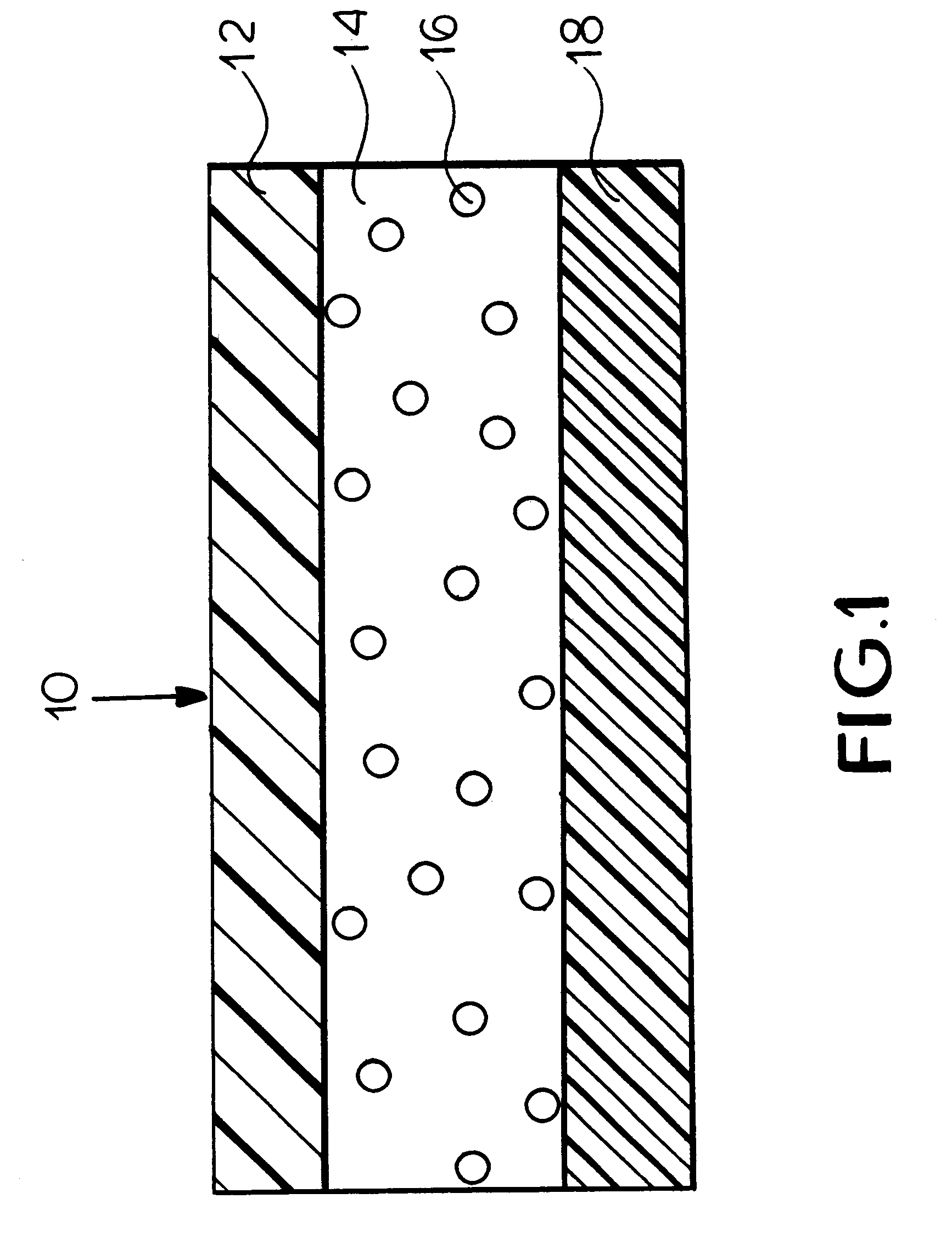
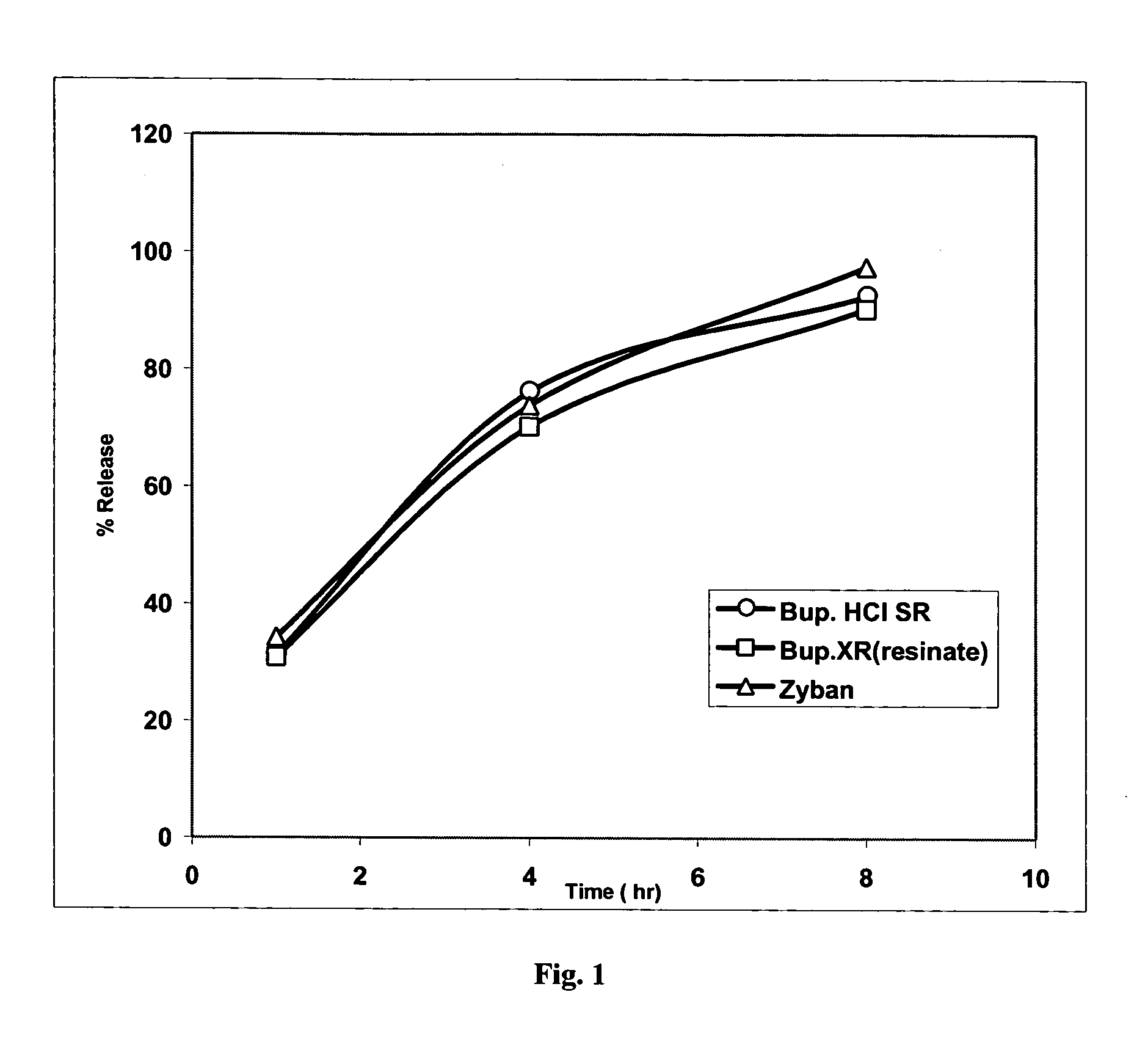
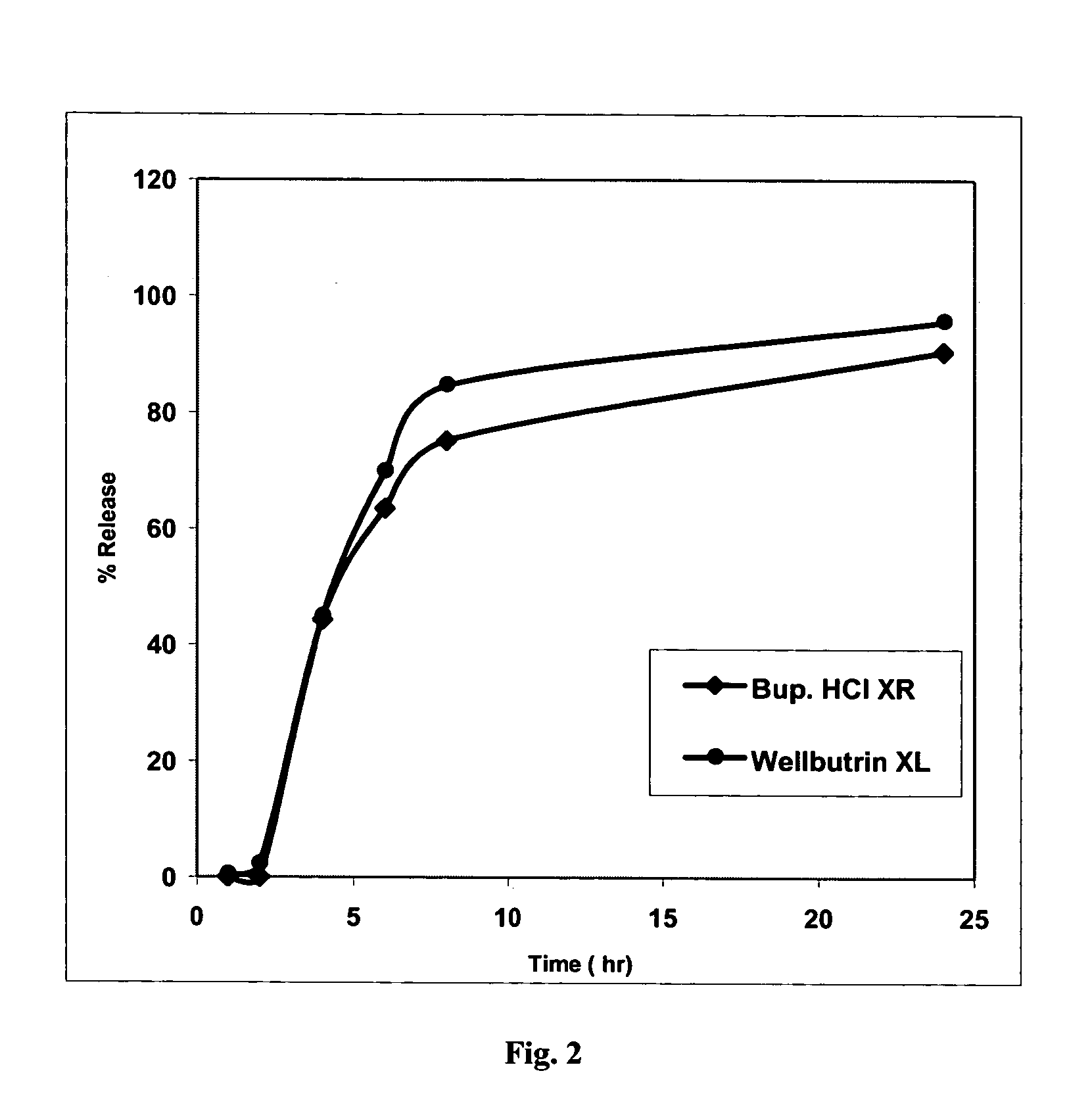
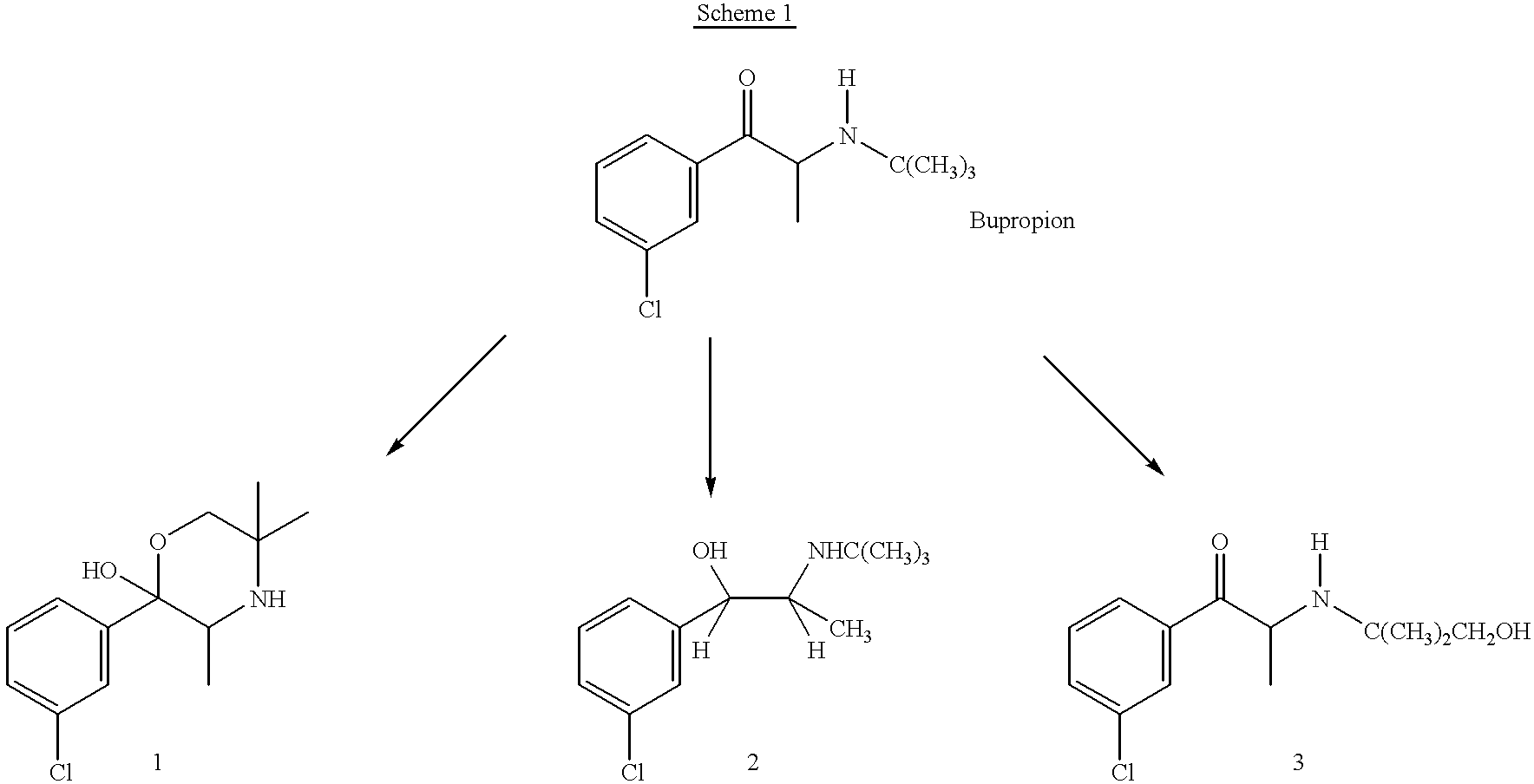
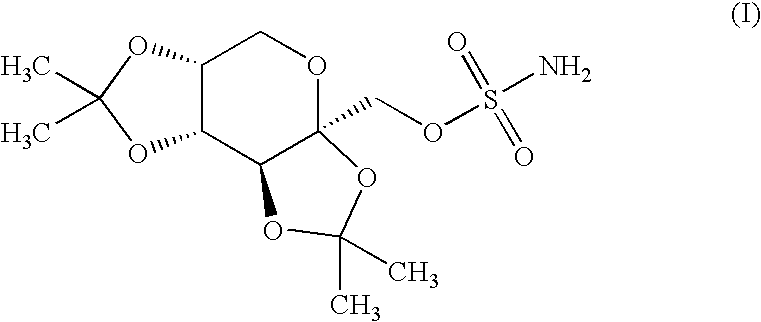
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
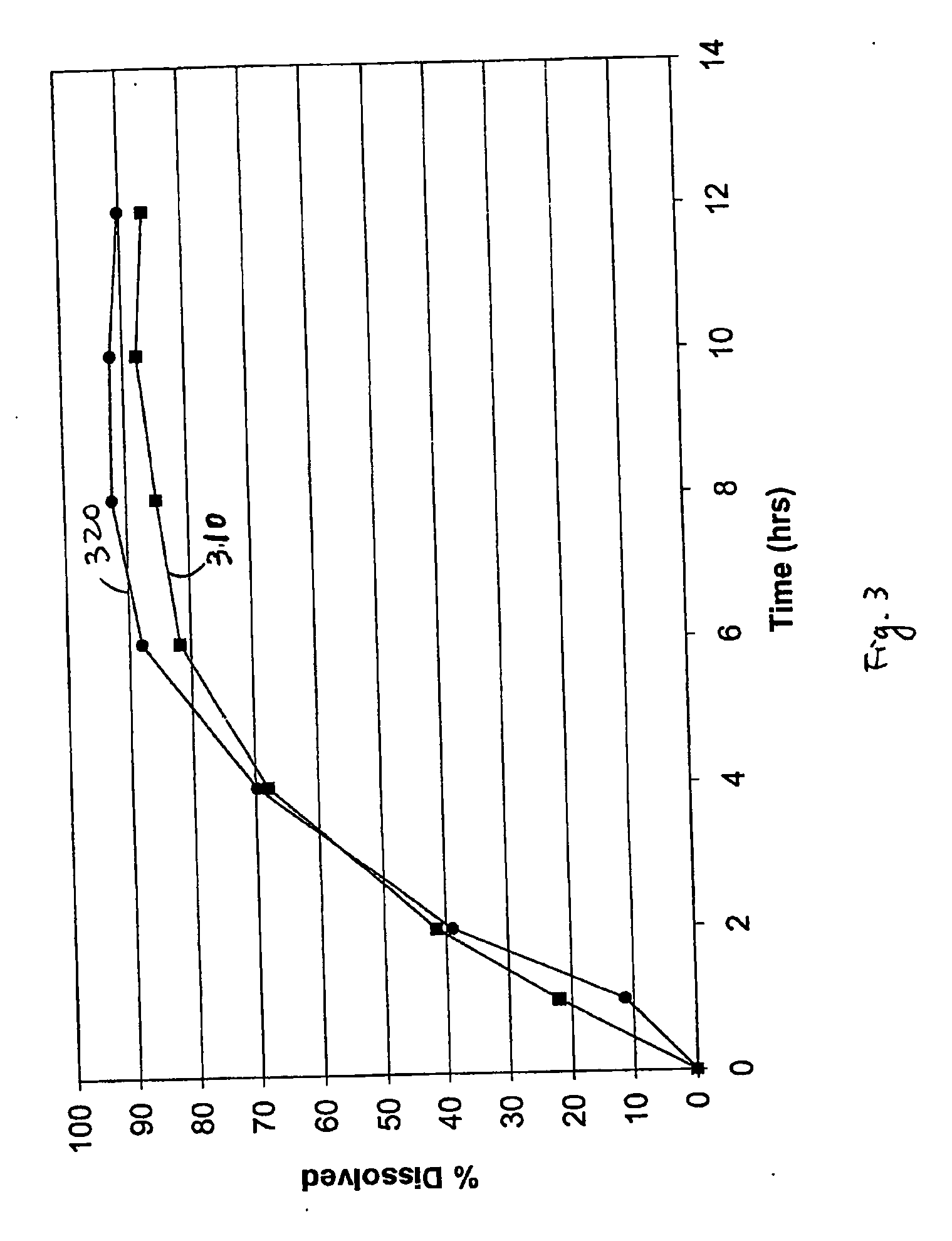
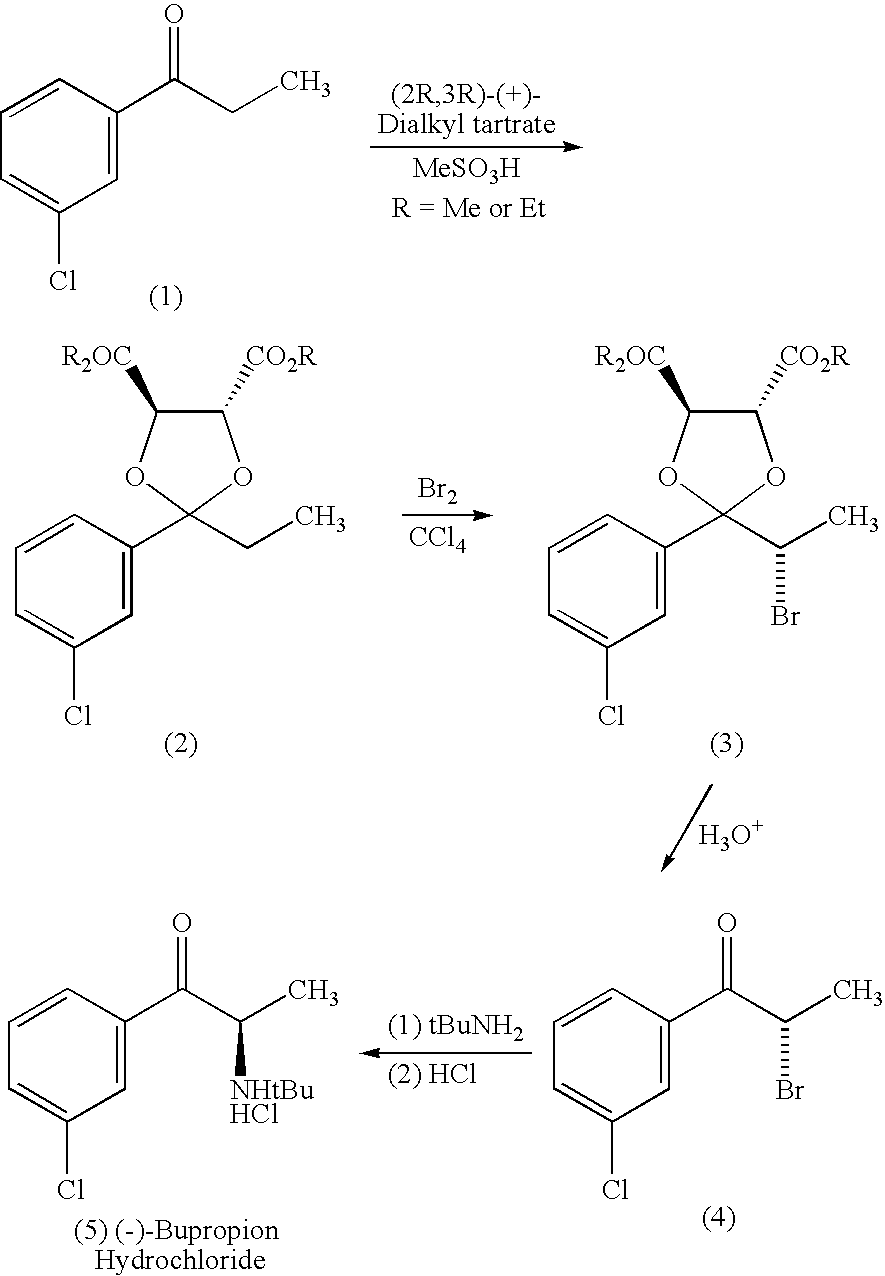
69 results about "Bupropion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
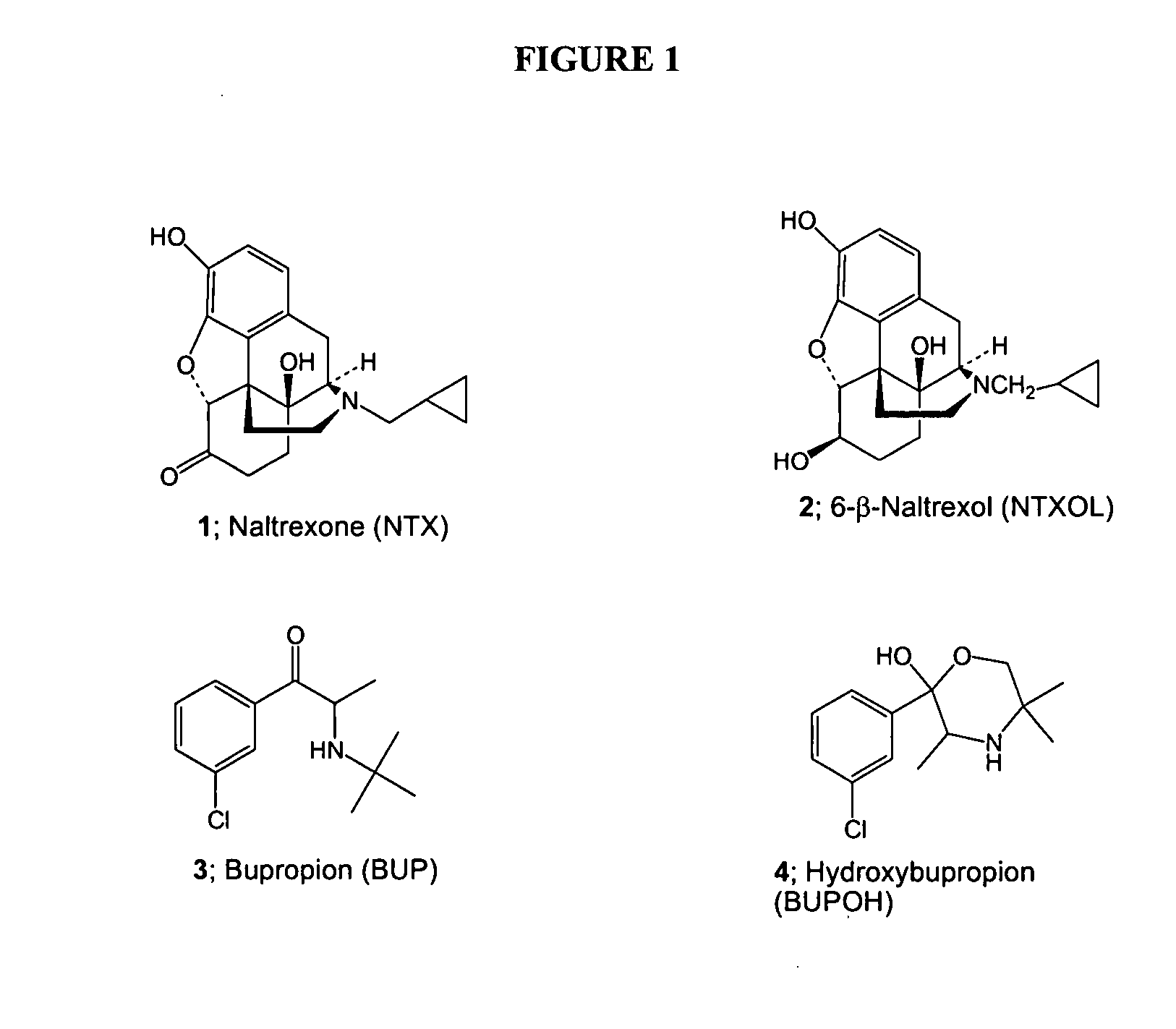
This medication is used to treat depression. It may also be used to prevent seasonal affective disorder (SAD), a type of depression that occurs each year at the same time (for example, during winter).
