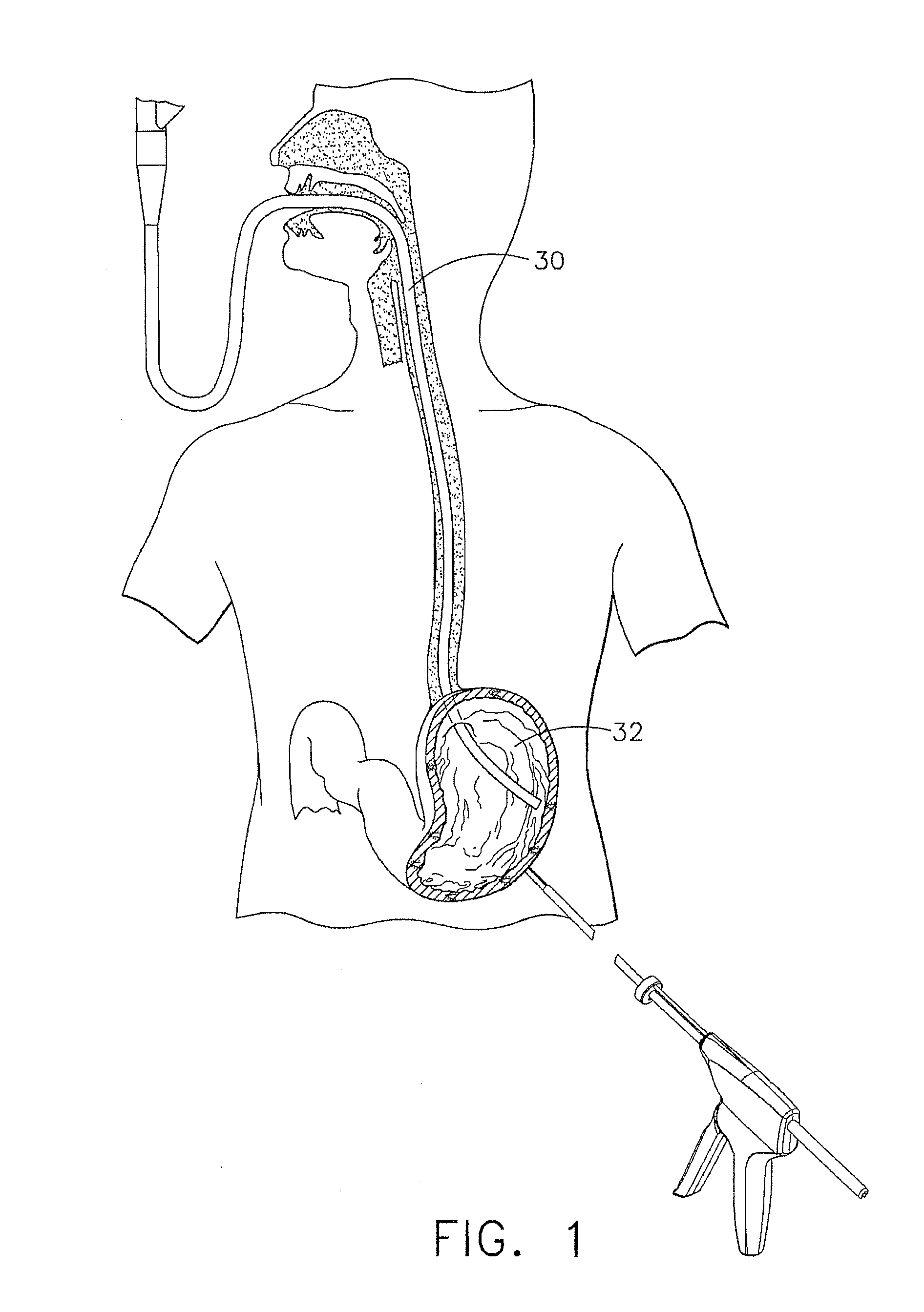Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Pyloric sphincter" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
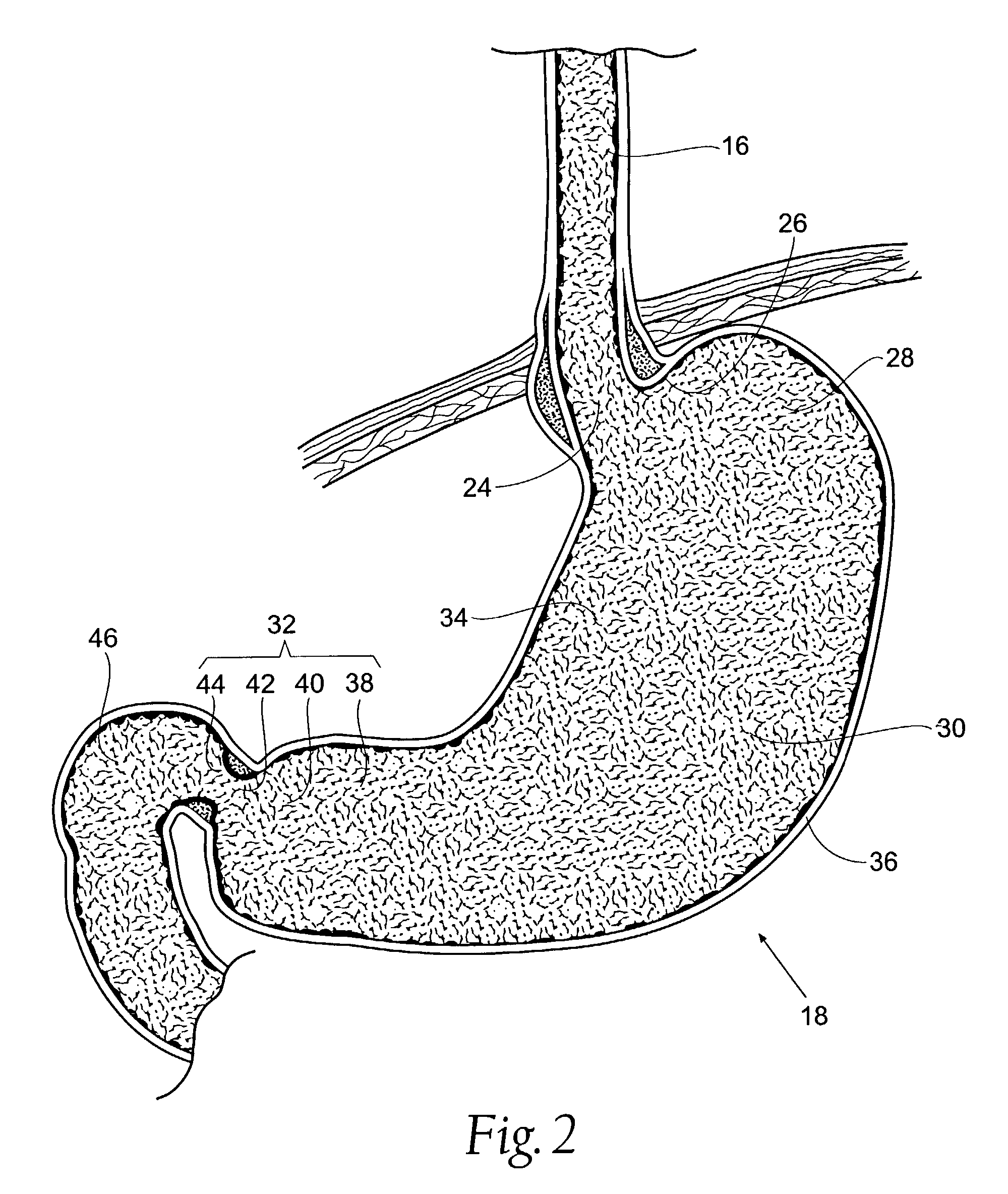
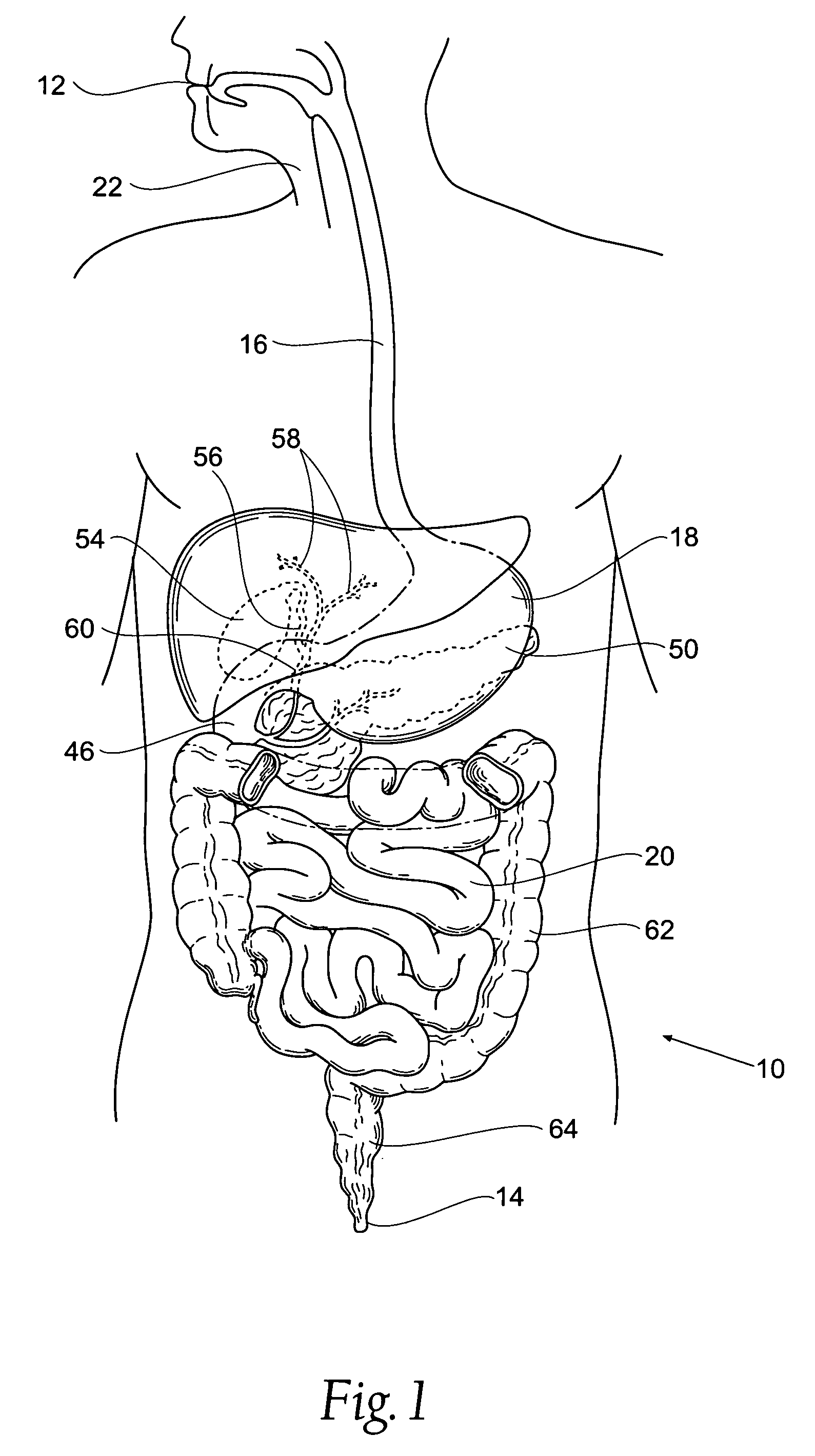
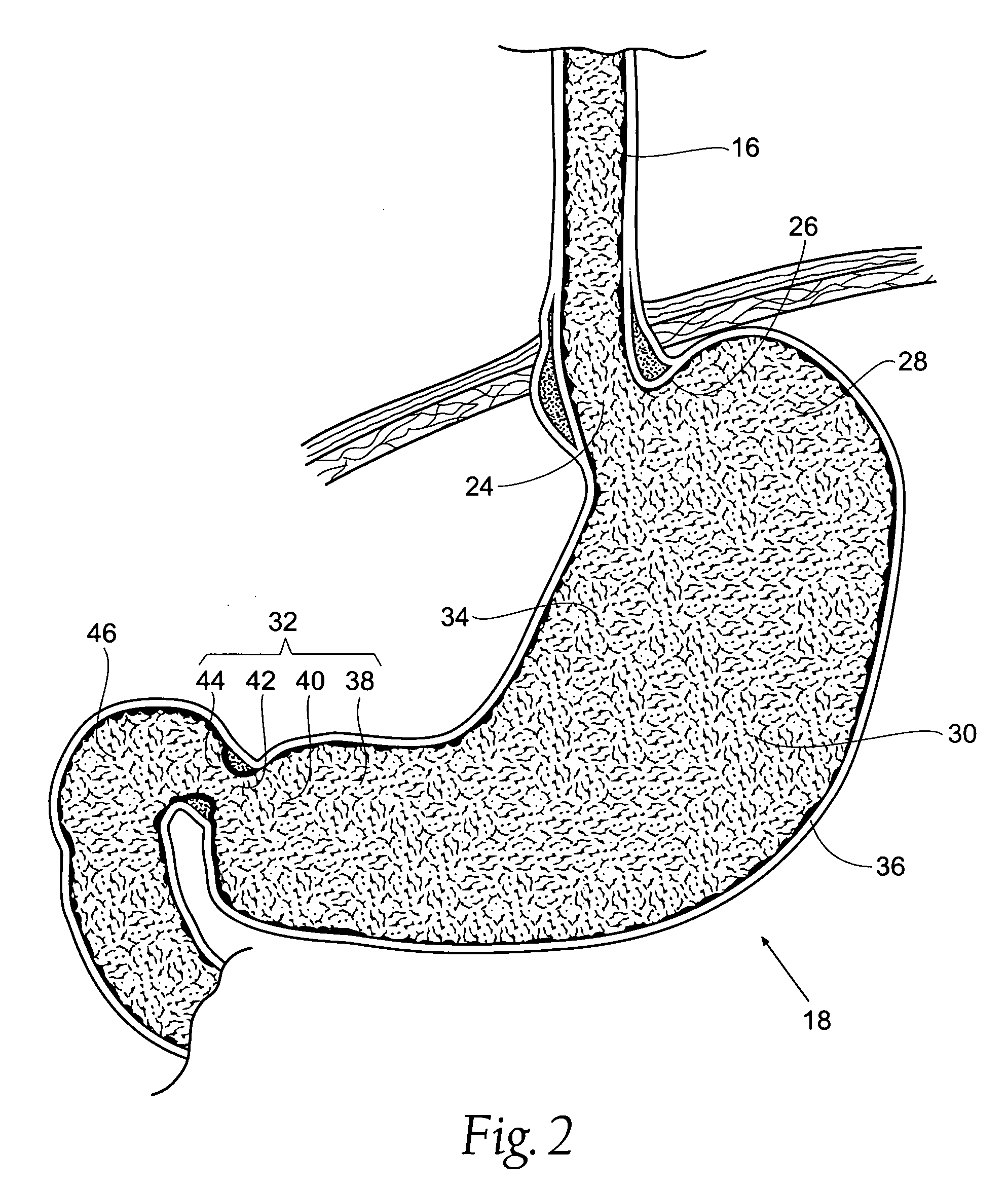
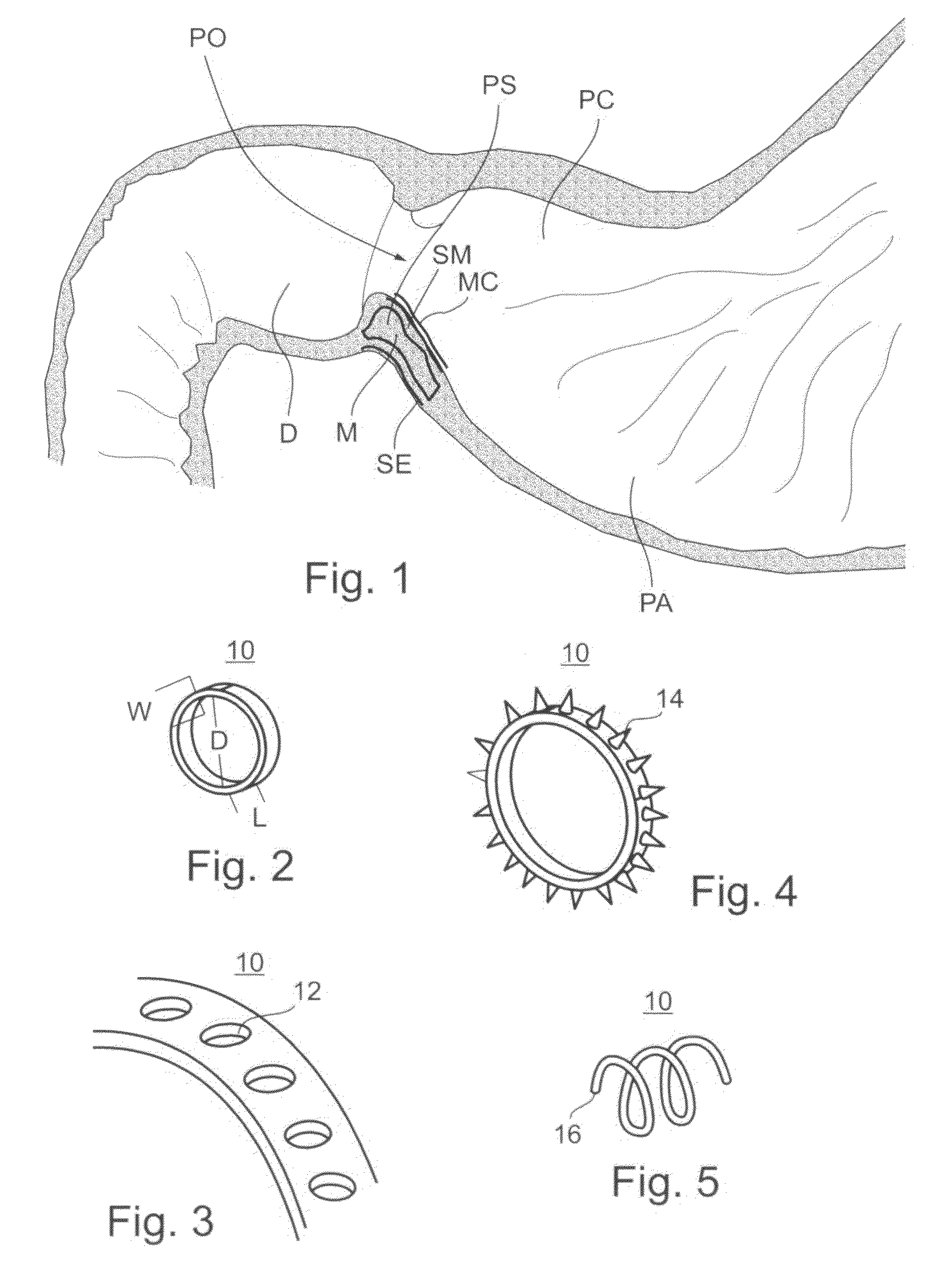
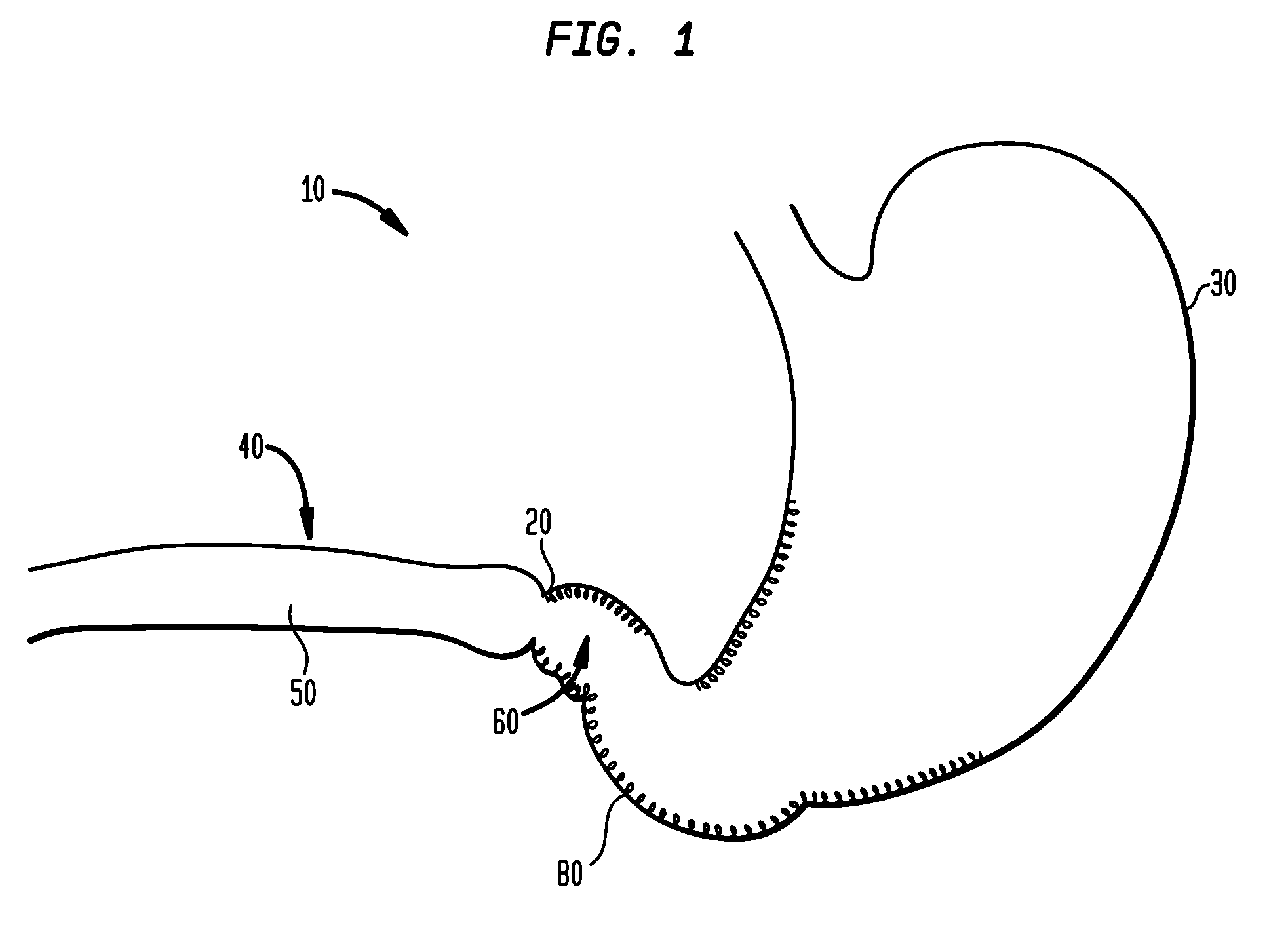
The pyloric sphincter is a band of smooth muscle that controls the movement of partially digested food and juices from the pylorus into the duodenum.
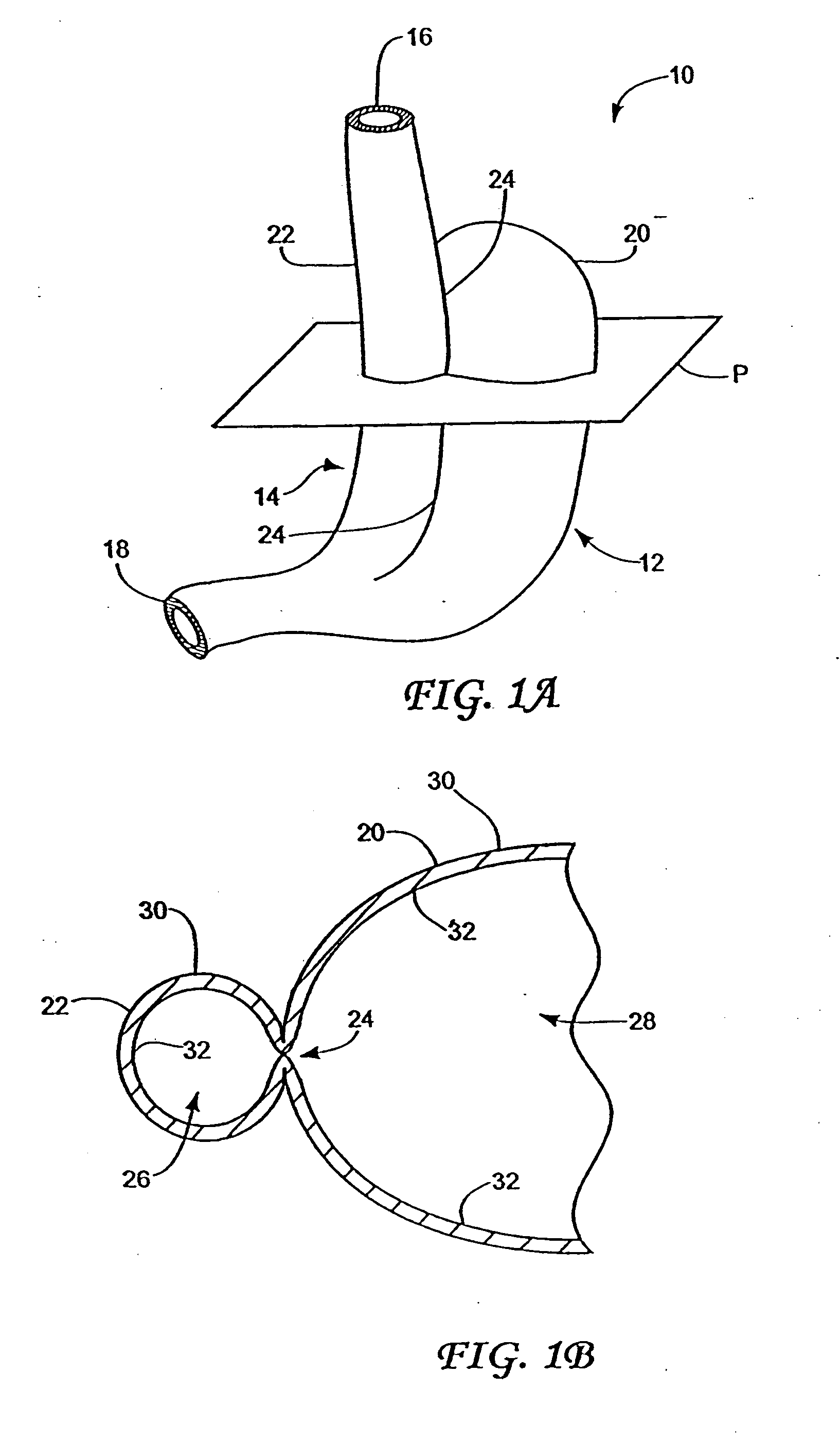
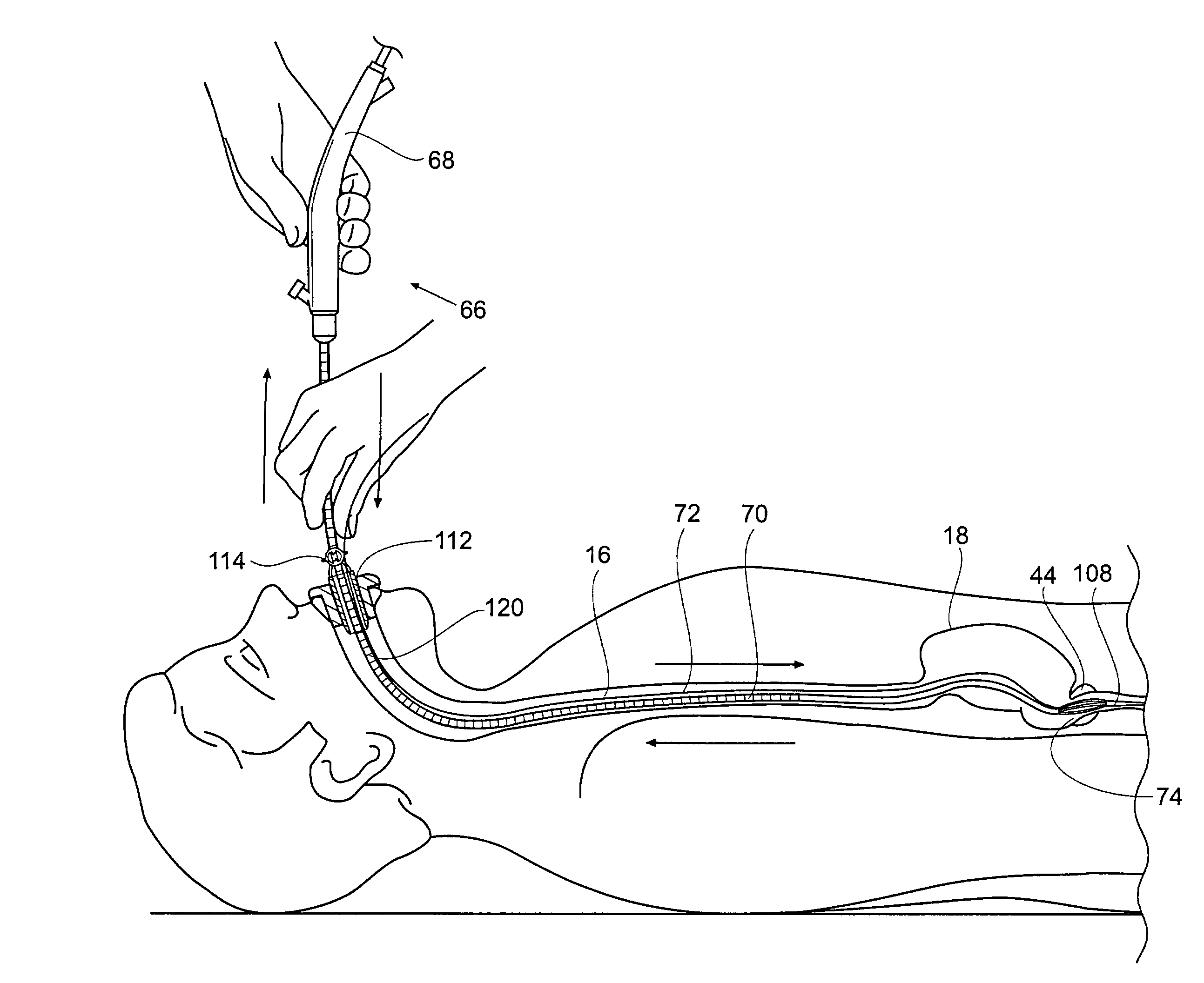
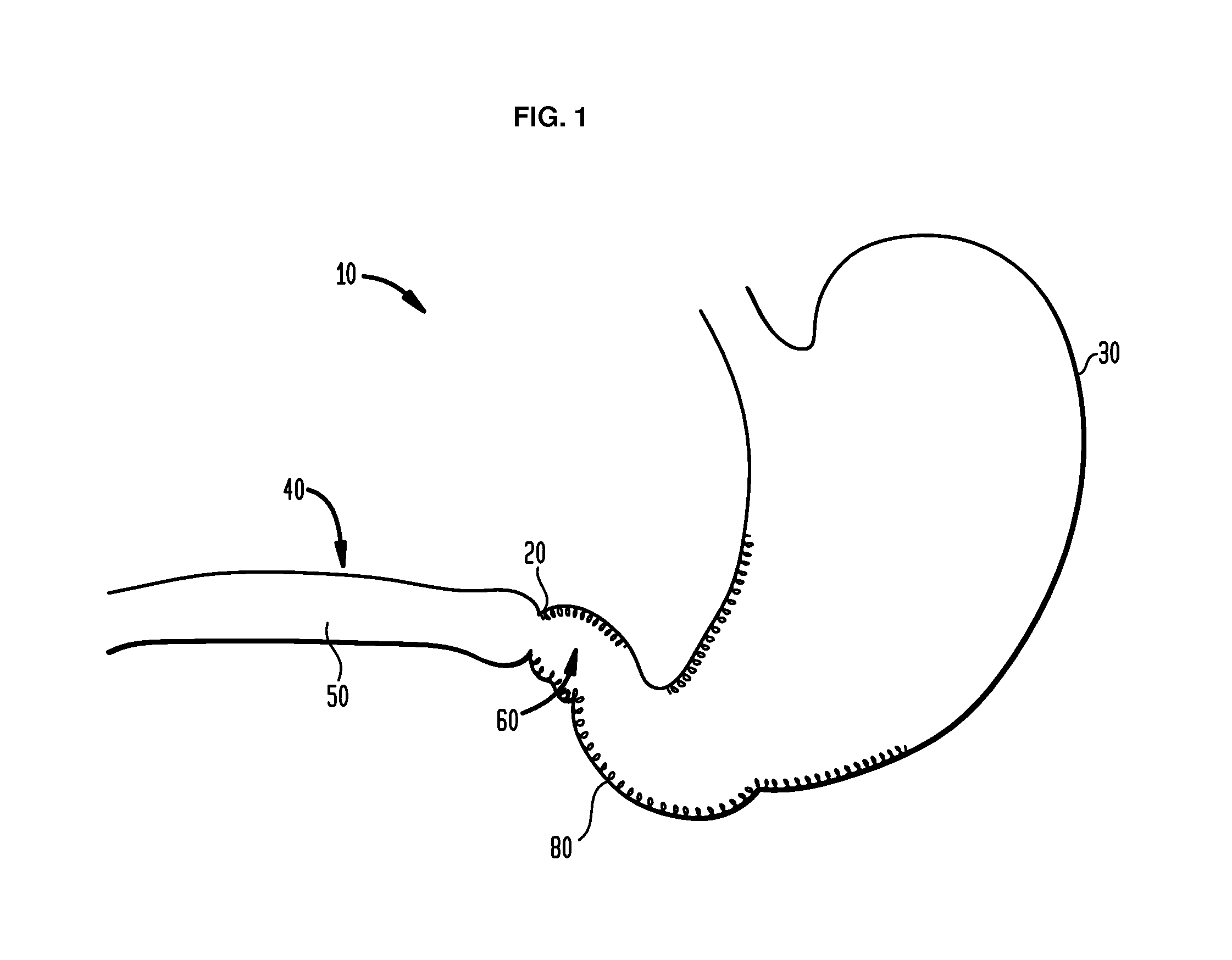
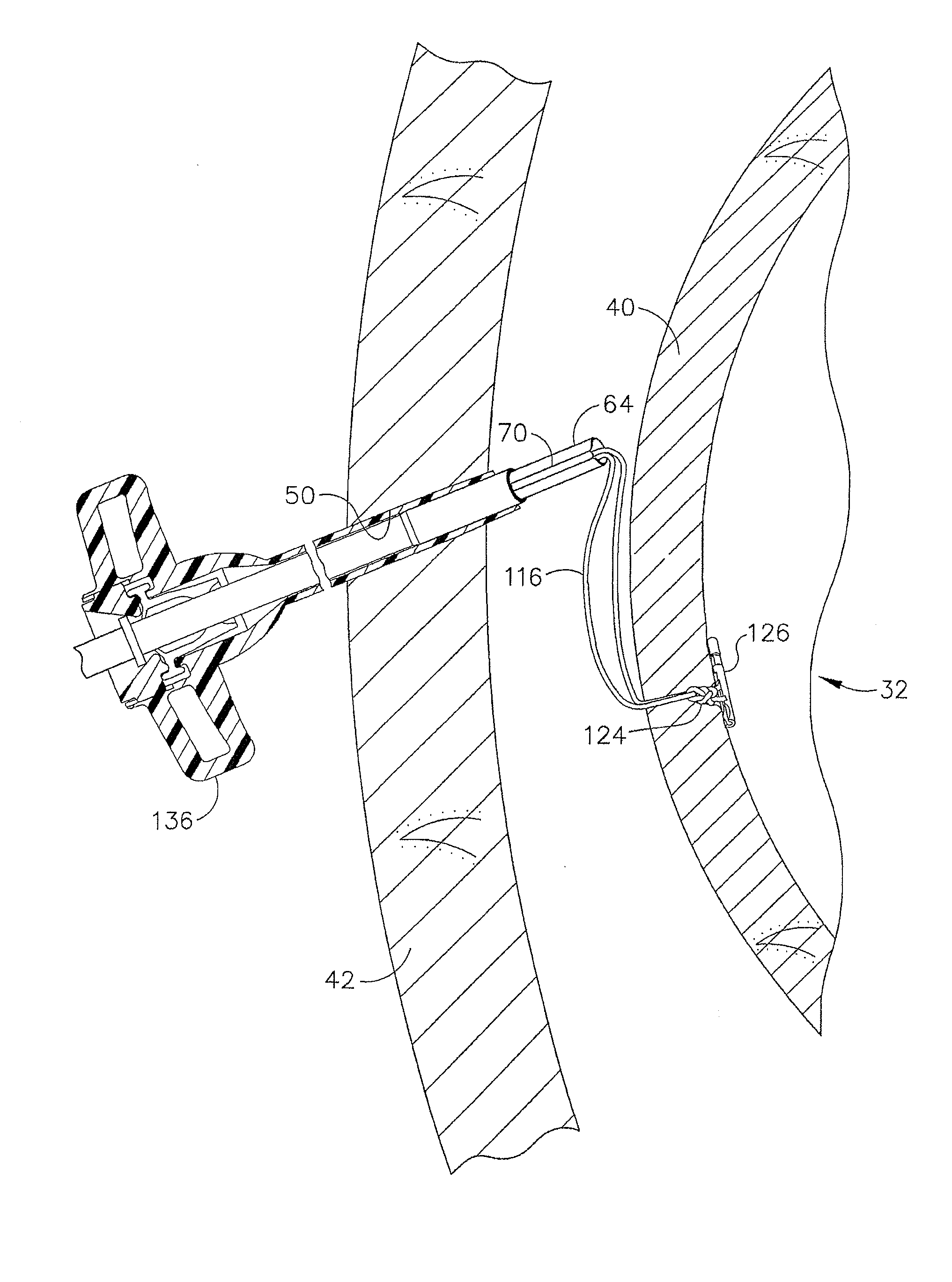
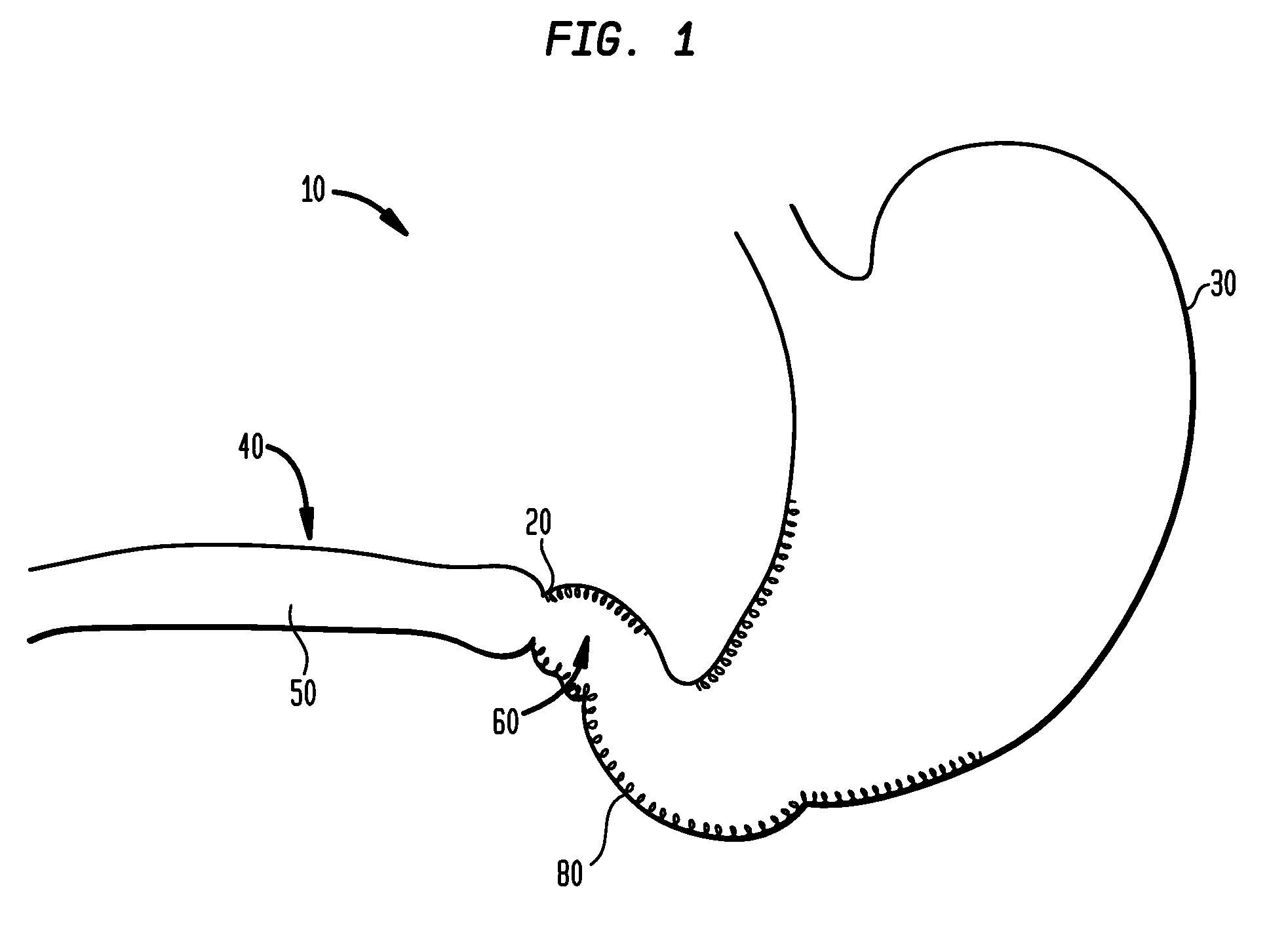
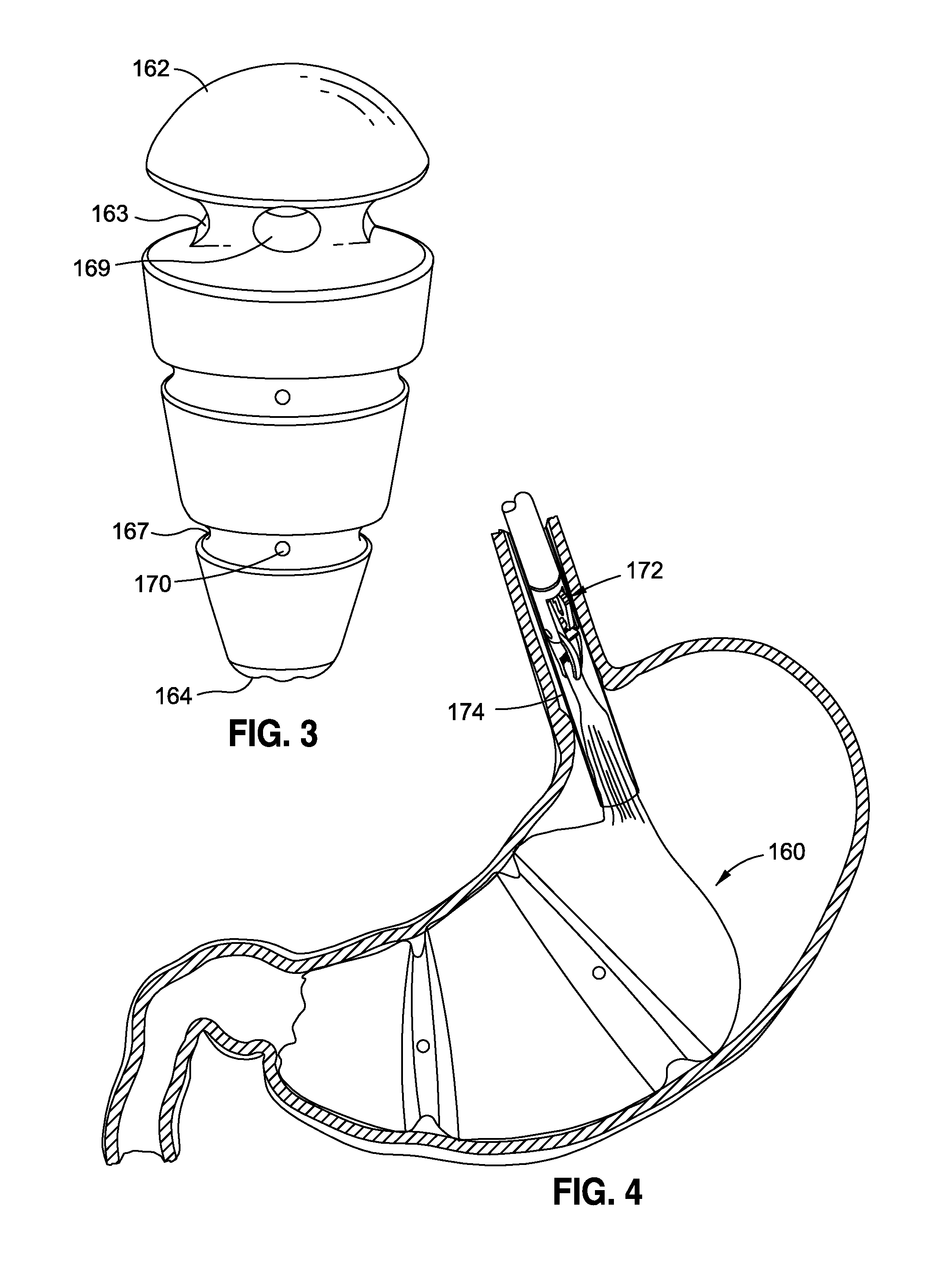
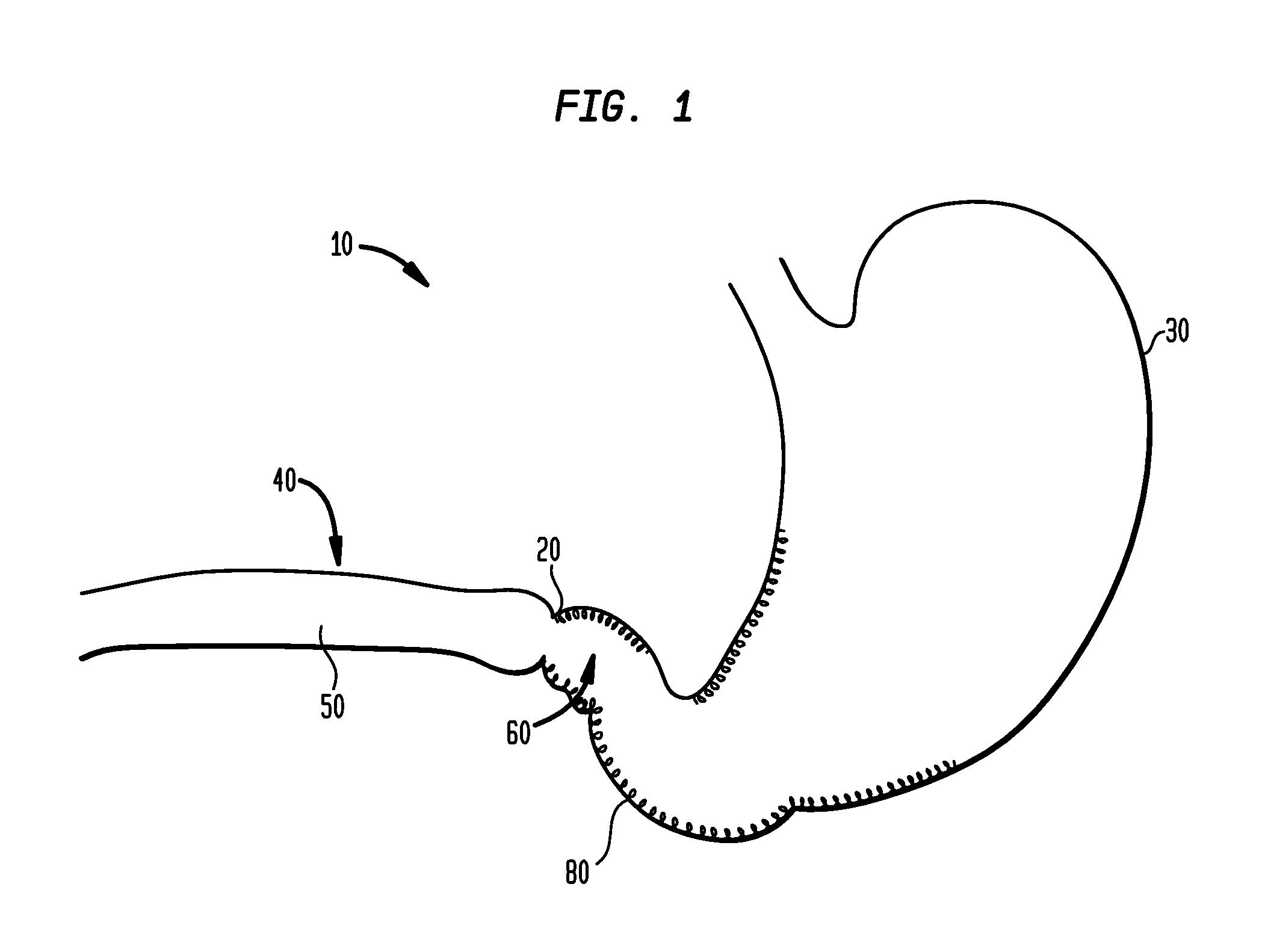
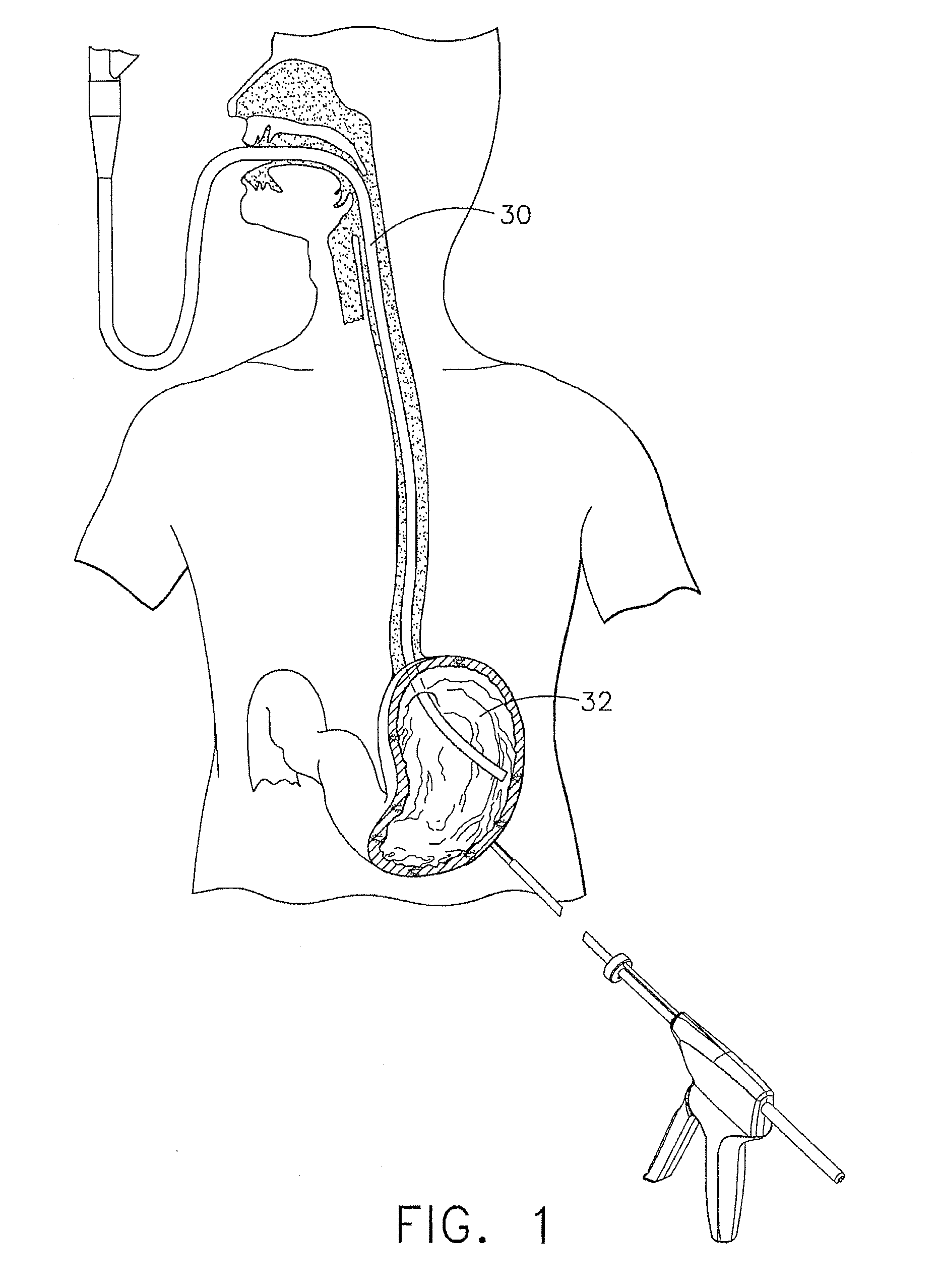
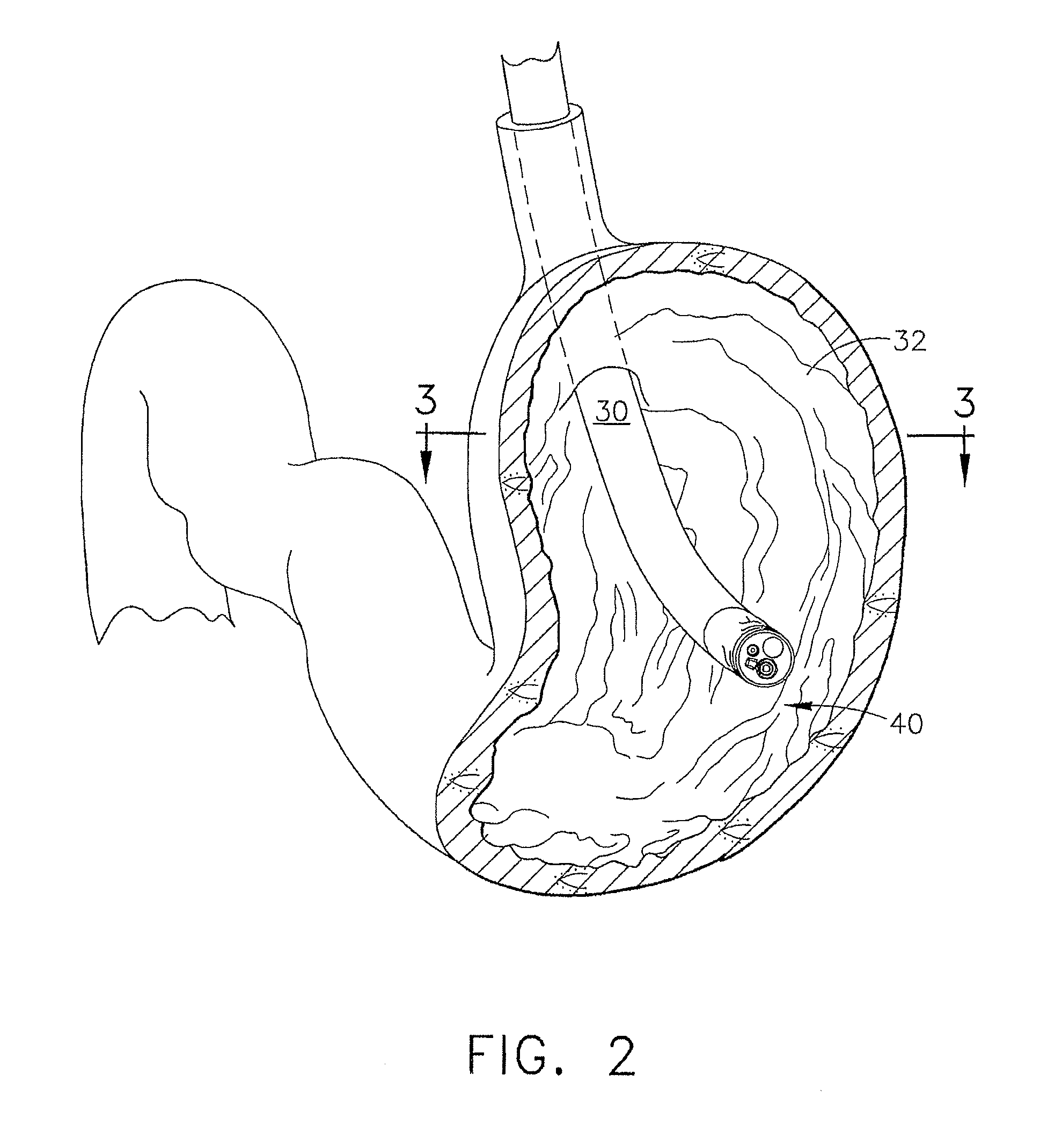
