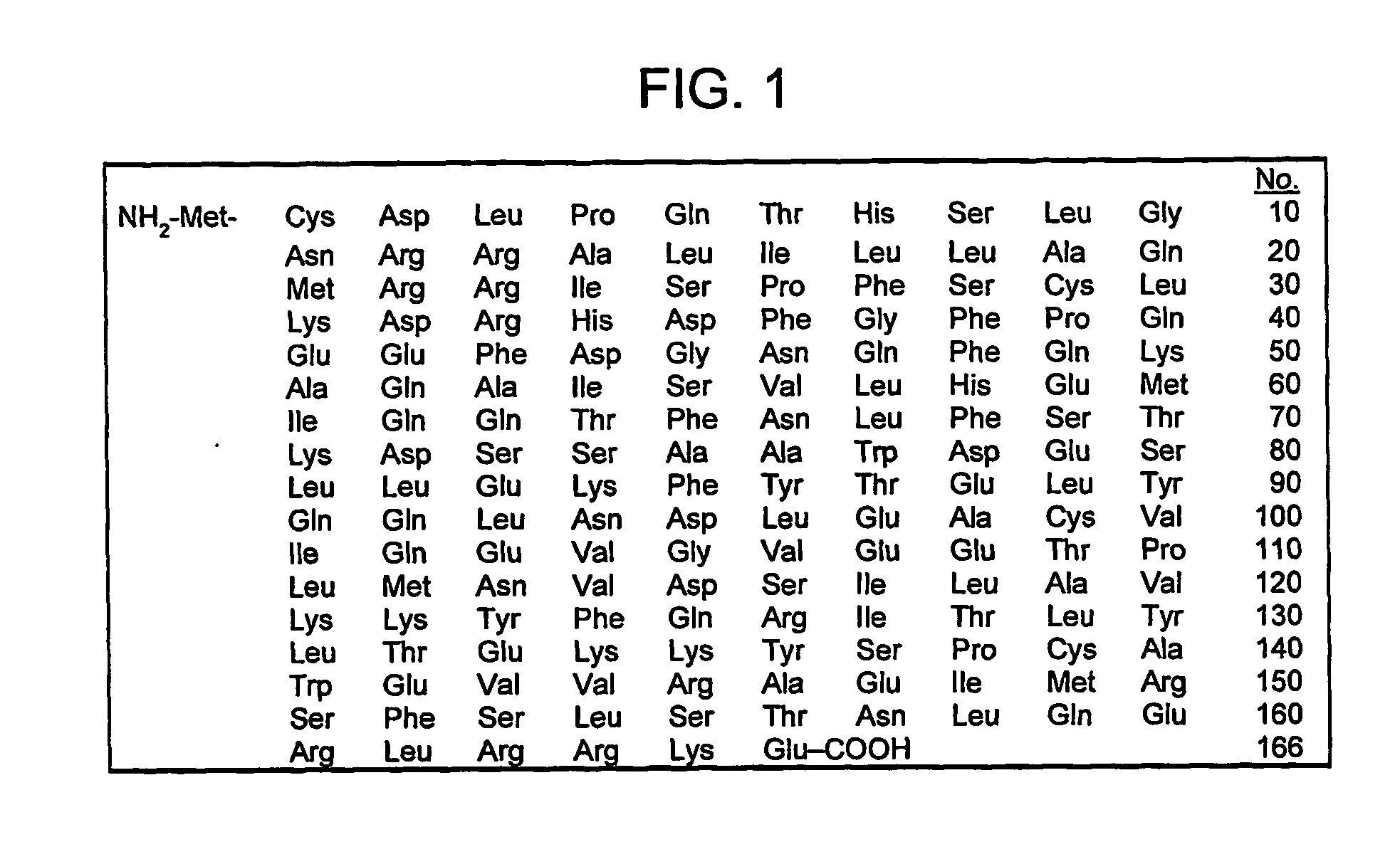
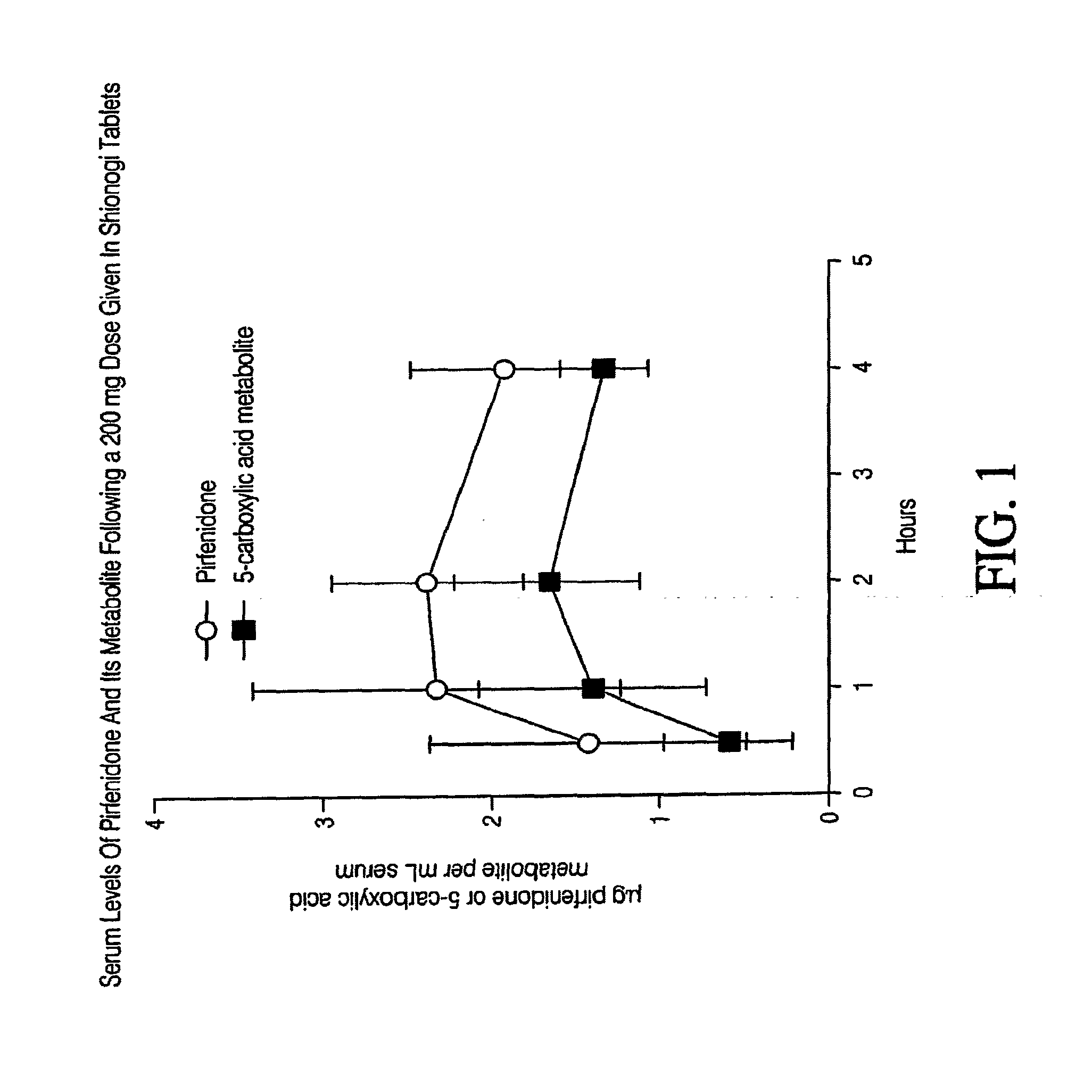
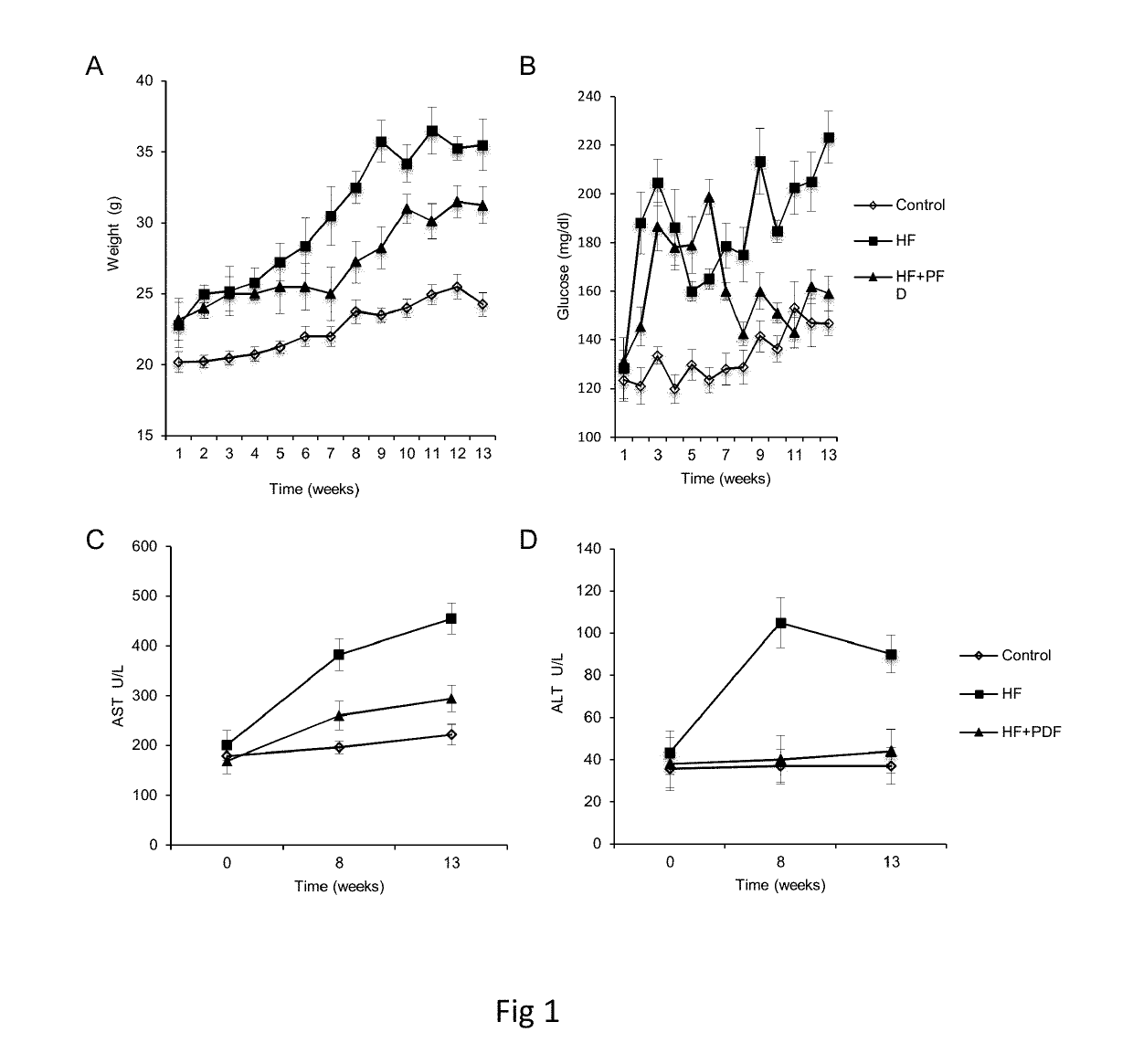
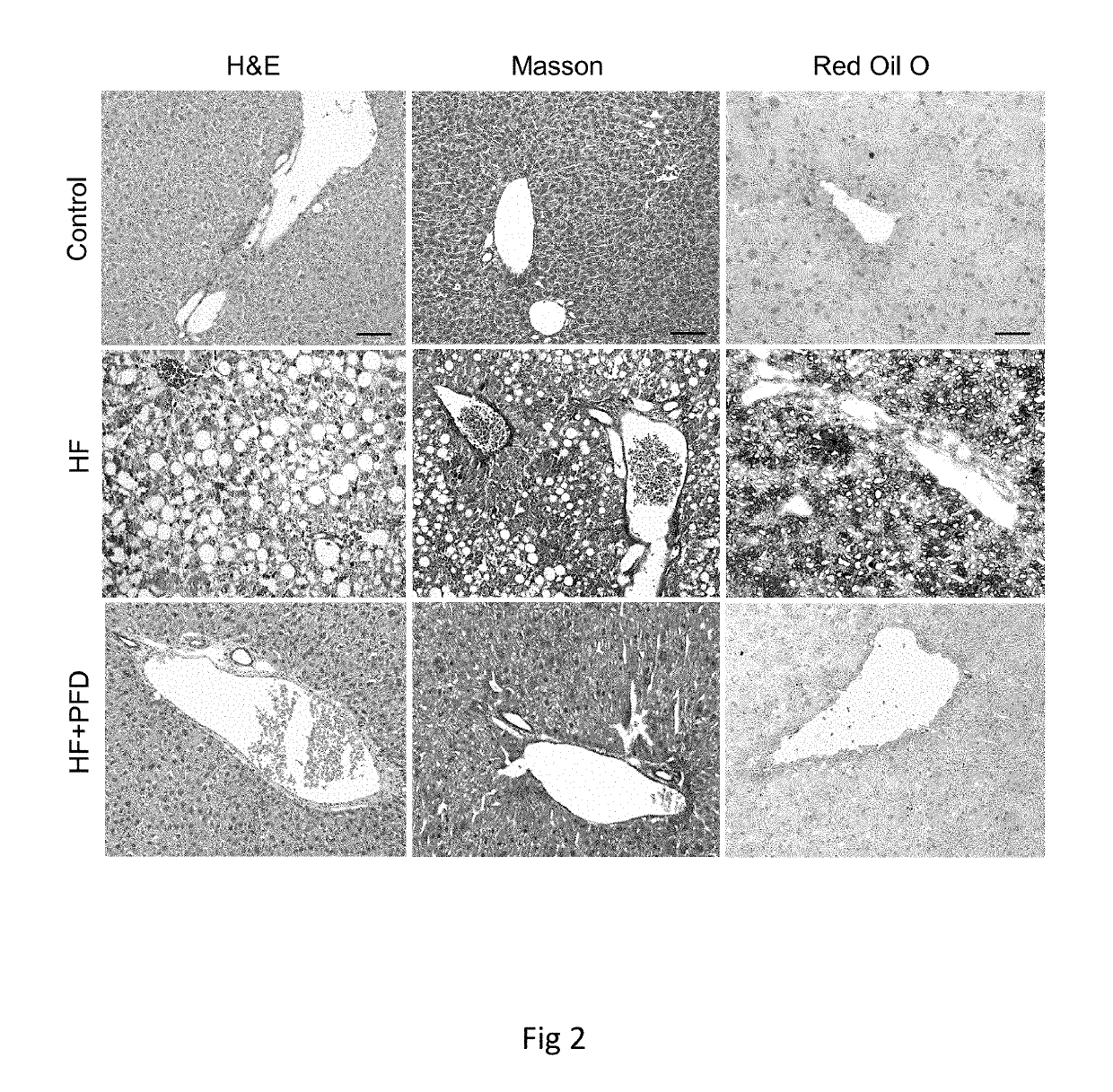
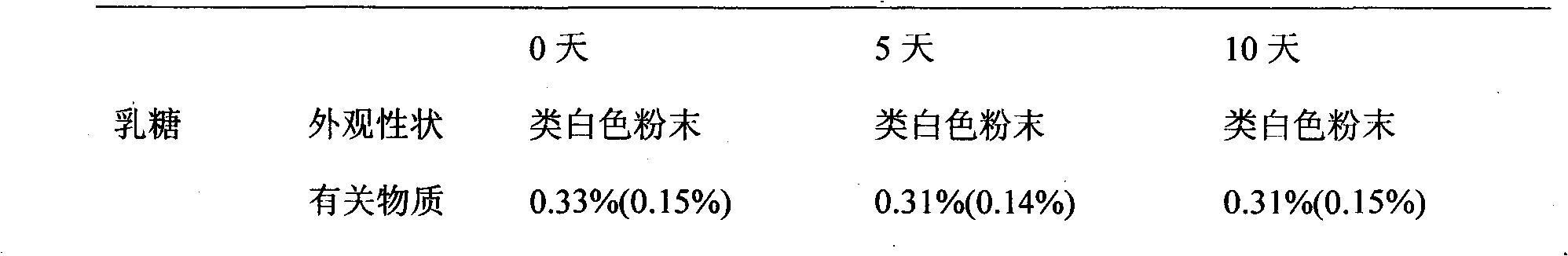
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
138 results about "Pirfenidone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
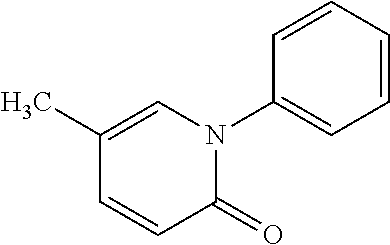
Pirfenidone is used to treat a certain lung disease called idiopathic pulmonary fibrosis (IPF).
