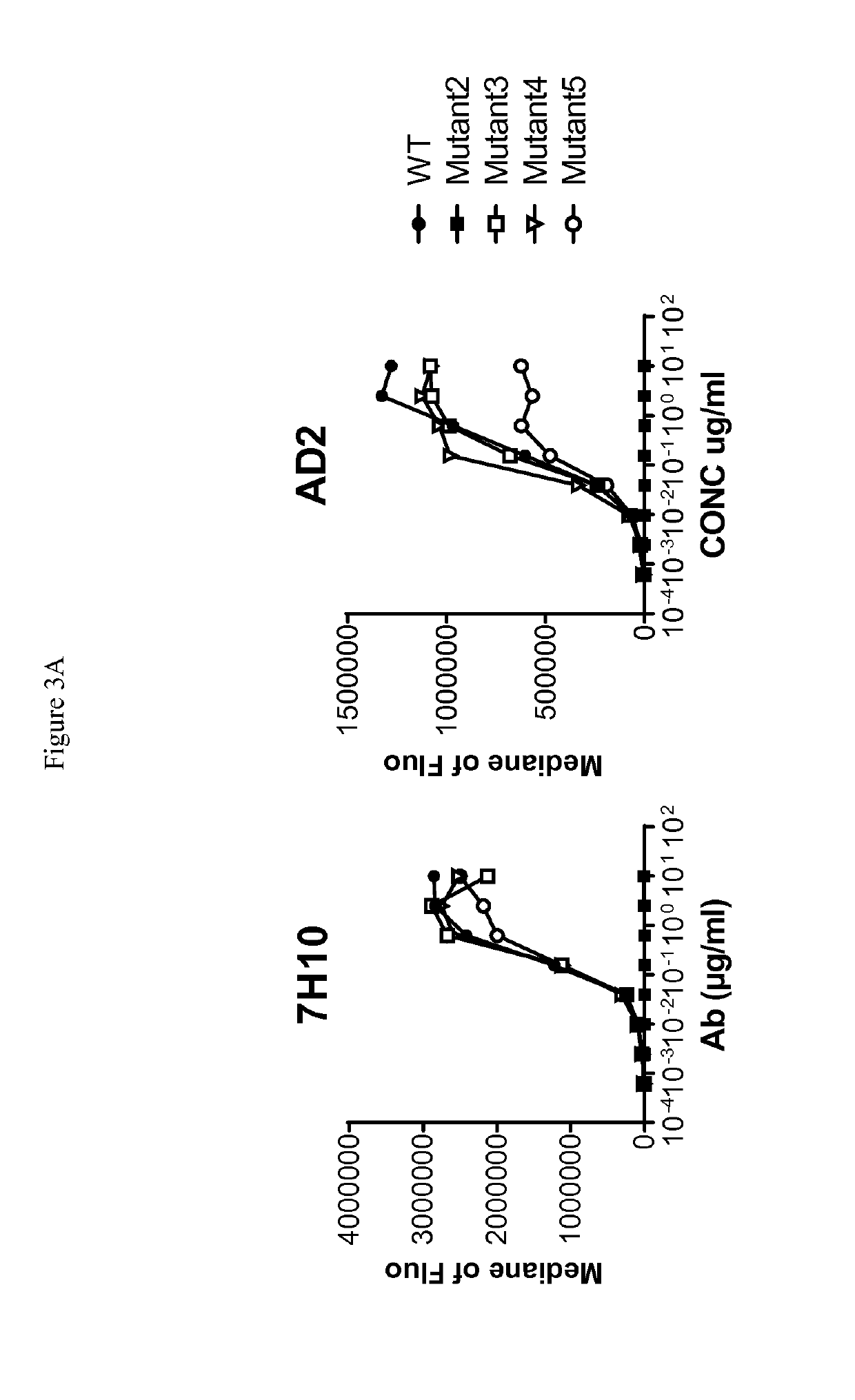
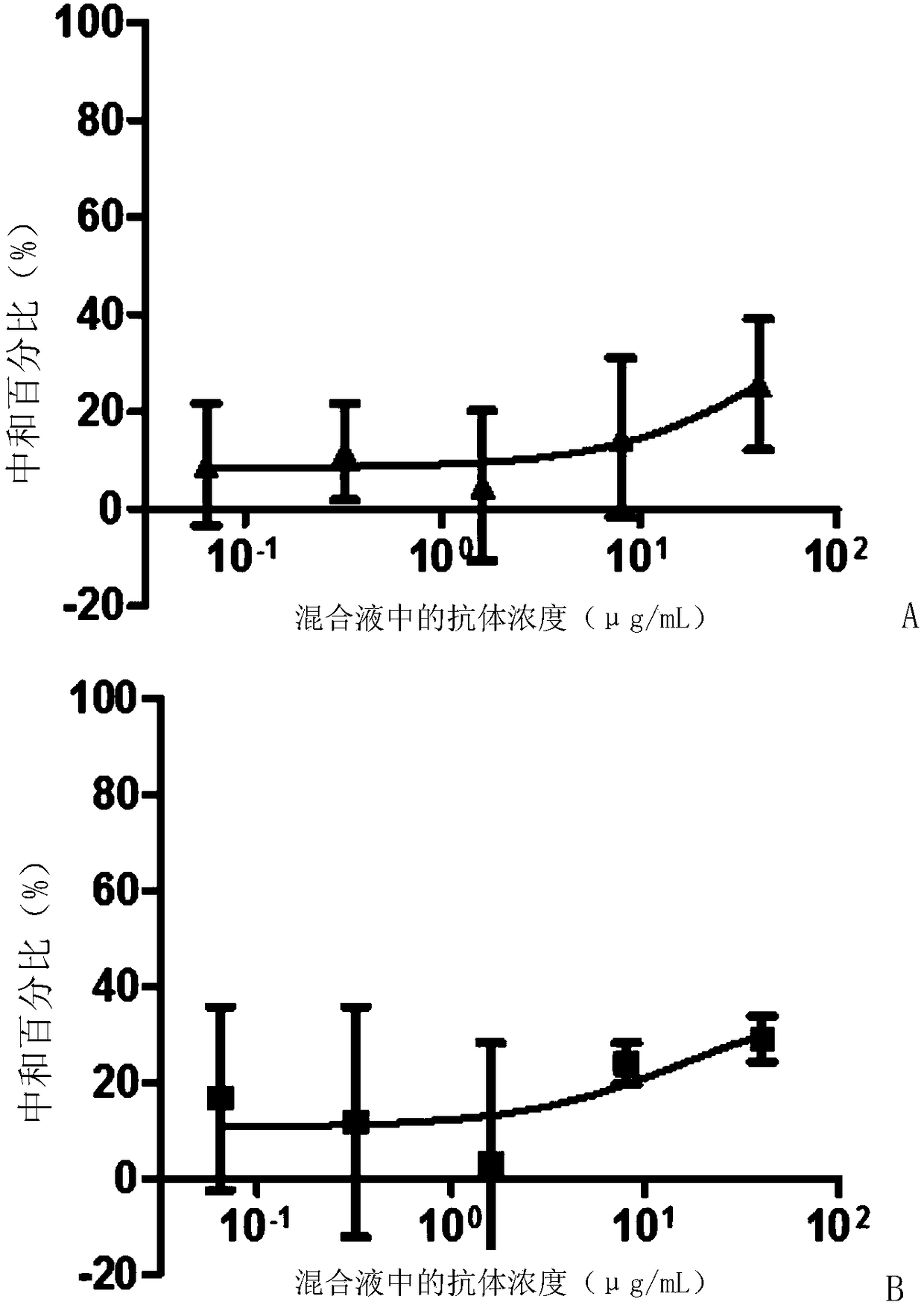
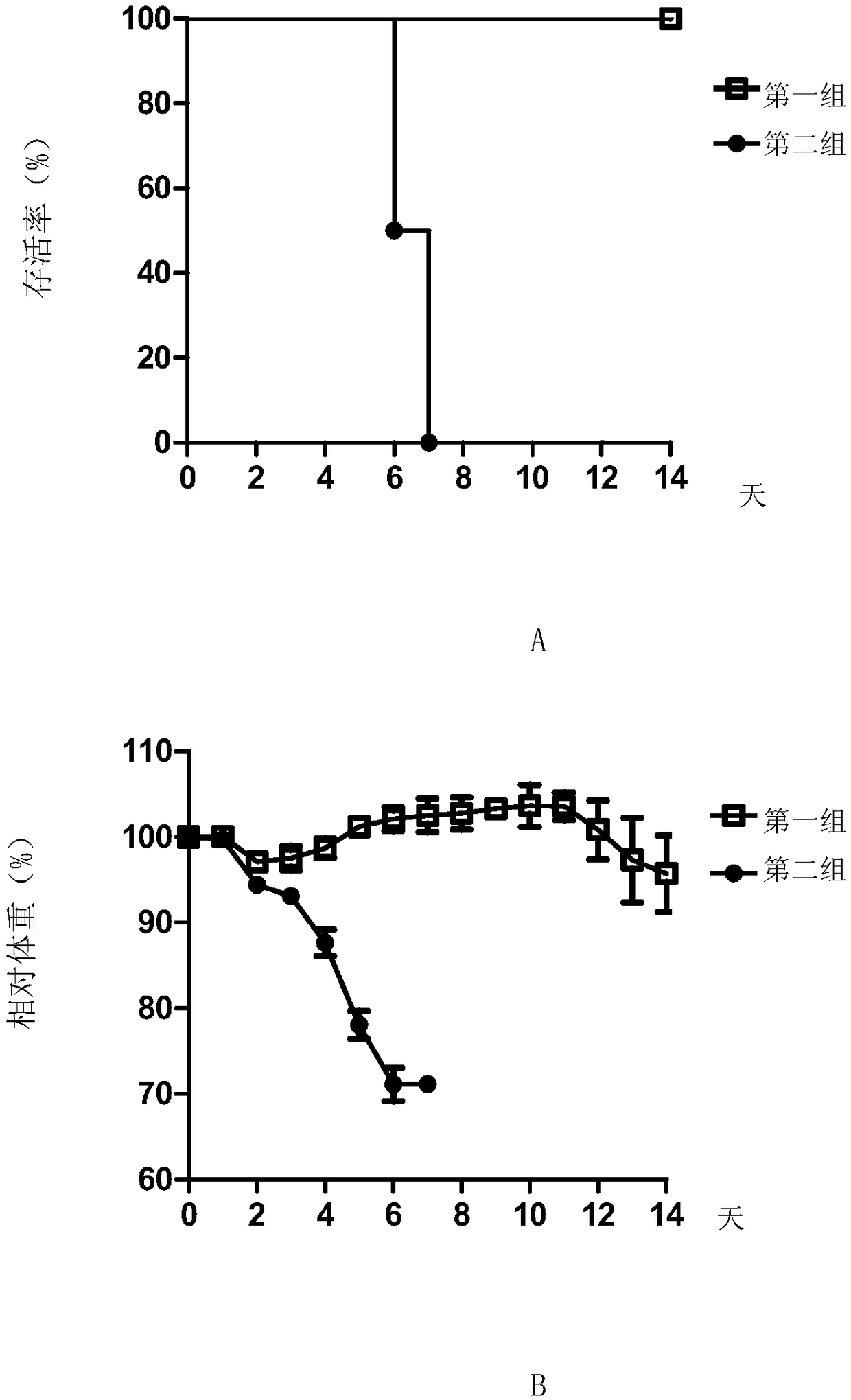
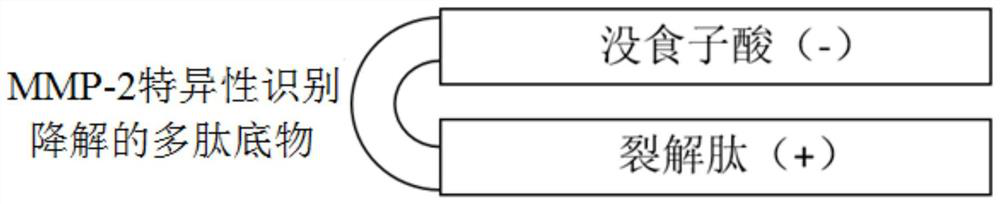
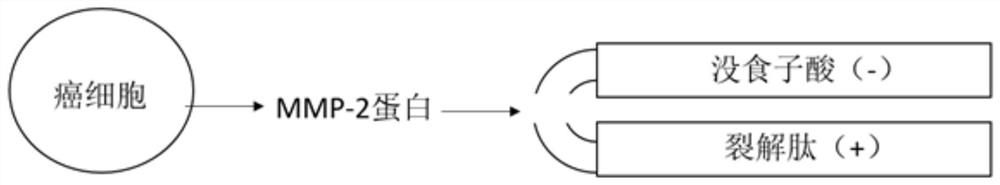
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
70results about How to "Neutralizing activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
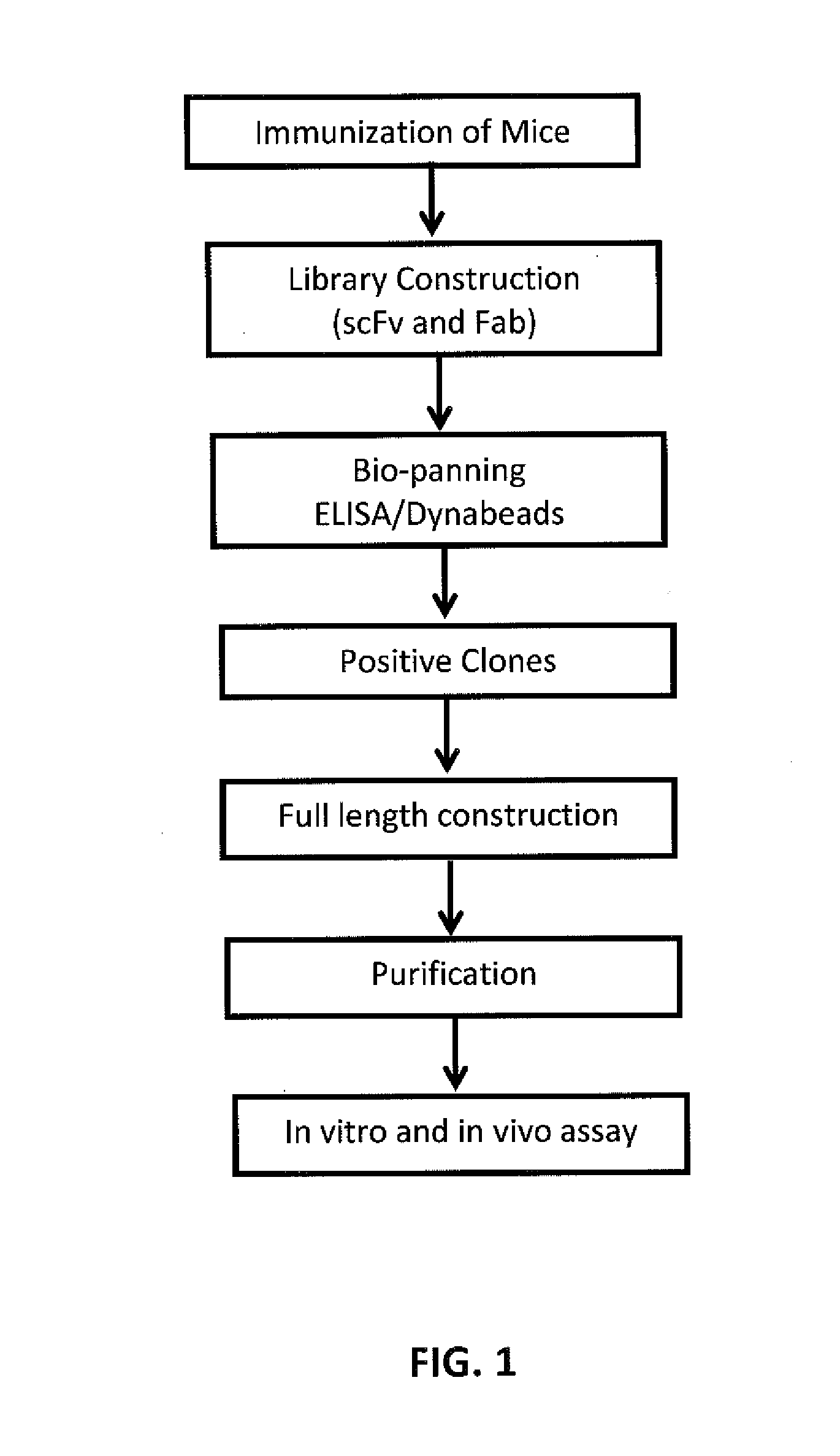
Human antibodies that bind human TNFalpha
InactiveUS20060024293A1High affinitySlow dissociation kineticsAntibacterial agentsOrganic active ingredientsHuman tumorAntigen binding
Human antibodies, preferably recombinant human antibodies, that specifically bind to human tumor necrosis factor α (hTNFα) are disclosed. These antibodies have high affinity for hTNFα (e.g., Kd=10−8 M or less), a slow off rate for hTNFα dissociation (e.g., Koff=10−3 sec−1 or less) and neutralize hTNFα activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. The antibodies, or antibody portions, of the invention are useful for detecting hTNFα and for inhibiting hTNFα activity, e.g., in a human subject suffering from a disorder in which hTNFα activity is detrimental. Nucleic acids, vectors and host cells for expressing the recombinant human antibodies of the invention, and methods of synthesizing the recombinant human antibodies, are also encompassed by the invention.
