Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
67results about How to "Meet the requirements of clinical use" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
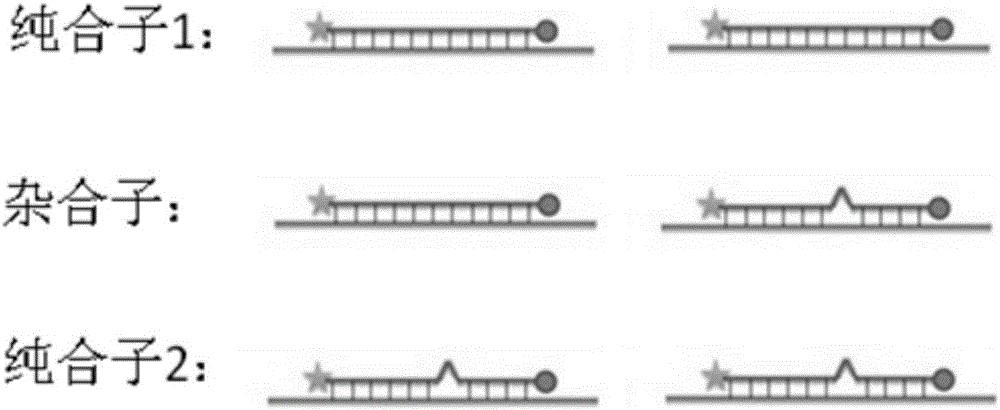
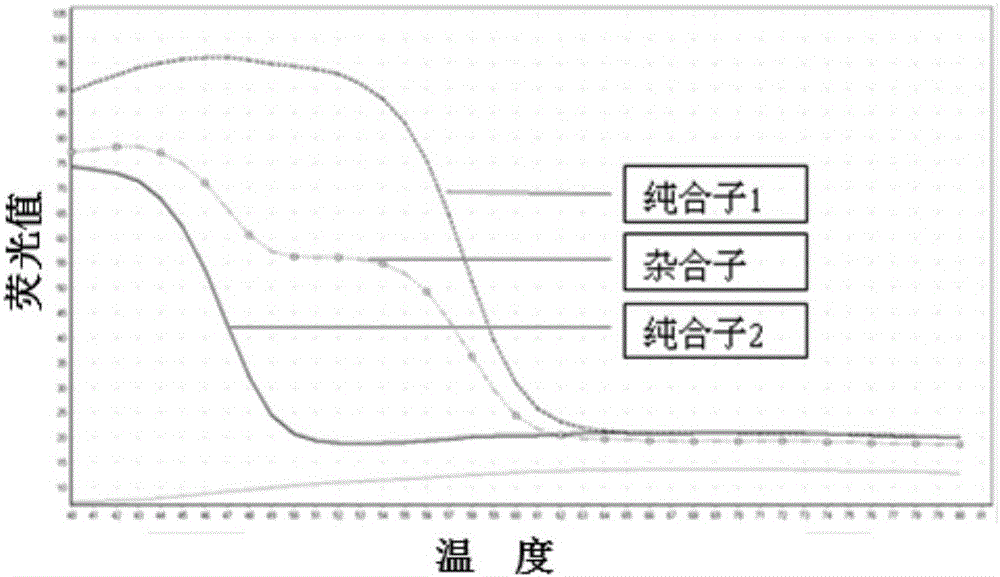
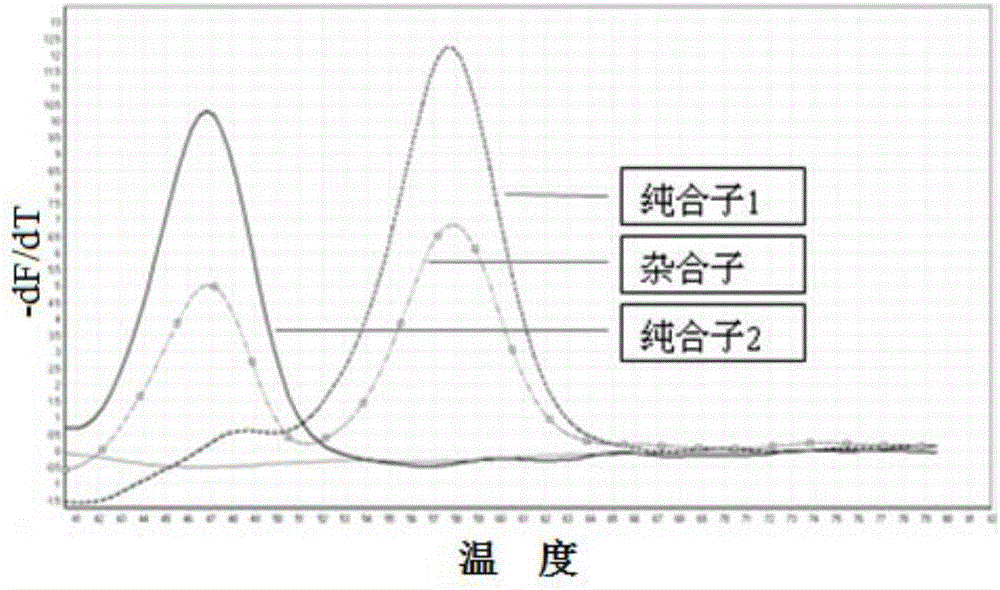
SNP (single nucleotide polymorphism) combination, detection method and kit for detecting liver damage susceptible genotype of antitubercular drug
ActiveCN106119363AReduce drug riskImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationGeneticsAntituberculous drugs
The present invention relates to an SNP (single nucleotide polymorphism) combination, detection method and kit for detecting liver damage susceptible genotype of an antitubercular drug and belongs to the technical field of medical molecular biological diagnosis; the SNP combination includes 7 SNP sites, and nucleotide sequences of the 7 SNP sites are shown sequentially as in SEQ ID NO. 1-7; the present invention also relates to an SNP detection method, comprising PCR (polymerase chain reaction) amplification and double-labeled probe melting curve analytical reaction, and primer pairs and double-labeled probe sequences for detection of the 7 SNP sites are shown as in SEQ ID NO. 8-20. The SNP site combination, detection method and kit provided herein enables quick, accurate, simple and high-throughput detection for a patient's genotype and prediction for the liver damage risk due to the patient using the antitubercular drug.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY +1
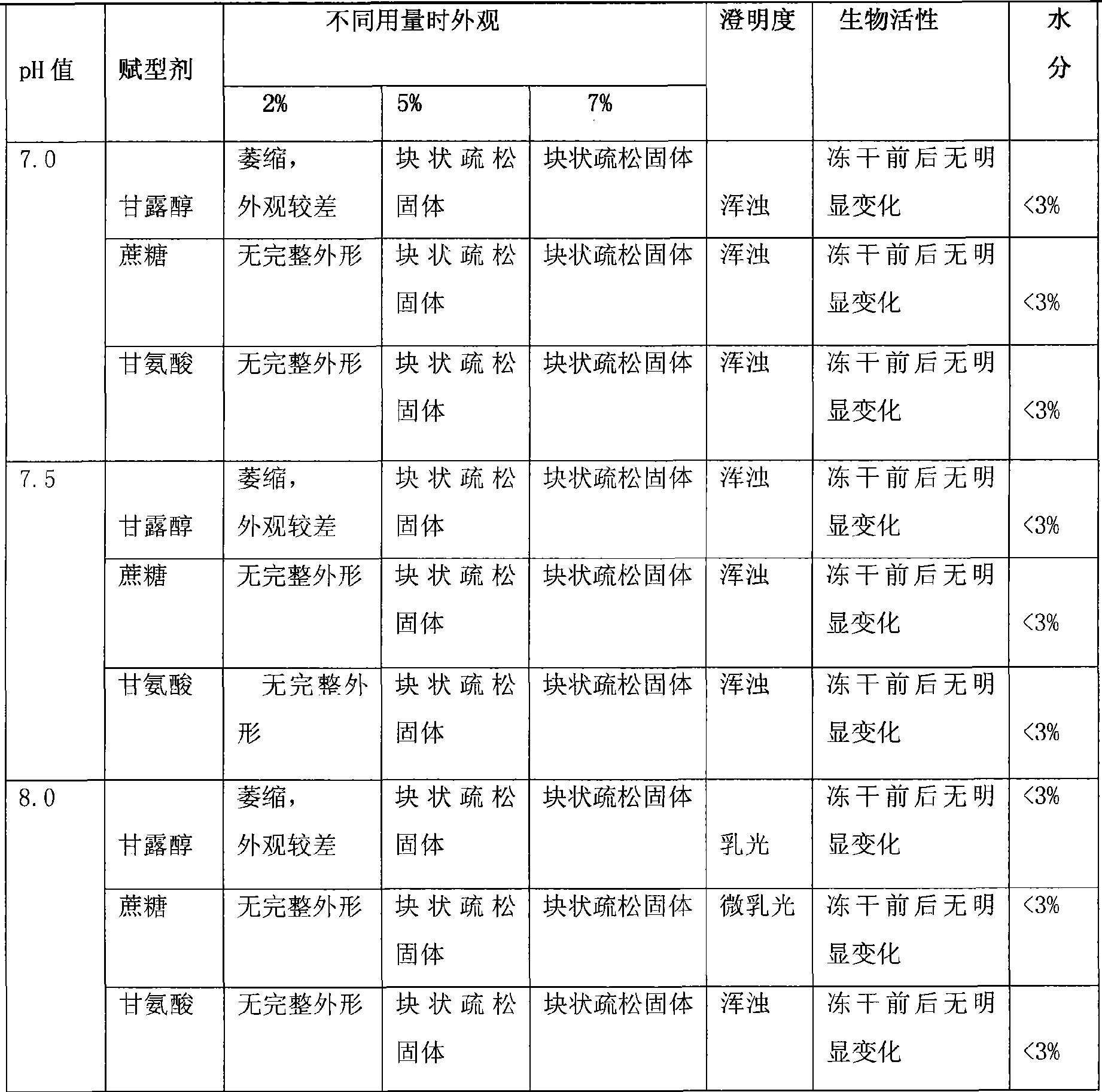
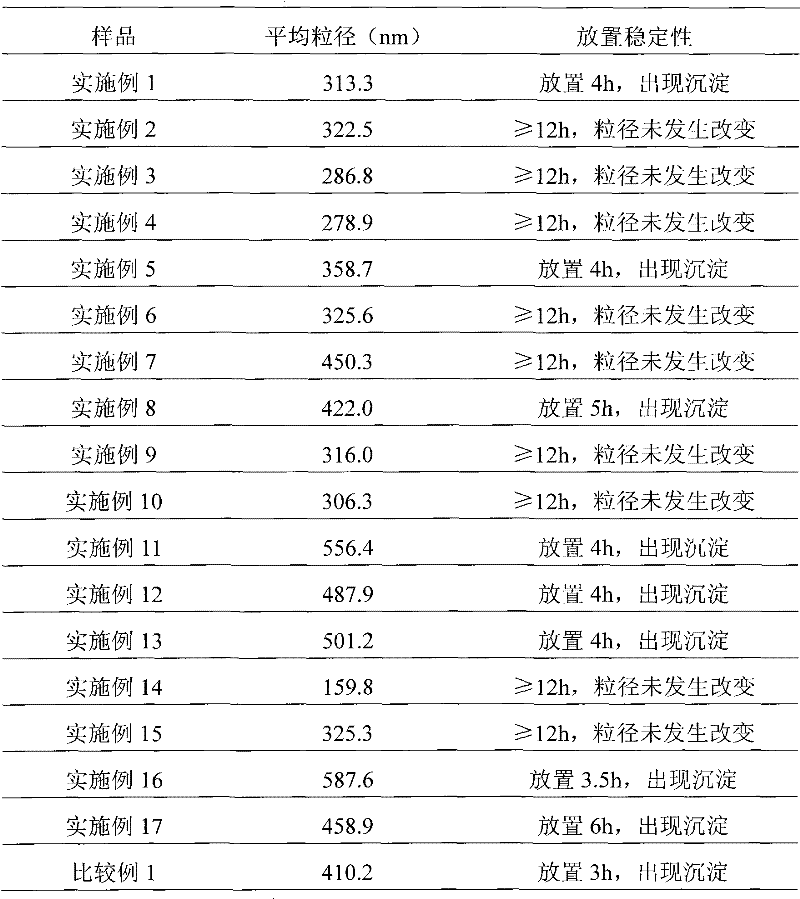
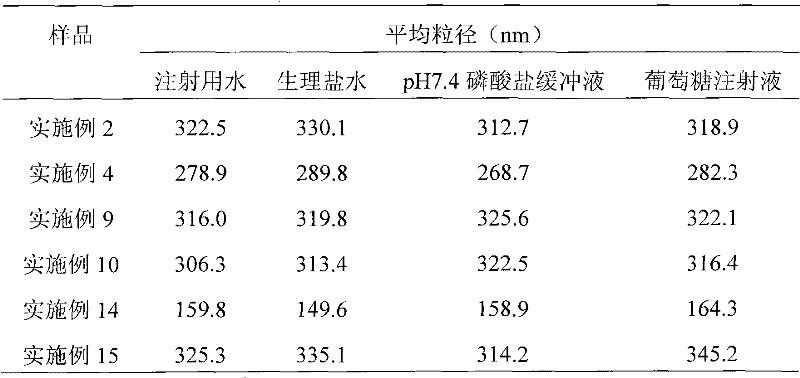
Paclitaxel albumin subparticles for injection and preparation method thereof
ActiveCN102274190ADenaturation does not produceMeet the requirements of clinical useOrganic active ingredientsPowder deliveryOrganic solventFreeze-drying
The invention discloses a paclitaxel alhumin submicron for injection. The paclitaxel alhumin submicron comprises 1-10% (W / W) of paclitaxel, 10-80%(W / W) of alhumin and 10-85% (W / W) of freeze-drying protective agent. The invention also discloses a preparation method of the paclitaxel alhumin submicron. The method comprises the following steps of 1) adding paclitaxel into tert-butyl alcohol, stirring and dissolving to be used as an organic phase; 2) adding the alhumin and the freeze-drying protective agent to water or a buffer with a pH within 6.0-8.5, stirring and dissolving to be used as a water phase; 3) adding the organic phase into water with stirring, stirring and dissolving, filtering and degerming through a millipore filtration method, and placing in a clean container; 4) freezing at-30- -50 DEG C, removing tert-butyl alcohol and water through freeze-drying by a lyophilizer to obtain freeze-drying powder with good profile and loosening quality. During usage, the freeze-drying powder is added to a solvent for injection and redissolved, so that paclitaxel alhumin submicrons with an average particle size of 100-600nm are formed. The paclitaxel alhumin submicron for injection ofthe invention has advantages of a simple preparation technology, no high toxic organic solvent and long-term storage, etc.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Disposable high speed turbine dental drill handpiece with light producing device
InactiveCN101658443AMeet clinical needsMeet the requirements of clinical usePoint-like light sourceElectric circuit arrangementsButton batteryElectrical battery
The invention relates to a disposable high speed turbine dental drill handpiece with a light producing device which is characterized in that a place of a front operating handle close to a handpiece isconnected with a lamp socket for installing a light-emitting diode, the head of the light-emitting diode is exposed from a hole of the lamp socket and opposites to a cutting position of a dill point,two electrodes at the tail part of the light-emitting diode are respectively connected with two light-emitting diode lead wires; a rear operation handle is provided with a battery bin which is provided with a battery bin cover, a space containing a illuminating gas switch is arranged below the battery bin, the bottom of the space is communicated with an air passage of the illuminating gas switchin the rear operating handle, the air passage of the illuminating gas switch is parallel to the air passage of a driving drill point and the two air passages are both communicated with a gas pipelineat the tail part of the rear operating handle, a button battery is put in the battery bin, the positive pole of the button battery is communicated with a lead wire of the light-emitting diode, and thenegative pole of the button battery is connected with the other lead wire of the light-emitting diode by the illuminating gas switch. The dental drill handpiece is characterized by low cost, safe andconvenient use, and good illuminating effect, and is applicable to be arranged in various dental units.
