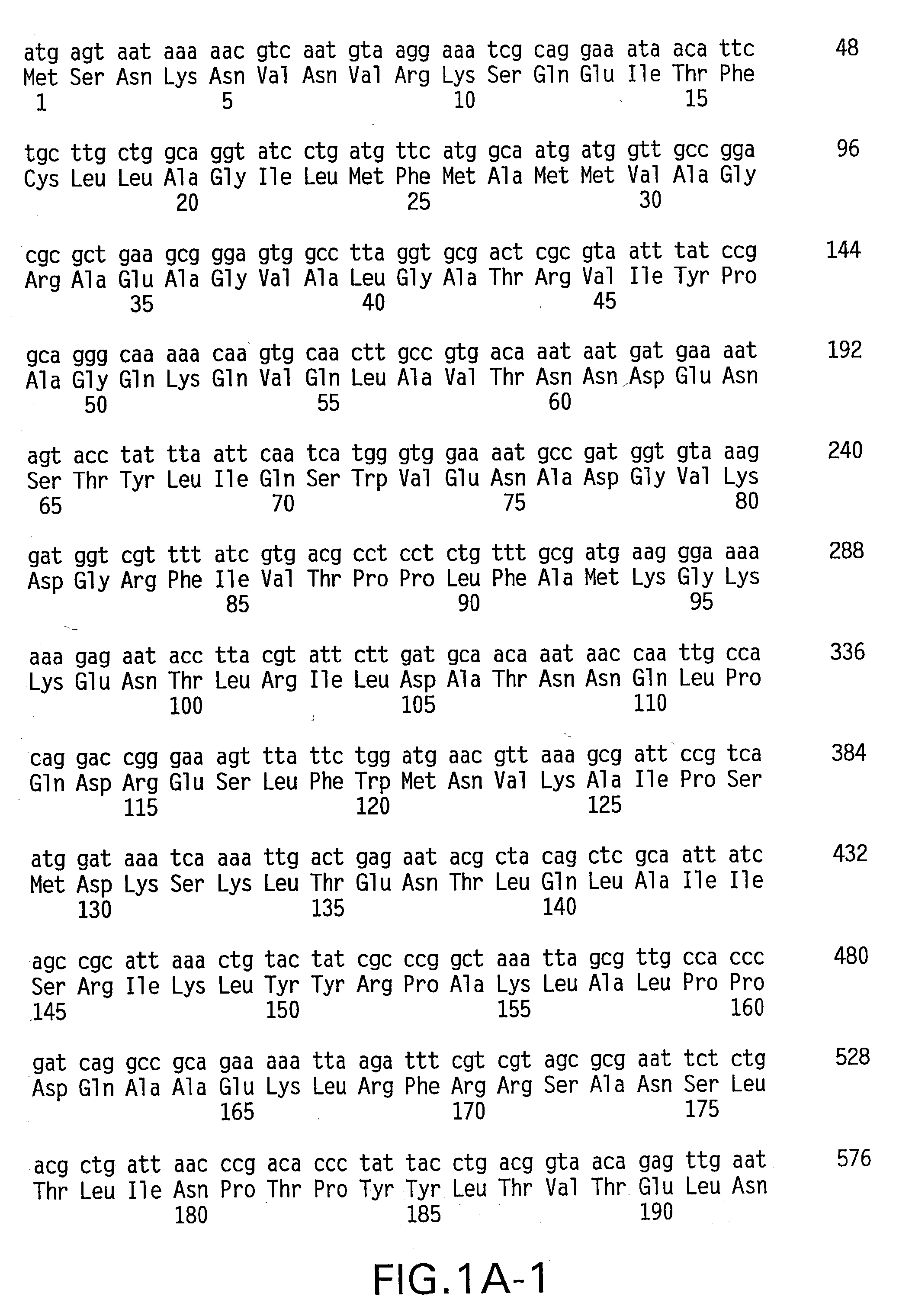
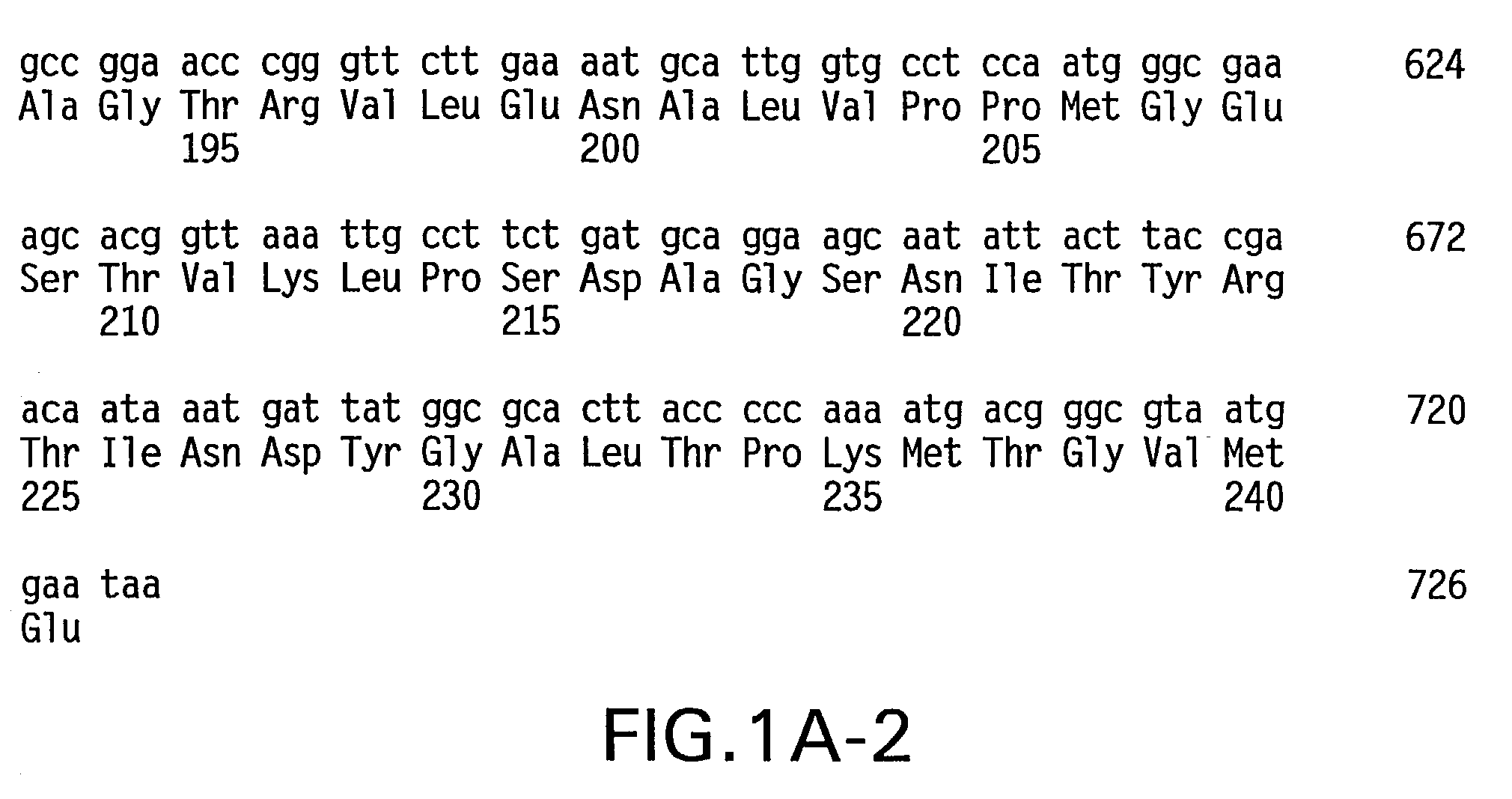
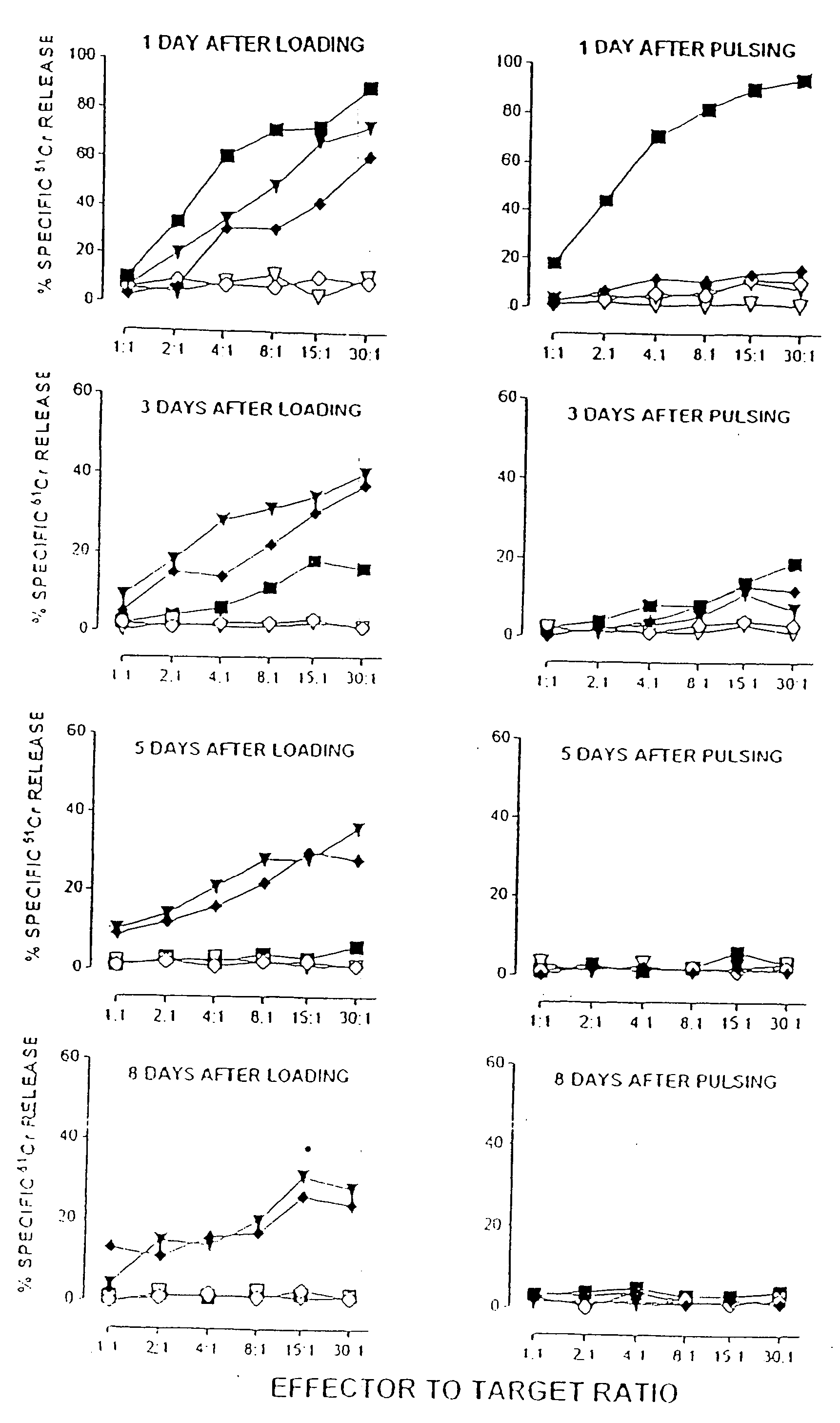
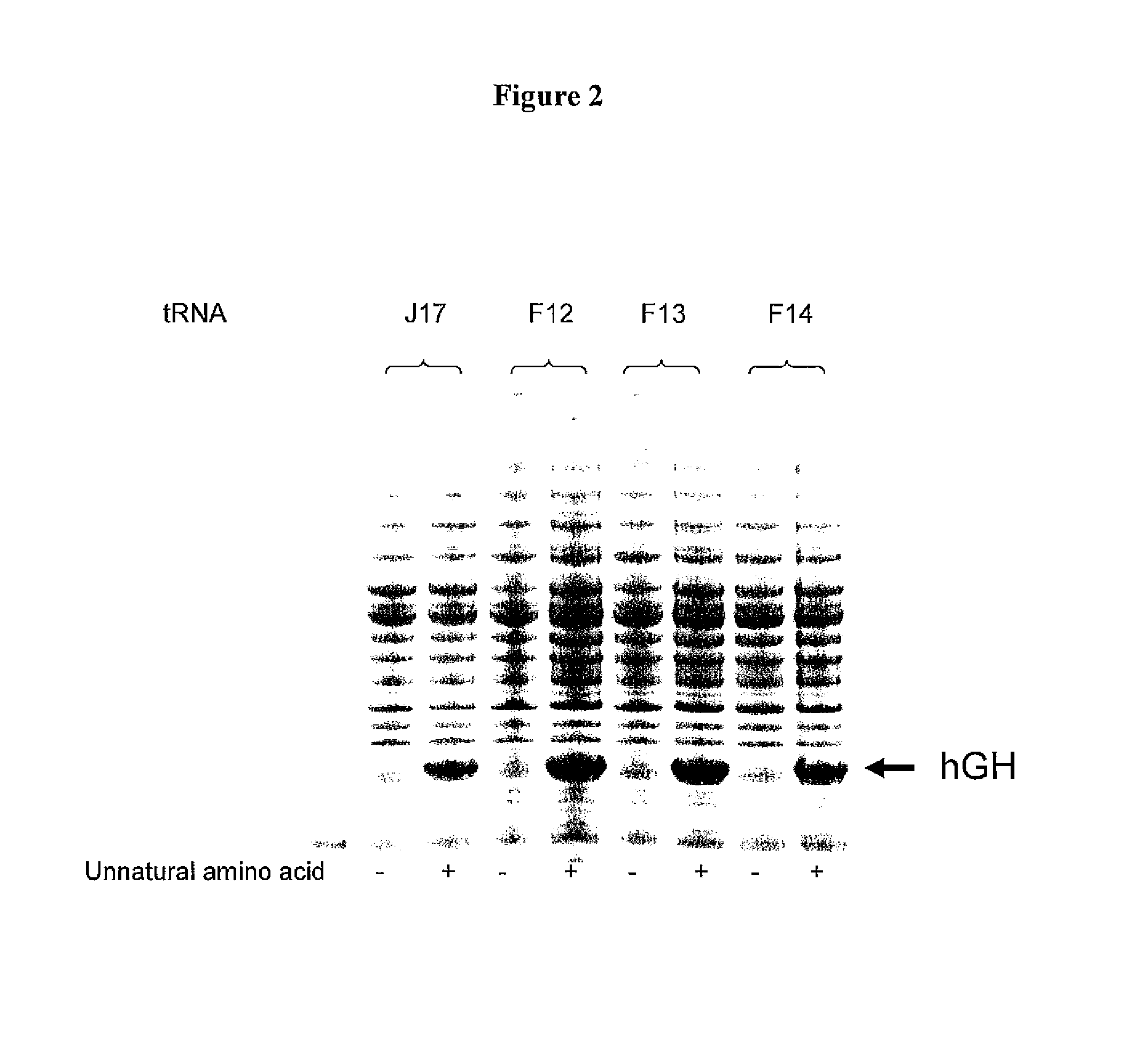
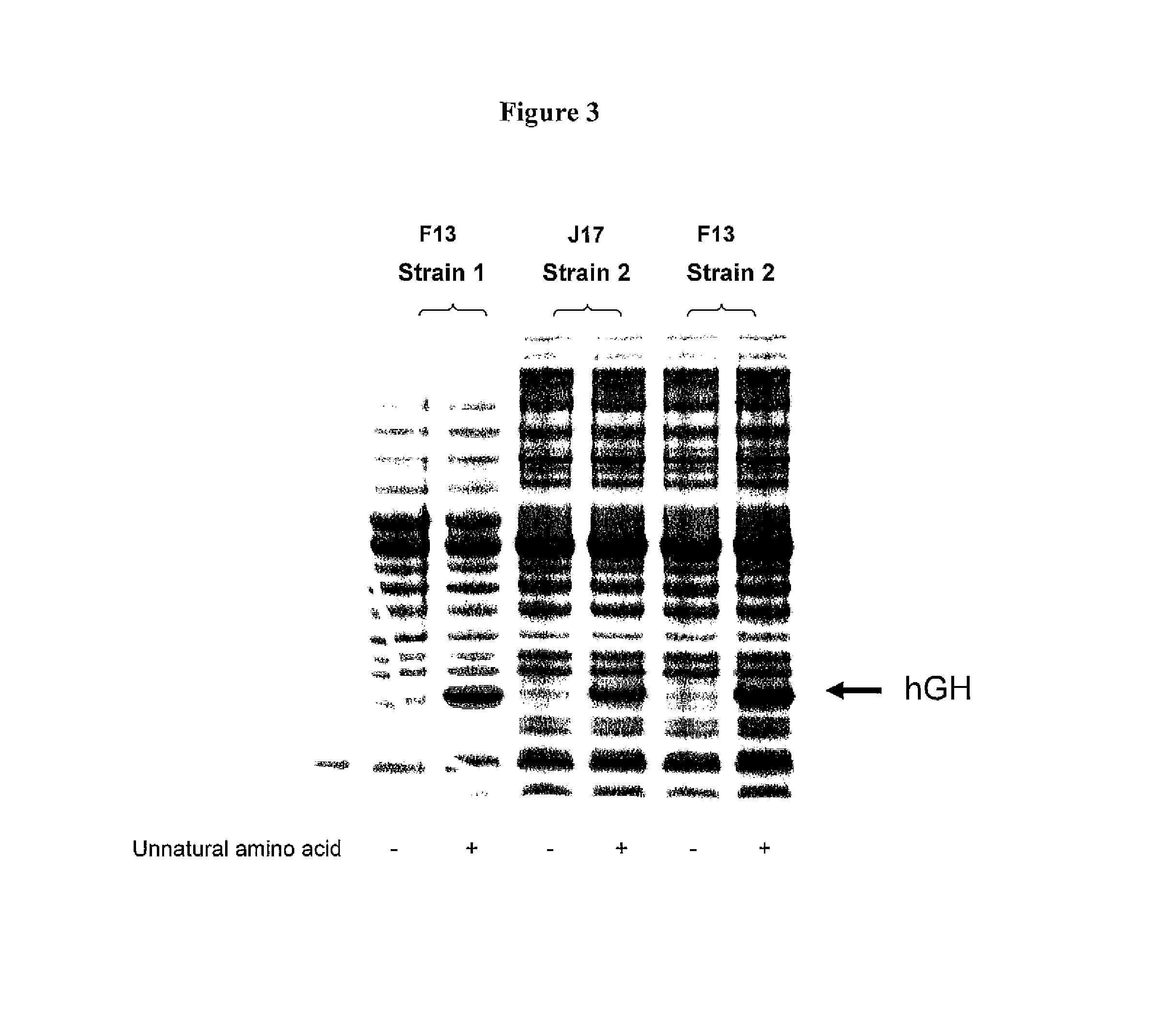
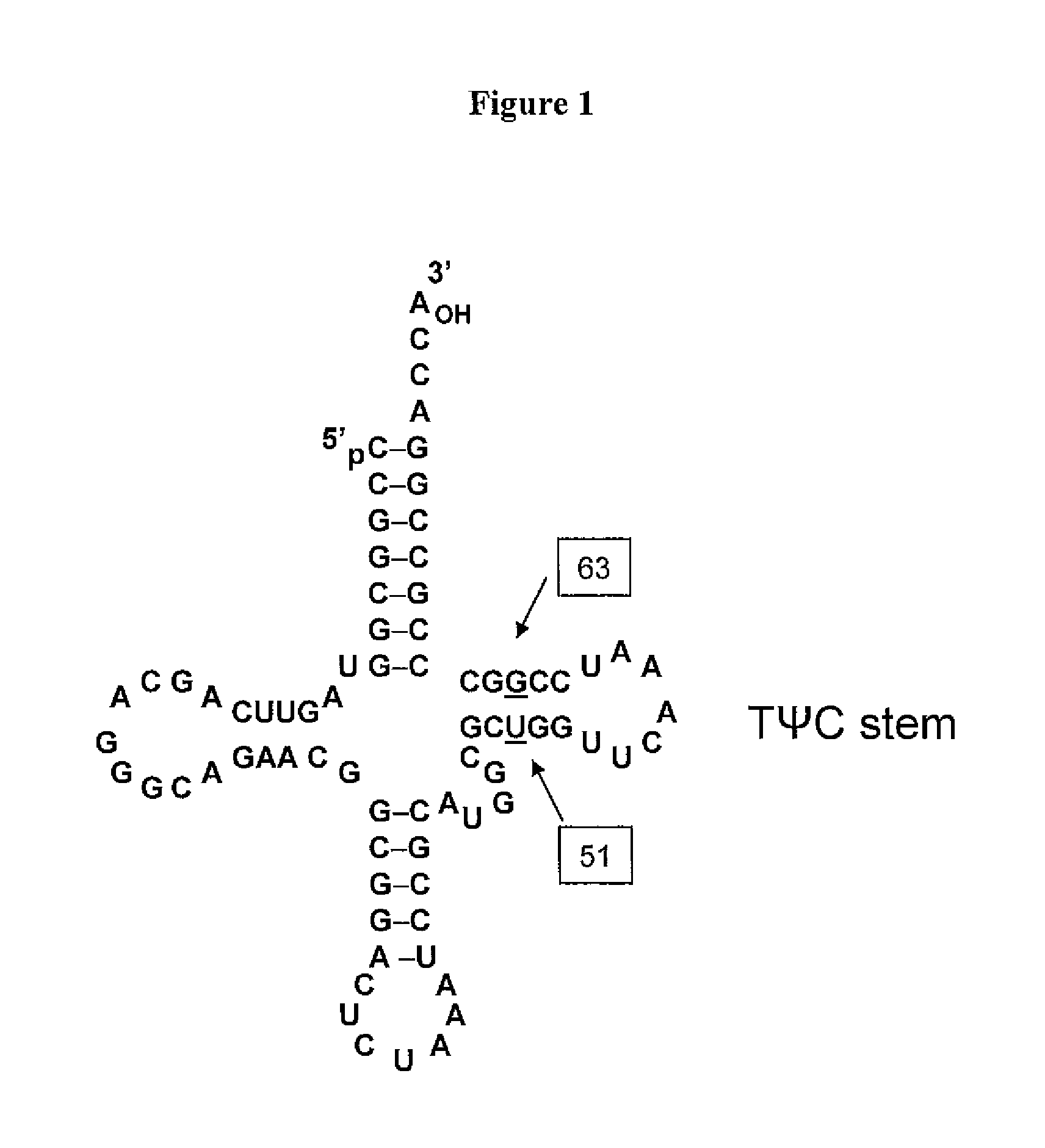
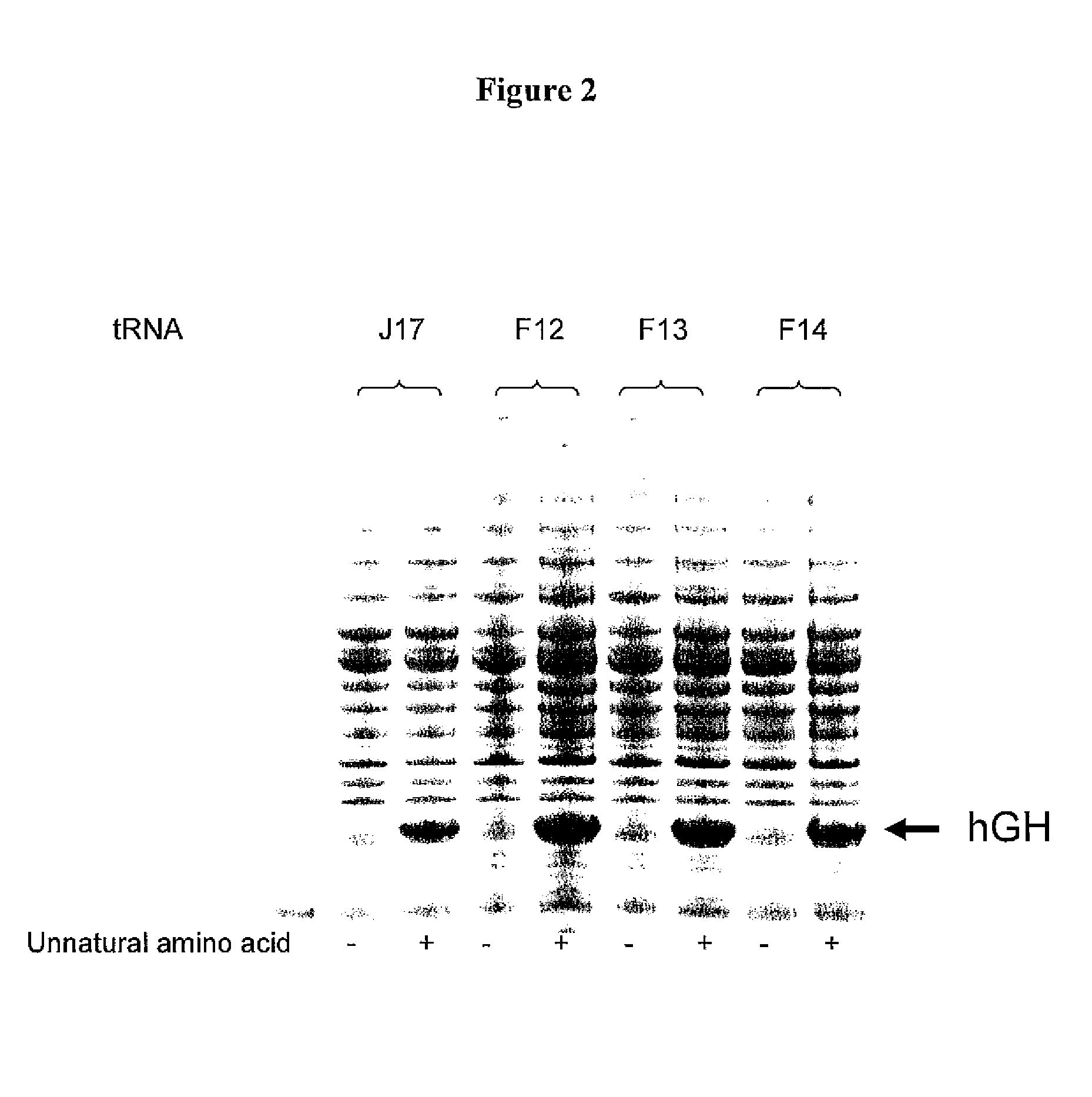
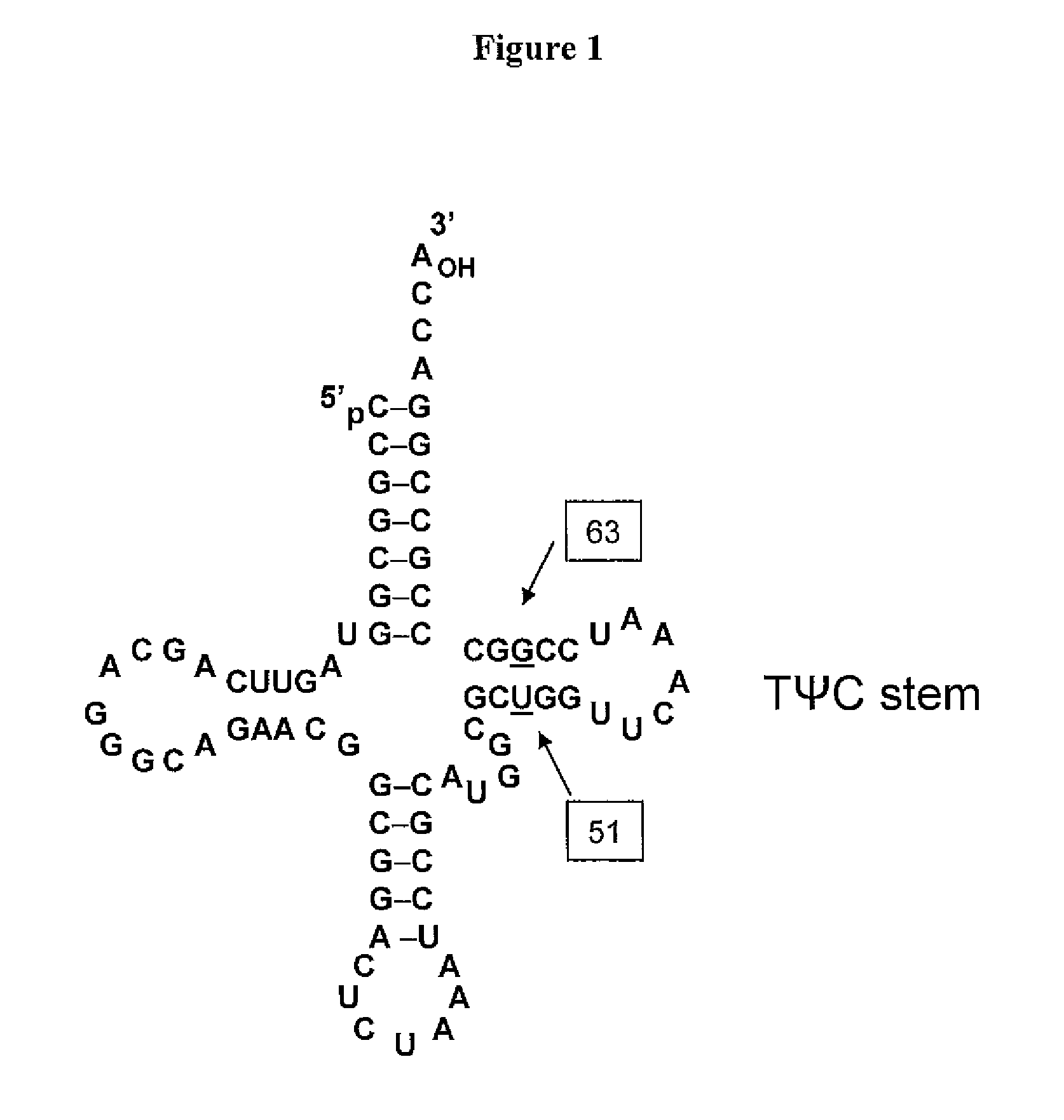
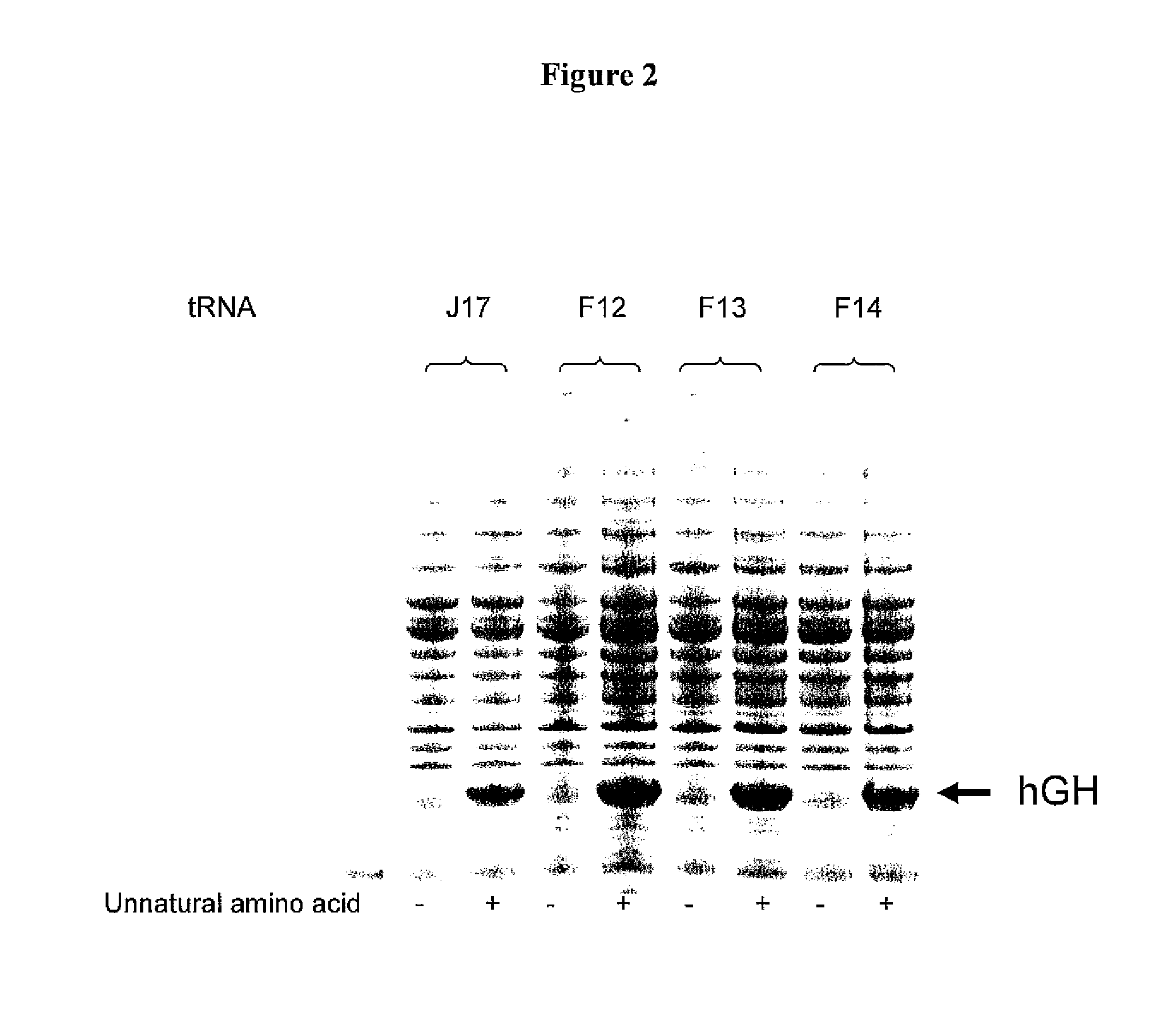
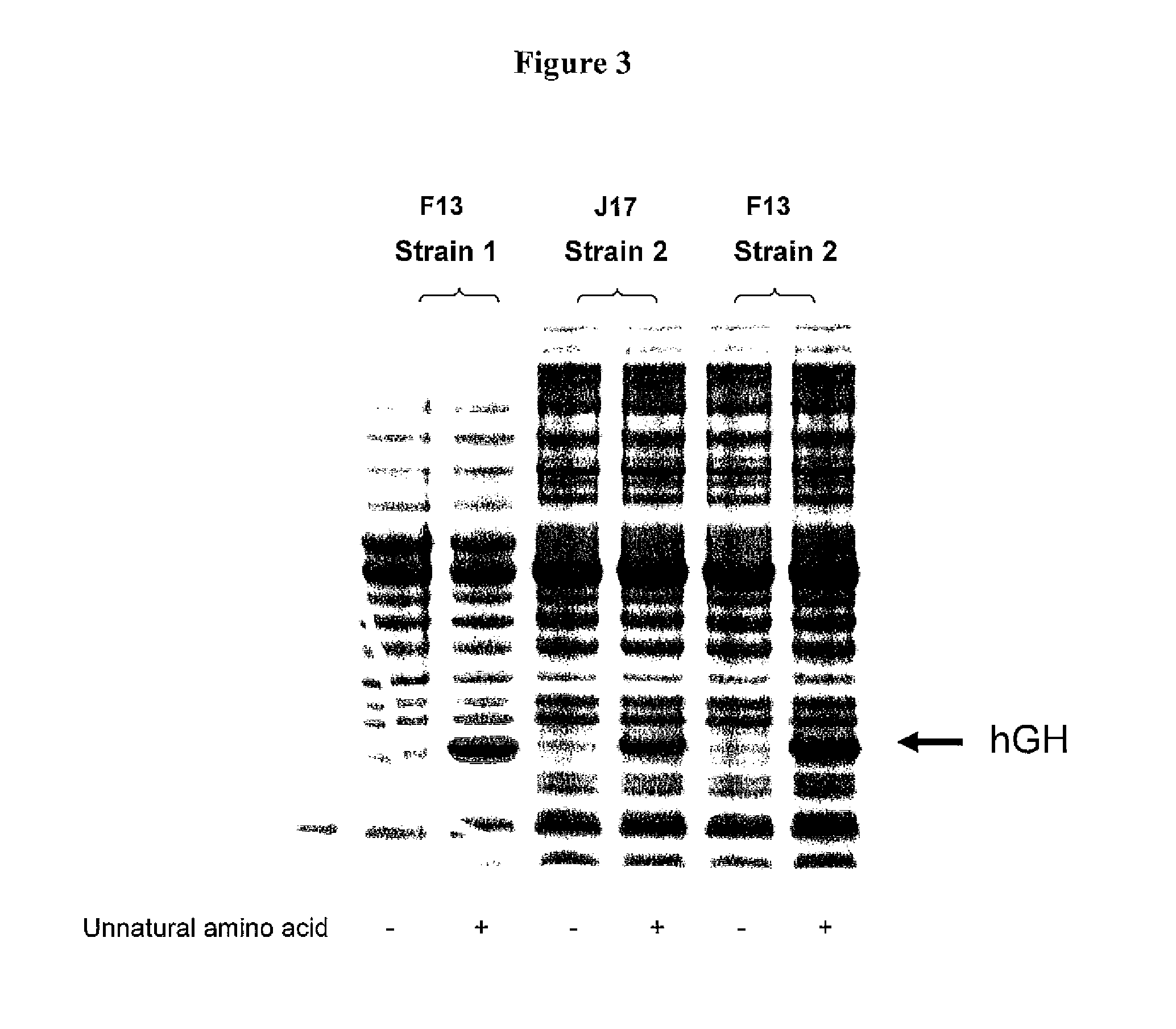
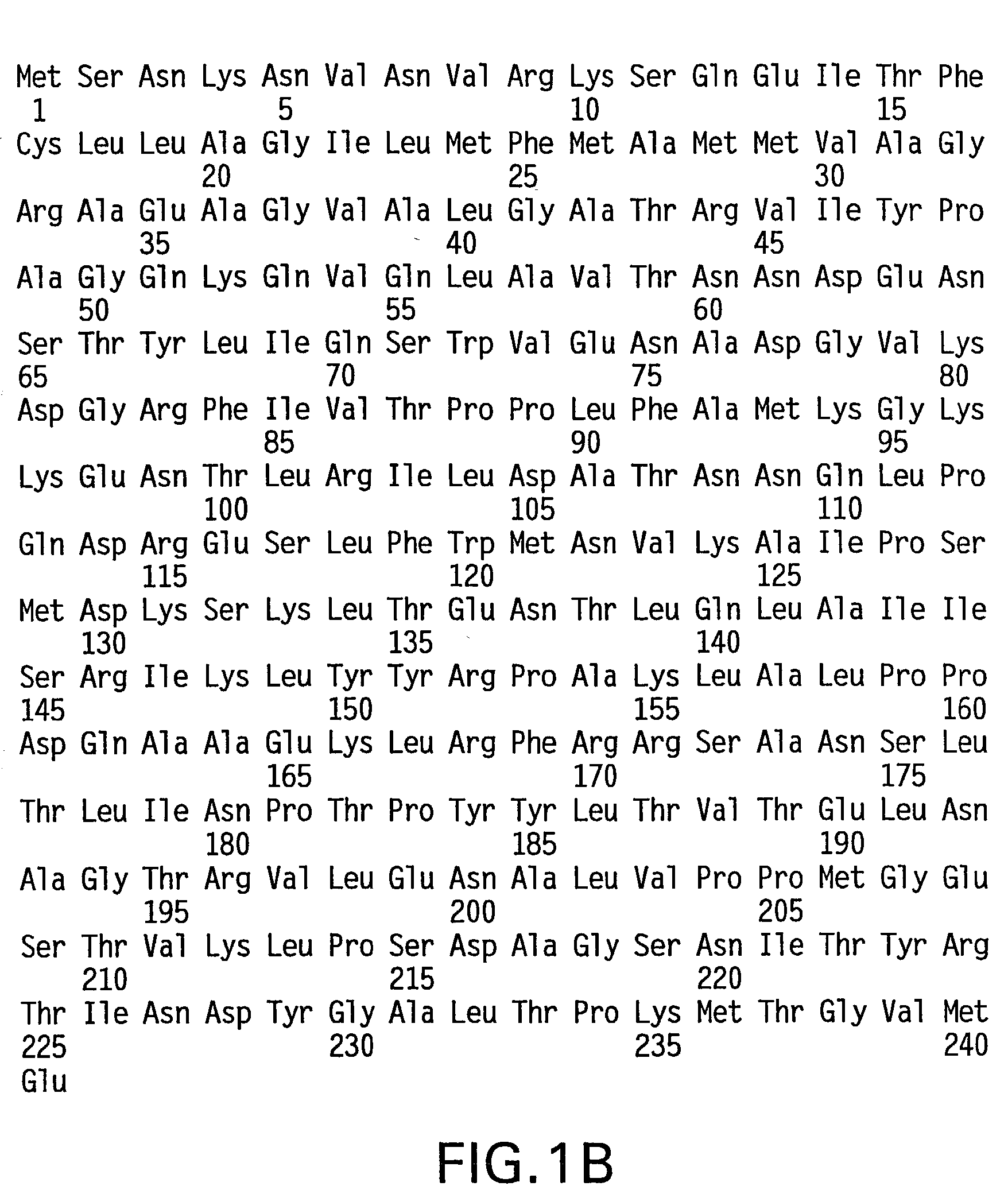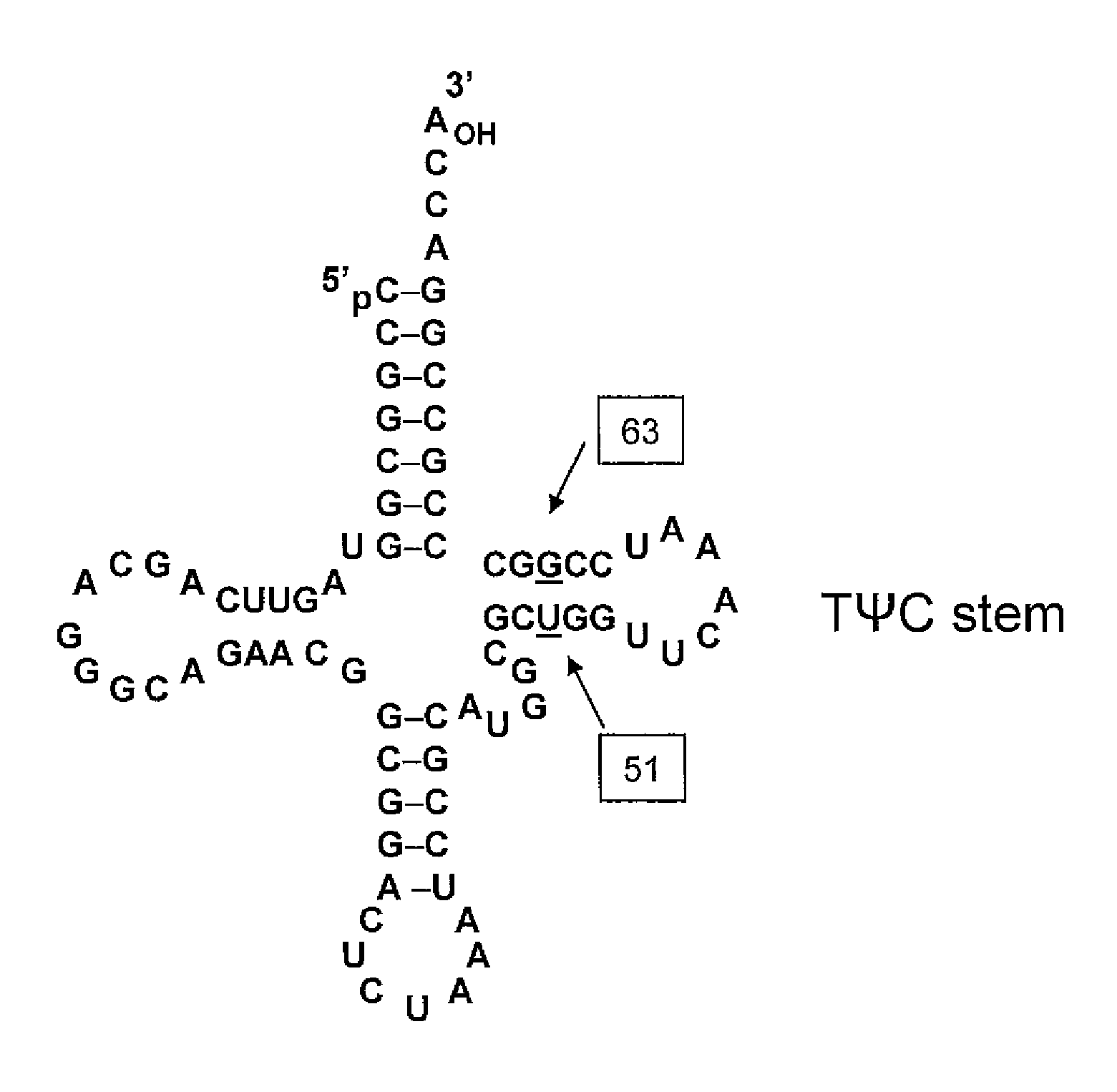Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57results about How to "Enhance antigen presentation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mutant proteins, high potency inhibitory antibodies and fimch crystal structure
InactiveUS20030199071A1Great functional inhibitory activityStrong inhibitory activityHydrolasesImmunoglobulins against bacteriaPassive ImmunizationsMutated protein
The present invention provides bacterial immunogenic agents for administration to humans and non-human animals to stimulate an immune response. It particularly relates to the vaccination of mammalian species, especially human patients, with variants of the E. coli FimCH protein that elicit antibodies that have better