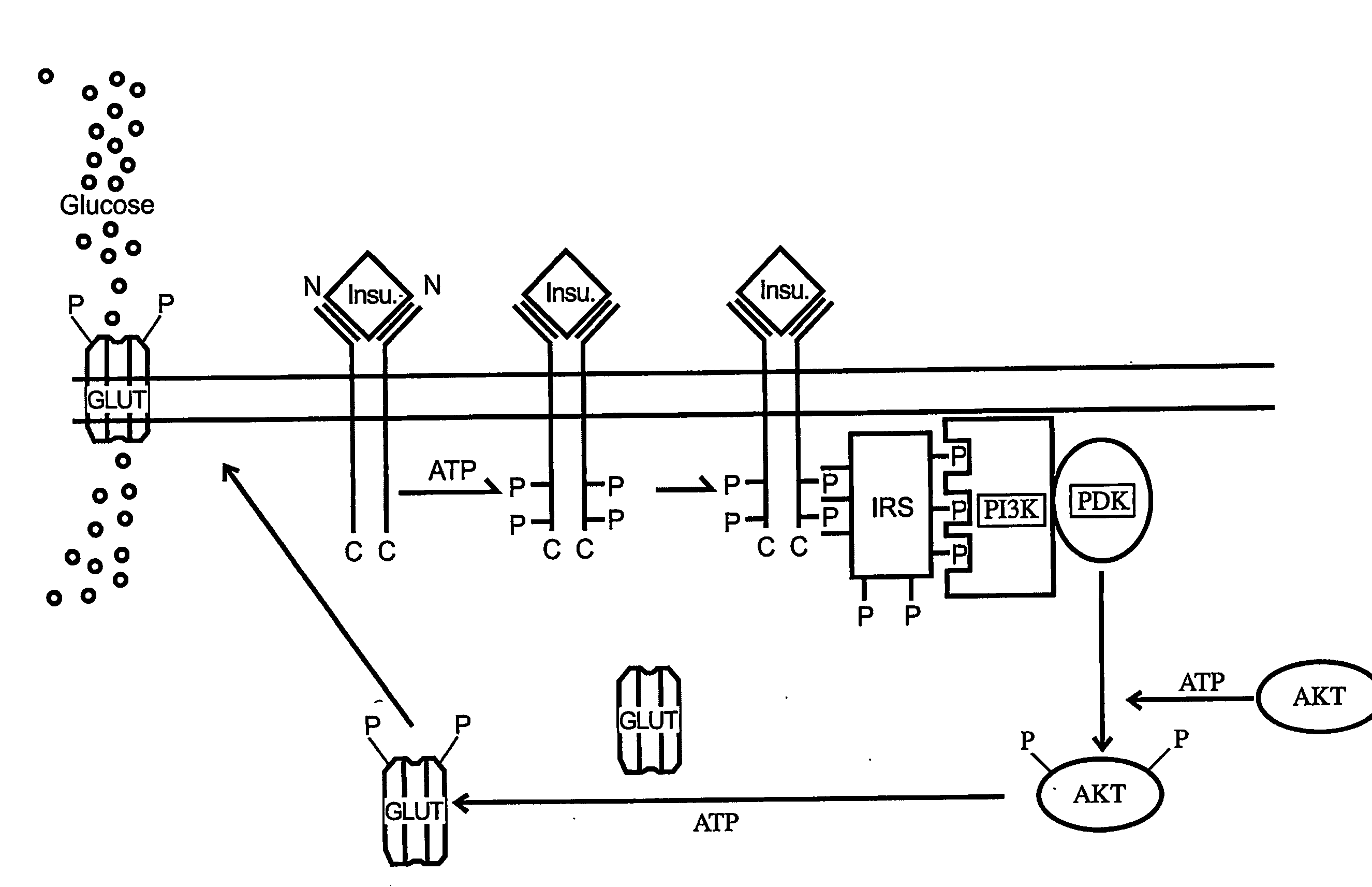
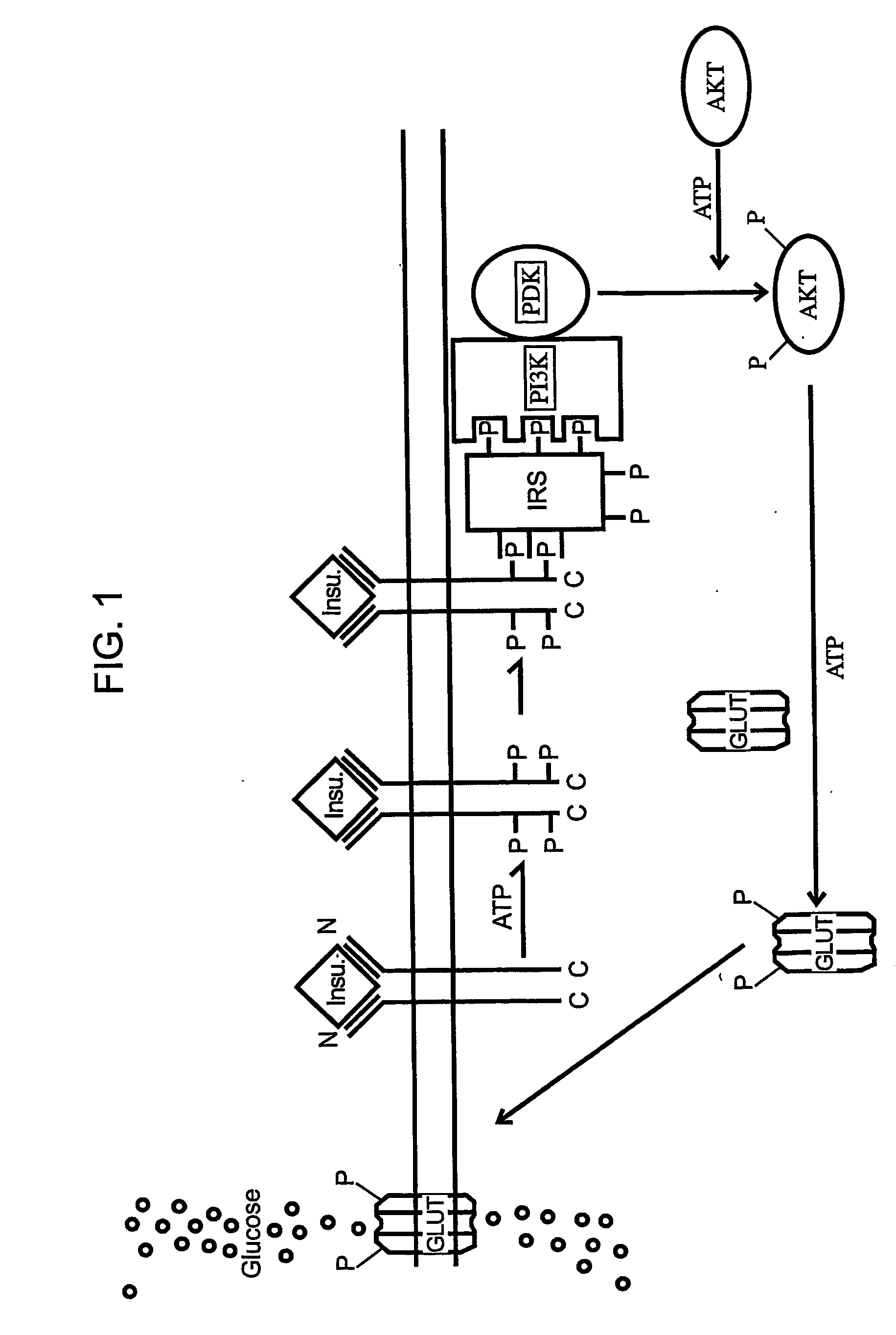
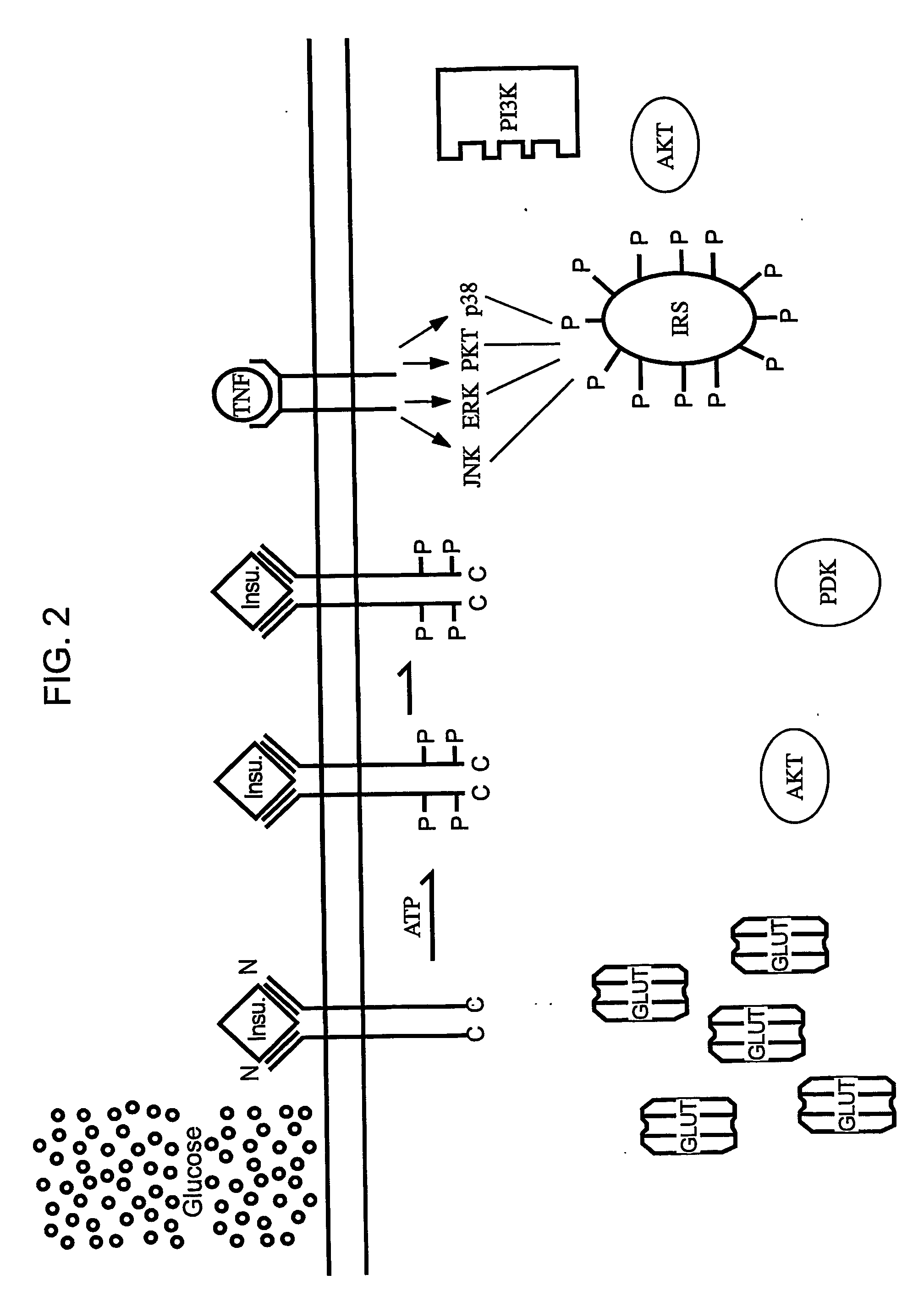
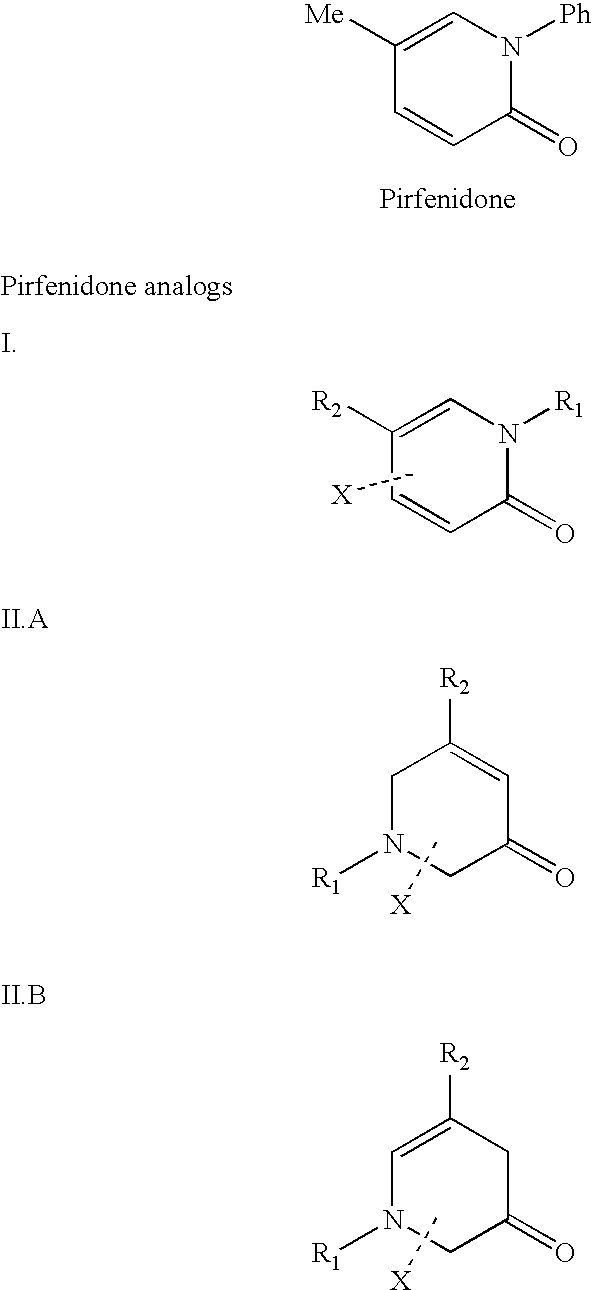
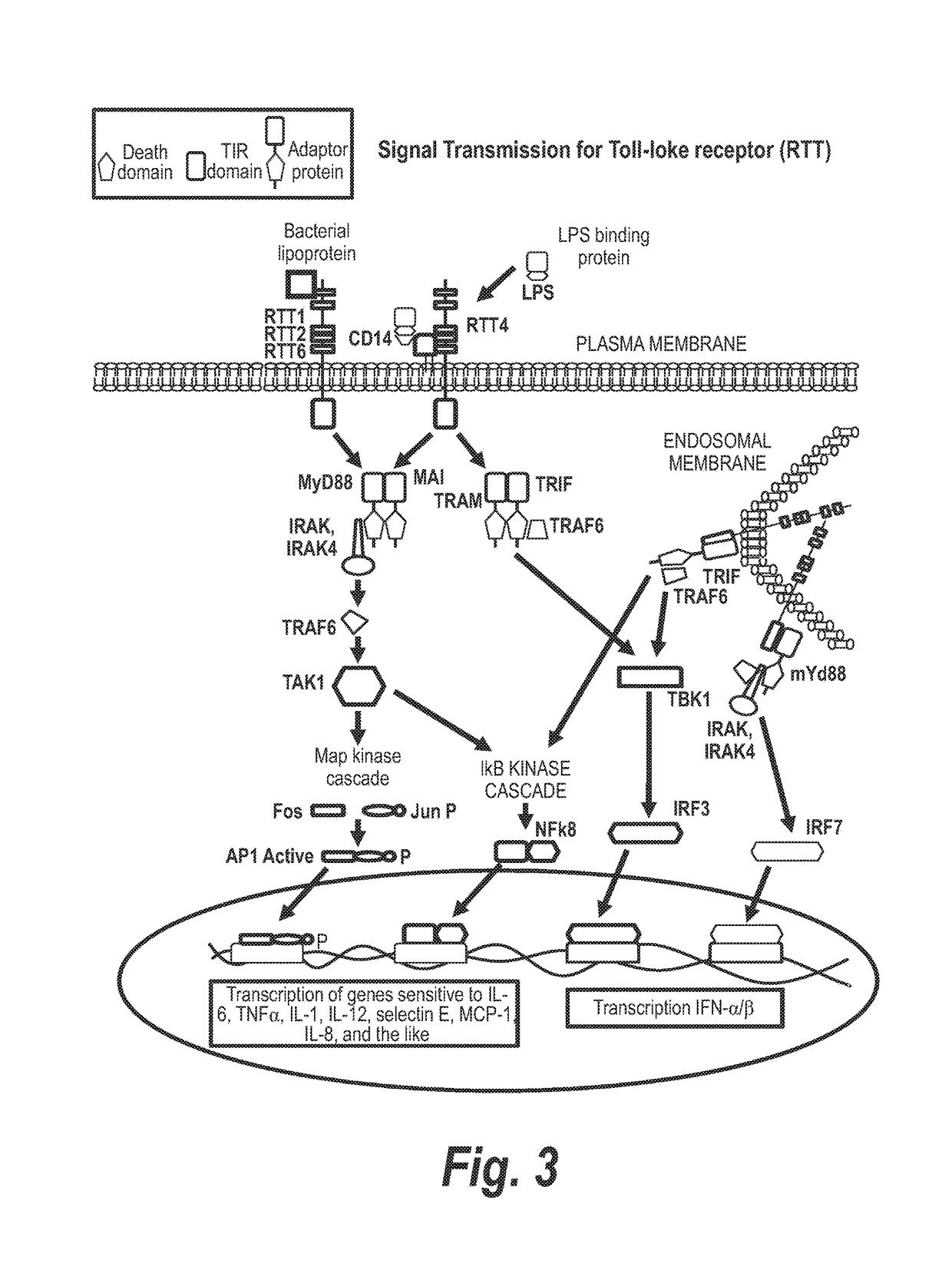
Methods of Treating Tnf-Mediated Disorders
The present invention provides methods of treating TNF-α-mediated disorders, the methods generally involving administering to an individual in need thereof effective amounts of pirfenidone or a pirfenidone analog and a second therapeutic agent that reduces TNF-α synthesis or that reduces TNF-α binding to a TNF receptor. The present invention further provides methods for treating non-alcoholic steatohepatitis, the method generally involving administering to an individual in need thereof an effective amount of pirfenidone. The present invention further provides methods of treating end-stage or advanced Type II diabetes, the methods generally involving administering to an individual in need thereof effective amounts of pirfenidone and insulin.
