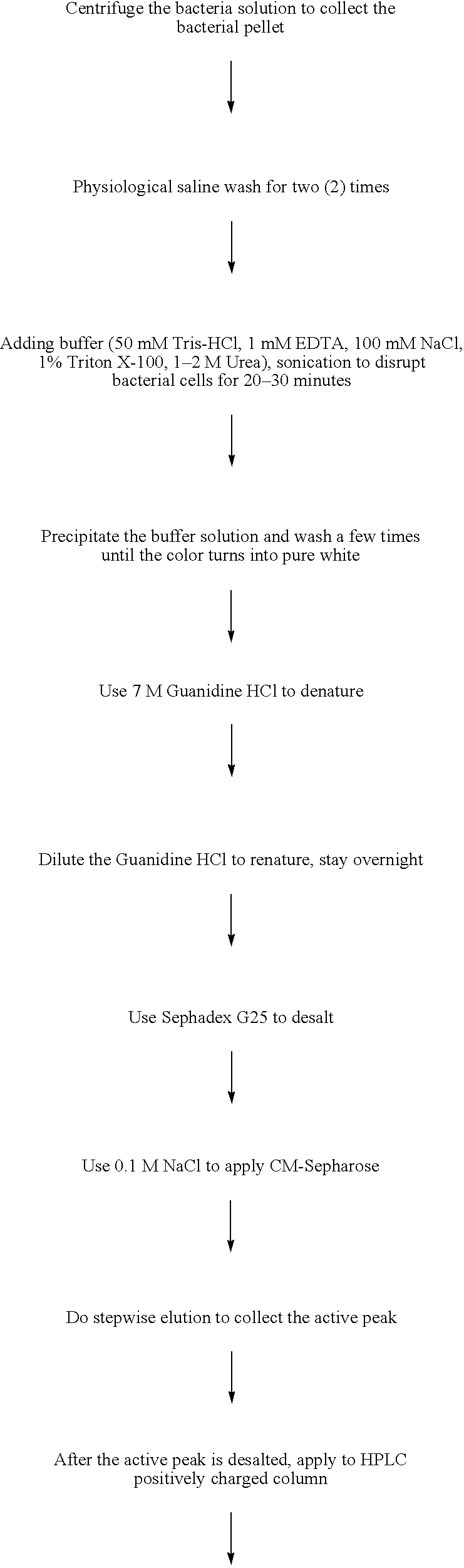
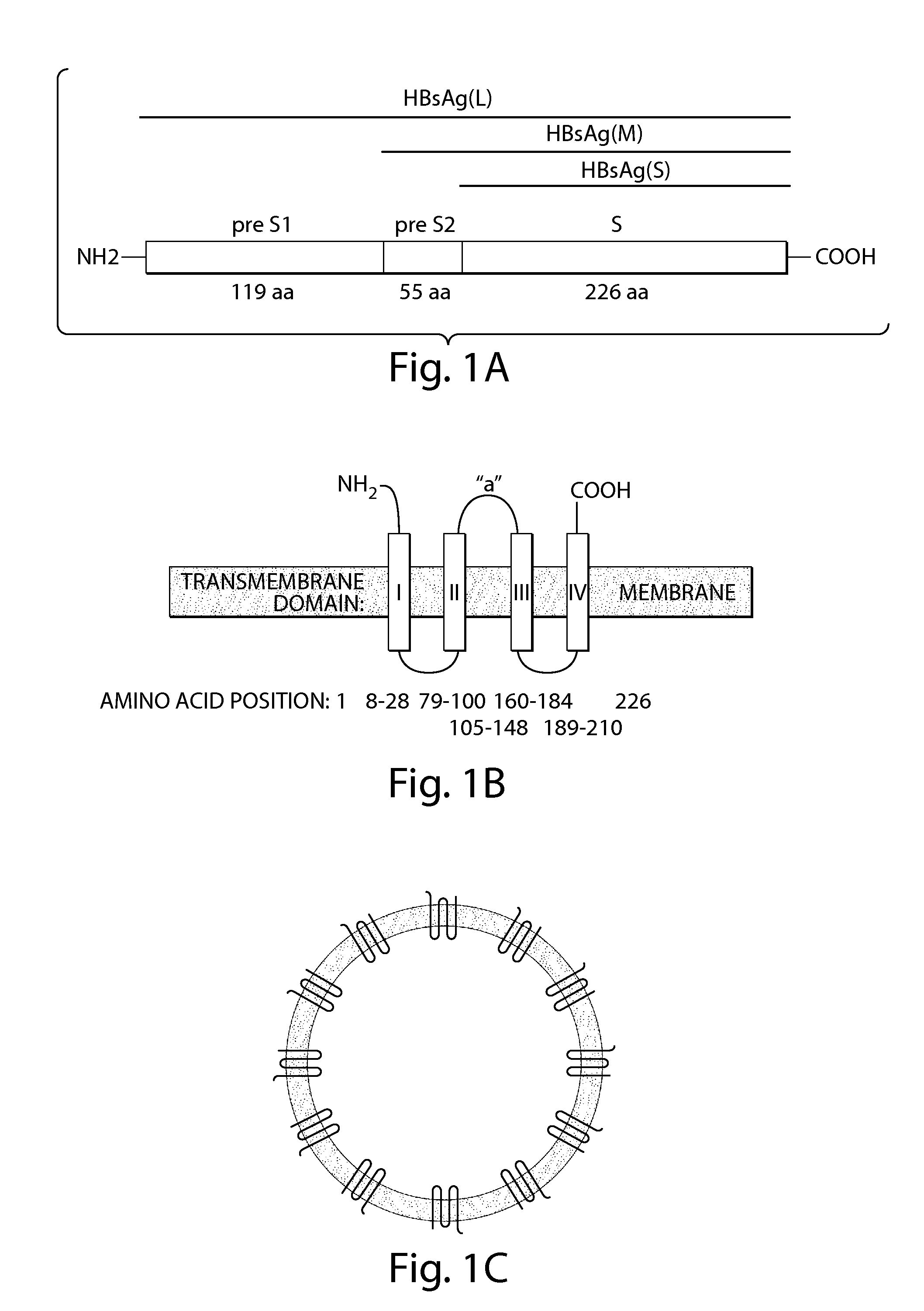
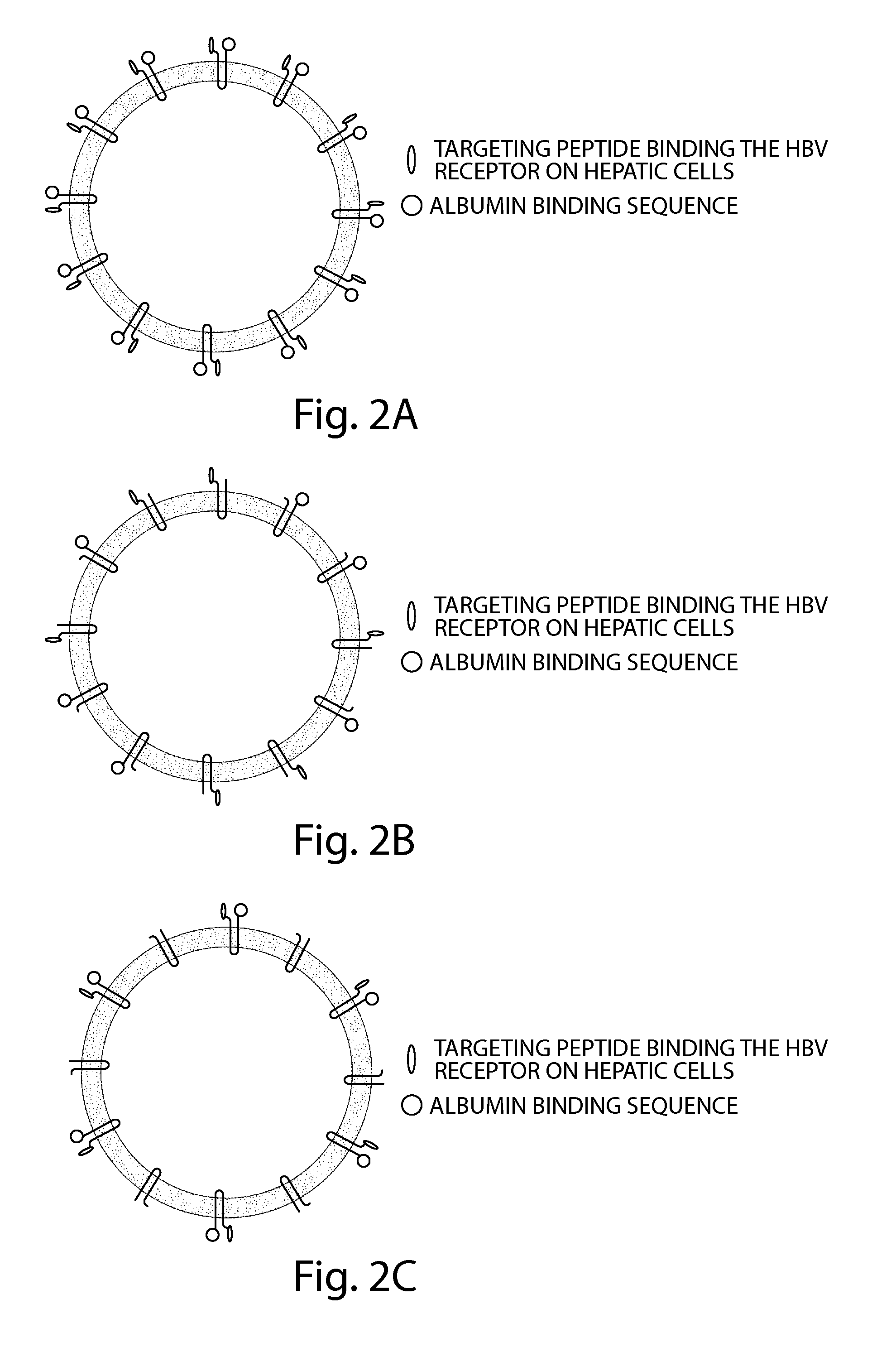
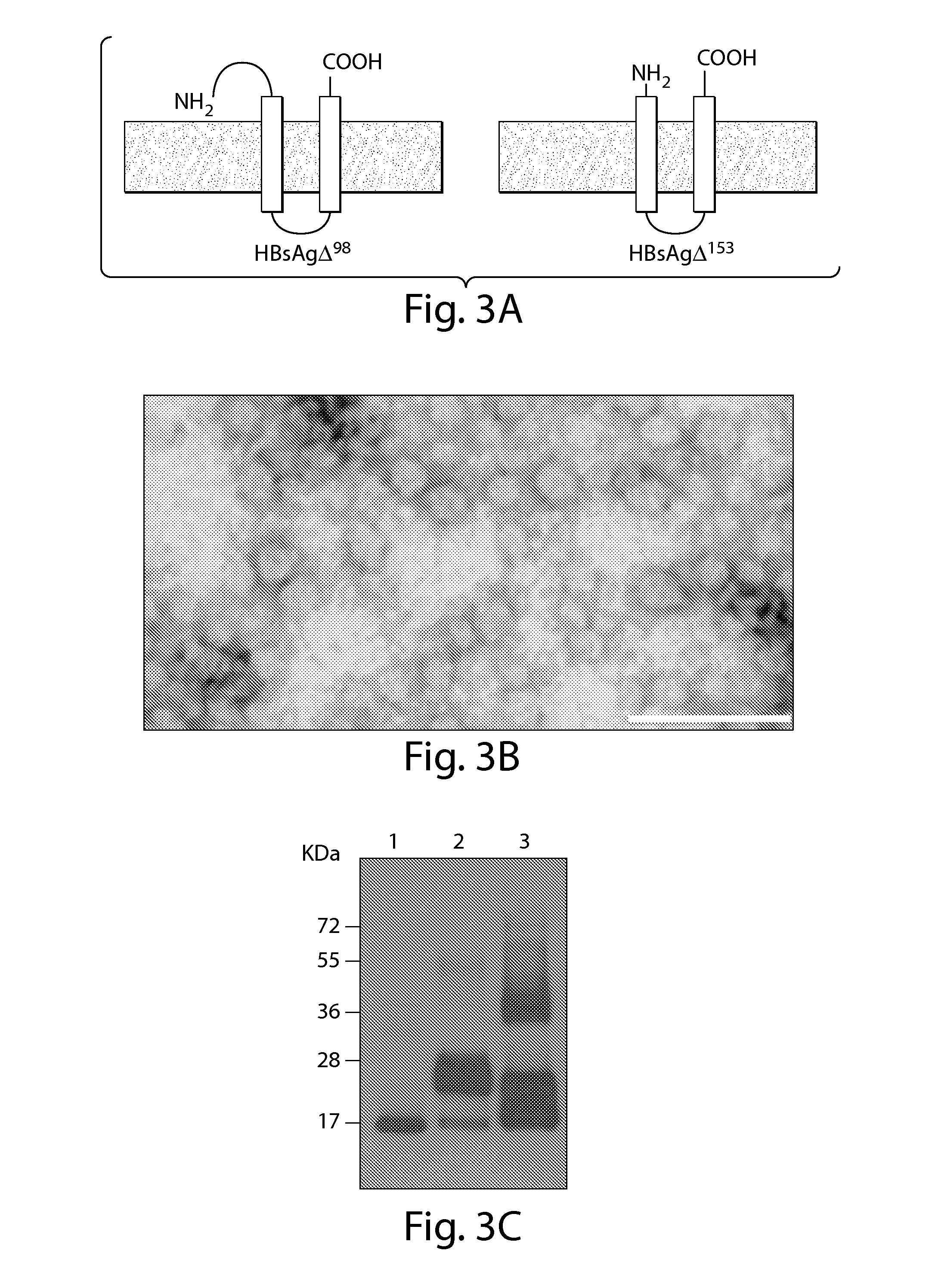
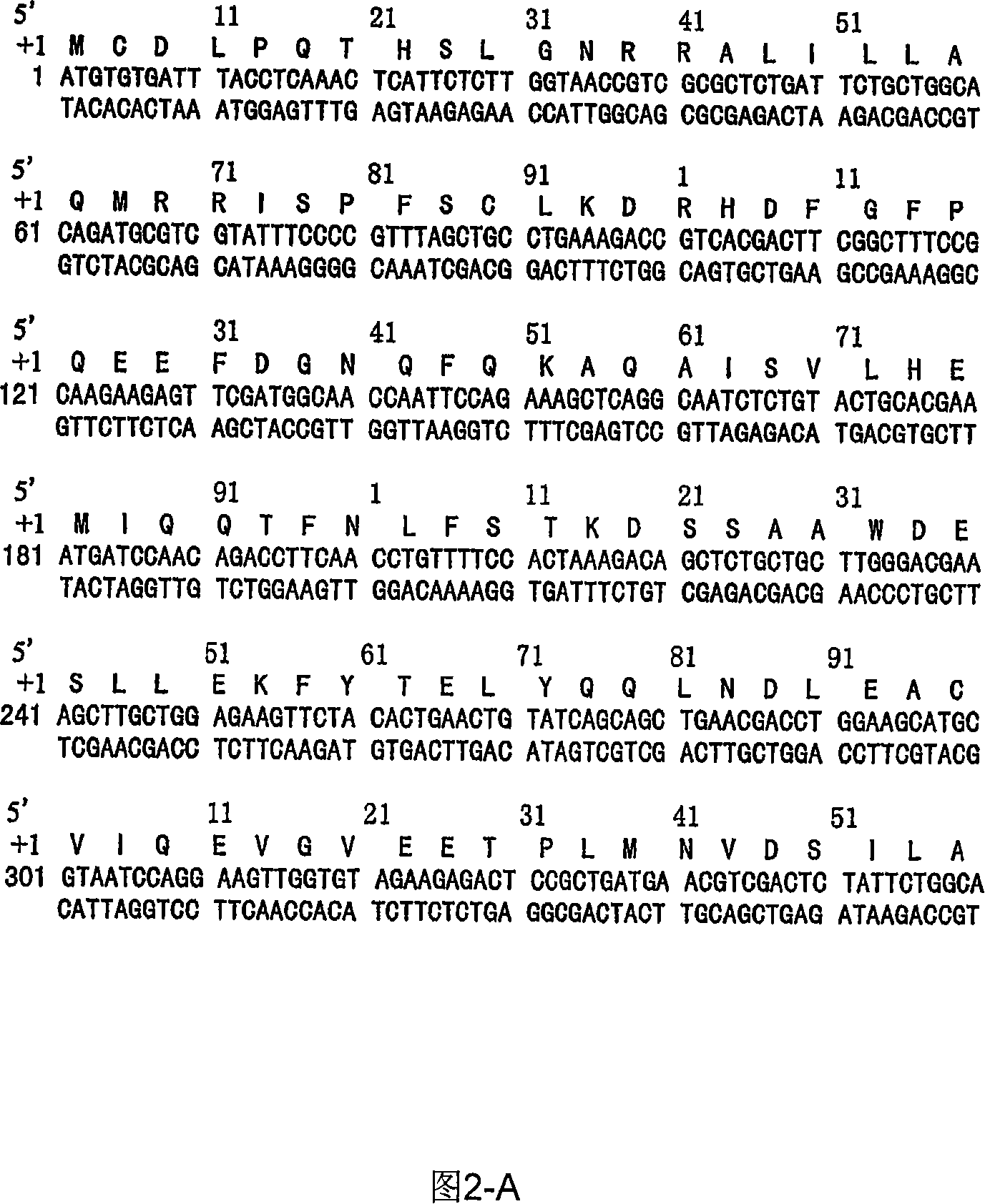
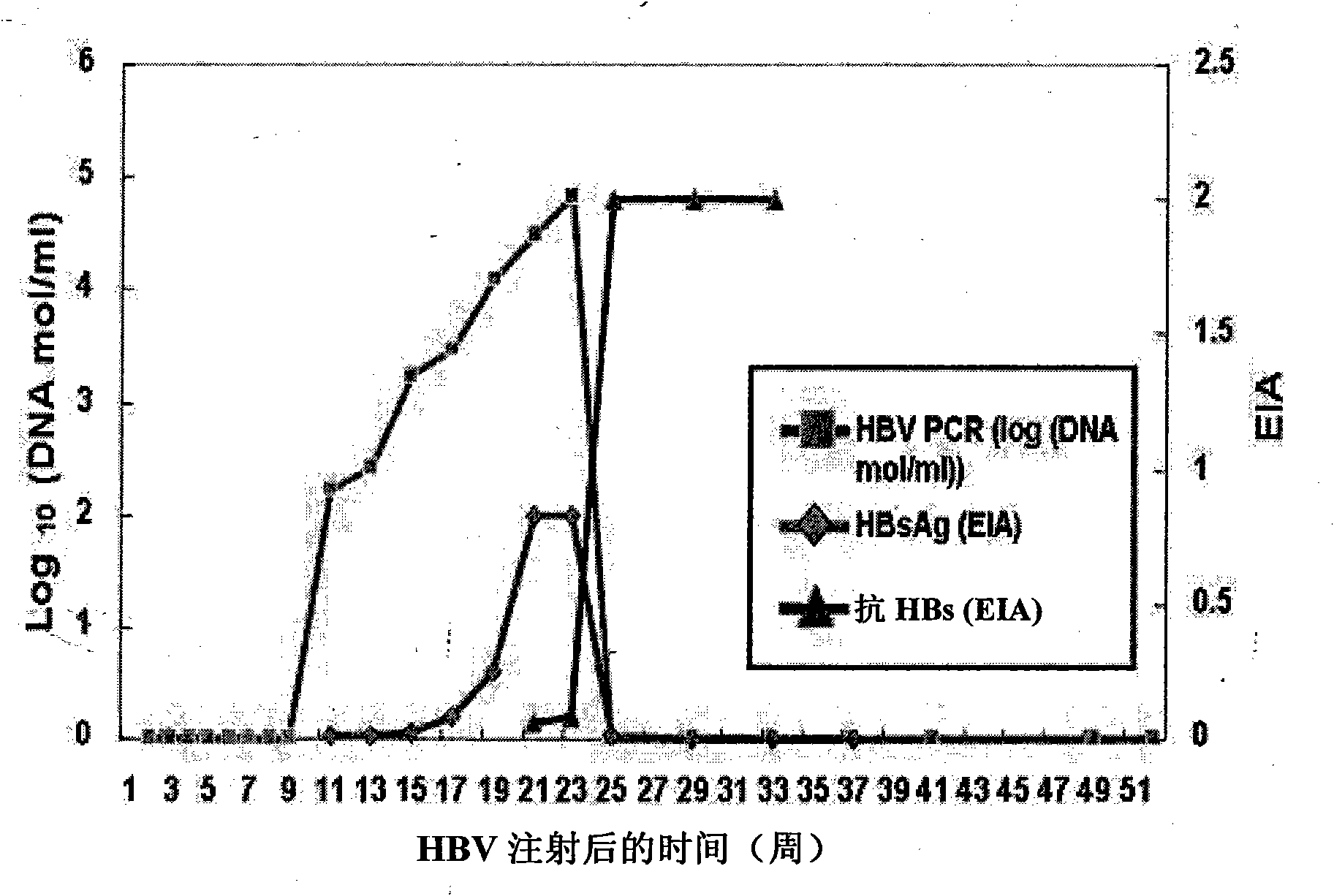
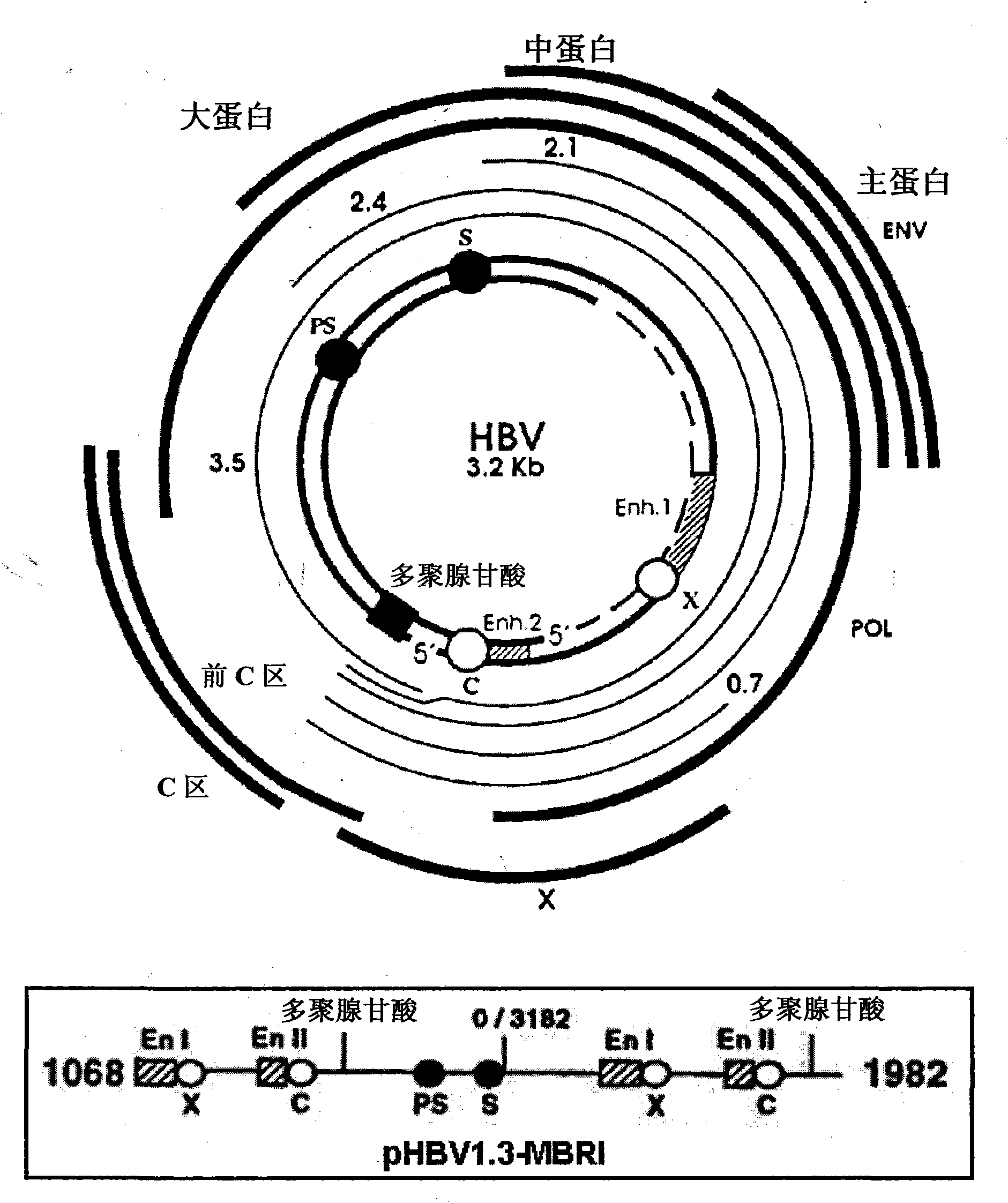
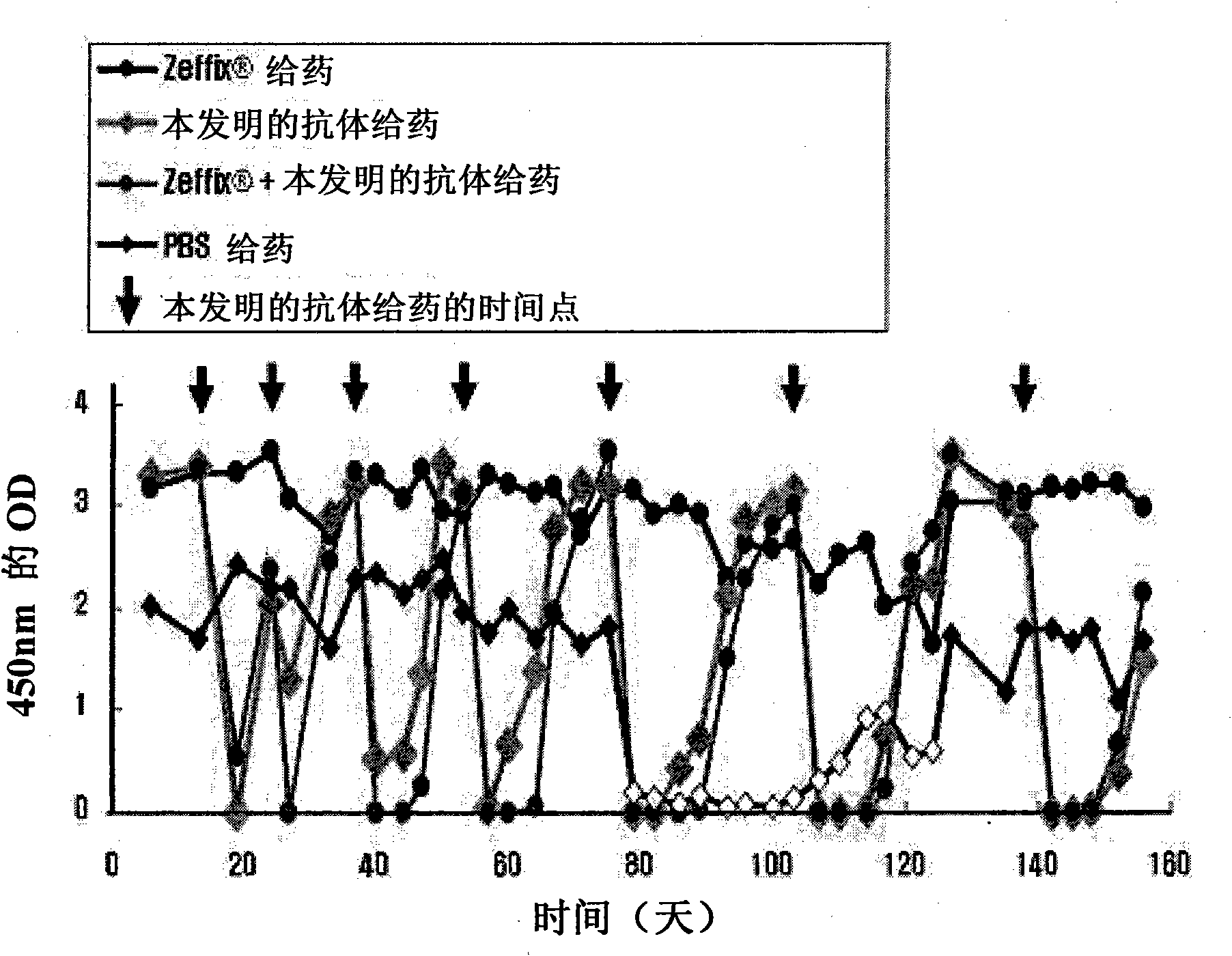
