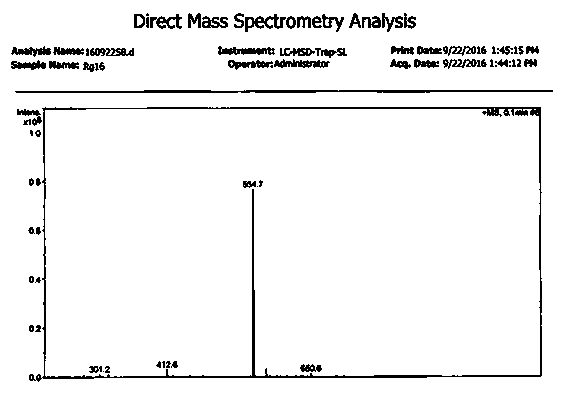
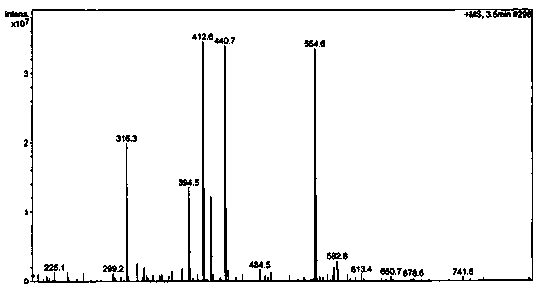
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
85 results about "Rotigotine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rotigotine is used alone or with other medications to treat Parkinson's disease.
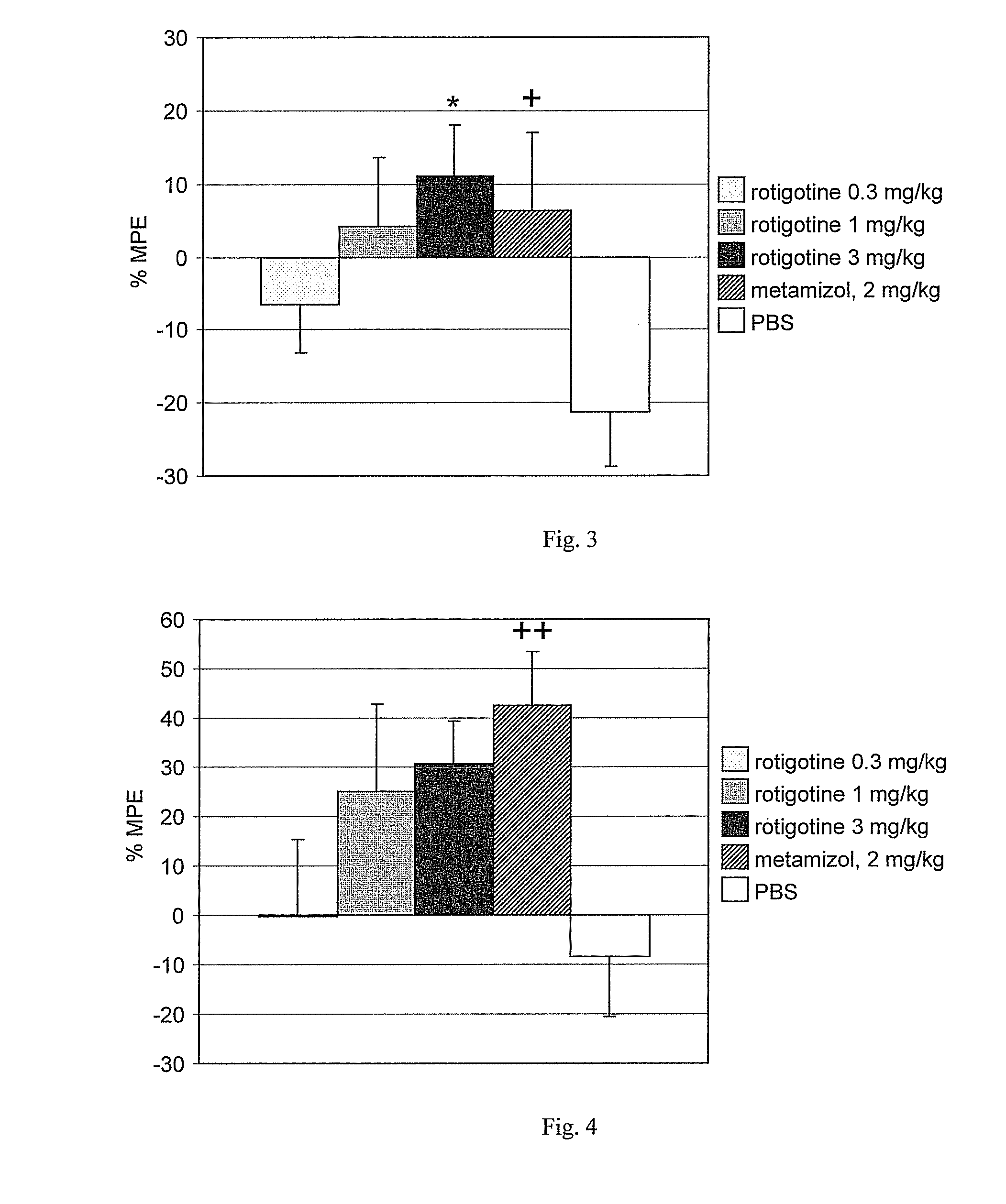
Method for treating pain using a substituted 2-aminotetralin compound
InactiveUS20080008748A1Reduce muscular hyperalgesia and/or muscular allodyniaReducing muscular hyperalgesia and/or muscular allodyniaBiocideNervous disorderCompound aChronic Widespread Pain
A method for treating pain, particularly non-inflammatory musculoskeletal pain such as fibromyalgia, myofascial pain or back pain, in a subject comprises administering to the subject a substituted 2-aminotetralin compound as defined herein, illustratively rotigotine.
Owner:UCB SA
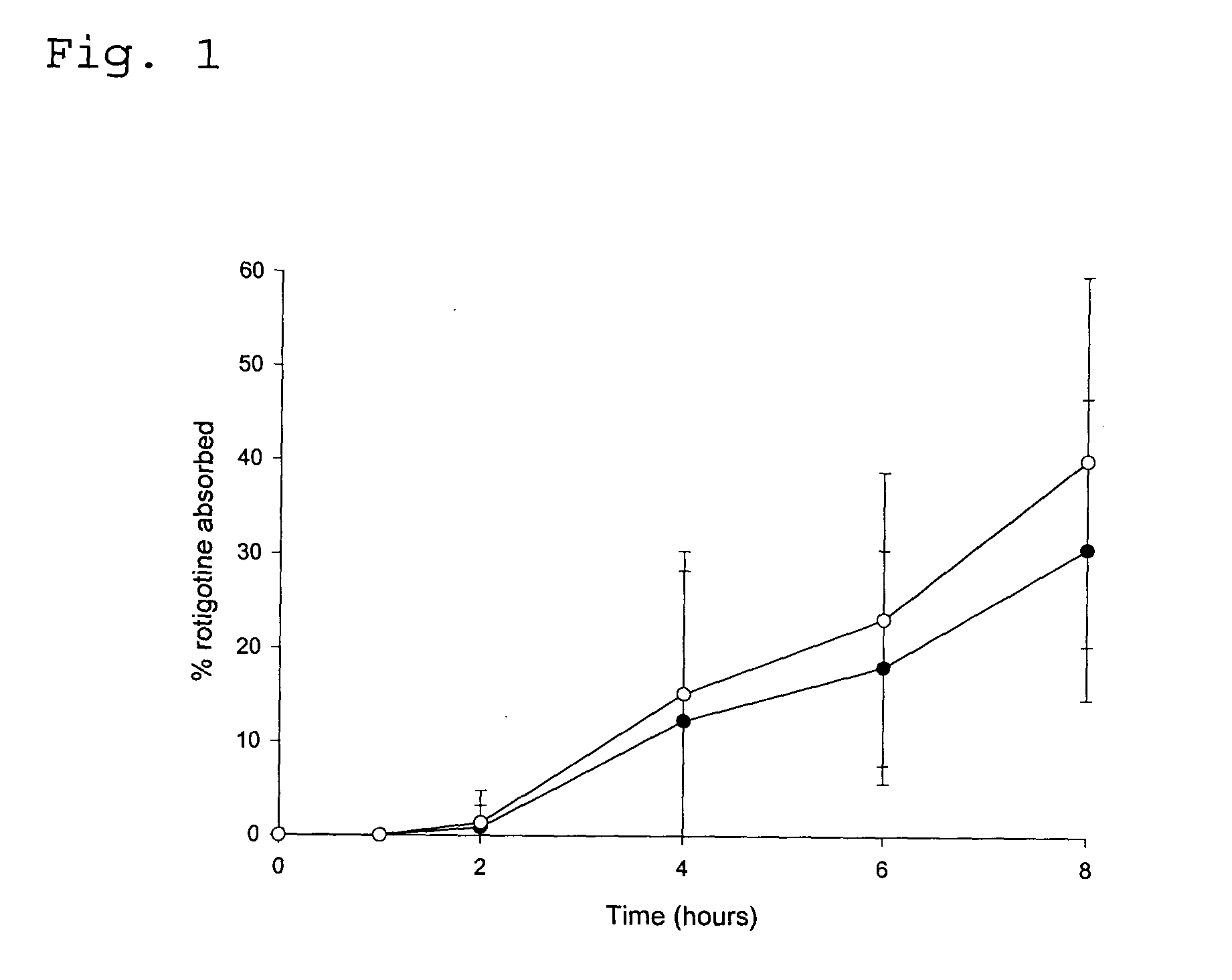
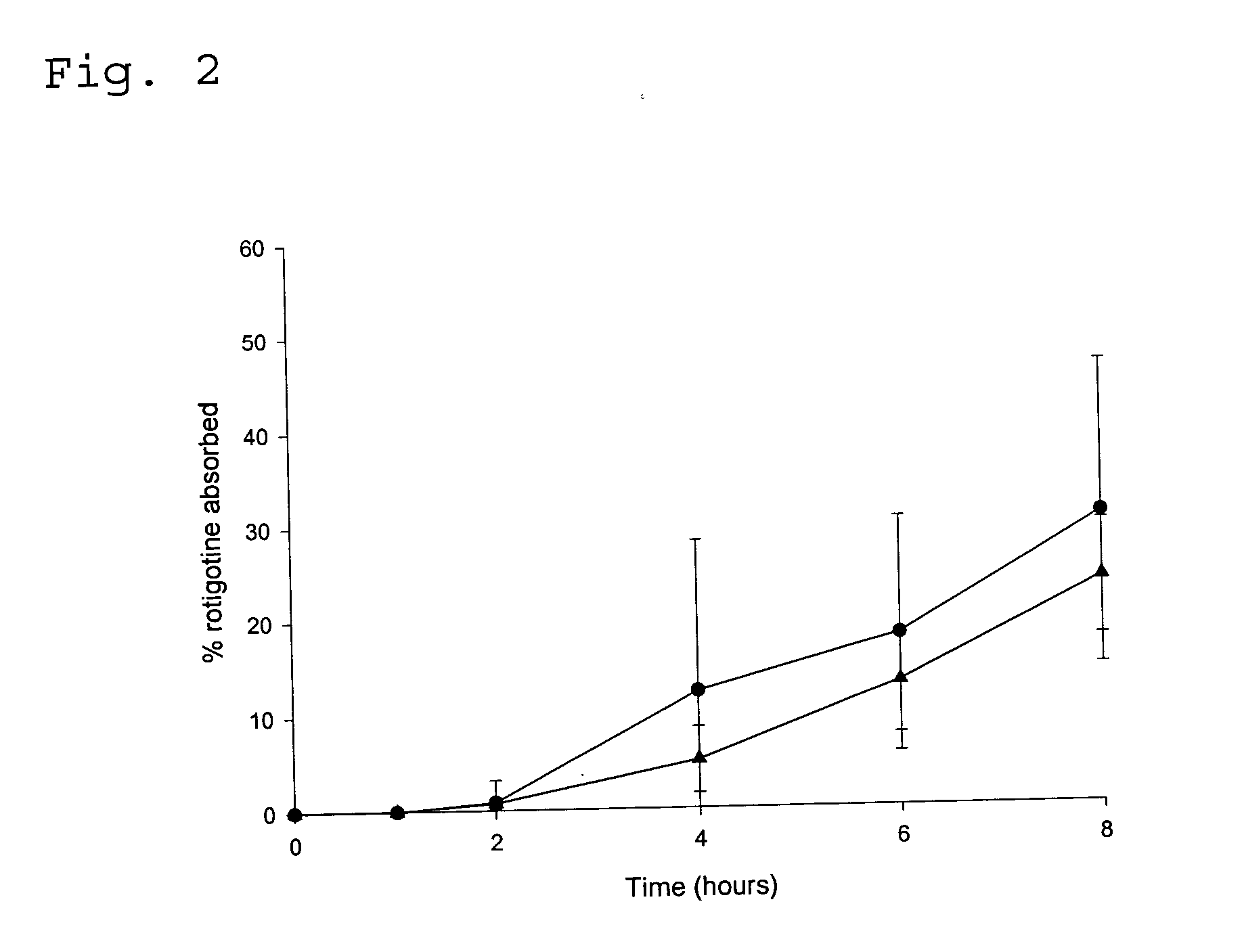
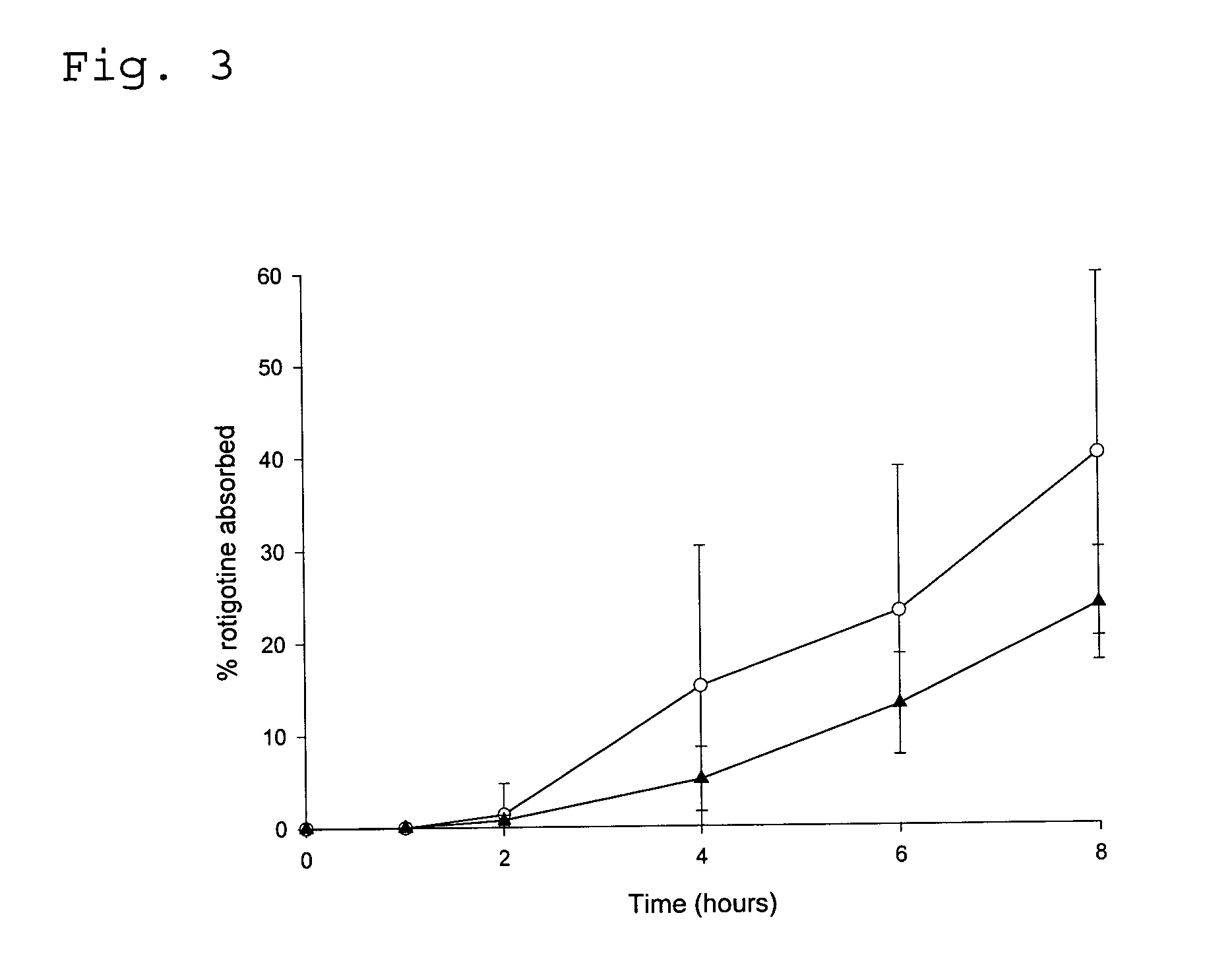
Transdermal delivery system for the administration of rotigotine
An improved Transdermal Delivery System (TDS) comprising a backing layer inert to the components of the matrix, a self-adhesive matrix containing rotigotine and a protective foil or sheet to be removed prior to use, characterized in that the self-adhesive matrix consists of a solid or semi-solid semi-permeable polymer (1) wherein rotigotine in its free base form has been incorporated, (2) which is saturated with rotigotine and contains said rotigotine as a multitude of microreservoirs within the matrix, (3) which is highly permeable for the free base of rotigotine, (4) which is impermeable for the protonated form of rotigotine, (5) wherein the maximum diameter of the microreservoirs is less than the thickness of the matrix. is provided. Said TDS provides for enhanced flux of rotigotine across the TDS / skin interface.
