Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1081results about "Optically-active compound separation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Polyelectrolyte complex films for analytical and membrane separation of chiral compounds
The present invention is directed to enantioselective polyelectrolyte complex films. Further, said films may be free or isolated membranes, or coatings on substrates such a porous substrates, capillary tubes, chromatographic packing material, and monolithic stationary phases and used to separate chiral compounds. The present invention is also directed to a method for forming such enantioselective polyelectrolyte complex films.
Owner:FLORIDA STATE UNIV RES FOUND INC
Bupropion metabolites and methods of use
Owner:SEPACOR INC
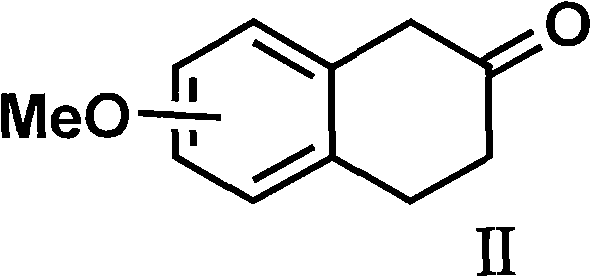
Method for preparing rotigotine and derivative thereof
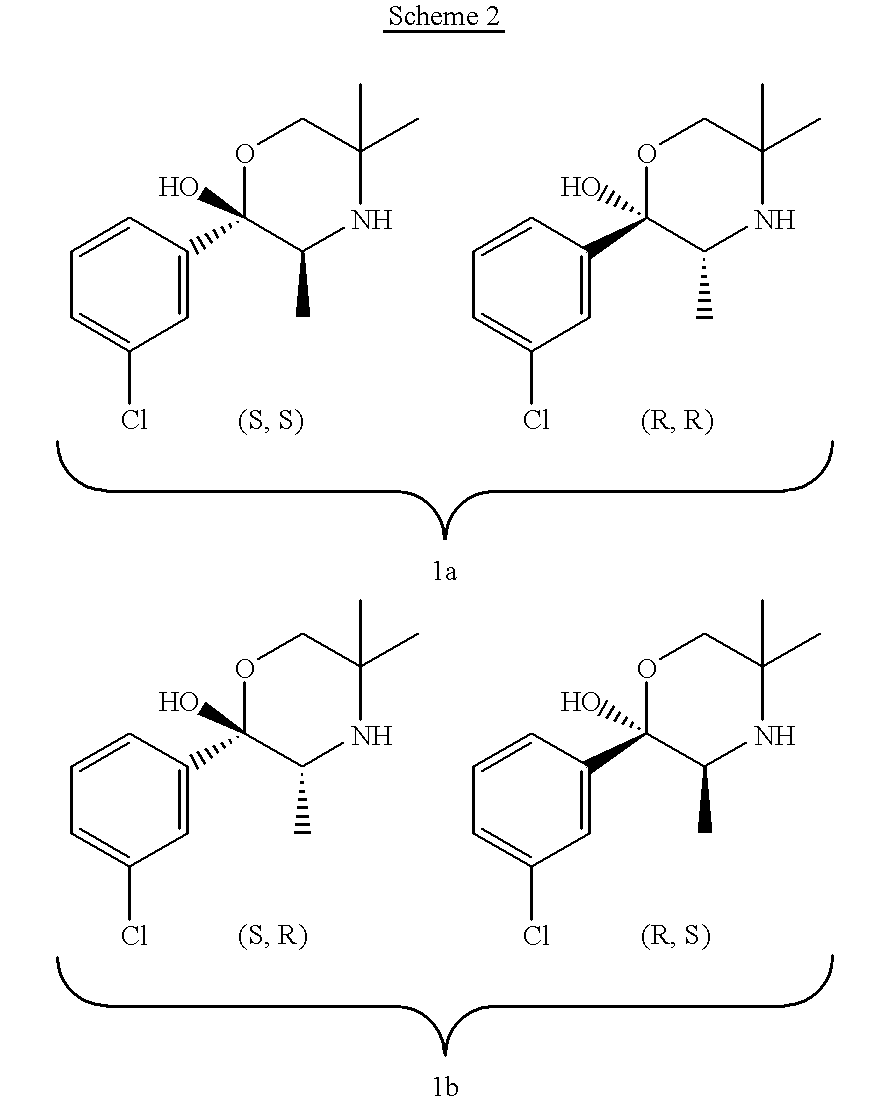
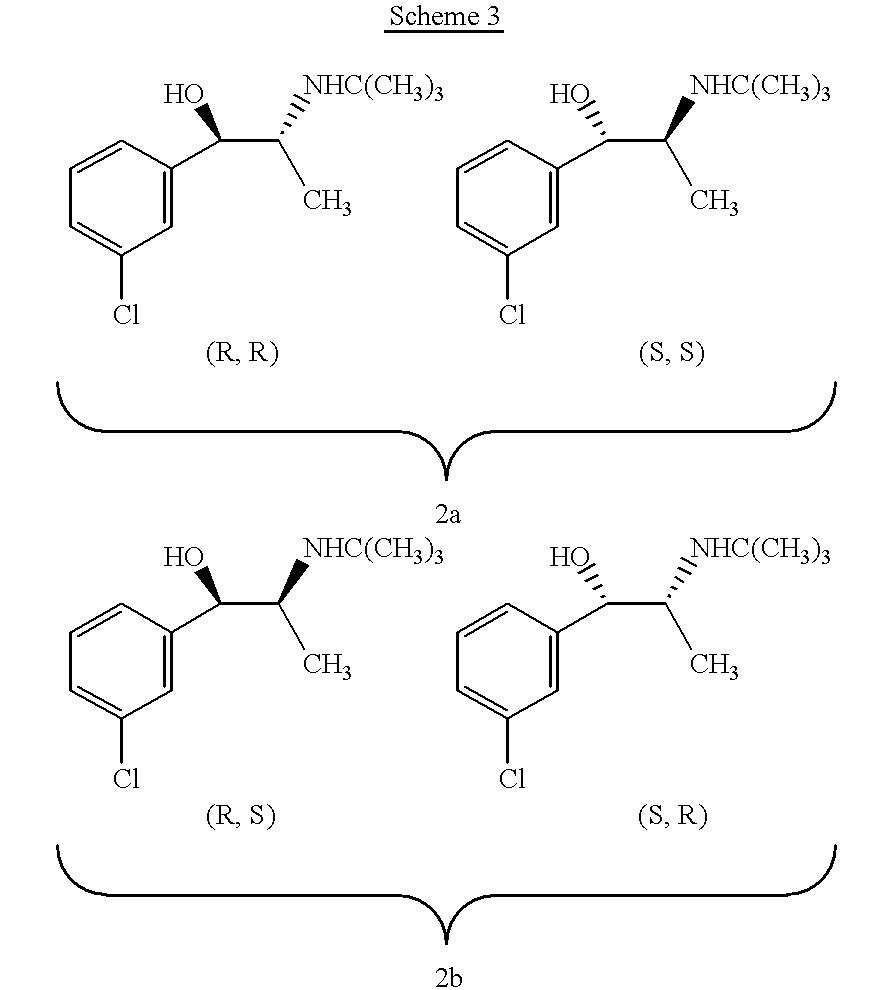
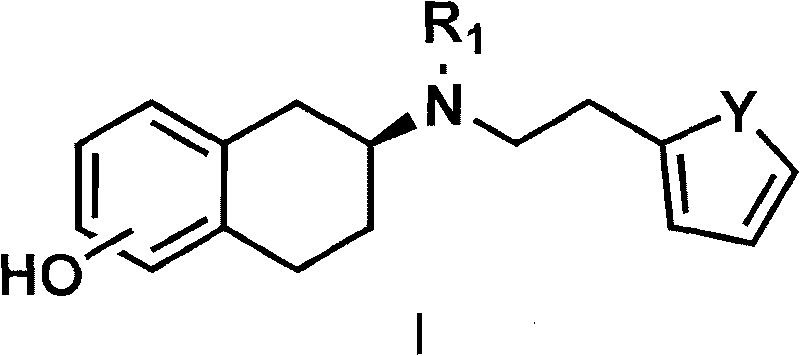
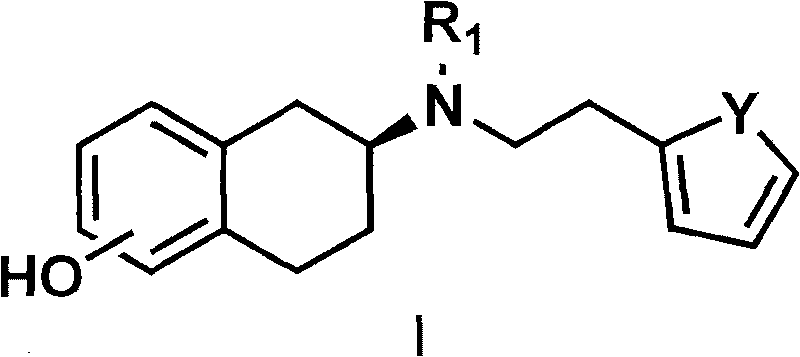
InactiveCN101717392AHigh optical puritySimple and fast operationOptically-active compound separationOrganic racemisationP-Toluenesulfonic acidSolvent
The invention discloses a method for preparing a compound of a formula (I) or pharmaceutically acceptable salts thereof. The method is characterized by comprising the following steps of: (1) using a compound of a formula (II) as a raw material and carrying out a reduction and amination reaction with an appropriate reducing agent to obtain a compound of a formula (III); (2) using 2-quinary heterocyclic substituted ethanol as a raw material and reacting to obtain a compound of a formula (IV) under the conditions of appropriate reagent, temperature and solvent; (3) after carrying out chiral separation on the compound of the formula (III), carrying out a condensation reaction with the compound of the formula (I) under an alkaline condition to obtain a compound of a formula (V); and (4) carrying out demethylation protection on the compound of the formula (V) under the condition of appropriate temperature and solvent to obtain the compound of the formula (I), wherein R1 in each formula is selected from C1-8 alkyl groups or aromatic bases which can be arbitrarily substituted, X is selected from halogen atoms or p-toluenesulfonic acid groups and methanesulfonic acid groups for protecting alcoholic extract hydroxyl groups, and Y is selected from O, S and N. A target product obtained by the method has high optical purity, convenient operation, lower cost, higher yield and less pollution and is suitable for industrialized production.