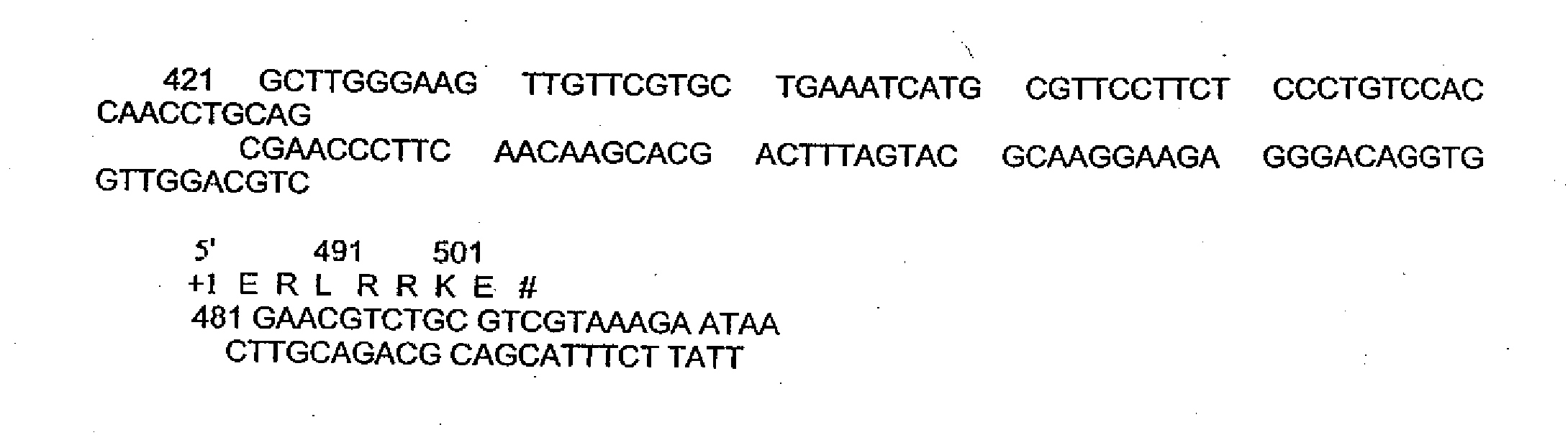Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
140 results about "HBsAg" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
HBsAg (also known as the Australia antigen) is the surface antigen of the hepatitis B virus (HBV). It indicates current hepatitis B infection.