Owner:UCB SA
Method for Treating a Restless Limb Disorder
A method for treating a restless limb disorder such as restless legs syndrome in a subject comprises administering, transmucosally in the oronasopharyngeal chamber of the subject, one or more doses of rotigotine or a pharmaceutically acceptable salt, prodrug or metabolite thereof, wherein each such dose comprises an amount effective to reduce occurrence and / or severity of one or more symptoms of the disorder, but wherein the total of all such doses in a 24-hour period does not exceed about 450μg rotigotine free base equivalent.
Owner:UCB SA
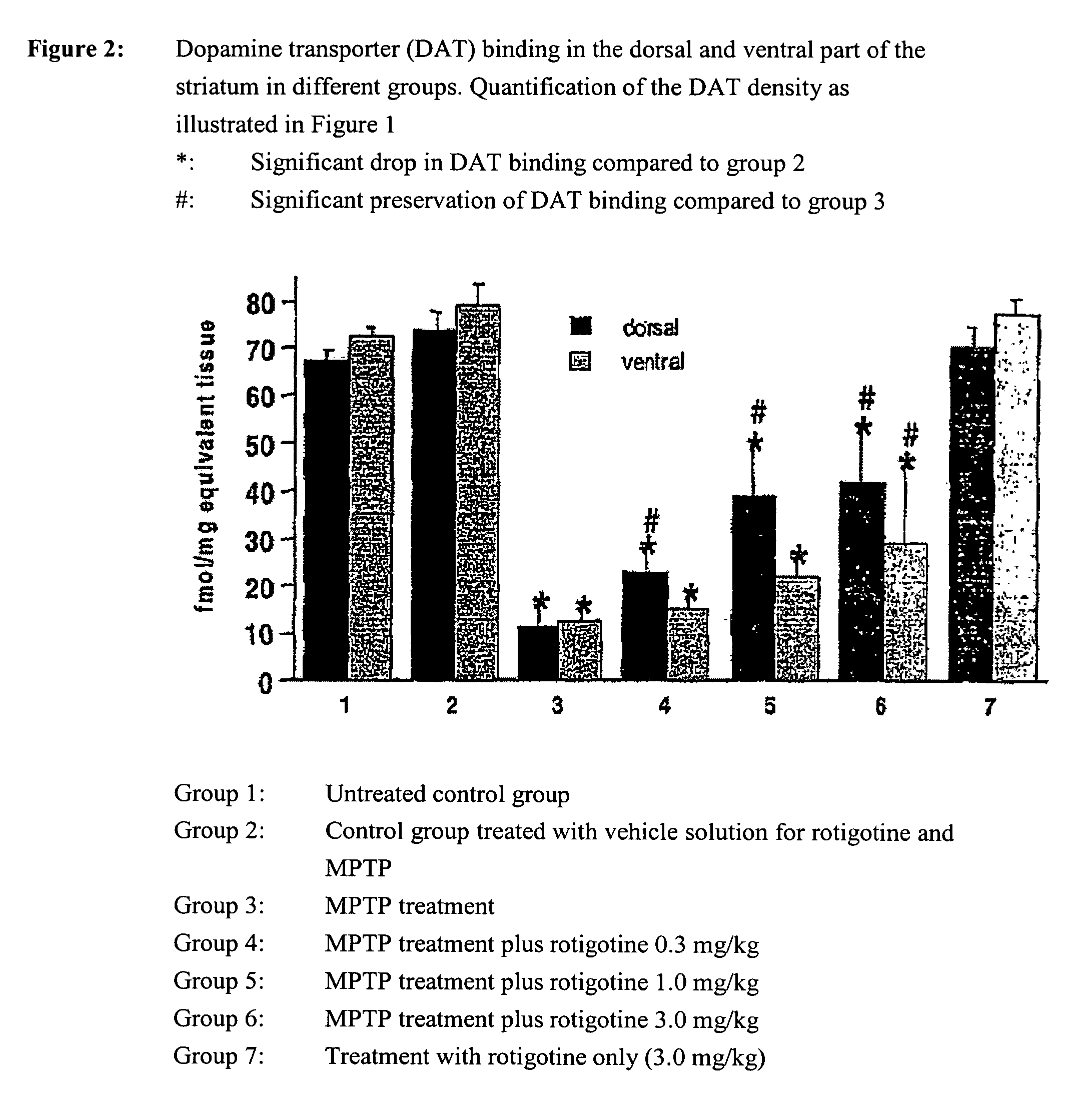
Use of rotigotine for treatment or prevention of dopaminergic neurone loss
The invention relates to the use of rotigotine or salts thereof and prodrugs for the production of a medicament for the treatment or prevention of dopaminergic cell destruction in diseases which are connected to increased