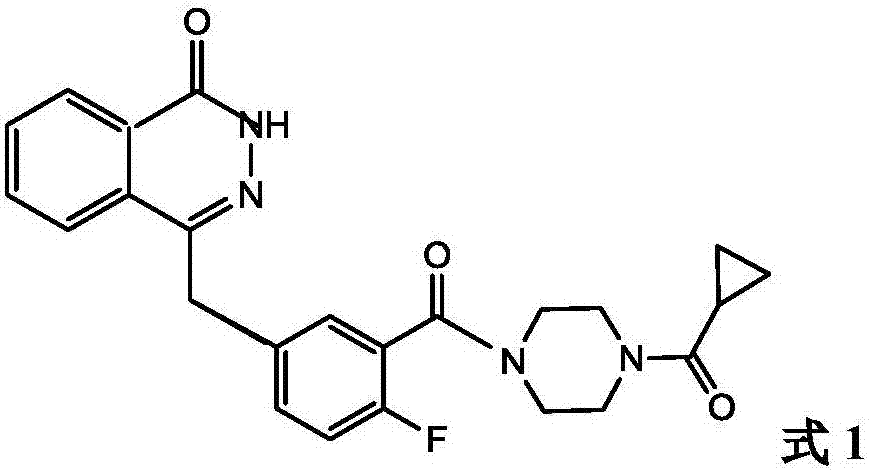
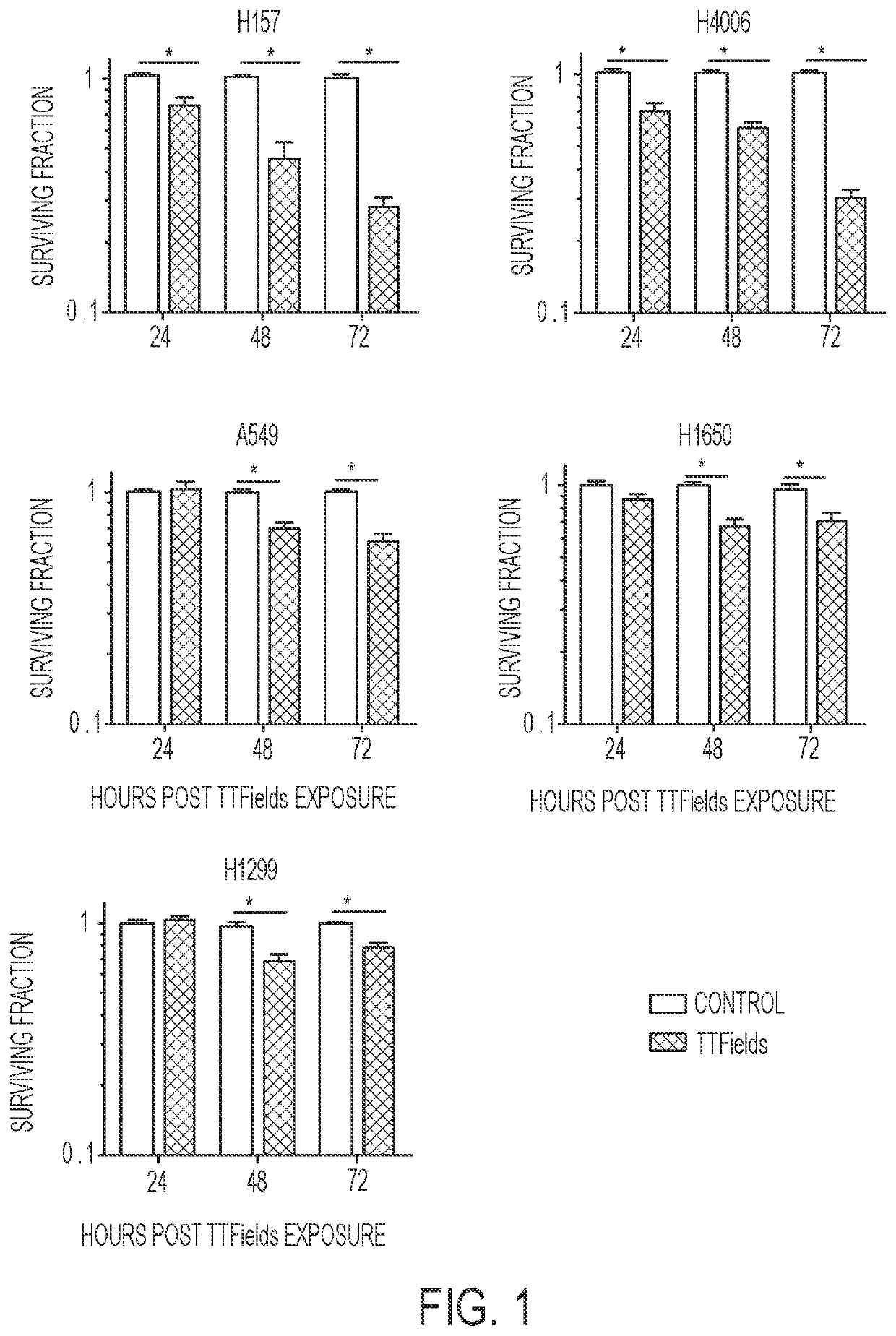
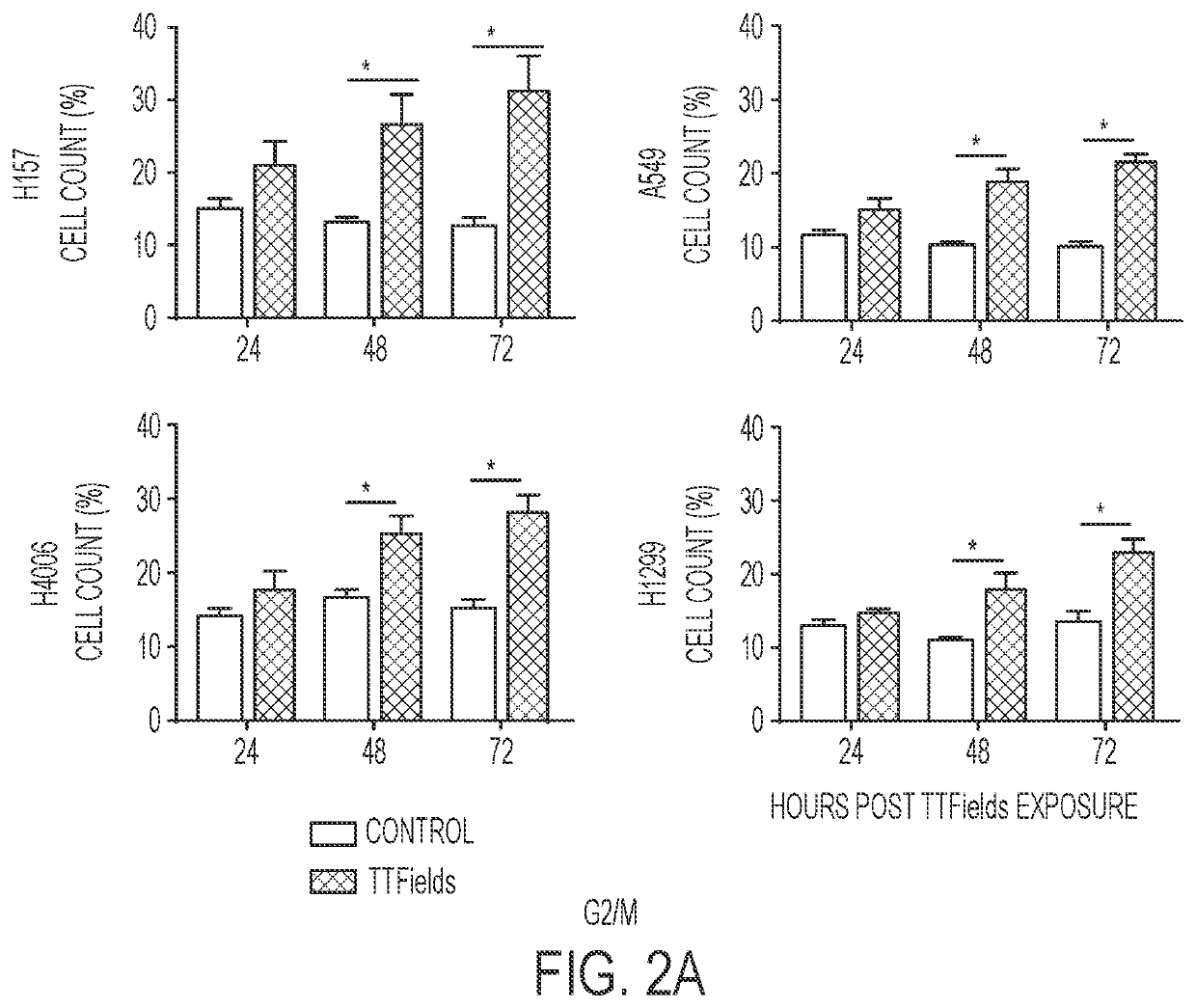
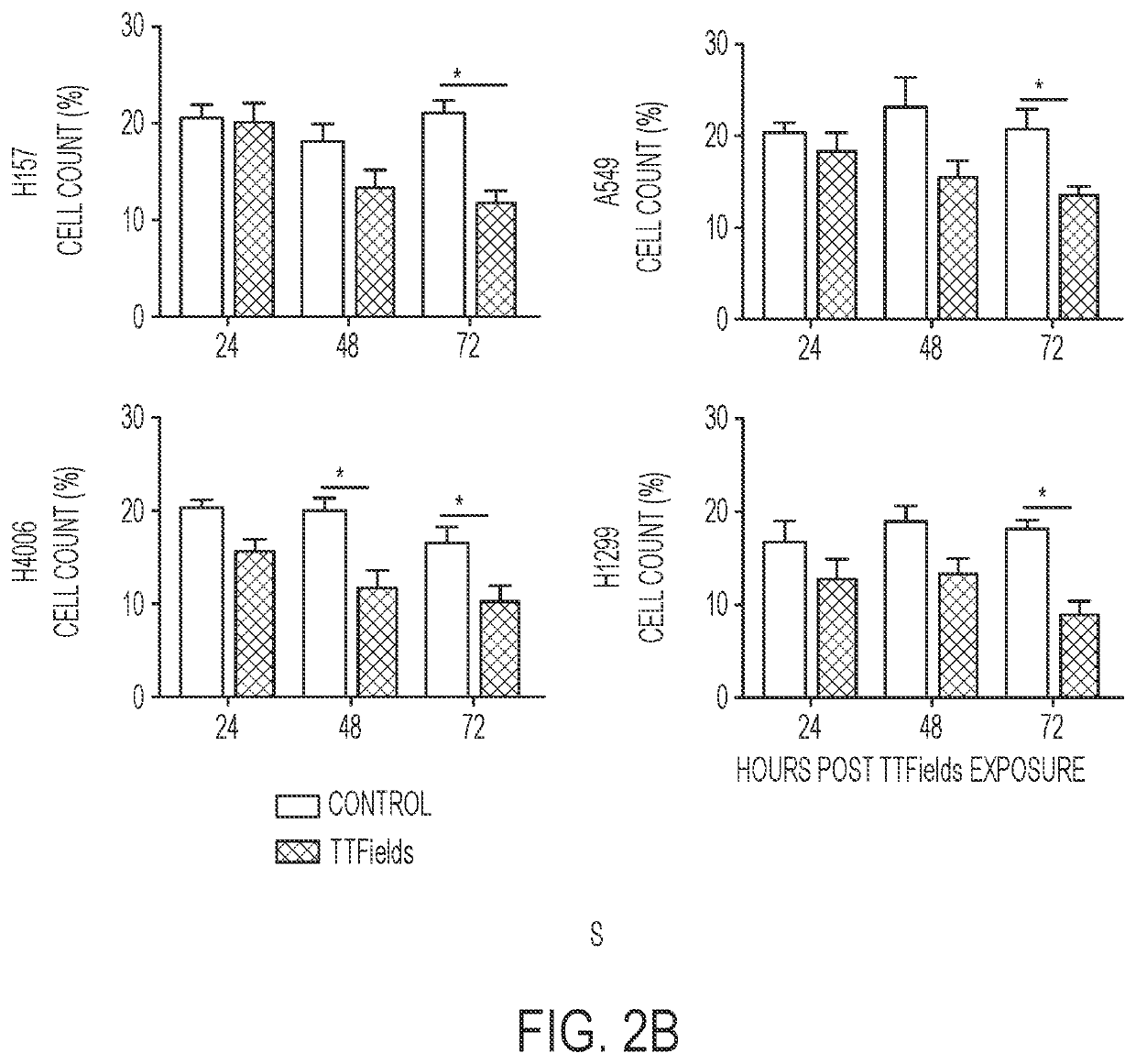
Treating Tumors Using TTFields Combined with a PARP Inhibitor
Tumor-Treating Fields elicit a conditional vulnerability to PARP inhibitors (e.g., Olaparib) in certain cancer cells such as non-small cell lung cancer (NSCLC) cell lines. This conditional vulnerability is exploited in a method of killing cancer cells that comprises delivering a PARP inhibitor to the cancer cells and applying an alternating 80-300 kHz electric field to the cancer cells. At least a portion of the applying step is performed simultaneously with at least a portion of the delivering step. In some embodiments, an additional step of treating the cancer cells with a radiation therapy is added to the method. In some embodiments, the frequency of the alternating electric field is between 100 and 200 kHz.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Olaparib compound refining method
InactiveCN107266370APrevent precipitationEfficient removalOrganic chemistryActivated carbonAcetic acid
The invention relates to an olaparib compound refining method. The method includes synthesis of a crude olaparib product and refining of olaparib. Refining includes steps: 1) adding ethyl acetate petroleum ether mixed liquid and the crude olaparib product into a reaction bottle, slowly heating to 50-55 DEG C, performing heat-preservation stirring for 20min, heating to 70-75 DEG C, stirring, and dissolving the crude product to obtain crude product solution; adding activated carbon into the crude product solution, decolorizing, filtering and collecting filtrate; 2) slowly cooling the filtrate to 20-25 DEG C, keeping the temperature and stirring; 3) cooling the filtrate to 0 DEG C or below, controlling a stirring speed at 15r / min, adding seed crystal, controlling the temperature and the stirring speed to grow the crystal for 