Owner:苏州凯达生物医药技术有限公司
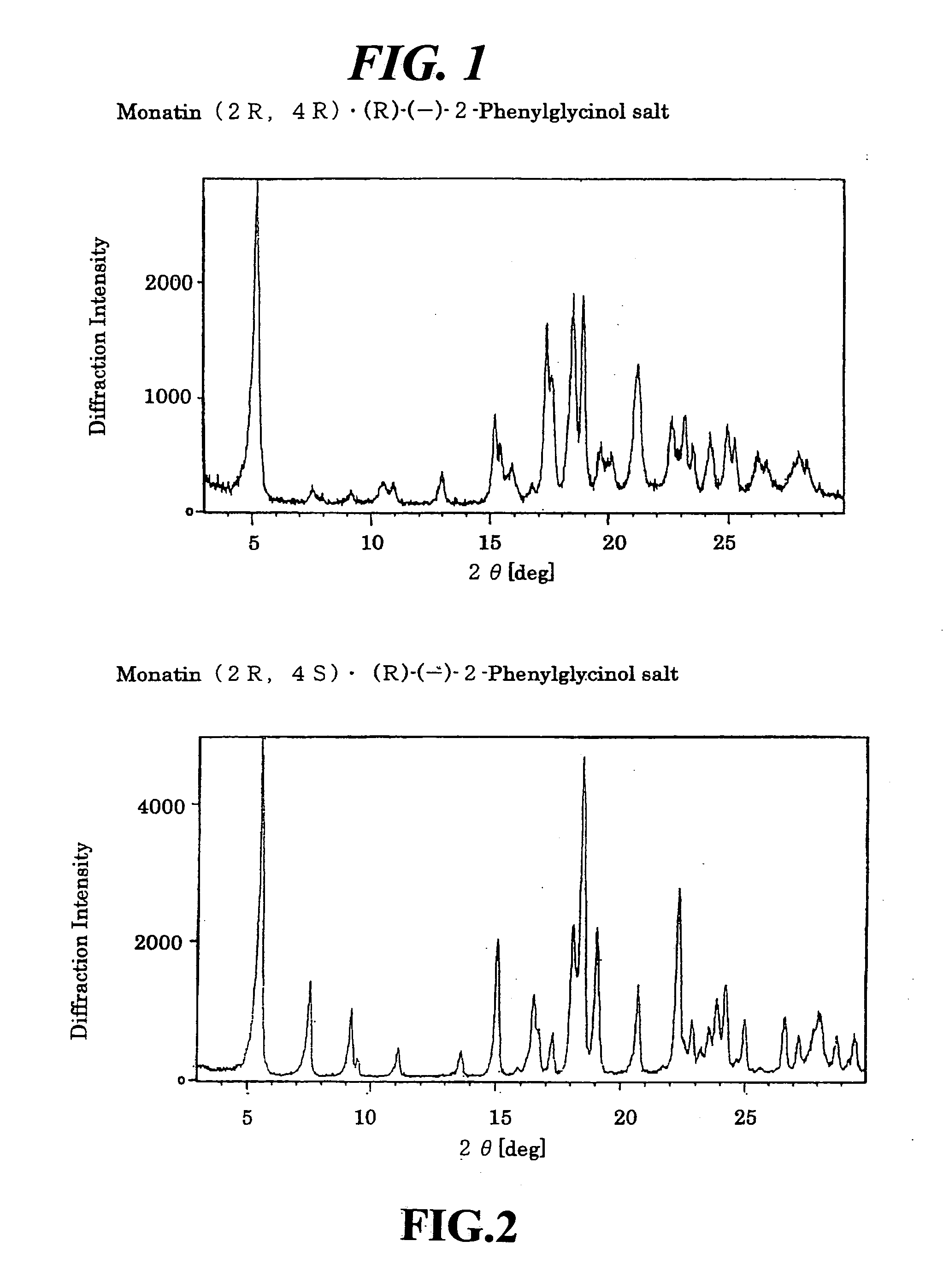
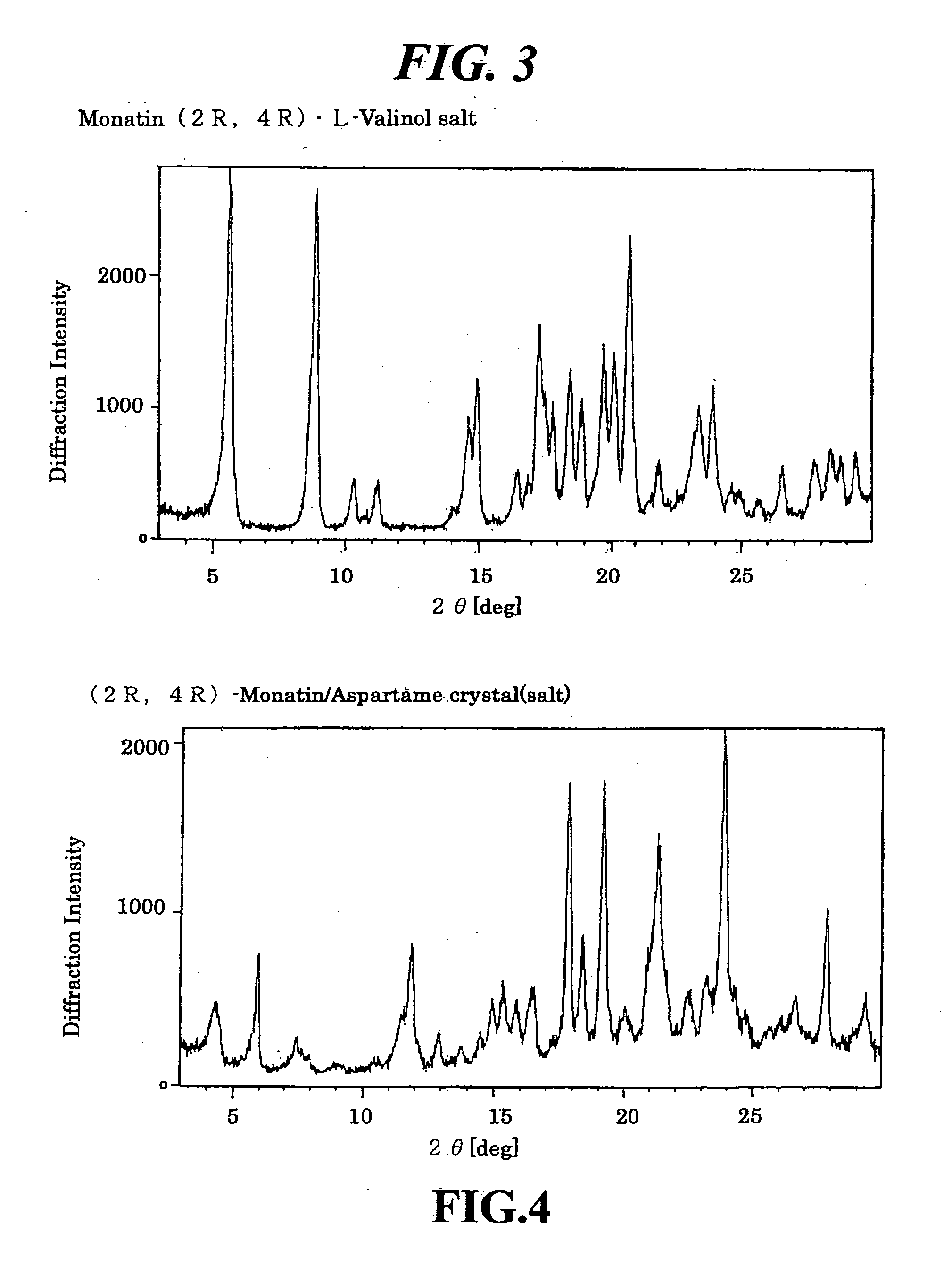
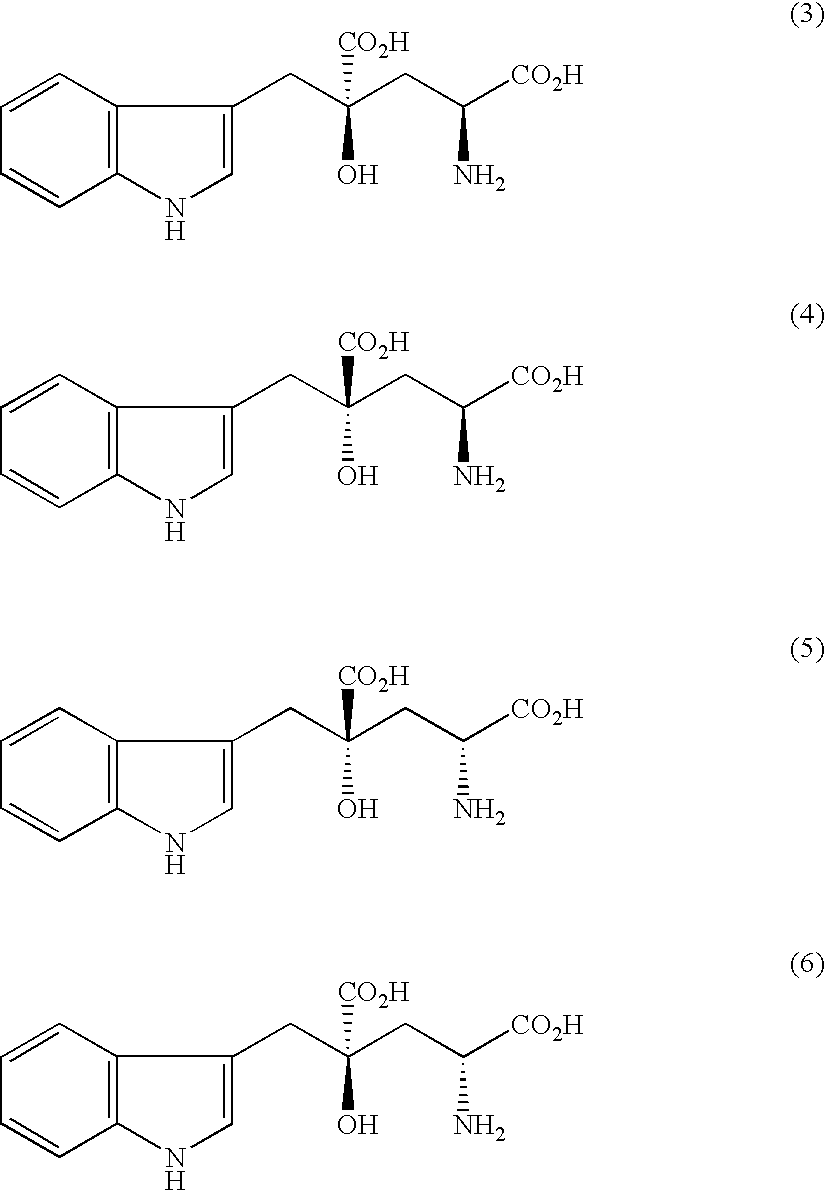
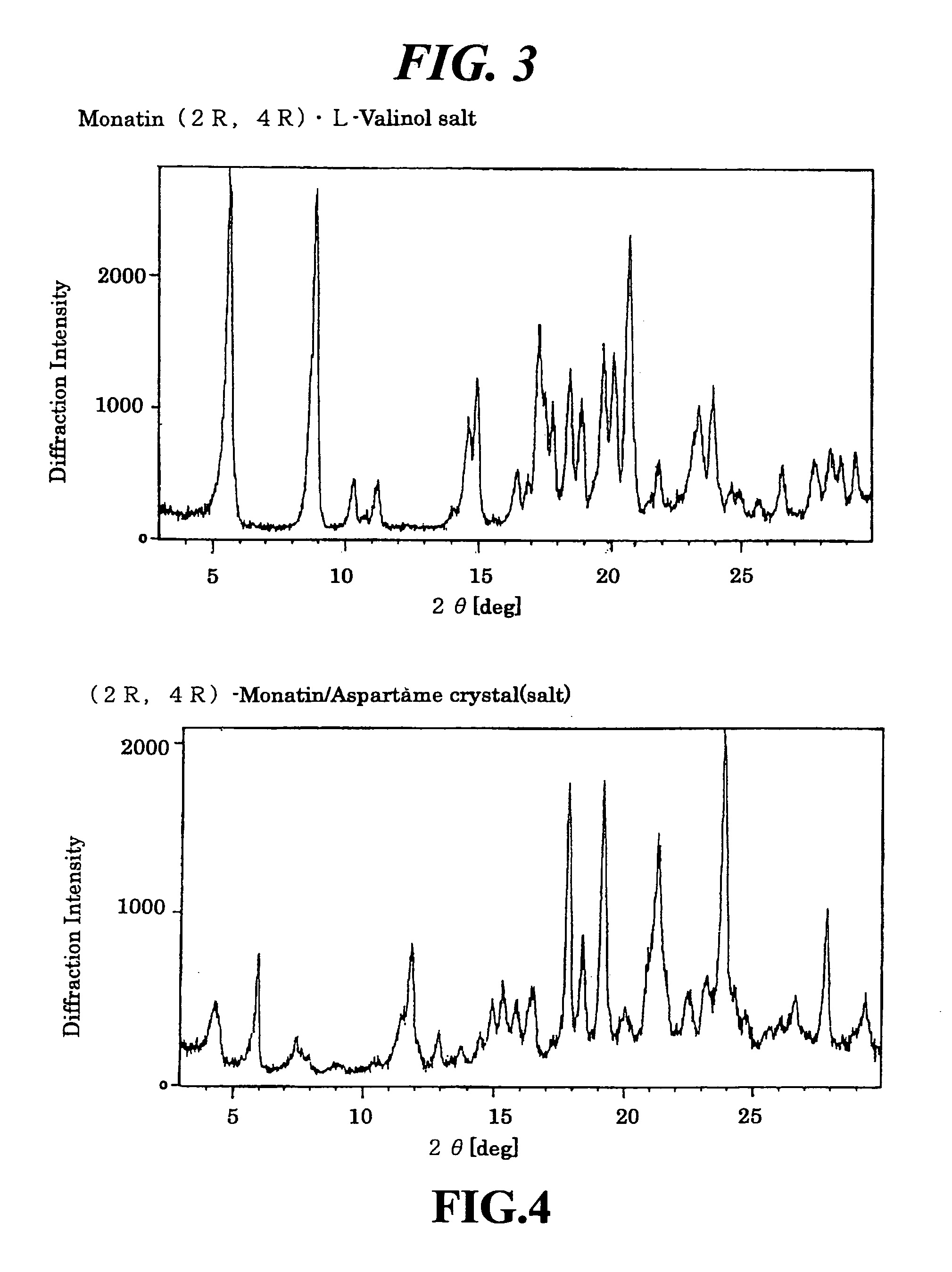
Organic amine salts of glutamic acid derivatives and their application
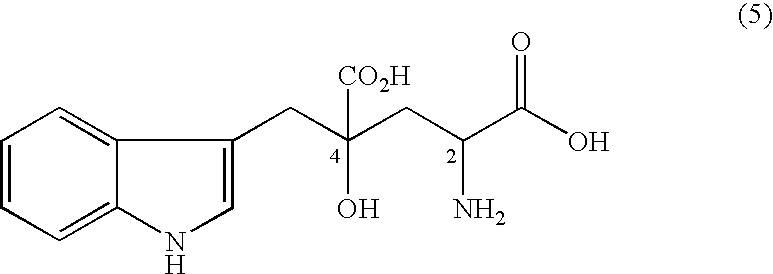
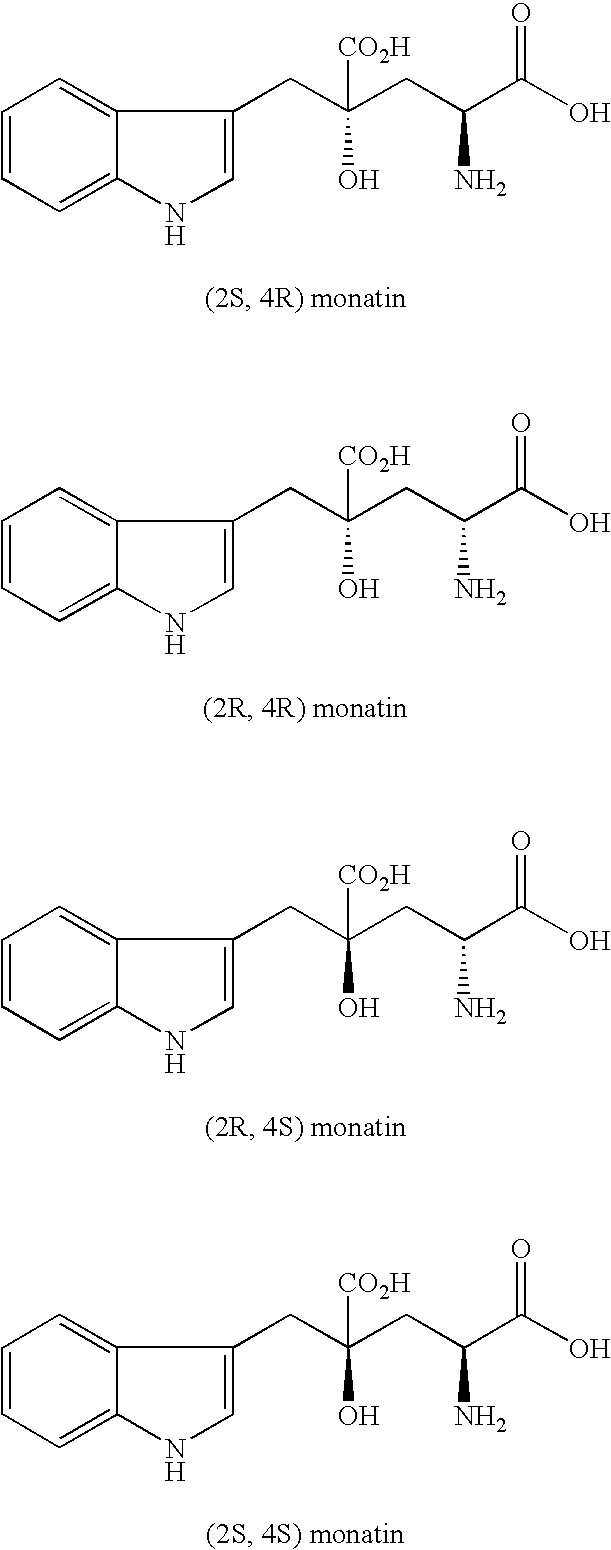
The present invention provides an organic amine salt of monatin, or crystal form thereof, and its application as well as a method for resolving the stereoisomers of monatin by forming its salt. The present invention further provides a salt form of monatin that is useful as a sweetening agent or as the active ingredient of a sweetener. The present invention also provides a method for preparing a salt of a particular stereoisomer of monatin with an organic amine by utilizing the difference of crystallinity or solubility of the salt of the stereoisomer of monatin with the organic amine. The present invention also relates to a use of the salt in a method for preparing a metal salt of monatin in which the organic amine is replaced by a metal such as sodium, potassium, or the like.
