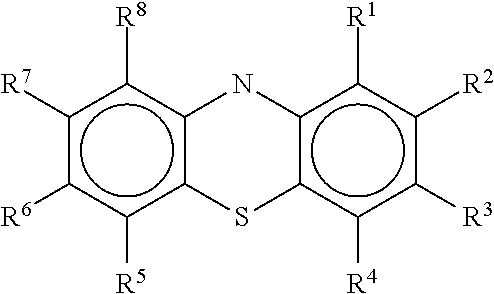
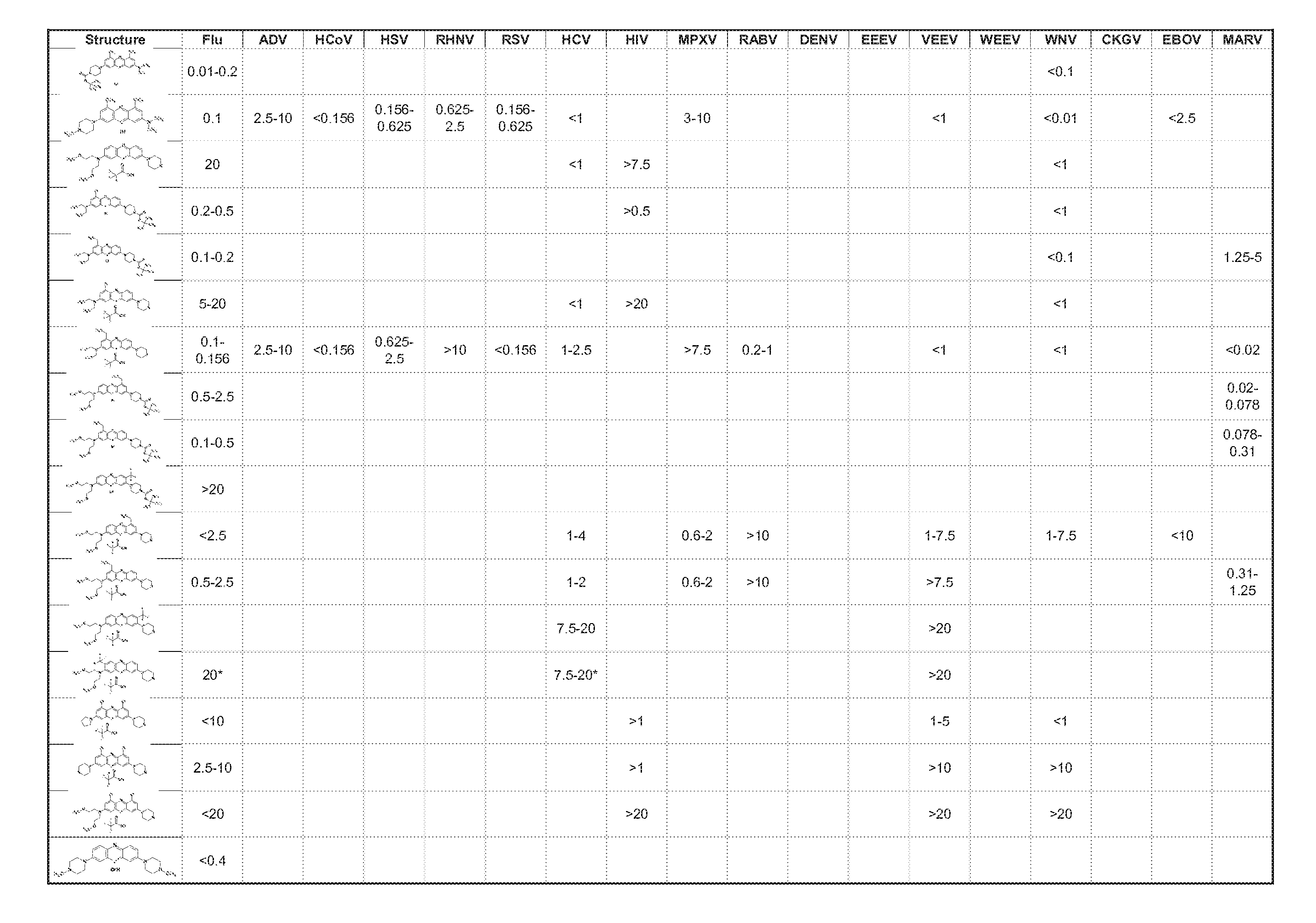
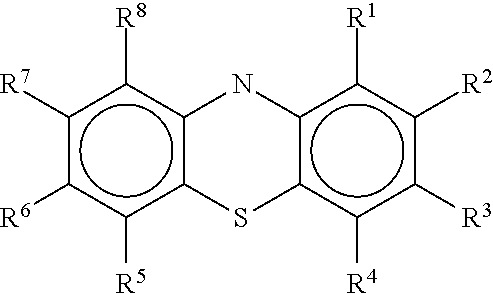
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49 results about "Venezuelan equine encephalitis virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Venezuelan equine encephalitis virus is a mosquito-borne viral pathogen that causes Venezuelan equine encephalitis or encephalomyelitis (VEE). VEE can affect all equine species, such as horses, donkeys, and zebras. After infection, equines may suddenly die or show progressive central nervous system disorders. Humans also can contract this disease. Healthy adults who become infected by the virus may experience flu-like symptoms, such as high fevers and headaches. People with weakened immune systems and the young and the elderly can become severely ill or die from this disease.