1.5h, filtering, flushing a filter cake with a small quantity of ethyl acetate petroleum ether mixed liquid, and drying to obtain a refined olaparib product. The refining method has advantages of simple conditions, reduction of olaparib disubstituted substances, high product purity and the like.
Owner:SHANDONG YUXIN PHARMA CO LTD
Olaparib oral controlled-release and sustained-release pharmaceutical composition and uses thereof
InactiveCN108201536AAchieve absorptionAccurate blood drug concentration in vivoOrganic active ingredientsPill deliveryControl releaseEnzyme inhibition
The present invention relates to an olaparib oral controlled-release and sustained-release pharmaceutical composition, which contains dissolution form improved olaparib and a matrix polymer for release rate adjustment. According to the present invention, the in vivo absorption behavior, the blood drug level and the PARP enzyme inhibition level of the pharmaceutical composition can be controlled, the pharmaceutical composition has the improved olaparib loading and / or oral absorption and / or bioavailability and / or blood drug concentration control and / or enzyme inhibition level control, and can beused as the sole preparation or can be combined with other treatment methods in the treatment of cancer.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Cocrystal of olaparib and urea and preparation method thereof
ActiveCN105753789BGood eutectic stabilityReduce humidityUrea derivatives preparationOrganic active ingredientsSolubilityX-ray
Disclosed are a co-crystal of olaparib and urea and a preparation method therefor. Specifically disclosed is a co-crystal form A, and an X-ray powder diffraction pattern of this crystal form has characteristic peaks at points where 2theta value is 17.4°±0.2°, 20.7°±0.2°, 10.5°±0.2°. The disclosed co-crystal has better stability, lower hygroscopicity and higher solubility as compared to existing olaparib free alkali crystal forms.
