Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68 results about "Chikungunya" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
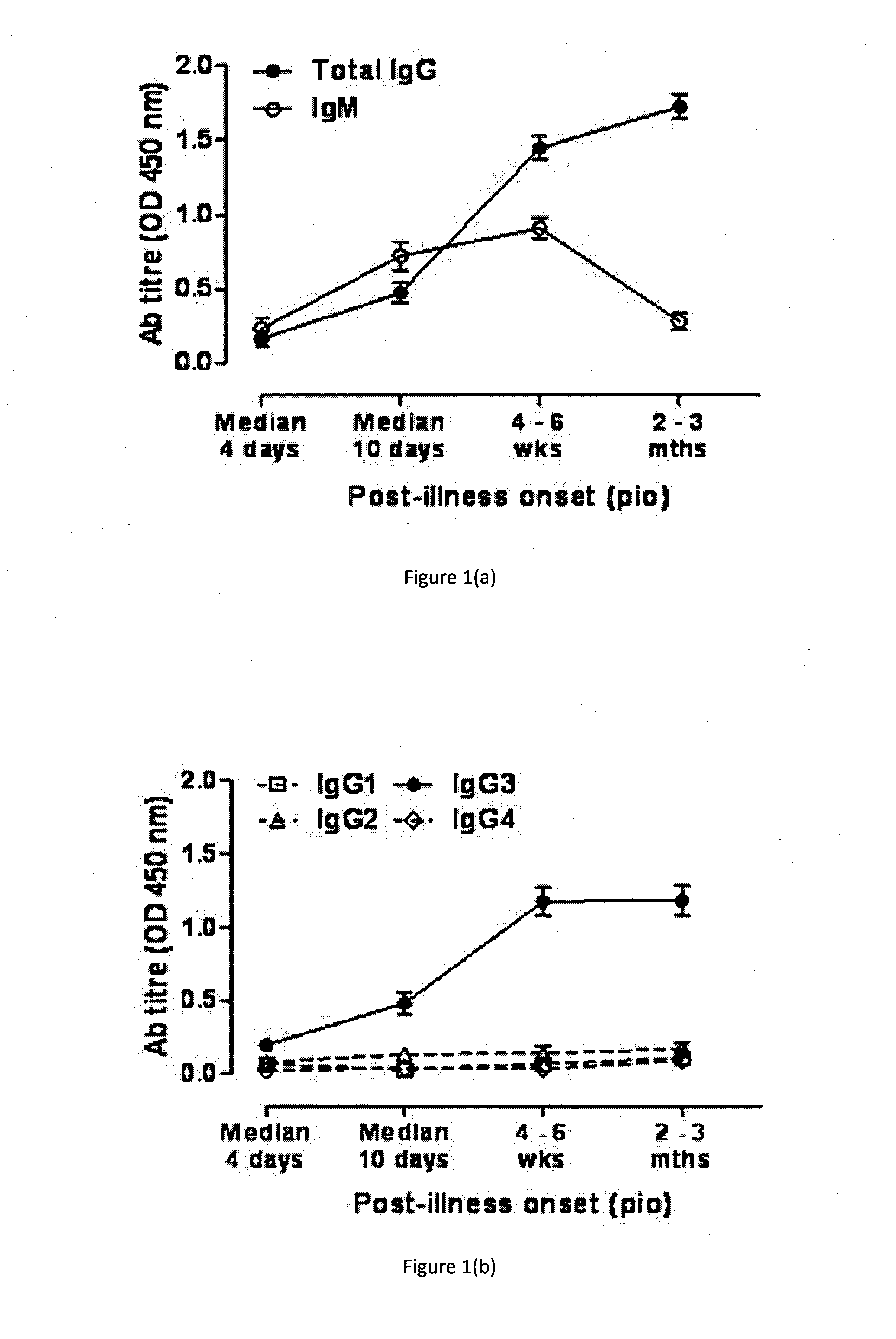
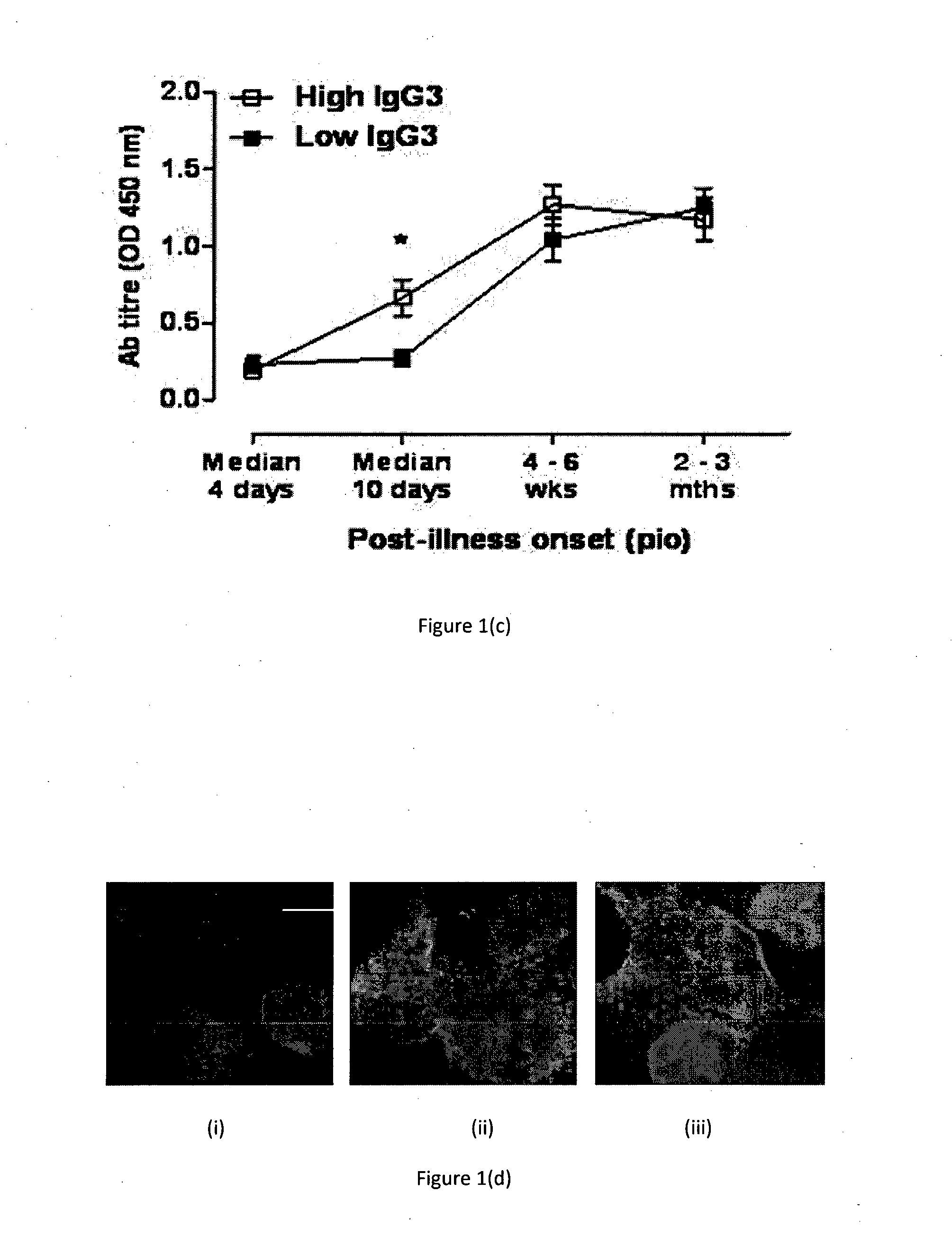
Chikungunya is a rare viral infection transmitted by the bite of an infected mosquito. It is characterized by a rash, fever, and severe joint pain (arthralgias) that usually lasts for three to seven days.
Vaccine compositions
ActiveUS20170014502A1SsRNA viruses positive-sensePharmaceutical delivery mechanismPathogenImmunogenicity
The present disclosure provides vaccine compositions for prophylaxis and treatment of Zika virus infections comprising Zika virus antigens in immunogenic compositions, and in combination of Zika antigens with one or more arbovirus antigens such as Chikungunya virus and Japanese encephalitis virus antigens, methods of preparation and production of such compositions for use as vaccines for eliciting immune response in mammals against the above mentioned pathogens.
Owner:BHARAT BIOTECH INTERNATIONAL
Preparation method of virus-like particles (VLPs) of Chikungunya virus (CHIKV) and its application
InactiveCN102321639ANeutralizing activityViral antigen ingredientsInactivation/attenuationImmune effectsVirus-like particle
The invention relates to a preparation method of virus-like particles (VLPs) of Chikungunya virus (CHIKV). The method comprises the steps of: modifying genetic elements of a structural protein encoding gene C-E3-E2-6K-E1 of CHIKV, cloning the modified genetic elements into the expression vector of an insect cell, then transfecting the obtained recombined expression vector and baculovirus linear DNA respectively to an SF9 insect cell and making the cell secrete and express CHIKV VLPs. Additionally, the invention also makes preliminary studies on the immune effects of CHIKV VLPs and applicationof CHIKV VLPs in virus specific antibody detection, thus laying a foundation for research and preparation of immunological detection reagents and even vaccines based on CHIKV VLPs.
Owner:中国疾病预防控制中心病毒病预防控制所
Virus like particle comprising modified envelope protein e3
ActiveUS20160200775A1Increase productionSsRNA viruses positive-senseAntibody mimetics/scaffoldsVirus-like particleChikungunya
A virus like particle comprising a viral structural protein which comprises modified envelope protein E3. The viral structural protein may be that derived from or alphavirus or flavivirus. Especially, the viral structural protein may be derived from Chikungunya virus or Venezuelan equine encephalitis virus.