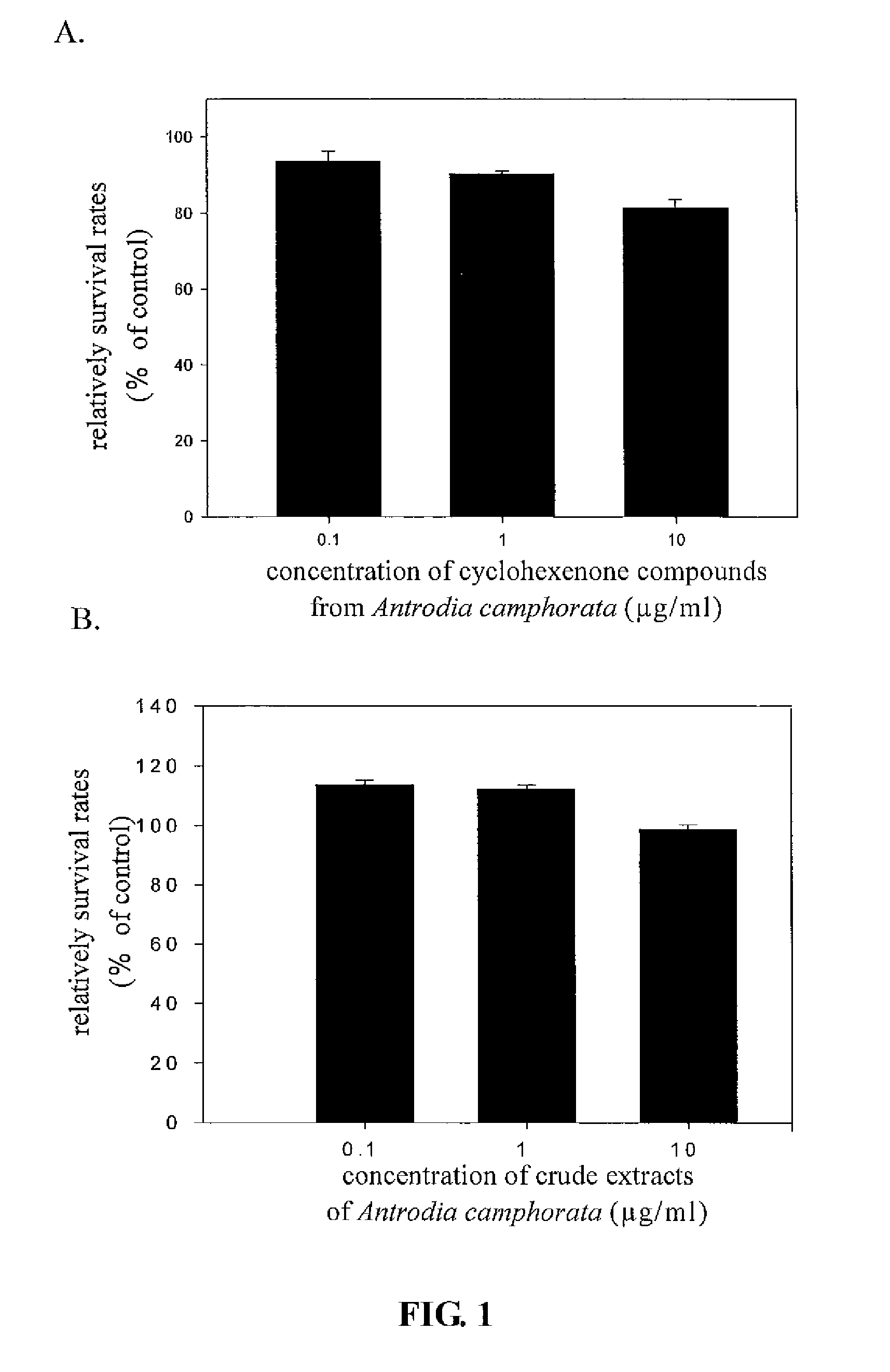
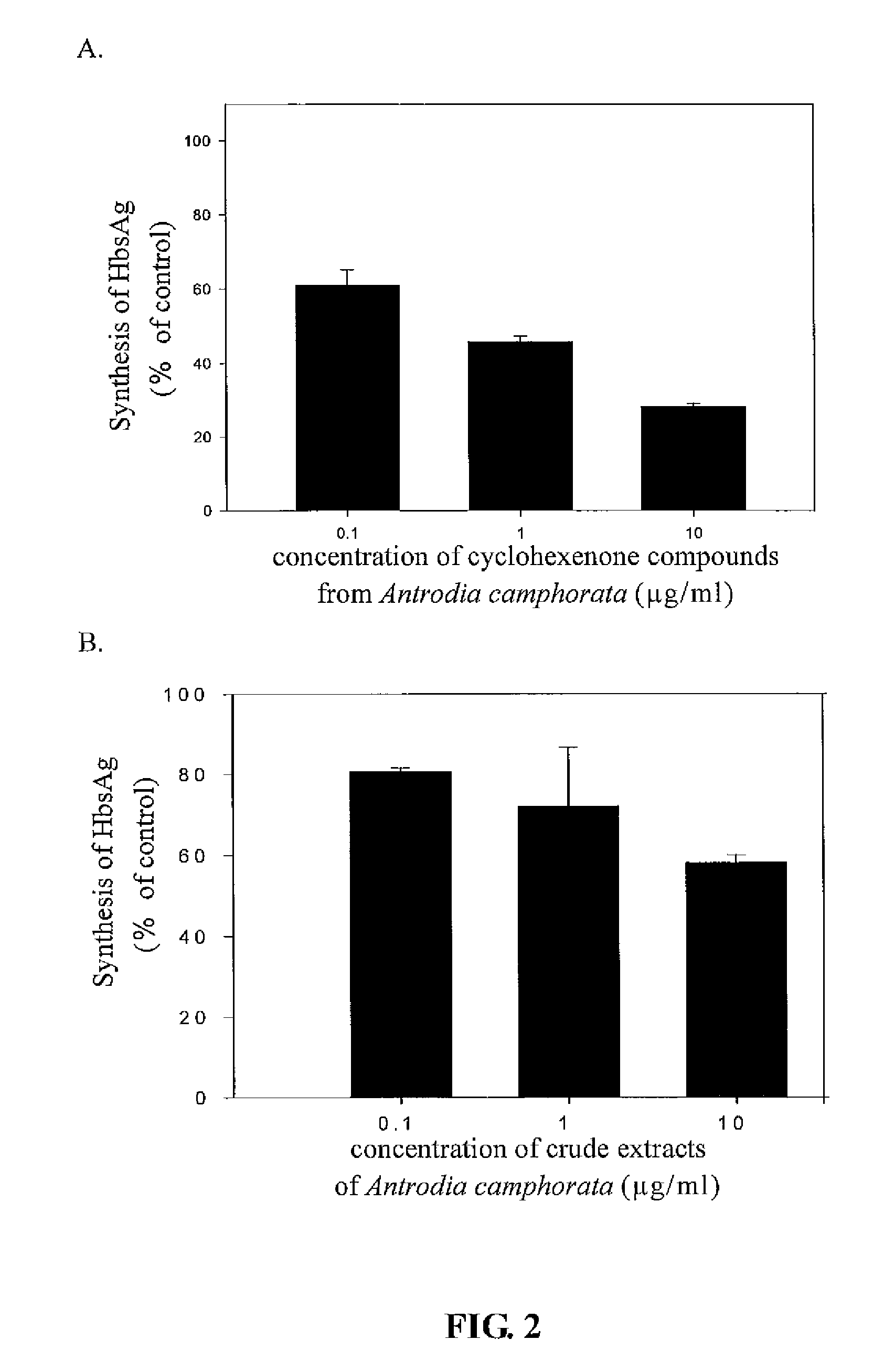
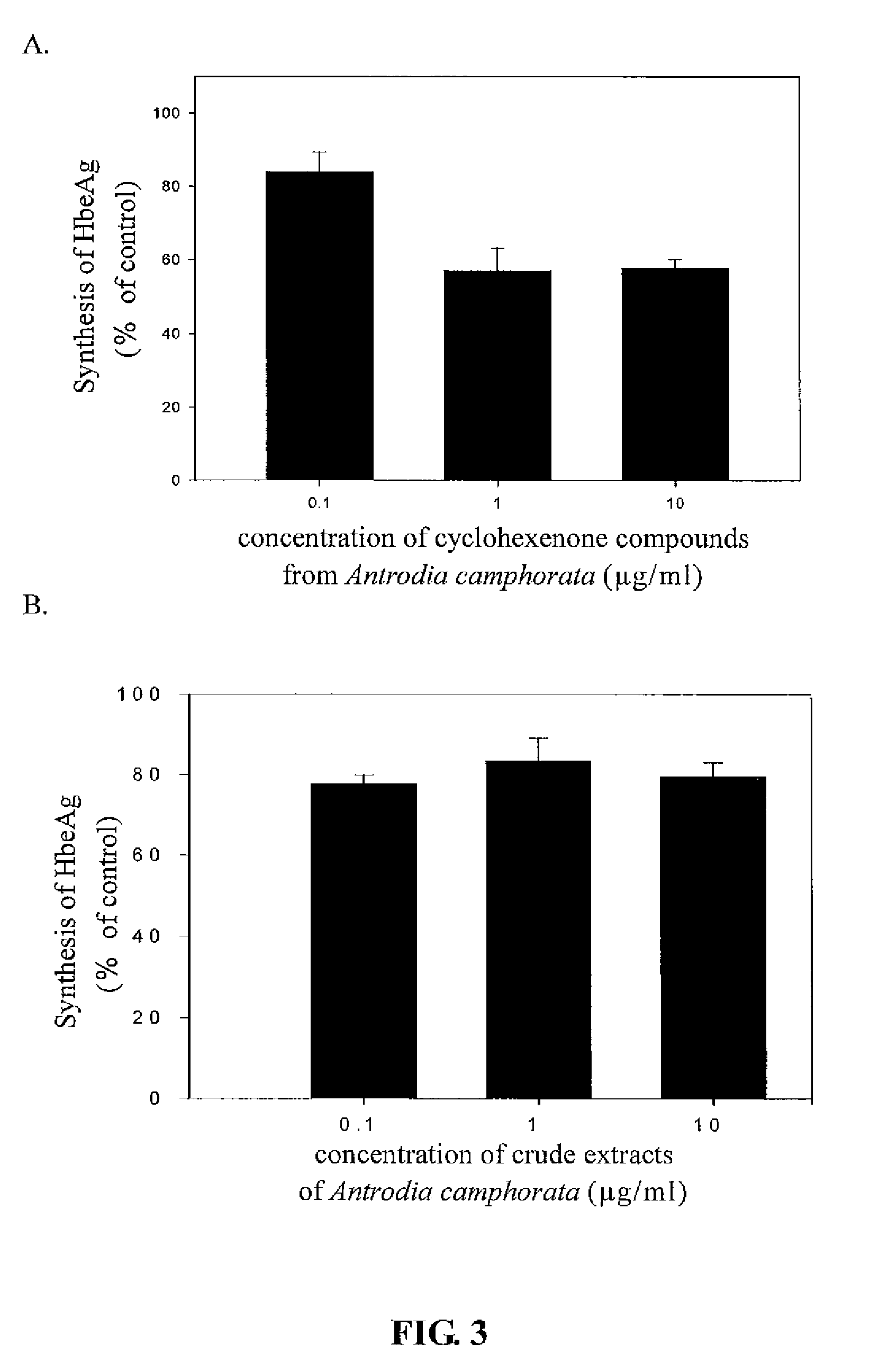
Inhibition of Hepatitis B virus by cyclohexenone compounds from Antrodia camphorata
ActiveUS7411003B1Good treatment effectAvoid survival rateBiocideOrganic chemistryHepatoma cell lineCyclohexenone
The present invention relates to a compound of Antrodia camphorata used to inhibit HBV, in particular to an extract, 4-hydroxy-2,3-dimethoxy-6-methyl-5(3,7,11-trimethyl-dodeca-2,6,10-trienyl)-cyclohex-2-enone which is isolated from Antrodia camphorata, and its use in inhibiting HBV effectively. The cyclohexenone compound according to the present invention showed cytotoxicity on HBV-secreting human hepatoma cell line HepG2 2.2.15, decreased synthesis of HBV particles, further inhibited synthesis of HbsAg and HbeAg effectively to achieve the goal of HBV inhibition.
