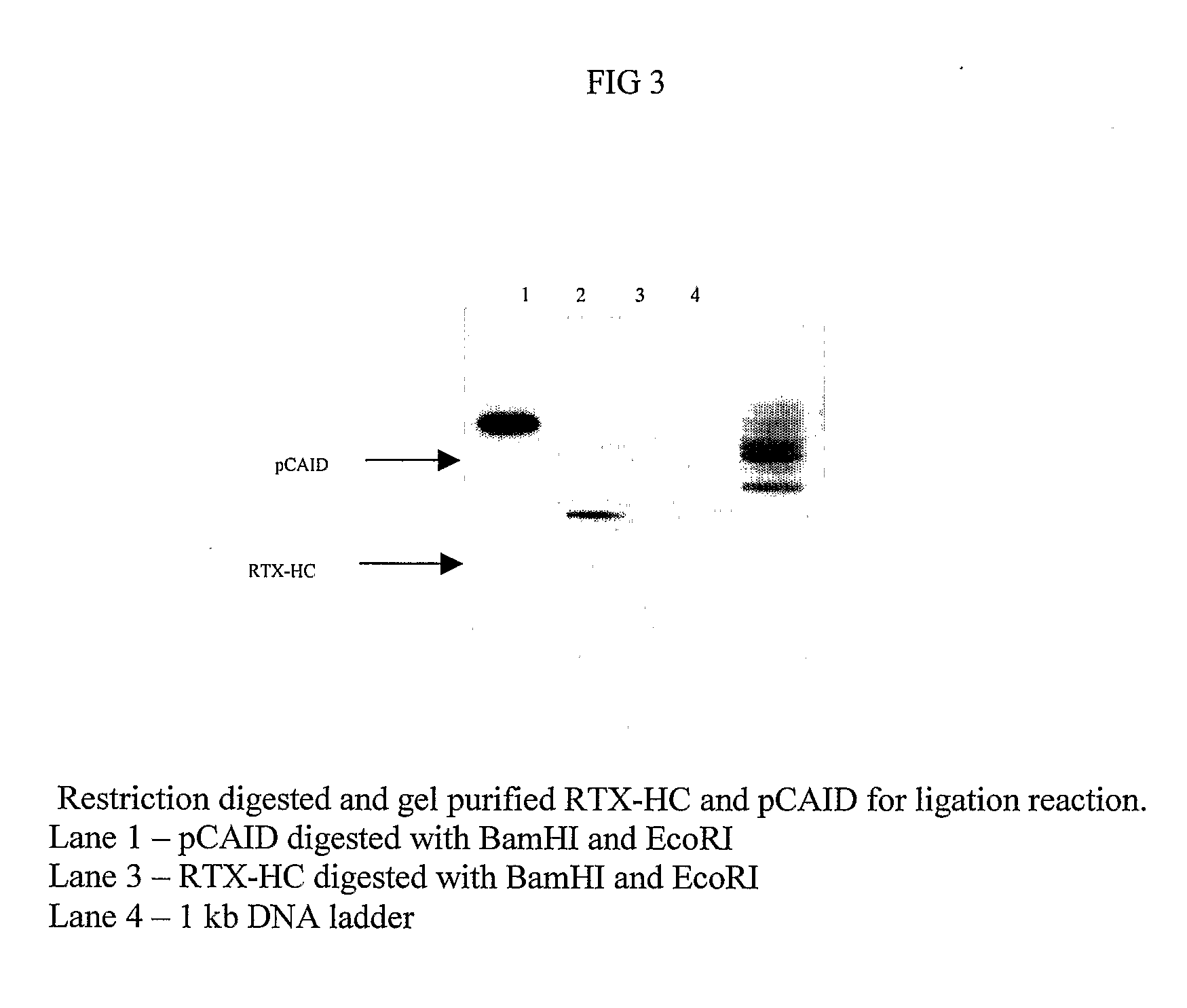
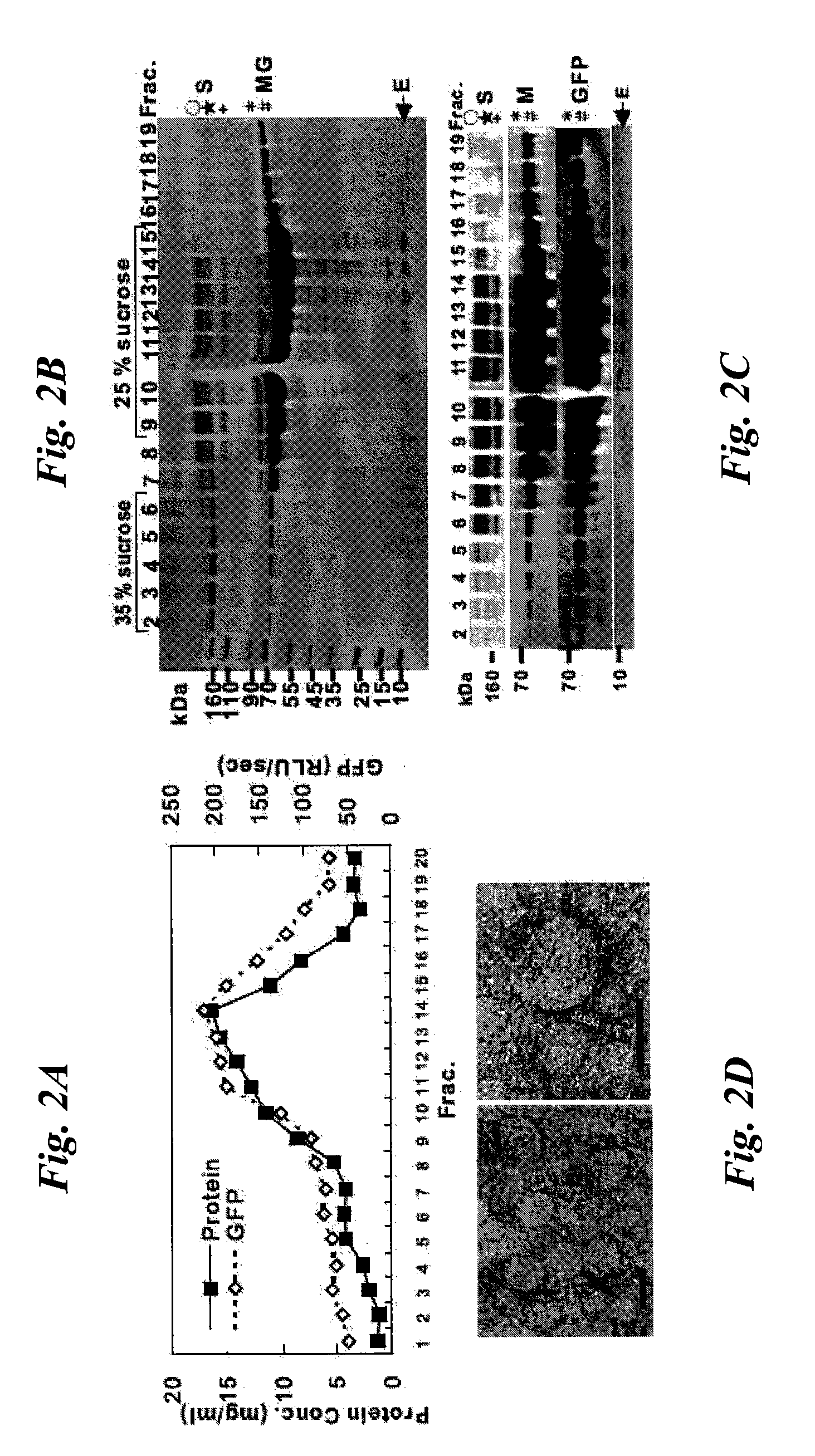
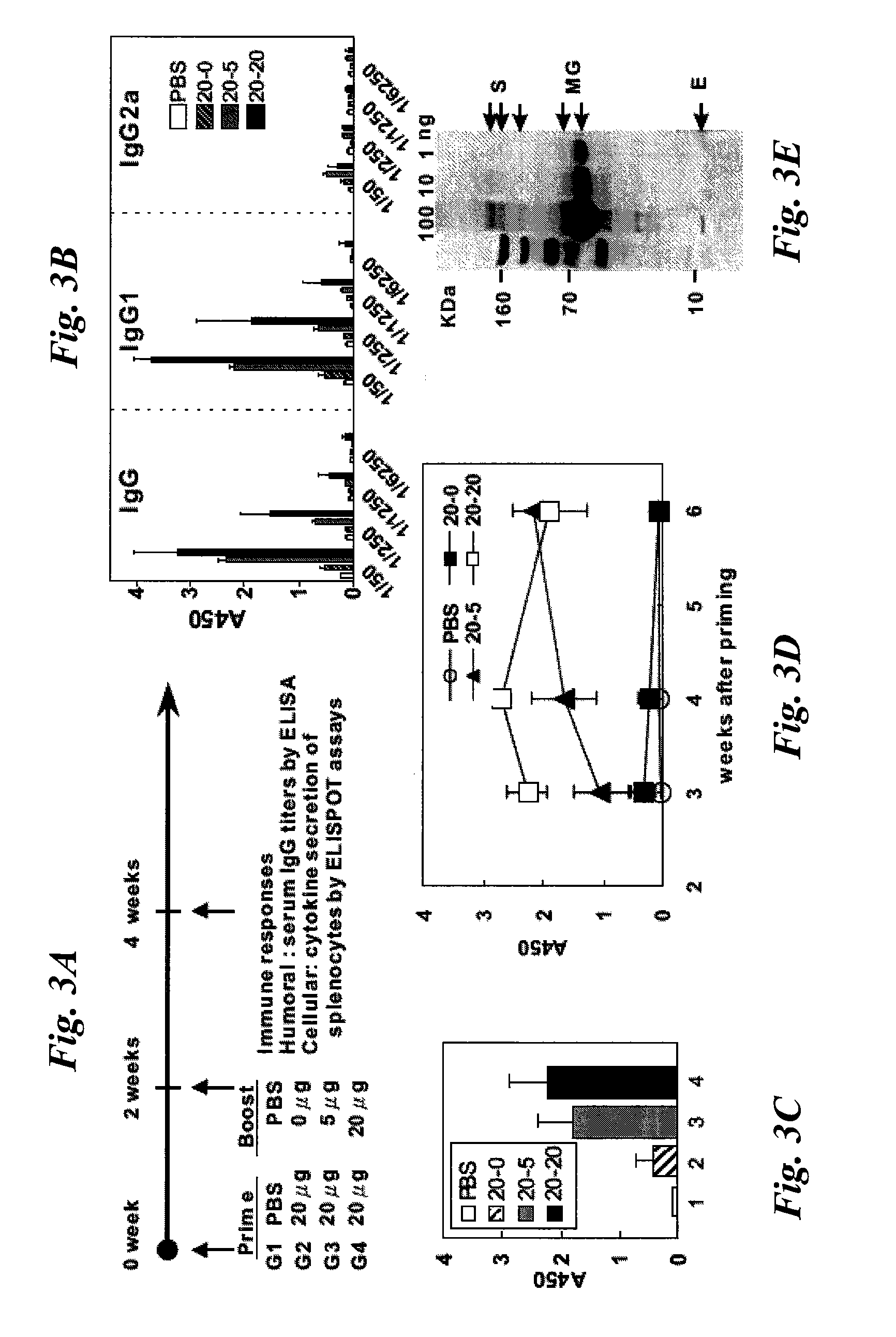
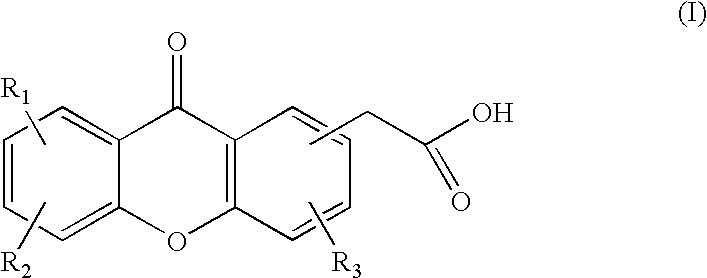
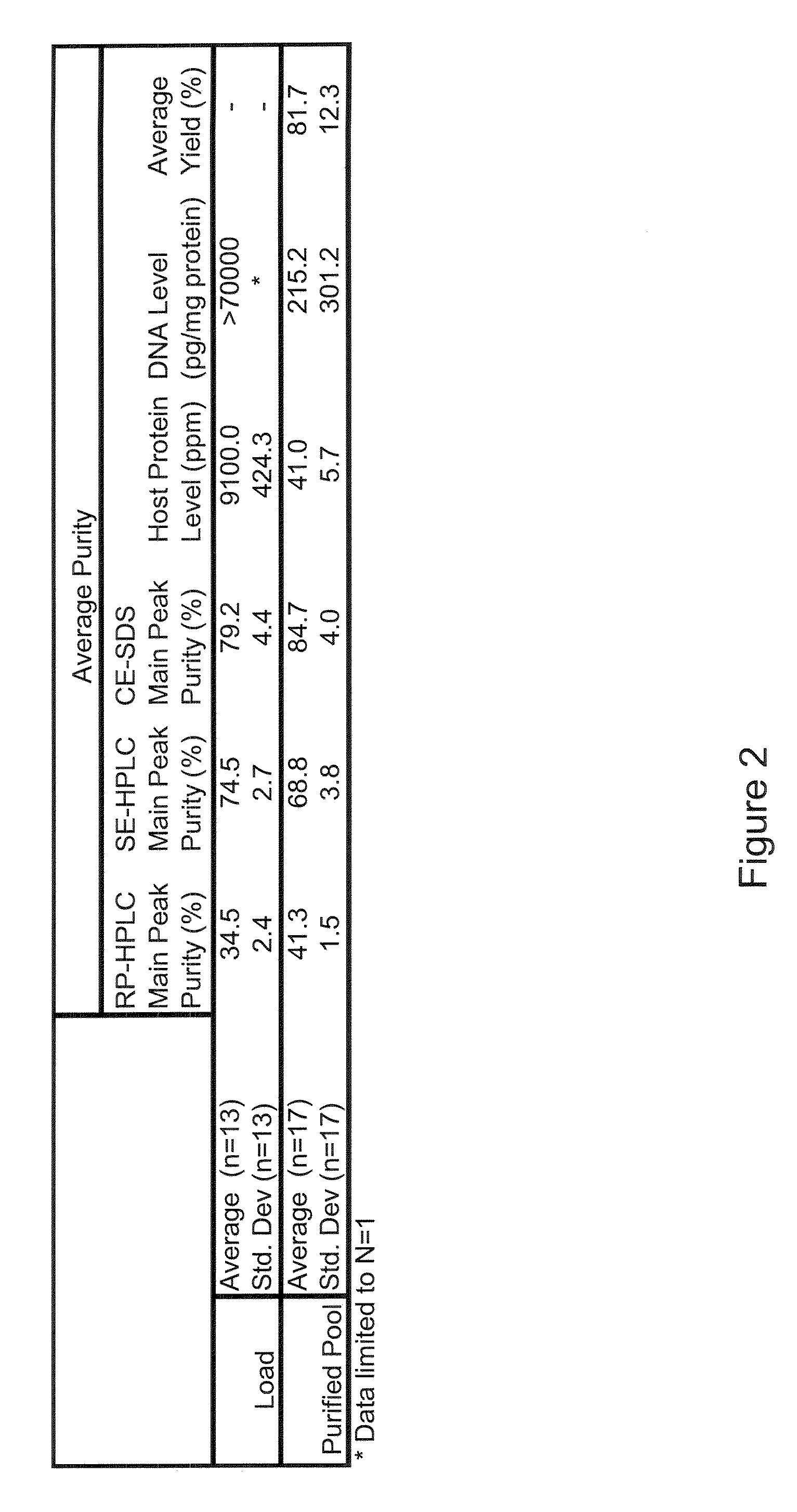
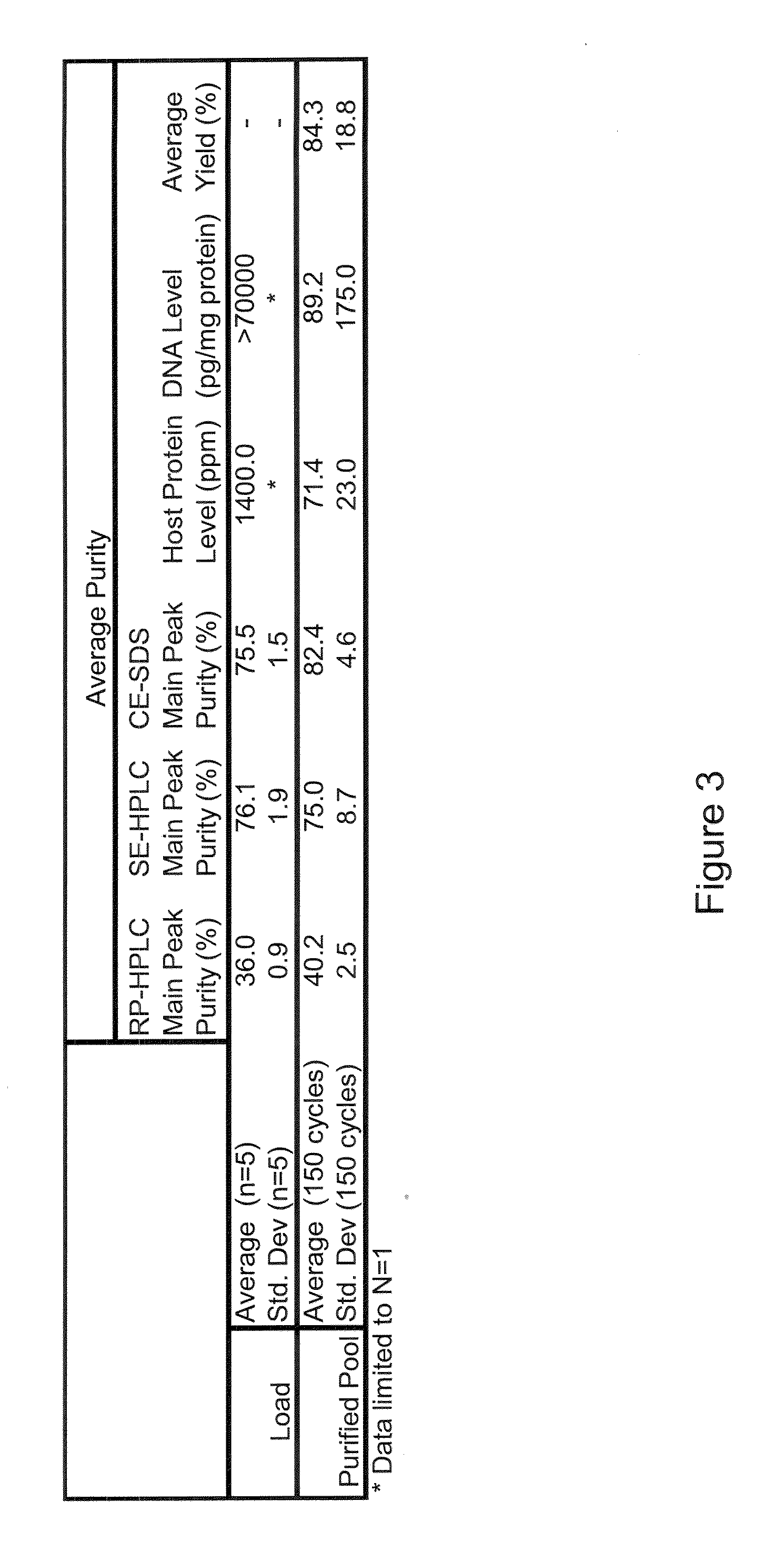
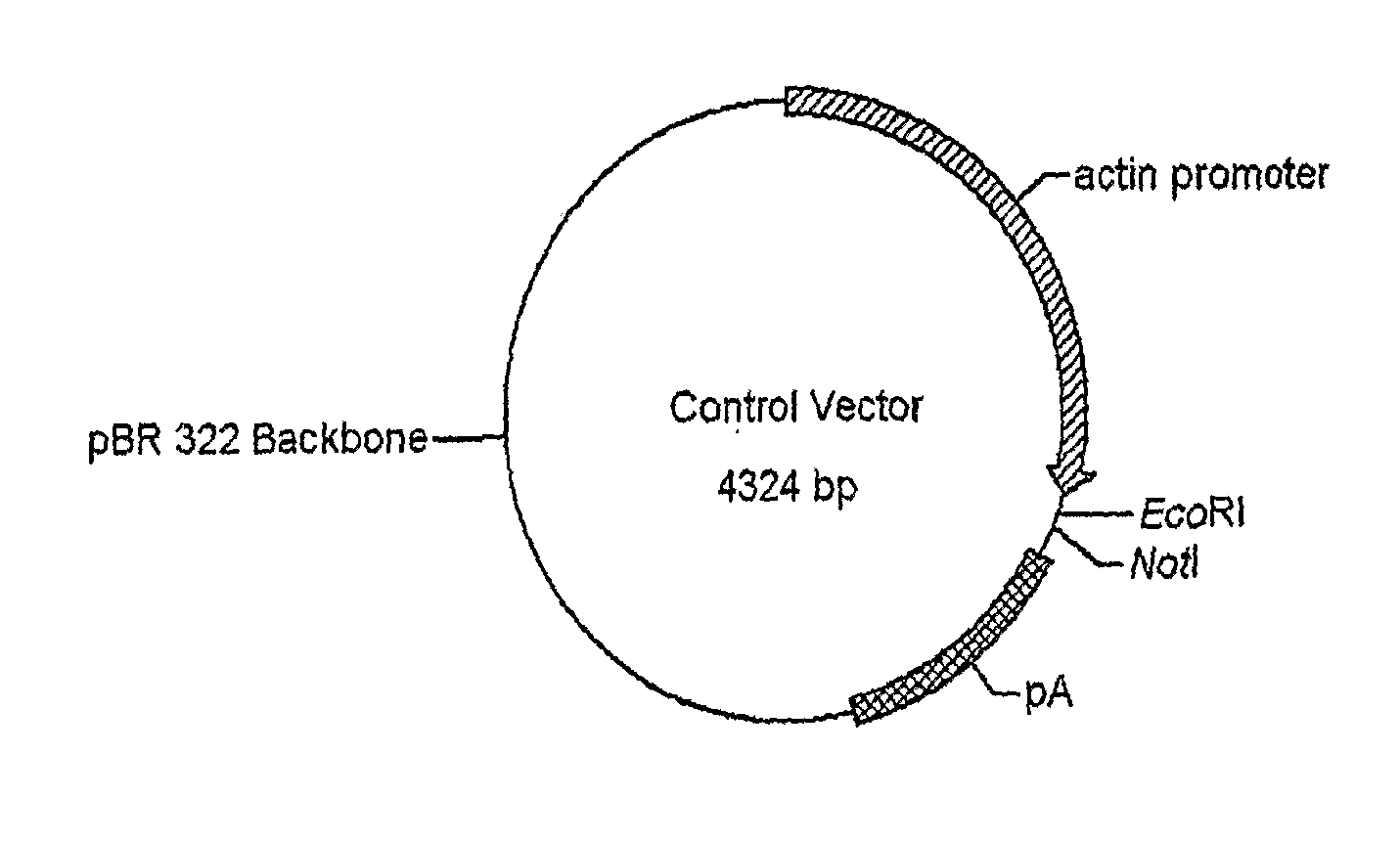
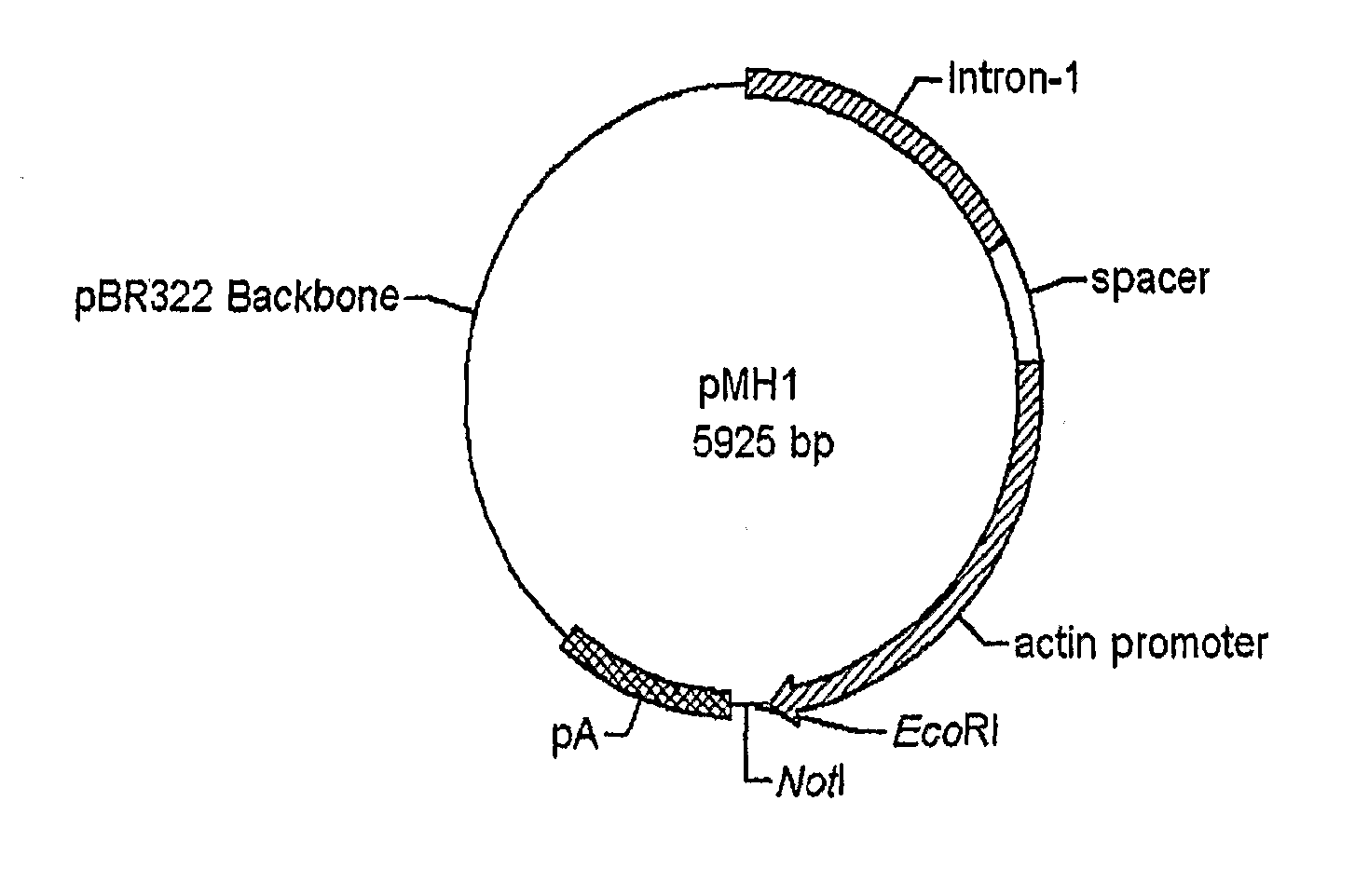
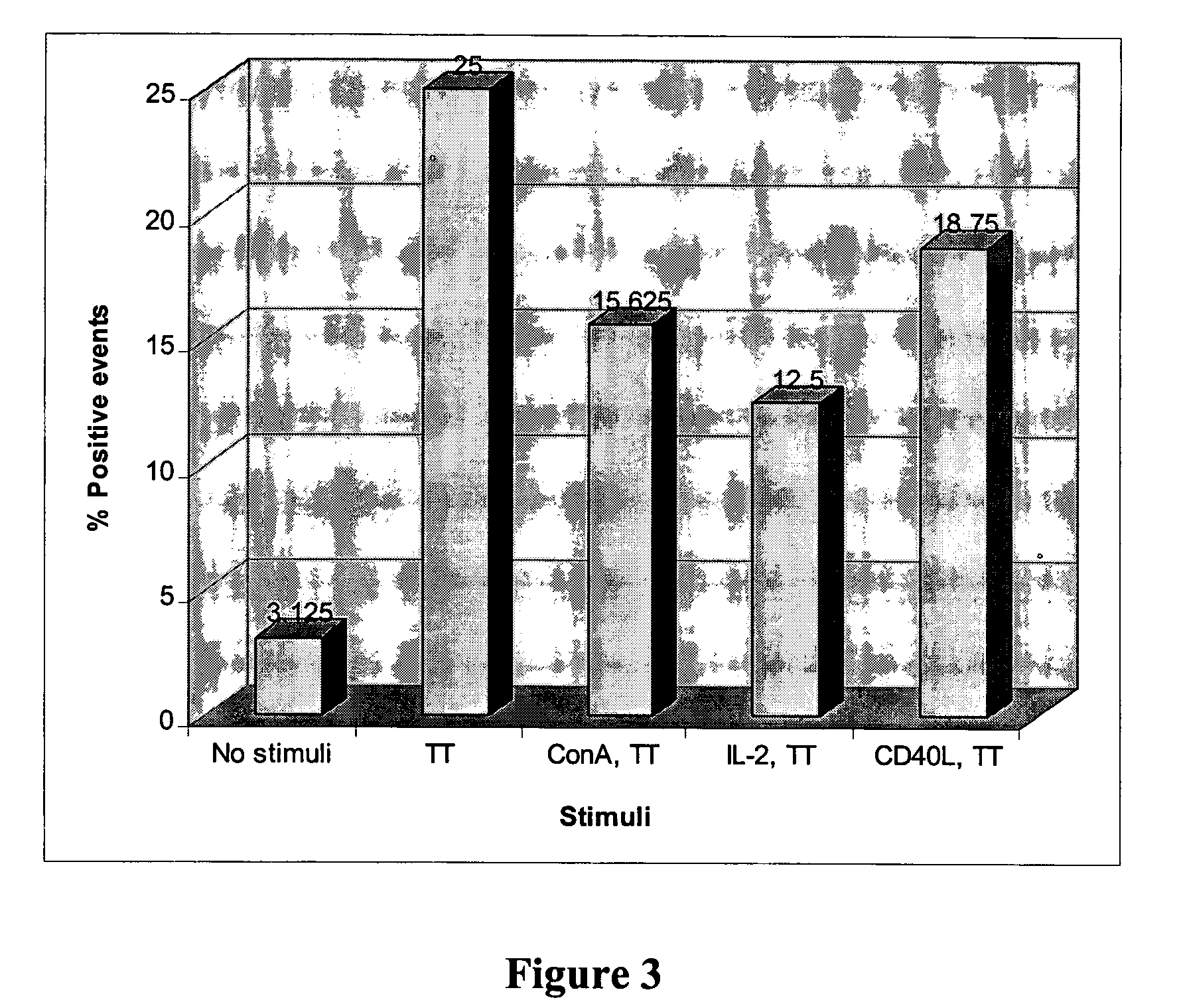
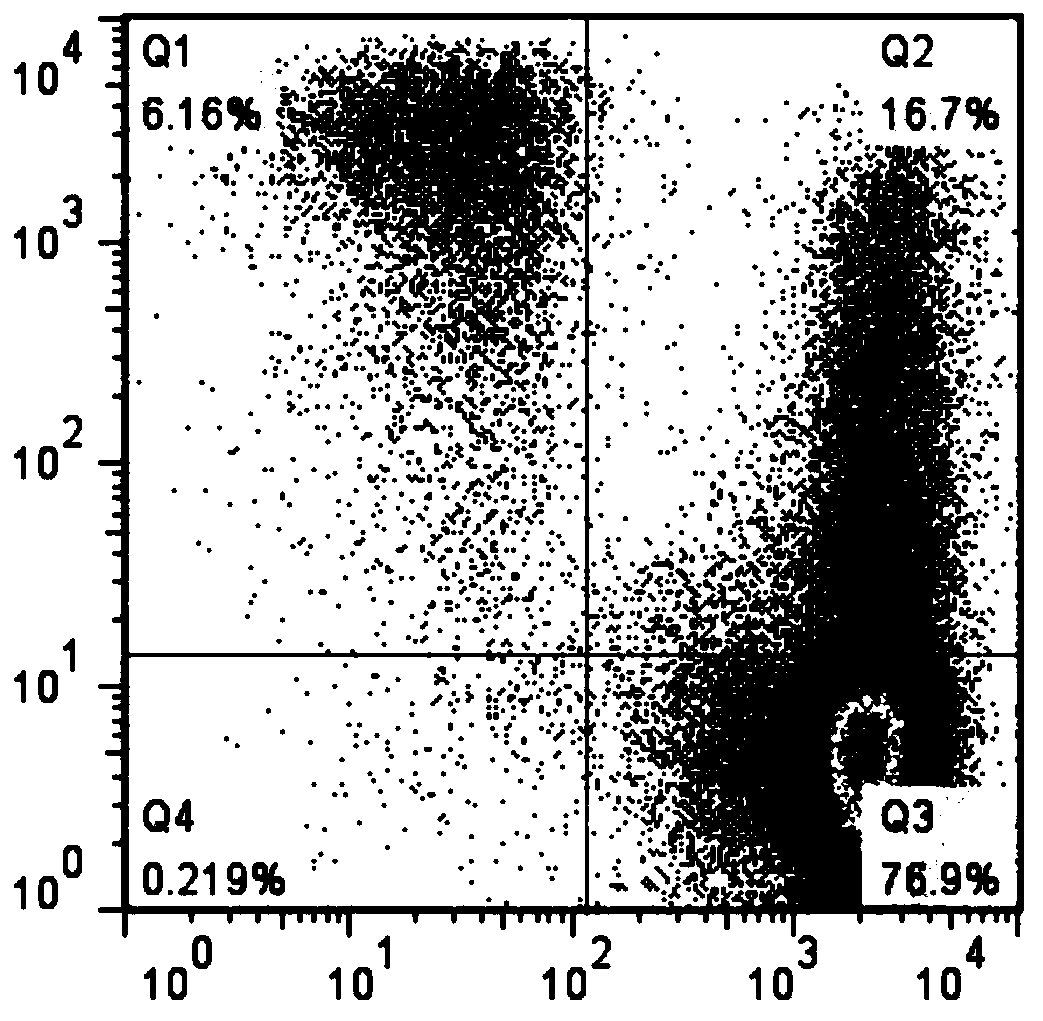
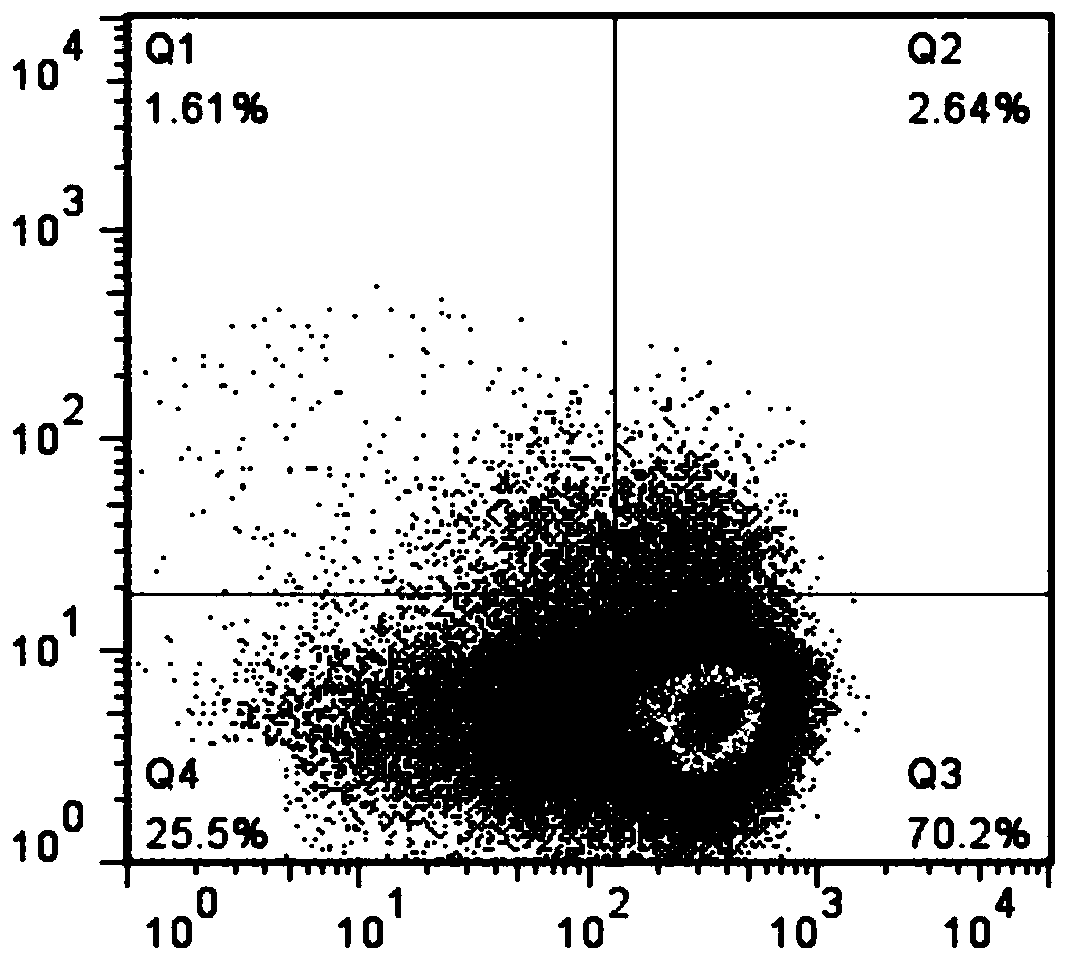
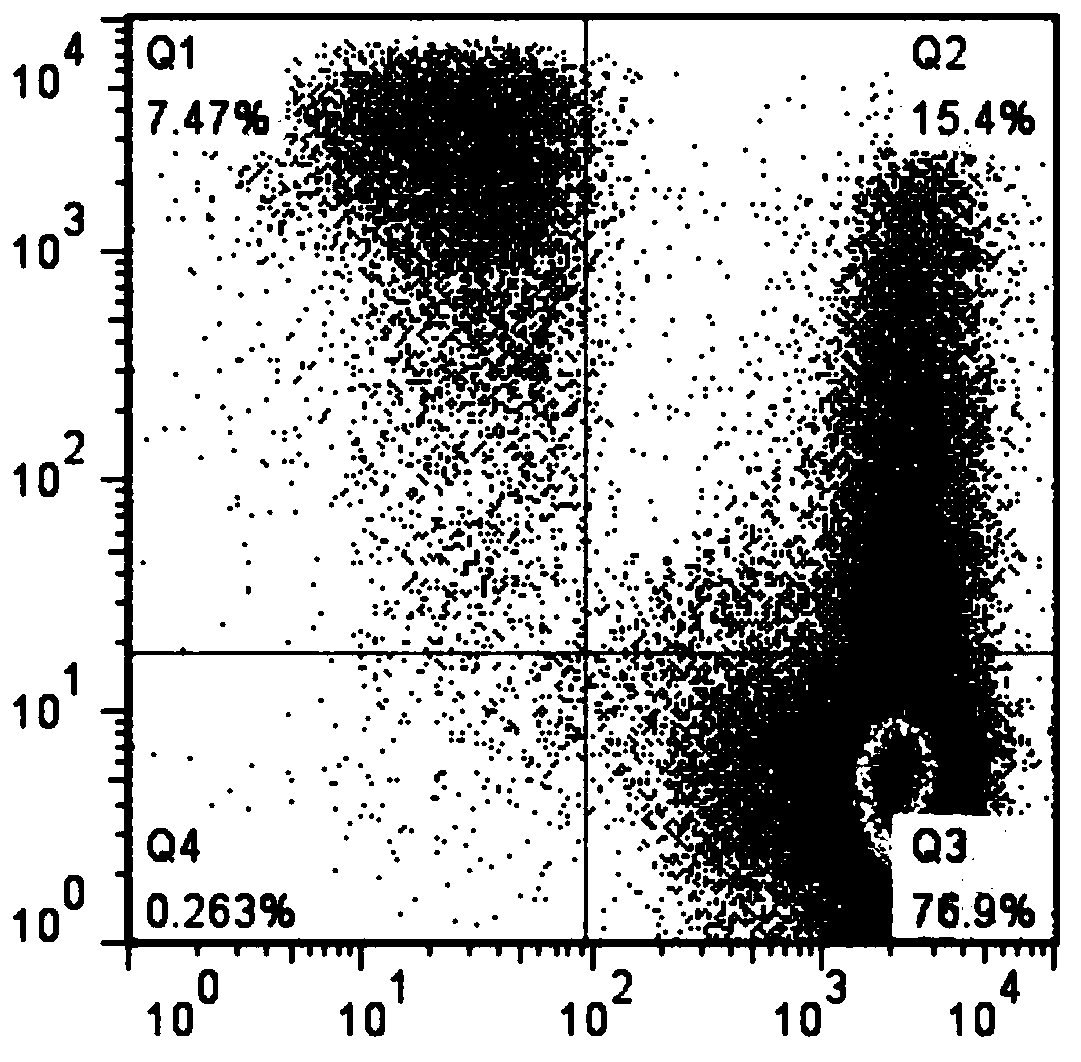
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
101 results about "Mammalian expression" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