Owner:VLP THERAPEUTICS LLC
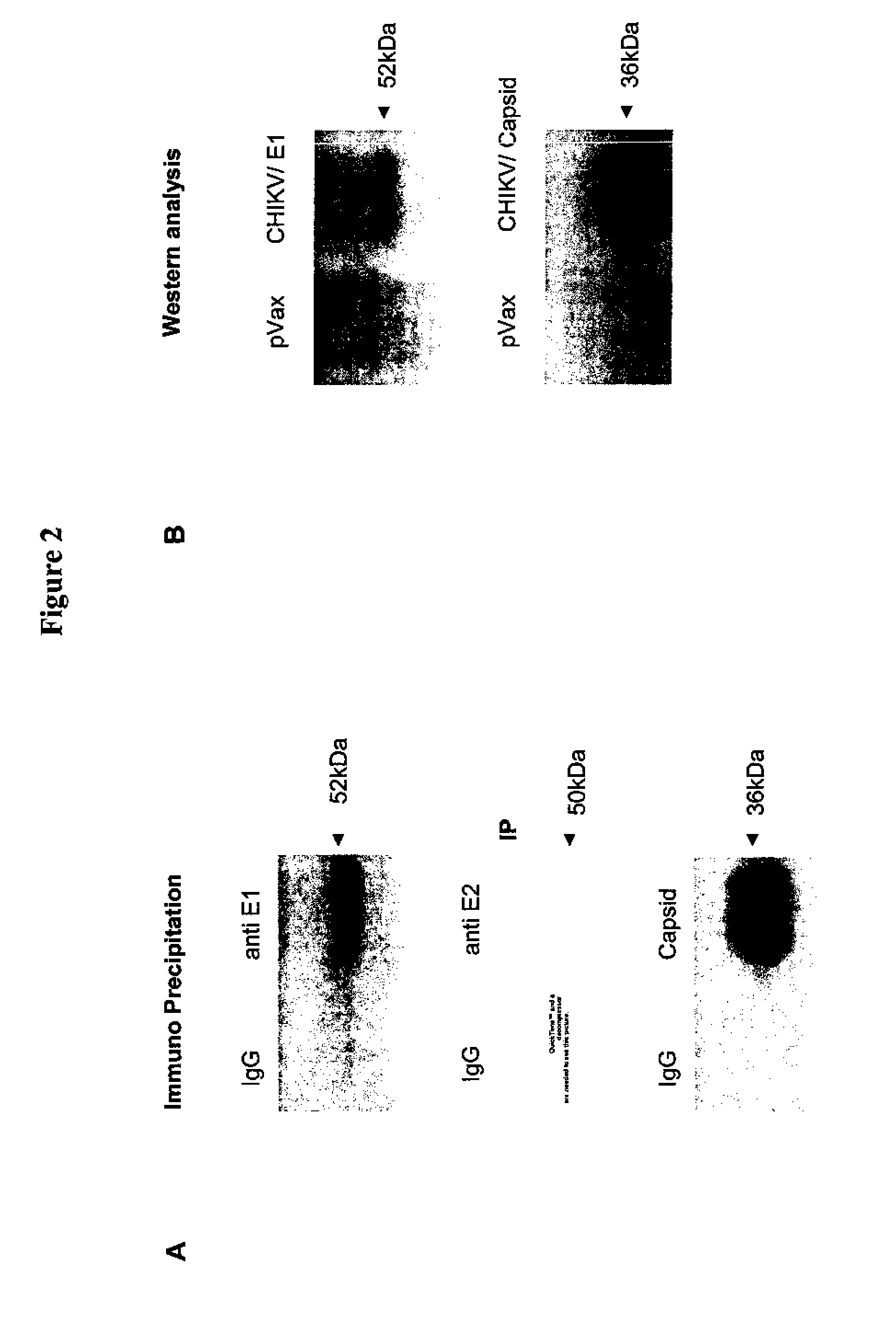
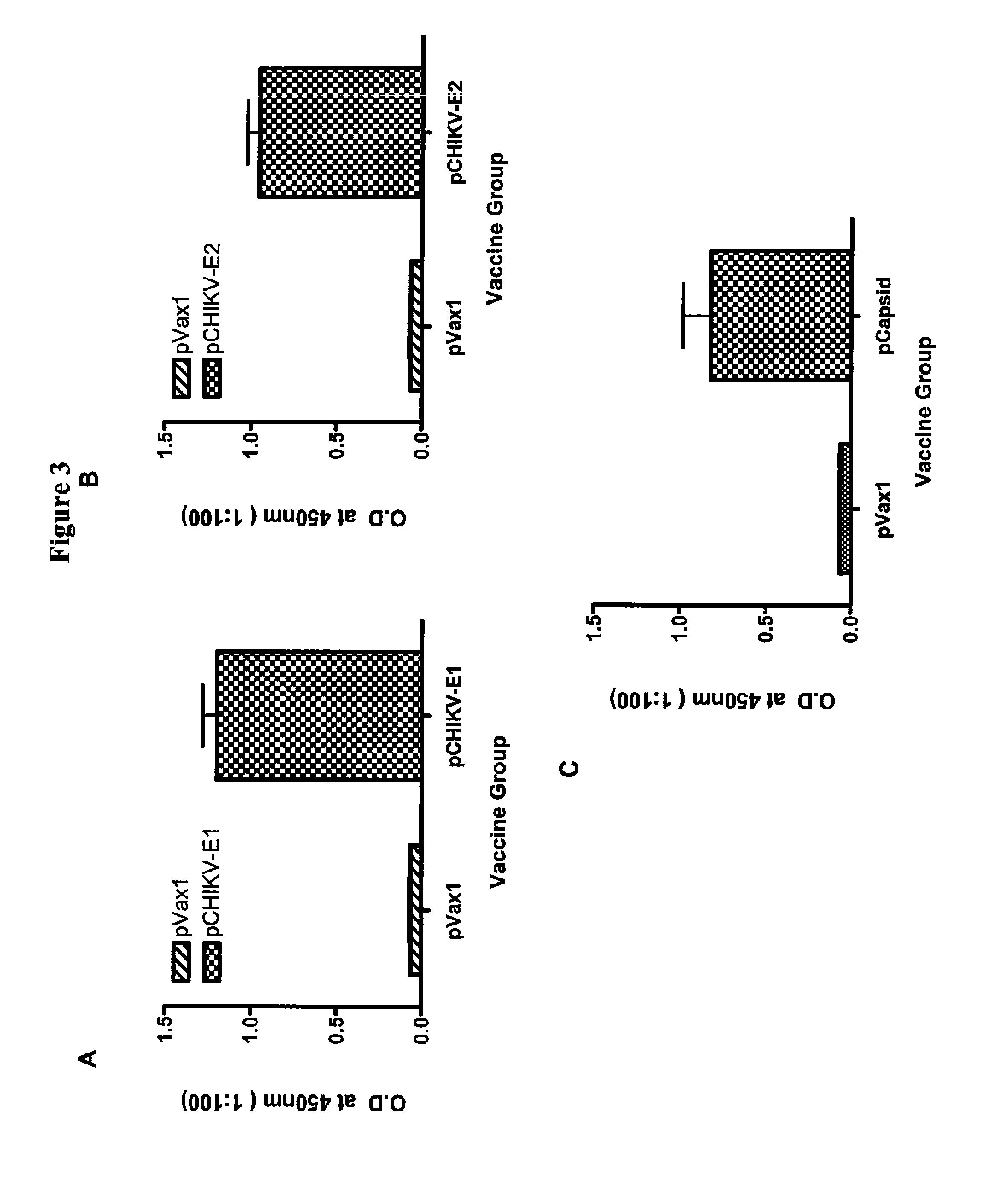
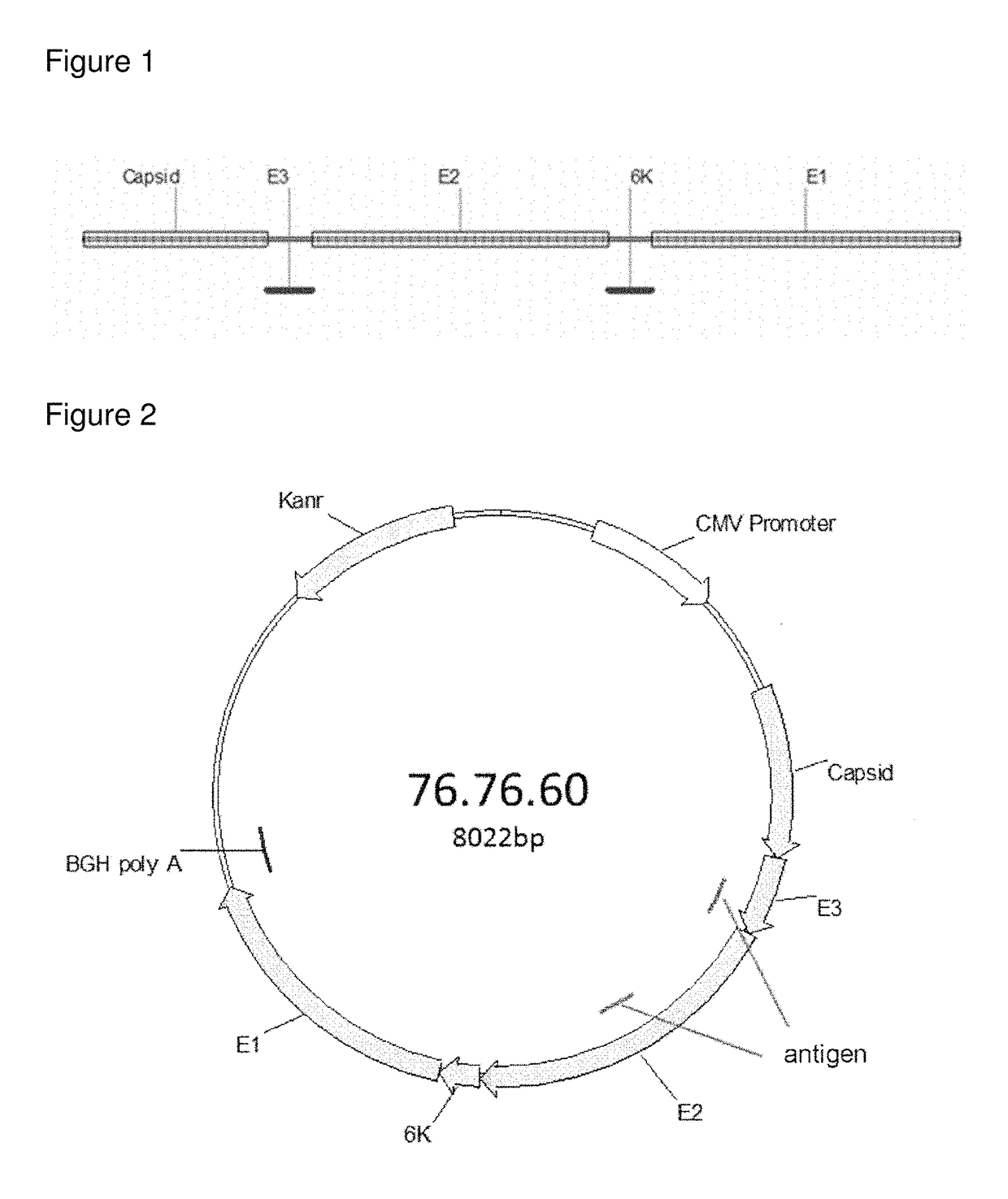
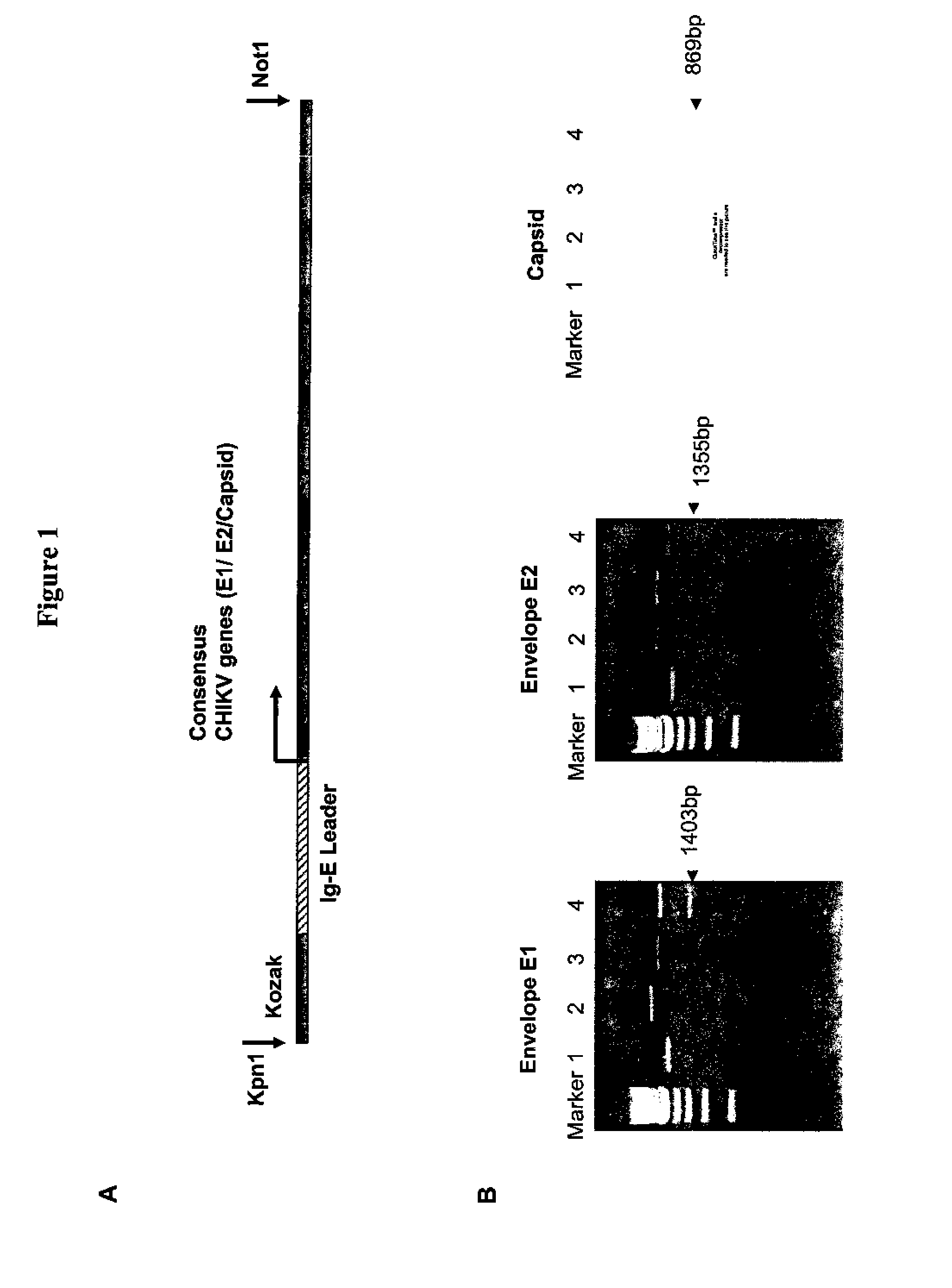
Consensus sequences of chikungunya viral proteins, nucleic acid molecules encoding the same, and compositions and methods for using the same
Consensus CHIKV E1 protein, consensus CHIKV E2 protein, consensus CHIKV capsid protein, or fragments and homologues thereof, and nucleic acid molecules that encode the same are disclosed. A consensus CHIKV Env protein which includes CHIKV E1 consensus protein, CHIKV E2 consensus protein, CHIKV E3 consensus protein, or fragments and homologues thereof and nucleic acid molecules that encode the same are also disclosed. Compositions and recombinant vaccines comprising CHIKV consensus proteins, and methods of using them are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
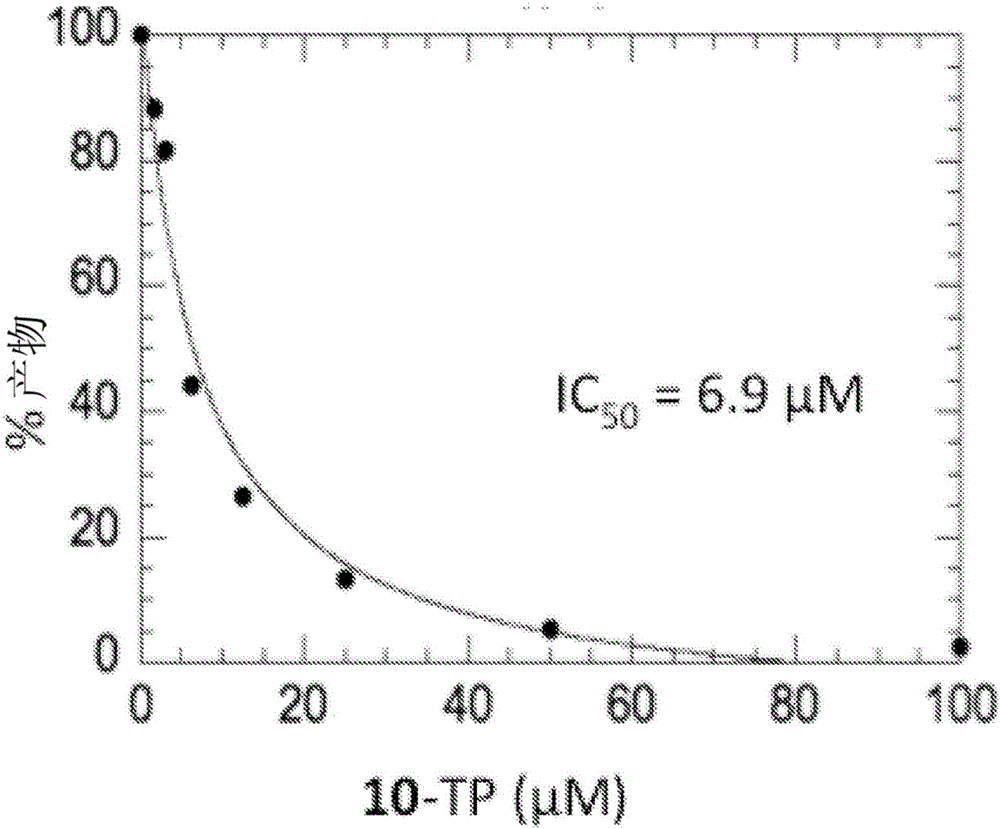
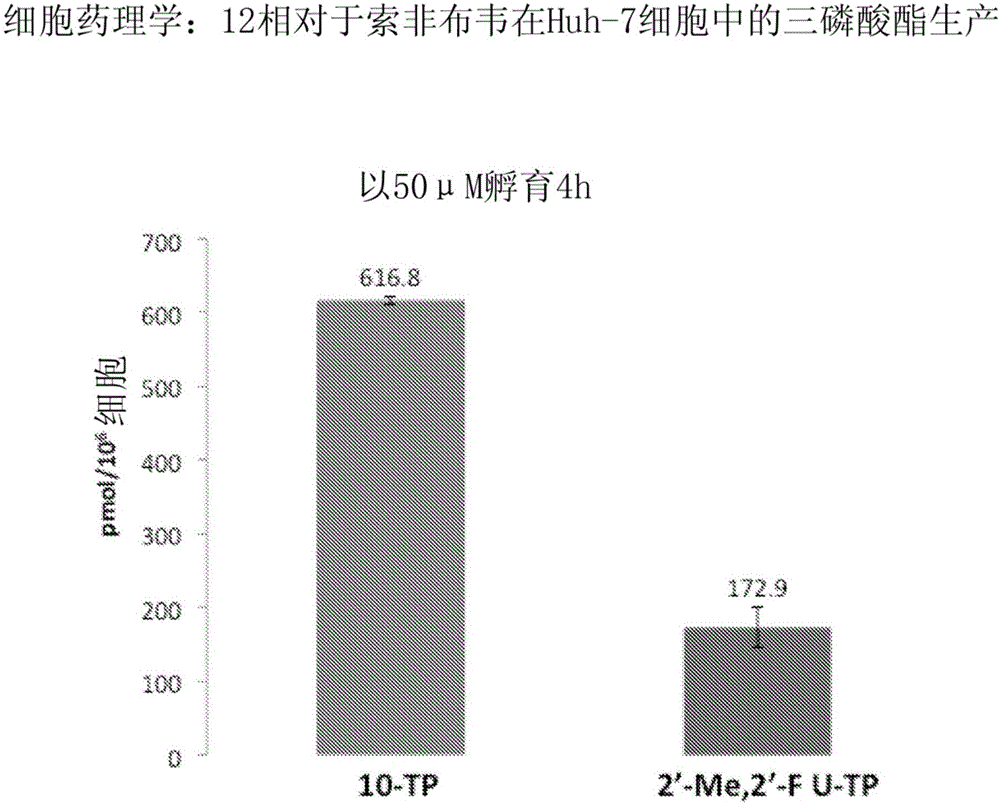
Purine monophosphate prodrugs for treatment of viral infections
The present invention is directed to compounds, compositions and methods for treating or preventing viral infections using nucleoside analog monophosphate prodrugs. More specifically, HCV, Norovirus, Saporovirus, Dengue virus, Chikungunya virus and Yellow fever in human patients or other animal hosts. The compounds are certain 2,6-diamino 2-C-methyl purine nucleoside monophosphate prodrugs and modified prodrug analogs, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HCV, Norovirus, Saporovirus, Dengue virus, Chikungunya virus and Yellow fever. This invention teaches how to modify the metabolic pathway of 2,6-diamino 2'-C-methyl purine and deliver nucleotide triphosphate(s) to polymerases at heretofore unobtainable therapeutically-relevant concentrations.
Owner:RFS PHARMA +1
Chimeric chikungunya virus and uses thereof
ActiveUS20110171249A1Avoid infectionSsRNA viruses positive-senseSugar derivativesSindbis virusChikungunya fever
The present invention discloses a chimeric Chikungunya virus comprising a heterologous alphavirus cDNA fragment and a Chikungunya virus cDNA fragment. The heterologous alphavirus may include but is not limited to Sindbis virus, Eastern equine encephalitis virus or Venezuelan equine encephalitis virus. The present invention also discloses the use of this chimeric Chikungunya virus as vaccines and in serological and diagnostic assays.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Attenuated recombinant alphaviruses incapable of replicating in mosquitoes and uses thereof
ActiveUS8426188B2Animal cellsSsRNA viruses positive-senseEastern equine encephalitis virusChikungunya
The present invention discloses an attenuated recombinant alphavirus that is incapable of replicating in mosquito cells and of transmission by mosquito vectors. These attenuated alphavirus may include but is not limited to Western Equine Encephalitis virus, Eastern equine encephalitis virus, Venezuelan equine encephalitis virus or Chikungunya virus. The present invention also discloses the method of generating such alphaviruses and their use as immunogenic compositions.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Virus purification
ActiveUS10537630B2Improving immunogenicityHighly cross-protectiveSsRNA viruses positive-senseViral antigen ingredientsYellow fever vaccineZika virus
Described herein are improved purification methods for virus vaccines and compositions. Also described are Zika, Chikungunya, dengue and yellow fever vaccines and methods of producing and administering said vaccines to subjects in need thereof.