Owner:CRYSTAL PHARMATECH CO LTD
Olaparib and malonic acid eutectic crystal and preparation method thereof
PendingCN111825621AImprove oral absorption efficiencyImprove apparent solubilityOrganic active ingredientsOrganic chemistry methodsMalonic acidPhysical chemistry
The invention discloses an olaparib and malonic acid eutectic crystal and a preparation method thereof. In the eutectic crystal, the molar ratio of olaparib to malonic acid is 1: 1; the eutectic X-raypowder diffraction pattern has characteristic peaks when the 2theta value is at the positions of 9.8 + / -0.2 degrees, 12.4 + / -0.2 degrees, 12.6 + / -0.2 degrees, 13.4 + / -0.2 degrees, 16.2 + / -0.2 degrees, 17.5 + / -0.2 degrees, 18.7 + / -0.2 degrees and 21.0 + / -0.2 degrees. The eutectic preparation method is simple in process, easy to control the crystallization process, good in reproducibility and suitable for industrial production. Compared with olaparib free alkali, the eutectic crystal has higher apparent solubility, and is beneficial to improving the oral absorption efficiency of olaparib.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Olaparib and maleic acid cocrystal and preparation method thereof
ActiveCN111689905AImprove oral absorption efficiencyImprove apparent solubilityOrganic active ingredientsOrganic chemistry methodsSolubilityCombinatorial chemistry
The invention discloses an olaparib and maleic acid cocrystal and a preparation method thereof. The molar ratio of olaparib to maleic acid in the cocrystal is 1:1, and an X-ray powder diffraction pattern of the cocrystal has characteristic peaks when the 2theta values are 5.1+ / -0.2 degrees, 9.8+ / -0.2 degrees, 13.7+ / -0.2 degrees, 16.0+ / -0.2 degrees, 17.7+ / -0.2 degrees and 20.0+ / -0.2 degrees. The cocrystal preparation method provided by the invention is simple in process, easy to control the crystallization process, good in reproducibility and suitable for industrial production. Compared with olaparib free alkali, the cocrystal has higher apparent solubility, and is beneficial to improving the oral absorption efficiency of olaparib.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Treating Tumors Using TTFields Combined with a PARP Inhibitor
Tumor-Treating Fields elicit a conditional vulnerability to PARP inhibitors (e.g., Olaparib) in certain cancer cells such as non-small cell lung cancer (NSCLC) cell lines. This conditional vulnerability is exploited in a method of killing cancer cells that comprises delivering a PARP inhibitor to the cancer cells and applying an alternating 80-300 kHz electric field to the cancer cells. At least a portion of the applying step is performed simultaneously with at least a portion of the delivering step. In some embodiments, an additional step of treating the cancer cells with a radiation therapy is added to the method. In some embodiments, the frequency of the alternating electric field is between 100 and 200 kHz.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