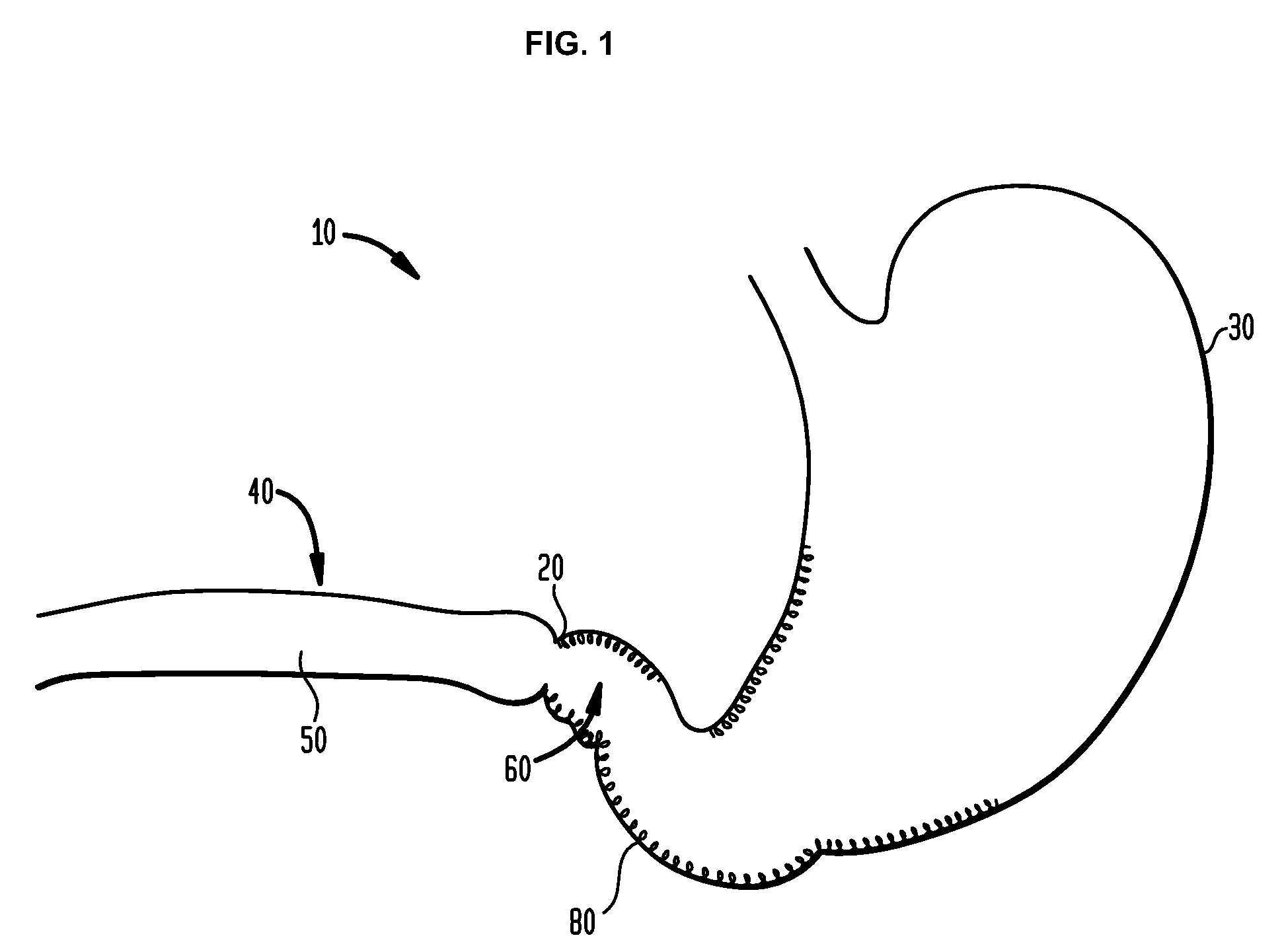
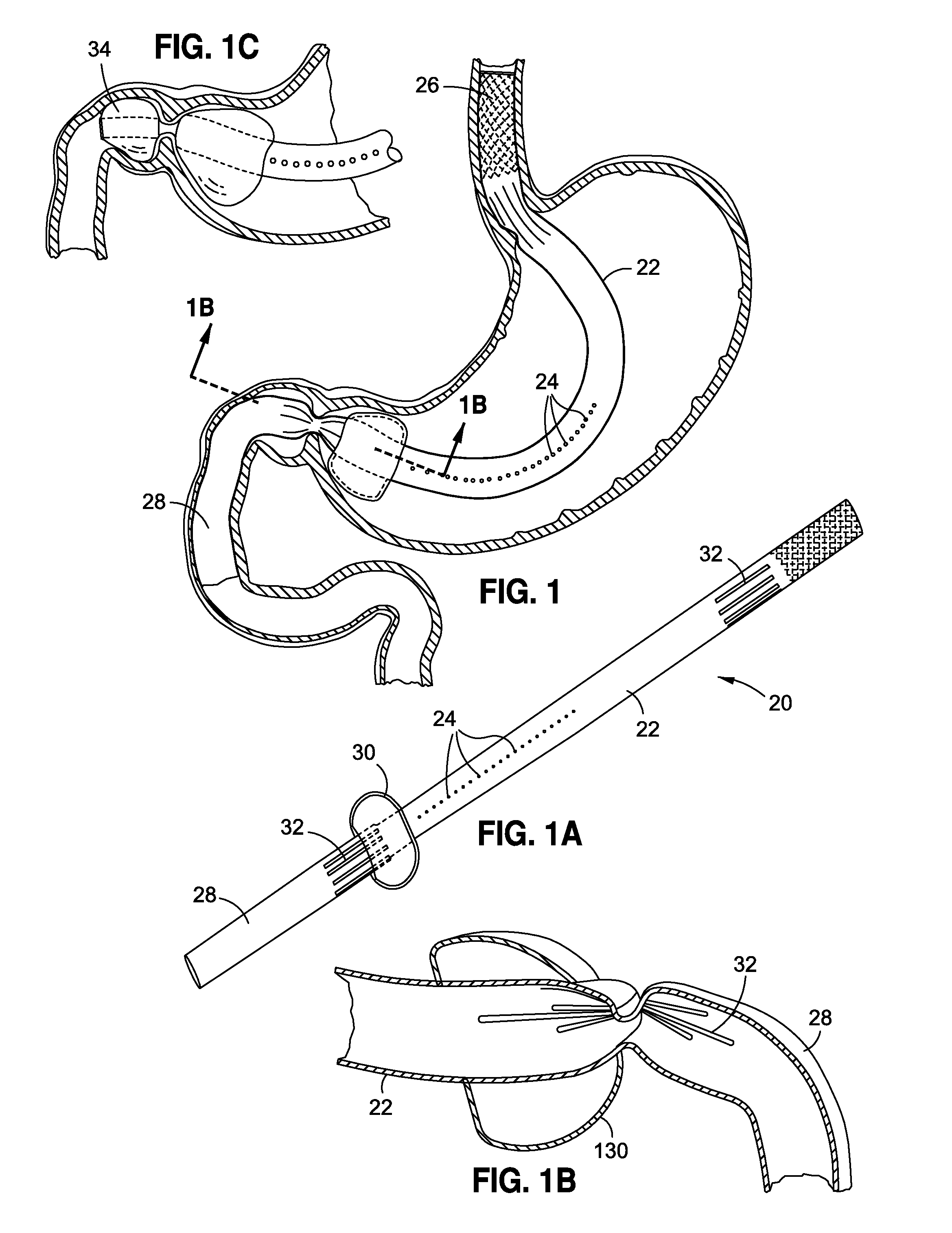
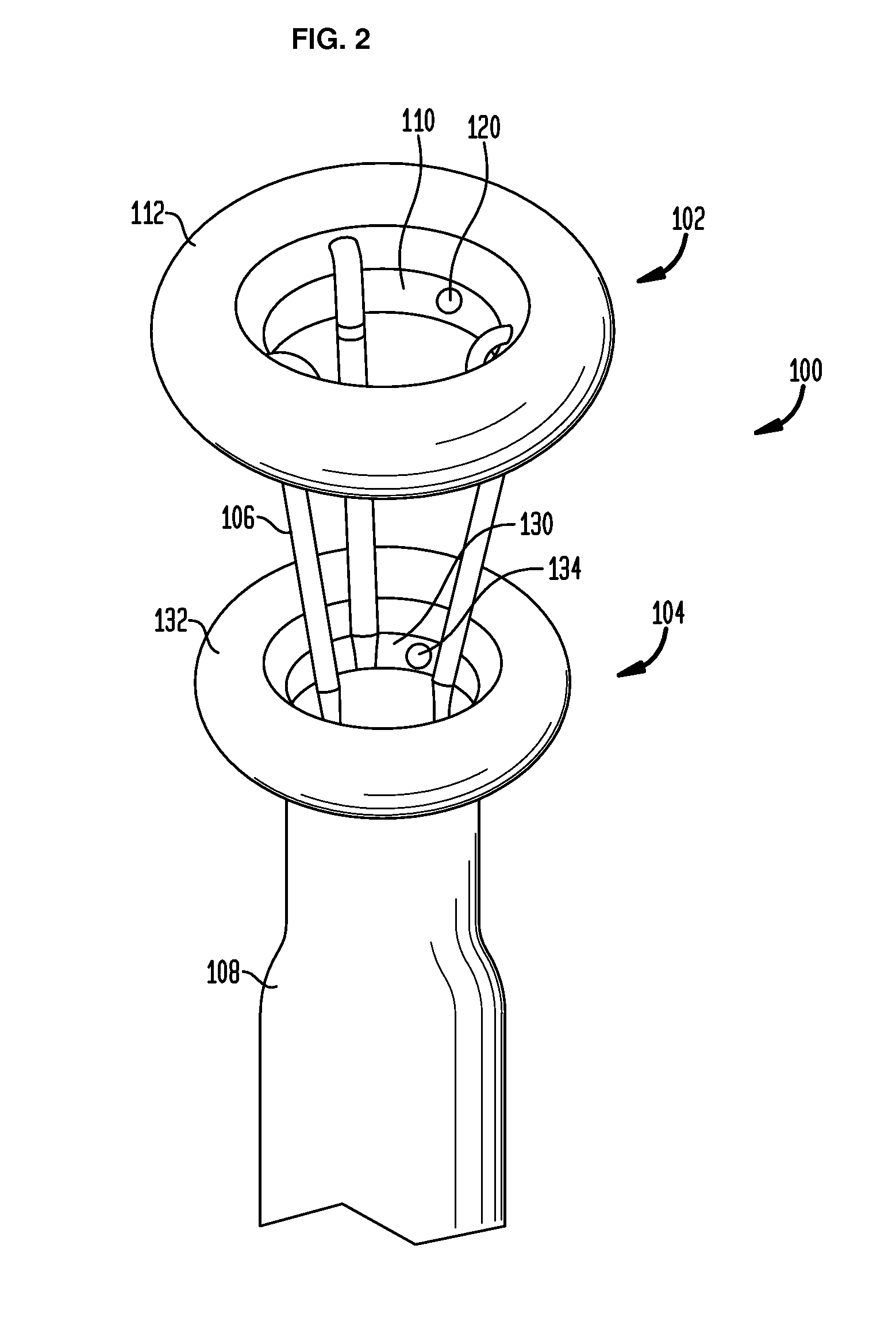
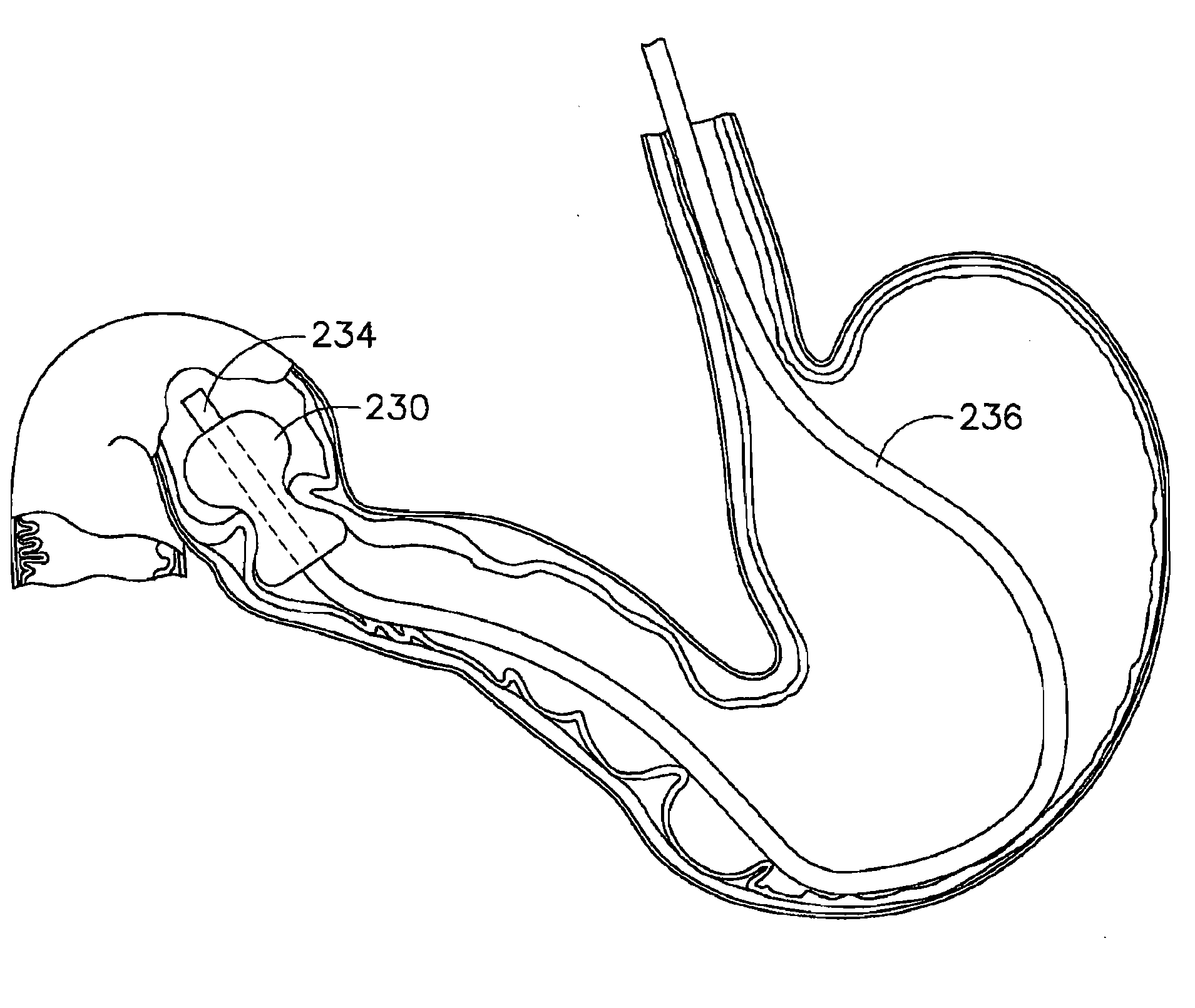
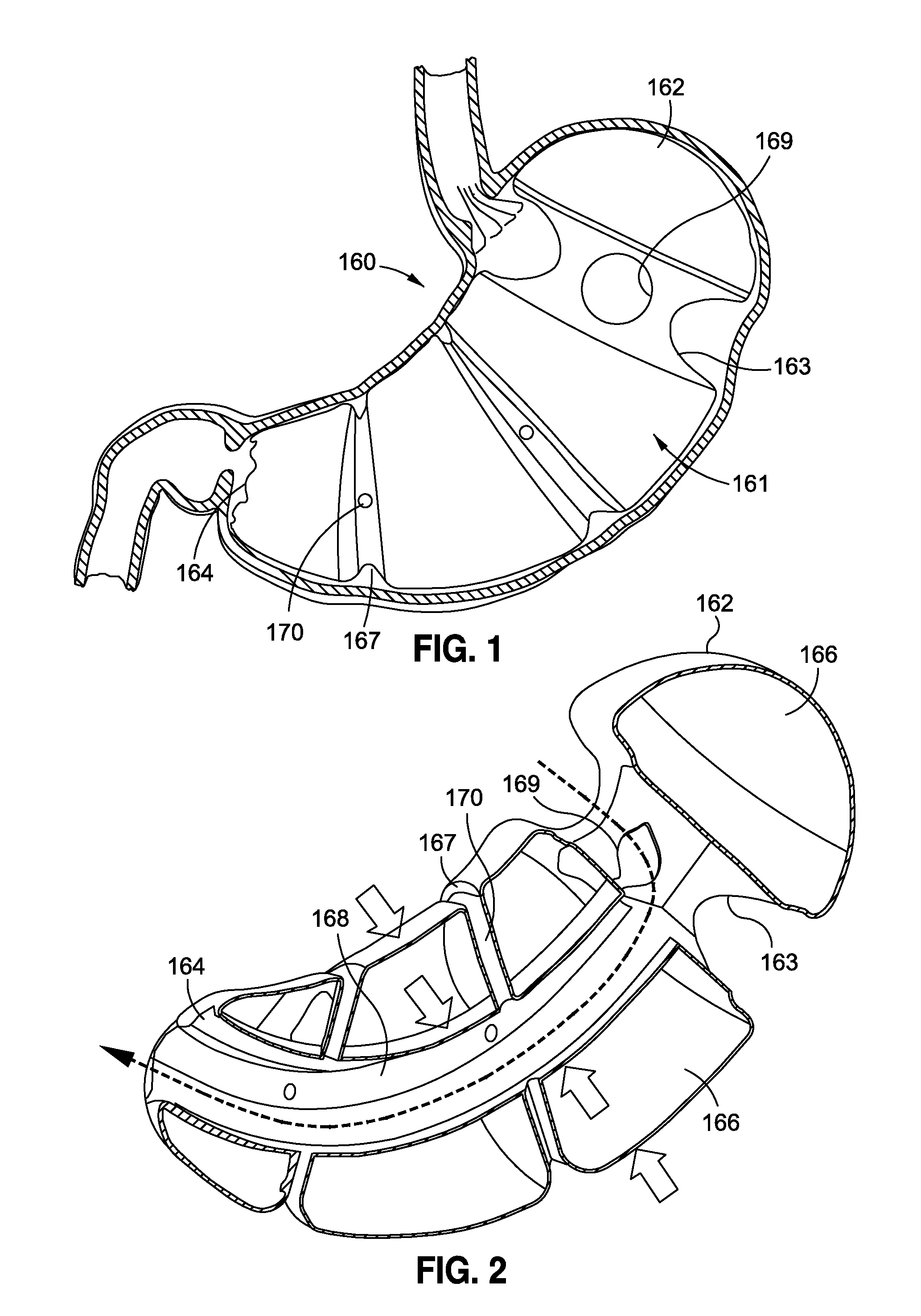
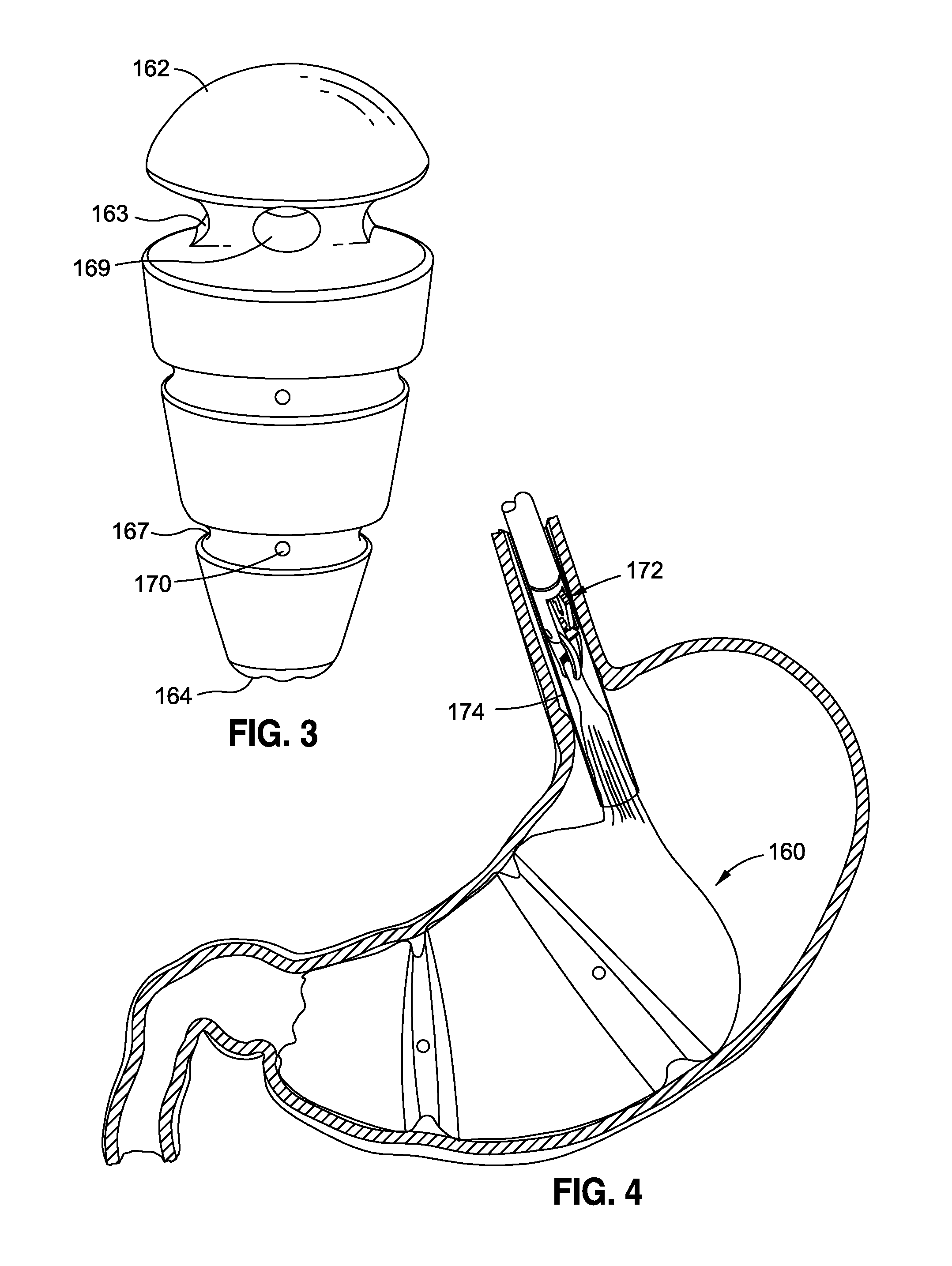
Method and apparatus for treatment of the gastrointestinal tract
InactiveUS20080086179A1Preventing and slowing esophageal emptyingIncreasing UES, esophageal or LES sphincter toneElectrotherapyPyloric sphincterGastric fundus
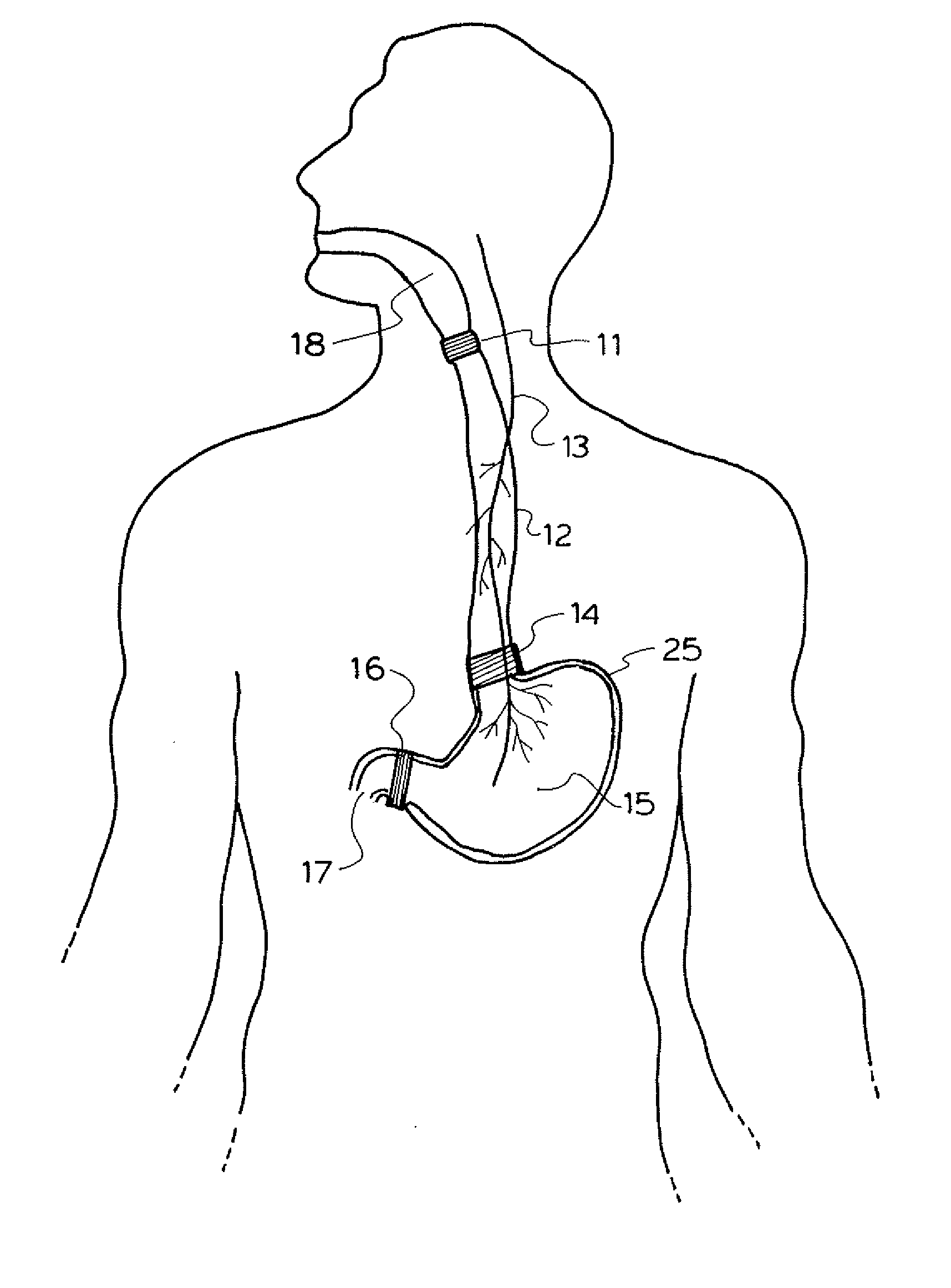
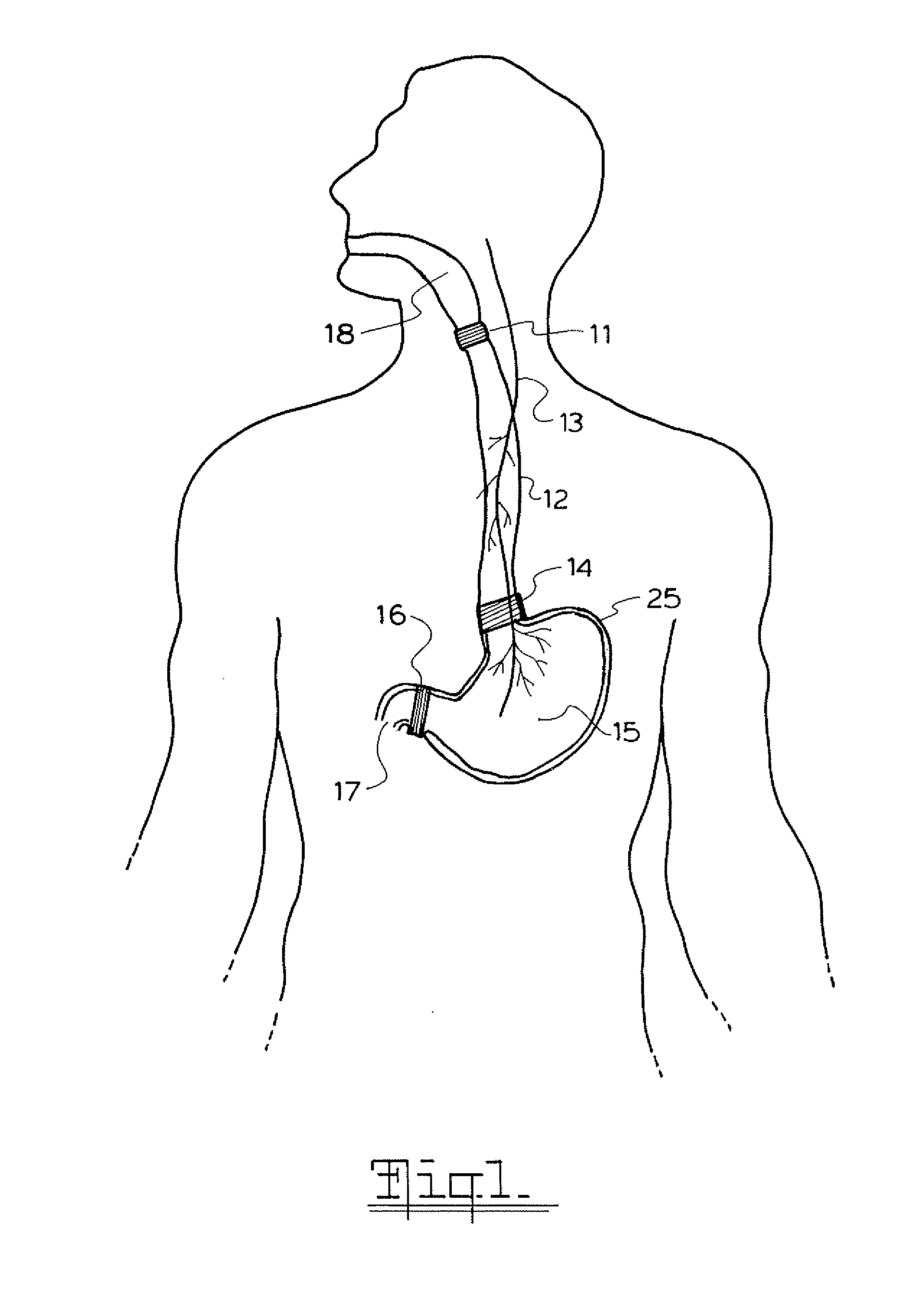
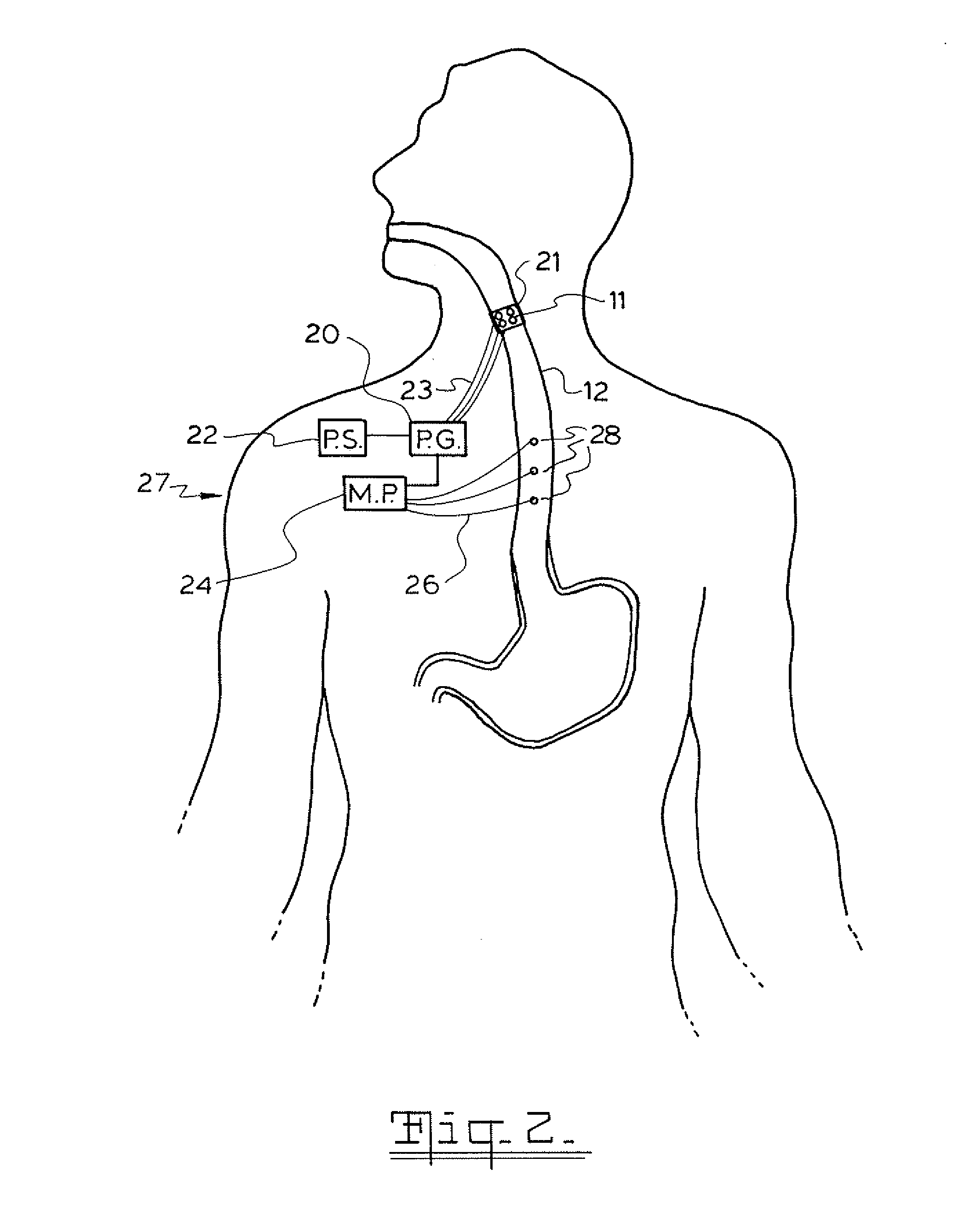
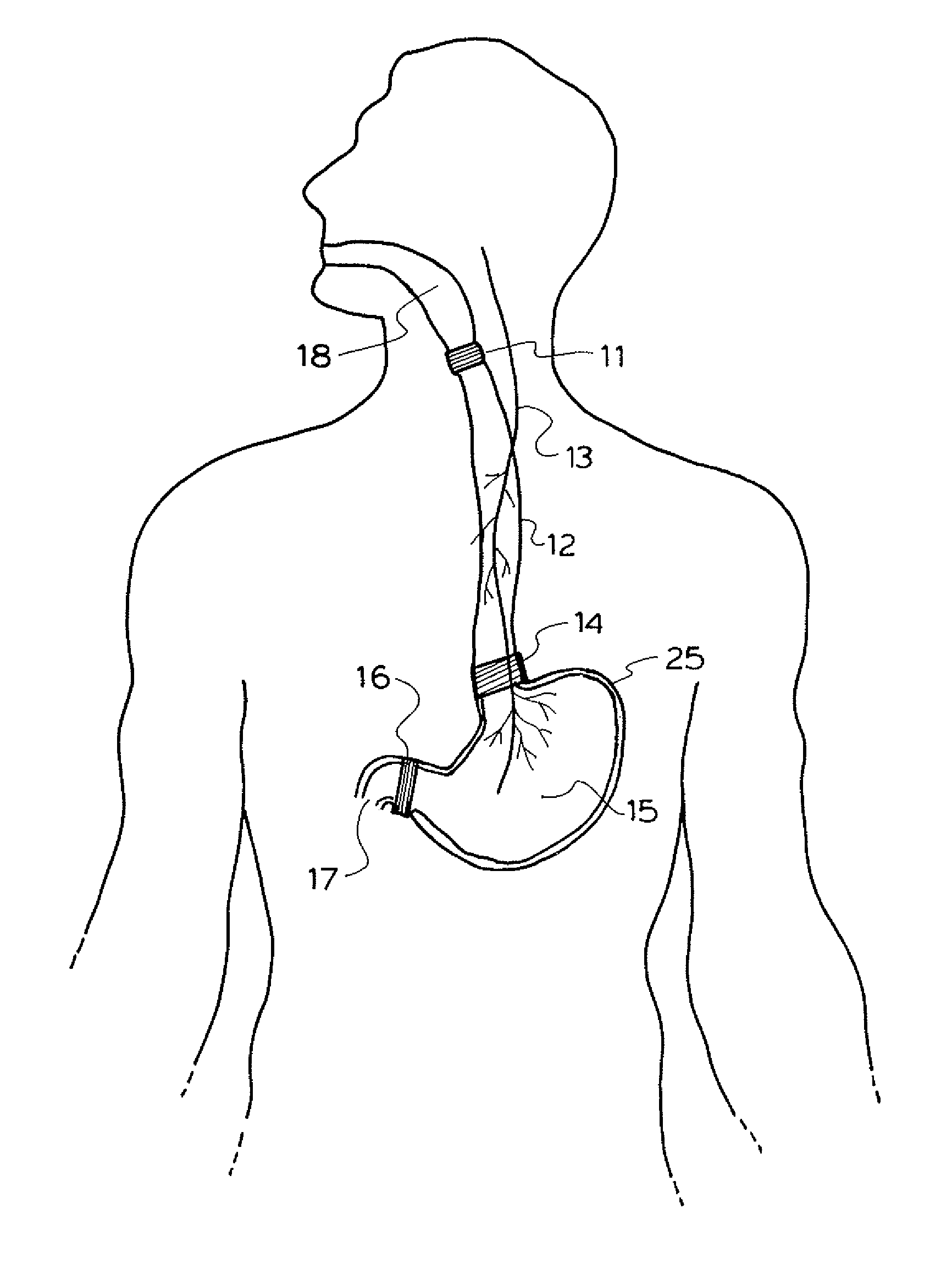
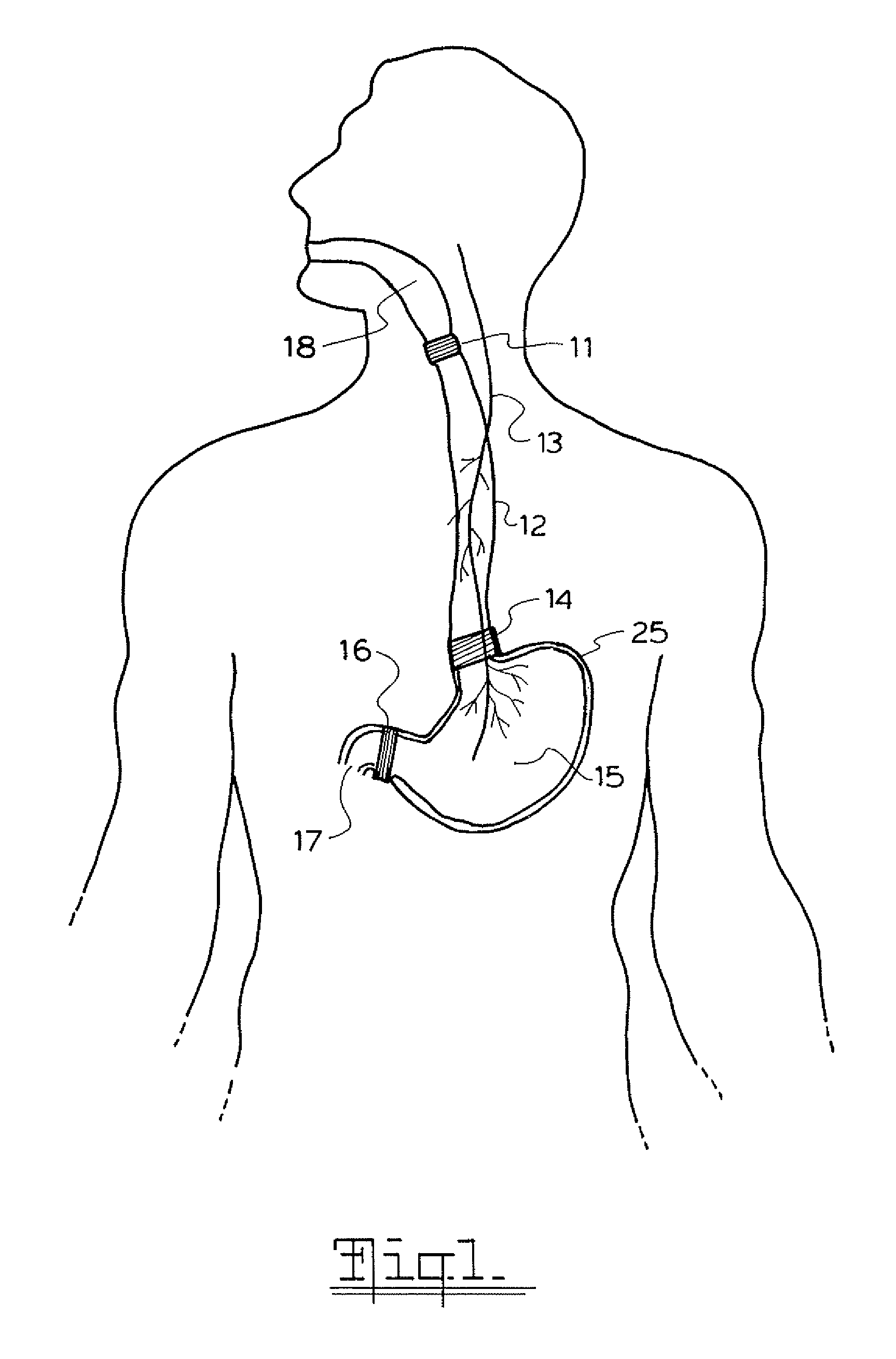
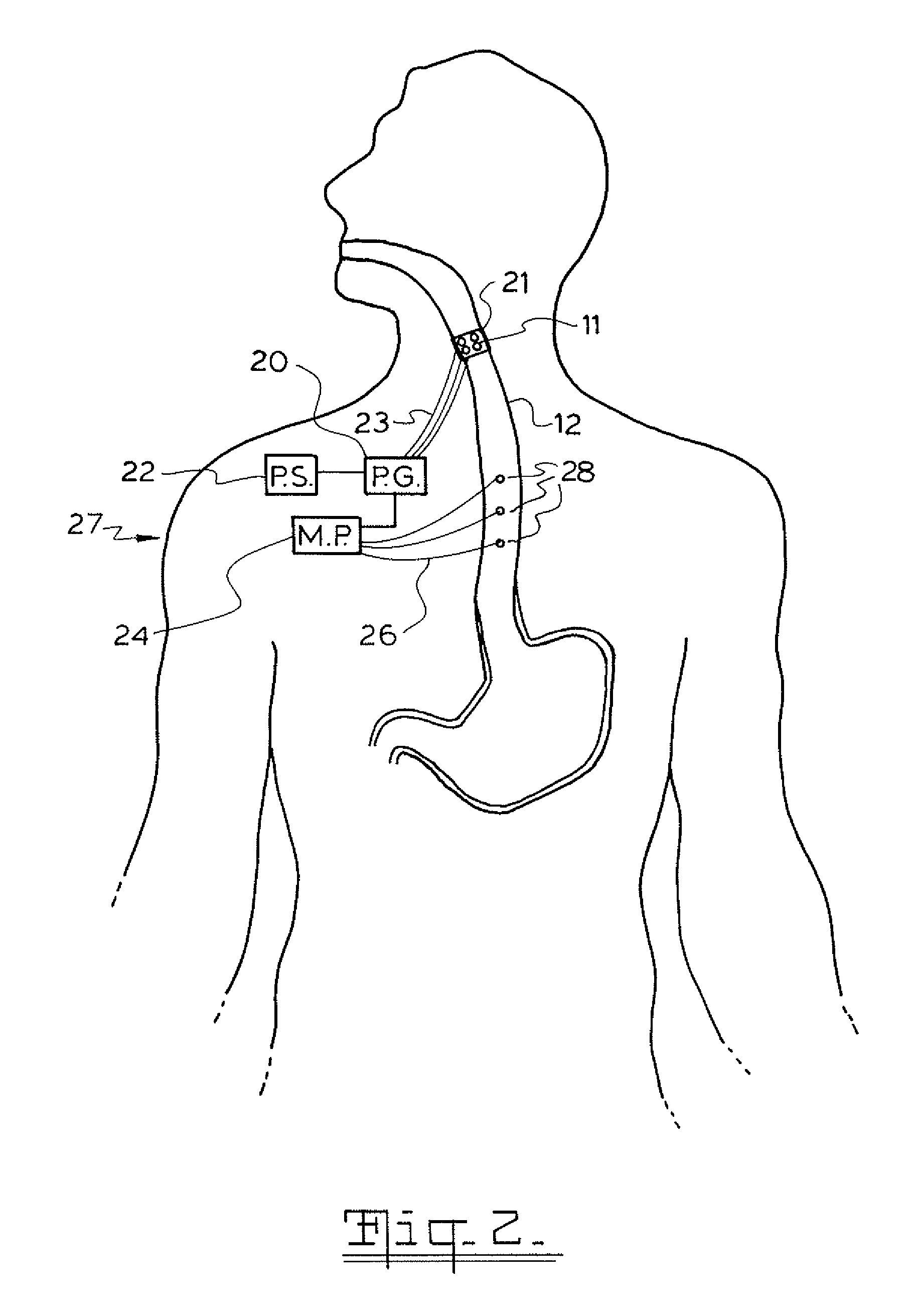
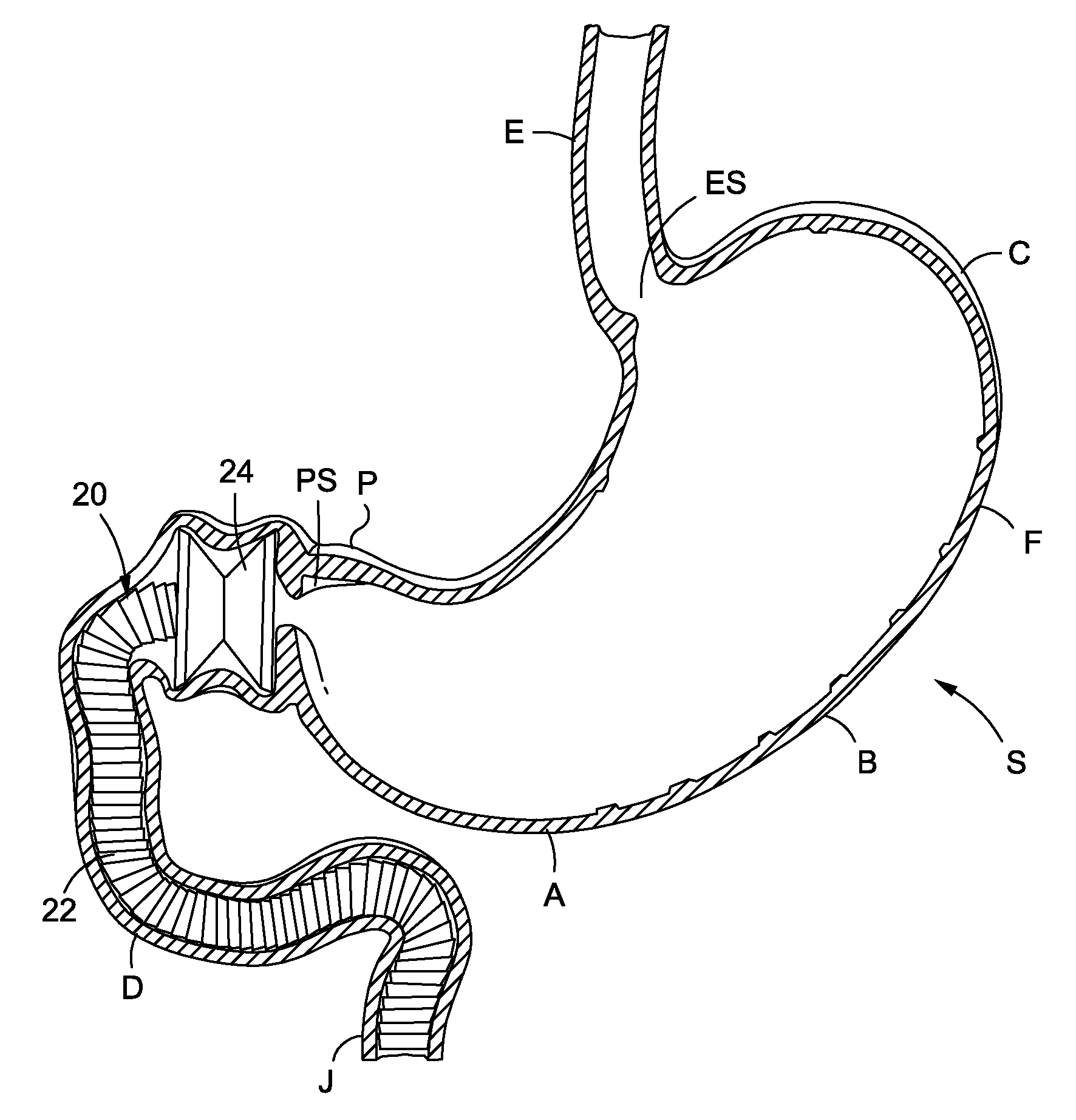
A method and device for electrically stimulating one or more structures in the gastrointestinal tract as described. The one or more structures are preferably selected from the upper esophageal sphincter, the esophagus and gastric fundus. The method may involve the step of arranging a plurality of stimulating electrodes adjacent one or more structures which may further include the lower esophageal sphincter, the stomach, the pyloric sphincter, the small intestine, the colon and the vagus. The method and device may further include sensing electrodes to detect change in one or more physiological parameters in the gastrointestinal tract and modulate the stimulating electrodes in response to the change. The device comprises a pulse generator, a power source, a plurality of stimulating electrode set, one or more sensing electrodes and means for varying activity of the stimulating electrodes in response to change detected in the gastrointestinal tract. The method and device may be used to treat obesity and / or GERD.
