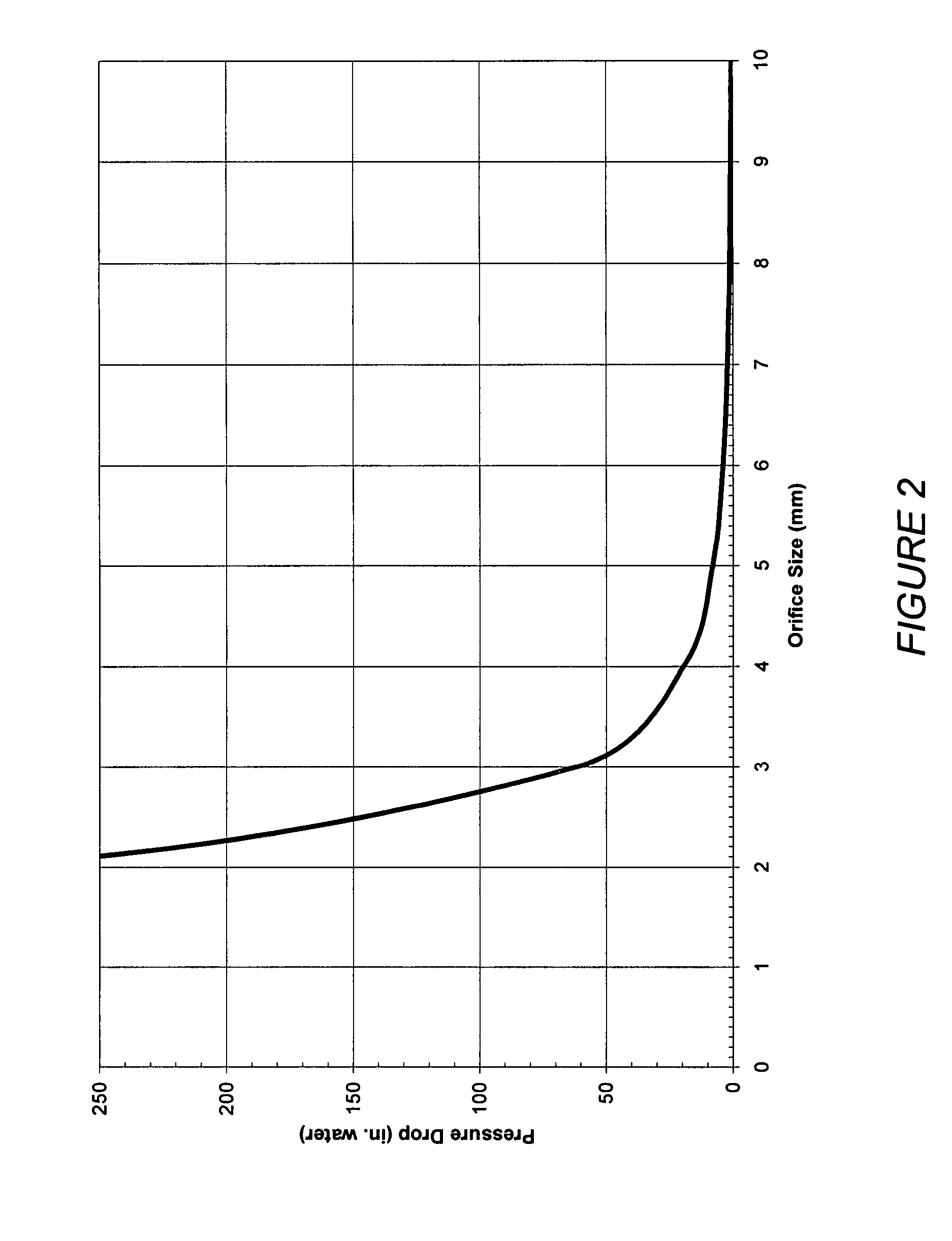
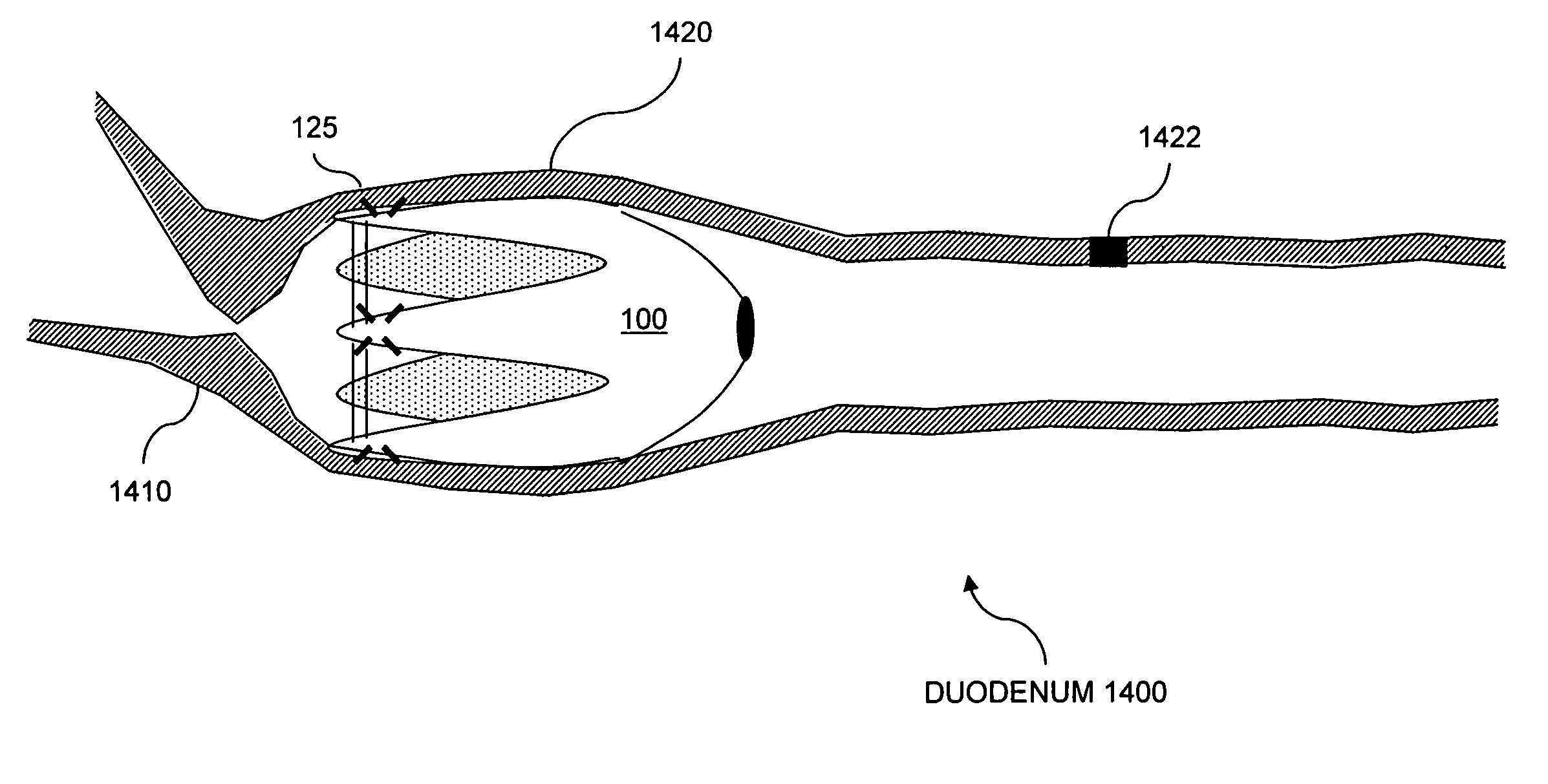
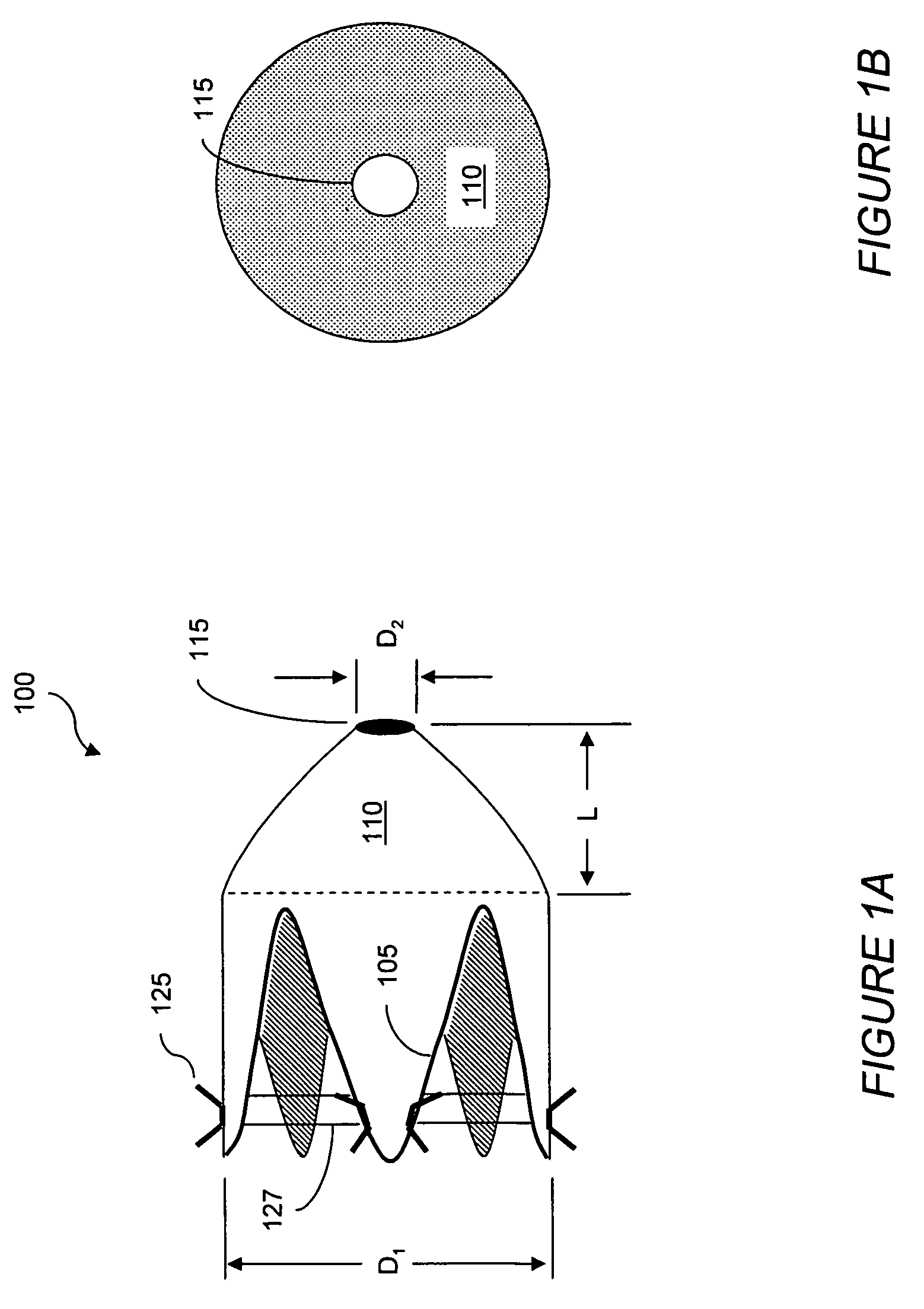
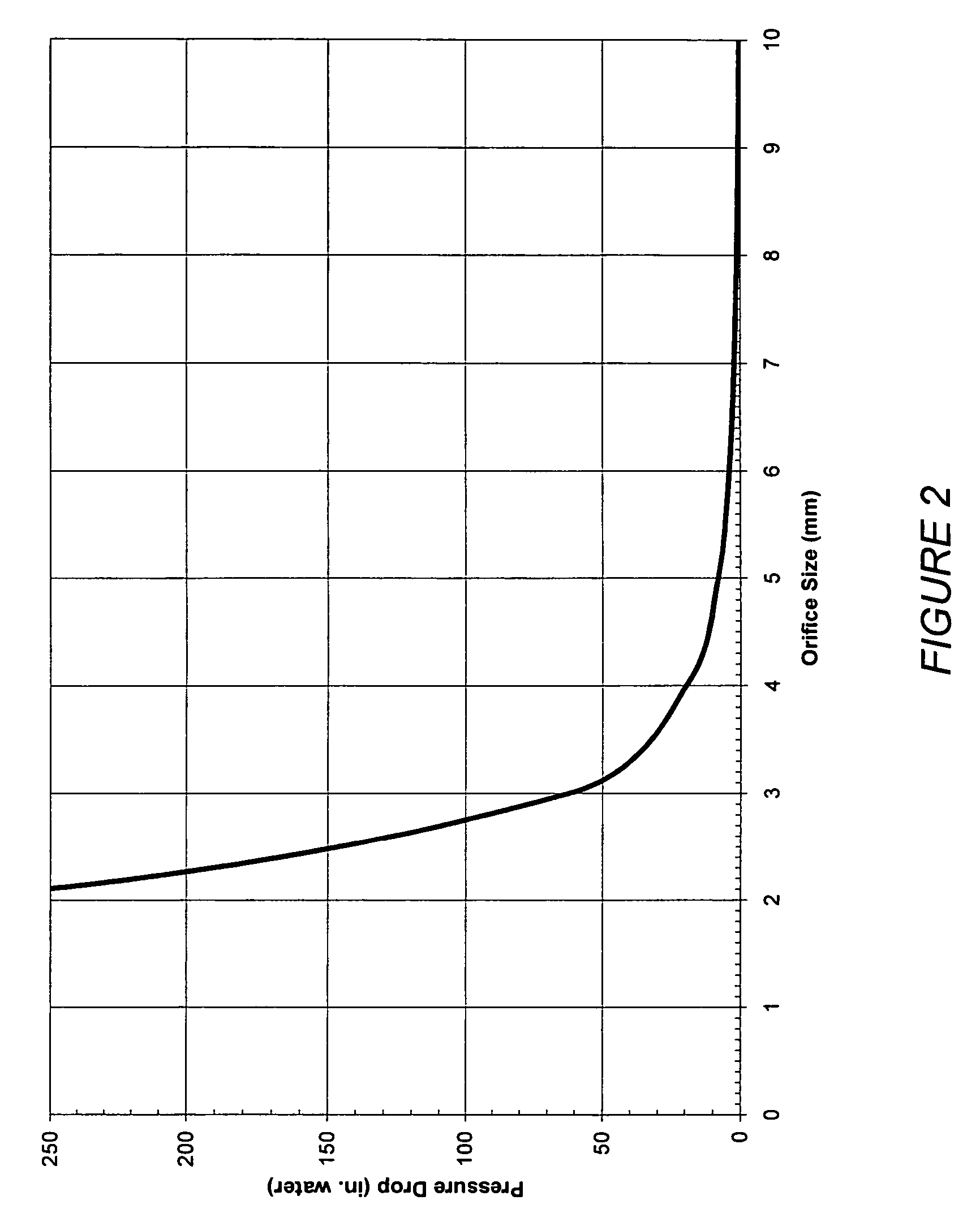
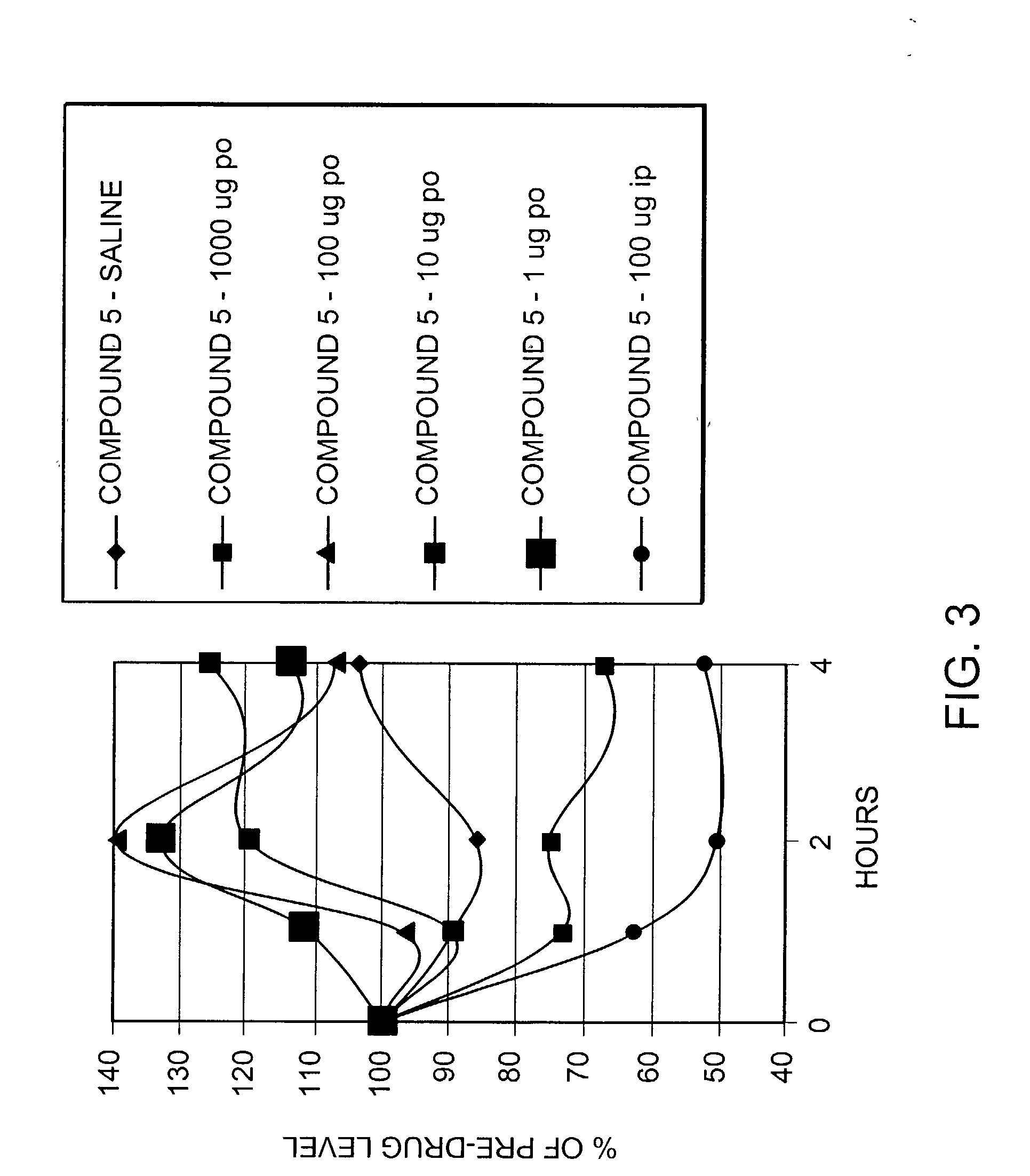
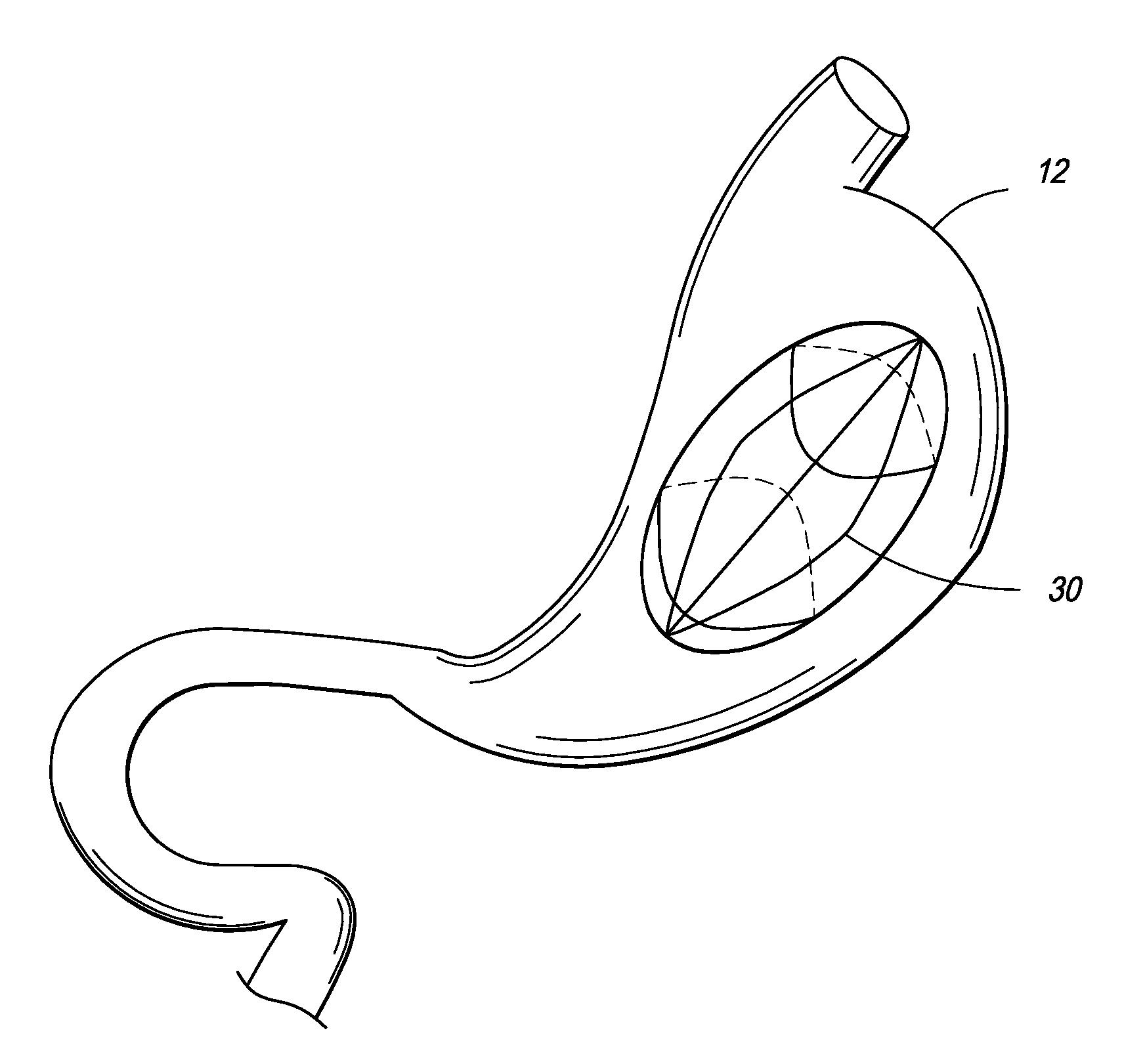
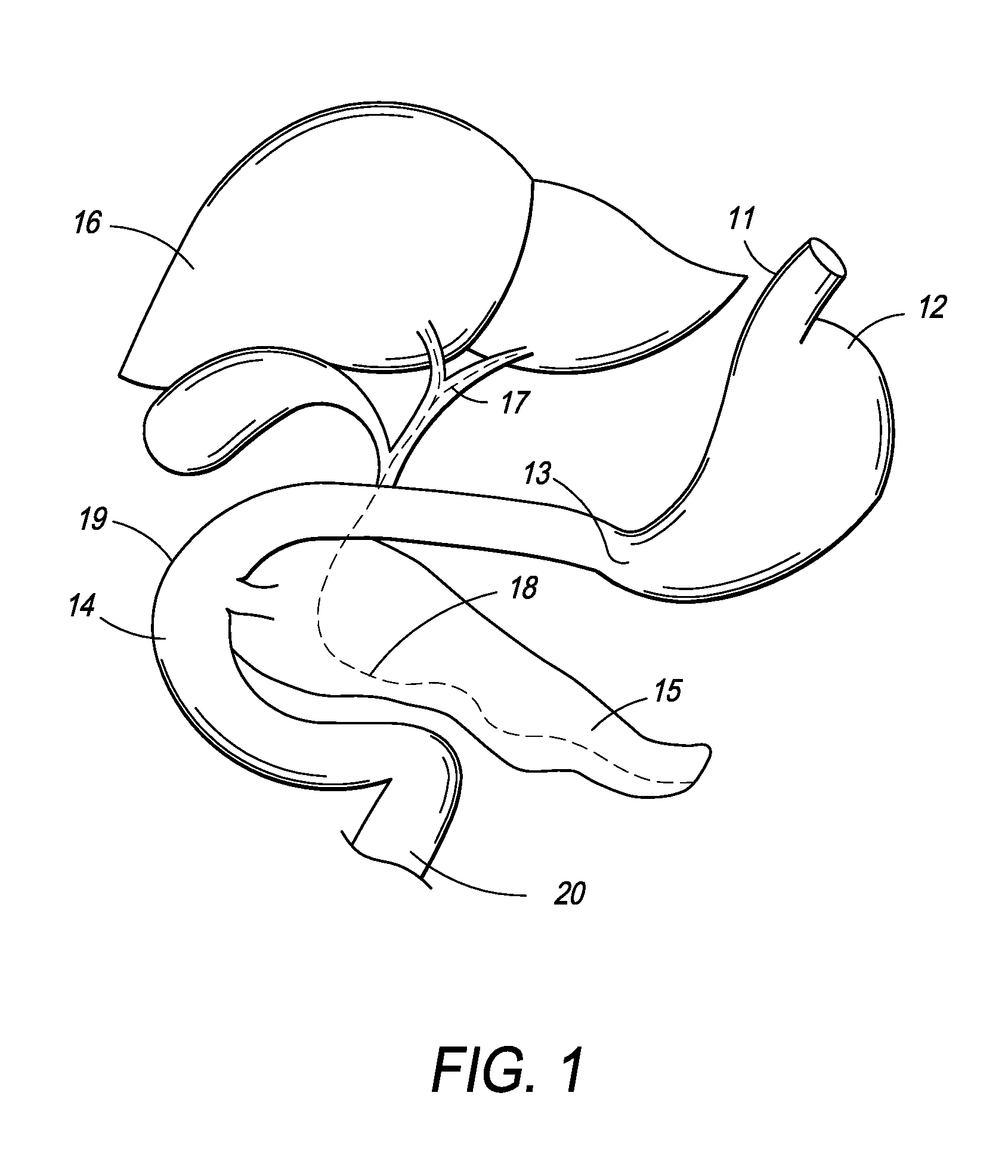
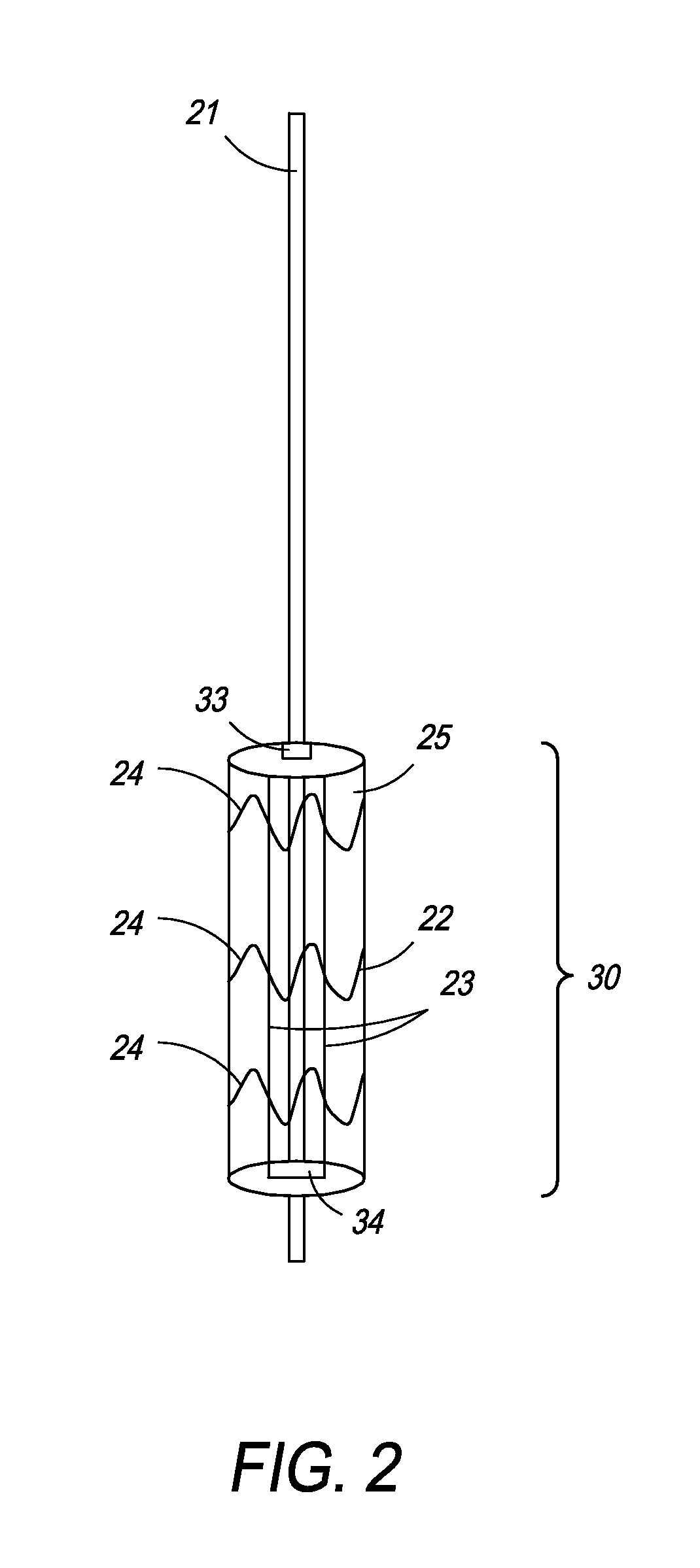
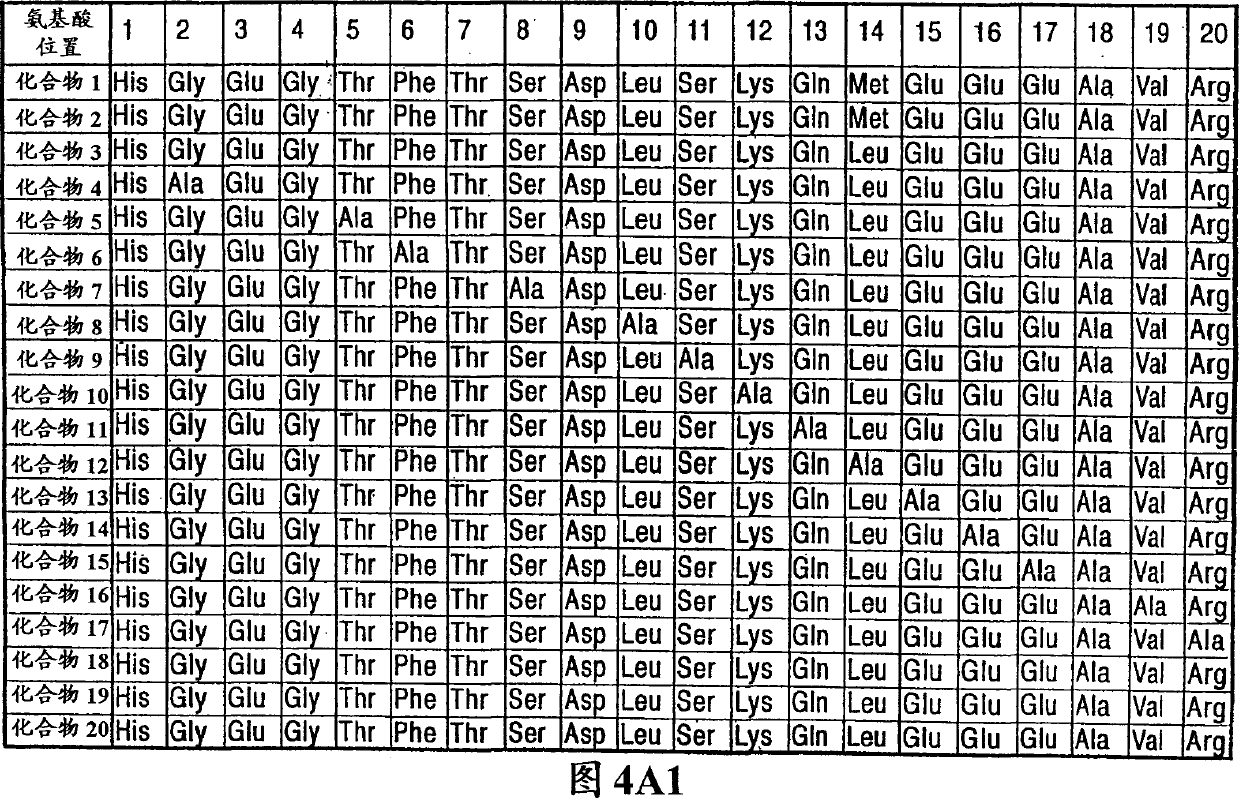
