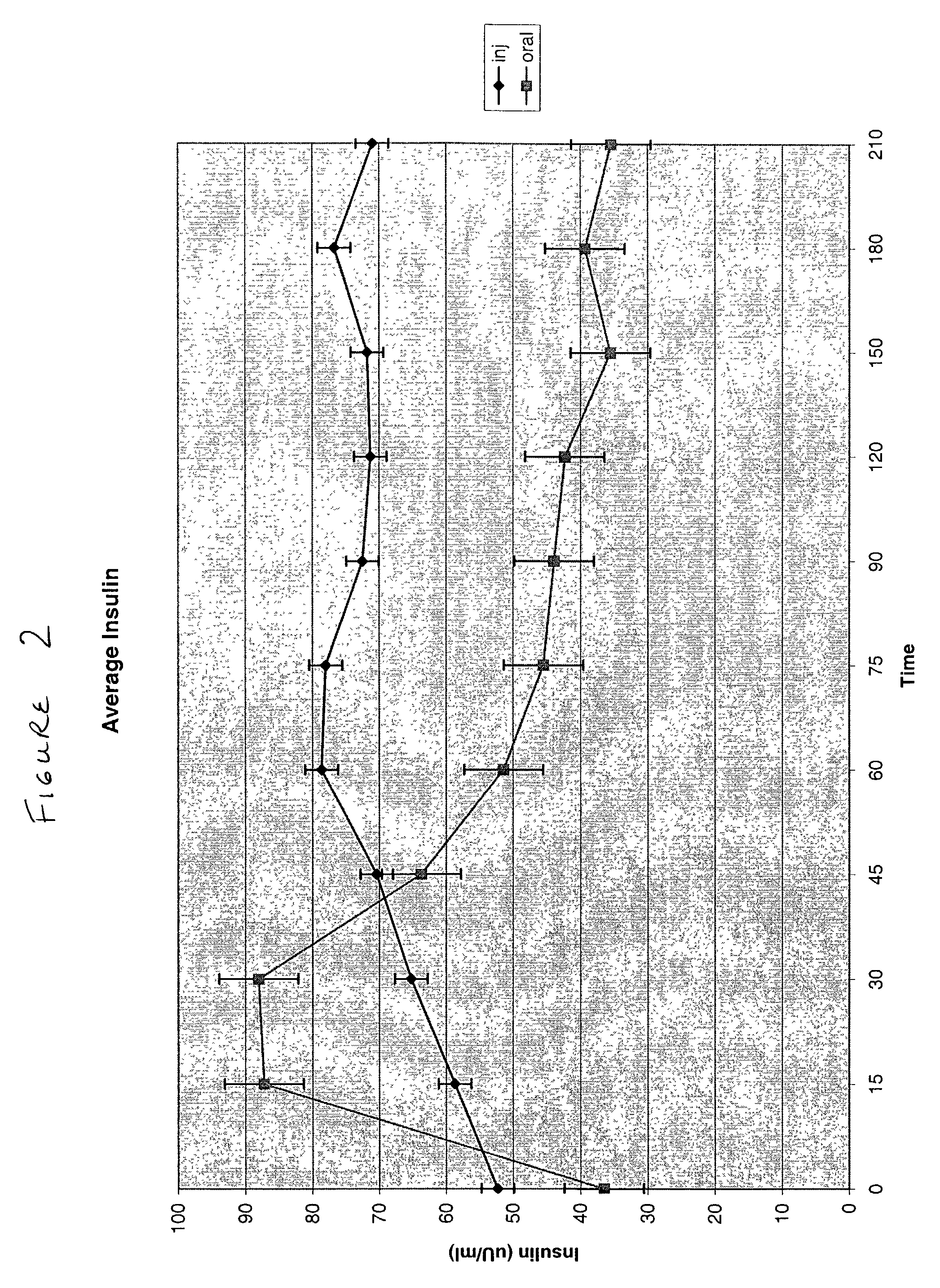
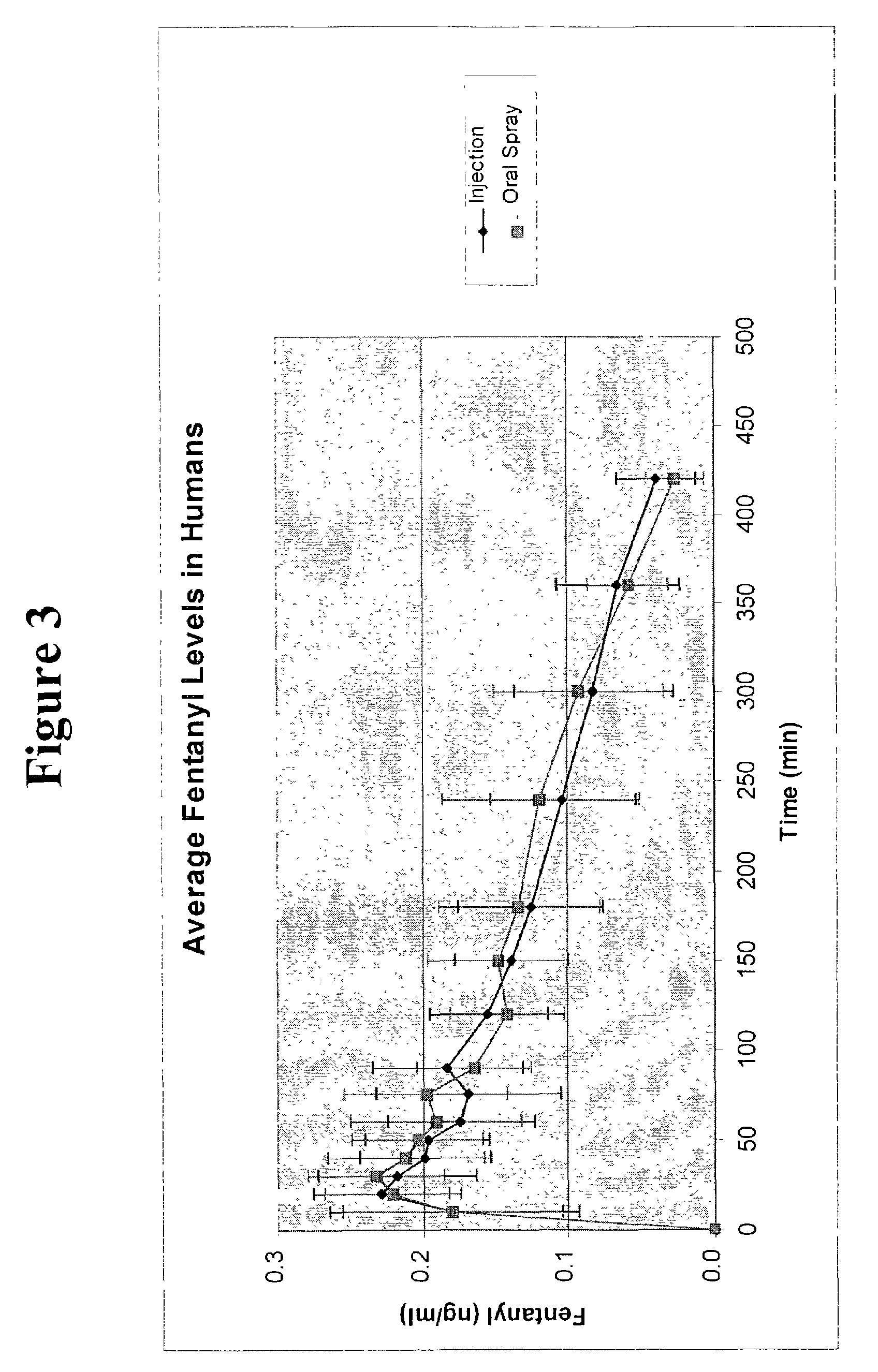
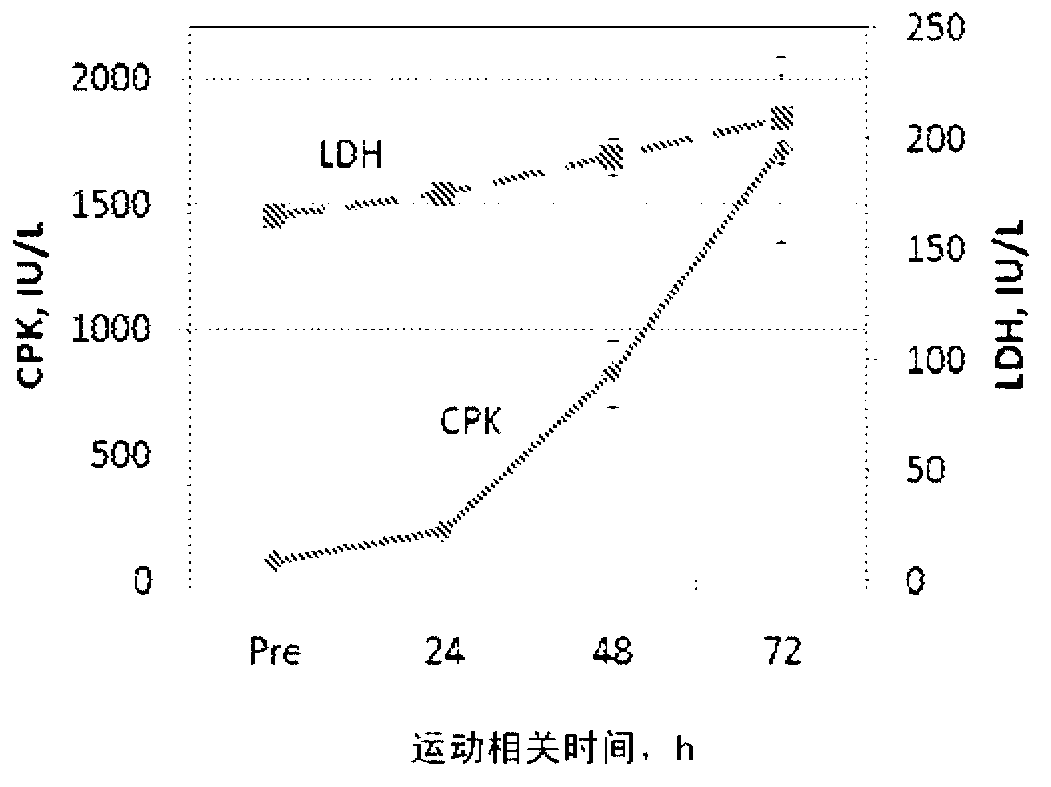
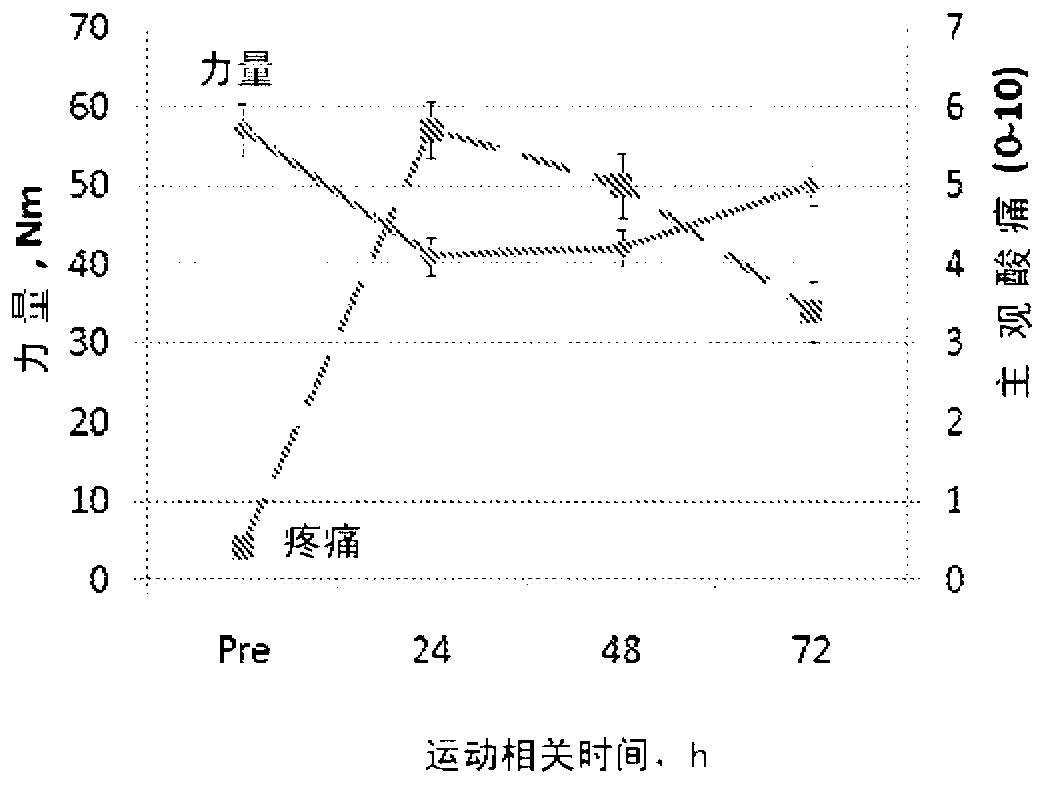
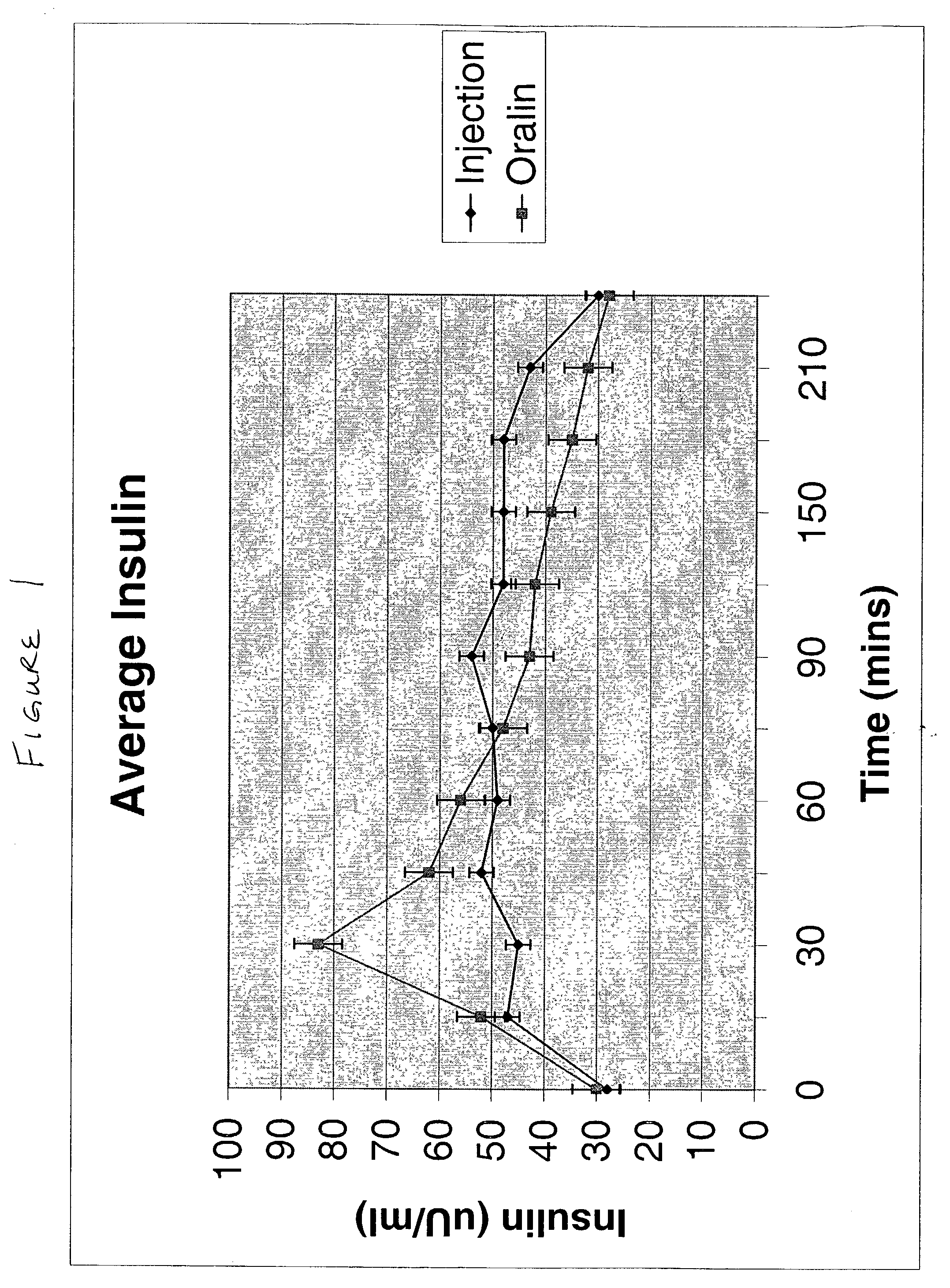
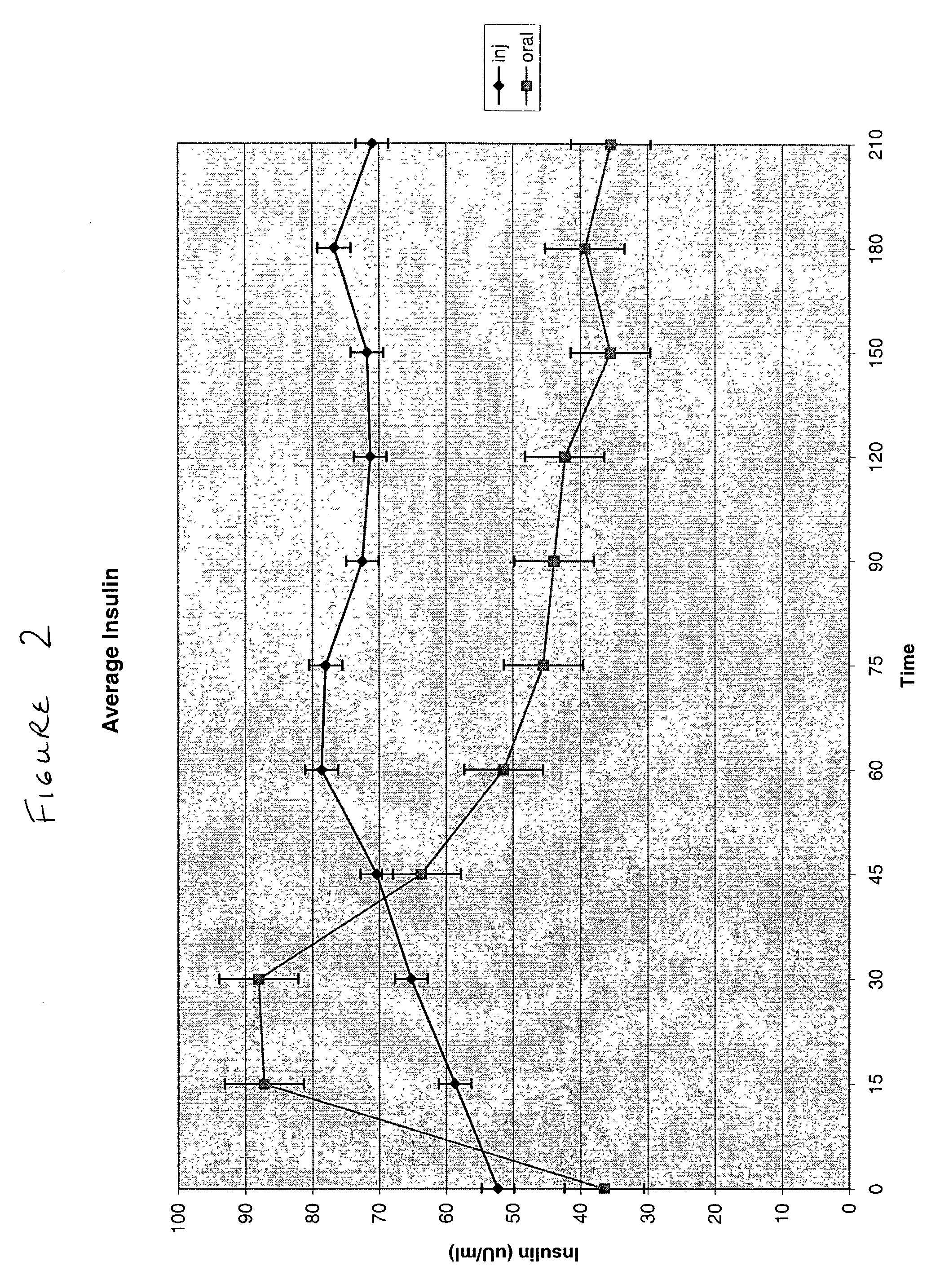
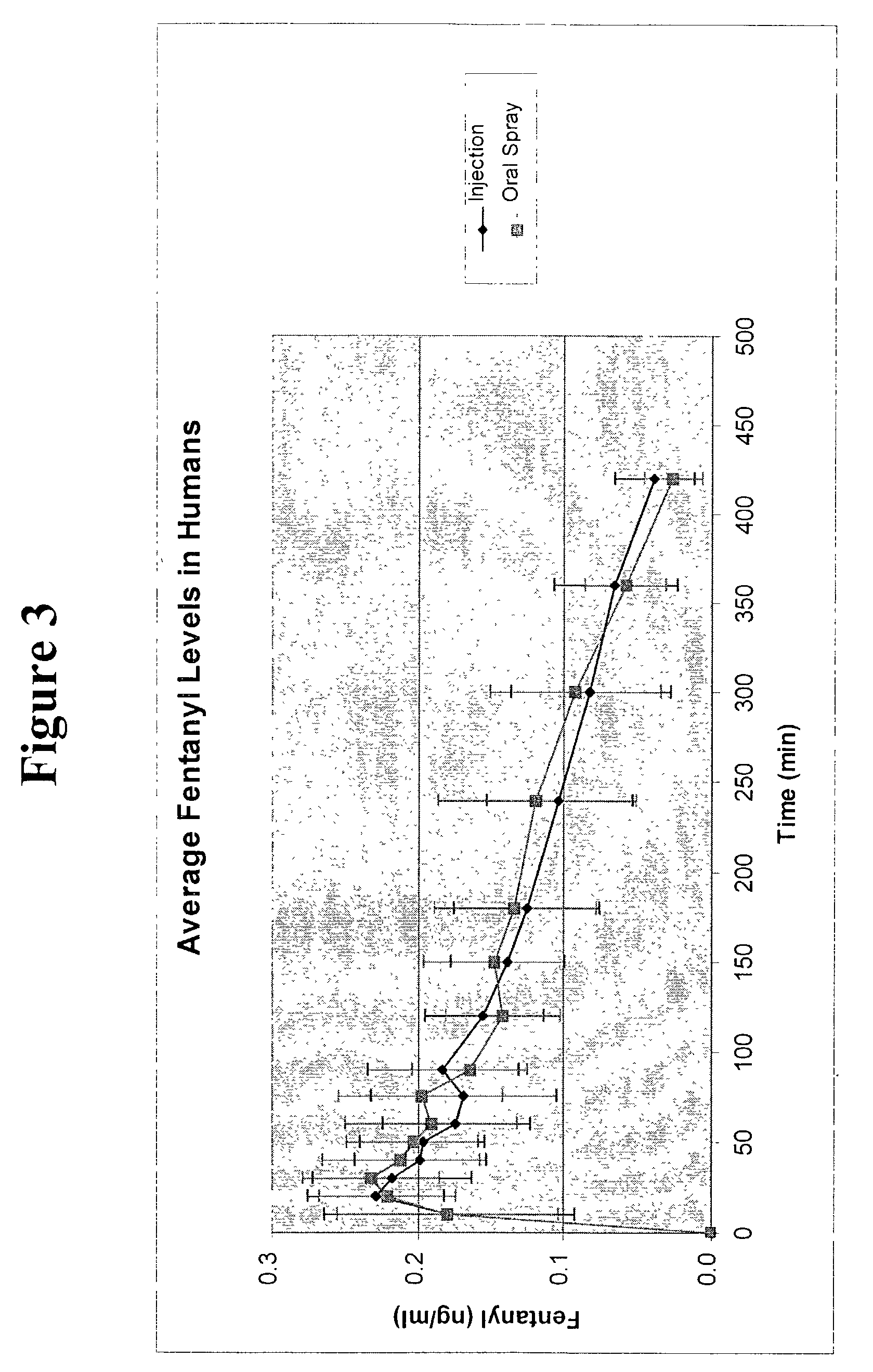
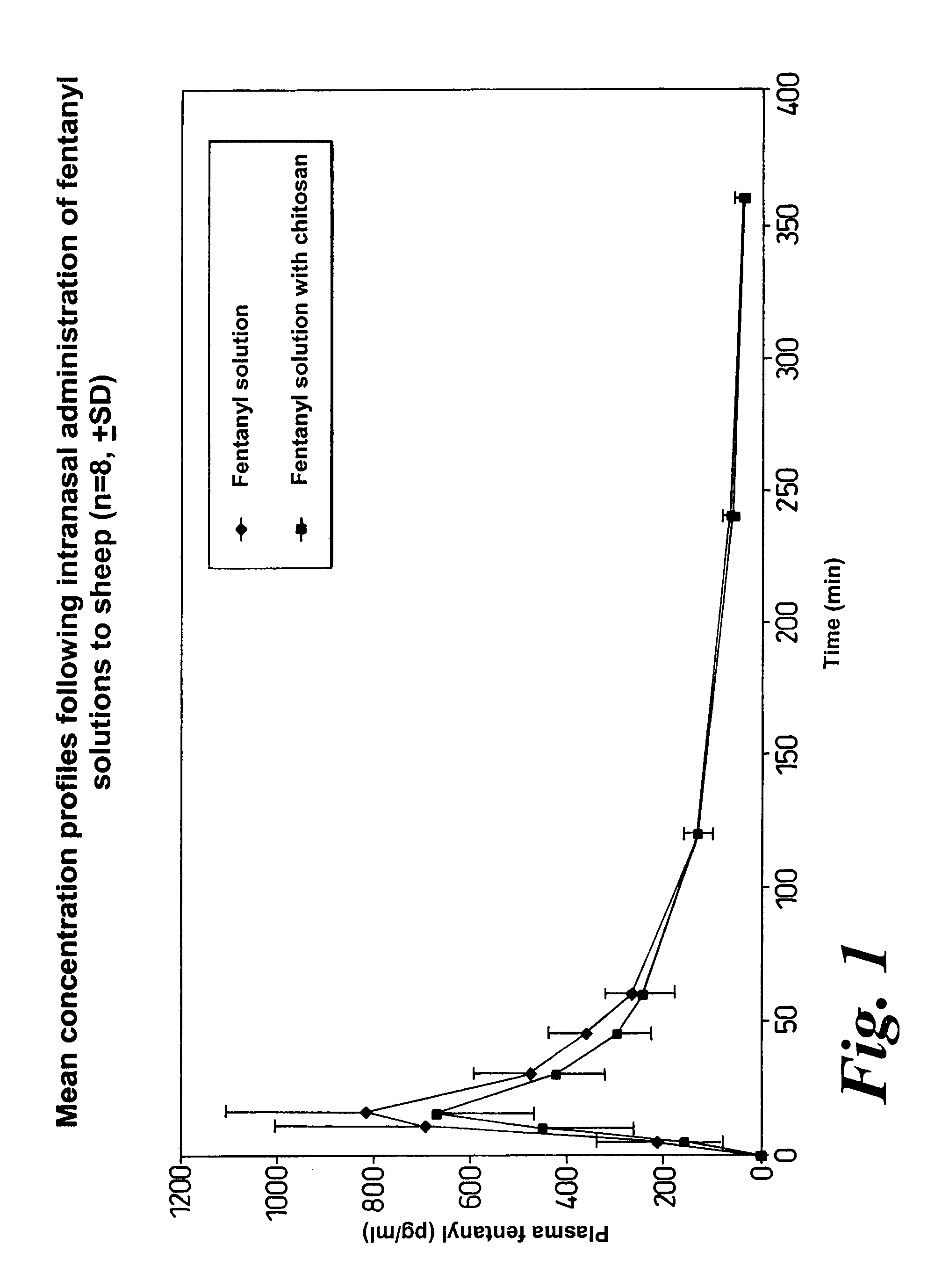
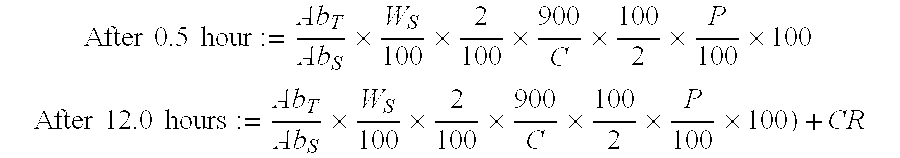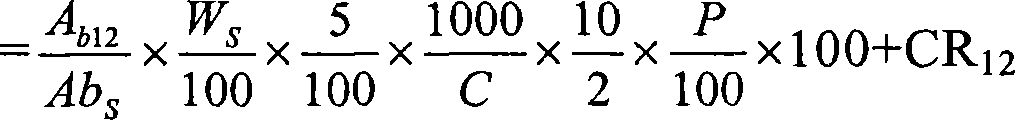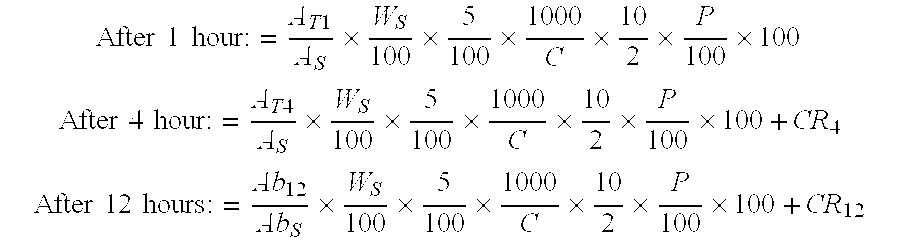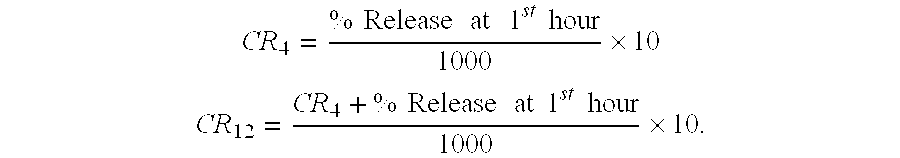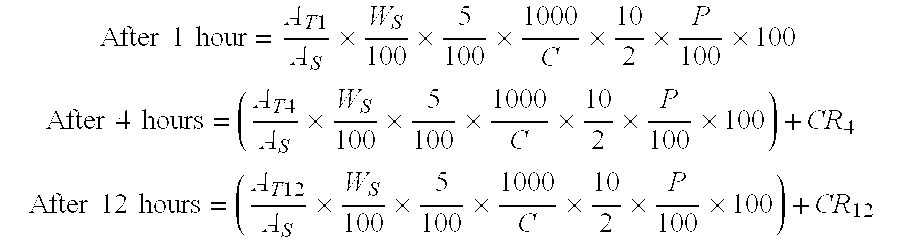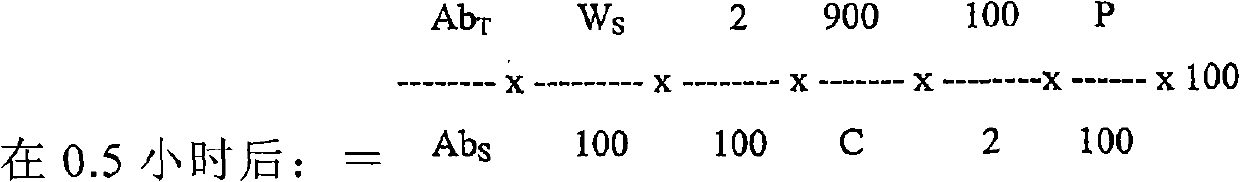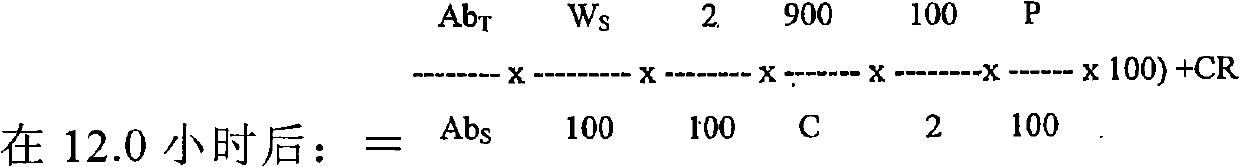Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
50 results about "Peak plasma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
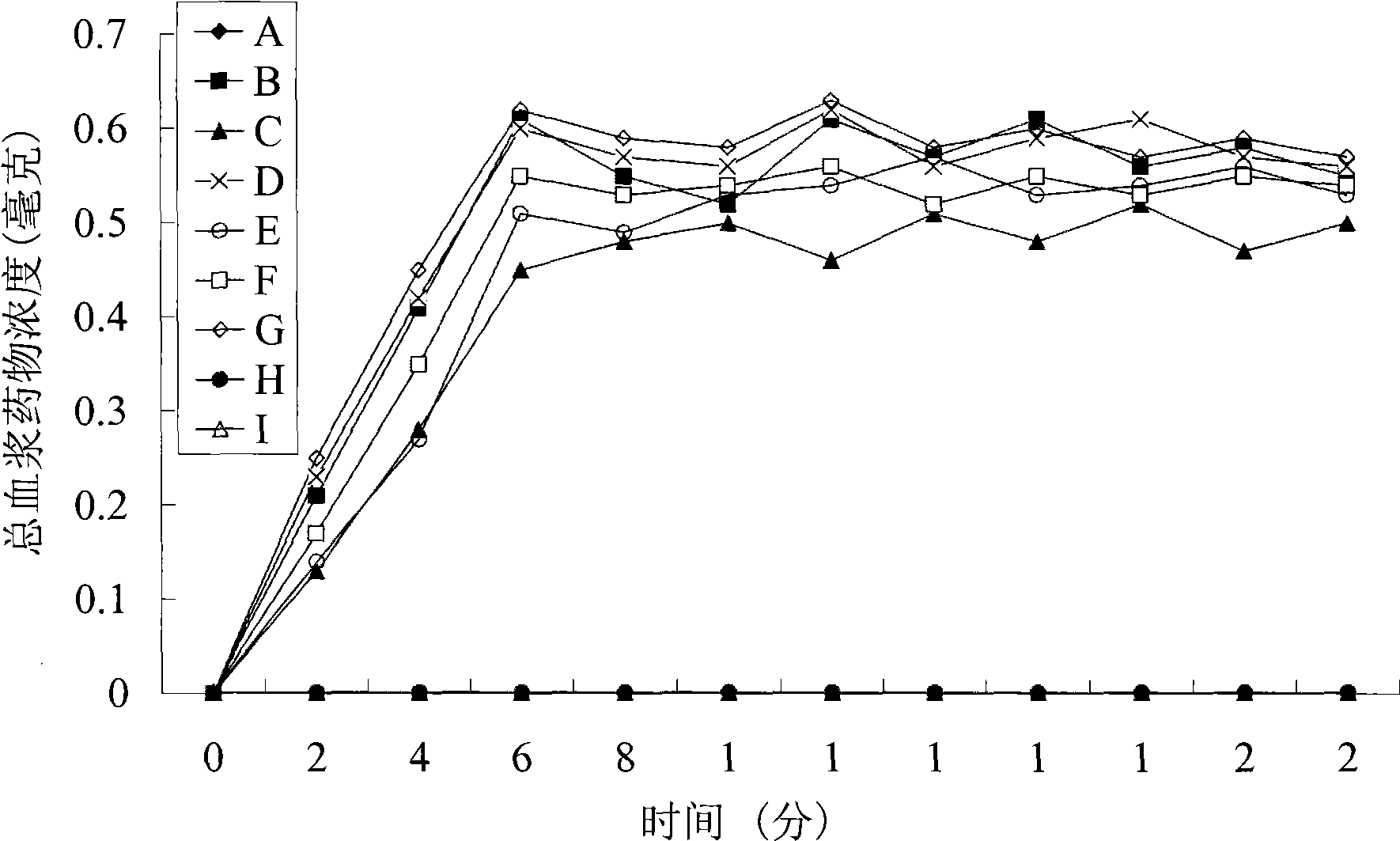
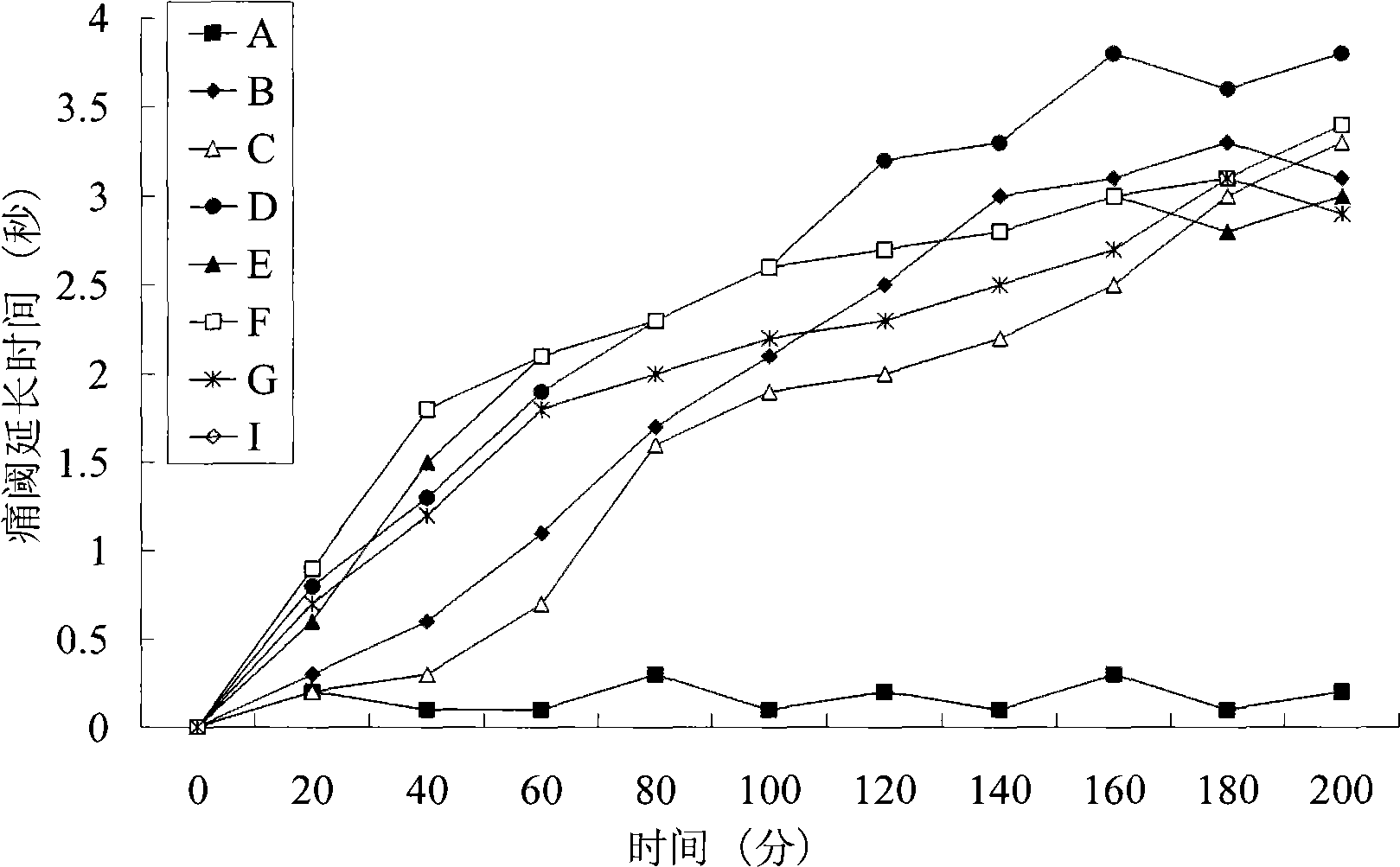
The maximum (peak) plasma drug concentration is the highest level of drug that occurs in the plasma portion of blood after administration of the drug. Another way to say this is that peak plasma concentrations of any drug occurs when the elimination rate equals the rate of absorption.