Host targeted inhibitors of dengue virus and other viruses
ActiveUS20150166532A1Delay or minimize one or more symptoms associatedReduces and avoids symptom and causeBiocideOrganic chemistryHerpes simplex diseaseDisease injury
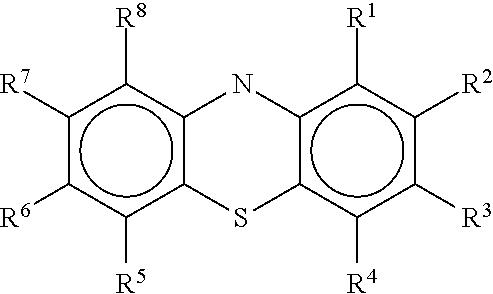
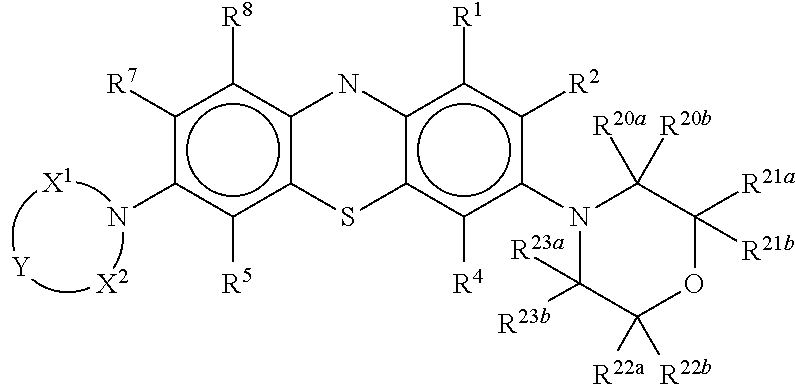
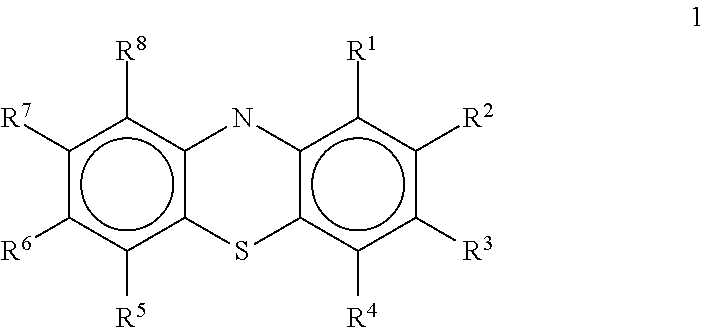
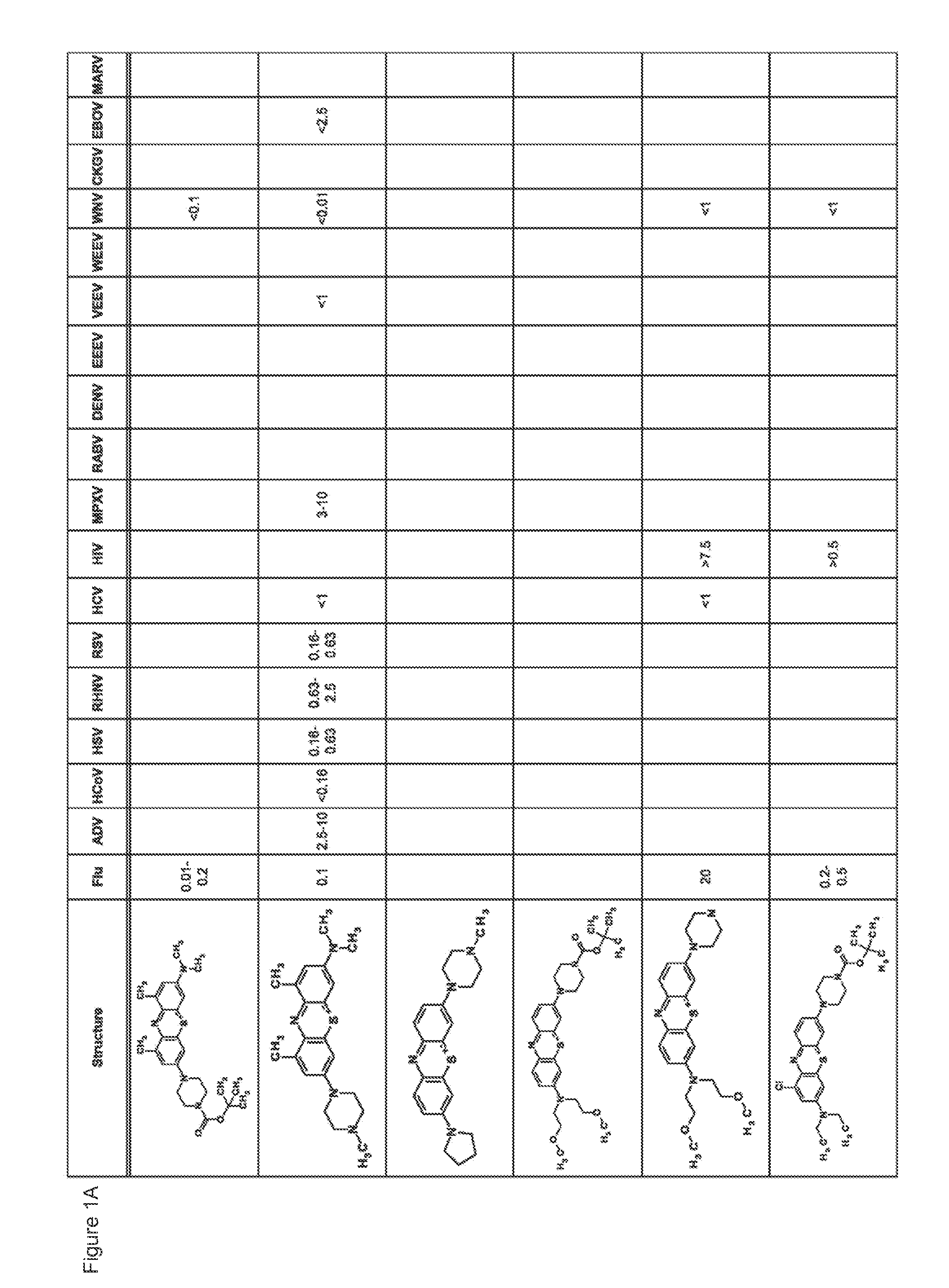
Novel antiviral compounds of Formulae (I)-(III) are provided: (I) (II) (III) The inventive compounds, pharmaceutical compositions thereof, and kits including the inventive compounds are useful for the prevention and treatment of infectious diseases caused by viruses, for example, by Flaviviridae virus (e.g., Dengue virus (DENY)), Kunjin virus, Japanese encephalitis virus, vesicular stomatitis virus (VSV), herpes simplex virus 1 (HSV-1), human cytomegalovirus (HCMV), poliovirus, Junin virus, Ebola virus, Marburg virus (MARV), Lassa fever virus (LASV), Venezuelan equine encephalitis virus (VEEV), or Rift Valley Fever virus (RVFV).
Owner:DANA FARBER CANCER INST INC +1
Fusogenic, self-propagating blebs as immunogenic compositions
InactiveUS20070128222A1Avoid infectionReduce severitySsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousCytopathic effect
Self-propagating, fusogenic blebs are produced from cells infected with a population of Venezuelan Equine Encephalitis virus replicon particles (VRP). The self-propagating, fusogenic nature of the blebs is derived from expression of heterologous genes encoding viral fusion proteins that are incorporated into the replication defective replicon particles. The resulting blebs can be harvested from supernatants of cells displaying severe cytopathic effects. The blebs are used to make immunogenic compositions and devise methods of immunizing mammals against paramyxoviruses such as parainfluenza virus type 3.
