Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
8624results about How to "Avoid infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
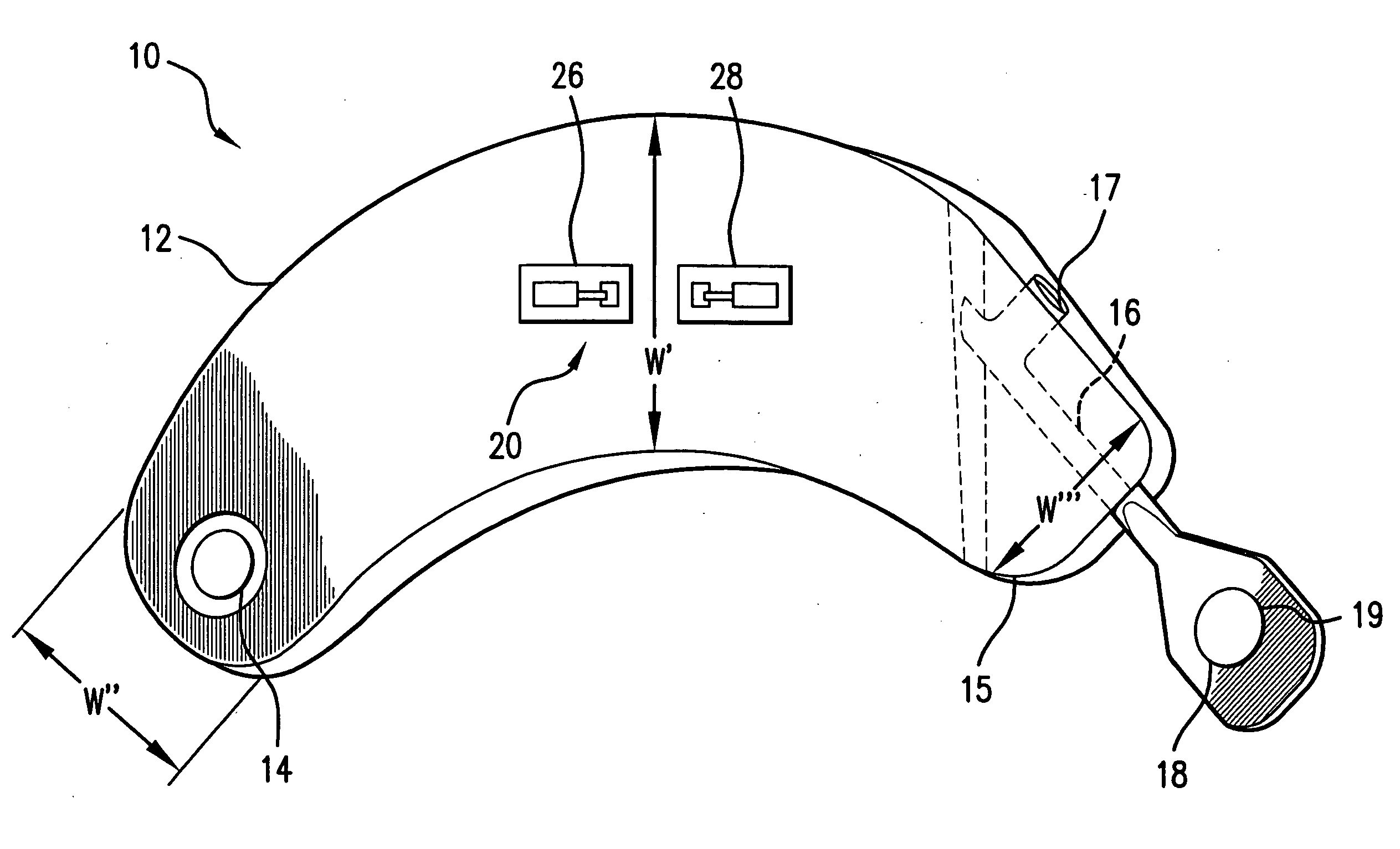
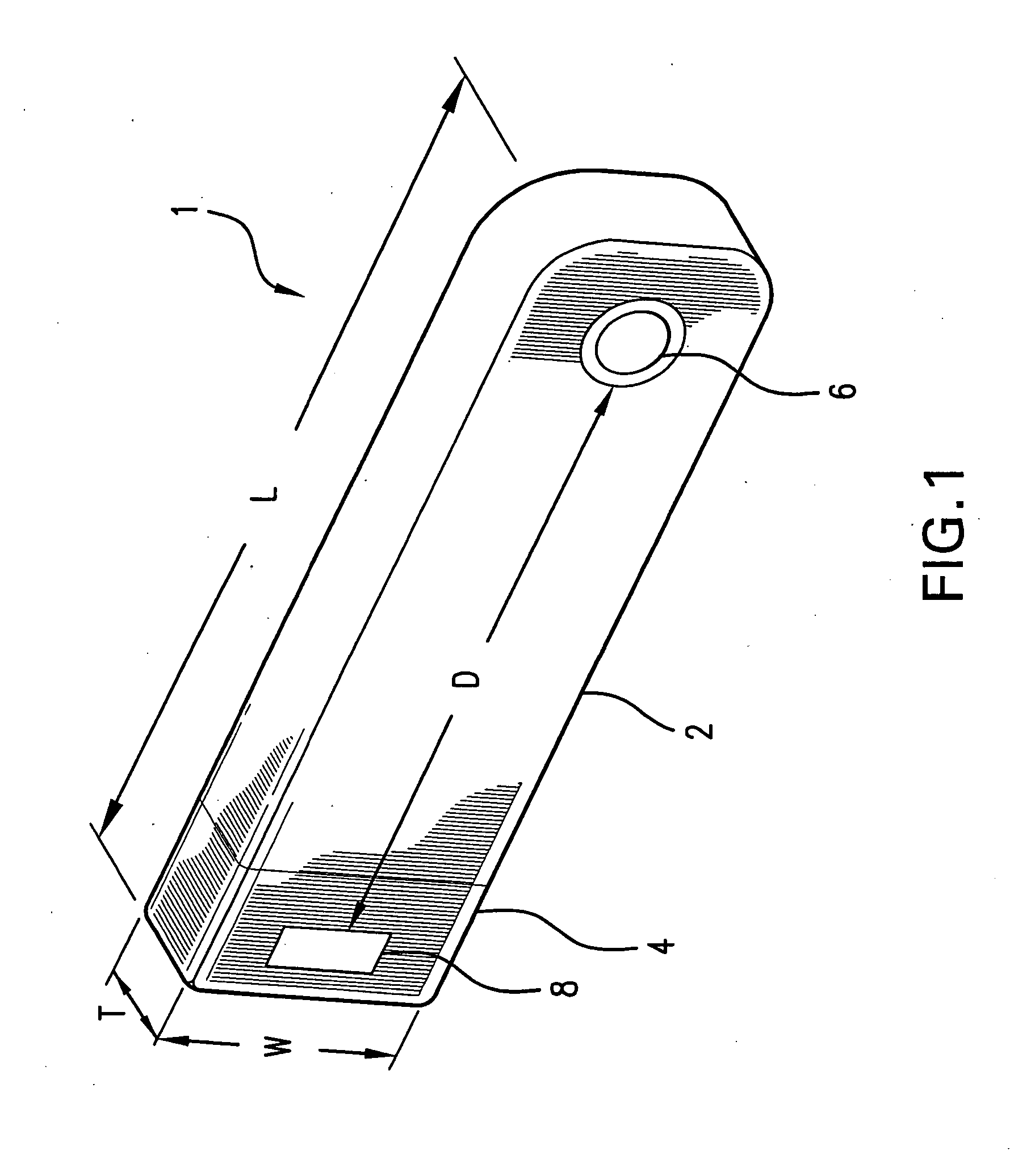
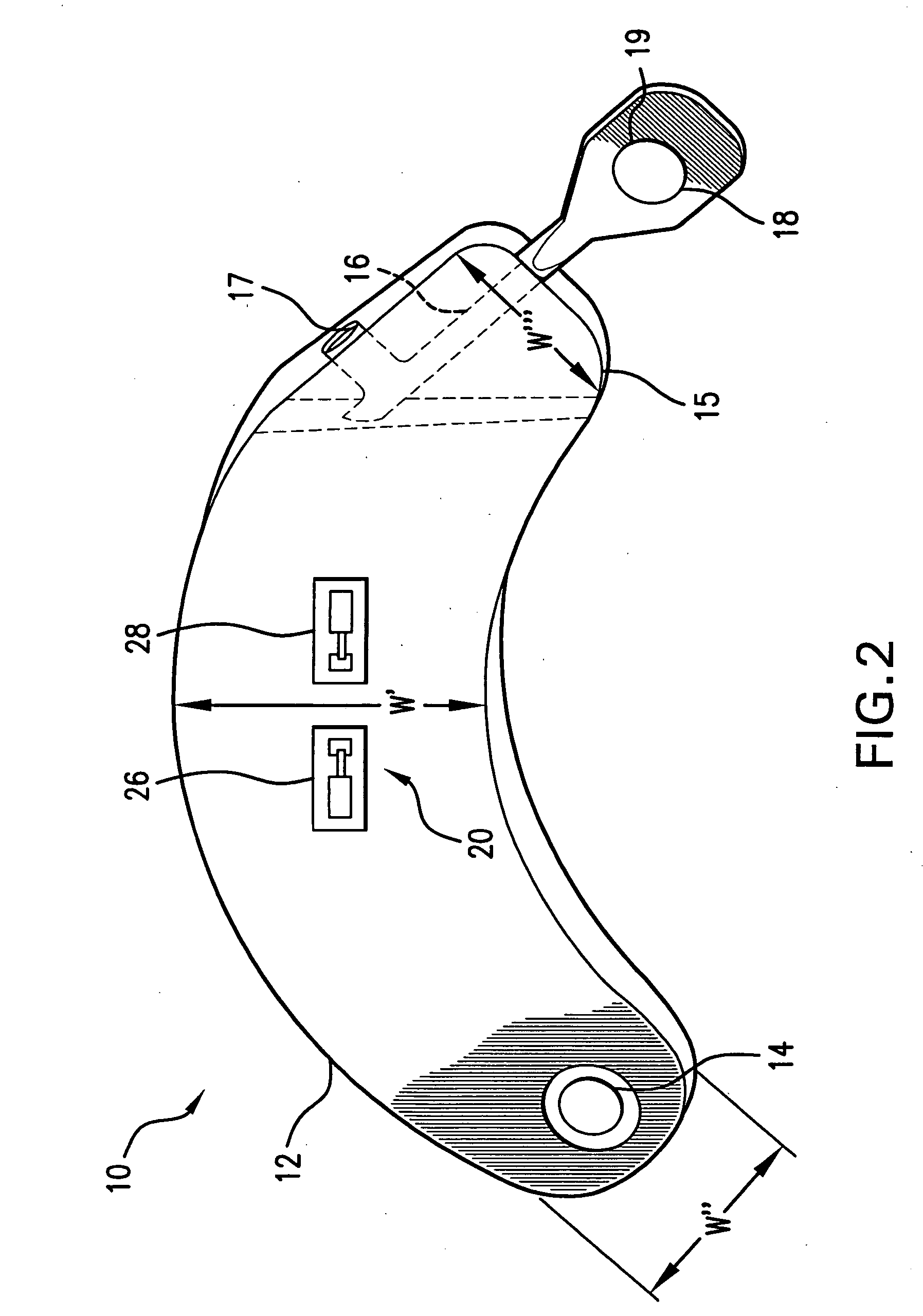
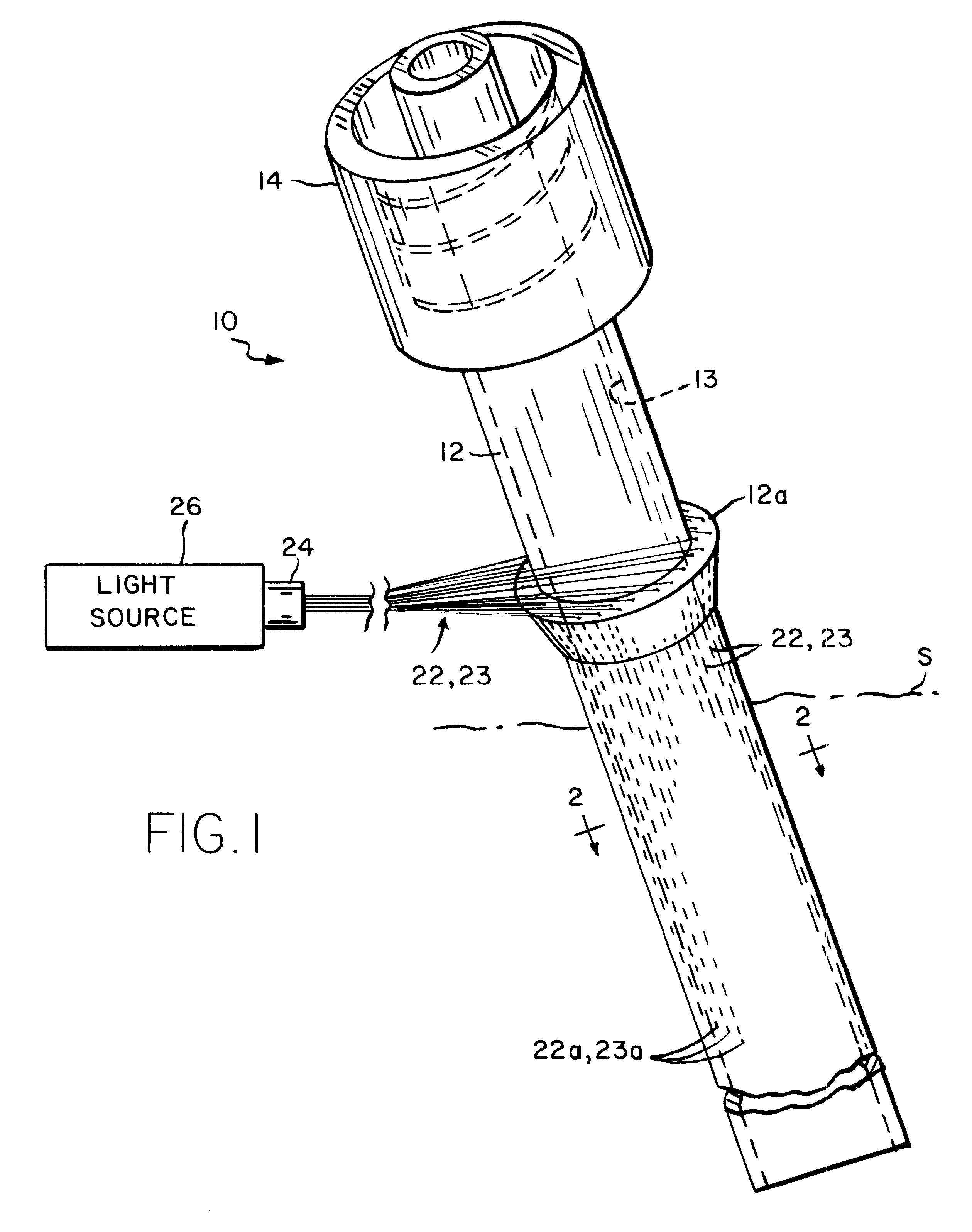
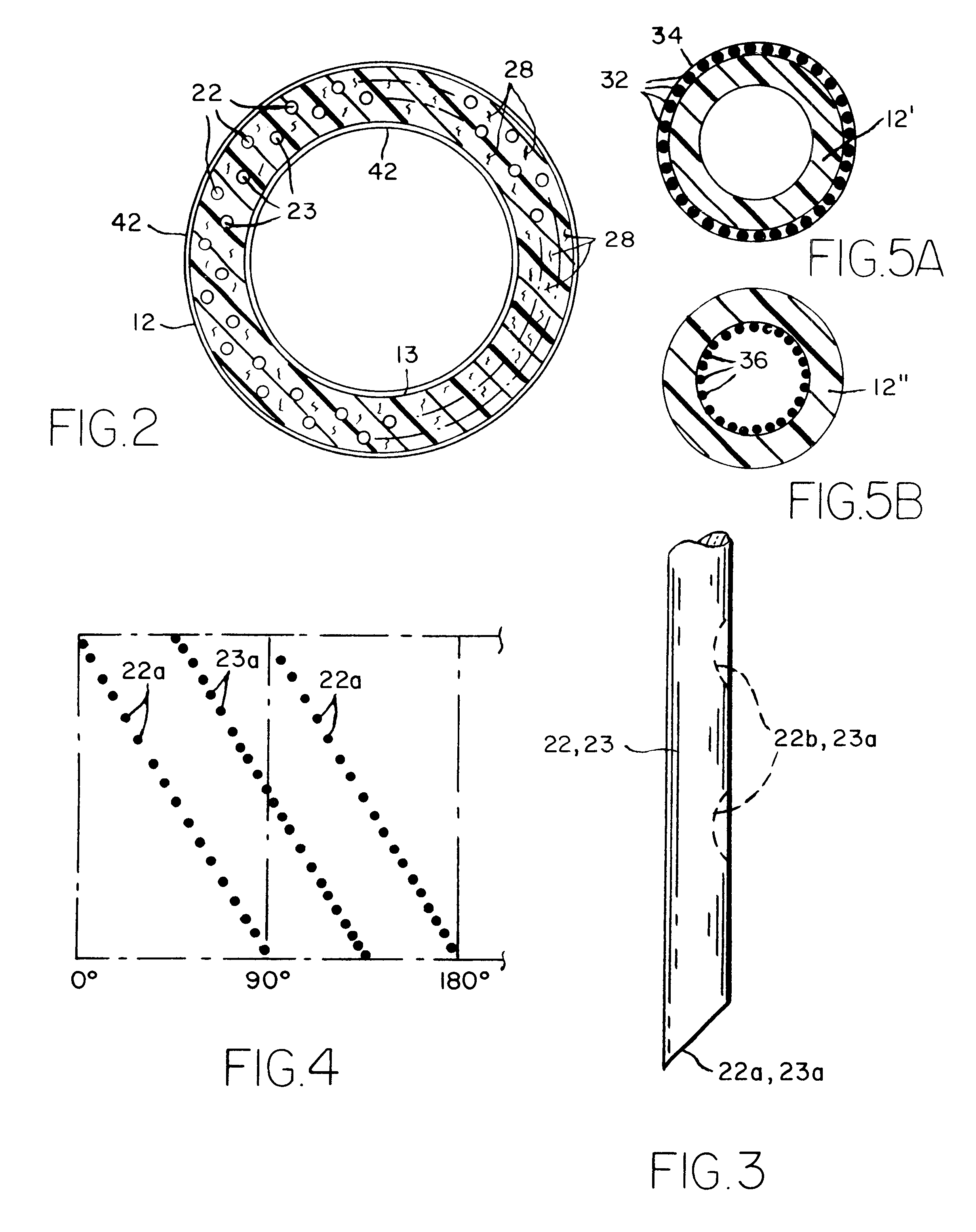
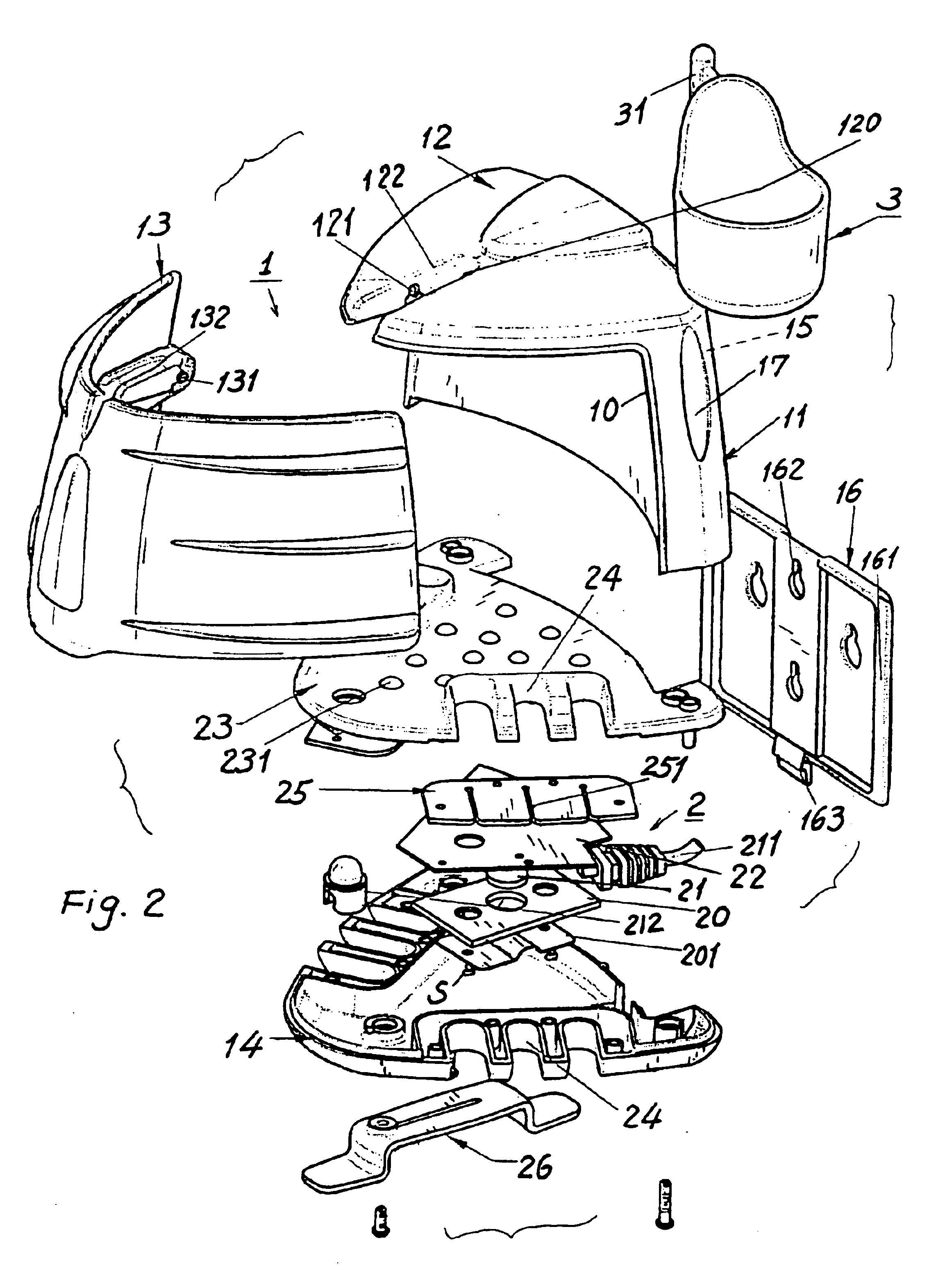
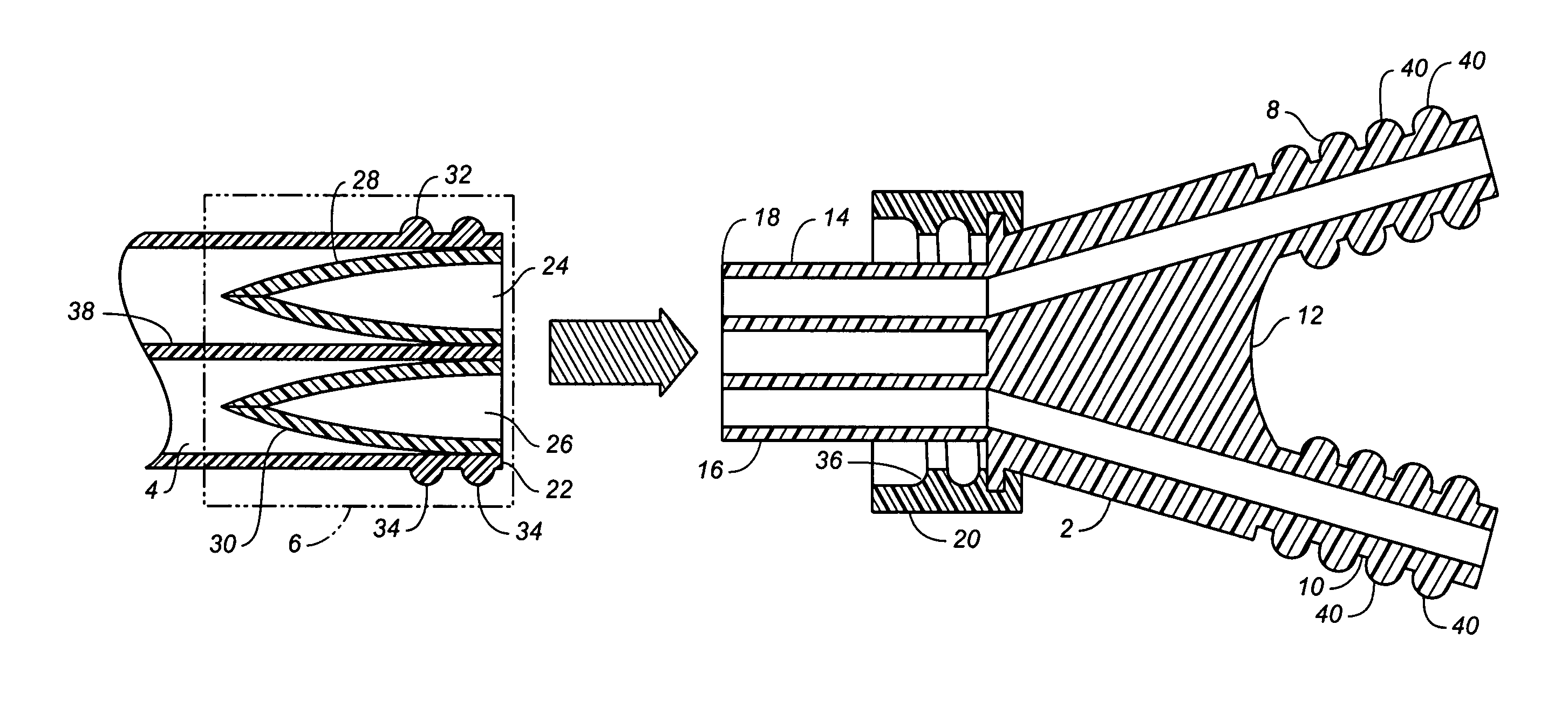
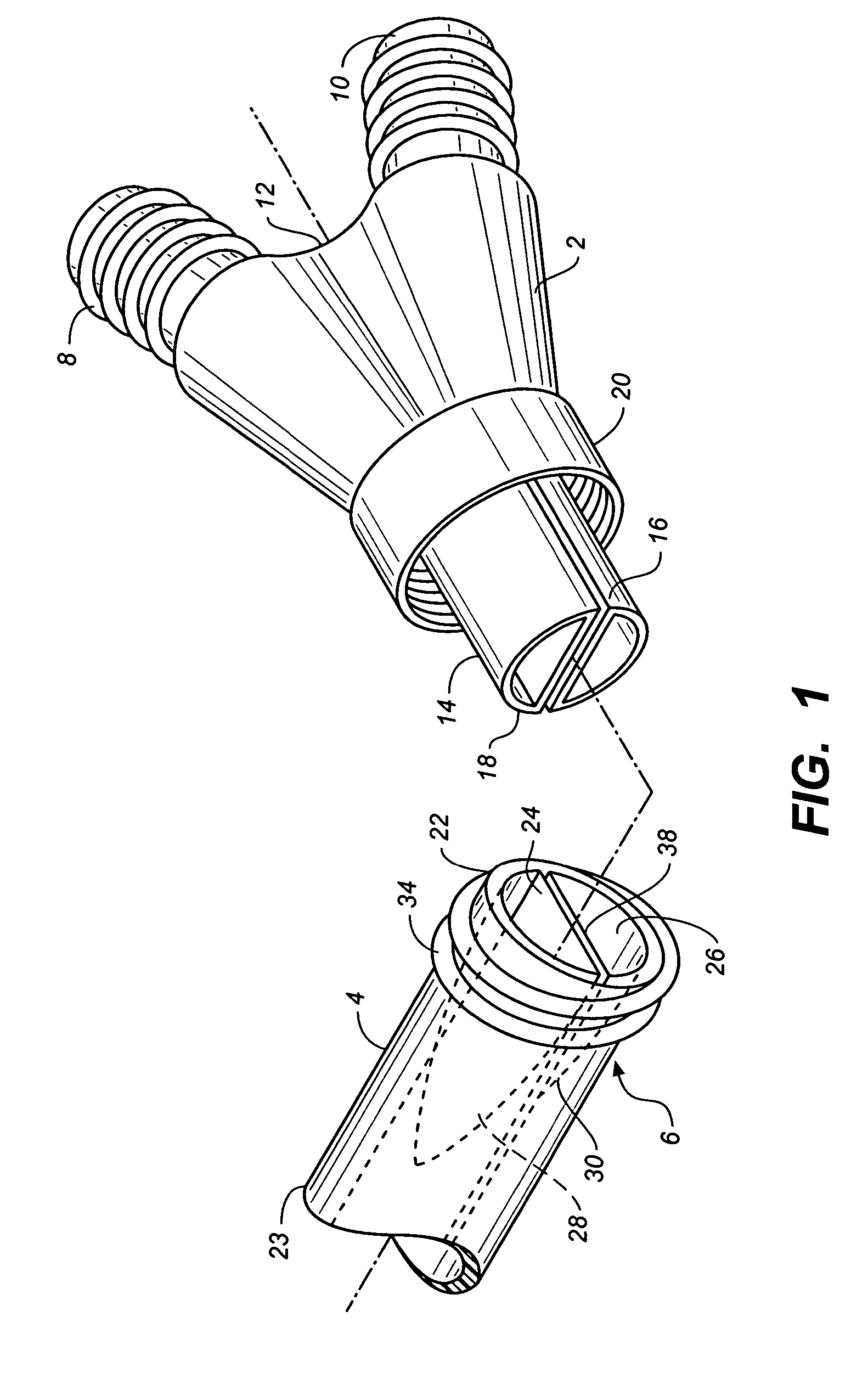
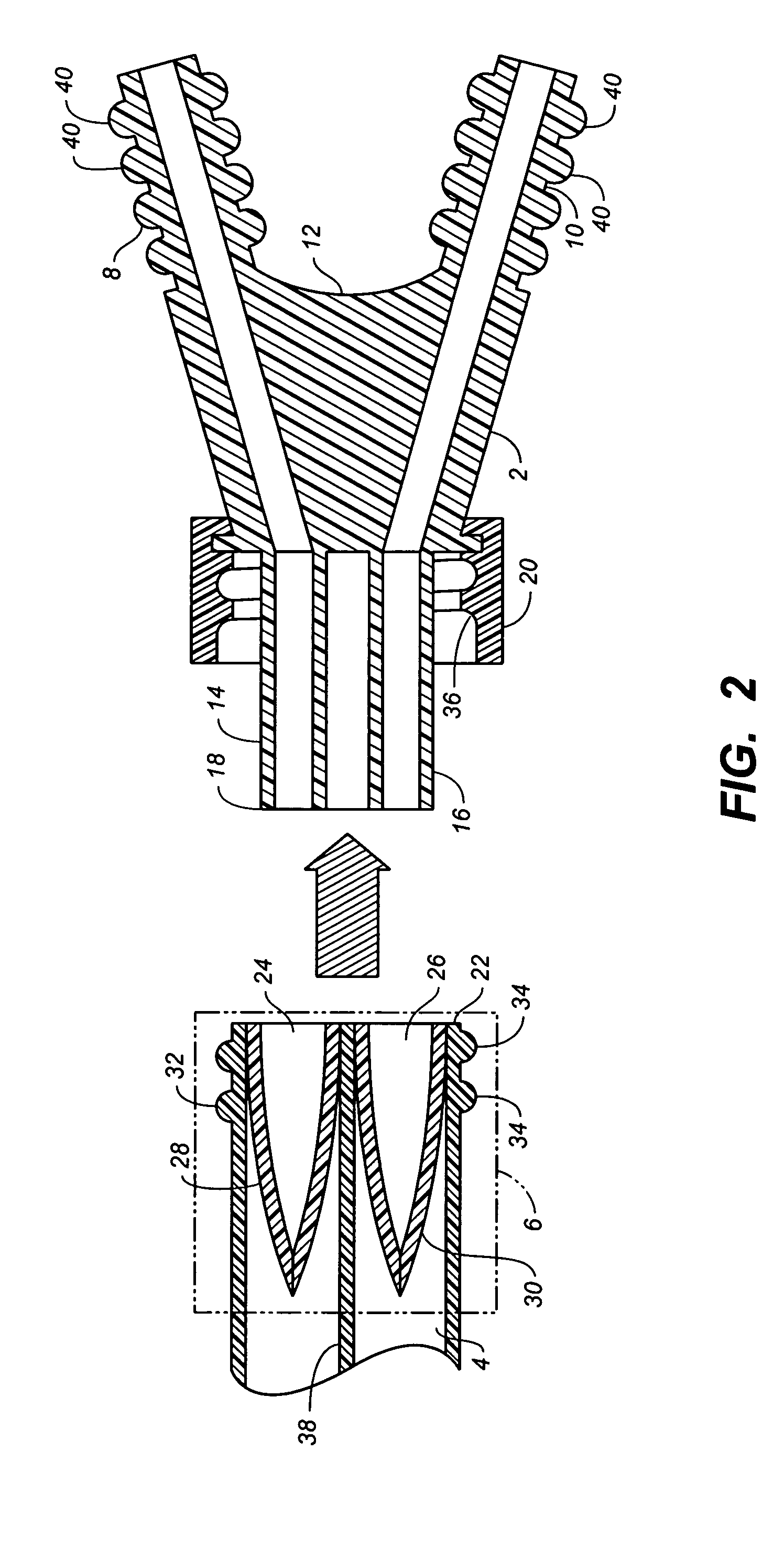
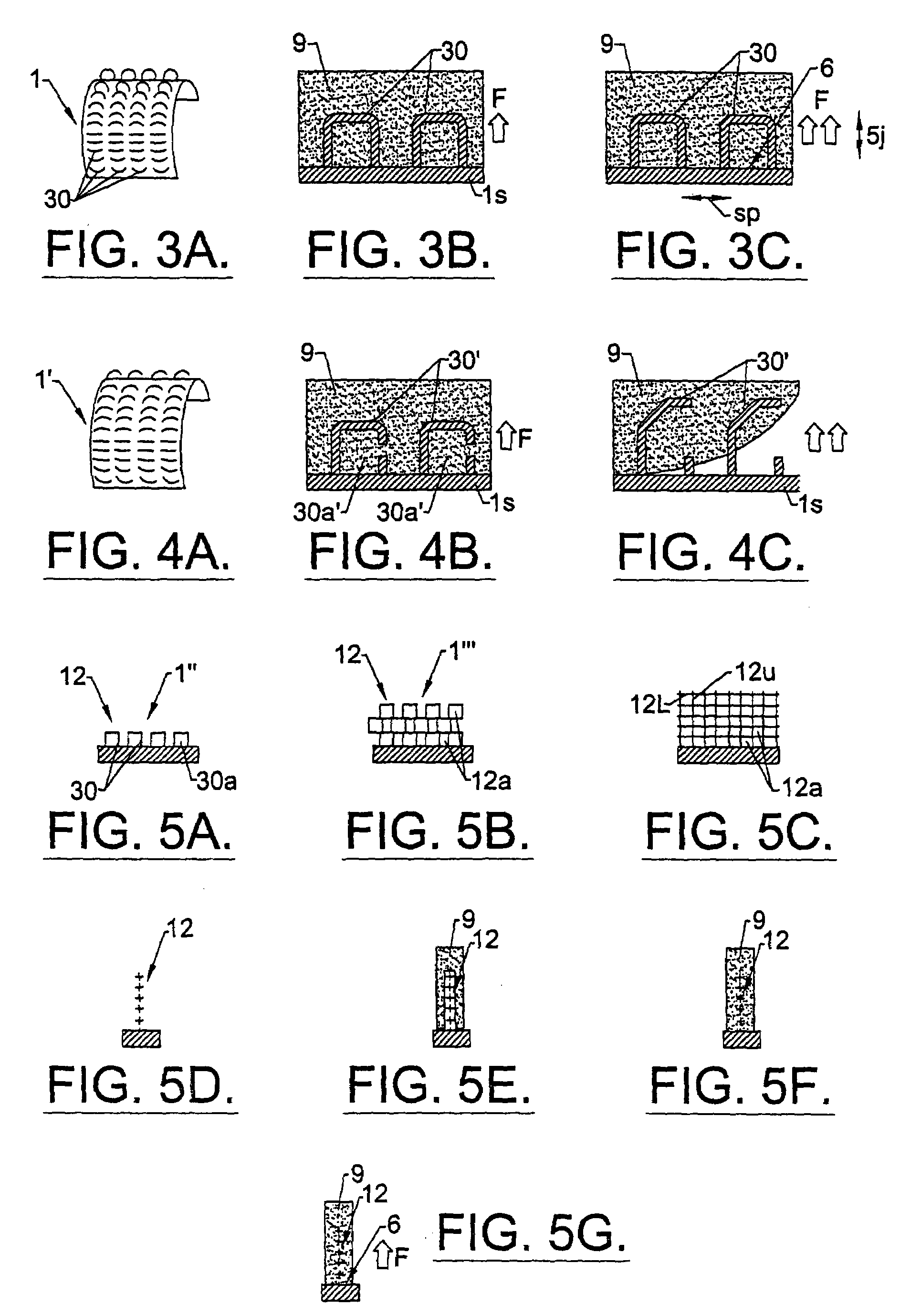
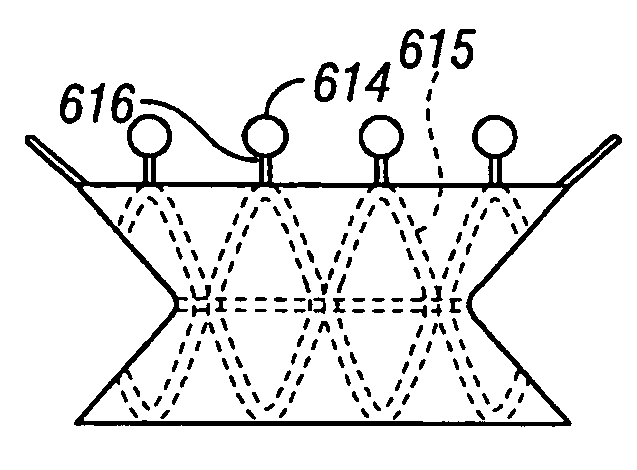
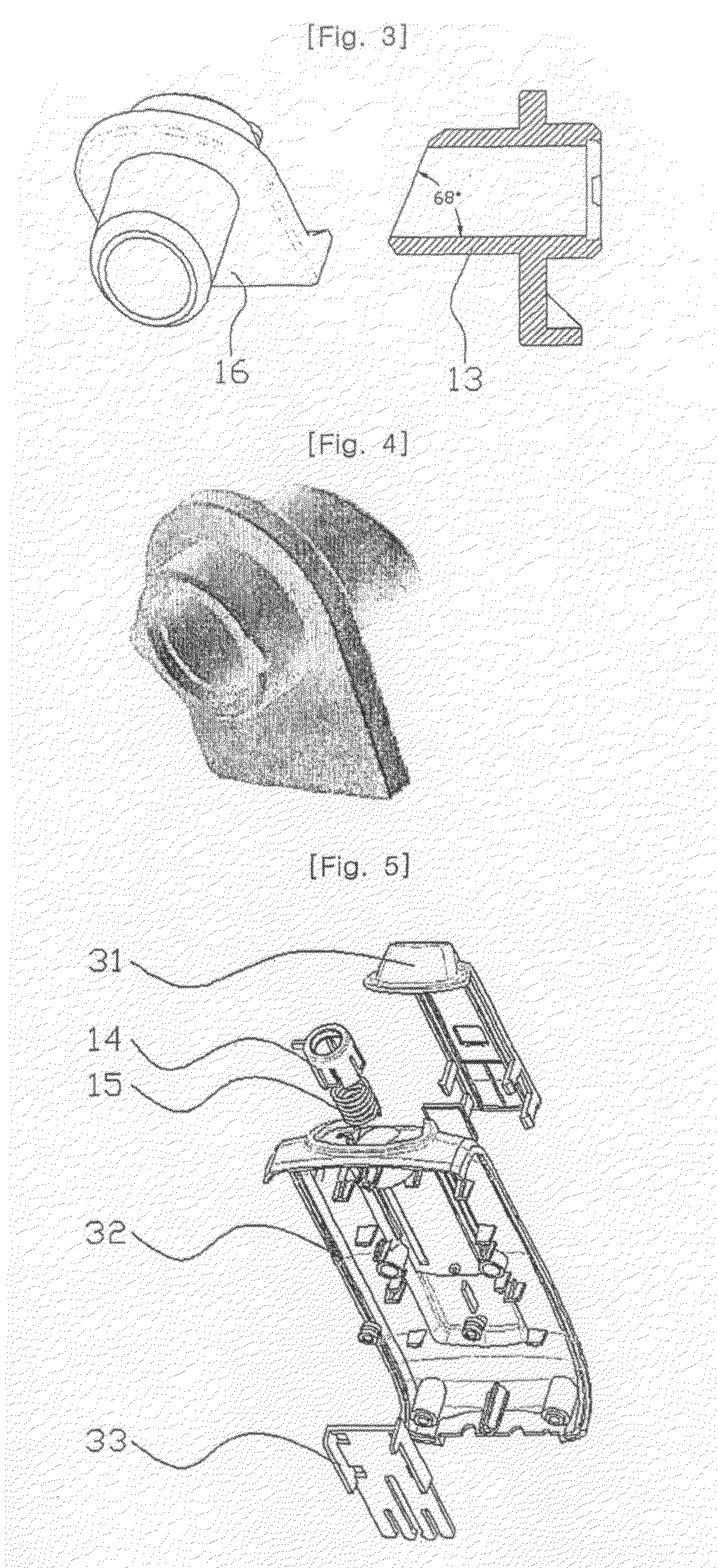
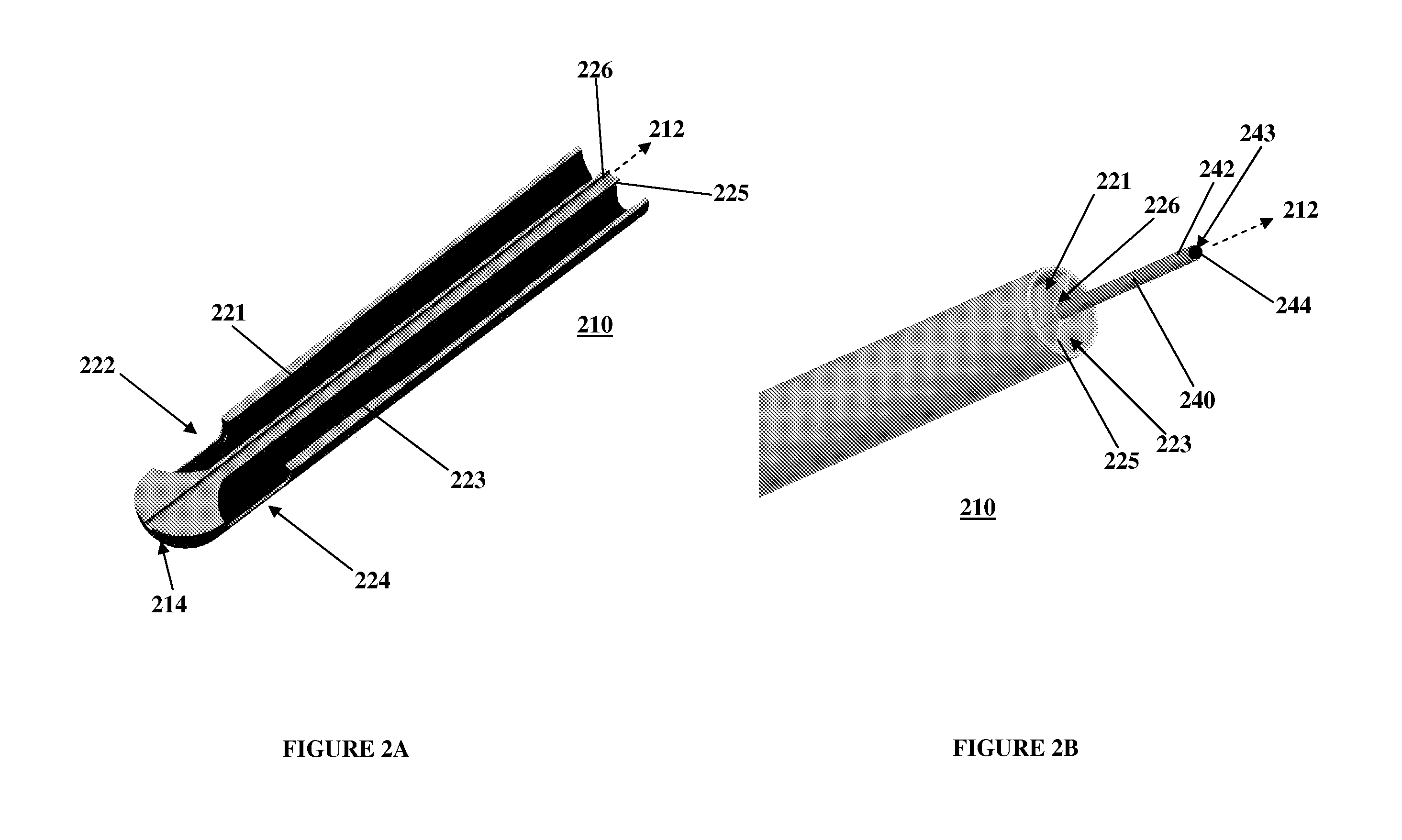
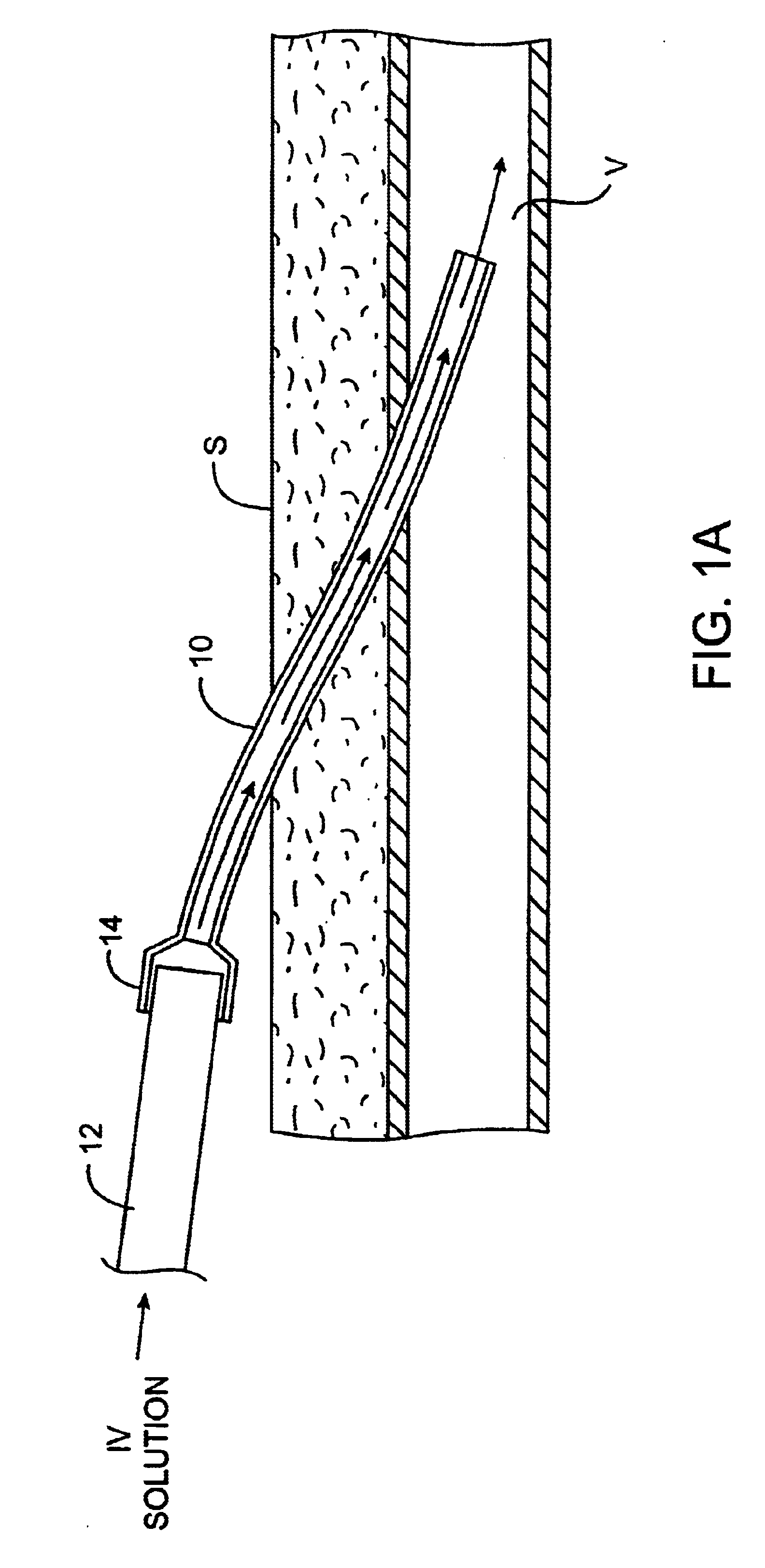
Sutures and surgical staples for anastamoses, wound closures, and surgical closures
InactiveUS8016881B2Improve featuresControlled release rateSuture equipmentsStentsSurgical stapleMicroparticle
The invention relates to sutures and surgical staples useful in anastomoses. Various aspects of the invention include wound closure devices that use amphiphilic copolymer or parylene coatings to control the release rate of an agent, such as a drug or a biological material, polymerizing a solution containing monomers and the agent to form a coating, using multiple cycles of swelling a polymer with a solvent-agent solution to increase loading, microparticles carrying the agent, biodegradable surgical articles with amphiphilic copolymer coatings, and sutures or surgical staples the deliver a drug selected from the group consisting of triazolopyrimidine, paclitaxol, sirolimus, derivatives thereof, and analogs thereof to a wound site.