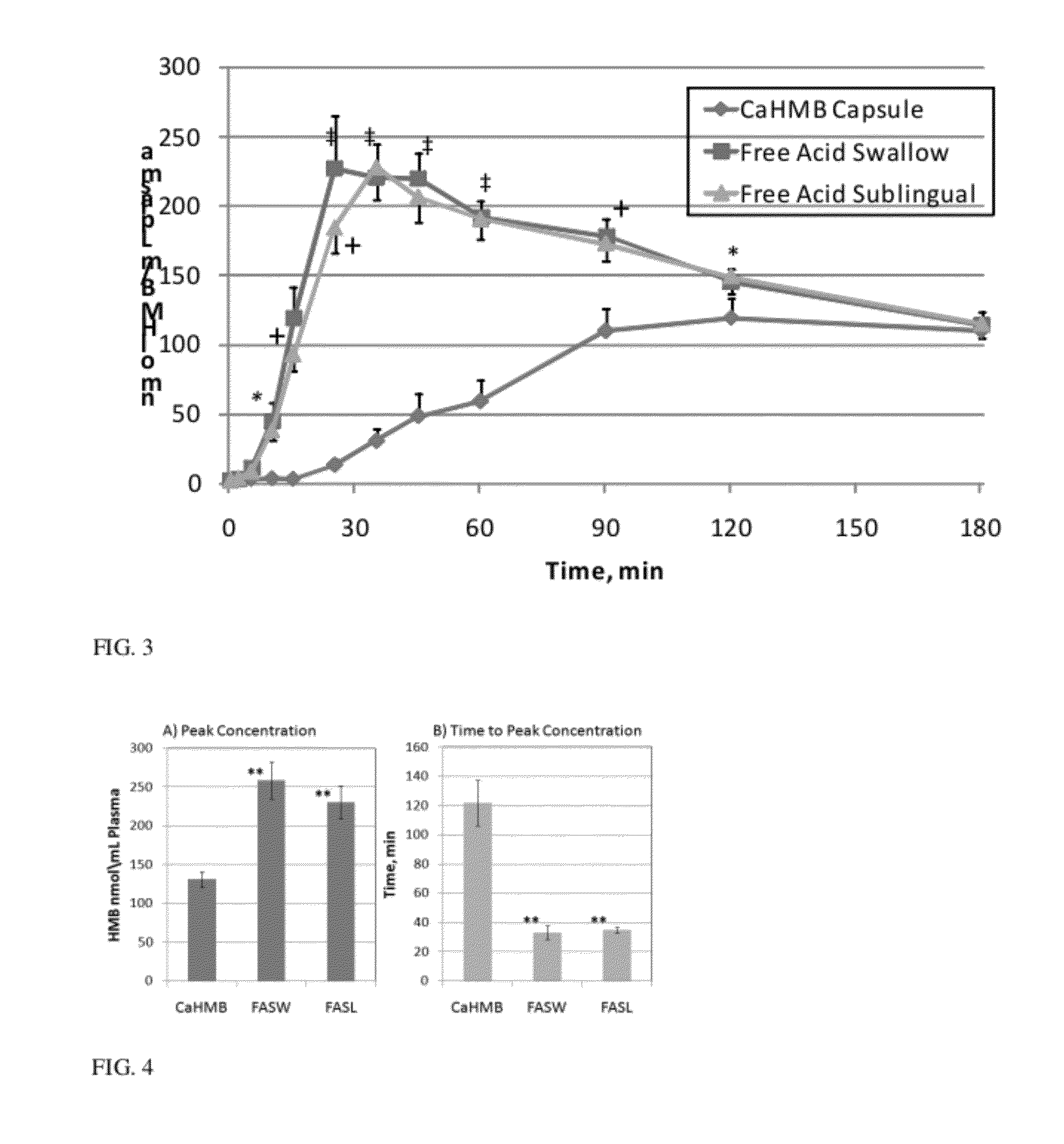
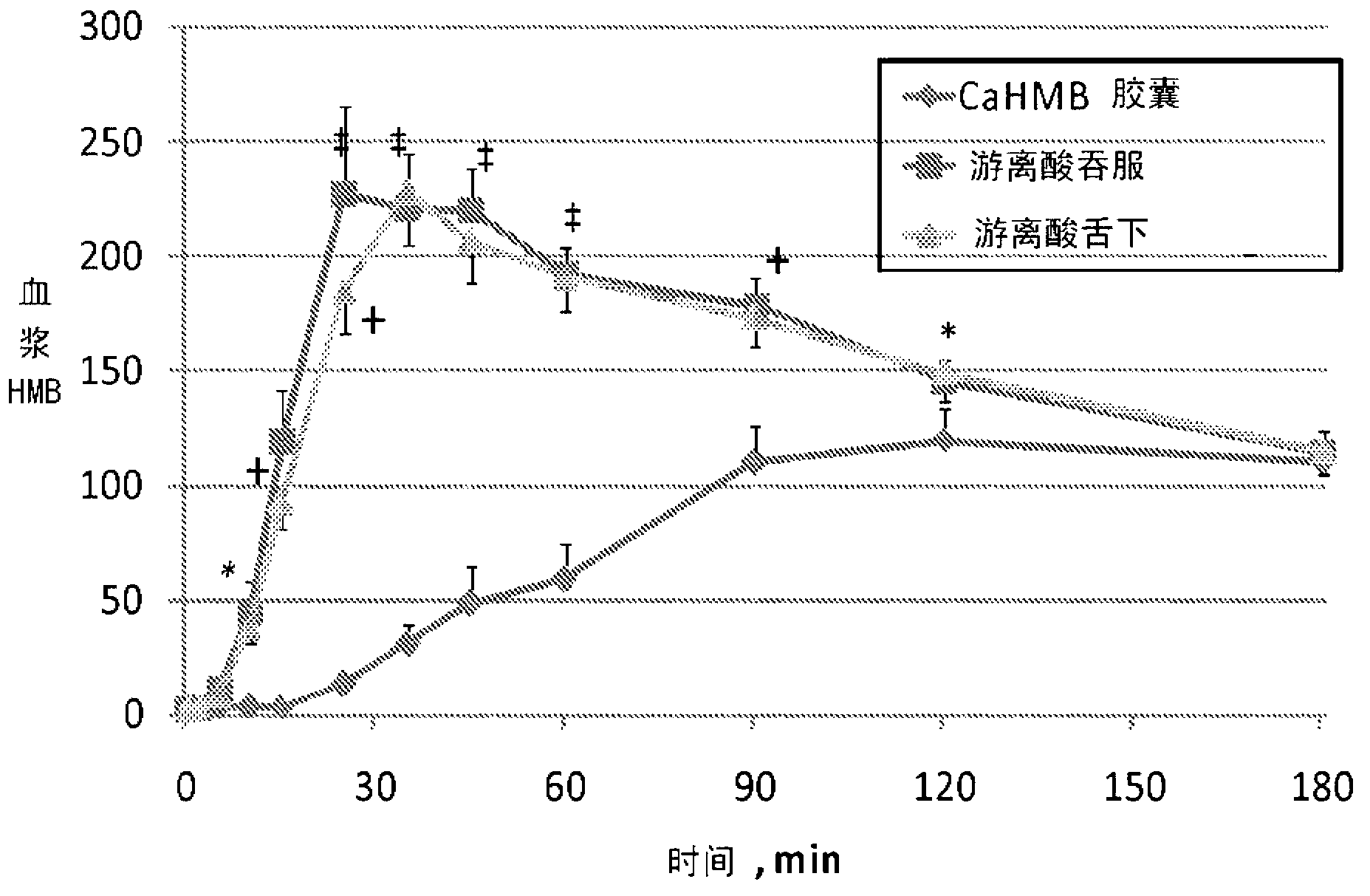
Method of Administering beta-hydroxy-beta-methylbutyrate (HMB)
InactiveUS20120053240A1Improves HMB availabilityFast wayBiocideNervous disorderBlood plasmaBeta-Hydroxy beta-methylbutyric acid
A method of administering beta-hydroxy-beta-methylbutyric acid (HMB) is described, and specifically administering HMB-acid to a person such that the administration of free acid HMB results in an increase in effectiveness of HMB over administration of other forms of HMB, including a calcium salt HMB composition is described. Administration of HMB-acid results in an increase in the peak level of HMB in plasma compared with administration of a similar dose of a calcium salt HMB composition. Administration of HMB-acid results in a faster time to reach peak plasma levels of HMB relative to administration of a similar dosage of a calcium salt HMB composition.