dopaminergic cell destruction. The invention also relates to the use of rotigotine as a medicament for the preventive treatment of Parkinson's disease.
Owner:UCB SA
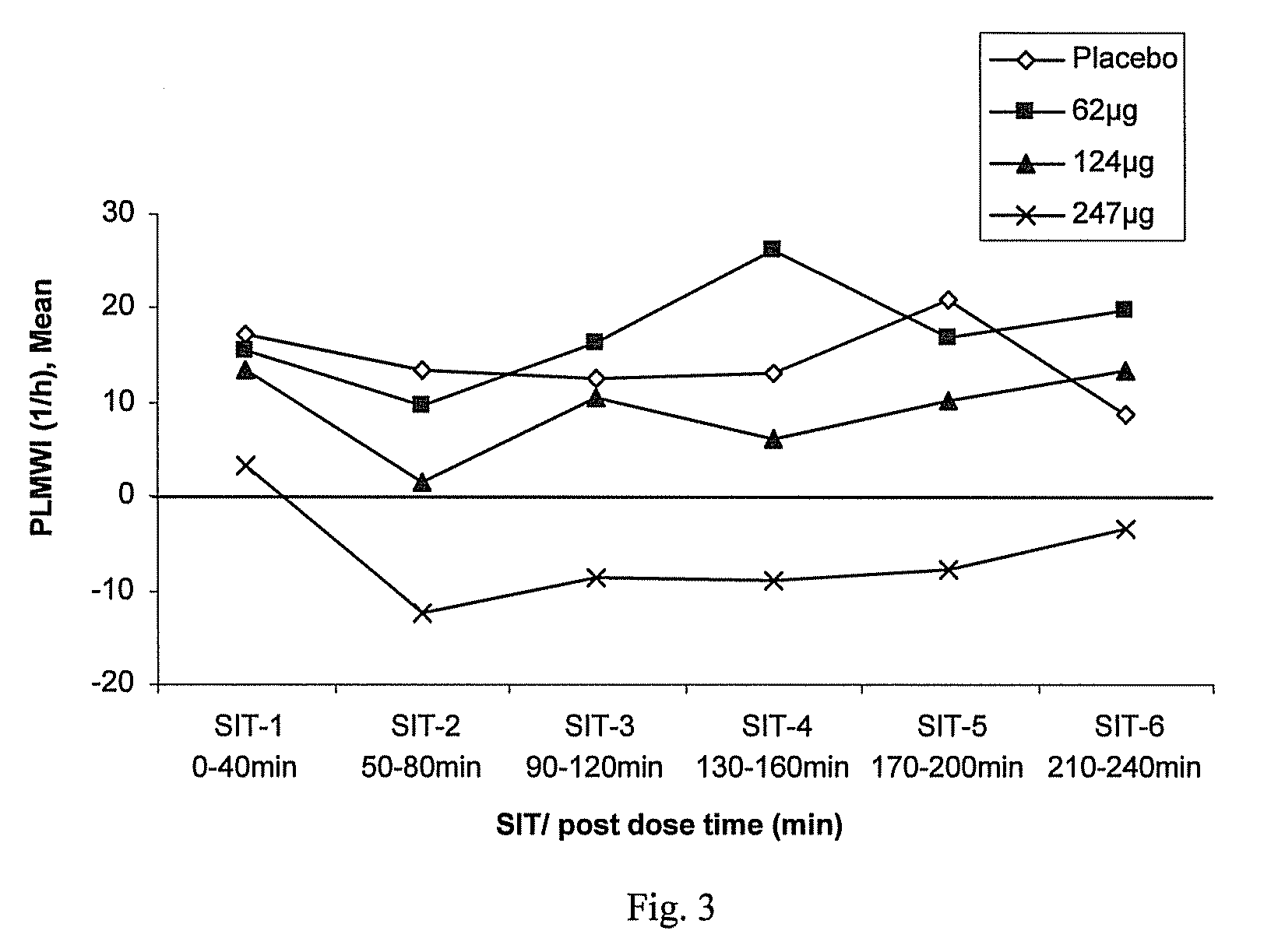
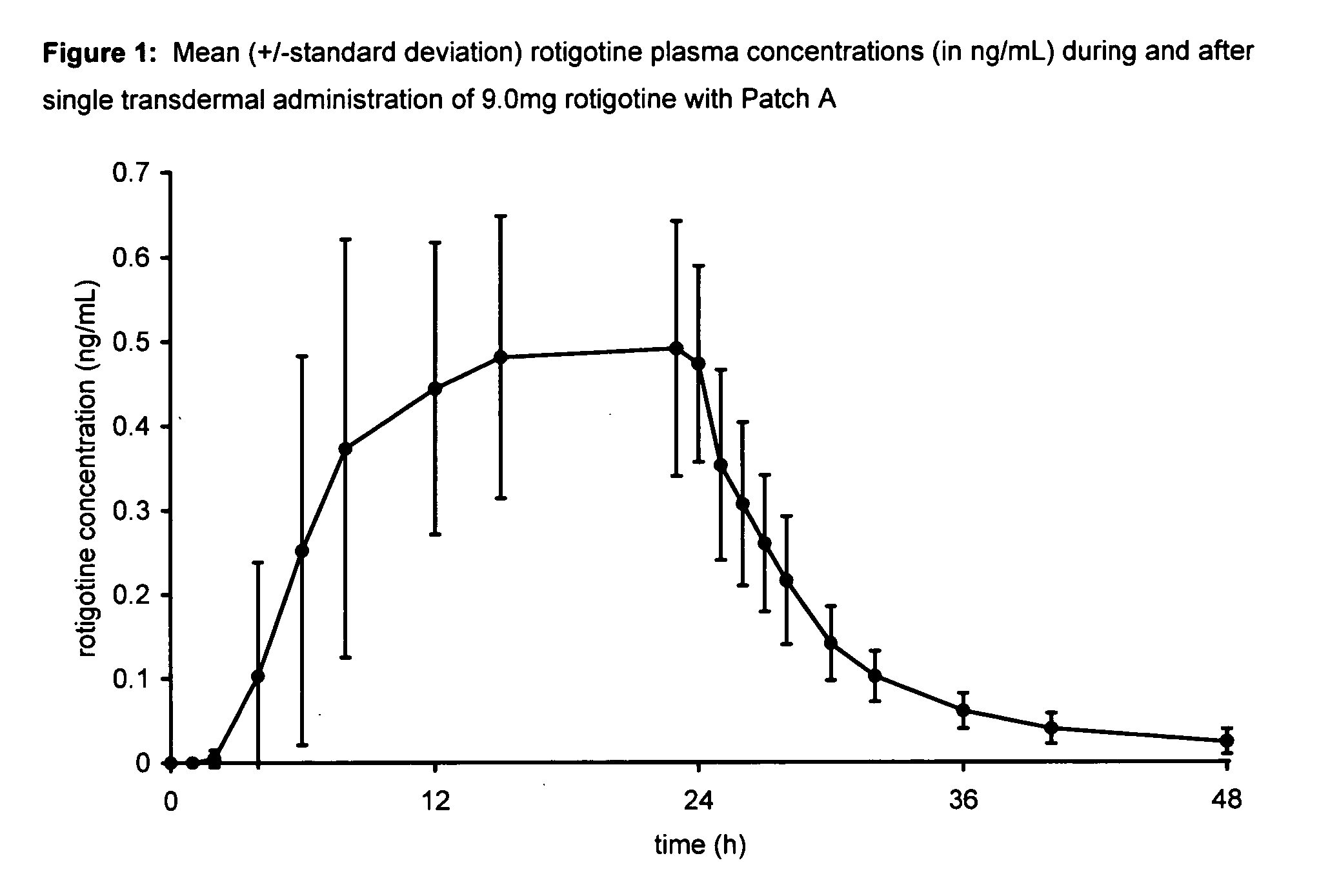
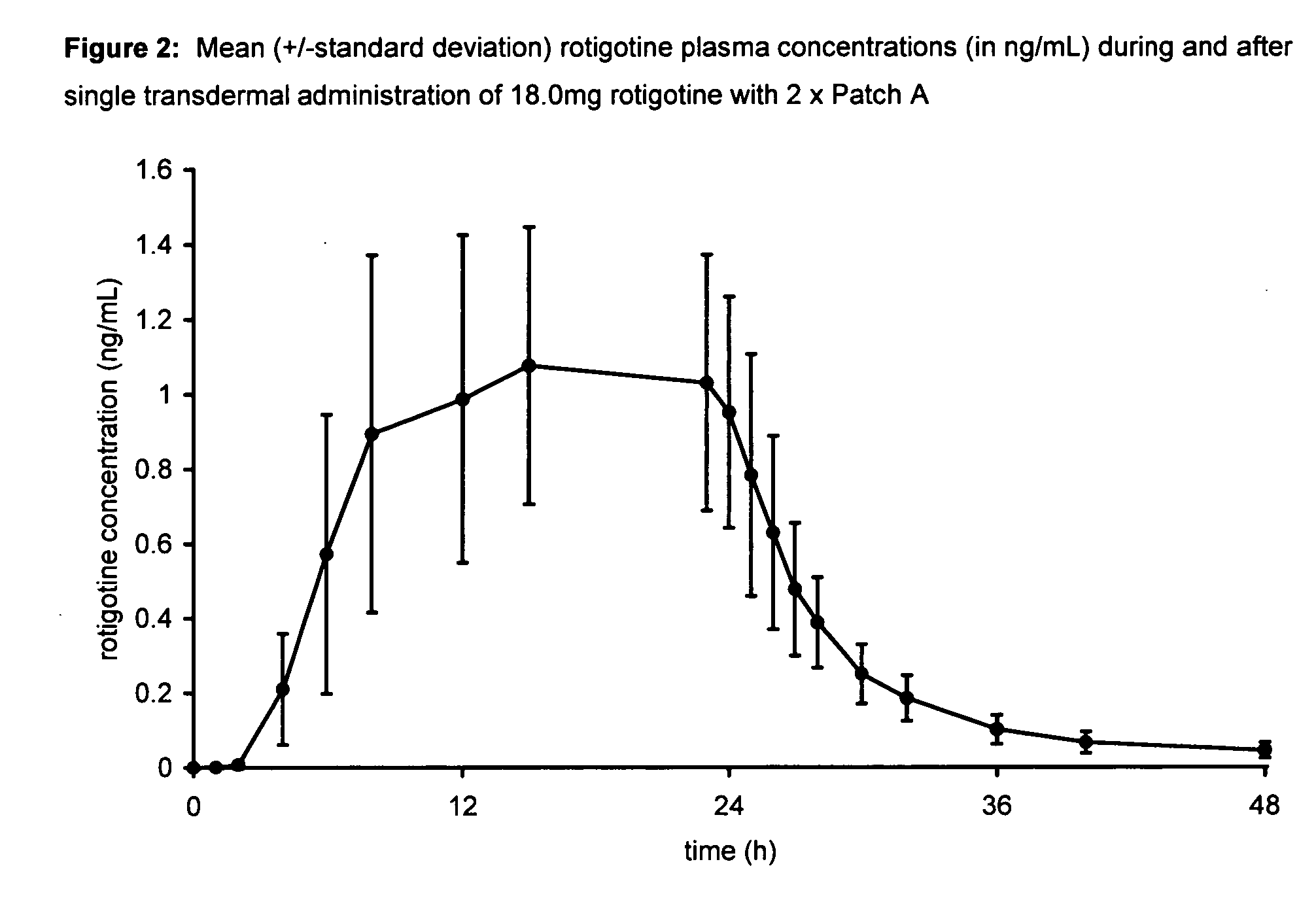
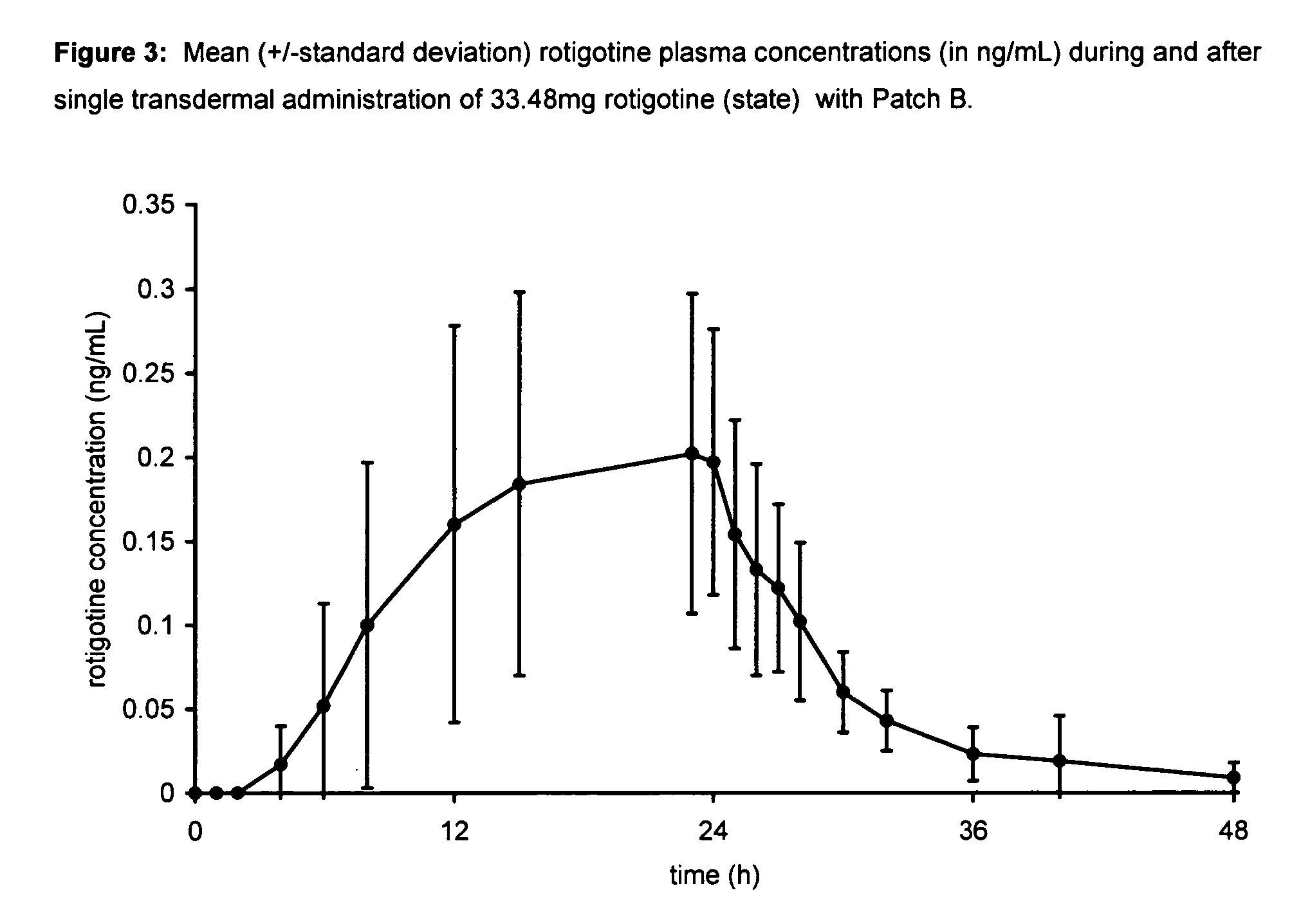
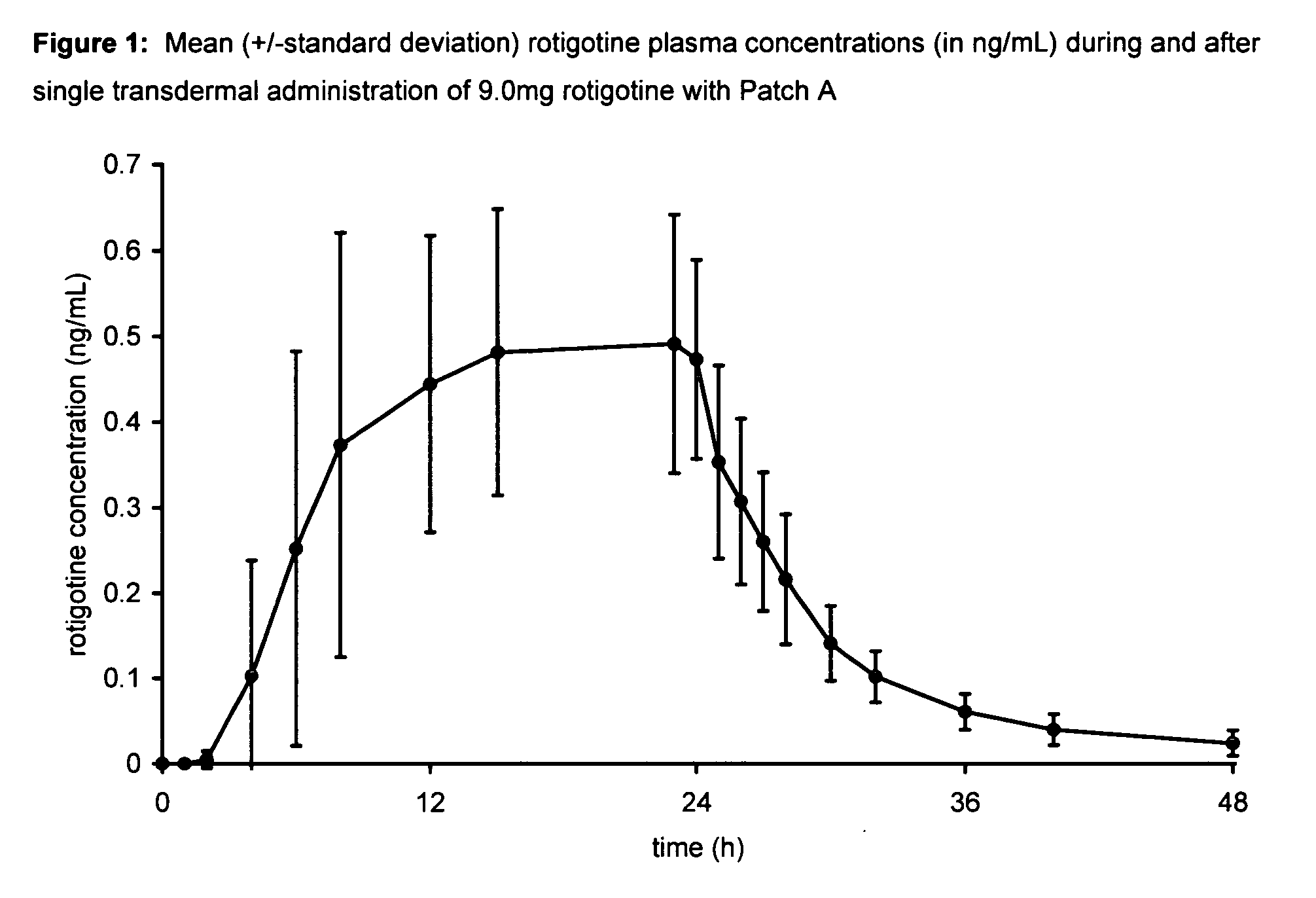
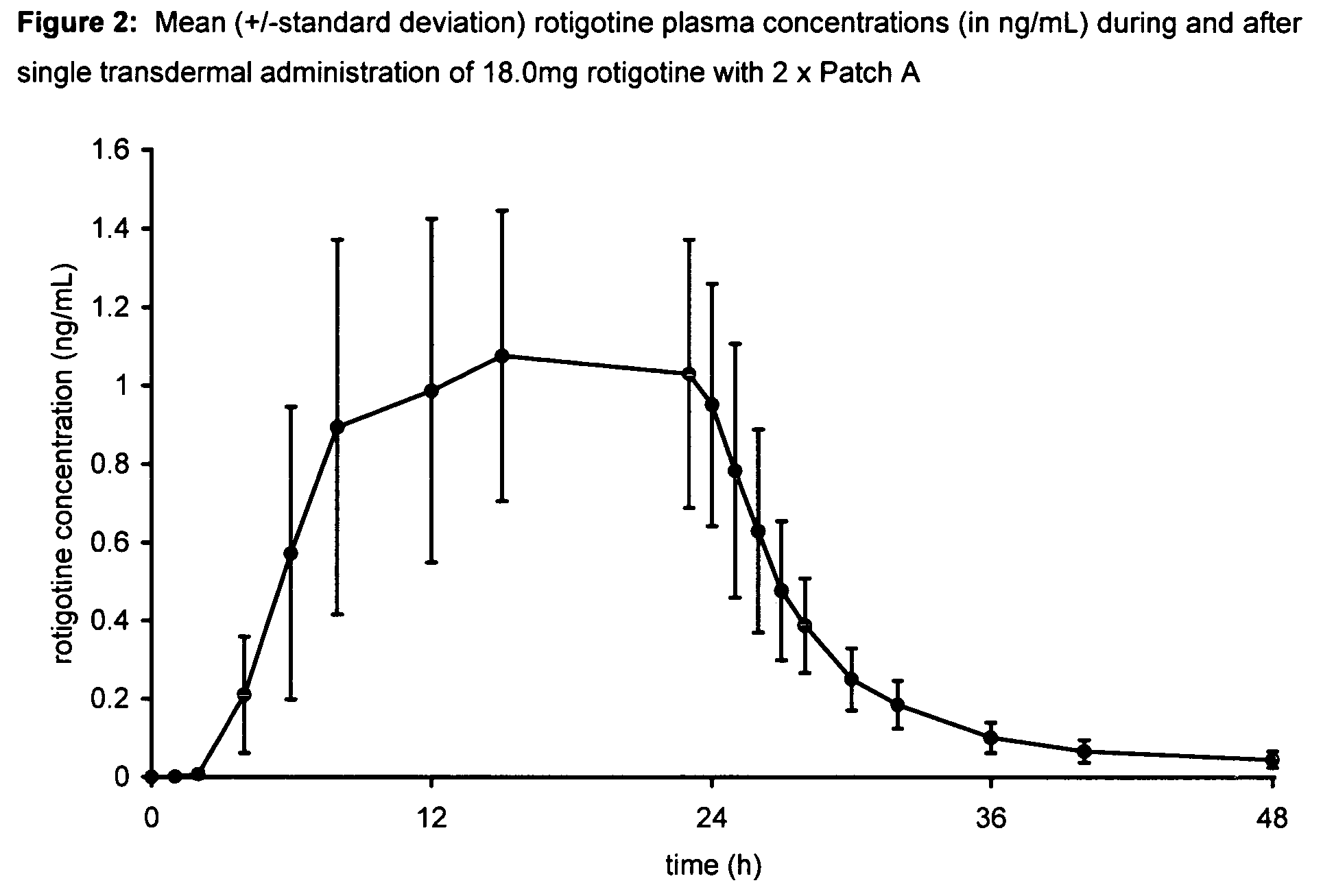
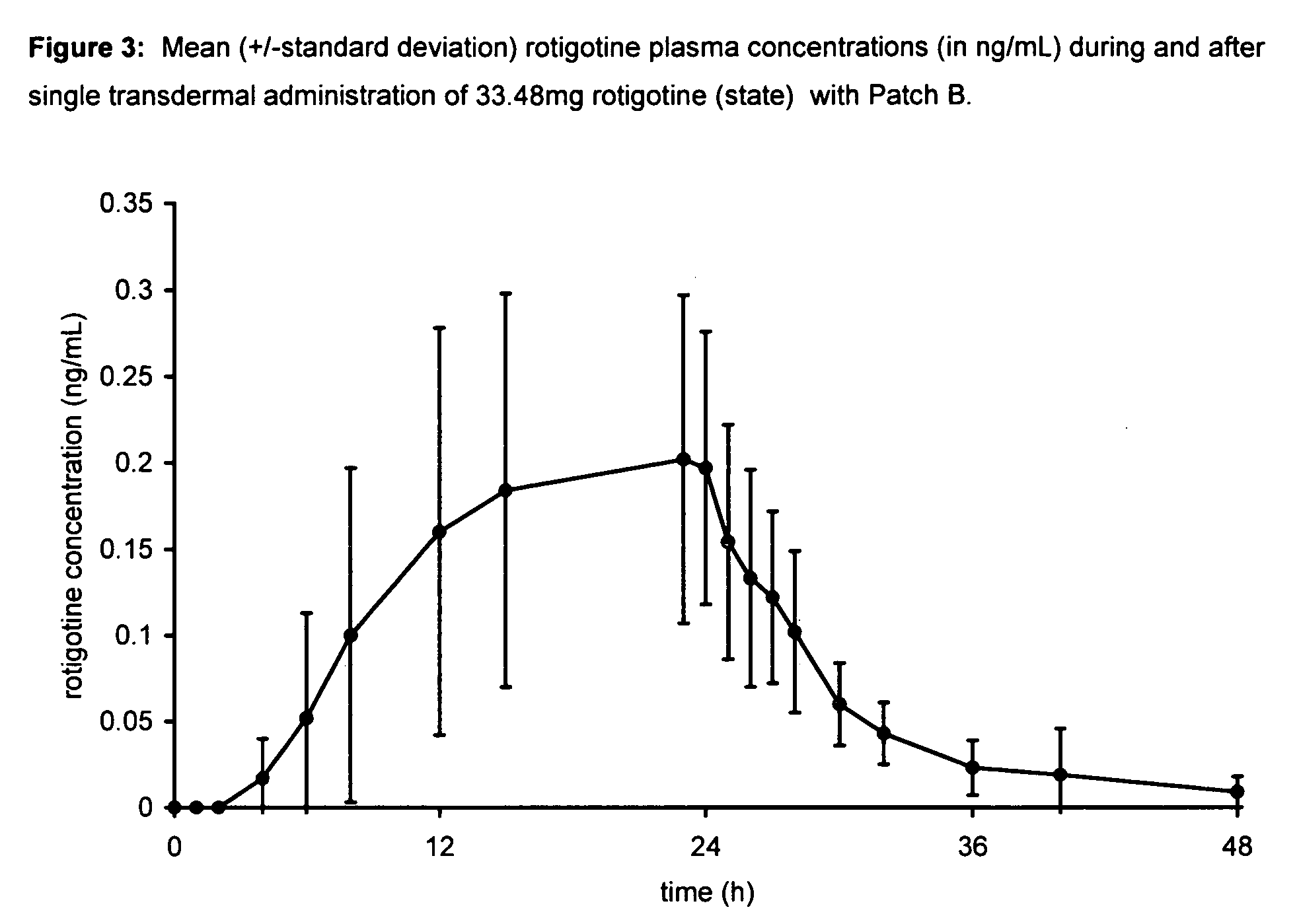
Transdermal therapeutic system for parkinson's disease inducing high plasma levels of rotigotine
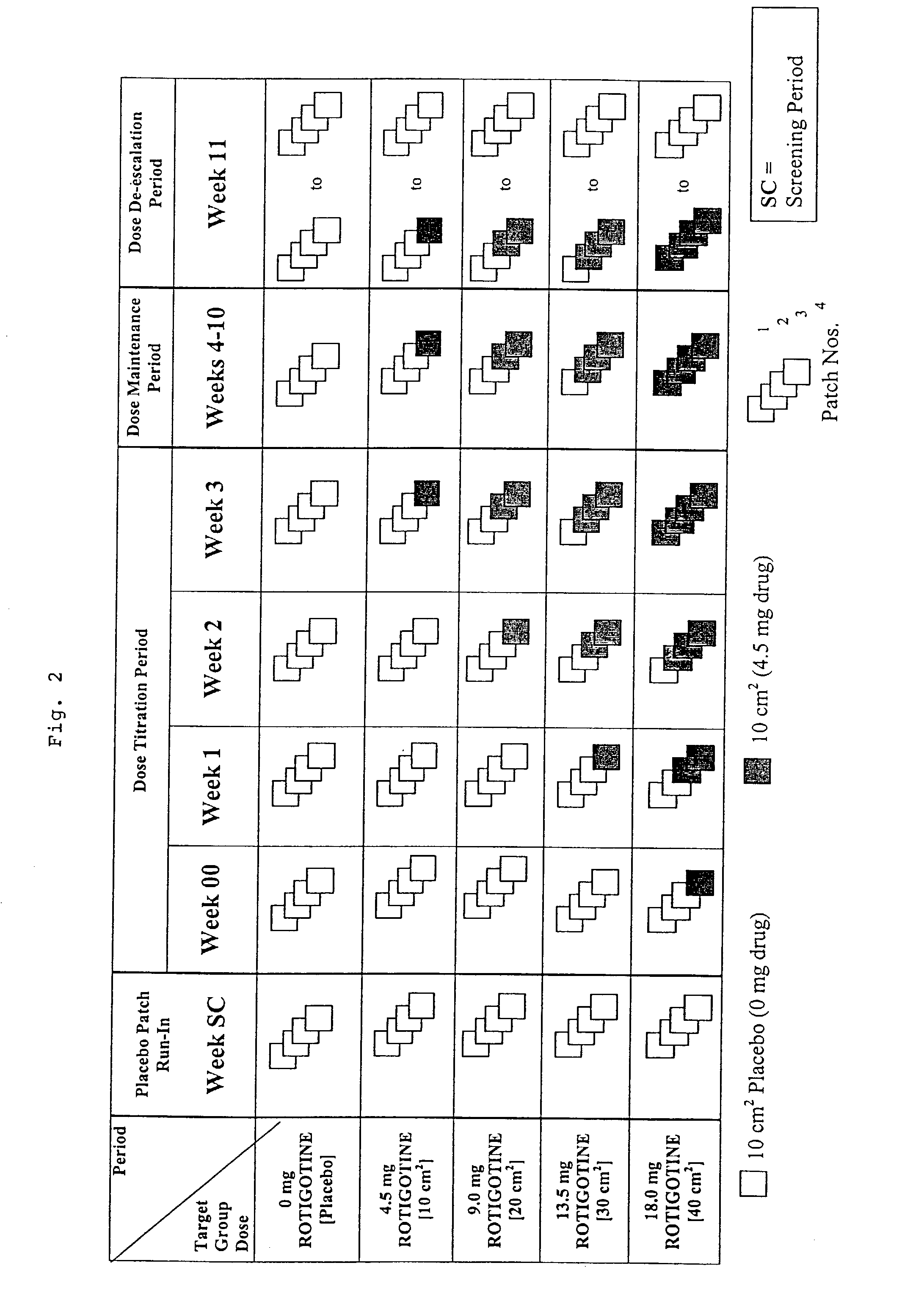
This invention provides the use of a silicone-based transdermal therapeutic system having an area of 10 to 40 cm2 and containing 0.1 to 3.15 mg / cm2 of rotigotine as active ingredient, for the preparation of an anti-Parkinson medicament which induces a mean plasma concentration of rotigotine in the range of 0.4 to 2 ng / ml 24 h after administration.
