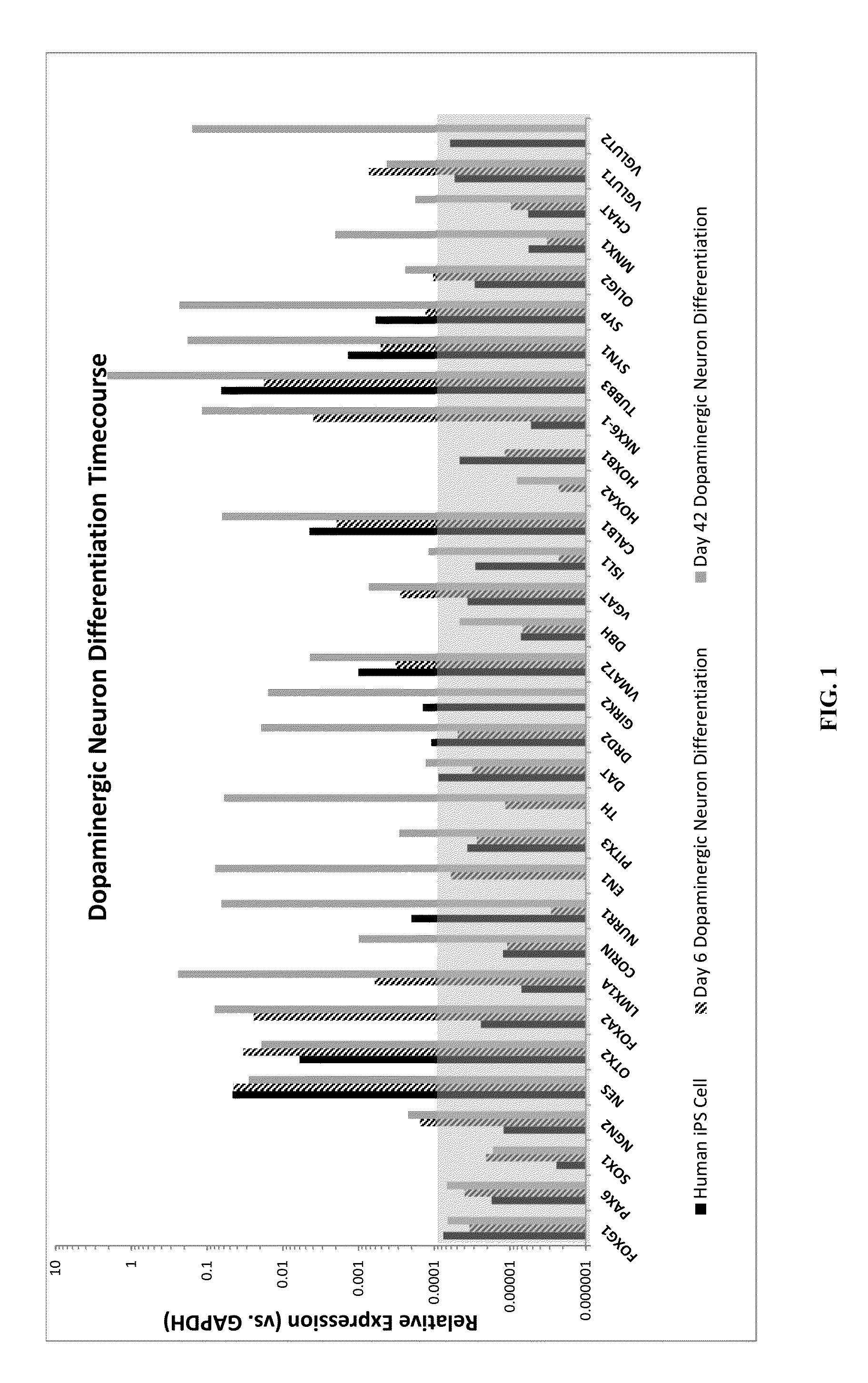
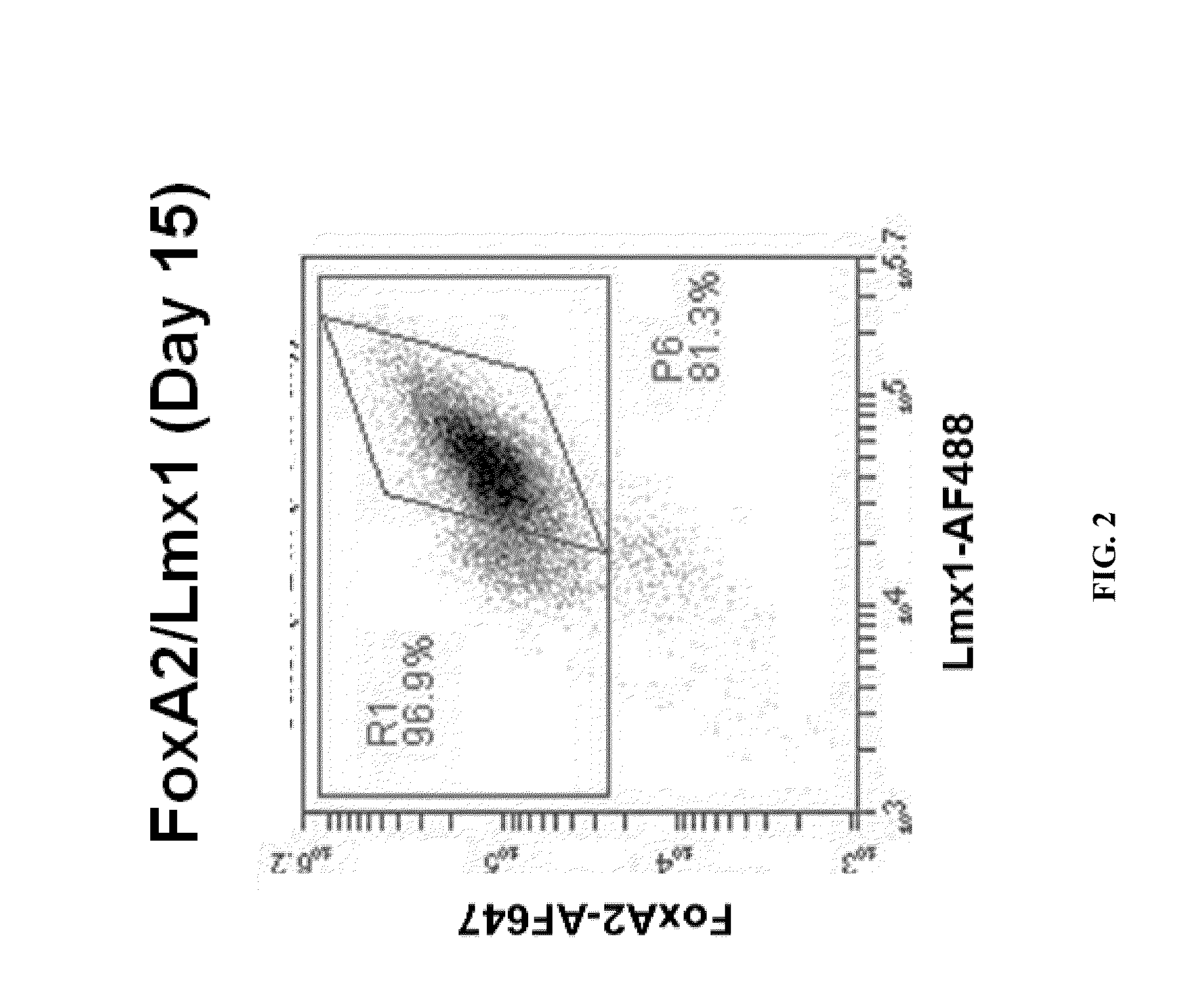
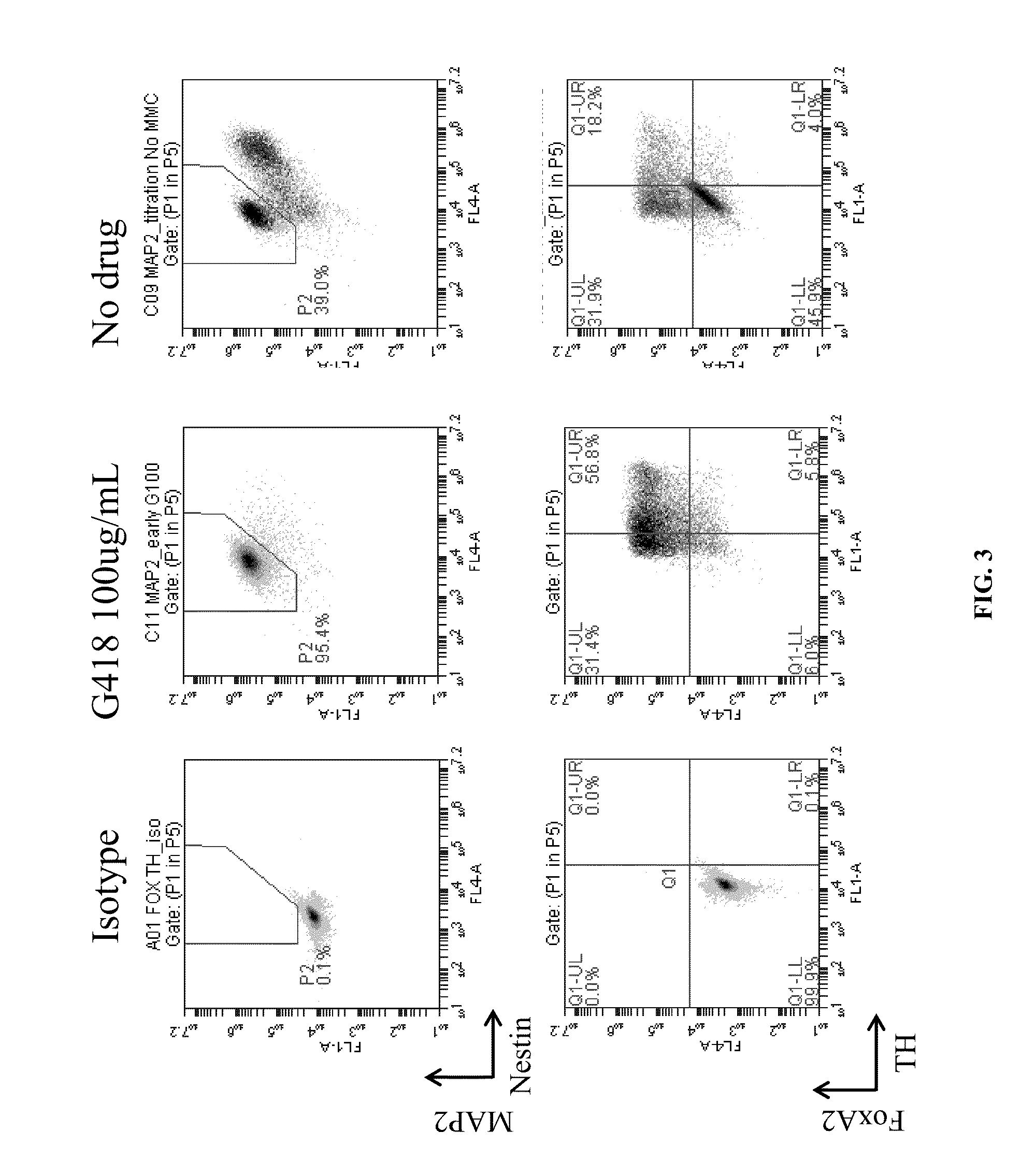
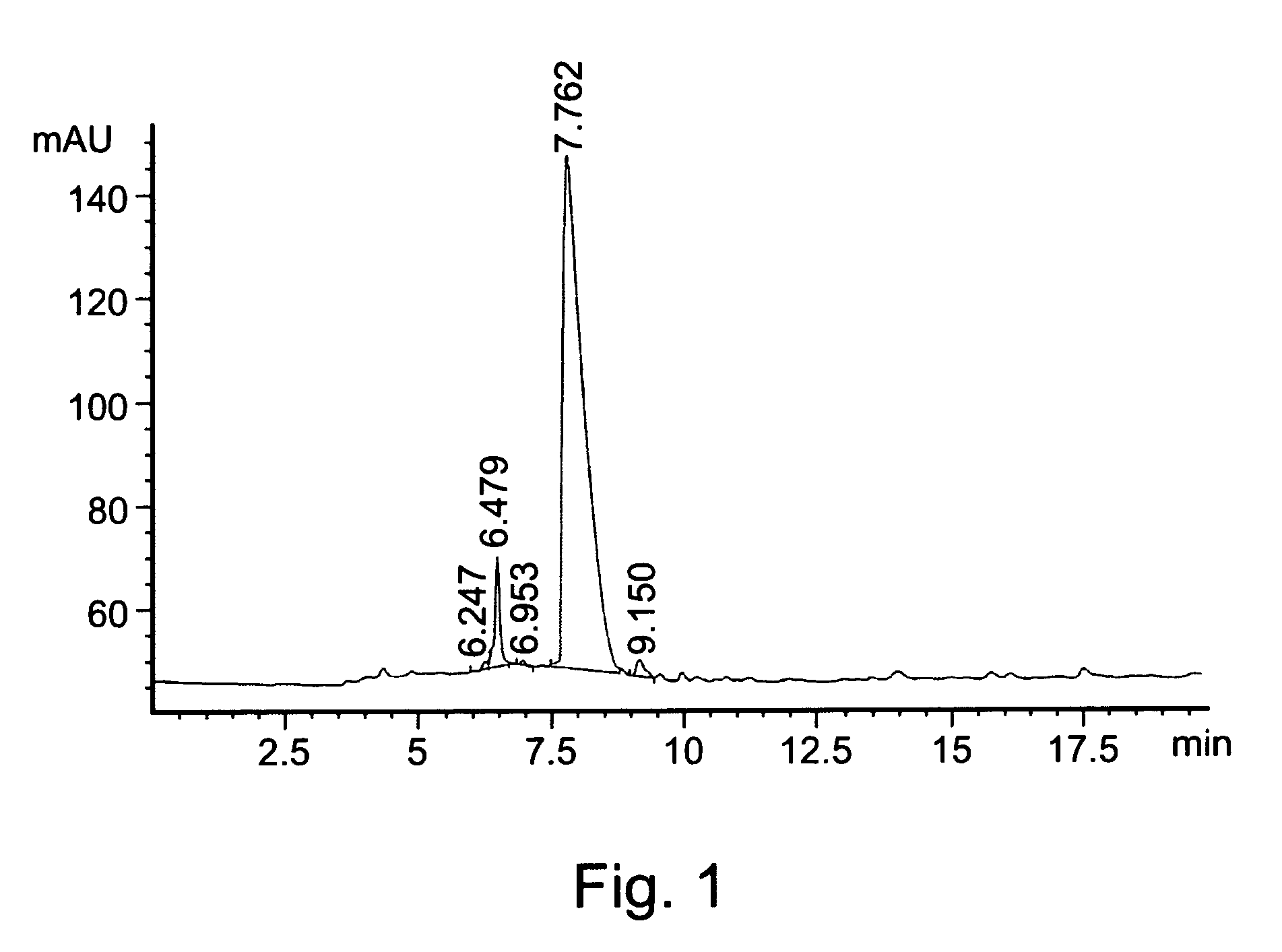
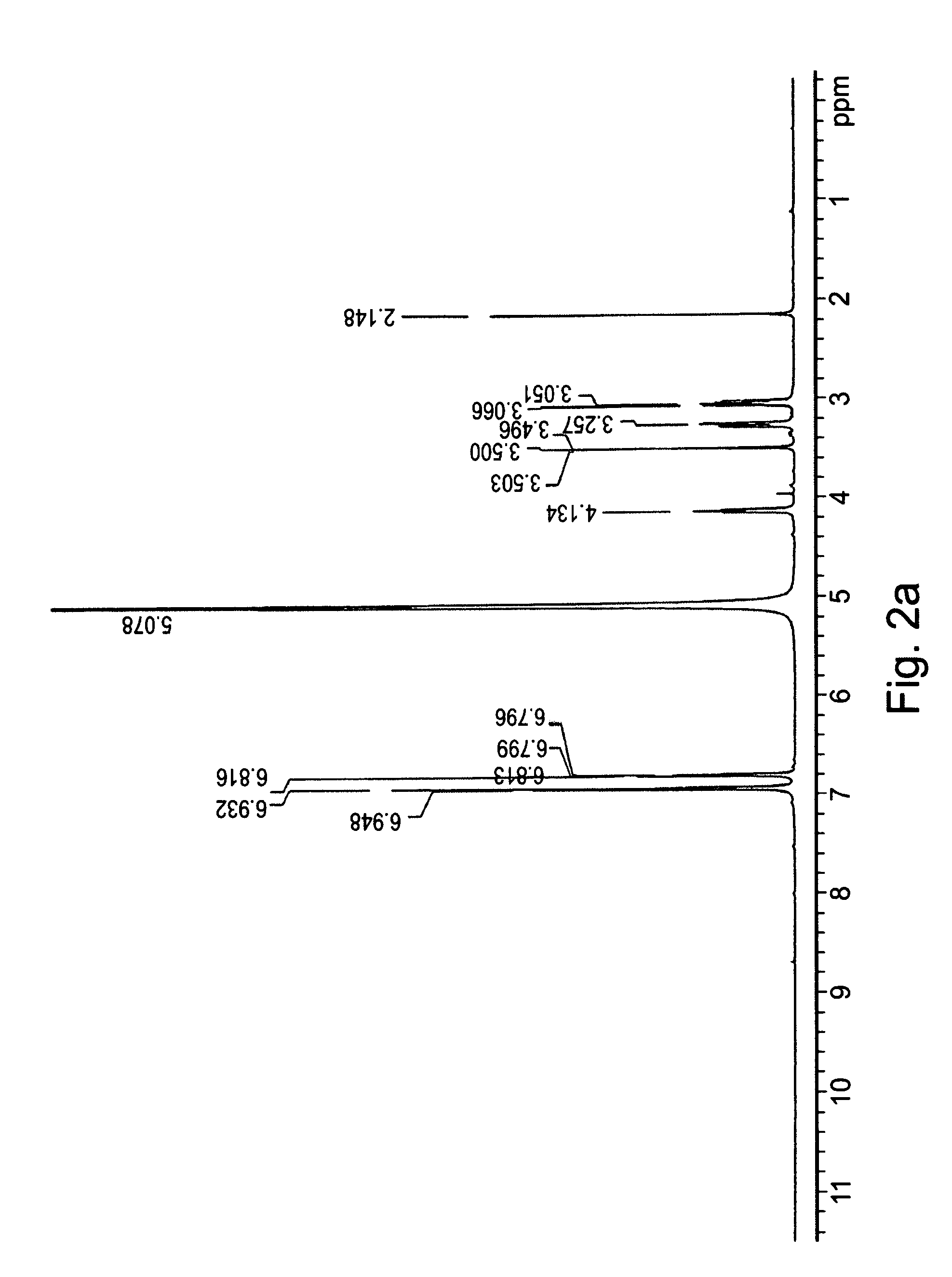
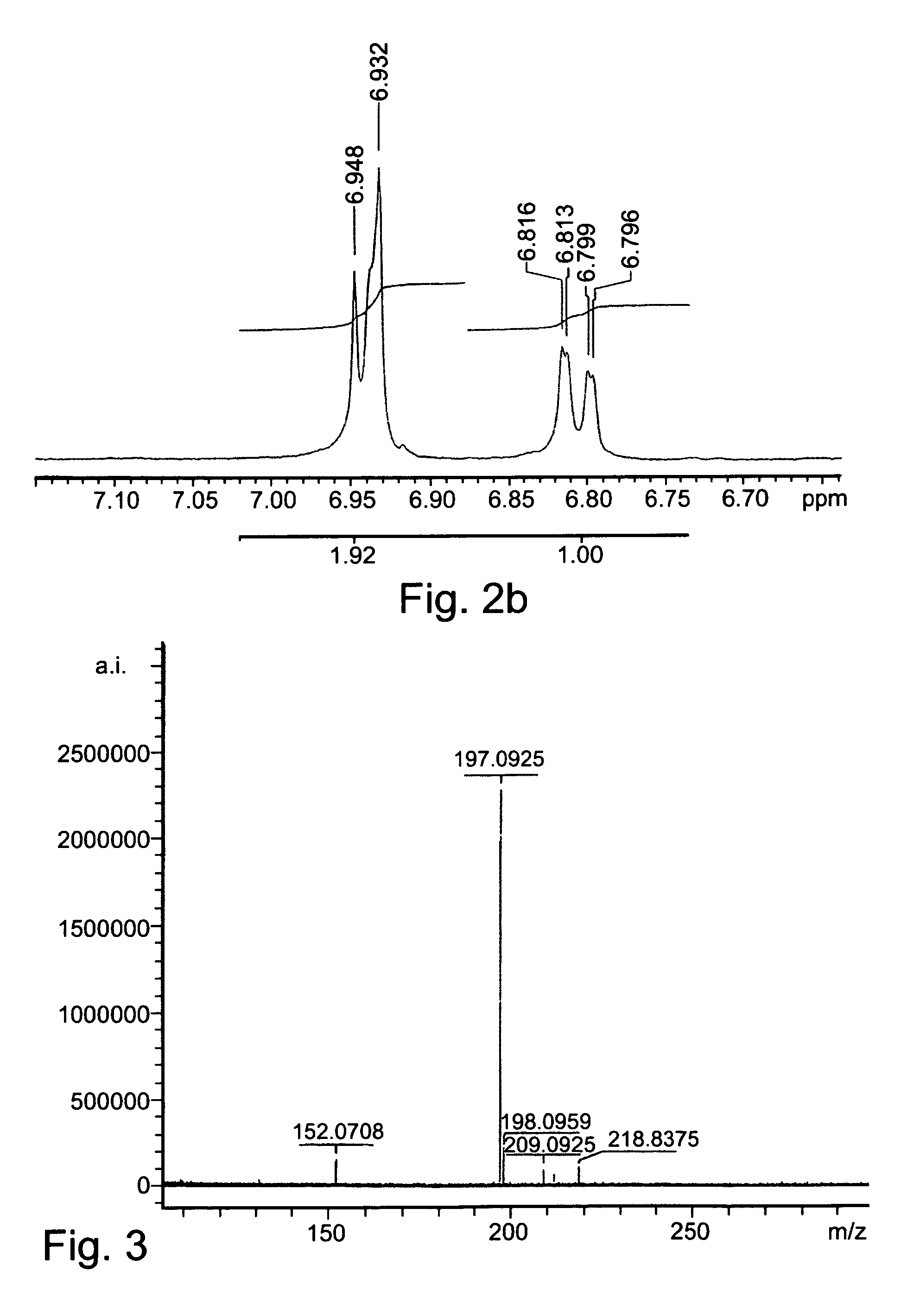
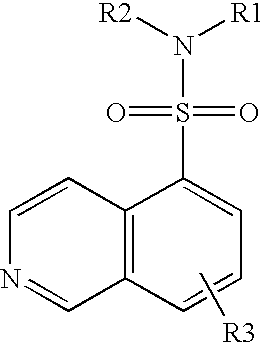
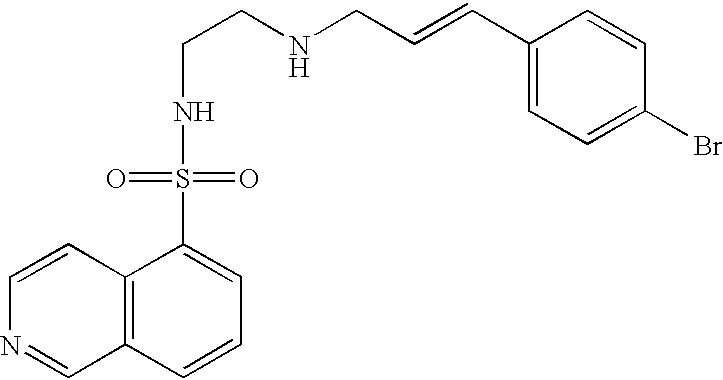
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
144 results about "Dopaminergic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dopaminergic means "related to dopamine" (literally, "working on dopamine"), dopamine being a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain. Dopaminergic brain structures facilitate dopamine-related activity. For example, certain proteins such as the dopamine transporter (DAT), vesicular monoamine transporter 2 (VMAT₂), and dopamine receptors can be classified as dopaminergic, and neurons that synthesize or contain dopamine and synapses with dopamine receptors in them may also be labeled as dopaminergic. Enzymes that regulate the biosynthesis or metabolism of dopamine such as aromatic L-amino acid decarboxylase or DOPA decarboxylase, monoamine