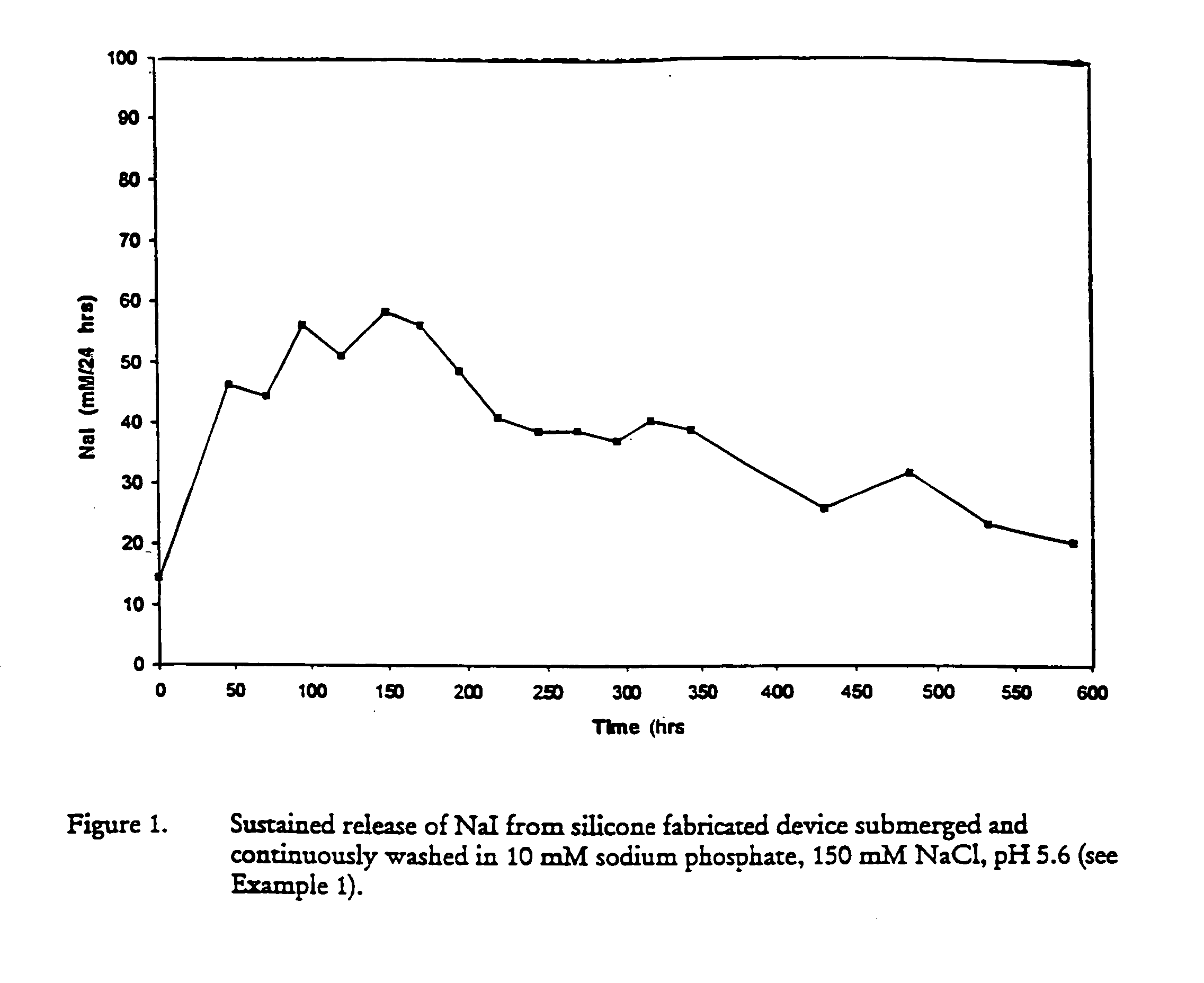
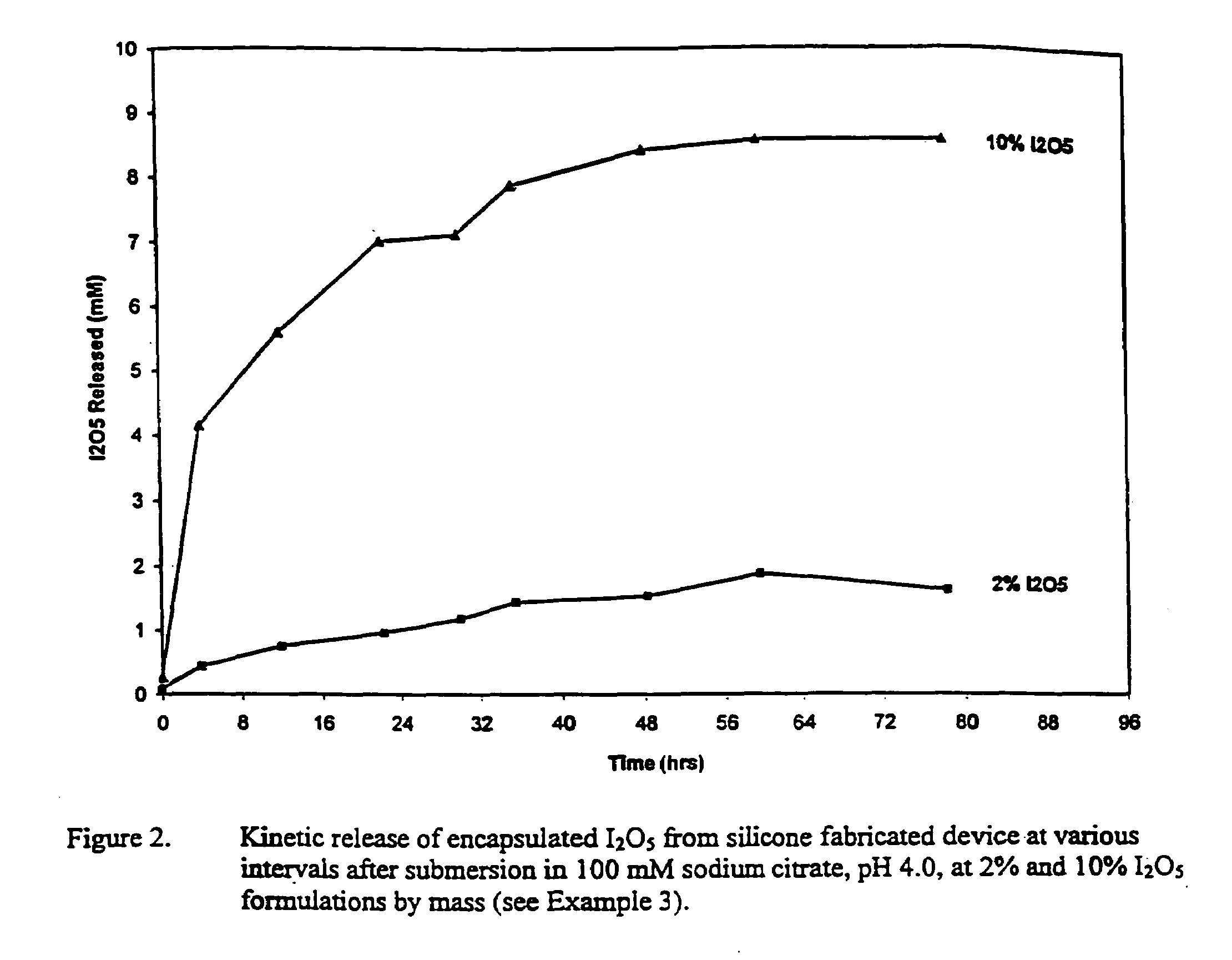
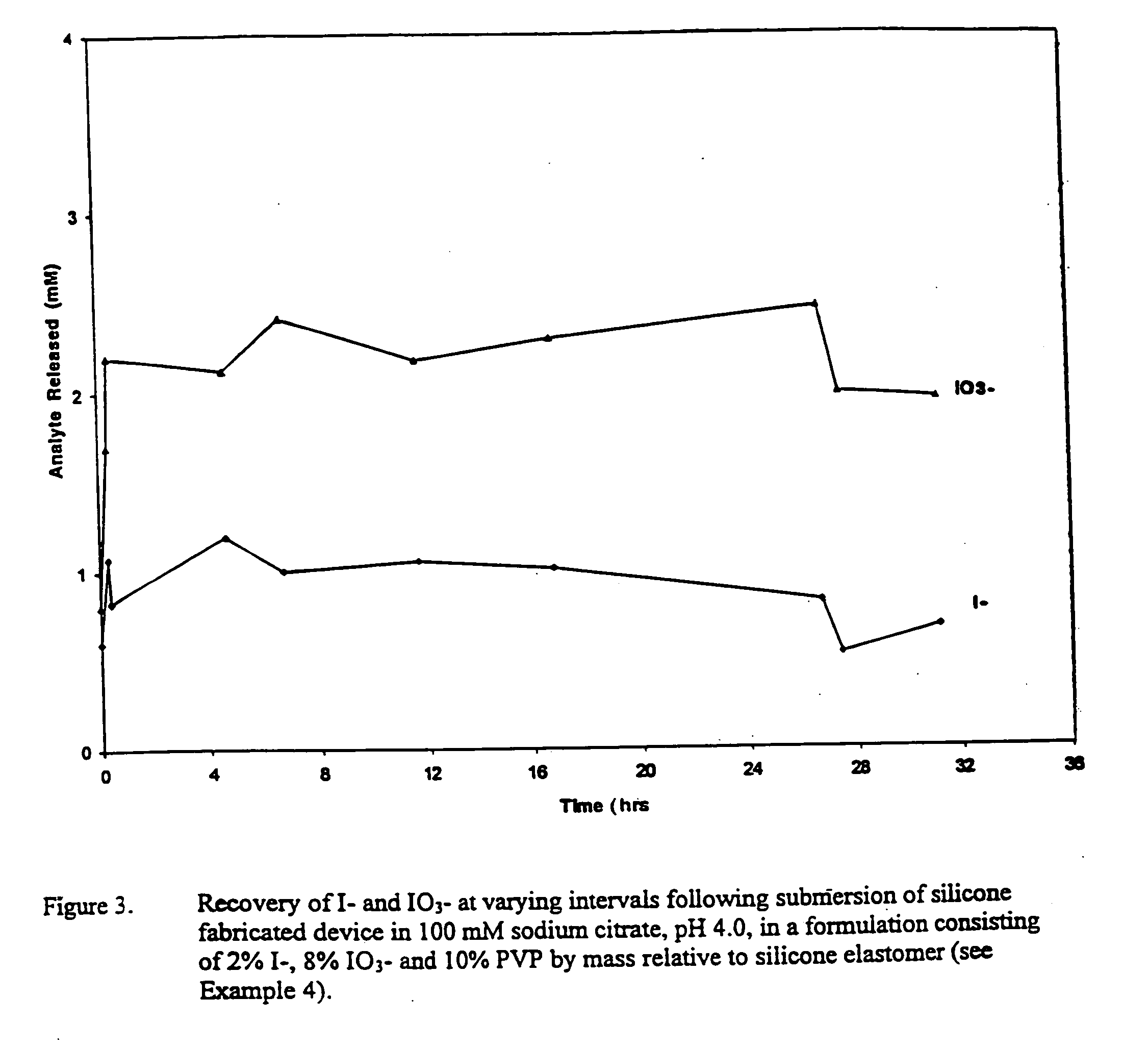
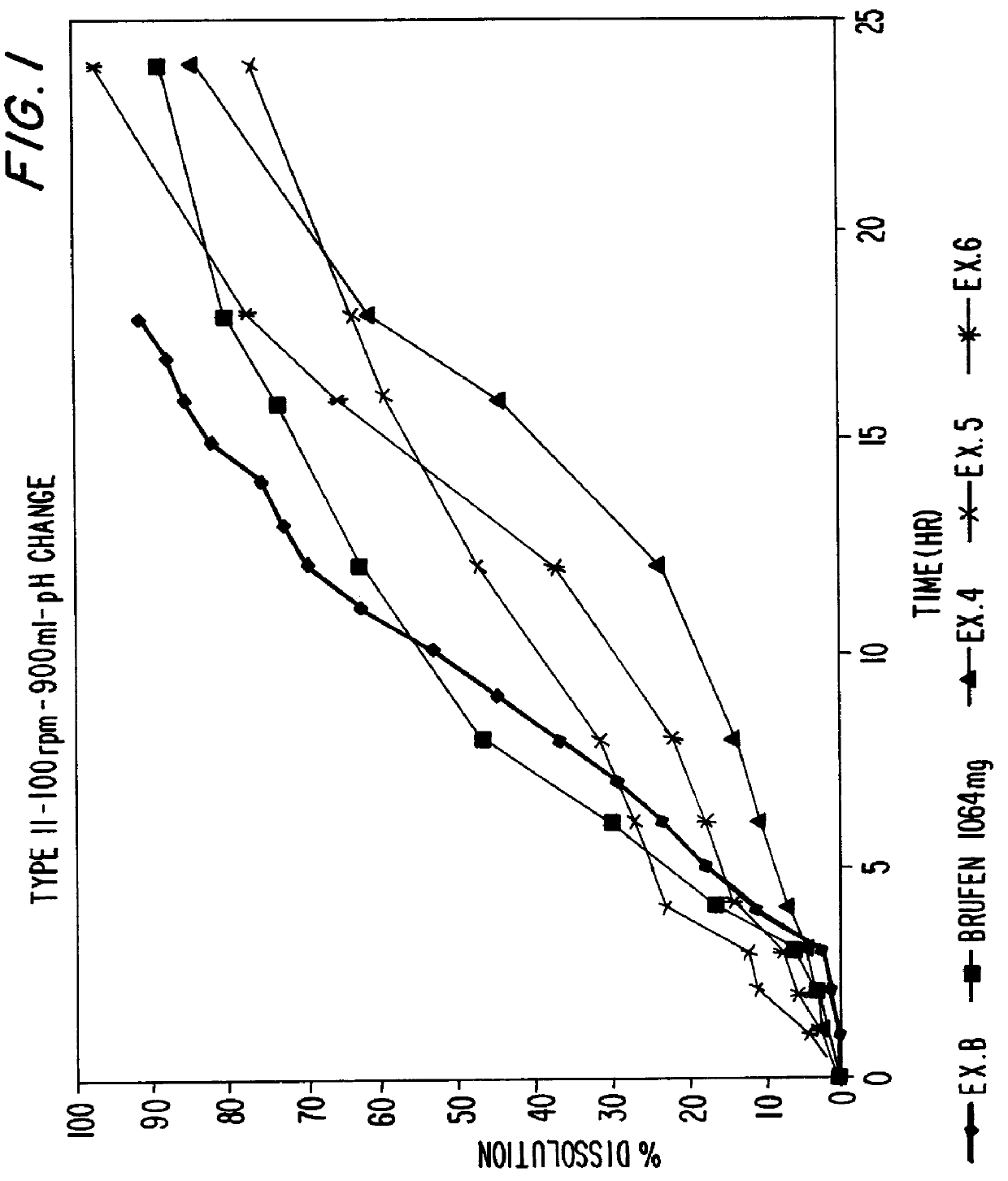
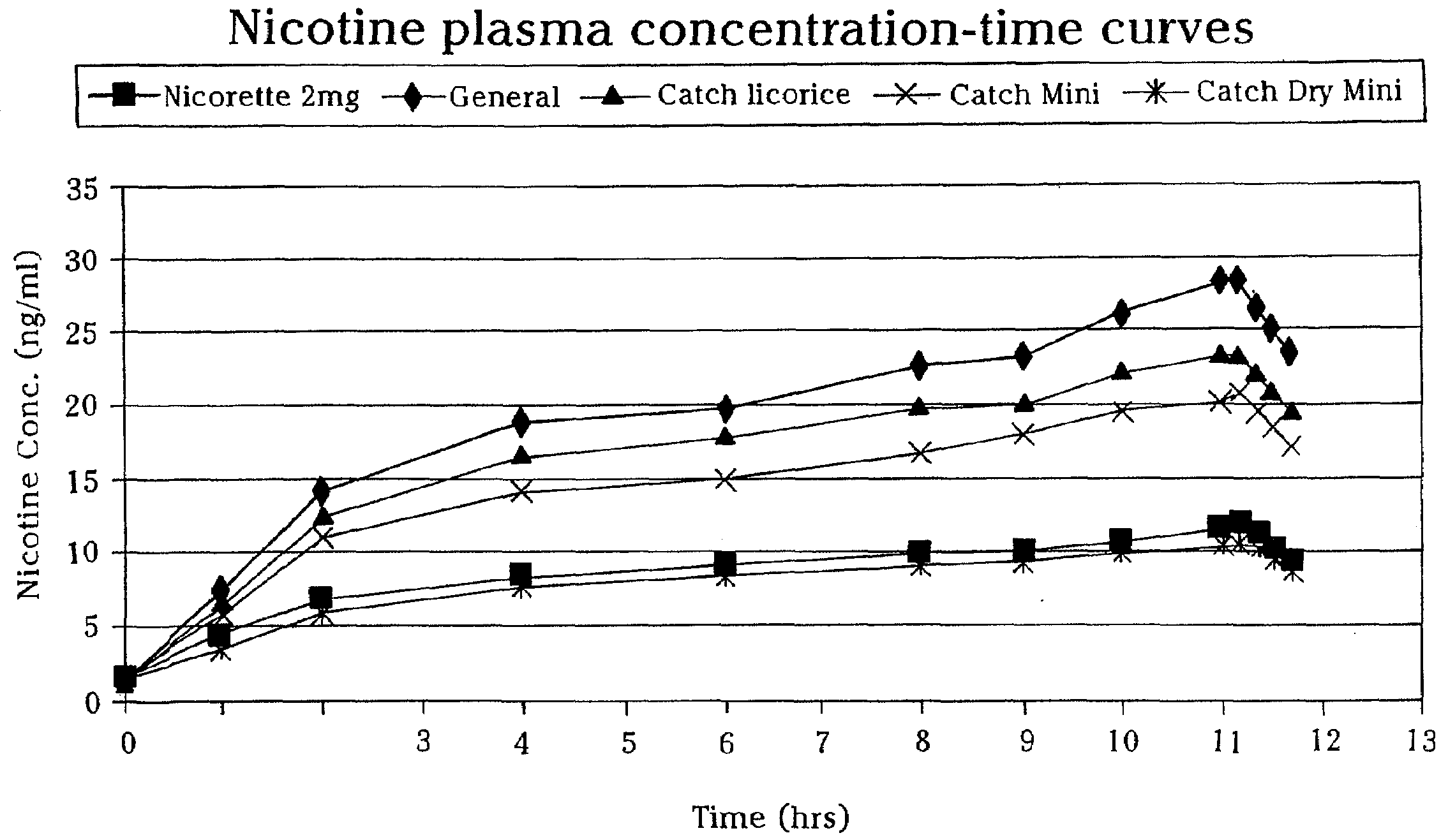
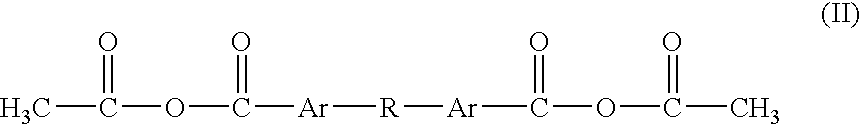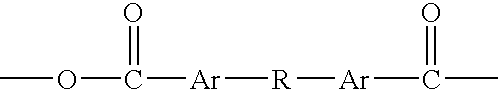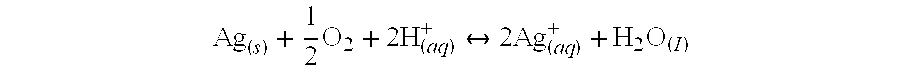Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
628results about How to "Sustained release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
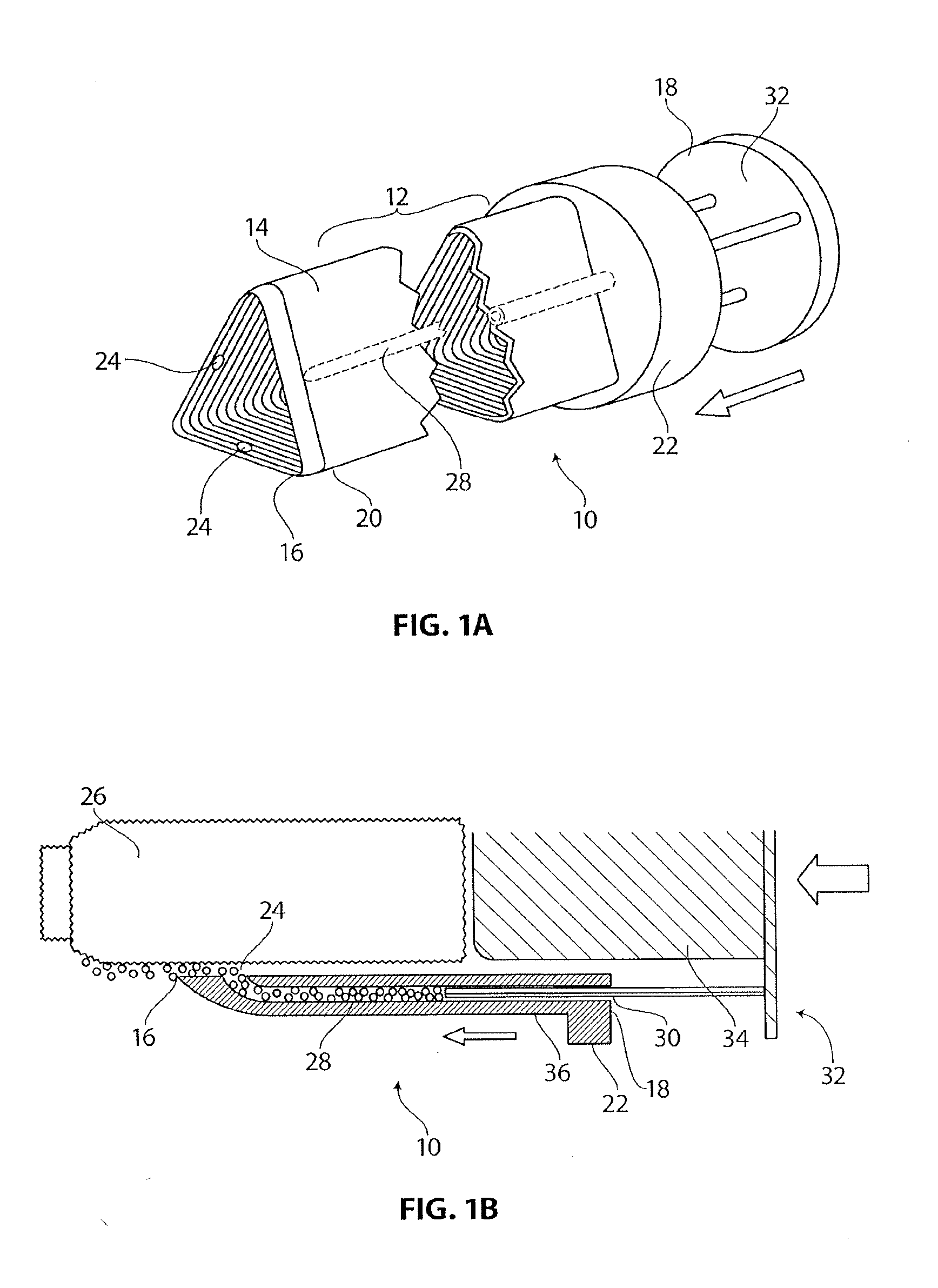
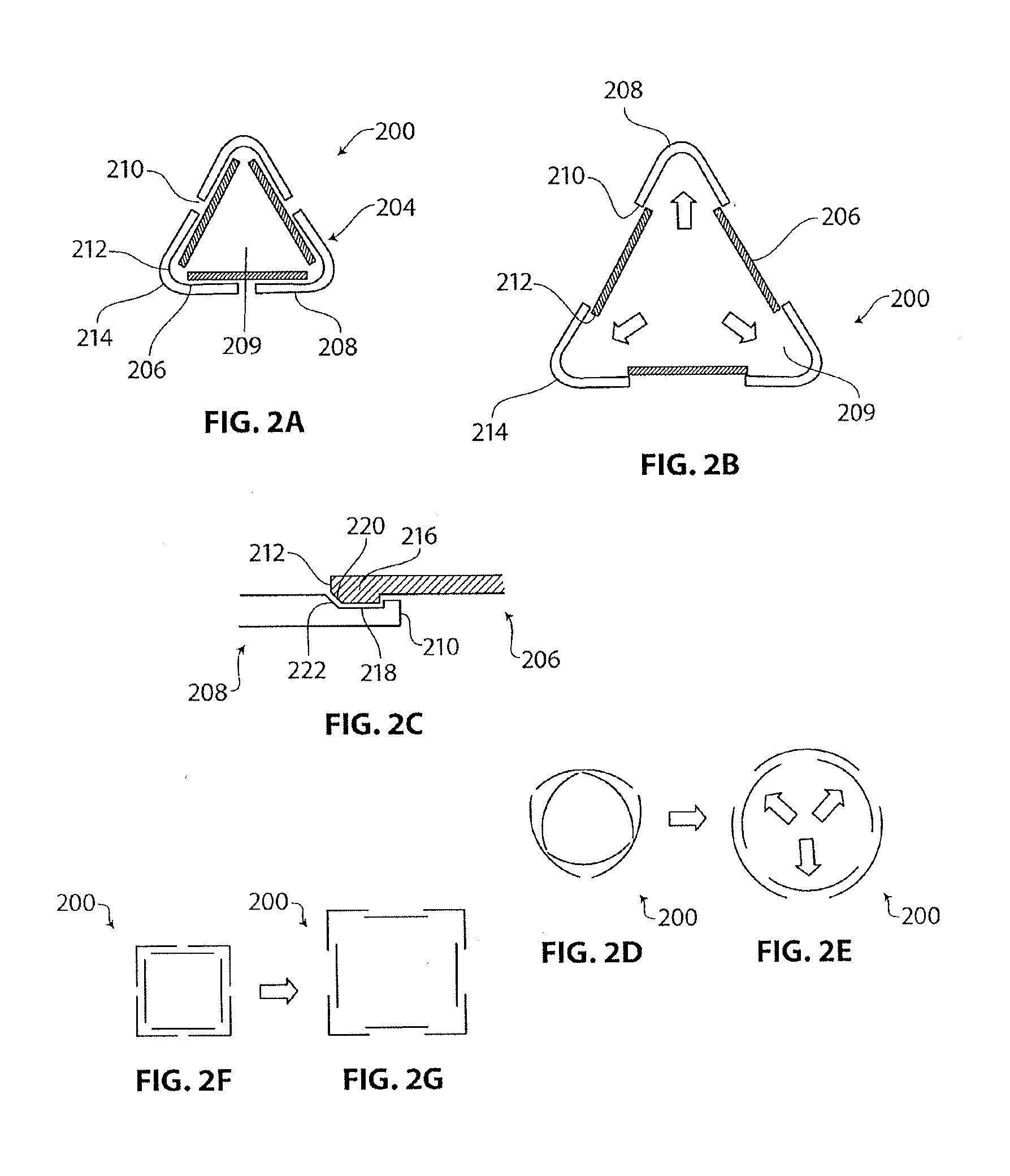
Medical device having anti-infective and contraceptive properties
There is disclosed a medical device for implantation that acts to prevent transmission of infectious agents. Specifically, the present invention provides implantable devices, such as catheters or living skin matrices or wound dressings, for insertion into various body cavities or over wound sites to confer to the site microbicidal or virucidal activity. Devices in the disclosure designed as vaginal inserts also exhibit contraceptive spermicidal activity. The coated devices or devices having the inventive polymeric material interspersed throughout are formed into appropriate shapes according to their contemplated uses (such as catheters or Foley catheters). Further, the present invention provides devices for providing therapeutic anti-microbial activity into an infected body cavity or on an infected wound site.
Owner:OXIBIO
Sustained release matrix for high-dose insoluble drugs
Sustained release dosage forms of high dose insoluble drugs such as ibuprofen and methods for their manufacture are disclosed.