Owner:INTERMUNE INC
Novel pharmaceutical composition of interferon gamma or pirfenidone with molecular diagnostics for the improved treatment of interstitial lung diseases
InactiveUS20090142301A1Bioreactor/fermenter combinationsBiocideInterstitial lung diseaseInterferon alpha
The present invention relates to a novel pharmaceutical composition of compounds having the biological activity of interferon gamma (IFN-γ) or pirfenidone in combination with a diagnostic array of candidate polynucleotides for the improved treatment of all forms of interstitial lung disease, in particular of idiopathic pulmonary fibrosis (IPF).
Owner:MONDOBIOTECH AG
Altering pharmacokinetics of pirfenidone therapy
InactiveUS20080287508A1Low toxicityDecreases the adverse events associatedBiocideDigestive systemPhenyl groupPirfenidone
The invention relates to methods for reducing adverse events in patients receiving pirfenidone (5-methyl-1-phenyl-2-(1H)-pyridone) therapy.
Owner:INTERMUNE INC
Methods of reducing adverse events associated with pirfenidone therapy
InactiveUS20070203202A1Reduces mean maximum plasma concentrationIncrease mean absorption half lifeBiocideAntipyreticPirfenidoneInternal medicine
The invention relates to methods for reducing adverse events in patients receiving pirfenidone (5-methyl-1-phenyl-2-(1H)-pyridone) therapy.
Owner:INTERMUNE INC
Combination therapy for treatment of fibrotic disorders
InactiveUS20060110358A1Good treatment effectProlong survival timeOrganic active ingredientsPeptide/protein ingredientsDiseaseFibrosis
The present invention provides methods of treating a fibrotic disorder. The methods generally involve administering an effective amount of IFN-γ and an effective amount of pirfenidone or a pirfenidone analog.
Owner:INTERMUNE INC
Pharmaceutical liquid composition containing pyridone derivative
A pharmaceutical liquid composition containing the Pirfenidone in a very high concentration of more or less 25% by weight can be obtained by dissolving the Pirfenidone in diethylene glycol monoethyl ether. Even when the liquid medicinal compositions are stored for a long period of time, the Pirfenidone will not be recrystallized with a good chemical and physical stability. Furthermore, the liquid compositions are little irritating to the wounds on the mucous membrane of the skin and suitable for the manufacture of pharmaceutical formulations to be administered either via the oral, percutaneous, nasal or vaginal routes or by means of spray, patch, inhalation, injection or intravenous drip.
Owner:KDL
Combination therapy for treating alphavirus infection and liver fibrosis
InactiveUS20070072181A1Improve liver functionReduce morbidityOrganic active ingredientsImpression capsMortality rateInterferon receptor
The present invention provides methods for treating alphavirus infections; methods of treating hepatitis C virus (HCV) infections; methods of treating West Nile virus infection; methods of reducing liver fibrosis; methods of increasing liver function in an individual suffering from liver fibrosis; methods of reducing the incidence of complications associated with HCV and cirrhosis of the liver; and methods of reducing viral load, or reducing the time to viral clearance, or reducing morbidity or mortality in the clinical outcomes, in patients suffering from viral infection. The methods generally involve administering effective amounts of an interferon receptor agonist and pirfenidone in combination therapy.
Owner:THREE RIVERS PHARMA LLC
Use of pyridone derivatives in the prevention or treatment of tissue or organ toxicity induced by cytotoxic agents and radiation
ActiveUS20080161361A1Preventing alleviating treating of damageRemarkable effectBiocideAntinoxious agentsCytotoxicityTherapeutic effect
The present invention is directed to a novel use of pyridone derivatives such as pirfenidone for the prevention and treatment of damages to tissues or organs induced by various cytotoxic agents, such as chemotherapeutic agents, biologics, immunosuppressants and radiation. Such prophylactic and / or therapeutic effects of the pyridone derivatives make it possible to increase therapeutic dosages of the cytotoxic agent, thereby enhancing the therapeutic efficacy of the cytotoxic agent and radiation therapy.
Owner:CATALYST BIOSCIENCES INC
Pharmaceutical composition containing pirfenidone in sustained-release tablet form
ActiveUS9408836B2Effective in regressionDeleterious effectNervous disorderAntipyreticHepatic fibrosisAnti fibrotic
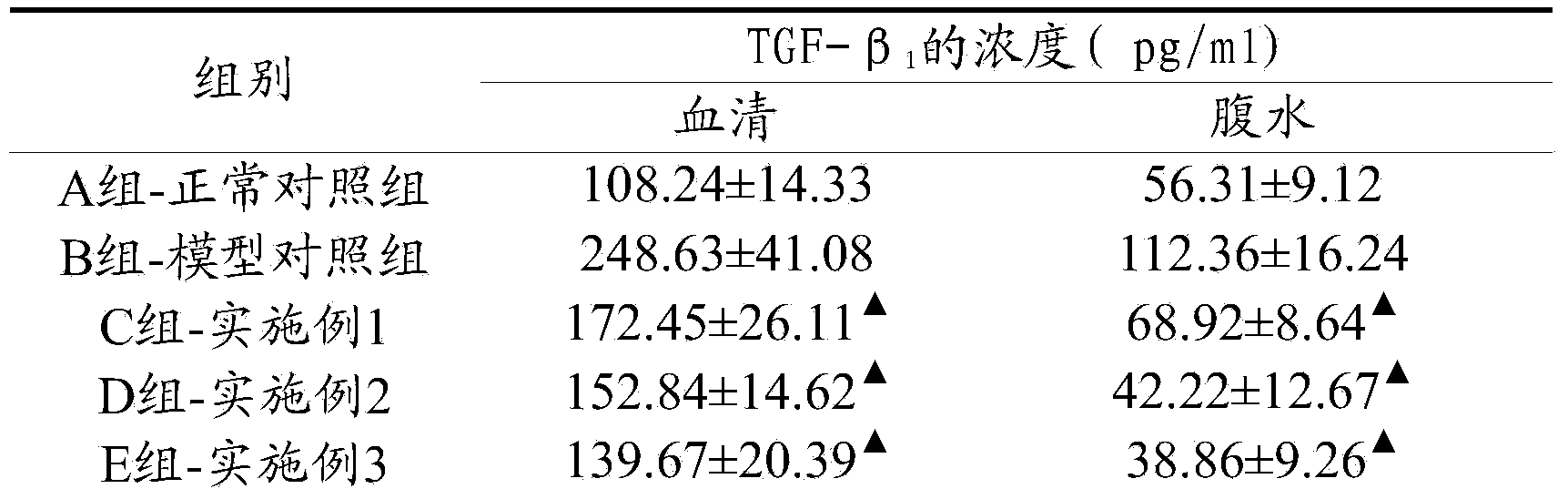
The instant invention relates to a process for the preparation of a pharmaceutical composition in sustained-release tablet form comprising from 600 milligrams to 2400 milligrams of Pirfenidone (PFD), in such a way that the drug is bioavailable during an extended period of time of 12 hours from its administration. In this way, the anti-fibrotic and anti-inflammatory action of the drug Pirfenidone is optimized. Moreover, the instant invention offers advantages and a higher therapeutic efficacy compared to other pharmaceutical forms of Pirfenidone for oral administration and its therapeutic application in the regression of chronic renal failure secondary to primary glomerulosclerosis; it shows a better activity with regard to the reduction and / or regression of deleterious effects in breast capsular contracture observed after the surgical implantation of breast implants in humans and has an important anti-TNF-α and anti-TGF-β1 action for the treatment of hepatic fibrosis.