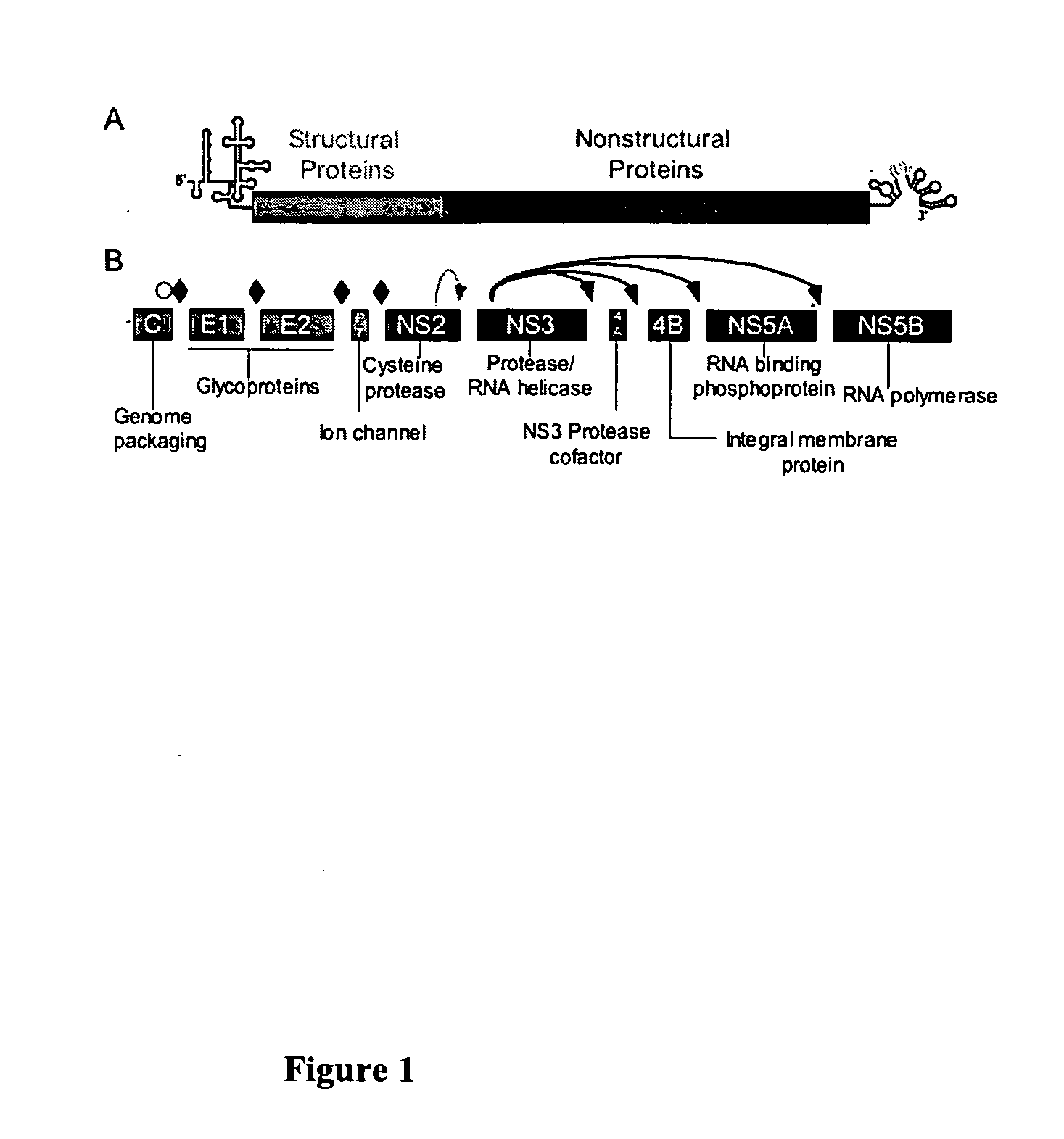
Mammalian Expression Systems. The main advantage of mammalian cell expression is that the mammalian cells can properly and efficiently recognize the signals for synthesis, processing and secretion of eukaryotic proteins. Therefore expression systems utilizing mammalian cells for recombinant protein manufacture are able to introduce proper protein...
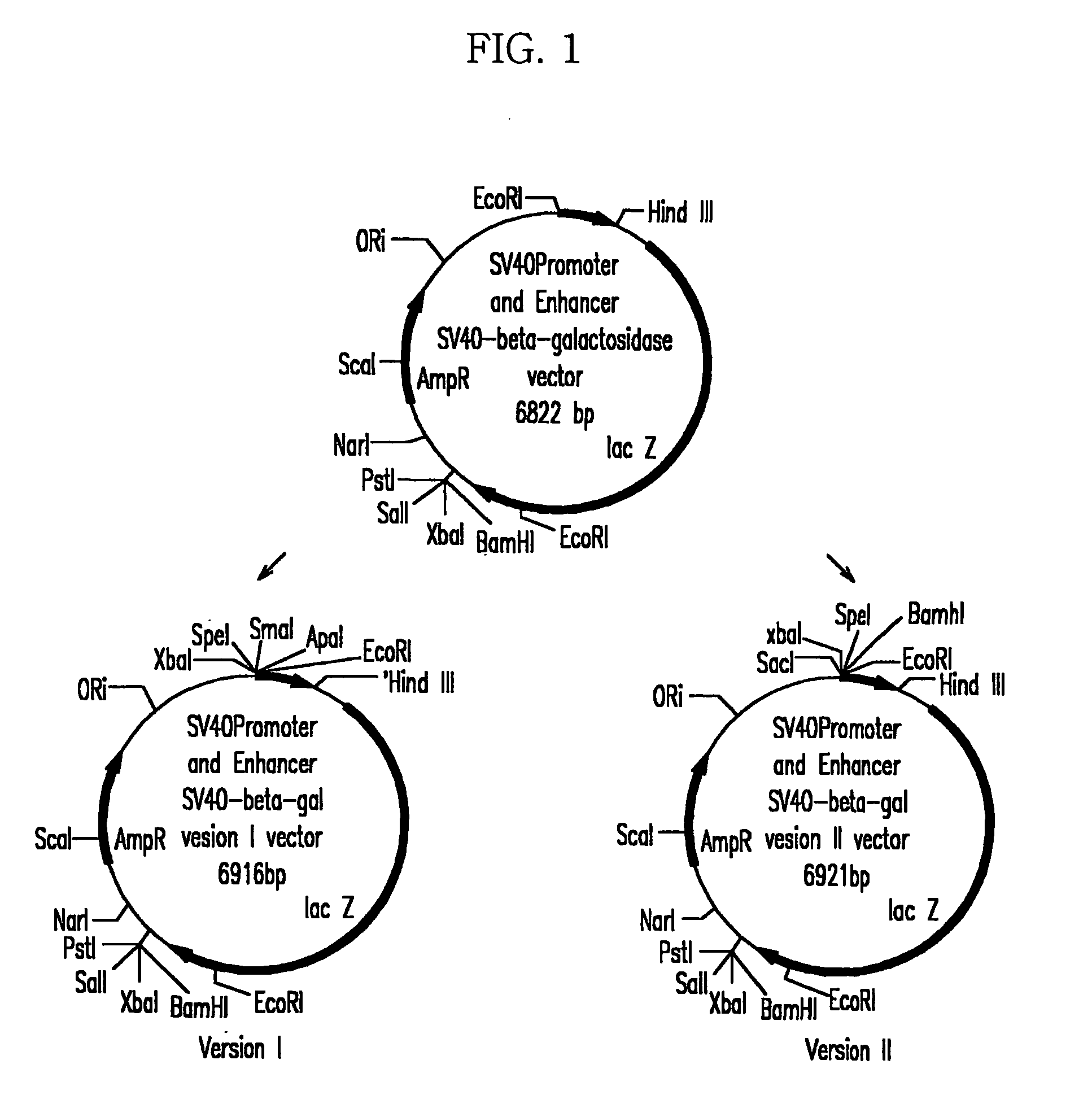
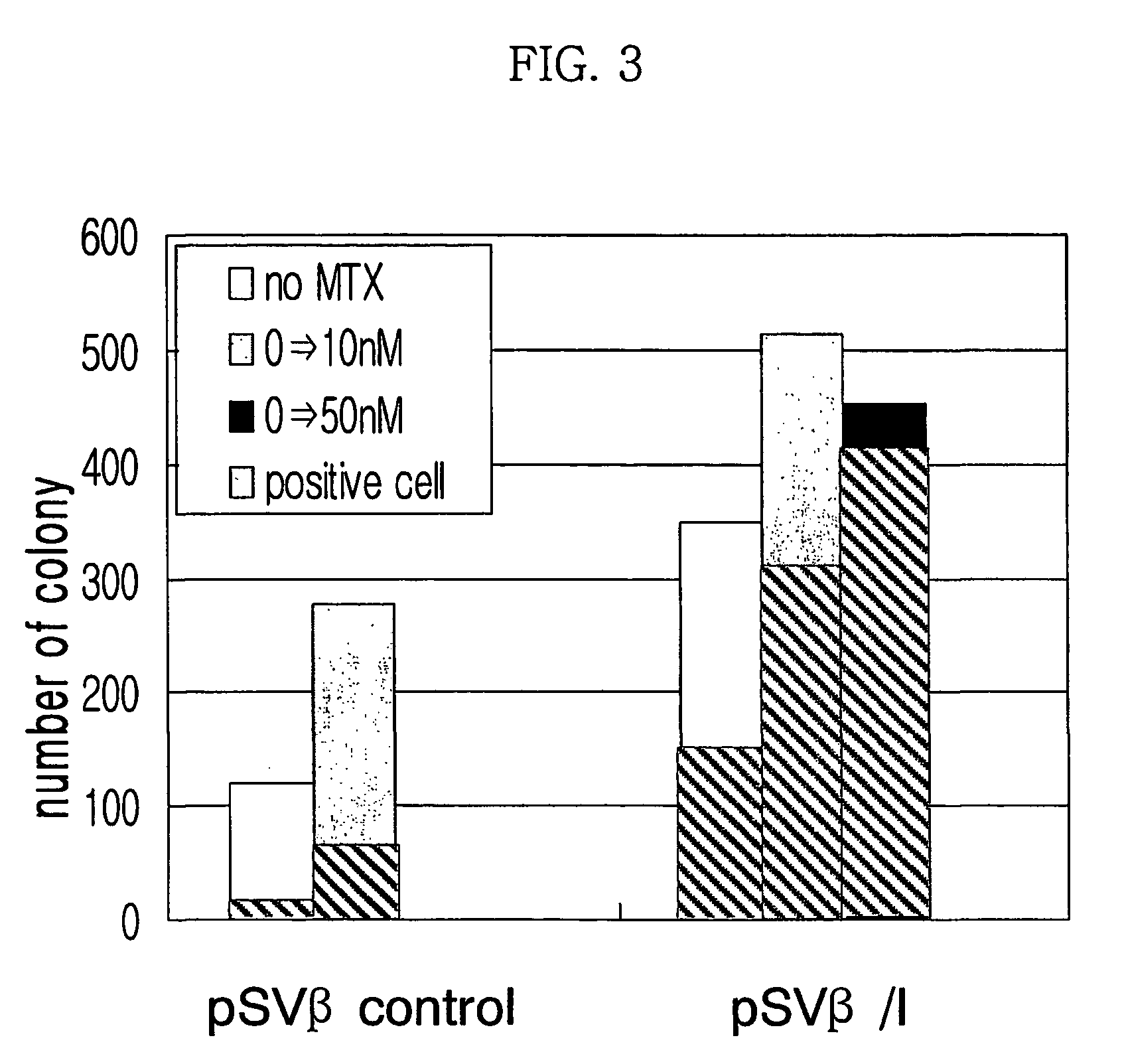
Expression vector for animal cell containing nuclear matrix attachment region of interferon beta
InactiveUS7259010B2Increased foreign protein expression efficiencyReduce the amount of solutionVectorsSugar derivativesMammalNuclear matrix
The present invention relates to mammalian expression vectors including nuclear matrix attachment region of human interferon β, and more particularly to pPGM-1, pPGM-2 and pPGM-3 including nuclear matrix attachment region of interferon β gene. Those expression vectors confer position independent expression of the introduced foreign gene, thus increasing the frequency of colonies which efficiently express the recombinant protein.