Owner:ABBVIE BIOTECHNOLOGY LTD
Human monoclonal antibodies against Bacillus anthracis protective antigen
ActiveUS7456264B2Avoid infectionNeutralizing activityAntibacterial agentsBacteriaAntigenV(D)J recombination
Isolated human monoclonal antibodies which bind to Anthrax protective antigen are disclosed. The human antibodies can be produced in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are derivatives of the human antibodies (e.g., bispecific antibodies and immunoconjugates), pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:ER SQUIBB & SONS INC
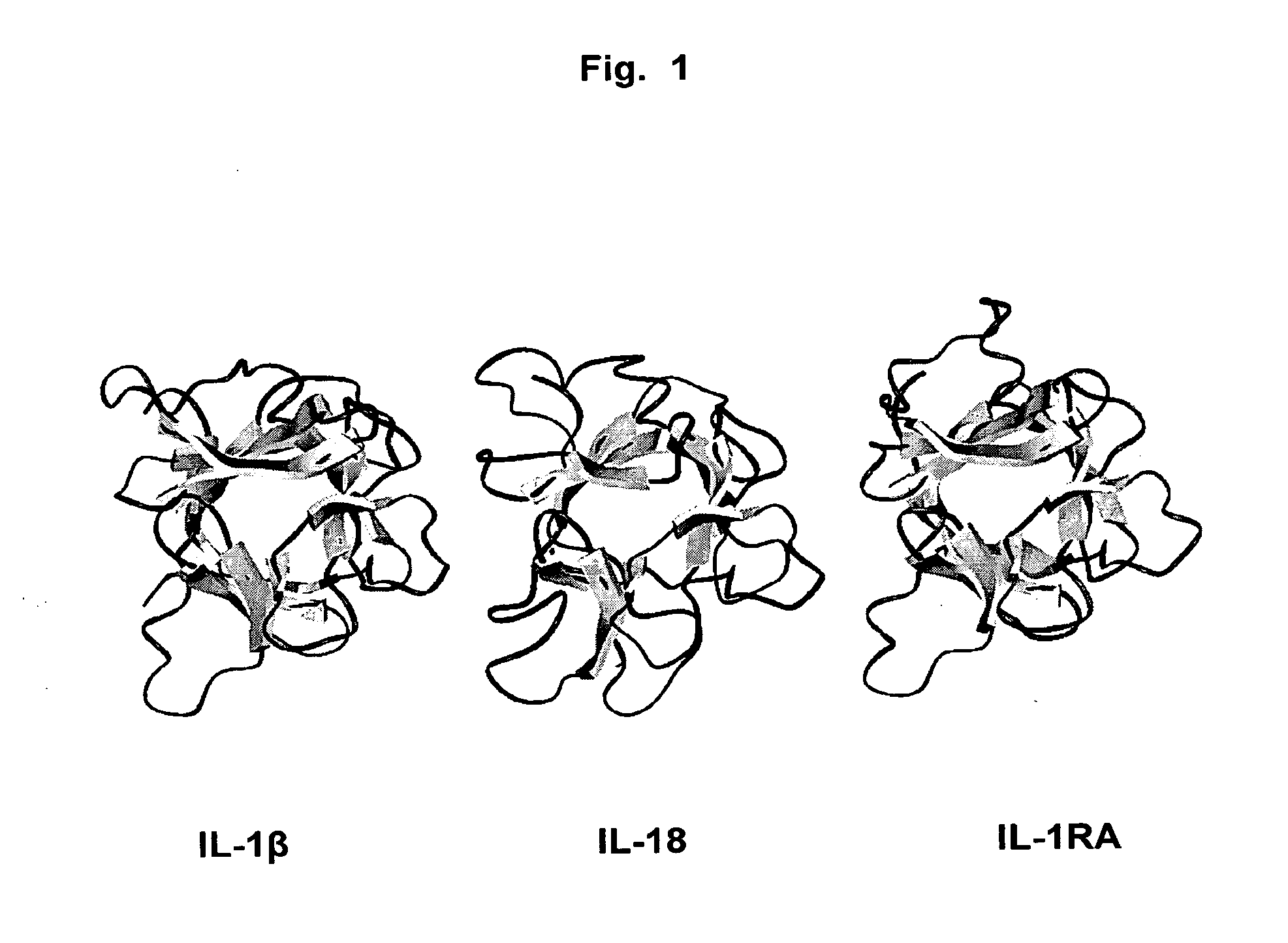
Antibodies that bind IL-18 and methods of inhibiting IL-18 activity
InactiveUS7767207B2Inhibit hIL-1 activityInhibitory activityAntibacterial agentsOrganic active ingredientsEpitopeAntigen binding
Antibodies that bind human interleukin-18 (hIL-18) are provided, in particular antibodies that bind epitope(s) of human IL-18. The antibodies can be, for example, entirely human antibodies, recombinant antibodies, or monoclonal antibodies. Preferred antibodies have high affinity for hIL-18 and neutralize hIL-18 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. Method of making and method of using the antibodies of the invention are also provided. The antibodies, or antibody portions, of the invention are useful for detecting hIL-18 and for inhibiting hIL-18 activity, e.g., in a human subject suffering from a disorder in which hIL-18 activity is detrimental.
Owner:ABBVIE INC
Antibodies for Botulinum Neurotoxins
The present disclosure provides antibodies that specifically bind to botulinum neurotoxins (e.g., BoNT / A, BoNT / B, BoNT / C, BoNT / D, BoNT / E, BoNT / F, BoNT / G, etc.) and the epitopes bound by those antibodies. The antibodies and derivatives thereof that specifically bind to the neutralizing epitopes provided herein can be used to neutralize botulinum neurotoxin and are therefore also useful in the treatment of botulism.
Owner:RGT UNIV OF CALIFORNIA
Antibodies that bind il-18 and methods of inhibiting il-18 activity
InactiveUS20100104563A1Inhibitory activityInhibit hIL-1 activityAntibacterial agentsOrganic active ingredientsEpitopeAntigen binding
Antibodies that bind human interleukin-18 (hIL-18) are provided, in particular antibodies that bind epitope(s) of human IL-18. The antibodies can be, for example, entirely human antibodies, recombinant antibodies, or monoclonal antibodies. Preferred antibodies have high affinity for hIL-18 and neutralize hIL-18 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. Method of making and method of using the antibodies of the invention are also provided. The antibodies, or antibody portions, of the invention are useful for detecting hIL-18 and for inhibiting hIL-18 activity, e.g., in a human subject suffering from a disorder in which hIL-18 activity is detrimental.
Owner:ABBVIE INC
Preparation method of virus-like particles (VLPs) of Chikungunya virus (CHIKV) and its application
InactiveCN102321639ANeutralizing activityViral antigen ingredientsInactivation/attenuationImmune effectsVirus-like particle
The invention relates to a preparation method of virus-like particles (VLPs) of Chikungunya virus (CHIKV). The method comprises the steps of: modifying genetic elements of a structural protein encoding gene C-E3-E2-6K-E1 of CHIKV, cloning the modified genetic elements into the expression vector of an insect cell, then transfecting the obtained recombined expression vector and baculovirus linear DNA respectively to an SF9 insect cell and making the cell secrete and express CHIKV VLPs. Additionally, the invention also makes preliminary studies on the immune effects of CHIKV VLPs and applicationof CHIKV VLPs in virus specific antibody detection, thus laying a foundation for research and preparation of immunological detection reagents and even vaccines based on CHIKV VLPs.
Owner:中国疾病预防控制中心病毒病预防控制所
Nano-particle metal treatment composition for creating a ceramic-metal layer
InactiveUS7304020B1Neutralizing activityExcellent plating characteristicMaterial nanotechnologyPigmenting treatmentNanoparticleMontmorillonite
A metal treatment composition including Tin (II) Chloride and processed montmorillonite clay. The addition of Tin (II) Chloride to the composition provides Tin for forming a ceramic-metal layer on the surfaces of the friction pair. Tin (II) Chloride provides Chlorine ions for forming Chloric films for protecting juvenile surfaces which form in the friction zone. The clay is heated and pulverized to produce a powder comprising both particles having crystalline layer structure and salts and oxides. The layered crystalline structure of the clay contains slip planes that transversely shift when tangential pressure from the friction pair is applied thereby lubricating the friction pair. The salts and oxides contribute to the formation of the ceramic-metal layer.