oxidase (MAO), and catechol O-methyl transferase (COMT) may be referred to as dopaminergic as well. Also, any endogenous or exogenous chemical substance that acts to affect dopamine receptors or dopamine release through indirect actions (for example, on neurons that synapse onto neurons that release dopamine or express dopamine receptors) can also be said to have dopaminergic effects, two prominent examples being opioids, which enhance dopamine release indirectly in the reward pathways, and some substituted amphetamines, which enhance dopamine release directly by binding to and inhibiting VMAT₂.
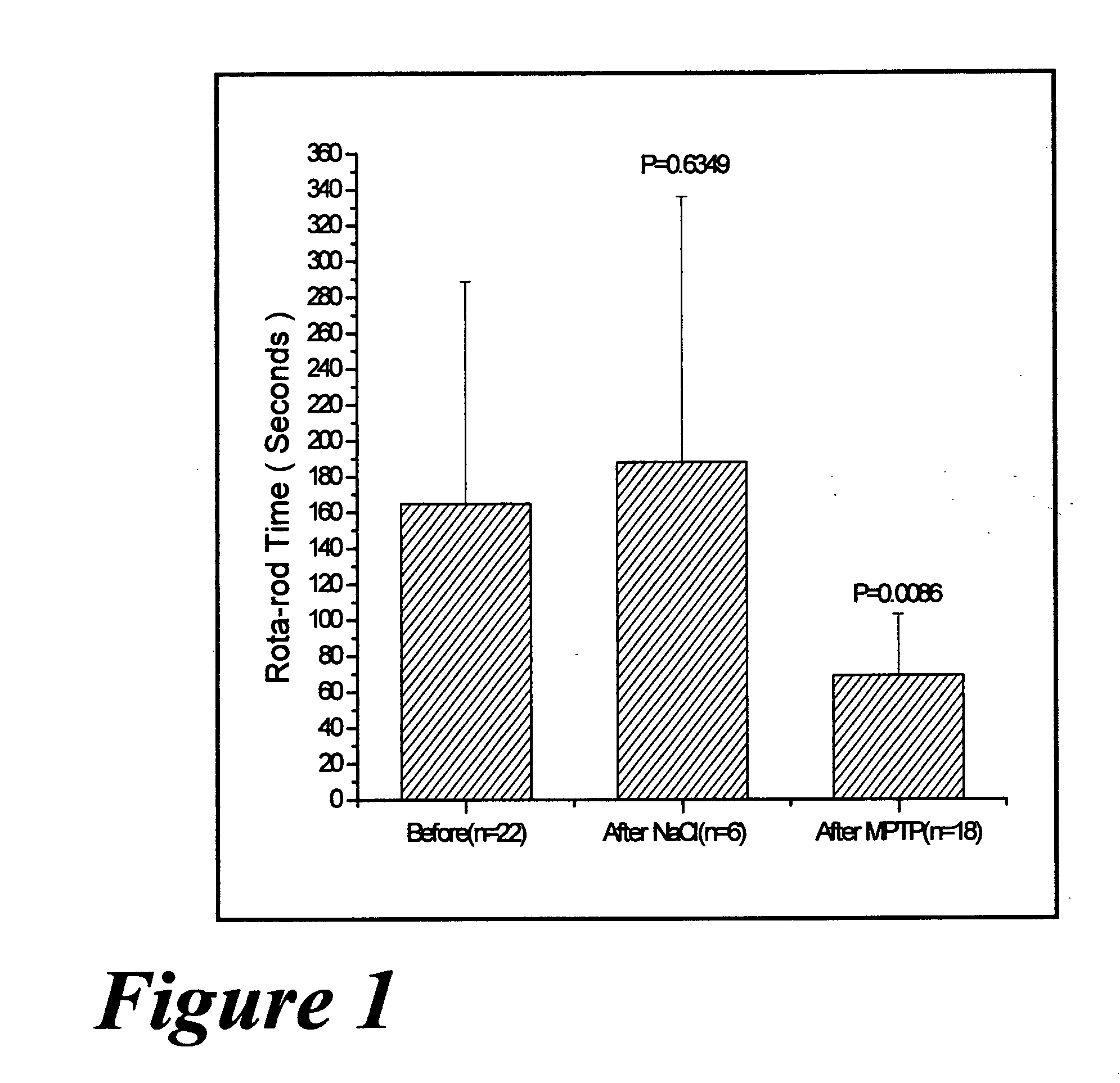
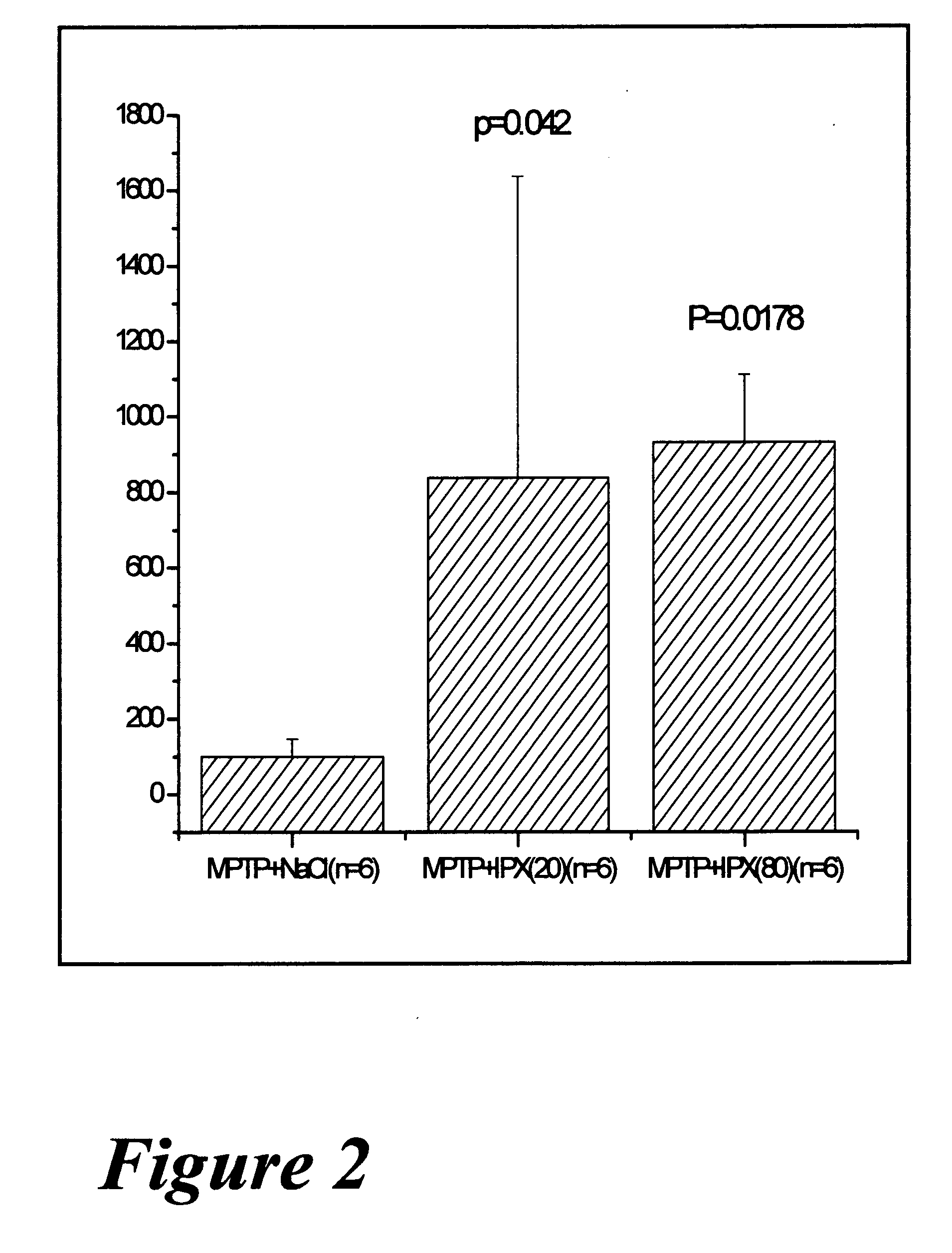
Use of rotigotine for treatment or prevention of dopaminergic neurone loss
The invention relates to the use of rotigotine or salts thereof and prodrugs for the production of a medicament for the treatment or prevention of dopaminergic cell destruction in diseases which are connected to increased dopaminergic cell destruction. The invention also relates to the use of rotigotine as a medicament for the preventive treatment of Parkinson's disease.
