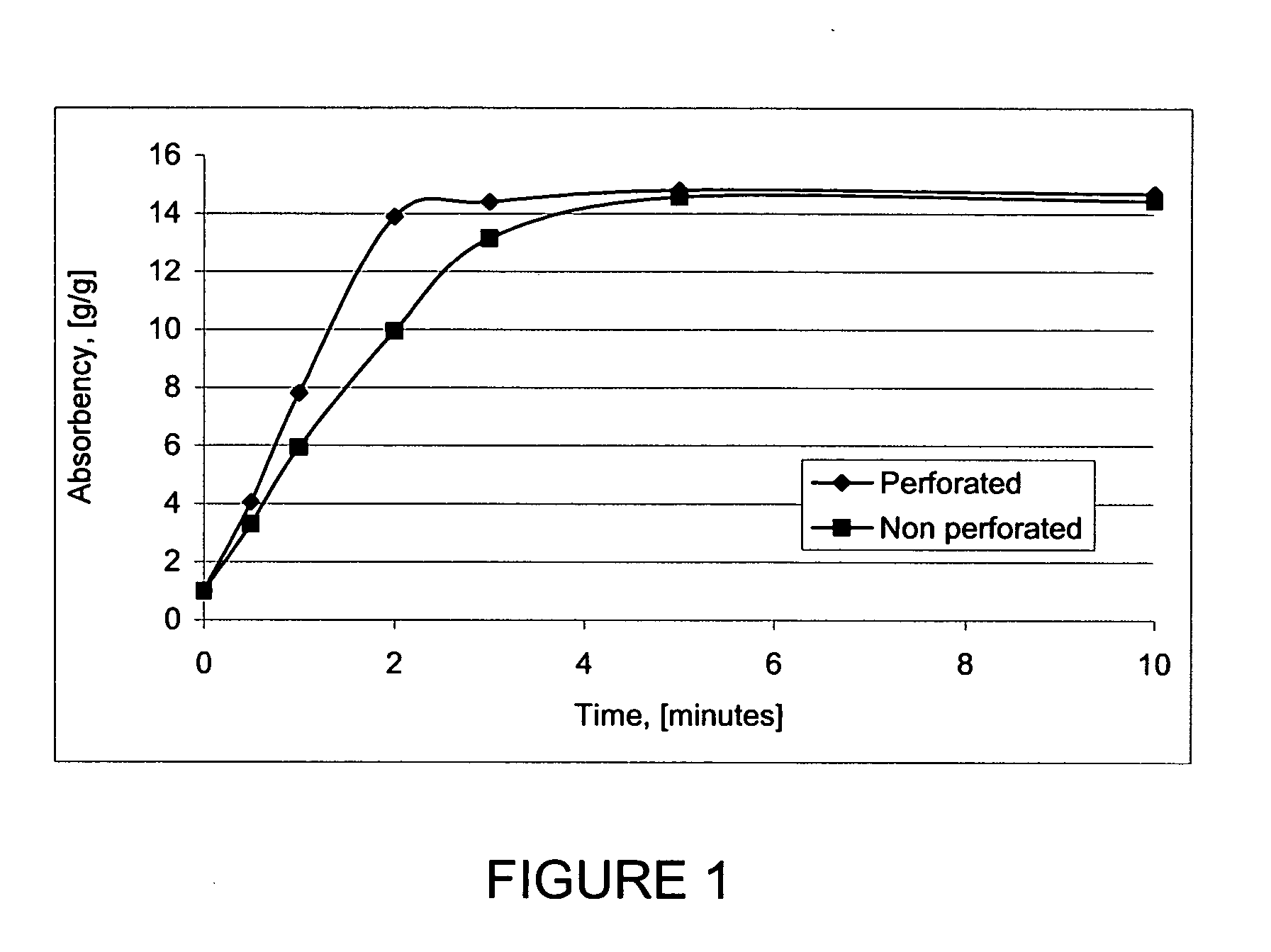
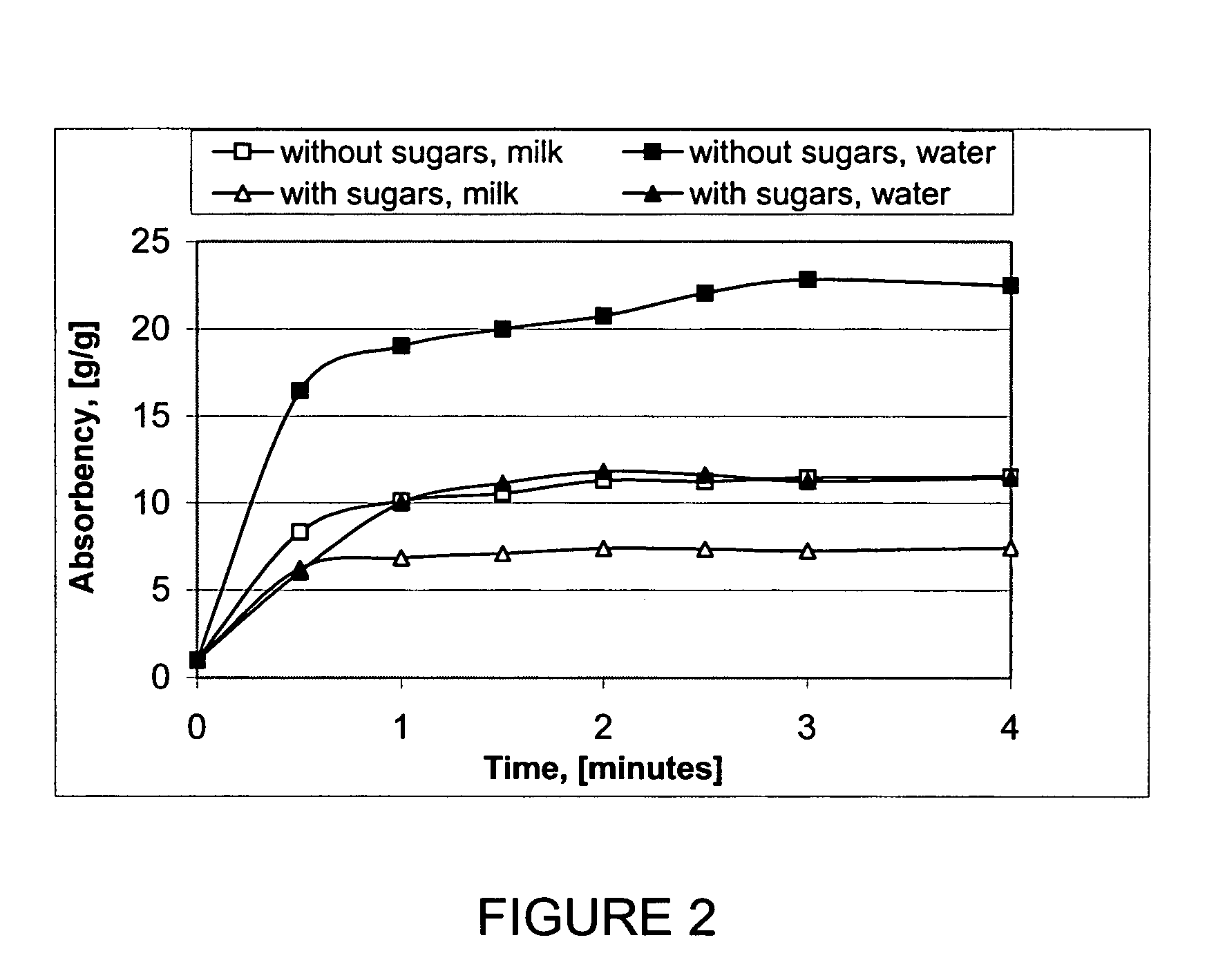
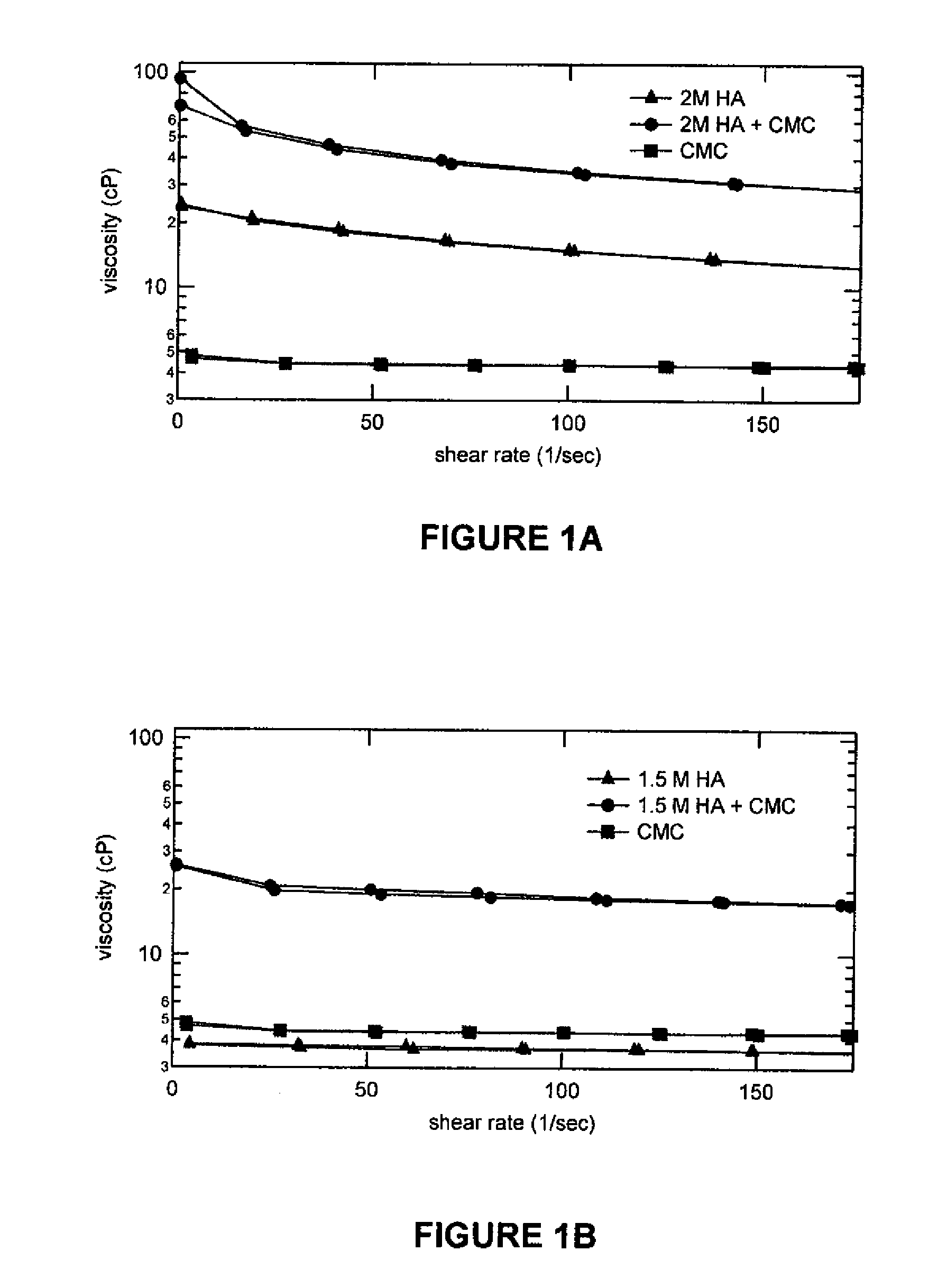
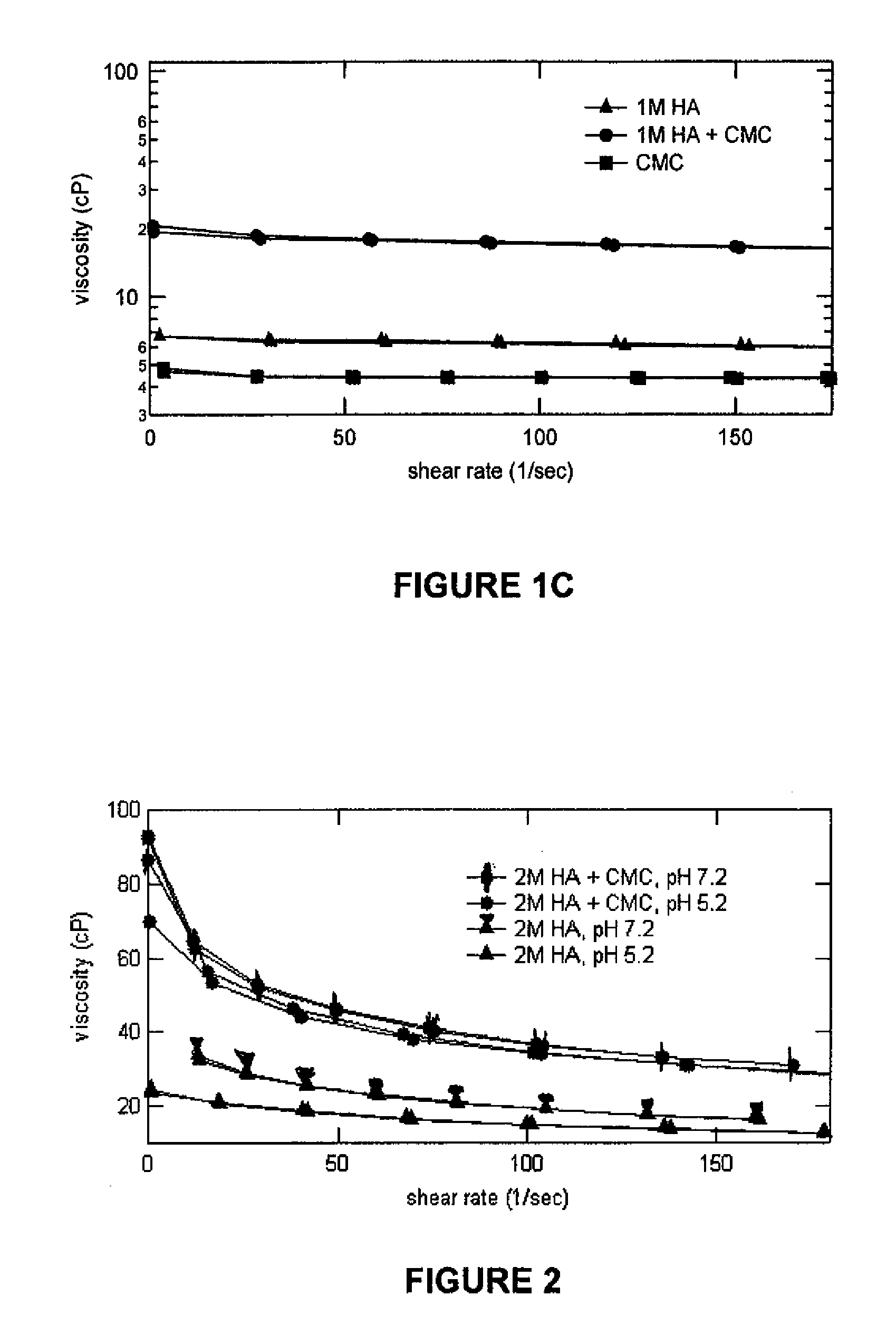
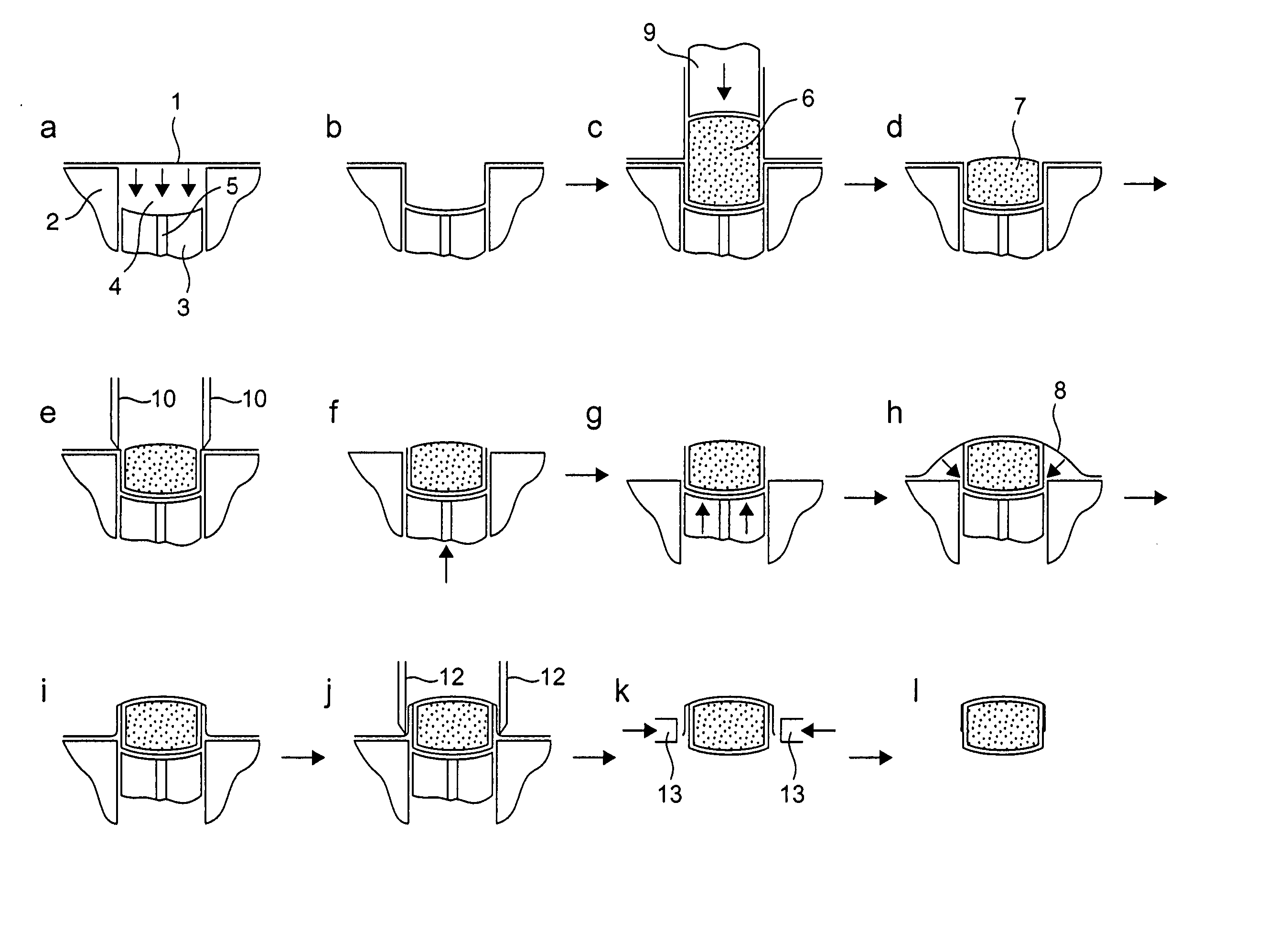
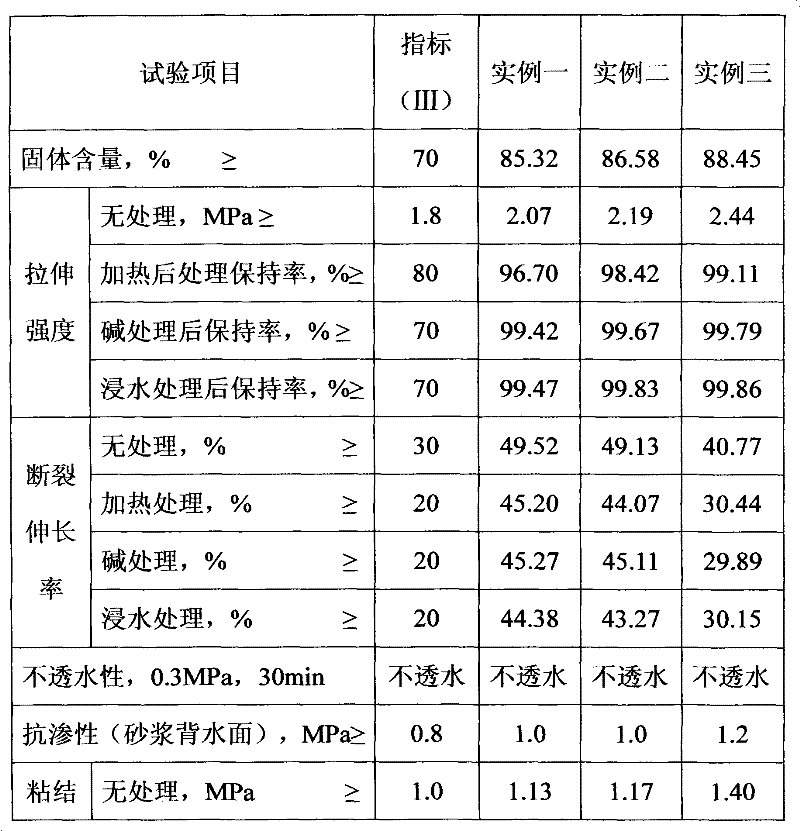
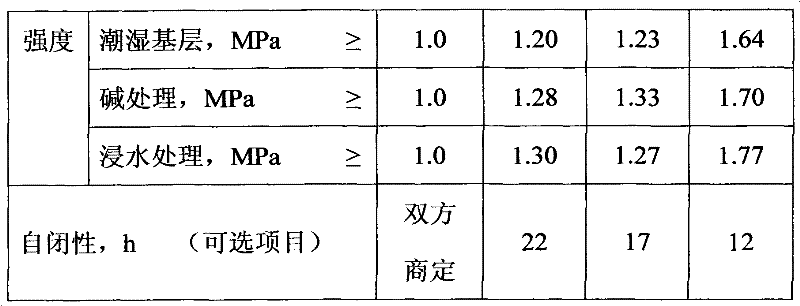
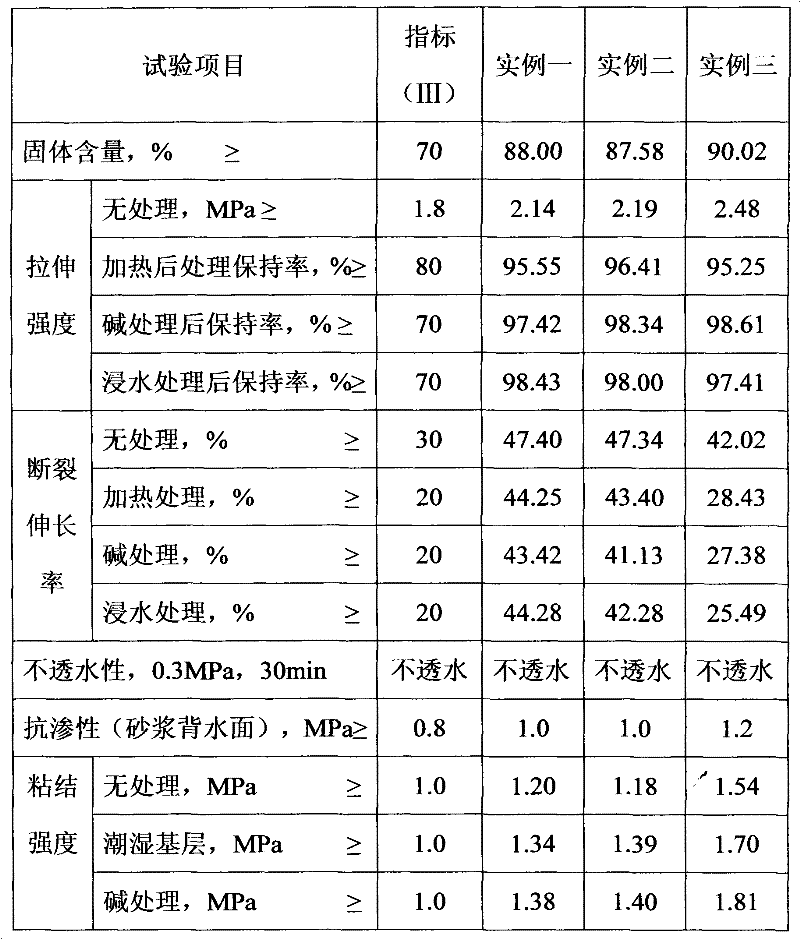
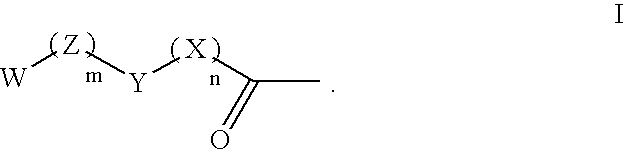Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4898 results about "Hydroxypropylmethyl cellulose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hydroxypropyl methyl cellulose (HPMC) is a polymer used for multiple applications in fields such as construction and ophthalmology.
Gelled biopolymer based foam
InactiveUS20050137272A1Improve water absorptionWet strengthCosmetic preparationsToilet preparationsPersonal careCross-link
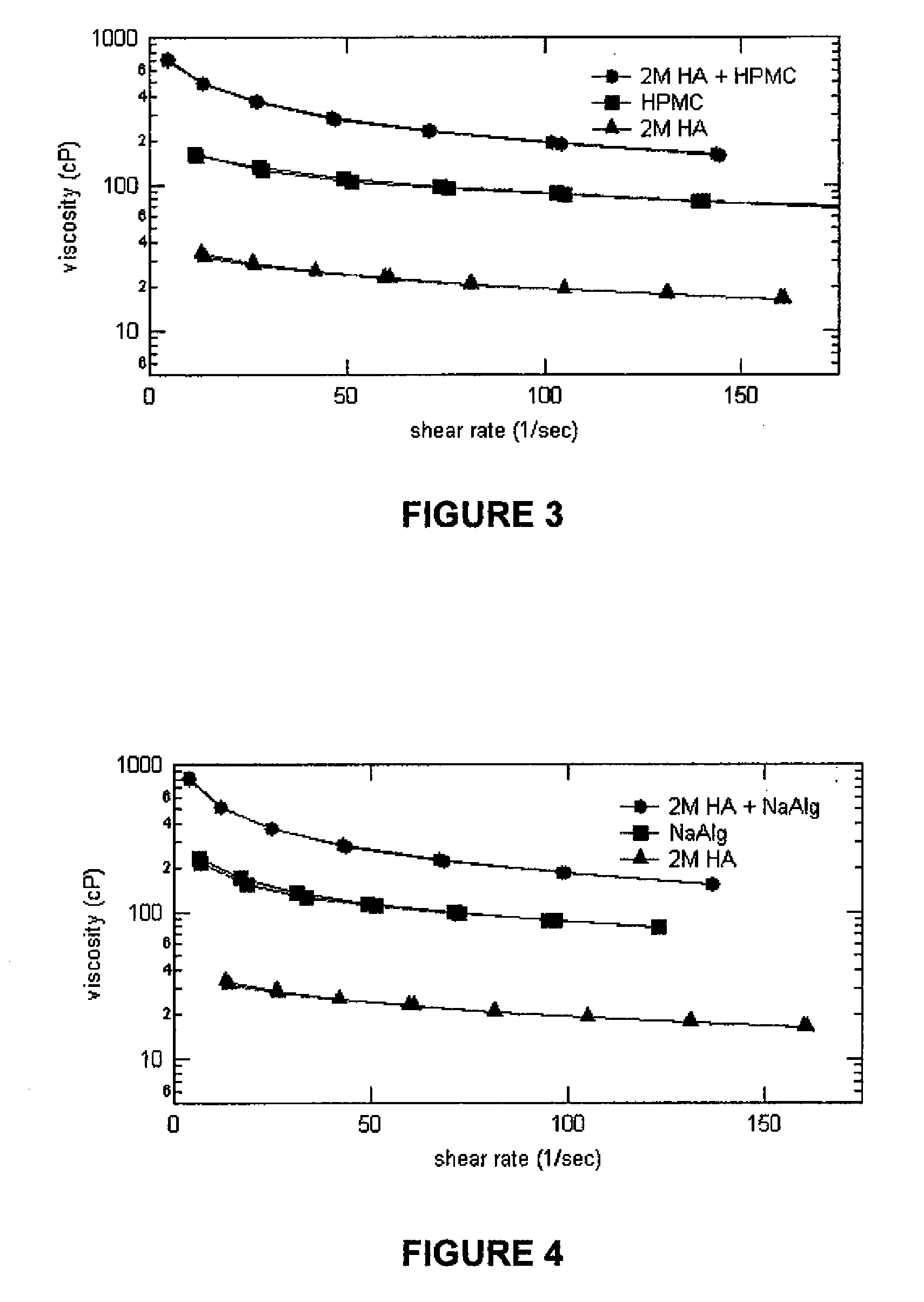
Gelled biopolymer based foams are disclosed. The gelled foams comprise a cross-linked biopolymer, preferably alginate; optionally, a foaming agent such as hydroxy propyl methyl cellulose; and a plasticizer, preferably glycerin sorbitol, or a mixture thereof, that forms the predominant portion of the gelled foam. The foams are soft and pliable and have high absorbency. They are used as wound dressing materials, controlled release delivery systems, cell