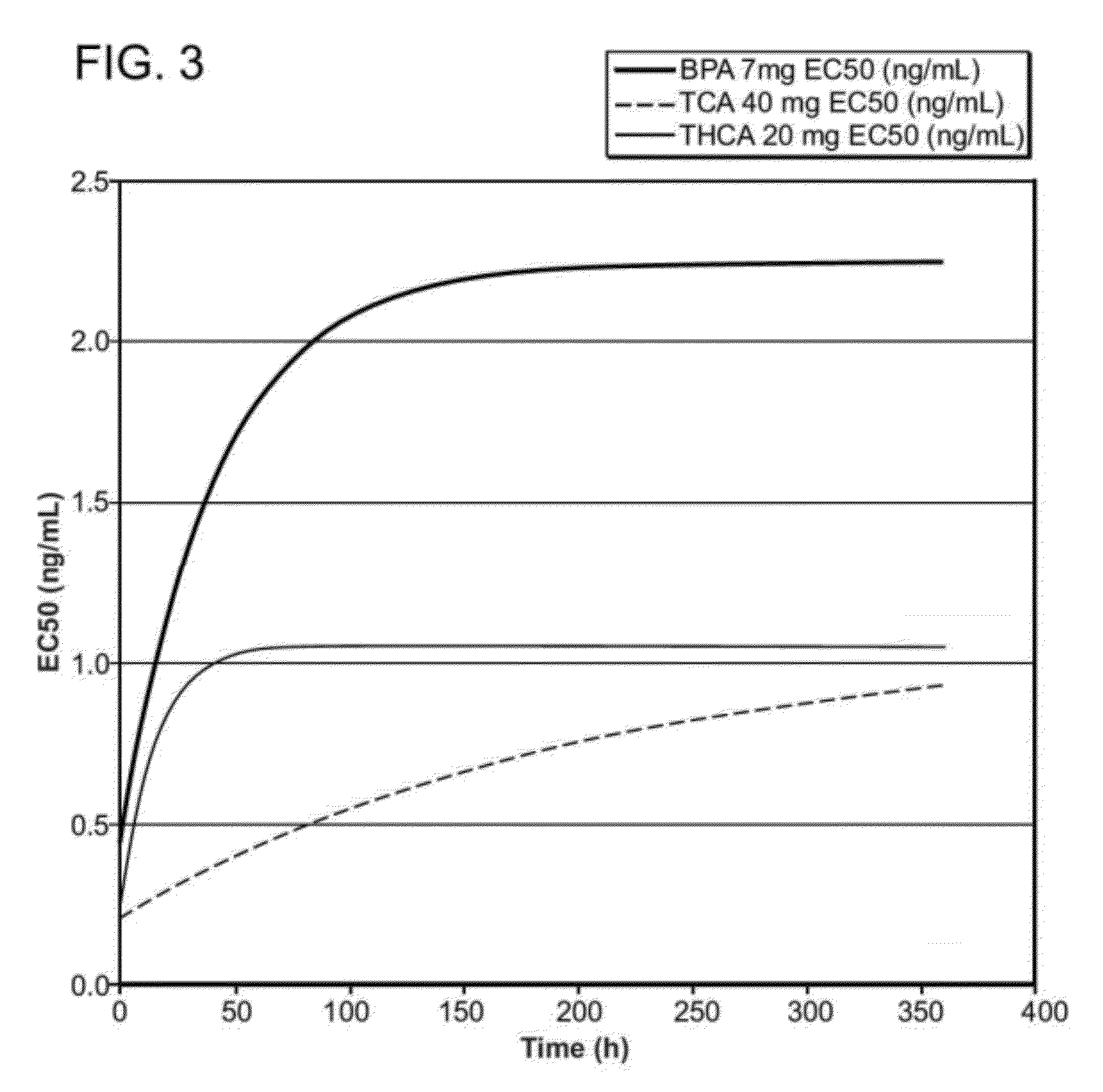
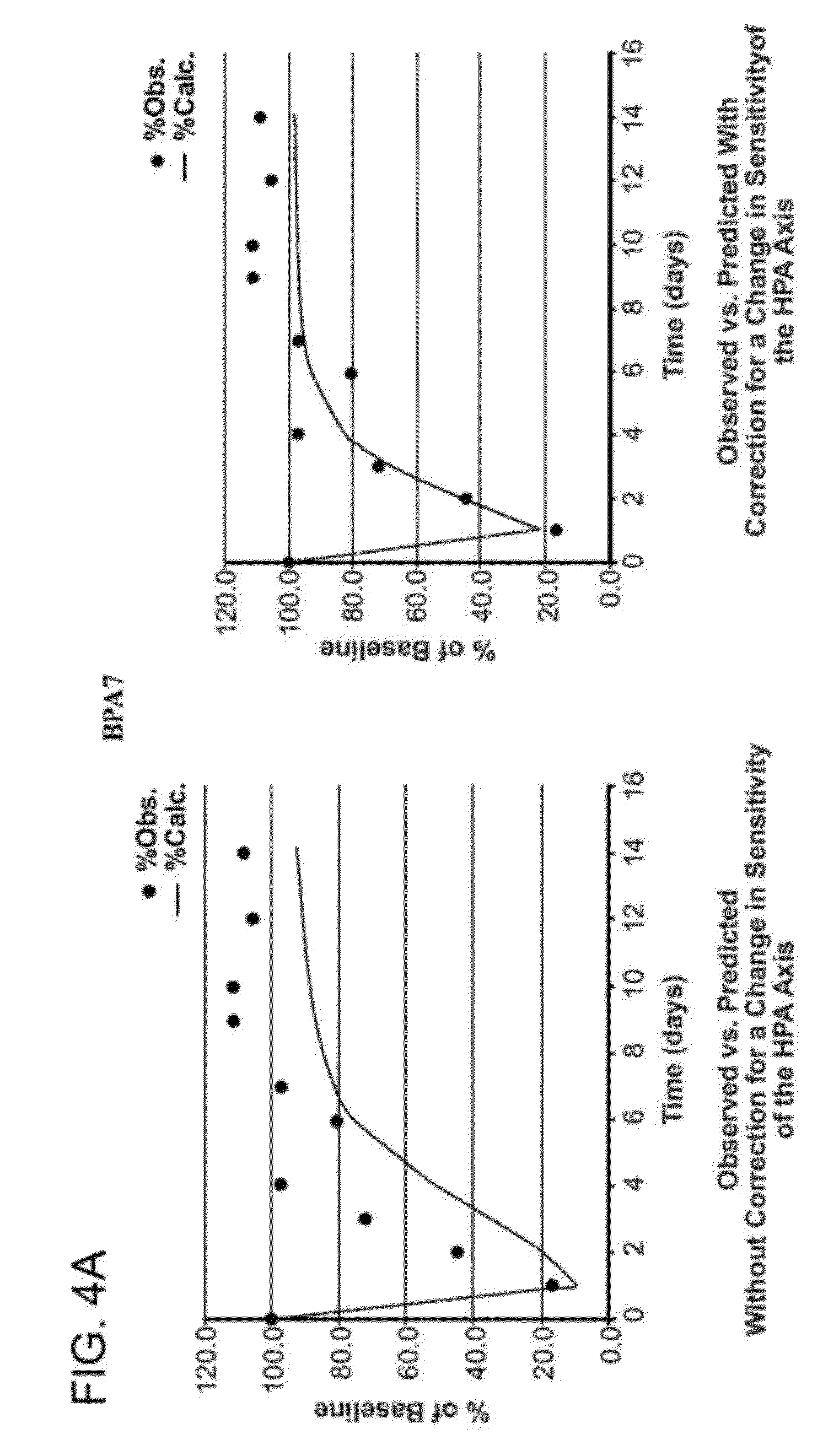
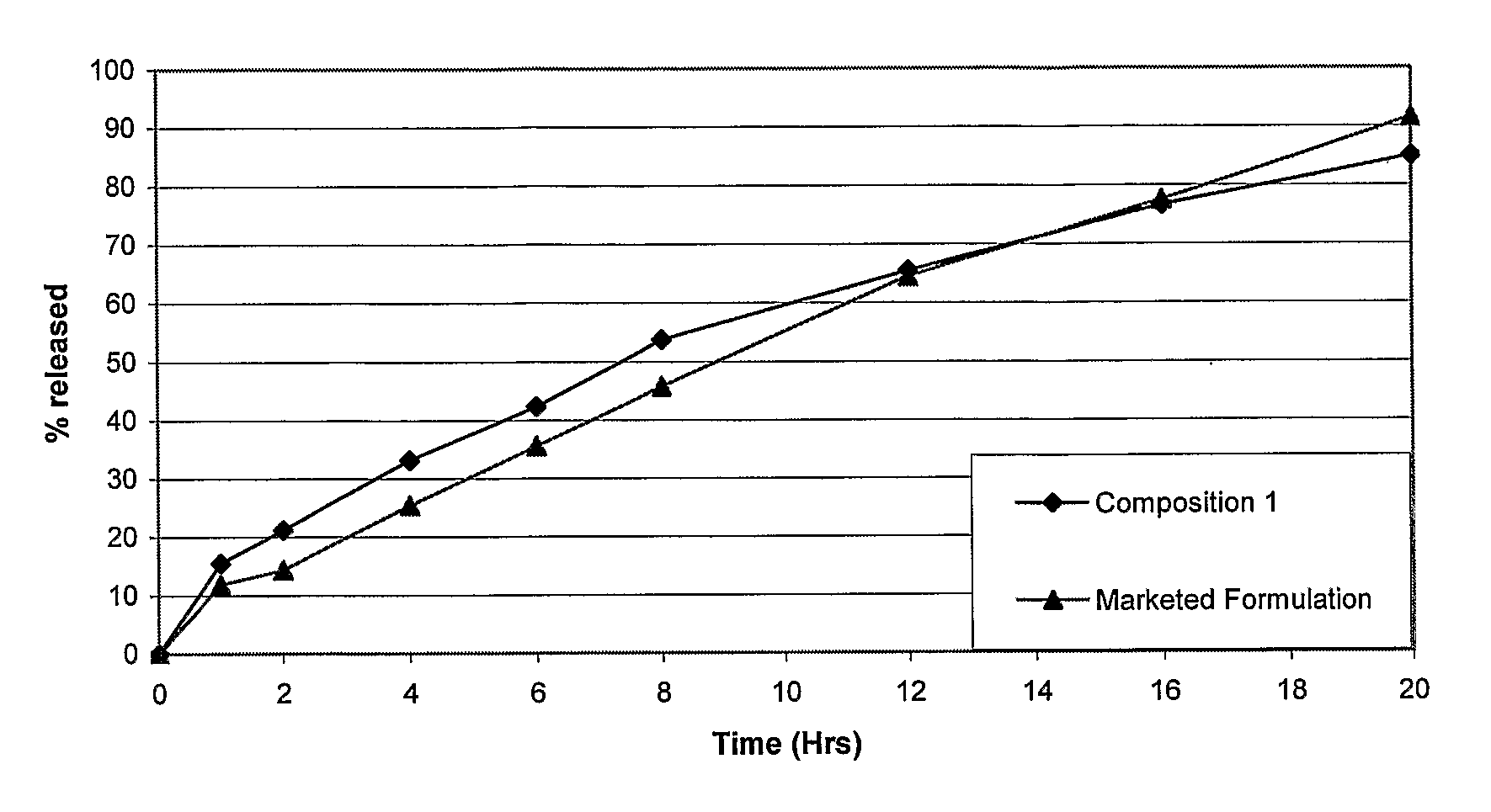
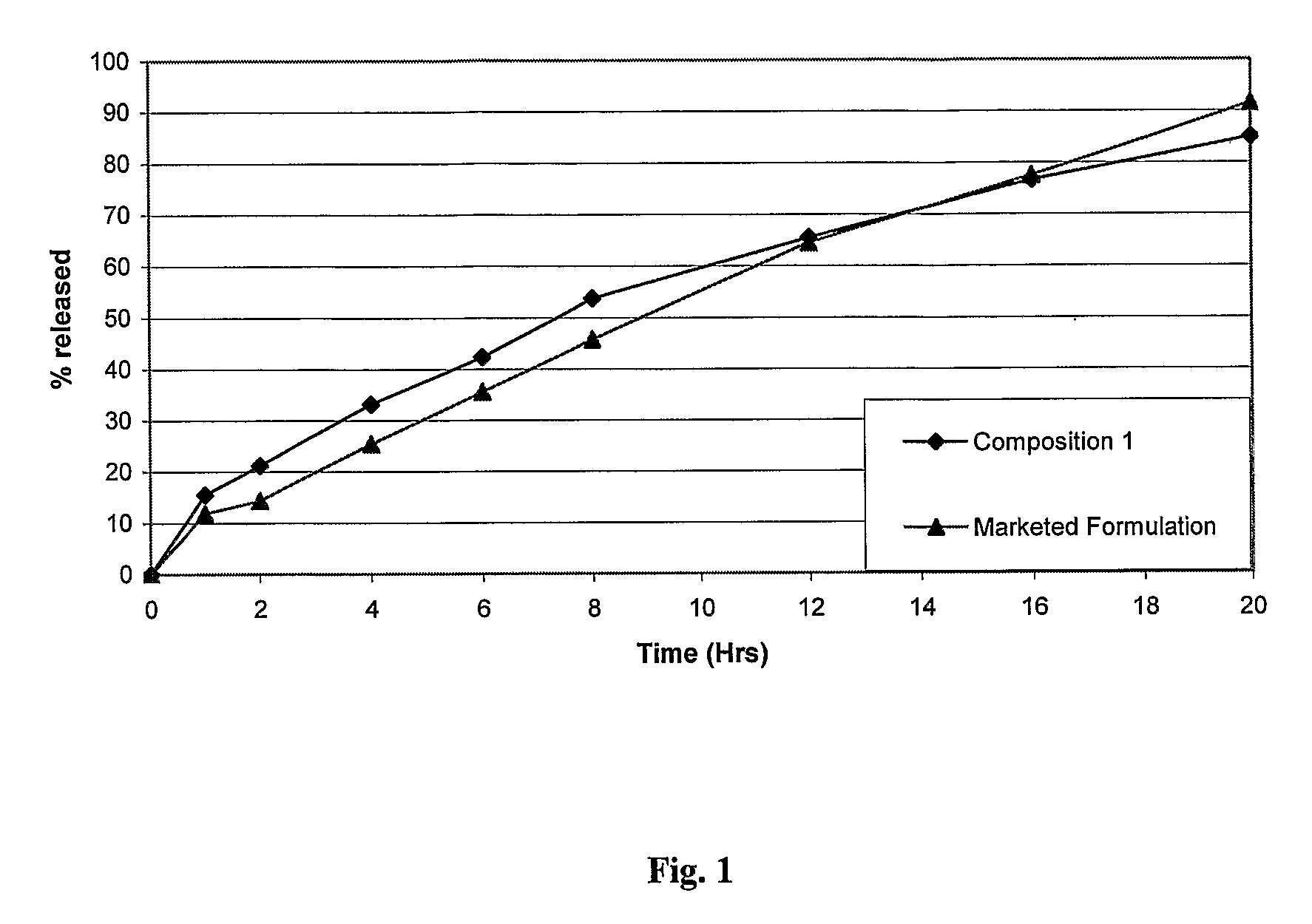
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
109 results about "Sustained release dosage forms" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
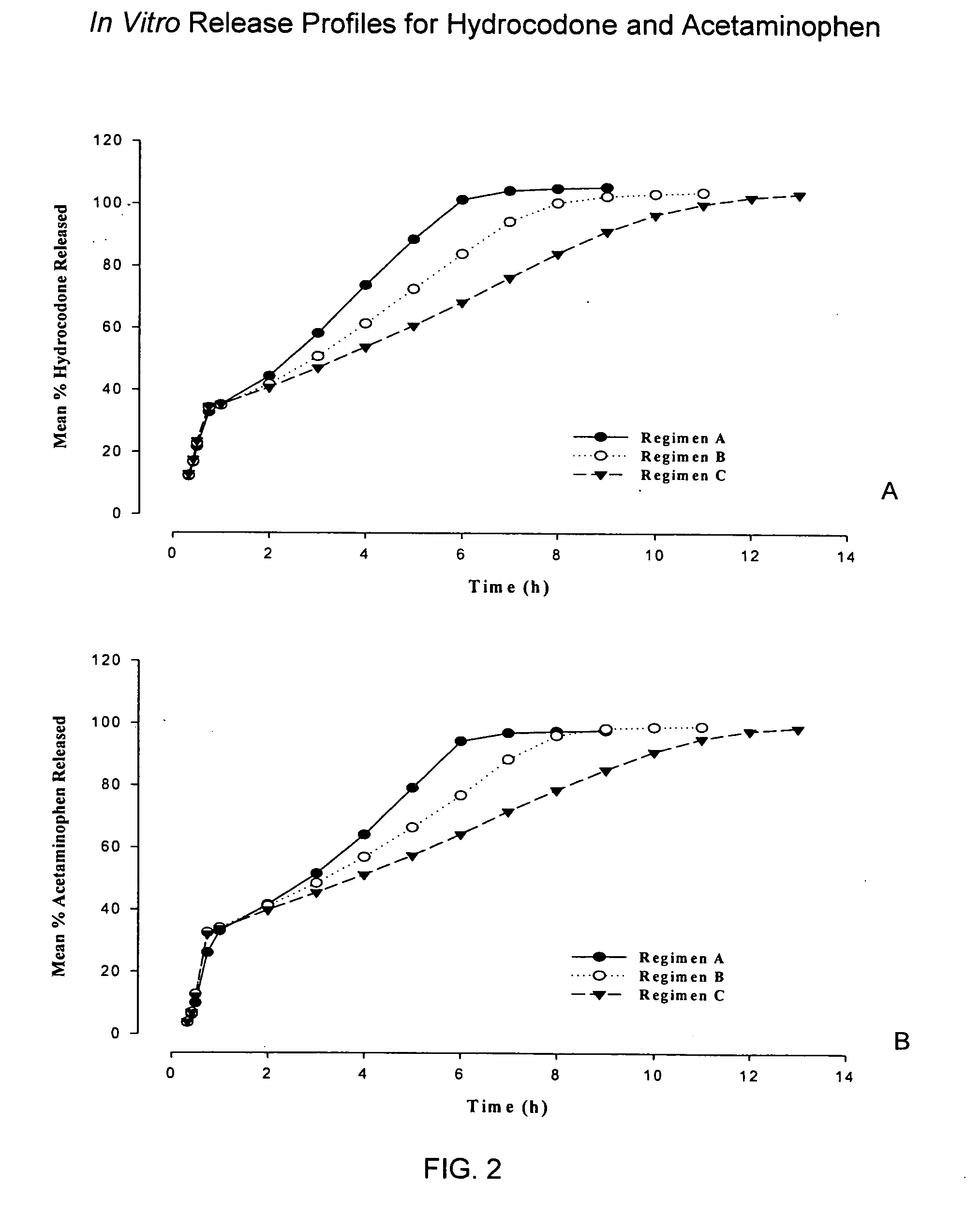
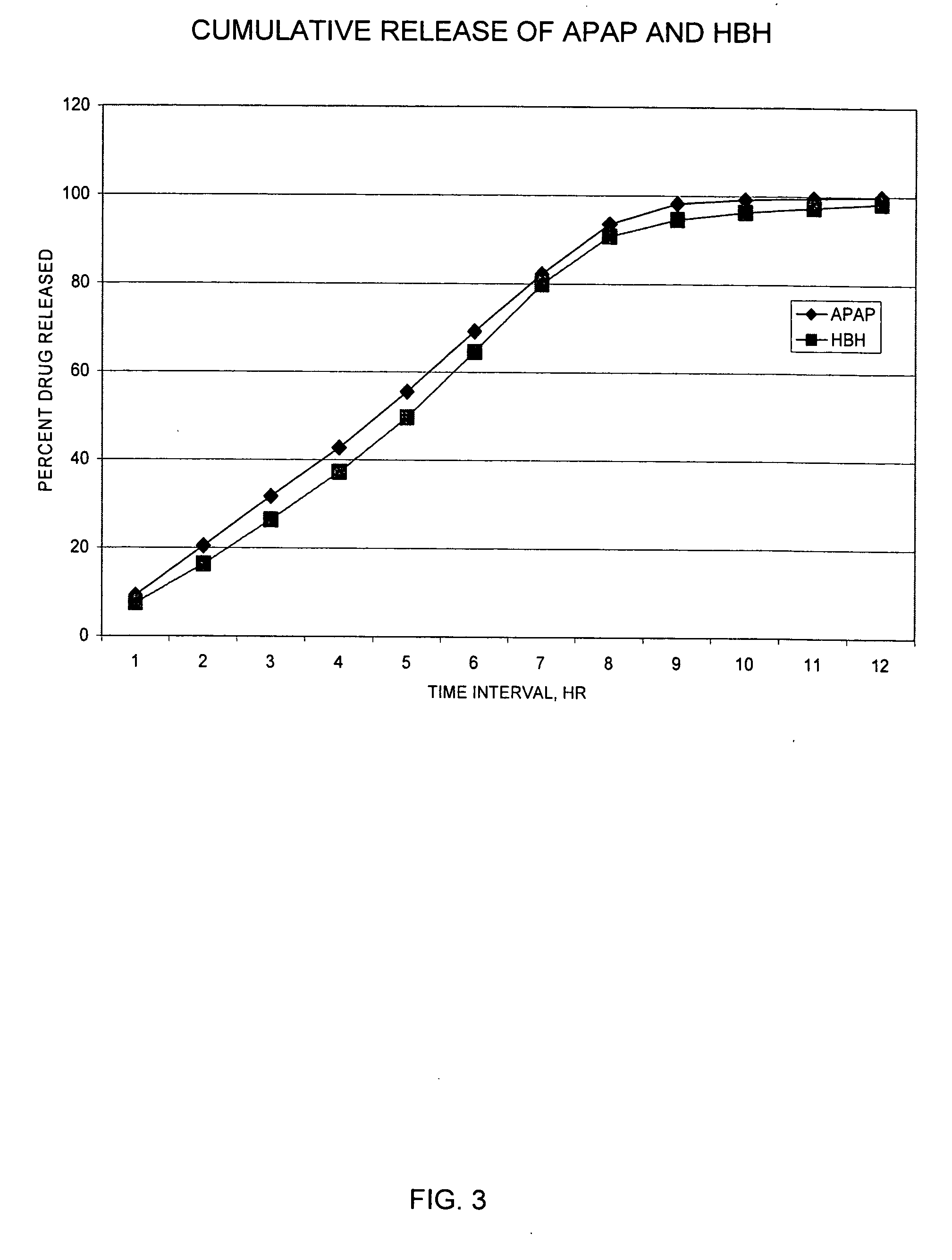
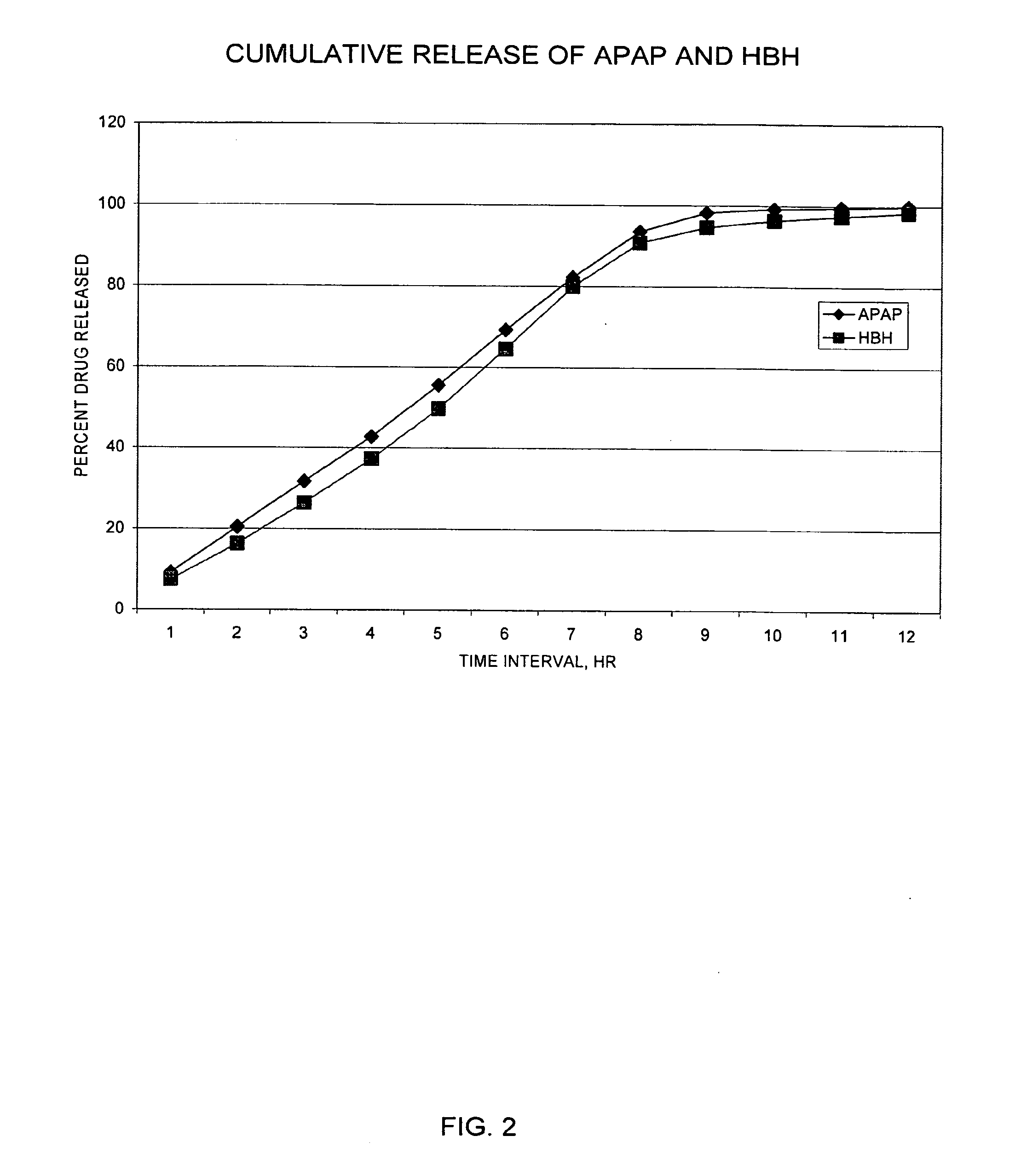
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
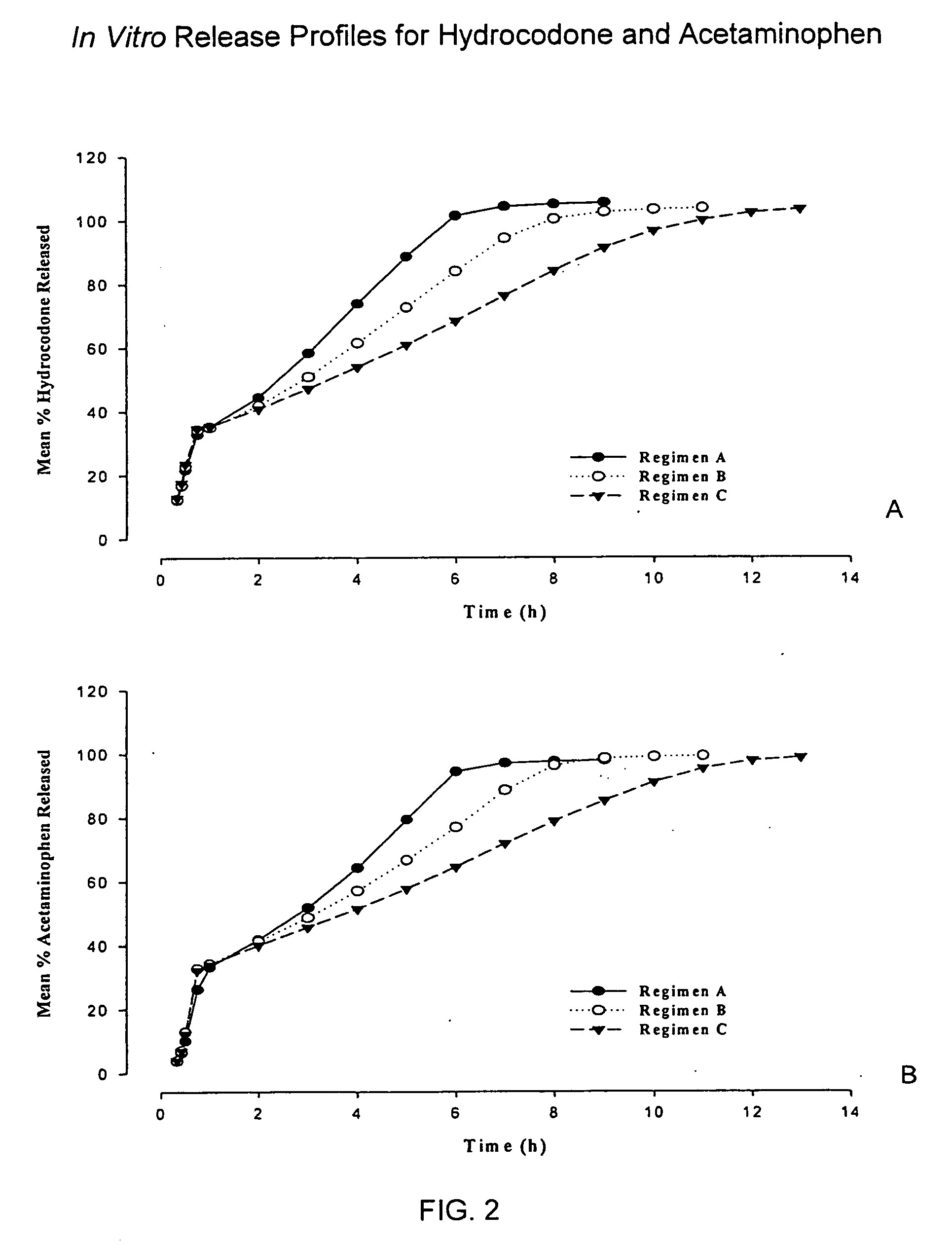
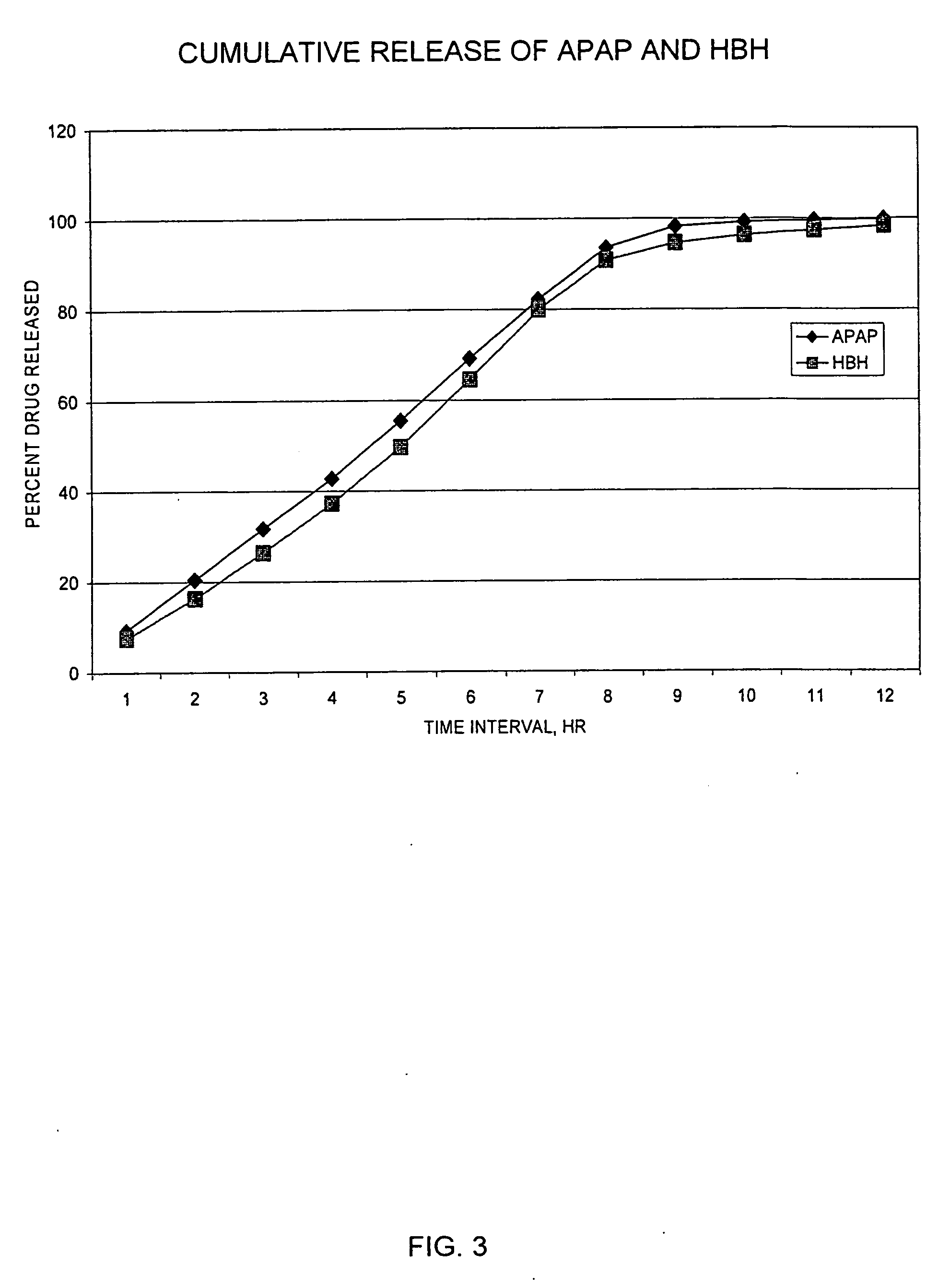
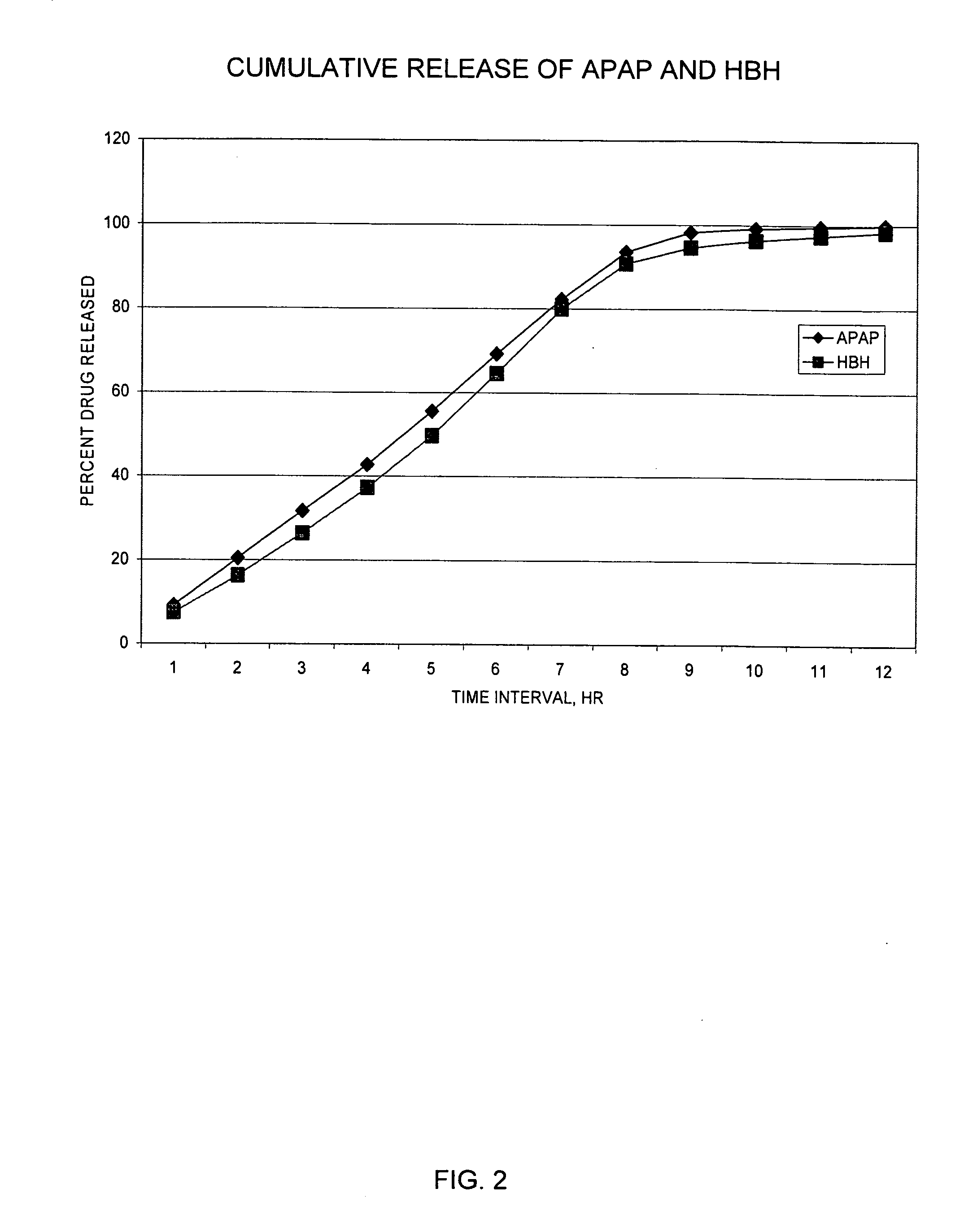