Owner:GOLDEN BIOTECH
Recombinant super-compound interferon and uses thereof
InactiveUS20090123417A1Eliminate side effectsIncrease dosePeptide/protein ingredientsAntiviralsSide effectHBsAg
This invention provides a recombinant super-compound interferon (rSIFN-co) and its equivalent with changed spatial configuration, high efficacy and low side effects. Therefore, high dose of rSIFN-co may be used. One characteristic of rSIFN-co is its ability to inhibit the HBV DNA duplication and secretion of HBsAg and HBeAg in in vitro pharmacological studies. The cytotoxic effect of rSIFN-co is only one-eighth (⅛) of currently clinically available interferons but its anti-viral effect is approximately five to twenty (5-20) times higher, and when used in vivo it has a broader spectrum of clinical applications and longer biofeedback response. This invention further provides super-compound interferon or its equivalent, synthesis of artificial gene with codon preference which codes for said rSIFN-co and its equivalent, vector comprising said gene and appropriate expression system for expression of said rSIFN-co. Finally this invention provides the super-compound interferon (rSIFN-co) and its equivalent, a process to produce same and uses thereof.
Owner:SUPERLAB FAR EAST LTD
Recombinant super-compound interferon and uses thereof
InactiveUS20060035327A1Eliminate side effectsIncrease doseSugar derivativesPeptide/protein ingredientsSide effectHBsAg
This invention provides a recombinant super-compound interferon (rSIFN-co) and its equivalent with changed spatial configuration, high efficacy and low side effects. Therefore, high dose of rSIFN-co may be used. One characteristic of rSIFN-co is its ability to inhibit the HBV DNA duplication and secretion of HBsAg and HBeAg in in vitro pharmacological studies. The cytotoxic effect of rSIFN-co is only one-eighth (⅛) of currently clinically available interferons but its anti-viral effect is approximately five to twenty (5-20) times higher, and when used in vivo it has a broader spectrum of clinical applications and longer biofeedback response. This invention further provides super-compound interferon or its equivalent, synthesis of artificial gene with codon preference which codes for said rSIFN-co and its equivalent, vector comprising said gene and appropriate expression system for expression of said rSIFN-co. Finally this invention provides the super-compound interferon (rSIFN-co) and its equivalent, a process to produce same and uses thereof.
Owner:WEI GUANGWEN
Hollow nanoparticles and uses thereof
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS
Methods and compositions for treating hepatitis b
InactiveUS20050106174A1Preventing and alleviatingBiocideFungiSerum glutamate pyruvate transaminaseHBsAg
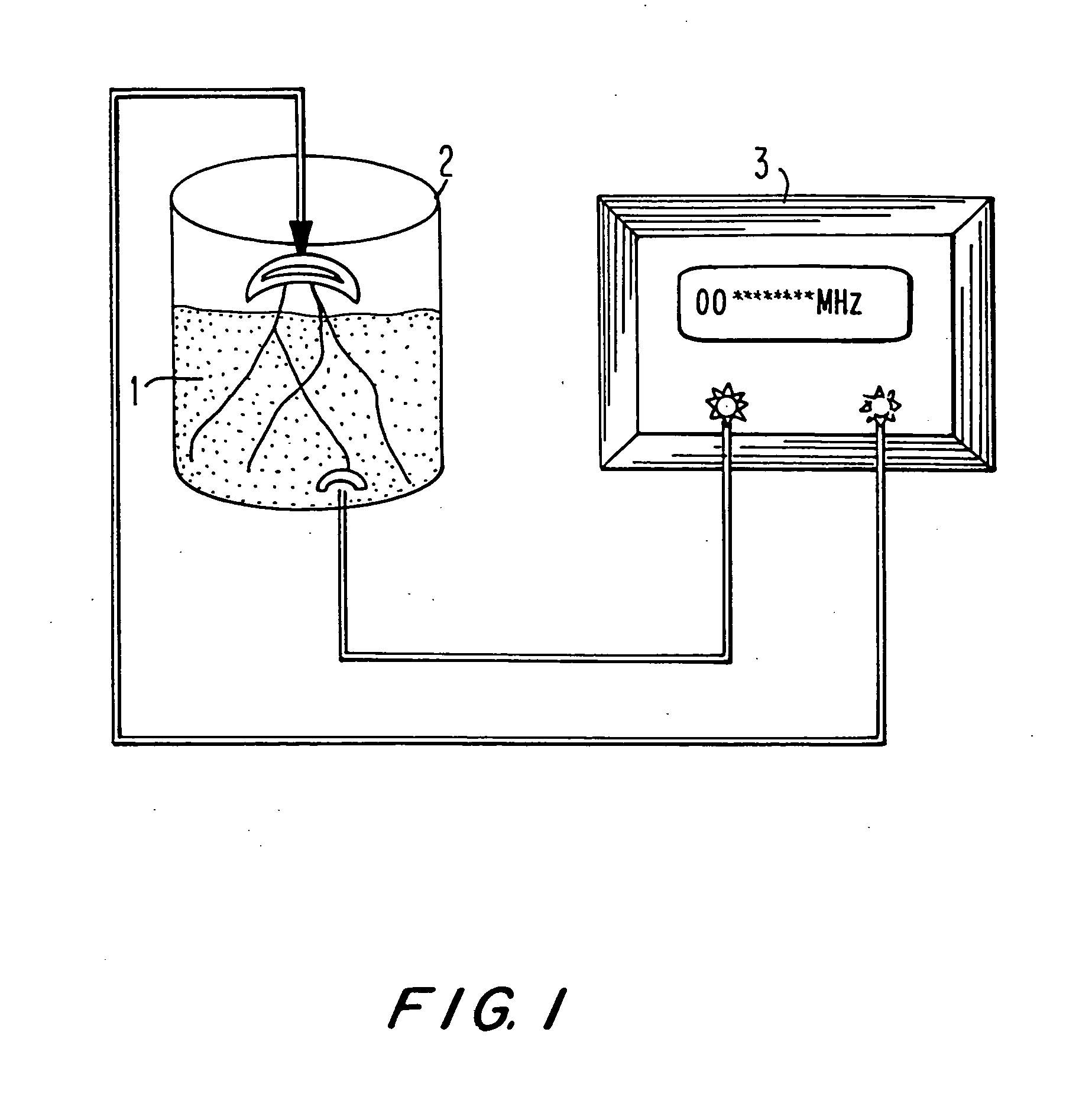
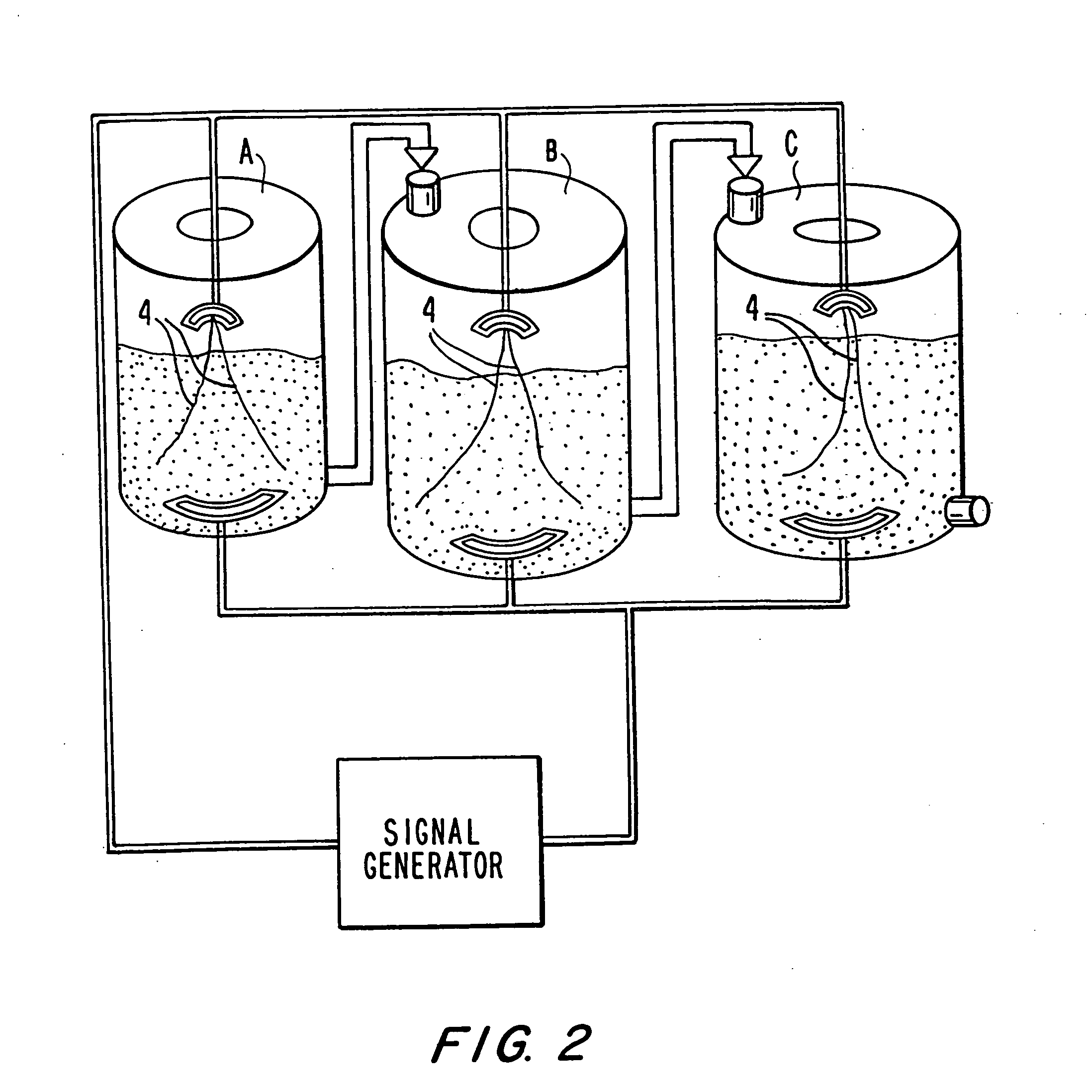
The invention provides compositions comprising a plurality of yeast cells, wherein said plurality of yeast cells are characterized by their ability to normalize the level of serum glutamate-pyruvate Transaminase (GPT), or reduce serum HBsAg levels in a subject, said ability resulting from their having been cultured in the presence of an alternating electric field having a specific frequency and a specific field strength. Also provided are methods of making and using these compositions.
Owner:ULTRA BIOTECH
Liver cancer resistant Antrodia camphorata and preparation method thererof
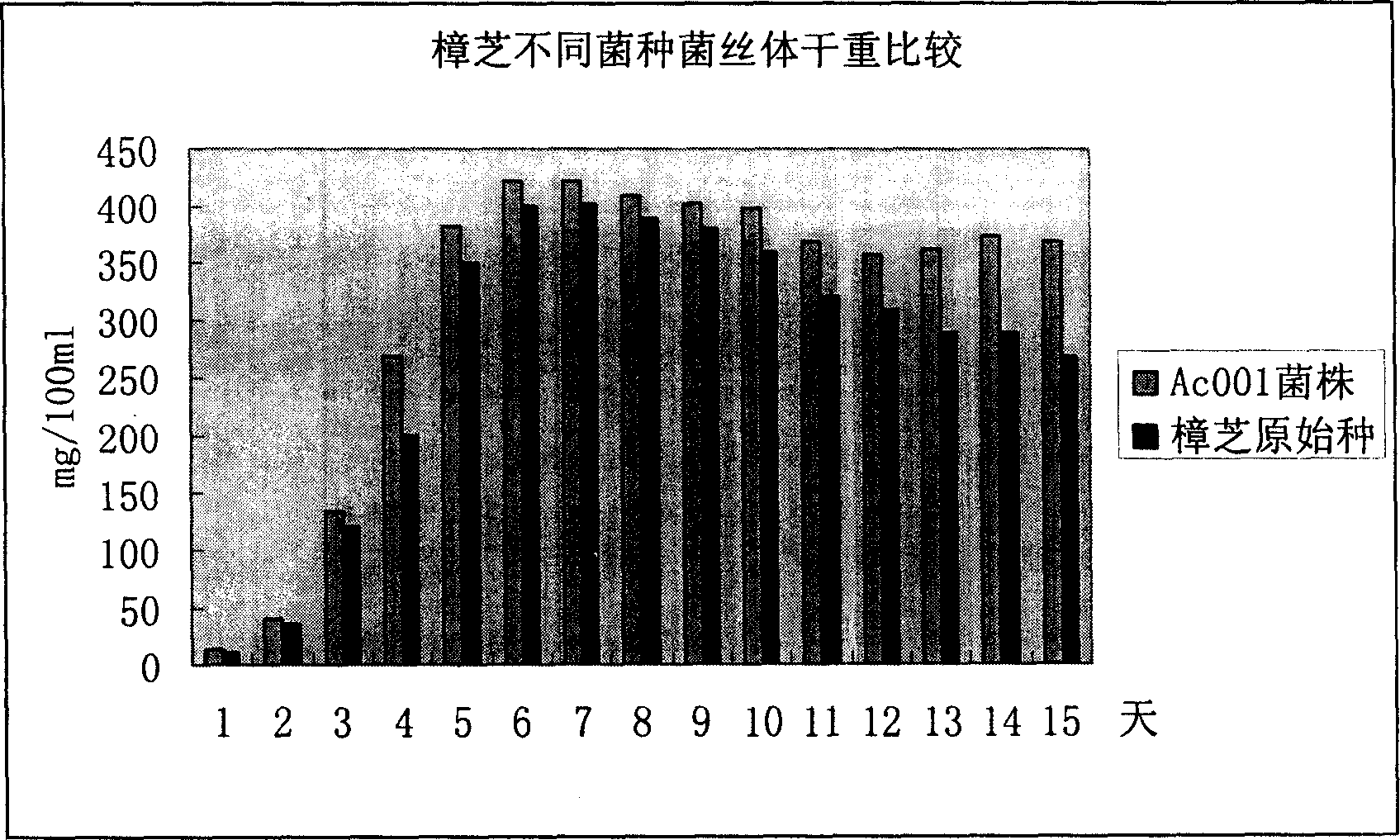
The invention discloses an Antrodia camphorata capsule for resisting hepatic carcinoma, which comprises the following constituents (by weight portions): Antrodia camphorate mycelium fermentation extract 20-100, protein-free maize starch or medicinal starch gum 200-480. The Antrodia camphorate mycelium fermentation extract is abstracted by ethanol and dried. The Antrodia camphorata bacterial strain Ac001 was preserved in the China General Microbiological Culture Collection Center with a docket number of CGMCC No.1460. The Antrodia camphorate mycelium fermentation extract in the hepatic carcinoma resisting Antrodia camphorate capsule has appreciable actions in inhibiting hepatitis B virus HbsAg, e antigen HbeAg and HBV DNA secretion and resisting cancers especially liver cancer.