culture, barrier media for preventing tissue adherence, and bioabsorbable implants. They also have various personal care applications, especially in oral hygiene, and can be used in food applications.
Owner:FMC BIOPOLYMER AS
Stabilized Glycosaminoglycan Preparations and Related Methods
Compositions comprising a glycosaminoglycan (e.g., a hyaluronan, hyaluronic acid, hyaluronate, sodium hyaluronate, dermatan sulfate, karatan sulfate, chondroitin 6-sulfate, heparin, etc.) in combination with at least one component selected from; i) polyglycols (e.g., polyethylene glycol), ii) long chain hydroxy polyanionic polysaccharides (e.g., dextran, sodium alginate, alginic acid, propylene glycol alginate, carboxymethyl cellulose and carboxyethyl cellulose, hydroxyl ethyl starch, hydroxyl propyl methyl cellulose, hydroxy propyl ethyl cellulose, hydroxy propyl cellulose, methyl cellulose, polylysine, polyhistidine, polyhydroxy proline, poly ornithine, polyvinyl pyrolidone, polyvinyl alcohol, chitosan, etc.) and iii) long chain Nitrogen containing polymers (e.g., Polylysine, Polyvinylpyrrolidone, and polyvinyl alcohol). The invention also includes methods for using such compositions (e.g., as substance delivery materials, tissue fillers or bulking agents, as moistening or hydrating agents, etc.)