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20060251721A1Improved ability to treat painLess attentionBiocideNervous disorderImmediate releasePharmaceutical medicine
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
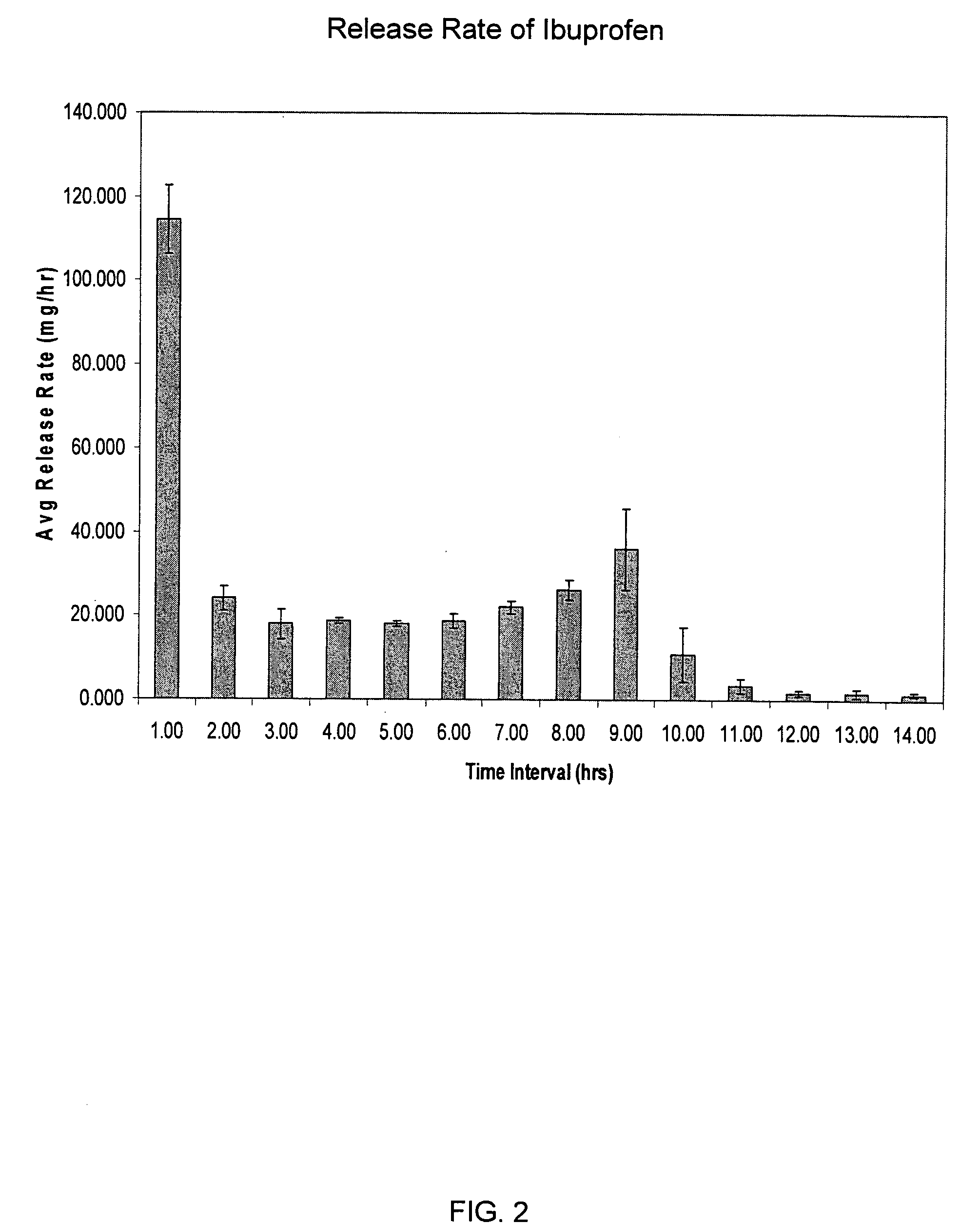
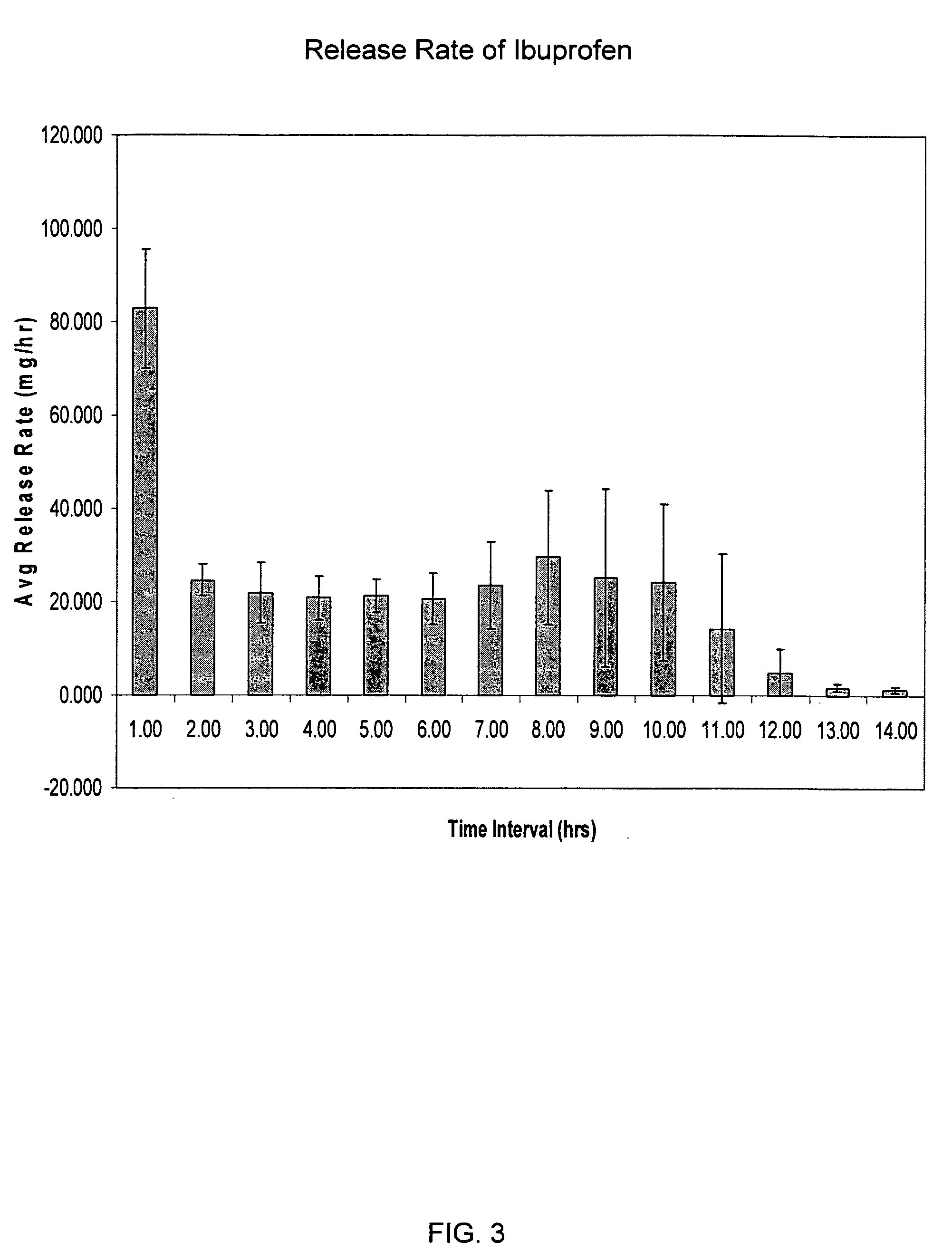
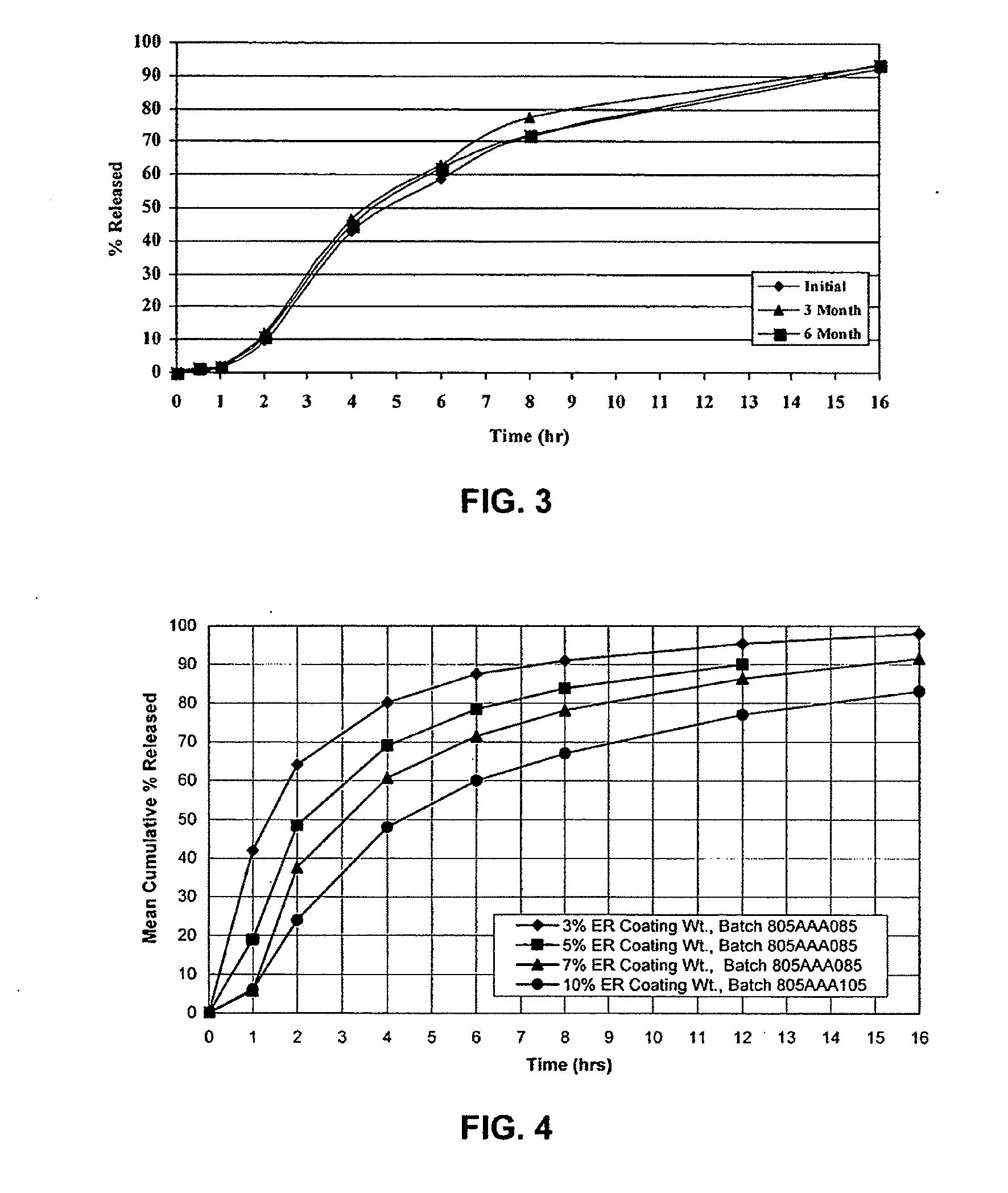
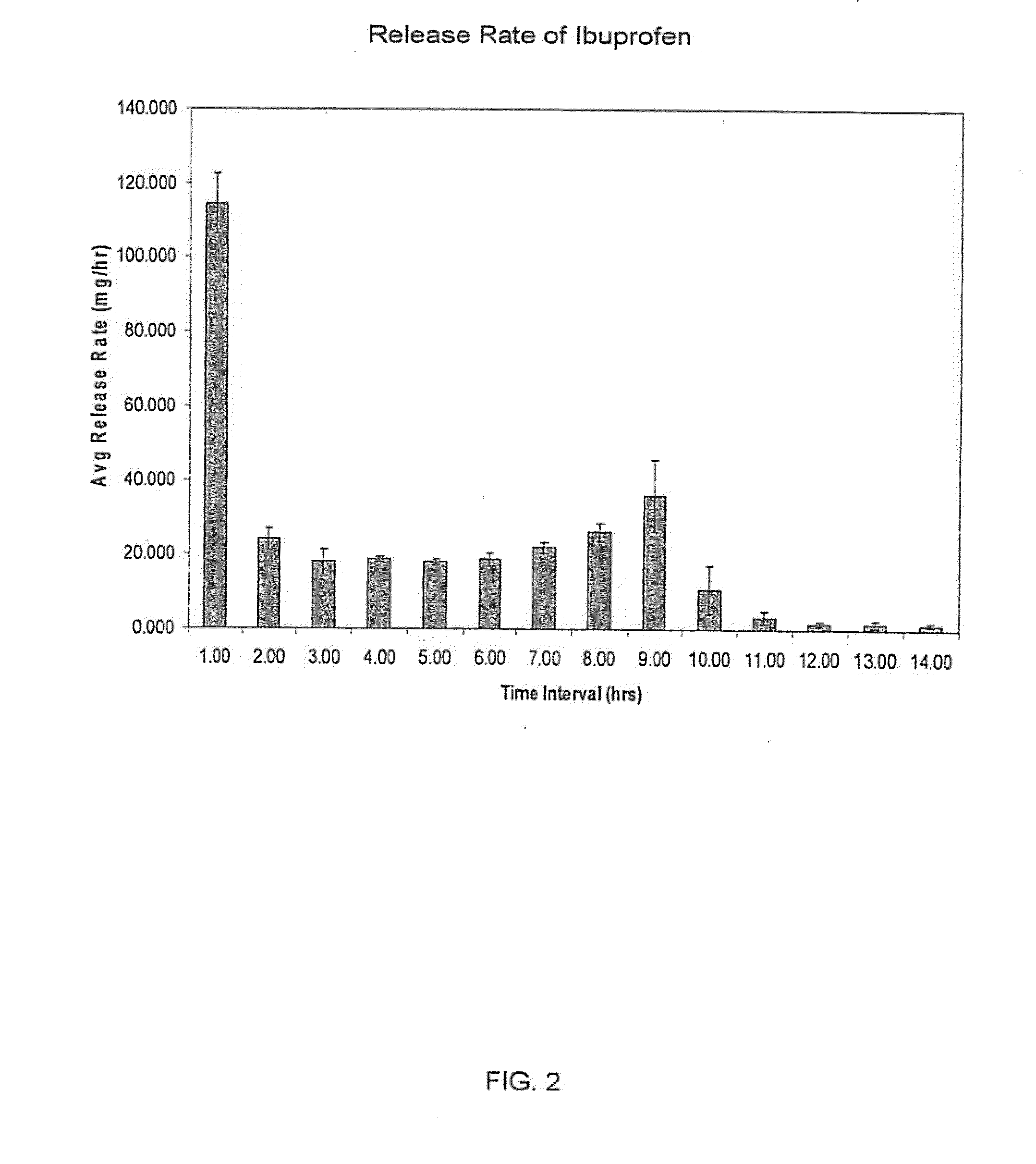
Sustained release matrix for high-dose insoluble drugs
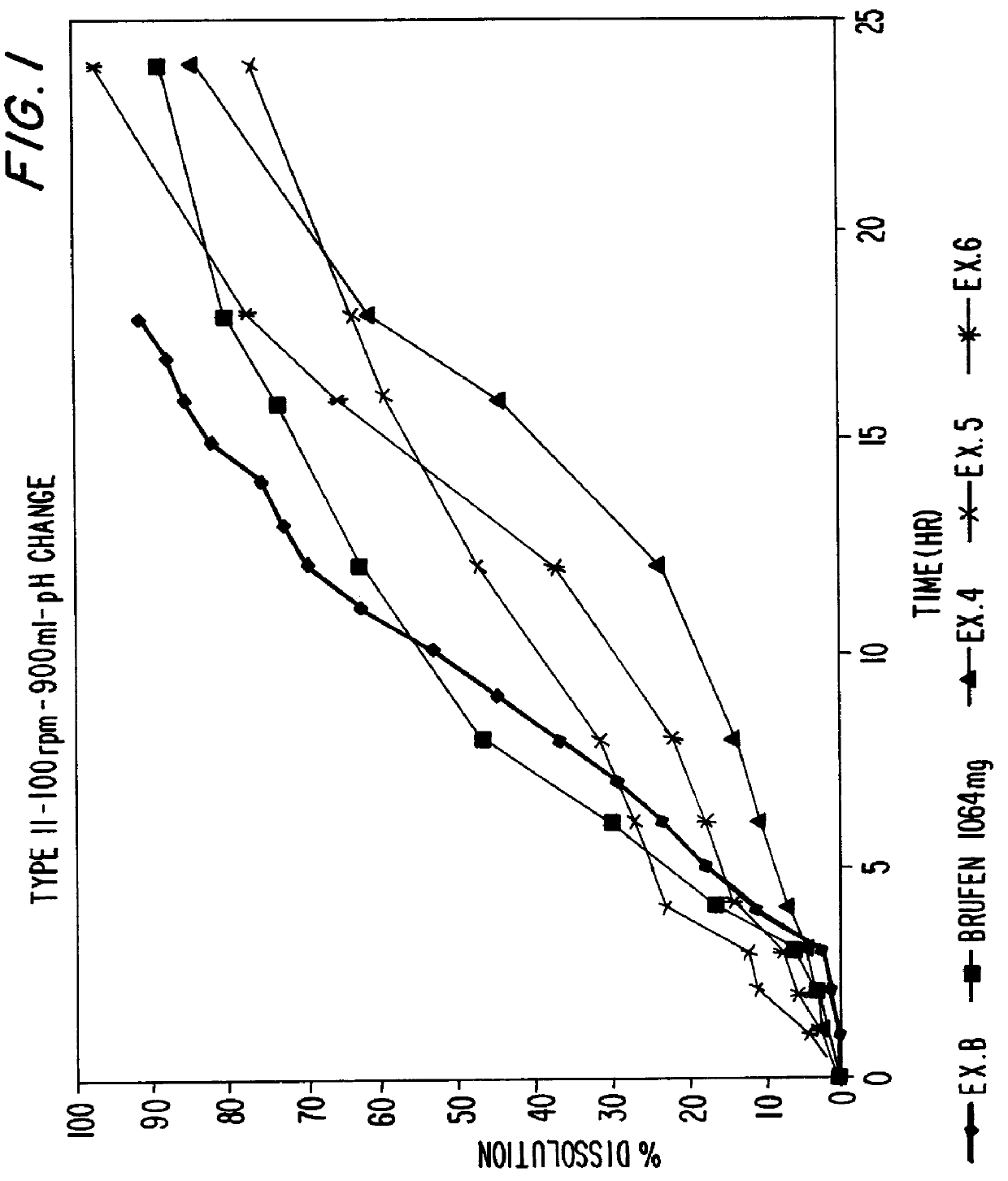
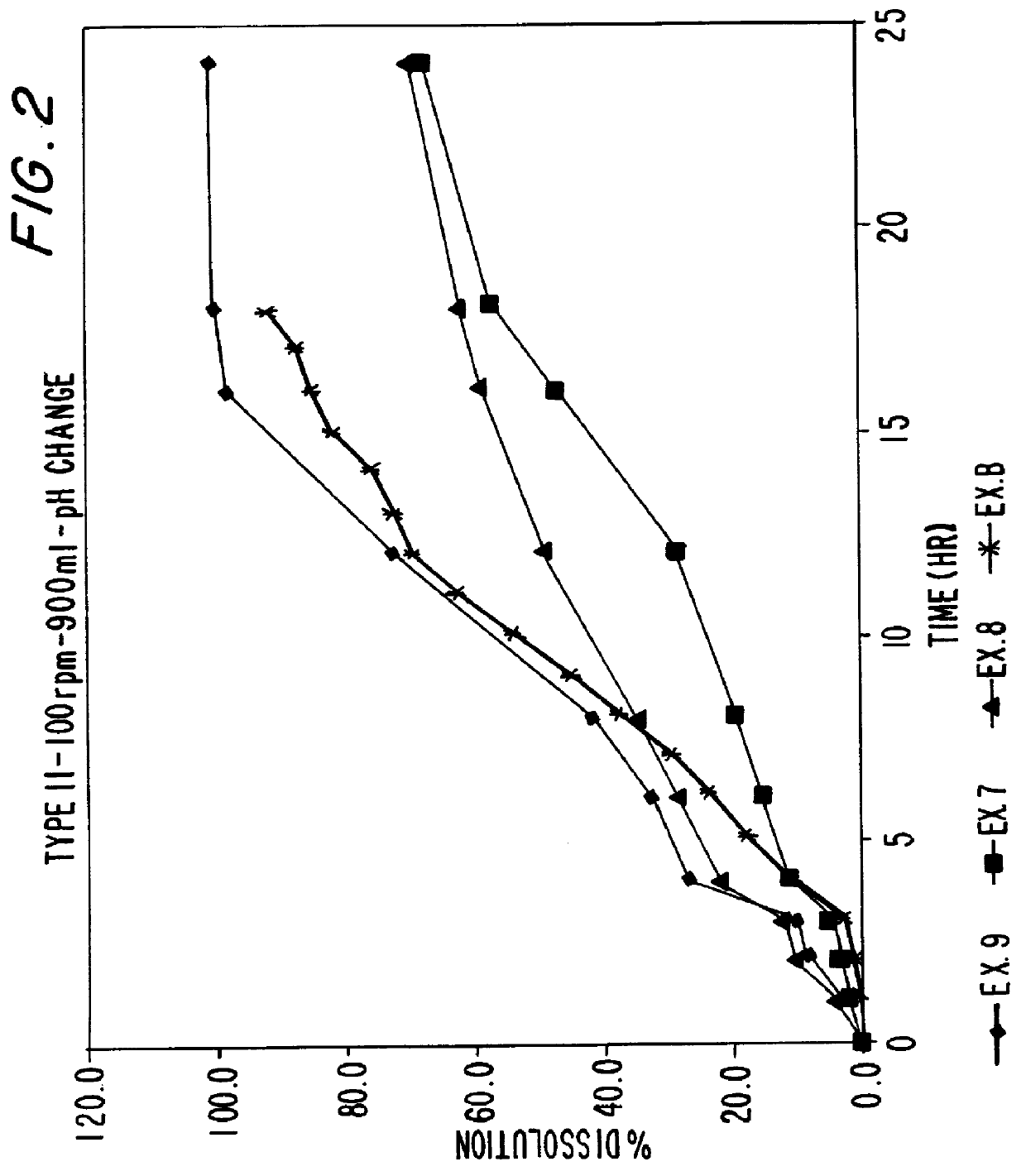
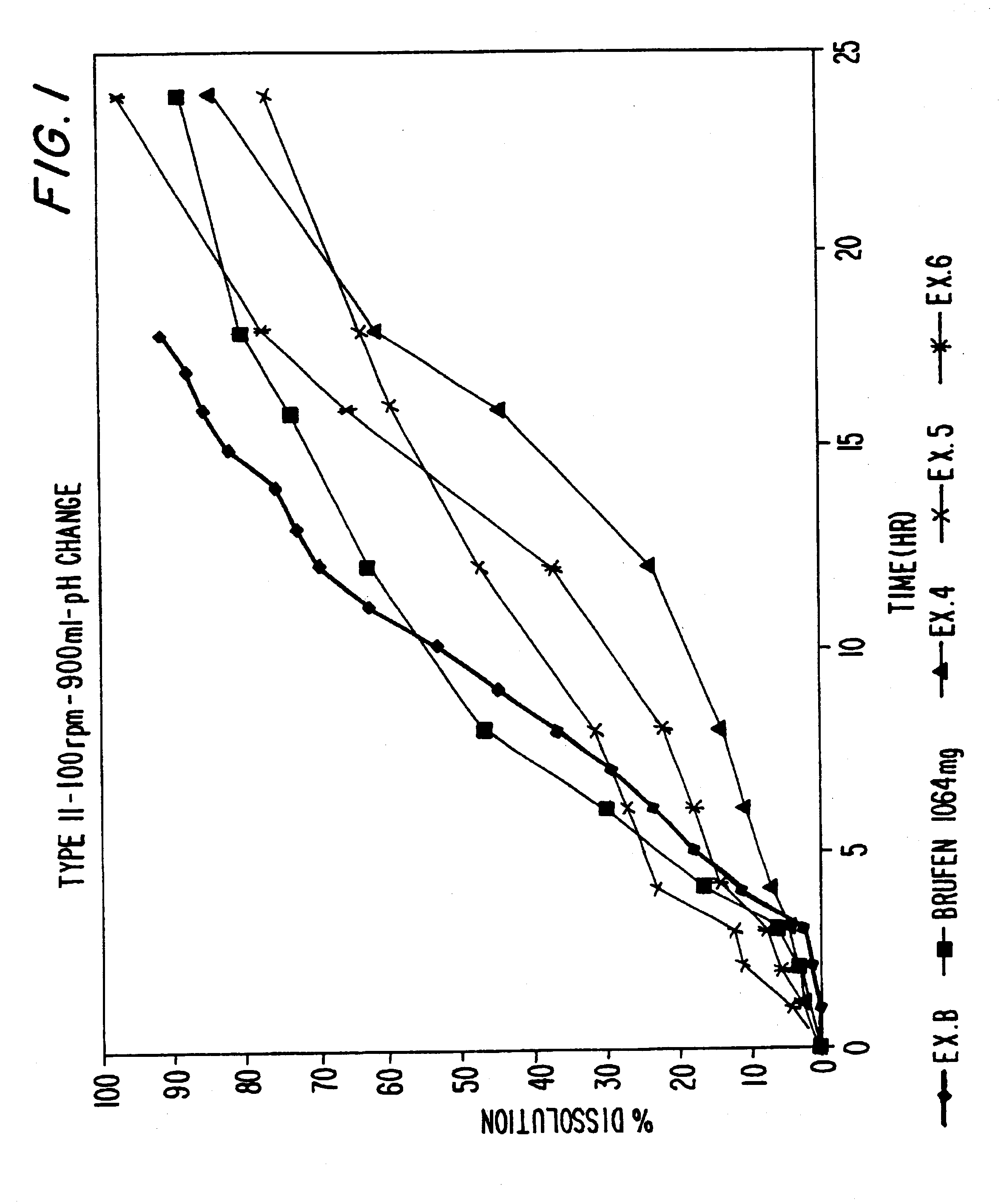
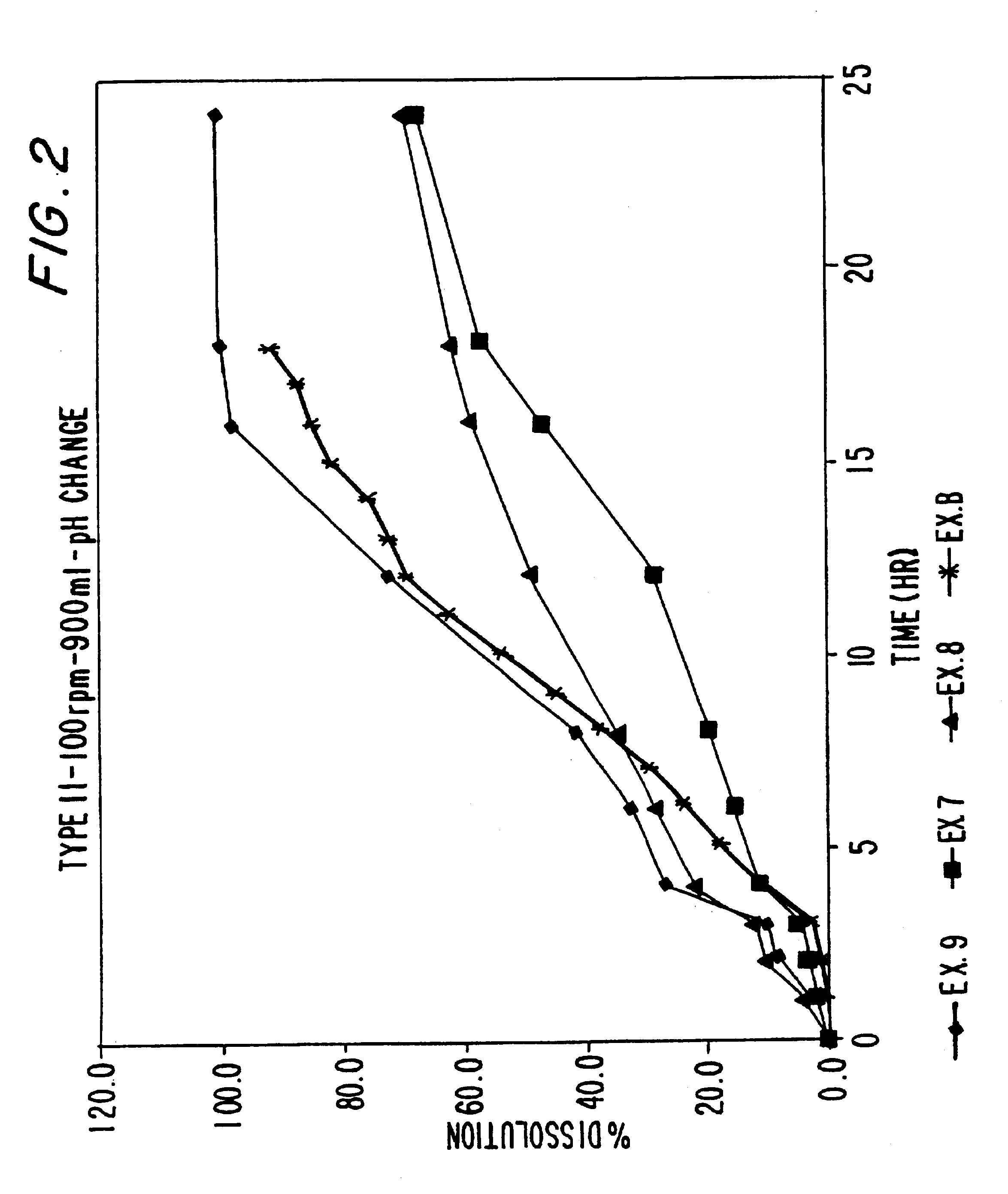
Sustained release dosage forms of high dose insoluble drugs such as ibuprofen and methods for their manufacture are disclosed.
