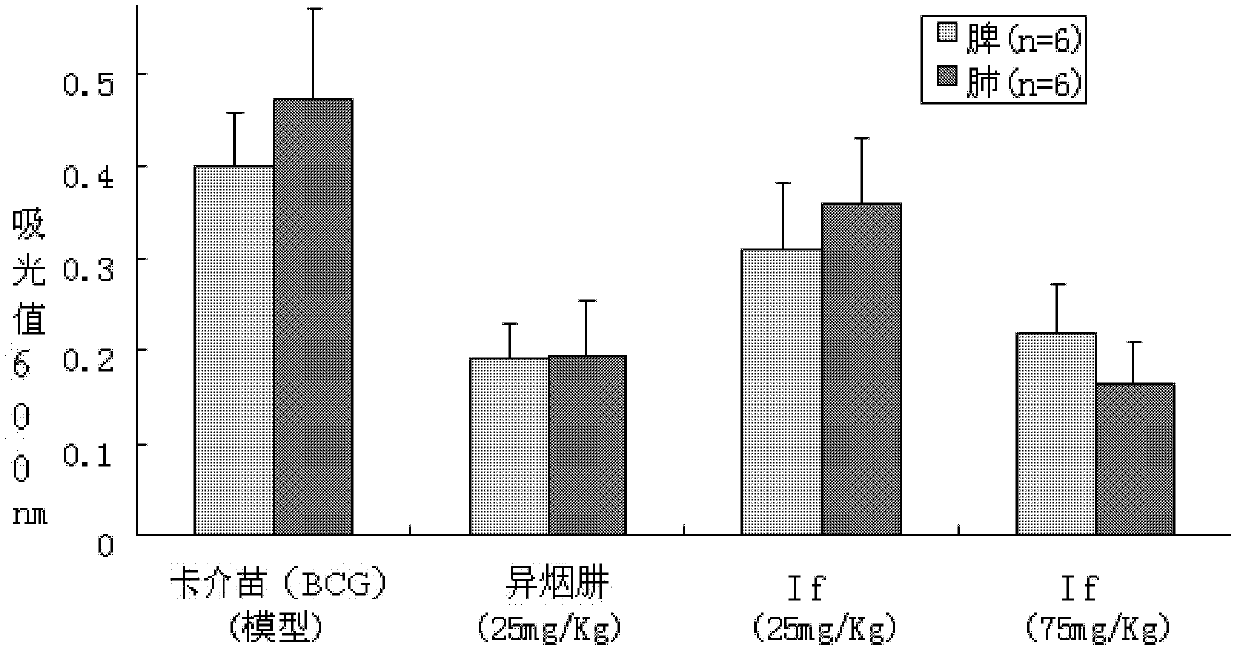
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
267 results about "Antituberculous drugs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmacologic issues and some clinical data related to use of antituberculous drugs are reviewed here; issues related to clinical use of antituberculous drugs in therapeutic regimens are discussed separately.
Anti tubercular drug: compositions and methods
Methods and compositions for treating disease caused by infectious agents, particularly tuberculosis. In particular, methods and compositions comprising substituted ethylene diamines for the treatment of infectious diseases are provided. In one embodiment, these methods and compositions are used for the treatment of mycobacterial infections, including, but not limited to, tuberculosis.
Owner:SEQUELLA +1
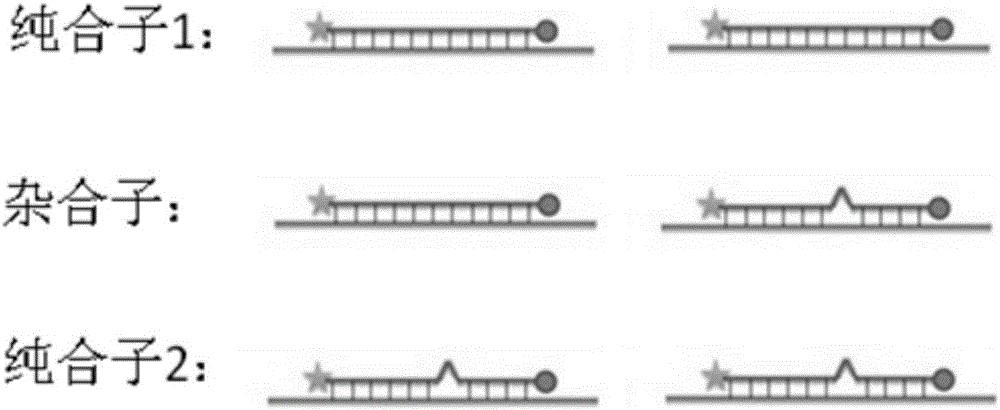
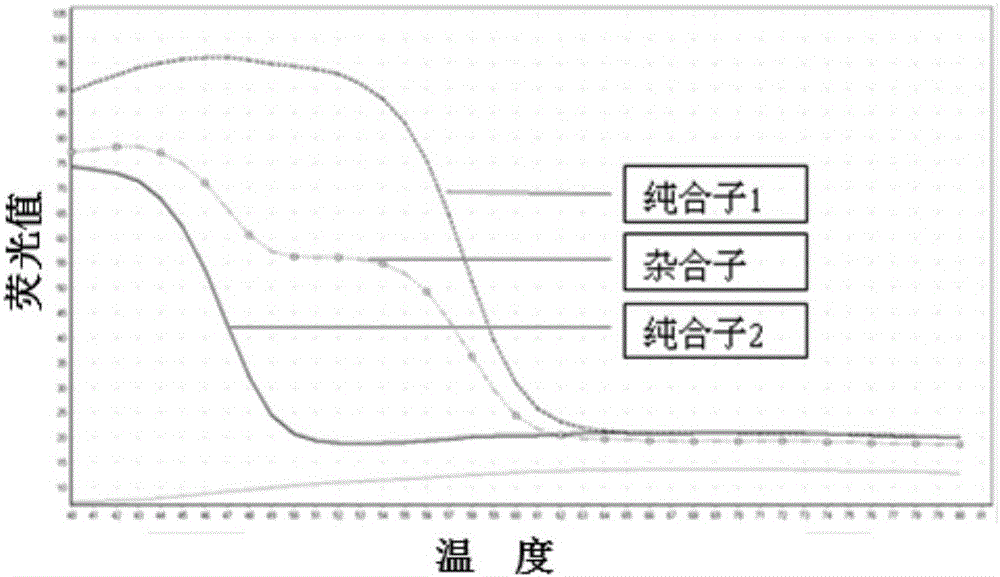
SNP (single nucleotide polymorphism) combination, detection method and kit for detecting liver damage susceptible genotype of antitubercular drug
ActiveCN106119363AReduce drug riskImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationGeneticsAntituberculous drugs
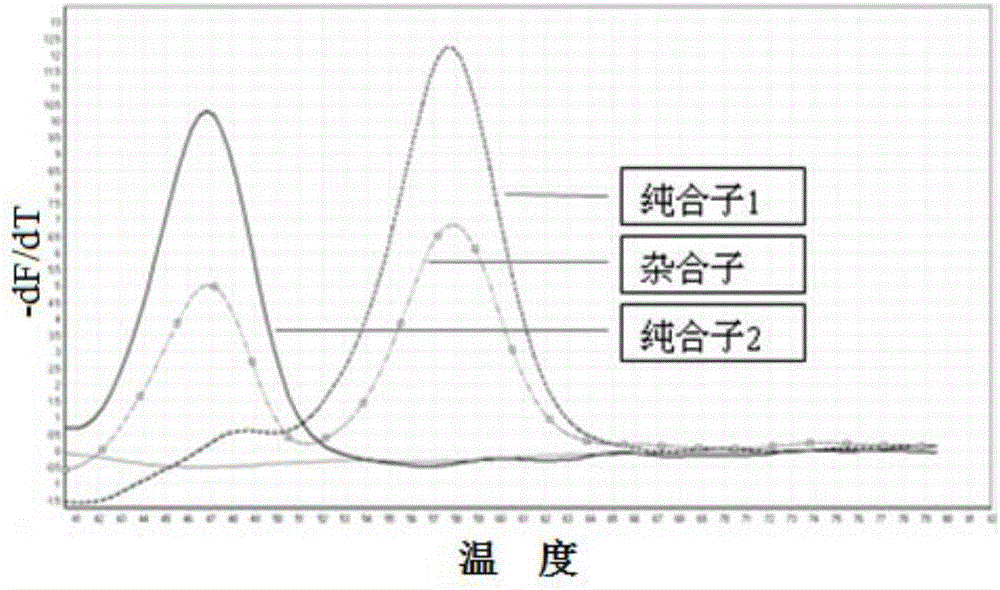
The present invention relates to an SNP (single nucleotide polymorphism) combination, detection method and kit for detecting liver damage susceptible genotype of an antitubercular drug and belongs to the technical field of medical molecular biological diagnosis; the SNP combination includes 7 SNP sites, and nucleotide sequences of the 7 SNP sites are shown sequentially as in SEQ ID NO. 1-7; the present invention also relates to an SNP detection method, comprising PCR (polymerase chain reaction) amplification and double-labeled probe melting curve analytical reaction, and primer pairs and double-labeled probe sequences for detection of the 7 SNP sites are shown as in SEQ ID NO. 8-20. The SNP site combination, detection method and kit provided herein enables quick, accurate, simple and high-throughput detection for a patient's genotype and prediction for the liver damage risk due to the patient using the antitubercular drug.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY +1