Owner:UCB SA
Methods and compositions for treating distress dysfunction and enhancing safety and efficacy of specific medications
InactiveUS20110159048A1Good treatment effectEliminate side effectsBiocideNervous disorderDiseaseNeurotransmitter systems
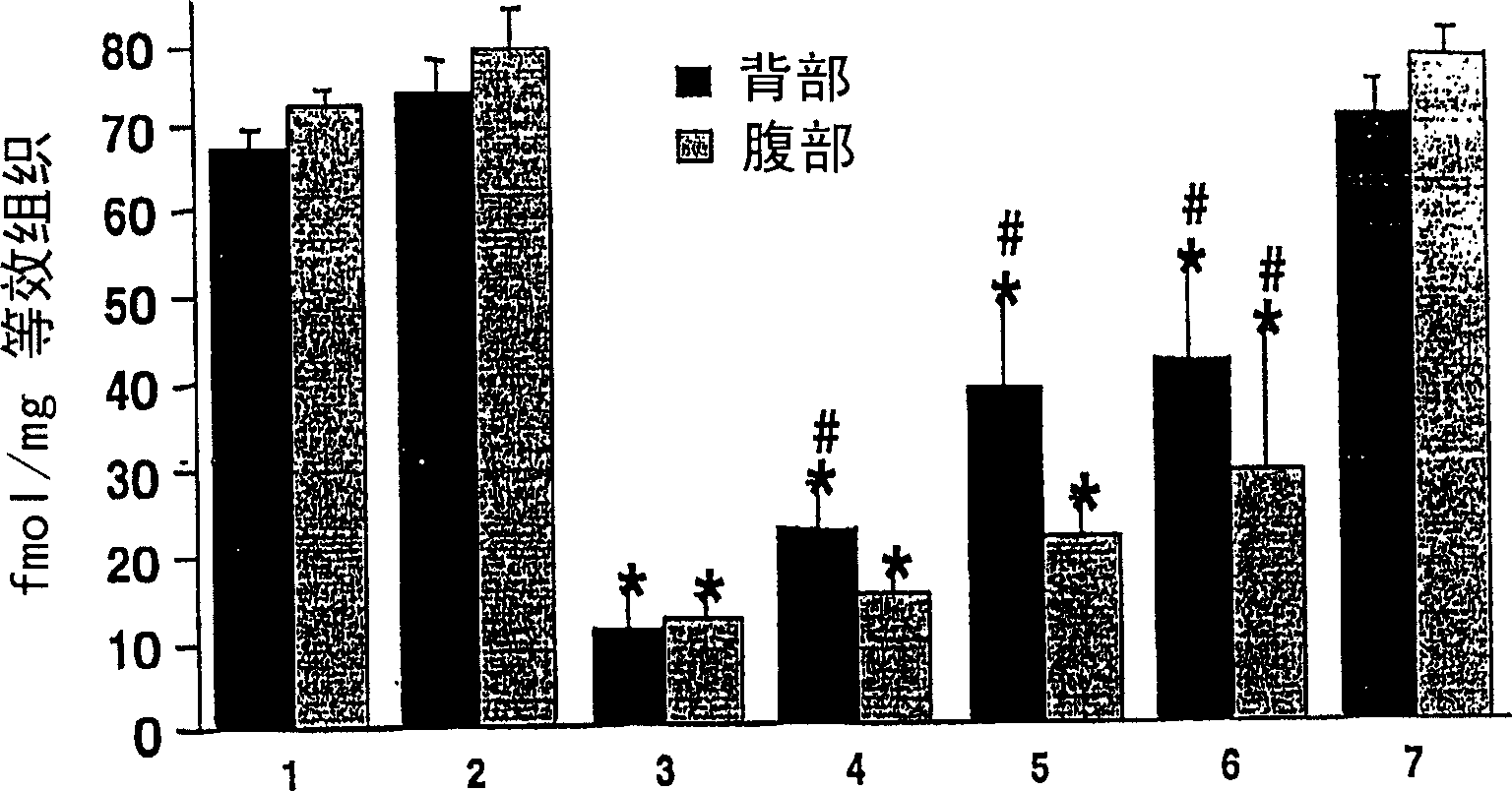
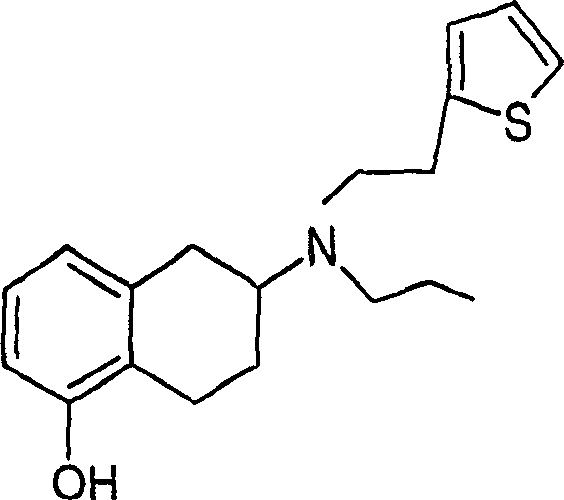
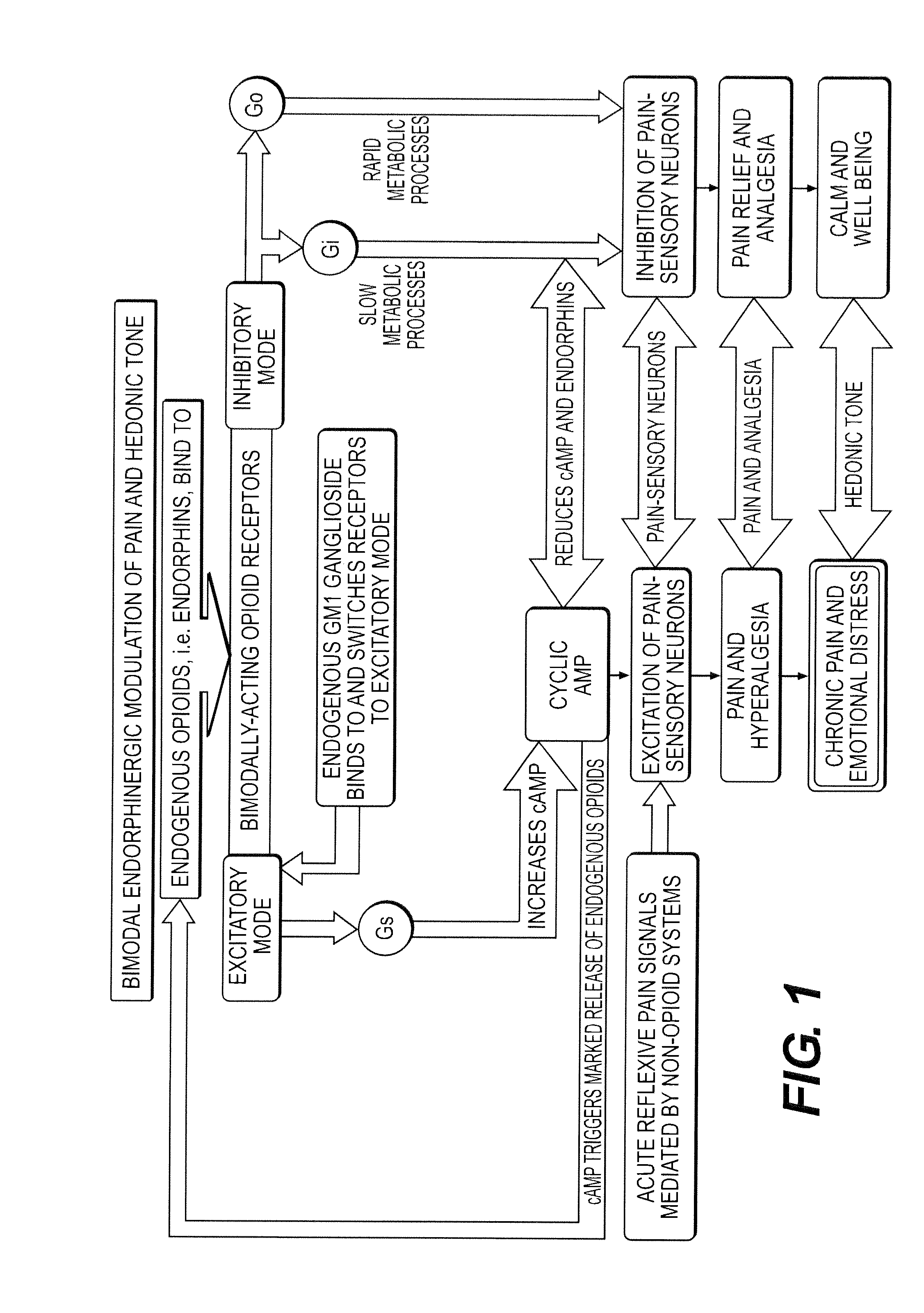
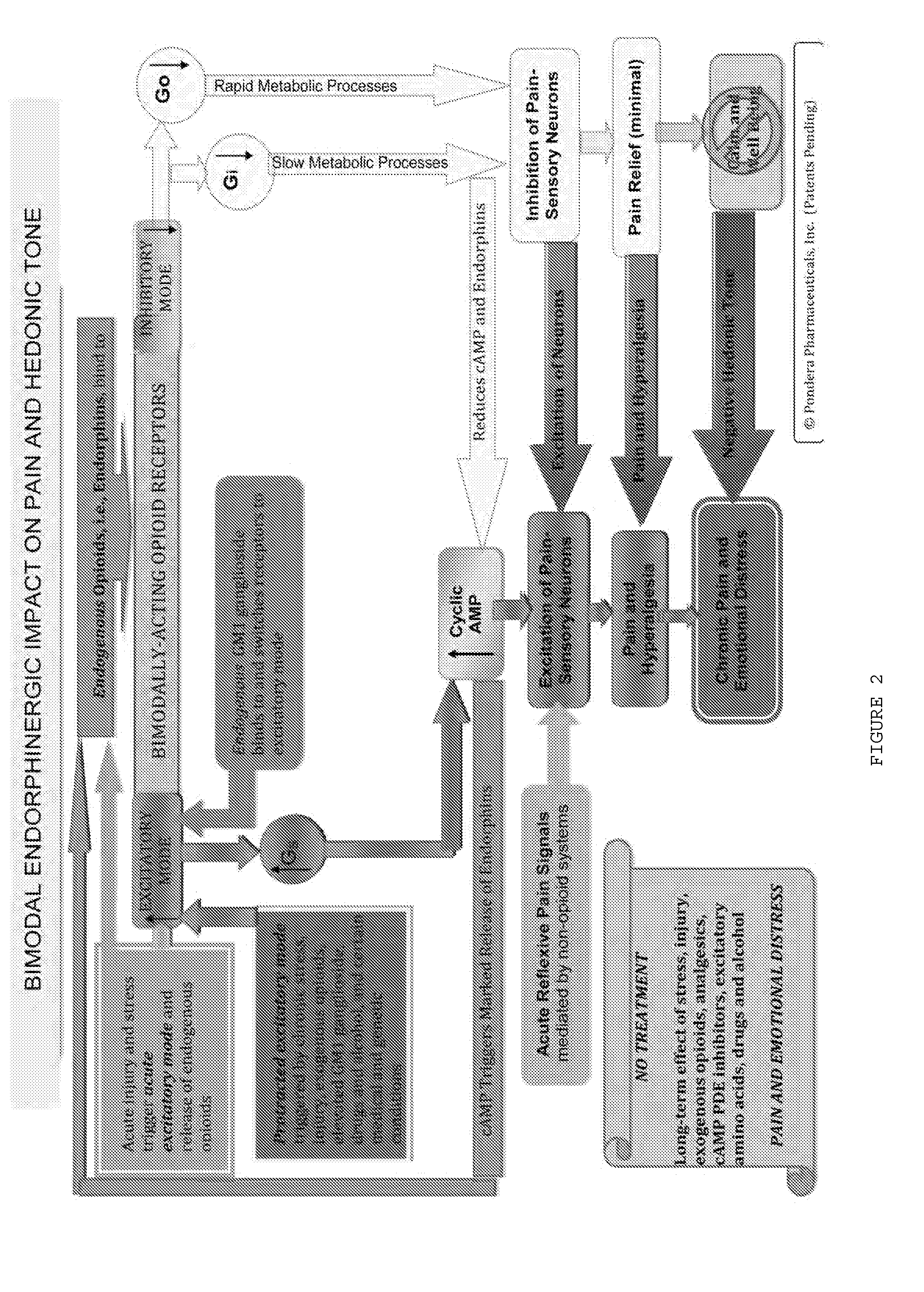
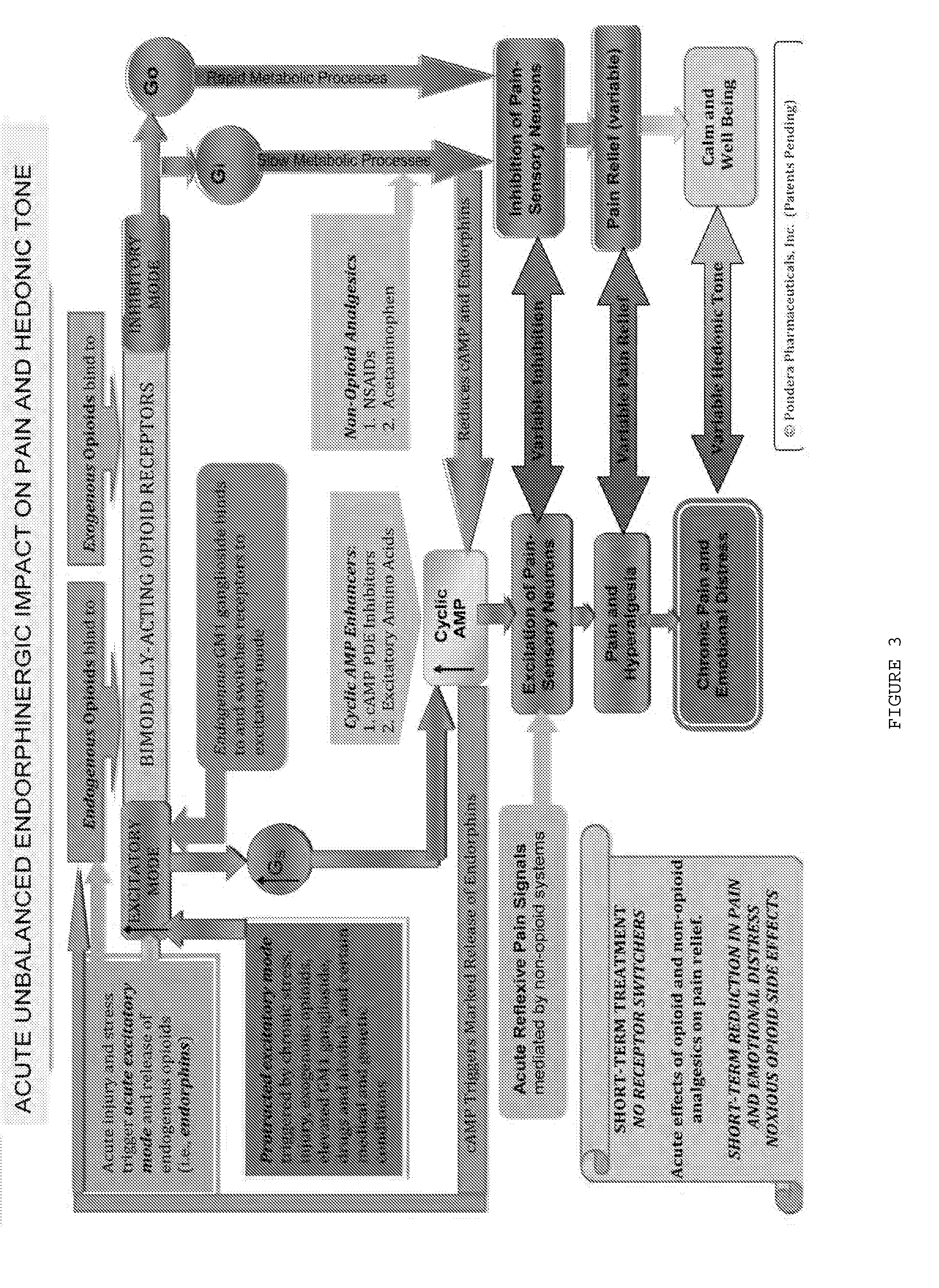
The present invention relates to methods and compositions for reducing Distress Dysfunction by restoring and maintaining homeostatic balance in the neurotransmitter systems underlying the Stress Response and the experience of distress and hedonic tone. Distress Dysfunction refers to the experience of dysfunctional emotional and physical distress that interferes with the individual's quality of life and functioning. A novel understanding of the bimodal opioid modulation of pain, and its impact, through serotonergic, dopaminergic, epinephrinergic, and norepinephrinergic processes, on hedonic tone, leads directly to new generation pharmaceutical formulations that are remarkably safe and effective for the treatment of a wide variety of Distress Dysfunctions, including anxiety, depression, anger, insomnia, mood disorders, eating disorders, sexual problems, pain, substance and behavioral addictions, gastrointestinal disorders, autistic spectrum disorders, attention-deficit and hyperactivity disorders, and other emotional and physical distress disorders. The foundation of this discovery is the power of Receptor Switchers, such as ultra-low-dose and very-low-dose opioid antagonists and GM1 ganglioside attenuators, in blocking acute and protracted excitatory opioid receptor signaling. Co-administration of Receptor Switchers with Endorphin Enhancers, such as specific cAMP PDE inhibitors and excitatory amino acids, is an excellent formulation for restoring healthy homeostatic balance to the endogenous opioid system, using the body's endorphins to reduce emotional and physical distress, and through synergistic and homeostatic processes, restoring positive hedonic tone. The addition of Synergistic Enhancers, such as amino acids, SSRI and SNRI agents, and non-opioid analgesics, as well as Exogenous Opioids, enhances and prolongs these therapeutic benefits. The novel principles discovered by this invention also teach a new generation of safe and effective formulations for the treatment of respiratory conditions, neuropathy, and nociceptive pain.