Owner:METABOLIC TECH
Methods and dosage forms for controlled delivery of paliperidone and risperidone
Dosage forms and methods for providing a substantially ascending rate of release of paliperidone or risperidone are provided. The sustained release dosage forms provide therapeutically effective average steady-state plasma paliperidone or risperidone concentrations when administered once per day. This once-a-day dosing regimen results in only one peak plasma paliperidone or risperidone concentration occurrence in each 24 hour period. In addition, the peak plasma paliperidone or risperidone concentration occurs at a later time following dose administration and exhibits a lesser magnitude than the peak plasma paliperidone or risperidone concentration that occurs following administration of paliperidone or risperidone in an immediate-release dosage form.
Owner:ALZA CORP
Method of treating insomnia
A method of treating insomnia comprising administering to a subject a formulation including zaleplon, wherein the formulation is adapted to release the zaleplon after a lag time of at least about one hour after administration of the formulation, and during which substantially no drug substance is released; provide a time of peak plasma concentration of about 3 hours to about 6 hours after administration; provide an elimination half-life after the time of peak plasma concentration of about 0.5 hours to about 0.3 hours; and provide an area under the curve of about 70 ng·h / mL to about 90 ng·h / mL.
Owner:SOMNUS THERAPEUTICS
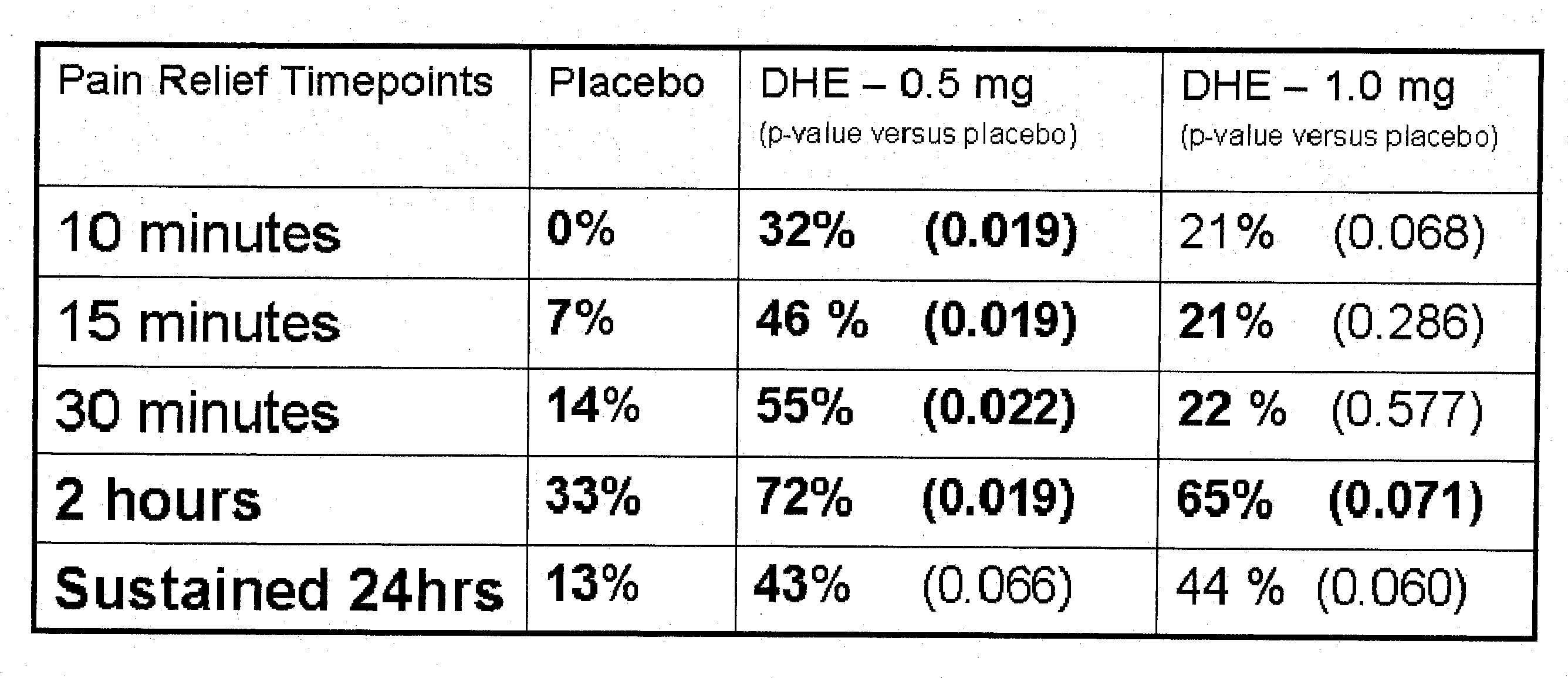
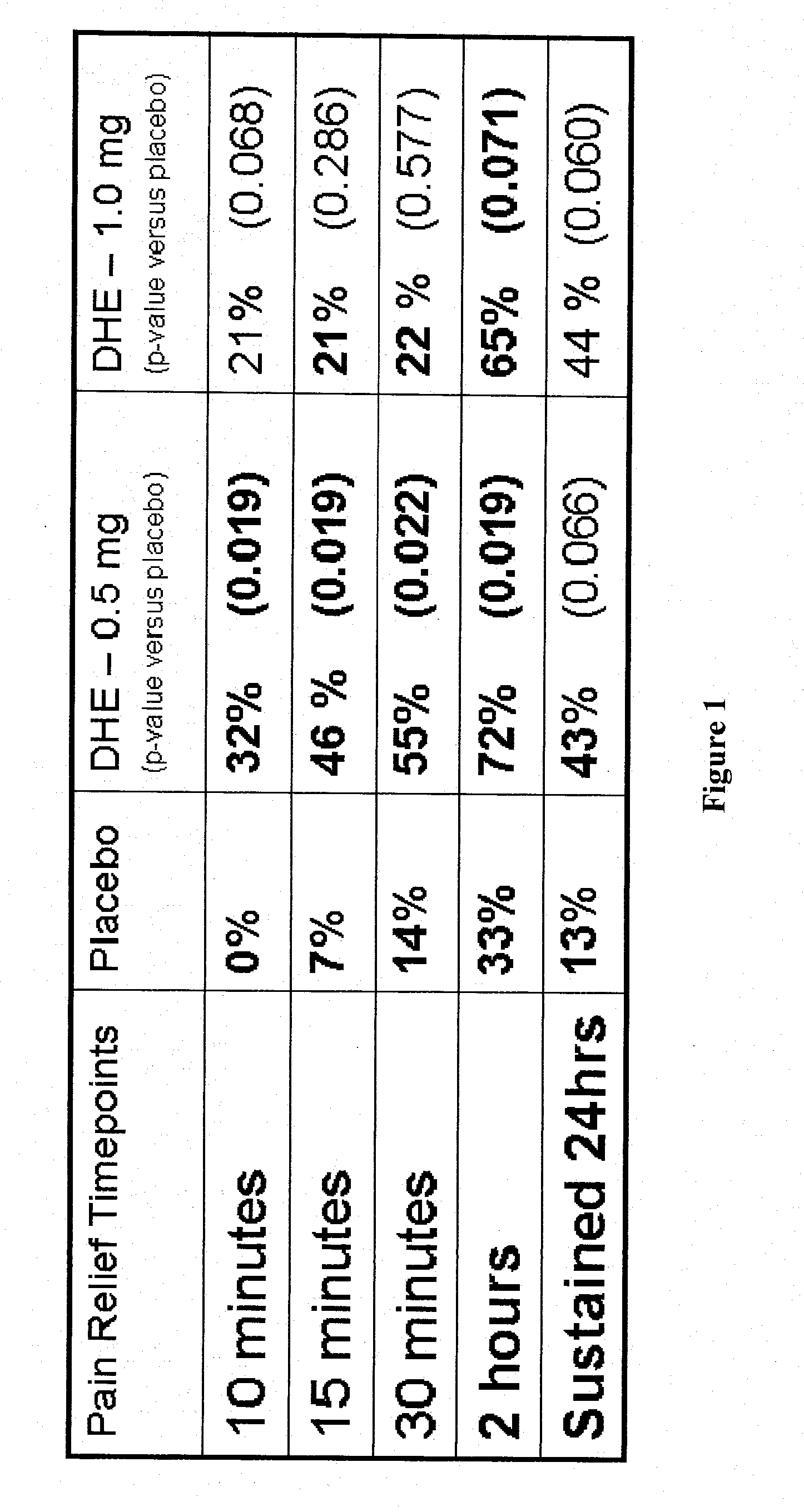
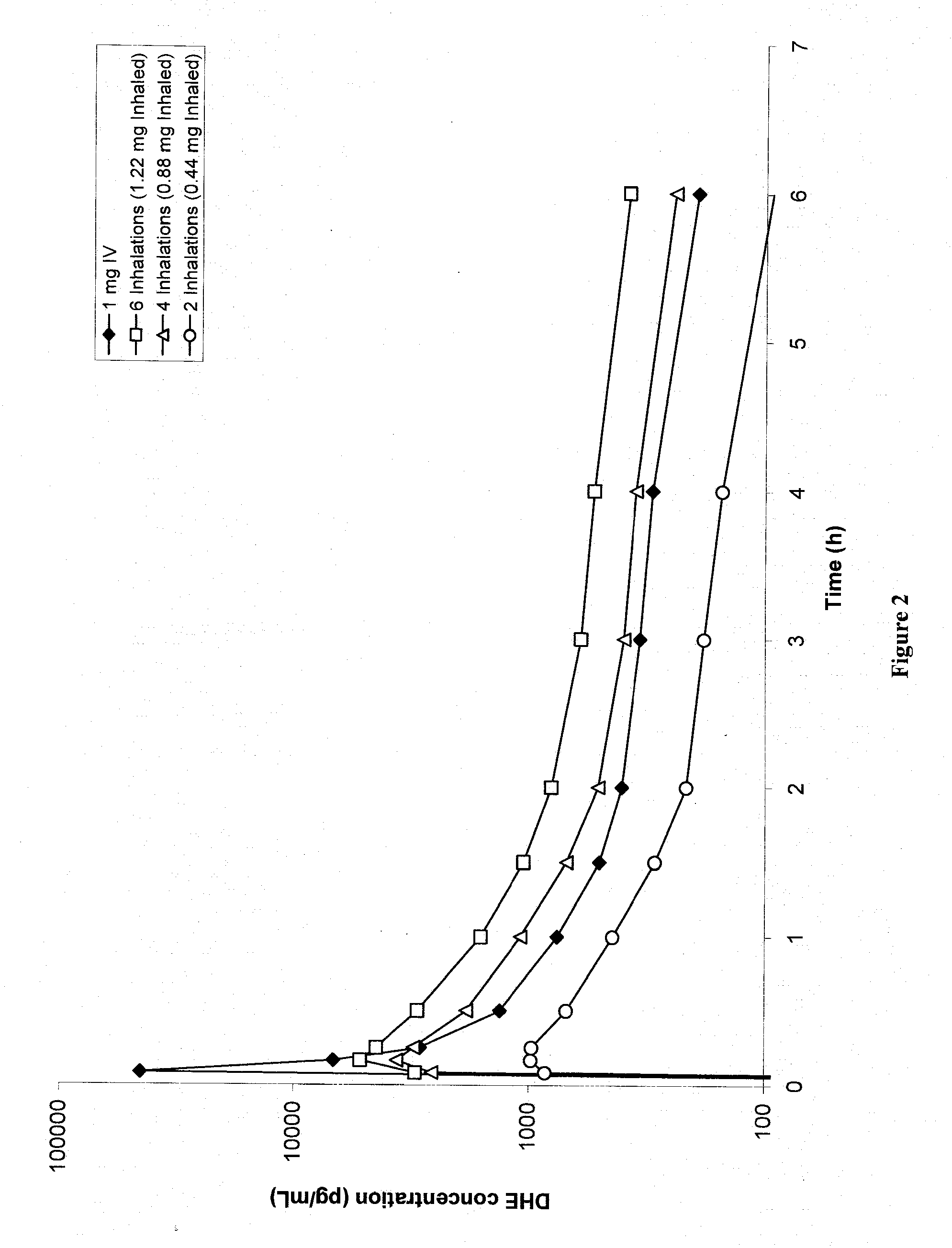
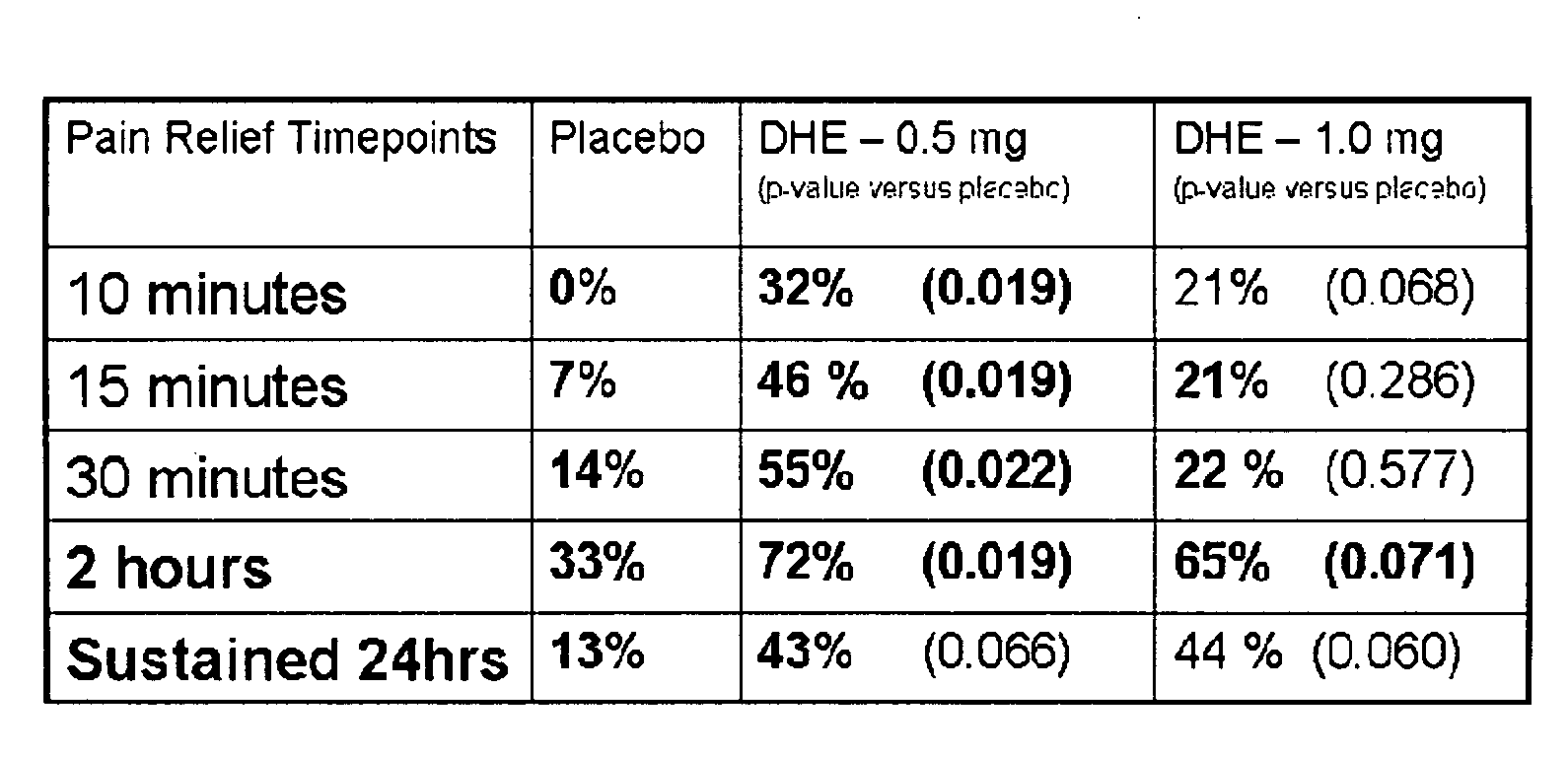
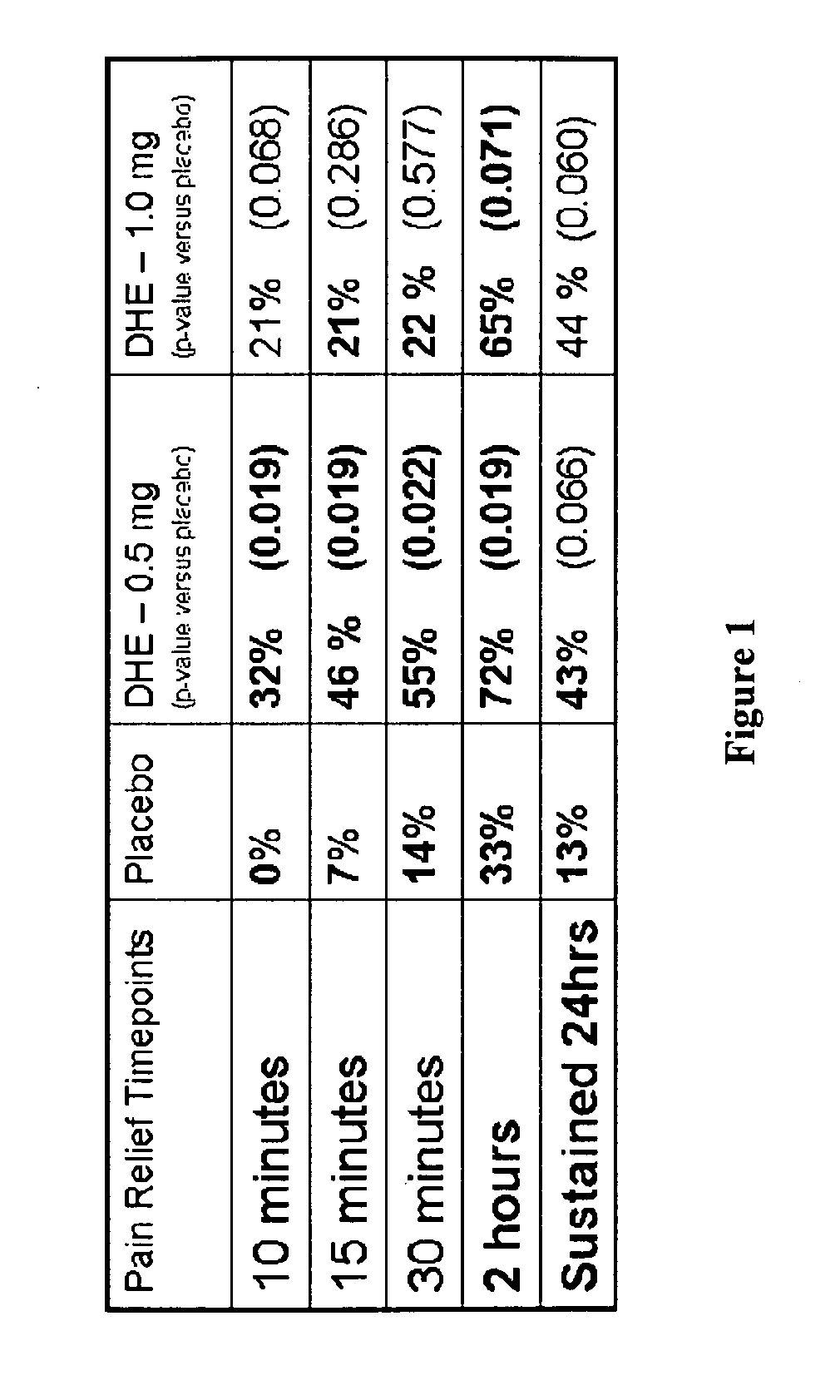
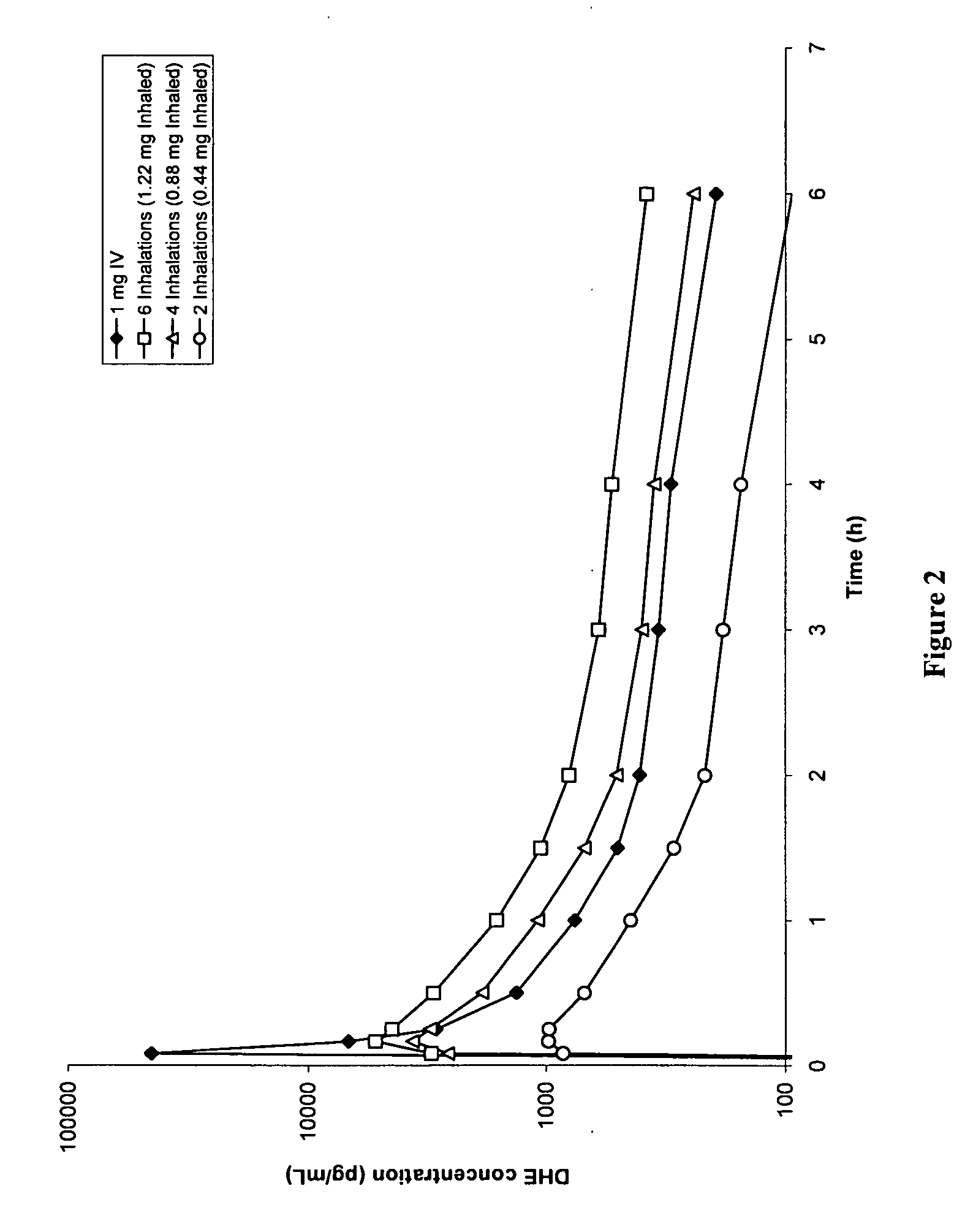
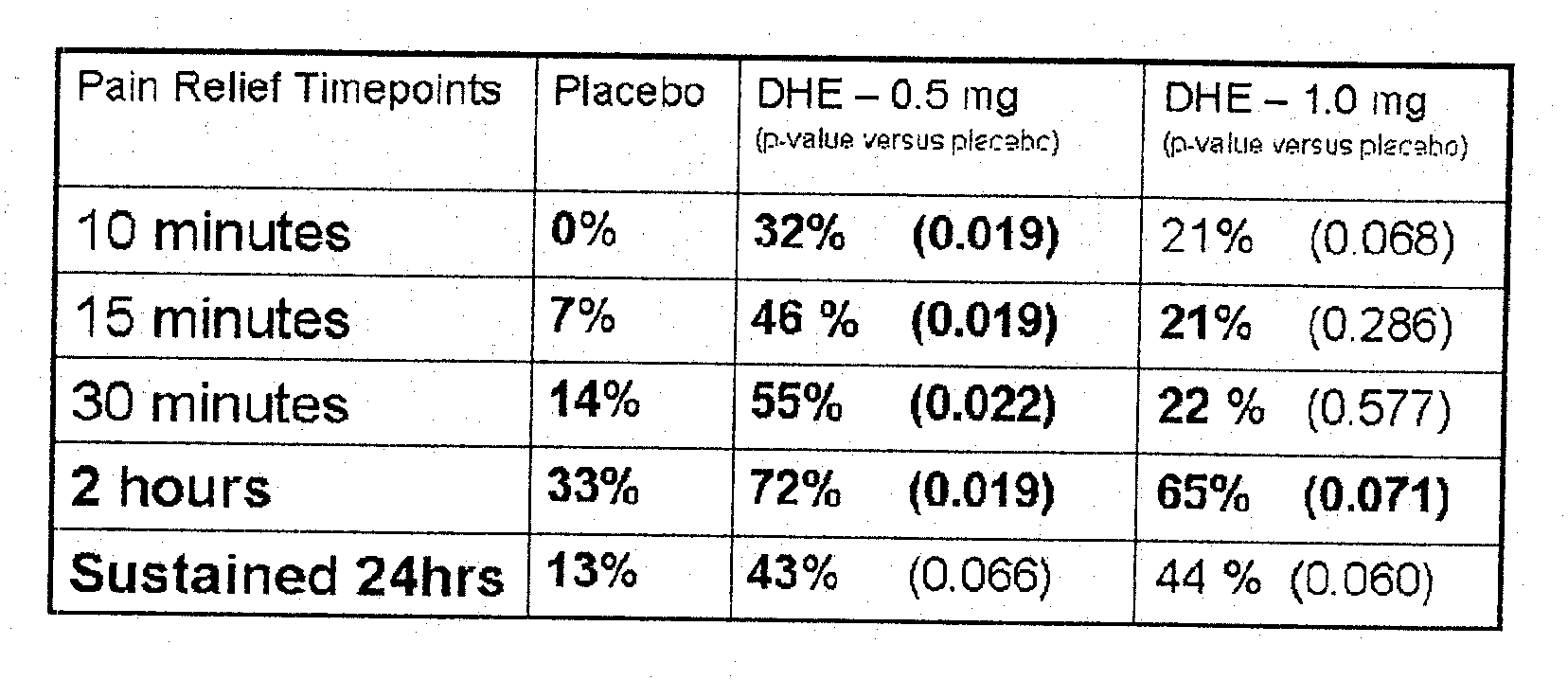
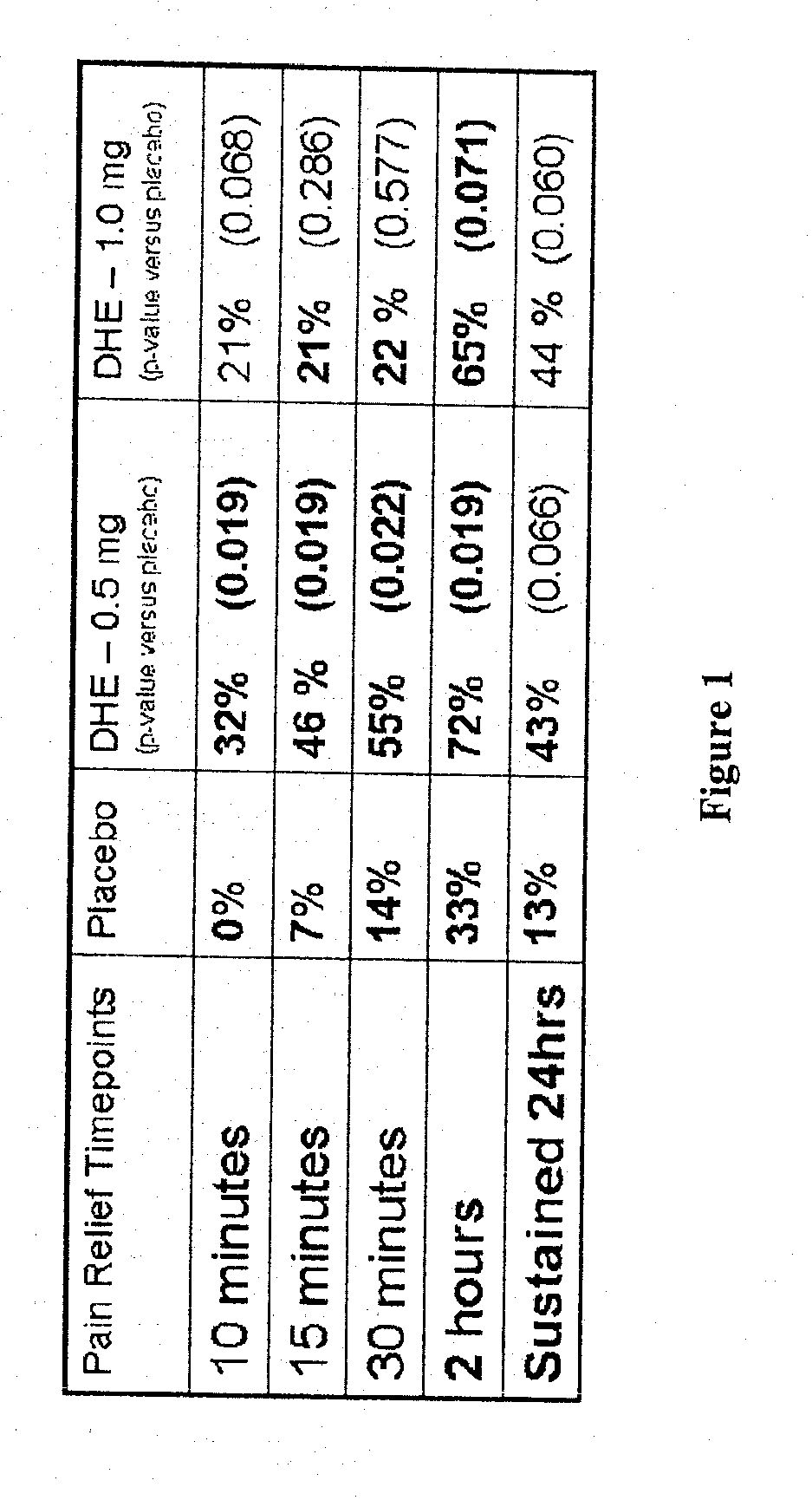
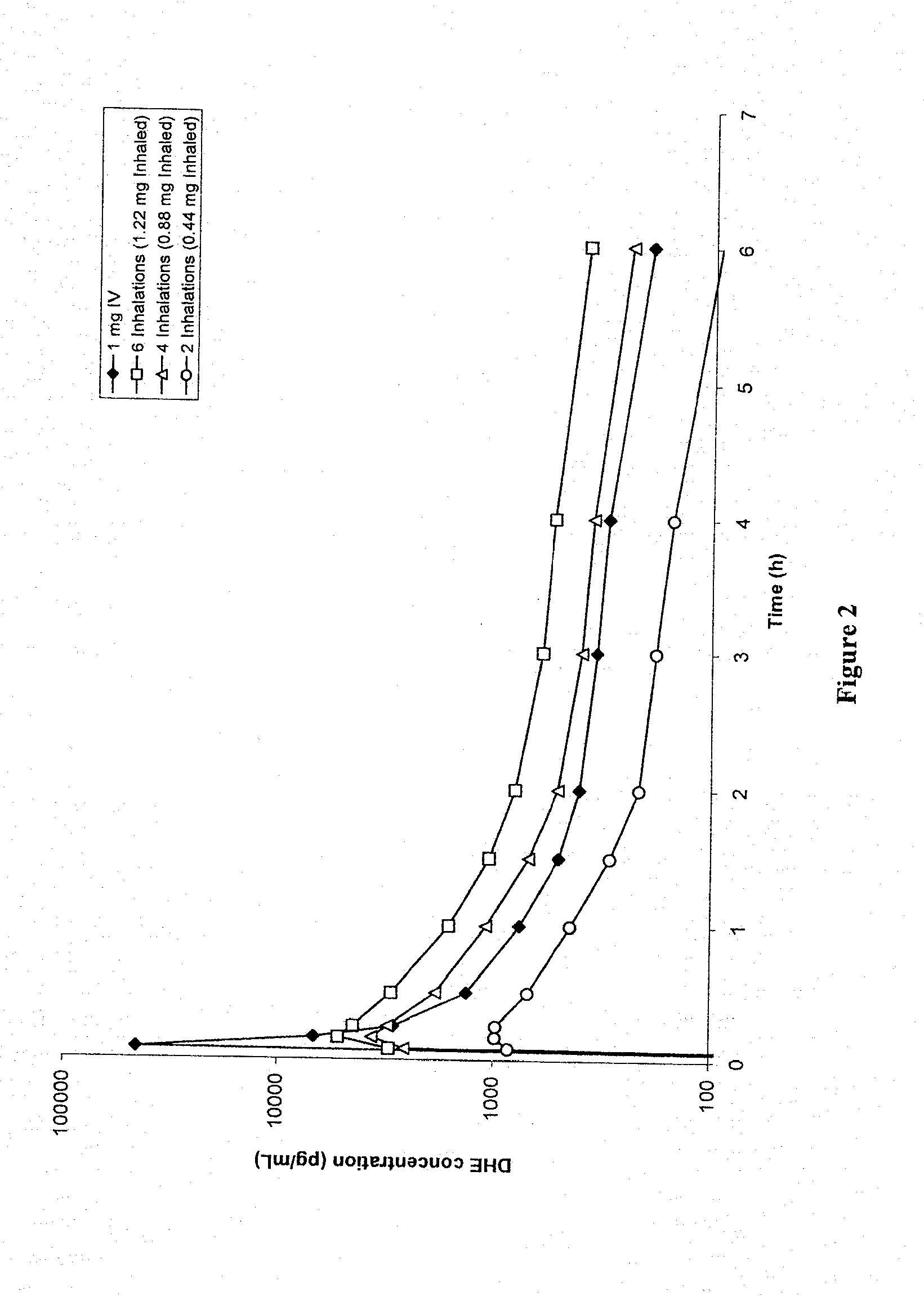
Method of therapeutic administration of dhe to enable rapid relief of migraine while minimizing side effect profile
Pharmaceutical compositions containing dihydroergotamine (DHE) and methods in which DHE is administered to patients for treatment of migraine without side effects or adverse effects are disclosed. Methods for rapid treatment of migraine with DHE are disclosed comprising: dampening the peak plasma concentration (Cmax) and slightly delaying the peak such as to avoid activating the dopaminergic and adrenergic receptors, while achieving sufficient active binding to the serotonin receptors to provide relief from migraine symptoms within a timeframe that permits rapid resolution of migraine symptoms. Inhaler devices suitable for the methods are disclosed. Kits for practicing the methods of invention are disclosed.