Chirality 7-(piperazine-substituted pyrazol aldehyde condensation isoniazide) fluoroquinolone carboxylic acid derivative as well as preparation method and application thereof
InactiveCN104402902AAchieve overlayReduce chance of drug resistanceAntibacterial agentsOrganic chemistryIsoniazidSide effect
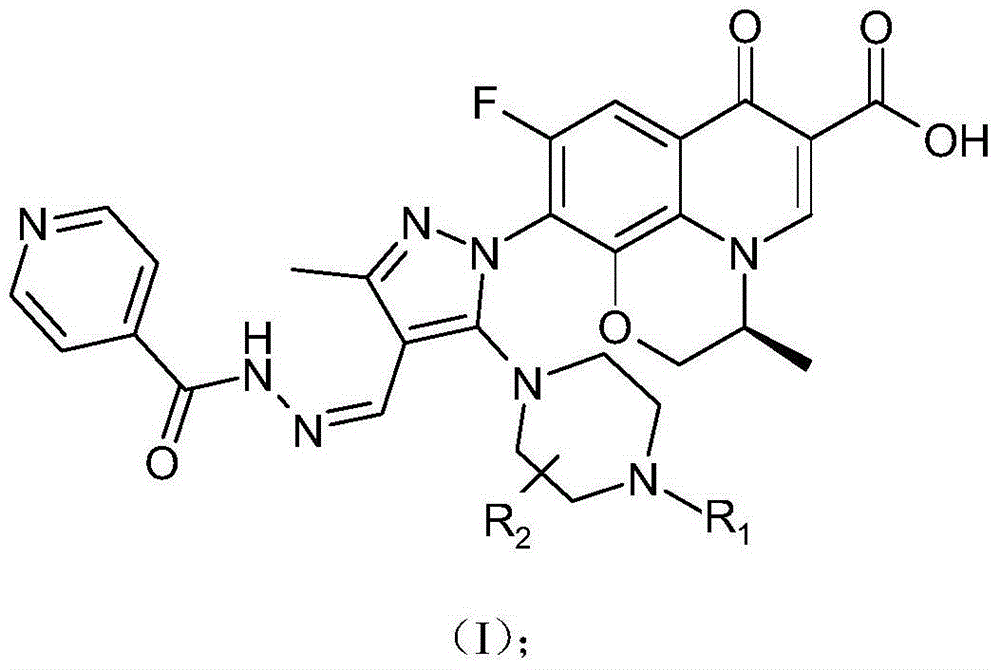
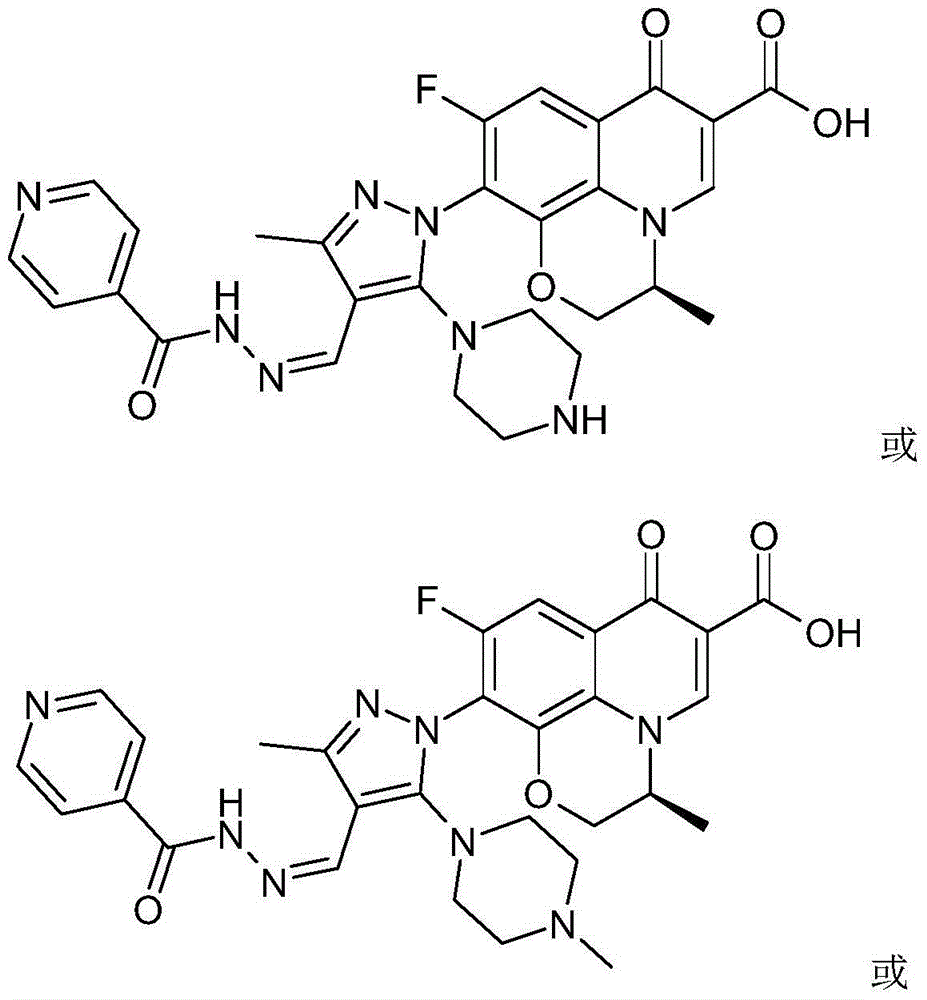
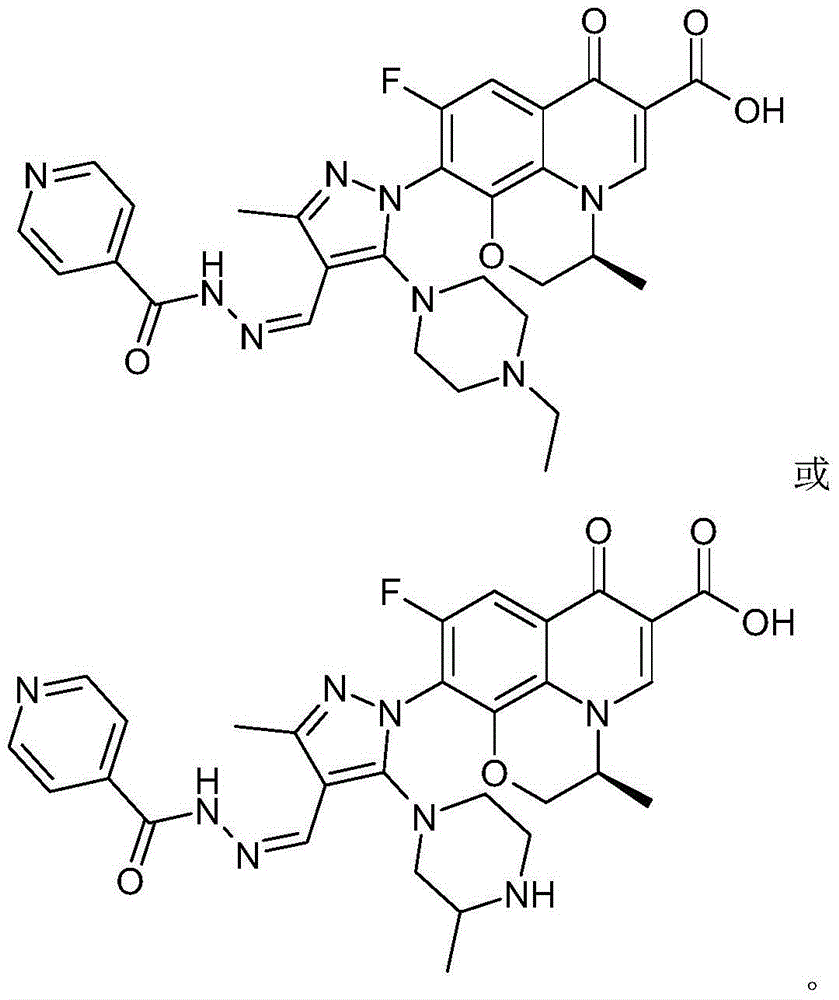
The invention discloses a chirality 7-(piperazine-substituted pyrazol aldehyde condensation isoniazide) fluoroquinolone carboxylic acid derivative as well as a preparation method and application thereof. The chirality 7-(piperazine-substituted pyrazol aldehyde condensation isoniazide) fluoroquinolone carboxylic acid derivative is a compound with the general structural formula (I), wherein R1 is H, methyl or ethyl, and R2 is H or methyl. According to the chirality 7-(piperazine-substituted pyrazol aldehyde condensation isoniazide) fluoroquinolone carboxylic acid derivative provided by the invention, fluoroquinolone, isoniazide and pyrazole aldehyde hydrazone are effectively combined to form a compound with a new structure; superposition and cooperation of activity are achieved; superposition of the three pharmacophores of fluoroquinolone, isoniazide and pyrazole aldehyde hydrazone is realized, the antituberculosis activity is improved, the toxic and side effects of fluoroquinolone and isoniazide to normal cells are decreased, and meanwhile, the probability that mycobacterium tuberculosis resists such drugs can be lowered; the chirality 7-(piperazine-substituted pyrazol aldehyde condensation isoniazide) fluoroquinolone carboxylic acid derivative can serve as an antituberculous active substance used for development of an antituberculous drug with a new structure.