Owner:LAIYANG AGRI COLLEGE
Immunogene therapeutic drug for chronic hepatitis B and preparation method for immunogene therapeutic drug
InactiveCN104940953AReduced viral copyImprove securityGenetic material ingredientsDigestive systemHBsAgChronic hepatitis
The invention relates to an immunogene therapeutic drug for chronic hepatitis B and a preparation method for the immunogene therapeutic drug. The active component of the drug is a replication-competent recombinant vector pSVK-dSFValpha-IRES-hIL-12 (for short, pSVK-HBVE), which is constructed by taking a pSVK carrier as a starting carrier and carries a fusion gene dSFValpha-IRES-hIL12. An antibody targeting interferon alpha and human IL-12 are co-expressed in the replication-competent recombinant vector pSVK to construct a humanized HBsAg dsFv antibody, human interferon-alpha and interleukin-12 fusion expression vector pSVK-dSFValpha-IRES-hIL-12, the fusion expression vector is efficiently expressed in an eukaryotic cell and in vitro, the combined application of human IL-12 and human IFN-alpha has an important synergistic effect during the tumor control process, and a safe and efficient immunogene therapeutic drug is provided for gene therapy of chronic hepatitis B.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Treatment of hepatitis B virus infection with human monoclonal antibodies
InactiveUS20050260195A1Eliminate infectionMicrobiological testing/measurementImmunoglobulins against virusesHBsAgCyrtanthus elatus virus A
Disclosed is a pharmaceutical composition for the treatment or prevention of hepatitis B virus infection, comprising a 1:3 mixture of two fully human anti HBsAg monoclonal antibodies 19.79.5 and 17.1.41. Also disclosed are preferred modes of administration. The pharmaceutical composition can be given as a monotherapy or in combination with other anti viral agents.
Owner:XTL BIOPHARMLS
siRNA for treating hbv
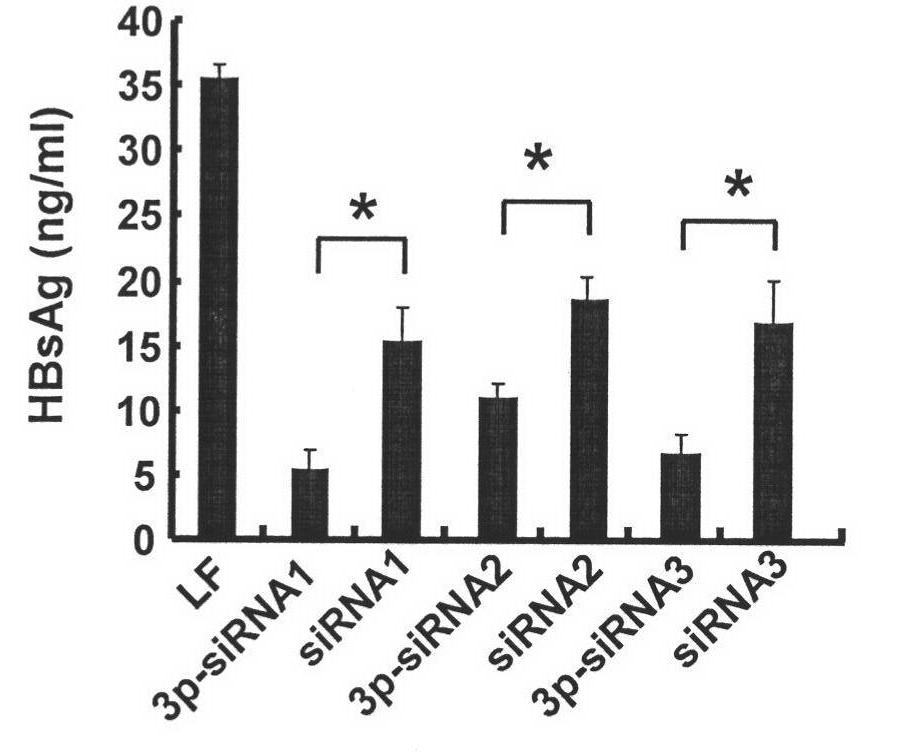
The invention discloses siRNA for treating HBV, which has a sequence shown in SEQ ID NO.1 or SEQ ID NO.2 or SEQ ID NO.3. The siRNA can be artificially synthesized or synthesized through in vivo transcription. After the siRNA transfects HepG2.2.15 cell for 24 hours and 48 hours, the siRNA can reduce the expression of HBx gene, the content of HBsAg and HBeAg in the cell and supernate is also obviously reduced, and the effect of 3p-siRNA is more obvious than that of chemically synthesized siRNA. The siRNA can be applied to medicaments for resisting the HBV.
Owner:SHANDONG UNIV
Methods for tailoring the immune response to an antigen or immunogen
The invention relates to methods and reagents for immunizing animals to elicit specific cellular and humoral immune-responses against specific antigens, such as viral antigens, including HBsAg antigen. The invention provides methods of using specifically prepared immunogen in fresh or lyophilized liposome, proper routes of administration of the immunogen, proper doses of the immunogen, and specific combinations of heterologous immunization including DNA priming in one administration route followed by liposome-mediated protein antigen boost in a different route to tailor the immune responses in respects of enhancing cell mediated immune response, cytokine secretion, humoral immune response, immune protection and selective skewing of T helper responses to be Th1, Th2, or a mixed or balanced Th response.
Owner:MUCOSAL VACCINE TECH LLC
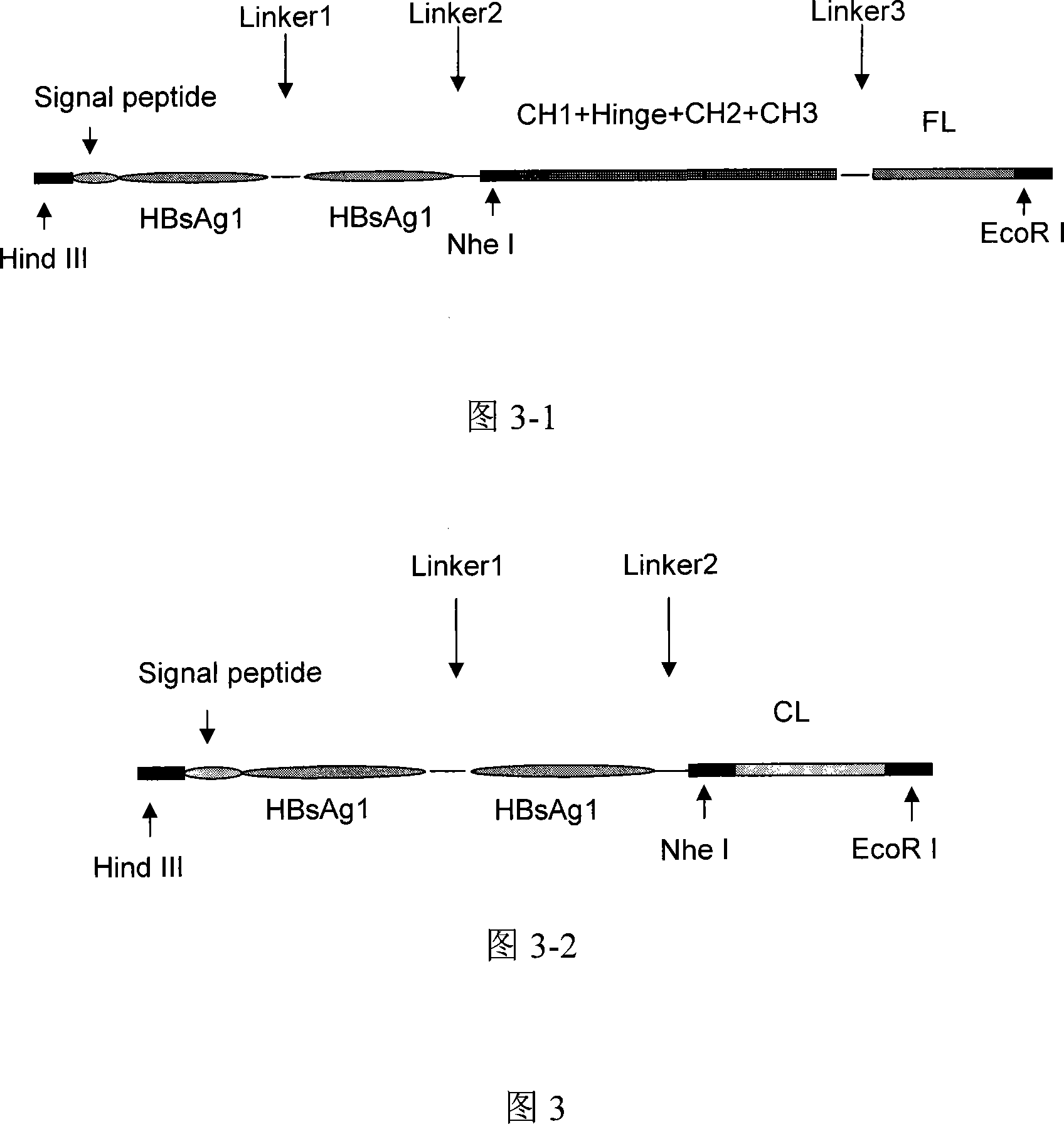
Hepatitis B fusion protein multiva lentvaccine, preparation method and uses thereof
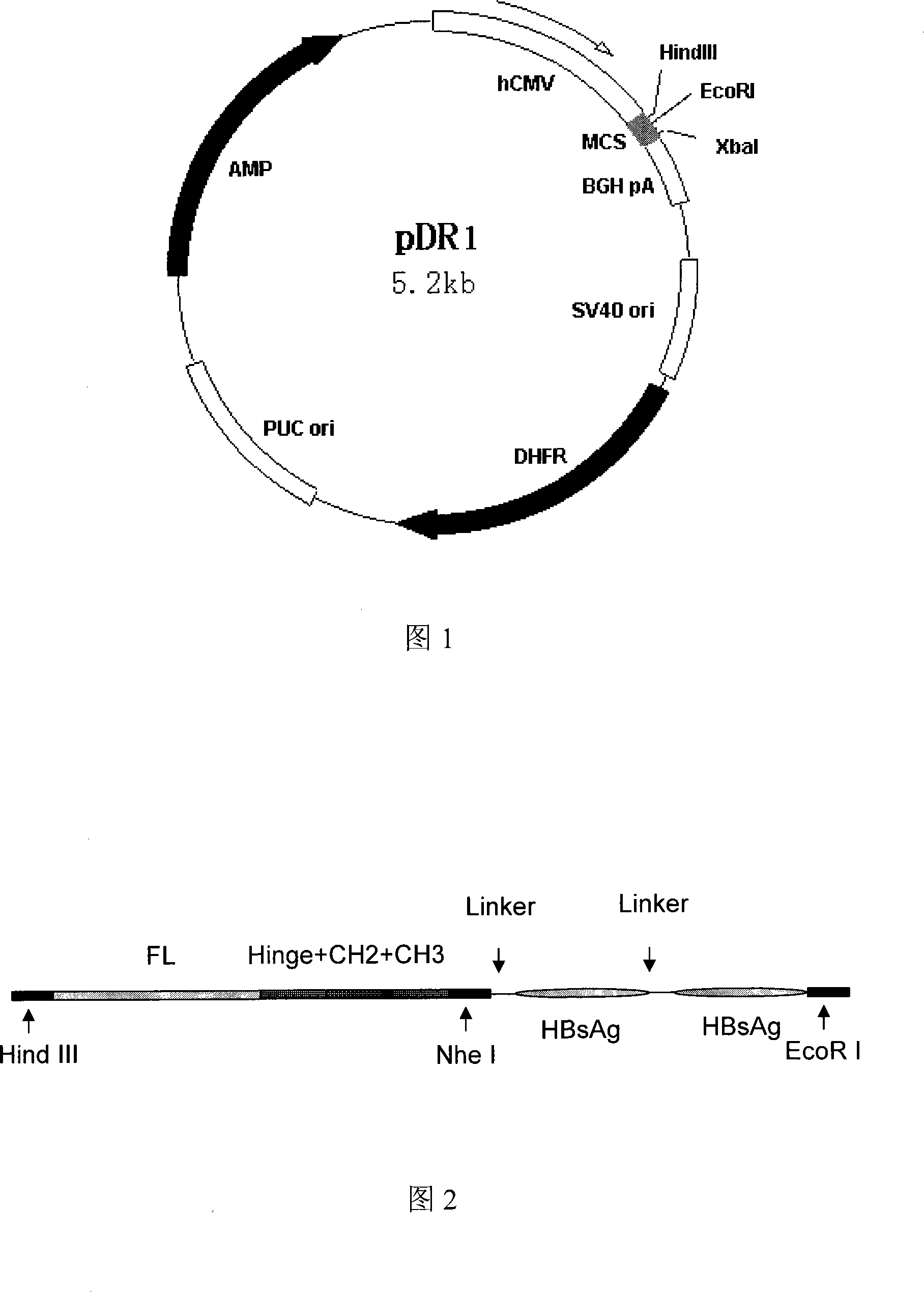
This invention discloses a multivalence hepatitis B fusion protein targeting adjuvant vaccine, a preparation method and an application thereof. Particularly, the invention discloses a quadrivalence hepatitis B fusion protein targeting adjuvant vaccine (FL-CH-HBsAg-HbsAg)2, an octivalence hepatitis B fusion protein targeting adjuvant vaccine (HBsAg-HBsAg-CH-FL, HBsAg-HBsAg-CL)2, a relevant preparation method, and an application in preparation of preventative pharmaceuticals, and in treatment and / or prevention of hepatitis B.
