Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1924 results about "Small intestine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
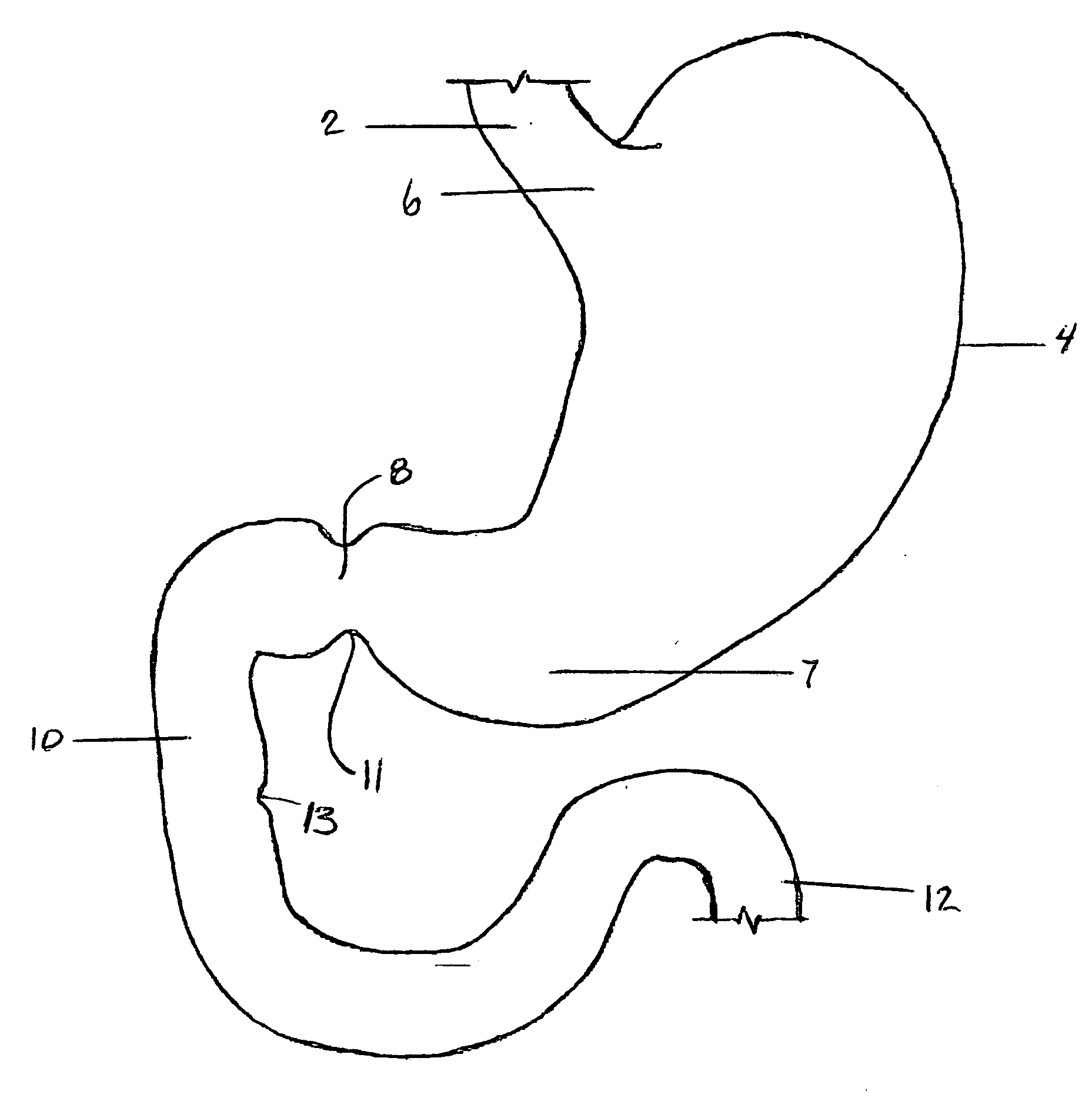
The small intestine or small bowel is an organ in the gastrointestinal tract where most of the end absorption of nutrients and minerals from food takes place. It lies between the stomach and large intestine, and receives bile and pancreatic juice through the pancreatic duct to aid in digestion.
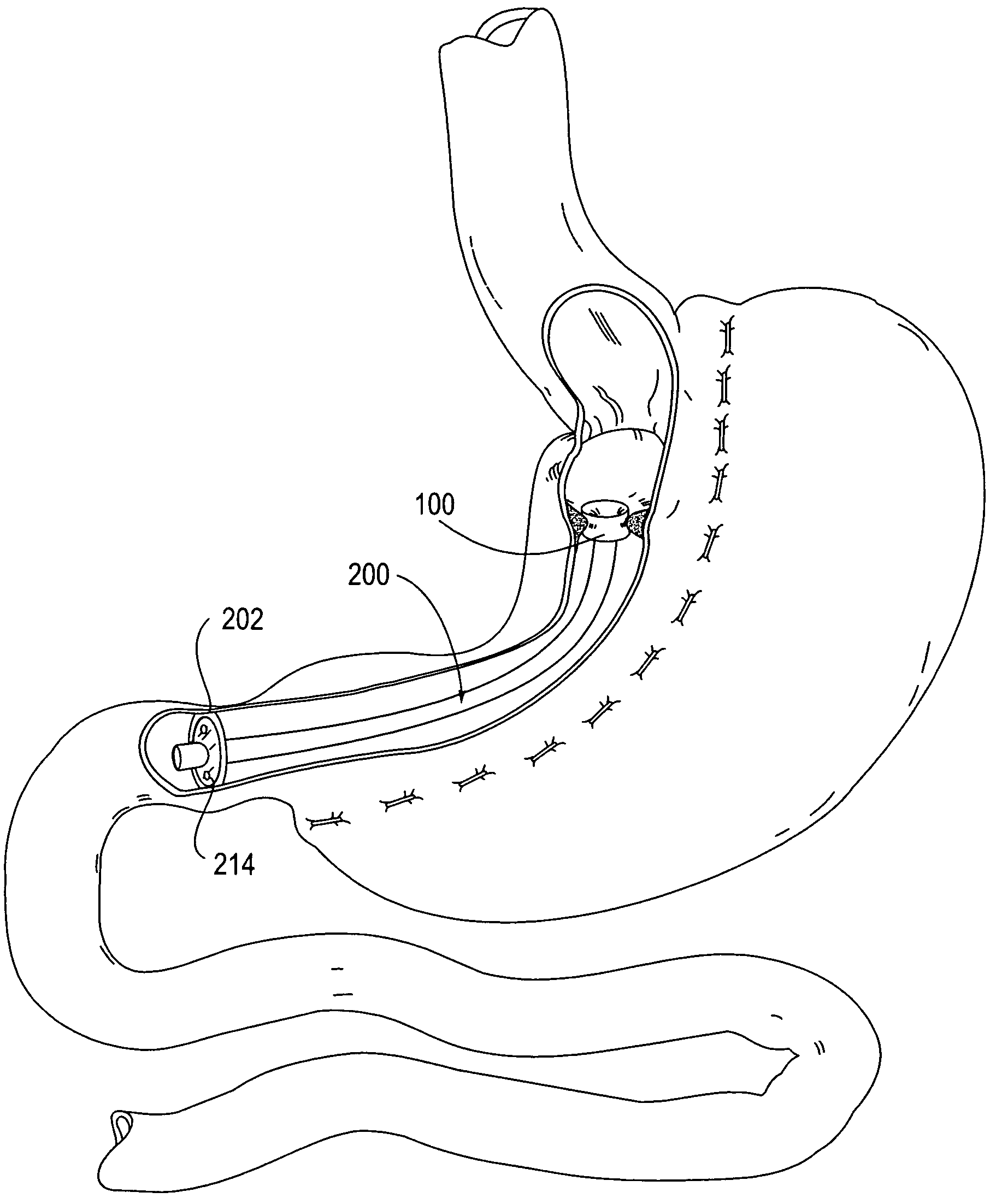
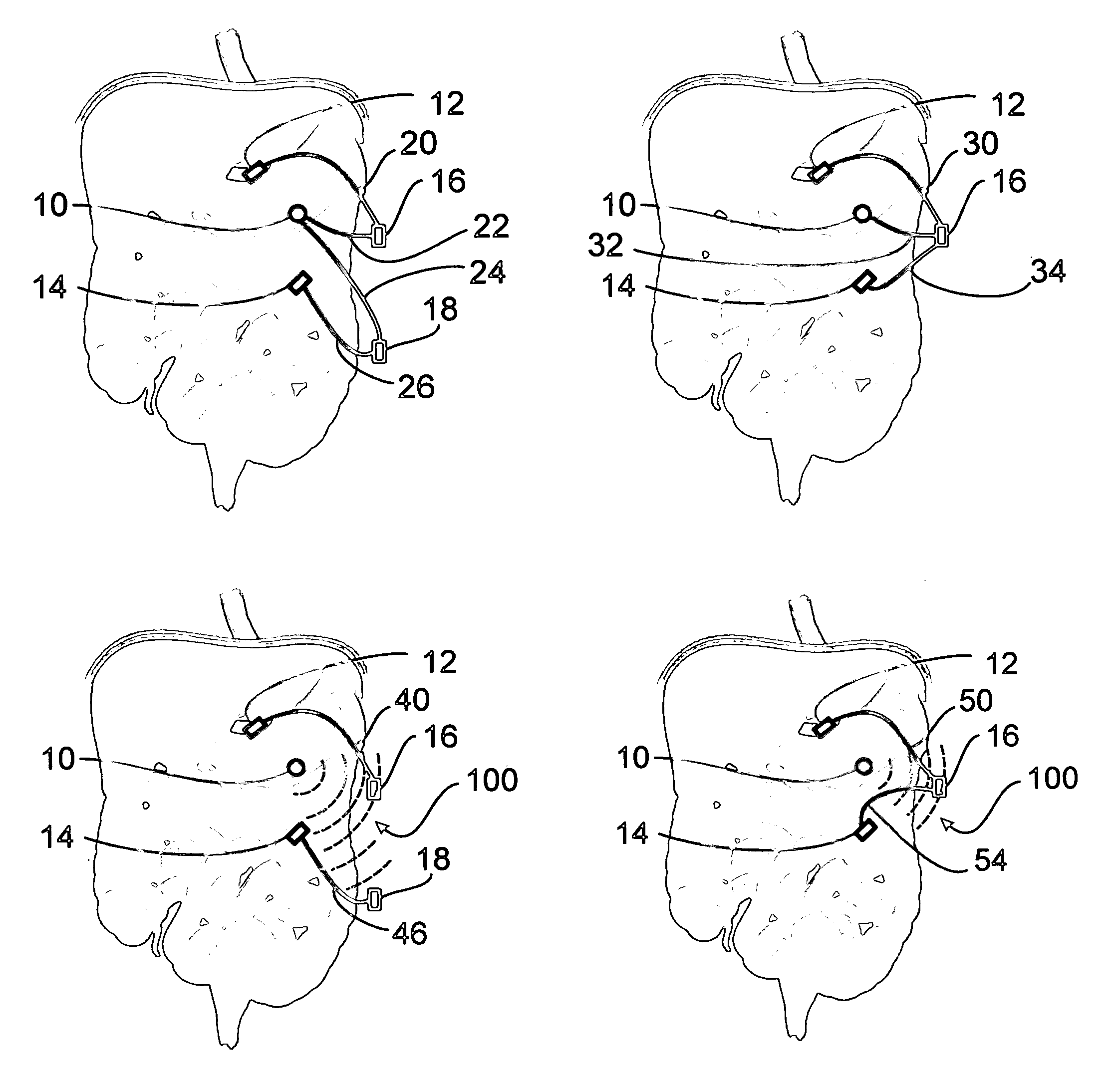
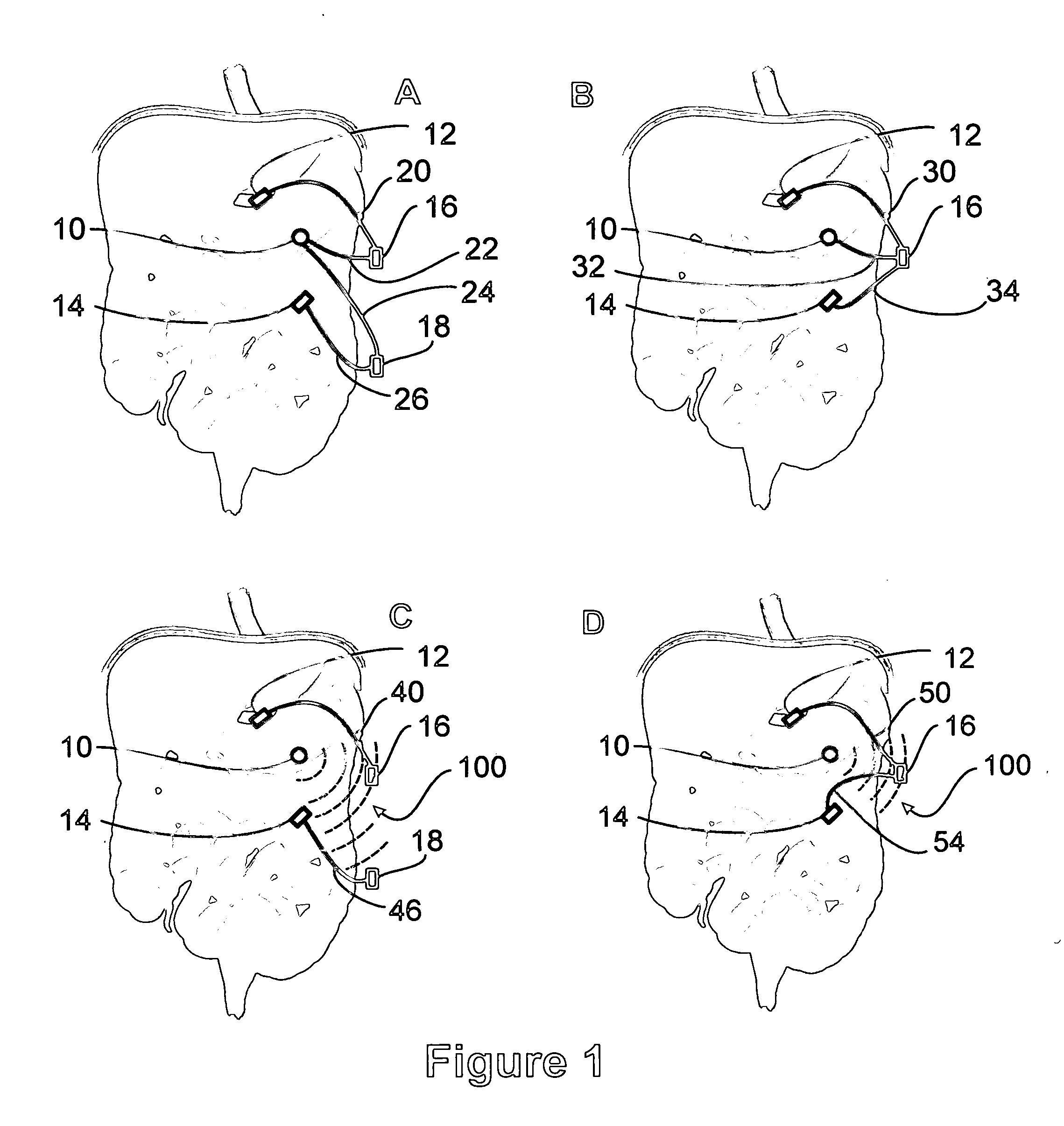
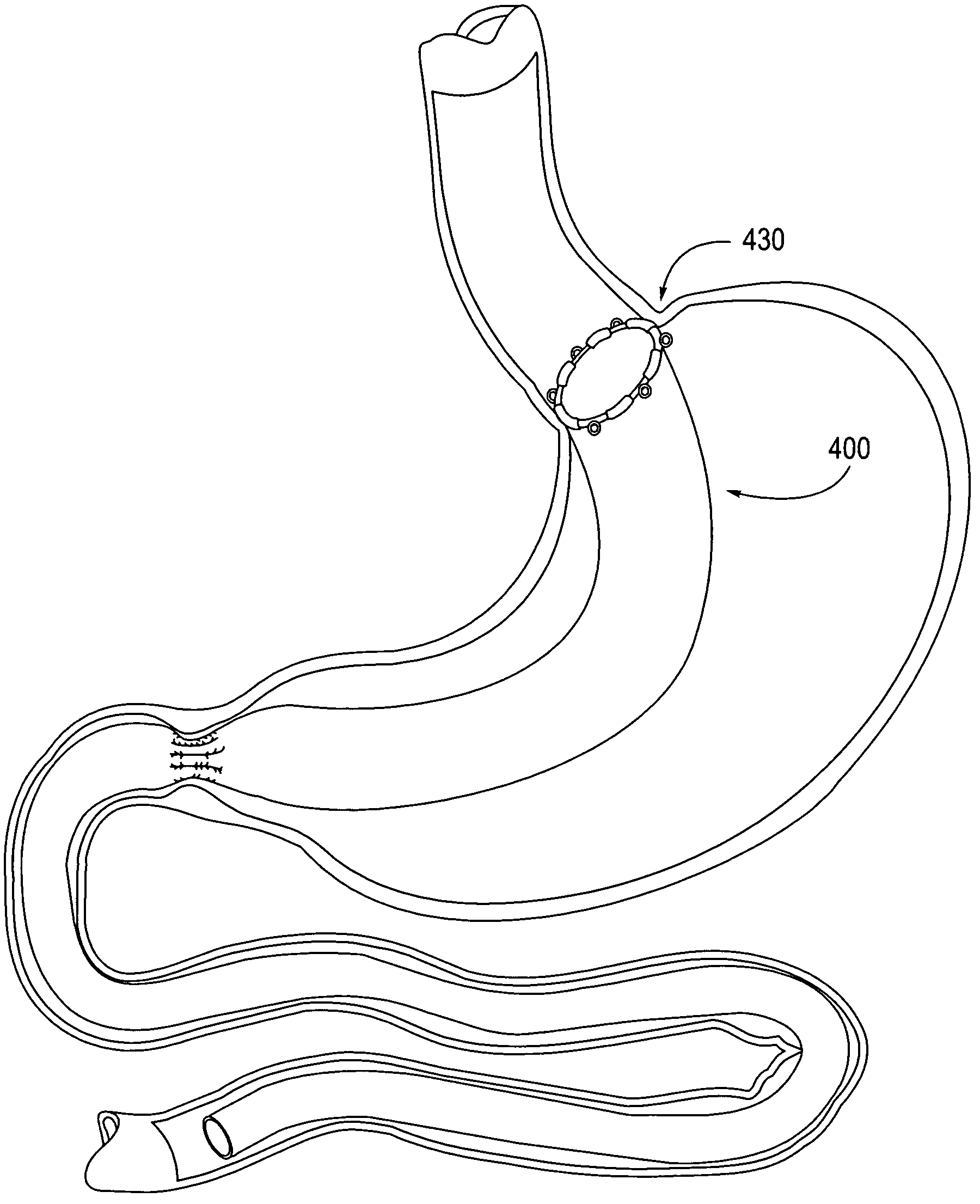
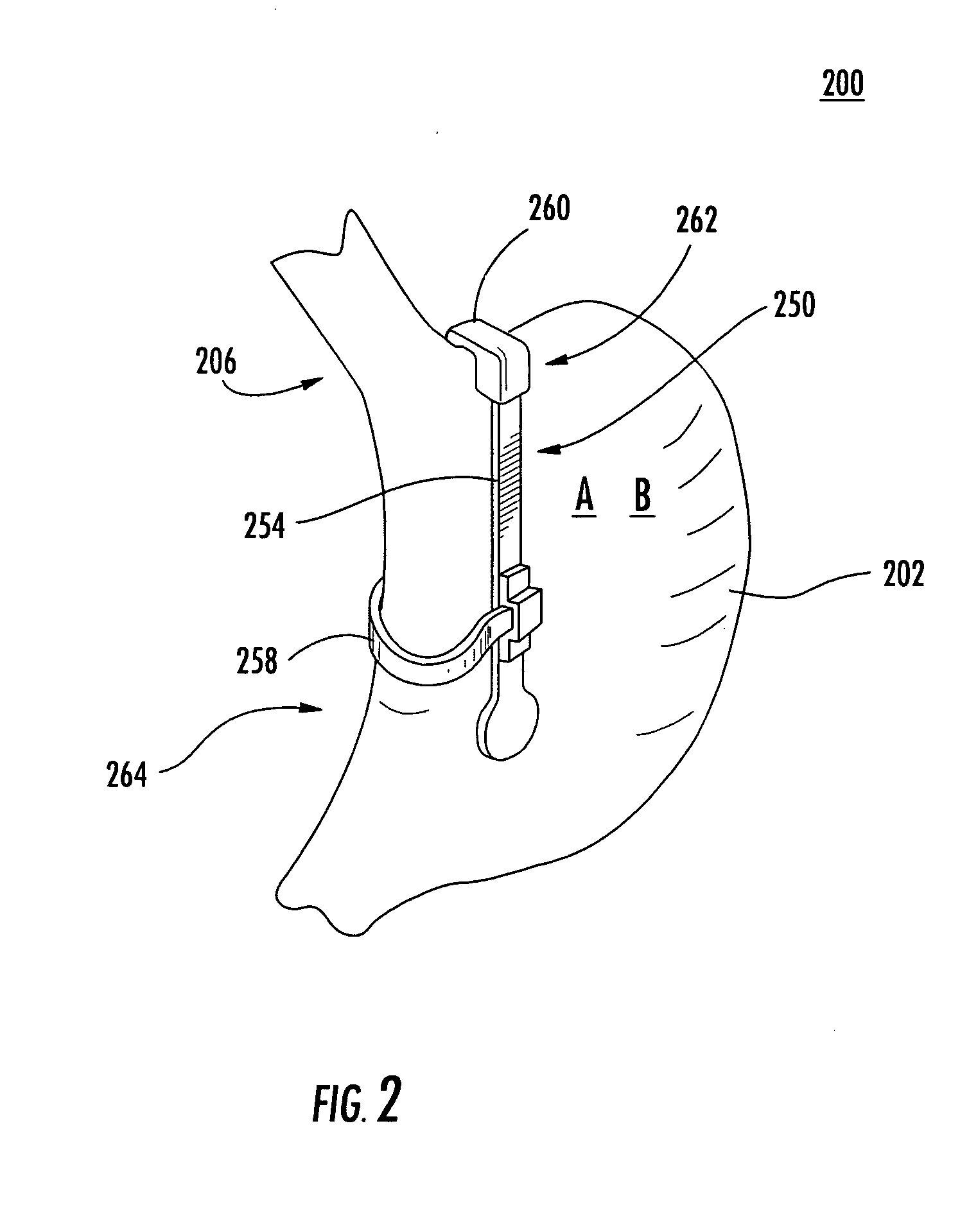
Apparatus and methods for treatment of morbid obesity
Apparatus and methods are described for treatment of morbid obesity using minimally invasive techniques. The apparatus includes a system of components that may be used separately or in combination for effectively reducing stomach volume, bypassing a portion of the stomach and / or small intestines, reducing nutrient absorption in the stomach and / or small intestines and / or depositing minimally or undigested food farther than normal into the intestines, thereby stimulating intestinal responses. The components described include an artificial stoma device, a gastric sleeve device, an intestinal sleeve device and a combined gastrointestinal sleeve device.