Owner:HENAN UNIVERSITY +1
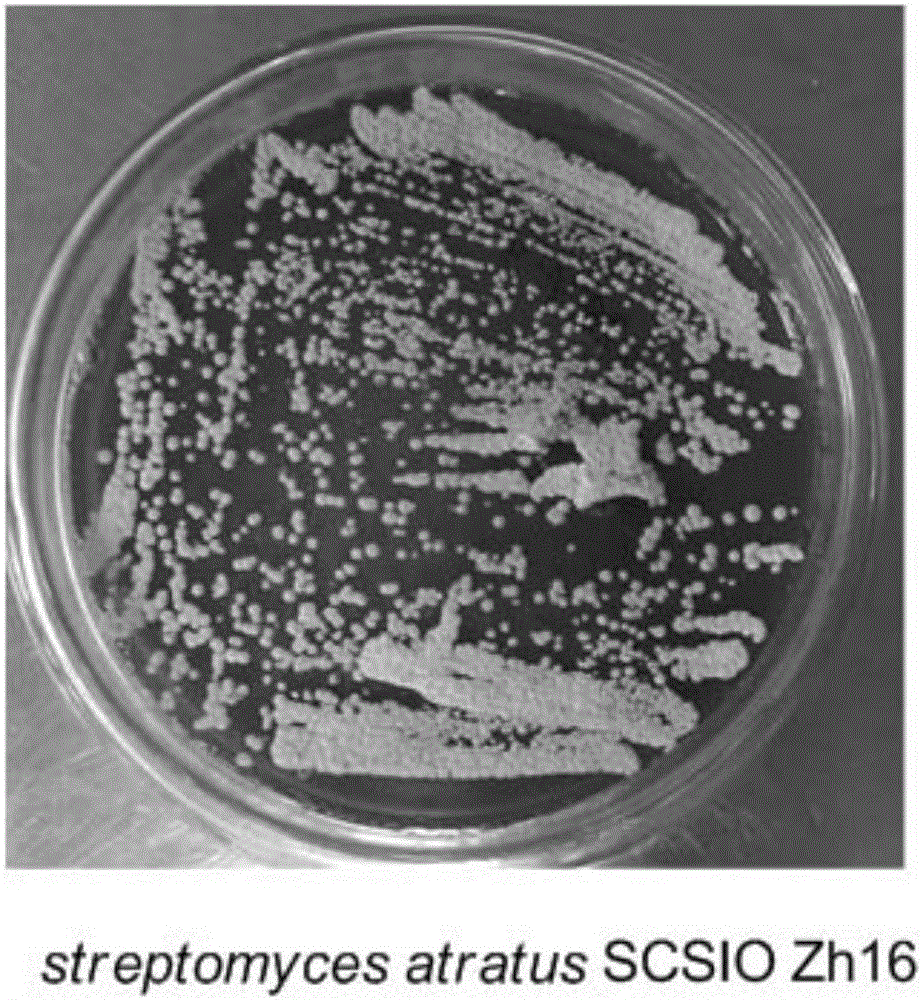
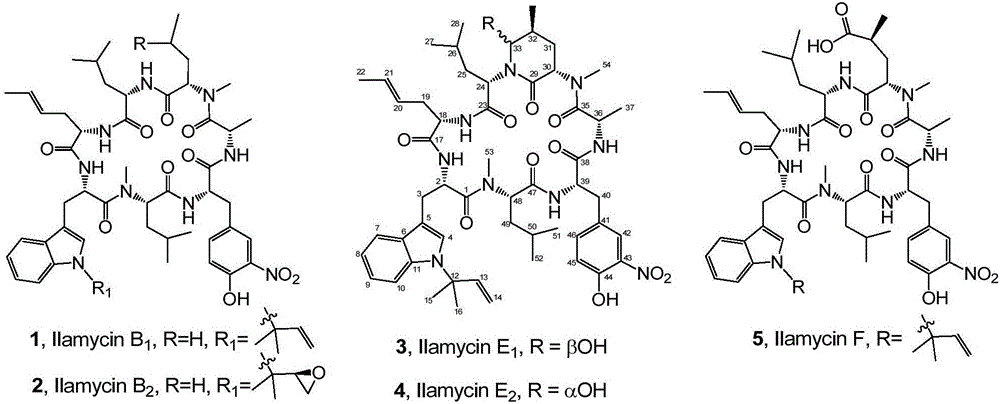
Streptomyces atratus and application of cyclic peptide compounds with same to preparing mycobacterium tuberculosis resistant medicines
ActiveCN106279370AGood inhibitory effectAntibacterial agentsBacteriaCyclic peptideAntituberculous drugs
The invention discloses streptomyces atratus and application of cyclic peptide compounds with the same to preparing mycobacterium tuberculosis resistant medicines. A structural formula of the cyclic peptide compounds is shown. A preservation number of the streptomyces atratus SCSIO Zh16 is CGMCC No.12198. The streptomyces atratus and the application have the advantages that the six cyclic peptide compounds are obtained from fermentation cultivation substances of the streptomyces atratus SCSIO Zh16 by means of separation, the cyclic peptide compound 6 is high in mycobacterium tuberculosis resistant activity, obvious effects of inhibiting mycobacterium tuberculosis can be realized by the cyclic peptide compound, accordingly, the cyclic peptide compounds can be used for preparing anti-tuberculosis medicines and can be used for treating tuberculosis, alternative compounds can be provided for developing novel anti-tuberculosis medicines, and the streptomyces atratus and the application have important significance on developing marine medicinal materials in China.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI +1
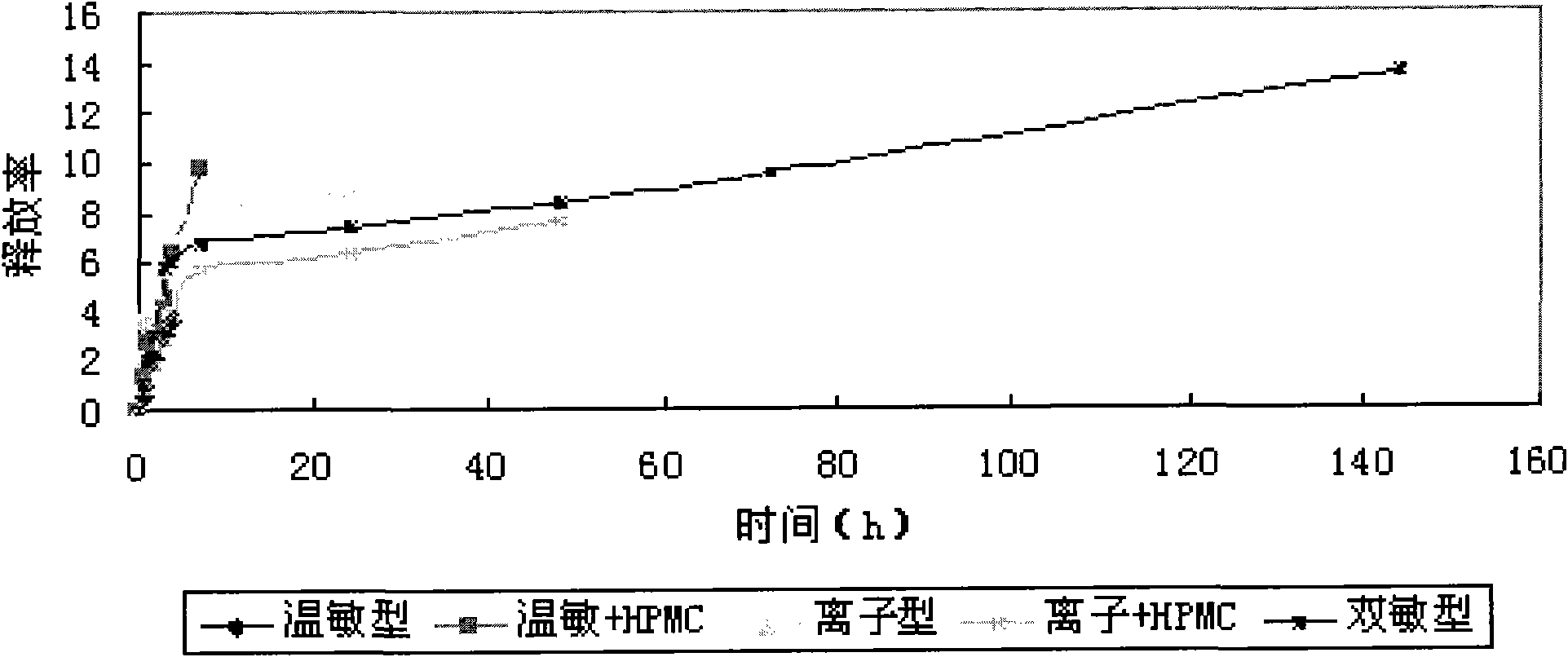
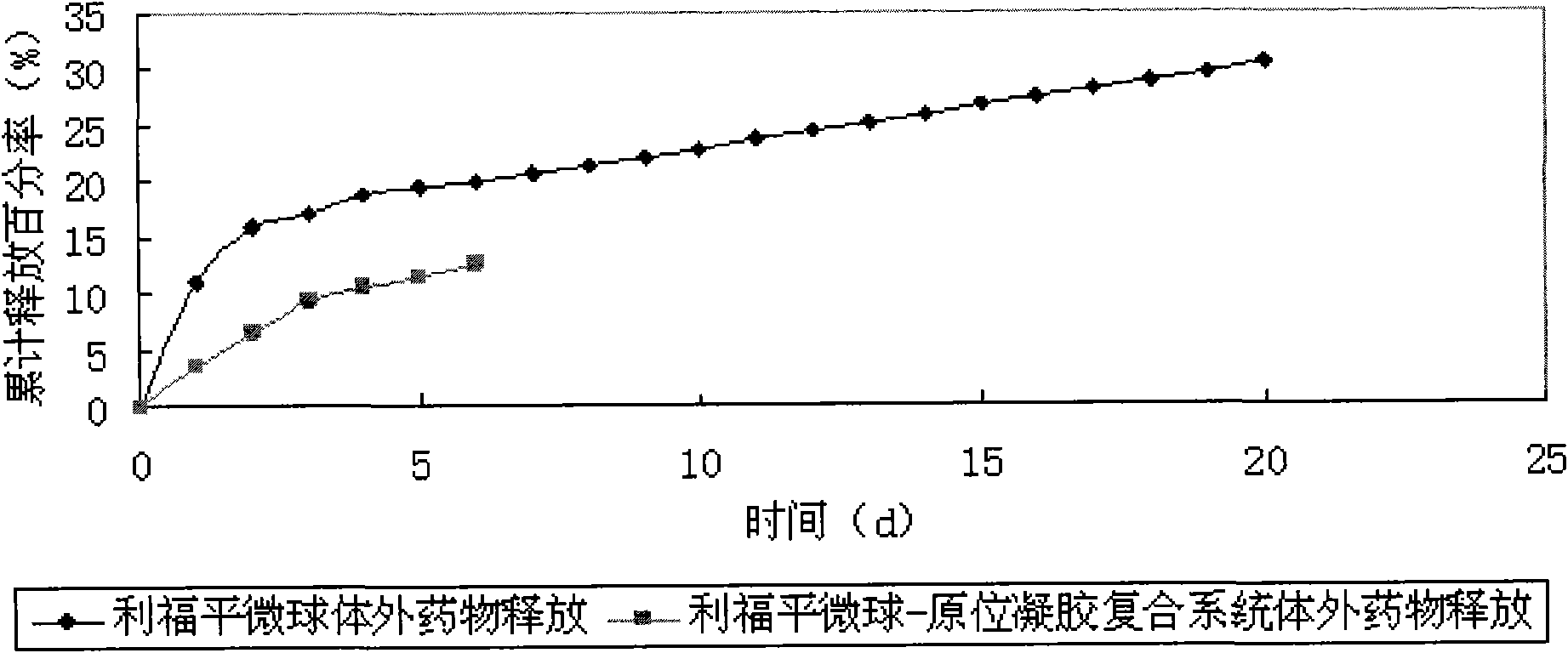
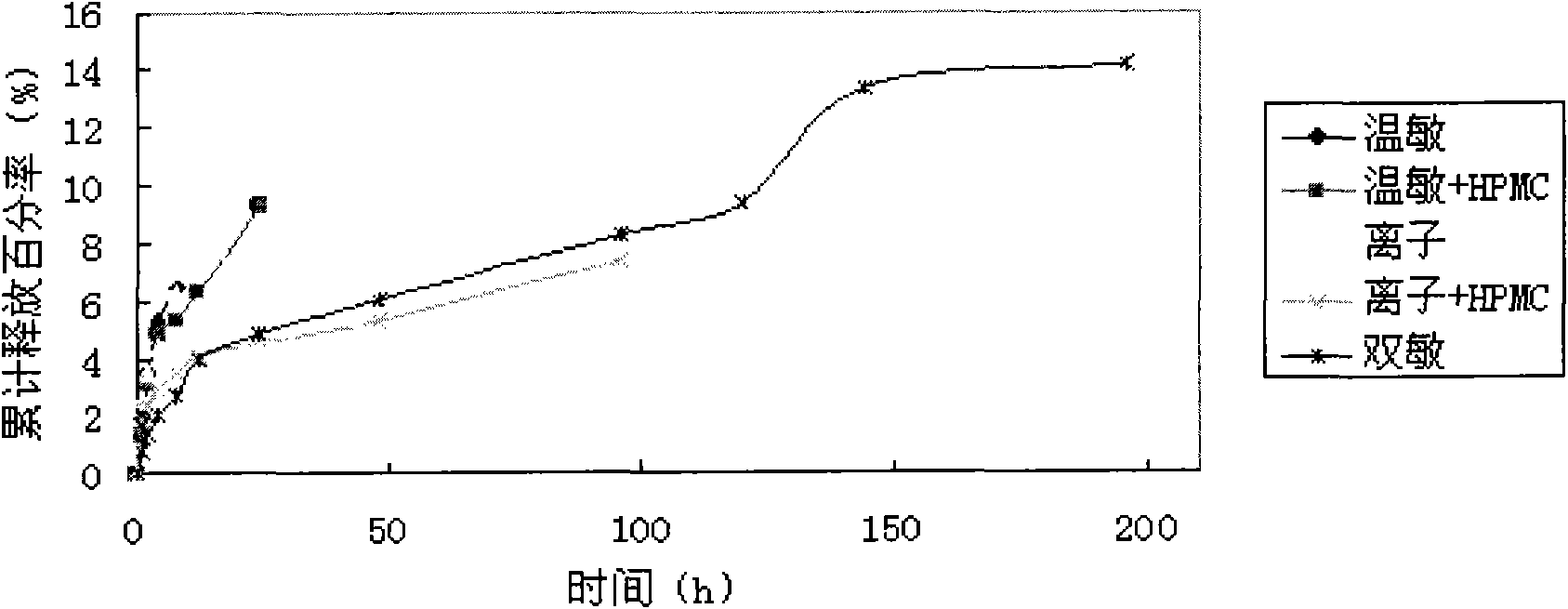
In-situ gel slow-release preparation for anti-tuberculosis drugs and preparation method thereof
The invention discloses an in-situ gel slow-release preparation for local drug delivery, which comprises drug active ingredient microspheres and in-situ gel. The in-situ gel slow-release preparation is characterized in that the gel forming temperature is kept within the scope of 15-25 DEG C and the calcium ion effect exists; the drug active ingredient is anti-tuberculosis drugs; the proportional relationship of the drug active ingredient microsphere and the temperature-ion sensitive gel is 5-50 mg:1-50 ml; the drug active ingredient microspheres are uniformly dispersed in the in-situ gel under the agitation condition; the in-situ gel slow-release preparation can restrain the excessive release of the drugs and lead the drugs to release stably and detain at the lung of a rat for 120 h at most; and a drug system detains at the local part for a long time, thereby realizing the treatment purpose.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Antituberculosis pharmaceutical composition
InactiveCN1602872AAvoid selective resistanceAvoid irregular medicationAntibacterial agentsOrganic active ingredientsPatient complianceHydrazine compound
The invention relates to an antituberculosis drug combination, prepared of rifampicin, isonicotinyl hydrazine, pyrazinamide, ethambutol hydrochloride and other auxiliaries, able to effectively overcome problems of drug resistance, improving compliance of patient, etc. It is applied in a compound form, which can avoid curing by single antituberculosis drug, thus avoiding selective drug resistance caused by single drug; the patients are willing to accept this, avoiding irregular drug application caused by simultaneously taking several drugs and different numbers of drug tablets, or taking more or less drugs.
