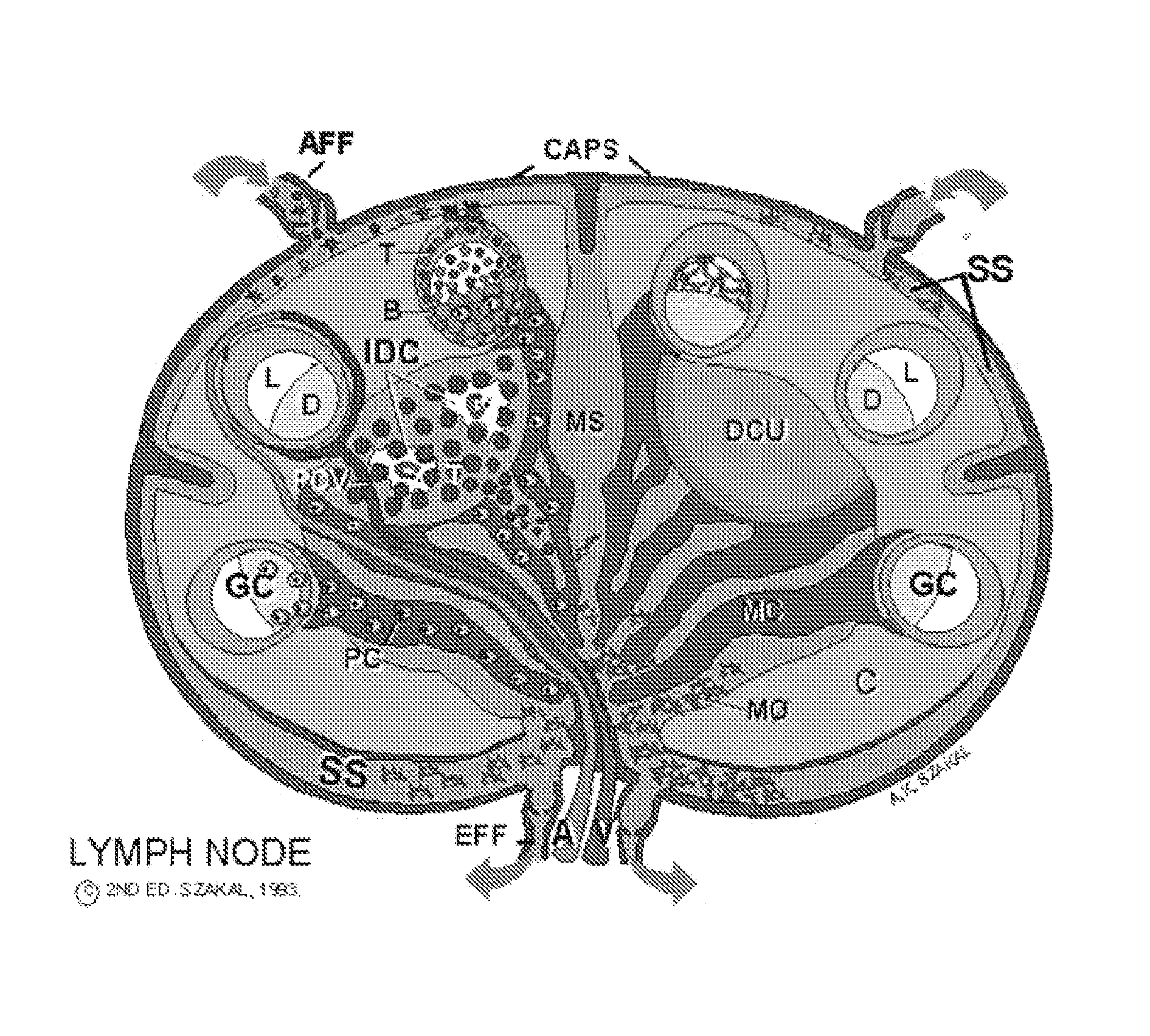
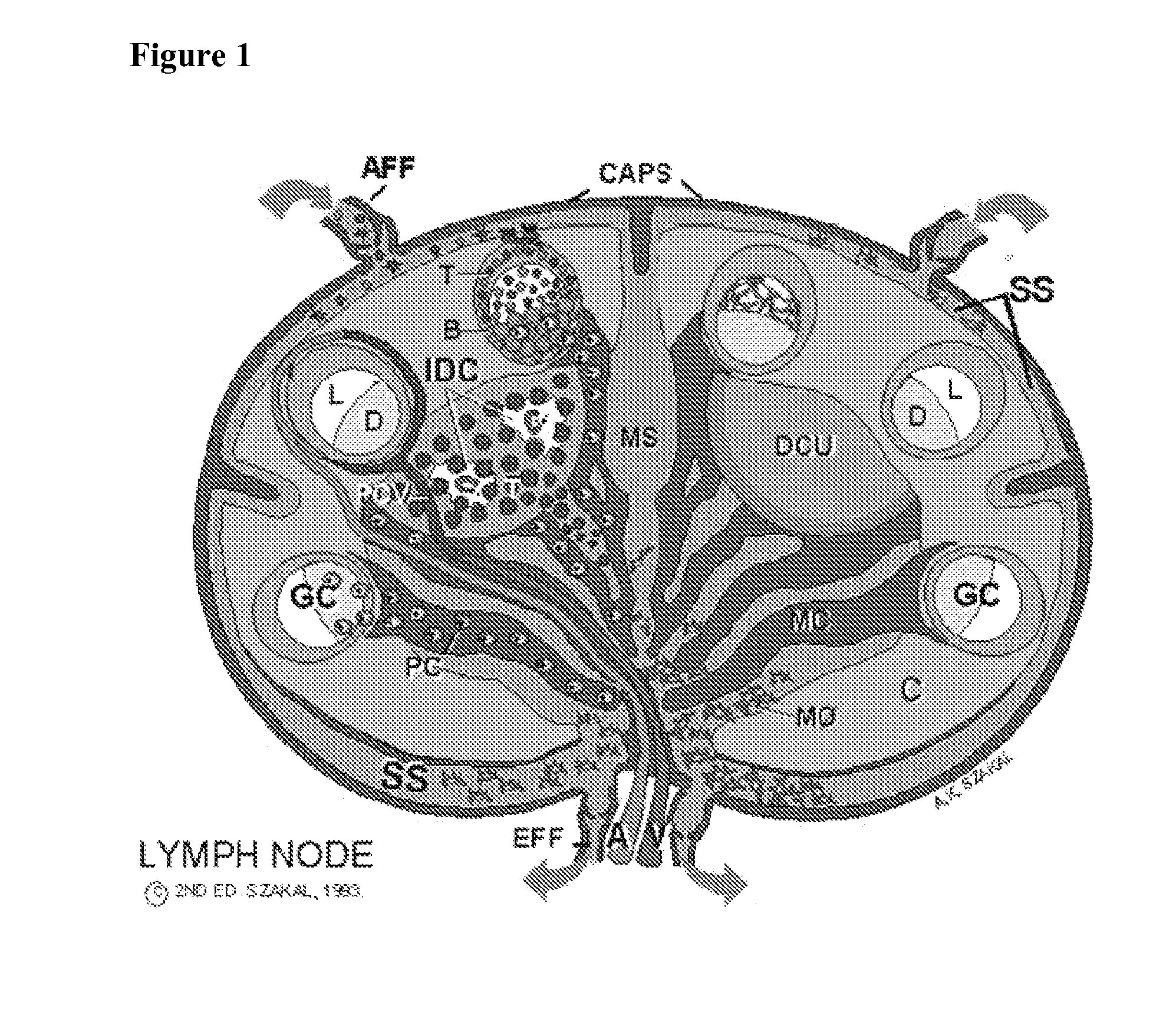
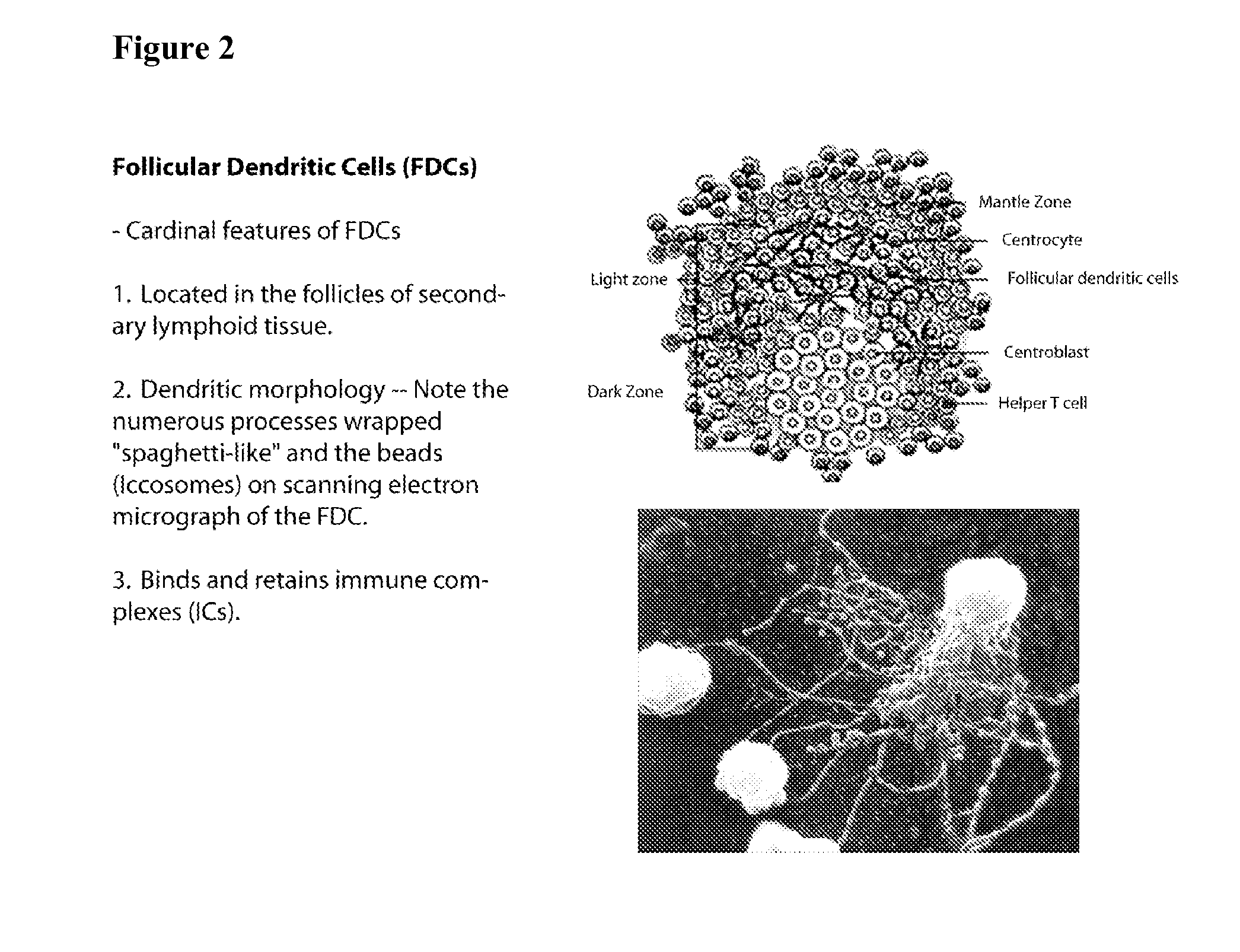
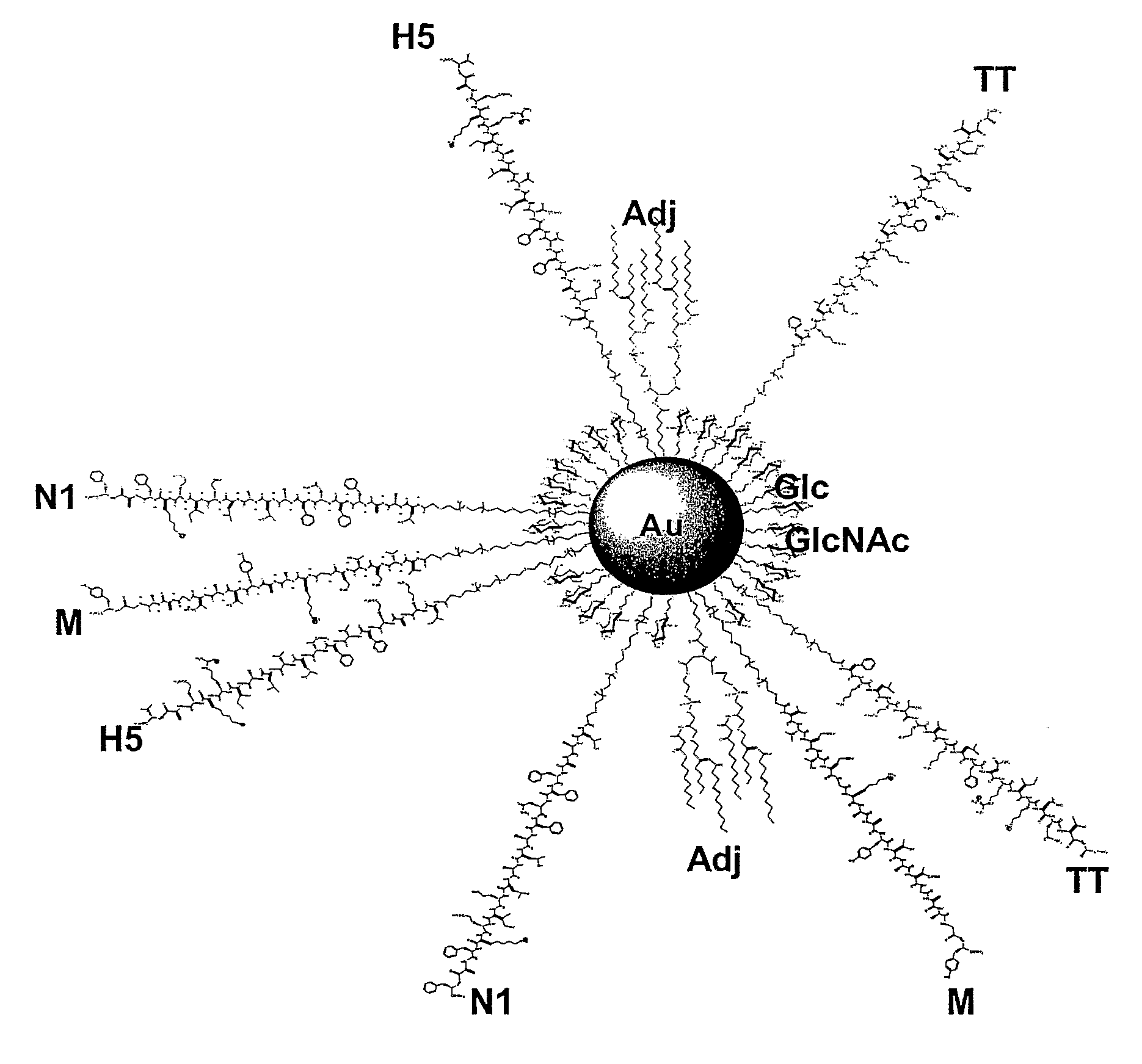
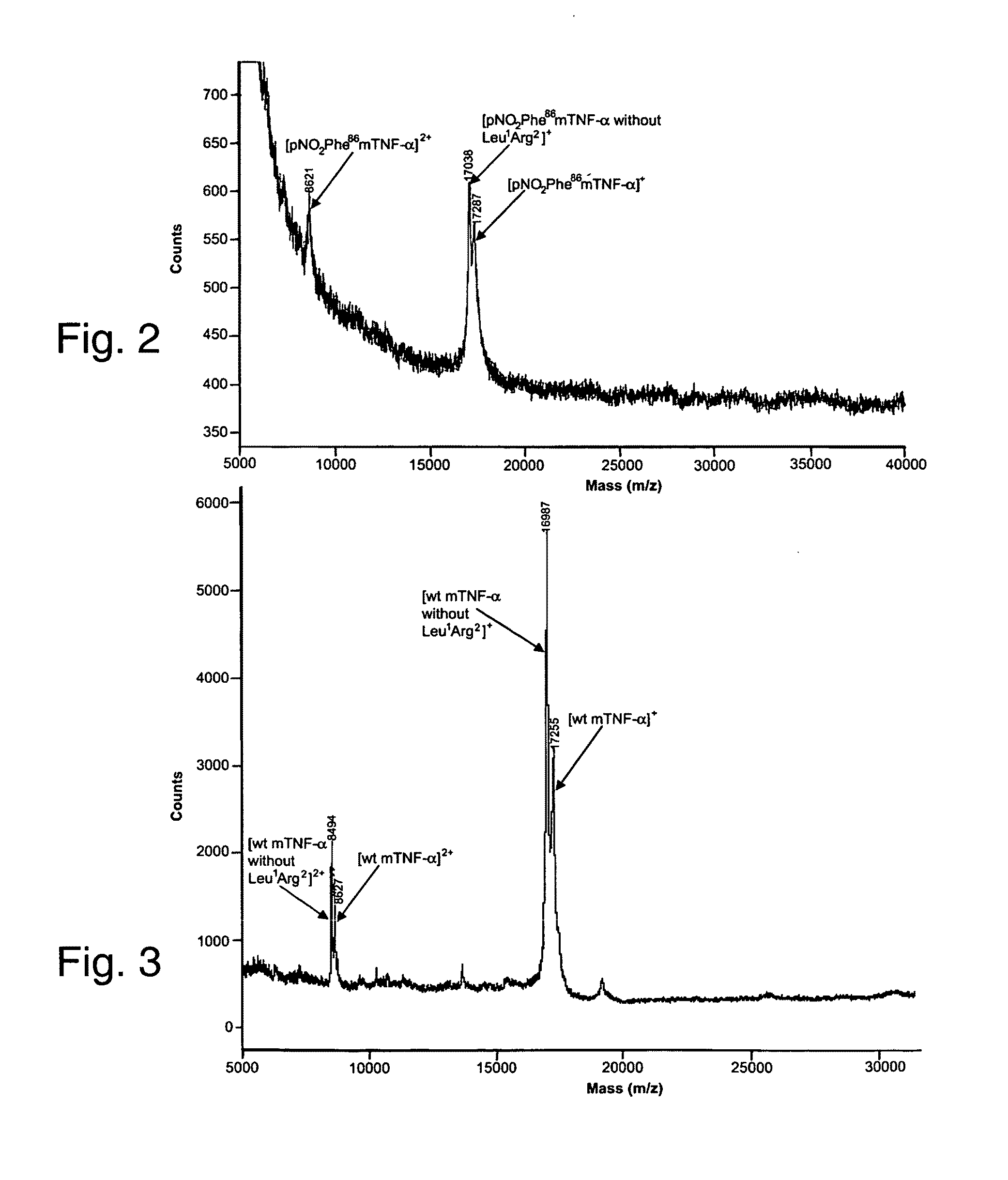
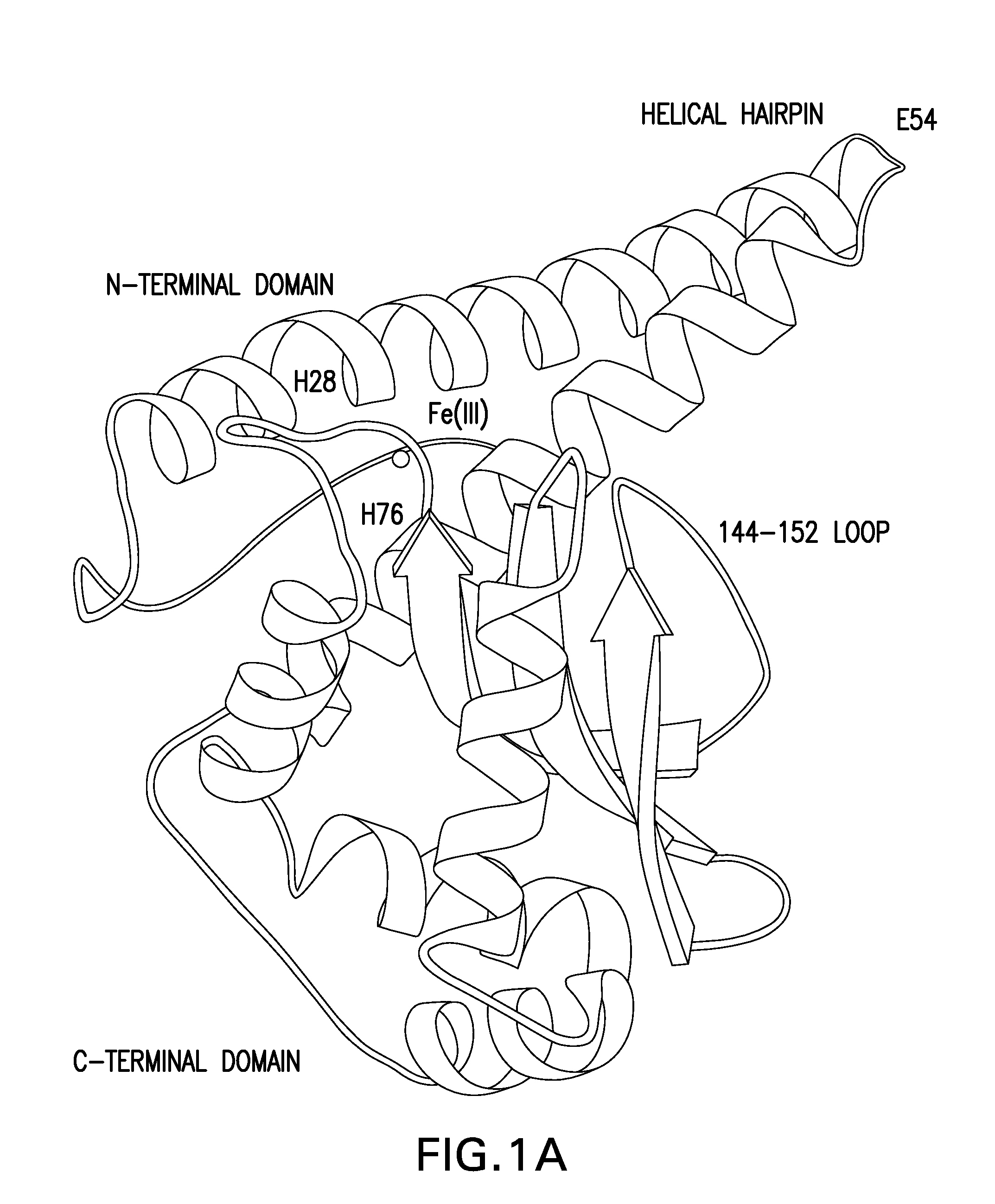
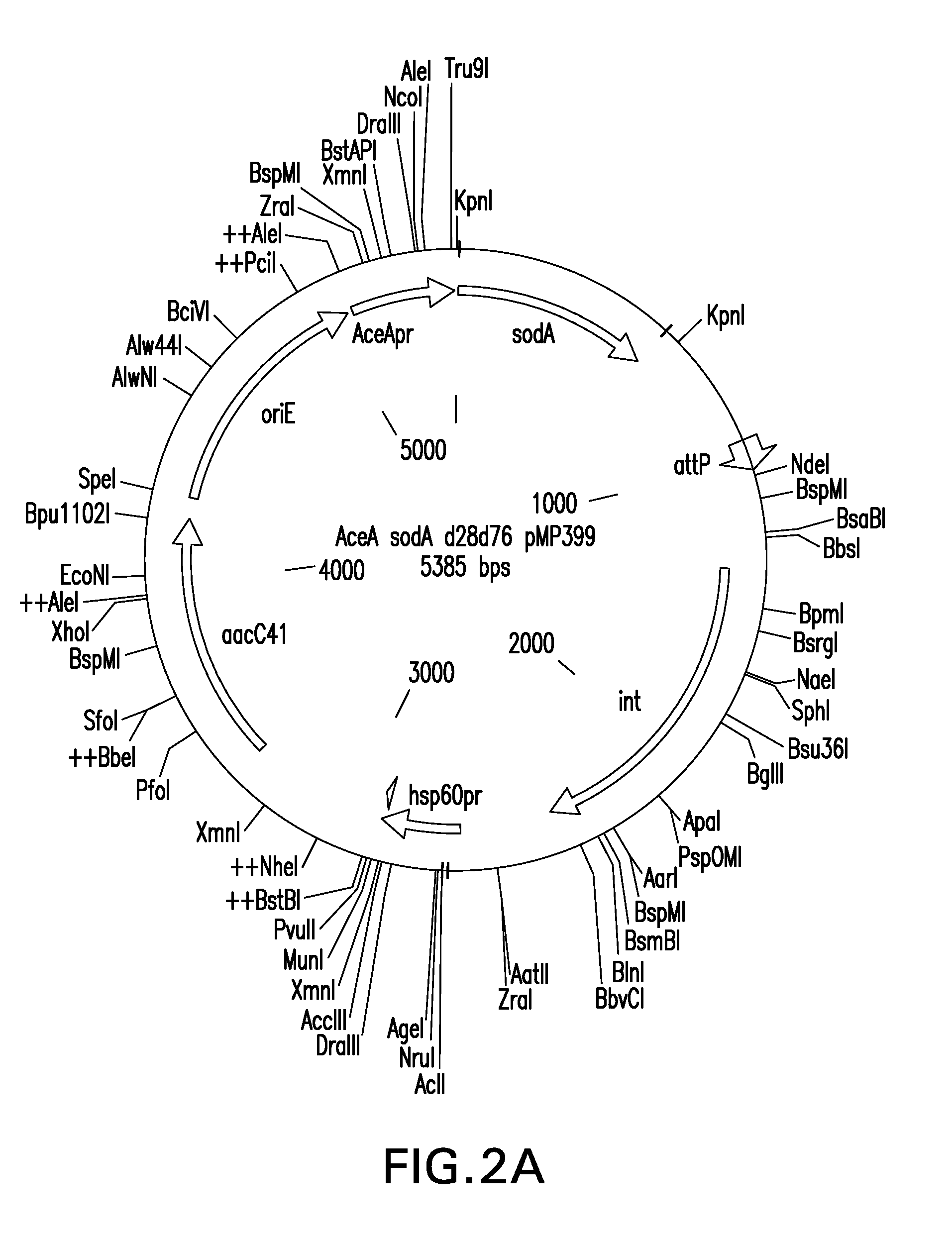
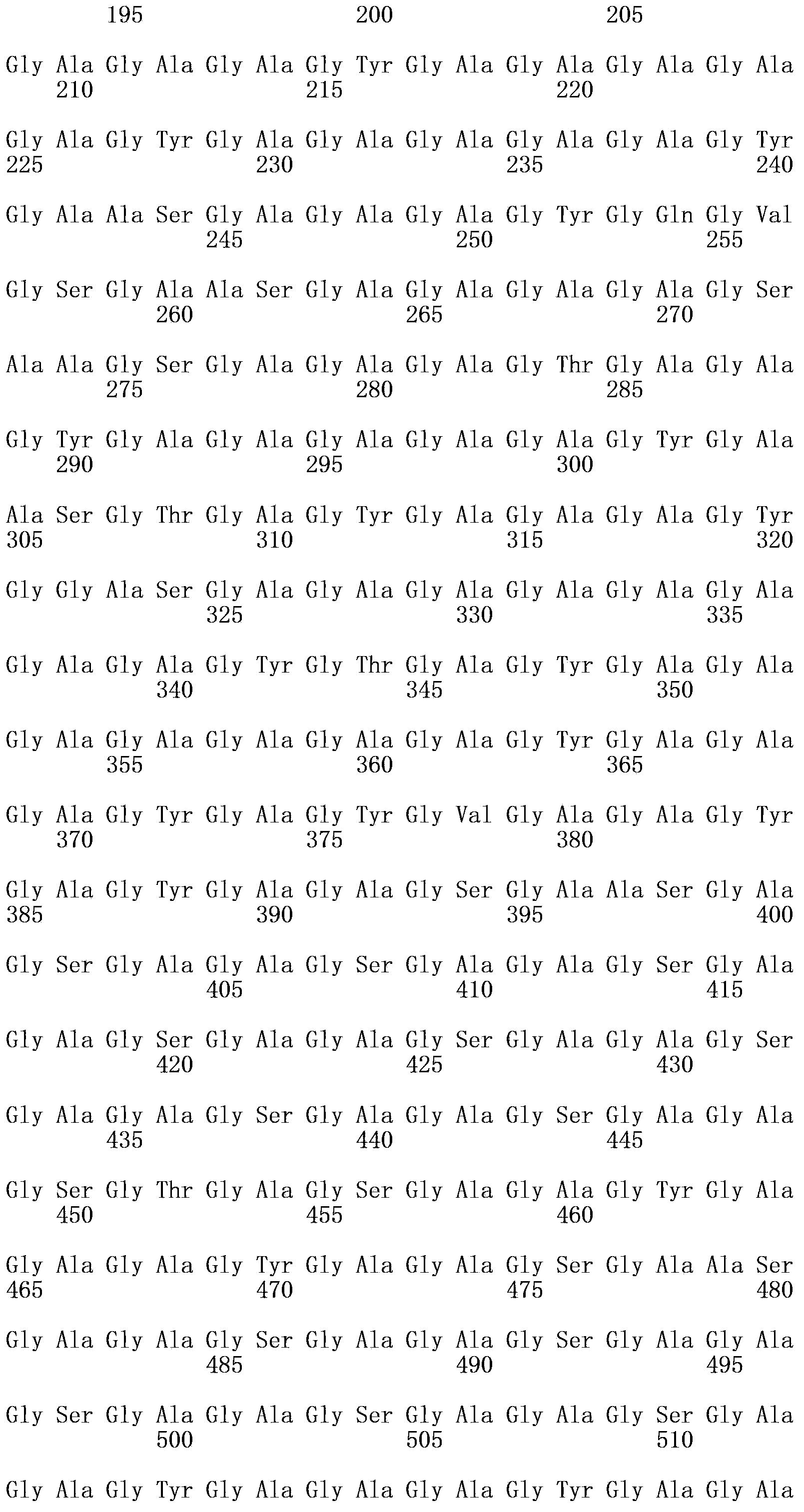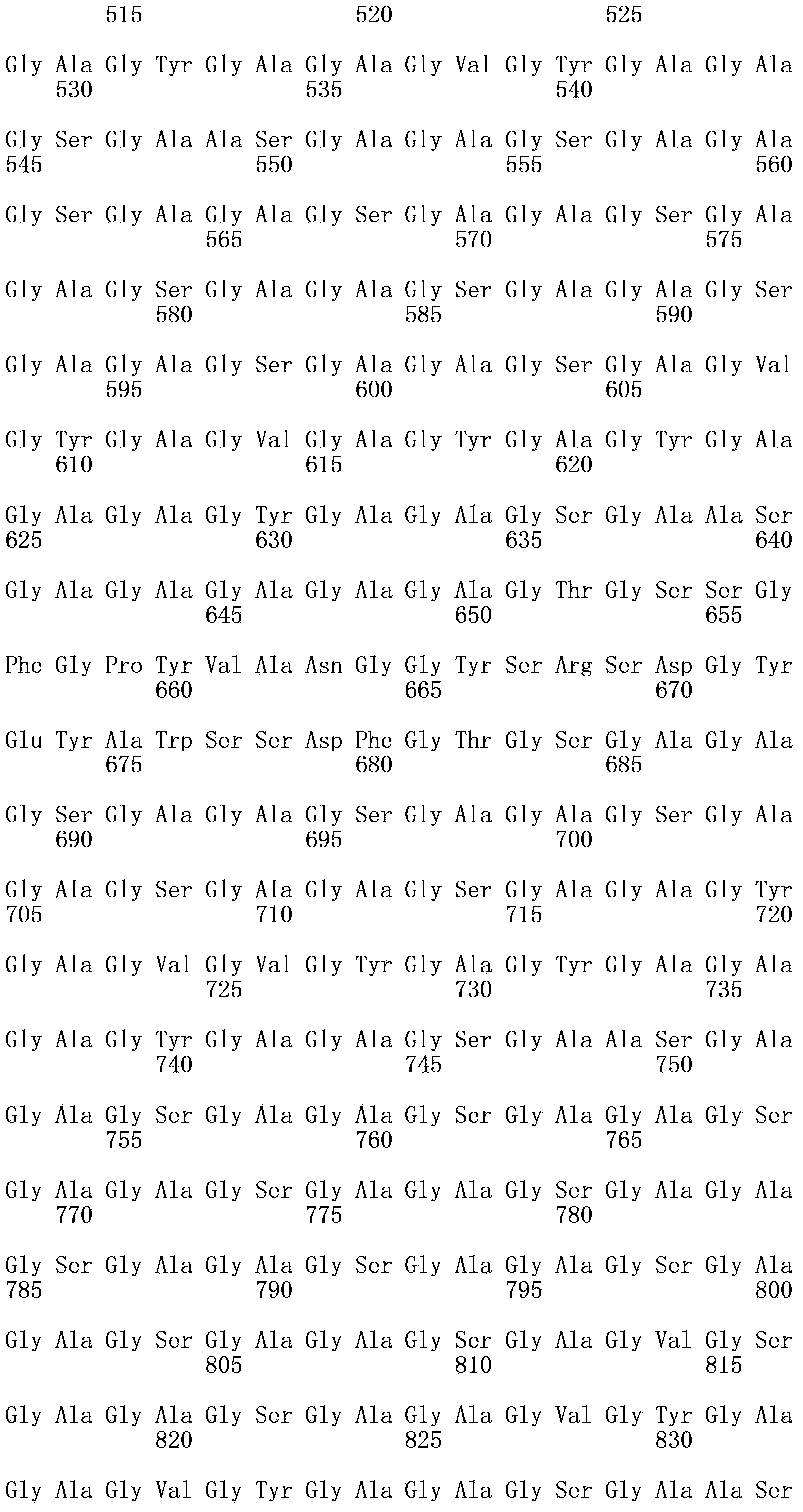Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
502results about How to "Strong immune response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
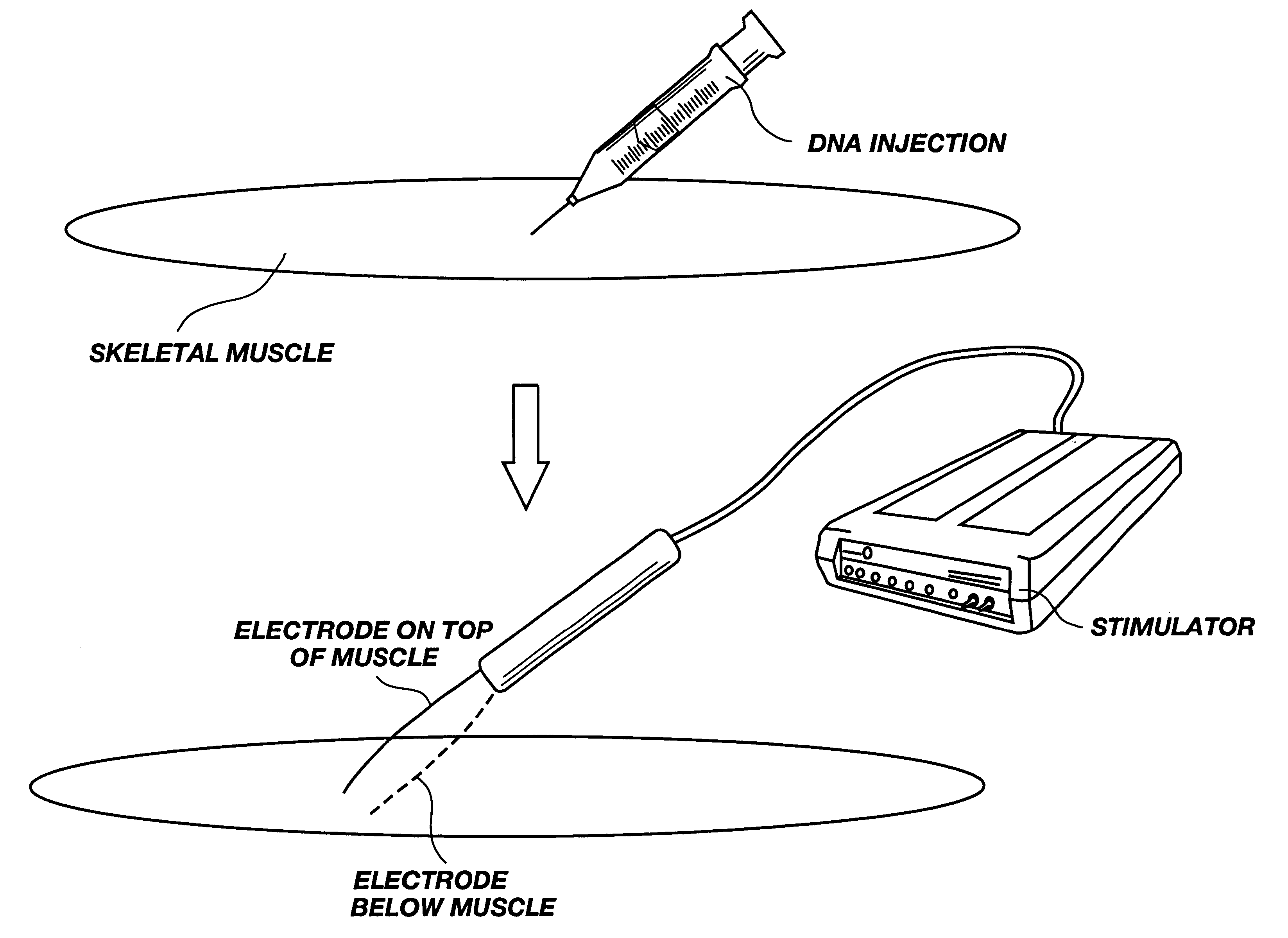