Owner:PENWEST PHARMA CO
Zero-order sustained release dosage forms and method of making same
InactiveUS20030133982A1High drug loadingReduce releasePowder deliveryBiocideSustained release drugHydrophobic polymer
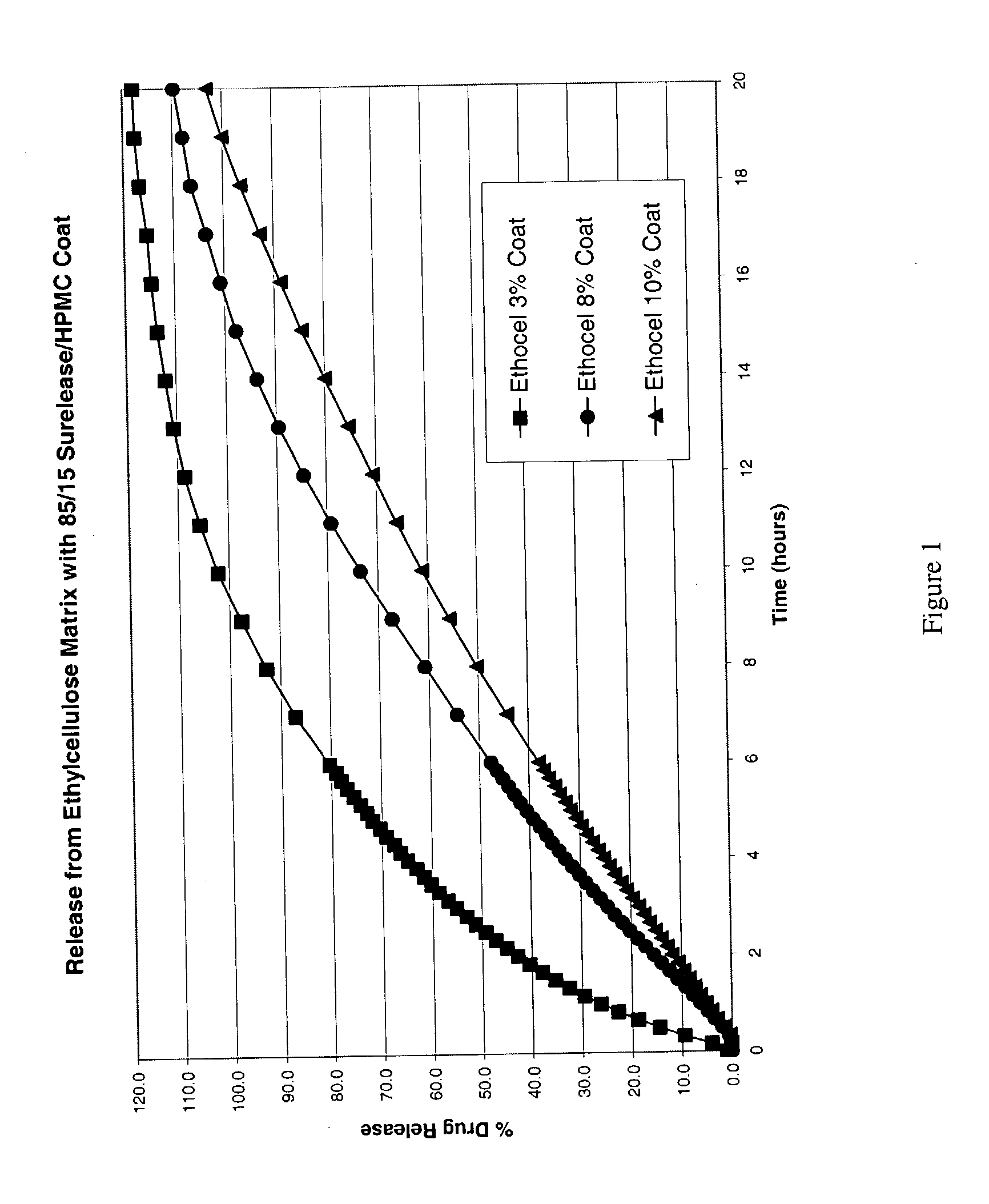
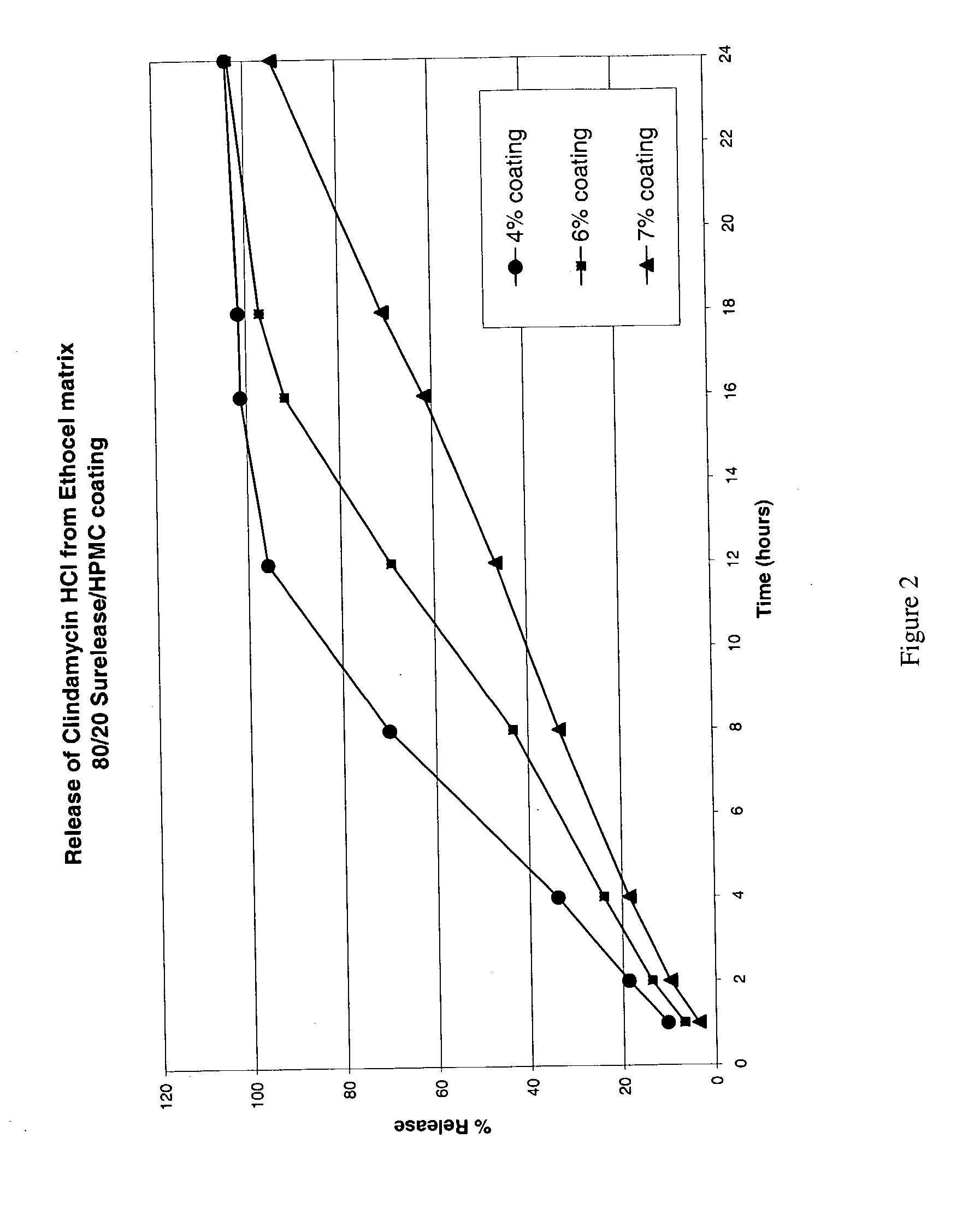
The present invention relates to zero-order sustained release solid dosage forms suitable for administration of a wide range of therapeutically active medicaments, especially those that are water-soluble, and to a process of making same. The solid dosage form comprises (a) a matrix core comprising ethylcellulose and the active agent and (b) a hydrophobic polymer coating encasing the entire matrix core.
Owner:PHARMACIA CORP
Sustained Release Dosage Forms For Delivery of Agents to an Oral Cavity of a User
Aspects of the invention include a sustained release dosage form that can be administered to an oral cavity, e.g., the mouth. In certain embodiments, the sustained release dosage form is formulated as a lozenge or gum that may be administered to an oral cavity of a user for the purpose of dissolving over a prolonged period of time and thereby delivering an essential oil component therein. In certain embodiments, the sustained release dosage form includes a beneficial agent and, therefore, not only provides for the prolonged delivery of an essential oil component to an oral cavity, but also provides for the sustained release of a beneficial agent thereto. In certain embodiments, the sustained release dosage form includes a biocompatible, water-insoluble polymer, e.g., ethylcellulose and an essential oil component, which are combined in such a manner so as to produce a dosage form that substantially dissolves over a prolonged period of time when positioned within an aqueous environment, such as an oral cavity of a user. In certain embodiments, the sustained release dosage form may include an additional water soluble agent, such as gum arabic, which may be included so as to further provide the dosage form with a desired dissolution characteristic. In certain embodiments, the dosage form may also include a beneficial agent to be delivered to the mouth. Methods of formulating such dosage forms and administering them to an oral cavity for the treatment of an adverse condition are also provided herein.
Owner:BENNES
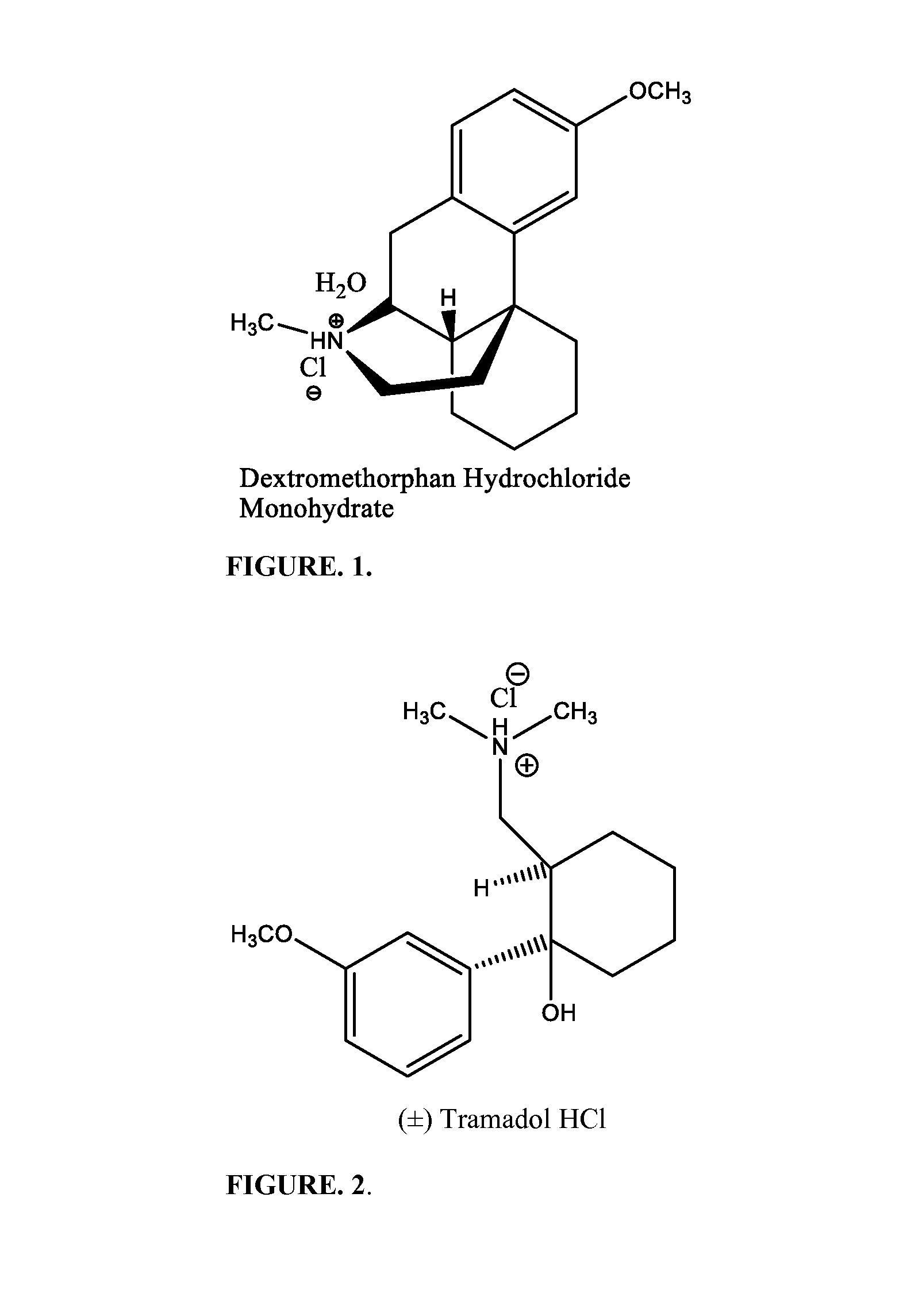
Novel pharmaceutical compositions for treating chronic pain and pain associated with neuropathy
InactiveUS20080058362A1Reduced plasma concentrationEffective pain managementBiocideAmide active ingredientsDextrorphanSustained release drug
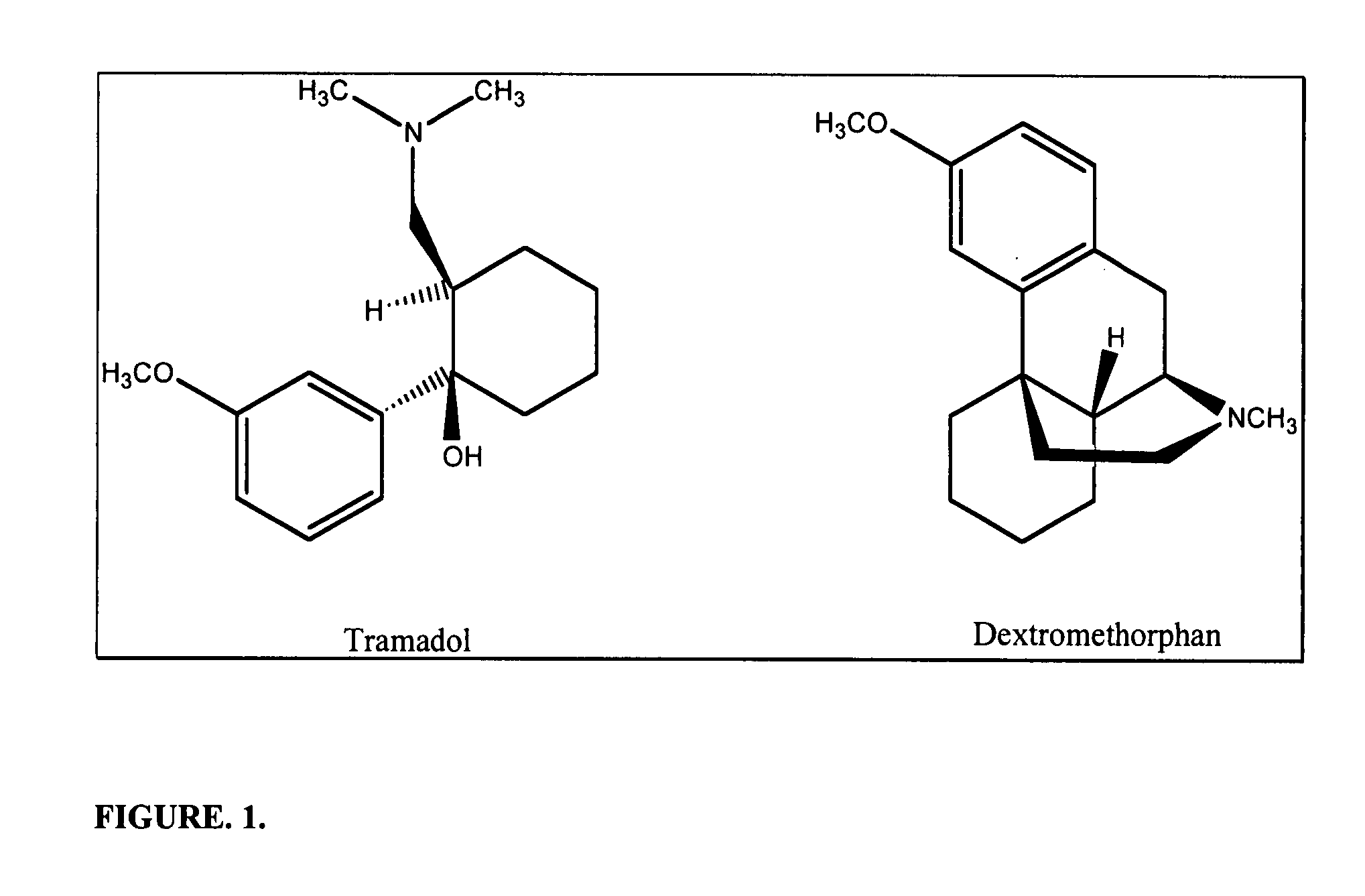
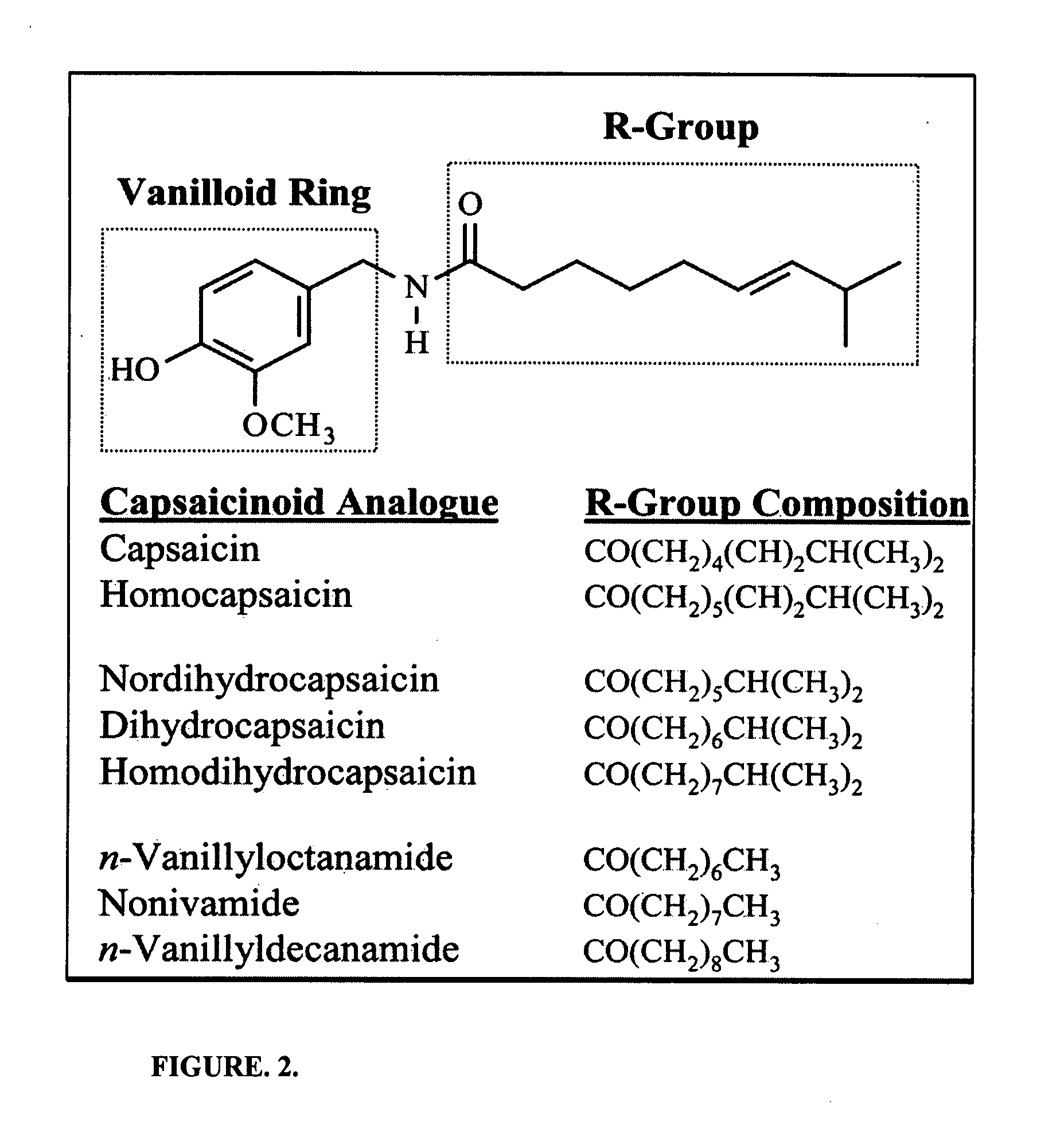
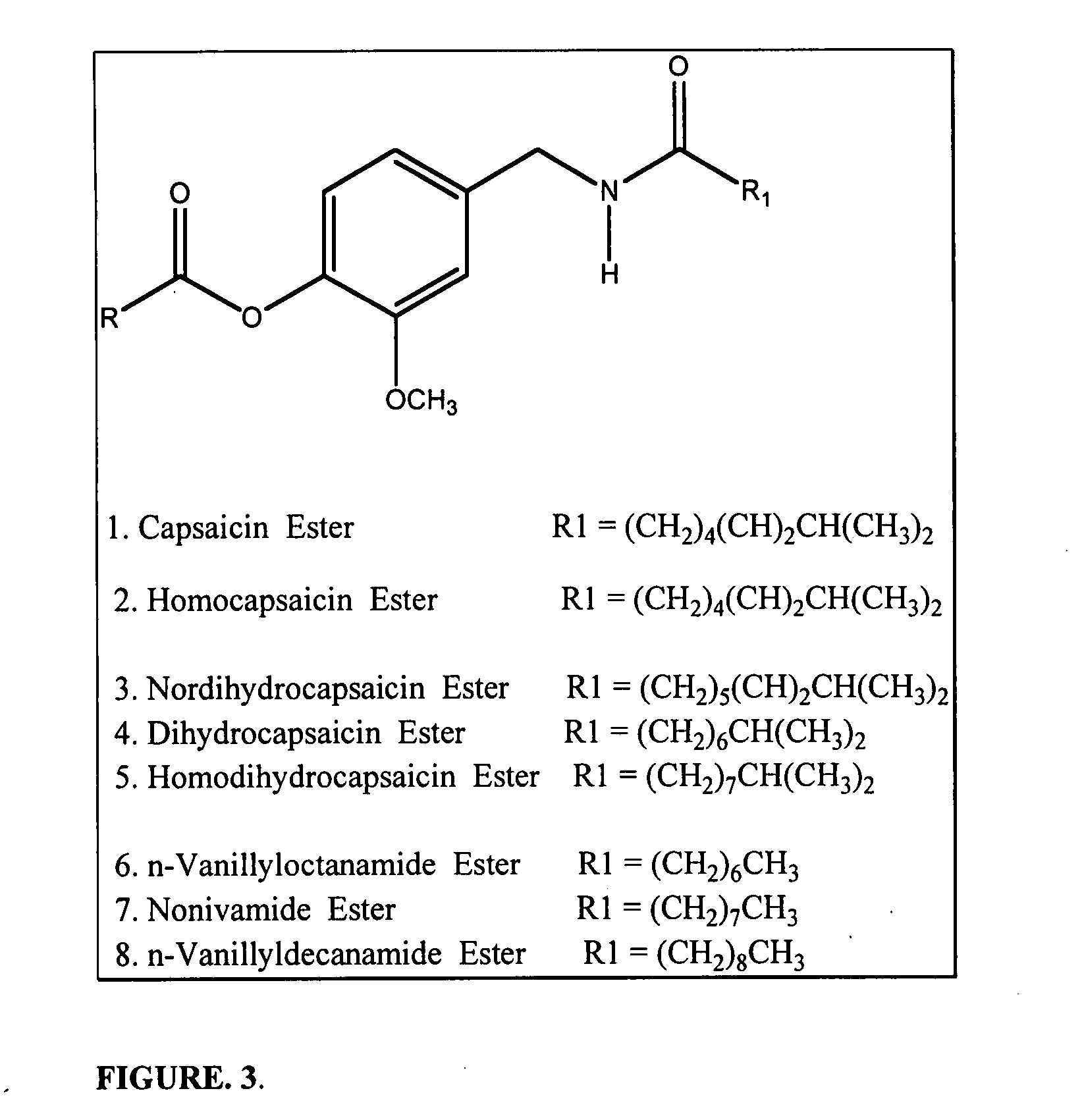
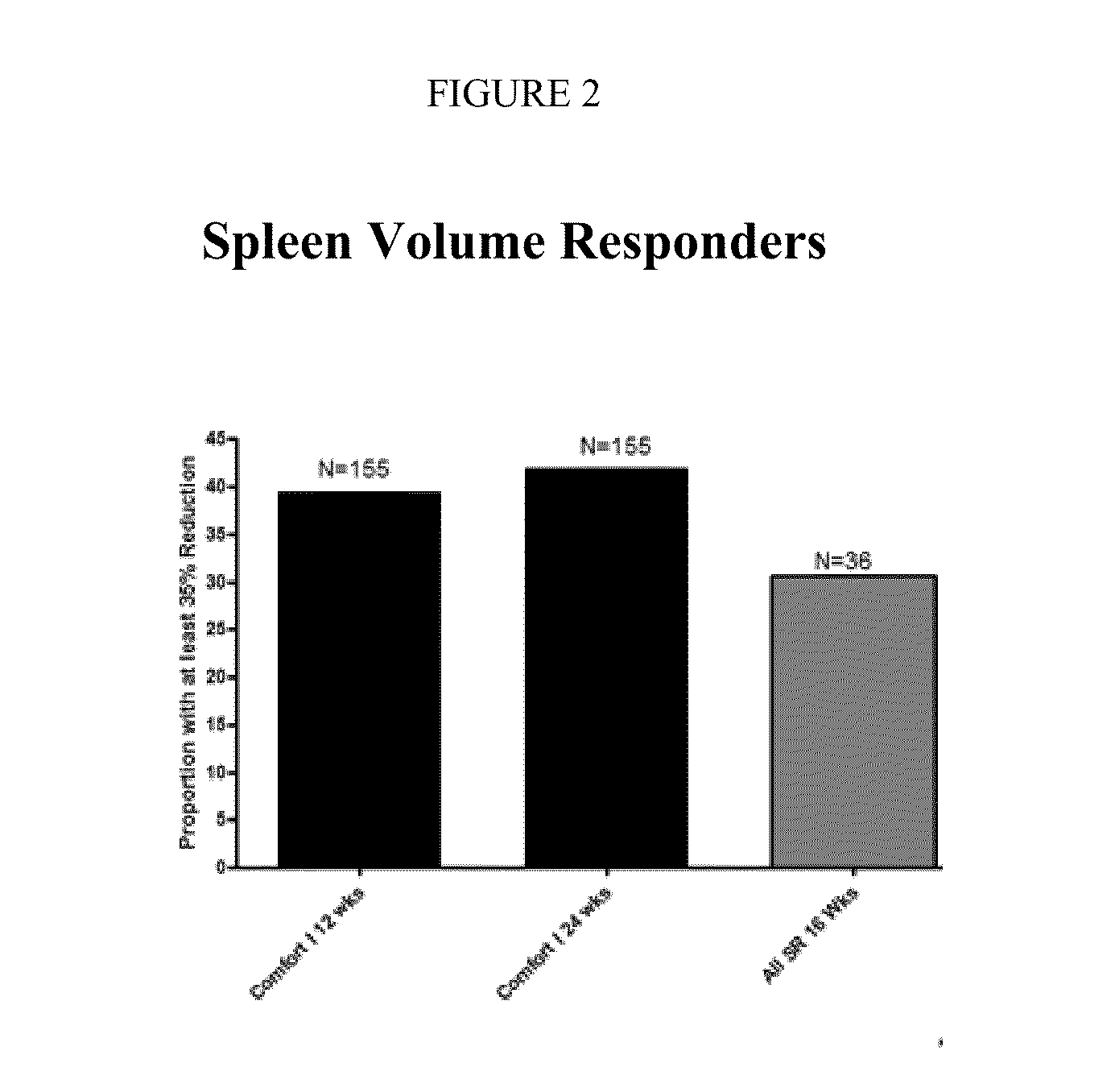
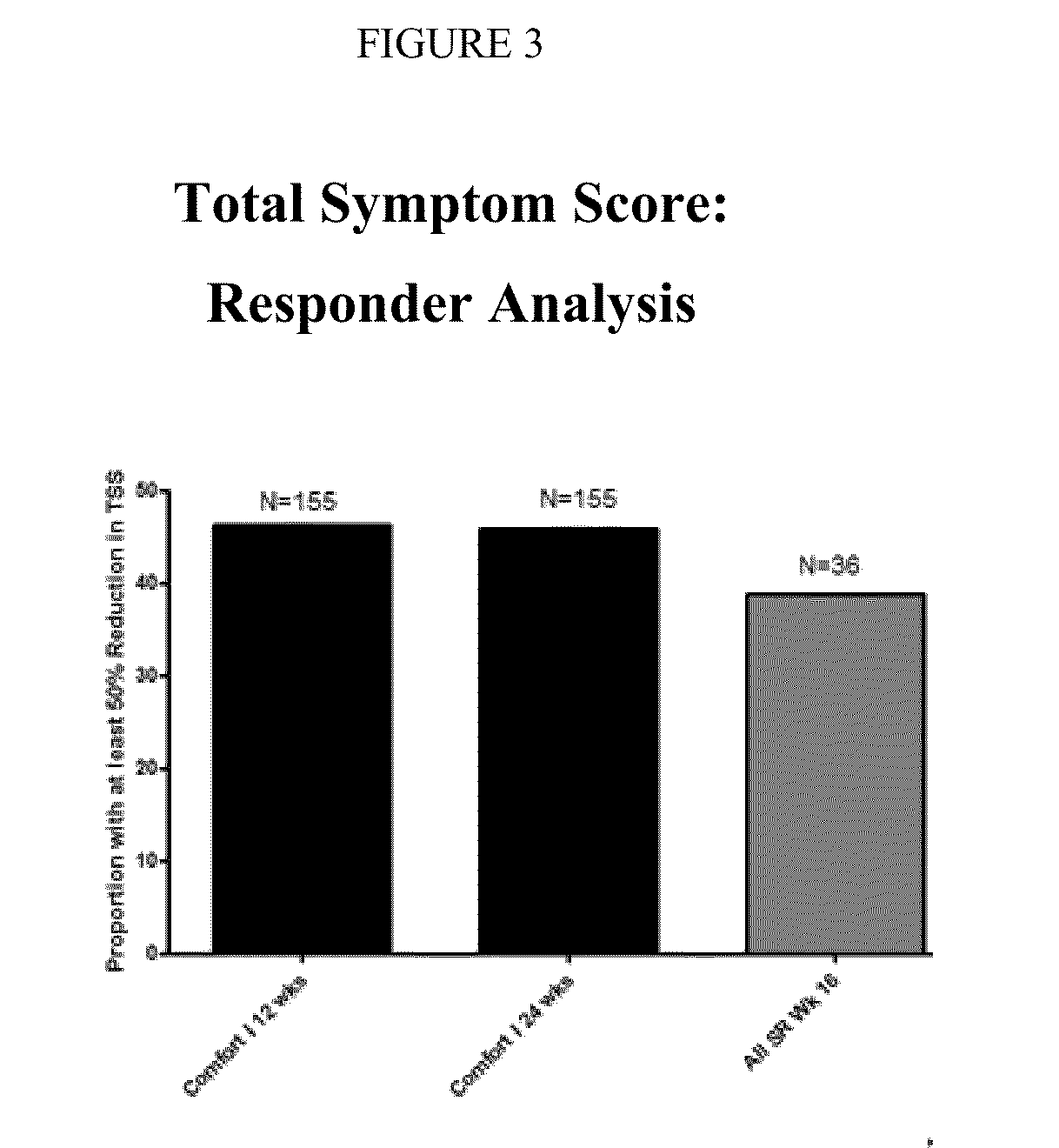
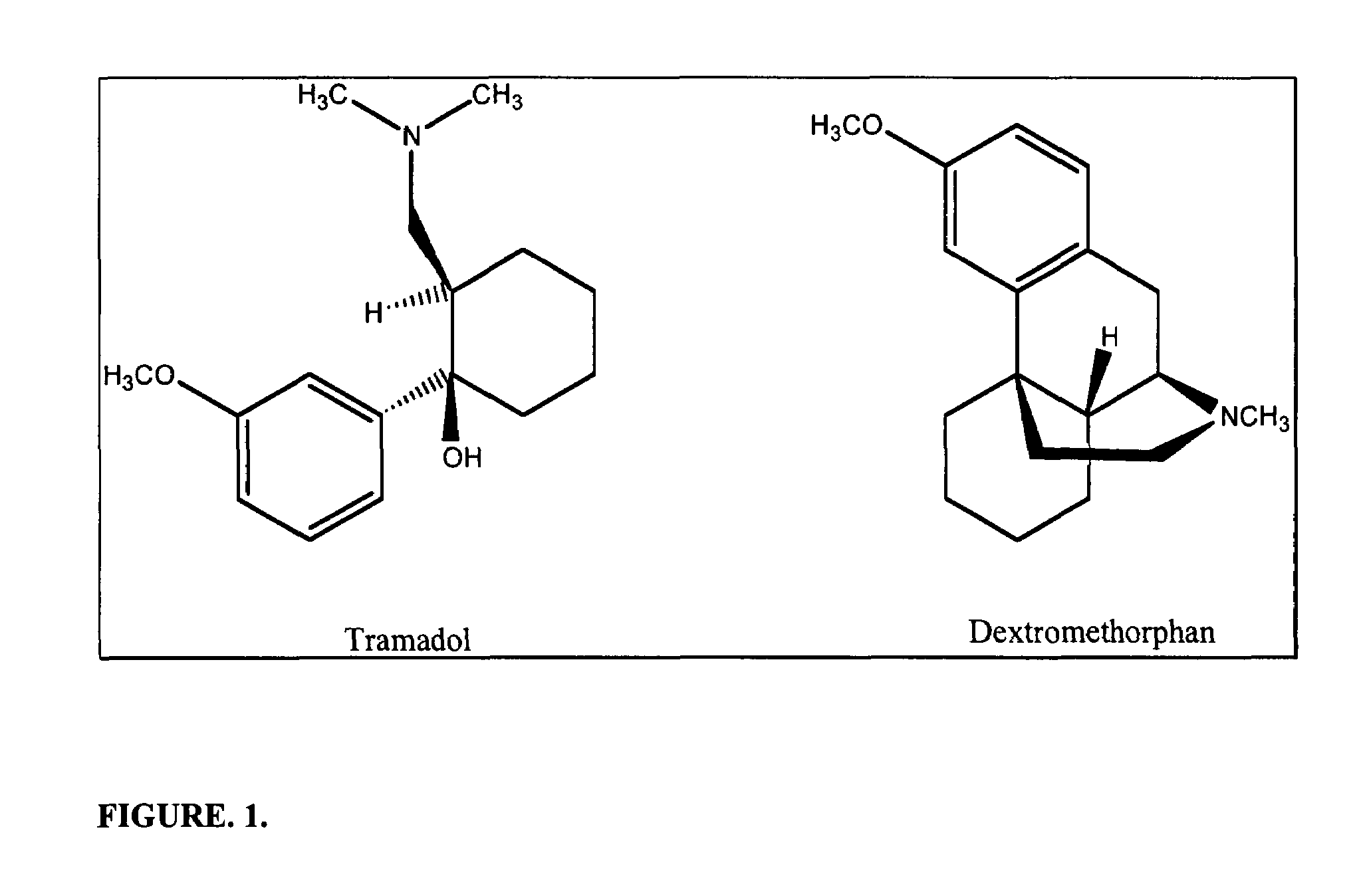
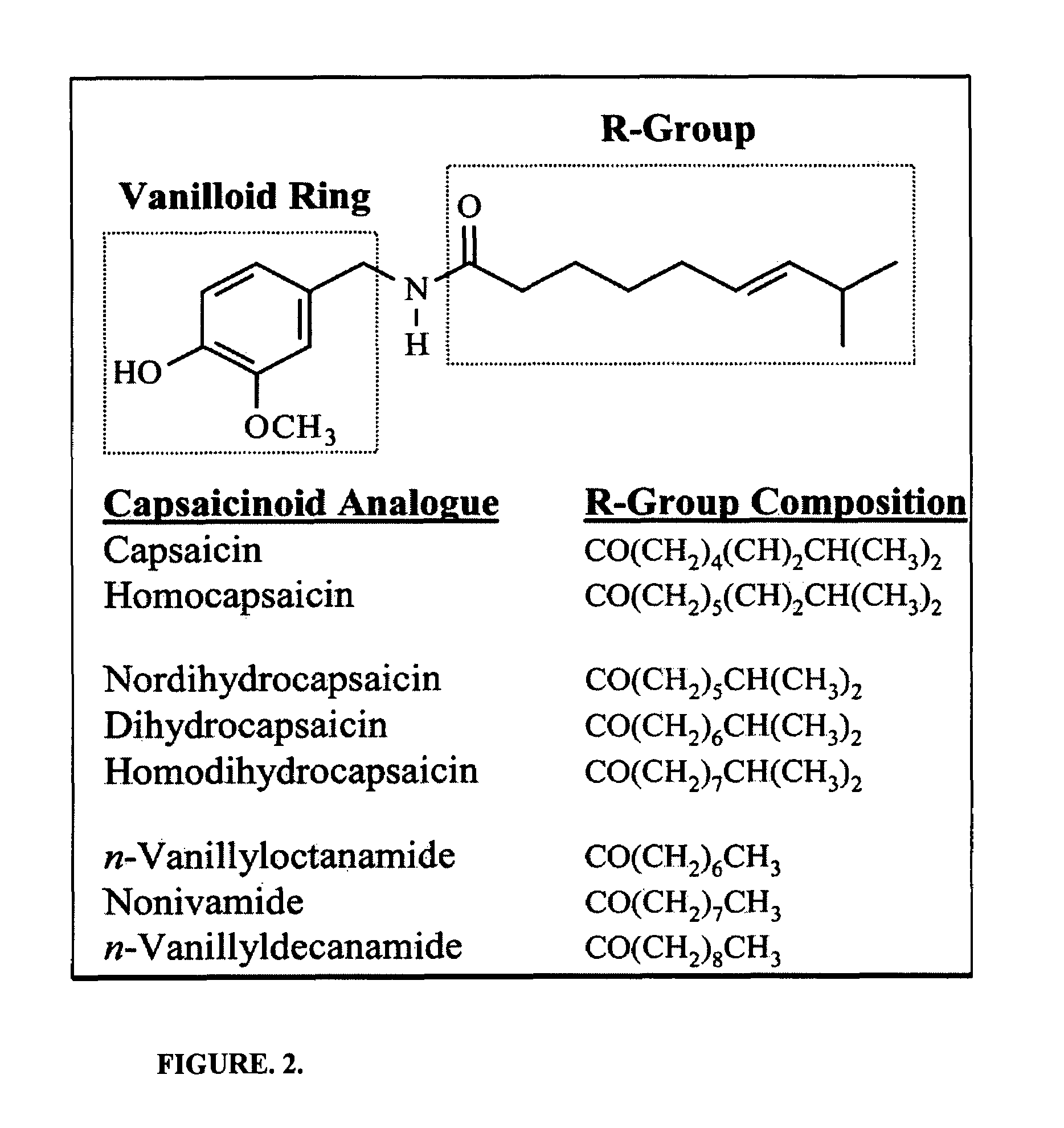
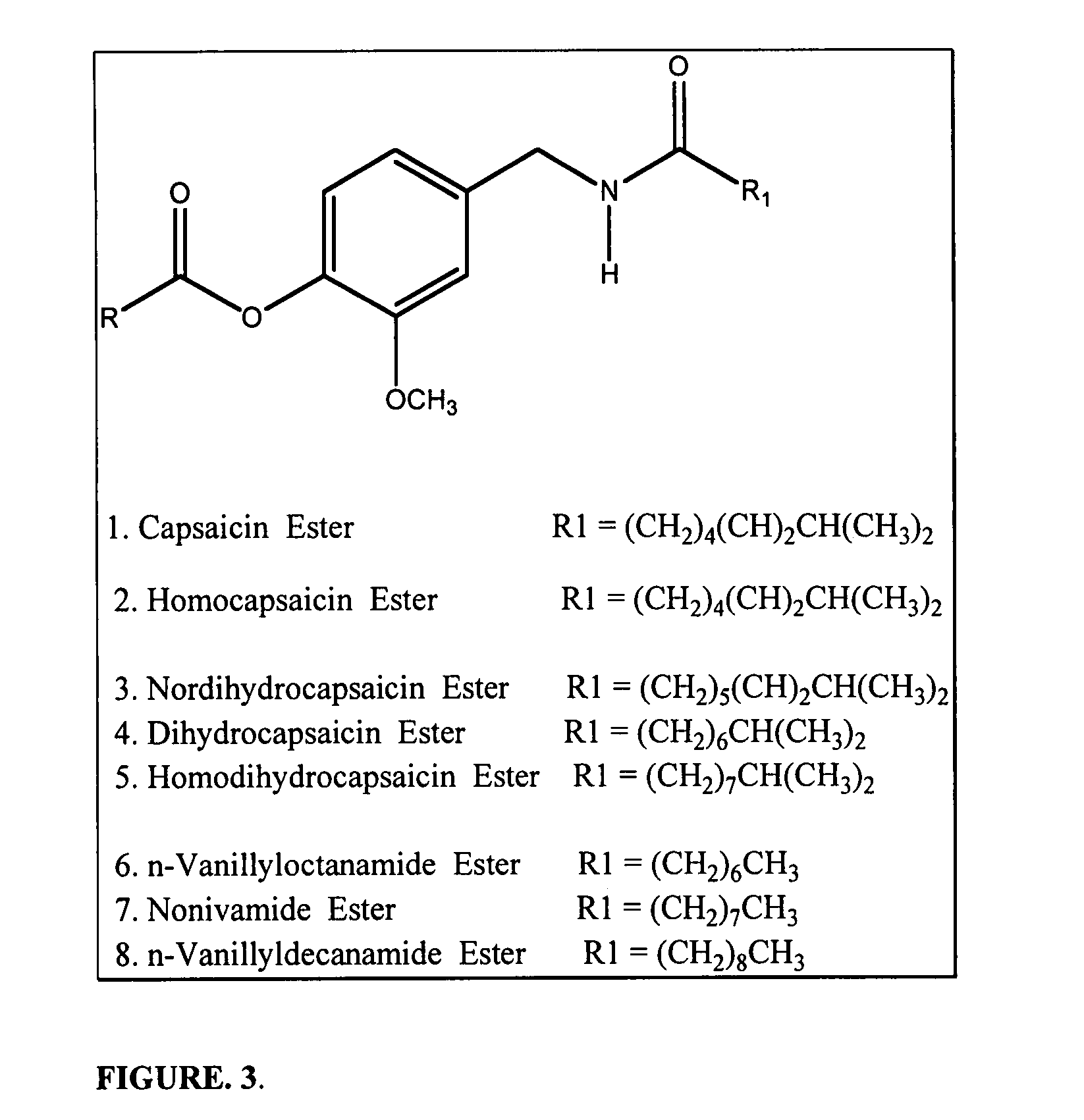
Chronic pain is alleviated in a mammal suffering there from by administering to the mammal a chronic pain alleviating amount of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, in combination with a μ-opiate analgesic such as tramadol or an analogously acting molecular entity, and a capsaicin or an ester of capsaicin, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