Owner:EXCALIBUR PHARM INC
Usage of pirfenidone for treating hepatic injury and necrosis and acute lung injury
ActiveCN1701793AEasy to useOrally effectiveDigestive systemTripeptide ingredientsMortality rateLower mortality
The invention relates to the usage of pirfenidone for treating hepatic injury and necrosis and acute lung injury, more specifically the invention relates to the application of pirfenidone in abatement of symptoms for patients with hepatic injury, necrosis and acute lung injury, and in lowering mortality rate. The invention also provides the medicinal composition for treating hepatic injury, necrosis and acute lung injury.
Owner:BEIJING CONTINENT PHARM CO LTD
Novel pharmaceutical composition of interferon gamma or pirfenidone with molecular diagnostics for the improved treatment of interstitial lung diseases
InactiveUS20060270618A1Faster and successful treatmentPromote circulationOrganic active ingredientsBioreactor/fermenter combinationsInterstitial lung diseaseMortality rate
The present invention relates to a novel pharmaceutical composition comprising interferon-γ or pirfenidone and a diagnostic array of candidate polynucleotides for the improved treatment of lung diseases, especially for all forms of interstitial lung diseases. This invention describes the combination of molecular diagnosis and clinical therapy as a novel medication principle for reduction of mortality and improvement of disease management in interstitial lung diseases.
Owner:MONDOBIOTECH LICENSING OUT AG +1
Pharmaceutical composition for treating hepatic fibrosis and preparation method thereof
ActiveCN103550242AQuality improvementConvenient for clinical operationOrganic active ingredientsDigestive systemDiseaseInosine
The invention relates to a pharmaceutical composition for treating hepatic fibrosis and a preparation method thereof. The pharmaceutical composition is a formulation prepared from a main ingredient and pharmaceutically acceptable auxiliary materials, wherein the main ingredient comprises 100-300 parts of pirfenidone and 50-150 parts of inosine. The formulation of pirfenidone and inosine is stable in quality, controllable, safe and effective. In clinic, the formulation is mainly used for the treatment of diseases of hepatic fibrosis, cirrhosis, liver cancer, and the like.
Owner:SICHUAN GUOKANG PHARMA
Process for the preparation of a pharmaceutical composition containing pirfenidone in sustained-release tablet form and its application in the regression of human chronic renal failure, breast capsular contracture and hepatic fibrosis
ActiveUS20160287567A1Effective in regressionDeleterious effectNervous disorderAntipyreticAnti fibroticLiver fibrosis
The instant invention relates to a process for the preparation of a pharmaceutical composition in sustained-release tablet form comprising from 600 milligrams to 2400 milligrams of Pirfenidone (PFD), in such a way that the drug is bioavailable during an extended period of time of 12 hours from its administration. In this way, the anti-fibrotic and anti-inflammatory action of the drug Pirfenidone is optimized. Moreover, the instant invention offers advantages and a higher therapeutic efficacy compared to other pharmaceutical forms of Pirfenidone for oral administration and its therapeutic application in the regression of chronic renal failure secondary to primary glomerulosclerosis; it shows a better activity with regard to the reduction and / or regression of deleterious effects in breast capsular contracture observed after the surgical implantation of breast implants in humans and has an important anti-TNF-α and anti-TGF-β1 action for the treatment of hepatic fibrosis.
Owner:EXCALIBUR PHARM INC
Method of providing pirfenidone therapy to a patient
The invention relates to methods for decreasing adverse events associated with pirfenidone (5-methyl-1-phenyl-2-(1H)-pyridone) therapy. The invention discloses an optimized dose escalation scheme that results in the patient having increased tolerance to adverse events associated with the administration of pirfenidone. The invention also discloses a starter pack that may be used in conjunction with the dose escalation scheme.
Owner:INTERMUNE INC
Capsule Formulation of Pirfenidone and Pharmaceutically Acceptable Excipients
A capsule formulation of pirfenidone is provided that includes pharmaceutically acceptable excipients. In one embodiment, this capsule formulation is capable of sustaining desirable pharmacokinetic responses in a patient. Further provided are methods of treating fibrotic conditions and other cytokine-mediated disorders by administering pirfenidone capsules of such formulation to a patient in need.
Owner:INTERMUNE INC
Methods of treating HIV patients with Anti-fibrotics
InactiveUS20120014917A1Good effectReduce areaBiocideOrganic chemistryImmunodeficiency virusAnti fibrotic
The invention relates to methods of treating patients infected with human immunodeficiency virus (HIV) with a therapeutic that has anti-fibrotic effects, for example, pirfenidone and analogs thereof.
Owner:INTERMUNE INC
Process for the preparation of a pharmaceutical composition containing pirfenidone in sustained-release tablet form and its application in the regression of human chronic renal failure, breast capsular contracture and hepatic fibrosis
ActiveUS20140296300A1Effective in regressionDeleterious effectBiocideNervous disorderAnti fibroticHepatic fibrosis
The instant invention relates to a process for the preparation of a pharmaceutical composition in sustained-release tablet form comprising from 600 milligrams to 2400 milligrams of Pirfenidone (PFD), in such a way that the drug is bioavailable during an extended period of time of 12 hours from its administration. In this way, the anti-fibrotic and anti-inflammatory action of the drug Pirfenidone is optimized. Moreover, the instant invention offers advantages and a higher therapeutic efficacy compared to other pharmaceutical forms of Pirfenidone for oral administration and its therapeutic application in the regression of chronic renal failure secondary to primary glomerulosclerosis; it shows a better activity with regard to the reduction and / or regression of deleterious effects in breast capsular contracture observed after the surgical implantation of breast implants in humans and has an important anti-TNF-α and anti-TGF-β1 action for the treatment of hepatic fibrosis.
Owner:EXCALIBUR PHARM INC
Pharmaceutical composition for treating hepatic fibrosis and preparation method thereof
The invention relates to a pharmaceutical composition for treating hepatic fibrosis and a preparation method thereof. The pharmaceutical composition is a formulation prepared from a main ingredient and pharmaceutically acceptable auxiliary materials, wherein the main ingredient comprises 100-300 parts of pirfenidone and 50-150 parts of inosine. The formulation of pirfenidone and inosine is stable in quality, controllable, safe and effective. In clinic, the formulation is mainly used for the treatment of diseases of hepatic fibrosis, cirrhosis, liver cancer, and the like.