Owner:浙江南洋药业有限公司
Anti tubercular drug: compositions and methods
Methods and compositions for treating disease caused by infectious agents, particularly tuberculosis. In particular, methods and compositions comprising substituted ethylene diamines for the treatment of infectious diseases are provided. In one embodiment, these methods and compositions are used for the treatment of mycobacterial infections, including, but not limited to, tuberculosis.
Owner:SEQUELLA INC +1
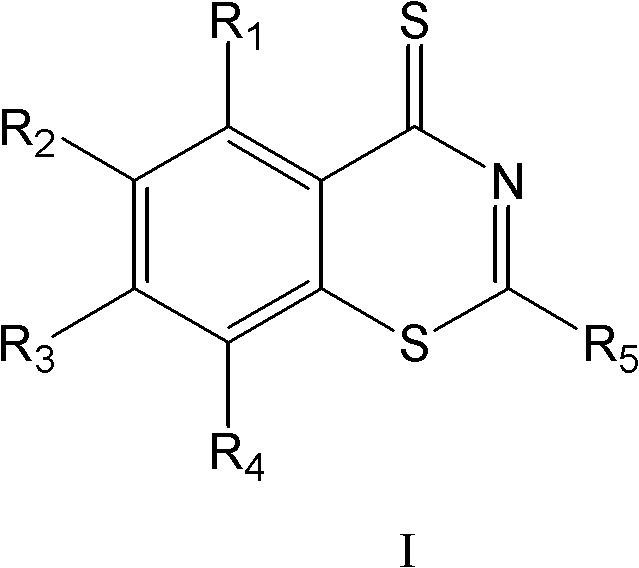
Benzothiazinethione derivatives and their preparation and use
ActiveCN102276598AHas anti-Mycobacterium tuberculosis activityAntibacterial agentsOrganic active ingredientsIsoniazidAntituberculous drugs
The invention belongs to the medicine field, and particularly relates to benzothiazinethione derivatives and preparation methods and uses thereof. In the aspect of the present invention, novel benzothiazinethione derivatives of formula I are provided, the benzothiazinethione derivatives of the invention are new compounds obtained based on extensive screening. Experimental results show that the benzothiazinethione derivatives of formula I have obvious inhibitory effects on mycobacterium tuberculosis, with effects equivalent to or even better than that of isoniazide (MIC 90 =0.8µM). The benzothiazinethione derivatives of formula I have anti-mycobacterium tuberculosis activities, and provide new choices for the development and application of antitubercular agents.
Owner:SICHUAN UNIV
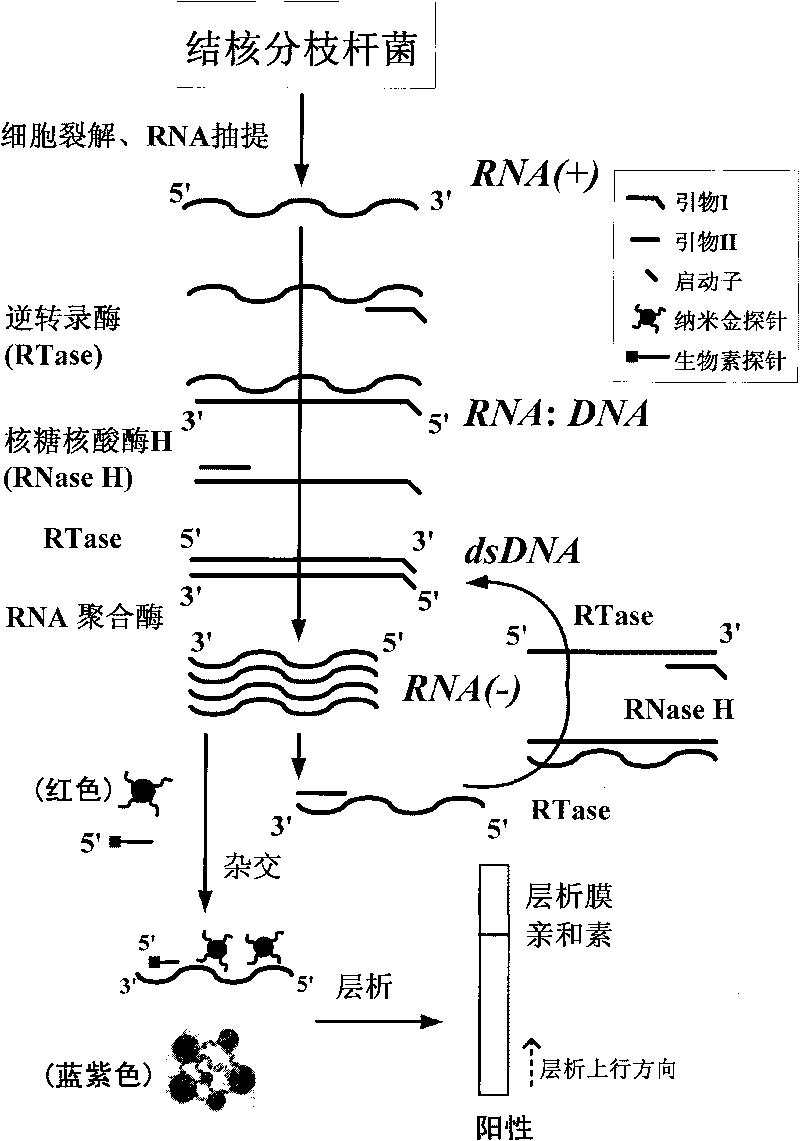
Method for detecting viable bacteria of Mycobacterium tuberculosis through isothermal amplification of nucleic acid and kit
InactiveCN101736078AEasy and intuitive to observeEasy to excludeMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementTreatment effectReverse transcriptase
The invention relates to a method for detecting viable bacteria of Mycobacterium tuberculosis through isothermal amplification of nucleic acid and a kit thereof. The method comprises the following detection steps: unlinking an mRNA template of the Mycobacterium tuberculosis at the temperature of between 60 and 70 DEG C and then reducing temperature; adding reverse transcriptase, RNaseH and RNA polymerase, and performing isothermal amplification at the temperature of between 37 and 42 DEG C under the guidance of a primer to obtain RNA amplicon; and then detecting the amplicon by utilizing a nanogold probe and a capture probe, and obtaining a detection result through chromatography hybridization color development reaction. That a color developing stripe appears on a hybrid membrane represents mRNA positive (the viable bacteria exist), no stripe represents negative. The kit adopting the method can detect the viable bacteria of the Mycobacterium tuberculosis, has the advantages of accuracy, sensitivity, simpleness, convenience, quickness and the like, can overcome the defects of long detection time, complex operation, low specificity and the like of the conventional methods, and can serve as an auxiliary experimental means for related researches such as diagnosis and prevention of tuberculosis, observation of treatment effect, screening of tuberculostatics, and sensitivity experiments.