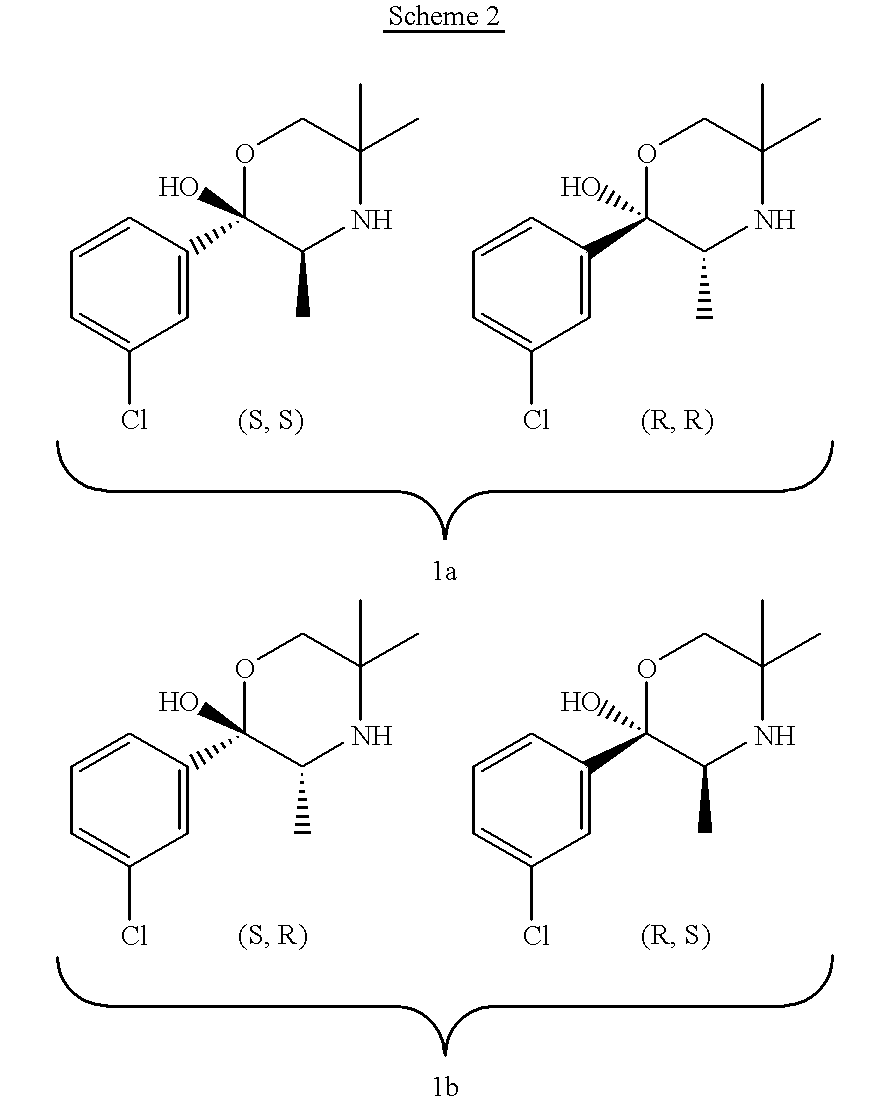
Combination therapy for depression
InactiveUS20090023744A1Good curative effectEliminate side effectsOrganic active ingredientsNervous disorderSerotoninMetabolite
A combination therapy for treating depressive disorders is provided herein. The combination therapy comprises administering an effective amount of bupropion or its metabolites together with at least one serotonin 5-HT1A partial agonist or its metabolites to a patient in need of treatment of the depressive disorder. Pharmaceutical formulations, including packaged pharmaceutical formulations, comprising this combination are also provided herein.