Owner:WYETH HOLDINGS CORP
Humanized Anti-venezuelan equine encephalitis virus recombinant antibody
A CDR grafted humanized rAb comprises a human Ig framework having CDRs from murine mAb 1A4A1 VH and VL. DNA sequences and vectors incorporating such sequences are also provided as are pharmaceutical preparations and methods of using the humanized rAbs.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
Host targeted inhibitors of dengue virus and other viruses
ActiveUS9879003B2Delay or minimize one or more symptoms associatedReduces and avoids symptom and causeOrganic chemistryHerpes simplex diseaseJunin virus
Novel antiviral compounds of Formulae (I)-(III) are provided: (I) (II) (III) The inventive compounds, pharmaceutical compositions thereof, and kits including the inventive compounds are useful for the prevention and treatment of infectious diseases caused by viruses, for example, by Flaviviridae virus (e.g., Dengue virus (DENY)), Kunjin virus, Japanese encephalitis virus, vesicular stomatitis virus (VSV), herpes simplex virus 1 (HSV-1), human cytomegalovirus (HCMV), poliovirus, Junin virus, Ebola virus, Marburg virus (MARV), Lassa fever virus (LASV), Venezuelan equine encephalitis virus (VEEV), or Rift Valley Fever virus (RVFV).
Owner:DANA FARBER CANCER INST INC +1
Venezuelan equine encephalitis virus replicons with adaptive mutations in the genome and uses thereof
ActiveUS7332322B2Reduced cytopathic effectReduce productionSsRNA viruses positive-senseViral antigen ingredientsMammalViral replication
The present invention provides a Venezuelan equine encephalitis virus replicon RNA useful in the development of stable lines of mammalian, avian and insect cells in which these replicons will persistently replicate. Venezuelan equine encephalitis (VEE) virus replicons contain a number of unique adaptive mutations that make the replicons noncytopathic. The replicons remain resistant to IFN-α / β. Replicon replication leads to high-level production of heterologous proteins, which are encoded by the replicons' genome and are under the control of a viral subgenomic promoter. Also provided are methods of screening for inhibitory compounds of Venezuelan equine encephalitis virus replication and eastern equine encephalitis virus replication.
Owner:BOARD OF RGT UNIV OF TEXAS THE
Attenuated recombinant alphaviruses incapable of replicating in mosquitoes and uses thereof
ActiveUS20110052634A1SsRNA viruses positive-senseMutant preparationEastern equine encephalitis virusEncephalitis Viruses
The present invention discloses an attenuated recombinant alphavirus that is incapable of replicating in mosquito cells and of transmission by mosquito vectors. These attenuated alphavirus may include but is not limited to Western Equine Encephalitis virus, Eastern equine encephalitis virus, Venezuelan equine encephalitis virus or Chikungunya virus. The present invention also discloses the method of generating such alphaviruses and their use as immunogenic compositions.
Virus like particle comprising modified envelope protein e3
ActiveUS20160200775A1Increase productionSsRNA viruses positive-senseAntibody mimetics/scaffoldsVirus-like particleChikungunya
A virus like particle comprising a viral structural protein which comprises modified envelope protein E3. The viral structural protein may be that derived from or alphavirus or flavivirus. Especially, the viral structural protein may be derived from Chikungunya virus or Venezuelan equine encephalitis virus.
Owner:VLP THERAPEUTICS LLC
Fusogenic, self-propagating blebs as immunogenic compositions
InactiveUS7541038B2SsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousCytopathic effect
Self-propagating, fusogenic blebs are produced from cells infected with a population of Venezuelan Equine Encephalitis virus replicon particles (VRP). The self-propagating, fusogenic nature of the blebs is derived from expression of heterologous genes encoding viral fusion proteins that are incorporated into the replication defective replicon particles. The resulting blebs can be harvested from supernatants of cells displaying severe cytopathic effects. The blebs are used to make immunogenic compositions and devise methods of immunizing mammals against paramyxoviruses such as parainfluenza virus type 3.