Owner:VALENTX
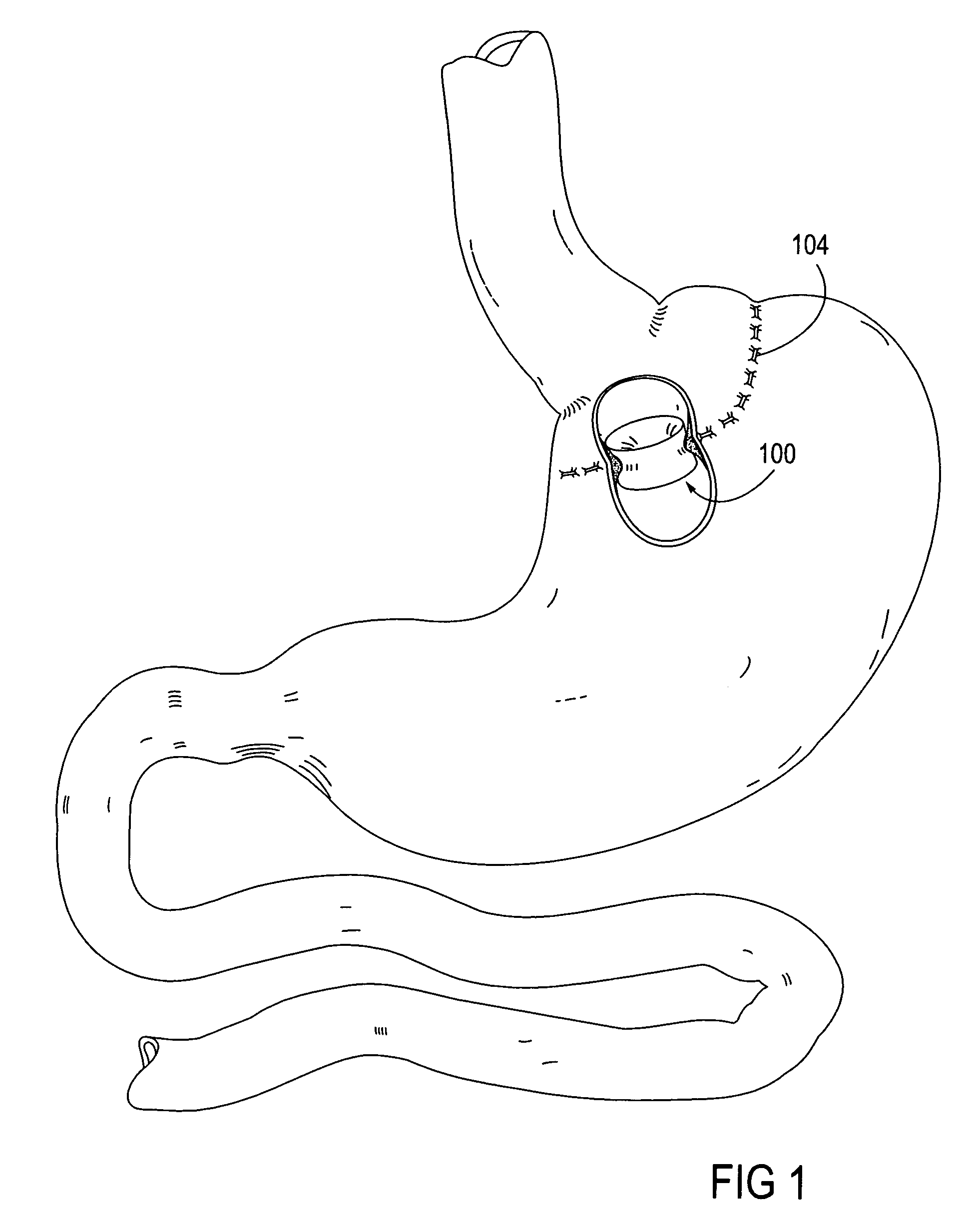
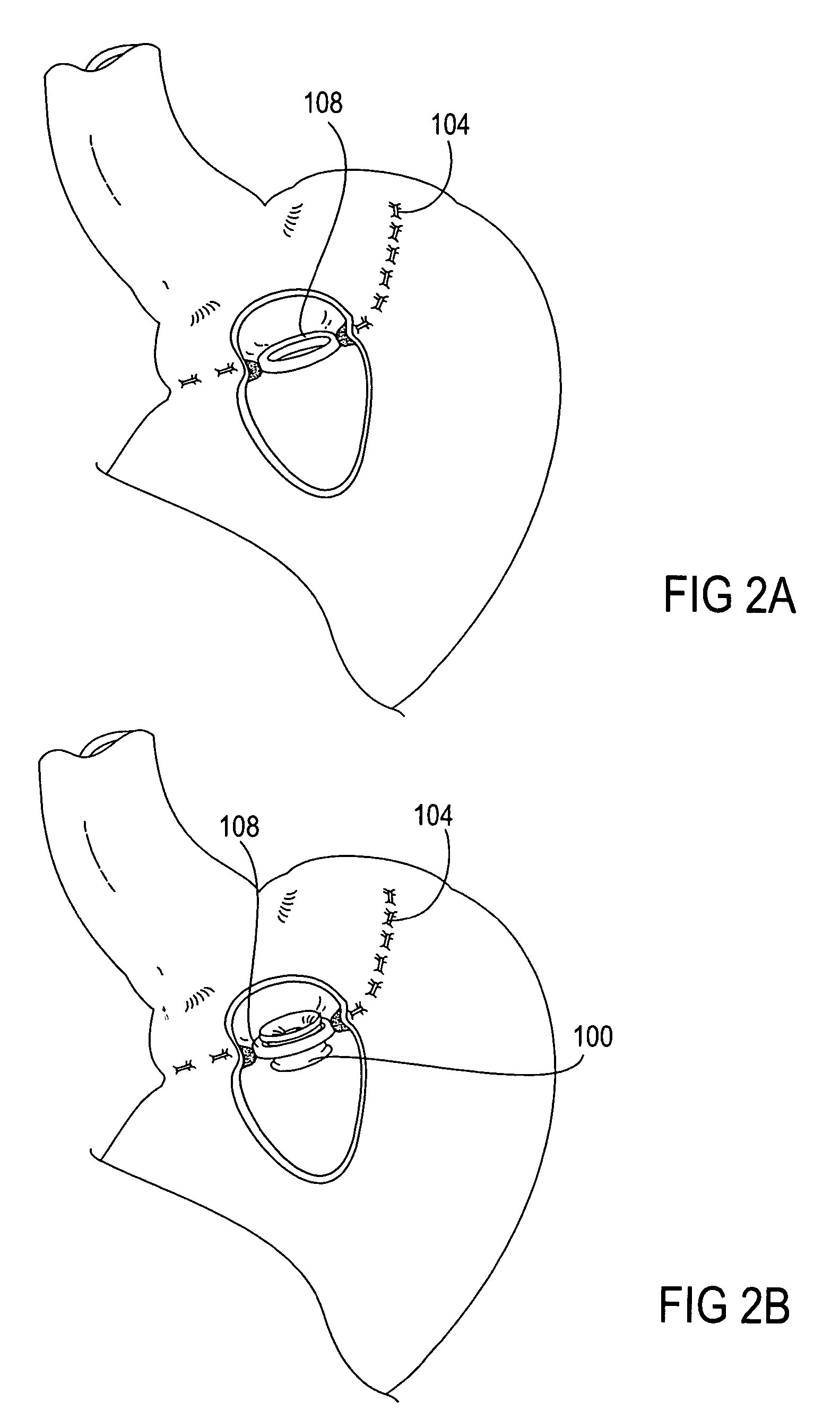
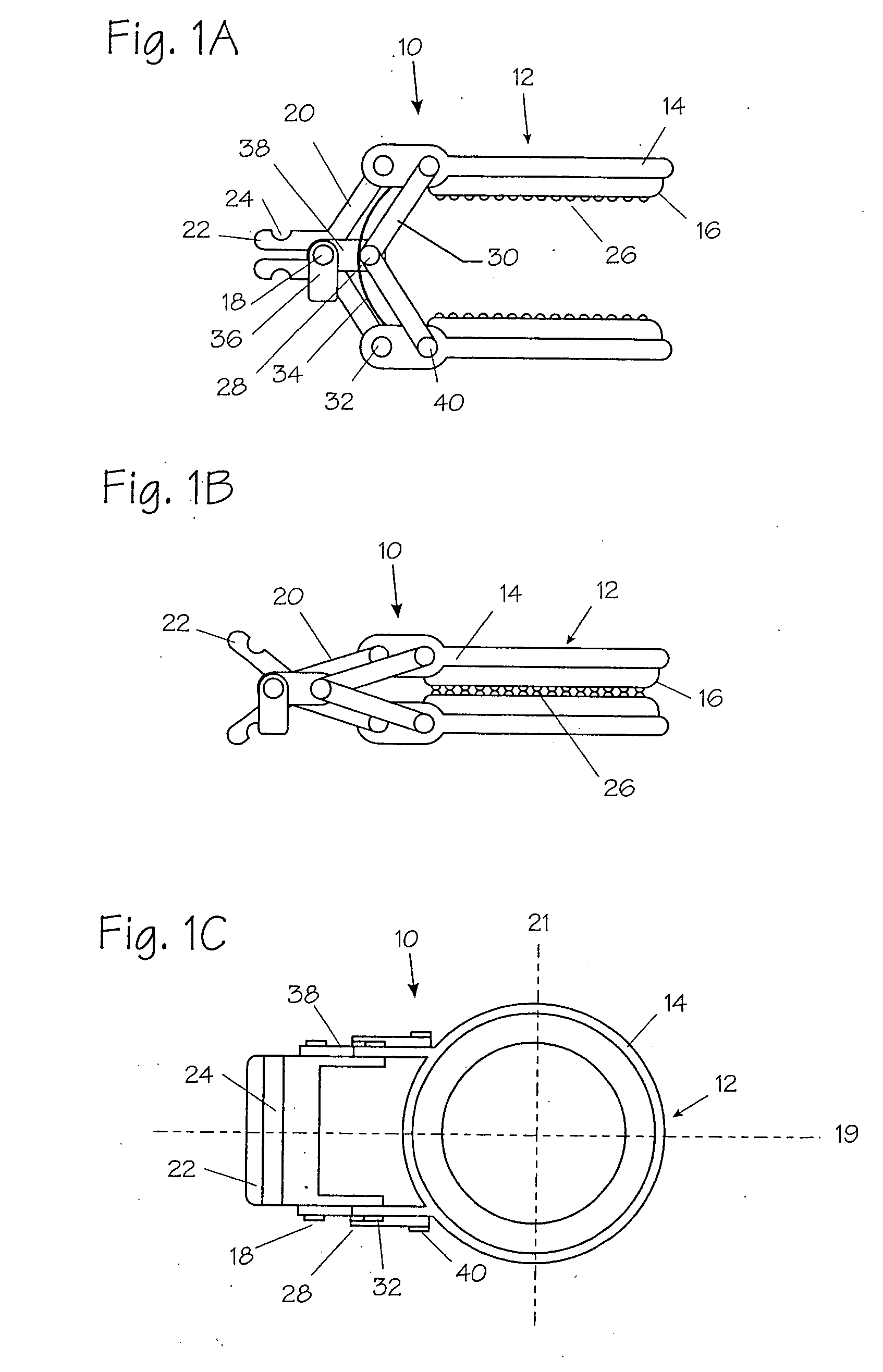
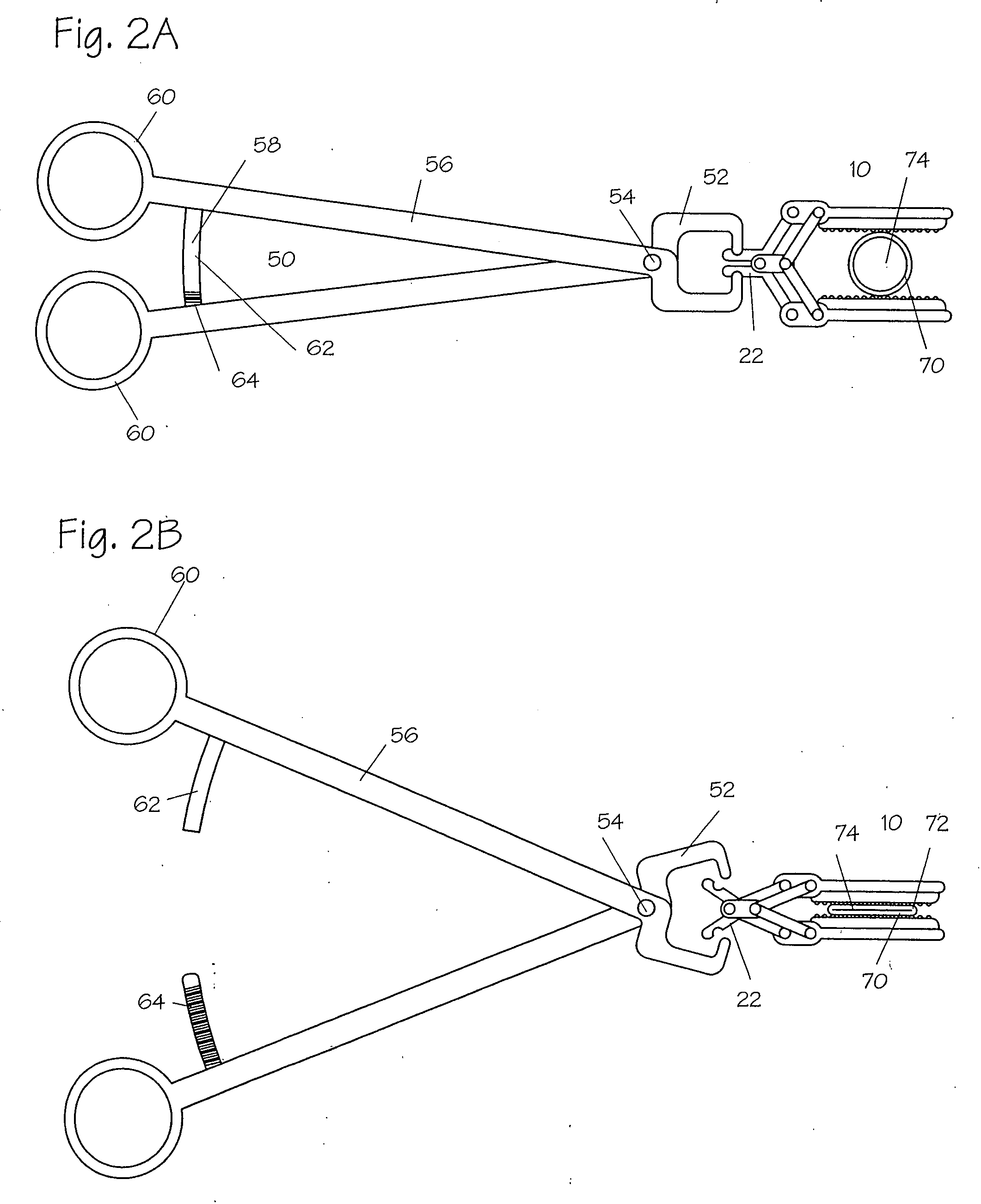
Adhesive Mechanical Fastener for Lumen Creation Utilizing Tissue Necrosing Means
A two piece anastomosis device for attaching two organs together and creating a passage between the organs is disclosed. The anastomosis device has a first tissue clamping ring and a second tissue clamping ring that are brought together to clamp tissue therebetween and cut off the flow of blood to the tissue. The tissue clamping rings are locked together with an adhesive, and over time, causes the clamped tissue to necrose and slough off. The sloughed tissue creates a passageway through the anastomosis device. A method of using the fastener to create a bypass passageway between the stomach and small intestine is disclosed.
Owner:ETHICON ENDO SURGERY INC
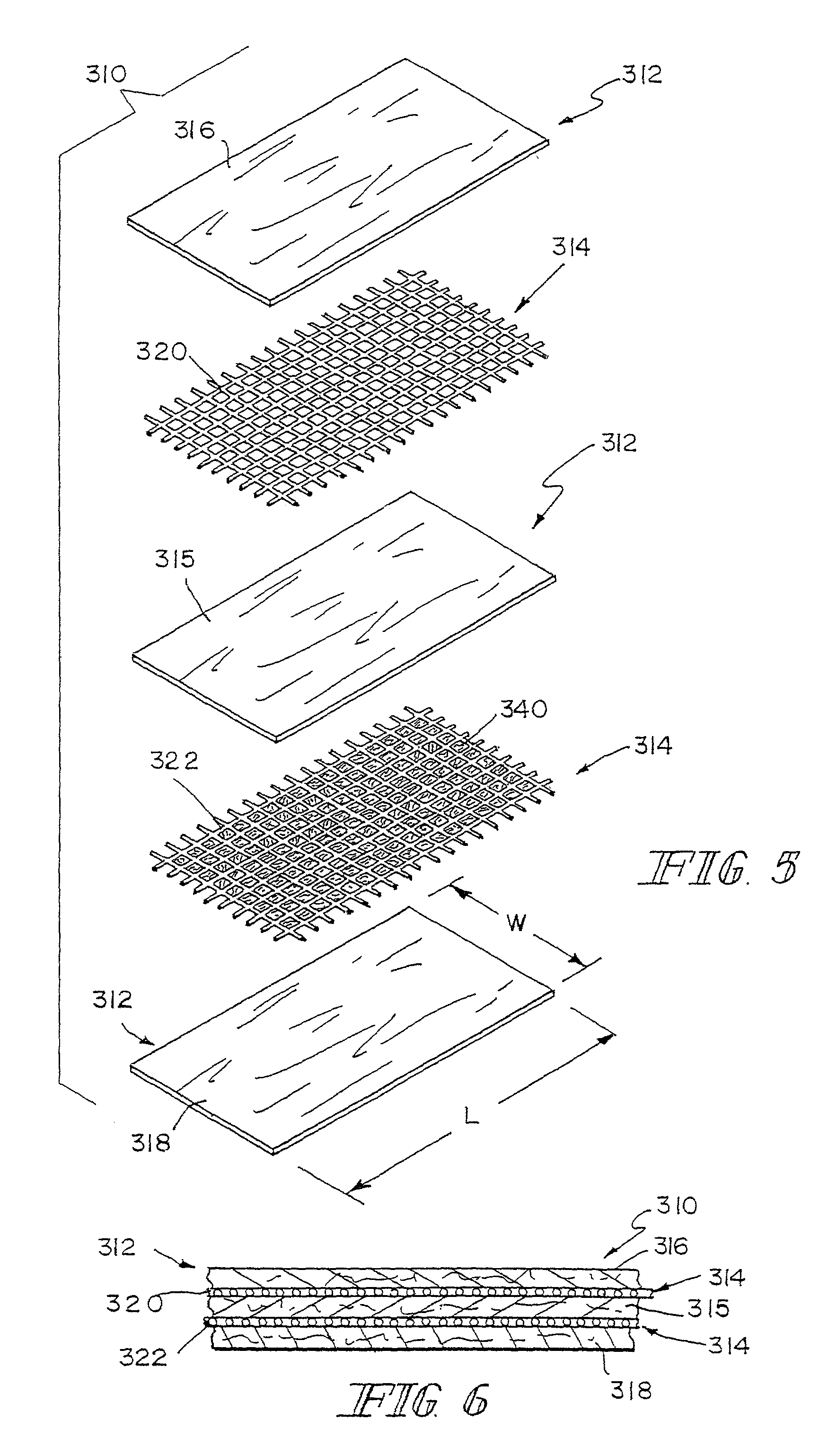
Hybrid biologic-synthetic bioabsorbable scaffolds
ActiveUS8366787B2Increase surface areaGood mechanical integritySuture equipmentsBone implantBioabsorbable scaffoldCell-Extracellular Matrix
A bioprosthetic device is provided for soft tissue attachment, reinforcement, and or reconstruction. The device comprises a naturally occurring extracellular matrix portion and a three-dimensional synthetic portion. In illustrated embodiments, the naturally occurring extracellular matrix portion comprises layers of small intestine submucosa, and the three-dimensional synthetic portion comprises a foam or a three-dimensional mesh, textile, or felt.
Owner:DEPUY SYNTHES PROD INC
Gastrointestinal sleeve device and methods for treatment of morbid obesity
InactiveUS20050049718A1Effectively reducing stomach volumeStimulating intestinal responseMedical devicesTubular organ implantsIntestinal structureMorbid obesity
Apparatus and methods are described for treatment of morbid obesity using minimally invasive techniques. The apparatus includes a system of components that may be used separately or in combination for effectively reducing stomach volume, bypassing a portion of the stomach and / or small intestines, reducing nutrient absorption in the stomach and / or small intestines and / or depositing minimally or undigested food farther than normal into the intestines, thereby stimulating intestinal responses. The components described include a gastric sleeve device, an intestinal sleeve device, and a combined gastrointestinal sleeve device.