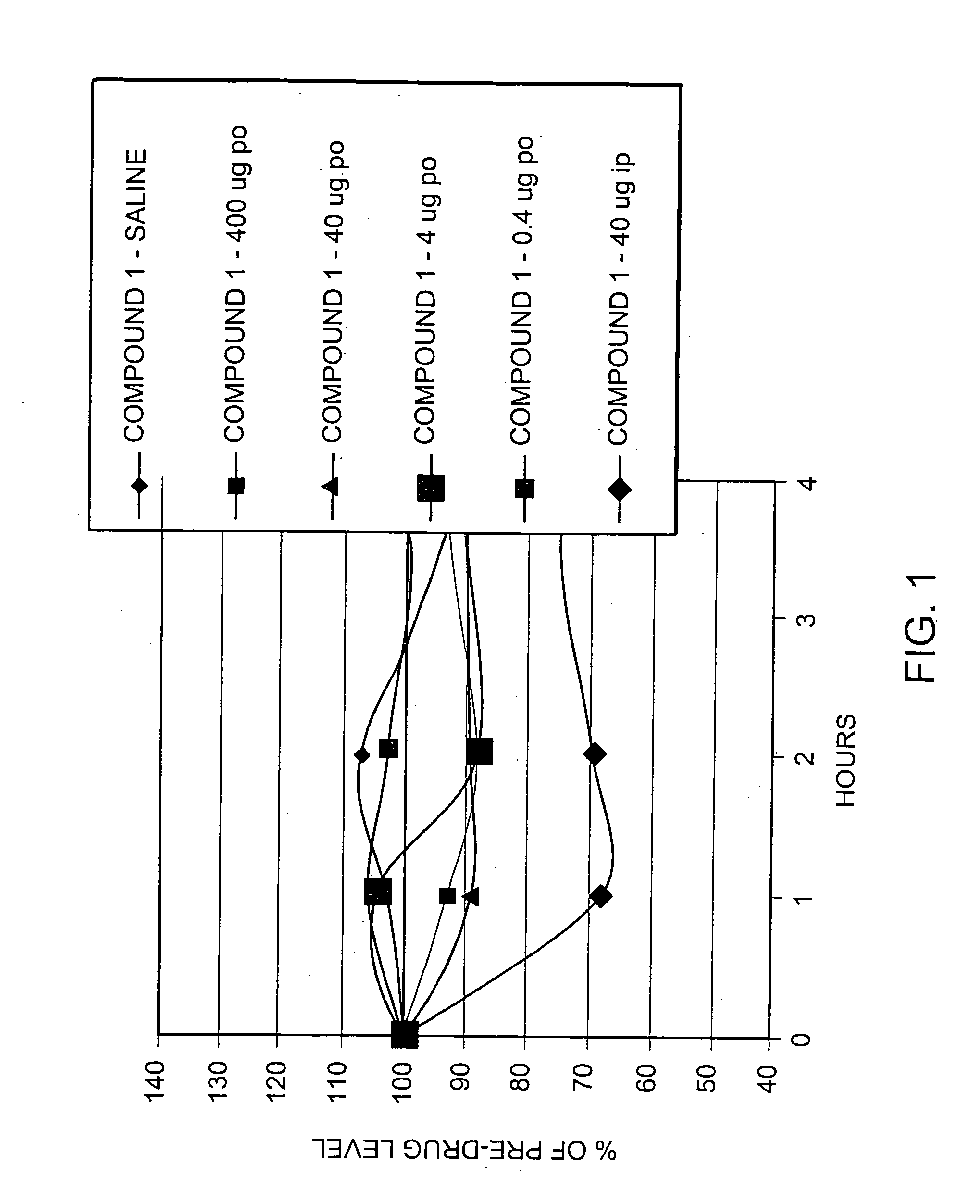
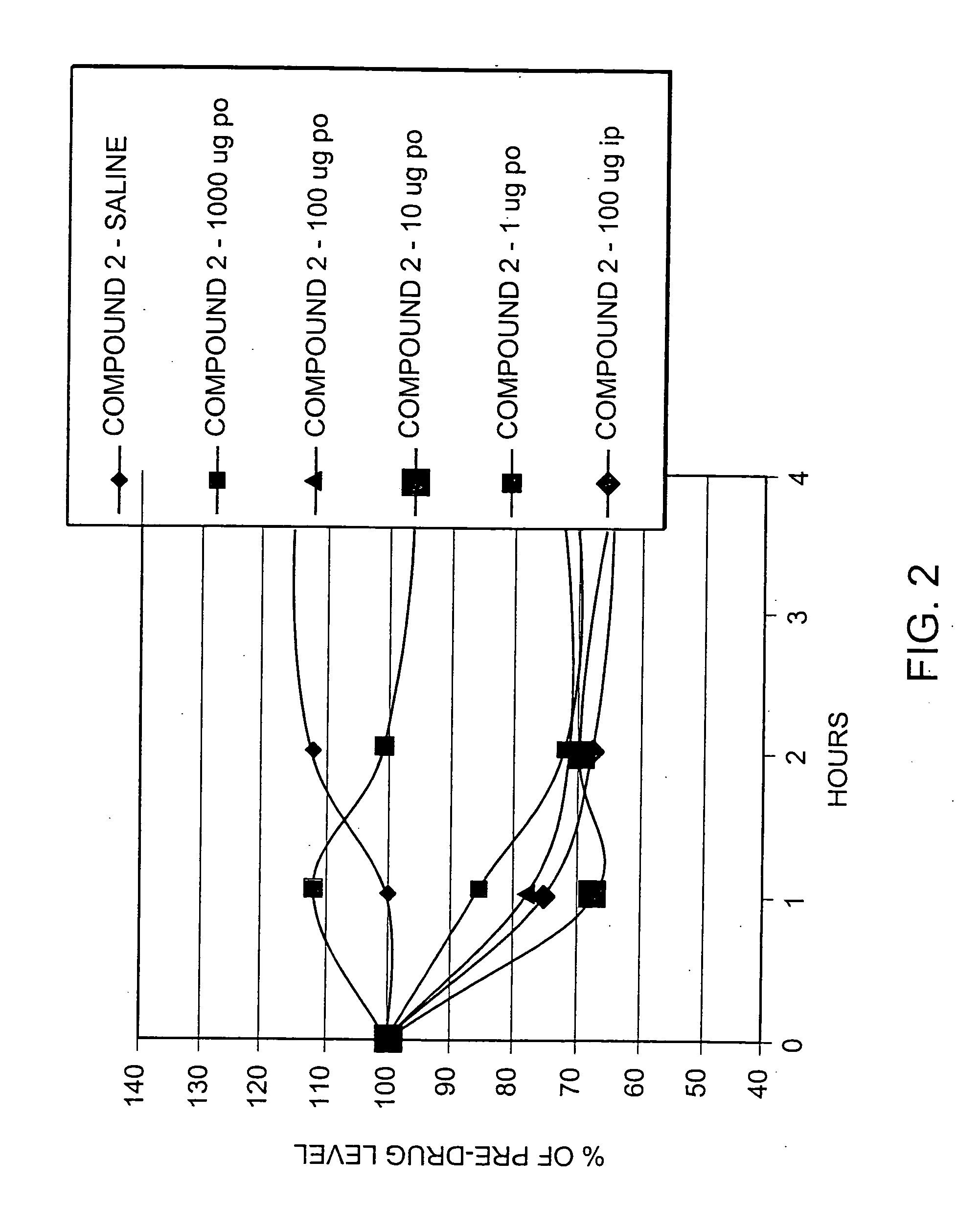
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
238 results about "Gastric emptying" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
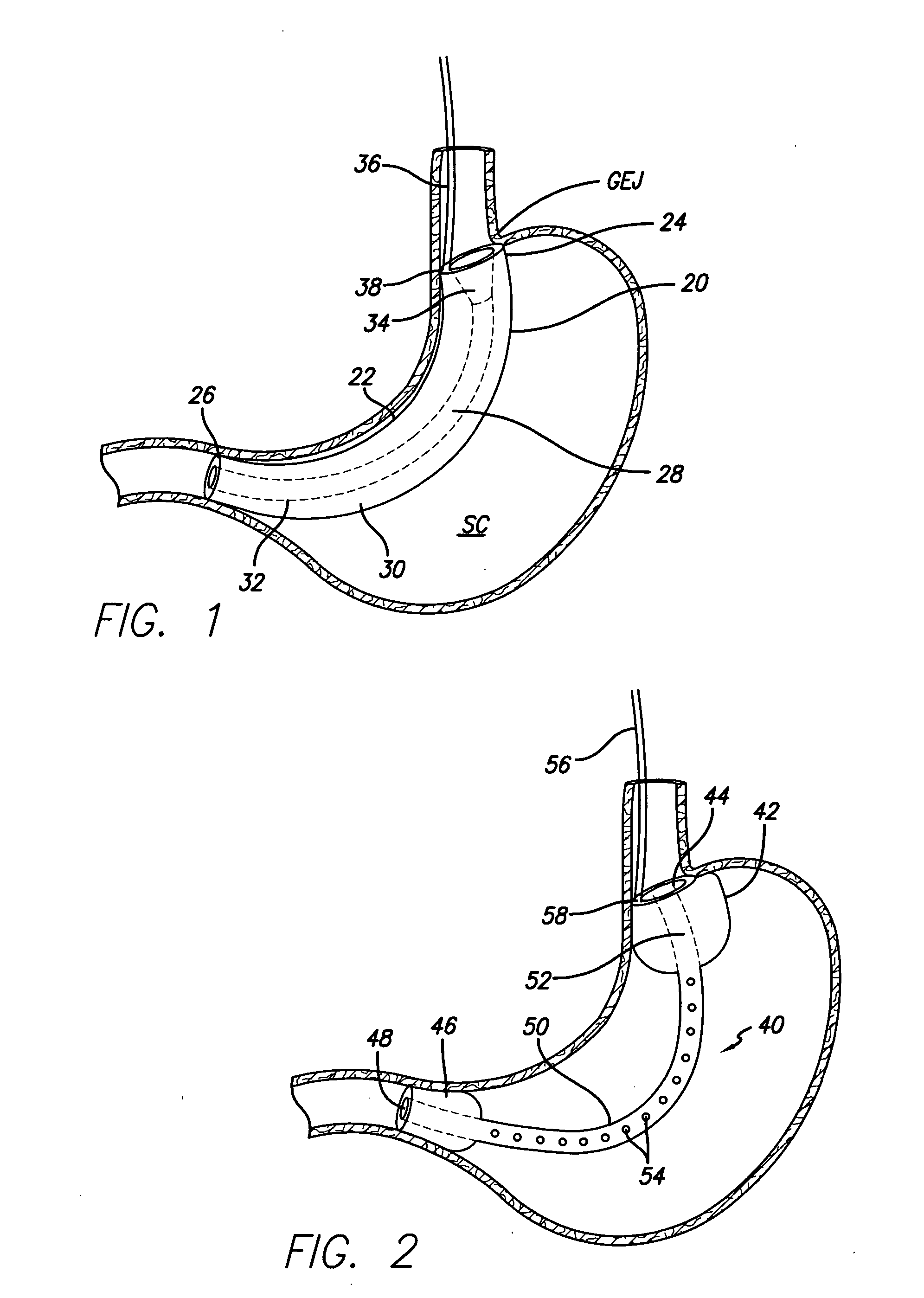
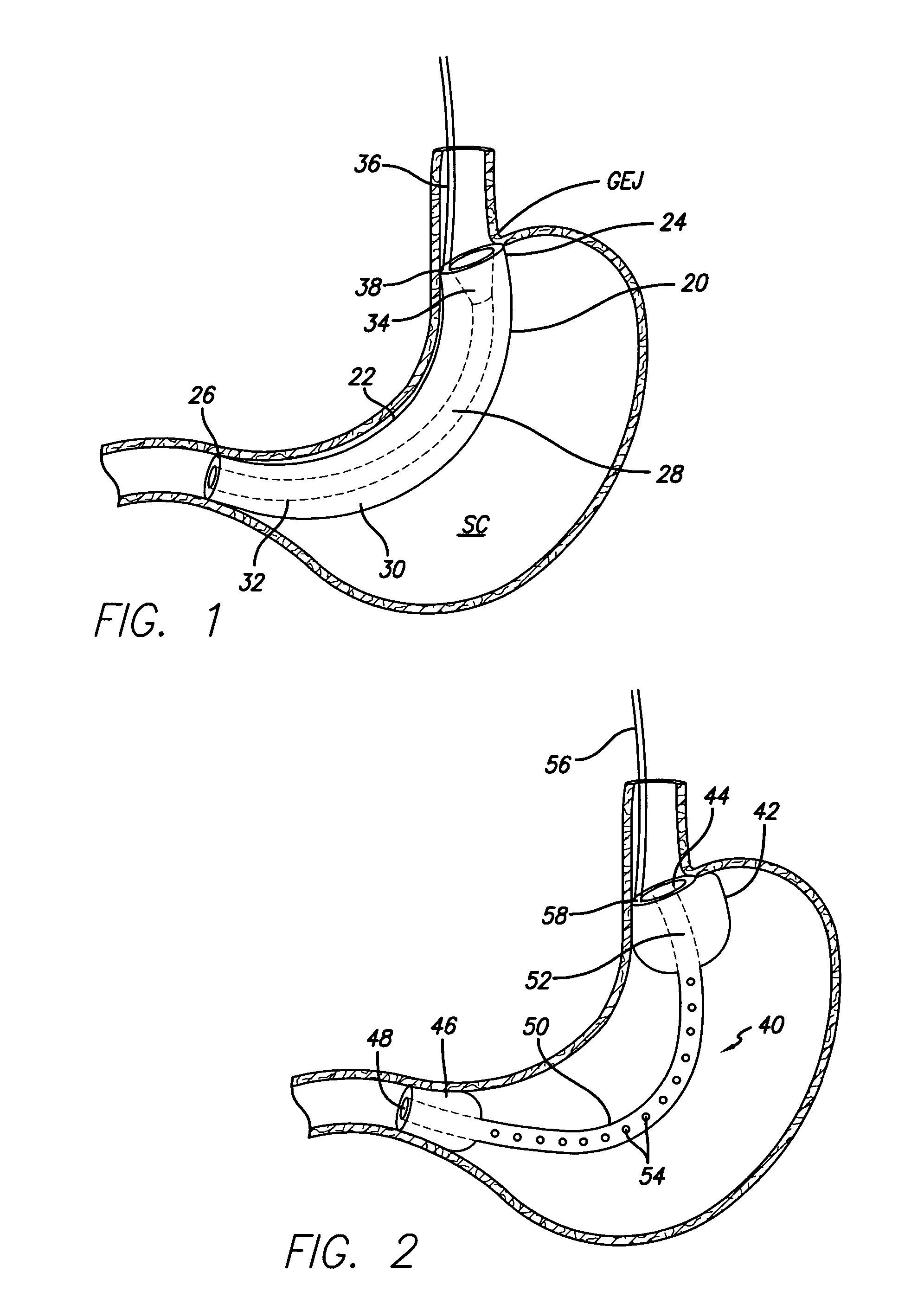
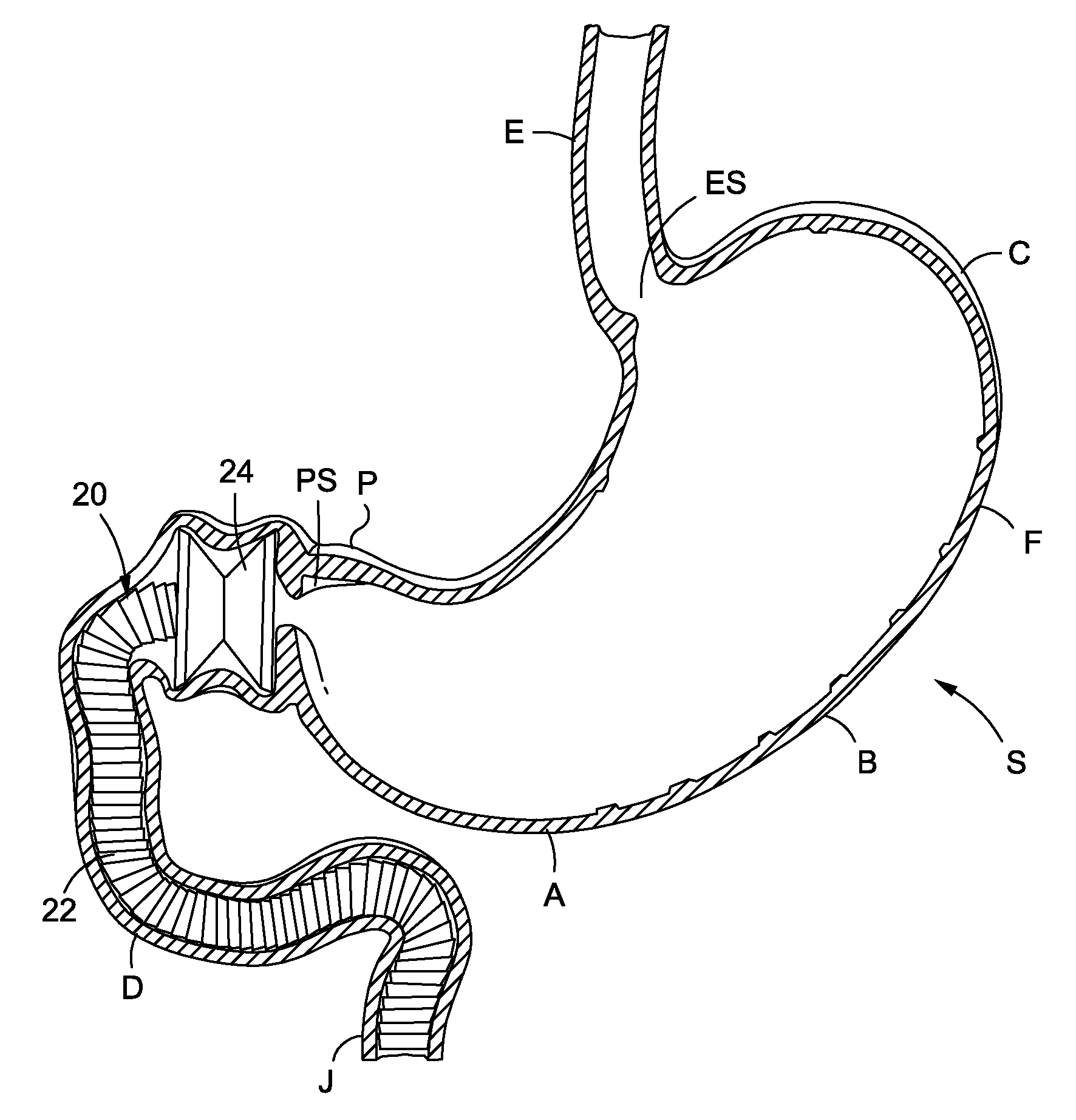