Owner:PONDERA BIOTECH
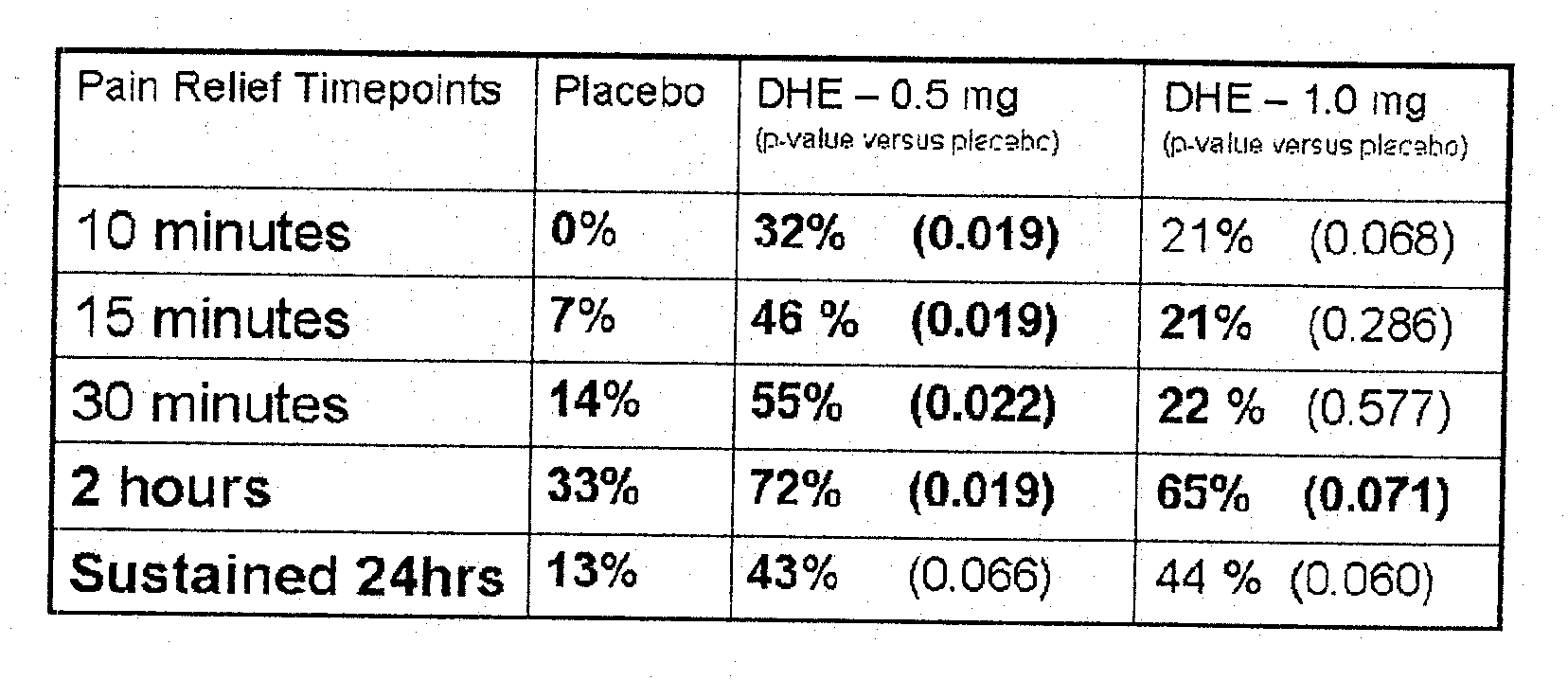
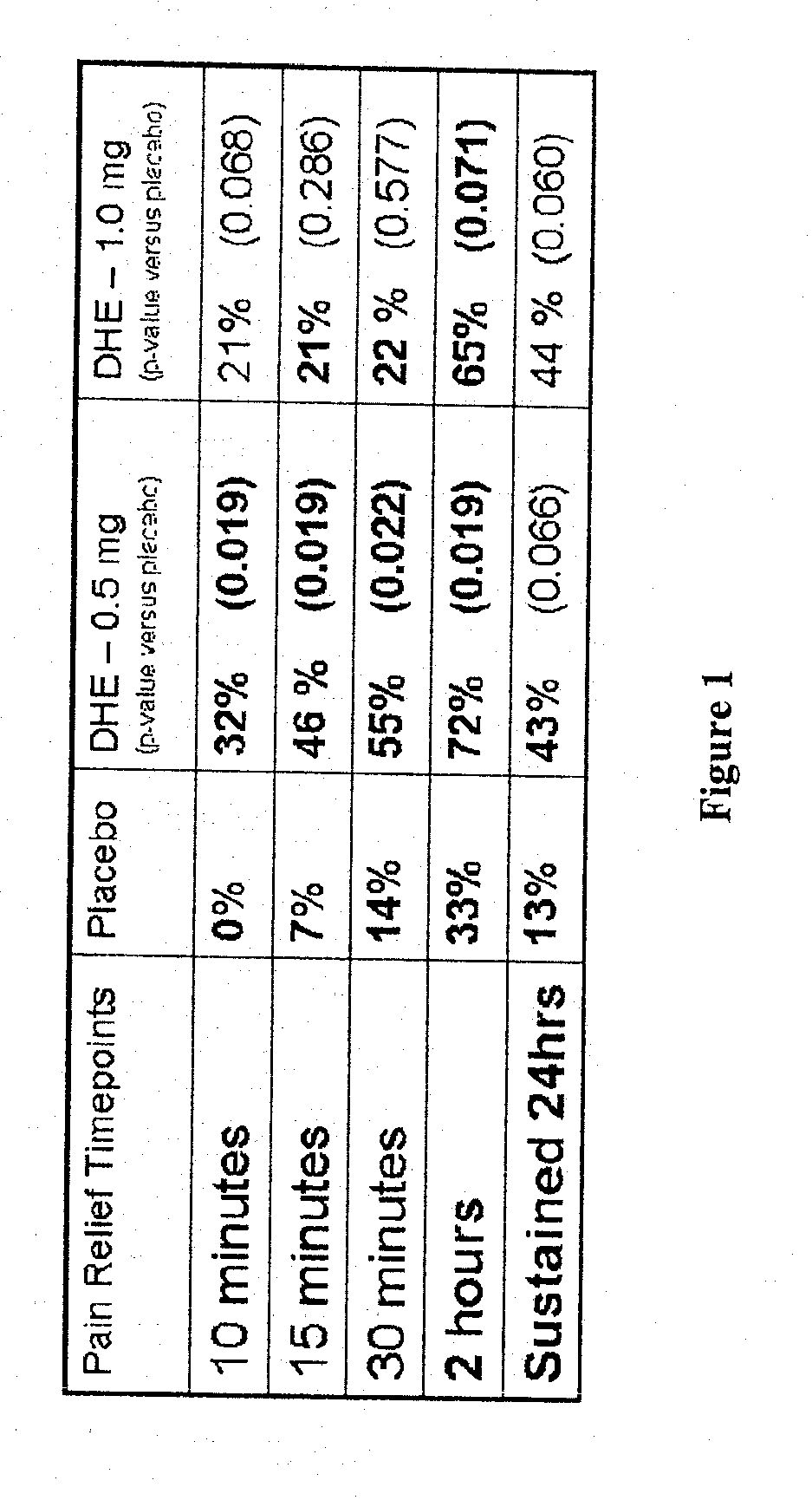
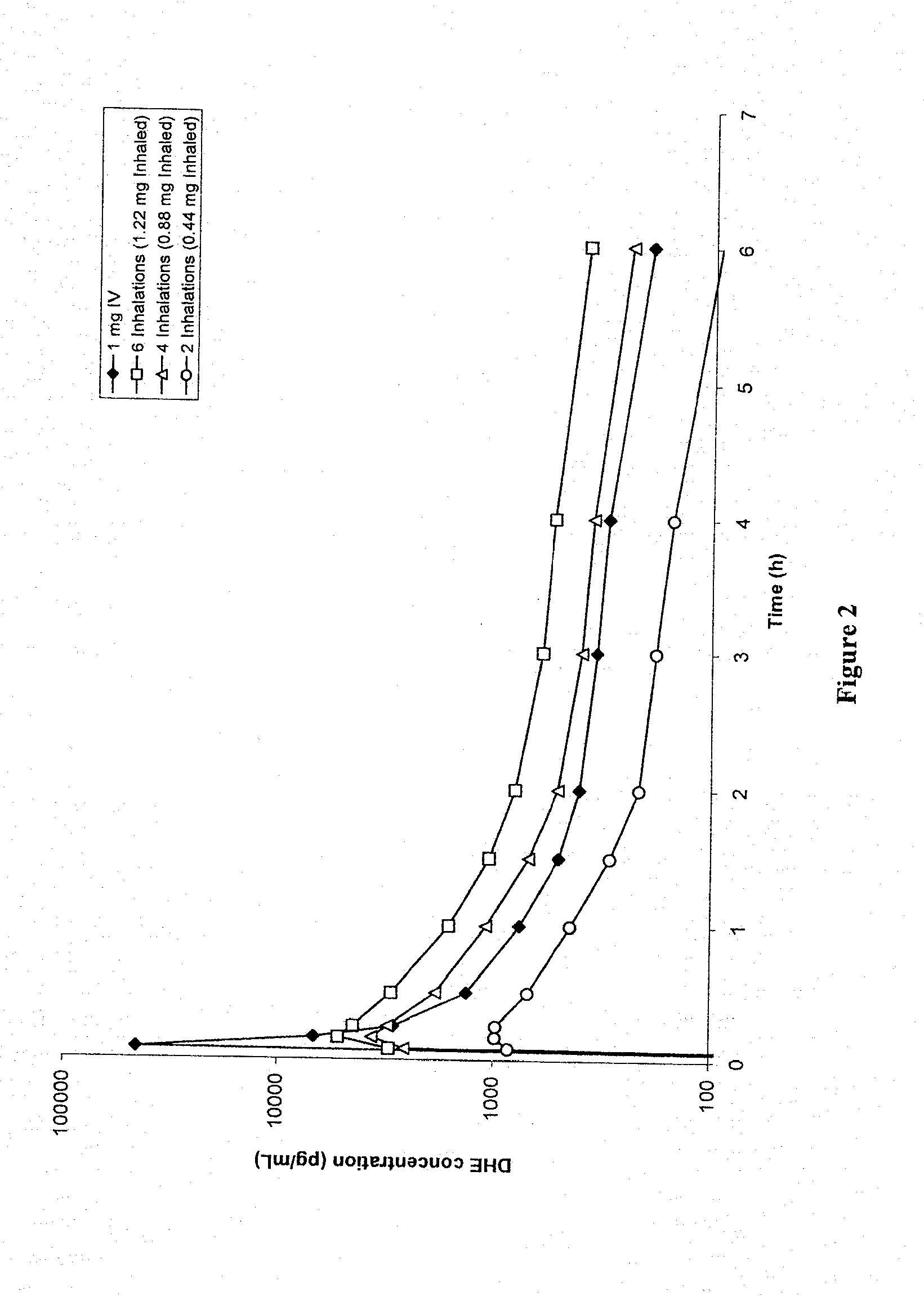
Method of therapeutic administration of dhe to enable rapid relief of migraine while minimizing side effect profile
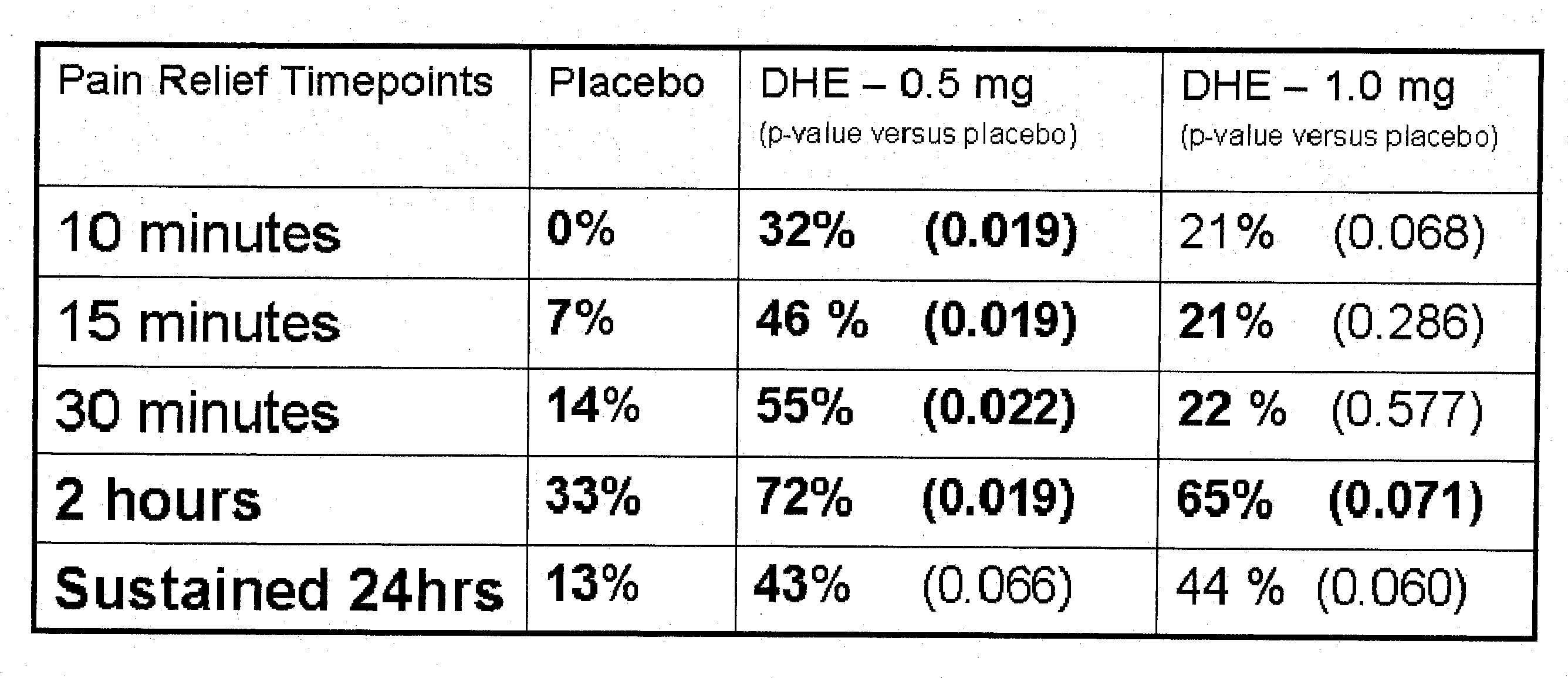
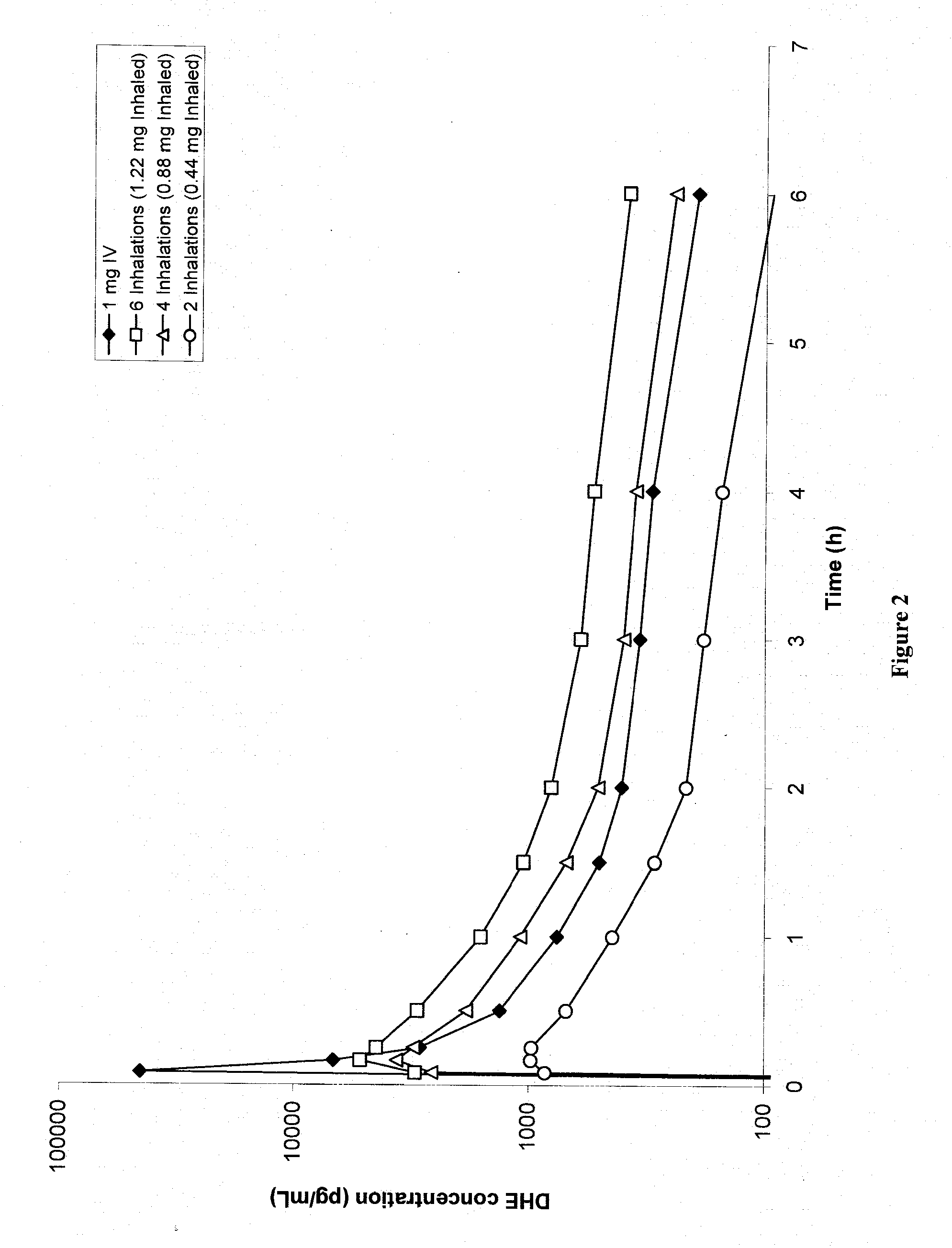
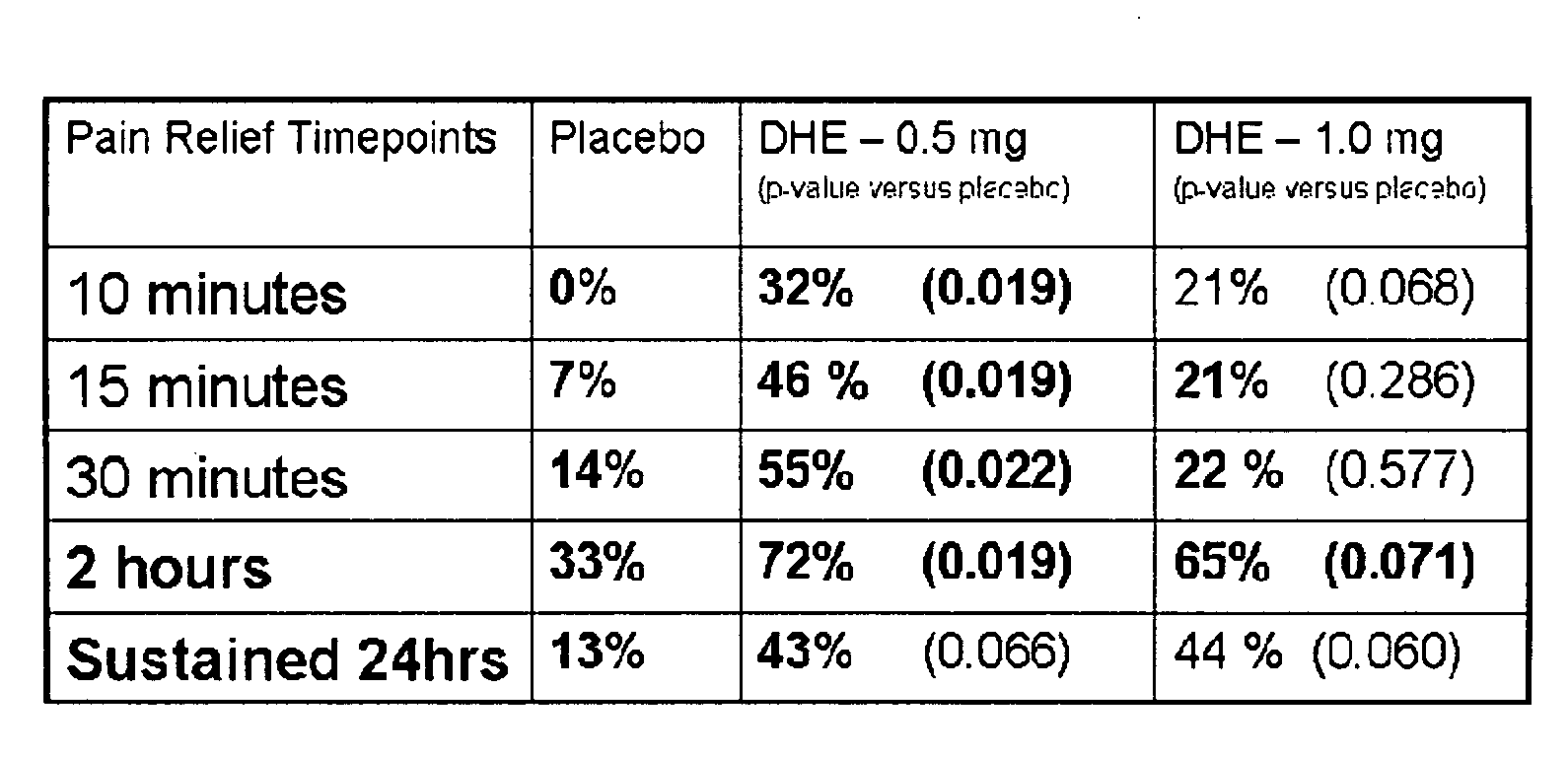
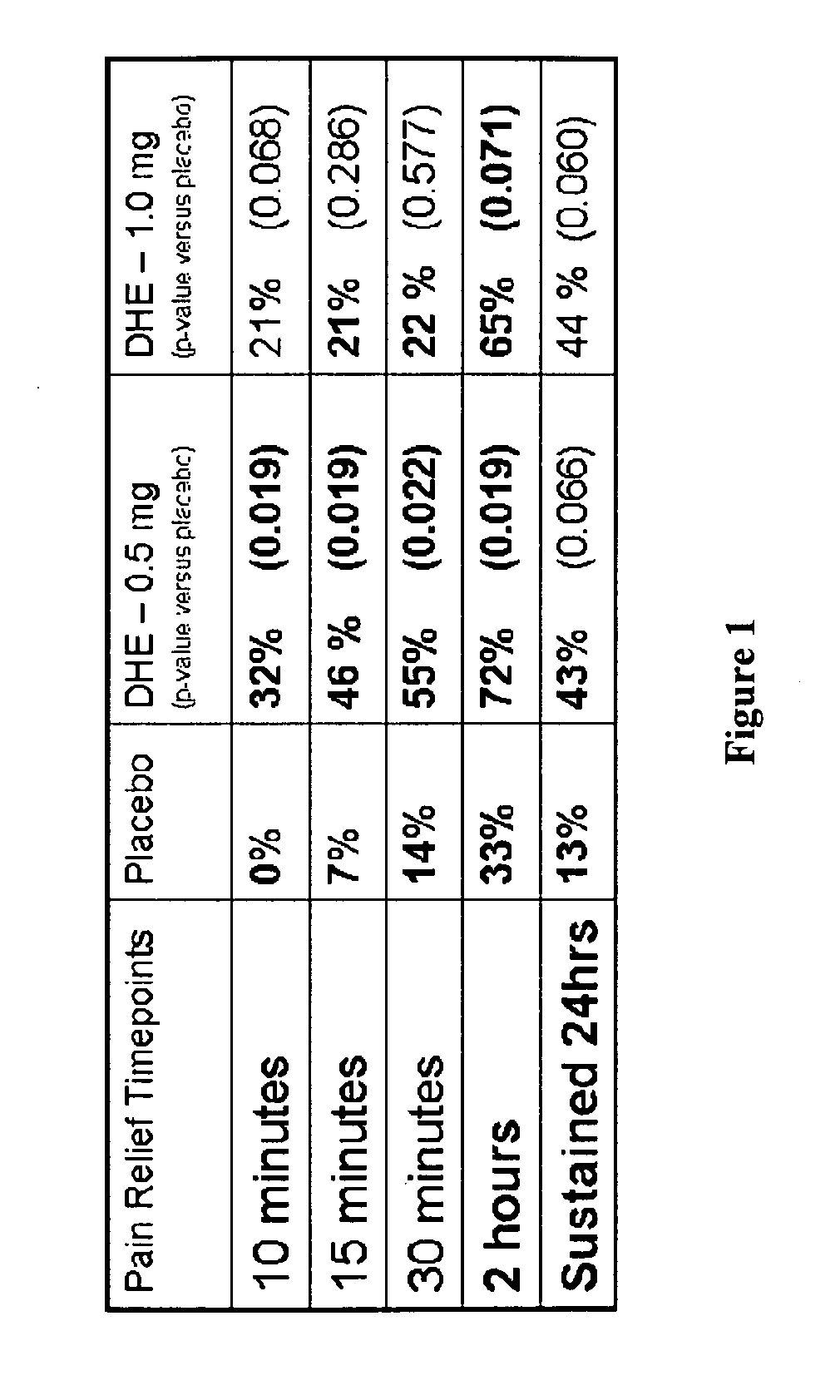
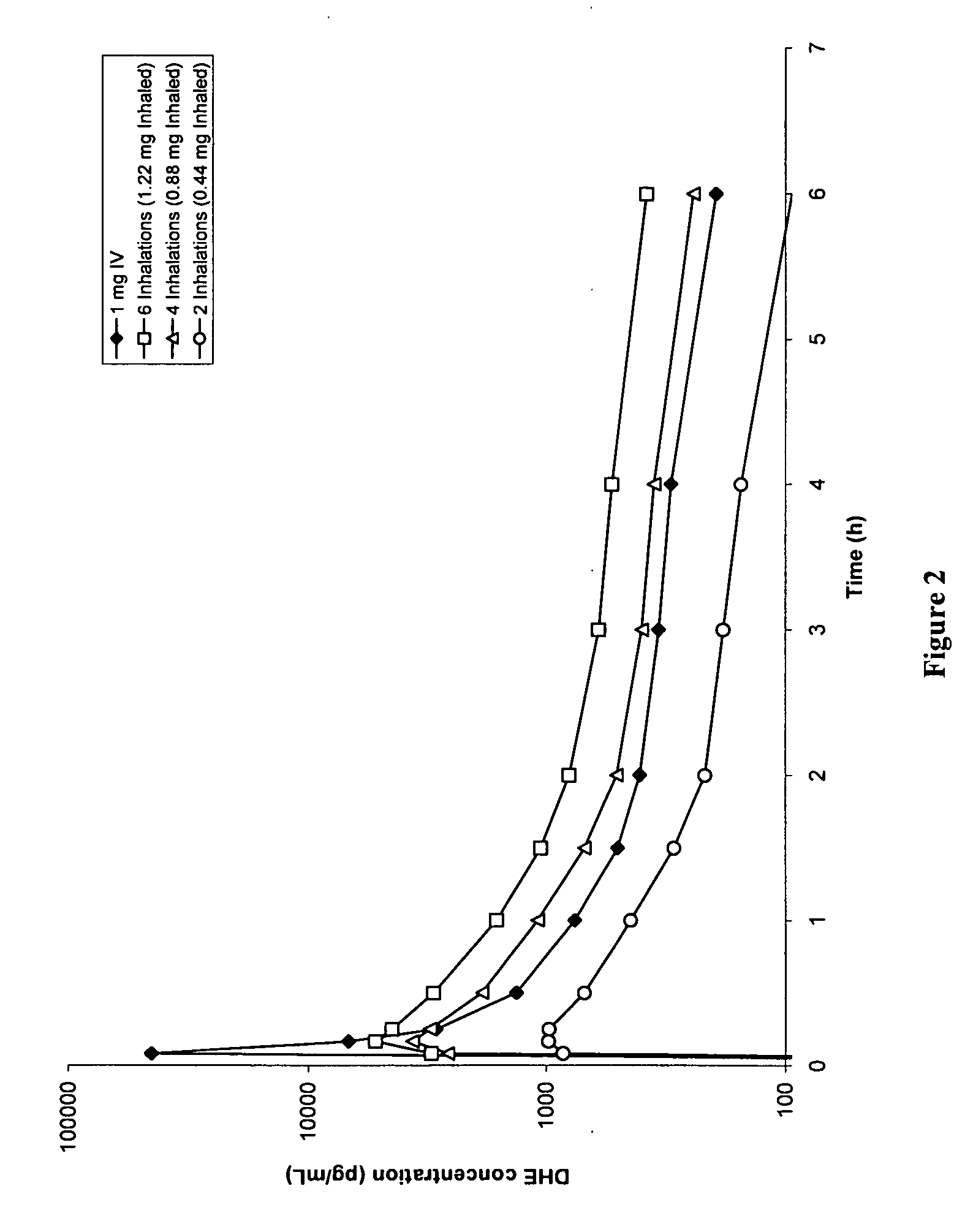
Pharmaceutical compositions containing dihydroergotamine (DHE) and methods in which DHE is administered to patients for treatment of migraine without side effects or adverse effects are disclosed. Methods for rapid treatment of migraine with DHE are disclosed comprising: dampening the peak plasma concentration (Cmax) and slightly delaying the peak such as to avoid activating the dopaminergic and adrenergic receptors, while achieving sufficient active binding to the serotonin receptors to provide relief from migraine symptoms within a timeframe that permits rapid resolution of migraine symptoms. Inhaler devices suitable for the methods are disclosed. Kits for practicing the methods of invention are disclosed.
Owner:MAP PHARMACEUTICAL INC
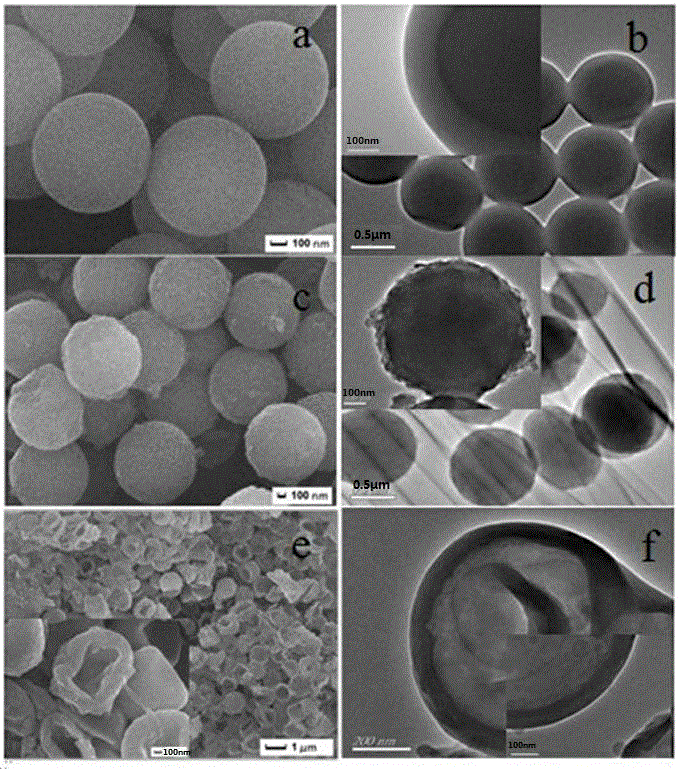
Phenylboronic-acid-functionalized graphene oxide composite nano material and preparation and application thereof
ActiveCN106475068AEasy to manufactureMild conditionsOther chemical processesAlkali metal oxides/hydroxidesPhenylboronic acidOxide composite
The present invention relates to a new phenylboronic-acid-functionalized graphene oxide composite nano material and preparation and application thereof. The material is prepared by immobilization of polydopamine-packed magnetic nanoparticles onto polyethyleneimine-modified graphene oxide and further introduction of phenylboronic acid monomers with carboxyl by amino of polyethyleneimine, and finally the material is used for enrichment of glycoproteins. The specific process is as follows: firstly, Fe3O4 magnetic nanoparticles are prepared by a solvothermal method, and auto polymerization of polydopamine can be performed on surface of the Fe3O4 magnetic nanoparticles under alkaline conditions; the magnetic graphene oxide composite nano material can be prepared by hydrogen bond and PI-PI interaction between the polydopamine and graphene oxide, then the positively charged polyethyleneimine is immobilized onto the surface of the negatively charged magnetic graphene oxide by electrostatic self-assembly; and finally the phenylboronic-acid-functionalized magnetic graphene oxide composite nano material can be prepared by introduction of the phenylboronic acid monomers by amidation reaction, and the phenylboronic-acid-functionalized magnetic graphene oxide composite nano material is successively used in the enrichment of the glycoproteins in bioanalysis.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Production of midbrain dopaminergic neurons and methods for the use thereof
Methods are provided for efficient production of midbrain dopaminergic (DA) neurons. In some aspects, methods involve differentiation and selection of DA neurons for a transgenic pluripotent cell population (e.g., cells comprising a selectable marker gene). Cell populations produced by the instant methods and methods of their use are likewise provided.
Owner:FUJIFILM CELLULAR DYNAMICS INC