functional inhibitory activity than antibodies raised against wild type protein. In particular, such variants include mutations that promote a more open confirmation of the FimH protein, particularly in regions involved in mannose binding, to expose regions previously poorly exposed and mutations that abolish a significantly reduce mannose binding. In another aspect, the invention provides antibodies against such proteins and protein complexes that may be used in passive immunization to protect or treat pathogenic bacterial infections. The present invention also provides machine readable media embedded with the three-dimensional atomic structure coordinates of FimCH bound to mannose, and subsets thereof, and methods of using the crystal structure to provide candidate amino acid residues for mutation.
Owner:WASHINGTON UNIV IN SAINT LOUIS +1
Use of polymeric nanoparticles for vaccine delivery
InactiveUS20080044484A1Enhance antigen presentationImprove efficiencyBiocidePowder deliveryCancer cellT lymphocyte
The invention relates generally to the treatment and prevention of human cancer and viral diseases. More specifically, this invention relates to development of a new generation of vaccines that rely on eliciting cellular immune responses, specifically induction of cytotoxic T lymphocytes (CTL), against cancer cells and virus-infected cells via administration of a polymeric nanoparticle containing a vaccine comprising a fusion peptide or a modified peptide. Such a fusion peptide is composed of an insertion signal sequence and a peptide derived from a tumor antigen or a viral antigen, which improves antigen presentation and induces CTL with higher efficiency against cancer cells and virus-infected cells. An exemplary peptide utilized in the invention is Mart-1:27-35 peptide.
Owner:RGT UNIV OF CALIFORNIA
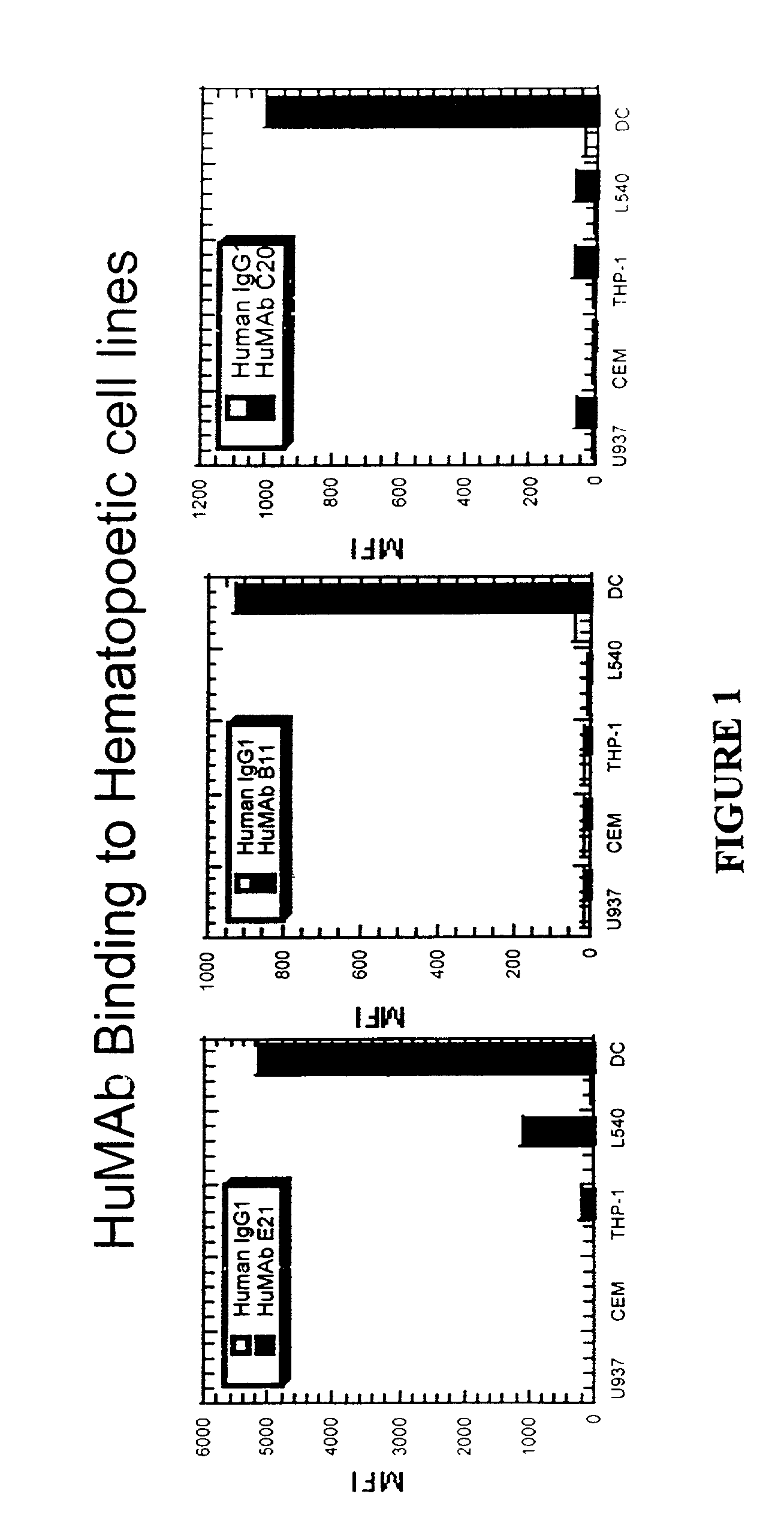
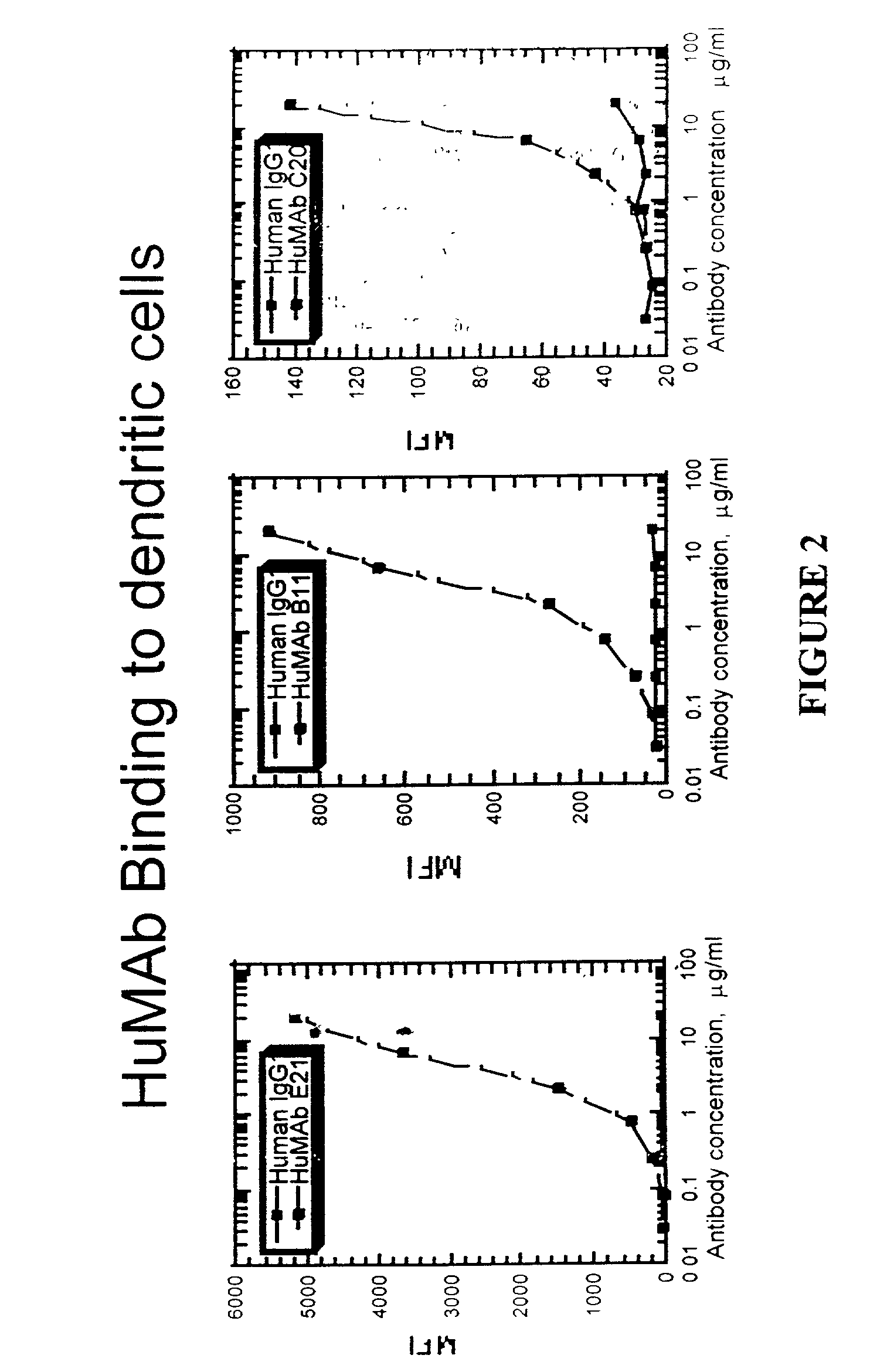
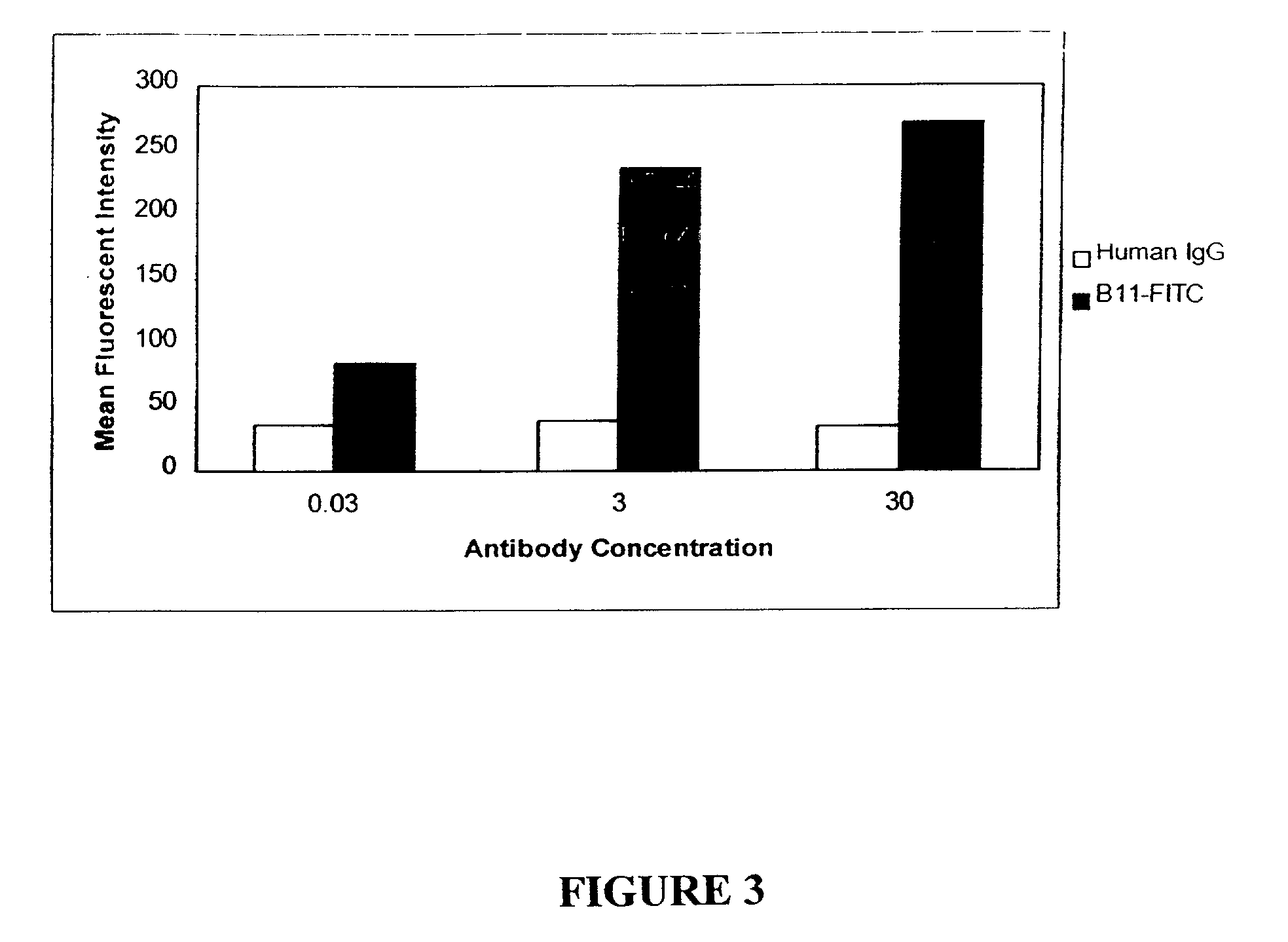
Molecular conjugates comprising human monoclonal antibodies to dendritic cells
InactiveUS7560534B2Enhance antigen presentationStrong immune responseNervous disorderAntipyreticDendritic cellMonoclonal antibody
Molecular conjugates comprising an antigen linked to a human monoclonal antibody that specifically binds to dendritic cells are disclosed. Also disclosed are pharmaceutical compositions comprising the molecular conjugates and therapeutic methods for using the conjugates.