Owner:S K PHARMA INC
Compositions and methods for treating obesity and related disorders by characterizing and restoring mammalian bacterial microbiota
ActiveUS20120058094A1Good for weight lossReduce the populationBiocideMetabolism disorderBiotechnologyDisease
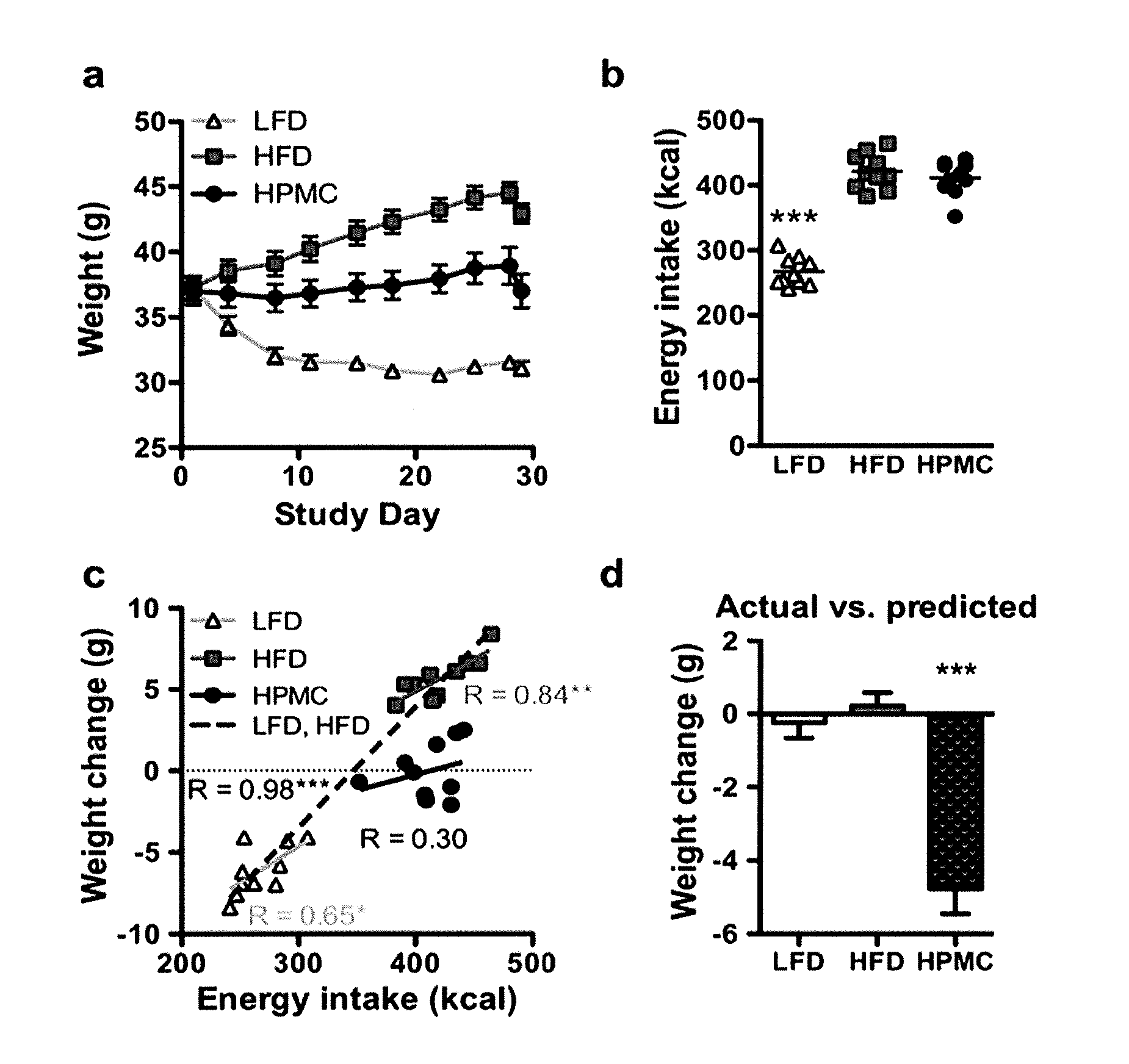
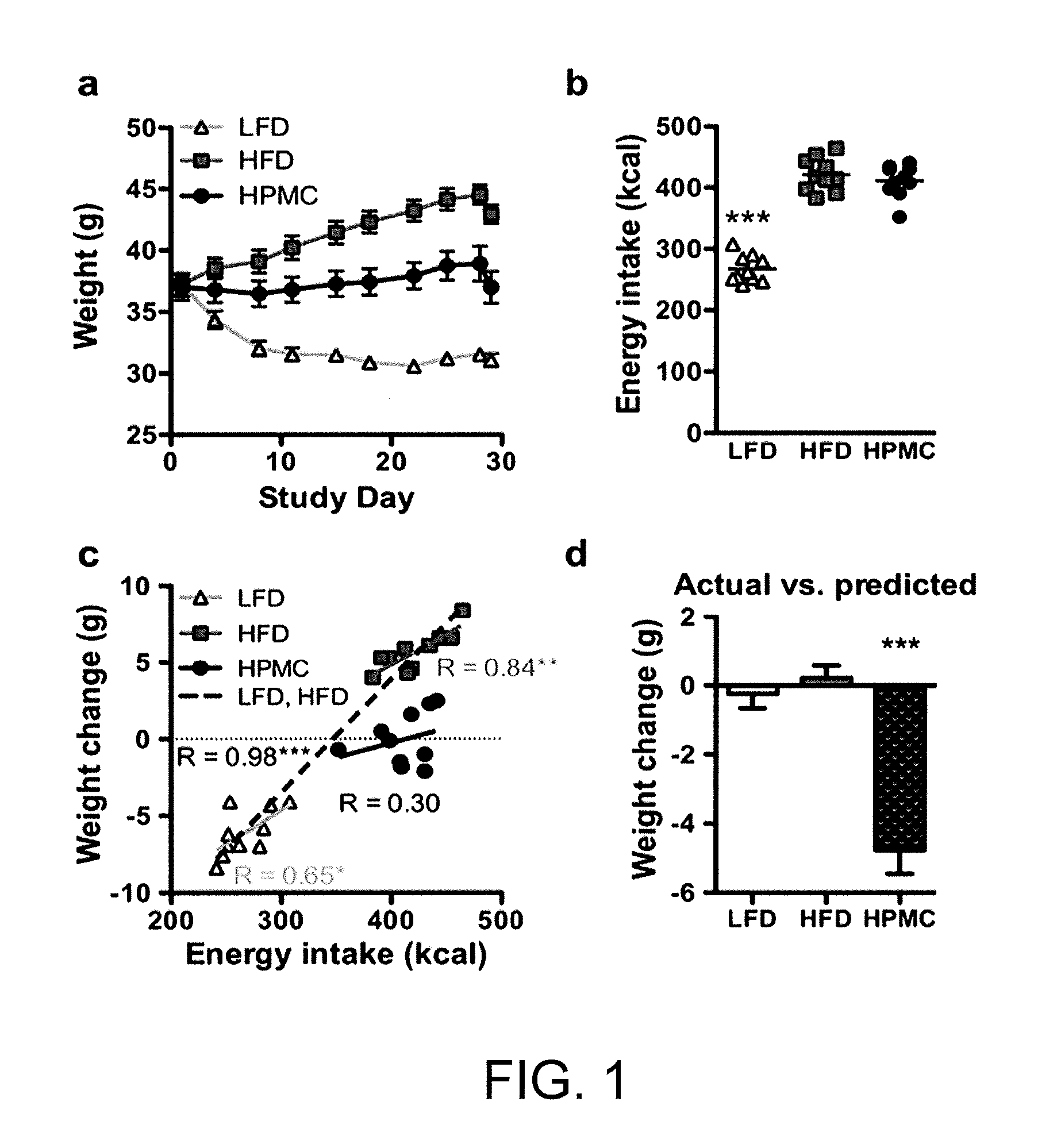
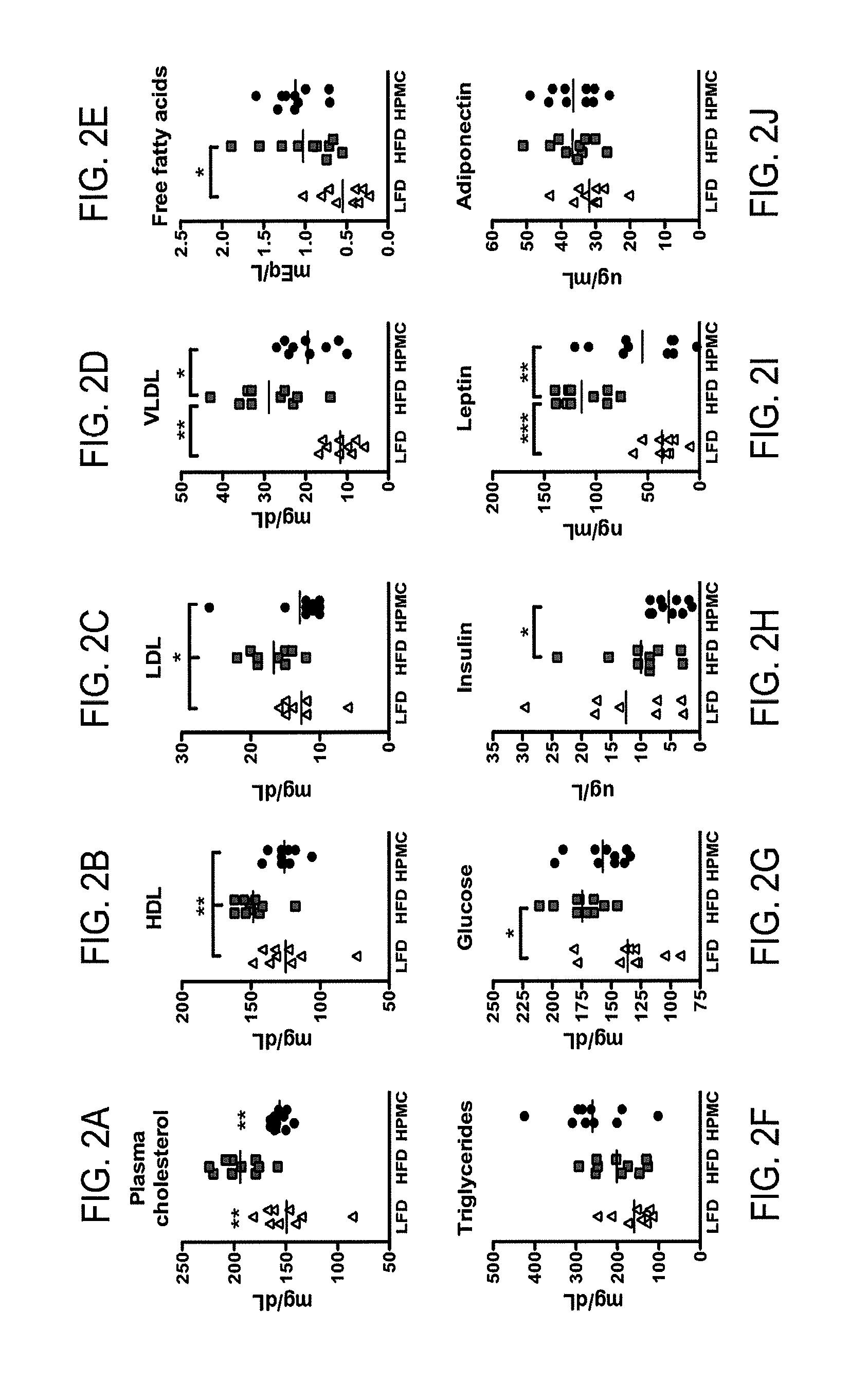
The present invention relates to characterizing changes in mammalian intestinal microbiota associated with associated with high-fat and low-fat diets and with diets containing hydroxypropylmethylcellulose (HPMC) and related methods for diagnosing, preventing and treating obesity and related conditions such as metabolic syndrome and diabetes mellitus. Therapeutic methods of the invention involve the use of probiotics, and / or prebiotics, and / or narrow spectrum antibiotics / anti-bacterial agents that are capable of restoring healthy mammalian bacterial intestinal microbiota.
Owner:NEW YORK UNIV
Acidified attapulgite clay
Owner:XUYI BOTU ATTAPULGITE CLAY HIGH TECH DEV
Compounds and methods for lowering the abuse potential and extending the duration of action of a drug
ActiveUS7230005B2Low abuse potentialProlong the action timeBiocideNervous disorderNasal cavityEster prodrug
The abuse potential of a bioavailable drug such as an opiate analgesic agent is reduced and its duration of action is extended by converting it to a poorly absorbed ester prodrug or other prodrug derivative prior to formulation. Unlike many existing sustained release formulations of active pharmaceutical agents wherein an active pharmaceutical agent can be released by chewing, crushing, or otherwise breaking tablets or capsule beads containing the active pharmaceutical agent, such mechanical processing of tablets or capsule beads containing a prodrug of this invention neither releases the active drug nor compromises the controlled conversion of prodrug to drug. Moreover, tablets and capsule beads containing prodrugs of this invention or other drugs can be formulated with a sufficient amount of a thickening agent such as hydroxypropylmethylcellulose or carboxymethylcellulose to impede inappropriate intravenous and nasal administration of formulations that are not indicated for these modes of administration.
Owner:CONTROLLED CHEM INC
Controlled release formulations using intelligent polymers
InactiveUS6893661B1Promote absorptionMaintenance of therapeutically effective blood levelPowder deliveryOrganic active ingredientsSmart polymerWater contact