Owner:UCB SA +1
Transdermal therapeutic system for Parkinson's Disease
InactiveUS20060263419A1Effective treatmentRelieve symptomsBiocideAdhesive dressingsBULK ACTIVE INGREDIENTBlood plasma
The invention provides a transdermal therapeutic system (TTS) containing rotigotine as the active ingredient. The TTS is useful in the treatment of Parkinson's Disease because it induces a pharmacokinetic profile where the rotigotine plasma level is high and stable.
Owner:LTS LOHMANN THERAPIE-SYST AG +1
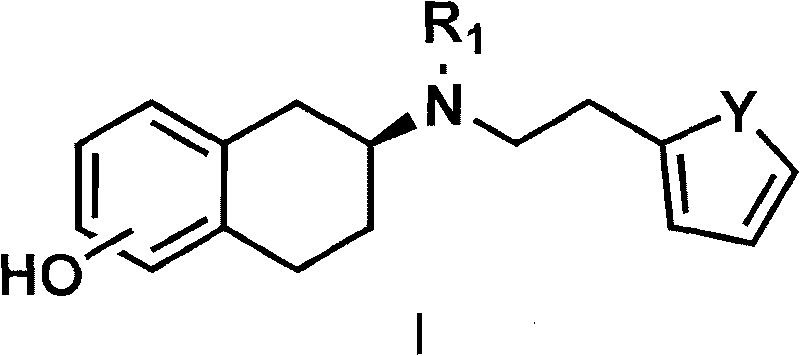
Method for preparing rotigotine and derivative thereof
InactiveCN101717392AHigh optical puritySimple and fast operationOptically-active compound separationOrganic racemisationP-Toluenesulfonic acidSolvent
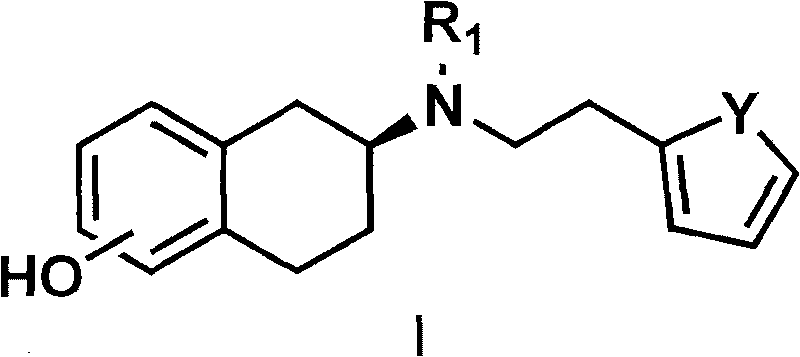
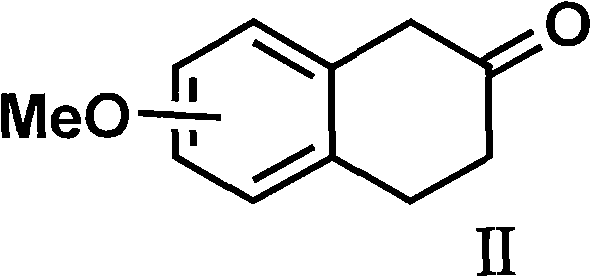
The invention discloses a method for preparing a compound of a formula (I) or pharmaceutically acceptable salts thereof. The method is characterized by comprising the following steps of: (1) using a compound of a formula (II) as a raw material and carrying out a reduction and amination reaction with an appropriate reducing agent to obtain a compound of a formula (III); (2) using 2-quinary heterocyclic substituted ethanol as a raw material and reacting to obtain a compound of a formula (IV) under the conditions of appropriate reagent, temperature and solvent; (3) after carrying out chiral separation on the compound of the formula (III), carrying out a condensation reaction with the compound of the formula (I) under an alkaline condition to obtain a compound of a formula (V); and (4) carrying out demethylation protection on the compound of the formula (V) under the condition of appropriate temperature and solvent to obtain the compound of the formula (I), wherein R1 in each formula is selected from C1-8 alkyl groups or aromatic bases which can be arbitrarily substituted, X is selected from halogen atoms or p-toluenesulfonic acid groups and methanesulfonic acid groups for protecting alcoholic extract hydroxyl groups, and Y is selected from O, S and N. A target product obtained by the method has high optical purity, convenient operation, lower cost, higher yield and less pollution and is suitable for industrialized production.
Owner:苏州凯达生物医药技术有限公司
Transdermal treatment of parkinson's disease
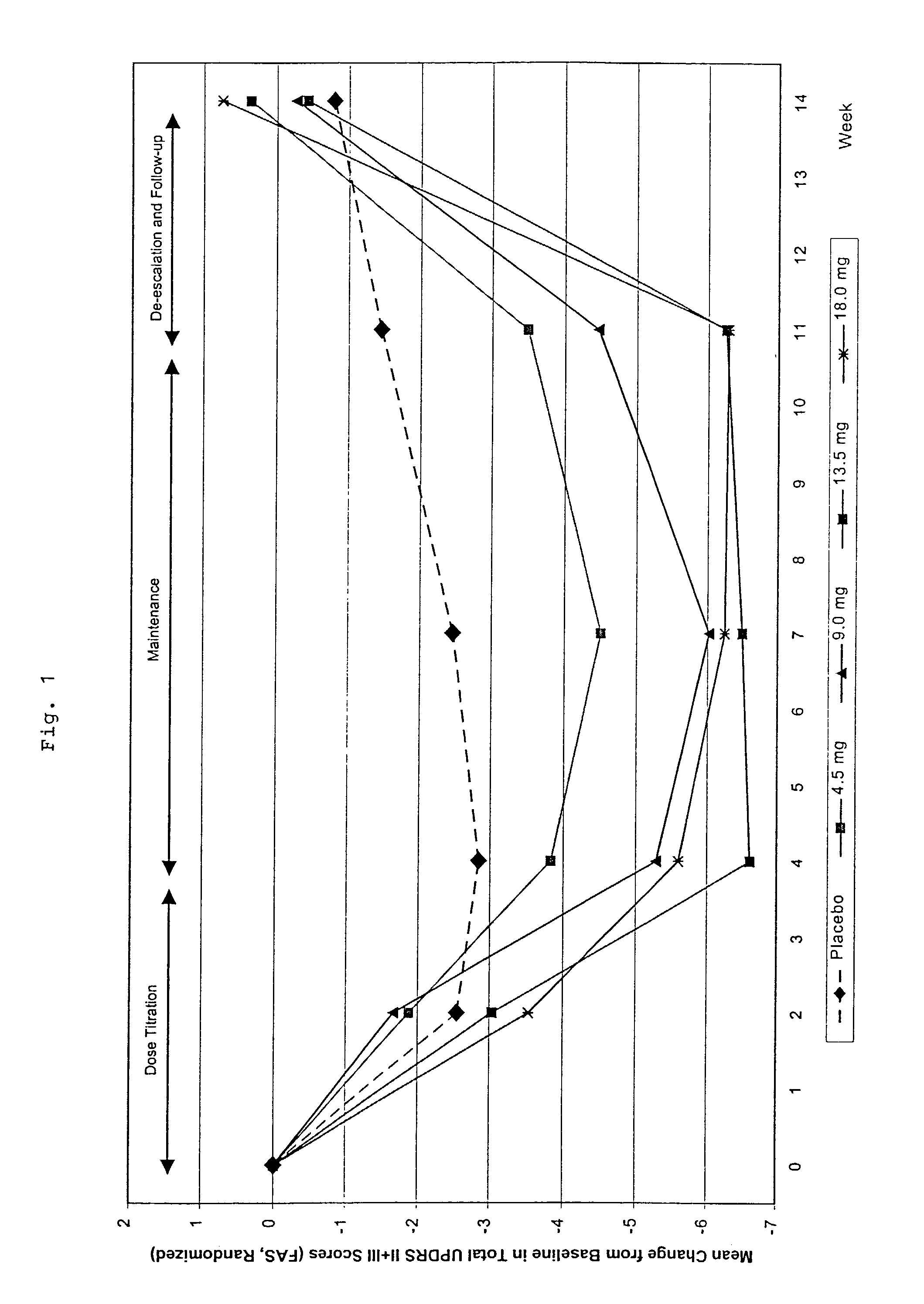
This invention provides the use of a silicone-based transdermal therapeutic system having an area of 10 to 40 cm2 and containing 0.1 to 3.15 mg / cm2 of Rotigotine as active ingredient, for the preparation of an anti-Parkinson medicament which effects an improvement, compared to a placebo treatment, of the condition of human Parkinson patients, measured according to the Unified Parkinson's Disease Rating Scale (UPDRS) parts II and III, of 2 units or more following administration for a time period of at least 7 weeks.
Owner:UCB SA +1
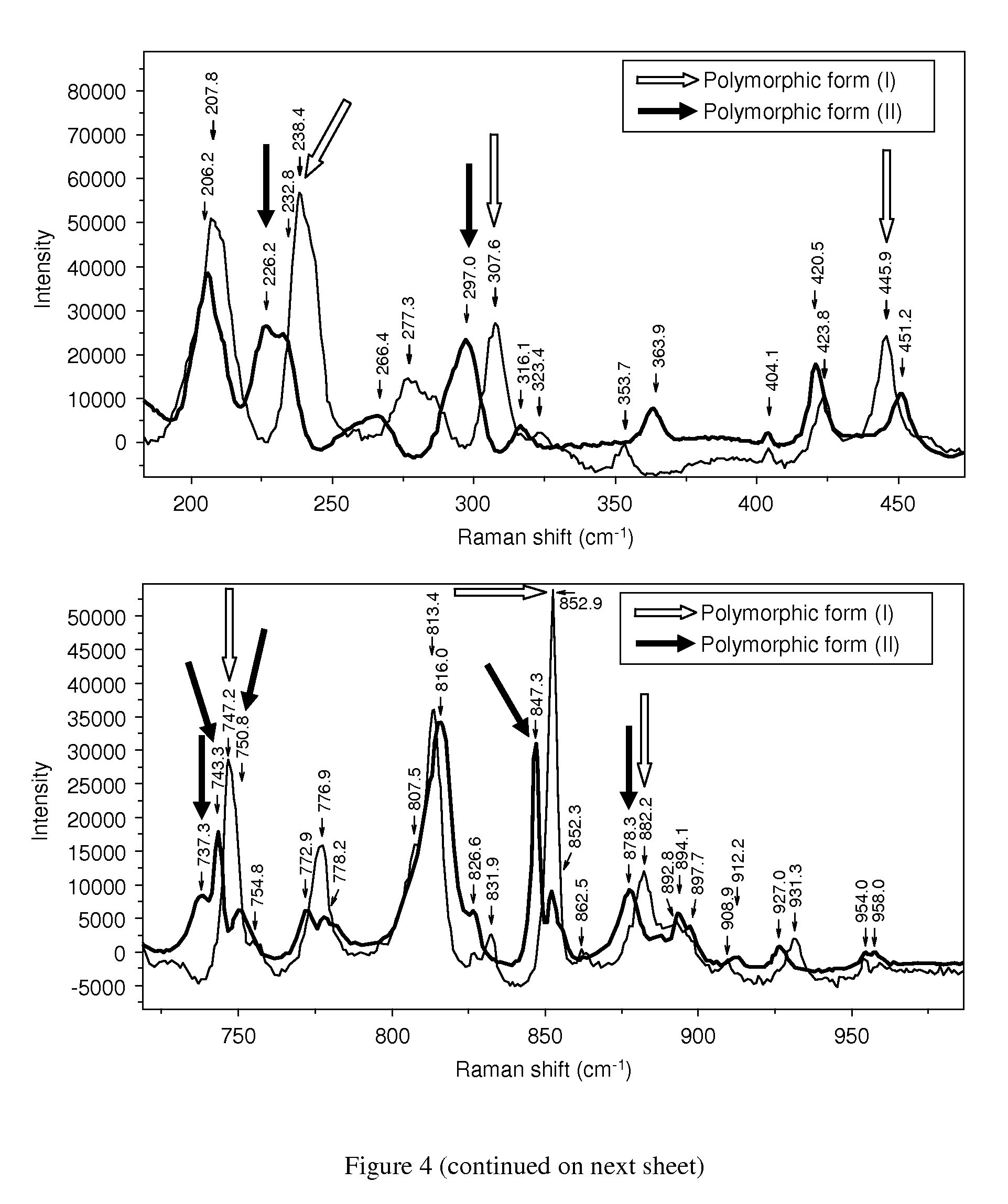
Novel polymorphic form of rotigotine and process for production
ActiveUS20090143460A1High thermodynamic stabilityExtended shelf lifeBiocideNervous disorderDiseaseX-ray
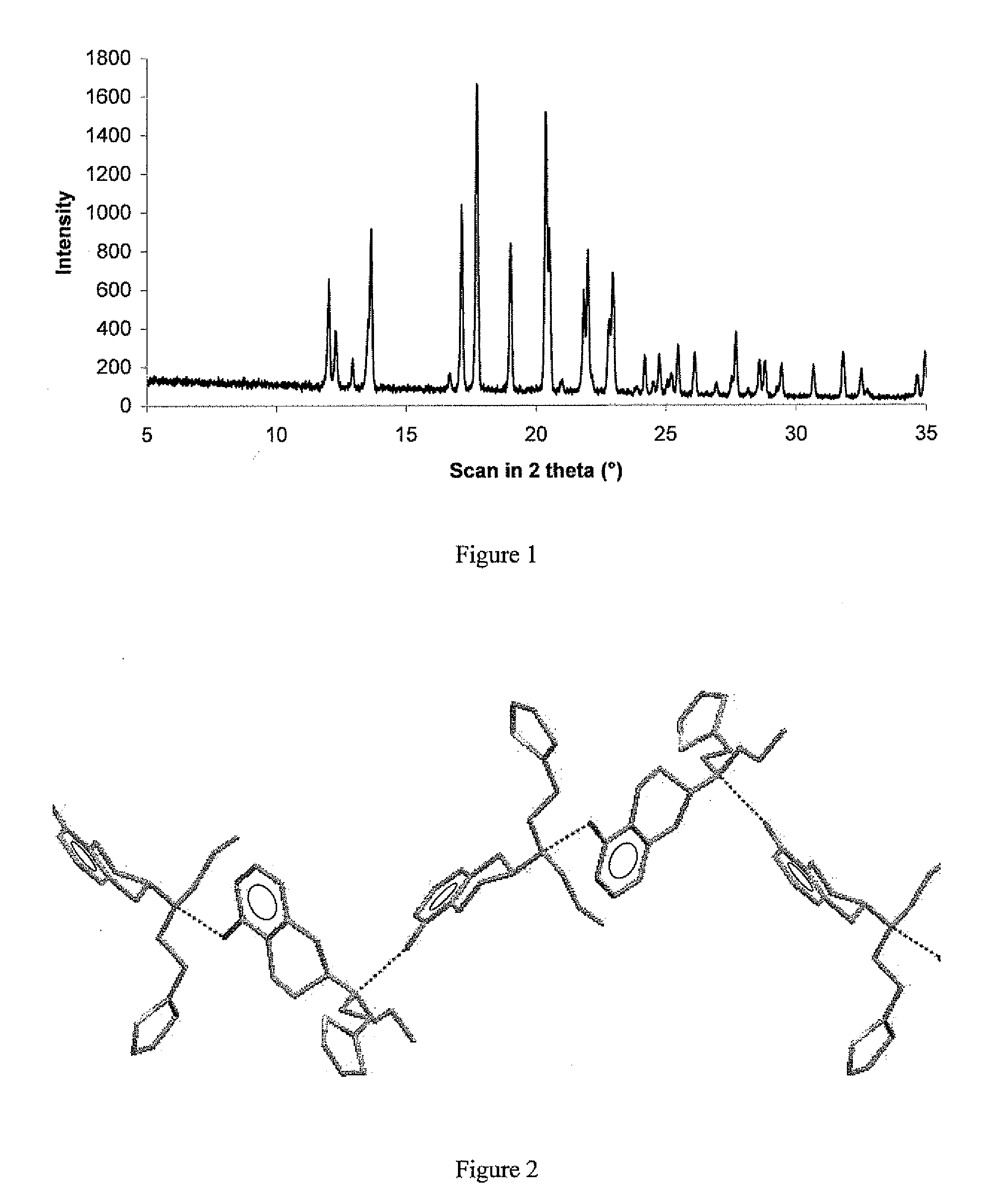
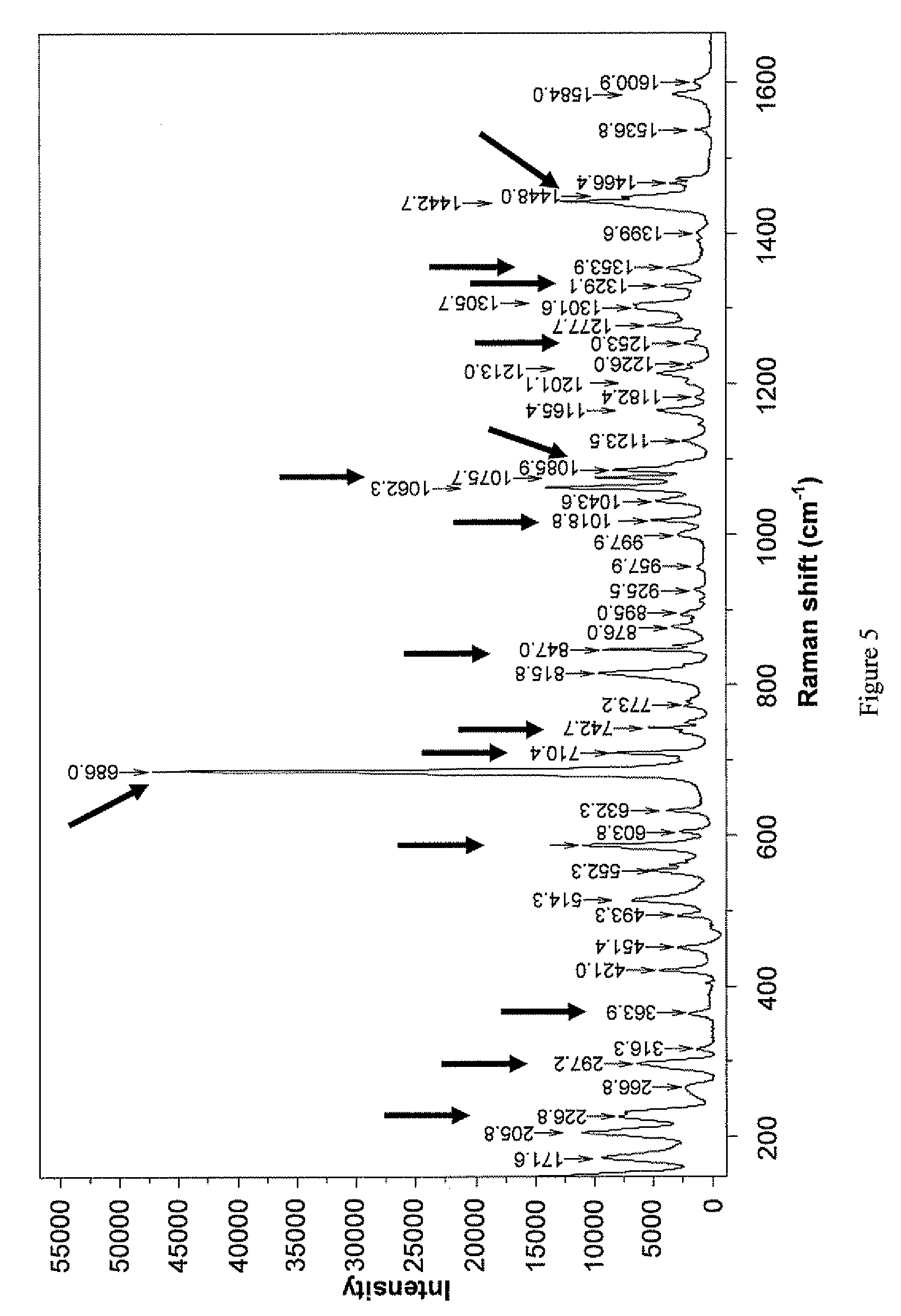
The present invention relates to a novel polymorphic form of rotigotine characterized by at least one of the following powder X-ray diffraction peaks: 12.04, 13.68, 17.72 and 19.01±0.2 (° 2θ), measured with a Cu—Kα irradiation (1.54060 Å), and a process for production thereof, which is useful for the manufacture of a stable medicament for treating or alleviating symptoms of Parkinson's disease and other dopamine-related disorders.