Owner:MIRUS LLC
Implantable device for vital signs monitoring
InactiveUS20070016089A1Few suture requirementKeep the distanceElectrocardiographyCatheterSubcutaneous implantationDigital storage
An implantable medical device is provided for subcutaneous implantation within a human being. The implantable medical device includes a pair of electrodes for sensing electrical signals from the human being's heart. Electronic circuitry having digital memory is provided with the electronic circuitry designed to record the electrical signals from the heart. The electronics of the electronic circuitry are housed in a case having a tapered shape to facilitate implantation and removal of the implantable medical device.
Owner:ANGEL MEDICAL SYST +1
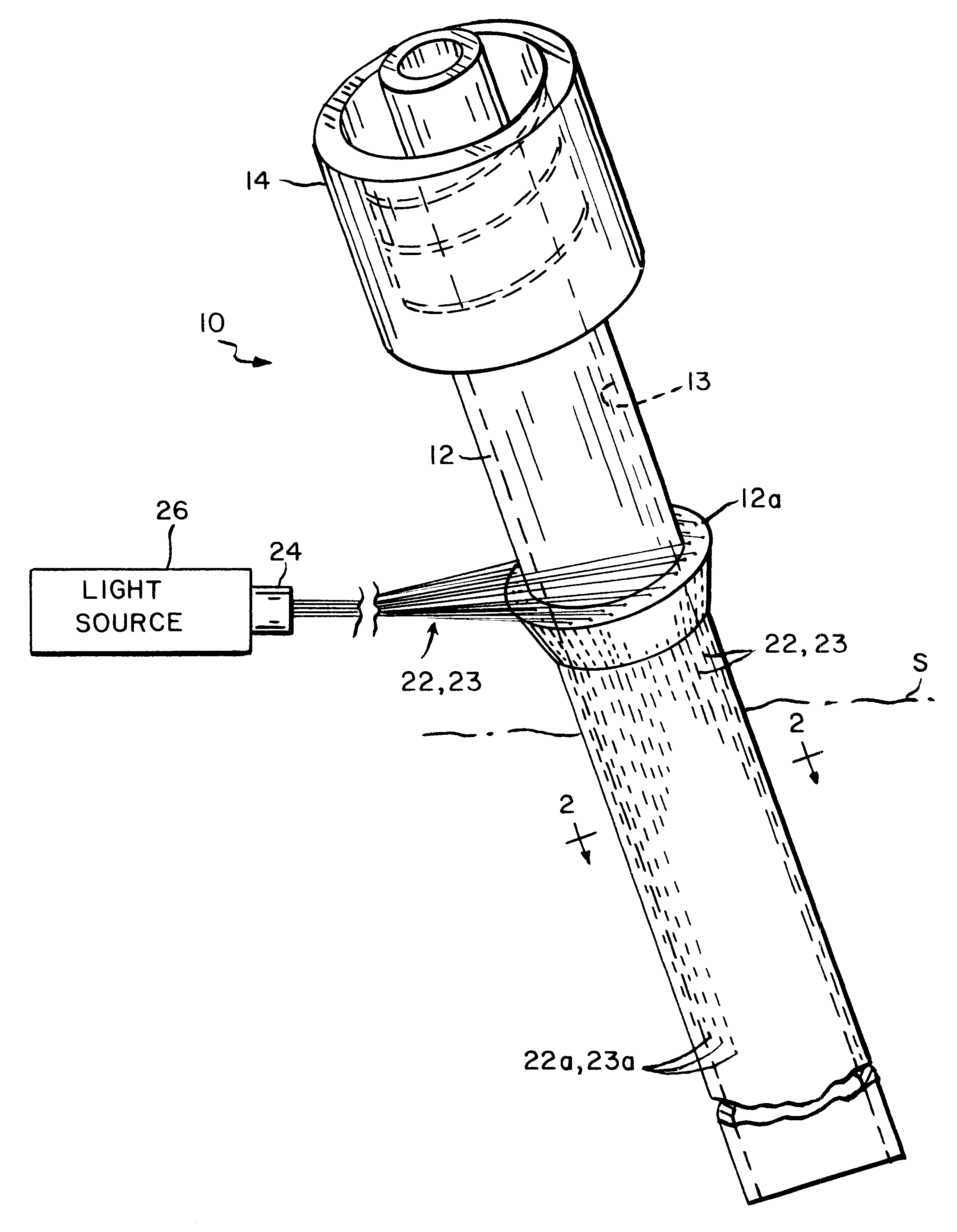
Method and apparatus to prevent infections
InactiveUS6551346B2Avoid infectionInhibition of colonizationElectrotherapySurgical instrument detailsPhotosensitizerLight energy
A photosensitizer together with complementary light energy are used to prevent the development of infection associated with an indwelling medical catheter or device. Light of a selected wavelength or wavelength band is coupled to the catheter or device and transmitted by a wall or walls thereof to one or both of the external and internal surfaces thereof. The catheter or device also incorporates at least one photosensitizer which releases a toxic substance when activated by the light energy which destroys bacteria on or around the catheter or device. A method of preventing infection using photosensitizers and complementary light energy is also disclosed.
Owner:CHAMPION ENT PROD INC
Toothbrush dryer
InactiveUS6874247B1Avoid contaminationAvoid infectionAgriculture tools and machinesWash-standsEngineeringContamination
A toothbrush dryer includes a housing formed as a heating chamber for storing toothbrush in the housing, and a heating device provided in the housing preferably at a bottom portion of the housing for warming the toothbrush for keeping a dried toothbrush for preventing bacteria, fungi or algae infection or contamination.
Owner:HSU TSANG HUNG
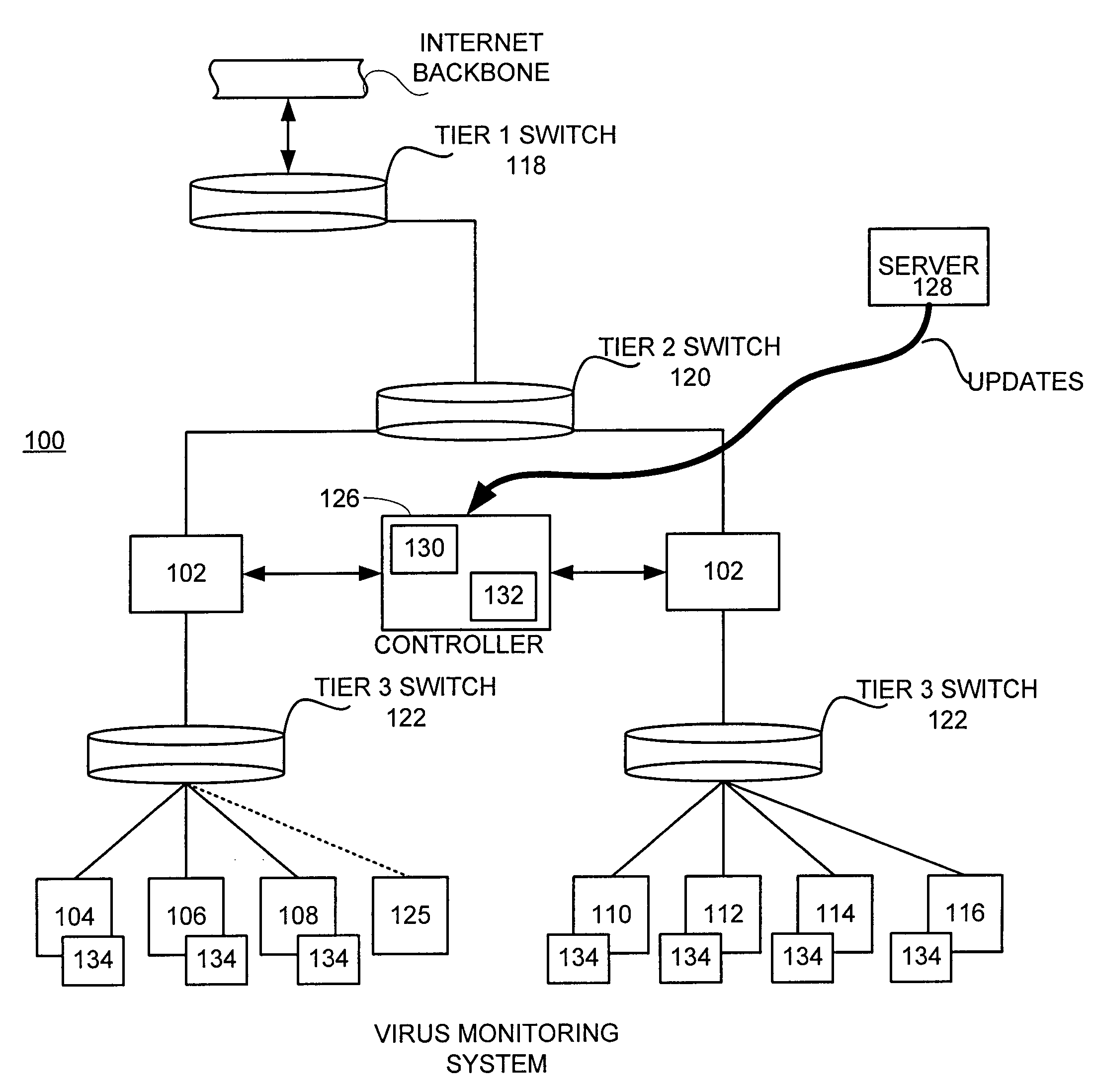
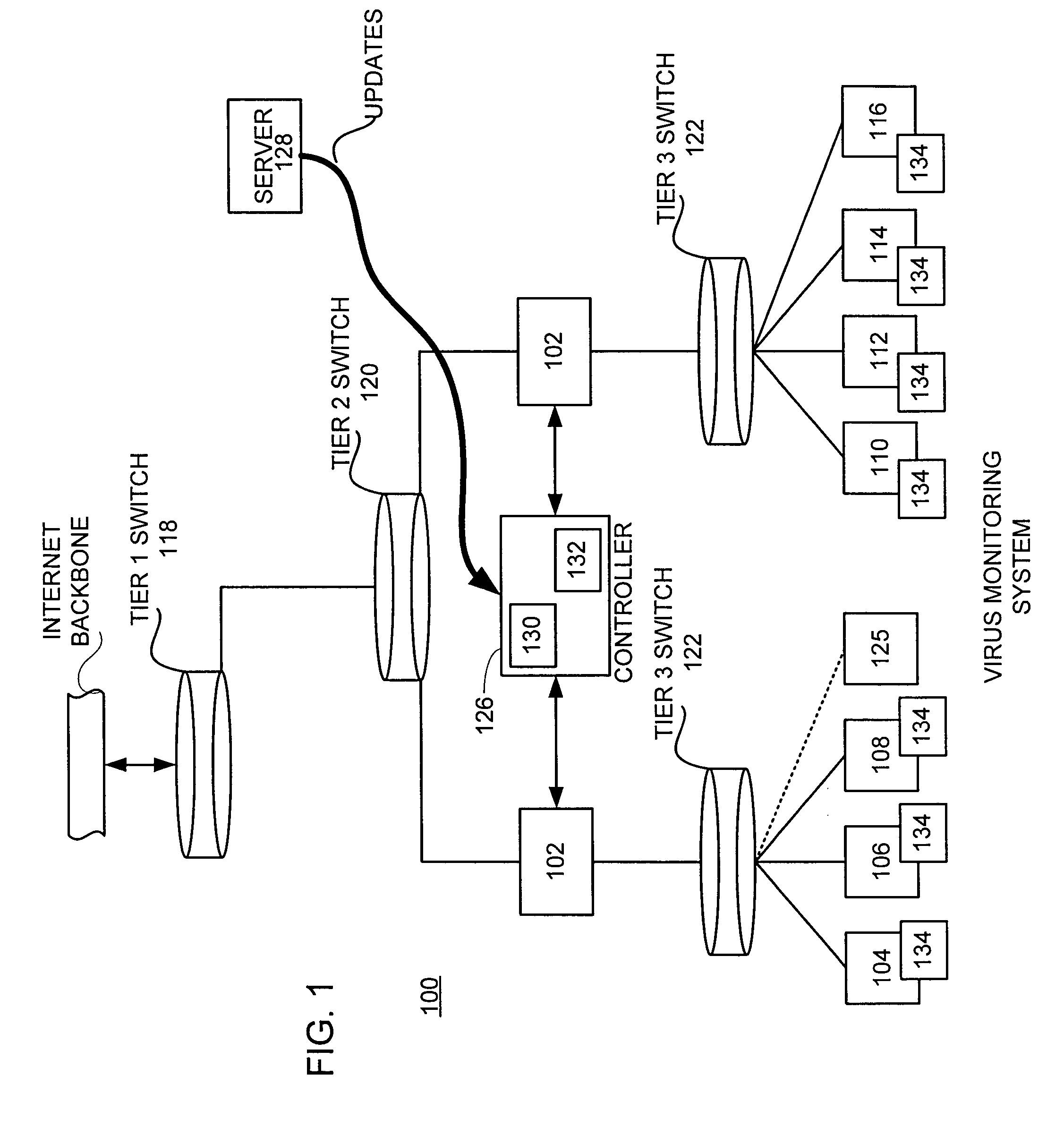
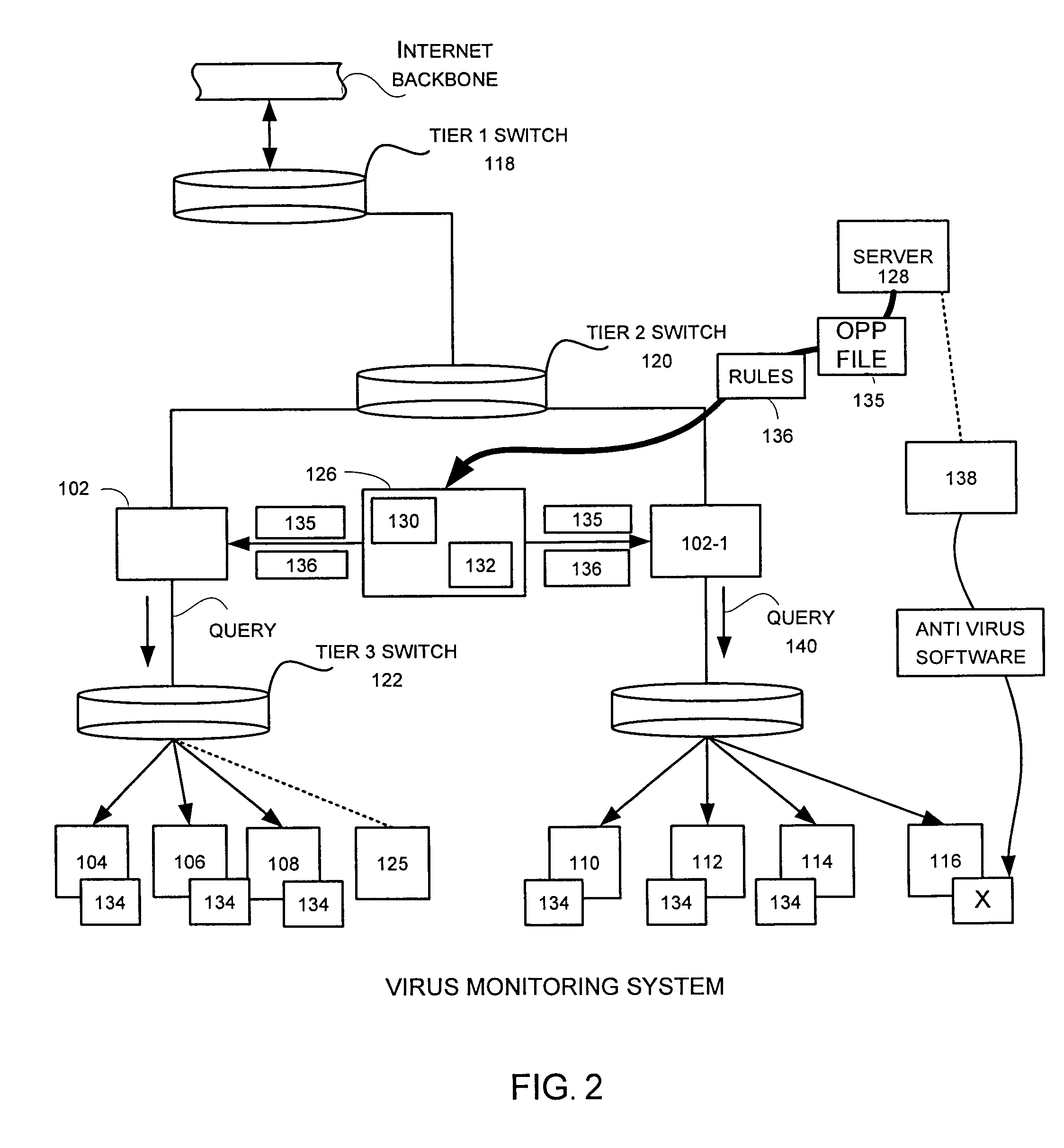
Inoculation of computing devices against a selected computer virus
ActiveUS7287278B2Avoid infectionMemory loss protectionUnauthorized memory use protectionComputer scienceVirus
In a distributed network having a number of server computers and associated client devices, method of creating an anti-computer virus agent is described. As a method, the inoculation is carried out by parsing a selected computer virus into a detection module that identifies a selected one of the client devices as a target client device, an infection module that causes the virus to infect those target client devices not infected by the selected virus, and a viral code payload module that infects the targeted client device modifying the infection module to infect those computers already infected by the selected virus; and incorporating inoculation viral code in the payload module that acts to prevent further infection by the selected virus.
Owner:TREND MICRO INC
Catheter with removable extension
ActiveUS20050256461A1Easy to disengageAvoid infectionInfusion devicesCatheterGuide tubeCatheter valves
Catheter valve assemblies and methods for connecting catheters and / or providing fluid access to catheters. In one variation, a device comprises a catheter valve assembly and an extension leg unit. The extension leg unit includes lumen inserts for engaging valves positioned within the catheter valve assembly. In another variation, the valve assembly comprises a depressable plunger which may be engaged by an access cannula. Various connectors with integrated valve assemblies are also disclosed.
Owner:CR BARD INC
Sutures and surgical staples for anastamoses, wound closures, and surgical closures
ActiveUS20050038472A1Good storage stabilityRemove moistureSuture equipmentsPowder deliverySurgical stapleMicroparticle
The invention relates to sutures and surgical staples useful in anastomoses. Various aspects of the invention include wound closure devices that use amphiphilic copolymer or parylene coatings to control the release rate of an agent, such as a drug or a biological material, polymerizing a solution containing monomers and the agent to form a coating, using multiple cycles of swelling a polymer with a solvent-agent solution to increase loading, microparticles carrying the agent, biodegradable surgical articles with amphiphilic copolymer coatings, and sutures or surgical staples the deliver a drug selected from the group consisting of triazolopyrimidine, paclitaxol, sirolimus, derivatives thereof, and analogs thereof to a wound site.
