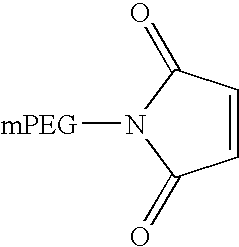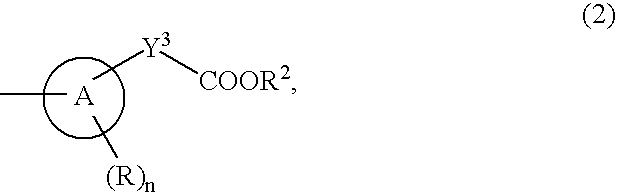Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
483 results about "Interferon alfa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
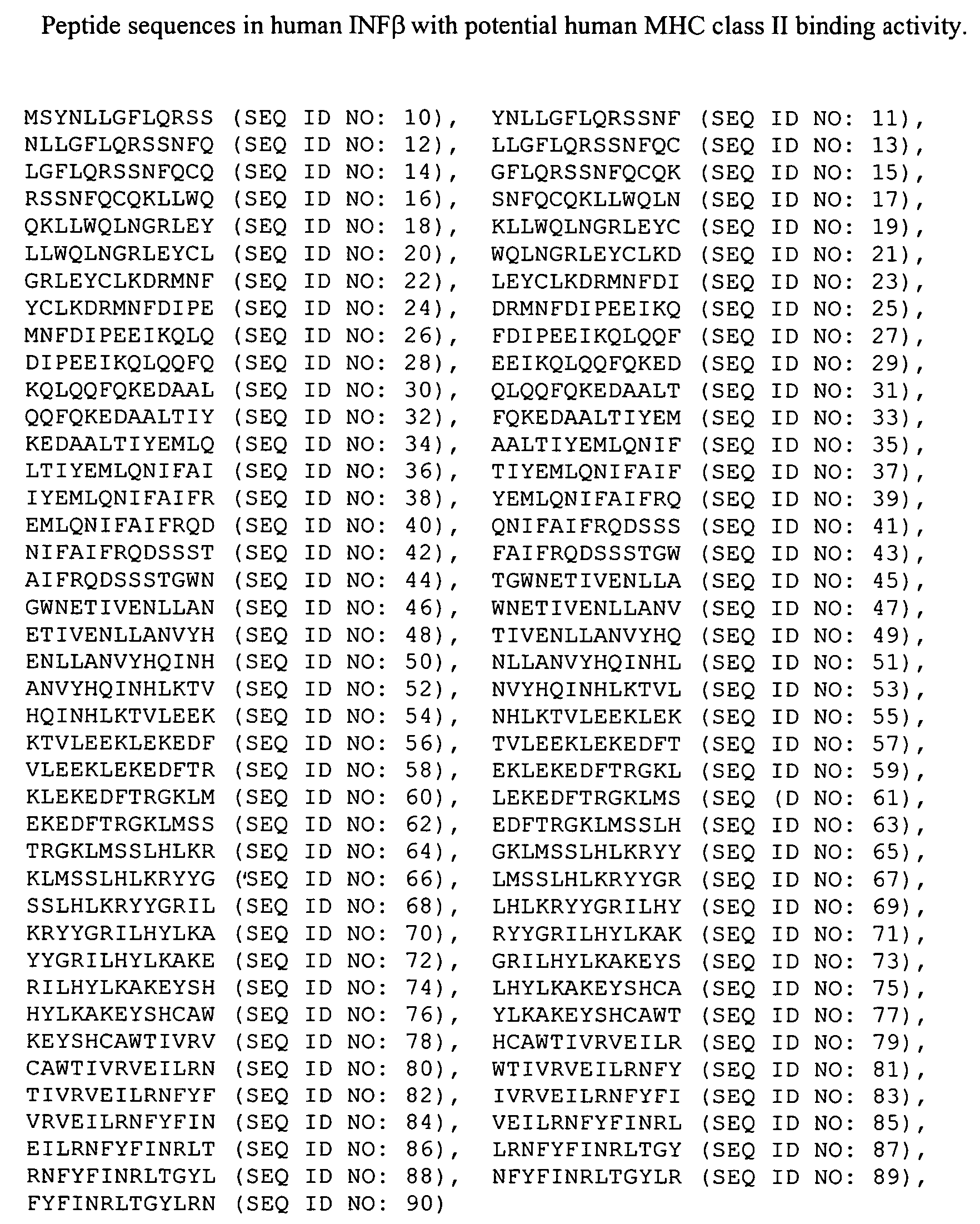
Interferon alfa (INN) or HuIFN-alpha-Le, trade name Multiferon, is a pharmaceutical drug composed of natural interferon alpha (IFN-α) obtained from the leukocyte fraction of human blood following induction with Sendai virus. Interferon alfa contains several naturally occurring IFN-α subtypes and is purified by affinity chromatography. Although the pharmaceutical product is often simply called "interferon alpha" or "IFN-α" like its endogenous counterpart, the product's International nonproprietary name (INN) is interferon alfa (the spelling of 'alfa' with 'f' reflects INN naming conventions).
Oxazolo, thiazolo and selenazolo [4,5-c]-quinolin-4-amines and analogs thereof
Thiazolo-, oxazolo- and selenazolo[4,5-c]quinolin-4-amines and analogs thereof are described including methods of manufacture and the use of novel intermediates. The compounds are immunomodulators and induce cytokine biosynthesis, including interferon and / or tumor biosynthesis, necrosis factor, and inhibit the T-helper-type 2 immune response. The compounds are further useful in the treatment of viral and neoplastic diseases.
Owner:3M INNOVATIVE PROPERTIES CO
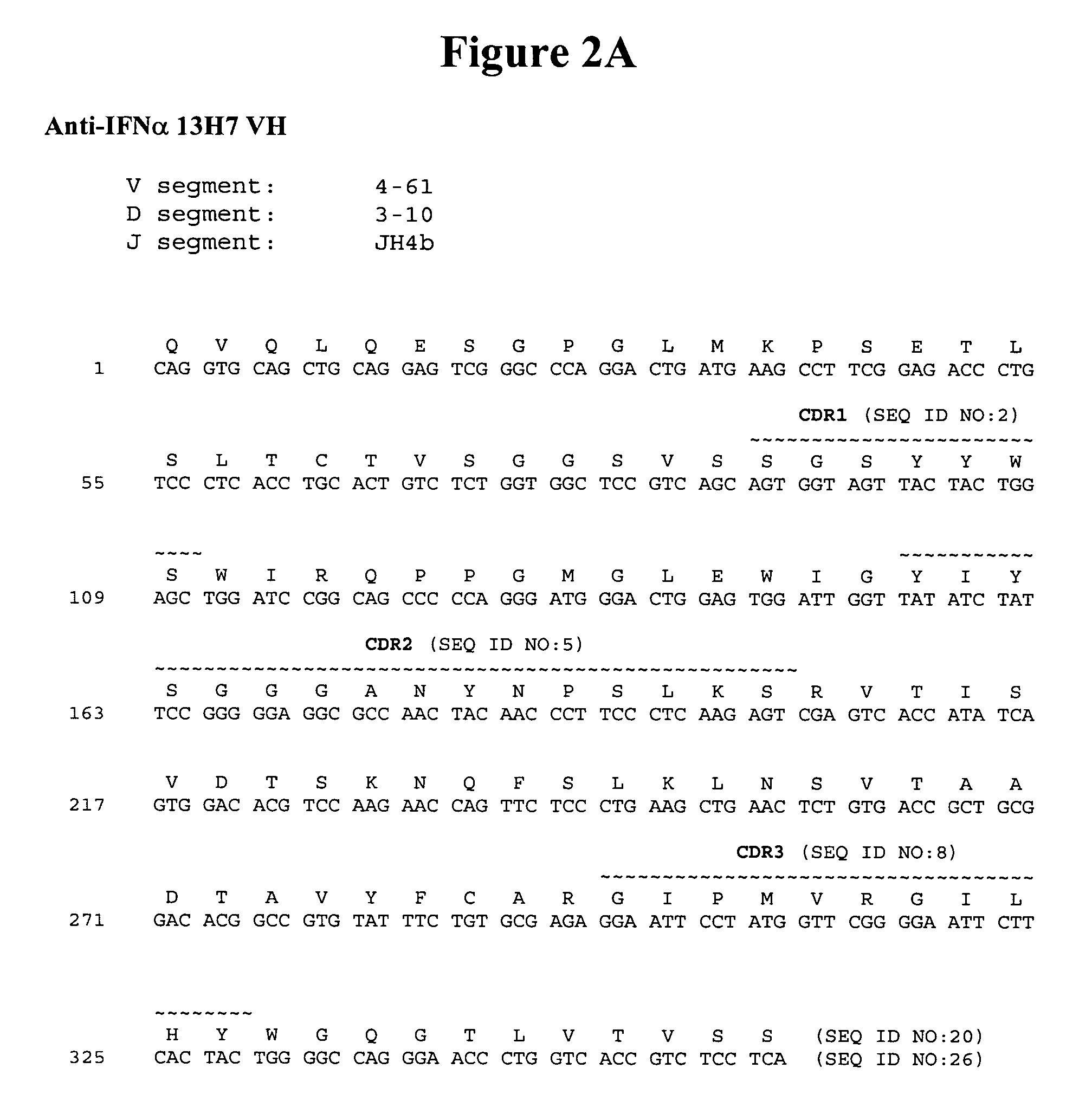
Humanized antibodies to gamma-interferon
The invention provides humanized immunoglobulins that bind to and neutralize gamma-interferon. The antibodies are useful for treatment of diseases of the immune system, particularly autoimmune diseases.
Owner:ABBOTT BIOTHERAPEUTICS CORP
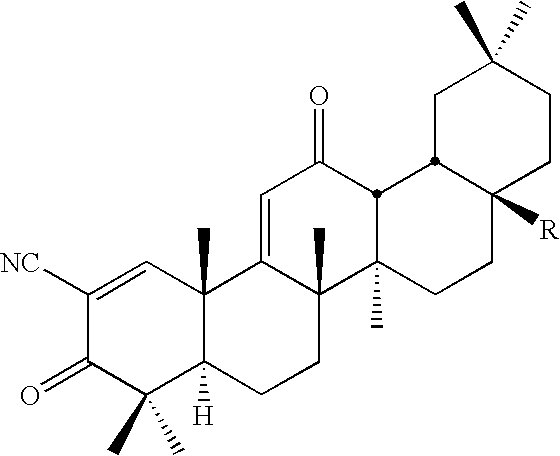
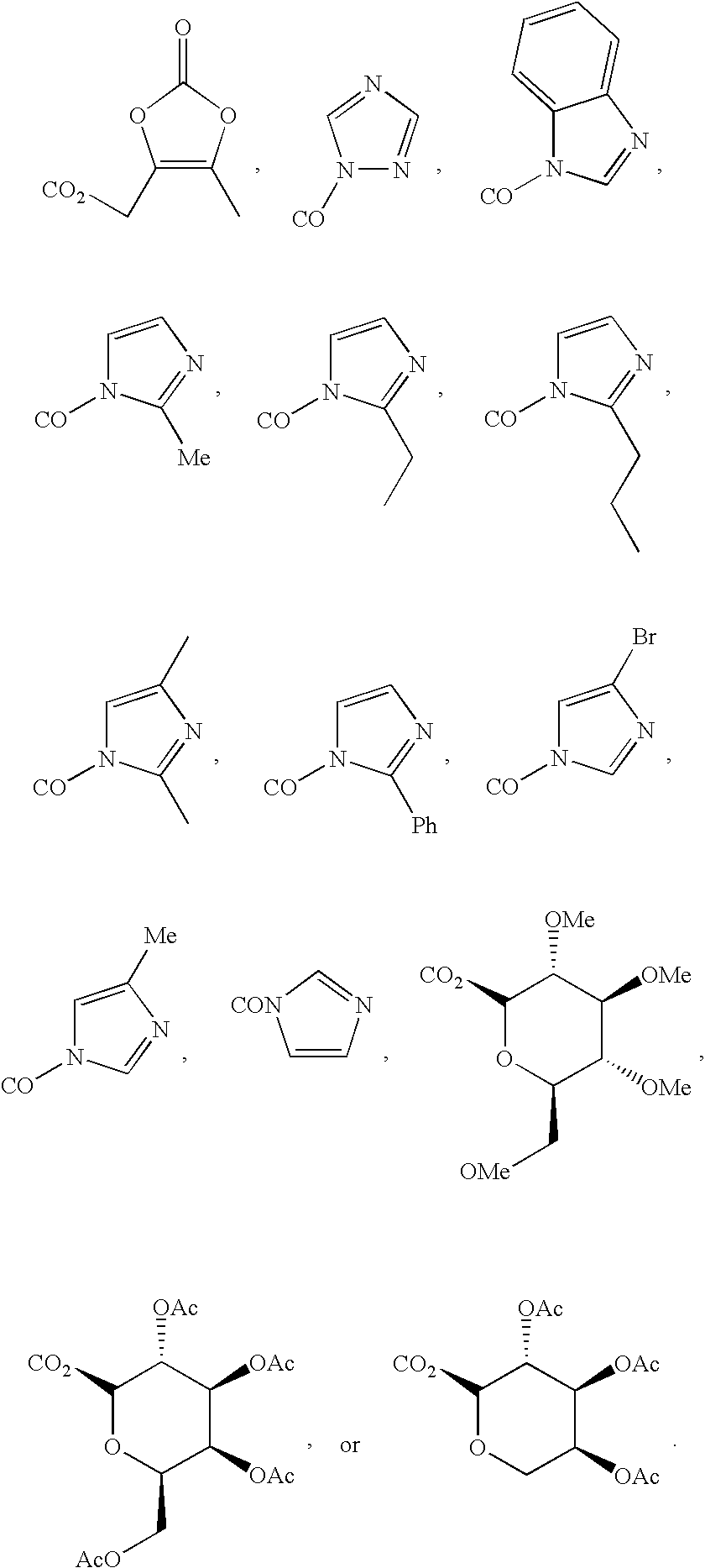
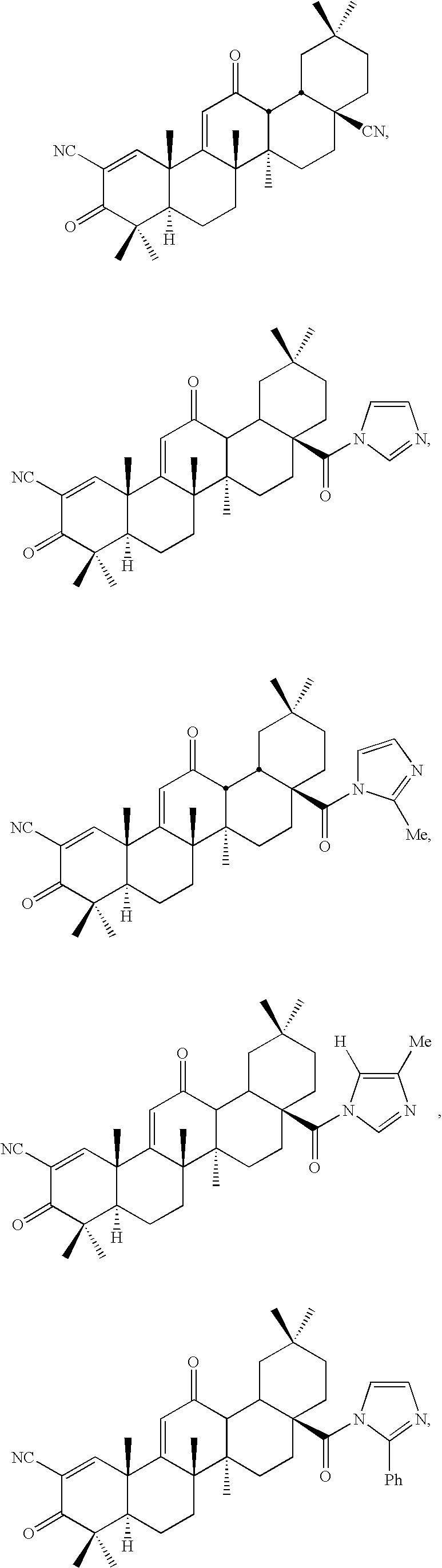
Inhibitors and methods of use thereof
New triterpenoid derivatives with various substituents at the C-17 position of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) were synthesized. Among them, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile (CNDDO), 1-(2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl) imidazole, 1-(2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl)-2-methylimidazole, 1-(2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl)-4-methylimidazole show extremely high inhibitory activity (IC50=0.01-1 pM level) against production of nitric oxide induced by interferon-γ in mouse macrophages. These compounds can be used in the prevention or treatment of diseases such as cancer, Alzheimer's disease, Parkinson's disease, multiple sclerosis, rheumatoid arthritis, and other inflammatory diseases. All the new triterpenoid derivatives are more potent than previously known CDDO.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Supplemented and unsupplemented tissue sealants, methods of their production and use
ActiveUS7189410B1Low antigenicityDecreasing thrombogenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant bandage, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, anti-inflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant bandage.
Owner:AMERICAN NAT RED CROSS
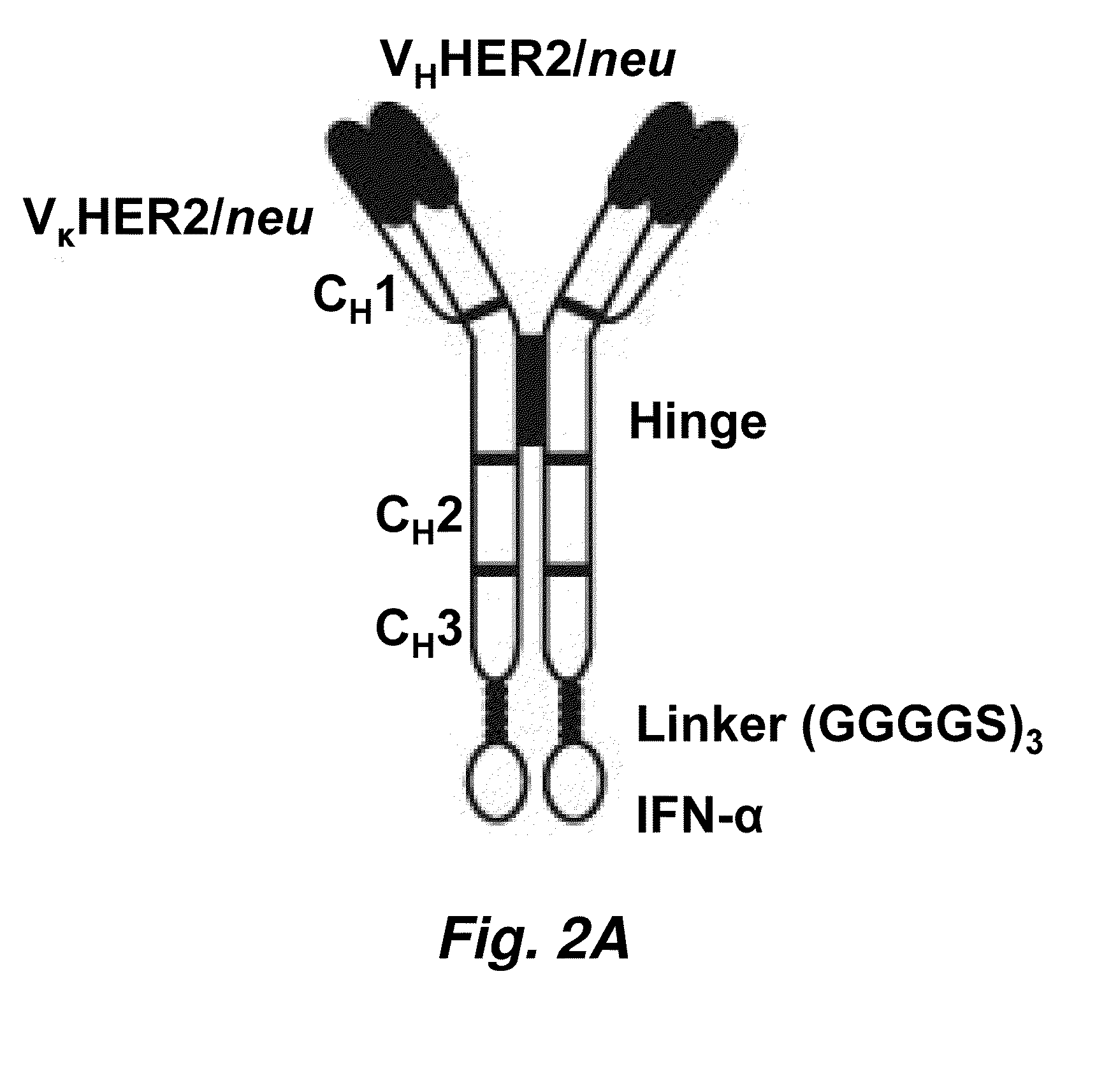
Targeted interferons demonstrate potent apoptotic and Anti-tumor activities
ActiveUS20100172868A1Peptide/protein ingredientsPharmaceutical delivery mechanismInterferon alphaAntitumor activity
Novel chimeric moieties that show significant efficacy against cancers are provided. In certain embodiments the chimeric moieties comprise a targeting moiety attached to an interferon. In certain embodiments, the chimeric moieties comprise fusion proteins where an antibody that specifically binds to a cancer marker is fused to interferon alpha (IFN-α) or interferon beta (IFN-β).