Owner:SHANGHAI NAT ENG RES CENT OF ANTIBODY MEDICINE +1
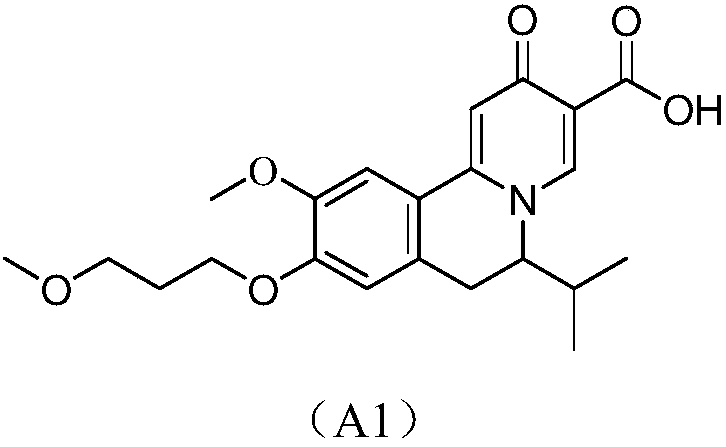
Compound for treating or preventing hepatitis B virus infection and preparation method and application thereof
ActiveCN108530449AInhibition of replicationImprove clearanceOrganic chemistryAntiviralsClearance rateHBsAg
The invention discloses a compound taking quinolizine ketone as a mother nucleus and for treating or preventing hepatitis B virus infection. The compound comprises optical isomer, raceme, cis-trans-isomer and any combination thereof or medicinal salt thereof. The invention further discloses a preparation method and application of the compound. The compound can remarkably lower in-vivo HBsAg leveland inhibit duplication of HBV, has good medicinal attribute and is low in toxicity, and pharmacokinetic and pharmacodynamic functions are improved; efficiency of combining with the HBV can be improved greatly, and clearance rate of in-vivo HBV can be further increased.
Owner:河南春风医药科技有限公司
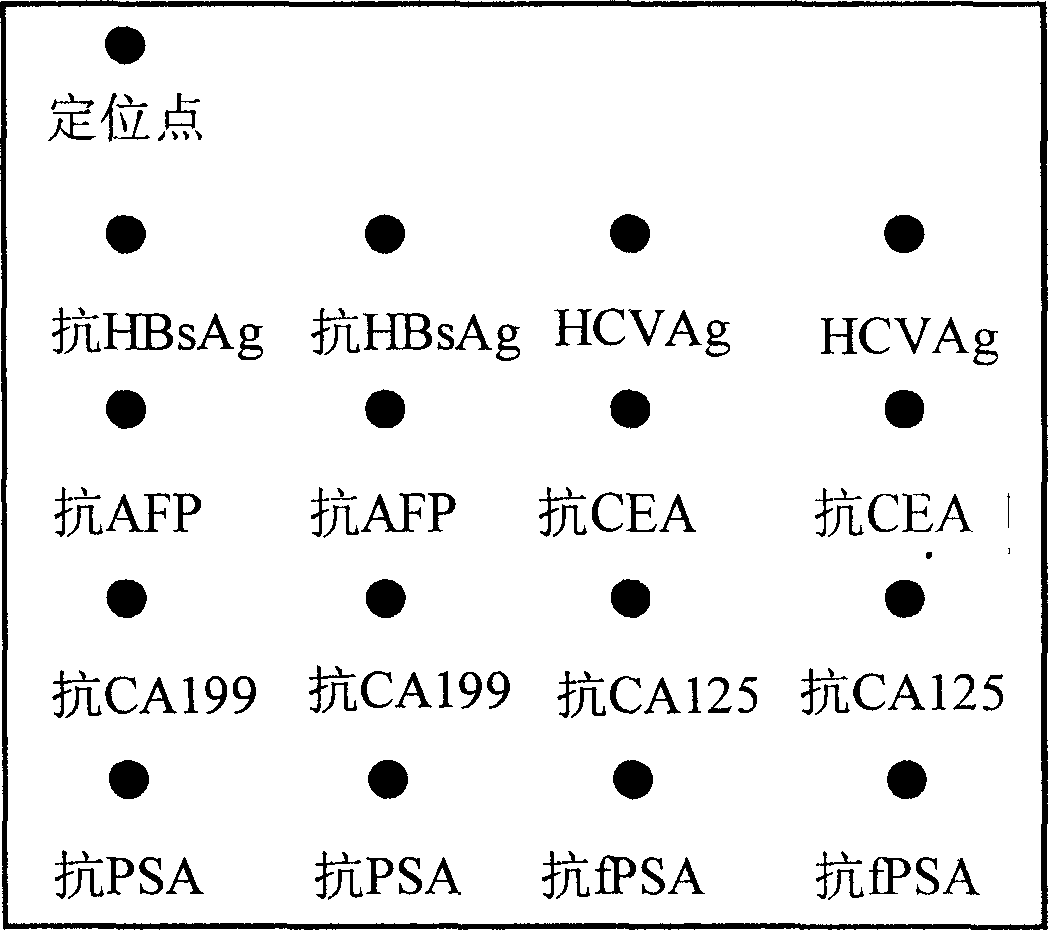
Hepatitis B and C and multiple tumor marker protein chip inspecting reagent unit
A detection kit of marker substance protein chip for hepatitis B and C as well as multiple type of tumor is prepared as having base plate and reaction holes on it set on reaction hole plate; including 12-200 sample holes and 5-8 standard piece holes in said reaction holes as each reaction hole bottom being set with solid phase carrier; enveloping micro lattice of eight antigen or and body as anti-HBsAg, anti-HCVAg, anti-AFP, anti-CEA, anti-CA199, anti-CA125, anti-PSA and anti-fPSA on said carrier for accurately diagnose said disease in multiple man-share.
Owner:穆海东
Production of mouse model of viral hepatitis B with high expression HBsAg
InactiveCN1679967AOvercome injectionOvercoming disadvantages of portal vein injection of naked DNAIn-vivo testing preparationsEscherichia coliPhosphorylation
A process for preparing VHB mouse model with high expression of HBsAg includes such steps as linearizing carrier pcDNA3 by EcoRV, dephosphorylating by alkaline phosphatase of ox's small intestine, recombining the clonal carrier p3.6II, enzyme severing by PvuII to obtain 1.1-time HBV gene fragment, linking it with linear pcDNA3, transforming colibacillus competent cell DH5 alpha, screening resistance, enzyme serving, sequencing to verify the recombinant plasmid, injecting it in the mouse body via tail vein, and verifying the model.
Owner:SHANDONG UNIV
HBsAg aptamer screening method based on magnetic separation
InactiveCN103667483AIncrease fixed amountFast magnetic separationMicrobiological testing/measurementDNA preparationAptamerHBsAg
Owner:SOUTHEAST UNIV
Therapeutic HBV DNA vaccines, method for preparing the same and application thereof
ActiveCN101095951ALower levelImprove immunityGenetic material ingredientsDigestive systemBALB/cAdjuvant
The invention relates to a kind of HBV bi-plasmid DNA vaccine, the preparation method and its application. Said HBV bi-plasmid DNA vaccine comprises vaccine plasmid pS2. S and its adjuvant plasmid pIIF, the eucaryon expression vector is pVAX1. The plasmid is transfected to Africa green monkey kidney cell(COS-7), and the detected target gene expression is higher. The compatibility of bi-plasmid is carried out with ratio being 1: 1 after crack and purification, the Balb / c mouse is injected with volume impulse technique and it is detected that the HBV DNA vaccine can induce body to generate higher HBsAg antibody performance and special cell immunity response. The invention also relates to the application of said HBV DNA vaccine for medicine preparation to treat hepatitis B.
Owner:广州广药益甘生物制品股份有限公司
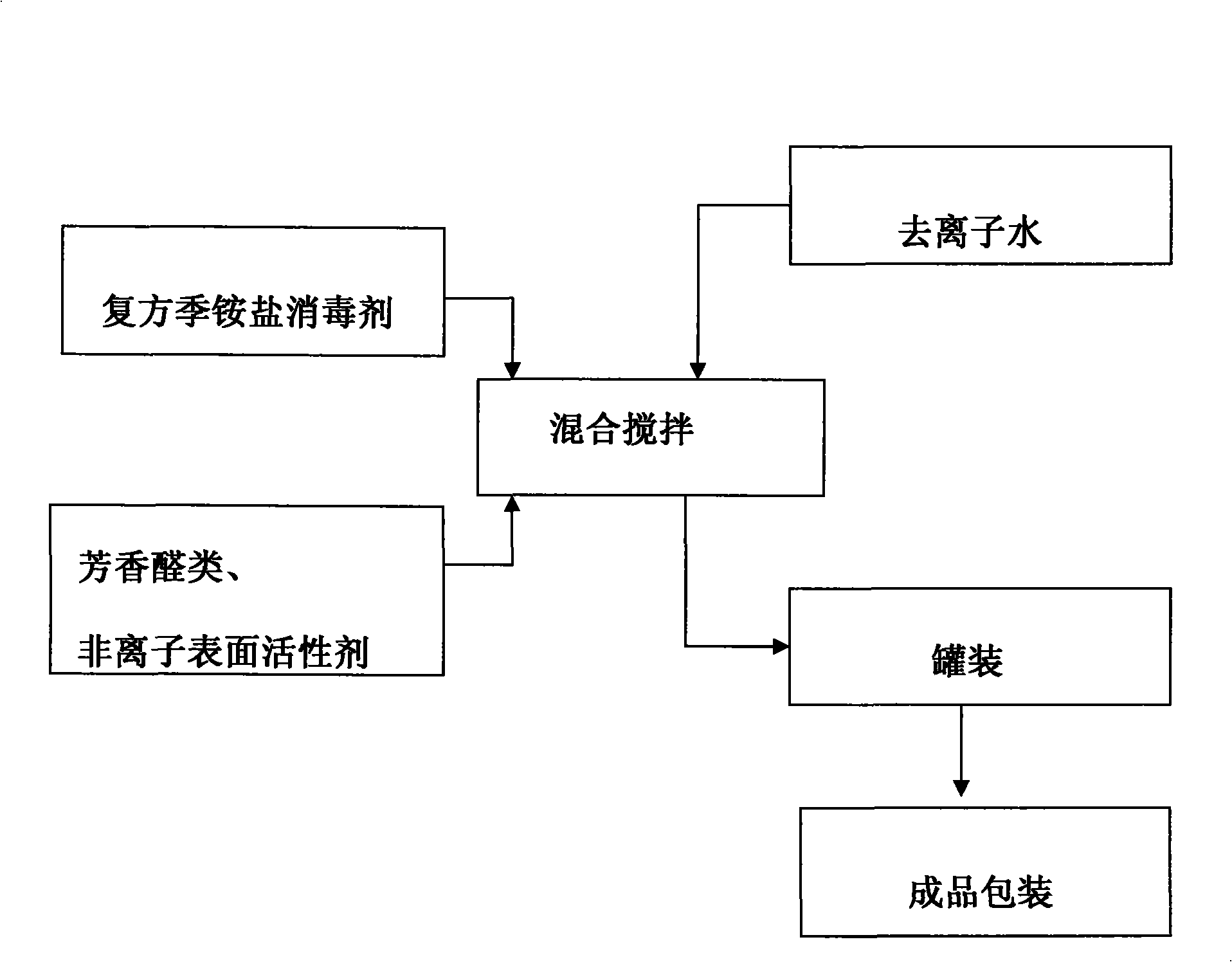
Novel compound quaternary ammonium salt disinfectant and preparation method
The invention discloses a novel compound quaternary ammonium disinfectant that can be prepared by mixing the component materials as below in proportion by share: 68-72 shares of dialkyl(C8-10) dimethyl ammonium chloride, 20-30 shares of dodecyl benzyl dimethyl ammonium chloride, 1-3 shares of glutaraldehyde and 2-6 shares of dialkyl(C8-10) dimethyl amine. The disinfectant can meet the requirement for disinfectant with high efficiency. The killing rate of the spores of bacillus subtilis var.niger and bacillus anthracis in 3-5 minutes is higher than 99% at 40ppm, and the disinfectant has preferable destructive effect on HBsAg at 50ppm. The killing rate of E.coli and Staphyloccocus aureus Rosenbach is higher than 99% at 30ppm. The killing rate of fowl plague virus is higher than 99% at 50ppm. The disinfectant can block the spread of H5N1 and H9N2 avian influenza virus as well as Newcastle disease virus among the chicken embryos effectively.
Owner:陈波
Application of miR-199a-3p in preparation of medicine for resisting hepatitis B virus
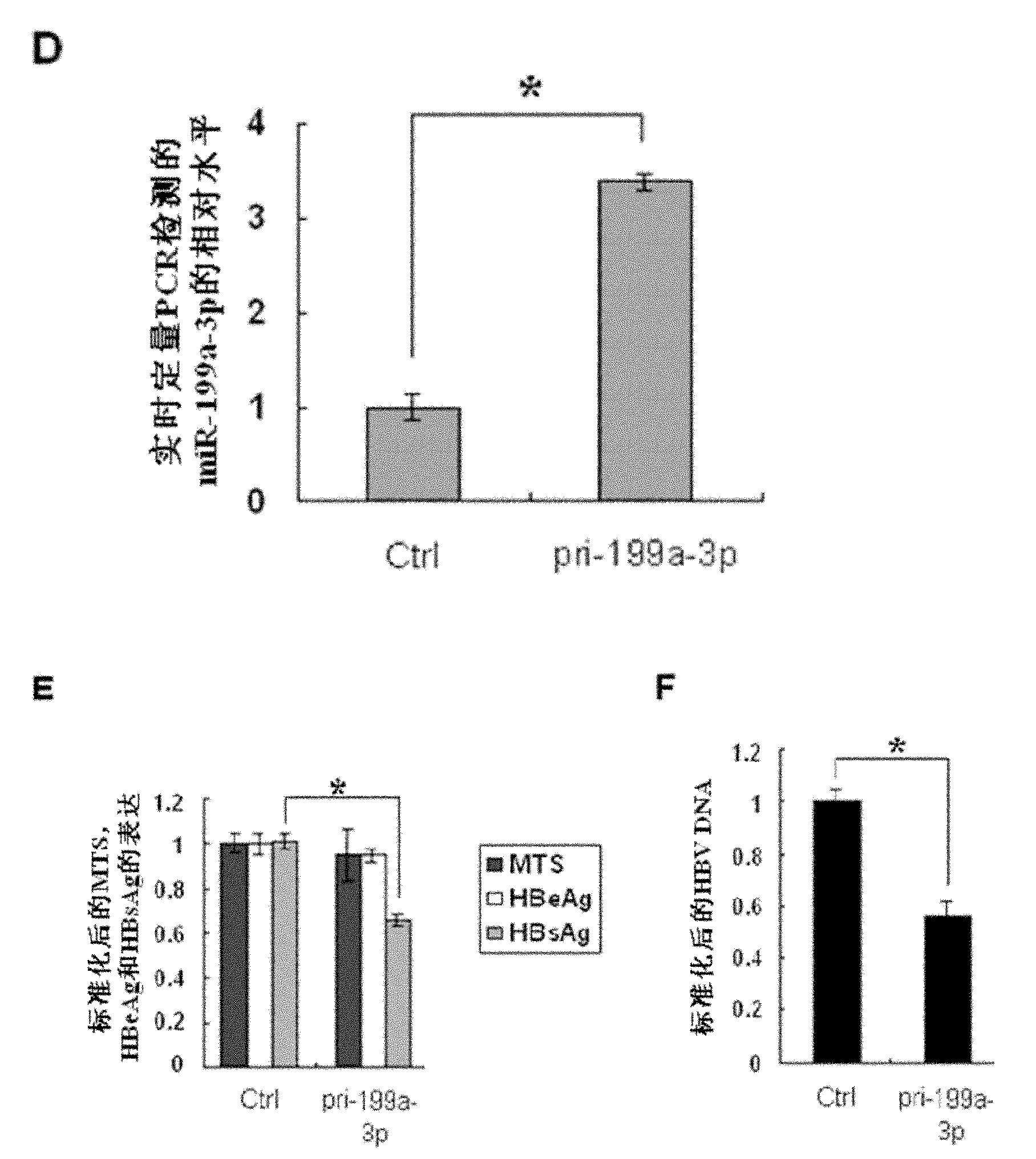
InactiveCN101954094AReduce expressionDoes not affect proliferationOrganic active ingredientsGenetic material ingredientsHBsAgFluorescence
The invention discloses application of miR-199a-3p in the preparation of a medicine for resisting hepatitis B virus (HBV). MiR-199a-3p is a nucleotide sequence described in the sequence table SEQ ID NO:1. Experimental results show that miR-199a-3p can effectively reduce the expression of HBsAg, but can not influence the proliferation of HepG2 2.2.15 cells. Real time PCR (Polymerase Chain Reaction) to HBV DNA quantitatively displays that miR-199a-3p inhibits the replication of the virus. Bioinformatic analysis shows that the HBsAg coding area has a binding site of miR-199a-3p; fluorescence report carrier experiments verify the influence of miR-199a-3p on HBV transcription products; and meanwhile, real time PCR and western blot are used for further verifying that miR-199a-3p has inhibition action on the expression of HBsAg. The results imply that miR-199a-3p plays an important role in regulating and controlling the replication of HBC and can be prepared into a medicine for resisting HBV.