Owner:UCB SA
Intranasal formulation of rotigotine
InactiveUS20070191308A1Improve temperature stabilityLess irritatingBiocideNervous disorderCyclodextrinAqueous solution
This invention pertains to a liquid intranasal pharmaceutical formulation comprising a pharmaceutically acceptable acid addition salt of rotigotine and α-cyclodextrin, preferably in the form of a buffered aqueous solution having a viscosity of 0.5-1.5 mm2 / s
Owner:UCB SA
Transdermal therapeutic system for Parkinson's Disease
InactiveUS20060216336A1Effective treatmentRelieve symptomsBiocideAdhesive dressingsBULK ACTIVE INGREDIENTBlood plasma
The invention provides a transdermal therapeutic system (TTS) containing rotigotine as the active ingredient. The TTS is useful in the treatment of Parkinson's Disease because it induces a pharmacokinetic profile where the rotigotine plasma level is high and stable.
Owner:SCHWARZ PHARM AG +1
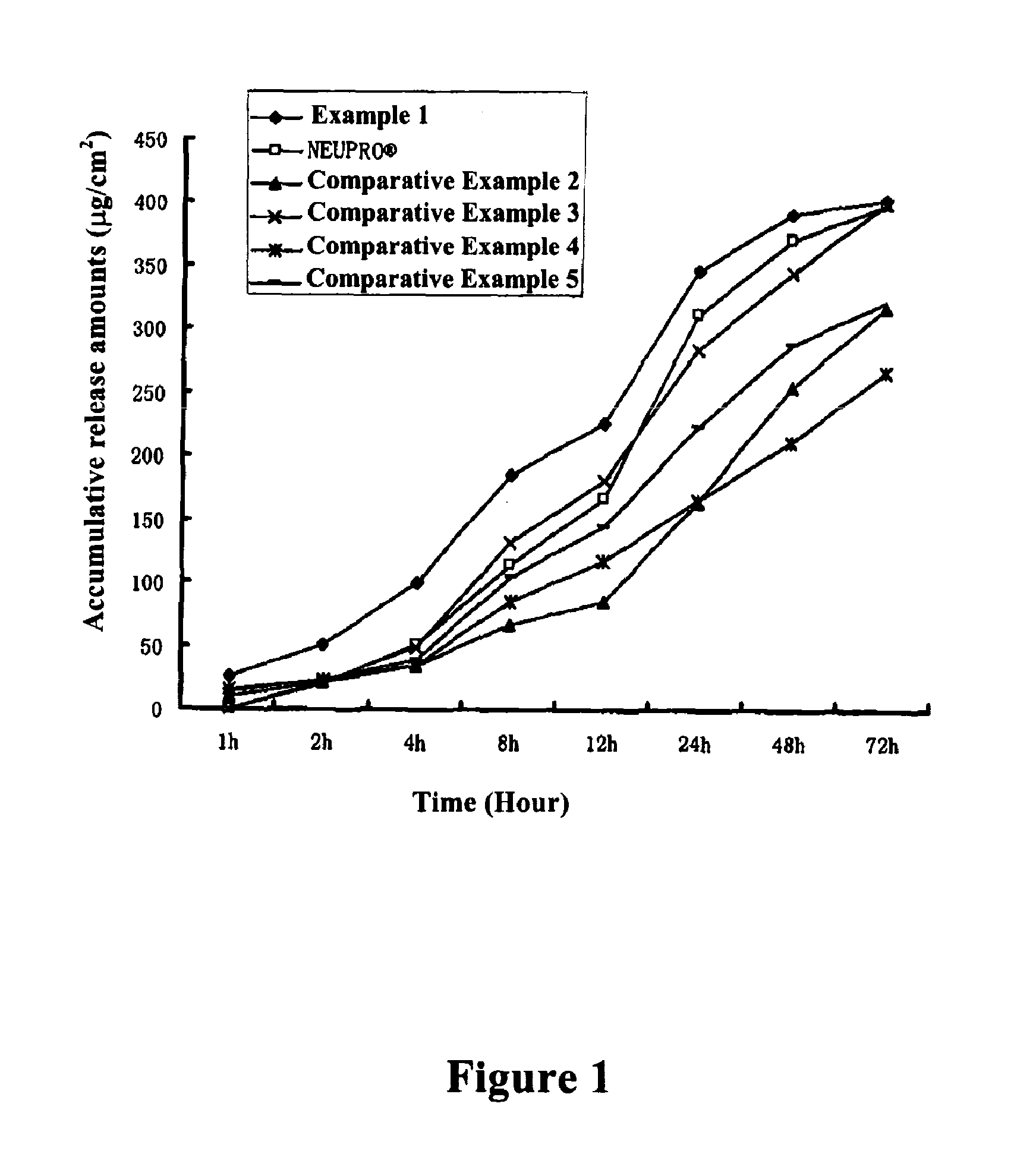
Composition containing rotigotine and use thereof and transdermal patch containing the composition
ActiveUS20110027345A1Improve solubilityIncreases initial dispersion ratePowder deliveryBiocideTransdermal patchSolubility
The present invention relates to a composition containing Rotigotine and the use thereof in the manufacture of a Rotigotine-containing transdermal patch, wherein said composition is based on a matrix mixture system formed from a combination of an acrylic pressure-sensitive adhesive with a silicone pressure-sensitive adhesive, and polyvinylpyrrolidone which are present in a particular weight ratio, wherein (1) the acrylic pressure-sensitive adhesive is present in an amount of about 1-25% by weight in the matrix mixture system, (2) the silicone pressure-sensitive adhesive is present in an amount of about 65-98% by weight in the matrix mixture system, and (3) the polyvinylpyrrolidone is present in an amount of about 1-10% by weight in the matrix mixture system, and comprises 1-40% of Rotigotine on the basis of the total weight of the composition. The present invention further relates to an improved transdermal patch containing Rotigotine comprising said composition. Said patch has improved properties in the solubility, release and initial penetration level of Rotigotine.
Owner:JIANGSU KANGBEIDE PHARMA
Polyvinylpyrrolidone for the stabilization of a solid dispersion of the non-crystalline form of rotigotine
ActiveUS20120322845A1Sufficient long-term storage stability propertyBiocideNervous disorderMedicineRotigotine
The present invention relates to a method for stabilizing rotigotine, the method comprising providing a solid dispersion comprising polyvinylpyrrolidone and a non-crystalline form of rotigotine, wherein the weight ratio of rotigotine to polyvinylpyrrolidone is in a range from about 9:3.5 to about 9:6. The present invention also relates to a solid dispersion comprising a dispersing agent and a dispersed phase, said dispersed phase comprising rotigotine and polyvinylpyrrolidone, wherein the weight ratio of rotigotine to polyvinylpyrrolidone is in a range from about 9:3.5 to about 9:6, a pharmaceutical composition comprising such a solid dispersion, in particular a transdermal therapeutic system, as well as a method for the preparation thereof.
Owner:UCB SA +1
Amorphous rotigotine transdermal system
The present invention refers to a transdermal delivery device comprising a backing layer, an adhesive matrix layer comprising a supersaturated concentration of rotigotine substantially in amorphous form within the adhesive matrix, and a release liner. The present invention also refers to a method of preparing an adhesive matrix containing a supersaturated amount of rotigotine substantially in amorphous form. Further, the present invention refers to a method of stabilizing and a method of reestablishing the meta-stable amorphous-drug transdermal system during its manufacturing, storing, shipping and handling process.
Owner:MYLAN TECH INC
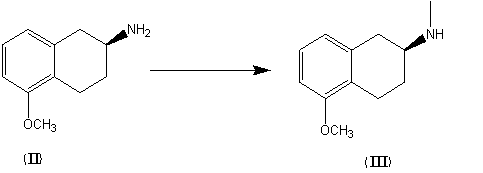
Preparation method of rotigotine
ActiveCN104130238AFew synthetic stepsHigh yieldOrganic compound preparationAmino-hyroxy compound preparationChemical reactionTetralin
The invention discloses a preparation method of rotigotine, and belongs to the technical field of medicine synthesis. According to the method, 5-methoxy-2-tetralone is used as a raw material for amination, asymmetric reduction, halogenation and methoxyl group removal four step reaction for synthesis of chiral rotigotine {(-)-(S)-2-(N-propyl-N-(2-(2-thiophene) ethyl] amino]-5-hydroxy-1, 2, 3, 4-tetralin}. According to the method, a simplex stereoscopic structural compound is synthesized by stereo selective chemical reaction, in the asymmetric reduction process, hantzsch ester 1, 4-dihydropyridine (HEH) is used as a reducing agent, and chiral phosphoric acid is used as a catalyst to synthesize an important intermediate (S)-2-(N-n-propyl) amido-5-methoxy tetralin (II) with a simplex stereoscopic structure, the use of a chiral reagent for splitting to obtain the simplex stereoscopic structural compound is avoided, the synthesis procedure is shortened, the yield is improveds, and the method is favorable for industrialized production.
Owner:HENAN ACADEMY OF SCI CHEM RES INST CO LTD
Use of rotigotine for treating and preventing Parkinson's plus syndrome
InactiveUS7872041B2Prevents and reduces rate of progressionBiocideNervous disorderGynecologyRotigotine
The invention relates to the use of rotigotine, its salts and prodrugs, as a medicament for preventing and / or treating Parkinson's plus syndrome.
Owner:UCB SA
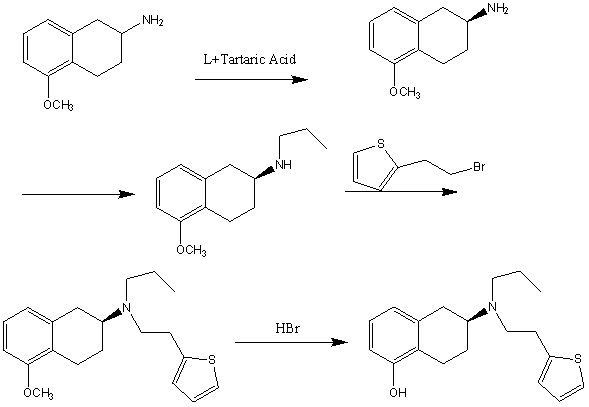
Novel process for preparing rotigotine
InactiveCN103058985AThe synthetic route is simpleSimple and fast operationOrganic chemistryBiochemical engineeringEthyl group
The invention discloses a novel process for preparing rotigotine based on 2-amino-5-methoxynaphthalene as a material. The process comprises the steps of splitting to obtain (S)-2-amino-5-methoxynaphthalene (II) by L-tartaric acid, reacting (S)-2-amino-5-methoxynaphthalene (II) with bromopropane to obtain (S)-2-(N-n-propyl)amido-5-methoxynaphthalene (III); reacting (S)-2-(N-n-propyl)amido-5-methoxynaphthalene (III) with 2-(2-bromethyl) thiophene to obtain (S)-2-[N-propyl-N-[2-(2-thiophene)ethyl]amino]-5-methoxyl-1,2,3,4-tetrahydronaphthalene (IV), and removing methyl of the (IV) under the effect of hydrobromic acid to obtain a target product rotigotine (-)-(S)-2-[N-propyl group-N-[2-(2-thiophene)ethyl]amino]-5-hydroxy-1,2,3,4-tetrahydronaphthalene (I), wherein the purity is higher than 99%. The novel process is simple in synthetic route, simple and convenient to operate and high in yield, and large-scale industrial production is easy to carry out.
Owner:SHANDONG LUBEI PHARMA
Transdermal patch containing Rotigotine
ActiveUS9265752B2Increased Rotigotine initial penetration levelEffective amountBiocidePowder deliveryTransdermal patchSolubility
Owner:JIANGSU KANGBEIDE PHARMA
Amorphous rotigotine transdermal system
The present invention relates to a transdermal drug delivery device comprising a backing layer, an adhesive matrix layer comprising a supersaturated concentration of rotigotine substantially in amorphous form in said adhesive matrix and a release liner . The invention also relates to a process for the preparation of an adhesive matrix containing a supersaturated concentration of rotigotine substantially in amorphous form. In addition, the present invention relates to methods of stabilizing and reconstituting metastable amorphous drug transdermal systems during their manufacture, storage, shipping and handling.
Owner:MYLAN TECH INC
Compositions of rotigotine, derivatives thereof, or pharmaceutically acceptable salts of rotigotine or its derivative
The disclosure provides a composition comprising rotigotine or a pharmaceutically acceptable salt thereof; at least one poly(lactide-co-glycolide) (PLGA); and at least one fatty acid, wherein the at least one fatty acid is at least 0.5% in weight relative to the total weight of the composition. The composition as disclosed herein has significantly reduced burse release effect. The disclosure also provides a method of treating Parkinson's disease comprising administering an effective amount of the composition as disclosed to a subject in need thereof.
Owner:SHANDONG LUYE PHARMA CO LTD
Sustained-release formulation of rotigotine
Provided herein are methods and compositions for producing formulations systemic delivery of dopamine agonists via the oral inhalation route. Specifically, provided herein are methods and compositions for a formulation of rotigotine that is suitable for administration via oral inhalation. Such methods and compositions are useful in the treatment or amelioration of one or more Parkinson's disease symptom(s).