Owner:VALNEVA SE
Method for detecting Zika virus, Chikungunya virus and Mayaro virus by triple real-time fluorescent quantitative RT-PCR
ActiveCN110305985AImprove universalityStrong specificityMicrobiological testing/measurementMicroorganism based processesZika virusChikungunya fever
The invention relates to the technical field of virus detection, and concretely relates to a method for detecting Zika virus, Chikungunya virus and Mayaro virus by triple real-time fluorescent quantitative RT-PCR. The method combines the probability of occurrence of related mosquito-borne pathogens, the risk of prevalence, and the operability of a laboratory testing procedure, the Mayaro virus isused to replace the dengue virus in an existing common combination detection scheme, and a new detection scheme using Zika virus, Chikungunya virus and Mayaro virus as detection targets is formed. Theinvention provides a specific primer, a probe and a triple real-time quantitative RT-PCR detection method for simultaneously identifying the viral pathogens Zika virus, Chikungunya virus and Mayaro virus which are transmitted by Aedes mosquito and cause similar clinical symptoms of diseases. The method has high specificity and sensitivity, good repeatability, simple and rapid detection, and costsaving, and can complement a traditional detection scheme and has high application value.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Virus like particle comprising modified envelope protein E3
ActiveUS9969986B2Increase productionSsRNA viruses positive-senseNervous disorderEncephalitisVirus-like particle
A virus like particle comprising a viral structural protein which comprises modified envelope protein E3. The viral structural protein may be that derived from or alphavirus or flavivirus. Especially, the viral structural protein may be derived from Chikungunya virus or Venezuelan equine encephalitis virus.
Owner:VLP THERAPEUTICS LLC
Consensus sequences of chikungunya viral proteins, nucleic acid molecules encoding the same, and compositions and methods for using the same
Consensus CHIKV E1 protein, consensus CHIKV E2 protein, consensus CHIKV capsid protein, or fragments and homologues thereof, and nucleic acid molecules that encode the same are disclosed. A consensus CHIKV Env protein which includes CHIKV E1 consensus protein, CHIKV E2 consensus protein, CHIKV E3 consensus protein, or fragments and homologues thereof and nucleic acid molecules that encode the same are also disclosed. Compositions and recombinant vaccines comprising CHIKV consensus proteins, and methods of using them are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
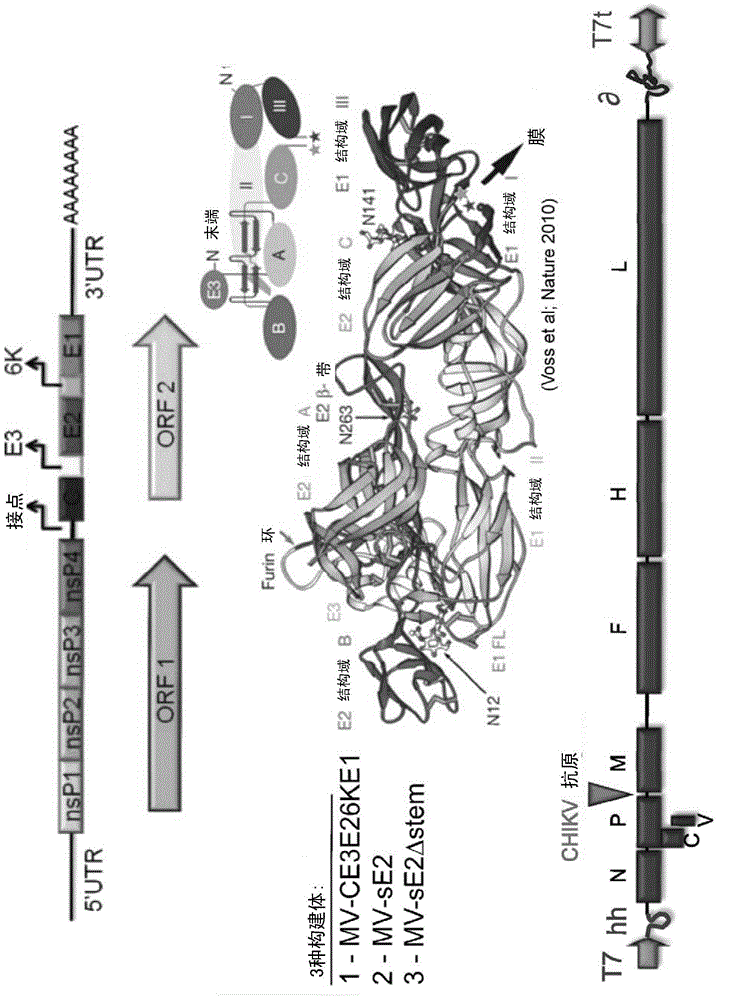
Recombinant measles virus expressing chikungunya virus polypeptides and their applications
ActiveCN104918952AAvoid infectionSsRNA viruses negative-senseSsRNA viruses positive-senseVirus-like particleChikungunya
The invention relates to recombinant Measles virus expressing Chikungunya virus polypeptides, and concerns in particular virus like particles (VLP) that contain envelope and capsid proteins of a Chikungunya virus at their surface. These particles are recombinant infectious particles able to replicate in a host after an administration. The invention provides means, in particular nucleic acids, vectors, cells and rescue systems to produce these recombinant infectious particles. The invention also relates to the use of these recombinant infectious particles, in particular under the form of a composition, more particularly in a vaccine formulation, for the treatment or prevention of an infection by Chikungunya virus.