Owner:PANGEN BIOTECH
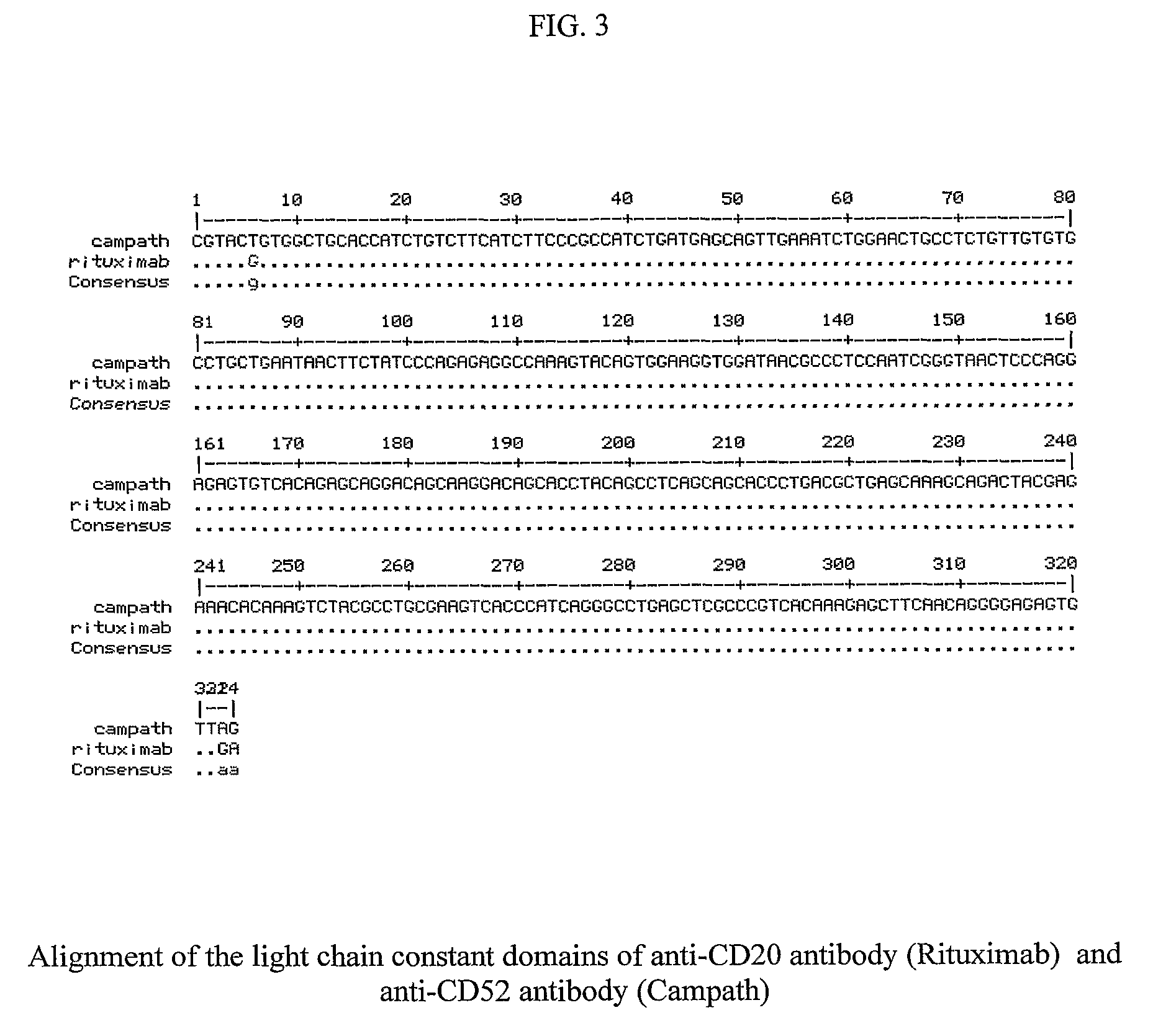
Method for the Production of a Monoclonal Antibody to CD20 for the Treatment of B-Cell Lymphoma
InactiveUS20090285795A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDNA constructMammalian expression
The present invention relates to the recombinant method used for the production of soluble form of an antibody that binds to CD20 for treatment of patients with relapsed or refractory, low-grade or follicular, CD20-positive, B-cell non-Hodgkin's lymphoma (NHL). The treatment will comprise the use of immunologically active anti-CD20 antibodies; or radiolabeled anti-CD20 antibodies and or cooperative strategies where both labeled and non-labeled antibodies will be used for treatment of NHL. The procedure describes the de novo synthesis of the nucleic acid sequence encoding anti-CD20, transformation of the constructed nucleic acid sequences into competent bacteria and the sub-cloning of the same into mammalian expression vectors for expression of the desired protein. DNA constructs comprising the control elements associated with the gene of interest has been disclosed. The nucleic acid sequence of interest has been codon optimized to permit expression in the suitable mammalian host cells.
Owner:AVESTHAGEN
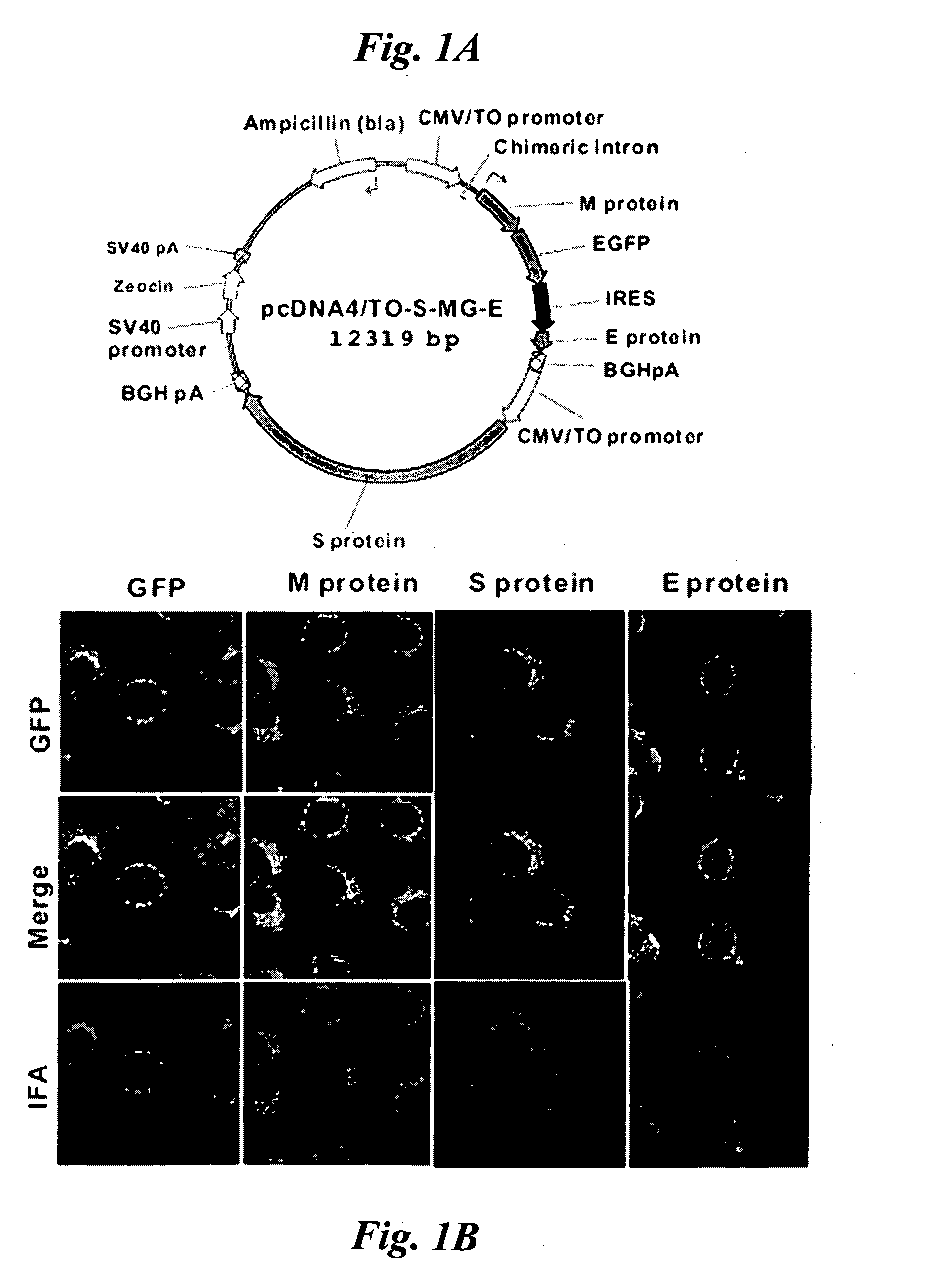
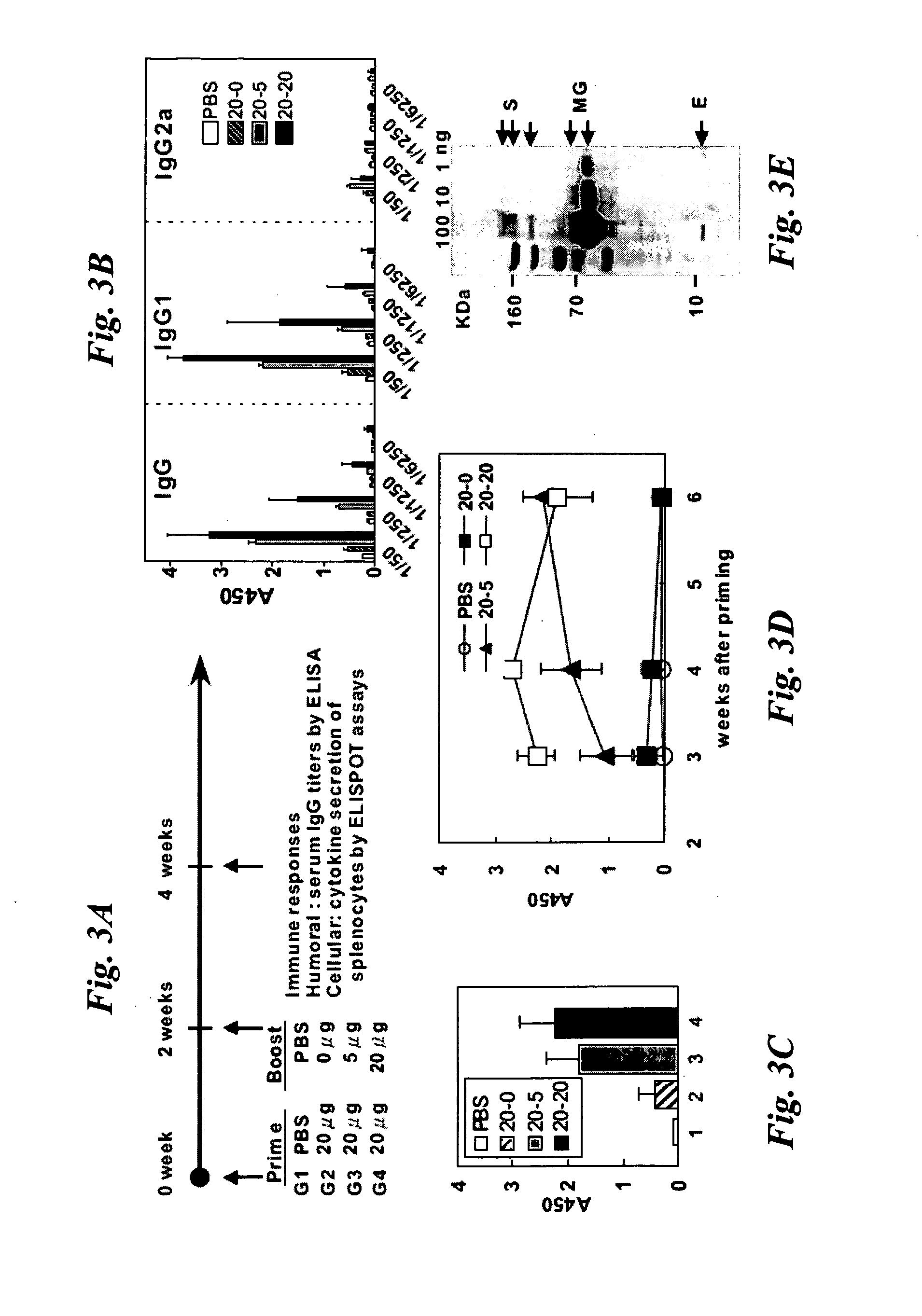
High-yield Transgenic Mammalian Expression System for Generating Virus-like Particles
ActiveUS20100166769A1Improving immunogenicityStimulate immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseMammalVirus-like particle
Virus-like particles (VLPs) of mammalian-hosted viruses, such as SARS-CoV and influenza viruses, have been recombinantly produced from Vero cells. The VLPs closely emulate the exterior of authentic virus particles and are highly immunogenic. They can elicit not only humoral but also cellular immune responses in a mammal. Compositions and methods related to the VLPs are also described.