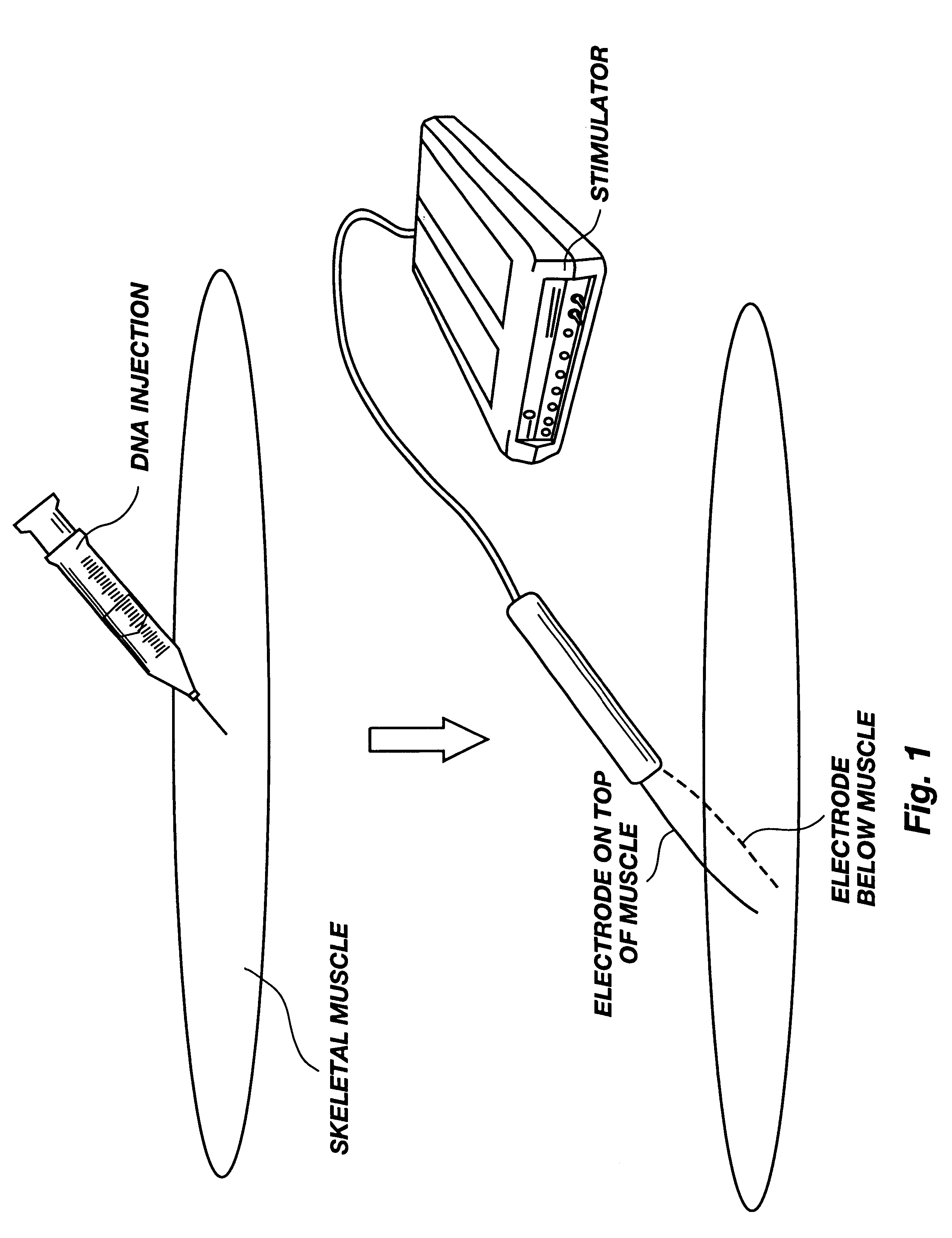
Method for genetic immunization and introduction of molecules into skeletal muscle and immune cells
InactiveUS6261281B1High transfection efficiencyGreat luciferace activityBacterial antigen ingredientsElectrotherapyVaccinationWhole body
A method is disclosed for enhanced vaccination and genetic vaccination of mammals. The vaccination is accomplished by delivering molecules such as proteins and nucleic acids into skeletal muscle and other cells residing in the skeletal muscle in vivo. The protein or nucleic acid is first injected into the muscle at one or multiple sites. Immediately or shortly after injection, electrodes are placed flanking the injection site and a specific amount of electrical current is passed through the muscle. The electrical current makes the muscle permeable, thus allowing the pharmaceutical drug or nucleic acid to enter the cell. The efficiency of transfer permits robust immune responses using DNA vaccines and produces sufficient secreted proteins for systemic biological activity to be observed.