Owner:BEIJING NORTH POLE DENTAL HANDPIECE
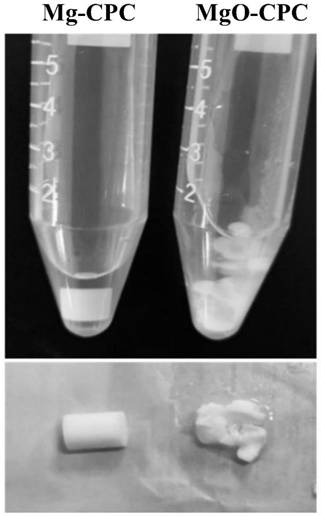
Preparation method of medical biodegradable zinc alloy capillary wire material
The invention provides a preparation method of a medical biodegradable zinc alloy capillary wire material. A medical biodegradable zinc alloy is sequentially subjected to vacuum melting and homogenizing treatment to obtain a zinc alloy cast ingot, then the zinc alloy cast ingot is sequentially subjected to skin turning and extrusion, a zinc alloy coarse bar is obtained and sequentially subjected to rotary swaging and annealing, a fine bar is obtained, the surface of the obtained fine bar is coated with a graphite lubricant coating, then the fine bar is subjected to cold drawing for wire drawing, and the medical biodegradable zinc alloy capillary wire material is obtained, wherein the diameter of the capillary wire material is smaller than 0.1 mm. The preparation method is a compound machining method comprising vacuum melting, thermal extrusion forming, rotary swaging and cold drawing for wire drawing, the purposes of enhancing the material performance and plastification can be achieved through surface lubrication and proper thermal treatment of the special procedures, the microstructure grain fragmentation can reach the nanoscale, the comprehensive mechanical performance is excellent, the surface quality is good, and corrosion resistance, degradation uniformity and degradation speed all meet the clinical application requirements.
Owner:XIAN ADVANCED MEDICAL TECH
Reagent for determining content of human cholyglycine by using latex immunoturbidimetry technology
InactiveCN108982860AImprove detection accuracyEliminate distractionsMaterial analysisSerum igePhosphate
The invention discloses a reagent for determining the content of human cholyglycine by using a latex immunoturbidimetry technology. The reagent is prepared from a reagent body 1 and a reagent body 2,wherein the reagent body 1 is prepared by adding 100 mM of an auxiliary agent, a phosphate buffer solution with the pH value of 8.0, a sodium chloride solution with the concentration of 0.9%, BSA withthe concentration of 0.1% and a stabilizer into a latex microsphere-BSA-cholyglycine conjugate with the concentration of 0.04%, and the reagent body 2 is prepared by adding 15 mM of an auxiliary agent, a phosphate buffer solution with the pH value of 7.4, a sodium chloride solution with the concentration of 0.9%, BSA with the concentration of 0.1%, a surfactant with the concentration of 0.1% anda stabilizer into an anti-mouse cholyglycine monoclonal antibody with the concentration of 5%. According to the reagent, latex microspheres are introduced into the reagent, due to the existence of thelatex microspheres, the sensitivity of the detection reagent is greatly improved, and the requirements of clinical use are met. Compared with homogeneous enzyme immunoassay, the reagent has great advantages in stability, under the acceleration condition, and the stability time of the reagent is at least 2 times or above of the stability time of a homogeneous enzyme immunoassay reagent.
Owner:北京安图生物工程有限公司
Preparation method for antibacterial liquid-absorbing gauze
InactiveCN103397509AIncreased polycationic propertiesImprove antibacterial propertiesPhysical treatmentAbsorbent padsYarnDegree of substitution
The invention relates to a preparation method for an antibacterial liquid-absorbing gauze, which belongs to the field of preparation of medical dressings. According to the invention, a quaternary ammonium salt group is introduced onto a cellulose molecular chain of a degreased gauze through an epoxide ring-opening or nucleophilic substitution reaction, so polycation characteristics of cellulose of the gauze are increased, and antibacterial properties of the gauze are improved; through controlling of a mol ratio of a hydroxyl group to a quaternary ammonium salt on the cellulose molecular chain of the degreased gauze and reaction conditions, the degree of substitution of the quaternary ammonium salt on the cellulose molecular chain is in a range of 5 to 80%, so the reacted gauze has high antibacterial properties and high liquid absorption performance and can maintain the basic form of yarns after absorption of liquid. The antibacterial liquid-absorbing gauze prepared by using the preparation method provided by the invention overcomes the defect of a single function of a conventional medical gauze, is applicable to an infectious wound with a great amount of exudate or an infectible wound and exerts the effects of killing of bacteria on the surface of the wound and absorption of exudate on the surface of the wound. The preparation method for the antibacterial liquid-absorbing gauze has the advantages of easiness, low cost and easy industrial production.