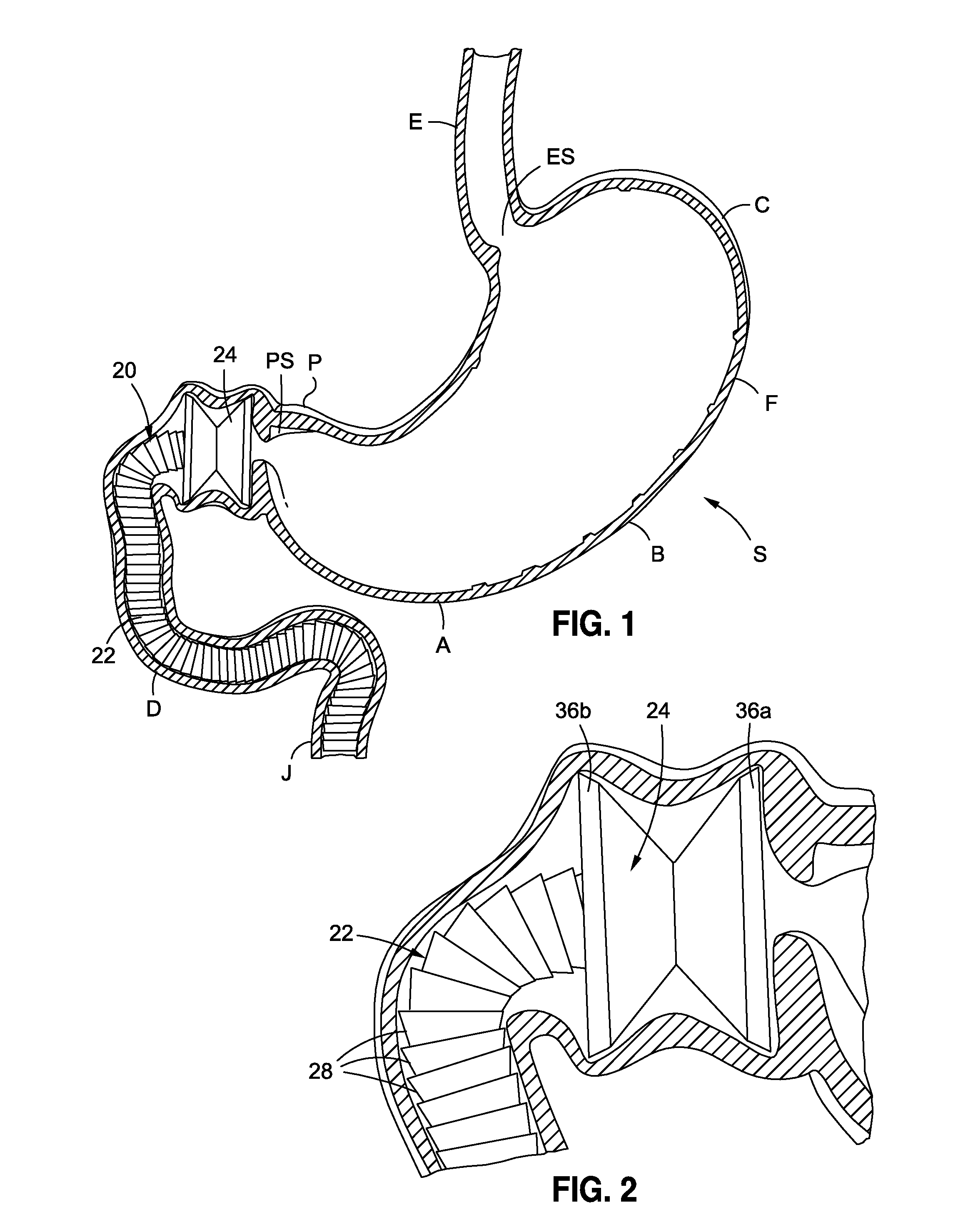
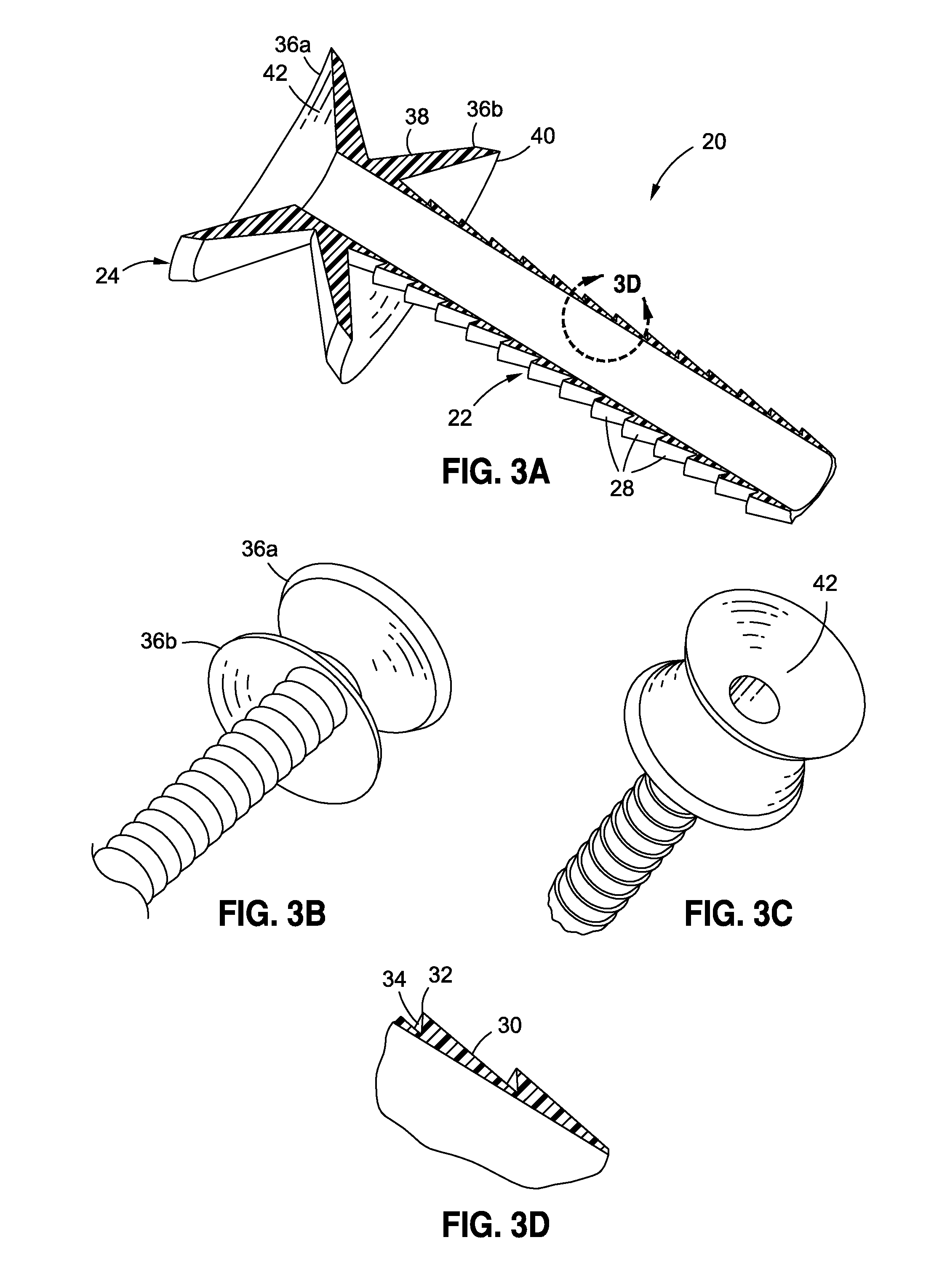
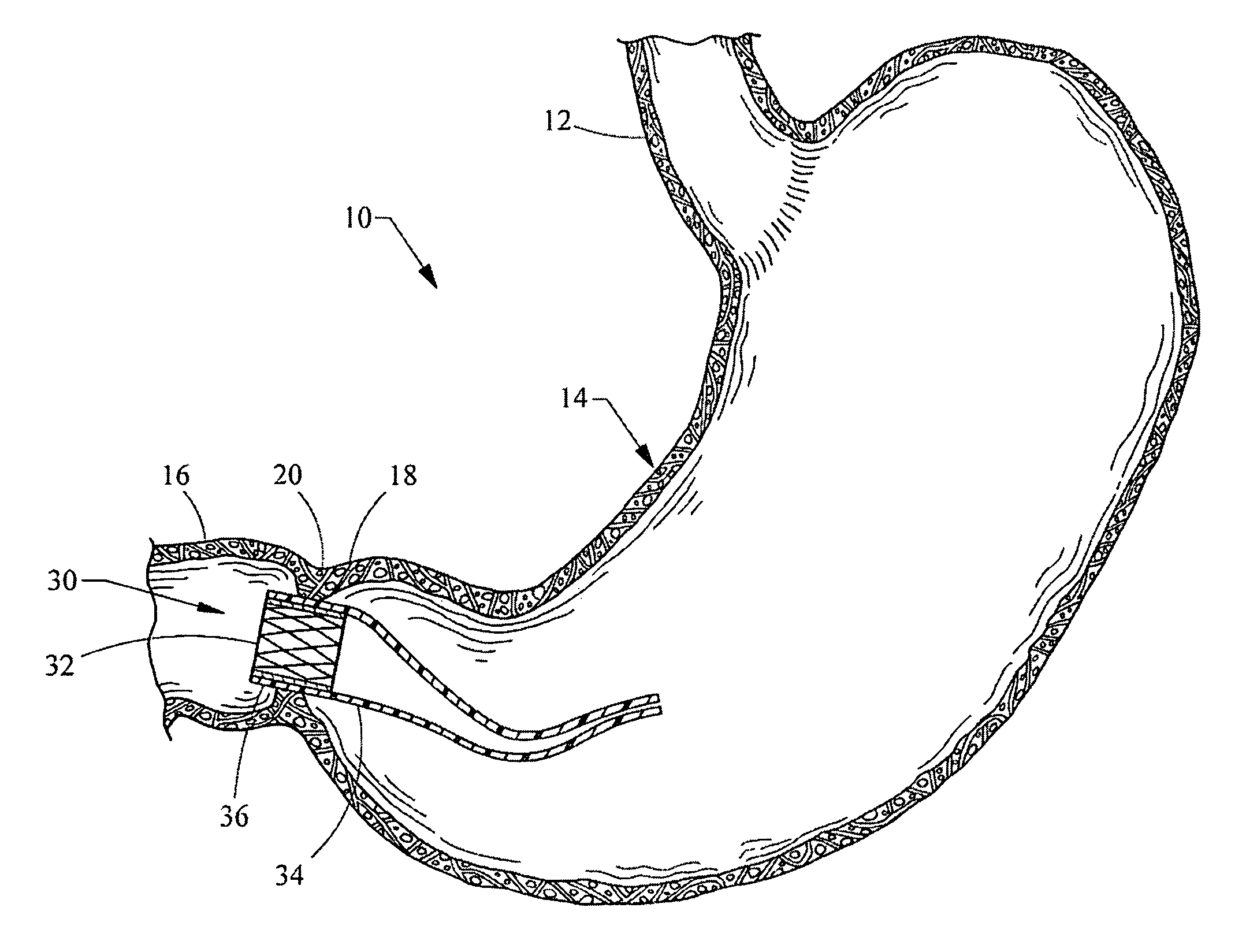
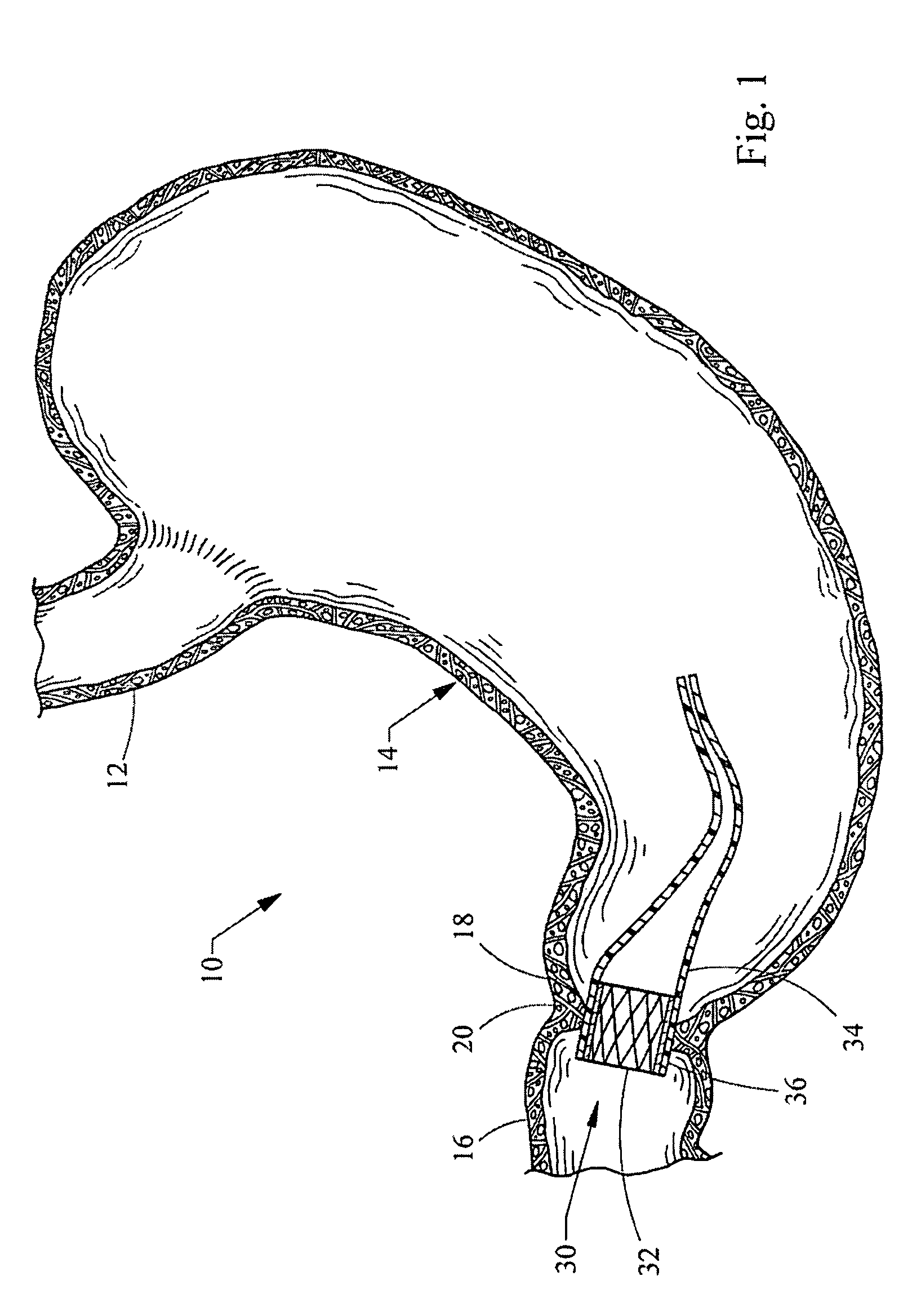
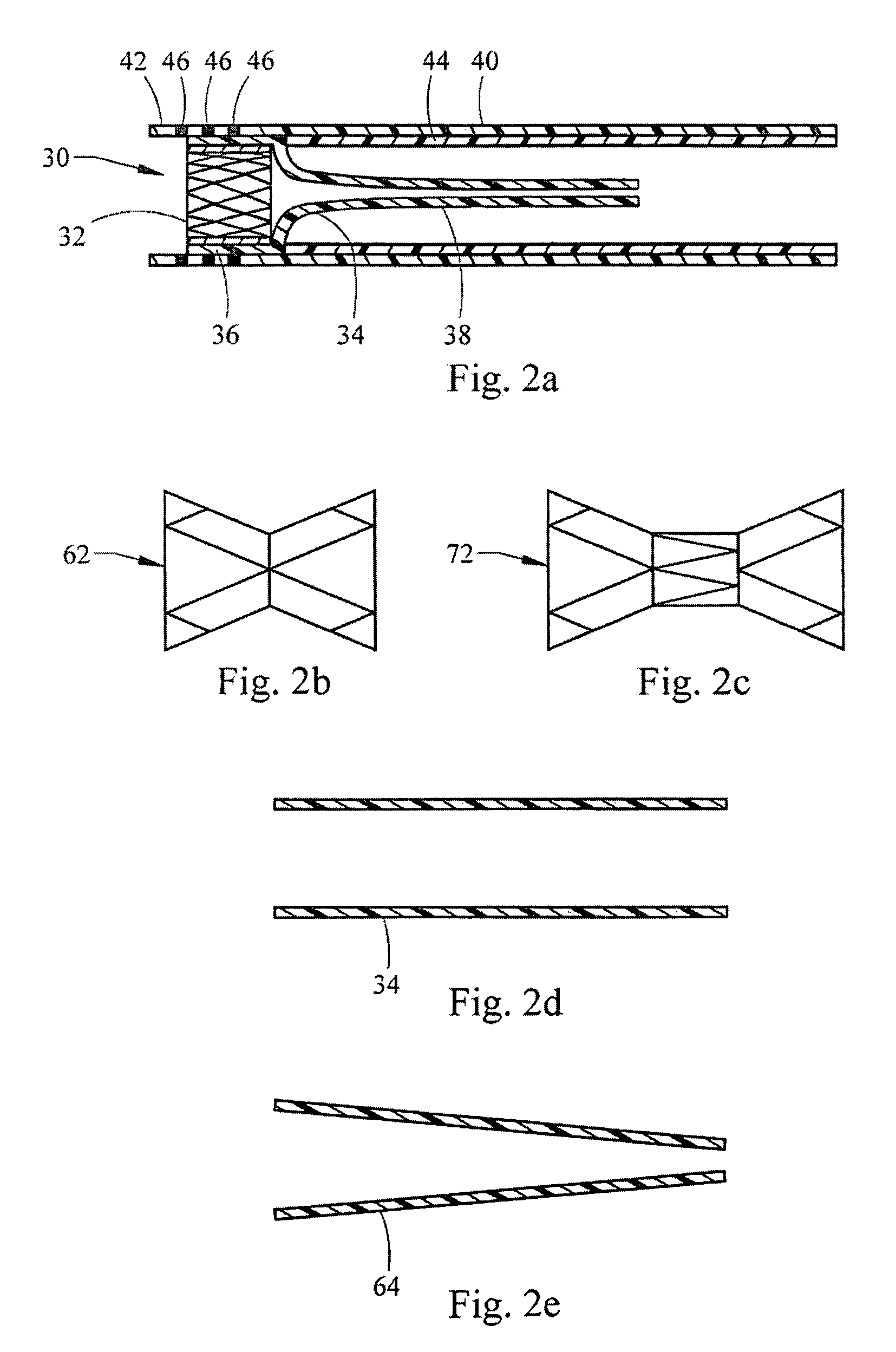
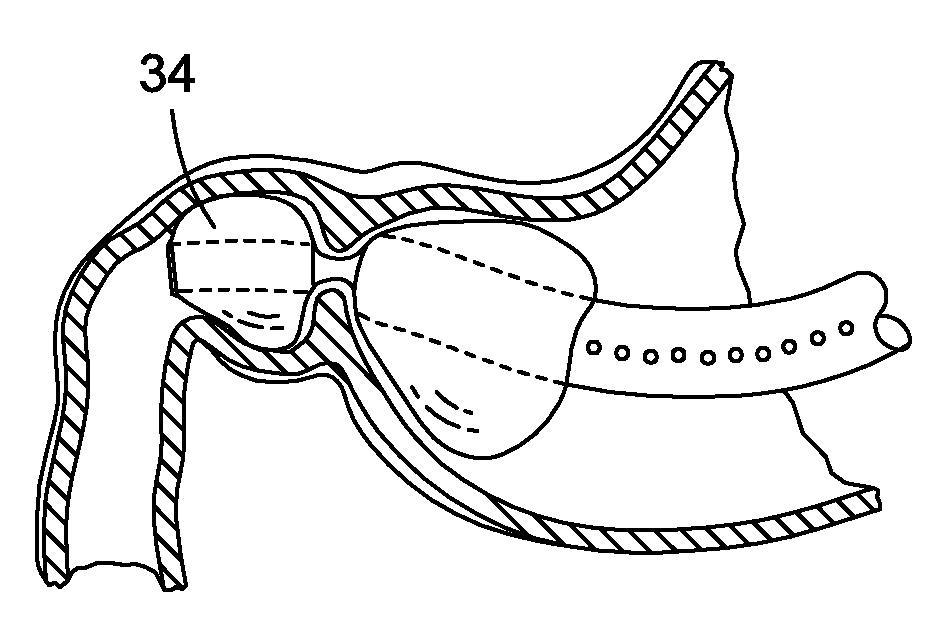
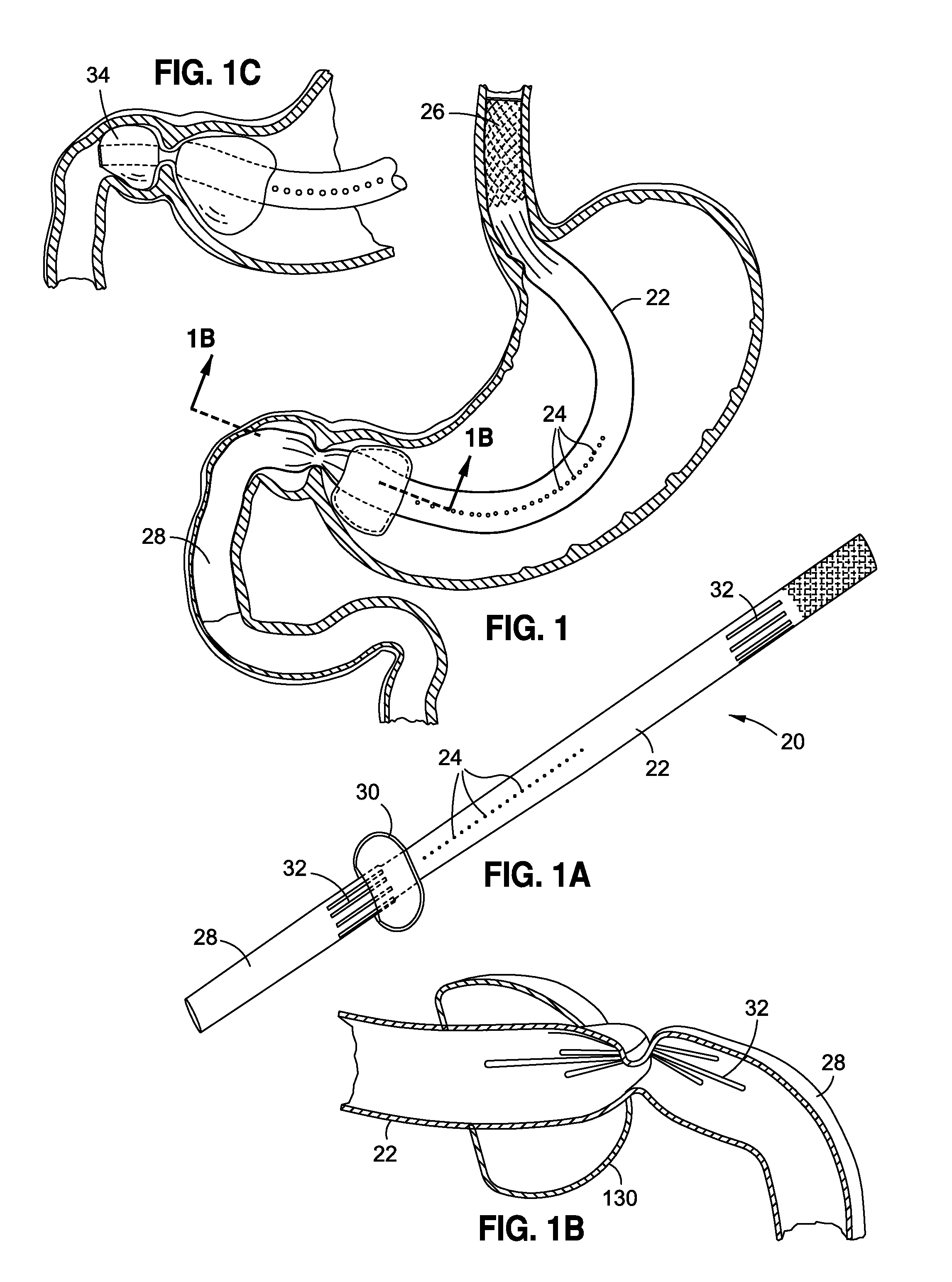
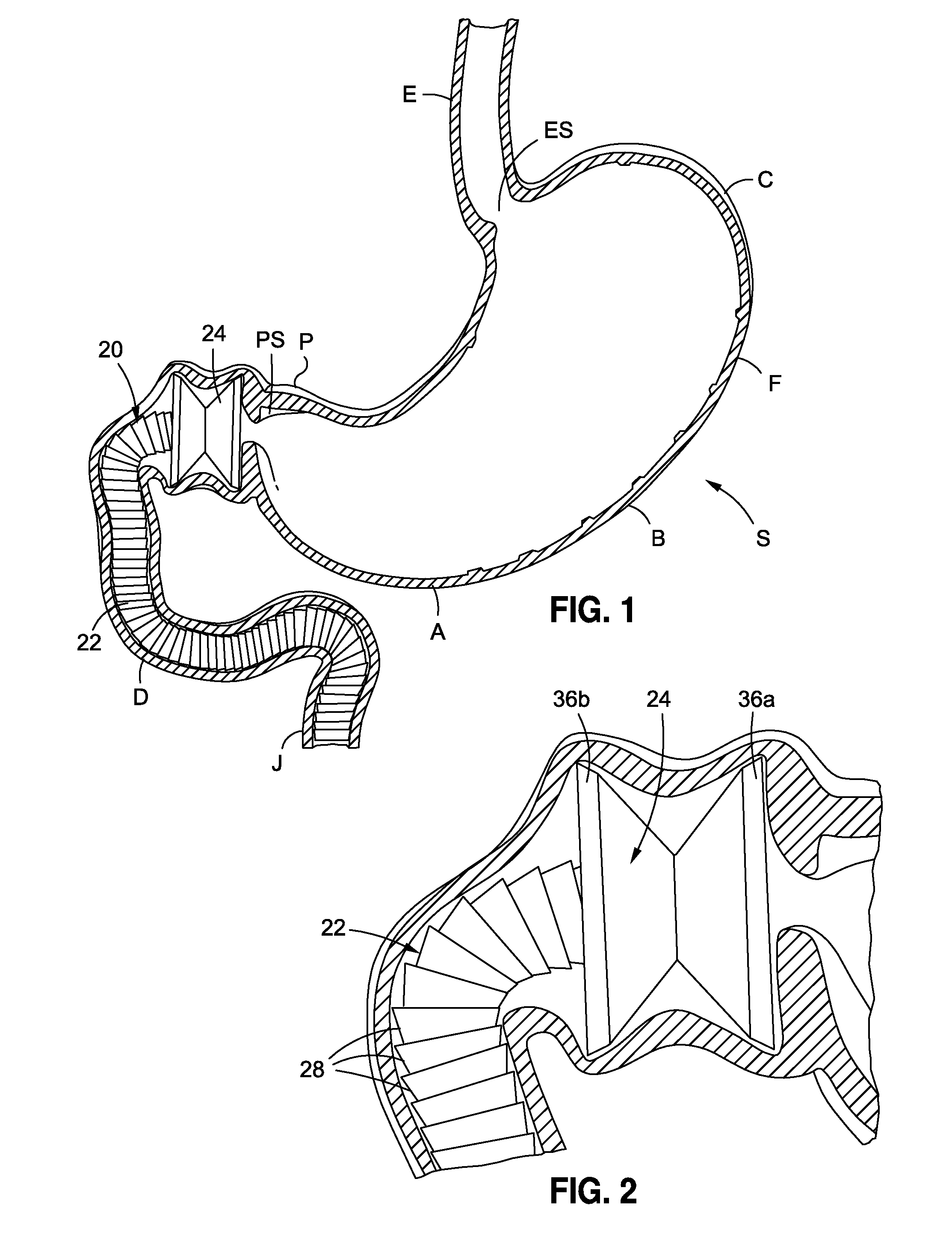
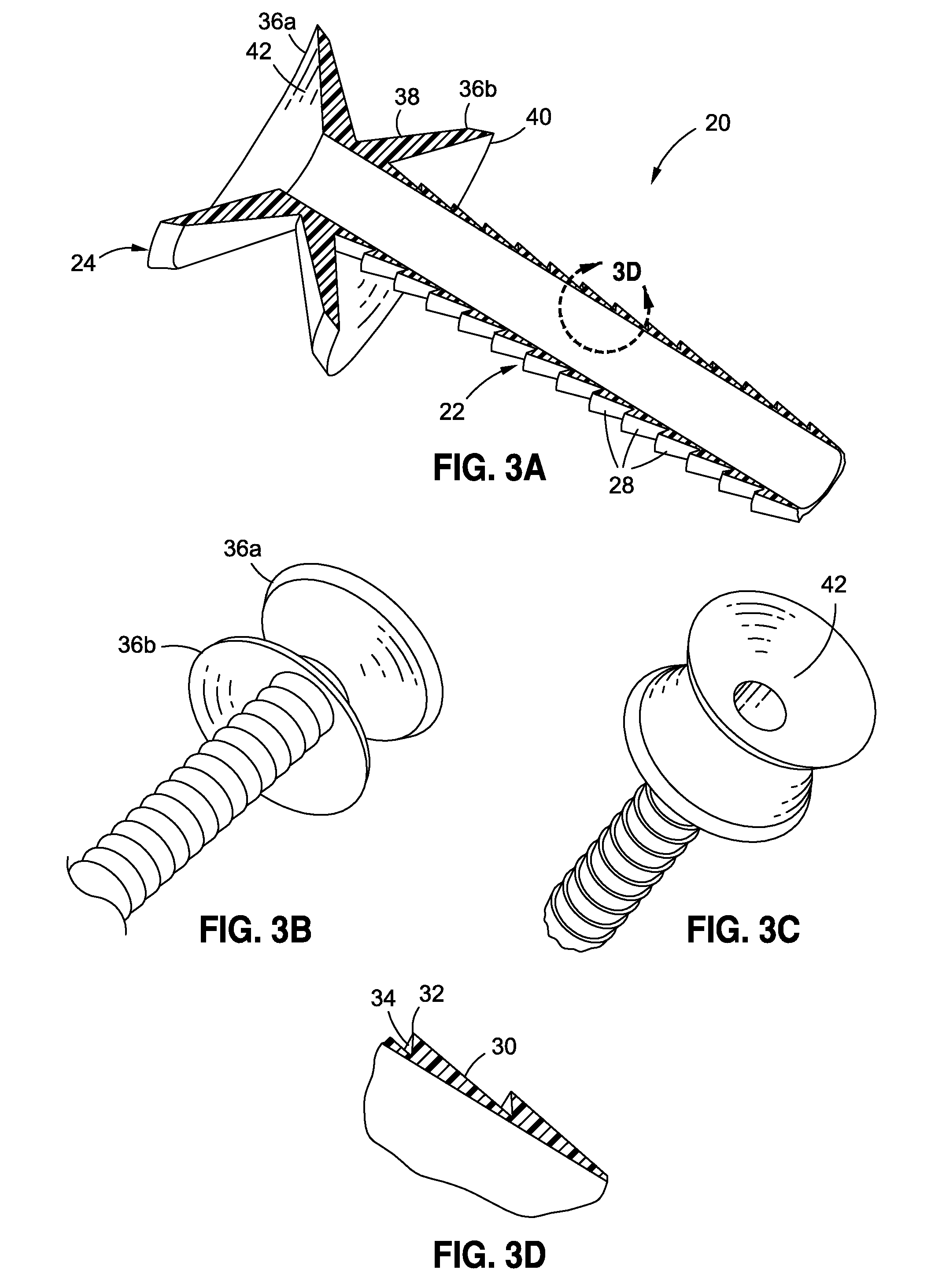
Endoscopic gastric restriction methods and devices
Endoscopic devices methods and devices are provided for attaching opposed walls of tissue. The methods and devices can be used to deliver a plurality of fasteners to opposed tissues, and suture extending through the fasteners can be used to pull the tissues together. The suture can be pre-woven through the fasteners, thus allowing for a quick and simply deployment. While the various methods and devices disclosed herein can be used in a variety of procedures, in certain exemplary embodiments the methods and devices are used to pull together opposed walls of the stomach to form a stomach pouch within the stomach which reduces the rate of gastric emptying.