Owner:THE GENERAL HOSPITAL CORP
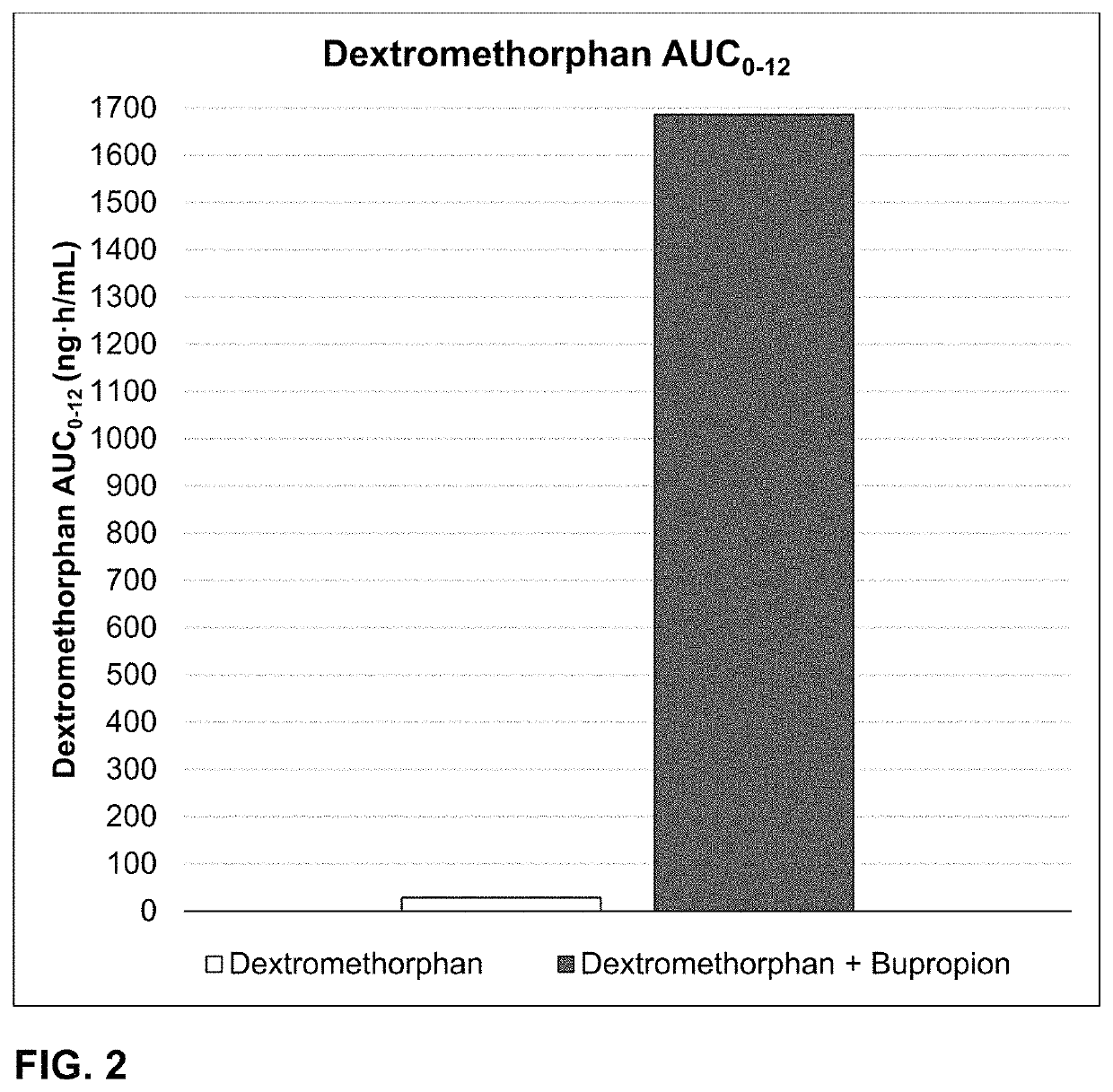
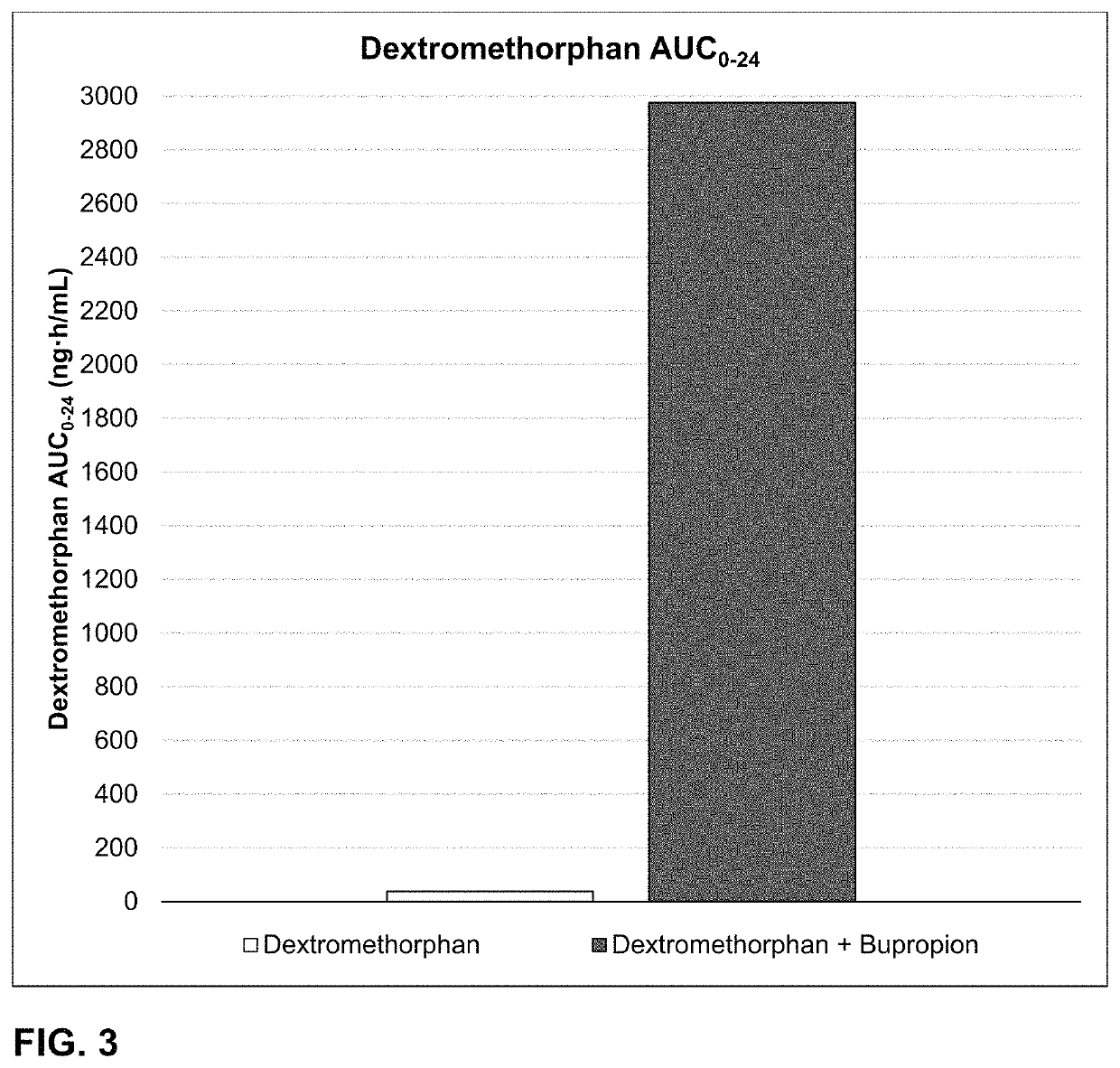
Bupropion and dextromethorphan for treating nicotine addiction
ActiveUS10688066B2Reduce in quantityOrganic active ingredientsNervous disorderPharmaceutical drugTherapeutic effect
Dosage forms, drug delivery systems, and methods related to sustained release of dextromethorphan or improved therapeutic effects are disclosed. Typically, bupropion or a related compound is orally administered to a human being to be treated with, or being treated with, dextromethorphan.
Owner:ANTECIP BIOVENTURES II
Combination of bupropion and a second compound for affecting weight loss
InactiveUS20060058293A1Increased energy expenditureAppetite suppressantBiocideNervous disorderMelanocortin 3 receptorMC4 Receptor
Disclosed are compositions for affecting weight loss comprising bupropion and a second compound, where the second compound causes increased agonism of a melanocortin 3 receptor (MC3-R) or a melanocortin 4 receptor (MC4-R) compared to normal physiological conditions, antagonizes cannabinoid receptor activity, or is useful in the treatment of bipolar disorders. Also disclosed are methods of affecting weight loss, increasing energy expenditure, increasing satiety in an individual, or suppressing the appetite of an individual, comprising identifying an individual in need thereof and treating that individual with a combination of bupropion and a compound that enhances α-MSH activity, antagonizes cannabinoid receptor activity, or is useful in the treatment of bipolar disorders.
Owner:OREXIGEN THERAPEUTICS INC
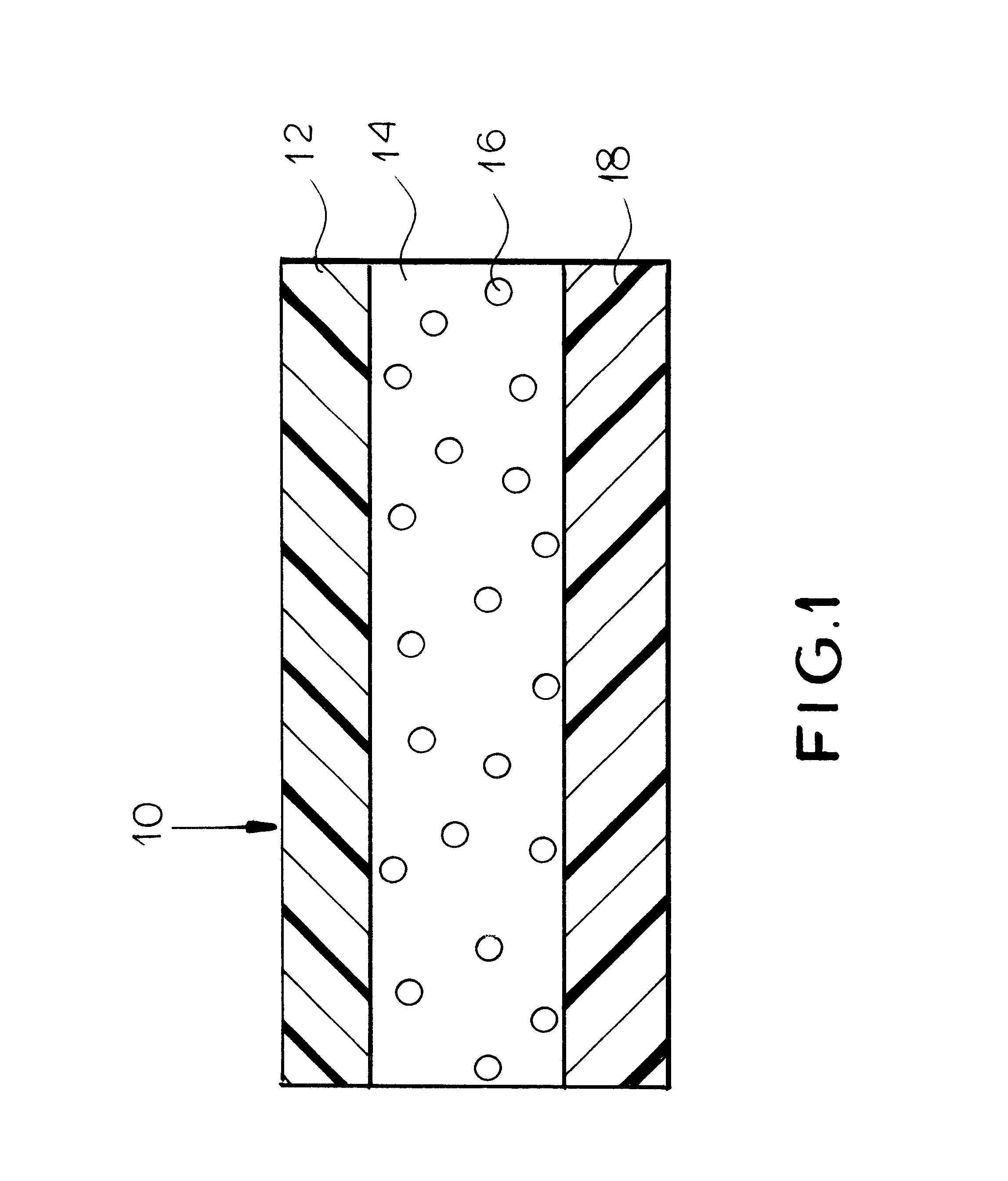
Patch and method for transdermal delivery of bupropion base
InactiveUS6312716B1Good for weight lossRelieve symptomsOrganic active ingredientsBiocideTransdermal patchDrug withdrawal symptoms
The invention includes a patch and method for transdermal delivery of bupropion base. In the method of this invention, a patient is administered a bupropion base in an amount effective to alleviate withdrawal symptoms and to prevent or reduce craving of nicotine in said patient. Alternatively, an effective amount of bupropion base is delivered to alleviate depression in a patient or to treat obesity. A transdermal patch includes a bupropion base. The bupropion base can be mixed with an acceptable pharmaceutical carrier.