Owner:WUHAN TEXTILE UNIV
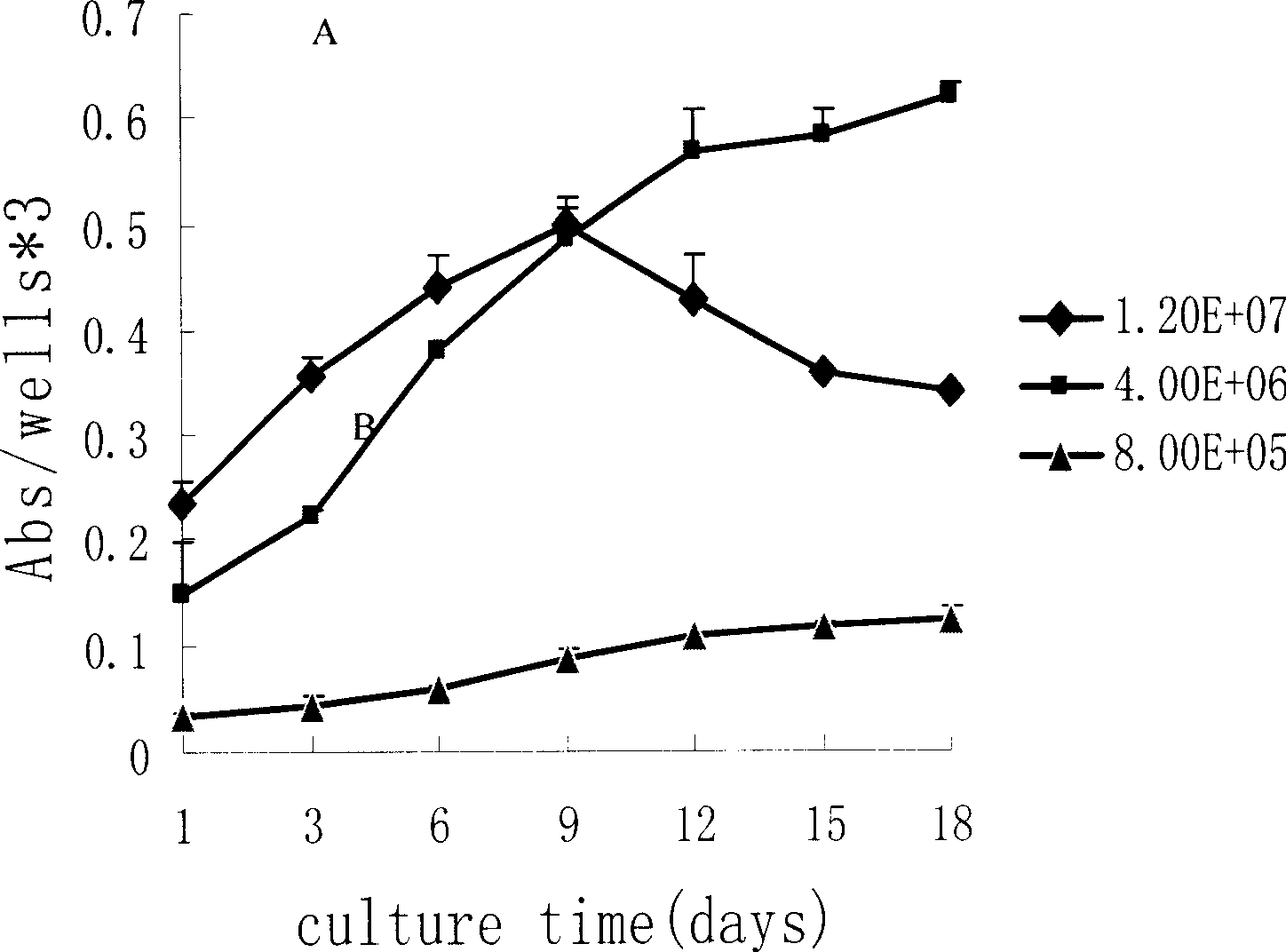
Method for amplifying candidate stem cell
InactiveCN101381700AEfficient amplificationImprove biological performanceBlood/immune system cellsHematopoietic stem cellStem cell
The invention relates to a method for amplification of hemopoietic stem cells. The method comprises the steps of separation of mononuclear cells, preparation and culture of microencapsulated mononuclear cells, and amplification of hemopoietic stem cells. The method can realize the amplification of hemopoietic stem cell as well as carries out culture on a large scale, and can play a significant role in blood biology and clinic application and research.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
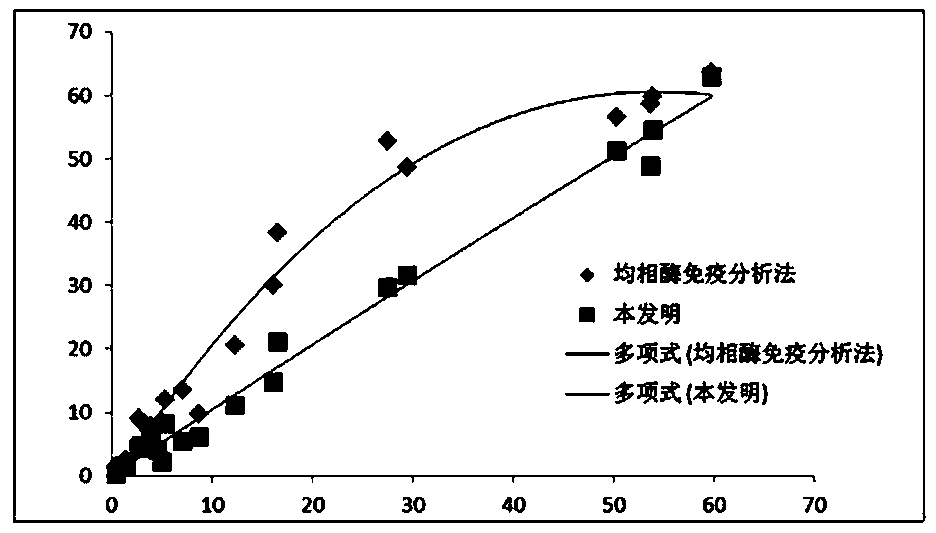
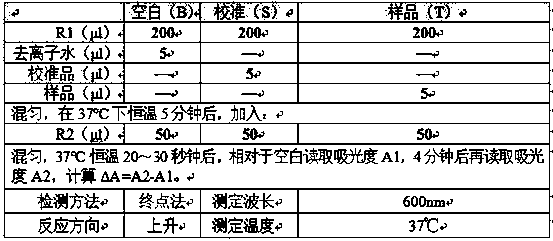
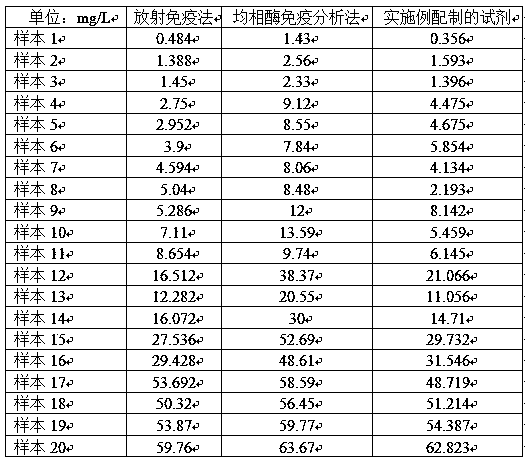
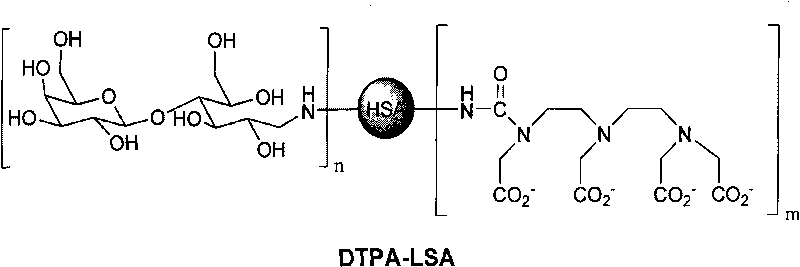
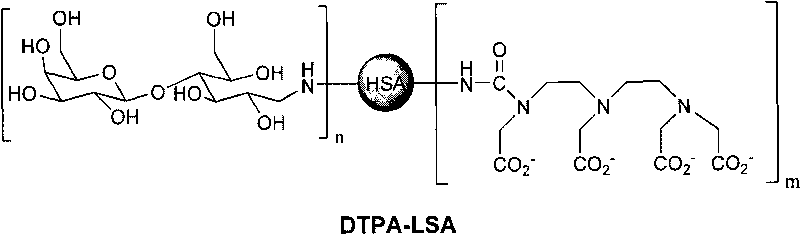
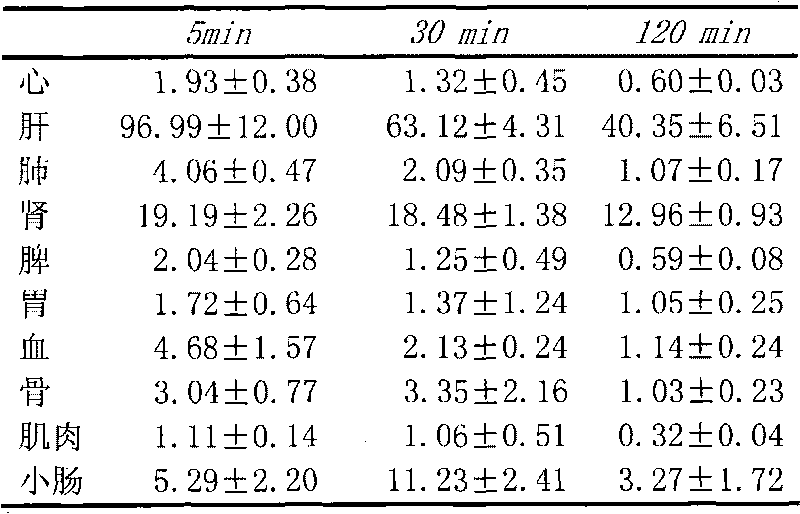
Medicine box for preparing technetium-99m labeled DTPA-LSA, preparation method and application thereof
ActiveCN101732736AThe synthesis steps are simpleQuick and easy kit preparationRadioactive preparation carriersChemistryCoordination complex
The invention discloses a medicine box for preparing technetium-99m (99mTc) labeled DTPA-LSA (99mTc-DTPA-LSA), a preparation method and application thereof. The medicine box is prepared by the method as follows: dissolving DTPA-LSA ligand in nitrogen-filled secondary water; adding buffer solution; mixing well; adding a reducing agent in a weight ratio of the reducing agent to the ligand of 1:10-800; performing sterile filtration on solution after dissolution is completely over; and putting the obtained product into containers separately. In the aspect of preparing 99mTc-DTPA-LSA, the medicine box has the advantages of use convenience, high labeling rate, good biological properties of prepared 99mTc-DTPA-LSA, low use cost and the like, and is beneficial to extensive clinical application. 99mTc-DTPA-LSA complex prepared by utilizing the medicine box can be sued as a novel liver imaging agent applied in the technical fields of radiopharmaceutical chemistry and clinical nuclear medicine.
Owner:BEIJING NORMAL UNIVERSITY +1
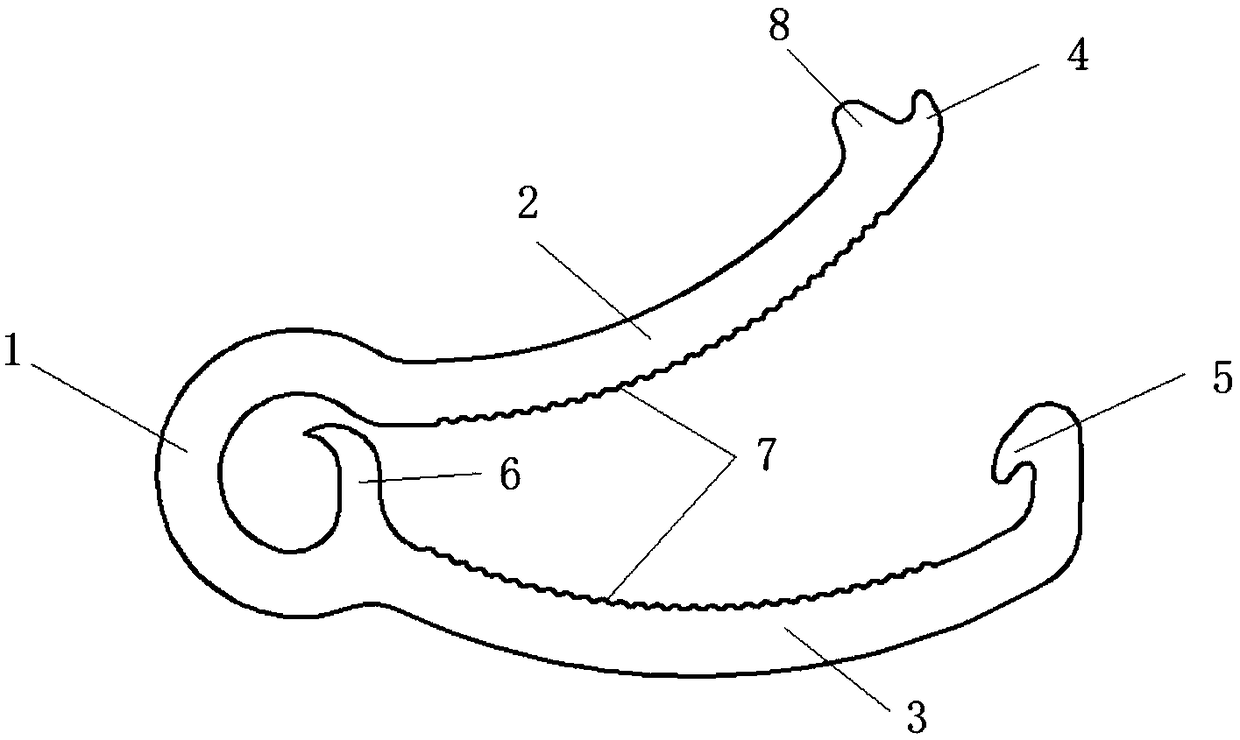
Degradable zinc alloy peritoneoscope hemostatic clip and preparing method thereof
Disclosed are a degradable zinc alloy peritoneoscope hemostatic clip and a preparing method thereof. The degradable zinc alloy peritoneoscope hemostatic clip is made of a zinc alloy. As for the components of the zinc alloy, an alloy element Mg is added into a pure zinc matrix. The degradable zinc alloy peritoneoscope hemostatic clip has the advantages that by introducing an arc structure and additionally arranging a lock catch, anti-slide insections and a limiting protrusion, stress concentration after the metal hemostatic clip is closed is effectively improved, meanwhile the clamping propertyof the hemostatic clip is effectively improved, and the risk of sliding off of the hemostatic clip after an operation is reduced; zinc is a necessary element of the human body, the good biocompatibility and absorbability are achieved by using the hemostatic clip prepared through the zinc alloy, and meanwhile, the service time of the hemostatic clip is increased obviously through the appropriate degradation in vivo characteristic of the hemostatic clip; the hemostatic clip is simple and easy in structure and simple in preparation technology, and the preparation cost is reduced effectively; andthe designed and prepared hemostatic clip fully meets the clinical use requirement.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Method for reducing endotoxin amount in bacterial cellulose shaping material
InactiveCN108329507ALess quantityMeet the requirements of clinical useSurgeryExisting TreatmentGamma ray
The invention provides a method for reducing endotoxin amount in a bacterial cellulose shaping material. The method comprises the following steps: separating and purifying a bacterial cellulose shaping material treated by alkali metal hydroxide by adopting a dialysis method through taking water as a solvent under a room-temperature condition; then drying and carrying out irradiation treatment by utilizing gamma rays with the dosage of 20 to 50kGy. Compared with an existing treatment method, the method provided by the invention has the greatest advantage that the amount of endotoxin in the bacterial cellulose shaping material can be effectively reduced to reach related standards of CFDA (China Food and Drug Administration); the requirements of clinical utilization are met; meanwhile, mechanical properties including strength of the shaping material and the like are basically not damaged.