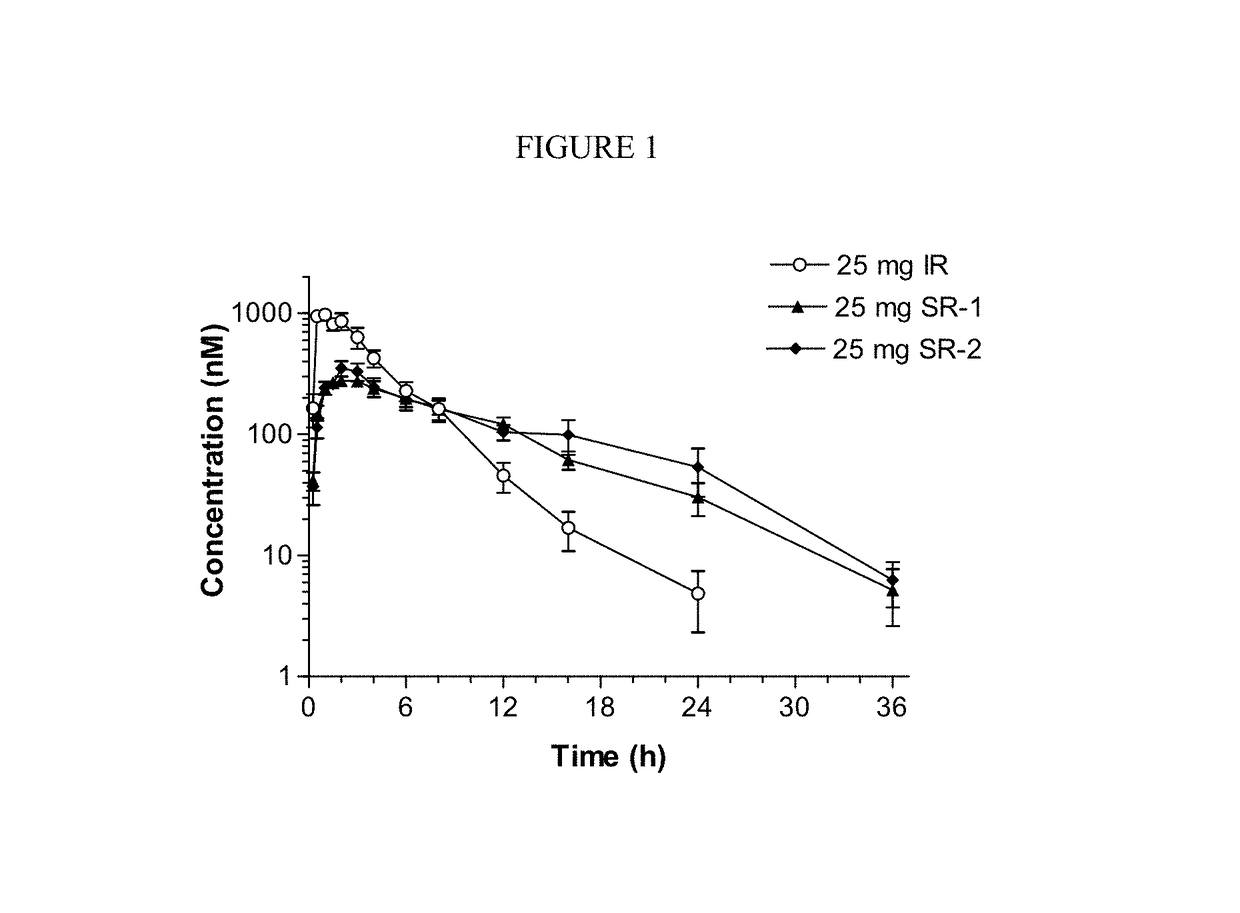
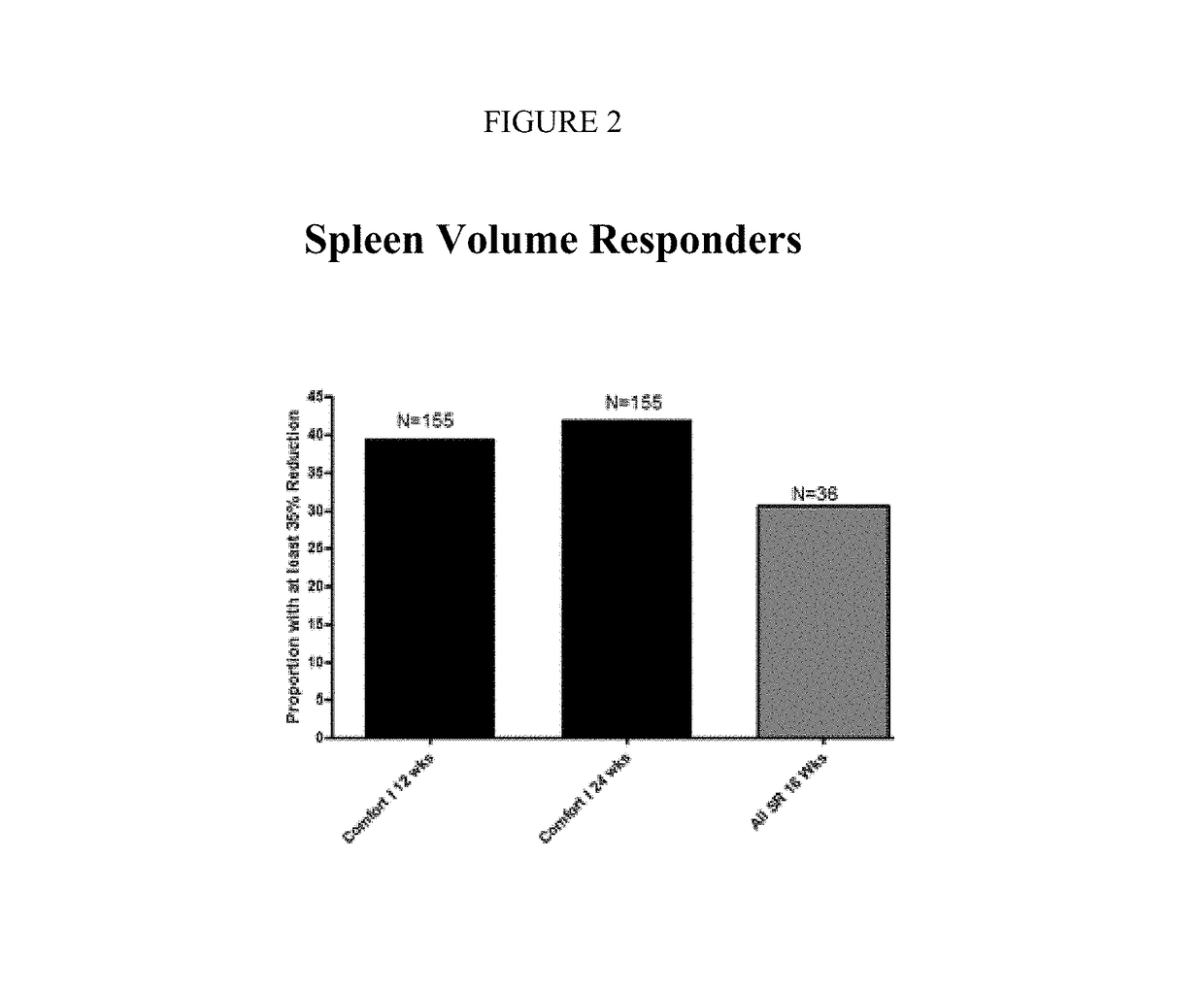
Sustained-release dosage forms of ruxolitinib
The present invention relates to sustained-release formulations and dosage forms of ruxolitinib, or a pharmaceutically acceptable salt thereof, which are useful in the treatment of Janus kinase-associated diseases such as myeloproliferative disorders.
Owner:INCYTE HLDG & INCYTE
Pharmaceutical compositions for treating chronic pain and pain associated with neuropathy
InactiveUS7645767B2Reduce concentrationEfficient managementBiocideAmide active ingredientsOpiatePharmaceutical medicine
Chronic pain is alleviated in a mammal suffering there from by administering to the mammal a chronic pain alleviating amount of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, in combination with a μ-opiate analgesic such as tramadol or an analogously acting molecular entity, and a capsaicin or an ester of capsaicin, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
Controlled release formulations exhibiting an ascending rate of release
InactiveUS20070259033A1Low profileReduce solubilityOrganic active ingredientsNervous disorderSustained release drugActive agent
A sustained release dosage form is comprising a pharmaceutically active agent and pharmaceutically acceptable salts thereof and adapted to release as an erodible solid over a prolonged period of time, wherein the dosage form provides an ascending rate of release of the pharmaceutically active agent for at least about 4 hours. The dosage form is able to deliver high doses of poorly soluble or slowly dissolving active agents. When additional pharmaceutically active agents are present, the agents are released from the dosage form at rates that are proportional to the respective weights of each active agent in the dosage form. Methods of using the dosage forms to treat disease or conditions in human patients are also disclosed.
Owner:ALZA CORP
Methods and dosage forms for controlled delivery of paliperidone and risperidone
Dosage forms and methods for providing a substantially ascending rate of release of paliperidone or risperidone are provided. The sustained release dosage forms provide therapeutically effective average steady-state plasma paliperidone or risperidone concentrations when administered once per day. This once-a-day dosing regimen results in only one peak plasma paliperidone or risperidone concentration occurrence in each 24 hour period. In addition, the peak plasma paliperidone or risperidone concentration occurs at a later time following dose administration and exhibits a lesser magnitude than the peak plasma paliperidone or risperidone concentration that occurs following administration of paliperidone or risperidone in an immediate-release dosage form.
Owner:ALZA CORP
Tofacitinib oral sustained release dosage forms
ActiveUS20140271842A1Safely ingestedMinimize biasBiocideNervous disorderSustained release drugTofacitinib
The present invention relates to oral sustained release formulations of tofacitinib and pharmaceutical acceptable salts thereof. The formulations described herein have desirable pharmacokinetic characteristics.