Owner:MAP PHARMACEUTICAL INC
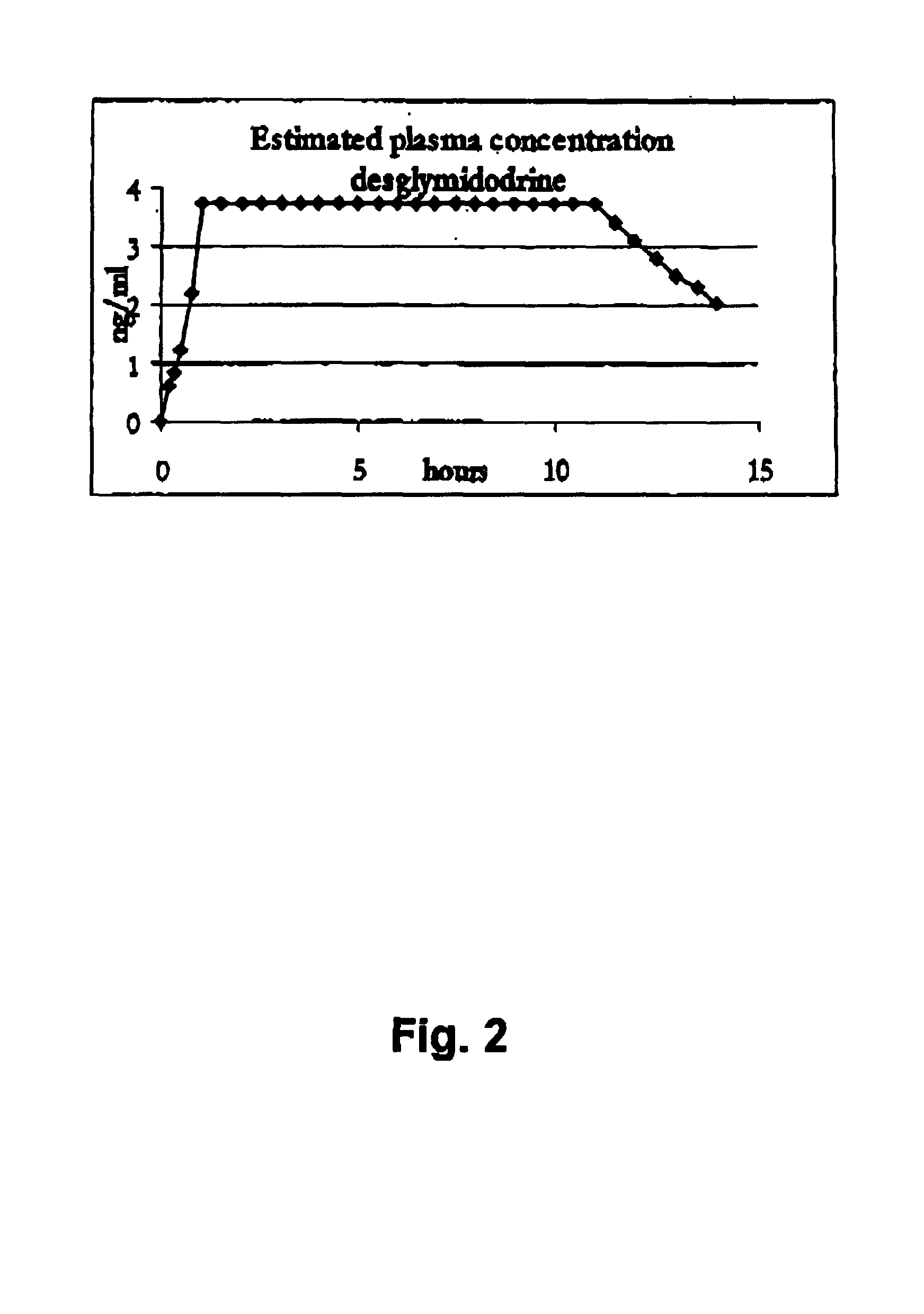
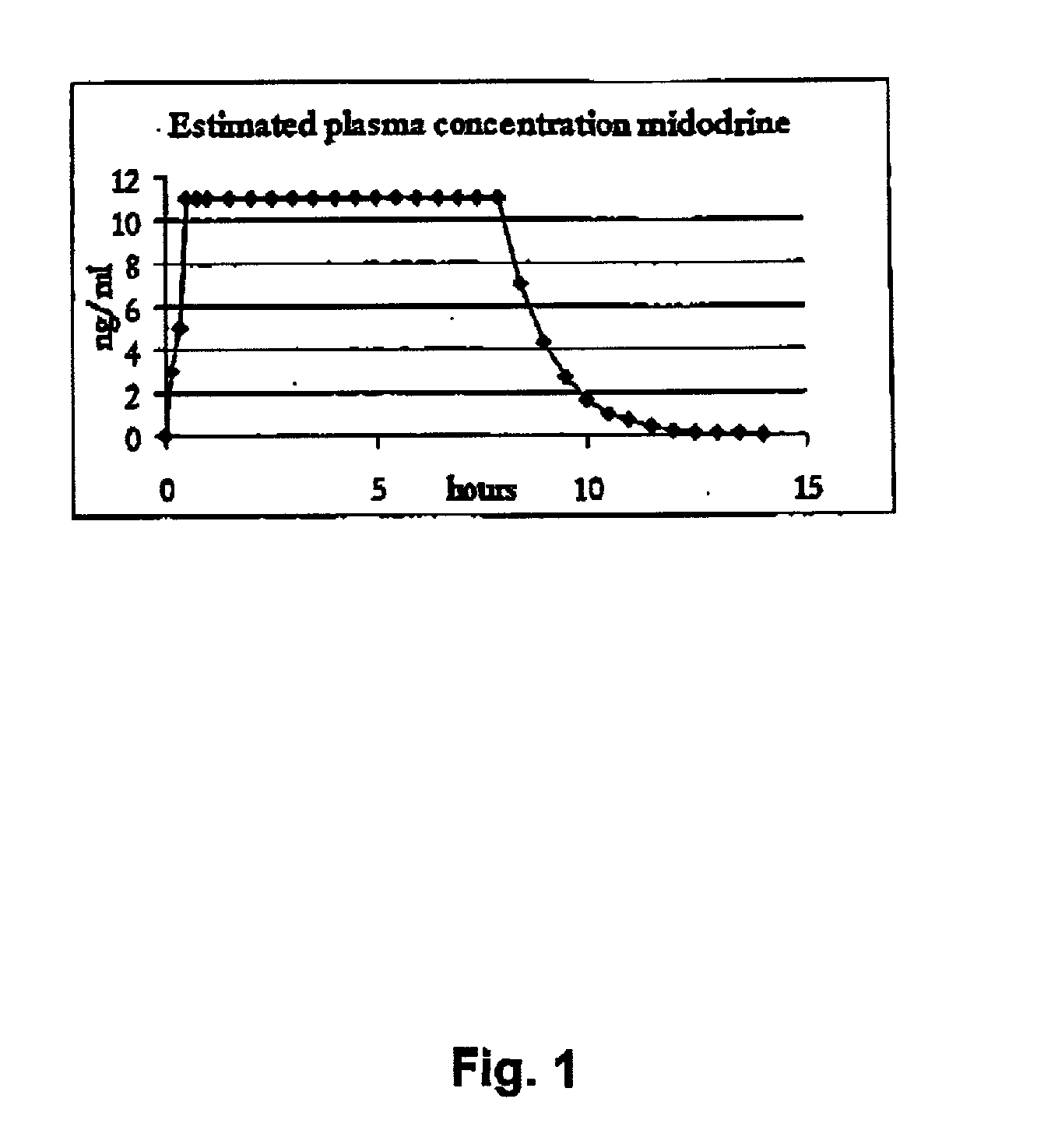
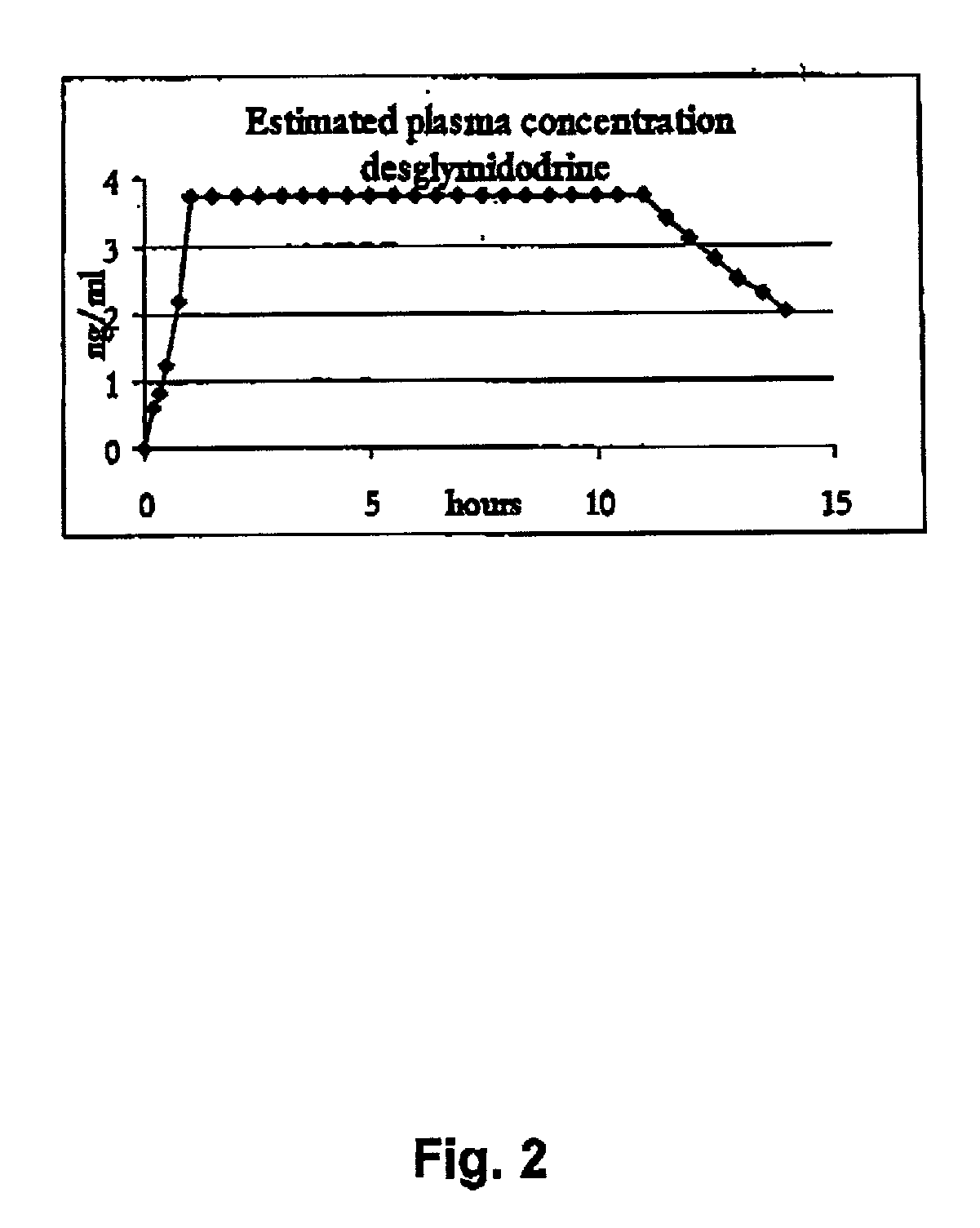
Controlled release pharmaceutical composition for oral use containing midodrine and/or active metabolite, desglymidodrine
InactiveUS7070803B2Increase surface areaEfficient processOrganic active ingredientsNanomedicineSide effectDesglymidodrine
Novel controlled release pharmaceutical compositions for oral use containing midodrine and / or its active metabolite desglymidodrine. The novel compositions are designed to release midodrine and / or desglymidodrine after oral intake in a manner which enables absorption to take place in the gastrointestinal tract so that a relatively fast peak plasma concentration of the active metabolite desglymidodrine is obtained followed by a prolonged and relatively constant plasma concentration of desglymidodrine.The novel compositions may be designed for administration once or twice daily, i.e. a therapeutically effective concentration of desglymidodrine is maintained for a period of at least 10-16 hours followed by a wash out period of about 8-12 hours in order to avoid the well-known midodrine related side effect with respect to supine hypertension. The therapeutically effective concentration of desglymidodrine is regarded as a plasma concentration of desglymidodrine of at least about 3 ng / ml. A composition is designed to release midodrine and / or desglymidodrine in at least the following consecutive steps: i) an initial relatively fast release of midodrine and / or desglymidodrine (in order to obtain a relatively fast onset of action), ii) a steady release or a slower release than in step 1 of midodrine and / or desglymidodrine (in order to maintain a plasma concentration of desglymidodrine which is prolonged and relatively constant), iii) a second rise in release of midodrine and / or desglymidodrine (in order to take advantage of absorption from the colon, i.e. such a second rise release is designed to take place when the composition (or the disintegrated parts of the composition) reaches the colon; normally this is regarded to take about 8 hours after oral intake, and iv) a decline in release rate corresponding to that essentially all midodrine and / or desglymidodrine have been released from the composition.Also disclosed is a method for treating orthostatic hypotension and / or urinary incontinence, the method comprising administration to a patient in need thereof of an effective amount of midodrine and / or desglymidodrine in a composition according to the invention.
