Olaparib compound and preparation method thereof
A technology of olaparib and its compound, which is applied in the field of olaparib compound and its preparation, can solve problems such as inability to break through, and achieve the effects of improved solubility, improved dissolution rate and stability, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: Preparation of olaparib compound
[0050] (1) Get 100 g of the crude product of olaparib, add 1500 ml of mixed solvent of chloroform / petroleum ether (0.5:1 by volume), stir and heat at 200 rpm until completely dissolved, decolorize activated carbon, and filter;
[0051] (2) Reduce the filtrate of step (1) from 3°C to 10°C every 10 minutes, add 1500ml of ethanol dropwise at 1.5mL / min, and continue to cool down to -5°C every 10 minutes to crystallize;
[0052] (3) Insulate and stir until the crystallization is complete, grow the crystal for 2 hours, filter with suction, wash with water, and dry at 40°C to obtain 99.92g of white crystalline powder with a yield of 99.92% and a purity of 99.99%.
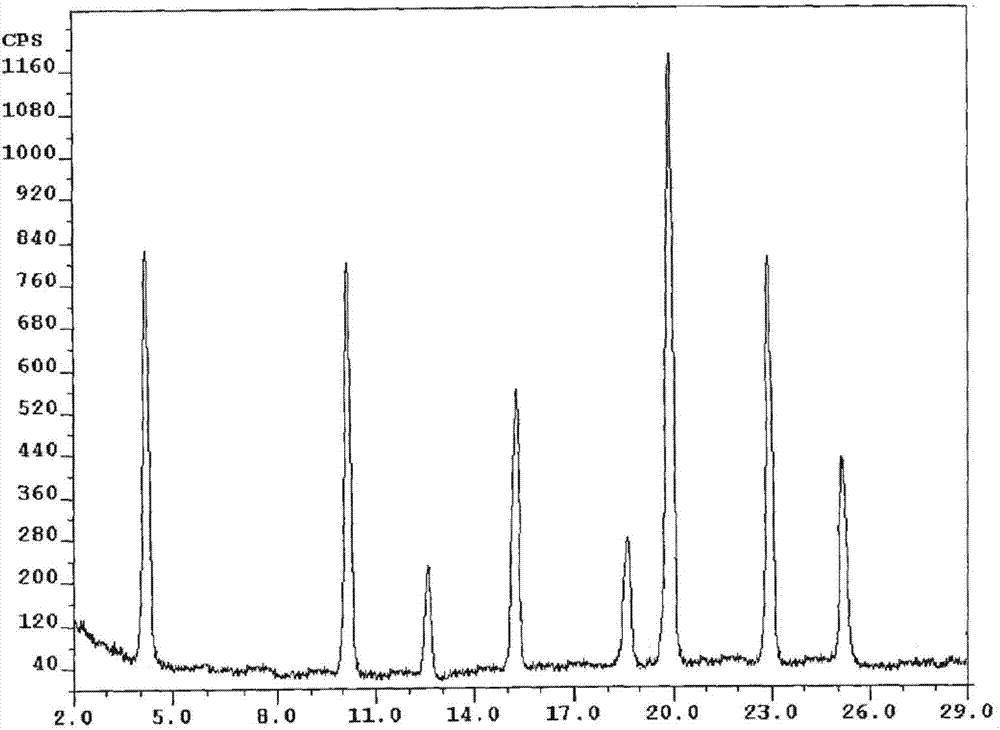
[0053] The X-ray powder diffraction spectrogram that the prepared white crystalline powder uses Cu-Kα ray measurement to obtain is shown in figure 1 .
Embodiment 2
[0054] Embodiment 2: Preparation of olaparib compound
[0055] (1) Get 100 g of the crude product of olaparib, add 1000 ml of mixed solvent of chloroform / petroleum ether (1:1 by volume), stir and heat at 200 rpm until completely dissolved, decolorize activated carbon, and filter;
[0056] (2) Reduce the filtrate of step (1) from 4°C to 10°C every 10 minutes, add 1200ml of ethanol dropwise at 2.0mL / min, and continue to cool down from 3°C to -3°C every 10 minutes to crystallize;
[0057] (3) Insulate and stir until crystallization is complete, grow crystals for 2.5 hours, filter with suction, wash with water, and dry at 45°C to obtain 99.91g of white crystalline powder with a yield of 99.91% and a purity of 99.98%.
[0058] The X-ray powder diffraction spectrum obtained by measuring the prepared white crystalline powder using Cu-Kα rays is similar to that of Example 1.
Embodiment 3
[0059] Embodiment 3: Preparation of olaparib compound
[0060] (1) Get 100g of the crude product of olaparib, add 1200ml of mixed solvent of chloroform / petroleum ether (1:1 by volume), stir and heat at 200 rpm until completely dissolved, decolorize activated carbon, and filter;
[0061] (2) Reduce the filtrate of step (1) from 2°C to 10°C every 10 minutes, add 1320ml of ethanol dropwise at 1.0mL / min, and continue to cool down from 3°C to 0°C every 10 minutes to crystallize;
[0062] (3) Insulate and stir until crystallization is complete, grow crystals for 3 hours, filter with suction, wash with water, and dry at 40°C to obtain 99.89g of white crystalline powder with a yield of 99.89% and a purity of 99.99%.
[0063] The X-ray powder diffraction spectrum obtained by measuring the prepared white crystalline powder using Cu-Kα rays is similar to that of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com