Therapeutic process for the treatment of the metabolic syndrome and associated metabolic disorders
Owner:VEROSCI
L-DOPA amide derivatives and uses thereof
L-DOPA amide derivatives, pharmaceutical compositions containing same and their use in the treatment of conditions associated with impaired dopaminergic activity / signaling (e.g., Parkinson disease) are disclosed.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Cell system for alleviating syndromes of Parkinson's disease in a mammal
A cell system for treating neurodegenerative disorders in a mammal is provided. The cell system includes a population of neurons differentiated from umbilical mesenchymal stem cells for expressing at least one of tyrosine hydroxylase (TH), dopamine-β-hydroxylase (DBH), glutamate decarboxylase (GAD), aromatic L-amino acid decarboxylase (AADC) and dopaminergic transporter (DAT) in a cell culture. A method for treating neurodegenerative disorders in a mammal is also provided. The method comprises the steps of differentiating umbilical mesenchymal stem cells into a population of neurons that express at least one of TH, DBH, GAD, AADC and DAT in a cell culture, and transplanting the population of neurons into the brain of the mammal.
Owner:FU YU SHOW +1
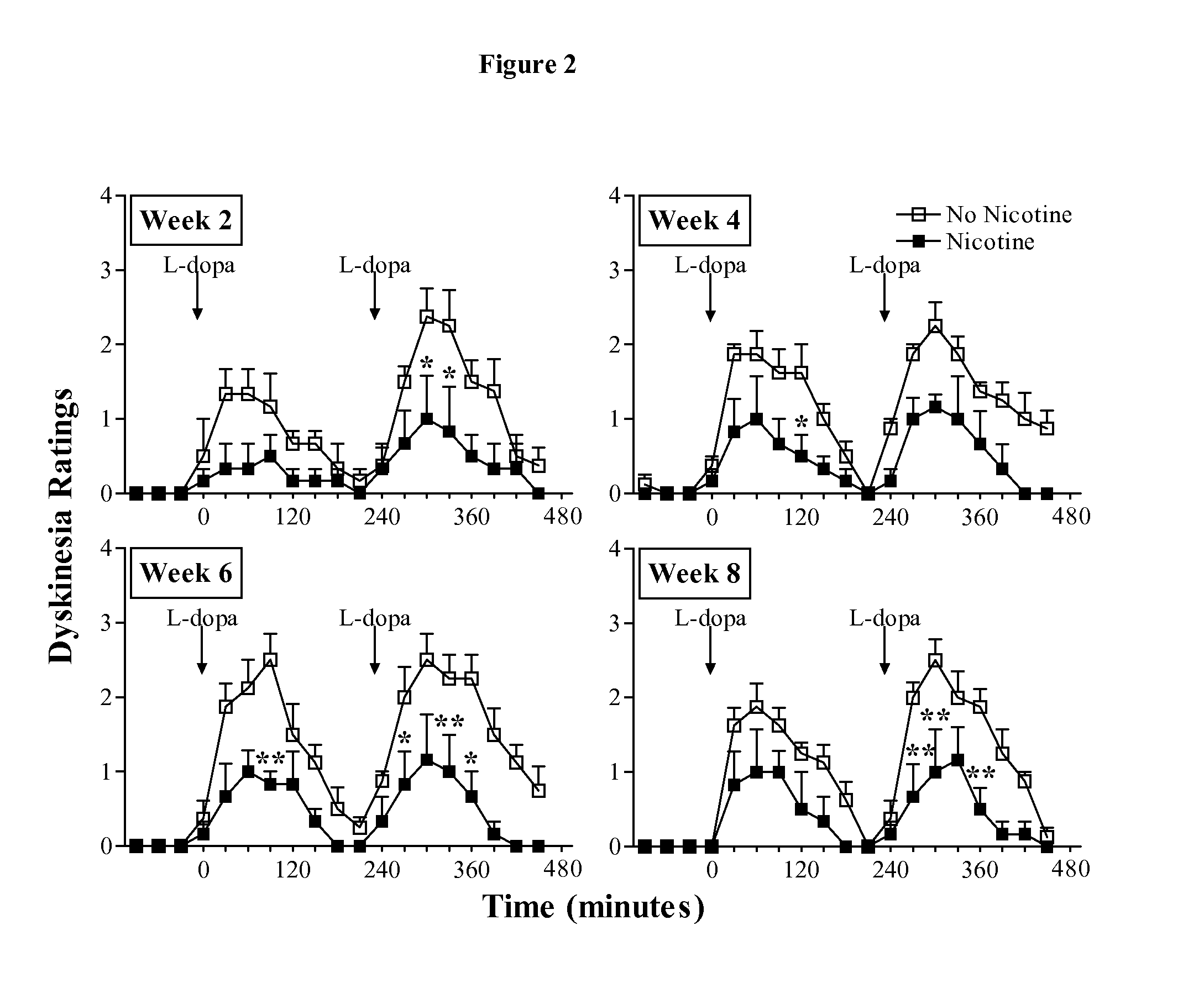
Methods and compositions for reduction of side effects of therapeutic treatments
The invention provides compositions and methods utilizing a nicotinic receptor modulator, e.g., to reduce or eliminate a side effect associated with dopaminergic agent treatment. In some embodiments, the invention provides compositions and methods utilizing a combination of a dopaminergic agent and a nicotinic receptor modulator that reduces or eliminates a side effect associated with dopaminergic agent treatment.
Owner:THE PARKINSONS INST
L-DOPA amide derivatives and uses thereof
L-DOPA amide derivatives, pharmaceutical compositions containing same and their use in the treatment of conditions associated with impaired dopaminergic activity / signaling (e.g., Parkinson disease) are disclosed.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Regulators of protein misfolding and aggregation and methods of using the same
InactiveUS20070204352A1Increase in protein misfoldingPromote aggregationCompound screeningNervous disorderCell AggregationsNucleotide
Polynucleotide molecules and the proteins encoded by the molecules, diagnostic and treatment methods for neurological disorders characterized by protein aggregation are provided. Genes are described herein that affect the misfolding of, and subsequent aggregation of, aggregation-prone proteins such as alpha-synuclein and have implications for the diagnosis and treatment of neurological diseases related to protein aggregation such as Parkinson's disease. Knockdown of expression of the genes described herein using RNAi results in alpha-synuclein protein aggregation in a C. elegans model of protein aggregation. Dopaminergic neuroprotection after exposure to the neurotoxin 6-OHDA or overexpression of alpha-synuclein may also be provided by overexpression of proteins. Knowledge of genes relating to protein misfolding and aggregation provides powerful means to develop diagnostic screening methods, mutation analysis and drug design information for the development of novel therapeutic and neuroprotective compounds to treat neurodegenerative diseases such as Parkinson's disease.