Owner:TANANKO DMITRY
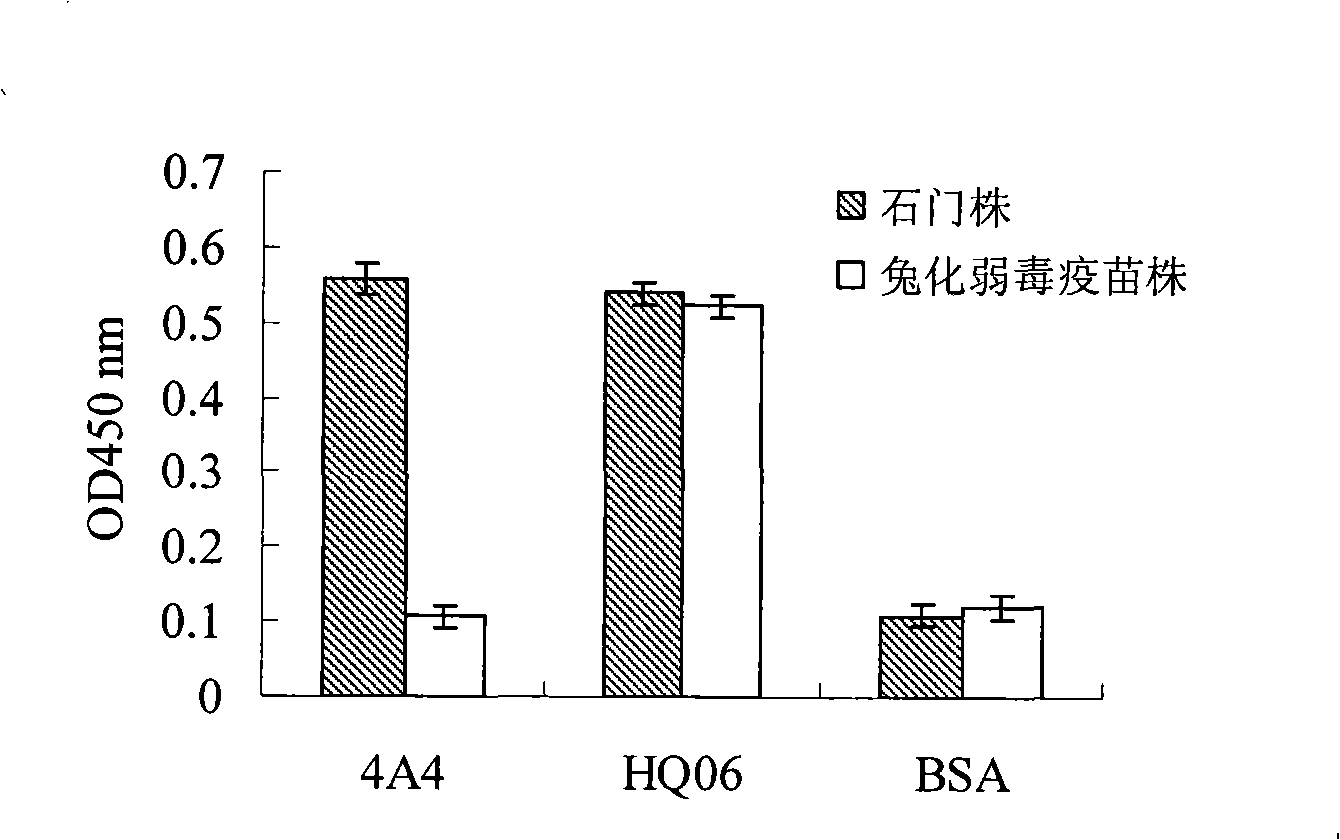
Monoclone antibody of swine fever virus resistant wild strain E2 protein, preparation method and application thereof
InactiveCN101294147ANeutralizing activityImmunoglobulins against virusesTissue cultureSwine Fever VirusCholera
The invention discloses a monoclonal antibody against virulent strain E2 protein of classical swine fever virus and a hybridoma cell strain secreting the monoclonal antibody. The hybridoma cell strain is obtained by using hog cholera lapinized virus vaccine strain E2 protein expressed by Baculovirus as tolerogen, selecting Shimen strain E2 protein as immunogen, immunizing mouse by cyclophosphamide immunosuppression method, carrying out cell fusion, and sieving hybridoma cell strain capable of stably secreting monoclonal antibody against E2 protein. The monoclonal antibody can react with Shimen strain and can produce specific reaction with virulent strain of classical swine fever viruses of 1.1, 2.1, 2.2 and 2.3 gene sub-groups. The monoclonal antibody has neutralization activity and does not react with hog cholera lapinized virus vaccine strain, so that the monoclonal antibody can be used for differentiating virulent strain of classical swine fever virus and hog cholera lapinized virus vaccine strain, which establishes the foundation for establishing a method for differentiating wild virus infection of classical swine fever and vaccine immunity and for researching the molecular difference between CSFV virulent strain and mild strain.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Single-chain antibody for resisting S1 protein on surface of new coronavirus SARS-CoV-2 and application of single-chain antibody
ActiveCN113264998ANeutralizing activityAvoid infectionHybrid immunoglobulinsBiological material analysisSingle-Chain AntibodiesMolecular virology
The invention provides a single-chain antibody for resisting S1 protein on the surface of a new coronavirus SARS-CoV-2, and relates to the technical fields of cellular immunology and molecular virology. The monoclonal antibody comprises heavy-chain variable regions of an scFv-22 sequence and with amino acid sequences shown as SEQ ID NO. 1-3 and light-chain variable regions of the scFv-22 sequence and with amino acid sequences shown as SEQ ID NO. 5-7, or heavy-chain variable regions of a scFv-24 sequence and with amino acid sequences shown as SEQ ID NO. 9-11 and light-chain variable regions of tje