Owner:CELLDEX THERAPEUTICS INC
Enhancing Class I Antigen Presentation With Synthetic Sequences
InactiveUS20080206270A1Enhance antigen presentationInduces antitumor and antiviral CTLTumor rejection antigen precursorsCell receptors/surface-antigens/surface-determinantsCancer cellT lymphocyte
The invention relates generally to the treatment and prevention of human cancer and viral diseases. More specifically, this invention relates to development of a new generation of vaccines that rely on eliciting cellular immune responses, specifically induction of cytotoxic T lymphocytes (CTL), against cancer cells and virus-infected cells via administration of a vaccine comprising a fusion peptide or a modified peptide. Such a fusion peptide is composed of an insertion signal sequence and a peptide derived from a tumor antigen or a viral antigen, which improves antigen presentation and induces CTL with higher efficiency against cancer cells and virus-infected cells. An exemplary antigen utilized in the invention is HER2 / neu. The peptides peptide vaccines of the invention are derived from the antigens PRAME, OFA / iLRP, STEAP and SURVIVIN.
Owner:RGT UNIV OF CALIFORNIA
Non-Natural Amino Acid Replication-Dependent Microorganisms and Vaccines
ActiveUS20130323821A1Low efficacyEnhance antigen presentationAntibacterial agentsBacterial antigen ingredientsMicroorganismBiological body
Compositions and methods of producing vaccines, including methods wherein whole organism vaccines are provided with limited replication abilities, thereby increasing vaccine safety and efficacy, through the use of non-natural, unnatural, or non-naturally encoded amino acids.
Owner:AMBRX
Non-Natural Amino Acid Replication-Dependent Microorganisms and Vaccines
ActiveUS20110195483A1Enhance vaccine efficiencyPromote cross presentationAntibacterial agentsBacterial antigen ingredientsWhole OrganismBiological body
Compositions and methods of producing vaccines, including methods wherein whole organism vaccines are provided with limited replication abilities, thereby increasing vaccine safety and efficacy, through the use of non-natural, unnatural, or non-naturally encoded amino acids.
Owner:AMBRX
Non-Natural Amino Acid Replication-Dependent Microorganisms and Vaccines
ActiveUS20130280301A1Low efficacyEnhance antigen presentationAntibacterial agentsBacterial antigen ingredientsMicroorganismBiological body
Compositions and methods of producing vaccines, including methods wherein whole organism vaccines are provided with limited replication abilities, thereby increasing vaccine safety and efficacy, through the use of non-natural, unnatural, or non-naturally encoded amino acids.
Owner:AMBRX
Dendritic cells, uses therefor, and vaccines and methods comprising the same
InactiveUS20090041792A1Improve abilitiesEnhance phagocytic activitySnake antigen ingredientsBlood/immune system cellsDendritic cellVaccination
Provided is a method of cross-priming CD8+ T cells to antigens using Dendritic Cells cultured in the presence of a type I Interferon and GM-CSF, and vaccines and methods of vaccination comprising said Dendritic Cells.
Owner:INST SUPERIORE DI SANITA
Recombinant adeno-associated virus as well as construction method and application thereof
InactiveCN105586320AImprove submission efficiencyEnhance antigen presentationTumor rejection antigen precursorsTumor specific antigensWilms' tumorGene
The invention relates to a recombinant adeno-associated virus. The recombinant adeno-associated virus is formed by inserting a tumor antigen gene into a shuttle expression vector pAAV-MCS. The invention also provides a construction method and an application of the recombinant adeno-associated virus. According to the invention, the recombinant adeno-associated virus carrying a tumor-associated antigen gene is utilized for infecting DC cells and expresses tumor-associated antigen protein in the DC cells, and a PD-1 gene is also combined for silencing CTL cells in the application method of the recombinant adeno-associated virus.
Owner:厚朴生物科技(苏州)有限公司
Modifications of HIV Env, Gag, and Pol enhance immunogenicity for genetic immunization
InactiveUS20040033487A1Low cytotoxicityEnhance humoral and CTL immunityOrganic active ingredientsPeptide/protein ingredientsVaccine ImmunogenicityDNA
Modified HIV Env, Gag, Pol, or Nef DNA with improved ability to elicit antibody and CTL responses to HIV antigens have been identified as prototype immunogens for the treatment and prevention of HIV infections.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Compositions and methods for prevention and treatment of fungal diseases
InactiveUS20070243209A1Significant clinical importanceEffective adjuvantBiocideOrganic active ingredientsDiseaseFungal disease