An extended release dosage composition of pharmaceutically active substances that have a water contact angle (θ) such that cos θ is between +0.9848 and −0.9848 presented as a matrix tablet containing the said pharmaceutically active substances, with / without suitable pharmaceutical excipients in intimate mixture with two groups of intelligent polymers having opposing wettability characteristics, one demonstrating a stronger tendency towards hydrophobicity and the other a stronger tendency towards hydrophilicity, the polymer combination being between the ratios of 1:50 and 50:1 amounts effective to control the release of said pharmaceutically active substances in a mathematically predictable manner, wherein the polymer demonstrating a stronger tendency towards hydrophobicity is not less than 5% wt / wt and preferably between 5-70% wt / wt of the final formulation composition. The intelligent polymers being ethylcellulose (EC) as a more strongly hydrophobic and hydroxyethylcellulose (HEC) and / or hydroxypropyl methylcellulose (HPMC) as more strongly hydrophilic (the ratio of HEC to HPMC being between 1:100 and 100:1). The matrix tablet is optionally coated with an enteric coat, 0-5%-15% wt / wt to prevent the initial burst effect seen in such systems and to impart gastrointestinal tract (GIT) “stealth” characteristics especially in the presence of food.
Owner:VALEANT INT BERMUDA
Powder compaction and enrobing
InactiveUS20050147710A1RobustQuicker release characteristicLayered productsConfectioneryMetallurgyMethyl cellulose
An apparatus and method is disclosed for forming a compacted powder slug coated with a film. The powder, e.g. of a medicament, is compacted and enrobed to produce compacted powder slugs by preferably mechanically compacting a powder and forming a film of a material, preferably hydroxy propyl methyl cellulose, by vacuum or pressure differential, about the surface of the powder thus compacted.
Owner:BIOPROGRESS TECH
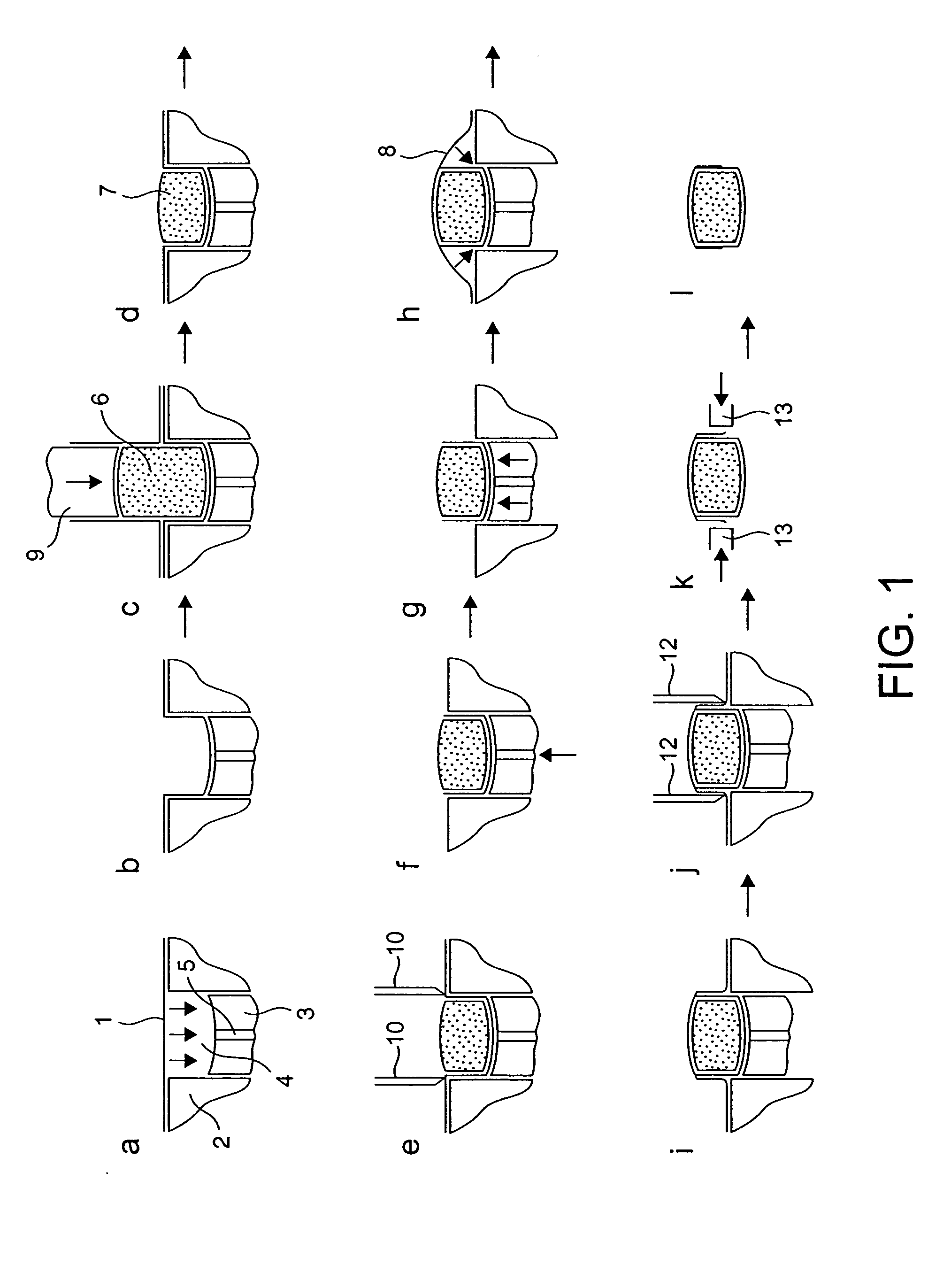
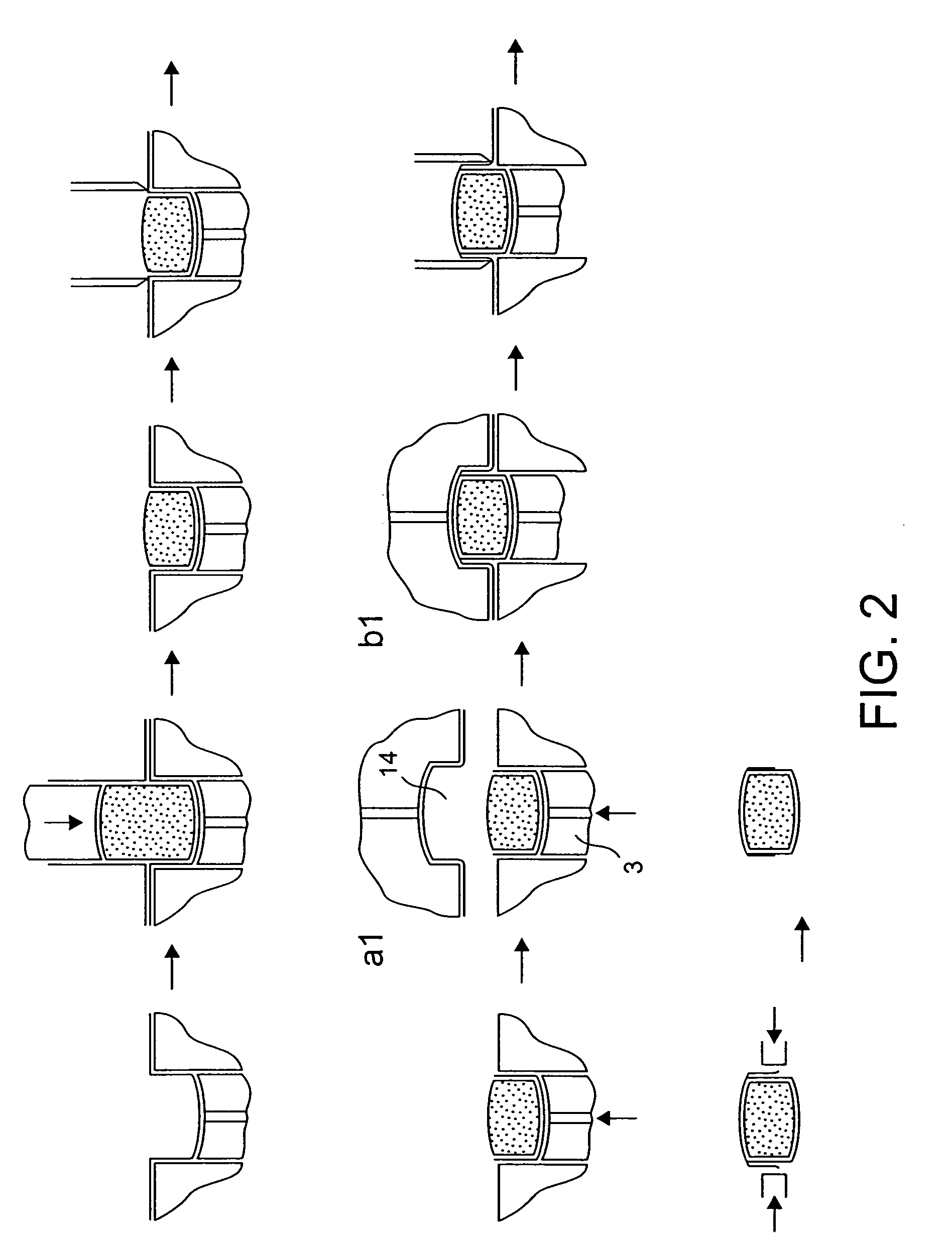
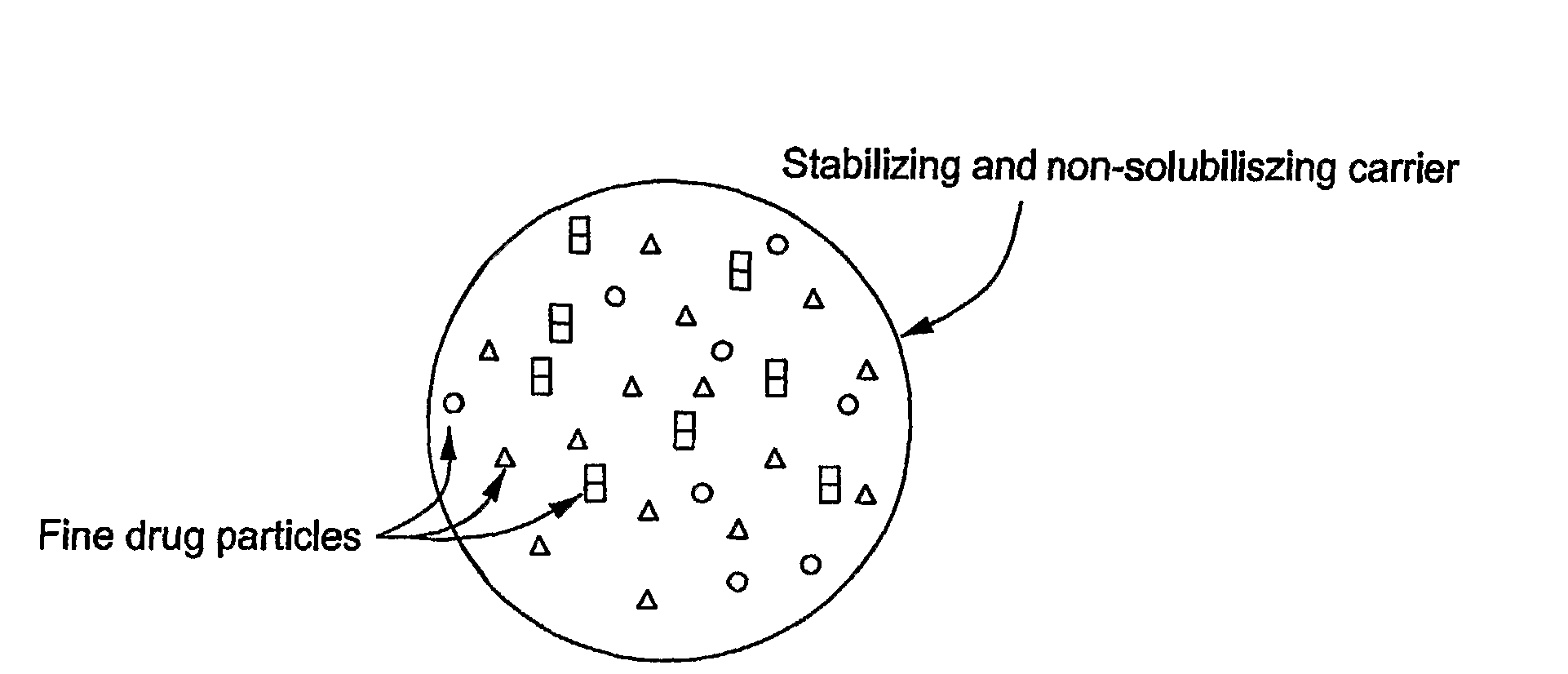
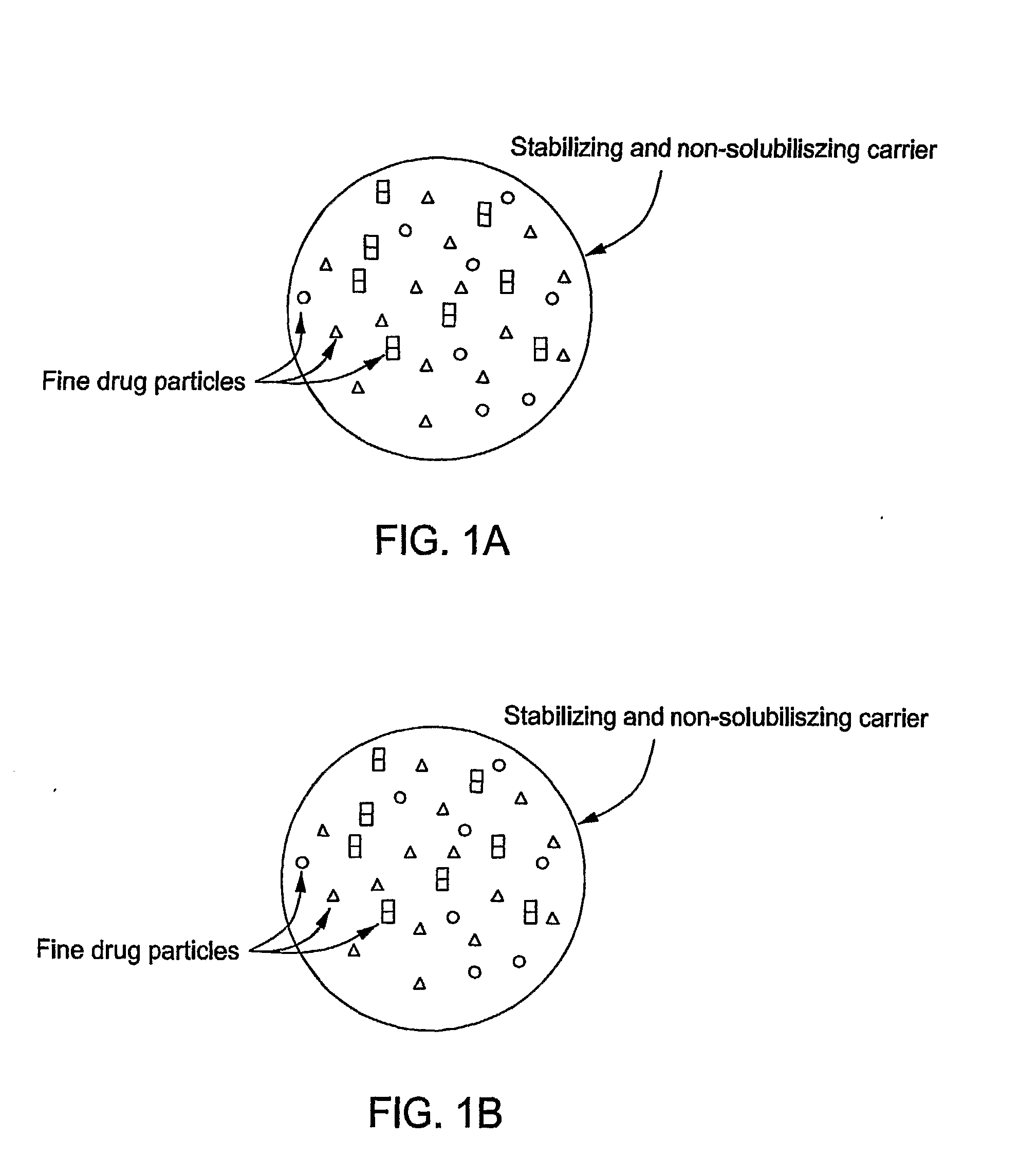
Stabilized Hme Composition With Small Drug Particles
ActiveUS20080274194A1Facilitated releaseReduce releasePowder deliveryCyclic peptide ingredientsAntioxidantCarrier system
A hot-melt extruded composition having finely divided drug-containing particles dispersed within a polymeric and / or lipophyllic carrier matrix is provided. The carrier softens or melts during hot-melt extrusion but it does not dissolve the drug-containing particles during extrusion. As a result, a majority or at least 90% wt. of the drug-containing particles in the extrudate are deaggregated during extrusion into essentially primary crystalline