Owner:ACAD SINIC
Cancer therapy
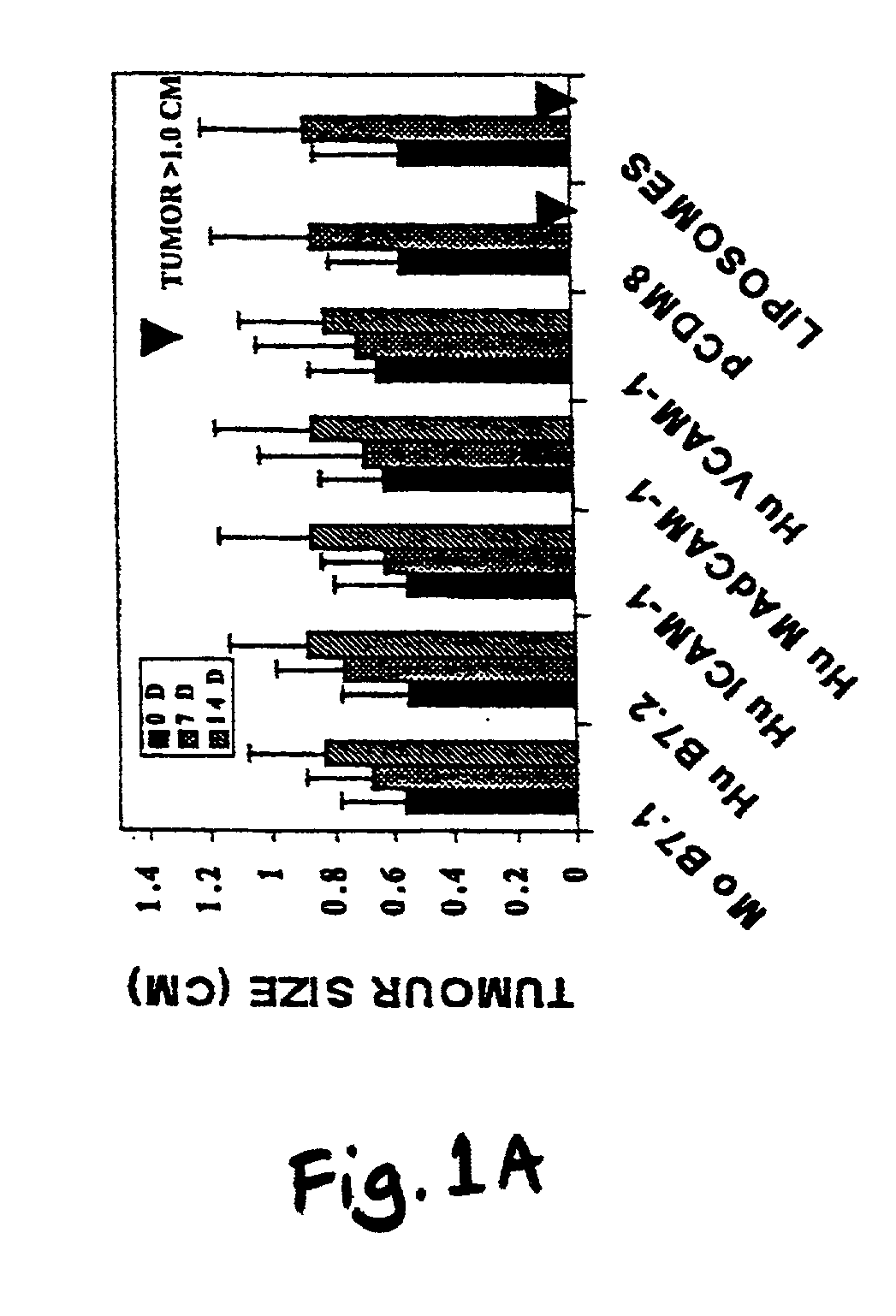
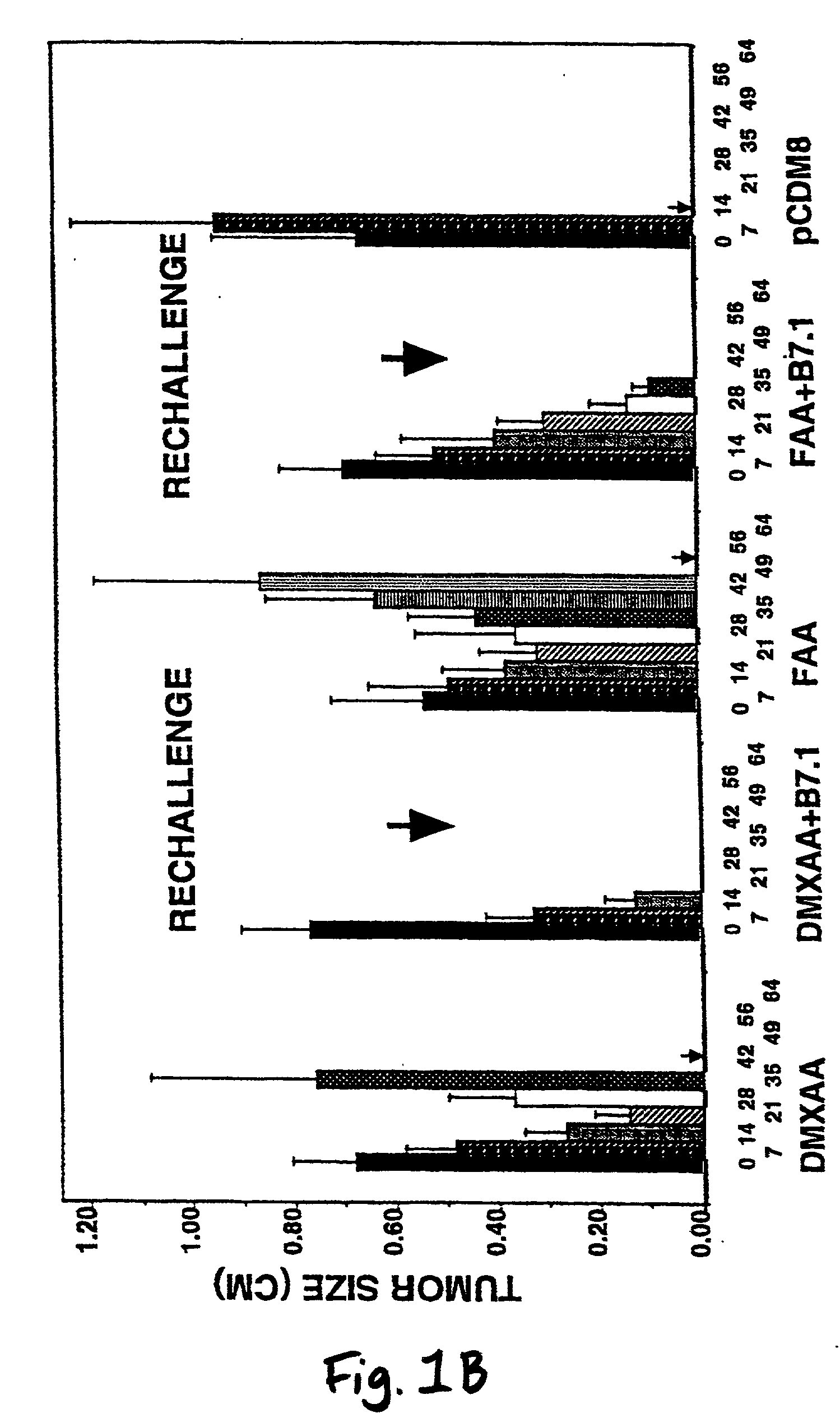
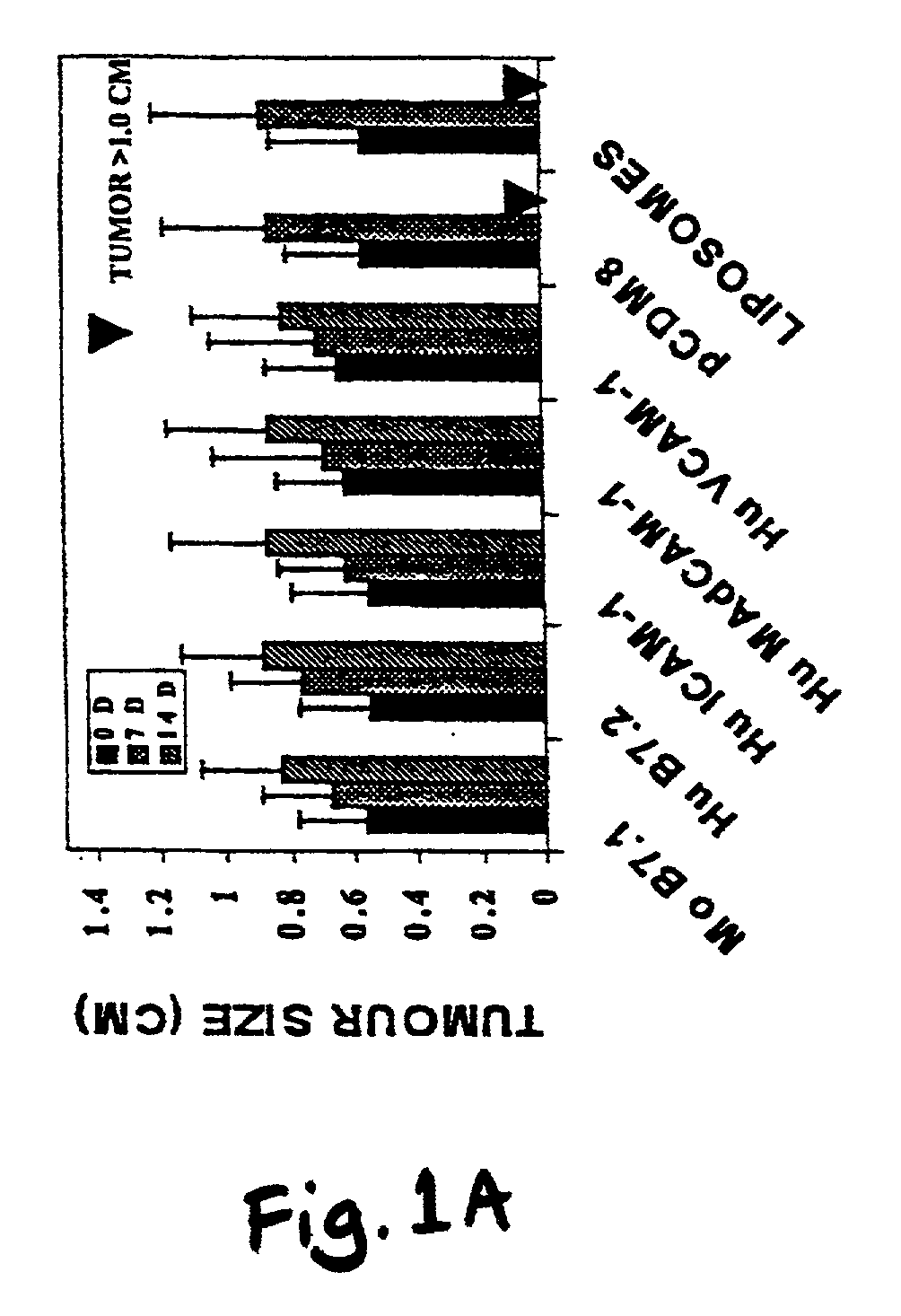
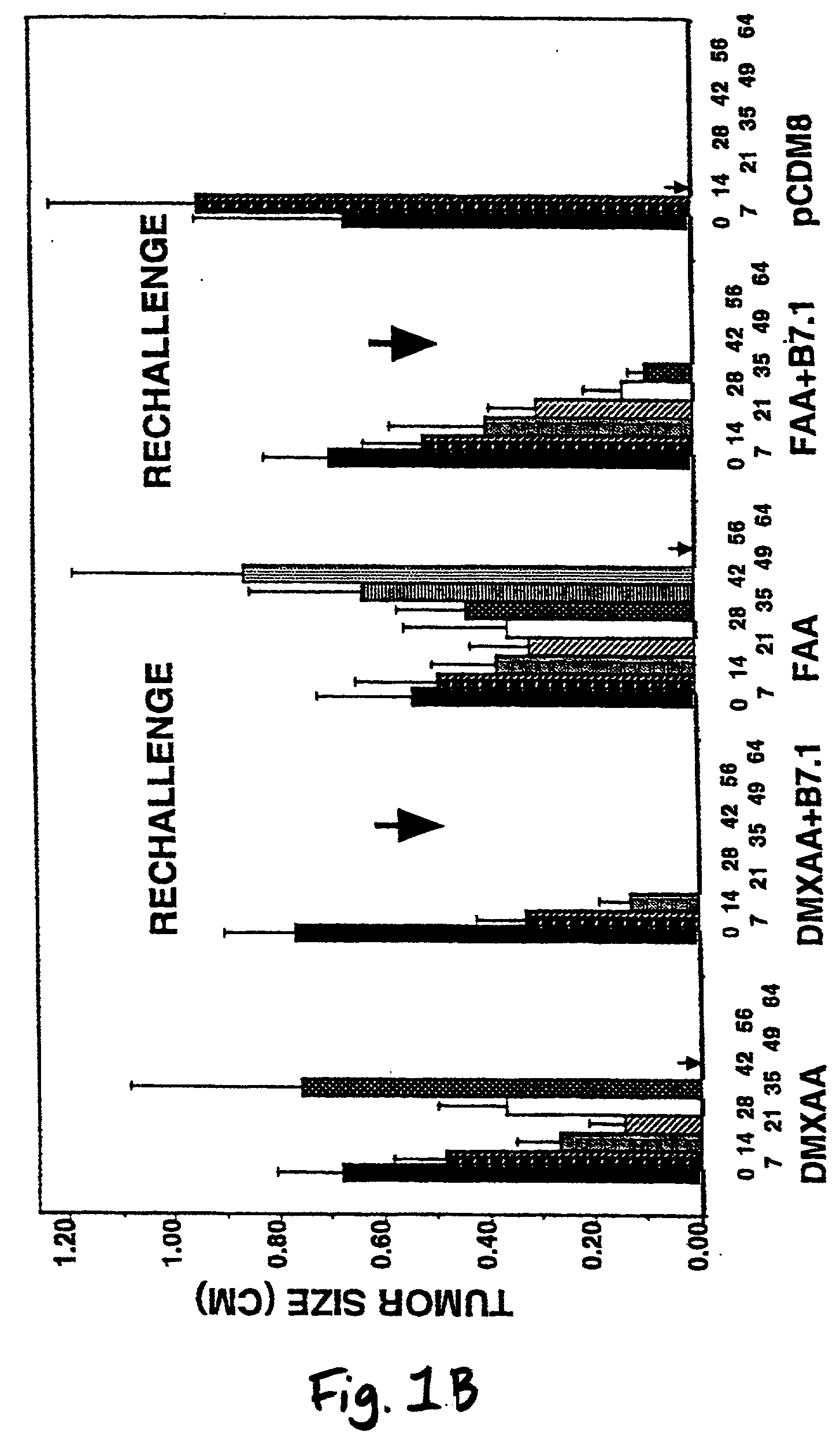
A method is provided for treating mammals, including humans, with advanced or large-tumour burdens. The method involves administering an immunotherapeautic agent in conjunction with a tumour growth restricting agent, in amounts effective to eradicate any advanced or large tumours present. In preferred embodiments, the immunotherapeautic agent comprises a T-cell co-stimulatory cell adhesion molecule (CAM) or a mammalian expression vector containing DNA which encodes a T-cell co-stimulatory CAM, such as B7.1, and the tumour growth restricting agent is flavone acetic acid, 5,6-dimenthyl-xanthenone-4-acetic acid, or an agent which disrupts the expression or activity of hypoxia-inducible factor-1 (HIF-1).
Owner:CANCER RES TECH LTD +1
DNA-based vaccine against the encephalitis alphaviruses
This invention relates to the development of a mammalian expression vector, under which expression of the structural genes of western equine encephalitis virus have been placed under the control of an eucaryotic promoter. When the recombinant vector is administered to mammalian cell culture or using a cell-free transcription / translation system, in vitro, authentic structural proteins of western equine encephalitis virus are produced as verified by reactivity with monoclonal antibodies developed to western equine encephalitis virus. When the recombinant DNA molecule is administered in vivo, a protective immune response is induced, thereby enhancing protection of the individual against subsequent infection by western equine encephalitis virus. In a similar manner, DNA vaccines to related alphaviruses (Venezuelan and eastern equine encephalitis viruses) could also be developed.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
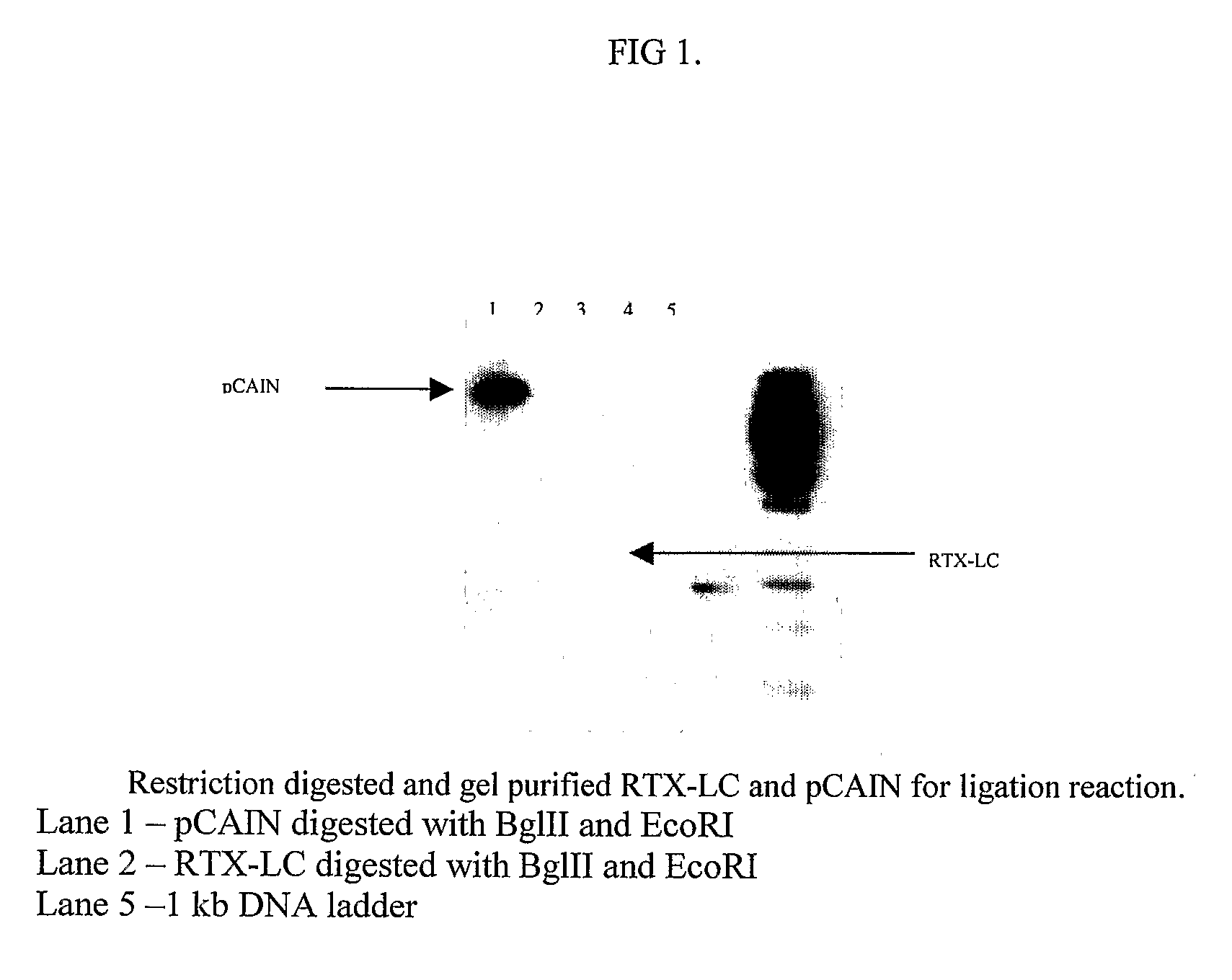
Process and methods for efficient manufacturing of highly pure asymmetric antibodies in mammalian cells
ActiveUS9499634B2Efficient preparationPromote formationAnimal cellsHybrid immunoglobulinsPurification methodsAsymmetric antibodies
The present invention provides a process and methods for producing asymmetric antibodies in a mammalian expression system. The asymmetric antibodies are transiently or stably expressed and in cells that stably express the asymmetric antibody, following a rapid 2-step process of stable pool to clone, a highly pure asymmetric antibody expressing clone can be identified at a success frequency that permits for screening of tens of clones rather than thousands. The asymmetric antibodies are produced at a high titre and with a high level of purity with no contaminating homodimer antibodies following protein A purification with a step yield of near 100%. Typical downstream purification processes employ standard hydrophobic interaction chromatography (HIC) and / or cation exchange (CEX) resins and the antibody is stable within a wide dynamic range of buffer pH (4-8) and within the requirements for manufacturing antibodies for pre-clinical and clinical applications.
Owner:ZYMEWORKS INC
Rapid immunoselection cloning method
InactiveUS7119183B2Large amount of proteinImprove efficiencyPeptide librariesPeptide/protein ingredientsAntigenCDNA library
A simple and highly efficient method for cloning cDNAs including CD27 (SEQ ID NO:28) from mammalian expression libraries based on transient expression in mammalian host cells has been discovered. Novel expression vectors allowing highly efficient construction of mammalian cDNA libraries are disclosed. The cloning method of the invention which has been used to clone genes for cell surface antigens of human lymphocytes, has general application in gene cloning. Cell surface antigens cloned according to the present invention have been purified, and the nucleotide and amino acid sequences determined. These antigens have diagnostic and therapeutic utility in immune-mediated infections in mammals, including humans.
Owner:THE GENERAL HOSPITAL CORP
Cancer therapy
A method is provided for treating mammals, including humans, with advanced or large-tumour burdens. The method involves administering an immunotherapeautic agent in conjunction with a tumour growth restricting agent, in amounts effective to eradicate any advanced or large tumours present. In preferred embodiments, the immunotherapeautic agent comprises a T-cell co-stimulatory cell adhesion molecule (CAM) or a mammalian expression vector containing DNA which encodes a T-cell co-stimulatory CAM, such as B7.1, and the tumour growth restricting agent is flavone acetic acid, 5,6-dimenthyl-xanthenone-4-acetic acid, or an agent which disrupts the expression or activity of hypoxia-inducible factor-1 (HIF-1).