Owner:WYETH HOLDINGS CORP
Attenuated recombinant alphaviruses incapable of replicating in mosquitoes and uses thereof
ActiveUS8426188B2Animal cellsSsRNA viruses positive-senseEastern equine encephalitis virusChikungunya
The present invention discloses an attenuated recombinant alphavirus that is incapable of replicating in mosquito cells and of transmission by mosquito vectors. These attenuated alphavirus may include but is not limited to Western Equine Encephalitis virus, Eastern equine encephalitis virus, Venezuelan equine encephalitis virus or Chikungunya virus. The present invention also discloses the method of generating such alphaviruses and their use as immunogenic compositions.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
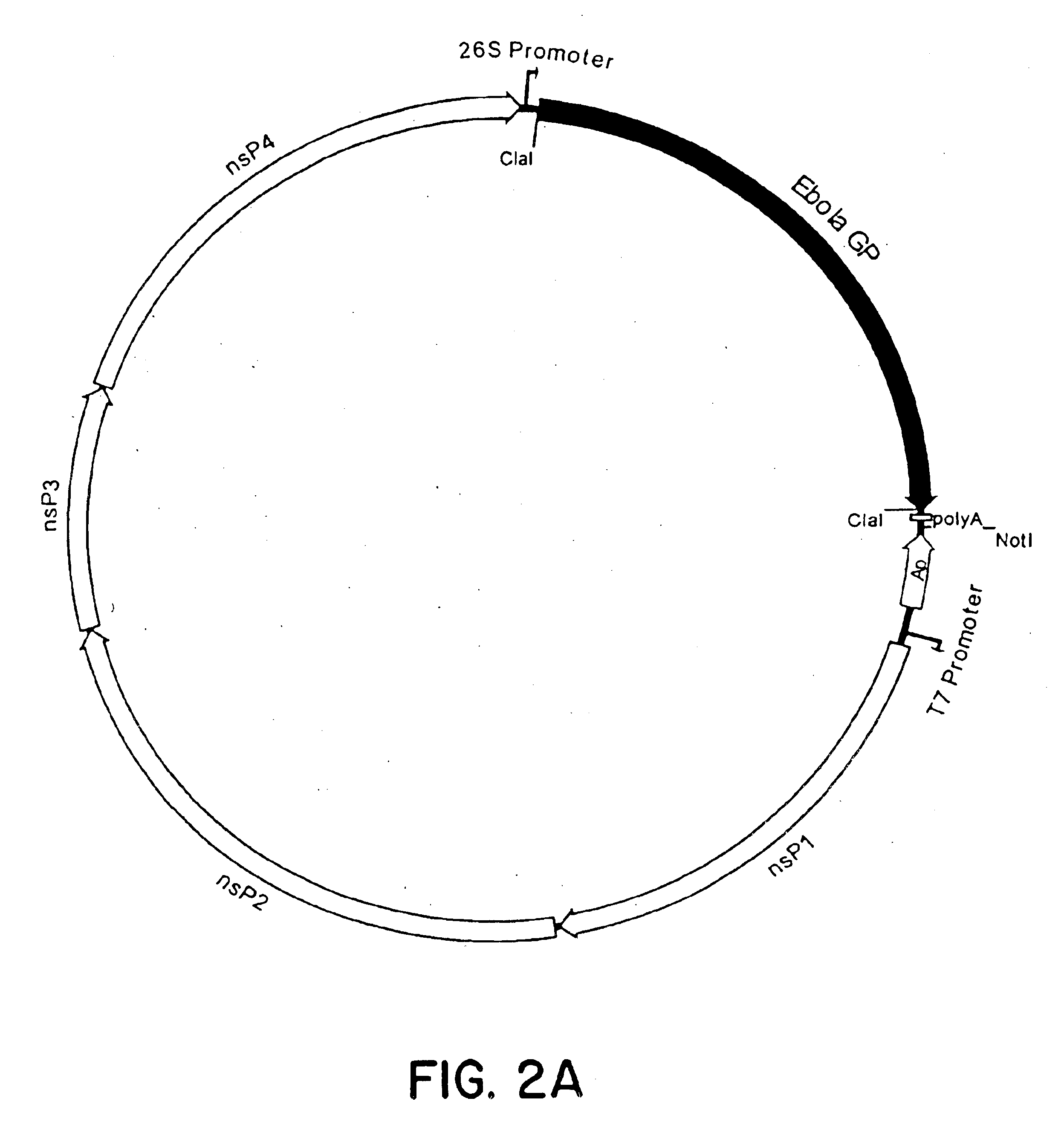
Ebola virion proteins expressed from venezuelan equine encephalitis (VEE) virus replicons
InactiveUS6984504B2SsRNA viruses negative-senseSsRNA viruses positive-senseVP40Venezuelan equine encephalitis virus
Using the Ebola GP, NP, VP24, VP30, VP35 and VP40 virion proteins, a method and composition for use in inducing an immune response which is protective against infection with Ebola virus is described.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Chimeric chikungunya virus and uses thereof
ActiveUS20110171249A1Avoid infectionSsRNA viruses positive-senseSugar derivativesSindbis virusChikungunya fever
The present invention discloses a chimeric Chikungunya virus comprising a heterologous alphavirus cDNA fragment and a Chikungunya virus cDNA fragment. The heterologous alphavirus may include but is not limited to Sindbis virus, Eastern equine encephalitis virus or Venezuelan equine encephalitis virus. The present invention also discloses the use of this chimeric Chikungunya virus as vaccines and in serological and diagnostic assays.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Humanized antibodies against the venezuelan equine encephalitis virus
InactiveUS20060024666A1Prevent and neutralize viral infectionFungiBacteriaHumanized antibodyViral infection
Owner:ALEXION PHARMA INC
Kit for detecting eastern equine encephalitis virus and west equine encephalitis virus by real-time fluorescence quantitative RT-PCR
InactiveCN101967524AMicrobiological testing/measurementFluorescence/phosphorescenceForward primerFluorescence
The invention discloses a kit for detecting eastern equine encephalitis virus and west equine encephalitis virus by real-time fluorescence quantitative RT-PCR. The kit provided by the invention comprises a primer pair as shown in the following (1) and (2): (1) EEEV forward primer: TGTGCGTACCTCCTCATCGTT, EEEV reverse primer: GACTGGCGTGAATCTCTGCT; (2) WEEV forward primer: AGGGATACCCCCGAAGGTT, WEEV reverse primer: GTGAATAGCACACGGGTGGTT. The kit can be applied to detecting EEEV and WEEV and has good sensitivity and specificity; in addition, the lowest EEEV virus titer that the kit can detect is 0.2TCID50 / ml and the WEEV virus titer that the kit can detect is 1TCID50 / ml.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Antibody dependent enhancement of Venezuelan Equine Encephalitis vector infection