Owner:INST PASTEUR +2
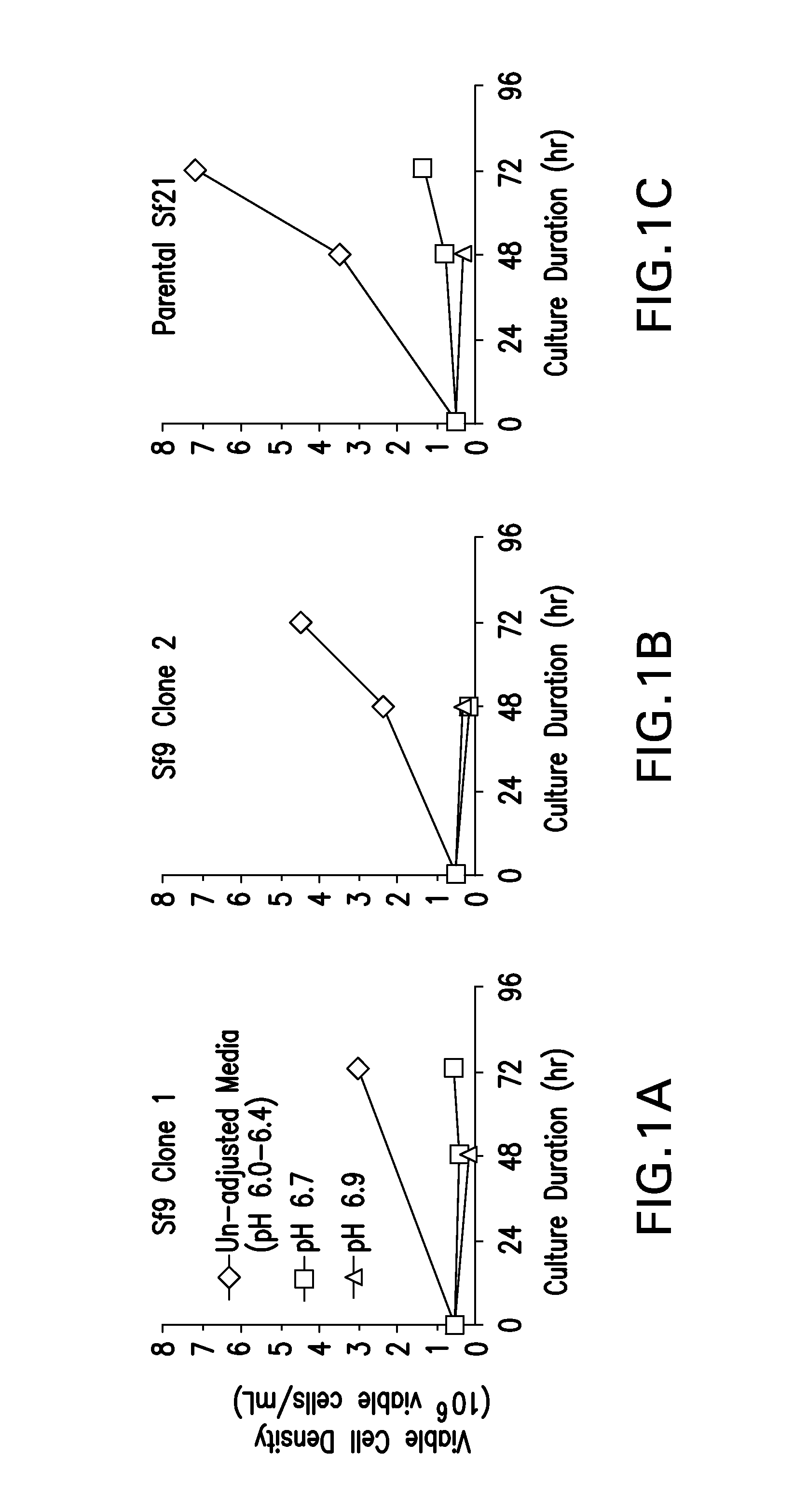
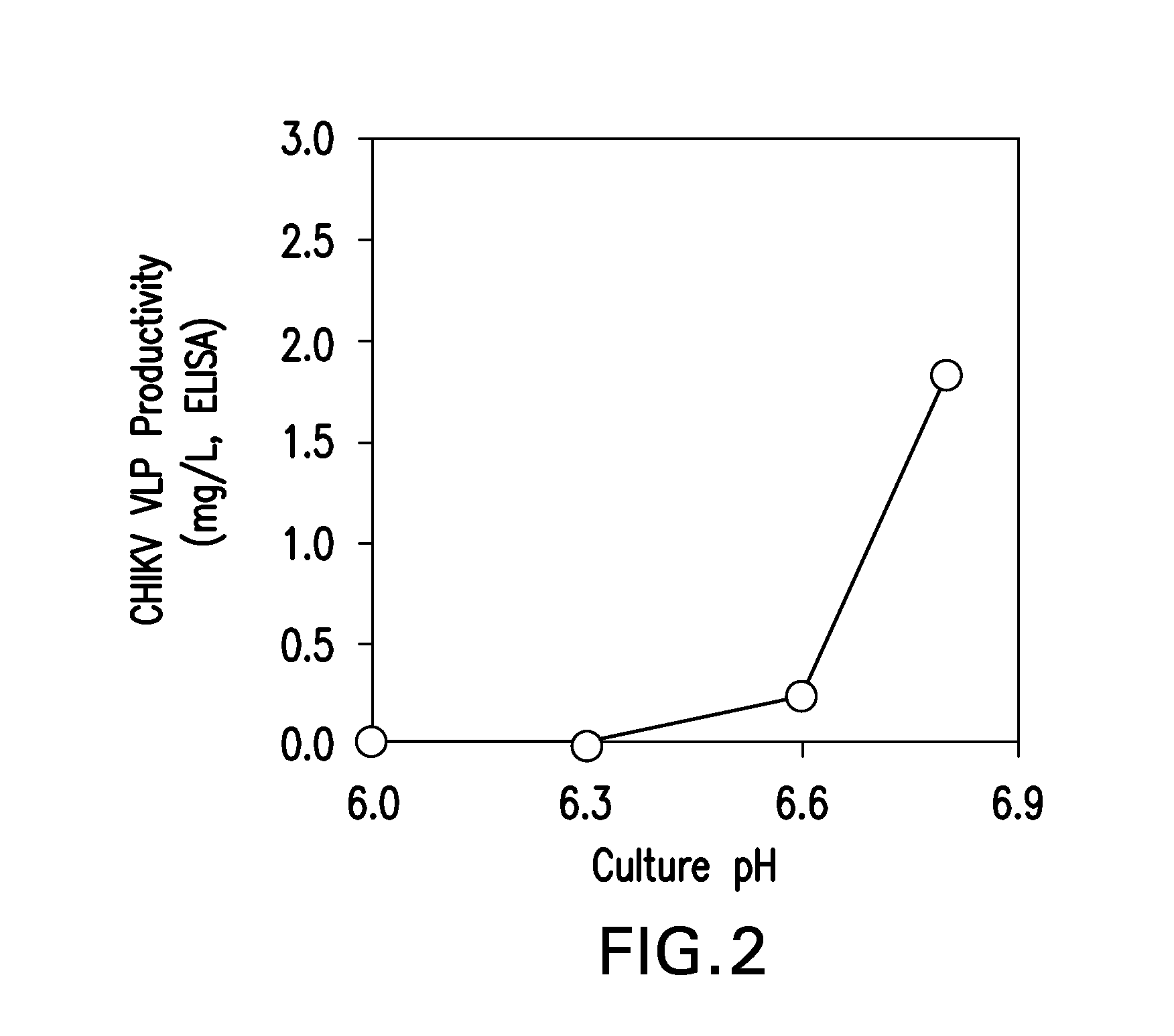
Adapted lepidopteran insect cells for the production of recombinant proteins
ActiveUS20160040124A1SsRNA viruses positive-senseInvertebrate cellsInsect cell cultureVirus-like particle
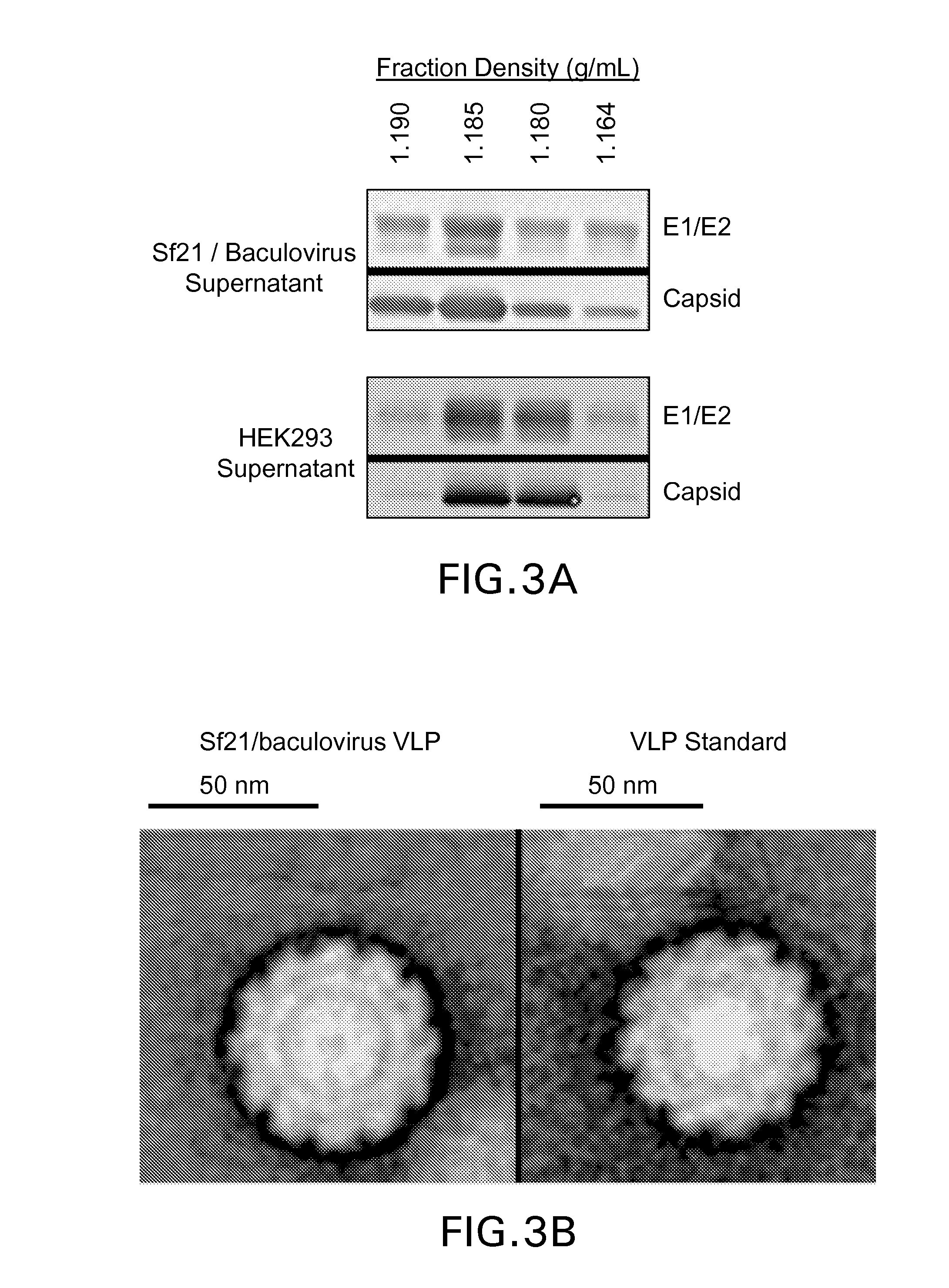
The present invention relates to the use of increased culture pH, relative to standard insect cell culture conditions, during baculovirus infection of lepidopteran insect cells to enable production of recombinant chikungunya (CHIKV) virus like particles (VLPs). The invention further relates to adapted insect cell lines derived from insect cells such as Sf21, which can grow robustly at elevated culture pH, the use of said cell lines to recombinantly produce pH sensitive proteins in the correct conformation and increase expression of recombinant proteins relative to standard insect cell lines. In some embodiments of the invention, the cells are useful for recombinant production of CHIKV VLPs. The invention also relates to a method for the production of a pH-adapted lepidopteran insect cell line. In some embodiments of said method, the cell line is produced and / or maintained in reduced phosphate serum-free insect cell media.
Owner:MERCK SHARP & DOHME LLC
Anti-chikv antibodies and uses thereof
ActiveUS20180127487A1Development of therapyNeed can be addressedImmunoglobulins against virusesAntiviralsDiseaseAntiendomysial antibodies
The present invention concerns antibodies and antigen-binding fragments of antibodies which specifically bind to and neutralize Chikungunya virus (CHIKV) and which are engineered to develop therapeutics in order to treat CHIKV disease or prevent CHIKV infection. The invention also relates to pharmaceutical compositions comprising antibodies of the invention and the use of the antibodies for the prevention and treatment of CHIKV disease.
Owner:SANOFI SA
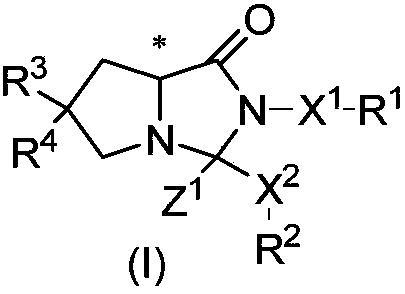
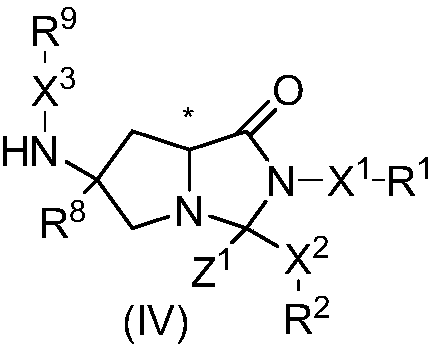
Cyclocompound of tetrahydropyrrole and dihydroimidazolone as well as preparation and pharmaceutical application thereof
The invention discloses a cyclocompound of tetrahydropyrrole and dihydroimidazolone as well as preparation and pharmaceutical application thereof. The cyclocompound is a compound shown in a formula (I), an isomer or pharmaceutically acceptable salt thereof. The compound, isomer or pharmaceutically acceptable salt thereof can be applied to preparation of medicines used for preventing or treating related diseases (such as dengue fever, dengue hemorrhagic fever, dengue shock syndrome, Zika, Chikungunya, Japanese encephalitis, yellow fever, hepatitis C and West Nile disease) caused by a dengue fever virus and related viruses. (The formula (I) is described in the specification).
Owner:NANJING TECH UNIV
2'-disubstituted nucleoside analogs for treatment of the flaviviridae family of viruses and cancer
The present invention is directed to compounds, compositions and methods for treating or preventing Flaviviridae family of viruses (including HCV, Yellow fever, Dengue, Chikungunya and West Nile virus), RSV and influenza infection and cancer in human subjects or other animal hosts. The compounds are as also pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof as pharmaceutical compositions and methods for treatment or prevention of HCV infection.
Owner:COCRYSTAL PHARMA INC +1
Method of producing pharmaceutical compositions comprising immunogenic chikungunya virus chikv-delta5nsp3
The present invention relates to a process for producing an immunogenic live attenuated Chikungunya virus, as well as pharmaceutical compositions comprising the same.
Owner:VALNEVA SE
Immunogenic chikungunya virus peptides
InactiveUS20140050754A1Reduce riskSsRNA viruses positive-sensePeptide/protein ingredientsChikungunya feverImmunogenic peptide
The present invention relates to immunogenic peptides of Chikungunya Virus and methods for vaccinating a subject using these peptides. Also disclosed are nucleic acids encoding these peptides and methods for their production.