Owner:RGT UNIV OF CALIFORNIA
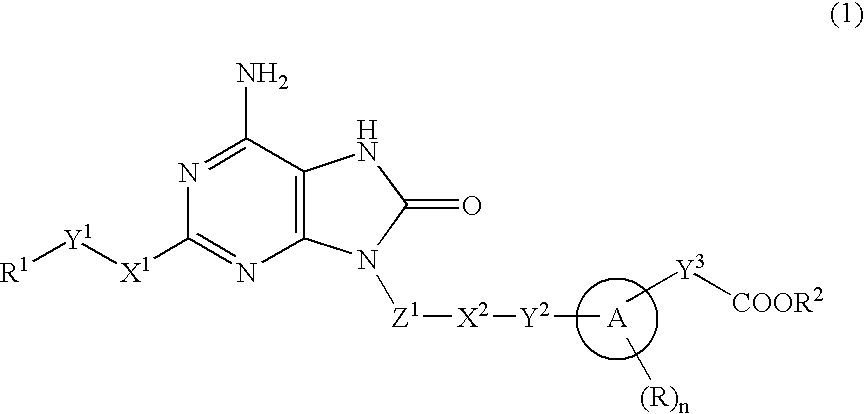
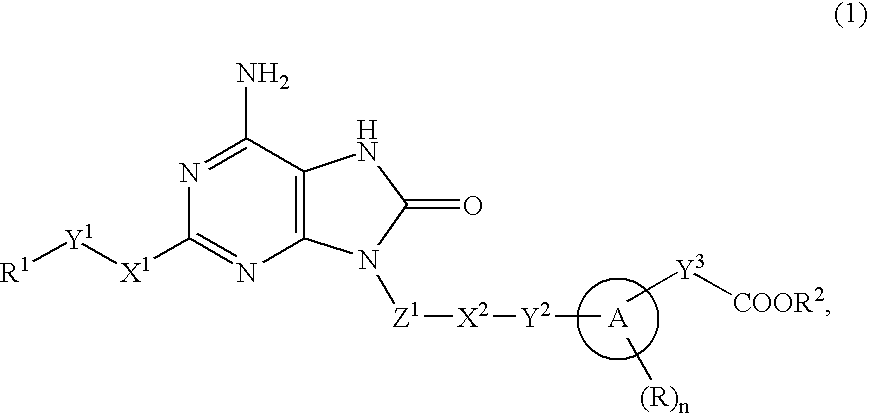
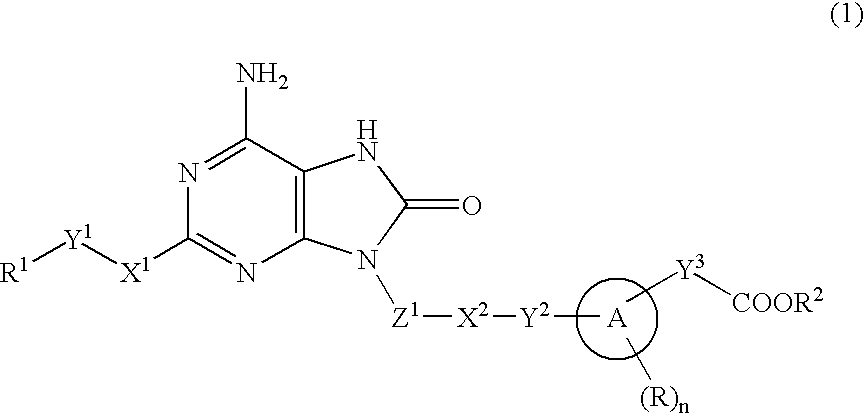
9-Substituted 8-oxoadenine compound
ActiveUS20070190071A1Inhibitory activityEffective therapyBiocideOrganic chemistryHydrogen atomHalogen
The present invention provides an 8-oxoadenine compound having immunemodulating activities such as an interferon inducing activity and useful as an antiviral agent and antiallergic agent, which is represented by the following formula (1): [wherein the ring A represents a 6-10 membered aromatic carbocyclic ring and the like, R represents a halogen atom, an alkyl group and the like, n represents an integer of 0-2, Z1 represents alkylene, X2 represents oxygen atom, sulfur atom, SO2, NR5, CO, CONR5, NR5CO and the like, Y1, Y2 and Y3 represent independently a single bond or an alkylene group, X1 represents oxygen atom, sulfur atom, NR4 (R4 is hydrogen atom or an alkyl group) or a single bond, R2 represents a substituted or unsubstituted alkyl group, R1 represents hydrogen atom, hydroxy group, an alkoxy group, an alkoxycarbonyl group or a haloalkyl group]or its pharmaceutically acceptable salt.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
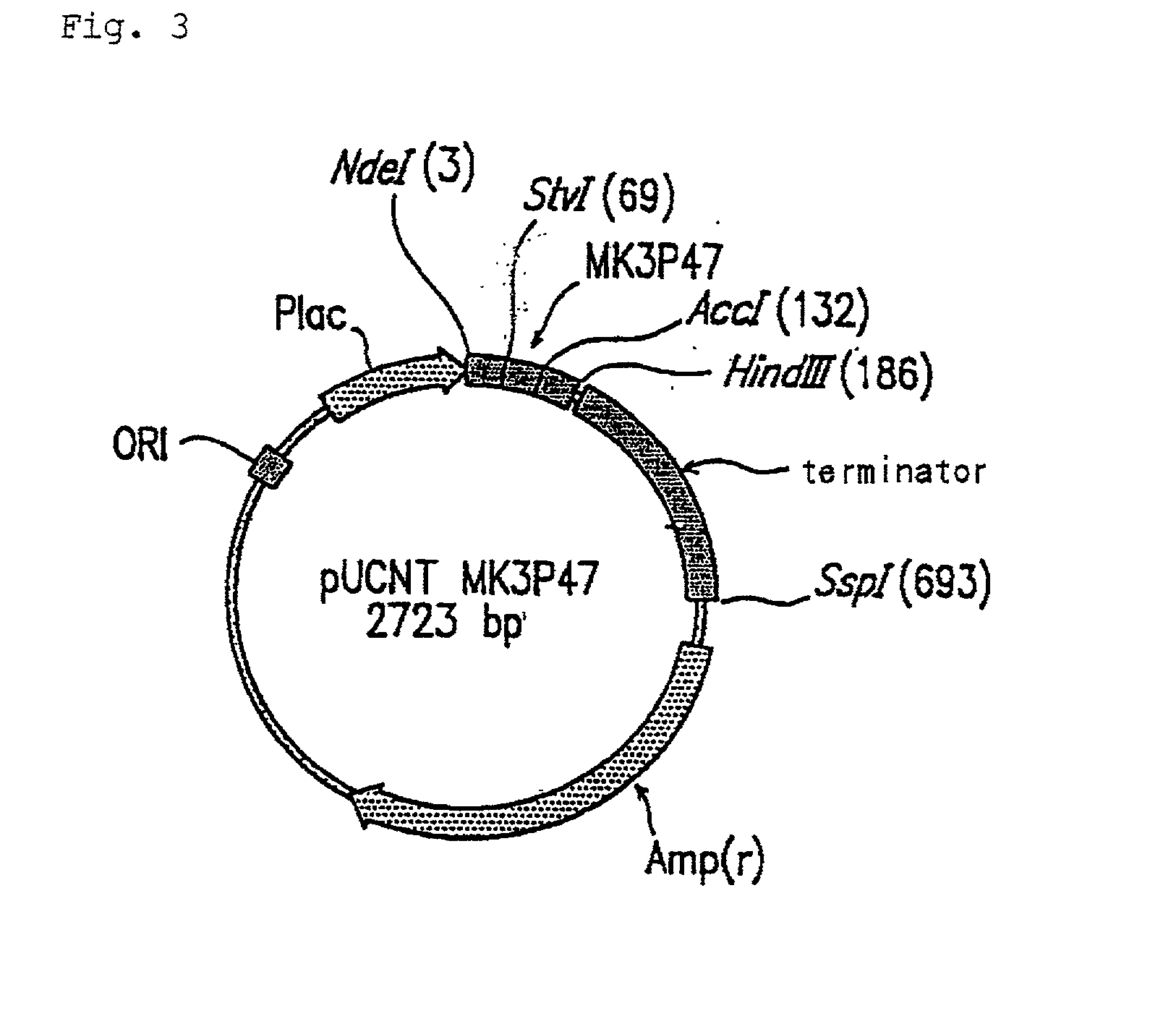
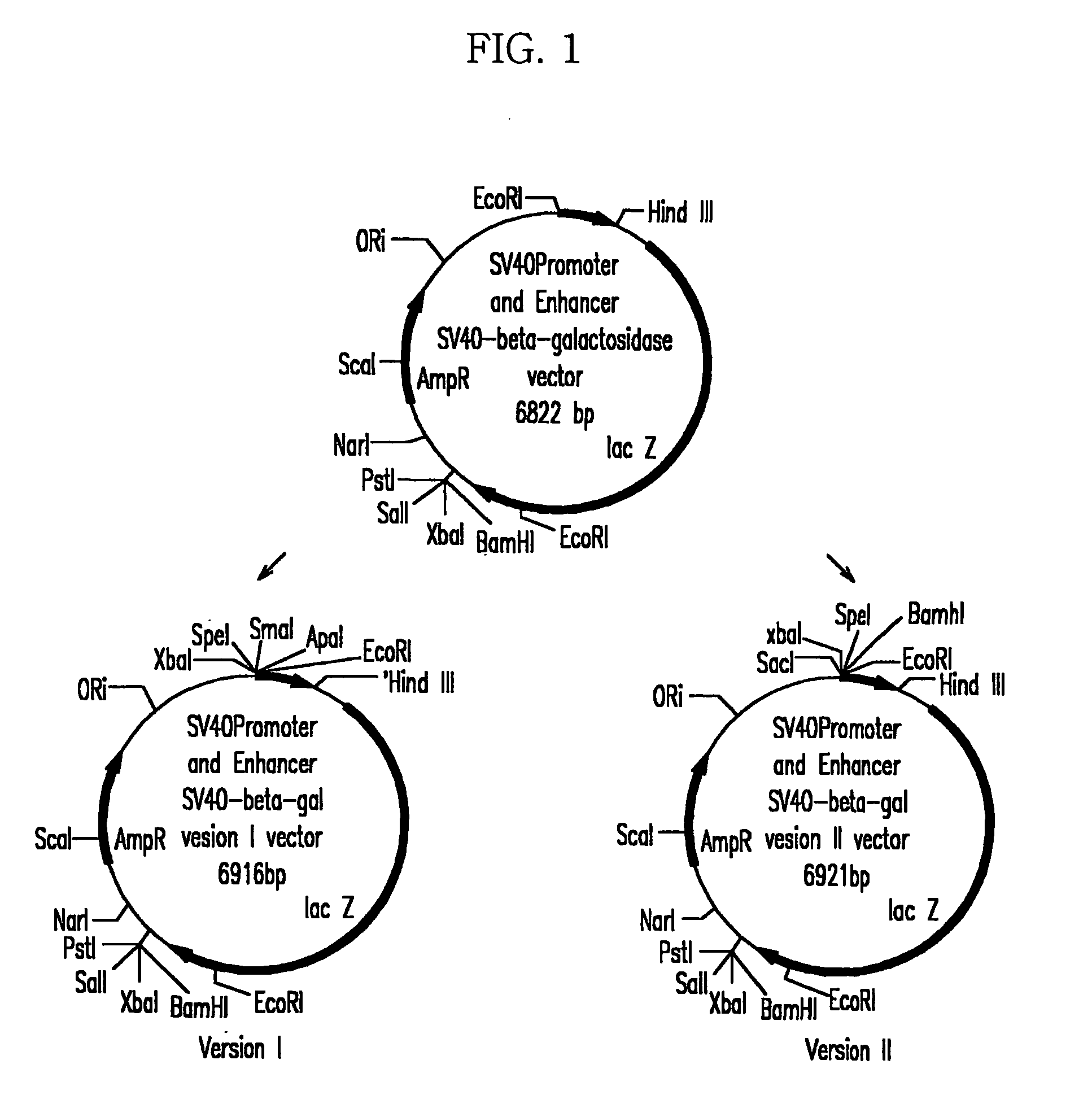
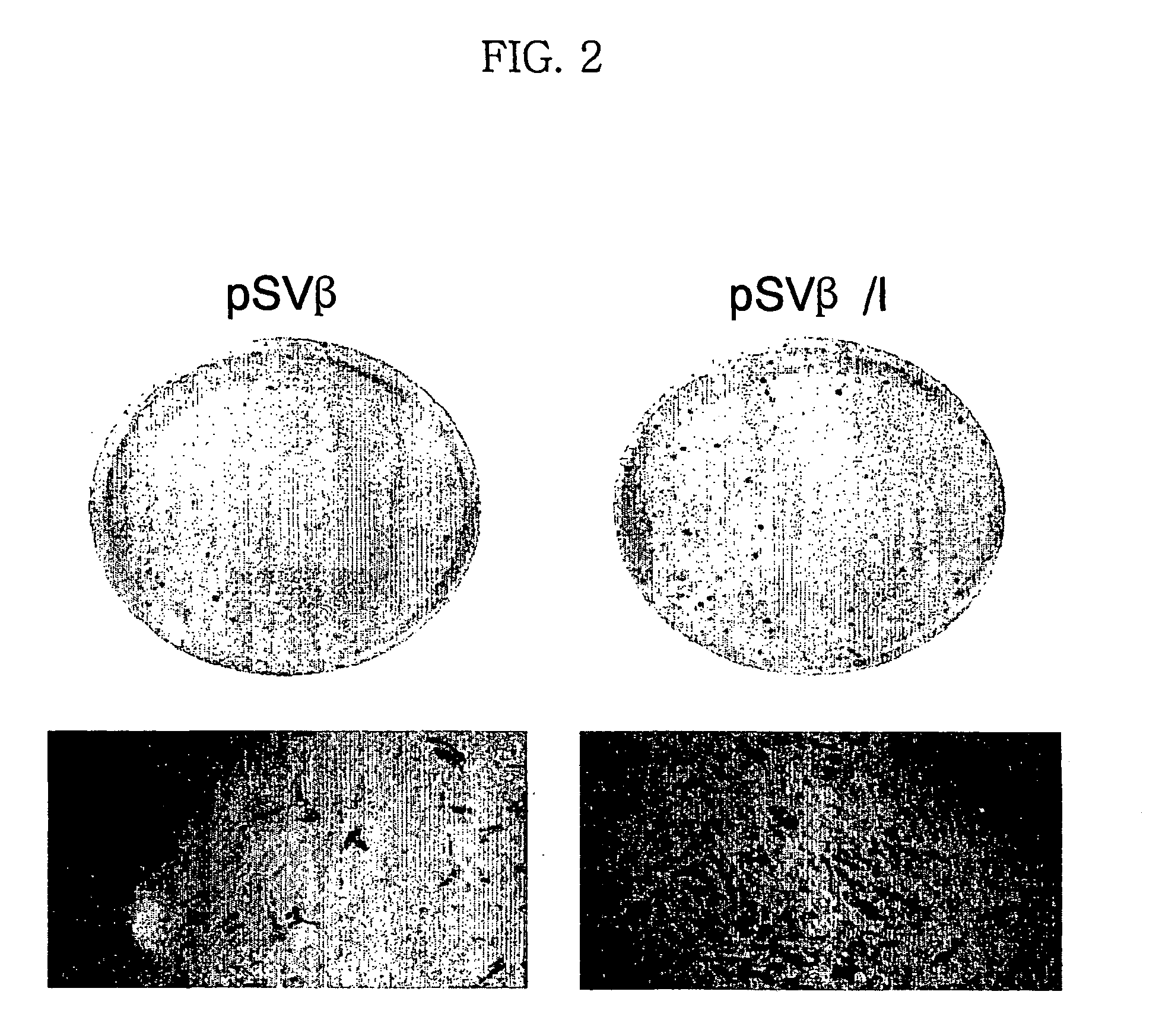
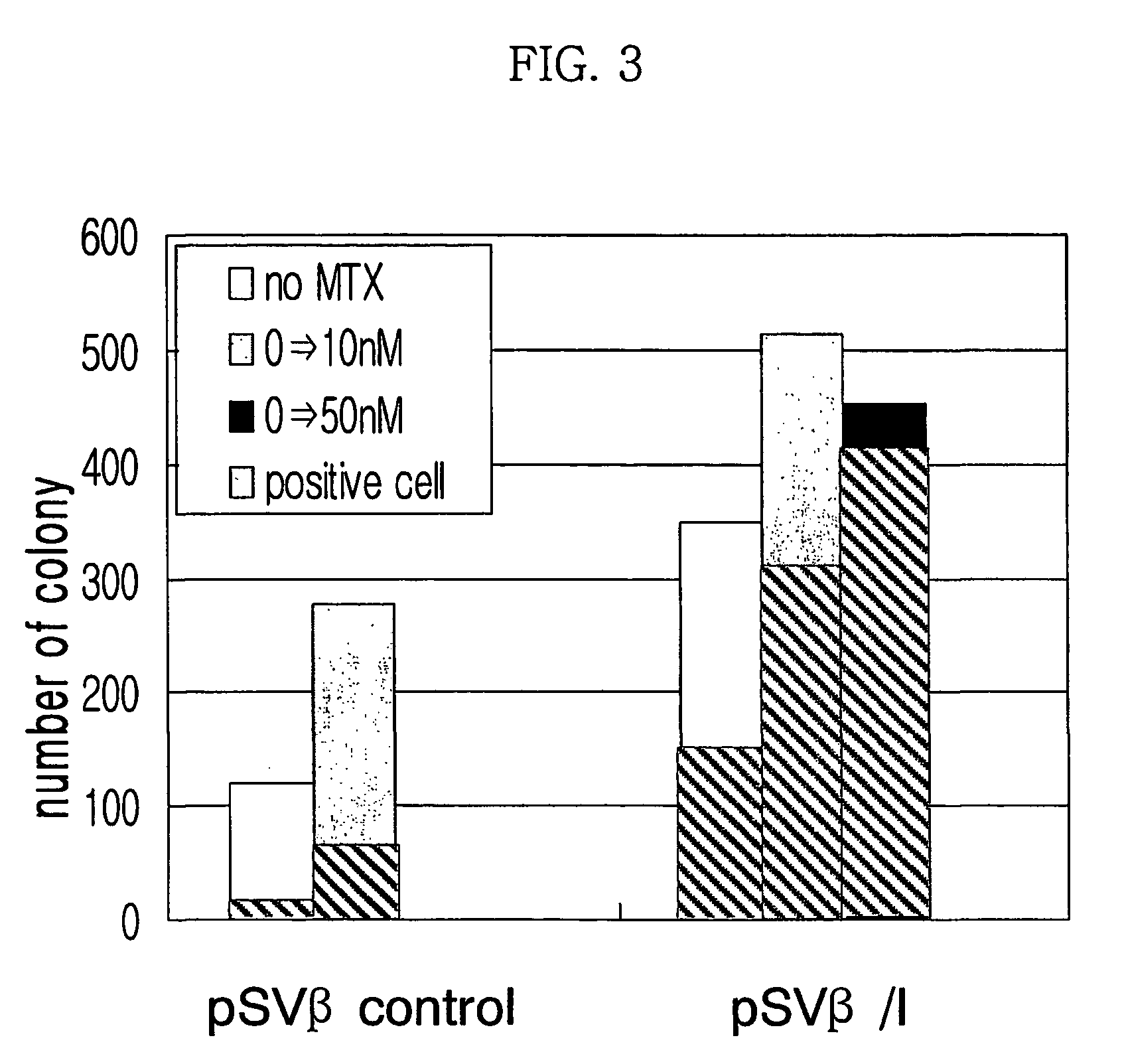
Expression vector for animal cell containing nuclear matrix attachment region of interferon beta
InactiveUS7259010B2Increased foreign protein expression efficiencyReduce the amount of solutionVectorsSugar derivativesMammalNuclear matrix
The present invention relates to mammalian expression vectors including nuclear matrix attachment region of human interferon β, and more particularly to pPGM-1, pPGM-2 and pPGM-3 including nuclear matrix attachment region of interferon β gene. Those expression vectors confer position independent expression of the introduced foreign gene, thus increasing the frequency of colonies which efficiently express the recombinant protein.