Owner:MIRUS LLC
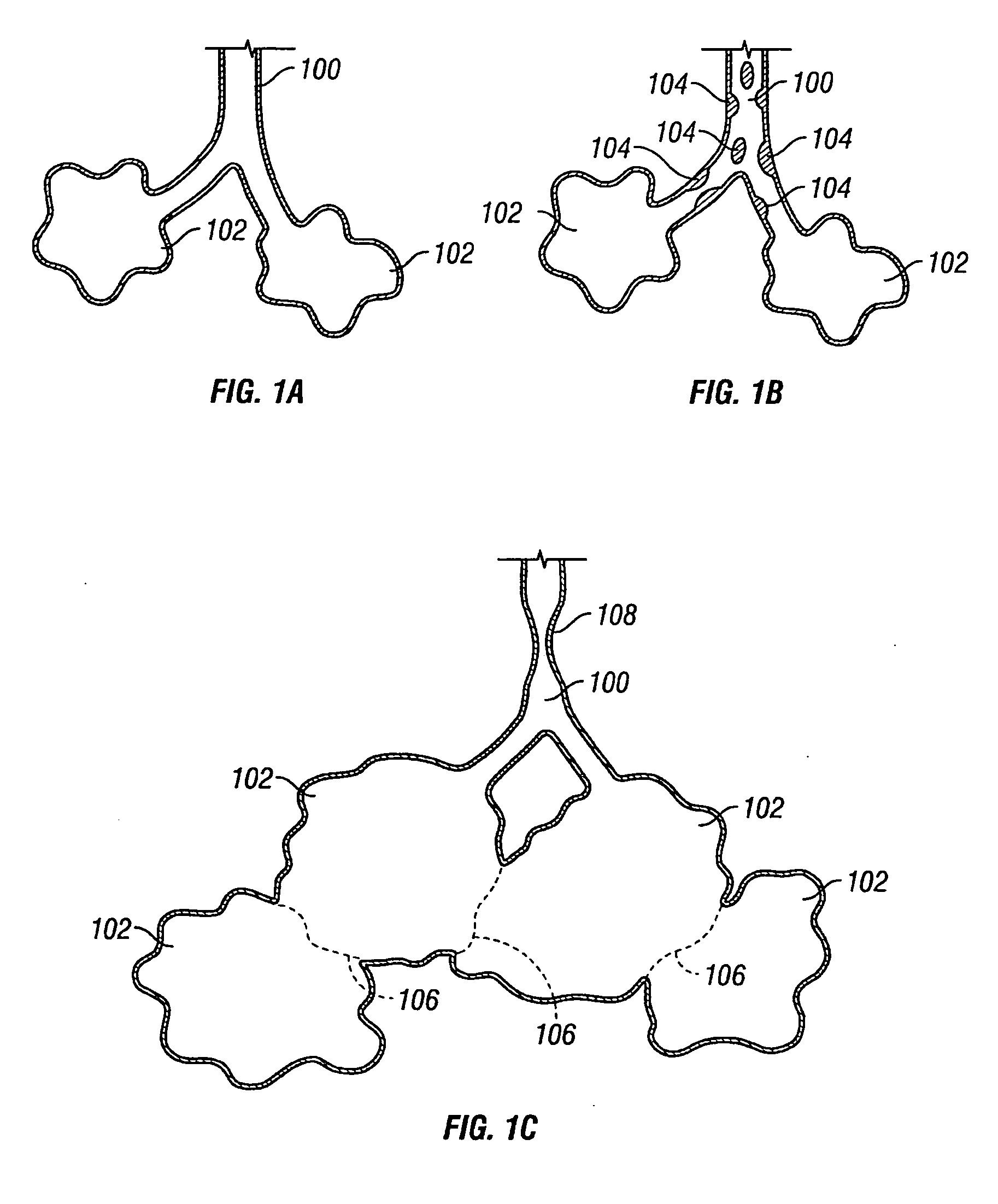
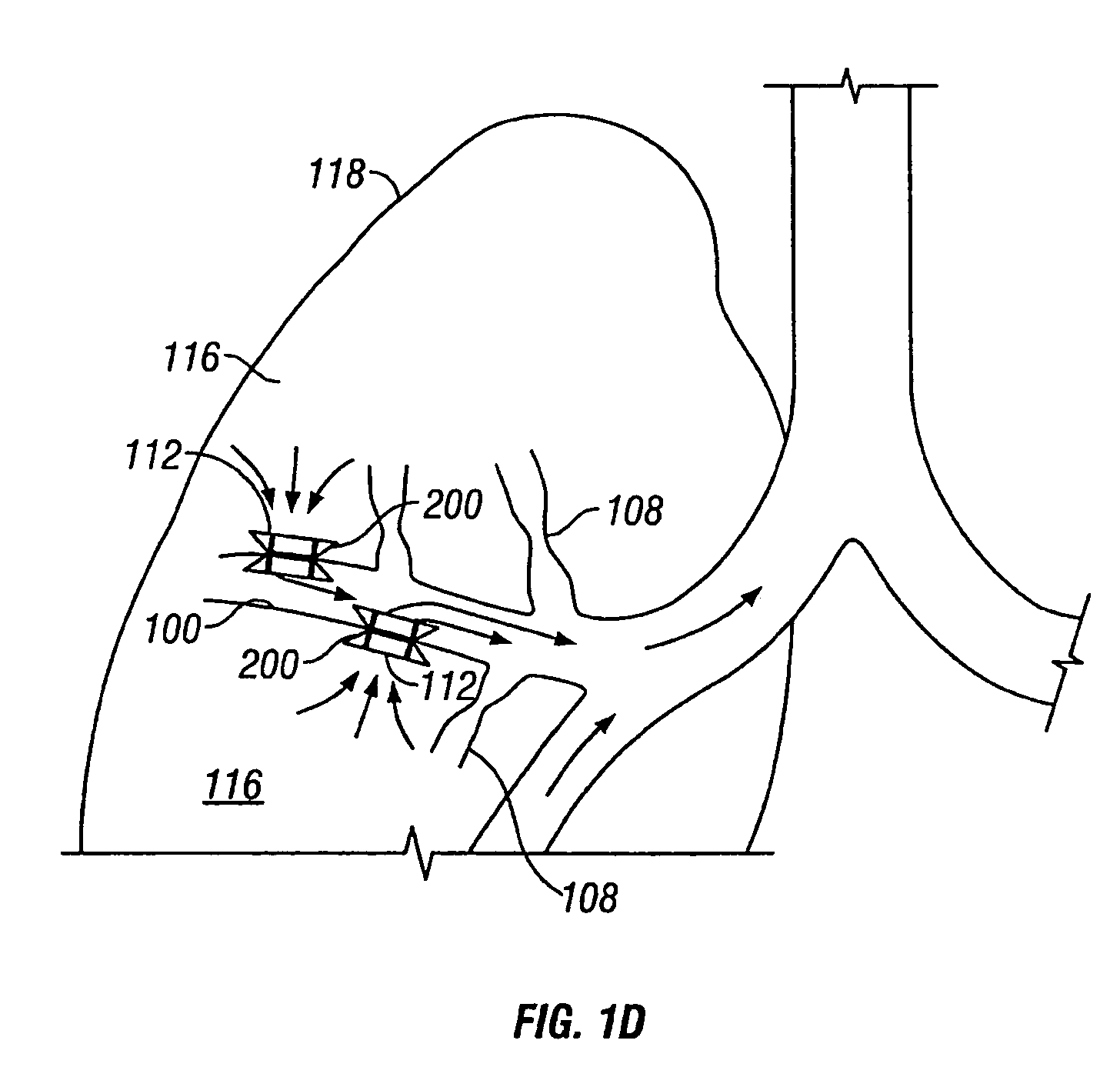
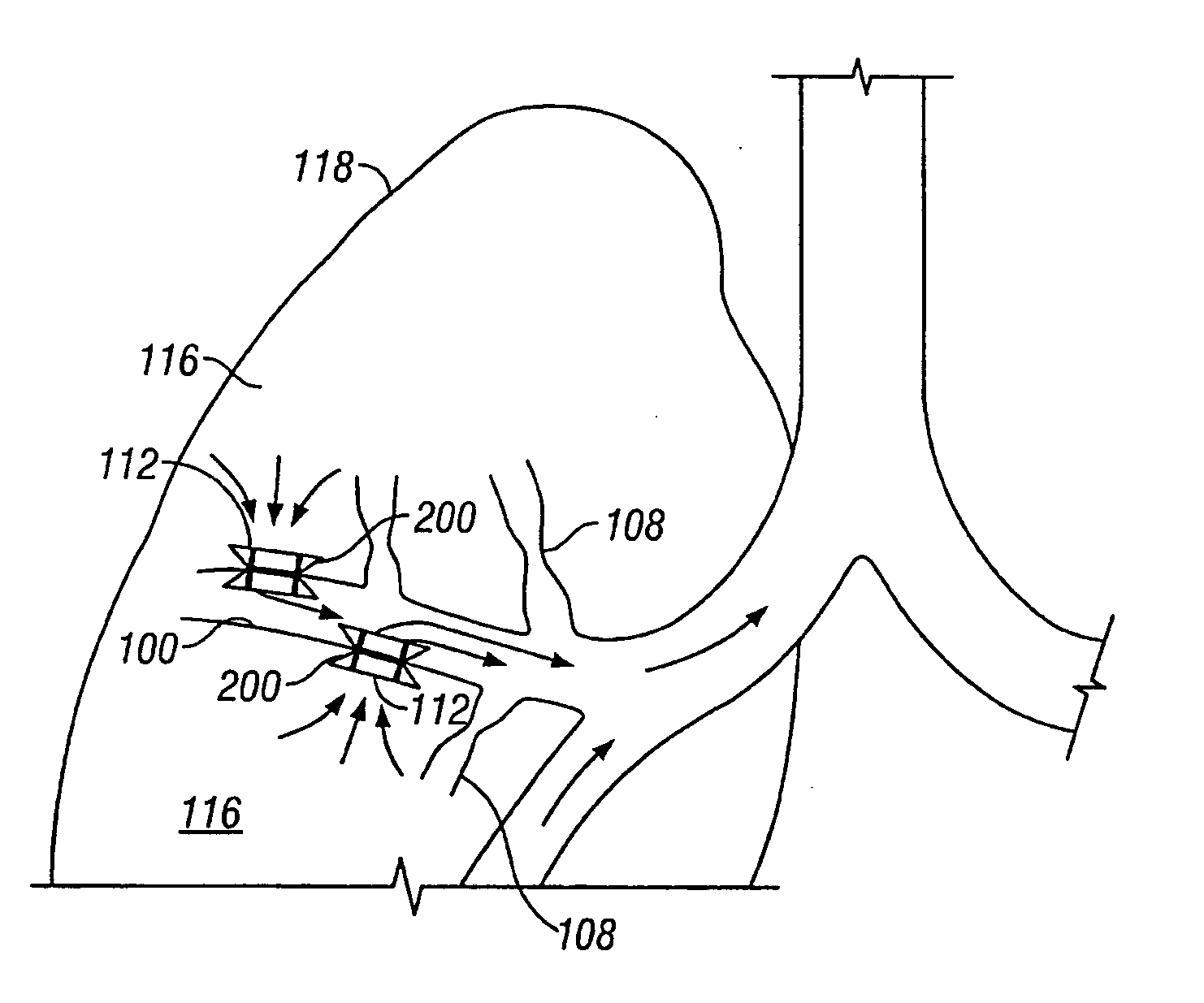
Devices for maintaining surgically created openings
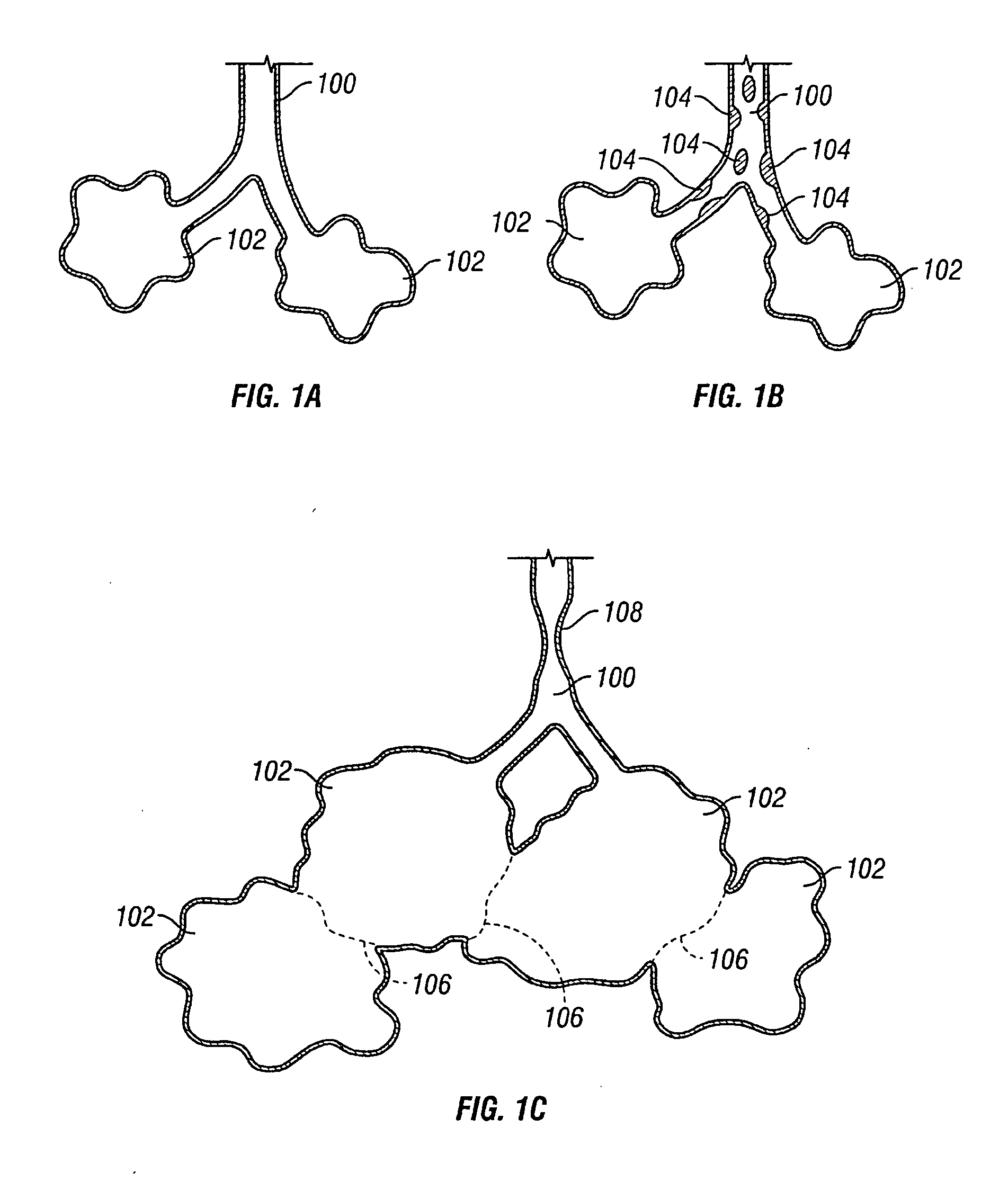
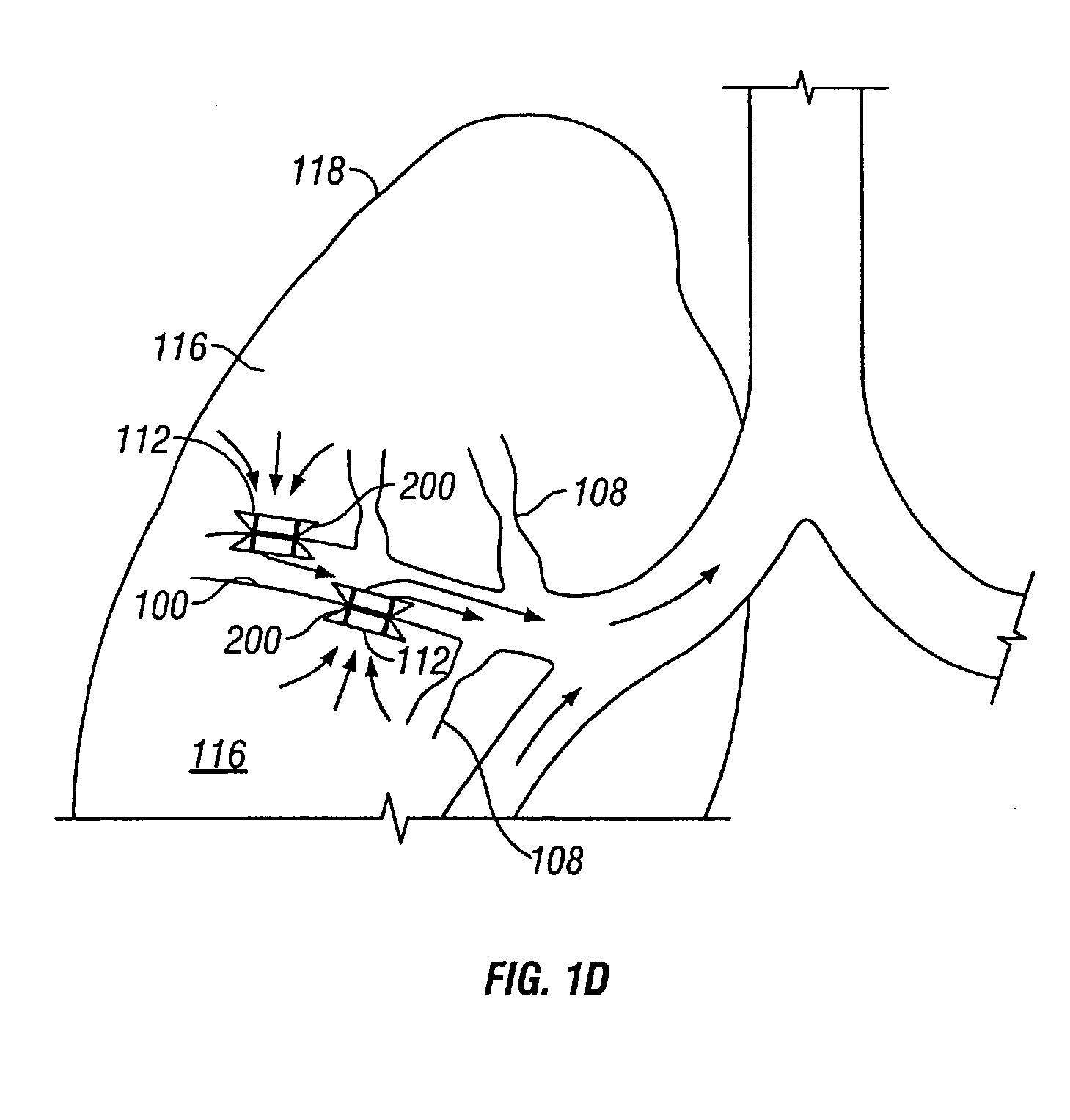
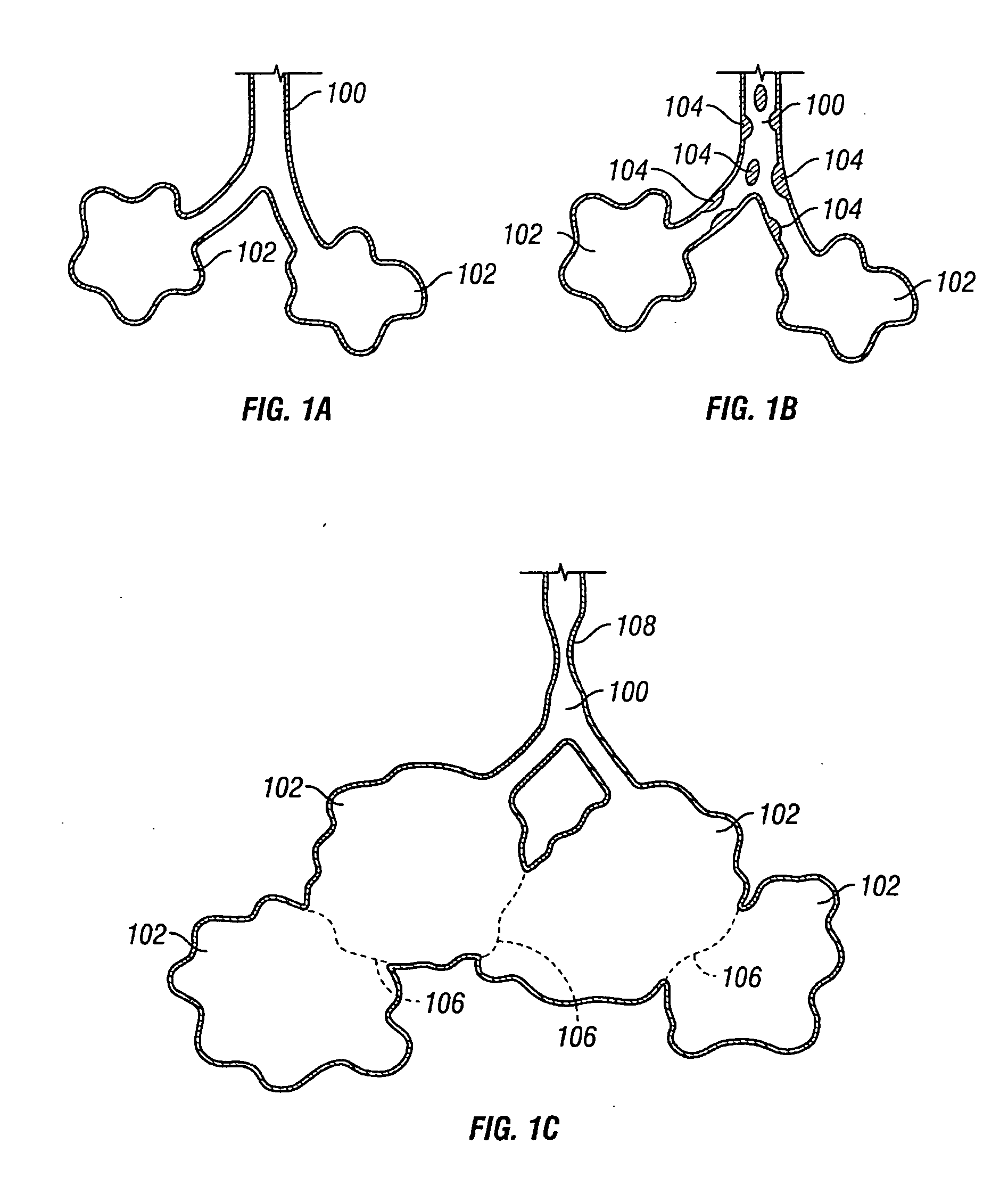
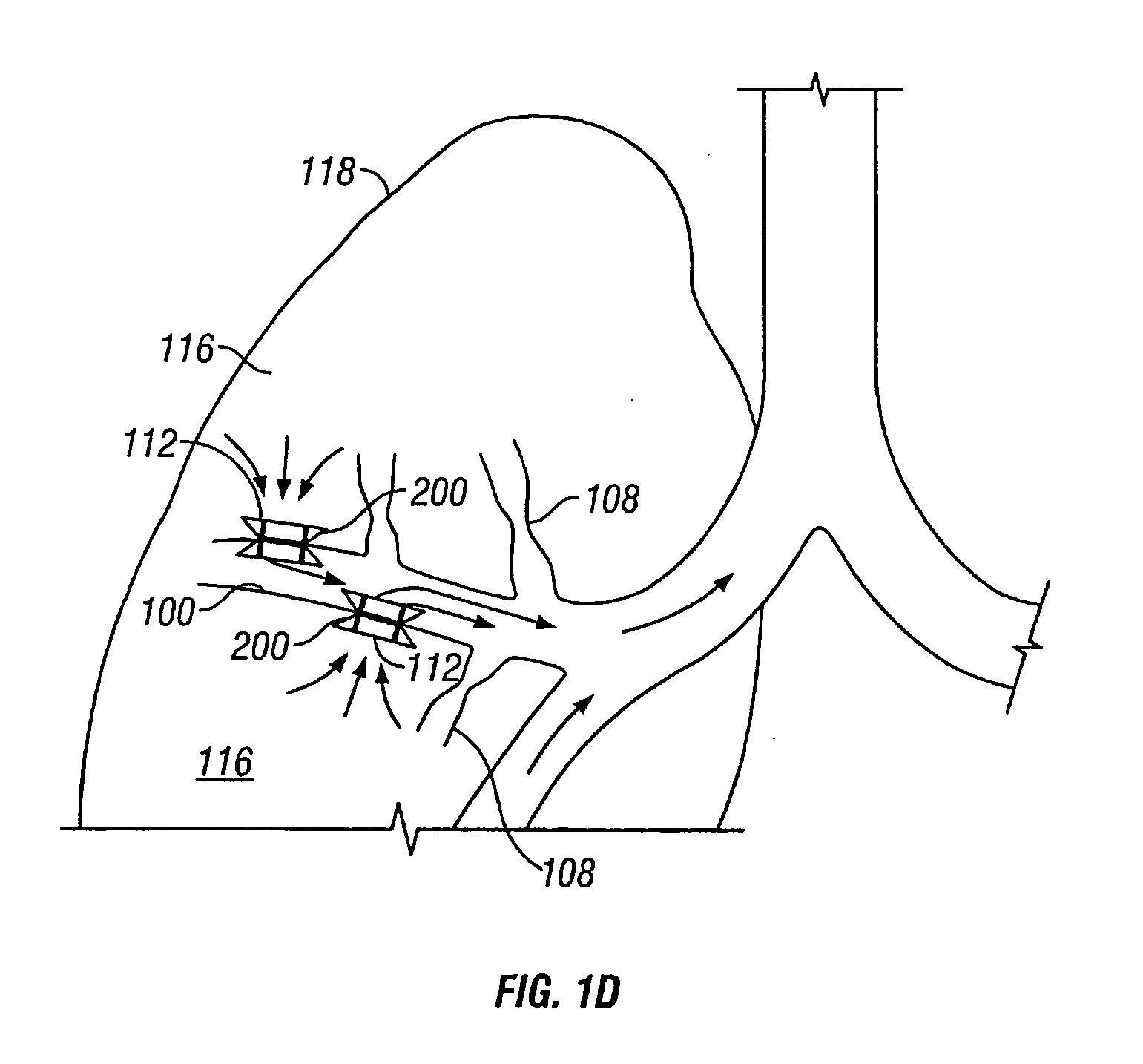
InactiveUS20050137518A1Simple processImprove scalabilityStentsBronchiCatheterObstructive Pulmonary Diseases
Devices and methods are directed to improving the gaseous exchange in a lung of an individual having, for instance, chronic obstructive pulmonary disease. More particularly, conduits may be deployed in the lung to maintain collateral openings (or channels) surgically created through airway walls. This tends to facilitate both the exchange of oxygen ultimately into the blood and decompress hyper-inflated lungs. The conduit includes a radially expandable center section having a first end, a second end, and a passageway extending from the first end to the second end. A control segment may be associated with the conduit to limit the degree of radial expansion. The conduit further includes a plurality of deflectable members extending from the ends of the center section. A tissue barrier may coaxially surround the conduit such that tissue ingrowth is prevented. The conduits may also include hold-down members and bioactive coatings that serve to prevent ejection of the conduit as well as prevent narrowing of the passageway due to tissue ingrowth.
Owner:BRONCUS TECH
Tissue lockable connecting structures
InactiveUS7083648B2Strengthen the mechanical connectionInhibits tissueDental implantsSkin implantsSoft tissue infectionSubcutaneous tissue
Percutaneous skin access devices include a plurality of locked connecting units mounted to the exterior surface of an implantable medical object which, in position, is configured to penetrate the skin of a subject. The locked connecting units may be mounted directly onto the desired surface of the exterior of the device or may be held on a substrate sheet, which is mounted to the exterior surface of the device. In position, the locked connecting units engage with soft tissue which can include the skin to form a bio-junction layer which includes mechanical and bio-sealing connection between the device body and the soft tissue. The configuration at the bio-junction layer secures the medical object in location in the subject even for long-term indwelling applications in a manner, which inhibits soft tissue infection.The locked connecting units may be rigid or semi-rigid for longer-term indwelling applications, and semi-rigid and / or resilient for shorter term indwelling applications. The locked connecting units may take on the form of rings, hooks, or loops having aperture or gap width / length sizes of from about 0.2–4 mm. The rings, loops, or hooks may connect with any soft tissue including skin as well subcutaneous tissue. The rings, hooks, or loops may be released from the skin / tissue without requiring surgical cutting procedures.The locked connecting units may be configured as a semi-rigid mesh collar arranged about the primary body providing access to the subject such that it resides in the subject and engages with the skin (epidermal / dermal layer). The mesh collar can be described as a particular type of ring or loop structure as the mesh defines the gap provided in individual loop configurations. The mesh collar may be used alone, or in combination with the loops, rings, or hooks. A skin stop collar having increased rigidity may be disposed under the mesh collar.