and / or amorphous particles. PEO is a suitable carrier material for drugs insoluble in the solid state in this carrier. Various functional excipients can be included in the carrier system to stabilize the particle size and physical state of the drug substance in either a crystalline and / or amorphous state. The carrier system is comprised of at least one thermal binder, and may also contain various functional excipients, such as: super-disintegrants, antioxidants, surfactants, wetting agents, stabilizing agents, retardants, or similar functional excipients. A hydrophilic polymer, such as hydroxypropyl methylcellulose (HPMC E15), polyvinyl alcohol (PVA), or poloxamer, and / or a surfactant, such as sodium lauryl sulfate (SLS), can be included in the composition. A process for preparing the extrudate is conducted at a temperature approximating or above the softening or melting temperature of the matrix and below the point of solubilization of drug-containing particles in the carrier system, and below the recrystallization point in the case of amorphous fine drug particles.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
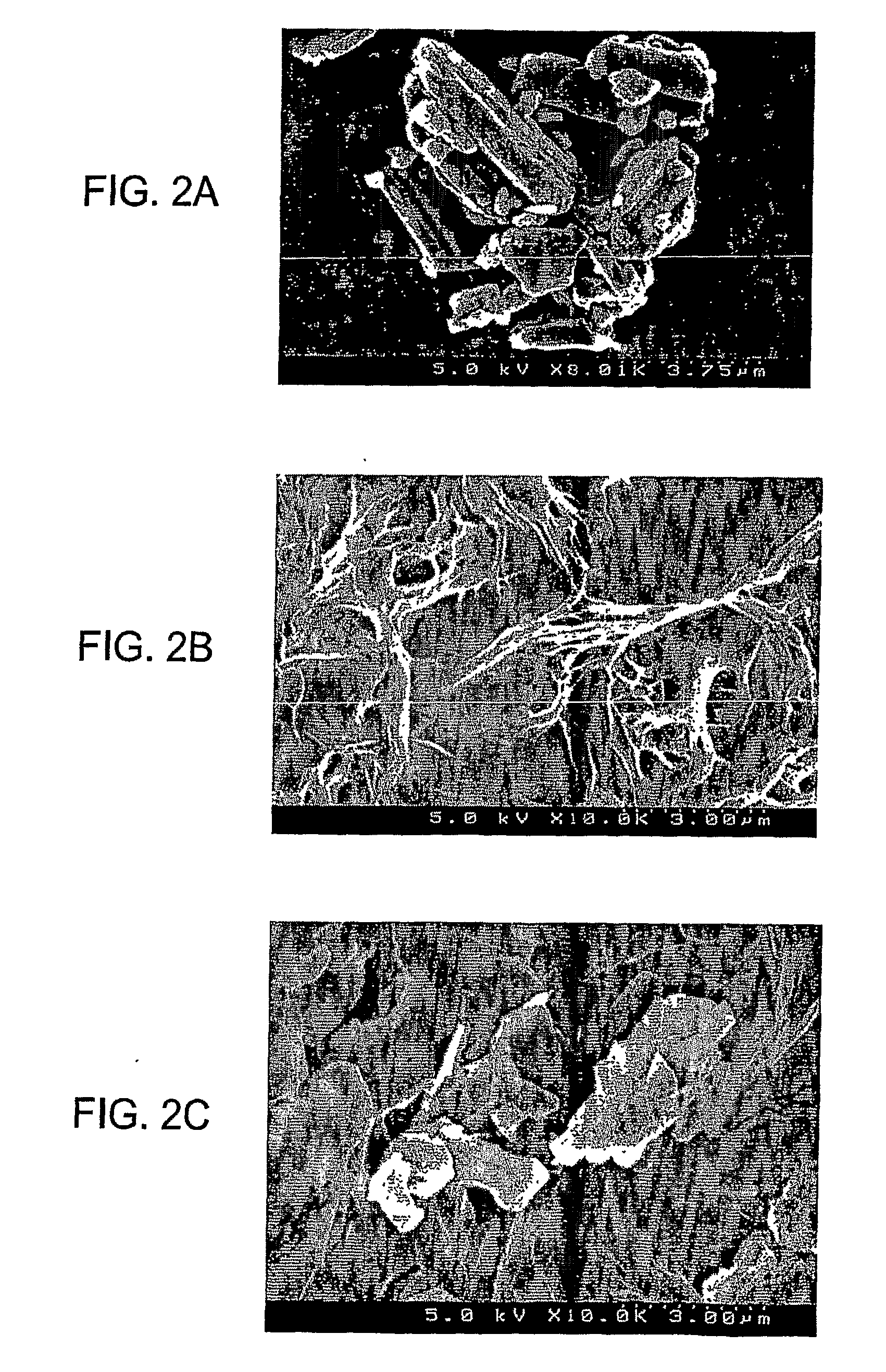
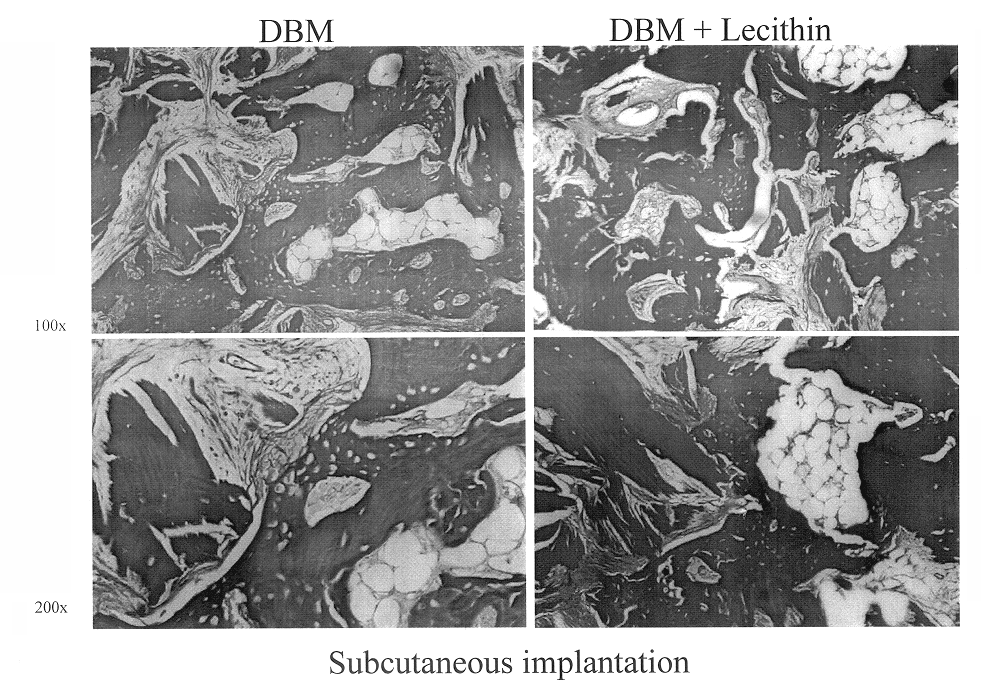
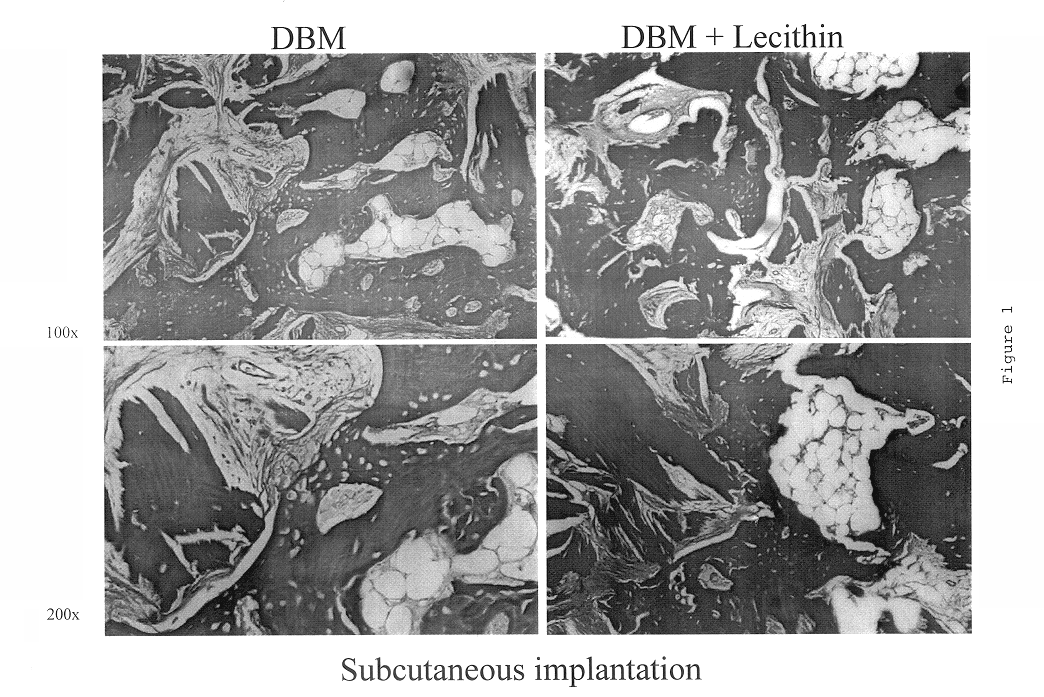
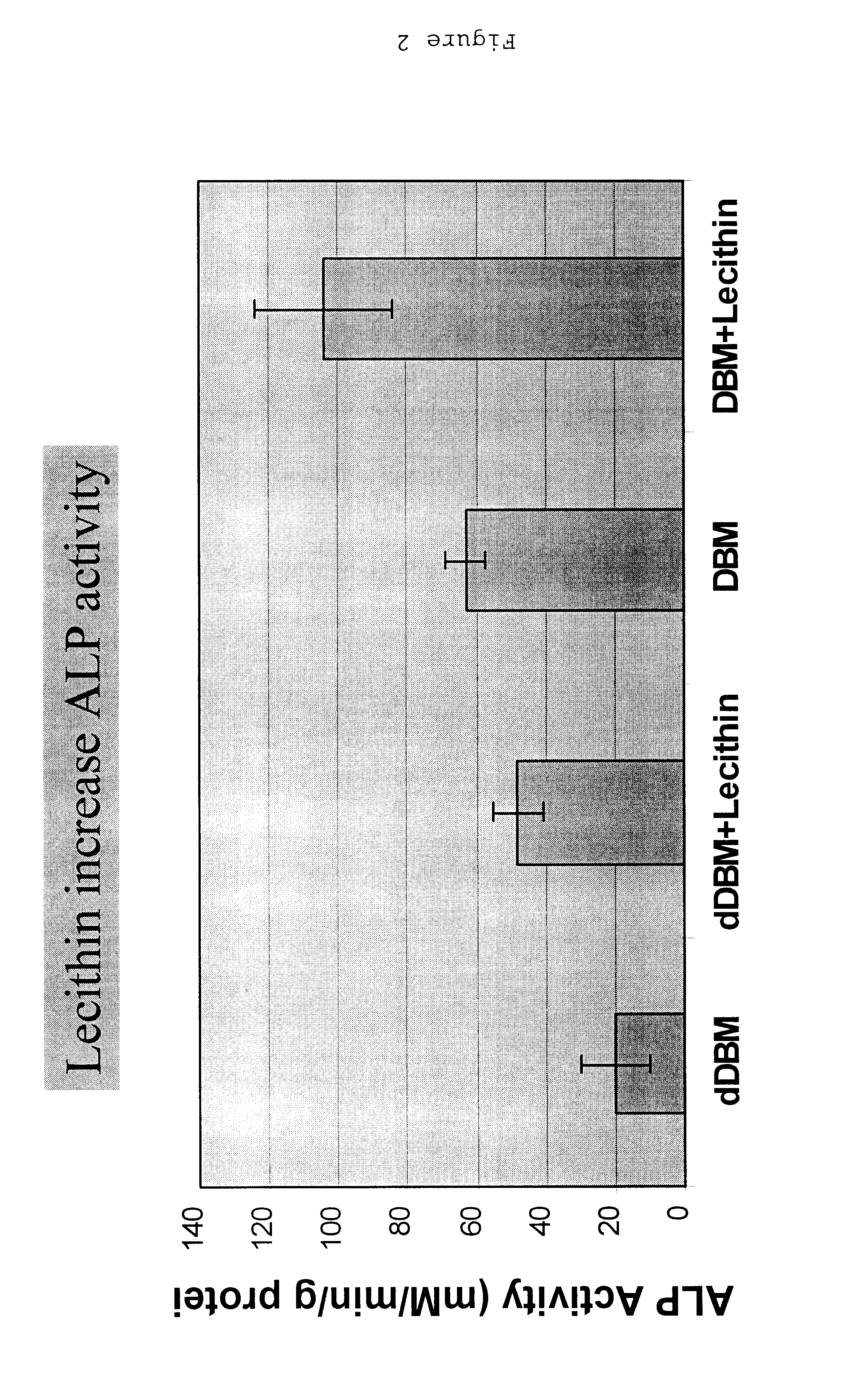
Bone graft material incorporating demineralized bone matrix and lipids
InactiveUS6565884B2Good osteoinductivityEasy to operateBiocidePowder deliveryHydrophilic polymersVitamin C
A demineralized bone putty composition comprises: (1) demineralized bone matrix (DBM); and (2) a lipid fraction selected from the group consisting of lecithin and a mixture of lecithin and triglycerides containing unsaturated fatty acids. The putty composition is moldable, biocompatible, slowly resorbable, and soluble in tissue fluids, and non-extrudable. The composition delivers a biologically active product to animals and humans that will enhance bone formation at sites where bone is lost, deficient, or present in suboptimal amounts. The composition can further comprise calcium, an antioxidant such as Vitamin E or Vitamin C, or a hydrophilic polymer such as methylcellulose or hydroxypropyl methylcellulose.
Owner:BIOMET MFG CORP
Dry powder inhaler system
InactiveUS20060254583A1Easy piercingRespiratorsPowder deliveryBULK ACTIVE INGREDIENTActive ingredient
An improved dry powder inhalation system comprising: at least one micronized active ingredient in an hydroxypropylmethylcellulose capsule (10), and an dry powder inhaler device equipped with piercing systems (12) having an equivalent diameter of not less than 0.8 mm.
Owner:GALEPHAR PHARMA RES