Owner:PENWEST PHARMA CO
Antioxidant-functionalized polymers
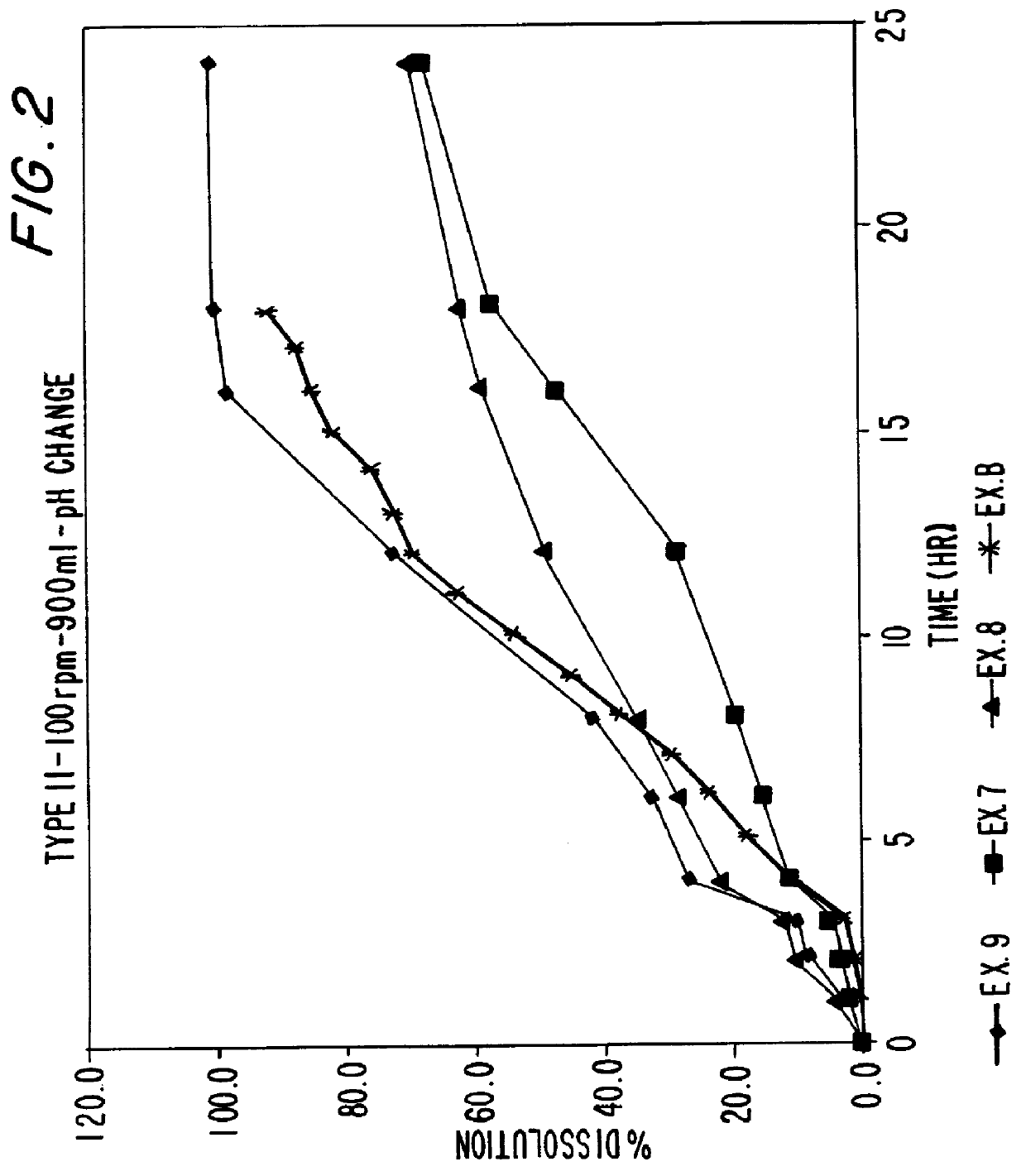
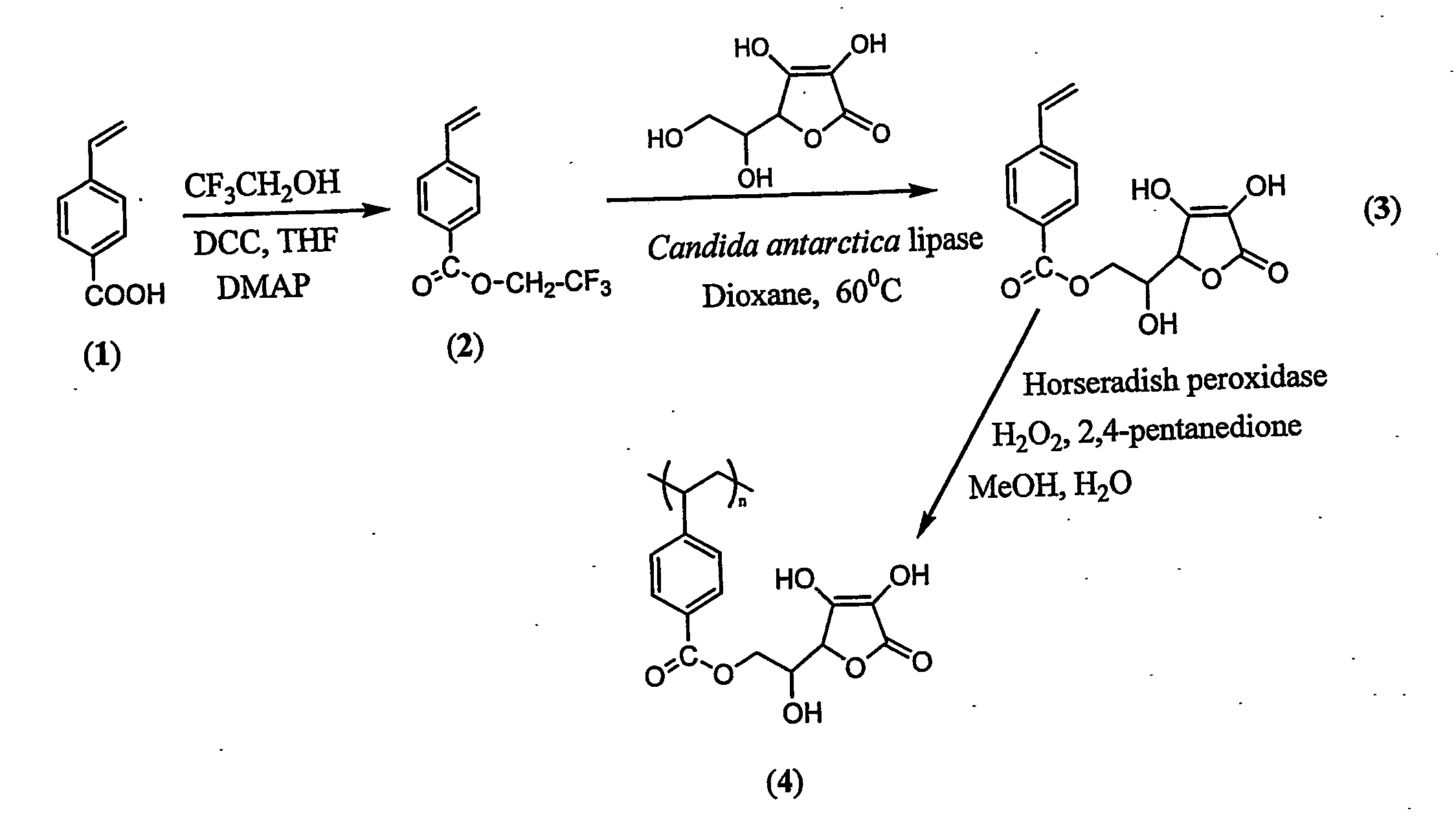
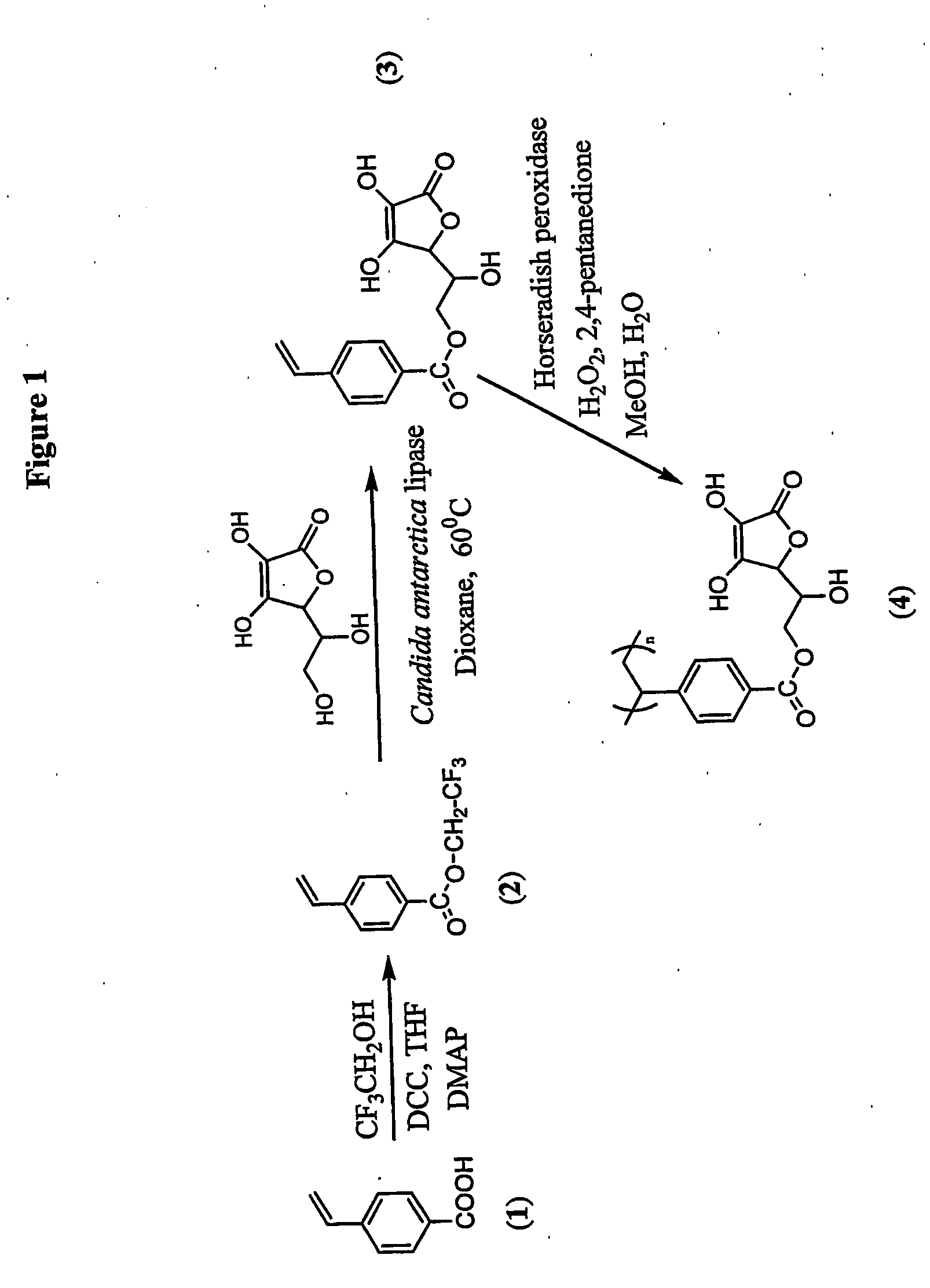
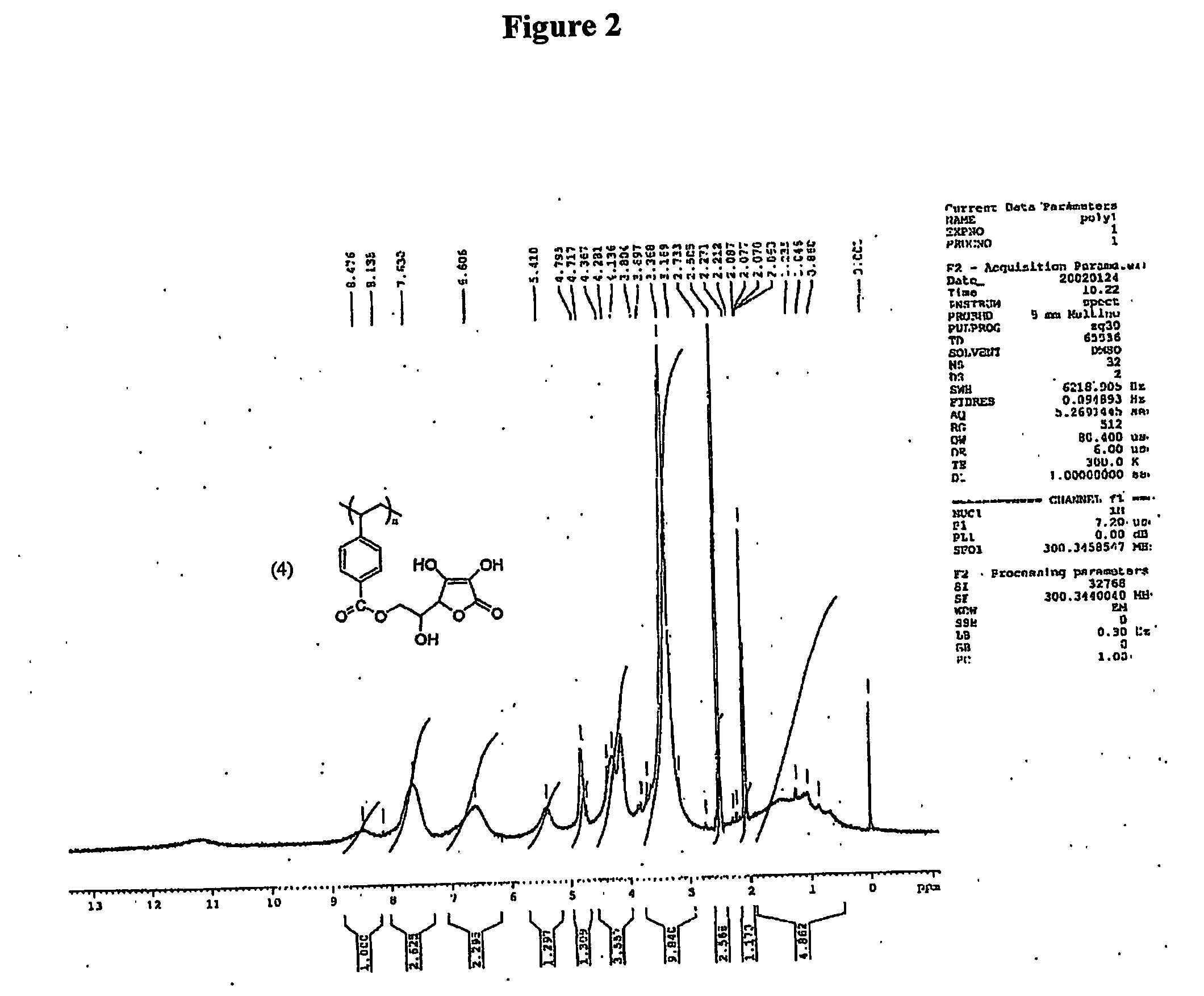
InactiveUS20070010632A1Low yieldReduce sensitivitySuture equipmentsOrganic chemistryAntioxidantOxygen
Methods and compositions are disclosed for the preparation of free radical scavenging polymers and polymer films functionalized with antioxidants. Enzymatic and chemical tailoring of monomers with antioxidants followed by enzymatic polymerization is described. These antioxidant functionalized polymers can increase shelf life and quality of food products, as well as, increase effectiveness of pharmaceutical agents when used as packaging or as coatings on packaging for oxygen sensitive materials. The novel enzymatic covalent coupling of antioxidants to a polymer enhances the free radical scavenging ability of packaging while also inhibiting the escape of the antioxidants, and thus limiting exposure and / or absorption by an individual. In addition to its use in food or pharmaceutical packaging, methods are disclosed for using the antioxidant coupled polymers in a variety of applications including as coatings on the inside of medical devices, such as stents and catheters, which would substantially reduce free radical damage and / or oxygen depletion during medical procedures. Furthermore, through the coupling of antioxidants to biodegradable polymers, controlled delivery and sustained release of an antioxidant to a subject is possible.