Owner:EAST CAROLINA UNIVERISTY
Image-type intubation-aiding device
InactiveUS20060004258A1Rapid positioningIncrease spot ratioTracheal tubesBronchoscopesDisplay deviceEndotracheal tube
An image-type intubation-aiding device comprises a small-size image sensor and a light source module both placed into an endotracheal tube to help doctors with quick intubation. Light from light emission devices in the light source module passes through a transparent housing and is reflected by a target and then focused. The optical signal is converted into a digital or analog electric signal by the image sensor for displaying on a display device after processing. Doctors can thus be helped to quickly find the position of trachea, keep an appropriate distance from a patient for reducing the possibility of infection, and lower the medical treatment cost. Disposable products are available to avoid the problem of infection. The intubation-aiding device can be used as an electronic surgical image examination instrument for penetration into a body. Moreover, a light source with tunable wavelengths can be used to increase the spot ratio of nidus.
Owner:MEDICAL INTUBATION TECH CORP
Devices for maintaining surgically created openings
InactiveUS20050192526A1Simple processImprove scalabilityStentsBronchiCatheterObstructive Pulmonary Diseases
Devices and methods are directed to improving the gaseous exchange in a lung of an individual having, for instance, chronic obstructive pulmonary disease. More particularly, conduits may be deployed in the lung to maintain collateral openings (or channels) surgically created through airway walls. This tends to facilitate both the exchange of oxygen ultimately into the blood and decompress hyper-inflated lungs.
Owner:BIGGS MICHAEL +12
Devices for maintaining surgically created openings
InactiveUS20050137712A1Simple processImprove scalabilityStentsBronchiCatheterObstructive Pulmonary Diseases
Devices and methods are directed to improving the gaseous exchange in a lung of an individual having, for instance, chronic obstructive pulmonary disease. More particularly, conduits may be deployed in the lung to maintain collateral openings (or channels) surgically created through airway walls. This tends to facilitate both the exchange of oxygen ultimately into the blood and decompress hyper-inflated lungs.
Owner:BIGGS MICHAEL +12
Glucose meter with er:yag laser lancing device
InactiveUS20100030110A1Avoid infectionImprove adhesionDiagnostic recording/measuringTemperature sensorsEr:YAG laserD-Glucose
A personal portable painless lancing device and a blood glucose meter using the same, which can lance at the minimum without any pain and use it to measure the blood glucose. The minimum painless lancing device using an Er:YAG laser includes an Er:YAG laser beam-generating section in which the laser beam is generated to punch skin and radiated in a single direction; an adapter installed at the front end of the Er:YAG laser beam-generating section so that the laser beam can be locally concentrated on a skin punch portion; a skin adhesion cap installed at the front end of the adapter so that it can be easily detached, and can reduce the contamination of the Er:YAG laser beam-generating section, which is produced at the time of the lancing, and the danger due to the reuse of it among the patients of lancing; and a case to accommodate the Er:YAG laser beam-generating section and the adapter therein.
Owner:ISOTECH
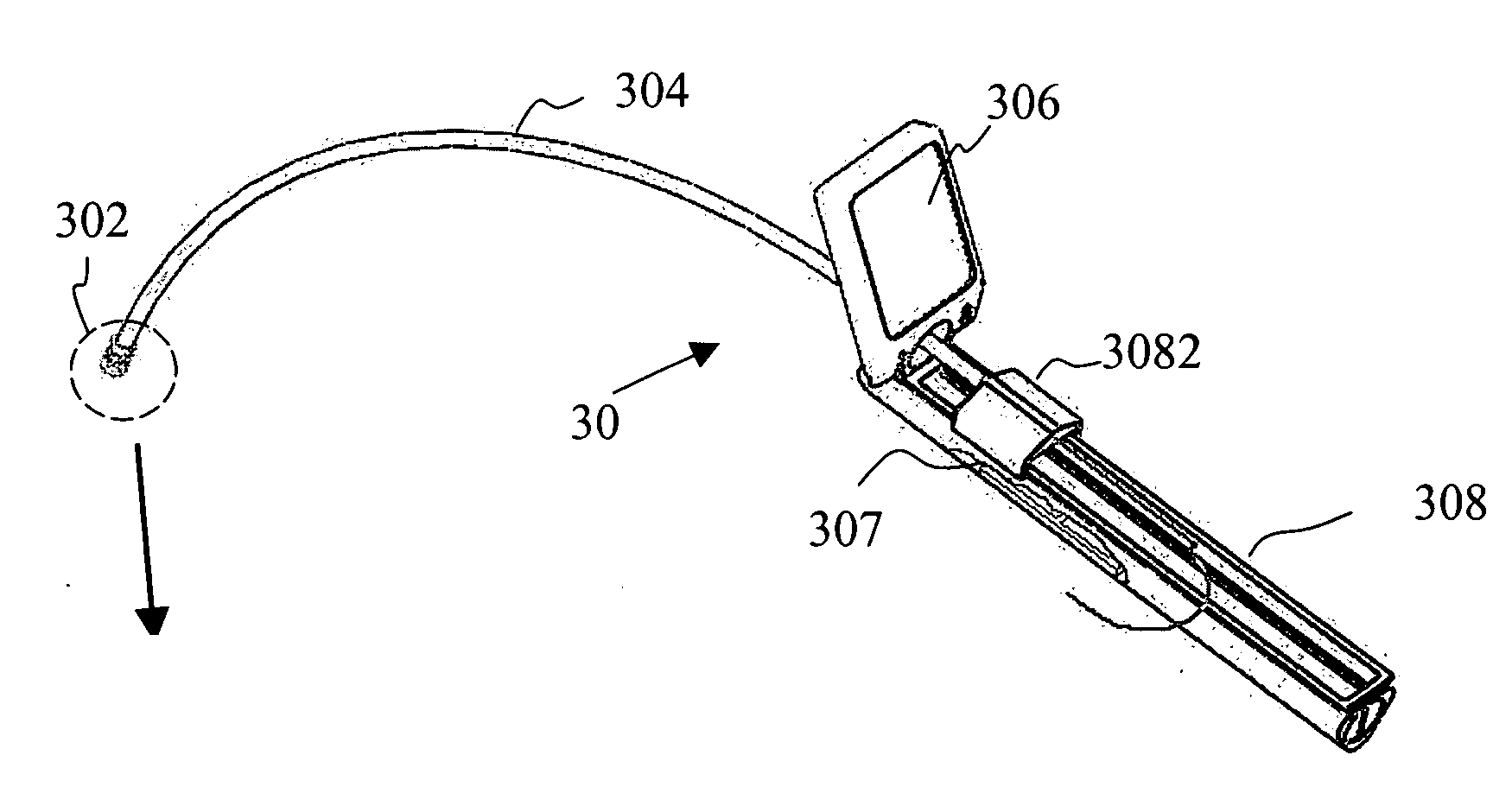
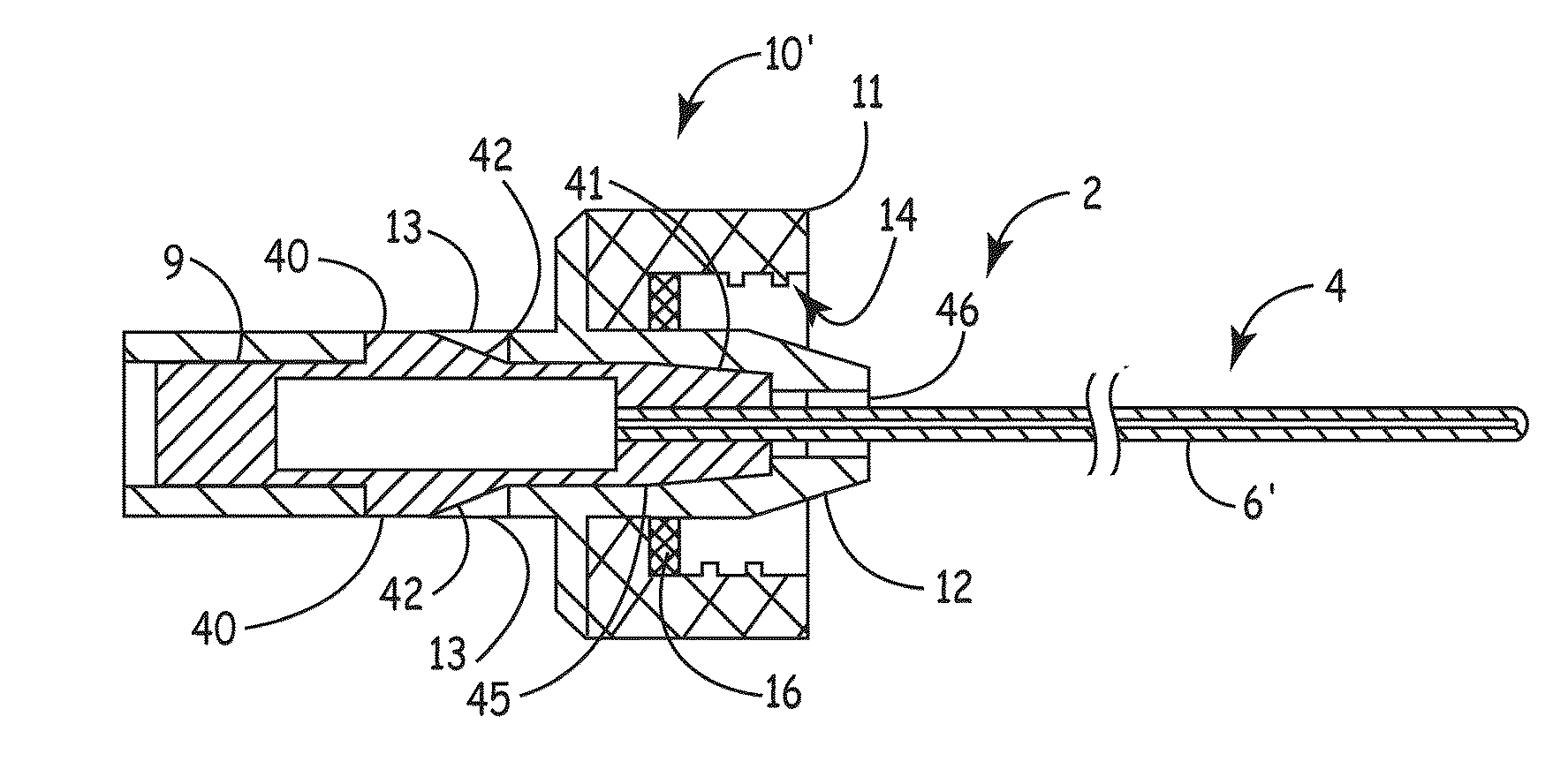
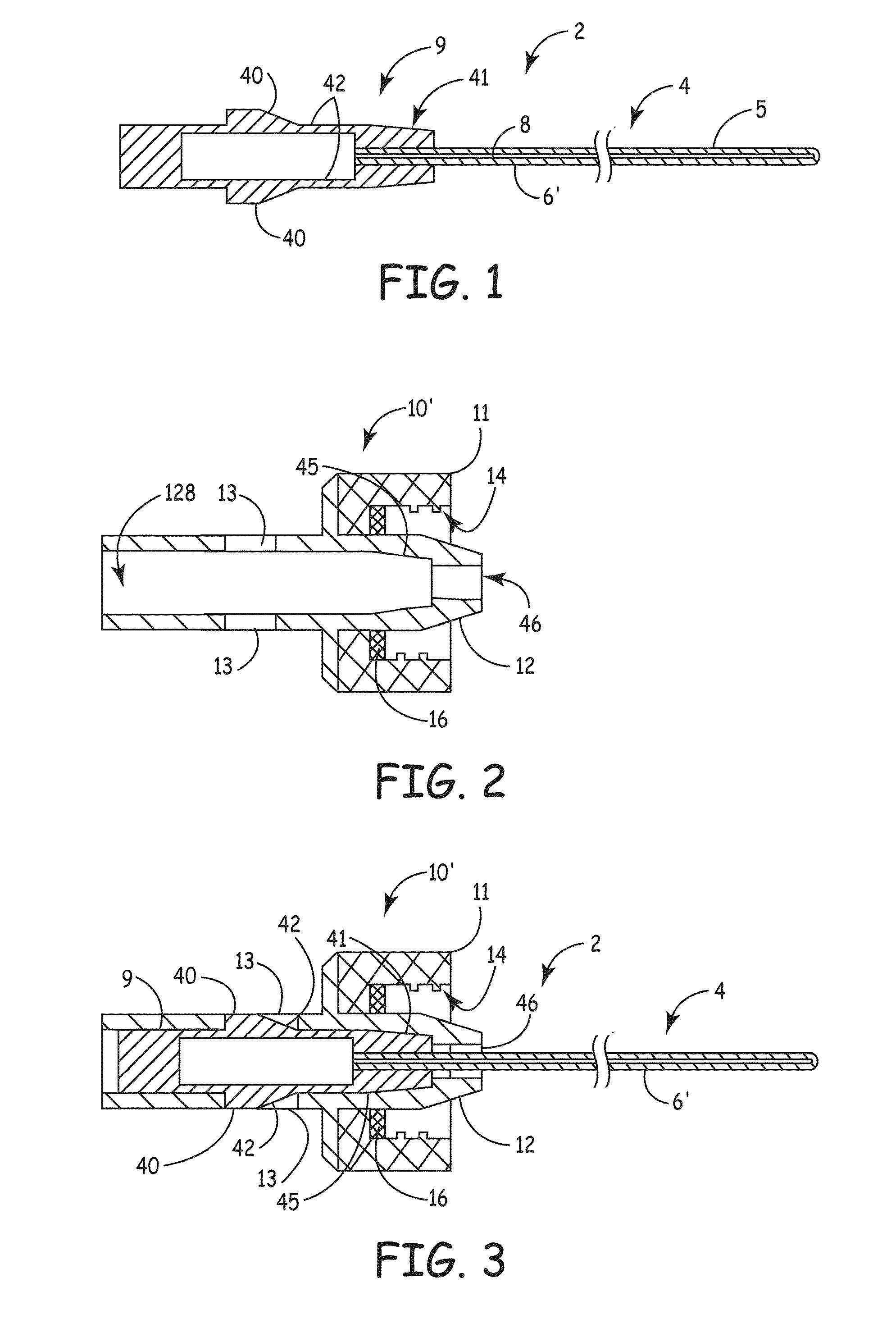
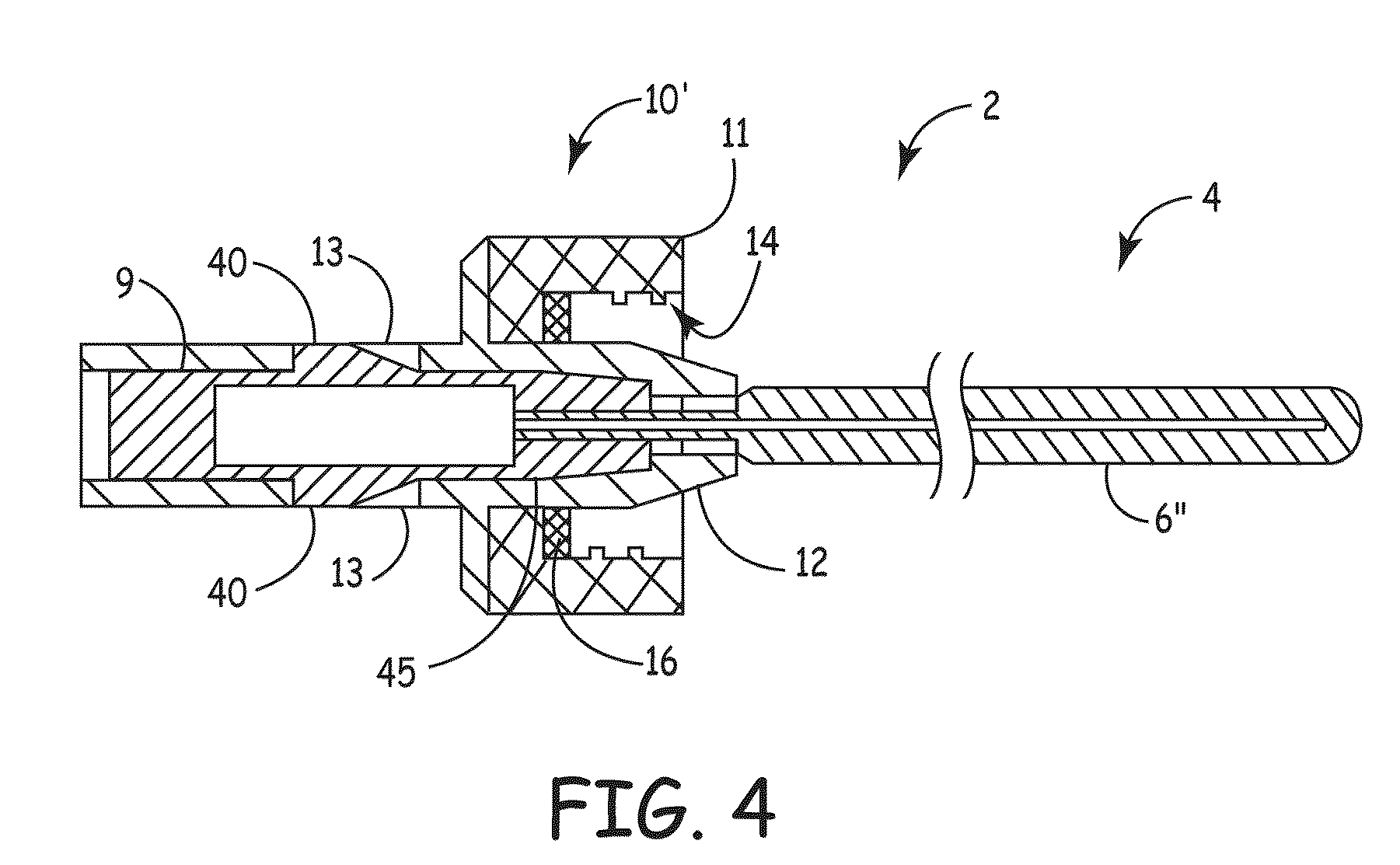
Apparatus for delivery of device and antimicrobial agent into trans-dermal catheter
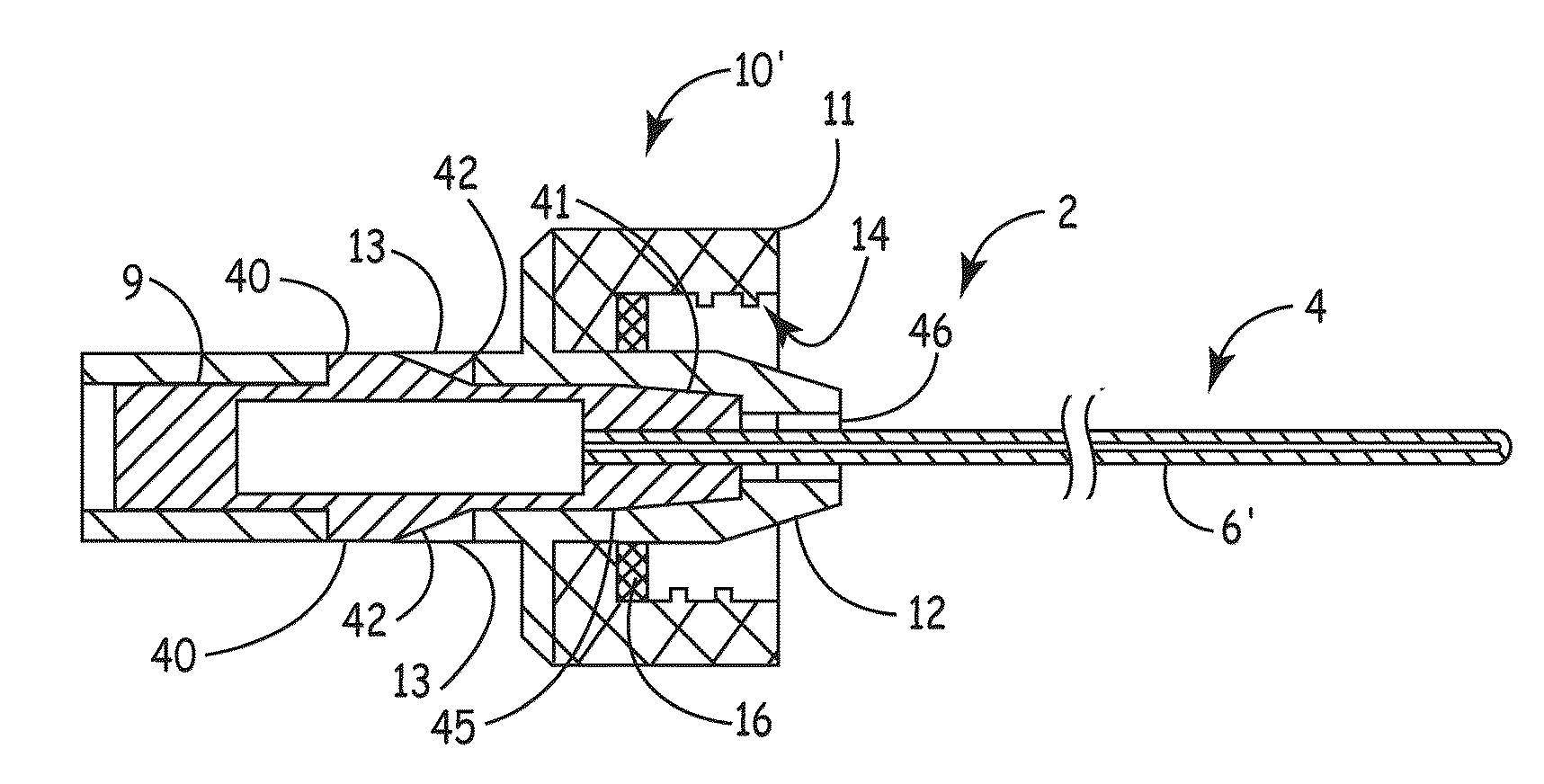
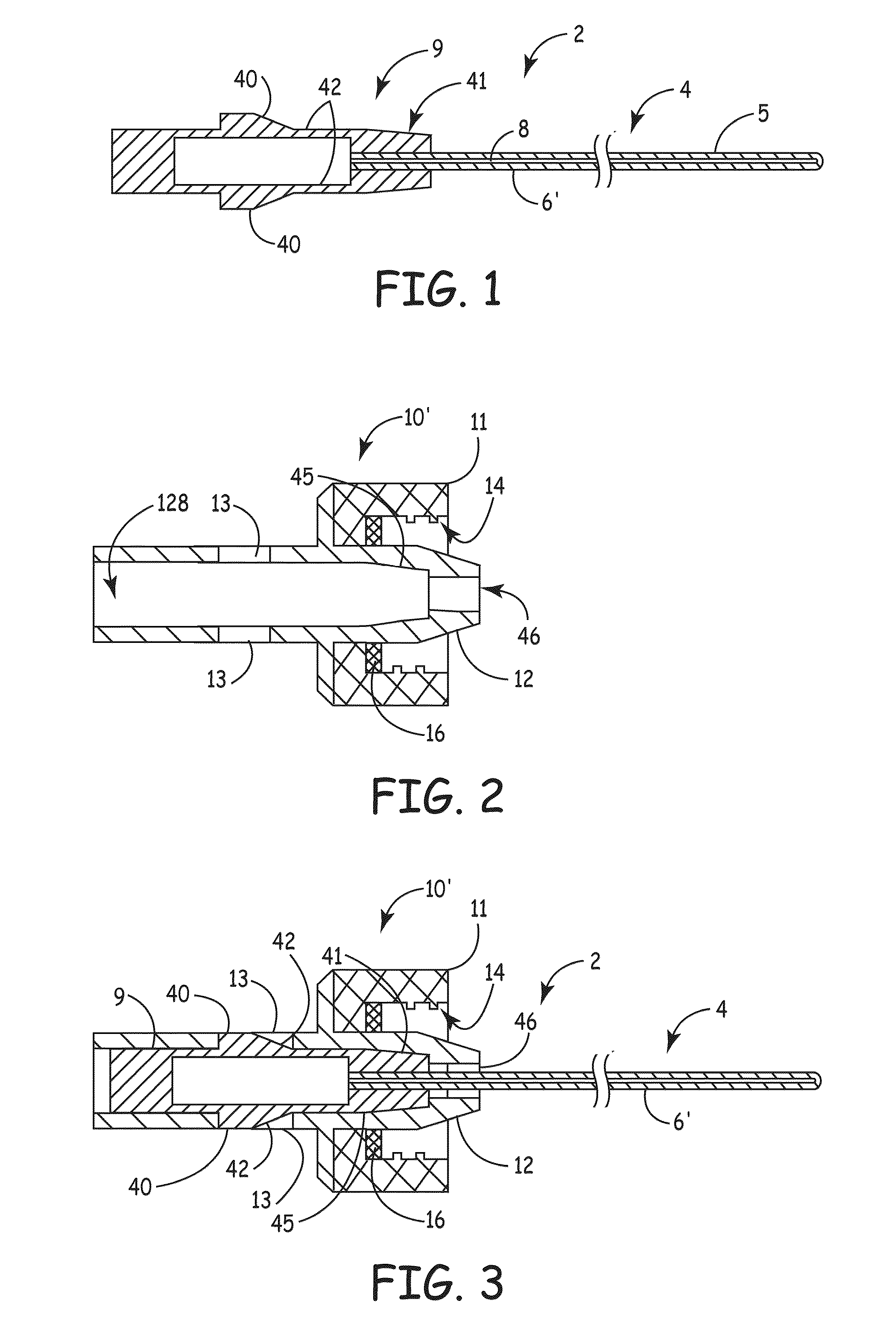
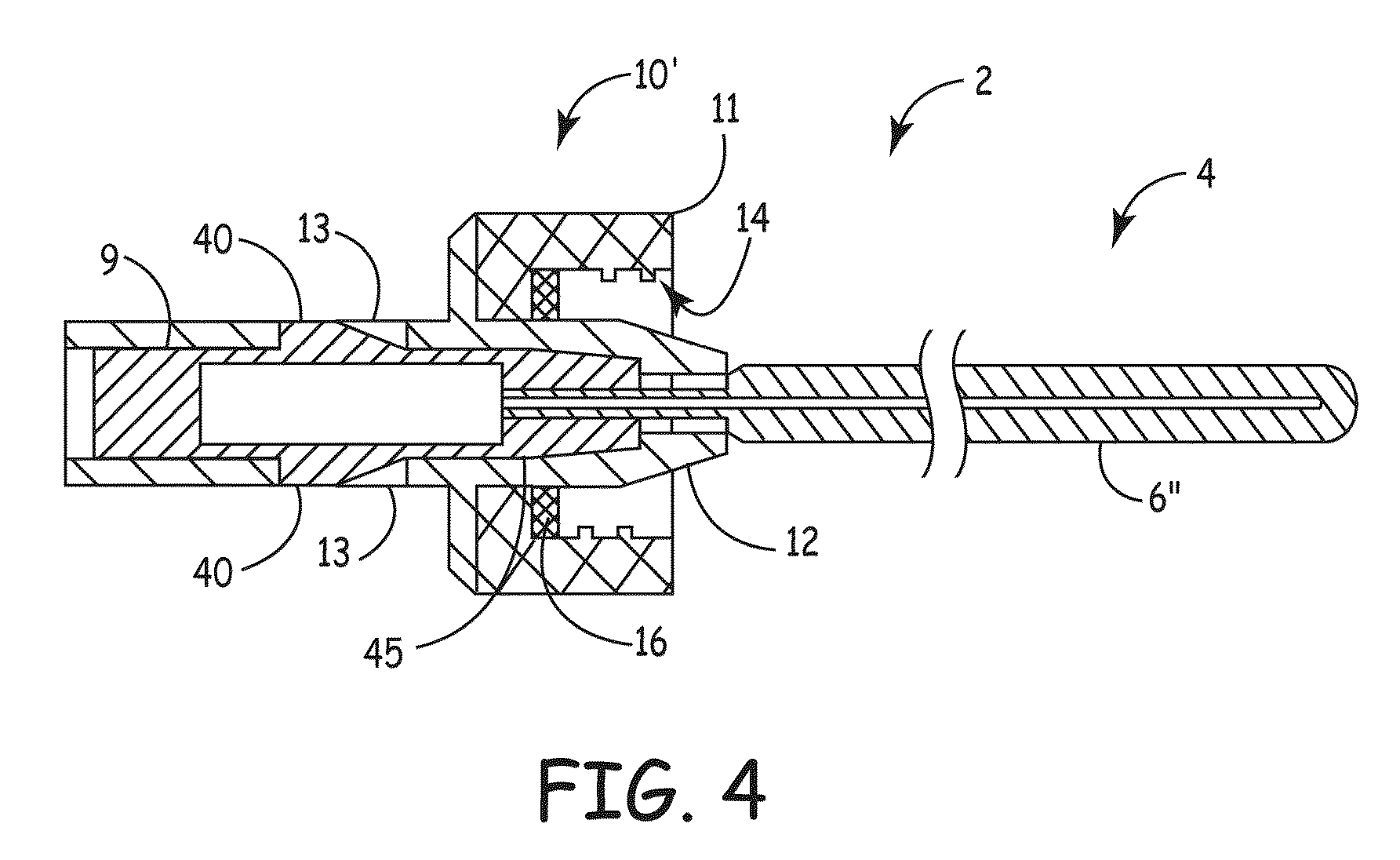
ActiveUS20100106102A1Reduce the risk of infectionReduce infection rateMedical devicesCatheterCatheter deviceAnti-Microbial Agents
An apparatus for delivery of an elongate member into the lumen of a trans-dermal catheter. In a preferred embodiment, the apparatus comprises a protective sheath configured to at least substantially surround an elongate member; and slidable member configured to travel along the protective sheath, the slidable member operatively coupled to the elongate member; wherein the elongate member is insertable into a lumen of a trans-dermal catheter by moving the slidable member along at least a part of the length of the protective sheath. In another embodiment, the apparatus comprises a protective sheath configured to at least substantially surround an elongate member; a slidable member configured to travel along the protective sheath, the slidable member operatively coupled to the elongate member; and a connecting member positioned on the distal end of the protective sheath, the connecting member configured to lock onto the distal end of a trans-dermal catheter; wherein the elongate member is insertable into the lumen of the trans-dermal catheter by moving the slidable member along at least a part of the length of the protective sheath.