Owner:VALENTX
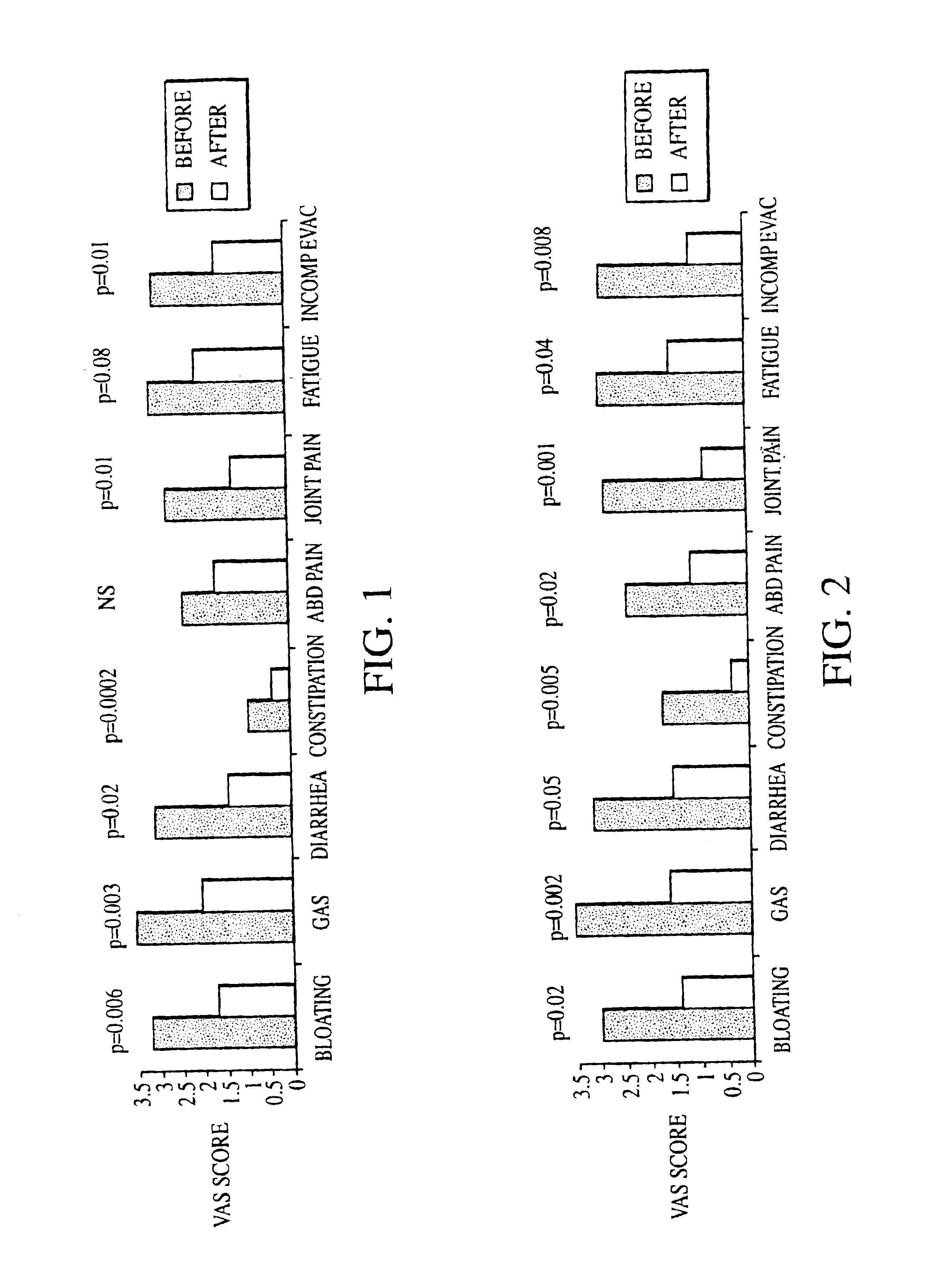
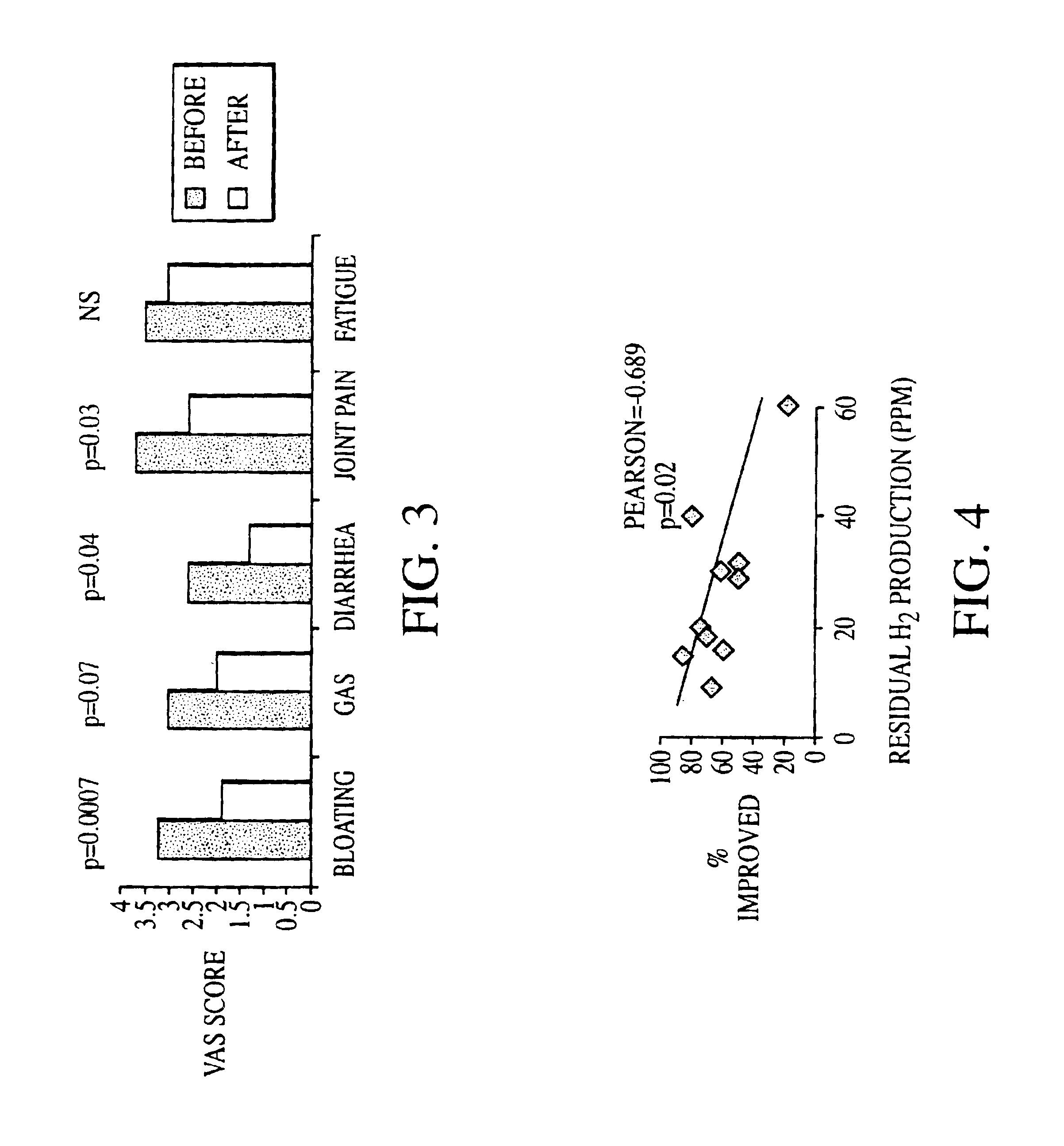
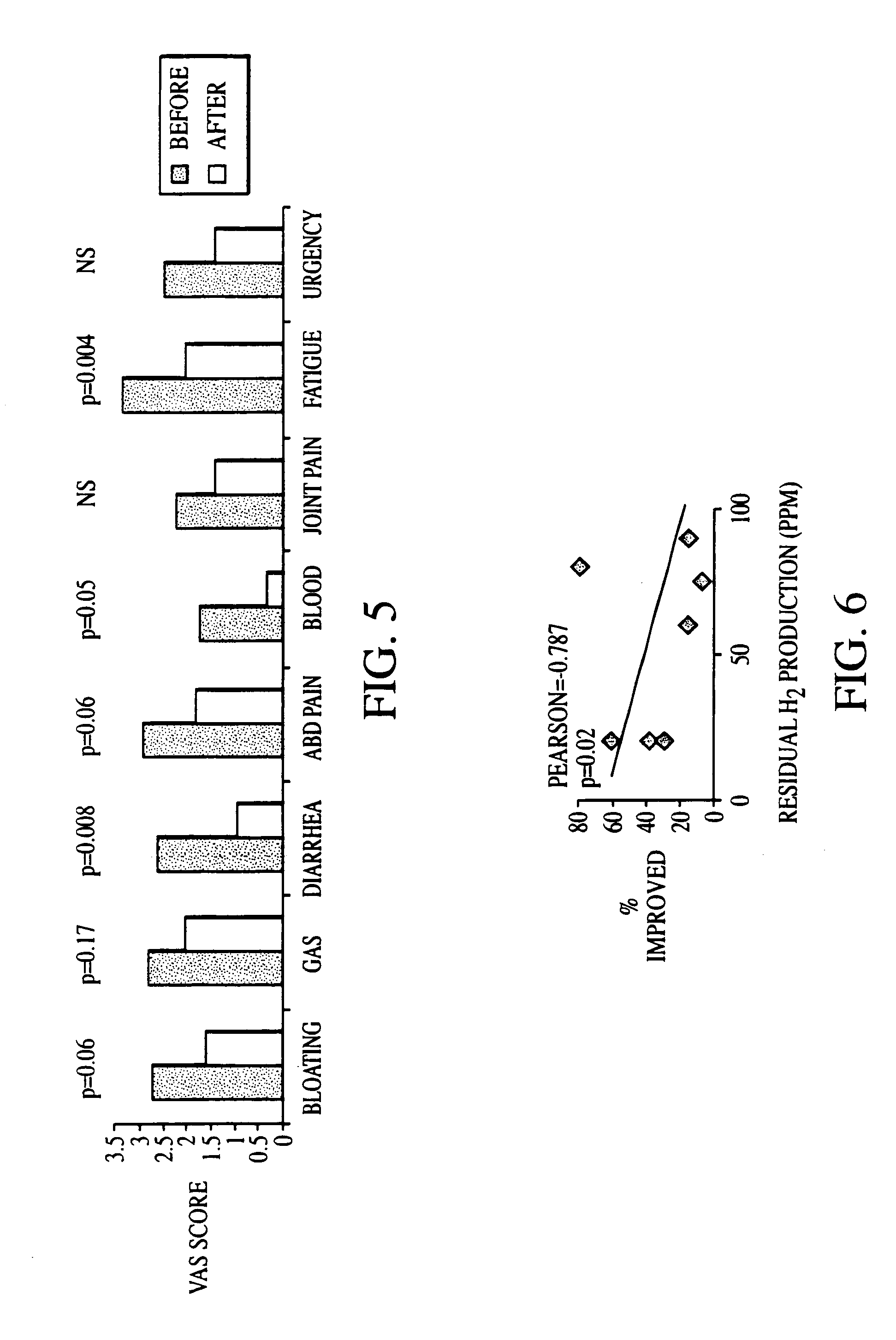
Methods of diagnosing or treating irritable bowel syndrome and other disorders caused by small intestinal bacterial overgrowth
InactiveUS6861053B1Eradicate small intestinal bacterial overgrowthSymptoms improvedAntibacterial agentsOrganic active ingredientsBacteroidesAutoimmune responses
Disclosed is a method of diagnosing irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome, depression, attention deficit / hyperactivity disorder, autoimmune diseases, such as multiple sclerosis and systemic lupus erythematosus, or Crohn's disease, which involves detecting the presence of small intestinal bacterial overgrowth (SIBO) in a human subject having at least one symptom associated with a suspected diagnosis of any of those diagnostic categories. Also disclosed is a method of treating these disorders, and other disorders caused by SIBO, that involves at least partially eradicating a SIBO condition in the human subject. The method includes administration of anti-microbial or probiotic agents, or normalizing intestinal motility by employing a prokinetic agent. The method improves symptoms, including hyperalgesia related to SIBO and disorders caused by SIBO. Also disclosed is a kit for the diagnosis or treatment of irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome, depression, attention deficit / hyperactivity disorder, autoimmune diseases, or Crohn's disease.
Owner:CEDARS SINAI MEDICAL CENT
Method and apparatus for vascular and visceral clipping
InactiveUS20050251183A1Convenient treatmentMinimizing chanceSnap fastenersClothes buttonsTrauma surgeryLarge intestine
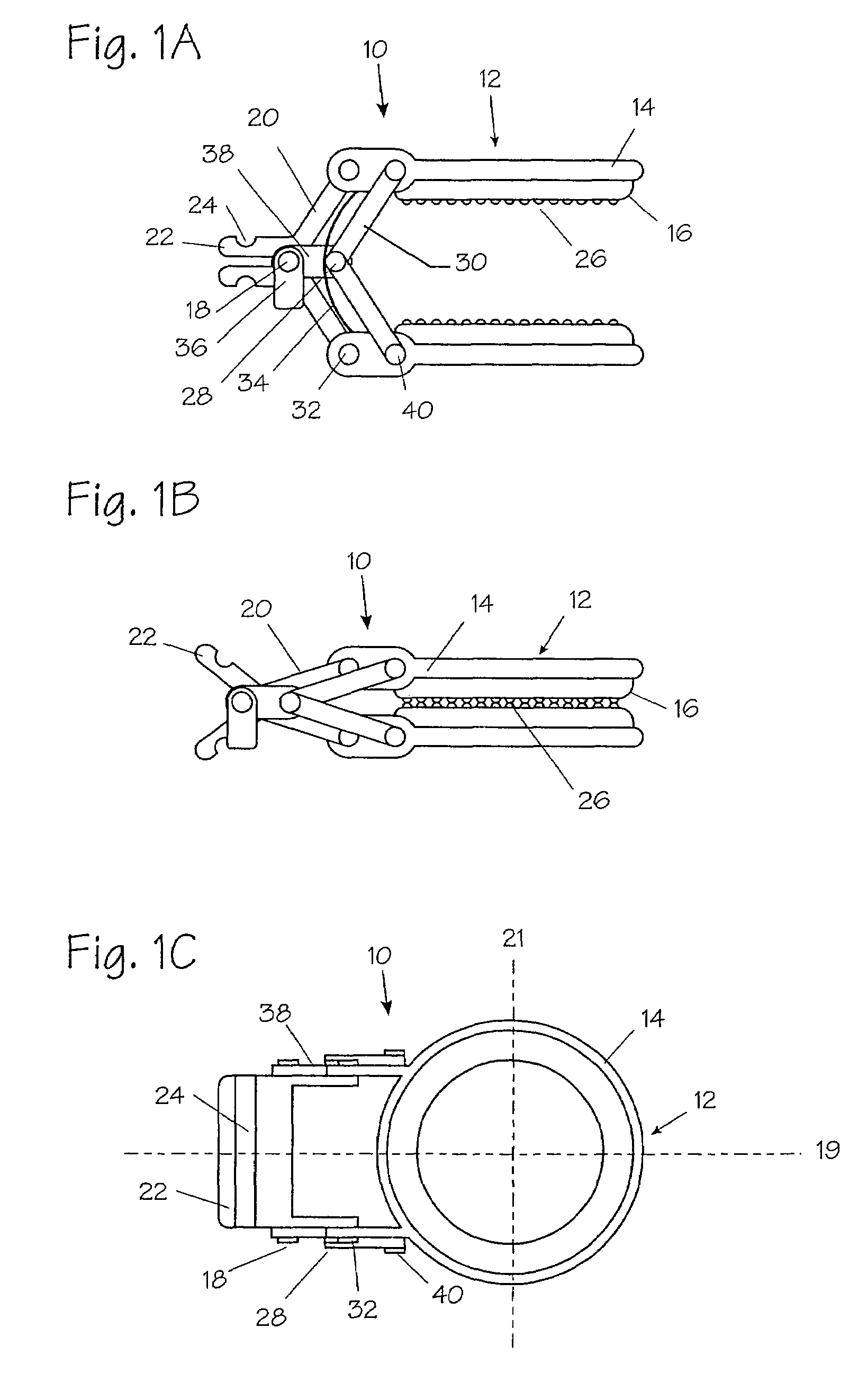
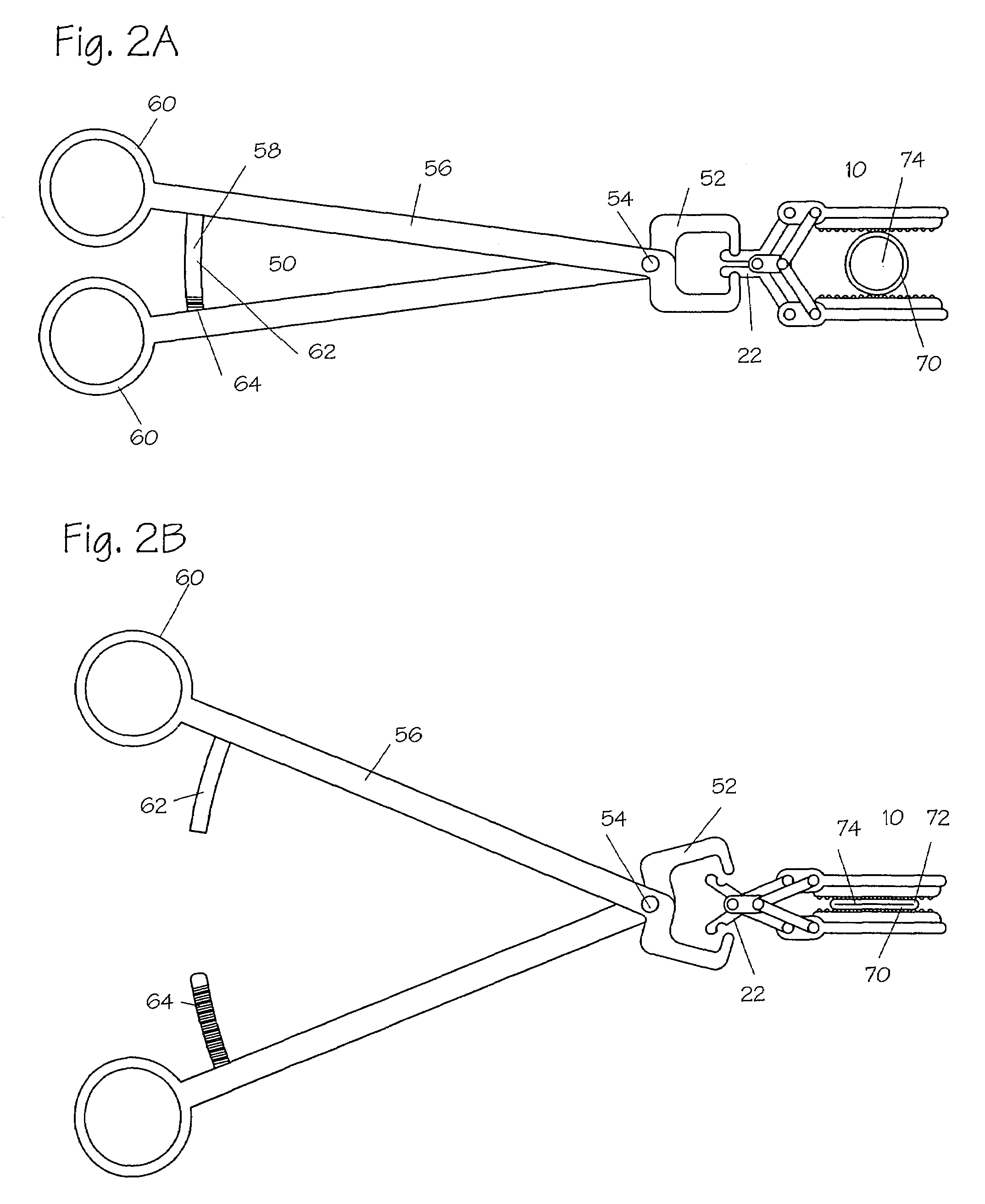
Devices and methods for achieving hemostasis and leakage control in hollow body vessels such as the small and large intestines, arteries and veins as well as ducts leading to the gall bladder and other organs. The devices and methods disclosed herein are especially useful in the emergency, trauma surgery or military setting, and most especially during damage control procedures. In such cases, the patient may have received trauma to the abdomen, extremities, neck or thoracic region. The devices utilize removable or permanently implanted, broad, soft, parallel jaw clips with minimal projections to maintain vessel contents without damage to the tissue comprising the vessel. These clips are applied using either standard instruments or custom devices that are subsequently removed leaving the clips implanted, on a temporary or permanent basis, to provide for hemostasis or leakage prevention, or both. These clips overcome the limitations of clips and sutures that are currently used for the same purposes. The clips come in a variety of shapes and sizes. The clips may be placed and removed by open surgery or laparoscopic access.
Owner:DAMAGE CONTROL SURGICAL TECH
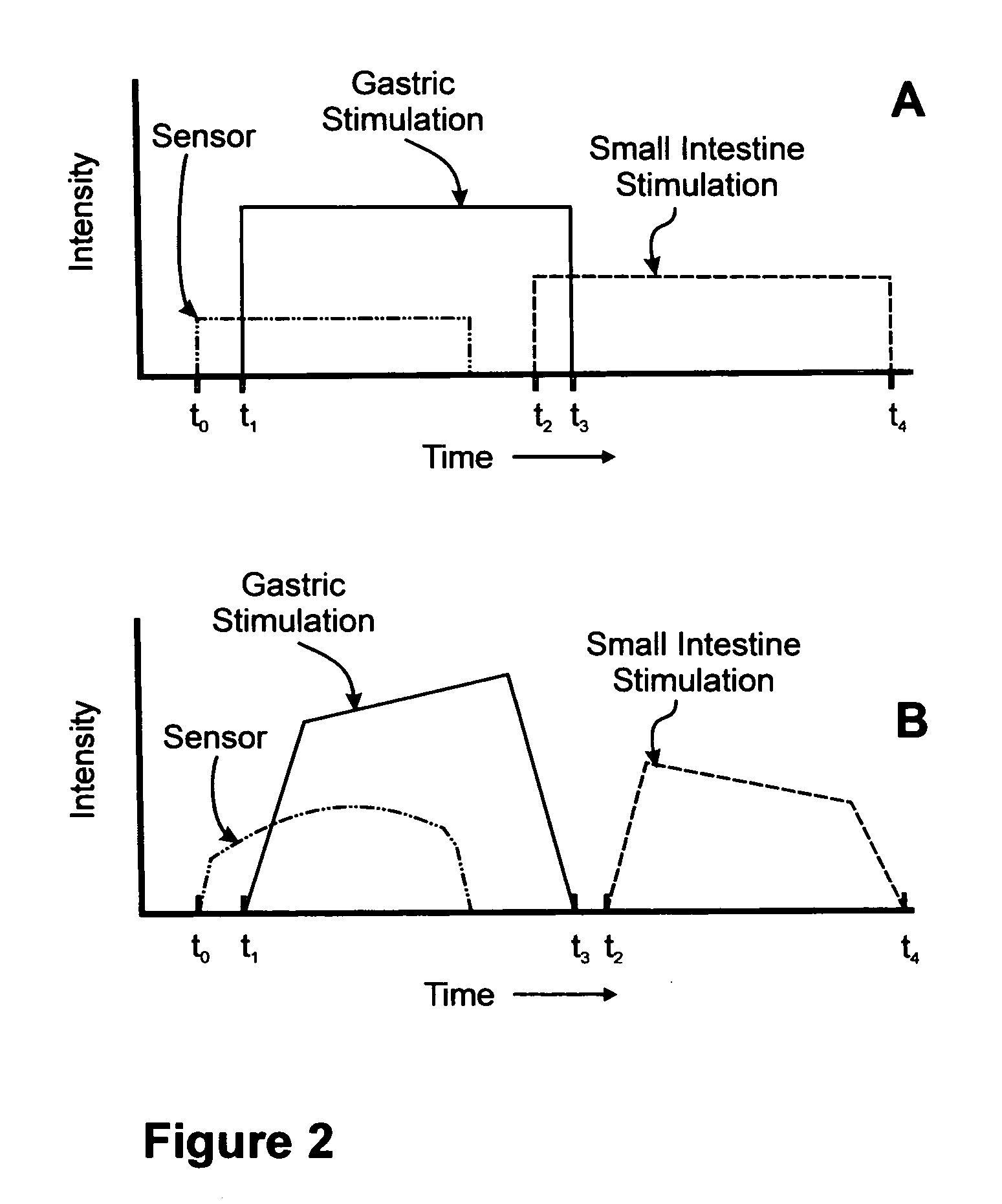
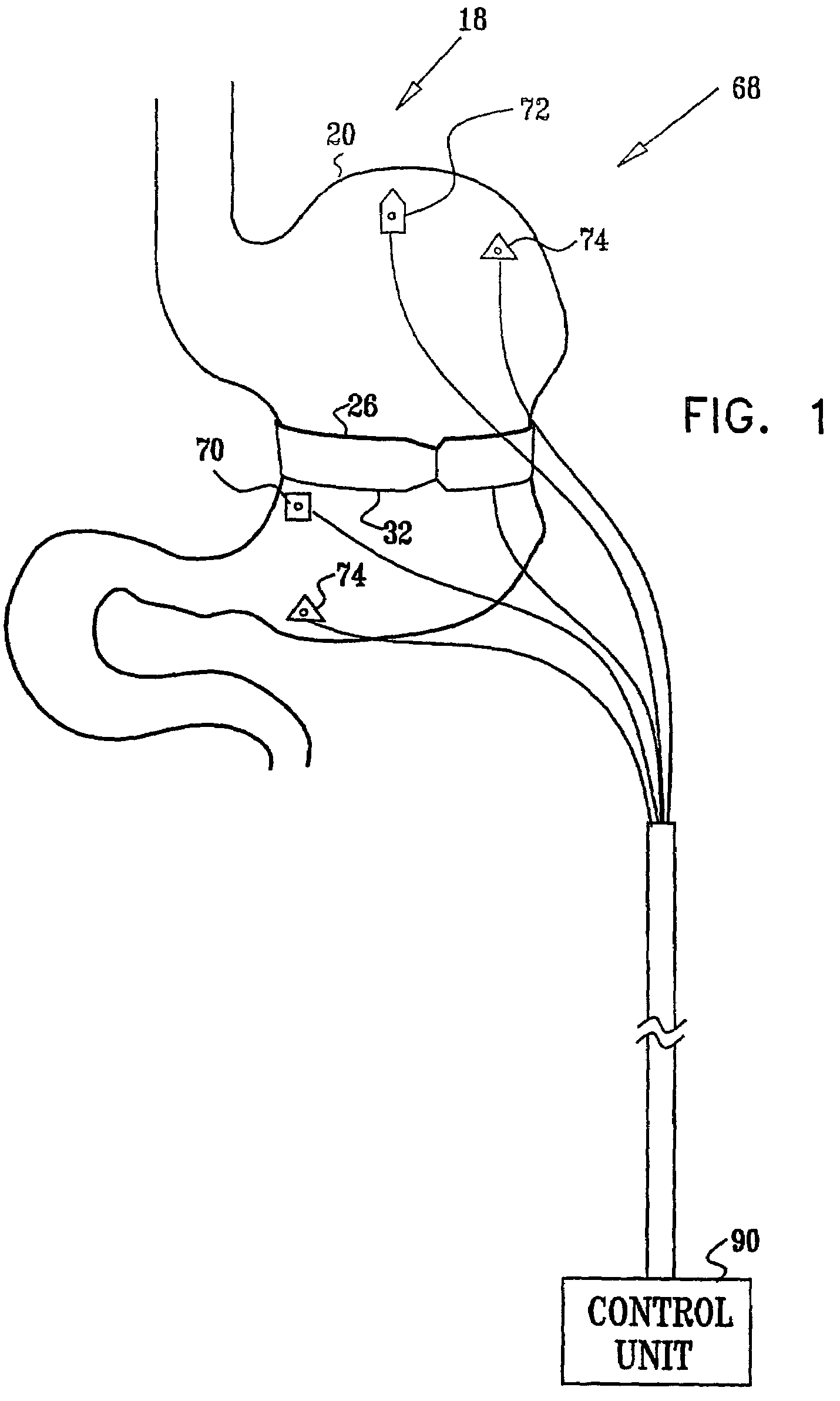
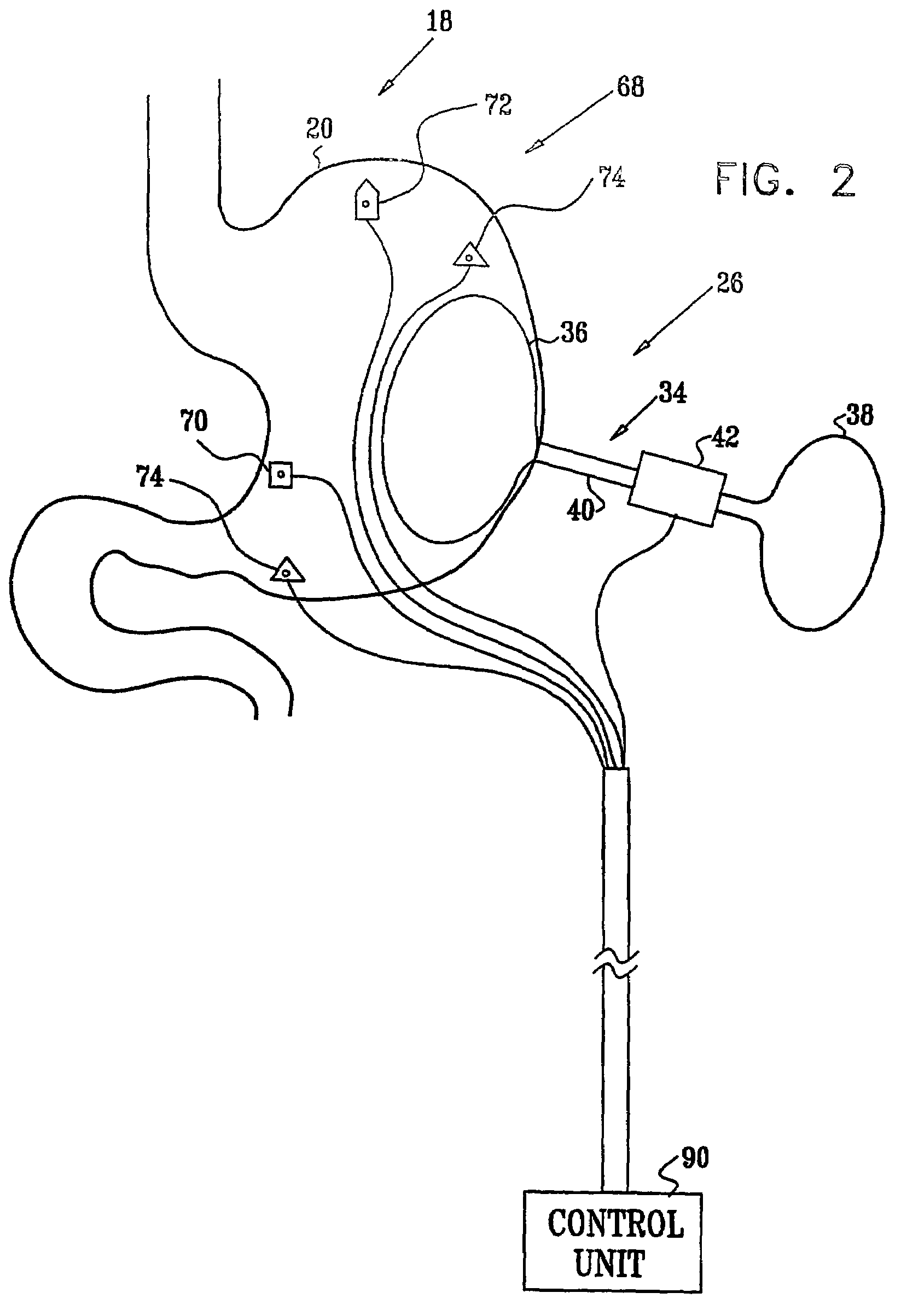
Sensor based gastrointestinal electrical stimulation for the treatment of obesity or motility disorders
InactiveUS20050222638A1Reduce riskAvoid stimulationElectrotherapyArtificial respirationGastroparesisMotility
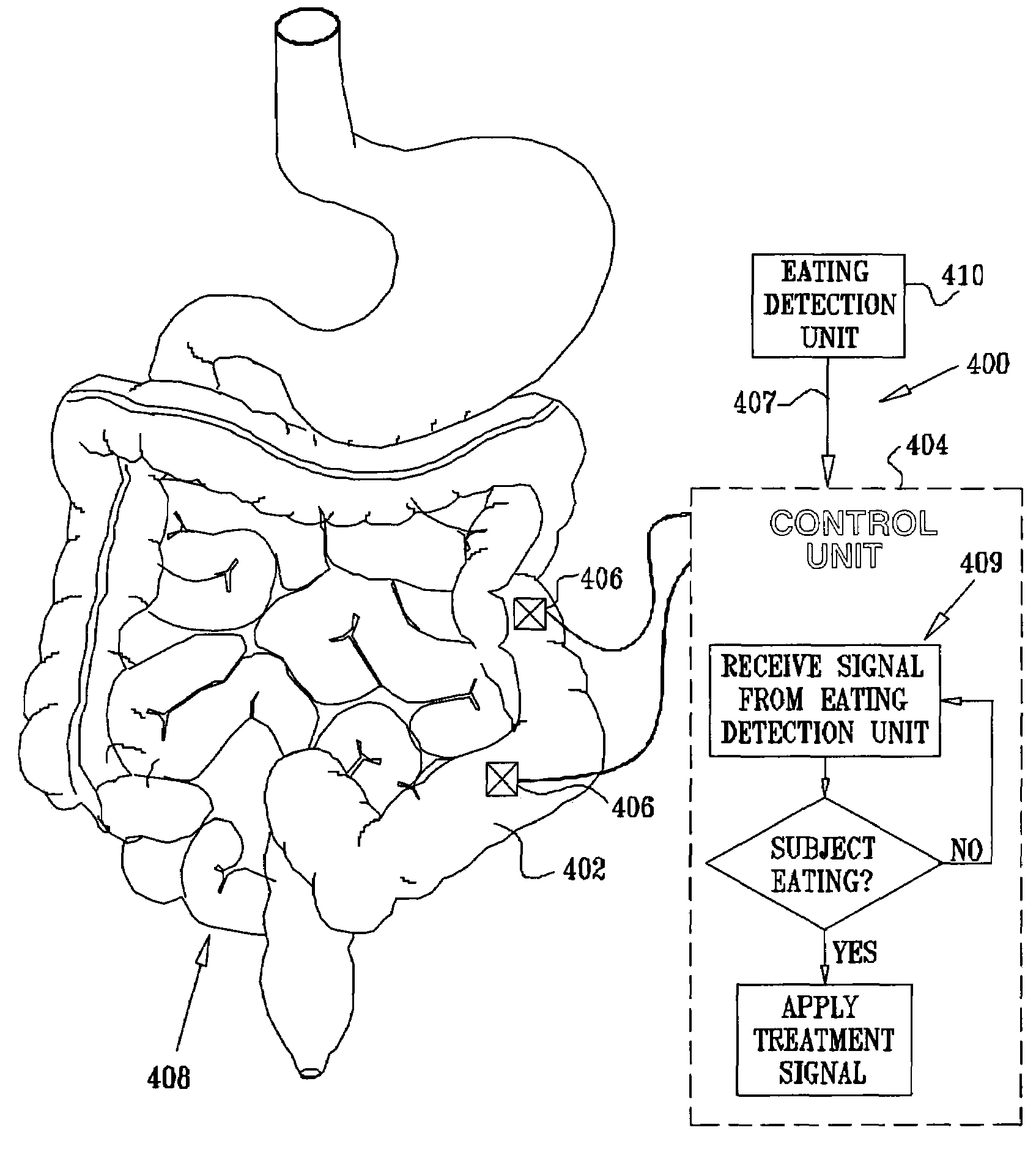
A method for treatment of obesity, especially morbid obesity, gastroparesis and other syndromes related to motor disorders of the stomach. The method of this invention utilizes a sensor to detect food entering the patient's stomach, thereby the sensor communicates with and activates at least one electrical stimulation device attached to either the stomach or the small intestine.
Owner:MEDTRONIC TRANSNEURONIX
Reinforced small intestinal submucosa
InactiveUS7160333B2Improve mechanical propertiesEnhanced handling propertyBone implantSurgeryCell-Extracellular MatrixSmall intestine
Bioprosthetic devices for soft tissue attachment, reinforcement, or construction are provided. The devices comprise a sheet of naturally occurring extracellular matrix and a sheet of synthetic mesh coupled to the naturally occurring extracellular matrix portion.
Owner:DEPUY SYNTHES PROD INC
Method and apparatus for reducing obesity
Method and apparatus for treatment of morbid obesity by placement of a series of flow reduction elements in the small intestine to induce satiety are disclosed. The flow reduction elements restrict the movement of partially digested food and reduce the flow rate through the small intestine which causes the emptying of the stomach and the duodenum to occur slower. The flow reduction elements are attached to an elongated tube and are constructed from various shapes and configurations. The flow reduction elements may be inflated with fluid or may be constructed from self-expandable materials. The device is anchored in the antrum of the stomach with an anchoring member. The transoral gastric device can be inserted with a delivery catheter through the working lumen of an endoscope or alongside an endoscope and may be removed with the aid of an endoscope if desired.