scFv-24 sequence and with amino acid sequences shown as SEQ ID NO. 13-16. The single-chain antibody disclosed by the invention can specifically bind to S1 protein of the novel coronavirus SARS-CoV-2, has neutralizing activity on the novel coronavirus, effectively inhibits infection of the novel coronavirus on target cells, and can be used for developing drugs for preventing or treating novel coronavirus infection.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Heat shock transcription factor-binding protein
InactiveUS6485936B1Rule out the possibilityImprove purification effectBacteriaMicroorganism based processesA-DNAHsp70
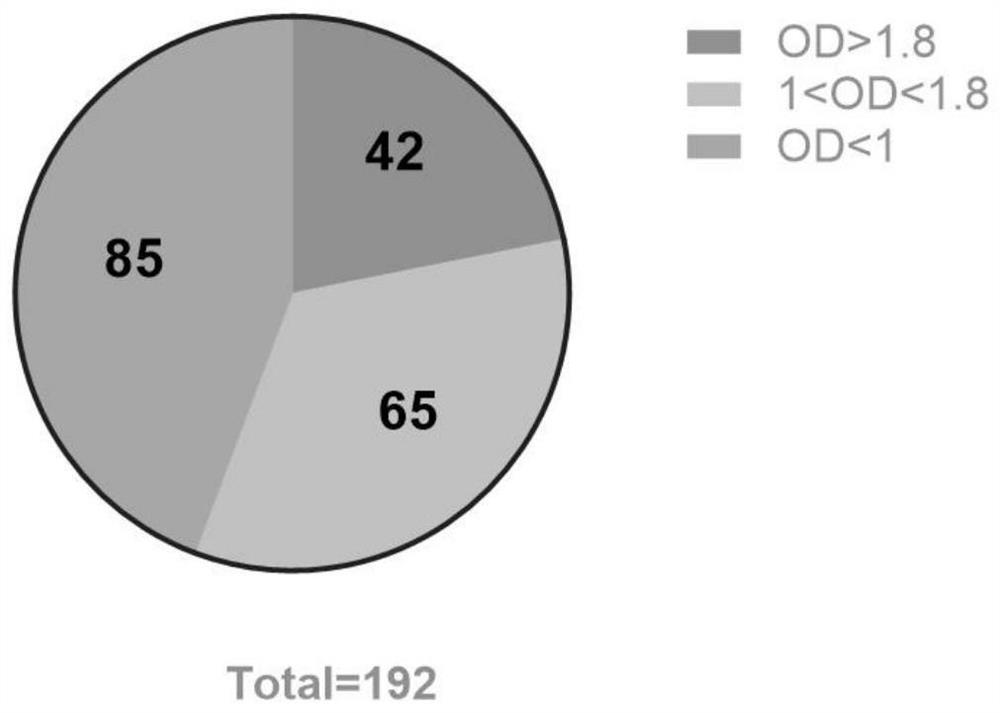
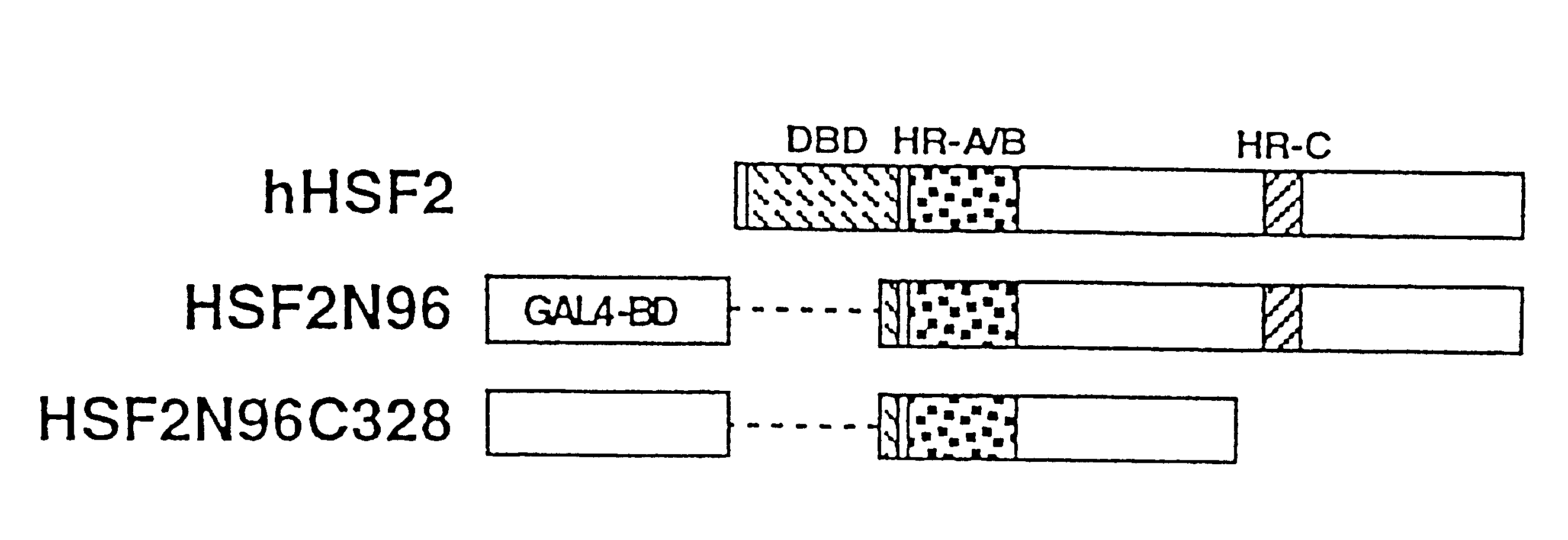
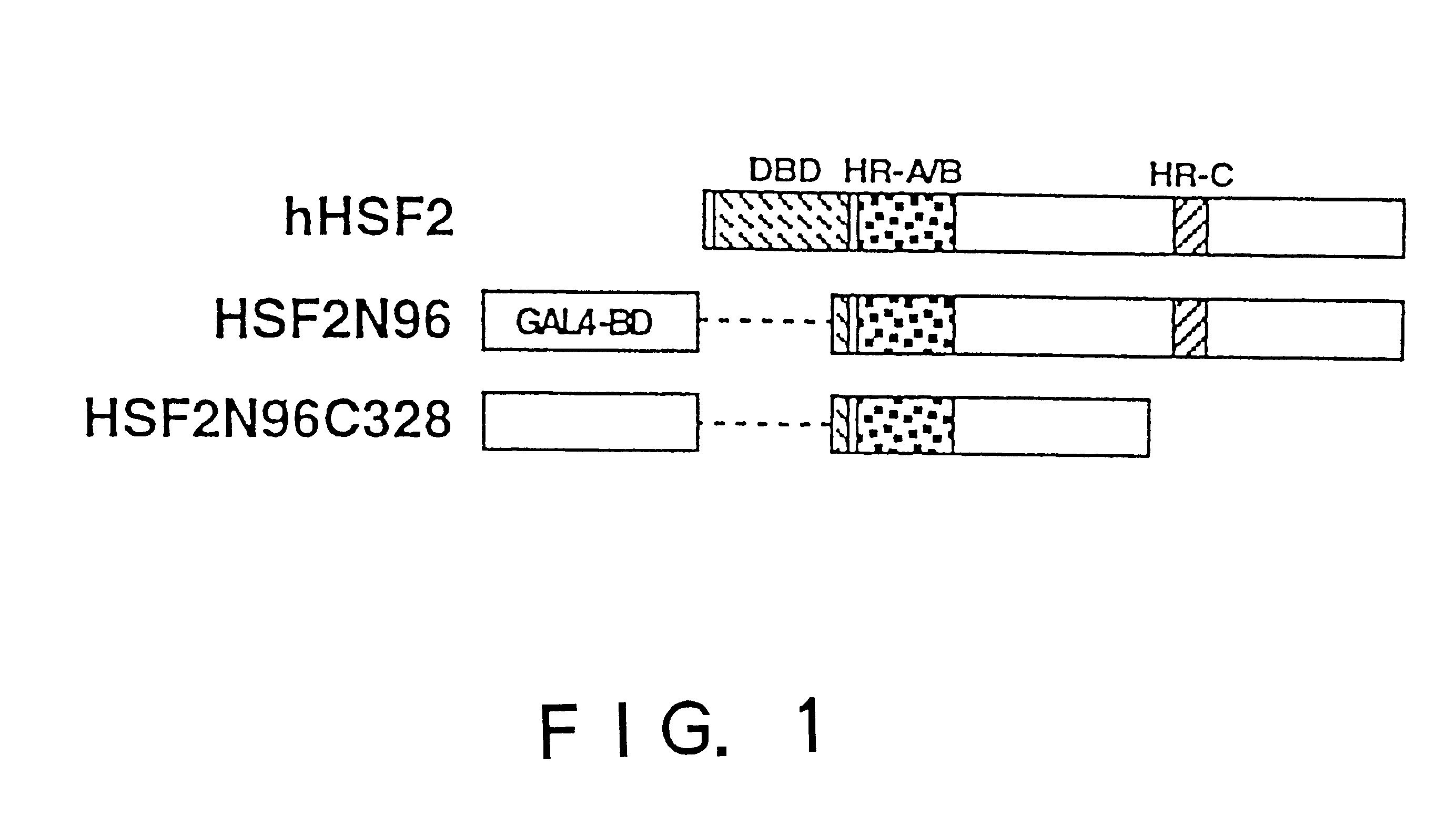
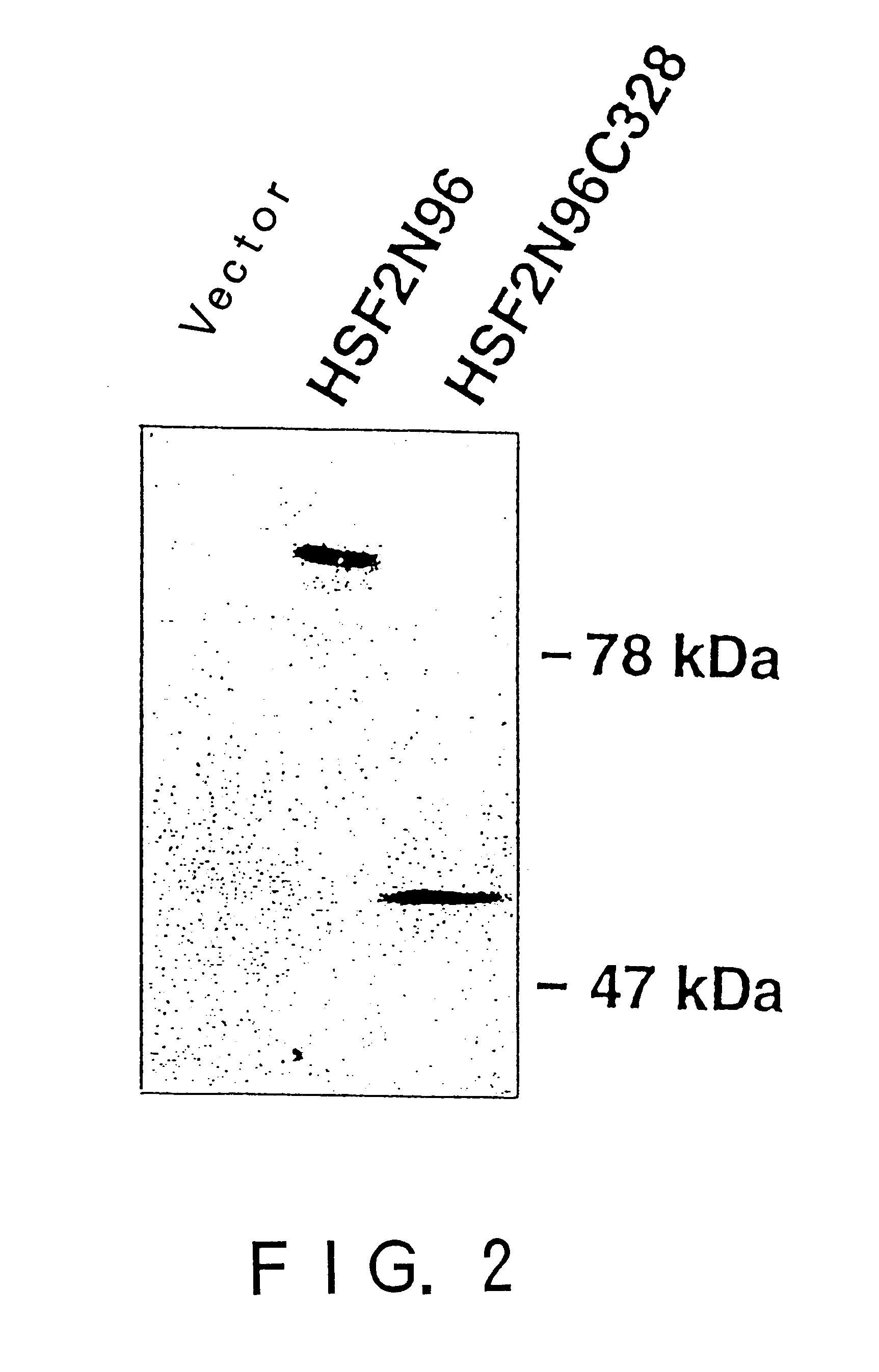
To provide a heat shock transcription factor (HSF) 2 binding factor, which can be involved in the transcriptional regulation of HSP70.2 playing an essential role in spermatogenesis; a DNA encoding the binding factor; an expression vector carrying the DNA; a transformant harboring the expression vector; a process for preparing a recombinant protein comprising the step of culturing the transformant; an antibody or a fragment thereof capable of specifically binding to the binding factor; an antisense DNA or antisense RNA complementary to the DNA; and an oligonudeotide probe or primer capable of specifically hybridizing to the DNA.
Owner:HSP RES INST
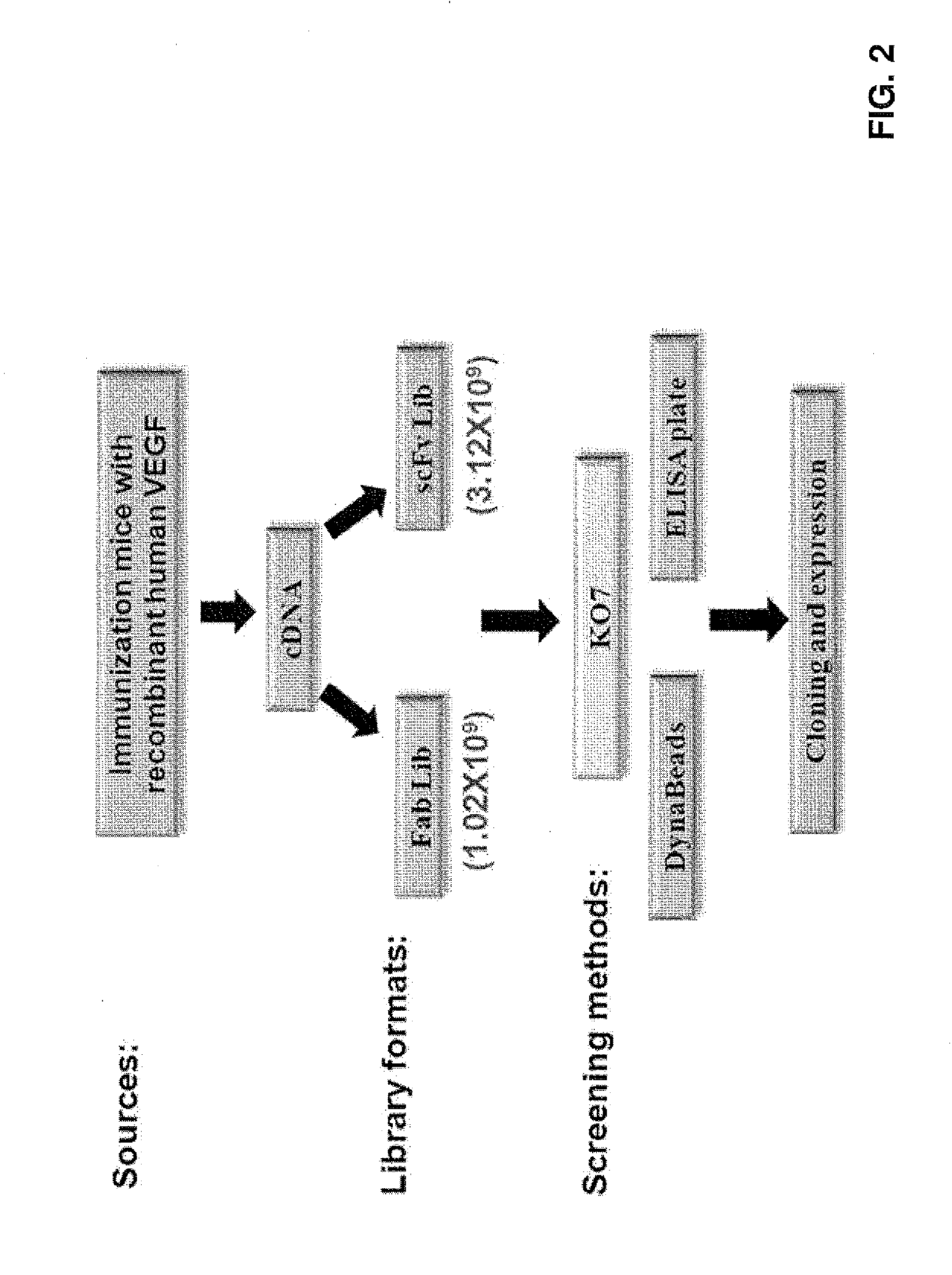
Anti-vegf antibodies and use thereof
InactiveUS20160130336A1Neutralizing activityAvoid confusionSenses disorderImmunoglobulins against growth factorsDiabetic retinopathyDisease
An anti-VEGF antibody, or a binding fragment thereof, includes a heavy-chain variable region that comprises: (1) a CDRH1 sequence selected from SEQ ID NO: 17, 20, 23, 26, 29, 32, 35, or 38), (2) a CDRH2 sequence selected from SEQ ID NO:18, 21, 24, 27, 30, 33, 36, or 39, and (3) a CDRH3 sequence selected from SEQ ID NO:19, 22, 25, 28, 31, 34, 37, or 40; and a light-chain variable region that comprises: (1) a CDRL1 sequence selected from SEQ ID NO: 41, 44, 47, 50, 53, 56, 59, or 62, (2) a CDRL2 sequence selected from SEQ ID NO: 42, 45, 48, 51, 54, 57, 60, or 63, and (3) a CDRL3 sequence selected from SEQ ID NO: 43, 46, 49, 52, 55, 58, 61, or 64. A method for treating or preventing a VEGF-related disorder, e.g., diabetic retinopathy, age-related macular degeneration, or cancer, uses the antibodies.