Owner:PARAS HLDG LLC +1
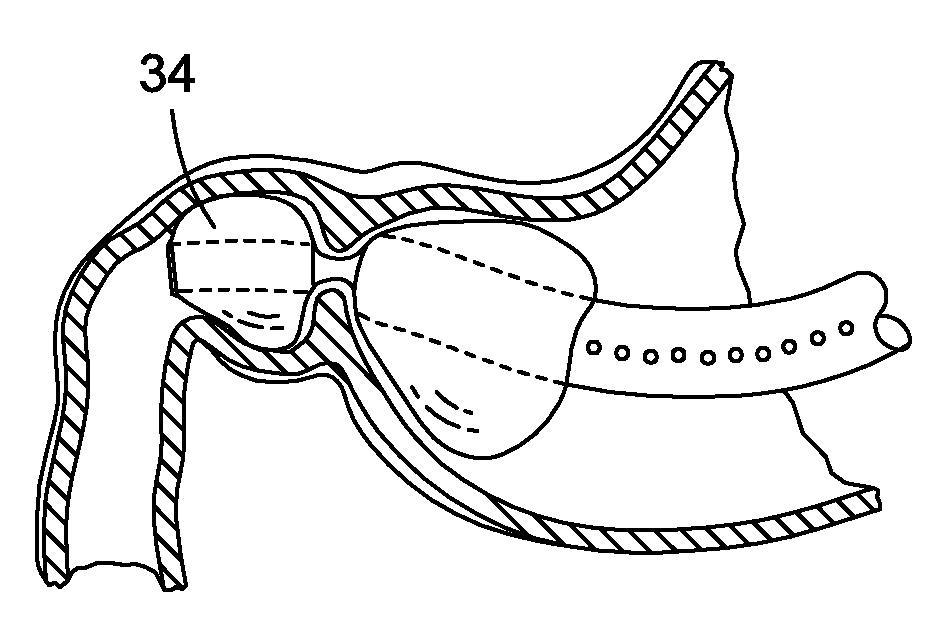
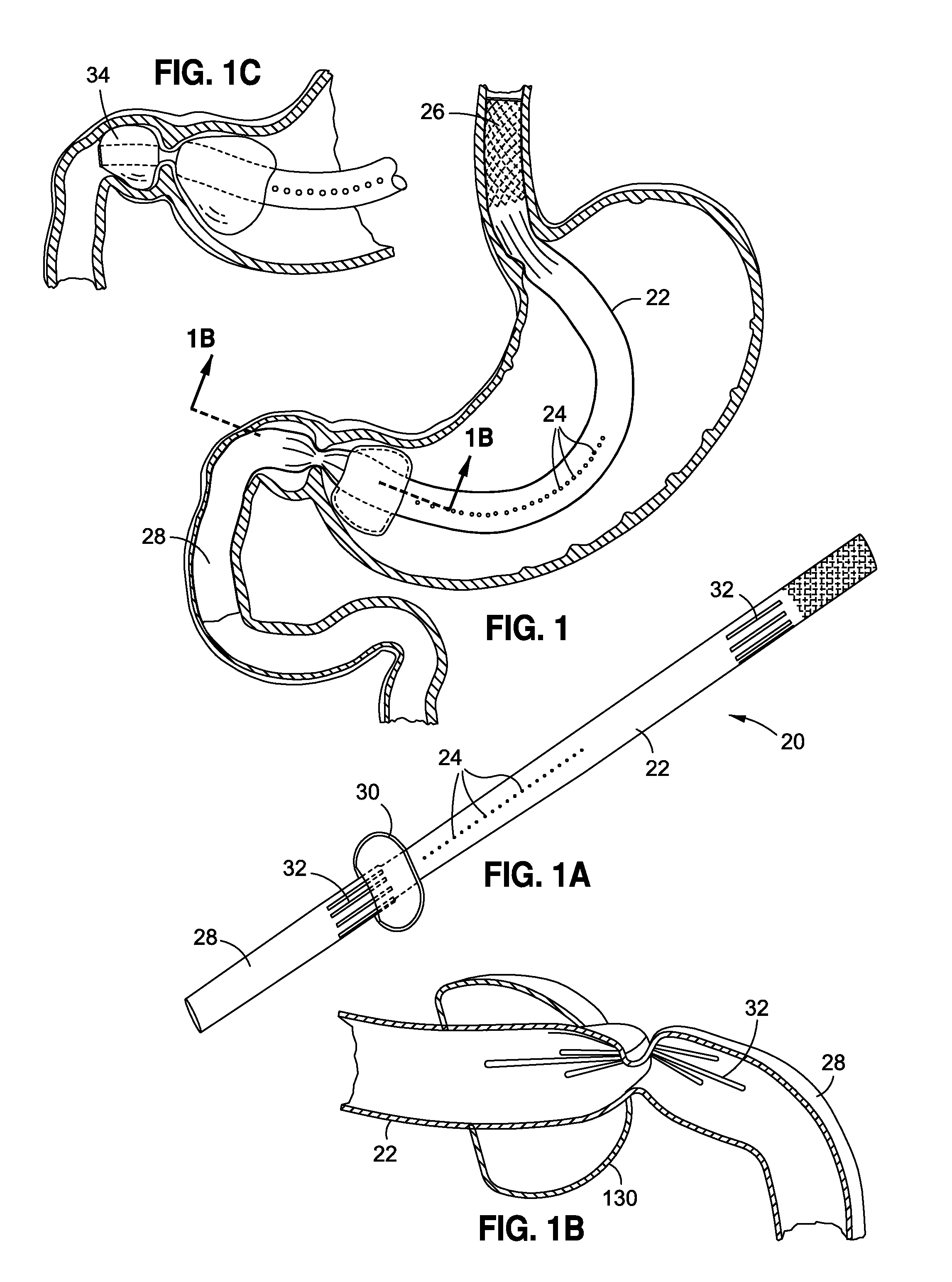
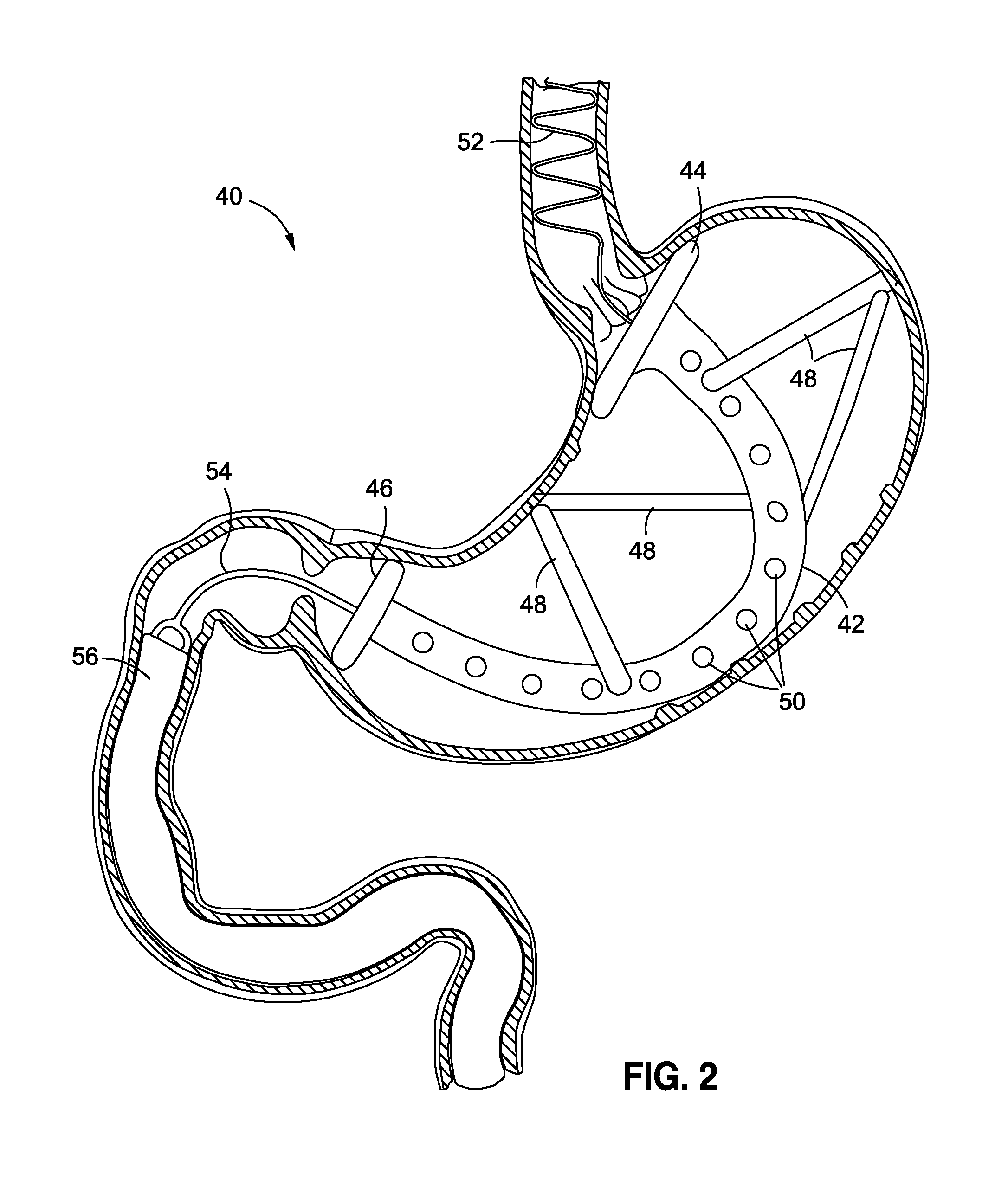
Obesity treatment tools and methods
Various obesity treatment tools and methods are described herein, as well as treatments for other gastric-related diseases, e.g., GERD. Treatment includes reducing the size of the stomach pouch to limit the caloric intake as well as to provide an earlier feeling of satiety. This may be done by creating a smaller gastric pouch within the stomach directly from the interior of the stomach itself. The smaller pouches may be made through the use of individual anchoring devices, rotating probes, or volume reduction devices. A pyloroplasty procedure may also be performed to render the pyloric sphincter incompetent. A gastric bypass procedure may additionally be performed using atraumatic magnetic anastomoses devices so that sugars and fats are passed directly to the bowel while bypassing the stomach. Many of these procedures may be done in a variety of combinations. Treatment may create enforced behavioral modifications by discouraging the ingestion of high-caloric foods.
Owner:ETHICON ENDO SURGERY INC
Systems and methods for treating obesity and other gastrointestinal conditions
InactiveUS7468060B2Increase satietyReduce and moderate incidenceElectrotherapyMetabolism disorderReflexOesophageal tube
Systems and methods affect tightening of the pyloric sphincter and / or serve to mediate or moderate receptive relaxation of muscles in the stomach, to treat or mitigate various physiologic conditions, such as obesity, biliary reflex, GERD, and / or Barrett's esophagus. The systems and methods may be used as either a primary treatment modality, or applied as a supplementary treatment before, during or after a primary intervention.
Owner:MEDERI THERAPEUTICS
Obesity treatment tools and methods
InactiveUS20070118159A1Convenient treatmentMinimal traumaSuture equipmentsStapling toolsDiseasePyloric sphincter
Various obesity treatment tools and methods are described herein, as well as treatments for other gastric-related diseases, e.g., GERD. Treatment includes reducing the size of the stomach pouch to limit the caloric intake as well as to provide an earlier feeling of satiety. This may be done by creating a smaller gastric pouch within the stomach directly from the interior of the stomach itself. The smaller pouches may be made through the use of individual anchoring devices, rotating probes, or volume reduction devices. A pyloroplasty procedure may also be performed to render the pyloric sphincter incompetent. A gastric bypass procedure may additionally be performed using atraumatic magnetic anastomoses devices so that sugars and fats are passed directly to the bowel while bypassing the stomach. Many of these procedures may be done in a variety of combinations. Treatment may create enforced behavioral modifications by discouraging the ingestion of high-caloric foods.