Owner:ENDOSPHERE
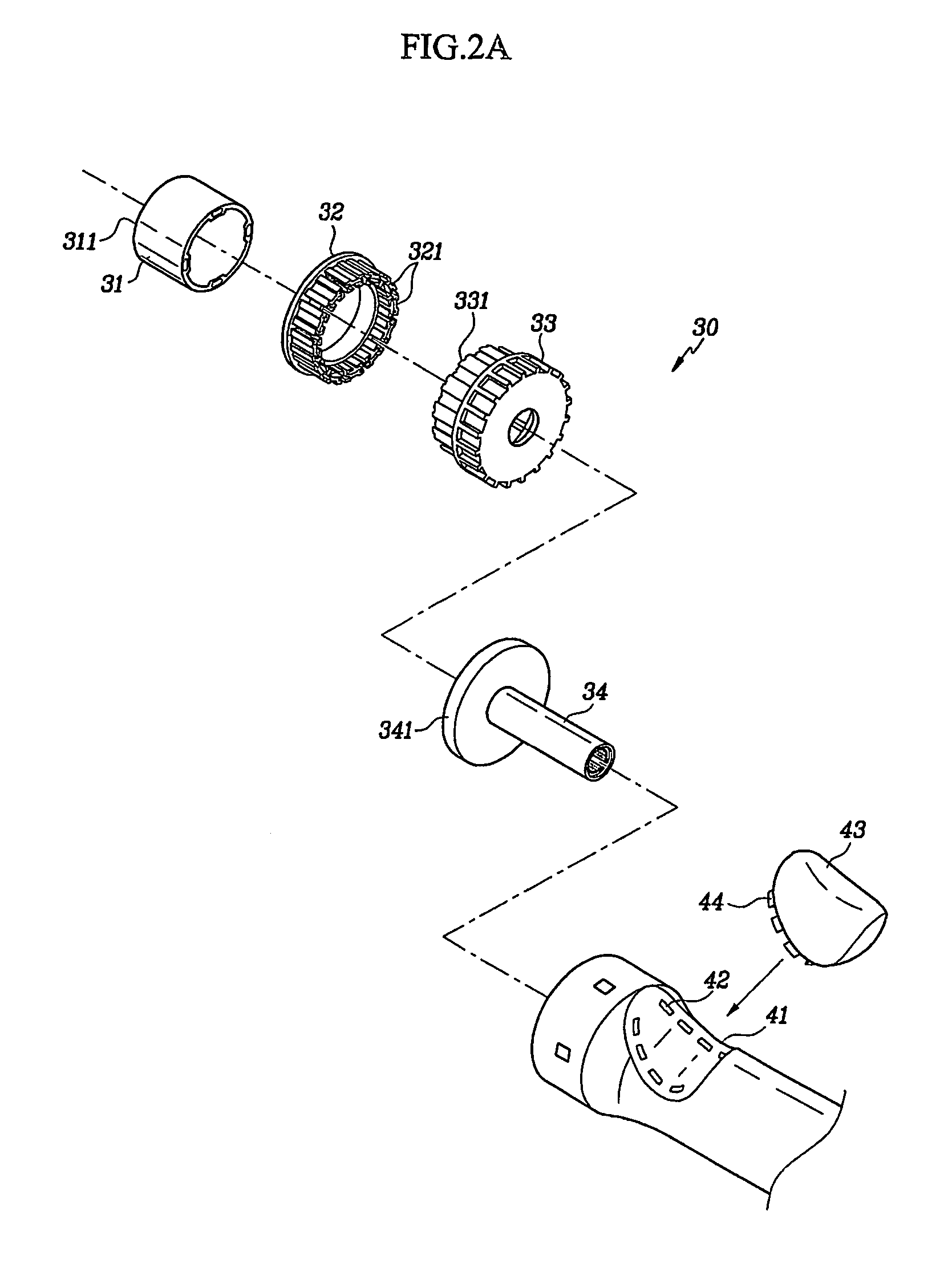
Circular surgical stapler with a detachable anvil
Owner:INHA UNIV RES & BUSINESS FOUNDATION
Gastrointestinal sleeve device and methods for treatment of morbid obesity
InactiveUS20050240279A1Effectively reducing stomach volumeStimulating intestinal responseTubular organ implantsNon-surgical orthopedic devicesIntestinal structureStoma
Apparatus and methods are described for treatment of morbid obesity using minimally invasive techniques. The apparatus includes a system of components that may be used separately or in combination for effectively reducing stomach volume, bypassing a portion of the stomach and / or small intestines, reducing nutrient absorption in the stomach and / or small intestines and / or depositing minimally or undigested food farther than normal into the intestines, thereby stimulating intestinal responses. The components described include an artificial stoma device, a gastric sleeve device, an intestinal sleeve device and a combined gastrointestinal sleeve device.
Owner:VALENTX
Method and apparatus for vascular and visceral clipping
InactiveUS7322995B2Convenient treatmentMinimizing chanceSnap fastenersClothes buttonsTrauma surgeryChest region
Devices and methods for achieving hemostasis and leakage control in hollow body vessels such as the small and large intestines, arteries and veins as well as ducts leading to the gall bladder and other organs. The devices and methods disclosed herein are especially useful in the emergency, trauma surgery or military setting, and most especially during damage control procedures. In such cases, the patient may have received trauma to the abdomen, extremities, neck or thoracic region. The devices utilize removable or permanently implanted, broad, soft, parallel jaw clips with minimal projections to maintain vessel contents without damage to the tissue comprising the vessel. These clips are applied using either standard instruments or custom devices that are subsequently removed leaving the clips implanted, on a temporary or permanent basis, to provide for hemostasis or leakage prevention, or both. These clips overcome the limitations of clips and sutures that are currently used for the same purposes. The clips come in a variety of shapes and sizes. The clips may be placed and removed by open surgery or laparoscopic access.
Owner:DAMAGE CONTROL SURGICAL TECH
Gastrointestinal methods and apparatus for use in treating disorders
InactiveUS7502649B2Reduce volumeCause a sensation of satiety felt by the patientElectrotherapyMetabolism disorderElectrical resistance and conductanceDisease
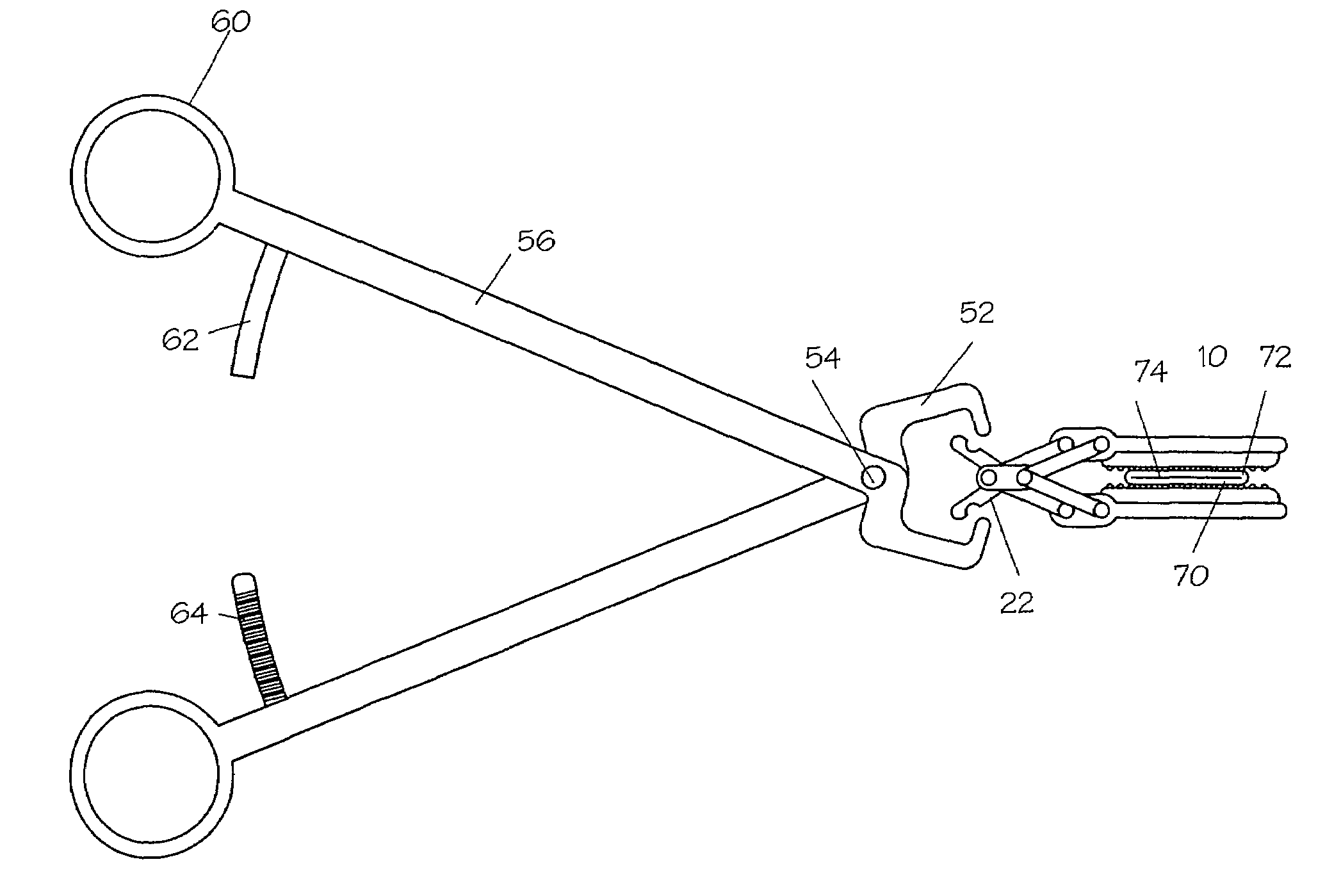
A method is provided for detecting a change in posture of a subject. An electrical impedance is measured between two or more sites on a stomach (20) of the subject, and an impedance signal is generated responsive thereto. The change in posture is detected by performing a posture analysis of the impedance signal. A method is also provided for treating a subject. The method includes applying an electrical signal to a site of the subject selected from the list consisting of: a colon (402) of the subject, and a distal small intestine (408) of the subject. The signal is configured to stimulate cells of the subject to increase secretion of glucagon-like-peptide-1 (GLP-1) or PYY, or to decrease secretion of ghrelin, in order to treat the subject.
Owner:TYLERTON INT INC
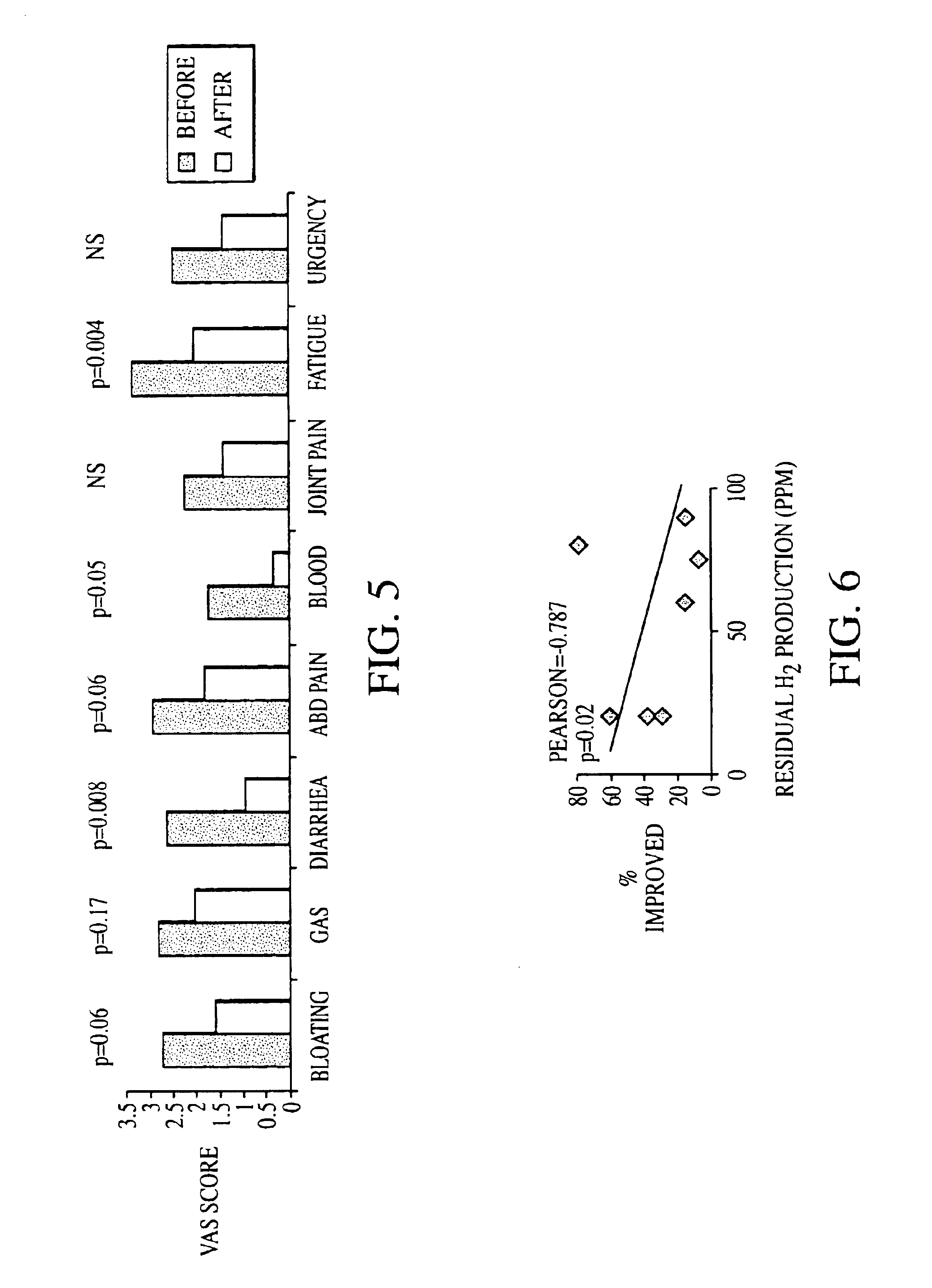
Methods of diagnosing and treating small intestinal bacterial overgrowth (SIBO) and SIBO-related conditions
InactiveUS7048906B2Prevent further growthReduced magnitudeAntibacterial agentsCompounds screening/testingImmunologic disordersPhysiology
Disclosed is a method of treating small intestinal bacterial overgrowth (SIBO) or a SIBO-caused condition in a human subject. SIBO-caused conditions include irritable bowel syndrome, fibromyalgia, chronic pelvic pain syndrome, chronic fatigue syndrome, depression, impaired mentation, impaired memory, halitosis, tinnitus, sugar craving, autism, attention deficit / hyperactivity disorder, drug sensitivity, an autoimmune disease, and Crohn's disease. Also disclosed are a method of screening for the abnormally likely presence of SIBO in a human subject and a method of detecting SIBO in a human subject. A method of determining the relative severity of SIBO or a SIBO-caused condition in a human subject, in whom small intestinal bacterial overgrowth (SIBO) has been detected, is also disclosed.