Owner:SHANGHAI FOSUN PHARMA (GROUP) CO LTD +2
Slow released compound antituberculotic preparation
The slow released compound antituberculotic preparation contains at least one of rifampicin, pyrazinamide, kanamycin, isoniazide, rifapentine, etc. The slow released preparation is slow released injection or slow released implanting agent. The slow released injection consists of slow released microsphere and solvent, the slow released microsphere contains slow releasing supplementary material and antituberculotic, and the solvent is special solvent containing suspending agent carboxymethyl cellulose sodium and of viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is EVAc, PLA, PLGA, sebacic acid copolymer, etc. The slow released compound antituberculotic preparation is set or injected into local tuberulosis focus to treat various kinds of intractable tuberulosis, and has medicine releasing period up to 30-40 days, less systemic toxicity and unique curative effect.
Owner:JINAN SHUAIHUA PHARMA TECH
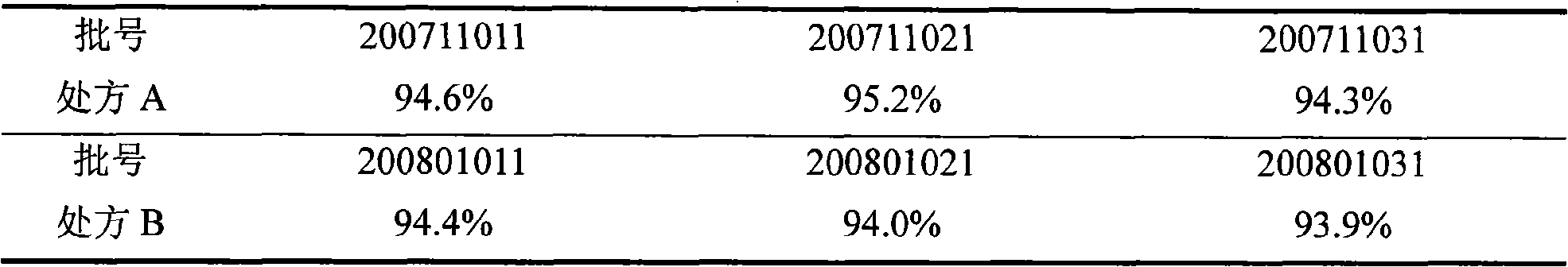
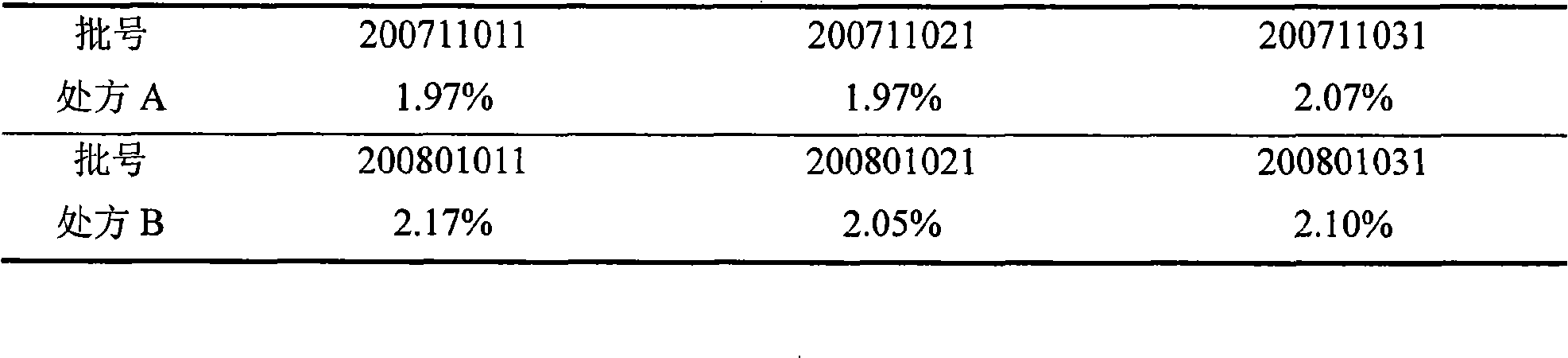
Compound preparation of antituberculosis medicaments, and preparation method thereof
ActiveCN101524355AEasy to measureAccurate measurementAntibacterial agentsOrganic active ingredientsDirect observationAntituberculous drugs
The invention belongs to the technical field of medicines, and provides a compound preparation of antituberculosis medicaments, and a preparation method thereof. Rifampicin, isoniazid, pyrazinamide and ethambutol hydrochloride as four leading antituberculosis medicaments are prepared into the compound preparation according to specific mixture ratio. The method comprises the steps of evenly mixing the leading drugs with an appropriate quantity of disintegrant and diluent, performing dry-process granulation, finishing granules, adding other auxiliary materials to the granules and preparing the obtained product into a suitable preparation. The invention solves the problems that patients are difficult to cooperate closely and complete normal chemotherapys because the antituberculosis medicaments are high in dosage, large in dosage form and troublesome in medicine-taking method, and the compound preparation and the preparation method accord with global tuberculosis direct-observation short-range supervision chemotherapy strategy (DOTS) recommended by the WHO. The antituberculosis fixed-dose compound preparation has important significance for simplifying medicine-taking method, carrying out the DOTS and controlling tuberculosis prevalence.
Owner:SHENYANG HONGQI PHARMA
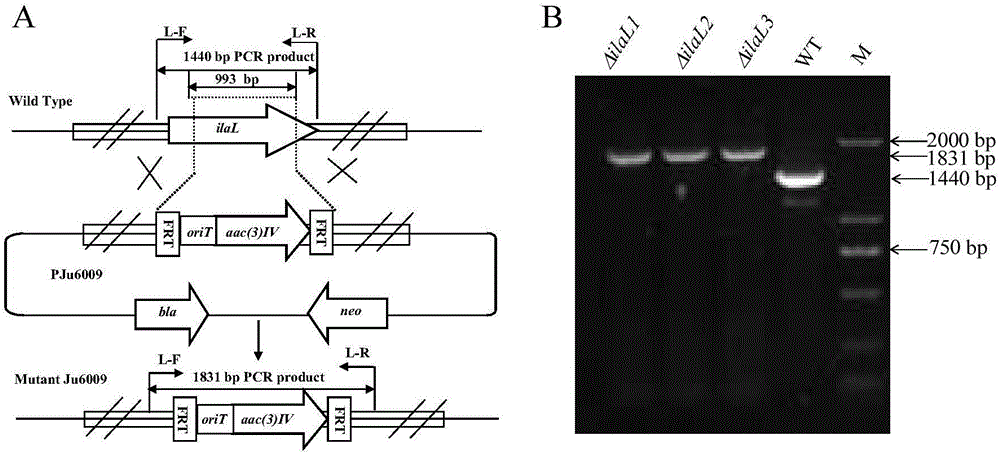
Genetic engineering bacterial strain for directionally producing compounds having anti-tuberculosis activity and anti-tumor activity and application thereof
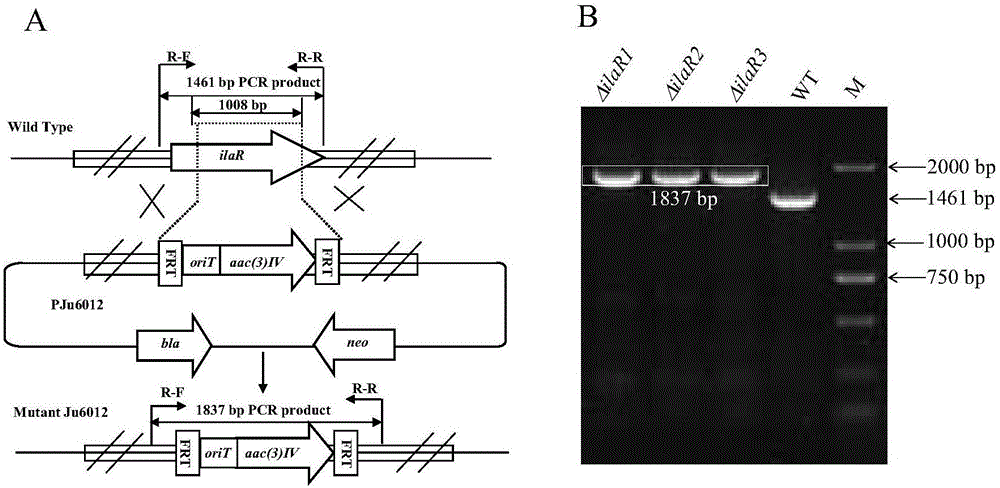
The invention discloses a genetic engineering bacterial strain for directionally producing compounds having anti-tuberculosis activity and anti-tumor activity and an application thereof. The genetic engineering bacterial strain is a genetic engineering bacterial strain obtained by knocking out an ialL gene or ilaR gene in a genome of streptomyces atratus SCSIO ZH16, wherein the ialL gene has the nucleotide sequence shown in SEQ ID NO.1, and the ialR gene has the nucleotide sequence shown in SEQ ID NO.2. The genetic engineering bacterial strain can produce the compounds 1, 2, 3, 4 and 5 having anti-tuberculosis activity and anti-tumor activity and shows great value in development of anti-tuberculosis drugs. Therefore, the successful construction of the genetic engineering bacterial strain for directionally producing the compounds having anti-tuberculosis activity and anti-tumor activity can accelerate the process of industrialization of the compounds and promote the development of Chinese marine drugs.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Innovative methods of treatmenting tuberculosis
InactiveUS20160074480A1Good effectEradicating diseaseAntibacterial agentsBiocideVitamin CAutohemotherapy
A Safe and effective treatment methods to cure and curtail tuberculosis affliction described using high dose Vitamin C, and other known anti-mycobacterium tuberculosis drugs especially rifampicin administered intravenously for 6 weeks instead of 6 to 24 months of conventional treatment. Insulin is administered to induce moderate hypoglycemia to augment and add effectiveness of anti-tuberculosis drugs and Vitamin C. Invention also delivers the drugs directly to a tuberculin lesion through a catheter. An embodiment of the invention uses a nebulizer and other methods of administration of Vitamin C with anti-tuberculosis drugs, interferon Y−, Coley's vaccine, dinitrophenol hyperthermia, ozone therapy, Hydrogen peroxide therapy and artemisinin, combined with oxygen supplementation including autohemotherapy with ozone, hyperbaric therapy, and hypertheramia to increase the respiration of the M. tuberculosis bacteria which has a killing effect.