Owner:ETHICON ENDO SURGERY INC
Method and apparatus for treatment of the gastrointestinal tract
InactiveUS7738961B2Increasing UES, esophageal or LES sphincter toneReduce delaysElectrotherapyArtificial respirationUpper esophageal sphincterSmall intestine
A method and device for electrically stimulating one or more structures in the gastrointestinal tract as described. The one or more structures are preferably selected from the upper esophageal sphincter, the esophagus and gastric fundus. The method may involve the step of arranging a plurality of stimulating electrodes adjacent one or more structures which may further include the lower esophageal sphincter, the stomach, the pyloric sphincter, the small intestine, the colon and the vagus. The method and device may further include sensing electrodes to detect change in one or more physiological parameters in the gastrointestinal tract and modulate the stimulating electrodes in response to the change. The device comprises a pulse generator, a power source, a plurality of stimulating electrode set, one or more sensing electrodes and means for varying activity of the stimulating electrodes in response to change detected in the gastrointestinal tract. The method and device may be used to treat obesity and / or GERD.
Owner:PARAS HLDG LLC +1
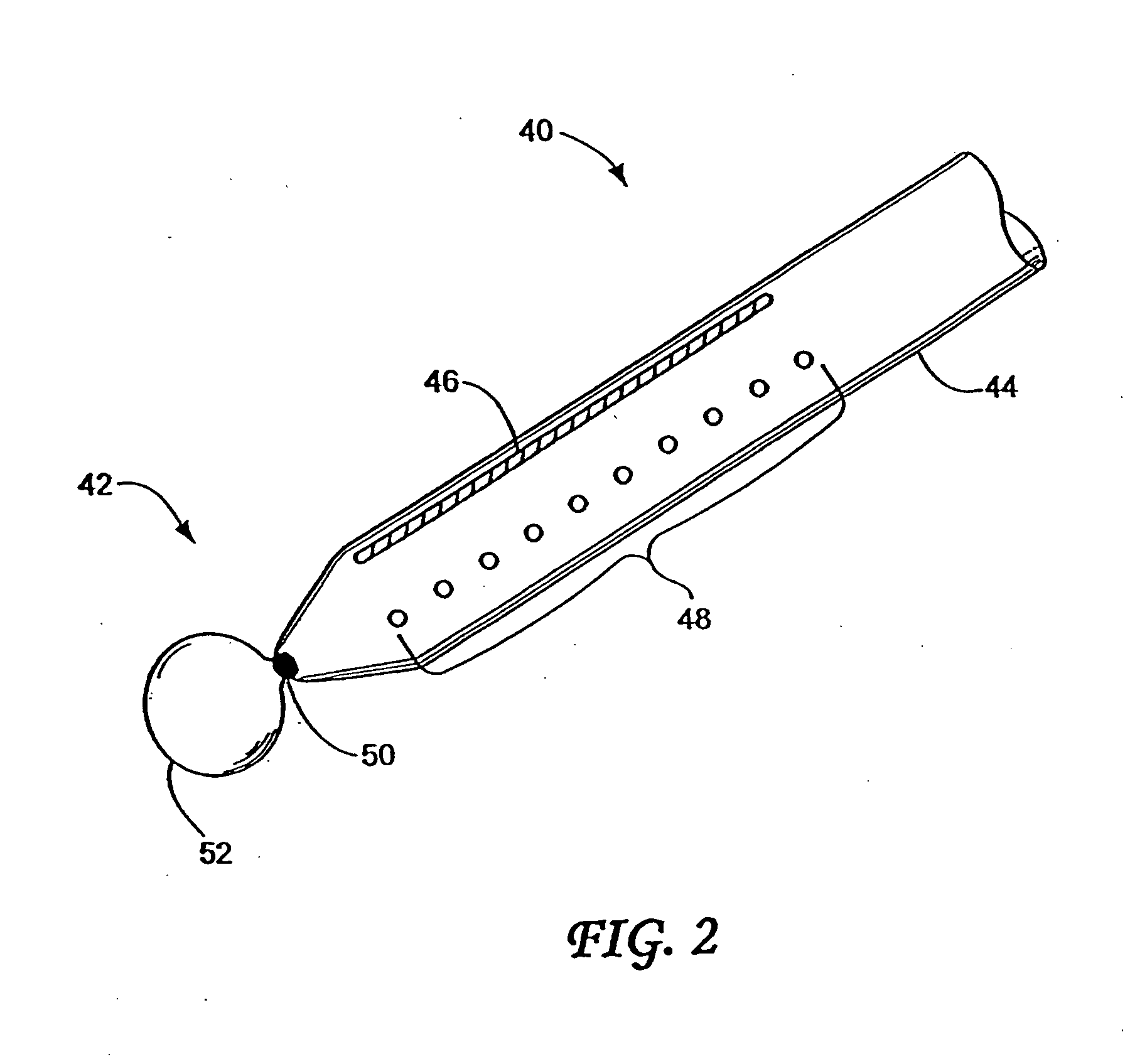
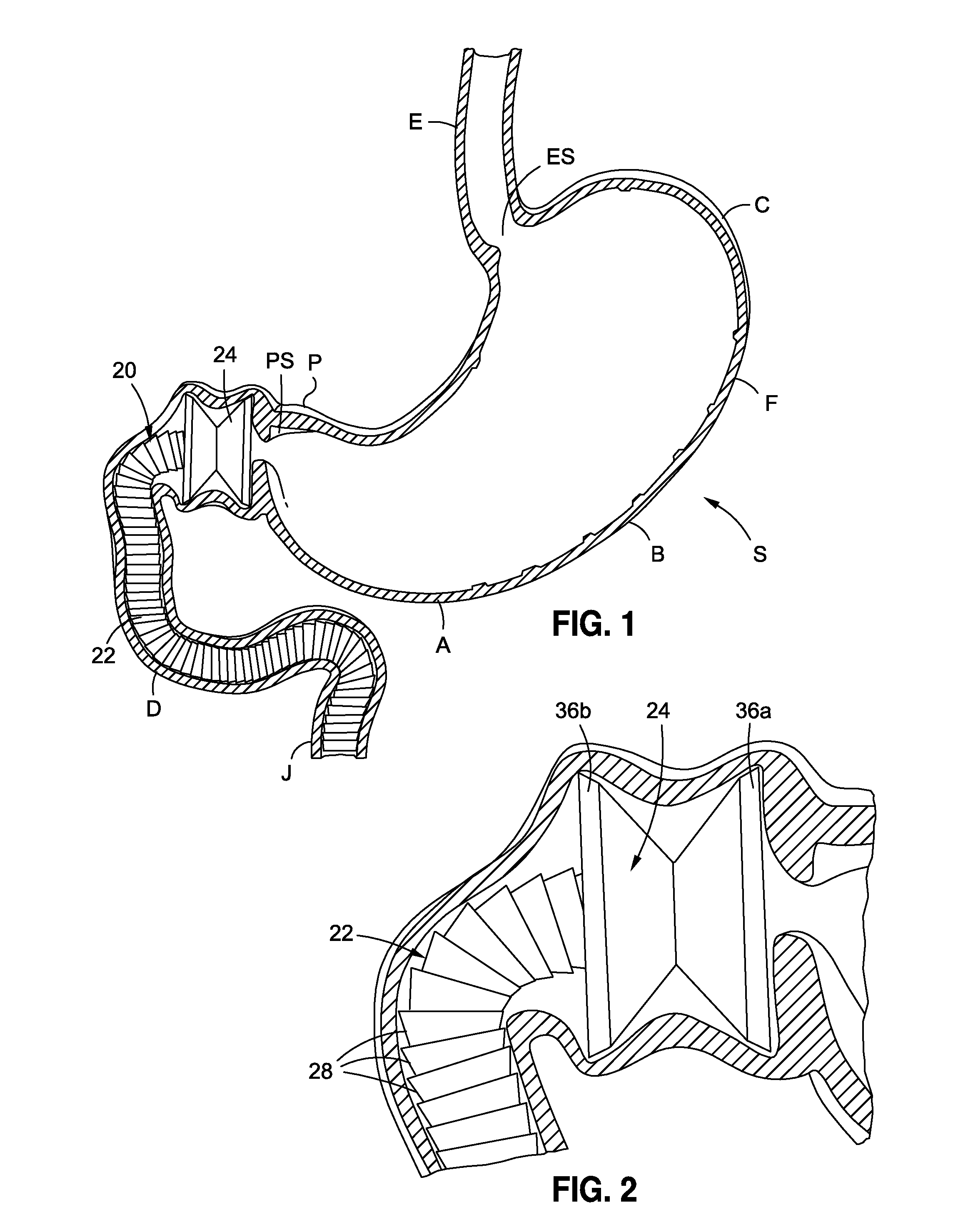
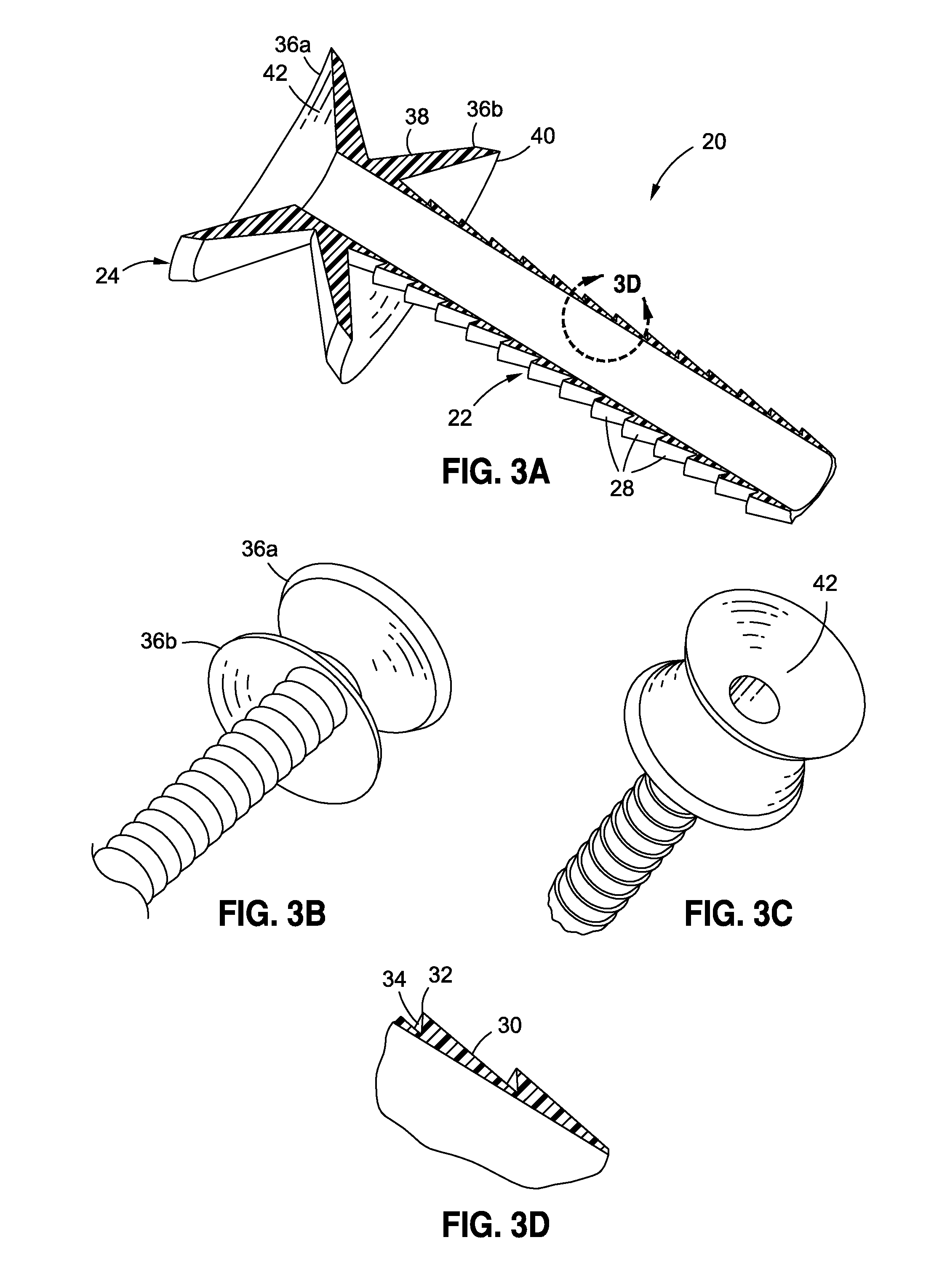
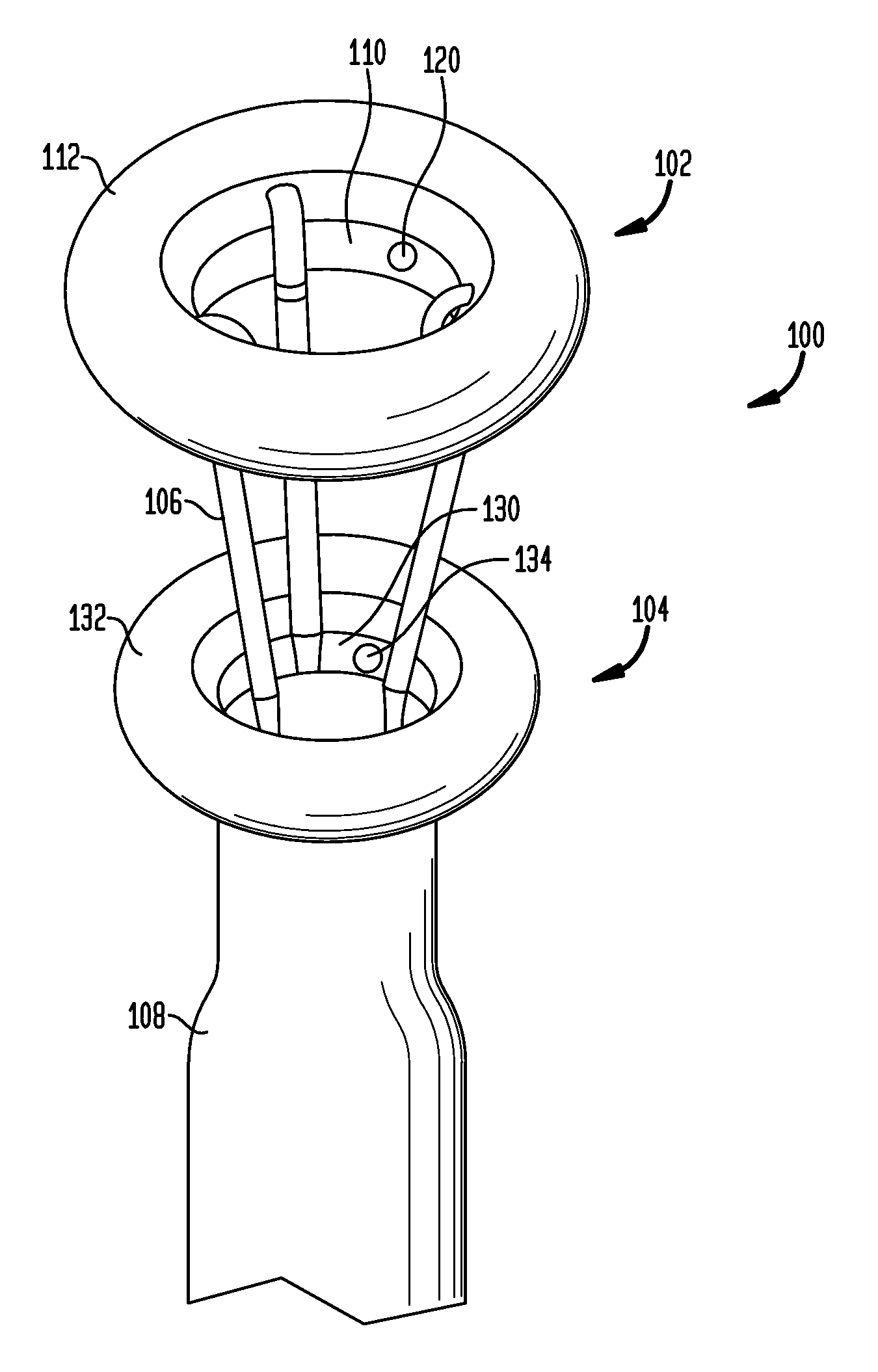
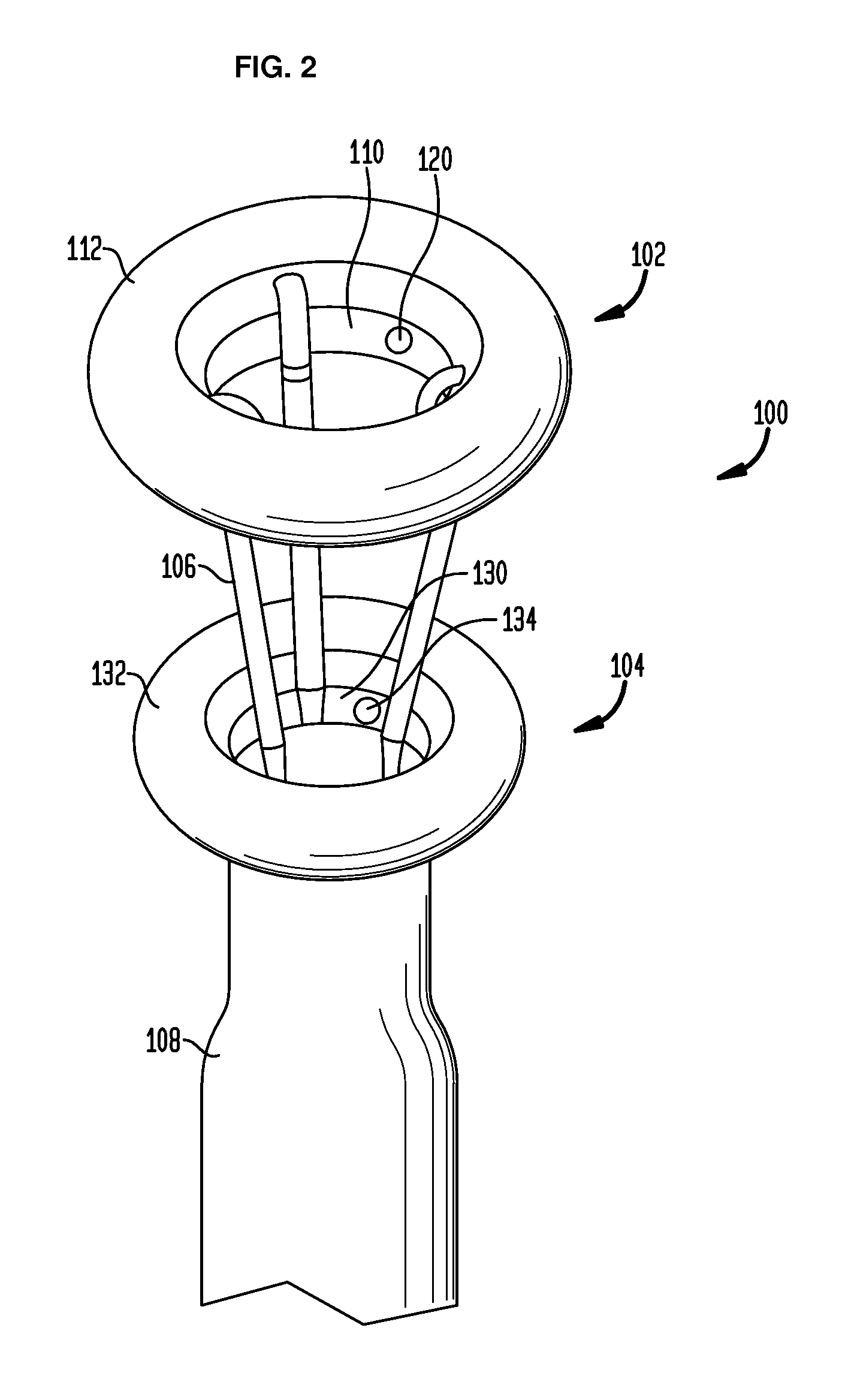
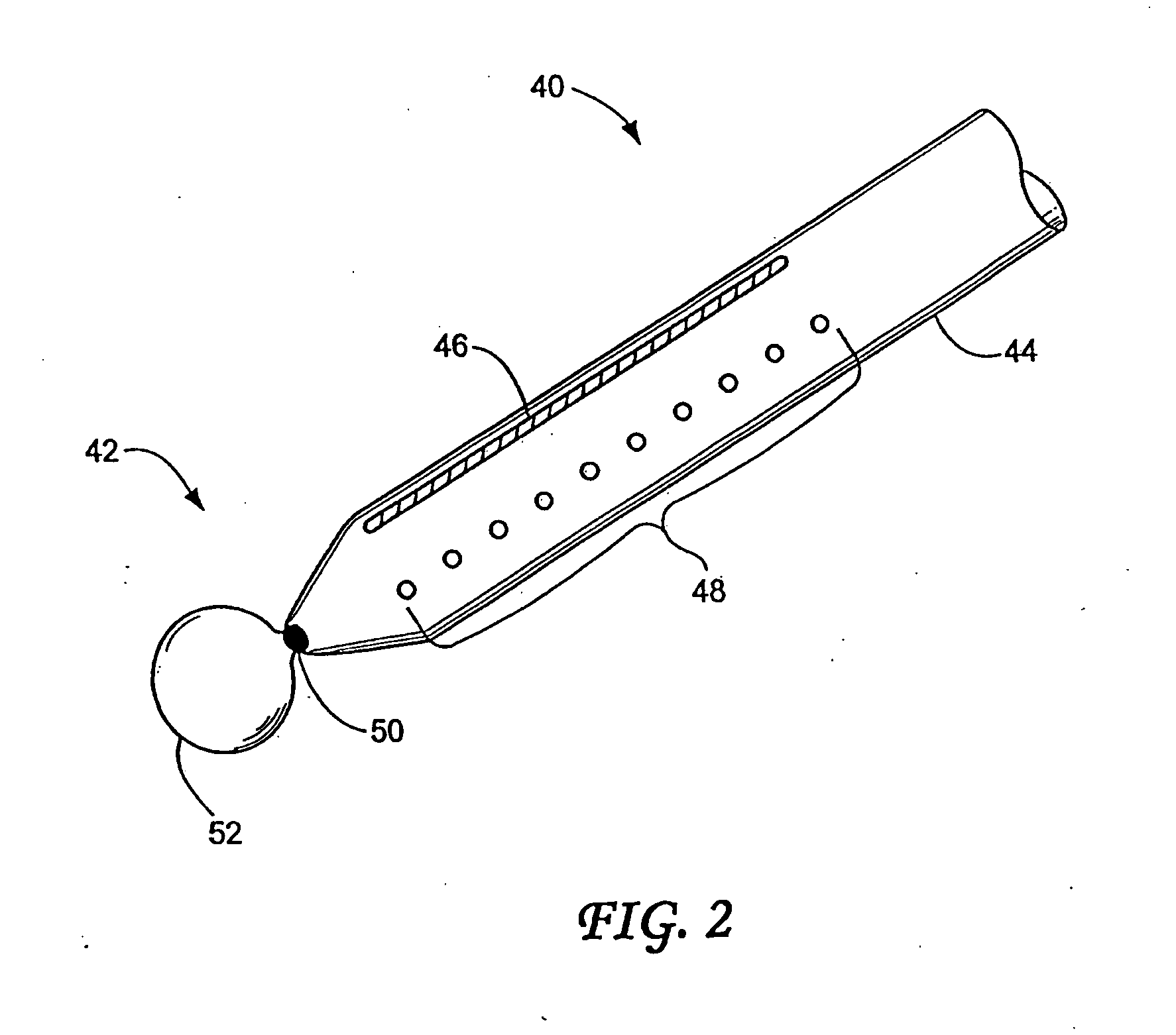
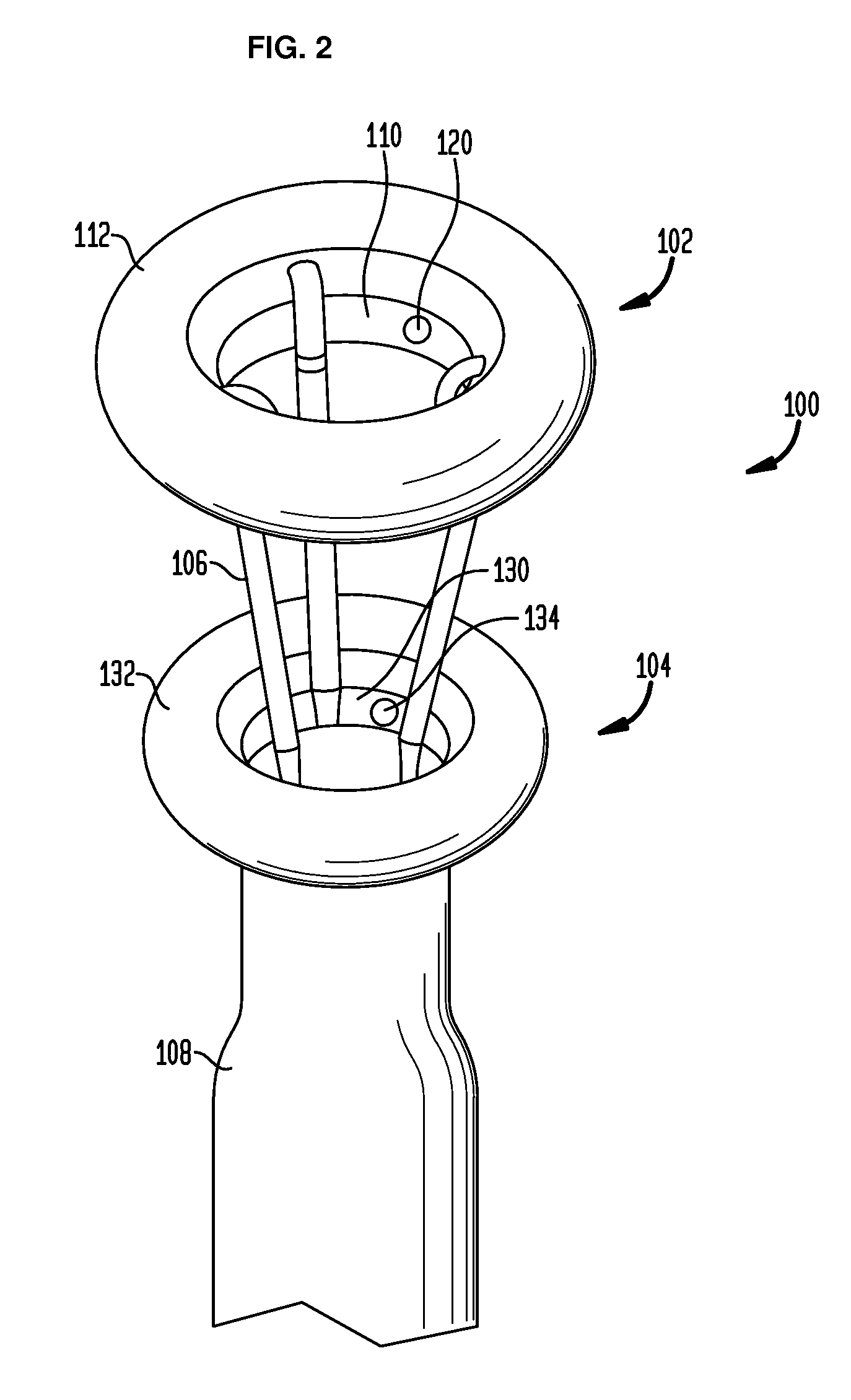
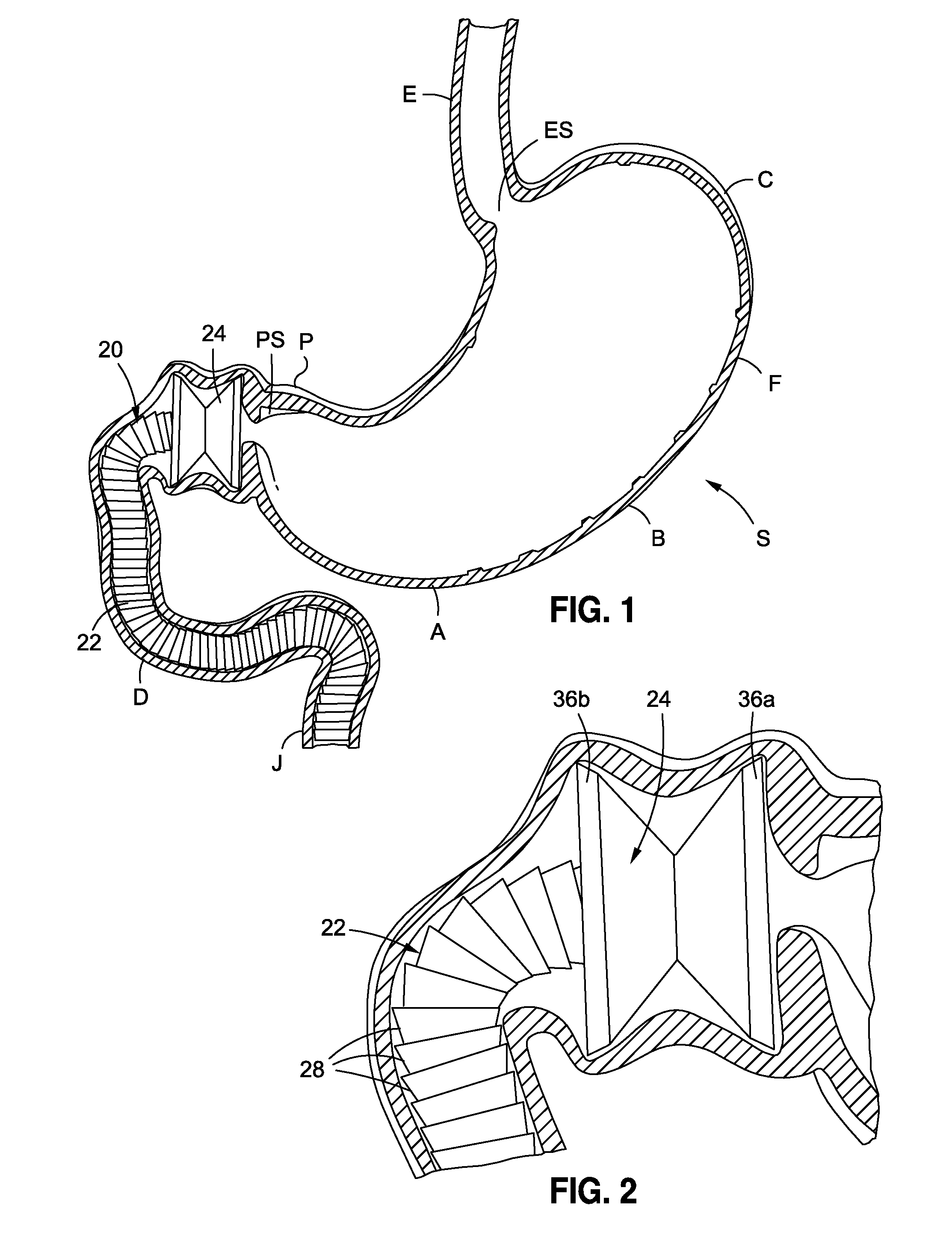
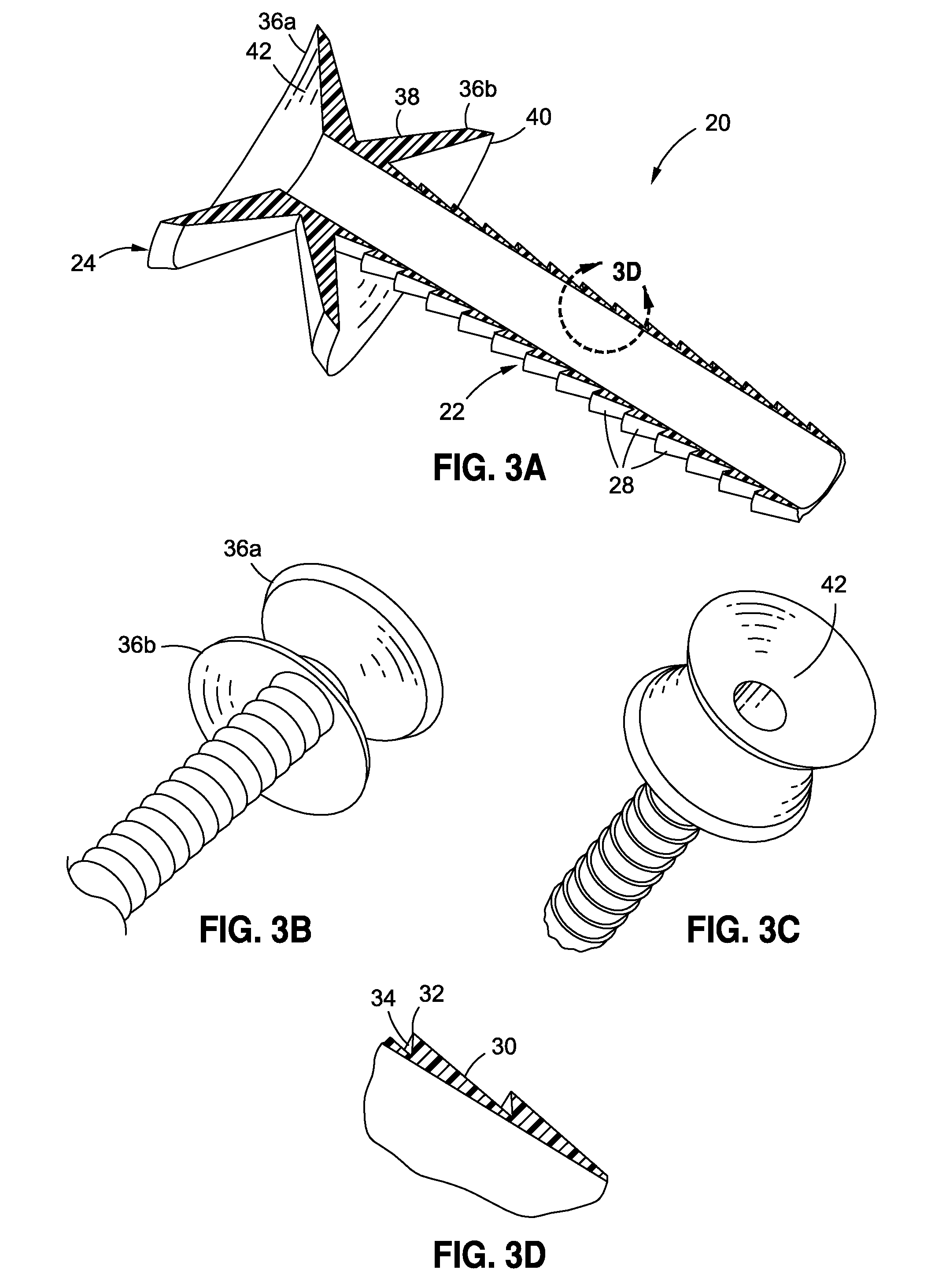
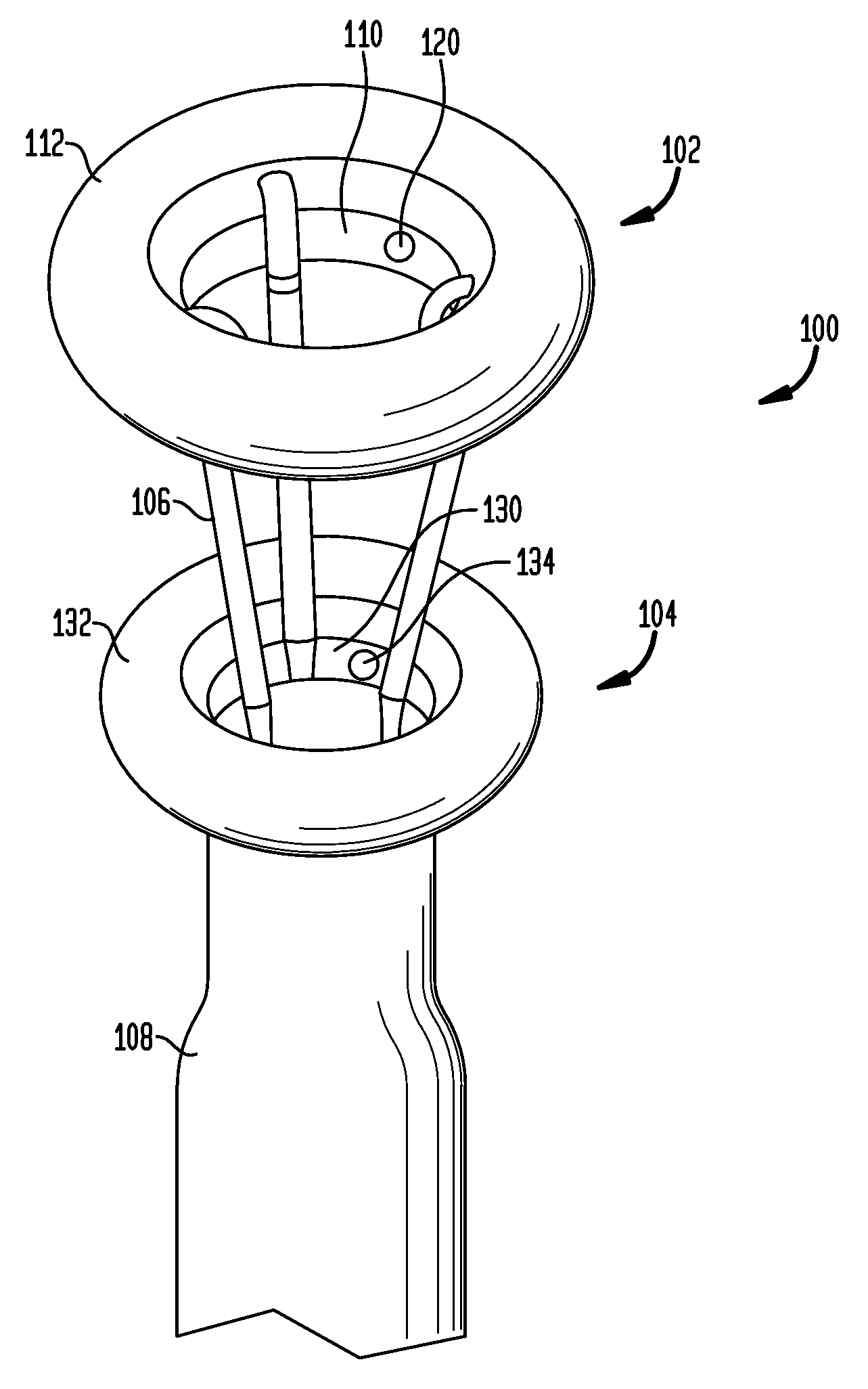
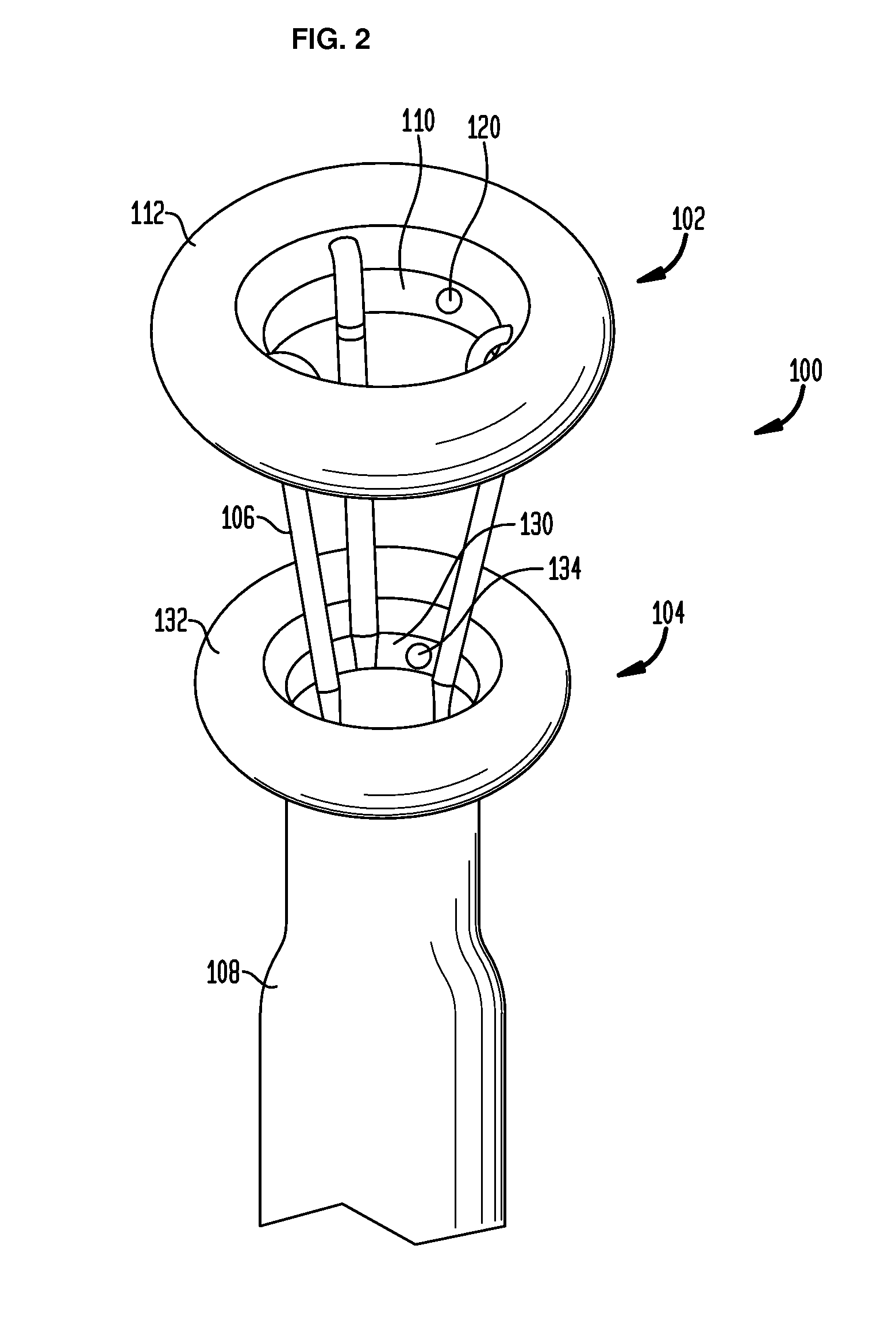
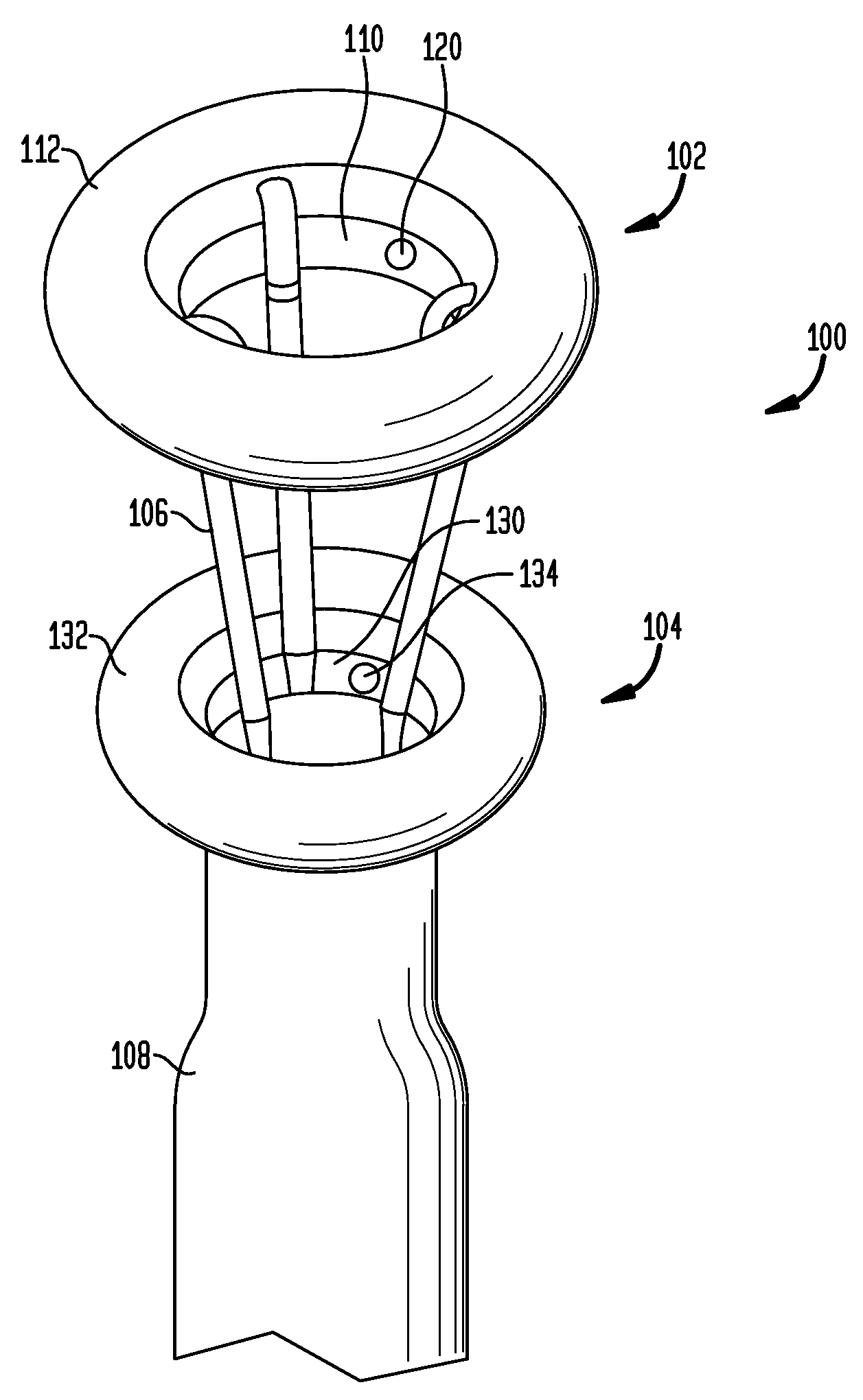
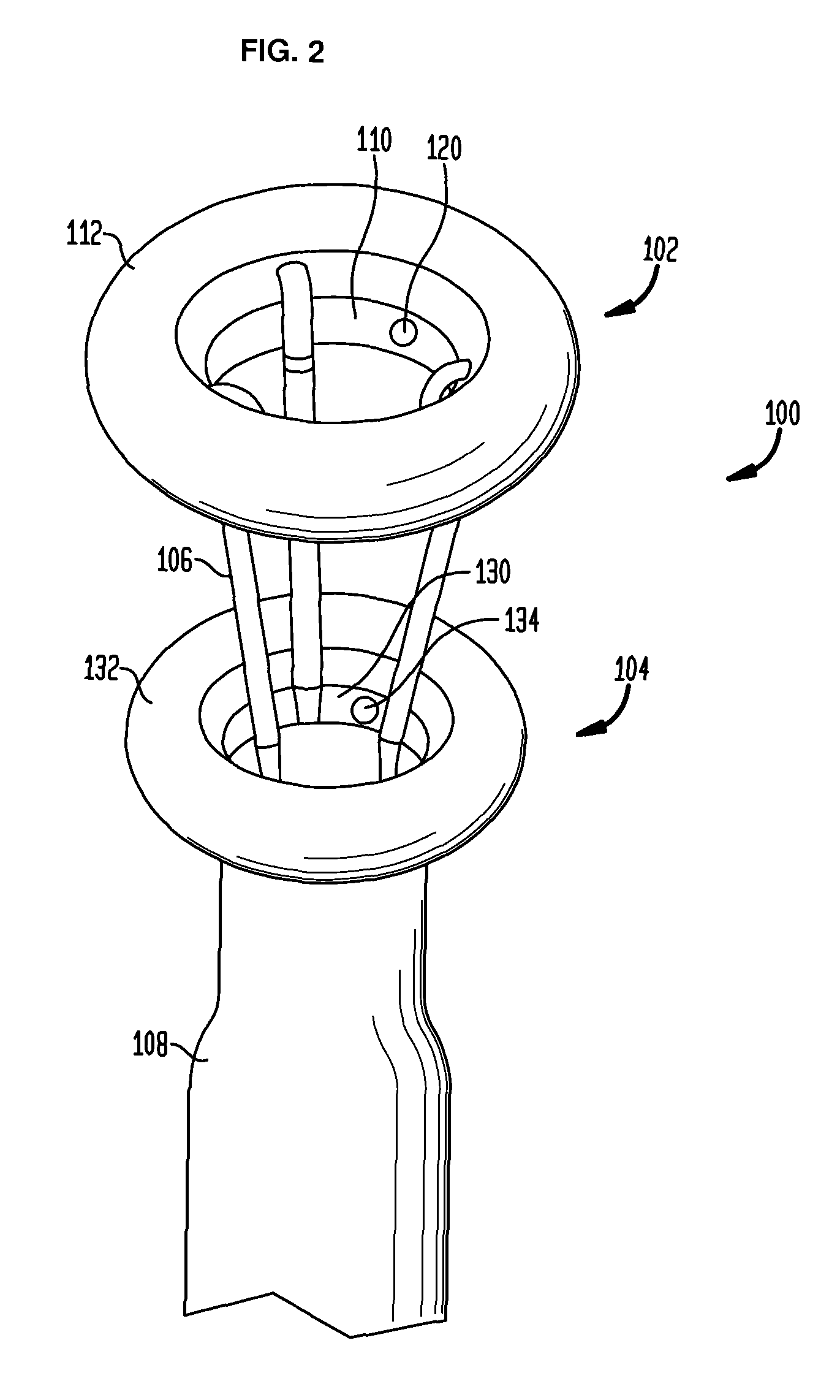
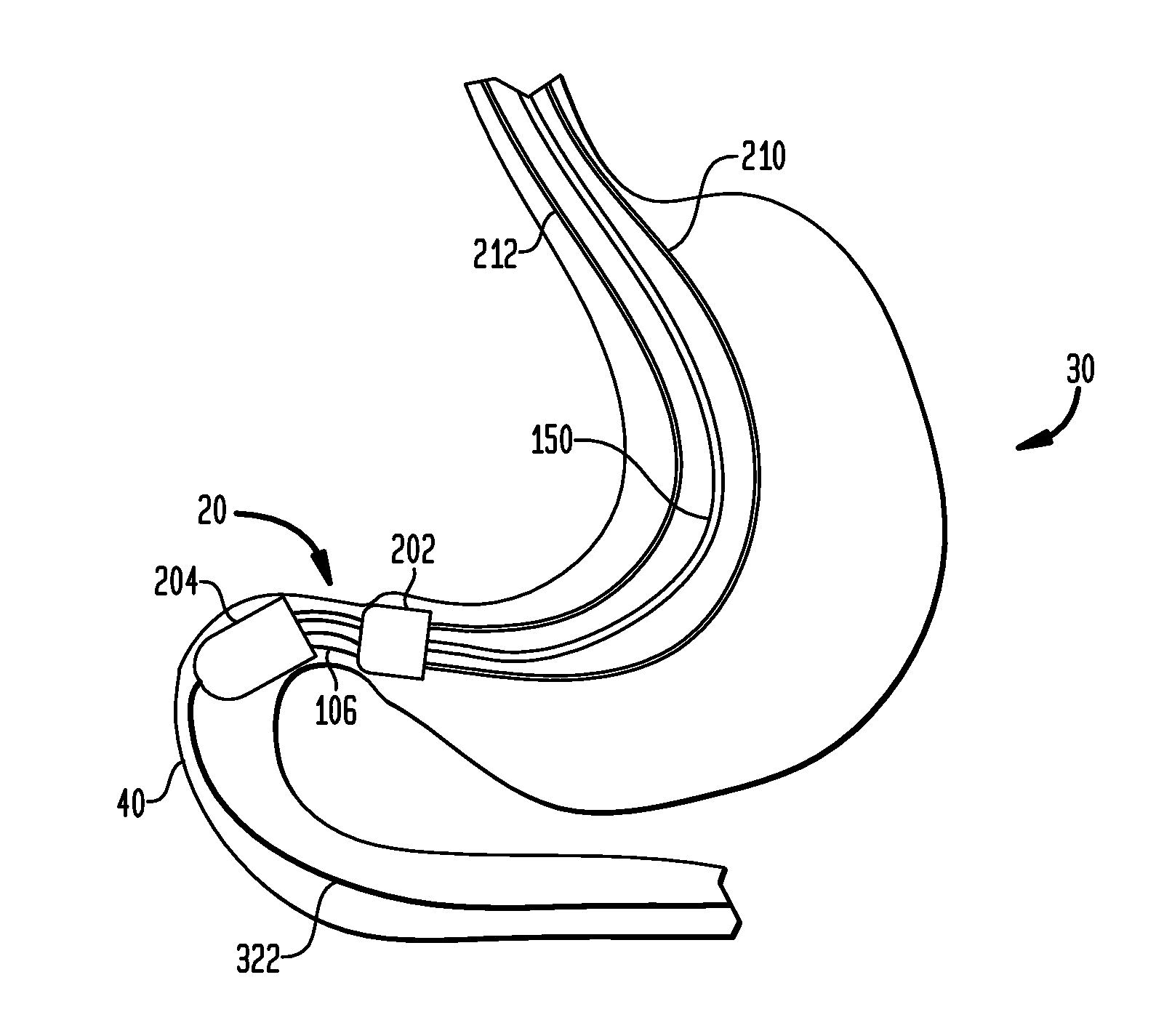
Anchored non-piercing duodenal sleeve and delivery systems
InactiveUS20120095483A1Easy to disassembleSimple structureObesity treatmentWound clampsGastric emptyingPyloric sphincter
An intragastric implant for obesity treatment is disclosed. The device delays digestion by providing a duodenal sleeve, and may also slows gastric emptying by limiting flow through the pyloric sphincter. The implant includes an elongated axially-compressible duodenal sleeve having a non-tissue-piercing anchor on a proximal end sized to lodge within the duodenal bulb. The anchor may have oppositely-directed anchoring flanges to resists migration in both directions. The sleeve may also have barbed ribs to resist proximal movement back up into the stomach. A method of implant includes collapsing / compressing the device and transorally advancing it through the esophagus to be deployed within the duodenum. A dissolvable jacket may constrain the implant for delivery and naturally dissolve upon implant. Removal of the implant may occur in the reverse.