Owner:TRUSTEES OF TUFTS COLLEGE
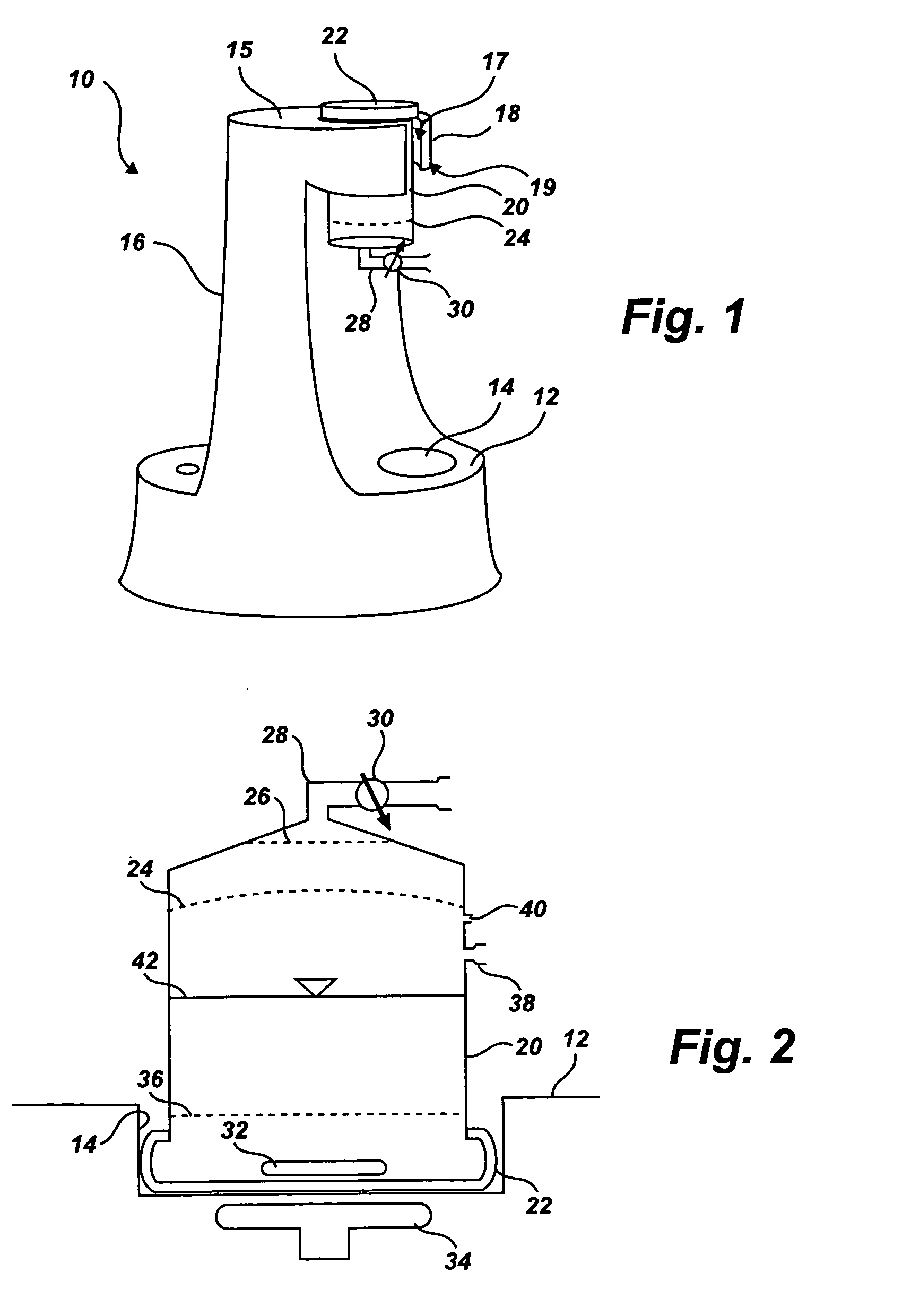
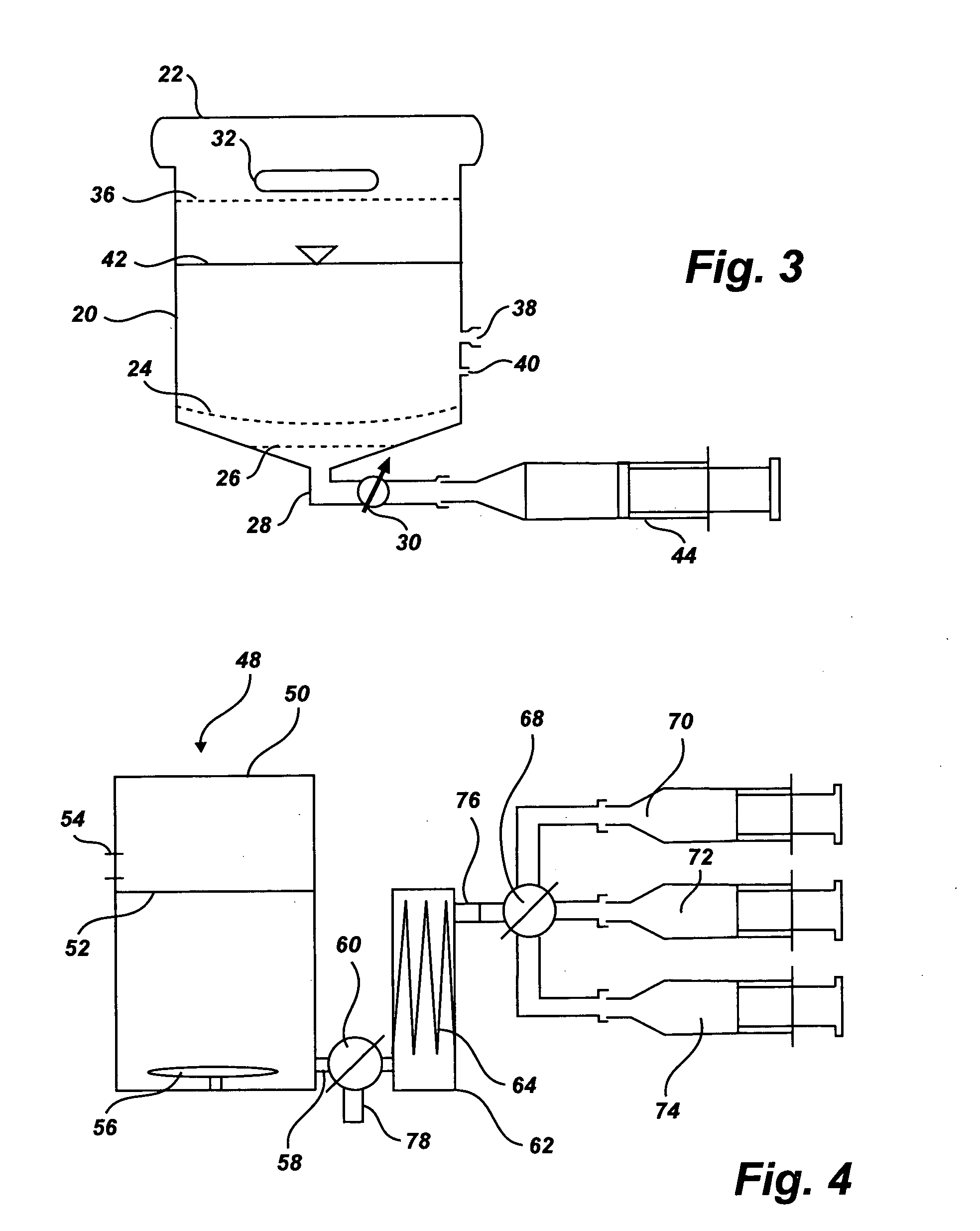
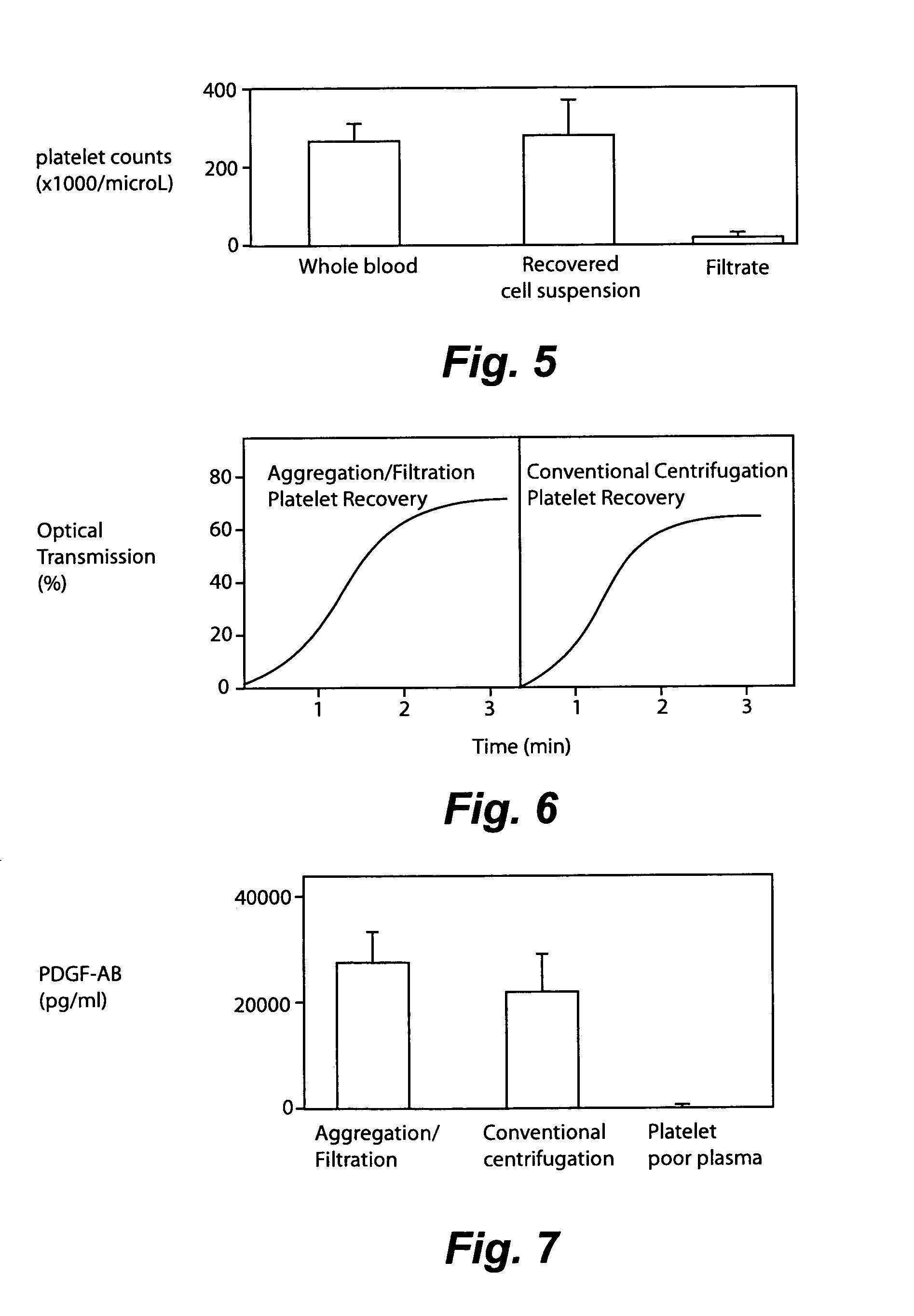
Separation of platelets from whole blood for use as a healant
InactiveUS7011852B2Rapidly and conveniently and cost-effectively harvestEliminate useSurgical adhesivesMammal material medical ingredientsFiltrationBlood plasma
Owner:MOHAMMAD S FAZAL +1
Natamycin dosage form, method for preparing same and use thereof
InactiveUS20050042341A1Sustained releaseReduce releaseDough treatmentEggs preservation by coatingFood productsDosage form
The present invention relates to a novel natamycin dosage form for the food industry, and more particularly to microcapsules where natamycin is encapsulated within a physiologically acceptable shell to provide a protected natamycin product. The present invention relates also to novel processes for preparing the capsules according to the invention, as well as to the use of the capsules of the present invention. The invention further relates to food products containing natamycin in encapsulated form.
Owner:AS DE DANSKE SUKKERFABRIKKER
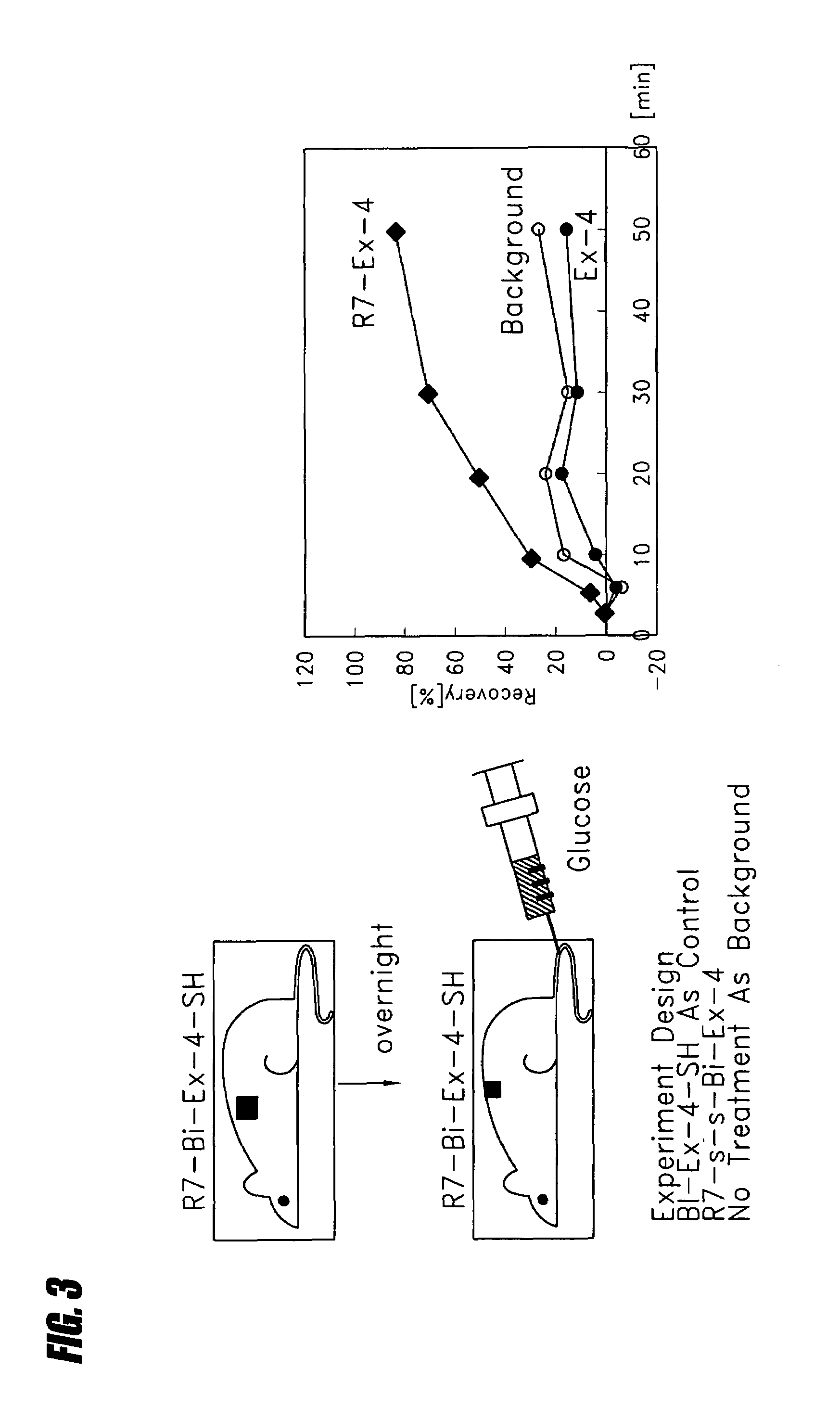
Transepithelial delivery of peptides with incretin hormone activities
InactiveUS20060084604A1Promote insulin secretionSustained releaseNervous disorderPeptide/protein ingredientsDiabetes mellitusIncretin Hormone
Compositions and methods are disclosed for the treatment of diabetes and related diseases using peptides with incretin hormone activity. Preferably, the peptide with incretin hormone activity is GLP-1, exendin or an analog of GLP-1 or exendin. The peptides with incretin hormone activity are administered transepithelially using a transepithelial carrier peptide. The transepithelial peptide contains sufficient amino, guanidine or amidino groups to stimulate transepithelial delivery. In some embodiments, the transepithelial carrier and the peptide with incretin hormone activity are embedded in a pressure sensitive adhesive layer of a plaster or patch.