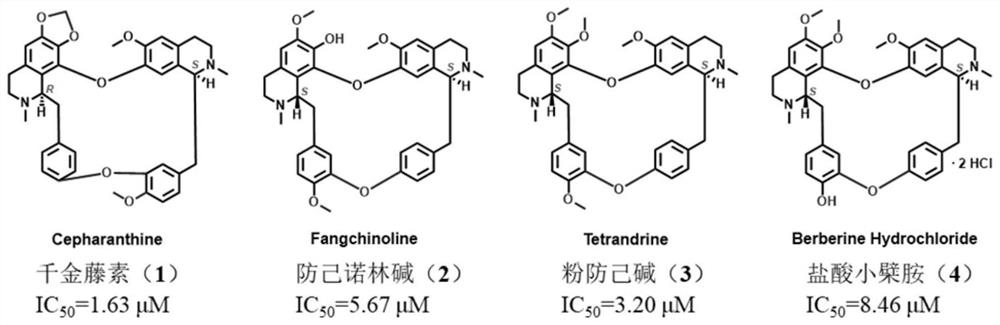
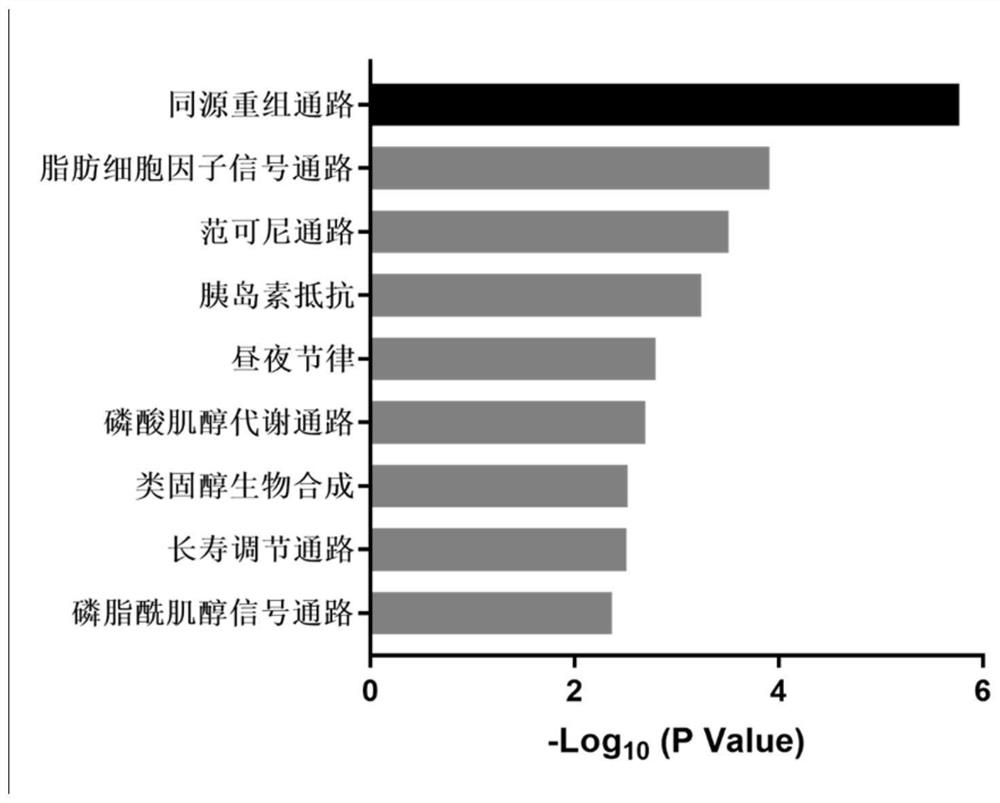
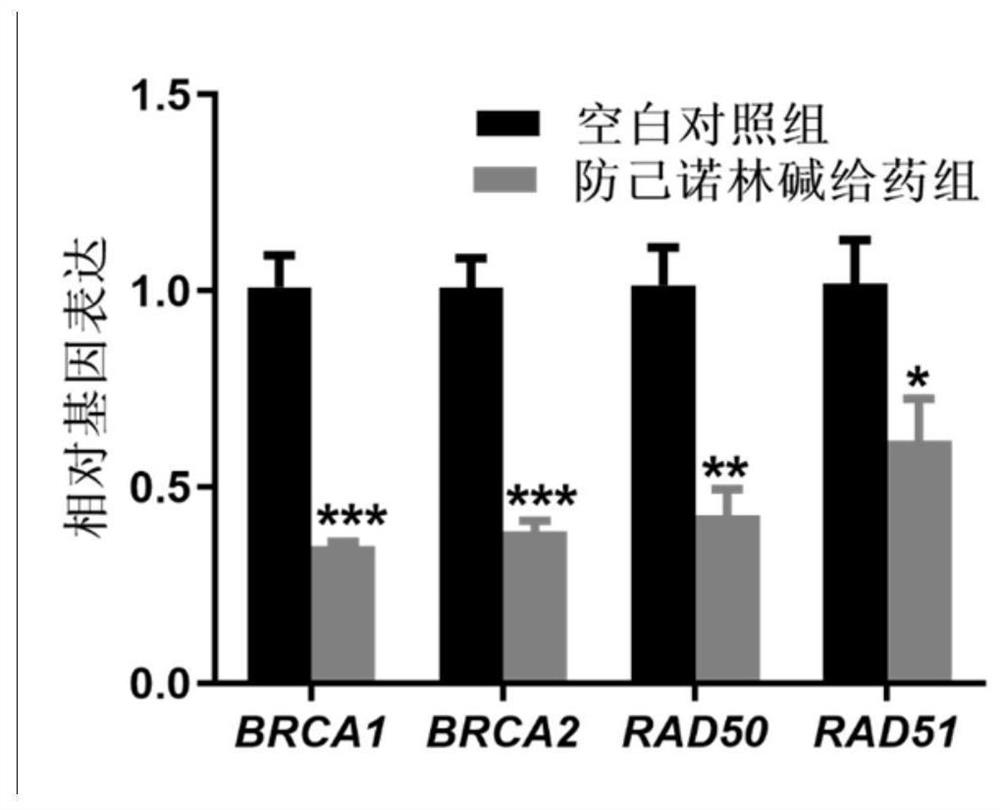
Action mechanism and application of fangchinoline in resisting conjunctival melanoma
The invention relates to application of a compound fangchinoline with a tetrahydroisoquinoline parent nucleus in the aspect of resisting conjunctival melanoma and research on an action mechanism of the compound fangchinoline. Specifically, experiments show that fangchinoline directly targets far-end upstream binding protein 2 (FUBP2), so that gene expression of c-Myc protein is reduced, expression of key proteins RAD51 and BRCA1 of c-Myc downstream homologous recombination (HR) pathways is reduced, and the purpose of inhibiting tumor replication is achieved. The compound disclosed by the invention can be administrated together with cisplatin or olaparib to increase the anti-conjunctival melanoma drug effect and reduce the drug toxicity, so that the compound has a good clinical application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH +1
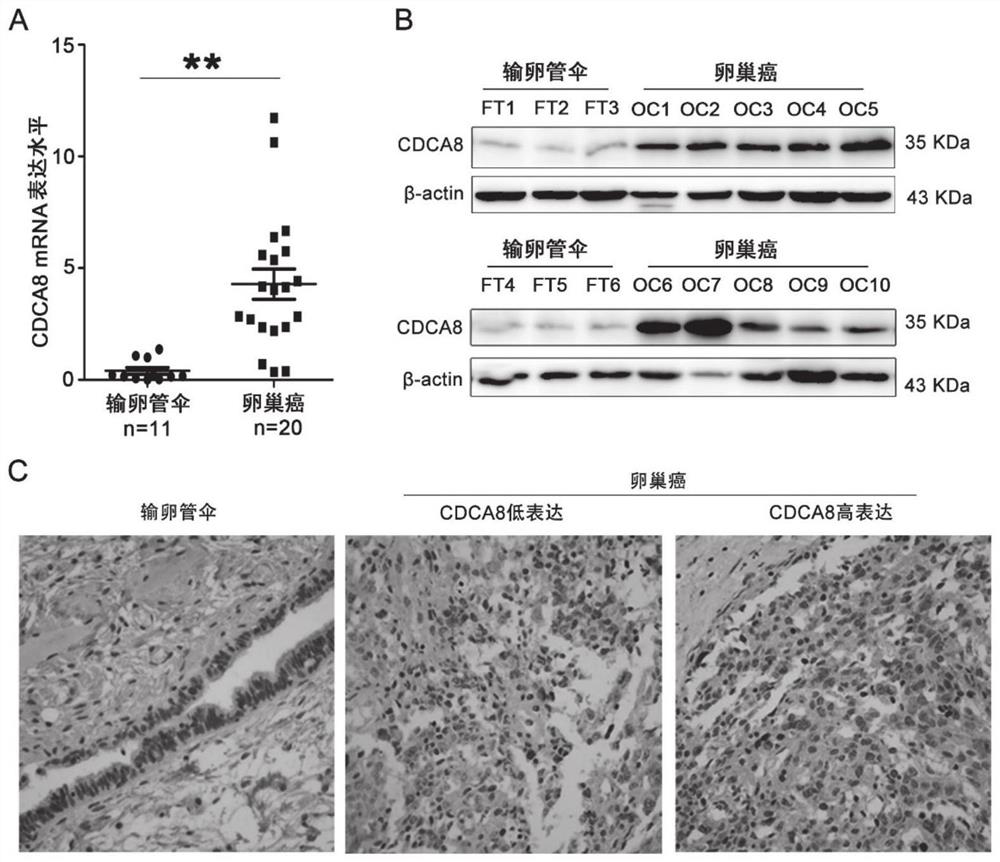
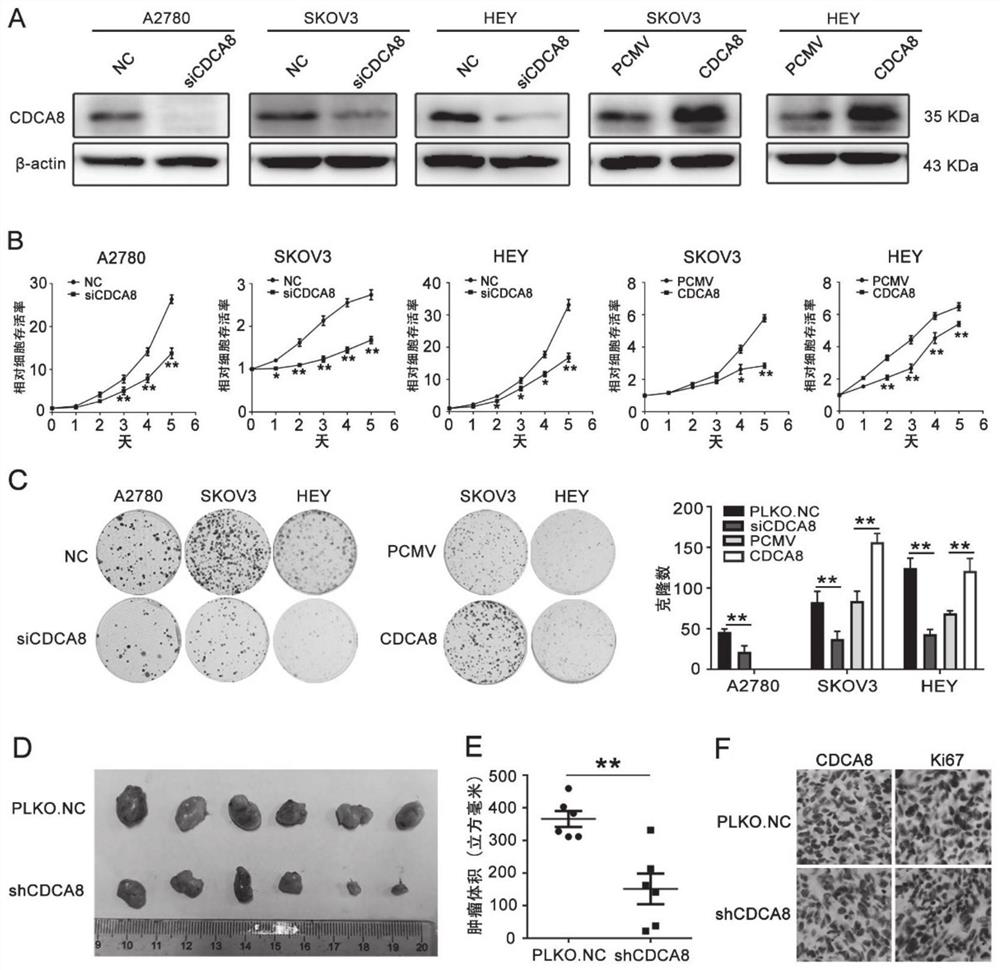
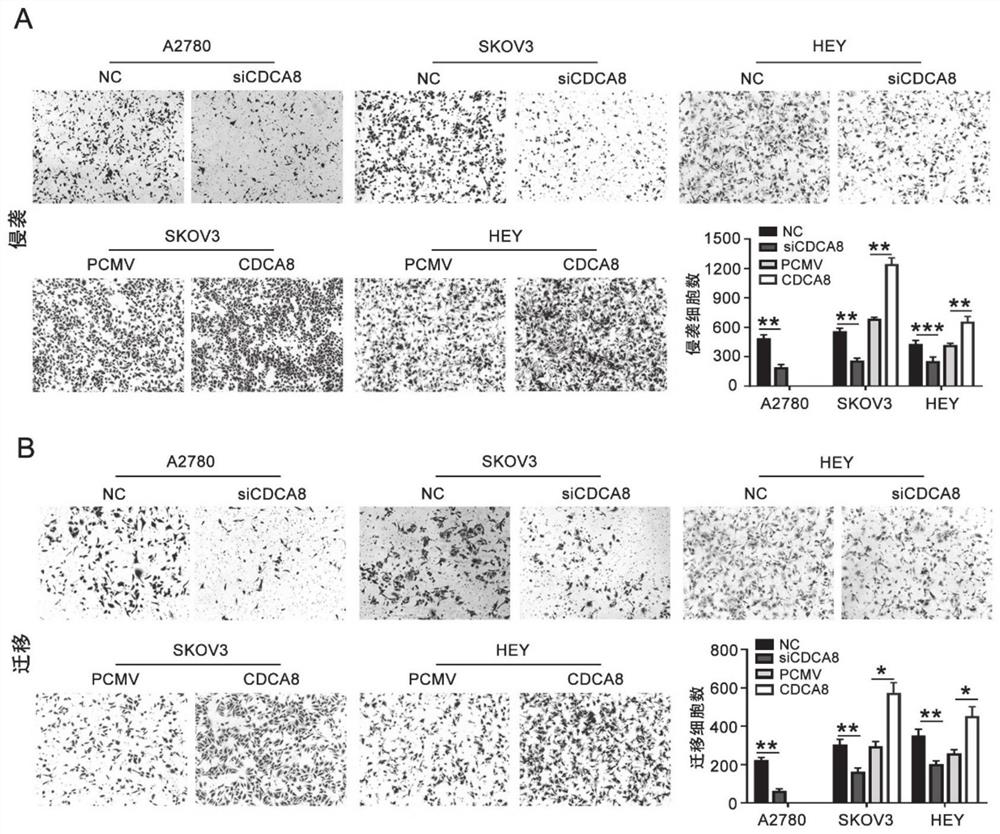
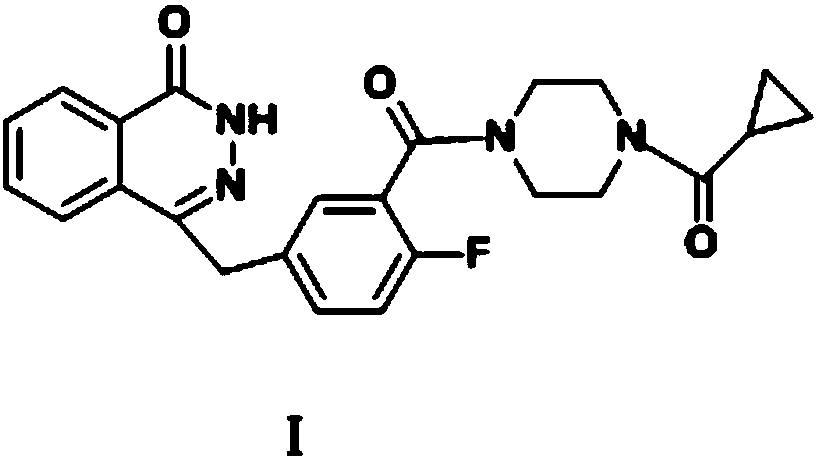
Application of CDCA8 in preparation of drug for treating ovarian cancer
ActiveCN112007161APromote proliferationPromote invasionOrganic active ingredientsInorganic active ingredientsCancer cellOncology
The invention belongs to the field of biological medicines, and particularly relates to an application of CDCA8 in the preparation of a drug for treating ovarian cancer. The invention provides an application of a reagent for inhibiting CDCA8 gene expression or inhibiting a CDCA8 gene expression product in the preparation of a drug for treating ovarian cancer and / or inhibiting proliferation, invasion and migration of ovarian cancer cells and at the same time promoting ovarian cancer cell cycle arrest and apoptosis. In addition, the inhibition of the expression of CDCA8 or the inhibition of theCDCA8 gene expression product can enhance the sensitivity of ovarian cancer cells to chemotherapeutic drugs cisplatin and olaparib by adjusting the cell cycle and inhibiting DNA damage repair. A new thought is provided for screening drugs for treating ovarian cancer, especially serous ovarian cancer, and a new method is provided for enhancing the sensitivity to traditional ovarian cancer chemotherapeutic drugs.
Owner:SHANDONG UNIV QILU HOSPITAL
Olaparib refining method
InactiveCN108586355ARaw materials are cheap and easy to getThe synthetic route is simpleOrganic chemistryAcetic acidEthyl ester