Owner:MAP PHARMACEUTICAL INC
Method for separating enantiomers of rotigotine and trihexyphenidyl
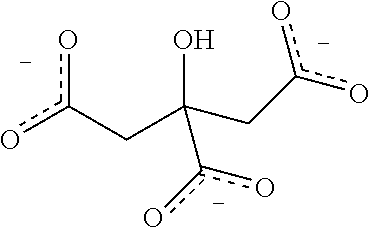
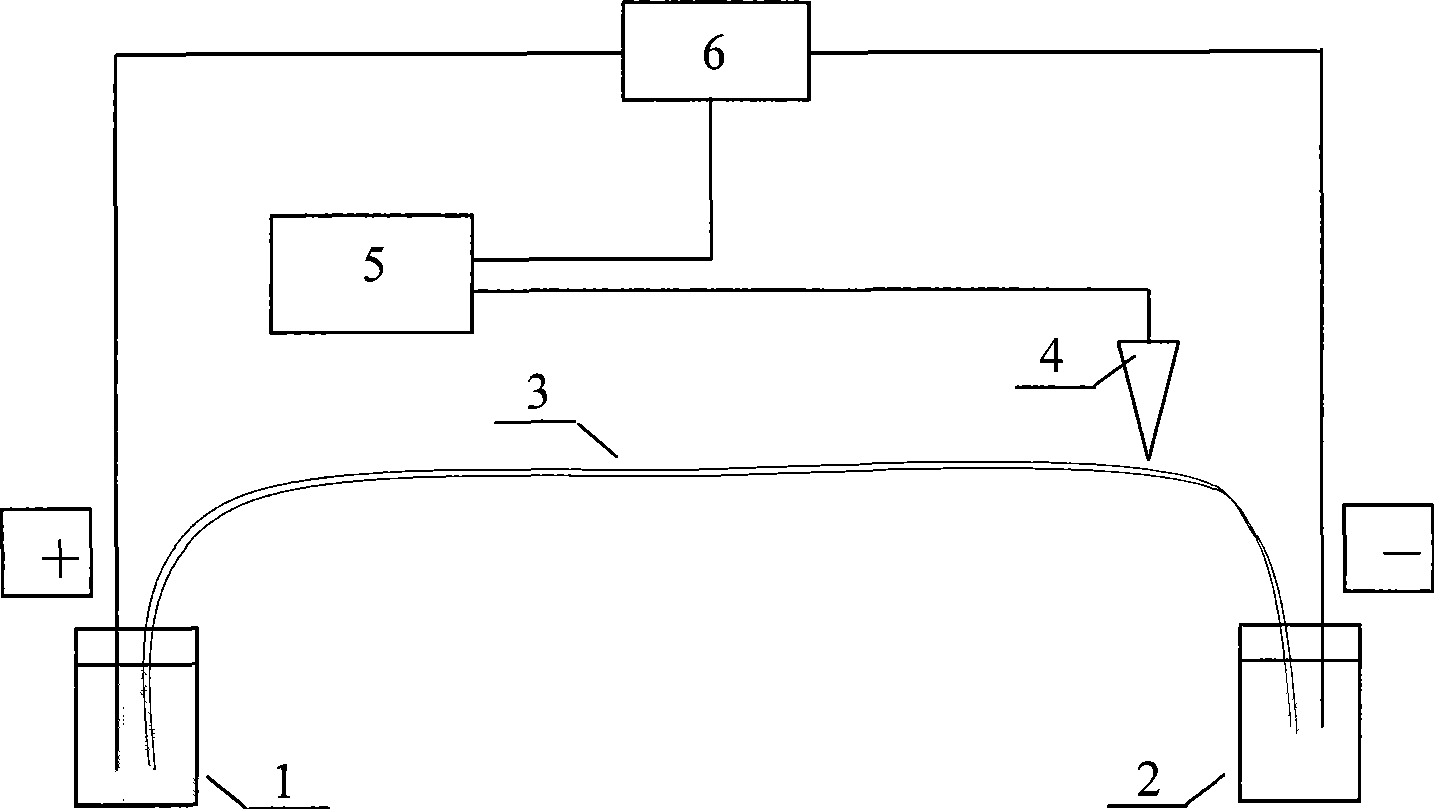
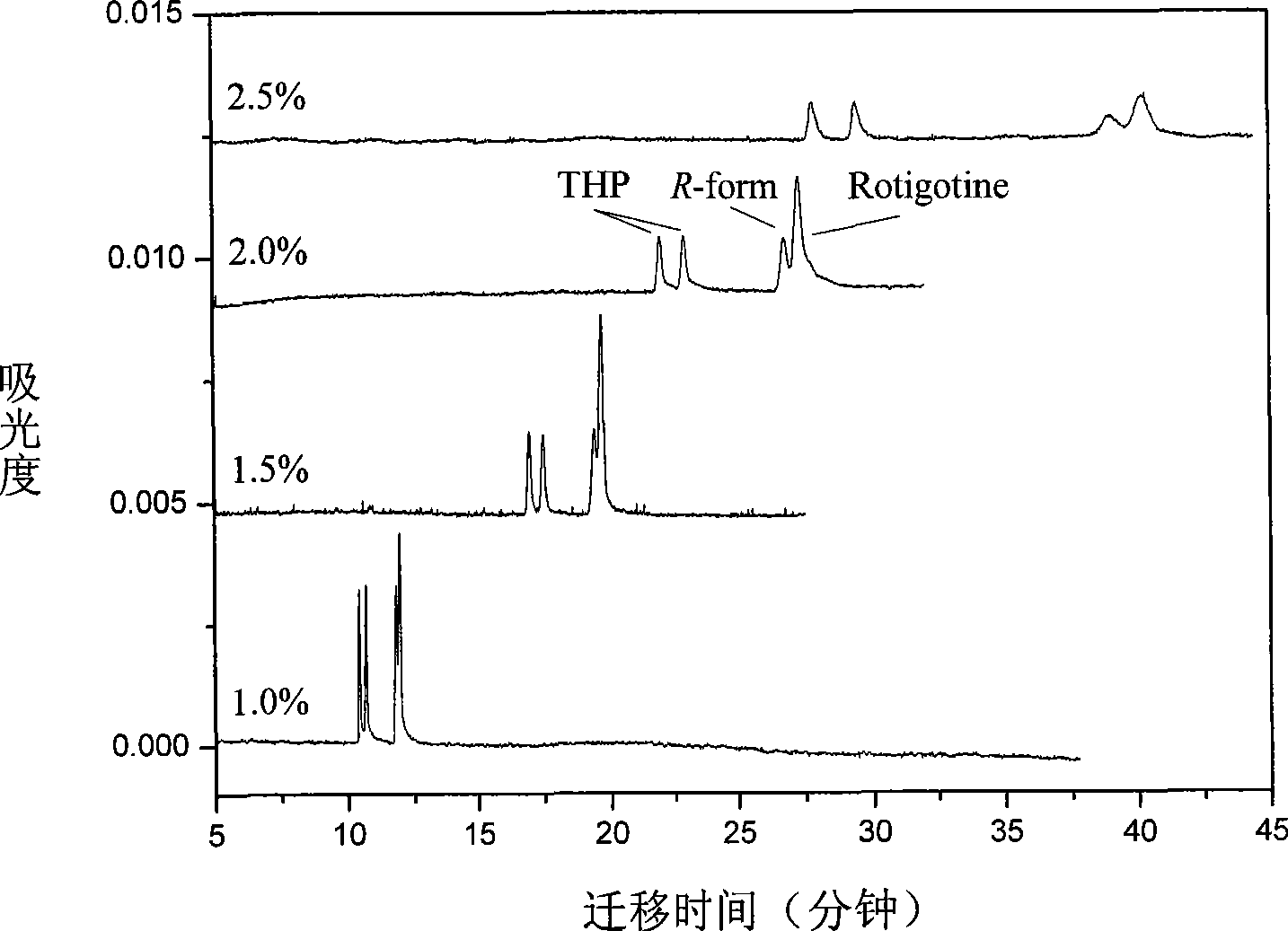
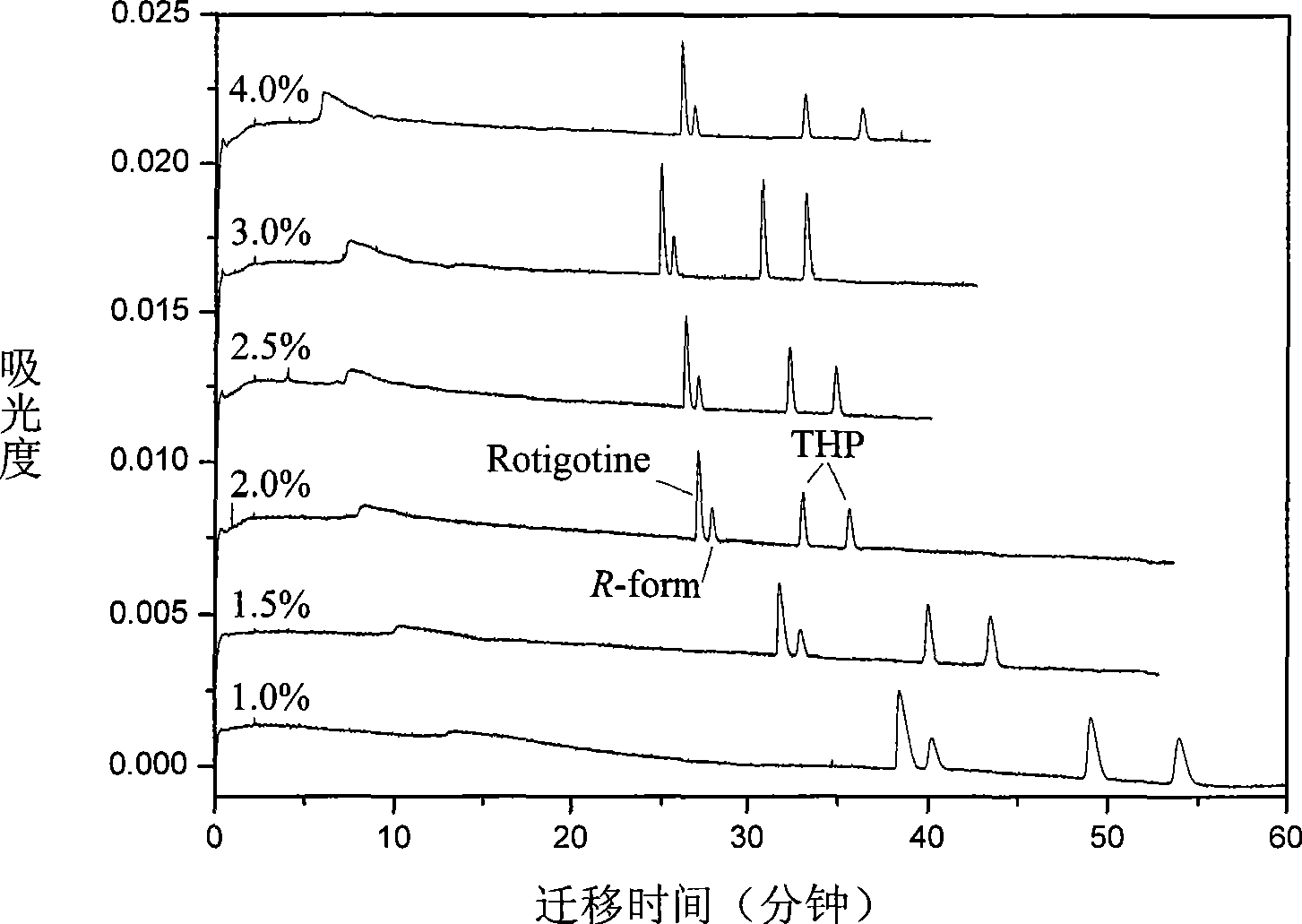
InactiveCN101544629ARich chiral centerReach flipMaterial analysis by electric/magnetic meansOptically-active compound separationCapillary electrophoresisEnantiomer
The invention provides a method for separating enantiomers of rotigotine and trihexyphenidyl. The method comprises the steps of dissolving dextran sulfate in a buffer solution, obtaining an operational buffer solution containing the dextran sulfate as a chiral selector, dissolving rotigotine to be separated and trihexyphenidyl as samples to be separated in the operational buffer solution and separating enantiomers of two medicaments through a capillary electrophoresis instrument. The method is characterized in that the adopted chiral selector is the dextran sulfate of which the molecular weight is 500,000 or 1,000,000, and is low in background ultraviolet absorption, cheap and readily available. The adopted method has the advantages of high separation efficiency, low reagent consumption, environment-friendly property, convenient operation, capability of realizing the chiral separation of the enantiomers in a forward or backward voltage mode, as well as opposite transfer order of the enantiomers in two modes. The method is high in the degree of separating the enantiomers of the two medicaments.
Owner:BEIJING UNIV OF CHEM TECH
Rotigotine percutaneous absorption patch, and preparation and application thereof
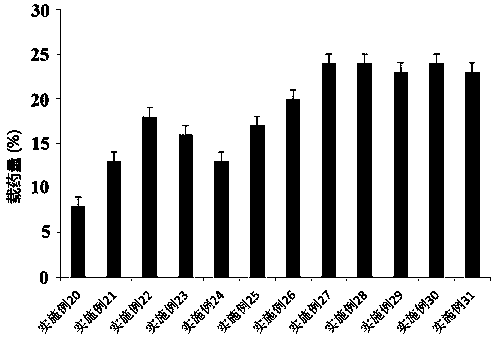
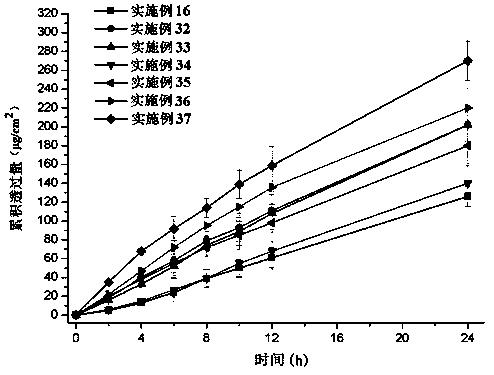
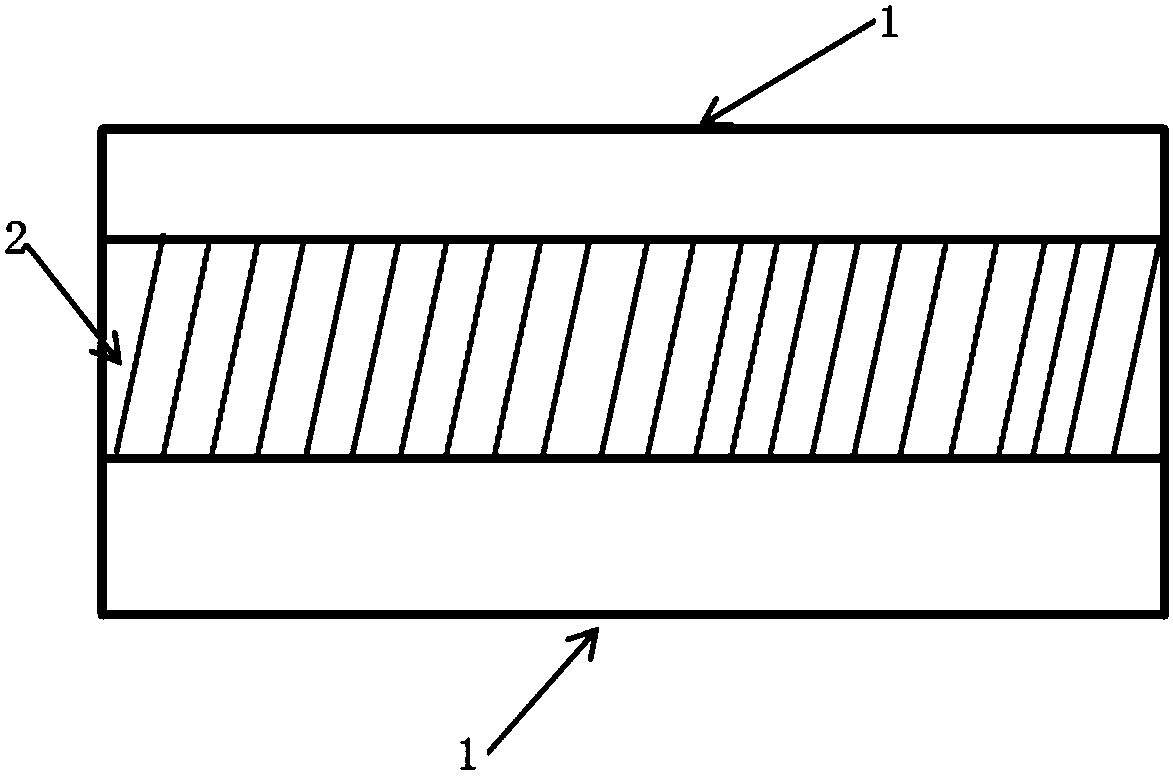
ActiveCN110638792APrevent the risk of devitrificationImprove percutaneous penetrationOrganic active ingredientsNervous disorderAdhesiveSkin penetration
The invention belongs to the technical field of medicine, and relates to a rotigotine percutaneous absorption patch, and preparation and application thereof. The patch can obviously relieve a crystalseparation phenomenon of a rotigotine patch and provides good skin penetration delivery performance. The rotigotine percutaneous absorption patch consists of a back lining layer, a medicine loaded pressure-sensitive adhesive layer and an anti-bonding layer, wherein the medicine loaded pressure-sensitive adhesive layer comprises rotigotine free alkali or rotigotine organic acid ionic liquid, pressure-sensitive adhesives and a percutaneous absorption enhancing agent, wherein the weight of the rotigotine free alkali or rotigotine organic acid ionic liquid accounts for 2.0 to 20 weight percent ofthe total weight of the medicine loaded pressure-sensitive adhesive layer; the weight of the pressure-sensitive adhesives accounts for 77 to 90 weight percent of the total weight of the pressure-sensitive adhesive layer; and the consumption of the percutaneous absorption enhancing agent accounts for 0 to 10 weight percent of the total weight of the pressure-sensitive adhesive layer. In the rotigotine organic acid ionic liquid, a mol ratio of the rotigotine free alkali to different organic acids is 0.5:1-2:1. The rotigotine percutaneous absorption patch has the advantages that the accumulated penetration amount of rotigotine in 24h can reach 0.5mg / cm<2>. The preparation technology of the patch is simple; the stability of the patch is high; and the use is convenient.