Owner:涂青山
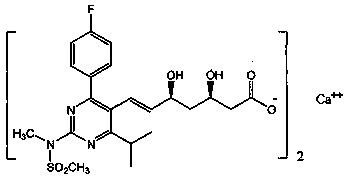
Stable rosuvastatin calcium pharmaceutical composition and preparation method thereof
InactiveCN103961354AGood disintegrationImprove featuresOrganic active ingredientsMetabolism disorderRosuvastatin CalciumMedicinal chemistry
The invention provides a stable rosuvastatin calcium pharmaceutical composition. The composition has high stability. A tablet containing the pharmaceutical composition has total impurity content of lower than 1.0% after accelerated testing, wherein lactone individual impurity content is lower than 0.10%.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
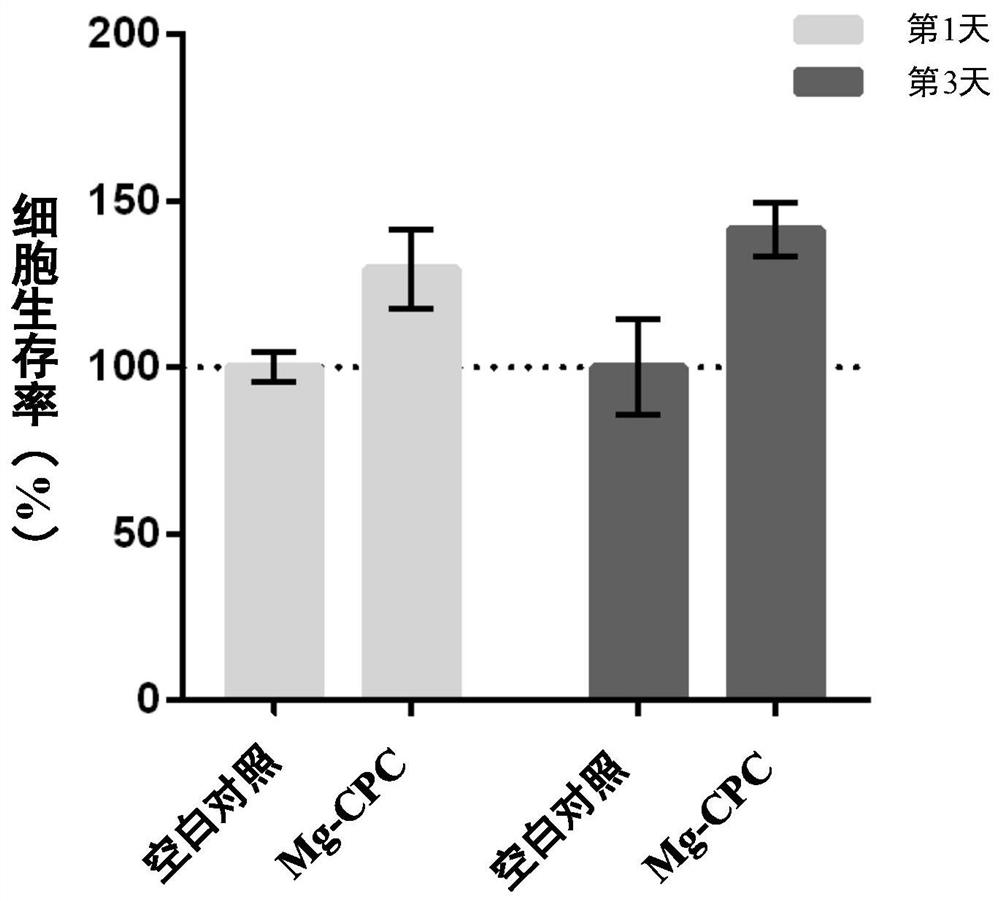
Preparation method of biomedical magnesium alloy wire
The invention relates to the technical field of metal material magnesium alloy preparation, and particularly provides a preparation method of a biomedical magnesium alloy wire. The preparation methodof the biomedical magnesium alloy wire comprises the steps that a magnesium-zinc-neodymium alloy is subjected to the processes of smelting, casting, rolling and the like to prepare a plate, the plateis subjected to a special mechanical stirring process to prepare a machining area having the same thickness with the plate, the machining area is subjected to machining treatment and then taken as a final product of the wire or subjected to multiple passes of drawing, and the wire with the needed diameter is finally formed. According to the preparation method of the biomedical magnesium alloy wire, the rolling and the mechanical stirring processes are introduced, therefore, the forming property of the wire is improved, alloy grains are significantly refined, the size of a second phase is significantly decreased, most of the second phase are solid soluble in a matrix, the strength, especially the elongation, of the obtained wire is significantly improved, the good corrosion resistance is achieved, and the property requirements of the medical magnesium alloy wire are met.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI +1
Bone repair material and preparation and use method thereof
InactiveCN104208747AGuaranteed DensityGuaranteed StrengthPharmaceutical non-active ingredientsProsthesisFilling materialsRepair material
Provided are a bone repair material and a preparation and use method thereof. The bone repair material is composed of a strontium compound and alpha-calcium sulfate hemihydrate. The molar weight of strontium occupies 0.1-10% of the total molar weight of calcium and the strontium. The preparation method includes 1) utilizing a co-precipitation method to prepare strontium CaSO4.2H2O and 2) utilizing a hydrothermal method to prepare strontium CaSO4.0.5H2O. Compared with the prior art, the bone repair material has the advantage that the bone repair material can serve as a bone filling material which is better in quality, degradable and good in bioactivity, can promote bone repair and can serve as an application of a drug delivery system.
Owner:佛山市乙太医疗用品有限公司 +1
Compound dichloroacetic acid diisopropylamin powder-injection and its preparation method
InactiveCN1596883AWell formedFlat surfacePowder deliveryPeptide/protein ingredientsChemistryMANNITOL/SORBITOL
A powder injection of compound diisopropylamine dichloroacetate is prepared from diisopropylamine dichloroacetate, sodium gluconate, mannitol and the water for injection through dissolving sodium gluconate in the water for injection, adding acetic acid to regulate pH-4.0-6.5, adding others, stirring and freeze drying.
Owner:王玫
Absorbable iron-based instrument
ActiveCN113116595AReduce consumptionDelays the point in time to initiate corrosionStentsCoatingsControlled releaseMedicine
The invention relates to an absorbable iron-based instrument which comprises an iron-based matrix, a zinc-containing protective layer, a corrosion promoting layer and a drug controlled release layer, the iron-based matrix is provided with an outer wall, an inner wall and a side wall, the zinc-containing protective layer at least covers the outer wall and the inner wall of the iron-based matrix, the corrosion promoting layer completely covers the zinc-containing protective layer, the drug controlled release layer at least partially covers the corrosion promoting layer, both the corrosion promoting layer and the drug controlled release layer contain degradable polymers, the weight-average molecular weight of the degradable polymer in the corrosion promoting layer is greater than that of the degradable polymer in the drug controlled release layer, and a thickness ratio of a portion of the zinc-containing protective layer located on the inner wall to a portion of the corrosion promoting layer located on the inner wall is greater than a thickness ratio of a portion of the zinc-containing protective layer located on the outer wall to a portion of the corrosion promoting layer located on the outer wall. The corrosion behavior of the absorbable iron-based instrument meets the clinical use requirement, and the adverse histological reaction is less or not generated.
Owner:BIOTYX MEDICAL (SHENZHEN) CO LTD
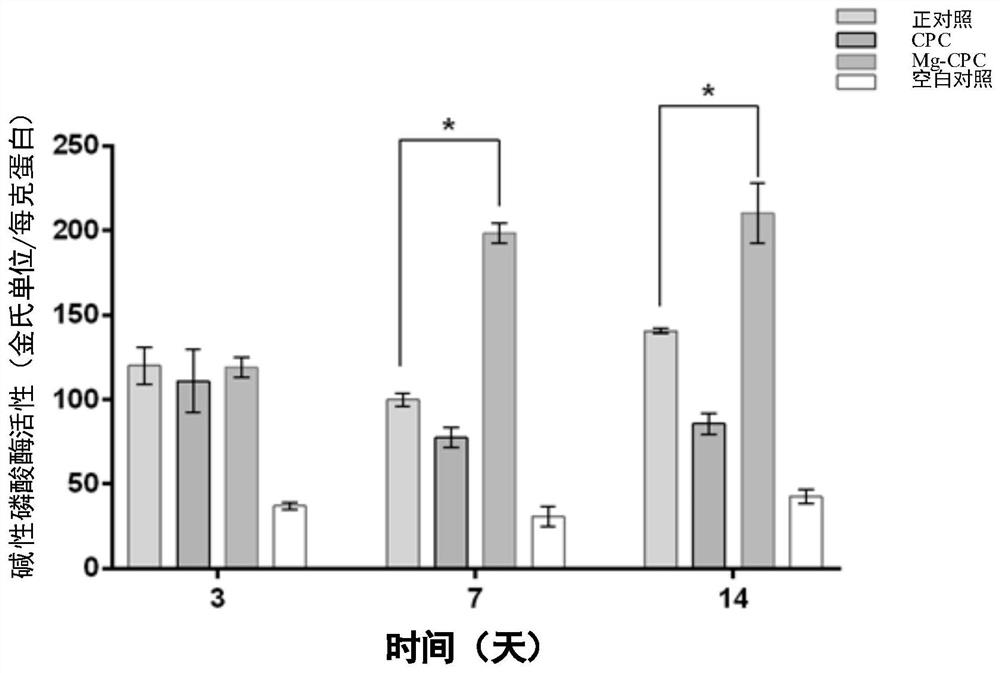
Magnesium slow-release bone cement with self-curing function and preparation method thereof
PendingCN112043862AAvoid disintegrationGood biocompatibilityTissue regenerationProsthesisMagnesium saltPyrrolidinones
The invention discloses magnesium slow-release bone cement with a self-curing function. The magnesium slow-release bone cement is characterized by being prepared by mixing and curing an aqueous solution and powder, wherein the powder comprises the following raw material components in percentage by mass: 60-95% of a calcium phosphate compound and 5-40% of a magnesium compound; and the aqueous solution comprises the following raw materials in percentage by mass: 10-30% of citric acid, 5-20% of polyvinylpyrrolidone and 40-84% of deionized water. According to the magnesium slow-release bone cementdisclosed by the invention, an organic crosslinking agent and specific magnesium salt are simultaneously added as donors for slow-release magnesium ions, so that the magnesium ions are slowly released, and calcium phosphate bone cement disintegration caused by too fast magnesium degradation in the prior art is avoided. In addition, the magnesium slow-release bone cement disclosed by the inventionhas excellent biocompatibility and a certain osteogenesis promoting ability and can well meet the clinical application requirements.