Owner:CEDARS SINAI MEDICAL CENT
Stomach prosthesis
InactiveUS7037343B2Treating obesityFacilitate expedite mixing breaking downDiagnosticsSurgeryPylorusSmall intestine
An implantable stomach prosthesis is provided for surgically replacing or augmenting all or part of the antrum and / or pylorus of a stomach. The prosthesis controls the passage of food from the stomach to the small intestine. The prosthesis may be configured to churn ingested material and release it from the stomach through a prosthetic pyloric valve. At least one expandable member is arranged to be expanded to control the passage of food and / or to mimic the churning action of a patient's stomach. The prosthesis includes an outer support structure, a flexible inner member forming a conduit for the movement of material, and at least one expandable member located between the outer support structure and inner member. An implantable pump system is provided for inflating and deflating the expandable member(s).
Owner:PYTHON MEDICAL
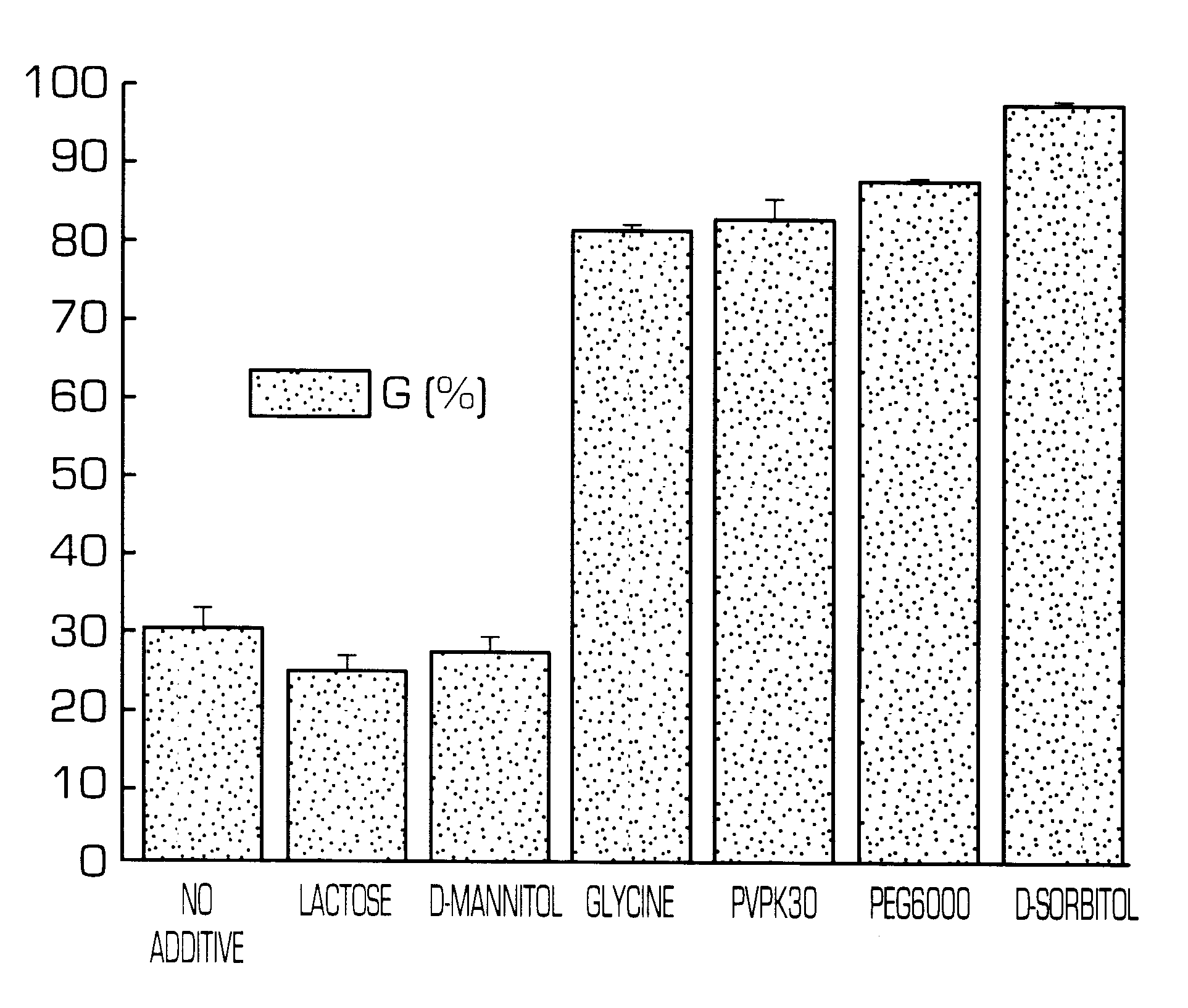
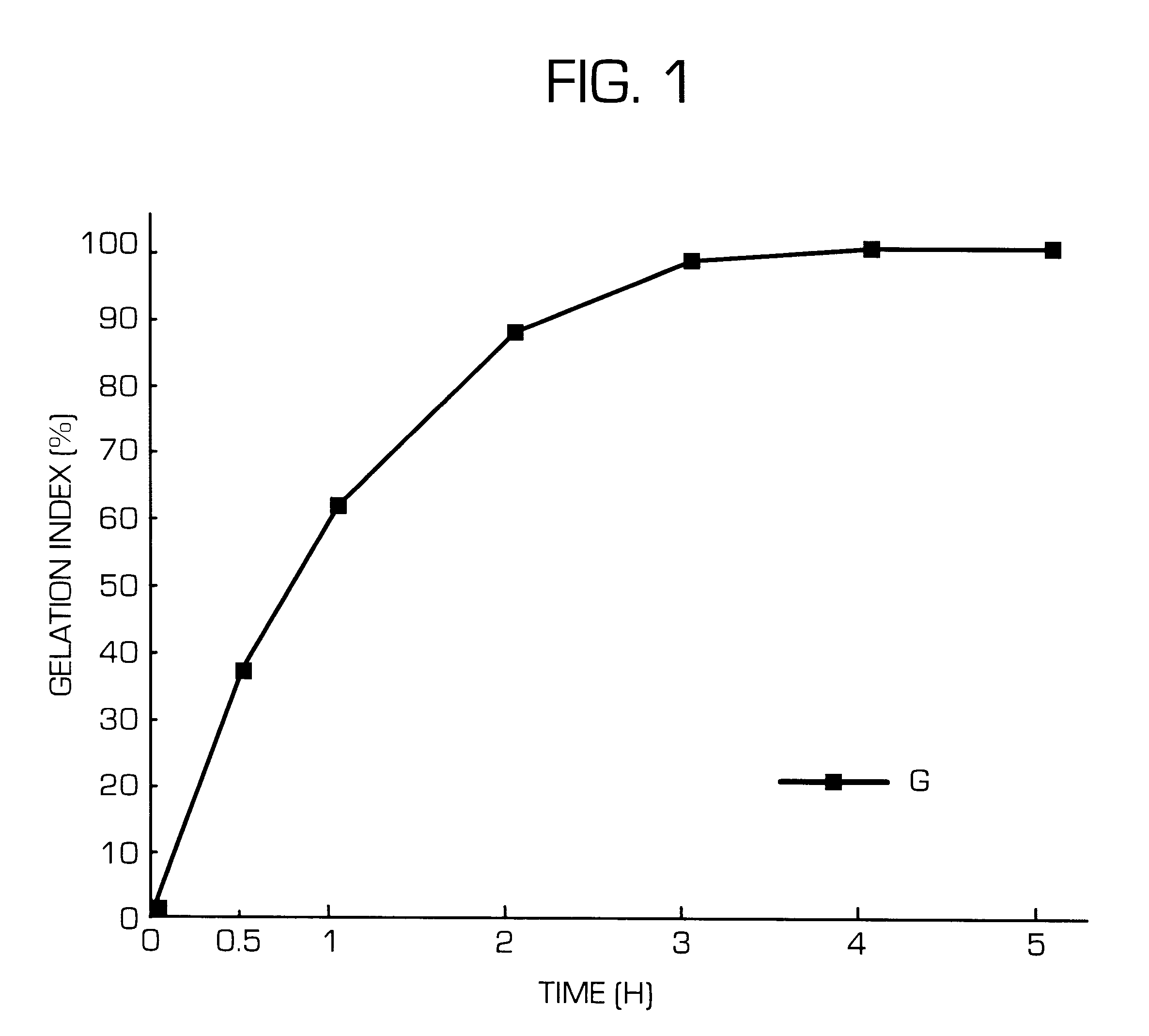
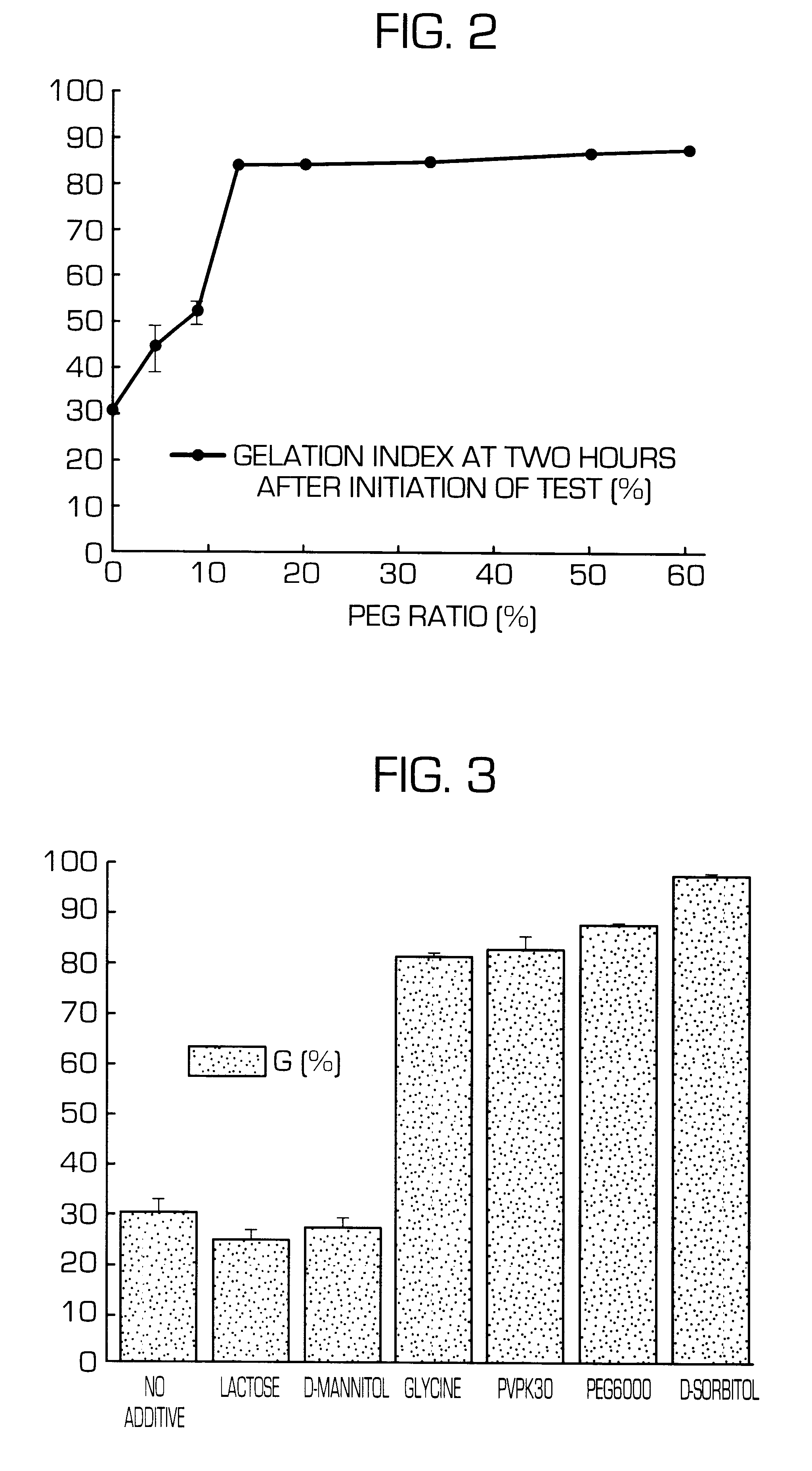
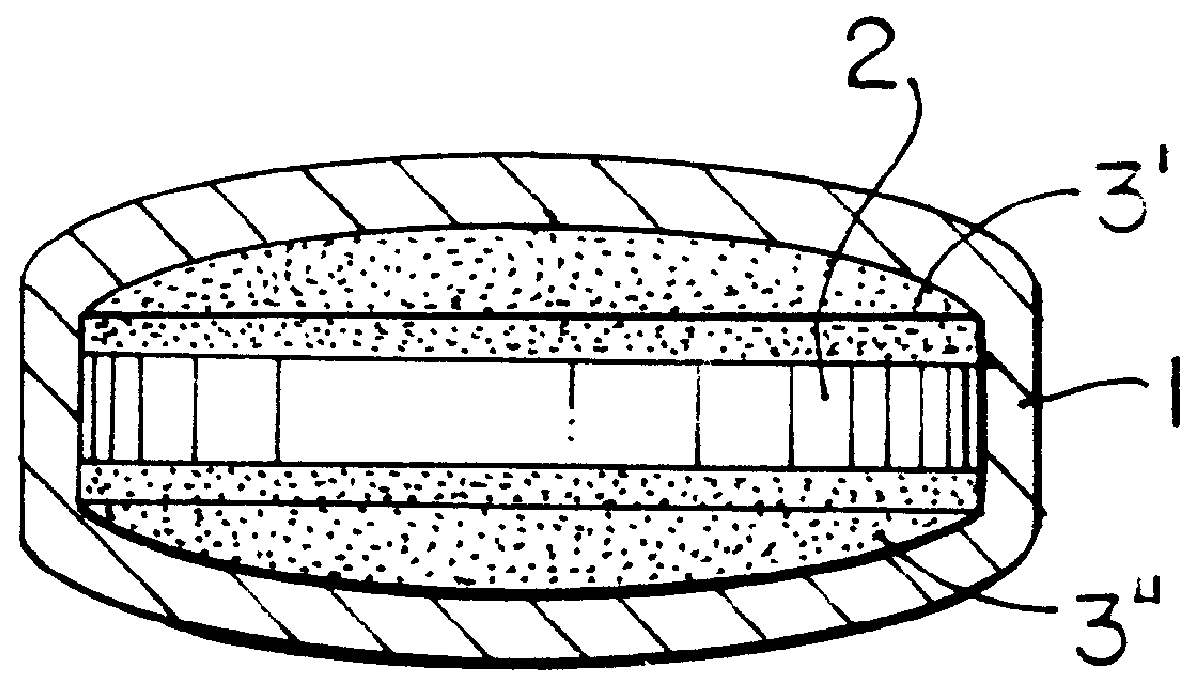
Hydrogel-forming sustained-release preparation
InactiveUS6436441B1Rapid drug releaseReduce the amount of solutionPowder deliveryPill deliverySmall intestinePolymer
The invention provides a hydrogel-type sustained-release preparation comprising (1) at least one drug, (2) an additive which insures a penetration of water into the core of the preparation and (3) a hydrogel-forming polymer, wherein said preparation is capable of undergoing substantially complete gelation during its stay in the upper digestive tract such as stomach and small intestine and is capable of releasing the drug in the lower digestive tract including colon.By the preparation of the invention, the drug is efficiently released and absorbed even in the colon so that a steady and sustained release effect can be achieved.
Owner:ASTELLAS PHARMA INC
Pharmaceutical tablet, completely coated, for controlled release of active principles that present problems of bio-availability linked to gastro-intestinal absorption
Described herein is a particular type of pharmaceutical tablet, for oral use, which is formed by one or more layers, and is specifically designed for controlled release of active principles that present problems of bio-availability linked to absorption in the gastro-intestinal tract, and in particular active principles that present an erratic and unpredictable absorption linked to the presence or absence of food at the level of the stomach and / or of the first portion of the small intestine, the said pharmaceutical form being characterized in that it is completely coated with one or more films of a biocompatible and biodegradable polymeric material.
Owner:JAGOTEC AG
Surgical adhesive and uses therefore
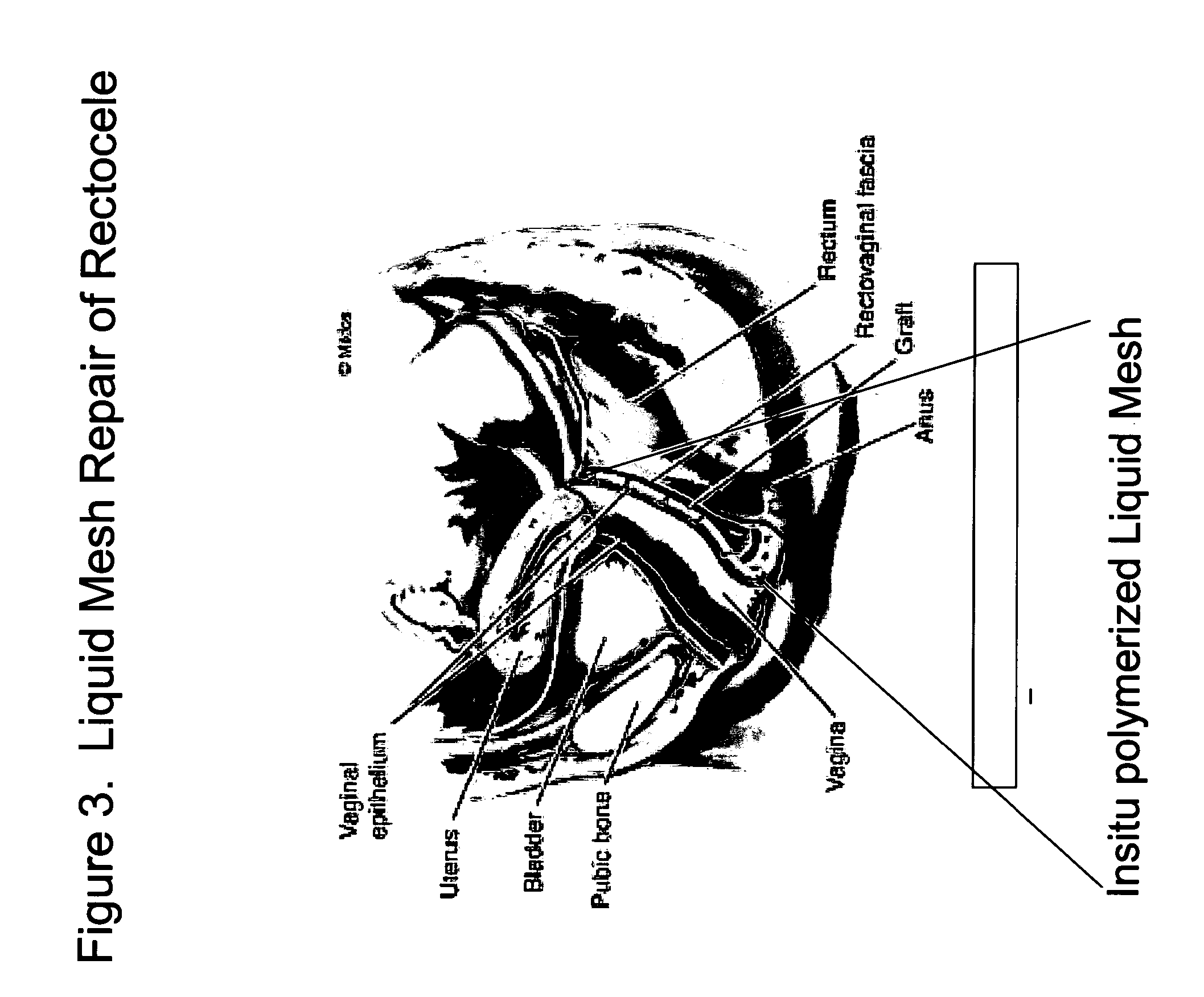
InactiveUS20050129733A1Controlled strengthLess erosiveSurgical adhesivesPharmaceutical delivery mechanismIn situ polymerizationEnteroceles
The present invention provides a liquid polymer composition which can be implanted into a living mammal and which forms a solid hydrogel by in situ polymerization upon contact with body fluid and tissue. The composition also can be used as a coating on a medical device, or for the formation of a medical device. Formation of a solid implant or coating involves crosslinking of the adhesive with itself and with surrounding tissue. The liquid implant, by itself or in conjunction with various prostheses, can be used for many purposed, including fixation of the urethra for providing treatment for incontinence, and repair of herniations in the abdominal cavity, including rectocele, cystocele, enterocele, and inguinal hernia. The adhesive may be used to establish adhesion prevention during such repairs, in part by coating or being the material of a repair mesh.
Owner:PROMETHEAN SURGICAL DEVICES
Reduced digestible carbohydrate food having reduced blood glucose response
ActiveUS20050118326A1Hypoglycemic responseReduced digestibleDough treatmentLeguminous plant bakery productsAdditive ingredientFood material
Reducing the digestion of digestible carbohydrates in a digestible carbohydrate-based material, and reducing the absorption of the digestion product(s) of digestible carbohydrates (that is, simple sugars) within the small intestine. The undigested digestible carbohydrate and the unabsorbed digestion products pass through the small intestines and into the colon, where they are fermented. In effect, the food materials made by practicing the present invention cause a controlled amount of digestible carbohydrate to by-pass the small intestine, resulting in the fermentation of digestible carbohydrates in the colon. The invention also provides for processing of a digestible carbohydrate-based ingredient with a non-digestible food film material, to form a reduced digestible carbohydrate food having a protective food film network, which can inhibit or prevent digestion of the digestible carbohydrate. The present invention also provides for processing of a digestible carbohydrate-based ingredient with a non-digestible food film material, to provide a resulting reduced digestible carbohydrate food containing a viscosity-building component that contributes to the formation of a viscous intestinal chyme that can inhibit or prevent digestion of the digestible carbohydrate and can inhibit adsorption of digestion products of digestible carbohydrates in the small intestine.
Owner:TECHCOM GRP LLC
Gastrointestinal sleeve device and methods for treatment of morbid obesity
InactiveUS7794447B2Reduce volumeReduce absorptionMedical devicesTubular organ implantsIntestinal structureSmall intestine
Owner:VALENTX
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS8734429B2Rapid drug releasePoor absorptionMedical devicesPressure infusionIntestinal wallsDrugs preparations
Owner:RANI THERAPEUTICS
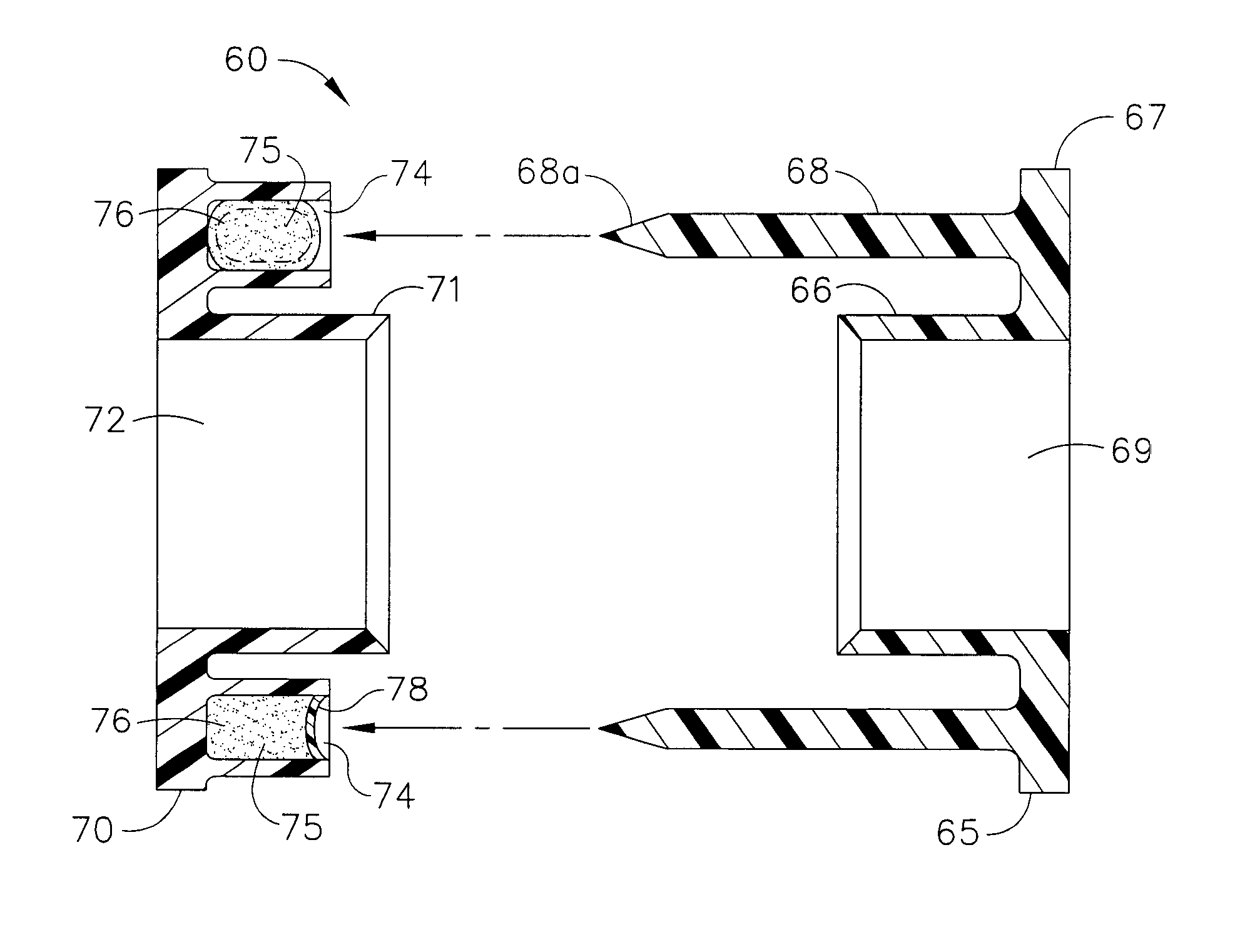
Bodily lumen closure apparatus and method
ActiveUS20050155608A1Improve sealingPrevent leakageStentsFallopian occludersCell-Extracellular MatrixSource material
An absorbable and expandable closure member used to occlude or exclude a body lumen or cavity, such as a blood vessel, fallopian tube, duct, aneurysmal sac, etc., comprising a closure member comprising one of more sheets of a biomaterial that are rolled, stacked, or folded to form a multilayer construct of a generally cylindrical configuration for deployment through a delivery system, either as a singularly or part of a multiplicity of closure members. The biomaterial is derived from a source material, such as small intestinal submucosa or another remodelable material (e.g., an extracellular matrix) having properties for stimulating ingrowth of adjacent tissue into the biomaterial deployed within the bodily lumen. The closure member is deployed to the bodily lumen from a delivery sheath, cartridge, and / or over a inner guiding member, such as a wire guide or catheter.