Owner:COLLEGIUM PHARMA INC
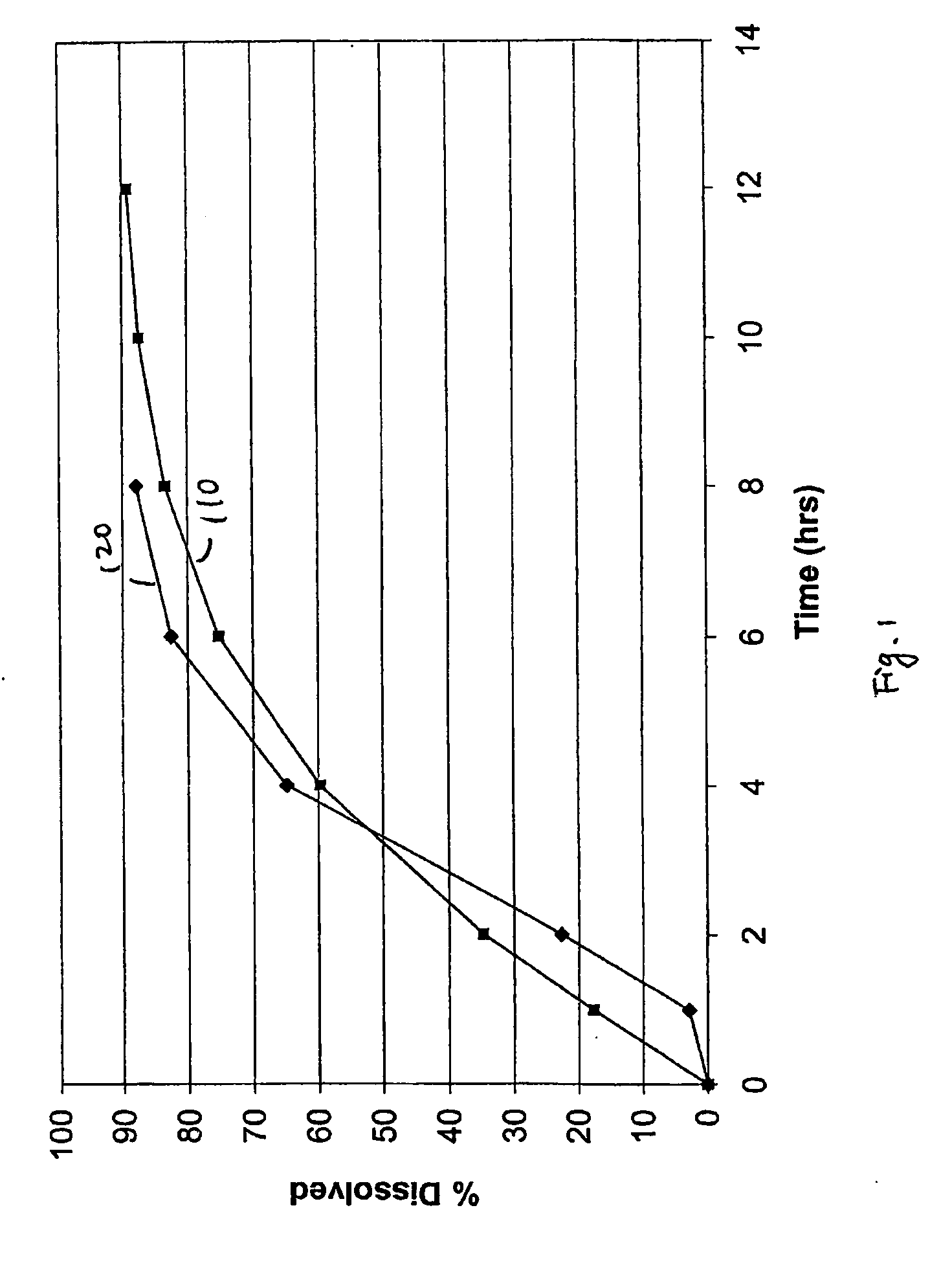
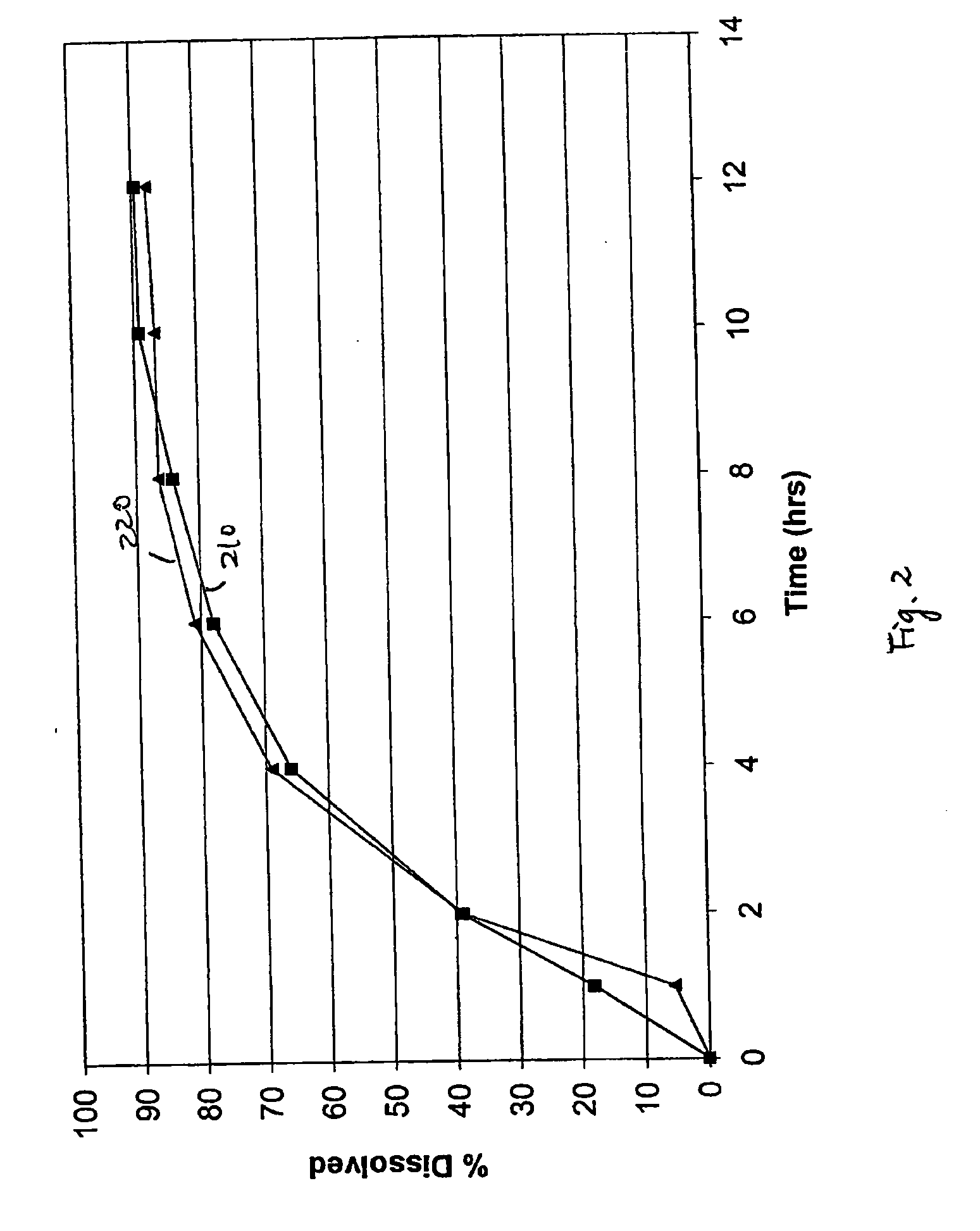
Bupropion formulation for sustained delivery
InactiveUS20050112198A1Improve stabilityProvide controlPharmaceutical non-active ingredientsPill deliveryAdditive ingredientPharmaceutical formulation
Disclosed is a pharmaceutical formulation for the stabilization and sustained delivery of an active pharmaceutical ingredient, such as the antidepressant, bupropion.
Owner:MURTY PHARMA
Bupropion metabolites and methods of use
Owner:SEPACOR INC
Method for treating obesity
InactiveUS20050143322A1Minimize risk factorHigh activityAntibacterial agentsBiocideAdrenergicCompound (substance)
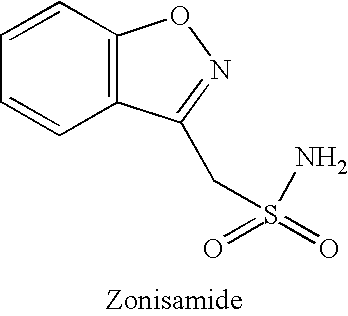
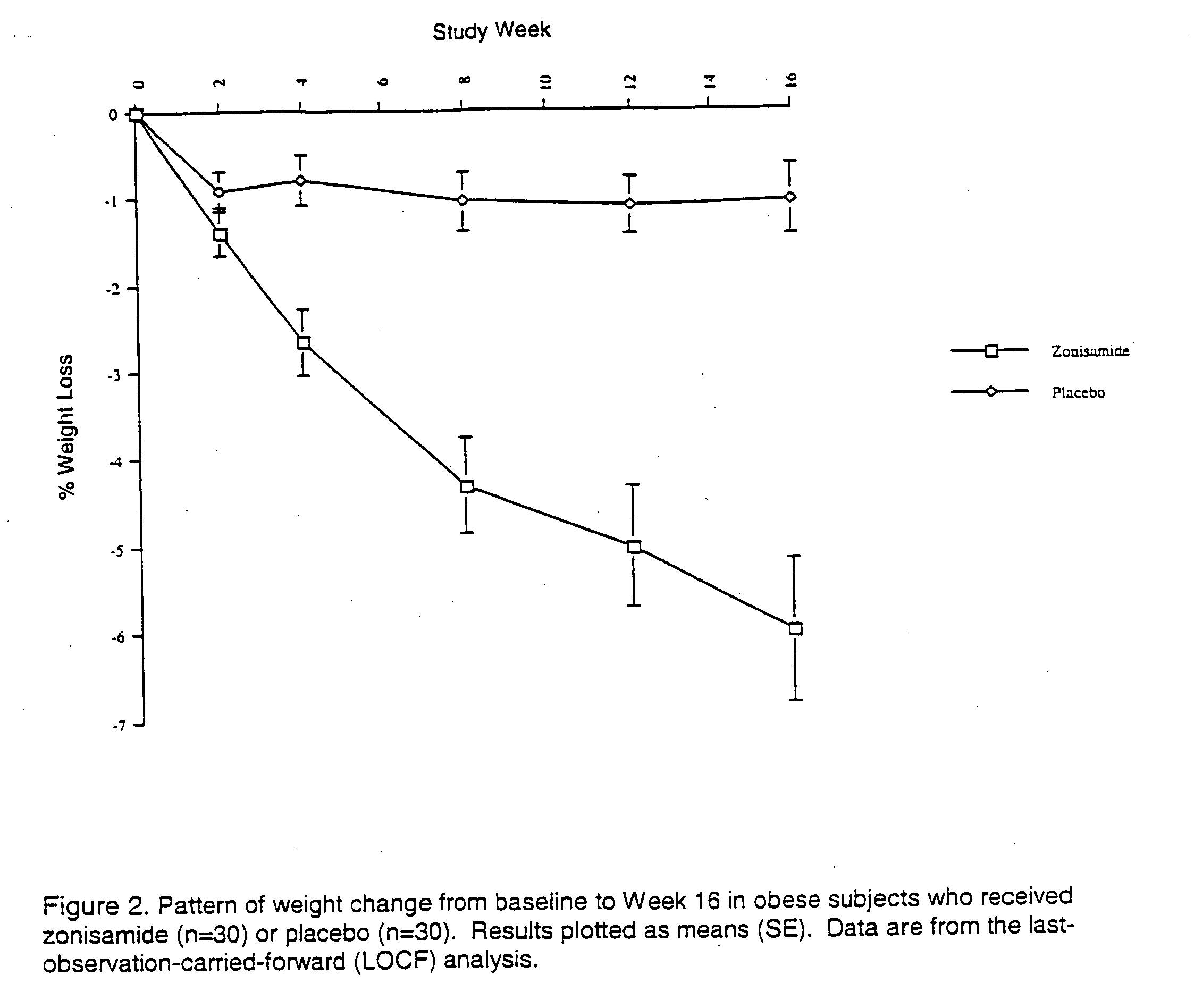
The present invention relates, in general, to obesity, and, in particular, to a method of treating obesity and minimizing metabolic risk factors associated therewith using, for example, zonisamide or other weight-loss promoting anticonvulsant either alone or in combination with bupropion or other compound that enhances the activity of norepinephrine and / or dopamine via uptake inhibition or other mechanism.
Owner:DUKE UNIV
Compositions and methods for treating obesity and related disorders
InactiveUS20080255093A1Reduces unwanted side effectGood for weight lossBiocideNervous disorderSYMPATHOMIMETIC AGENTSSerotonin receptor agonist
The present invention is drawn to combinations of pharmaceutical agents having similar chemical and / or pharmacological properties, wherein the combinations maximize the therapeutic effect of the drug while minimizing their adverse effects. The methods and compositions of the invention are particularly useful in the treatment of obesity and related conditions which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) or bupropion in combination with an anti-epileptic agent (e.g., topiramate, zonisamide), CB1 antagonists (e.g., rimonabant), or a 5HT2C-selective serotonin receptor agonist, (e.g., lorcaserin) for the treatment of obesity and related conditions. The invention also features kits for use in the practice of these novel therapies.
Owner:VIVUS
Method for treating obesity
InactiveUS20060009514A1Minimize risk factorHigh activityAntibacterial agentsBiocideAdrenergicMetabolic risk
The present invention relates, in general, to obesity, and, in particular, to a method of treating obesity and minimizing metabolic risk factors associated therewith using, for example, zonisamide or other weight-loss promoting anticonvulsant either alone or in combination with bupropion or other compound that enhances the activity of norepinephrine and / or dopamine via uptake inhibition or other mechanism.
Owner:GADDE KISHORE M +1
Methods for reducing the side effects associated with mirtzapine treatment
InactiveUS20060122127A1Eliminate side effectsBiocideCarbohydrate active ingredientsDiseaseNorepinephrine reuptake inhibitor
Compositions, and methods of use thereof, are provided for the prevention or treatment of side effects associated with the use of drugs that act as 5HT2 / 5HT3 serotonin receptor antagonists and alpha-2 adrenergic receptor antagonists (5HT2 / 5HT3 antagonist / alpha-2 antagonist). The method involves using dopamine-releasing compounds, such as amantadine, anticonvulsants, such as zonisamide, or dopamine / norepinephrine reuptake inhibitors, such as bupropion, in combination with 5HT2 / 5HT3 antagonist / alpha-2 antagonists, such as mirtazapine, to reduce the excessive daytime drowsiness and / or weight gain associated with 5HT2 / 5HT3 antagonist / alpha-2 antagonist use for the treatment of disorders, such as, depression, schizophrenia, anxiety disorders, sleep-related breathing disorders, insomnia, migraine headache, chronic tension-type headache, hot flashes, lower back pain, neuropathic pain and functional somatic syndromes. Formulations of dopamine-releasing compounds or anticonvulsants with 5HT2 / 5HT3 antagonist / alpha-2 antagonists are provided. In particular embodiments, combination therapy with mirtazapine and zonisamide provides relief from chronic low back pain, while reducing or avoiding side effects associated with monotherapy with mirtazapine or zonisamide.
Owner:CYPRESS BIOSCI
Method for treating obesity
InactiveUS20050215552A1Minimizing metabolic risk factorHigh activityBiocideMetabolism disorderMetaboliteAdrenergic
The present invention relates generally to methods of treating obesity and minimizing metabolic risk factors associated therewith using, for example, zonisamide or other weight loss-promoting anti-convulsant either alone or in combination with bupropion or metabolites thereof or other compound that enhances the activity of norepinephrine and / or dopamine via uptake inhibition or other mechanism.