The present invention relates to various pharmaceutical compositions that can be used as active or passive vaccines for the treatment or prevention of fungal disease. Methods for prevention and treatment of infectious and allergic fungal diseases in subjects using the pharmaceutical compositions of the present invention are also disclosed.
Owner:HEALTH RES INC
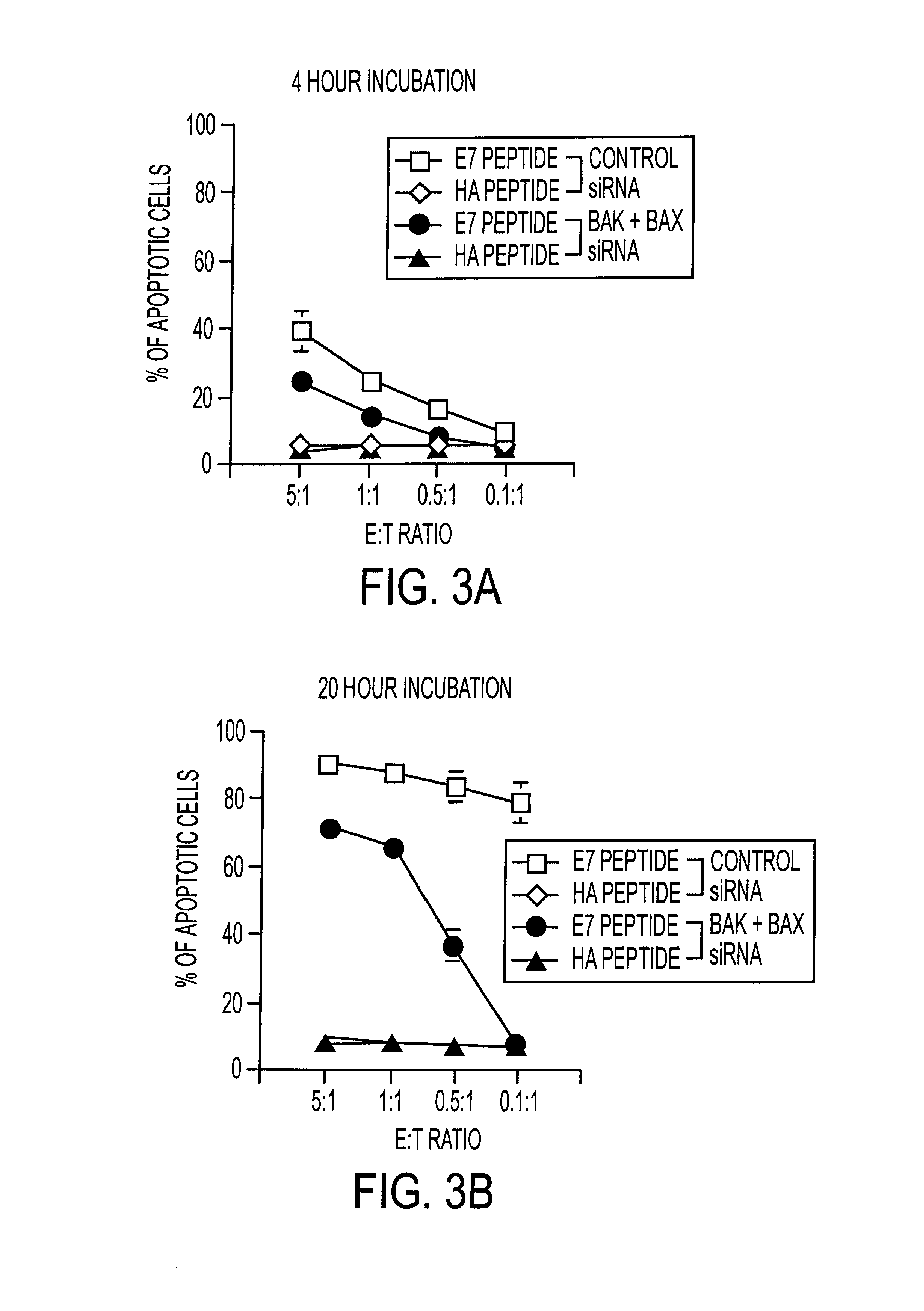
RNA Interference That Blocks Expression of Pro-Apoptotic Proteins Potentiates Immunity Induced by DNA and Transfected Dendritic Cell Vaccines
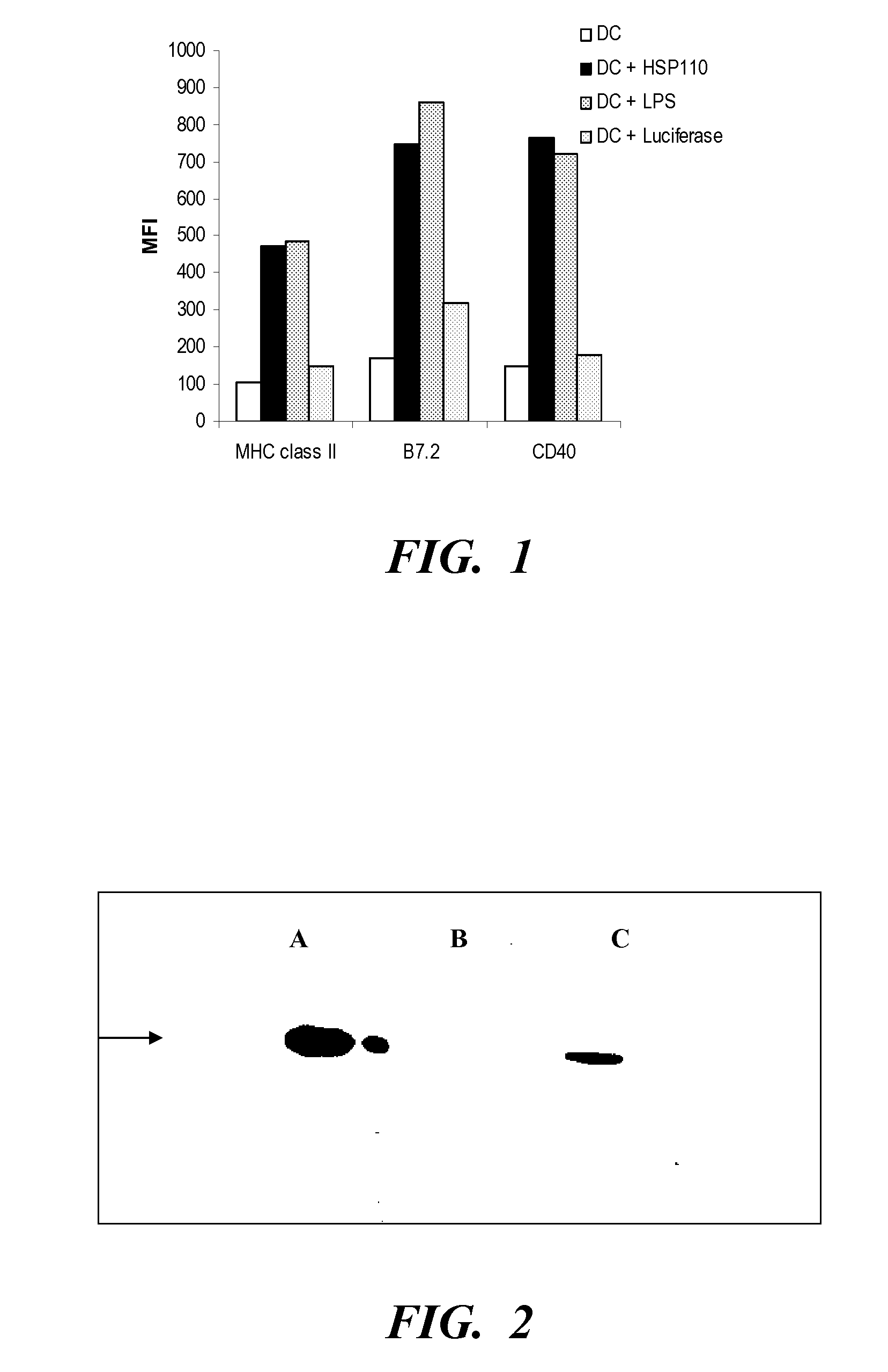
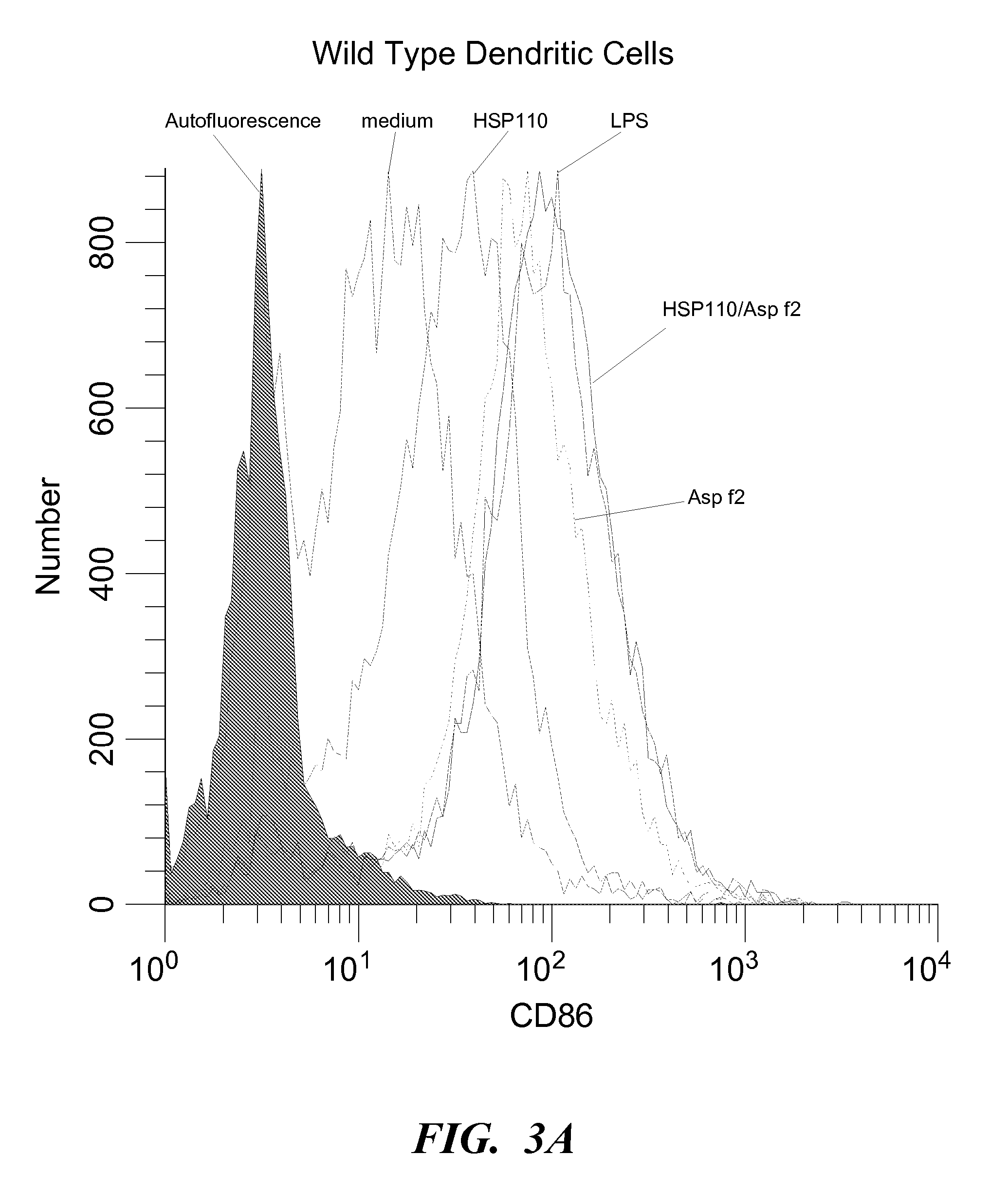
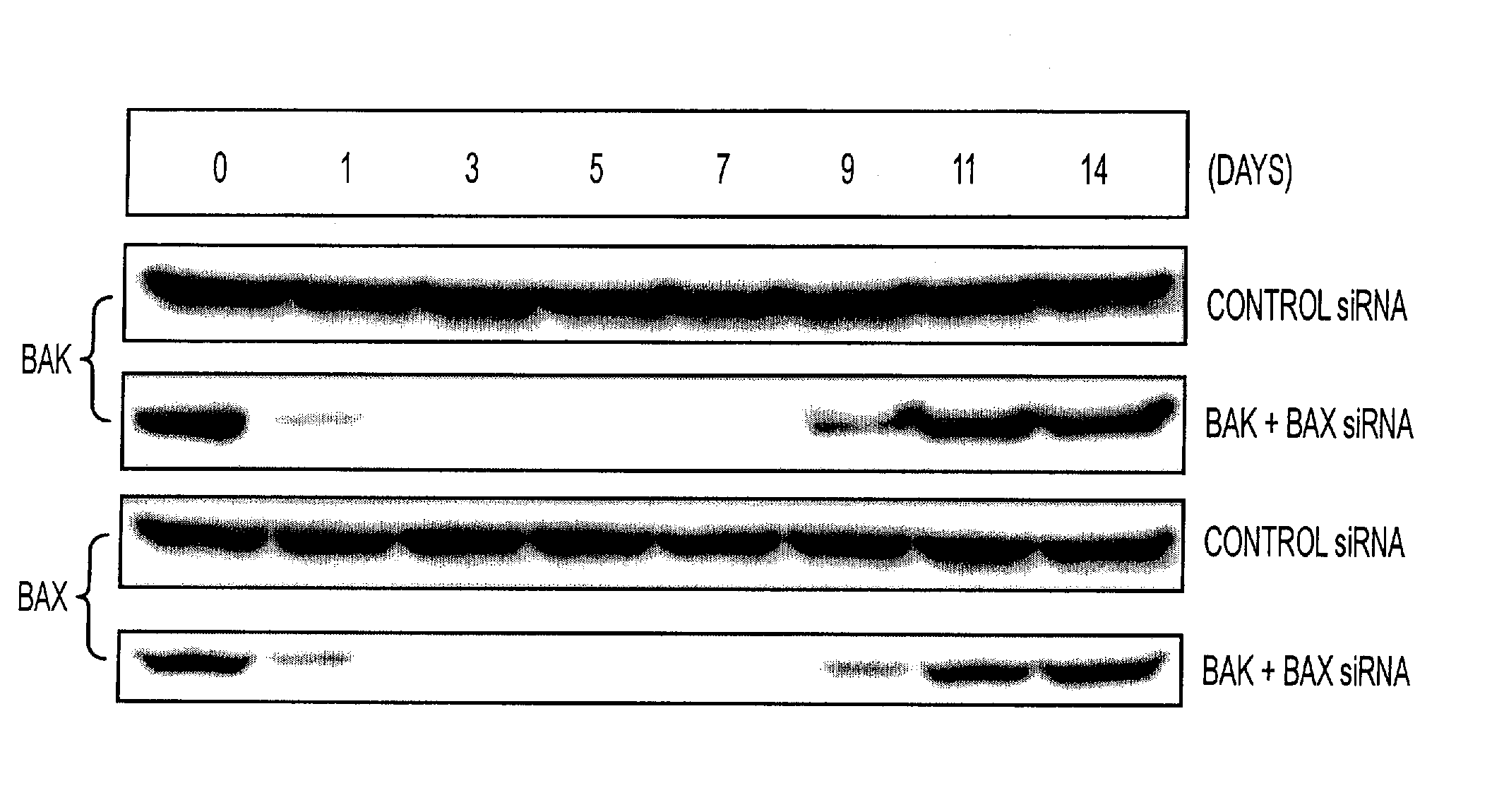
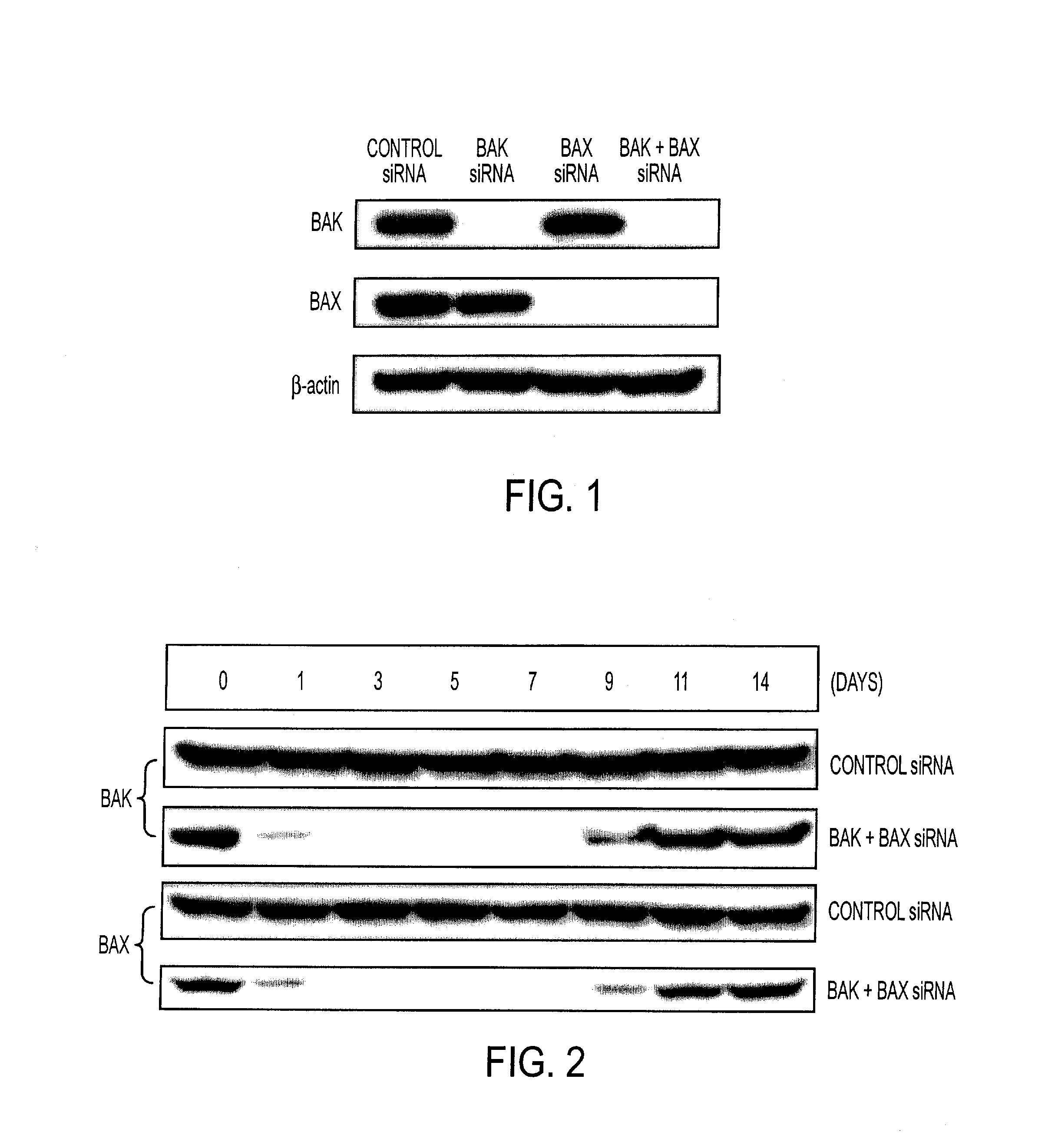
InactiveUS20080069840A1Enhance antigen presentationConducive to survivalOrganic active ingredientsBiocideAbnormal tissue growthImmunotherapeutic agent
An immunotherapeutic strategy is disclosed that combines antigen-encoding DNA vaccine compositions combined with siRNA directed to pro-apoptotic genes, primarily Bak and Bax, the products of which are known to lead to apoptotic death. Gene gun delivery (particle bombardment) of siRNA specific for Bak and / or Bax to antigen-expressing DCs prolongs the lives of such DCs and lead to enhanced generation of antigen-specific CD8+ T cell-mediated immune responses in vivo. Similarly, antigen-loaded DC's transfected with siRNA targeting Bak and / or Bax serve as improved immunogens and tumor immunotherapeutic agents.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Veterinary vaccine immunologic adjuvant as well as preparation and application method thereof
InactiveCN105797153AFacilitates multiple antigen and antibody bindingEnhance immune response level and antigen presentation abilityImmunological disordersAntibody medical ingredientsIonAntibody
Owner:ZHEJIANG FORESTRY UNIVERSITY
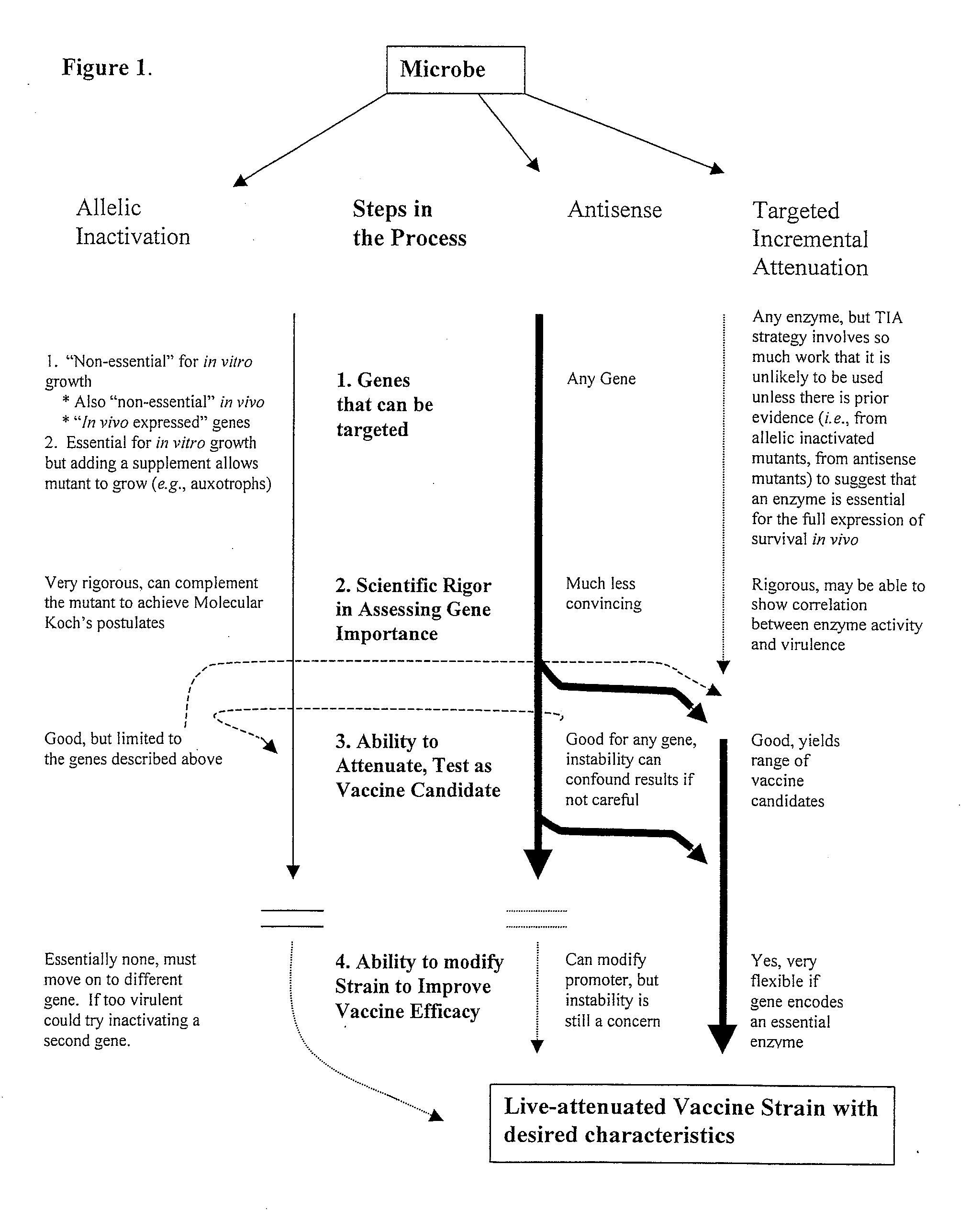
Pro-Apoptotic Bacterial Vaccines To Enhance Cellular Immune Responses
InactiveUS20120276144A1Diminishing intracellular survivalReduced activityAntibacterial agentsBacterial antigen ingredientsVaccine PotencyVaccine efficacy
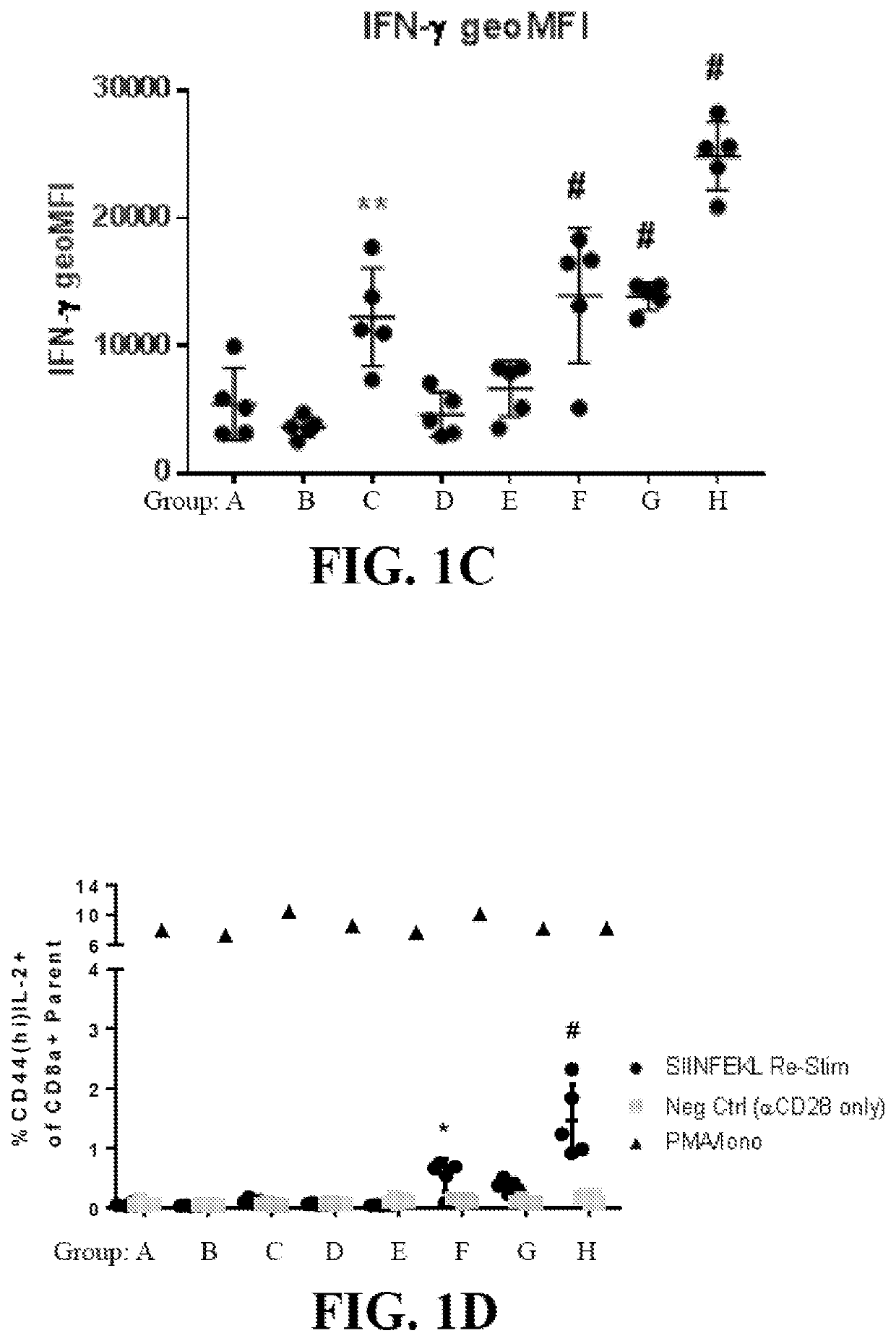
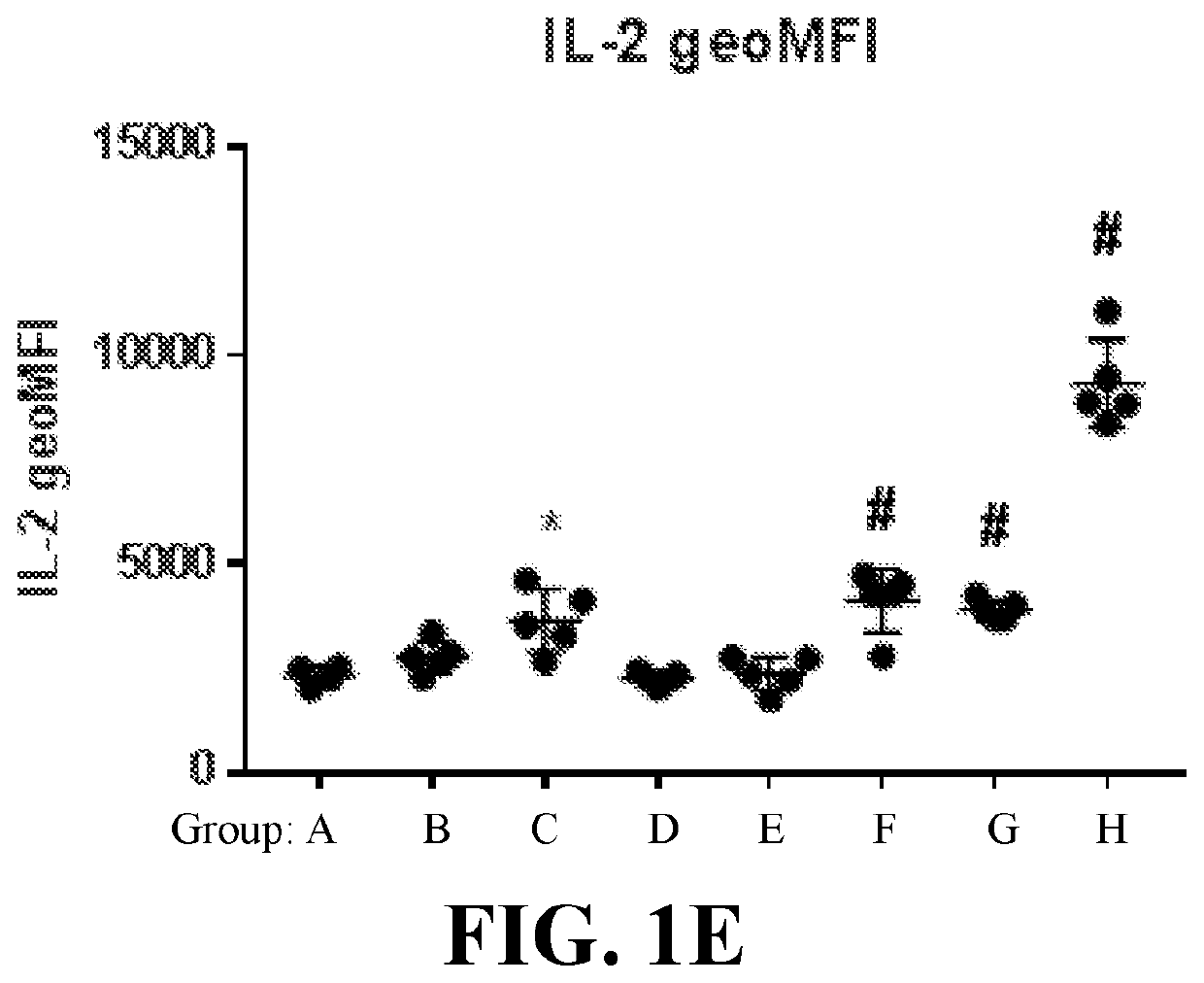
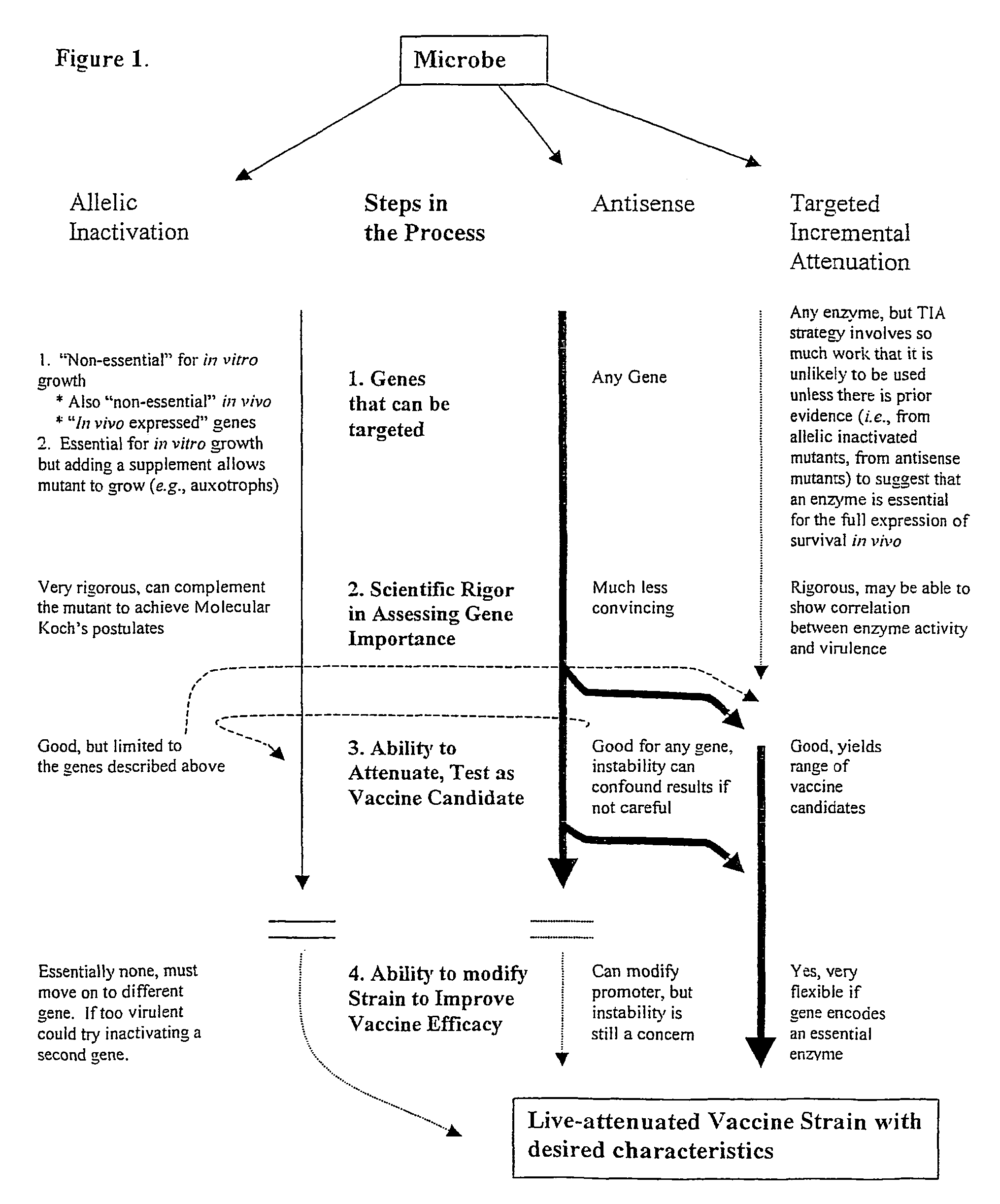
Whole-cell vaccines and methods for their use in producing protective immune responses in vertebrate hosts subsequently exposed to pathogenic bacteria. The present invention involves a method of enhancing antigen presentation by intracellular bacteria in a manner that improves vaccine efficacy. After identifying an enzyme that has an anti-apoptotic effect upon host cells infected by an intracellular microbe, the activity of the enzyme is reduced, thereby modifying the microbe so that it increases immunogenicity. Also, the present invention provides a method of incrementally modifying enzyme activity to produce incrementally attenuated mutants of the microbe from which an effective vaccine candidate can be selected.
Owner:VANDERBILT UNIV
Methods For Producing Dendritic Cells That Have Acquired Ctl-Inducing Ability
InactiveUS20080194020A1Promotion of antigenic protein polyubiquitinationEnhance antigen presentationSugar derivativesPeptide preparation methodsProteasomeCross-presentation
The present inventors worked on elucidating the mechanism of antigen cross-presentation by dendritic cells, and revealed that foreign antigens taken up in dendritic cells are degraded by proteasomes after undergoing polyubiquitination. Based on the novel finding that polyubiquitination is involved in cross-presentation, the promotion of polyubiquitination was attempted by a number of methods, and the promotion of antigen presentation was confirmed. These methods enable the production of dendritic cells effective for inducing CTL activation.
Owner:KEIO UNIV +1
Preparation method and application of efficient immunocompetent cell CpG-DCIK
InactiveCN104651312AIncrease proliferative activityStrong proliferative activityMammal material medical ingredientsBlood/immune system cellsTumour-associated antigenPopulation
The invention relates to a preparation method and an application of efficient immunocompetent cell CpG-DCIK. The preparation method comprises the following steps: inducing DC cell by use of CpG and cytokine, inducing homologous CIK cell by use of cytokine, and performing mixed culture of the DC cell and CIK cell to obtain a new immune effector cell population, namely oligonucleotide induced dendritic cell and cytokine induced killer cell co-culture cell which is named CpG-DCIK. According to the invention, the DC is induced by use of in-vitro CpG-ODN in combination with cytokine, and then the DC is co-cultured with homologous CIK to obtain an immune effector cell population with higher proliferation activity and cytotoxic activity, and an immune cell population with relatively high antitumor activity can still be induced and amplified for the cases in which a tumor antigen is hardly acquired since the operation opportunity is lost or the cancer is in the late stage and the like, thereby widening the application range of tumor resistance.