Owner:NITTO DENKO CORP
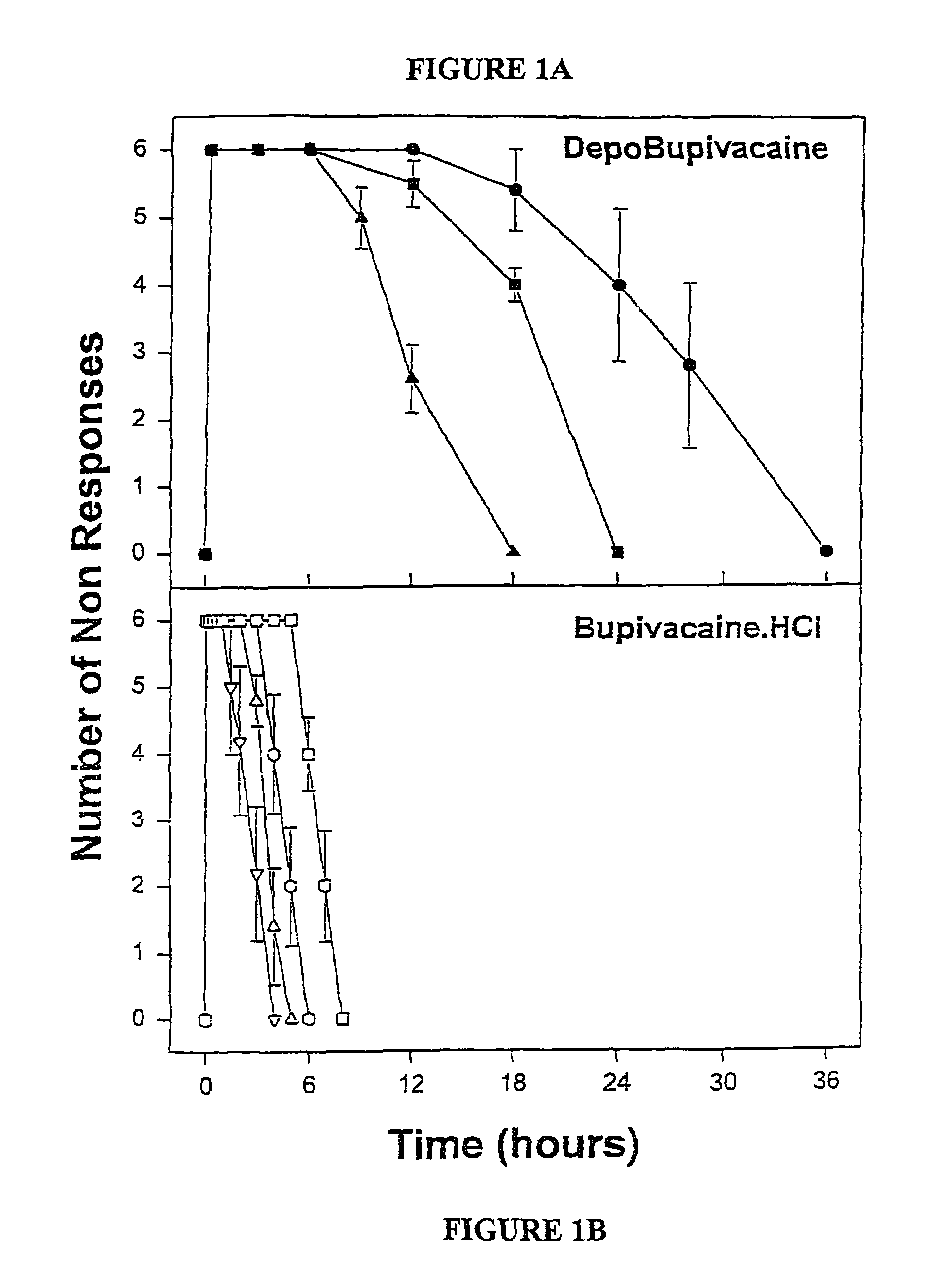
Sustained-release liposomal anesthetic compositions
InactiveUS8182835B2High acceptabilityImprove encapsulationInorganic non-active ingredientsAnaestheticsHalf-lifeMaximum tolerated dose
Owner:PACIRA PHARMA INC
Dressing material containing medicine chitoholosida and its preparation method
InactiveCN1579559AHas therapeutic effectSustained releaseAbsorbent padsBandagesSolid componentPhosphate
The invention produces polyethylene alcohol hydrogel dressing containing medicine and chitosan with 60Co gamma-radial or high energy electron beam radial cross linking. Additional, adds in some humectant, plasticizer, medicine, the solvent is the secondary distilled water, physiological saline or phosphate neutral buffer liquid. The product can release medicine slowly and has natural amylose chitosan with biology sterilization activity, it has active sterilization function, at the same time, it has high water quantity, and good water reserving performance, the mechanical intensity is moderate, and the light penetration and air penetration are excellent. It can accord the demands for curing each kind of wound. It can used as the permanent dressing for light skin injuries, and it also can be applied to the temporally close of severe skin organization wound or burn wound.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Sustained release medicinal compositions
InactiveUS6328979B1Improve hydrophobicityGood sustained release effectElcosanoid active ingredientsAerosol deliverySolubilityAcid derivative
The present invention relates to sustained-release pharmaceutical compositions for ionic pharmaceutically active substances (excluding ionic prostanoic acid derivatives) containing ionic compounds having opposite charges to those of the active substances and increasing hydrophobicity of the active substances. More specifically, the invention relates to sustained-release pharmaceutical compositions comprising the ionic pharmaceutically active substances and the ionic compounds having opposite charges to those of the active substances and increasing hydrophobicity of the active substances that contain hydrophobic groups in the molecule thereof. The pharmaceutical composition of the invention can exhibit excellent sustained release effect of the active substance, irrespective of water solubility possessed by the ionic pharmaceutically active substances.
Owner:ASTELLAS PHARMA INC
Drug-delivery stent formulations for restenosis and vulnerable plaque
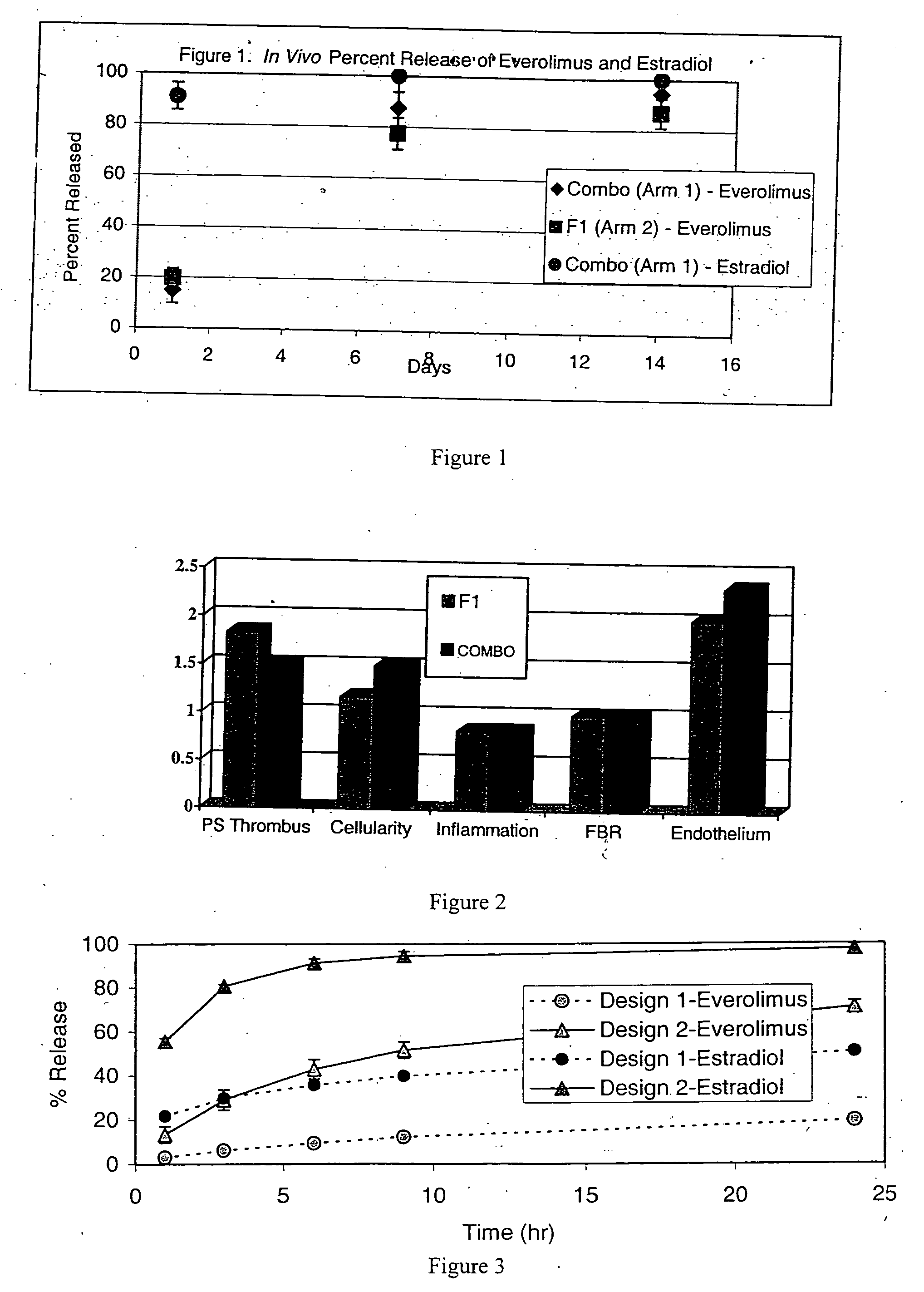
Drug-delivery stents capable of providing release of two or more drugs such as everolimus and estradiol are provided. The stents can be used for treating a disease such as restenosis and vulnerable plaque.
Owner:ABBOTT CARDIOVASCULAR
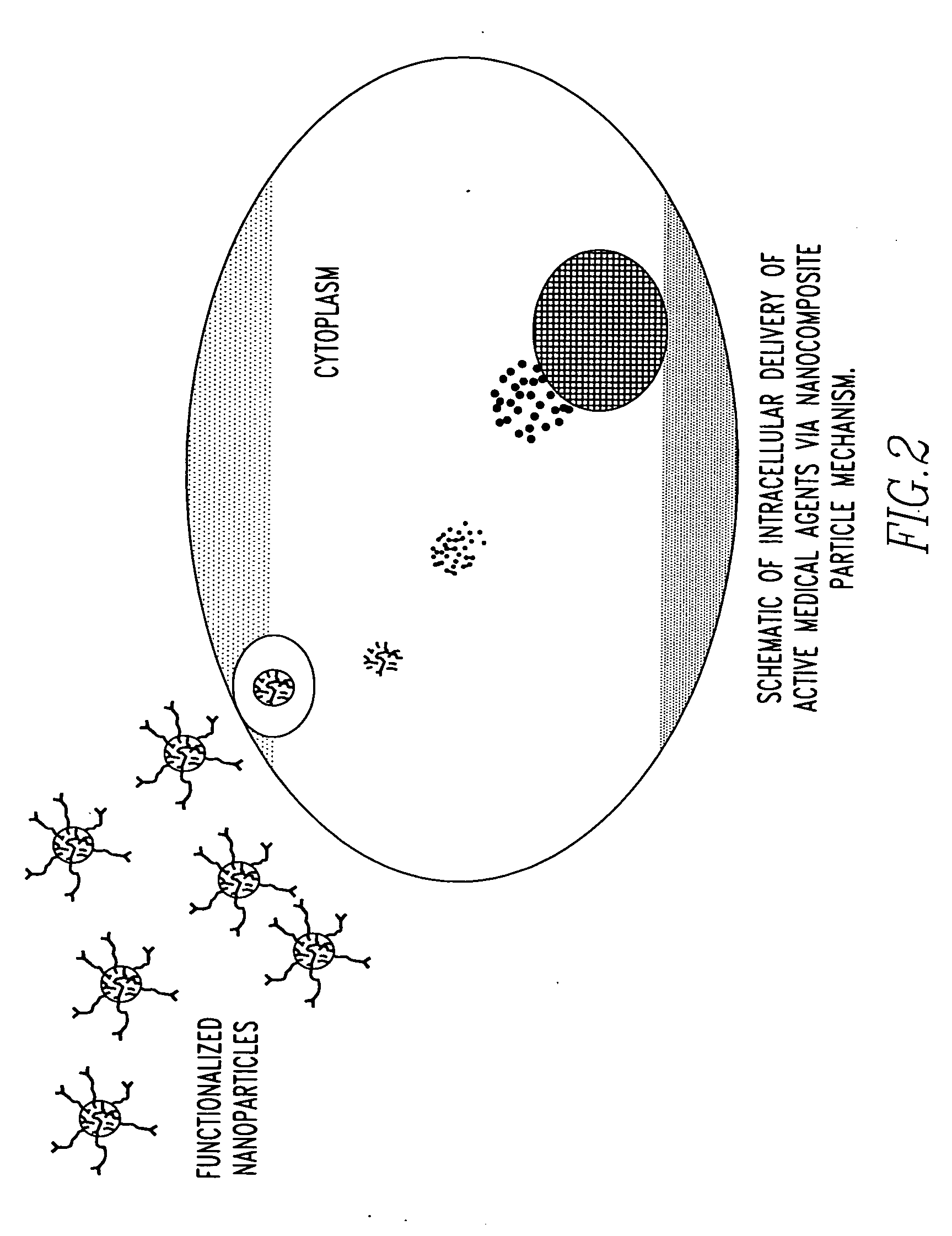
Unagglomerated core/shell nanocomposite particles
ActiveUS20050281884A1Improve stabilityPromote absorptionPowder deliveryMaterial nanotechnologyAlcoholMicroemulsion
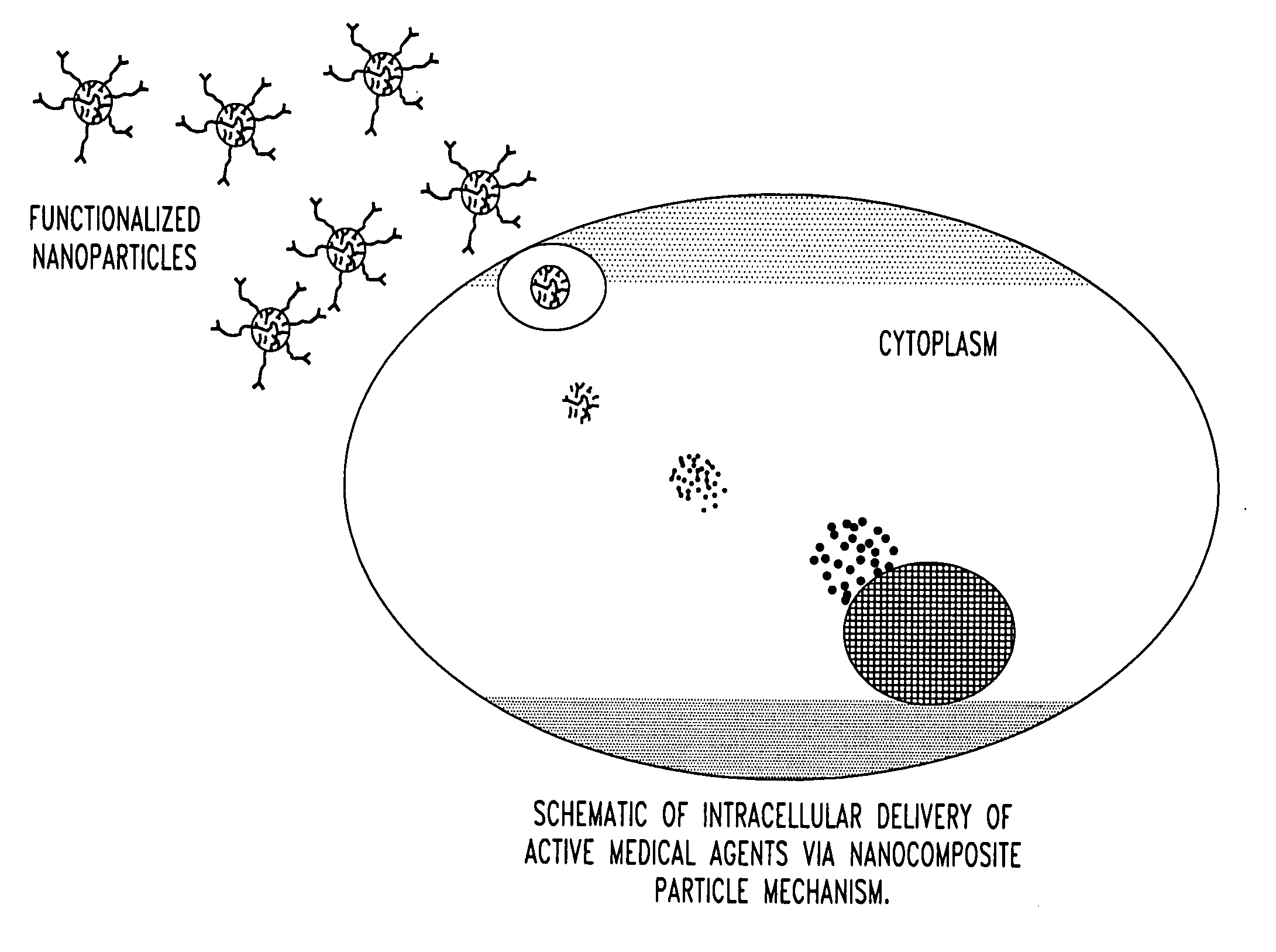
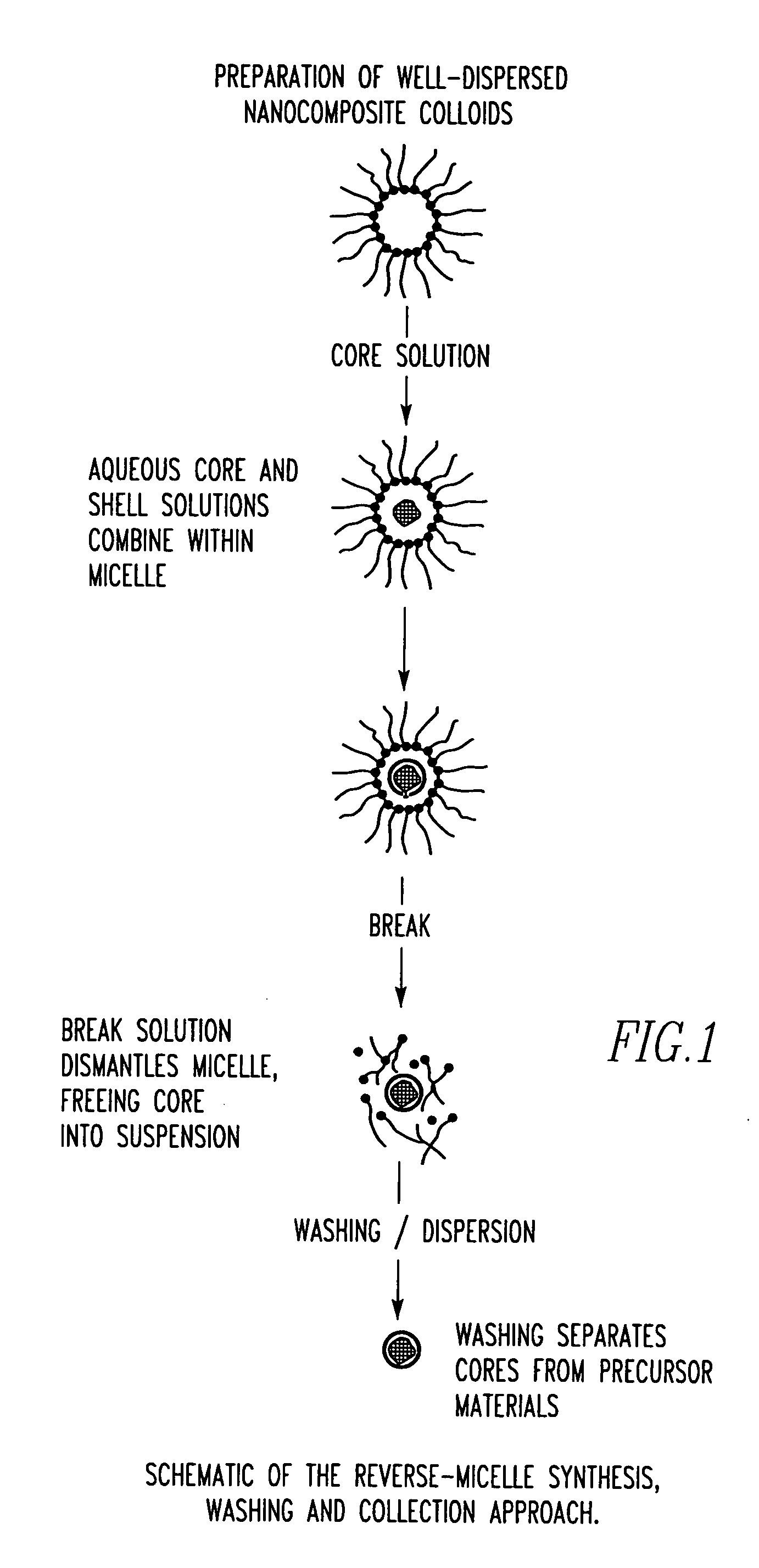
The present invention provides a method for the synthesis of unagglomerated, highly dispersed, stable core / shell nanocomposite particles comprised of preparing a reverse micelle microemulsion that contains nanocomposite particles, treating the microemulsion with a silane coupling agent, breaking the microemulsion to form a suspension of the nanocomposite particles by adding an acid / alcohol solution to the microemulsion that maintains the suspension of nanocomposite particles at a pH of between about 6 and 7, and simultaneously washing and dispersing the suspension of nanocomposite particles, preferably with a size exclusion HPLC system modified to ensure unagglomeration of the nanocomposite particles. The primary particle size of the nanocomposite particles can range in diameter from between about 1 to 100 nm, preferably from between about 10 to 50 nm, more preferably about 10 to 20 nm, and most preferably about 20 nm.