Owner:UNIVERSITY OF ALABAMA
Pharmaceutical dopamine glycoconjugate compositions and methods of their preparation and use
Owner:GLYCON
Intranasal administration of pharmaceutical agents for treatment of neurological diseases
Pharmaceutical formulations for treating neurological diseases are described, wherein the formulations comprise a pharmaceutically active agent-transport moiety complex. The formulations are suitable for administration via an intranasal route. Neurological diseases and conditions are associated with reduced brain insulin signaling (i.e., CNS insulin insensitivity), reduced dopaminergic signaling, reduced serotonergic signaling, reduced cholinergic signaling, or reduced GABAergic signaling, and include Alzheimer's disease, Parkinson's disease, epilepsy, neuropathic pain, fibromyalgia, post-herpetic neuralgia, insomnia, or anxiety. Neurological diseases also include cancers of the central nervous system (CNS).
Owner:WONG PATRICK SL
Excitant of dopamine transport protein and usage
The present invention discloses an application of flavone compound or its derivative in preparation of medicine for curing psychic disease and nervous disease. Said invention also discloses a medicine composition containing flavone compound or its derivative. Said invention also provides an excitant of dopamine transport protein firstly, and the tests show that the dopamine transport protein can be excited.
Owner:上海国联干细胞技术有限公司
Method for preparing porous difunctional adsorption material
InactiveCN105289540AImprove stabilityAchieve simultaneous removalIon-exchange process apparatusOther chemical processesCypermethrinGlycidyl methacrylate
The invention relates to a method for preparing a porous difunctional adsorption material, which belongs to the technical field of the environment functional material. According to the invention, the method uses Hypermer 2296 to obtain a stabilize high internal phase emulsion, an oil phase comprises a monomer methyl acrylic glycidyl ester, a cross-linking agent divinyl benzene, a solvent toluene and an initiator azodiisobutyronitrile, a water phase is a potassium sulfate aqueous solution; after the water phase and oil phase are mixed, a stable water-in-oil emulsion is prepared after stirring preparation, a macroporous foam polymer is prepared after thermal initiation polymerization; then dopamine microspheres are grafted to a macroporous foamed material by a biology adhesion method; and finally, tetrahydrofuran is used for removing sulfonated styrene core and shell to obtain the macroporous material adhered with the hollow dopamine. The product has the characteristics of water-soluble performance and oil solubility of two materials, the hollow dopamine can effectively increase a specific surface area of the material and metal ions can be absorbed; the macroporous foam contains a lot of ester groups and are convenient for adhesion of trifluoro-cypermethrin, and two harmful substances can be removed simultaneously.
Owner:JIANGSU UNIV
Pharmaceutical dopamine glycoconjugate compositions and methods of their preparation and use
Hydrophilic transportable N-linked glycosyl dopaminergic prodrug compounds according to FORMULA V and methods of their use, wherein, Ring 1 comprises an aryl or heteroaryl ring having 4 to 8 carbon atoms, among which atoms are counted “X” and “Y”; each of X and Y is optional; X, when present is either —C(R1)2— or —C(R1)2—; Y, when present, is either —CH2— or —CH2—CH2—; z, R5 and R5′. are optional, and when present z, R5 and R5′ together form a lower alkyl or a substituted lower alkyl moiety; N is part of either an amine or an amide linkage; E is a saccharide which forms a linkage with N through a single bond from a carbon or oxygen atom thereof; R1 and R4 are selected form the group consisting of hydrogen, hydroxyl, halogen, halo-lower alkyl, alkoxyl, alkoxyl-lower alkyl, halo-alkoxy, thioamido, amidosulfonyl, alkoxylcarbonyl, carboxamide, aminocarbonyl, and alkylamino-carbonyl; R2 and R3 are hydroxyl; R5 and R6, when present, are selected from the group consisting of hydrogen, hydroxyl, alkoxyl, carbonyl, alkoxylcarbonyl, aminocarbonyl, alkylamino-carbonyl and dialkylamino-carbonyl; and, R6 and R6′ are selected from the group consisting of hydrogen, hydroxyl, alkoxyl, carboxyl, alkoxylcarbonyl, aminocarbonyl, alkylamino-carbonyl and dialylamino-carbonyl, with the proviso that Ring 1 is capable of binding to any of: a dopaminergic receptor selected from the group consisting of a D1 receptor and a D5 receptor; a DAT transporter; a VMAT transporter; and, with the proviso that E is capable of binding to a GLUT transporter selected from the group consisting of a GLUT1 receptor and a GLUT3 receptor.
Owner:GLYCON
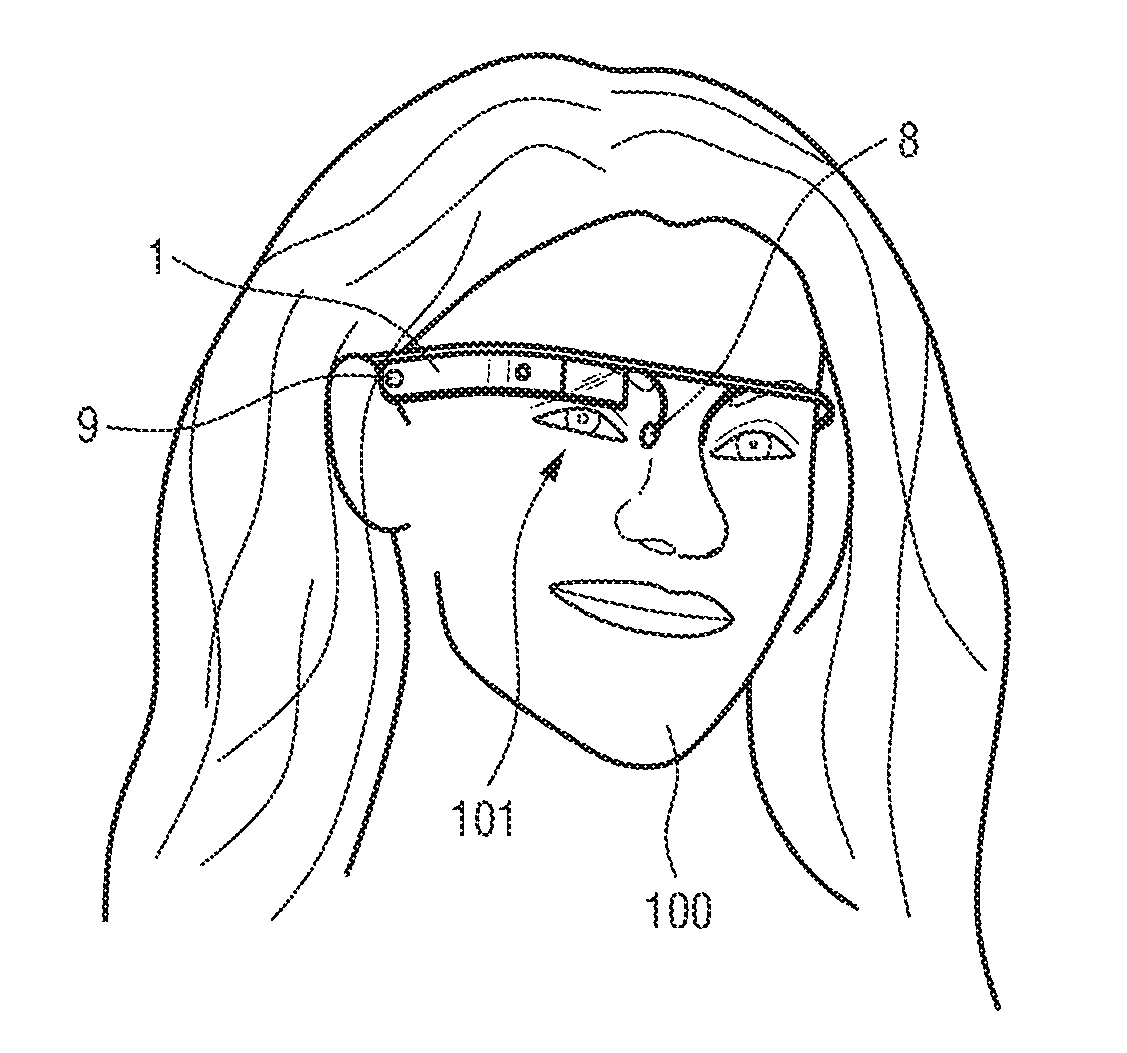
System for monitoring a dopaminergic activity
InactiveUS20160374594A1Easy to adjustEarly screeningElectro-oculographySensorsDopaminergicContinuous monitoring
The present invention relates to a system for monitoring a dopaminergic activity of a subject, wherein the system comprises a detection unit for detecting an eye blink signal relating to eye blinks of the subject; an analysis unit for determining, based on the detected eye blink signal, an eye blink feature indicative of the dopaminergic activity of the subject and for comparing the eye blink feature with a reference eye blink feature; and a feedback unit for providing feedback relating to the dopaminergic activity of the subject based on the comparison of the eye blink feature and the reference eye blink feature. Further aspects of the present invention relate to a corresponding method and computer program. The approach permits early screening of a dopaminergic activity and continuous monitoring of the patient's response to dopamine drugs, for example.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Method of therapeutic administration of DHE to enable rapid relief of migraine while minimizing side effect profile
Pharmaceutical compositions containing dihydroergotamine (DHE) and methods in which DHE is administered to patients for treatment of migraine without side effects or adverse effects are disclosed. Methods for rapid treatment of migraine with DHE are disclosed comprising: dampening the peak plasma concentration (Cmax) and slightly delaying the peak such as to avoid activating the dopaminergic and adrenergic receptors, while achieving sufficient active binding to the serotonin receptors to provide relief from migraine symptoms within a timeframe that permits rapid resolution of migraine symptoms. Inhaler devices suitable for the methods are disclosed. Kits for practicing the methods of invention are disclosed.
Owner:MAP PHARMACEUTICAL INC
Method of therapeutic administration of dhe to enable rapid relief of migraine while minimizing side effect profile
Pharmaceutical compositions containing dihydroergotamine (DHE) and methods in which DHE is administered to patients for treatment of migraine without side effects or adverse effects are disclosed. Methods for rapid treatment of migraine with DHE are disclosed comprising: dampening the peak plasma concentration (Cmax) and slightly delaying the peak such as to avoid activating the dopaminergic and adrenergic receptors, while achieving sufficient active binding to the serotonin receptors to provide relief from migraine symptoms within a timeframe that permits rapid resolution of migraine symptoms. Inhaler devices suitable for the methods are disclosed. Kits for practicing the methods of invention are disclosed.
Owner:MAP PHARMACEUTICAL INC
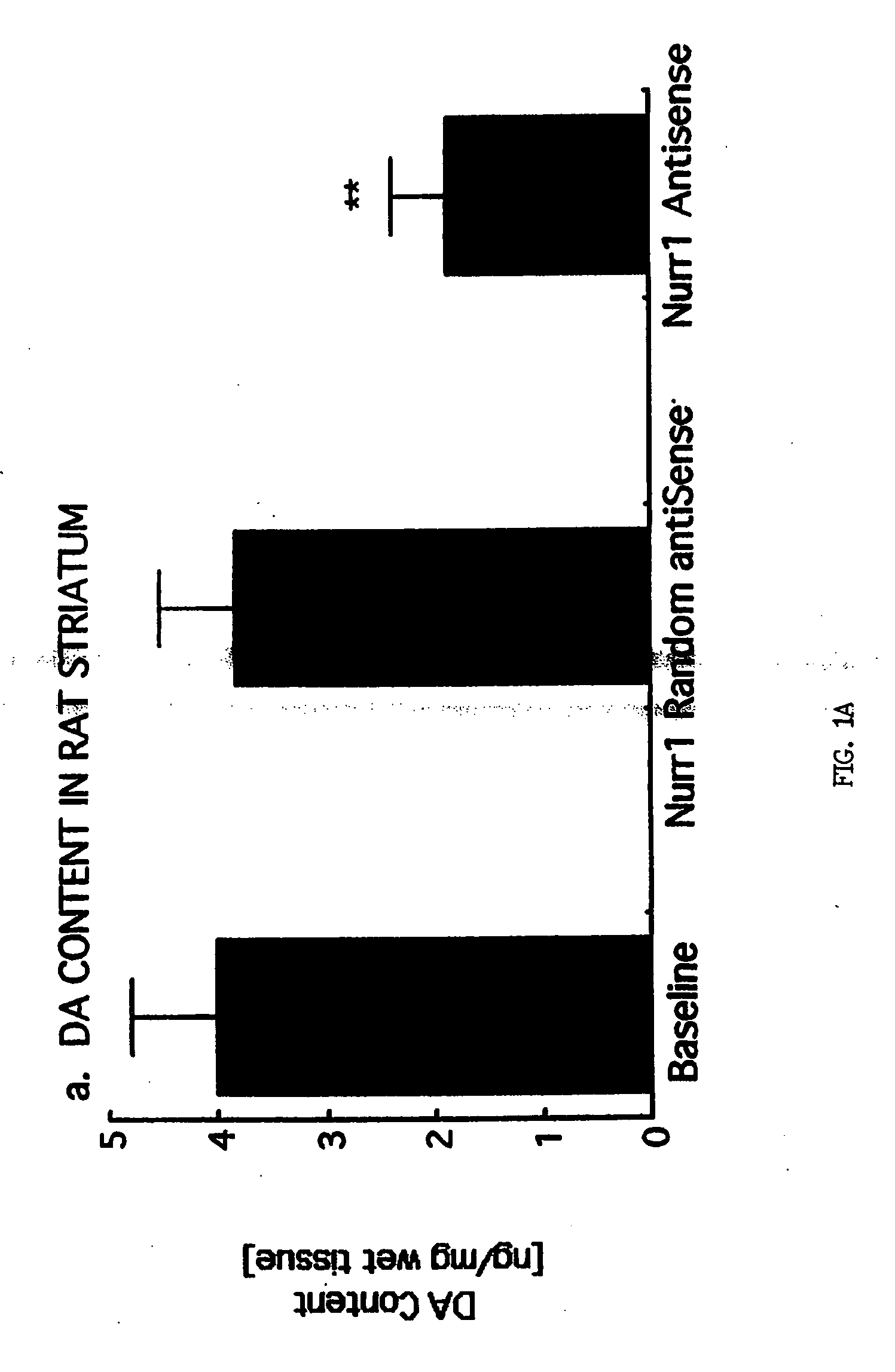
Methods and compositions for treating Parkinson's disease
InactiveUS20050070493A1Lower Level RequirementsIncrease in tyrosine hydroxylase activityNervous disorderPeptide/protein ingredientsMedicineDopaminergic
The present invention relates to novel methods and compositions for gene therapy. The invention also provides methods for treating diseases or disorders of the central nervous system associated with dopaminergic hypoactivity, disease, injury or chemical lesioning, including Parkinson's disease, manic depression, and schizophrenia.
Owner:BAYLOR COLLEGE OF MEDICINE +1
Regulation of differentiation into dopaminergic neurons by metalloprotease
InactiveUS20160040126A1Reduce expressionReduced activityOrganic active ingredientsNervous disorderProgenitorDisease
The present invention relates to a method for regulating the differentiation of neural stem cells or neural progenitor cells into dopaminergic neural cells, the method comprising increasing or inhibiting the activity of ADAM17 and / or ADAM10 in neural stem cells or neural progenitor cells, and to the use of an activator or inhibitor of ADMA17 and / or ADAM10.The method and composition according to the invention can regulate the activity of ADAM17 and / or ADAM10 in neural stem cells or neural progenitor cells to increase dopaminergic neural cells in the midbrain area, and thereby providing the effect of treating diseases induced by the death of dopaminergic neural cells such as Parkinson's disease. In addition, the method and composition according to the invention can inhibit the activity of ADAM17 and / or ADAM10, and thereby improving effect of treating diseases such as tumor. Thus, the present invention is very useful.
Owner:KOREA UNIV RES & BUSINESS FOUND
Dopaminergic stimulatory factor
InactiveUS20050148500A1Increasing dopamine levelImprove the level ofHormone peptidesBiocideNeuronal damageMedicine
The present invention provides a novel proteinaceous composition that increases the levels of dopamine (DA) in neurons. Therefore, provided are methods for treating conditions caused by the deficiency of dopamine and / or by the loss of DA neurons or injury to DA neurons. An example includes Parkinson's disease. Also provided are methods for isolating and partially purifying the proteins and further characterizing the same.
Owner:UNIVERSITY OF CHICAGO
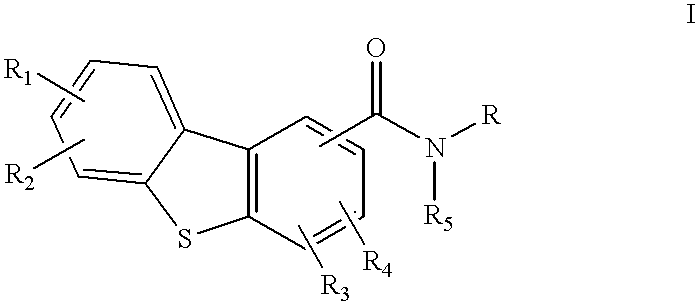
N-aminoalkyldibenzothiopencarboxamide receptor subtype specific ligands
Disclosed are compounds of the formula:or the pharmaceutically acceptable acid addition salts thereof, wherein:R1, R2, R3, R4 are the same or different and represent hydrogen, C1-C6 alkyl, halogen, hydroxy, amino, cyano, nitro, trifluoromethyl, trifluoromethoxy, C1-C6 alkoxy, -O2CR', -NHCOR', -COR', -SOmR', where R' is C1-C6 alkyl and wherein m is 0, 1 or 2; orR1, R2, R3, R4 independently represent -CONR'R'', or -NR'R'' where R' and R'' independently represent hydrogen or C1-C6 alkyl;R5 is hydrogen or C1-C6 alkyl; andR represents an aminoalkyl group,which compounds are useful in the treatment of affective disorders such as schizophrenia, depression, Alzheimer's disease, movement disorders such as Parkinsonism and dystonia, and other disorders which respond to dopaminergic blockade such as substance abuse and obsessive compulsive disorders. Further, compounds of this invention may be useful in treating the extrapyramidal side effects associated with the use of conventional neuroleptic agents.