Owner:SHANTHA TOTADA R
Markers for screening anti-mycobacterial treatment efficacy
ActiveUS8580490B1Effectively resolving the diseaseBacterial antigen ingredientsMicrobiological testing/measurementAnti mycobacterialAntituberculous drugs
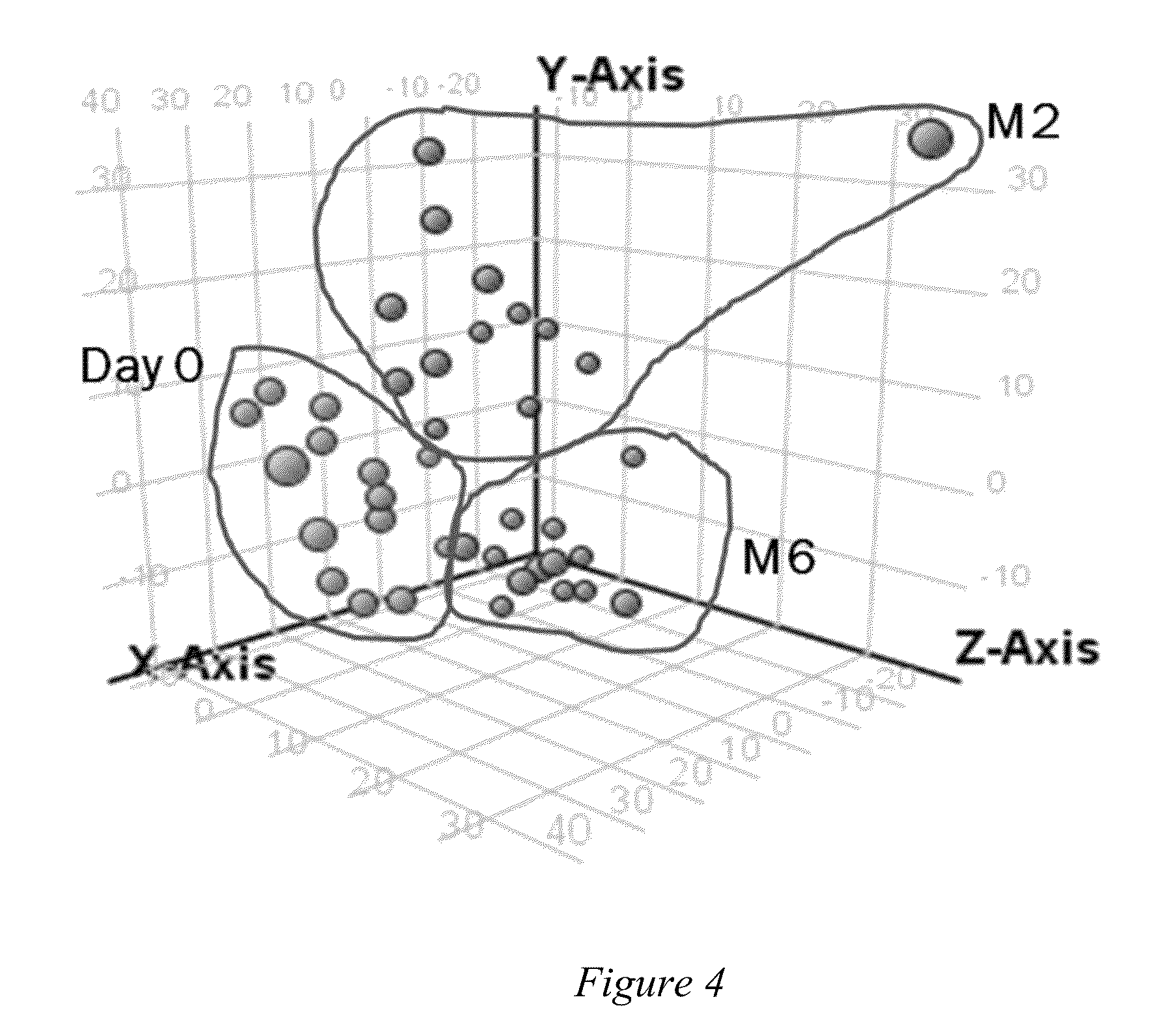
A method for metabolomically evaluating a subject's response to an anti-mycobacterial agent. The method includes the steps of generating multiple small molecule profiles using samples collected from the subject at or immediately prior to the start of treatment and at a times subsequent to the start of treatment with the anti-mycobacterial agent, identifying predetermined biomarkers in the small molecule profiles of the subject and comparing to a known standard established for the agent as an indication of whether the human is benefiting from treatment with the agent. Also provided are methods of monitoring treatment compliance, methods for establishing biomarkers indicative of treatment efficacy and validated biomarkers shown to be effective in assessing efficacy of anti-tuberculosis drugs.
Owner:COLORADO STATE UNIVERSITY
Application of clinafloxacin amino derivatives and medicinal salts thereof in preparing antitubercular medicaments
ActiveCN103405435AHas inhibitory effectStrong anti-tuberculosis activityAntibacterial agentsOrganic active ingredientsThioureaMoxifloxacin
The invention discloses an application of clinafloxacin amino derivatives and medicinal salts thereof in preparing antitubercular medicaments. In the structural general formula of the clinafloxacin amino derivatives, R is -(CH2)nNR<1>R<2>, -CH(CH2CH2XCH3)NH2, 2-pyrrolidyl or -OR3; n is 0 or 1; R1 and R2 are separately hydrogen, methyl, 2-hydroxyethyl, (R)-1-ethyl-2-hydroxyethyl, 2-aminoethyl, 3-(dimethylamino)propyl, hydroxyl, amino, methylamino, methoxyl or thiourea group; X is S or SO2; R3 is methyl or isobutyl; the compounds have certain inhibitory effect on standard sensitive strains, clinically isolated sensitive strains and clinically isolated drug-resistant strains of mycobacterium tuberculosis, the antitubercular activity of a part of compounds is stronger than that of ofloxacin and clinafloxacin and weaker than that of moxifloxacin, thus providing a new research direction for the antitubercular medicaments, and being beneficial to clinical treatment of tuberculosis.
Owner:SOUTHWEST UNIVERSITY
Benzothiazine-4-ketone compounds containing basic nitrogen heterocyclic fragments and preparing methods of benzothiazine-4-ketone compounds
ActiveCN105669664AHigh activityGood curative effectAntibacterial agentsOrganic active ingredientsBenzeneNitrogen
The invention relates to benzothiazine-4-ketone compounds (shown in the formula I and formula I' in the description) containing basic nitrogen heterocyclic fragments, preparing methods and medical application of the benzothiazine-4-ketone compounds and antituberculosis drug compositions with the benzothiazine-4-ketone compounds as effective constituents, in particular to a 6-trifluoromethyl-8-nitryl-4H-benzo[e][1,3] thiazine-4-ketone compound. A 2-substituent group is 1-nitrogen heterocyclic alkyl or dinitrogen heterocyclic alkyl. R represents H, an alkyl group of 1-4 C atoms, heterocyclic alkyl of 4-7 C atoms, a phenyl group and a substituted phenyl; R1 represents H, an alkyl group of 1-3 C atoms and heterocyclic alkyl of 3-6 C atoms; R2 represents H, an alkyl group of 1-4 C atoms, heterocyclic alkyl of 4-7 C atoms, a phenyl group and substituted phenyl; n1 represents 0-1; n2 represents 1-3; n3 represents 1 and 3.
Owner:ZHEJIANG STARRY PHARMA +1
Tubercle bacillus drug tolerance detection reagent kit and tubercle bacillus drug tolerance detection method
PendingCN111172303AWide range of drug resistance detectionEasy constructionMicrobiological testing/measurementLibrary creationAntituberculosis drugTuberculosis bacillus
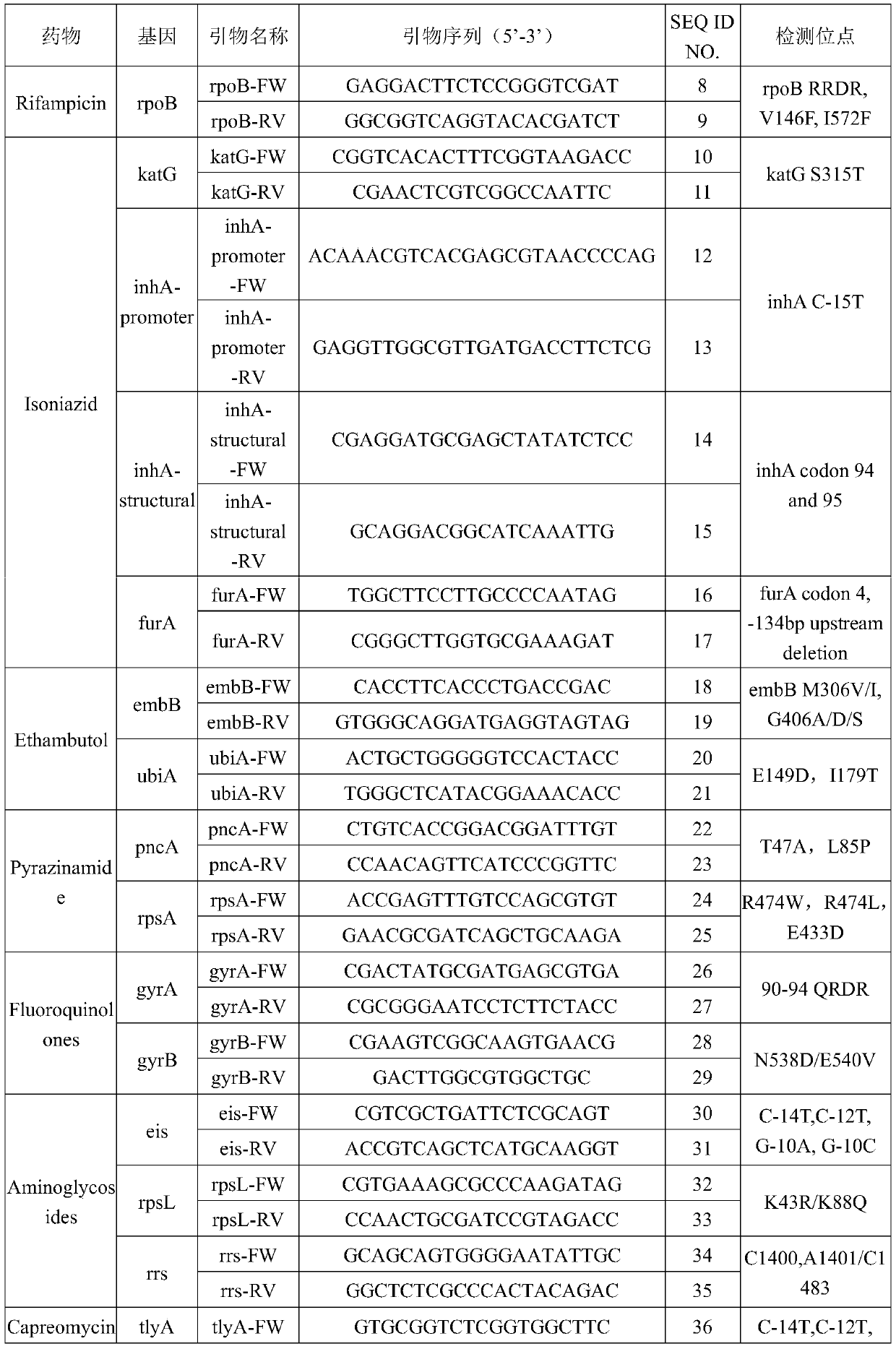
The invention provides a tubercle bacillus drug tolerance detection reagent kit and method. The tubercle bacillus drug tolerance detection reagent kit comprises a tubercle bacillus drug tolerance detection reagent, wherein the tubercle bacillus drug tolerance detection reagent comprises a sequencing primer in accordance with a tubercle bacillus drug tolerance gene; the tubercle bacillus drug tolerance gene comprises one or more genes of rpoB, katG, inhA-promoter, inhA-structural, furA, embB, ubiA, pncA, rpsA, gyrA, gyrB, eis, rpsL, rrs, tlyA, rplC and rrl; and further, the reagent kit also contains a tubercle bacillus nucleic acid detection reagent, and the tubercle bacillus nucleic acid detection reagent comprises a primer pair 1 in accordance with IS6110, a primer pair 2 in accordance with the IS6110 and a probe primer in accordance with the IS6110. Through the adoption of the tubercle bacillus drug tolerance detection reagent kit disclosed by the invention, tubercle bacillus nucleicacid in samples can be quickly detected, and positive samples can be further subjected to drug tolerance detection; and the tubercle bacillus drug tolerance detection reagent kit has good sensitivity, good specificity and good accuracy, and can perform mutation detection on 48 sites of 17 drug tolerance genes of common antituberculosis drugs and fragment deficiency detection of an intergenic region, so that the tuberculosis medication can be more accurately and comprehensively guided.