Owner:PANGEN BIOTECH
Oxazolo, thiazolo and selenazolo [4,5-c] quinolin-4-amines and analogs thereof
Thiazolo-, oxazolo- and selenazolo[4,5-c]quinolin-4-amines and analogs thereof are described including methods of manufacture and the use of novel intermediates. The compounds are immunomodulators and induce cytokine biosynthesis, including interferon and / or tumor biosynthesis, necrosis factor, and inhibit the T-helper-type 2 immune response. The compounds are further useful in the treatment of viral and neoplastic diseases.
Owner:3M INNOVATIVE PROPERTIES CO
Method and composition for treatment of renal failure with antibodies and their equivalents as partial or complete replacement for dialysis
InactiveUS7504106B2Lower Level RequirementsSlow onsetBiocidePeptide/protein ingredientsInterleukin 6Creatinine rise
A method for treating patients with renal failure includes administering to them an effective amount of antibody or of a functional equivalent thereof to at least two of urea, creatinine, tumor necrosis factor alpha, interferon gamma, interleukin 6 and interleukin 1 beta. Soluble cytokine receptors also can be employed. The method can be used as a supplement to or as partial or complete replacement for dialysis. A pharmaceutical composition includes antibody or functional equivalent thereof to urea, creatinine, or both; antibody, functional equivalent or soluble cytokine receptor to tumor necrosis factor alpha, interferon gamma, interleukin 6, interleukin 1 beta or any combination thereof The composition can be included in a kit.
Owner:SKURKOVICH BORIS +2
HCV combination therapy
InactiveUS6849254B1Ameliorate ribavirin-related hemolysisLow viral-RNABiocidePeptide/protein ingredientsChronic viral hepatitis CHemolysis
Methods of treating patients having susceptible viral infections, especially chronic hepatitis C infection by administering to said patient a therapeutically effective amount of a combination therapy of interferon-alfa and ribavirin for a time sufficient to lower HCV-RNA in association with a therapeutically effective amount of an antioxidant for a time sufficient to ameliorate ribavirin-related hemolysis are disclosed.
Owner:MERCK SHARP & DOHME CORP
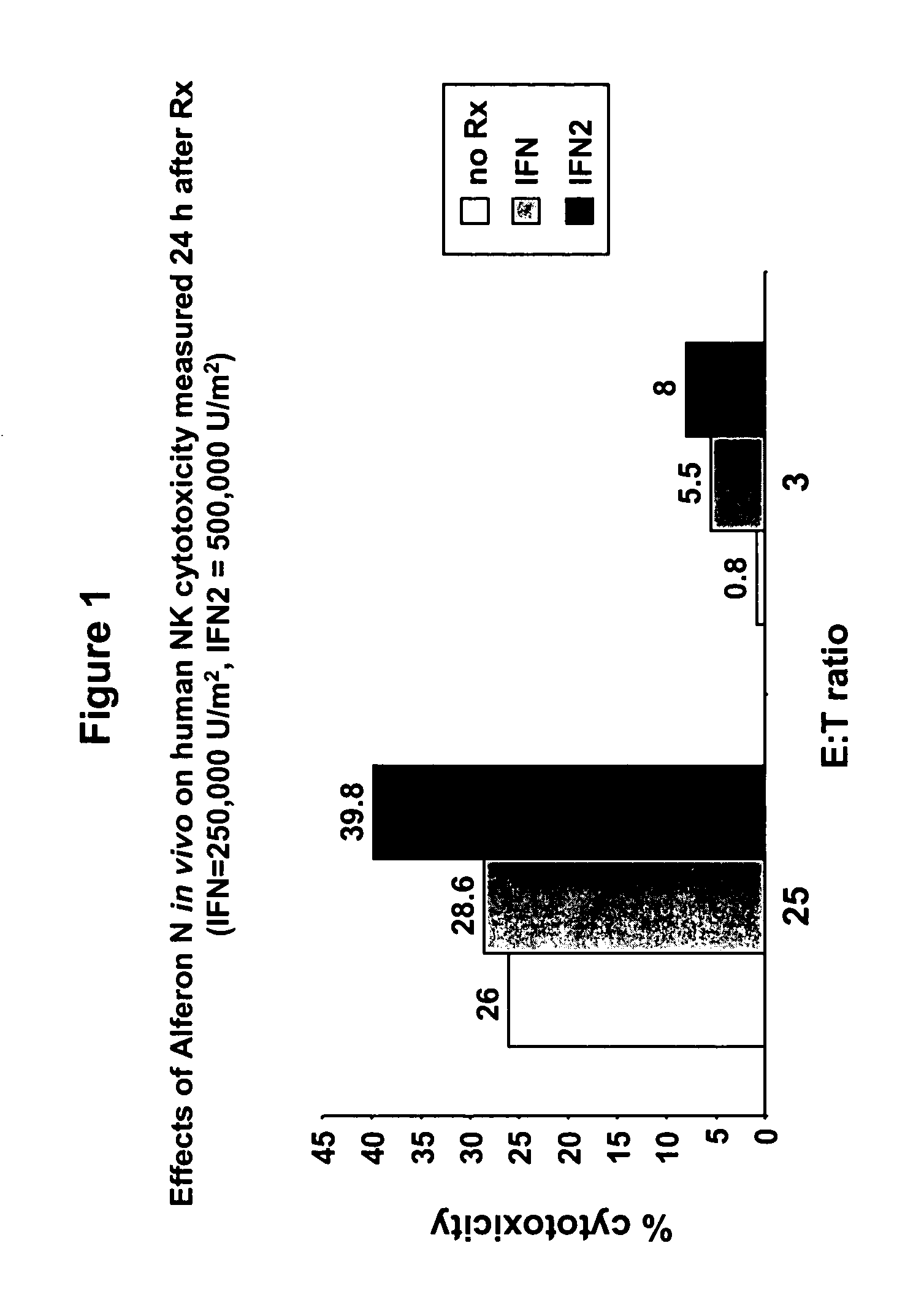
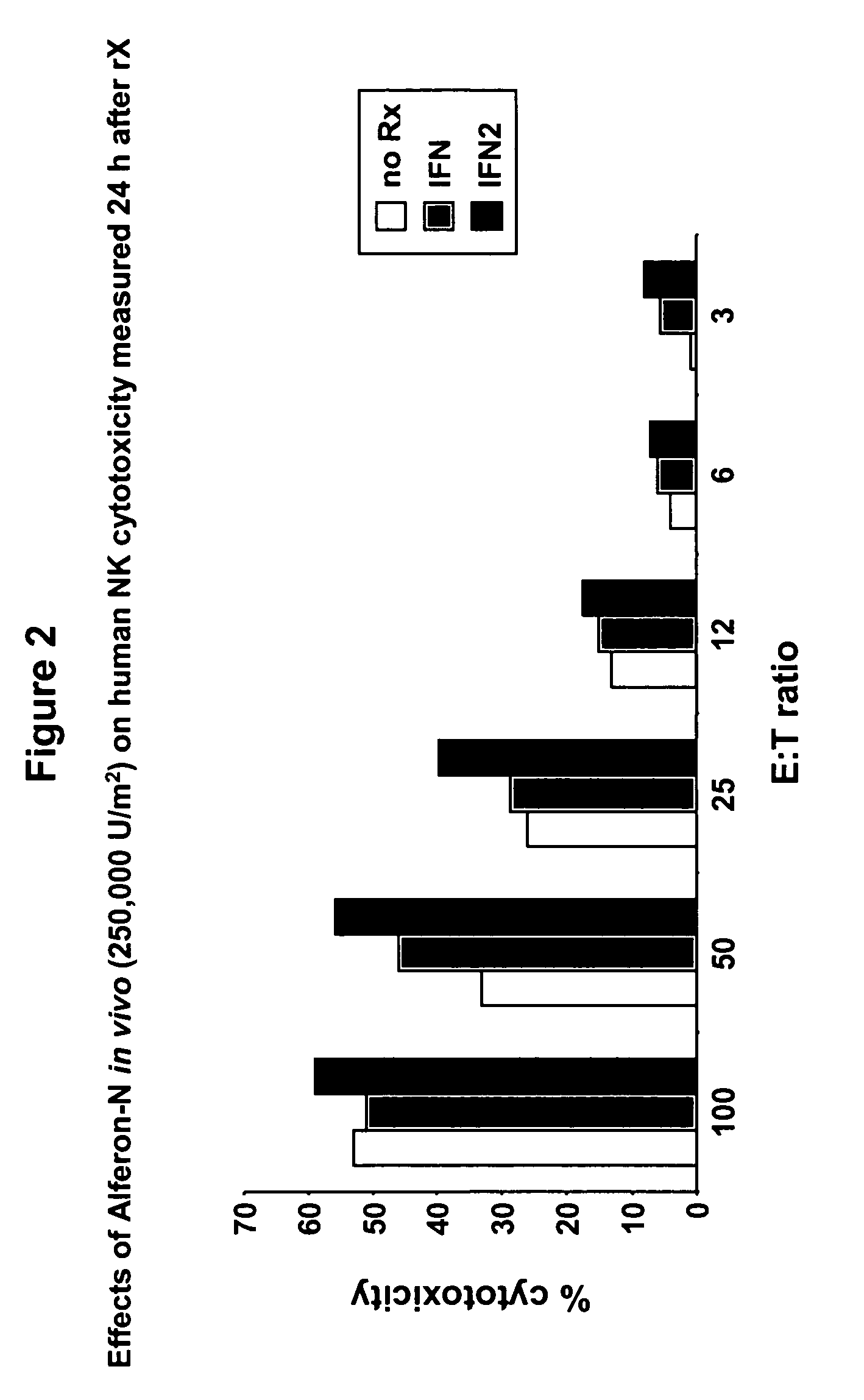
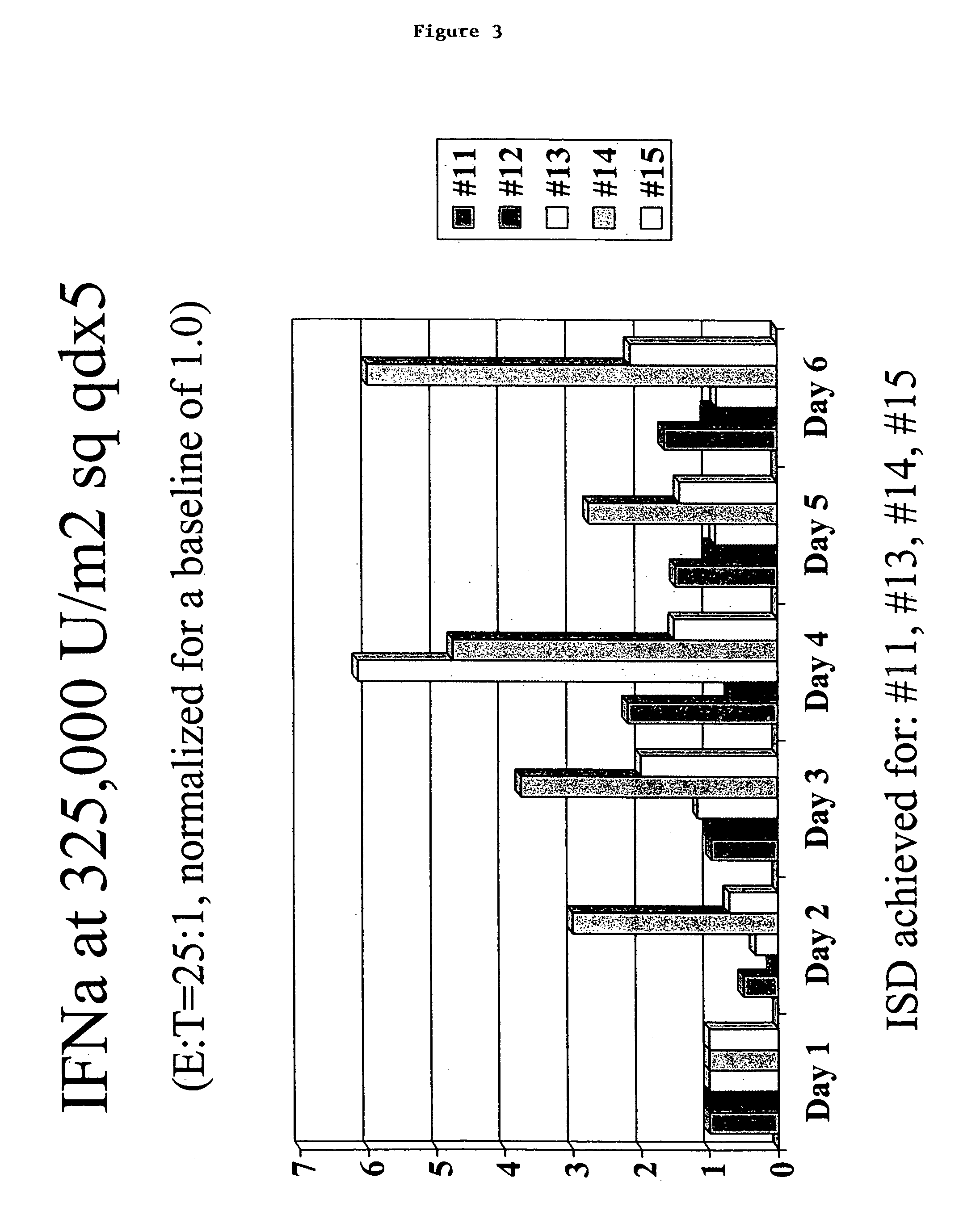
Interferon immunotherapy
InactiveUS7041301B1Inhibit progressStrong cytotoxicityPeptide/protein ingredientsPeptide preparation methodsHuman patientInterferon alpha
A method for reducing the recurrence of a resectable malignant tumor includes administering an immunostimulatory dosage of an α-interferon composition, then surgically resecting the malignant tumor. A method for treating a human patient having a non-resectable malignant tumor includes administering an immunostimulatory dosage of an α-interferon composition to the patient and treating the patient with effective non-surgical medical methodologies to diminish the tumor. An article of manufacture combines an α-interferon composition within a packaging material and a package label or insert indicating that administration of an immunostimulatory dosage of an α-interferon composition followed by surgical resection of a malignant tumor can be effective for treating a human patient having the malignant tumor.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Humanized antibodies to gamma-interferon
The invention provides humanized immunoglobulins that bind to and neutralize gamma-interferon. The antibodies are useful for treatment of diseases of the immune system, particularly autoimmune diseases.
Owner:ABBOTT BIOTHERAPEUTICS CORP
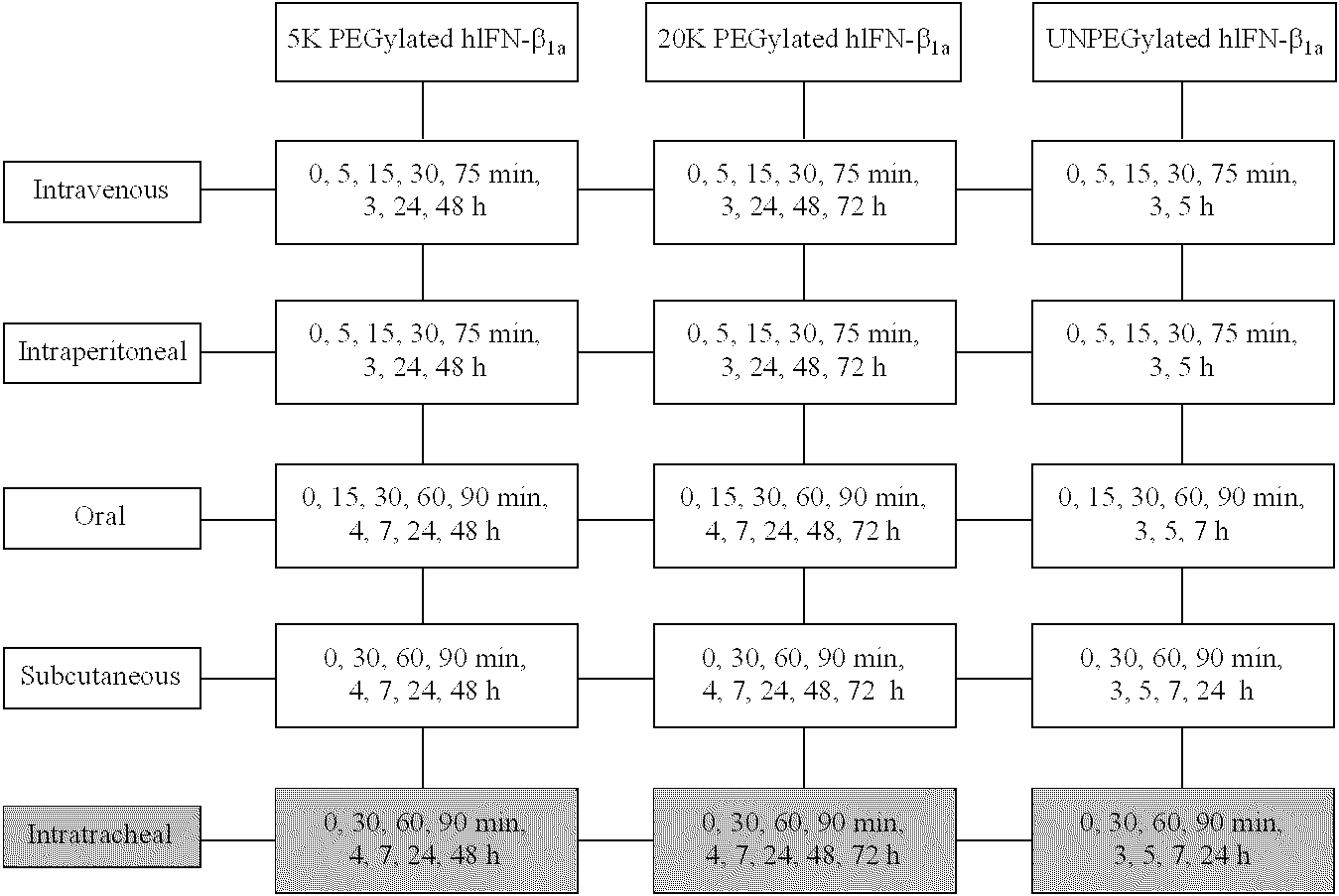
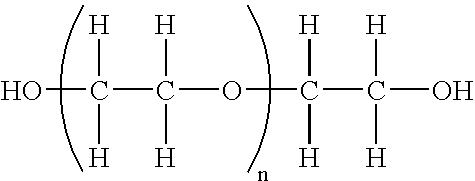
Polymer conjugates of interferon beta-1a and uses
InactiveUS6962978B2High activityNo effective loss in activitySenses disorderNervous disorderBeta interferonsBiochemistry
An interferon beta polypeptide comprising interferon-beta 1a coupled to a polymer containing a polyalkylene glycol moiety wherein the interferon-beta-1a and the polyalkylene glycol moiety are arranged such that the interferon-beta-1a has an enhanced activity relative to another therapeutic form of interferon beta (interferon-beta-1b) and exhibits no decrease in activity as compared to non-conjugated interferon-beta-1a. The conjugates of the invention are usefully employed in therapeutic as well as non-therapeutic, e.g., diagnostic, applications.
Owner:BIOGEN MA INC
Pegylated interferon alpha-1b
The invention provides PEG-IFN α-1b conjugates, where a PEG moiety is covalently bound to Cys86 of human IFN α-1b conjugates. A pharmaceutical composition and a method for treating inflammatory diseases, infections, and cancer are also provided. The invention further relates to a method for the modification of interferons by conjugation of a PEG moiety to free cysteine residues in interferon molecules.