Owner:PENN STATE RES FOUND
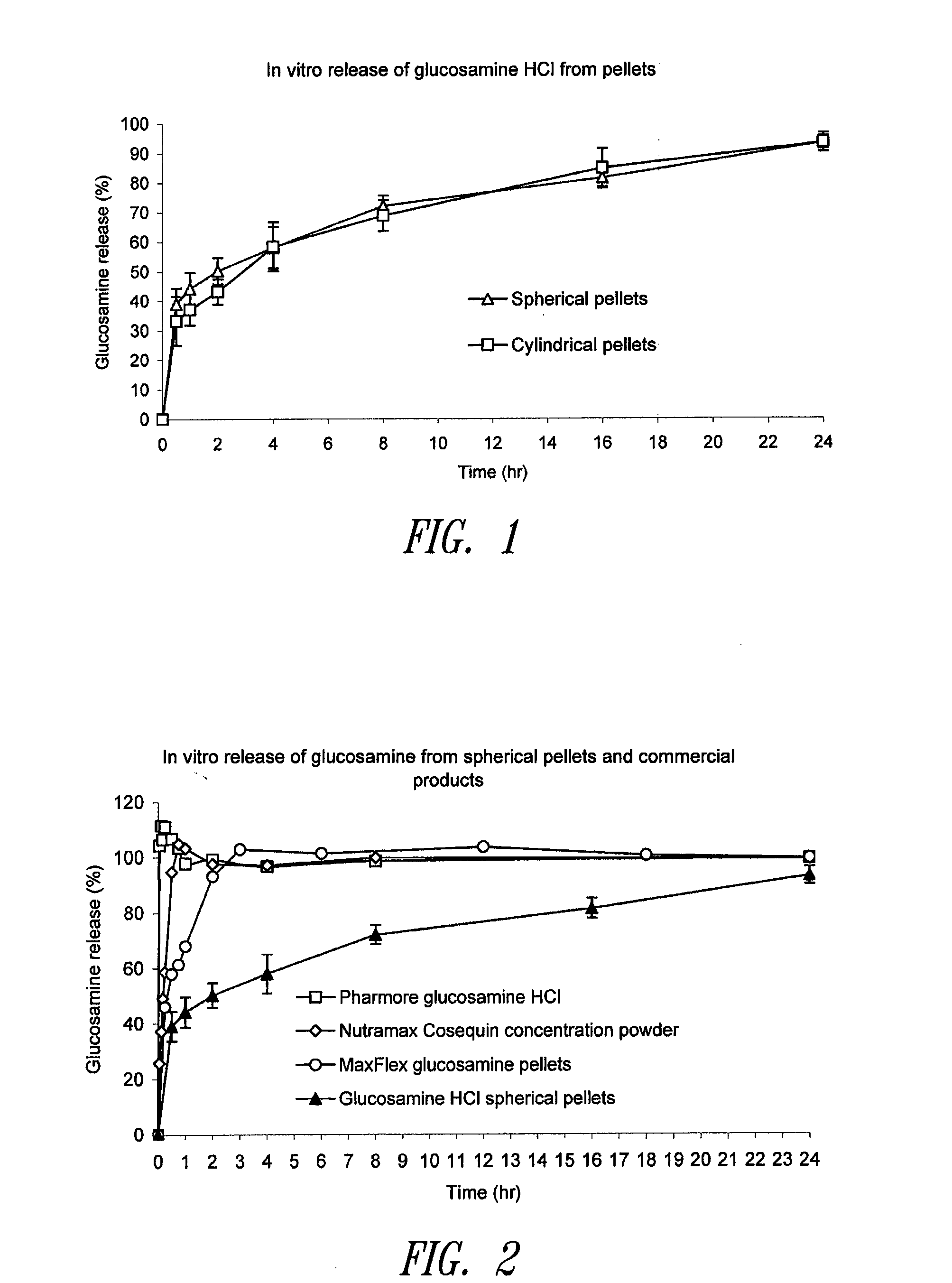
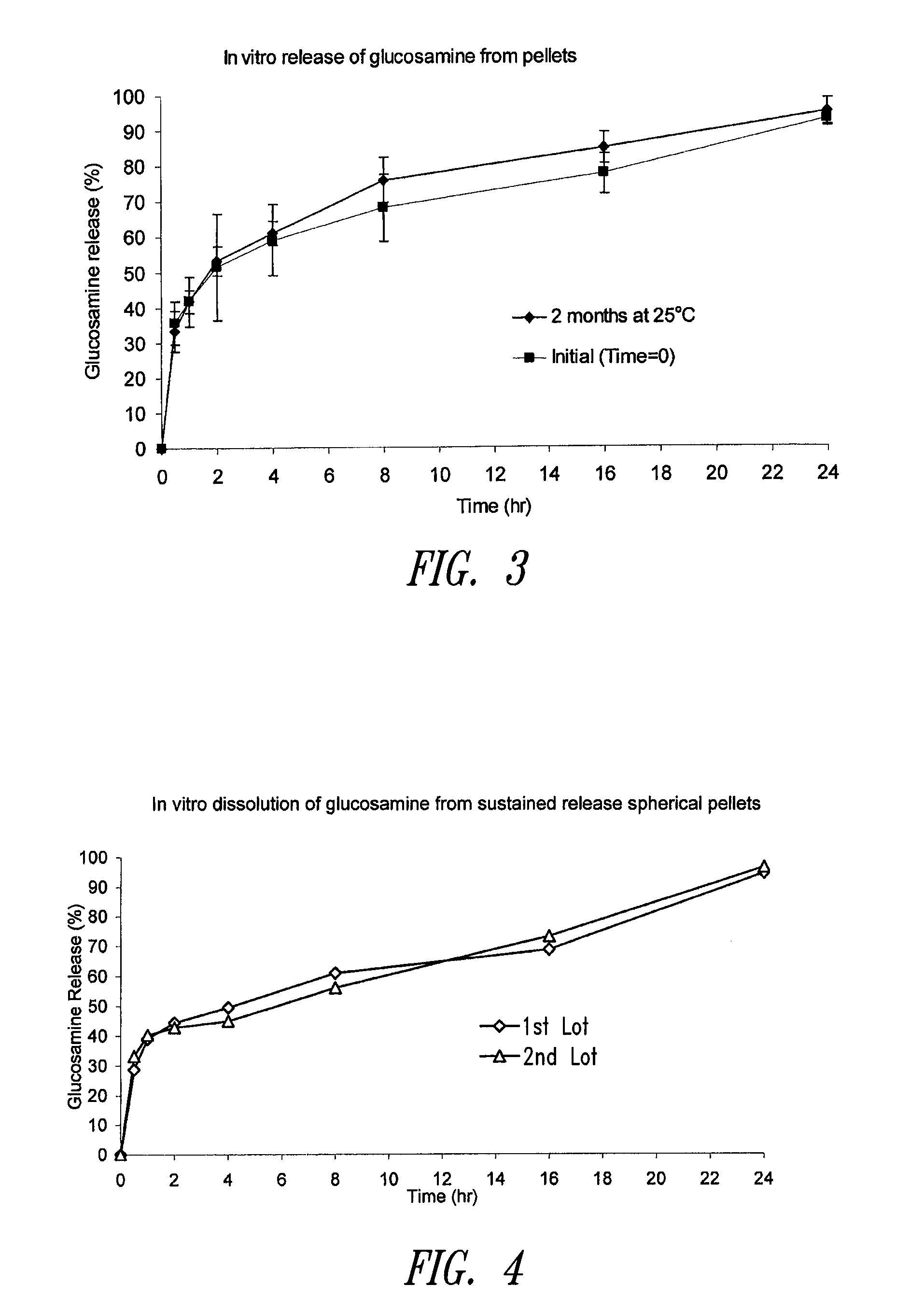
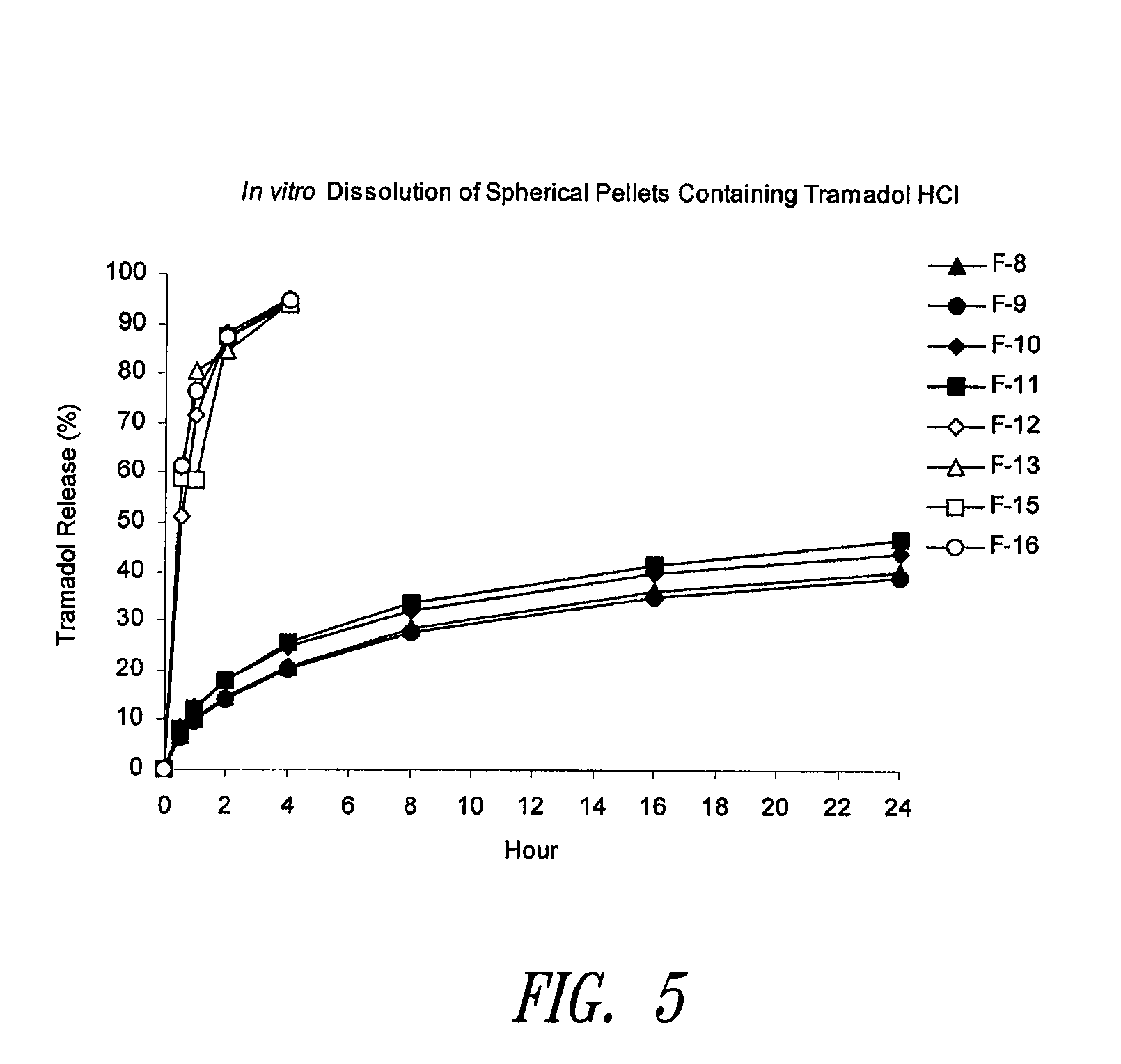
Sustained release compositions using wax-like materials
ActiveUS20080220079A1Low costDecrease scale-up complexityAntibacterial agentsPowder deliveryWaxEngineering
Sustained release spherical or non-spherical pellets comprising (a) an active ingredient (b) a wax-like agent, and (c) a spheronizing agent are provided. Oral dosage forms comprising said pellets and methods for preparing and using such pellets and dosage forms are also provided.
Owner:FARNAM
Nanoscale particle formulations and methods
InactiveUS20140205546A1Reduce the populationSustained releasePowder deliveryMaterial nanotechnologyDermatologySustained Release Formulations
Provided herein in some embodiments is a formulation comprising (a) one or more nanoscale particle; and (b) a film-forming polymer. In some instances, the formulation is an immediate and sustained released formulation suitable for topical administration or administration to surfaces. Also provided here in certain embodiments is a method of reducing the population of pathogenic microorganisms on skin or surfaces, the method comprising applying to the skin or surface a composition, the composition comprising (a) one or more nanoscale particle; and (b) a film-forming polymer.
Owner:ANNUARY HEALTHCARE
Transepithelial delivery of peptides with incretin hormone activities
InactiveUS7442682B2Sustained releasePromote secretionNervous disorderPeptide/protein ingredientsDiabetes mellitusDisease
Compositions and methods are disclosed for the treatment of diabetes and related diseases using peptides with incretin hormone activity. Preferably, the peptide with incretin hormone activity is GLP-1, exendin or an analog of GLP-1 or exendin. The peptides with incretin hormone activity are administered transepithelially using a transepithelial carrier peptide. The transepithelial peptide contains sufficient amino, guanidine or amidino groups to stimulate transepithelial delivery. In some embodiments, the transepithelial carrier and the peptide with incretin hormone activity are embedded in a pressure sensitive adhesive layer of a plaster or patch.