Owner:ETHICON ENDO SURGERY INC
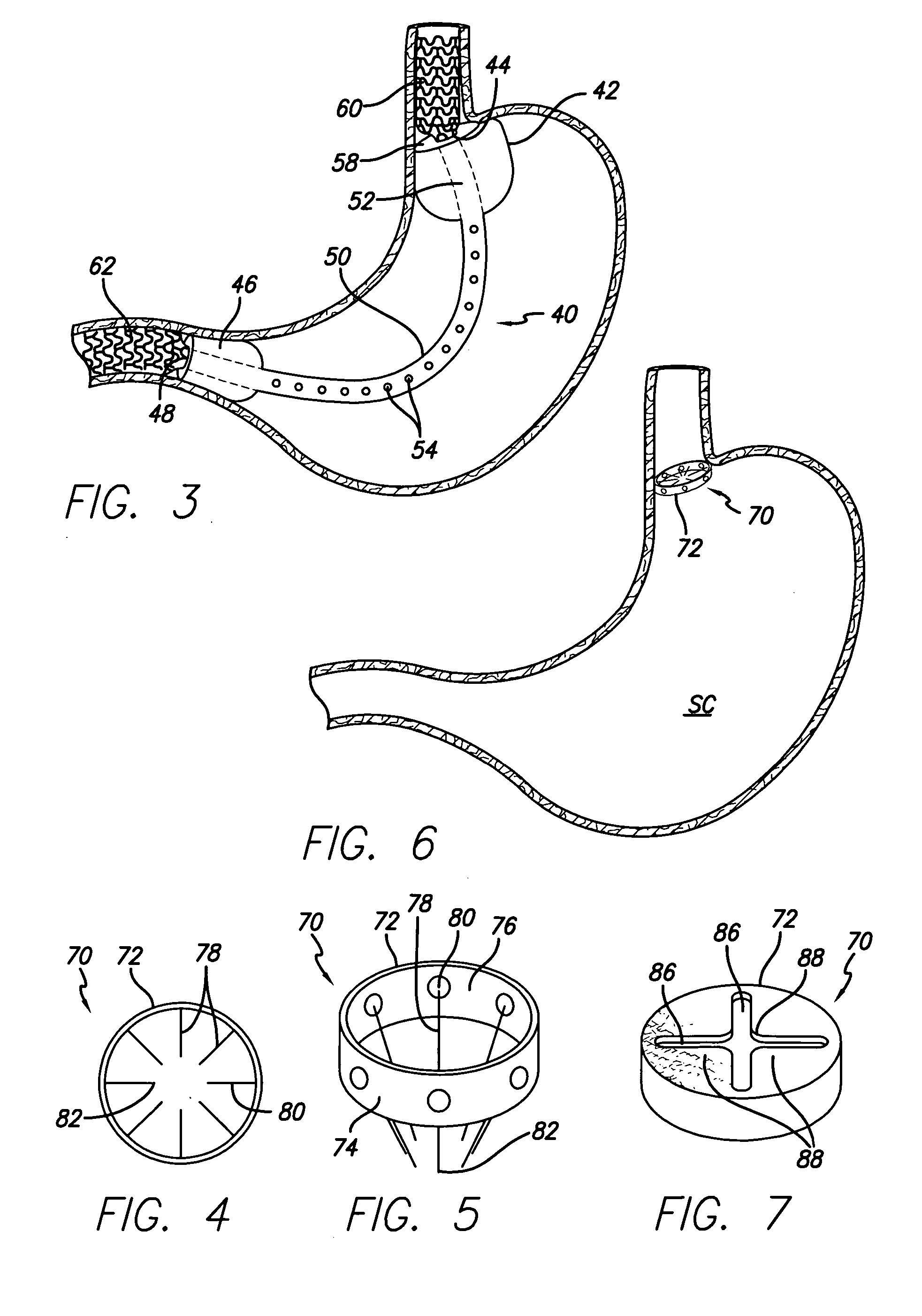
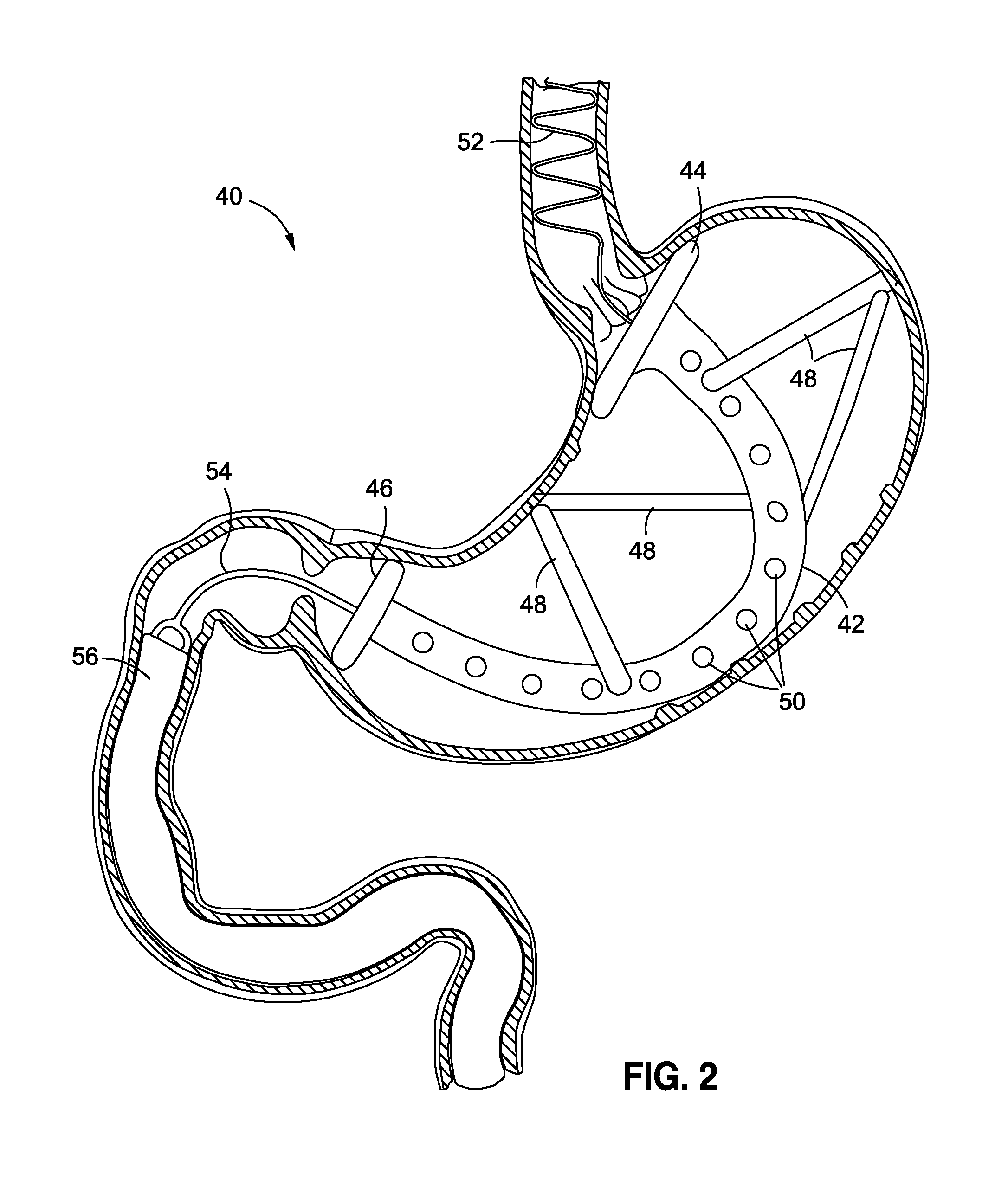
Systems and methods for treating obesity
ActiveUS20050228504A1Good for weight lossStimulatesIntravenous devicesTubular organ implantsPylorusGastric emptying
Methods and devices for simulating a gastric bypass and reducing the volume of the stomach involve placing a tubular liner along the lesser curve of the stomach cavity. Also, methods and devices for slowing gastric emptying involve placing valves within the stomach cavity near the gastrointestinal junction and / or the pylorus. These methods and devices may prevent a patient from drinking and eating large volumes at one time and from eating slowly all day.
Owner:ETHICON ENDO SURGERY INC
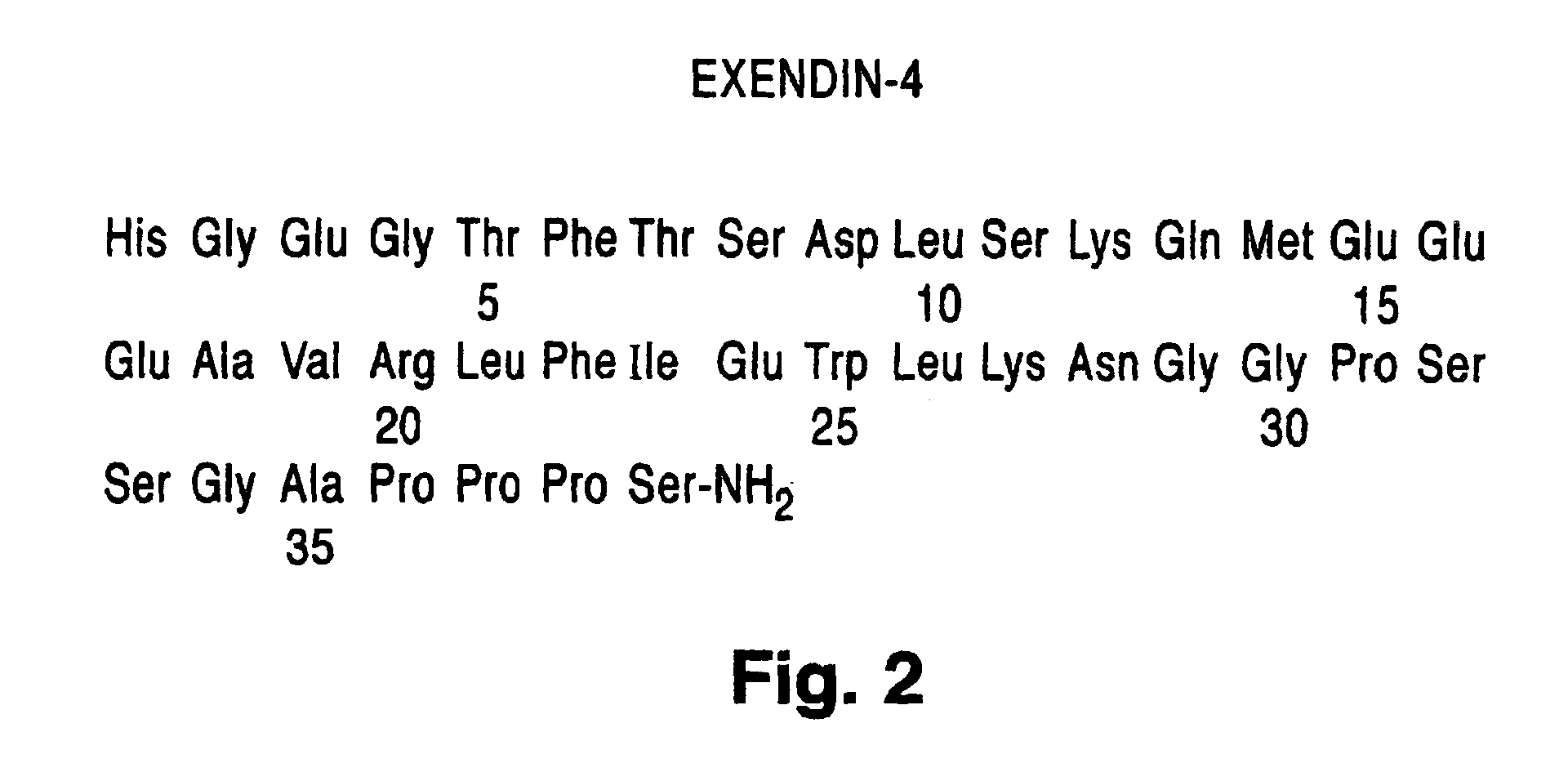
Modified exendins and exendin agonists
InactiveUS6924264B1Increase ratingsDecrease amount of potassiumMetabolism disorderSaccharide peptide ingredientsGastric emptyingPolyethylene glycol
Novel modified exendins and exendin agonist analogs having an exendin or exendin agonist analog linked to one or more polyethylene glycol polymers, for example, and related formulations and dosages and methods of administration thereof are provided. These modified exendins and exendin agonist analogs, compositions and methods are useful in treating diabetes and conditions that would be benefited by lowering plasma glucose or delaying and / or slowing gastric emptying or inhibiting food intake.
Owner:ASTRAZENECA PHARMA LP
Resistive anti-obesity devices
ActiveUS20060161139A1Improve overall senseReduce food consumptionSurgeryOesophagiIntestinal structureGastric emptying
A patient is provided with an increased sense of satiety by increasing resistance to the outflow of food from the stomach and through the intestines. Stomach emptying may be slowed with devices implantable within the gastrointestinal tract below the stomach. Implants are preferably removable and can include artificial strictures that may be adjustable to vary the rate of stomach emptying. Slowing gastric emptying may induce satiety for a longer period and may therefore reduce food consumption. Many of the embodiments include intestinal liners or sleeves, but they need not. The resistor concept may be applied to a simple anchor and resistor without a long liner.
Owner:GI DYNAMICS
Exendin agonist formulations and methods of administration thereof
InactiveUS6902744B1Slow gastric emptyingLowering plasma glucose levelPowder deliveryPeptide/protein ingredientsGastric emptyingPlasma glucose
Novel exendin and exendin agonist compound formulations and dosages and methods of administration thereof are provided. These compositions and methods are useful in treating diabetes and conditions that would be benefited by lowering plasma glucose or delaying and / or slowing gastric emptying or inhibiting food intake.