Owner:OREGON HEALTH & SCI UNIV +2
Stimulation of Satiety Hormone Release
The present invention provides, among other things, a site specific way to enhance a natural hormonal response to nutrients entering the small intestine after gastric emptying, thereby providing therapeutic value for obesity or diabetic patients. In one aspect, the present invention provides methods of stimulating the release of satiety hormone in a subject comprising applying a first electrical stimulus to a tissue in the lumen of the gastrointestinal system of the subject contemporaneously with the contacting of L-cells of the tissue with a nutrient stimulus. In another aspect, the present invention provides methods for predicting patient response to a weight loss surgery comprising applying a first electrical stimulus to a tissue of the gastrointestinal system of said patient contemporaneously with the contacting of L-cells of the tissue with a nutrient stimulus, assessing the effect of the electrical stimulus in said patient, and, correlating said effect to said patient's response to a weight loss surgery.
Owner:CENTOCOR ORTHO BIOTECH
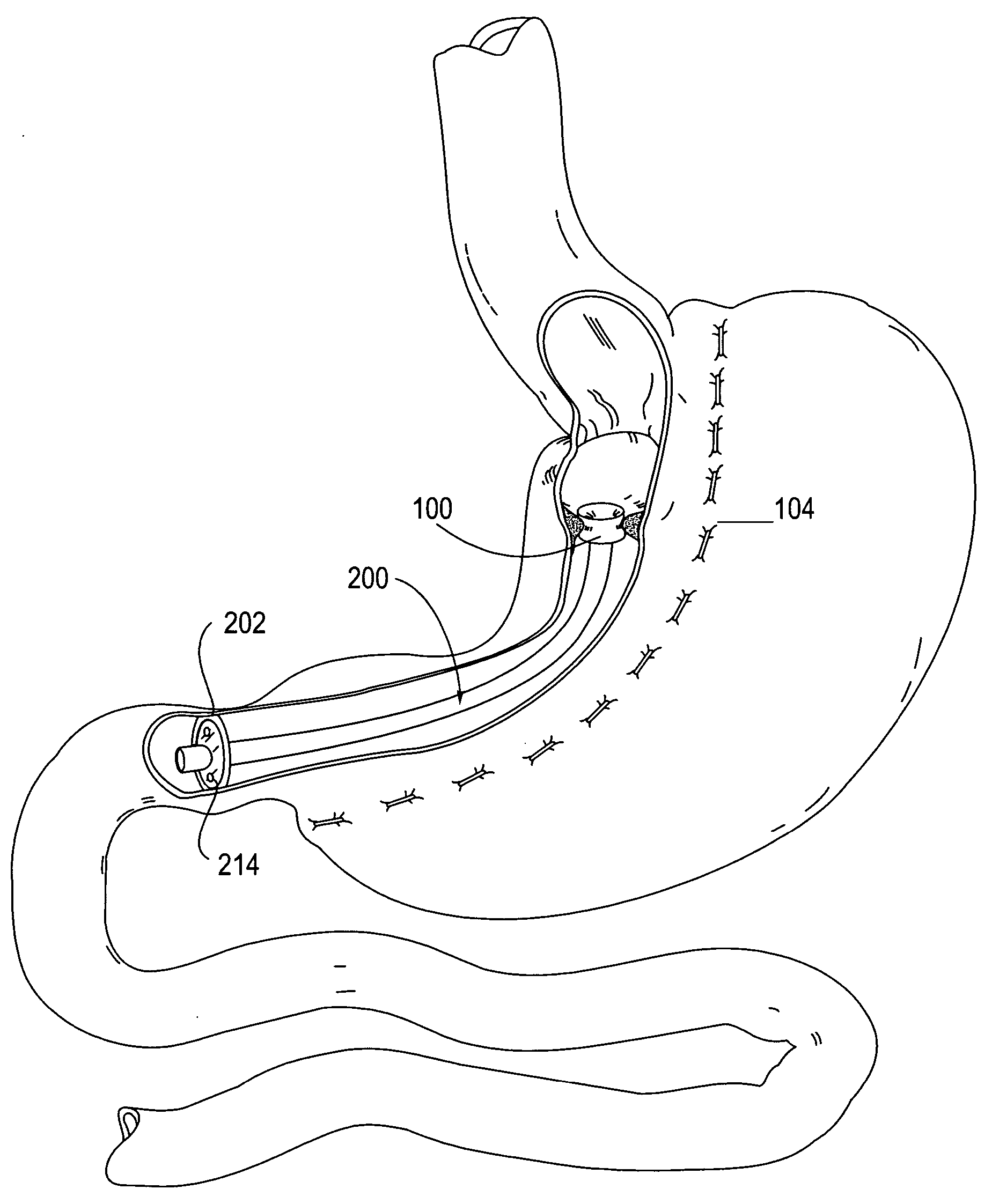
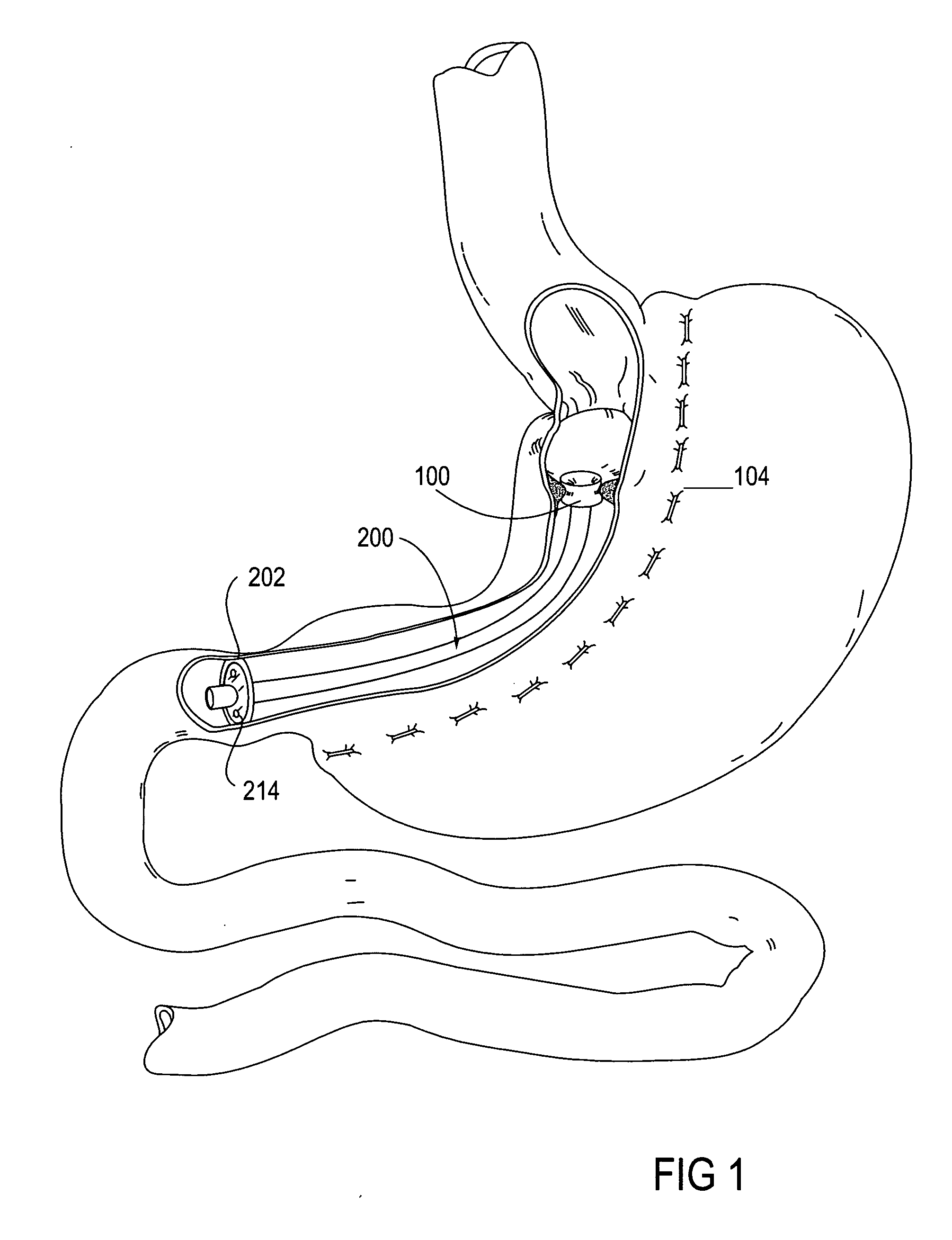
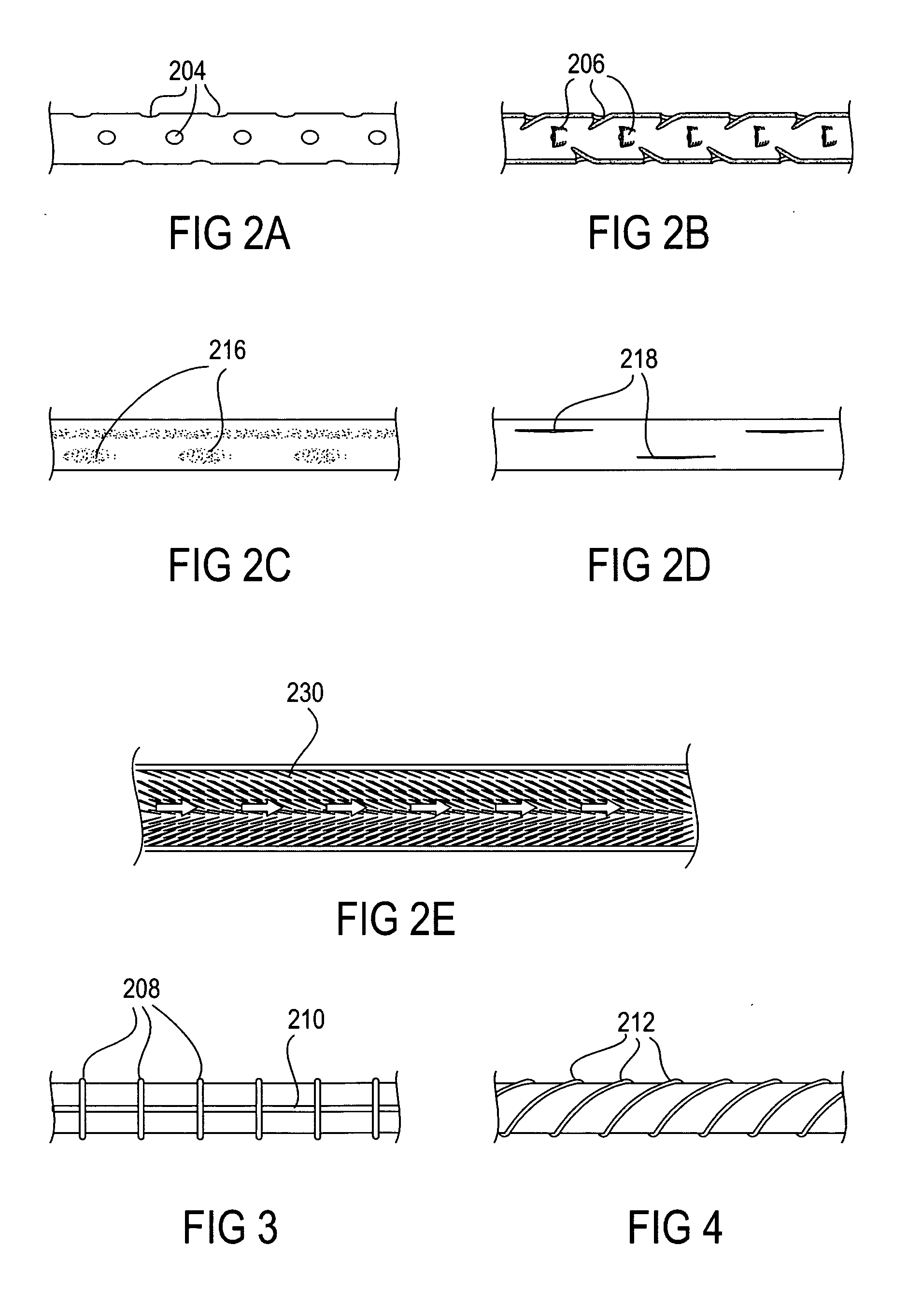