ActiveUS7651998B1Good curative effectIncrease infectivitySsRNA viruses positive-sensePeptide/protein ingredientsAntibody-dependent enhancementDendritic cell
The present invention provides compositions and methods for delivering a nucleotide sequence to a cell using an alphavirus vector that is complexed with an enhancing antibody that specifically binds to the alphavirus vector. Venezuelan Equine Encephalitis vectors are preferred. The cell may be a cell in vitro or in vivo. Alternatively, the cell may be removed from a subject, administered the alphavirus vector ex vivo and then administered to a subject. Antigen-presenting cells are preferred, with dendritic cells being more preferred. Also provided are methods of producing an immune response in a subject, e.g., for producing an immune response against an antigen associated with a pathogen or for immunotherapy of cancer of tumors.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Methods of treating zika virus, mers-cov, chikungunya, venezuelan equine encephalitus, and rhinovirus in mammalian patients
InactiveUS20170157219A1Inhibition formationHydrolasesPeptide/protein ingredientsZika virusMiddle East respiratory syndrome coronavirus
Viral infections in mammals can be treated and prophylactically prevented by systemic administration of ranpirnase and three other ribonucleases that are highly homologous with it and that have activities that are highly similar to it. Experimental results against Zika virus, Middle East Respiratory Syndrome Coronavirus (“MERS-CoV”), Chikungunya virus, Venezuelan equine encephalitis, and rhinovirus-14 are disclosed.
Owner:TAMIR BIOTECH
Virus like particle comprising modified envelope protein E3
ActiveUS9969986B2Increase productionSsRNA viruses positive-senseNervous disorderEncephalitisVirus-like particle
A virus like particle comprising a viral structural protein which comprises modified envelope protein E3. The viral structural protein may be that derived from or alphavirus or flavivirus. Especially, the viral structural protein may be derived from Chikungunya virus or Venezuelan equine encephalitis virus.
Owner:VLP THERAPEUTICS LLC
Method and kit for detecting multiple encephalitis related viruses
ActiveCN102796827AImprove throughputAddresses issues with reduced sensitivityMicrobiological testing/measurementDNA/RNA fragmentationEastern equine encephalitis virusMicrosphere
The invention provides a method and a kit for detecting multiple encephalitis related viruses. In particular, the invention discloses a method for simultaneously detecting multiple encephalitis related viruses. The method comprises the following steps of: performing polymerase chain reaction (PCR) by using a specific primer set aiming at the encephalitis related viruses in a polymerase reaction system to obtain an amplification product; and detecting with specific probes or probe microspheres. The invention also provides the corresponding kit. The method and the kit can be used for sensitively and simply detecting and identifying multiple encephalitis related viruses comprising eastern equine encephalitis viruses, western equine encephalitis viruses, Venezuelan equine encephalitis viruses, forest encephalitis viruses and Japanese encephalitis viruses.