Owner:NEUROGEN
Derivation of neural stem cells from embryonic stem cells and methods of use thereof
Provided is a method for the derivation of neural stem cells (NSCs) from embryonic stem cells (ESCs) and the use of the NSCs for treatment of various neural disorders. The NSCs that are derived from the ESCs are tissue-specific multipotent NSCs with a stable growth rate, unlimited self-renewal capacity, and a predictable differentiation profile. Being both non-tumorigenic and engraftable, the NSCs of the present invention have utility in repopulation stroke-damaged tissue. The NSCs of the present invention may be differentiated to produce tyrosine-hydroxylase expressing neurons, which may be used as a source of dopaminergic neurons for subjects suffering from a condition characterized by dopaminergic dysfunction, such as Parkinson's disease.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Method
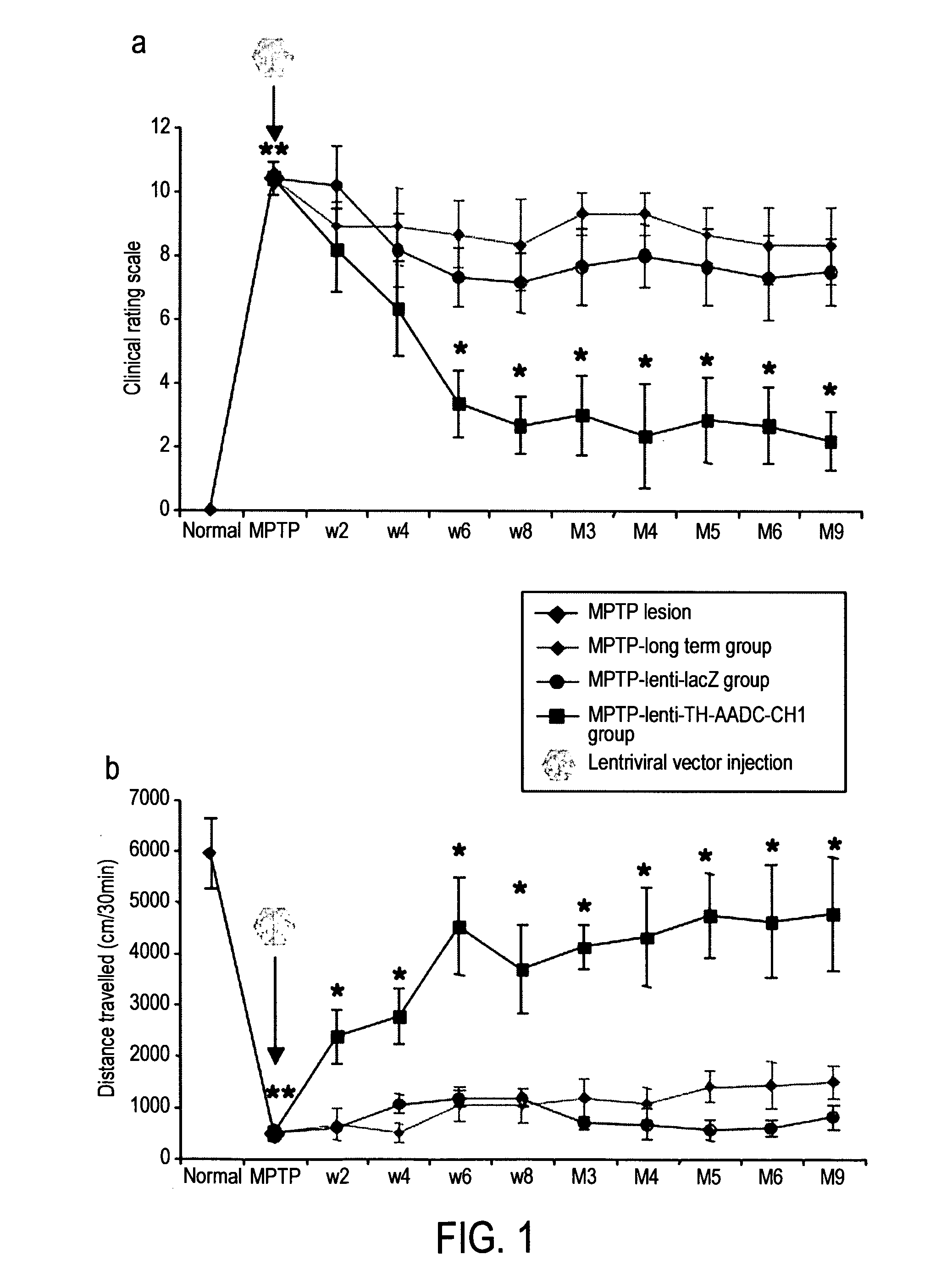
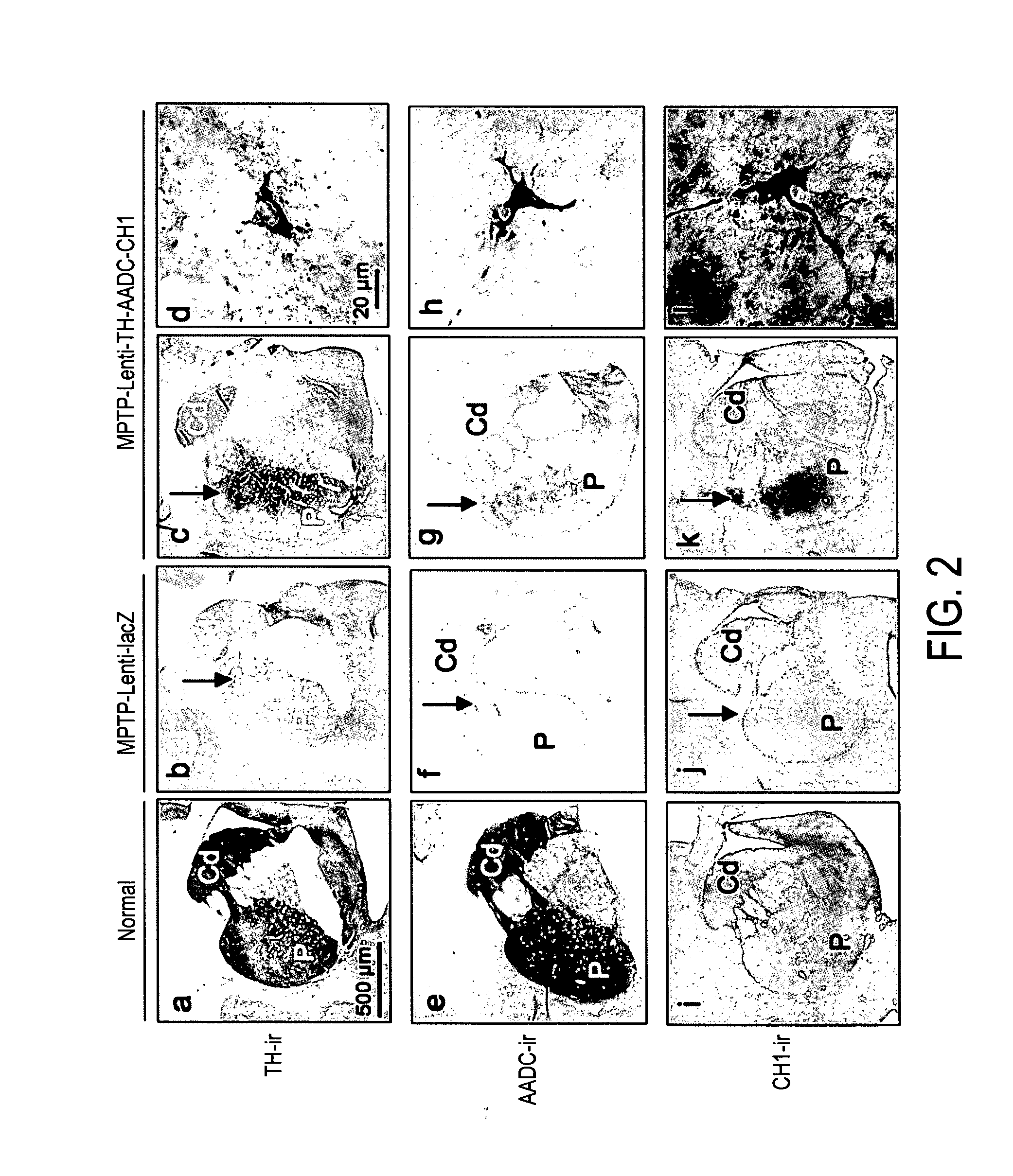
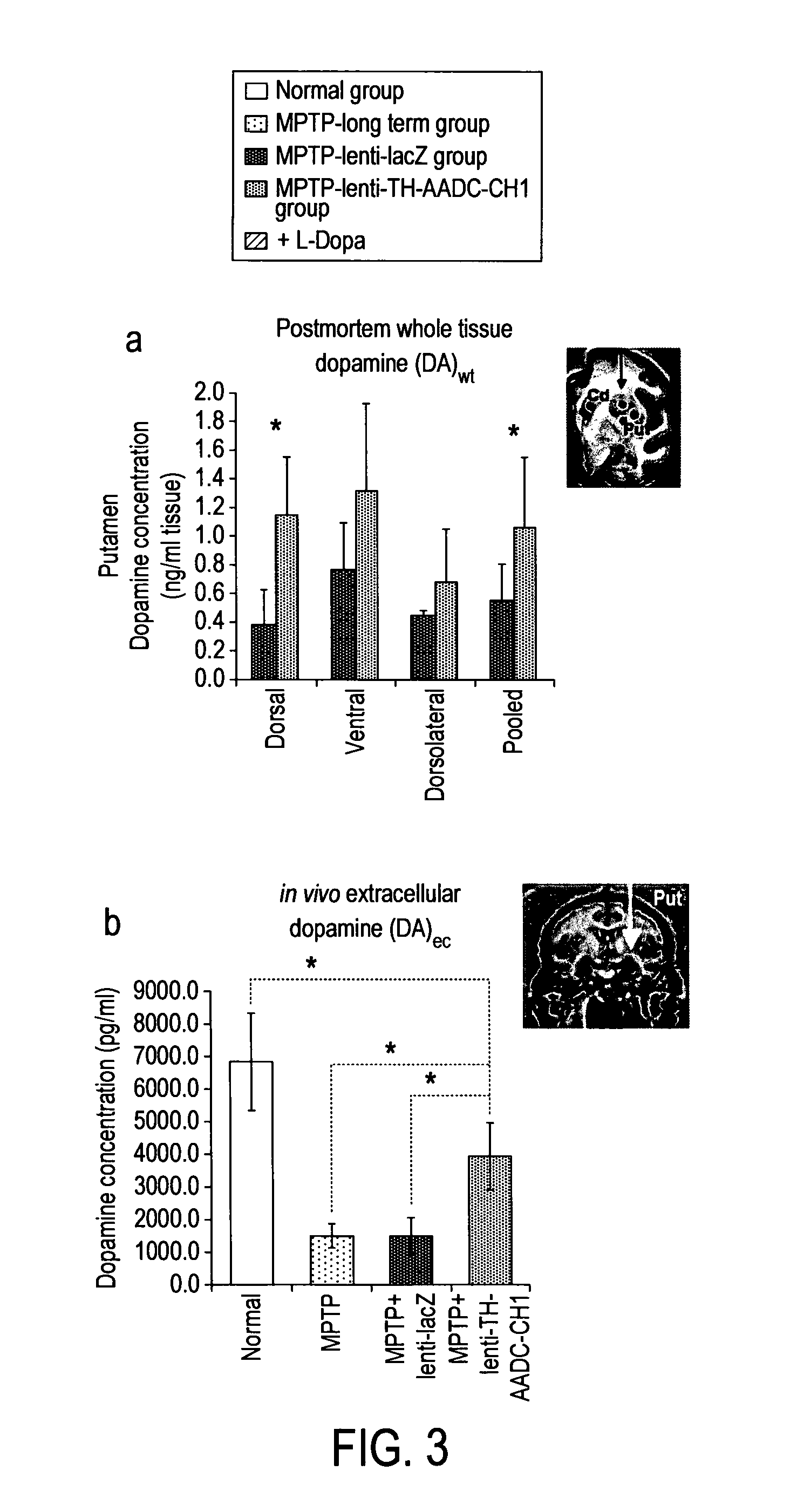
InactiveUS20110269826A1Good curative effectHigh expressionNervous disorderVirusesVector systemMedicine
The present invention provides methods for: (i) treating and / or preventing Parkinson's disease in a subject without causing cognitive impairment by using dopamine replacement gene therapy to maintain or restore constant physiological dopaminergic tone in both the dorsal and ventral striatum of the subject; (ii) normalising neuronal electrical activity in basal ganglia and / or subthalamic nucleus in a Parkinson's disease subject; and (iii) treating and / or preventing dyskinesias associated with oral L-dopa administration in a Parkinson's disease subject by administration of a vector system for dopamine replacement gene therapy to the subject.
Owner:OXFORD BIOMEDICA (UK) LTD
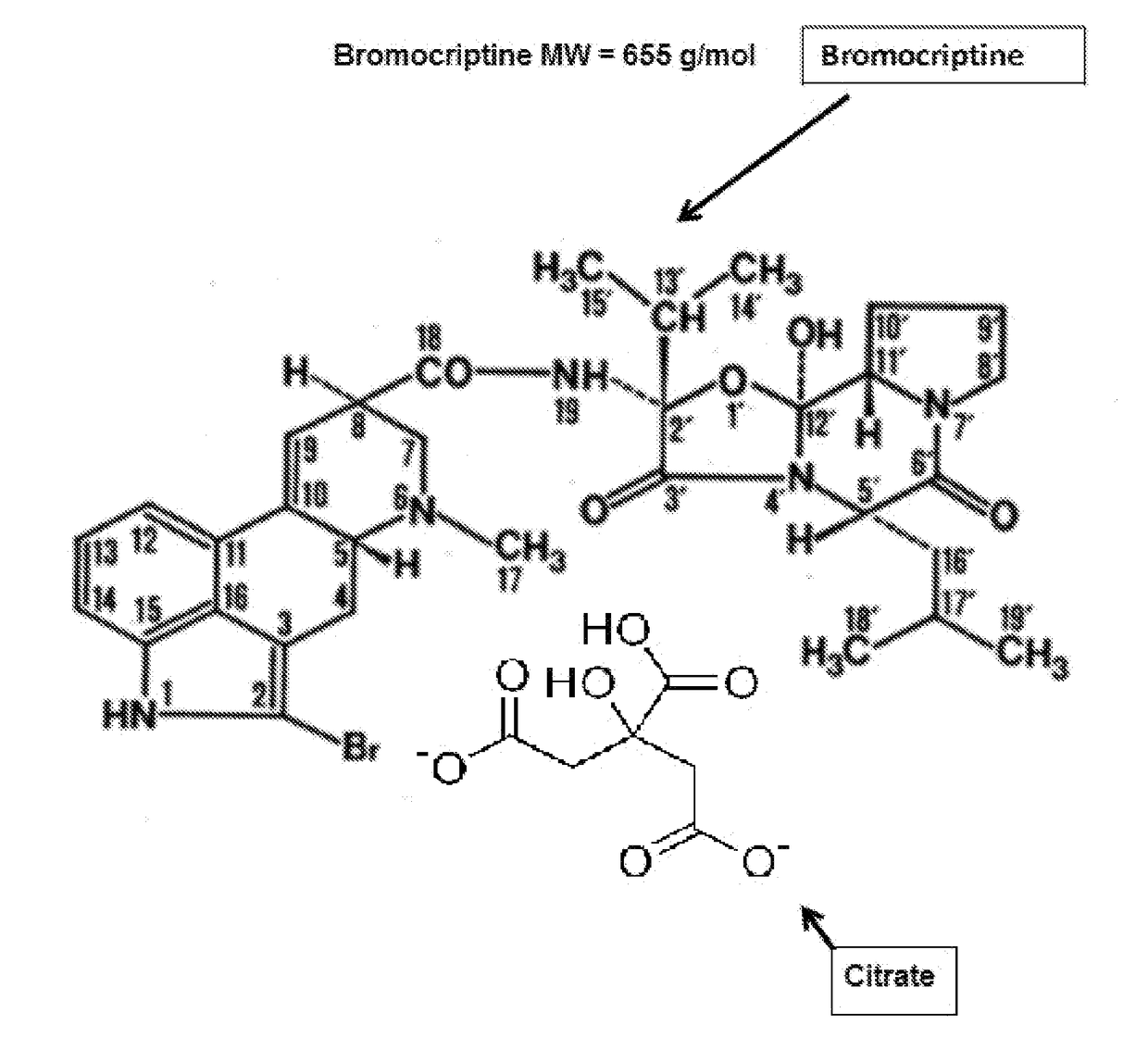
Composition and Method for Treating Metabolic Disorders
Bromocriptine citrate administered to a vertebrate, animal or human, can be used for any purpose including, e.g., the long-term modification and regulation of metabolic disorders, including prediabetes, obesity, insulin resistance, hyperinsulinemia, hyperglycemia and type 2 diabetes mellitus (T2DM) and / or, e.g., the treatment of other medical disorder(s) including immune or endocrine disorders or diseases. Bromocriptine citrate is administered over a limited or extended period at a time of day dependent on re-establishing the normal circadian rhythm of central dopaminergic activity of healthy members of a similar species and sex. Insulin resistance, hyperinsulinemia and hyperglycemia, T2DM, prediabetes, MS or all, can be controlled in humans on a long term basis by such treatment inasmuch as the daily administration of bromocriptine citrate resets neuronal activity timing in the neural centers of the brain to produce long term effects.
Owner:VEROSCI
Method for inducing umbilical cord mesenchymal stem cells to be differentiated into dopaminergic neurons
InactiveCN104789531AHigh differentiation efficiencyHigh selectivityNervous system cellsSkeletal/connective tissue cellsMedicineDopaminergic
The invention discloses a method for inducing umbilical cord mesenchymal stem cells to be differentiated into dopaminergic neurons. According to the method, a two-step combination method of pre-induction and induction is adopted, optimized pre-induction culture solution and induction culture solution are prepared, and a glycoside compound improved in structure is added as an inducer, so that the umbilical cord mesenchymal stem cells are successfully induced to be differentiated into the dopaminergic neurons and the differentiation ratio is relatively high.