Owner:TAN TOCK SENG HOSPITAL PTE LTD +1
Triple detection method of arbovirus liquid chips
InactiveCN104120191AEasy to operateLow costMicrobiological testing/measurementMicroorganism based processesMultiplexMicrosphere
The invention discloses a triple detection method of arbovirus liquid chips. The method comprises the following steps: respectively designing specific primers and probes aiming at three types of arboviruses comprising Chikungunya viruses, Crimean-Congo hemorrhagic fever and Rift Valley fever viruses, determining the specificity of the primers and the probes via a single PCR and clone sequencing, and then carrying out multiplex-PCR amplification under the condition that the concentration ratio of the forward and reverse primers in an amplification reaction system is 1:1-1:8; then coupling the probes with fluorescent coding microspheres so as to prepare a microsphere mixing liquid and carrying out coupling quality control by coupling the probes; finally, carrying out a hybridization reaction on multiplex-PCR products and the microsphere mixing liquid, simultaneously adding streptomycin-phycoerythrin diluted by a nucleic acid detection buffer solution, and analyzing reaction products by utilizing a liquid chip detecting instrument. The method is good in detection specificity and can be used for simultaneously detecting the three types of arboviruses, and has high detection sensitivity due to the adoption of an asymmetric PCR amplification method in the process of the multiplex-PCR amplification.
Owner:李云峰
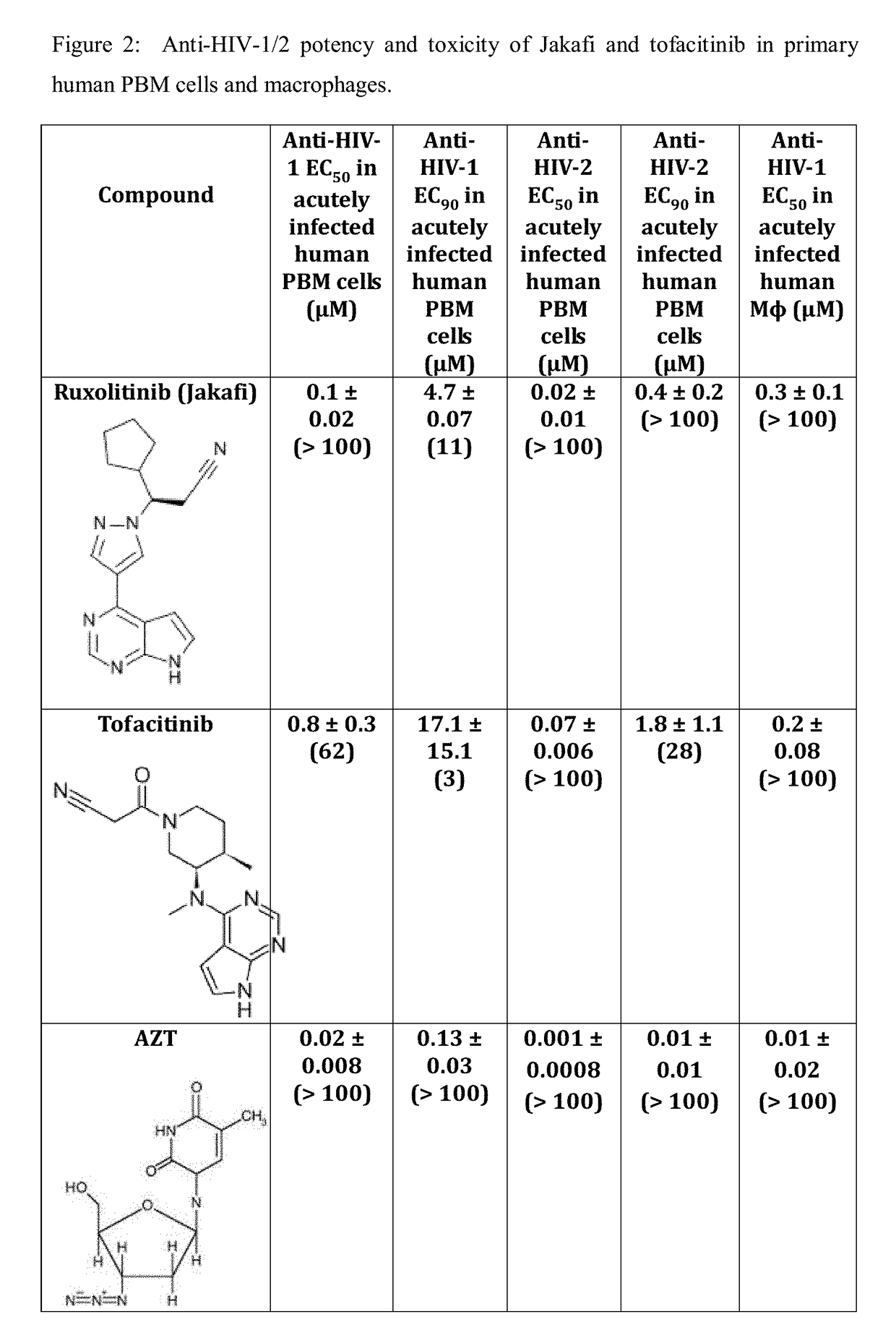
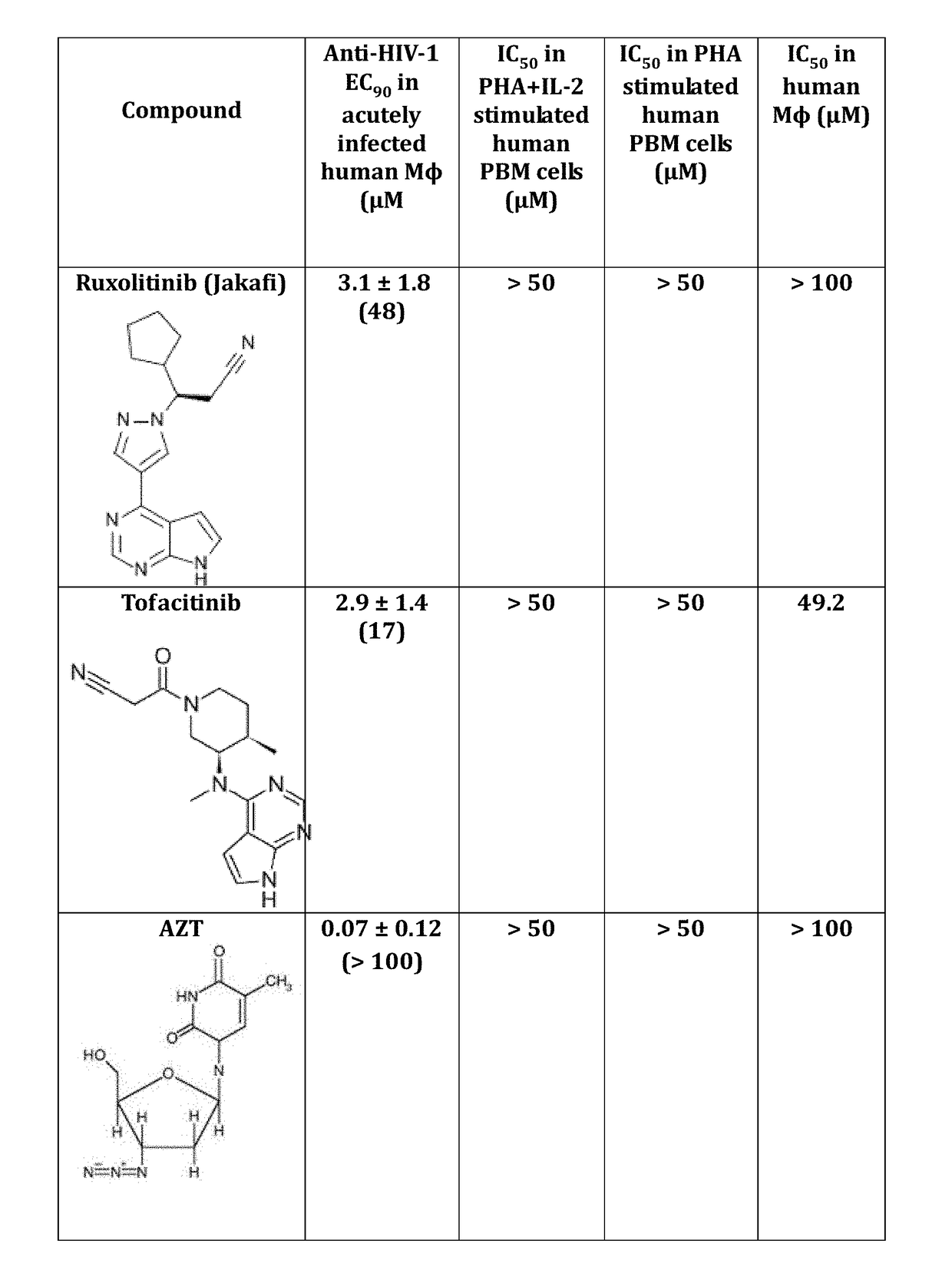
Use of trem-1 inhibitors for treatment, elimination and eradication of hiv-1 infection
ActiveUS20180185372A1Reduce inflammationMinimize abilityOrganic active ingredientsPeptide/protein ingredientsChikungunyaVirus inhibitors
Compounds, compositions, and methods of treatment and prevention of HIV, including HIV-1 and HIV-2, Dengue, and Chikungunya infection are disclosed. The compounds are TREM-1 inhibitors. Combinations of these TREM-1 inhibitors and additional antiretroviral compounds, such as NRTI, NNRTI, integrase inhibitors, entry inhibitors, protease inhibitors, JAK inhibitors, macrophage depleting agents, and the like, are also disclosed. In one embodiment, the combinations include a combination of adenine, cytosine, thymidine, and guanine nucleoside antiviral agents, optionally in further combination with at least one additional antiviral agent that works via a different mechanism than a nucleoside analog. This combination has the potential to eliminate the presence of HIV, Dengue, or Chikungunya virus in an infected patient.