Owner:SHENYANG PHARMA UNIVERSITY
Polymorphic form of rotigotine and process for production
ActiveUS8232414B2High thermodynamic stabilityExtended shelf lifeBiocideNervous disorderX-rayDopamine
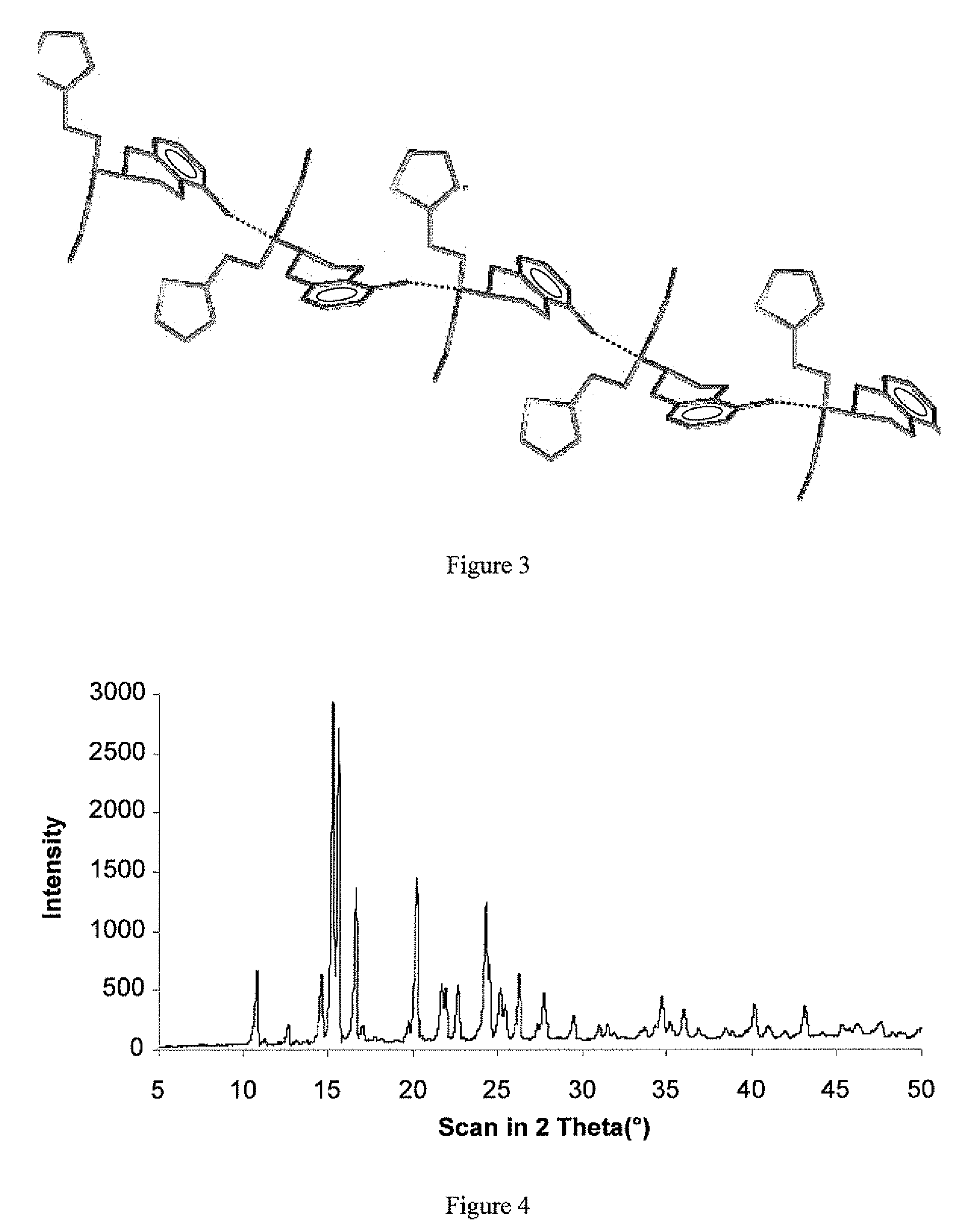
The present invention relates to a novel polymorphic form of rotigotine characterized by at least one of the following powder X-ray diffraction peaks: 12.04, 13.68, 17.72 and 19.01±0.2 (° 2θ), measured with a Cu—Kα irradiation (1.54060 Å), and a process for production thereof, which is useful for the manufacture of a stable medicament for treating or alleviating symptoms of Parkinson's disease and other dopamine-related disorders.
Owner:UCB SA
Transdermal patch containing rotigotine, and preparation method thereof
ActiveCN108451934AImprove transdermal release performanceGood weather resistanceOrganic active ingredientsNervous disorderTransdermal patchDouble bond
The invention discloses a transdermal patch composition containing rotigotine, and more specifically discloses a transdermal patch containing rotigotine, and a preparation method thereof. The transdermal patch composition contains effective doses of rotigotine and a self-adhesive matrix; the self-adhesive matrix comprises a polyisobutylene pressure-sensitive adhesive and a colloid softener. According to the preparation method, the polyisobutylene pressure-sensitive adhesive is adopted, the C-H skeleton of the polyisobutylene pressure-sensitive adhesive is long and straight, only the terminal groups contain unsaturated bonds, double bond content is low, and reaction sites are few, so that the transdermal patch containing rotigotine is extremely stable, is excellent in weatherability, heat resistance, and aging resistance, is low in cost; the materials are easily available; the operationality is high; the colloid softener is added, so that the stability of the transdermal patch containing rotigotine is improved, and precipitation of drug active components out from the transdermal patch containing rotigotine is prevented; and in addition, an adhesiveness conditioning agent is added, so that the permeability of the transdermal patch containing rotigotine is excellent.
Owner:BEIJING TIDE PHARMA
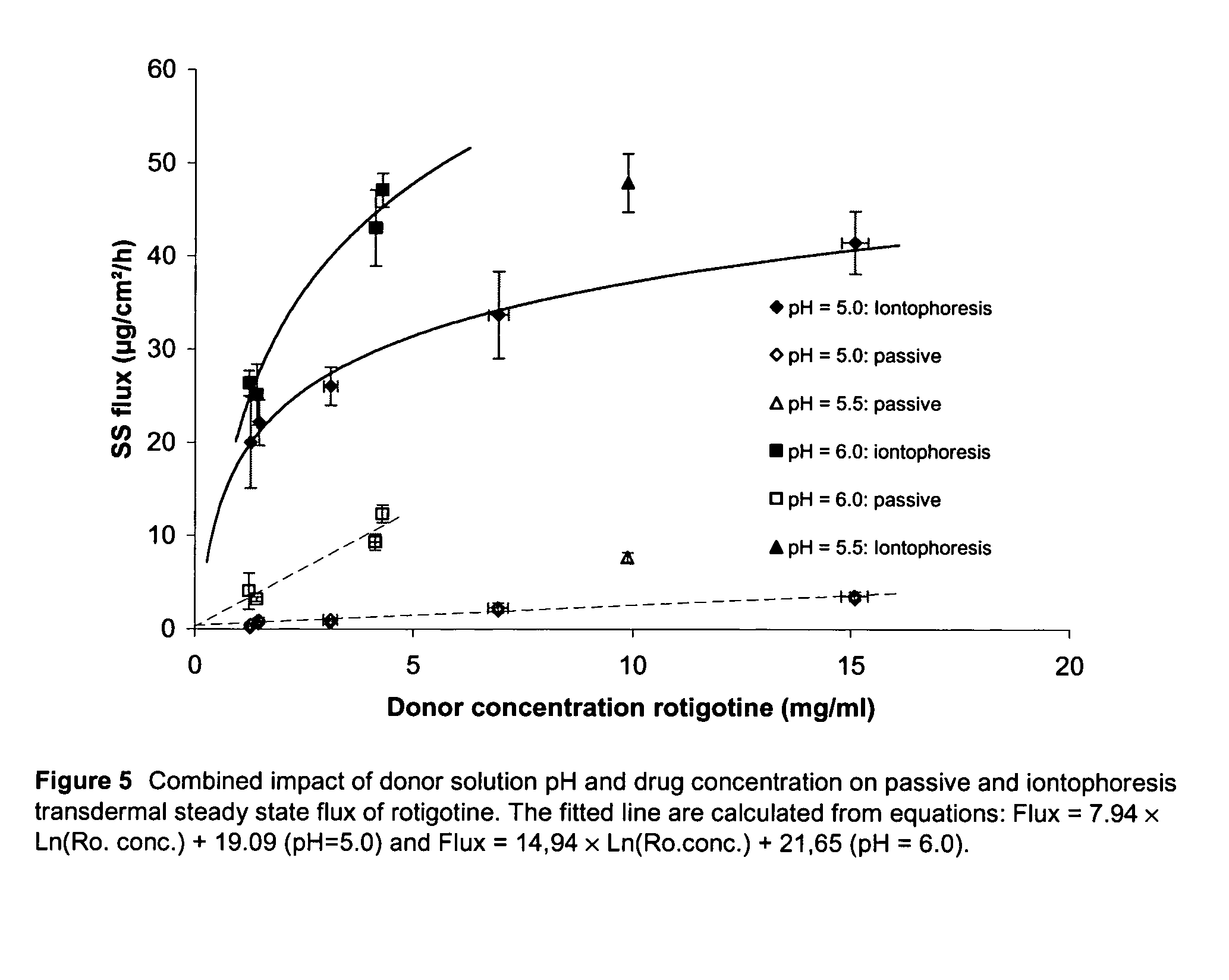
Pharmaceutical composition comprising rotigotine salts (acid or Na), especially for iontophoresis
InactiveUS8754120B2Enhanced iontophoretic deliveryGood to excellent solubilityBiocideNervous disorderDiseaseSolubility
Owner:UCB SA
Intranasal formulation of rotigotine
InactiveUS7683040B2Less irritatingStable and safe and effectiveBiocideNervous disorderPharmaceutical formulationAqueous solution
This invention pertains to a liquid intranasal pharmaceutical formulation comprising a pharmaceutically acceptable acid addition salt of rotigotine and α-cyclodextrin, preferably in the form of a buffered aqueous solution having a viscosity of 0.5-1.5 mm2 / s.
Owner:UCB SA
Polyvinylpyrrolidone for the stabilization of a solid dispersion of the non-crystalline form of rotigotine
ActiveUS9925150B2Sufficient long-term storage stability propertyBiocideNervous disorderMedicineRotigotine
The present invention relates to a method for stabilizing rotigotine, the method comprising providing a solid dispersion comprising polyvinylpyrrolidone and a non-crystalline form of rotigotine, wherein the weight ratio of rotigotine to polyvinylpyrrolidone is in a range from about 9:3.5 to about 9:6. The present invention also relates to a solid dispersion comprising a dispersing agent and a dispersed phase, said dispersed phase comprising rotigotine and polyvinylpyrrolidone, wherein the weight ratio of rotigotine to polyvinylpyrrolidone is in a range from about 9:3.5 to about 9:6, a pharmaceutical composition comprising such a solid dispersion, in particular a transdermal therapeutic system, as well as a method for the preparation thereof.
Owner:UCB SA +1
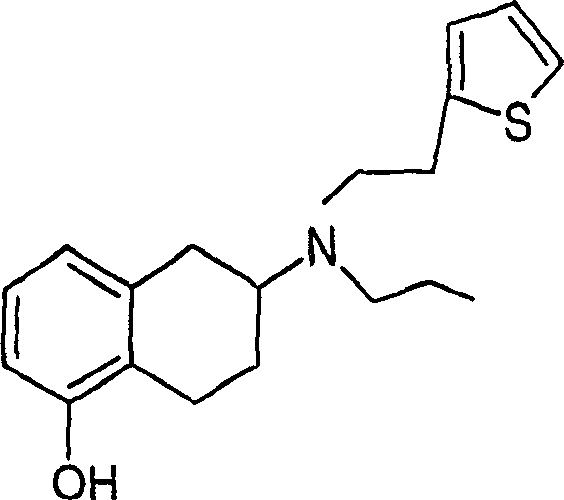
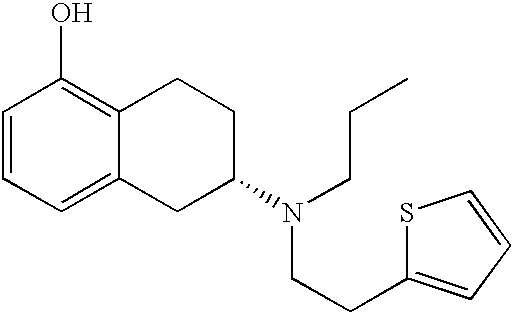
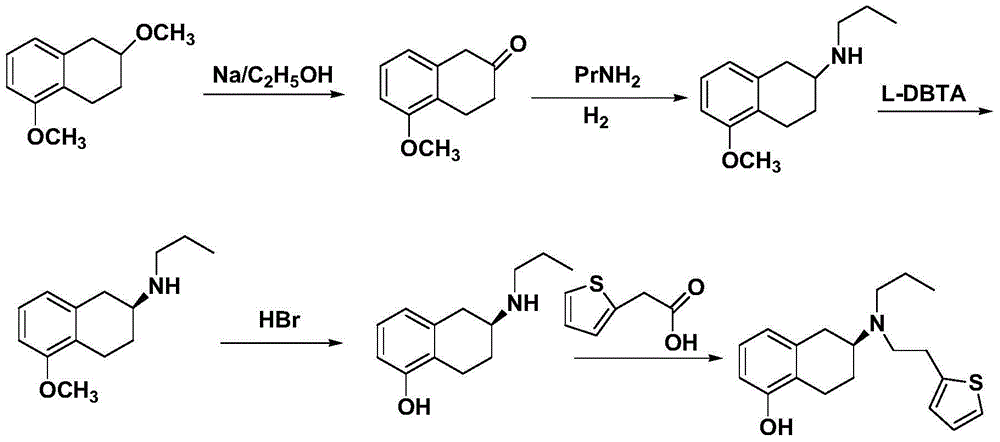
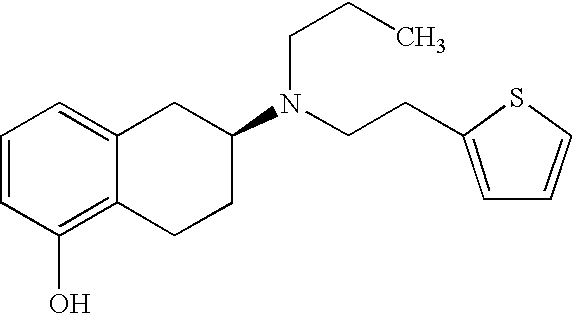
Process for the preparation of (6S)-(-)-5,6,7,8-tetrahydro-6-[propyl-(2-thienyl)ethyl]amino-1-naphthol (rotigotine)
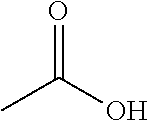
ActiveUS20110306776A1Avoid reactionEasy to useOrganic active ingredientsOrganic chemistryAcetic acidAcetylation
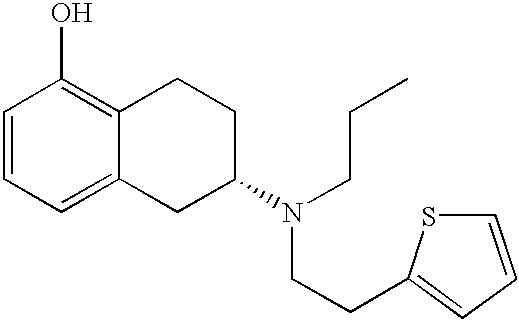
The present invention describes a novel process for the preparation of (6S)-(−)-5,6,7,8-tetrahydro-6-[propyl-(2-thienyl)ethyl]amino-1-naphthol (Rotigotine) comprising: (a) acetylating (S)-(−)-5-hydroxy-N-n-propyl-2-aminotetraline to afford the acetate; (b) reacting this acetate, (−)-5-acetoxy-N-n-propyl-2-aminotetraline, with 2-(2-thienyl)ethanol 2-nitrobenzenesul-fonate; (d) hydrolyzing (6S)-(−)-1-acetoxy-5,6,7,8-tetrahydro-6-[propyl-(2-thienyl)ethyl]amino-1-naphthalene to afford (6S)-(−)-5,6,7,8-tetrahydro-6-[propyl-(2-thienypethyl]amino-1-naphthol (Rotigotine) and (d) purifying rotigotine either by the acetylation reaction and subsequent hydrolysis of the formed acetate or by salification of rotigotine through hydrochloride or hydrobromide formation and subsequent base release. Rotigotine is a dopamine agonist and is indicated for the treatment of Parkinson's disease.