Owner:AJINOMOTO CO INC
Method for producing monatin
InactiveUS7396941B2Efficient productionOptically-active compound separationFood preparationIsomerizationOrganic solvent
By simultaneously carrying out an isomerization reaction at position 2 of monatin in different configurations at positions 2 and 4 in the presence of an aldehyde under a condition of pH 4 to 11 in a mixture solvent of water and an organic solvent, and the crystallization of monatin in the same configurations at positions 2 and 4 or a salt thereof, monatin useful as a sweetener, particularly optically active monatin can efficiently be produced.
Owner:AJINOMOTO CO INC
Axially chiral enantiomers of drug Lesinurad
InactiveCN105399694AEnhanced inhibitory effectAddress nephrotoxicityOrganic active ingredientsSkeletal disorderMedicineEnantiomer
The invention discloses an axially chiral R-enantiomer and an axially chiral S-enantiomer of the drug Lesinurad. The axially chiral R-enantiomer shows excellent URAT1 inhibition effect, overcomes the problems that a high dosage of Lesinurad racemate leads to renal toxicity and a low dosage Lesinurad racemate cannot produce additional drug effect, and can be applied to treatment or prevention of symptoms of abnormal tissue uric acid levels.
Owner:ZHEJIANG JINGXIN PHARMA +1
Main-group metal-based asymmetric catalysts and applications thereof
InactiveUS6844448B2Lactams preparationCarbamic acid derivatives preparationTetradentate ligandNucleophile
The present invention relates to a method and catalysts for the stereoselective addition of a nucleophile to a reactive π-bond of a substrate. The chiral, non-racemic catalysts of the present invention constitute the first examples of catalysts for nucleophilic additions that comprise a main-group metal and a tri- or tetra-dentate ligand.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Method for racemate splitting of 2-hydroxypropionic acids
InactiveUS6559338B1Easy to carryOrganic compound preparationOptically-active compound separationEthylaminePhotochemistry
A process for racemate resolution of 2-hydroxypropionic acids by reacting the racemic acid with an optically active base and subsequently separating off a diastereomeric salt of acid and base comprises using 1-(4-chlorophenyl)ethylamine as optically active base.
Owner:ROYALTY PHAMA COLLECTION TRUST
Resolution of alpha-(phenoxy)phenylacetic acid derivatives
ActiveUS20050033084A1Increase productionOrganic compound preparationCarboxylic compound preparationPhenylacetic acidEnantiomer
The present invention provides a method for producing an enantiomerically enriched α-(phenoxy)phenylacetic acid compound of the formula: from its enantiomeric mixture, where R1 is alkyl or haloalkyl and X is halide.
Owner:DIATEX INC (US)
Separating agent for optical isomers and process for producing the same
InactiveUS6217769B1Ion-exchanger regenerationCarboxylic acid amides optical isomer preparationSolventPolysaccharide
The invention provides separating agents for optical isomers which have a high optical resolving power inherent in polysaccharide derivatives and high solvent resistance, and which can be produced through short process steps; a process for producing the same, and a method for separating optical isomers. The invention also provides separating agents for optical isomers, wherein the surface of a polysaccharide derivative supported on a carrier or the surface of a pulverized or granulated polysaccharide derivative is coated with a polymer, which are produced by supporting the polysaccharide derivative on the carrier and then coating the surface thereof with the polymer to thereby immobilize the polysaccharide derivative on the substrate, or by grinding or spheroidizing the polysaccharide derivative and then coating the surface thereof with a polymer.
Owner:DAICEL CHEM IND LTD
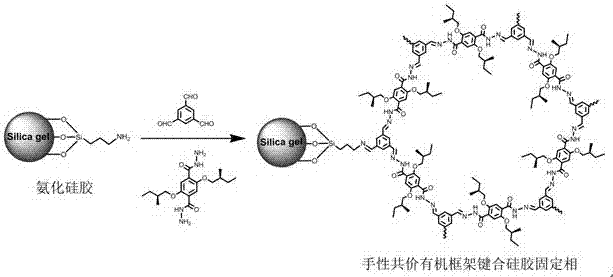
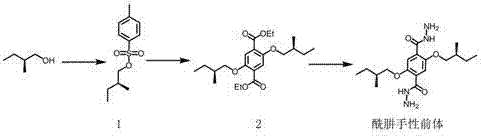
Hydrazone bond-connected chiral covalent organic framework bonded silica gel stationary phase and application thereof
ActiveCN107362785AClear structureEasy to prepareOther chemical processesSolid sorbent liquid separationSilica gelHplc mass spectrometry
The invention relates to a hydrazone bond-connected chiral covalent organic framework bonded silica gel stationary phase and an application thereof. The stationary phase is prepared by a method comprising the following steps: uniformly mixing a hydrazide chiral precursor, 1,3,5-benzenetricarboxaldehyde and ammoniated silica gel in an organic solvent in an inert atmosphere, carrying out a reaction under the catalysis of acetic acid, and filtering, washing and drying the obtained reaction product to obtain the chiral covalent organic framework bonded silica stationary phase. The structure of the hydrazide chiral precursor is represented by formula (I) shown in the description, a mass ratio of the ammoniated silica gel to the hydrazide chiral precursor is (2-12):1, and molar ratio of the hydrazide chiral precursor to the 1,3,5-benzenetricarboxaldehyde is 1:(0.5-2). The chiral covalent organic framework-bonded silica gel stationary phase is uniform in particle size; and the stationary phase has the advantages of high column efficiency, moderate column pressure and good separation effect on cis-trans isomers and position isomers when used in high performance liquid chromatography, and also has the advantages of definite structure, simple preparation method and good batch reproducibility.
Owner:SOUTH CHINA NORMAL UNIVERSITY
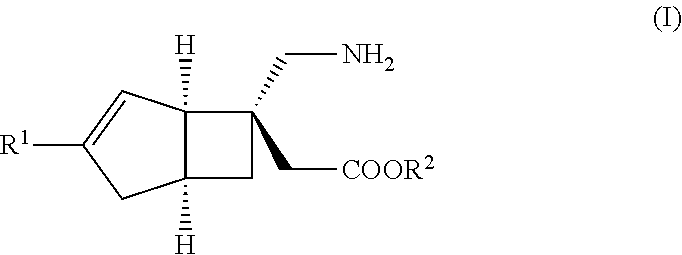
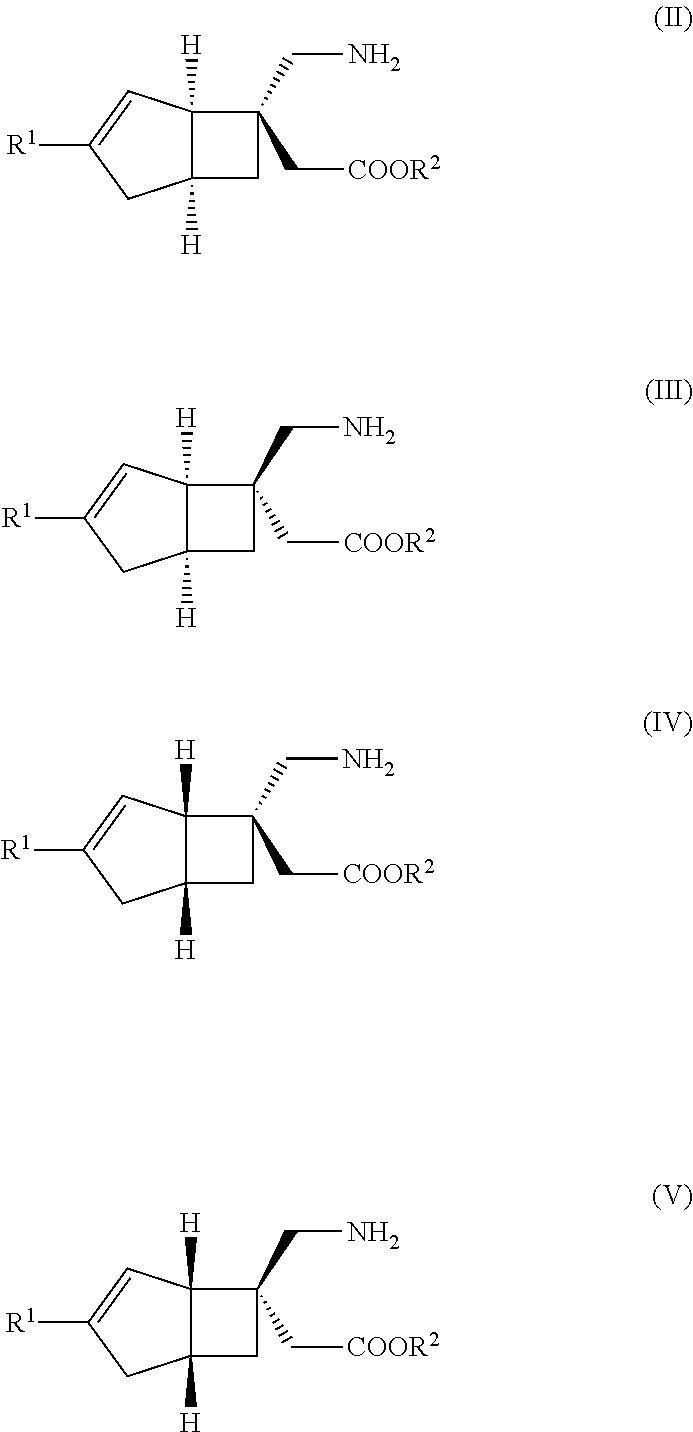
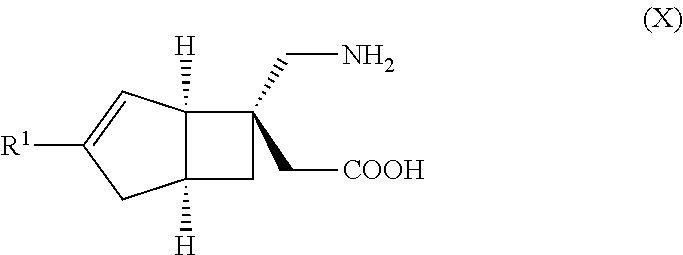
METHOD FOR PRODUCING BICYCLIC gamma-AMINO ACID DERIVATIVE
ActiveUS20120071685A1High activityOrganic active ingredientsNervous disorderMedicinal chemistryAmino acid derivative
Owner:DAIICHI SANKYO CO LTD
Organic amine salts of glutamic acid derivatives and their application
The present invention provides an organic amine salt of monatin, or crystal form thereof, and its application as well as a method for resolving the stereoisomers of monatin by forming its salt. The present invention further provides a salt form of monatin that is useful as a sweetening agent or as the active ingredient of a sweetener. The present invention also provides a method for preparing a salt of a particular stereoisomer of monatin with an organic amine by utilizing the difference of crystallinity or solubility of the salt of the stereoisomer of monatin with the organic amine. The present invention also relates to a use of the salt in a method for preparing a metal salt of monatin in which the organic amine is replaced by a metal such as sodium, potassium, or the like.