Owner:OREXIGEN THERAPEUTICS INC
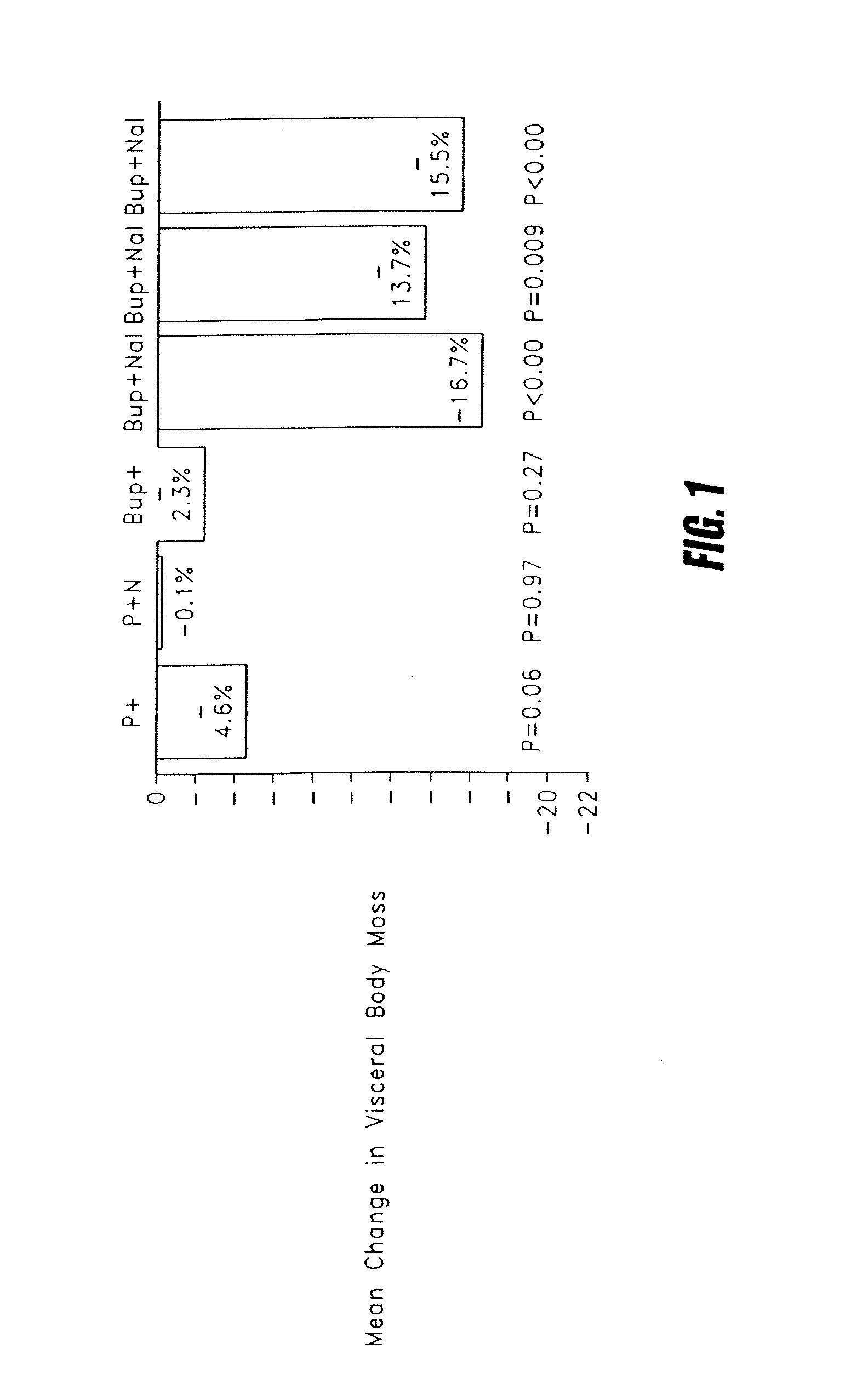
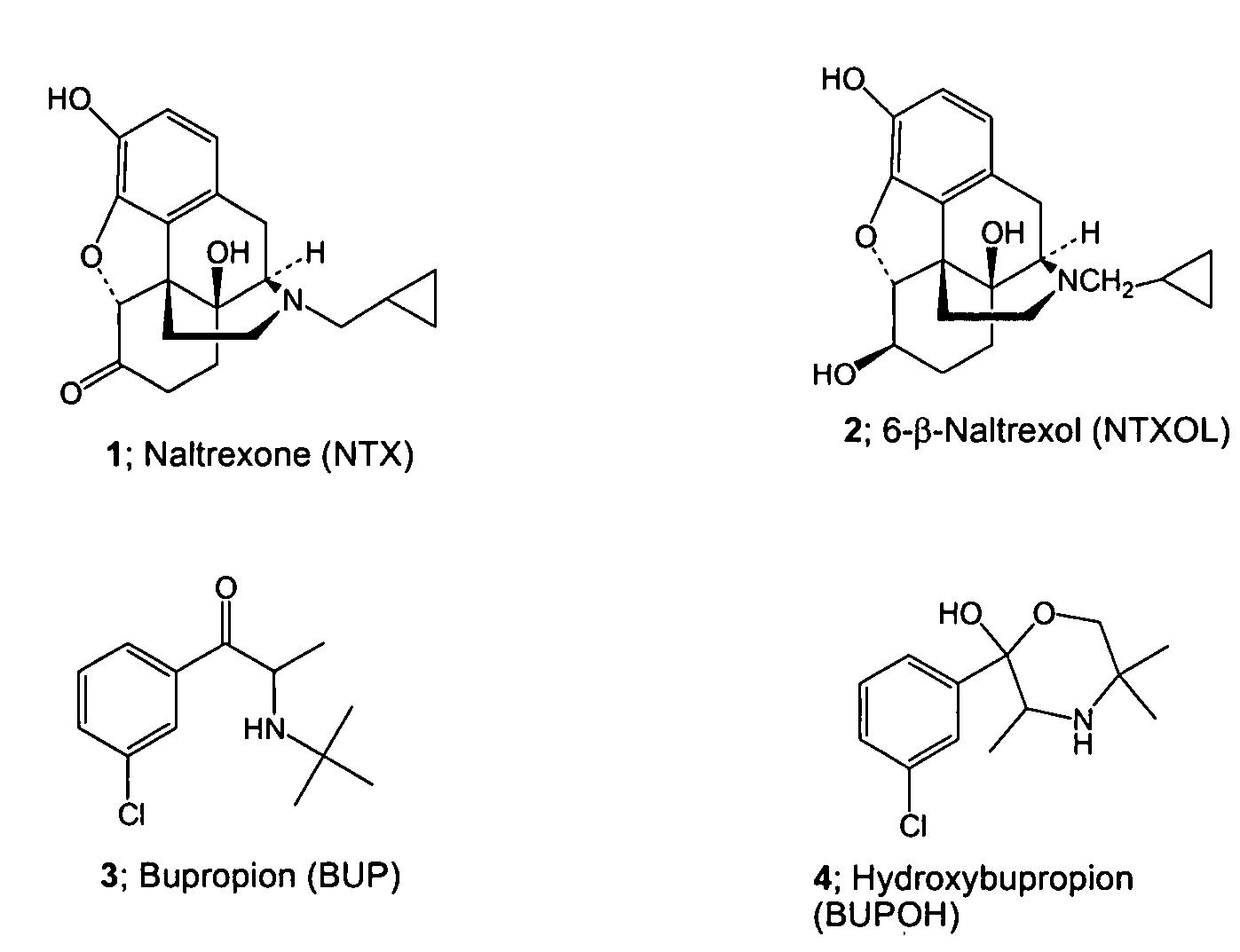
Methods for treating visceral fat conditions
Disclosed are methods and compositions for treating visceral fat conditions and / or metabolic syndrome using combinations of naltrexone and bupropion.
Owner:OREXIGEN THERAPEUTICS INC
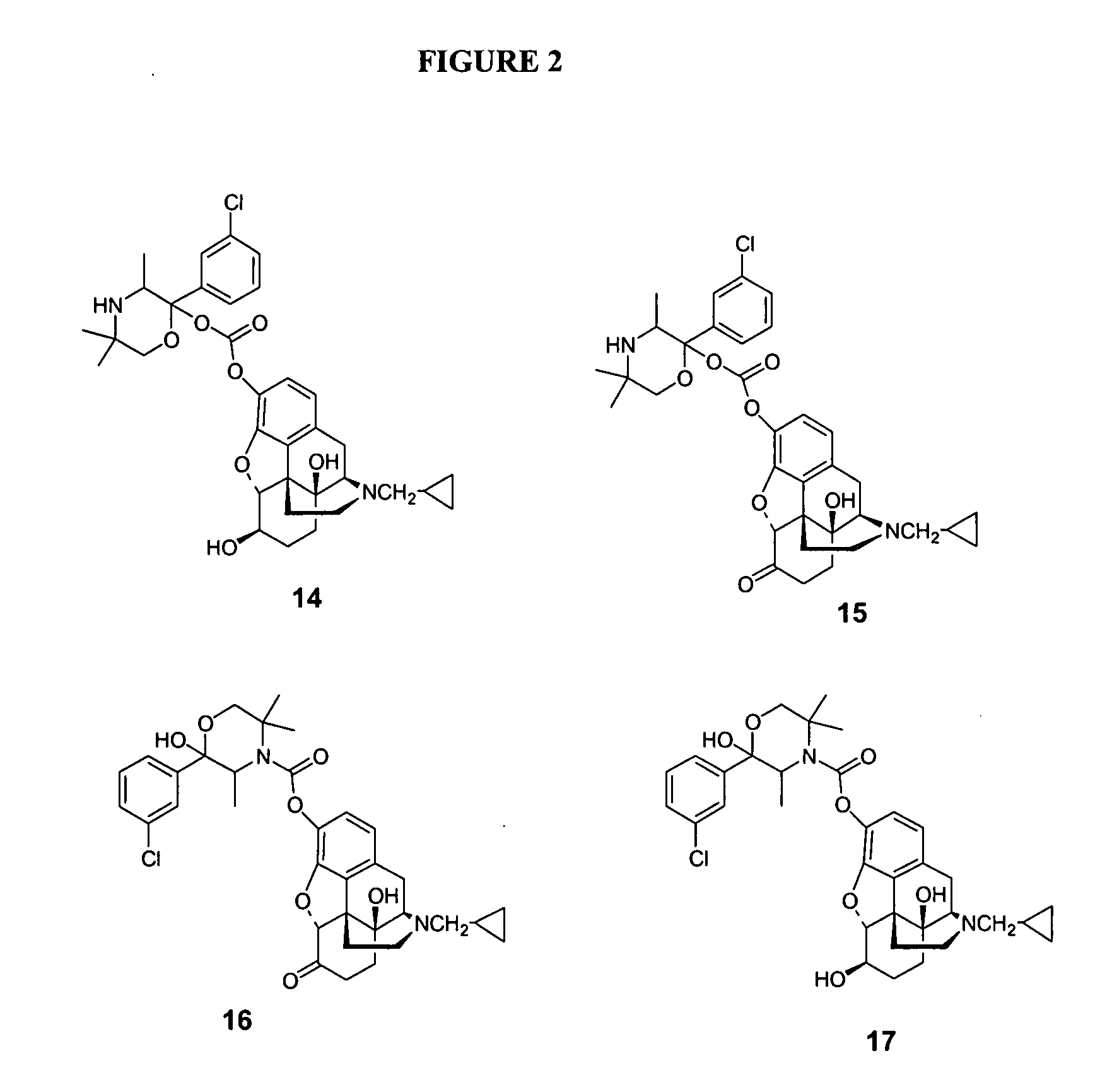
Enhancing transdermal delivery of opiod antagonists and agonistis using codrugs links to bupropion or hydroxybupropion
InactiveUS20090017102A1Improve oral bioavailabilityBiocideNervous disorderOpioid antagonistOpioid Agonist
The present invention is directed to novel codrugs comprising bupropion or hydroxybupropion and an opioid antagonist or an opioid agonist joined together by chemical bonding. The codrugs provide a significant increase in the transdermal flux across human skin, as compared to the basic opioid antagonist or opioid agonist.