Owner:NYCOMED AUSTRIA
Oral controlled release pharmaceutical composition containing metaxalone as active agent
The present invention provides an oral controlled release pharmaceutical composition comprising metaxalone, a pharmaceutically acceptable release rate controlling excipient, and pharmaceutically acceptable excipients, wherein the oral controlled release pharmaceutical composition provides peak plasma levels at a time of about 3 hours or more after oral administration of the composition.
Owner:SUN PHARMA INDS
Pharmaceutical kit comprising midodrine as active drug substance
InactiveUS20020193445A1Fast curative effectRapid onsetBiocidePowder deliveryHigh concentrationSide effect
Novel phannaceutcal kit comprising a controlled release pharmaceutical compositions for oral use containing midodrine and / or its active metabolite desglymidodrine and a relatively fast onset composition. The controlled release compositions are designed to release midodrine and / or desglymidodrine after oral intake in a manner which enables absorption to take place in the gastrointestinal tract so that a relatively fast peak plasma concentration of the active metabolite desglymidodrine is obtained followed by a prolonged and relatively constant plasma concentration of desglymidodrine. The controlled release compositions may be designed for administration once or twice daily, i.e. a therapeutically effective concentration of desglymidodrine is maintained for a period of at least 10-16 hours followed by a wash out period of about 8-12 hours in order to avoid the well-known midodrine related side effect with respect to supine hypertension. The therapeutically effective concentration of desglymidodrine is regarded as a plasma concentration of desglymidodrine of at least about 3 ng / ml. A composition is designed to release midodrine and / or desglymidodrine in at least the following consecutive steps; i) an initial relatively fast release of midodrine and / or desglymidodrine (in order to obtain a relatively fast onset of action), ii) a steady release or a slower release than in step 1 of midodrine and / or desglymidodrine (in order to maintain a plasma concentration of desglymidodrine which is prolonged and relatively constant), iii) a second rise in release of midodrine and / or desglymidodrine (in order to take advantage of absorption from the colon, i.e. such a second rise release is designed to take place when the composition (or the disintegrated parts of the composition) reaches the colon; normally this is regarded to take about 8 hours after oral intake, and iv) a decline in release rate corresponding to that essentially all midodrine and / or desgtymidodrine have been released from the composition. One of the advantages of the invention is that the controlled release composition provides a base line plasma concentration, which during most of the day is therapeutically effective. When a higher concentration is needed, only a minor supply of active drug substance is necessary to obtain a very fast relief from symptoms. If the constant base line plasma concentration was absent, it would be necessary to use a relative higher fast onset dose to reach the high therapeutically effective level. The kit according to the present invention is a superior tool for obtaining an optimal treatment with a minimum of active drug substance. Also disclosed is a method for treating orthostaic hypotension and / or urinary incontinence, the method comprising administration to a patient in need thereof of an effective amount of midodrine and / or desglymidodrine in a kit according to the invention.
Owner:NYCOMED AUSTRIA
Method of therapeutic administration of DHE to enable rapid relief of migraine while minimizing side effect profile
Pharmaceutical compositions containing dihydroergotamine (DHE) and methods in which DHE is administered to patients for treatment of migraine without side effects or adverse effects are disclosed. Methods for rapid treatment of migraine with DHE are disclosed comprising: dampening the peak plasma concentration (Cmax) and slightly delaying the peak such as to avoid activating the dopaminergic and adrenergic receptors, while achieving sufficient active binding to the serotonin receptors to provide relief from migraine symptoms within a timeframe that permits rapid resolution of migraine symptoms. Inhaler devices suitable for the methods are disclosed. Kits for practicing the methods of invention are disclosed.
Owner:MAP PHARMACEUTICAL INC
Methods and dosage forms for controlled delivery of paliperidone and risperidone
InactiveUS20050232995A1Eliminate side effectsImprove development of toleranceOrganic active ingredientsBiocideDosing regimenRegimen
Dosage forms and methods for providing a substantially ascending rate of release of paliperidone or risperidone are provided. The sustained release dosage forms provide therapeutically effective average steady-state plasma paliperidone or risperidone concentrations when administered once per day. This once-a-day dosing regimen results in only one peak plasma paliperidone or risperidone concentration occurrence in each 24 hour period. In addition, the peak plasma paliperidone or risperidone concentration occurs at a later time following dose administration and exhibits a lesser magnitude than the peak plasma paliperidone or risperidone concentration that occurs following administration of paliperidone or risperidone in an immediate-release dosage form.
Owner:ALZA CORP
Method of therapeutic administration of dhe to enable rapid relief of migraine while minimizing side effect profile
Pharmaceutical compositions containing dihydroergotamine (DHE) and methods in which DHE is administered to patients for treatment of migraine without side effects or adverse effects are disclosed. Methods for rapid treatment of migraine with DHE are disclosed comprising: dampening the peak plasma concentration (Cmax) and slightly delaying the peak such as to avoid activating the dopaminergic and adrenergic receptors, while achieving sufficient active binding to the serotonin receptors to provide relief from migraine symptoms within a timeframe that permits rapid resolution of migraine symptoms. Inhaler devices suitable for the methods are disclosed. Kits for practicing the methods of invention are disclosed.
Owner:MAP PHARMACEUTICAL INC
Methods of administering and enhancing absorption of pharmaceutical agents
InactiveUS7087215B2Improve bioavailabilityEffective penetrationAntibacterial agentsOrganic active ingredientsSulfateMixed micelle
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in mixed micellar form are disclosed. The mixed micelles are formed from an alkali metal alkyl sulfate, and at least three different micelle-forming compounds as described in the specification. Micelle size ranges between about 1 and 10 nanometers. Methods for administering the compositions are also disclosed. A preferred method for administering the present composition is through the buccal region of the mouth, which has been demonstrated to achieve peak plasma levels of the pharmaceutical agent in about thirty minutes.
Owner:GENEREX PHARMA INC
Improved method of administering beta-hydroxy-beta-methylbutyrate (hmb)
A method of administering beta-hydroxy-beta-methylbutyric acid (HMB) is described, and specifically administering HMB-acid to a person such that the administration of free acid HMB results in an increase in effectiveness of HMB over administration of free acid HMB results in an increase in effectiveness of HMB over administration of other forms of HMB, including a calcium salt HMB composition is described. Administration of HMB-acid results in an increase in the peak level of HMB in plasma compared with administration of a similar dose of a calcium salt HMB composition. Administration of HMB-acid results in a faster time to reach peak plasma levels of HMB relative to administration of a similar dosage of a calcium salt HMB composition.
Owner:TSI PHARMA (JIANGYIN) CO LTD
Method of treating humans with opioid formulations having extended controlled release
InactiveUS7740881B1No difference in analgesic efficacyGood effectBiocidePowder deliveryControlled releaseAqueous buffer
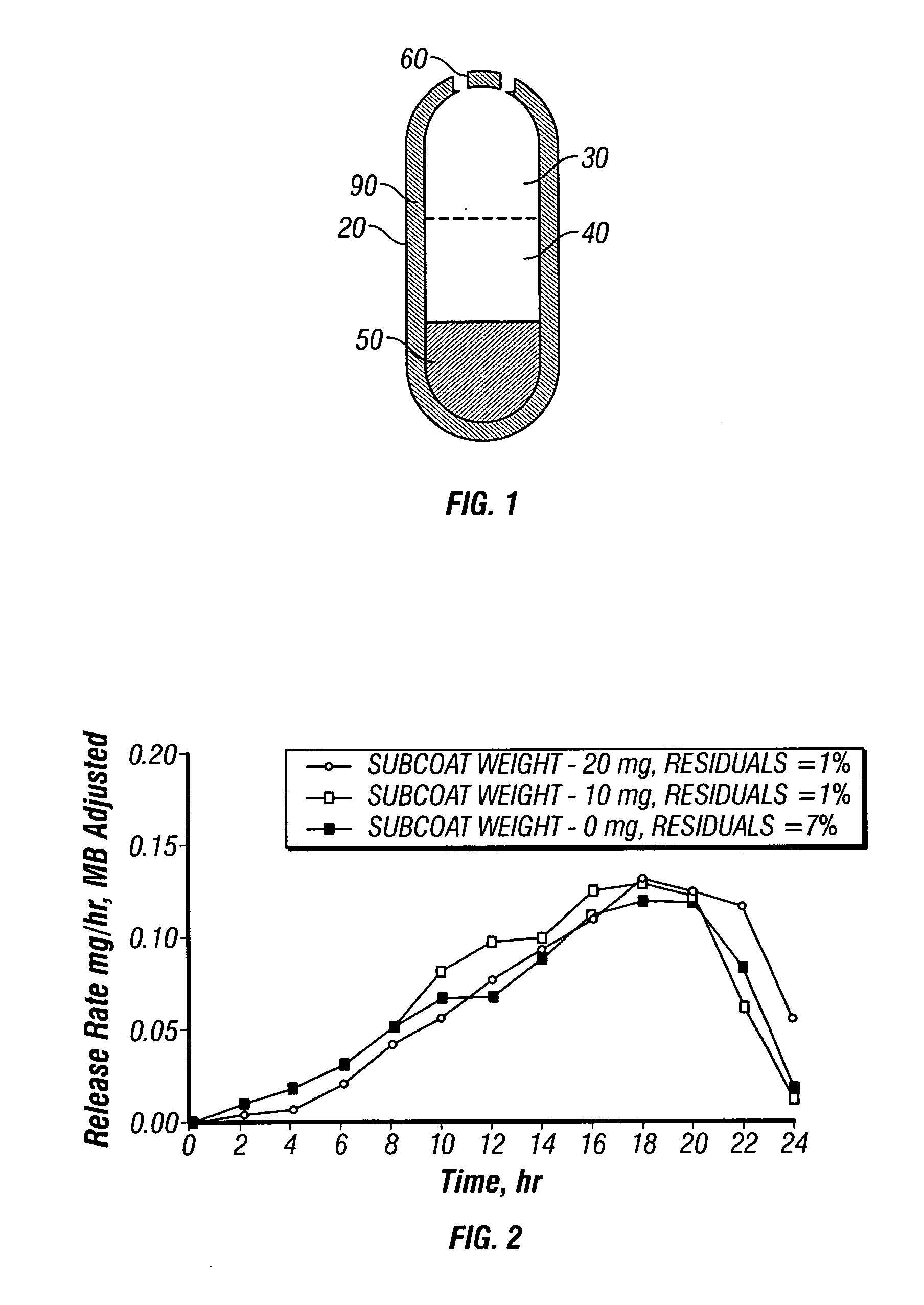
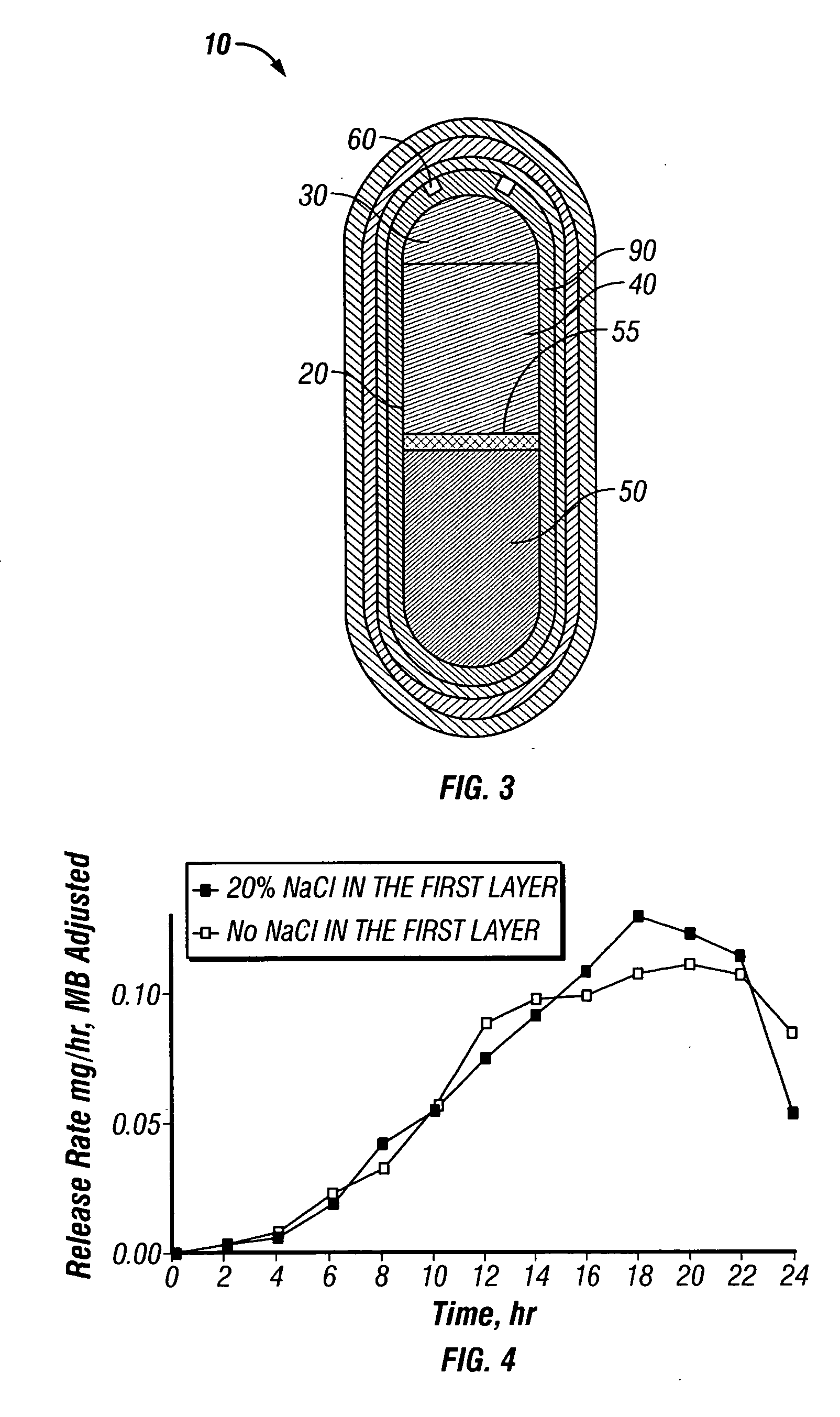
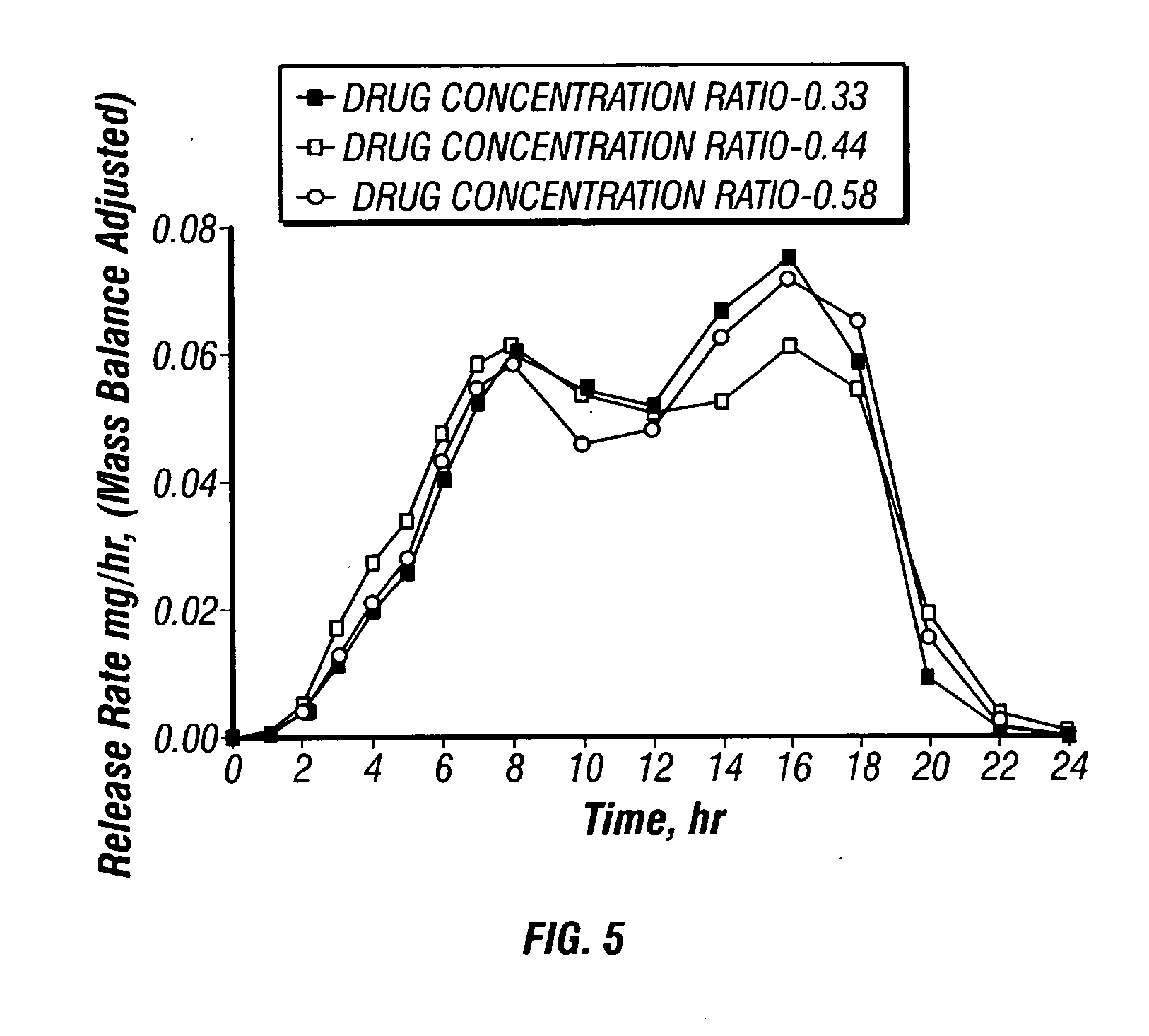
Solid controlled-release oral dosage forms comprising a therapeutically effective amount of an opioid analgesic or a salt thereof which provide an extended duration of pain relief of about 24 hours, have a dissolution rate in-vitro of the dosage form, when measured by the USP Paddle Method at 100 rpm at 900 ml aqueous buffer (pH between 1.6 and 7.2) at 37° C. of from about 12.5% to about 42.5% (by wt) opioid released after 1 hour, from about 25% to about 65% (by wt) opioid released after 2 hours, from about 45% to about 85% (by wt) opioid released after 4 hours, and greater than about 60% (by wt) opioid released after 8 hours, the in-vitro release rate being substantially independent of pH and chosen such that the peak plasma level of said opioid analgesic obtained in-vivo occurs from about 2 to about 8 hours after administration of the dosage form.