Owner:TIANJIN MEDICAL UNIV
Application of wogonin for preparing medicine to treat or prevent hepatitis B
ActiveCN1785174AInhibition of replicationSignificant effectOrganic active ingredientsDigestive systemE AntigensSerum ige
Owner:CHINA PHARM UNIV +1
Synthesis of human virus antigens by yeast
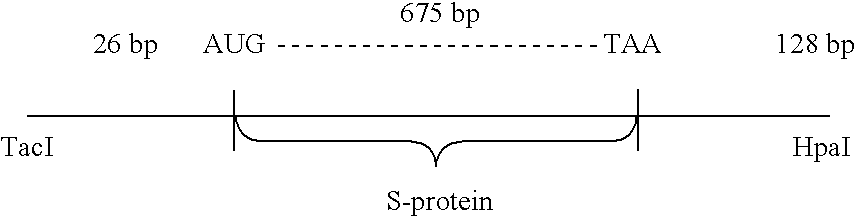
The present invention relates to synthesis of HBsAg in yeast. Yeast expression vectors comprising a yeast promoter, ADH1, have been constructed. The region of the HBV genome coding for the S-protein, excluding a possible 163 amino acid presequence, has been transferred to the yeast expression vector. Using the described yeast vector, the successful synthesis of HBsAg by yeast has been achieved. The product is antigenic (reactive with anti-HBsAg), and a substantial portion is found associated with particles identical in electron microscopic appearance to those found in the serum of HBV-infected patients and in Alexander cells but having a smaller particle size diameter. The HBSAg synthesized by yeast has identical sedimentation behavior to purified, naturally-occurring HBsAq particles purified from Alexander cells as measured by sucrose gradient sedimentation. The present invention demonstrates synthesis and assembly of a higher ordered multi-component structure resulting from expression of a heterologous DNA coding segment in a microorganism.
Owner:RGT UNIV OF CALIFORNIA
Hybridized tumour cell strain of anti hpatitis B virus surface antigen and its monoclonal antibody
InactiveCN1693454AConvenient for clinical operationAdvancing EpidemiologyImmunoglobulins against animals/humansTissue cultureAntigenHBsAg
A hybridoma cell strain (CCTCC-C200505) is disclosed, which can secrete the monoclonal antibody of HBV surface antigen. An EL1SA method created by enveloping the monoclonal antibody can detect wild-type HBsAg and 13 variant HBsAgs.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
Iridoid glycoside composition
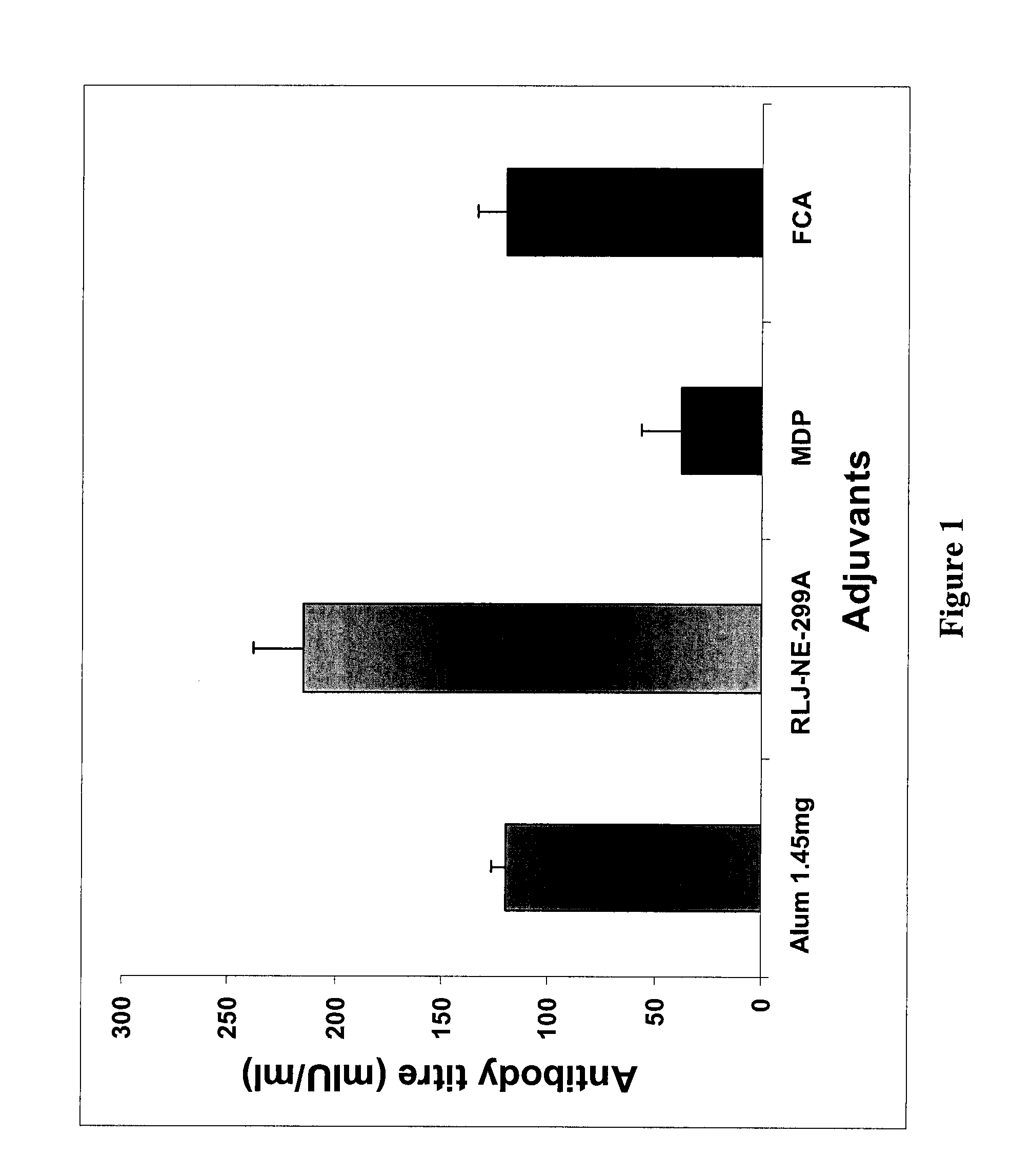
The present invention relates to an adjuvants, particularly to the use of a well-characterized plant based iridoid glycoside adjuvant from plant Picrorhiza kurroa, acting as an adjuvant against T-dependent antigen and specifically against HBsAg and typhoid antigens. The present invention also relates to the method of producing the iridoid glycoside adjuvant and the products utilizing such adjuvants for induction of cellular immunity. The adjuvants may be used alone or with specific antigens. The two antigens used in the study represents HBsAg, a recombinant antigen expressed in Pichia pastoris, and typhoid Vi polysaccharide purified from Salmonella typhi broth. These antigens are studied for their immunogenicity with the adjuvant iridoid glycoside adjuvant
Owner:COUNCIL OF SCI & IND RES +1
Tiantan remocined vaccine virus of IFN-alpha receptor gene (B8R) deletion and application thereof
InactiveCN1560248AImprove securityImproving immunogenicityViruses/bacteriophagesAntibody medical ingredientsIfn alphaHBsAg
The invention relates to an IFN-gama receptor gene (B8R) deleted attenuating carrier of Tiantan recombinant t vaccinia virus as well as a Tiantan recombinant t vaccinia virus AIDS vaccinum deleting IFN-gama receptor gene (B8R) and expressing various HIV-1 antigens, which is constructed based on this carrier, a Tiantan recombinant t vaccinia virus VTKgpe, recombinant t vaccinia virus VTKgpe CGMCC.No.1099 and another a hepatitis-B virus HBSAg antigen, and a hepatitis-B vaccinum of Tiantan recombinant t vaccinia virus of IL-2.
Owner:NAT CENT FOR AIDSSTD CONTROL & PREVENTION CHINESE CENT FOR DISEASE CONTROL & PREVENTION
Recombinant super-compound interferon and uses thereof
ActiveCN101137391AObvious cytopathicReduce reflected lightPeptide/protein ingredientsDigestive systemSide effectHBsAg
This invention provides different uses of recombinant super-compound interferons (rSIFN-cos) and its equivalent with changed spatial configuration, high efficacy and low side effects. Therefore, high dose of rSIFN-co may be used. One characteristic of rSIFN-co is its ability to inhibit the HBV DNA duplication and secretion of HBsAg and HBeAg in in vitro pharmacological studies. The cytotoxic effect of rSIFN-co is only one-eighth (1 / 8) of currently clinically available interferons but its anti-viral effect is approximately five to twenty (5-20) times higher, and when used in vivo it has a broader spectrum of clinical applications and longer biofeedback response. This invention further provides super-compound interferon or its equivalent, synthesis of artificial gene with codon preference which codes for said rSIFN-co and its equivalent, vector comprising said gene and appropriate expression system for expression of said rSIFN-co. Finally this invention provides the super-compound interferon (rSIFN-co) and its equivalent, a process to produce same and uses thereof.
Owner:SUPERLAB FAR EAST LTD
Use of human antibody capable of neutralizing hepatitis b virus for the prevention or treatment of hepatitis b virus infection
Owner:GREEN CROSS CORP THE
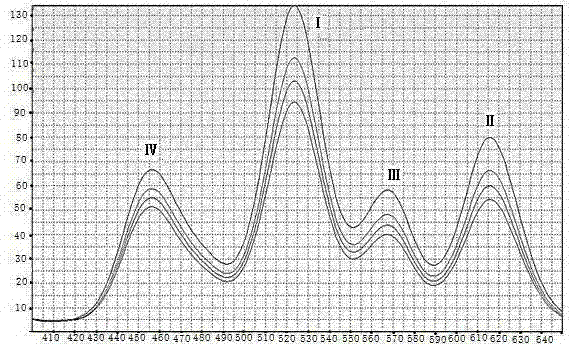
Enzyme-linked immunosorbent assay detection kit and preparation method thereof
The invention relates to an enzyme-linked immunosorbent assay detection kit and a preparation method thereof. The enzyme-linked immunosorbent assay kit comprises an avidin-coated microplate, a biotin-labeled HbsAb, a biotin-labeled HCV gene recombinant antigen, a biotin-labeled HIV gene recombination antigen, a horseradish peroxidase-labeled HbsAb, a horseradish peroxidase-labeled mouse anti-humanIgG, a horseradish peroxidase-labeled HIV gene recombinant antigen, a substrate solution A, a substrate solution B, a washing concentrate and a stop buffer. The enzyme-linked immunosorbent assay detection kit of the invention can be used to simultaneously detect an HBsAg, an HCV virus IgG antibody and an HIV virus antibody in a sample. The enzyme-linked immunosorbent assay detection kit for bloodsource screening has the advantages of quickness, simplicity, high accuracy, high sensitivity and wide application range. With the detection kit adopted, results can be judged and read with only 2 hours of whole operation time needed. The enzyme-linked immunosorbent assay detection kit is very suitable for blood source screening.