Owner:UNIV OF KENTUCKY RES FOUND +1
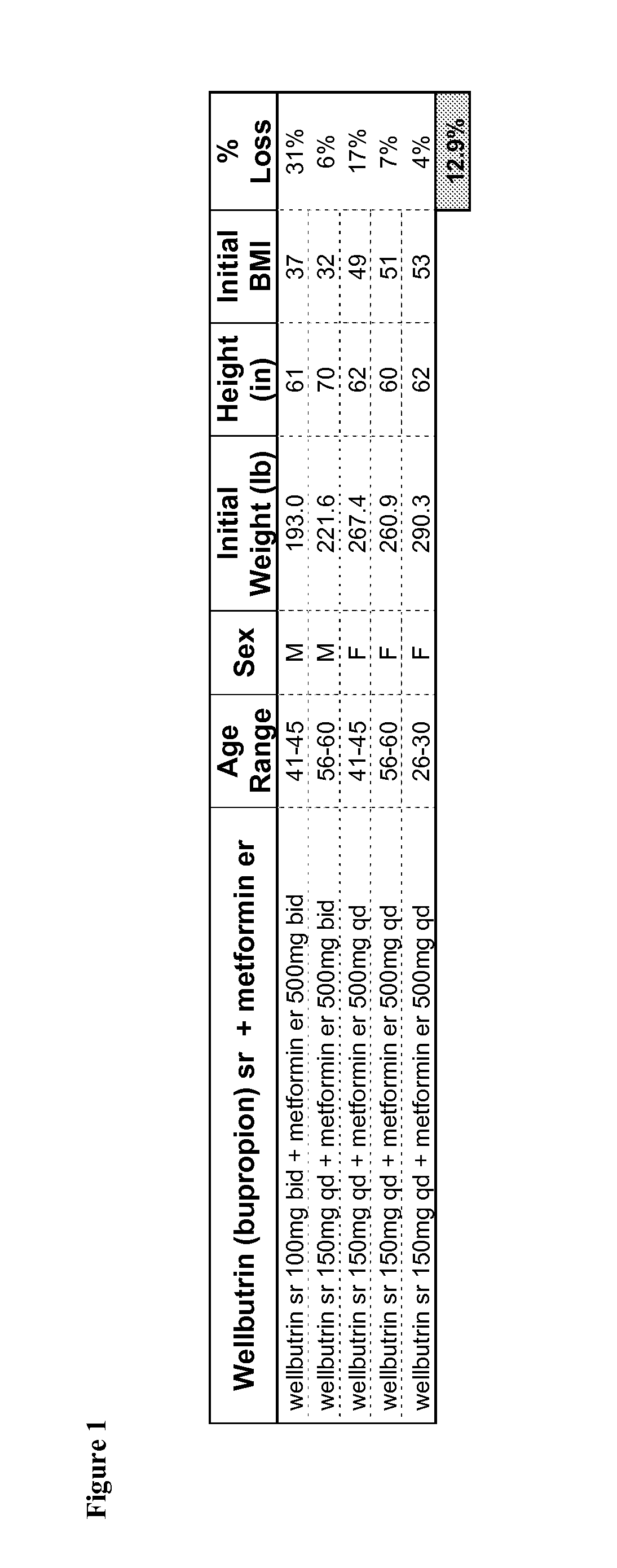
Combination Therapies for the Treatment of Obesity
InactiveUS20100331419A1Reduce weightOrganic active ingredientsBiocideCombined Modality TherapyObesity
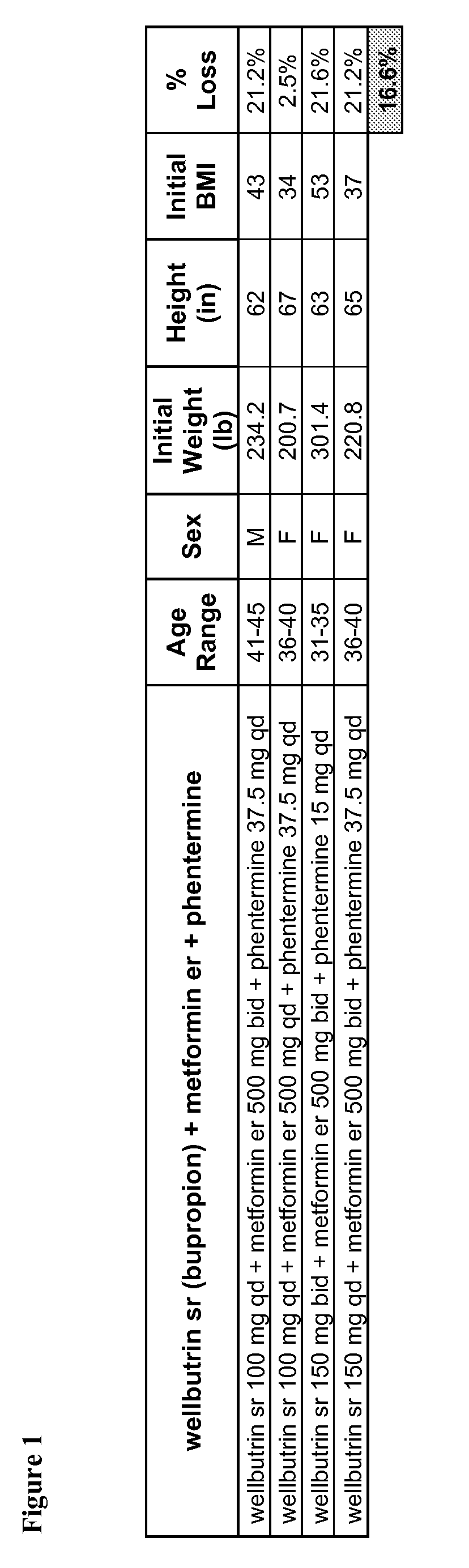
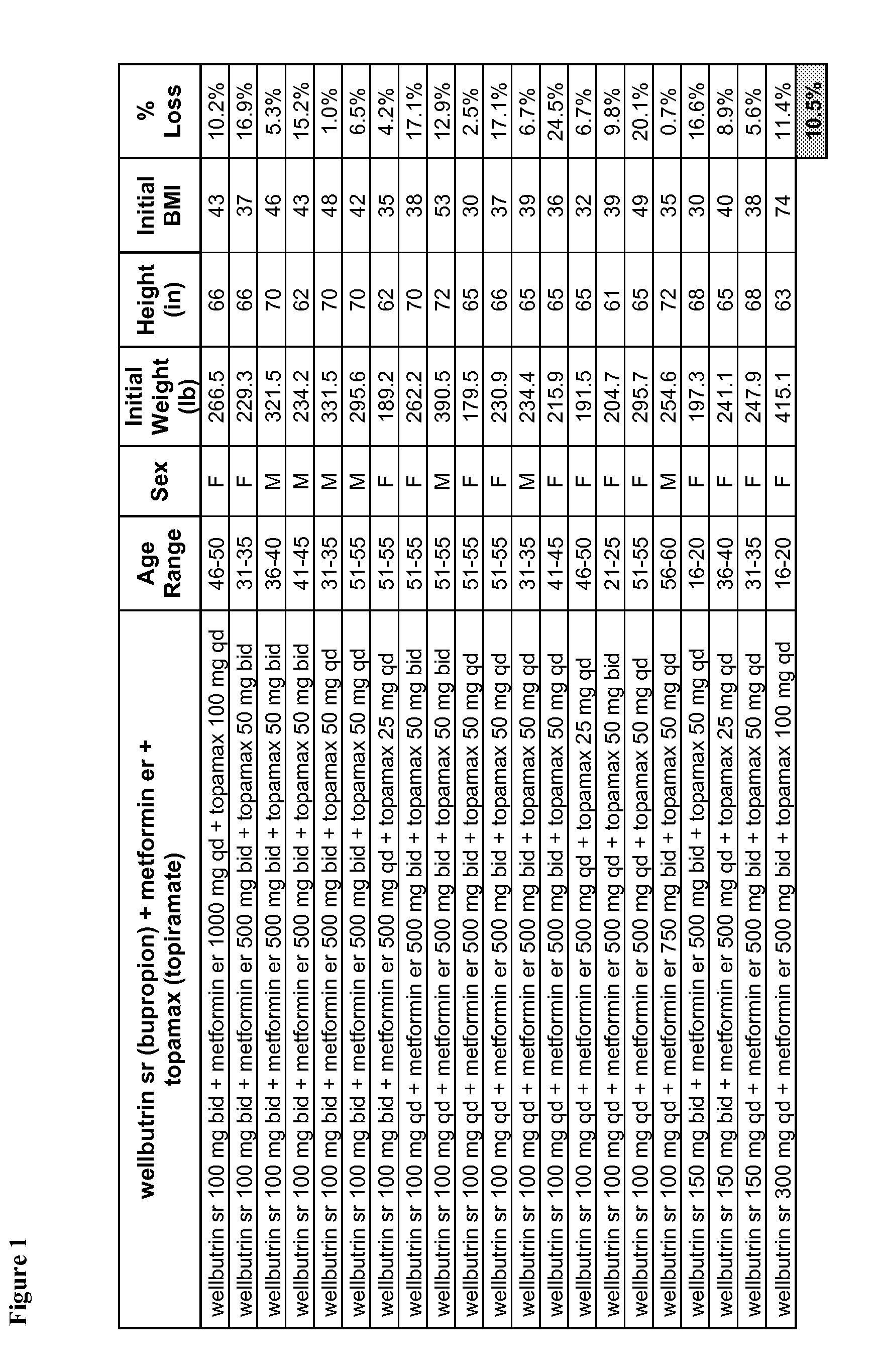
Described are pharmaceutical compositions comprising bupropion, metformin, and at least one pharmaceutically acceptable carrier or excipient. Another aspect of the present invention relates to a method of treating a patient suffering from obesity or needing to lose weight, comprising the step of co-administering to said patient a therapeutically effective amount of bupropion and metformin. In certain embodiments, an aforementioned method is practiced in conjunction or tandem with a medical procedure or the use of a medical device or both.
Owner:METABOLOUS PHARMA
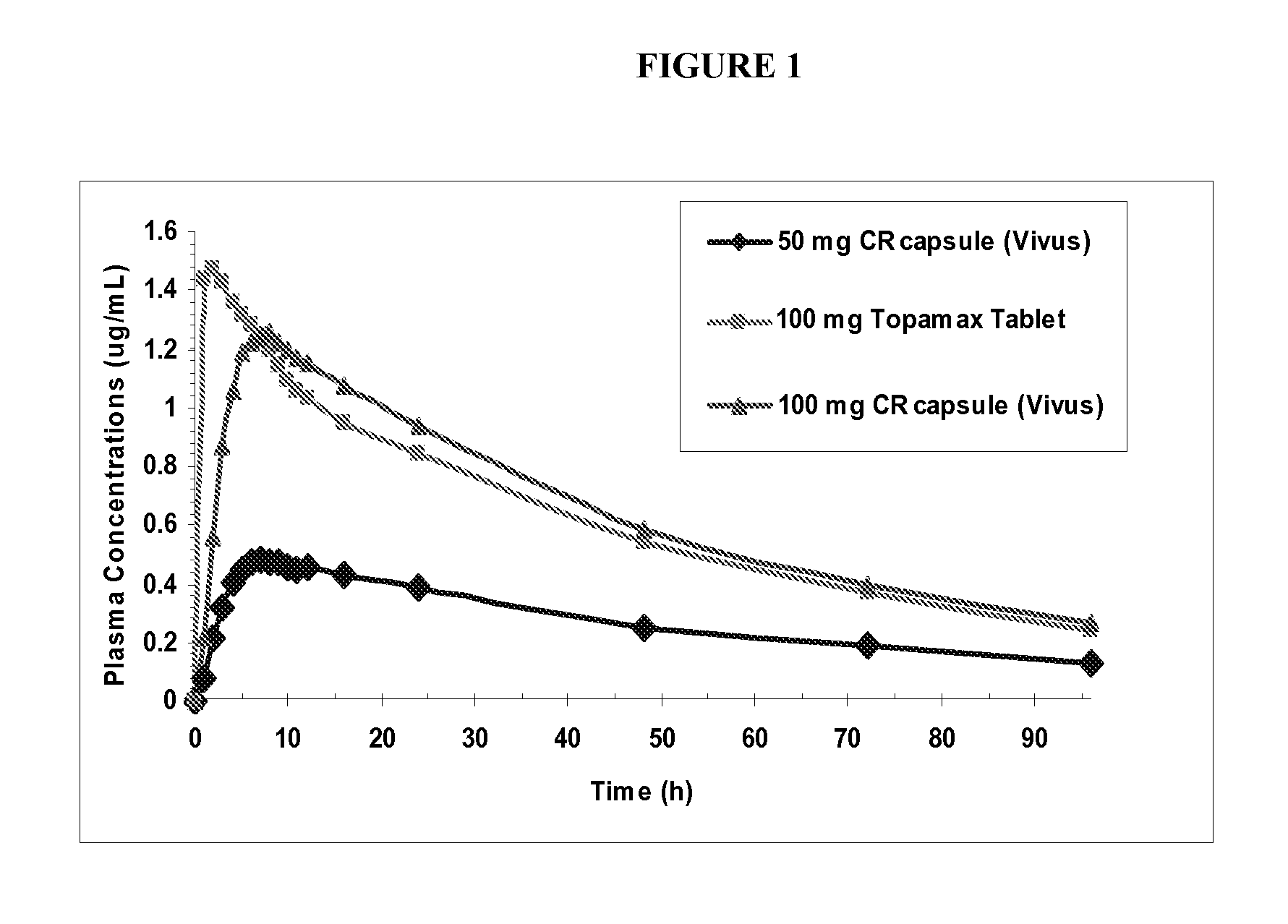
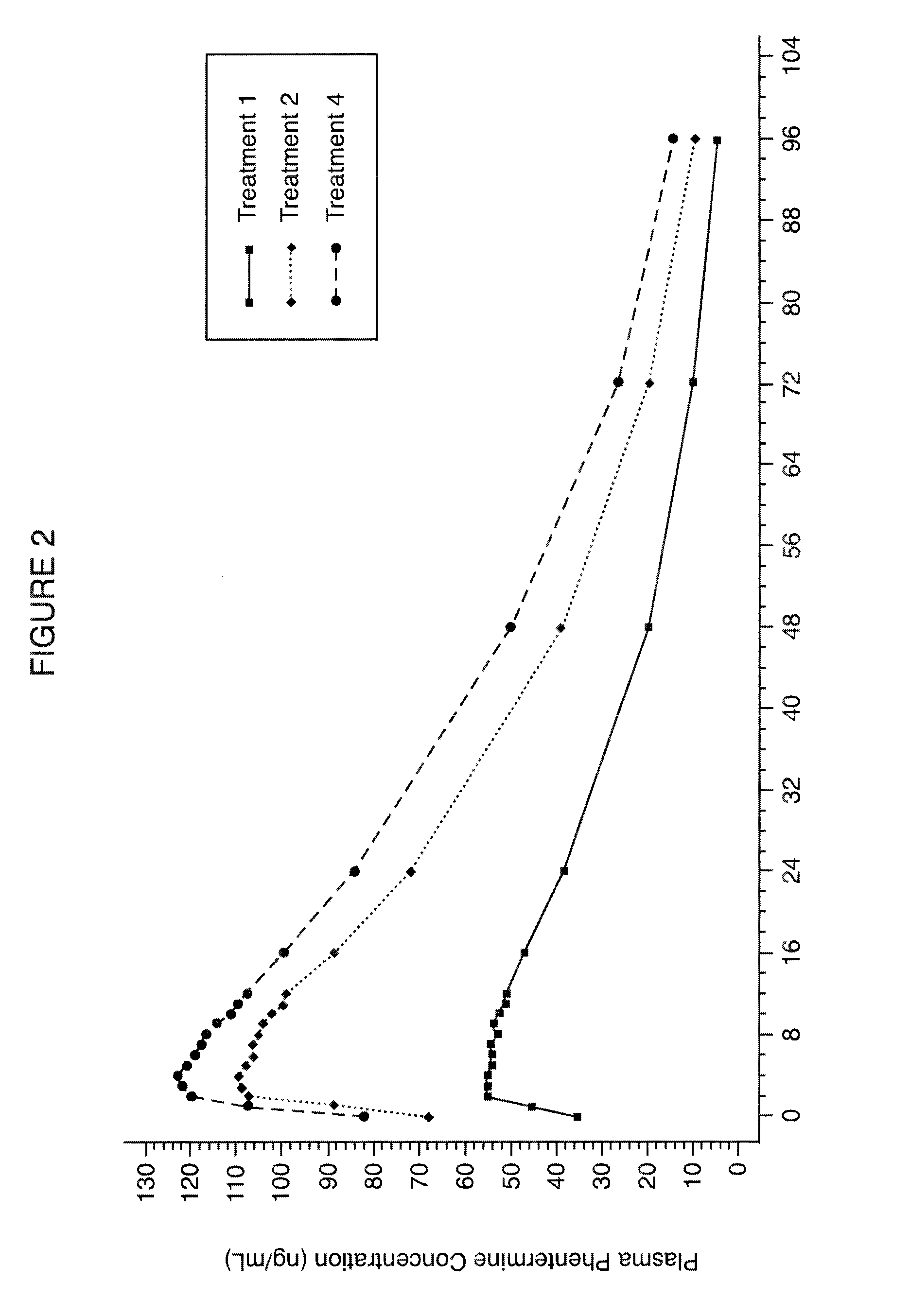
Escalating dosing regimen for effecting weight loss and treating obesity
The present invention is drawn to novel topiramate compositions as well as methods for effecting weight loss, e.g., in the treatment of obesity and related conditions, including conditions associated with and / or caused by obesity per se. The present invention features an escalating dosing regimen adapted for the administration of topiramate and optionally a sympathomimetic agent such as phentermine or bupropion, in the treatment of obesity and related conditions.