Owner:SHEN CHUN +6
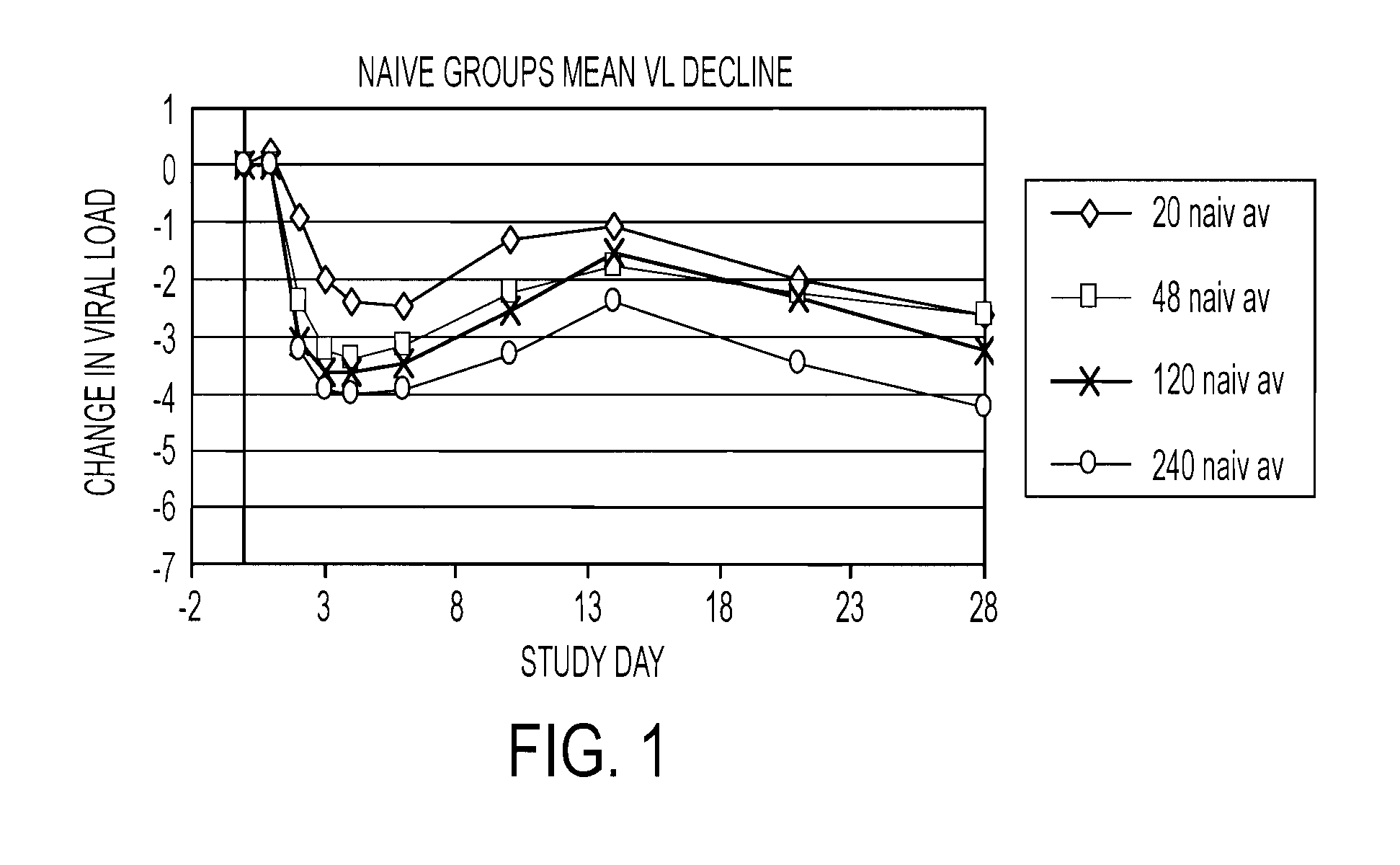
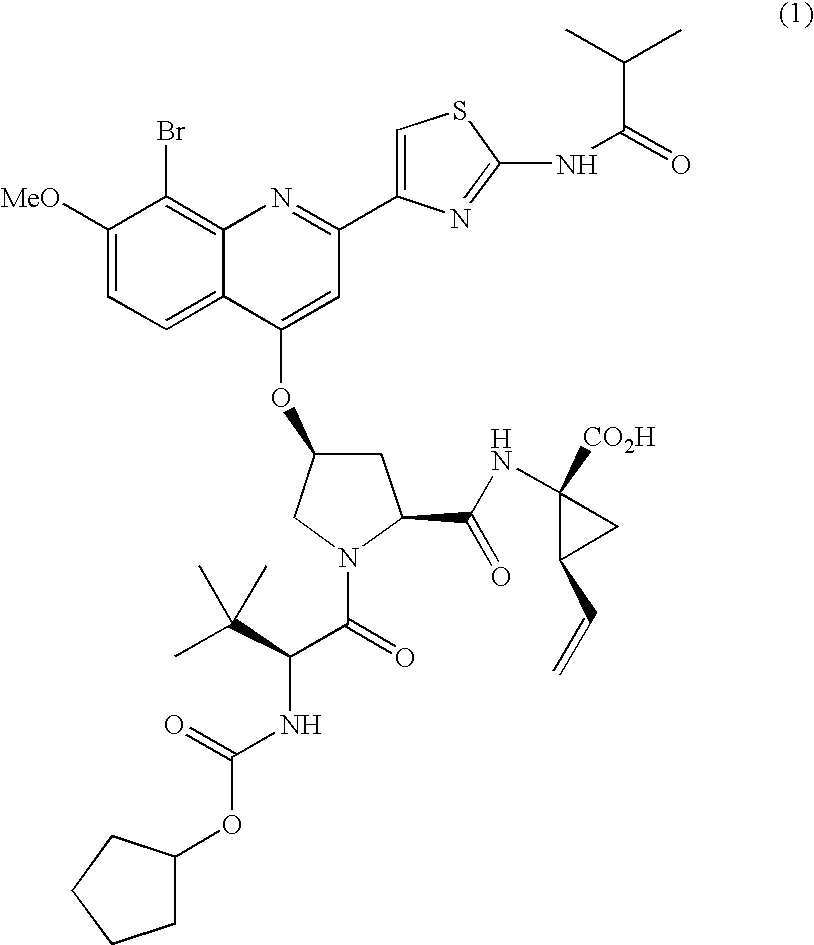
Combination therapy for treating hcv infection
The present invention relates to therapeutic combinations comprising (a) Compound (1), or a pharmaceutically acceptable salt thereof, as herein described, (b) an interferon alfa and (c) ribavirin. Compound (1) is a selective and potent inhibitor of the HCV NS3 serine protease. The present invention also relates to methods of using such therapeutic combinations for treating HCV infection or alleviating one or more symptoms thereof in a patient.
Owner:BOEHRINGER INGELHEIM INT GMBH
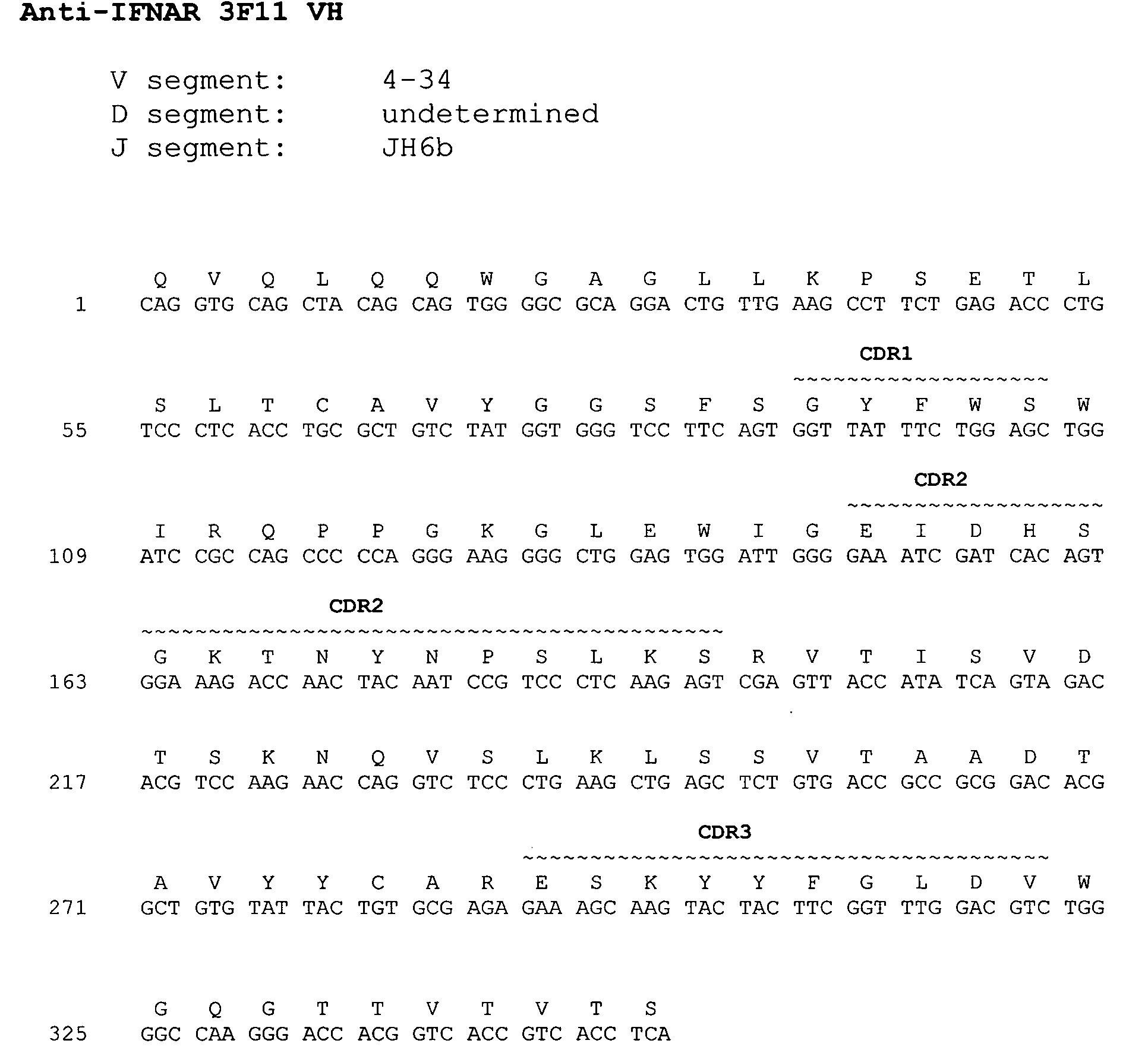
Interferon alpha receptor 1 antibodies and their uses
ActiveUS20060029601A1Inhibit biological activityNervous disorderAntipyreticTransplant rejectionGraft versus host disease induction
The present invention provides isolated human monoclonal antibodies that bind to IFNAR-1 and that are capable of inhibiting the biological activity of Type I interferons. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for inhibiting Type I interferon-mediated disorders using the antibodies of the invention, including methods for treating autoimmune disorders, transplant rejection or Graft Versus Host Disease using the antibodies of the invention.
Owner:ER SQUIBB & SONS INC
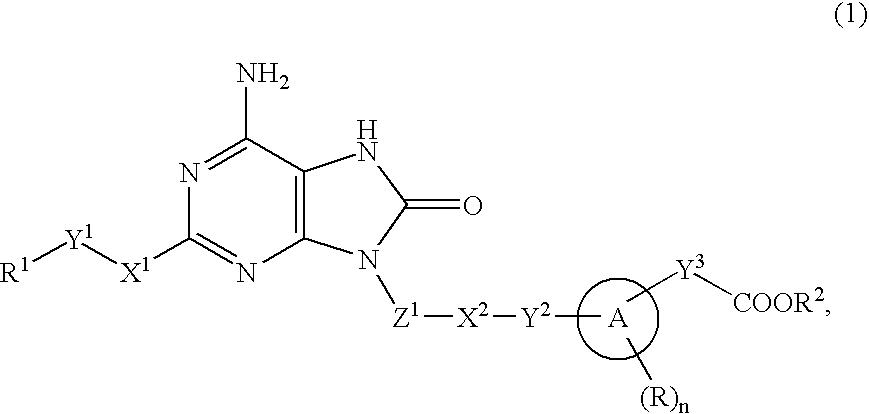
9-substituted 8-oxoadenine compound
The present invention provides an 8-oxoadenine compound having immunemodulating activities such as an interferon inducing activity and useful as an antiviral agent and antiallergic agent, which is represented by the following formula (1):[wherein the ring A represents a 6-10 membered aromatic carbocyclic ring and the like, R represents a halogen atom, an alkyl group and the like, n represents an integer of 0-2, Z1 represents alkylene, X2 represents oxygen atom, sulfur atom, SO2, NR5, CO, CONR5, NR5CO and the like, Y1, Y2 and Y3 represent independently a single bond or an alkylene group, X1 represents oxygen atom, sulfur atom, NR4 (R4 is hydrogen atom or an alkyl group) or a single bond, R2 represents a substituted or unsubstituted alkyl group, R1 represents hydrogen atom, hydroxy group, an alkoxy group, an alkoxycarbonyl group or a haloalkyl group]or its pharmaceutically acceptable salt.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