Owner:AJINOMOTO CO INC
Argatroban separation method
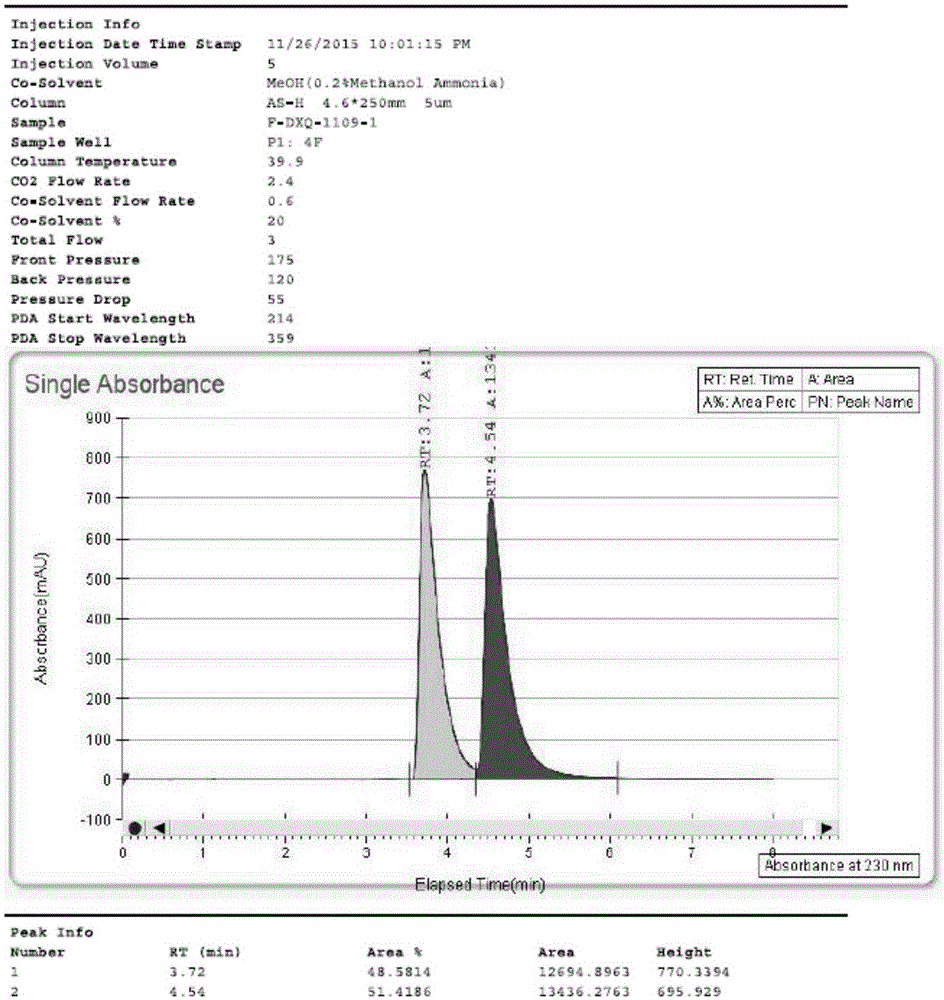
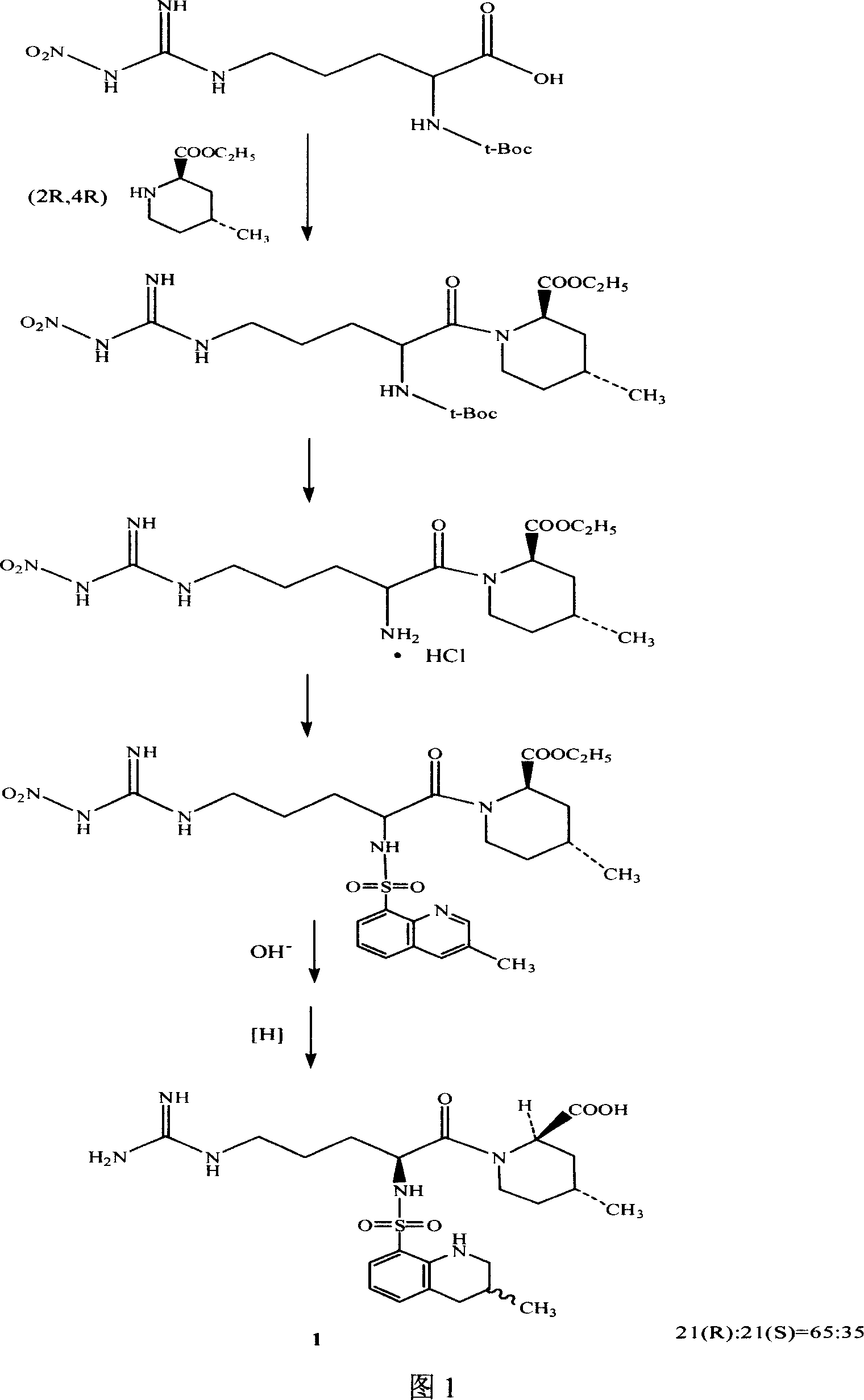
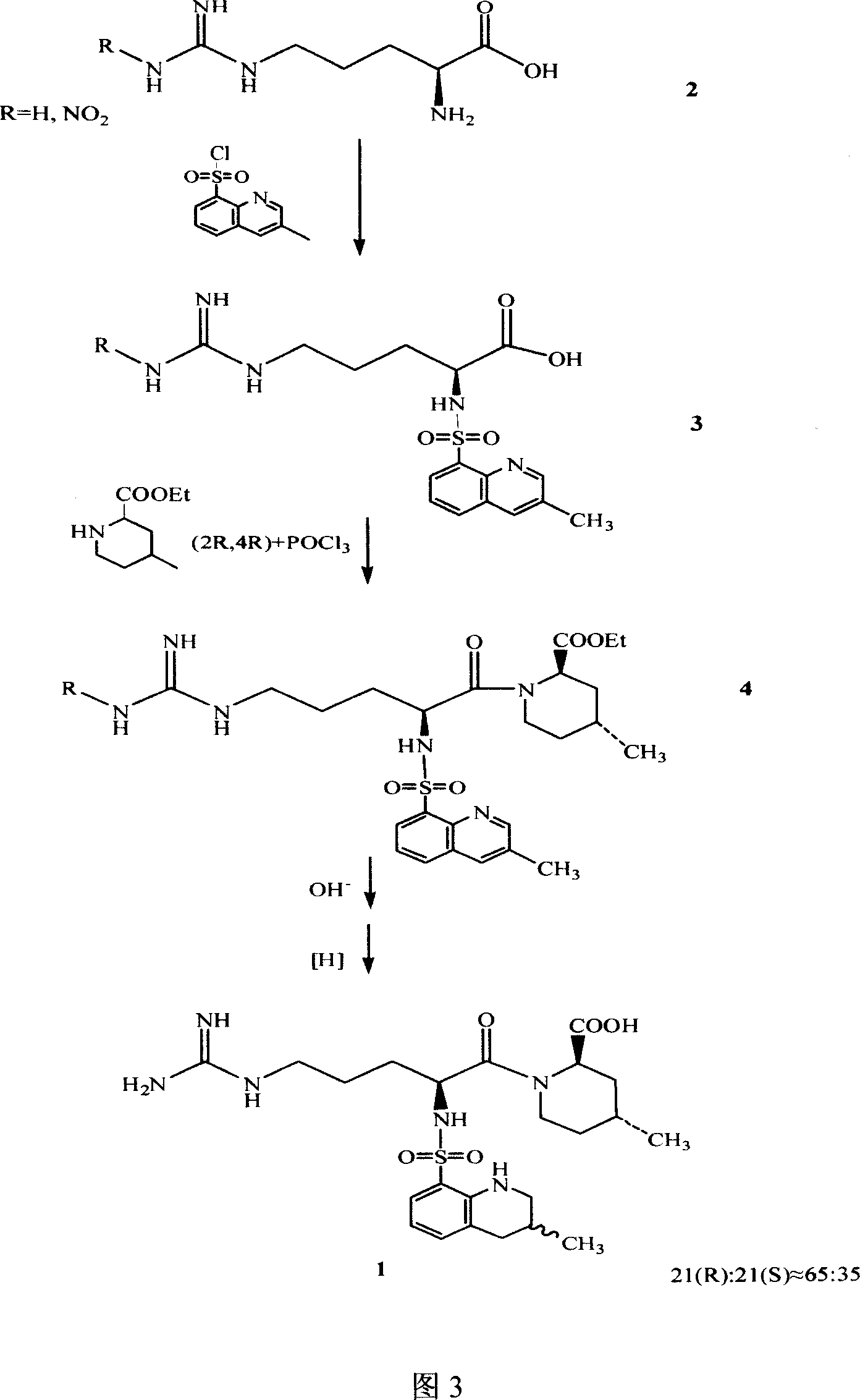
The invention discloses a separating method of optically-active pure isomer 21 (S)-Argatroban, which comprises the following steps: adopting 21(S) and 21(R) Argatroban as raw material; heating to reflux based on mixture of alcohol and water as solvent for 5-10h; cooling; stewing; filtering; obtaining white crystal product; drying; repeating 2-6 times; combining mother liquid; stripping solvent; detecting the content of 21 (S) through high-effect liquid-phase chromatography not less than 98%.