Owner:INOVIO
Fc fusion proteins for enhancing the immunogenicity of protein and peptide antigens
InactiveUS20050261229A1Stimulate productionEnhanceVirusesPeptide/protein ingredientsFc receptorAdjuvant
Disclosed herein are methods and compositions for enhancing the immunogenicity of a preselected protein or peptide antigen in a mammal. Immunogenicity is enhanced by fusing the preselected antigen to an immunoglobulin heavy chain constant region to produce an Fc-antigen fusion protein. The Fc-antigen fusion proteins bind Fc receptors on the surface of antigen presenting cells, thereby targeting the antigen to the antigen presenting cells in the mammal. In addition, disclosed is a family of adjuvants, for example, an Fc-adjuvant fusion protein, for use in combination with the Fc-antigen fusion proteins to enhance or modulate a particular immune response against the preselected antigen.
Owner:MERCK PATENT GMBH
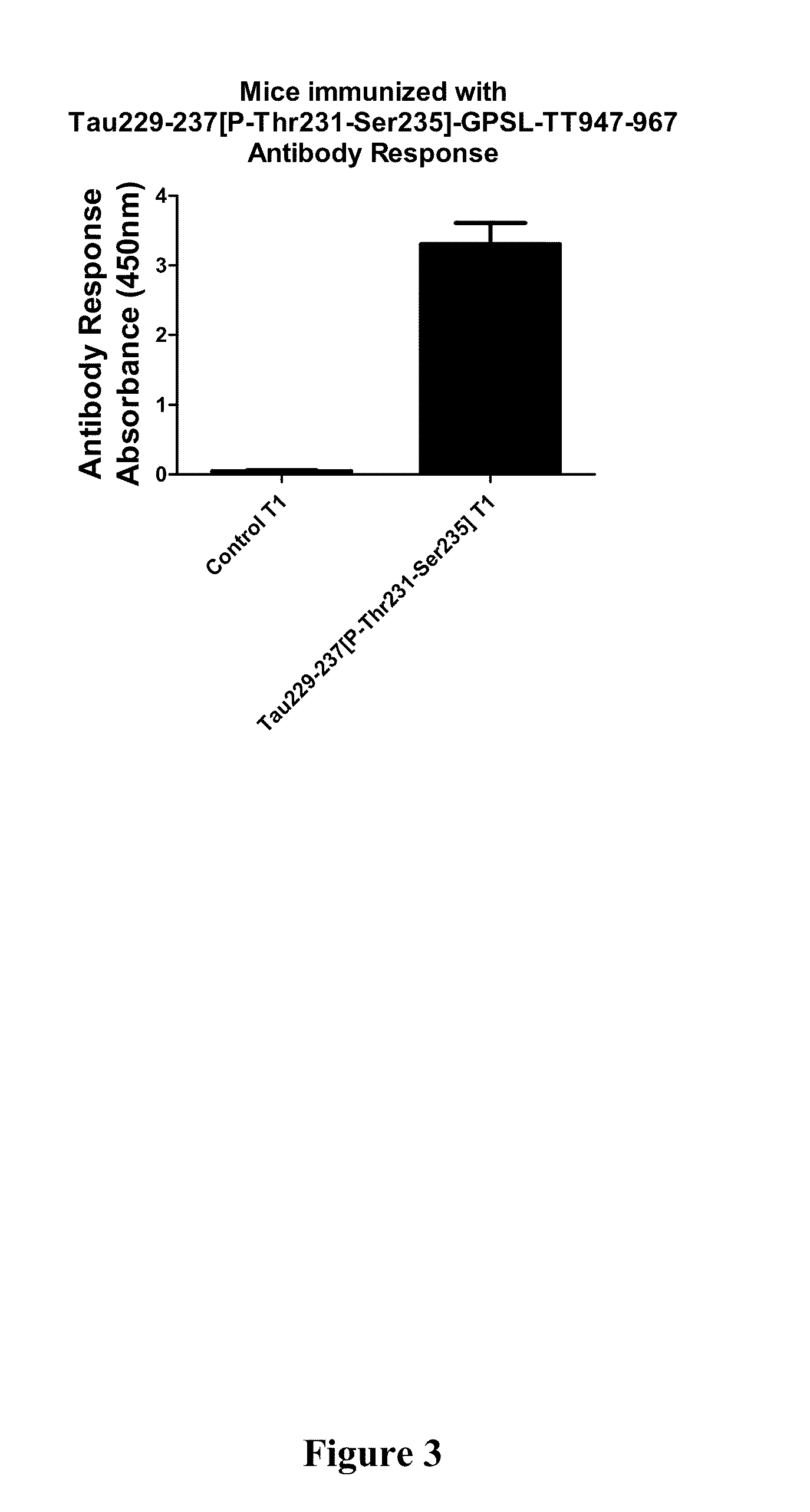
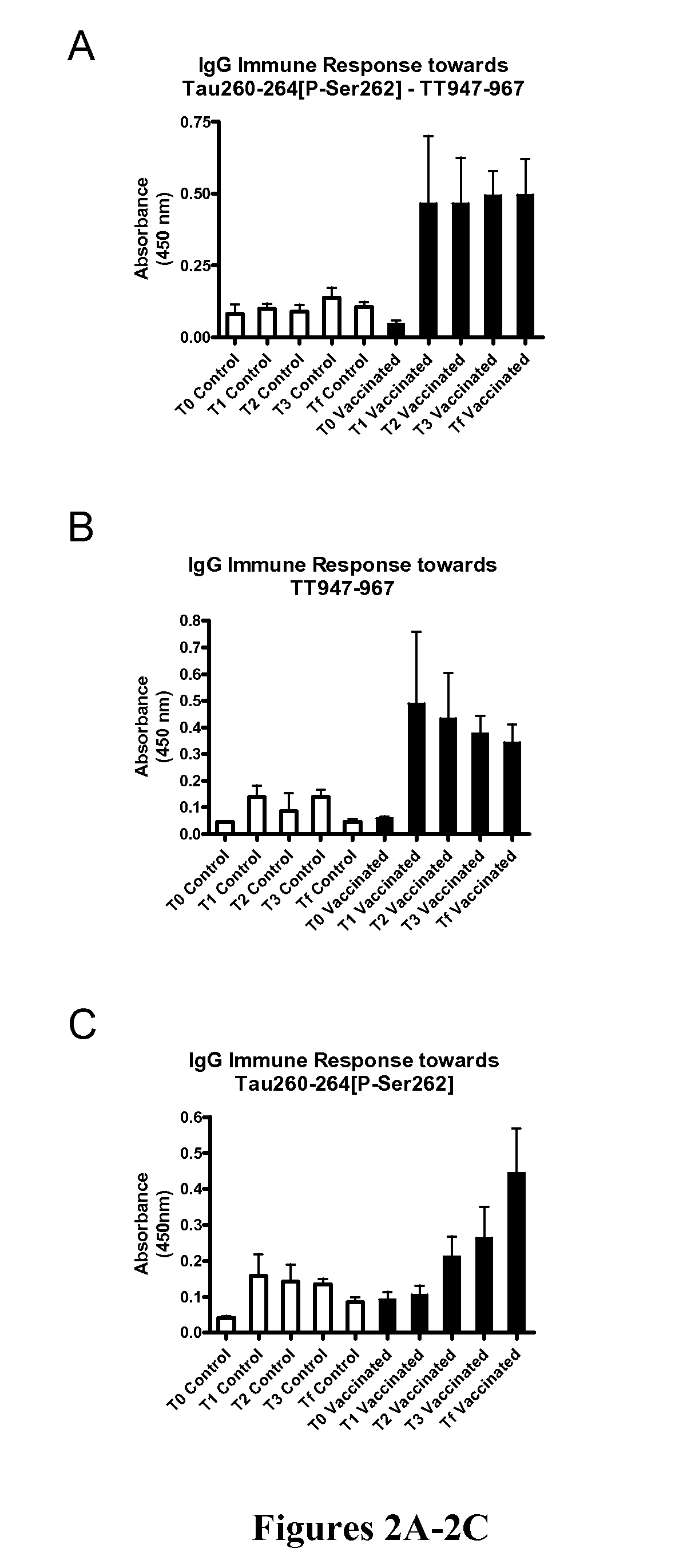
Immunological targeting of pathological tau proteins
ActiveUS20100316564A1Promote clearanceSlow progressionNervous disorderMuscular disorderPeptideImmunogenicity
The present invention relates to methods and compositions for treating, preventing, and diagnosing Alzheimer's Disease or other tauopathies in a subject by administering an immunogenic tau peptide or an antibody recognizing the immunogenic tau epitope under conditions effective to treat, prevent, or diagnose Alzheimer's Disease or other tauopathies. Also disclosed are methods of promoting clearance of aggregates from the brain of the subject and of slowing progression of tau-pathology related behavioral phenotype in a subject.
Owner:NEW YORK UNIVERSITY
Fc fusion proteins for enhancing the immunogenicity of protein and peptide antigens
InactiveUS7067110B1Improving immunogenicityStrengthIn-vivo radioactive preparationsAntibody mimetics/scaffoldsPeptide antigenFc(alpha) receptor
Disclosed herein are methods and compositions for enhancing the immunogenicity of a preselected protein or peptide antigen in a mammal. Immunogenicity is enhanced by fusing the preselected antigen to an immunoglobulin heavy chain constant region to produce an Fc-antigen fusion protein. The Fc-antigen fusion proteins bind Fc receptors on the surface of antigen presenting cells, thereby targeting the antigen to the antigen presenting cells in the mammal. In addition, disclosed is a family of adjuvants, for example, an Fc-adjuvant fusion protein, for use in combination with the Fc-antigen fusion proteins to enhance or modulate a particular immune response against the preselected antigen.
Owner:MERCK PATENT GMBH
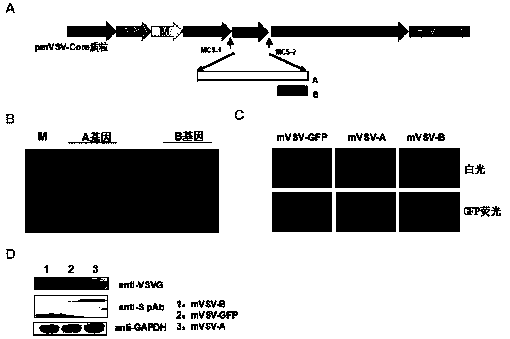
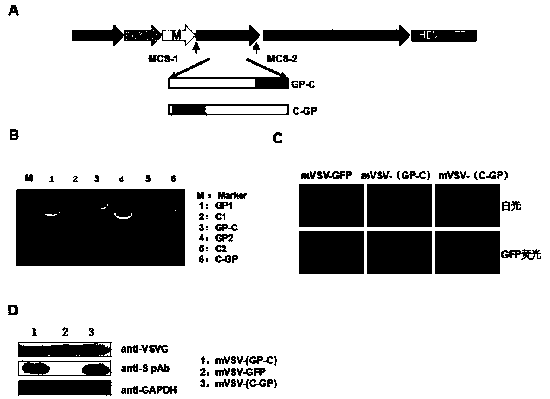
mVSV virus vector and virus vector vaccine, and COVID-19 vaccine based on mVSV mediation
ActiveCN111088283AEnhance immune responseStrong immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousReceptor
The invention provides an mVSV virus vector, i.e., attenuated mVSV obtained after multiple modification mutations occur to an M protein amino acid site of a wild Indiana strain VSV, and an optimized heterologous antigen gene is preferentially integrated to a double cloning site area of an mVSV packaging core plasmid pmVSV-Core at the same time. The mVSV virus vector vaccine comprises a heterologous antigen gene which fuses or embeds a target virus between G and L genes of an mVSV vector envelope, wherein the antigen gene comprises an enveloped and embedded antigen gene encoding the target virus, an embedded combination antigen gene or a fused antigen gene; the mVSV virus vector is embedded or fused with a dominant antigen of spike protein S of an SARS-CoV-2 pathogen; the dominant antigen is preferably selected from a receptor binding domain of spike protein S, namely RBD; and a COVID-19 vaccine based on mVSV mediation is formed. The vaccine has good prevention or treatment effect on COVID-19 infected people.