Owner:PURDUE PHARMA LP
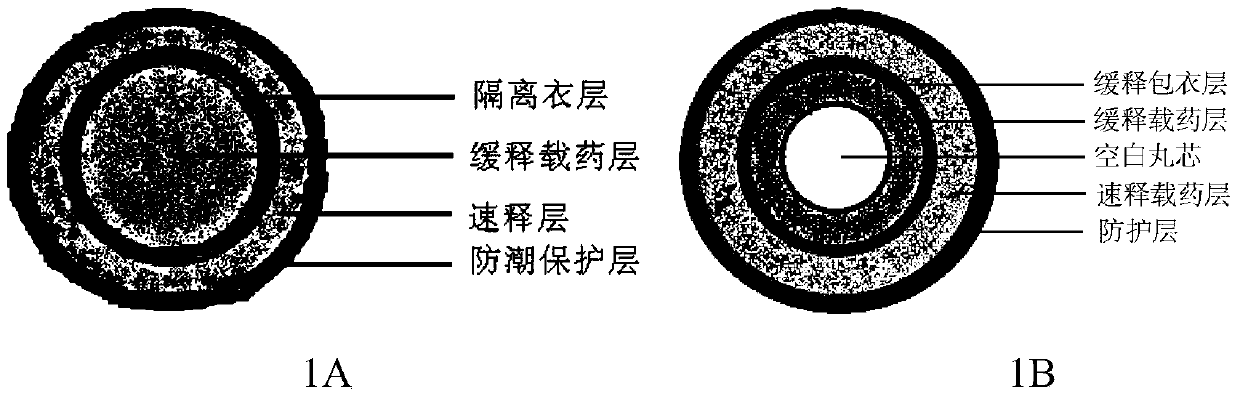
Montelukast sodium pulse release preparation
The invention relates to an oral solid drug dosage form containing montelukast sodium as a single active drug. The dosage form is prepared from a slow-release kernel and a quick-release outer layer, wherein the slow-release kernel can be prepared from blank-pellets coated by slow-release garments after loading a drug, also can be prepared from a main drug and other proper slow-release macromolecule after extrusion and spheronization, and then the quick-release outer layer is prepared on the slow-release kernel in a manner of loading the drug; the pellets can be filled into the capsules or tabletted. Compared with a common preparation, the novel pulsed release capsules disclosed by the invention achieve the peak plasma concentration at the moment that asthma easily breaks out before dawn; the target of enough time to treat can be met; the medication availability and security of an asthma patient are improved.
Owner:TIANYAO PHARMA TECH DEV SHANGHAI
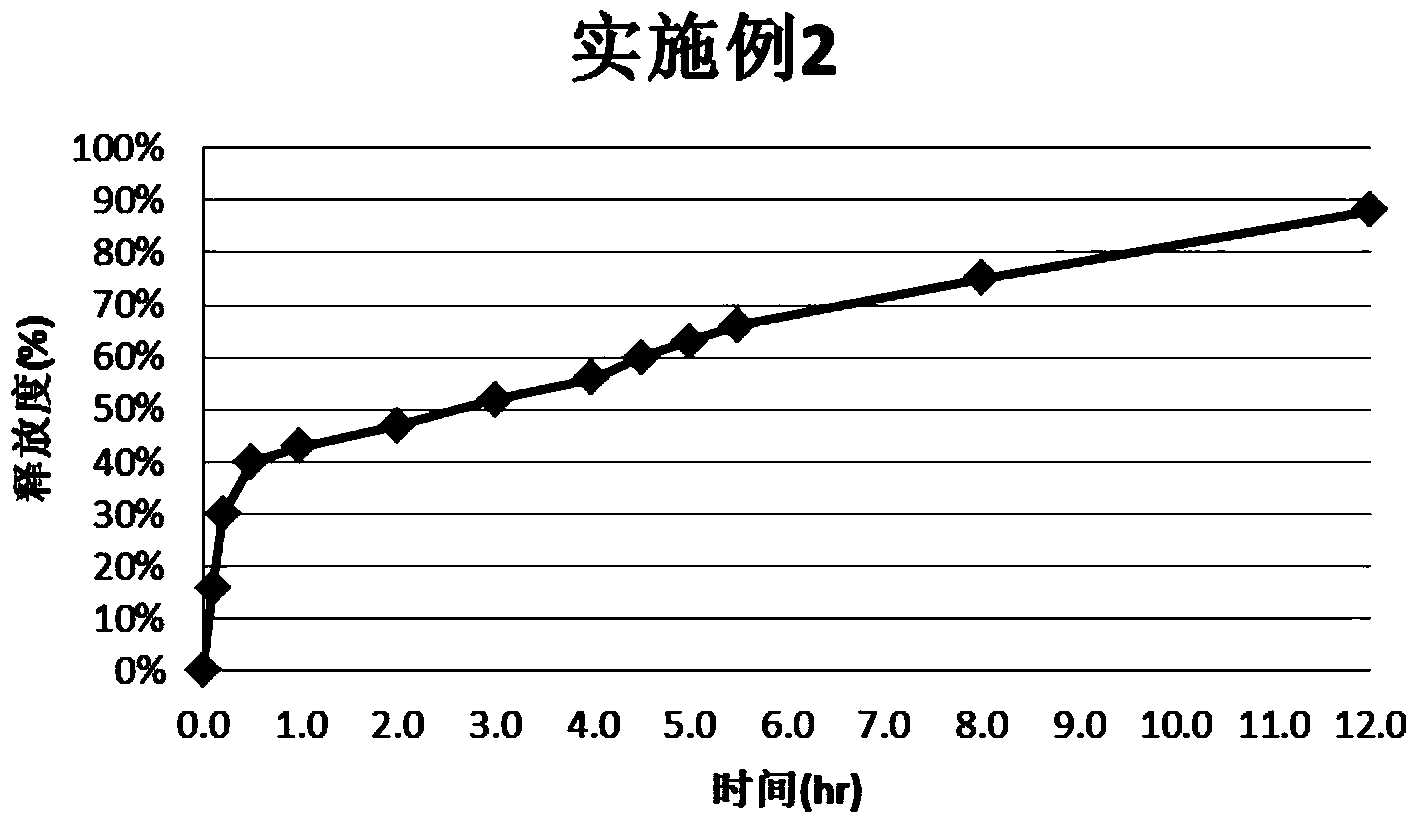
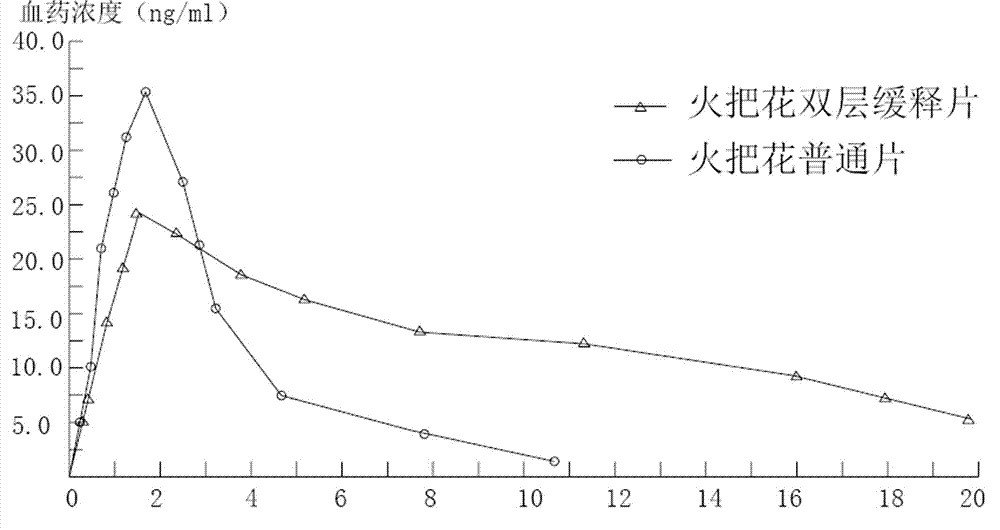
Tripterygium Hypoglaucum Hutch root extract, and bi-layer extended release tablet and application thereof
InactiveCN102727558AReduce adverse reactionsEasy to useAntipyreticAnalgesicsHalf-lifeTherapeutic effect
To solve the problem of an obvious side effect existing in the traditional Tripterygium Hypoglaucum Hutch root tablet, the invention provides a bi-layer extended release tablet which can reduce medicine peak plasma concentration. A Tripterygium Hypoglaucum Hutch root extract is any one extract of the whole Tripterygium Hypoglaucum Hutch root, Tripterygium Hypoglaucum Hutch root bark, or Tripterygium Hypoglaucum Hutch root core with the bark removed. The Tripterygium Hypoglaucum Hutch extract bi-layer extended release tablet has the characteristics of little untoward effect, and is convenient and safe to use. At the same time, a medicine-time curve is stable, the plasma concentration has small fluctuations, and the half life is prolonged. The Tripterygium Hypoglaucum Hutch root extract extended release bi-layer tablet using the root core with the bark removed has good therapeutic effects on asthma, lupus erythematosus, nephritis, and rheumatoid arthritis.
Owner:CHONGQING ACAD OF CHINESE MATERIA MEDICA +1
Antibiotic compositions of modified release and process of production thereof
InactiveUS20090111788A1Minimize adverse effectsEasy and cost-effectiveAntibacterial agentsBiocideAntibiotic YBULK ACTIVE INGREDIENT
Novel modified release pharmaceutical compositions wherein the composition comprises at least one antibiotic(s) preferably amoxicillin or its pharmaceutically acceptable salts, esters, polymorphs, isomers, prodrugs, solvates, hydrates, or derivatives thereof either alone or in combination with other antibiotic(s) as active ingredient, with at least one release modifying agent(s) for controlling the release of the beta lactam antibiotic optionally with one or more other pharmaceutically acceptable excipient(s) is provided, wherein the dosage form provides a release of not more than about 60% of the antibiotic in about 30 minutes and not less than about 70% of the antibiotic after 8 hours when subjected to in vitro dissolution study or when tested in vivo. Further, the compositions of the present invention which when tested in a group of healthy humans provide a mean peak plasma concentration (Cmax) after at least about 0.5 hour of administration of the dosage form. The present invention also provides process of preparing such dosage form and methods of using such dosage form.
Owner:PANACEA BIOTEC
Positively charged water-soluble prodrugs of oxicams and related compounds with very high skin penetration rate
InactiveCN101522692AAvoid side effectsImprove absorption rateSenses disorderNervous disorderSolubilitySide effect
The novel positively charged pro-drugs of oxicams and related compounds in the general formula (1) 'Structure 1' were designed and synthesized. The positively charged amino groups of these pro-drugs not only largely increases the solubility of the drugs, but also bonds to the negative charge on the phosphate head group of membranes and pushes the pro-drug into the cytosol. The results suggest that the pro-drugs diffuses through human skin approximately 100 times faster than do oxicams and related compounds. It takes 1-2 hours for oxicams and related compounds to reach the peak plasma level when they are taken orally, but these prodrugs only took about approximately 50 minutes to reach the peak plasma level when they are taken transdermally. In plasma, more than 90% of these pro-drugs can change back to the parent drugs in a few minutes. The prodrugs can be used medicinally in treating any oxicams-treatable conditions in humans or animals. Second, the prodrugs can be administered not only orally, but also transdermally for any kind of medical treatments and avoid most of the side effects of oxicams. Controlled transdermal administration systems of the prodrugs enable oxicams and related compounds to reach constantly optimal therapeutic blood levels to increase effectiveness and reduce the side effects of oxicams and related compounds. Another great benefit of the transdermal administration of these pro-drugs is that administering medication, especially to children, will be much easier.
Owner:于崇曦 +1
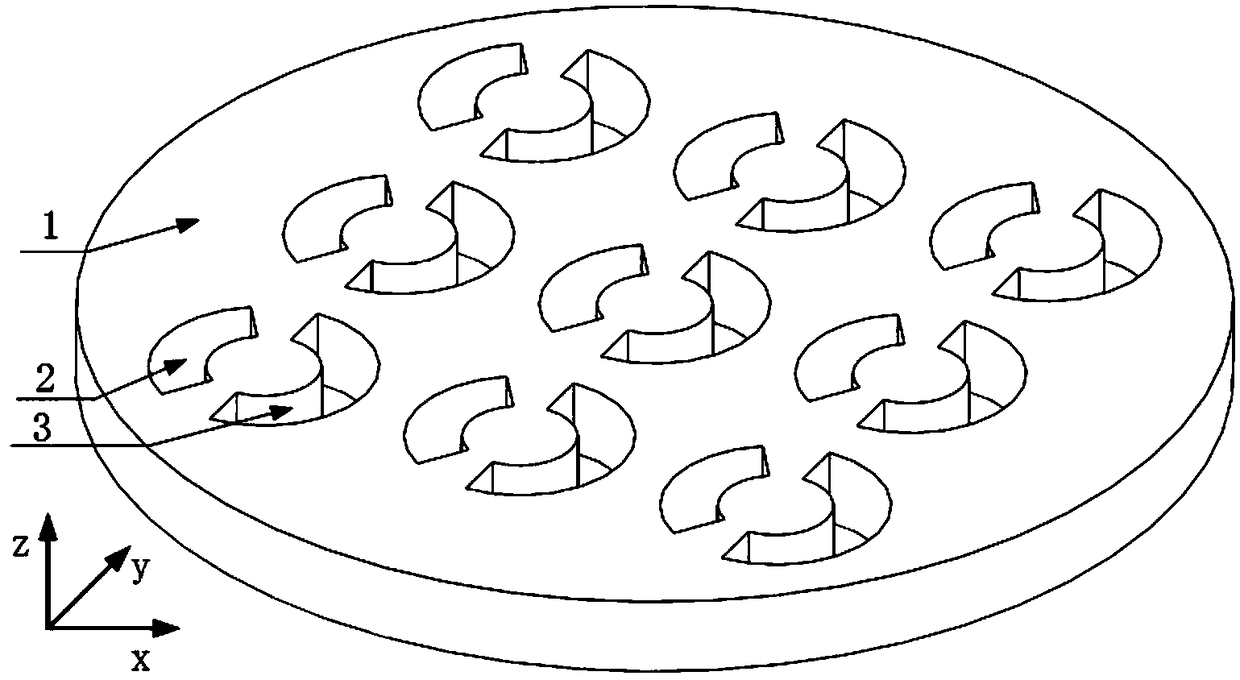
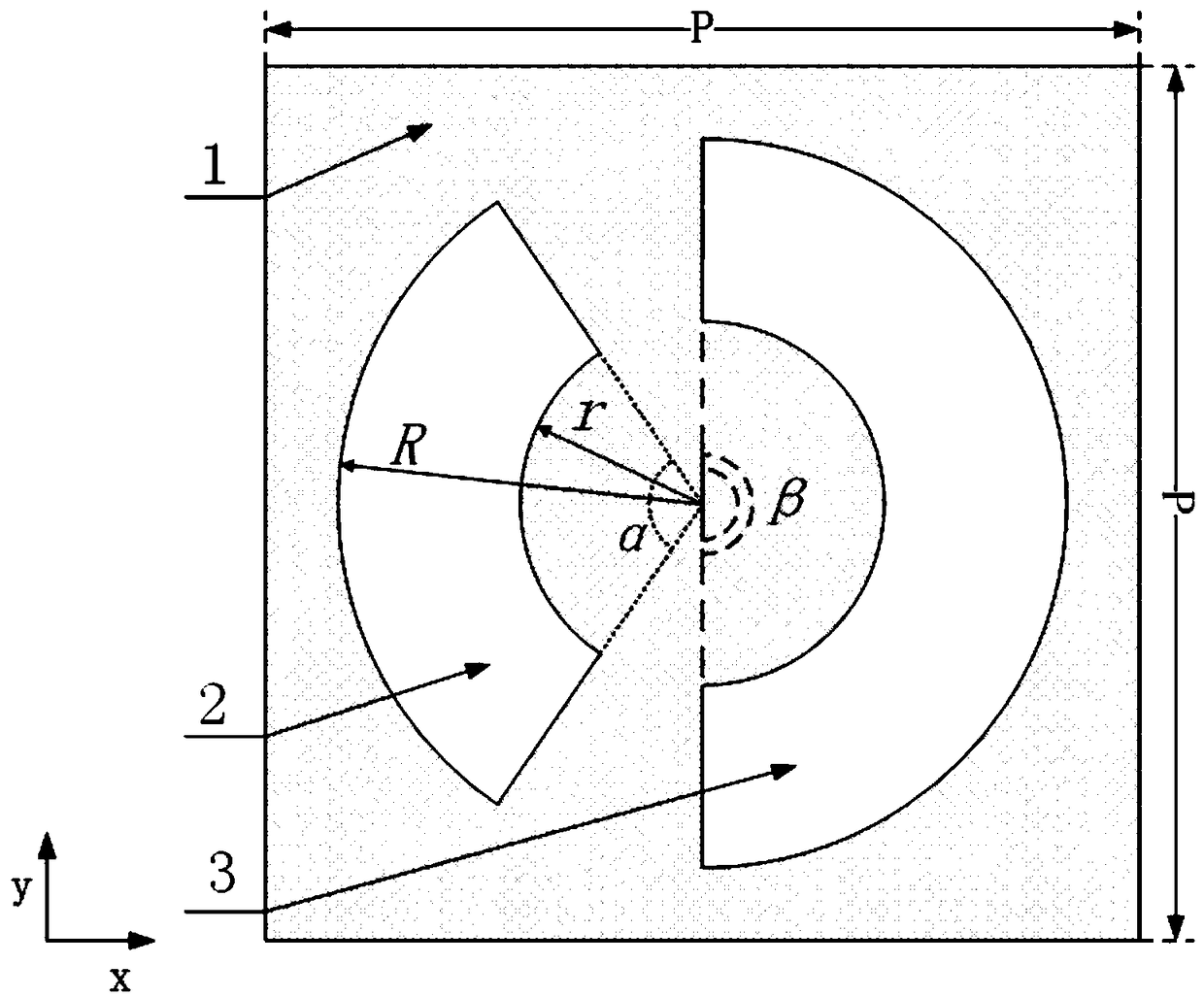
Dual transmission peak plasma optical fiber sensor based on asymmetric opening ring structure
ActiveCN109100332AExpand the measurement rangeIncrease profitMaterial analysis by optical meansFrequency spectrumMetal
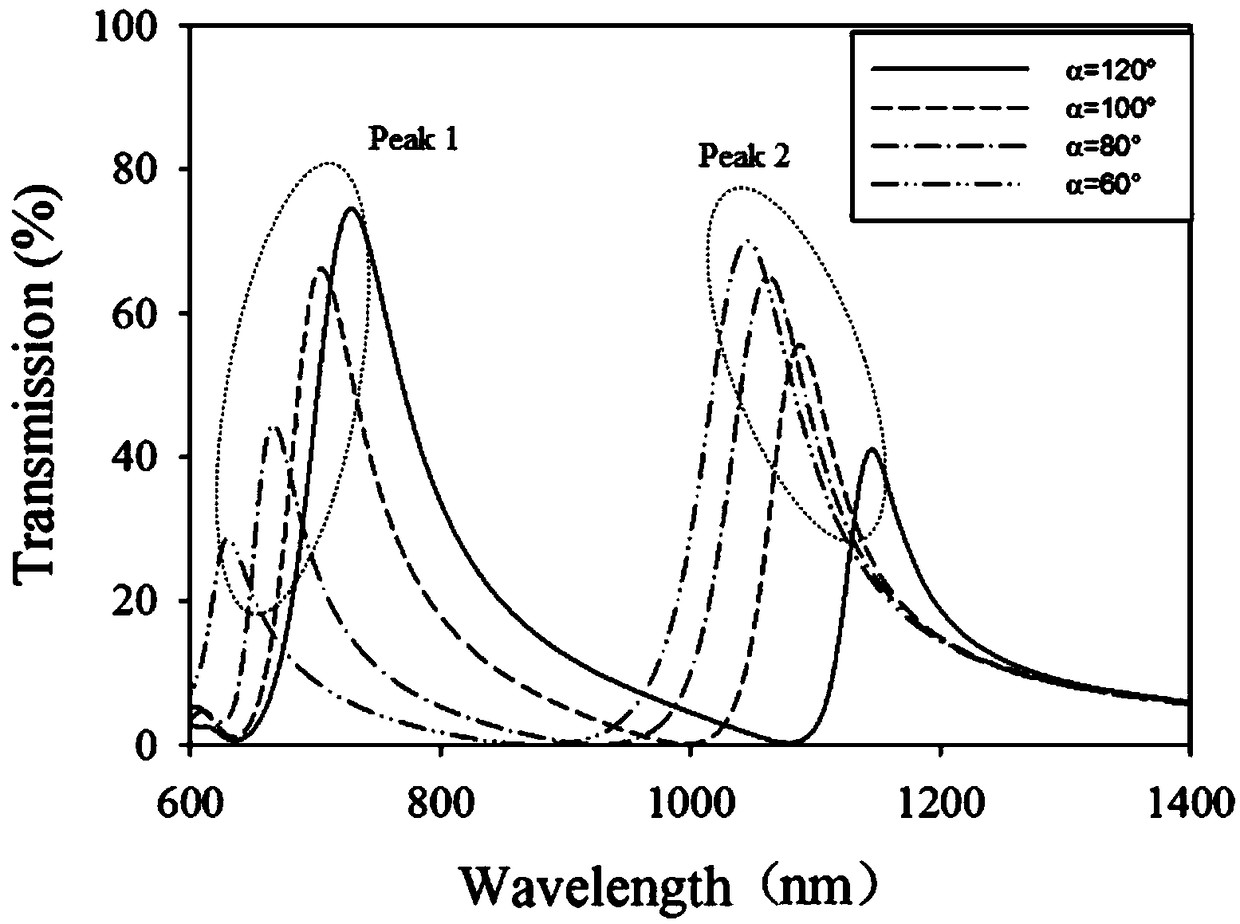
The invention discloses a dual transmission peak plasma optical fiber sensor based on an asymmetric opening ring structure. The sensor comprises a metal film and a periodic opening ring slit array structure formed on the metal film, wherein a single periodic structure is composed of left and right opening ring slits, and two opening ring slits having the same inner and outer radius but different central angles are horizontally located on the left and right sides in the middle of the unit. The sensor structure provided by the invention has a dual transmission peak characteristic of a high quality factor and high transmittance in a near-infrared frequency band, and the sensitivity of the sensor can be further improved and the sensor can be operated in two different frequency bands by using the characteristic. At the same time, the purpose of adjusting the spectral position of the dual transmission peak can be achieved by modifying the relevant structural parameters, thereby realizing a plasma optical fiber sensor with a wide working frequency band, wide application range, high sensitivity and easy processing.