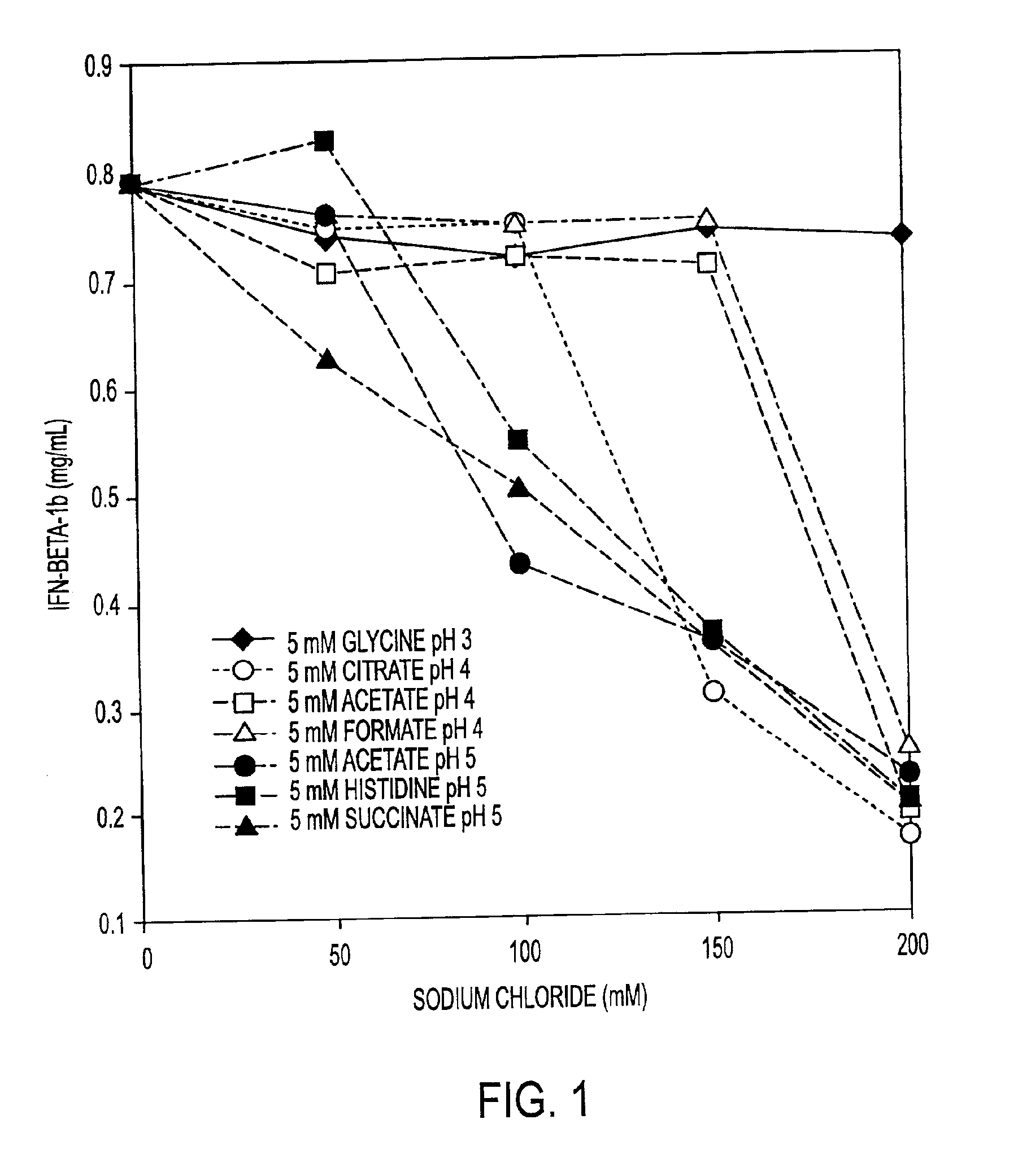
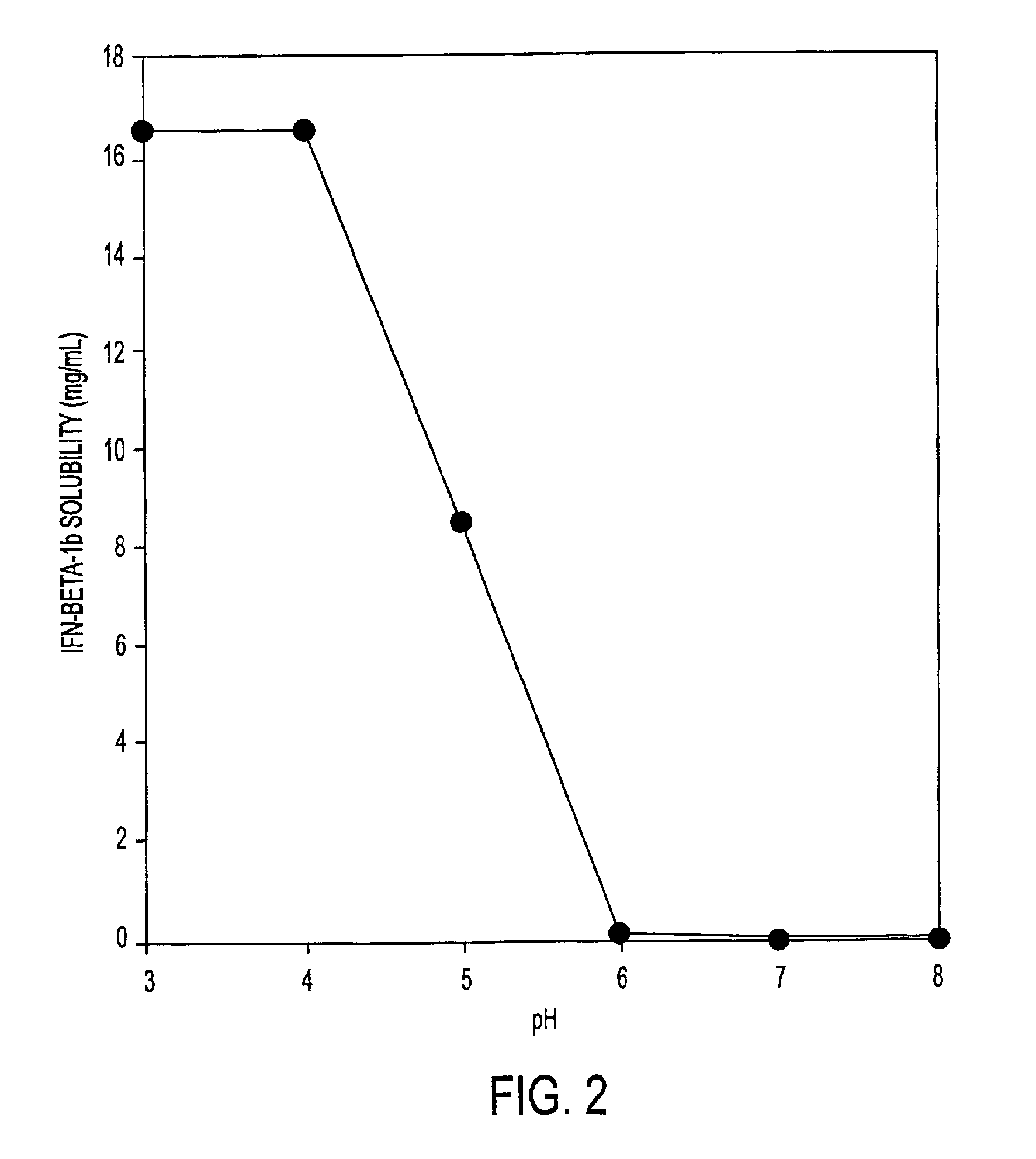
HSA-free formulations of interferon-beta
InactiveUS6887462B2Improve solubilityIncreasing the amount of monomeric IFN-βPowder deliveryNervous disorderSolubilityLow ionic strength
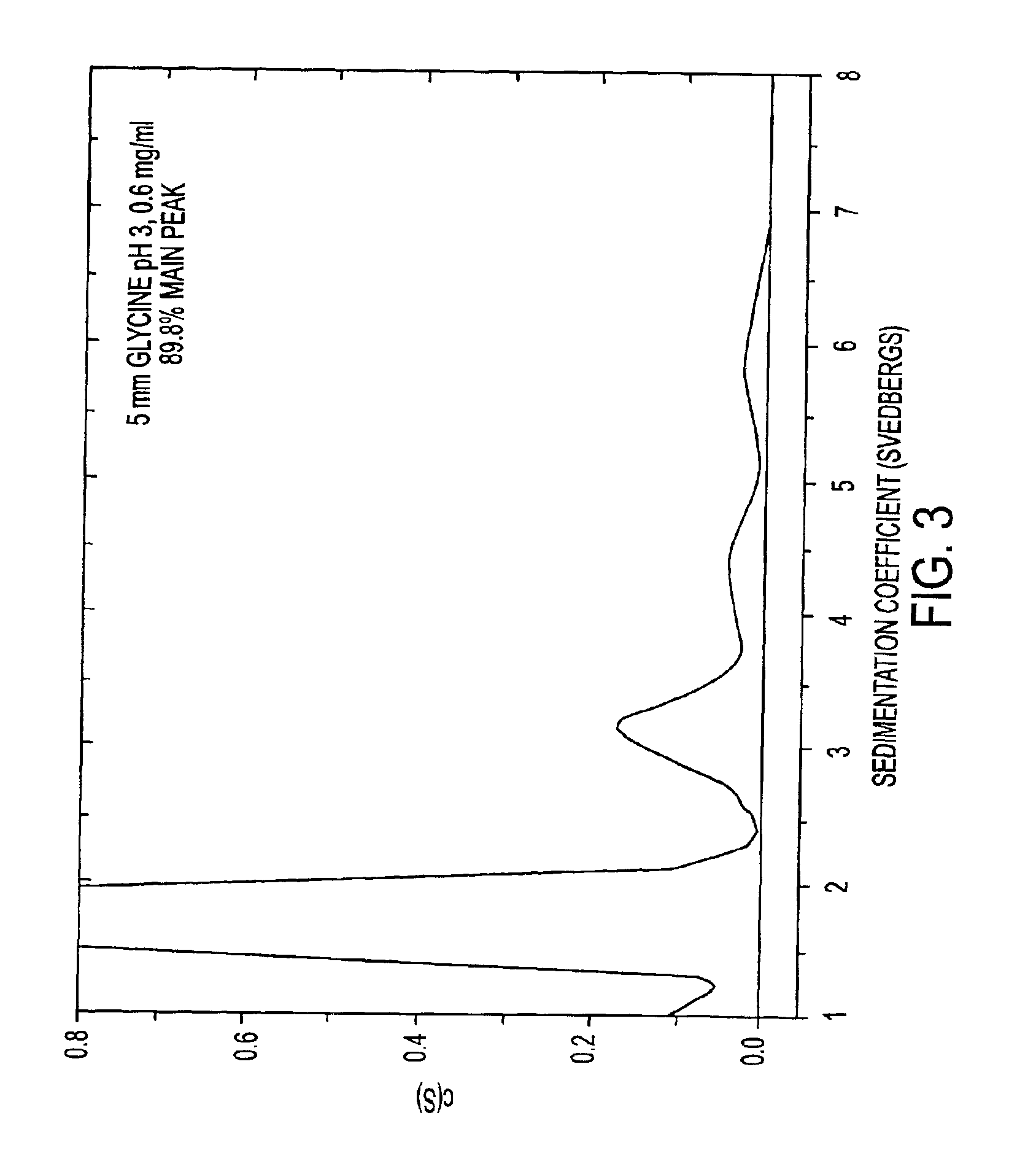
Stabilized pharmaceutical compositions comprising substantially monomeric interferon-beta (IFN-β) and methods useful in their preparation are provided. The compositions comprise the IFN-β solubilized in a low-ionic-strength formulation that maintains the composition at a pH of about 3.0 to about 5.0. Methods for preparing these compositions, and for increasing solubility of IFN-β in pharmaceutical compositions, are provided.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
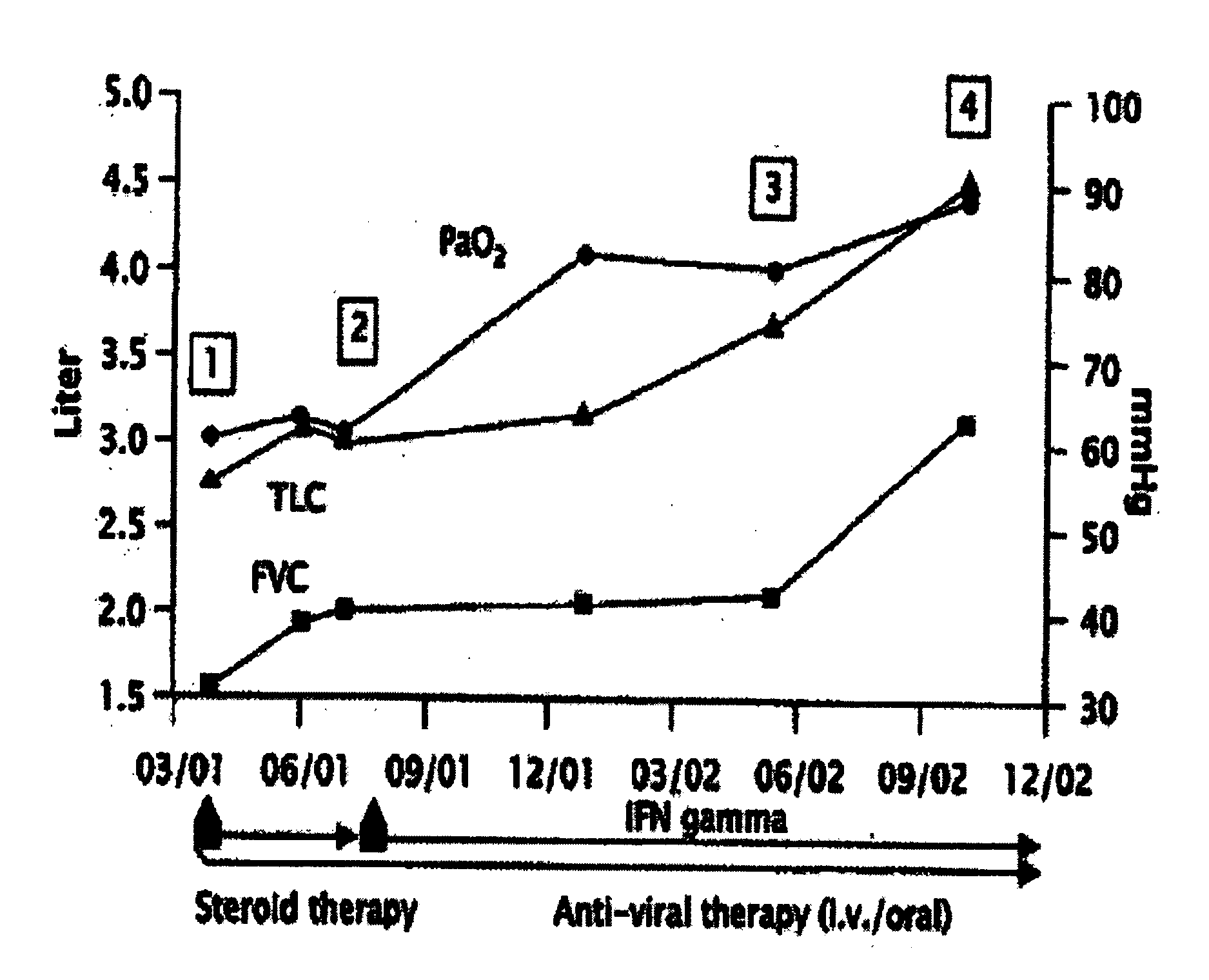
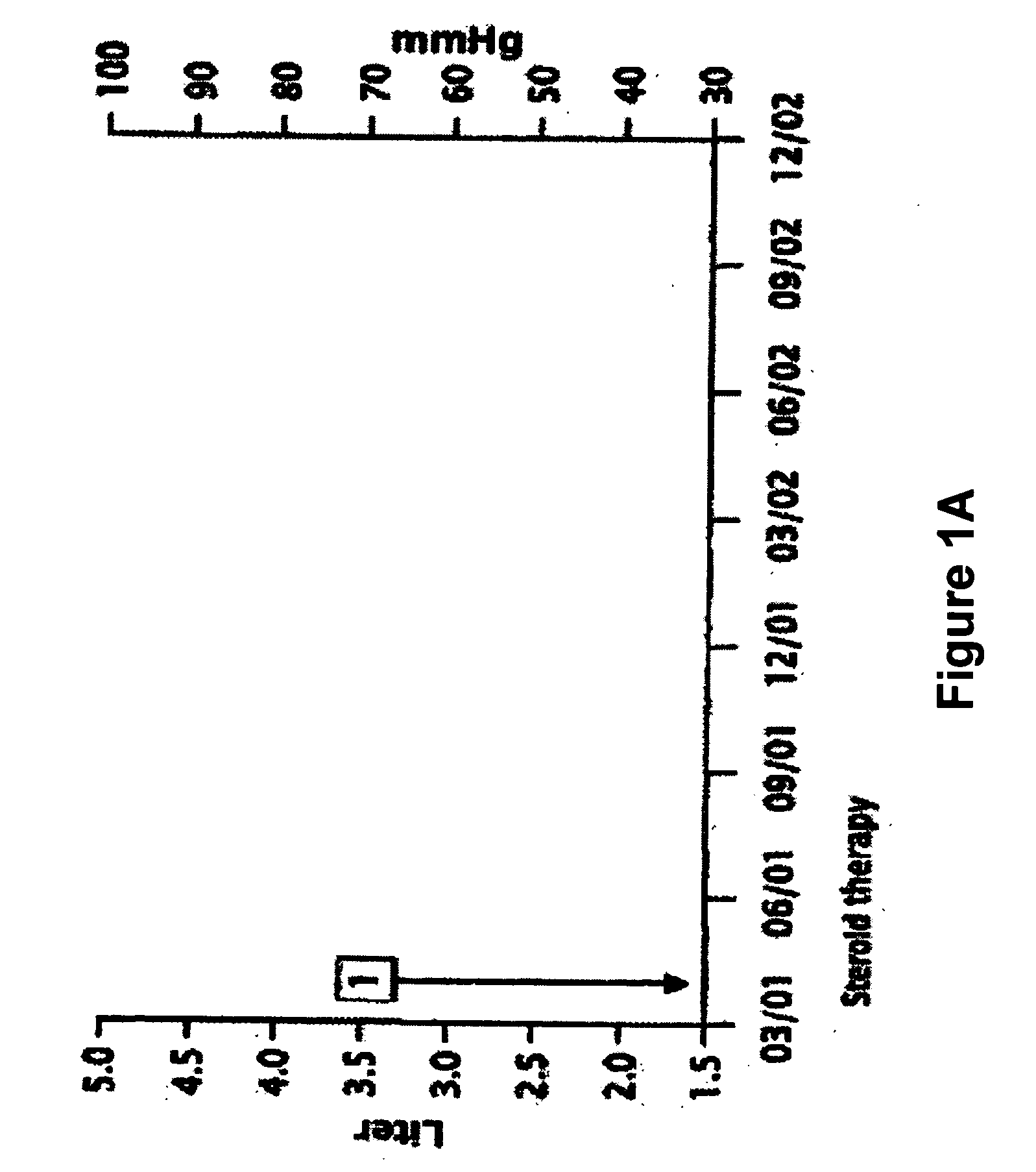
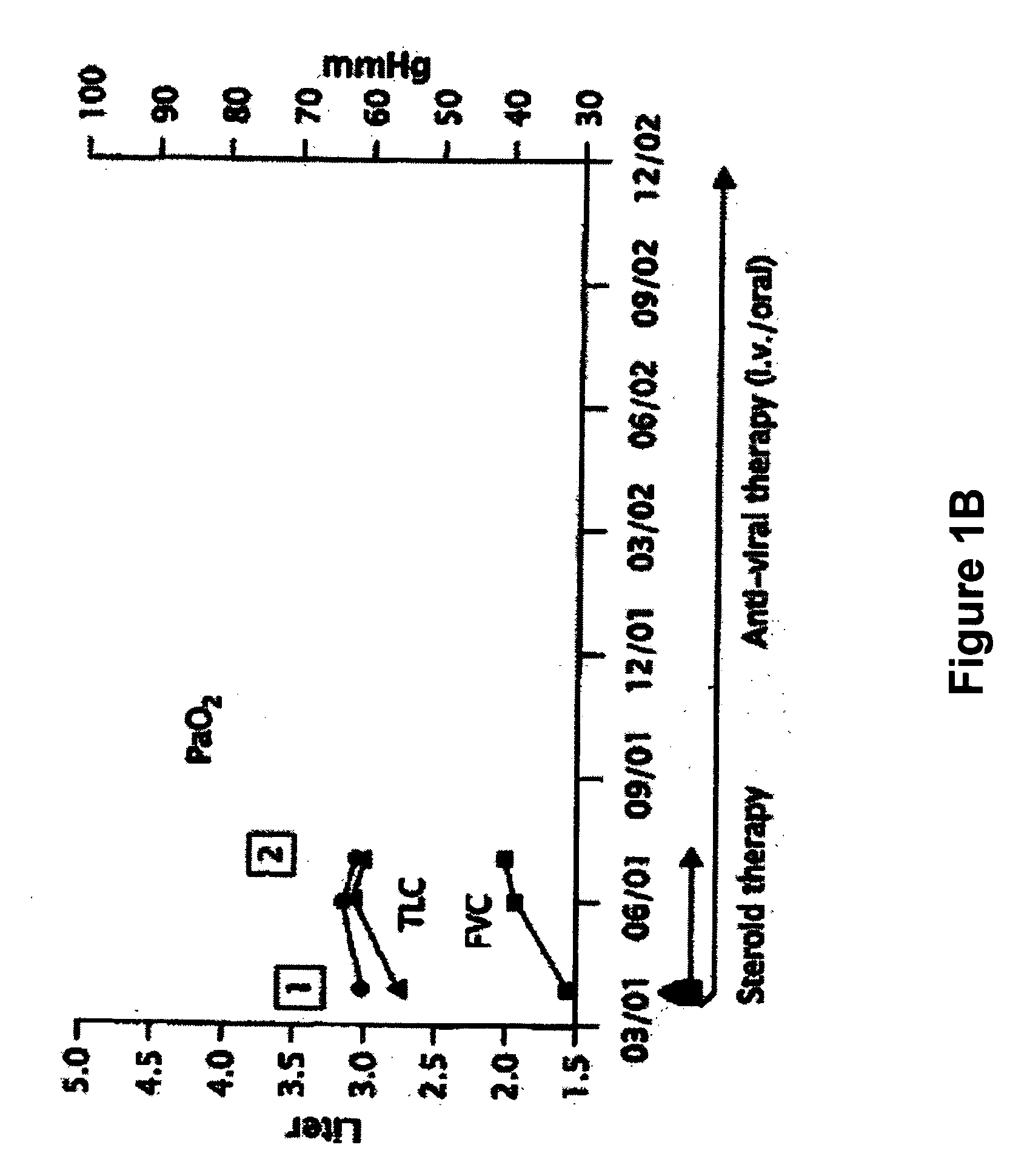
Novel pharmaceutical composition of interferon gamma or pirfenidone with molecular diagnostics for the improved treatment of interstitial lung diseases
InactiveUS20090142301A1Bioreactor/fermenter combinationsBiocideInterstitial lung diseaseInterferon alpha
The present invention relates to a novel pharmaceutical composition of compounds having the biological activity of interferon gamma (IFN-γ) or pirfenidone in combination with a diagnostic array of candidate polynucleotides for the improved treatment of all forms of interstitial lung disease, in particular of idiopathic pulmonary fibrosis (IPF).
Owner:MONDOBIOTECH AG
Single domain antibodies directed against interferron-gamma and uses therefor
InactiveUS20060034833A1Improve breathabilityImmunoglobulins against blood coagulation factorsImmunoglobulins against cytokines/lymphokines/interferonsSingle-domain antibodyInterferon gamma
The present invention relates to polypeptides derived from single domain heavy chain antibodies directed to interferon gamma. It further relates to single domain antibodies that are Camelidae VHHs. It further relates to methods of administering said polypeptides. It further relates to protocols for screening for agents that modulate the IFN-gamma receptor, and the agents resulting from said screening.