Owner:SHANGHAI LIWO BIOTECH
Method for preparing HPV (human papillomavirus) antigen specific CTL (cytotoxic T lymphocyte)
ActiveCN108300692AEnhance antigen presentationInduced proliferationMammal material medical ingredientsBlood/immune system cellsHPV AntigenDisease
The invention discloses a method for preparing HPV (human papillomavirus) antigen specific CTL (cytotoxic T lymphocyte) and particularly discloses a preparation method of HLA-A2402 restrictive anti-HPV antigen specific CTL. According to the method, a peripheral blood mononuclear cell is collected through single blood collection or venous blood collection, the antigen presentation function of B cells is enhanced with CpG ODN 2395, the B cells supporting HLA-A2402 restrictive HPV antigen peptide are used for stimulating the peripheral blood mononuclear cell, rhIL-2, rhIL-7, rhIL-15 and rhIL-21 are jointly used to promote growth of the T cell. The target CTL prepared with the method has the characteristics that preparation is simple, the preparation cycle is short, the cost is low, the multiplication capacity and the killing activity are high, the cell viability is high and the like, and can be used for immunotherapy of HPV infection related diseases including cervical cancer.
Owner:JILIN TUO HUA BIOTECH
Artocarpus lingnanensis lectin capable of inducing dentritic cells to mature and proliferate in vitro
InactiveCN102690783AEnhance antigen presentationOvercome expensiveBlood/immune system cellsBiotechnologyPeripheral blood mononuclear cell
The invention discloses Artocarpus lingnanensis lectin capable of inducing dentritic cells (DC) to mature and proliferate in vitro, a special culture medium for inducing DC to mature and proliferate, and a preparation method for the DC. The Artocarpus lingnanensis lectin is prepared from red cassia tree seeds through separation and purification by the conventional separation method. According to the special culture medium, inducing factors and the Artocarpus lingnanensis lectin are added into a DC culture medium. The preparation method comprises the following steps: adding precursor cells of the DC and the inducing factors into the DC culture medium, and adding the Artocarpus lingnanensis lectin when the cells are cultured in the sixth day; and continuously culturing to obtain mature DC, wherein the precursor cells are peripheral blood mononuclear cells; and the inducing factors are granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). A stable culture technical system for inducing the DC to mature and proliferate is established, and is easy to operate, convenient and practical; the required cell factors are a few, and the cost is low; and the dentritic cells (DC) have high maturity and strong functions. The Artocarpus lingnanensis lectin provides a novel effective way for further developing vaccine enhancers.
Owner:GUANGXI MEDICAL UNIVERSITY
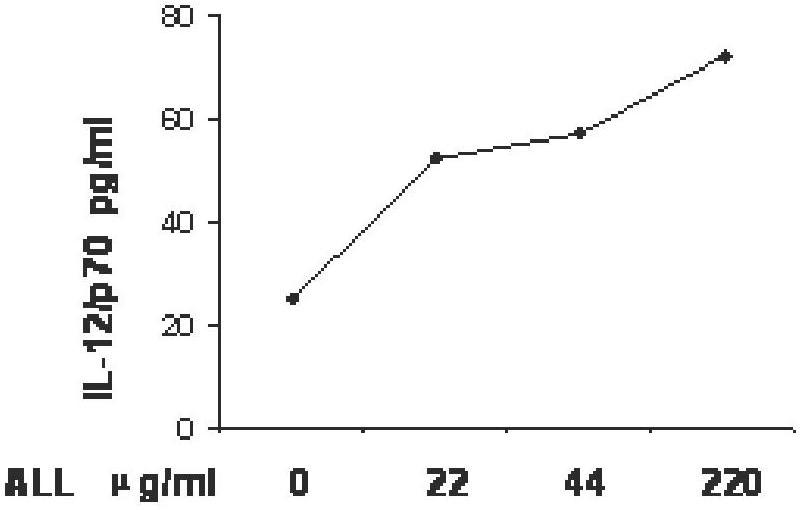
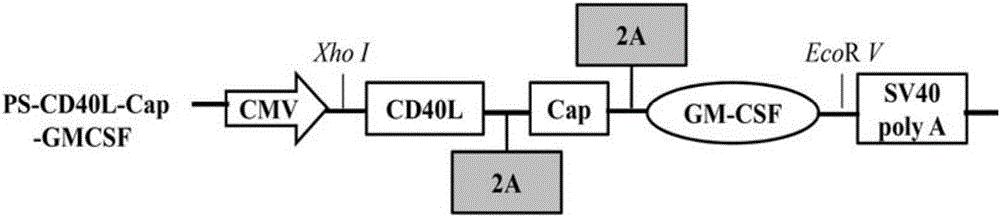
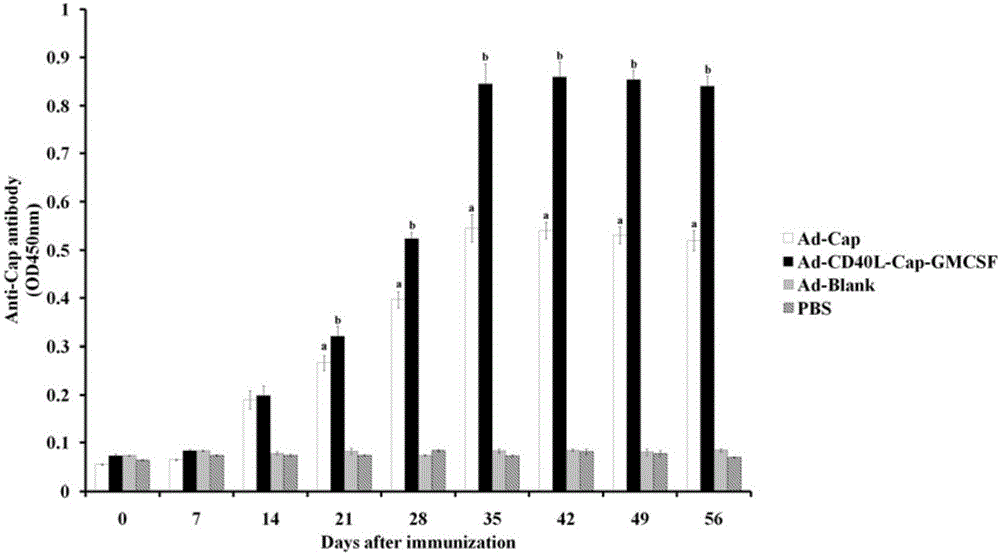
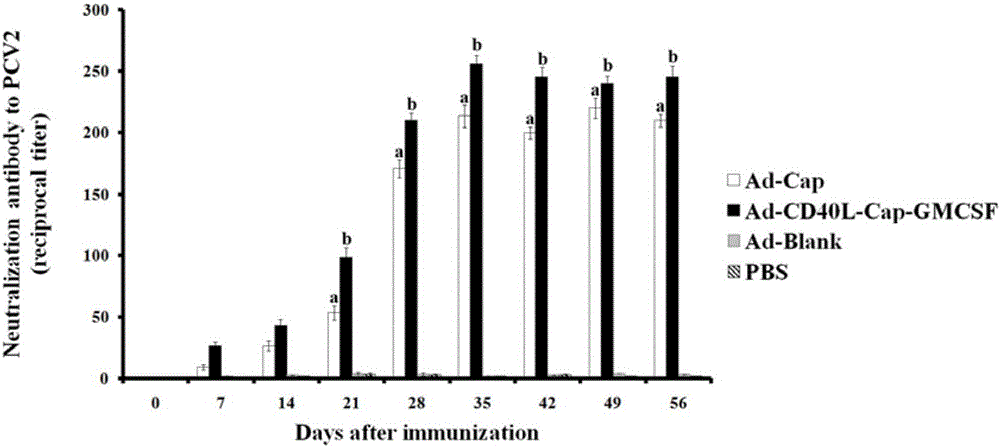
Construction, amplification and purification method of porcine CD40L/GMCSF/PCV2Cap recombinant adenovirus
InactiveCN105838684AImprove the level ofPromote secretionMicrobiological testing/measurementVirus peptidesEnzyme digestionPurification methods
The invention relates to a construction, amplification and purification method of a porcine CD40L / GMCSF / PCV2Cap recombinant adenovirus. The method comprises the steps of: S1. connecting CD40L, GM-CSF and Cap to a vector pUC57 in order, and naming the product as pUC-CD40L-Cap-GMCSF; S2. connecting CD40L, Cap and GM-CSF to pShuttle-CMV, converting DH5a, conducting bacteria picking, bacteria shaking and plasmid extraction, and naming the product as PS-CD40L-Cap-GMCSF; S3. linearizing the constructed PS-CD40L-Cap-GMCSF, then conducting electric transformation on BJ5183 with the linearized PS-CD40L-Cap-GMCSF and skeleton plasmid pAdEasy-1, and then carrying out bacteria picking, bacteria shaking and plasmid extraction, and performing single enzyme digestion identification; S4. when the identification result is right, using a kit to extract plasmid, transfecting HEK293A cell, when cell lesion appears, collecting cells, performing centrifugation, then resuspending the precipitate in autoclaving PBS; S5. carrying out repeated freezing and thawing, performing centrifugation to extract the supernatant, thus obtaining recombinant adenovirus; 6. amplifying the obtained recombinant adenovirus; and 7. purifying the recombinant adenovirus. The method provided by the invention for the first time adds porcine tumor necrosis factor related activation protein gen and porcine granulocyte-macrophage colony stimulating factor into the adenovirus vector simultaneously to improve expression of the PCV2 Cap recombinant adenovirus immunogenicity.