Owner:SHANGHAI TELLGEN LIFE SCI CO LTD
Antiviral Compounds
InactiveUS20120302556A1Easy to PlatingEliminate residual PBS.Organic chemistryAntiviralsViral infectionWest Nile virus RNA
Compounds and methods for preventing and treating viral infections are provided. In some embodiments, novel compounds broad-spectrum antiviral activity are provided. In more specific embodiments, the compounds and methods are effective against viruses such as Venezuelan Equine Encephalitis, West Nile Virus, and Hepatitis C.
Owner:PROSETTA ANTIVIRAL
Lphavirus replicon vector-based rabies virus infectious clone, and preparation method and application thereof
ActiveCN110643632AControllable positioningEasy way to getSsRNA viruses negative-senseViral antigen ingredientsViral glycoproteinTGE VACCINE
The invention belongs to the technical field of biology, and particularly discloses an alphavirus replicon vector-based rabies virus infectious clone, and a preparation method and application thereof.The infectious clone of the strain is constructed by taking Venezuelan Equine Encephalitis Virus (VEEV) vaccine strain TC-83 replicon which lacks structural protein genes as a vector and inserting rabies virus Glycoprotein (G) genes into a deletion position of the vector, and the rescued strain serving as an attenuated live vaccine not only provides effective immune protection, but also is saferto use.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI +1
Immunogenic compositions comprising venezuelan equine encephalitis virus replicon vectors and paramyxovirus protein antigens
InactiveCN1798760ASsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousCytopathic effect
Self-propagating, fusogenic blebs are produced from cells infected with a population of Venezuelan Equine Encephalitis virus replicon particles (VRP). The self-propagating, fusogenic nature of the blebs is derived from expression of heterologous genes encoding viral fusion proteins that are incorporated into the replication defective replicon particles. The resulting blebs can be harvested from supernatants of cells displaying severe cytopathic effects. The blebs are used to make immunogenic compositions and devise methods of immunizing mammals against paramyxoviruses such as parainfluenza virus type 3.
Owner:WYETH HOLDINGS CORP
Venezuelan equine encephalitis virus replicon encoding marburg virus proteins
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Antiviral Compounds
Compounds and methods for preventing and treating viral infections are provided. In some embodiments, novel compounds broad-spectrum antiviral activity are provided. In more specific embodiments, the compounds and methods are effective against viruses such as Venezuelan Equine Encephalitis, West Nile Virus, and respiratory viruses including the common cold.
Owner:PROSETTA ANTIVIRAL
Antiviral Compounds
ActiveUS20120270854A1Easy to PlatingEliminate residual PBS.BiocideOrganic chemistryViral infectionWest Nile virus RNA
Compounds and methods for preventing and treating viral infections are provided. In some embodiments, novel compounds broad-spectrum antiviral activity are provided. In more specific embodiments, the compounds and methods are effective against viruses such as Venezuelan Equine Encephalitis, West Nile Virus, and Hepatitis C.
Owner:PROSETTA ANTIVIRAL
Alphavirus Replicon Particles Expressing TRP2
InactiveUS20120128714A1Reduce the possibilityProlong survival timeViral antigen ingredientsCancer antigen ingredientsAdjuvantLymphatic Spread
The immune response to melanoma cells and tumors can be induced or significantly increased by administering to a subject a pharmaceutical composition comprising alphavirus particles, especially Venezuelan equine encephalitis virus replicon particles, which express the melanoma antigen dopachrome tautomerase (DCT, TRP2) in cells of the subject, with the result of tumor regression and / or inhibition of metastasis of a melanoma subject, or a decreased risk of the occurrence or recurrence of melanoma and / or decreased severity of melanoma in a subject not suffering from melanoma at the time of administration. The pharmaceutical composition described herein can be used in conjunction with other therapeutic agents, it can be administered on more than one occasion and it can be combined with administrations of other compositions such as protein or other immunogenic compositions, and / or adjuvants, with beneficial effects to the human or animal subject to which it has been administered.