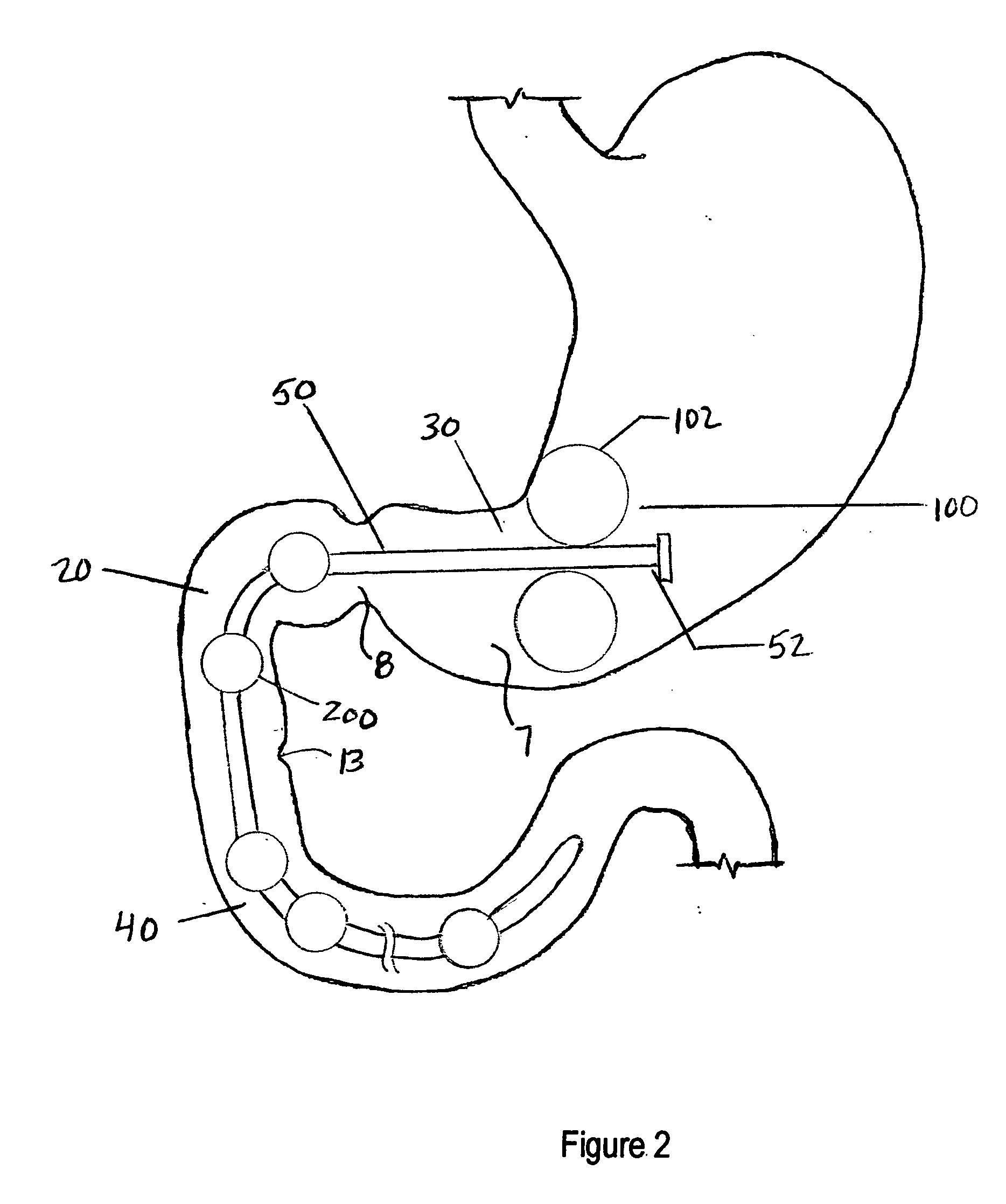
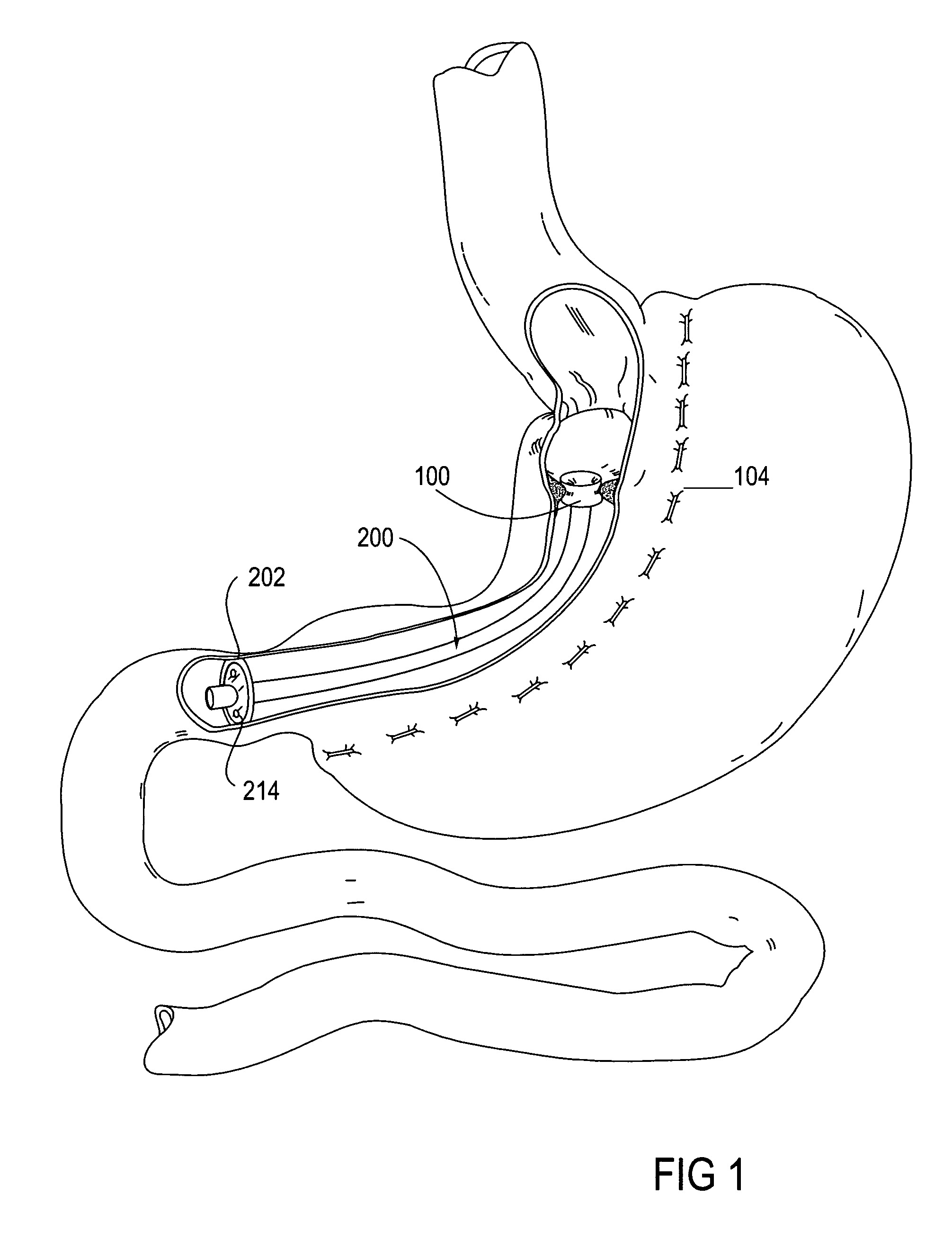
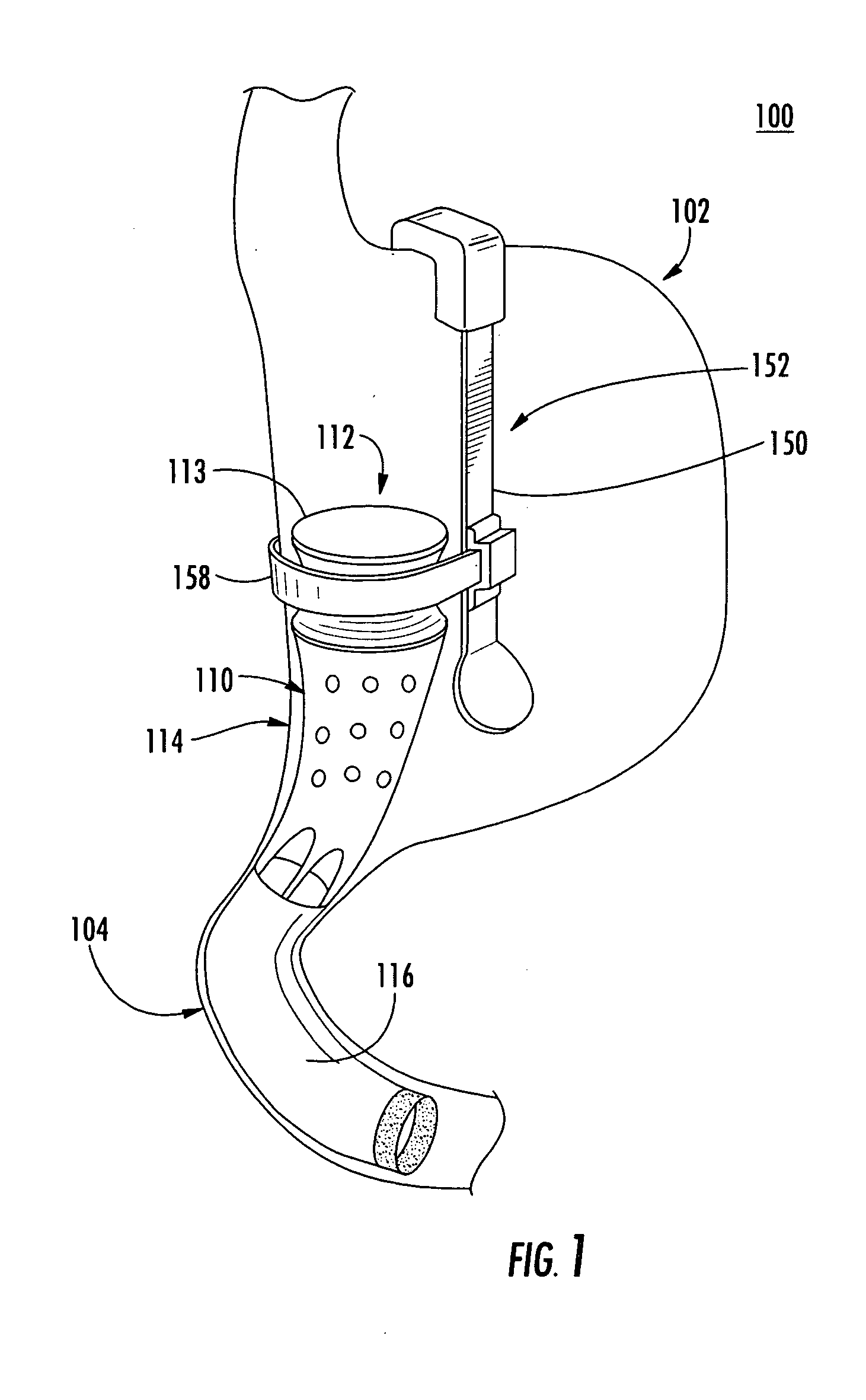
System, system devices, and methods for regulating nutrient absorption and caloric intake
InactiveUS20050197714A1Easy to superviseRegulate nutrient absorption and caloric intakeTubular organ implantsObesity treatmentSmall intestineNon invasive
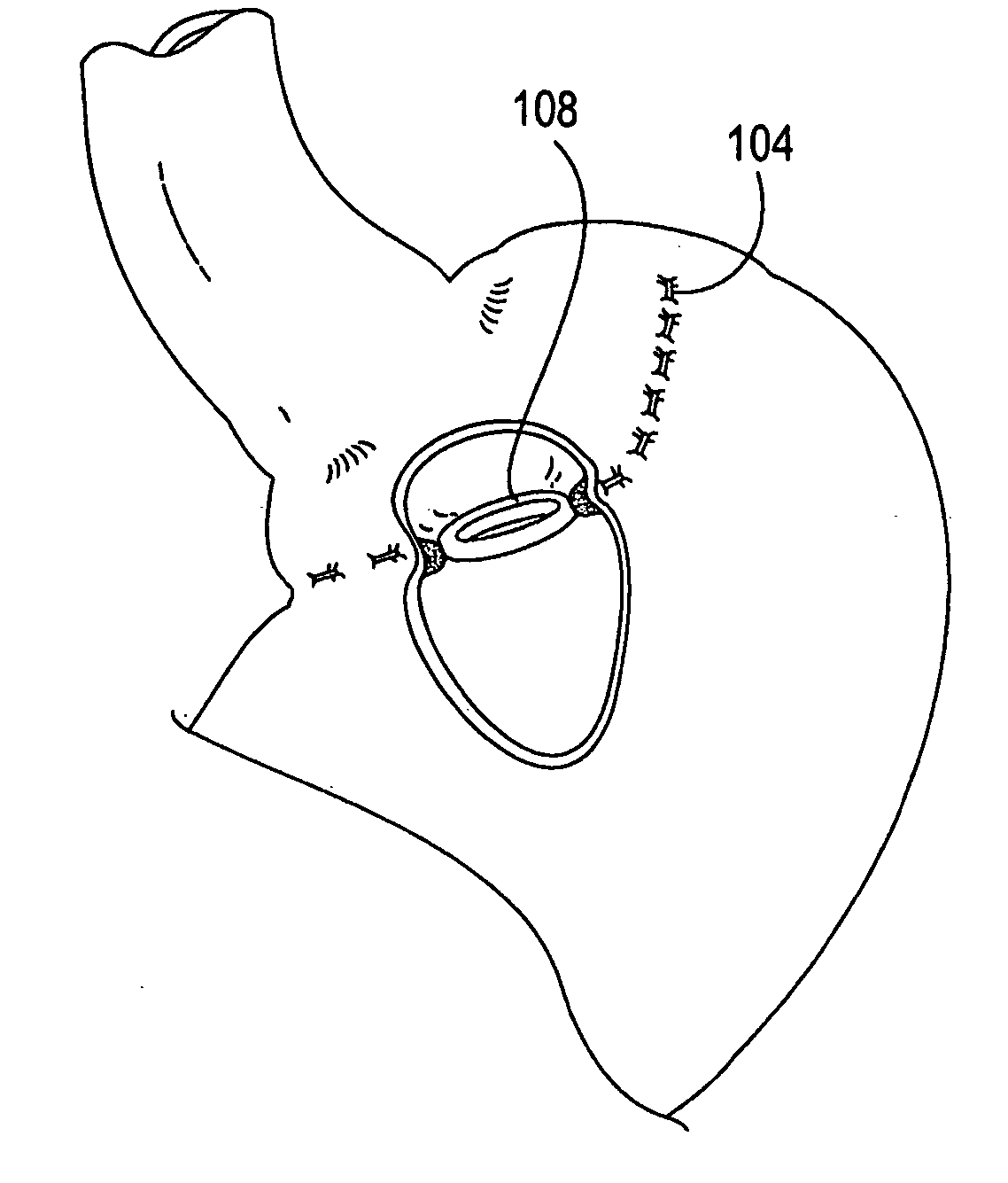
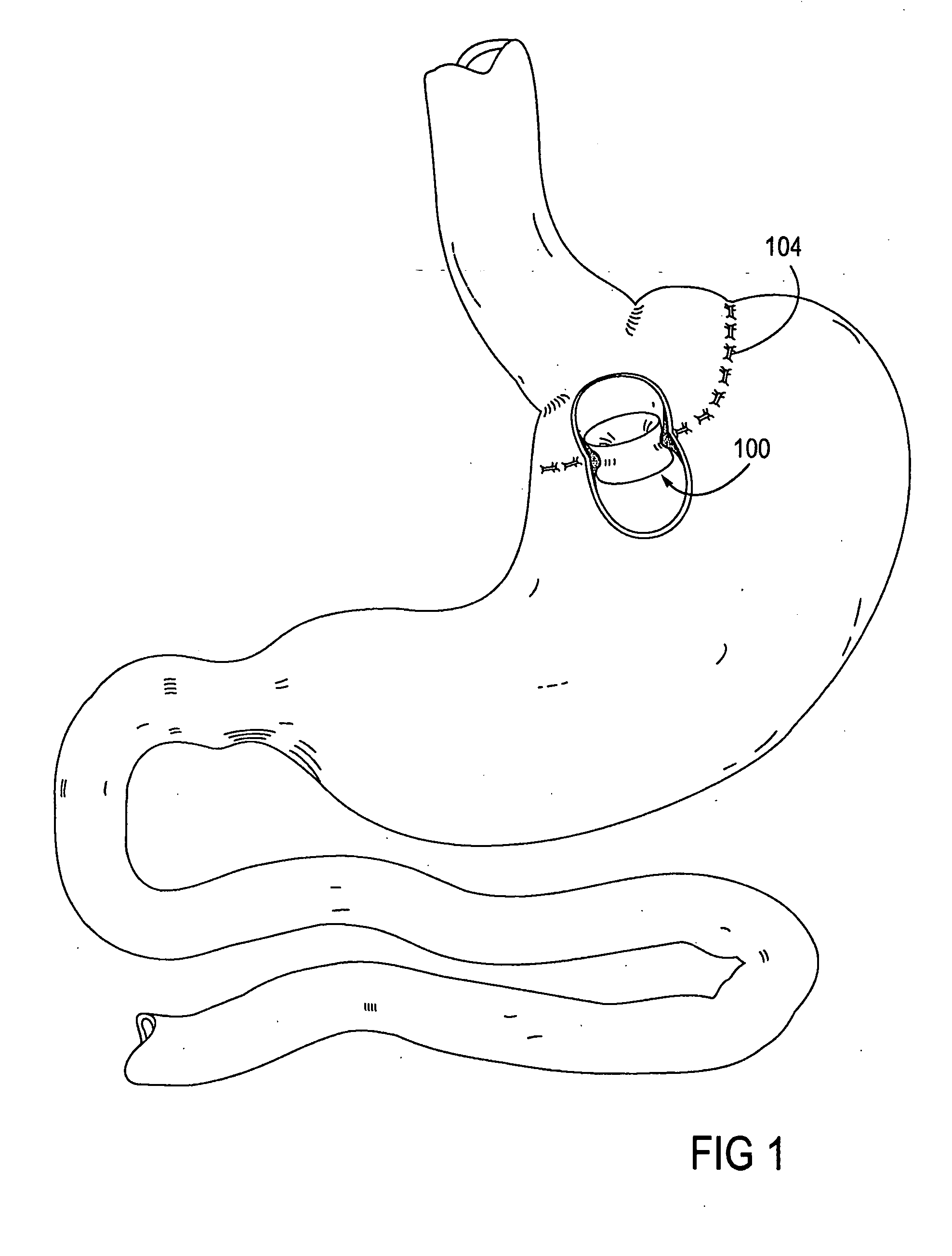
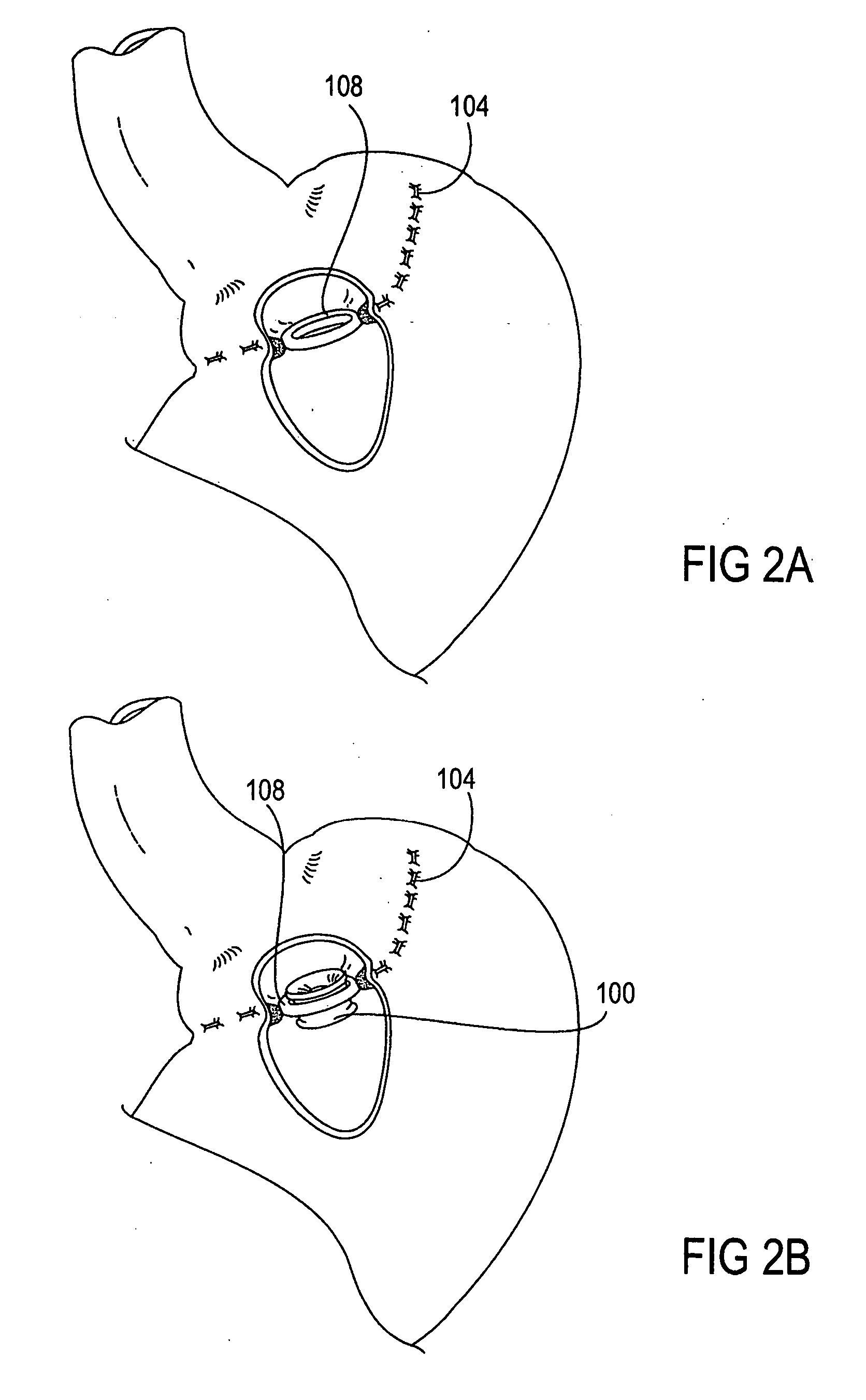
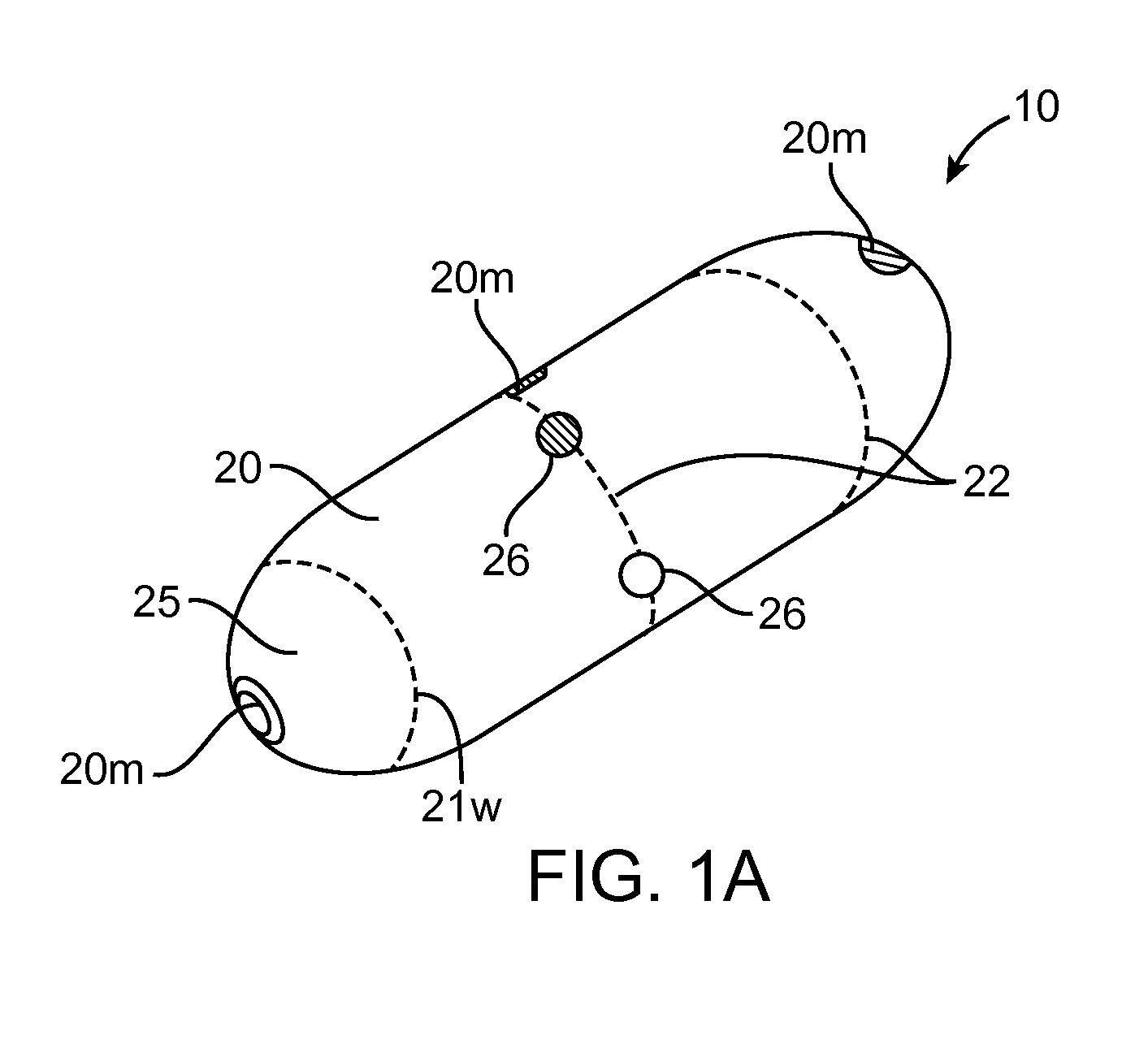
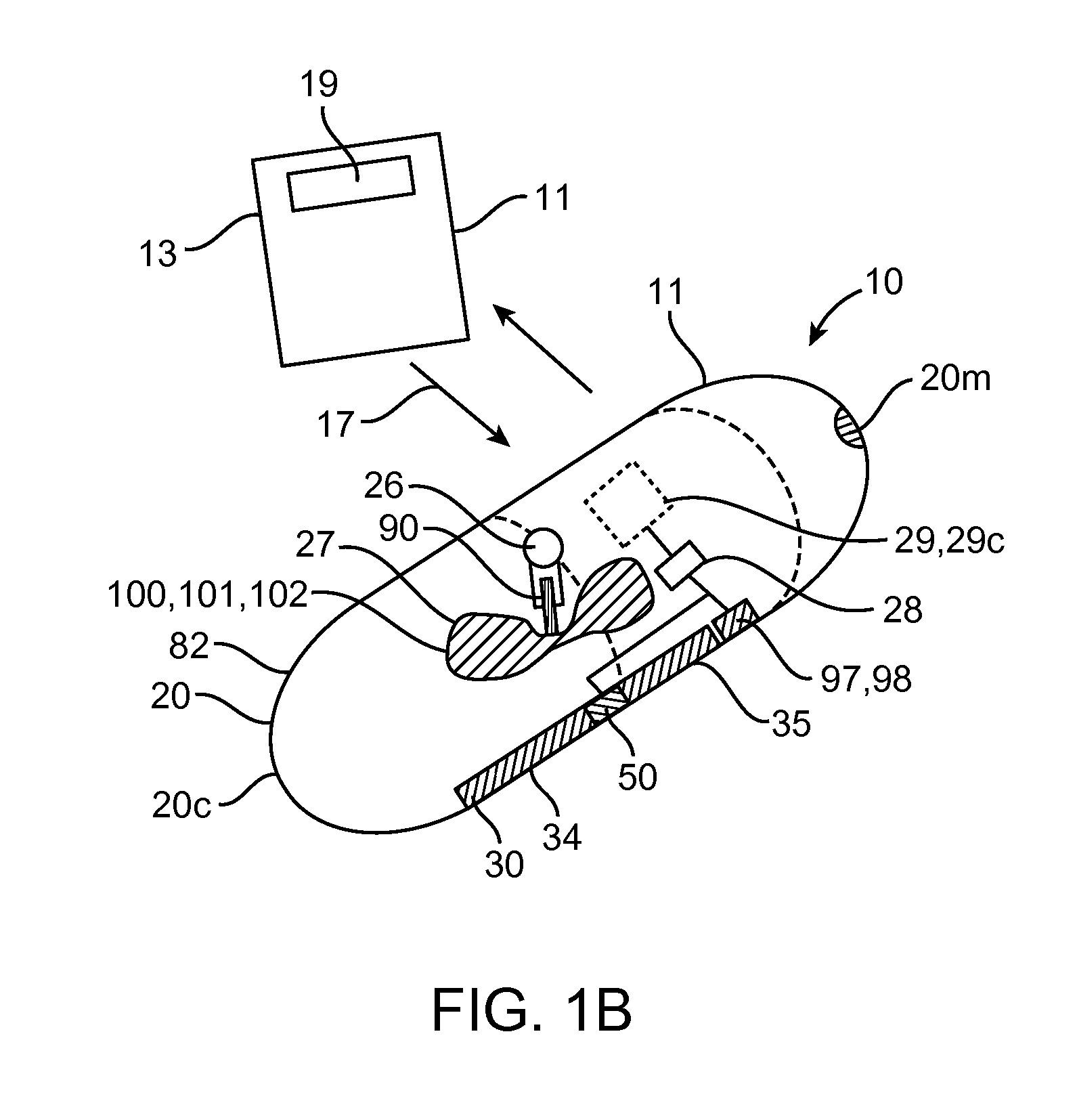
A system (100) for regulating absorption and caloric intake can include an elongated tube (110) having a stomach portion (114) and a lower intestine portion (116) where the elongated tube defines a passage (114) to guide ingested material through the stomach (102) and through a portion of the small intestine (104). The system also includes a non-invasive stomach stricture device (150) having a clamping structure (152) for regulating the rate of flow of ingested material through the elongated tube where the clamping structure reduces the capacity of the passage by clamping a portion of the elongated tube through the exterior of the stomach.