Owner:江苏伯纳德生物科技发展有限公司
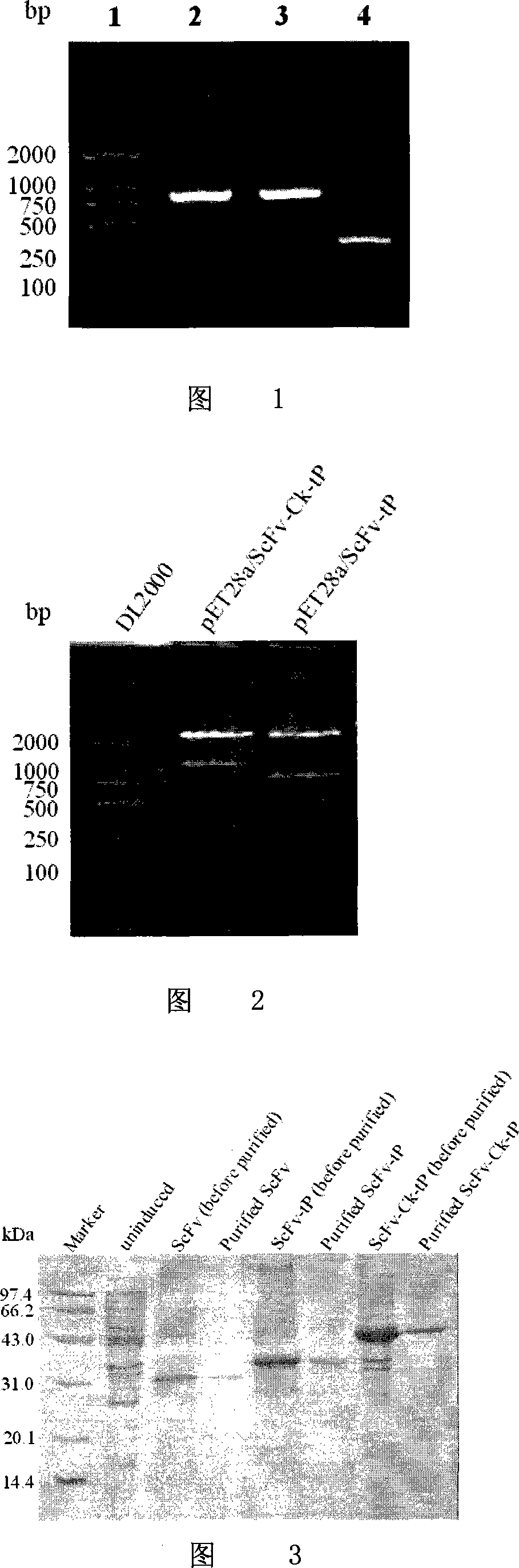
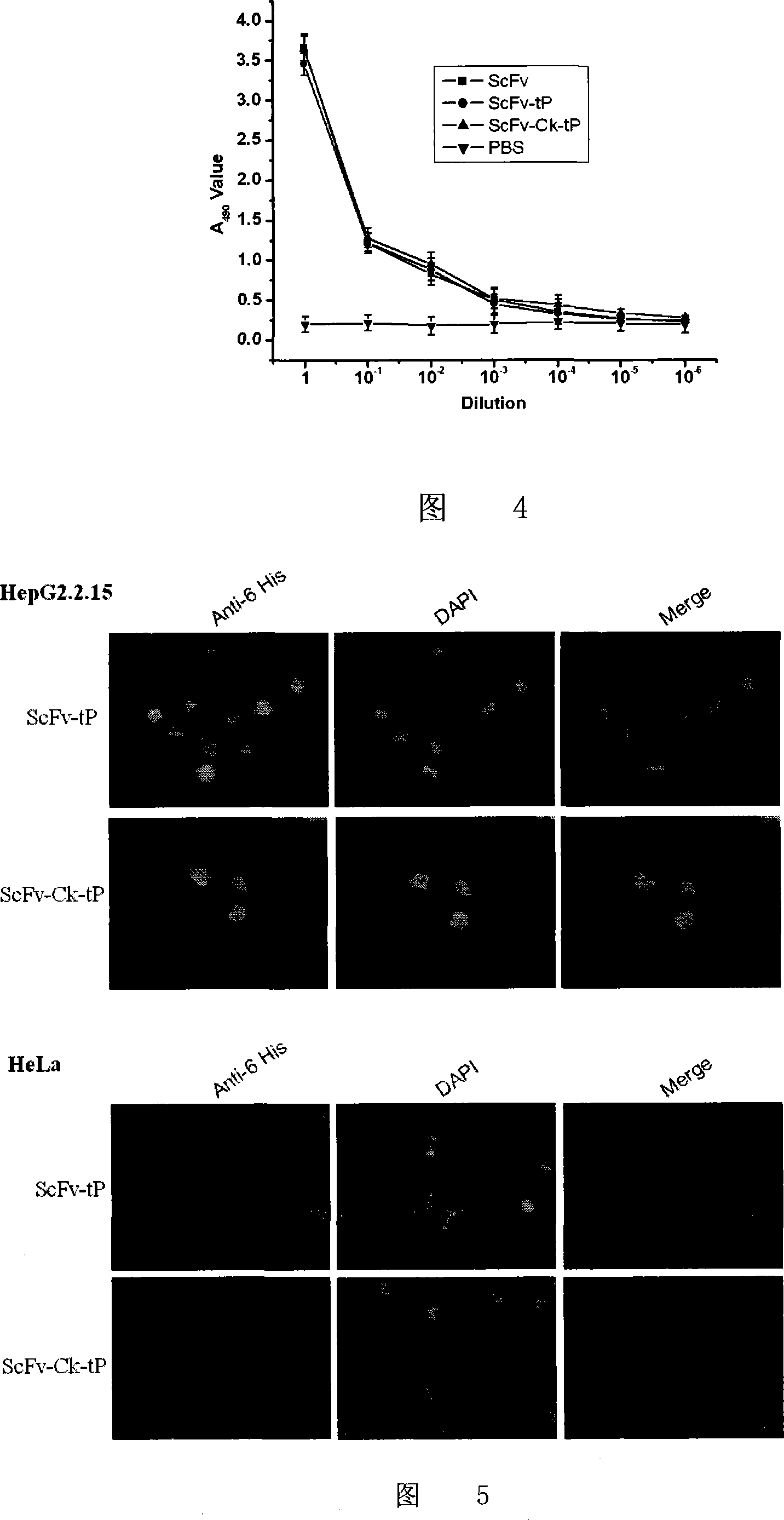
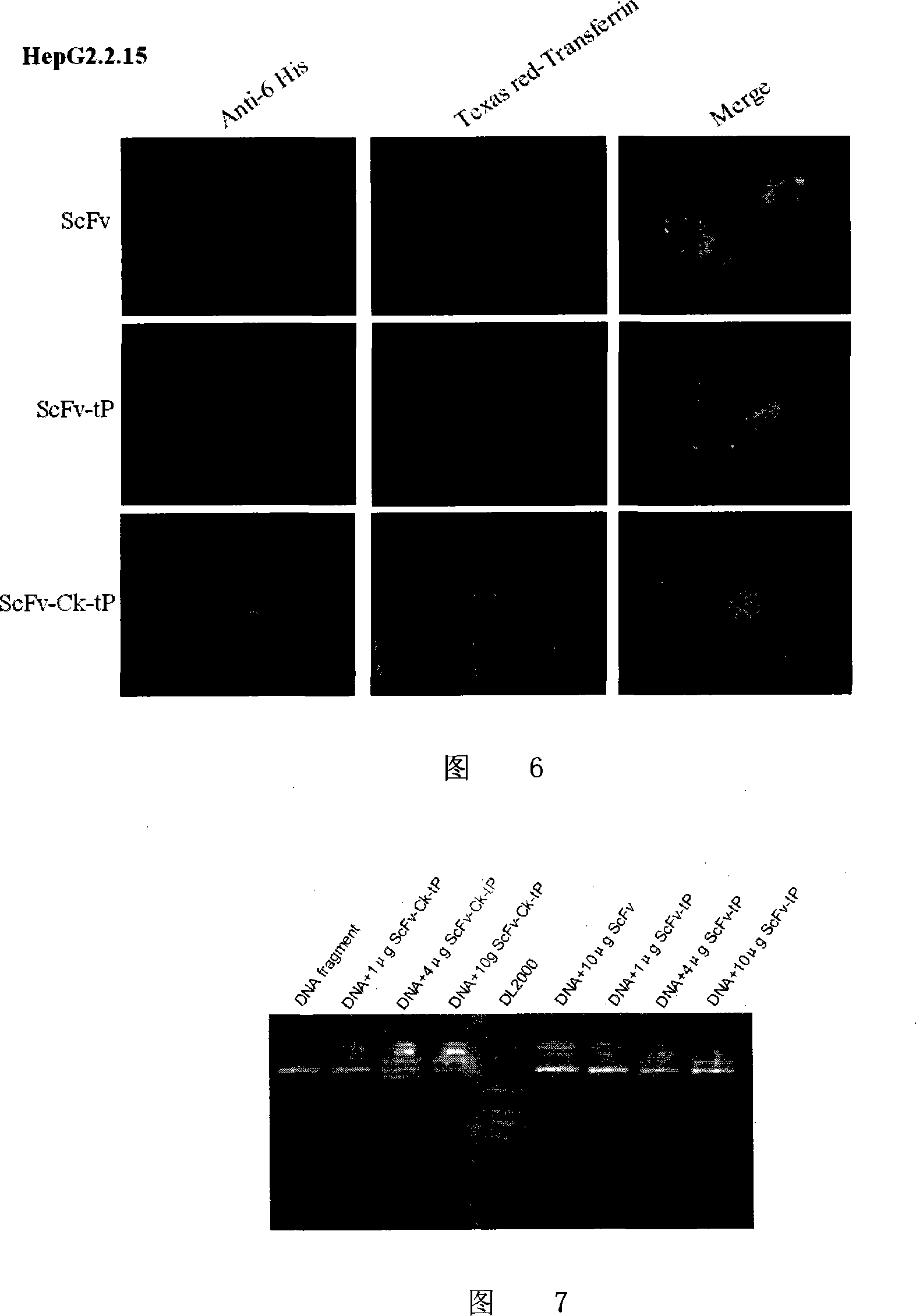
Human anti-HBsAg single-chain antibody/human antibody light chain constant region/protamine truncated recombination gene, coding protein and application
InactiveCN101100671AAvoid side effectsAvoid degradationPeptide/protein ingredientsGenetic material ingredientsDiseaseAntigen
A human anti-HBsAg mono-chain antibody / human antibody light chain constant region / protamine truncate recombinant gene, encoding protein and use are disclosed. The protein has antigen and nucleic acid combined activities. The process is carried out by: combining HBV siRNA with siRNA expression plasmid, transferring to HBV infectious cell specifically, and inhibiting HBV gene expression and copy. It has excellent specificity, efficiency, continuity and safety.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
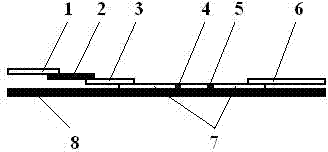
Quantum-dot marking test strip capable of quantitatively determining multiple indexes of blood infectious diseases and preparation method and quantitative determination method thereof
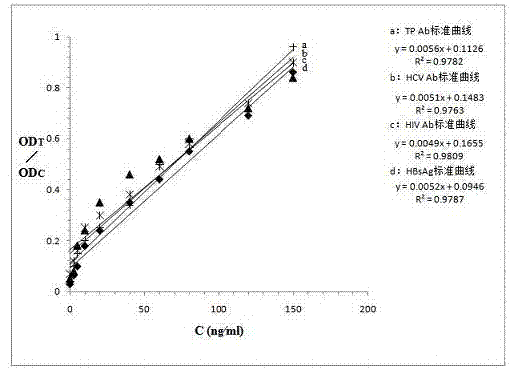
The invention belongs to the field of in-vitro diagnosis, and concretely relates to a quantum-dot marking test strip capable of quantitatively determining multiple indexes of blood infectious diseases and a preparation method and a quantitative determination method. A marking pad (3) of the test strip is coated with a quantum-dot marked mixture of HBsAg monoclonal antibody, HIVAg, HCVAg and TPAg, a T band (4) of an analysis membrane (7) is coated with a mixture of HBsAg monoclonal antibody, HIVAg, mouse anti-human antibody and TPAg, and a C band (5) of the analysis membrane (7) is coated with secondary antibody. The standard curves of all detected substances are stored and installed on the test strip by employing electron labels. The test strip employs a detector with signal detection function to read the standard curves stored in the electron labels and combines with the to-be-tested sample corresponding fluorescence intensity measured by the detector, so as to synchronously quantitatively determine the concentration of HBsAg, HIVAb, HCVAb and TPAb in the samples.
Owner:CHENGDU LINGYU BIOTECH
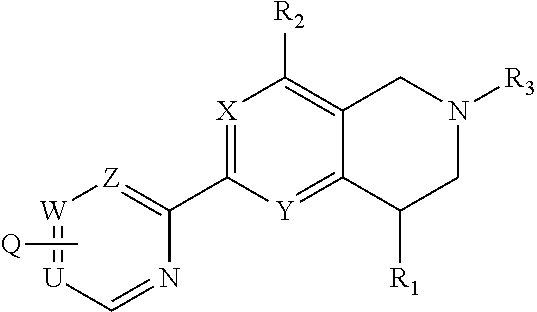
Novel tetrahydropyridopyimidines and tetrahydropridopyridopyridines for the treatment and prophylaxis of hepatitis b virus infection
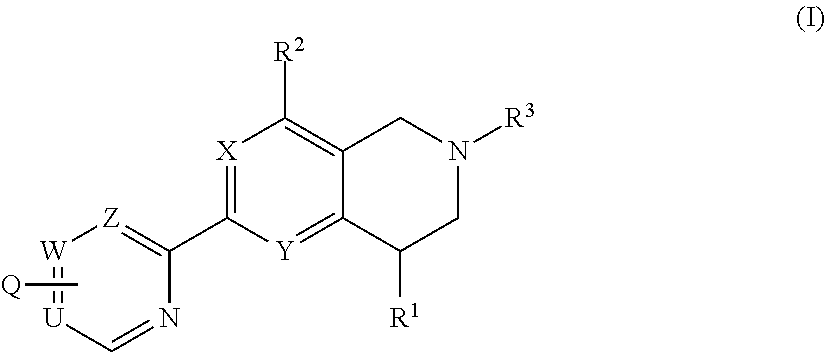
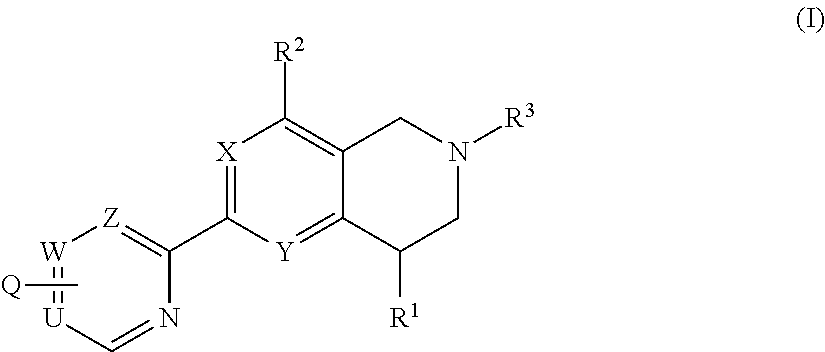
The invention provides novel compounds having the general formula:wherein R1, R2, R3, Q, U,W, Z, X and Y are as described herein, compositions including the compounds and methods of using the compounds. These compounds are HbsAg inhibitors and are useful as medicaments for the treatment or prophylaxis of HBV infection.
Owner:F HOFFMANN LA ROCHE INC
Use of human antibody capable of neutralizing hepatitis b virus for the prevention or treatment of hepatitis b virus infection
Owner:THE GREEN CROSS CORP
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com