Owner:SICHUAN GUOKANG PHARMA
Semi-solid topical composition containing pirfenidone and modified diallyl disulfide oxide (m-ddo) for eliminating or preventing acne
ActiveUS20160228424A1Eliminating and reducing and preventing occurrenceReducing and preventing acneAerosol deliverySulfur/selenium/tellurium active ingredientsAntiseptic AgentDisulfide bond
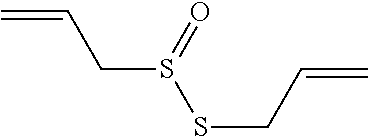
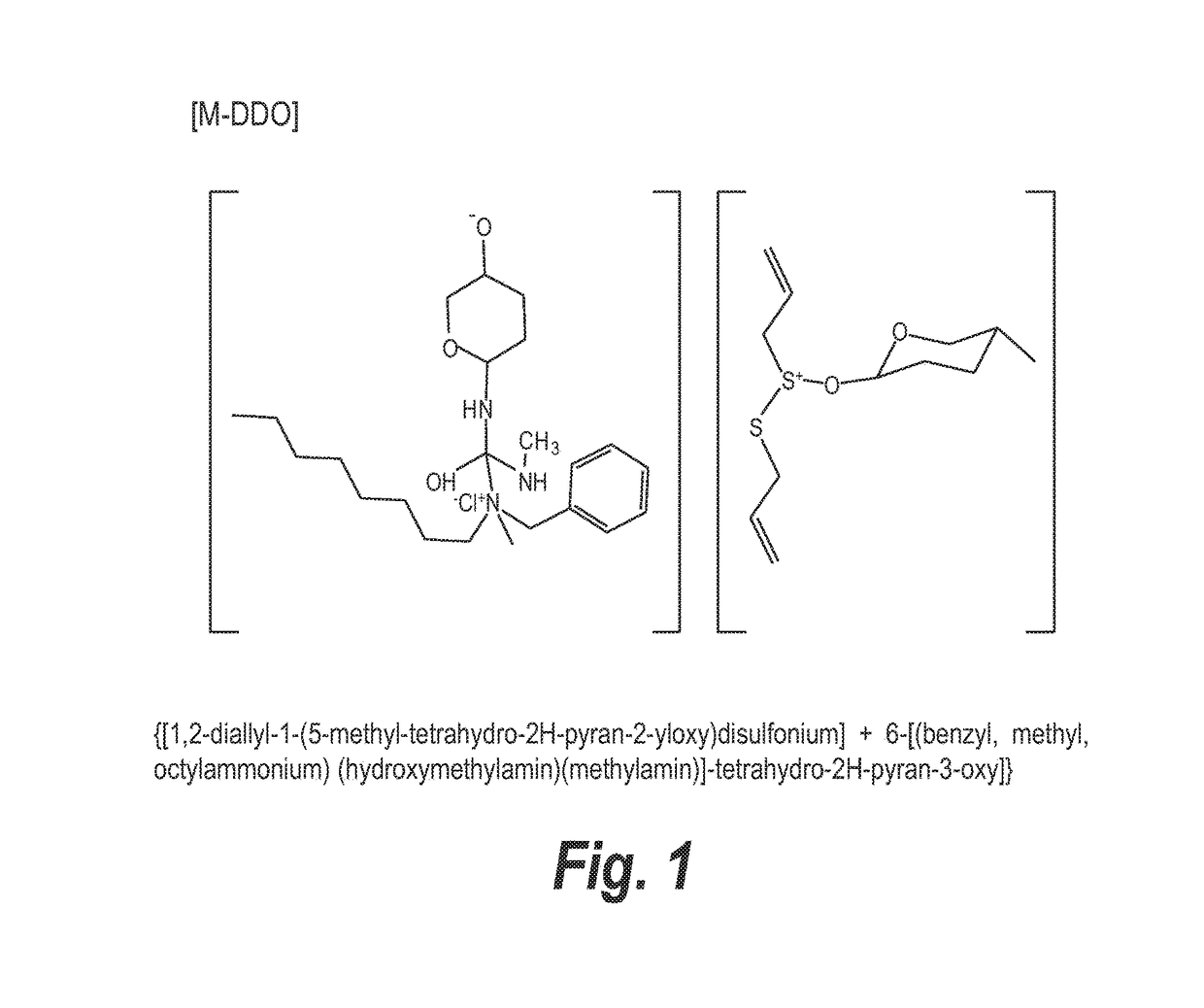
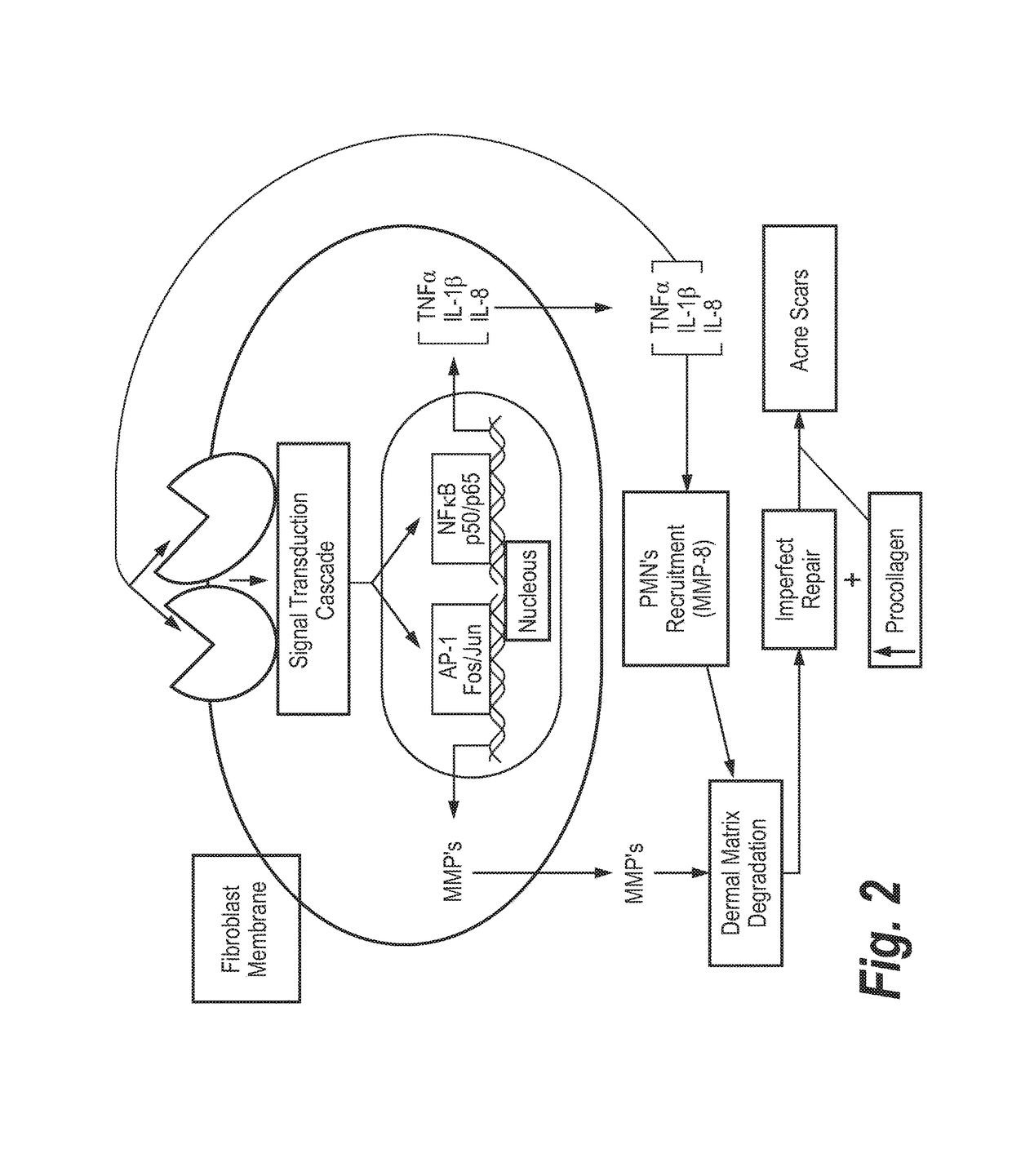
The instant invention relates to a semi-solid topical composition containing Pirfenidone and an antimicrobial / antiseptic agent such as Modified Diallyl Disulfide Oxide (M-DDO) and its preparation process, offering advantages compared to other pharmaceutical forms of topical administration known in the state of the art, useful as antifibrotic, anti-inflammatory and antiseptic agent in the prevention, treatment and reversion of acne and post acne lesions. Said compositions is also useful for reducing skin redness, detaining the formation of new acne outbreaks, reversing already existing outbreaks and regenerating skin damage caused by acne.
Owner:EXCALIBUR PHARM INC
Semi-solid topical composition containing pirfenidone and modified diallyl disulfide oxide (M-DDO) for eliminating or preventing acne
ActiveUS9949959B2Eliminating and reducing and preventing occurrenceReducing and preventing acneAerosol deliverySulfur/selenium/tellurium active ingredientsDiallyl disulfide-oxidePreservative
The instant invention relates to a semi-solid topical composition containing Pirfenidone and an antimicrobial / antiseptic agent such as Modified Diallyl Disulfide Oxide (M-DDO) and its preparation process, offering advantages compared to other pharmaceutical forms of topical administration known in the state of the art, useful as antifibrotic, anti-inflammatory and antiseptic agent in the prevention, treatment and reversion of acne and post acne lesions. Said compositions is also useful for reducing skin redness, detaining the formation of new acne outbreaks, reversing already existing outbreaks and regenerating skin damage caused by acne.
Owner:EXCALIBUR PHARM INC
Drug composition containing pirfenidone, and preparation method thereof
InactiveCN102846569AReduce contentImprove stabilityOrganic active ingredientsRespiratory disorderCombinatorial chemistryPharmaceutical Substances
The present invention belongs to the technical field of medicine, and particularly relates to a solid drug composition containing pirfenidone, and a preparation process thereof. The drug composition contains pirfenidone, a binder and a disintegrating agent, and can be prepared into tablets, granules and capsules.