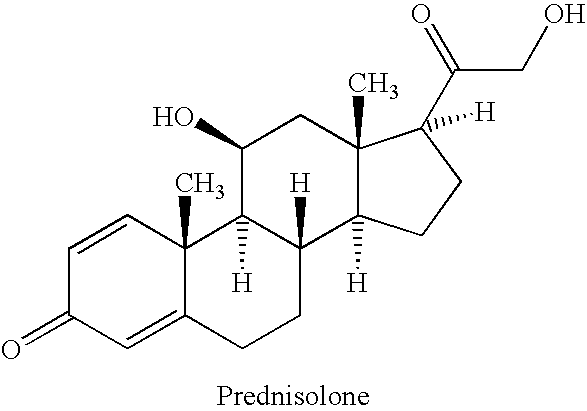
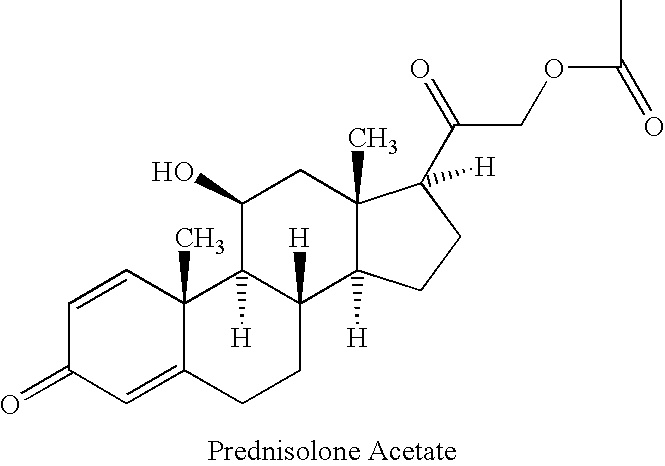
Prednisolone compositions
Disclosed herein are compositions comprising cyclodextrin derivatives and prednisolone and prodrugs thereof, and methods related thereto. The use of soluble polyanionic polymers such as hydroxypropylmethylcellulose and others in relation to these compositions is also disclosed. Delivery of these prednisolone-related compounds to the back of the eye via topical ophthalmic administration is also disclosed.
Owner:ALLERGAN INC
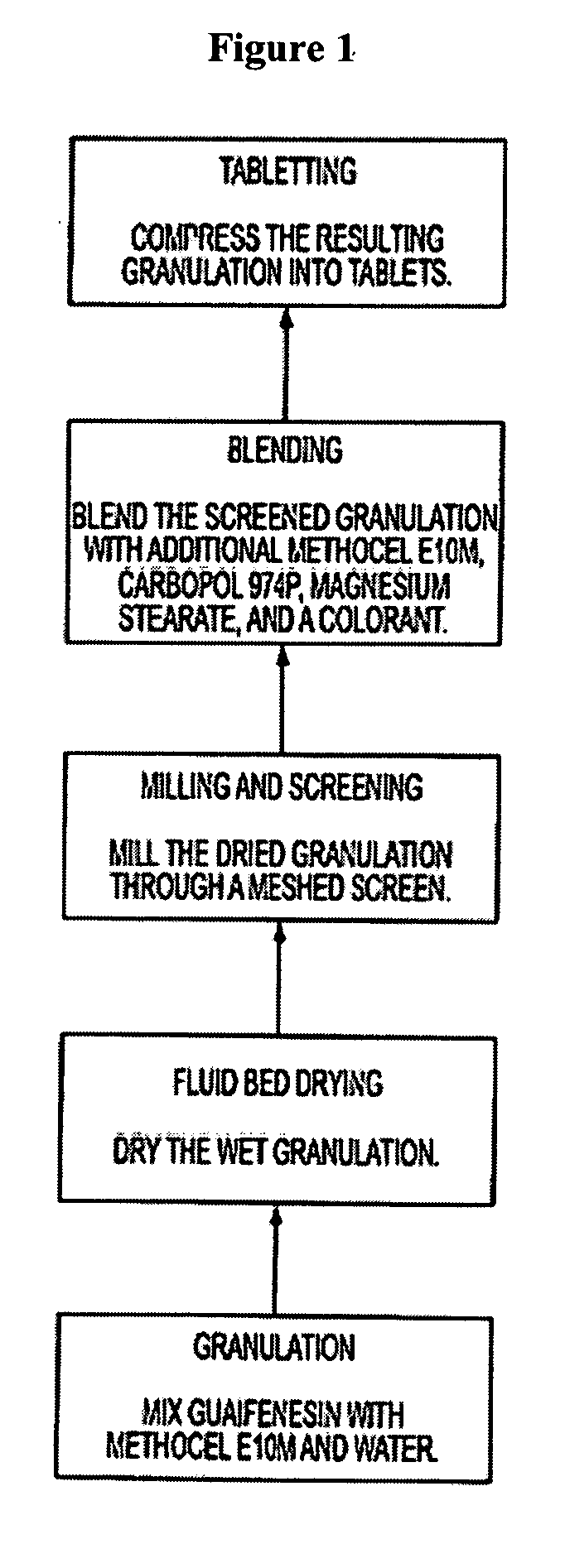
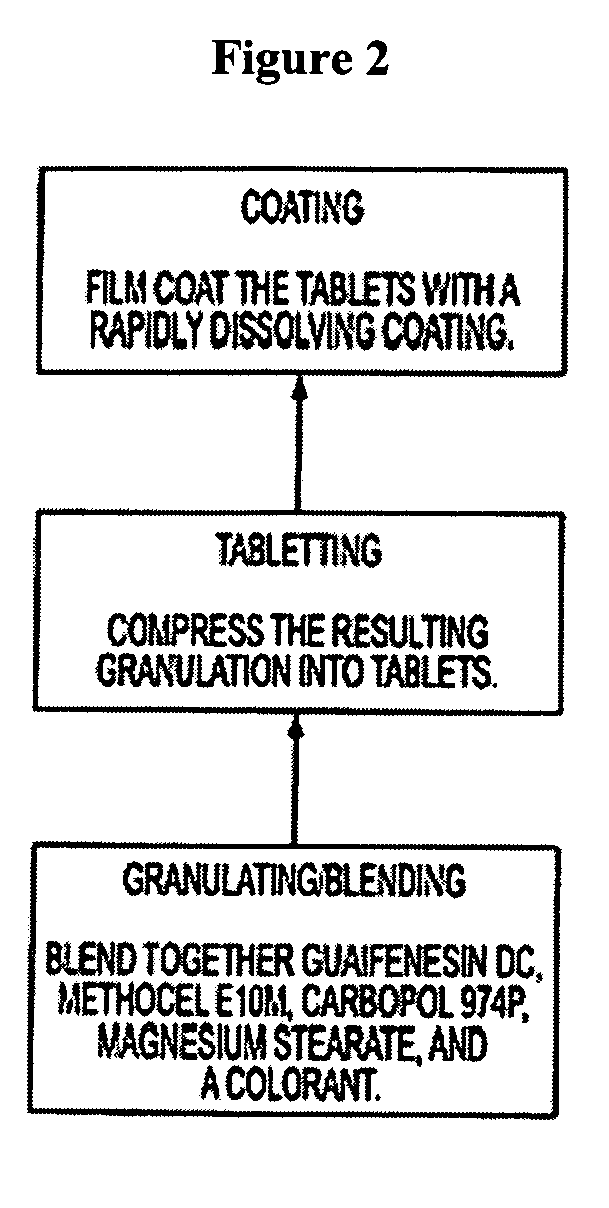
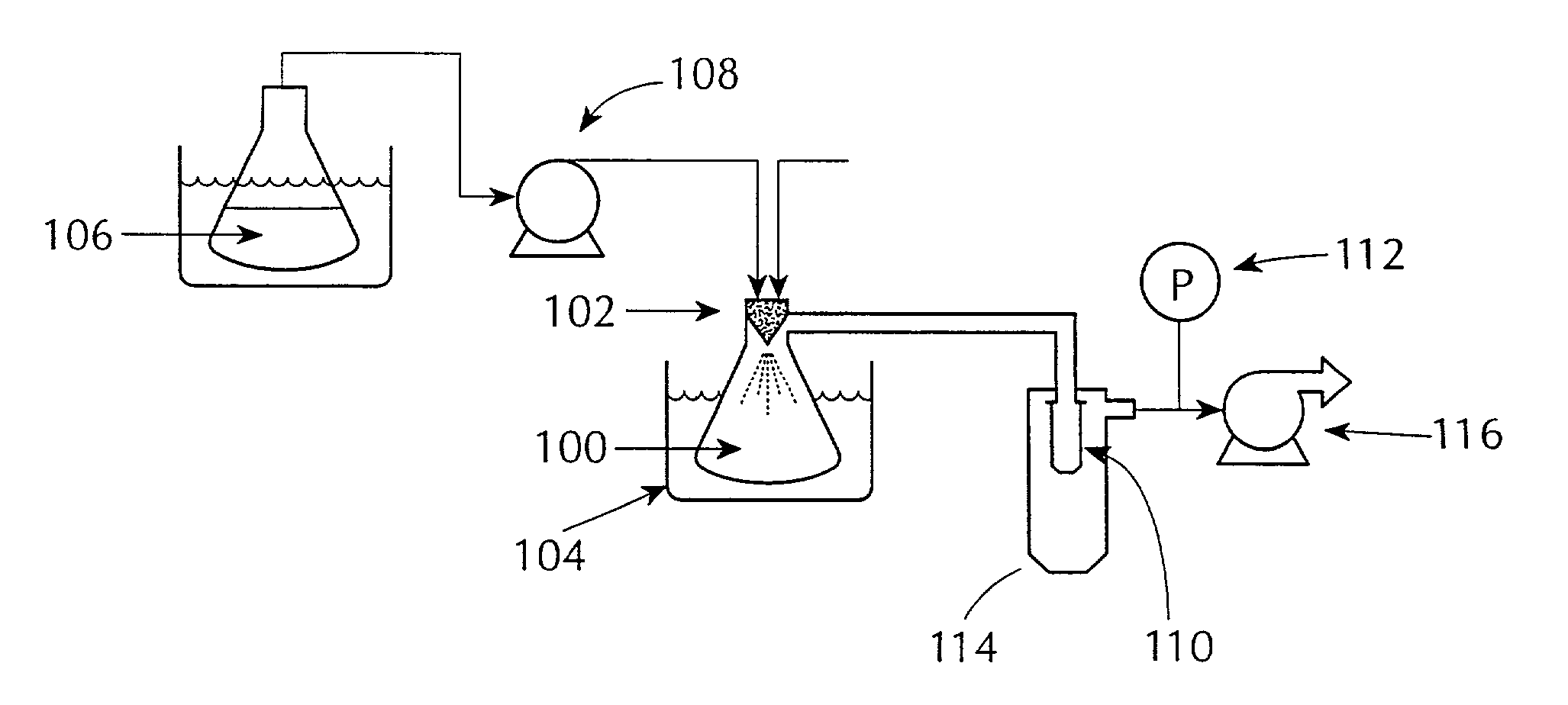
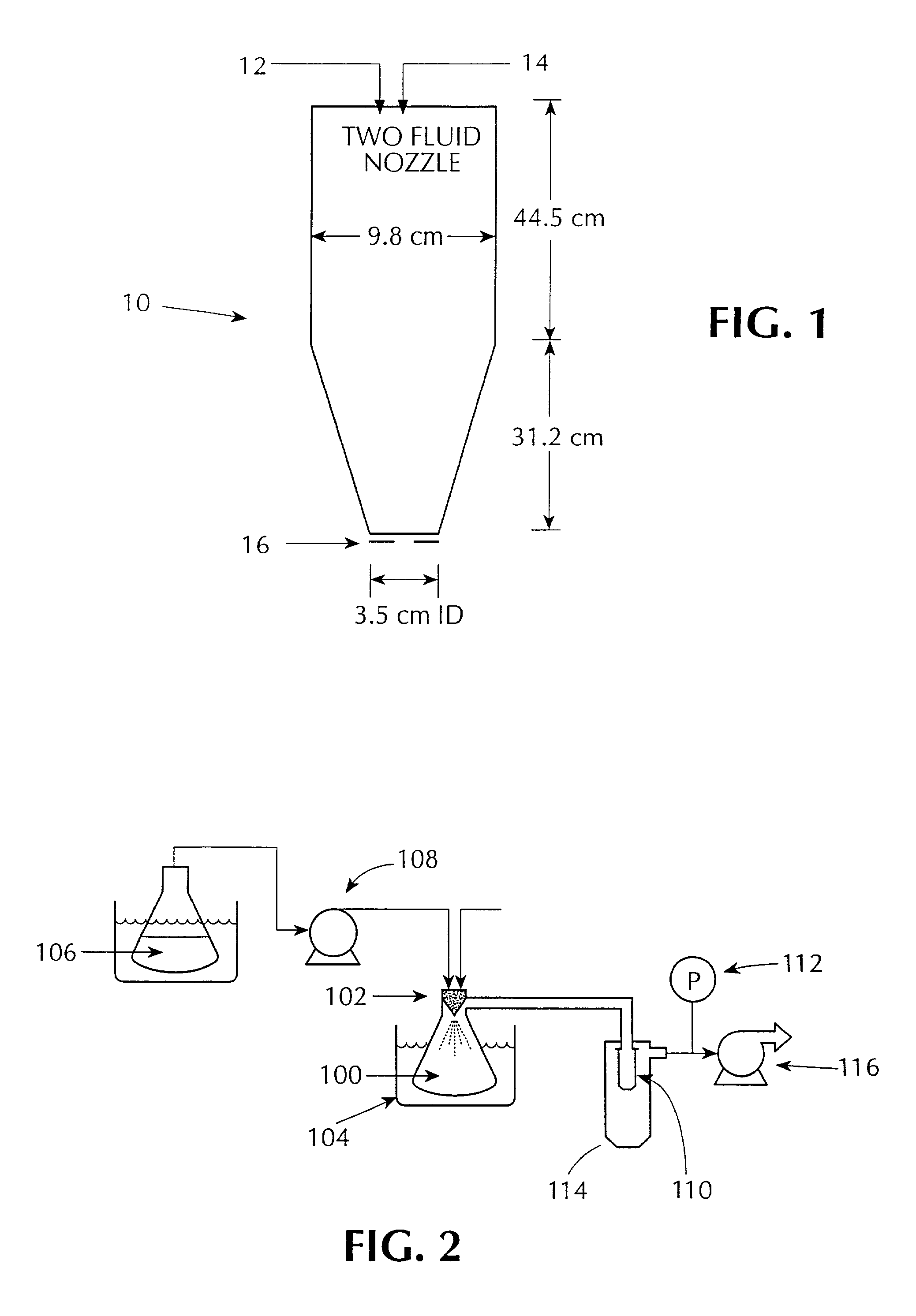
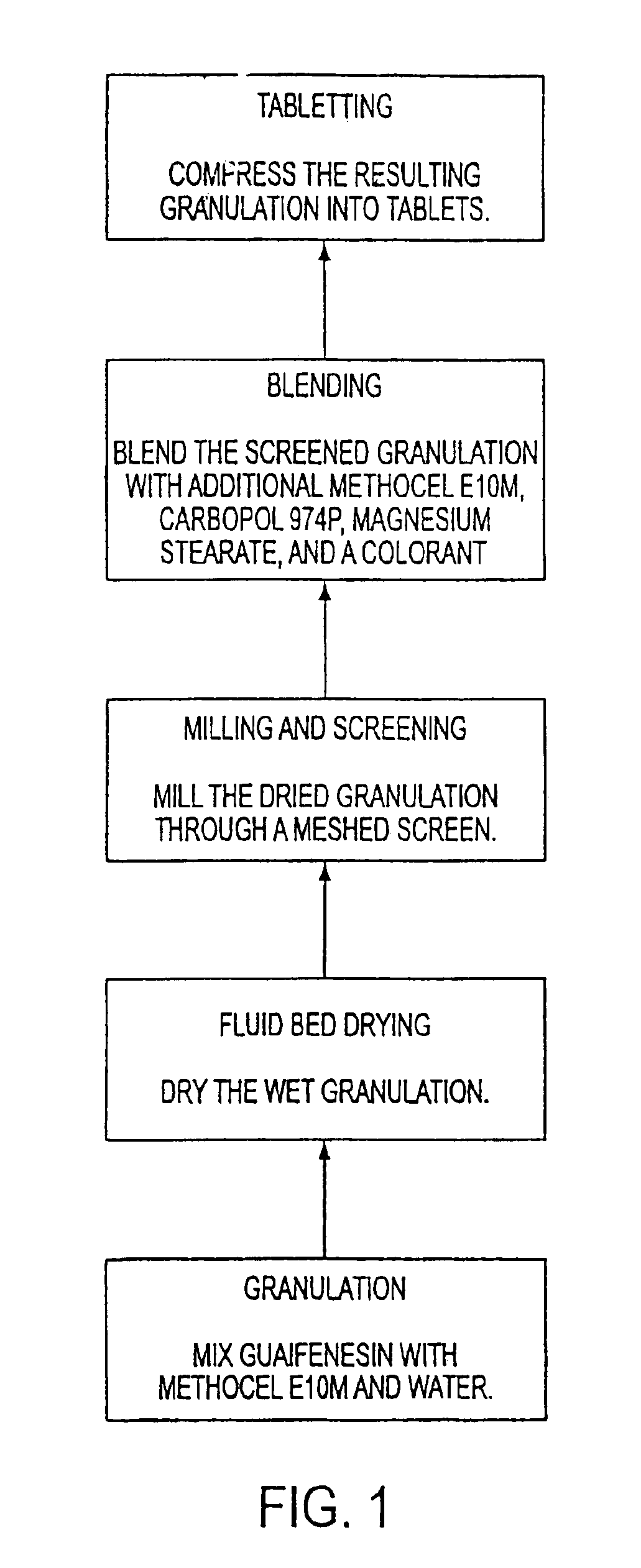
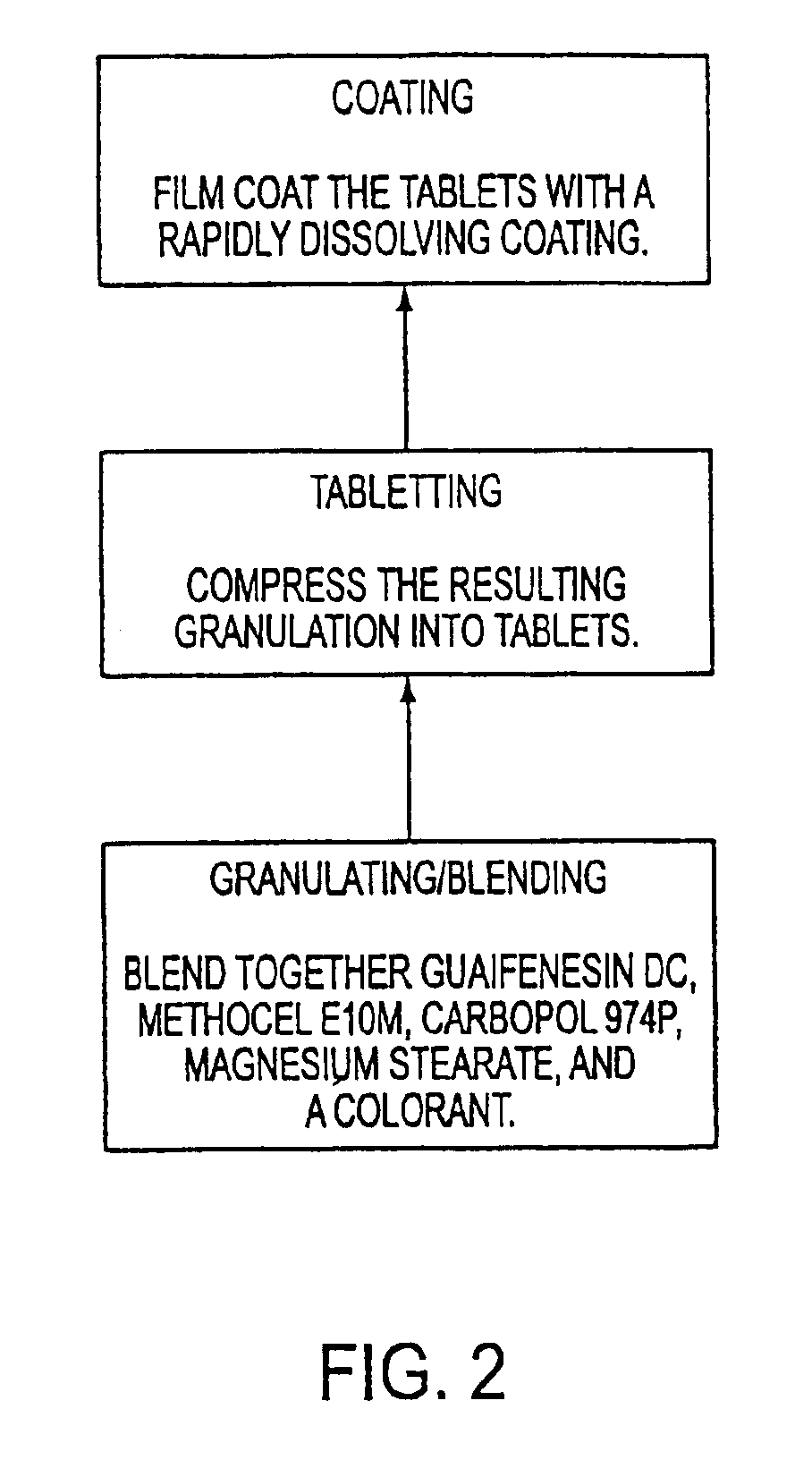
Sustained release formulations of guaifenesin and additional drug ingredients
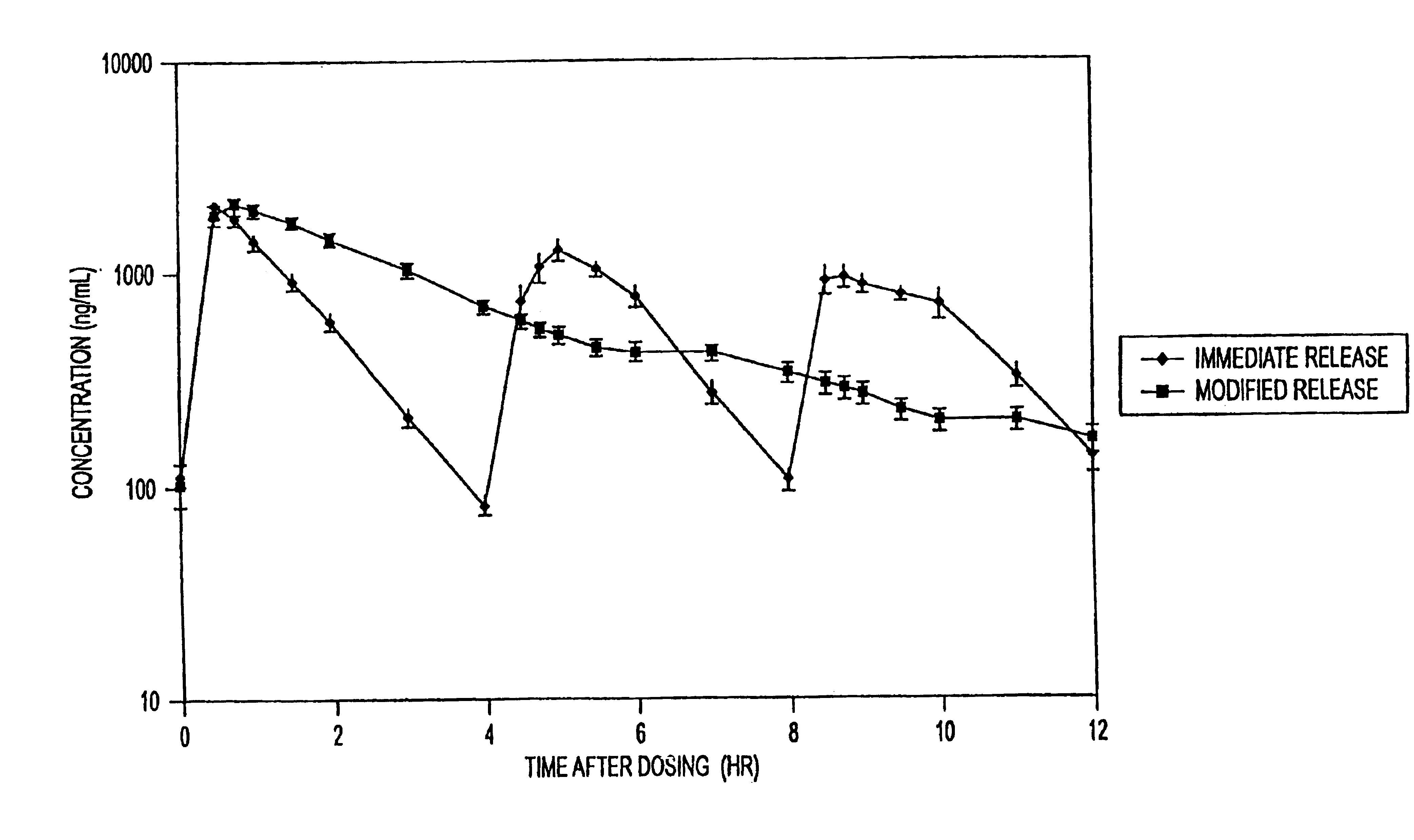
InactiveUS20050276852A1Increase in drug strengthSustained releaseOrganic non-active ingredientsEther/acetal active ingredientsSerum concentrationImmediate release
The invention relates to a novel pharmaceutical sustained release formulation of guaifenesin and at least one additional drug ingredient. The formulation may comprise a hydrophilic polymer, preferably a hydroxypropyl methylcellulose, and a water-insoluble polymer, preferably an acrylic resin, in a ratio range of about one-to-one (1:1) to about nine-to-one (9:1), more preferably a range of about three-to-two (3:2) to about six-to-one (6:1), and most preferably in a range of about two-to-one (2:1) to about four-to-one (4:1) by weight. This formulation capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject. The invention also relates to a modified release product which has two portions: a first portion having an immediate release formulation of guaifenesin and a second portion having a sustained release formulation of guaifenesin, wherein one or both portions has at least one additional drug ingredient. The modified release product has a maximum guaifenesin serum concentration equivalent to that of an immediate release guaifenesin tablet, and is capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject.
Owner:RB HEALTH US LLC
Solid pharmaceutical dispersions with enhanced bioavailability
InactiveUS8263128B2Improve bioavailabilityPreventing and retarding ratePowder deliveryBiocideAcetic acidHydroxypropylmethylcellulose acetate succinate
Spray dried solid dispersions comprising a sparingly soluble drug and hydroxypropylmethylcellulose acetate succinate (HPMCAS) provide increased aqueous solubility and / or biavailability in a use environment.