Owner:VIVUS LLC
Combination therapies for the treatment of obesity
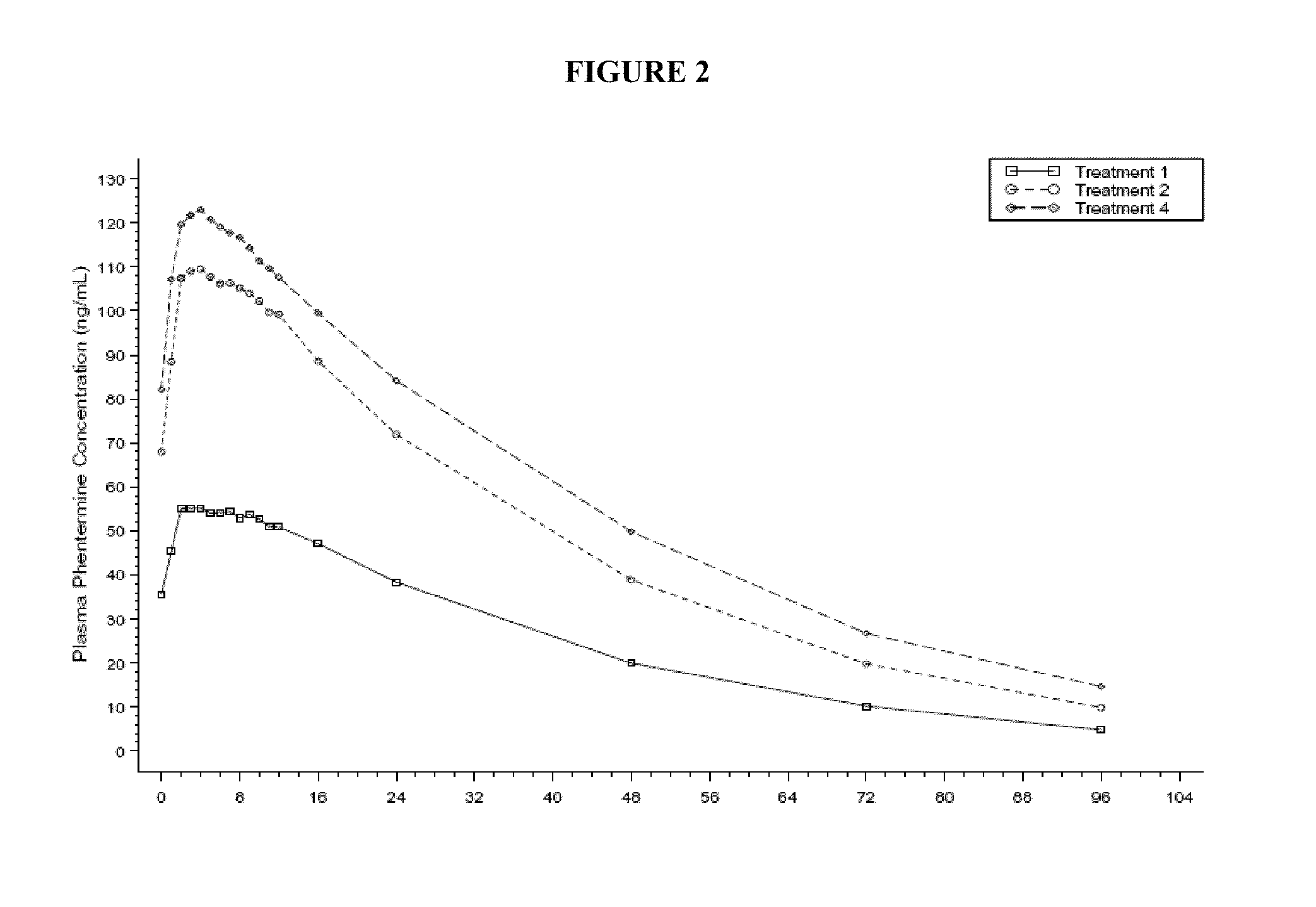
Described are pharmaceutical compositions comprising bupropion, metformin, phentermine, and at least one pharmaceutically acceptable carrier or excipient. Another aspect of the present invention relates to a method of treating a patient suffering from obesity or needing to lose weight, comprising the step of co-administering to said patient a therapeutically effective amount of bupropion, metformin, and phentermine. In certain embodiments, an aforementioned method is practiced in conjunction or tandem with a medical procedure or the use of a medical device or both.
Owner:METABOLOUS PHARMA
Method for treating obesity
InactiveUS20090076108A1Minimize risk factorHigh activityBiocideMetabolism disorderAdrenergicMetabolite
Owner:OREXIGEN THERAPEUTICS INC
Methods and formulations for making pharmaceutical compositions containing bupropion
InactiveUS20060099260A1Organic active ingredientsNervous disorderPh independentPharmaceutical formulation
Embodiments of the invention generally provide pharmaceutical drug compositions, methods of preparing oral drug compositions, such as extended release dosage compositions, and methods for treating antidepressant or smoking cessation. In one aspect, the invention provides a pharmaceutical formulation comprising a core, including bupropion and its salt derivatives, and a coating. The coating may include from about 5% to about 99% by weight of the coating of a pharmaceutically acceptable pH-independent polymer. The coating may further include from about 0.001% to about 30% by weight of the coating of a surfactant. In another aspect, the invention provides methods for preparing and administering a pharmaceutical composition in oral dosage form, such as a tablet.
Owner:BIOKEY
Application of bakuchiol compound
InactiveCN101088498AAchieve therapeuticAchievement or effect of neurological diseaseNervous disorderHydroxy compound active ingredientsDiseaseCytotoxicity
The present invention discloses the application of bakuchiol compound in preparing medicine for treating psychogenic diseases and neurogenic diseases. Extracorporeal experiment and animal experiment show that the bakuchiol compound can suppressing dopamine transport protein, noradrenalin transport protein and 5-hydroxy tryptamine transport protein selectively, and no influence on gamma-aminobutyric acid transport protein and L-glytamic acid transport protein, no cytotoxicity, and antidepressant effect higher than available antidepressant medicine amfebutamoe hydrochloride. Therefore, the bakuchiol compound has excellent marketability in preparing medicine for treating psychogenic diseases and neurogenic diseases.
Owner:上海国联干细胞技术有限公司
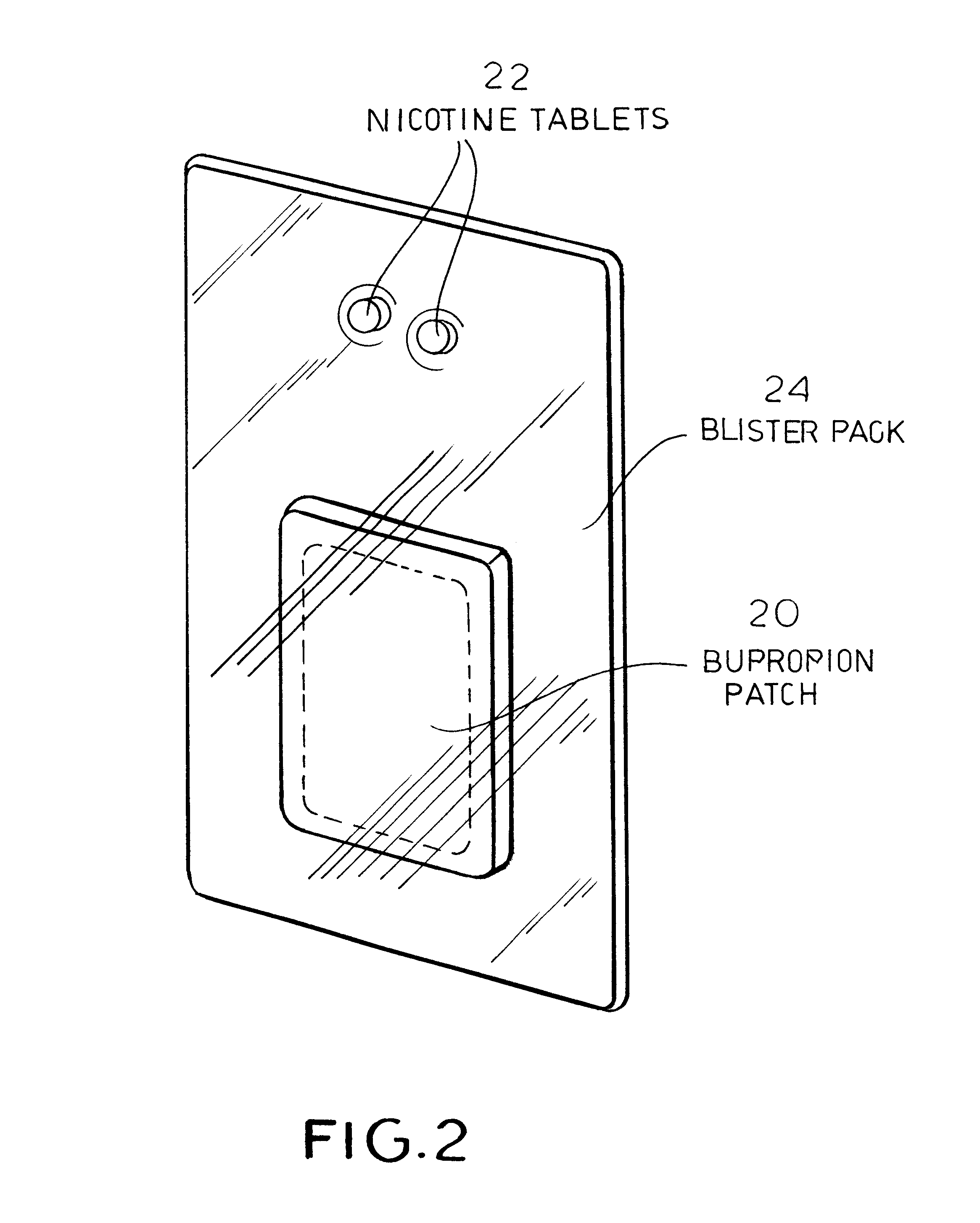
Pharmaceutical composition containing two active ingredients for smoking cessation
InactiveUS6582737B2Slowly weaning the patientEliminating depressive stateBiocideNervous disorderMetaboliteBULK ACTIVE INGREDIENT
A kit to alleviate tobacco-smoking withdrawal symptoms in a patient is disclosed which comprises:(a) a therapeutically effective amount of nicotine, at least one active nicotine metabolite, a combination of nicotine and an active nicotine metabolite, or an azapirone, or a pharmaceutically acceptable salt thereof;(b) a transdermal delivery system consisting essentially of a bupropion base in a therapeutically effective amount; and(c) a packaging material surrounding (a) and (b).
Owner:COLLEGIUM PHARMA INC
Escalating dosing regimen for effecting weight loss and treating obesity
The present invention is drawn to novel topiramate compositions as well as methods for effecting weight loss, e.g., in the treatment of obesity and related conditions, including conditions associated with and / or caused by obesity per se. The present invention features an escalating dosing regimen adapted for the administration of topiramate and optionally a sympathomimetic agent such as phentermine or bupropion, in the treatment of obesity and related conditions.
Owner:VIVUS LLC
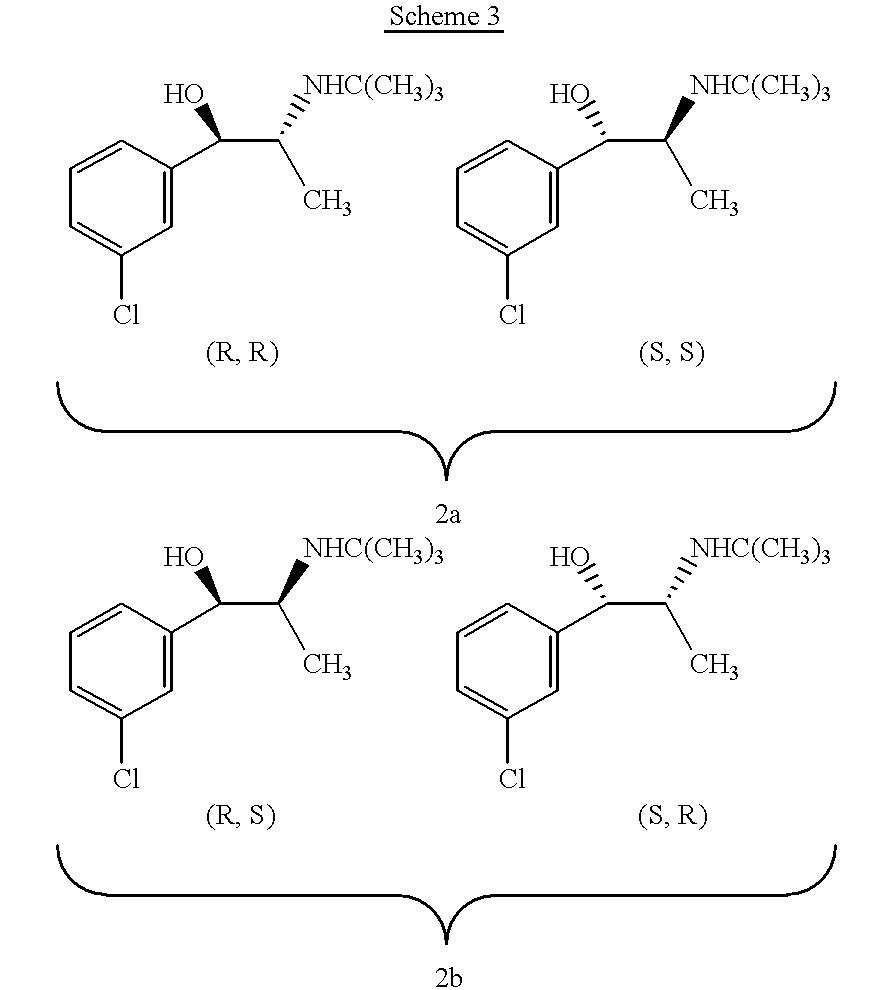
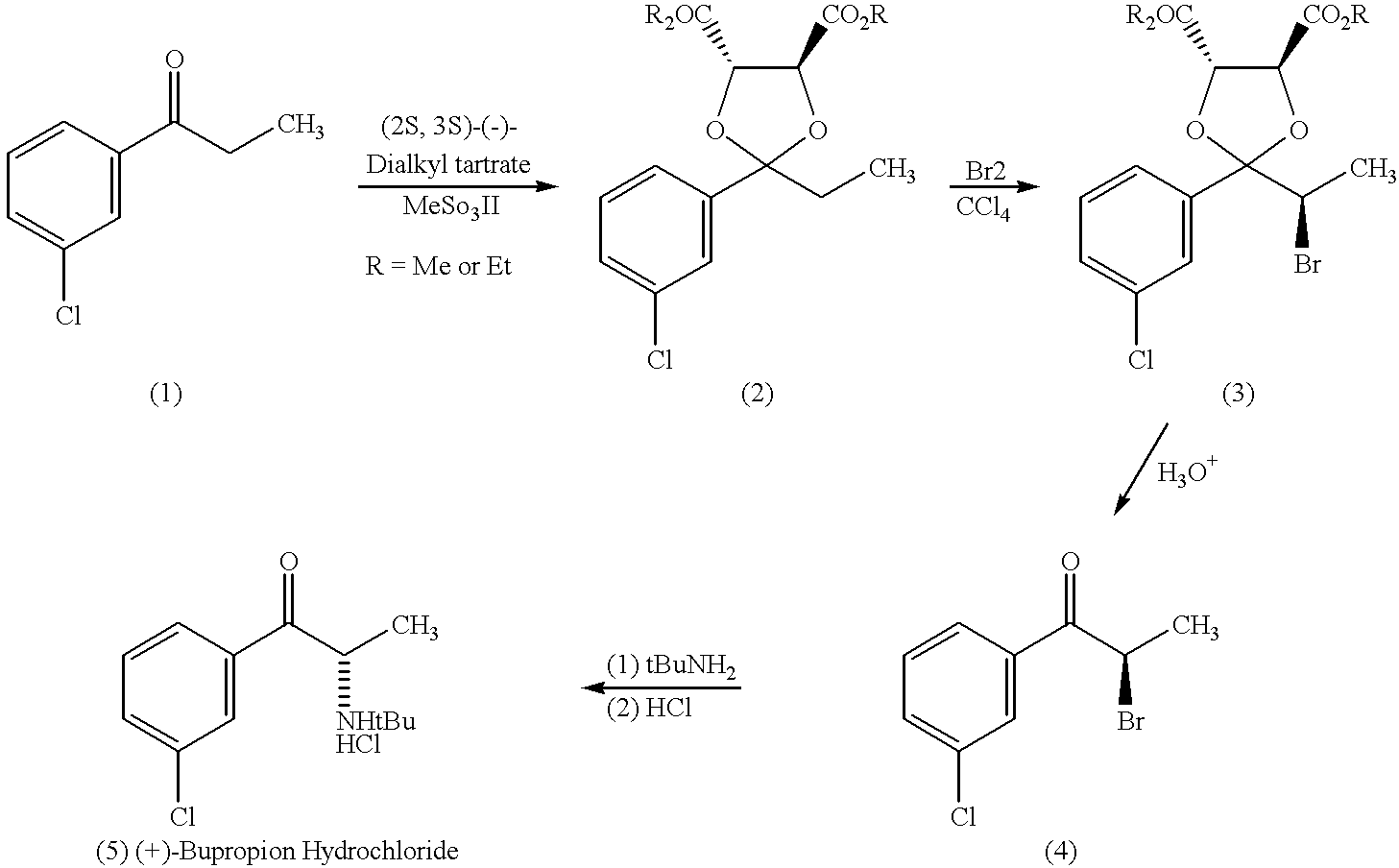
Methods and compositions for aiding in smoking cessation and for treating pain and other disorders using optically pure (+)-bupropion
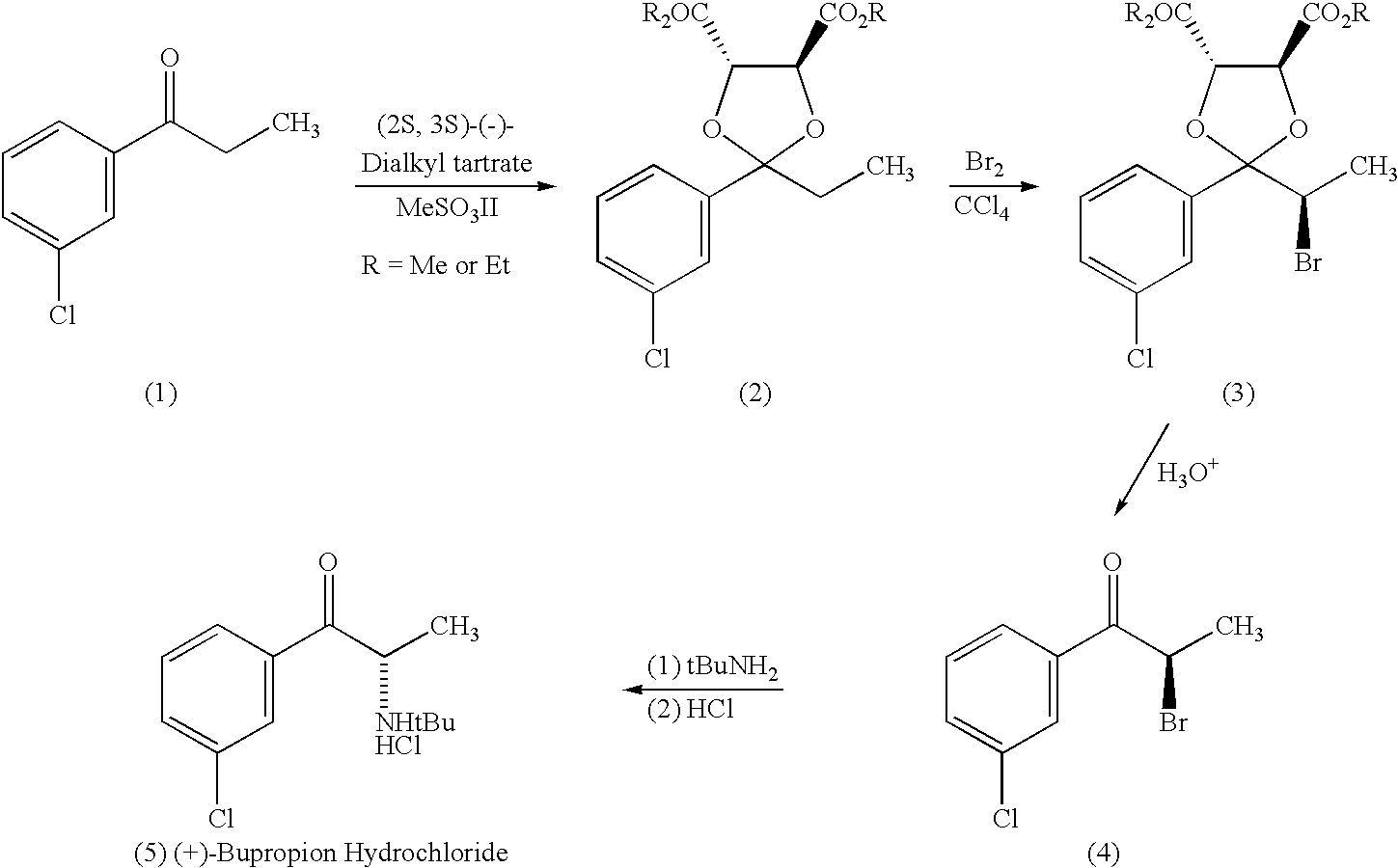
InactiveUS20010011103A1Reduce adverse effectsEffective treatmentOrganic active ingredientsBiocideReflexSeasonal Affective Disorders
Methods and compositions are disclosed utilizing the optically pure (+)-isomer of bupropion to assist in smoking cessation, for treating smoking and nicotine addiction, and for treating pain, including, but not limited to, chronic pain, neuropathetic pain and reflex sympathetic dystrophy, and other disorders such as narcolepsy, chronic fatigue syndrome, fibromyalgia, seasonal affective disorder and premenstrual syndrome, while avoiding adverse affects associated with racemic bupropion.
Owner:SEPACOR INC
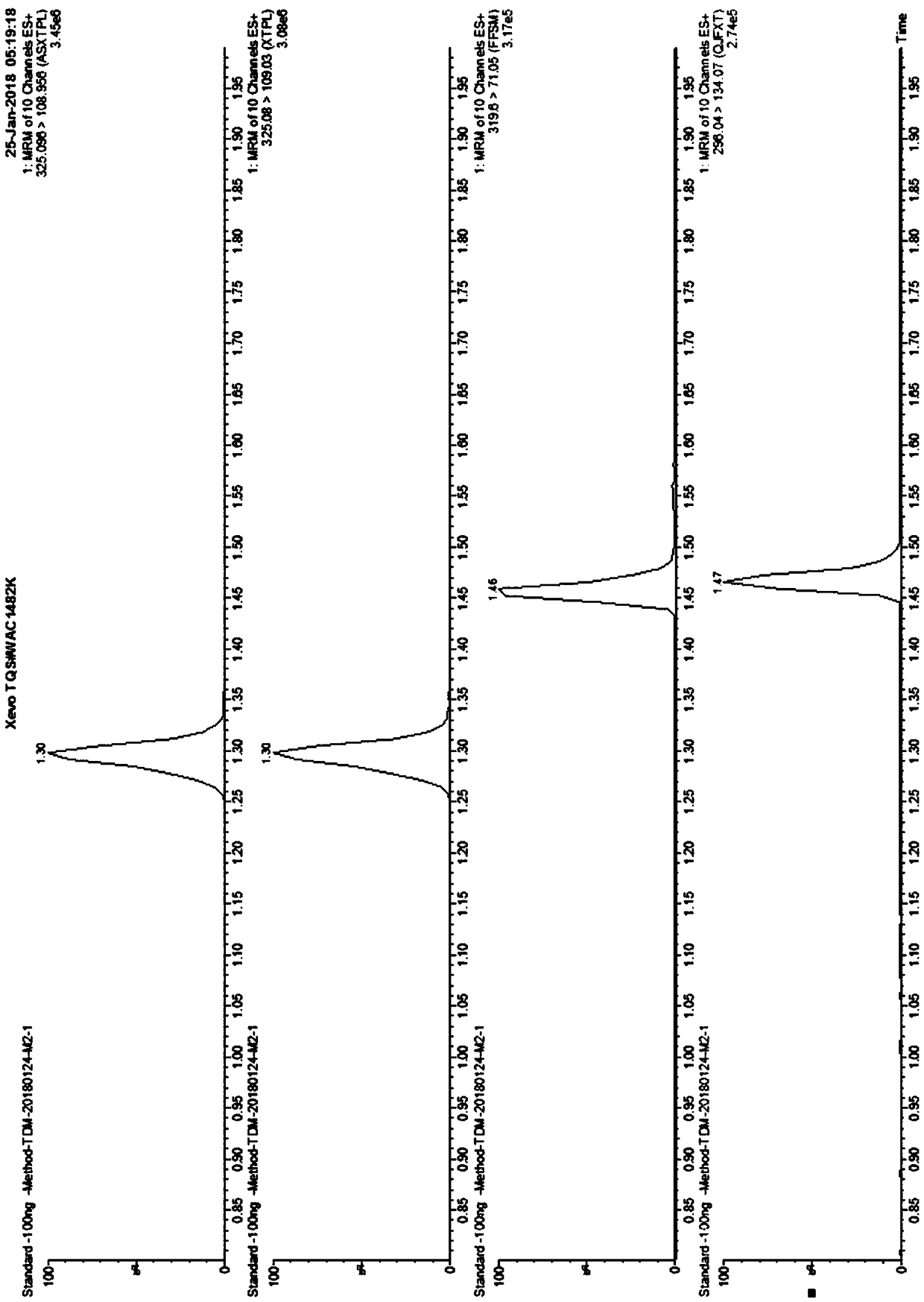
Kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry and application thereof
InactiveCN109085264APharmacologically activeInterpret blood levelsComponent separationSertralineTandem mass spectrometry