Owner:THE UNIV OF HONG KONG SHENZHEN HOSPITAL
Preparation method for small-diameter artificial silk fibroin blood vessel with high unblocked rate
InactiveCN104922728AImprove adhesionGood biocompatibilityProsthesisBiocompatibility TestingAnimal body
The invention discloses a preparation method for a small-diameter artificial silk fibroin blood vessel with high unblocked rate, and belongs to the field of biomedical materials. The small-diameter artificial silk fibroin blood vessel is obtained by uniformly coating a specific metal mould with an anticoagulant modified silk fibroin solution, performing rotary forming and heat drying, and soaking in an ethanol aqueous solution. Silk fibroin blood vessels different in performance and shape can be prepared as needed; the mechanical performance of the blood vessel is controlled by regulating the rotational speed and the drying temperature of the mould; the thickness of the blood vessel is controlled by the number of coating layers; the diameter of the blood vessel is controlled by regulating the diameter of the mould. The silk fibroin blood vessel obtained by the preparation method has good biocompatibility, blood compatibility and mechanical performance; the diameter of the silk fibroin blood vessel is smaller than 6 mm, the silk fibroin blood vessel is formed once, and the preparation process is simple; the silk fibroin blood vessel is good in adaptability and free of obvious stress defects, keeps unblocked in an animal body within 6 months, and lays a certain foundation for clinical application of small-diameter blood vessels.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
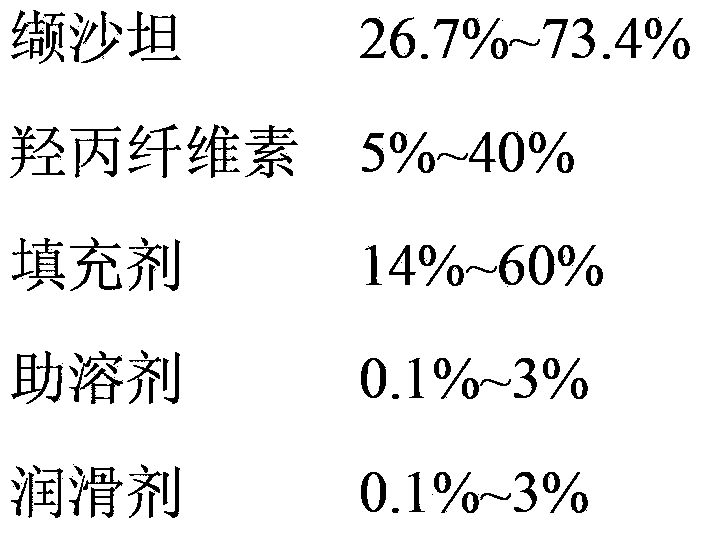
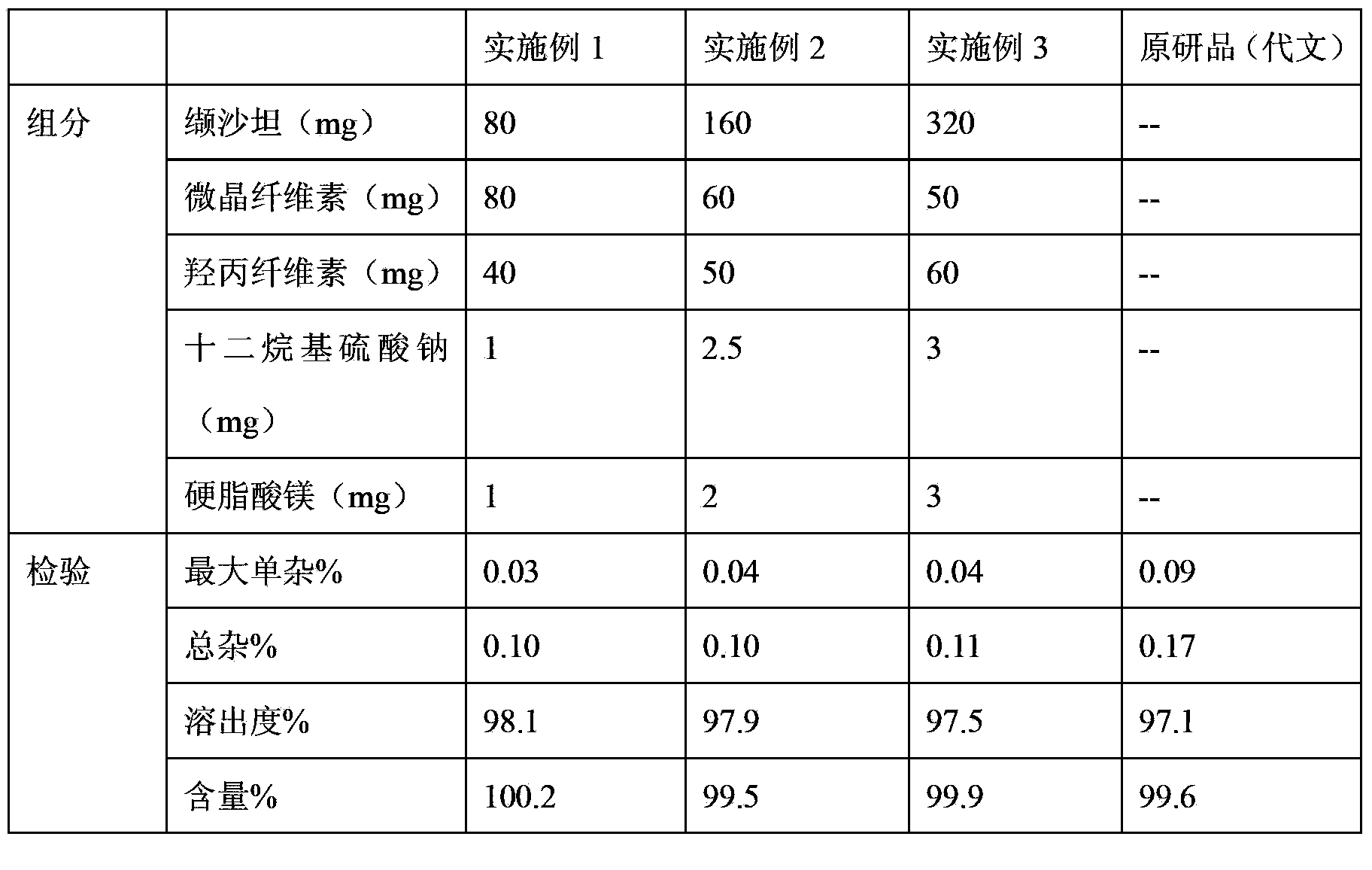
Valsartan capsule and preparation method thereof
ActiveCN103349656AQuality improvementReduce humidityMetabolism disorderDigestive systemAlcoholValsartan
The invention relates to a valsartan capsule and a preparation method thereof. The valsartan capsule comprises an active component valsartan and hydroxypropylcellulose serving as a disintegrating agent and an adhesive. The preparation method of the valsartan capsule comprises the following steps that 1), valsartan is sieved by a 200-mesh sieve; a filling agent and hydroxypropylcellulose are sieved by an 80-mesh sieve, and mixed uniformly; 2), an alcohol solution with the concentration of 5-60% is prepared, and accounts for 45-55% of the gross weight of valsartan, hydroxypropylcellulose, the filling agent, a cosolvent and a lubricant, and the cosolvent is dissolved by a half of the alcohol solution; 3), valsartan premix powder is placed in a wet method granulator, the alcohol solution of the cosolvent is added, and the left alcohol solution and purified water are added according to a granulation situation of a material, and stirred to form wet particles sieved by a 14-mesh sieve; 4), the wet particles are dried and granulated; and 5), the particles are uniformly mixed with the lubricant sieved by the 80-mesh sieve and filled to form the valsartan capsule. The valsartan capsule can be disintegrated and dissolved out quickly, and is simple in technology and stable in quality.
Owner:TIANDA PHARMA ZHUHAI
Method for preparing high-strength hydroxylapatite and chitosan three-dimensional composite rod material
The invention discloses a method for preparing a high-strength hydroxylapatite and chitosan three-dimensional composite rod material, which comprises the following steps: adding a calcium salt and a phosphate into solution of acetic acid to form solution of a hydroxylapatite precursor; dissolving chitosan powder in the solution of the precursor and standing the solution for deaeration; filling the mixed solution in a mold and obtaining a hydroxylapatite and chitosan three-dimensional composite gel rod material through the pervasion of alkaline coagulating liquid and self-assembly; and placing the hydroxylapatite and chitosan three-dimensional composite gel rod material in aqueous solution of glutaraldehyde and standing the solution for crosslinking, soaking and washing the resulting product with deionized water and drying the resulting product. The bending strength and bending modulus of the hydroxylapatite and chitosan three-dimensional composite gel rod material can reach 178MPa and 5.2GPa respectively, so the hydroxylapatite and chitosan three-dimensional composite gel rod material can meet clinic use requirements for fracture internal fixation.