Owner:BEND RES
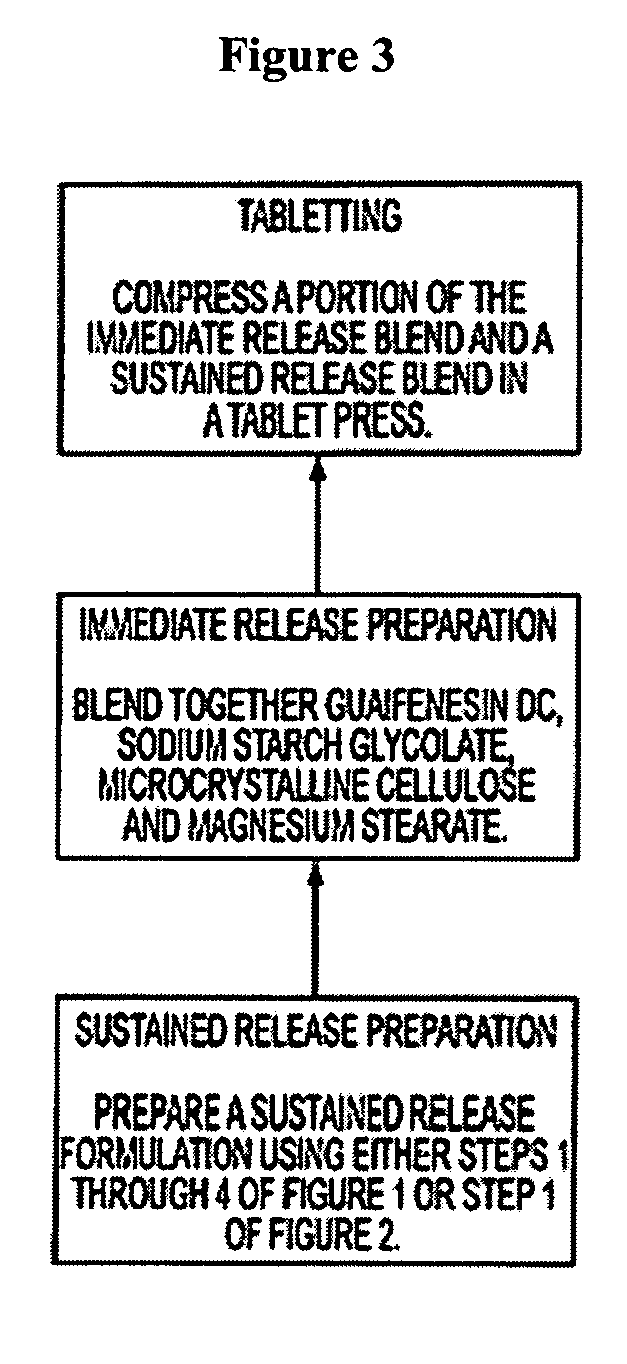
Sustained release formulations of guaifenesin and additional drug ingredients
InactiveUS6955821B2Increase in drug strengthSustained releaseEther/acetal active ingredientsOrganic non-active ingredientsSerum concentrationImmediate release
The invention relates to a novel pharmaceutical sustained release formulation of guaifenesin and at least one additional drug ingredient. The formulation may comprise a hydrophilic polymer, preferably a hydroxypropyl methylcellulose, and a water-insoluble polymer, preferably an acrylic resin, in a ratio range of about one-to-one (1:1) to about nine-to-one (9:1), more preferably a range of about three-to-two (3:2) to about six-to-one (6:1), and most preferably in a range of about two-to-one (2:1) to about four-to-one (4:1) by weight. This formulation capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject. The invention also relates to a modified release product which has two portions: a first portion having an immediate release formulation of guaifenesin and a second portion having a sustained release formulation of guaifenesin, wherein one or both portions has at least one additional drug ingredient. The modified release product has a maximum guaifenesin serum concentration equivalent to that of an immediate release guaifenesin tablet, and is capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject.
Owner:RB HEALTH US LLC
Slowly-released compound acidifier for poultry and livestock feed, preparation method thereof and feed
ActiveCN102578387AMatching scienceDefinitelyClimate change adaptationAnimal feeding stuffDiseaseFeed conversion ratio
The invention discloses a slowly-released compound acidifier for poultry and livestock feed, a preparation method thereof and feed containing the same. The compound acidifier comprises the following compositions in part by weight: 30 to 80 parts of complex organic acid, 15 to 50 parts of supplementary materials and 5 to 20 parts of coating agents. The complex organic acid is citric acid, fumaric acid, malic acid, lactic acid, linoleic acid, crataegolic acid, ursolic acid, chlorogenic acid, glycyrrhizic acid and oleanolic acid, and the supplementary materials are silicon dioxide, hydroxypropyl methylcellulose and ethyl cellulose. The acidifier is scientifically proportioned, has strong efficiency and is long-acting and slow-releasing, nutrients in the feed are free from being damaged, the acidifier has good fluidity and is easy to be uniformly mixed with the feed, the digestive absorption of nutrients can be promoted, the conversion rate of the feed can be improved, the productivity of poultry and livestock can be improved, the time for domestic animals for sale can be effectively shortened, the health of gastrointestinal mucosa of the animals can be protected, the immunologic function of the animals can be strengthened, and diseases can be prevented.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH +1
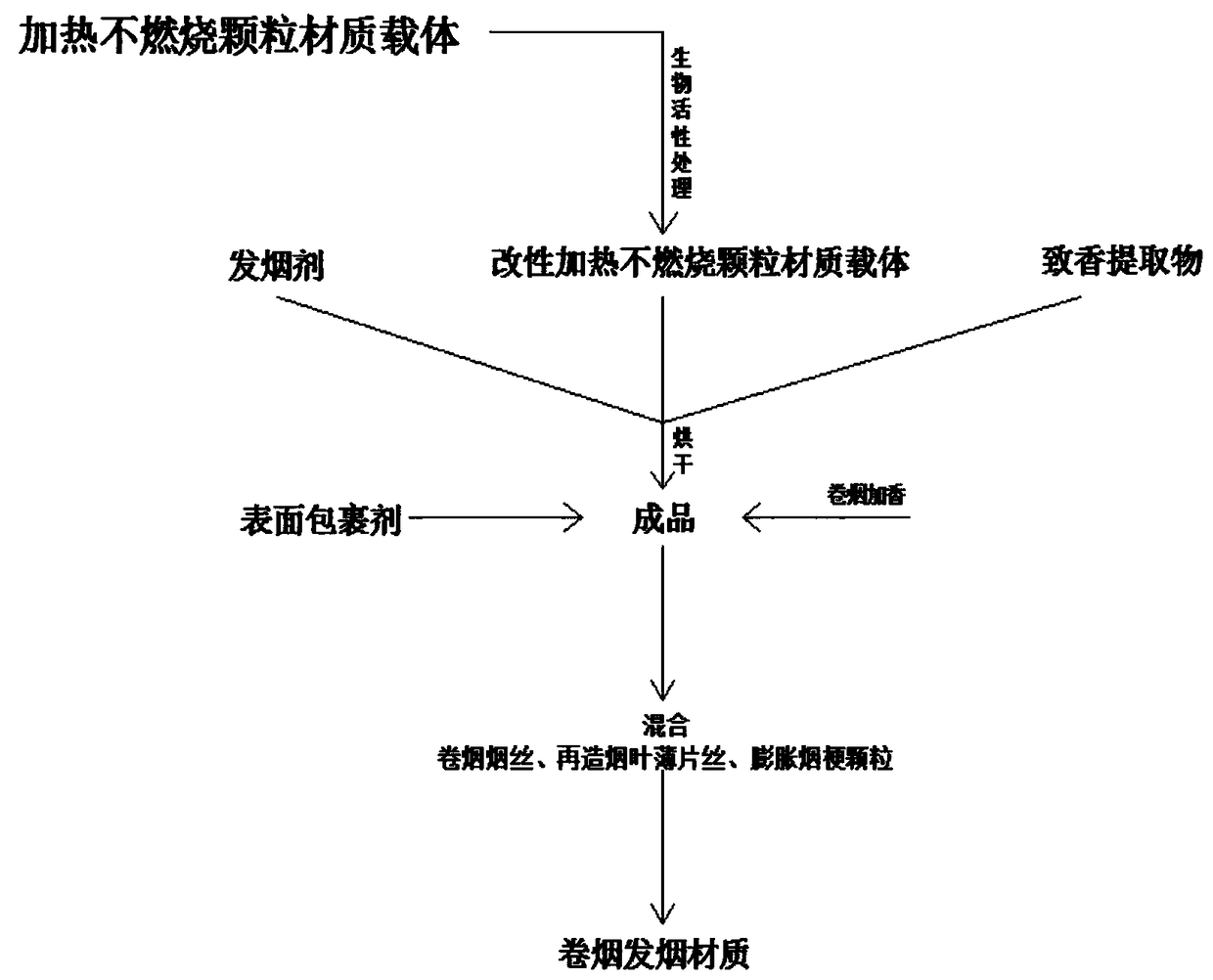
Heating-non-burning cigarette fuming granular material and preparation method thereof
The invention discloses a heating-non-burning cigarette fuming granular material and a preparation method thereof. The granular material includes a heating-non-burning granular material carrier, fuming agents, a surface coating agent and a perfuming extract. The heating-non-burning granular material carrier includes juncus roemerianus, gramineous plant granules, radix puerariae, eupatorium adenophorum, tobacco powder, tobacco stem powder, hydroxypropyl methyl cellulose, beta cyclodextrin, gelatin, sodium alginate, diatom ooze, chitosan, methylcellulose, lignin and other natural plant granules.The preparation method of the granular material includes the following step of preparing the heating-non-burning granular material carrier at first, wherein the carrier is preferably selected from domestic high-quality granules or natural mineral compound granules and powder thereof for pelleting or compounding. The fuming granules are integrally loaded according to volume and then sealed, finished products of a fuming unit are mixed with the fuming agents of perfuming groups different in taste, and the problems that cigarette containers in the heating-non-burning field are monotonous in typeand processing technologies are cumbersome are solved.
Owner:YUNNAN XIKE TECH CO LTD
Printable Composition of a Liquid or Gel Suspension of Diodes
ActiveUS20120161195A1Solid-state devicesSemiconductor/solid-state device manufacturingOctanolSolvent
An exemplary printable composition of a liquid or gel suspension of diodes comprises a plurality of diodes, a first solvent and / or a viscosity modifier. In other exemplary embodiments a second solvent is also included, and the composition has a viscosity substantially between about 100 cps and about 25,000 cps at about 25° C. In an exemplary embodiment, a composition comprises: a plurality of diodes or other two-terminal integrated circuits; one or more solvents comprising about 15% to 99.9% of any of N-propanol, isopropanol, dipropylene glycol, diethylene glycol, propylene glycol, 1-methoxy-2-propanol, N-octanol, ethanol, tetrahydrofurfuryl alcohol, cyclohexanol, and mixtures thereof; a viscosity modifier comprising about 0.10% to 2.5% methoxy propyl methylcellulose resin or hydroxy propyl methylcellulose resin or mixtures thereof; and about 0.01% to 2.5% of a plurality of substantially optically transparent and chemically inert particles having a range of sizes between about 10 to about 50 microns.
Owner:NTHDEGREE TECH WORLDWIDE
Solid pharmaceutical dispersions with enhanced bioavailability
InactiveUS8257741B2Improve solubilityEffective dispersionPowder deliveryBiocideAcetic acidHydroxypropylmethylcellulose acetate succinate
Spray dried solid dispersions comprising a sparingly soluble drug and hydroxypropylmethylcellulose acetate succinate (HPMCAS) provide increased aqueous solubility and / or biavailability in a use environment.
Owner:BEND RES
Methyl ester-based microemulsions for cleaning hard surfaces
A cleaning composition containing: (a) from about 1.0 to about 15.0% by weight of a monoethanolamine salt of an alkyl sulfonic acid; (b) from about 3 to about 50% by weight of a C6-C14 methyl ester primary solvent; (c) from about 1.0 to about 15.0% by weight of a short-chain cosurfactant; (d) from about 1 to about 25% by weight of a polar solvent having a water solubility of from about 1 to 5 g / 100 ml; (e) up to about 10.0% by weight of a nonionic surfactant; (f) from about 0.05 to about 3.0% by weight of a thickening agent selected from the group consisting of hydroxypropyl cellulose, hydroxypropyl methylcellulose, and mixtures thereof; and (g) remainder, water, all weights being based on the total weight of the composition, and wherein the composition is terpene-free.
Owner:COGNIS IP MANAGEMENT GMBH
Printable composition of a liquid or gel suspension of diodes
An exemplary printable composition of a liquid or gel suspension of diodes comprises a plurality of diodes, a first solvent and / or a viscosity modifier. In other exemplary embodiments a second solvent is also included, and the composition has a viscosity substantially between about 100 cps and about 25,000 cps at about 25° C. In an exemplary embodiment, a composition comprises: a plurality of diodes or other two-terminal integrated circuits; one or more solvents comprising about 15% to 99.9% of any of N-propanol, isopropanol, dipropylene glycol, diethylene glycol, propylene glycol, 1-methoxy-2-propanol, N-octanol, ethanol, tetrahydrofurfuryl alcohol, cyclohexanol, and mixtures thereof; a viscosity modifier comprising about 0.10% to 2.5% methoxy propyl methylcellulose resin or hydroxy propyl methylcellulose resin or mixtures thereof; and about 0.01% to 2.5% of a plurality of substantially optically transparent and chemically inert particles having a range of sizes between about 10 to about 50 microns.
Owner:NTHDEGREE TECH WORLDWIDE
Grinding aid for slag cement
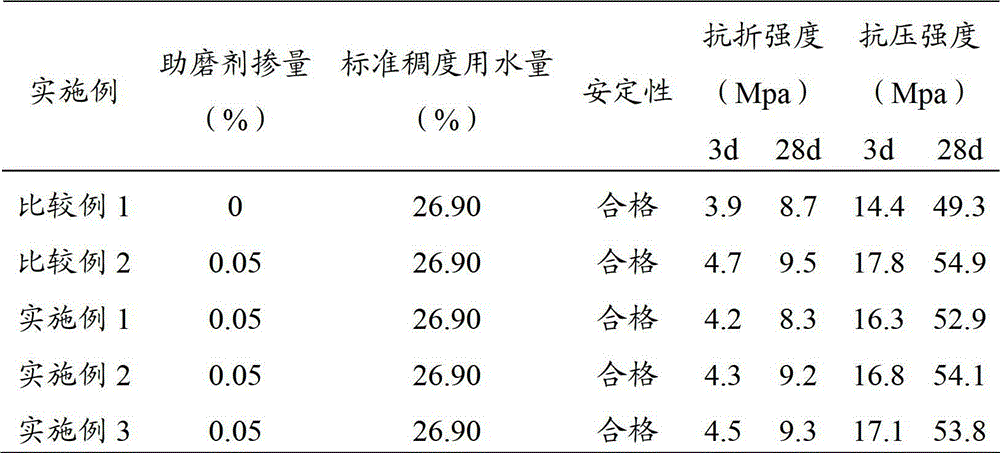
The invention provides a grinding aid for slag cement. The grinding aid provided by the invention comprises the following components by weight percent: 5-20wt% of alcohol amine compound, 0.1-5wt% of hydroxypropyl methyl cellulose, 5-30wt% of polyhydric alcohol compound, 0.5-10wt% of sodium hexametaphosphate, 1-10wt% of soluble sulfate, 1-8wt% of sodium dodecyl benzene sulfonate and the balance of water. In the grinding agent provided by the invention, the hydroxypropyl methyl cellulose is beneficial for stimulating the activity of the slag, forming stable hydrous products in the reaction process, improving the property of the cement, improving the strength of the cement and improving the stability of the liquid grinding aid at the same time. Experimental result shows that the grinding aid is capable of increasing the breaking strength of the cement to 9.5MPa and increasing the compressive strength to about 55MPa.
Owner:SHANDONG HONGYI TECH +1
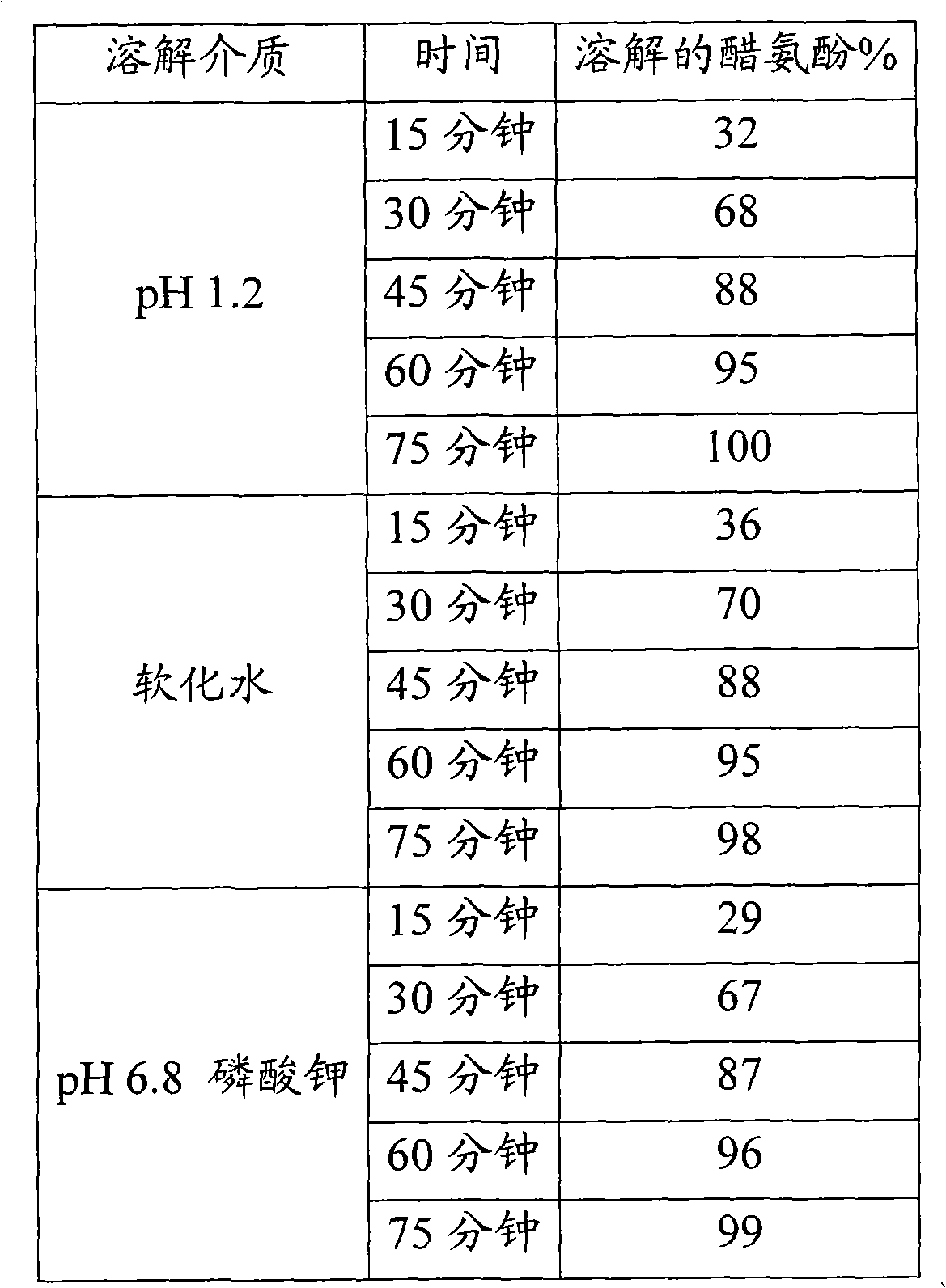
Pantoprazole multiparticulate formulations
ActiveUS20050129761A1Low variabilityProlong the action timeBiocideDispersion deliveryMedicineEnantiomer
Pantoprazole sodium multiparticulates are described which avoid sticking to nasogastric and gastronomy tubes. The pantoprazole multiparticulates have a spheroid core of pantoprazole or an enantiomer thereof, or a salt thereof, a surfactant, and a distintegrant; a sub coat which is comprised of hydroxypropyl methylcellulose (hypromellose) and water, an enteric coat on the sub-coat, and a final seal coat over the enteric coat, which is composed of hydroxypropyl methylcellulose (hypromellose) and water.