Owner:EMORY UNIVERSITY
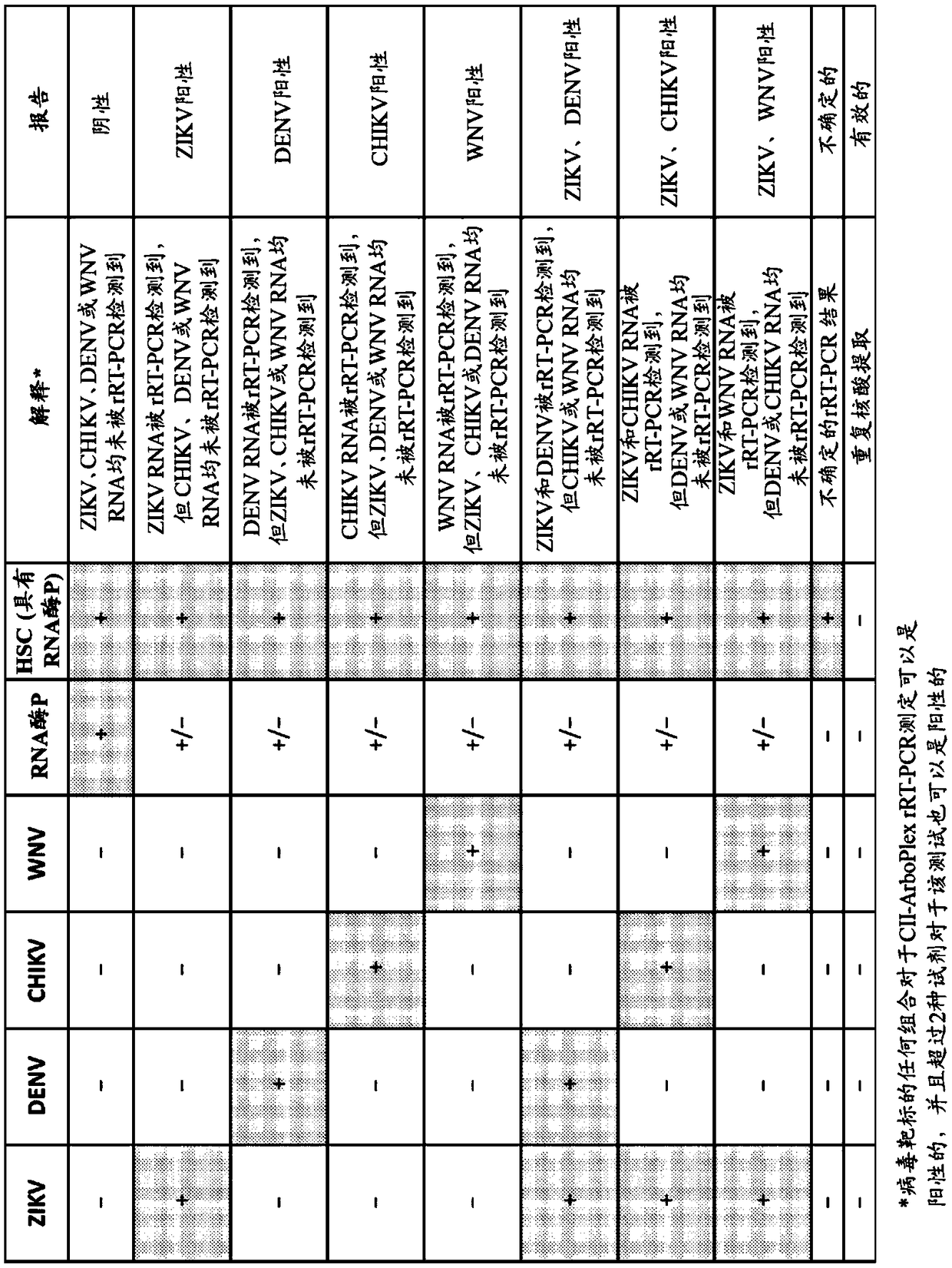
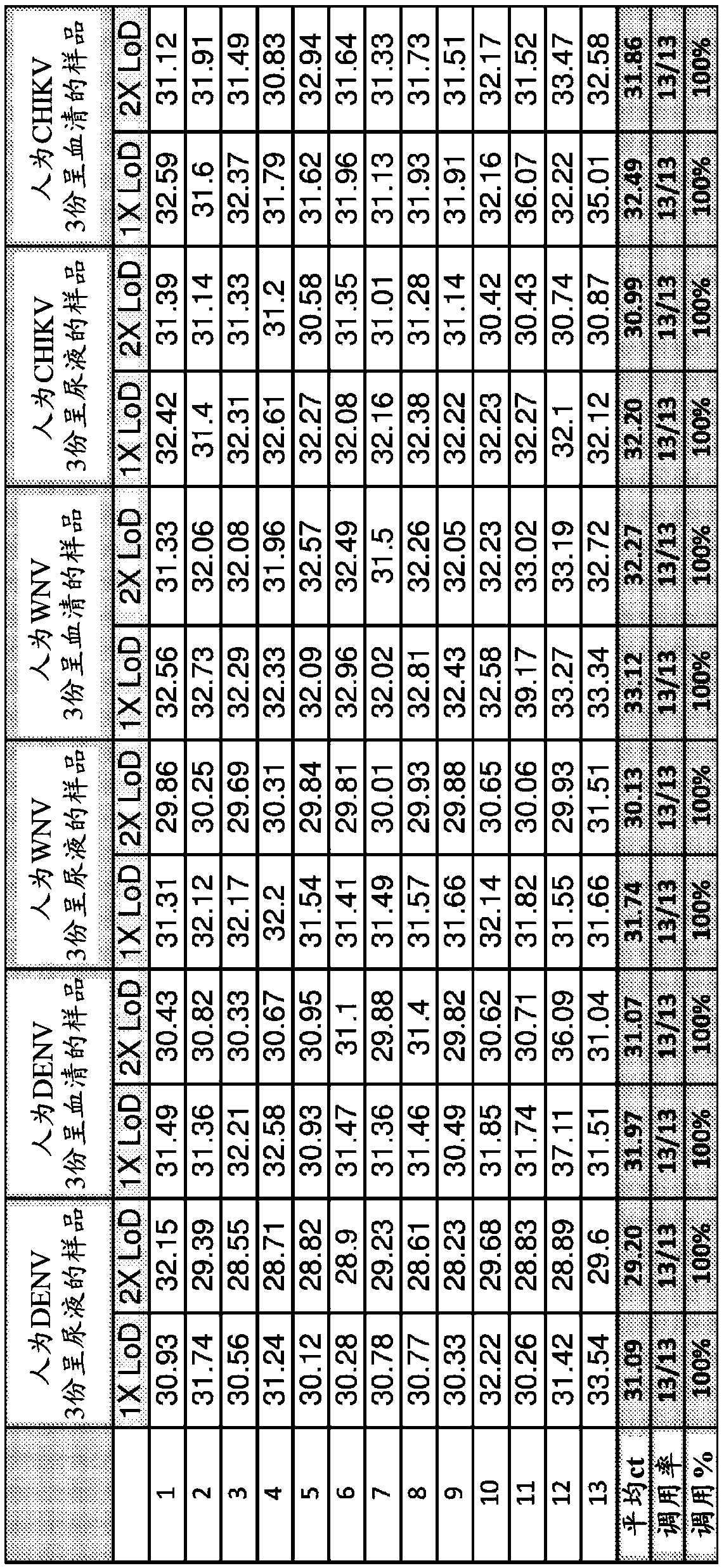
Compositions and methods for the rapid differential detection of zika virus
This invention relates to compositions and methods for the differential detection of multiple viruses using a one-step assay. The viruses to be detected include Zika, West Nile, dengue (genotype 1-4)and chikungunya viruses. In particular, the invention relates to a method of and assay for differential detection of the viruses using specific primers and probes designed to detect and differentiatebetween the viruses.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
DNA molecule containing Chikungunya virus whole genome cDNA, and uses thereof
ActiveCN105483139AImprove efficiencyShort build processMicrobiological testing/measurementMicroorganism based processesChikungunyaA-DNA
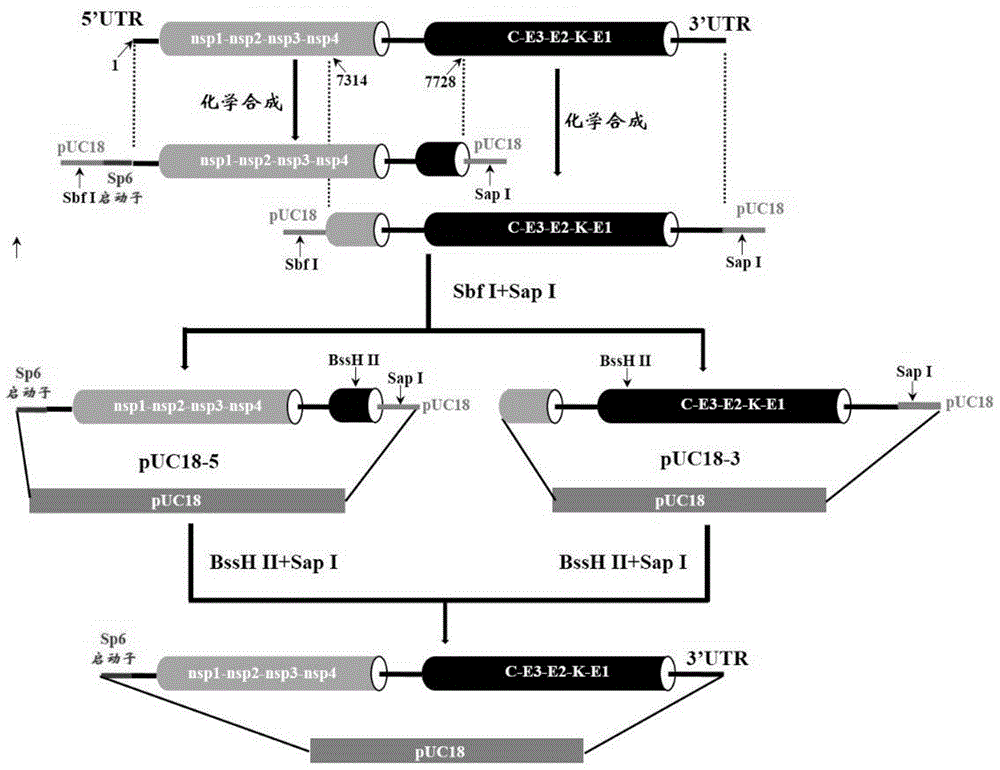
The present invention discloses a DNA molecule having a nucleotide sequence represented by SEQ ID NO:1, and uses thereof, and further discloses a Chikungunya virus DRDE-06 strain labeling method, recombinant plasmid containing the DNA molecule, a construction method of the recombinant plasmid, infectious clone of the Chikungunya virus having the genomic RNA corresponding cDNA nucleotide sequence represented by SEQ ID NO:1, and a construction method of the infectious clone. According to the present invention, the DNA molecule or the recombinant plasmid can be used for constructing the Chikungunya virus infectious clone having the performance similar to the wild-type virus.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Chikungunya virus E2 protein rabbit monoclonal antibody and application thereof
The invention relates to the technical field of biological medicines, and provides an amino acid sequence of a chikungunya virus (CHIKV) E2 protein rabbit monoclonal antibody and application thereof. The amino acid sequence of the CHIKV E2 protein rabbit monoclonal antibody comprises an amino acid sequence of a heavy chain and an amino acid sequence of a light chain of the antibody. The antibody expressed by mammalian cells can