Owner:GUANGZHOU KINGMED DIAGNOSTICS GRP CO LTD +1
Near infrared spectrum damage-free analysis method for anti-tuberculosis drugs
InactiveCN101101260AQuick checkNon-destructive testingColor/spectral properties measurementsSpecial data processing applicationsInfraredAction spectrum
The invention discloses method of testing effective constituent of near infrared light detection anti tubercular agent. Using the basis of anti tubercular agent action spectrum near infrared light and the measurand information, getting effective constituent (rifampicin,isoniazide or pyrazinamide ) of which by anti tubercular agent action spectrum near infrared light and their background information; achieving the lossless detection the content of effective constituent (rifampicin,isoniazide or pyrazinamide ) in the anti tubercular agent near infrared light complex background by the multi element normalized method of chemometrics. It settles the problem of high cost, long period, medicament cannot use after analyzing etc when analyzing the anti tubercular agent effective constituent in existence, establishing the fast, high pass mete, lossless and needed on-line analysis green medicament analytic method for the anti tubercular agent. The advantages is that the sample pre-processing is easy, the detection is fast and undamaged, the detection time of each sample is shortage of two min; the result is credibility, the error is less than5%.
Owner:JILIN UNIV
Method for extracting live bacteria RNA in Mycobacterium tuberculosis and detection kit thereof
InactiveCN101760518AEasy to detectImprove featuresMicrobiological testing/measurementDNA preparationRNA extractionAntituberculous drugs
The invention relates to a method for extracting live bacteria RNA in Mycobacterium tuberculosis and a detection kit thereof, and particularly provides a method for extracting live bacteria RNA in Mycobacterium tuberculosis and a detection kit which is used for the live bacteria RNA in the Mycobacterium tuberculosis and is obtained by applying the method and combining fluorescent quantitative PCR technology. The kit can accurately, sensitively and quickly identify dead bacteria and the live bacteria of the Mycobacterium tuberculosis, reduce the cost and shorten the detection time, and more importantly, the kit is the basis of studies such as the diagnosis of tuberculosis, medicinal susceptibility experiments, the monitoring of chemotherapy response, the screening of new antitubercular medicaments, the prevention of the tuberculosis and the like.
Owner:SHANGHAI FOSUN PHARMA (GROUP) CO LTD +1
Applications of Mycobacterium tuberculosis antigen protein Rv0446c and T-cell epitope peptide thereof
ActiveCN106248934AReduce false positivesStrong immune responseAntibacterial agentsBacterial antigen ingredientsAntigenStimulant
The present invention relates to applications of Mycobacterium tuberculosis antigen protein Rv0446c and a T-cell epitope peptide thereof in preparation of tuberculosis detection reagents, vaccines and medicines, wherein the amino acid sequences of the antigen protein Rv0446c and the T-cell epitope peptide thereof are respectively represented by SEQ ID NO:1-5. According to the present invention, the Mycobacterium tuberculosis antigen protein Rv0446c and the T-cell epitope peptide thereof are used as the stimulants for the specific T cell and B cell immune response caused by Mycobacterium tuberculosis infection, and the false positive caused by the impure antigen can be reduced compared with the use of the complete antigen in the prior art; and the detection reagents prepared from the antigen protein Rv0446c and the T-cell epitope peptide thereof can be widely used for assisted diagnosis of tuberculosis, epidemiological surveillance and other related fields, and the tuberculosis vaccines and the anti-tuberculosis drugs prepared from the antigen protein Rv0446c and the T-cell epitope peptide thereof can be used for prevention and treatment of tuberculosis.
Owner:ICDC CHINA CDC
Bone restoration support frame for bone tuberculosis treatment and preparation method thereof
ActiveCN109432498APromote regenerationInduced regenerationCapsule deliveryProsthesisMicrosphereBiocompatibility Testing
The invention relates to a bone restoration support frame for bone tuberculosis treatment and a preparation method thereof, and belongs to the technical field of biomedicine engineering. The bone restoration support frame contains strontium-doped nanometer hydroxyapatite, gelatin and sodium alginate medicine carrying microspheres. The bone restoration support frame has an antituberculous medicineslow release system of a three-dimensional porous support frame structure. Compared with implementing bodies with other forms, the support frame has the advantages that the cell and blood vessel growth is facilitated; the new bone regeneration is promoted; the antituberculous medicine is effectively released for a long time in the local part of the tuberculosis disease; the good local environmentis provided for bone defect healing. The medicine carrying anti-bone tuberculosis restoration support frame has good mechanical property and biocompatibility; the antituberculous medicine can be continuously released; good bone induction capability and bone conduction capability are realized; the regeneration of the bone tissue defect position can be induced.
Owner:GUANGZHOU CHUANGSAI BIOLOGICAL MEDICAL MATERIALS CO LTD
Method for simultaneously measuring content of three antitubercular agents in blood and tissues
InactiveCN102384949AWide applicabilityReliable detection meansComponent separationSodium 1-heptanesulfonateColumn temperature
The invention provides a method for simultaneously measuring content of three antitubercular agents (retozide, rifampicin and pyrazinamide) in blood and tissues. The method has the characteristics of simplicity and convenience in operation, high precision, high detection speed, stability and reliability. A high-efficient liquid-phase chromatographic instrument (LC2010C), a column temperature control box AT-130 and an Lc-solution spectrum work station of the Japan Shimadzu Company are adopted. The chromatographic column is an analysis column and adopts a C8 column, the protection column adopts a C18 column, and the fluid phase adopts sodium 1-heptanesulfonate solution to prepare.
Owner:蒋晖 +2
Mycobacterium tuberculosis surface lipolysaccharide-antistatic nucleic acid aptamer and application thereof
ActiveCN102373213AEasy to makeLow priceAntibacterial agentsOrganic active ingredientsSide effectNucleotide
The invention discloses a mycobacterium tuberculosis surface lipolysaccharide-antistatic nucleic acid aptamer and application thereof. The aptamer is a small molecular single-stranded deoxyribonucleic acid (ssDNA) which is specific to toxic mycobacterium tuberculosis surface lipolysaccharide ManLAM (Mannosylated Lipoarabinomannan) and has tuberculosis infection resisting and cellular immunity enhancing functions, and the nucleotide sequence of the ssDNA is shown as SEQIDNo.1. The small molecular ssDNA has a novel target spot and a novel structure which are different from those of the conventional anti-tuberculosis medicament, and has the immunologic suppression function of directly enclosing the ManLAM. The aptamer is easy to prepare and has low price; the obtained ssDNA aptamer is specifically combined on the surface of toxic mycobacterium tuberculosis; and the treatment targeting is further enhanced. The problems of increasing medicament tolerance and large side effect existing in the conventional treatment of tuberculosis are solved, and the aptamer can be taken as an effective novel anti-tuberculosis medicament or a tuberculosis diagnosis reagent.
Owner:武汉顺可达生物科技有限公司
Anti tubercular drug: compositions and methods
Methods and compositions for treating disease caused by infectious agents, particularly tuberculosis. In particular, methods and compositions comprising substituted ethylene diamines for the treatment of infectious diseases are provided. In one embodiment, these methods and compositions are used for the treatment of mycobacterial infections, including, but not limited to, tuberculosis.