Owner:DEV CENT FOR BIOTECHNOLOGY +1
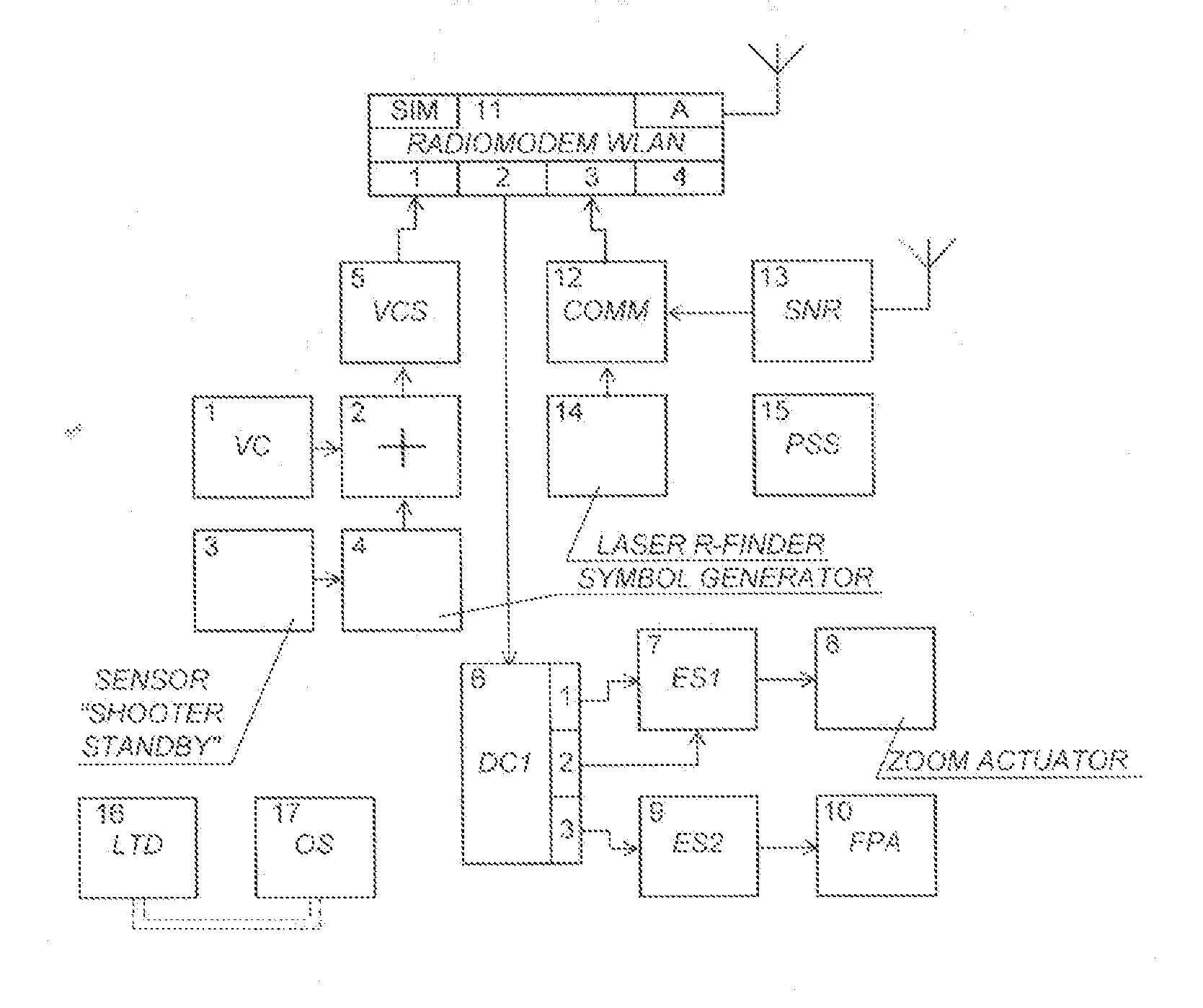
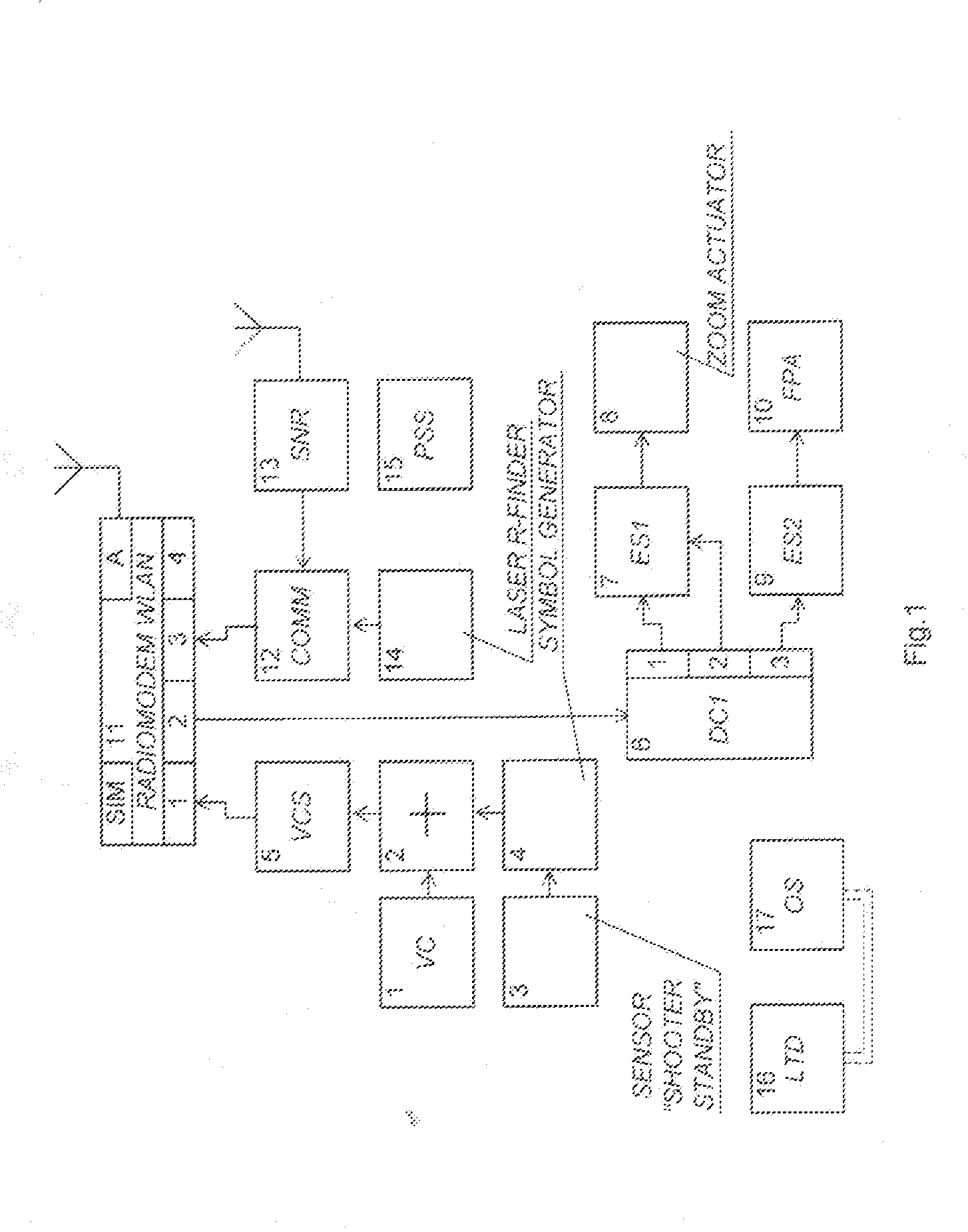
Management system of several snipers
InactiveUS20130118341A1Increasing target hit accuracyImplementation of operationFiring/trigger mechanismsAiming meansElectronic switchEngineering
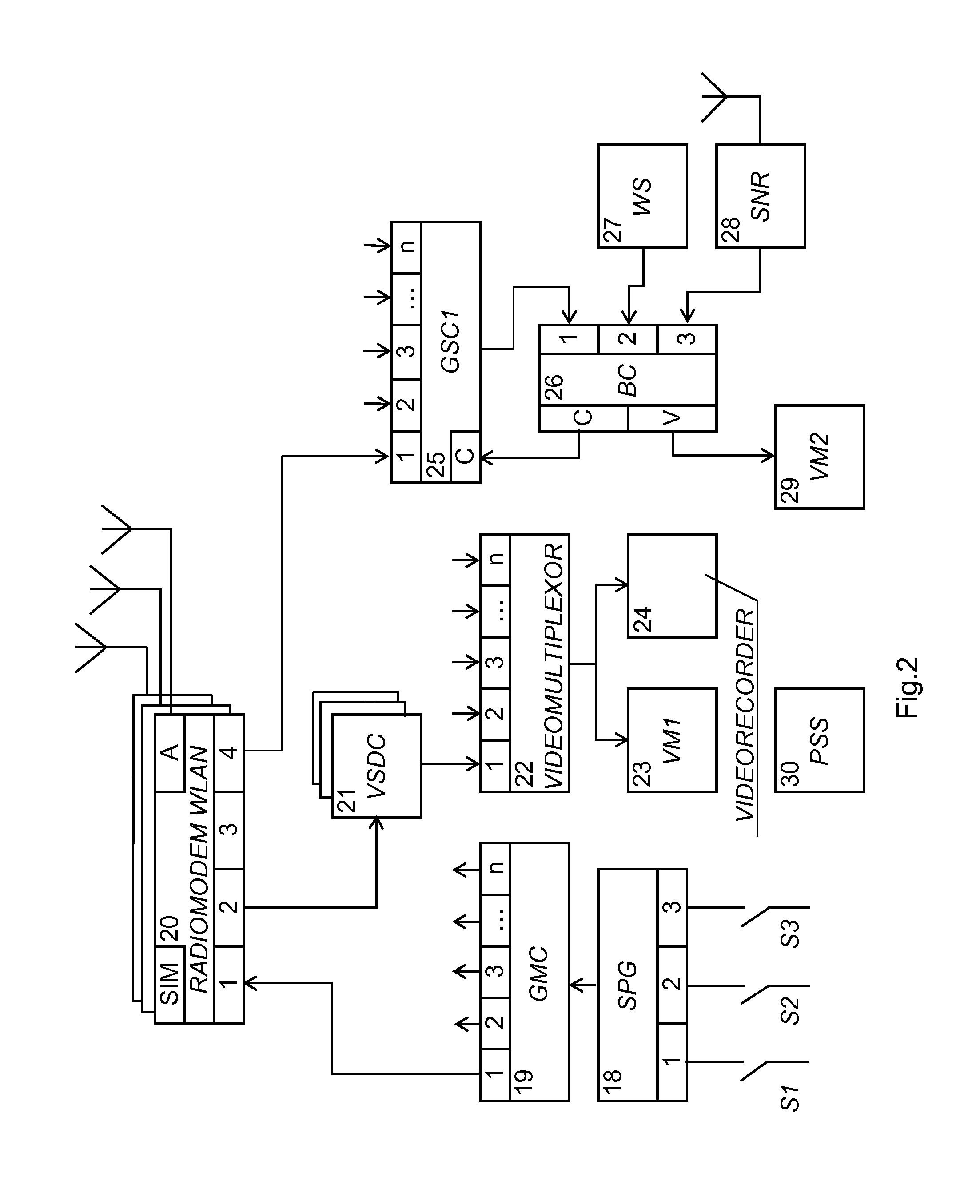
There is proposed a system for managing fire of snipers, including a central station (CS) and a plurality of N individual kits (IK), each operated by one sniper. Preferably, the IK includes: --a rifle, --an optical sight (OS) mounted on the rifle with a mechanism for correction of the OS, --equipment including devices activating the rifle's firing pin; --a laser target designator having an axis coinciding with the OS axis, satellite navigation receiver (SNR), video-camera, symbol generator, adding device summarizing output signals, readiness sensor installed on the rifle's trigger, command decoder; zoom-lens actuator; electronic switches controlling the zoom-lens actuator, laser rangefinder; commutator receiving output signals from the laser rangefinder and SNR, and radio-modem module (IK-RM) provided with a two-way communication with the CS furnished with certain devices specified therein. The system enhances the synchronousness and target hit accuracy, and is capable of counteraction to acoustic counter-sniper systems.
Owner:BRYLEV SERGEY FEDOROVICH
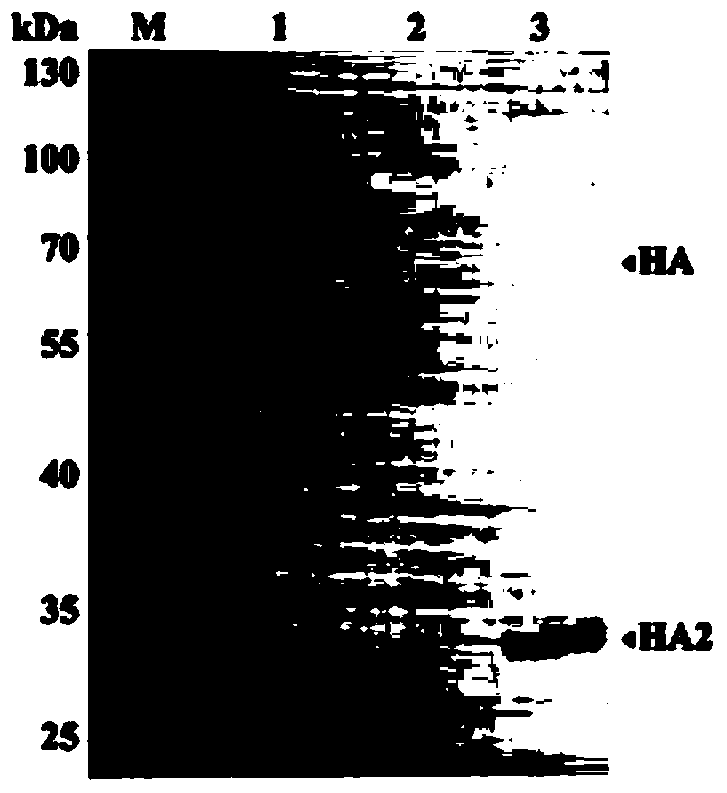
Single clone antibody aiming at H3N2 dog flu virus HA2 protein
ActiveCN104293739AJudgment of toxicityStrong specificityImmunoglobulins against virusesAntiviralsAntigenNeutralizing antibody
The invention provides a single clone antibody aiming at H3N2 dog flu virus HA2 protein, belonging to the technical field of biologics. The single clone antibody is prepared by inoculating an H3N2 dog flu virus Jiangsu separated strain JS / 10 separated in 2010 with SPF chicken embryos of 10 days old, collecting allantoic fluid of which the blood clotting titer is greater than or equal to 6, purifying virus inoculation MDCK cells, performing intracutaneous multipoint injection to inoculate mice, after cell fusion, performing three times of ELISA to detect cell supernate that cell colonies are generated, selecting an anti-H3 type hybridization tumor cell strain mAbD7, and recognizing and identifying the antigen so as to verify that the antibody aims at conservative H2A protein, wherein the neutralizing antibody titer is up to 1:12800, and the antibody subclass is IgG2b. The single clone antibody aiming at H3N2 dog flu virus and HA2 protein, which is provided by the invention, provides an important immunological preparation for treating H3N2 dog flu virus infection and has a potential application prospect.