neutralize infection of CHIKV genotypes which are mainly popular in the world on the cells in vitro, and can protect type I interferon receptor genes from knocking out mice to resist fatal CHIKV infection in vivo. The antibody is modified, such as humanized modification or preparation of a single-chain antibody, and is expected to be used for treating severe CHIKV infection.
Owner:THE NAVAL MEDICAL UNIV OF PLA
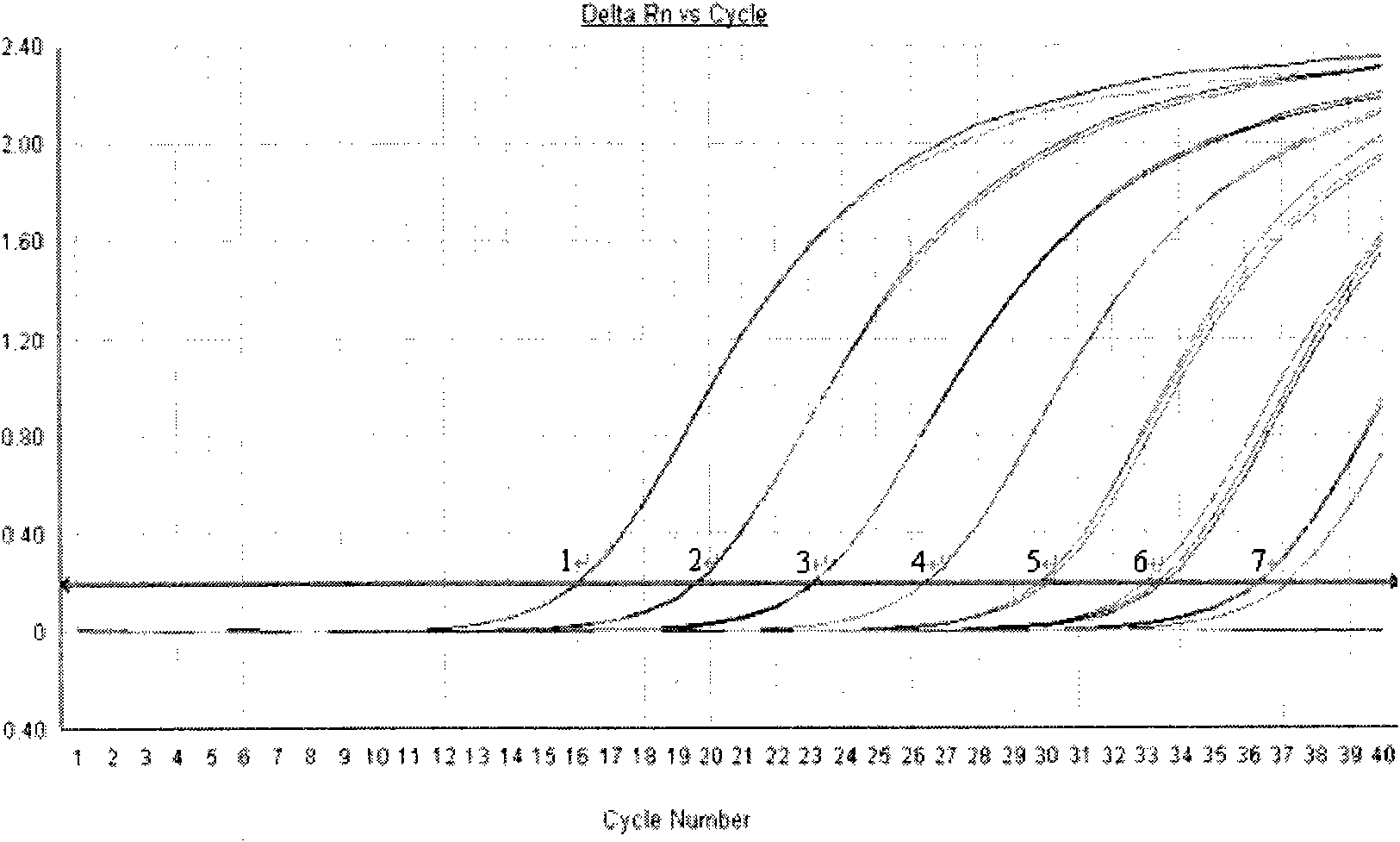
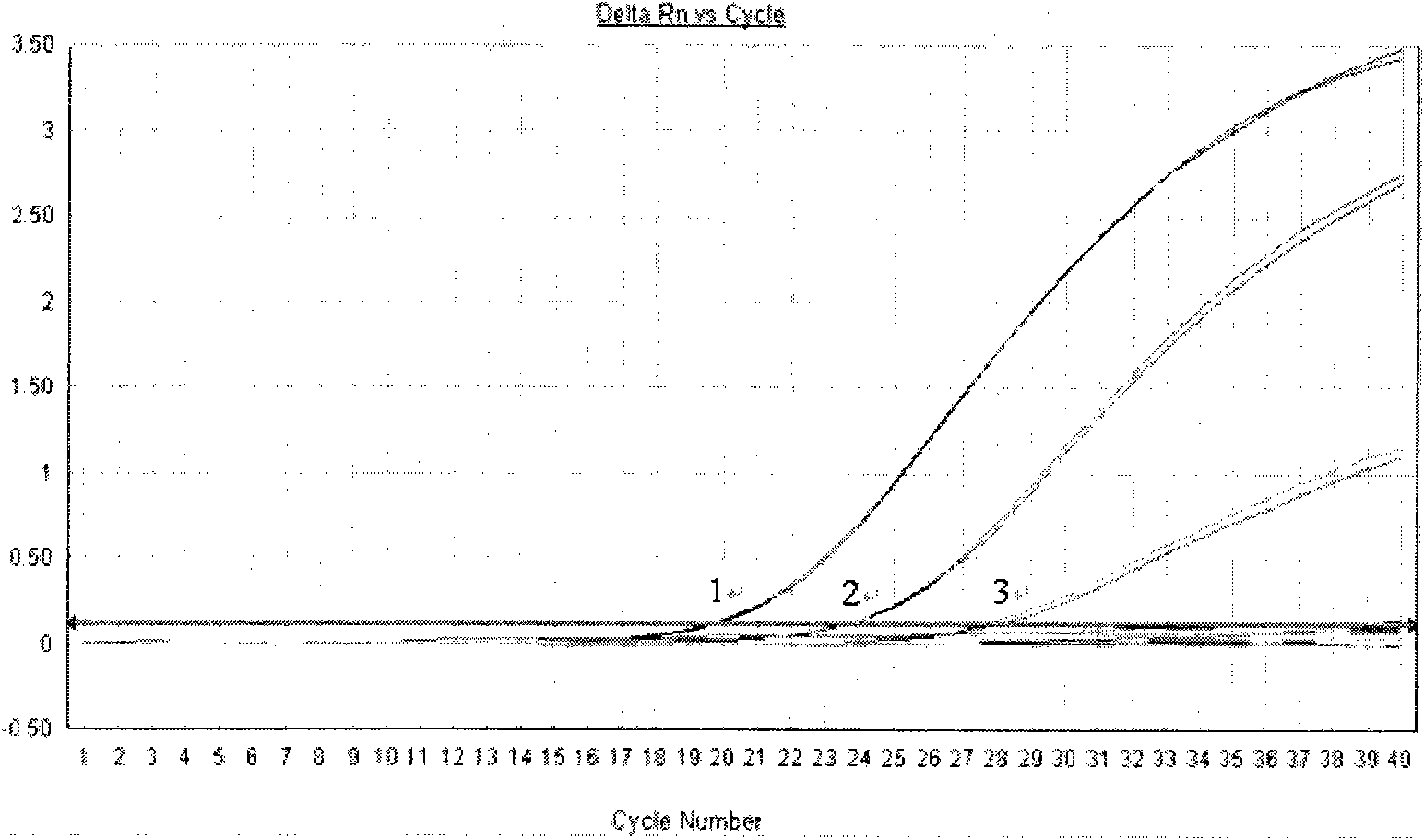
Method for detecting Chikungunya virus nucleic acid by real-time fluorescent quantitative PCR
InactiveCN101935715AHigh detection specificityHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceChikungunyaBiology
The invention discloses a method for detecting Chikungunya virus nucleic acid by real-time fluorescent quantitative PCR, comprising: obtaining a primer and a probe, and detecting the Chikungunya virus nucleic acid. The invention has the advantages of good specificity, high sensitivity and the like.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
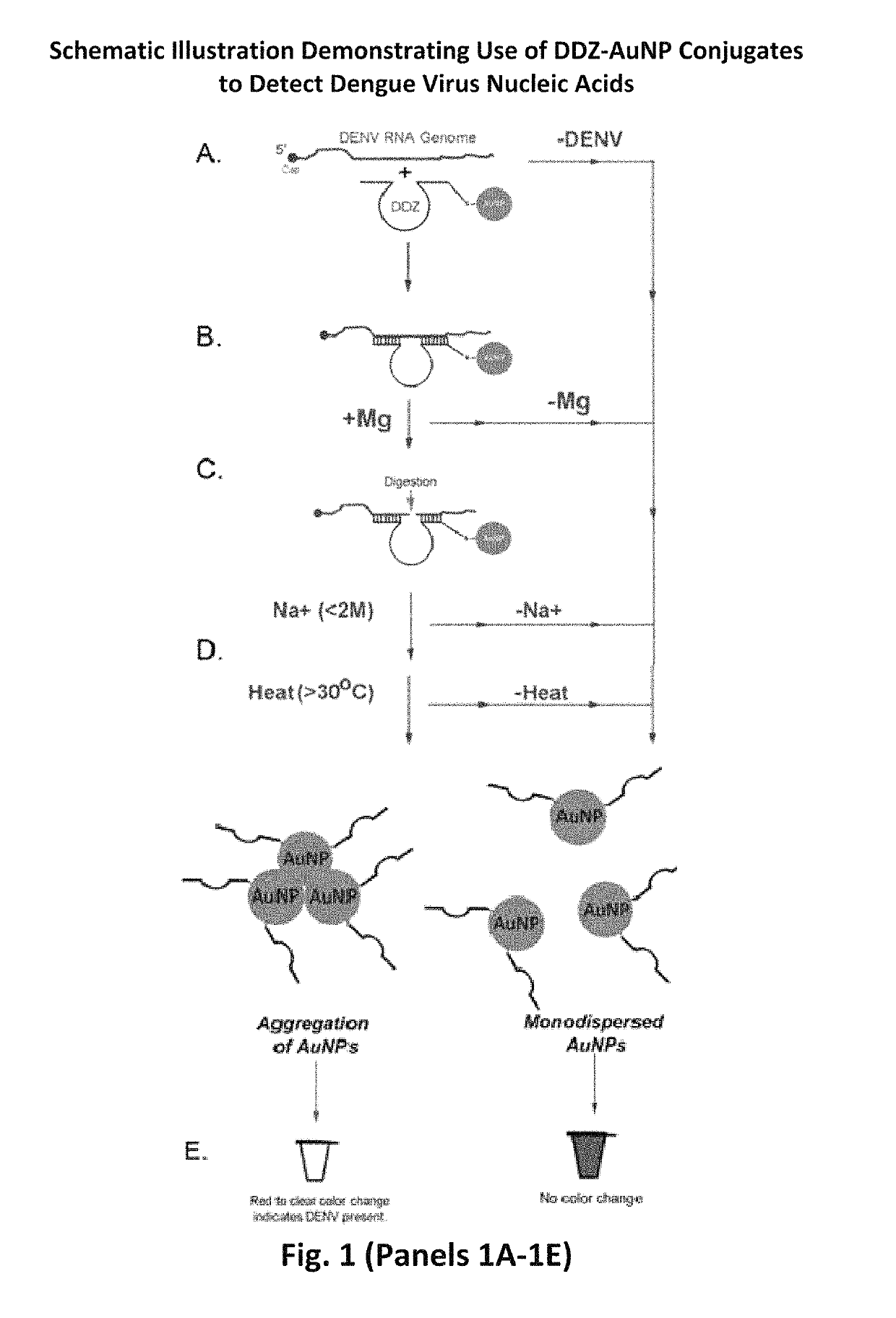
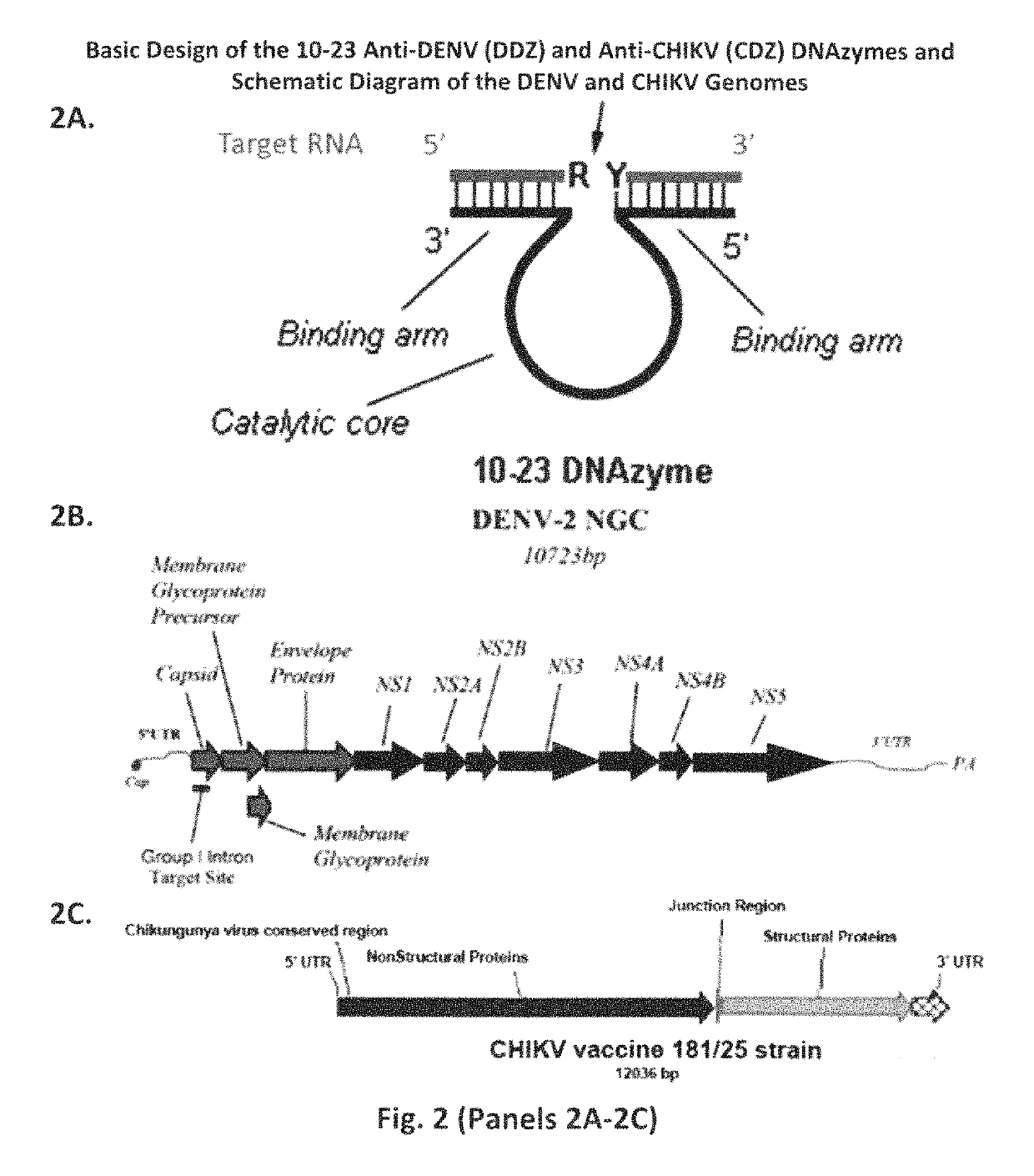
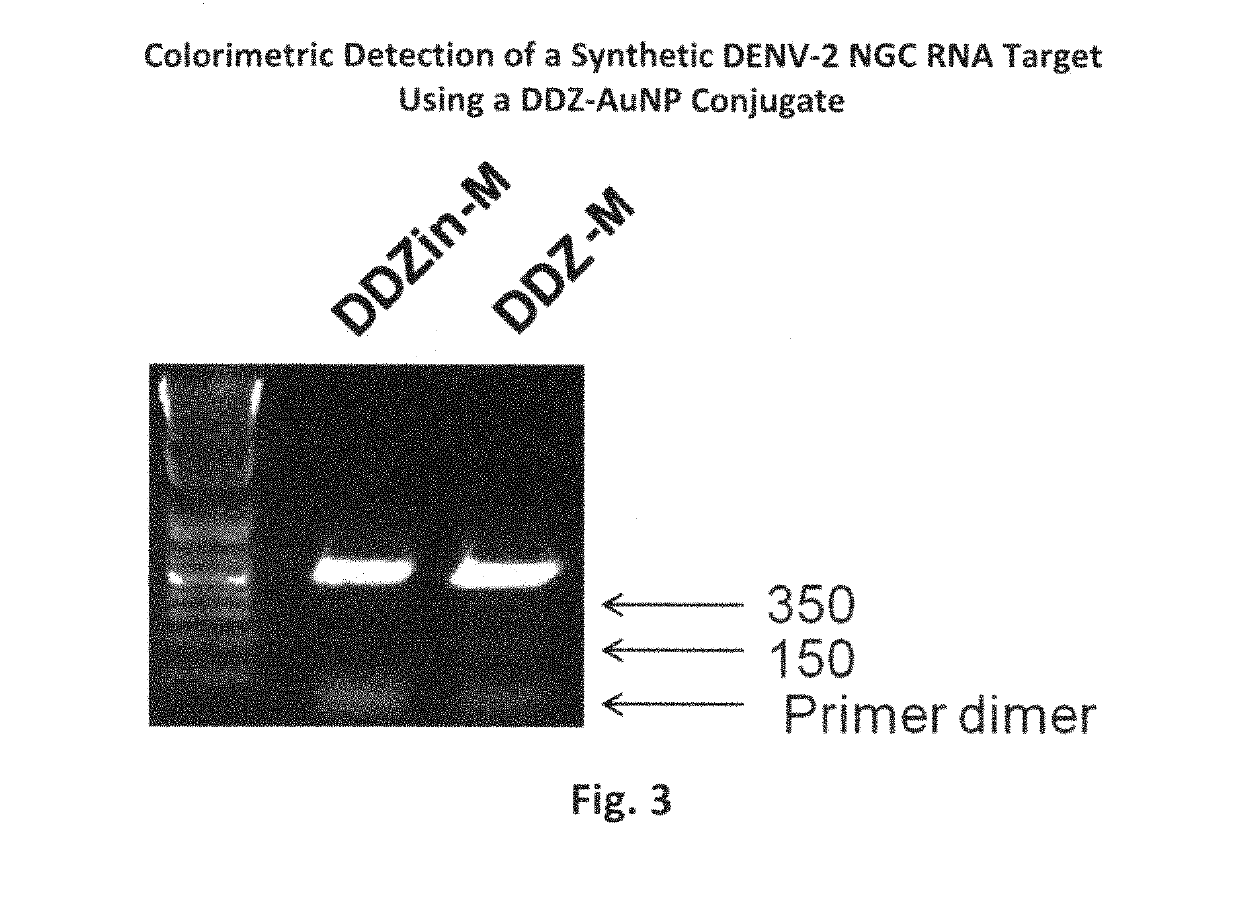
DNAzyme-nanoparticle conjugates and methods of use thereof
ActiveUS10287578B2SsRNA viruses positive-senseMicrobiological testing/measurementChikungunyaNanoparti cles
The present invention relates to DNAzymes (also known as deoxyribozymes, DNA enzymes, catalytic DNA, or DZ), which are conjugated to nanoparticles (NP) to facilitate the detection of nucleic acids. One aspect of the invention relates to compounds comprising DNAzymes conjugated to nanoparticles (DZ-NP), such as metallic or gold nanoparticles, and methods for their synthesis. Another aspect of the invention relates to methods of using the conjugated compounds to detect nucleic acids, such as genomic material or transcripts of infectious agents, such as viruses, exemplified by applications demonstrating visual detection of Flavivirus RNA molecules, such as dengue virus, or Alphavirus RNA molecules, such as chikungunya virus, in short time periods, using compositions comprising stable components.