Owner:ZHEJIANG UNIV
Method for amplifying candidate stem cell in vitro
InactiveCN101381701ARealize large-scale cultivationInhibition/delay towards differentiationBlood/immune system cellsHematopoietic stem cellStem cell
The invention relates to a method for in vitro amplification of hemopoietic stem cells. The method comprises the steps of separation of mononuclear cells, preparation and culture of microencapsulated mononuclear cells and amplification of hemopoietic stem cells. The method can realize the effective amplification of hemopoietic stem cell as well as carries out culture on a large scale, thereby stimulating the development of blood biology and clinic application and research.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
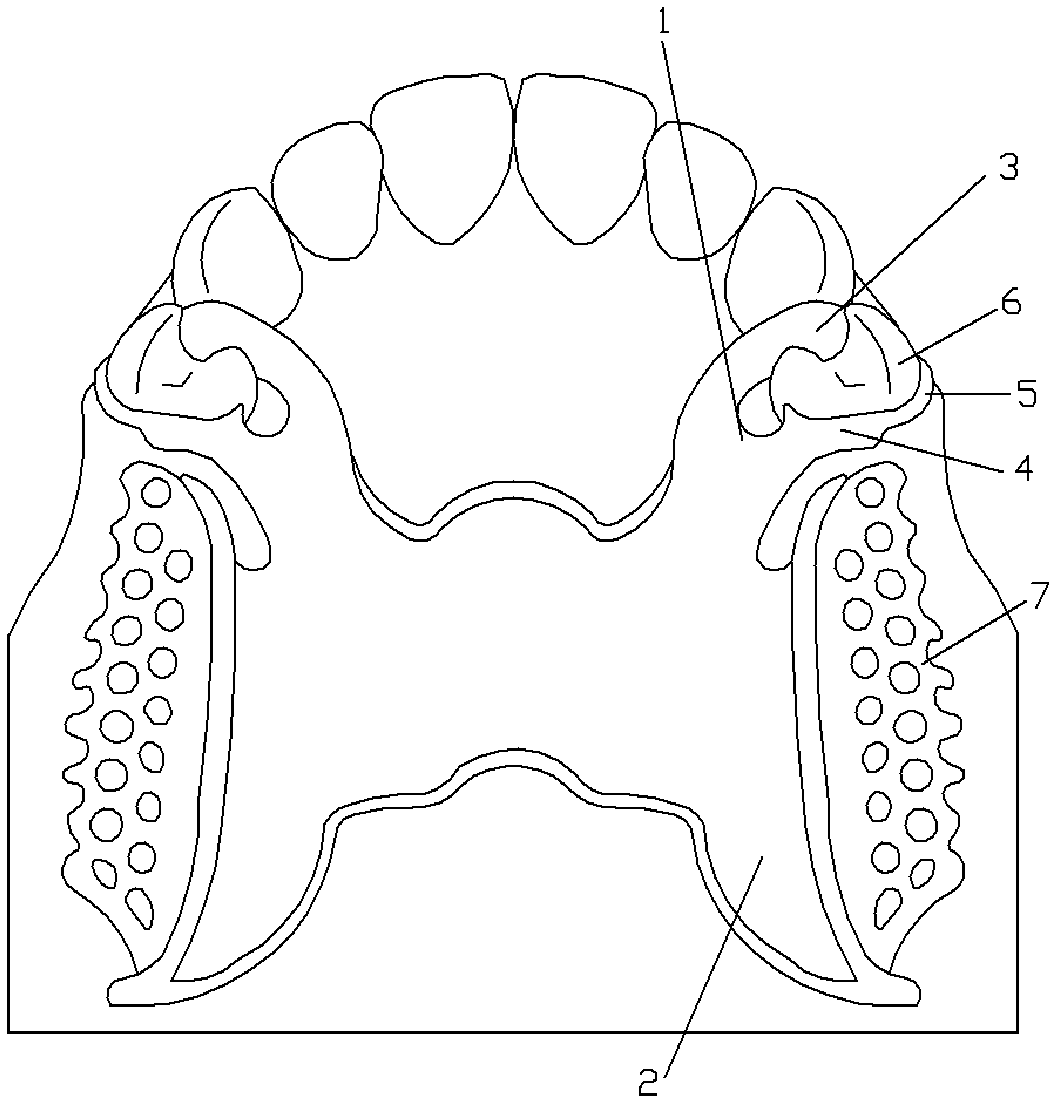
Plate rod snap ring for removable partial denture
ActiveCN102488562ADoes not affect the beauty of the faceMeet the requirements of clinical useFastening prosthesisRemovable partial dentureEngineering
The invention discloses a plate rod snap ring for a removable partial denture. According to the plate rod snap ring, the bottom of a U-shaped minor connector is connected with a connector; one end of the top of the U-shaped minor connector is connected with an occlusal rest on the mesial side of an oeclusal of an abutment, and the other end of the top of the U-shaped minor connector is connected with a distal adjacent panel; the distal adjacent panel is attached to a guide plane prepared from distal adjacent surfaces of the abutment; the cheek side upper end of the distal adjacent panel is connected with a retention arm; and the tail of the retention arm is clamped in an inverted concave area of the cheek side of the abutment and does not exceed a cheek axial ridge of the abutment. The plate rod snap ring for the removable partial denture does not influence the face and attractiveness of the patient, does not disturb the arrangement of artificial teeth and has a wide application range.
Owner:SICHUAN UNIV
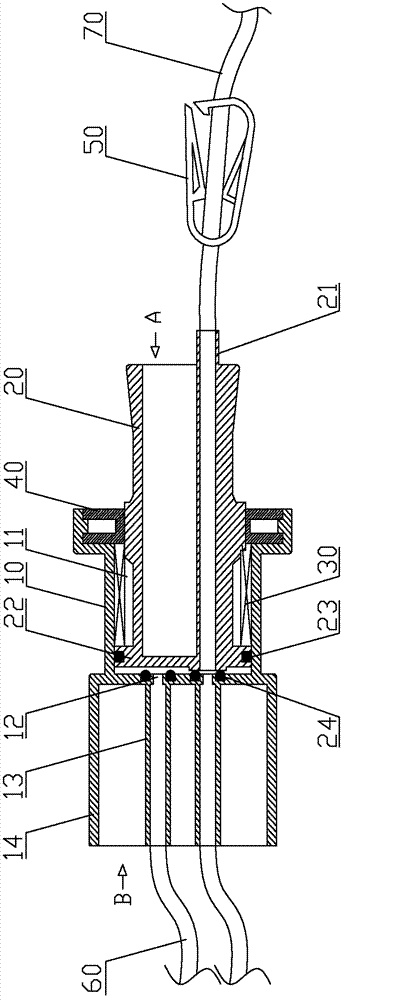
Micro-infusion multi-flow control device and disposable infusion pump
ActiveCN103357087ARealize multi-level controlMeet the requirements of clinical usePressure infusionMedicineEngineering
The invention discloses a micro-infusion multi-flow control device. The micro-infusion multi-flow control device comprises a base, a flow control member, a spring and a fixing member. The base is provided with a piston cavity. The closed end of the piston cavity is provided with a plurality of circularly distributed liquid outlets. The flow control member is provided with at least one front-back through runner. The front end of the flow control member is provided with a piston head and is fitted into the piston cavity of the base, and the rear end of the flow control member is provided with an operating handle. The runner can be in one-to-one but joint with the liquid outlets when the flow control member rotates. The fixing member is arranged at the open end of the piston cavity and is provided with a rotary positioning structure matching with the flow control member. The spring is sleeved to the flow control member. One end of the spring is supported on the piston head of the flow control member, and the other end of the spring is supported on the fixing member. The control device allows for multi-position control for the flow of a disposable infusion pump, so that the clinical application requirement is met. The invention discloses the disposable infusion pump with the control device.
Owner:北京科联升华医疗科技有限公司
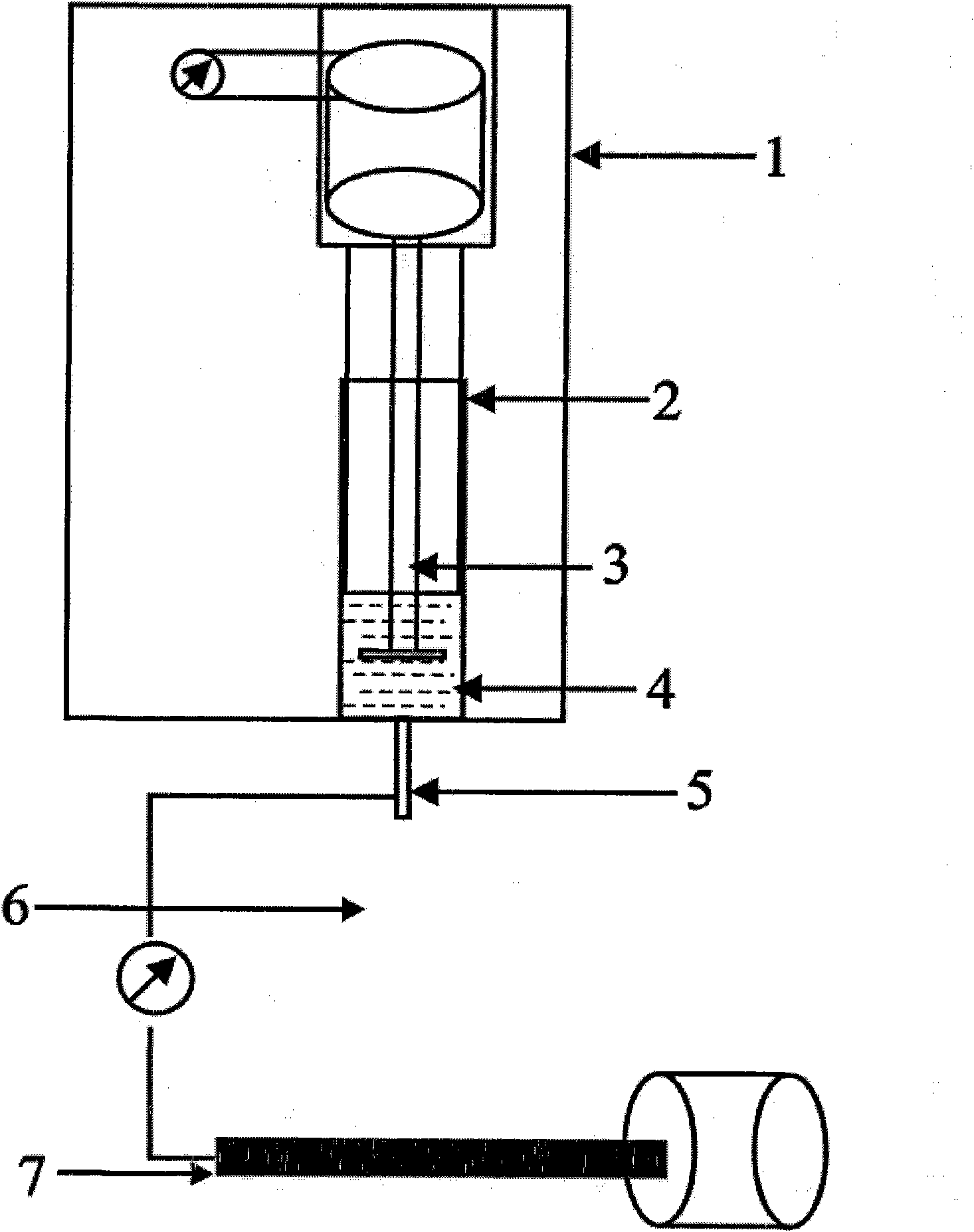
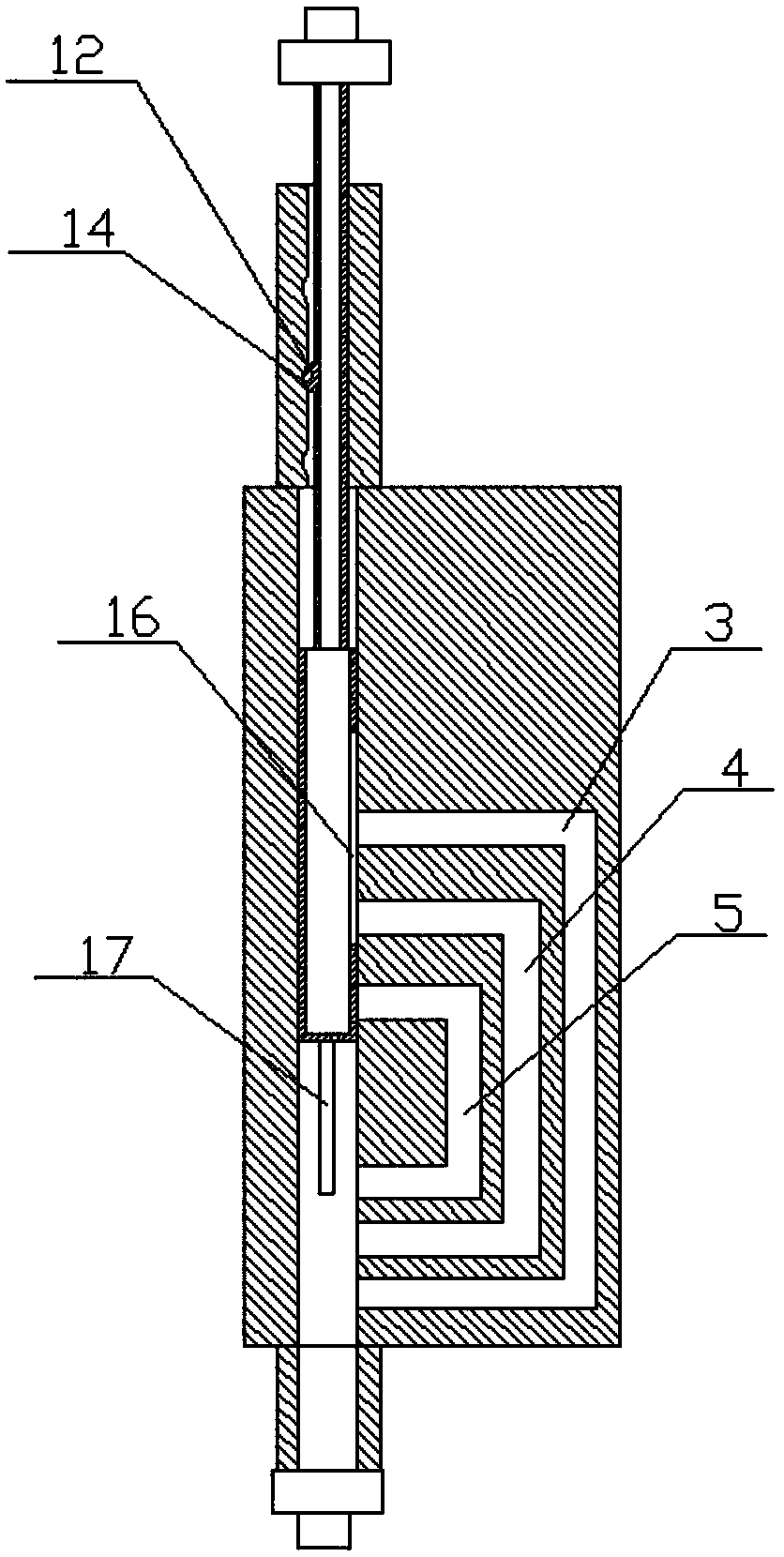
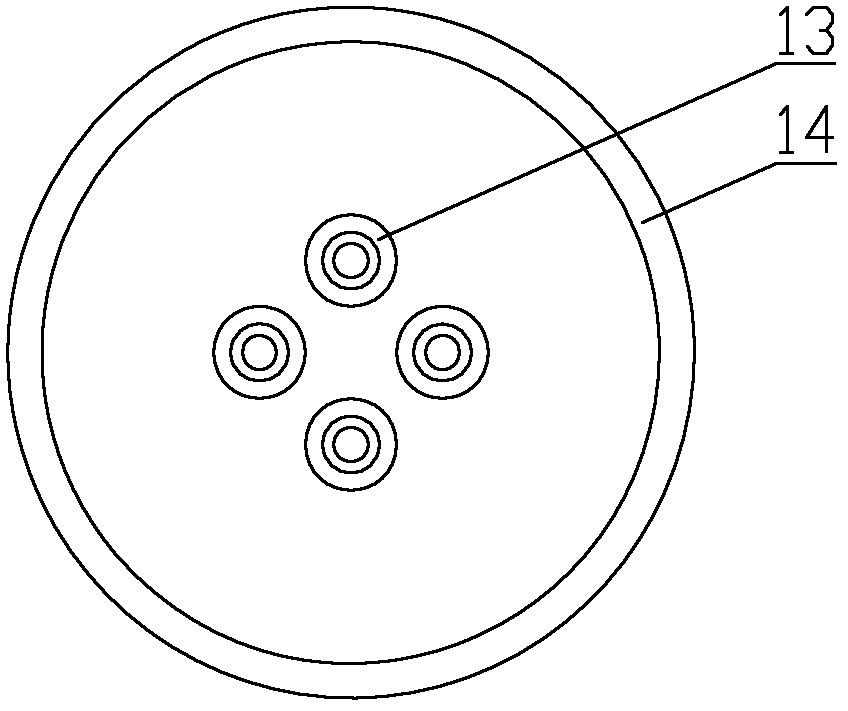
Automatic synthesis device and method of sodium fluoride [< 18 > F] injection
PendingCN113332142ASimple and fast operationImprove efficiencyPharmaceutical product form changeSodium Chloride InjectionHigh doses
The invention discloses an automatic synthesis device and method of a sodium fluoride [< 18 > F] injection. The synthesis device comprises an < 18 > F <-> supply device, a sodium chloride injection supply device, a sterilization injection water supply device, a first three-way valve group, a QMA column, a second three-way valve group, a sodium fluoride [< 18 > F] injection receiving device and a waste liquid recovery bottle group, the <18>F <-> supply device, the sodium chloride injection supply device and the sterilization injection water supply device are respectively connected with the first three-way valve group, the QMA column, the second three-way valve group and the waste liquid recovery bottle group in series, and are selectively communicated under the control of the first three-way valve group and the second three-way valve group. Compared with a traditional manual mode, the automatic synthesis device of the sodium fluoride [< 18 > F] injection has the advantages that the efficiency is high, the operation is simple and convenient, the clinical use requirement of sterility and no pyrogen can be ensured through a clamping sleeve type structural design, and operators can be prevented from receiving high-dose radioactive radiation.