Owner:FANTASIA BIOPHARMA ZHEJIANG CO LTD
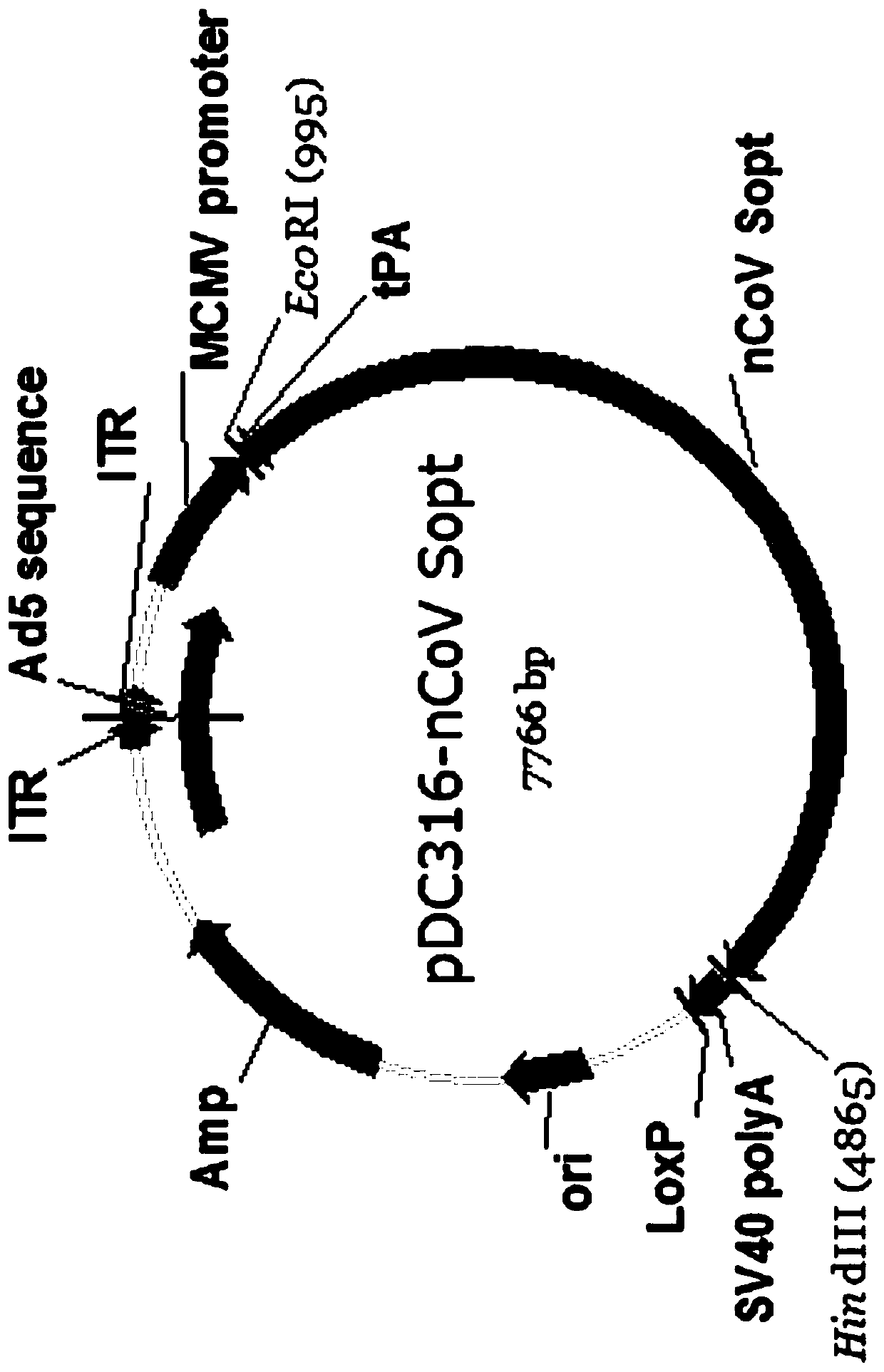
Recombinant SARS-CoV-2 vaccine using human replication-defective adenovirus as vector
ActiveCN111218459AReduce loadSimple manufacturing methodSsRNA viruses positive-senseViral antigen ingredientsProtective antigenCoronavirus vaccination
The invention provides a SARS-CoV-2 vaccine using human type-5 replication-defective adenovirus as a vector. The vaccine uses E1 and E3 to be combined with replication-defective human type-5 adenovirus as the vector and HEK293 cells integrating adenovirus E1 gene as a packaging cell line, and a protective antigen gene carried is the 2019 SARS-CoV-2 S protein gene (Ad5-nCoV) which is subjected to optimization design. After the S protein gene is optimized, the expression level in transfected cells is increased significantly. The vaccine has good immunogenicity in mouse and guinea pig models, andcan induce a body to produce a strong cellular and humoral immune response in a short time. Studies on the protective effect of hACE2 transgenic mice show that after 14 days of single immunization ofAd5-nCoV, the viral load in lung tissue can be significantly reduced, and it is indicated that the vaccine has a good immunoprotective effect on the 2019 SARS-CoV-2. In addition, the vaccine is quick, simple and convenient to prepare, and can be mass-produced in a short period of time to respond to sudden outbreaks.
Owner:ACADEMY OF MILITARY MEDICAL SCI +1
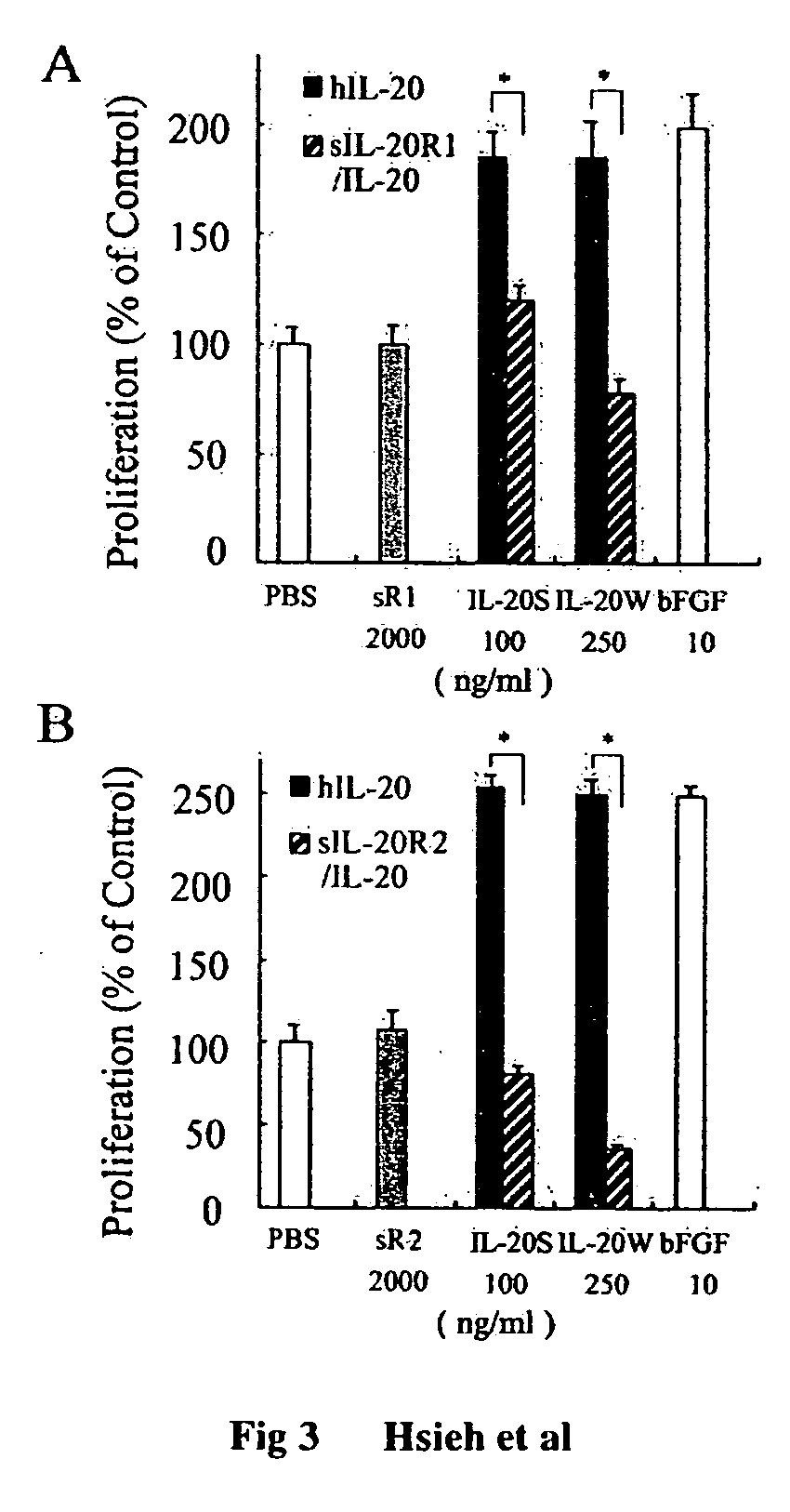
Antibodies to interleukin-20 and method for inhibiting interleukin-20 induced cell proliferation
ActiveUS20060142550A1Stimulate tumor growthStimulate angiogenesisImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationWhite blood cellProliferation activity
Antibody to human IL-20 protein, a method for generation thereof and cells producing this antibody are disclosed. The antibody of the present invention has specificity to neutralizing hIL-20W-induced CPAE proliferation activity, and is useful for treating IL-20-induced inflammation, such as artheriosclerosis and rheumatoid arthritis.
Owner:LBL BIOTECH INC
Adenoviral Vectors and Uses Thereof
The present invention relates to recombinant adenoviral vectors based on adenoviruses that encounter pre-existing immunity in a minority of the human population and which harbour a chimeric capsid. The chimeric capsid comprises fiber proteins that have at least the knob domain of a human adenovirus that binds to the Coxsackievirus and Adenovirus Receptor (CAR) and a hexon protein from an adenovirus serotype that encounters pre-existing immunity in a low percentage of the human population.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +1
Immunostimulatory compositions and methods of use thereof
ActiveUS20130295129A1Enhance immune responseIncreased cellular responsePeptide/protein ingredientsImmunoglobulinsLipid formationAdjuvant
Lipid conjugates for enhanced delivery of cargo to the lymph nodes are disclosed. The lipid conjugates typically include three domains: a lipophilic domain that binds to albumin, a polar block domain, and a cargo such as a molecular adjuvant or immunostimulatory compound (such as an oligonucleotide) or antigenic peptide. Depending on the cargo, the length and compositions of the polar block can be tailored to push the equilibrium toward albumin binding, stable micelle formation, or cell insertion. The conjugates can be administered to a subject, for example, a subject with cancer or an infection, to induce or enhance a robust immune response in the subject.
Owner:MASSACHUSETTS INST OF TECH
Immunogenic Compositions Comprising Hmgb 1 Polypeptides
InactiveUS20080305120A1Improving immunogenicityMinimizing unnecessary inflammationAntibacterial agentsSugar derivativesAntigenActivation cells
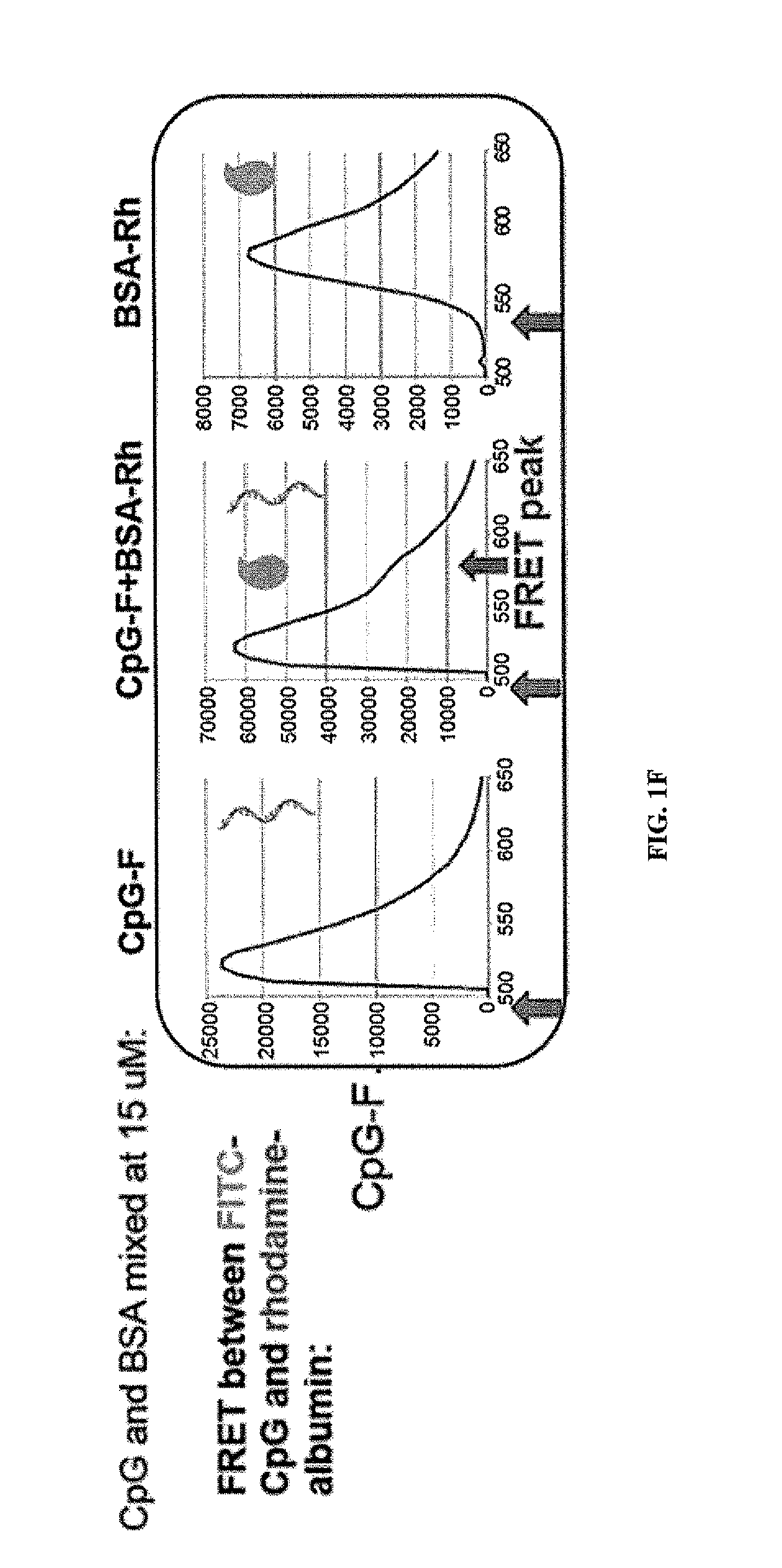
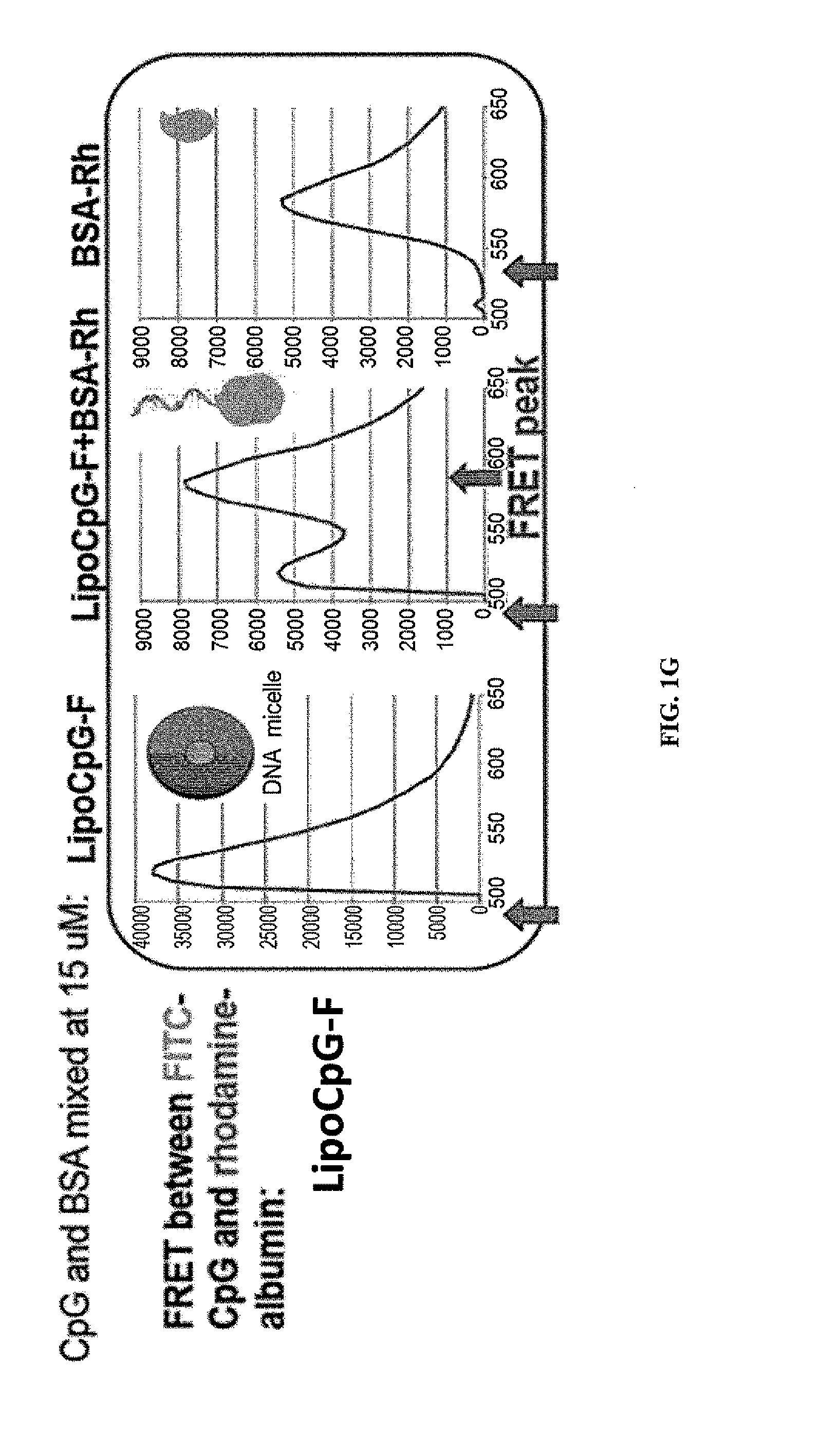
The present invention relates to novel immunogenic compositions (e.g., vaccines), the production of such immunogenic compositions and methods of using such compositions. More specially, this invention provides unique immunogenic molecules comprising an HMGB1 polypeptide (e.g., an HMGB1 B-box polypeptide) and an antigen. Even more specifically, this invention provides novel fusion proteins comprising an isolated HMGB1 polypeptide and an antigen such that administration of these fusion proteins provides the two signals required for native T-cell activation.