Owner:ALPHAVAX INC +1
Equine Encephalitis Virus Vaccines and Methods of Using Thereof
ActiveUS20150056230A1% survivabilitySsRNA viruses positive-senseViral antigen ingredientsEquine EncephalitisVenezuelan equine encephalitis virus
Disclosed herein are nucleotide sequences which encode a plurality of structural proteins, except the capsid, of an equine encephalitis virus, wherein the nucleotide sequence is codon-optimized for mammalian expression. The nucleotide sequences are codon-optimized for expression in humans. As disclosed herein, the nucleotide sequences confer protection against Venezuelan equine encephalitis virus (VEEV), western equine encephalitis virus (WEEV), and / or eastern equine encephalitis virus (EEEV).
Owner:THE UNITED STATES OF AMERICA AS REPESENTED BY THE SEC OF ARMY ON BEHALF OF THE U S ARMY MEDICAL RES INST OF INFECTIONS DISEASES
Genetically biotinylated recombinant antibody in immunofiltration assay by light addressable potentiometric sensor for identification of Venezuelan equine encephalitis virus
InactiveUS7309566B2Microbiological testing/measurementImmunoglobulins against virusesBiotin-streptavidin complexFiltration
A genetically biotinylated single chain fragment variable (scFv) antibody against Venezuelan equine encephalitis virus (VEE) being applied in a system consisting of an immunofiltration-enzyme assay (IFA) with a light addressable potentiometric sensor (LAPS) for the rapid identification of VEE is disclosed. The IFA entails formation of an immunocomplex sandwich consisting of VEE, biotinylated antibody, fluoresceinated antibody and streptavidin, capturing the sandwich by filtration on biotinylated membrane, and detecting the sandwich by anti-fluorescein urease conjugate. The concentration ratio of biotinylated to fluoresceinated antibodies is investigated and optimized. The IFA / LAPS assay sensitivity was approximately equal to that of a conventional enzyme-linked immunosorbant assay utilizing polystyrene plates and a chromogenic substrate, however, less time and effort were required for performance of the IFA / LAPS assay.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
Antiviral compounds
InactiveUS8809317B2Easy to PlatingEliminate residual PBS.BiocideOrganic chemistryMedicineViral infection
Compounds and methods for preventing and treating viral infections are provided. In some embodiments, novel compounds broad-spectrum antiviral activity are provided. In more specific embodiments, the compounds and methods are effective against viruses such as Venezuelan Equine Encephalitis, West Nile Virus, and Hepatitis C.
Owner:PROSETTA ANTIVIRAL
Monoclonal antibody EEEV-6E2 resisting EEEV E2 protein, B-cell epitope peptide recognized by EEEV-6E2 as well as application of EEEV-6E2 and B-cell epitope peptid
The invention discloses a monoclonal antibody EEEV-6E2 resisting EEEV (Eastern Equine Encephalitis Virus) E2 protein, B-cell epitope peptide recognized by EEEV-6E2 as well as the application of the EEEV-6E2 and the B-cell epitope peptide. The invention further discloses a hybridoma cell strain which can stably secrete the EEEV-6E2 resisting the EEEV E2 protein and is named as EEEV-6E2. The microbial preservation number of the hybridoma cell strain is CGMCC No. 7007. An idiosyncratic reaction can occur between the EEEV-6E2 secreted by the hybridoma cell strain and the EEEV E2 protein while the EEEV-6E2 does not react with WEEVes / VEEVes (Western Equine Encephalitis Viruses / Venezuelan Equine Encephalitis Viruses). Secondly, the invention further discloses the B-cell epitope peptide which is specially recognized by the EEEV-6E2. The EEEV-6E2 and the specific B-cell epitope peptide, recognized by the EEEV-6E2, of the EEEV E2 protein can be used for preparing reagents for identifying and diagnosing EEEVes and other alphaviruses and lay a foundation for creating identifying and diagnosing methods of EEEV antigen clusters and other alphaviruses as well as further studying prevention and treatment measures.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Antiviral compounds
Compounds and methods for preventing and treating viral infections are provided. In some embodiments, novel compounds broad-spectrum antiviral activity are provided. In more specific embodiments, the compounds and methods are effective against viruses such as Venezuelan Equine Encephalitis, West Nile Virus, and respiratory viruses including the common cold.
Owner:PROSETTA ANTIVIRAL
Antiviral compounds
ActiveUS8828986B2Easy to PlatingEliminate residual PBS.BiocideOrganic chemistryVenezuelan equine encephalitis VEEViral infection
Compounds and methods for preventing and treating viral infections are provided. In some embodiments, novel compounds broad-spectrum antiviral activity are provided. In more specific embodiments, the compounds and methods are effective against viruses such as Venezuelan Equine Encephalitis, West Nile Virus, and Hepatitis C.
Owner:PROSETTA ANTIVIRAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com