Owner:UNIV OF NOTRE DAME DU LAC
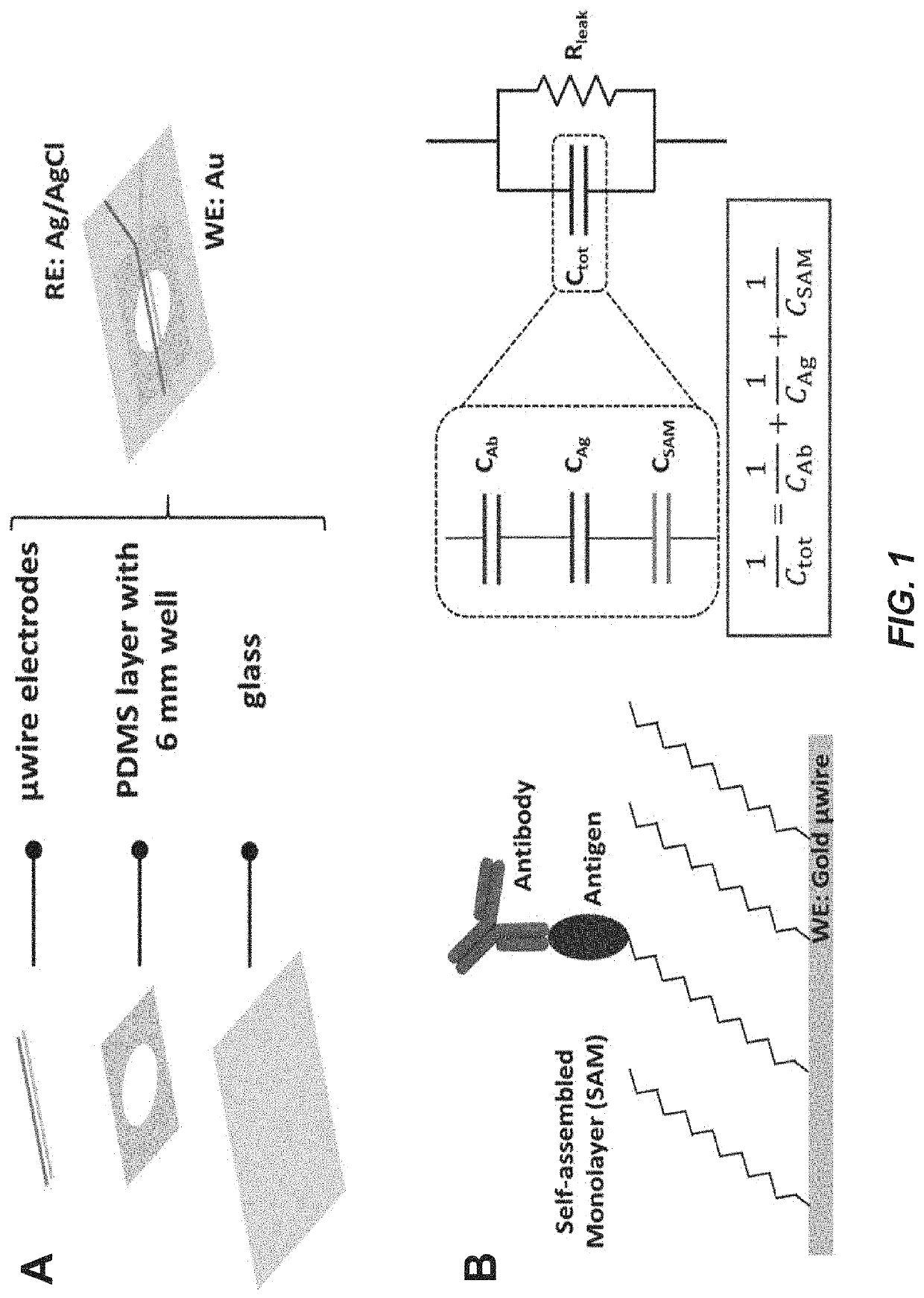
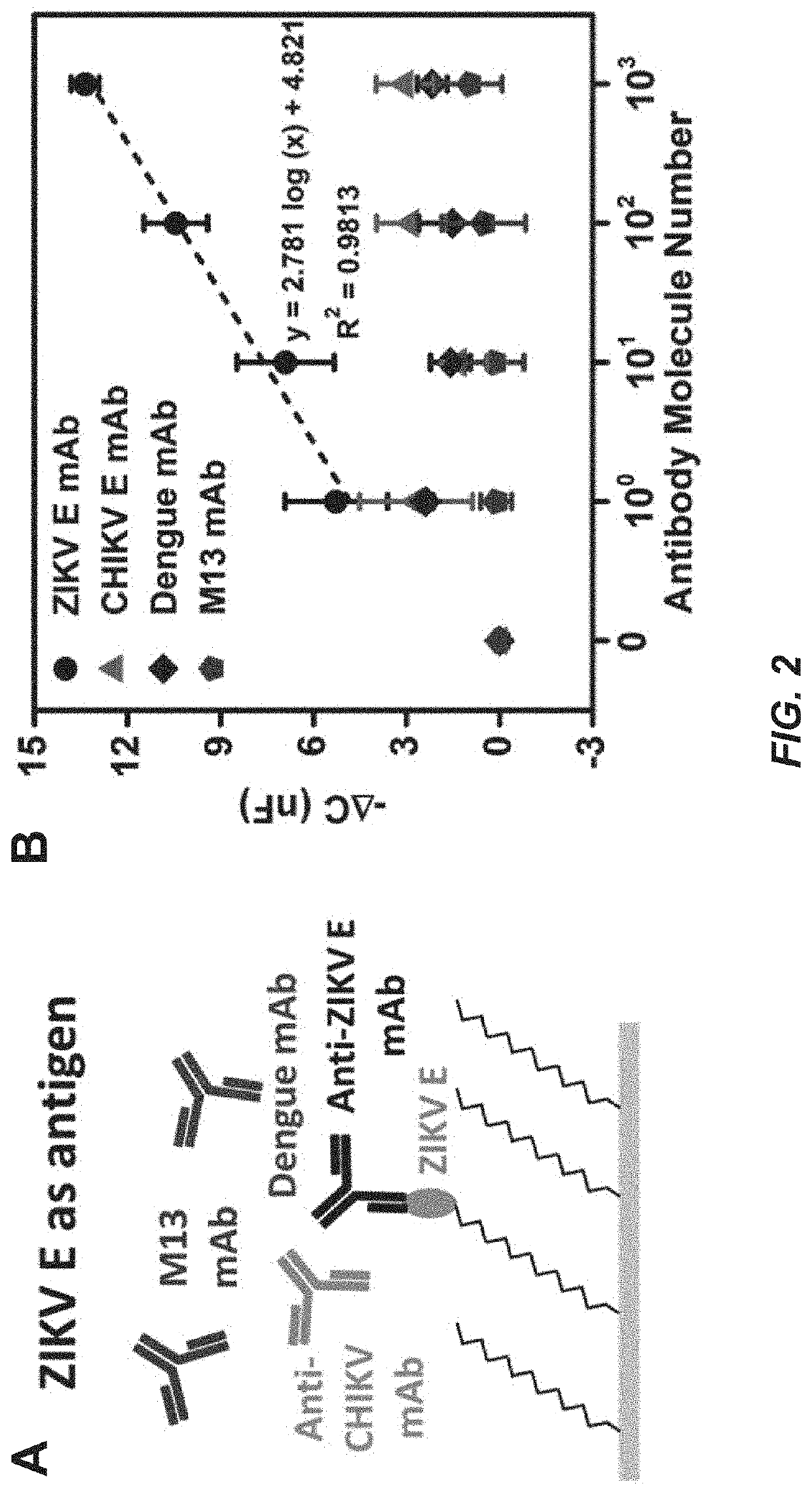
Capacitive micro-sensor for pathogen-specific antibody responses
A novel technique for label-free, rapid detection of ultra-low concentrations of virus specific antibodies is described. We have developed a simple, robust capacitive biosensor using microwires coated with Zika or Chikungunya virus envelope antigen. With little discernable nonspecific binding, the sensor can detect as few as 10 antibody molecules in a small volume (10 molecules / 30 μL) within minutes. It can also be used to rapidly, specifically, and accurately determine the isotype of antigen-specific antibodies. Finally, we demonstrate that anti-Zika virus antibody can be sensitively and specifically detected in dilute mouse serum and can be isotyped using the sensor. Overall, our findings indicate that our microwire sensor platform can be used as a reliable, sensitive, and inexpensive diagnostic tool to detect immune responses at the point of care.
Owner:COLORADO STATE UNIVERSITY
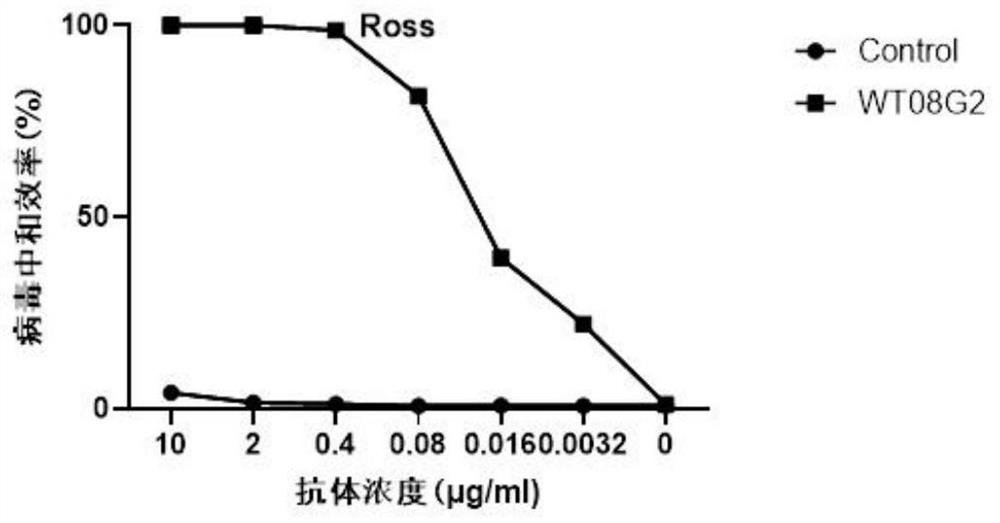
Antibody-mediated neutralization of chikungunya virus
ActiveCN107708726AViral antigen ingredientsMicrobiological testing/measurementChikungunyaBioinformatics
The present disclosure is directed to antibodies binding to and neutralizing Chikungunya virus (CHIKV) and methods for use thereof.
Owner:VANDERBILT UNIV
Chikungunya virus specific detection antigen and application thereof
ActiveCN105906694AStrong specificitySsRNA viruses positive-senseVirus peptidesAntigenSpecific detection
The invention discloses a Chikungunya virus specific detection antigen and an application thereof. The Chikungunya virus specific detection antigen provided by the invention is a polypeptide shown as any of (a1), (a2) and (a3), wherein (a1) is a polypeptide shown as a sequence 1 or a 166-378 site of the sequence 1; (a2)is a polypeptide shown as a sequence 2 or a 166-392 site of the sequence 2; and (a3) is a polypeptide which is obtained by performing substitution and / or deletion and / or addition of one or more amino acid residues on an amino acid sequence of the polypeptide defined by any of (a1) and (a2)and has the same function as the polypeptide defined by any of (a1) and (a2). The Chikungunya virus specific detection antigen has relatively high specificity and sensitivity in detection of Chikungunya virus serum infection.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Vaccine compositions
The present disclosure provides vaccine compositions for prophylaxis and treatment of Zika virus infections comprising Zika virus antigens in immunogenic compositions, and in combination of Zika antigens with one or more arbovirus antigens such as Chikungunya virus and Japanese encephalitis virus antigens, methods of preparation and production of such compositions for use as vaccines for eliciting immune response in mammals against the above mentioned pathogens.
Owner:BHARAT BIOTECH INTERNATIONAL
Nanocapsules carrying chikungunya-associated peptides
The present invention refers to a composition comprising a viral protein or fragment thereof, wherein the viral protein or fragment thereof is enclosed within a self-assembling protein nanocapsule, preferably ferritin, and wherein the viral protein, or fragment thereof is selected from a virus of the Togaviridae family. The viral protein or fragment thereof may also further be selected from a virus of the alphavirus subfamily.
Owner:NANYANG TECH UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com