Owner:NORTHWEST A & F UNIV
Construction method of humanized immune system mouse with myeloid immune cells
InactiveCN111705082AIncreased developmental levelEnhance antigen presentationFermentationVector-based foreign material introductionAntigenLymphocyte
The invention provides a construction method of a humanized immune system mouse with myeloid immune cells. The construction method is characterized by comprising the step of artificially supplementingexogenous cytokines GM-CSF, IL-3 and SCF. By the construction method, the humanized immune system mouse is further perfected, so that the humanized immune system mouse has a more complete immune system. The antigen presentation capability of the humanized immune system mouse is greatly improved by promoting development of myeloid immune cell subgroups including mononuclear-macrophages, DC cells and the like. On the basis of an original immune system mainly containing T and B lymphocytes, the capabilities of generating antibodies, performing specific cytotoxic killing and the like are increased. After the exogenous human cytokines are supplemented, the development level of the myeloid immune cells in the humanized immune system mouse is greatly improved, the level of the corresponding immune cell subgroups in normal human peripheral blood is achieved, and the corresponding functions are fulfilled.
Owner:澎立生物医药技术(上海)股份有限公司
DC cell culture reagent and culture method thereof
ActiveCN105087489APromote proliferationGood cell viabilityBlood/immune system cellsCytokineMolecular biology
The invention relates to the technical field of cell culture, and in particular relates to a DC cell culture reagent and a culture method thereof. The DC cell culture reagent includes a first DC cell culture reagent and a second DC cell culture reagent, wherein the first DC cell culture reagent consists of GM-SCF and IL-4; and the second DC cell culture reagent consists of GM-SCF, IL-4, TNF-alpha and MDC. The method disclosed by the invention, by improving the type and the concentration of cell factors in a DC cell culture process, can be used for promoting the proliferation in the quantity of DC cells and keeping a relatively good cell viability, so that the antigen presentation capacity of the DC cells is enhanced and a better antitumor effect is guaranteed.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
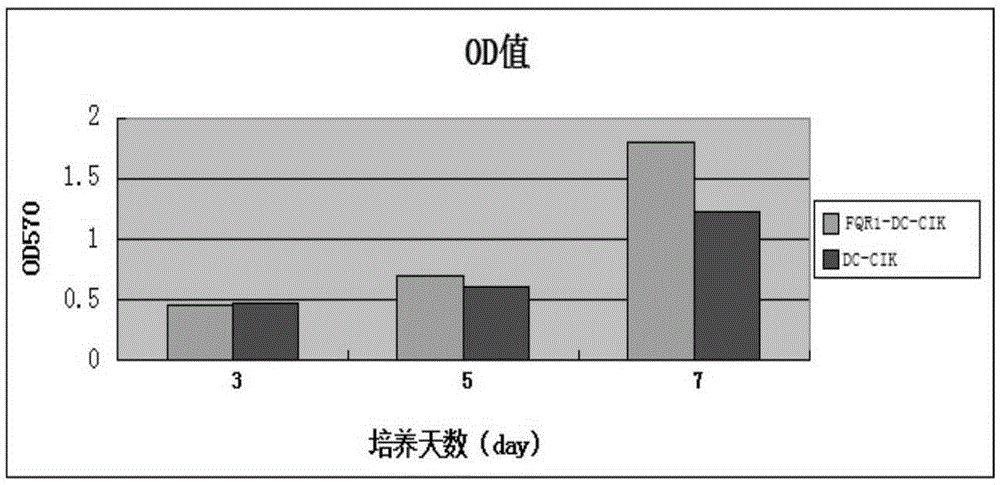
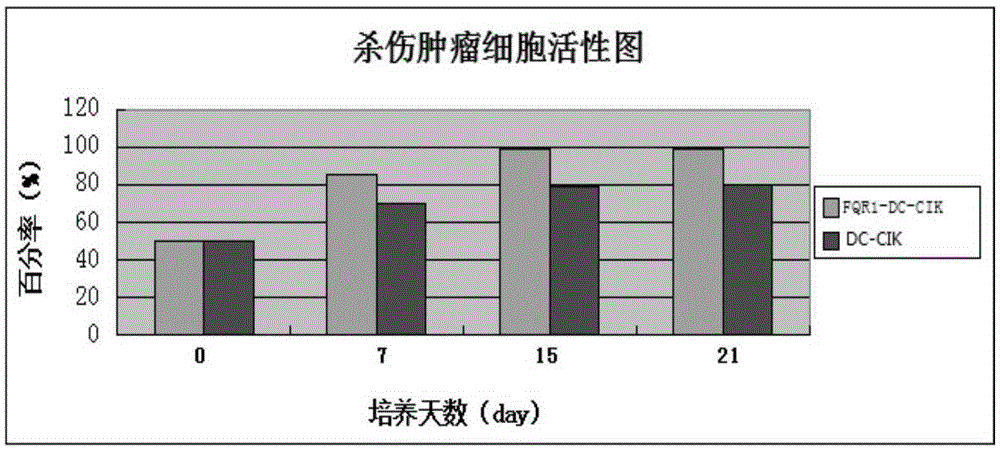
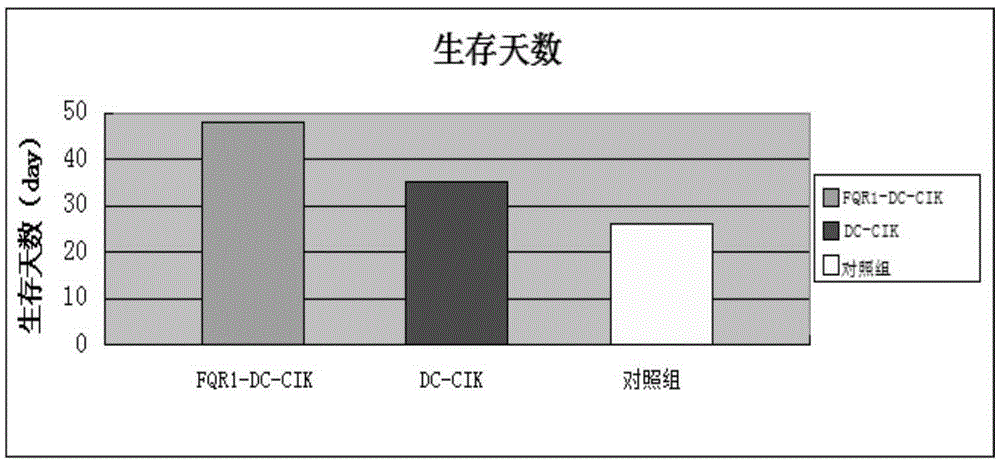
Gingseng extract product FQR1 and application thereof in tumour immunotherapy
InactiveCN105238755AEnhance phagocytosisEnhance antigen presentationBlood/immune system cellsAntineoplastic agentsAbnormal tissue growthTumour immunotherapy
The invention provides a gingseng extract product FQR1 as well as a preparation and an application thereof, and provides a FQR1 induced culture method for DC-CIK cell. The method comprises the following steps: a collection of peripheral blood, an acquisition of tumor antigens, a separation of mononuclear cells, a washing, an induction of DC-CIK cells, a culture, etc. The obtained gingseng extract product FQR1 can effectively increase propagation of DC-CIK cells, improve tumor killing activity of DC-CIK cells, and prolong the survival time with tumor of tumor-bearing mice with the DC-CIK cell therapy.
Owner:东营凤起生物科技发展有限公司
Anucleate cell-derived vaccines
PendingUS20220105166A1Decreased hemoglobin levelImprove the level ofCell dissociation methodsPeptide/protein ingredientsDiseaseAntigen delivery
The present invention provides methods for stimulating an immune response to an antigen comprising administering to an individual, an anucleate cell-derived vesicle comprising an antigen and / or an adjuvant. In some embodiments, the anucleate cell-derived vesicle comprising the antigen and / or adjuvant is generated by passing a cell suspension containing an input anucleate cell through a constriction, wherein the constriction deforms the input anucleate cell thereby causing a perturbation of the cell to form an anucleate cell-derived vesicle such that an antigen and / or an adjuvant enters the anucleate cell-derived vesicle. In some embodiments, the anucleate cell-derived vesicle comprising the antigen and / or adjuvant is delivered to an individual and the antigen is delivered to and processed in an immunogenic environment to treat a disease, prevent a disease, and / or vaccinate an individual against an antigen.
Owner:SQZ BIOTECH CO
Health care method for improving vaccine immunity effect and reducing immunity stress response of livestock and poultry
InactiveCN102908608AImprove immunityImprove autoimmunityAntibacterial agentsPeptide/protein ingredientsDiseaseLivestock
The invention relates to a health care method for improving vaccine immunity effect and reducing immunity stress response of livestock and poultry, which is an immunity-enhancing health care method implemented during vaccine inoculation of livestock and poultry. The method is specifically as follows: for each time of vaccine inoculation, the medicament taking method comprises the following specific steps of: taking a first medicament one day before immunization; taking a second medicament on the day of immunization; and taking a third medicament three days after immunization. According to the invention, the body immunity and disease resistance of livestock and poultry can be obviously enhanced, the vaccine immunization effect can be enhanced, the immunization success is ensured, and the vaccine stress response is reduced.
Owner:SHIJIAZHUANG GUANGHUA PHARMA
Culture solution and culture method of DC cells
InactiveCN113249322AFast maturing timeIncrease the number ofCulture processBlood/immune system cellsAntigenVitamin B12
The invention discloses a culture solution and a culture method of DC cells. The culture solution of the DC cells is added in batches according to the growth condition of the DC cells, namely an adherent cell culture solution, a subsequent first supplemented DC cell culture solution and a subsequent second supplemented DC cell culture solution, wherein the adherent cell culture solution comprises a serum-free DC culture medium, GM-SCF and IL-13; the subsequent first supplemented DC cell culture solution comprises a serum-free DC culture medium, GM-CSF, IL-4, an anti-CD 83 antibody and autoserum with the concentration of 1%; and the subsequent second supplemented DC cell culture solution comprises TNF-a, vitamin B12 and nicotinamide. By applying the culture solution and the culture method disclosed by the invention, the DC cells are cultured to be mature in a short time, the DC cells can be mature in only 3 days, the number and purity of the cells are high, the antigen presentation ability of the DC cells is strong, and the culture solution has an excellent anti-tumor effect.
Owner:蓝莲(杭州)生物科技有限公司
Pro-apoptotic bacterial vaccines to enhance cellular immune responses
InactiveUS8021671B2Improves vaccine efficacyDiminishing intracellular survivalAntibacterial agentsBiocideBacteroidesApoptosis
Whole-cell vaccines and methods for their use in producing protective immune responses in vertebrate hosts subsequently exposed to pathogenic bacteria. The present invention involves a method of enhancing antigen presentation by intracellular bacteria in a manner that improves vaccine efficacy. After identifying an enzyme that has an anti-apoptotic effect upon host cells infected by an intracellular microbe, the activity of the enzyme is reduced, thereby modifying the microbe so that it increases immunogenicity. Also, the present invention provides a method of incrementally modifying enzyme activity to produce incrementally attenuated mutants of the microbe from which an effective vaccine candidate can be selected.