Owner:APOLLO ENDOSURGERY INC
Obesity treatment tools and methods
Various obesity treatment tools and methods are described herein, as well as treatments for other gastric-related diseases, e.g., GERD. Treatment includes reducing the size of the stomach pouch to limit the caloric intake as well as to provide an earlier feeling of satiety. This may be done by creating a smaller gastric pouch within the stomach directly from the interior of the stomach itself. The smaller pouches may be made through the use of individual anchoring devices, rotating probes, or volume reduction devices. A pyloroplasty procedure may also be performed to render the pyloric sphincter incompetent. A gastric bypass procedure may additionally be performed using atraumatic magnetic anastomoses devices so that sugars and fats are passed directly to the bowel while bypassing the stomach. Many of these procedures may be done in a variety of combinations. Treatment may create enforced behavioral modifications by discouraging the ingestion of high-caloric foods.
Owner:ETHICON ENDO SURGERY INC
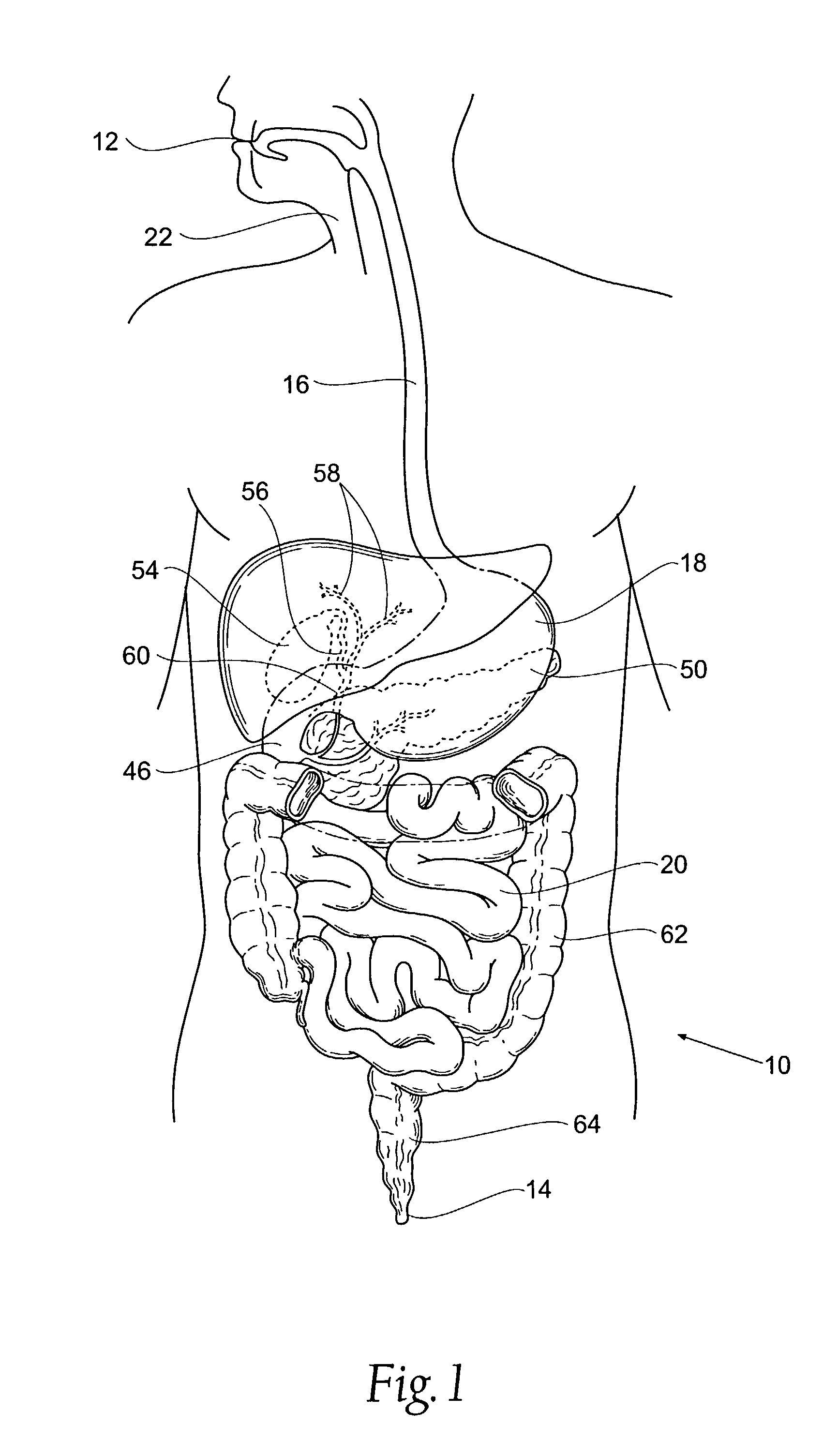
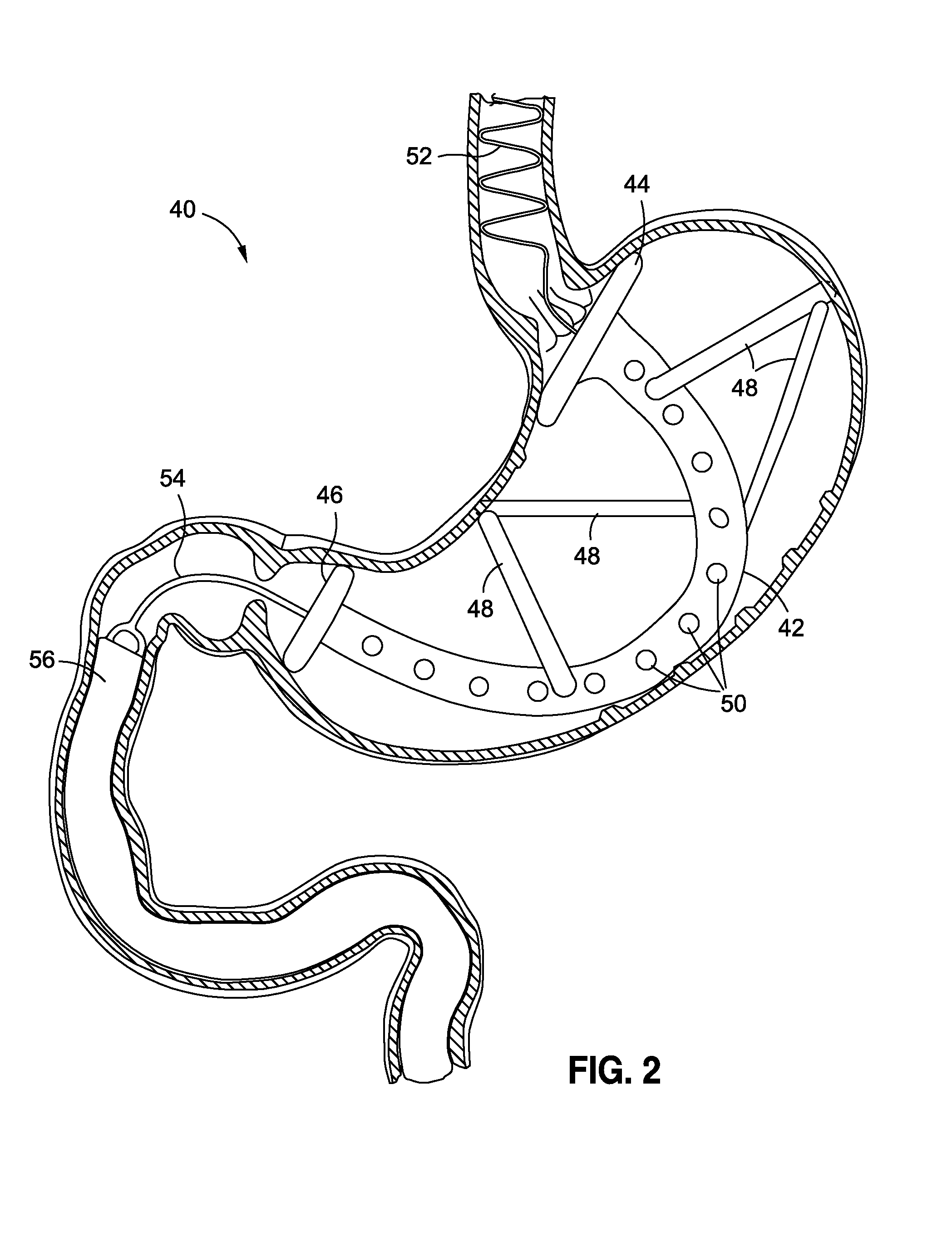
Systems and methods for treatment of obesity and type 2 diabetes
InactiveUS20110004320A1Reducing collateral tissue damageMinimize impactIntravenous devicesStomachPyloric sphincterEndoscopy
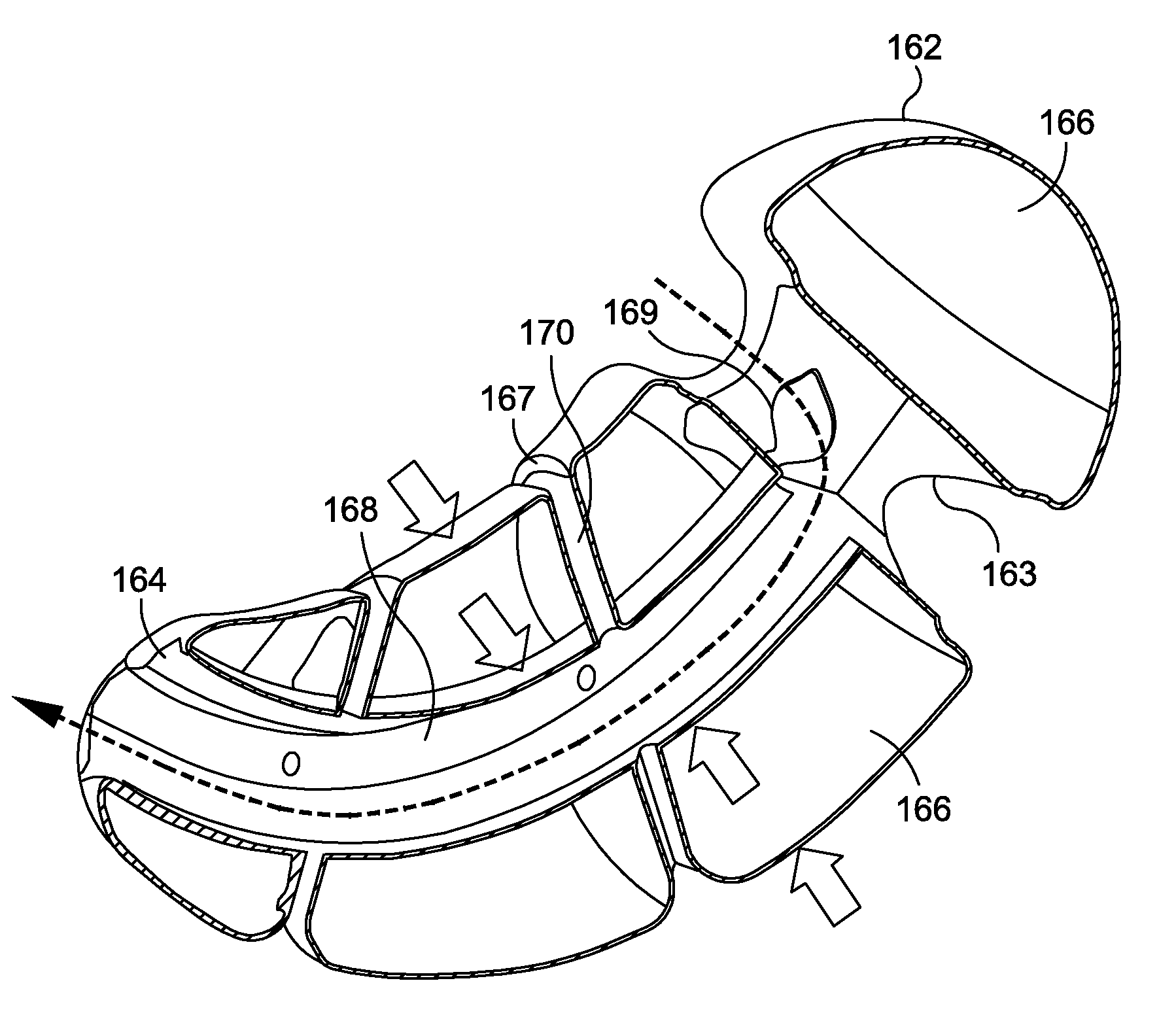
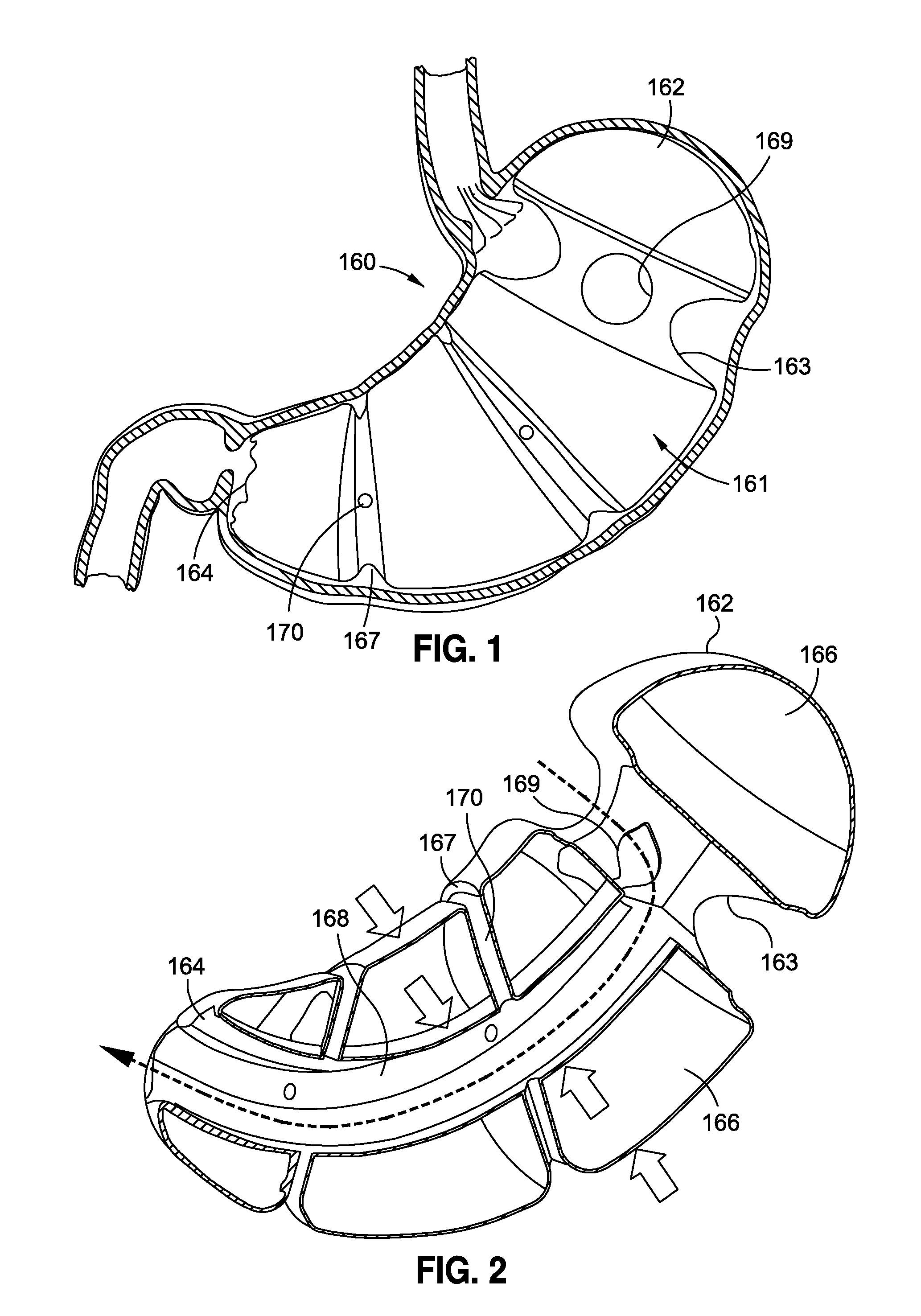
The present invention provides systems and methods for treating and controlling obesity and / or type II diabetes. In one aspect of the invention, an internal bypass device includes gastric and duodenal anchors coupled to each other and positioned on either side of the pylorus and a hollow sleeve designed to extend from the pylorus through at least a proximal portion of a patient's small intestine. The gastric and duodenal anchors are movable between collapsed configurations for advancement through the esophagus and an expanded configuration for inhibiting movement of the anchors through the pyloric sphincter. Thus, the bypass device can be placed and removed endoscopically through the patient's esophagus in a minimally invasive outpatient procedure and it is “self-anchoring” and does not require invasive tissue fixation within the patient's GI tract, thereby reducing collateral tissue damage and minimizing its impact on the digestive process.
Owner:E2 LLC DENTONS
Obesity treatment tools and methods
Various obesity treatment tools and methods are described herein, as well as treatments for other gastric-related diseases, e.g., GERD. Treatment includes reducing the size of the stomach pouch to limit the caloric intake as well as to provide an earlier feeling of satiety. This may be done by creating a smaller gastric pouch within the stomach directly from the interior of the stomach itself. The smaller pouches may be made through the use of individual anchoring devices, rotating probes, or volume reduction devices. A pyloroplasty procedure may also be performed to render the pyloric sphincter incompetent. A gastric bypass procedure may additionally be performed using atraumatic magnetic anastomoses devices so that sugars and fats are passed directly to the bowel while bypassing the stomach. Many of these procedures may be done in a variety of combinations. Treatment may create enforced behavioral modifications by discouraging the ingestion of high-caloric foods
Owner:ETHICON ENDO SURGERY INC
Systems and methods for treating obesity and other gastrointestinal conditions
InactiveUS20090118699A1Reduce morbidityIncrease satietyElectrotherapyPeptide/protein ingredientsReflexREFLEX DECREASE
Owner:RESPIRATORY DIAGNOSTICS
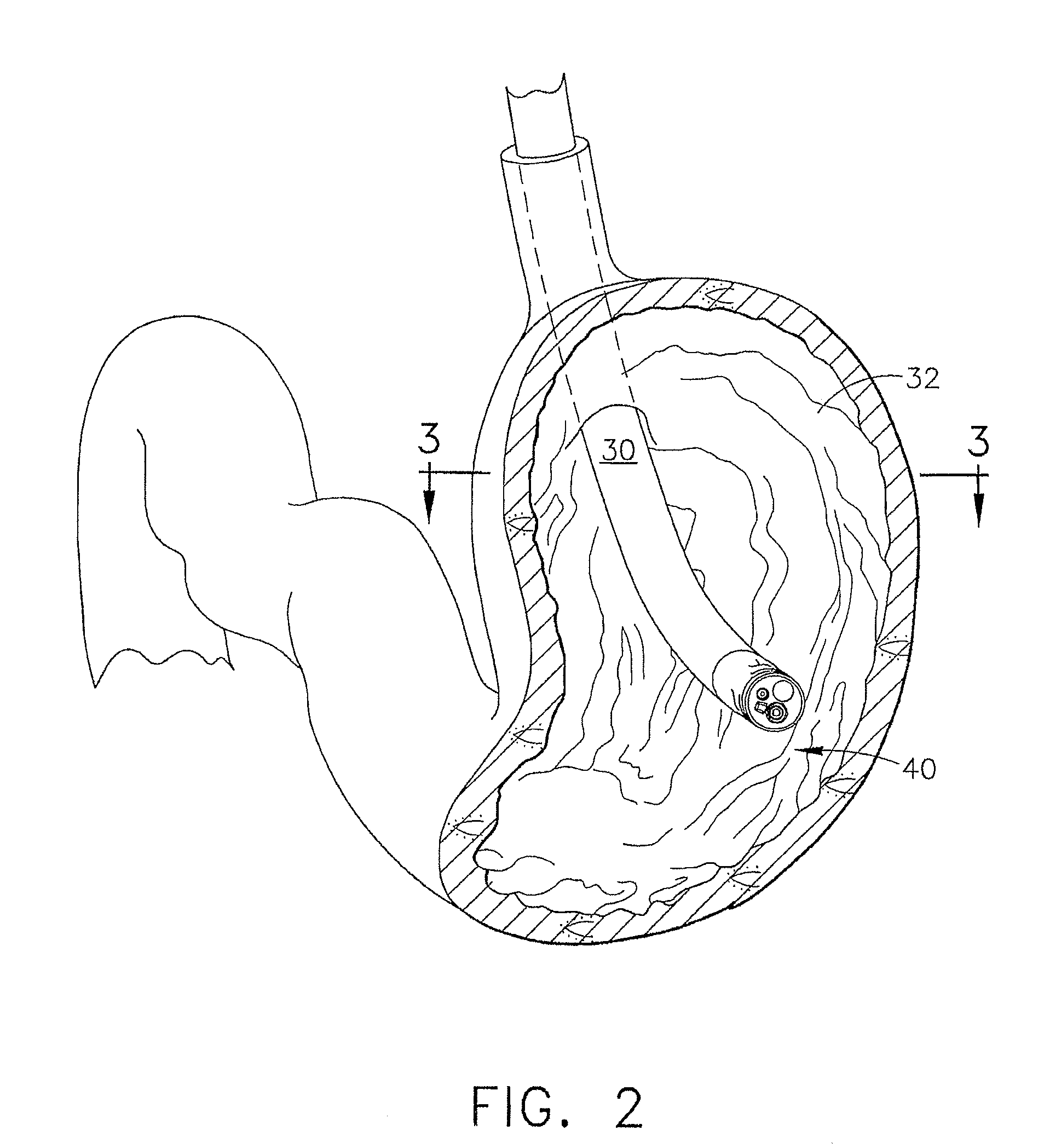
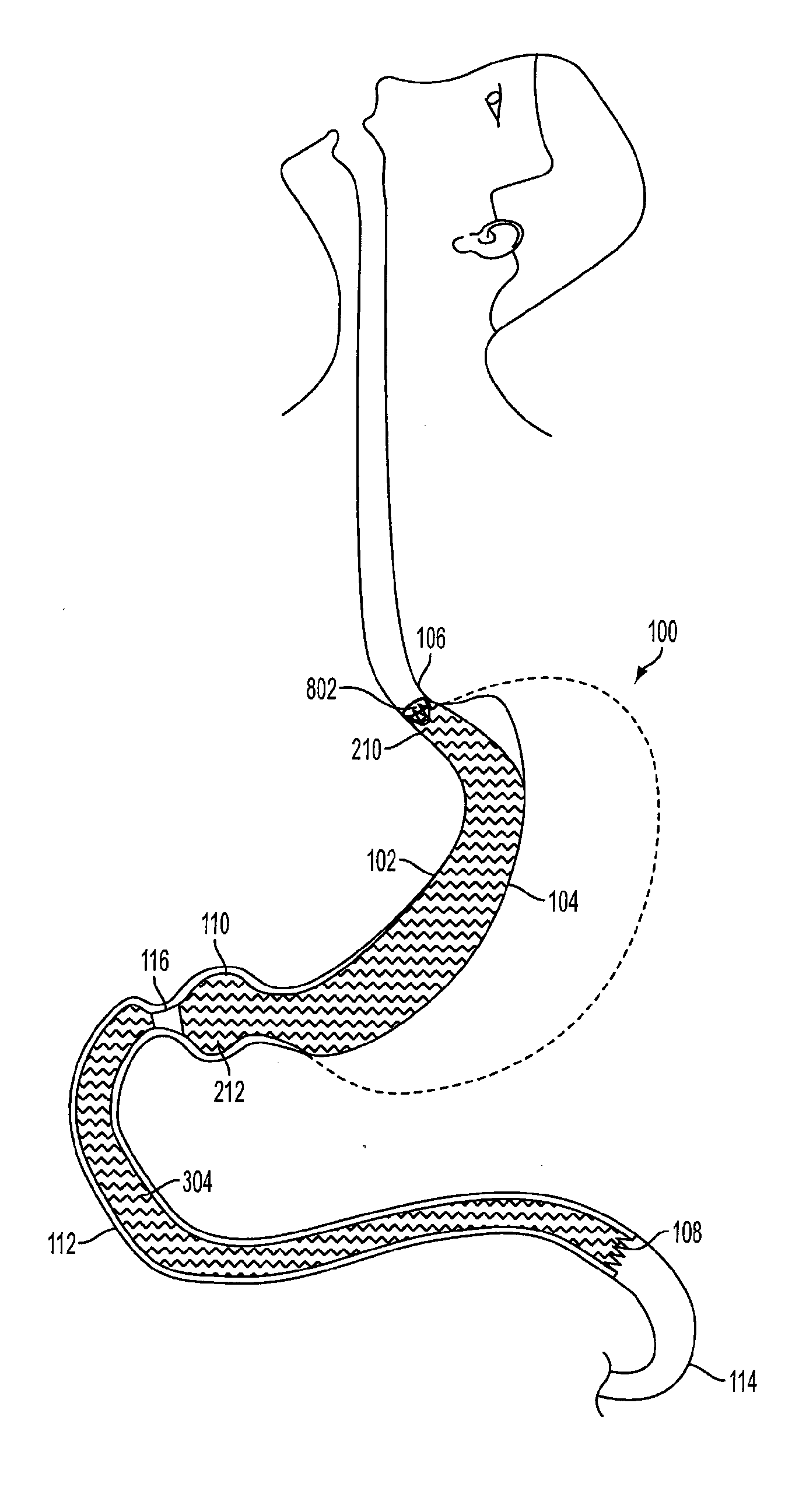
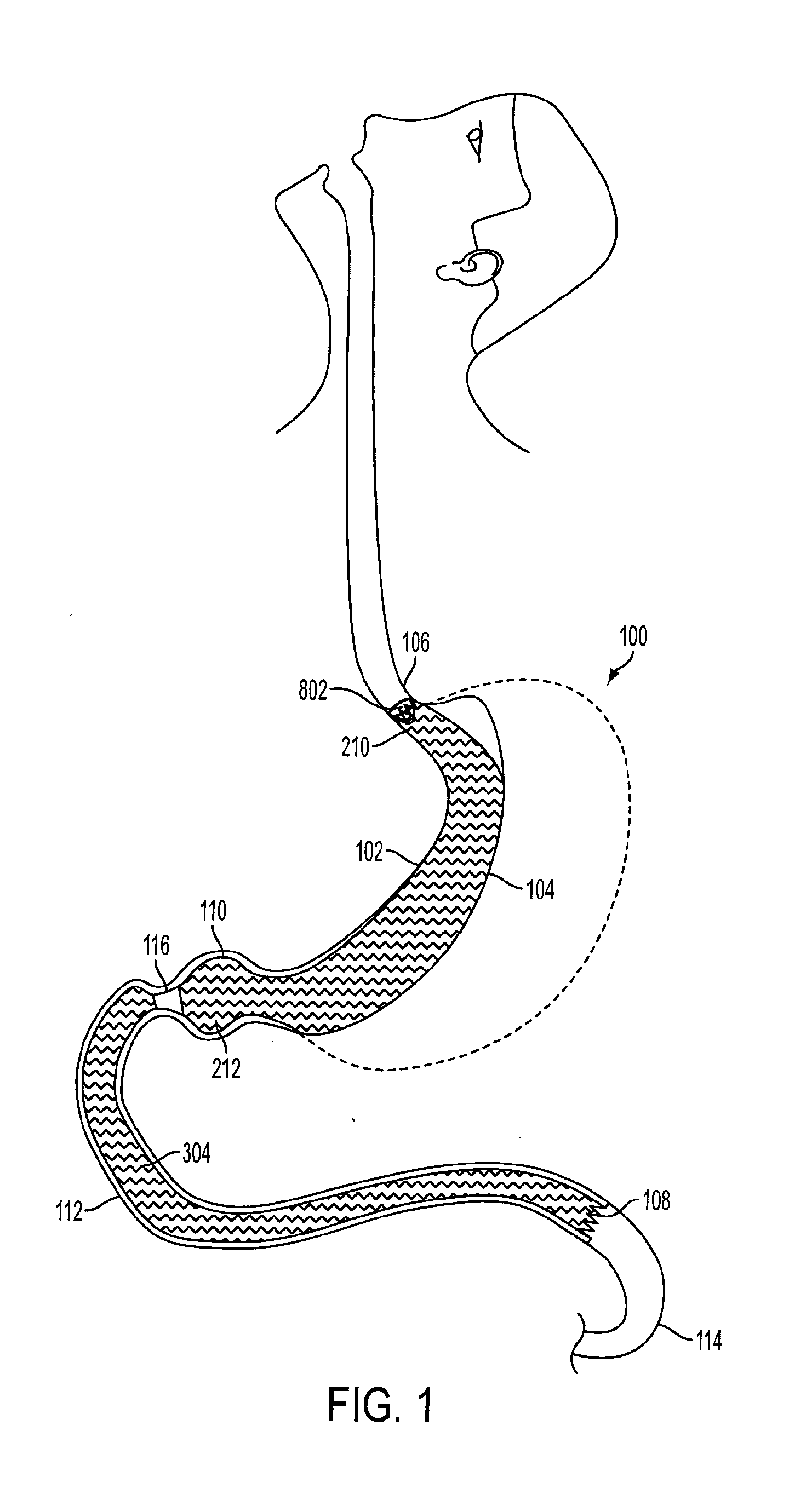
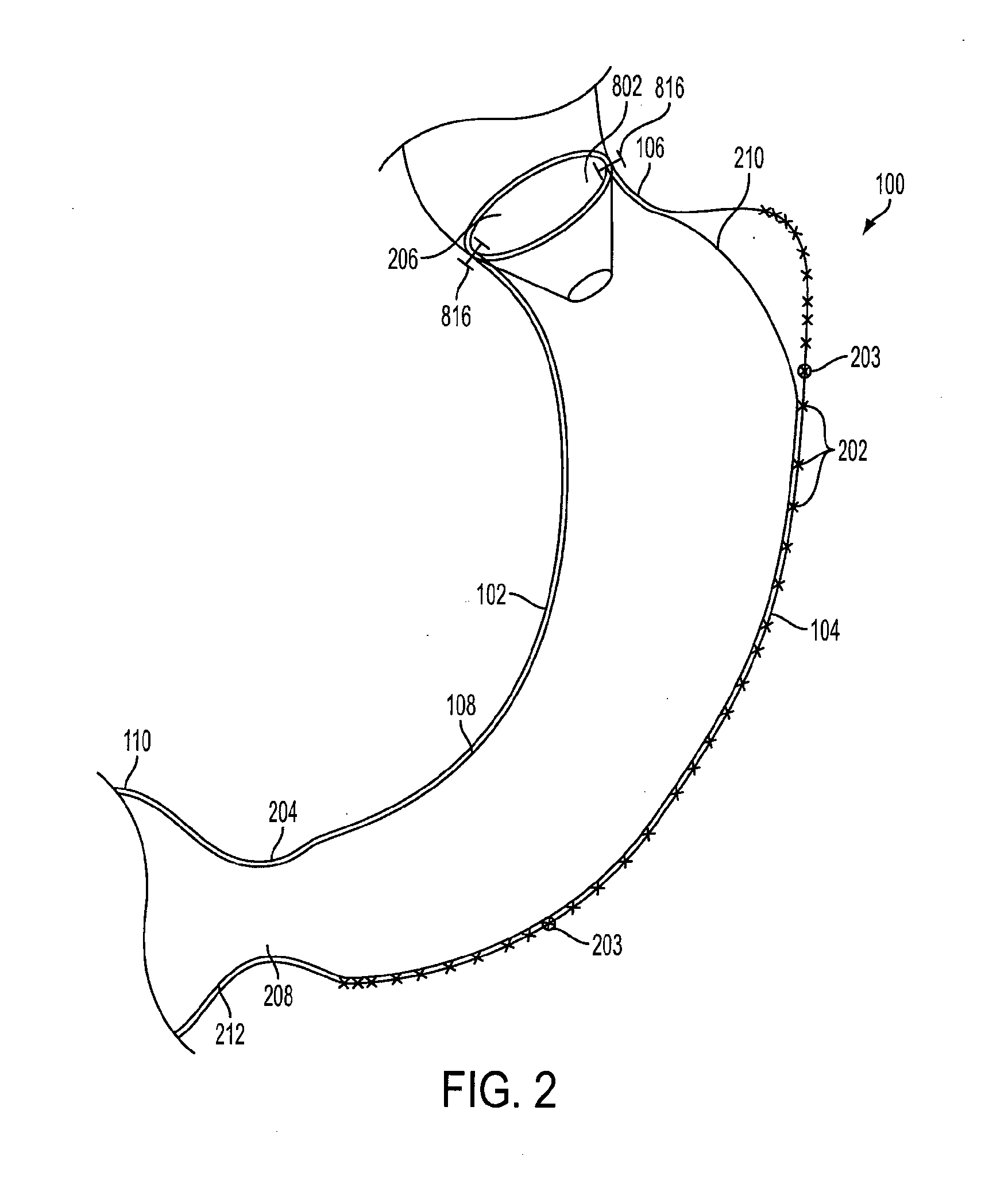
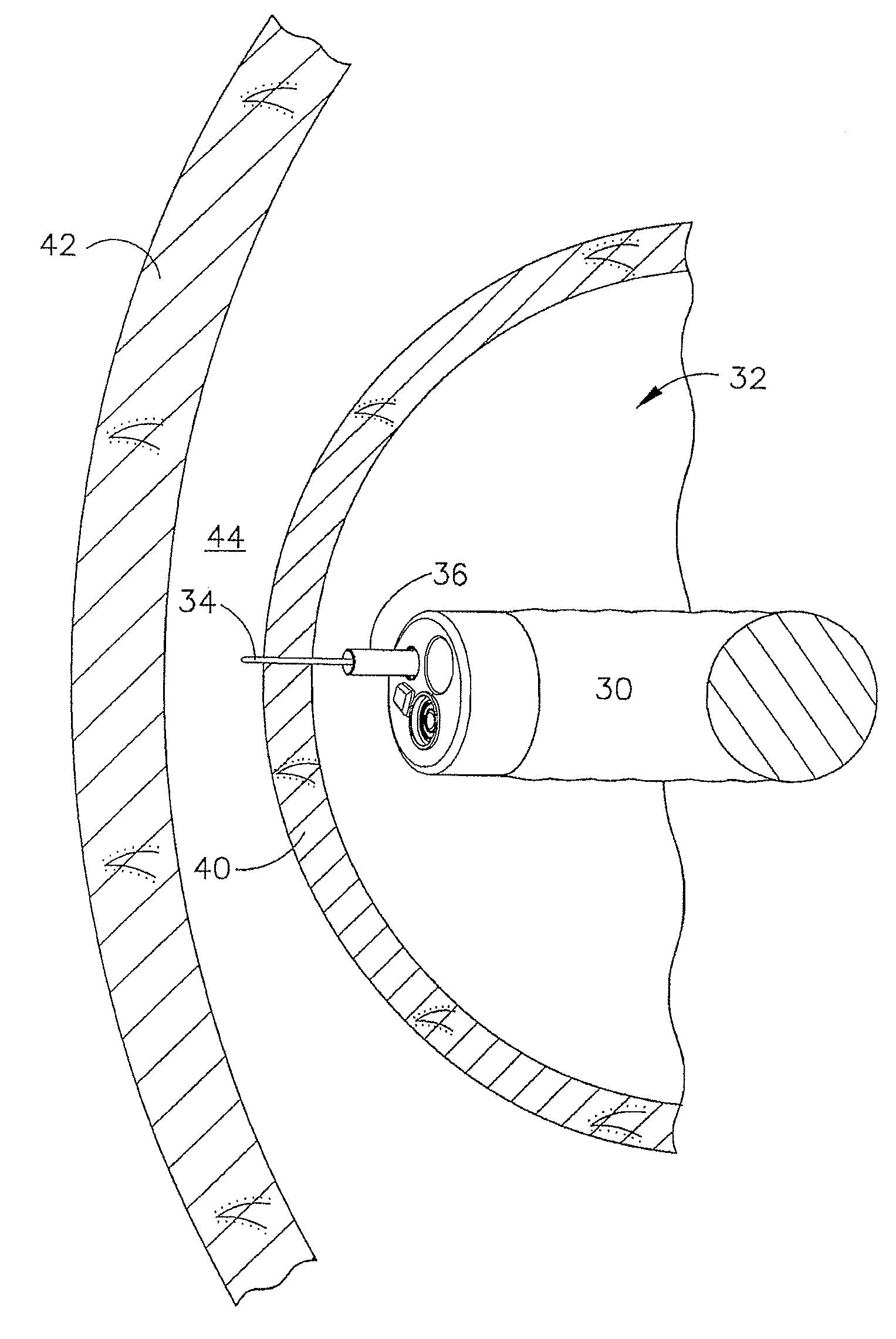
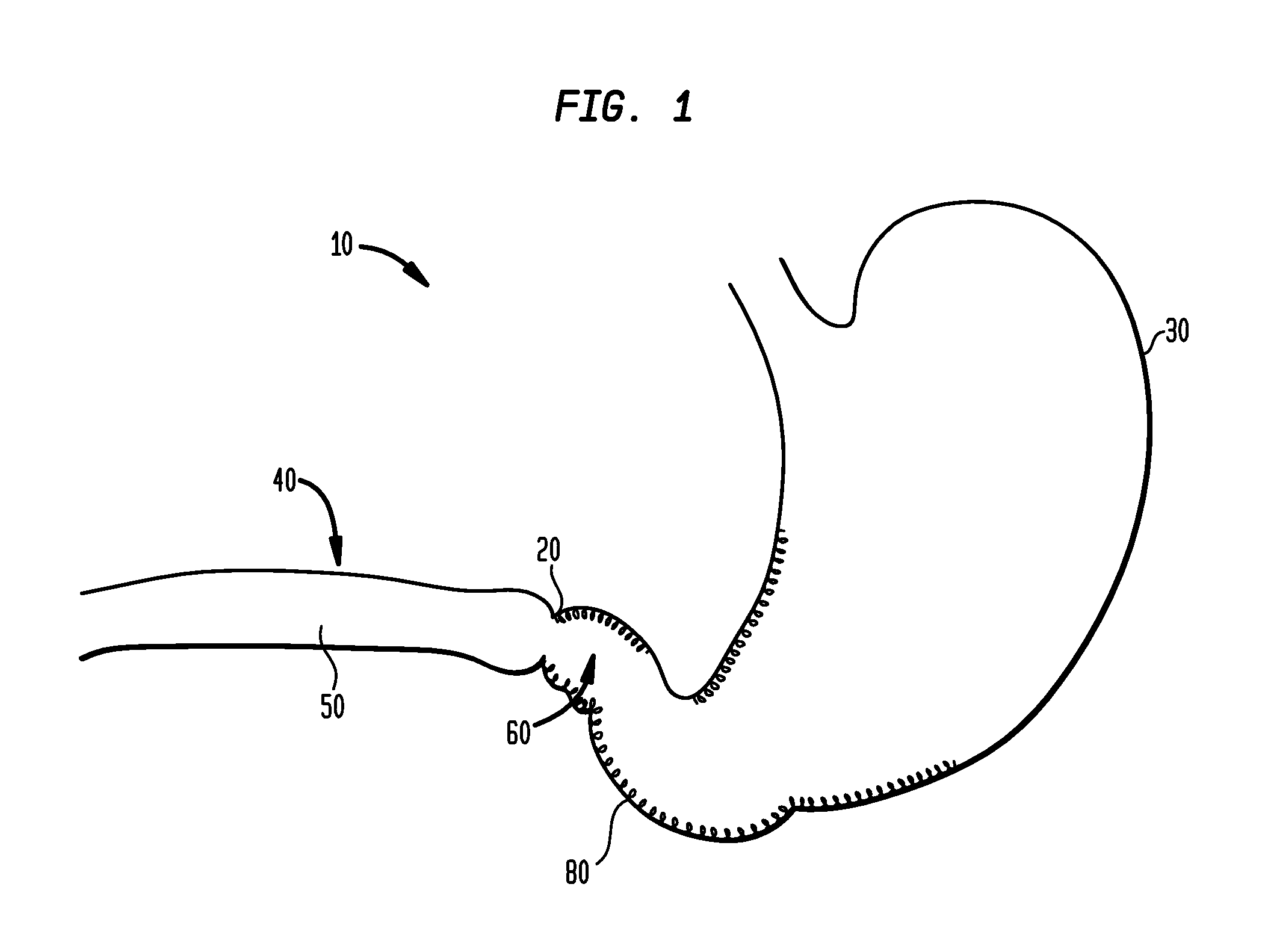
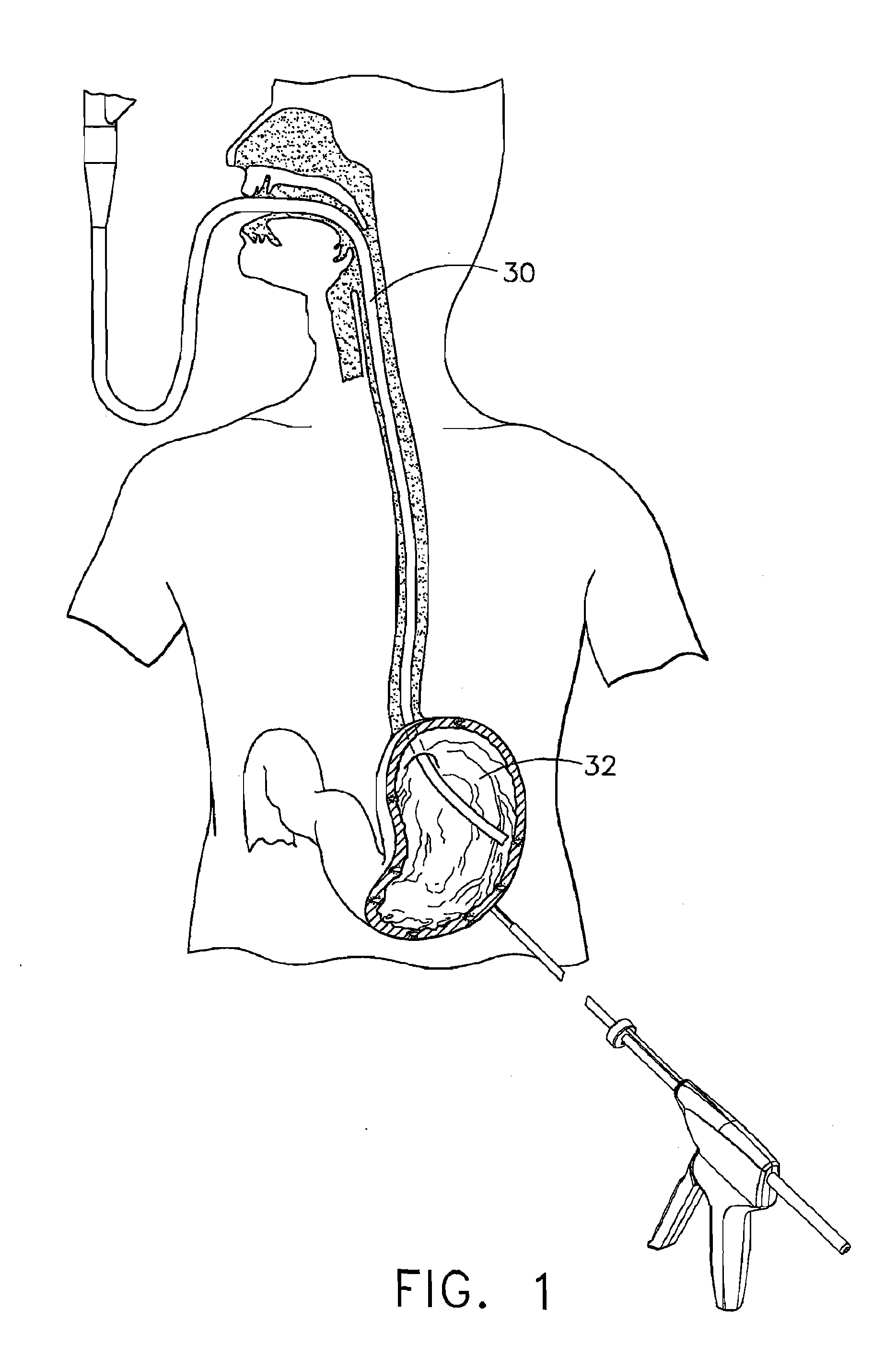
Device for insufflating the interior of a gastric cavity of a patient
InactiveUS20090023984A1Preventing insufflationSuture equipmentsGastroscopesGastric cavityPERITONEOSCOPE
A method for laparoscopically preventing insufflation of the small bowel during gastric procedures includes applying an obstruction member at the pyloric sphincter to block the passage of gas from the gastric cavity into the small bowel and insufflating the gastric cavity.
Owner:ETHICON ENDO SURGERY INC
Obesity controlling method
InactiveUS20100204673A1Minimally invasiveOrganic active ingredientsMetabolism disorderMuscle tissueSatiations