Owner:CANCER RES TECH LTD +1
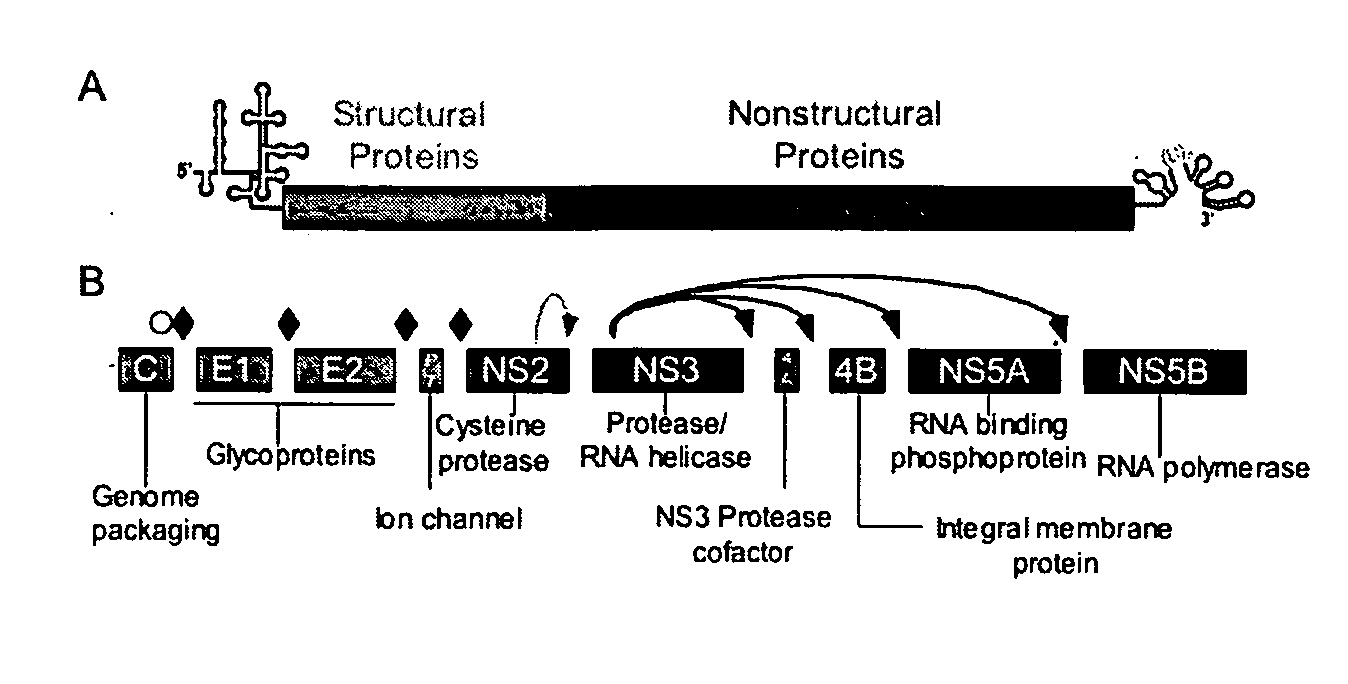
Rapid method to determine inhibitor sensitivity of NS3/4A protease sequences cloned from clinical samples
InactiveUS20090203008A1Microbiological testing/measurementEnzymesProteinase activityMammalian expression
A method for measuring HCV NS3 / 4A activity from a HCV NS3 / 4A sequence, comprising obtaining and cloning the sequence into a mammalian expression vector, transiently transfecting a mammalian cell with the vector, which includes a reporter construct encoding a HCV NS3 / 4A cleavage site fused to a detectable label, measuring signal production from the label resulting from cleavage at the cleavage site, and measuring effects on signal production by addition of a test compound.
Owner:MERCK SHARP & DOHME CORP
Novel DNA-based vaccine against the encephalitis alphaviruses
InactiveUS20050118251A1BiocideSsRNA viruses positive-senseCell freeEastern equine encephalitis virus
This invention relates to the development of a mammalian expression vector, under which expression of the structural genes of western equine encephalitis virus have been placed under the control of an eucaryotic promoter. When the recombinant vector is administered to mammalian cell culture or using a cell-free transcription / translation system, in vitro, authentic structural proteins of western equine encephalitis virus are produced as verified by reactivity with monoclonal antibodies developed to western equine encephalitis virus. When the recombinant DNA molecule is administered in vivo, a protective immune response is induced, thereby enhancing protection of the individual against subsequent infection by western equine encephalitis virus. In a similar manner, DNA vaccines to related alphaviruses (Venezuelan and eastern equine encephalitis viruses) could also be developed.
Owner:HER MAJESTY THE QUEEN AS REPRESENTED BY THE MINIST OF NAT DEFENCE OF HER MAJESTYS CANADIAN GOVERNMENT
Process and methods for efficient manufacturing of highly pure asymmetric antibodies in mammalian cells
ActiveUS20170158779A1Efficient preparationPromote formationHybrid immunoglobulinsFermentationAsymmetric antibodiesMammalian expression
The present invention provides a process and methods for producing asymmetric antibodies in a mammalian expression system. The asymmetric antibodies are transiently or stably expressed and in cells that stably express the asymmetric antibody, following a rapid 2-step process of stable pool to clone, a highly pure asymmetric antibody expressing clone can be identified at a success frequency that permits for screening of tens of clones rather than thousands. The asymmetric antibodies are produced at a high titre and with a high level of purity with no contaminating homodimer antibodies following protein A purification with a step yield of near 100%. Typical downstream purification processes employ standard hydrophobic interaction chromatography (HIC) and / or cation exchange (CEX) resins and the antibody is stable within a wide dynamic range of buffer pH (4-8) and within the requirements for manufacturing antibodies for pre-clinical and clinical applications.
Owner:ZYMEWORKS INC
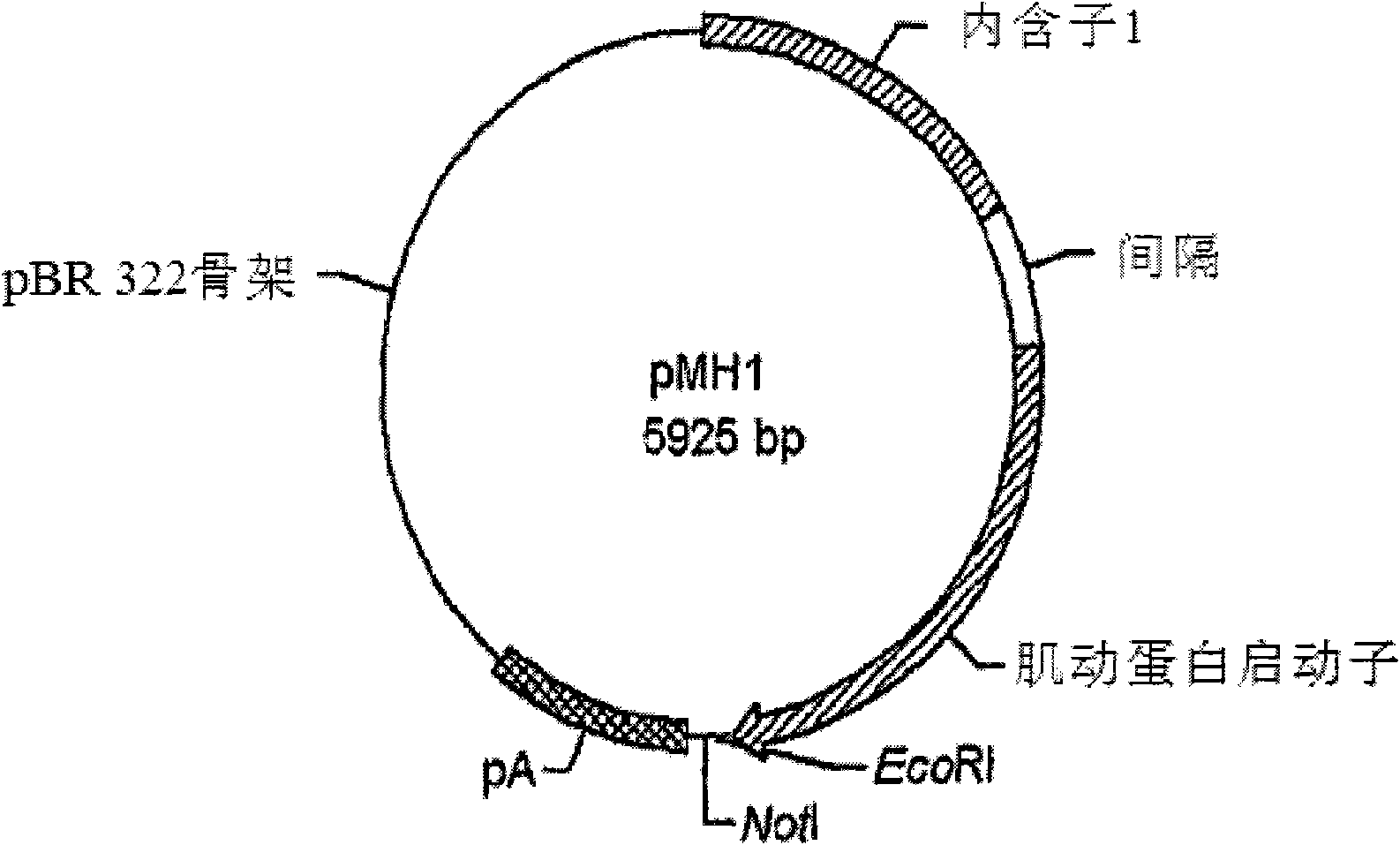
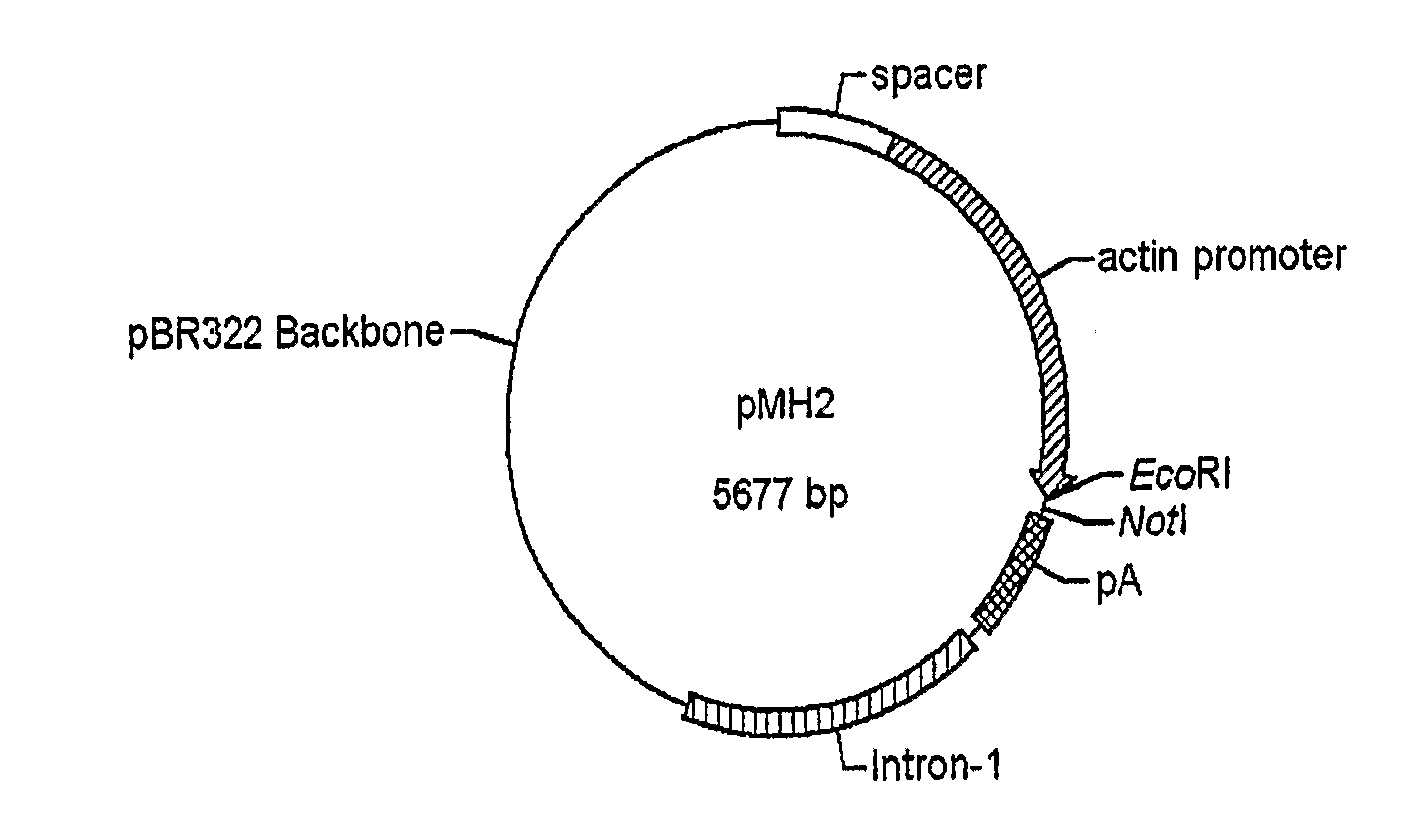
Use of chick beta actin gene intron-1
A method to use chick beta actin gene intron-1 or functional equivalent as a gene expression enhancer eiement or a gene expression 'hot spot' sequence for constructing or reconstructing a mammalian expression vector for extremely high expression of recombinant proteins is disclosed. Composition of a set of extremely strong gene expression vectors is also disclosed.
Owner:QINGDAO HUINUODE BIOLOGICAL TECH CO LTD
Hcv e2 construct compositions and methods
InactiveUS20110091495A1Strong immune responseStrong specificitySsRNA viruses positive-senseAntibody mimetics/scaffoldsFc domainThrombin activity
A construct comprising the ectodomain of the Hepatitis C Virus (HCV) E2 sequence and a mammalian expression system therefor is disclosed. The construct comprises a CMV promoter, prolactin signal sequence, the ectodomain of HCV E2 sequence truncated at aa 664, a thrombin cleavage site and a human Fc domain. The method also relates to an expression system for the construct, which is stably expressed in human embryonic kidney cells 293T. Continuous protein expression in a bioreactor allows for 4 mg of purified protein per liter of cell supernatant.
Owner:RUTGERS THE STATE UNIV +1
Efficient clone selection expression vector, and preparation method and use thereof
ActiveCN103834686AHigh transfection efficiencyImprove screening rateVector-based foreign material introductionMammalian expressionGenome
The invention discloses an efficient clone selection expression vector, which is obtained by an ITRs sequence of a piggyBac transposition system inserted into a mammal expression vector. The invention also discloses a preparation method and use thereof. The efficient clone selection expression vector is built by using the ITRs sequence of the piggyBac transposition system, and meanwhile, a transcriptase gene which is optimized by a codon and can be efficiently expressed in mice is cloned to another eukaryotic expression vector. A selection marker and a target gene are integrated into a genome of a host cell in common by coexpression of an expression vector containing the ITRs sequence and the vector containing transcriptase. Thus, the transfection efficiency and the positive clone screening rate are greatly improved.
Owner:SHANGHAI WUXI BIOLOGIC TECH CO LTD
Capture purification processes for proteins expressed in a non-mammalian system
Methods of purifying proteins expressed in non-mammalian expression systems in a non-native soluble form directly from cell lysate are disclosed. Methods of purifying proteins expressed in non-mammalian expression systems in a non-native limited solubility form directly from a refold solution are also disclosed. Resin regeneration methods are also provided.
Owner:AMGEN INC
Use of chick beta actin gene intron-1
A method to use chick beta actin gene intron-1 or functional equivalent as a gene expression enhancer element or a gene expression “hot spot” sequence for constructing or reconstructing a mammalian expression vector for extremely high expression of recombinant proteins is disclosed. Composition of a set of extremely strong gene expression vectors is also disclosed.
Owner:AMPROTEIN CORP
Mammalian cell expression vectors and utilization
An improved mammalian expression vector system which allows: (1) highly expressing exogenous proteins in host mammalian cells; (2) rapidly and efficiently screening recombinant stable cell lines expressing the gene of interest; (3) maintain sustainable expression and prevent gene silencing; and (4) effectively secreting proteins into media in some of cases. The entire expression vector system includes optimized promoters and core promoters, the use of special internal ribosome entry sites, integration of the bacterial backbone into the mammalian expression unit, multiple choices of selection markers, artificial matrix attachment region elements, effective secreting lead sequences and their 5′ and 3′ UTRs, and proper combinations of these expression elements.
Owner:ATGCELL
High-yield transgenic mammalian expression system for generating virus-like particles
InactiveUS20080063664A1Improving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsMammalVirus-like particle
The present invention provides a method utilizing mammalian expression system for generating virus-like particles (VLPs) of mammalian-hosted viruses, particularly SARS-CoV. The method of the present invention involves expression of viral structural proteins in Vero cells and thereby obtaining recombinant VLPs in the culture medium. SARS-VLPs generated by the method of the present invention are highly immunogenic and can elicit not only humoral but also cellular immune responses in a mammal.