Owner:NITTO DENKO CORP
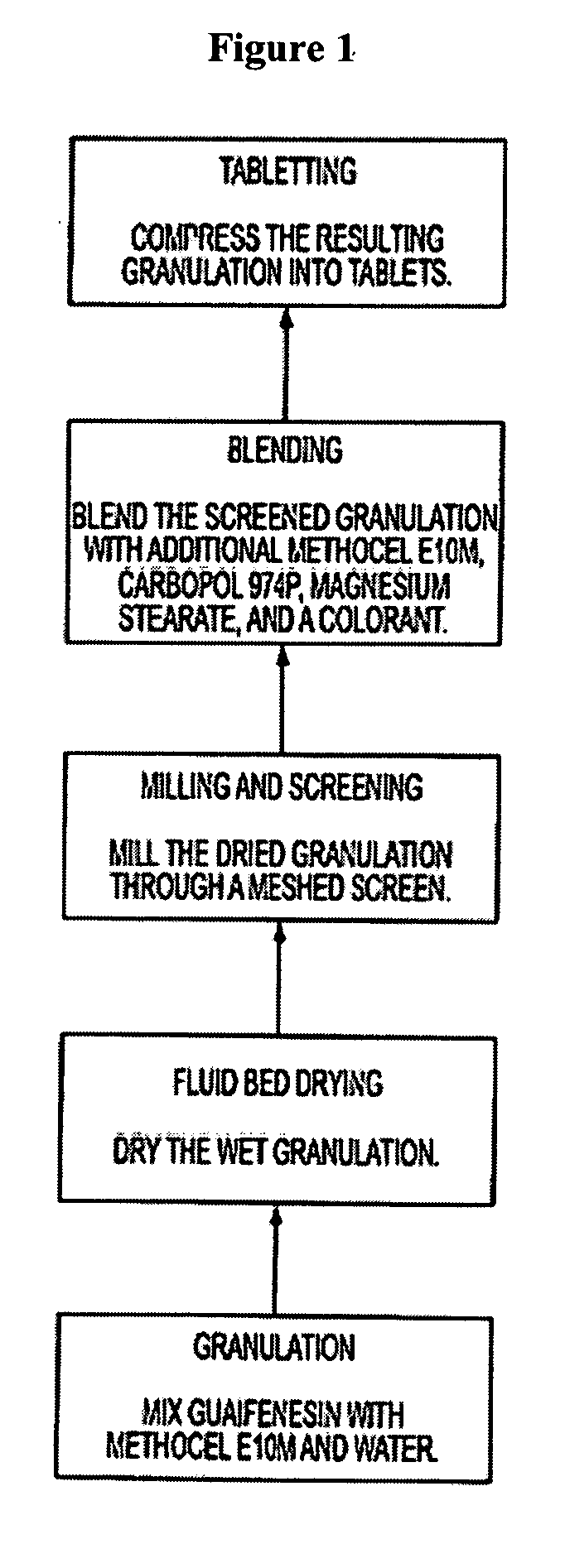
Sustained release formulations of guaifenesin and additional drug ingredients
InactiveUS20050276852A1Increase in drug strengthSustained releaseOrganic non-active ingredientsEther/acetal active ingredientsSerum concentrationImmediate release
The invention relates to a novel pharmaceutical sustained release formulation of guaifenesin and at least one additional drug ingredient. The formulation may comprise a hydrophilic polymer, preferably a hydroxypropyl methylcellulose, and a water-insoluble polymer, preferably an acrylic resin, in a ratio range of about one-to-one (1:1) to about nine-to-one (9:1), more preferably a range of about three-to-two (3:2) to about six-to-one (6:1), and most preferably in a range of about two-to-one (2:1) to about four-to-one (4:1) by weight. This formulation capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject. The invention also relates to a modified release product which has two portions: a first portion having an immediate release formulation of guaifenesin and a second portion having a sustained release formulation of guaifenesin, wherein one or both portions has at least one additional drug ingredient. The modified release product has a maximum guaifenesin serum concentration equivalent to that of an immediate release guaifenesin tablet, and is capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject.
Owner:RB HEALTH US LLC
Pharmaceutical compositions for controlled release delivery of biologically active compounds
InactiveUS20060034923A1Reduce solubilitySlow degradationBiocidePeptide/protein ingredientsControlled releaseWater insoluble
The present invention provides compositions and methods for the controlled release delivery of one or more biologically active compounds to a subject. Specifically, the invention provides for a pharmaceutical composition for the controlled release delivery of biologically active compounds to a subject comprising: a) a complex of a biologically active compound having at least one basic functional group and a polyanion derived from hexahydroxycyclohexane having at least two negatively charged functional groups; and b) a pharmaceutically acceptable carrier comprising a biodegradable, water-insoluble polymer. By complexing a biologically active compound with a polyanion, the tight, stable complex may be incorporated into a long-acting dosage system having a more desired drug release curve over time than that is found in the prior art. The invention also provides the methods of making such compositions and the methods of use thereof.
Owner:FORESEE PHARMA CO LTD
Compositions and methods for dermally treating infections
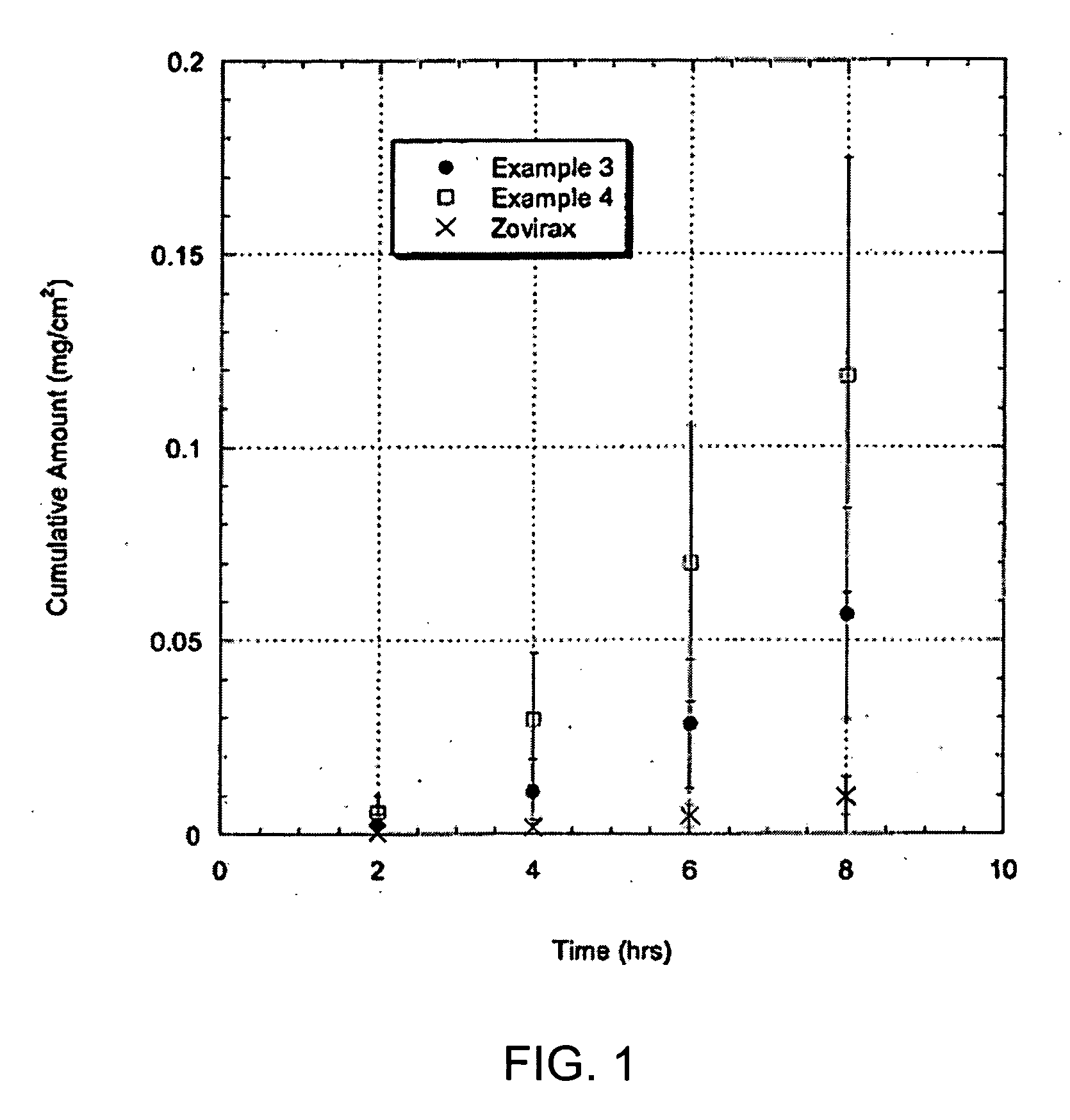
InactiveUS20070196325A1Sustained release of drugReduce deliveryAntimycoticsAntiviralsSkin treatmentsSkin surface
The present invention is drawn to solidifying adhesive formulations, methods of drug delivery, and solidified layers for dermal delivery of a drug which can treat various skin infections, such as fungal, bacterial, and / or viral skin infections. The formulation can include an anti-infective drug, a solvent vehicle, and a solidifying agent. The solvent vehicle can include a volatile solvent system including at least one volatile solvent, and a non-volatile solvent system including at least one non-volatile solvent. The non-volatile solvent system can facilitate the delivery of the drug at therapeutically effective rates for sustained period of time. The non-volatile solvent system can also act as a plasticizer for the solidifying agent. The formulation can have a viscosity suitable for application to a skin surface prior to evaporation of the volatile solvents system. When applied to the skin, the formulation can form a solidified layer after at least a portion of the volatile solvent system is evaporated.
Owner:NUVO RES
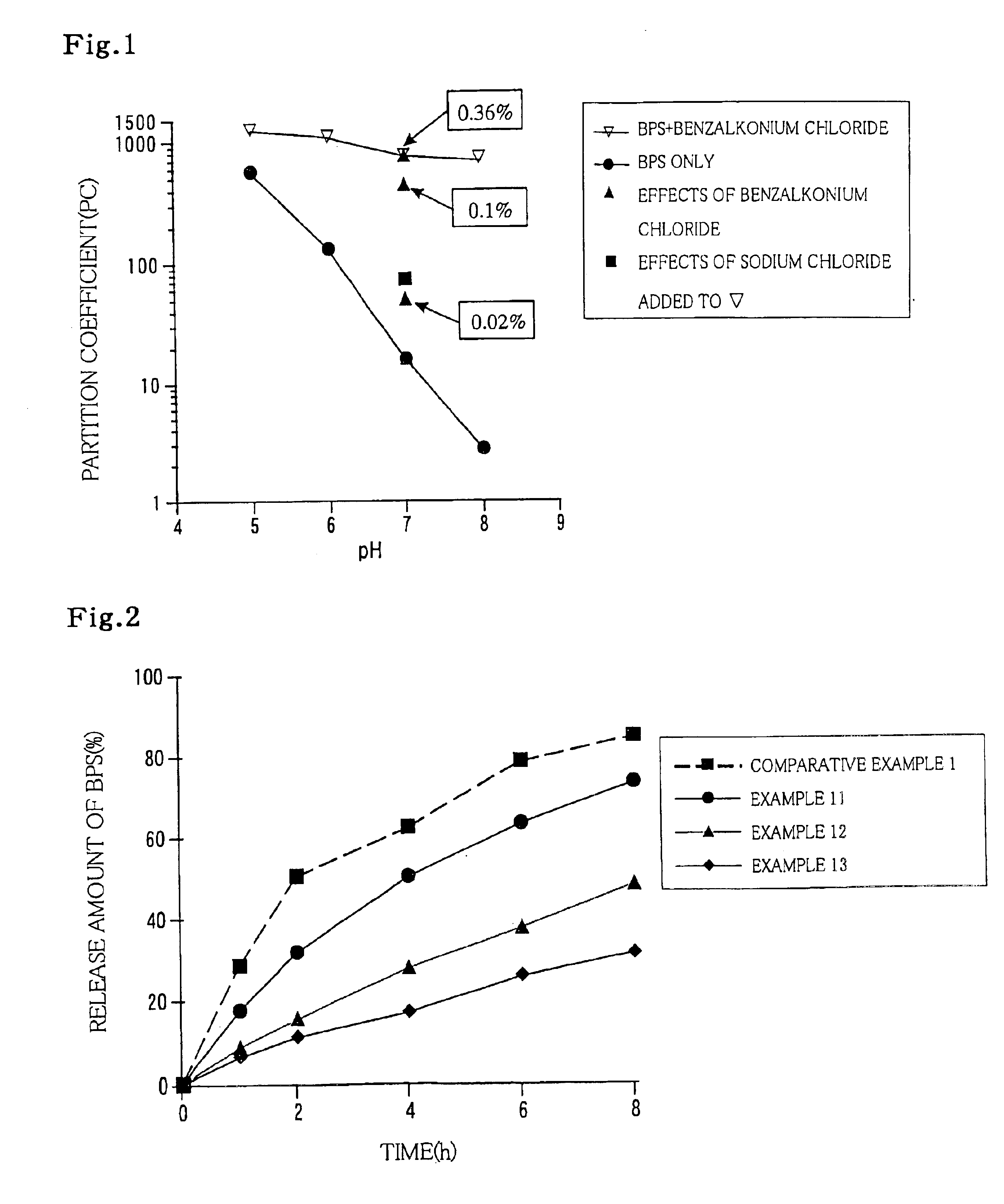
Sustained release pharmaceutical compositions
InactiveUS6919372B1Improve hydrophobicityGood sustained release effectBiocideAerosol deliverySolubilityAcid derivative
The present invention relates to sustained-release pharmaceutical compositions for ionic pharmaceutically active substances containing ionic compounds having opposite charges to those of ionic prostanoic acid derivatives and increasing hydrophobicity of the active substances. More specifically, the invention relates to sustained-release pharmaceutical compositions comprising the ionic prostanoic acid derivatives and the ionic compounds having opposite charges to those of the prostanoic acid derivatives and increasing hydrophobicity of these derivatives that contain hydrophobic groups in the molecule thereof.The pharmaceutical composition of the invention can exhibit excellent sustained release effect of the ionic prostanoic acid derivatives, irrespective of water solubility possessed by the ionic prostanoic acid derivatives.
Owner:TORAY IND INC +1
Prodrug comprising beta-keto carboxylic acid, beta-keto carboxylic acid salt or beta-keto carboxylic acid ester for drug delivery
InactiveUS20120065179A1Improve bioavailabilityGood water solubilityBiocideOrganic compound preparationActive agentCarboxylic acid
There is provided a prodrug of a pharmaceutically active agent, such prodrug comprising a beta-keto carboxylic acid, a beta-keto carboxylic acid salt or a beta-keto carboxylic acid ester functional group, a pharmaceutical composition comprising the prodrug, and to the use of the prodrug or composition for treatment of a mammalian subject suffering from a condition which can be cured or alleviated by administration of the pharmaceutically active agent. There is further provided a method of inhibiting decarboxylation of a compound comprising a beta-keto carboxylic acid or a salt thereof with a monovalent cation, characterized in that a dry salt of the beta-keto carboxylic acid with a divalent or polyvalent cation is prepared.
Owner:INFINITON
Extrudable and Extruded Compositions for Delivery of Bioactive Agents, Method of Making Same and Method of Using Same
A nonaqueous, extrudable composition includes at least one thermoplastic polymer in an amount of more than 20 wt % of the whole composition and tobacco. An extruded bioactive product in the form of a sheet can be made by extruding or hot melt shaping a nonaqueous composition comprising at least one thermoplastic polymer and a bioactive agent, the sheet being soluble in a user's mouth and resulting in sustained release of bioactive to the user. The sheet can be in a form that may be placed in contact with the mucosa of the user, and have an average dissolution time of 5 to 50 minutes for delivering the bioactive to the user.
Owner:PHILIP MORRIS PROD SA
Polyanhydrides with therapeutically useful degradation products
InactiveUS7122615B1Sufficient quantitySustained releasePowder deliveryDigestive systemTissue reconstructionDicarboxylic acid
An aromatic polyanhydride having a repeating unit with structure (I) wherein Ar is a substituted or unsubstituted aromatic ring and R is a difunctional organic moiety substituted on each Ar ortho to the anhydride group. Ortho-substituted bis-aromatic dicarboxylic acid anhydride monomers and ortho-substituted bis-aromatic dicarboxylic acid intermediates thereof are also disclosed, as well as implantable medical devices, such as scaffolding implants for tissue reconstruction, drug delivery systems prepared from the aromatic polyanhydrides, as well as therapeutic oral dosage forms and treatment methods.