Owner:INTERQUIM SA
Rotigotine derivatives and preparation and application thereof
ActiveCN108341798AHigh purityHigh reaction conversion rateOrganic active ingredientsNervous disorderAlkanePatient compliance
The invention relates to preparation and application of rotigotine derivatives, specifically to synthesis of a series of rotigotine derivatives. The rotigotine derivatives are applied to preparation of micrometer or nanometer drug suspension and are prepared by directly or indirectly acylating rotigotine with alkanes of different chain lengths or fatty acid thereof, olefins or fatty acid thereof,vitamins, polyethylene glycol, polylactic acid, amino acid and other groups containing hydroxyl groups, amino groups or carboxyl groups. According to the invention, the micrometer or nanometer drug suspensions of the rotigotine derivatives are prepared by using industrially common equipment used for reducing the particle sizes of drugs, e.g., a high-pressure homogenizer or a ball mill; and when used in an in-vivo administration manner, the prepared drug suspensions of the rotigotine derivatives greatly improve the biological half-life of rotigotine and prolong the action time of rotigotine, and can effectively reduce administration frequency and improve patient compliance during clinical administration.
Owner:SHENYANG PHARMA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
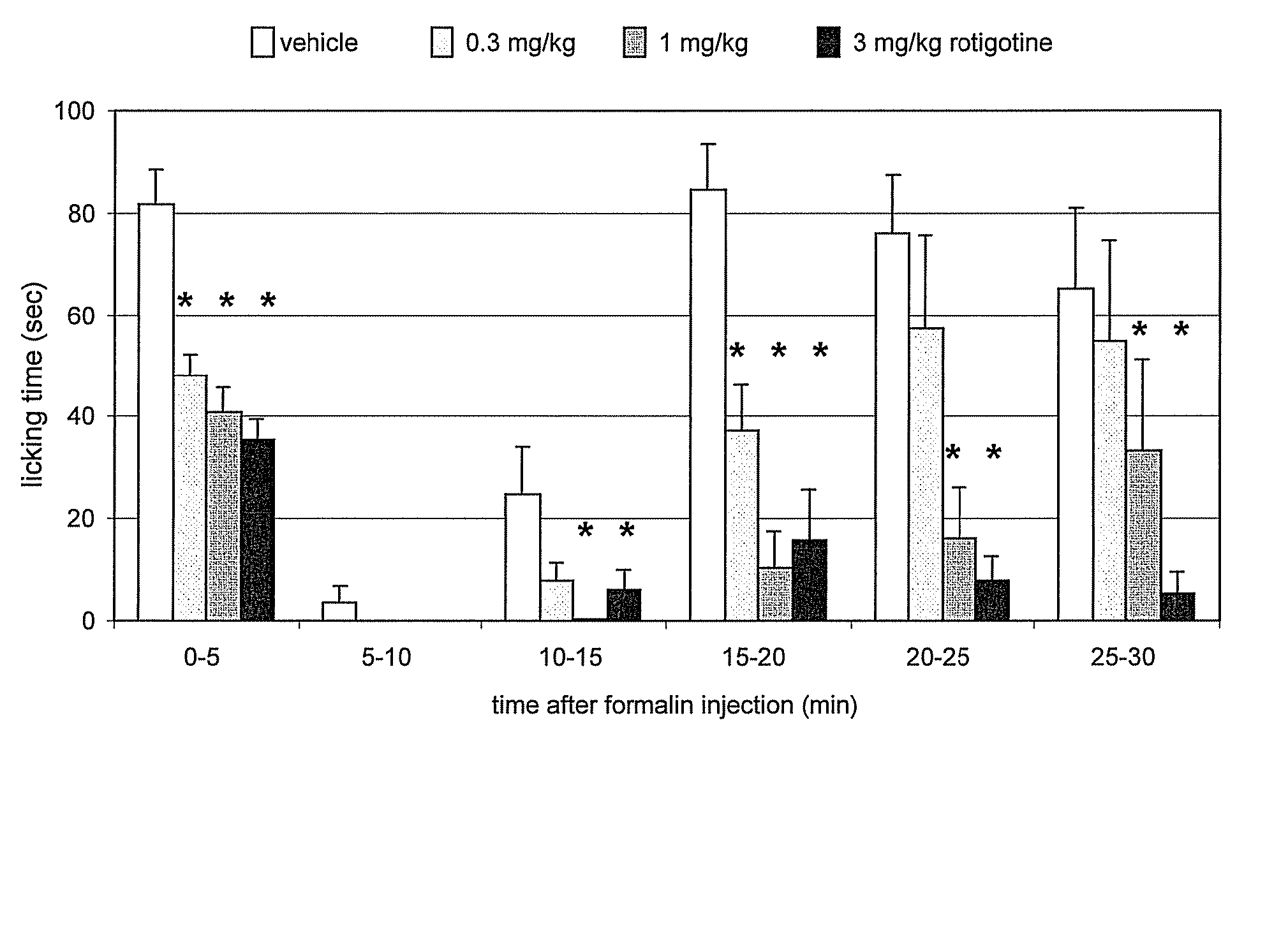
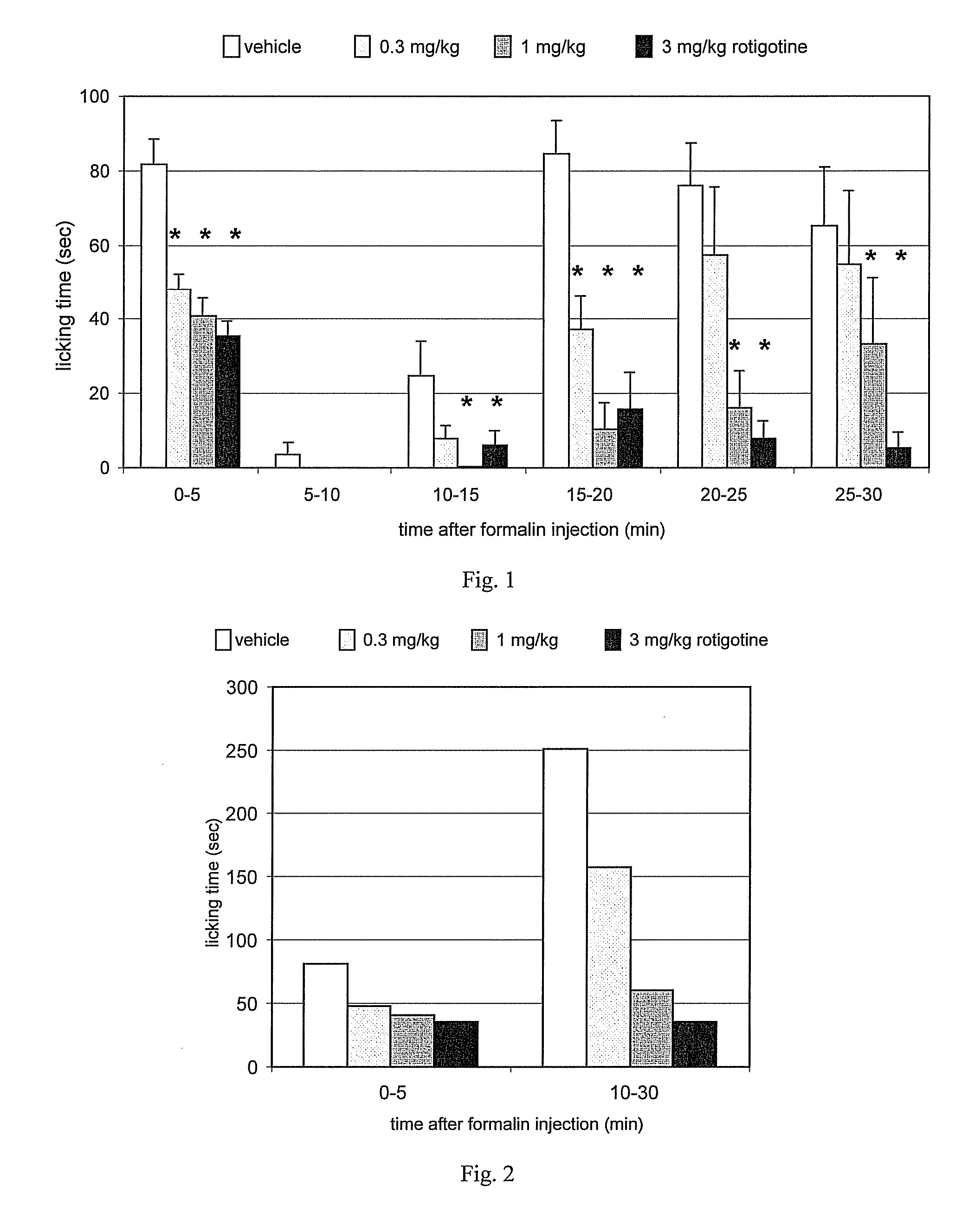









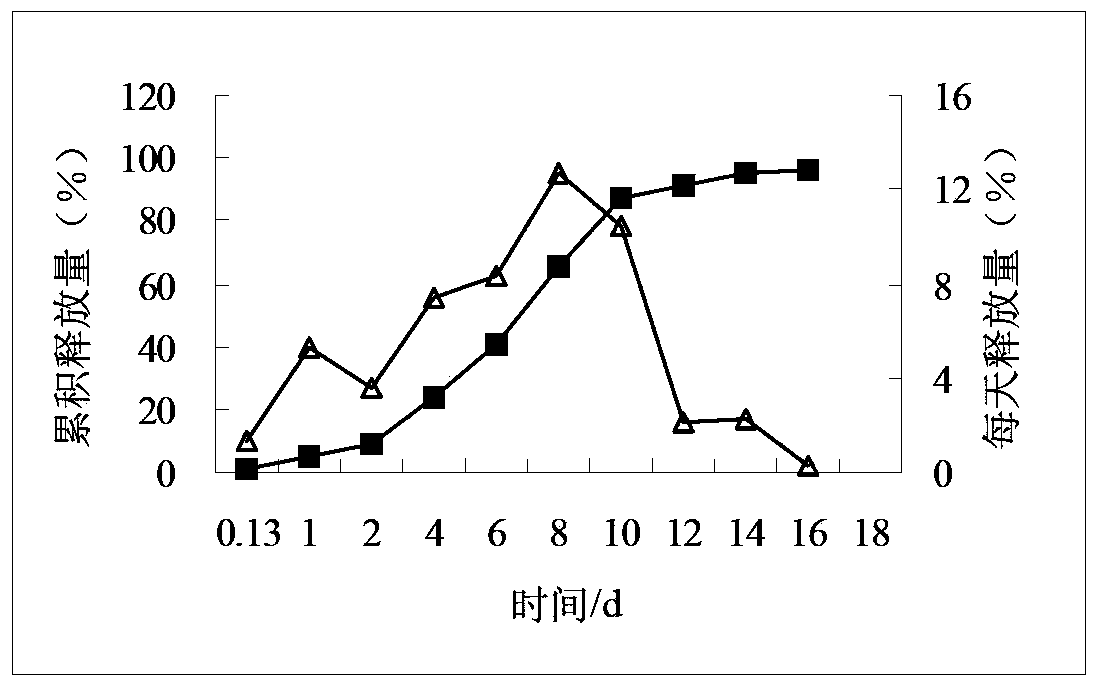









![Process for the preparation of (6S)-(-)-5,6,7,8-tetrahydro-6-[propyl-(2-thienyl)ethyl]amino-1-naphthol (rotigotine) Process for the preparation of (6S)-(-)-5,6,7,8-tetrahydro-6-[propyl-(2-thienyl)ethyl]amino-1-naphthol (rotigotine)](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cb5f4fd9-e258-4d61-84da-2cec7c2fc7e9/US20110306776A1-20111215-C00001.png)
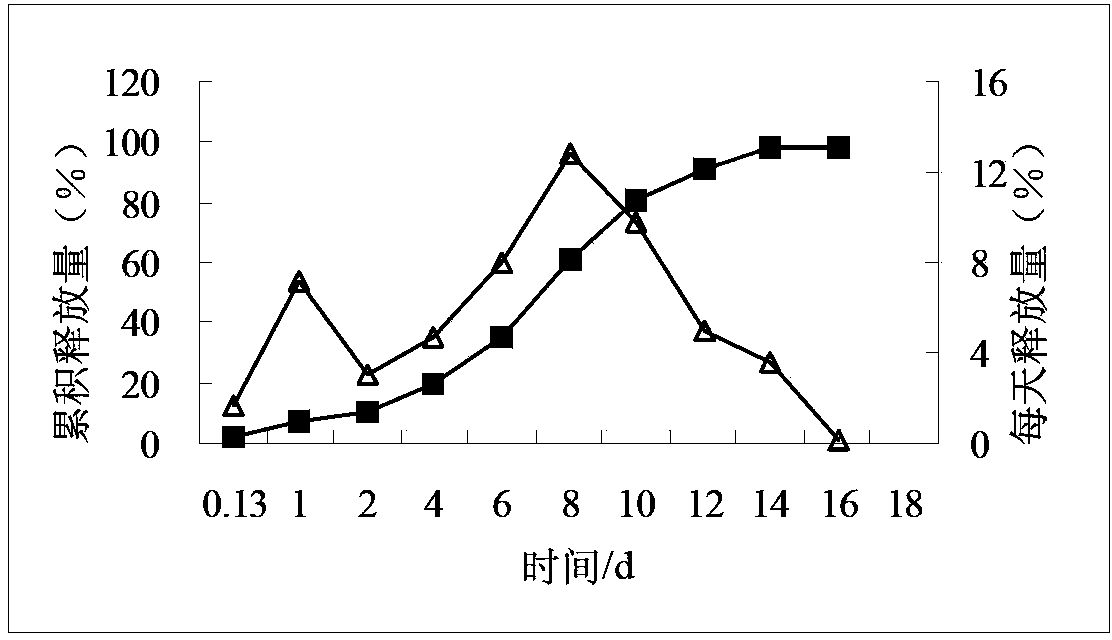
![Process for the preparation of (6S)-(-)-5,6,7,8-tetrahydro-6-[propyl-(2-thienyl)ethyl]amino-1-naphthol (rotigotine) Process for the preparation of (6S)-(-)-5,6,7,8-tetrahydro-6-[propyl-(2-thienyl)ethyl]amino-1-naphthol (rotigotine)](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cb5f4fd9-e258-4d61-84da-2cec7c2fc7e9/US20110306776A1-20111215-C00002.png)
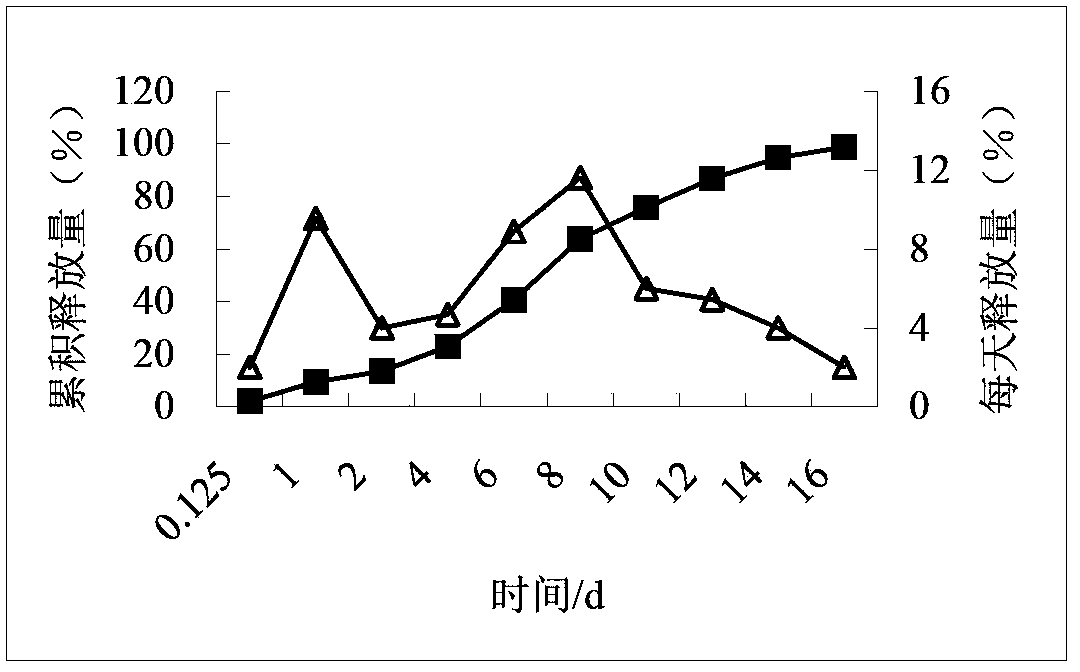
![Process for the preparation of (6S)-(-)-5,6,7,8-tetrahydro-6-[propyl-(2-thienyl)ethyl]amino-1-naphthol (rotigotine) Process for the preparation of (6S)-(-)-5,6,7,8-tetrahydro-6-[propyl-(2-thienyl)ethyl]amino-1-naphthol (rotigotine)](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cb5f4fd9-e258-4d61-84da-2cec7c2fc7e9/US20110306776A1-20111215-C00003.png)