Owner:TIANJIN WEIJIE TECH
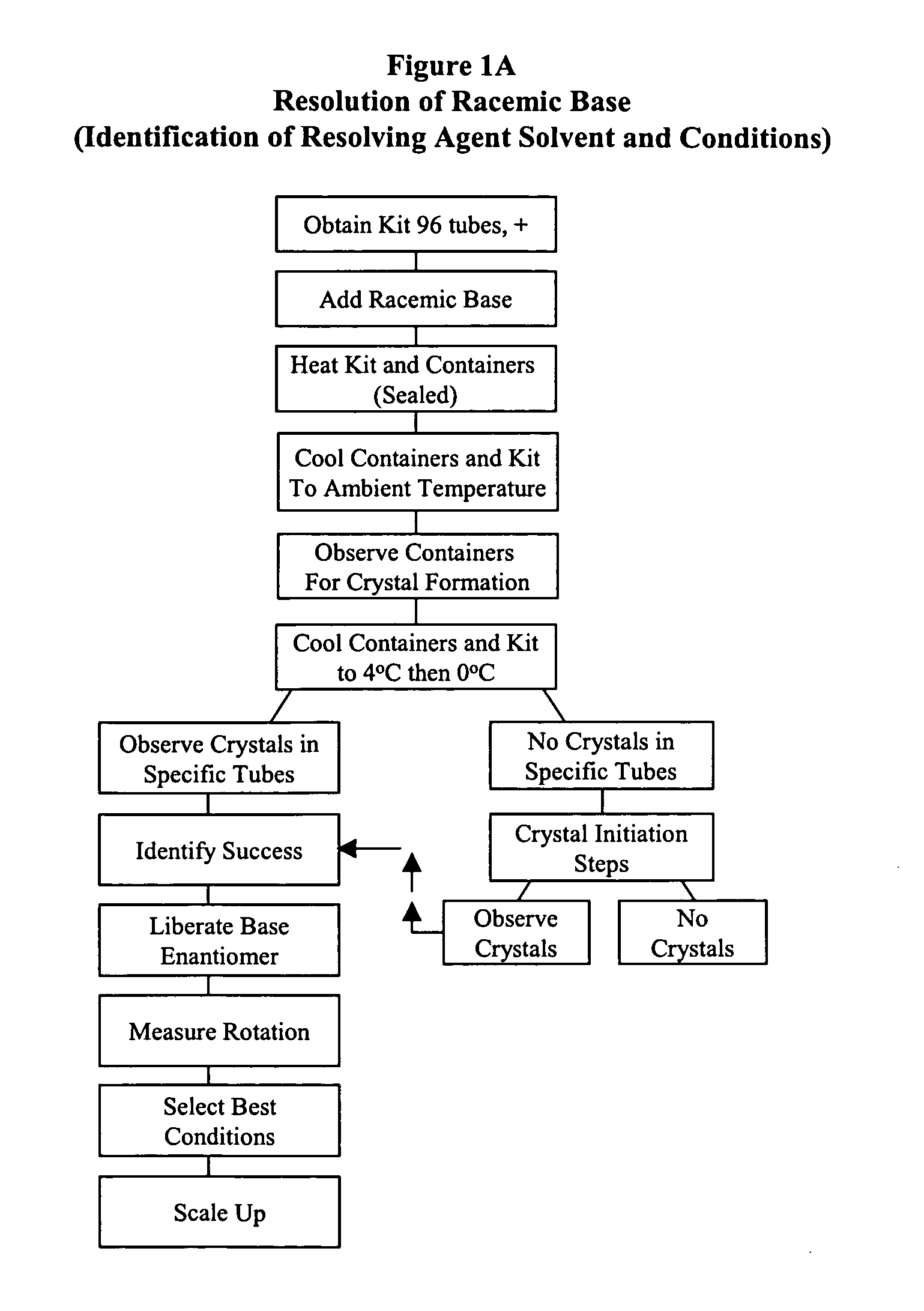
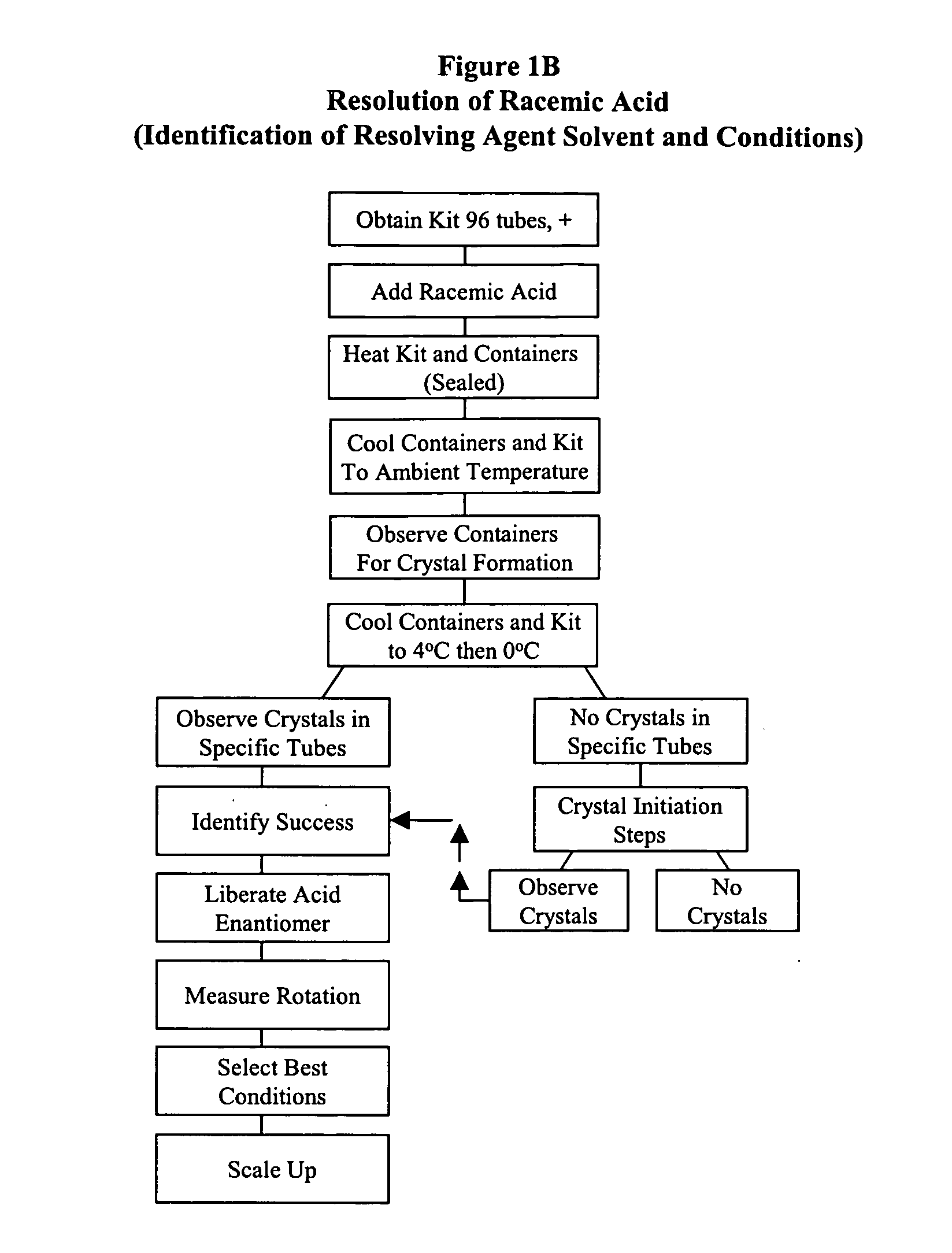
Kit for automated resolving agent selection and method thereof
InactiveUS20070185346A1Avoid lostOrganic compound preparationAmino-carboxyl compound preparationImproved methodBiomedical engineering
The present invention concerns an improved method and a tray or kit, which is useful to select quickly the optimum resolution agents, combinations and conditions to separate optical isomers. The tray of 24, 48, 96 or more samples is examined simultaneously visually or by standard analytical techniques.
Owner:VAIDYA NITEEN A
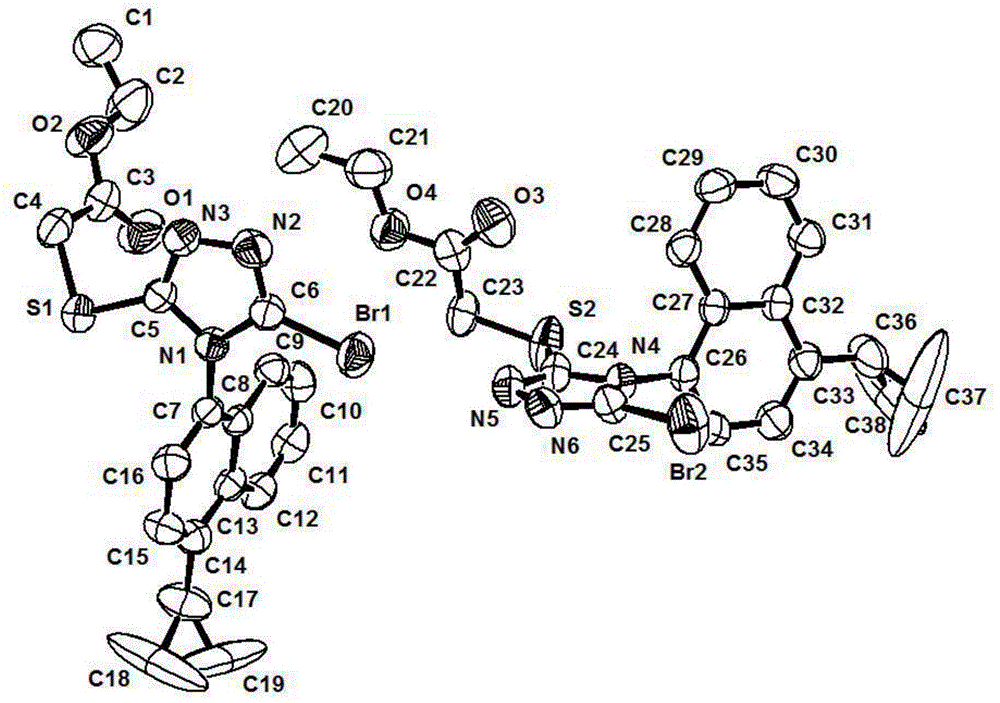
Axial chiral isomers and preparation method and pharmaceutical application thereof
InactiveCN105622531ALow costOrganic active ingredientsSkeletal disorderMedicinal chemistryCombinatorial chemistry
Owner:MEDSHINE DISCOVERY INC
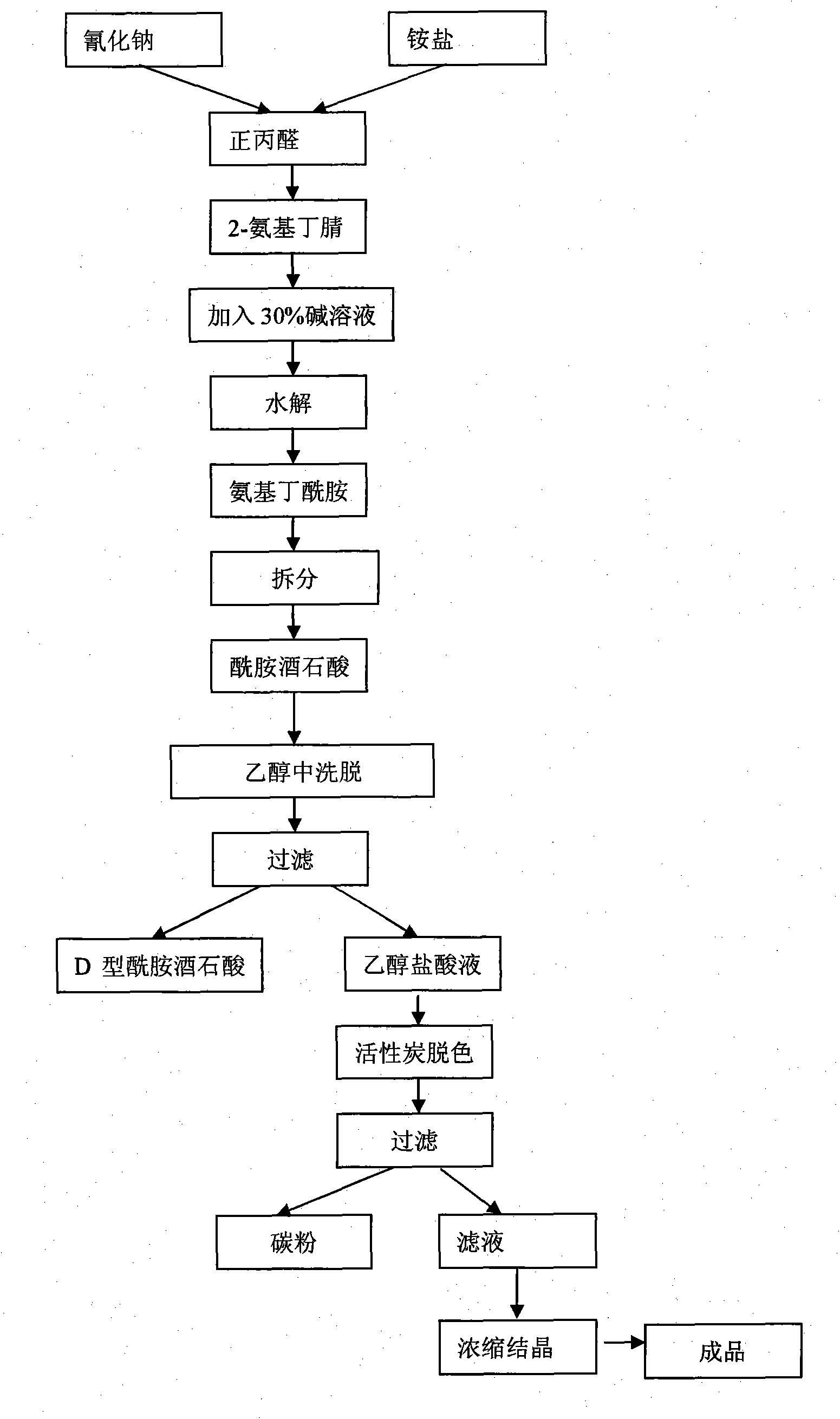
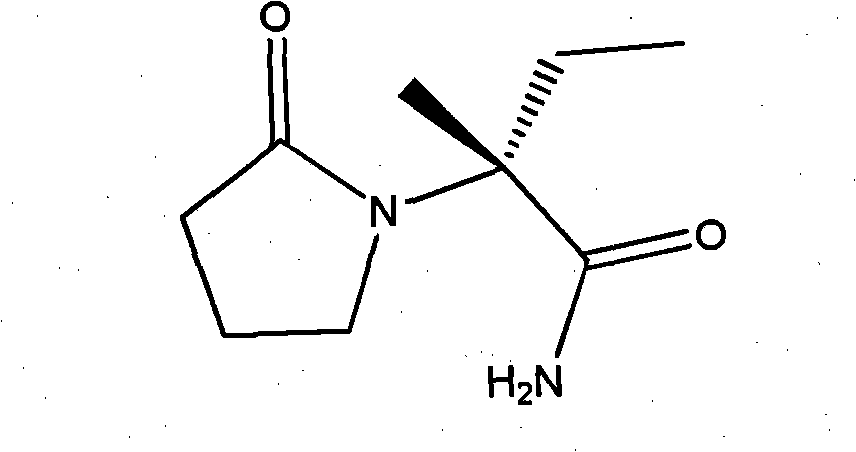
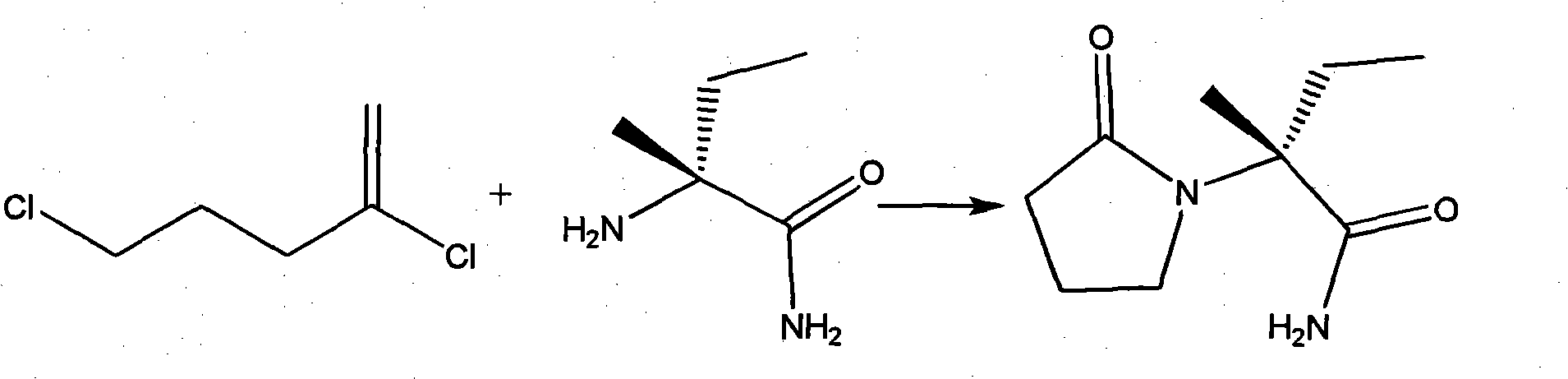
Process method for producing L-2-aminobutanamide hydrochloride serving as intermediate of levetiracetam
InactiveCN101928229ALow costShort synthesis cycleOrganic compound preparationCarboxylic acid amides preparationSodium cyanideHydrolysis
The invention discloses a process method for producing L-2-aminobutanamide hydrochloride serving as an intermediate of levetiracetam, which solves the problem that the conventional produced product has excessively high impurity content or production cost is too high for an enterprise to bear and the like. The method is characterized by comprising the following steps of: reacting propionaldehyde with ammonia water, ammonium chloride and sodium cyanide to obtain 2-amino butyronitrile; hydrolyzing the 2-amino butyronitrile under an alkali condition to obtain 2-aminobutanamide; and splitting the 2-aminobutanamide with L-tartaric acid to obtain the L-2-aminobutanamide hydrochloride. An L-2-amino amides product of which the content is over 99.5 percent is obtained by removing a byproduct, namely, sodium chloride by recrystallization so as to meet the use requirement of foreign customers. The process has the advantages of high yield, high safety and low cost and can be widely suitable for industrialized production of medium-sized and small enterprises.