Owner:PFIZER INC
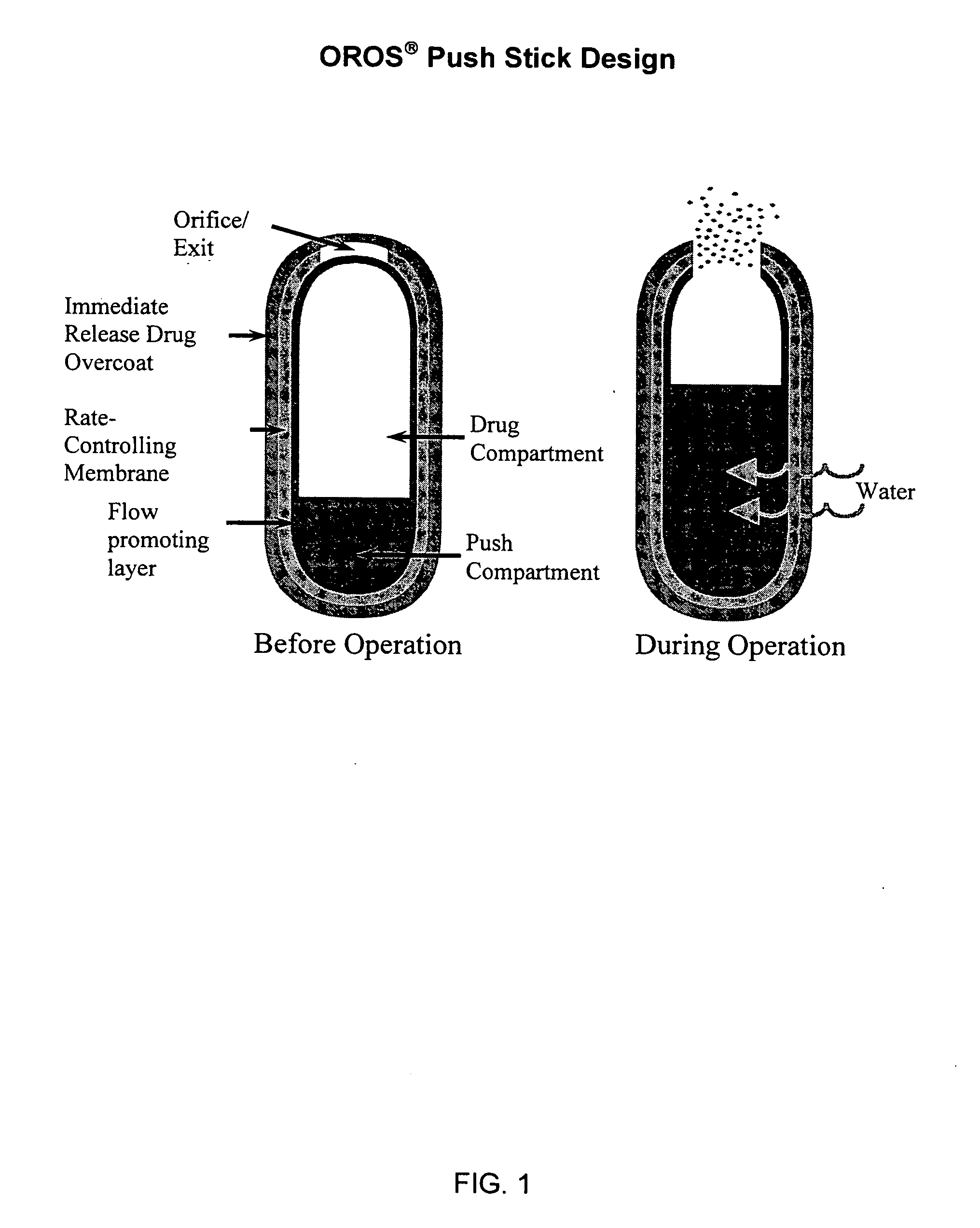
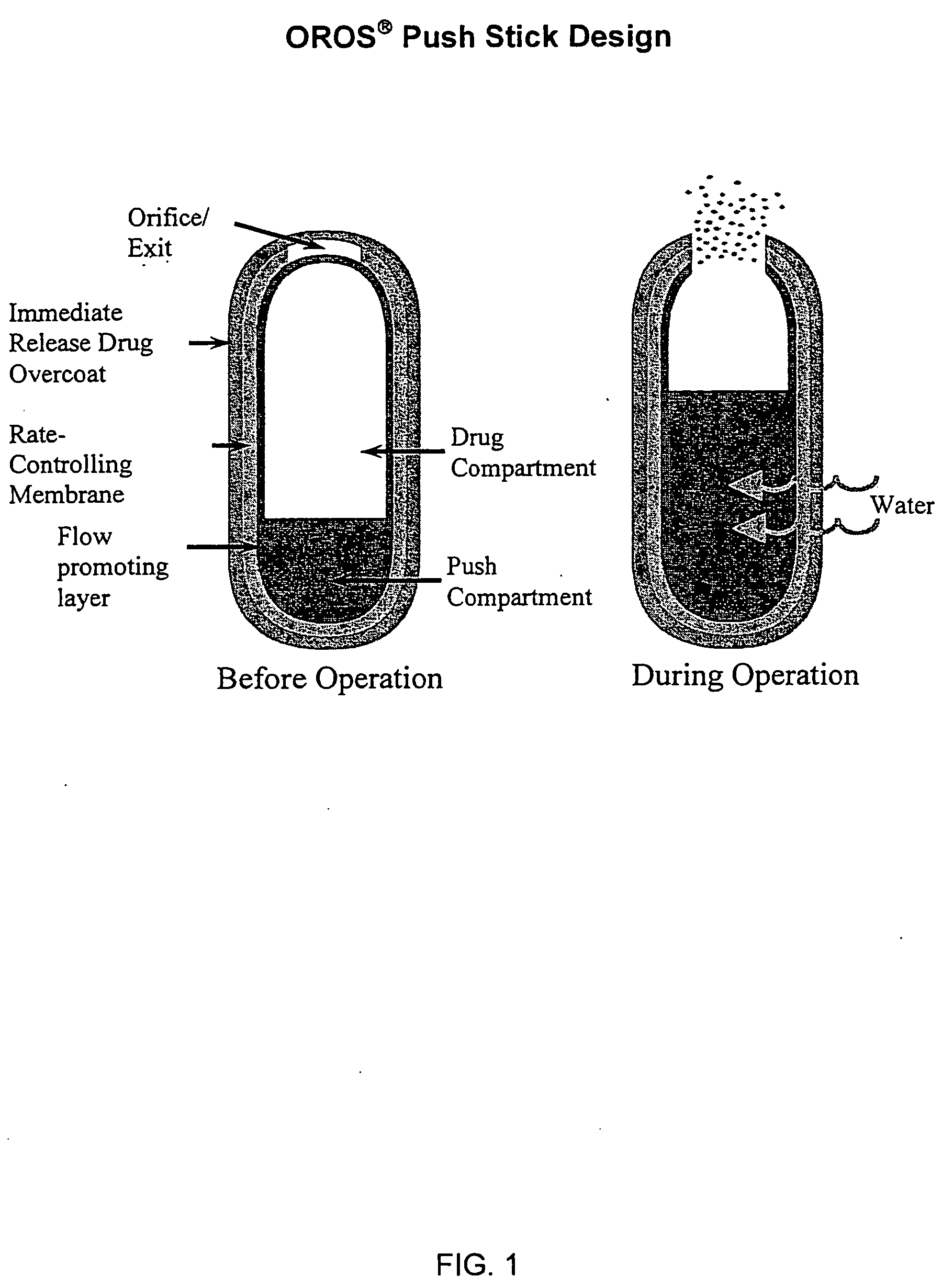
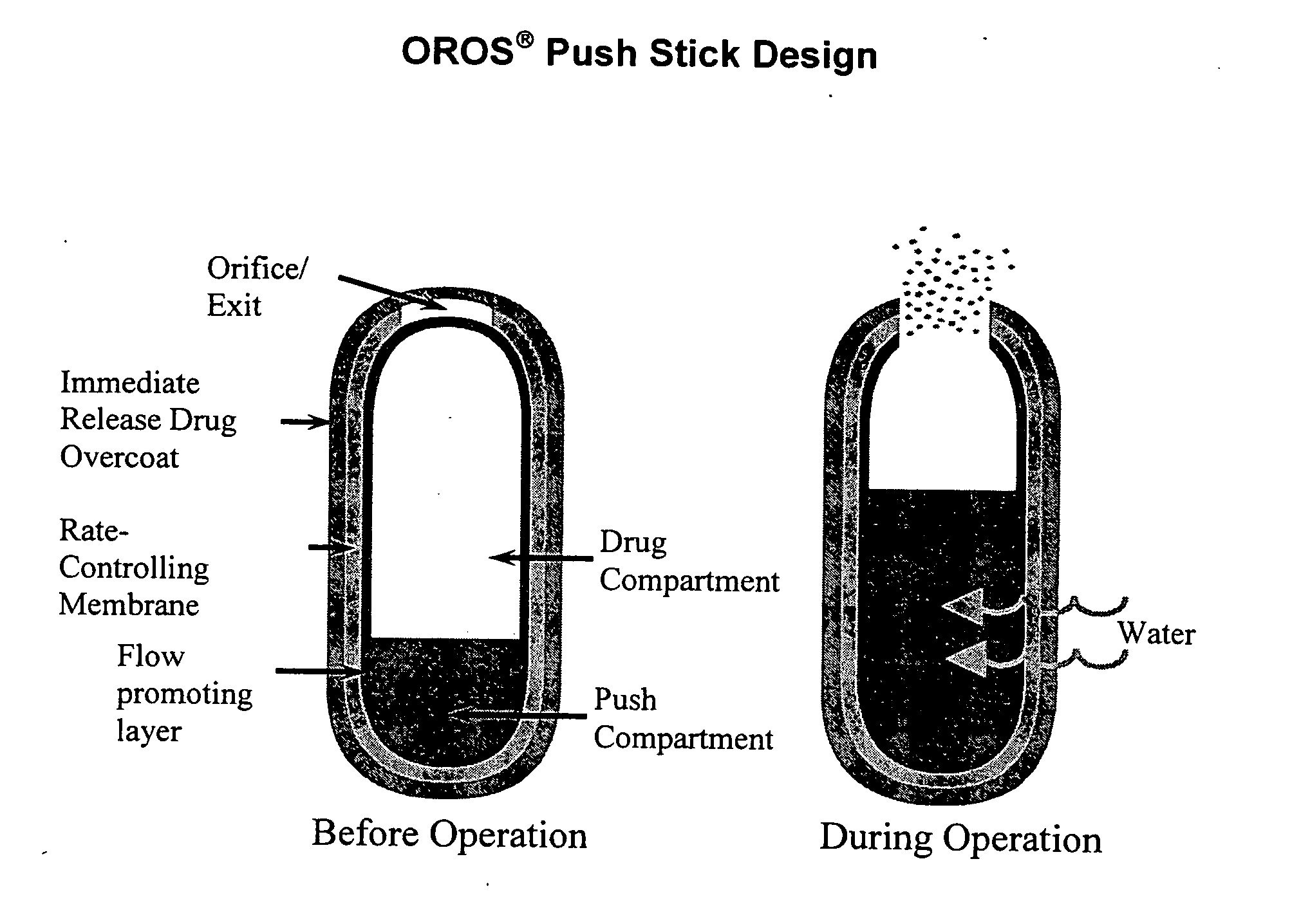
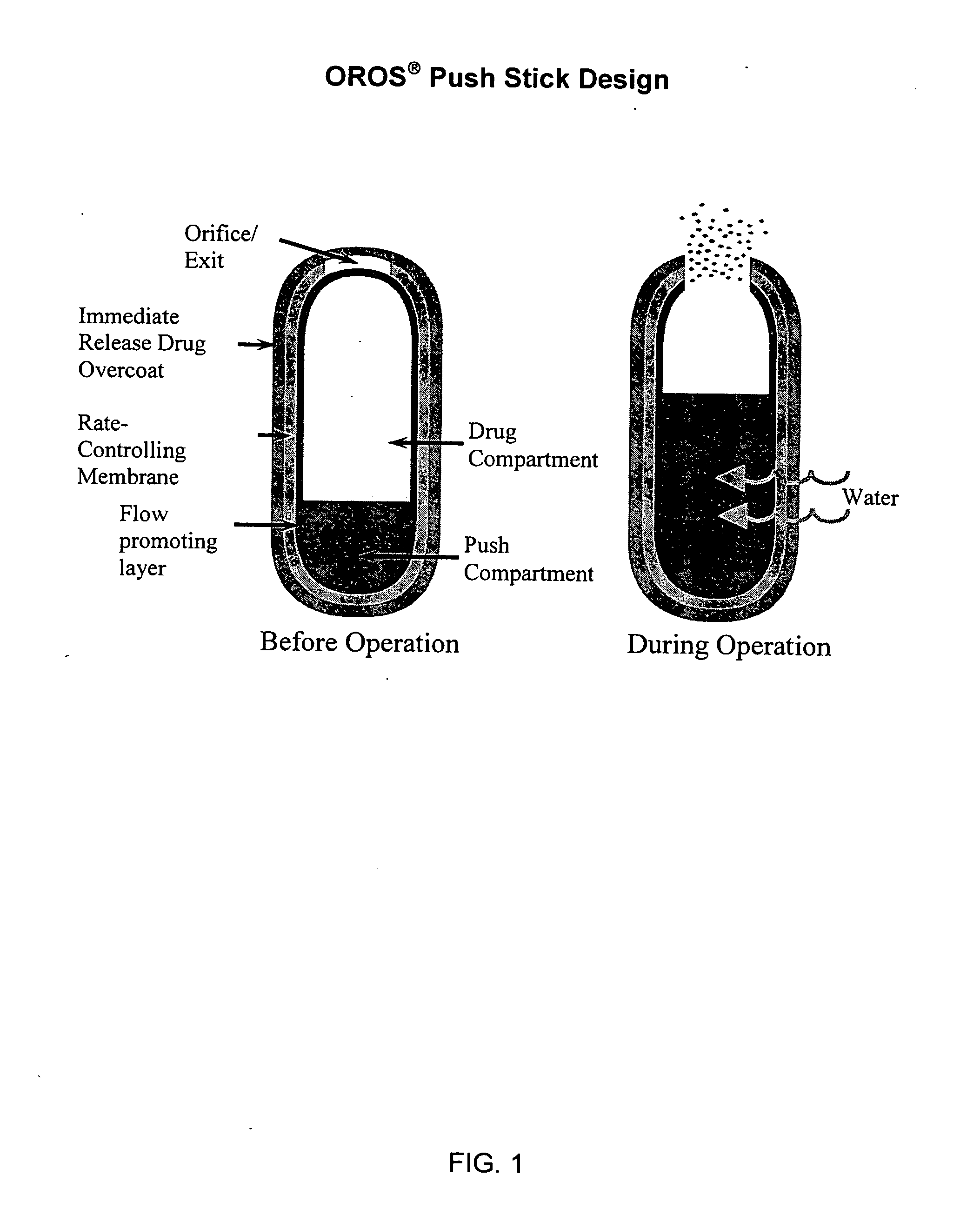
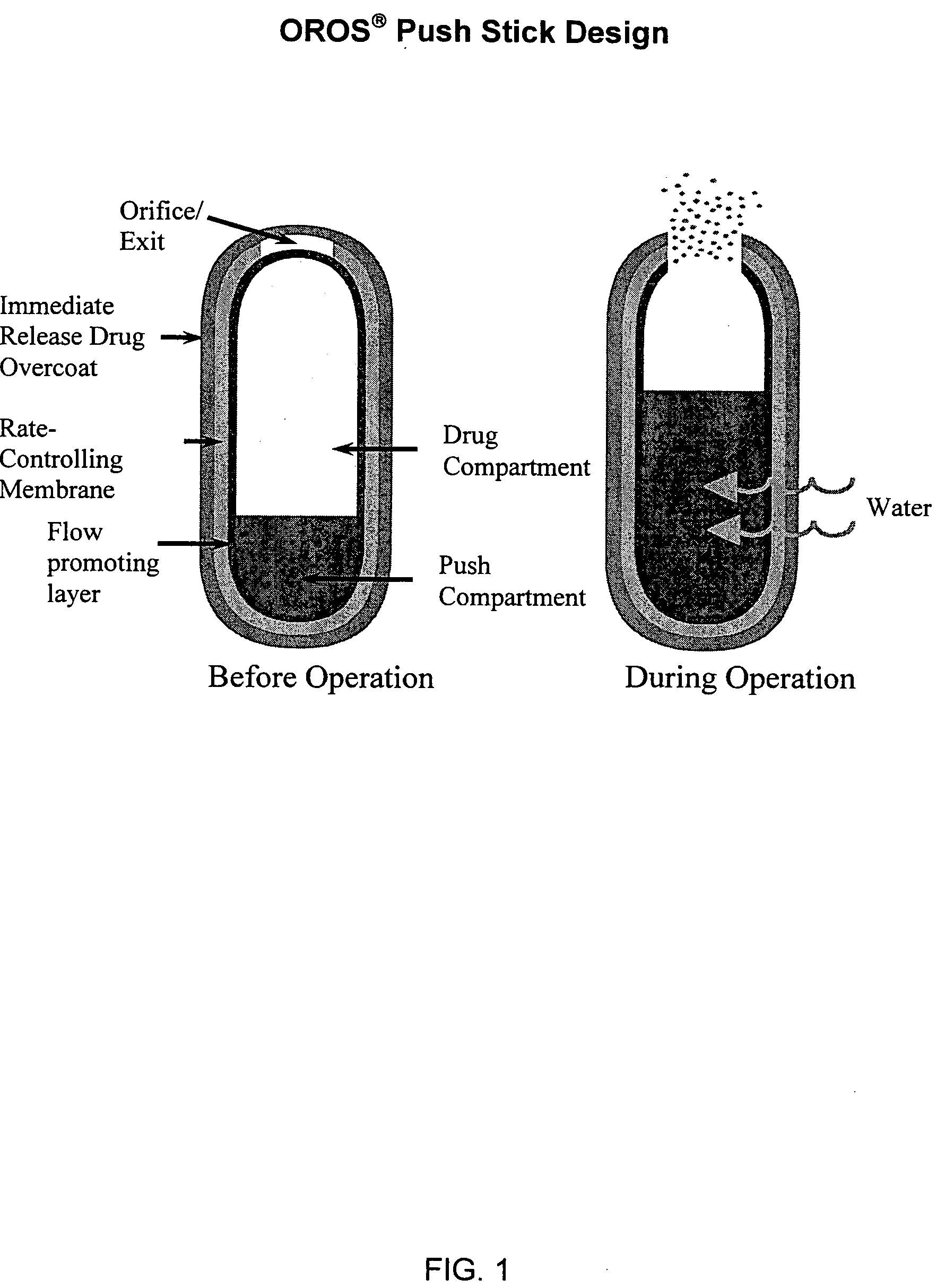
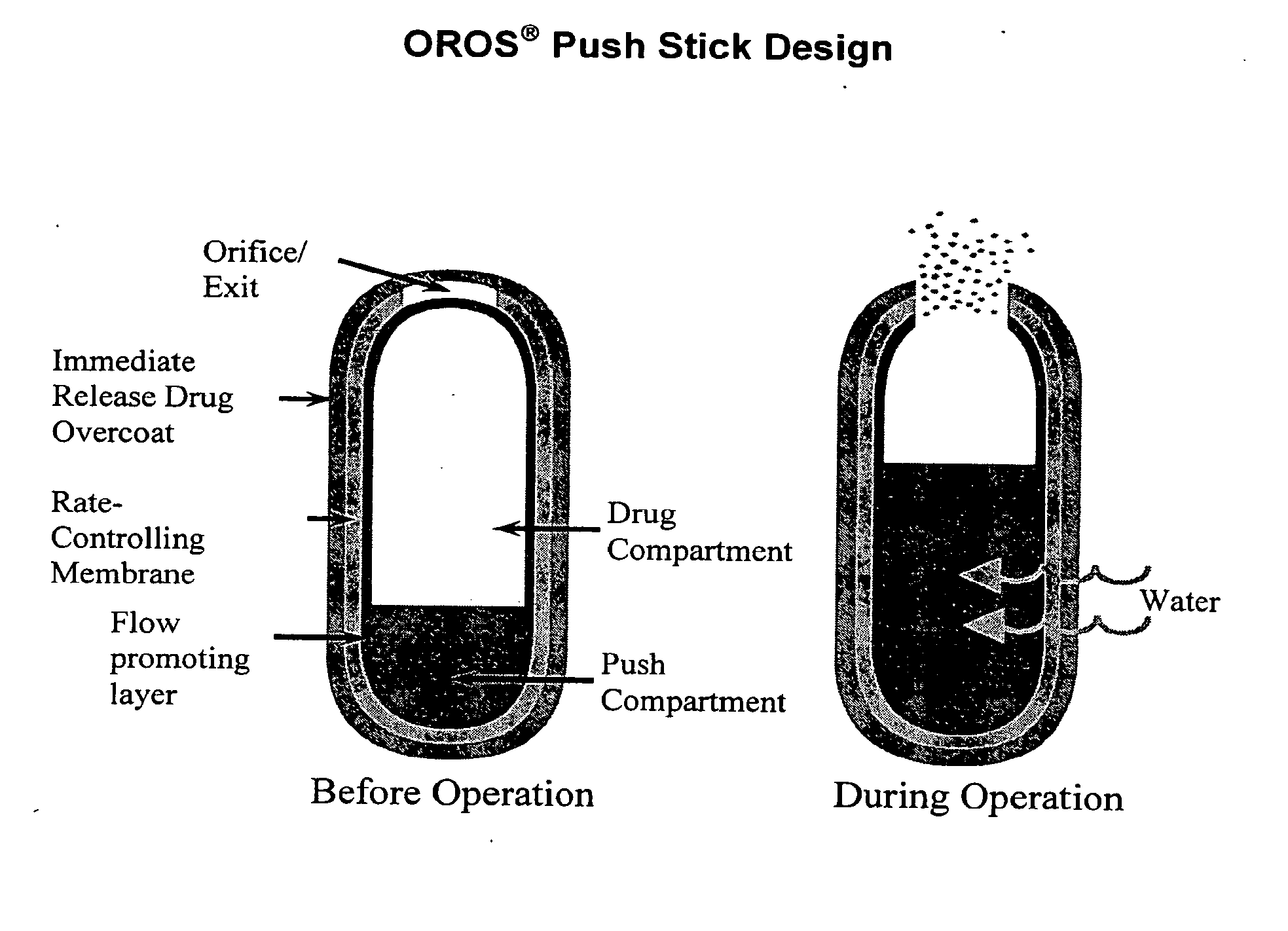
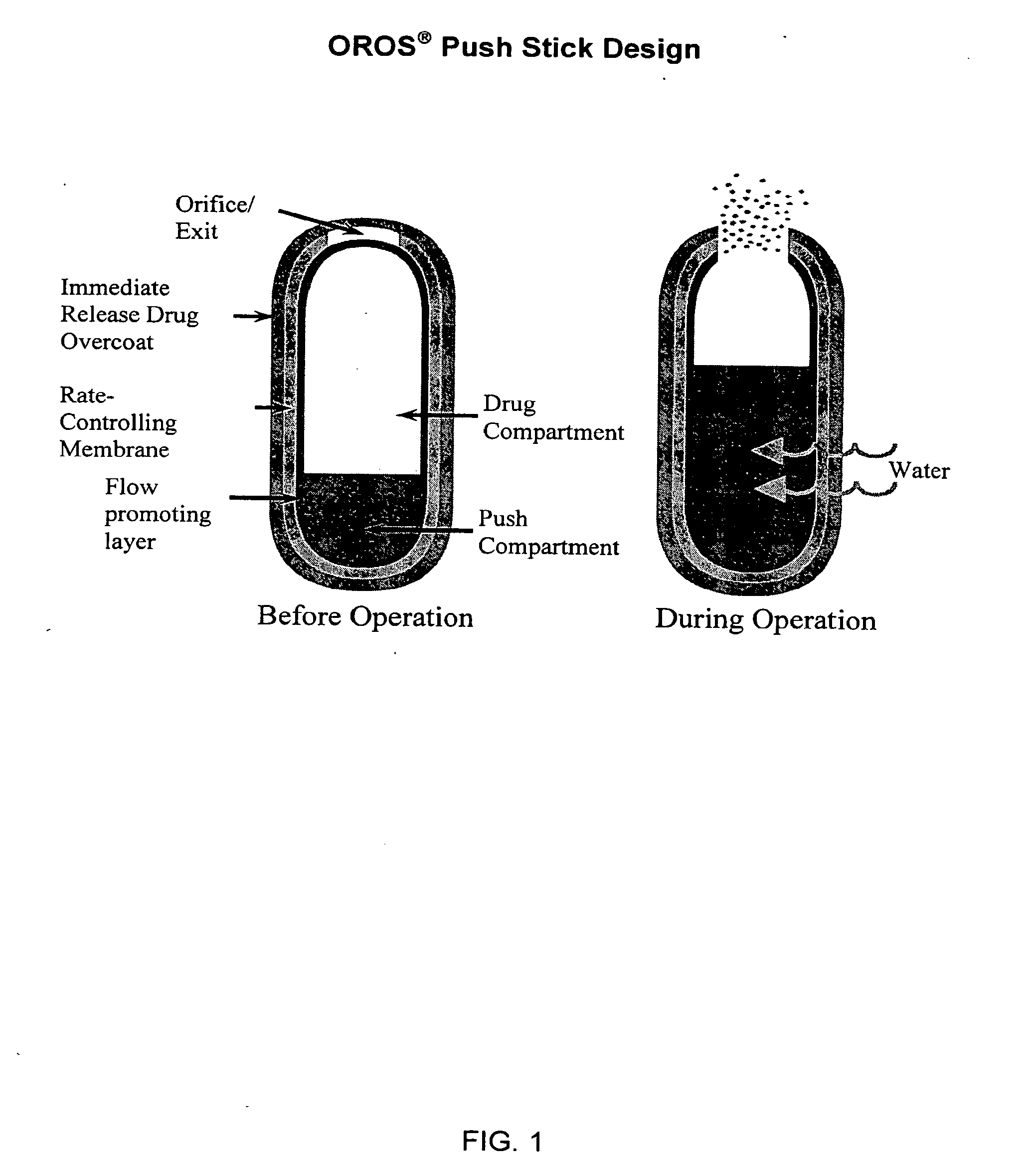
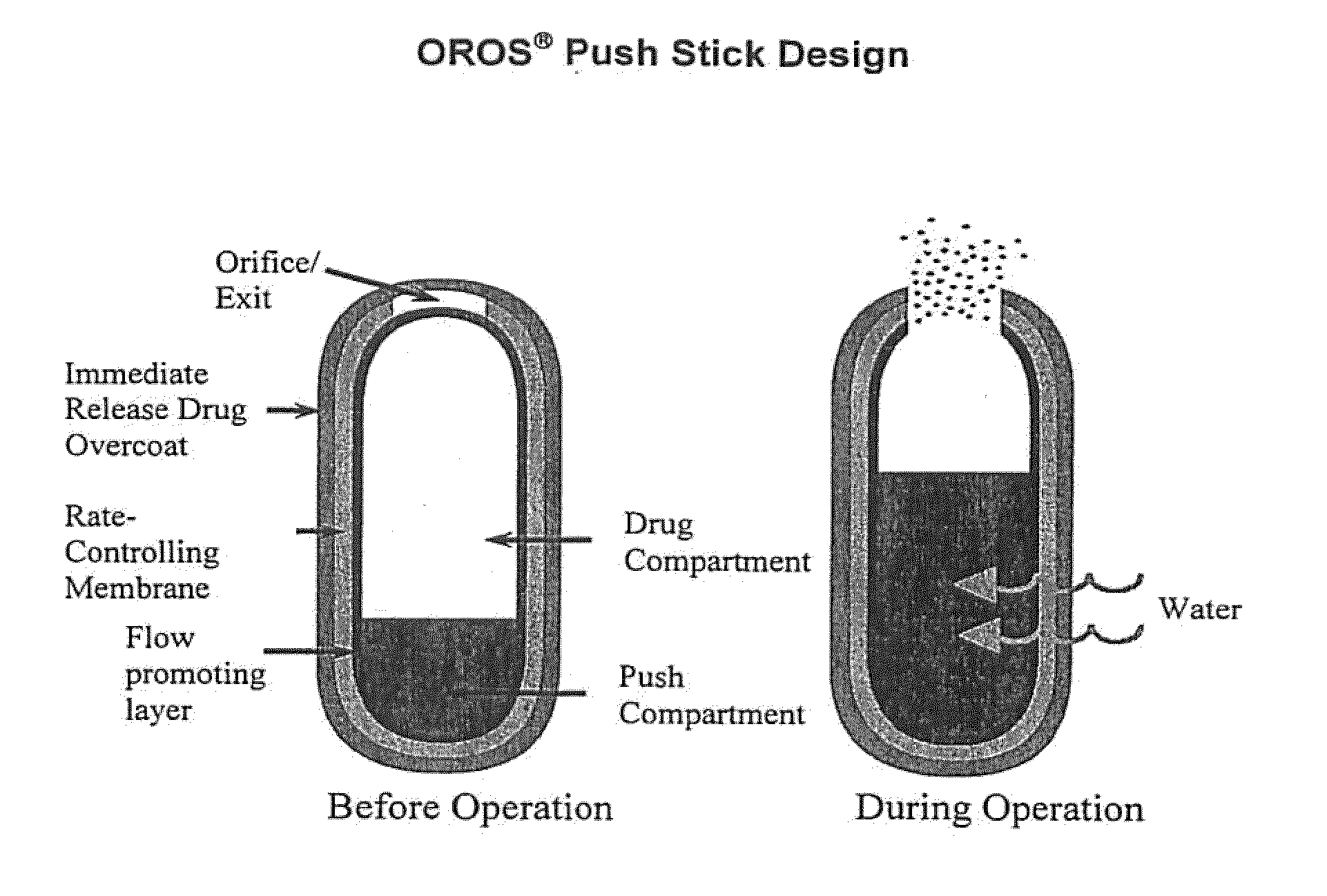
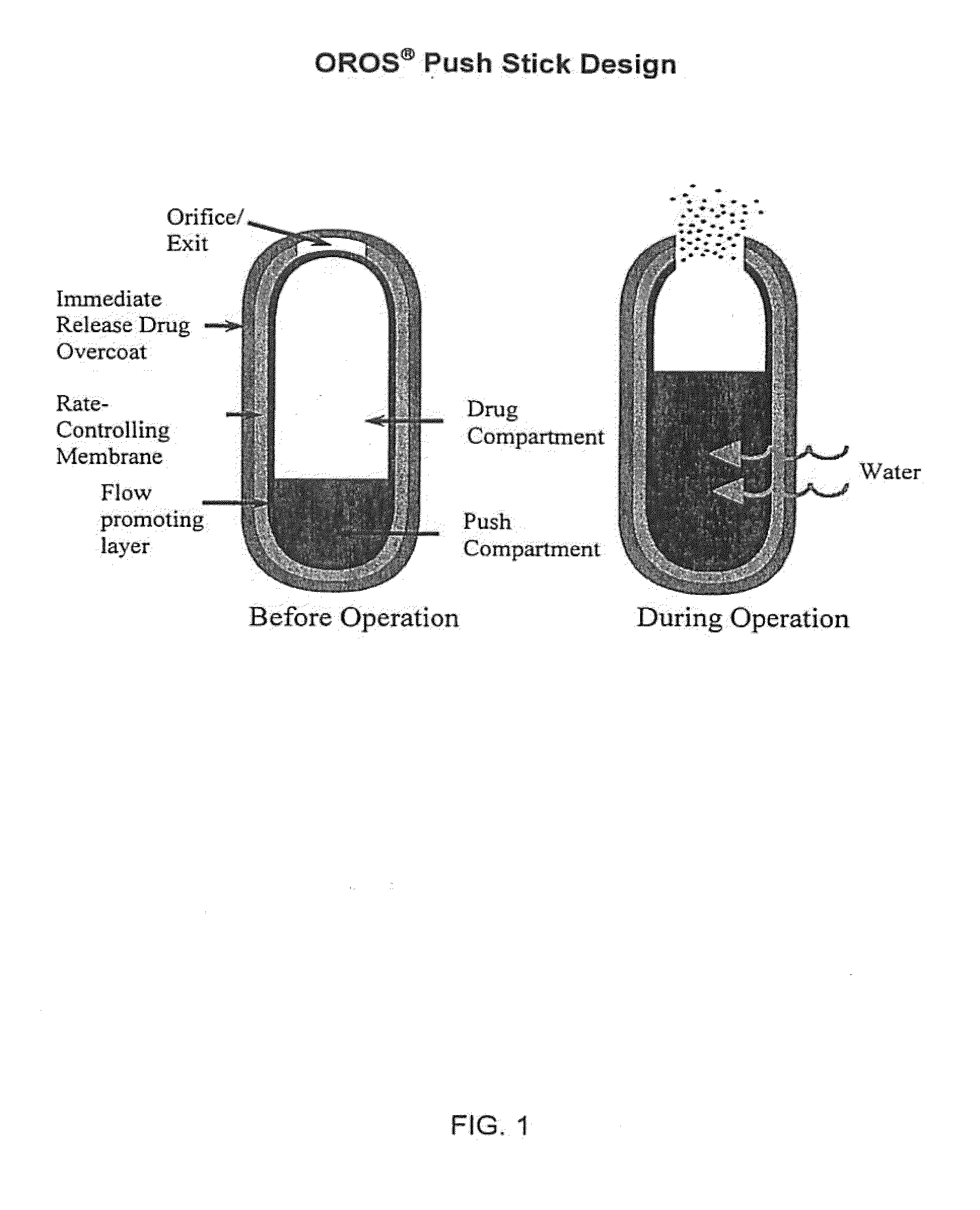
Oros push-stick for controlled delivery of active agents
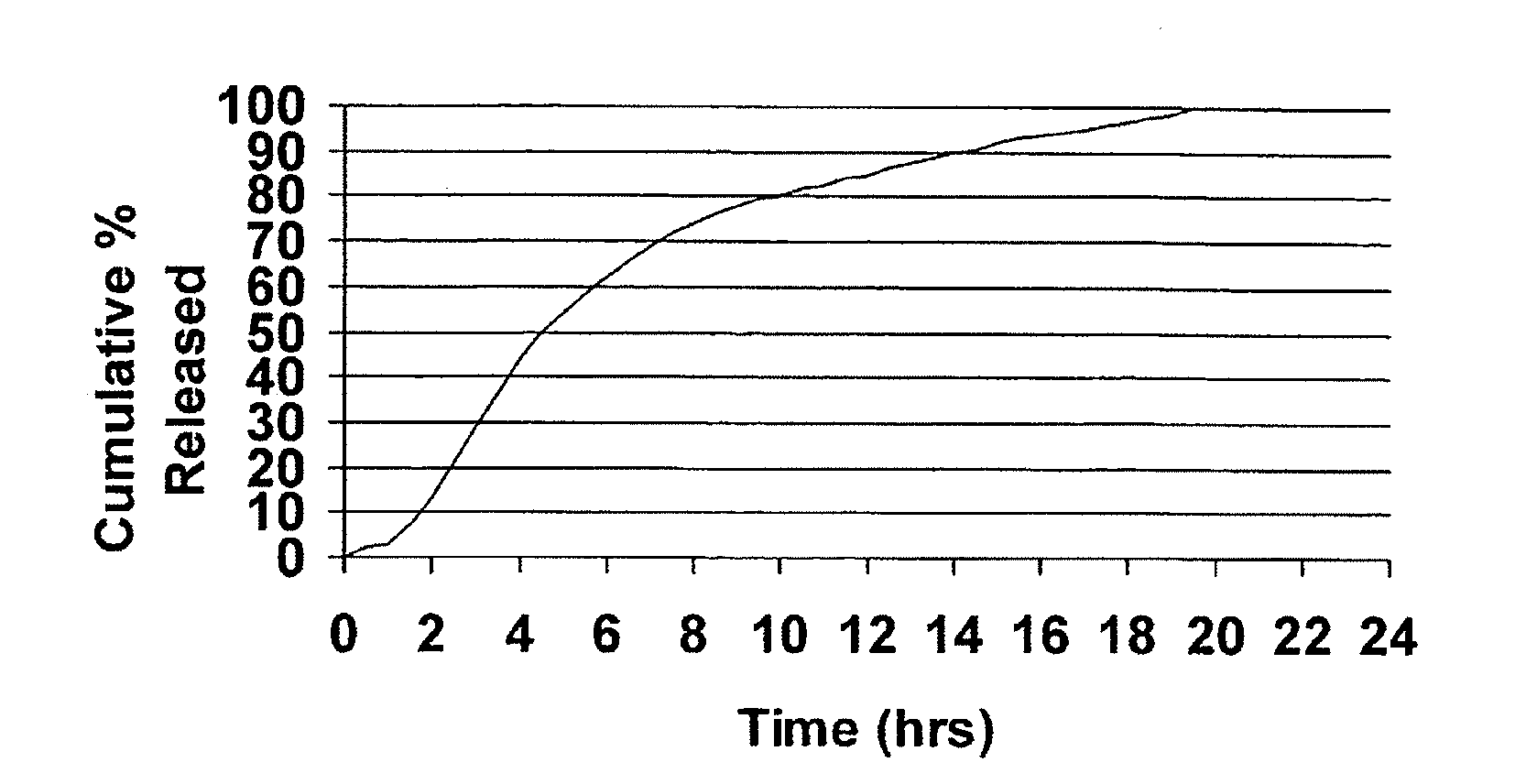
InactiveUS20050089570A1Faster initial rate of releaseFast releaseOrganic active ingredientsPill deliveryDiseaseSustained release drug
A sustained release dosage form is provided comprising a pharmaceutically active agent and pharmaceutically acceptable salts thereof and adapted to release as an erodible solid over a prolonged period of time, wherein the dosage form provides burst release of the pharmaceutically active agent without the use of an immediate release drug coating. The dosage form is able to deliver high doses of poorly soluble or slowly dissolving active agents at a controlled rate. Methods of using the dosage forms to treat disease or conditions in human patients are also disclosed.
Owner:ALZA CORP
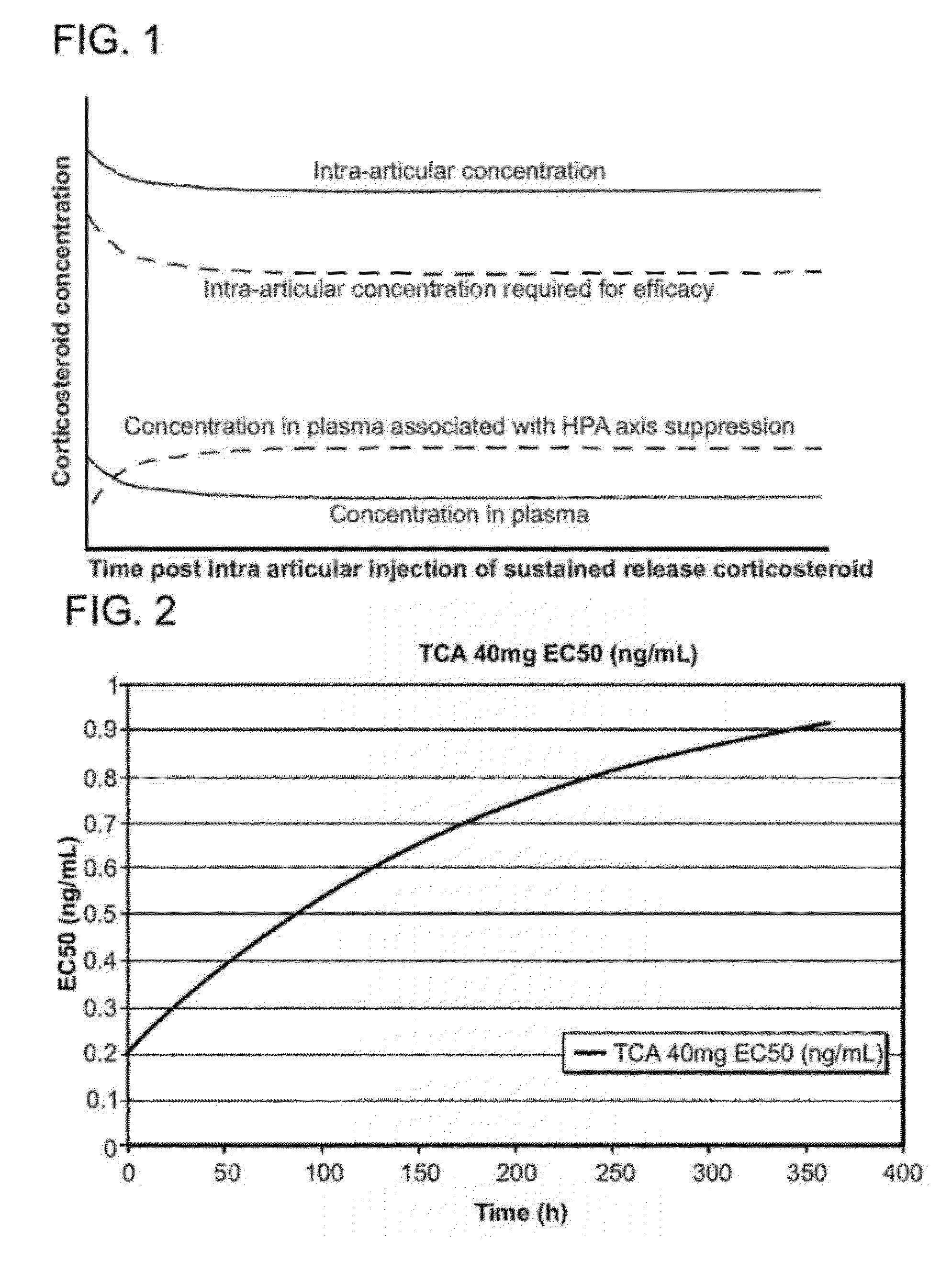
Corticosteroids for the treatment of joint pain
InactiveUS20120282298A1Minimal long-term side effectTreat painPowder deliveryOrganic active ingredientsDiseaseImmediate release
Corticosteroid microparticle formulations are provided for use for treating pain, including pain caused by inflammatory diseases such as osteoarthritis or rheumatoid arthritis, and for slowing, arresting or reversing structural damage to tissues caused by an inflammatory disease, for example damage to articular and / or peri-articular tissues caused by osteoarthritis or rheumatoid arthritis. Corticosteroid microparticle formulations are administered locally as a sustained release dosage form (with or without an immediate release component) that results in efficacy accompanied by clinically insignificant or no measurable effect on endogenous cortisol production.