The invention provides a kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry. The kit comprises drug standard substances, drug internal standardization compounds, drug extraction compositions, negative plasma and a diluent. The drug standard substances comprise amfebutamone, oxybupropion, citalopram, Escitalopram, venlafaxine, O-desmethylvenlafaxine, duloxetine, fluoxetine, norfloxetine, fluvoxamine, mirtazapine, paroxetine, sertraline and trazodone. The drug internal standardization compounds comprise amfebutamone-d9, oxybupropion-d6,citalopram-d6, venlafaxine-d6, O-desmethylvenlafaxine-d6, duloxetine-d3, fluoxetine-d6, norfloxetine-d6, fluvoxamine-d4, mirtazapine-d3, paroxetine-d6, sertraline-d3 and trazodone-d6. The drug extraction compositions comprise, by volume, 60% of methanol solution, 20% of acetonitrile solution, 10% of isopropyl alcohol solution and 10% of purified water. The diluent comprises 50 % of methanol waterfluid. The kit can be used for simultaneous detection of the anti-depressant drugs and active metabolites, the detection time is short, and flux is high.
Owner:HANGZHOU BAICHEN MEDICAL INSTR CO LTD +1
Methods and compositions for aiding in smoking cessation and for treating pain and other disorders using optically pure (+)-bupropion
InactiveUS6495605B2Reduce adverse effectsEffective treatmentBiocideOrganic active ingredientsReflexSeasonal Affective Disorders
Methods and compositions are disclosed utilizing the optically pure (+)-isomer of bupropion to assist in smoking cessation, for treating smoking and nicotine addiction, and for treating pain, including, but not limited to, chronic pain, neuropathetic pain and reflex sympathetic dystrophy, and other disorders such as narcolepsy, chronic fatigue syndrome, fibromyalgia, seasonal affective disorder and premenstrual syndrome, while avoiding adverse affects associated with racemic bupropion.
Owner:SEPACOR INC
Methods for treating visceral fat conditions
Disclosed are methods and compositions for treating visceral fat conditions and / or metabolic syndrome using combinations of naltrexone and bupropion.
Owner:OREXIGEN THERAPEUTICS INC
Methods and formulations for making controlled release oral dosage form
InactiveUS20060099262A1Organic active ingredientsCoatingsControlled releasePharmaceutical formulation
Embodiments of the invention generally provide pharmaceutical drug compositions, methods of preparing oral drug compositions, such as extended release dosage compositions, and methods for treating antidepressant or smoking cessation. In one aspect, the invention provides a pharmaceutical formulation comprising a core, including bupropion and its salt derivatives, and a coating. The coating may include one or more surfactants and aqueous dispersions of one or more insoluble pharmaceutical acceptable polymers. The core may also include aqueous dispersions of one or more insoluble pharmaceutical acceptable polymers. In another aspect, the invention provides methods for preparing and administering a pharmaceutical composition in oral dosage form, such as a tablet.
Owner:BIOKEY
Methods of treating central nervous system disorders with a low dose combination of escitalopram and bupropion
The present invention relates to a method of treating a central nervous system disorder, such as a mood disorder (e.g., major depressive disorder) or an anxiety disorder (e.g., general anxiety disorder, social anxiety disorder, post traumatic stress disorder, and panic disorder) with a low dose combination of escitalopram and bupropion.
Owner:H LUNDBECK AS +1
Weight reduction or weight controlling composition
InactiveUS20050031670A1Improvement ingredientsOrganic active ingredientsDispersion deliveryFood ComponentWeight decreasing
A composition having weight reduction capability, weight controlling activity, or both comprising (a) a bupropion compound and (b) a food component and a method of treatment associated with it.
Owner:SMITHKLINE BECKMAN CORP
Methods and compositions for aiding in smoking cessation and for treating pain and other disorders using optically pure (-) -bupropion
InactiveUS20040225020A1Reduce adverse effectsEffective treatmentBiocideOrganic active ingredientsReflexSeasonal Affective Disorders
Methods and compositions are disclosed utilizing the optically pure (-)-isomer of bupropion to assist in smoking cessation, for treating smoking and nicotine addiction, and for treating pain, including, but not limited to, chronic pain, neuropathetic pain and reflex sympathetic dystrophy, and other disorders such as narcolepsy, chronic fatigue syndrome, fibromyalgia, seasonal affective disorder and premenstrual syndrome, while avoiding adverse affects associated with racemic bupropion.
Owner:SEPACOR INC
Combination therapies for the treatment of obesity
Described are pharmaceutical compositions comprising bupropion, metformin, topiramate, and at least one pharmaceutically acceptable carrier or excipient. Another aspect of the present invention relates to a method of treating a patient suffering from obesity or needing to lose weight, comprising the step of co-administering to said patient a therapeutically effective amount of bupropion, metformin, and topiramate. In certain embodiments, an aforementioned method is practiced in conjunction or tandem with a medical procedure or the use of a medical device or both.
Owner:METABOLOUS PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com