Owner:HUANGGANG HUAYANG PHARMA
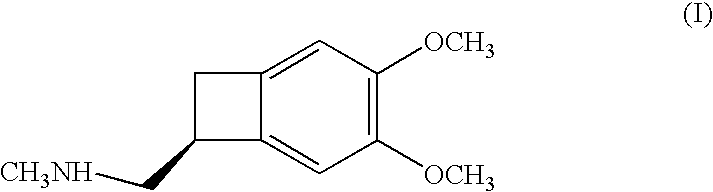
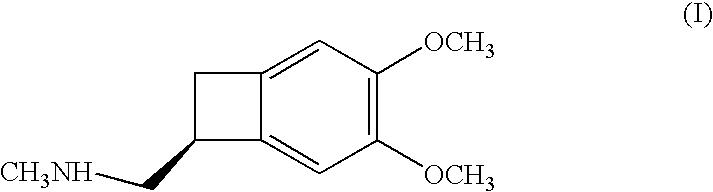
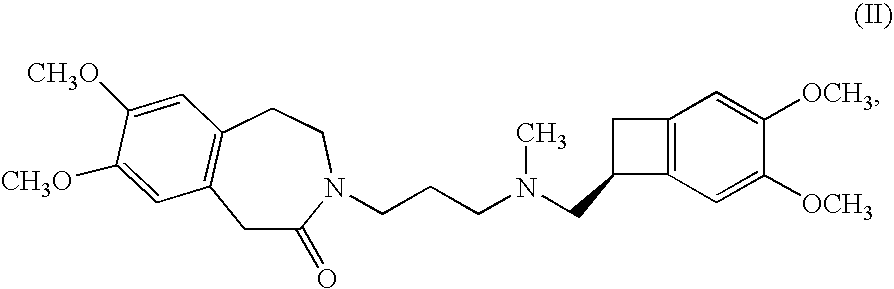
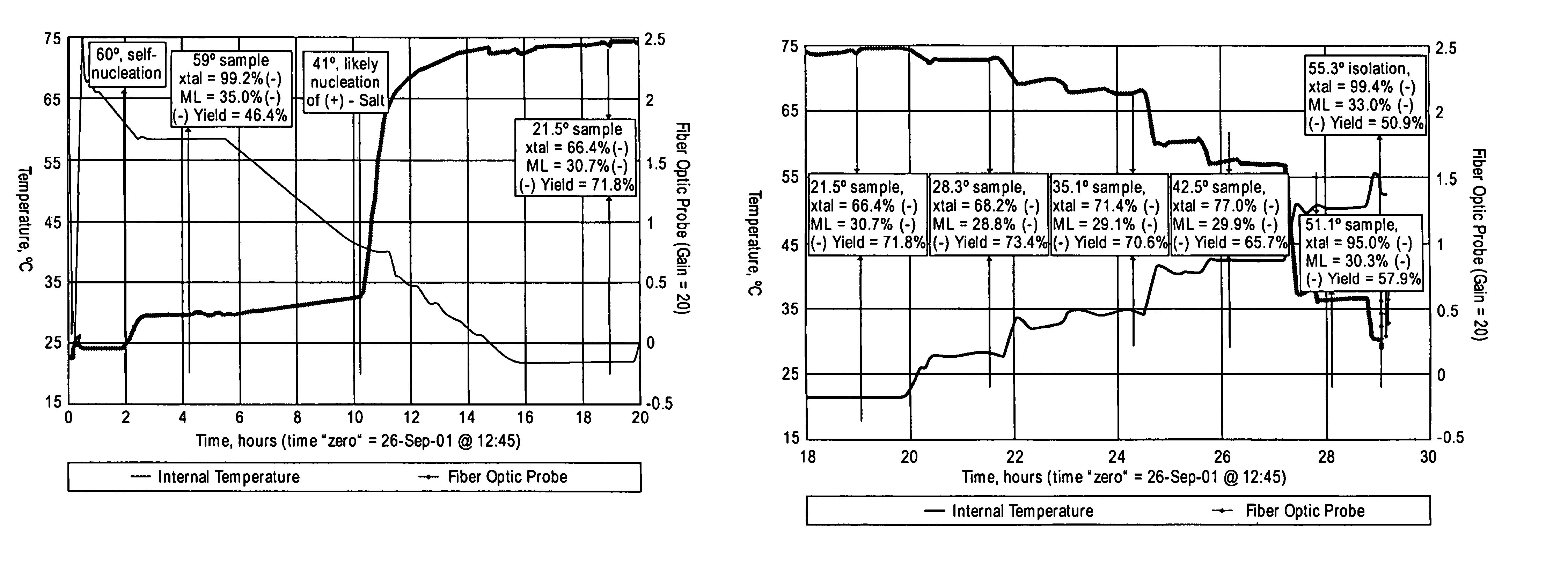
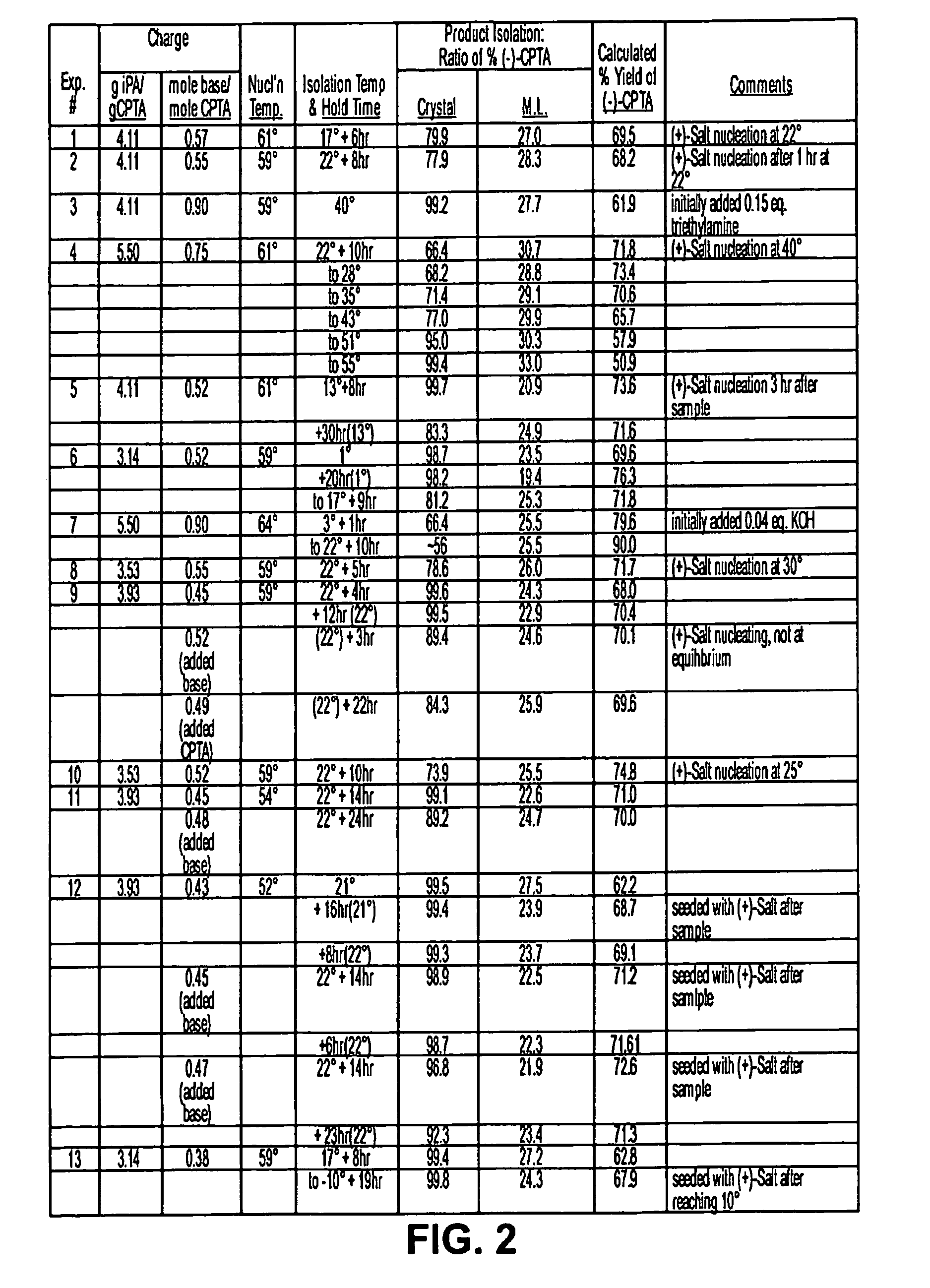
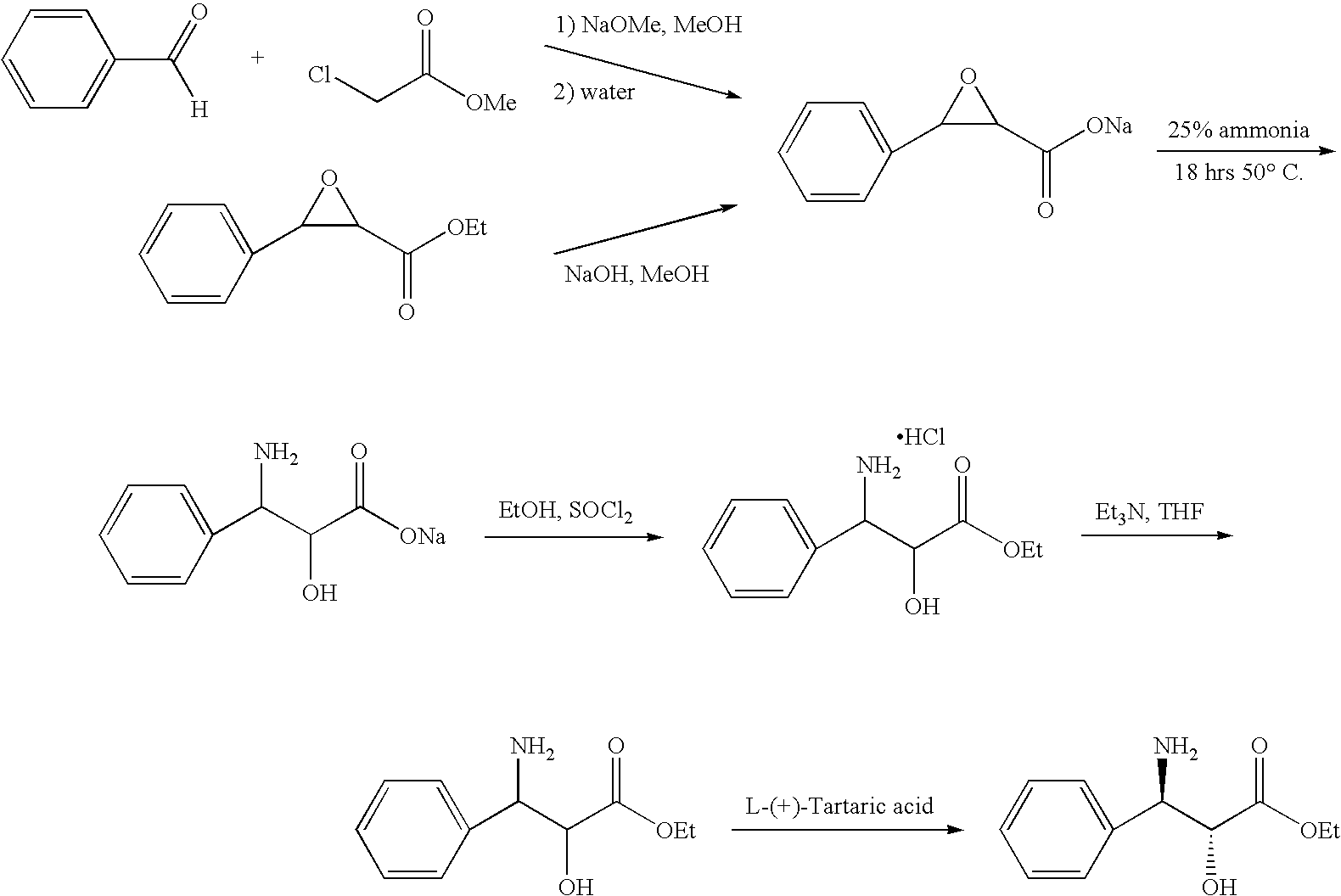
Process for the synthesis of (IS)-4,5-dimethoxy-1-(methylaminomethyl)-benzocyclobutane and addition salts thereof, and to the application thereof in the synthesis of ivabradine and addition salts thereof with a pharmaceutically acceptable acid
Process for the synthesis of the compound of formula (I): Application in the synthesis of ivabradine, addition salts thereof with a pharmaceutically acceptable acid, and hydrates thereof.