Owner:NANJING AGRICULTURAL UNIVERSITY
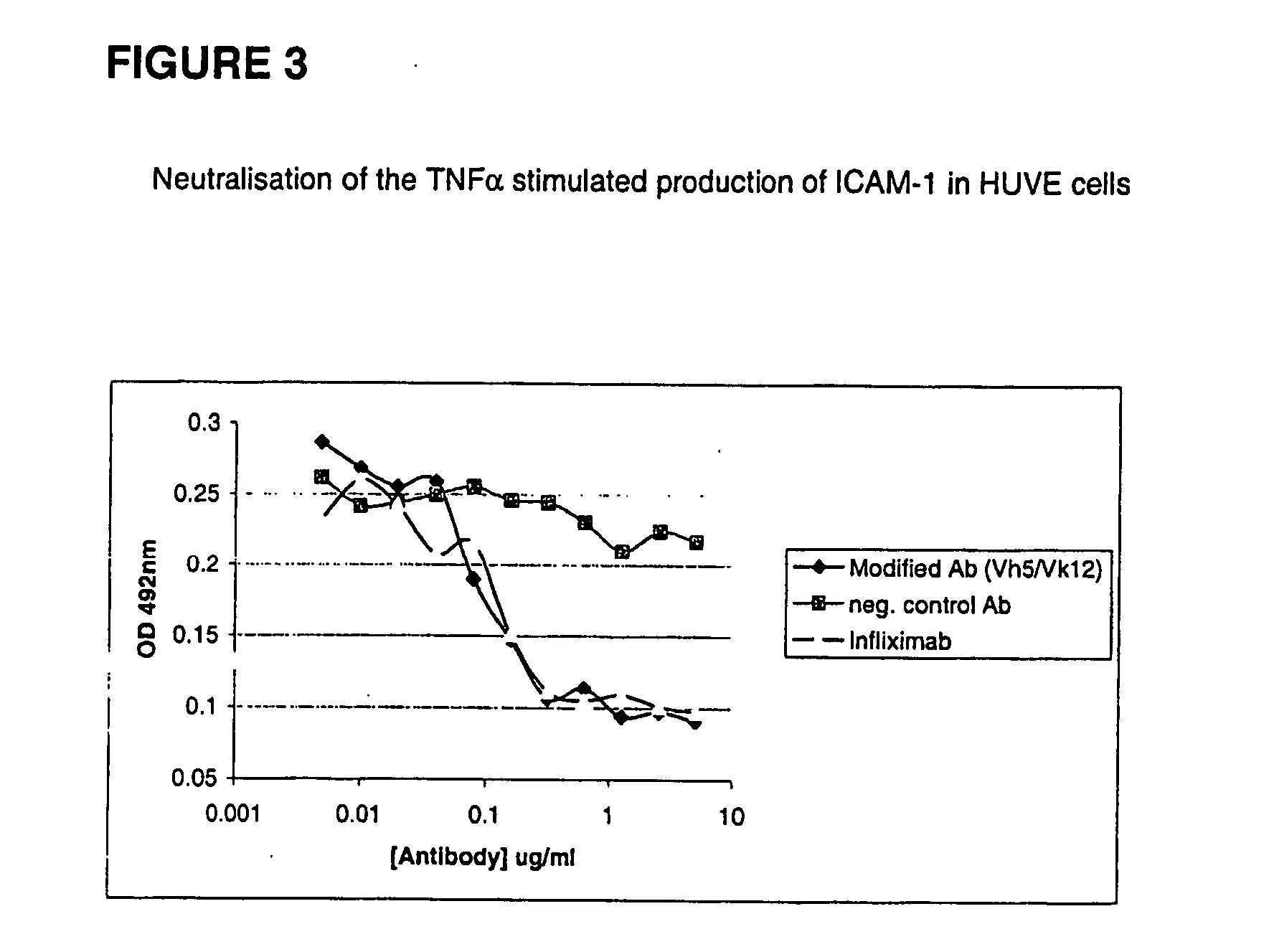
Modified anti-tnf aplha antibody
InactiveUS20040260069A1Modify characteristicLow immunogenicityPeptide/protein ingredientsAntipyreticHuman tumorV region
The invention relates to the modification of antibodies reactive to human tumor necrosis factor alpha (TNF alpha) to result in anti-TNF alpha antibodies that are substantially non-immunogenic or less immunogenic than any non-modified parental antibody when used in vivo. The invention relates also to peptide molecules comprising T-cell epitopes of the V-regions of the parental antibody which are modified by amino acid alteration in order to reduce or eliminate said T-cell epitopes.
Owner:MERCK PATENT GMBH
Carbonated dairy nutrient beverage and method of making a carbonated dairy nutrient beverage to supply the same nutrition of skim milk in the human diet
InactiveUS20110244076A1Increase attractivenessFast absorptionMilk preparationCocoaAdditive ingredientPasteurization
Carbonated dairy nutrient beverage solutions that supply essential nutrients in the human diet are disclosed. The solutions may contain per 8 oz., calcium, magnesium and potassium ions in the form of salts, vitamins A, D, C, and optionally lutein, zeaxanthin and folic acid in specified amounts to provide dietary supplementation. Sweeteners, stabilizers and flavors can also be added to enhance flavor, sensory appeal, mouth-feel, ingredient solubilization and product appearance. A method of making the beverages is also described. A method of using Carbon Dioxide and Nitrogen to reduce bacterial counts and reduce degradation of essential nutrients in dairy beverages with or without pasteurization is also disclosed.
Owner:CLARK GEORGE H +1
Monoclonal antibody targeting at Zika virus envelop protein conserved epitope and application thereof
ActiveCN109081868ASensitive and specific recognitionAvoid infectionImmunoglobulins against virusesAntiviralsZika virusViral Vaccine
The invention provides a monoclonal antibody targeting at Zika virus envelop protein conserved epitope and application thereof. specifically, a recombinant expressed Zika virus E-protein immune mouseis used herein to attain a murine monoclonal antibody specific to E-protein. Research results show that the antibody herein is an ideal Zika virus detection antibody and is applicable to the development antibody drugs against Zika virus. Identification of a conserved neutralizing epitope can also guide the development of broad-spectrum Zika virus vaccines.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Monoclonal antibody ZK2B10 and application
ActiveCN108727490ANeutralizing activityStrong specificityImmunoglobulins against virusesAntiviralsHeavy chainMonoclonal antibody
The invention discloses a monoclonal antibody ZK2B10 and application. The invention provides an IgG antibody named as ZK2B10 and composed of light chains and heavy chains. CDR1, CDR2 and CDR3 in a heavy chain variable region of the heavy chains are sequentially the No. 45-58 amino acid residues, No. 76-83 amino acid residues and No. 122-142 amino acid residues from N terminal in sequence 3 of a sequence table; CDR1, CDR2 and CDR3 in a light chain variable region of the light chains are sequentially the No. 45-52 amino acid residues, No. 70-72 amino acid residues and No. 109-120 amino acid residues from N terminal in sequence 5 of the sequence table. The invention further provides application of the IgG antibody in preparation of a medicine for inhibiting Zika virus. The antibody disclosedby the invention has significant application value on prevention and control of Zika virus diseases and produces profound social significance.
Owner:TSINGHUA UNIV +1
Piperazine derivatives for influenza virus inhibitions
ActiveUS20200010459A1Neutralizing activityCompetitive binding activityOrganic chemistryAntiviralsHemagglutininPharmaceutical drug
The present invention provides piperazine derivatives exhibiting high affinity to the stem region (viral membrane proximal part) of influenza hemagglutinin as determined through competition binding and high virus neutralization activity while having low cytotoxity. Furthermore, the present invention relates to pharmaceutical compositions comprising said piperazine derivatives, methods of preparing said piperazine derivatives, as well as said piperazine derivatives for use in medical prevention or treatment, especially for preventing or treating influenza.
Owner:JANSSEN VACCINES & PREVENTION BV
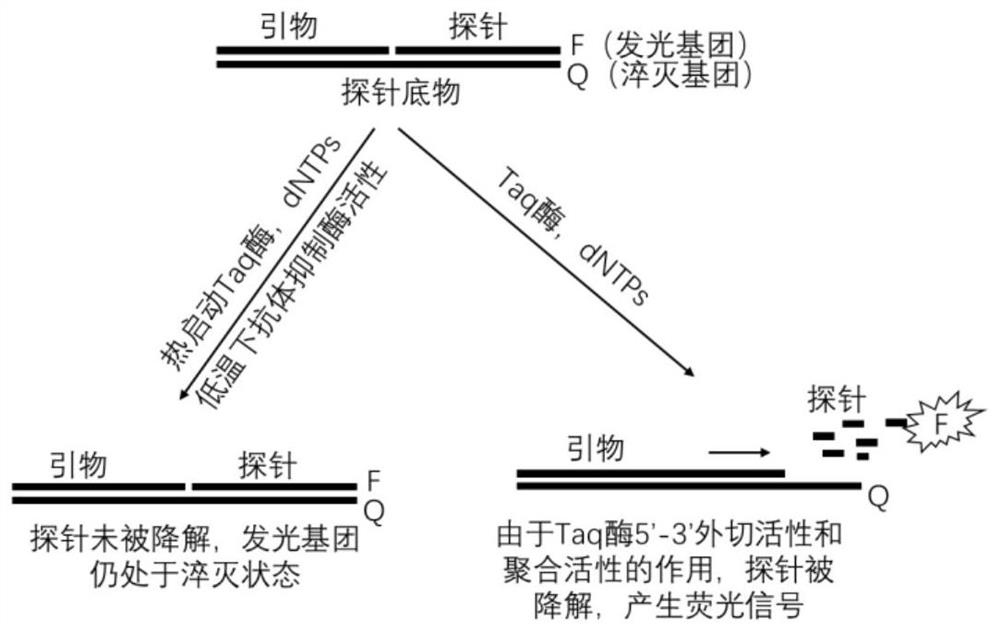
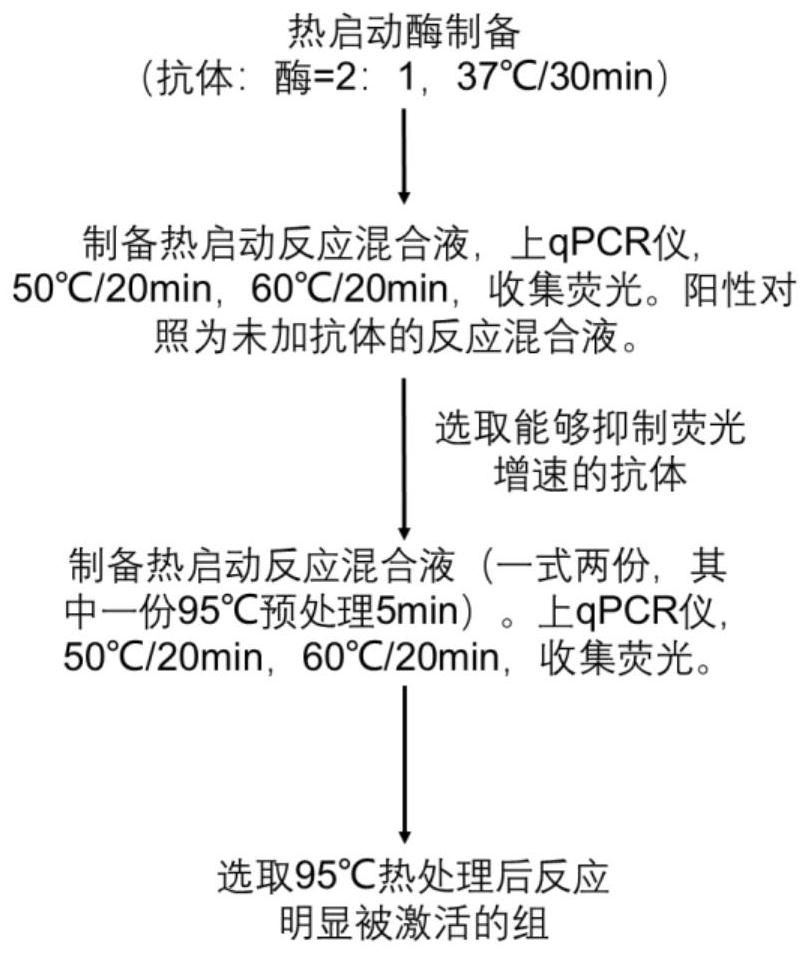
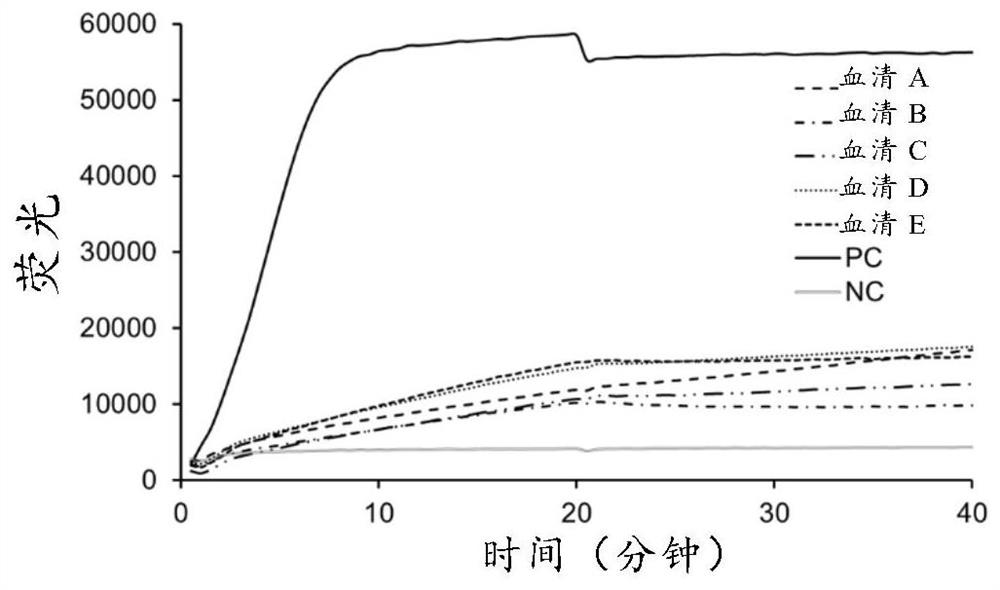
Monoclonal antibody specifically combined with Taq DNA polymerase and application thereof
ActiveCN114685671ANeutralization aggregationNeutralizing activityMicrobiological testing/measurementTransferasesAntiendomysial antibodiesBiochemistry
The invention relates to the field of antibodies, in particular to a monoclonal antibody specifically combined with Taq DNA polymerase and application of the monoclonal antibody. The monoclonal antibody provided by the invention is specifically combined with Taq DNA polymerase to neutralize the excision and / or polymerization activity of the Taq DNA polymerase, and releases the combined Taq DNA polymerase under a thermal activation condition, so that the excision and / or polymerization activity of the Taq DNA polymerase is recovered.