Owner:ASTRAZENECA PHARMA LP
Gip analog and hybrid polypeptides with selectable properties
ActiveUS20080312157A1Increased insulin secretionDecreasing bone loss bonePeptide/protein ingredientsMetabolism disorderDyslipidemiaFeeding disability
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
Stimulation of Satiety Hormone Release
The present invention provides, among other things, a site specific way to enhance a natural hormonal response to nutrients entering the small intestine after gastric emptying, thereby providing therapeutic value for obesity or diabetic patients. In one aspect, the present invention provides methods of stimulating the release of satiety hormone in a subject comprising applying a first electrical stimulus to a tissue in the lumen of the gastrointestinal system of the subject contemporaneously with the contacting of L-cells of the tissue with a nutrient stimulus. In another aspect, the present invention provides methods for predicting patient response to a weight loss surgery comprising applying a first electrical stimulus to a tissue of the gastrointestinal system of said patient contemporaneously with the contacting of L-cells of the tissue with a nutrient stimulus, assessing the effect of the electrical stimulus in said patient, and, correlating said effect to said patient's response to a weight loss surgery.
Owner:CENTOCOR ORTHO BIOTECH
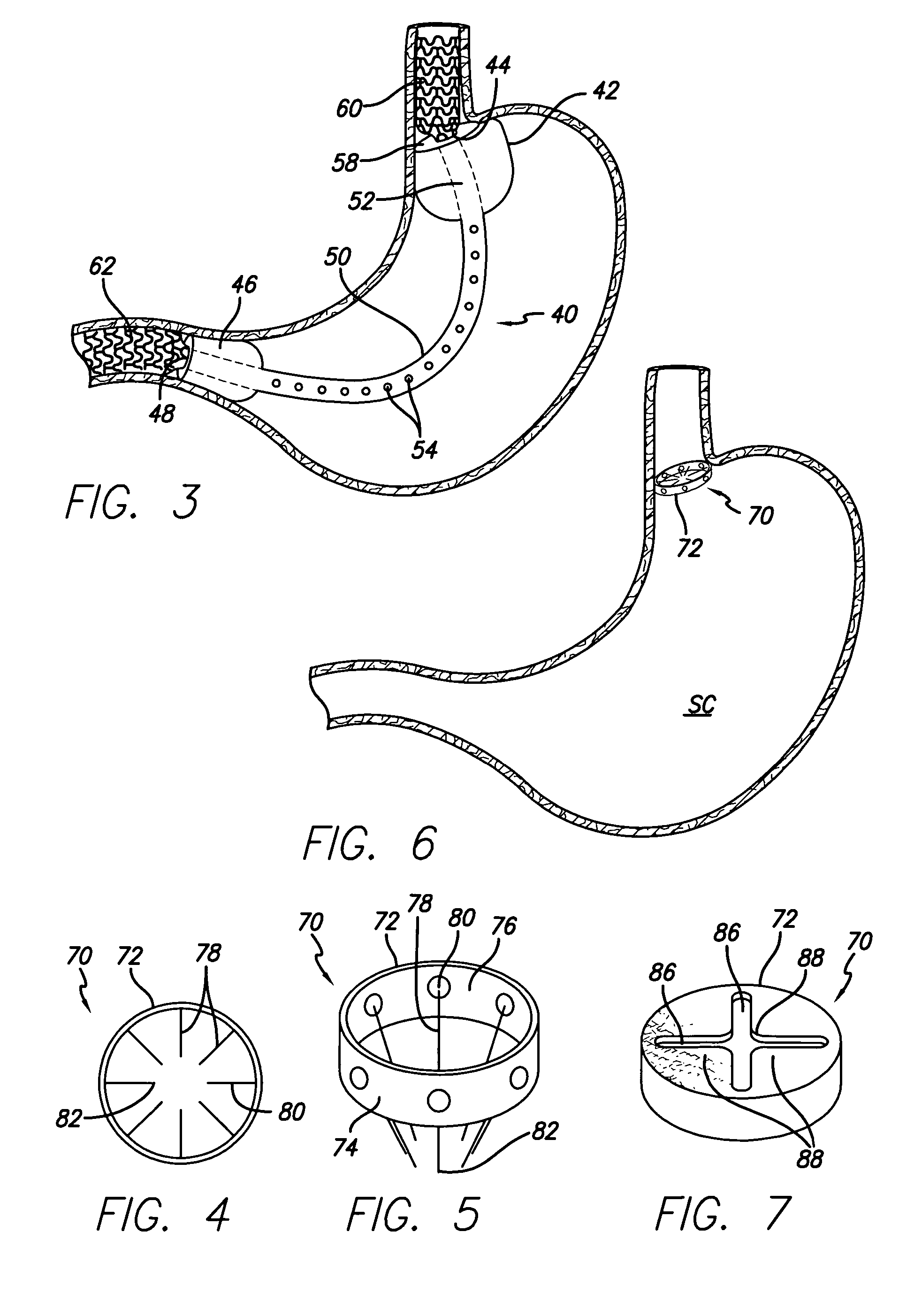
Systems and methods for treating obesity
ActiveUS7753870B2Reduce food intakeGood for weight lossIntravenous devicesTubular organ implantsPylorusGastric emptying
Methods and devices for simulating a gastric bypass and reducing the volume of the stomach involve placing a tubular liner along the lesser curve of the stomach cavity. Also, methods and devices for slowing gastric emptying involve placing valves within the stomach cavity near the gastro-intestinal junction and / or the pylorus. These methods and devices may prevent a patient from drinking and eating large volumes at one time and from eating slowly all day.
Owner:ETHICON ENDO SURGERY INC
Resistive anti-obesity devices
ActiveUS7819836B2Control outflowSurgical instrument detailsIntravenous devicesIntestinal structureGastric emptying
A patient is provided with an increased sense of satiety by increasing resistance to the outflow of food from the stomach and through the intestines. Stomach emptying may be slowed with devices implantable within the gastrointestinal tract below the stomach. Implants are preferably removable and can include artificial strictures or apertures that may be adjustable or elastic to vary the rate of stomach emptying. Slowing gastric emptying may induce satiety for a longer period and may therefore reduce food consumption. Many of the embodiments include intestinal sleeves or sleeves, but they need not. The resistor concept may be applied to a simple anchor and resistor without a long sleeve.
Owner:GI DYNAMICS
Resistive anti-obesity devices
ActiveUS7771382B2Improve overall senseImprove the immunityOesophagiIntravenous devicesIntestinal structureGastric emptying
A patient is provided with an increased sense of satiety by increasing resistance to the outflow of food from the stomach and through the intestines. Stomach emptying may be slowed with devices implantable within the gastrointestinal tract below the stomach. Implants are preferably removable and can include artificial strictures that may be adjustable to vary the rate of stomach emptying. Slowing gastric emptying may induce satiety for a longer period and may therefore reduce food consumption. Many of the embodiments include intestinal liners or sleeves, but they need not. The resistor concept may be applied to a simple anchor and resistor without a long liner.
Owner:GI DYNAMICS
Resistive anti-obesity devices
ActiveUS20080071383A1Improve overall senseReduce food consumptionSurgical instrument detailsIntravenous devicesIntestinal structureGastric emptying
A patient is provided with an increased sense of satiety by increasing resistance to the outflow of food from the stomach and through the intestines. Stomach emptying may be slowed with devices implantable within the gastrointestinal tract below the stomach. Implants are preferably removable and can include artificial strictures or apertures that may be adjustable or elastic to vary the rate of stomach emptying. Slowing gastric emptying may induce satiety for a longer period and may therefore reduce food consumption. Many of the embodiments include intestinal sleeves or sleeves, but they need not. The resistor concept may be applied to a simple anchor and resistor without a long sleeve.
Owner:GI DYNAMICS
Gip analog and hybrid polypeptides with selectable properties
InactiveUS20090036364A1Increased insulin secretionDecreasing bone loss boneSenses disorderNervous disorderDyslipidemiaPhysiology
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
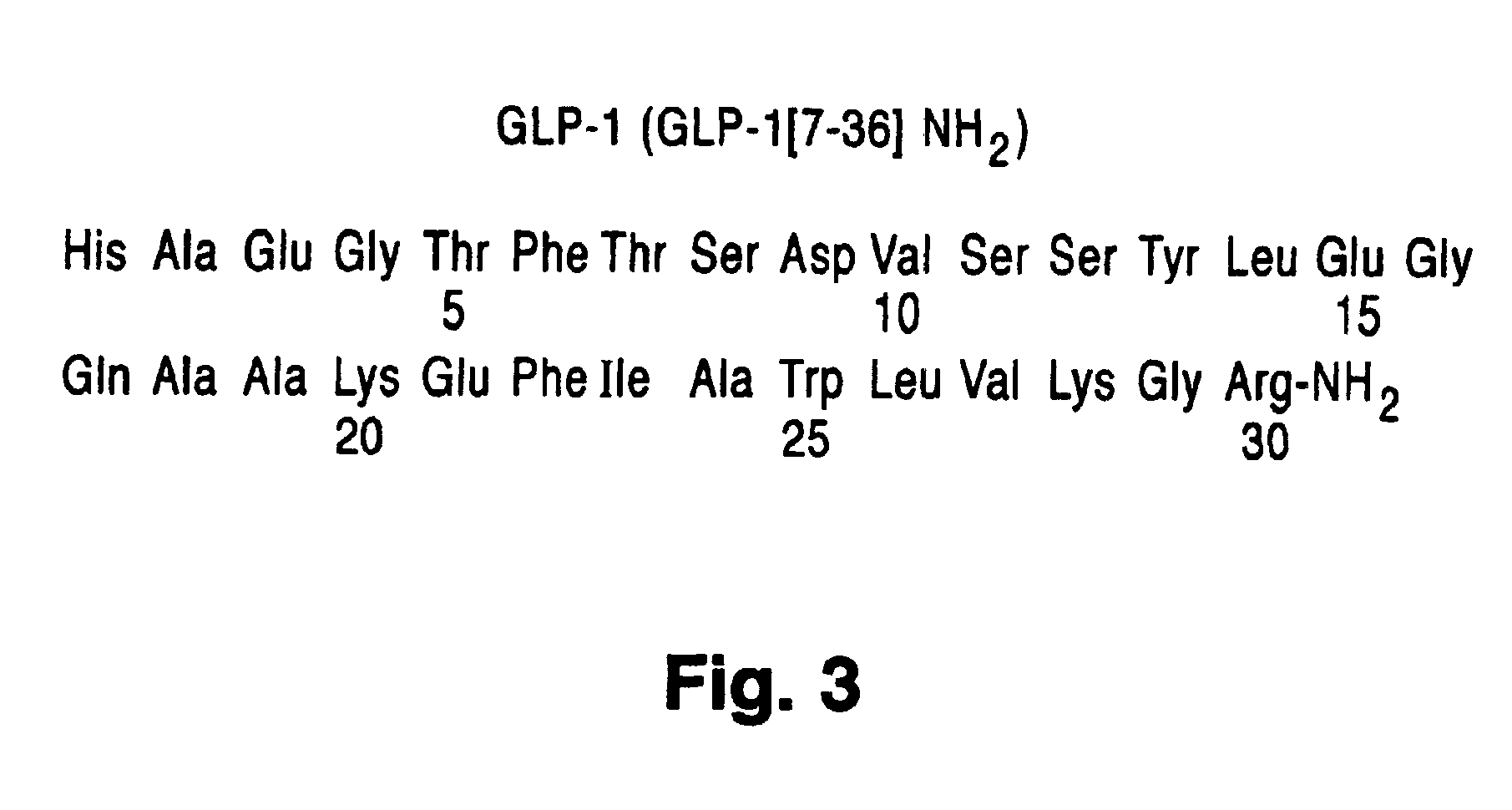
Novel peptide agonists of GLP-1 activity
InactiveUS20040106547A1Lower blood sugar levelsEffective and stablePeptide/protein ingredientsVasoactive intestinal peptideDiseaseGastric emptying
Owner:ZEALAND PHARM AS
Dietary supplement for supressing appetite, enhancing and extending satiety, improving glycemic control, and stimulant free
This invention relates to a nutritional intervention composition for enhancing satiety prior to a meal and extending satiety after a meal. The nutritional intervention composition decreases food intake producing weight loss over time. The composition consists of Niacin, Vitamin B6, Calcium, Phosphorous, Magnesium, Chromium, Chitosan, Fenugreek, Ginseng, White willow bark, Garcinia cambogia, Aloe Vera gel powder, Momordica charantia, Griffonia simplicifolia, Lagerstroemia speciosa and Vanadyl sulfate. The invention does not require stimulants or anabolic ingredients. There are three phases of activity within the composition. One, enhanced satiety through elevated serotonin. Two, improved carbohydrate metabolism, reduced blood glucose and slowed gastric emptying. Three, enhanced fiber binding of lipids and excess bile acids.
Owner:NEEDLEMAN ALVIN +1
Intragastric Device for Treating Obesity
The present invention is directed toward an intragastric device used to treat obesity that includes a wire mesh structure capable of changing from a compressed pre-deployment shape to an expanded post-deployment shape with a greatly increased volume. The post-deployment shape contains a light weight at the top and a heavier weight at the bottom to ensure proper positioning within the stomach. In the post-deployment shape, the device contains larger spaces in the upper portion and smaller spaces in the lower portion to sequester food and delay gastric emptying. Alternatively, the device can be enveloped by a membrane containing larger holes at the top and smaller holes at the bottom to sequester food and delay gastric emptying. The device has a dynamic weight where the weight of the device in the pre-feeding stage is less than the weight of the device in feeding or post-feeding stage.
Owner:SYNERZ MEDICAL
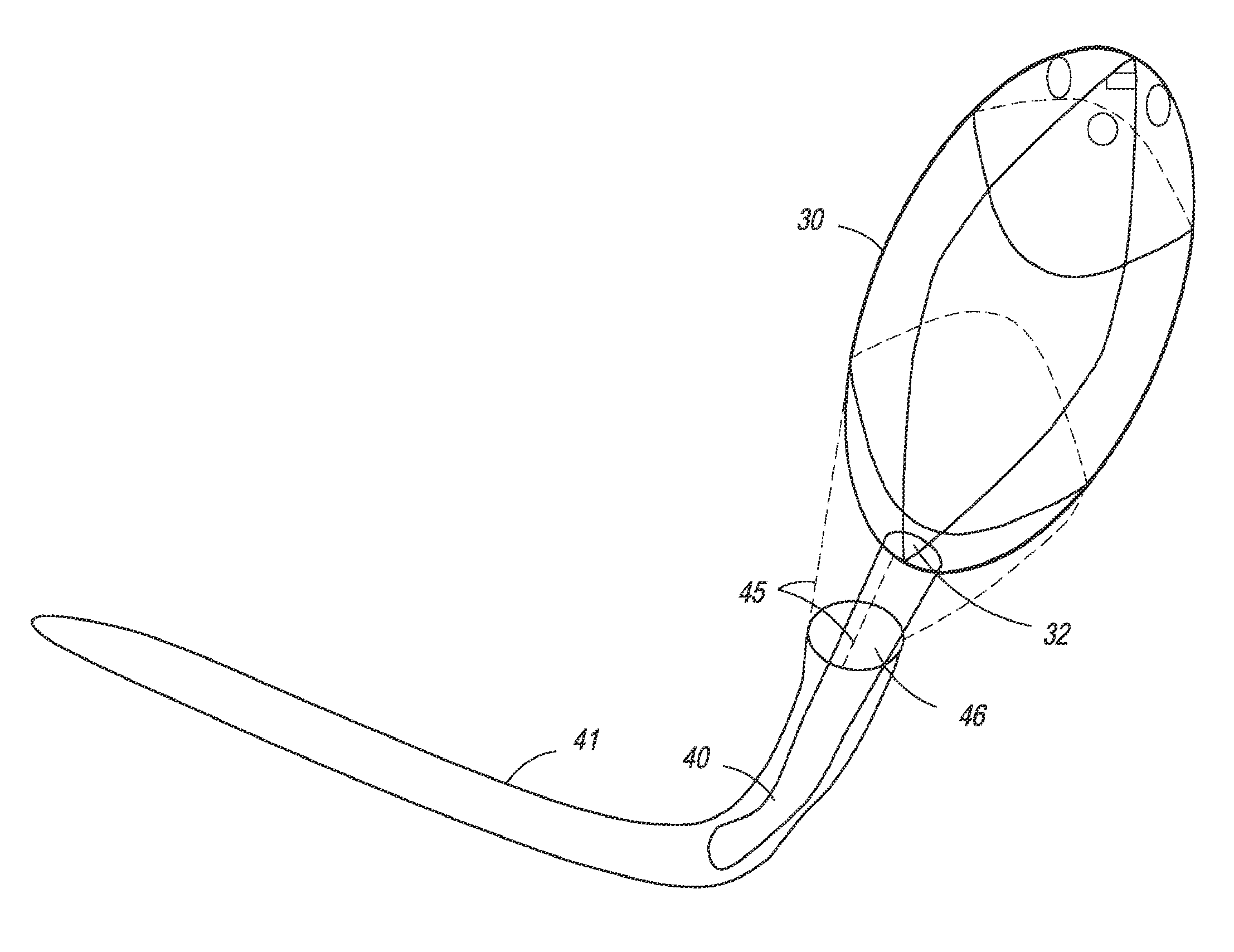
Anchored non-piercing duodenal sleeve and delivery systems
InactiveUS20120095483A1Easy to disassembleSimple structureObesity treatmentWound clampsGastric emptyingPyloric sphincter
An intragastric implant for obesity treatment is disclosed. The device delays digestion by providing a duodenal sleeve, and may also slows gastric emptying by limiting flow through the pyloric sphincter. The implant includes an elongated axially-compressible duodenal sleeve having a non-tissue-piercing anchor on a proximal end sized to lodge within the duodenal bulb. The anchor may have oppositely-directed anchoring flanges to resists migration in both directions. The sleeve may also have barbed ribs to resist proximal movement back up into the stomach. A method of implant includes collapsing / compressing the device and transorally advancing it through the esophagus to be deployed within the duodenum. A dissolvable jacket may constrain the implant for delivery and naturally dissolve upon implant. Removal of the implant may occur in the reverse.