Owner:LES LAB SERVIER
Resolution of alpha-(phenoxy)phenylacetic acid derivatives
ActiveUS7199259B2Increase productionOrganic compound preparationCarboxylic compound preparationPhenylacetic acidAlkyl
The present invention provides a method for producing an enantiomerically enriched α-(phenoxy)phenylacetic acid compound of the formula (I):from its enantiomeric mixture, where R1 is alkyl or haloalkyl R7 is heteroalkyl and X is halide.
Owner:DIATEX INC (US)
Process for the production of 3-phenylisoserine
This invention is drawn to a process for the production of (R,R)-phenylisoserine or a 1–4C-alkyl ester thereof.
Owner:ALTANA PHARMA
Non-interpenetrating chiral MOF stationary phase, its preparation method and application in enantiomer separation in HPLC
InactiveCN103331151ARaw materials are cheap and easy to getEasy to operateAmino compound purification/separationOther chemical processesEnantiomerStructural formula
The invention relates to a non-interpenetrating chiral MOF (metal organic framework) stationary phase, its preparation method and application in enantiomer separation in HPLC (high-performance liquid chromatography). The stationary phase is a non-interpenetrating chiral three-dimensional porous framework complex with a structural formula as {[ZnL].H2O}n. An asymmetric structural unit {[ZnL].H2O} of the complex is composed of a Zn<2+>, an L ligand and a guest water molecule. The L ligand is -NH- containing chiral pyridine carboxylic acid, its chemical composition is [(N-(4-pyridylmethyl)-L-leucine.HBr)], and its molecular formula is C12H19BrN2O2. Chiral amino acid and 4-pyridylaldehyde are selected as raw materials to synthesize the-NH- containing pyridine carboxylic acid chiral ligand by a one-step process. The ligand and zinc acetate are adopted as raw materials to undergo room temperature diffusion so as to obtain the MOF stationary phase. The material provided in the invention has uniform chiral helical channel, uniform aperture and orifice, and can be used for separation of chiral drugs and other enantiomers. The separation is selectively dependent on the size of a separated enantiomer molecular size, but is not dependent on the functional group of the separated enantiomer. Thus, the non-interpenetrating chiral MOF stationary phase has the characteristics of traditional zeolite molecular sieve separation.
Owner:SHANDONG NORMAL UNIV
Processes and intermediates
The invention relates to processes and compounds useful for producing modified aspartic acid derivatives, such as aspartic acid aldehyde moieties. Aspartic acid derivatives are useful for preparing caspase inhibitors and / or prodrugs thereof.
Owner:VERTEX PHARMA INC
Method for the Production of Enriched Isopulegol
ActiveUS20080214877A1Easy to disassembleIncrease in the enantiomeric excessOxygen-containing compound preparationOrganic compound preparationMentholPolymer science
The present invention relates to a process for preparing enriched isopulegol by crystallization from a melt comprising isopulegol. The invention relates specifically to a process for preparing enantiomerically enriched n-isopulegol proceeding from optically active isopulegol having a relatively low enantiomeric excess by crystallization from the melt. The invention further relates to a process for preparing menthol proceeding from enantiomerically and / or diastereomerically enriched n-isopulegol prepared by crystallization from the melt.
Owner:BASF AG
Resolution of alpha-(phenoxy) phenylacetic acid derivatives with naphthyl-alkylamines
InactiveUS7432394B2Reduce the ratioOrganic compound preparationCarboxylic acid amides preparationPhenylacetic acidEnantiomer
The present invention provides a methods and compounds for producing an enantiomerically enriched α-(phenoxy)phenylacetic acid compound of the formula:from a mixture of its enantiomers, where R1 is alkyl or haloalkyl and X is halide.
Owner:DIATEX INC (US)
Enantioselective resolution process for arylpropionic acid drugs from the racemic mixture
InactiveUS6093830AOrganic compound preparationOptically-active compound separationCelsius DegreeOrganic solvent
The invention relates to a novel non-catalytic enantioselective resolution process for the separation of enantiomer of arylpropionic acid drugs from the racemic mixture, which comprises dissolving the racemic mixture of the said drug an organic solvent, reacting this solution with an aqueous phase containing an ionic surfactant with or without a suitable co-surfactant, and an electrolyte in microemulsion / micellar / biphasic medium, reacting this mixture with an appropriate chiral amine at a temperature in the range of 0 to 70 degrees Celsius to obtain a diastereomeric salt, acid hydrolysing the diastereomeric salt to result in the pure enantiomer of the drug which is extracted by known methods.