Owner:FLEXION THERAPEUTICS
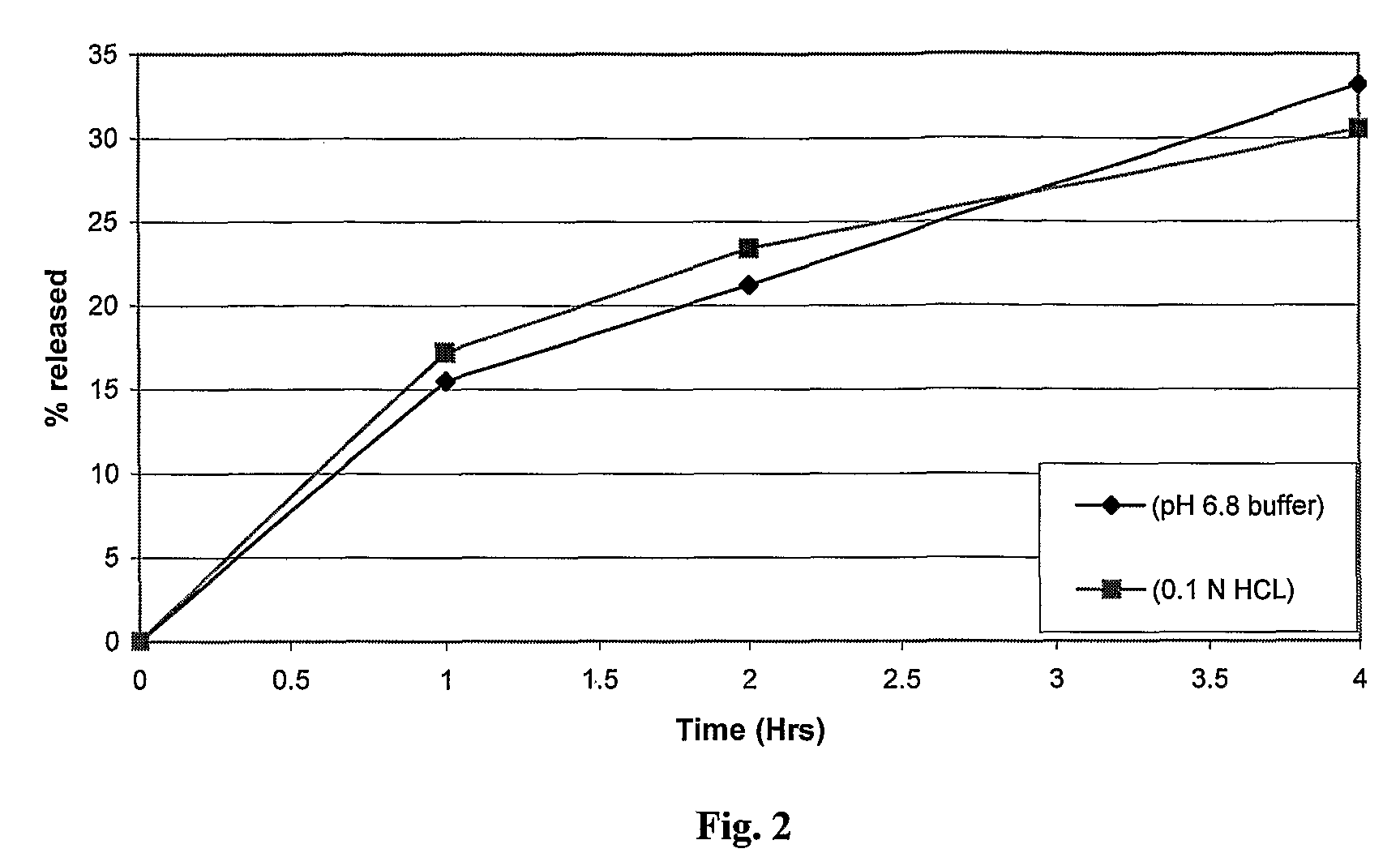
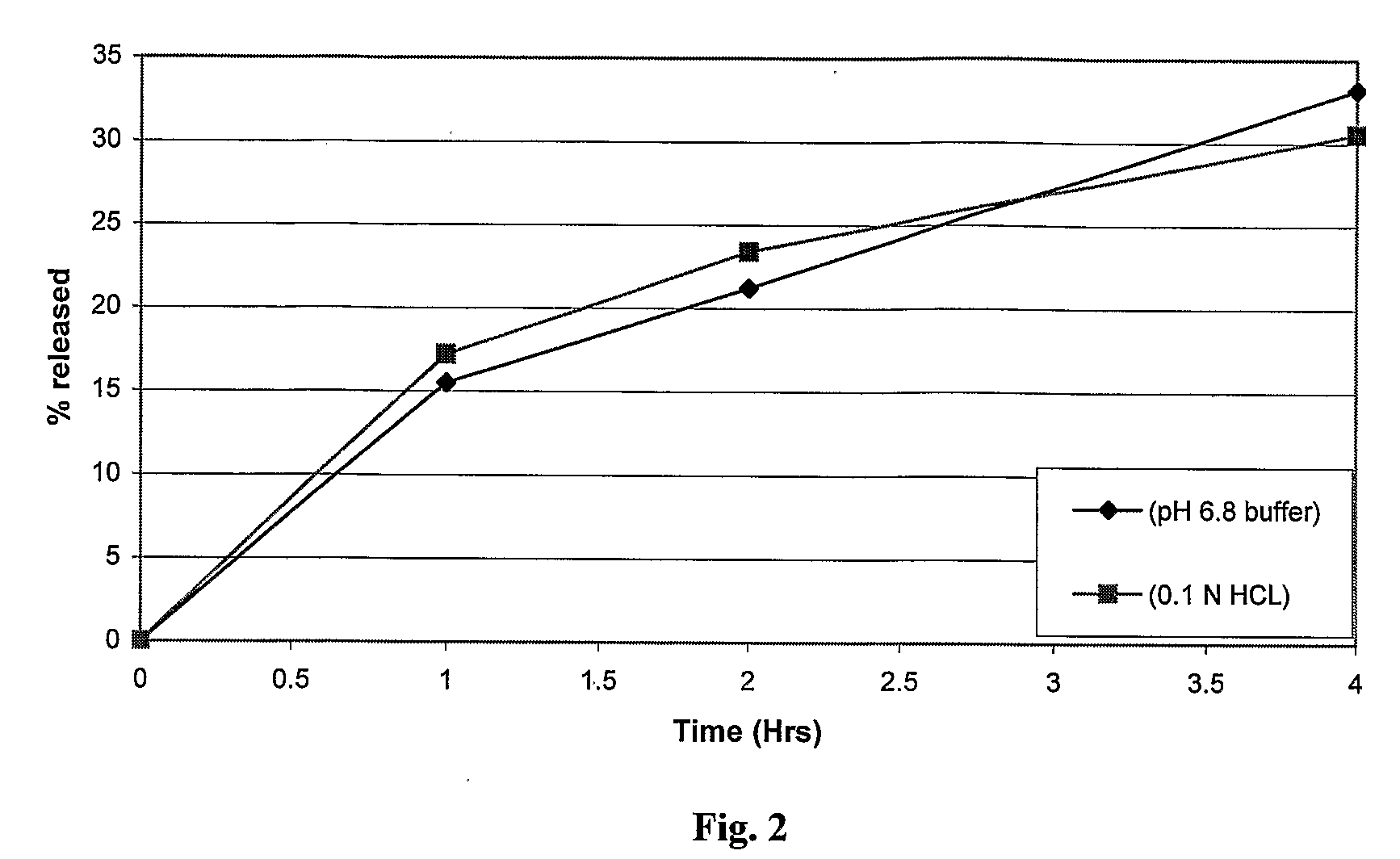
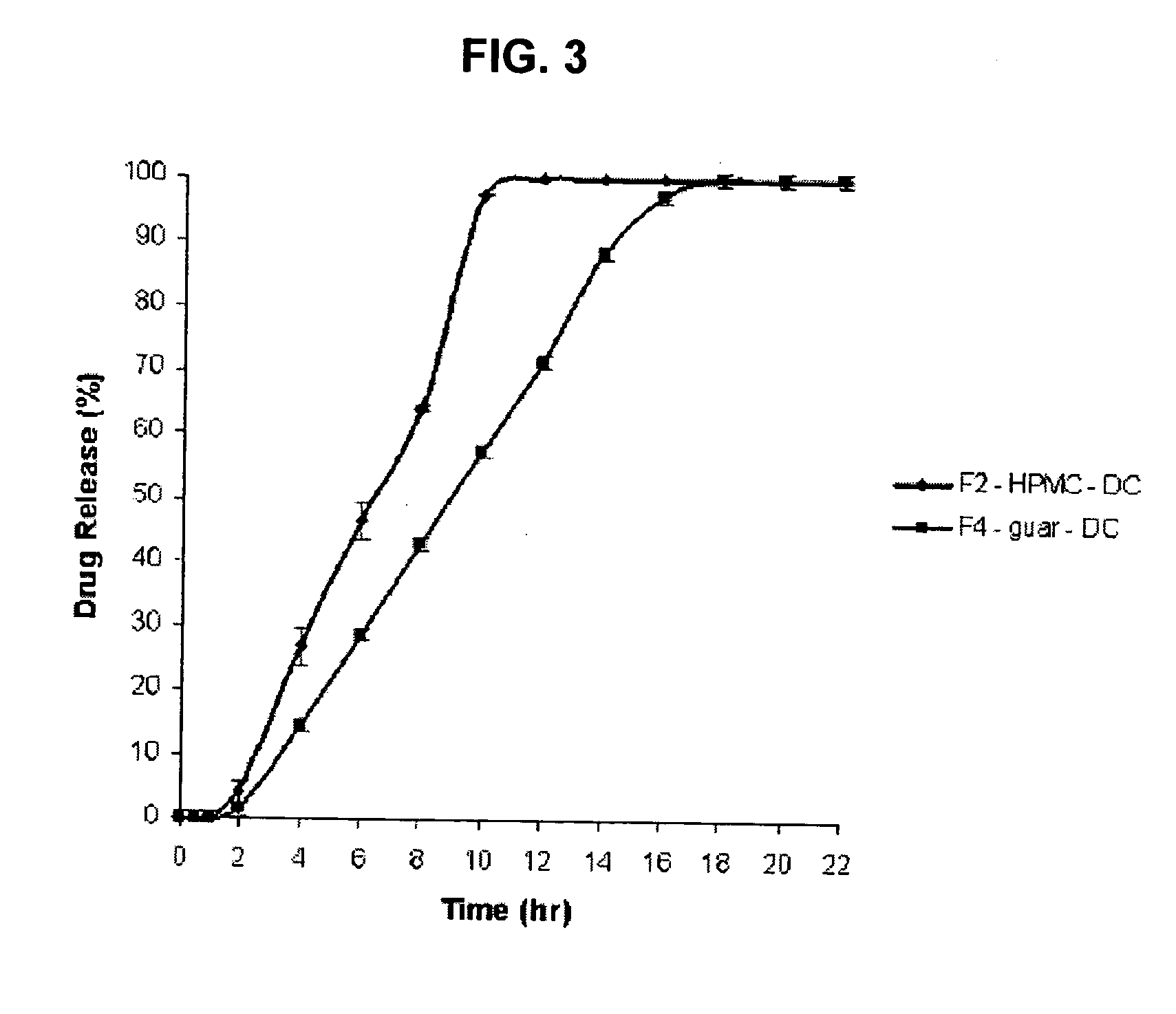
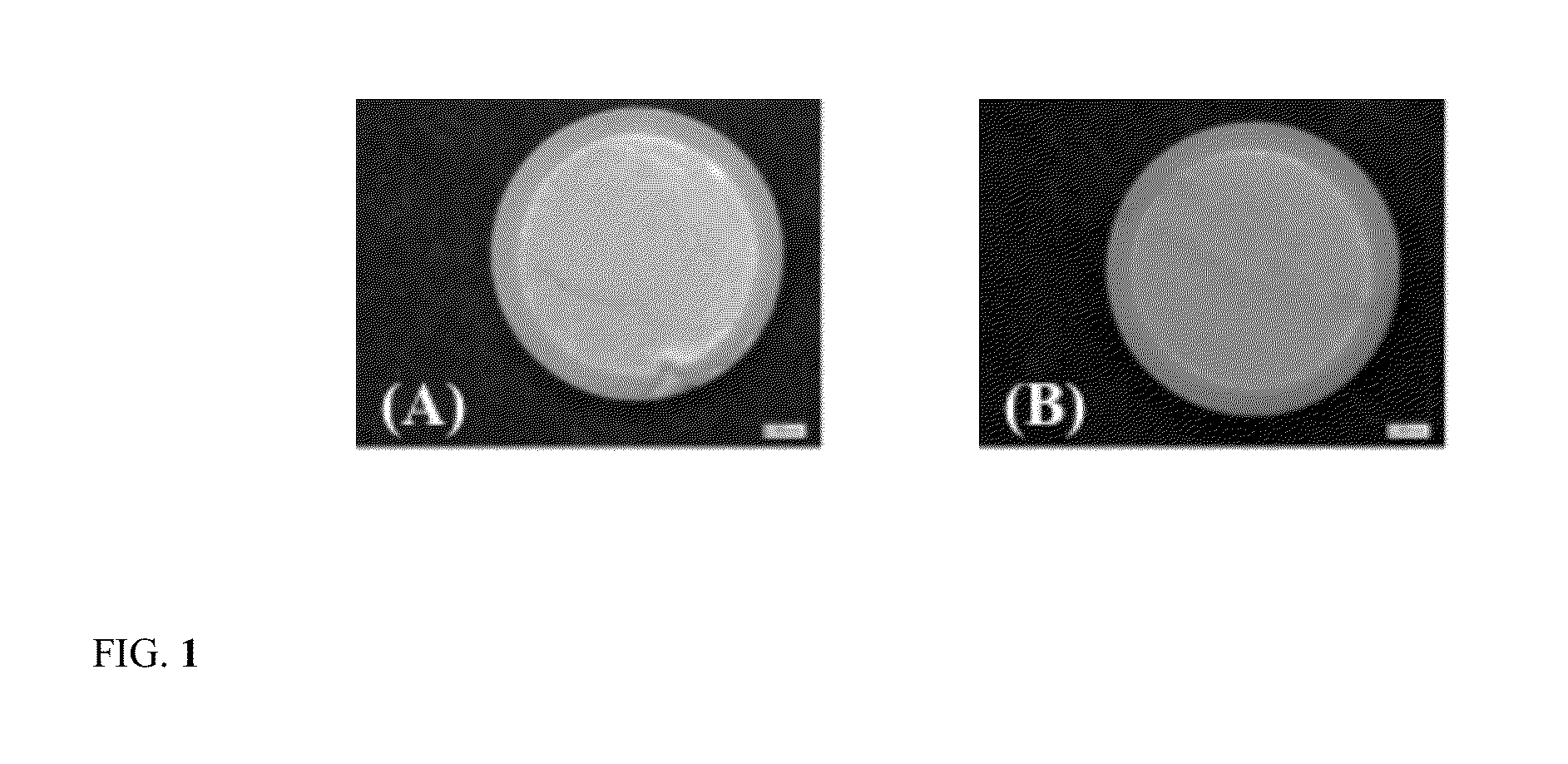
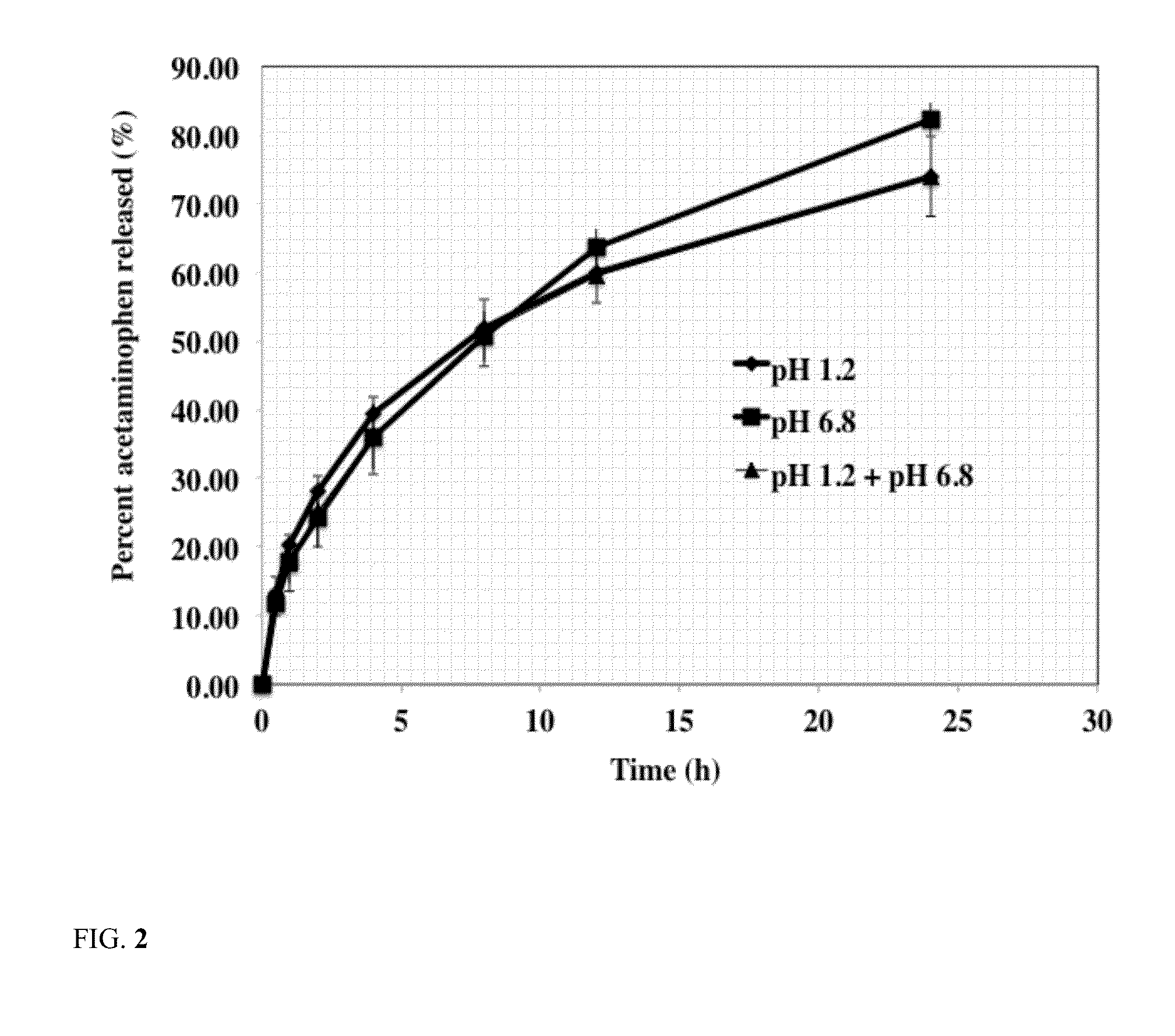
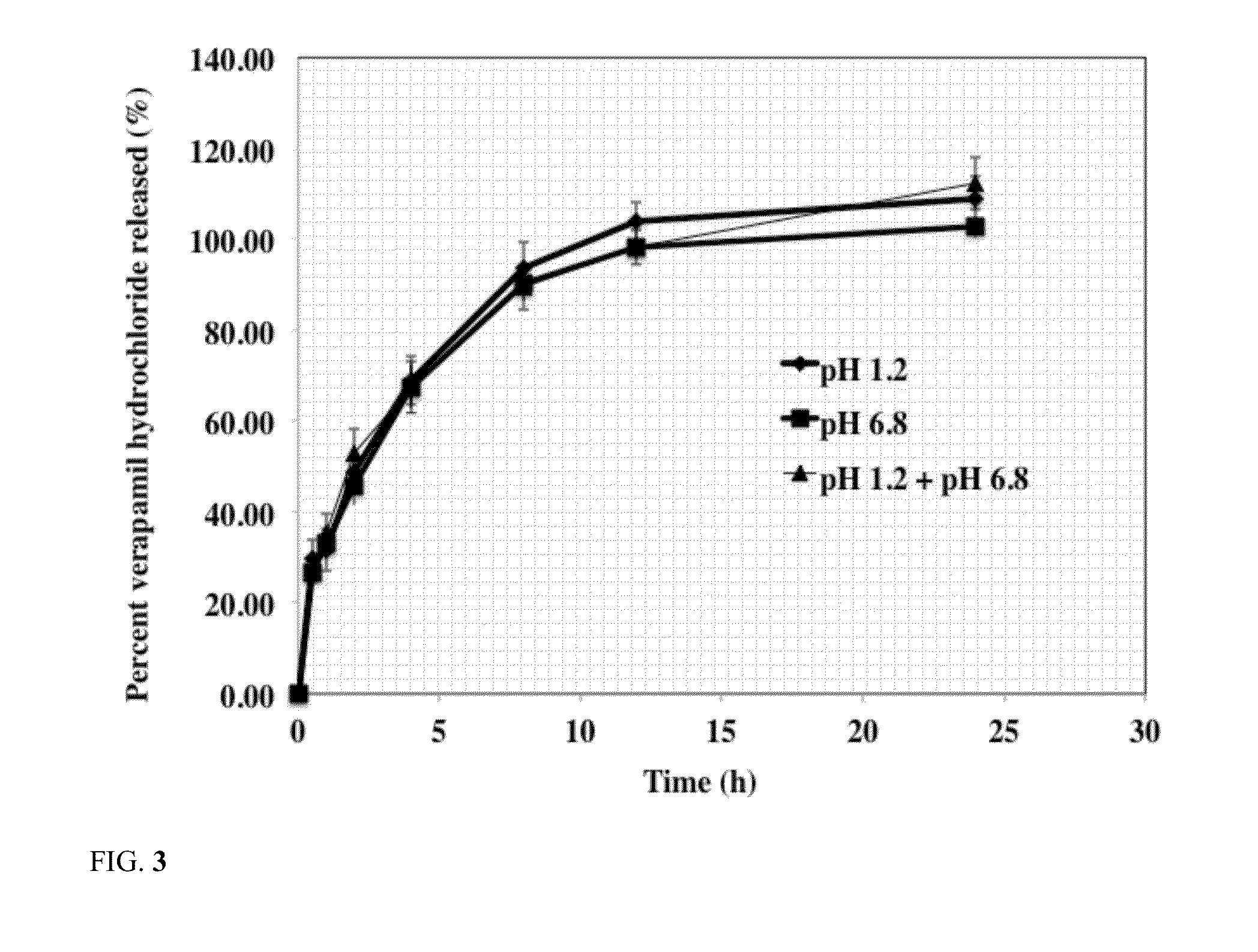
Sustained release dosage form
The novel sustained release dosage form comprising an active agent and a combination of a non-swelling pH dependent release retardant and a non swelling pH independent release retardant polymer which provides pH-independent drug release for a considerable period of time after administration.
Owner:RUBICON RES PTY LTD
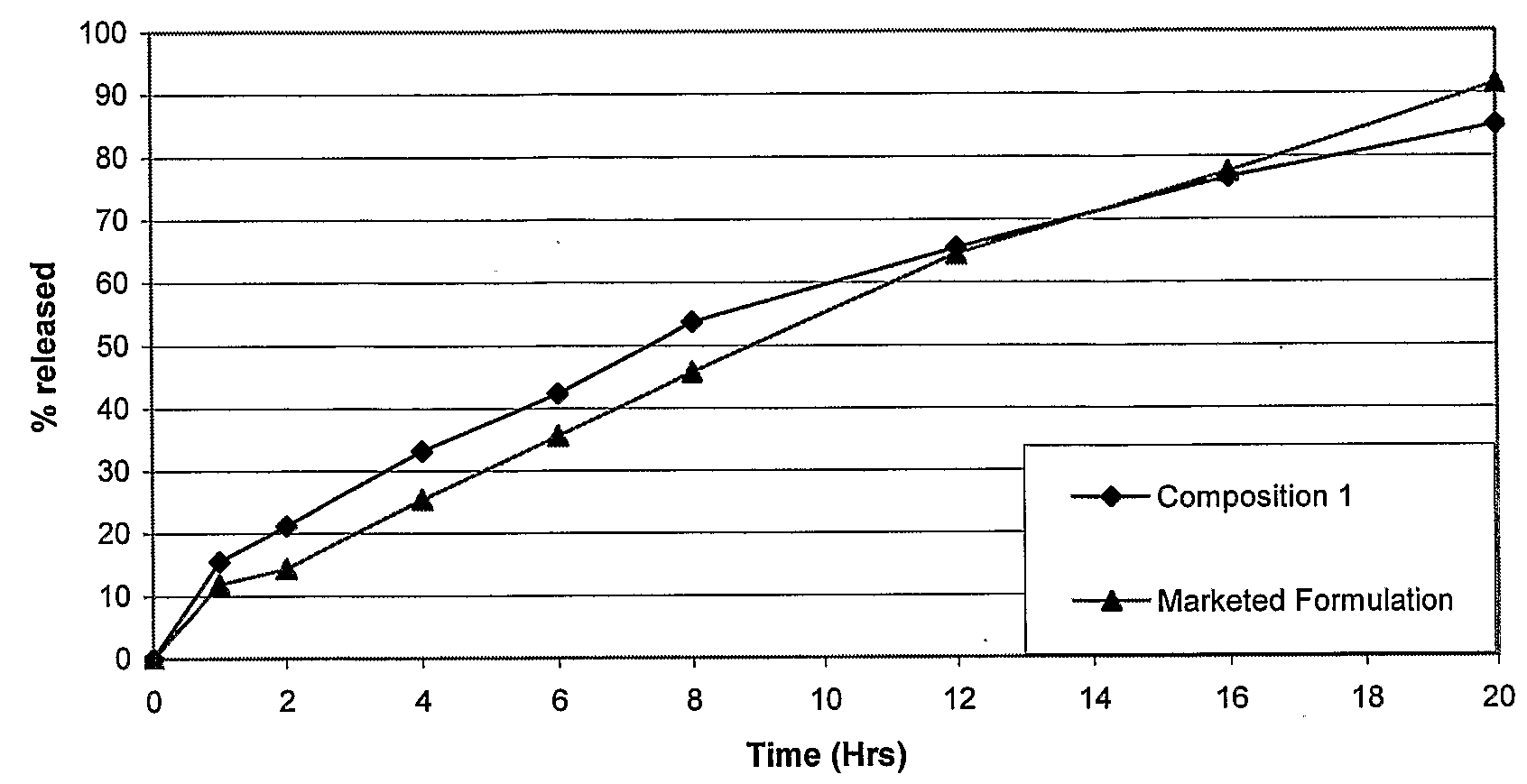
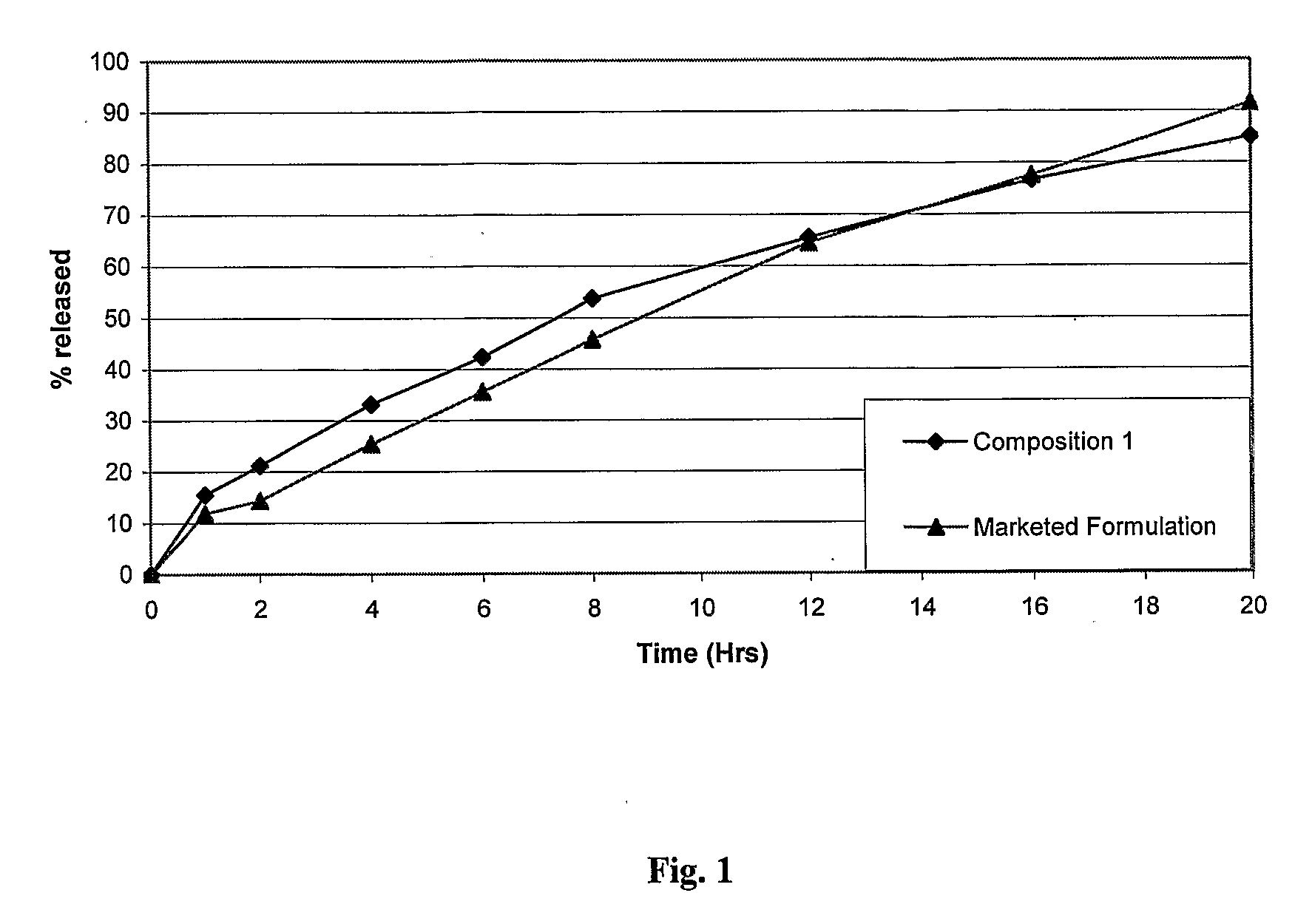
Novel sustained release dosage form
The novel sustained release dosage form comprising an active agent and a combination of a non-swelling pH dependent release retardant and a non swelling pH independent release retardant polymer which provides pH-independent drug release for a considerable period of time after administration.
