Olaparib capsule and preparation method thereof
A technology of olaparib and capsules, which is applied in the field of medicine, can solve the problems of easy stickiness of olaparib raw materials, unstable quality of finished products, and slow dissolution rate, etc., to meet the needs of clinical use, simple production process, and stable quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
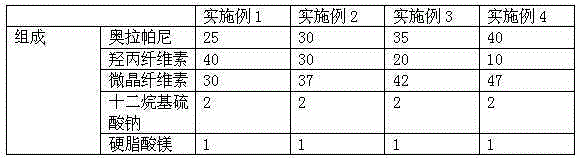
Embodiment 1~3
[0028] The weight percent of each component of embodiment 1~3
[0029]
[0030] The preparation method of olaparib capsules:
[0031] 1) Pass olaparib through a 200-mesh sieve, filler and hydroxypropyl cellulose through a 80-mesh sieve, and mix evenly to obtain olaparib premixed powder;
[0032] 2) Prepare an ethanol solution with a concentration of 60%, and its dosage is 55% of the total weight of olaparib, hydroxypropyl cellulose, filler, cosolvent and lubricant; dissolve the cosolvent with half the weight of ethanol solution to obtain Ethanol solution of co-solvent;
[0033] 3) Put the olaparib premixed powder in the wet granulator, add the ethanol solution of the co-solvent, add the remaining ethanol solution and purified water according to the granulation of the material, and stir to obtain the wet granulator passing through a 14-mesh sieve. particles;
[0034] 4) Dry the wet granules; granulate;
[0035] 5) Mix the granules with the lubricant passed through a 80-m...
experiment example 1
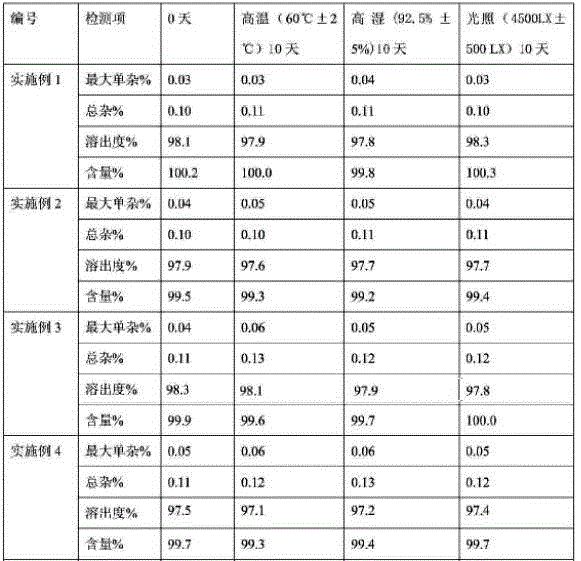
[0036] Experimental example 1 In order to investigate the quality stability of the above-mentioned different formula products under different conditions, they were placed under the conditions of high temperature (60°C±2°C), high humidity (92.5%±5%), and light (4500LX±500LX) for 10 days. Day, to investigate the influencing factors.
[0037]
[0038] From the above test results, the products produced under the process conditions are less affected by temperature, humidity and light, and have good quality stability.
experiment example 2
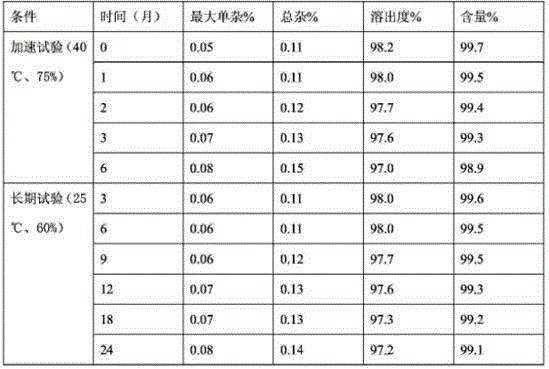
[0039] Experimental example 2 In order to investigate the quality stability of the product during the long-term storage process, we selected the product of Example 1 and placed it under the condition of 40°C and 75% humidity for 6 months, and placed 24 samples under the condition of 25°C and 60% humidity. Month, inspect the stability of product quality.
[0040]
[0041] From the results of product stability investigation in the following examples, the product produced under the process conditions has good quality stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com