Owner:ACAD SINIC
Mammalian expression vector pUHAB
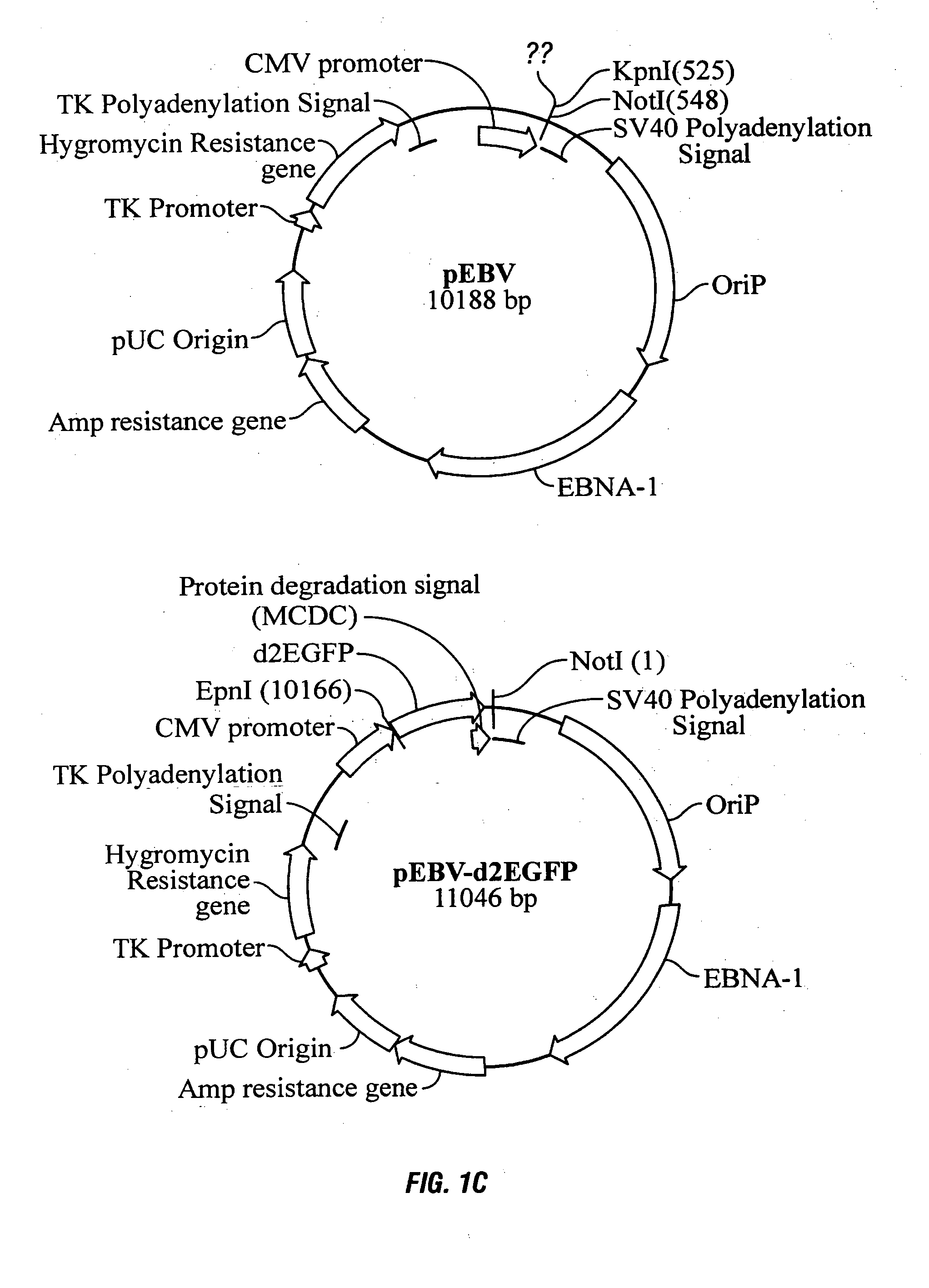
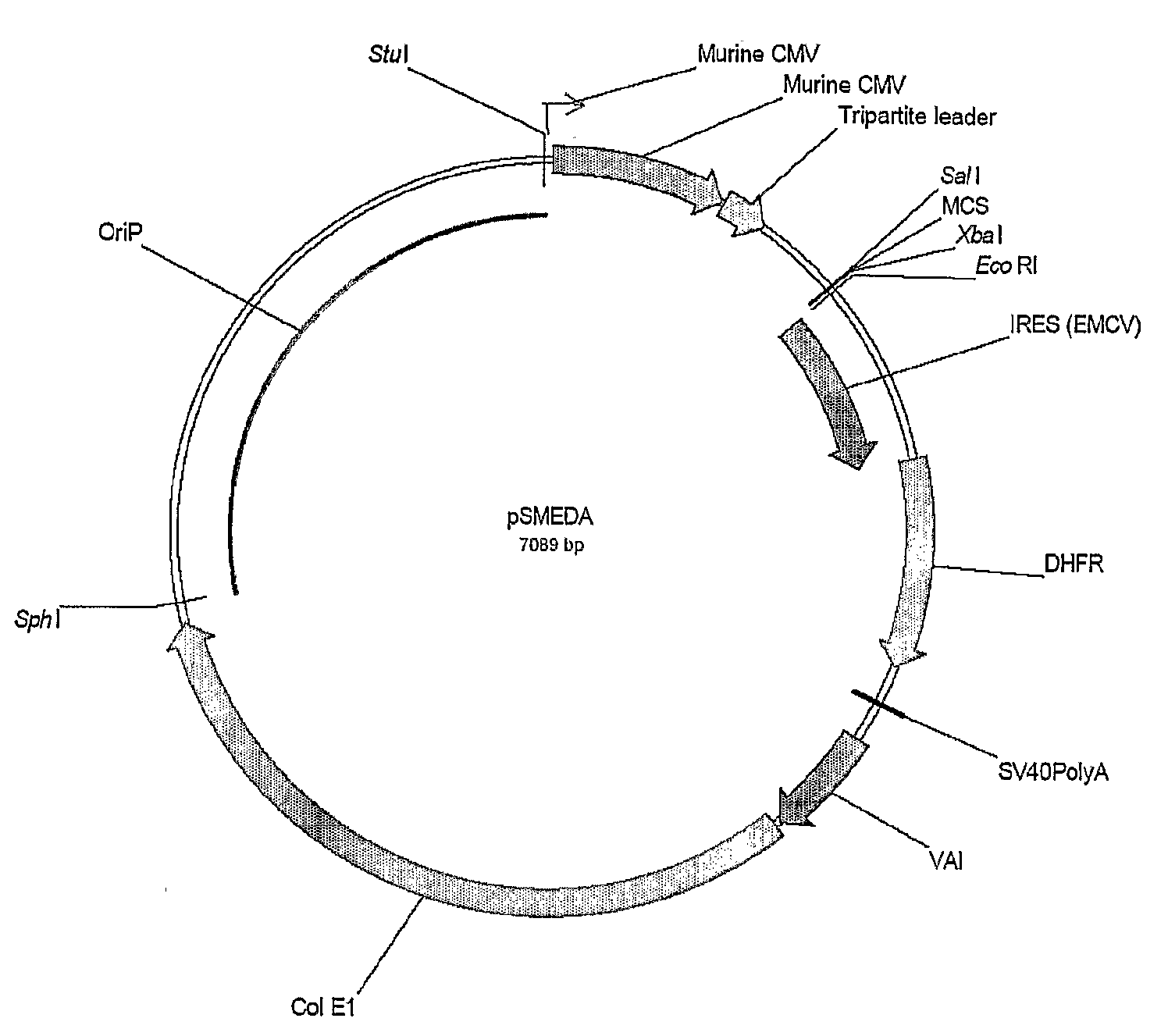
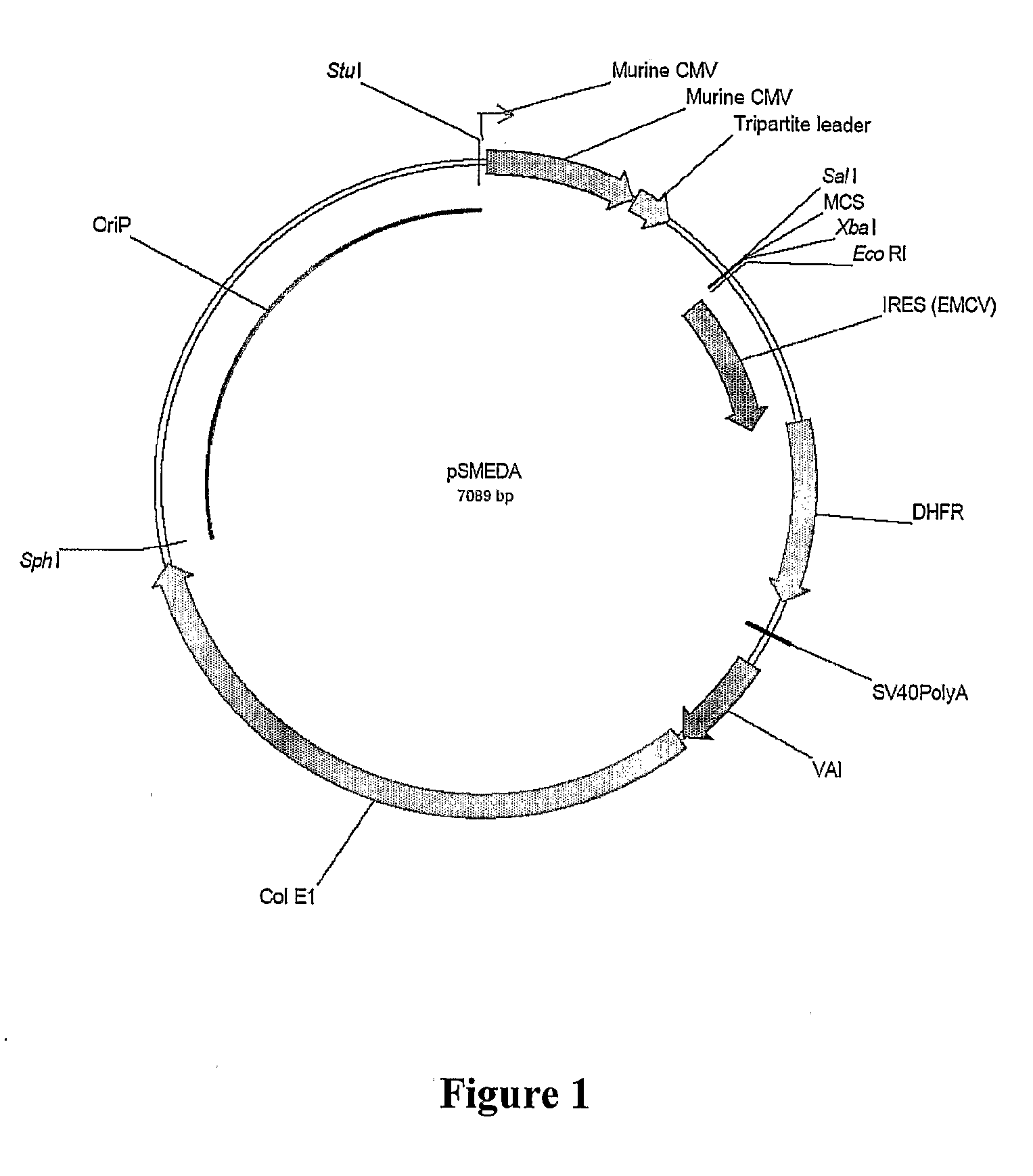
The present invention relates to the construction and utilization of a new mammalian expression vector that contains a unique multiple cloning site (MCS), designated pUHAB. The pUHAB vector comprises a high copy replication origin (ColE1), a drug resistance gene (TK-Hygromycin), and a human cytomegalovirus promoter operably associated with a unique intron (hCMV / intron). Further, pUHAB comprises a selectable marker conferring resistance to kanamycin in bacterial cells, and a phage f1(+) region. pUHAB can be used to transiently or stably express cloned genes when transfected into mammalian cells. The invention also encompasses kits and host cells and cell lines comprising pUHAB, and methods of producing a recombinant protein using pUHAB.
Owner:MERCK SHARP & DOHME CORP
OB polypeptides, modified forms and derivatives
The present invention relates generally to the control of body weight of animals including mammals and humans, and more particularly to materials identified herein as modulators of weight, and to the diagnostic and therapeutic uses to which such modulators may be put. In its broadest aspect, the present invention relates to the elucidation and discovery of nucleotide sequences, and proteins putatively expressed by such nucleotides or degenerate variations thereof, that demonstrate the ability to participate in the control of mammalian body weight. The nucleotide sequences in object represent the genes corresponding to the murine and human ob gene, that have been postulated to play a critical role in the regulation of body weight and adiposity. Preliminary data, presented herein, suggests that the polypeptide product of the gene in question functions as a hormone. The present invention further provides nucleic acid molecules for use as molecular probes, or as primers for polymerase chain reaction (PCR) amplification, i.e., synthetic or natural oligonucleotides. In further aspects, the present invention provides a cloning vector, which comprises the nucleic acids of the invention; and a bacterial, insect, or a mammalian expression vector, which comprises the nucleic acid molecules of the invention, operatively associated with an expression control sequence. Accordingly, the invention further relates to a bacterial or a mammalian cell transfected or transformed with an appropriate expression vector, and correspondingly, to the use of the above mentioned constructs in the preparation of the modulators of the invention. Also provided are antibodies to the ob polypeptide. Moreover, a method for modulating body weight of a mammal is provided. In specific examples, genes encoding two isoforms of both the murine and human ob polypeptides are provided.
Owner:THE ROCKEFELLER UNIV
Expression Vectors with Improved Safety
ActiveUS20080153770A1High level of transcriptionBiocideGenetic material ingredientsPlasmid VectorTiter
The present invention relates to the use of internal promoters in mammalian expression vectors including plasmid vectors and enhancer-deleted retroviral vectors. The retroviral vectors have improved safety and optimal levels of transgene expression and vector titers.
Owner:VIROMED CO LTD
Mammalian expression system
InactiveUS20050227317A1High transfection efficiencyHigh expressionAnimal cellsSugar derivativesHigh level expressionMammal
The present invention relates to protein expression systems and in particular to a mammalian protein expression system. Specifically, the present invention provides a rodent cell line with enhanced protein production capabilities. The invention also relates to eukaryotic cloning and expression vectors and related methods, and in particular to DNA vectors capable of high level expression of a protein of interest. The invention allows for long-term episomal maintenance of expression vectors in mammalian cells.
Owner:ACYTE BIOTEC
Recombinant method for the production of a monoclonal antibody to CD52 for the treatment of chronic lymphocytic leukemia
InactiveUS20090220520A1Stable expressionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDNA constructChronic lymphocytic leukemia
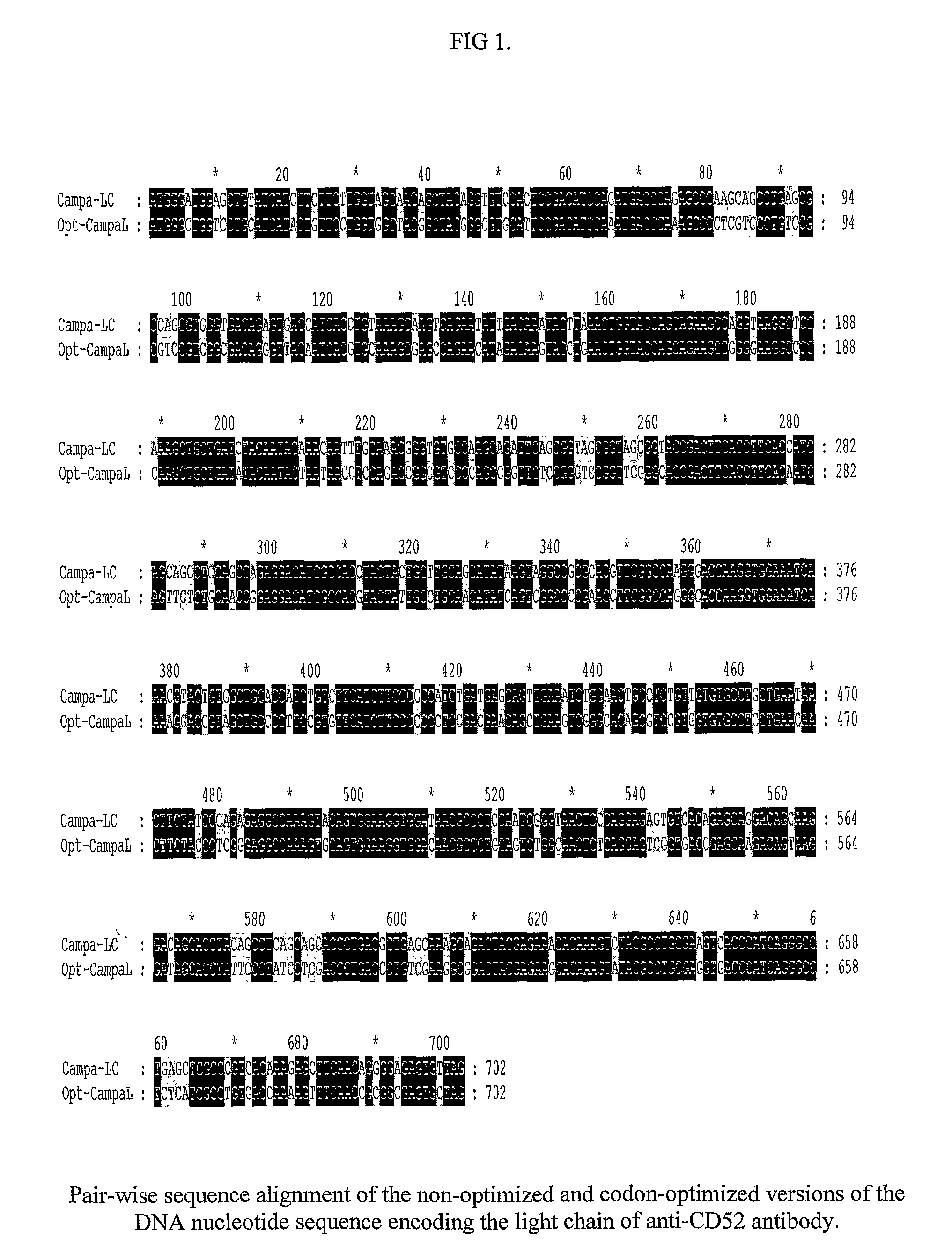
The present invention relates to the recombinant method used for the production of soluble form monoclonal antibody that binds to CD52. The procedure describes the de novo synthesis of the nucleic acid sequence encoding anti-CD 52, transformation of the constructed nucleic acid sequences into competent bacteria and the sub-cloning of the same into mammalian expression vectors for expression of the desired protein. DNA constructs comprising the control elements associated with the gene of interest has been disclosed. The nucleic acid sequence of interest has been codon optimized to permit expression in the suitable mammalian host cells.