The invention discloses an olaparib refining method, which comprises: (1) dissolving an olaparib crude product in a mixed solvent of ethyl acetate and acetone, and controlling the temperature at 45-50DEG C until olaparib is completely dissolved; (2) adding active carbon, decolorizing, filtering, cooling the filtrate to a temperature of -10-0 DEG C, crystallizing, and growing the crystal; and (3)filtering, washing the filtered solid by using acetone, and carrying out vacuum drying to obtain the refined olaparib. With the refining method of the present invention, the purity of the obtained olaparib can reach more than 99.9%, the total impurity and the single impurity are respectively controlled within 0.1% and 0.05%, the quality of the product is remarkably improved, the refining process is easy to operate, and the refining method is suitable for industrial production.
Owner:SHANDONG YUXIN PHARMA CO LTD
A kind of preparation method of olaparib
ActiveCN105820126BEasy to operateSimple processing capacityOrganic chemistryState of artCatecholborane
The invention discloses a preparing method for Olaparib. 5-bromomethyl-2-fluorobenzaote serves as a raw material and is subjected to a boric acid reaction with catecholborane, and a compound 3 is obtained; the compound 3 is subjected to a Suzuki coupling reaction, and a compound 5 is obtained; the compound 5 is subjected to a hydrolysis reaction, and a compound 6 is obtained; the compound 6 reacts with a compound 7 under the action of a CDI catalyst, and Olaparib is obtained. According to the preparing method, the raw material is easy to obtain, the course is short, operation and posttreatment are simple, the reaction conditions in all the steps are mild, the reaction yields of all the steps reach 90% or above, the total yield is increased to 82.3% from 49% achieved in the prior art, and the preparing method is environmentally friendly and suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
Combination drug composition for treating liver cancer
InactiveCN109512822APrevent proliferationLow growth inhibitionOrganic active ingredientsAntineoplastic agentsA-DNAOncology
The invention relates to a combination drug composition for treating liver cancer. The combination drug composition is prepared from a PARP1 protein inhibitor Olaparib and a DNA-PKcs protein inhibitorNU7441. A DNA double strand breakage repair passage in a liver cancer cell is highly activated by protein inhibited by the combination drug composition, and accordingly an effective target spot is provided for treating liver cancer. Compared with the prior art, the micromolecular drug Olaparib can interdict homologous recombination repairing and selective non-homologous end joining repairing in liver cancer tissue, while NU7441 can interdict the non-homologous end jointing repairing, so that a combination of the two drugs can completely interdict the DNA double strand breakage repairing passage and thereby effectively inhibit proliferation of liver cancer, and the tumor increasing inhibition rate is as lowest as 62.3%, and as highest as 83.9%.