Owner:SEQUELLA +1
Triple compound microsphere vascular targeted embolization sustained-release preparation containing antituberculous drug as well as preparation method and application of preparation
ActiveCN104324032AExcellent anti-tuberculosis effect in vitro and in vivoReduce concentrationAntibacterial agentsOrganic active ingredientsAntituberculous drugHemoptyses
The invention relates to a triple compound microsphere vascular targeted embolization sustained-release preparation containing an antituberculous drug as well as a preparation method and application of the preparation. The sustained-release agent comprises a carrier and drugs, wherein the drugs are coated with the carrier; the carrier is sodium alginate or chitosan, and the drugs are triple antituberculous compound drugs including rifampicin, isoniazid and pyrazinamide or moxifloxacin. The three antituberculous drugs are matrix drug solutions, the sodium alginate or chitosan is a carrier solution, the matrix drug solutions and the carrier solutiona are mixed to prepare a solution, the polymer solution containing drugs is dispersed into fogdrops with a certain diameter by adopting a high-voltage electrostatic droplet mode, and the fogdrops are sprayed into a solidifying liquid to prepare antituberculous drug microspheres under the action of calcium ions. The embolization sustained-release preparation can be used for treating tuberculosis, massive hemoptysis of pulmonary tuberculosis, tuberculosis cavity, renal tuberculosis, osteoarticular tuberculosis, genital tuberculosis, tuberculosis of thyroid gland, tuberculosis of cervical lymph nodes, tuberculosis of pericardium, tuberculosis of chest wall, pleural tuberculosis and other kinds of tuberculosis in a body.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY +1
Method for screening drugs for targeting synthesis and assembly of bacillus tubercle cell wall core
ActiveCN101906461ASimple and fast operationImprove throughputMicrobiological testing/measurementMicroorganism based processesMicroorganismHigh flux
The invention relates to a method for screening drugs for targeting bacillus tubercle, in particular to a method for screening drugs for targeting synthesis and assembly of a bacillus tubercle cell wall. The invention comprises the following steps of establishing a screening model for targeting the synthesis and the assembly of the bacillus tubercle cell wall core by adopting Corynebacterium glutamicum as a test organism and an BHI (Brain-Heart Infusion) and BHIS (Brain-Heart Infusion Supplemented) medium as a screening medium, and screening novel anti-tuberculous drugs and lead compounds of different enzyme systems and target spots, which act on the synthesis and assembly process of the bacillus tubercle cell wall core, from microorganism metabolic products and samples of other sources. The anti-tuberculous drugs for targeting the whole synthesis and assembly process of the bacillus tubercle cell wall core can be screened by the screening model of the invention. The model is a cell-level high-flux screening model.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Porous calcium phosphate support loaded microsphere composite material, as well as preparation method and application thereof
InactiveCN109432020AReduce recurrenceAntibacterial agentsPowder deliveryCalcium biphosphateAntituberculosis drug
The invention provides a porous calcium phosphate support loaded microsphere composite material, as well as a preparation method and application thereof. The porous calcium phosphate support loaded microsphere composite material comprises a porous calcium phosphate support, wherein a polylactic acid-glycolic acid copolymer microsphere is loaded in each hole of the porous calcium phosphate supportand the polylactic acid-glycolic acid copolymer microsphere coats one or more antituberculosis drugs. The experiment proves that the porous calcium phosphate support loaded microsphere composite material has a filling bone remodeling and bone inducing osteogenesis function, further has an effect of locally continuously and three-dimensionally slowly releasing the antituberculosis drugs, and achieves an effect of treating osteoarticular tuberculosis and reducing recurrence. The invention further provides the preparation method and the application of the porous calcium phosphate support loaded microsphere composite material.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
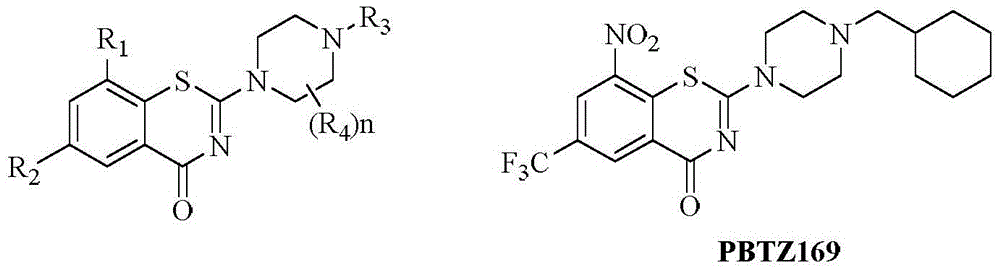
Benzothiazinone compound, preparation method thereof and application of benzothiazinone compound as antituberculosis drug
ActiveCN112409293AGood antibacterial effectWork around the bug with high cLogP valuesAntibacterial agentsOrganic active ingredientsAntituberculosis drugAntituberculous drugs
The invention discloses a benzothiazinone compound and a preparation method and application of the benzothiazinone compound as an antituberculosis drug, and particularly relates to a novel compound with a benzothiazinone skeleton. The compound has an inhibition effect on tubercle bacillus, especially tubercle bacillus with clinical drug resistance. Results show that the compound shows an obvious antibacterial effect, the antibacterial effect far exceeds that of a positive control isoniazide, and particularly, compared with a positive control pBTZ169, the compound has an obvious and good cLogPvalue.
Owner:SUZHOU UNIV
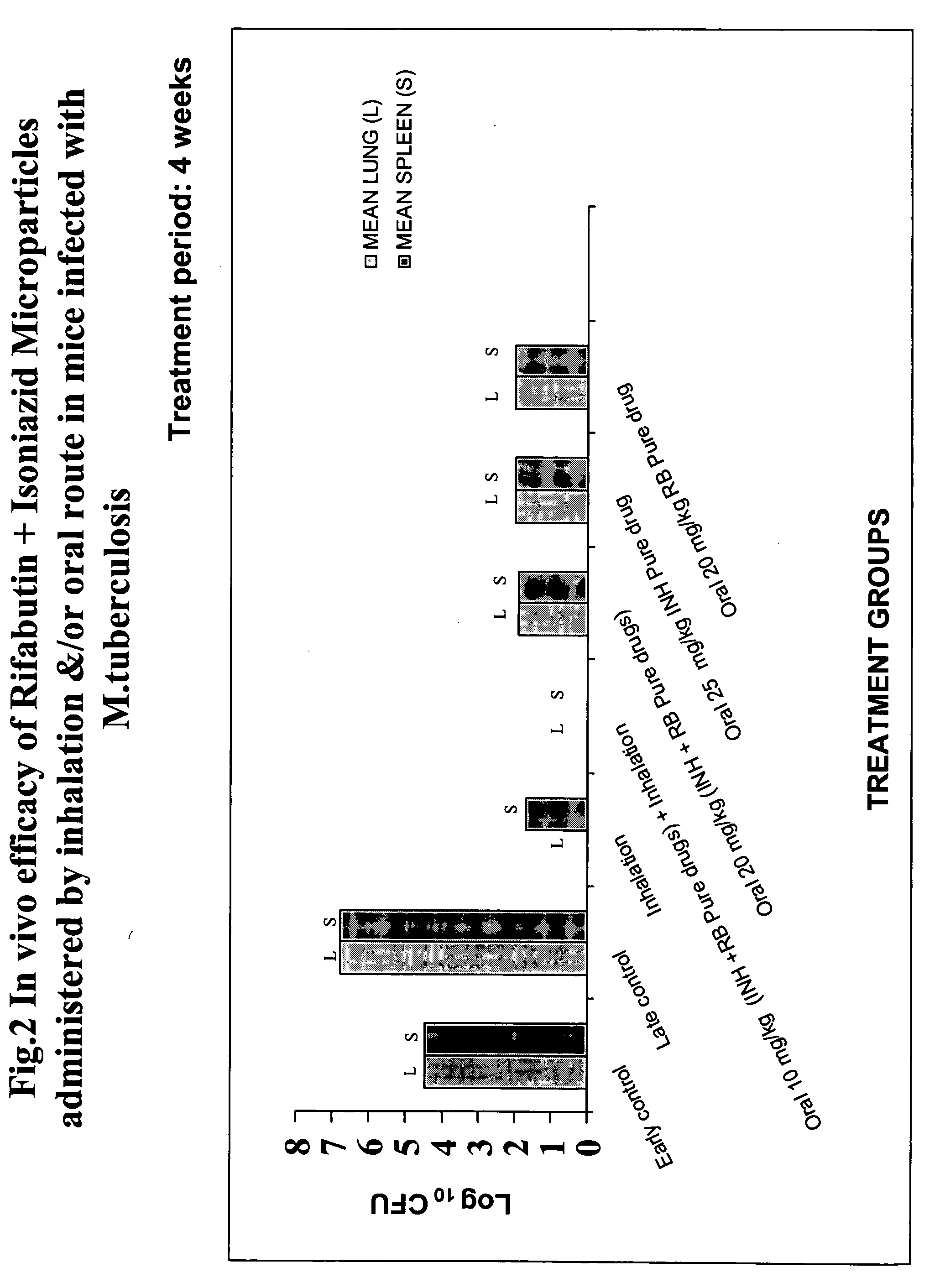
Inhalable biodegradable microparticles for target-specific drug delivery in tuberculosis and a process thereof
InactiveUS20050084455A1Reduce the amount of solutionReduce the amount requiredPowder deliveryBiocideMicroparticleAntituberculous drugs
The present invention relates to a biodegradable microparticle composition useful for the target specific drug delivery to manage pulmonary tuberculosis, said composition comprising two anti-tuberculosis drugs, and a biodegradable polymer for drug delivery in a ratio of about 1:2 to 2:1, wherein the anti-tubercular drugs are in the ratio of 1:2 to 2:1, also, a process for the preparation of the composition, and lastly, a method of treating pulmonary tuberculosis in a subject, said method comprising administering by inhalation alone or in combination with oral route, pharmaceutically effective amount of the composition to the subject in need thereof, wherein the dosage for inhalation is ranging between 0.5 to 10 mg / kg body weight / day and that for oral route is ranging between 4 to 32 mg / kg body weight / day.
Owner:COUNCIL OF SCI & IND RES +1
Diphasic quick differential medium of mycobacterium tuberculosis and application of medium
InactiveCN103993065APromote growthHigh speedMicrobiological testing/measurementMicroorganism based processesLiquid mediumAntituberculosis drug
The invention relates to a preparation method of a diphasic quick differential medium of mycobacterium tuberculosis, a method for quickly differentiating the mycobacterium tuberculosis by using the medium, a method for testing the drug resistance on antituberculous drugs caused by the mycobacterium tuberculosis and a method for quickly culturing a bacillus Calmette and Guerin vaccine by using a liquid medium in the medium. The diphasic medium prepared by adopting the method can be used for quickly culturing the mycobacterium tuberculosis, the diphasic quick culture medium is capable of differentiating the mycobacterium tuberculosis within 2-5 days, the differentiated mycobacterium tuberculosis can be subjected to drug resistance detection of the antituberculous drugs such as rifampicin, isoniazide and streptomycin by virtue of a reverse transcription PCR (polymerase chain reaction) method, and the liquid medium prepared by adopting the method is capable of finishing the culture of the bacillus Calmette and Guerin vaccine within 6-9 days.
Owner:JINING MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com