Owner:WYETH LLC
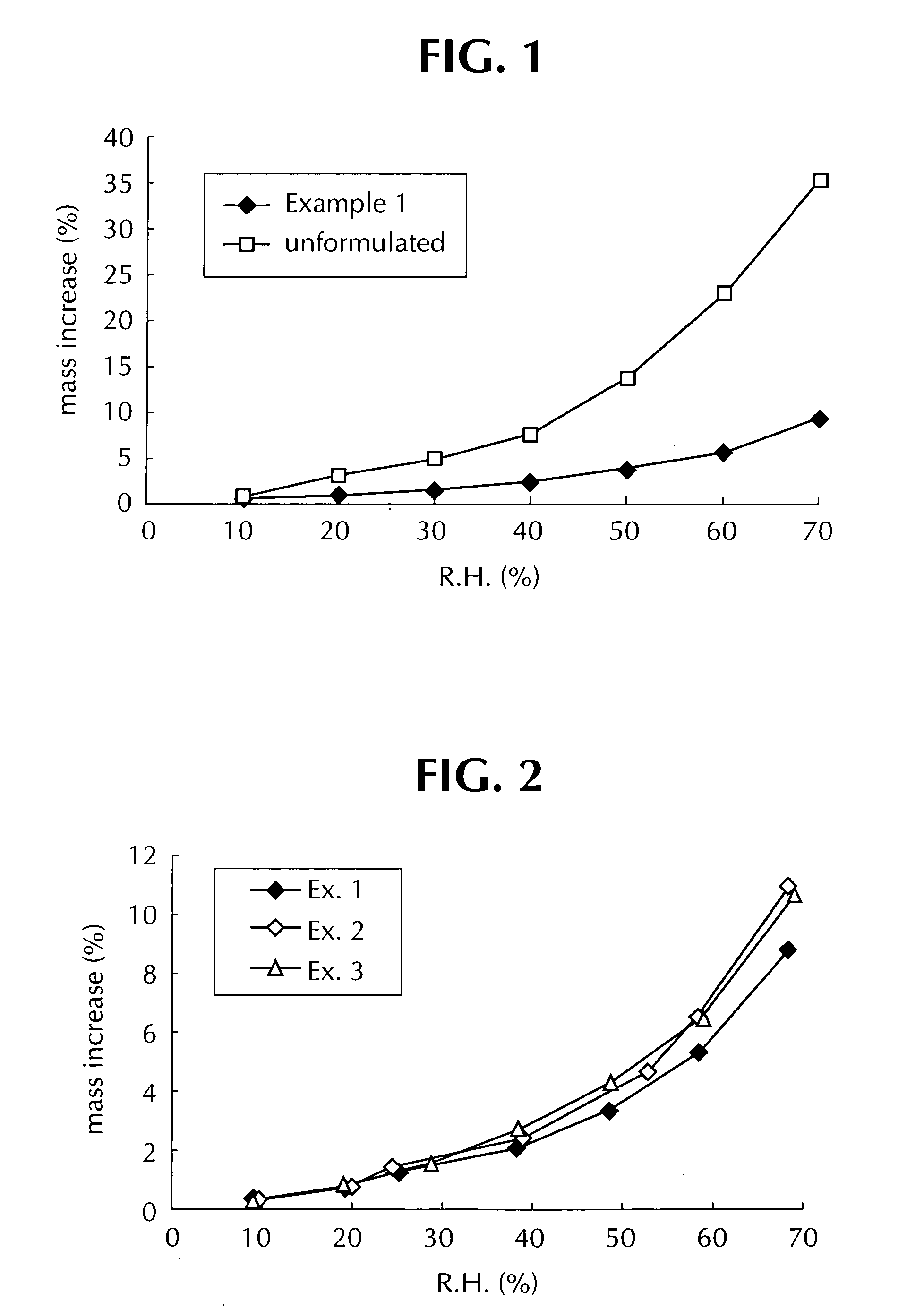
Solid dispersions comprising a hygroscopic and/or deliquescent drug
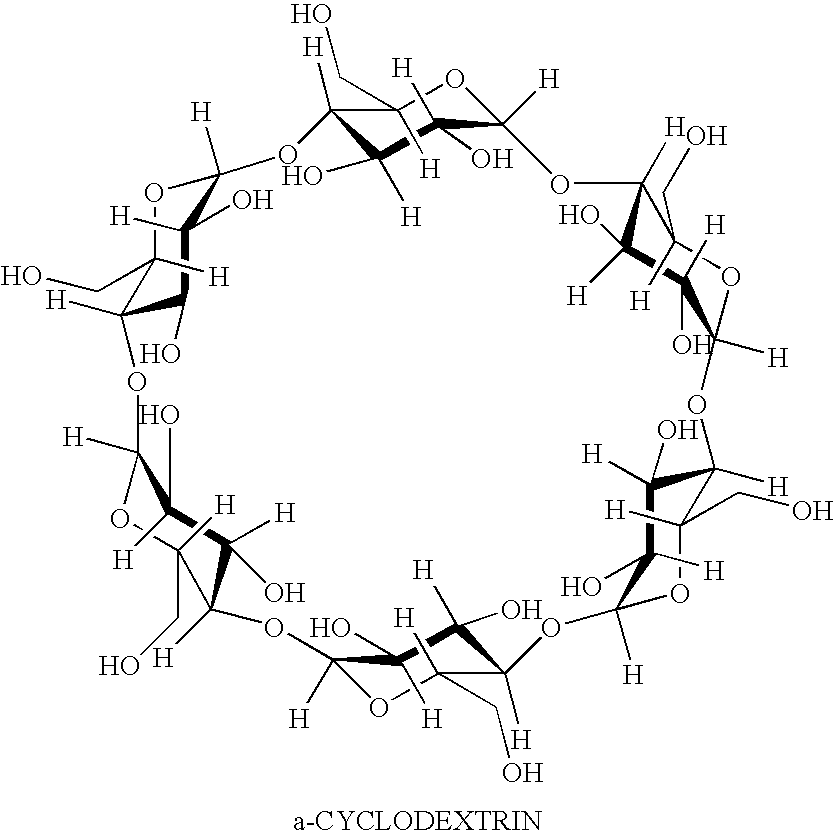
InactiveUS20050013856A1Easy to mergeAbsorb morePowder deliveryOrganic active ingredientsPolyethylene glycolCyclodextrin
A pharmaceutical composition is provided comprising a drug and a carrier medium, wherein the carrier medium comprisese (a) a matrix forming agent selected from the group consisting of hydroxyethylcelluloses, hydroxypropylcelluloses, hydroxypropylmethylcelluloses, hydroxypropylmethylcellulose phthalates, polyvinylpyrrolidones, polyethylene glycols, polyglycolized glycerides, cyclodextrins, carbomers and combinations thereof, and (b) a filler; and wherein the drug is hygroscopic and / or deliquescent and is dispersed in the carrier medium, and wherein the composition is a solid dispersion and is acceptably non-hygroscopic.
Owner:PHARMACIA CORP
Cementitious capillary crystalline waterproofing agent
InactiveCN101759414AImprove impermeabilityPromotes self-healingSolid waste managementSelf-healingSodium Bentonite
The invention discloses a cementitious capillary crystalline waterproofing agent, which contains the following raw materials in parts by weight: 50 parts of Portland cement; 25 parts of quartz sand; 9 parts of siliceous dust; 3 parts of calcium hydroxide; 2 parts of sodium bentonite; 1 part of kieselguhr; 3.9 parts of instant sodium silicate; 2.5 parts of aluminum potassium sulfate; 0.3 parts of zinc fluosilicate; 0.20 parts of sodium polyacrylate; 0.2 parts of hydroxypropyl methyl cellulose; 2 parts of zinc stearate; 0.5 parts of naphthalene water reducer; 0.2 parts of sugar lime; 0.1 parts of citric acid; and 0.1 parts of antifoaming agent. After the raw materials are weighed according to the proportions, the raw materials are ground into particles with grain size more than or equal to 200-mesh sieve in a ball mill to obtain the finished product. The invention has the advantages that the cost is low, the anti-permeability is good, the self-healing performance is good, the bonding power is strong, the steel bar corrosion is prevented, no toxicity and no harmfulness are caused and the construction is easy.
Owner:河南奥思达新材料有限公司
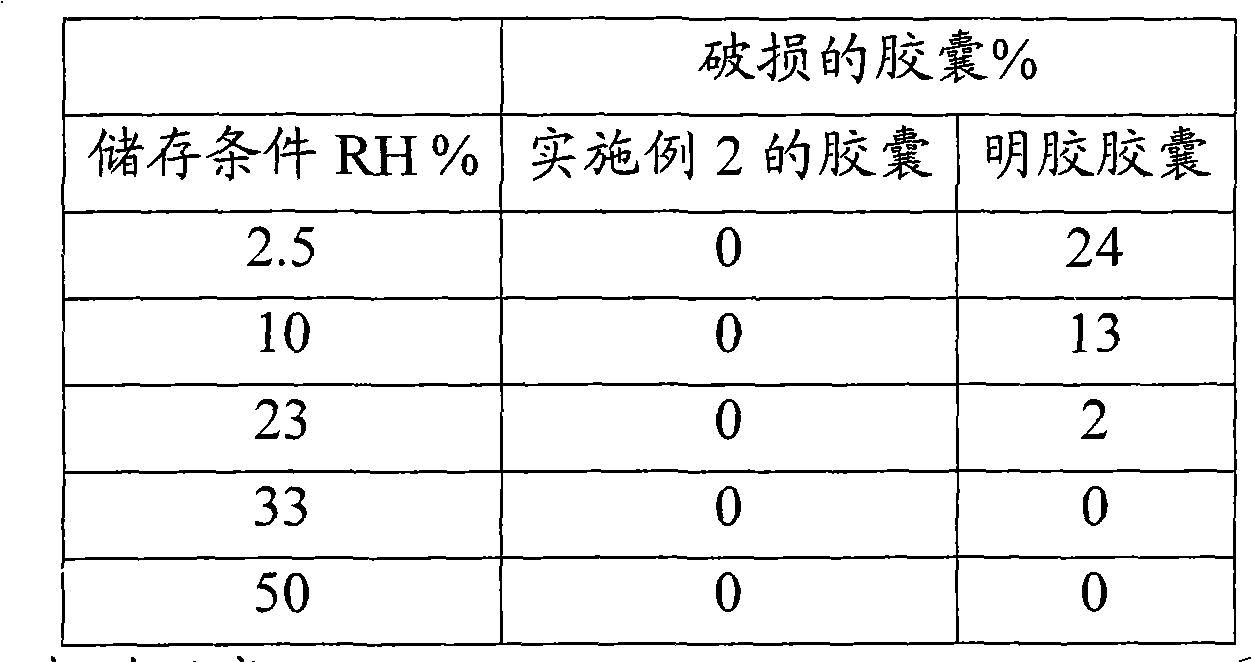
Hydroxypropyl methyl cellulose hard capsules and process of manufacture
ActiveCN101595133ASolubility effectCost effectivePowder deliveryCapsule deliveryPolymer scienceHard Capsule
A composition for manufacture of hard hydroxypropyl methyl cellulose capsules comprising a film forming material of hydroxypropyl methyl cellulose having a methoxy content of 27.0-30.0% (w / w), and a hydroxypropoxy content of 4.0 - 7.5% and as a 2% weight solution, a viscosity of 3.5 - 6.0 cPs at 20 DEG C, dipping compositions, process for manufacture of hard hydroxypropyl methyl cellulose capsules according to a dip coating process and hard capsule shells.
Owner:CAPSUGEL BELGIUM NV
Compounds and methods for lowering the abuse potential and extending the duration of action of a drug
InactiveUS20070203165A1Low affinityLow abuse potentialBiocideNervous disorderNasal cavityEster prodrug
Owner:CONTROLLED CHEM INC
Self-healing polymer cement waterproofing coating and preparation process thereof
The invention relates to a self-healing polymer cement waterproofing coating. The self-healing polymer cement waterproofing coating comprises a liquid material component A and a powder material component B in a weight ratio of 1:(1.0-1.2), wherein the liquid material component A comprises the following components in percentage by weight: 15 to 50 percent of ethylene-vinyl acetate copolymer emulsion, 35 to 65 percent of crylic acid emulsion, 0.2 to 0.8 percent of acrylic resin, and 14.20 to 19.80 percent of water. The powder material component B comprises the following components in percentage by weight: 19.50 to 37.50 percent of low alkali rapid hardening sulphate aluminium cement, 62.10 to 80.35 percent of quartz sand, 0.05 to 0.15 percent of hydroxypropyl methyl cellulose, 0.05 to 0.10 percent of water reducing agent, and 0.05 to 0.15 percent of antifoaming agent. The waterproofing coating is bonded firmly, has high tensile strength, a good permeability resistance effect, is not easy to crack and has the function of repairing fine cracks.
Owner:BEIJING ORIENTAL YUHONG WATERPROOF TECH CO LTD +2
Baked products containing rice flour
A mix for the preparation of rice-containing baked products, containing 100% rice flour (containing about 15 to about 26% amylose), about 0 to about 20% waxy rice flour, about 1 to about 4% hydroxypropyl methylcellulose food gum, about 1 to about 5% yeast, about 0 to about 10% rice bran, about 4 to about 20% sugar, and about 1 to about 4% salt. A method for preparing a rice-containing baked product, involving mixing 100% rice flour (containing about 15 to about 26% amylose), about 0 to about 20% waxy rice flour, about 1 to about 4% hydroxypropyl methylcellulose food gum, about 1 to about 5% yeast, about 0 to about 10% rice bran, about 4 to about 20% sugar, and about 1 to about 4% salt with an amount of water and oil and for a time sufficient to wet all the particles, and baking.
Owner:US SEC AGRI
Enveloping acidifier for feed and preparation method thereof
ActiveCN102106461AHigh total acid contentImprove protectionAnimal feeding stuffAccessory food factorsMaterial consumptionMethyl cellulose
The invention relates to enveloping acidifier and the preparation method thereof. The enveloping acidifier comprises acidifier solid microparticles and an enveloping layer on the surface of the acidifier solid microparticles, wherein the enveloping layer comprises an ethyecellulose (EC) and hydroxypropyl methyl cellulose (HPMC) composite layer and an enteric-coated polyacrylic resin layer. In theinvention, sustained-release coating material with good film forming property and the coating process are adopted, so that excessive enveloping material consumption is avoided, the weight of the acidifier solid microparticles is increased slightly, the large total effective acid of the acidifier is kept, and the acidifier has good sustained-release effect.
Owner:安徽泰格生物技术股份有限公司
Acidified zeolite
The invention discloses an acidified zeolite. The technical scheme is as follows: the acidified zeolite is composed of zeolite, attapulgite clay, magnesia, hydrochloric acid, instant sodium silicate, polyvinyl alcohol, hydroxypropyl methylcellulose and sodium carbonate. The acidified zeolite materials are input into a mill and milled, and the milled powder is the acidified zeolite. The production method of acidified red mud adopts acidification before composite proportioning, thereby avoiding the chemical reaction between the sulfuric acid and the instant sodium silicate, polyvinyl alcohol, hydroxypropyl methylcellulose and sodium carbonate; and the acidified zeolite can effectively remove ammonia, iron, fluorine, phosphides and micro pollutants in domestic sewage, and can be used for removing or recovering heavy metal ions and treating radioactive waste. The acidified rear has the characteristics of favorable thixotropy, favorable heat stability, favorable plasticity and favorable binding property, and is suitable for producing drying agents, adsorptive separation agents, molecular sieves, catalysts, defluorination soil improvers, deodorizers and firefighting products.
Owner:江苏世澳非金属应用科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com