Owner:RUBICON RES PTY LTD
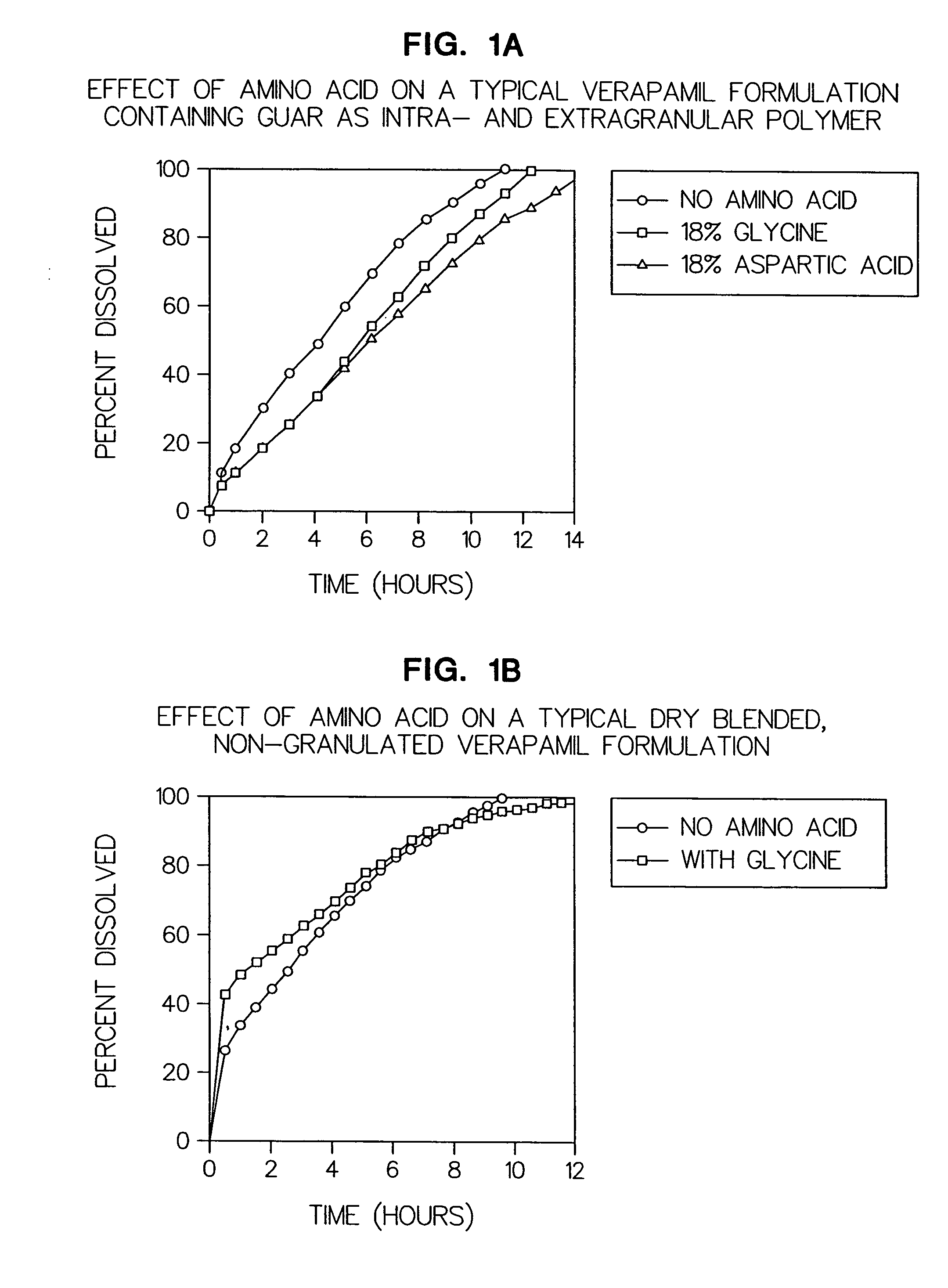
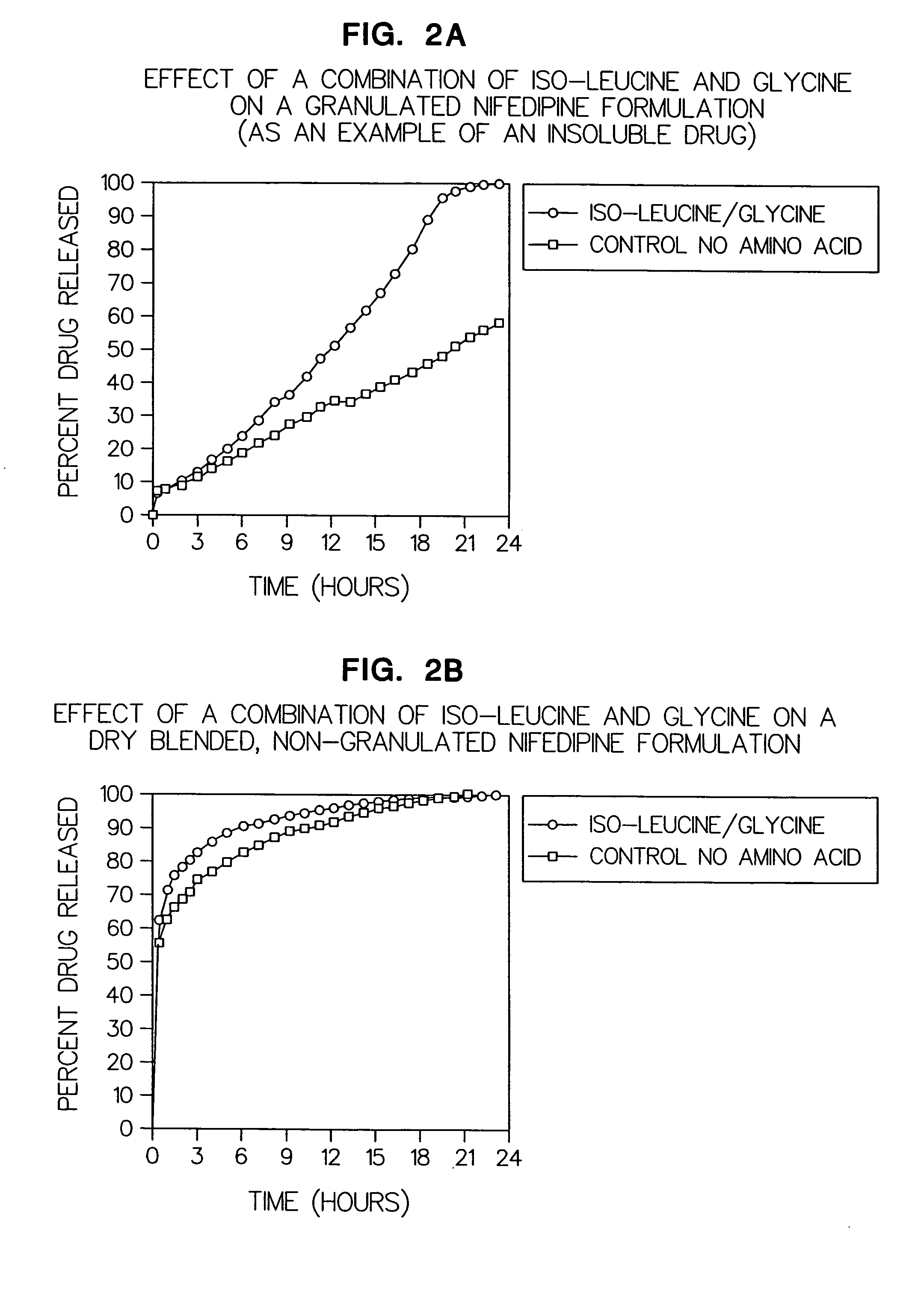
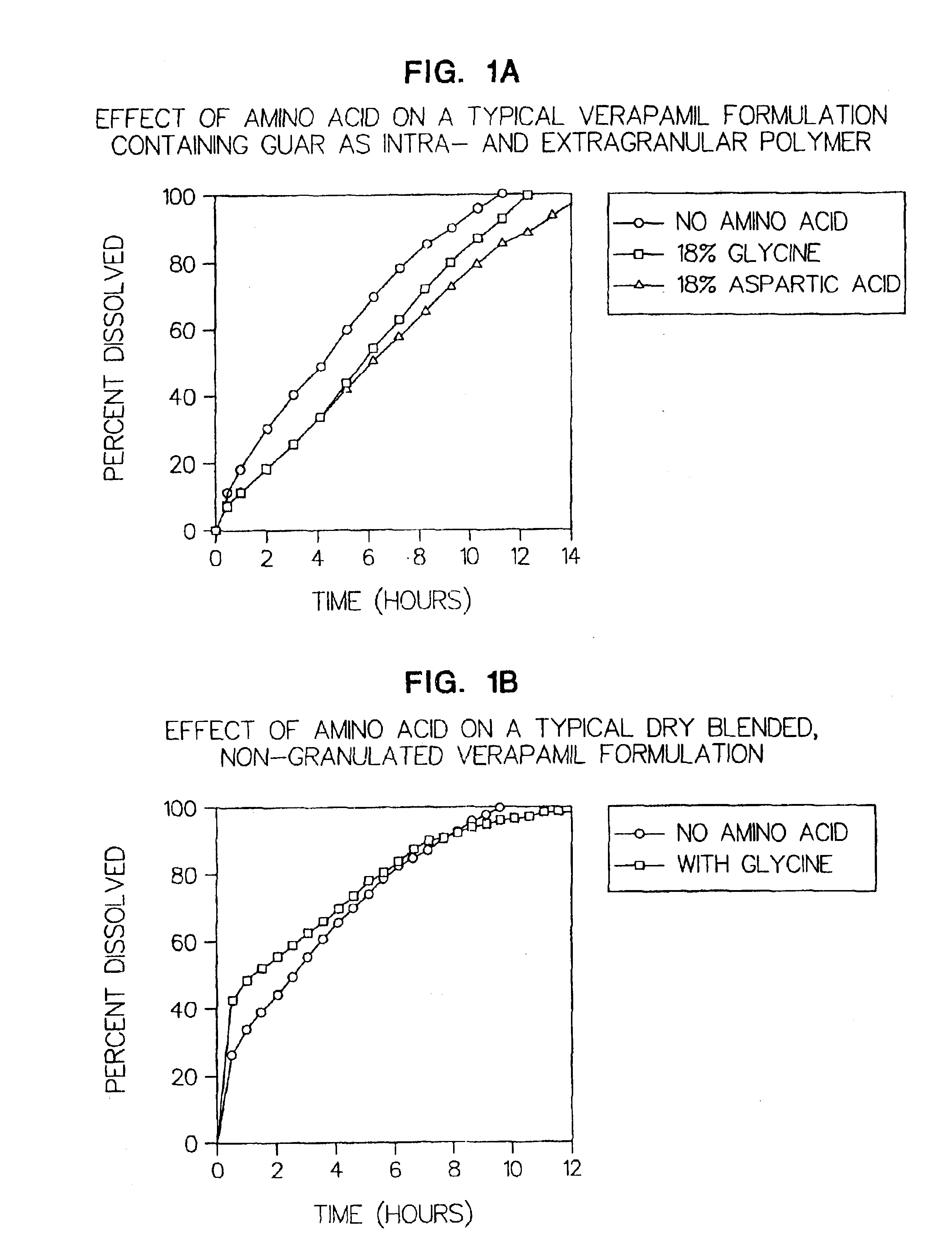
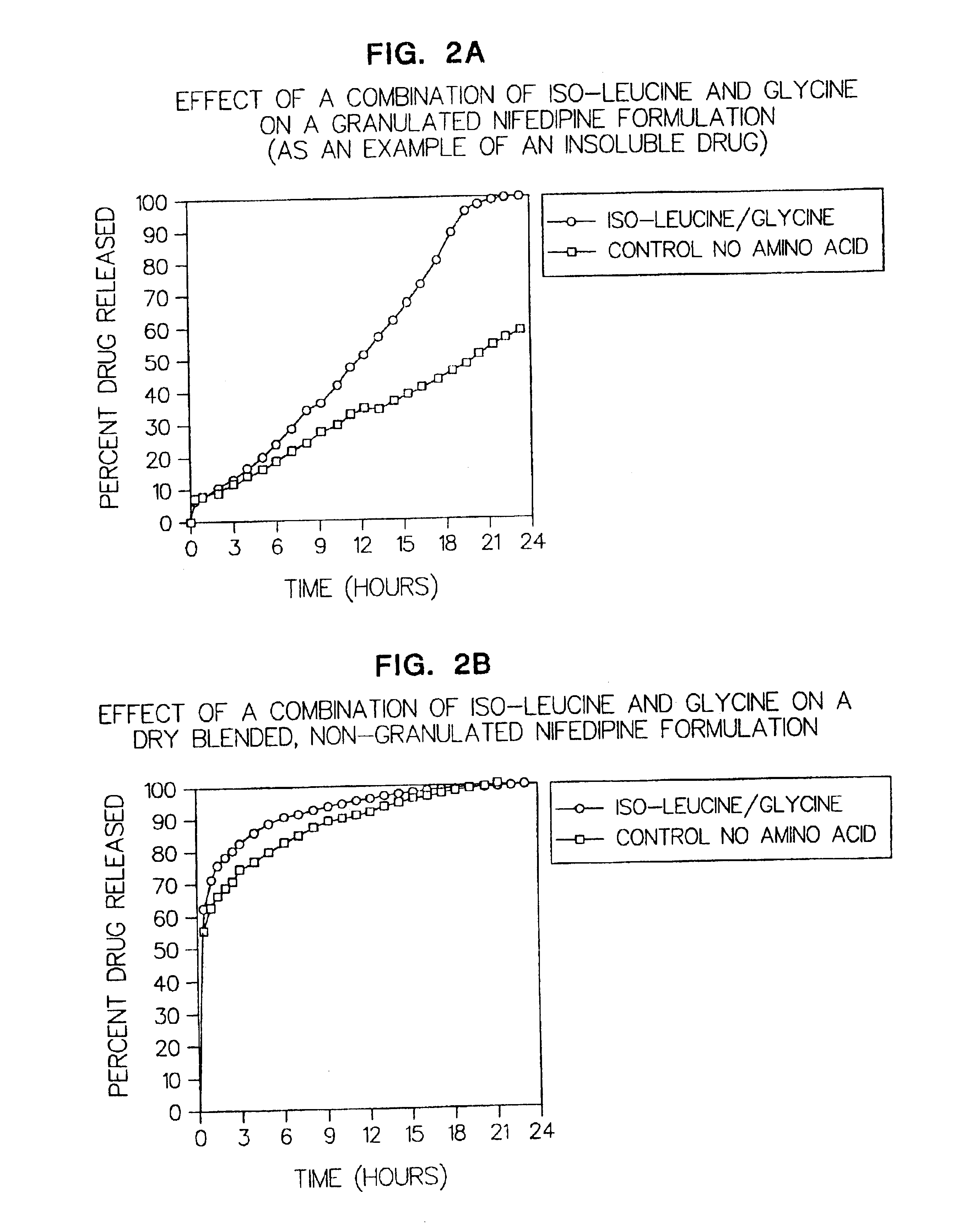
Amino acid modulated extended release dosage form
Disclosed herein is a oral extended release dosage form comprising a plurality of granules of an effective amount of a pharmaceutically active compound, at least one amino acid, and an intragranular polymer in which the granule is dispersed within a hydrophilic extragranular polymer matrix which is more rapidly hydrating than the intragranular polymer. The amino acid is selected for hydropathy characteristics depending on solubility characteristics of the active compound.
Owner:SCOLR PHARMA
Amino acid modulated extended release dosage form
InactiveUS6936275B2Minimizing complicationReduce manufacturing costPowder deliveryBiocideSolubilityPolymer
Disclosed herein is a oral extended release dosage form comprising a plurality of granules of an effective amount of a pharmaceutically active compound, at least one amino acid, and an intragranular polymer in which the granule is dispersed within a hydrophilic extragranular polymer matrix which is more rapidly hydrating than the intragranular polymer. The amino acid is selected for hydropathy characteristics depending on solubility characteristics of the active compound.
Owner:SCOLR PHARMA
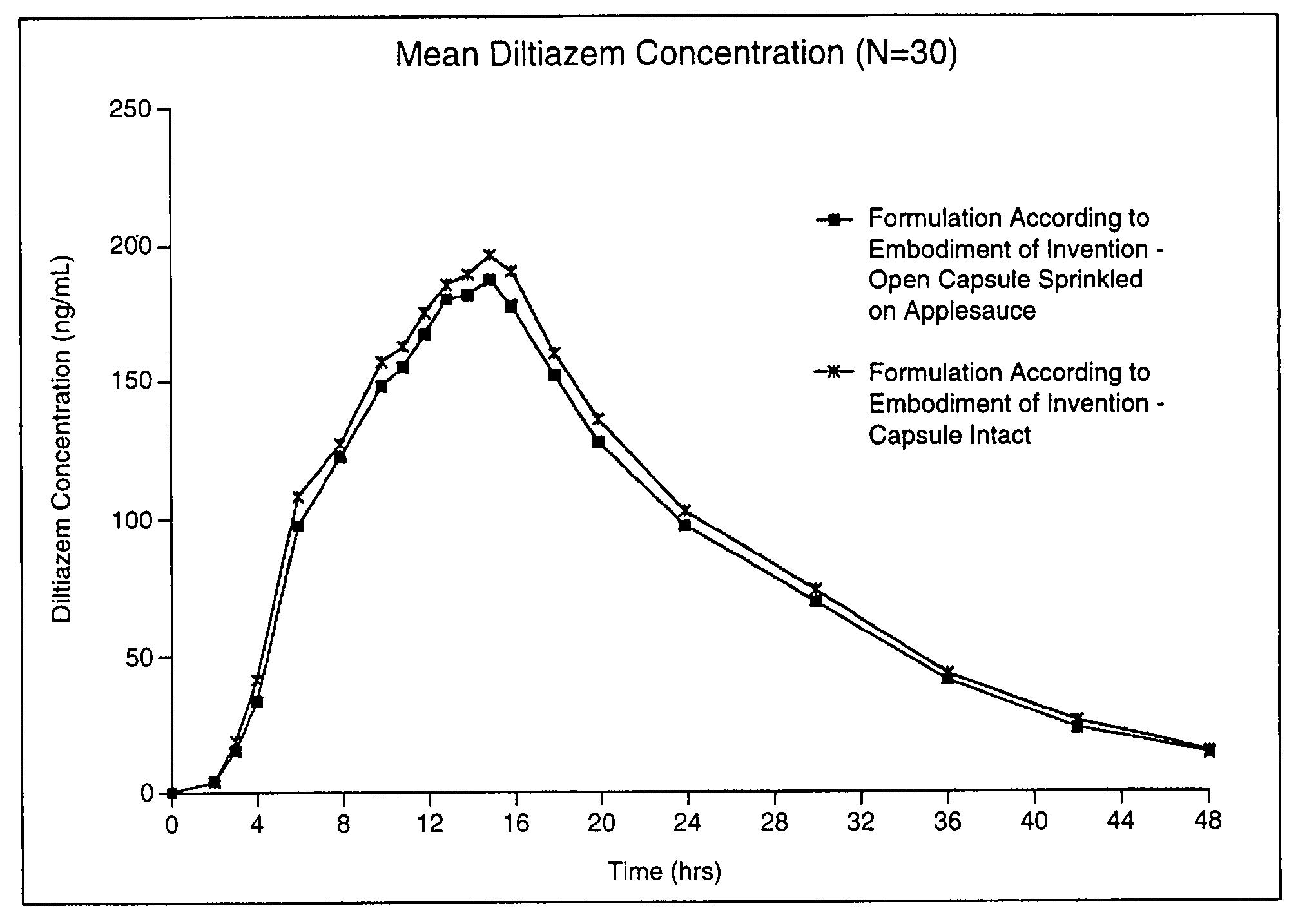
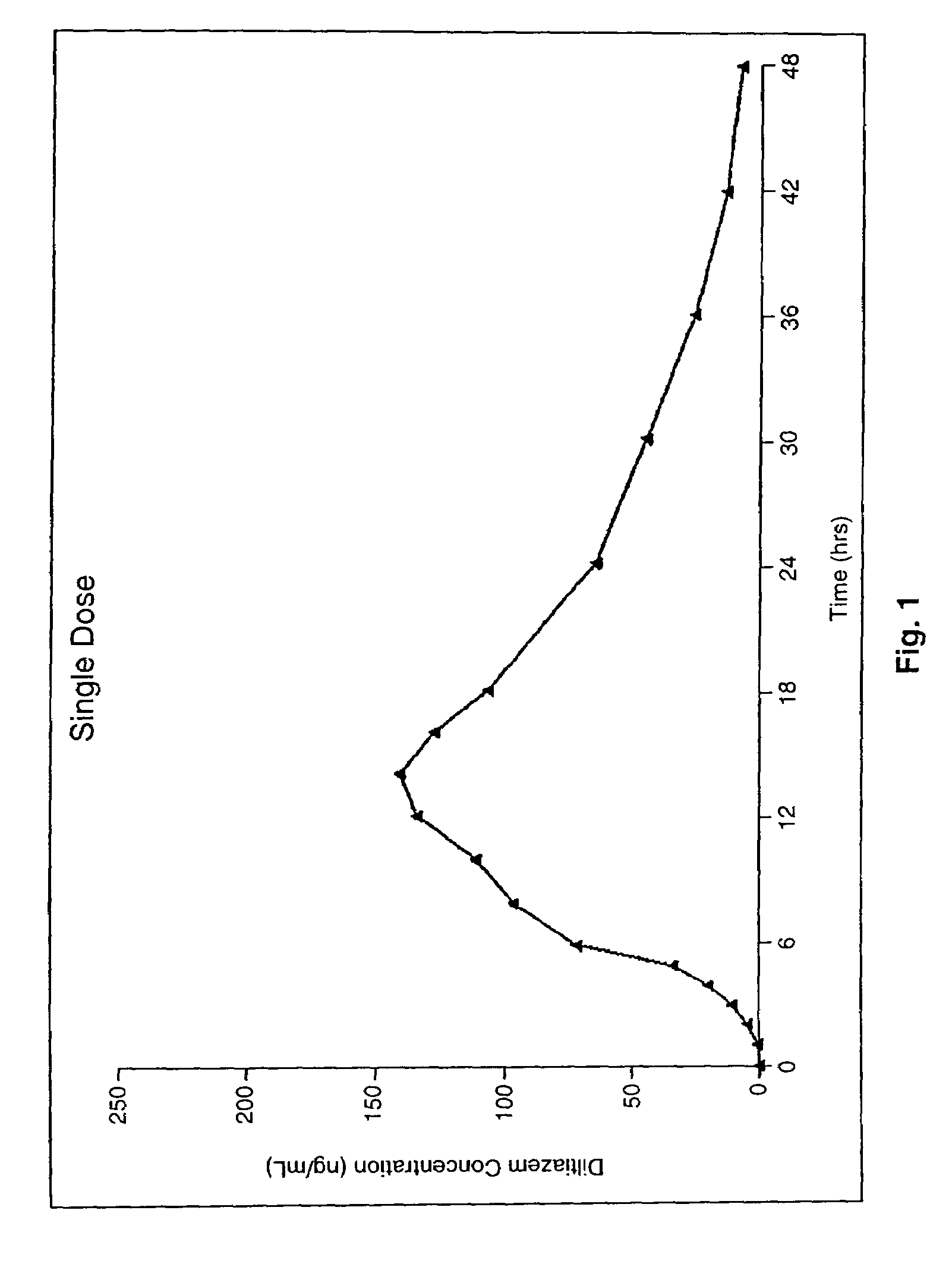
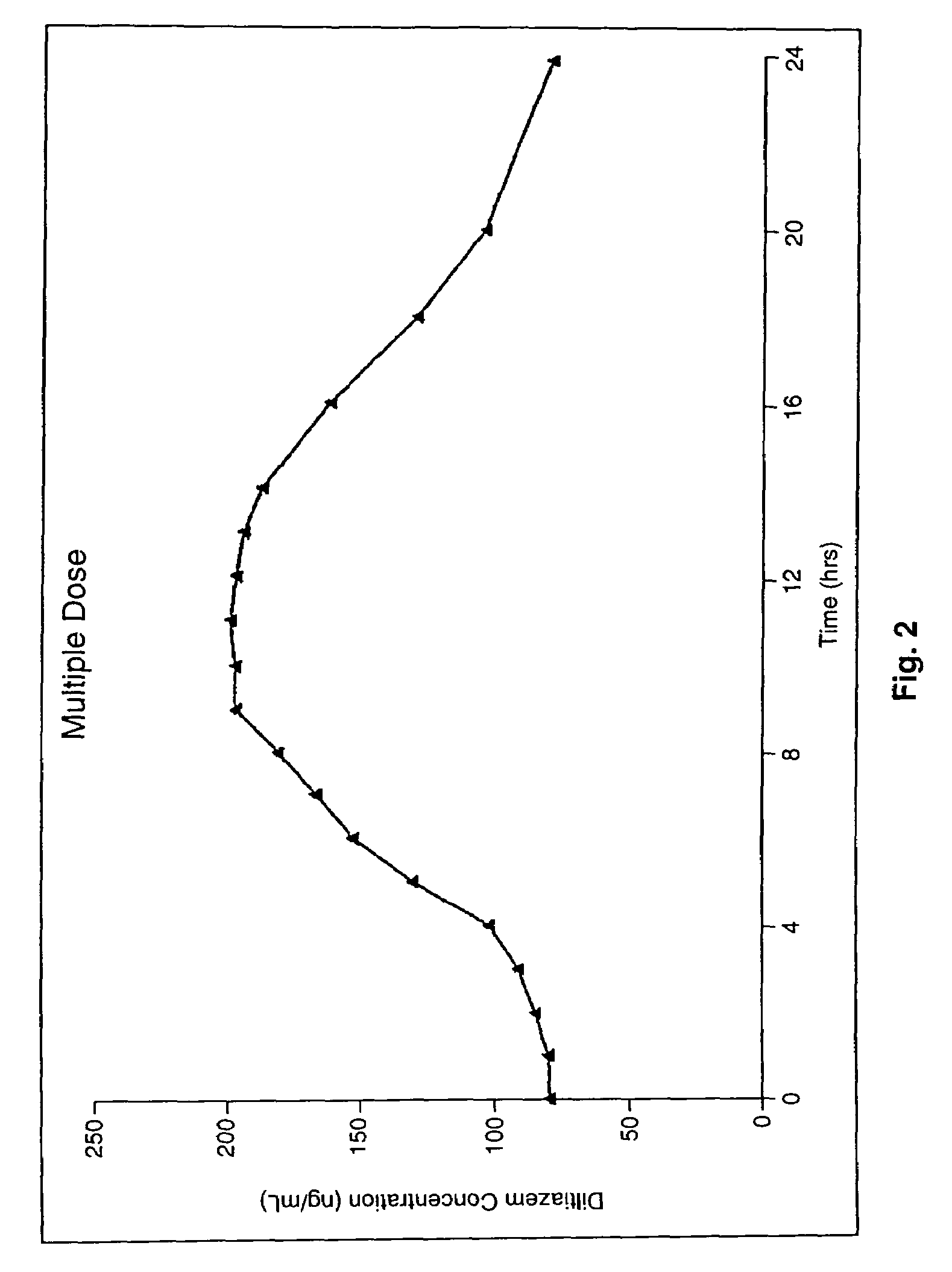
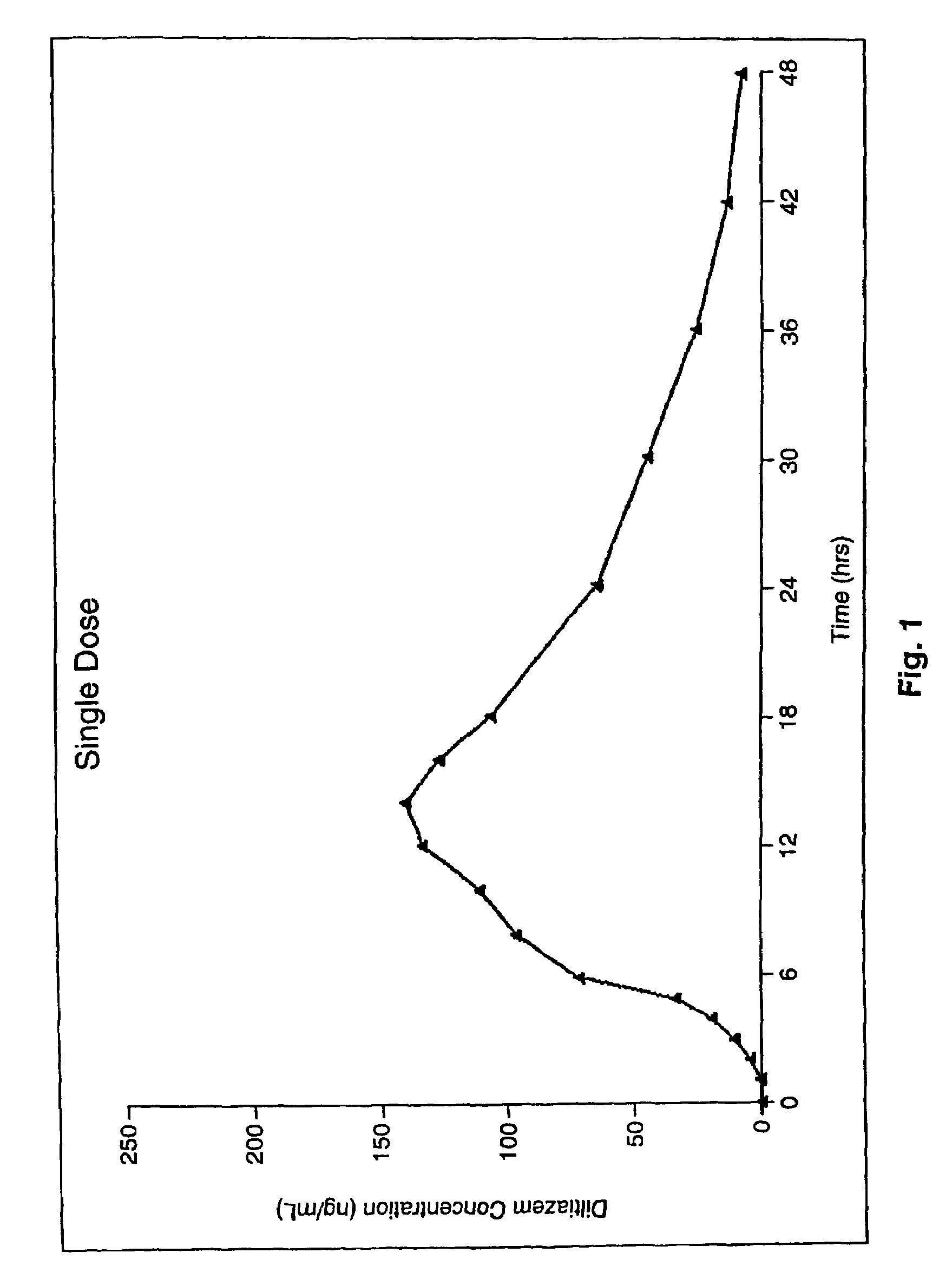
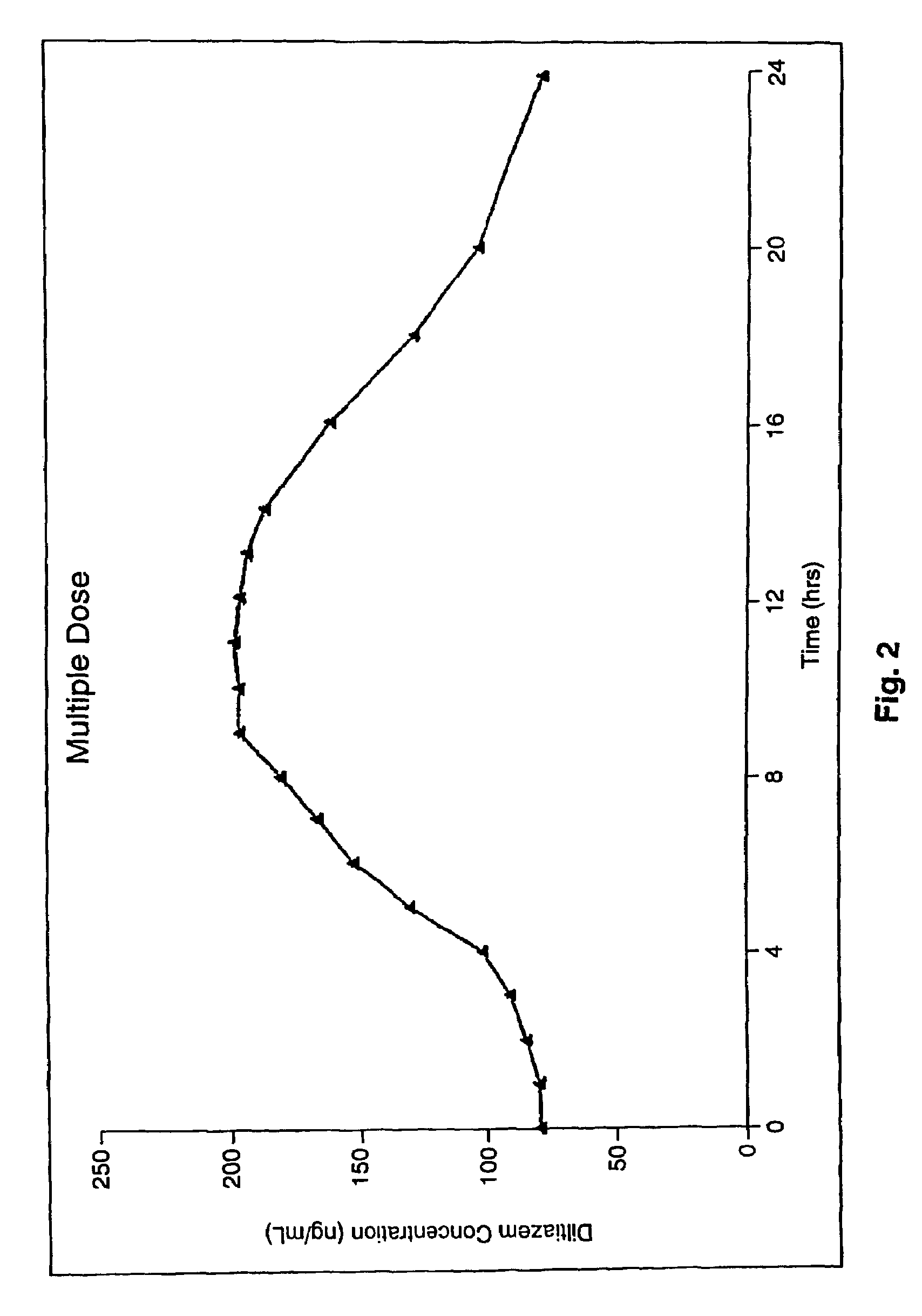
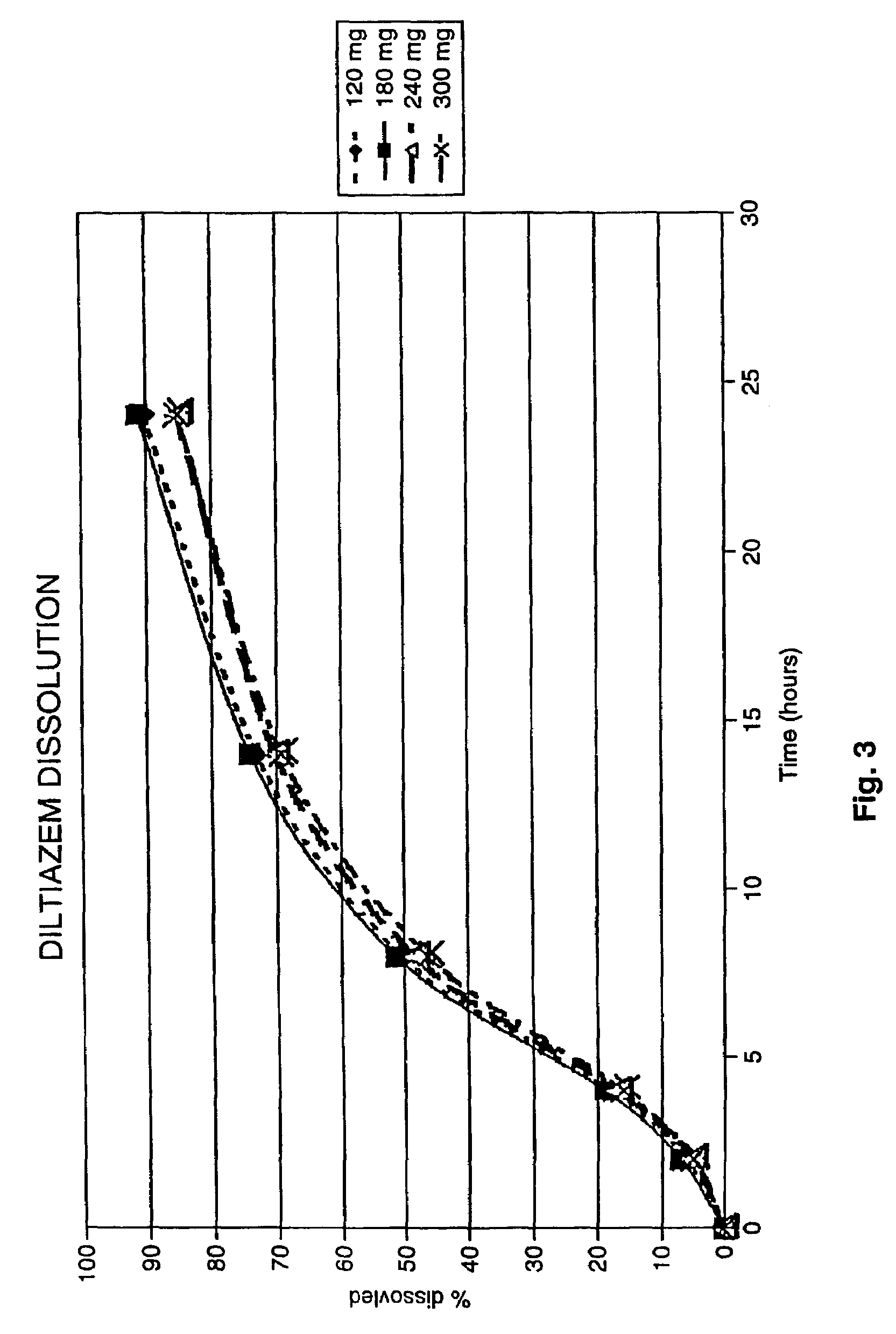
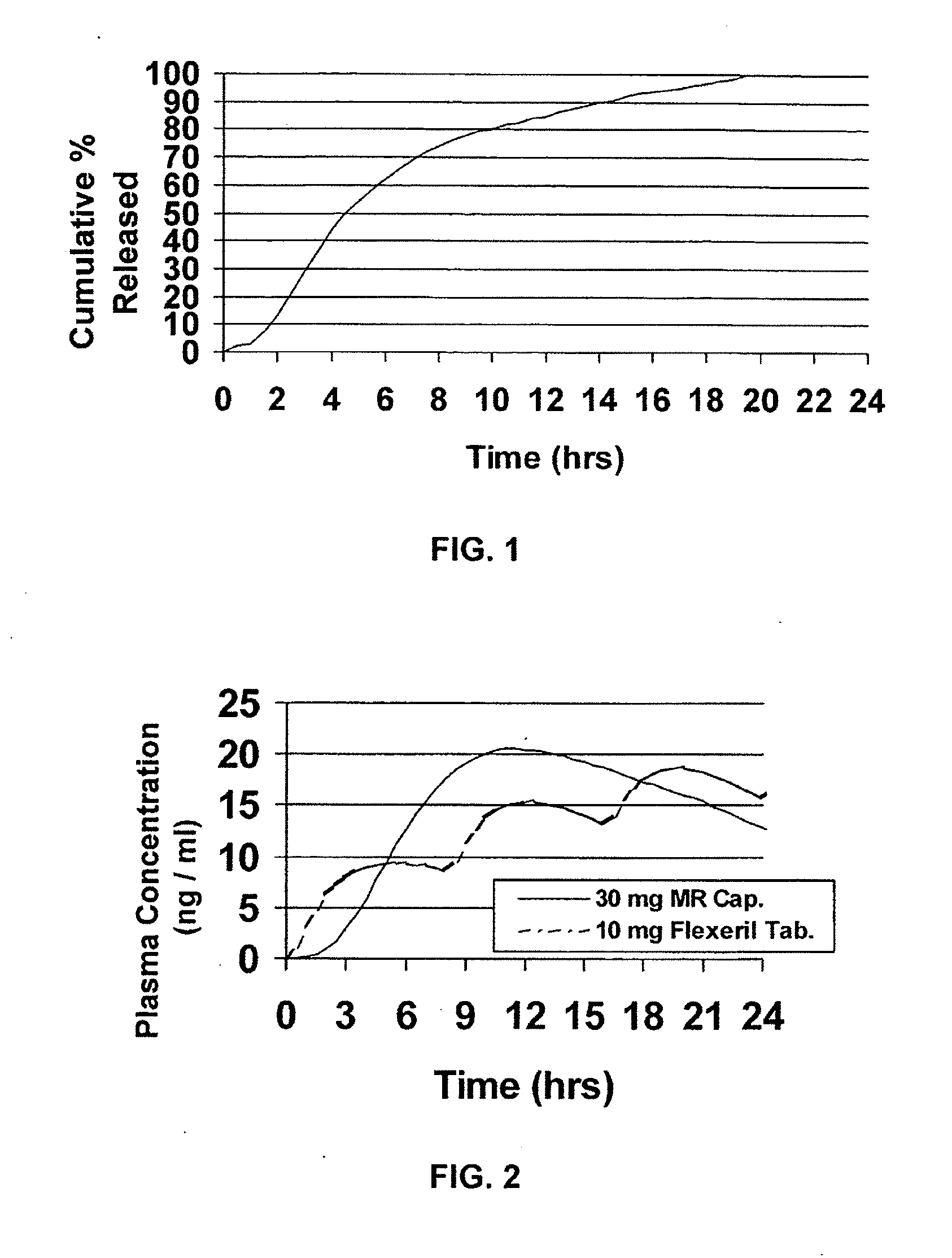
Chronotherapeutic diltiazem formulations and the administration thereof
InactiveUS7108866B1Maintain the solubility of the DiltiazemImprove bioavailabilityInorganic non-active ingredientsPill deliveryPharmacyControlled release
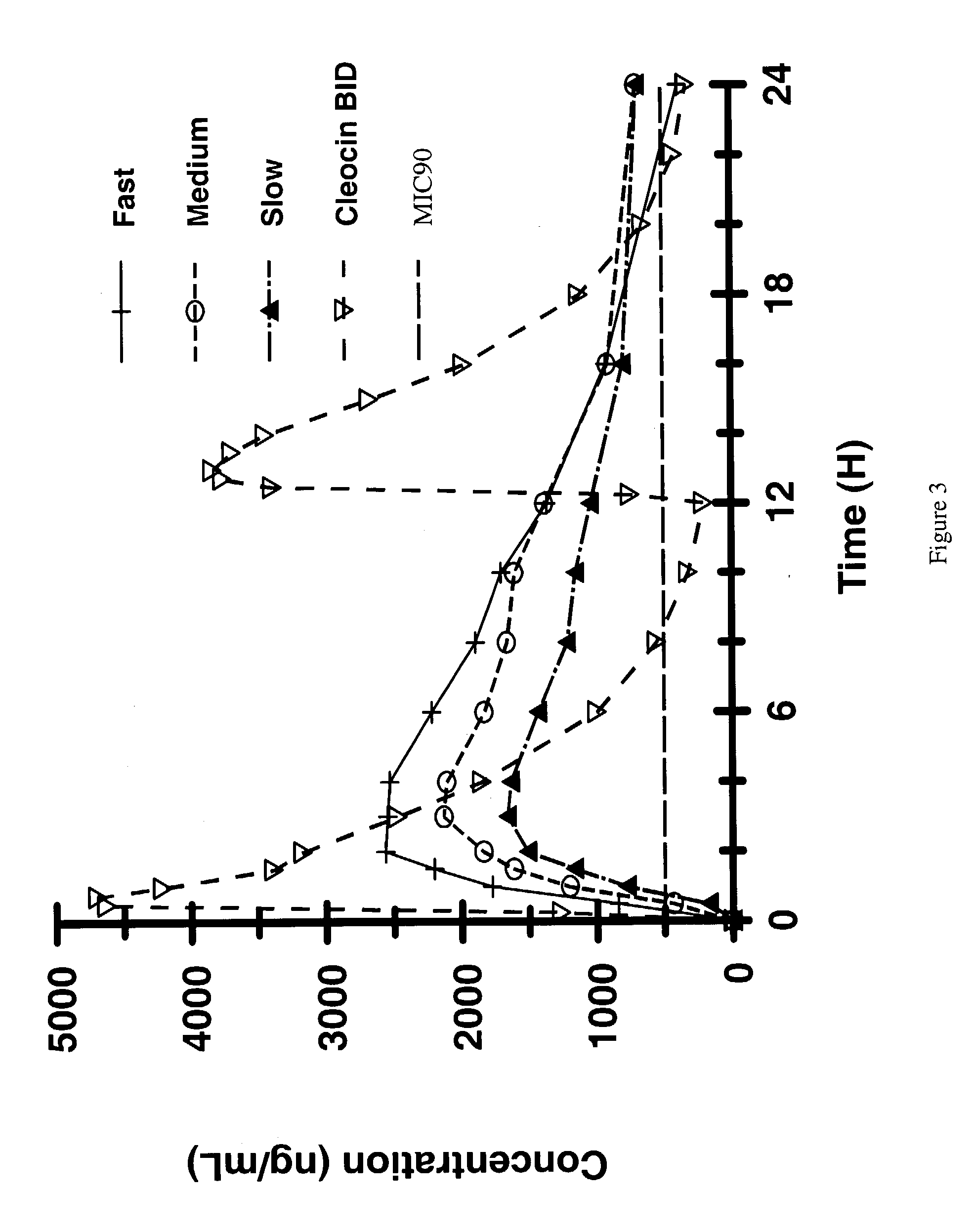
A controlled-release Galenical preparation of pharmaceutically acceptable Diltiazem including the pharmaceutically acceptable salts thereof, suitable for evening dosing every 24 hours containing from about 120 mg to about 540 mg or more (as desired) of the form of Diltiazem associated with excipients to provide controlled (sustained) release of the form of Diltiazem for providing a Cmax of Diltiazem in the blood at between about 10 hours and about 15 hours after administration, the preparation comprising the form of Diltiazem in oral sustained-release dosage form in which the Diltiazem is adapted to be released after administration over a prolonged period of time and exhibits when given to humans(i) a higher bioavailability when given at night compared to when given in the morning without food according to FDA guidelines or criteria and(ii) bioequivalence when given in the morning with and without food according to the same FDA guidelines or criteria.
Owner:VALEANT INT BERMUDA
Pharmaceutical compositions for treating pain associated with dysmenorrhea
InactiveUS20150313892A1Reduced plasma concentrationEfficient managementBiocidePeptide/protein ingredientsMolecular entityN-methyl-D-aspartate Receptor Antagonists
Pain associated with primary and secondary dysmenorrhea is relieved in a human suffering there from by administering to the human a pain relieving amount of a synergistically acting sub-therapeutic combination of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, magnesium, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, tramadol or its analog such as recemic tramadol or an analogously acting molecular entity or pharmaceutically acceptable salt thereof, and an anticonvulsant and / or a tricyclic anti-depressant or pharmaceutically acceptable salt thereof, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
Sustained release dosage form for lubricating an oral cavity
InactiveUS20090081294A1Increase dissolution characteristicExtended durationOrganic active ingredientsBiocideSustained release drugWater insoluble
Aspects of the invention include a sustained release dosage form that can be administered to an oral cavity, e.g., the mouth. In certain embodiments, the sustained release dosage form is formulated as a lozenge or gum that may be administered to an oral cavity for the purpose of providing lubrication therein. In certain embodiments, the sustained release dosage form not only provides lubrication to a mucosal surface of an oral cavity, but also provides for the sustained release of a flavoring and / or beneficial agent. Accordingly, in certain embodiments, the sustained release dosage form includes a water-insoluble polymer, e.g., ethylcellulose, an essential oil component, and an effective amount of a film forming binder, e.g., xanthan gum. In certain embodiments, the effective amount of the film forming binder and the type of film forming binder are selected so as to provide the sustained release dosage with the capability of lubricating one or more mucosal surfaces within an oral cavity when the dosage form is positioned therein. In certain embodiments, the sustained release dosage form is formulated in a manner sufficient to form a matrix that includes the various components of the sustained release dosage form, such that when positioned in an oral cavity the matrix slowly dissolves and thereby lubricates the oral cavity and / or delivers a flavoring and / or beneficial agent thereto. Methods of formulating such dosage forms and administering them to an oral cavity for the treatment of an adverse condition are also provided.
Owner:BENNES
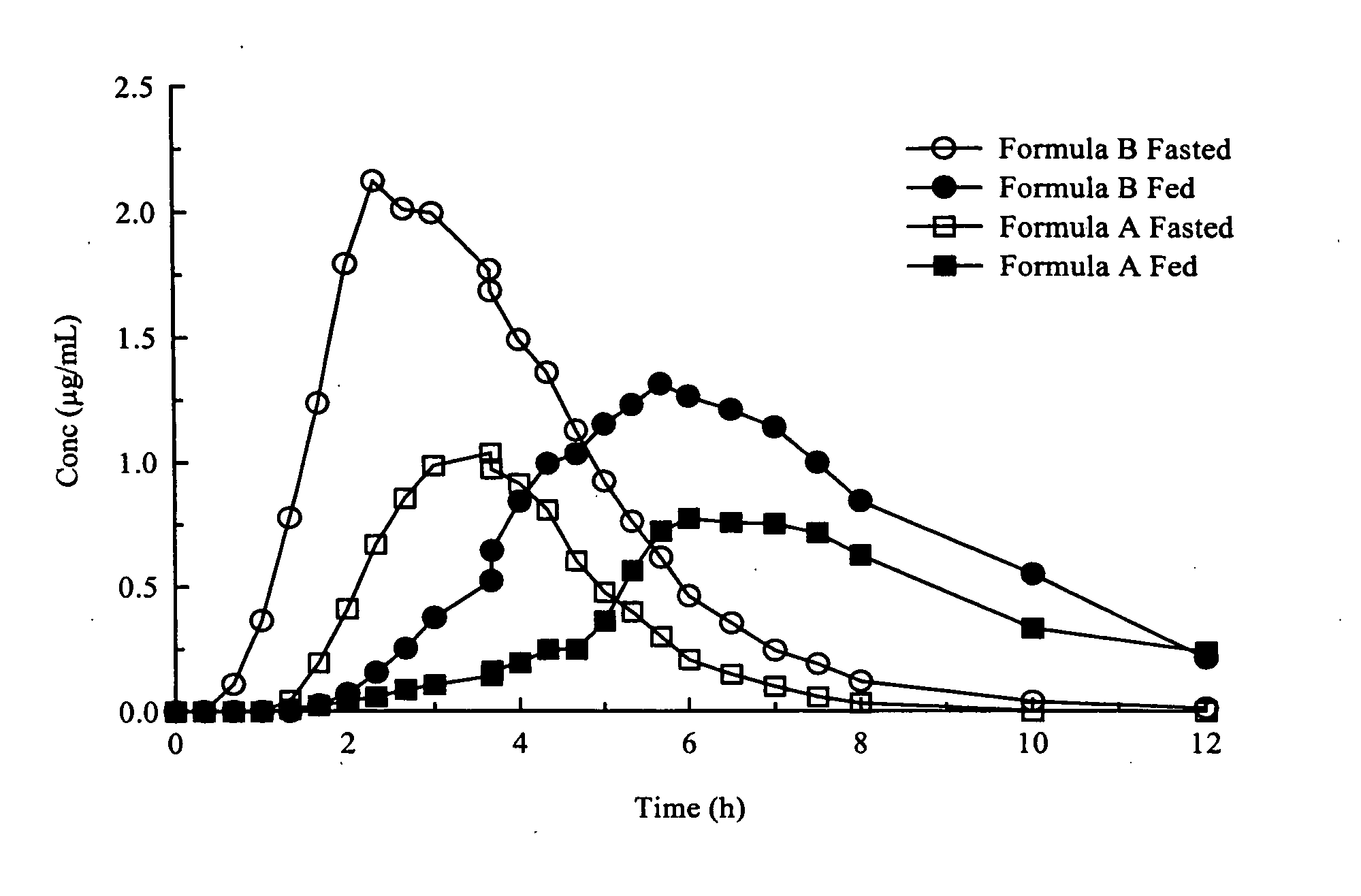
Enhanced absorption of modified release dosage forms
InactiveUS20050142187A1Effective for bacterial infectionEffective treatmentPill deliveryGranular deliveryIntestinal structureModified Release Dosage Form
Disclosed are products and methods for improving the plasma profile in a patient being treated with a pharmaceutical active agent that is subject to a limited window of absorption, which products and methods comprise orally administering the active agent in multiparticulate form, such that at least a portion thereof is delivered to the intestine while the patient is in the fed condition.
Owner:SHIONOGI INK
Chronotherapeutic diltiazem formulations and the administration thereof
InactiveUS7348028B2Improve bioavailabilityMaintain the solubility of the DiltiazemPowder deliveryPill deliveryPharmacyControlled release