A method of controlling obesity by injecting bio-compatible bulking material into the pyloric sphincter area of the stomach. The injection of this material bulks the pyloric sphincter, retarding stomach emptying and producing a feeling of satiation in the patient. The method may be supplemental and augmented by inducing flacid paralysis of the stomach by injecting botulinum toxin into the muscle tissue of the antrum or fundus of the stomach.
Owner:TEMPLE UNIV
Systems and Methods for Treating Obesity and Type 2 Diabetes
InactiveUS20110004228A1Reducing collateral tissue damageMinimize impactSurgical needlesWound drainsPyloric sphincterProlongation
The present invention provides systems and methods for treating and / or controlling obesity and type II diabetes. In one aspect of the invention, a device for treating obesity includes a flow restrictor and an anchor coupled to the flow restrictor. The flow restrictor is movable between a first or collapsed configuration sized and shaped for endoscopic advancement through the patient's esophagus and into a distal region of the stomach and a second or operative configuration sized and shaped for inhibiting a flow of chyme from the stomach to the pyloric sphincter. It is believed that this will cause the prolongation of satiety, and result in fewer meals being eaten and / or smaller meals being ingested. The anchor is movable between a first or collapsed configuration sized and shaped for advancement through the esophagus, stomach and pyloric sphincter and into the proximal region of the duodenum and a second or operative configuration sized and shaped to inhibit proximal movement of the anchor through the pyloric sphincter.
Owner:E2 LLC DENTONS
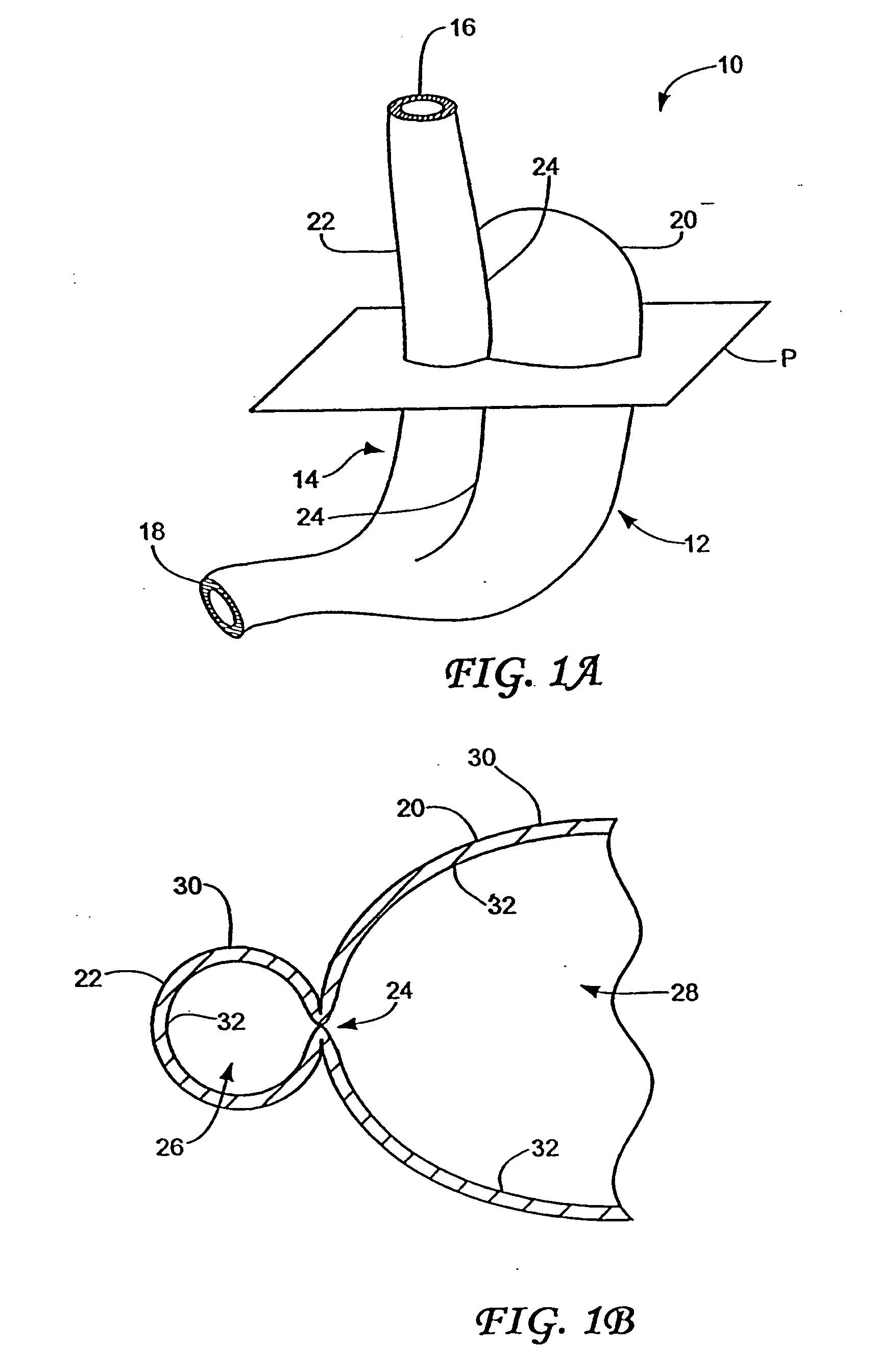
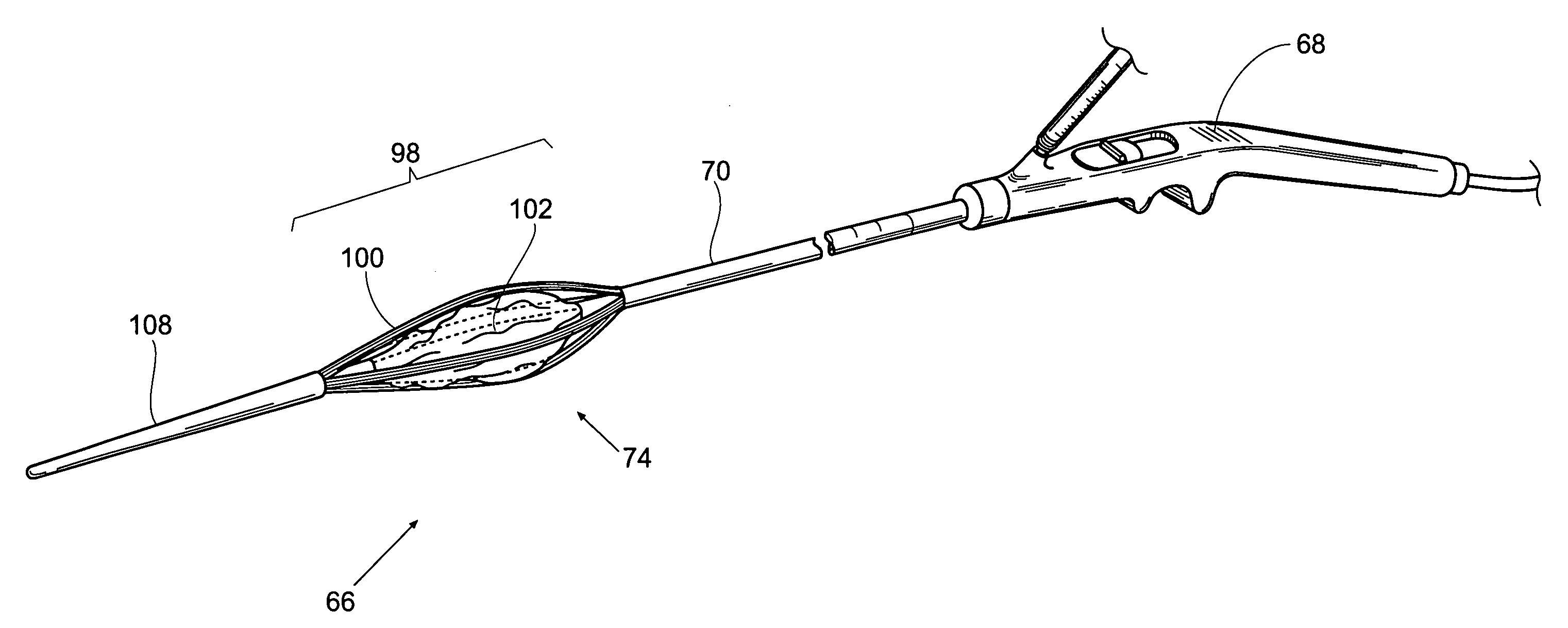
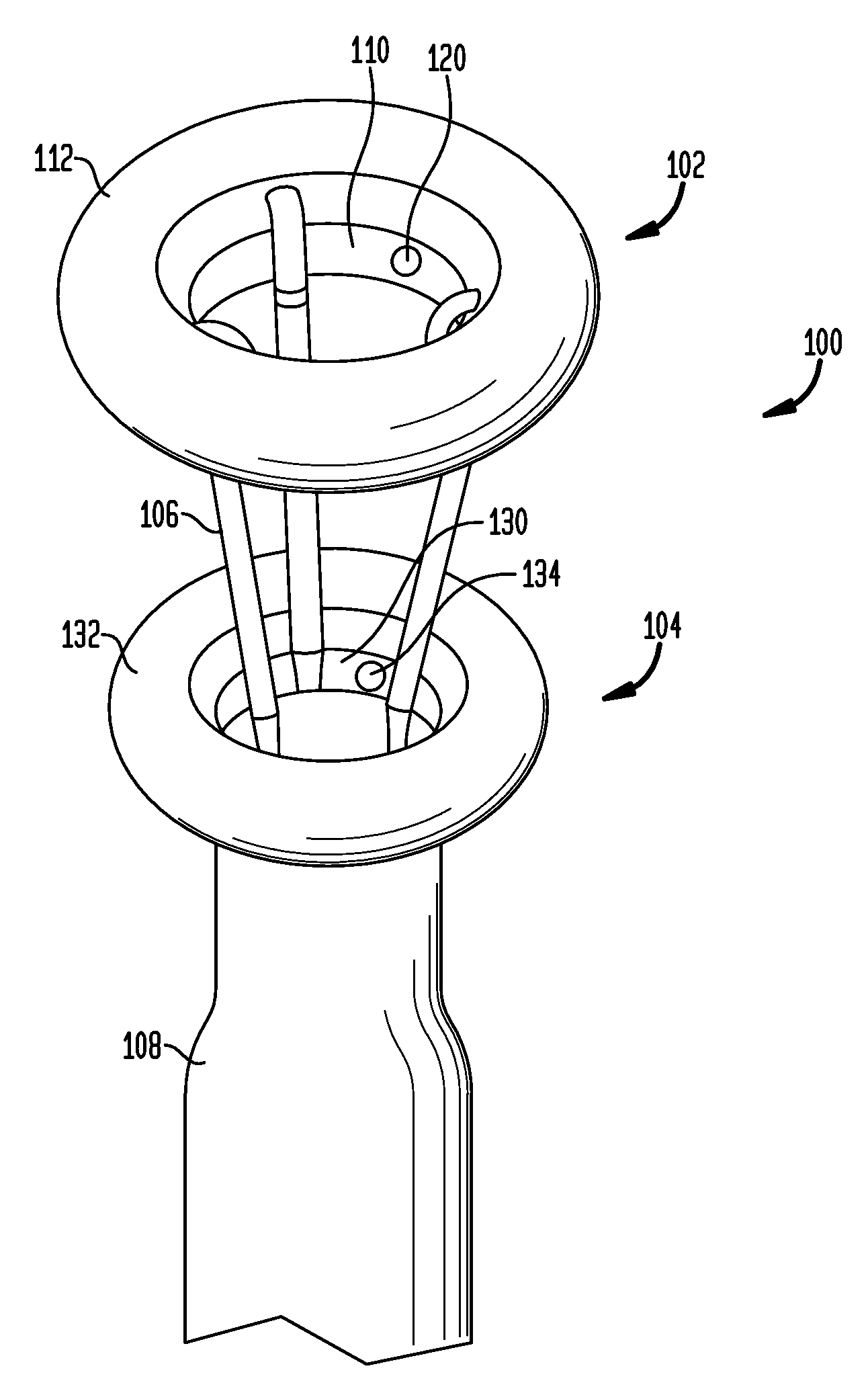
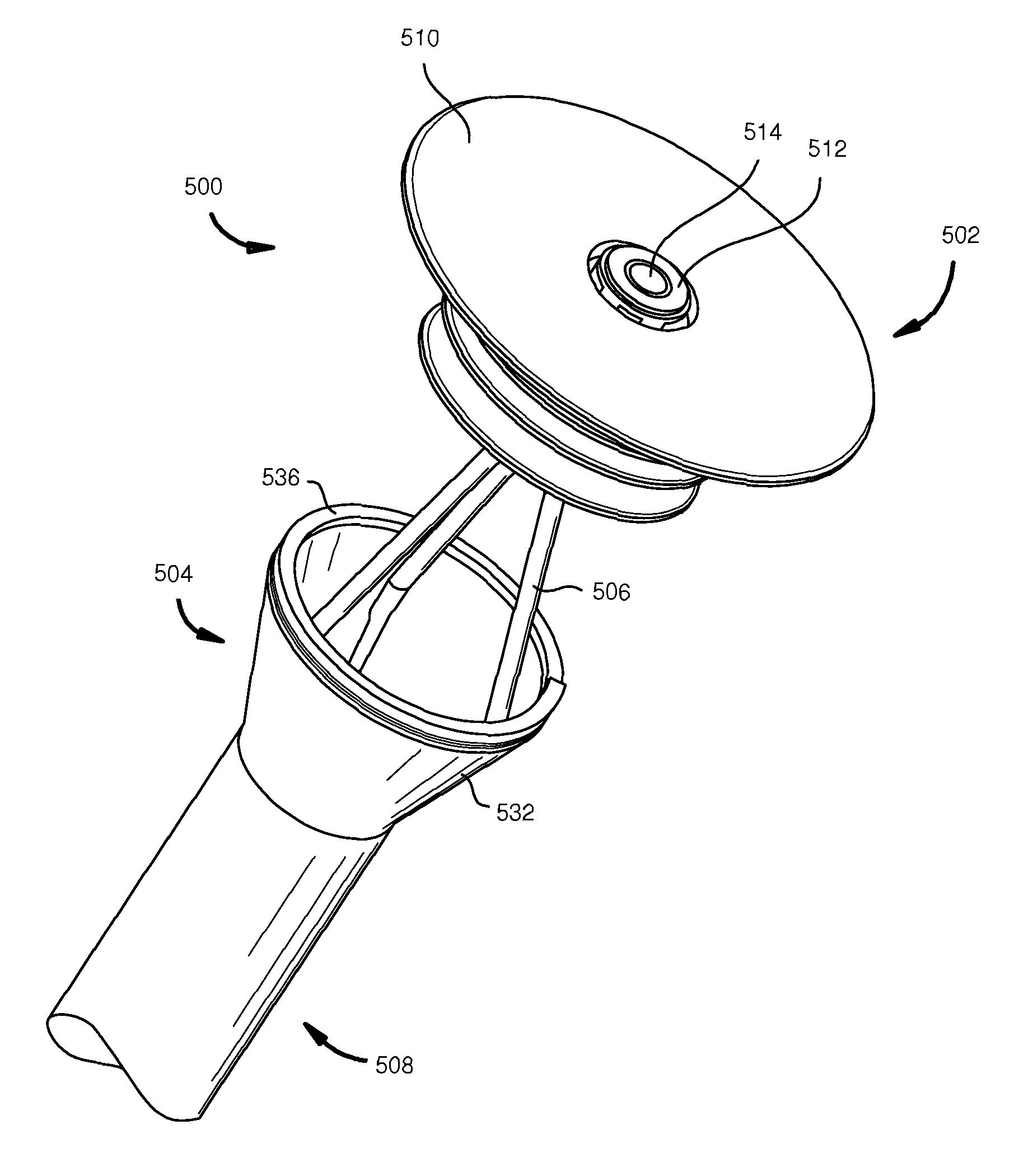
Stomach-spanning gastric implants
A variety of passive intragastric implant devices for obesity treatment are disclosed. Such passive implants do not autonomously change shape, but instead react within the stomach to induce satiety. The implants may take up volume within the stomach, thus reducing the digestive capacity. Additionally, the implants may contact areas within the stomach, such as the cardia surrounding the esophageal sphincter, to stimulate satiety-inducing nerves. Also, a number of implants slow gastric emptying by blocking or otherwise impeding flow through the pyloric sphincter. Other implants delay digestion by providing a duodenal sleeve. A number of implants combine two or more of these satiety-inducing features. Methods of implant are disclosed including compressing the implants within a delivery tube and transorally advancing the implants through the esophagus to be deployed within the stomach. Removal of the implants occurs in the reverse.
Owner:ALLERGAN INC
Obesity controlling method
InactiveUS7608578B2Minimally invasiveOrganic active ingredientsElectrotherapyMuscle tissueSatiations
A method of controlling obesity by injecting bio-compatible bulking material into the pyloric sphincter area of the stomach. The injection of this material bulks the pyloric sphincter, retarding stomach emptying and producing a feeling of satiation in the patient. The method may be supplemental and augmented by inducing flaccid paralysis of the stomach by injecting botulinum toxin into the muscle tissue of the antrum or fundus of the stomach.
Owner:TEMPLE UNIVERSITY
Anchored non-piercing duodenal sleeve and delivery systems
ActiveUS20130281911A1Easy to disassembleSimple structureIntravenous devicesNon-surgical orthopedic devicesAxial compressionGastric emptying
An intragastric implant for obesity treatment is disclosed. The device delays digestion by providing a duodenal sleeve, and may also slows gastric emptying by limiting flow through the pyloric sphincter. The implant includes an elongated axially-compressible duodenal sleeve having a non-tissue-piercing anchor on a proximal end sized to lodge within the duodenal bulb. The anchor may have oppositely-directed anchoring flanges to resists migration in both directions. The sleeve may also have barbed ribs to resist proximal movement back up into the stomach. A method of implant includes collapsing / compressing the device and transorally advancing it through the esophagus to be deployed within the duodenum. A dissolvable jacket may constrain the implant for delivery and naturally dissolve upon implant. Removal of the implant may occur in the reverse.