Owner:SHENZHEN HUADA GENE INST
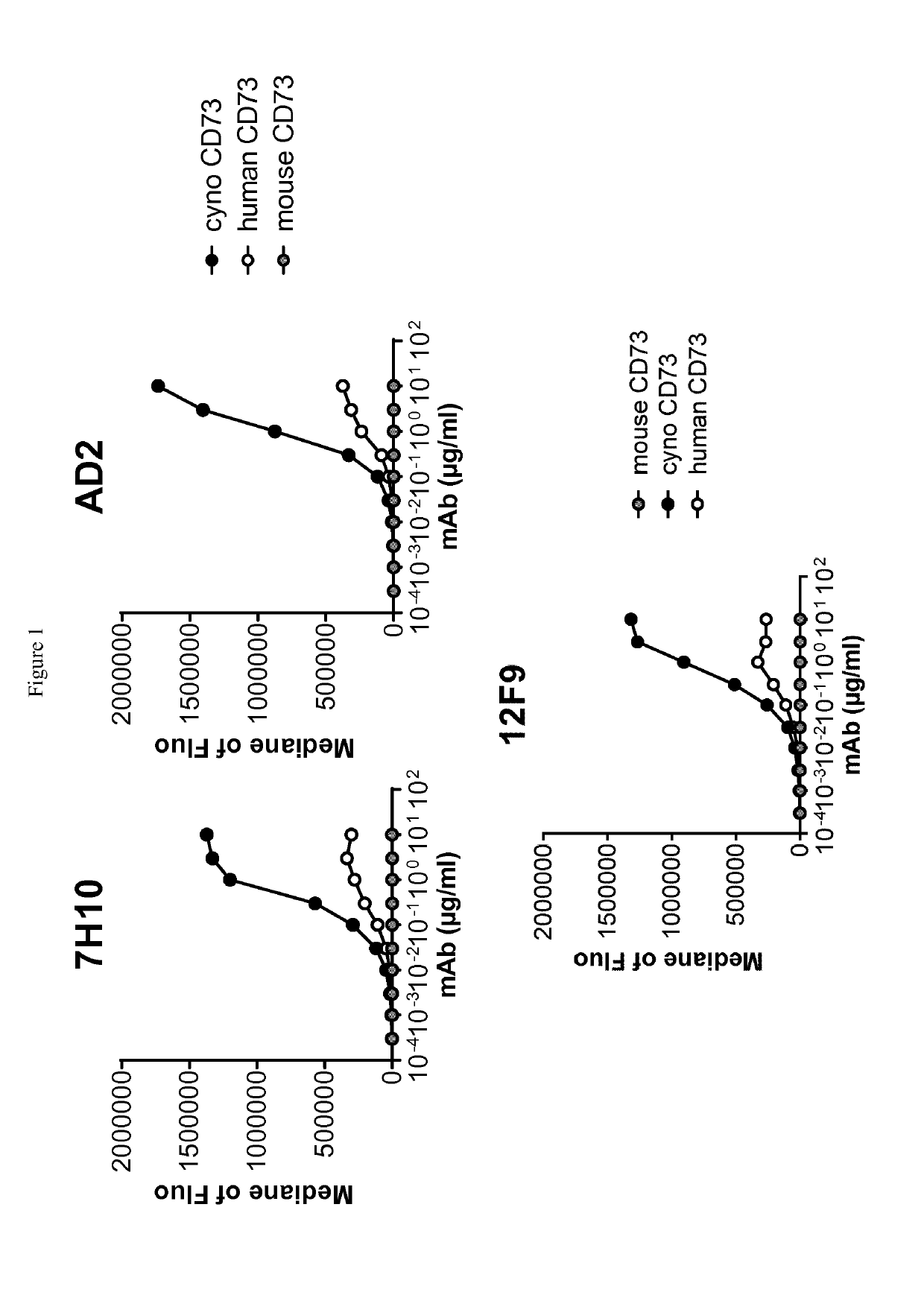
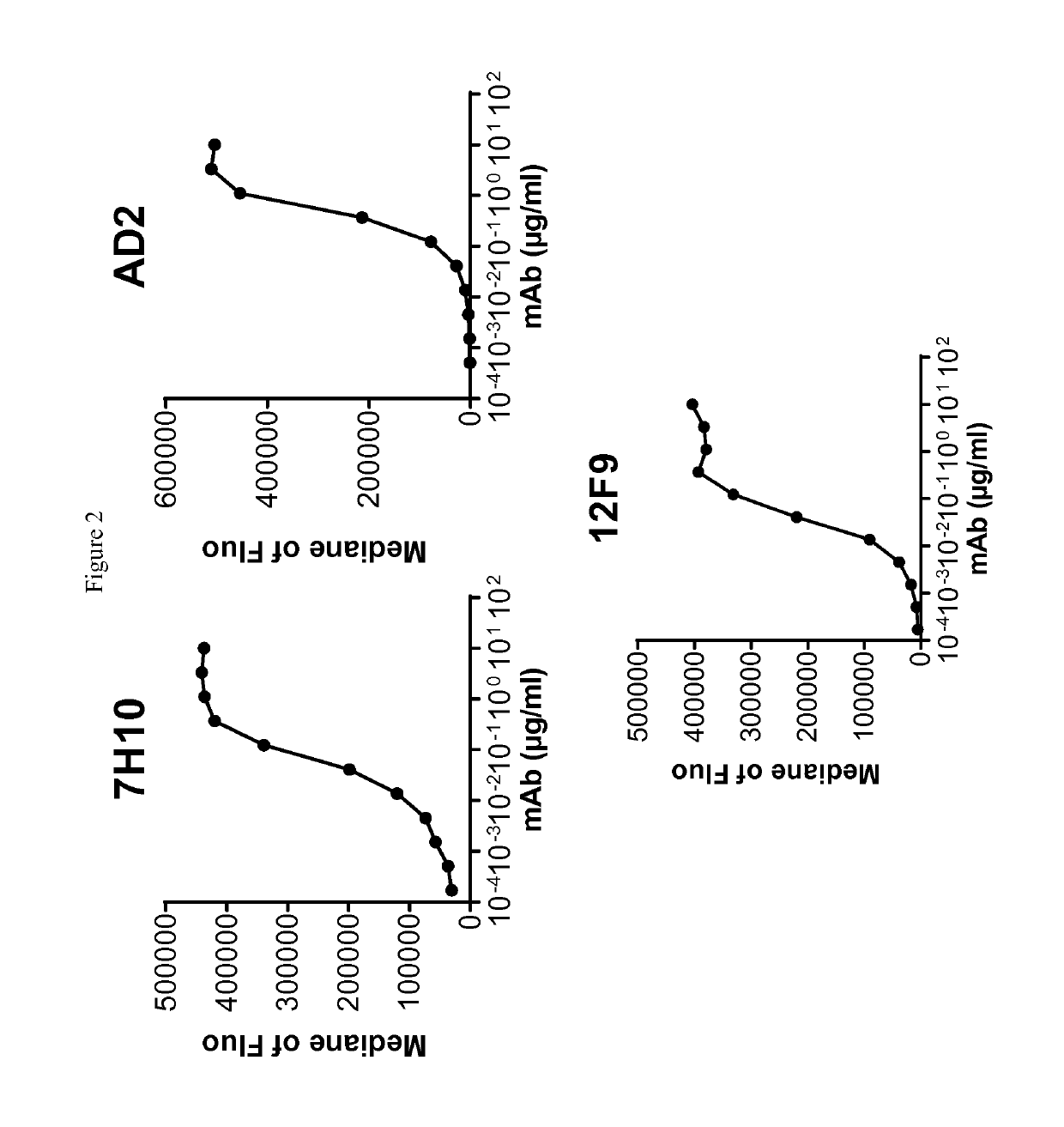
Cd73 blocking agents
ActiveUS20190225703A1Increasing cell proliferationInhibitory activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntineoplastic agentsCancer preventionT cell
Provided are antibodies that bind and inhibit CD73, are capable of increasing the proliferation of T cells in the presence of CD39-expressing B cells and ATP. The invention also relates to cells producing such compounds; methods of making such compounds, and antibodies, fragments, variants, and derivatives thereof; pharmaceutical compositions comprising the same; methods of using the compounds to diagnose, treat or prevent cancer.
Owner:CENT LEON BERARD +1
Anti-Japanese encephalitis virus monoclonal antibody and its application
InactiveCN102286431ANeutralizing activityFast detection methodImmunoglobulins against virusesMicroorganism based processesMicroorganismEncephalitis Viruses
The invention belongs to the field of biology, and relates to a monoclonal antibody for resisting the Japanese encephalitis virus (JEV) and application thereof. The hybridoma cell strain 2B4 which can excrete the monoclonal antibody for resisting the JEV is preserved in the China General Microbiological Culture Collection Center on August 3, 2011, and the preservation number is CGMCC No. 5124. The monoclonal antibody for resisting the JEV is produced by the hybridoma cell strain 2B4 with the preservation number of CGMCC No. 5124. The monoclonal antibody can specifically identify the JEV, and both cell and animal experiments show that the monoclonal antibody has neutralizing activity and can be applied in the preparation of detecting reagents for detecting the JEV. The successful preparation of the anti-JEV monoclonal antibody lays a foundation for the further research on the JEV, the establishment of a faster and more sensitive and convenient detection method and the development of related diagnostic reagent kits, test paper and vaccines.
Owner:NANJING AGRICULTURAL UNIVERSITY
Monoclonal antibody Q206 and application
ActiveCN105542002ASignificant application valueNeutralizing activityImmunoglobulins against virusesAntiviralsComplementarity Determining Region 1Ebola virus
The invention relates to a monoclonal antibody Q206 and application. The protected monoclonal antibody Q206 is an IgG antibody, and consists of a light chain and a heavy chain, wherein a CDR1 (complementarity determining region 1), a CDR2 and a CDR3 in a variable area of the heavy chain are sequentially 45th to 52nd amino acid residues, 70th to 79th amino acid residues and 118th to 128th amino acid residues from an N-terminal in a No.3 sequence of a sequence table; a CDR1, a CDR2 and a CDR3 in a variable area of the light chain are sequentially 45th to 53rd amino acid residues, 77th to 73rd amino acid residues and 110th to 123rd amino acid residues from an N-terminal in a No.5 sequence of the sequence table. The monoclonal antibody Q206 has the advantage that the important application value on the prevention and control of a Zaire subtype of an Ebola virus is realized.