Owner:ICU MEDICAL INC
Ventilator device
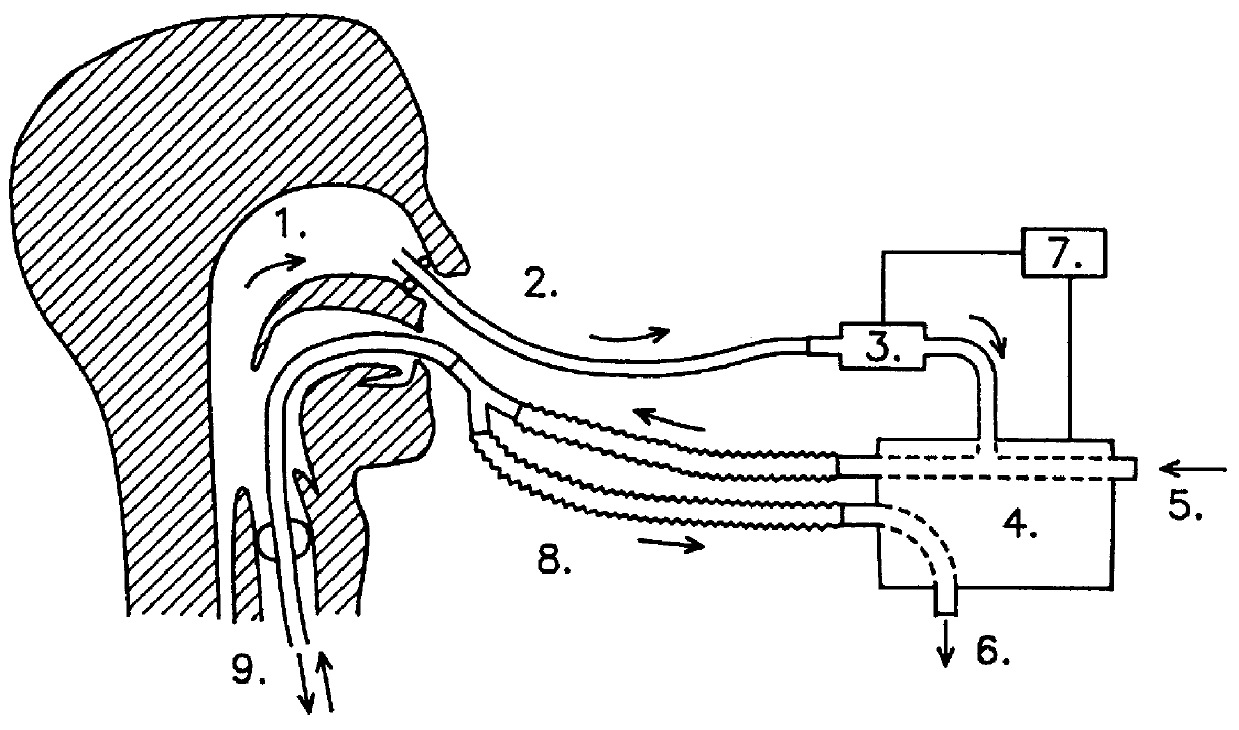
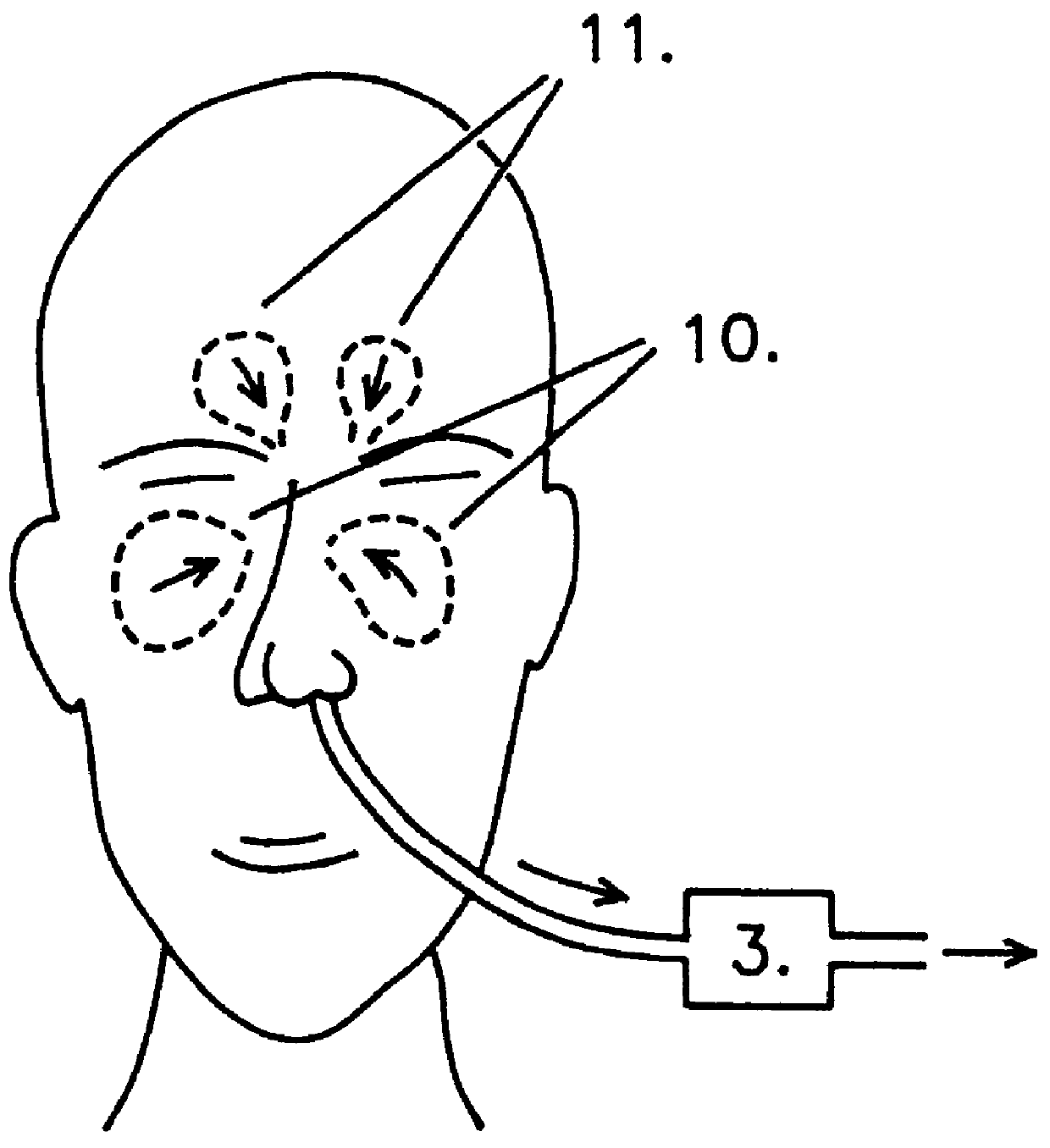
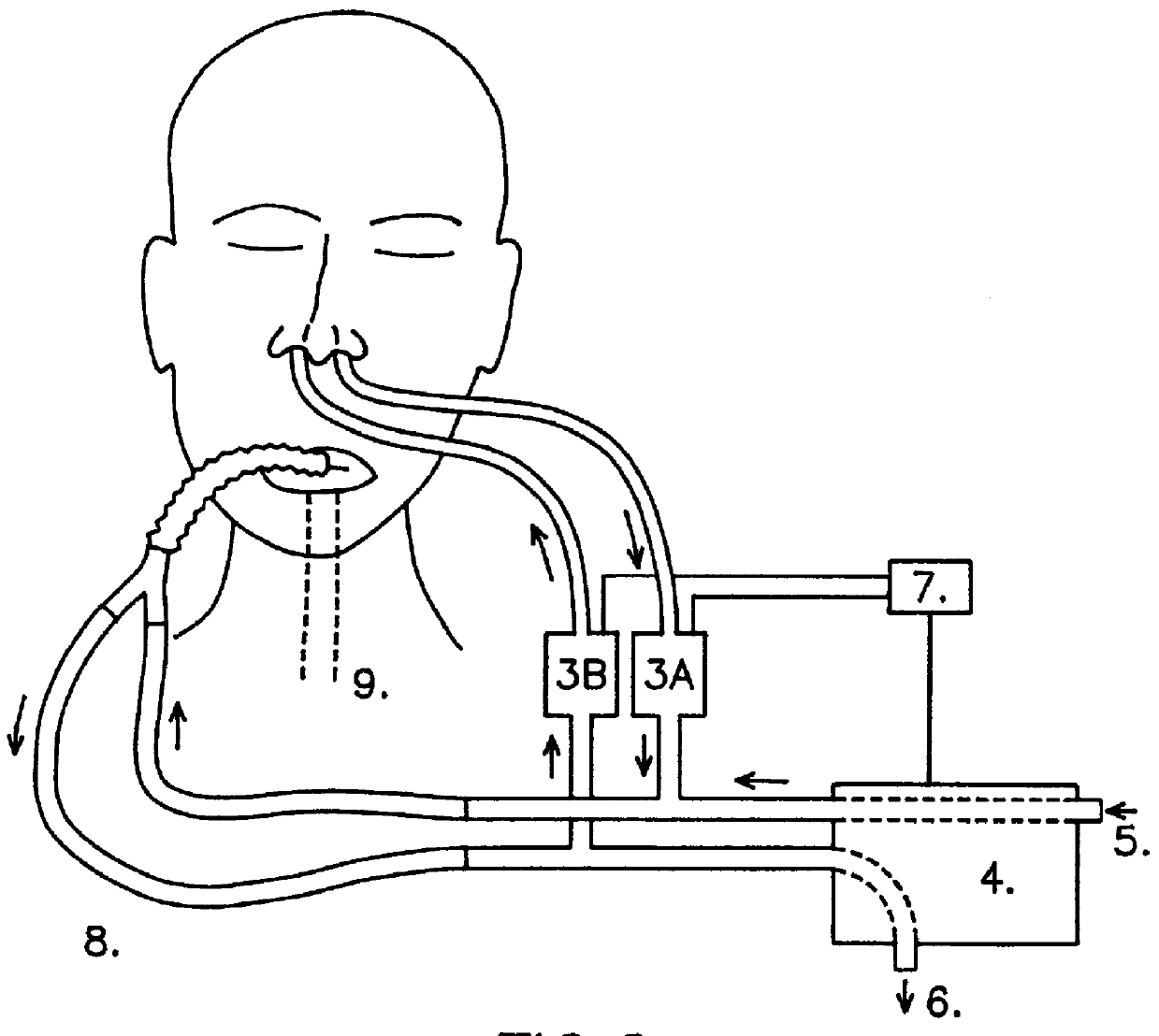
InactiveUS6019100AIncrease ciliary beat frequencyRestore natural nasal breathing patternTracheal tubesOperating means/releasing devices for valvesActive agentNitric oxide
The present invention restores the normal low-dose flushing of the lower airways with air from the upper airways, containing nitric oxide (NO) and possible other biologically active agents by aspiration of air from the upper airways and introducing said air in the inspiratory airflow of a ventilator. The inventive apparatus and method is free from the risks associated with traditional administration of exogenous NO.
Owner:ALVING KJELL +3
Image-type intubation-aiding device
InactiveUS20090118580A1Improve medical qualityIncrease spot ratioBronchoscopesTracheal tubesDisplay deviceEndotracheal tube
An image-type intubation-aiding device comprises a small-size image sensor and a light source module both placed into an endotracheal tube to help doctors with quick intubation. Light from light emission devices in the light source module passes through a transparent housing and is reflected by a target and then focused. The optical signal is converted into a digital or analog electric signal by the image sensor for displaying on a display device after processing. Doctors can thus be helped to quickly find the position of trachea, keep an appropriate distance from a patient for reducing the possibility of infection, and lower the medical treatment cost. Disposable products are available to avoid the problem of infection. The intubation-aiding device can be used as an electronic surgical image examination instrument for penetration into a body. Moreover, a light source with tunable wavelengths can be used to increase the spot ratio of nidus.
Owner:MEDICAL INTUBATION TECH CORP
Catheter device
ActiveUS20070232981A1Avoid infectionMinimize developmentMulti-lumen catheterSurgeryEngineeringMechanical engineering
A catheter device has an elongate body and a chamber positioned within the elongate body. The chamber has a chamber opening, and a barrier, such as a gate, moves over the chamber opening. The barrier moves between an uncovered position uncovering the chamber opening and a covered position covering the chamber opening. The catheter has at least one cutting edge by the chamber opening. In particular, the at least one cutting edge may be on the barrier, where the at least one cutting edge is guided over the chamber opening with movement of the barrier between the uncovered position and the covered position. The cutting edge cuts away a fibrin sheath blocking the chamber opening. The catheter may have multiple chambers connected by channels that allow the chambers to be flushed to prevent microbial colonization.
Owner:PHASE ONE MEDICAL
Methods of reducing microbial contamination
Methods of delaying the onset of an infection or preventing an infection caused by a microbial organism in an internal cavity of a subject are provided. Methods of killing or inactivating microorganisms in at least a portion of the urethra of a subject are provided.