Owner:TONGJI UNIV
Olaparib dihydrate and preparation method thereof
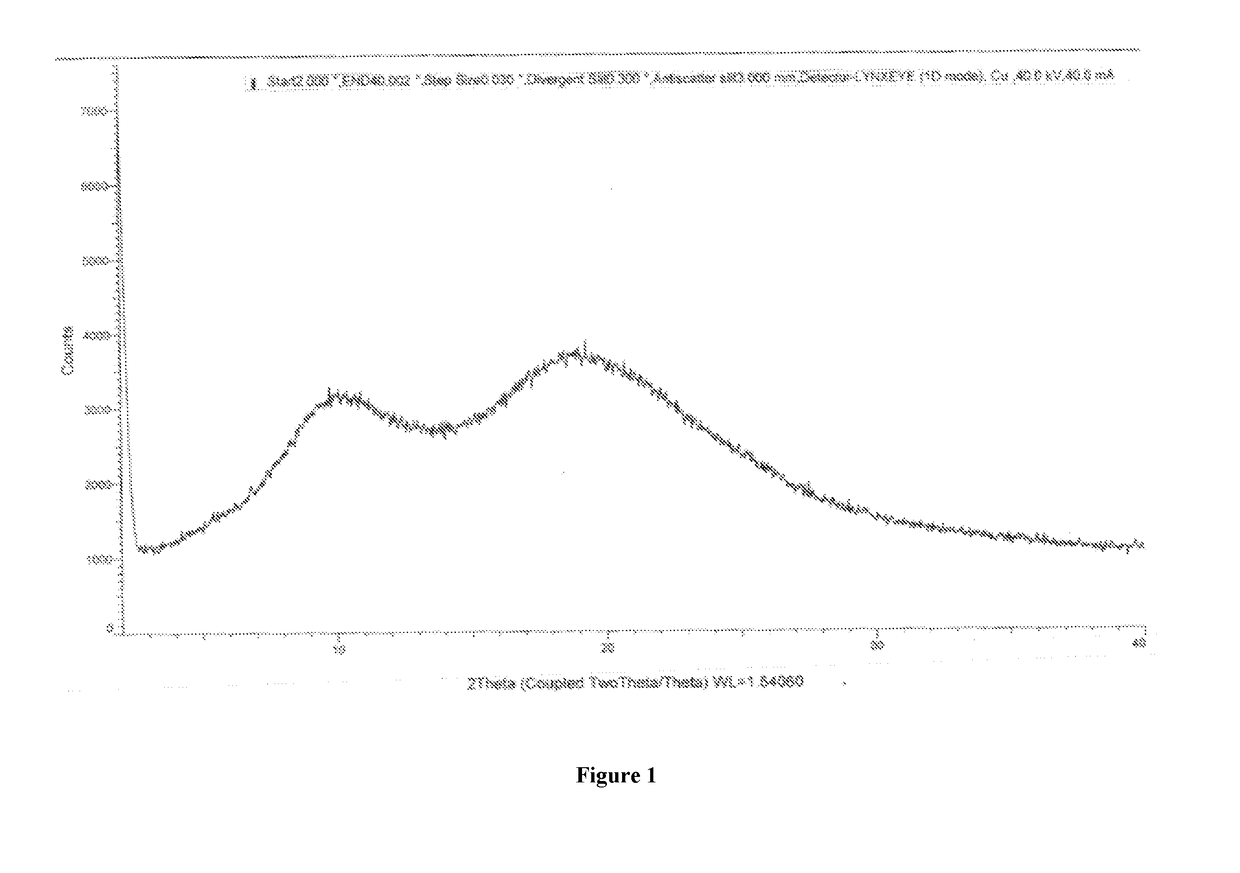
InactiveCN107098862AHigh purityImprove stabilityOrganic active ingredientsOrganic chemistry methodsSolubilityOlaparib
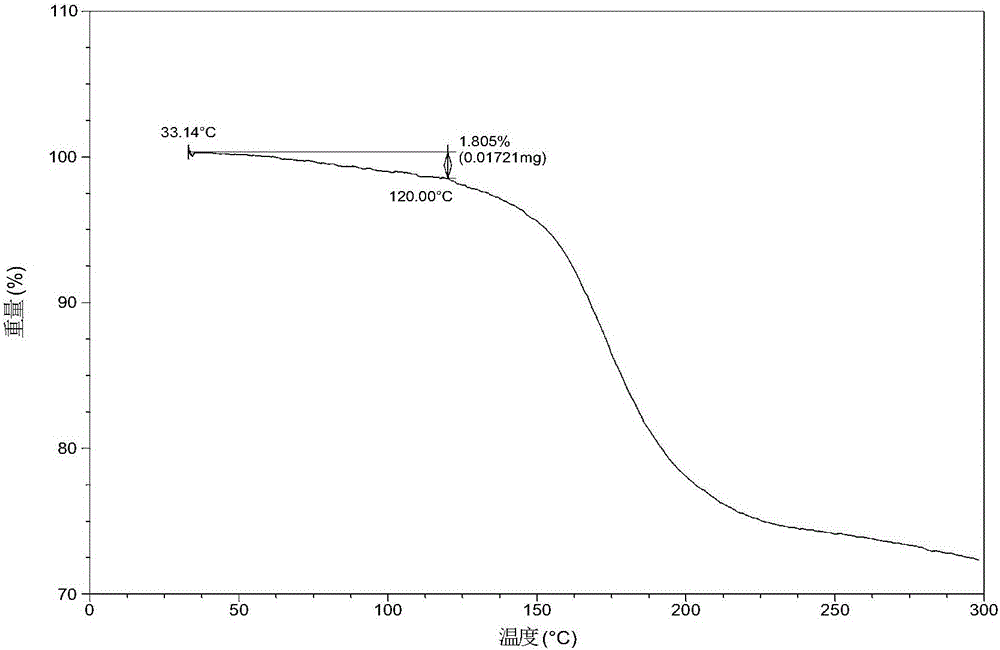
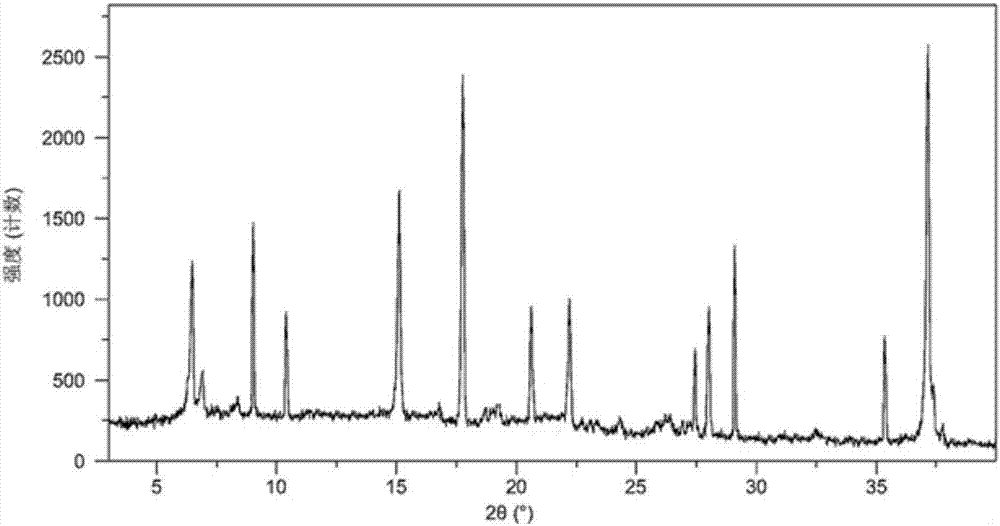
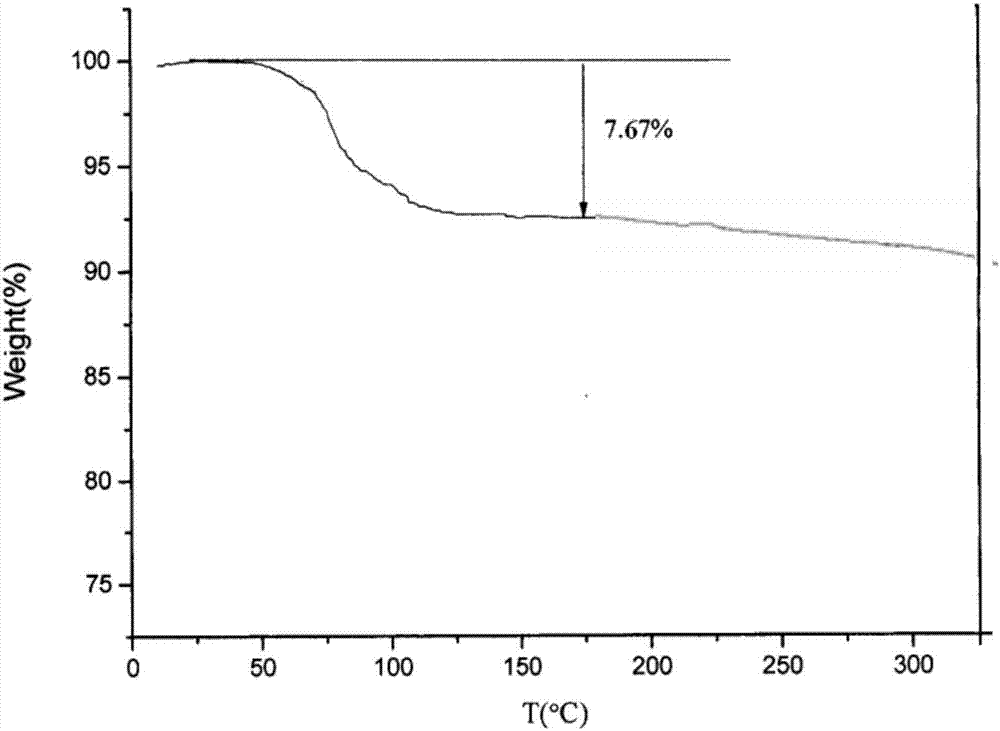
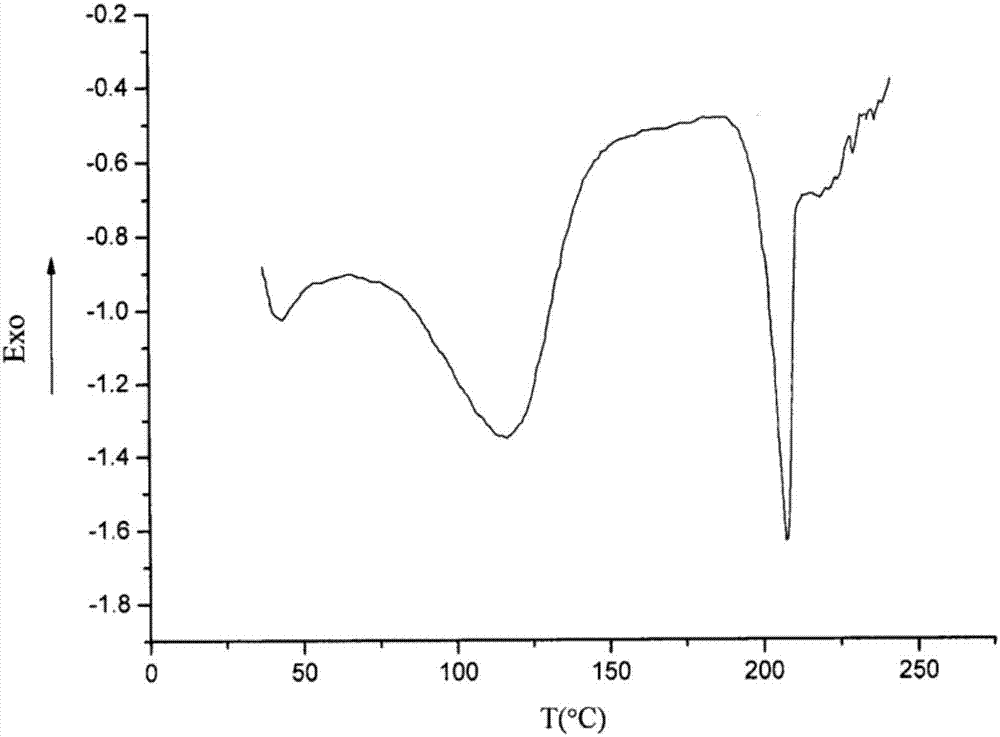
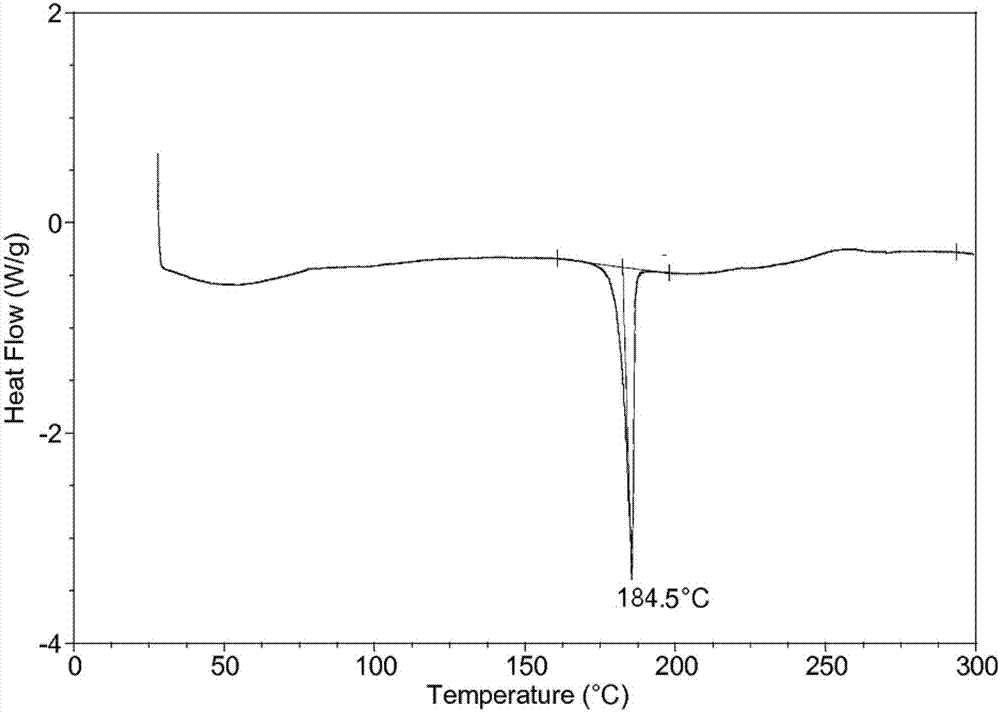
The invention belongs to the technical field of medicine, and discloses an olaparib dihydrate and a preparation method thereof. Characteristic diffraction peaks are displayed at 6.75 DEG, 7.04 DEG, 9.16 DEG, 10.53 DEG, 15.20 DEG, 17.46 DEG, 20.68 DEG, 22.21 DEG, 27.43 DEG, 28.62 DEG, 29.30 DEG, 35.23 DEG and 37.14 DEG of X-ray powder diffraction spectra of the crystalline compound expressed at diffraction angles of 2Theta+ / -0.2 DEG, and the X-ray powder diffraction spectra measured by the aid of Cu-K alpha rays are shown as a graph 1 and are totally different from X-ray powder diffraction spectra in the prior art. The experiment proves that the dissolvability of the olaparib dihydrate is obviously increased. The invention also discloses the preparation method of the olaparib dehydrate, the preparation method is simple and easy to carry out, the yield and purity are high, the reaction condition is mild, and the method is suitable for large scale production. A dissolution rate and the stability of the capsule prepared by the olaparib dihydrate are obviously increased, and the capsule is very suitable for clinical application.
Owner:SHANDONG YUXIN PHARMA CO LTD
Preparation method of medicine Olaparib for treating ovarian cancer
InactiveCN107382873APrevent precipitationEfficient removalOrganic chemistryMethyl carbonatePharmaceutical drug
The invention relates to a preparation method of medicine Olaparib for treating ovarian cancer. The preparation method comprises the following steps of 1, adding Olaparib crude product into mixed solvent of dimethyl carbonate and isopropyl ether, slowly heating to 45-50 DEG C, keeping warm and stirring for 30min, continuously raising to 60-65 DEG C, stirring until the crude product is dissolved to acquire crude production solution; 2, adding