Owner:THE FEINSTEIN INST FOR MEDICAL RES +1
Flavivirus vaccines
InactiveUS20050002968A1Improve security levelDecreased viscerotropismSsRNA viruses positive-senseSugar derivativesVirologyFlavivirus
The invention provides attenuated flavivirus vaccines and methods of making and using these vaccines.
Owner:ACAMBIS INC
Models for vaccine assessment
ActiveUS20080008653A1Improve accuracyImprove predictabilityCompounds screening/testingCrankshaftsAdjuvantBiological Immunotherapy
The present invention is directed to methods for constructing and using in vivo and in vitro models of aspects of human immunity and, in particular, construction of a human immune system model for the testing of, for example, vaccines, adjuvants, immunotherapy candidates, cosmetics, drugs, biologics and other chemicals. The present invention comprises both in vivo and in vitro models of aspects of human immunity that are useful for assessing the interaction of substances with the immune system, and thus can be used to accelerate and improve the accuracy and predictability of, for example, vaccine, drug, biologic, immunotherapy, cosmetic and chemical development. The invention is also useful for the generation of human monoclonal and polyclonal antibodies.
Owner:VIRGINIA COMMONWEALTH UNIV +1
Sucking feed for leech
The invention discloses a sucking feed for leeches, which relates to the technical field of feedstuff. The sucking feed is prepared from the following components in percentage by weight: 55-80% of fishmeal, 5-15% of conch meal, 5-10% of soymeal, 1-5% of germ meal, 3-5% of blood meal, 1-5% of yeast and 1-2% of chitosan and colloid protein. The preparation method comprises the following steps: preparing raw materials, tedding, preliminary cleaning, grinding and mixing. The sucking feed provided by the invention significantly improves the feeding strength of leeches, promotes the growth of leeches, improves the yield of leeches and the survival rate of young leeches, improves the utilization rate of the feed, improves intestinal micro environment of the leeches, stimulates the leech bodies to emerge strong immune and stress response and improves the immunity and quality of the leeches.
Owner:邵汝强
Nanoparticles for providing immune responses against infectious agents
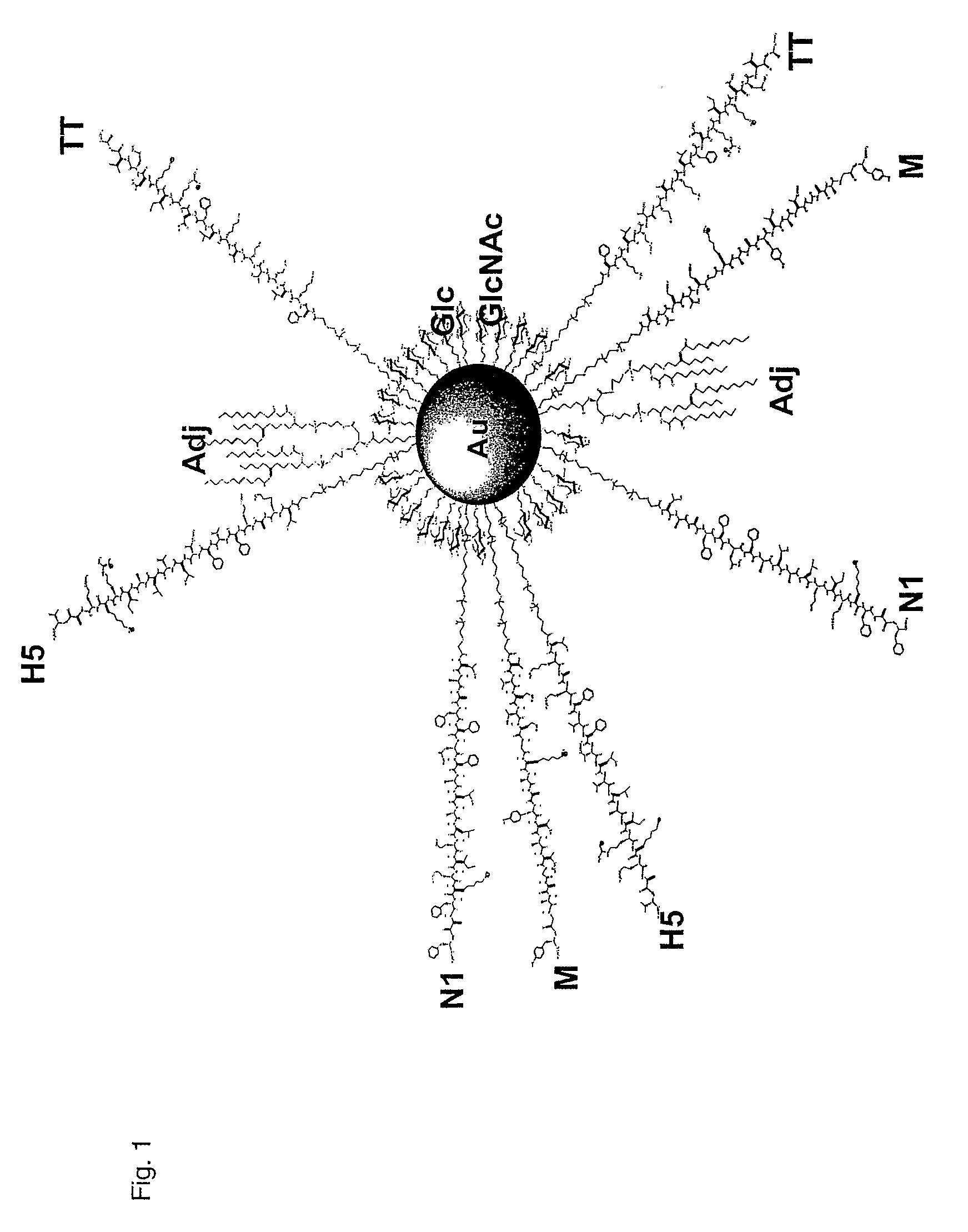
ActiveUS20090297614A1Strong immune responseConvenience needsPowder deliveryHeavy metal active ingredientsNanoparticleT cell
Nanoparticles for providing immune responses for the treatment or prophylaxis of infection by infectious agents such as viruses, parasites, bacteria, prions and fungi are described which comprises a core including metal and / or semiconductor atoms, wherein the core is covalently linked to a plurality of ligands, the ligands including a carbohydrate residue capable of stimulating an innate immune response, a T cell helper peptide and a danger signal. This platform may then be adapted by including one or more further ligands capable of producing a specific response to a target infectious agent.
Owner:MIDATECH LTD
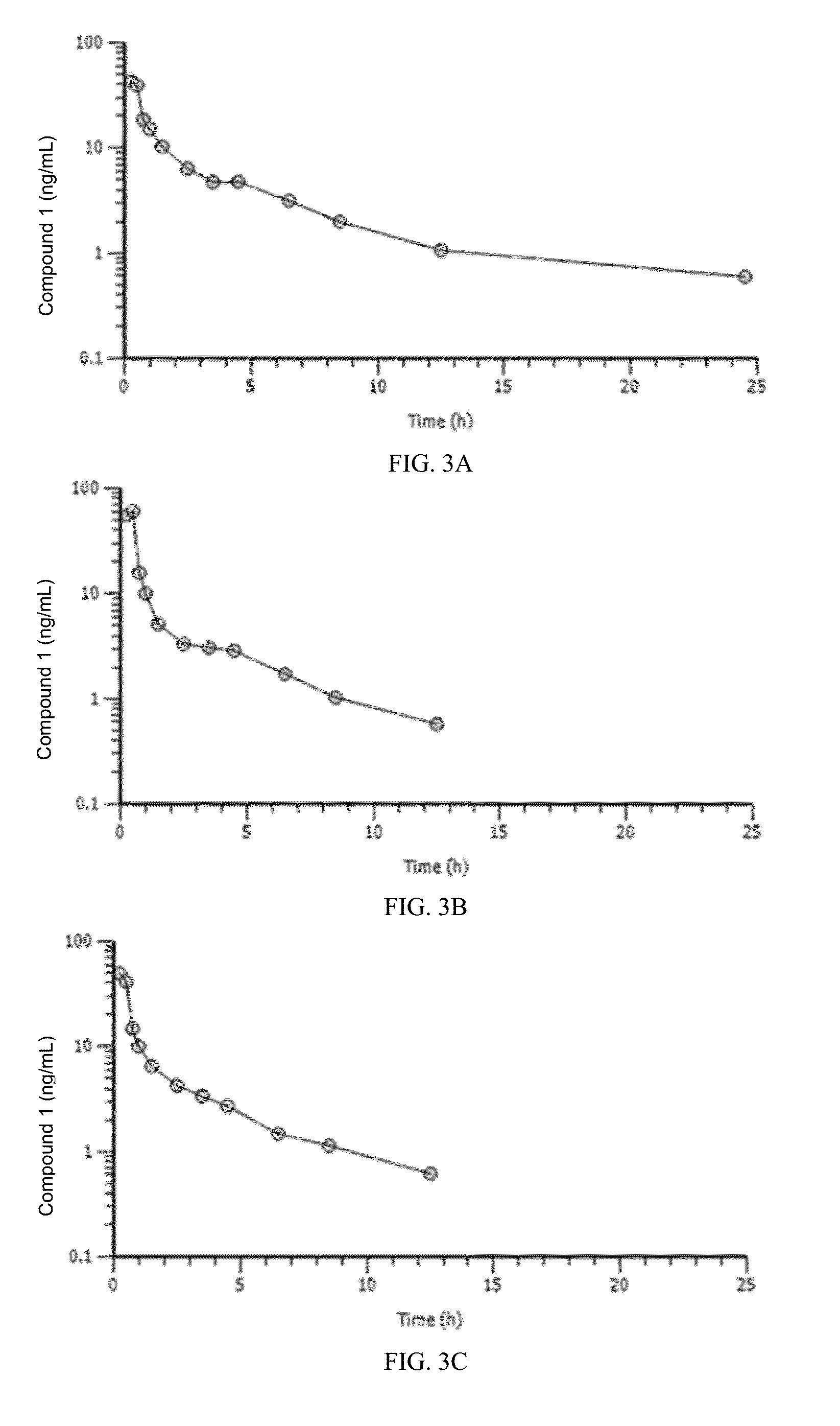
CDK4/6 Inhibitor Dosage Formulations For The Protection Of Hematopoietic Stem And Progenitor Cells During Chemotherapy
InactiveUS20160220569A1Reduction in therapeutically effective doseFaster hematopoietic recoveryPowder deliveryOrganic active ingredientsProgenitorDNA
This invention is in the area of dosage formulations and methods of administering a CDK4 / 6 inhibitor for the transient protection of healthy cells, and in particular hematopoietic stem and progenitor cells (HSPC), from damage associated with DNA damaging chemotherapeutic agents in subjects undergoing DNA damaging chemotherapeutic therapies for the treatment of proliferative disorders. In one aspect, improved protection of healthy cells is disclosed using a dosage that provides desirable pharmacokinetic and pharmacodynamic characteristics, including AUC, Tmax, Cmax, dosage-corrected AUC, and dosage-corrected Cmax. In another aspect, a method of treating a subject undergoing chemotherapy for the treatment of a CDK 4 / 6-replication independent cellular proliferation disorder by administering Compound 1 is provided.
Owner:G1 THERAPEUTICS INC
Adjuvant compositions and methods for delivering vaccines
InactiveUS20080292663A1Strong immune responseReduce adverse reactionsInorganic non-active ingredientsPharmaceutical delivery mechanismAntigenAdjuvant
An adjuvant composition is provided that is comprised of a calcium compound, lecithin, and an acrylic polymer. Pharmaceutical compositions are provided which include an antigen and the adjuvant. Methods are provided for stimulating an immune response in a human or animal subject by administering a composition comprising an antigen and the adjuvant composition to human or animal subjects.