Owner:HTA CO LTD
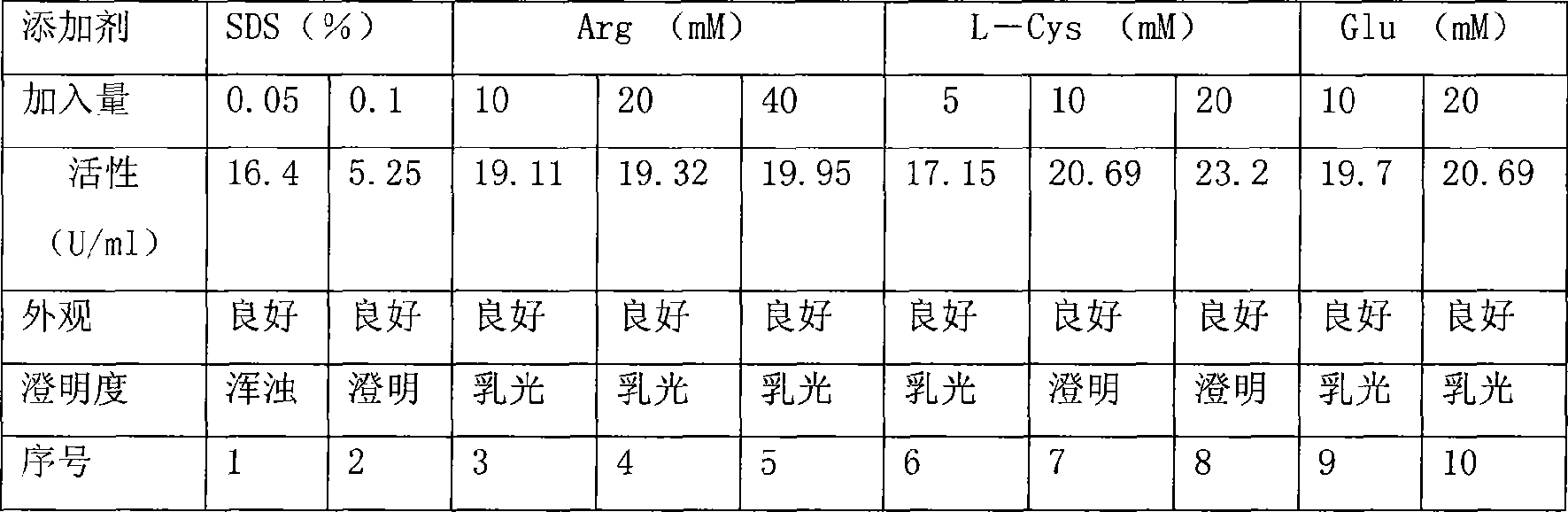
Pharmaceutical formulation containing candida urate oxidase
InactiveCN101485882AMeet the requirements of clinical useReduce medical expensesPowder deliveryPeptide/protein ingredientsGlycineIntramuscular injection
The invention relates to a medicinal preparation containing Candida urate oxidase, in particular to a liquid or freeze-dried composition medicinal preparation containing the Candida urate oxidase. The composition comprises the following active ingredients: the Candida urate oxidase, glycine, cysteine and a buffer system. The composition is aseptic, and can be injected into human body or animal body to take effect through hypodermic injection, intravenous injection or intramuscular injection.
Owner:BEIJING SL PHARMA
Polyethylene terephthalate and polyurethane composite artificial blood vessel and preparation thereof
ActiveCN102008755BIncrease elasticityImprove mechanical propertiesNew-spun product collectionFilament/thread formingPolyethylene terephthalate glycolPolyethylene terephthalate
The invention discloses a polyethylene terephthalate and polyurethane composite artificial blood vessel and a preparation method thereof, belonging to the field of biomedical engineering. The artificial blood vessel is prepared from polyethylene terephthalate and polyurethane by means of an electrostatic spinning device with a stirrer. The prepared artificial blood vessel is a tubular object witha microporous structure. The polyurethane added in the artificial blood vessel can improve the elasticity of the blood vessel under the condition of not damaging the strength of the polyethylene terephthalate material, increase the compliance of the artificial blood vessel and achieve the requirements of clinical use. The polyethylene terephthalate and polyurethane composite artificial blood vessel has the advantages of high strength, large elasticity and compliance, good biocompatibility, strong anticoagulant property and large cell adhesion, and can meet the requirements of the clinical useof the artificial blood vessels with large diameter and small diameter. The preparation process is simple and practical, and applicable to preparing the artificial blood vessels with various different requirements on mechanical properties.
Owner:宁波贝昂生物材料有限公司
Preparation method of medical composite glass fiber strontium containing enhanced bone cement product
Calcium nitrate, diammonium phosphate and urea are mixed and added with strontium phosphate to be mixed, stirred and heated, to prepare strontium containing hydroxyapatite; the hydroxyapatite is added into magnesium chloride and magnesium oxide, added with water to be dissolved, to prepare magnesium oxychloride adhesive; a hand lay-up bone pre-fabricated piece is paved with pulp and glass fiber cloth, flattened, paved uniformly and brushed with glue; and then the prefabricated outer skin is paved and compacted, the seam is sealed by plastic thin film, cured and stripped; the product is trimmed within one day after stripping, and a medical composite glass fiber strontium containing enhanced bone cement product is prepared. The composite material prepared by the invention has the advantages of uniform glass fiber dispersion and good combination of the strontium containing hydroxyapatite and a matrix interface, and biomedical composite material with very good application prospect.
Owner:李胜
Multi-level flow control device applied to infusion pump
InactiveCN108295338ARealize micro-precise controlRealize the supplyMedical devicesPressure infusionEngineeringInfusion pump
The invention relates to an infusion pump assembly, in particular to a multi-level flow control device applied to an infusion pump. Through cooperation among arc-shaped protrusions, an upper groove, amiddle groove and a lower groove, a liquid outlet is matched with a lateral channel A, a lateral channel B and a lateral channel C, and therefore flow adjustment is achieved. The device has the advantages that the structure is simple, control is simple, microscale accurate control and supply of medicine are achieved, multi-level flow control can also be achieved, and clinical use requirements aremet.