Owner:APOLLO ENDOSURGERY INC
GIP analog and hybrid polypeptides with selectable properties
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
Modified exendin and exendin agonists
Novel modified exendins and exendin agonists having an exendin or exendin agonist linked to one or more polyethylene glycol polymers, for example, and related formulations and dosages and methods of administration thereof are provided. These modified exendins and exendin agonists, compositions and methods are useful in treating diabetes and conditions that would be benefited by lowering plasma glucose or delaying and / or slowing gastric emptying or inhibiting food intake.
Owner:AMYLIN PHARMA INC
Peptide agonists of GLP-1 activity
InactiveUS20070111940A1Improve glucose toleranceLower blood sugar levelsPeptide/protein ingredientsVasoactive intestinal peptideGastric emptyingAgonist
Novel peptide agonists of GLP-1 activity useful for lowering blood glucose levels. The novel peptides comprise variants of the GLP-1 or the exendin-4 polypeptide sequence and are pharmacologically active and stable. These peptides are useful in the treatment of diseases that benefit from regulation of excess levels of blood glucose and / or regulation of gastric emptying, such as diabetes and eating disorders.
Owner:LARSEN BJARNE DUE +2
Exendin-4 and analog fusion protein thereof
ActiveCN101891823APromote regenerationPromote repairPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseMotility
The invention discloses exendin-4, an analog fusion protein thereof, the corresponding polynucleotide sequence, carrier, host cell and pharmaceutical composite and a preparation method and applications of the fusion protein. The fusion protein is prepared by fusing exendin-4 and analog thereof with human immunoglobulin IgG2-Fc through special connecting peptide and has better stability and loner half-life in vivo. The fusion protein can be administered by performing local delivery, using aerosol and using injection. The fusion protein can promote the regeneration and repair of islet beta cells, increase islet beta cells, promote the secretion of insulin and improve the sensitivity of organism to insulin. The fusion protein is used to cure diabetes, adiposity and other diseases which can be benefited by reducing plasma glucose and inhibiting gastrointestinal motility and gastric emptying.
Owner:BEIJING DONGFANG BIOTECH
Novel exendin agonist formulations and methods administration thereof
Owner:AMYLIN PHARMACEUTICALS LLC
Apparatus and methods for delaying gastric emptying to treat obesity
ActiveUS8114045B2Small sizeConstricting pylorusIntravenous devicesTubular organ implantsPylorusGastric emptying
Medical devices and methods for the treatment of obesity. The medical devices generally include an attachment portion for attaching the medical devices on or adjacent the pylorus and a limitation portion for limiting the passage of stomach contents through the pylorus to delay emptying the stomach. The limitation portion may be responsive to pressure from stomach contents to substantially close or to open the passageway.
Owner:COOK MEDICAL TECH LLC
Novel exendin agonist formulations and methods of administration thereof
InactiveUS20060183677A1Slow gastric emptyingLower levelPowder deliveryPeptide/protein ingredientsGastric emptyingPlasma glucose
Novel exendin and exendin agonist compound formulations and dosages and methods of administration thereof are provided. These compositions and methods are useful in treating diabetes and conditions that would be benefited by lowering plasma glucose or delaying and / or slowing gastric emptying or inhibiting food intake.
Owner:AMYLIN PHARMA INC
Stomach-spanning gastric implants
A variety of passive intragastric implant devices for obesity treatment are disclosed. Such passive implants do not autonomously change shape, but instead react within the stomach to induce satiety. The implants may take up volume within the stomach, thus reducing the digestive capacity. Additionally, the implants may contact areas within the stomach, such as the cardia surrounding the esophageal sphincter, to stimulate satiety-inducing nerves. Also, a number of implants slow gastric emptying by blocking or otherwise impeding flow through the pyloric sphincter. Other implants delay digestion by providing a duodenal sleeve. A number of implants combine two or more of these satiety-inducing features. Methods of implant are disclosed including compressing the implants within a delivery tube and transorally advancing the implants through the esophagus to be deployed within the stomach. Removal of the implants occurs in the reverse.
Owner:ALLERGAN INC
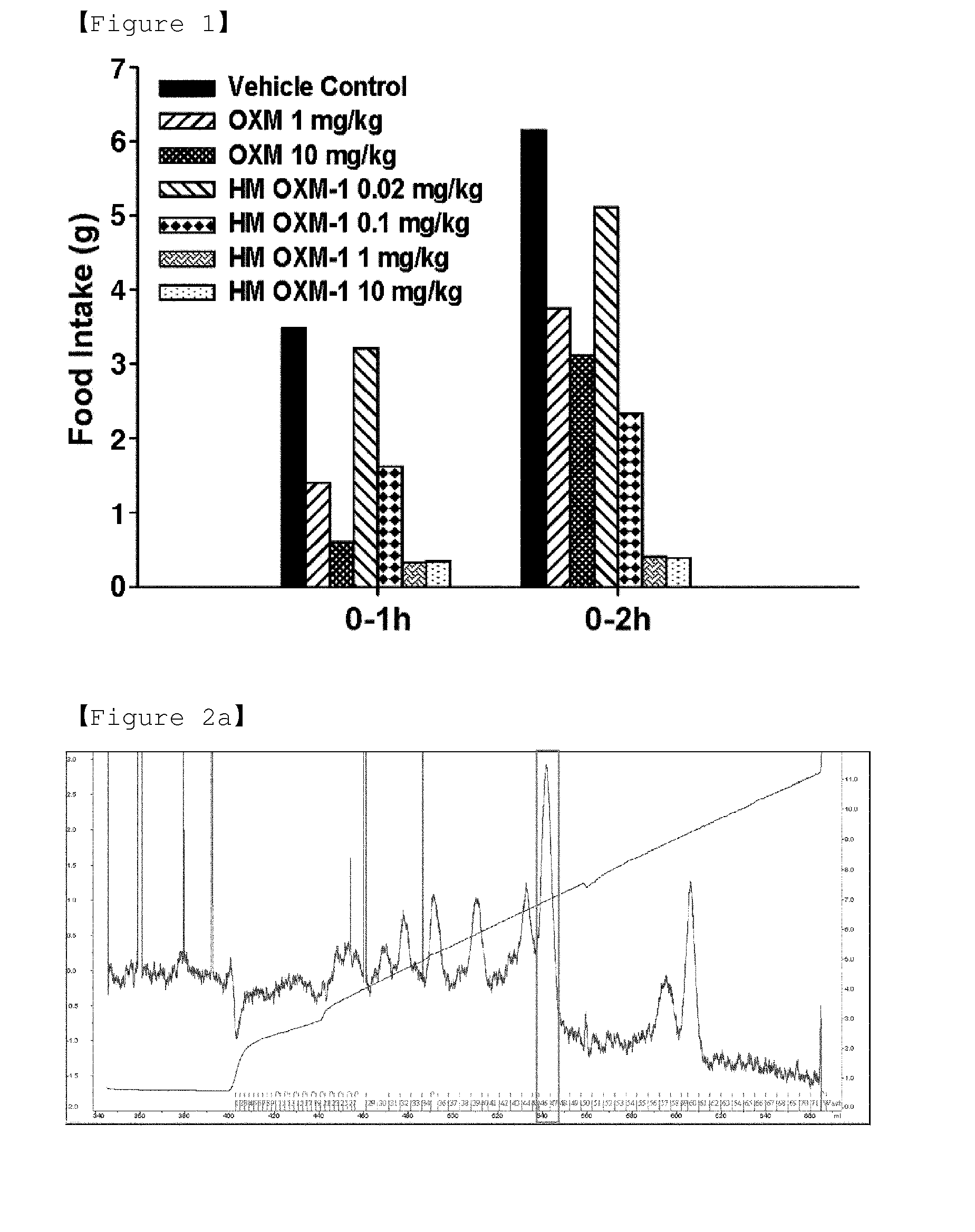
Conjugate comprising oxyntomodulin and an immunoglobulin fragment, and use thereof
ActiveUS20140212440A1Reduces food intakeSuppresses gastric emptyingPeptide/protein ingredientsMetabolism disorderSide effectReceptor activation
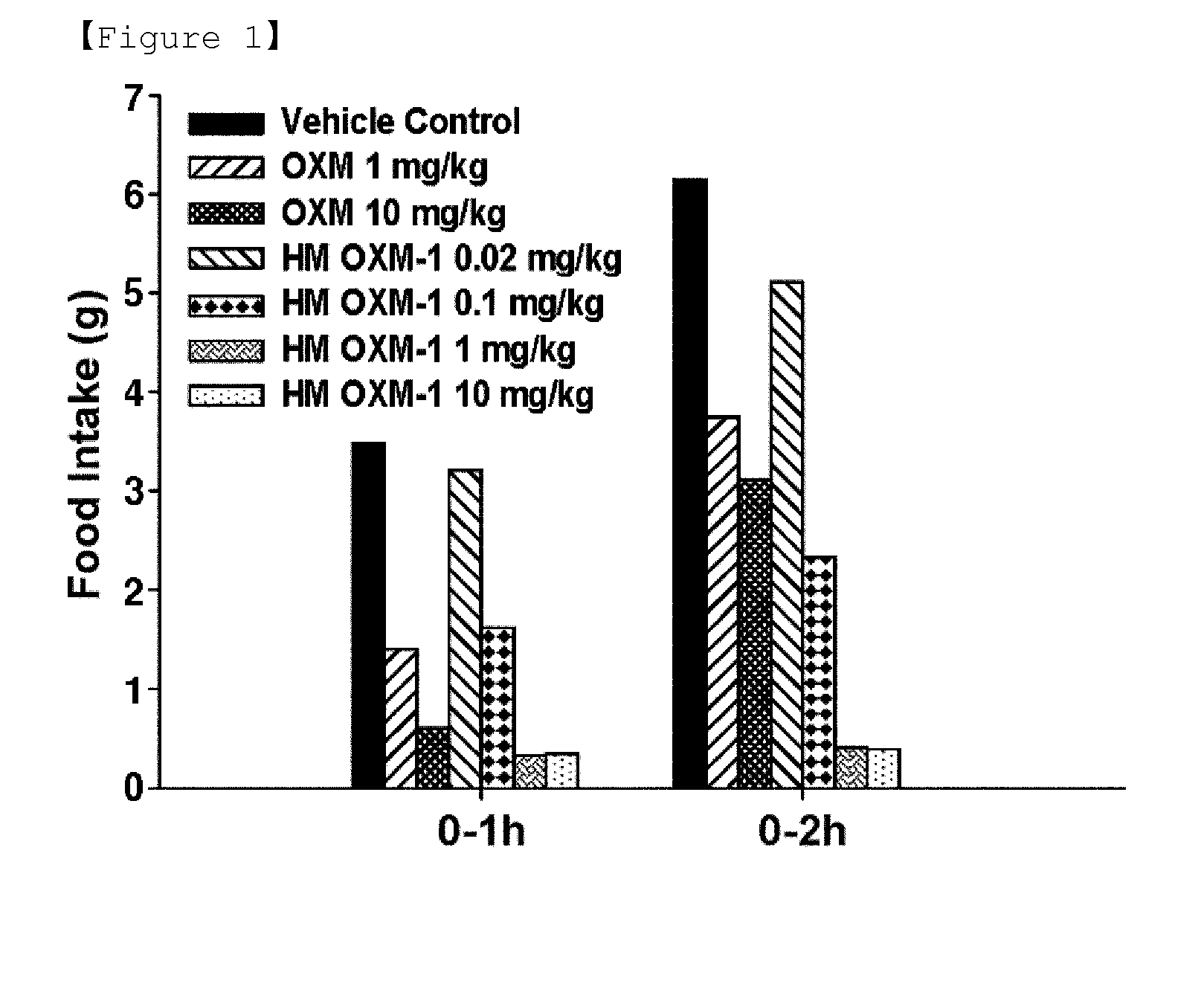
The present invention relates to a conjugate comprising oxyntomodulin, an immunoglobulin Fc region, and non-peptidyl polymer wherein the conjugate being obtainable by covalently linking oxyntomodulin to immunoglobulin Fc region via non-peptidyl polymer, and a pharmaceutical composition for the prevention or treatment of obesity comprising the conjugates. The conjugate comprising oxyntomodulin and the immunoglobulin Fc of the present invention reduces food intake, suppresses gastric emptying, and facilitates lipolysis without side-effects, unlike native oxyntomodulin, and also shows excellent receptor-activating effects and long-term sustainability, compared to native oxyntomodulin. Thus, it can be widely used in the treatment of obesity with safety and efficacy.
Owner:HANMI SCI CO LTD
DPP-IV Resistant GIP Hybrid Polypeptides with Selectable Properties
ActiveUS20110136737A1Increased insulin secretionDecreasing bone loss bonePeptide/protein ingredientsAntibody mimetics/scaffoldsDyslipidemiaFeeding disability
The Present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
Anchored non-piercing duodenal sleeve and delivery systems
ActiveUS20130281911A1Easy to disassembleSimple structureIntravenous devicesNon-surgical orthopedic devicesAxial compressionGastric emptying
An intragastric implant for obesity treatment is disclosed. The device delays digestion by providing a duodenal sleeve, and may also slows gastric emptying by limiting flow through the pyloric sphincter. The implant includes an elongated axially-compressible duodenal sleeve having a non-tissue-piercing anchor on a proximal end sized to lodge within the duodenal bulb. The anchor may have oppositely-directed anchoring flanges to resists migration in both directions. The sleeve may also have barbed ribs to resist proximal movement back up into the stomach. A method of implant includes collapsing / compressing the device and transorally advancing it through the esophagus to be deployed within the duodenum. A dissolvable jacket may constrain the implant for delivery and naturally dissolve upon implant. Removal of the implant may occur in the reverse.
Owner:APOLLO ENDOSURGERY INC
Intragastric device for treating obesity
The present invention is directed toward an intragastric device used to treat obesity that includes a wire mesh structure capable of changing from a compressed pre-deployment shape to an expanded post-deployment shape with a greatly increased volume. The post-deployment shape contains a light weight at the top and a heavier weight at the bottom to ensure proper positioning within the stomach. In the post-deployment shape, the device contains larger spaces in the upper portion and smaller spaces in the lower portion to sequester food and delay gastric emptying. Alternatively, the device can be enveloped by a membrane containing larger holes at the top and smaller holes at the bottom to sequester food and delay gastric emptying. The device has a dynamic weight where the weight of the device in the pre-feeding stage is less than the weight of the device in feeding or post-feeding stage.
Owner:SYNERZ MEDICAL
Dpp-iv resistant gip hybrid polypeptides with selectable properties
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:LEVY ODILE ESTHER +15
Novel oxyntomodulin derivatives and pharmaceutical composition for treating obesity comprising the same
ActiveUS20140128318A1Reduces food intakeSuppresses gastric emptyingCell receptors/surface-antigens/surface-determinantsSugar derivativesSide effectReceptor activation
The present invention relates to a novel peptide showing more excellent activities on a glucagon like peptide-1 receptor and a glucagon receptor than native oxyntomodulin, and a composition for the prevention or treatment of obesity comprising the peptide as an active ingredient. Unlike native oxyntomodulin, the novel peptide of the present invention reduces food intake, suppresses gastric emptying, and facilitates lipolysis with reduced side-effects, and also shows excellent receptor-activating effects. Thus, it can be widely used in the treatment of obesity with safety and efficacy.
Owner:HANMI SCI CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com