Owner:BEIJING VENTUREPHARM BIOTECH
Altering Pharmacokinetics of Pirfenidone Therapy
InactiveUS20110136876A1Decreases the adverse events associatedLow toxicityBiocideDigestive systemMethyl groupPirfenidone
The invention relates to methods for reducing adverse events in patients receiving pirfenidone (5-methyl-1-phenyl-2-(1H)-pyridone) therapy.
Owner:INTERMUNE INC
Peritoneal dialysis solution
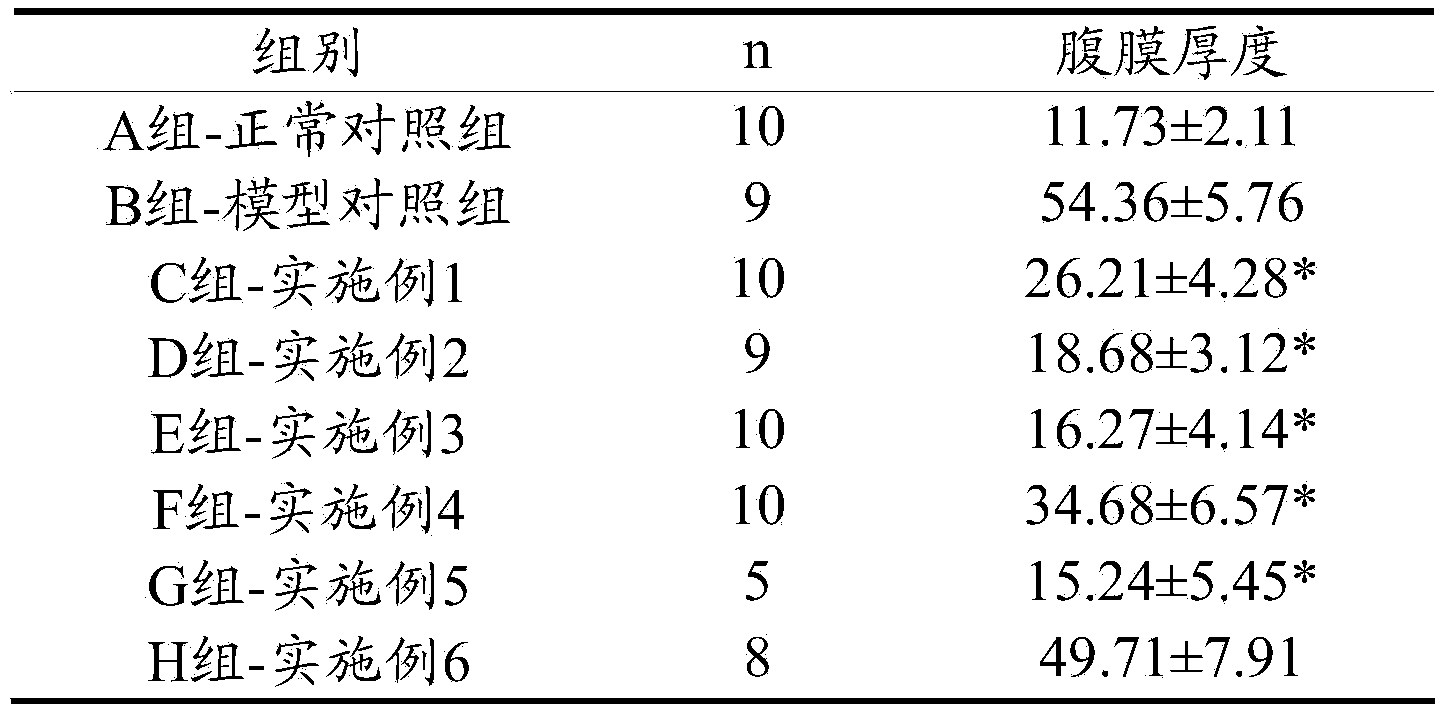
ActiveCN103463081ABlock and delay the process of peritoneal fibrosisOrganic active ingredientsAluminium/calcium/magnesium active ingredientsHigh concentrationExperimental research
The invention discloses a peritoneal dialysis solution which comprises 0.01-5%w / v pirfenidone. Through a good deal of experimental research and analysis, the process that the 0.01-5%w / v pirfenidone is added to a conventional peritoneal dialysis solution so that peritoneum fibrosis caused by high-concentration glucose in an existing peritoneal dialysis solution can be remarkably blocked and delayed is found out.
Owner:HUAREN PHARMACEUTICAL CO LTD
Pirfenidone-contained gel composition
InactiveCN101972225APromote absorptionImprove toleranceOrganic active ingredientsAerosol deliveryMedicinePirfenidone
The invention provides a pirfenidone-contained gel composition and a preparation method thereof. The pirfenidone-contained gel composition comprises pirfenidone and other pharmaceutically acceptable accessories. The invention further illuminates the clinical use method and the applications of the preparation mode.
Owner:BEIJING TRADE STAR MEDICAL TECH
Capsule formulation of pirfenidone and pharmaceutically acceptable excipients
A capsule formulation of pirfenidone is provided that includes pharmaceutically acceptable excipients. In one embodiment, this capsule formulation is capable of sustaining desirable pharmacokinetic responses in a patient. Further provided are methods of treating fibrotic conditions and other cytokine-mediated disorders by administering pirfenidone capsules of such formulation to a patient in need.
Owner:INTERMUNE INC
Preparing method for pirfenidone
ActiveCN105330598ASimple and fast operationGood reaction selectivityOrganic chemistryAlkoxy groupAniline
The invention relates to a preparing method for pirfenidone. The method includes the steps that under the action of an acid catalyst, 2-pentenenitrile is used for reacting with trimethyl / triethyl orthoformate to generate 2-methyl-1-alkoxy-4-cyano-1,3-butadiene (II), and 2-methyl-1-alkoxy-4-cyano-1,3-butadiene (II) and aniline are condensed and then hydrolyzed to obtain pirfenidone. The used raw materials are low in price and easy to obtain, the process flow is short, and the preparing method is easy to operate, environmentally friendly, high in reaction selectivity and high in product yield and purity.
Owner:XINFA PHARMA
Pharmaceutical use of an extended-release composition containing pirfenidone for the treatment and reversal of human steatohepatitis (nafld/nash)
InactiveUS20190262325A1Reduce contentReduce expressionOrganic active ingredientsDigestive systemFactor iiTG - Triglyceride
The present invention relates to the use of a pharmaceutical composition in the form of extended-release tablets containing Pirfenidone for treating NAFLD / NASH and advanced liver fibrosis by decreased serum cholesterol and triglycerides as well as reducing the content of hepatic fat accumulation, both in the form of macrosteatosis and microsteatosis. Additionally, its use as an agonist for PPARgamma (peroxisome proliferation receptor activated gamma), PPARalpha (peroxisome proliferation receptor activated alpha), LXR and CPT1, key molecules in the metabolism of fatty degradation and inflammation of the liver. In addition, another use is the induction of decreased expression of NFkB master gene, transcriptional inducer of hepatic inflammatory process factor. All of these events results in the reversal of NAFLD / NASH and advanced liver fibrosis.
Owner:EXCALIBUR PHARM INC
Solid preparation comprising pirfenidone as active component and application thereof
ActiveCN102846555ADisintegrates quicklyImprove bioavailabilityOrganic active ingredientsGranular deliveryDiseaseActive component
The invention relates to a solid preparation comprising pirfenidone as an active component and application thereof. The solid preparation is an oral solid preparation prepared by carrying out pharmaceutical technology on pirfenidone and pharmaceutically acceptable auxiliary materials. The solid preparation disclosed herein has the advantages of stable and controllable quality, safety and effectiveness, and is mainly clinically used as a medicine for treating idiopathic pulmonary fibrosis to mitigate the symptoms and reduce the mortality.
Owner:ZHUHAI EBANG PHARMA
Medical composite containing pirfenidone and preparation method thereof
ActiveCN101912395ADissolution rate is fastIncrease concentrationOrganic active ingredientsPharmaceutical non-active ingredientsPharmacologyPirfenidone
The invention provides a medical composite containing pirfenidone, which is characterized by containing 20-90% of pirfenidone, 10-50% of filler, 1-10% of disintegrating agent, 0.1-8% of adhesive and 0.2-3% of lubricant.
Owner:BEIJING KAWIN TECH SHARE HLDG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com