Owner:BEIRNAERT ELS
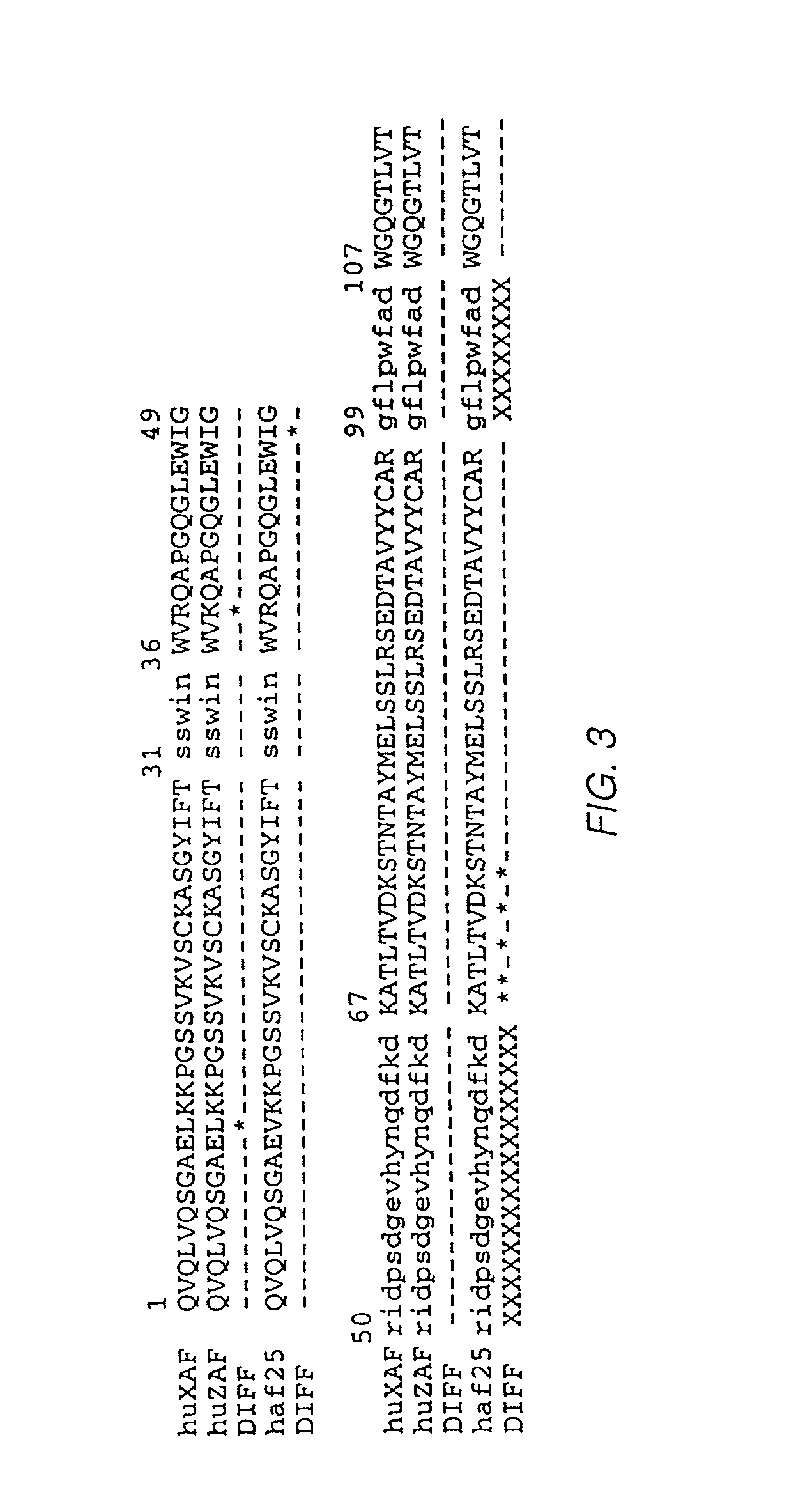
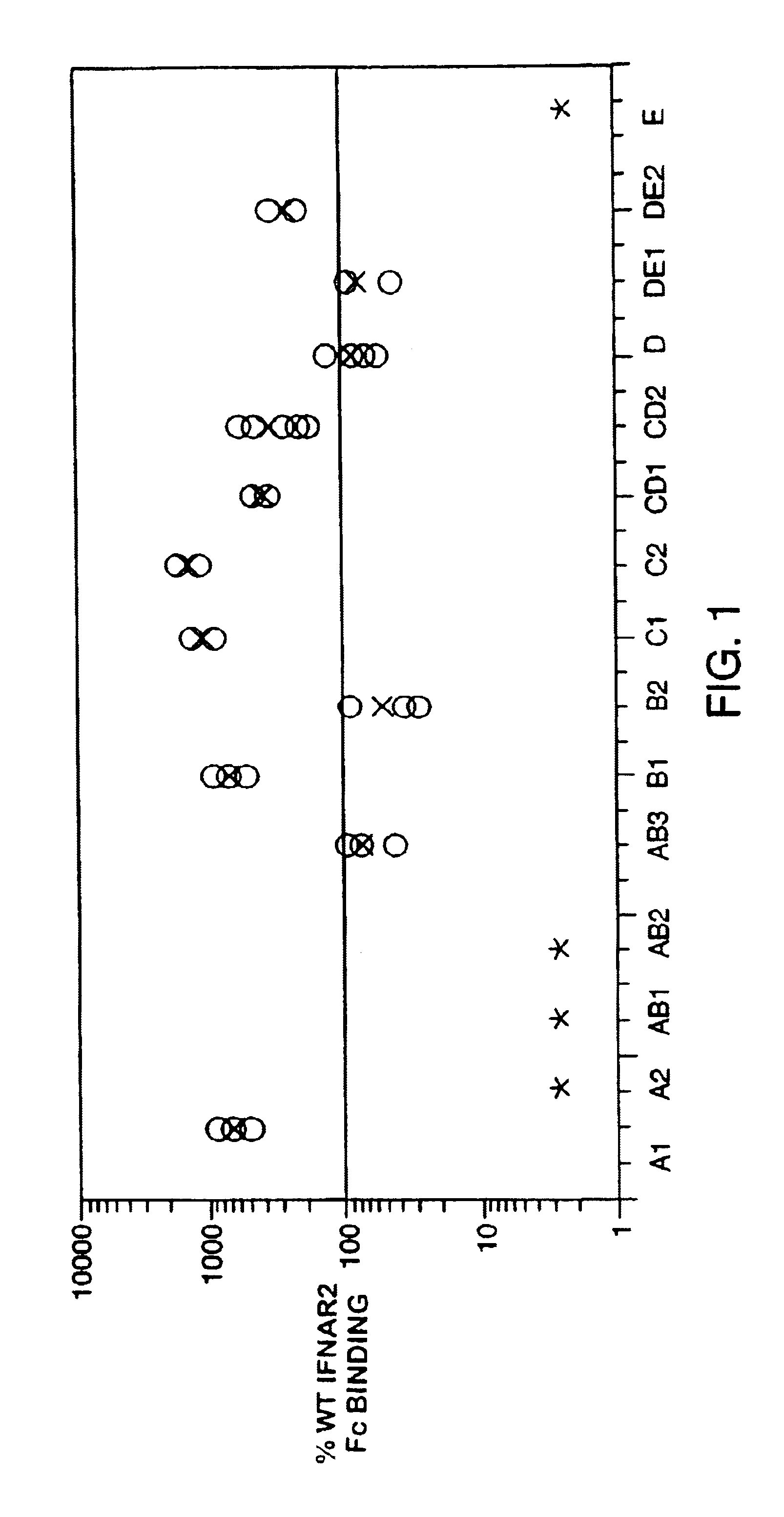
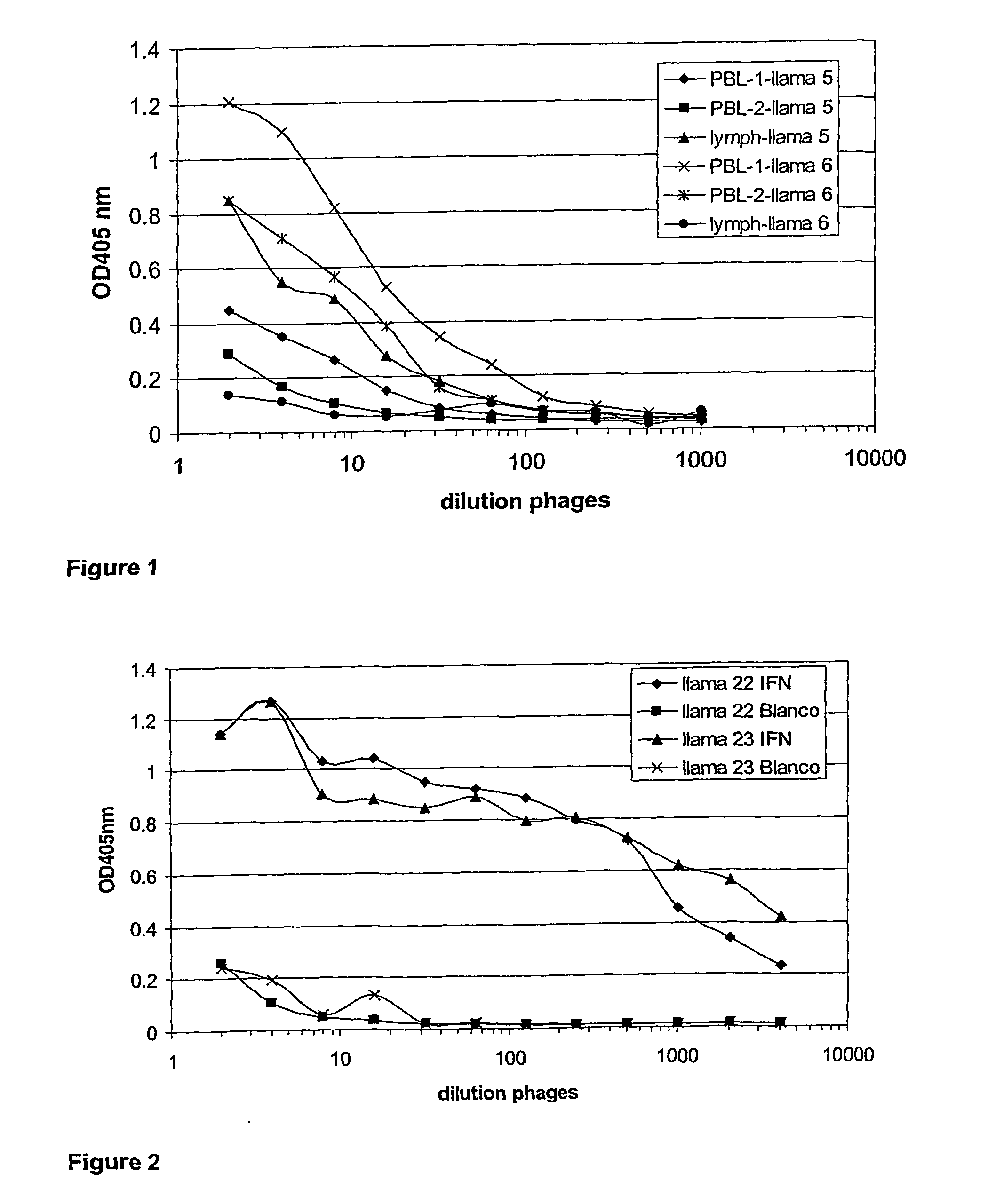
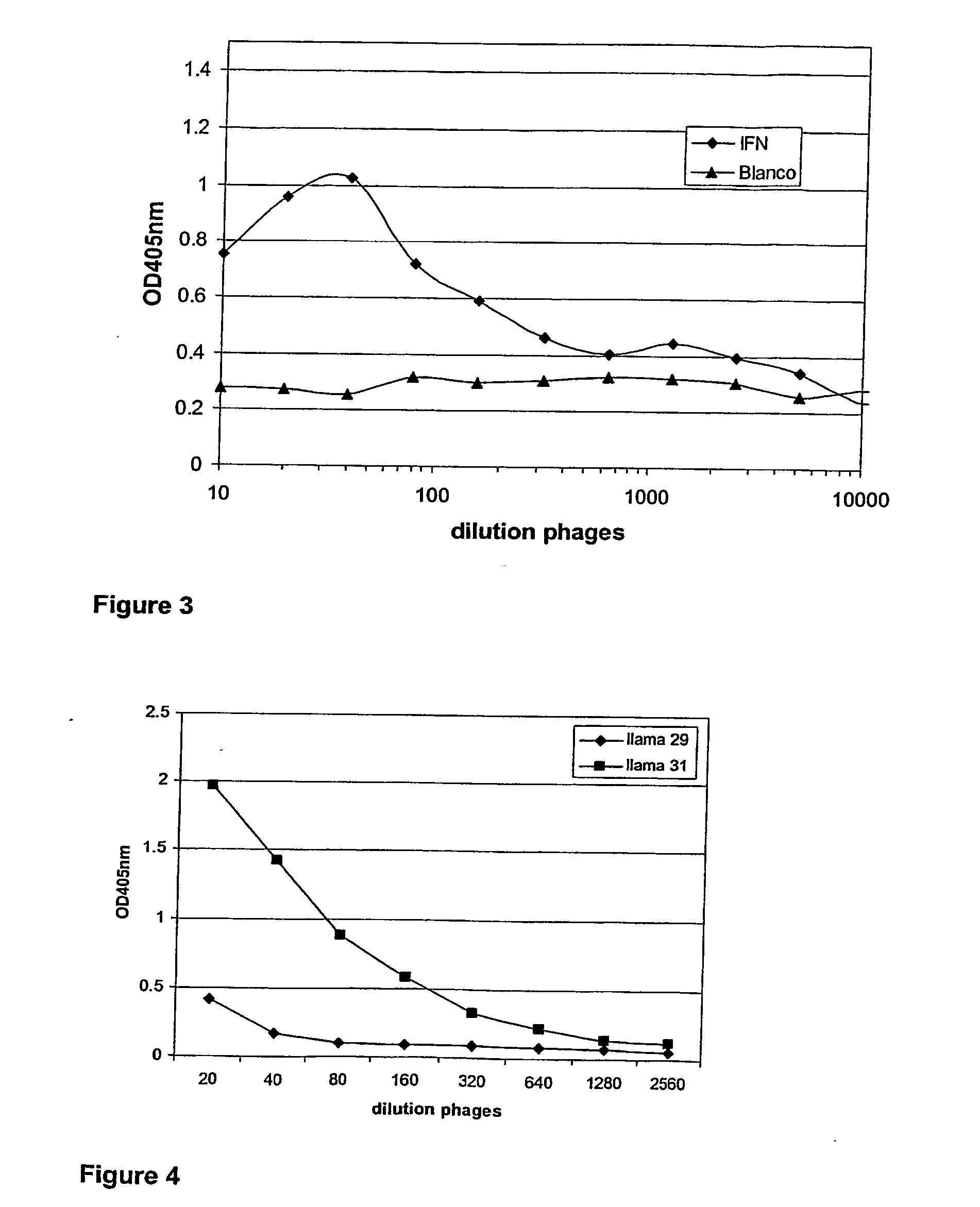
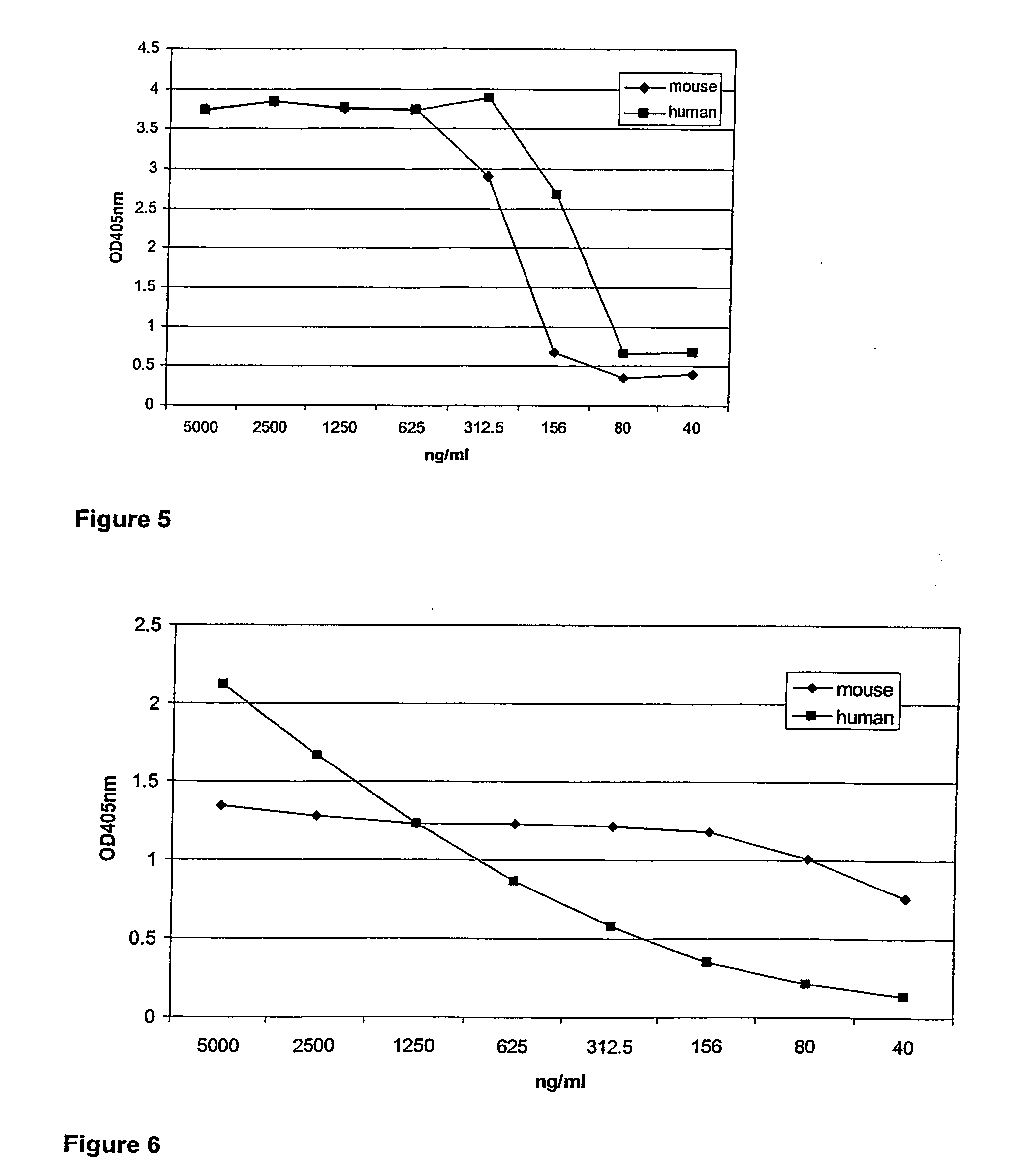
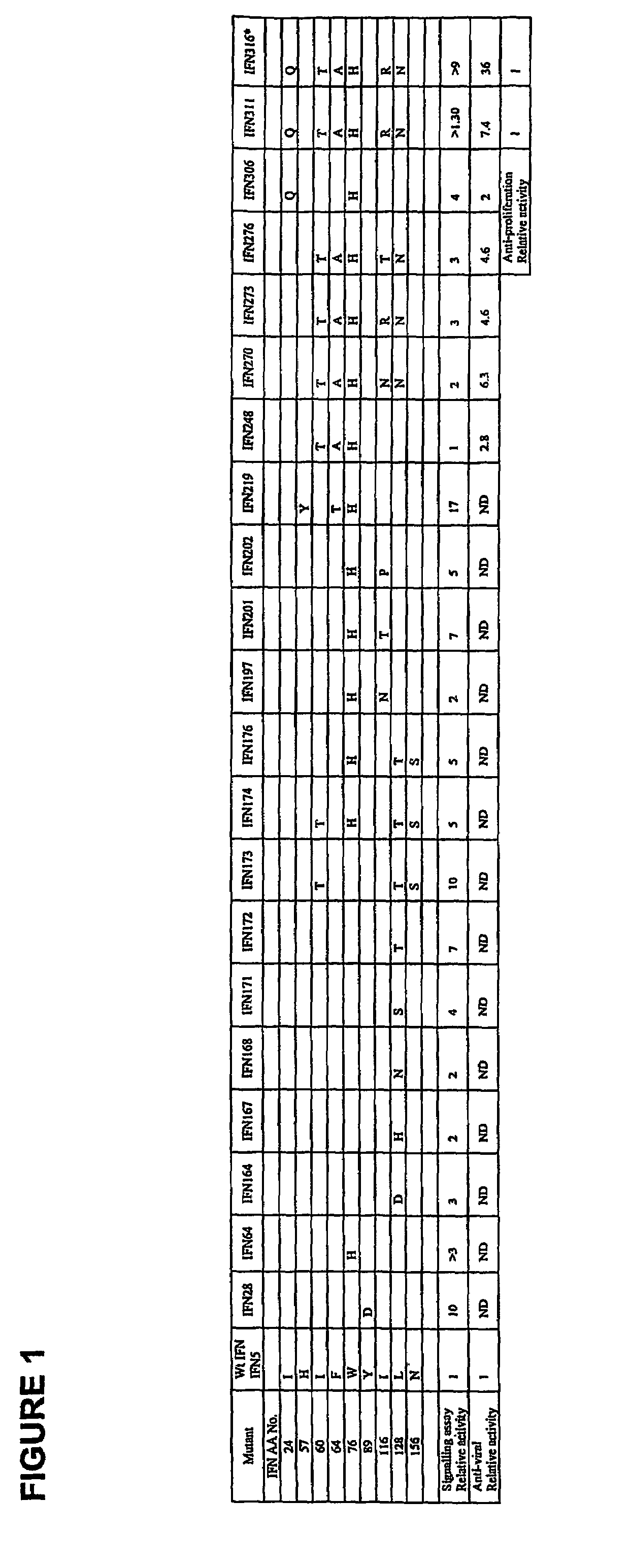
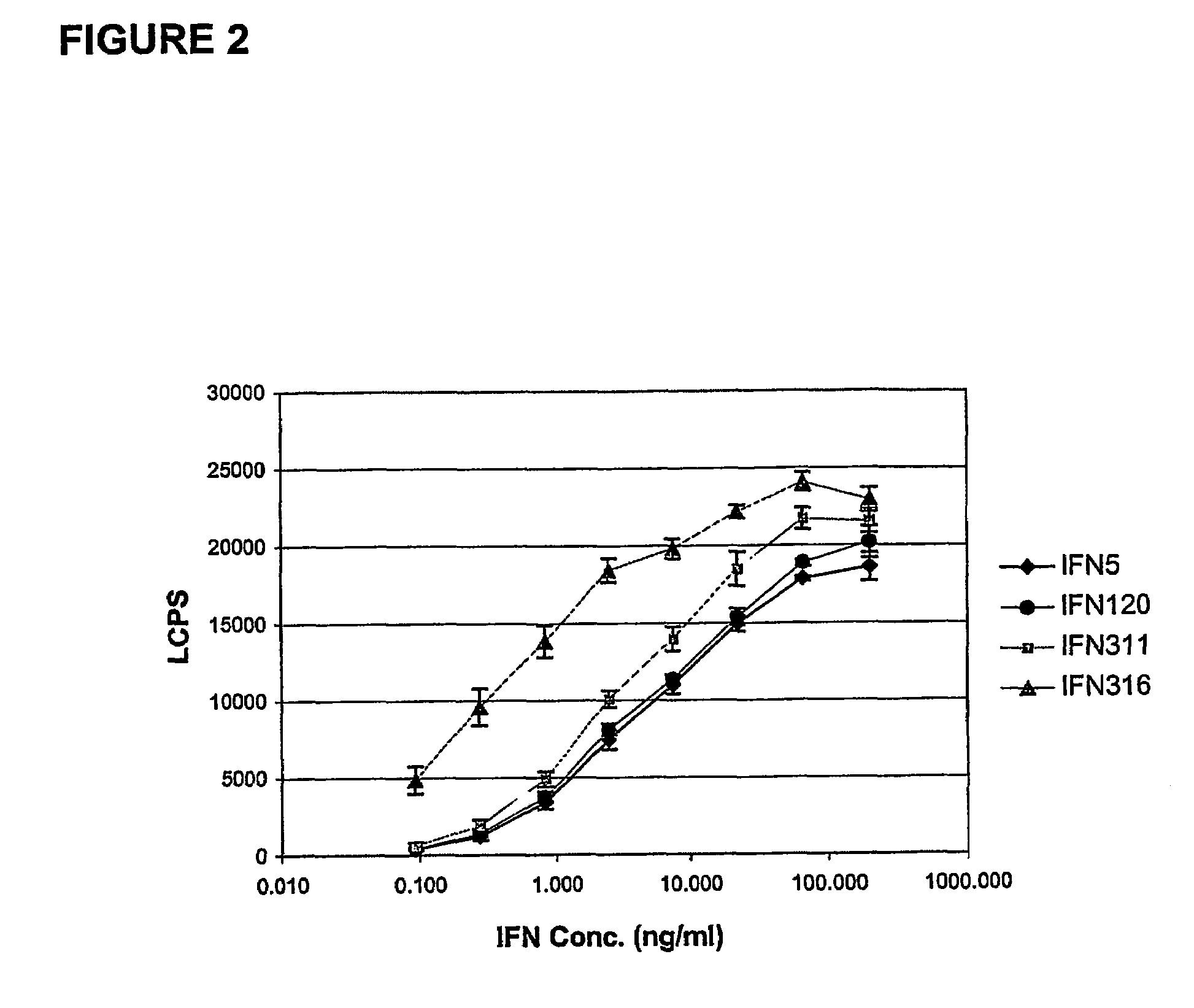
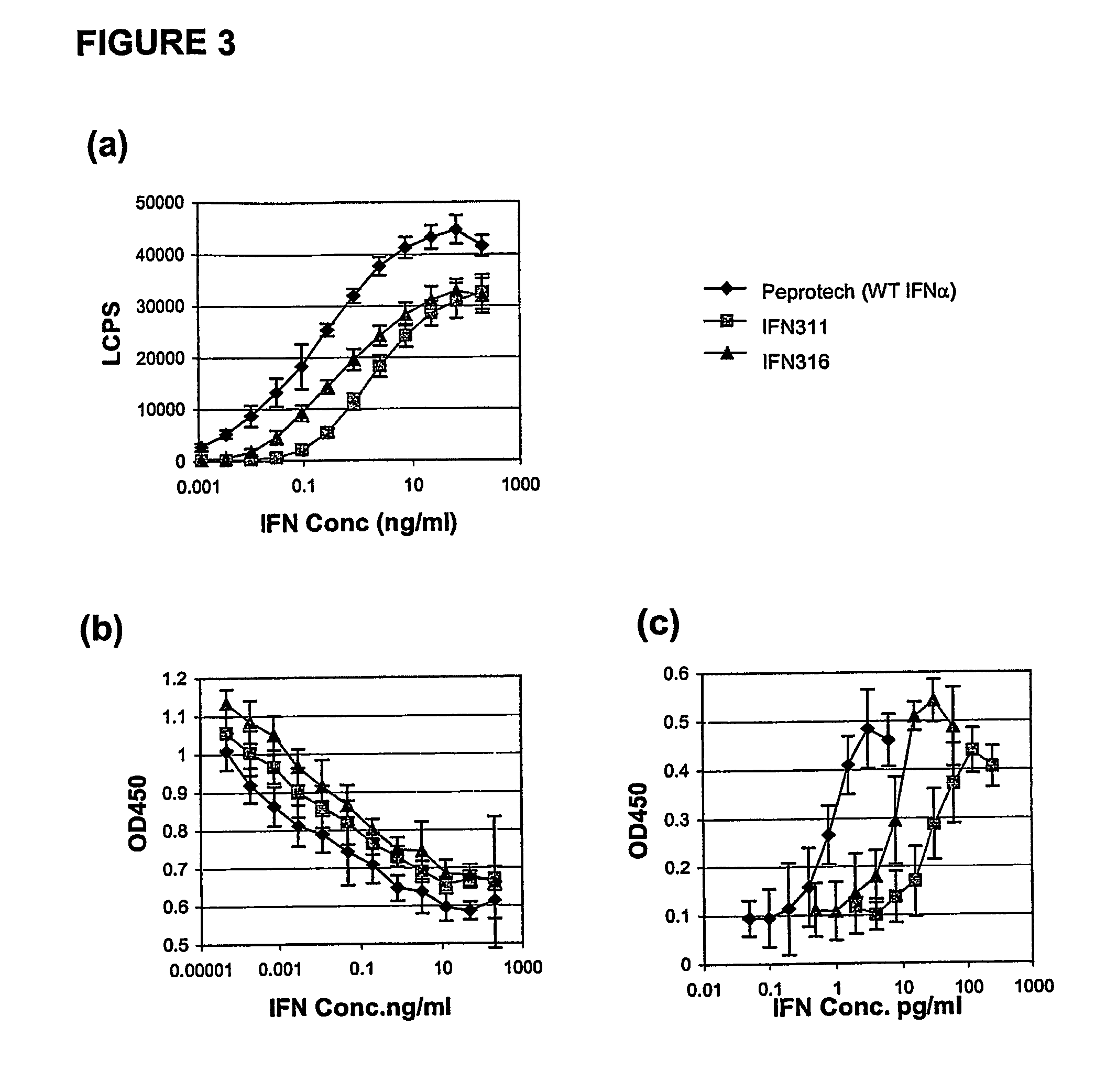
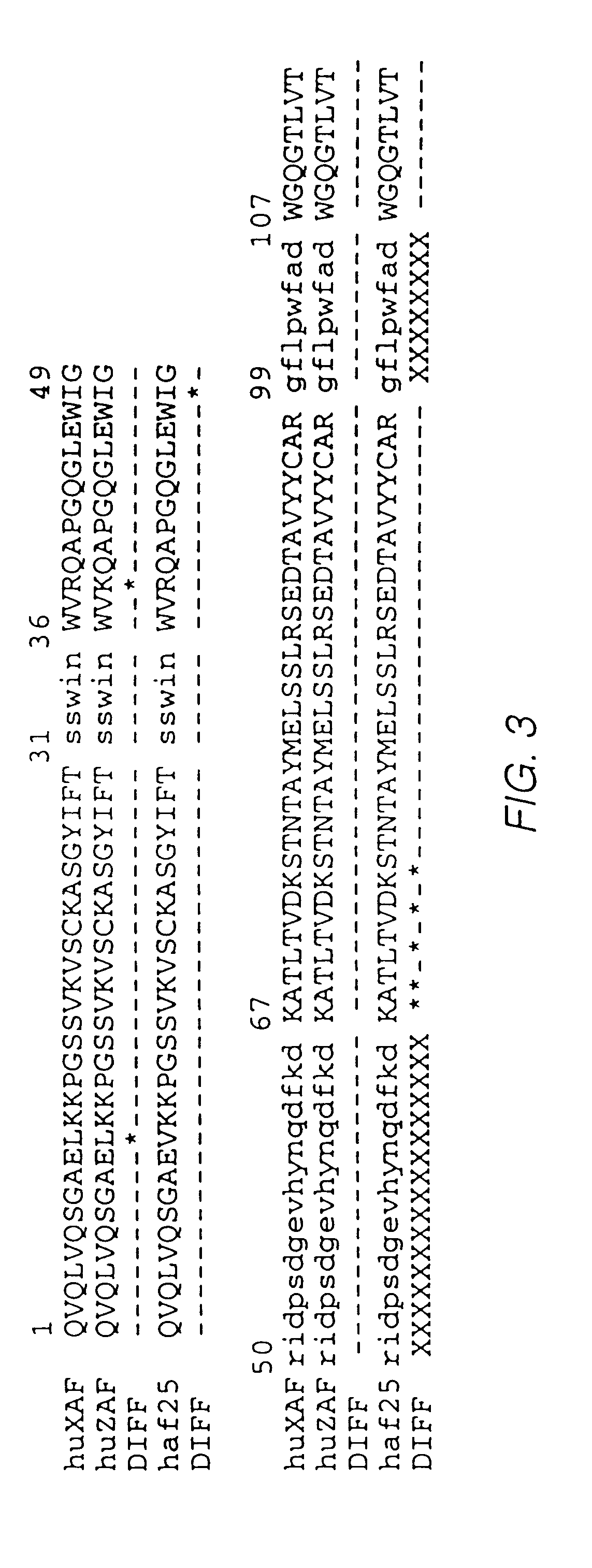
Fusion proteins of interferon alpha muteins with improved properties
InactiveUS7456257B2Low immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseMutated protein
The invention concerns human interferon alpha and in particular modified forms of interferon alpha 2 with improved properties. The improved proteins contain amino acid substitutions at specific positions that confer increased relative activity in biological assays. The invention provides also modified interferon alpha with improved biological activity concomitant with reduced immunogenic potential in the protein. The improved proteins are intended for therapeutic use in the treatment of diseases in humans.
Owner:MERCK PATENT GMBH
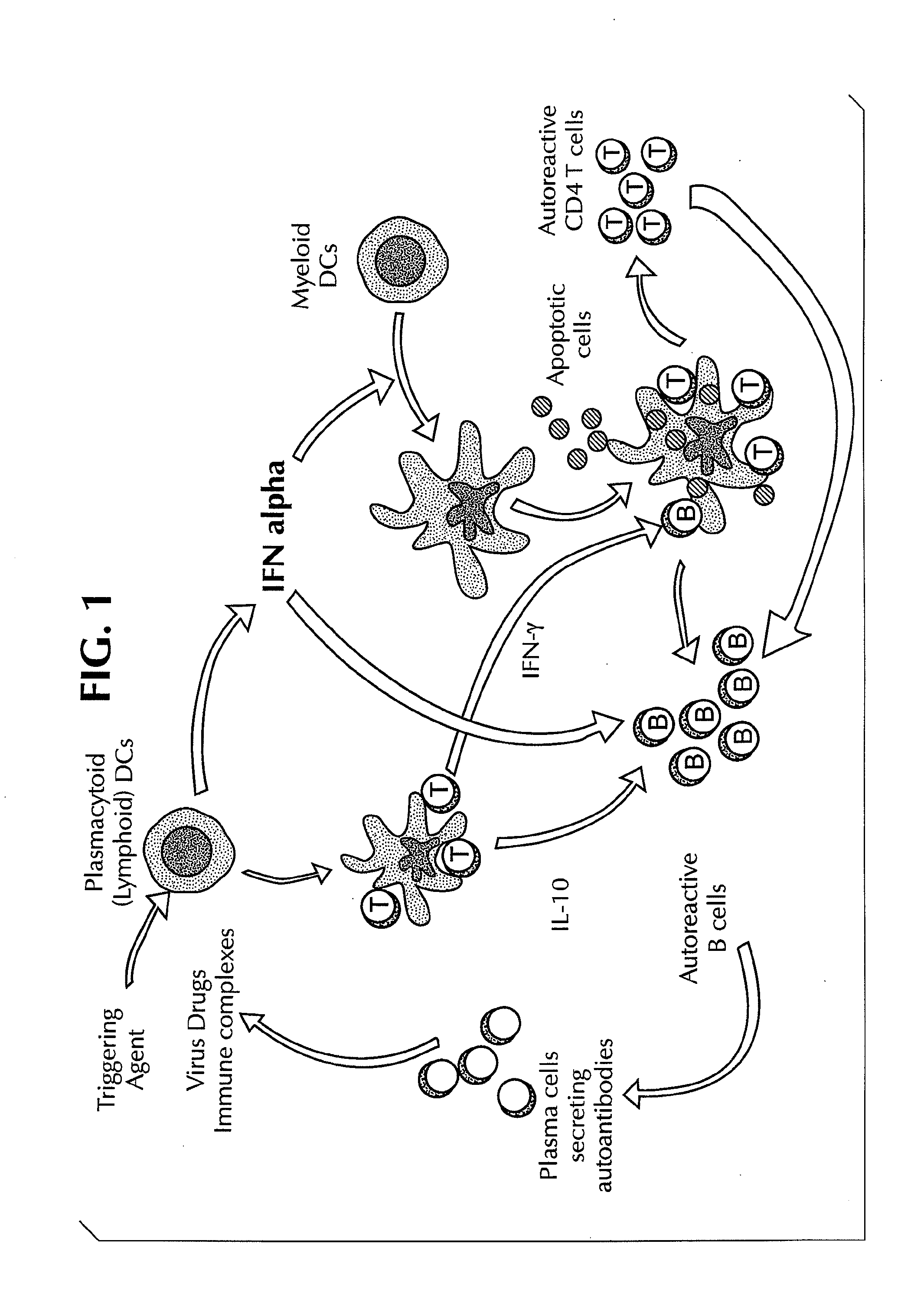
Methods for Treating Autoimmune Diseases in a Subject and In Vitro Diagnostic Assays
InactiveUS20090263474A1Organic active ingredientsPeptide/protein ingredientsDiseaseAutoimmune responses
The invention provides a method for treating an autoimmune disease in a subject by administering an interferon antagonist and a Flt3 ligand (Flt3L) antagonist. The invention also provides compositions containing one or more interferon antagonists, and one or more Flt3L antagonists, an in vitro assay for determining a subject's risk for developing an autoimmune disease, and kits for use, inter alia, with the assay.
Owner:BANCHEREAU JACQUES F +2