Owner:APOLLO ENDOSURGERY INC
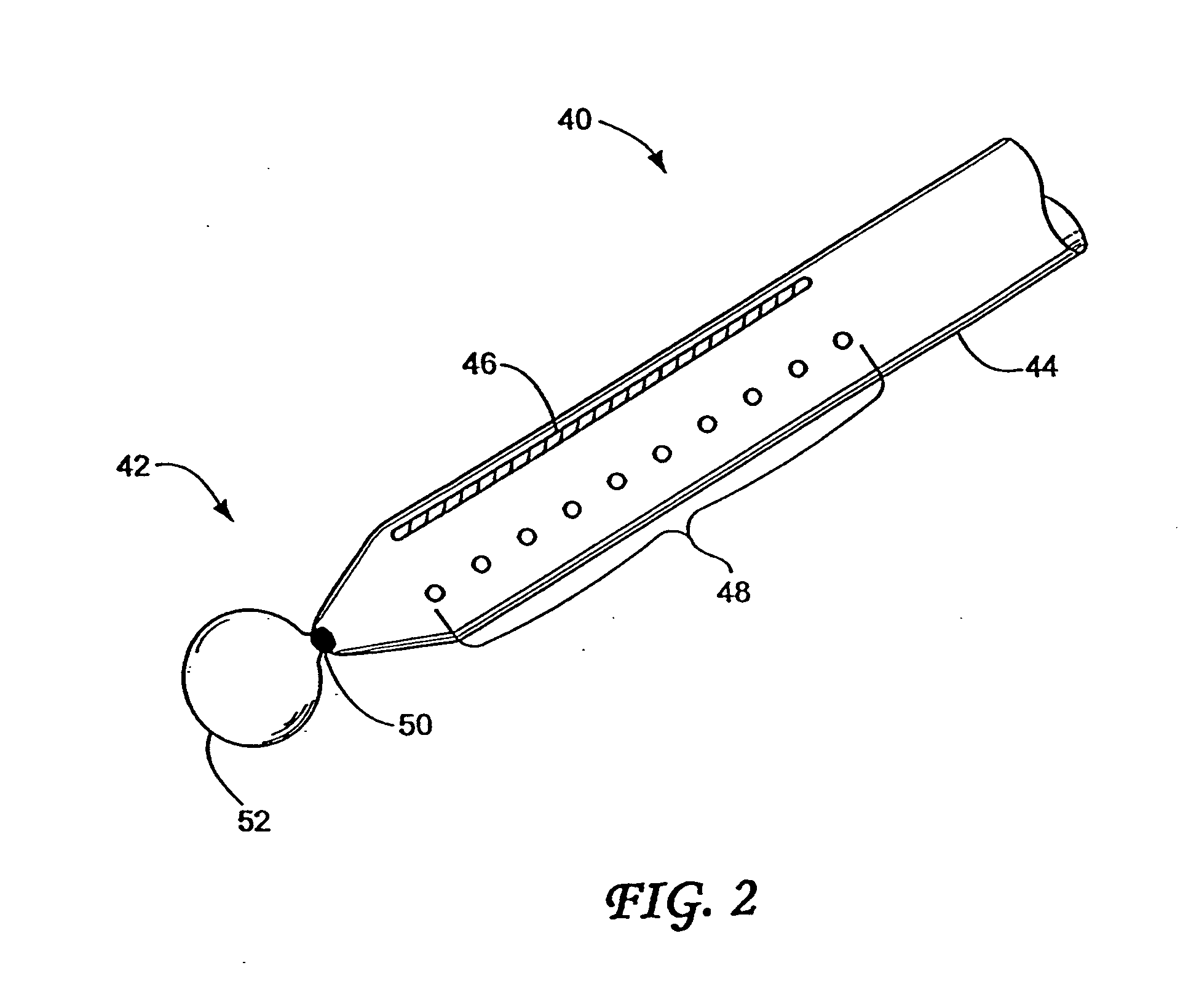
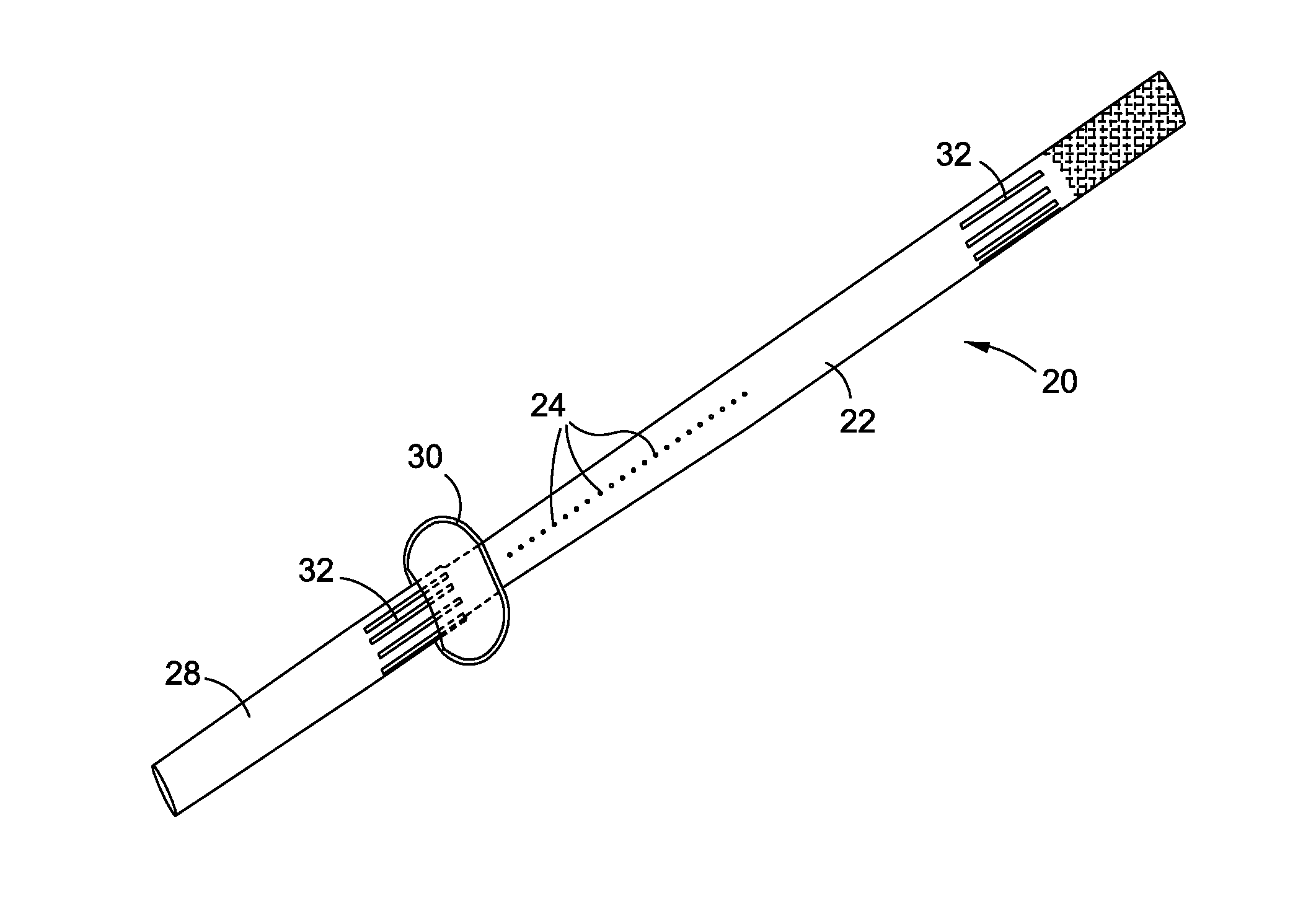
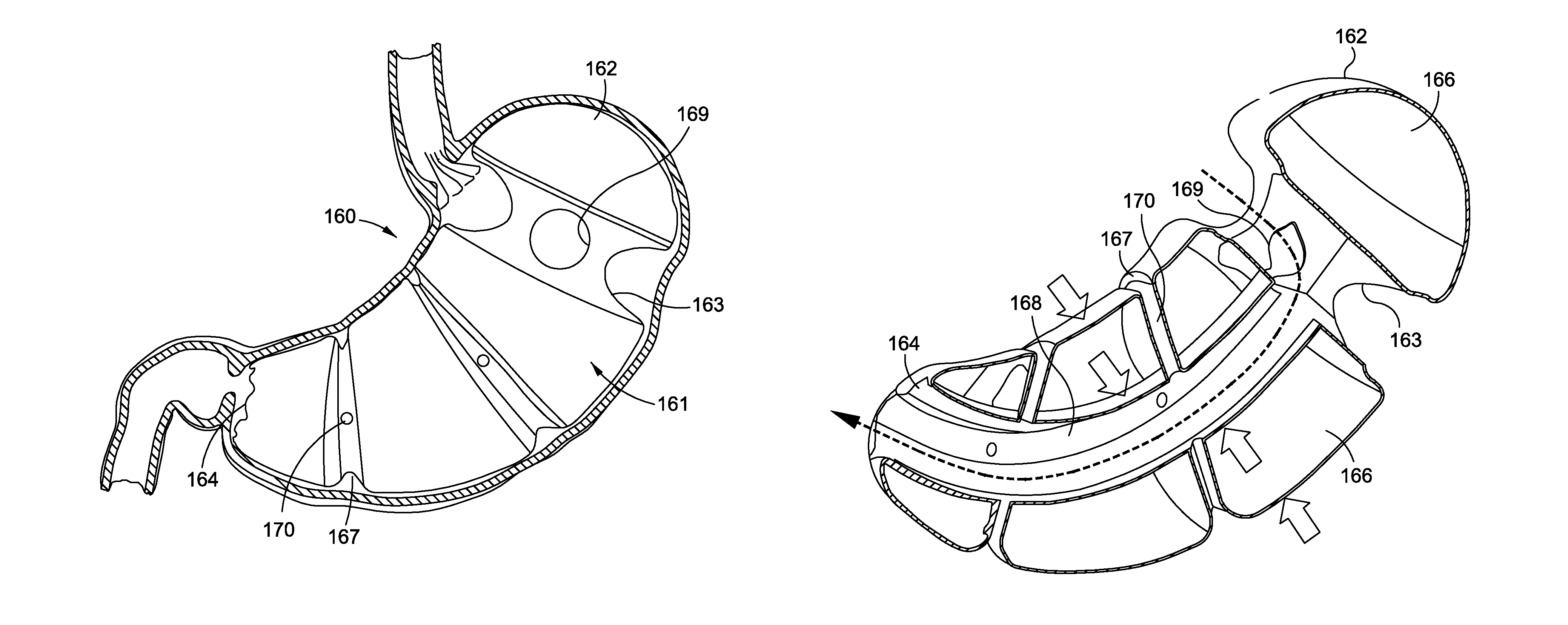
Pyloric Devices and Methods
InactiveUS20090118749A1Shorten the lag periodEffectively alter satietyObesity treatmentWound clampsGastrointestinal devicePyloric sphincter
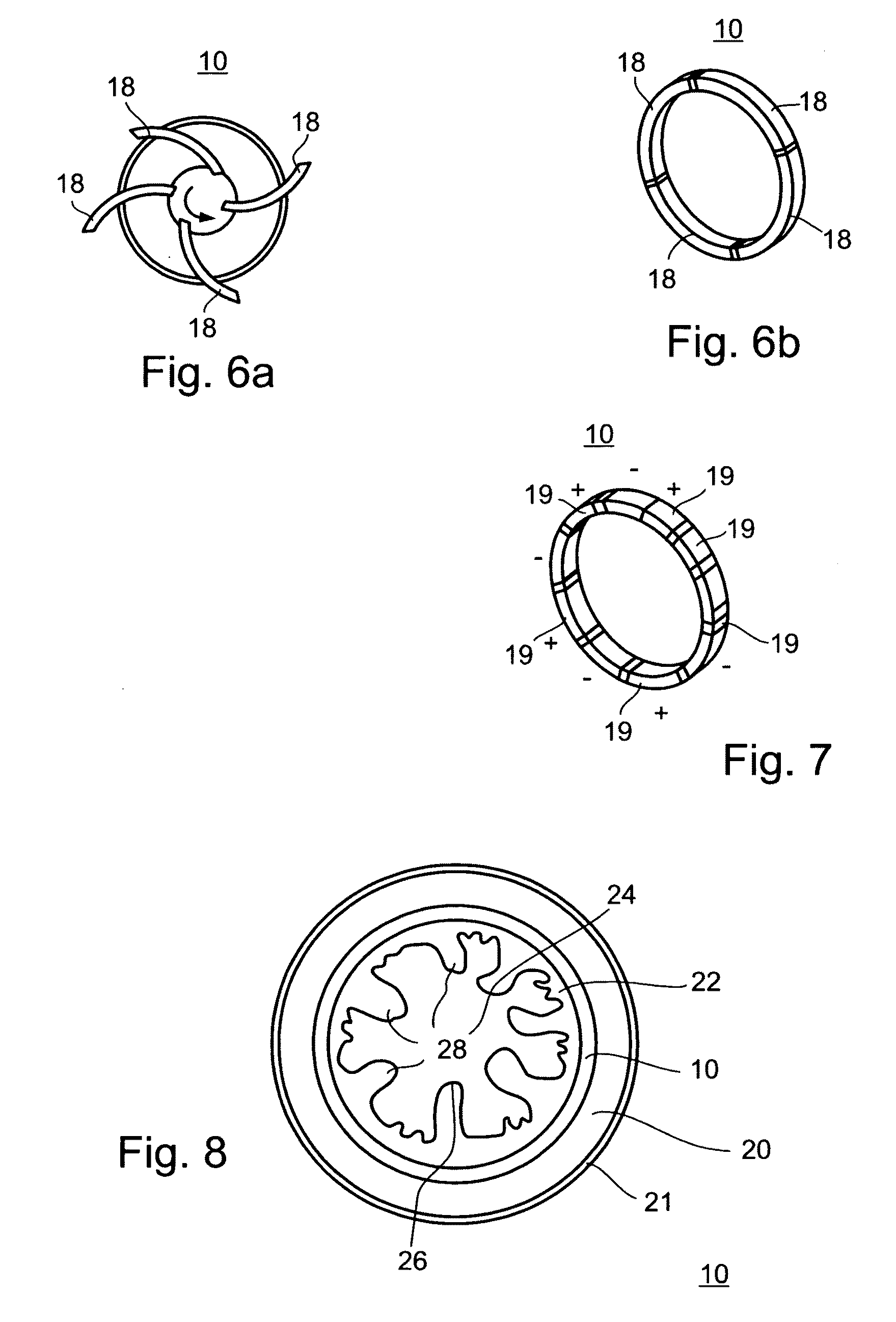
A gastrointestinal device is provided. The device includes a band sized and configured for residing in or around a pyloric sphincter region of the subject. The band is functional in maintaining the pyloric sphincter at a fixed opening size.
Owner:SVIP 2
Space-filling intragastric implants with fluid flow
Owner:APOLLO ENDOSURGERY INC
Method and apparatus for treating obesity and controlling weight gain and absorption of glucose in mammals
InactiveUS20110118650A1Reduce leakageIncrease volumeSuture equipmentsOesophagiPyloric sphincterPyloric Antrum
The present invention includes an endoluminal sleeve having a stomach sleeve portion, an antral sleeve portion, and an intestine sleeve portion. The endoluminal sleeve includes a flexible core layer which is formed from a self-expanding material. The flexible core layer in the stomach sleeve portion can self expand to grip the stomach. The antral sleeve is located in the pyloric antrum and is connected to the stomach sleeve portion and the intestine sleeve portion. The intestine sleeve portion is connected to the antral sleeve through a junction. The junction contains non-self-expanding material allowing the pyloric sphincter to properly function. The intestine sleeve portion is located in the small intestines. The intestine sleeve portion can have fenestrations, roots, a nutrient delivery layer, a nutrient delivery cover, an outer shell with a plurality of holes, and / or a semi-porous skin for nutrient delivery to the small intestines.
Owner:ONCIOMED
Systems and methods for treating obesity and type 2 diabetes
InactiveUS8475401B2Reduce tissue damageImpact digestive processSurgical needlesWound drainsPyloric sphincterEndoscopy
The present invention provides systems and methods for treating and controlling obesity and / or type II diabetes. In one aspect of the invention, a bypass device includes gastric and duodenal anchors coupled to each other and positioned on either side of the pylorus and a hollow sleeve designed to extend from the pylorus through at least a proximal portion of a patient's small intestine. The gastric and duodenal anchors are movable between collapsed configurations for advancement through the esophagus and an expanded configuration for inhibiting movement of the anchors through the pyloric sphincter. Thus, the bypass device can be placed and removed endoscopically through the patient's esophagus in a minimally invasive outpatient procedure and it is “self-anchoring” and does not require invasive tissue fixation within the patient's GI tract, thereby reducing collateral tissue damage and minimizing its impact on the digestive process.
Owner:E2 LLC DENTONS
Device for insufflating the interior of a gastric cavity of a patient
InactiveUS20090023997A1Preventing insufflationSuture equipmentsGastroscopesGastric cavitySmall intestine
A method for endoscopically preventing insufflation of the small bowel during gastric procedures includes applying an obstruction member at the pyloric sphincter to block the passage of gas from the gastric cavity into the small bowel and insufflating the gastric cavity.
Owner:ETHICON ENDO SURGERY INC
Systems and methods for treatment of obesity and type 2 diabetes
InactiveUS8403877B2Reduce tissue damageImpact digestive processIntravenous devicesStomachPyloric sphincterEndoscopy
The present invention provides systems and methods for treating and controlling obesity and / or type II diabetes. In one aspect of the invention, an internal bypass device includes gastric and duodenal anchors coupled to each other and positioned on either side of the pylorus and a hollow sleeve designed to extend from the pylorus through at least a proximal portion of a patient's small intestine. The gastric and duodenal anchors are movable between collapsed configurations for advancement through the esophagus and an expanded configuration for inhibiting movement of the anchors through the pyloric sphincter. Thus, the bypass device can be placed and removed endoscopically through the patient's esophagus in a minimally invasive outpatient procedure and it is “self-anchoring” and does not require invasive tissue fixation within the patient's GI tract, thereby reducing collateral tissue damage and minimizing its impact on the digestive process.
Owner:E2 LLC DENTONS
Systems and methods for treating obesity and type 2 diabetes
InactiveUS8591452B2Reduce tissue damageImpact digestive processSurgical needlesWound drainsPyloric sphincterProlongation
The present invention provides systems and methods for treating and / or controlling obesity and type II diabetes. In one aspect of the invention, a device for treating obesity includes a flow restrictor and an anchor coupled to the flow restrictor. The flow restrictor is movable between a first or collapsed configuration sized and shaped for endoscopic advancement through the patient's esophagus and into a distal region of the stomach and a second or operative configuration sized and shaped for inhibiting a flow of chyme from the stomach to the pyloric sphincter. It is believed that this will cause the prolongation of satiety, and result in fewer meals being eaten and / or smaller meals being ingested. The anchor is movable between a first or collapsed configuration sized and shaped for advancement through the esophagus, stomach and pyloric sphincter and into the proximal region of the duodenum and a second or operative configuration sized and shaped to inhibit proximal movement of the anchor through the pyloric sphincter.
Owner:E2 LLC DENTONS
Stomach-spanning gastric implants
A variety of passive intragastric implant devices for obesity treatment are disclosed. Such passive implants do not autonomously change shape, but instead react within the stomach to induce satiety. The implants may take up volume within the stomach, thus reducing the digestive capacity. Additionally, the implants may contact areas within the stomach, such as the cardia surrounding the esophageal sphincter, to stimulate satiety-inducing nerves. Also, a number of implants slow gastric emptying by blocking or otherwise impeding flow through the pyloric sphincter. Other implants delay digestion by providing a duodenal sleeve. A number of implants combine two or more of these satiety-inducing features. Methods of implant are disclosed including compressing the implants within a delivery tube and transorally advancing the implants through the esophagus to be deployed within the stomach. Removal of the implants occurs in the reverse.
Owner:APOLLO ENDOSURGERY INC
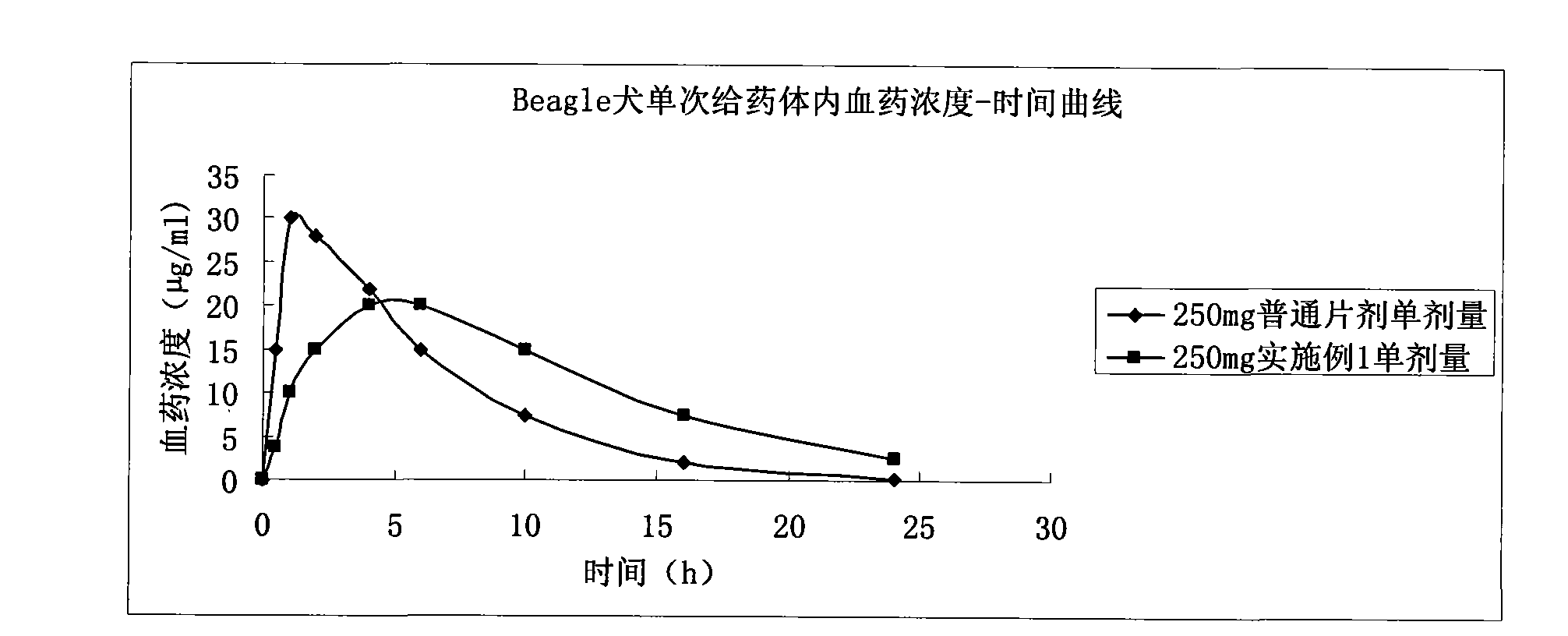
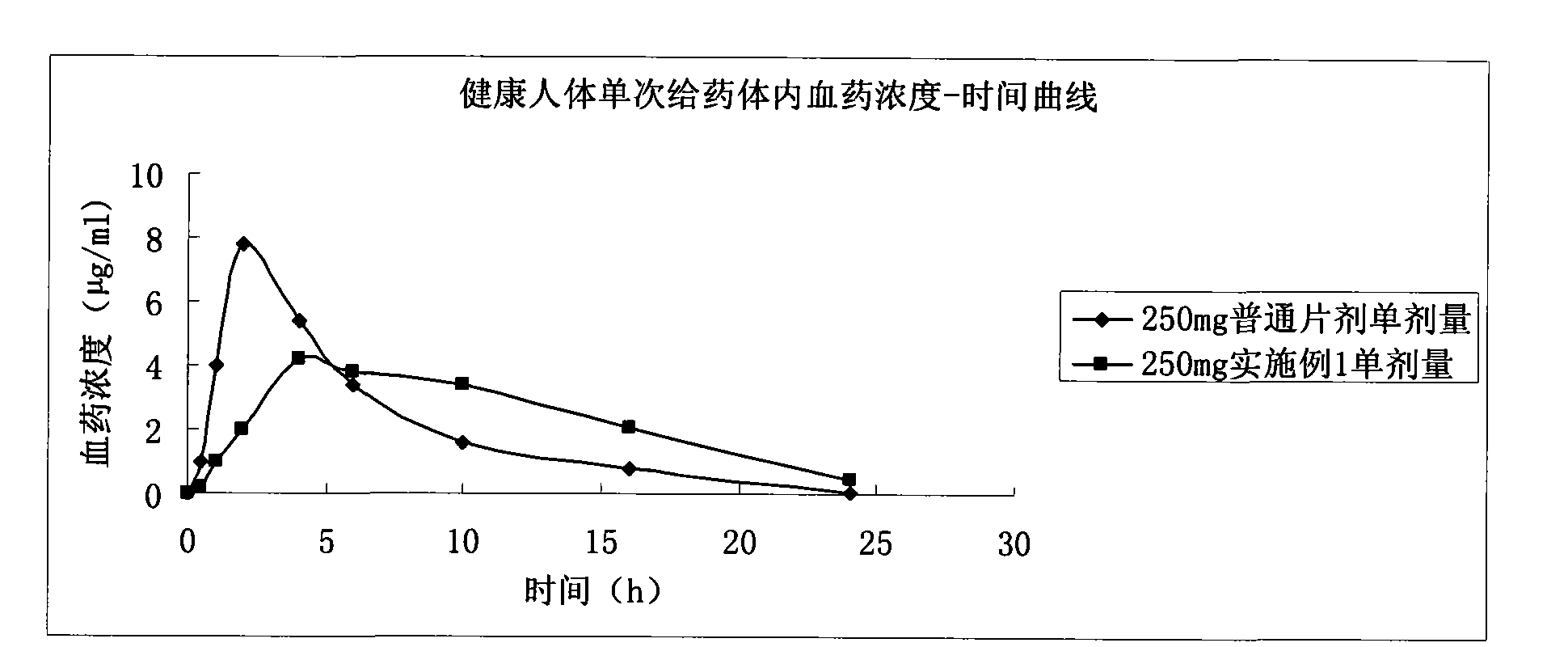
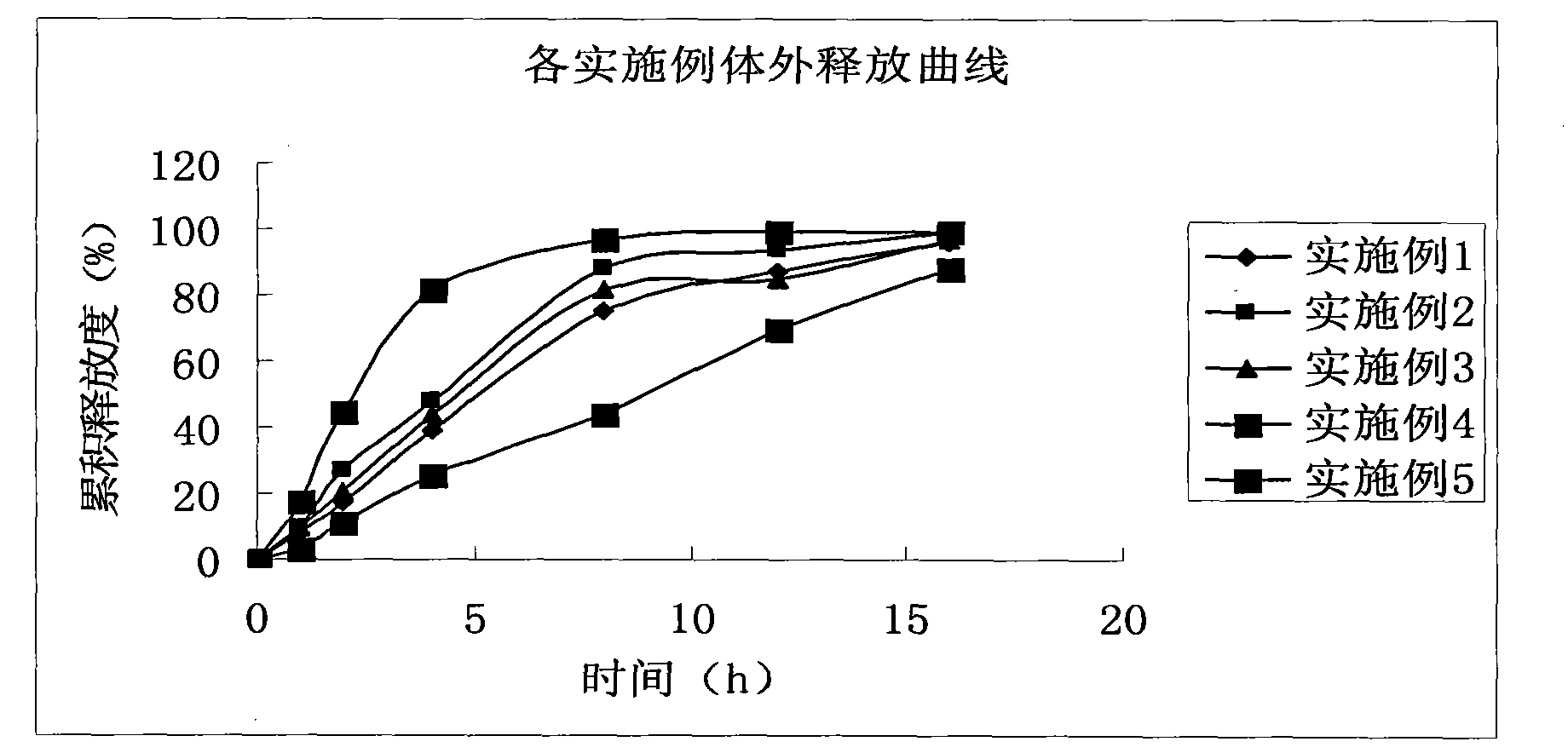
Levetiracetam slow release pellet capsule preparation and preparation method thereof
InactiveCN101647789AImprove liquidityReduce or eliminate irritationNervous disorderPharmaceutical delivery mechanismMass compositionSide effect
The invention relates to a levetiracetam slow release pellet capsule preparation and a preparation method thereof. The levetiracetam slow release pellet capsule preparation comprises the following components in percentage by mass: 50-70 percent of levetiracetam, 15-30 percent of blank pellet, 5-10 percent of hydroxypropylmethyl cellulose or polyvidone, 7-15 percent of ethylcellulose or acrylic resin, 1-8 percent of talc powder and 0.5-2 percent of cataloid. The preparation method comprises the following steps: coating levetiracetam fine powder on the blank pellet by a binding agent to be madeinto a medicine-contained pellet; coating an isolating layer on the medicine-contained pellet; coating a slow release coating film on the medicine-contained pellet coated with the isolating layer to prepare a slow release pellet; and mixing the slow release pellet, the talc powder and the cataloid and filling the mixture into a capsule. The levetiracetam is made into the slow release capsule preparation by a pellet and slow release technology, and the slow release preparation can stabilize blood medicine concentration, reduce the generating frequency and degree of the side effect and fundamentally solve the problems of great influence on a tablet by gastric pyloric sphincter and big differences of gastric emptying individuals.
Owner:天津药物研究院药业有限责任公司
Obesity controlling method
InactiveUS7737109B2Slow gastric emptyingMinimally invasiveElectrotherapyPeptide/protein ingredientsMuscle tissueSatiations
Owner:TEMPLE UNIVERSITY
Systems and Methods for Treating Obesity and Type 2 Diabetes
InactiveUS20110004229A1Reducing collateral tissue damageMinimize impactSurgical needlesWound drainsPyloric sphincterDuodenum length
The present invention provides systems and methods for treating and controlling obesity and / or type II diabetes. In one aspect of the invention, a bypass device includes gastric and duodenal anchors coupled to each other and positioned on either side of the pylorus and a hollow sleeve designed to extend from the pylorus through at least a proximal portion of a patient's small intestine. The gastric and duodenal anchors are movable between collapsed configurations for advancement through the esophagus and an expanded configuration for inhibiting movement of the anchors through the pyloric sphincter. Thus, the bypass device can be placed and removed endoscopically through the patient's esophagus in a minimally invasive outpatient procedure and it is “self-anchoring” and does not require invasive tissue fixation within the patient's GI tract, thereby reducing collateral tissue damage and minimizing its impact on the digestive process.
Owner:E2 LLC DENTONS
Method of insufflating the interior of a gastric cavity of a patient.
A method of insufflating the interior of a gastric cavity of a patient by accessing the gastric cavity of a patient, sealing the pyloric sphincter so as to prevent passage of fluids therethrough, and introducing a fluid within said gastric cavity proximal to the pyloric sphincter.
Owner:ETHICON ENDO SURGERY INC
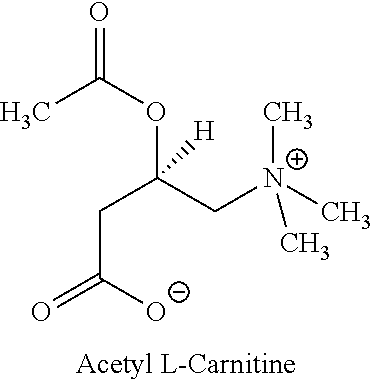
Multiparticulate L-carnitine compositions and related methods
ActiveUS10085947B2Minimize side effectsLess frequent, more predictable, and reliable dosingOrganic active ingredientsNervous disorderParticulatesCellulose
An oral controlled-release multiparticulate dosage form comprises a plurality of individually enteric coated particulates containing an L-carnitine that independently disperse in a patient's stomach after oral ingestion and travel through the stomach and past the pyloric sphincter without substantially releasing the L-carnitine in the stomach. The individual particulates contain (a) a solid core containing the L-carnitine, (b) a subcoating containing a cellulosic water soluble polymer over the core, and (c) an enteric coating over the subcoating. The dosage form may be used to treat conditions associated with a reduction of the amount of L-carnitine in the body.
Owner:SOC DES PROD NESTLE SA
Space-filling intragastric implants with fluid flow
A variety of passive intragastric implant devices for obesity treatment are disclosed. Such passive devices do not autonomously change shape, but instead react within the stomach to induce satiety. The devices may take up volume within the stomach, thus reducing the intake capacity. Additionally, the devices may contact areas within the stomach, such as the cardia surrounding the esophageal sphincter, to stimulate satiety-inducing nerves. Also, certain devices slow gastric emptying by blocking or otherwise impeding flow through the pyloric sphincter. A number of devices combine two or more of these satiety-inducing features. Methods of implant are disclosed including compressing the devices within a delivery tube and transorally advancing the devices through the esophagus to be deployed within the stomach. Removal of the devices occurs in the reverse.
Owner:APOLLO ENDOSURGERY INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com