Owner:ADVANCED BIOADJUVANTS
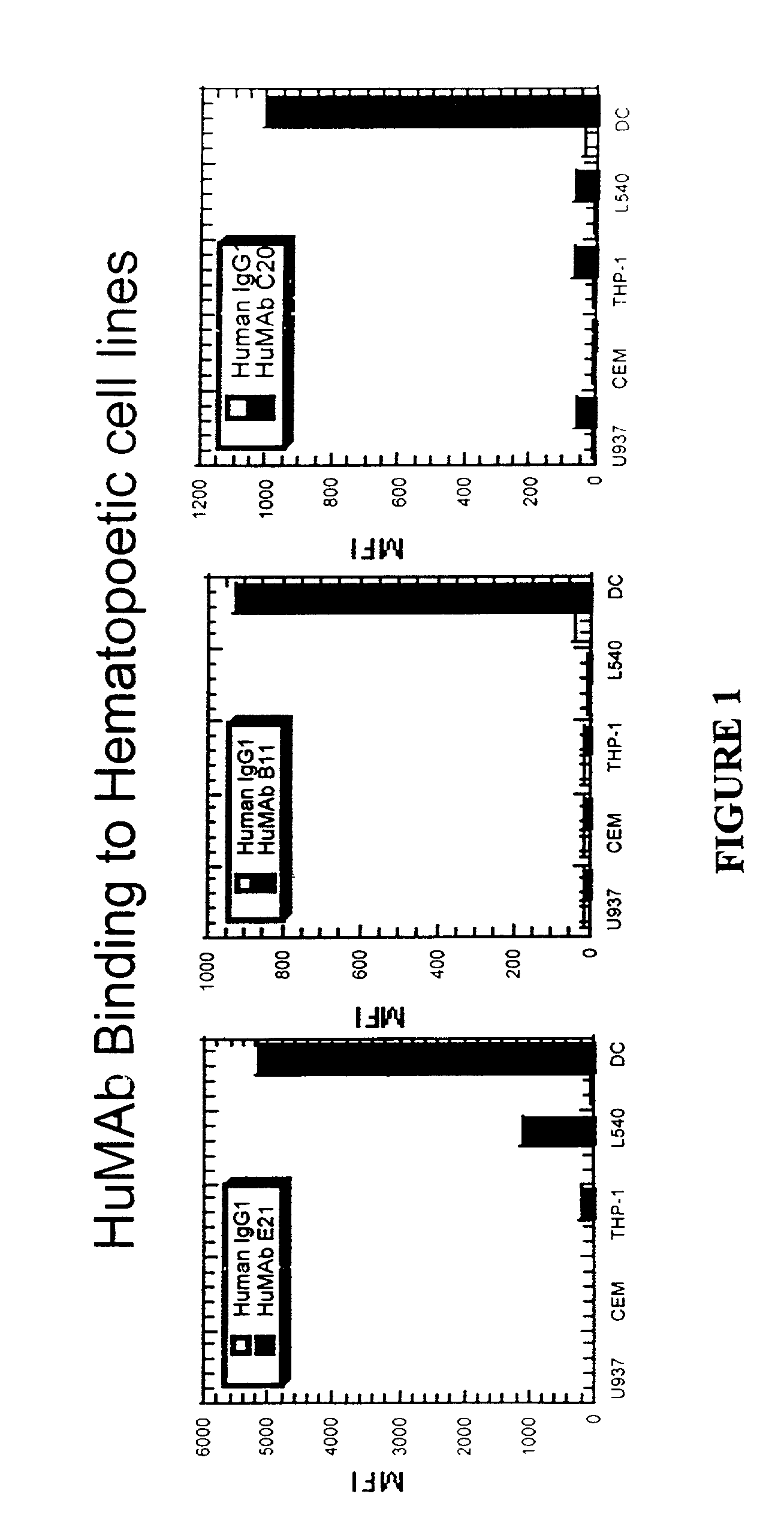
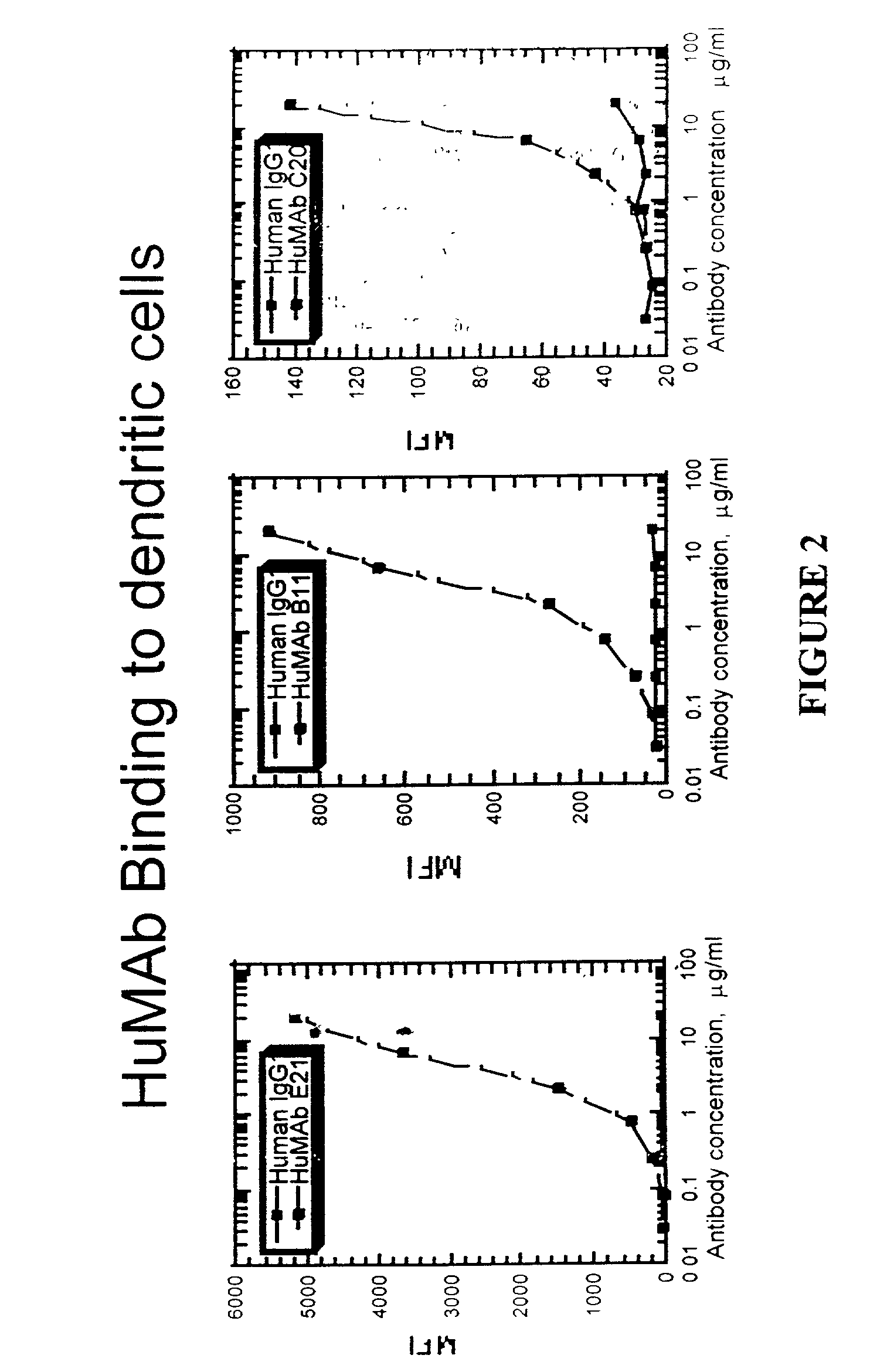
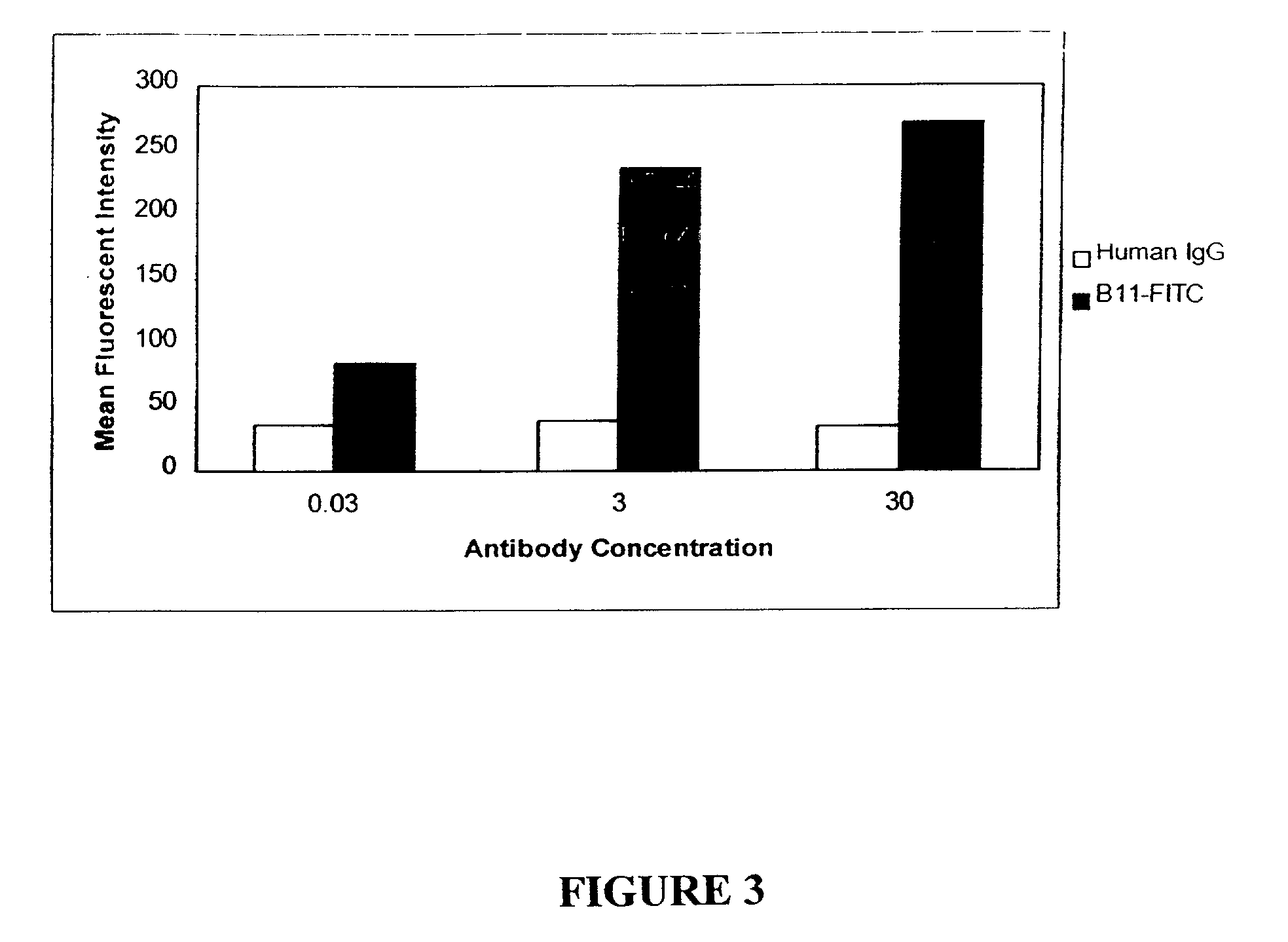
Molecular conjugates comprising human monoclonal antibodies to dendritic cells
InactiveUS7560534B2Enhance antigen presentationStrong immune responseNervous disorderAntipyreticDendritic cellMonoclonal antibody
Molecular conjugates comprising an antigen linked to a human monoclonal antibody that specifically binds to dendritic cells are disclosed. Also disclosed are pharmaceutical compositions comprising the molecular conjugates and therapeutic methods for using the conjugates.
Owner:CELLDEX THERAPEUTICS INC
Immunological Targeting of Pathological Tau Proteins
InactiveUS20140302046A1Easy to removeShorten the progressNervous disorderImmunoglobulins against animals/humansEpitopeImmunogenicity
The present invention relates to methods and compositions for treating, preventing, and diagnosing Alzheimer's Disease or other tauopathies in a subject by administering an immunogenic tau peptide or an antibody recognizing the immunogenic tau epitope under conditions effective to treat, prevent, or diagnose Alzheimer's Disease or other tauopathies. Also disclosed are methods of promoting clearance of aggregates from the brain of the subject and of slowing progression of tau-pathology related behavioral phenotype in a subject.
Owner:NEW YORK UNIV
Chimeric Influenza Virus-Like Particles Comprising Hemagglutinin
ActiveUS20120189658A1Easy to captureStrong immune responseSsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininVirus-like particle
A method for synthesizing chimeric influenza virus-like particles (VLPs) within a plant or a portion of a plant is provided. The method involves expression of chimeric influenza HA in a plant or a portion of a plant. The invention is also directed towards a VLP comprising chimeric influenza HA protein and plants lipids. The invention is also directed to a nucleic acid encoding chimeric influenza HA as well as vectors. The VLPs may be used to formulate influenza vaccines, or may be used to enrich existing vaccines.
Owner:MEDICAGO INC
Neisseria meningitidis serogroup a capsular polysaccharide acetyltransferase, methods and compositions
InactiveUS20060073168A1Easy to optimizeComplete acetylationAntibacterial agentsBacteriaSalmonella serotype typhiAcetyltransferase
Provided are recombinant DNA molecules that do not occur in nature encoding an O-acetyltransferase, vectors that direct expression of an O-acetyltransferase, recombinant host cells which express an O-acetyltransferase, methods for recombinant production of an O-acetyltransferase, methods for acetylating capsular polysaccharides, especially those of a Serogroup A Neisseria meningitidis using a recombinant O-acetyltransferase, and immunogenic compositions comprising the acetylated capsular polysaccharide.