A method of treating or preventing myocardial ischemia in a patient in need thereof comprising administration of a controlled-release Galenical preparation of pharmaceutically acceptable Diltiazem including the pharmaceutically acceptable salts thereof, suitable for evening dosing every 24 hours containing from about 180 mg to about 420 mg of the form of Diltiazem associated with excipients to provide controlled (sustained) release of the form of Diltiazem for providing a Cmax of Diltiazem in the blood at between about 10 hours and about 17 hours after administration, the preparation comprising the form of Diltiazem in oral sustained-release dosage form in which the Diltiazem is adapted to be released after administration over a prolonged period of time and exhibits when given to humans(i) a higher bioavailability when given at night compared to when given in the morning without food according to FDA guidelines or criteria and(ii) bioequivalence when given in the morning with and without food according to the same FDA guidelines or criteria.
Owner:CHANTILLY BIOPHARMA
Modified release dosage forms of skeletal muscle relaxants
InactiveUS20080124398A1Patient compliance is goodEfficient ConcentrationPowder deliveryOrganic active ingredientsDiseaseModified Release Dosage Form
A unit dosage form, such as a capsule or the like, for delivering a skeletal muscle relaxant, such as cyclobenzaprine hydrochloride, into the body in an extended or sustained release fashion comprising one or more populations of drug-containing particles (beads, pellets, granules, etc.) is disclosed. At least one bead population exhibits a pre-designed sustained release profile. Such a drug delivery system is designed for once-daily oral administration to maintain an adequate plasma concentration—time profile, thereby providing relief of muscle spasm associated with painful musculoskeletal conditions over a 24 hour period.
Owner:ADARE PHARM INC
Controlled release formulations exhibiting an ascending rate of release
InactiveUS20110129507A1Reduce solubilityPoor dissolution rateBiocideNervous disorderDiseaseSustained release drug
A sustained release dosage form is comprising a pharmaceutically active agent and pharmaceutically acceptable salts thereof and adapted to release as an erodible solid over a prolonged period of time, wherein the dosage form provides an ascending rate of release of the pharmaceutically active agent for at least about 4 hours. The dosage form is able to deliver high doses of poorly soluble or slowly dissolving active agents. When additional pharmaceutically active agents are present, the agents are released from the dosage form at rates that are proportional to the respective weights of each active agent in the dosage form. Methods of using the dosage forms to treat disease or conditions in human patients are also disclosed.
Owner:CRUZ EVANGELINE
Modified release dosage form
The present invention relates to a medicinal dosage form having a first core, a second core, and a shell that surrounds a first portion of each core and a fill material that covers a second portion of at least one core, wherein the fill material that is provided over at least one core is not in contact with any portion of the other core. e. The inventive dosage forms provide modified release of one or more active ingredients contained therein. The present invention also relates to methods for manufacturing such medicinal dosage forms.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Sustained release matrix for high-dose insoluble drugs
Sustained release dosage forms of high dose insoluble drugs such as ibuprofen and methods for their manufacture are disclosed.
Owner:PENWEST PHARMA CO
Sustained-release dosage forms of ruxolitinib
ActiveUS10166191B2Lower the volumeOrganic active ingredientsSenses disorderDiseaseSustained release drug
The present invention relates to sustained-release formulations and dosage forms of ruxolitinib, or a pharmaceutically acceptable salt thereof, which are useful in the treatment of Janus kinase-associated diseases such as myeloproliferative disorders.
Owner:INCYTE HLDG & INCYTE
Oros push-stick for controlled delivery of active agents
InactiveUS20100196425A1Faster initial rate of releaseFast releaseBiocidePill deliveryDiseaseSustained release drug
A sustained release dosage form is provided comprising a pharmaceutically active agent and pharmaceutically acceptable salts thereof and adapted to release as an erodible solid over a prolonged period of time, wherein the dosage form provides burst release of the pharmaceutically active agent without the use of an immediate release drug coating. The dosage form is able to deliver high doses of poorly soluble or slowly dissolving active agents at a controlled rate. Methods of using the dosage forms to treat disease or conditions in human patients are also disclosed.
Owner:ALZA CORP
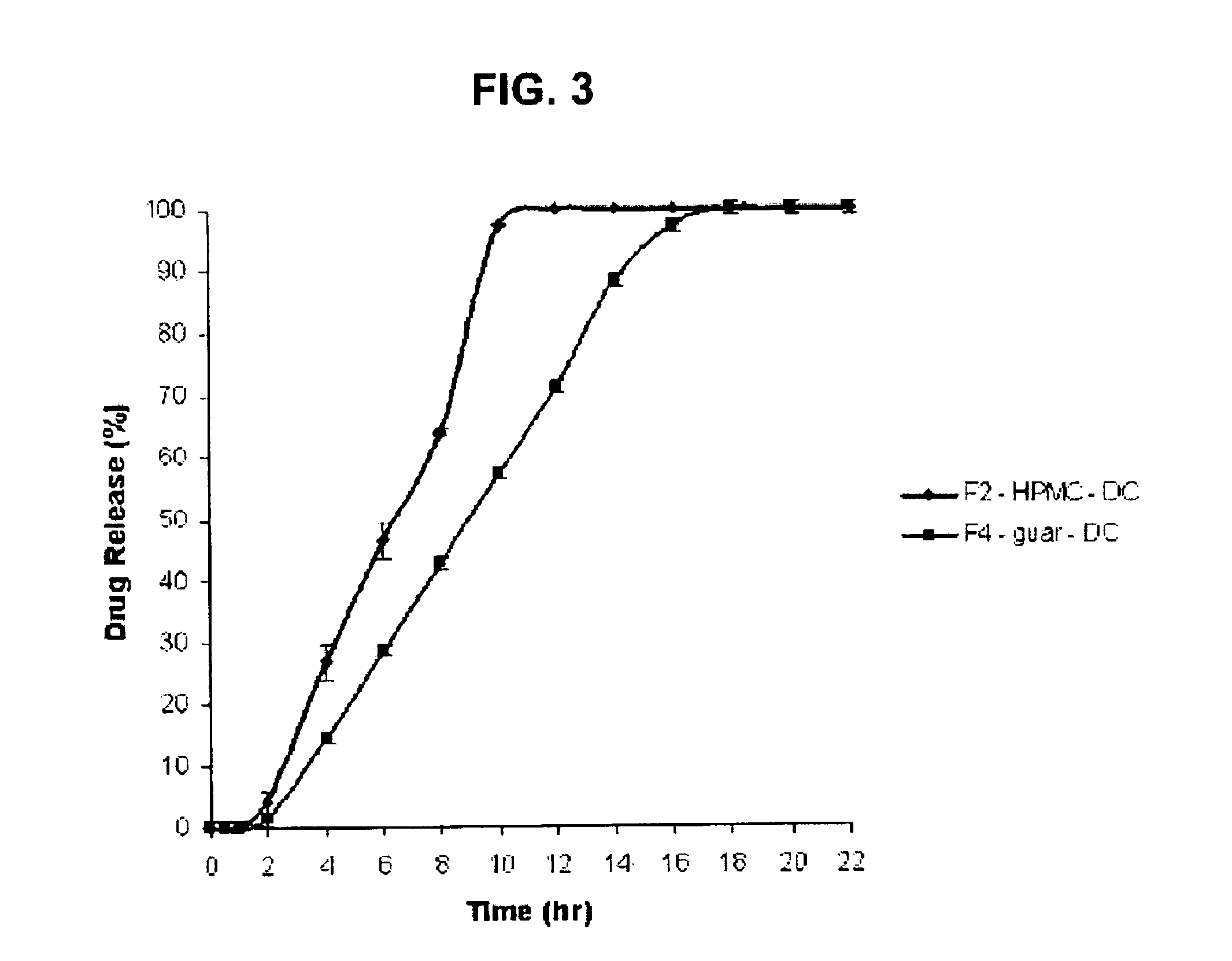
Sustained release oral matrix and methods of making thereof
InactiveUS20130028955A1Sustained releaseAntibacterial agentsBiocideCompressible materialSustained release drug
A solid dosage form suitable for forming a tablet for the containment and delivery of medicament is provided wherein the matrix forming material is a pressure sensitive adhesive, present in the amount from about 0.1 to about 40 weight %, based on the total weight of the composition. The dosage form is comprised of the medicament and a water-insoluble polymer silicone pressure sensitive adhesive and allows release of the medicament in a controlled fashion depending on simple parameters such as weight percent of the polymer silicone adhesive. A sustained release dosage form is provided for delivery of medicament wherein the release rate of medicament does not depend on the dissolution medium of the pH. Another aspect of invention is formation of solid tablets of poorly compressible material and the method for making the solid composition. The dosage form for this invention s particularly suitable for oral dosage forms.
Owner:TOLIA GAURAV THAKERSI
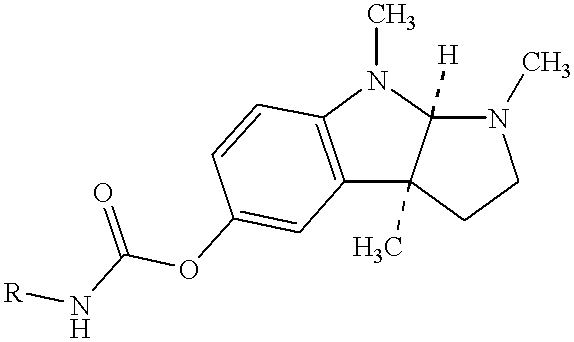
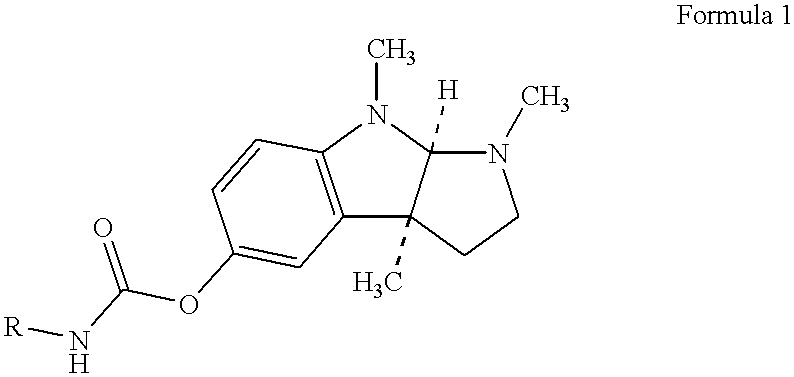
Buccal and sublingual administration of physostigmine
Physostigmine, 1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethylpyrrolo[2,3-b] indol-5ol methylcarbamate, administered buccally or sublingually in non-sustained release dosage form provides extremely prolonged blood levels. This active agent is physically compounded with materials of some or all of classes of ingredients that function as pH controls, preservative agents, viscosity control agents, absorption enhancers, stabilizing agents, solvents, and carrier vehicles. This compounding will produce a pharmaceutical composition in the form of a liquid, tablet, gel, patch or lozenge for administration of the active agent, Physostigmine, by absorption through the buccal or sublingual mucosa of the patient. This method of delivery of Physostigmine and similar compounds is useful for treatment of cognitive deficiencies and / or neurological function deficits, mood and / or mental disturbances in mammals including human beings.
Owner:MADHAT MAHER N
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com