Owner:TSINGHUA UNIV
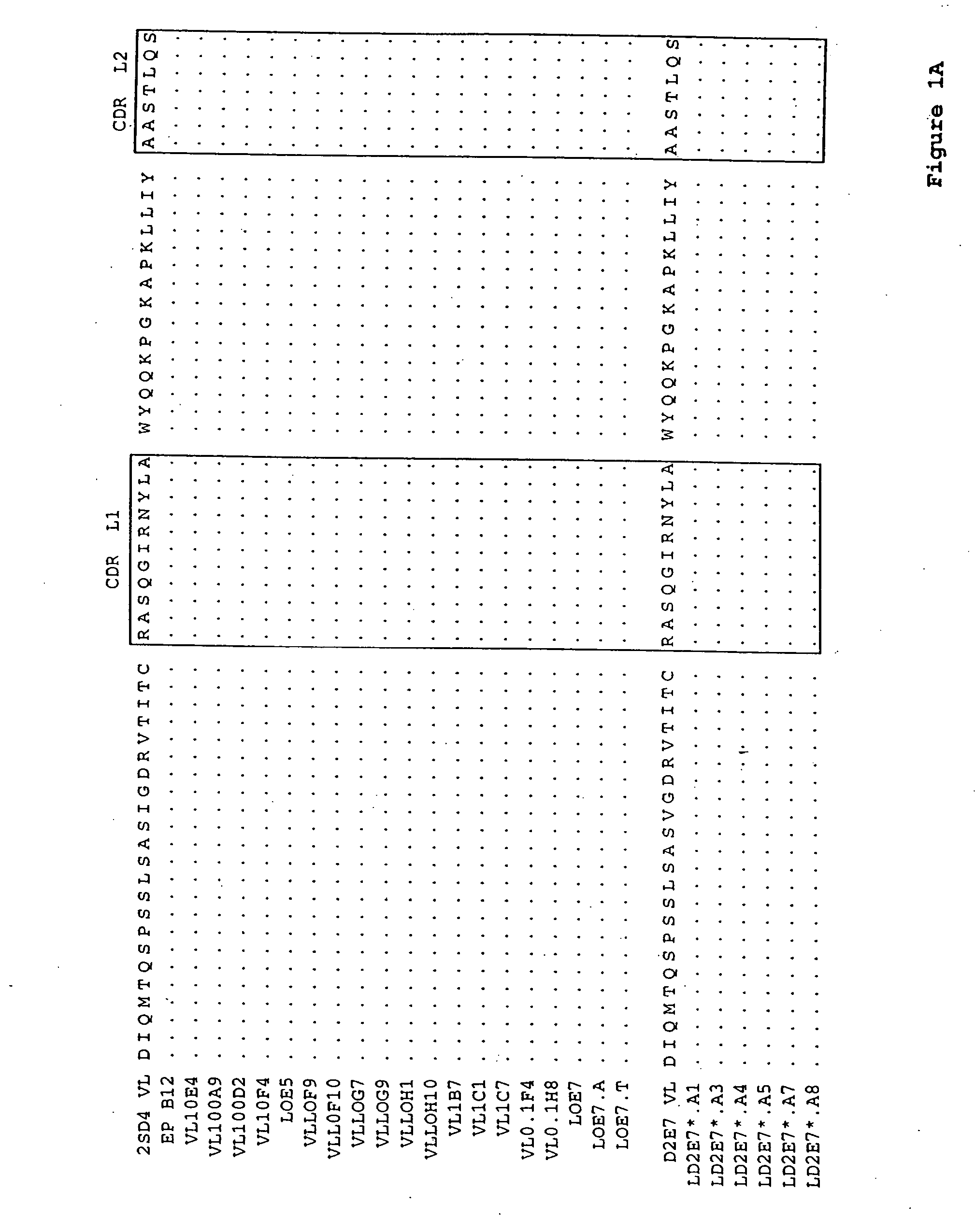
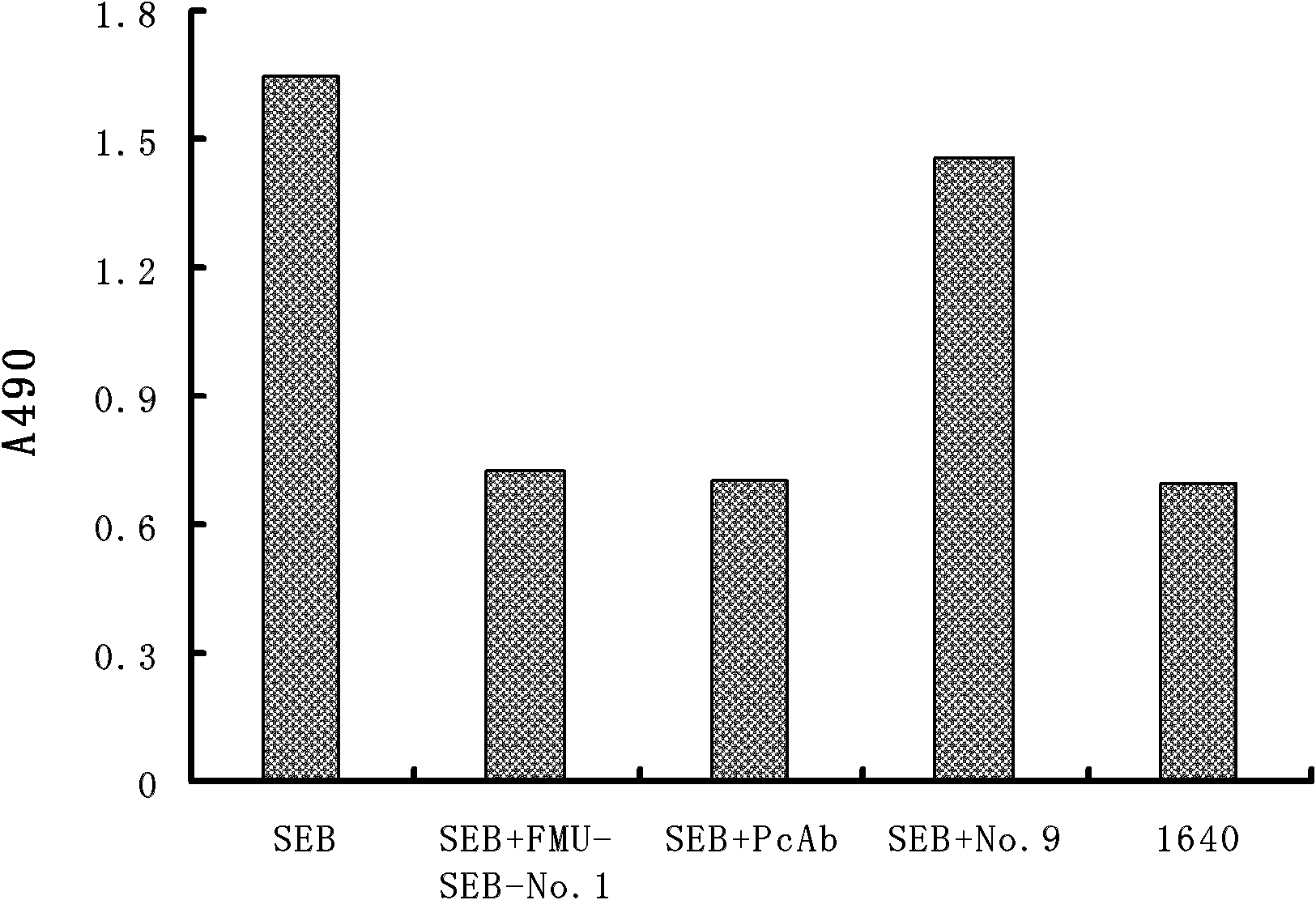
Light chain and heavy chain variable regions of anti-SEB (Staphylococcal Enterotoxin B) monoclonal antibody FMU-SEB-No.1 with high neutralizing activity
ActiveCN101955533AHigh affinityStrong neutralization abilityImmunoglobulins against bacteriaMaterial analysisBALB/cGenetic engineering
The invention discloses light chain and heavy chain variable regions of an anti-SEB (Staphylococcal Enterotoxin B) monoclonal antibody FMU-SEB-No.1 with a high neutralizing activity. The amino acid sequence of an FMU-SEB-No.1 mAb light chain variable region is shown in SEQ.ID.NO.1, and the amino acid sequence of an FMU-SEB-No.1 mAb heavy chain variable region is shown in SEQ.ID.NO.2. A method for the invention comprises the following steps of: preparing a group of mouse anti-SEB mAbs by using a natural SEB immune Balb / c mouse, screening hybridoma cell lines which can stably secrete high-affinity anti-SEB mAb; and preparing ascitic fluid to obtain a high-affinity anti-SEB mAb FMU-SEB-No.1. The invention is beneficial to confirming the uniqueness of a gene sequence and a corresponding protein sequence as well as a CDR sequence thereof, and providing support for researching and developing an anti-SEB mosaic or humanized genetic engineering antibody.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Antibodies that bind il-18 and methods of inhibiting il-18 activity
InactiveUS20110008357A1Inhibit hIL-1 activityInhibitory activityAntibacterial agentsOrganic active ingredientsEpitopeAntigen binding
Antibodies that bind human interleukin-18 (hIL-18) are provided, in particular antibodies that bind epitope(s) of human IL-18. The antibodies can be, for example, entirely human antibodies, recombinant antibodies, or monoclonal antibodies. Preferred antibodies have high affinity for hIL-18 and neutralize hIL-18 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. Method of making and method of using the antibodies of the invention are also provided. The antibodies, or antibody portions, of the invention are useful for detecting hIL-18 and for inhibiting hIL-18 activity, e.g., in a human subject suffering from a disorder in which hIL-18 activity is detrimental.
Owner:ABBOTT LAB INC
Enterovirus 71 (EV71) monoclonal antibody and application thereof
ActiveCN102409028AHigh school and activeStrong guiding significanceImmunoglobulins against virusesMicroorganism based processesLaboratory culturePassive Treatment
The invention provides an enterovirus 71 (EV71) monoclonal antibody and an application thereof. The monoclonal antibody is derived from an EV71 hybrid tumor cell strain, which has a preservation number of CGMCC (China General Microbiological Culture Collection) No.5330. The monoclonal antibody and related reagents thereof have important significance in promoting the treatment and research of severe cases and death cases caused by EV71 in China, and have extremely important value in further promoting clinical research, epidemic development and passive treatment of EV71.
Owner:SINOVAC BIOTECH
Monoclonal antibody ZK2C2 and application
ActiveCN108727489ASignificant application valueNeutralizing activityImmunoglobulins against virusesAntiviralsHeavy chainMonoclonal antibody
The invention discloses a monoclonal antibody ZK2C2 and application. The invention provides an IgG antibody named as ZK2C2 and composed of light chains and heavy chains. CDR1, CDR2 and CDR3 in a heavychain variable region of the heavy chains are sequentially the No. 45-52 amino acid residues, No. 70-77 amino acid residues and No. 116-122 amino acid residues from N terminal in sequence 3 of a sequence table; CDR1, CDR2 and CDR3 in a light chain variable region of the light chains are sequentially the No. 45-52 amino acid residues, No. 70-72 amino acid residues and No. 109-119 amino acid residues from N terminal in sequence 5 of the sequence table. The invention further provides application of the IgG antibody in preparation of a medicine for inhibiting Zika virus. The antibody disclosed bythe invention has significant application value on prevention and control of Zika virus diseases and produces profound social significance.
Owner:GUANGZHOU EIGHTH PEOPLES HOSPITAL +1
Varicella-zoster virus mRNA vaccine as well as preparation method and application thereof
PendingCN114854772AStable structureImprove translation efficiencyVirus peptidesAntiviralsChickenpoxTGE VACCINE
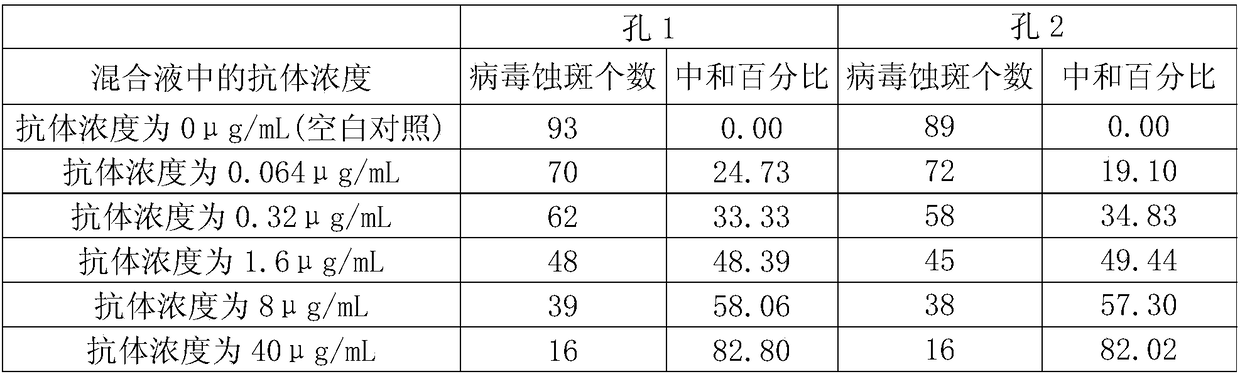
The invention relates to the field of nucleic acid vaccines, in particular to a varicella-herpes zoster mRNA vaccine and a preparation method and application thereof. The mRNA for coding the varicella-zoster virus antigen provided by the invention contains a coding region for coding glycoprotein E of the varicella-zoster virus, and the mRNA can induce specific antibody response and CD4 + T cell response aiming at the glycoprotein E when being delivered into a body.
Owner:浙江君怡生物科技有限公司
Monoclonal antibody ZK7C3 and application
ActiveCN108727491ANeutralizing activityStrong specificityImmunoglobulins against virusesAntiviralsAntiendomysial antibodiesHeavy chain
The invention discloses a monoclonal antibody ZK7C3 and application. The invention provides an IgG antibody named as ZK7C3 and composed of light chains and heavy chains. CDR1, CDR2 and CDR3 in a heavychain variable region of the heavy chains are sequentially the No. 45-52 amino acid residues, No. 70-77 amino acid residues and No. 116-129 amino acid residues from N terminal in sequence 3 of a sequence table; CDR1, CDR2 and CDR3 in a light chain variable region of the light chains are sequentially the No. 45-50 amino acid residues, No. 68-70 amino acid residues and No. 107-117 amino acid residues from N terminal in sequence 5 of the sequence table. The invention further provides application of the IgG antibody in preparation of a medicine for inhibiting Zika virus. The antibody disclosed bythe invention has significant application value on prevention and control of Zika virus diseases and produces profound social significance.
Owner:GUANGZHOU EIGHTH PEOPLES HOSPITAL +1
Compound polypeptide molecule for targeted killing of cancer cells and preparation method thereof
PendingCN112724258ANeutralize electropositivityNeutralizing activityAntibody mimetics/scaffoldsPeptide preparation methodsLytic peptideGallic acid ester
The invention belongs to the technical field of biological medicines, and particularly relates to a compound polypeptide molecule for targeted killing of cancer cells and a preparation method thereof. The compound polypeptide molecule comprises gallic acid, a cracking peptide and a polypeptide substrate which is connected between the gallic acid and the cracking peptide and is used for MMP-2 specific recognition degradation. In the composite polypeptide molecule, the electronegative gallic acid small molecule can effectively neutralize the electropositivity and the split membrane activity of the split peptide, so that the damage of the split peptide to normal cells in the in-vivo circulation process can be effectively prevented, and after the composite polypeptide molecule enters cancer tissues, the cancer cells secrete a large amount of MMP-2 protein, the MMP-2 protein can recognize and degrade a polypeptide substrate connected between gallic acid and lytic peptide, so that free lytic peptide is released, and the effect of killing cancer cells in a targeted manner can be achieved.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
AEG-1 (Astrocyte Elevated Gene-1)/1E3 monoclonal antibody with high affinity
ActiveCN102585004AHigh affinityProving uniquenessAntibody ingredientsImmunoglobulinsHeavy chainMonoclonal antibody
The invention discloses an AEG-1 (Astrocyte Elevated Gene-1) / 1E3 monoclonal antibody with high affinity. The amino acid sequence of a variable region of a light chain of AEG-1 / 1E3mAb is shown as SEQ ID. NO. 1, and the amino acid sequence of a variable region of a heavy chain is shown as SEQ ID. NO. 2. The uniqueness for the gene sequence and corresponding protein sequence of a variable region of the antibody and CDR (Complementary Determined Region) sequence of the antibody are determined; the AEG-1 / 1E monoclonal antibody has high affinity with AEG-1, and the AEG-1 can be specifically identified and immunological reaction can be performed; and the titer of ascites is 1*10<-7>. Support is provided for researching and developing anti-AEG-1 gene engineering antibody and a diagnostic reagent.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com