Owner:EMORY UNIVERSITY +1
Protein-chaperoned t-cell vaccines
InactiveUS20170252417A1Increase lymph node uptakeEnhance immune responseViral antigen ingredientsPharmaceutical delivery mechanismPeptide antigenTolerability
Protein antigens are provided. The protein antigens typically include a peptide antigen conjugated or fused to a chaperone protein to form a “chaperone-antigen” that increases lymph node uptake; improves an immune response; or a combination thereof relative to the peptide antigen alone. The immune response can be, for example, increased antigen-specific proliferation, enhanced cytokine production, stimulation of differentiation and / or effector functions, promotion of survival, rescue from exhaustion and / or anergy of T cells, or a combination thereof. Chaperon-antigens can also be used to induce tolerance and increase immune suppressive responses. In the most preferred embodiments, the peptide antigen is fused to the chaperone protein to form a fusion protein. The “chaperone-antigen” can be combined with an adjuvant to form a vaccine and administered to a subject to modulate an immune response to the antigen. Methods of increasing immune responses, treating cancer and infectious and inducing tolerance are also provided.
Owner:MASSACHUSETTS INST OF TECH
Virus-like particles comprising composite capsid amino acid sequences for enhanced cross reactivity
ActiveUS8841120B2Strong immune responseImprove protectionVirusesMicroorganismsEpitopeVirus-like particle
The present invention provides polypeptides having a composite amino acid sequence derived from a consensus sequence representing the capsid proteins of two or more circulating strains of a non-enveloped virus. In particular, the invention provides virus-like particles comprising at least one composite polypeptide. Such virus-like particles have antigenic epitopes of two or more circulating strains of a non-enveloped virus and produce an increase in antisera cross-reactivity to one or more circulating strains of the non-enveloped virus. Methods of making composite virus-like particles and vaccine formulations comprising composite virus-like particles are also disclosed.
Owner:TAKEDA VACCINES INC
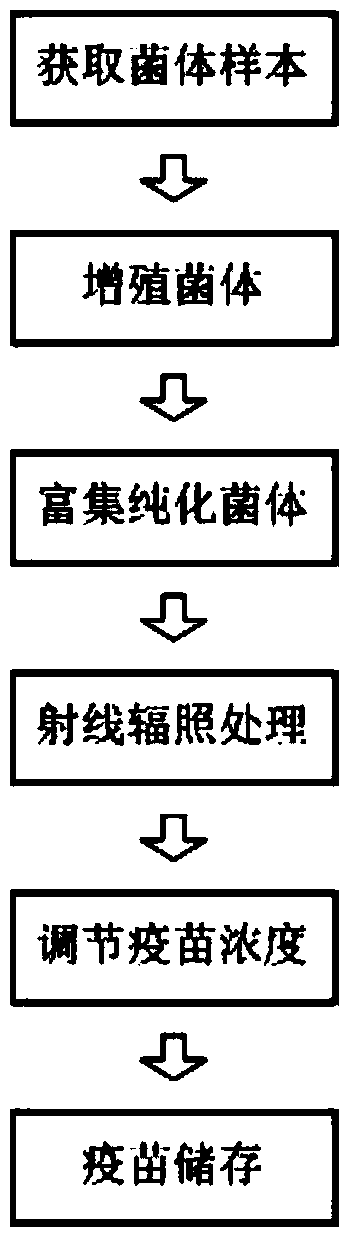
Pseudomonas aeruginosa vaccine and preparation method thereof
ActiveCN104189898ARetain metabolic activityPreserve immunogenicityAntibacterial agentsElectrical/wave energy microorganism treatmentBacteroidesDisease
The invention relates to the technical field of biology and specifically relates to a pseudomonas aeruginosa vaccine and a preparation method thereof. The pseudomonas aeruginosa vaccine is used for solving the technical problem that the existing pseudomonas aeruginosa vaccine at the development and clinical test stages are low in protective efficacy, high in toxicity to organisms and incapable of meeting the requirements of clinical immunology due to failure in curing infection. The technical scheme adopted to solve the technical problem is as follows: a preparation method of a novel vaccine is provided. The preparation method comprises the steps of firstly obtaining pseudomonas aeruginosa bacteria and then irradiating the bacteria by use of rays to obtain the pseudomonas aeruginosa vaccine. The pseudomonas aeruginosa vaccine is used for either preventing diseases or treating diseases, and has good application prospect.
Owner:SICHUAN UNIV
Microemulsions as precursors to solid nanoparticles
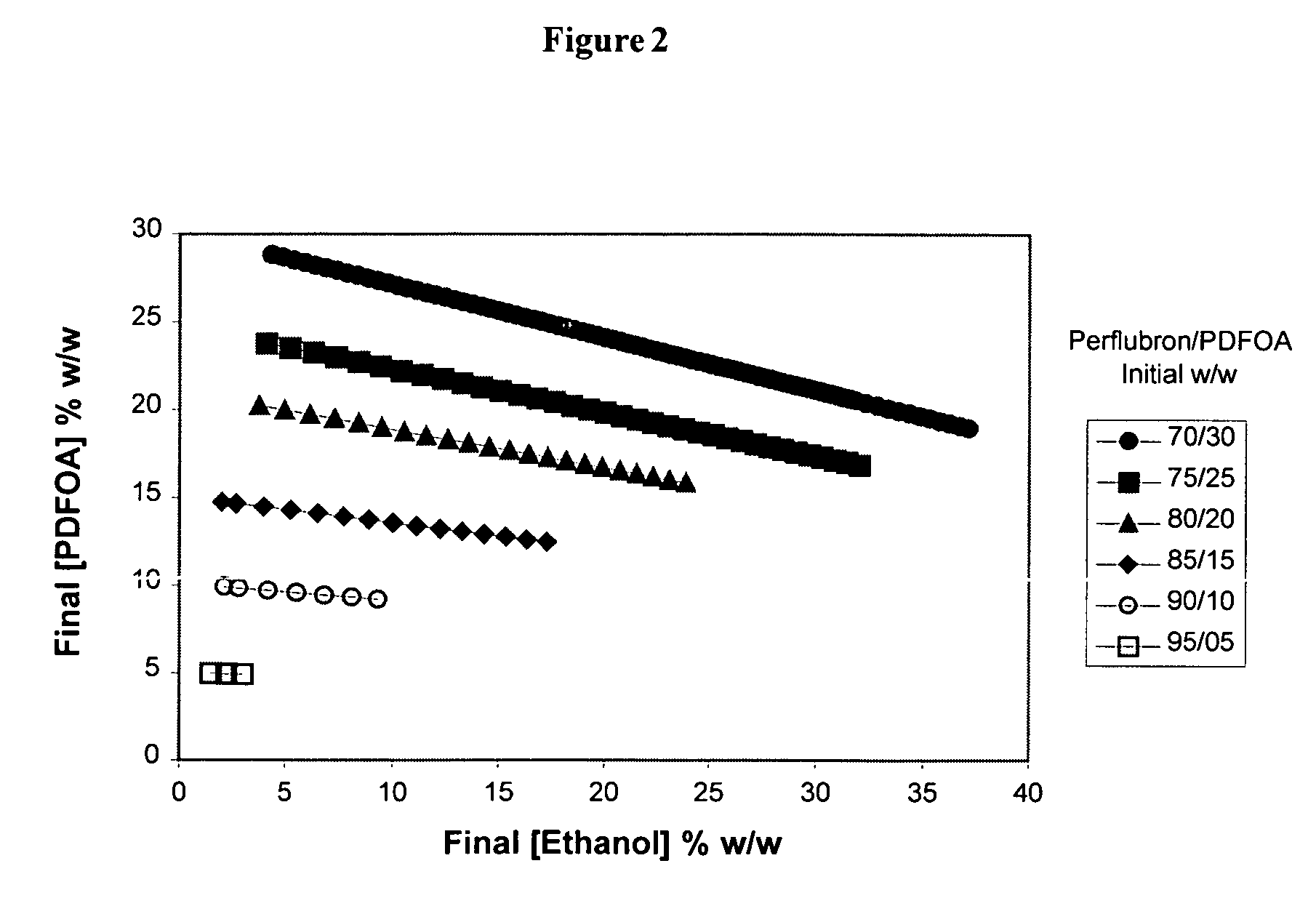
InactiveUS7153525B1Robust immune responseSimple curing processBiocideOrganic active ingredientsDrugNanometre
The preparation of novel microemulsions to be used as precursors for solid nanoparticles is described. The microemulsion precursors consist of either alcohol-in-fluorocarbon microemulsions, liquid hydrocarbon-in-fluorocarbon microemulsions, or liquid hydrocarbon-in-water microemulsions. The formed solid nanoparticles have diameters below 200 nanometers and can be made to entrap various materials including drugs, magnets, and sensors. The solid nanoparticles can be made to target different cells in the body by the inclusion of a cell-specific targeting ligand. Methods of preparing the novel microemulsion precursors and methods to cure solid nanoparticles are provided.
Owner:PARTICLE SCI
Virus-like particles comprising composite capsid amino acid sequences for enhanced cross reactivity
ActiveUS20110195113A1Enhance immune responseHigh IgG levelSsRNA viruses positive-senseMicroorganismsEpitopeVirus-like particle
The present invention provides polypeptides having a composite amino acid sequence derived from a consensus sequence representing the capsid proteins of two or more circulating strains of a non-enveloped virus. In particular, the invention provides virus-like particles comprising at least one composite polypeptide. Such virus-like particles have antigenic epitopes of two or more circulating strains of a non-enveloped virus and produce an increase in antisera cross-reactivity to one or more circulating strains of the non-enveloped virus. Methods of making composite virus-like particles and vaccine formulations comprising composite virus-like particles are also disclosed.
Owner:TAKEDA VACCINES INC
Breaking immunological toterance with a genetically encoded unnatural amino acid
InactiveUS20090263376A1Improving immunogenicityStrong immune responseAntibacterial agentsNervous disorderDiseaseAntigenic variation
The present invention comprises methods and compositions for producing and / or enhancing an immunological response in a subject against a target moiety such as a disease-related moiety by administration of an antigenic version of the target moiety having one or more unnatural amino acid and / or by administration of an antibody against a version of a target moiety having one or more unnatural amino acid which antibody is cross reactive with the natural target moiety.
Owner:THE SCRIPPS RES INST
Methods of enhancing the immunogenicity of mycobacteria and compositions for the treatment of cancer, tuberculosis, and fibrosing lung diseases
InactiveUS20110243992A1Enhance recruitmentIncreased activationAntibacterial agentsBacteriaMycobacterium immunogenumCancer cell
Whole-cell vaccines and methods for enhancing the immunogenicity of cellular microorganisms for use in producing protective immune responses in vertebrate hosts subsequently exposed to pathogenic bacteria or for use as vectors to express exogenous antigens and induce responses against other infectious agents or cancer cells. The present invention involves an additional method of enhancing antigen presentation by intracellular bacteria in a manner that improves vaccine efficacy. After identifying an enzyme that has an anti-apoptotic effect upon host cells infected by an intracellular microbe, the activity of the enzyme produced by the intracellular microbe is reduced by expressing a mutant copy of the enzyme, thereby modifying the microbe so that it increases immunogenicity.
Owner:VANDERBILT UNIV
Flexible vaccine assembly and vaccine delivery platform
ActiveUS7939318B2Modulate host immune responseEfficient identificationVirusesAntibody mimetics/scaffoldsVaccine deliveryVirus-like particle
Herein-described are various methods for making a vaccine that are made of re-assembled virus like particles (VLP). First, the VLPs are disassembled into encapsidation intermediate populations. Each encapsidation intermediate population undergoes, for instance, chemical conjugation of unique peptide or nucleic moieties to form separate populations. Thereafter, a predetermined amount of each of the several (one or more) different encapsidation intermediates from the different populations is mixed and joined, forming intact VLPs, surrounding a nucleic acid core, that are composed of different encapsidation intermediate such that the reassembled VLP displays more than one peptide or nucleic acid. The nucleic acid can function either as a scaffold alone or can be engineered for the expression of an immunomodulatory protein in a eukaryotic cell.
Owner:KBIO HLDG LTD
Method of preparing bombyx mori silk fibroin specific antibody by utilizing characteristic dodecapeptide
InactiveCN103509108AStrong specificityStrong immune responseSerum immunoglobulinsImmunoglobulins against animals/humansKeyhole-limpet haemocyaninPrimary immunization
The invention discloses a method of preparing a bombyx mori silk fibroin specific antibody by utilizing a characteristic dodecapeptide. The method comprises the following steps: synthesizing a polypeptide with a "CGYGAGAGAGYGA" sequence, coupling the polypeptide with keyhole limpet hemocyanin (KLH) so as to obtain a complete antigen; diluting the complete antigen with normal saline, mixing the diluted complete antigen with a complete Freund's adjuvant, carrying out an emulsion treatment so as to obtain primary immunized antigen emulsion, subjecting a rabbit to a primary immunization by using the primary immunized antigen emulsion, then subjecting the rabbit to a strengthened immunization, wherein the strengthened immunization uses a strengthened immunized antigen emulsion, which is prepared by the following steps: mixing the diluted complete antigen with an incomplete Freund's adjuvant, and then carrying out an emulsion treatment so as to obtain the target product; collecting the blood of the immunized rabbit, when the antiserum titer of rabbit arrives at 1 / 10000; making the blood blocks fully contract to completely separate out the antiserum, then collecting the antiserum, and subjecting the antiserum to a centrifugation treatment so as to obtain a supernate. The antibody prepared by the invention has a strong specificity, and can be used for detection and analysis of silk fibroin in textile, and the like.
Owner:ZHEJIANG UNIV +1
Asia1 type foot-and-mouth disease recombinant virus and preparation method and application thereof
The invention relates to an Asia1 type foot-and-mouth disease recombinant virus without pathogenicity for a host and a preparation method and application thereof. A saving system is efficient eukaryotic plasmids which are constructed by gene engineering and can express exact foot-and-mouth disease virus genome RNA (Ribonucleic Acid), and therefore the foot-and-mouth disease recombinant virus can be constructed and prepared; vaccine strains with high titer and good antigen matching property can be prepared by using the plasmids, can be prepared into live vaccines or inactivated vaccines and can effectively stimulate bodies to produce immune response after being used for immunizing pigs and cattle, provide an immune protective effect on the pigs and the cattle and effectively protect GV and GII prevalent strains, the immune protection rate can reach 100 percent, and the median protective dose (PD50) is 6.34 to 13.59; and the recombinant virus has the advantages of high titer, high antigen matching property with the prevalent strains, wide antigen spectrum and high immune protection rate, does not have pathogenicity for pig and cattle hosts, does not form toxemia or expel toxin, and can be applied to prevention and control of Asia1 type foot-and-mouth disease viruses of China and neighboring countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com