Owner:AVESTHAGEN
High-yield transgenic mammalian expression system for generating virus-like particles
ActiveUS8980281B2Improving immunogenicityStimulate immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseMammalVirus-like particle
Owner:ACAD SINIC
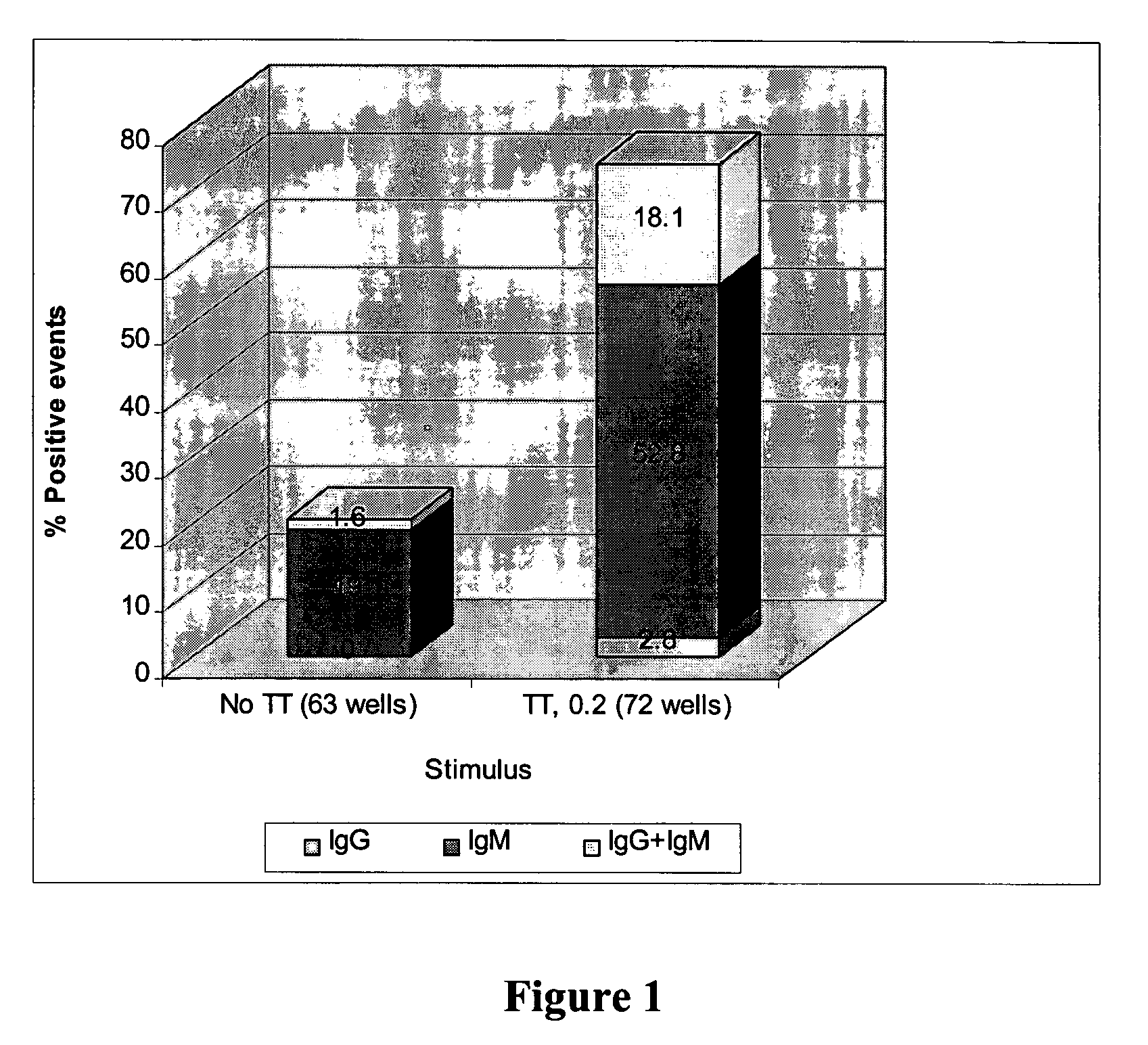
Methods of generating high-production of antibodies from hybridomas created by in vitro immunization
The invention provides methods for generating high titers of high-affinity antibodies from hybridoma cells produced by fusing myeloma cells with in vitro immunized donor cells. The hybridoma cells or mammalian expression cells with cloned antibody genes from the hybridomas producing the high-affinity antibodies may be mismatch repair defective due to defects of endogenous mismatch repair subunits of through expression of a dominant negative allele of a mismatch repair gene which allows the hybridoma cell to be hypermutable, may be rendered hypermutable by chemical means, or may be naturally mismatch repair deficient. High-affinity antibodies and high titer producer cells producing antibodies may be prepared by the methods of the invention.
Owner:EISAI INC
Mammalian expression systems
InactiveUS20090214513A1High protein yieldIncrease productionPeptide/protein ingredientsSkeletal disorderBiotechnologyADAMTS Proteins
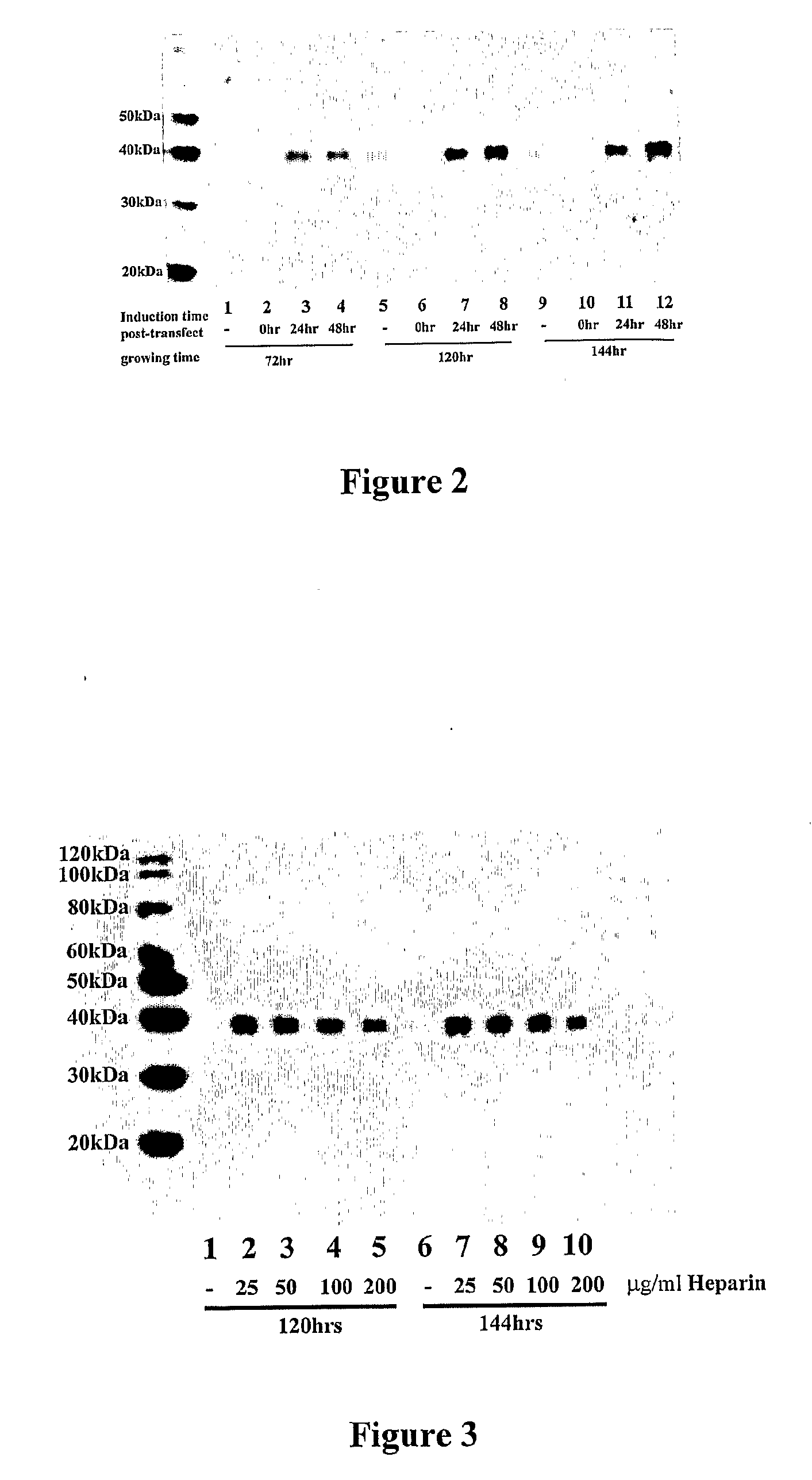
The present invention features mammalian expression systems with improved production yields, and method of using these systems to produce desired proteins. In one embodiment, the expression systems of the present invention comprise genetically-engineered mammalian host cells cultured in a medium that contains an effective amount of heparin or heparin-like molecules. The presence of heparin or heparin-like molecules significantly increases protein production by the cultured cells. The present invention also features the use of constitutively-active components of FGFR-I-mediated signal transduction pathways to improve protein production by cultured mammalian cells. Co-expression of such a component with a protein of interest markedly increases the production yield of the protein of interest.
Owner:WYETH LLC
Gene expression regulation and control method and system based on Type I-F CRISPR/ Cas
ActiveCN111979240AHave binding activityHigh expressionHydrolasesStable introduction of DNARegulation of gene expressionTranscription Repressor
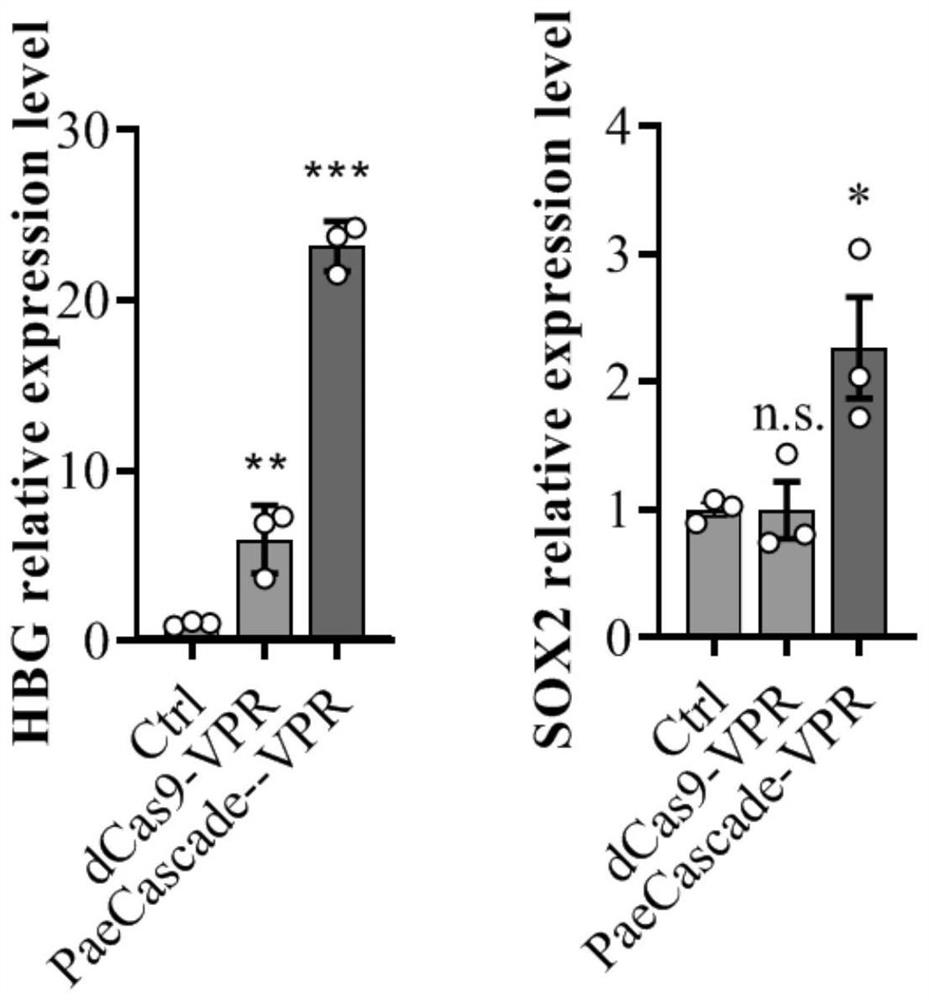
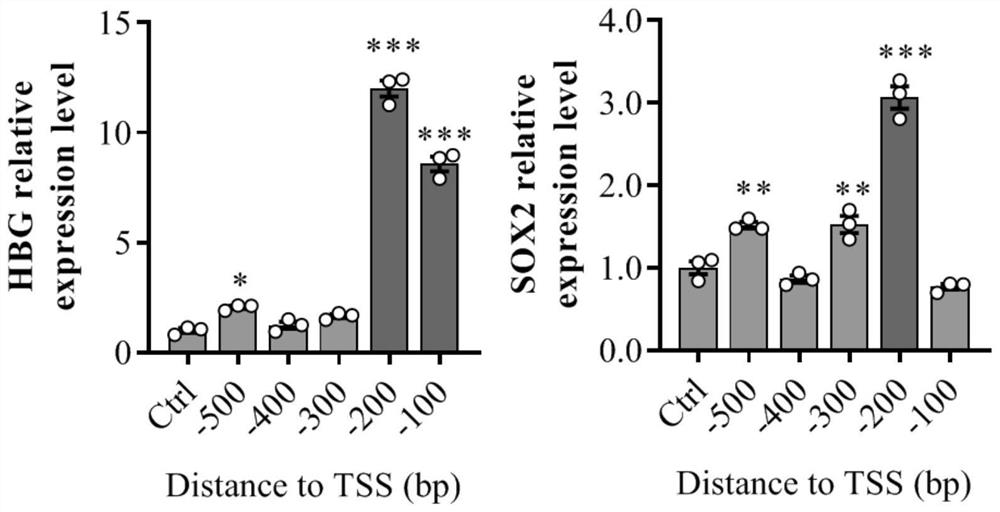
The invention discloses a gene expression regulation and control method and system based on Type I-F CRISPR / Cas. The first study shows that a Cascade compound, such as a PaeCascade compound, belonging to a Type I-F CRISPR / Cas system can be efficiently combined with a target site in a mammalian cell; and meanwhile, the Type I-F CRISPR / Cas system is subjected to mammalian expression system optimization, so that a transcription activation factor or a transcription inhibition factor can be efficiently recruited to the target site in the mammalian cell, and a transcription activation system anda transcription inhibition system are successfully constructed. The application of the Type I-F CRISPR / Cas system in the aspect of gene regulation and control of the mammalian cell becomes possible,and a necessary tool is provided for gene transcription regulation and control based on the Type I-F CRISPR / Cas system.
Owner:SUN YAT SEN UNIV
Methods of generating high-production of antibodies from hybridomas created by in vitro immunization
InactiveUS7754450B2Stabilizing genomeHigh mutation rateImmunoglobulins against bacteriaMutant preparationAlleleMammalian expression
The invention provides methods for generating high titers of high-affinity antibodies from hybridoma cells produced by fusing myeloma cells with in vitro immunized donor cells. The hybridoma cells or mammalian expression cells with cloned antibody genes from the hybridomas producing the high-affinity antibodies may be mismatch repair defective due to defects of endogenous mismatch repair subunits of through expression of a dominant negative allele of a mismatch repair gene which allows the hybridoma cell to be hypermutable, may be rendered hypermutable by chemical means, or may be naturally mismatch repair deficient. High-affinity antibodies and high titer producer cells producing antibodies may be prepared by the methods of the invention.
Owner:EISAI INC
Preparation method of dendritic cells and application thereof
ActiveCN110195042ASpecific killing effect is obviousIncrease binding rateGenetically modified cellsOncogene translation productsDendritic cellKras mutation
The invention provides a preparation method of dendritic cells, which belongs to the technical field of tumor treatment. The preparation method comprises the steps of: 1) ligating a fusion gene of human leukocyte antigen (HLA) and KRAS mutation to pCDH or a mammalian expression vector to obtain a recombinant plasmid; 2) transforming the dendritic cells (DC) derived from peripheral blood by electroporation with the recombinant plasmid to obtain the recombinant cells (i-DC); and 3) inducing maturation of the recombinant cells to obtain the mature dendritic cells. In the prior art, antigen-presenting cells are generally loaded by mutating polypeptides, or mutant antigens are transferred into antigen-presenting cells, but the efficiency of both is low, The method converts the fusion gene of KRAS antigen and HLA into the antigen-presenting cells and overexpresses two genes, increases the binding rate of HLA and KRAS mutant antigen, improves the efficiency of presentation, and more easily obtains CTL with specific killing effect.
Owner:焦顺昌 +1
Batroxobin and its preparing process and specific coding gene
The invention discloses batroxobin, its preparation method and special coding gene. The batroxobin coding gene is one of the following nucleotide sequences: 1) sequence 2 in the sequence listing; 2) sequence 4 in the sequence listing; 3) sequence 6 in the sequence listing; 4) sequence 8 in the sequence listing . The method for preparing batroxobin comprises introducing the coding gene described in any one of claims 1-3 into yeast or mammalian cell line for expression through a eukaryotic expression vector to obtain batroxobin protein. The method of the present invention has successfully expressed the batroxobin protein with high biological activity, and the yeast yield after purification reaches 10 μg / ml or 14 batroxobin units (14BU / ml) per milliliter of fermentation broth, and the specific activity is 1400BU / mg . This yield exceeds other published and reported expression levels and is suitable for large-scale production.
Owner:JOINN LAB (SUZHOU) INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com