Modified interferon beta with reduced immunogenicity
InactiveUS20050054052A1Extended circulation timeImproved propertyPeptide/protein ingredientsSerum albuminBiochemistryHuman Interferon Beta
The present invention relates to polypeptides to be administered especially to humans and in particular for therapeutic use. The polypeptides are modified polypeptides whereby the modification results in a reduced propensity for the polypeptide to elicit an immune response upon administration to the human subject. The invention in particular relates to the modification of human interferon beta to result in proteins that are substantially non-immunogenic or less immunogenic than any non-modified counterpart when used in vivo.
Owner:MERCK PATENT GMBH
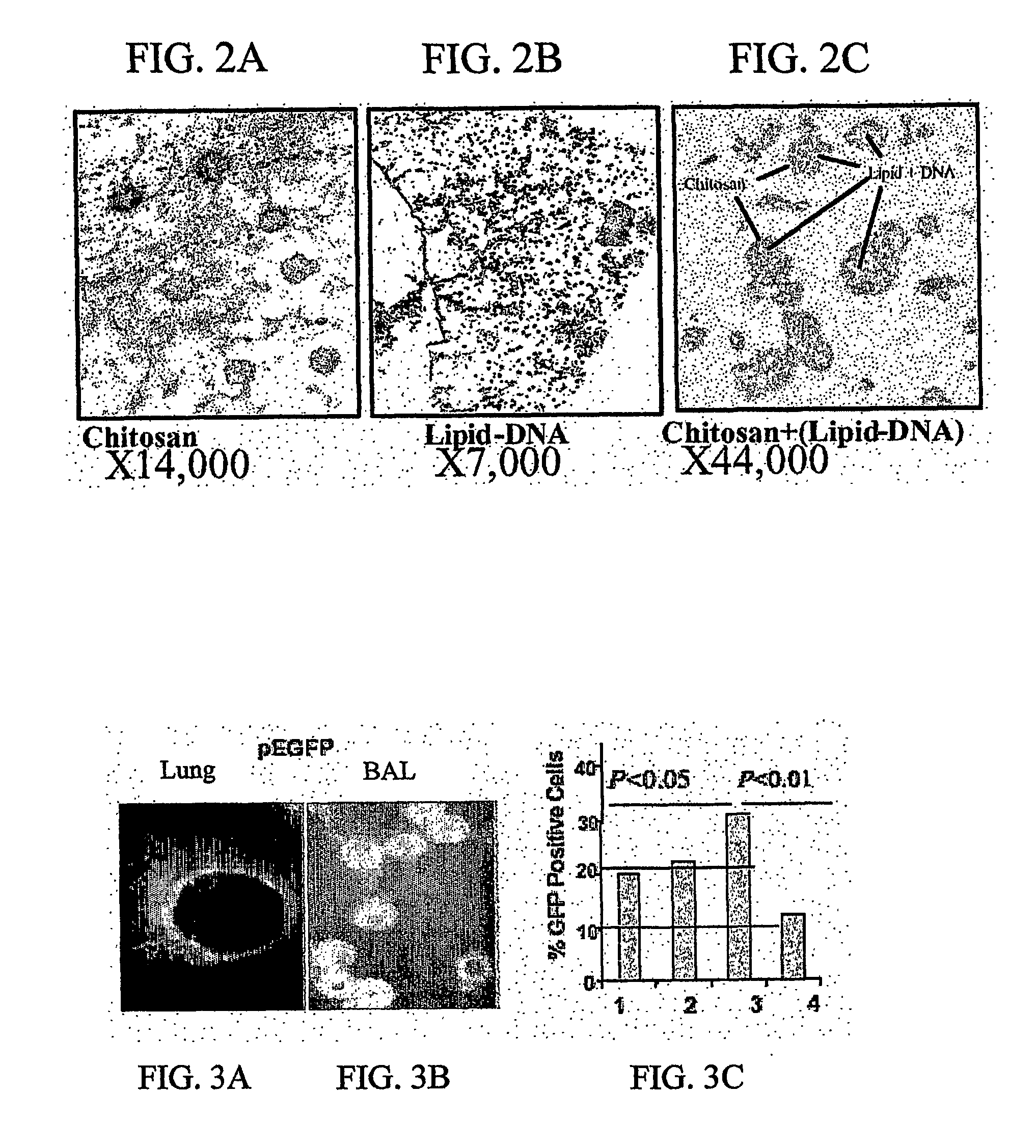
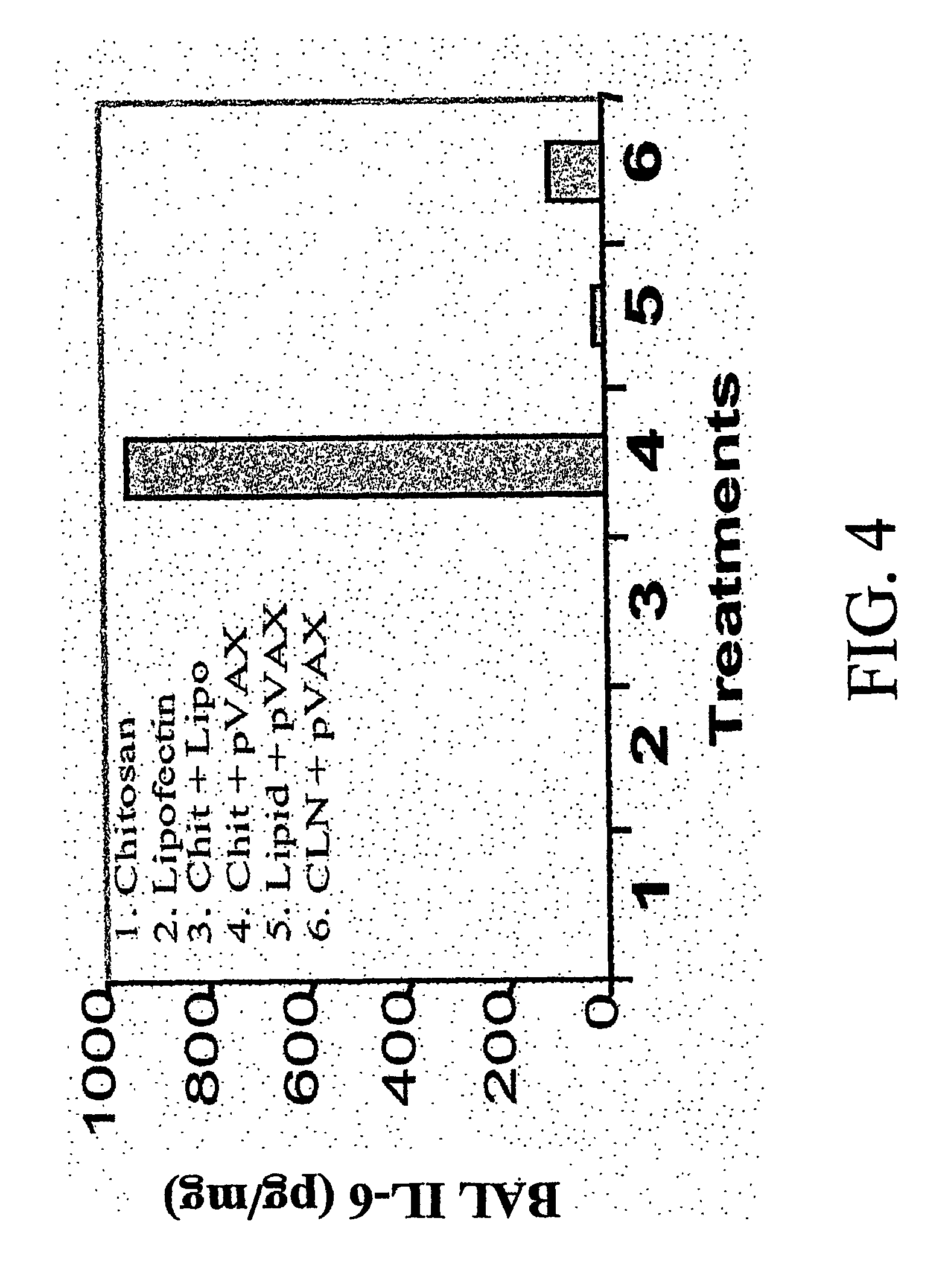
Chitosan-microparticles for ifn gene delivery
InactiveUS20070116767A1Enhancing interferon-gamma expressionProduction process specificationPowder deliveryBiocideGene deliveryDelivery vehicle
The present invention provides particles comprising chitosan, or a derivative thereof, useful as delivery vehicles for polynucleotides encoding polypeptides, compositions comprising such particles and a pharmaceutically acceptable carrier, and methods for delivering polynucleotides using such particles. Optionally, the particles of the invention further comprise a lipid component. The present invention further provides a method for enhancing interferon-gamma expression to regulate the production of cytokines secreted by T-helper type 2 (Th2) cells within a subject by administering an effective amount of a particle of the subject invention to the subject, wherein the particle comprises a polynucleotide encoding interferon-gamma.