Owner:AMT CAPITAL +1
Pharmaceutical Formulation Containing Opioid Agonist, Opioid Antagonist and Gelling Agent
InactiveUS20150031718A1Avoid abuseReduce releaseBiocidePharmaceutical non-active ingredientsOpioid antagonistPharmaceutical formulation
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Hydrogel-forming sustained-release preparation
InactiveUS20030203024A1Prolongs absorption periodStable levelPharmaceutical non-active ingredientsPill deliverySmall intestinePharmaceutical Substances
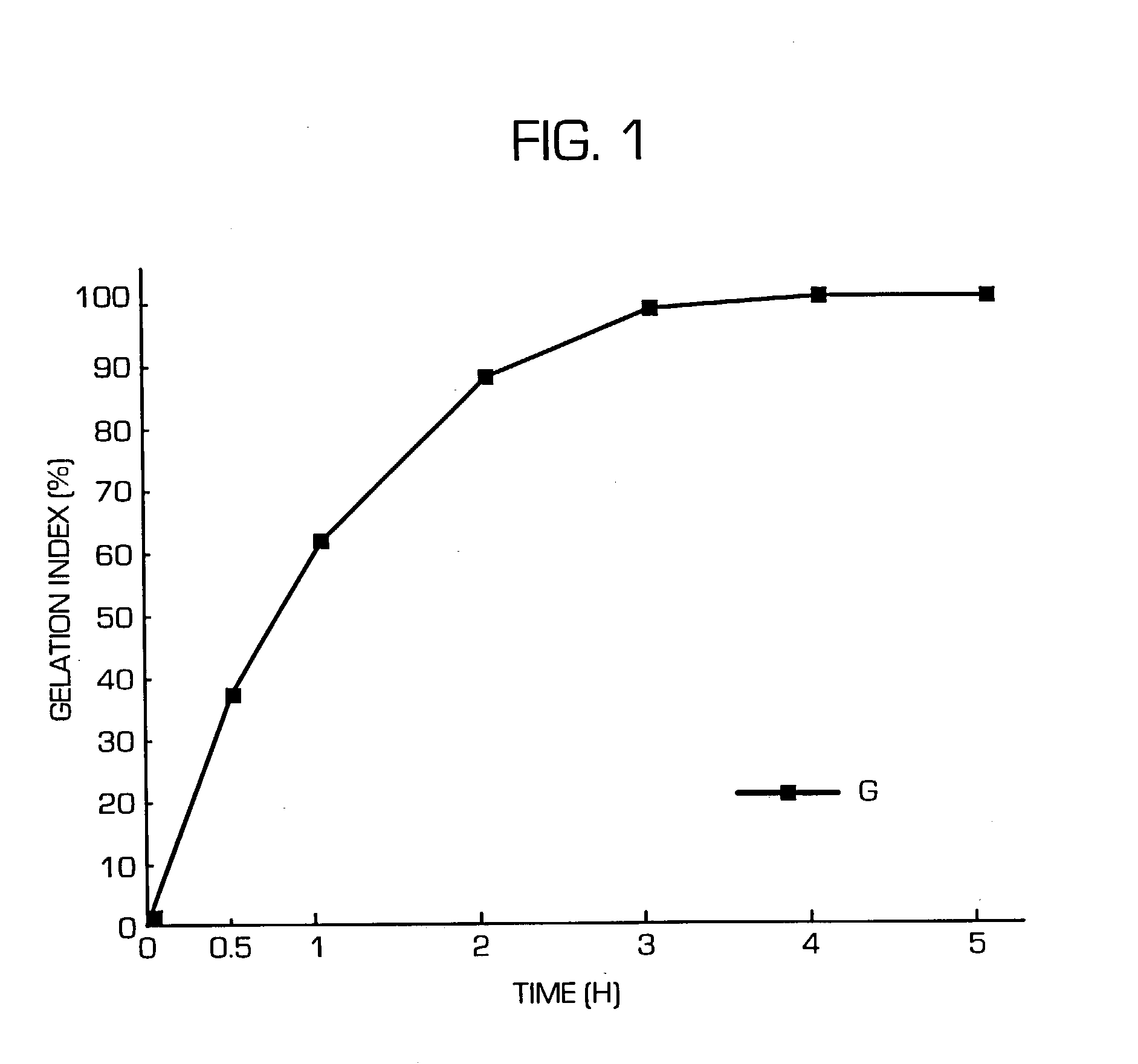
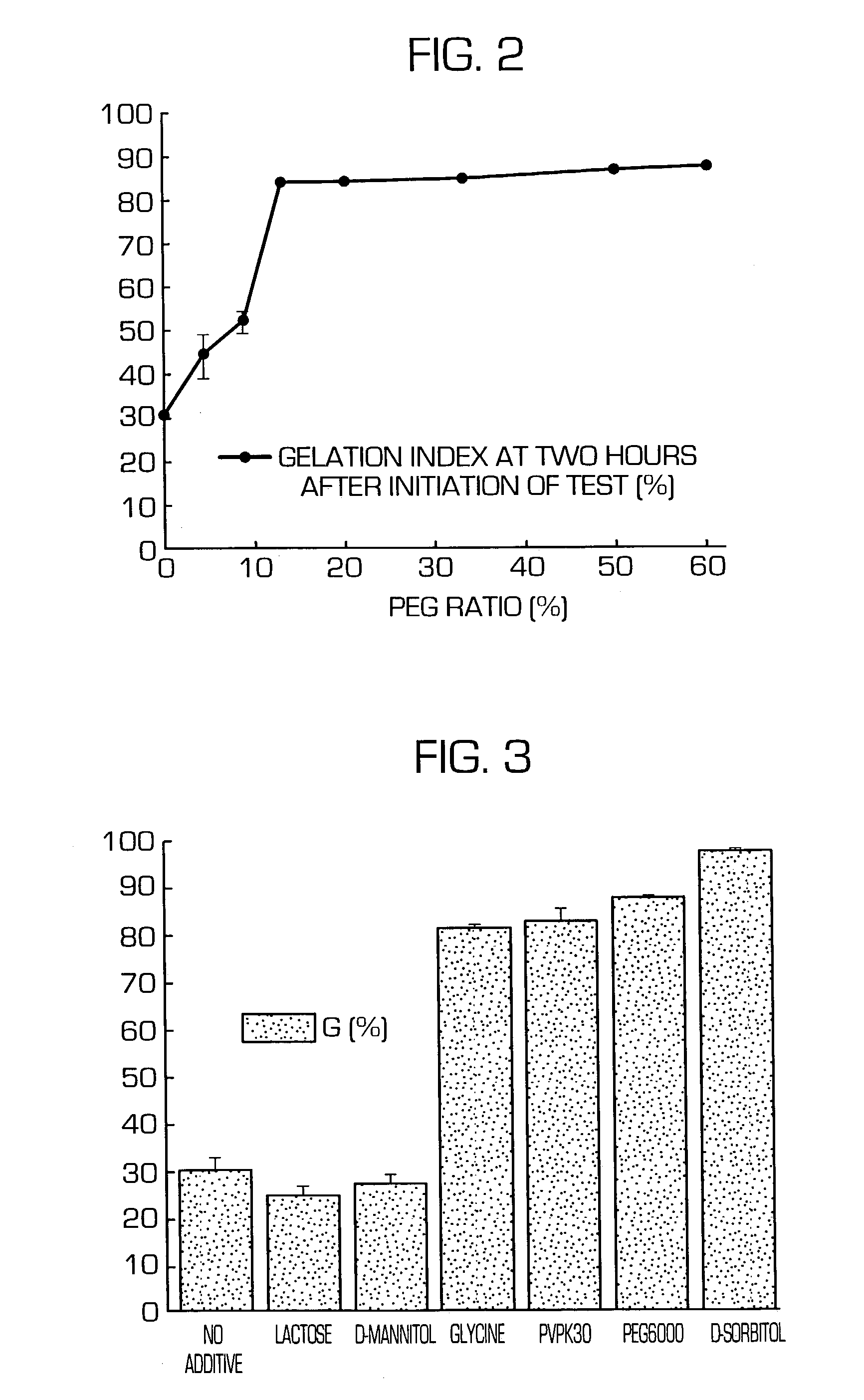
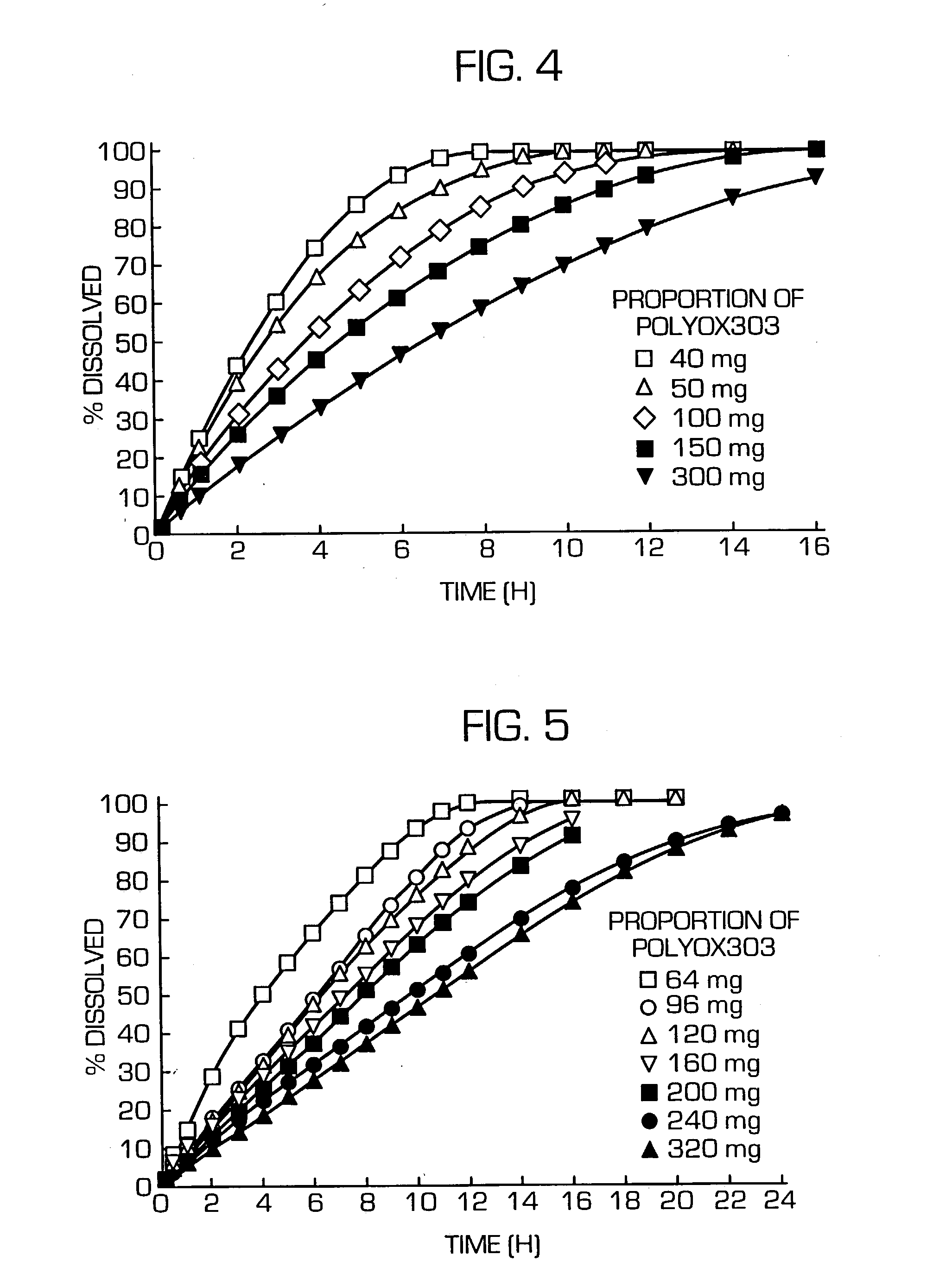
The invention provides a hydrogel-type sustained-release preparation comprising (1) at least one drug, (2) an additive which insures a penetration of water into the core of the preparation and (3) a hydrogel-forming polymer, wherein said preparation is capable of undergoing substantially complete gelation during its stay in the upper digestive tract such as stomach and small intestine and is capable of releasing the drug in the lower digestive tract including colon. By the preparation of the invention, the drug is efficiently released and absorbed even in the colon so that a steady and sustained release effect can be achieved.
Owner:ASTELLAS PHARMA INC
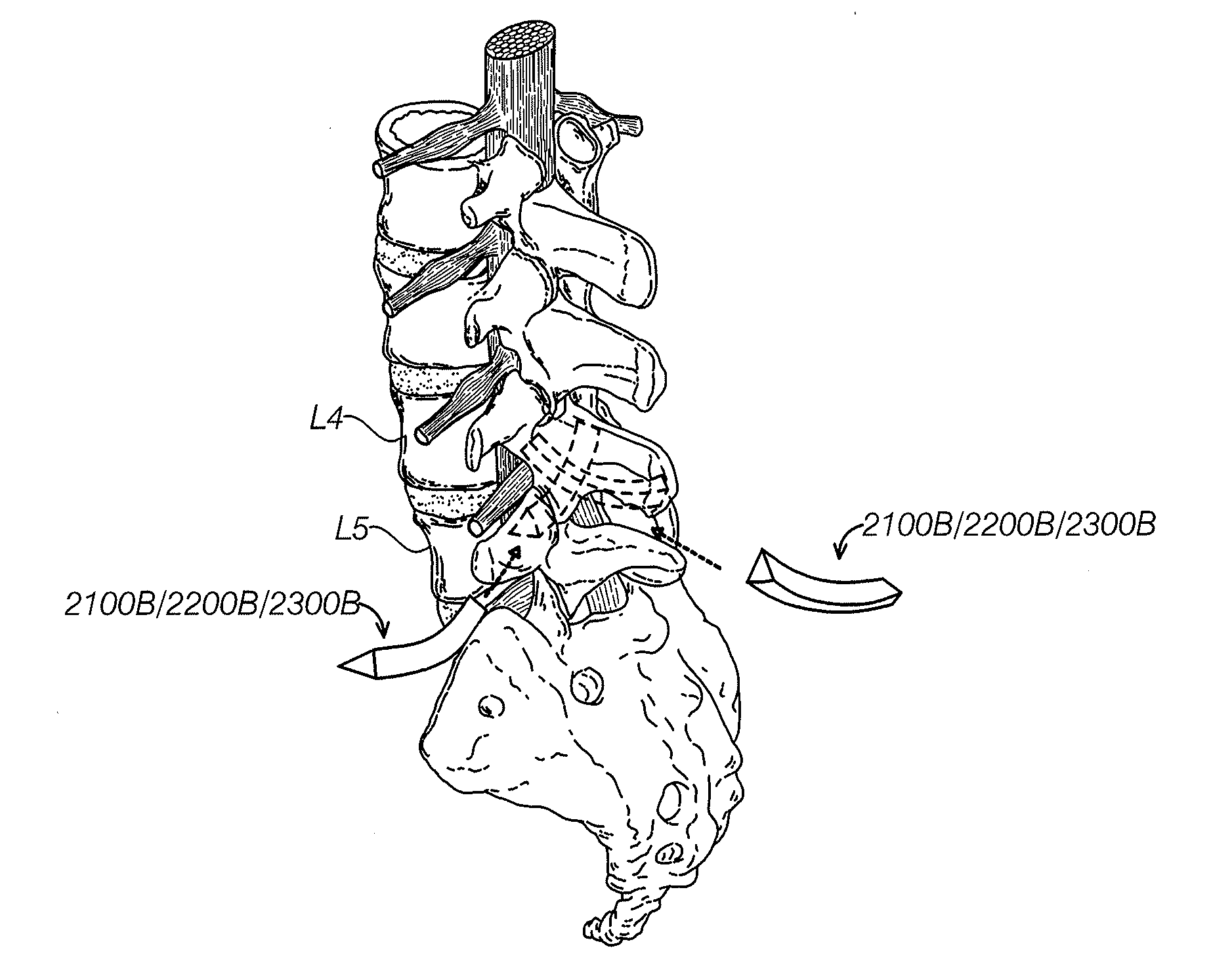
Systems, device, and methods for joint fusion
ActiveUS20170007409A1Sustained releaseSpeed up fusion and stabilization processInternal osteosythesisCannulasVertebraNon invasive
The present invention relates generally to implants and tools for the fixation or fusion of joints or bone segments. These tools include tissue dilators and protectors. Other tools include broaches used to shape bores in bone. The tools can also include a system for removing an implant from bone. Implants can include assemblies of one or more implant structures that make possible the achievement of diverse interventions involving the fusion and / or stabilization of lumbar and sacral vertebra in a non-invasive manner, with minimal incision, and without the necessitating the removing the intervertebral disc. Implants for fusing both sacroiliac joints of a patient include a long implant that extends across both sacroiliac joints.
Owner:SI BONE
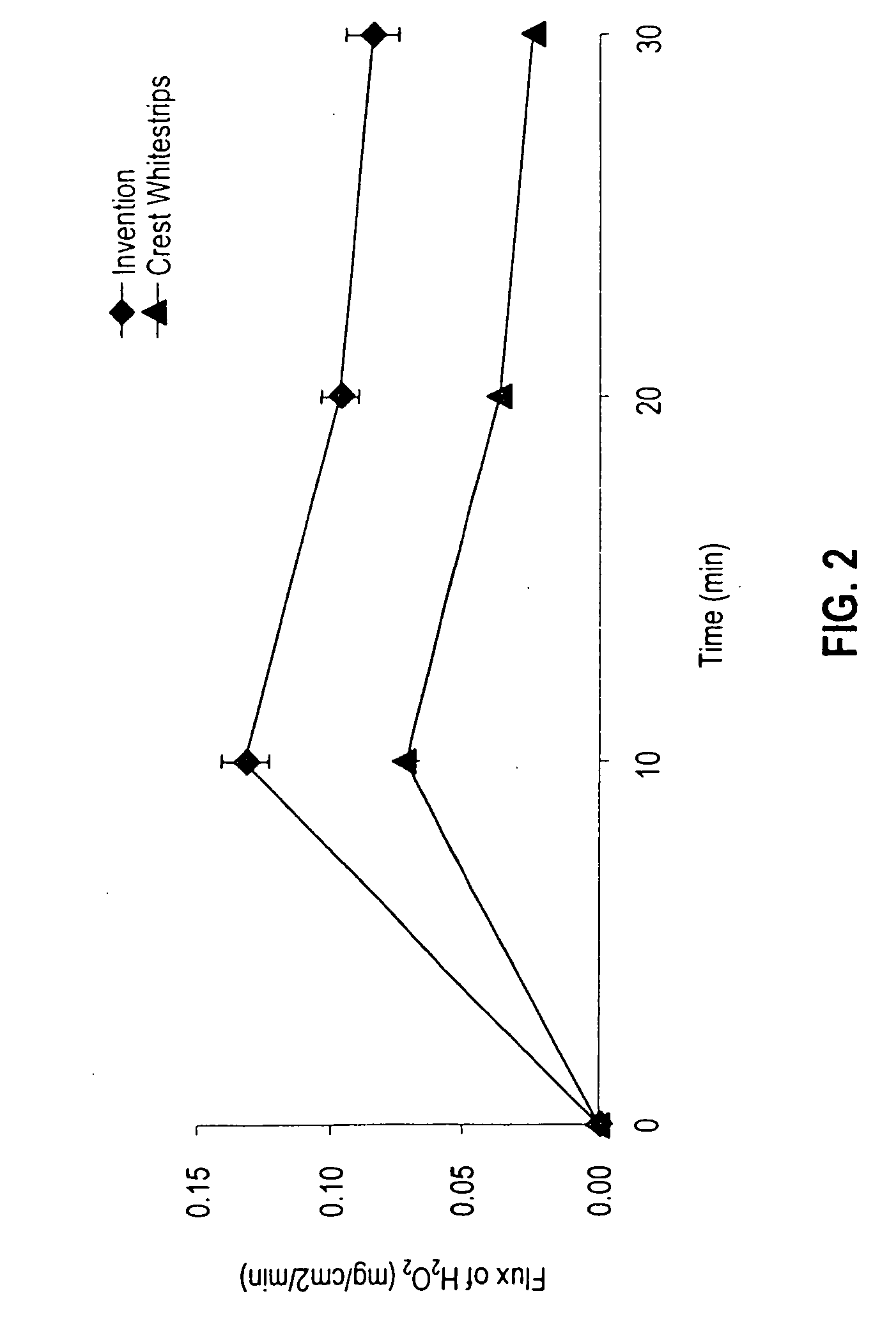
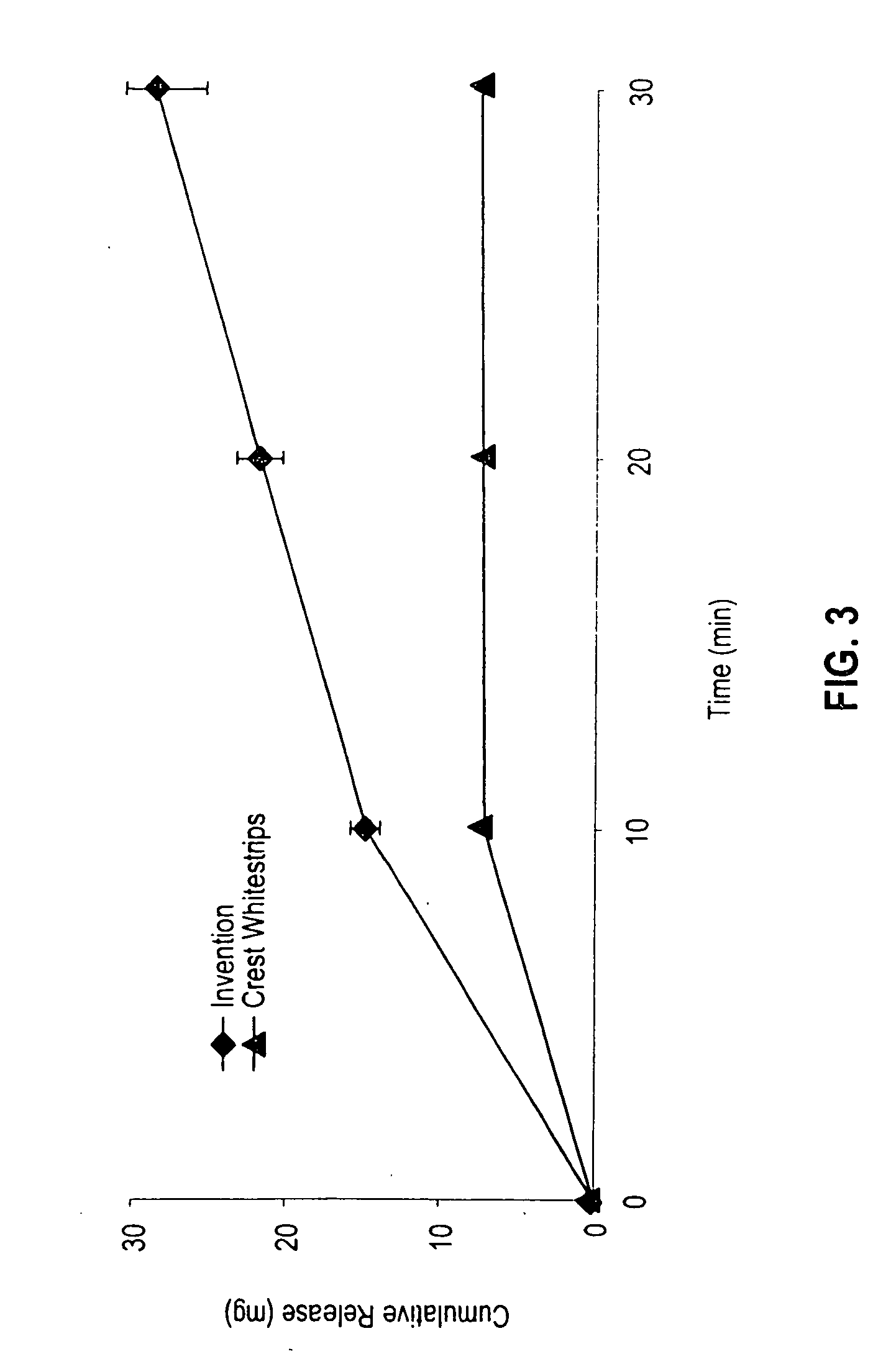
Sustained release tooth whitening formulations and systems
InactiveUS20060171906A1Improve the level ofWell-tolerated by usersCosmetic preparationsToilet preparationsWhitening AgentsTolerability
A new tooth whitening composition provides sustained release of high levels of whitening agent and is moisture-activated without significant swelling. A preferred system for applying the composition to the teeth is flexible, self-adhesive, and well-tolerated by users. In certain embodiments the tooth whitening composition comprises a mixture of tooth whitening agents, with a first whitening agent selected so as to release peroxide gradually upon contact with moisture and produce an alkaline pH, and a second whitening agent selected so as to release peroxide rapidly upon contact with moisture.