Owner:GUILIN UNIV OF ELECTRONIC TECH
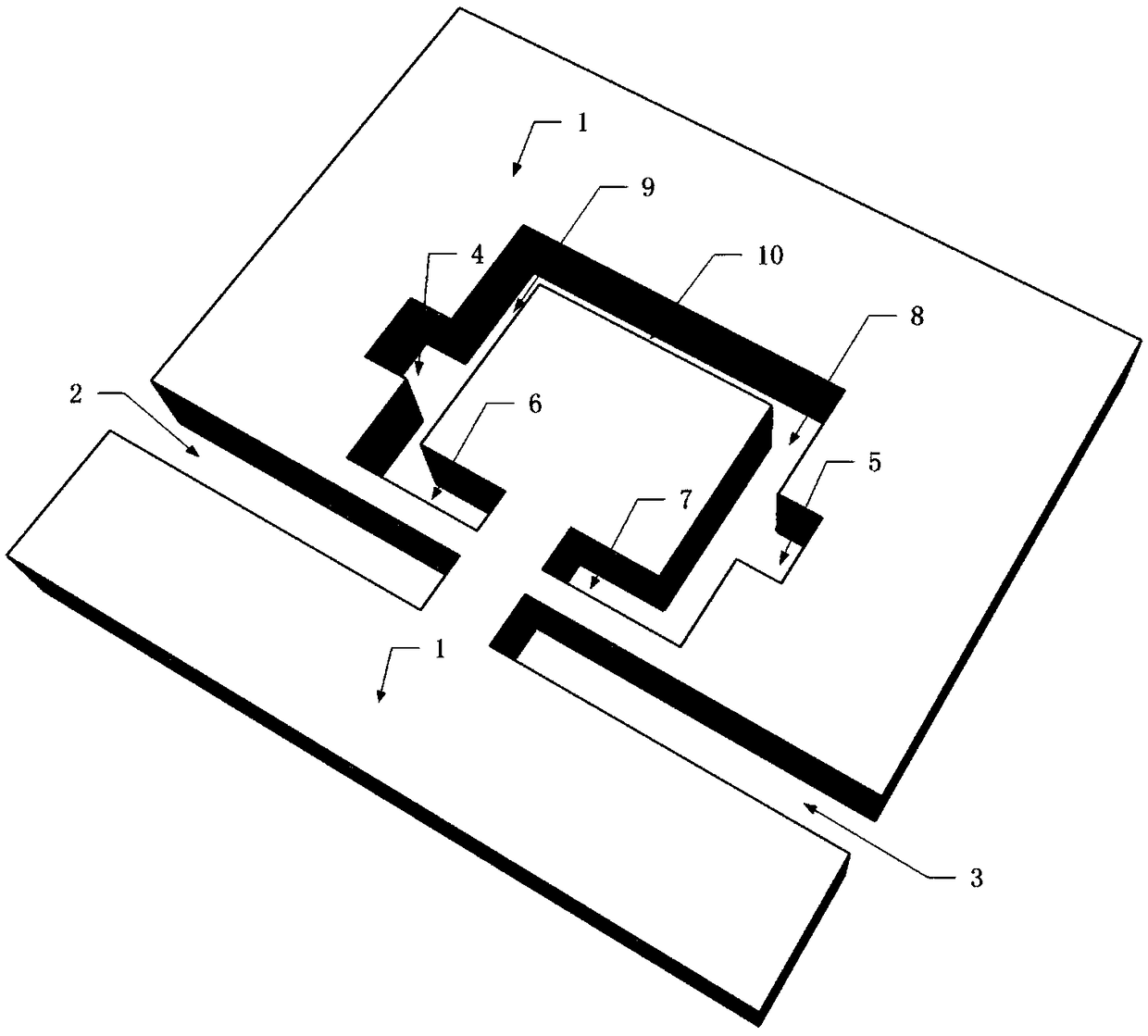
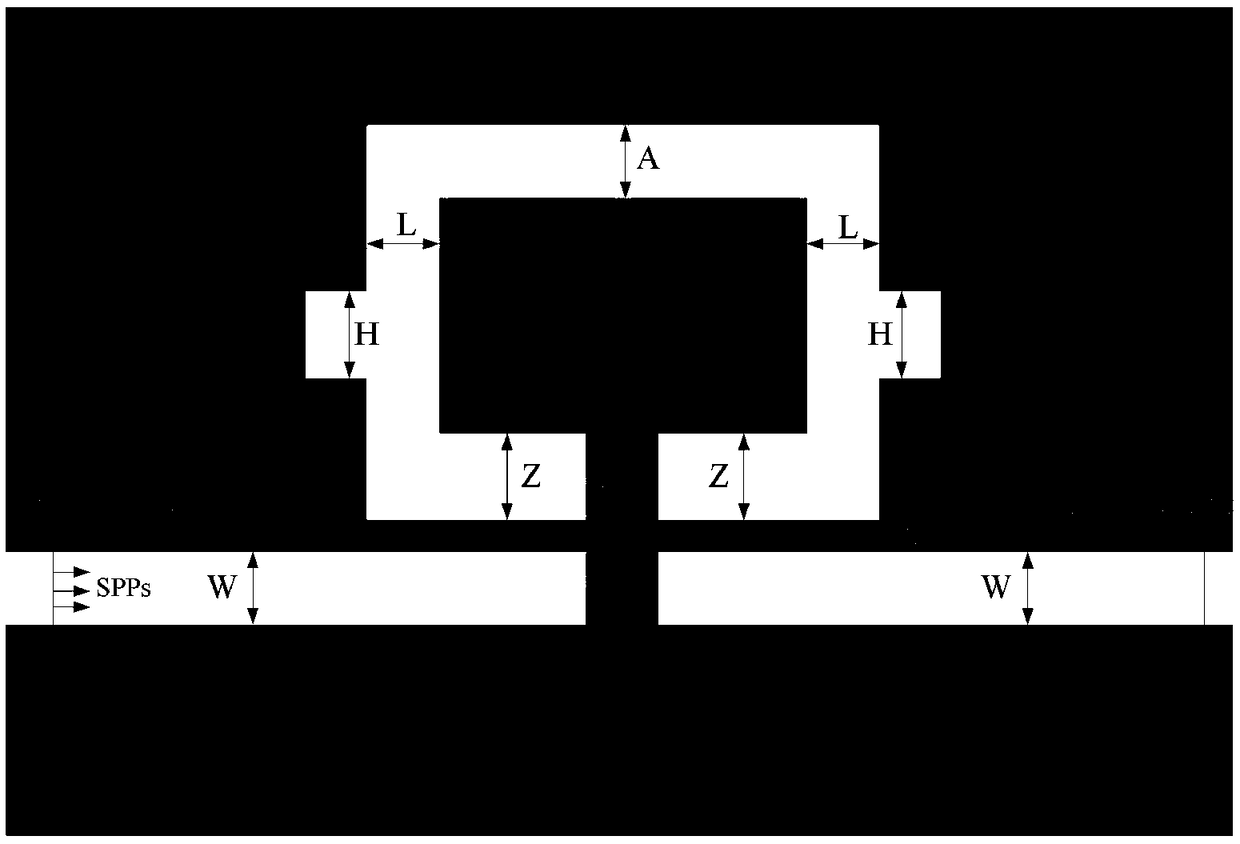
Multi-transmission peak plasma filter based on MIM waveguide coupling cavity structure
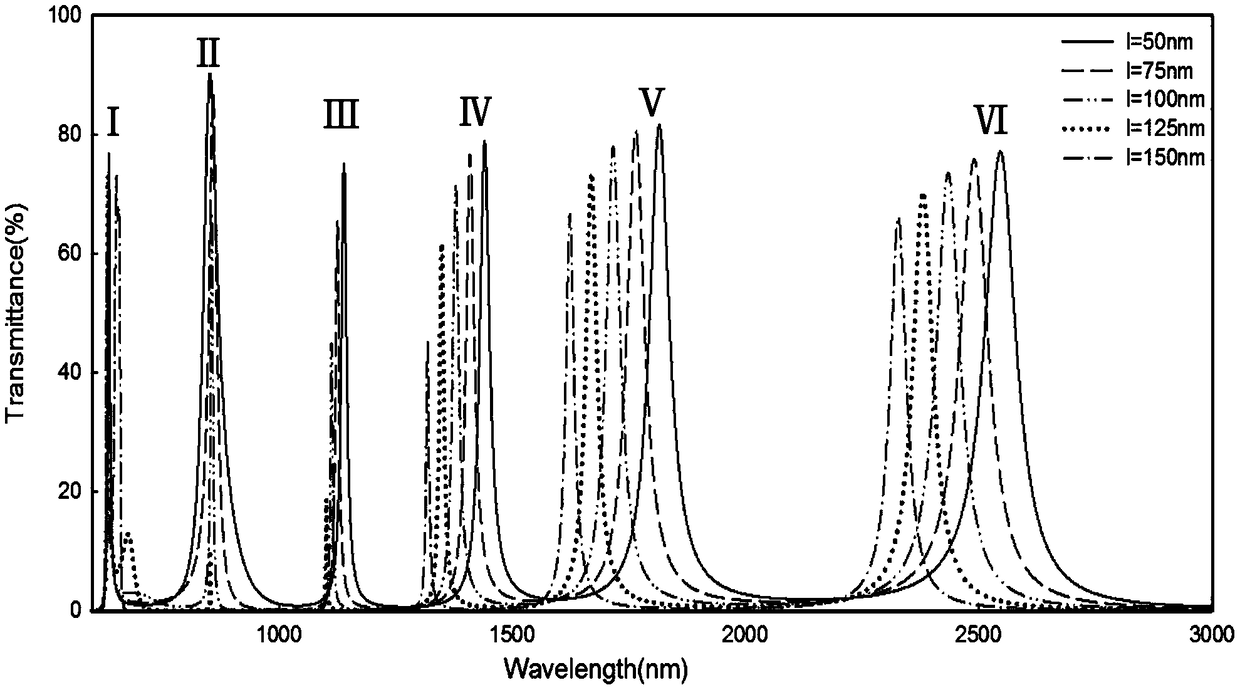
The invention discloses a multi-transmission peak plasma filter based on a MIM waveguide coupling cavity structure, which is characterized in that the multi-transmission peak plasma filter mainly comprises a metal film and a plurality of slit structural units formed on the metal film; the slit structural units are composed of a plurality of lateral rectangular slits and a plurality of longitudinalrectangular slits which pass through upper and lower surfaces of the metal film; two lateral rectangular slit structural units at the same level of the lower end of the metal film are an incident tube and an exit tube of a waveguide; and three lateral rectangular slits and four longitudinal rectangular slit structural units above the waveguide in the metal film form a unified overall resonant cavity. The filter structure of the invention has tunable and high transmittance characteristics in the infrared band; the size of a transmission spectrum and a frequency selection position can be effectively adjusted by changing relevant parameters of the structure, so that the output characteristics of the filter are changed. The research results show that the highest transmittance can reach 93%, the transmittance of each transmission peak is 75% or more, the half-height width can reach below 10nm, and the quality factor can reach 122.81. The optimal filtering effect can be obtained by changingthe relevant parameters of the structure. The multi-transmission peak filter device has the advantages of wide application range, high utilization rate, easy integration, flexible application, etc.,and thus has good application prospect in all-optical networks.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Methods of administering and enhancing absorption of pharmaceutical agents
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in mixed micellar form are disclosed. The mixed micelles are formed from an alkali metal alkyl sulfate, and at least three different micelle-forming compounds as described in the specification. Micelle size ranges between about 1 and 10 nanometers. Methods for administering the compositions are also disclosed. A preferred method for administering the present composition is through the buccal region of the mouth, which has been demonstrated to achieve peak plasma levels of the pharmaceutical agent in about thirty minutes.
Owner:GENEREX PHARMA
Method of managing or treating pain
ActiveUS8216604B2Effectively treat and manageBiocideNervous disorderDivalent metal ionsPain controlling
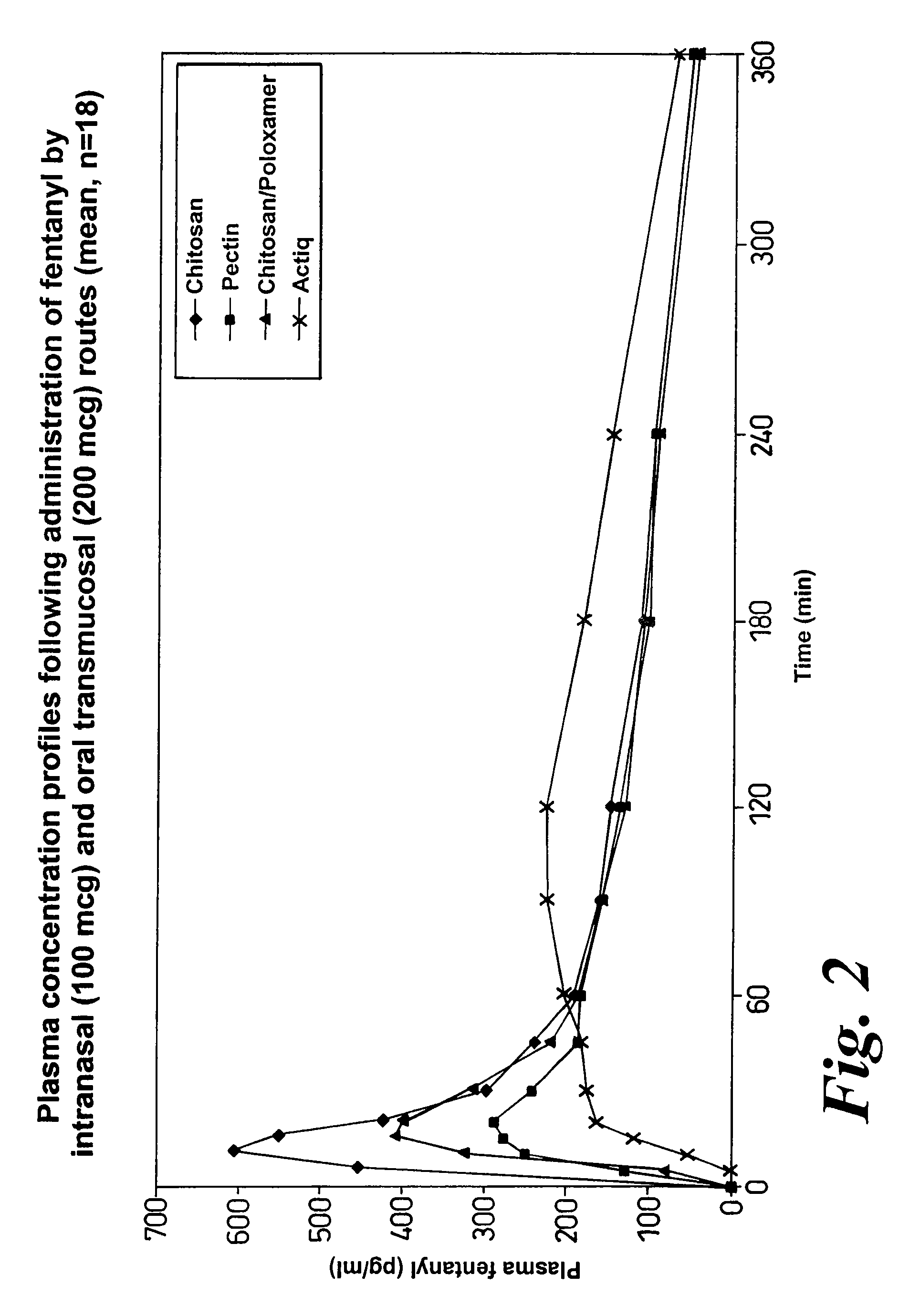
A composition for the intranasal delivery of fentanyl or a pharmaceutically acceptable salt thereof to an animal includes an aqueous solution of fentanyl or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable additive selected from (i) a pectin and (ii) a poloxamer and chitosan or a salt or derivative thereof; provided that when the composition comprises a pectin it is substantially free of divalent metal ions; and which, in comparison to a simple aqueous solution of fentanyl administered intranasally at the same dose, provides a peak plasma concentration of fentanyl (Cmax) that is from 10 to 80% of that achieved using a simple aqueous solution of fentanyl administered intranasally at an identical fentanyl dose. A method for treating or managing pain by intranasally administering the composition is also disclosed.
Owner:BTCP PHARMA LLC
Improved release dosage of febuxostat and preparation method of release dosage
InactiveCN107224431AExcellent xanthine oxidase inhibitory activityOrganic active ingredientsSkeletal disorderSide effectPatient compliance
The invention relates to an improved release dosage form of febuxostat and a preparation method thereof. Specifically, the febuxostat preparation of the present invention consists of febuxostat granules or pellets with different release properties. The preparation can effectively reduce the peak value of drug concentration in blood plasma, prolong drug action time, reduce side effects and improve patient compliance.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
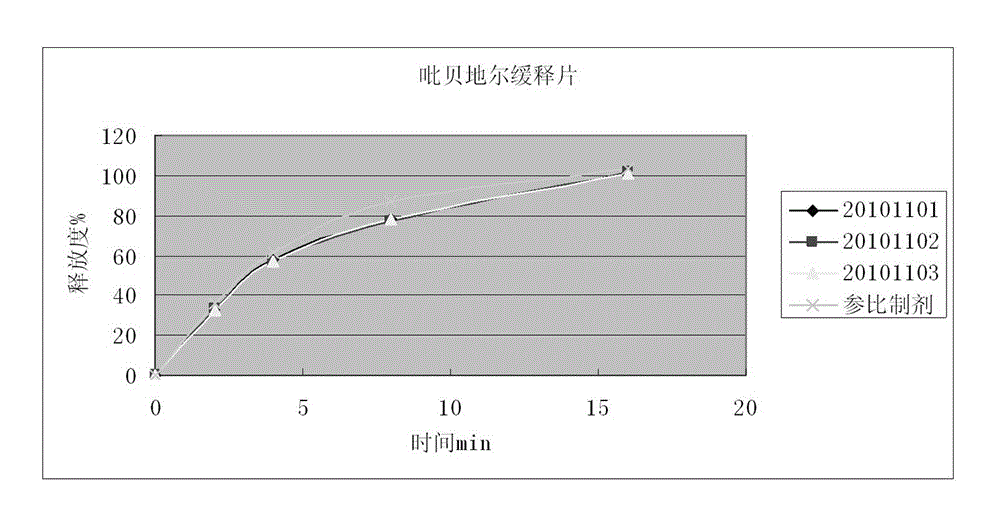
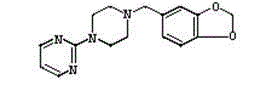
Piribedil sustained release preparation and preparation method thereof
InactiveCN102940605ALong duration of actionProlong the action timeOrganic active ingredientsNervous disorderProlonged-release tabletPiribedil
The invention discloses a piribedil sustained release preparation and a preparation method thereof. The piribedil sustained release preparation comprises 40-50 weight parts of piribedil, 90-120 weight parts of filling agent, 25-40 weight parts of sustained release material, 0.1-0.2 weight parts of binding agent, and 0.25-0.5 weight parts of lubricant. The preparation method comprises the following steps: firstly respectively crushing piribedil, the filling agent and the sustained release material and fully mixing to obtain mixed powder; then adding the binding agent and uniformly mixing to obtain a soft material; preparing the soft material into wet particles, and drying to obtain dry particles; and adding the lubricant to prepare into piribedil sustained release tablets. According to the invention, the piribedil sustained release preparation disclosed herein can effectively reduce the peak plasma concentration and prolong the action time of the drug, so as to improve the pathological cognition and sensory nerve dysfunction of patients and improve the pain symptoms caused by arterial disease of patients, and the curative effect is significant.
Owner:KAIFENG BAIYUN PHARMA CO LTD
Novel pharmaceutical modified release dosage form composition comprising cyclooxygenase enzyme inhibitor
InactiveCN101227893AExtension of timeNo toxicityNervous disorderAntipyreticModified Release Dosage FormEster prodrug
Pharmaceutical modified release dosage form comprising at least one cyclooxygenase enzyme inhibitor or its pharmaceutically acceptable salts, esters, prodrugs, solvates, hydrates, or derivatives thereof as active agent, with a pharmaceutically acceptable carrier for controlling the release of the cyclooxygenase enzyme inhibitor is provided. The dosage form preferably provides a release of not more than about 60 % of the cyclooxygenase enzyme inhibitor in 1 hour and not less than about 75 % of the cyclooxygenase enzyme inhibitor after 12 hours when tested in accordance with the dissolution method (I) described herein employing Distilled water with 2.0 % Sodium lauryl sulphate as the dissolution medium or in accordance with the dissolution method (II) described herein employing pH 7.0 Phosphate buffer with 2.0% Sodium lauryl sulphate as the dissolution medium or in accordance with the dissolution method (III) described herein employing 0.001 N Hydrochloric acid with 1.0 % Sodium lauryl sulphate as dissolution medium. Further, the pharmaceutical composition of the present invention when tested in a group of healthy humans preferably achieves a mean peak plasma concentration (Cmax) after at least about 1 hour of administration of the dosage form,. The present invention also provides process of preparing such dosage form compositions and prophylactic and / or therapeutic methods of using such dosage form.