Owner:中国人民解放军总医院第三医学中心
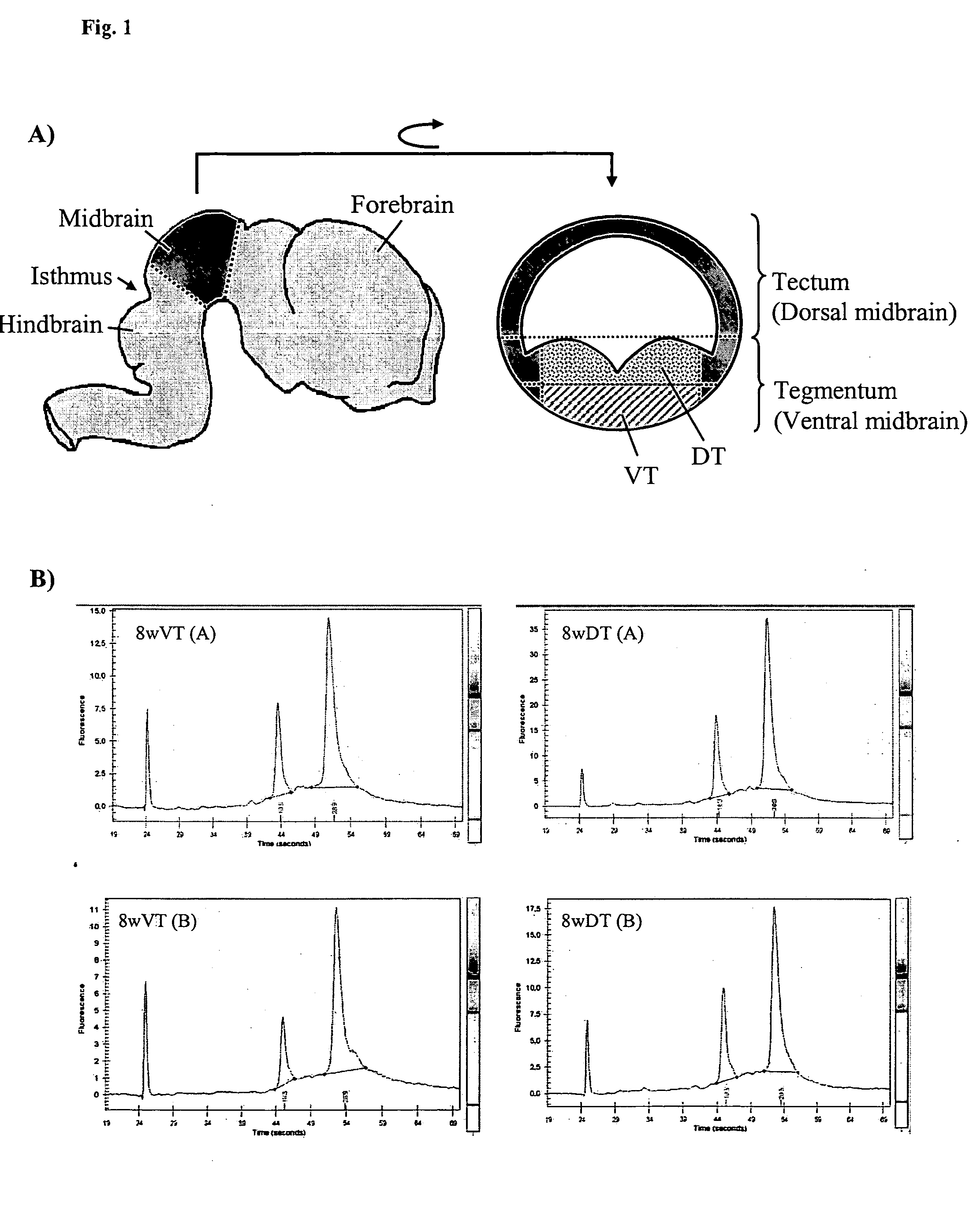
Novel genes regulated in the developing human ventral mesencephalon
A human embryonal stem cell, neural stem cell, neural precursor cell, neural cell or dopaminergic neuron is genetically modified to overexpress at least one of certain genes identified as regulated in the developing human ventral mesencephalon, and more particularly, up-regulated in the ventral tegmentum. The genes are associated with dopaminergic differentiation.
Owner:NSGENE AS
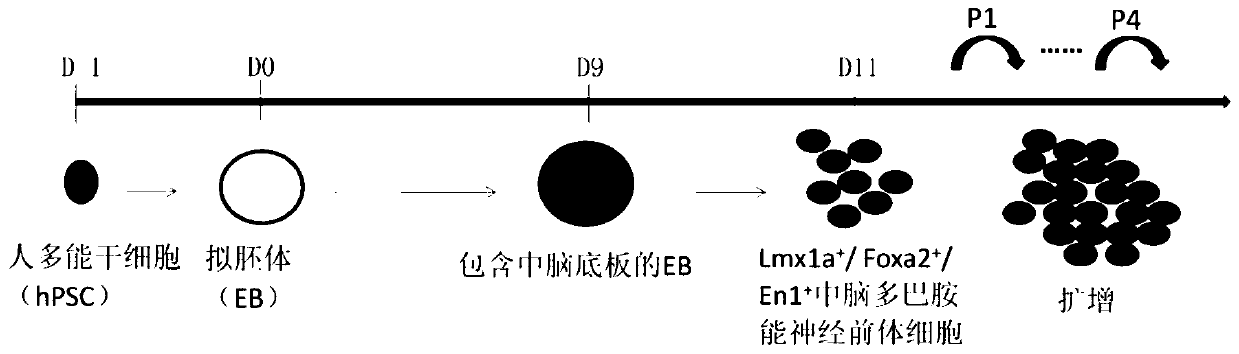
Midbrain dopaminergic nerve precursor cells and preparation method and application thereof
InactiveCN109880800AFunctionalClinical majorNervous disorderNervous system cellsClinical gradeMidbrain
The invention relates to the field of stem cell biology, in particular to midbrain dopaminergic neural precursor cells and a preparation method and application thereof. The midbrain dopaminergic neural precursor cell expresses Lmx1a+, Foxa2+, En1+ and Otx2+, wherein the midbrain dopaminergic neural precursor cell does not express one or more of Nkx2.1, DBX1 and GBX2. The method for preparing the mesencephalic dopaminergic neural precursor cell comprises the following steps: embryoid bodies are formed, midbrain floor cells are obtained, and midbrain dopaminergic neural precursor cells are obtained. By adopting the preparation method, the mesencephalic dopaminergic nerve cells can be rapidly and efficiently differentiated from the human pluripotent stem cells, and the problems are solved that an existing differentiation method is long in time, unstable in effect and low in efficiency, the culture system containing serum or the trophoblast cells are not conducive to the production of subsequent clinical-grade cell preparations and the like.
Owner:安徽中盛溯源生物科技有限公司
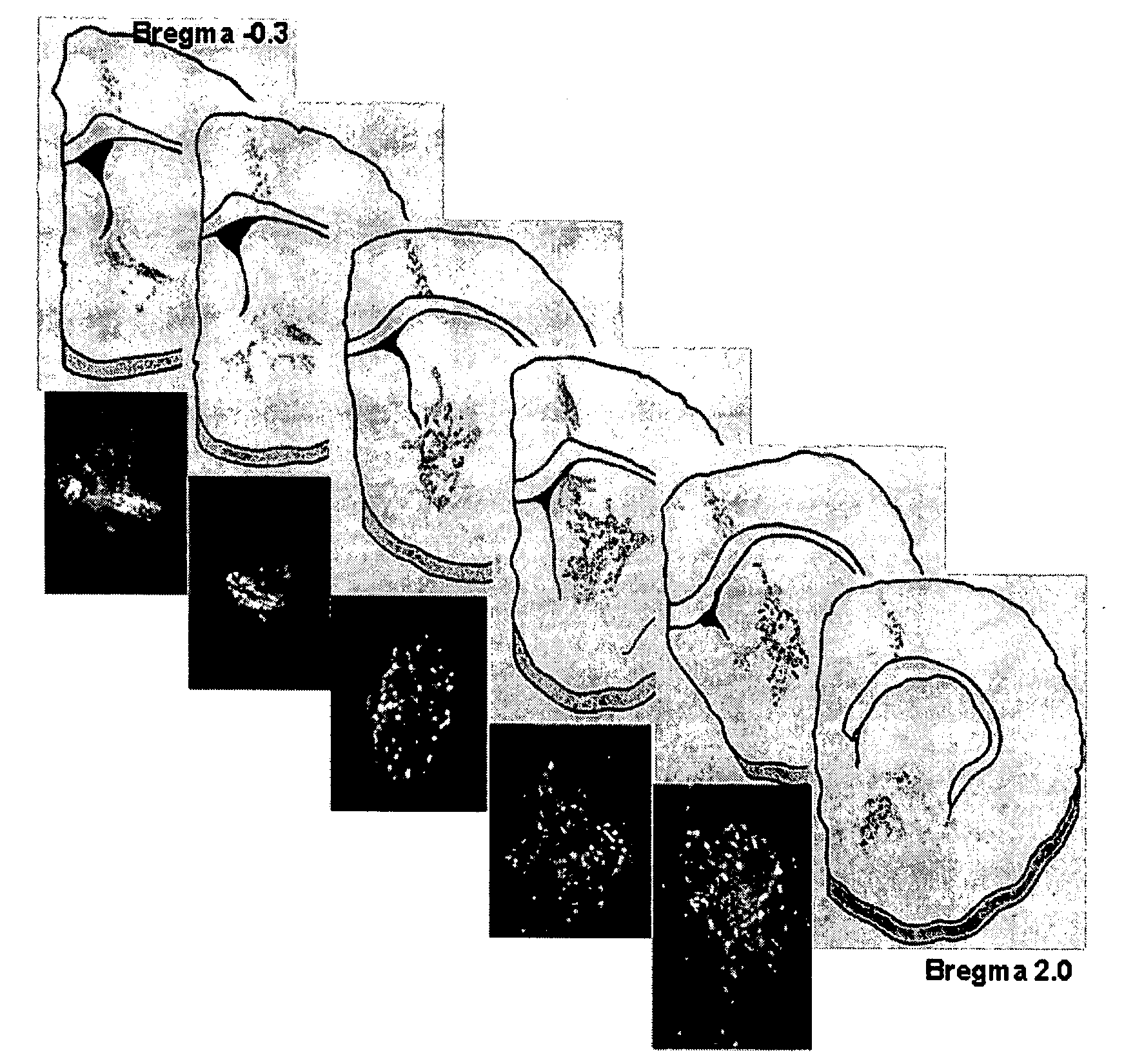
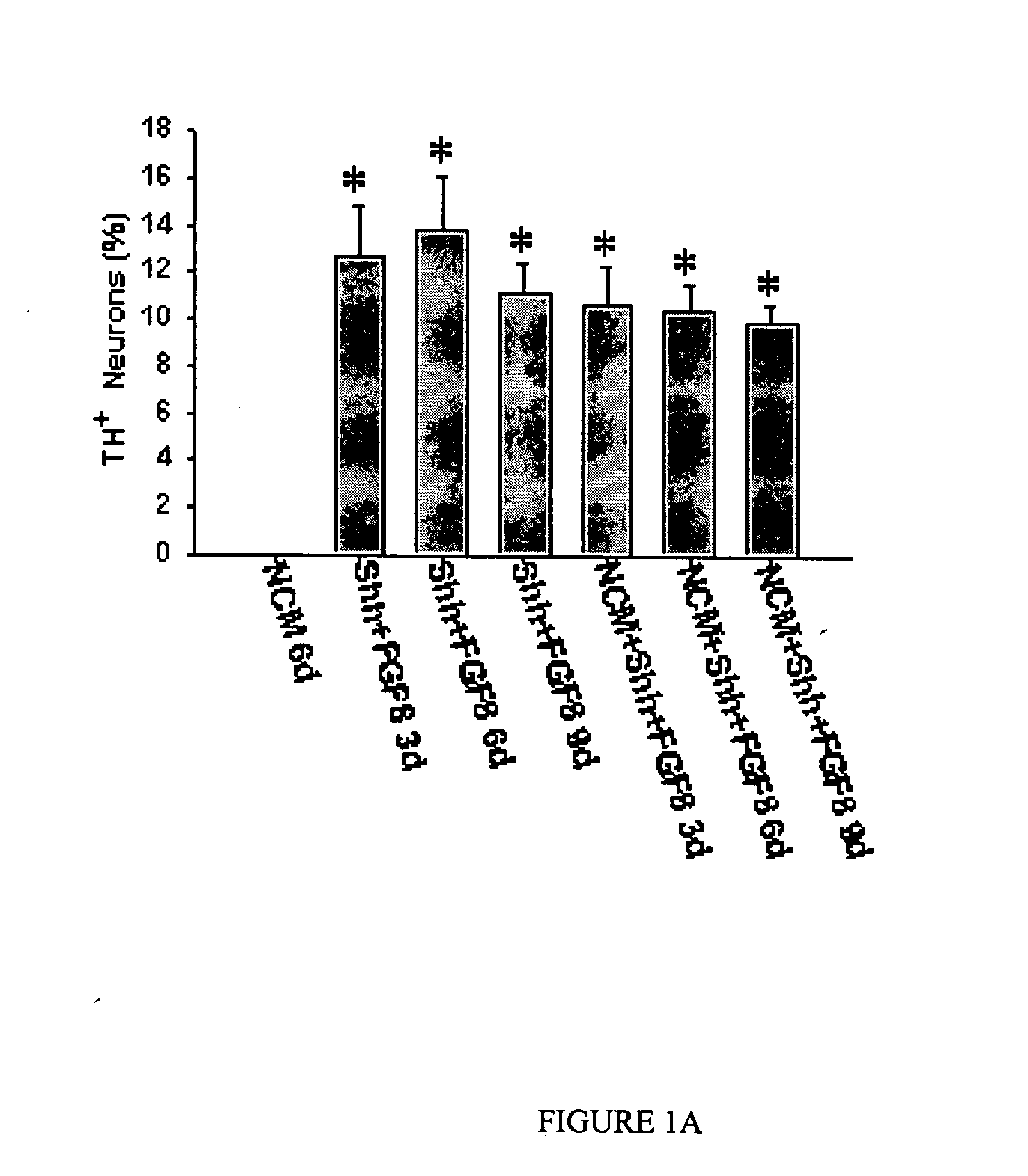
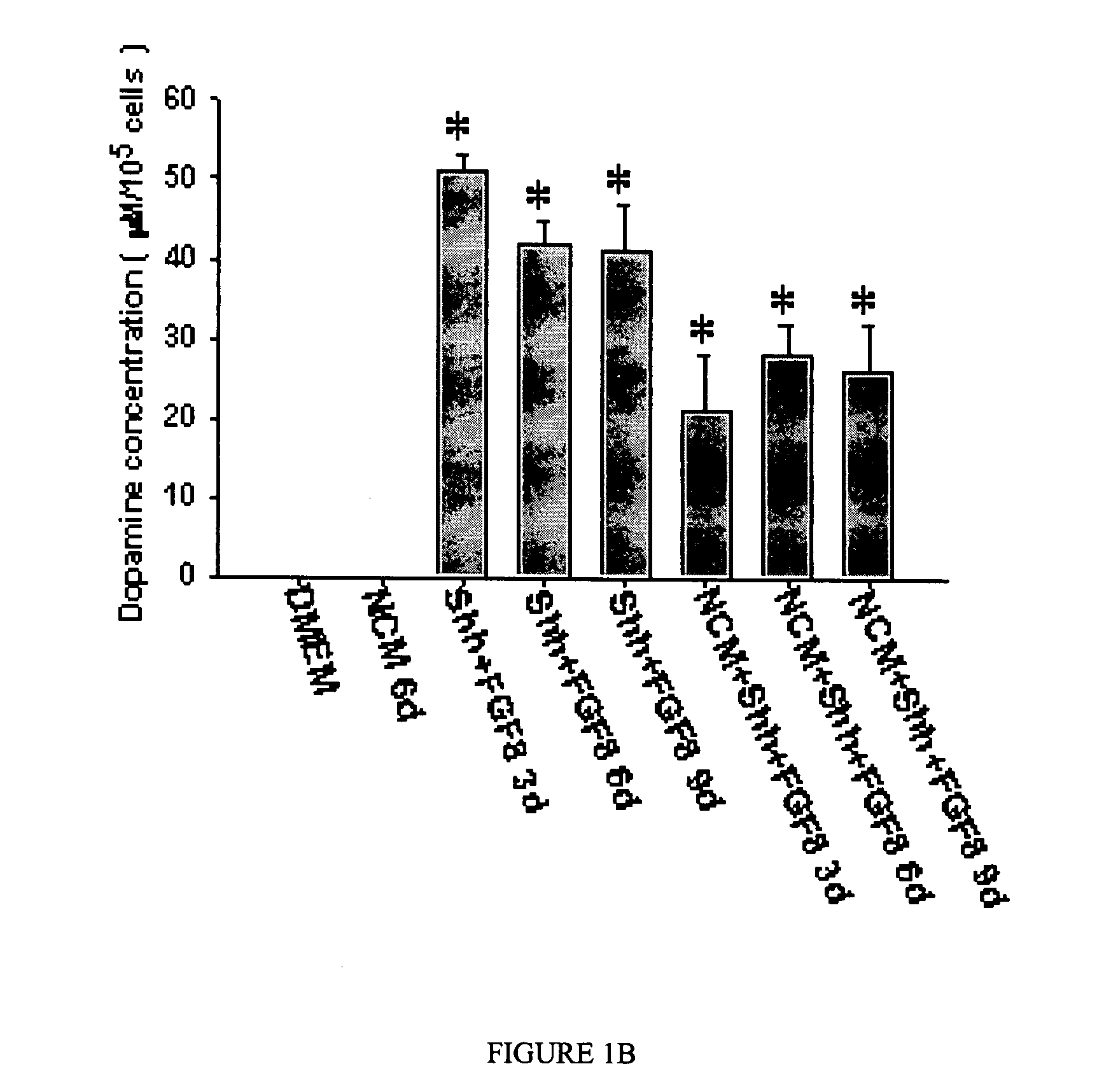
Method of treating dopaminergic and GABA-nergic disorders
InactiveUS7138492B2Improve survival rateConducive to survivalOrganic active ingredientsFungiMidbrainDopaminergic
It is shown here that hedgehog proteins possess novel activities beyond phenotype specification. Using cultures derived from the embryonic day 14.5 (E14.5) rat ventral mesencephalon, we show that hedgehog is also trophic for dopaminergic neurons. Interestingly, hedgehog not only promotes dopaminergic neuron survival, but also promotes the survival of midbrain GABA-immunoreactive (GABA-ir) neurons.
Owner:CURIS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com