active carbon, into the crude product solution, decoloring, filtering and collecting filtrate; 3, slowly cooling to reduce the temperature of the filtrate to 30-35 DEG C, keeping warm and stirring; and 4, further cooling the filtrate to be 5 DEG C and below, controlling a stirring speed to be 150 turns / min, adding seed crystal, filtering after controlling the temperature and the steering speed to grow the crystal for 2h, washing a filter cake with a small amount of mixed solvent of dimethyl carbonate and isopropyl ether, and drying to acquire the Olaparib. The preparation method provided by the invention is simple in technology, high in yield and purity, particularly significantly reduced in bisubstituted product, and meanwhile an applicant is surprised to find that the prepare Olaparib is significantly reduced in hygroscopicity.
Owner:HUNAN QIWEI TECH CO LTD
Olaparib compound and preparation method thereof
InactiveCN107162985AEasy to prepareEasy to operateOrganic active ingredientsOrganic chemistry methodsX-rayDissolution
The present invention belongs to the technical field of medicine, and discloses an olaparib compound and a preparation method thereof, wherein the olaparib of the present invention has advantages of high purity and good stability, and has characteristic diffraction peaks in the X-ray powder diffraction pattern when the 2[theta]+ / -0.2 DEG C diffraction angle is 4.2679 DEG, 10.1345 DEG, 12.6058 DEG, 15.3421 DEG, 18.6022 DEG, 19.8103 DEG, 22.9050 DEG and 25.2146 DEG, and the X-ray powder diffraction pattern obtained through Cu-K[alpha] ray measurement is represented by Fig. 1 and is completely different from the X-ray powder diffraction pattern in the prior art. The experiment results show that the obtained olaparib has significantly improved solubility. The invention further discloses a preparation method of the olaparib, wherein the preparation method has advantages of simple and easy operation, high yield, high product purity and mild reaction conditions, and is suitable for large-scale production. The dissolution and the stability of the capsules prepared from the olaparib of the present invention are remarkably improved, and the obtained capsules are suitable for clinical use.