Owner:3M INNOVATIVE PROPERTIES CO
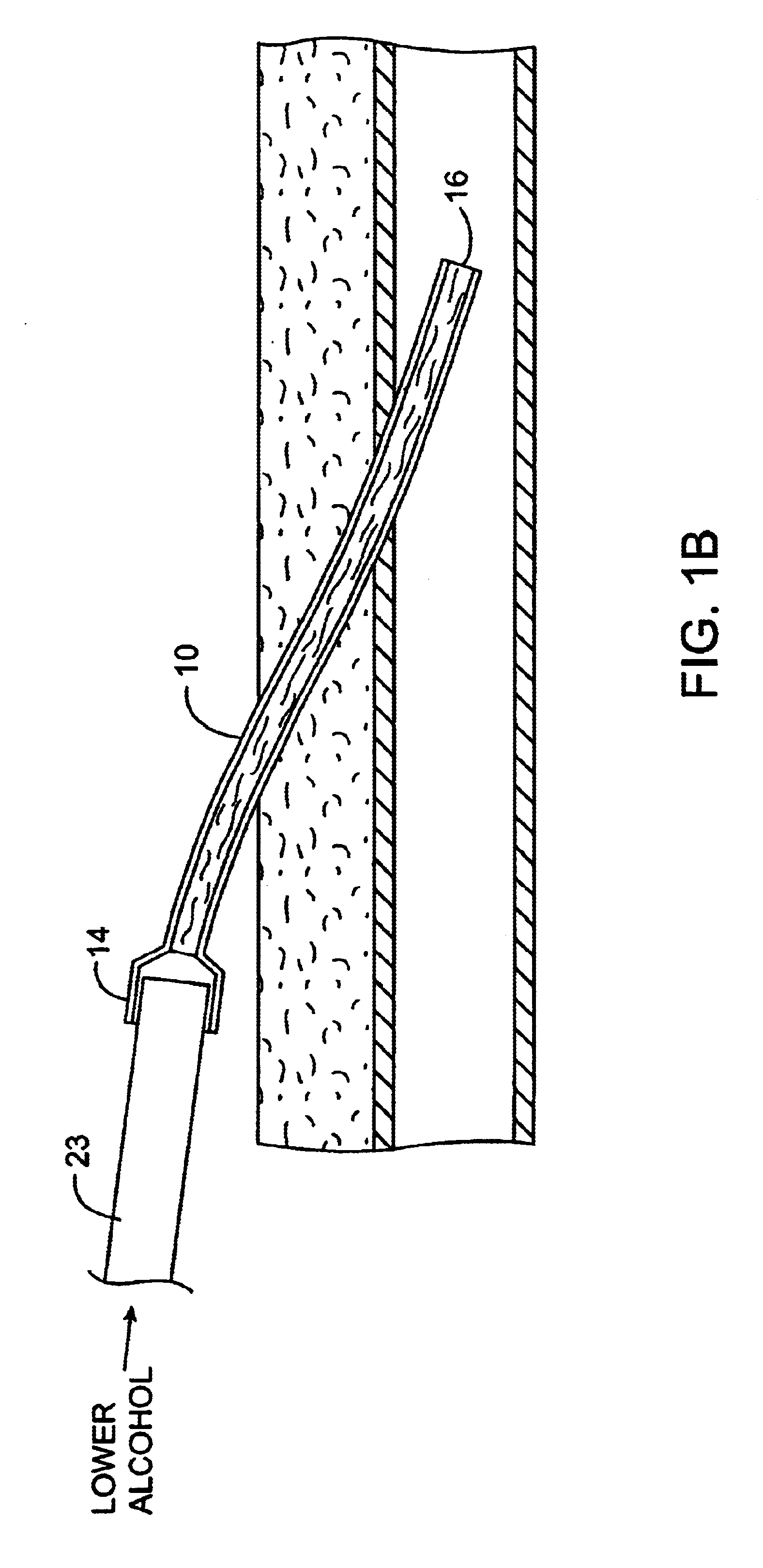
Methods and kits for locking and disinfecting implanted catheters
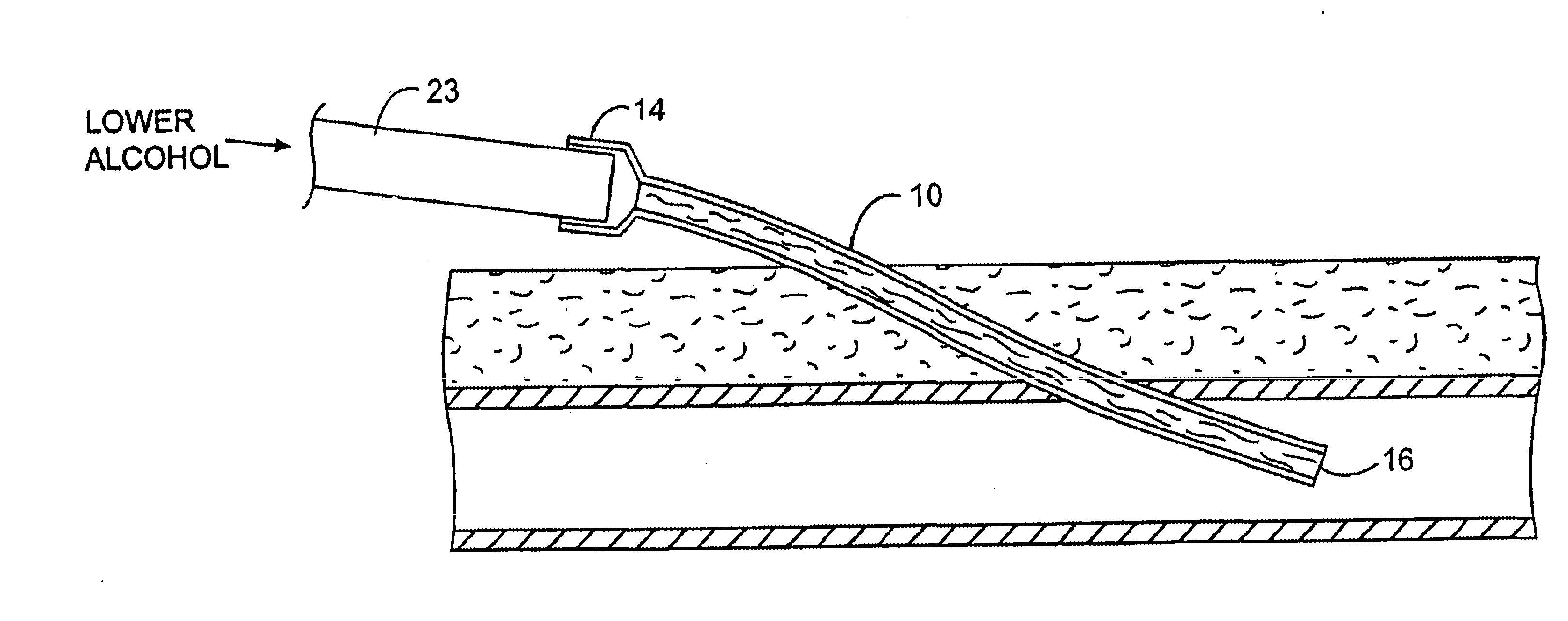
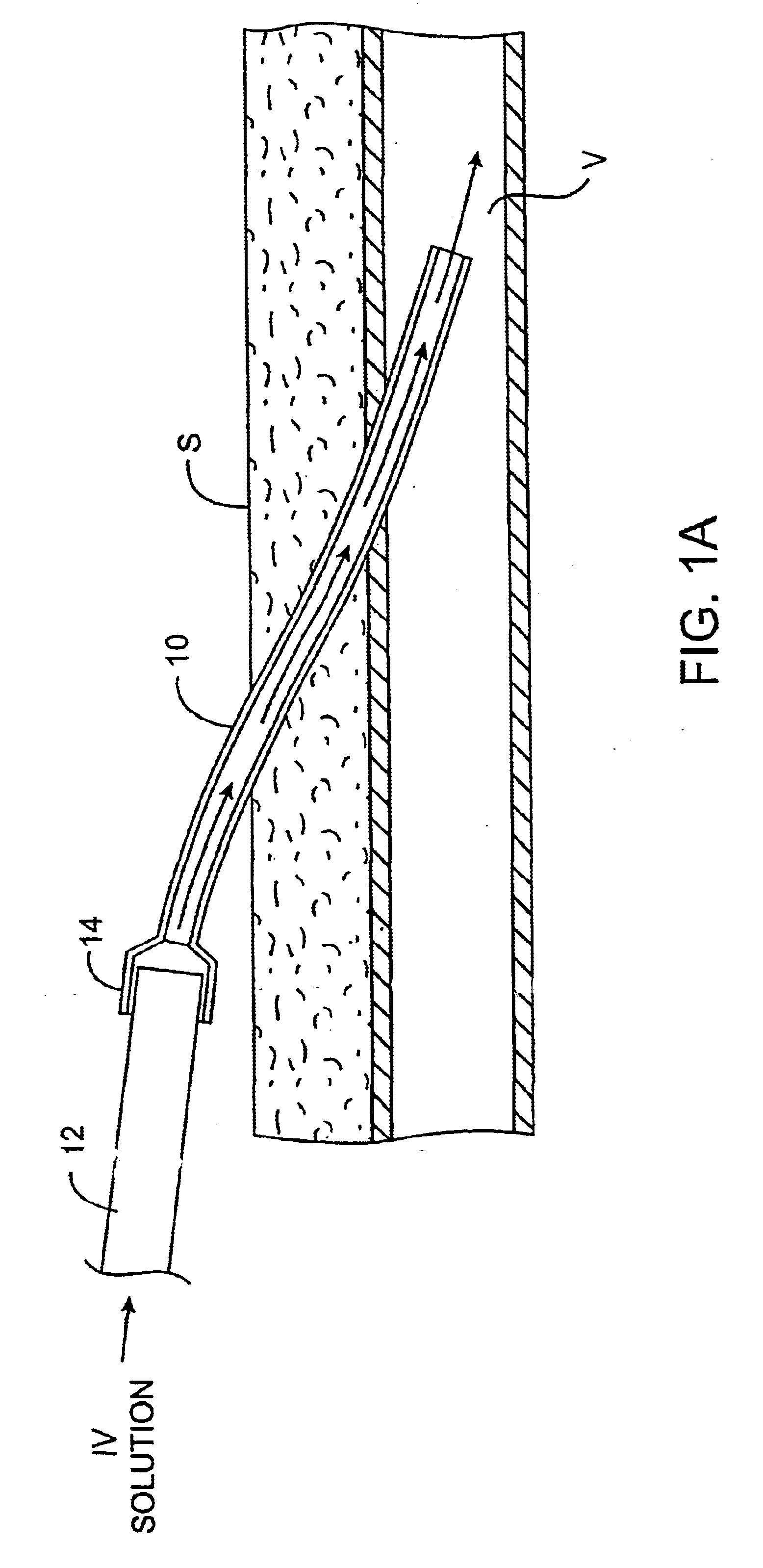
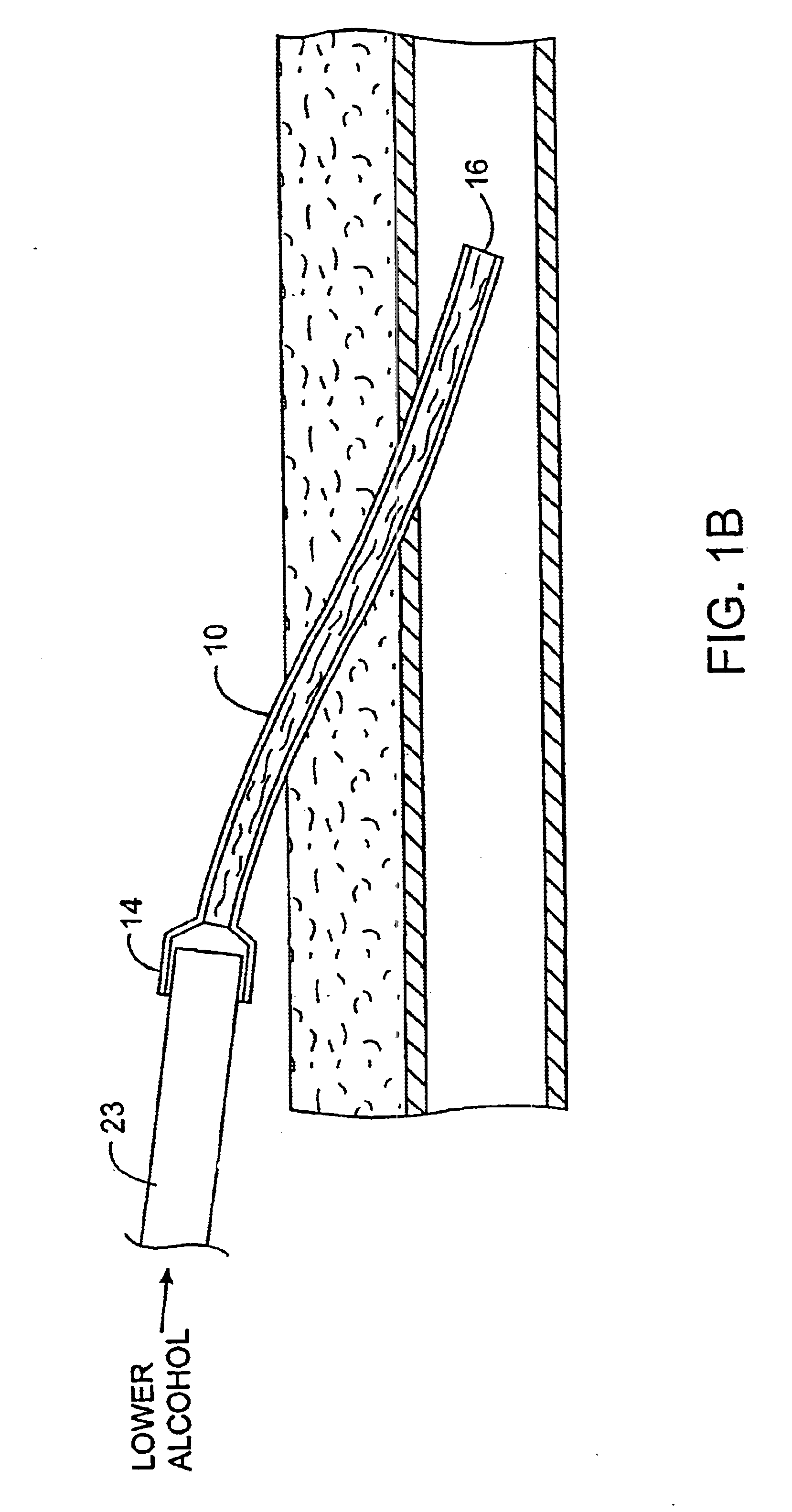
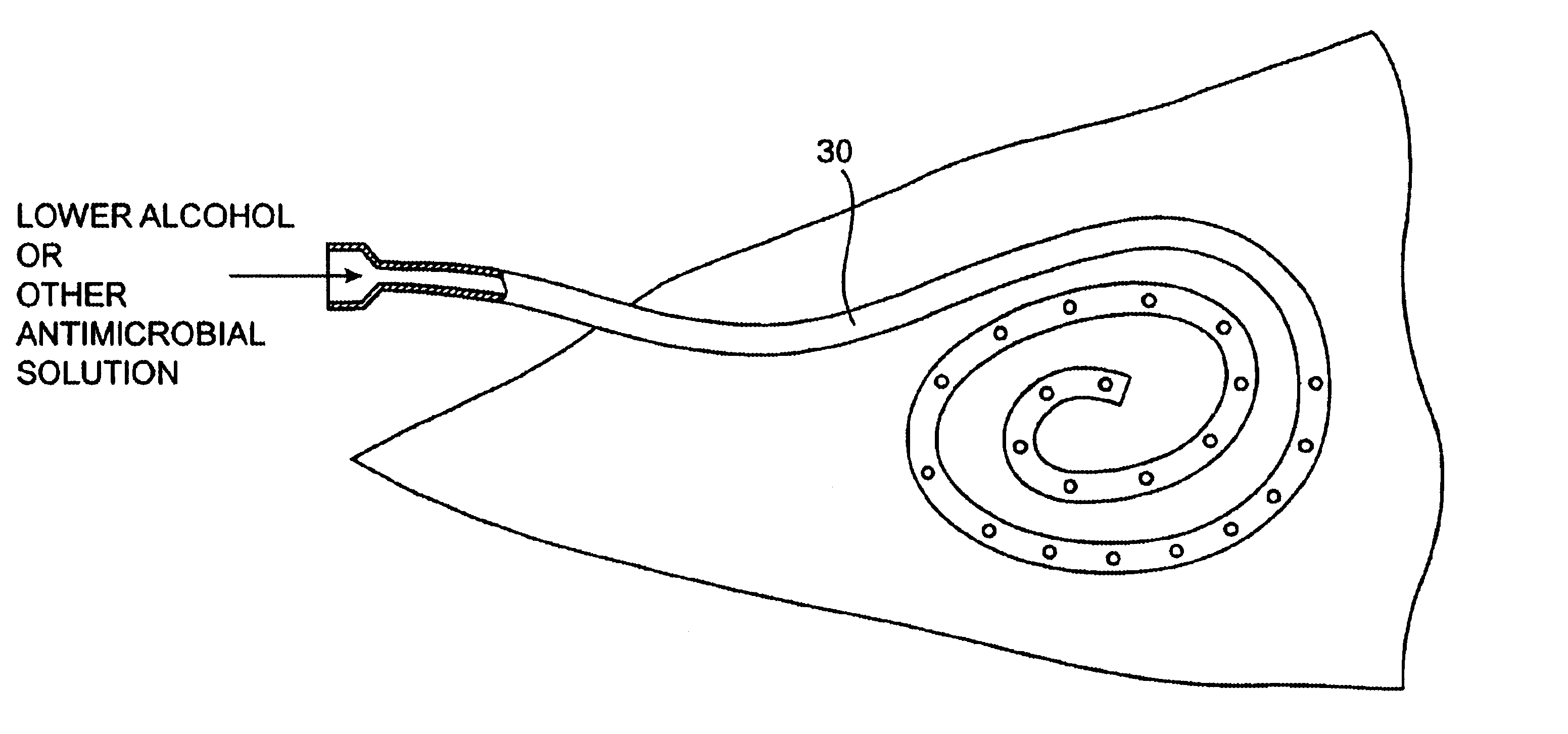
InactiveUS6679870B1Reduce riskInhibiting fouling and plugging of the lumenMedical devicesCatheterPropanolTrisodium citrate
Implanted catheters are locked with a solution comprising a lower alcohol, typically ethanol, propanol, or butanol, most preferably isopropanol, and an additive, the additive comprising an anti-microbial, typically taurolidine or triclosan, or an anti-coagulant, typically riboflavin, sodium citrate, ethylene diamine tetraacetic acid, or citric acid. The use of an alcohol and additive solution can effectively reduce fouling of the catheter, particularly clotting and thrombus in intravascular catheters, as well as reduce the risk of infection. The risk of infection can be further reduced by employing a catheter body which is sufficiently porous to permit the anti-microbial solution of a lower alcohol and another anti-microbial or anti-coagulant compound to penetrate into the catheter body and preferably through the catheter into tissue surrounding the implanted catheter.
Owner:EXCELSIOR MEDICAL
Aqueous humor drainage implant for treatment glaucoma
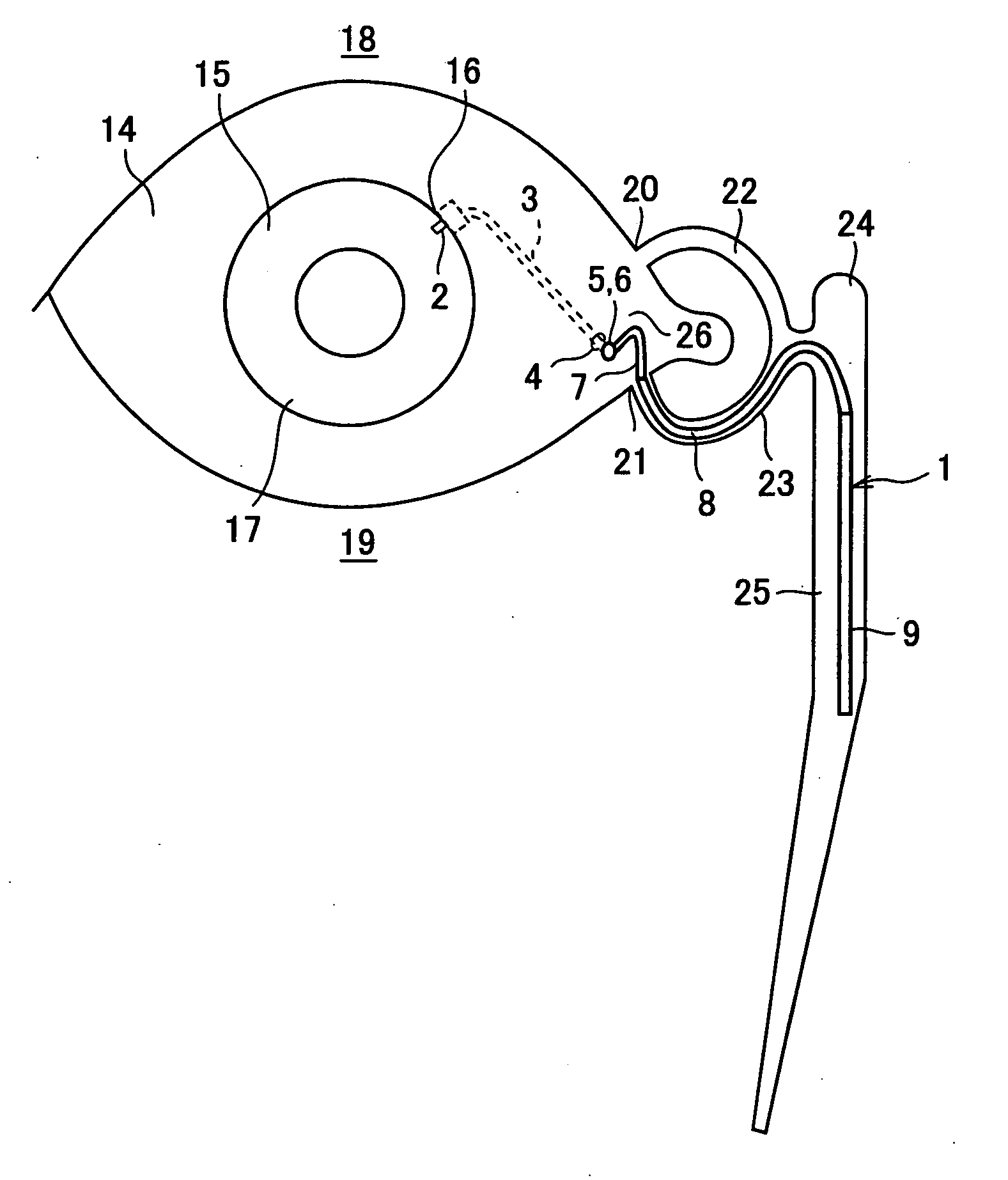
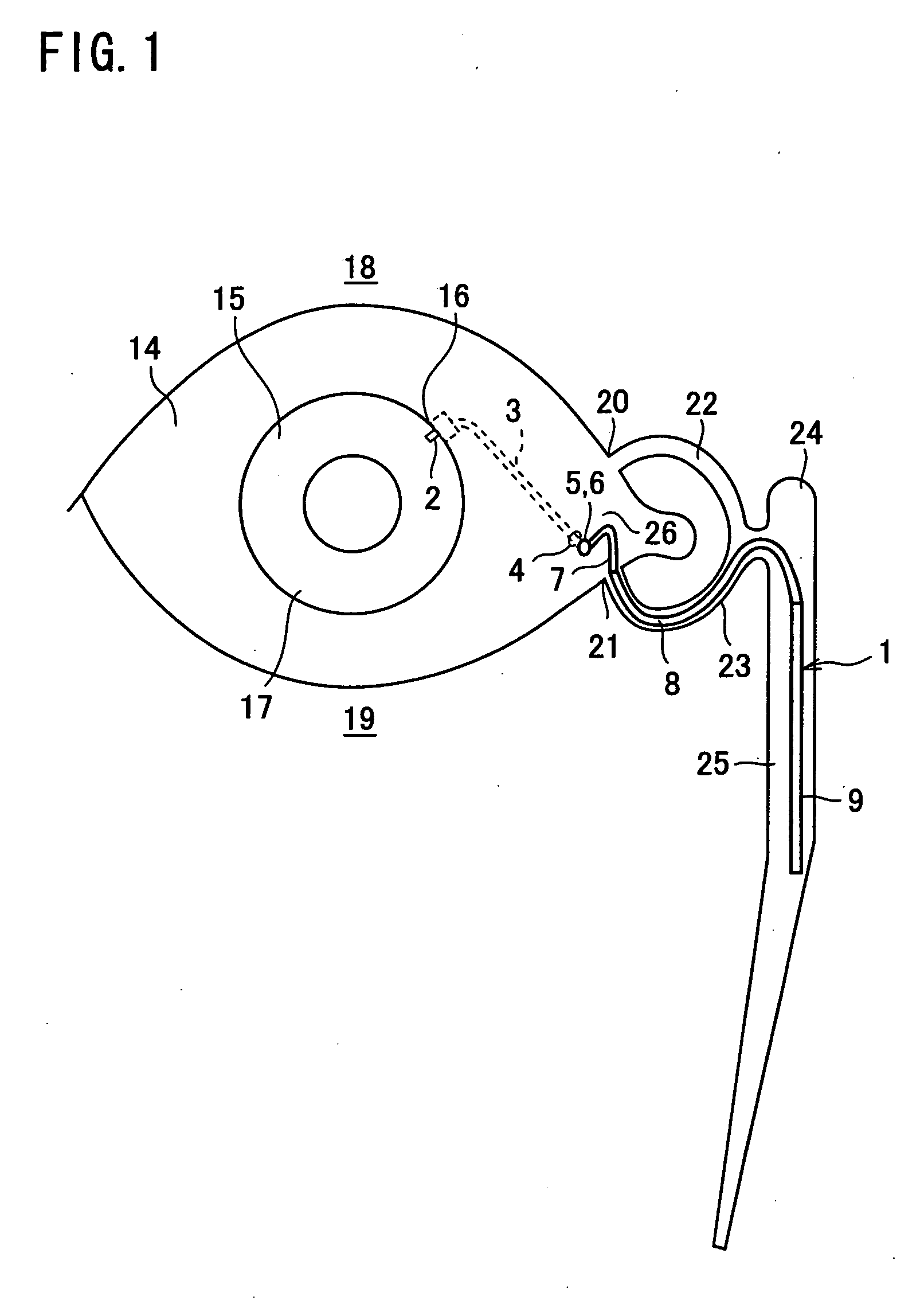
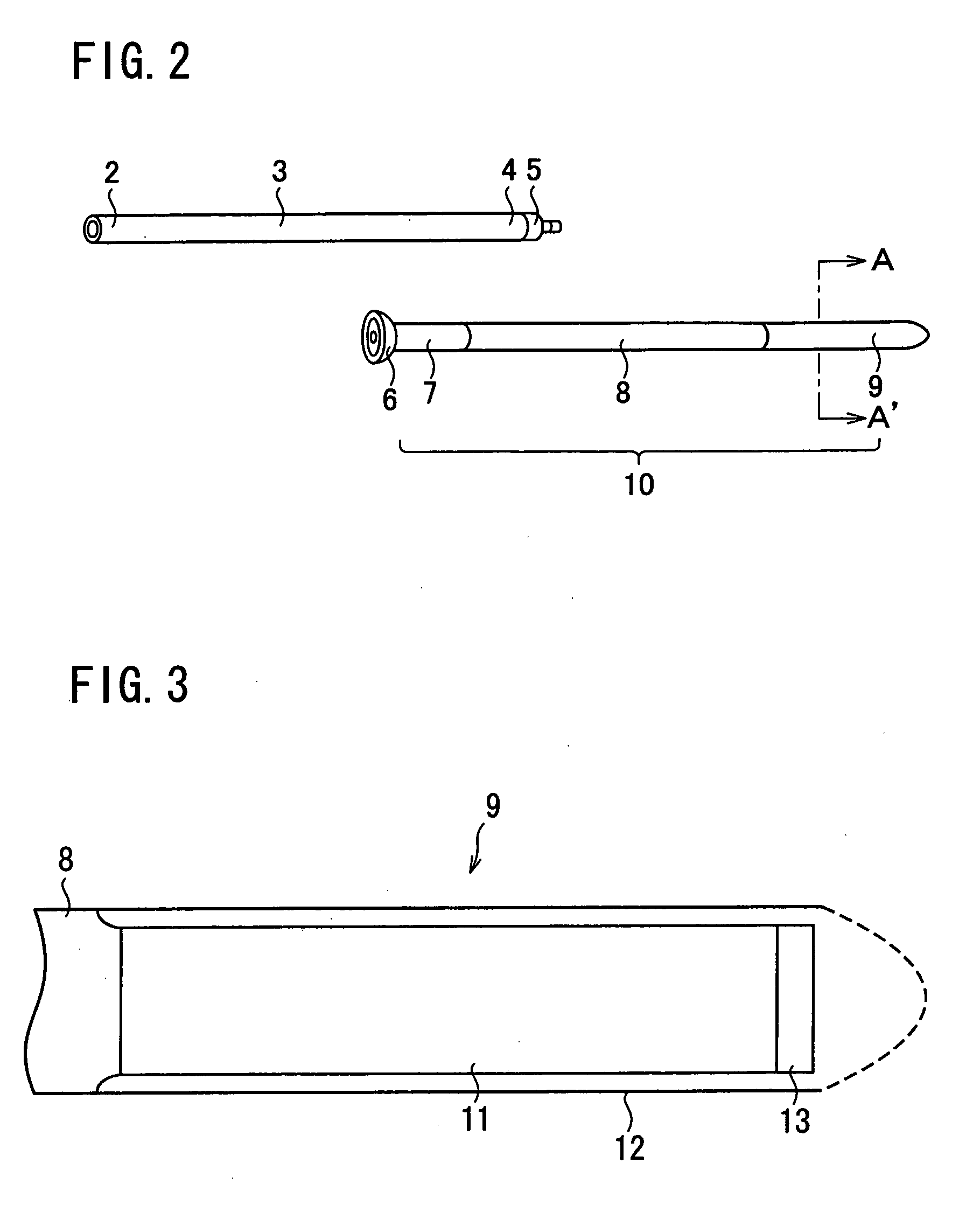
A first tube (3) and second tube (7) for guiding aqueous humor to the exterior of the eye are connected to each other in the vicinity of a surface of conjunctiva (14) via a first joint (5) and second joint (6). A filter part (9) provided to prevent reflux infection from the exterior to interior of the eye is connected to the second tube (7) via a third tube (8) positioned inside a lower lacrimal canaliculus (23). This enables an aqueous humor drainage implant (1) to be positioned in the eye and the exterior of the conjunctiva with reduced invasiveness. With the aqueous humor drainage implant for glaucoma treatment, the aqueous humor in the eye can be drained to the exterior of the conjunctiva while preventing reflux infection at the viral level, and the intraocular pressure reducing effect can be sustained for extended time periods over the lifespan of the patient. Further, the aqueous humor drainage implant can be readily positioned with reduced surgical invasiveness, while posing no danger of damaging the eye or nasolacrimal duct after the installation.