Owner:UNIV OF SOUTH FLORIDA
Methods of treating gastrointestinal inflammation
InactiveUS20070004654A1Improve the level ofBiocidePeptide/protein ingredientsGastrointestinal inflammationCrohn's disease
The present invention provides methods of treating gastrointestinal inflammation, methods of treating inflammatory bowel disease, methods of treating Crohn's Disease, and methods of treating ulcerative colitis in an individual. The methods generally involve administering an effective amount of an agent that increases the level of a Type I interferon and / or that activates a Type I interferon signaling pathway in the individual.
Owner:RGT UNIV OF CALIFORNIA
Anti-interferon alpha antibodies
Owner:ER SQUIBB & SONS INC
Application of diamine formyl dehydrogenated silybin serving as medicament for curing viral hepatitis B
InactiveCN101829090AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
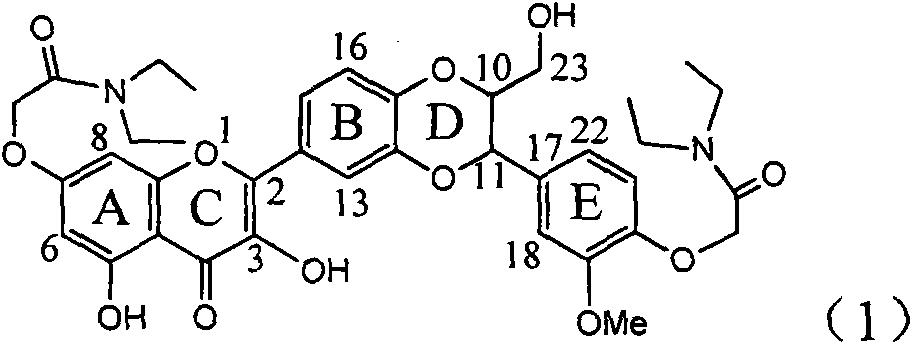
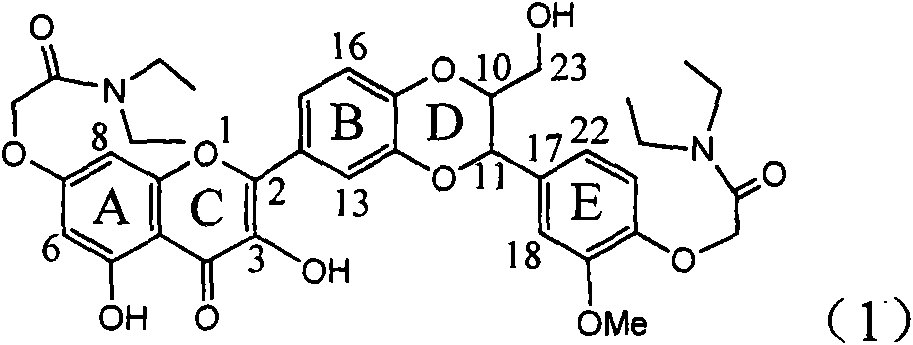
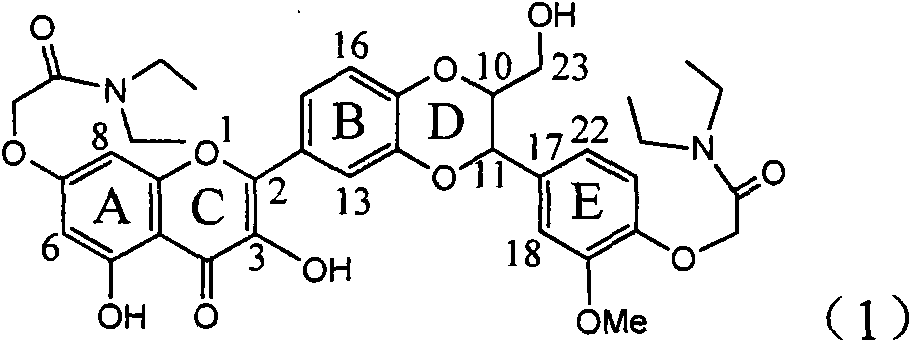
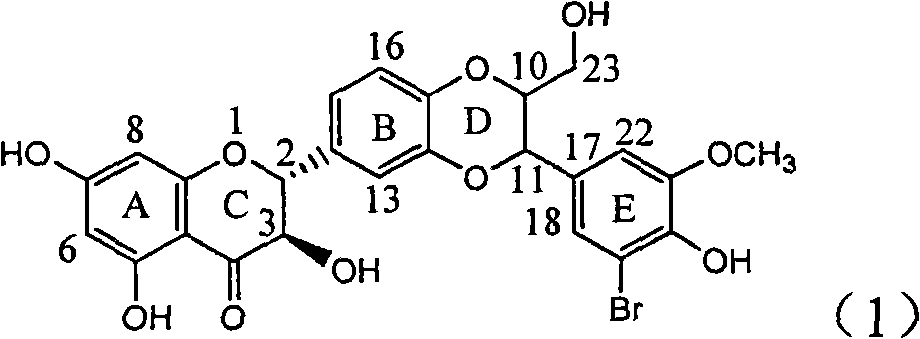
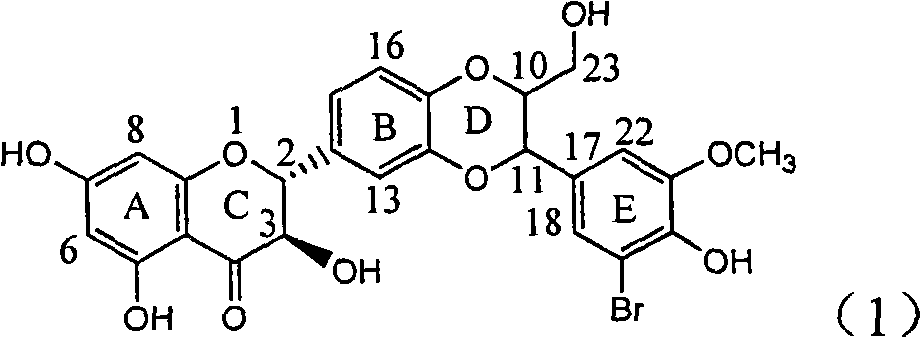
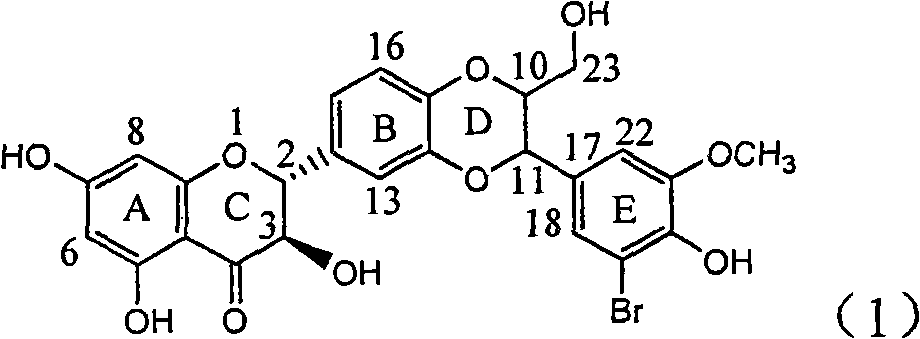
The invention relates to application of diamine formyl dehydrogenated silybin serving as a medicament for curing viral hepatitis B, in particular to application of a flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or pharmaceutically acceptable salts thereof in preparation of a medicament for clearing HBsAg and HBeAg and a medicament for inhibiting HBV DNA replication. The flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents has extremely high HBsAg and HBeAg inhibiting activities; when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at a concentration of 20 mu g / ml, the inhibition rates of the HBsAg and the HBeAg are respectively 94.4 percent and 95.7 percent which exceed 5.9 times and 5.7 times those of a positive control alpha-interferon; and simultaneously the inhibition rate of the HBV DNA is 99.7 percent when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at the same concentration, and the inhibition activity of the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is higher than that of lamivudine and the alpha-interferon. In summary, the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or the pharmaceutically acceptable salts thereof can be expected for preparing non-nucleoside medicaments for clearing the HBsAg and the HBeAg, inhibiting the HBV DNA replication, and curing the hepatitis B virus infection diseases.
Owner:DALI UNIV
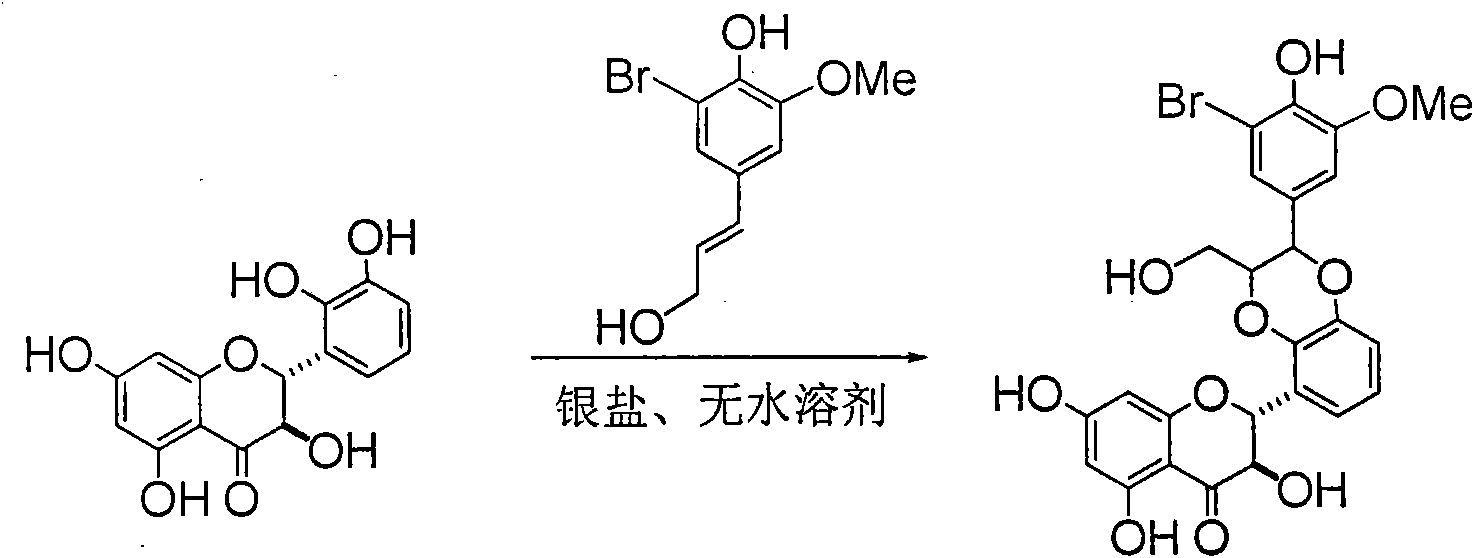
Preparation of brominated flavanonollignan and application in medicine for treating viral hepatitis B
InactiveCN101955478AConvenient sourceThe source is easy to getOrganic active ingredientsOrganic chemistryPositive controlInterferon alpha
The invention relates to the preparation of brominated flavanonollignan and an application in medicines for treating viral hepatitis B, in particular to a B cyclo-dioxane flavanonollignan compound and a preparation method thereof as well as the application of the compound or pharmaceutically acceptable salts thereof in the preparation of medicines for eliminating hepatitis B surface antigens (HBsAg) and hepatitis B e antigens (HBeAg) and medicines for inhibiting HBV DNA replication. The compound has obvious activity of inhibiting HBsAg and HBeAg, and the intensities of the compound for eliminating HBsAg and HBeAg under the concentration of 20 microgram / millimeter are respectively 2.1 times and 1.2 times larger than the corresponding activity of a positive control medicine alpha-interferon; meanwhile, the compound displays high inhibition ratio more than 57% on HBV DNA at the concentration. The results show that the favonolignan or pharmaceutically acceptable salts thereof can be expected to be used for preparing non-nucleoside type medicines for eliminating HBsAg and HBeAg, inhibiting HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
Adsorbent for eliminating hepatitis c virus, adsorber, and adsorption method
InactiveUS20030044769A1High adsorbing affinityImprove performanceOrganic active ingredientsBiocideInterferon therapySorbent
An adsorbent for removing hepatitis C virus which has the ability to adsorb HCV particles, particularly immune-complex HCV particles, from a patient's body blood safely and with high efficiency and high selectivity for enhancing the efficacy of interferon therapy, an HCV adsorption apparatus including said adsorbent, and a adsorbing method for removing HCV are provided. An adsorbent for removing hepatitis C virus which comprises a compound capable of adsorbing hepatitis C virus as immobilized on a water-insoluble carrier, an adsorption apparatus including said adsorbent, and an adsorbing method for removing HCV.
Owner:KANEKA CORP
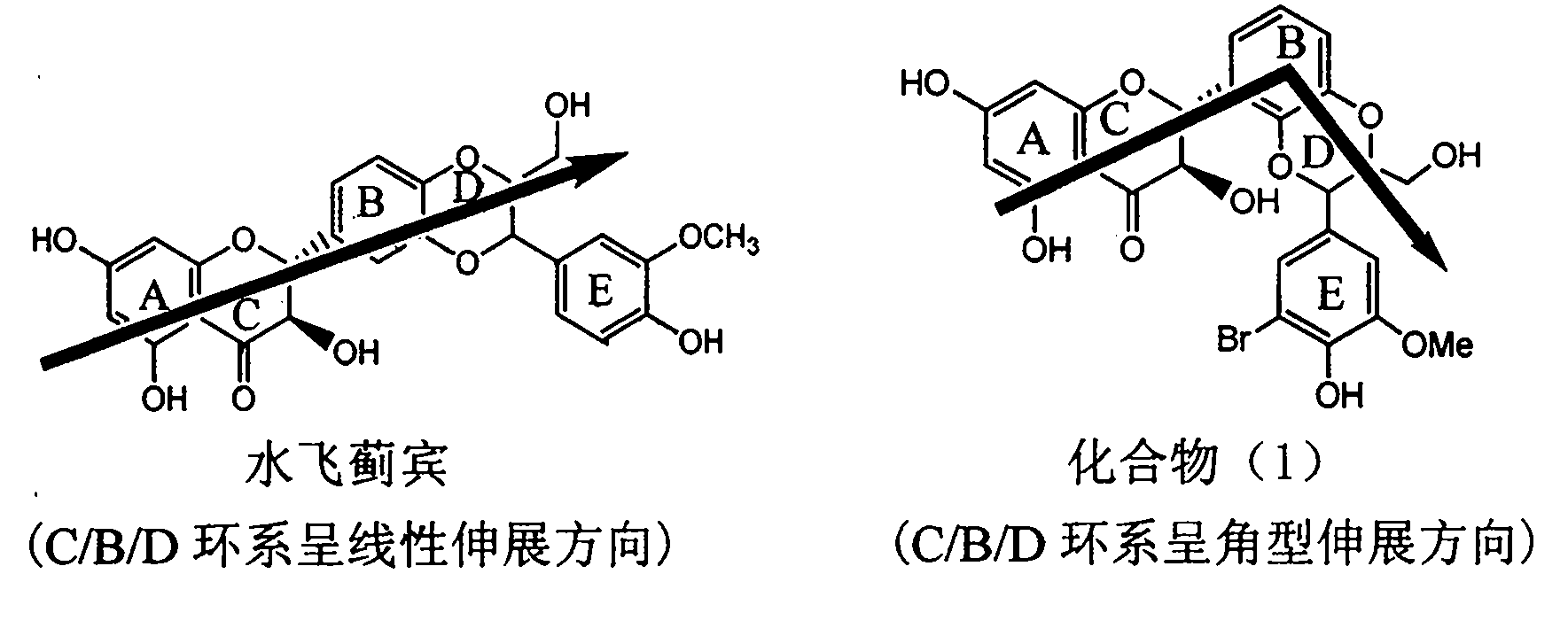
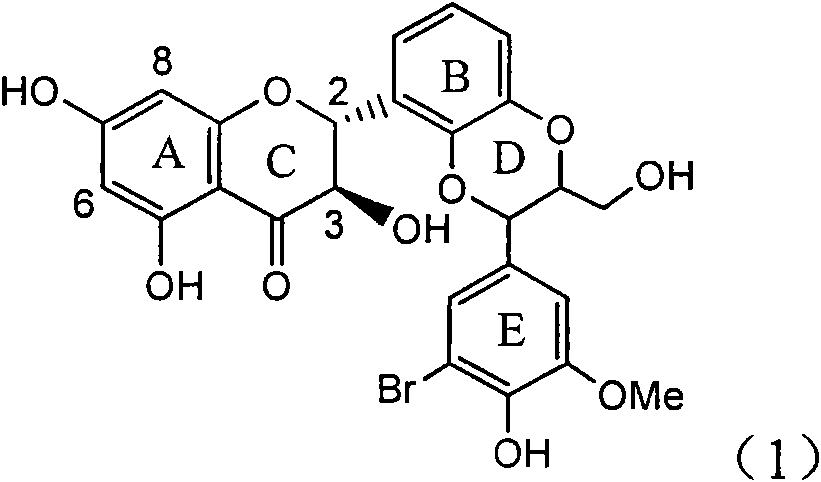
Application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B
InactiveCN101829094AInhibitory activityInhibition of replicative activityOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of ring E bromine substituted silybin in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of a formula (1) and a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B surface antigens (HBsAg) and hepatitis e antigens (HBeAg) and suppressing the HBV (Hepatitis B Virus) DNA replication. The compound has definite activity on suppressing the HBsAg and the HBeAg, and in the presence of a concentration of 100 micrograms / milliliter, the intensities of the compound for clearing away the HBsAg and the HBeAg are respectively 38.2 percent and 39.1 percent which are respectively 2.4 times and 2.3 times of that of a positive control medicament (10,000 units / milliliter of alpha-interferon). Meanwhile, in the presence of the concentration, the suppression ratio of the compound on the HBV DNA is 36 percent which is close to that of the alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically acceptable salt thereof are indicated to be capable of being used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



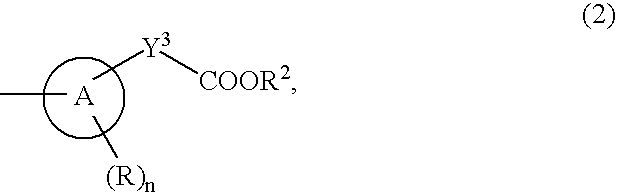
![Oxazolo, thiazolo and selenazolo [4,5-c] quinolin-4-amines and analogs thereof Oxazolo, thiazolo and selenazolo [4,5-c] quinolin-4-amines and analogs thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/74ac572b-d35d-49e9-8ac6-b860065b1a3c/US06323200-20011127-C00001.png)
![Oxazolo, thiazolo and selenazolo [4,5-c] quinolin-4-amines and analogs thereof Oxazolo, thiazolo and selenazolo [4,5-c] quinolin-4-amines and analogs thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/74ac572b-d35d-49e9-8ac6-b860065b1a3c/US06323200-20011127-C00002.png)
![Oxazolo, thiazolo and selenazolo [4,5-c] quinolin-4-amines and analogs thereof Oxazolo, thiazolo and selenazolo [4,5-c] quinolin-4-amines and analogs thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/74ac572b-d35d-49e9-8ac6-b860065b1a3c/US06323200-20011127-C00003.png)