Owner:常州千手纺织机械科技有限公司
Vascular Imaging Enhancer
ActiveCN105561347BQuality improvementHigh precisionEchographic/ultrasound-imaging preparationsTime lagIrritation
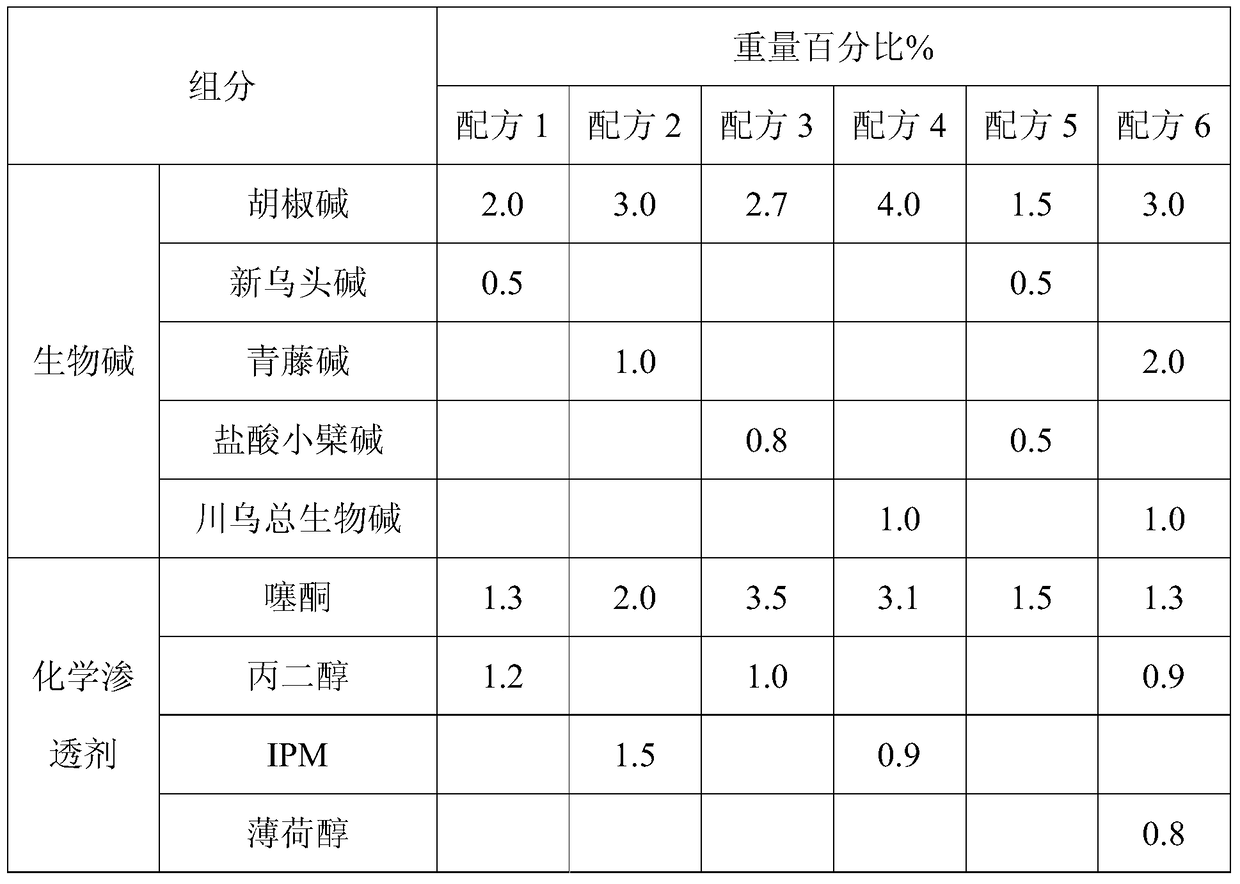
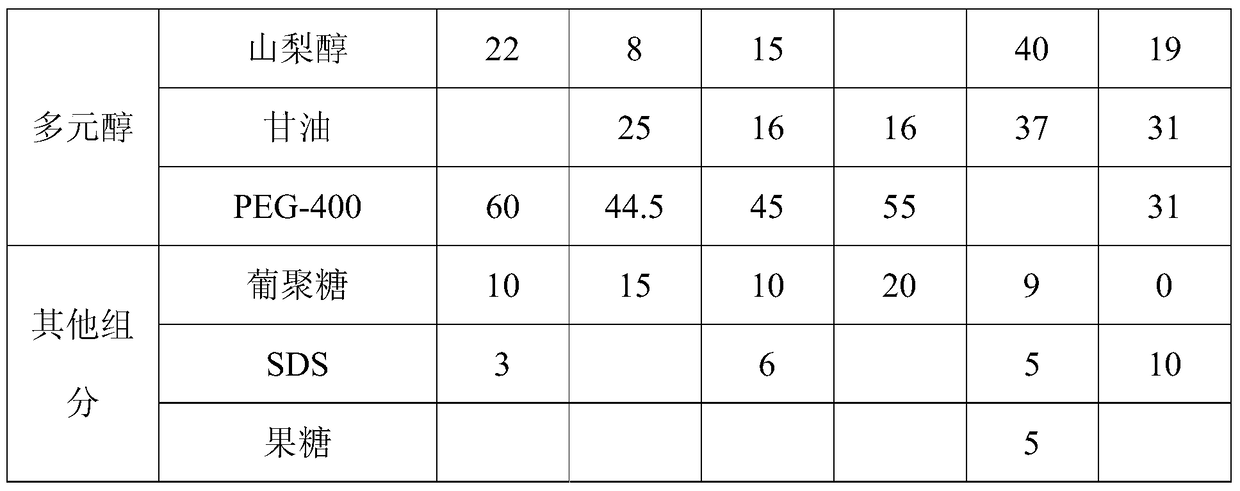
The invention provides an angiography enhancer. The angiography enhancer comprises 2.5-5.0% of alkaloids, 1.5-4.5% of a chemical penetrating agent, 70-85% of polyol and 10-20% of other components. The alkaloids comprise two or more of berberine hydrochloride, piperine, sinomenine, radix aconite total alkaloids and mesaconitine, the chemical penetrating agent is one or more of thiazone, isopropyl myristate, propylene glycol and menthol, and the polyol is one or more of sorbitol, glycerin and polyethylene glycol-400. The angiography enhancer compounding natural alkaloid penetration promoters with chemical penetration promoters increases the amount of infrared lights penetrating through skins, reduces the penetration promotion time lag, makes skin tissues be transparent in 1-2min, greatly improves the contrast between blood vessels and surrounding tissues thereof under the infrared lights, substantially improves the quality and the precision of development, contains no skin sensitizing or high-irritation components, is prepared through a simple technology according to a simple ratio, and is suitable for large-scale industrial production.
Owner:SHANGHAI YUKING WATER SOLUBLE MATERIAL TECH
Postoperative washing agent and preparation method thereof
The invention belongs to the field of medicines, and in particular relates to a postoperative washing agent and a preparation method thereof; the postoperative washing agent is composed of sodium hyaluronate, a chitin derivative and a cross-linking agent, wherein the mass ratio of the sodium hyaluronate, the chitin derivative to the cross-linking agent is 1:(0.3-1):(0.1-0.5). The preparation method disclosed by the invention comprises the following steps: weighing and mixing the sodium hyaluronate, the chitin derivative and the cross-linking agent according to the formula, grinding into fine powder, sieving fine powder through a 200-mesh sieve, packaging sieved fine powder by using an aluminium foil bag, sterilizing through ethylene oxide or microwave, and obtaining the postoperative washing agent. The postoperative washing agent and the preparation method thereof disclosed by the invention have the advantages that various components of medicines are matched with each other and used by being prepared into the washing agent; the postoperative washing agent has the obvious effects for preventing postoperative joint tissue adhesion, recovering and healing wound surfaces and easing pain; and furthermore, the postoperative washing agent disclosed by the invention is steady in quality, low in cost, convenient for use and good in tissue adhesion and accords with clinical use requirements.
Owner:陈凯
Metformin and fenofibric acid complex and preparation thereof
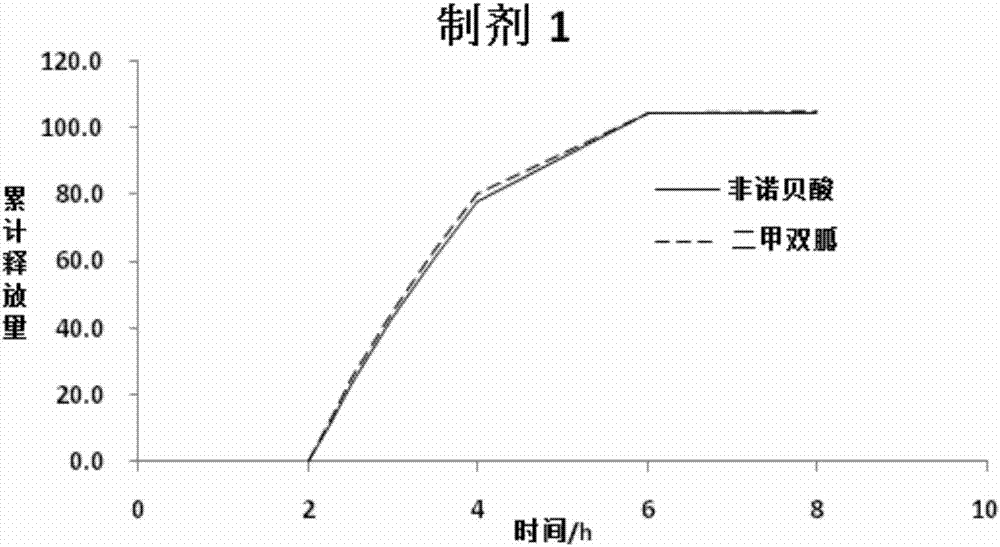
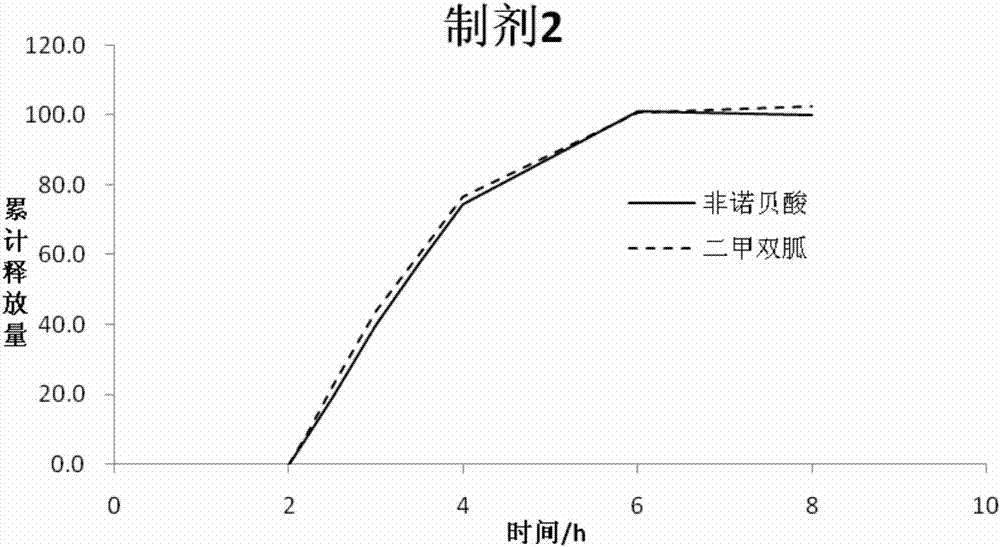
InactiveCN107496397AGood release consistencyAvoid food effectOrganic active ingredientsMetabolism disorderRelease consistencyFENOFIBRIC ACID
The present invention discloses a metformin and fenofibric acid complex and a preparation thereof, wherein metformin and fenofibric acid are subjected to a direct salt forming reaction to obtain the complex. According to the present invention, the experiment results prove that the complex is the enteric sustained-release preparation prepared from the active components, and can release the two active components such as metformin and fenofibric acid in a simulated artificial intestinal juice, wherein the metformin and the fenofibric acid have good release consistency, such that the complex can be used for preparing pharmaceutical preparations for treatment of hyperlipoidemia, diabetes and other metabolic diseases; with the complex, the problem of the release inconsistency caused by the inconsistent pharmacokinetics between the two active components is solved, the significant sustained-release property is provided, the food effect and the early release condition do not exist, and the clinical use requirements of pharmaceutical preparations can be met; and the advantages of good compressibility, simple preparation process, easy scalization, low cost, stable quality and the like are provided.
Owner:重庆瑞泊莱医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

















![Automatic synthesis device and method of sodium fluoride [< 18 > F] injection Automatic synthesis device and method of sodium fluoride [< 18 > F] injection](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/824c29d6-5a5c-44a6-bbb3-11ecaa864f83/HDA0003108529710000011.png)
![Automatic synthesis device and method of sodium fluoride [< 18 > F] injection Automatic synthesis device and method of sodium fluoride [< 18 > F] injection](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/824c29d6-5a5c-44a6-bbb3-11ecaa864f83/HDA0003108529710000012.png)
![Automatic synthesis device and method of sodium fluoride [< 18 > F] injection Automatic synthesis device and method of sodium fluoride [< 18 > F] injection](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/824c29d6-5a5c-44a6-bbb3-11ecaa864f83/HDA0003108529710000021.png)