Owner:COUNCIL OF SCI & IND RES
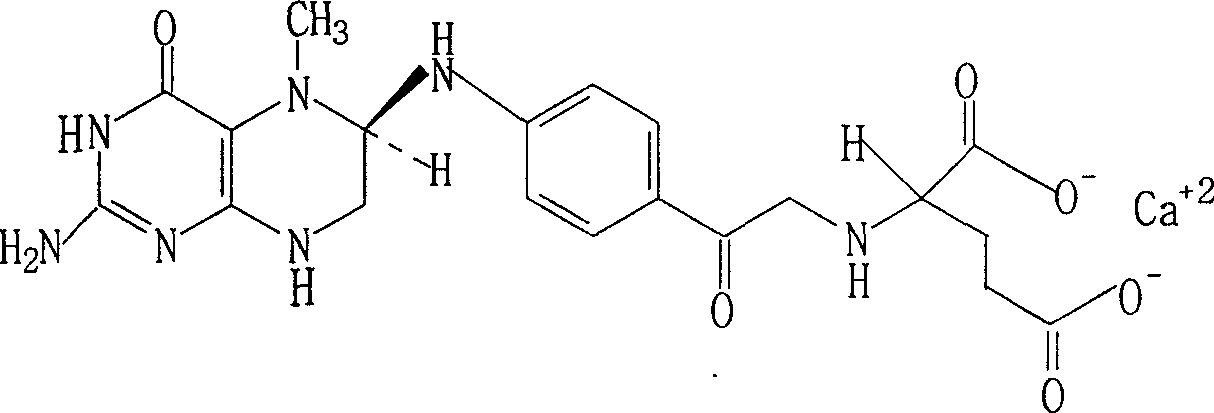
Resolution for 5-methyltetrahydrofolic acid and salifying method thereof
InactiveCN101143863AOptically-active compound separationOrganic racemisationCalcium hydroxideAlkaline earth metal
The invention brings forward a preparation method, which utilizes organic alkali Alpha-phenylethylamine to obtain (6S)-5-methyltetrahydrofolate by splitting racemate (6R, S)-5-methyltetrahydrofolate, and utilizes the hydroxide of alkaline earth, particularly calcium hydroxide, to generate (6S)-5-methyltetrahydrofolate calcium salt.
Owner:NAN JING RHINE PHARM TECH
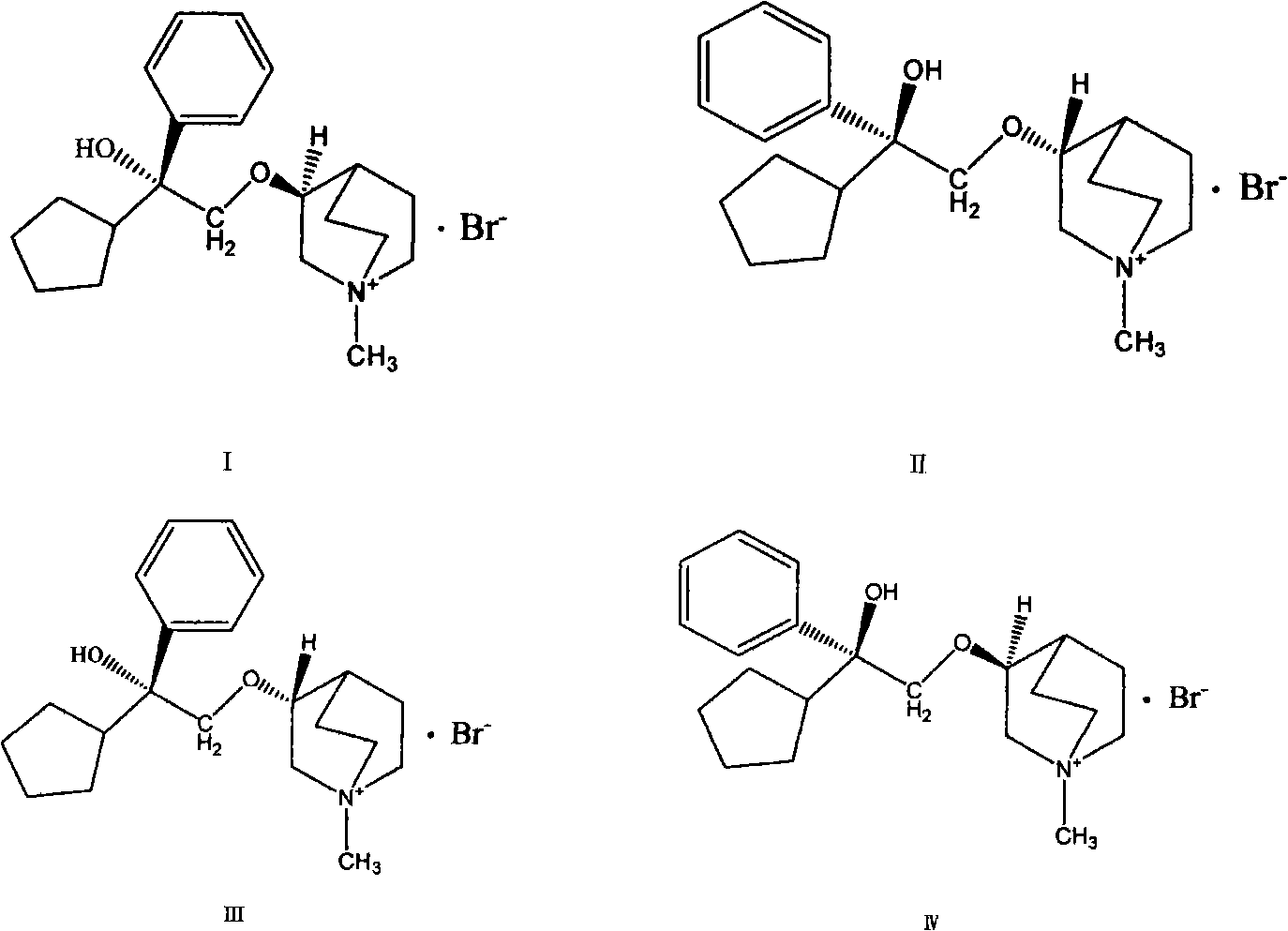
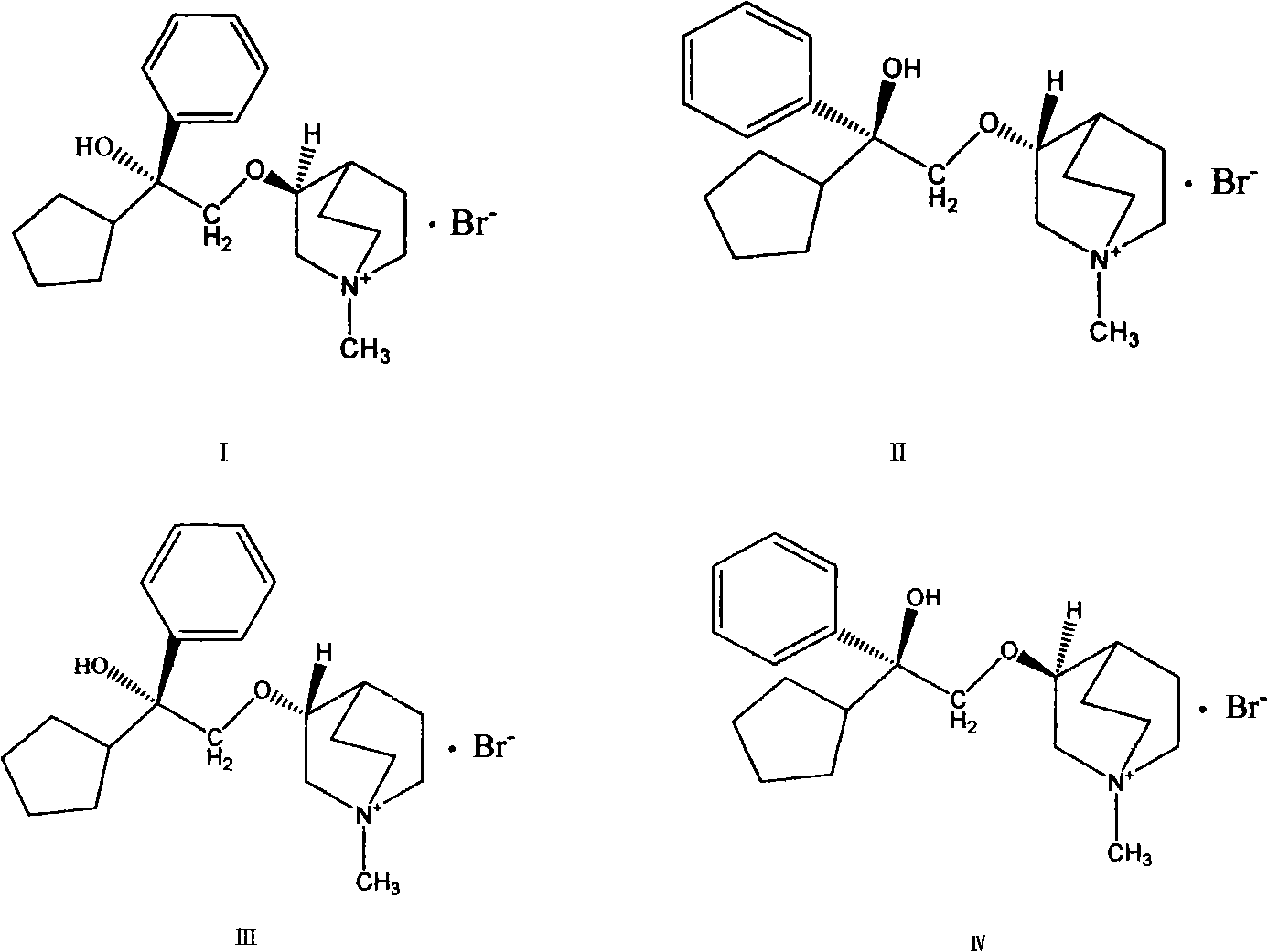
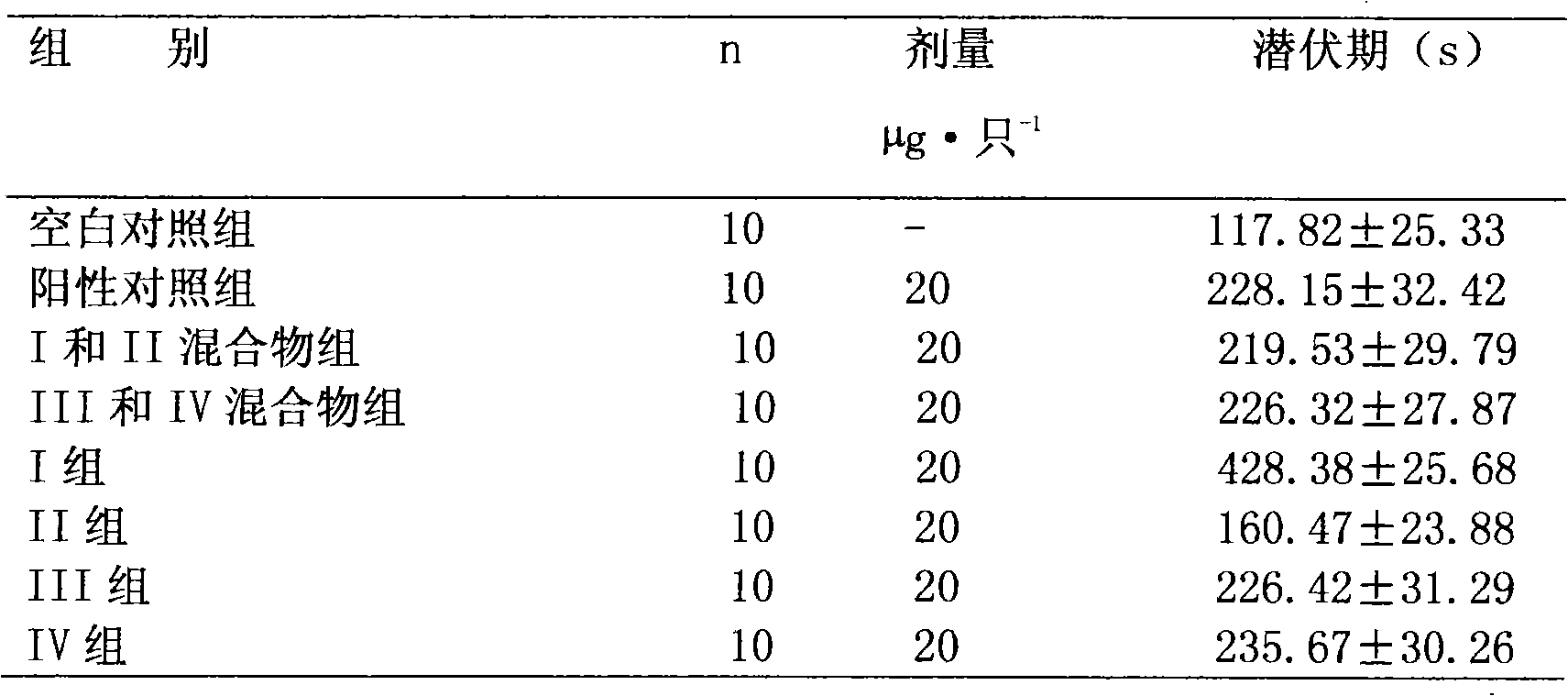
Preparation method and application of bencycloquidium bromide optical isomer and composition of bencycloquidium bromide optical isomer
The invention relates to the technical field of medicaments, in particular to a preparation method and application of a bencycloquidium bromide optical isomer. The isomer is obtained by a chemical resolution method, the activity of the isomer is far beyond that of other isomers and racemic forms; when the isomer is taken as a medicament to be taken, the taking dosage is reduced, and side effects caused by other isomers are simultaneously eliminated; moreover, the bencycloquidium bromide optical isomer has a romurtide cyclodextrin inclusion compound with higher pharmaceutical value and a preparation thereof. The romurtide is coated by the cyclodextrin, so that the problems of low water-solubility, unstable preparation prepared by the romurtide cyclodextrin inclusion compound and the like are solved. The romurtide cyclodextrin inclusion compound has the characteristics of high stability, good water-solubility, and low toxic and side effects, and is a quite good immunomodulator.
Owner:YINGU PHARMA
Rho-kinase inhibitors
InactiveUS6924290B2Conveniently preparedOrganic active ingredientsBiocideSexual impotenceCoronary heart disease
Disclosed are compounds and derivatives thereof, their synthesis, and their use as Rho-kinase inhibitors. These compounds are useful for inhibiting tumor growth, treating erectile dysfunction, and treating other indications mediated by Rho-kinase, e.g., coronary heart disease.
Owner:BAYER HEALTHCARE LLC
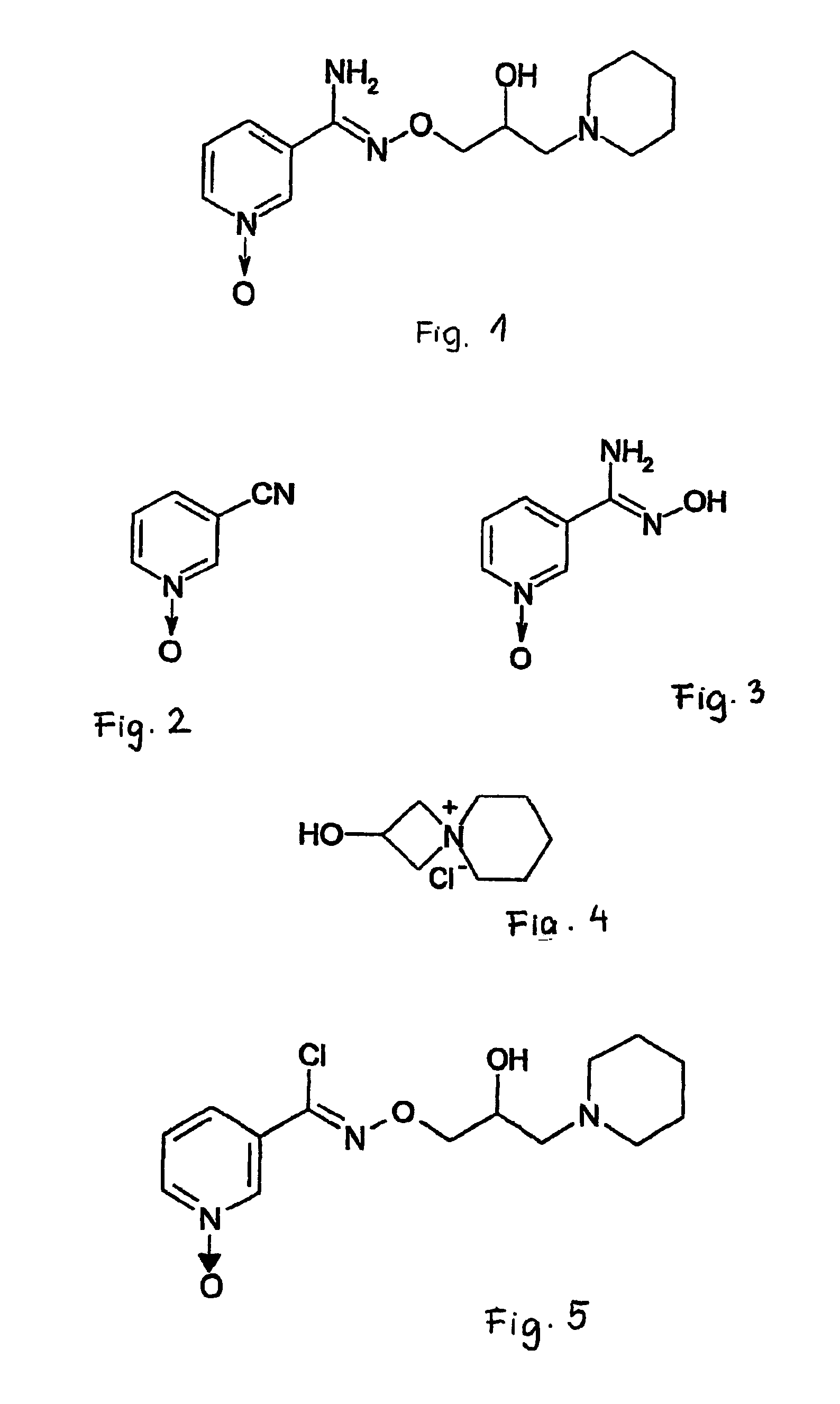
Pyridine-1-oxide derivative, and process for its transformation into pharmaceutically effective compounds
The invention relates to N-[2-hydroxy-3-(1-piperidinyl)-propoxy]-pyridine-1-oxide-3-carboxamidine and its optically active enantiomers. (R)-(−)-N-[2-hydroxy-3-(1-piperidinyl)-propoxy]-pyridine-1-oxide-3-carboxamidine and (S)-(+)-N-[2-hydroxy-3-(1-piperidinyl)-propoxy]-pyridine-1-oxide-3-carboxamidine. Furthermore, the invention relates to the preparation of N-[2-hydroxy-3-(1-piperidinyl)-propoxy]-pyridine-1-oxide-3-carboxyimidoyl chloride, which may be used as an active ingredient of medicaments, and the preparation of the optically active enantiomers of this compound using the compounds of the invention as intermediate substances.
Owner:KEMPHARM DENMARK AS
Beta-cyclodextrin functionalized chiral stationary phase, preparation and application thereof
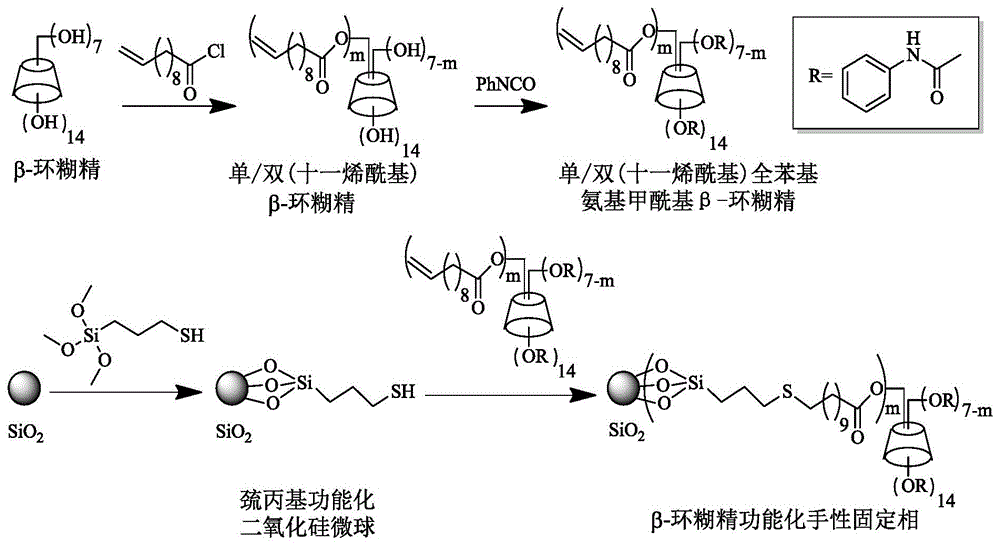
ActiveCN105312039AFew stepsSynthesis fastOther chemical processesOptically-active compound separationFunctional monomerMicrosphere
The invention relates to a beta-cyclodextrin functionalized silica gel microsphere chiral stationary phase, preparation, and application thereof in enantiomer separation. The preparation method comprises the following steps: introducing 3-mercapto into the surface of silica gel microspheres at first, and then adding beta-cyclodextrin derivative functional monomers and an initiator, wherein the functional monomers carry out mercapto-alkene addition reactions under the regulation of the initiator so as to obtain the surface modified beta-cyclodextrin functional monomer chiral stationary phase. The preparation of chiral stationary phase is simple, the reaction conditions are mild, and the chiral stationary phase is successfully applied to the enantiomer separation.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com