Owner:PANACEA BIOTEC
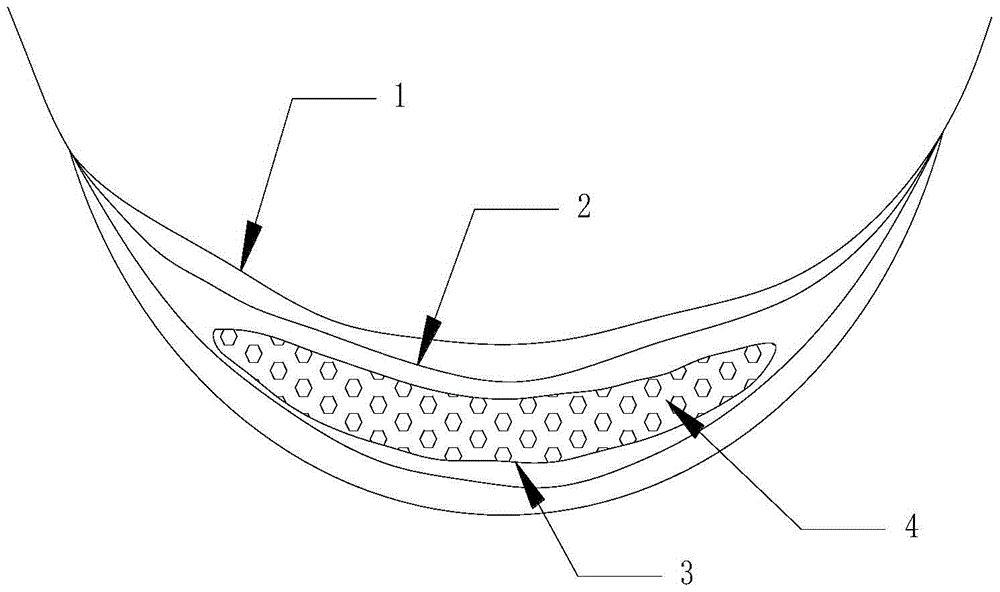
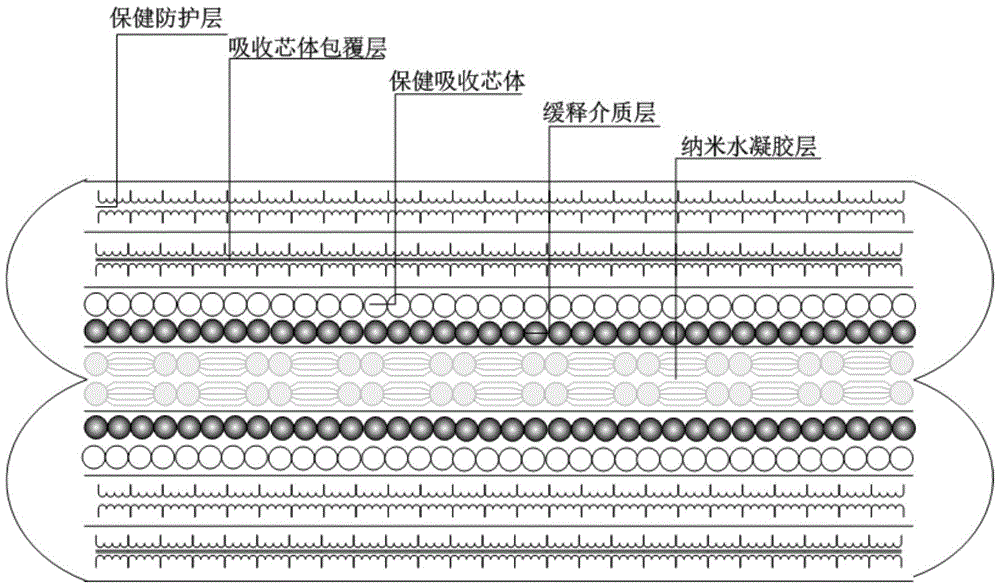
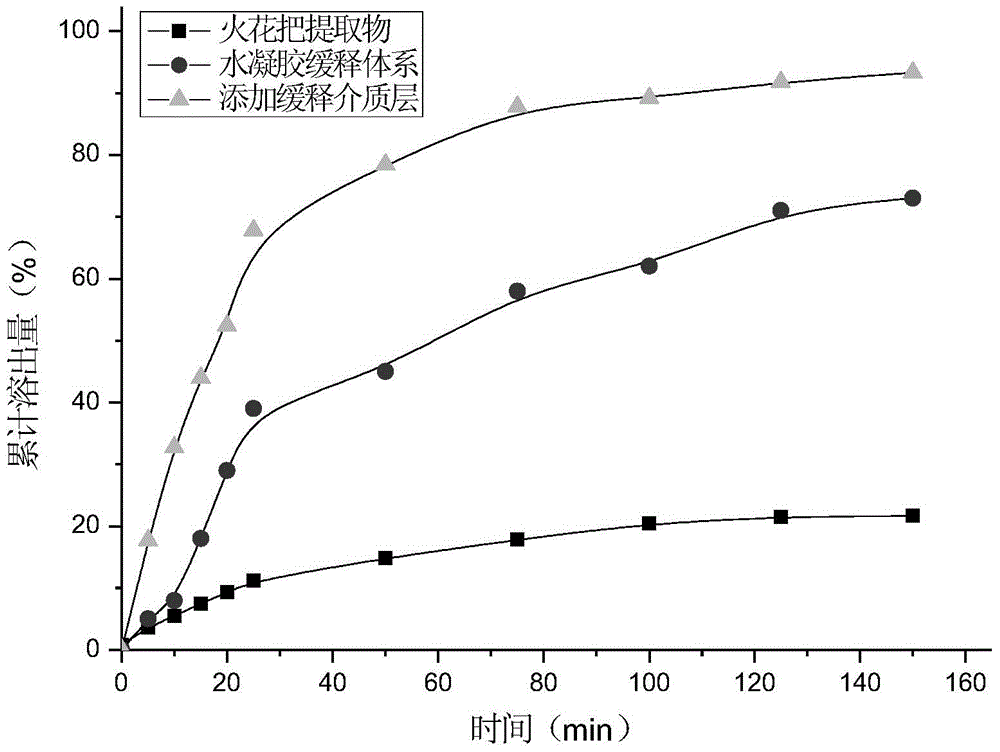
Hydrogel for slow release of tripterygium hypoglaucum hutch extract and paper diaper for the aged made of same
InactiveCN105726610ALong duration of pharmacological effectsEasy to useAntipyreticAerosol deliveryPatient complianceTherapeutic effect
The invention belongs to the field of traditional Chinese medical care, and particularly relates to hydrogel for slow release of tripterygium hypoglaucum hutch extract and a paper diaper for the aged made of the same.Esterified chitosan serves as the water-soluble nano-porous frame carrier of the hydrogel, and the tripterygium hypoglaucum hutch extract serves as the solubilization slow release object.The paper diaper for the aged comprises a healthcare protection layer, an adsorption core coating layer, an adsorption core layer and a nano-hydrogel layer from outside to inside in sequence, wherein the nano-hydrogel layer is mainly formed by the hydrogel for slow release of the tripterygium hypoglaucum hutch extract.The hydrogel for slow release of the tripterygium hypoglaucum hutch extract can effectively reduce the peak plasma concentration of drugs and is a slow release preparation with a remarkable treatment effect.By the adoption of the paper diaper for the aged, the trouble of frequent clinical medication everyday and problems caused thereby are avoided, and medication efficiency and safety and patient compliance are improved.The paper diaper for the aged is mainly used for adjuvant treatment of rheumatoid arthritis, lupus erythematosus, chronic nephritis and autoimmune hemolytic anemia.
Owner:CHONGQING ACAD OF CHINESE MATERIA MEDICA
Novel Pharmaceutical Modified Release Dosage Form Cyclooxygenase Enzyme Inhibitor
InactiveUS20100204333A1Easy and cost-effectiveBiocideNervous disorderModified Release Dosage FormPhosphate
Pharmaceutical modified release dosage form comprising at least one cyclooxygenase enzyme inhibitor or its pharmaceutically acceptable salts, esters, prodrugs, solvates, hydrates, or derivatives thereof as active agent, with a pharmaceutically acceptable carrier for controlling the release of the cyclooxygenase enzyme inhibitor is provided. The dosage form preferably provides a release of not more than about 60% of the cyclooxygenase enzyme inhibitor in 1 hour and not less than about 75% of the cyclooxygenase enzyme inhibitor after 12 hours when tested in accordance with the dissolution method (I) described herein employing Distilled water with 2.0% Sodium lauryl sulphate as the dissolution medium or in accordance with the dissolution method (II) described herein employing pH 7.0 Phosphate buffer with 2.0% Sodium lauryl sulphate as the dissolution medium or in accordance with the dissolution method (III) described herein employing 0.001 N Hydrochloric acid with 1.0% Sodium lauryl sulphate as dissolution medium. Further, the pharmaceutical composition of the present invention when tested in a group of healthy humans preferably achieves a mean peak plasma concentration (Cmax) after at least about 1 hour of administration of the dosage form. The present invention also provides process of preparing such dosage form compositions and prophylactic and / or therapeutic methods of using such dosage form.
Owner:PANACEA BIOTEC
Positively charged water-soluble prodrugs of n-arylanthranilic acids with very fast skin penetration rate
ActiveCN101506168AGood absorption rateGood curative effectOrganic compound preparationAntipyreticSolubilityFlufenamic acid
The novel positively charged pro-drugs of arylanthranilic acids in the general formula (1) 'Structure 1' were designed and synthesized. The compounds of the general formula (1) 'Structure 1' indicated above can be prepared from mefenamic acid, meclofenamic acid, flufenamic acid, niflumic acid, flunixin, and related compounds, by reaction with suitable alcohols, thiols, or amines and coupling reagents, such as N, N'-Dicyclohexylcarbodiimide, N, N'-Diisopropylcarbodiimide, O-(Benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate, O-(Benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate, Benzotriazol-1-yl-oxy-tris (dimethylamino)phosphonium hexafluorophosphate, et al. The positively charged amino groups of these pro-drugs not only largely increases the solubility of the drugs, but also bonds to the negative charge on the phosphate head group of membranes and pushes the pro-drug into the cytosol. The results suggest that the pro-drugs diffuses through human skin ~200 times faster than does mefenamic acid, meclofenamic acid, flufenamic acid, niflumic acid, flunixin, and related compounds. It takes 2-4 hours for mefenamic acid, meclofenamic acid, flufenamic acid, niflumic acid, flunixin, and related compounds to reach the peak plasma level when they are taken orally, but these prodrugs only took about ~50 minutes to reach the peak plasma level when they are taken transdermally. In plasma, more than 90% of these pro-drugs can change back to the parent drugs in a few minutes. The prodrugs can be used medicinally in treating any NSAIAs-treatable conditions in humans or animals. The prodrugs can be administered not only orally, but also transdermally for any kind of medical treatments and thus avoid most of the side effects of NSAIAs, most notably GI disturbances such as dyspepsia, gastroduodenal bleeding, gastric ulcerations, and gastritis.
Owner:TECHFIELDS BIOCHEM CO LTD
Antibiotic compositions of modified release and process of production thereof
Novel modified release pharmaceutical compositions wherein the composition comprises at least one antibiotic(s) preferably amoxicillin or its pharmaceutically acceptable salts, esters, polymorphs, isomers, prodrugs, solvates, hydrates, or derivatives thereof either alone or in combination with other antibiotic(s) as active ingredient, with at least one release modifying agent(s) for controlling the release of the beta lactam antibiotic optionally with one or more other pharmaceutically acceptable excipient(s) is provided, wherein the dosage form provides a release of not more than about 60 % of the antibiotic in about 30 minutes and not less than about 70 % of the antibiotic after 8 hours when subjected to in vitro dissolution study or when tested in vivo. Further, the compositions of the present invention which when tested in a group of healthy humans provide a mean peak plasma concentration (Cmax) after at least about 0.5 hour of administration of the dosage form. The present invention also provides process of preparing such dosage form and methods of using such dosage form.
Owner:PANACEA BIOTEC
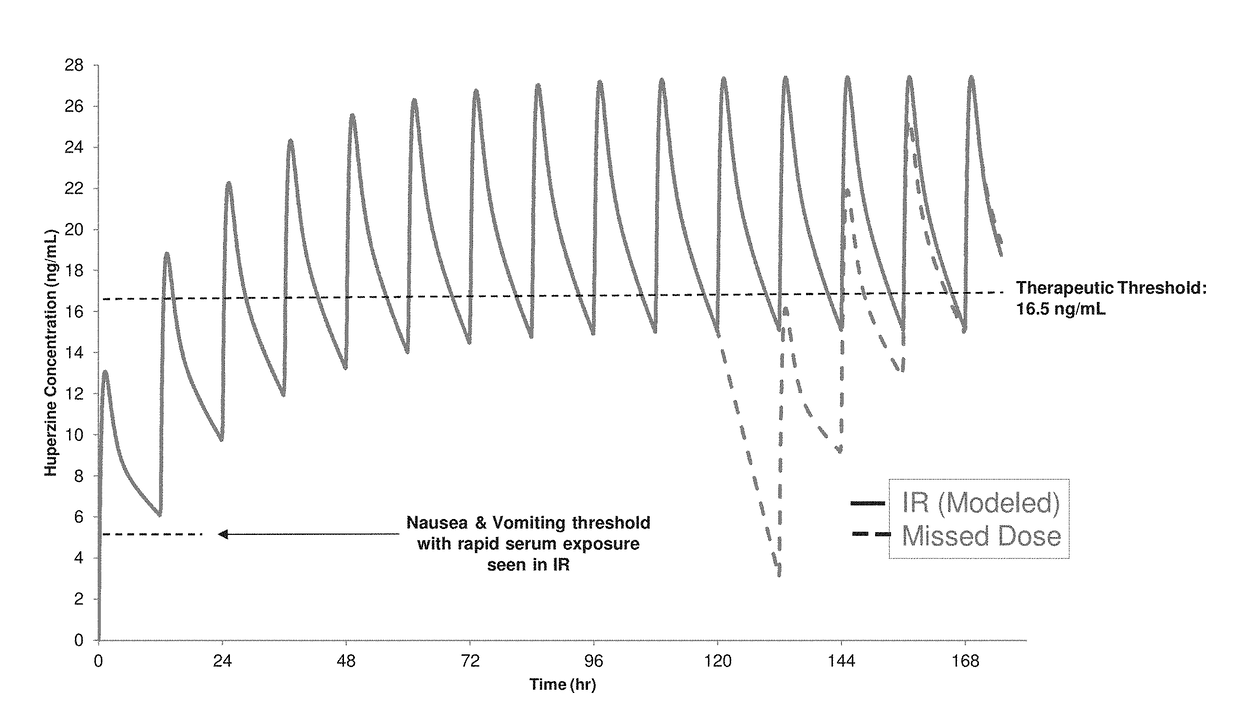
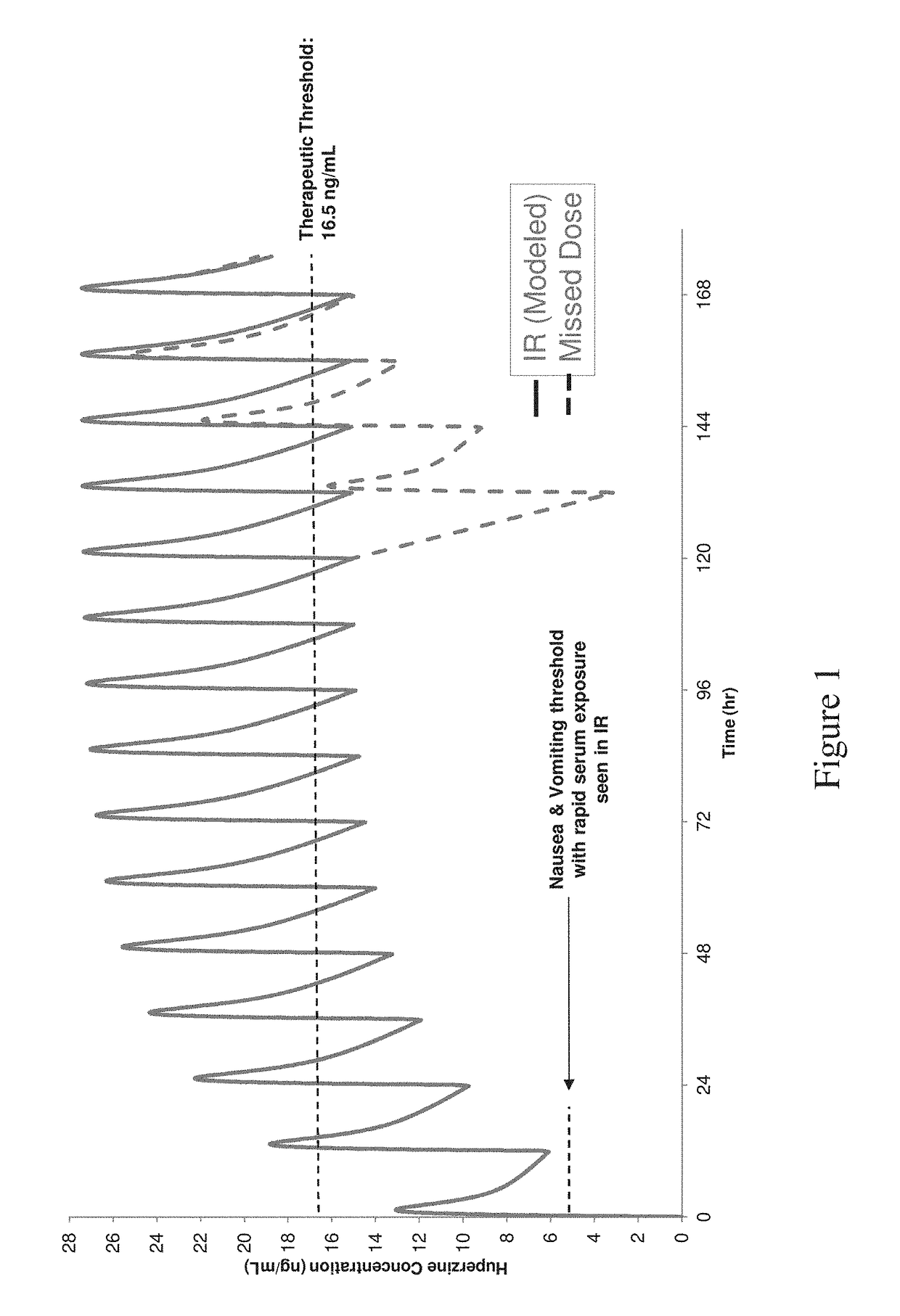
Modified release pharmaceutical compositions of huperzine and methods of using the same
InactiveUS20180333365A1Side effect profileHigh levelOrganic active ingredientsNervous disorderImmediate releaseMedicine
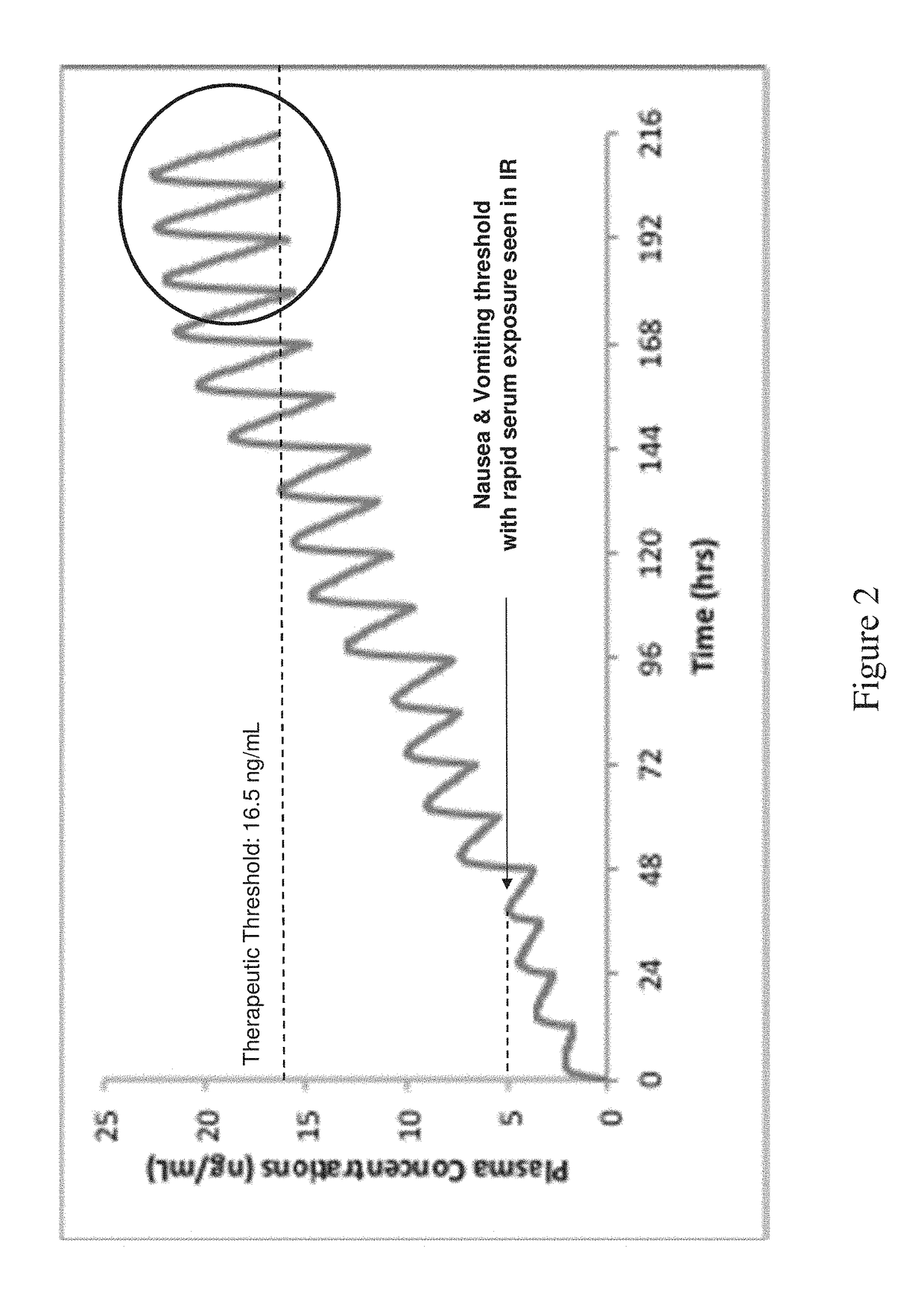
The present application discloses pharmaceutical compositions for modified release of huperzine. The pharmaceutical compositions and methods described herein, allow for dosing of huperzine at higher therapeutic thresholds, while avoiding rapid serum peak plasma levels, thereby avoiding the adverse nausea and vomiting associated with the immediate release pharmaceutical compositions. Methods of treating neurological disorders and / or seizure disorders with the modified release compositions is also described.
Owner:BISCAYNE NEUROTHERAPEUTICS INC
A kind of montelukast sodium pulse release preparation
The invention relates to an oral solid drug dosage form containing montelukast sodium as a single active drug. The dosage form is prepared from a slow-release kernel and a quick-release outer layer, wherein the slow-release kernel can be prepared from blank-pellets coated by slow-release garments after loading a drug, also can be prepared from a main drug and other proper slow-release macromolecule after extrusion and spheronization, and then the quick-release outer layer is prepared on the slow-release kernel in a manner of loading the drug; the pellets can be filled into the capsules or tabletted. Compared with a common preparation, the novel pulsed release capsules disclosed by the invention achieve the peak plasma concentration at the moment that asthma easily breaks out before dawn; the target of enough time to treat can be met; the medication availability and security of an asthma patient are improved.
Owner:TIANYAO PHARMA TECH DEV SHANGHAI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com