Owner:JAPAN SCI & TECH CORP
Methods and kits for locking and disinfecting implanted catheters
InactiveUS6685694B2Reduce riskInhibiting fouling and plugging of the lumenDialysis systemsMedical devicesTriclosanThrombus
Implanted catheters are locked with a solution comprising a lower alcohol, typically ethanol, propanol, or butanol, in a range from 1% to 99% by volume, and an additive in a range from 1% to 99% by volume, the additive comprising an anti-microbial, typically taurolidine or triclosan, or an anti-coagulant, typically riboflavin, sodium citrate, ethylene diamine tetraacetic acid, or citric acid. The use of an alcohol and additive solution can effectively reduce fouling of the catheter, particularly clotting and thrombus in intravascular catheters, as well as eradicate existing infections and / or reduce the risk of potential infections. Existing infections and / or potential infections can be further reduced by employing a catheter body which permits an anti-microbial solution to penetrate into the catheter body and preferably through the catheter into tissue surrounding the implanted catheter.
Owner:EXCELSIOR MEDICAL
Electro spun nanofibrous wound dressing and a method of synthesizing the same
InactiveUS20130150763A1Improve mechanical propertiesGood chemical propertiesNon-adhesive dressingsAdhesive dressingsCross-linkWound dressing
The various embodiments herein provide an electro spun wound dressing comprising three nano fibrous layers and a method of synthesizing the same. The outer polymeric layer acts as a support layer for the other two layers and is hydrophilic in nature. The middle layer comprises a genipin cross linked reservoir layer loaded with a herbal extract of Melilotus officinalis and is hydrophobic or hydrophilic in nature. The second layer is cross-linked to control a release of the Melilotus officinalis extract in an aqueous environment. The third layer is a wound contacting inner layer comprising a non-cross-linked chitosan based nanofibrous layer that is hydrophilic in nature. Various additives are provided to enhance the mechanical or the chemical properties of the wound dressing and to control a release profile of the herbal extract.
Owner:MIRZAEI ESMAEIL +3
Human monoclonal antibodies against Bacillus anthracis protective antigen
ActiveUS7456264B2Avoid infectionNeutralizing activityAntibacterial agentsBacteriaAntigenV(D)J recombination
Isolated human monoclonal antibodies which bind to Anthrax protective antigen are disclosed. The human antibodies can be produced in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are derivatives of the human antibodies (e.g., bispecific antibodies and immunoconjugates), pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:ER SQUIBB & SONS INC
Rabies vaccine
ActiveUS20160166711A1Easy to identifyFaster and strong attackSsRNA viruses negative-senseVirus peptidesCoding regionRabies vaccine
The present invention relates to an mRNA sequence, comprising a coding region, encoding at least one antigenic peptide or protein of Rabies virus or a fragment, variant or derivative thereof. Additionally the present invention relates to a composition comprising a plurality of mRNA sequences comprising a coding region, encoding at least one antigenic peptide or protein of Rabies virus or a fragment, variant or derivative thereof.Furthermore it also discloses the use of the mRNA sequence or the composition comprising a plurality of mRNA sequences for the preparation of a pharmaceutical composition, especially a vaccine, e.g. for use in the prophylaxis or treatment of Rabies virus infections. The present invention further describes a method of treatment or prophylaxis of rabies using the mRNA sequence.
Owner:CUREVAC AG
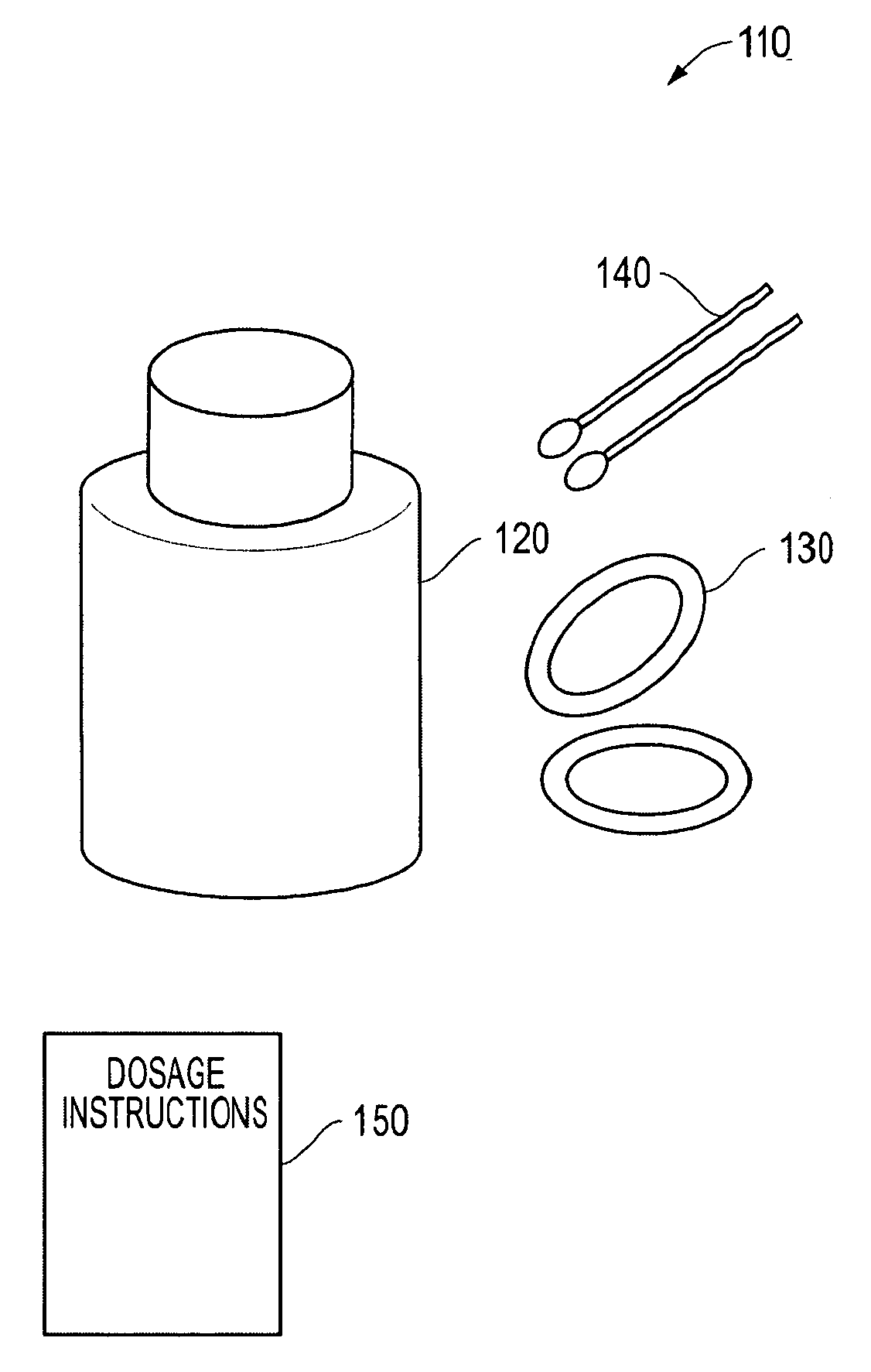
Convenience Kit for Eyelid Treatment
The present invention offers an eyelid treatment kit used for convenient combination therapy for improving overall eyelid hygiene while also providing for adjunctive eyelid therapy. The eyelid treatment kit comprises low dose doxycycline hyclate tablets, a non-irritating eyelid cleansing composition, an anti-bacterial eyelid preparation and at least one pair of moist heat goggles and / or one pair of moisture chamber goggles. The eyelid treatment kit further comprises instruction sheets containing dosage and administration information on the doxycycline hyclate coupled with information on improving eyelid hygiene. The various embodiments of the eyelid treatment kit of the invention facilitate treatment of dry eyes due to infected eyelids, and proper cleansing of the eyelids to prevent recurring infections.
Owner:OCUSOFT
Compositions and methods for targeted inactivation of HIV cell surface receptors
InactiveUS20110262406A1Avoid infectionReduce viral loadBiocideOrganic active ingredientsImmunodeficiency virusTail
Compositions for targeted mutagenesis of cell surface receptors for HIV and methods of their use are provided herein. The compositions include triplex-forming molecules that displace the polypyrimidine strand of target duplex and form a triple-stranded structure and hybrid duplex in a sequence specific manner with the polypurine strand of the target duplex. The triplex-forming molecules include a mixed-sequence “tail” which increases the stringency of binding to the target duplex, improves the frequency of modification at the target site, and reduces the requirement for a polypurine:polypyrimidine stretch. Methods for using the triplex-forming molecules in combination with one or more donor oligonucleotides for targeted modification of sites within or adjacent to genes that encodes cell surface receptors for human immunodeficiency virus (HIV) are also disclosed. Methods for ex vivo and in vivo prophylaxis and therapy of HIV infection using the disclosed compositions are also provided.
Owner:YALE UNIV
Device for delivery of antimicrobial agent into trans-dermal catheter
ActiveUS20100106103A1Reduce the risk of infectionReduce infection rateMedical devicesCatheterGuide tubeGeneral surgery
A system for delivering an antimicrobial agent into the lumen of a trans-dermal catheter. In an embodiment, the system comprises an elongate member configured for insertion into a lumen of a catheter; an expandable portion of the elongate member, said expandable portion configured to increase in diameter upon exposure to an aqueous fluid; and an antimicrobial composition positioned to be delivered into the catheter. In another embodiment, the system comprises an elongate member configured for insertion into a lumen of a trans-dermal catheter, said elongate member comprising a hydrogel; and an antimicrobial composition positioned to be delivered into the catheter; wherein the elongate member defines a volume of liquid that is at least substantially contained within the lumen of the trans-dermal catheter.
Owner:ICU MEDICAL INC
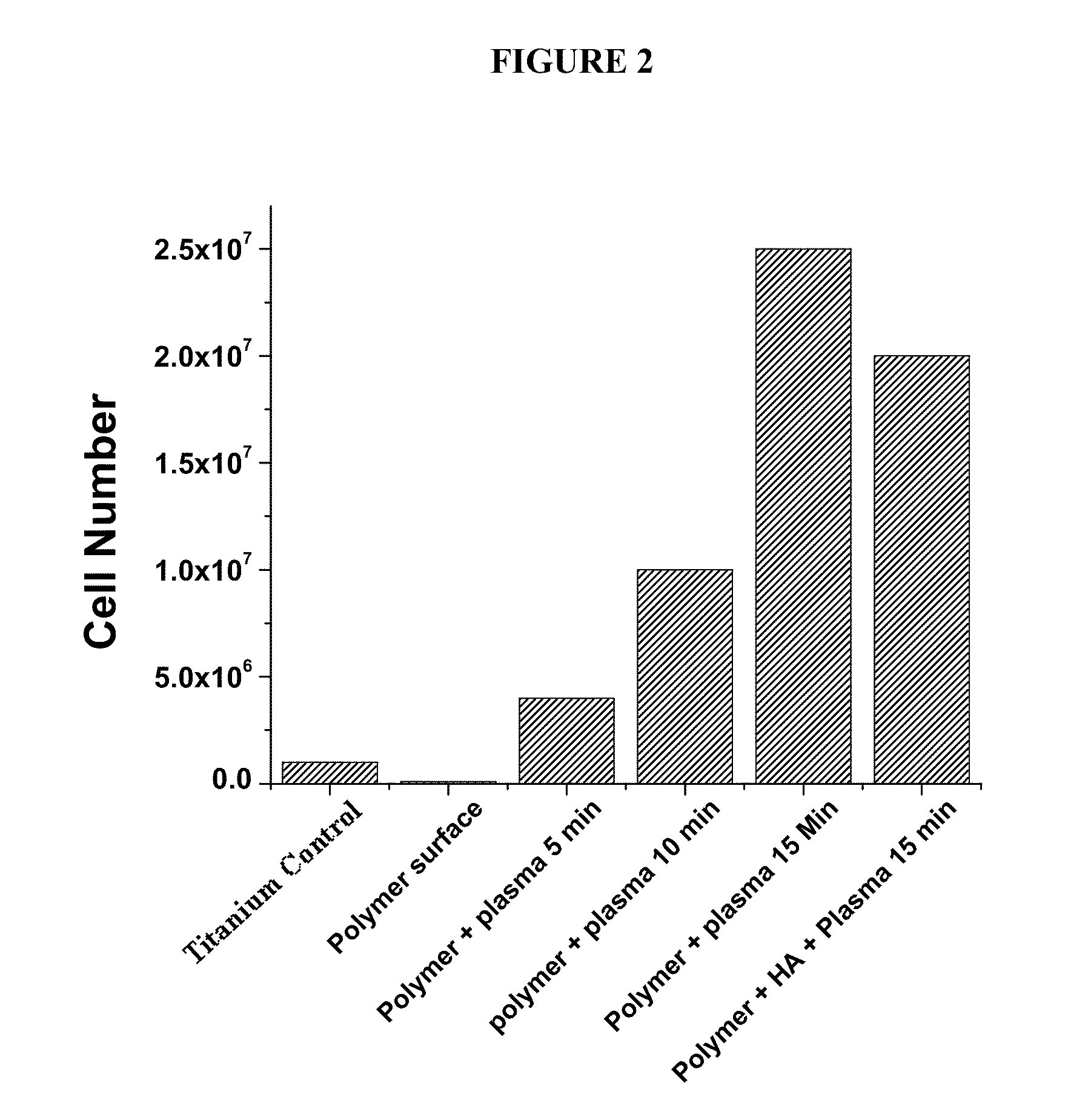
Advanced bio-compatible polymer surface coatings for implants and tissue engineering scaffolds
InactiveUS20100255447A1Improve bindingPromote cell adhesionOrganic active ingredientsDental implantsTissue engineering scaffoldChemistry
Disclosed herein are methodologies and compositions for coating materials, which can be used in a variety of biological applications.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Anti-infective functionalized surfaces and methods of making same
InactiveUS20100215643A1Preventing pin-site infectionReduce morbidityBiocideHeavy metal active ingredientsPolymer scienceMoiety
Devices are provided which are functionalized to include surface regions having anti-infective agents. Methods are provided for functionalizing various material surfaces to include active surface regions for binding anti-infective agents. Methods are provided by which anti-infective moieties or agents are bonded to functionalized surfaces.
Owner:ORTHOBOND
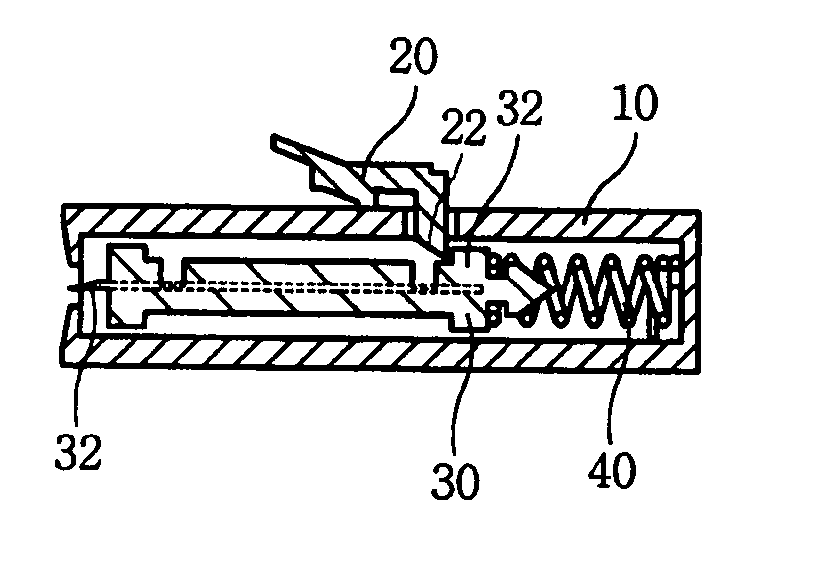
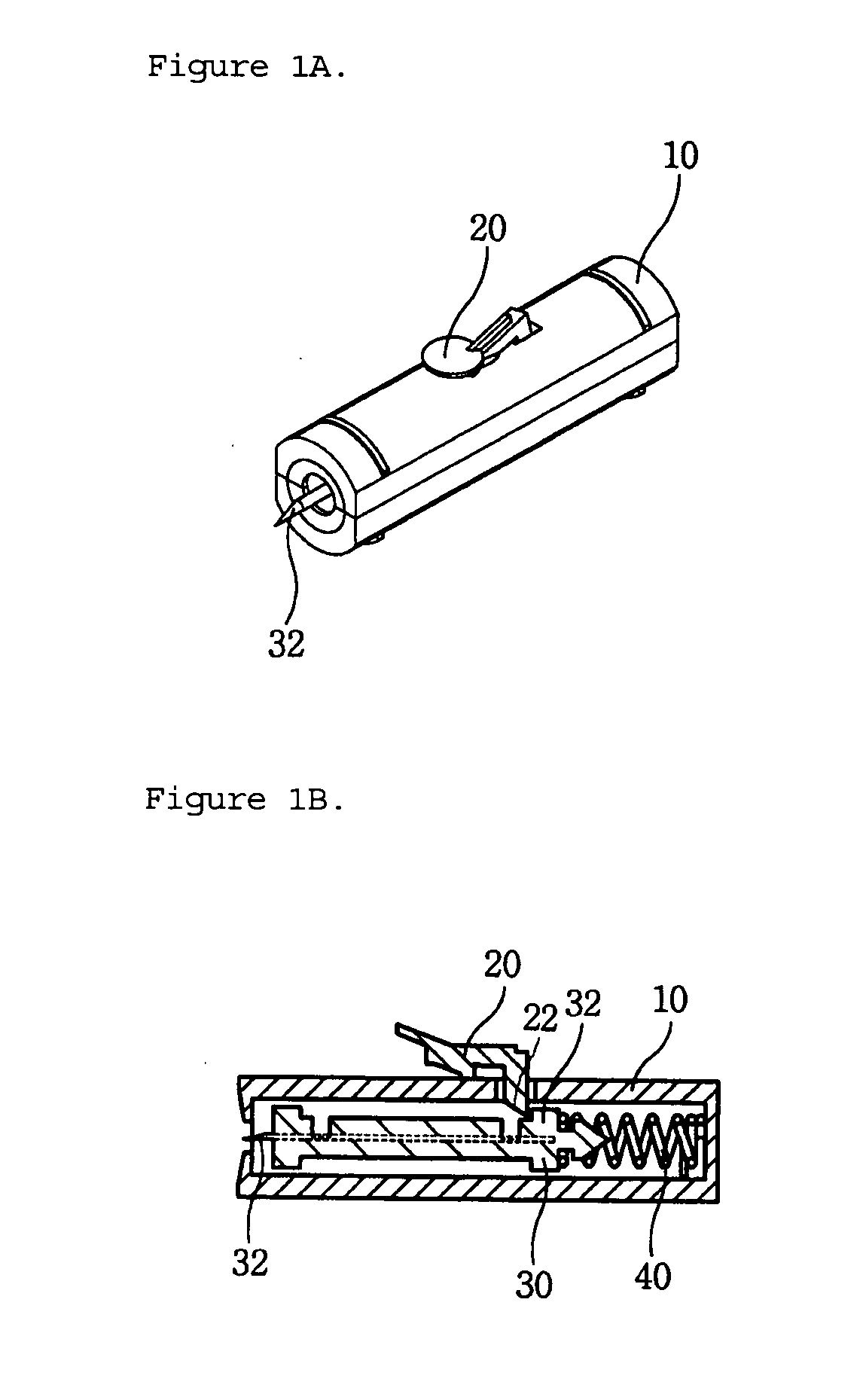
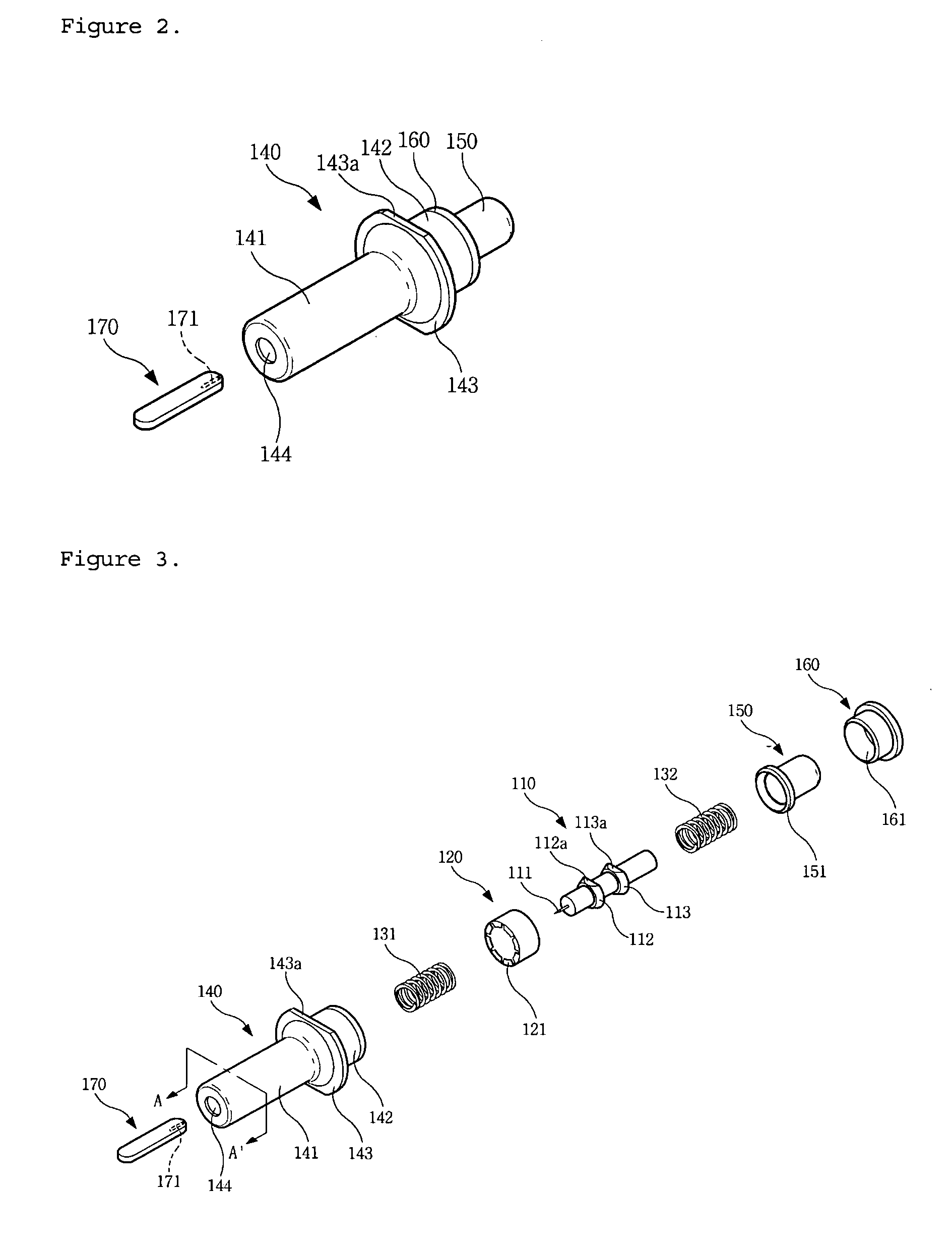
Single-use lancet device
InactiveUS20070078474A1Reduce component countEasy to assembleDiagnostic recording/measuringSensorsEngineeringMechanical engineering
Owner:KIM YONG PIL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com