Owner:PRECISION MEDICAL DEVICES INC
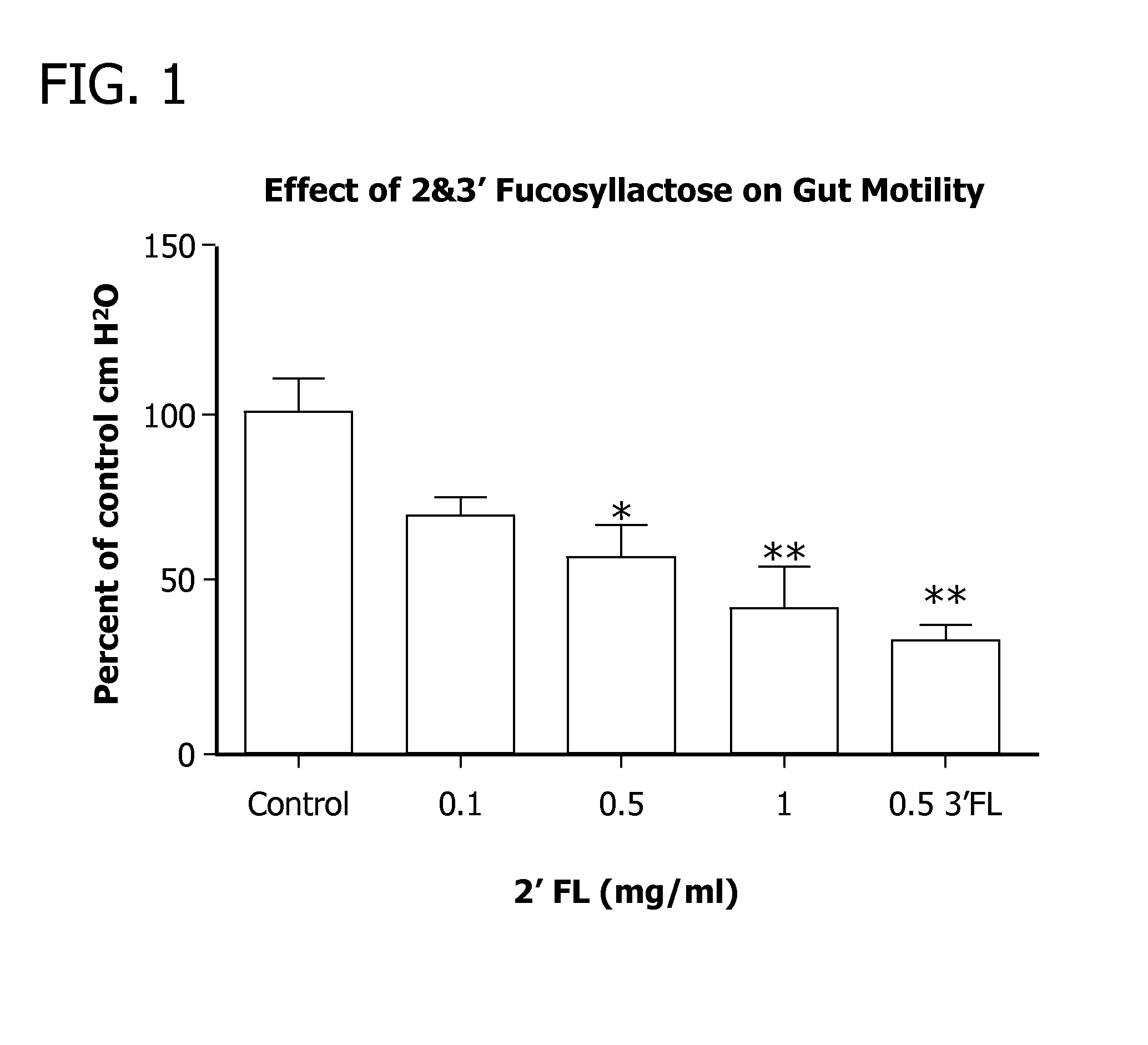
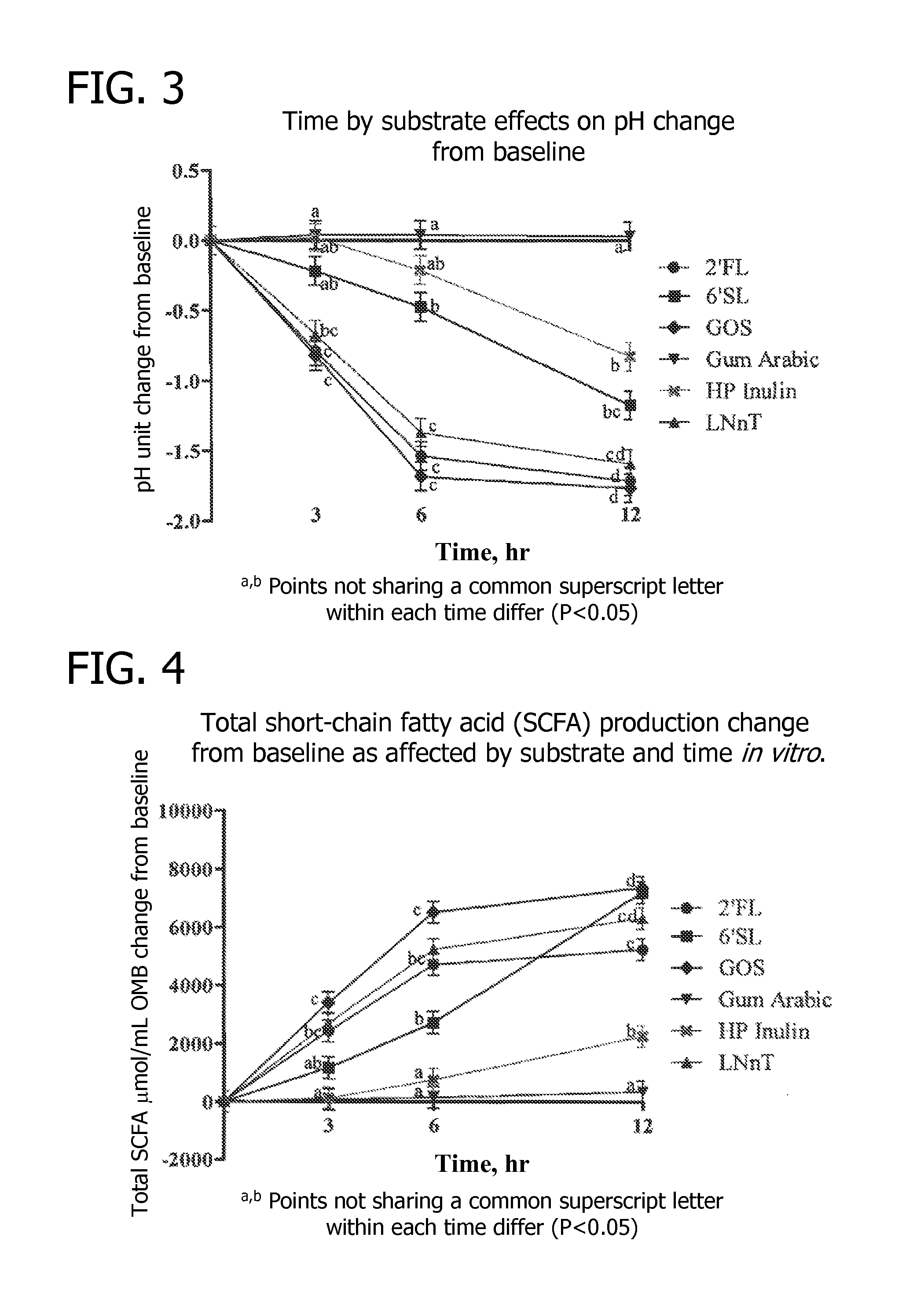
Methods for decreasing the incidence of necrotizing enterocolitis in infants, toddlers, or children using human milk oligosaccharides
ActiveUS20120172319A1Improve immune system systemImprove system enteric nervous systemOrganic active ingredientsBiocideDiseaseMorbidity aspects
Disclosed are methods of reducing the incidence of necrotizing enterocolitis in an infant, toddler, or child using nutritional compositions including human milk oligosaccharides. The nutritional compositions including the human milk oligosaccharides are effective in reducing inflammation and the incidence of inflammatory diseases.
Owner:ABBOTT LAB INC
Methods and devices for intervertebral augmentation using injectable formulations and enclosures
Devices and methods for treating diseased or damaged portions of an intervertebral region are provided. In particular, intervertebral implants that can include use of a tissue regeneration structure having small intestine submucosa are described. The intervertebral implants can be utilized with any combination of load bearing structures for supporting loading on the implant, shaping structures for biasing the configuration of the implant, collapsible support structures for shaping the implant, and other features. Implants can also be formed with an enclosure to contain a filling material, such as an injectable small intestine submucosa formulation. Methods of delivering and utilizing the various implants are also discussed.
Owner:DEPUY SPINE INC (US) +1
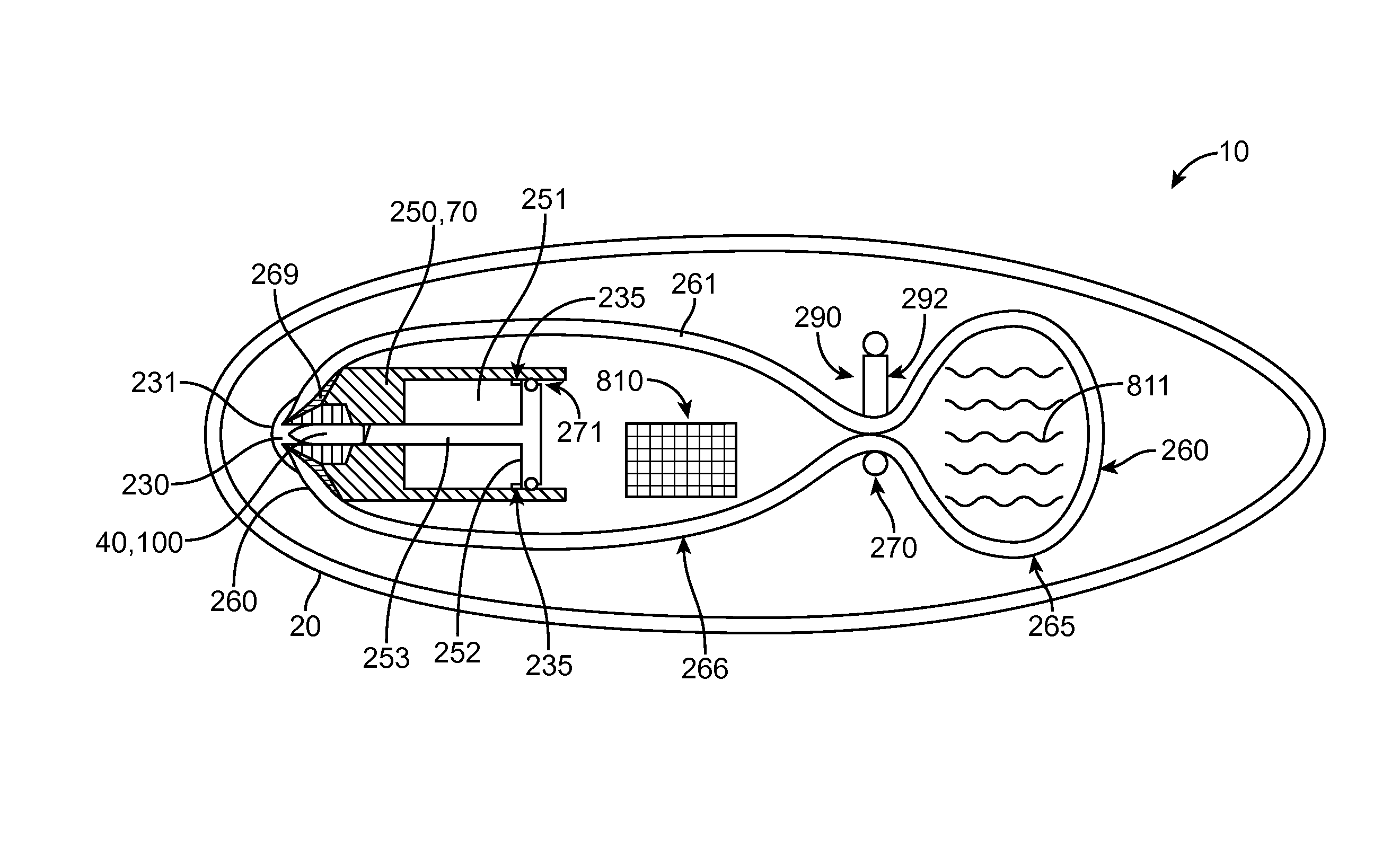
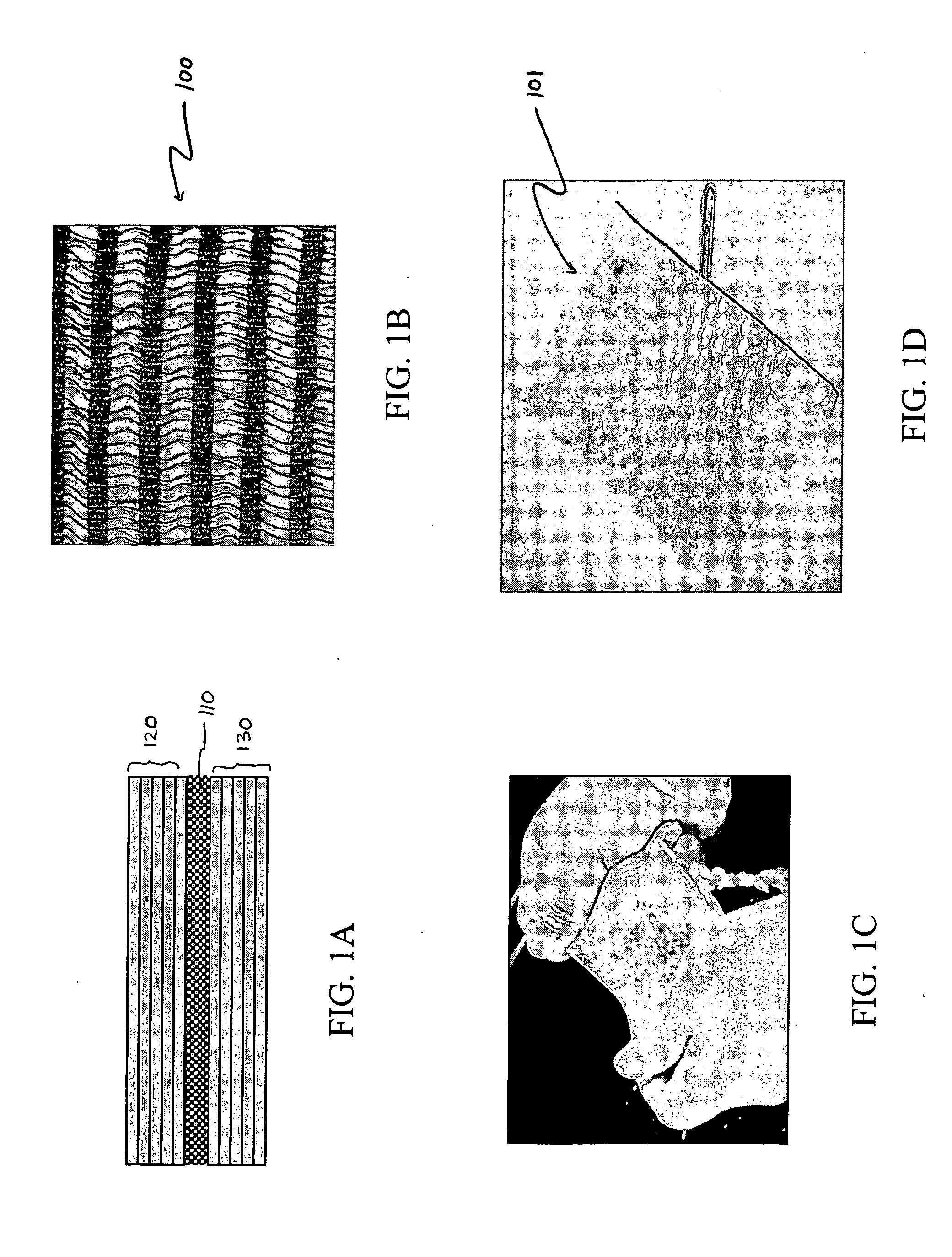
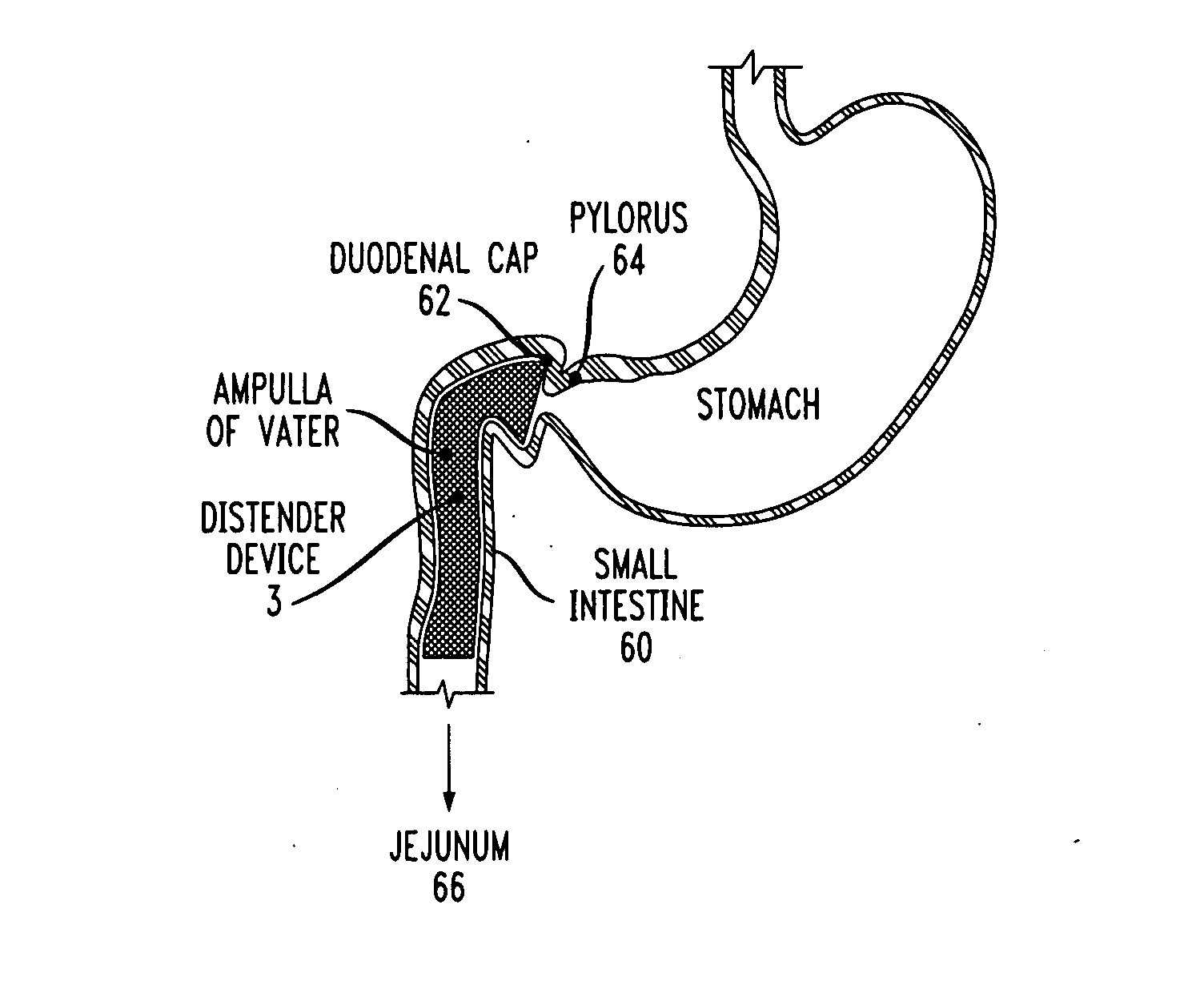
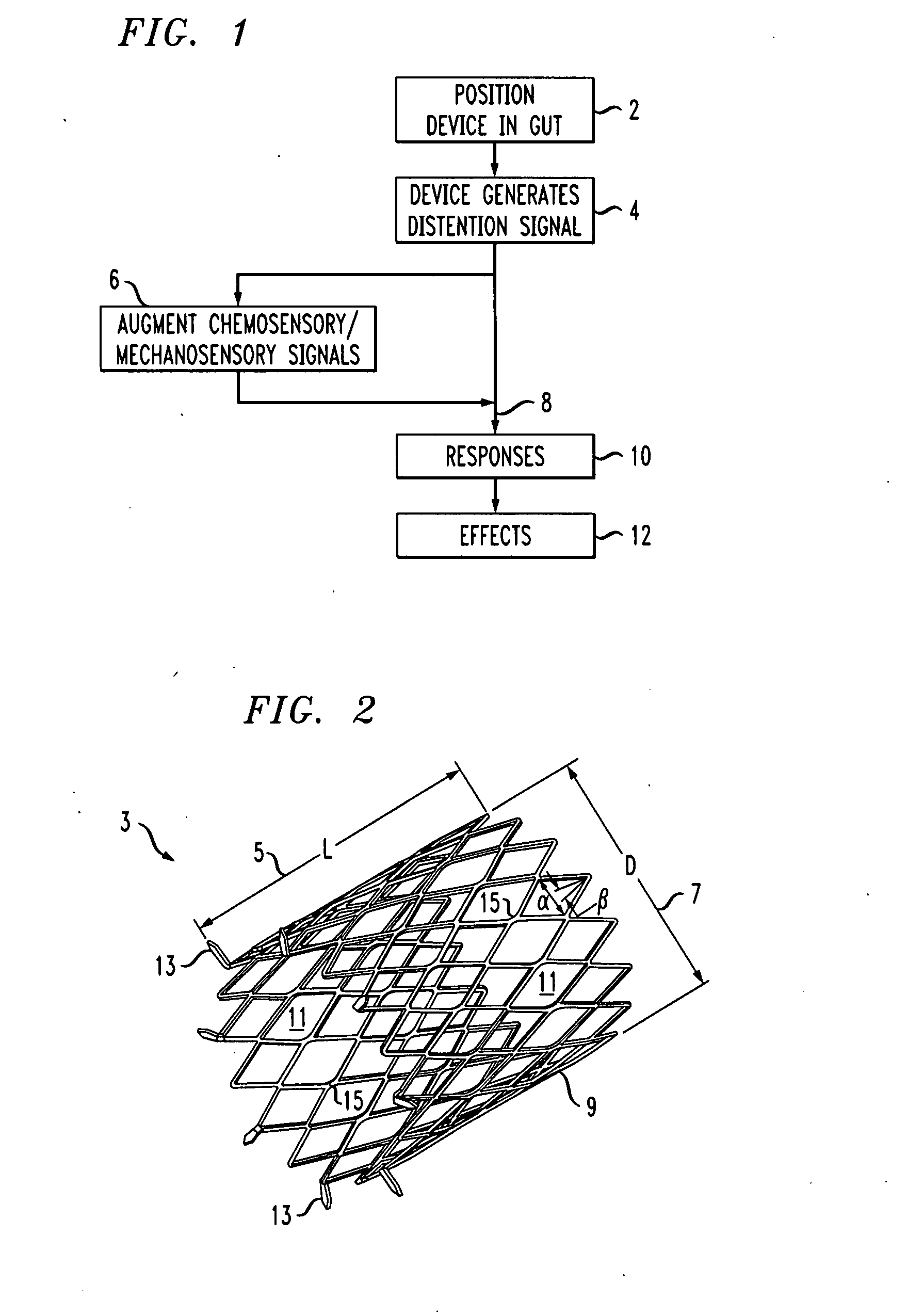
Distender device and method for treatment of obesity and metabolic and other diseases
A gastrointestinal implant device is positioned in a patient's small intestine or rectum and produces an outward force that itself produces a distension signal which is a therapeutically useful neural or humoral signal that evokes satiogenic or weight loss effects by itself. The device may advantageously be placed in the duodenum adjacent the pylorus or in the jejunum, ileum or rectum. The distension signals may amplify chemosensory or mechanosensory signals such as enteroendocrine secretions within the patient. The device may be a mesh and include a low material density that allows for unrestricted chyme absorption within the small intestine and unrestricted chyme flow through the gastrointestinal system. A method includes inserting the device into the patient then either retrieving the device after treatment is complete or allowing a device formed of a biodegradable material to degrade in time after treatment is complete.
Owner:ADVANCED NEUROMODULATION SYST INC
Devices for intervertebral augmentation and methods of controlling their delivery
InactiveUS20070150063A1Promote tissue growthHigher compressive modulusBone implantLigamentsFilling materialsSmall intestine
Devices and methods for treating diseased or damaged portions of an intervertebral region are provided. In particular, intervertebral implants that can include use of a tissue regeneration structure having small intestine submucosa are described. The intervertebral implants can be utilized with any combination of load bearing structures for supporting loading on the implant, shaping structures for biasing the configuration of the implant, collapsible support structures for shaping the implant, and other features. Implants can also be formed with an enclosure to contain a filling material, such as an injectable small intestine submucosa formulation. Methods of delivering and utilizing the various implants are also discussed.
Owner:DEPUY SYNTHES PROD INC +1
Ingestible device platform for the colon
ActiveUS20050266074A1Enhance the imageUltrasonic/sonic/infrasonic diagnosticsSurgeryAbnormal tissue growthOptical fluorescence
An ingestible pill platform for colon imaging is provided, designed to recognize its entry to the colon and expand in the colon, for improved imaging of the colon walls. On approaching the external anal sphincter muscle, the ingestible pill may contract or deform, for elimination. Colon recognition may be based on a structural image, based on the differences in diameters between the small intestine and the colon, and particularly, based on the semilunar fold structure, which is unique to the colon. Additionally or alternatively, colon recognition may be based on a functional image, based on the generally inflammatory state of the vermiform appendix. Additionally or alternatively, pH, flora, enzymes and (or) chemical analyses may be used to recognize the colon. The imaging of the colon walls may be functional, by nuclear-radiation imaging of radionuclide-labeled antibodies, or by optical-fluorescence-spectroscopy imaging of fluorescence-labeled antibodies. Additionally or alternatively, it may be structural, for example, by visual, ultrasound or MRI means. Due to the proximity to the colon walls, the imaging in accordance with the present invention is advantageous to colonoscopy or virtual colonoscopy, as it is designed to distinguish malignant from benign tumors and detect tumors even at their incipient stage, and overcome blood-pool background radioactivity.
Owner:SPECTRUM DYNAMICS MEDICAL LTD
Device, system and methods for the oral delivery of therapeutic compounds
ActiveUS9149617B2Rapid drug releasePoor absorptionPeptide/protein ingredientsMedical devicesIntestinal wallsSmall intestine
Embodiments of the invention provide swallowable devices, preparations and methods for delivering drugs and other therapeutic agents within the GI tract. Particular embodiments provide a swallowable device such as a capsule for delivering drugs into the intestinal wall or other GI lumen. Embodiments also provide various drug preparations that are configured to be contained within the capsule, advanced from the capsule into the intestinal wall and degrade within the wall to release the drug to produce a therapeutic effect. The preparation can be coupled to a delivery mechanism having one or more balloons or other expandable devices which are expandable responsive to a condition in the small intestine or other GI lumen to advance the preparation out of the capsule into the intestinal wall. Embodiments of the invention are particularly useful for the delivery of drugs which are poorly absorbed, tolerated and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com