Owner:VANDERBILT UNIV +1
Immunity enhancing therapeutic vaccine for HPV and related diseases
ActiveUS20160324952A1Easy to degradeEasy to presentViral antigen ingredientsAntibody mimetics/scaffoldsDiseaseHPV Antigen
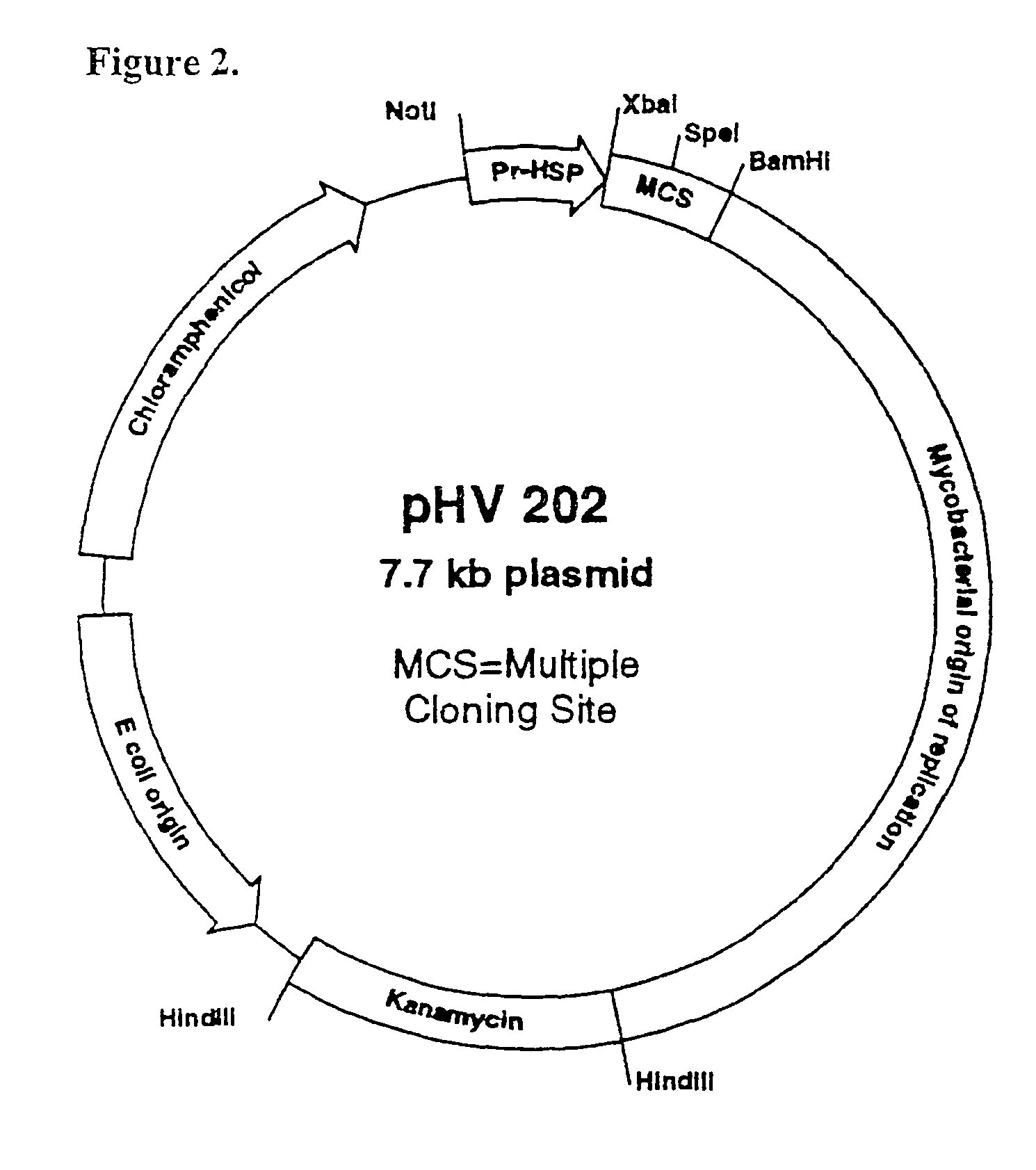
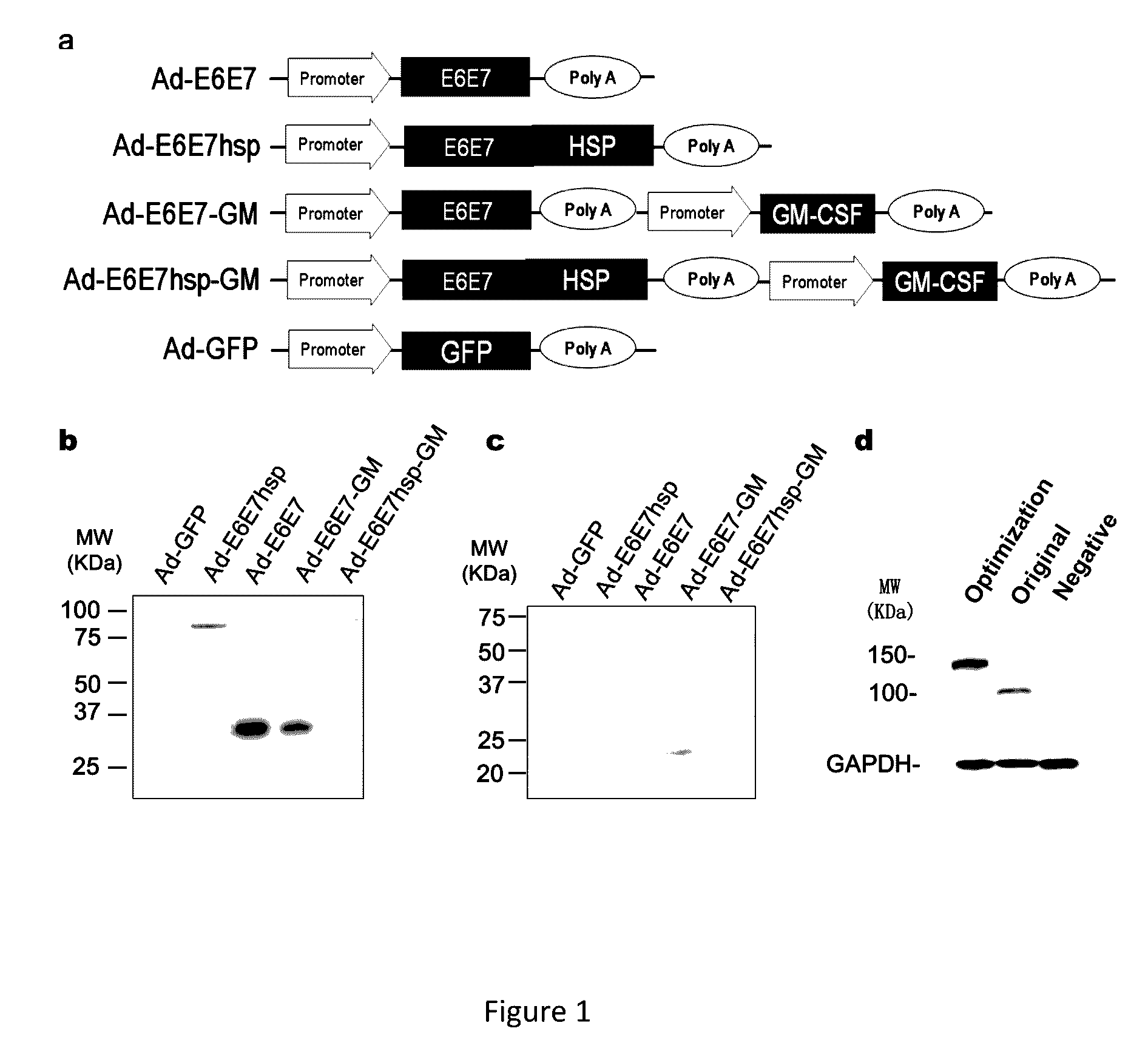
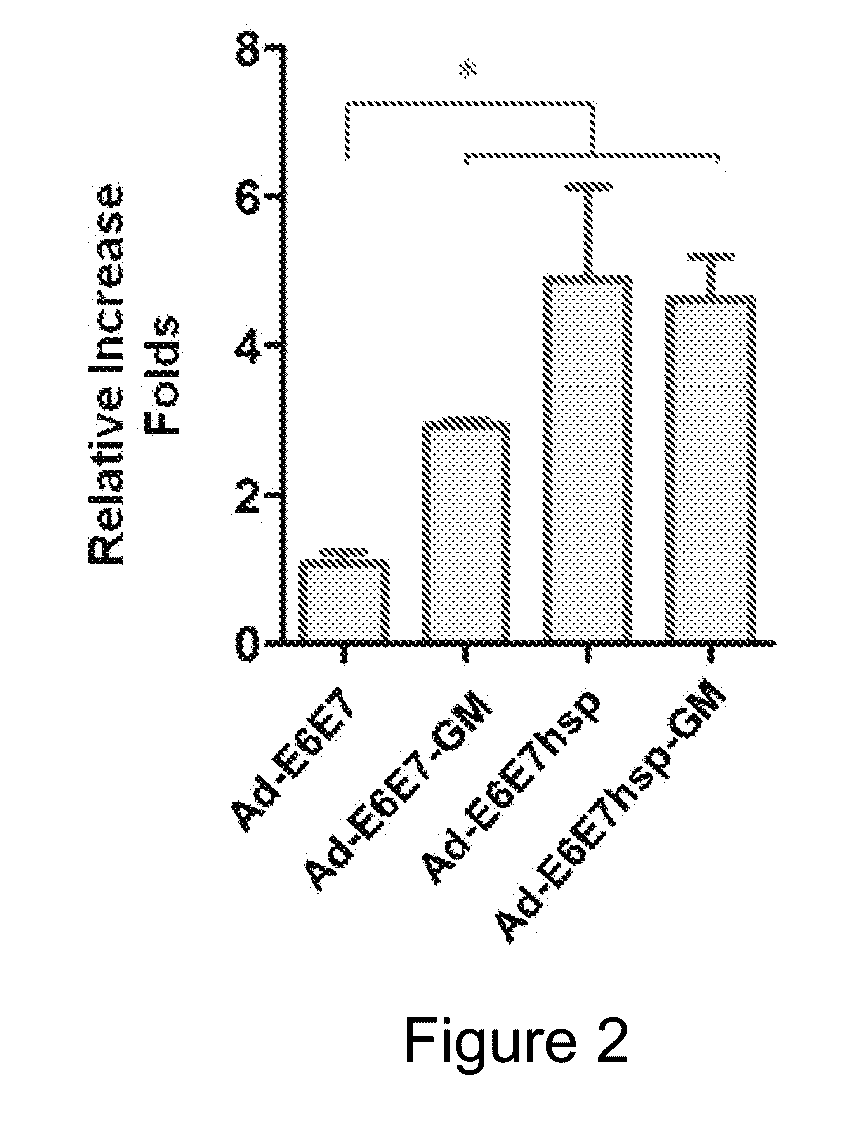
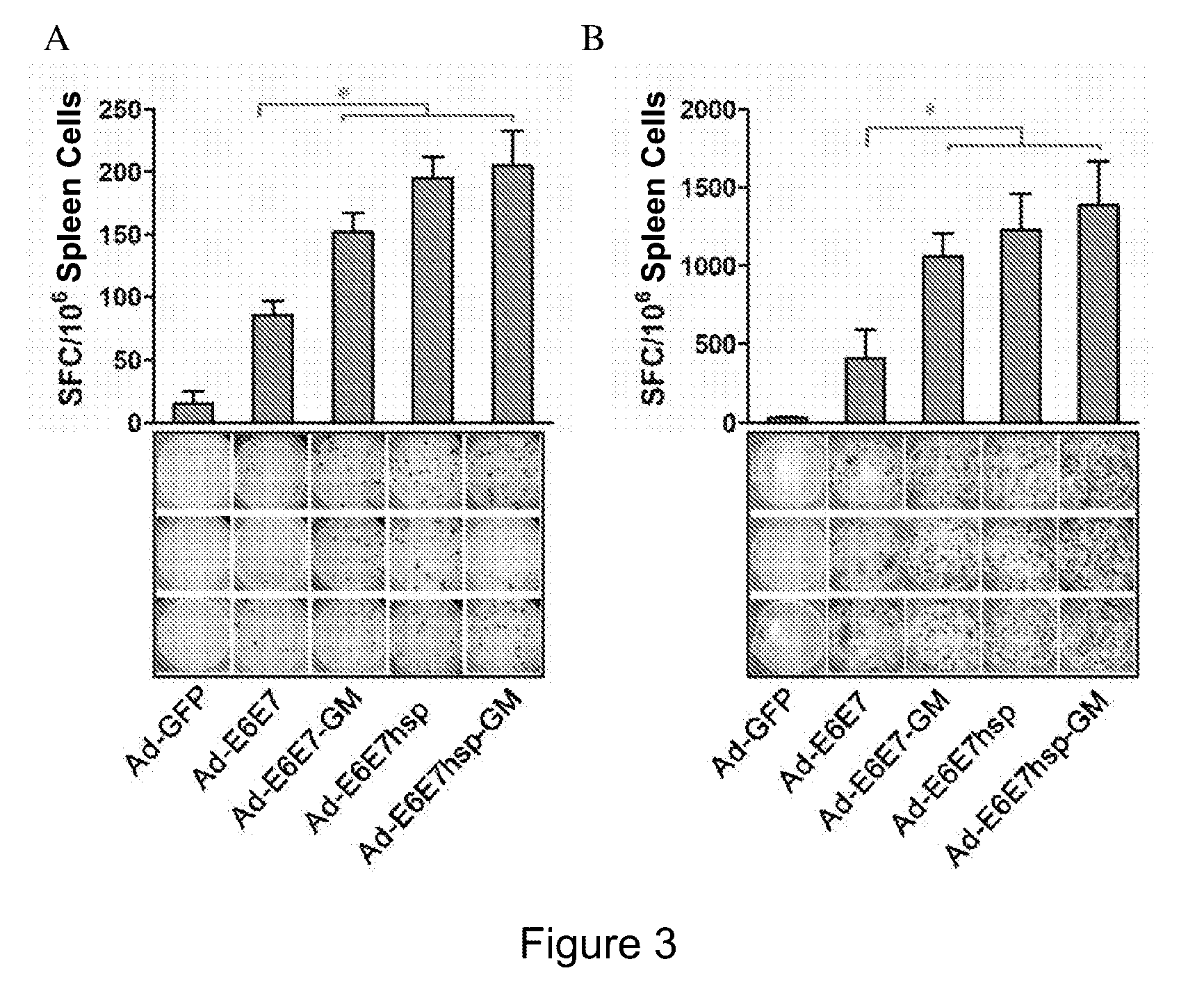
An immunity enhancing therapeutic vaccine includes a gene vector based on recombinant adenovirus and three elements: 1) an HPV antigen including the E6 and E7 multivalent fusion proteins of HPV types 16 and 18, 2) an immunologic adjuvant protein fused with said antigen, which protein may be a heat shock protein (HSP) of Mycobacterium tuberculosis, and 3) an immunostimulant, which may be granulocyte-macrophage colony stimulating factor (GM-CSF). The vaccine is used for the treatment of human papilloma virus infections and related diseases.
Owner:MYGT BIOPHARM LLC
Methods and compositions comprising an nfkb inhibitor and an adjuvant
PendingUS20210346495A1Reduce systemic inflammationIncreasing adaptive immune responseSsRNA viruses positive-senseHydroxy compound active ingredientsAdjuvantPharmaceutical drug
The current disclosure describes the use of NFkB inhibitors as immune potentiators in vaccine compositions comprising adjuvants. Accordingly, aspects of the disclosure relate to a method for vaccinating a subject comprising administering a NFkB inhibitor and an adjuvant (or a composition of the disclosure comprising NFkB and an adjuvant) to the subject. Further aspects relate to a method for inhibiting an inflammatory reaction associated with an adjuvant in a subject, the method comprising co-administering a NFkB inhibitor and an adjuvant (or a composition of the disclosure comprising NFkB and an adjuvant) to the subject. Yet further aspects relate to a pharmaceutical composition comprising a NFkB inhibitor and an adjuvant.
Owner:UNIVERSITY OF CHICAGO
DC (dendritic cell) inducer and application thereof
InactiveCN105670994AImprove cell activityIncrease value-added rateBlood/immune system cellsCell culture active agentsMultiplication rateDendritic cell
The present invention relates to a DC inducer, comprising: rhGM-CSF, GM-CSF, IL-4, rhIL-4 and IL-13. The present invention further relates to the application of the above-mentioned DC inducer in the process of inducing DC maturation. Compared with the prior art, the DC inducer provided by the present invention can increase the cell activity of dendritic cells, increase the proliferation rate and maturation rate of dendritic cells, and further improve the antigen presentation of dendritic cells.
Owner:SHEN ZHEN ISTEM REGENERATIVE MEDICINE SCI TECH CO LTD
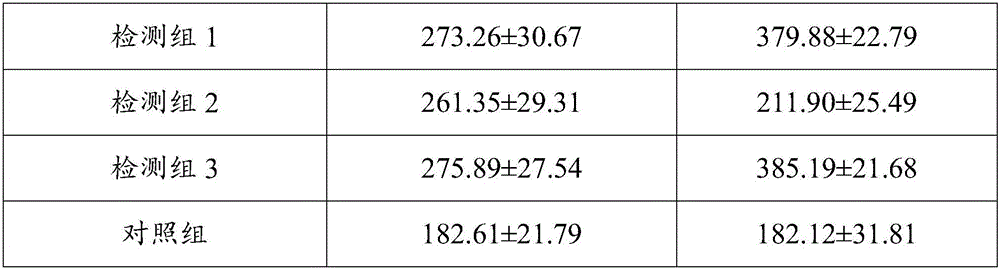
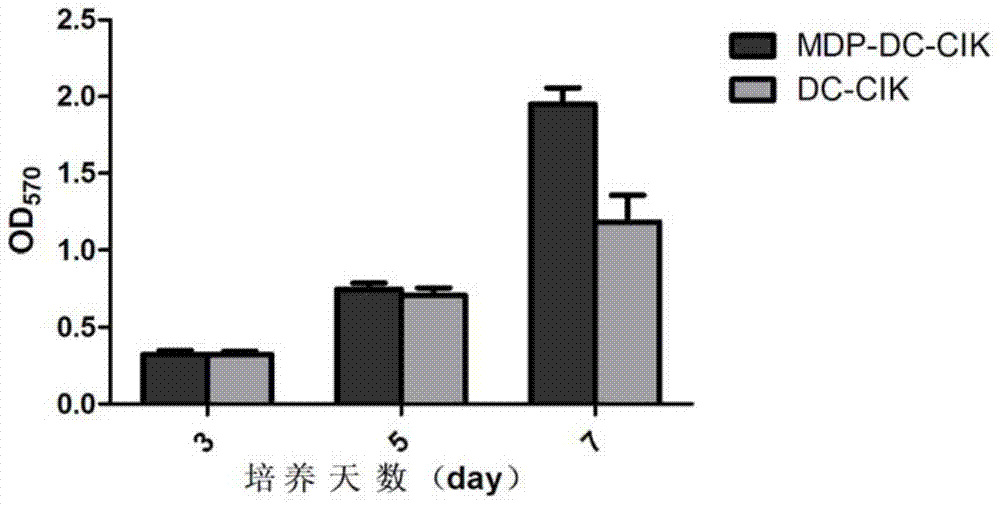
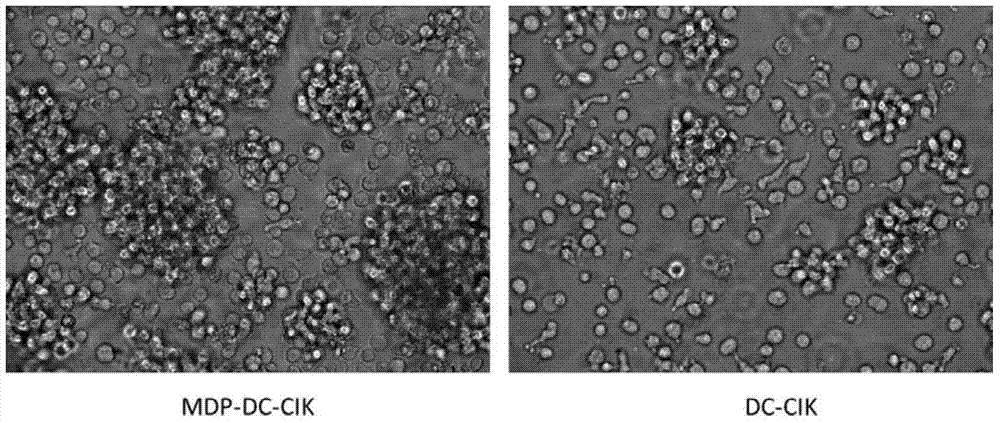
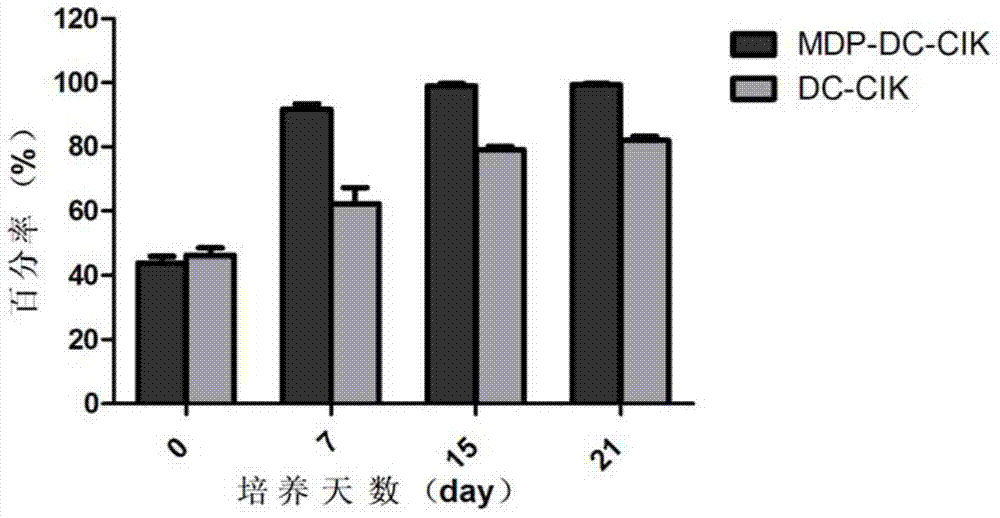
Method for inducing DC-CIK by utilizing muramyl dipeptide
ActiveCN103710308AEnhance phagocytosisEnhance antigen presentationMammal material medical ingredientsBlood/immune system cellsLaboratory Test ResultMHC restriction
The invention provides a method for inducing DC-CIK by utilizing muramyl dipeptide, that is to say, the muramyl dipeptide is added into a CIK or DC-CIK culture solution to induce proliferation and differentiation of CIK or DC-CIK cells, so as to improve the tumor-killing activity of the CIK or DC-CIK cells. The method comprises the following steps: peripheral blood collection, tumor antigen acquisition, mononuclear cell separation, mononuclear cell collection, mononuclear cell washing, MDP-DC-CIK cell induction, and culture. MDP-DC-CIK cells introduced and cultured by the method are detected by a flow cytometry, the ratio of CD3+CD56+ non-MHC restricted NKT cells is found to be up to 80% or more; and at the same time, the activity of tumor killer cells is much higher than that of DC-CIK cells by conventional induction culture, and a laboratory test result of the tumor-killing percentage reaches 99% or more.
Owner:中海峡福建细胞生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com