Owner:SHANDONG YUXIN PHARMA CO LTD
Radiolabeled tracers for poly (adp-ribose) polymerase-1 (parp-1), methods and uses therefor
ActiveUS20160339124A1High radiochemical purityExcellent specific activityOrganic chemistryAntipyreticPolymerase LKetone
Disclosed arc PARP-1 inhibitors, which can be 18P-labeled for use as tracers in positron emission tomographic (PET) imaging. Further disclosed are methods of synthesis. Of the compounds synthesized, 2-[p-(2-Fluoroethoxy)phenyl]-1,3,10-triazatricyclo[6.4.1.04,13]trideca-2,4(13),5,7-tetraen-9-one (12) had the highest inhibition potency for PARP-1 (IC50=6.3 nM). Synthesis of [18F]-12 is disclosed under conventional conditions in high specific activity with 40-50% decay-corrected yield, MicroPET imaging using [18F]-12 in MDA-MB-436 tumor-bearing mice demonstrated accumulation of [18F]-12 in a tumor. Binding, can be blocked by olaparib. The compounds have utility for tumor imaging.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Olaparib-urea eutectic crystal and preparation method thereof
ActiveCN111995582AImprove oral absorption efficiencyImprove apparent solubilityUrea derivatives preparationOrganic active ingredientsPhysical chemistryPowder diffraction
The invention discloses an olaparib-urea eutectic crystal and a preparation method thereof. In the eutectic crystal, a molar ratio of olaparib to urea is 1: 2; in an X-ray powder diffraction pattern of the eutectic crystal, characteristic peaks occur when the value of 2theta is 6.4 + / - 0.2 degrees, 14.3 + / - 0.2 degrees, 15.0 + / - 0.2 degrees, 18.6 + / - 0.2 degrees, 19.2 + / - 0.2 degrees, 24.3 + / -0.2degrees and 24.8 + / -0.2 degrees. The preparation method of the eutectic crystal provided by the invention is simple in process, a crystallization process is easy to control, good in reproducibility and suitable for industrial production. Compared with olaparib free alkali, the eutectic crystal has higher apparent solubility and is beneficial to improving the oral absorption efficiency of olaparib.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Antineoplastic drug hydrate and preparation method thereof
PendingCN107056712AHigh purityImprove stabilityOrganic active ingredientsOrganic chemistry methodsSolubilityX-ray
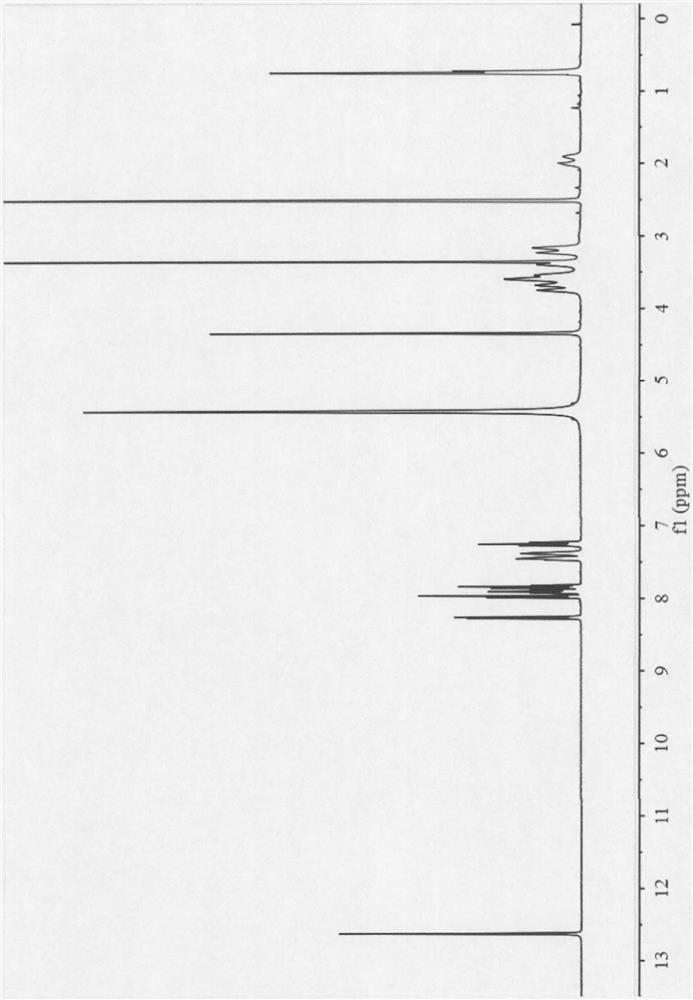
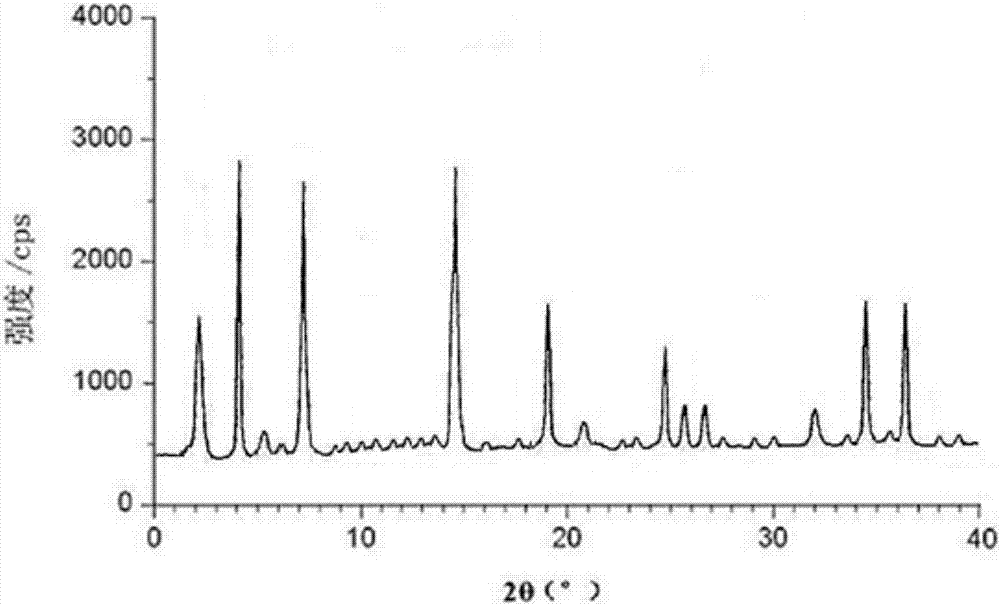
The invention belongs to the technical field of medicines, discloses an antineoplastic drug hydrate and a preparation method thereof, and particularly discloses an Olaparib hydrate and a preparation method thereof. An X-ray powder diffraction pattern, represented by the diffraction angle of 2theta+ / -0.2 degrees, of the Olaparib hydrate shows characteristic diffraction peaks at 2.228 degrees, 4.309 degrees, 7.125 degrees, 14.613 degrees, 19.134 degrees, 24.672 degrees, 25.448 degrees, 26.317 degrees, 32.115 degrees, 34.451 degrees and 36.228 degrees, the X-ray powder diffraction pattern obtained by using Cu-Kalpha rays for measurement is shown in figure 1, and the Olaparib hydrate is completely different from the prior art. It is surprisingly found through experiments that the solubility of the Olaparib hydrate is remarkably improved. The preparation method of the antineoplastic drug hydrate is also disclosed and simple and easy to operate, the yield and the purity are high, the reaction condition is mild, and the antineoplastic drug hydrate is suitable for large-scale production. The dissolution and stability of capsules made from the Olaparib hydrate are significantly improved, and the Olaparib hydrate is very suitable for clinical application.
Owner:刘德鹏
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com