Owner:CORIUM INT +1
Nanoparticles for drug delivery
InactiveUS20070237827A1Sustained releaseReduce resistanceBiocidePowder deliveryHMG-CoA reductaseMedicine
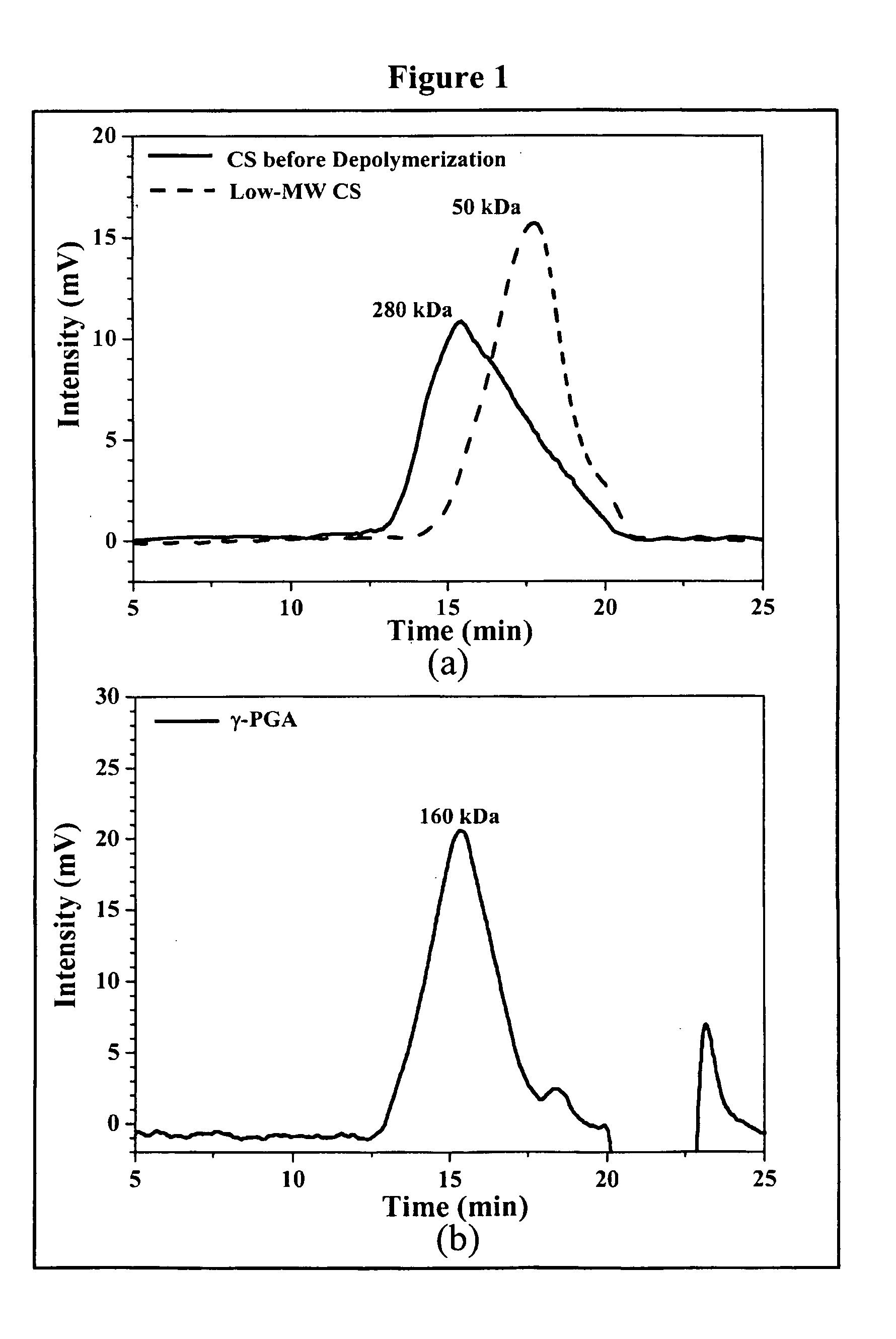
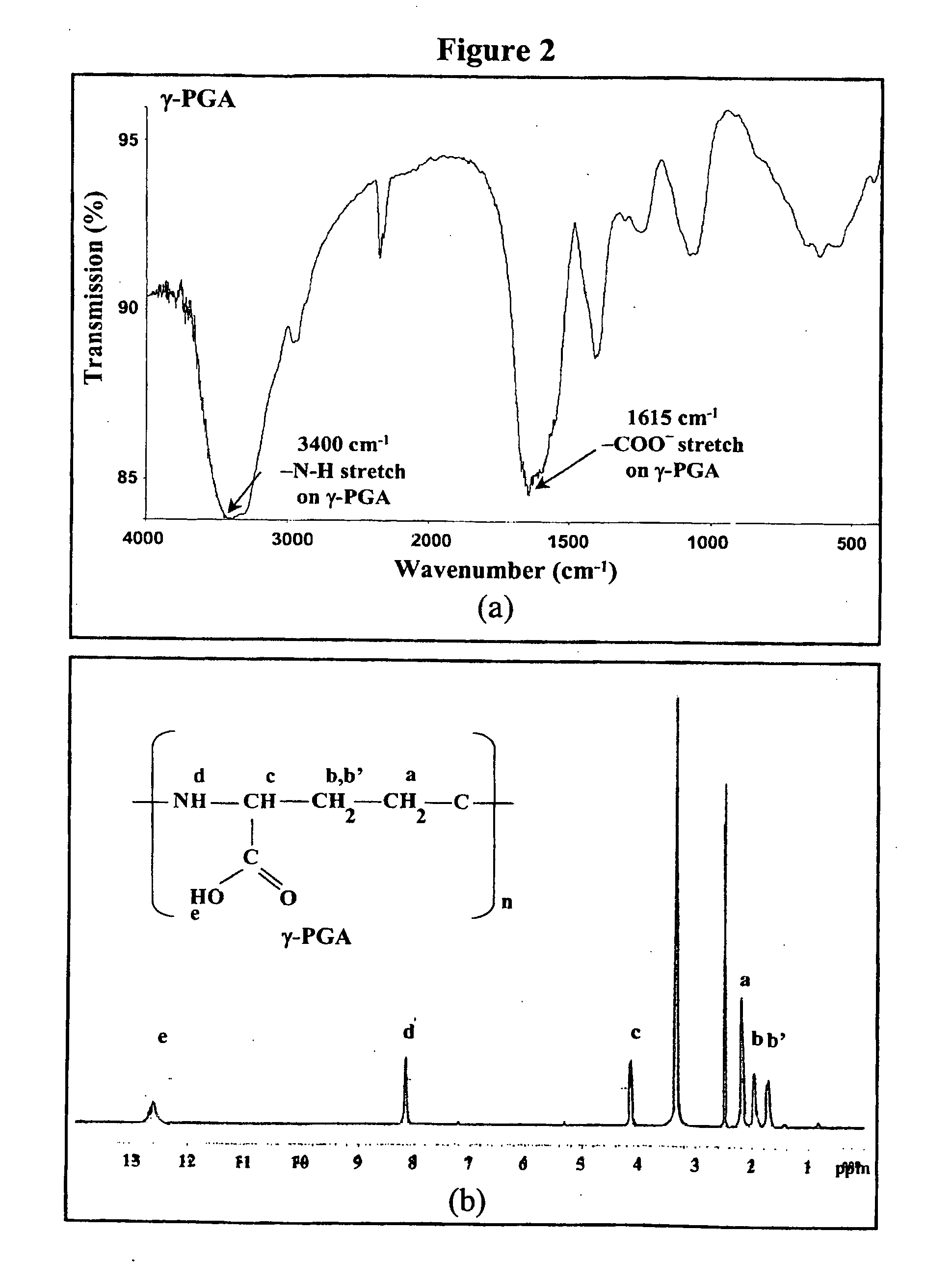
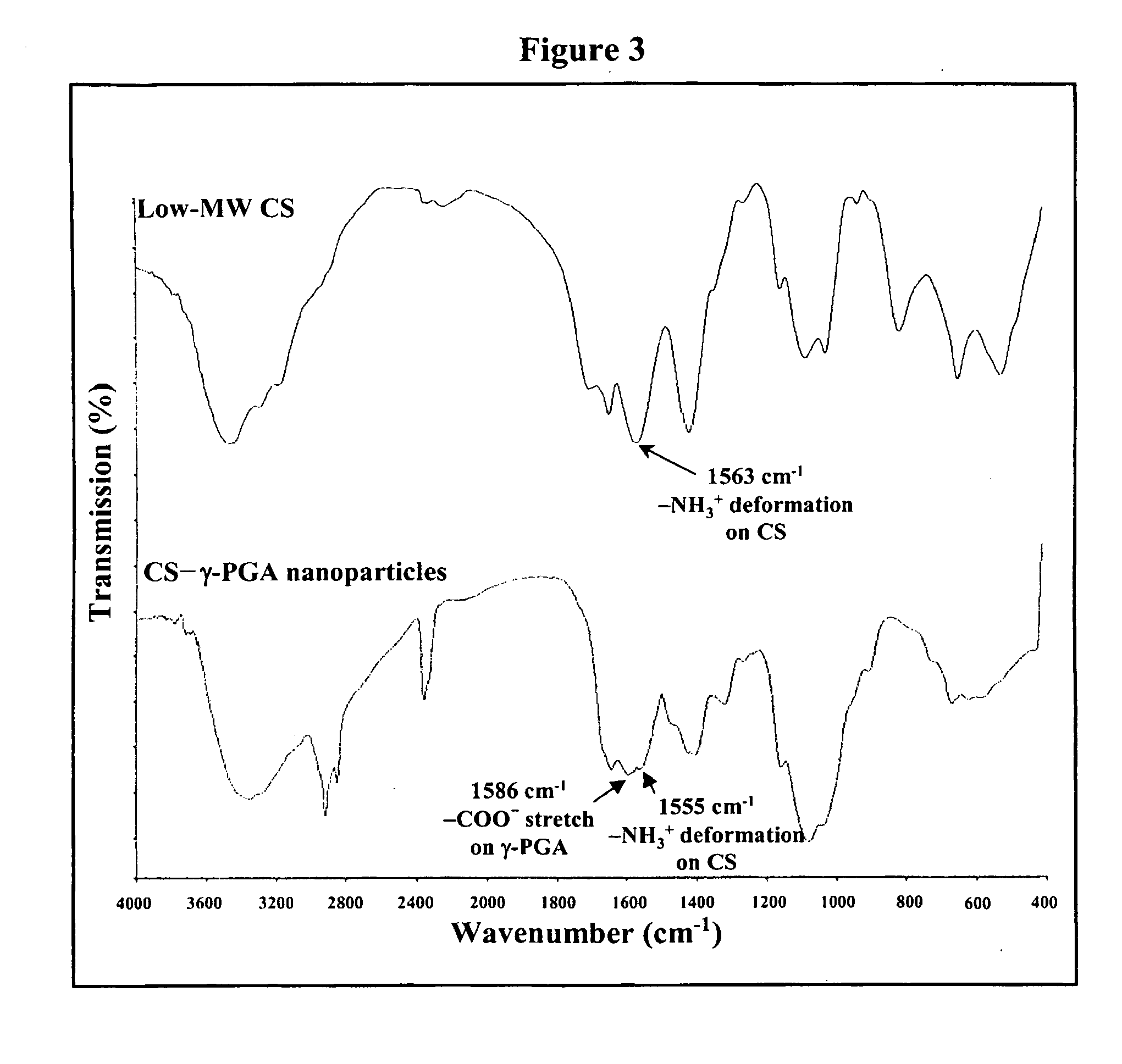
The invention discloses the nanoparticles composed of chitosan, poly-glutamic acid, and at least one bioactive agent of HMG-CoA reductase inhibitors or erythropoietin. The nanoparticles are characterized with a positive surface charge and their enhanced permeability for paracellular drug delivery.
Owner:NANOMEGA MEDICAL CORP
Nanoscale Particle Formulations and Methods
InactiveUS20130177504A1Reduce the populationSustained releaseBiocideHeavy metal active ingredientsDermatologySustained Release Formulations
Provided herein in some embodiments is a formulation comprising (a) one or more nanoscale particle; and (b) a film-forming polymer. In some instances, the formulation is an immediate and sustained released formulation suitable for topical administration or administration to surfaces. Also provided here in certain embodiments is a method of reducing the population of pathogenic microorganisms on skin or surfaces, the method comprising applying to the skin or surface a composition, the composition comprising (a) one or more nanoscale particle; and (b) a film-forming polymer.
Owner:ANNUARY HEALTHCARE
Antimicrobial coating methods
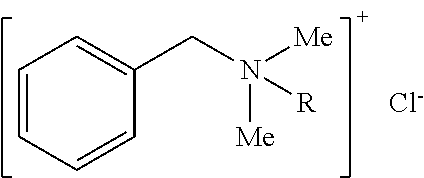
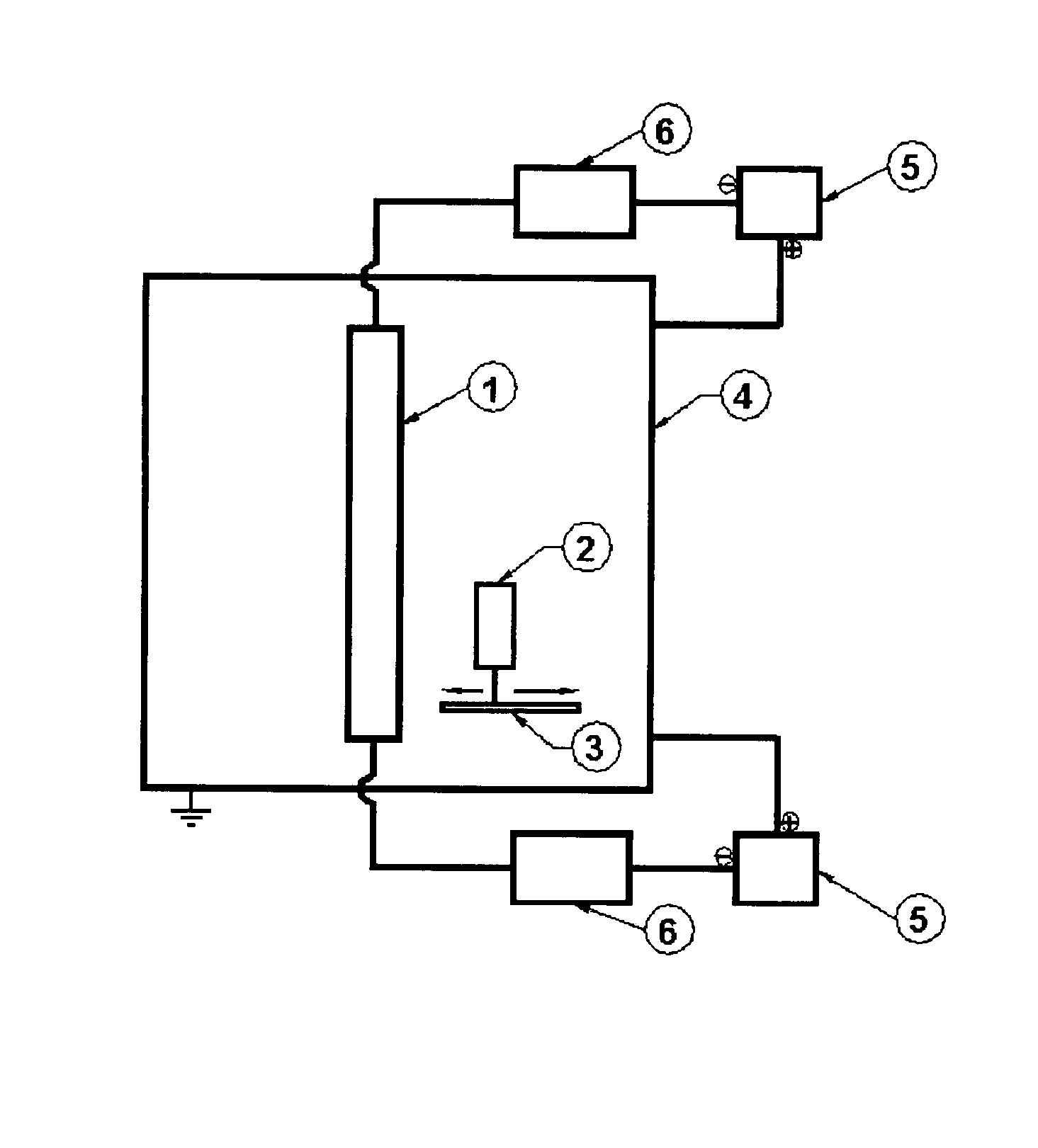
ActiveUS8066854B2Eliminate needSustained releaseBiocideHeavy metal active ingredientsMetallurgyIn vivo
The invention is directed to efficient methods for depositing highly adherent anti-microbial materials onto a wide range of surfaces. A controlled cathodic arc process is described, which results in enhanced adhesion of silver oxide to polymers and other surfaces, such as surfaces of medical devices. Deposition of anti-microbial materials directly onto the substrates is possible in a cost-effective manner that maintains high anti-microbial activity over several weeks when the coated devices are employed in vivo.
Owner:NORTHEASTERN UNIV
Floating type pellets in stomach and preparation method thereof
ActiveCN101288659ASustained releaseLow densityCapsule deliveryMacromolecular non-active ingredientsIntestinal absorptionSustained release pellets
The invention relates to the technical field of medicine, in particular to a stomach floating type pellet and a preparation method thereof. The stomach floating type pellet comprises a pellet core, a drug layer and a sustained-release layer; the density of the pellet is smaller than 1g / cm<3>, so that the drug can float in the stomach, sustainably release the drug ingredients; stomach floating type capsules can be prepared by loading the pellets into capsules. The preparation type is suitable for drugs mainly absorbed in the stomach, in particular to the drugs that are absorbed better in the stomach than in the enteric canal. When the drugs are prepared into stomach floating type pellets or stomach floating type capsules, compared with ordinary preparation, the times of administration is reduced and the relatively stable blood concentration of the drugs is kept in vivo, thus ensuring the sustained release of the drugs, improving the bioavailability and reducing the dosage. At the same time, as the stomach floating type pellet is a granose system, the drug release behavior is the collective behavior of each pellet, so that coating failure of a plurality of pellets can not lead to failure of the whole dosage, thus having higher safety. The sustained-release pellet is prepared by adopting general technique and is easy for realizing industrial production.
Owner:特康药业集团有限公司
Detergent pack
ActiveUS7304025B2Easy to detectSustained releaseDetergent materialsDetergent perfumesEngineeringWater soluble
Detergent pack comprising a combination of a malodour-generating water-soluble cleaning pouch, comprising a liquid composition and an enveloping film material, and a packaging container therefor wherein:a) the liquid composition comprises a first perfume; andb) the packaging container comprises a hot melt adhesive adhered to an internal wall thereof, the hot melt comprising an aldehyde-comprising perfume.There is also provided a method of preventing or reducing malodour in the interior of a packaging container containing a malodour-generating water-soluble pouch.
Owner:THE PROCTER & GAMBLE COMPANY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com